WO2012043488A1 - ポリエステルの製造方法 - Google Patents
ポリエステルの製造方法 Download PDFInfo
- Publication number
- WO2012043488A1 WO2012043488A1 PCT/JP2011/071910 JP2011071910W WO2012043488A1 WO 2012043488 A1 WO2012043488 A1 WO 2012043488A1 JP 2011071910 W JP2011071910 W JP 2011071910W WO 2012043488 A1 WO2012043488 A1 WO 2012043488A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- polyester
- mass
- contact
- acid
- esterification reaction
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G63/00—Macromolecular compounds obtained by reactions forming a carboxylic ester link in the main chain of the macromolecule
- C08G63/88—Post-polymerisation treatment
- C08G63/90—Purification; Drying
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/18—Stationary reactors having moving elements inside
- B01J19/1862—Stationary reactors having moving elements inside placed in series
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/18—Stationary reactors having moving elements inside
- B01J19/20—Stationary reactors having moving elements inside in the form of helices, e.g. screw reactors
Definitions
- the present invention relates to a method for producing a polyester mainly comprising an aliphatic diol and an aliphatic dicarboxylic acid. More specifically, the present invention relates to a method for producing a polyester having a low oligomer content such as a cyclic dimer.
- Polyester having aliphatic diol represented by polybutylene succinate and aliphatic dicarboxylic acid as main raw materials (hereinafter sometimes abbreviated as “polyester”), the raw material can be obtained from plant resources, Since it has good physical properties and degradability, it is processed into products such as agricultural materials, civil engineering materials, vegetation materials, and packaging materials, and is expected to be widely used.
- Patent Document 1 describes a method for reducing the CD content by treating polyester with acetone.
- Patent Document 2 describes a method for reducing the CD content with a water / alcohol mixture containing alcohol as a main component of alcohol in the state of polyester, powder, pellets, or molded article. Methanol and isopropanol are described as applicable alcohols. Furthermore, a treatment method using a water / alcohol mixture mainly composed of water is described.
- polyester When the polyester is treated with acetone to reduce the CD content, problems such as an unpleasant odor due to the acetone remaining in the treated pellets may occur. There was a problem of requiring. Further, when polyester is treated with alcohol or a water / alcohol mixture containing alcohol as a main component in the form of powder, pellets or molded products, particularly when methanol or isopropanol is used as alcohol, the content of CD The reduction effect is not always satisfactory, and there are problems such as a long processing time. Further, when isopropanol is used, there is a problem that isopropanol tends to remain in the pellets in drying after the contact treatment, and the melt viscosity is lowered when the pellets are melt-molded.
- the present invention is a method for producing a polyester having a reduced CD content and elution amount, and produces a polyester having good moldability with little deterioration in physical properties of the polyester even after the production method. It is an object of the present invention to provide a method for producing polyester that can be used.
- the present inventors have mixed polyester pellets obtained by pelletizing polyester obtained through an esterification reaction step in which an aliphatic diol and an aliphatic dicarboxylic acid are reacted. That the CD contained in the polyester can be efficiently removed by contact treatment with a mixed solution containing ethanol and water containing 10% by mass to 99% by mass of water with respect to the entire solution.
- the headline and the present invention have been completed.
- the gist of the present invention resides in the following [1] to [7].
- An esterification reaction step of reacting an aliphatic diol with an aliphatic dicarboxylic acid, a step of pelletizing polyester obtained through the esterification reaction step, a polyester pellet obtained, and ethanol and water are contained.
- a method for producing a polyester comprising: a contact treatment step for bringing a mixed solution into contact with the polyester, wherein the mixed solution contains 10% by mass to 99% by mass of water with respect to the entire mixed solution.
- the present invention it is possible to provide a method for producing a polyester having a reduced CD content and an elution amount, and having excellent polyester physical properties and also good moldability.
- mass% “mass ppm”, and “mass part” and “wt%”, “weight ppm”, and “part by weight” have the same meaning, respectively.
- the polyester obtained through an esterification reaction step in which at least an aliphatic diol and an aliphatic dicarboxylic acid are reacted is pelletized, and the resulting polyester pellet is mixed with a mixed solution containing ethanol and water.
- a manufacturing method of polyester which has the contact treatment process made to contact Comprising: It is a manufacturing method of polyester in which this liquid mixture contains 10 mass% or more and 99 mass% or less of water with respect to the whole liquid mixture.
- the production method of the present invention has an esterification reaction step in which at least an aliphatic diol and an aliphatic dicarboxylic acid are reacted as main raw materials, but “with an aliphatic diol and an aliphatic dicarboxylic acid as main raw materials”
- the diol component used as a main component is an aliphatic diol
- the dicarboxylic acid component used as a raw material is a main component containing an aliphatic dicarboxylic acid.
- the total of aliphatic diols is preferably 50 mol% or more, more preferably 60 mol% or more, still more preferably, based on the total of raw material diols. It is 70 mol% or more, and particularly preferably 90 mol% or more.
- “having an aliphatic dicarboxylic acid as a main raw material” means having at least one of an aliphatic dicarboxylic acid and an aliphatic dicarboxylic acid alkyl ester as a main component
- “The main component is at least one of the aliphatic dicarboxylic acid alkyl esters” means that the total molar ratio of the aliphatic dicarboxylic acid and the aliphatic dicarboxylic acid alkyl ester is the highest among the total of the raw material dicarboxylic acid and the dicarboxylic acid alkyl ester. It means "much".
- the total of the aliphatic dicarboxylic acid and the aliphatic dicarboxylic acid alkyl ester is preferably 50 mol% or more based on the total of the raw material dicarboxylic acids, and more preferably, It is 60 mol% or more, more preferably 70 mol% or more, and particularly preferably 90 mol% or more.
- each reaction step in producing the polyester can be carried out by a batch method or a continuous method.
- the raw materials are continuously supplied, and the polyester is continuously produced. The so-called continuous method for obtaining is preferred.
- ⁇ Diol component As the diol component used in the present invention, as described above, at least an aliphatic diol is used, and if the total molar ratio is most frequently used in the raw material diol, those usually used for a polyester raw material are used without any particular limitation. be able to.
- ethylene glycol 1,2-propanediol, 1,3-propanediol, 1,4-butanediol, 1,5-pentanediol, 1,6-hexanediol, 1,7- Hexanediol, 1,8-octanediol, 1,9-nonanediol, 1,10-decanediol, alkylene diols such as neopentyl glycol, oxyalkylenes such as diethylene glycol, polyethylene glycol, polypropylene glycol, and polytetramethylene ether glycol And cycloalkylene diols such as diol, 1,2-cyclohexane diol, 1,4-cyclohexane diol, 1,2-cyclohexane dimethanol, 1,4-cyclohexane dimethanol and the like.
- alkylene diols having 6 or less carbon atoms such as ethylene glycol, 1,3-propanediol and 1,4-butanediol, or cycloalkylene having 6 or less carbon atoms such as 1,4-cyclohexanedimethanol Diols are preferred.
- 1,4-butanediol is particularly preferable. Two or more of these may be used in combination.
- 1,4-butanediol When 1,4-butanediol is used as the diol component, the amount of 1,4-butanediol used is based on the total aliphatic diol from the viewpoint of the melting point (heat resistance), biodegradability, and mechanical properties of the resulting polyester. It is preferably 50 mol% or more, more preferably 70 mol% or more, and particularly preferably 90 mol% or more. Further, ethylene glycol, 1,3-propanediol and 1,4-butanediol can be derived from plant raw materials.
- ⁇ Dicarboxylic acid component As the dicarboxylic acid component used in the present invention, as described above, at least aliphatic dicarboxylic acid is used, and if the total molar ratio is most frequently used in the raw material carboxylic acid, those that are usually used for the raw material of the polyester are particularly limited. It can be used without.
- oxalic acid More specifically, for example, oxalic acid, malonic acid, succinic acid, succinic anhydride, glutaric acid, adipic acid, pimelic acid, suberic acid, azelaic acid, sebacic acid, undecadicarboxylic acid, dodecadicarboxylic acid, dimer acid
- Aromatic dicarboxylic acids such as aliphatic dicarboxylic acids such as hexahydrophthalic acid, hexahydroisophthalic acid, hexahydroterephthalic acid, etc.
- succinic acid and succinic anhydride are preferred in view of the physical properties of the resulting polyester.
- Aliphatic dicarboxylic acids such as acids, adipic acid and sebacic acid are preferred.
- aliphatic dicarboxylic acids having 4 or less carbon atoms such as succinic acid and succinic anhydride are preferable. Two or more of these may be used in combination.
- the amount of succinic acid used is 50 mol% or more based on the total aliphatic dicarboxylic acid from the viewpoint of the melting point (heat resistance), biodegradability and mechanical properties of the resulting polyester. It is preferably 70 mol% or more, particularly preferably 90 mol% or more.
- aromatic dicarboxylic acid as dicarboxylic acid components other than aliphatic dicarboxylic acid. Specific examples of the aromatic dicarboxylic acid include terephthalic acid, isophthalic acid, naphthalenedicarboxylic acid, and diphenyldicarboxylic acid.
- succinic acid succinic anhydride, adipic acid, etc. can use the thing derived from a plant raw material.
- the polyester of the present invention may be copolymerized with other components other than aliphatic diols and aliphatic dicarboxylic acids.
- copolymer components that can be used in this case include lactic acid, glycolic acid, hydroxybutyric acid, hydroxycaproic acid, 2-hydroxy3,3-dimethylbutyric acid, 2-hydroxy-3-methylbutyric acid, and 2-hydroxyisocaprone.
- Oxycarboxylic acids such as acids, malic acid, maleic acid, citric acid and fumaric acid, and esters or lactones of these oxycarboxylic acids, oxycarboxylic acid polymers, etc., or trifunctional or more functional groups such as glycerin, trimethylolpropane and pentaerythritol Or polyhydric carboxylic acids having three or more functional groups such as propanetricarboxylic acid, pyromellitic acid, trimellitic acid benzophenone tetracarboxylic acid and anhydrides thereof, or the like.
- tri- or higher-functional oxycarboxylic acid, tri- or higher-functional alcohol, tri- or higher-functional carboxylic acid, and the like can be easily obtained by adding a small amount of polyester.
- oxycarboxylic acids such as malic acid, citric acid, and fumaric acid are preferable, and malic acid is particularly preferably used.
- the trifunctional or higher functional compound is preferably 0.001 to 5 mol%, more preferably 0.05 to 0.5 mol%, based on the total dicarboxylic acid component. If the upper limit of this range is exceeded, gel (unmelted product) is likely to be formed in the obtained polyester, and if it is less than the lower limit, it is possible to increase the viscosity of the resulting polyester (usually by using a polyfunctional compound). ) Is difficult to obtain.
- the lower limit of the intrinsic viscosity (IV, dL / g) of the polyester pellets to be contact-treated with a mixed solution containing ethanol and water is preferably 1.4 dL / g, particularly preferably 1.6 dL. / G.
- the upper limit is preferably 2.8 dL / g, more preferably 2.5 dL / g, and particularly preferably 2.3 dL / g.
- the amount of terminal carboxyl groups of the polyester subjected to contact treatment with a mixed solution containing ethanol and water is usually 80 (equivalent / ton) or less, preferably 60 (equivalent / ton) or less, more preferably 40 (equivalent). / Ton) or less, particularly preferably 25 (equivalent / ton) or less.
- the lower the lower limit the better the thermal stability and hydrolysis resistance, but it is usually 5 (equivalent / ton) or more.
- the upper limit is exceeded, the viscosity drop due to hydrolysis becomes remarkable, and the quality may be significantly impaired.
- polyester pellets are continuously obtained.
- the method is not limited to the continuous method as long as the effects of the present invention are not hindered, and conventionally known polyester production methods can be employed.
- the polyester pellets are contact-treated with a mixed solution containing ethanol and water and then dried.
- the polyester to be used is produced through an esterification reaction step in which at least a dicarboxylic acid component and a diol component are reacted.
- the esterification reaction step and other subsequent steps can be carried out in a plurality of continuous reaction tanks or in a single reaction tank, but in order to reduce fluctuations in the physical properties of the resulting polyester, It is preferable to carry out in the reaction tank.
- the reaction temperature in the esterification reaction step is not particularly limited as long as it is a temperature at which the esterification reaction can be performed, but is preferably 200 ° C. or higher, more preferably 210 ° C. in that the reaction rate can be increased. In order to prevent the coloring of the polyester and the like, it is preferably 250 ° C. or lower, more preferably 245 ° C. or lower, and particularly preferably 240 ° C. or lower. If the reaction temperature is too low, the esterification reaction rate is slow, requiring a long reaction time, and undesirable reactions such as dehydration decomposition of aliphatic diols increase.
- the esterification temperature is preferably a constant temperature.
- the esterification rate is stabilized due to the constant temperature.
- the constant temperature is a set temperature ⁇ 5 ° C., preferably ⁇ 2 ° C.
- the reaction atmosphere is preferably an inert gas atmosphere such as nitrogen or argon.
- the reaction pressure is preferably 50 kPa to 200 kPa, more preferably 60 kPa or more, further preferably 70 kPa or more, more preferably 130 kPa or less, and still more preferably 110 kPa or less. If it is less than the lower limit, the amount of scattered matter in the reaction tank increases and the haze of the reaction product increases, which tends to cause an increase in foreign matter, and the distillation of the aliphatic diol to the outside of the reaction system increases and the polycondensation reaction rate tends to decrease. . If the upper limit is exceeded, the dehydration decomposition of the aliphatic diol increases, and the polycondensation rate tends to decrease.
- the reaction time is preferably 1 hour or longer, and the upper limit is preferably 10 hours or shorter, more preferably 4 hours or shorter.
- the molar ratio of the aliphatic diol component to the aliphatic dicarboxylic acid component in which the esterification reaction is performed is based on the aliphatic dicarboxylic acid and the esterified aliphatic dicarboxylic acid present in the gas phase and the reaction liquid phase of the esterification reaction tank.
- the fact that it is decomposed and does not contribute to the esterification reaction does not include, for example, those obtained by decomposing 1,4-butanediol, which is an aliphatic diol, into tetrahydrofuran, in this molar ratio.
- the lower limit of the molar ratio is usually 1.10 or more, preferably 1.12 or more, more preferably 1.15 or more, and particularly preferably 1.20 or more.
- the upper limit is usually 2.00 or less, preferably 1.80 or less, more preferably 1.60 or less, and particularly preferably 1.55 or less. If it is less than the lower limit, the esterification reaction tends to be insufficient, and the polycondensation reaction which is a reaction in the subsequent step is difficult to proceed, and it is difficult to obtain a polyester having a high degree of polymerization.
- the upper limit is exceeded, the decomposition amount of the aliphatic diol and the aliphatic dicarboxylic acid tends to increase. In order to keep this molar ratio within a preferable range, it is a preferable method to appropriately supplement the esterification reaction system with an aliphatic diol.
- an esterification reaction product having an esterification rate of 80% or more is subjected to a polycondensation reaction.
- the polycondensation reaction means a high molecular weight reaction of polyester carried out at a reaction pressure of 50 kPa or less
- the esterification reaction is 50 to 200 kPa, usually carried out in an esterification reaction tank
- the polycondensation reaction is preferably 50 kPa or less, preferably Is carried out in a polycondensation reaction tank at 10 kPa or less.
- the esterification rate indicates the ratio of the esterified acid component to the total acid component in the esterification reaction product sample, and is represented by the following formula.
- Esterification rate (%) (saponification value ⁇ acid value) / saponification value ⁇ 100
- the esterification rate of the esterification reaction product is preferably 85% or more, more preferably 88% or more, and particularly preferably 90% or more. If it is less than the lower limit, the polycondensation reactivity, which is a reaction in the subsequent step, is deteriorated. Further, the amount of scattered matter during the polycondensation reaction increases, adheres to the wall surface and solidifies, and further, this scattered matter falls into the reaction material, causing haze deterioration (foreign matter generation).
- the upper limit is preferably 99% for the polycondensation reaction, which is a post-process reaction, but is usually 99%.
- the haze is reduced by performing a continuous reaction with the molar ratio of dicarboxylic acid and diol in the esterification reaction, reaction temperature, reaction pressure and reaction rate within the above ranges and continuously subjecting to a polycondensation reaction.
- a high-quality polyester with few foreign substances can be obtained efficiently.
- ⁇ Polycondensation reaction step> In the production of the polyester used in the contacting step of the present invention, it is preferable to carry out the polycondensation reaction in the polycondensation reaction step following the esterification reaction step.
- the polycondensation reaction can be performed under reduced pressure using a plurality of continuous reaction vessels.
- the reaction pressure of the final polycondensation reaction tank the lower limit is usually 0.01 kPa or more, preferably 0.03 kPa or more, and the upper limit is usually 1.4 kPa or less, preferably 0.4 kPa or less.
- the method of producing using an ultra-high vacuum polycondensation equipment with a reaction pressure of less than 0.01 kPa is a preferred embodiment from the viewpoint of improving the polycondensation reaction rate, but requires extremely expensive equipment investment. Therefore, it is economically disadvantageous.
- the lower limit of the reaction temperature is usually 215 ° C, preferably 220 ° C
- the upper limit is usually 270 ° C, preferably 260 ° C. If it is less than the lower limit, the polycondensation reaction rate is slow, and not only a long time is required for producing a polyester having a high degree of polymerization, but also a high power stirrer is required, which is economically disadvantageous.
- the amount exceeds the upper limit thermal decomposition of the polyester during production tends to be caused, and it tends to be difficult to produce polyester having a high degree of polymerization.
- the lower limit of the reaction time is usually 1 hour, and the upper limit is usually 15 hours, preferably 10 hours, more preferably 8 hours. If the reaction time is too short, the reaction is insufficient and it is difficult to obtain a polyester having a high degree of polymerization, and the mechanical properties of the molded product tend to be inferior. On the other hand, if the reaction time is too long, the molecular weight drop due to the thermal decomposition of the polyester becomes remarkable, the mechanical properties of the molded product tend to be inferior, and the terminal amount of the carboxyl group that adversely affects the durability of the polyester is thermally decomposed. May increase due to
- Polyester having a desired intrinsic viscosity can be obtained by controlling the polycondensation reaction temperature, time and pressure.
- ⁇ Reaction catalyst> The esterification reaction and polycondensation reaction are promoted by using a reaction catalyst.
- a sufficient reaction rate can be obtained without an esterification reaction catalyst.
- the catalyst may cause insoluble precipitates in the reaction product due to water generated by the esterification reaction, which may impair the transparency of the resulting polyester (ie, increase haze).
- it since it may become a foreign material, it is preferable not to use the reaction catalyst during the esterification reaction.
- the catalyst is added to the gas phase part of the reaction vessel, the haze may be increased, and the catalyst may be converted into a foreign substance. Therefore, it is preferable to add the catalyst to the reaction solution.
- a catalyst In the polycondensation reaction, it is difficult to proceed without a catalyst, and it is preferable to use a catalyst.
- a catalyst As the polycondensation reaction catalyst, a compound containing at least one of the metal elements of Groups 1 to 14 of the periodic table is generally used. Specific examples of metal elements include scandium, yttrium, samarium, titanium, zirconium, vanadium, chromium, molybdenum, tungsten, tin, antimony, cerium, germanium, zinc, cobalt, manganese, iron, aluminum, magnesium, calcium, Examples include strontium, sodium and potassium.
- scandium, yttrium, titanium, zirconium, vanadium, molybdenum, tungsten, zinc, iron, and germanium are preferable, and titanium, zirconium, tungsten, iron, and germanium are particularly preferable.
- a metal element belonging to Group 3 to 6 of the periodic table showing Lewis acidity is preferable.
- scandium, titanium, zirconium, vanadium, molybdenum, and tungsten are preferable, and titanium and zirconium are particularly preferable from the viewpoint of availability, and titanium is more preferable from the viewpoint of reaction activity.
- a compound containing an organic group such as a carboxylate salt, an alkoxy salt organic sulfonate salt or a ⁇ -diketonate salt containing these metal elements, and the above-described metal oxide, halide, etc.
- organic groups such as a carboxylate salt, an alkoxy salt organic sulfonate salt or a ⁇ -diketonate salt containing these metal elements, and the above-described metal oxide, halide, etc.
- Inorganic compounds and mixtures thereof are preferably used as a catalyst.
- the catalyst is preferably a liquid at the time of polymerization or a compound that dissolves in an ester low polymer or polyester because the polymerization rate is increased when the catalyst is melted or dissolved at the time of polymerization.
- the polycondensation is preferably carried out without a solvent. Alternatively, a small amount of solvent may be used to dissolve the catalyst.
- Examples of the solvent for dissolving the catalyst include alcohols such as methanol, ethanol, isopropanol, and butanol, diols such as ethylene glycol, butanediol, and pentanediol, ethers such as diethyl ether and tetrahydrofuran, and nitriles such as acetonitrile. , Hydrocarbon compounds such as heptane and toluene, water and mixtures thereof, and the like. The amount used is such that the catalyst concentration is usually 0.0001 mass% or more and 99 mass% or less.
- a tetraalkyl titanate and a hydrolyzate thereof are preferable.
- tetra-n-propyl titanate, tetraisopropyl titanate, tetra-n-butyl titanate, tetra-t-butyl titanate, tetraphenyl titanate examples include tetracyclohexyl titanate, tetrabenzyl titanate and mixed titanates thereof, and hydrolysates thereof.
- titanium (oxy) acetylacetonate, titanium tetraacetylacetonate, titanium (diisoproxide) acetylacetonate, titanium bis (ammonium lactate) dihydroxide, titanium bis (ethylacetoacetate) diisopropoxide, titanium (triethanolaminate) ) Isopropoxide, polyhydroxytitanium stearate, titanium lactate, titanium triethanolamate, and butyl titanate dimer are also preferably used.
- alcohol a Group 2 metal compound in the long-period periodic table (Nomenclature of Inorganic Chemistry IUPAC Recommendations 2005) (hereinafter sometimes referred to as a Group 2 metal compound in the long-period periodic table), a phosphate ester compound, and A liquid material obtained by mixing a titanium compound is also used.
- tetra-n-propyl titanate, tetraisopropyl titanate and tetra-n-butyl titanate titanium (oxy) acetylacetonate, titanium tetraacetylacetonate, titanium bis (ammonium lactate) dihydroxide, polyhydroxytitanium stearate
- tetra-n-butyl titanate polyhydroxy titanium stearate, titanium ( Oxy) acetylacetonate, titanium tetraacetylacetonate, and a liquid obtained by mixing alcohol, a Group 2 metal compound in a long-period periodic table, a phosphate ester compound, and a titanium compound are preferred.
- zirconium compound examples include zirconium tetraacetate, zirconium acetylate hydroxide, zirconium tris (butoxy) stearate, zirconyl diacetate, zirconium oxalate, zirconyl oxalate, potassium potassium oxalate, and polyhydroxyzirconium.
- germanium compounds include inorganic germanium compounds such as germanium oxide and germanium chloride, and organic germanium compounds such as tetraalkoxygermanium. From the viewpoint of price and availability, germanium oxide, tetraethoxygermanium and mu and tetrabutoxygermanium are preferable, and germanium oxide is particularly preferable.
- iron compound examples include inorganic chlorides such as ferric chloride, inorganic oxides such as triiron tetroxide, and organic iron complexes such as ferrocene. Of these, inorganic oxides are preferred.
- metal-containing compounds include scandium carbonate, scandium acetate, scandium chloride, scandium compounds such as scandium acetylacetonate, yttrium carbonate, yttrium chloride, yttrium acetate, yttrium acetate such as yttrium acetylacetonate, vanadium chloride, and vanadium trichloride.
- the lower limit is usually 0.1 mass ppm or more, preferably 0.5 mass ppm or more, more preferably 1 as the metal amount relative to the polyester to be produced.
- the upper limit is usually 3000 ppm or less, preferably 1000 ppm or less, more preferably 250 ppm or less, and particularly preferably 130 ppm or less. If too much catalyst is used, it is not only economically disadvantageous, but the reason is not yet clear, but the carboxyl group terminal concentration in the polyester may increase, so the carboxyl group terminal amount and residual catalyst concentration. The increase in the temperature may reduce the thermal stability and hydrolysis resistance of the polyester. On the other hand, if the amount is too small, the polymerization activity is lowered, and accordingly, thermal decomposition of the polyester is induced during the production of the polyester, making it difficult to obtain a polyester having practically useful physical properties.
- the position of addition of the catalyst to the reaction system is not particularly limited as long as it is before the polycondensation reaction step, and may be added at the time of charging the raw material, but the catalyst is used in a situation where a lot of water is present or generated. If it coexists, the catalyst is deactivated and foreign matter may be precipitated, which may impair the quality of the product. Therefore, it is preferably added after the esterification reaction step.
- ⁇ Reaction tank> As the esterification reaction tank used in the present invention, known ones can be used, and any of a vertical stirring complete mixing tank, a vertical heat convection mixing tank, a tower type continuous reaction tank, etc. may be used. A single tank or a plurality of tanks in which similar or different tanks are connected in series may be used. Among them, a reaction tank having a stirrer is preferable.
- a stirrer in addition to a normal type including a power unit, a receiver, a shaft, and a stirring blade, a turbine stator type high-speed rotating stirrer, a disk mill type stirrer, a rotor mill type stirrer, and the like The type that rotates at a high speed can also be used.
- stirring there is no limitation on the form of stirring, and in addition to the normal stirring method in which the reaction liquid in the reaction tank is directly stirred from the top, bottom, side, etc. of the reaction tank, a part of the reaction liquid is piped outside the reaction tank, etc.
- the reaction solution can be circulated using a line mixer and stirred with a line mixer.
- stirring blades can be selected, and specific examples include propeller blades, screw blades, turbine blades, fan turbine blades, disk turbine blades, fouller blades, full zone blades, Max blend blades, and the like.
- the type of the polycondensation reaction tank used in the present invention is not particularly limited, and examples thereof include a vertical stirring polymerization tank, a horizontal stirring polymerization tank, and a thin film evaporation polymerization tank.
- the number of polycondensation reaction tanks can be one, or a plurality of tanks of the same or different types can be connected in series. It is preferable to select a horizontal stirring polymerization machine having a thin film evaporation function excellent in renewability, plug flow property, and self-cleaning property.
- the polyester obtained through the polycondensation reaction is pelletized and contact-treated with a mixed solution containing ethanol and water in the form of pellets.
- the pelletizing method is a strand cutting method in which molten polyester is extruded from a nozzle hole of a die head using a gear pump or an extruder, and cooled or solidified with water or the like, and the strand is cut with a cutter.
- An underwater hot cut method that cuts immediately in a molten state is widely used. In the polyester of the present invention, underwater hot cut because there are few chips in the pellet, the angle of repose of the resulting pellet is low, the pellet transport stability, and the stability of the feed to the molding machine during molding are good A method is preferably employed.
- the lower limit of the cooling water temperature in the underwater cut method is preferably 10 ° C or higher, more preferably 20 ° C or higher, and the upper limit is preferably 70 ° C or lower, more preferably 60 ° C or lower, and further preferably 50 ° C or lower.
- the size of the nozzle hole is usually one having a hole diameter of 1 mm to 30 mm.
- the shape of the opening is not particularly limited, but a shape such as a circle, an ellipse, an oval, a square, or a star is used.
- the discharge amount per opening is usually 5 to 100 kg / hour, preferably 10 to 70 kg / hour, more preferably 20 to 50 kg / hour.
- the shape of the pellet includes a spherical shape, a cylindrical shape, an elliptical columnar shape, a long cylindrical shape, a prismatic shape, a jasper shape, etc., and those in which these are flattened.
- the underwater hot cut method often has a spherical shape, a jade shape and a flat shape thereof.
- the weight per pellet is 1 to 50 mg, preferably 3 to 40 mg, more preferably from the viewpoint of the extraction efficiency of CD by contact treatment, the drying efficiency of the drying process, pellet transfer operability, etc. Is 5 to 30 mg.
- a larger pellet surface area per mass is preferable from the viewpoint of extraction efficiency in the contact treatment step.
- ⁇ Contact treatment process> (Mixture containing ethanol and water)
- the obtained pellet is treated with a mixed solution containing ethanol and water (hereinafter sometimes referred to as contact treatment), and the CD contained in the pellet is extracted into the mixed solution. This reduces the CD content in the pellet.
- the contact treatment step is usually performed continuously after pelletization, but the obtained pellets may be temporarily stored in a storage tank and then contact-treated.
- the ratio of water to the entire mixed liquid containing ethanol and water to be brought into contact with the polyester pellets is usually 10% by mass or more, preferably 20% by mass or more, more preferably 25% by mass. % Or more. Moreover, it is 99 mass% or less normally, Preferably it is 95 mass% or less, More preferably, it is 90 mass% or less, Most preferably, it is 85 mass% or less.
- the polyester quality tends to decrease due to a decrease in molecular weight due to alcohol decomposition.
- the proportion of alcohol used is increased, handling is required from the viewpoint of safety, such as the risk of explosion of the liquid used and the gas generated from the liquid increases.
- the ratio of water exceeds the upper limit, oligomer removal is not sufficient, the CD content cannot be sufficiently reduced, and a polyester having a desired quality may not be obtained.
- the contact treatment liquid used in the present invention can contain isopropanol.
- the content is usually 20% by mass or less, preferably 15% by mass or less, and more preferably 10% by mass or less. If the isopropanol content exceeds the upper limit, oligomer removal may not be sufficient, and a desired quality polyester may not be obtained.
- the lower limit of the temperature of the contact liquid for bringing the polyester pellets into contact with the contact treatment liquid is 25 ° C, preferably 30 ° C, more preferably 35 ° C, and particularly preferably 40 ° C.
- the upper limit is usually the melting point of the polyester, preferably 95 ° C, more preferably 90 ° C, particularly preferably 85 ° C.
- the contact temperature is less than the lower limit, a long treatment time is required, which is not only economically disadvantageous, but also a desirable polyester may not be obtained due to a decrease in the oligomer removal effect.
- the contact temperature exceeds the upper limit, the viscosity decreases greatly due to hydrolysis and alcohol decomposition, which not only impairs the quality, but also causes difficulty in operation, such as causing fusion between pellets and defective pellet extraction.
- the lower limit of the time for contacting the polyester pellet and the contact treatment liquid is usually 0.1 hour, preferably 1 hour, more preferably 3 hours.
- the upper limit is usually 24 hours, preferably 18 hours, and more preferably 10 hours. If the contact time is less than the lower limit, oligomer removal may not be sufficient, and a polyester of desired quality may not be obtained. On the other hand, when the contact time exceeds the upper limit, the decrease in viscosity increases due to hydrolysis and alcohol decomposition, which may impair quality.
- the lower limit of the ratio of the polyester pellets to be contacted and the contact treatment liquid is usually 1.0 or more, preferably 1.5 or more, and more preferably 2.0 or more in terms of mass ratio.
- the upper limit is usually 50 or less, preferably 30 or less, more preferably 20 or less.
- the mass ratio of the pellet to be contacted exceeds the upper limit, it is disadvantageous in terms of process and cost, such as an increase in the size of equipment due to the large amount of contact treatment liquid used and an increase in the cost of the contact treatment liquid.
- Contact method As an aspect which makes a polyester pellet and a contact processing liquid contact, there exist a batch type and a continuous type, and any aspect is employable.
- a batch-type aspect in this invention the method of extracting after putting a pellet and a contact processing liquid into a processing tank and carrying out contact processing for predetermined temperature and time is mentioned. While the pellets and the contact treatment liquid are contact-treated, the contact treatment liquid can be circulated or non-circulated.
- the pellets are continuously supplied to the pipe or the treatment tank, and the contact treatment liquid at a predetermined temperature is brought into contact with the flow of the pellets in parallel or countercurrent, and the predetermined contact is made. There is a method of continuously extracting pellets while maintaining time.
- the contact treatment liquid after the contact treatment can be recovered by distillation and recycled.
- the oligomers containing the extracted cyclic dimer are separated from the contact treatment liquid by cooling or the like, or concentrated, and then supplied to the esterification reaction process or the polycondensation reaction process, so that the polyester Can be used as a raw material.
- the esterification reaction tank in the esterification reaction step or the slurry tank of aliphatic dicarboxylic acid and aliphatic diol is a preferable method.
- the extracted contact treatment liquid can be recycled as it is, or a part of the extracted contact treatment liquid can be discarded and a new amount of contact treatment liquid added. You can also.
- the oligomer containing CD separated from the contact treatment liquid by distillation concentration and / or cooling is once melted or dissolved in an aliphatic diol as a raw material, and then used as a raw material for polyester. It can be recovered.
- the recovered oligomers can be directly supplied to the esterification reaction tank, or can be supplied to the aliphatic diol recycle line (2) and the esterification reaction product extraction line (4) illustrated in FIG. it can. Moreover, it can also supply to the polycondensation tank (a) illustrated in FIG. Moreover, it can also supply to the slurry tank of aliphatic dicarboxylic acid and aliphatic diol.
- the contact-treated polyester pellets contain a contact treatment solution such as ethanol and water, they are dried in a drying step in order to remove them.
- the dryer used in the drying process circulates an inert gas such as heated air or heated nitrogen as a drying gas, a shelf-type dryer, a band dryer, a horizontal cylindrical rotary dryer, a horizontal dryer with rotary blades, There are vertical dryers with rotating blades (so-called hopper dryer type dryers), moving bed type vertical dryers, fluidized bed type dryers, and the like.
- hopper dryer type dryers there are vertical dryers with rotating blades (so-called hopper dryer type dryers), moving bed type vertical dryers, fluidized bed type dryers, and the like.
- a dryer different from the above gas distribution method there are a double cone type rotary vacuum dryer, a tumbler type rotary vacuum dryer, a microwave dryer and the like.
- the lower limit of the drying temperature as the gas temperature is 25 ° C, preferably 30 ° C, more preferably 35 ° C, and particularly preferably 40 ° C.
- the upper limit is usually the melting point of the polyester, preferably the melting point of the polyester minus 5 ° C, more preferably the melting point of the polyester minus 8 ° C, and particularly preferably the melting point of the polyester minus 10 ° C. If the temperature is lower than the lower limit, a long time is required for drying, which is economically disadvantageous. On the other hand, when the temperature exceeds the upper limit, it may be difficult to operate such as fusing the pellets together or causing unsatisfactory extraction when the pellets are extracted from the dryer.
- the dry gas that has passed through the drier contains contact treatment liquid components such as water and ethanol, and the contact treatment liquid components can be reduced by cooling or adsorption of the dry gas and reused as the dry gas.
- the drying time is usually 0.1 to 100 hours, preferably 1 to 80 hours, more preferably 5 to 50 hours.
- the flow rate of the drying gas is usually 0.05 to 1.0 m / sec (superficial velocity).
- the pellet after drying has an upper limit of ethanol content of usually 1000 ppm by mass, preferably 800 ppm by mass, more preferably 500 ppm by mass.
- the amount of ethanol contained is large, a decrease in melt viscosity tends to be remarkably inferior in moldability when the present pellet is melt-molded. The lower the lower the better, the better, but the concentration that is reasonably obtained industrially is usually 50 ppm by mass.
- the moisture content of the pellets after drying is less than the ethanol content.
- the moisture content of the pellet is 1000 mass ppm or less, preferably 500 mass ppm or less, more preferably 250 mass ppm or less.
- the moisture content is 1000 mass ppm or less, preferably 500 mass ppm or less, more preferably 250 mass ppm or less.
- BG 1,4-butanediol
- malic acid as a polyfunctional compound
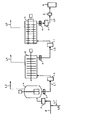
- FIG. 1 is an explanatory diagram of an example of an esterification reaction step employed in the present invention
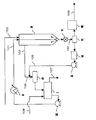
- FIG. 2 is an explanatory diagram of an example of a polycondensation step employed in the present invention.
- succinic acid and malic acid which are raw materials
- BG a raw material mixing tank
- A esterification reaction tank
- the catalyst supply line (3) is connected to the recycle 1,4-butanediol recycle line (2), mixed and then fed to the liquid phase part of the esterification reaction tank (A). showed that.
- the gas distilled from the esterification reaction tank (A) is separated into a high-boiling component and a low-boiling component in the rectifying column (C) through the distillation line (5).
- the main component of the high boiling point component is 1,4-butanediol
- the main component of the low boiling point component is water and tetrahydrofuran which is a decomposition product of BG (hereinafter sometimes abbreviated as THF).
- THF tetrahydrofuran which is a decomposition product of BG
- the condenser (G) is connected to an exhaust device (not shown) via a vent line (14).
- the esterification reaction product generated in the esterification reaction tank (A) passes through the extraction pump (B) and the extraction line (4) of the esterification reaction product, and the first polycondensation reaction tank (a) shown in FIG. To be served.
- the catalyst supply line (3) is connected to the recirculation line (2), but both may be independent.
- the raw material supply line (1) may be connected to the liquid phase part of the esterification reaction tank (A).
- a catalyst is added to the esterification reaction product before the polycondensation tank, it is prepared to a predetermined concentration in a catalyst preparation tank (not shown), and then passed through a catalyst supply line (L7) in FIG. ) And further diluted with BG, and then supplied to the esterification reaction product extraction line (4).
- the esterification reaction product supplied to the first polycondensation reaction tank (a) from the extraction line (4) of the esterification reaction product through the filter (p) is polycondensed under reduced pressure to reduce the polyester low weight. Thereafter, they are combined and supplied to the second polycondensation reaction tank (d) through the extraction gear pump (c), the extraction line (L1), and the filter (q). In the second polycondensation reaction tank (d), the polycondensation reaction usually proceeds further at a lower pressure than in the first polycondensation reaction tank (a).
- the obtained polycondensate is supplied to the third polycondensation tank (k) through the extraction gear pump (e), the extraction line (L3) serving as the outlet channel, and the filter (r).
- the third polycondensation reaction tank (k) is a horizontal reaction tank composed of a plurality of stirring blade blocks and equipped with a biaxial self-cleaning type stirring blade.
- the polycondensation reaction product introduced from the second polycondensation reaction tank (d) to the third polycondensation reaction tank (k) through the extraction line (L3) is further pelletized after the polycondensation reaction is further advanced here. It is transferred to the process.
- molten polyester is discharged in the form of strands melted from the die head (g) through the extraction gear pump (m), the outlet channel filter (s) and the extraction line (L5). After being extracted and cooled with water or the like, it is cut with a rotary cutter (h) to form polyester pellets. Further, it can be extracted in the form of a strand into the water without being extracted into the atmosphere, and cut into a pellet by a rotary underwater cutter.
- Reference numerals (L2), (L4), and (L6) in FIG. 2 denote vents of the first polycondensation reaction tank (a), the second polycondensation reaction tank (d), and the third polycondensation reaction tank (k), respectively.
- the filters (p), (q), (r), and (s) are not necessarily all installed, and can be appropriately installed in consideration of the foreign matter removing effect and operation stability.
- FIG. 3 is an explanatory diagram of an example of a mixed liquid contact treatment step containing ethanol and water employed in the present invention.
- Ethanol / water as the contact treatment liquid is temperature-controlled by the pump (IX) from the circulation tank (I) via the heat exchanger (II), and supplied to the treatment tank (III) from the contact treatment liquid supply line (101).
- Pellets to be subjected to the contact treatment are continuously supplied from the pellet supply line (103), contact-treated with ethanol / water for a predetermined time, and then adjusted from the extraction line (104) while adjusting the extraction amount with the rotary valve (V). Extract continuously.
- the contact treatment liquid extracted along with the pellets is separated by the preliminary solid-liquid separator (VI), passes through the recovery tank (VII), and then passes through the supply line (105) by the pump (X) to the recovery line ( 106).
- the contact treatment liquid is continuously extracted from the circulation tank (I) through the extraction line (107).
- An amount of ethanol / water equivalent to the extracted contact treatment liquid is supplied from the supply line (108).
- the pellets continuously extracted are separated from the contact treatment liquid entrained by the preliminary solid-liquid separator, and then continuously supplied to the drying process via the solid-liquid separator (VIII).
- FIG. 4 is an explanatory diagram of an example of a drying process employed in the present invention. Shown is an example provided with two drying towers (I) and (K).
- the polyester pellets after the contact treatment step are continuously supplied to the first drying tower (I) through the pellet supply line (201). Heated and dried nitrogen gas is continuously introduced into the first drying tower from the supply line (208) and discharged from the gas recovery line (207). The discharged gas is heated by the heat exchanger (N) through the condenser (L), and is circulated and used to the first drying tower through the supply line (208).
- the contact treatment liquid condensed in the condenser (L) and the heat exchanger (M) is extracted from the extraction line (210).
- New dry nitrogen gas is supplied from the new dry gas supply line (209).
- the pellets are continuously sent from the first drying tower to the cooling tower (J) through the rotary valve (O). Dry air is introduced into the cooling tower from the cooling gas supply line (212) and discharged from the cooling gas extraction line (211).
- the pellets cooled to a temperature lower than the drying temperature of the first drying tower are supplied to the second drying tower (K) through the pellet extraction line (204), the rotary valve (P), and the pellet supply line (205). .
- the drying gas normal air
- the air temperature supplied to the second drying tower is usually lower than the nitrogen gas temperature supplied to the first drying tower, for example, a nitrogen gas temperature of 80 ° C. and an air temperature of 50 ° C.
- the dried polyester pellets are continuously or intermittently extracted via a rotary valve (Q) pellet extraction line (206), and become a product through a storage tank, a fine powder remover, a packaging machine, and the like.
- the storage tank can also be used as the second drying tower. In this figure, the storage tank and the subsequent parts are not shown.
- the lower limit of the cyclic dimer content of the polyester obtained in the present invention is preferably 1 mass ppm or more, more preferably 100 mass ppm or more, still more preferably 500 mass ppm or more, and particularly preferably 1000 mass ppm or more. is there.
- the upper limit is usually 6,000 mass ppm or less, preferably 5000 mass ppm or less, more preferably 4,000 mass ppm or less, and particularly preferably 3,500 mass ppm or less. If the amount of the cyclic dimer is less than the lower limit, the quality is good, but it is economically disadvantageous because the equipment is enlarged due to the longer time required for oligomer removal.
- problems such as cloudiness (synonymous with bleeding out and whitening phenomenon) occur on the surface and the surface gloss is lost.
- the upper limit is preferably 30 ⁇ g / mL or less, more preferably 28 ⁇ g. / ML or less, more preferably 25 ⁇ g / mL or less.
- Exceeding the upper limit does not satisfy the standards for equipment and containers / packaging among the standards for food additives, etc., so application to containers and packaging for food is not permitted.
- the amount of elution is a value measured by the method shown in the Examples section.
- the polyester of the present invention has an intrinsic viscosity of 1.4 dL / g or more and 2.8 dL / g or less and a cyclic dimer content of 500 mass ppm or more and 5000 mass ppm or less. Thereby, it can become the raw material of the favorable polyester molded product which does not impair the quality after a shaping
- polyester composition An aromatic-aliphatic copolymer polyester and an aliphatic oxycarboxylic acid may be blended with the polyester of the present invention.
- other biodegradable resins such as polycaprolactone, polyamide, polyvinyl alcohol, cellulose ester, and the like, as long as the effects of the present invention are not impaired, starch Cellulose, paper, wood powder, chitin / chitosan, animal / plant substance fine powder such as coconut shell powder, walnut shell powder, or a mixture thereof can be blended.
- additives such as heat stabilizers, plasticizers, lubricants, antiblocking agents, nucleating agents, inorganic fillers, colorants, pigments, ultraviolet absorbers, light stabilizers, etc., are used for the purpose of adjusting the physical properties and processability of the molded product.
- a modifier, a crosslinking agent, etc. may be included.
- the method for producing the polyester composition of the present invention is not particularly limited, but is a method in which raw material chips of blended polyester are melt-mixed in the same extruder, a method in which each is melted in separate extruders, and a single-screw extruder is mixed. , Mixing by kneading using a normal kneader such as a twin screw extruder, Banbury mixer, roll mixer, Brabender plastograph, kneader blender, and the like. In addition, it is possible to obtain a molded body at the same time as preparing a composition by directly supplying each raw material chip to a molding machine.
- a normal kneader such as a twin screw extruder, Banbury mixer, roll mixer, Brabender plastograph, kneader blender, and the like.
- Equation (1) ((1 + 4K H ⁇ sp ) 0.5 ⁇ 1) / (2K H C) (1)
- ⁇ SP ⁇ / ⁇ 0 ⁇ 1
- ⁇ is the sample solution dropping time
- ⁇ 0 is the solvent dropping time
- C is the sample solution concentration (g / dL)
- K H Is the constant of Haggins.
- K H adopted the 0.33.
- the acid value was determined by heating 0.3 g of a sample of the esterification reaction product to 40 mL of benzyl alcohol at 180 ° C. for 20 minutes, cooling for 10 minutes, and titrating with a 0.1 mol / L potassium hydroxide / methanol solution.
- the saponification value was determined by hydrolyzing the oligomer with a 0.5 mol / L potassium hydroxide / ethanol solution and titrating with 0.5 mol / L hydrochloric acid.
- Esterification rate (%) (saponification value ⁇ acid value) / saponification value ⁇ 100 (2)
- Terminal carboxyl group amount (equivalent / ton) (ab) ⁇ 0.1 ⁇ f / W (3)
- a is the amount of benzyl alcohol solution of 0.1 mol / L sodium hydroxide required for titration ( ⁇ L)
- b is benzyl of 0.1 mol / L sodium hydroxide required for titration with a blank.
- the amount ( ⁇ L) of the alcohol solution, W is the amount (g) of the polyester sample, and f is the titer of the benzyl alcohol solution of 0.1 mol / L sodium hydroxide.
- the titer (f) of the benzyl alcohol solution of 0.1 mol / L sodium hydroxide was calculated
- the pellet was separated by filtration, and acetone was volatilized from the acetone solution from which the oligomer component was extracted to obtain a solid.
- This solid was dissolved in acetone at 50 ° C. to become a saturated solution, then slowly cooled, the supernatant was discarded, the acicular precipitate was taken out, and further purified by repeating this recrystallization operation several times.
- This acicular precipitate was confirmed to be a cyclic dimer by 1H-NMR analysis and high performance liquid chromatographic analysis.
- ⁇ Solution haze> 2.70 g of a polyester sample was placed in 20 mL of a mixture of phenol / tetrachloroethane 3/2 (weight ratio), dissolved in 110 ° C. for 30 minutes, and then cooled in a constant temperature water bath at 30 ° C. for 15 minutes. Using a turbidimeter (NDH-300A) manufactured by Nippon Denshoku Co., Ltd., the turbidity of the solution was measured with a cell having an optical path length of 10 mm to obtain a solution haze. It shows that transparency is so favorable that a value is low.
- ⁇ Measurement of elution amount ⁇ g / mL> A Teflon (registered trademark) sheet and a 6 mm thick metal frame (outside: 240 ⁇ 240 mm, inside: 200 ⁇ 200 mm) are placed on a metal plate, and 30 g of resin is uniformly spread in the frame, and Teflon (registered trademark) is placed on it. ) A sheet and a metal plate were placed in this order, and the set was put in an electric heating press machine heated to 200 ° C.
- the metal plate set After preheating without applying pressure for 2 minutes, after heating for 2 minutes at a pressure of 100 kg / cm 2 , the metal plate set is taken out, put into a cooling water circulation type cooling press, pressurized to 100 kg / cm 2 and pressurized for 2 minutes. Cooled down.
- the cooled metal plate set was taken out, a resin press piece (200 mm ⁇ 200 mm ⁇ 6 mm) was taken out, and the sample was cut out to a size of 50 mm ⁇ 70 mm.
- the cut out 0.6 mm thick press sheet was immersed in a 20% by mass aqueous ethanol solution at a rate of 2 mL per 1 cm 2 and heated at 60 ° C. for 30 minutes.
- the press sheet was removed by filtration, and the filtrate was evaporated to dryness, followed by heat drying at 105 ° C. for 2 hours.
- the mass after drying was weighed, and the elution amount ( ⁇ g / mL) was calculated.
- ⁇ Quantitative determination of moisture in pellet mass ppm> About 1 g of the sample is heated in a nitrogen gas stream at 210 ° C. for about 10 minutes with a moisture vaporizer (“VA-200” manufactured by Mitsubishi Chemical Analytech Co., Ltd.) to vaporize moisture contained in the material into the nitrogen stream. The amount of water contained in the nitrogen stream was measured by a coulometric titration method using a Karl Fischer moisture meter (“CA-200” manufactured by Mitsubishi Chemical Analytech Co., Ltd.) and expressed in ppm by mass with respect to the pellets.
- VA-200 moisture vaporizer manufactured by Mitsubishi Chemical Analytech Co., Ltd.
- ⁇ Melt viscosity MVR (cm 3/10 min)> The measurement method uses the method defined in JIS K7210. The measurement temperature was 190 ° C., the working load was 2.16 kg, the orifice diameter was 1.0 mm, and the measurement was performed using a melt indexer “L242” manufactured by Techno Seven Co., Ltd. The higher the value, the lower the melt viscosity.
- ⁇ Color b value> The pelletized polyester is filled in a cylindrical powder measurement cell having an inner diameter of 30 mm and a depth of 12 mm, and described in Reference Example 1 of JIS Z8730 using a colorimetric color difference meter Z300A (Nippon Denshoku Industries Co., Ltd.).
- the b value by the color coordinate of Hunter's color difference formula in the Lab display system was obtained as an arithmetic mean value of values measured by rotating the measurement cell 90 degrees by 90 degrees by the reflection method.
- Example 1 [Preparation of catalyst for polycondensation] Into a glass eggplant-shaped flask equipped with a stirrer, 100 parts by mass of magnesium acetate tetrahydrate was added, and 1500 parts by mass of absolute ethanol (purity 99% by mass or more) was further added. Furthermore, 65.3 parts by mass of ethyl acid phosphate (mixing mass ratio of monoester and diester was 45:55) was added and stirred at 23 ° C. After confirming that magnesium acetate was completely dissolved after 15 minutes, 122 parts by mass of tetra-n-butyl titanate was added. Stirring was further continued for 10 minutes to obtain a uniform mixed solution.
- This mixed solution was transferred to an eggplant-shaped flask and concentrated under reduced pressure by an evaporator in an oil bath at 60 ° C. After 1 hour, most of ethanol was distilled off to obtain a translucent viscous liquid. The temperature of the oil bath was further raised to 80 ° C., and further concentrated under a reduced pressure of 5 Torr to obtain a viscous liquid.
- This liquid catalyst was dissolved in 1,4-butanediol to prepare a titanium atom content of 3.36% by mass. The storage stability of this catalyst solution in 1,4-butanediol was good, and no precipitate was observed in the catalyst solution stored at 40 ° C. under a nitrogen atmosphere for at least 40 days. The catalyst solution had a pH of 6.3.
- a polyester was produced as follows by the esterification step shown in FIG. 1 and the polycondensation step shown in FIG. First, with respect to 1.00 mol of succinic acid containing 0.18% by mass of malic acid, 1.30 mol of 1,4-butanediol and malic acid were mixed at a total amount of 0.0033 mol.
- a stirrer in which a 50 ° C. slurry was previously filled with a low molecular weight polyester (esterification reaction product) having an esterification rate of 99 mass% in a nitrogen atmosphere through a raw material supply line (1) from a slurry preparation tank (not shown). It was continuously supplied to the esterification reaction tank (A) having 45.5 kg / hour.
- the esterification reaction tank (A) is set to an internal temperature of 230 ° C. and a pressure of 101 kPa, and water, tetrahydrofuran and excess 1,4-butanediol produced are distilled from the distillation line (5), and a rectifying column (C) It separated into a high boiling component and a low boiling component. A part of the high-boiling component at the bottom of the column after the system was stabilized was extracted to the outside through an extraction line (8) so that the liquid level of the rectification column (C) was constant.
- the low boiling component mainly composed of water and tetrahydrofuran is extracted from the top of the column in the form of gas, condensed by the condenser (G), and the extraction line (13) so that the liquid level of the tank (F) becomes constant. ) Extracted outside.
- the total amount of the bottom component of the rectification column (C) at 100 ° C.
- the esterification reaction product produced in the esterification reaction tank (A) is continuously extracted from the esterification reaction product extraction line (4) using the pump (B), and the liquid in the esterification reaction tank (A).
- the liquid level was controlled so that the average residence time in terms of succinic acid unit was 3 hours.
- the esterification reaction product extracted from the extraction line (4) was continuously supplied to the first polycondensation reaction tank (a) in FIG. After the system was stabilized, the esterification rate of the esterification reaction product collected at the outlet of the esterification reaction tank (A) was 92.4%, and the amount of terminal carboxyl groups was 884 equivalent / ton.
- a catalyst solution prepared in advance by the above-described method was prepared by diluting a catalyst solution diluted with 1,4-butanediol so that the concentration as a titanium atom was 0.12% by mass in a catalyst preparation tank. Through L8), the esterification reaction product was continuously fed to the extraction line (4) at 1.4 kg / h (the catalyst was added to the liquid phase of the reaction solution). The supply was stable during the operation period.
- the liquid level was controlled so that the internal temperature of the first polycondensation reaction tank (a) was 240 ° C., the pressure was 2.7 kPa, and the residence time was 120 minutes.
- An initial polycondensation reaction was carried out while extracting water, tetrahydrofuran and 1,4-butanediol from a vent line (L2) connected to a decompressor (not shown). The extracted reaction liquid was continuously supplied to the second polycondensation reactor (d).
- the second polycondensation reactor (d) has an internal temperature of 240 ° C., a pressure of 400 Pa, liquid level control so that the residence time is 120 minutes, and a vent line (not shown) connected to a decompressor (not shown)
- the polycondensation reaction was further advanced while extracting water, tetrahydrofuran and 1,4-butanediol from L4).
- the obtained polyester was continuously supplied to the third polycondensation reactor (k) via the extraction line (L3) by the extraction gear pump (e).
- the internal temperature of the third polycondensation reactor (k) was 240 ° C., the pressure was 130 Pa, the residence time was 120 minutes, and the polycondensation reaction was further advanced.
- the obtained polyester was continuously extracted in the form of a strand from the die head (g) and cooled with water, and cut with a rotary cutter (h) to obtain pellets.
- the esterification reaction and polycondensation reaction were carried out for 7 consecutive days, and the physical properties of the polyester obtained by sampling every 8 hours after the lapse of 16 hours from the start of the reaction were measured. The average value and the width of each sample are shown.
- Intrinsic viscosity is 1.85 ⁇ 0.02 dL / g
- terminal carboxyl group concentration is 19 ⁇ 1 equivalent / ton
- color b value is 1.9 ⁇ 0.1
- solution haze is 0.4 ⁇ 0.1%
- pellet The weight was 19 ⁇ 1 mg / grain, and the polyester pellets were stable in quality.
- the pellet weight was measured per 100 grains by measuring the weight of 100 grains.
- the obtained polyester pellets were subjected to contact treatment by the contact treatment step shown in FIG.
- the mixed liquid of ethanol and water used as the contact treatment liquid is controlled to 70 ° C. from the circulation tank (I) via the heat exchanger (II) by the pump (IX), and from the supply line (101) to the treatment tank ( To III).
- the ratio of ethanol (hereinafter sometimes abbreviated as EtOH) and water in the contact treatment liquid was 90% by mass of water with respect to the entire contact treatment liquid.
- the mass ratio of the contact treatment liquid and pellets in the treatment tank was 5 (treatment liquid / pellet ratio).
- the contact treatment liquid was countercurrently contacted with the pellets in the treatment tank, extracted from the extraction line (102), and collected into the circulation tank (I) via the fine powder remover (IV).
- the pellets used for the contact treatment were continuously supplied from the supply line (103), and after being brought into contact with the contact treatment liquid for 8 hours, were continuously extracted from the extraction line (104) by the rotary valve (V).
- the contact treatment liquid extracted along with the pellets is separated by the preliminary solid-liquid separator (VI), passes through the recovery tank (VII), and then passes through the supply line (105) by the pump (X) to the recovery line ( 106).
- the contact treatment liquid was continuously extracted from the circulation tank (I) through the extraction line (107), and the amount thereof was set to 1/20 (flow rate ratio) of the total circulation flow rate of the contact treatment liquid.
- a quantity of ethanol and water corresponding to the contact treatment liquid extracted from the supply line (108) was supplied to the circulation tank (I).
- the pellets continuously extracted are separated from the contact treatment liquid entrained in the preliminary solid-liquid separator (IV), and then dried from the solid-liquid separator (VIII) via the extraction line (109). was fed continuously.
- Drying was performed by the drying process shown in FIG.
- the dry nitrogen gas in the first drying tower has a purity of 99% or more (dew point minus 40 ° C.), the gas temperature is 80 ° C., the gas (empty tower) speed is 0.125 m / sec, the pellet residence time is 15 hours, and the drying air in the second drying tower. (Dew point minus 40 ° C.)
- the temperature was 50 ° C.
- the gas (superficial velocity) was 0.125 m / sec
- the pellet residence time was 24 hours.
- Table 1 shows the ratio of the intrinsic viscosity measured for the dried sample to the intrinsic viscosity before the contact treatment (represented by% retention), the cyclic dimer content of the dried sample, and the pellet weight.
- Example 2 (Example 2) to (Example 7), (Comparative Example 1)
- Table 1 shows the results of the contact treatment performed in the same manner as in Example 1 except that the composition of the contact treatment liquid and the contact treatment time were changed as shown in Table 1 in Example 1.
- Example 8 Polyester pellets obtained by polycondensation in the same manner as in Example 1 except that the length of the extraction line (L3) for extracting the polyester from the third triple condensation tank in Example 1 was changed to 4 times longer piping. Got.
- the obtained polyester has an intrinsic viscosity of 1.85 ⁇ 0.02 dL / g, a terminal carboxyl group amount of 30 ⁇ 1 equivalent / ton, a color b value of 2.0 ⁇ 0.1, and a solution haze of 0.4 ⁇ 0.
- the polyester pellets were stable in quality.
- Table 1 shows the results of contact treatment performed in the same manner as in Example 1 except that the composition of the contact treatment liquid and the contact treatment time were changed as shown in Table 1, respectively.
- Example 9 The same procedure as in Example 3 was performed except that the pellet speed was changed to 30 ⁇ 1 mg / grain by changing the cutter speed during pelletization after melt polycondensation in Example 3. The results are shown in Table 1.
- Example 10 Examples 10 and 11
- Comparative Example 4 Comparative Example 4
- Example 1 except that the contact treatment liquid composition and the contact treatment liquid temperature were changed as shown in Table 1, the contact treatment was performed in the same manner as in Example 1, and the intrinsic viscosity contact treatment was performed in the same manner as in Example 1.
- the ratio to the previous intrinsic viscosity (represented by% retention), the CD content, the elution amount, the ethanol content in the pellets after drying (isopropanol in Comparative Example 4) and the melt viscosity MVR were measured, and the results are shown in Table 2. It was shown to.
- the production method of the present invention can efficiently obtain a polyester having a reduced CD content and a small amount of elution, and can provide a raw material polyester for a molded product in which the surface does not fog when formed into a molded product. .
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Polyesters Or Polycarbonates (AREA)
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP11829042.8A EP2623540B1 (en) | 2010-09-27 | 2011-09-26 | Method for producing polyester |
| CN2011800461657A CN103124761A (zh) | 2010-09-27 | 2011-09-26 | 聚酯的制造方法 |
| US13/851,592 US8680229B2 (en) | 2010-09-27 | 2013-03-27 | Method for producing polyester |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2010-215641 | 2010-09-27 | ||
| JP2010215641 | 2010-09-27 |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US13/851,592 Continuation US8680229B2 (en) | 2010-09-27 | 2013-03-27 | Method for producing polyester |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2012043488A1 true WO2012043488A1 (ja) | 2012-04-05 |
Family
ID=45892932
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2011/071910 Ceased WO2012043488A1 (ja) | 2010-09-27 | 2011-09-26 | ポリエステルの製造方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8680229B2 (enExample) |
| EP (1) | EP2623540B1 (enExample) |
| JP (1) | JP5834696B2 (enExample) |
| CN (1) | CN103124761A (enExample) |
| WO (1) | WO2012043488A1 (enExample) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2689666A1 (de) * | 2012-07-27 | 2014-01-29 | Bühler GmbH | Verfahren und Vorrichtung zum Darren von Darrgut |
| JP6325529B2 (ja) * | 2013-04-19 | 2018-05-16 | 株式会社武蔵野化学研究所 | ポリ乳酸の精製方法 |
| JP2018145221A (ja) * | 2017-03-01 | 2018-09-20 | 三菱ケミカル株式会社 | ポリエステルの製造方法 |
| CN111372972A (zh) | 2017-11-20 | 2020-07-03 | 巴斯夫欧洲公司 | 纯化脂族聚酯的方法 |
| US11401373B2 (en) | 2017-11-20 | 2022-08-02 | Basf Se | Continuous method for producing an aliphatic polyester |
| WO2019169296A1 (en) | 2018-03-01 | 2019-09-06 | Tepha, Inc. | Medical devices containing poly(butylene succinate) and copolymers thereof |
| US20200390944A1 (en) | 2018-03-01 | 2020-12-17 | Tepha, Inc. | Medical devices containing compositions of poly(butylene succinate) and copolymers thereof |
| AT521534A2 (de) * | 2018-07-03 | 2020-02-15 | Next Generation Recyclingmaschinen Gmbh | Verfahren zur Herstellung einer Polykondensatschmelze aus einem Primärmaterial und einem Sekundärmaterial |
| PT3891208T (pt) | 2018-12-06 | 2023-05-02 | Basf Se | Processo para produzir um (co) poliéster |
| CN110229378B (zh) * | 2019-05-20 | 2021-11-19 | 安徽东锦资源再生科技有限公司 | 一种回收聚酯瓶片料的结晶干燥工艺 |
| KR20220012897A (ko) | 2019-05-22 | 2022-02-04 | 바스프 에스이 | 지방족-방향족 폴리에스테르의 정제 방법 |
| WO2020234294A1 (en) | 2019-05-22 | 2020-11-26 | Basf Se | Continuous process for producing an aliphatic-aromatic polyester |
| BR112022003620A2 (pt) | 2019-08-29 | 2022-05-24 | Tepha Inc | Dispositivos médicos contendo poli(succinato de butileno) e copolímeros do mesmo |
| CN113121801A (zh) * | 2021-06-04 | 2021-07-16 | 山东大学 | 一种聚酯材料及其制备方法与其在医药罩泡中的应用 |
| CN114957201B (zh) * | 2022-06-15 | 2023-10-20 | 万华化学集团股份有限公司 | 一种低环状副产物聚丁二酸丁二醇聚酯的制备方法 |
| EP4596603A1 (en) * | 2022-09-29 | 2025-08-06 | Mitsubishi Chemical Corporation | Method for producing polyester |
| CN115785413B (zh) * | 2022-12-08 | 2024-07-12 | 金发科技股份有限公司 | 一种可生物降解脂肪族聚酯及其制备方法和应用 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07316276A (ja) * | 1994-05-30 | 1995-12-05 | Showa Highpolymer Co Ltd | 脂肪族ポリエステルまたは組成物の洗浄処理方法 |
| JP2004107457A (ja) * | 2002-09-18 | 2004-04-08 | Mitsubishi Chemicals Corp | 脂肪族或いは脂環式ポリエステル及びその製造方法 |
| JP2005162890A (ja) * | 2003-12-03 | 2005-06-23 | Mitsubishi Chemicals Corp | 脂肪族ポリエステル樹脂及びその製造方法 |
| JP2010195989A (ja) * | 2009-02-26 | 2010-09-09 | Mitsubishi Chemicals Corp | 脂肪族ポリエステルの製造方法 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4006342B2 (ja) * | 2003-01-21 | 2007-11-14 | 三菱化学株式会社 | ポリエステル粒体の処理方法 |
| WO2004104067A1 (ja) * | 2003-05-21 | 2004-12-02 | Mitsubishi Chemical Corporation | 脂肪族ポリエステル及びその製造方法 |
| JP2005325259A (ja) * | 2004-05-14 | 2005-11-24 | Mitsubishi Chemicals Corp | 脂肪族或いは脂環式ポリエステルの洗浄処理方法 |
-
2011
- 2011-09-26 JP JP2011209173A patent/JP5834696B2/ja active Active
- 2011-09-26 CN CN2011800461657A patent/CN103124761A/zh active Pending
- 2011-09-26 WO PCT/JP2011/071910 patent/WO2012043488A1/ja not_active Ceased
- 2011-09-26 EP EP11829042.8A patent/EP2623540B1/en active Active
-
2013
- 2013-03-27 US US13/851,592 patent/US8680229B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07316276A (ja) * | 1994-05-30 | 1995-12-05 | Showa Highpolymer Co Ltd | 脂肪族ポリエステルまたは組成物の洗浄処理方法 |
| JP2004107457A (ja) * | 2002-09-18 | 2004-04-08 | Mitsubishi Chemicals Corp | 脂肪族或いは脂環式ポリエステル及びその製造方法 |
| JP2005162890A (ja) * | 2003-12-03 | 2005-06-23 | Mitsubishi Chemicals Corp | 脂肪族ポリエステル樹脂及びその製造方法 |
| JP2010195989A (ja) * | 2009-02-26 | 2010-09-09 | Mitsubishi Chemicals Corp | 脂肪族ポリエステルの製造方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2623540A4 (en) | 2016-05-18 |
| JP5834696B2 (ja) | 2015-12-24 |
| US20130211037A1 (en) | 2013-08-15 |
| CN103124761A (zh) | 2013-05-29 |
| JP2012092310A (ja) | 2012-05-17 |
| US8680229B2 (en) | 2014-03-25 |
| EP2623540B1 (en) | 2018-03-28 |
| EP2623540A1 (en) | 2013-08-07 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5834696B2 (ja) | ポリエステルの製造方法 | |
| US9714320B2 (en) | Process for preparing a high molecular weight heteroaromatic polyester or copolyester | |
| JP2018145221A (ja) | ポリエステルの製造方法 | |
| EP2256145B1 (en) | Process for production of aliphatic polyester | |
| JP7718406B2 (ja) | 脂肪族芳香族ポリエステルの製造方法 | |
| JP2022136023A (ja) | 脂肪族芳香族ポリエステル組成物及びその製造方法 | |
| WO2005044892A1 (ja) | ポリエチレンテレフタレート樹脂およびポリエステル樹脂成形体の製造方法 | |
| JP2018145413A (ja) | 脂肪族芳香族ポリエステルの製造方法 | |
| JP2010195989A (ja) | 脂肪族ポリエステルの製造方法 | |
| JP5200531B2 (ja) | 脂肪族ポリエステルの製造方法 | |
| JP2012144744A (ja) | 脂肪族ポリエステルの製造方法 | |
| JP2009067897A (ja) | 脂肪族ポリエステルの製造方法 | |
| JP5045216B2 (ja) | ポリエステル重縮合用触媒の製造方法、該触媒を用いたポリエステルの製造方法 | |
| WO2024204351A1 (ja) | 生分解性樹脂組成物及び成形体 | |
| JP5412893B2 (ja) | ポリエステルの製造方法 | |
| JP5729217B2 (ja) | 脂肪族ポリエステルの製造方法 | |
| EP4596603A1 (en) | Method for producing polyester | |
| JP2018145220A (ja) | 脂肪族ポリエステルの製造方法 | |
| WO2024204552A1 (ja) | ポリエステルの製造方法、組成物および成形体 | |
| JP5678667B2 (ja) | 脂肪族ポリエステルの製造方法 | |
| JP2014141633A (ja) | 脂肪族ポリエステルの製造方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 201180046165.7 Country of ref document: CN |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11829042 Country of ref document: EP Kind code of ref document: A1 |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2011829042 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |