WO1995026397A1 - Alkaline bacillus amylase - Google Patents
Alkaline bacillus amylase Download PDFInfo
- Publication number
- WO1995026397A1 WO1995026397A1 PCT/DK1995/000142 DK9500142W WO9526397A1 WO 1995026397 A1 WO1995026397 A1 WO 1995026397A1 DK 9500142 W DK9500142 W DK 9500142W WO 9526397 A1 WO9526397 A1 WO 9526397A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- amylase
- bacillus
- ncib
- determined
- activity
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/38—Products with no well-defined composition, e.g. natural products
- C11D3/386—Preparations containing enzymes, e.g. protease or amylase
- C11D3/38609—Protease or amylase in solid compositions only
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/38—Products with no well-defined composition, e.g. natural products
- C11D3/386—Preparations containing enzymes, e.g. protease or amylase
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/38—Products with no well-defined composition, e.g. natural products
- C11D3/386—Preparations containing enzymes, e.g. protease or amylase
- C11D3/38618—Protease or amylase in liquid compositions only
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
- C12N9/2402—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1)
- C12N9/2405—Glucanases
- C12N9/2408—Glucanases acting on alpha -1,4-glucosidic bonds
- C12N9/2411—Amylases
- C12N9/2414—Alpha-amylase (3.2.1.1.)
- C12N9/2417—Alpha-amylase (3.2.1.1.) from microbiological source
-
- D—TEXTILES; PAPER
- D06—TREATMENT OF TEXTILES OR THE LIKE; LAUNDERING; FLEXIBLE MATERIALS NOT OTHERWISE PROVIDED FOR
- D06L—DRY-CLEANING, WASHING OR BLEACHING FIBRES, FILAMENTS, THREADS, YARNS, FABRICS, FEATHERS OR MADE-UP FIBROUS GOODS; BLEACHING LEATHER OR FURS
- D06L1/00—Dry-cleaning or washing fibres, filaments, threads, yarns, fabrics, feathers or made-up fibrous goods
- D06L1/12—Dry-cleaning or washing fibres, filaments, threads, yarns, fabrics, feathers or made-up fibrous goods using aqueous solvents
- D06L1/14—De-sizing
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C5/00—Other processes for obtaining cellulose, e.g. cooking cotton linters ; Processes characterised by the choice of cellulose-containing starting materials
- D21C5/005—Treatment of cellulose-containing material with microorganisms or enzymes
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/005—Microorganisms or enzymes
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21C—PRODUCTION OF CELLULOSE BY REMOVING NON-CELLULOSE SUBSTANCES FROM CELLULOSE-CONTAINING MATERIALS; REGENERATION OF PULPING LIQUORS; APPARATUS THEREFOR
- D21C5/00—Other processes for obtaining cellulose, e.g. cooking cotton linters ; Processes characterised by the choice of cellulose-containing starting materials
- D21C5/02—Working-up waste paper
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H11/00—Pulp or paper, comprising cellulose or lignocellulose fibres of natural origin only
- D21H11/14—Secondary fibres
-
- D—TEXTILES; PAPER
- D21—PAPER-MAKING; PRODUCTION OF CELLULOSE
- D21H—PULP COMPOSITIONS; PREPARATION THEREOF NOT COVERED BY SUBCLASSES D21C OR D21D; IMPREGNATING OR COATING OF PAPER; TREATMENT OF FINISHED PAPER NOT COVERED BY CLASS B31 OR SUBCLASS D21G; PAPER NOT OTHERWISE PROVIDED FOR
- D21H17/00—Non-fibrous material added to the pulp, characterised by its constitution; Paper-impregnating material characterised by its constitution
- D21H17/63—Inorganic compounds
- D21H17/67—Water-insoluble compounds, e.g. fillers, pigments
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02W—CLIMATE CHANGE MITIGATION TECHNOLOGIES RELATED TO WASTEWATER TREATMENT OR WASTE MANAGEMENT
- Y02W30/00—Technologies for solid waste management
- Y02W30/50—Reuse, recycling or recovery technologies
- Y02W30/64—Paper recycling
Definitions
- the present invention relates to amylases having improved dishwashing and/or washing performance.
- ⁇ -amylase enzymes have been used for a variety of different purposes, the most important of which are starch liquefaction, textile desizing, starch modification in the paper and pulp industry, and for brewing and baking.
- a further use of ⁇ -amylases, which is becoming increasingly important is the removal of starchy stains during washing and dishwashing.
- Examples of commercial ⁇ -amylase products are Termamyl ® , BAN ® and Fungamyl ® , all available from Novo Nordisk A/S, Denmark. These and similar products from other commercial sources have an acidic to a neutral pH optimum, typically in the range of from pH 5 to pH 7.5, which means that they do not display optimal activity in detergent solutions owing to the alkaline character of the detergents.
- the present invention provides ⁇ -amylases with a very high specific activity at pH 8-10 and at temperatures of from 30°C to around 60°C, conditions normal in detergent solutions.
- the present invention relates to an ⁇ -amylase having a specific activity at least 25% higher than the specific activity of Termamyl ® at a temperature in the range of25°C to 55°C and at a pH value in the range of pH 8 to pH 10, measured by the ⁇ -amylase activity assay as described herein.
- Fig. 1 shows the relation between pH and the ⁇ -amylase activity of a novel amylase (obtained from Bacillus strain NCIB 12289), determined as described in Example 2.
- Fig. 2 shows the pH profile of an ⁇ -amylase obtained from Bacillus strain NCIB 12512 (I), of an ⁇ -amylase obtained from Bacillus strain NCIB 12513 (II) and of Termamyl ® (III) determined at 55°C in the pH interval of from 4 to 10.5, the test being performed as described in Example 3.
- Fig. 3 shows the temperature profile of an ⁇ -amylase obtained from Bacillus strain NCIB 12512 (I), of an ⁇ -amylase obtained from Bacillus strain NCIB 12513 (II) and of Termamyl ® (III) determined at pH 10.0 in the temperature interval of from 25°C to 95°C, the test being performed as described in Example 3.
- Fig. 4 shows the RSF-rating - removal of starch film from dish- and glassware, as a function of the dosage of a novel ⁇ -amylase (obtained from Bacillus strain NCIB 12289) at 55°C, the test being performed as described in Example 4.
- Fig. 5 shows the RSF-rating - removal of starch film from dish- and glassware, as a function of the dosage of a novel ⁇ -amylase (obtained from Bacillus strain NCIB 12512) at45°C (•), at 55°C (*) and at 65°C (x), the test being performed as described in Example 4.
- a novel ⁇ -amylase obtained from Bacillus strain NCIB 12512
- One embodiment of the present invention provides an ⁇ -amylase having a specific activity at least 25% higher or at least 35% higher or at least 45% higher or at least 55% higher or at least 65% higher or at least 75% or at least 25-75% higher than the specific activity of Termamyl ® at a temperature in the range of 25°C to 55°C or at a temperature in the range of 25°C to 35°C or at a temperature in the range of 35°C to 45°C or at a temperature in the range of 45°C to 55°C and at a pH value in the range of pH 8 to pH 10 or at a pH value in the range of pH 8 to 8.5 or at a pH value in the range of pH 8.5 to 9.0 or at a pH value in the range of pH 9.0 to 9.5 or at a pH value in the range of pH 9.5 to 10.0, measured by the ⁇ .-amylase activity assay as described herein.
- preferred novel ⁇ -amylases of the invention may be characterized by having a specific activity at least 25% higher than the specific activity of Termamyl ® at any temperature in the range of 25°C to 55°C and at any pH value in the range of from pH 8 to pH 10, measured by the ⁇ -amylase activity assay as described herein.
- the ⁇ -amylases of the invention Compared with known o.-amylases it is very remarkable how well the ⁇ -amylases of the invention perform at pH 10; accordingly in a preferred embodiment the ⁇ -amylase is characterized by having a specific activity at least 25% higher than the specific activity of Termamyl ® at any temperature in the range of 25°C to 55°C and at pH 10, using the ⁇ -amylase activity assay as described herein.
- the invention relates to an ⁇ -amylase comprising the amino acid sequence shown in SEQ ID No.
- an ⁇ -amylase being at least 80% homologous with the amino acid sequence (SEQ ID No.1), preferably being at least 85% homologous with SEQ ID No. 1, more preferably being at least 90% homologous with SEQ ID No.1.
- a polypeptide is considered to be X% homologous to the parent ⁇ -amylase if a comparison of the respective amino acid sequences, performed via known algorithms, such as the one described by Lipman and Pearson in Science 227, 1985, p. 1435, reveals an identity of X% .
- the invention relates to an ⁇ -amylase comprising the amino acid sequence shown in SEQ ID No.
- an ⁇ -amylase being at least 80% homologous with the amino acid sequence (SEQ ID No.2), preferably being at least 85% homologous with SEQ ID No. 2, more preferably being at least 90% homologous with SEQ ID No.2.
- the invention relates to an ⁇ -amylase comprising an N-terminal amino acid sequence identical to that shown in SEQ ID No. 3 or an ⁇ -amylase being at least 80% homologous with SEQ ID No.3 in the N-terminal, preferably being at least 90% homologous with SEQ ID No.3 in the N-terminal.
- Preferred ⁇ -amylases of the invention are obtainable from an alkaliphilic Bacillus species, particularly from one of the Bacillus strains NCIB 12289, NCIB 12512, NCIB 12513 and DSM 9375.
- the term "obtainable from” is intended not only to indicate an ⁇ -amylase produced by a Bacillus strain but also an ⁇ -amylase encoded by a DNA sequence isolated from such a Bacillus strain and produced in a host organism transformed with said DNA sequence.
- strain NCIB 12289 has been deposited according to the Budapest Treaty on the International Recognition of the Deposits of Microorganisms for the Purpose of Patent Procedures, on 8 July 1986 at The National Collection of Industrial Bacteria (NCIB) under accession no. NCIB 12289.
- the strain NCIB 12512 is described in detail in EP 0 277 216.
- the strain NCIB 12512 has been deposited according to the Budapest Treaty on the International Recognition of the Deposits of Microorganisms for the Purpose of Patent Pro ⁇ cedures, on 5 August 1987 at The National Collection of Industrial Bacteria (NCIB) under accession no. NCIB 12512.
- the strain NCIB 12513 is described in detail in EP 0 277 216.
- the strain NCIB 12513 has been deposited according to the Budapest Treaty on the International Recognition of the Deposits of Microorganisms for the Purpose of Patent Procedures, on 5 August 1987 at The National Collection of Industrial Bacteria (NCIB) under accession no. NCIB 12513.
- the strain DSM 9375 has been deposited according to the Budapest Treaty on the International Recognition of the Deposits of Microorganisms for the Purpose of Patent Procedures, on 16 August 1994 at Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSM) under Accession No. DSM Cloning a DNA sequence encoding an ⁇ -amylase
- the DNA sequence encoding an ⁇ - amylase of the invention may be isolated from any cell or microorganism producing the ⁇ -amylase in question, using various methods well known in the art.
- a genomic DNA and/or cDNA library should be constructed using chromosomal DNA or messenger RNA from the organism that produces the ⁇ -amylase to be studied.
- homologous, labelled oligonucleotide probes may be synthesized and used to identify ⁇ -amylase-encoding clones from a genomic library prepared from the organism in question.
- a labelled oligonucleotide probe containing sequences homologous to a known ⁇ -amylase gene could be used as a probe to identify ⁇ -amylase-encoding clones, using hybridization and washing conditions of lower stringency.
- preferred probes may be constructed on the basis of SEQ ID No. 1 or on the basis of SEQ ID No. 2 or on the basis of SEQ ID No. 4 or on the basis of SEQ ID No 5.
- Yet another method for identifying ⁇ -amylase-encoding clones would involve inserting fragments of genomic DNA into an expression vector, such as a plasmid, transforming ⁇ -amylase-negative bacteria with the resulting genomic DNA library, and then plating the transformed bacteria onto agar containing a substrate for ⁇ -amylase, thereby allowing clones expressing the ⁇ -amylase to be identified.
- an expression vector such as a plasmid
- transforming ⁇ -amylase-negative bacteria with the resulting genomic DNA library
- the DNA sequence encoding the enzyme may be prepared synthetically by established standard methods, e.g. the phosphoamidite method described by S.L. Beaucage andM.H. Caruthers in Tetrahedron Letters 22, 1981, pp. 1859-1869 or the method described by Matthes et al. in The EMBO J. 3, 1984, pp. 801-805.
- phosphoamidite method oligonucleotides are synthesized, e.g. in an automatic DNA synthesizer, purified, annealed, ligated and cloned in appropriate vectors.
- the DNA sequence may be of mixed genomic and synthetic origin, mixed synthetic and cDNA origin or mixed genomic and cDNA origin, prepared by ligating fragments of synthetic, genomic or cDNA origin (as appropriate, the fragments corresponding to various parts of the entire DNA sequence), in accordance with standard techniques.
- the DNA sequence may also be prepared by polymerase chain reaction (PCR) using specific primers, for instance as described in US 4,683,202 or R.K. Saiki et al. in Science 239, 1988, pp. 487-491.
- an ⁇ -amylase-encoding DNA sequence produced by methods described above, or by any alternative methods known in the art can be expressed, in enzyme form, using an expression vector which typically includes control sequences encoding a promoter, operator, ribosome binding site, translation initiation signal, and, optionally, a repressor gene or various activator genes.
- the recombinant expression vector carrying the DNA sequence encoding an ⁇ -amylase of the invention may be any vector which may conveniently be subjected to recombinant DNA procedures, and the choice of vector will often depend on the host cell into which it is to be introduced.
- the vector may be an autonomously replicating vector, i.e. a vector which exists as an extrachromosomal entity, the replication of which is independent of chromosomal replication, e.g., a plasmid, a bacteriophage or an extrachromosomal element, minichromosome or an artificial chromosome.
- the vector may be one which, when introduced into a host cell, is integrated into the host cell genome and replicated together with the chromosome(s) int) which it has been integrated.
- the DNA sequence should be operably connected to a suitable promoter sequence.
- the promoter may be any DNA sequence which shows transcriptional activity in the host cell of choice and may be derived from genes encoding proteins either homologous or heterologous to the host cell.
- suitable promoters for directing the transcription of the DNA sequence encoding an ⁇ -amylase of the invention, especially in a bacterial host are the promoter of the lac operon of E.
- coli the Streptomyces coelicolor agarase gene dagA promoters, the promoters of the Bacillus licheniformis ⁇ - amylase gene (amyL), the promoters of the Bacillus stearothermophilus maltogenic amylase gene (amyM), the promoters of the Bacillus Amyloliquefaciens ⁇ -amylase (amyO), the promoters of the Bacillus subtilis xylA and xylB genes etc.
- useful promoters are those derived from the gene encoding A. oryzae TAKA amylase, Rhizo ⁇ mucor miehei aspartic proteinase, A.
- niger neutral ⁇ -amylase A. niger acid stable ⁇ -amylase, A. niger glucoamylase, Rhizomucor miehei lipase, A. oryzae alkaline protease, A. oryzae triose phosphate isomerase or A. nidulans acetamidase.
- the expression vector of the invention may also comprise a suitable transcription terminator and, in eukaryotes, polyadenylation sequences operably connected to the DNA sequence encoding the ⁇ -amylase of the invention. Termination and polyadenylation sequences may suitably be derived from the same sources as the promoter.
- the vector may further comprise a DNA sequence enabling the vector to replicate in the host cell in question.
- a DNA sequence enabling the vector to replicate in the host cell in question. Examples of such sequences are the origins of replication of plasmids pUC19, pACYC177, pUB110, pE194, pAMB1 and pIJ702.
- the vector may also comprise a selectable marker, e.g., a gene the product of which complements a defect in the host cell, such as the dal genes from B. subtilis or B. licheniformis, or one which confers antibiotic resistance such as ampicillin, kanamycin, chloramphenicol or tetracyclin resistance.
- a selectable marker e.g., a gene the product of which complements a defect in the host cell, such as the dal genes from B. subtilis or B. licheniformis, or one which confers antibiotic resistance such as ampicillin, kanamycin, chloramphenicol or tetracyclin resistance.
- the vector may comprise Aspergillus selection markers such as amdS, argB, niaD and sC, a marker giving rise to hygromycin resistance, or the selection may be accomplished by co-transformation, e.g., as described in WO 91/17243.
- the cell of the invention is advantageously used as a host cell in the recombinant production of an ⁇ -amylase of the invention.
- the cell may be transformed with the DNA construct of the invention encoding the ⁇ -amylase conveniently by integrating the DNA construct (in one or more copies) in the host chromosome. This integration is generally considered to be an advantage as the DNA sequence is more likely to be stably maintained in the cell. Integration of the DNA constructs into the host chromosome may be performed according to conventional methods, e.g., by homologous or heterologous recombination. Alternatively, the cell may be transformed with an expression vector as described above in connection with the different types of host cells.
- the cell of the invention may be a cell of a higher organism such as a mammal or an insect, but is preferably a microbial cell, e.g., a bacterial or a fungal (including yeast) cell.
- a microbial cell e.g., a bacterial or a fungal (including yeast) cell.
- suitable bacteria are grampositive bacteria such as Bacillus subtilis, Bacillus licheniformis, Bacillus lentus, Bacillus brevis, Bacillus stearothermophilus, Bacillus alkalophilus, Bacillus amyloliquefaciens, Bacillus coagulans, Bacillus circulans, Bacillus lautus, Bacillus megaterium. Bacillus thuringiensis, or Streptomyces lividans or
- Streptomyces murinus or gramnegative bacteria such as E. coli.
- the transformation of the bacteria may, for instance, be effected by protoplast transformation or by using competent cells in a manner known per se.
- the yeast organism may favourably be selected from a species of Saccharomyces or Schizosaccharomyces, e.g., Saccharomyces cerevisiae.
- the filamentous fungus may advan tageously belong to a species of Aspergillus, e.g., Aspergillus oryzae or Aspergillus niger.
- Fungal cells may be transformed by a process involving protoplast formation and transformation of the protoplasts followed by regeneration of the cell wall in a manner known per se. A suitable procedure for transformation of Aspergillus host cells is described in EP 238 023.
- the present invention relates to a method of producing an ⁇ -amylase of the invention, which method comprises cultivating a host cell as described above under conditions conducive to the production of the ⁇ -amylase and recovering the ⁇ -amylase from the cells and/or culture medium.
- the medium used to cultivate the cells may be any conventional medium suitable for growing the host cell in question and obtaining expression of the ⁇ -amylase of the invention. Suitable media are available from commercial suppliers or may be prepared according to published recipes (e.g., as described in catalogues of the American Type Culture Collection).
- the ⁇ -amylase secreted from the host cells may conveniently be recovered from the culture medium by well-known procedures, including separating the cells from the medium by centrifugation or filtration, and precipitating proteinaceous components of the medium by means of a salt such as ammonium sulphate, followed by the use of chromatographie procedures such as ion exchange chromatography, affinity chromatography, or the like.
- Phadebas ® tablets Phadebas tablets (Phadebas ® Amylase Test, supplied by Pharmacia Diagnostic) contain a cross-linked insoluble blue-coloured starch polymer which has been mixed with bovine serum albumin and a buffer substance and tabletted.
- the measured 620 nm absorbance after 10 or 15 minutes of incubation is in the range of 0.2 to 2.0 absorbance units at 620 nm. In this absorbance range there is linearity between activity and absorbance (Lambert-Beer law). The dilution of the enzyme must therefore be adjusted to fit this criterion.
- the specific activity of each of the ⁇ -amylases at a given temperature and at a given pH can be compared directly, and the ratio of the specific activity of each of the ⁇ -amylases of interest relative to the specific activity of Termamyl ® can be determined.
- the ⁇ -amylases of the invention are well suited for use in a variety of industrial processes, in particular the enzyme finds potential applications as a component in washing, dishwashing and hard surface cleaning detergent compositions, but it may also be useful in the production of sweeteners and ethanol from starch.
- Conditions for conventional starch-converting processes and liquefaction and/or saccharification processes are described in, for instance, US Patent No. 3,912,590 and EP patent publications Nos. 252,730 and 63,909.
- the ⁇ -amylases of the invention also possess valuable properties in the production of lignocellulosic materials, such as pulp, paper and cardboard, from starch reinforced waste paper and cardboard, especially where repulping occurs at pH above 7 and where amylases can facilitate the disintegration of the waste material through degradation of the reinforcing starch.
- the ⁇ -amylases of the invention are especially useful in the deinking/recycling processes of making paper out of old starch-coated or starch-containing printed paper. It is usually desirable to remove the printing ink in order to produce new paper of high brightness; examples of how the ⁇ -amylases of the invention may be used in this way are described in PCT/DK 94/00437.
- the ⁇ -amylases of the invention may also be very useful in modifying starch where enzymatically modified starch is used in papermaking together with alkaline fillers such as calcium carbonate, kaolin and clays. With the alkaline ⁇ -amylases of the invention it becomes possible to modify the starch in the presence of the filler thus allowing for a simpler integrated process.
- ⁇ -amylases of the invention may also be very useful in textile desizing.
- ⁇ -amylases are traditionally used as auxiliaries in the desizing process to facilitate the removal of starch-containing size which has served as a protective coating on weft yarns during weaving.
- non-enzymatic auxiliaries such as alkali or oxidation agents are typically used to break down the starch, because traditional ⁇ -amylases are not very compatible with high pH levels and bleaching agents.
- alkali or oxidation agents are typically used to break down the starch, because traditional ⁇ -amylases are not very compatible with high pH levels and bleaching agents.
- the non-enzymatic breakdown of the starch size does lead to some fibre damage because of the rather aggressive chemicals used.
- ⁇ -amylases of the invention may be used alone or in combination with a cellulase when desizing cellulose-containing fabric or textile.
- the ⁇ -amylases of the invention may also be very useful in a beer-making process; the ⁇ -amylases will typically be added during the mashing process.
- the ⁇ -amylases may typically be a component of a detergent composition, e.g., a laundry detergent composition or a dishwashing detergent composition. As such, it may be included in the detergent composition in the form of a non-dusting granulate, a stabilized liquid, or a protected enzyme. Non-dusting granulates may be produced, e.g., as disclosed in US 4,106,991 and 4,661,452 (both to Novo Industri A/S) and may optionally be coated by methods known in the art.
- waxy coating materials are poly (ethylene oxide) products (polyethyleneglycol, PEG) with mean molecular weights of 1000 to 20000; ethoxylated nonylphenols having from 16 to 50 ethylene oxide units; ethoxylated fatty alcohols in which the alcohol contains from 12 to 20 carbon atoms and in which there are 15 to 80 ethylene oxide units; fatty alcohols; fatty acids; and mono- and di- and triglycerides of fatty acids.
- PEG poly (ethylene oxide) products
- PEG polyethyleneglycol
- Liquid enzyme preparations may, for instance, be stabilized by adding a polyol such as propylene glycol, a sugar or sugar alcohol, lactic acid or boric acid according to established methods.
- a polyol such as propylene glycol, a sugar or sugar alcohol, lactic acid or boric acid according to established methods.
- Other enzyme stabilizers are well known in the art.
- Protected enzymes may be prepared according to the method disclosed in EP 238,216.
- the detergent composition of the invention may be in any convenient form, e.g. as powder, granules, paste or liquid.
- a liquid detergent may be aqueous, typically containing up to 70% water and 0-30% organic solvent, or nonaqueous.
- the detergent composition comprises one or more surfactants, each of which may be anionic, nonionic, cationic, or amphoteric (zwitterionic).
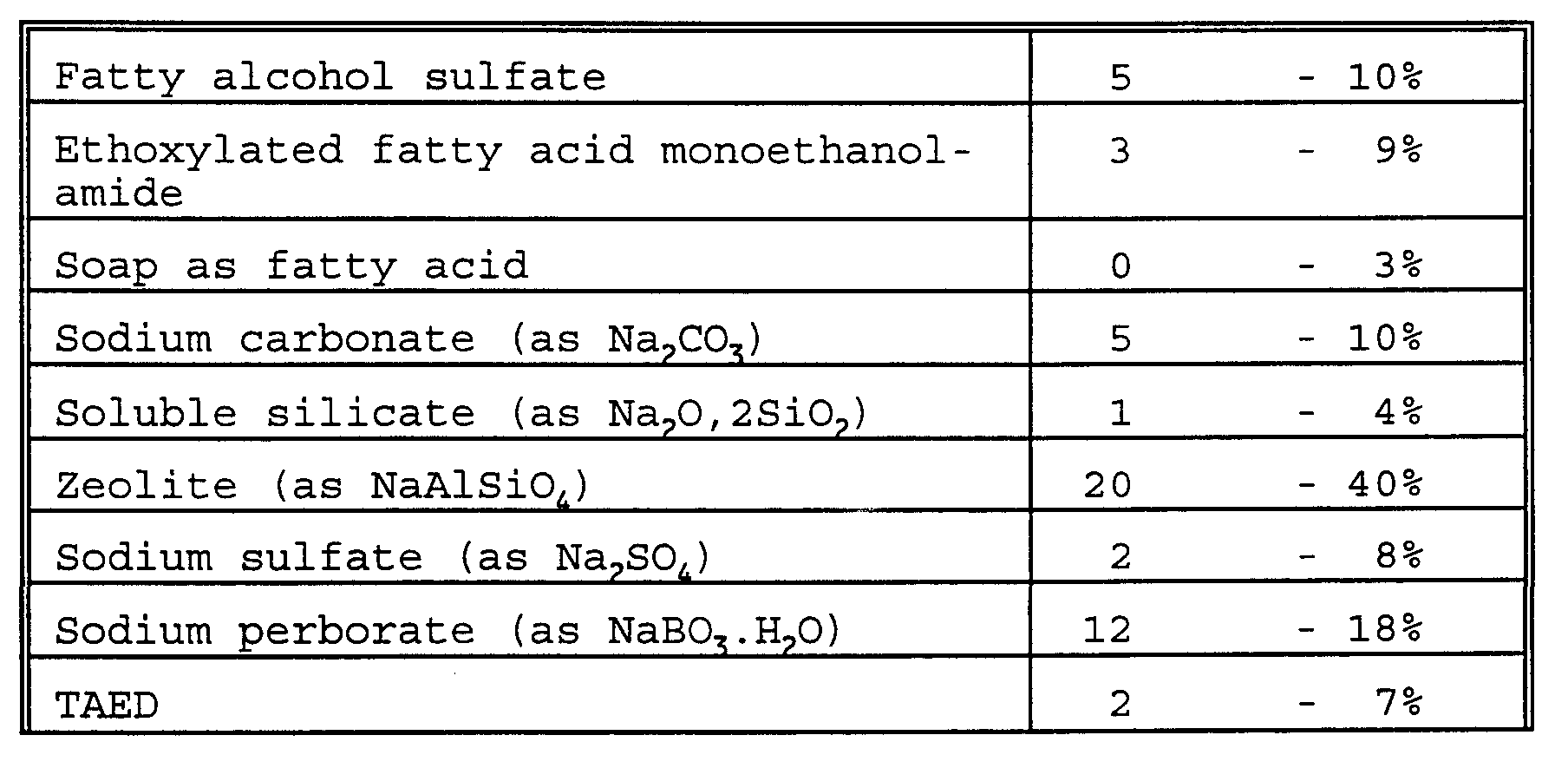
- the detergent will usually contain 0-50% of anionic surfactant such as linear alkylbenzenesulfonate (LAS), alpha-olefinsulfonate (AOS), alkyl sulfate (fatty alcohol sulfate) (AS), alcohol ethoxysulfate (AEOS or AES), secondary alkanesulfonates (SAS), alpha-sulfo fatty acid methyl esters, alkyl- or alkenylsuccinic acid, or soap.
- anionic surfactant such as linear alkylbenzenesulfonate (LAS), alpha-olefinsulfonate (AOS), alkyl sulfate (fatty alcohol sulfate) (AS), alcohol ethoxysulfate (AEOS or AES), secondary alkanesulfonates (SAS
- nonionic surfactant such as alcohol ethoxylate (AEO or AE), alcohol propoxylate, carboxylated alcohol ethoxylates, nonylphenol ethoxylate, alkylpolyglycoside, alkyldimethylamine oxide, ethoxylated fatty acid monoethanolamide, fatty acid monoethanolamide, or polyhydroxy alkyl fatty acid amide (e.g. as described in WO 92/06154).
- the detergent composition may additionally comprise one or more other enzymes, such as pullulanase, esterase, lipase, cutinase, protease, cellulase, peroxidase, or oxidase, e.g., laccase.
- enzymes such as pullulanase, esterase, lipase, cutinase, protease, cellulase, peroxidase, or oxidase, e.g., laccase.
- the detergent contains 1-65% of a detergent builder, but some dishwashing detergents may contain even up to 90% of a detergent builder, or complexing agent such as zeolite, diphosphate, triphosphate, phosphonate, citrate, nitrilotriacetic acid (NTA), ethylenediaminetetraacetic acid
- EDTA diethylenetriaminepentaacetic acid
- DTMPA diethylenetriaminepentaacetic acid
- alkyl- or alkenylsuccinic acid soluble silicates or layered silicates
- the detergent builders may be subdivided into phosphorus-containing and non-phosphorous-containing types.
- phosphorus-containing inorganic alkaline detergent builders include the water-soluble salts, especially alkali metal pyrophosphates, orthophosphates, polyphosphates and phosphonates.
- non-phosphorus-containing inorganic builders include water-soluble alkali metal carbonates, borates and silicates as well as layered disilicates and the various types of water-insoluble crystalline or amorphous alumino silicates of which zeolites is the best known representative.
- suitable organic builders include alkali metal, ammonium or substituted ammonium salts of succinates, malonates, fatty acid malonates, fatty acid sulphonates, carboxymethoxy succinates, polyacetates, carboxylates, polycarboxylates, aminopolycarboxylates and polyacetyl carboxylates.
- the detergent may also be unbuilt, i.e. essentially free of detergent builder.
- the detergent may comprise one or more polymers.
- CMC carboxymethylcellulose
- PVP poly(vinyl-pyrrolidone)
- PEG polyethyleneglycol
- PVA poly(vinyl alcohol)
- polycarboxylates such as polyacrylates, polymaleates, maleic/acrylic acid copolymers and lauryl methacrylate/acrylic acid copolymers.
- the detergent composition may contain bleaching agents of the chlorine/bromine-type or the oxygen-type.
- the bleaching agents may be coated or incapsulated.
- examples of inorganic chlorine/bromine-type bleaches are lithium, sodium or calcium hypochlorite or hypobromite as well as chlorinated trisodium phosphate.
- the bleaching system may also comprise a H 2 O 2 source such as perborate or percarbonate which may be combined with a peracid-forming bleach activator such as tetraacetylethylenediamine (TAED) or nonanoyloxybenzenesulfonate (NOBS).
- TAED tetraacetylethylenediamine
- NOBS nonanoyloxybenzenesulfonate
- organic chlorine/bromine-type bleaches are heterocyclic N-bromo and N-chloro imides such as trichloroisocyanuric, tribromoisocyanuric, dibromoisocyanuric and dichloroisocyanuric acids, and salts thereof with water solubilizing cations such as potassium and sodium.
- Hydantoin compounds are also suitable.
- the bleaching system may also comprise peroxyacids of, e.g., the amide, imide, or sulfone type .
- oxygen bleaches are preferred, for example in the form of an inorganic persalt, preferably with a bleach precursor or as a peroxy acid compound.
- suitable peroxy bleach compounds are alkali metal perborates, both tetrahydrates and monohydrates, alkali metal percarbonates, persilicates and perphosphates.
- Preferred activator materials are TAED or NOBS.
- the enzymes of the detergent composition of the invention may be stabilized using conventional stabilizing agents, e.g. a polyol such as propylene glycol or glycerol, a sugar or sugar alcohol, lactic acid, boric acid, or a boric acid derivative such as, e.g., an aromatic borate ester, and the composition may be formulated as described in, e.g., WO 92/19709 and WO 92/19708.
- the enzymes of the invention may also be stabilized by adding reversible enzyme inhibitors, e.g., of the protein type as described in EP 0 544 777 B1.
- the detergent may also contain other conventional detergent ingredients such as, e.g., fabric conditioners including clays, deflocculant material, foam boosters/foam depressors (in dishwashing detergents foam depressors), suds suppressors, anti-corrosion agents, soil-suspending agents, anti-soil-redeposition agents, dyes, dehydrating agents, bactericides, optical brighteners, or perfume.
- fabric conditioners including clays, deflocculant material, foam boosters/foam depressors (in dishwashing detergents foam depressors), suds suppressors, anti-corrosion agents, soil-suspending agents, anti-soil-redeposition agents, dyes, dehydrating agents, bactericides, optical brighteners, or perfume.
- the pH (measured in aqueous solution at use concentration) will usually be neutral or alkaline, e.g. in the range of 7-11.
- laundry detergent compositions within the scope of the invention include: 1) A detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- a detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- a detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- a detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- An aqueous liquid detergent compos ition comprising 6) An aqueous structured liquid detergent composition comprising
- a detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- a detergent composition formulated as a granulate comprising
- a detergent composition formulated as a granulate comprising
- An aqueous liquid detergent composition comprising
- An aqueous liquid detergent composition comprising
- a detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- a detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- a detergent composition formulated as a granulate having a bulk density of at least 600 g/l comprising
- the manganese catalyst may, e.g., be one of the compounds described in "Efficient manganese catalysts for low-temperature bleaching", Nature 369, 1994, pp. 637-639.
- the detergent may also comprise anionic surfactant and/or a bleach system.
- Particular forms of dishwashing detergent compositions within the scope of the invention include:
- the manganese catalyst may, e.g., be one of the compounds described in "Efficient manganese catalysts for low-temperature bleaching", Nature 369, 1994, pp. 637-639.
- the ⁇ -amylases of the invention may be incorporated in concentrations conventionally employed in detergents. It is at present contemplated that, in the detergent composition of the invention, the ⁇ -amylase may be added in an amount corresponding to 0.00001-1 mg (calculated as pure enzyme protein) of ⁇ -amylase per liter of wash/dishwash liquor.
- the starch in the medium was liquified by slowly heating the medium from 60°C to 85°C for 30 minutes. After this the temperature of the medium was quickly raised to 95°C for 10 minutes and then cooled. Lastly the medium was sterilized by heating at 121°C for 40 minutes.
- the culture broth was filtrated and concentrated using a FiltronTM ultrafiltration module with 3KD membranes and washed with deionized water until the conductivity was 1 mS/cm.
- the pH was adjusted to pH 5.9 with 10% (v/v) acetic acid.
- a S-sepharose FF column was equilibrated in EKV-buffer, pH 5.9. If not otherwise stated, the purification buffer was 100 mM boric acid, 10 mM succinic acid, 2 mM CaCl 2 , (EKV-buffer) adjusted to the indicated pH with NaOH.
- the enzyme solution was applied to the column, the column was washed with EKV-buffer, pH 5.9, and the amylase was eluted with a linear NaCl gradient (0-> 500 mM NaCl). Amylase containing fractions were pooled and the pH adjusted to pH 7 with 3% (w/v) NaOH.
- a chelate agarose column was loaded with Cu++ and equilibrated in the following manner: 50 mM CuSO 4 , pH 5 was pumped on to the column until the whole column was blue, then excess of Cu++-ions were removed by washing the column with 500 mM imidazol, pH 7, and finally the column was equilibrated with EKV-buffer, pH 7.
- the amylase pool from the S-sepharose column was applied to the Cu++-loaded Chelate agarose column, the column was washed with EKV-buffer, pH 7, and the enzyme was eluted with a linear gradient of imidazol (0-> 500 mM imidazol).
- Amylase containing fractions were pooled and a solution of saturated ammonium sulphate was added to give a final concentration of 1M (NH 4 ) 2 SO 4 in the pool.
- a phenyl sepharose column was equilibrated in EKV-buffer + 1M (NH 4 ) 2 SO 4 , pH 7.
- the amylase pool from the Cu++-column was applied to the hydrophobic interaction column. Binding experiments had shown that the amylase is a rather hydrophobic enzyme, and hence binds tightly to the phenyl column. Protein which did not bind as tightly to the column was washed off the column with EKV-buffer, pH 7.
- the amylase was step-eluted from the column with EKV-buffer + 25% (v/v) isopropanol.
- the amylase containing pool was adjusted to pH 9.5 with 3% (w/v) NaOH and diluted 5 times with deionized water.
- a Q-sepharose HP column was equilibrated in 20 mM Tris-HCl, pH 9.5.
- the amylase pool from the phenyl sepharose column was applied to the column and the column was washed with 20 mM Tris-HCl, pH 9.5.
- the amylase was eluted with a linear gradient of NaCl (0 -> 250 mM NaCl).
- the amylase peak was adjusted to pH 7 with 10% (v/v) acetic acid.
- a Cu++-loaded chelating sepharose FF column (loaded with Cu++ as described for the chelate agarose column) was equilibrated with EKV-buffer, pH 7.
- the amylase peak from the Q-sepharose column was applied to the column, and the column was washed thoroughly with EKV-buffer, pH 7.
- the amylase was eluted with a steep linear gradient of imidazol (0 -> 500 mM imidazol).
- the purified amylase was purity checked by SDS-PAGE electrophoresis.
- the coomassie stained gel had only one band. Purification of ⁇ -amylase from NCIB 12513
- the culture broth was filtrated and concentrated using a FiltronTM ultrafiltration module with 3KD membranes.
- the concentrated solution was filtrated and saturated to 20% w/w with ammoniumsulfate.
- the solution was then batch absorbed using a AFFI-TTM matrix from Kem-En-Tec A/S.
- the amylase was eluted using 25% isopropanol in 20 mM Tris pH 7.5 after wash of the matrix with deionized water.
- the eluted enzyme was subjected to dialysis (20 mM Tris pH 8.5) and a stepwise batch adsorption on Q-sepharose FF for colour removal was made.
- a chelate agarose column was loaded with Cu++ and equilibrated in the following manner: 50 mM CuSO 4 , pH 5 was pumped on to the column until the whole column was blue, then excess of Cu++-ions was removed by washing the column with 500 mM imidazol, pH 7, and finally the column was equilibrated with 50 mM borate buffer, pH 7.
- the run through from the Q-sepharose FF column was applied on the Cu-chelating agarose and eluted using 250 mM imidazol, 20 mM Tris pH 7.0 and the eluted column was dialysed against 50 mM borate buffer pH 7.0.
- the pH was adjusted to pH 9.5 and the dialysed solution was bound on a Q-sepharose HP and eluted over 10 columns using a linear gradient from 0-250 mM NaCl.
- Amylase containing fractions were pooled and a solution of saturated ammonium sulphate was added to give a final concentration of 20% w/w, and the fractions were applied on a phenyl sepharose column.
- the column was washed using deionized water and eluted using 25% isopropanol in 50 mM borate buffer pH 7.0.
- the purified amylase was purity checked by SDS-PAGE electrophoresis.
- the coomassie stained gel had only one band.
- FIG. 1 A pH profile as shown in Fig. 1, which was determined at 37°C in the pH range of from 4 to 10.5.
- the assay for ⁇ -amylase activity described previously was used, using Britton-Robinson buffer adjusted to predetermined pH values. It appears from Fig. 1 that the enzyme possesses ⁇ -amylase activity at all pH values of from 4 to 10.5, having optimum at pH 7.5-8.5, and at least 60% of the maximum activity at pH 9.5.
- Amino acid sequence of the ⁇ -amylase was determined using standard methods for obtaining and sequencing peptides, for reference see Findlay & Geisow (Eds.), Protein Seguencing - a Practical Approach, 1989, IRL Press.
- N-terminal amino acid sequence was found to be : His-His-Asn-Gly-Thr-Asn-Gly-Thr-Met-Met-Gln-Tyr-Phe-Glu-Trp-Tyr-Leu-Pro-Asn-Asp (SEQ ID No. 3).
- Example 1 fermented and purified as described in Example 1, was found to possess a pI of about 5.8 and a molecular weight of approximately 55 kD.
- the full amino acid sequence of the Bacillus strain NCIB 12513 ⁇ -amylase is disclosed in SEQ ID No. 2 of the present invention.
- the full DNA sequence of the Bacillus strain NCIB 12513 ⁇ -amylase is disclosed in SEQ ID No. 5 of the present invention.
- a pH profile of an ⁇ -amylase obtained from Bacillus strain NCIB 12512 (I), of an ⁇ -amylase obtained from Bacillus strain NCIB 12513 (II) and of Termamyl ® (III) were determined at 55°C in the pH interval of from 4 to 10.5.
- the ⁇ -amylases of the invention were fermented and purified as described in Example 1 and Termamyl ® was obtained from Novo Nordisk A/S.
- the assay for ⁇ -amylase activity described previously was used, using 50 mM Britton-Robinson buffer adjusted to predetermined pH values and a reaction time of 15 minutes. The results are presented in Fig. 2. It appears from Fig. 2 that the ⁇ -amylases of the invention possess ⁇ -amylase activity at all pH values of from pH 4 to pH 10.5, having optimum at pH 7.5-8.5.
- a temperature profile of an ⁇ -amylase obtained from Bacillus strain NCIB 12512 (I), of an ⁇ -amylase obtained from Bacillus strain NCIB 12513 (II) and of Termamyl ® (III) were determined at pH 10.0 in the temperature interval of from 25°C to 95°C.
- the ⁇ -amylases of the invention were fermented and purified as described in Example 1 and Termamyl ® was obtained from Novo Nordisk A/S.
- the assay for ⁇ -amylase activity described previously was used, using 50 mM Britton-Robinson buffer adjusted to pH 10.0 and a reaction time of 10 minutes. The results are presented in Fig. 3. It appears from Fig.
- the ⁇ -amylases of the invention possess ⁇ -amylase activity at all temperature values of from 25°C to 85°C, having optimum at 45°C-55°C, and that the specific activity of the ⁇ -amylase of the invention is 25% higher than the specific activity of Termamyl ® at any temperature in the temperature interval of from 25°C to 55°C.
- ⁇ -amylases of the invention obtained from Bacillus strain NCIB 12289 and from Bacillus strain 12512 as described in Example 1, were tested using the following test for detergent amylases for automatic dishwashing:
- Plates were dipped in hot corn starch and glasses were soiled by pouring corn starch from one glass to another. The plates and glasses were left to dry overnight and then washed in a dishwasher under the following conditions:
- Amylase dosage 0-0.50 mg of enzyme protein per litre of washing liquor
- Detergent dosage 4.0 g per litre of washing liquor
- Dishwashing 45°C, 55°C or 65°C program, Cylinda pH: 10.1 during dishwashing.
- Bacillus strain NCIB 12289 ⁇ -amylase This ⁇ -amylase was tested at 55°C and the results are shown in Fig. 4. It can be seen from Fig. 4 that an RSF value of between 3 and 4 is obtained at an enzyme dosage of 0.1 mg of ⁇ -amylase protein per litre of washing liquor.
- Bacillus strain NCIB 12512 ⁇ -amylase This ⁇ -amylase was tested at 45°C (•), at 55°C (*) and at 65°C (x), and the results are shown in Fig. 5. It can be seen from Fig. 5 that an RSF value of between 3 and 4.5 is obtained at an enzyme dosage of 0.1 mg of ⁇ -amylase protein per litre of washing liquor (the RSF-value increasing with increasing temperature).
- the following mini dishwashing assay was used: A suspension of starchy material was boiled and cooled to 20°C. The cooled starch suspension was applied on small, individually identified glass plates (approx. 2 ⁇ 2 cm) and dried at a temperature in the range of 60-140°C in a drying cabinet. The individual plates were then weighed.
- a solution of standard European-type automatic dishwashing detergent (5 g/l) having a temperature of 55°C was prepared. The detergent was allowed a dissolution time of 1 minute, after which the amylase in question was added to the detergent solution (contained in a beaker equipped with magnetic stirring) so as to give an enzyme concentration of 0.5 mg/l.
- the weighed glass plates held in small supporting clamps, were immersed in a substantially vertical position in the amylase/detergent solution, which was then stirred for 15 minutes at 55°C.
- the glass plates were then removed from the amylase/detergent solution, rinsed with distilled water, dried at 60°C in a drying cabinet and re-weighed.
- the performance of the amylase in question [expressed as an index relative to Termamyl (index 100)] was then determined from the difference in weight of the glass plates before and after treatment, as follows:
- NCIB 12512 Index: 163 ⁇ -amylase (NCIB 12513) Index: 175
- Termamyl ® Spec activity: 2200 U/mg ⁇ -amylase (NCIB 12512) Spec, activity: 4400 U/mg ⁇ -amylase (NCIB 12513) Spec, activity: 5200 U/mg.
- Laundry washing Detergent Commercial US heavy duty granulate detergent (HDG)
- Soil Potato starch colored with Cibacron Blue
- the delta reflectance was calculated from the reflectance obtained for a swatch having been washed with the relevant enzyme and the reflectance obtained for a swatch washed without enzyme. More specifically, the delta reflectance is the reflectance obtained with enzyme minus the reflectance obtained without enzyme.
- the ⁇ -amylases of the invention exert a considerably improved starch removal capacity relative to Termamyl, in other words that the ⁇ -amylases of the invention have an improved laundry washing performance compared to that of Termamyl.
- the kinetics of hydrolysis catalyzed by the ⁇ - amylases of the invention and by Termamyl ® at various substrate concentrations were determined using the Somogyi-Nelson method (described below) with amylose (Merck 4561) and amylopectin (Sigma A7780) as substrates.
- hydrolysis velocities were measured under different substrate concentrations (1%, 0.5%, 0.3%, 0.25% and 0.2%).
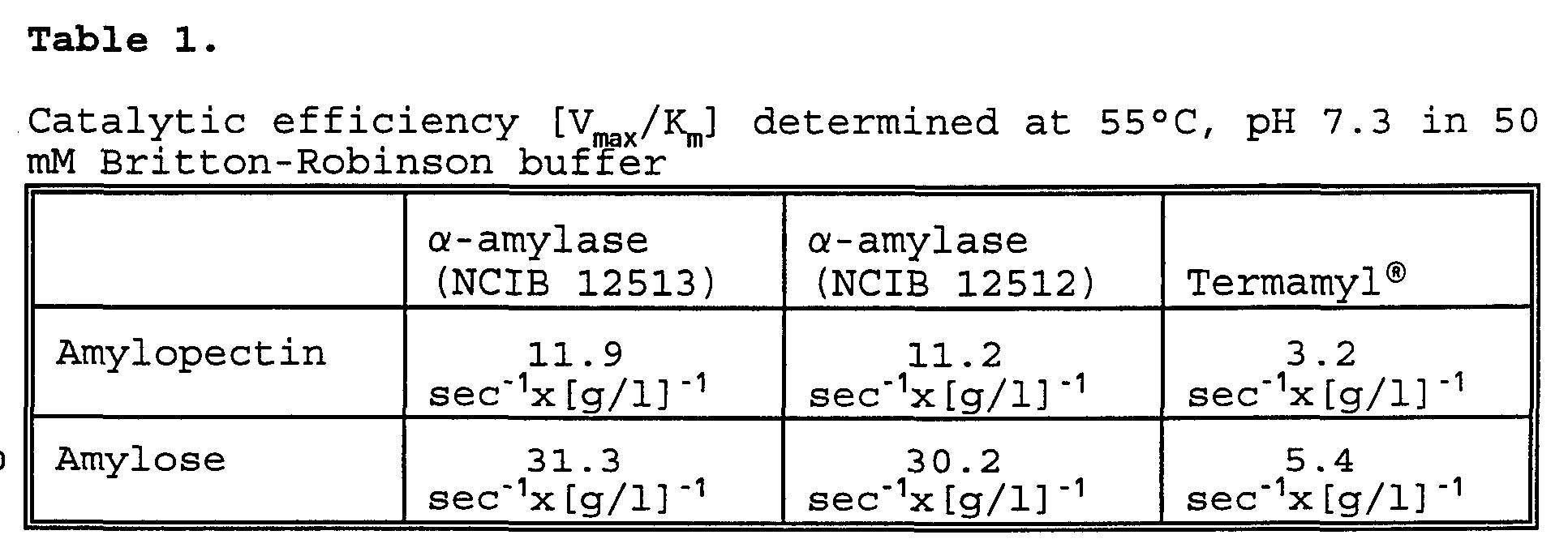
- V max /K m is equivalent to the catalytic efficiency of a given ⁇ - amylase.
- Table 1 below V max /K m is calculated for three different ⁇ -amylases.
- the catalytic efficiency of ⁇ -amylase (NCIB 12513) and ⁇ -amylase (NCIB 12512) have shown to be surprisingly high towards both Amylopectin and Amylose compared to Termamyl. Especially the high catalytic efficienty towards amylose is considered to be of significant importance for the improved specific activities and dishwash/laundry performance compared to Termamyl.
- Linear amylose molecules can align themselves next to each other and form interchain hydrogenbonds through the hydroxyl groups.
- This network of amylose molecules has crystalline characteristics and are difficult to solubilize and hydrolyze by any known amylase.
- the method is based on the principle that the sugar reduces cupric ions to cuprous oxide which reacts with arsenate molybdate reagent to produce a blue colour which is measured spectrophotometrically.
- the solution which is to be examined must contain between 50 and 600 mg of glucose per litre.
- 1 ml of sugar solution is mixed with 1 ml of copper reagent and placed in a boiling water bath for 20 minutes. The resulting mixture is cooled and admixed with 1 ml of Nelson's colour reagent and 10 ml of deionized water. The absorbancy at 520 nm is measured.
- the absorbance is proportional to the amount of sugar, which may thus be calculated as follows:
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Health & Medical Sciences (AREA)
- Wood Science & Technology (AREA)
- Organic Chemistry (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Microbiology (AREA)
- Zoology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Textile Engineering (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Detergent Compositions (AREA)
- Enzymes And Modification Thereof (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Distillation Of Fermentation Liquor, Processing Of Alcohols, Vinegar And Beer (AREA)
- Polysaccharides And Polysaccharide Derivatives (AREA)
Abstract
Description
Claims
Priority Applications (13)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| BR9507229A BR9507229A (en) | 1994-03-29 | 1995-03-29 | Amylase detergent additive detergent composition use of a detergent and an amylase construction of a recombinant cell expression vector dna and process to produce amylase |
| EP95913062A EP0753057B1 (en) | 1994-03-29 | 1995-03-29 | Alkaline bacillus amylase |
| AU20677/95A AU2067795A (en) | 1994-03-29 | 1995-03-29 | Alkaline bacillus amylase |
| CNB951923129A CN1326994C (en) | 1994-03-29 | 1995-03-29 | Alkaling bacillus amylase |
| AT95913062T ATE305031T1 (en) | 1994-03-29 | 1995-03-29 | ALKALINE AMYLASE FROM BACELLUS |
| US08/446,803 US5824531A (en) | 1994-03-29 | 1995-03-29 | Alkaline bacilus amylase |
| MX9604313A MX196038B (en) | 1994-03-29 | 1995-03-29 | Alkaline bacillus amylase. |
| CA 2186592 CA2186592C (en) | 1994-03-29 | 1995-03-29 | Alkaline bacillus amylase |
| KR1019960705305A KR970702363A (en) | 1994-03-29 | 1995-03-29 | Alkaline Bacillus Amylase |
| JP52491395A JPH09510617A (en) | 1994-03-29 | 1995-03-29 | Alkaline bacillus amylase |
| DE1995634464 DE69534464T2 (en) | 1994-03-29 | 1995-03-29 | ALKALIC AMYLASE FROM BACELLUS |
| FI963861A FI119693B (en) | 1994-03-29 | 1996-09-27 | Alkaline Bacillus amylase |
| FI20080645A FI120910B (en) | 1994-03-29 | 2008-12-02 | Alkaline Bacillus amylase |
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DK35394 | 1994-03-29 | ||
| DK353/94 | 1994-03-29 | ||
| DK127194 | 1994-11-03 | ||
| DK1271/94 | 1994-11-03 | ||
| DK12395 | 1995-02-03 | ||
| DK123/95 | 1995-02-03 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1995026397A1 true WO1995026397A1 (en) | 1995-10-05 |
Family
ID=27220422
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/DK1995/000142 WO1995026397A1 (en) | 1994-03-29 | 1995-03-29 | Alkaline bacillus amylase |
Country Status (15)
| Country | Link |
|---|---|
| US (2) | US5824531A (en) |
| EP (2) | EP1637596B1 (en) |
| JP (2) | JPH09510617A (en) |
| KR (1) | KR970702363A (en) |
| CN (1) | CN1326994C (en) |
| AT (2) | ATE510010T1 (en) |
| AU (1) | AU2067795A (en) |
| BR (1) | BR9507229A (en) |
| CA (1) | CA2186592C (en) |
| DE (1) | DE69534464T2 (en) |
| DK (1) | DK0753057T3 (en) |
| ES (1) | ES2250969T3 (en) |
| FI (2) | FI119693B (en) |
| MX (1) | MX196038B (en) |
| WO (1) | WO1995026397A1 (en) |
Cited By (398)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1996023873A1 (en) * | 1995-02-03 | 1996-08-08 | Novo Nordisk A/S | Amylase variants |
| WO1997000324A1 (en) * | 1995-06-14 | 1997-01-03 | Kao Corporation | Gene encoding alkaline liquefying alpha-amylase |
| US5635468A (en) * | 1993-05-19 | 1997-06-03 | Kao Corporation | Liquefying alkaline α-amylase, process for producing the same, and detergent composition containing the same |
| WO1997032961A2 (en) * | 1996-03-07 | 1997-09-12 | The Procter & Gamble Company | Detergent compositions comprising improved amylases |
| WO1997041213A1 (en) | 1996-04-30 | 1997-11-06 | Novo Nordisk A/S | α-AMYLASE MUTANTS |
| WO1997043386A1 (en) * | 1996-05-15 | 1997-11-20 | The Procter & Gamble Company | Detergent compositions comprising improved amylases, cellulase and cationic surfactant |
| WO1998005748A1 (en) * | 1996-08-01 | 1998-02-12 | The Procter & Gamble Company | Detergent compositions comprising improved amylase for dingy fabric clean-up |
| WO1998044126A1 (en) | 1997-03-31 | 1998-10-08 | Kao Corporation | MUTATED α-AMYLASES |
| WO1999020723A2 (en) * | 1997-10-23 | 1999-04-29 | The Procter & Gamble Company | Multiply-substituted protease variant and amylase variant-containing cleaning compositions |
| WO1999020768A1 (en) * | 1997-10-17 | 1999-04-29 | The Procter & Gamble Company | Methods for producing amylase enzymes |
| WO2000041522A2 (en) * | 1999-01-11 | 2000-07-20 | The Procter & Gamble Company | Cleaning compositions containing a multi-function component and method for using |
| US6093562A (en) * | 1996-02-05 | 2000-07-25 | Novo Nordisk A/S | Amylase variants |
| WO2000060058A2 (en) | 1999-03-31 | 2000-10-12 | Novozymes A/S | Polypeptides having alkaline alpha-amylase activity and nucleic acids encoding same |
| US6187576B1 (en) | 1997-10-13 | 2001-02-13 | Novo Nordisk A/S | α-amylase mutants |
| US6194370B1 (en) | 1996-12-31 | 2001-02-27 | The Procter & Gamble Company | Cost effective stain and soil removal aqueous heavy duty liquid laundry detergent compositions |
| US6197070B1 (en) | 1996-05-15 | 2001-03-06 | The Procter & Gamble Company | Detergent compositions comprising alpha combination of α-amylases for malodor stripping |
| US6204232B1 (en) | 1997-10-30 | 2001-03-20 | Novo Nordisk A/S | α-amlase mutants |
| US6221825B1 (en) | 1996-12-31 | 2001-04-24 | The Procter & Gamble Company | Thickened, highly aqueous liquid detergent compositions |
| JP2001507727A (en) * | 1996-12-20 | 2001-06-12 | ザ、プロクター、エンド、ギャンブル、カンパニー | Dishwashing detergent composition containing organic diamine |
| WO2001066712A2 (en) | 2000-03-08 | 2001-09-13 | Novozymes A/S | Variants with altered properties |
| US6361989B1 (en) | 1997-10-13 | 2002-03-26 | Novozymes A/S | α-amylase and α-amylase variants |
| WO2002040370A1 (en) | 2000-11-17 | 2002-05-23 | The Procter & Gamble Company | Water soluble packages |
| US6410295B1 (en) | 1999-03-30 | 2002-06-25 | Novozymes A/S | Alpha-amylase variants |
| EP1241112A2 (en) | 2001-03-15 | 2002-09-18 | The Procter & Gamble Company | Flexible multiple compartment pouch |
| WO2002092797A2 (en) | 2001-05-15 | 2002-11-21 | Novozymes A/S | Alpha-amylase variant with altered properties |
| WO2002099091A2 (en) | 2001-06-06 | 2002-12-12 | Novozymes A/S | Endo-beta-1,4-glucanase from bacillus |
| DE10036752C2 (en) * | 2000-07-28 | 2003-02-20 | Henkel Kgaa | Detergent and cleaning agent with a new amylolytic enzyme from Bacillus sp. A 7-7 (DSM 12368) |
| US6583095B1 (en) | 1998-11-20 | 2003-06-24 | Procter & Gamble Company | Synthesis of bleach activators |
| US6623948B1 (en) * | 1999-03-31 | 2003-09-23 | Novozymes A/S | Nucleic acid sequences encoding alkaline alpha-amylases |
| WO2004003187A2 (en) | 2002-07-01 | 2004-01-08 | Novozymes A/S | Mpg added to fermentation |
| WO2004050820A1 (en) | 2002-12-05 | 2004-06-17 | Novozymes A/S | Beer mashing process |
| WO2004053039A2 (en) | 2002-12-11 | 2004-06-24 | Novozymes A/S | Detergent composition comprising endo-glucanase |
| WO2004111221A1 (en) | 2003-06-19 | 2004-12-23 | Novozymes A/S | Proteases |
| WO2004113551A1 (en) | 2003-06-25 | 2004-12-29 | Novozymes A/S | Process for the hydrolysis of starch |
| WO2005005646A2 (en) | 2003-06-10 | 2005-01-20 | Novozymes North America, Inc. | Fermentation processes and compositions |
| US6887986B1 (en) | 1998-11-16 | 2005-05-03 | Novozymes A/S | α-amylase variants |
| US6953587B2 (en) | 2000-09-13 | 2005-10-11 | Proacter & Gamble Company | Process for making a water-soluble foam component |
| WO2005123911A2 (en) | 2004-06-21 | 2005-12-29 | Novozymes A/S | Proteases |
| WO2006002643A2 (en) | 2004-07-05 | 2006-01-12 | Novozymes A/S | Alpha-amylase variants with altered properties |
| US7005288B1 (en) | 1999-11-10 | 2006-02-28 | Novozymes A/S | Fungamyl-like alpha-amylase variants |
| WO2006063594A1 (en) * | 2004-12-15 | 2006-06-22 | Novozymes A/S | Alkaline bacillus amylase |
| WO2006066596A2 (en) | 2004-12-22 | 2006-06-29 | Novozymes A/S | Hybrid enzymes consisting of an endo-amylase first amino acid sequence and a carbohydrate -binding module as second amino acid sequence |
| US7078213B1 (en) | 1998-02-18 | 2006-07-18 | Novozymes A/S | Alkaline Bacillus amylase |
| US7153820B2 (en) | 2001-08-13 | 2006-12-26 | Ecolab Inc. | Solid detergent composition and method for solidifying a detergent composition |
| US7189552B2 (en) | 2002-12-17 | 2007-03-13 | Novozymes A/S | Thermostable alpha-amylases |
| WO2007035730A2 (en) | 2005-09-20 | 2007-03-29 | Novozymes North America, Inc. | Process of producing a fermentation product |
| EP1818396A2 (en) | 1999-03-30 | 2007-08-15 | Novozymes A/S | Alpha-Amylase variants |
| US7300782B2 (en) | 2001-12-21 | 2007-11-27 | B.R.A.I.N. Biotechnology Research And Information Network Ag | Glycosyl hydrolases |
| US7319112B2 (en) | 2000-07-14 | 2008-01-15 | The Procter & Gamble Co. | Non-halogenated antibacterial agents and processes for making same |
| WO2008021761A2 (en) | 2006-08-11 | 2008-02-21 | Novozymes Biologicals, Inc. | Bacteria cultures and compositions comprising bacteria cultures |
| CN100374557C (en) * | 1998-11-16 | 2008-03-12 | 诺维信公司 | Alpha-amylse variant |
| EP1923455A2 (en) | 2003-02-18 | 2008-05-21 | Novozymes A/S | Detergent compositions |
| WO2008118749A2 (en) | 2007-03-23 | 2008-10-02 | Novozymes Biologicals, Inc. | Preventing and reducing biofilm formation and planktonic proliferation |
| EP1978081A2 (en) | 2000-10-27 | 2008-10-08 | The Procter and Gamble Company | Stabilized liquid compositions |
| EP1980614A2 (en) | 1999-11-10 | 2008-10-15 | Novozymes A/S | Fungamyl-like Alpha-Amylase Variants |
| EP2011864A1 (en) | 1999-03-31 | 2009-01-07 | Novozymes A/S | Polypeptides having alkaline alpha-amylase activity and nucleic acids encoding same |
| WO2009050684A2 (en) | 2007-10-18 | 2009-04-23 | Ecolab Inc. | Pressed, waxy, solid cleaning compositions and methods of making them |
| EP2135931A1 (en) | 2008-06-16 | 2009-12-23 | The Procter and Gamble Company | Use of soil release polymer in fabric treatment compositions |
| EP2135934A1 (en) | 2008-06-16 | 2009-12-23 | Unilever PLC | Use of a laundry detergent composition |
| EP2149786A1 (en) | 2008-08-01 | 2010-02-03 | Unilever PLC | Improvements relating to detergent analysis |
| US7713723B1 (en) | 2000-08-01 | 2010-05-11 | Novozymes A/S | Alpha-amylase mutants with altered properties |
| EP2202290A1 (en) | 2008-12-23 | 2010-06-30 | Unilever PLC | A flowable laundry composition and packaging therefor |
| EP2204446A1 (en) | 2000-08-01 | 2010-07-07 | Novozymes A/S | Alpha-amylase mutants with altered properties |
| EP2213732A1 (en) | 2003-10-28 | 2010-08-04 | Novozymes North America, Inc. | Hybrid glucoamylases |
| WO2010108000A1 (en) | 2009-03-18 | 2010-09-23 | The Procter & Gamble Company | Structured fluid detergent compositions comprising dibenzylidene polyol acetal derivatives and detersive enzymes |
| WO2010108002A1 (en) | 2009-03-18 | 2010-09-23 | The Procter & Gamble Company | Structured fluid detergent compositions comprising dibenzylidene sorbitol acetal derivatives |
| US7803604B2 (en) | 2000-07-28 | 2010-09-28 | Henkel Ag & Co. Kgaa | Amylolytic enzyme extracted from Bacillus sp. A 7-7 (DSM 12368) and washing and cleaning agents containing this novel amylolytic enzyme |
| WO2010127919A1 (en) | 2009-05-05 | 2010-11-11 | Unilever Plc | Shading composition |
| EP2258837A1 (en) | 2004-09-10 | 2010-12-08 | Novozymes North America, Inc. | Methods for preventing, removing, reducing, or disrupting biofilm |
| EP2261359A1 (en) | 1998-06-10 | 2010-12-15 | Novozymes A/S | Mannanases |
| US7888104B2 (en) | 2000-11-28 | 2011-02-15 | Henkel Ag & Co. Kgaa | Cyclodextrin glucanotransferase (CGTase), obtained from<I>Bacillus agaradherens<λ>(DSM 9948) and detergents and cleaning agents containing said novel cyclodextrin glucanotransferase |
| EP2284259A2 (en) | 2003-10-10 | 2011-02-16 | Novozymes A/S | Protease variants |
| EP2295545A1 (en) | 2002-09-26 | 2011-03-16 | Novozymes North America, Inc. | Fermentation methods and compositions |
| WO2011042372A1 (en) | 2009-10-08 | 2011-04-14 | Unilever Plc | Shading composition |
| WO2011045195A1 (en) | 2009-10-13 | 2011-04-21 | Unilever Plc | Dye polymers |
| WO2011047987A1 (en) | 2009-10-23 | 2011-04-28 | Unilever Plc | Dye polymers |
| WO2011049945A2 (en) | 2009-10-23 | 2011-04-28 | Danisco Us Inc. | Methods for reducing blue saccharide |
| WO2011080267A2 (en) | 2009-12-29 | 2011-07-07 | Novozymes A/S | Polypetides having detergency enhancing effect |
| WO2011080354A1 (en) | 2010-01-04 | 2011-07-07 | Novozymes A/S | Alpha-amylases |
| WO2011082889A1 (en) | 2010-01-07 | 2011-07-14 | Unilever Plc | Natural shading agents |
| EP2357220A1 (en) | 2010-02-10 | 2011-08-17 | The Procter & Gamble Company | Cleaning composition comprising amylase variants with high stability in the presence of a chelating agent |
| WO2011098356A1 (en) | 2010-02-12 | 2011-08-18 | Unilever Plc | Laundry treatment composition comprising bis-azo shading dyes |
| WO2011098531A1 (en) | 2010-02-10 | 2011-08-18 | Novozymes A/S | Variants and compositions comprising variants with high stability in presence of a chelating agent |
| WO2011098355A1 (en) | 2010-02-09 | 2011-08-18 | Unilever Plc | Dye polymers |
| WO2011104339A1 (en) | 2010-02-25 | 2011-09-01 | Novozymes A/S | Variants of a lysozyme and polynucleotides encoding same |
| WO2011134809A1 (en) | 2010-04-26 | 2011-11-03 | Novozymes A/S | Enzyme granules |
| WO2011134685A1 (en) | 2010-04-29 | 2011-11-03 | Unilever Plc | Bis-heterocyclic azo dyes |
| US20110274676A1 (en) * | 1997-04-18 | 2011-11-10 | Sean Farmer | Topical Use Of Probiotic Bacillus Spores To Prevent Or Control Microbial Infections |
| US8084240B2 (en) | 2008-06-06 | 2011-12-27 | Danisco Us Inc. | Geobacillus stearothermophilus α-amylase (AmyS) variants with improved properties |
| WO2011161135A1 (en) | 2010-06-22 | 2011-12-29 | Novozymes A/S | Enzyme dehairing of skins and hides |
| DE212009000119U1 (en) | 2008-09-12 | 2011-12-30 | Unilever N.V. | Dispenser and pretreatment agent for viscous liquids |
| WO2012019169A1 (en) | 2010-08-06 | 2012-02-09 | Danisco Us Inc. | Production of isoprene under neutral ph conditions |
| WO2012019159A1 (en) | 2010-08-06 | 2012-02-09 | Danisco Us Inc. | Neutral ph saccharification and fermentation |
| WO2012035103A1 (en) | 2010-09-16 | 2012-03-22 | Novozymes A/S | Lysozymes |
| WO2012038144A1 (en) | 2010-09-20 | 2012-03-29 | Unilever Plc | Fabric treatment compositions comprising target benefit agents |
| US8153412B2 (en) | 2007-11-05 | 2012-04-10 | Danisco Us Inc. | Variants of Bacillus sp. TS-23 alpha-amylase with altered properties |
| EP2441822A1 (en) | 2010-10-14 | 2012-04-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Laundry detergent particles |
| EP2441825A1 (en) | 2010-10-14 | 2012-04-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Process for preparing laundry detergent particles |
| EP2441820A1 (en) | 2010-10-14 | 2012-04-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Laundry detergent particles |
| WO2012049053A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Package comprising a laundry composition, dispenser for said package and method for washing using said dispenser and said package |
| WO2012048951A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012049032A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Refill and refillable packages of concentrated particulate detergent compositions |
| WO2012049034A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaging and dispensing of detergent compositions |
| WO2012048910A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaged particulate detergent composition |
| WO2012048950A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012048945A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Particulate detergent compositions comprising fluorescer |
| WO2012048947A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012049178A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012048949A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particle |
| WO2012048909A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaged particulate detergent composition |
| WO2012049055A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Transparent packaging of detergent compositions |
| WO2012048956A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaged concentrated particulate detergent composition |
| WO2012048948A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012049033A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Top-loading laundry vessel method |
| WO2012048955A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaging and dispensing of detergent compositions |
| WO2012052306A1 (en) | 2010-10-22 | 2012-04-26 | Unilever Plc | Externally structured aqueous detergent liquid |
| US8206966B2 (en) | 2007-11-05 | 2012-06-26 | Danisco Us Inc. | Alpha-amylase variants with altered properties |
| EP2476743A1 (en) | 2011-04-04 | 2012-07-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Method of laundering fabric |
| WO2012098046A1 (en) | 2011-01-17 | 2012-07-26 | Unilever Plc | Dye polymer for laundry treatment |
| US8236545B2 (en) | 2008-02-04 | 2012-08-07 | Danisco Us Inc., Genencor Division | TS23 alpha-amylase variants with altered properties |
| WO2012104159A1 (en) | 2011-01-31 | 2012-08-09 | Unilever Plc | Alkaline liquid detergent compositions |
| WO2012112718A1 (en) | 2011-02-15 | 2012-08-23 | Novozymes Biologicals, Inc. | Mitigation of odor in cleaning machines and cleaning processes |
| EP2495316A2 (en) | 2006-06-21 | 2012-09-05 | Novozymes North America, Inc. | Desizing and scouring process of starch |
| WO2012119859A1 (en) | 2011-03-10 | 2012-09-13 | Unilever Plc | Dye polymer |
| WO2012130492A1 (en) | 2011-03-25 | 2012-10-04 | Unilever Plc | Dye polymer |
| WO2012149275A1 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Use of cellulase and glucoamylase to improve ethanol yields from fermentation |
| WO2012149288A1 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Single ph process for starch liquefaction and saccharification for high-density glucose syrups |
| EP2522715A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| EP2522714A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| WO2012156250A1 (en) | 2011-05-13 | 2012-11-22 | Unilever Plc | Aqueous concentrated laundry detergent compositions |
| WO2012159778A1 (en) | 2011-05-26 | 2012-11-29 | Unilever Plc | Liquid laundry composition |
| WO2012160498A2 (en) | 2011-05-20 | 2012-11-29 | Ecolab Usa Inc. | Acid formulations for use in a system for warewashing |
| US8323946B2 (en) | 1995-02-03 | 2012-12-04 | Novozymes A/S | Alpha-amylase mutants |
| US8323945B2 (en) | 2008-06-06 | 2012-12-04 | Danisco Us Inc. | Variant alpha-amylases from Bacillus subtilis and methods of uses, thereof |
| EP2537918A1 (en) | 2011-06-20 | 2012-12-26 | The Procter & Gamble Company | Consumer products with lipase comprising coated particles |
| WO2012175401A2 (en) | 2011-06-20 | 2012-12-27 | Novozymes A/S | Particulate composition |
| EP2540824A1 (en) | 2011-06-30 | 2013-01-02 | The Procter & Gamble Company | Cleaning compositions comprising amylase variants reference to a sequence listing |
| WO2013001087A2 (en) | 2011-06-30 | 2013-01-03 | Novozymes A/S | Method for screening alpha-amylases |
| WO2013006756A2 (en) | 2011-07-06 | 2013-01-10 | Novozymes A/S | Alpha amylase variants and polynucleotides encoding same |
| WO2013011071A1 (en) | 2011-07-21 | 2013-01-24 | Unilever Plc | Liquid laundry composition |
| WO2013022799A1 (en) | 2011-08-05 | 2013-02-14 | Danisco Us Inc. | PRODUCTION OF ISOPRENOIDS UNDER NEUTRAL pH CONDITIONS |
| WO2013023938A1 (en) | 2011-08-12 | 2013-02-21 | Novozymes A/S | Reduction of culture viscosity by manganese addition |
| WO2013026796A1 (en) | 2011-08-19 | 2013-02-28 | Novozymes A/S | Polypeptides having protease activity |
| WO2013057143A2 (en) | 2011-10-17 | 2013-04-25 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2013057141A2 (en) | 2011-10-17 | 2013-04-25 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2013092052A1 (en) | 2011-12-20 | 2013-06-27 | Unilever Plc | Isotropic liquid detergents comprising soil release polymer |
| WO2013098185A1 (en) | 2011-12-28 | 2013-07-04 | Novozymes A/S | Polypeptides having protease activity |
| EP2617804A1 (en) | 2007-02-15 | 2013-07-24 | Ecolab Inc. | Fast dissolving solid detergent |
| US8507243B2 (en) | 2008-09-25 | 2013-08-13 | Danisco Us Inc. | Alpha-amylase blends and methods for using said blends |
| EP2639291A1 (en) | 2012-03-13 | 2013-09-18 | Unilever PLC | Packaged particulate detergent composition |
| WO2013139702A1 (en) | 2012-03-21 | 2013-09-26 | Unilever Plc | Laundry detergent particles |
| WO2013149753A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particles |
| WO2013149755A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particles |
| WO2013149754A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particle |
| WO2013149752A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particles |
| WO2013160025A1 (en) | 2012-04-23 | 2013-10-31 | Unilever Plc | Structured aqueous liquid detergent |
| WO2014006040A1 (en) | 2012-07-06 | 2014-01-09 | Novozymes A/S | Inactivation of a production strain using a fatty acid |
| WO2014048857A1 (en) | 2012-09-25 | 2014-04-03 | Unilever Plc | Laundry detergent particles |
| WO2014068083A1 (en) | 2012-11-01 | 2014-05-08 | Novozymes A/S | Method for removal of dna |
| WO2014086659A2 (en) | 2012-12-06 | 2014-06-12 | Ahmedabad Textile Industry's Research Association | Method for enzymatical preparation of textiles |
| US8809031B2 (en) | 2007-03-23 | 2014-08-19 | Danisco Us Inc. | Enhanced amylase production by N-terminal addition to mature amylase protein |
| EP2792737A1 (en) | 2011-05-20 | 2014-10-22 | Ecolab USA Inc. | Non-phosphate detergents and non-phosphoric acids in an alternating alkali/acid system for warewashing |
| WO2014194032A1 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| WO2014191170A1 (en) | 2013-05-30 | 2014-12-04 | Novozymes A/S | Particulate enzyme composition |
| WO2014194054A1 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| WO2014194034A2 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| WO2014194117A2 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| WO2014198840A1 (en) | 2013-06-12 | 2014-12-18 | Earth Alive Clean Technologies Inc. | Dust suppressant |
| WO2015057517A1 (en) | 2013-10-17 | 2015-04-23 | Danisco Us Inc. | Use of hemicellulases to improve ethanol production |
| US9040279B2 (en) | 2008-06-06 | 2015-05-26 | Danisco Us Inc. | Saccharification enzyme composition and method of saccharification thereof |
| US9040278B2 (en) | 2008-06-06 | 2015-05-26 | Danisco Us Inc. | Production of glucose from starch using alpha-amylases from Bacillus subtilis |
| WO2015089447A1 (en) | 2013-12-13 | 2015-06-18 | Danisco Us Inc. | Serine proteases of the bacillus gibsonii-clade |
| WO2015089441A1 (en) | 2013-12-13 | 2015-06-18 | Danisco Us Inc. | Serine proteases of bacillus species |
| WO2015095358A1 (en) | 2013-12-18 | 2015-06-25 | E. I. Du Pont De Nemours And Company | Cationic poly alpha-1,3-glucan ethers |
| WO2015123323A1 (en) | 2014-02-14 | 2015-08-20 | E. I. Du Pont De Nemours And Company | Poly-alpha-1,3-1,6-glucans for viscosity modification |
| WO2015138283A1 (en) | 2014-03-11 | 2015-09-17 | E. I. Du Pont De Nemours And Company | Oxidized poly alpha-1,3-glucan as detergent builder |
| WO2015195960A1 (en) | 2014-06-19 | 2015-12-23 | E. I. Du Pont De Nemours And Company | Compositions containing one or more poly alpha-1,3-glucan ether compounds |
| WO2015195777A1 (en) | 2014-06-19 | 2015-12-23 | E. I. Du Pont De Nemours And Company | Compositions containing one or more poly alpha-1,3-glucan ether compounds |
| WO2016041676A1 (en) | 2014-09-18 | 2016-03-24 | Unilever Plc | Whitening composition |
| WO2016061438A1 (en) | 2014-10-17 | 2016-04-21 | Danisco Us Inc. | Serine proteases of bacillus species |
| WO2016069569A2 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| WO2016069552A1 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| WO2016069548A2 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| WO2016069557A1 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases of bacillus species |
| WO2016069544A1 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| WO2016079305A1 (en) | 2014-11-20 | 2016-05-26 | Novozymes A/S | Alicyclobacillus variants and polynucleotides encoding same |
| WO2016106011A1 (en) | 2014-12-23 | 2016-06-30 | E. I. Du Pont De Nemours And Company | Enzymatically produced cellulose |
| WO2016110378A1 (en) | 2015-01-09 | 2016-07-14 | Unilever Plc | Laundry treatment composition comprising a dye |
| WO2016128466A1 (en) | 2015-02-13 | 2016-08-18 | Unilever Plc | Laundry liquid composition |
| US9434932B2 (en) | 2011-06-30 | 2016-09-06 | Novozymes A/S | Alpha-amylase variants |
| WO2016155993A1 (en) | 2015-04-02 | 2016-10-06 | Unilever Plc | Composition |
| WO2016180792A1 (en) | 2015-05-08 | 2016-11-17 | Novozymes A/S | Alpha-amylase variants having improved performance and stability |
| WO2016180748A1 (en) | 2015-05-08 | 2016-11-17 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2016201069A1 (en) | 2015-06-09 | 2016-12-15 | Danisco Us Inc | Low-density enzyme-containing particles |
| WO2016201044A1 (en) | 2015-06-09 | 2016-12-15 | Danisco Us Inc | Osmotic burst encapsulates |
| WO2016201040A1 (en) | 2015-06-09 | 2016-12-15 | Danisco Us Inc. | Water-triggered enzyme suspension |
| WO2016205755A1 (en) | 2015-06-17 | 2016-12-22 | Danisco Us Inc. | Bacillus gibsonii-clade serine proteases |
| US9540669B2 (en) | 2012-08-14 | 2017-01-10 | Danisco Us Inc. | Trichoderma reesei glucoamylase variants resistant to oxidation-related activity loss and the use thereof |
| WO2017036915A1 (en) | 2015-08-28 | 2017-03-09 | Unilever N.V. | Liquid detergency composition comprising protease and non-protease enzyme |
| WO2017079756A1 (en) | 2015-11-05 | 2017-05-11 | Danisco Us Inc | Paenibacillus and bacillus spp. mannanases |
| WO2017079751A1 (en) | 2015-11-05 | 2017-05-11 | Danisco Us Inc | Paenibacillus sp. mannanases |
| WO2017083226A1 (en) | 2015-11-13 | 2017-05-18 | E. I. Du Pont De Nemours And Company | Glucan fiber compositions for use in laundry care and fabric care |
| WO2017083229A1 (en) | 2015-11-13 | 2017-05-18 | E. I. Du Pont De Nemours And Company | Glucan fiber compositions for use in laundry care and fabric care |
| WO2017083228A1 (en) | 2015-11-13 | 2017-05-18 | E. I. Du Pont De Nemours And Company | Glucan fiber compositions for use in laundry care and fabric care |
| WO2017106676A1 (en) | 2015-12-18 | 2017-06-22 | Danisco Us Inc | Polypeptides with endoglucanase activity and uses thereof |
| EP3190167A1 (en) | 2016-01-07 | 2017-07-12 | Unilever PLC | Bitter pill |
| WO2017121714A1 (en) | 2016-01-15 | 2017-07-20 | Unilever Plc | Dye |
| WO2017133879A1 (en) | 2016-02-04 | 2017-08-10 | Unilever Plc | Detergent liquid |
| WO2017140391A1 (en) | 2016-02-17 | 2017-08-24 | Unilever Plc | Whitening composition |
| WO2017140392A1 (en) | 2016-02-17 | 2017-08-24 | Unilever Plc | Whitening composition |
| WO2017162378A1 (en) | 2016-03-21 | 2017-09-28 | Unilever Plc | Laundry detergent composition |
| WO2017173324A2 (en) | 2016-04-01 | 2017-10-05 | Danisco Us Inc. | Alpha-amylases, compositions & methods |
| WO2017173190A2 (en) | 2016-04-01 | 2017-10-05 | Danisco Us Inc. | Alpha-amylases, compositions & methods |
| WO2017174251A1 (en) | 2016-04-08 | 2017-10-12 | Unilever Plc | Laundry detergent composition |
| WO2017192300A1 (en) | 2016-05-05 | 2017-11-09 | Danisco Us Inc | Protease variants and uses thereof |
| WO2017192692A1 (en) | 2016-05-03 | 2017-11-09 | Danisco Us Inc | Protease variants and uses thereof |
| US9833761B2 (en) | 2013-08-05 | 2017-12-05 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| WO2017210295A1 (en) | 2016-05-31 | 2017-12-07 | Danisco Us Inc. | Protease variants and uses thereof |
| WO2017211697A1 (en) | 2016-06-09 | 2017-12-14 | Unilever Plc | Laundry products |
| WO2017219011A1 (en) | 2016-06-17 | 2017-12-21 | Danisco Us Inc | Protease variants and uses thereof |
| EP3282004A1 (en) | 2011-12-13 | 2018-02-14 | Ecolab USA Inc. | Warewashing method |
| US9895673B2 (en) | 2015-12-01 | 2018-02-20 | Twist Bioscience Corporation | Functionalized surfaces and preparation thereof |
| WO2018072979A1 (en) | 2016-10-18 | 2018-04-26 | Unilever Plc | Whitening composition |
| WO2018085524A2 (en) | 2016-11-07 | 2018-05-11 | Danisco Us Inc | Laundry detergent composition |
| US9981239B2 (en) | 2015-04-21 | 2018-05-29 | Twist Bioscience Corporation | Devices and methods for oligonucleic acid library synthesis |
| WO2018108382A1 (en) | 2016-12-15 | 2018-06-21 | Unilever Plc | Laundry detergent composition |
| WO2018118950A1 (en) | 2016-12-21 | 2018-06-28 | Danisco Us Inc. | Bacillus gibsonii-clade serine proteases |
| WO2018118917A1 (en) | 2016-12-21 | 2018-06-28 | Danisco Us Inc. | Protease variants and uses thereof |
| EP3354792A1 (en) | 2011-06-01 | 2018-08-01 | Unilever PLC, a company registered in England and Wales under company no. 41424 of | Liquid detergent composition containing dye polymer |
| US10053688B2 (en) | 2016-08-22 | 2018-08-21 | Twist Bioscience Corporation | De novo synthesized nucleic acid libraries |
| WO2018169750A1 (en) | 2017-03-15 | 2018-09-20 | Danisco Us Inc | Trypsin-like serine proteases and uses thereof |
| WO2018184004A1 (en) | 2017-03-31 | 2018-10-04 | Danisco Us Inc | Alpha-amylase combinatorial variants |
| WO2018183662A1 (en) | 2017-03-31 | 2018-10-04 | Danisco Us Inc | Delayed release enzyme formulations for bleach-containing detergents |
| EP2940116B1 (en) | 2014-04-30 | 2018-10-17 | The Procter and Gamble Company | Detergent |
| WO2018206197A1 (en) | 2017-05-10 | 2018-11-15 | Unilever Plc | Laundry detergent composition |
| WO2018206202A1 (en) | 2017-05-10 | 2018-11-15 | Unilever Plc | Laundry detergent composition |
| WO2018234056A1 (en) | 2017-06-20 | 2018-12-27 | Unilever N.V. | Particulate detergent composition comprising perfume |
| WO2018234003A1 (en) | 2017-06-21 | 2018-12-27 | Unilever Plc | Packaging and dispensing of detergent compositions |
| WO2019006077A1 (en) | 2017-06-30 | 2019-01-03 | Danisco Us Inc | Low-agglomeration, enzyme-containing particles |
| WO2019008035A1 (en) | 2017-07-07 | 2019-01-10 | Unilever Plc | Laundry cleaning composition |
| WO2019008036A1 (en) | 2017-07-07 | 2019-01-10 | Unilever Plc | Whitening composition |
| WO2019036721A2 (en) | 2017-08-18 | 2019-02-21 | Danisco Us Inc | Alpha-amylase variants |
| WO2019038187A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| WO2019038186A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| EP3470516A1 (en) | 2012-09-10 | 2019-04-17 | Ecolab USA Inc. | Stable liquid manual dishwashing compositions containing enzymes |
| WO2019105675A1 (en) | 2017-11-30 | 2019-06-06 | Unilever Plc | Detergent composition comprising protease |
| WO2019108599A1 (en) | 2017-11-29 | 2019-06-06 | Danisco Us Inc | Subtilisin variants having improved stability |
| US10316275B2 (en) | 2015-05-08 | 2019-06-11 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2019125683A1 (en) | 2017-12-21 | 2019-06-27 | Danisco Us Inc | Enzyme-containing, hot-melt granules comprising a thermotolerant desiccant |
| WO2019156670A1 (en) | 2018-02-08 | 2019-08-15 | Danisco Us Inc. | Thermally-resistant wax matrix particles for enzyme encapsulation |
| EP3533858A1 (en) * | 2018-02-28 | 2019-09-04 | The Procter & Gamble Company | Cleaning composition comprising a glycogen-debranching enzyme and methods of cleaning |
| US10417457B2 (en) | 2016-09-21 | 2019-09-17 | Twist Bioscience Corporation | Nucleic acid based data storage |
| US10428321B2 (en) | 2014-06-12 | 2019-10-01 | Novozymes A/S | Alpha-amylase variants |
| EP3550015A1 (en) | 2014-04-10 | 2019-10-09 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2019219302A1 (en) | 2018-05-17 | 2019-11-21 | Unilever Plc | Cleaning composition comprising rhamnolipid and alkyl ether carboxylate surfactants |
| WO2019219531A1 (en) | 2018-05-17 | 2019-11-21 | Unilever Plc | Cleaning composition |
| WO2019238423A1 (en) | 2018-06-12 | 2019-12-19 | Novozymes A/S | Less added sugar in baked products |
| WO2019245705A1 (en) | 2018-06-19 | 2019-12-26 | Danisco Us Inc | Subtilisin variants |
| WO2019245704A1 (en) | 2018-06-19 | 2019-12-26 | Danisco Us Inc | Subtilisin variants |
| EP3587569A1 (en) | 2014-03-21 | 2020-01-01 | Danisco US Inc. | Serine proteases of bacillus species |
| WO2020016097A1 (en) | 2018-07-17 | 2020-01-23 | Unilever Plc | Use of a rhamnolipid in a surfactant system |
| WO2020020703A1 (en) | 2018-07-27 | 2020-01-30 | Unilever N.V. | Laundry detergent |
| WO2020028443A1 (en) | 2018-07-31 | 2020-02-06 | Danisco Us Inc | Variant alpha-amylases having amino acid substitutions that lower the pka of the general acid |
| WO2020047215A1 (en) | 2018-08-30 | 2020-03-05 | Danisco Us Inc | Enzyme-containing granules |
| WO2020058024A1 (en) | 2018-09-17 | 2020-03-26 | Unilever Plc | Detergent composition |
| WO2020068486A1 (en) | 2018-09-27 | 2020-04-02 | Danisco Us Inc | Compositions for medical instrument cleaning |
| WO2020077331A2 (en) | 2018-10-12 | 2020-04-16 | Danisco Us Inc | Alpha-amylases with mutations that improve stability in the presence of chelants |
| WO2020104158A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104155A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104156A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104157A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104159A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| US10669304B2 (en) | 2015-02-04 | 2020-06-02 | Twist Bioscience Corporation | Methods and devices for de novo oligonucleic acid assembly |
| WO2020112599A1 (en) | 2018-11-28 | 2020-06-04 | Danisco Us Inc | Subtilisin variants having improved stability |
| WO2020114965A1 (en) | 2018-12-03 | 2020-06-11 | Novozymes A/S | LOW pH POWDER DETERGENT COMPOSITION |
| US10696965B2 (en) | 2017-06-12 | 2020-06-30 | Twist Bioscience Corporation | Methods for seamless nucleic acid assembly |
| WO2020151959A1 (en) | 2019-01-22 | 2020-07-30 | Unilever N.V. | Laundry detergent |
| WO2020151992A1 (en) | 2019-01-22 | 2020-07-30 | Unilever N.V. | Laundry detergent |
| WO2020229535A1 (en) | 2019-05-16 | 2020-11-19 | Unilever Plc | Laundry composition |
| US10844373B2 (en) | 2015-09-18 | 2020-11-24 | Twist Bioscience Corporation | Oligonucleic acid variant libraries and synthesis thereof |
| WO2020242858A1 (en) | 2019-05-24 | 2020-12-03 | Danisco Us Inc | Subtilisin variants and methods of use |
| WO2020247582A1 (en) | 2019-06-06 | 2020-12-10 | Danisco Us Inc | Methods and compositions for cleaning |
| WO2020249546A1 (en) | 2019-06-13 | 2020-12-17 | Basf Se | Method of recovering a protein from fermentation broth using a divalent cation |
| WO2020260040A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259949A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259948A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020264552A1 (en) | 2019-06-24 | 2020-12-30 | The Procter & Gamble Company | Cleaning compositions comprising amylase variants |
| WO2020259947A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020260038A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020260006A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent compositions |
| WO2020260223A1 (en) | 2019-06-24 | 2020-12-30 | Novozymes A/S | Alpha-amylase variants |
| WO2021004830A1 (en) | 2019-07-05 | 2021-01-14 | Basf Se | Industrial fermentation process for microbial cells using a fed-batch pre-culture |
| US10894959B2 (en) | 2017-03-15 | 2021-01-19 | Twist Bioscience Corporation | Variant libraries of the immunological synapse and synthesis thereof |
| US10894242B2 (en) | 2017-10-20 | 2021-01-19 | Twist Bioscience Corporation | Heated nanowells for polynucleotide synthesis |
| US10907274B2 (en) | 2016-12-16 | 2021-02-02 | Twist Bioscience Corporation | Variant libraries of the immunological synapse and synthesis thereof |
| WO2021022045A1 (en) | 2019-07-31 | 2021-02-04 | Ecolab Usa Inc. | Personal protective equipment free delimer compositions |
| US10936953B2 (en) | 2018-01-04 | 2021-03-02 | Twist Bioscience Corporation | DNA-based digital information storage with sidewall electrodes |
| WO2021043764A1 (en) | 2019-09-02 | 2021-03-11 | Unilever Global Ip Limited | Detergent composition |
| WO2021053122A1 (en) | 2019-09-19 | 2021-03-25 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2021069516A1 (en) | 2019-10-07 | 2021-04-15 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021080948A2 (en) | 2019-10-24 | 2021-04-29 | Danisco Us Inc | Variant maltopentaose/maltohexaose-forming alpha-amylases |
| CN112844816A (en) * | 2021-03-18 | 2021-05-28 | 河南金源黄金矿业有限责任公司 | Gravity separation regrinding process and equipment for treating Nielsen products |
| WO2021151536A1 (en) | 2020-01-29 | 2021-08-05 | Unilever Ip Holdings B.V. | Laundry detergent product |
| EP3872174A1 (en) | 2015-05-13 | 2021-09-01 | Danisco US Inc. | Aprl-clade protease variants and uses thereof |
| WO2021185956A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021185870A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021219297A1 (en) | 2020-04-29 | 2021-11-04 | Henkel Ag & Co. Kgaa | Highly alkaline textile detergent containing protease |
| WO2021219296A1 (en) | 2020-04-29 | 2021-11-04 | Henkel Ag & Co. Kgaa | Highly alkaline textile washing agent comprising protease |
| WO2021249927A1 (en) | 2020-06-08 | 2021-12-16 | Unilever Ip Holdings B.V. | Method of improving protease activity |
| WO2022043138A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022043045A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022043042A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022042989A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022042977A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022047149A1 (en) | 2020-08-27 | 2022-03-03 | Danisco Us Inc | Enzymes and enzyme compositions for cleaning |
| WO2022074037A2 (en) | 2020-10-07 | 2022-04-14 | Novozymes A/S | Alpha-amylase variants |
| WO2022090562A1 (en) | 2020-11-02 | 2022-05-05 | Novozymes A/S | Baked and par-baked products with thermostable amg variants from penicillium |
| US11332738B2 (en) | 2019-06-21 | 2022-05-17 | Twist Bioscience Corporation | Barcode-based nucleic acid sequence assembly |
| EP4011256A1 (en) | 2020-12-14 | 2022-06-15 | Henkel AG & Co. KGaA | Method for cleaning an electric motorised kitchen appliance |
| EP4012011A1 (en) | 2020-12-14 | 2022-06-15 | Henkel AG & Co. KGaA | Cleaning agent, particularly for a kitchen appliance |
| WO2022122481A1 (en) | 2020-12-07 | 2022-06-16 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2022122480A1 (en) | 2020-12-07 | 2022-06-16 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2022128781A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Cleaning composition |
| WO2022128786A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Use and cleaning composition |
| WO2022128620A1 (en) | 2020-12-14 | 2022-06-23 | Henkel Ag & Co. Kgaa | Method for cleaning a food processor that is driven by an electric motor |
| US11377676B2 (en) | 2017-06-12 | 2022-07-05 | Twist Bioscience Corporation | Methods for seamless nucleic acid assembly |
| WO2022165107A1 (en) | 2021-01-29 | 2022-08-04 | Danisco Us Inc | Compositions for cleaning and methods related thereto |
| US11407837B2 (en) | 2017-09-11 | 2022-08-09 | Twist Bioscience Corporation | GPCR binding proteins and synthesis thereof |
| WO2022171780A2 (en) | 2021-02-12 | 2022-08-18 | Novozymes A/S | Alpha-amylase variants |
| WO2022199418A1 (en) | 2021-03-26 | 2022-09-29 | Novozymes A/S | Detergent composition with reduced polymer content |
| US11492728B2 (en) | 2019-02-26 | 2022-11-08 | Twist Bioscience Corporation | Variant nucleic acid libraries for antibody optimization |
| US11492727B2 (en) | 2019-02-26 | 2022-11-08 | Twist Bioscience Corporation | Variant nucleic acid libraries for GLP1 receptor |
| US11492665B2 (en) | 2018-05-18 | 2022-11-08 | Twist Bioscience Corporation | Polynucleotides, reagents, and methods for nucleic acid hybridization |
| US11512347B2 (en) | 2015-09-22 | 2022-11-29 | Twist Bioscience Corporation | Flexible substrates for nucleic acid synthesis |
| WO2022268885A1 (en) | 2021-06-23 | 2022-12-29 | Novozymes A/S | Alpha-amylase polypeptides |
| WO2023278297A1 (en) | 2021-06-30 | 2023-01-05 | Danisco Us Inc | Variant lipases and uses thereof |
| US11550939B2 (en) | 2017-02-22 | 2023-01-10 | Twist Bioscience Corporation | Nucleic acid based data storage using enzymatic bioencryption |
| WO2023034486A2 (en) | 2021-09-03 | 2023-03-09 | Danisco Us Inc. | Laundry compositions for cleaning |
| WO2023039270A2 (en) | 2021-09-13 | 2023-03-16 | Danisco Us Inc. | Bioactive-containing granules |
| WO2023041694A1 (en) | 2021-09-20 | 2023-03-23 | Unilever Ip Holdings B.V. | Detergent composition |
| EP4163305A1 (en) | 2013-12-16 | 2023-04-12 | Nutrition & Biosciences USA 4, Inc. | Use of poly alpha-1,3-glucan ethers as viscosity modifiers |
| WO2023057367A1 (en) | 2021-10-08 | 2023-04-13 | Unilever Ip Holdings B.V. | Laundry composition |
| DE102021213462A1 (en) | 2021-11-30 | 2023-06-01 | Henkel Ag & Co. Kgaa | Method for cleaning a food processor operated by an electric motor |
| WO2023114932A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2023114936A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2023114939A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2023114988A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Variant maltopentaose/maltohexaose-forming alpha-amylases |
| WO2023168234A1 (en) | 2022-03-01 | 2023-09-07 | Danisco Us Inc. | Enzymes and enzyme compositions for cleaning |
| WO2023213424A1 (en) | 2022-05-04 | 2023-11-09 | Novozymes A/S | Brewing with thermostable amg variants |
| WO2023225459A2 (en) | 2022-05-14 | 2023-11-23 | Novozymes A/S | Compositions and methods for preventing, treating, supressing and/or eliminating phytopathogenic infestations and infections |
| WO2023227375A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Laundry liquid composition comprising a surfactant, an aminocarboxylate, an organic acid and a fragrance |
| WO2023227331A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Composition comprising a specific methyl ester ethoxylate surfactant and a lipase |
| WO2023227335A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Liquid composition comprising linear alkyl benzene sulphonate, methyl ester ethoxylate and alkoxylated zwitterionic polyamine polymer |
| WO2023227332A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Laundry liquid composition comprising a surfactant, an alkoxylated zwitterionic polyamine polymer and a protease |
| WO2023227421A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Laundry liquid composition comprising a surfactant, an alkoxylated zwitterionic polyamine polymer, and a fragrance |
| WO2023227356A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Composition containing enzyme |
| WO2023233028A1 (en) | 2022-06-03 | 2023-12-07 | Unilever Ip Holdings B.V. | Laundry detergent product |
| DE102022205591A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | DETERGENT AND CLEANING AGENTS WITH IMPROVED ENZYME STABILITY |
| WO2023232192A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | Detergent and cleaning agent with improved enzyme stability |
| DE102022205594A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | PERFORMANCE-IMPROVED AND STORAGE-STABLE PROTEASE VARIANTS |
| DE102022205588A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | DETERGENT AND CLEANING AGENTS WITH IMPROVED ENZYME STABILITY |
| WO2023250301A1 (en) | 2022-06-21 | 2023-12-28 | Danisco Us Inc. | Methods and compositions for cleaning comprising a polypeptide having thermolysin activity |
| WO2024028160A1 (en) | 2022-08-04 | 2024-02-08 | Unilever Ip Holdings B.V. | Packaged homecare product |
| WO2024028161A1 (en) | 2022-08-04 | 2024-02-08 | Unilever Ip Holdings B.V. | Packaged homecare product |
| WO2024028159A1 (en) | 2022-08-04 | 2024-02-08 | Unilever Ip Holdings B.V. | Packaged homecare product |
| EP4324900A1 (en) | 2022-08-17 | 2024-02-21 | Henkel AG & Co. KGaA | Detergent composition comprising enzymes |
| US11920170B2 (en) | 2015-12-09 | 2024-03-05 | Danisco Us Inc. | Alpha-amylase combinatorial variants |
| WO2024050339A1 (en) | 2022-09-02 | 2024-03-07 | Danisco Us Inc. | Mannanase variants and methods of use |
| WO2024050343A1 (en) | 2022-09-02 | 2024-03-07 | Danisco Us Inc. | Subtilisin variants and methods related thereto |
| WO2024050346A1 (en) | 2022-09-02 | 2024-03-07 | Danisco Us Inc. | Detergent compositions and methods related thereto |
| WO2024046595A1 (en) | 2022-09-01 | 2024-03-07 | Novozymes A/S | Baking with thermostable amyloglucosidase (amg) variants (ec 3.2.1.3) and low added sugar |
| WO2024056334A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| WO2024056332A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| WO2024056333A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| WO2024056278A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| EP4349948A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349947A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349943A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349946A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Unit dose fabric treatment product |
| EP4349945A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349942A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349944A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4361239A1 (en) | 2022-10-25 | 2024-05-01 | Unilever IP Holdings B.V. | Laundry liquid composition |
| WO2024088716A1 (en) | 2022-10-25 | 2024-05-02 | Unilever Ip Holdings B.V. | Composition |
| WO2024088549A1 (en) | 2022-10-24 | 2024-05-02 | Novozymes A/S | Baking method with thermostable amg variant and alpha-amylase |
| WO2024088550A1 (en) | 2022-10-24 | 2024-05-02 | Novozymes A/S | Baking method for pulse protein fortified bread employing thermostable amyloglucosidase variante (ec 3.2.1.3) |
| WO2024088706A1 (en) | 2022-10-25 | 2024-05-02 | Unilever Ip Holdings B.V. | Composition |
| WO2024102698A1 (en) | 2022-11-09 | 2024-05-16 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2024118096A1 (en) | 2022-11-30 | 2024-06-06 | Novozymes A/S | Baking at low-ph with thermostable glucoamylase variants |
| DE102022131732A1 (en) | 2022-11-30 | 2024-06-06 | Henkel Ag & Co. Kgaa | Improved washing performance through the use of a protease fused with a special adhesion promoter peptide |
| WO2024115106A1 (en) | 2022-11-29 | 2024-06-06 | Unilever Ip Holdings B.V. | Composition |
| WO2024163584A1 (en) | 2023-02-01 | 2024-08-08 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2024186819A1 (en) | 2023-03-06 | 2024-09-12 | Danisco Us Inc. | Subtilisin variants and methods of use |
| US12091777B2 (en) | 2019-09-23 | 2024-09-17 | Twist Bioscience Corporation | Variant nucleic acid libraries for CRTH2 |
| WO2024191711A1 (en) | 2023-03-16 | 2024-09-19 | Nutrition & Biosciences USA 4, Inc. | Brevibacillus fermentate extracts for cleaning and malodor control and use thereof |
| WO2024194190A1 (en) | 2023-03-17 | 2024-09-26 | Unilever Ip Holdings B.V. | Composition |
| WO2024193937A1 (en) | 2023-03-17 | 2024-09-26 | Unilever Ip Holdings B.V. | Machine dishwash filter cleaner |
| WO2024194098A1 (en) | 2023-03-21 | 2024-09-26 | Unilever Ip Holdings B.V. | Detergent unit dose |
Families Citing this family (232)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6165770A (en) * | 1996-09-26 | 2000-12-26 | Novo Nordisk A/S | Alkaline stable amylase from Thermoalcalibacter |
| US6060442A (en) * | 1998-02-24 | 2000-05-09 | Novo Nordisk A/S | Laccase mutants |
| US6172020B1 (en) * | 1998-03-20 | 2001-01-09 | Colgate-Palmolive Company | Powdered automatic dishwashing tablets |
| GB0004130D0 (en) * | 2000-02-23 | 2000-04-12 | Procter & Gamble | Detergent tablet |
| US20020183226A1 (en) * | 2001-02-28 | 2002-12-05 | Chandrika Kasturi | Liquid detergent composition exhibiting enhanced alpha-amylase enzyme stability |
| US7226771B2 (en) | 2002-04-19 | 2007-06-05 | Diversa Corporation | Phospholipases, nucleic acids encoding them and methods for making and using them |
| CA2481411C (en) | 2002-04-19 | 2016-06-14 | Diversa Corporation | Phospholipases, nucleic acids encoding them and methods for making and using them |
| NZ537597A (en) | 2002-06-14 | 2008-07-31 | Diversa Corp | Xylanases, nucleic acids encoding them and methods for making and using them |
| JP2006516889A (en) * | 2002-10-10 | 2006-07-13 | ダイヴァーサ コーポレイション | Protease, nucleic acid encoding the same, and method for producing and using the same |
| EP3508578A1 (en) | 2003-03-06 | 2019-07-10 | BASF Enzymes, LLC | Amylases, nucleic acids encoding them and methods for making and using them |
| DK2853593T3 (en) | 2003-03-07 | 2018-01-08 | Dsm Ip Assets Bv | Hydrolases, nucleic acids encoding them, and processes for their preparation and use |
| EP1608766A4 (en) * | 2003-03-20 | 2006-11-02 | Diversa Corp | Glucosidases, nucleic acids encoding them and methods for making and using them |
| WO2004090099A2 (en) | 2003-04-04 | 2004-10-21 | Diversa Corporation | Pectate lyases, nucleic acids encoding them and methods for making and using them |
| US7960148B2 (en) | 2003-07-02 | 2011-06-14 | Verenium Corporation | Glucanases, nucleic acids encoding them and methods for making and using them |
| CA2535526C (en) | 2003-08-11 | 2015-09-29 | Diversa Corporation | Laccases, nucleic acids encoding them and methods for making and using them |
| CA2558266C (en) | 2004-03-05 | 2017-10-17 | Gen-Probe Incorporated | Reagents, methods and kits for use in deactivating nucleic acids |
| US7531490B2 (en) * | 2004-10-01 | 2009-05-12 | Kao Corporation | Detergent composition comprising calcium gluconate and a mixture of calcium ion sequestering agents |
| JP4714457B2 (en) * | 2004-11-18 | 2011-06-29 | ライオン株式会社 | Amylase-containing granular detergent composition |
| JP2006143855A (en) * | 2004-11-18 | 2006-06-08 | Lion Corp | Amylase-containing bleaching composition with improved effect for removing drink stain |
| JP5343212B2 (en) | 2005-03-15 | 2013-11-13 | ヴェレニウム コーポレイション | Cellulases, nucleic acids encoding them, and methods of making and using them |
| WO2007092314A2 (en) | 2006-02-02 | 2007-08-16 | Verenium Corporation | Esterases and related nucleic acids and methods |
| MY160710A (en) | 2006-02-10 | 2017-03-15 | Verenium Corp | Cellulolytic enzymes, nucleic acids encoding them and methods for making and using them |
| CA2638801C (en) | 2006-02-14 | 2016-12-13 | Verenium Corporation | Xylanases, nucleic acids encoding them and methods for making and using them |
| US7629158B2 (en) * | 2006-06-16 | 2009-12-08 | The Procter & Gamble Company | Cleaning and/or treatment compositions |
| CN101528766A (en) | 2006-08-04 | 2009-09-09 | 维莱尼姆公司 | Glucanases, nucleic acids encoding them and methods for making and using them |
| MX2009003034A (en) | 2006-09-21 | 2009-11-18 | Verenium Corp | Phospholipases, nucleic acids encoding them and methods for making and using them. |
| DK2479266T3 (en) | 2006-12-21 | 2016-06-20 | Basf Enzymes Llc | Amylases and glucoamylases, nucleic acids encoding them, and methods of making and using the same |
| NZ598285A (en) | 2007-01-30 | 2013-10-25 | Syngenta Participations Ag | Enzymes for the treatment of lignocellulosics, nucleic acids encoding them and methods for making and using them |
| NZ601191A (en) | 2007-10-03 | 2014-01-31 | Verenium Corp | Xylanases, nucleic acids encoding them and methods for making and using them |
| US8066818B2 (en) | 2008-02-08 | 2011-11-29 | The Procter & Gamble Company | Water-soluble pouch |
| US20090209447A1 (en) * | 2008-02-15 | 2009-08-20 | Michelle Meek | Cleaning compositions |
| US20090233830A1 (en) | 2008-03-14 | 2009-09-17 | Penny Sue Dirr | Automatic detergent dishwashing composition |
| EP2100947A1 (en) | 2008-03-14 | 2009-09-16 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP2100948A1 (en) * | 2008-03-14 | 2009-09-16 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| US8357503B2 (en) | 2008-08-29 | 2013-01-22 | Bunge Oils, Inc. | Hydrolases, nucleic acids encoding them and methods for making and using them |
| US8198062B2 (en) | 2008-08-29 | 2012-06-12 | Dsm Ip Assets B.V. | Hydrolases, nucleic acids encoding them and methods for making and using them |
| US8153391B2 (en) | 2008-08-29 | 2012-04-10 | Bunge Oils, Inc. | Hydrolases, nucleic acids encoding them and methods for making and using them |
| EP2166092A1 (en) | 2008-09-18 | 2010-03-24 | The Procter and Gamble Company | Detergent composition |
| EP2166076A1 (en) | 2008-09-23 | 2010-03-24 | The Procter & Gamble Company | Cleaning composition |
| EP2166073A1 (en) | 2008-09-23 | 2010-03-24 | The Procter & Gamble Company | Cleaning composition |
| EP2166075A1 (en) | 2008-09-23 | 2010-03-24 | The Procter and Gamble Company | Cleaning composition |
| US20100267304A1 (en) * | 2008-11-14 | 2010-10-21 | Gregory Fowler | Polyurethane foam pad and methods of making and using same |
| US20100125046A1 (en) * | 2008-11-20 | 2010-05-20 | Denome Frank William | Cleaning products |
| EP2216393B1 (en) | 2009-02-09 | 2024-04-24 | The Procter & Gamble Company | Detergent composition |
| BRPI1010238A2 (en) | 2009-04-01 | 2015-08-25 | Danisco Us Inc | Compositions and methods comprising alpha-amylase variants with altered properties |
| WO2011005730A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | A catalytic laundry detergent composition comprising relatively low levels of water-soluble electrolyte |
| WO2011005913A1 (en) | 2009-07-09 | 2011-01-13 | The Procter & Gamble Company | A catalytic laundry detergent composition comprising relatively low levels of water-soluble electrolyte |
| HUE029942T2 (en) | 2009-08-13 | 2017-04-28 | Procter & Gamble | Method of laundering fabrics at low temperature |
| UA109884C2 (en) | 2009-10-16 | 2015-10-26 | A POLYPEPTIDE THAT HAS THE ACTIVITY OF THE PHOSPHATIDYLINOSYTOL-SPECIFIC PHOSPHOLIPASE C, NUCLEIC ACID, AND METHOD OF METHOD | |
| UA111708C2 (en) | 2009-10-16 | 2016-06-10 | Бандж Ойлз, Інк. | METHOD OF OIL REFINING |
| CA2782613C (en) | 2009-12-09 | 2016-08-23 | The Procter & Gamble Company | Fabric and home care products |
| EP2333039B2 (en) | 2009-12-10 | 2020-11-11 | The Procter & Gamble Company | Method and use of a dishwasher composition |
| EP2333040B2 (en) | 2009-12-10 | 2019-11-13 | The Procter & Gamble Company | Detergent composition |
| EP2333041B1 (en) | 2009-12-10 | 2013-05-15 | The Procter & Gamble Company | Method and use of a dishwasher composition |
| ES2548772T3 (en) | 2009-12-10 | 2015-10-20 | The Procter & Gamble Company | Dishwasher product and use of the same |
| EP2361964B1 (en) | 2010-02-25 | 2012-12-12 | The Procter & Gamble Company | Detergent composition |
| EP2380481B1 (en) | 2010-04-23 | 2014-12-31 | The Procter and Gamble Company | Automatic dishwashing product |
| EP2383329A1 (en) | 2010-04-23 | 2011-11-02 | The Procter & Gamble Company | Particle |
| ES2579217T3 (en) | 2010-04-23 | 2016-08-08 | The Procter & Gamble Company | Particle |
| EP2380478A1 (en) | 2010-04-23 | 2011-10-26 | The Procter & Gamble Company | Automatic dishwashing product |
| PL2380961T3 (en) | 2010-04-23 | 2018-10-31 | The Procter & Gamble Company | Detergent composition |
| ES2565192T3 (en) | 2010-04-23 | 2016-04-01 | The Procter & Gamble Company | Method to perfume |
| CN108410585A (en) | 2010-05-06 | 2018-08-17 | 宝洁公司 | The consumer goods with ease variants |
| EP2420558B1 (en) | 2010-08-17 | 2017-08-02 | The Procter & Gamble Company | Stable sustainable hand dish-washing detergents |
| CA2806265C (en) | 2010-08-17 | 2016-10-18 | The Procter & Gamble Company | Method for hand washing dishes having long lasting suds |
| EP2625268B1 (en) | 2010-10-06 | 2015-12-30 | BP Corporation North America Inc. | Variant cbh i polypeptides |
| WO2012057781A1 (en) | 2010-10-29 | 2012-05-03 | The Procter & Gamble Company | Cleaning and/or treatment compositions comprising a fungal serine protease |
| CN106065381B (en) | 2011-05-05 | 2019-07-26 | 宝洁公司 | Composition and method comprising serine protease variants |
| CN103764823B (en) | 2011-05-05 | 2018-05-11 | 丹尼斯科美国公司 | Composition and method comprising serine protease variants |
| US20140371435A9 (en) | 2011-06-03 | 2014-12-18 | Eduardo Torres | Laundry Care Compositions Containing Thiophene Azo Dyes |
| EP2551335A1 (en) | 2011-07-25 | 2013-01-30 | The Procter & Gamble Company | Enzyme stabilized liquid detergent composition |
| ES2633292T3 (en) | 2011-10-19 | 2017-09-20 | The Procter & Gamble Company | Particle |
| CN104080902B (en) | 2012-02-03 | 2018-08-03 | 宝洁公司 | The composition and method for surface treatment with lipase |
| FI124202B (en) | 2012-02-22 | 2014-04-30 | Kemira Oyj | Process for improvement of recycled fiber material utilizing the manufacturing process of paper or paperboard |
| TR201900214T4 (en) | 2012-03-19 | 2019-02-21 | Milliken & Co | Carboxylate Dyes |
| CN104204198B (en) | 2012-04-02 | 2018-09-25 | 诺维信公司 | Lipase Variant and the polynucleotides for encoding it |
| ES2646416T3 (en) | 2012-05-11 | 2017-12-13 | The Procter & Gamble Company | Detergent composition |
| EP2674475A1 (en) | 2012-06-11 | 2013-12-18 | The Procter & Gamble Company | Detergent composition |
| MX2015000312A (en) | 2012-07-12 | 2015-04-10 | Novozymes As | Polypeptides having lipase activity and polynucleotides encoding same. |
| EP2700703B1 (en) | 2012-08-24 | 2018-05-02 | The Procter and Gamble Company | Dishwashing method |
| ES2678543T3 (en) | 2012-08-24 | 2018-08-13 | The Procter & Gamble Company | Dishwashing method |
| MX2015007802A (en) | 2012-12-20 | 2015-09-04 | Procter & Gamble | Detergent composition with silicate coated bleach. |
| EP2746381A1 (en) | 2012-12-21 | 2014-06-25 | The Procter & Gamble Company | Cleaning pack |
| CA2902279C (en) | 2013-03-05 | 2019-05-28 | The Procter & Gamble Company | Mixed sugar amine or sugar amide surfactant compositions |
| US9631164B2 (en) | 2013-03-21 | 2017-04-25 | Novozymes A/S | Polypeptides with lipase activity and polynucleotides encoding same |
| PL2978830T3 (en) | 2013-03-28 | 2019-08-30 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| US9206382B2 (en) | 2013-05-28 | 2015-12-08 | The Procter & Gamble Company | Surface treatment compositions comprising photochromic dyes |
| EP3047008B1 (en) | 2013-09-18 | 2018-05-16 | The Procter and Gamble Company | Laundry care composition comprising carboxylate dye |
| US9834682B2 (en) | 2013-09-18 | 2017-12-05 | Milliken & Company | Laundry care composition comprising carboxylate dye |
| EP3047009B1 (en) | 2013-09-18 | 2018-05-16 | The Procter and Gamble Company | Laundry care composition comprising carboxylate dye |
| AR098668A1 (en) | 2013-09-18 | 2016-06-08 | Procter & Gamble | COMPOSITIONS CONTAINING COLORS FOR CLOTHING CARE |
| EP2857487A1 (en) | 2013-10-07 | 2015-04-08 | WeylChem Switzerland AG | Multi-compartment pouch comprising cleaning compositions, washing process and use for washing and cleaning of textiles and dishes |
| EP2857485A1 (en) | 2013-10-07 | 2015-04-08 | WeylChem Switzerland AG | Multi-compartment pouch comprising alkanolamine-free cleaning compositions, washing process and use for washing and cleaning of textiles and dishes |
| EP2857486A1 (en) | 2013-10-07 | 2015-04-08 | WeylChem Switzerland AG | Multi-compartment pouch comprising cleaning compositions, washing process and use for washing and cleaning of textiles and dishes |
| EP3097174A1 (en) | 2014-01-22 | 2016-11-30 | The Procter & Gamble Company | Method of treating textile fabrics |
| EP3097175B1 (en) | 2014-01-22 | 2018-10-17 | The Procter and Gamble Company | Fabric treatment composition |
| WO2015112339A1 (en) | 2014-01-22 | 2015-07-30 | The Procter & Gamble Company | Fabric treatment composition |
| WO2015112338A1 (en) | 2014-01-22 | 2015-07-30 | The Procter & Gamble Company | Method of treating textile fabrics |
| WO2015130669A1 (en) | 2014-02-25 | 2015-09-03 | The Procter & Gamble Company | A process for making renewable surfactant intermediates and surfactants from fats and oils and products thereof |
| WO2015130653A1 (en) | 2014-02-25 | 2015-09-03 | The Procter & Gamble Company | A process for making renewable surfactant intermediates and surfactants from fats and oils and products thereof |
| EP2915872A1 (en) | 2014-03-06 | 2015-09-09 | The Procter and Gamble Company | Dishwashing composition |
| EP2915873A1 (en) | 2014-03-06 | 2015-09-09 | The Procter and Gamble Company | Dishwashing composition |
| US9719052B2 (en) | 2014-03-27 | 2017-08-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| EP3122850A1 (en) | 2014-03-27 | 2017-02-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| WO2015148890A1 (en) | 2014-03-27 | 2015-10-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| EP2924105A1 (en) | 2014-03-28 | 2015-09-30 | The Procter and Gamble Company | Water soluble unit dose article |
| EP2924106A1 (en) | 2014-03-28 | 2015-09-30 | The Procter and Gamble Company | Water soluble unit dose article |
| WO2015171592A1 (en) | 2014-05-06 | 2015-11-12 | Milliken & Company | Laundry care compositions |
| CN106793781B (en) * | 2014-05-28 | 2020-07-28 | 拜耳作物科学有限合伙公司 | Compositions and methods for controlling fungal and bacterial diseases in plants |
| EP3152288A1 (en) | 2014-06-06 | 2017-04-12 | The Procter & Gamble Company | Detergent composition comprising polyalkyleneimine polymers |
| US9617502B2 (en) | 2014-09-15 | 2017-04-11 | The Procter & Gamble Company | Detergent compositions containing salts of polyetheramines and polymeric acid |
| EP3197988B1 (en) | 2014-09-25 | 2018-08-01 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| US20160090552A1 (en) | 2014-09-25 | 2016-03-31 | The Procter & Gamble Company | Detergent compositions containing a polyetheramine and an anionic soil release polymer |
| US9388368B2 (en) | 2014-09-26 | 2016-07-12 | The Procter & Gamble Company | Cleaning compositions containing a polyetheramine |
| WO2016081437A1 (en) | 2014-11-17 | 2016-05-26 | The Procter & Gamble Company | Benefit agent delivery compositions |
| EP3026103B1 (en) | 2014-11-26 | 2018-07-25 | The Procter and Gamble Company | Cleaning pouch |
| ES2690335T3 (en) | 2014-11-26 | 2018-11-20 | The Procter & Gamble Company | Cleaning bag |
| EP3026099B1 (en) | 2014-11-26 | 2021-02-17 | The Procter and Gamble Company | Cleaning pouch |
| PL3026102T3 (en) | 2014-11-26 | 2019-06-28 | The Procter & Gamble Company | Cleaning pouch |
| EP3034596B2 (en) | 2014-12-17 | 2021-11-10 | The Procter & Gamble Company | Detergent composition |
| EP3034589A1 (en) | 2014-12-17 | 2016-06-22 | The Procter and Gamble Company | Detergent composition |
| EP3034597A1 (en) | 2014-12-17 | 2016-06-22 | The Procter and Gamble Company | Detergent composition |
| EP3034588B1 (en) | 2014-12-17 | 2019-04-24 | The Procter and Gamble Company | Detergent composition |
| EP3034590A1 (en) | 2014-12-17 | 2016-06-22 | The Procter and Gamble Company | Method of automatic dishwashing |
| EP3034591A1 (en) | 2014-12-17 | 2016-06-22 | The Procter and Gamble Company | Method of automatic dishwashing |
| EP3034592A1 (en) | 2014-12-17 | 2016-06-22 | The Procter and Gamble Company | Method of automatic dishwashing |
| ES2668504T3 (en) | 2014-12-22 | 2018-05-18 | The Procter & Gamble Company | Process for recycling detergent bags |
| EP3050955B2 (en) | 2015-02-02 | 2023-11-08 | The Procter & Gamble Company | Detergent pack |
| EP3050951A1 (en) | 2015-02-02 | 2016-08-03 | The Procter and Gamble Company | Method of dishwashing |
| EP3050952A1 (en) | 2015-02-02 | 2016-08-03 | The Procter and Gamble Company | Method of dishwashing |
| ES2714130T3 (en) | 2015-02-02 | 2019-05-27 | Procter & Gamble | Detergent composition |
| EP3050947A1 (en) | 2015-02-02 | 2016-08-03 | The Procter and Gamble Company | Detergent pack |
| EP3050950B1 (en) | 2015-02-02 | 2018-09-19 | The Procter and Gamble Company | New use of sulfonated polymers |
| EP3050948B1 (en) | 2015-02-02 | 2018-09-19 | The Procter and Gamble Company | New use of complexing agent |
| EP3050954A1 (en) | 2015-02-02 | 2016-08-03 | The Procter and Gamble Company | New use of sulfonated polymers |
| ES2683906T3 (en) | 2015-04-29 | 2018-09-28 | The Procter & Gamble Company | Method of treating a tissue |
| EP3088503B1 (en) | 2015-04-29 | 2018-05-23 | The Procter and Gamble Company | Method of treating a fabric |
| CN117683589A (en) | 2015-04-29 | 2024-03-12 | 宝洁公司 | Method for washing fabrics |
| DK3088505T3 (en) | 2015-04-29 | 2020-08-03 | Procter & Gamble | PROCEDURE FOR TREATMENT OF A TEXTILE FABRIC |
| WO2016176241A1 (en) | 2015-04-29 | 2016-11-03 | The Procter & Gamble Company | Detergent composition |
| CN107532007B (en) | 2015-05-04 | 2020-06-30 | 美利肯公司 | Leuco triphenylmethane colorants as bluing agents in laundry care compositions |
| EP3101102B2 (en) | 2015-06-05 | 2023-12-13 | The Procter & Gamble Company | Compacted liquid laundry detergent composition |
| EP3101103B1 (en) | 2015-06-05 | 2019-04-24 | The Procter and Gamble Company | Compacted liquid laundry detergent composition |
| EP3101107B1 (en) | 2015-06-05 | 2019-04-24 | The Procter and Gamble Company | Compacted liquid laundry detergent composition |
| HUE036591T2 (en) | 2015-06-05 | 2018-08-28 | Procter & Gamble | Compacted liquid laundry detergent composition |
| EP3124586A1 (en) | 2015-07-29 | 2017-02-01 | The Procter and Gamble Company | Process for reducing malodour in a pack |
| EP3124587B1 (en) | 2015-07-29 | 2019-03-20 | The Procter and Gamble Company | Multi-phase unit-dose cleaning product |
| CA3002668A1 (en) | 2015-11-26 | 2017-06-01 | Neil Joseph Lant | Liquid detergent compositions comprising protease and encapsulated lipase |
| EP3178917A1 (en) | 2015-12-08 | 2017-06-14 | The Procter and Gamble Company | Cleaning pouch |
| EP3228690B1 (en) | 2016-04-08 | 2020-05-13 | The Procter and Gamble Company | Automatic dishwashing cleaning composition |
| EP3228687B1 (en) | 2016-04-08 | 2019-05-22 | The Procter and Gamble Company | Dishwashing cleaning composition |
| WO2017176501A1 (en) | 2016-04-08 | 2017-10-12 | The Procter & Gamble Company | Automatic dishwashing cleaning composition |
| EP3228686B1 (en) | 2016-04-08 | 2021-10-27 | The Procter & Gamble Company | Automatic dishwashing |
| EP3241891B1 (en) | 2016-05-03 | 2019-04-03 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3452569A1 (en) | 2016-05-03 | 2019-03-13 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3241889B1 (en) | 2016-05-03 | 2019-03-20 | The Procter and Gamble Company | Cleaning composition |
| EP3241890B1 (en) | 2016-05-03 | 2019-06-26 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3257931A1 (en) | 2016-06-17 | 2017-12-20 | The Procter and Gamble Company | Detergent composition |
| EP3266860B1 (en) | 2016-07-08 | 2020-04-08 | The Procter and Gamble Company | Process for making a particle |
| EP3275988B1 (en) | 2016-07-26 | 2020-07-08 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3275986B1 (en) | 2016-07-26 | 2020-07-08 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3275987A1 (en) | 2016-07-26 | 2018-01-31 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3275985A1 (en) | 2016-07-26 | 2018-01-31 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3275989A1 (en) | 2016-07-26 | 2018-01-31 | The Procter and Gamble Company | Automatic dishwashing detergent composition |
| EP3290503A3 (en) | 2016-09-01 | 2018-05-30 | The Procter & Gamble Company | Automatic dishwashing cleaning composition |
| EP3312265A1 (en) | 2016-10-18 | 2018-04-25 | The Procter and Gamble Company | Detergent composition |
| JP6790257B2 (en) | 2016-11-01 | 2020-11-25 | ザ プロクター アンド ギャンブル カンパニーThe Procter & Gamble Company | Leuco colorants as bluish agents in laundry care compositions, their packaging, kits and methods |
| BR112019006576A2 (en) | 2016-11-01 | 2019-07-02 | Milliken & Co | leuco dyes as bleaching agents in laundry care compositions |
| CA3038855A1 (en) | 2016-11-01 | 2018-05-11 | The Procter & Gamble Company | Leuco colorants as bluing agents in laundry care compositions |
| US20180119056A1 (en) | 2016-11-03 | 2018-05-03 | Milliken & Company | Leuco Triphenylmethane Colorants As Bluing Agents in Laundry Care Compositions |
| DE102016221851A1 (en) * | 2016-11-08 | 2018-05-09 | Henkel Ag & Co. Kgaa | Amylase for washing and cleaning agent applications |
| US10550443B2 (en) | 2016-12-02 | 2020-02-04 | The Procter & Gamble Company | Cleaning compositions including enzymes |
| WO2018102478A1 (en) | 2016-12-02 | 2018-06-07 | The Procter & Gamble Company | Cleaning compositions including enzymes |
| EP3330349B1 (en) | 2016-12-02 | 2024-06-19 | The Procter & Gamble Company | Cleaning compositions including enzymes |
| EP3339410A1 (en) | 2016-12-22 | 2018-06-27 | The Procter & Gamble Company | Automatic dishwashing composition |
| EP3339423A1 (en) | 2016-12-22 | 2018-06-27 | The Procter & Gamble Company | Automatic dishwashing detergent composition |
| EP3415592A1 (en) | 2017-06-15 | 2018-12-19 | The Procter & Gamble Company | Water-soluble unit dose article comprising a solid laundry detergent composition |
| EP3418365A1 (en) | 2017-06-19 | 2018-12-26 | The Procter & Gamble Company | Automatic dishwashing cleaning composition |
| EP3418364A1 (en) | 2017-06-19 | 2018-12-26 | The Procter & Gamble Company | Automatic dishwashing cleaning composition |
| EP3418366A1 (en) | 2017-06-19 | 2018-12-26 | The Procter & Gamble Company | Automatic dishwashing cleaning composition |
| EP3441450A1 (en) | 2017-08-11 | 2019-02-13 | The Procter & Gamble Company | Automatic dishwashing composition |
| EP3456808A1 (en) | 2017-09-13 | 2019-03-20 | The Procter & Gamble Company | Automatic dishwashing cleaning composition |
| EP3467085A1 (en) | 2017-10-05 | 2019-04-10 | The Procter & Gamble Company | Dishwashing cleaning composition |
| EP3467086B1 (en) | 2017-10-05 | 2021-03-24 | The Procter & Gamble Company | Dishwashing cleaning composition |
| WO2019075144A1 (en) | 2017-10-12 | 2019-04-18 | The Procter & Gamble Company | Leuco colorants in combination with a second whitening agent as bluing agents in laundry care compositions |
| WO2019075148A1 (en) | 2017-10-12 | 2019-04-18 | The Procter & Gamble Company | Leuco colorants as bluing agents in laundry care compositions |
| MX2020003103A (en) | 2017-10-12 | 2020-07-28 | Procter & Gamble | Leuco colorants as bluing agents in laundry care composition. |
| TWI715878B (en) | 2017-10-12 | 2021-01-11 | 美商美力肯及公司 | Leuco colorants and compositions |
| BR112020008476B1 (en) | 2017-11-01 | 2023-11-21 | Milliken & Company | LEUCO COMPOUND |
| EP3530723B1 (en) | 2018-02-21 | 2023-03-29 | The Procter & Gamble Company | Automatic dishwashing composition |
| CA3154330A1 (en) * | 2018-05-11 | 2019-11-14 | Ecochem Australia Pty Ltd | Compositions, methods and systems for removal of starch |
| CN113439116B (en) | 2019-03-14 | 2023-11-28 | 宝洁公司 | Enzyme-containing cleaning compositions |
| EP3938503A1 (en) | 2019-03-14 | 2022-01-19 | The Procter & Gamble Company | Method for treating cotton |
| MX2021011104A (en) | 2019-03-14 | 2021-10-22 | Procter & Gamble | Cleaning compositions comprising enzymes. |
| EP3741283A1 (en) | 2019-05-22 | 2020-11-25 | The Procter & Gamble Company | Automatic dishwashing method |
| EP3760699A1 (en) | 2019-07-02 | 2021-01-06 | The Procter & Gamble Company | Automatic dishwashing detergent composition |
| EP3835396A1 (en) | 2019-12-09 | 2021-06-16 | The Procter & Gamble Company | A detergent composition comprising a polymer |
| MX2022013878A (en) | 2020-06-05 | 2022-11-30 | Procter & Gamble | Detergent compositions containing a branched surfactant. |
| ES2956240T3 (en) | 2020-09-29 | 2023-12-15 | Procter & Gamble | Dishwasher cleaning composition |
| US20230392018A1 (en) | 2020-10-27 | 2023-12-07 | Milliken & Company | Compositions comprising leuco compounds and colorants |
| MX2023004260A (en) | 2020-10-29 | 2023-04-25 | Procter & Gamble | Cleaning compositions containing alginate lyase enzymes. |
| EP4001385A1 (en) | 2020-11-17 | 2022-05-25 | The Procter & Gamble Company | Automatic dishwashing composition |
| EP4006131A1 (en) | 2020-11-30 | 2022-06-01 | The Procter & Gamble Company | Method of laundering fabric |
| JP2023551014A (en) | 2020-12-23 | 2023-12-06 | ビーエーエスエフ ソシエタス・ヨーロピア | Amphiphilic alkoxylated polyamines and their uses |
| CA3199985A1 (en) | 2021-03-15 | 2022-09-22 | Lars Lehmann Hylling Christensen | Cleaning compositions containing polypeptide variants |
| EP4095223A1 (en) | 2021-05-05 | 2022-11-30 | The Procter & Gamble Company | Methods for making cleaning compositions and for detecting soils |
| EP4086330A1 (en) | 2021-05-06 | 2022-11-09 | The Procter & Gamble Company | Surface treatment |
| EP4108767A1 (en) | 2021-06-22 | 2022-12-28 | The Procter & Gamble Company | Cleaning or treatment compositions containing nuclease enzymes |
| EP4108150B1 (en) | 2021-06-22 | 2024-10-16 | The Procter & Gamble Company | A method of treating dishware in a domestic automatic dishwashing machine |
| EP4123007A1 (en) | 2021-07-19 | 2023-01-25 | The Procter & Gamble Company | Fabric treatment using bacterial spores |
| EP4123006A1 (en) | 2021-07-19 | 2023-01-25 | The Procter & Gamble Company | Composition comprising spores and pro-perfume materials |
| CA3228918A1 (en) | 2021-08-10 | 2023-02-16 | Nippon Shokubai Co., Ltd. | Polyalkylene-oxide-containing compound |
| WO2023064749A1 (en) | 2021-10-14 | 2023-04-20 | The Procter & Gamble Company | A fabric and home care product comprising cationic soil release polymer and lipase enzyme |
| EP4194537A1 (en) | 2021-12-08 | 2023-06-14 | The Procter & Gamble Company | Laundry treatment cartridge |
| EP4194536A1 (en) | 2021-12-08 | 2023-06-14 | The Procter & Gamble Company | Laundry treatment cartridge |
| EP4273210A1 (en) | 2022-05-04 | 2023-11-08 | The Procter & Gamble Company | Detergent compositions containing enzymes |
| EP4273209A1 (en) | 2022-05-04 | 2023-11-08 | The Procter & Gamble Company | Machine-cleaning compositions containing enzymes |
| EP4279571A1 (en) | 2022-05-19 | 2023-11-22 | The Procter & Gamble Company | Laundry composition comprising spores |
| EP4286501A1 (en) | 2022-06-01 | 2023-12-06 | The Procter & Gamble Company | Dishwashing detergent composition comprising xylanase and block co-polymer |
| EP4286499A1 (en) | 2022-06-01 | 2023-12-06 | The Procter & Gamble Company | Dishwashing detergent composition comprising xylanase and sulphonated carboxylate polymer |
| EP4286500A1 (en) | 2022-06-01 | 2023-12-06 | The Procter & Gamble Company | Use of xylanase in a dishwashing process |
| EP4321604A1 (en) | 2022-08-08 | 2024-02-14 | The Procter & Gamble Company | A fabric and home care composition comprising surfactant and a polyester |
| EP4339268A1 (en) | 2022-09-16 | 2024-03-20 | The Procter & Gamble Company | Methods and apparatuses for automatic dishwashing chemical distribution |
| EP4361061A1 (en) | 2022-10-26 | 2024-05-01 | The Procter & Gamble Company | Detergent product |
| WO2024094790A1 (en) | 2022-11-04 | 2024-05-10 | Clariant International Ltd | Polyesters |
| WO2024094803A1 (en) | 2022-11-04 | 2024-05-10 | The Procter & Gamble Company | Fabric and home care composition |
| WO2024094802A1 (en) | 2022-11-04 | 2024-05-10 | The Procter & Gamble Company | Fabric and home care composition |
| WO2024119298A1 (en) | 2022-12-05 | 2024-06-13 | The Procter & Gamble Company | Fabric and home care composition comprising a polyalkylenecarbonate compound |
| WO2024129520A1 (en) | 2022-12-12 | 2024-06-20 | The Procter & Gamble Company | Fabric and home care composition |
| EP4386074A1 (en) | 2022-12-16 | 2024-06-19 | The Procter & Gamble Company | Fabric and home care composition |
| WO2024151572A1 (en) | 2023-01-09 | 2024-07-18 | The Procter & Gamble Company | Superposed multi-sectioned water-soluble unit dose automatic dishwashing detergent pouch |
| EP4410941A1 (en) | 2023-02-01 | 2024-08-07 | The Procter & Gamble Company | Detergent compositions containing enzymes |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1989005863A1 (en) * | 1987-12-23 | 1989-06-29 | Gist-Brocades N.V. | Purified industrial enzyme and process for the preparation thereof |
| WO1991000353A2 (en) * | 1989-06-29 | 1991-01-10 | Gist-Brocades N.V. | MUTANT MICROBIAL α-AMYLASES WITH INCREASED THERMAL, ACID AND/OR ALKALINE STABILITY |
| EP0516553A2 (en) * | 1991-05-31 | 1992-12-02 | Colgate-Palmolive Company | Powdered automatic dishwashing composition containing enzymes |
| WO1994002597A1 (en) * | 1992-07-23 | 1994-02-03 | Novo Nordisk A/S | MUTANT α-AMYLASE, DETERGENT, DISH WASHING AGENT, AND LIQUEFACTION AGENT |
Family Cites Families (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS519033B1 (en) * | 1969-09-12 | 1976-03-23 | ||
| US3912590A (en) | 1973-01-03 | 1975-10-14 | Novo Industri As | Procedure for liquefying starch |
| GB1483591A (en) | 1973-07-23 | 1977-08-24 | Novo Industri As | Process for coating water soluble or water dispersible particles by means of the fluid bed technique |
| GB1590432A (en) | 1976-07-07 | 1981-06-03 | Novo Industri As | Process for the production of an enzyme granulate and the enzyme granuate thus produced |
| JPS57174089A (en) | 1981-04-20 | 1982-10-26 | Novo Industri As | Chain dividing enzyme product |
| DK263584D0 (en) | 1984-05-29 | 1984-05-29 | Novo Industri As | ENZYMOUS GRANULATES USED AS DETERGENT ADDITIVES |
| US4683202A (en) | 1985-03-28 | 1987-07-28 | Cetus Corporation | Process for amplifying nucleic acid sequences |
| EG18543A (en) | 1986-02-20 | 1993-07-30 | Albright & Wilson | Protected enzyme systems |
| DK122686D0 (en) | 1986-03-17 | 1986-03-17 | Novo Industri As | PREPARATION OF PROTEINS |
| ATE80657T1 (en) | 1986-07-09 | 1992-10-15 | Novo Nordisk As | MIXTURES OF ALPHA-AMYLASE FOR LIQUEFYING STARCH. |
| DK386586D0 (en) | 1986-08-14 | 1986-08-14 | Novo Industri As | PROTEASE, PROCEDURE FOR PREPARING IT AND USING THEREOF |
| US5147796A (en) * | 1989-09-19 | 1992-09-15 | Kao Corporation | Alkaline pullulanase Y having α-amylase activity |
| JPH0632613B2 (en) * | 1989-09-19 | 1994-05-02 | 花王株式会社 | Novel alkaline pullulanase Y having α-amylase activity, microorganism producing the same, and novel method for producing alkaline pullulanase Y |
| AU639570B2 (en) | 1990-05-09 | 1993-07-29 | Novozymes A/S | A cellulase preparation comprising an endoglucanase enzyme |
| US5401657A (en) * | 1990-08-06 | 1995-03-28 | Gist-Brocades, N.V. | Gram-positive alkaliphilic microorganisms |
| US5459062A (en) * | 1990-08-06 | 1995-10-17 | Gist-Brocades, N.V. | Gram-negative alkaliphilic microorganisms |
| DK204290D0 (en) | 1990-08-24 | 1990-08-24 | Novo Nordisk As | ENZYMATIC DETERGENT COMPOSITION AND PROCEDURE FOR ENZYME STABILIZATION |
| CA2092556C (en) | 1990-09-28 | 1997-08-19 | Mark Hsiang-Kuen Mao | Polyhydroxy fatty acid amide surfactants to enhance enzyme performance |
| US5482849A (en) * | 1990-12-21 | 1996-01-09 | Novo Nordisk A/S | Subtilisin mutants |
| EP0511456A1 (en) | 1991-04-30 | 1992-11-04 | The Procter & Gamble Company | Liquid detergents with aromatic borate ester to inhibit proteolytic enzyme |
| ATE136055T1 (en) | 1991-04-30 | 1996-04-15 | Procter & Gamble | LIQUID DETERGENTS CONTAINING BRACKETS WITH BORIC ACID-POLYOL COMPLEX FOR PTOTEOLYTIC ENZYMIN INHIBITION |
| US5635468A (en) * | 1993-05-19 | 1997-06-03 | Kao Corporation | Liquefying alkaline α-amylase, process for producing the same, and detergent composition containing the same |
| US6486113B1 (en) * | 1997-03-31 | 2002-11-26 | Kao Corporation | Mutant α-amylases |
| JP4426716B2 (en) * | 2000-10-11 | 2010-03-03 | 花王株式会社 | High productivity α-amylase |
-
1995
- 1995-03-29 AU AU20677/95A patent/AU2067795A/en not_active Abandoned
- 1995-03-29 DE DE1995634464 patent/DE69534464T2/en not_active Expired - Lifetime
- 1995-03-29 CN CNB951923129A patent/CN1326994C/en not_active Expired - Lifetime
- 1995-03-29 DK DK95913062T patent/DK0753057T3/en active
- 1995-03-29 EP EP20050020499 patent/EP1637596B1/en not_active Expired - Lifetime
- 1995-03-29 CA CA 2186592 patent/CA2186592C/en not_active Expired - Lifetime
- 1995-03-29 EP EP95913062A patent/EP0753057B1/en not_active Expired - Lifetime
- 1995-03-29 MX MX9604313A patent/MX196038B/en not_active IP Right Cessation
- 1995-03-29 JP JP52491395A patent/JPH09510617A/en not_active Withdrawn
- 1995-03-29 AT AT05020499T patent/ATE510010T1/en not_active IP Right Cessation
- 1995-03-29 BR BR9507229A patent/BR9507229A/en not_active IP Right Cessation
- 1995-03-29 US US08/446,803 patent/US5824531A/en not_active Expired - Lifetime
- 1995-03-29 AT AT95913062T patent/ATE305031T1/en active
- 1995-03-29 ES ES95913062T patent/ES2250969T3/en not_active Expired - Lifetime
- 1995-03-29 WO PCT/DK1995/000142 patent/WO1995026397A1/en active Application Filing
- 1995-03-29 KR KR1019960705305A patent/KR970702363A/en active IP Right Grant
-
1996
- 1996-09-27 FI FI963861A patent/FI119693B/en not_active IP Right Cessation
-
1997
- 1997-05-22 US US08/861,837 patent/US5856164A/en not_active Expired - Lifetime
-
2005
- 2005-12-27 JP JP2005375802A patent/JP4057613B2/en not_active Expired - Lifetime
-
2008
- 2008-12-02 FI FI20080645A patent/FI120910B/en not_active IP Right Cessation
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1989005863A1 (en) * | 1987-12-23 | 1989-06-29 | Gist-Brocades N.V. | Purified industrial enzyme and process for the preparation thereof |
| WO1991000353A2 (en) * | 1989-06-29 | 1991-01-10 | Gist-Brocades N.V. | MUTANT MICROBIAL α-AMYLASES WITH INCREASED THERMAL, ACID AND/OR ALKALINE STABILITY |
| EP0410498A2 (en) * | 1989-06-29 | 1991-01-30 | Gist-Brocades N.V. | Mutant microbial alpha-amylases with increased thermal, acid and/or alkaline stability |
| EP0516553A2 (en) * | 1991-05-31 | 1992-12-02 | Colgate-Palmolive Company | Powdered automatic dishwashing composition containing enzymes |
| WO1994002597A1 (en) * | 1992-07-23 | 1994-02-03 | Novo Nordisk A/S | MUTANT α-AMYLASE, DETERGENT, DISH WASHING AGENT, AND LIQUEFACTION AGENT |
Non-Patent Citations (2)
| Title |
|---|
| BIOCHEM BIOPHYS RES COMMUN, vol. 151, no. 1, 29 February 1988 (1988-02-29), pages 25 - 31 * |
| DATABASE MEDLINE [online] TSUKAMOTO A ET AL: "Nucleotide sequence of the maltohexaose-producing amylase gene froom an alkalophilic Bacillus sp. #707 and structural similarity to liquefying type alpha-amylases", accession no. Dialog Database accession no. 88162814 * |
Cited By (607)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5635468A (en) * | 1993-05-19 | 1997-06-03 | Kao Corporation | Liquefying alkaline α-amylase, process for producing the same, and detergent composition containing the same |
| US6867031B2 (en) | 1995-02-03 | 2005-03-15 | Novozymes A/S | Amylase variants |
| US6297038B1 (en) | 1995-02-03 | 2001-10-02 | Novozymes A/S | Amylase variants |
| EP2465930A3 (en) * | 1995-02-03 | 2013-04-24 | Novozymes A/S | Amylase variants |
| EP2455465A2 (en) | 1995-02-03 | 2012-05-23 | Novozymes A/S | Amylase Variants |
| EP1752525A1 (en) * | 1995-02-03 | 2007-02-14 | Novozymes A/S | Amylase variants |
| EP2455468A2 (en) | 1995-02-03 | 2012-05-23 | Novozymes A/S | Amylase variants |
| WO1996023873A1 (en) * | 1995-02-03 | 1996-08-08 | Novo Nordisk A/S | Amylase variants |
| EP2455468A3 (en) * | 1995-02-03 | 2013-01-23 | Novozymes A/S | Amylase variants |
| US8323946B2 (en) | 1995-02-03 | 2012-12-04 | Novozymes A/S | Alpha-amylase mutants |
| EP2465930A2 (en) | 1995-02-03 | 2012-06-20 | Novozymes A/S | Amylase variants |
| US6638748B2 (en) | 1995-06-14 | 2003-10-28 | Kao Corporation | Gene encoding alkaline liquifying alpha-amylase |
| US6979731B1 (en) | 1995-06-14 | 2005-12-27 | Kao Corporation | Gene encoding alkaline liquefying α-amylase |
| WO1997000324A1 (en) * | 1995-06-14 | 1997-01-03 | Kao Corporation | Gene encoding alkaline liquefying alpha-amylase |
| US6093562A (en) * | 1996-02-05 | 2000-07-25 | Novo Nordisk A/S | Amylase variants |
| WO1997032961A2 (en) * | 1996-03-07 | 1997-09-12 | The Procter & Gamble Company | Detergent compositions comprising improved amylases |
| WO1997032961A3 (en) * | 1996-03-07 | 1997-10-30 | Procter & Gamble | Detergent compositions comprising improved amylases |
| WO1997041213A1 (en) | 1996-04-30 | 1997-11-06 | Novo Nordisk A/S | α-AMYLASE MUTANTS |
| US9487769B2 (en) | 1996-04-30 | 2016-11-08 | Novozymes A/S | Alpha-amylase mutants |
| US8999908B2 (en) | 1996-04-30 | 2015-04-07 | Novozymes A/S | Alpha-amylase mutants |
| US8263382B2 (en) | 1996-04-30 | 2012-09-11 | Novozymes A/S | Alpha-amylase mutants |
| US7960161B2 (en) | 1996-04-30 | 2011-06-14 | Novozymes A/S | Alpha-amylase mutants |
| US8513175B2 (en) | 1996-04-30 | 2013-08-20 | Novozymes A/S | Alpha-amylase mutants |
| US7625737B2 (en) | 1996-04-30 | 2009-12-01 | Novozymes A/S | Alpha-amylase mutants |
| WO1997043386A1 (en) * | 1996-05-15 | 1997-11-20 | The Procter & Gamble Company | Detergent compositions comprising improved amylases, cellulase and cationic surfactant |
| US6197070B1 (en) | 1996-05-15 | 2001-03-06 | The Procter & Gamble Company | Detergent compositions comprising alpha combination of α-amylases for malodor stripping |
| WO1998005748A1 (en) * | 1996-08-01 | 1998-02-12 | The Procter & Gamble Company | Detergent compositions comprising improved amylase for dingy fabric clean-up |
| JP2001507727A (en) * | 1996-12-20 | 2001-06-12 | ザ、プロクター、エンド、ギャンブル、カンパニー | Dishwashing detergent composition containing organic diamine |
| US6194370B1 (en) | 1996-12-31 | 2001-02-27 | The Procter & Gamble Company | Cost effective stain and soil removal aqueous heavy duty liquid laundry detergent compositions |
| US6221825B1 (en) | 1996-12-31 | 2001-04-24 | The Procter & Gamble Company | Thickened, highly aqueous liquid detergent compositions |
| US6486113B1 (en) | 1997-03-31 | 2002-11-26 | Kao Corporation | Mutant α-amylases |
| WO1998044126A1 (en) | 1997-03-31 | 1998-10-08 | Kao Corporation | MUTATED α-AMYLASES |
| US20110274676A1 (en) * | 1997-04-18 | 2011-11-10 | Sean Farmer | Topical Use Of Probiotic Bacillus Spores To Prevent Or Control Microbial Infections |
| EP2302027A2 (en) | 1997-10-13 | 2011-03-30 | Novozymes A/S | Alpha-amylase mutants |
| US6361989B1 (en) | 1997-10-13 | 2002-03-26 | Novozymes A/S | α-amylase and α-amylase variants |
| US6528298B1 (en) | 1997-10-13 | 2003-03-04 | Novozymes, A/S | α-amylase mutants |
| US6187576B1 (en) | 1997-10-13 | 2001-02-13 | Novo Nordisk A/S | α-amylase mutants |
| US7566561B2 (en) | 1997-10-13 | 2009-07-28 | Novozymes A/S | Alpha-amylase mutants |
| US8263368B2 (en) | 1997-10-13 | 2012-09-11 | Novozymes A/S | Alpha-amylase mutants |
| WO1999020768A1 (en) * | 1997-10-17 | 1999-04-29 | The Procter & Gamble Company | Methods for producing amylase enzymes |
| JP2001520305A (en) * | 1997-10-23 | 2001-10-30 | ザ、プロクター、エンド、ギャンブル、カンパニー | Cleaning compositions containing polysubstituted protease variants and amylase variants |
| WO1999020723A3 (en) * | 1997-10-23 | 2001-01-04 | Procter & Gamble | Multiply-substituted protease variant and amylase variant-containing cleaning compositions |
| WO1999020723A2 (en) * | 1997-10-23 | 1999-04-29 | The Procter & Gamble Company | Multiply-substituted protease variant and amylase variant-containing cleaning compositions |
| US6673589B2 (en) | 1997-10-30 | 2004-01-06 | Novozymes A/S | α-amylase mutants |
| EP2388267A1 (en) | 1997-10-30 | 2011-11-23 | Novozymes A/S | Alpha-amylase mutants |
| EP2386568A1 (en) | 1997-10-30 | 2011-11-16 | Novozymes A/S | Alpha-amylase mutants |
| US6204232B1 (en) | 1997-10-30 | 2001-03-20 | Novo Nordisk A/S | α-amlase mutants |
| EP2386569A1 (en) | 1997-10-30 | 2011-11-16 | Novozymes A/S | Alpha-amylase mutants |
| US7078213B1 (en) | 1998-02-18 | 2006-07-18 | Novozymes A/S | Alkaline Bacillus amylase |
| EP2287318A1 (en) | 1998-06-10 | 2011-02-23 | Novozymes A/S | Mannanases |
| EP2261359A1 (en) | 1998-06-10 | 2010-12-15 | Novozymes A/S | Mannanases |
| EP2284272A1 (en) | 1998-06-10 | 2011-02-16 | Novozymes A/S | Mannanases |
| US8465957B2 (en) | 1998-11-16 | 2013-06-18 | Novozymes A/S | Alpha-amylase variants |
| CN100374557C (en) * | 1998-11-16 | 2008-03-12 | 诺维信公司 | Alpha-amylse variant |
| US7601527B2 (en) | 1998-11-16 | 2009-10-13 | Novozymes A/S | α-amylase variants |
| US6887986B1 (en) | 1998-11-16 | 2005-05-03 | Novozymes A/S | α-amylase variants |
| US6583095B1 (en) | 1998-11-20 | 2003-06-24 | Procter & Gamble Company | Synthesis of bleach activators |
| WO2000041522A3 (en) * | 1999-01-11 | 2001-09-07 | Procter & Gamble | Cleaning compositions containing a multi-function component and method for using |
| WO2000041522A2 (en) * | 1999-01-11 | 2000-07-20 | The Procter & Gamble Company | Cleaning compositions containing a multi-function component and method for using |
| US8124395B2 (en) | 1999-03-30 | 2012-02-28 | Novozymes A/S | Alpha-amylase variants |
| US8859255B2 (en) | 1999-03-30 | 2014-10-14 | Novozymes A/S | Alpha-amylase variants |
| US7306936B2 (en) | 1999-03-30 | 2007-12-11 | Novozymes A/S | Alpha-amylase variants |
| EP1818396A3 (en) * | 1999-03-30 | 2007-12-19 | Novozymes A/S | Alpha-Amylase variants |
| EP2290060A1 (en) | 1999-03-30 | 2011-03-02 | Novozymes A/S | Alpha-amylase variants |
| EP1818396A2 (en) | 1999-03-30 | 2007-08-15 | Novozymes A/S | Alpha-Amylase variants |
| US7867746B2 (en) | 1999-03-30 | 2011-01-11 | Novozymes A/S | Alpha-amylase variants |
| US6410295B1 (en) | 1999-03-30 | 2002-06-25 | Novozymes A/S | Alpha-amylase variants |
| US10113157B2 (en) | 1999-03-30 | 2018-10-30 | Novozymes A/S | Alpha-amylase variants |
| US9464279B2 (en) | 1999-03-30 | 2016-10-11 | Novozymes A/S | Alpha-amylase variants |
| US8420370B2 (en) | 1999-03-30 | 2013-04-16 | Novozymes A/S | Alpha-amylase variants |
| WO2000060058A2 (en) | 1999-03-31 | 2000-10-12 | Novozymes A/S | Polypeptides having alkaline alpha-amylase activity and nucleic acids encoding same |
| EP2011864A1 (en) | 1999-03-31 | 2009-01-07 | Novozymes A/S | Polypeptides having alkaline alpha-amylase activity and nucleic acids encoding same |
| US6623948B1 (en) * | 1999-03-31 | 2003-09-23 | Novozymes A/S | Nucleic acid sequences encoding alkaline alpha-amylases |
| EP2889375A1 (en) | 1999-03-31 | 2015-07-01 | Novozymes A/S | Polypeptides having alkaline alpha-amylase activity and nucleic acids encoding same |
| US7341858B2 (en) | 1999-11-10 | 2008-03-11 | Bisgaard-Frantzen Henrik | Fungamyl-like alpha-amylase variants |
| US7160710B2 (en) | 1999-11-10 | 2007-01-09 | Novozymes A/S | Fungamyl-like alpha-amylase variants |
| EP1980614A2 (en) | 1999-11-10 | 2008-10-15 | Novozymes A/S | Fungamyl-like Alpha-Amylase Variants |
| US7919586B2 (en) | 1999-11-10 | 2011-04-05 | Novozymes A/S | Fungamyl-like alpha-amylase variants |
| US7005288B1 (en) | 1999-11-10 | 2006-02-28 | Novozymes A/S | Fungamyl-like alpha-amylase variants |
| US8883970B2 (en) | 2000-03-08 | 2014-11-11 | Novozymes A/S | Alpha-amylase variants |
| EP2302048A1 (en) | 2000-03-08 | 2011-03-30 | Novozymes A/S | Variants with altered properties |
| EP2298875A2 (en) | 2000-03-08 | 2011-03-23 | Novozymes A/S | Variants with altered properties |
| WO2001066712A2 (en) | 2000-03-08 | 2001-09-13 | Novozymes A/S | Variants with altered properties |
| EP3594334A2 (en) | 2000-03-08 | 2020-01-15 | Novozymes A/S | Variants with altered properties |
| EP2221365A1 (en) | 2000-03-08 | 2010-08-25 | Novozymes A/S | Variants with altered properties |
| US8609811B2 (en) | 2000-03-08 | 2013-12-17 | Novozymes A/S | Amylase variants |
| US9856437B2 (en) | 2000-03-08 | 2018-01-02 | Novozymes A/S | Amylase variants |
| US7319112B2 (en) | 2000-07-14 | 2008-01-15 | The Procter & Gamble Co. | Non-halogenated antibacterial agents and processes for making same |
| US7803604B2 (en) | 2000-07-28 | 2010-09-28 | Henkel Ag & Co. Kgaa | Amylolytic enzyme extracted from Bacillus sp. A 7-7 (DSM 12368) and washing and cleaning agents containing this novel amylolytic enzyme |
| DE10036752C2 (en) * | 2000-07-28 | 2003-02-20 | Henkel Kgaa | Detergent and cleaning agent with a new amylolytic enzyme from Bacillus sp. A 7-7 (DSM 12368) |
| EP2204446A1 (en) | 2000-08-01 | 2010-07-07 | Novozymes A/S | Alpha-amylase mutants with altered properties |
| US7713723B1 (en) | 2000-08-01 | 2010-05-11 | Novozymes A/S | Alpha-amylase mutants with altered properties |
| EP2308980A2 (en) | 2000-08-01 | 2011-04-13 | Novozymes A/S | Alpha-amylase mutants with altered properties |
| EP2308979A2 (en) | 2000-08-01 | 2011-04-13 | Novozymes A/S | Alpha-amylase mutants with altered properties |
| EP2298903A2 (en) | 2000-08-01 | 2011-03-23 | Novozymes A/S | Alpha-amylase mutants with altered properties |
| US6953587B2 (en) | 2000-09-13 | 2005-10-11 | Proacter & Gamble Company | Process for making a water-soluble foam component |
| EP1978081A2 (en) | 2000-10-27 | 2008-10-08 | The Procter and Gamble Company | Stabilized liquid compositions |
| WO2002040370A1 (en) | 2000-11-17 | 2002-05-23 | The Procter & Gamble Company | Water soluble packages |
| US7888104B2 (en) | 2000-11-28 | 2011-02-15 | Henkel Ag & Co. Kgaa | Cyclodextrin glucanotransferase (CGTase), obtained from<I>Bacillus agaradherens<λ>(DSM 9948) and detergents and cleaning agents containing said novel cyclodextrin glucanotransferase |
| EP1241112A2 (en) | 2001-03-15 | 2002-09-18 | The Procter & Gamble Company | Flexible multiple compartment pouch |
| US10612012B2 (en) | 2001-05-15 | 2020-04-07 | Novozymes A/S | Alpha-amylase variant with altered properties |
| US8252573B2 (en) | 2001-05-15 | 2012-08-28 | Novozymes A/S | Alpha-amylase variant with altered properties |
| EP2264160A2 (en) | 2001-05-15 | 2010-12-22 | Novozymes A/S | Alpha-amylase variant with altered properties |
| US7498158B2 (en) * | 2001-05-15 | 2009-03-03 | Novozymes A/S | Alpha-amylase variant with altered properties |
| US9796968B2 (en) | 2001-05-15 | 2017-10-24 | Novozymes A/S | Alpha-amylase variant with altered properties |
| EP2159279A2 (en) | 2001-05-15 | 2010-03-03 | Novozymes A/S | Alpha-amylase variant with altered properties |
| US8617837B2 (en) | 2001-05-15 | 2013-12-31 | Novozymes A/S | Method of hydrolyzing soluble starch with an alpha-amylase variant |
| US9670471B2 (en) | 2001-05-15 | 2017-06-06 | Novozymes A/S | Alpha-amylase variant with altered properties |
| EP3000881A2 (en) | 2001-05-15 | 2016-03-30 | Novozymes A/S | Alpha-amylase variant with altered properties |
| US8486681B2 (en) | 2001-05-15 | 2013-07-16 | Novozymes A/S | Alpha-amylase variant with altered properties |
| US9080137B2 (en) | 2001-05-15 | 2015-07-14 | Novozymes A/S | Alpha-amylase variant with altered properties |
| WO2002092797A2 (en) | 2001-05-15 | 2002-11-21 | Novozymes A/S | Alpha-amylase variant with altered properties |
| WO2002099091A2 (en) | 2001-06-06 | 2002-12-12 | Novozymes A/S | Endo-beta-1,4-glucanase from bacillus |
| US7153820B2 (en) | 2001-08-13 | 2006-12-26 | Ecolab Inc. | Solid detergent composition and method for solidifying a detergent composition |
| US7300782B2 (en) | 2001-12-21 | 2007-11-27 | B.R.A.I.N. Biotechnology Research And Information Network Ag | Glycosyl hydrolases |
| WO2004003187A2 (en) | 2002-07-01 | 2004-01-08 | Novozymes A/S | Mpg added to fermentation |
| EP2295545A1 (en) | 2002-09-26 | 2011-03-16 | Novozymes North America, Inc. | Fermentation methods and compositions |
| WO2004050820A1 (en) | 2002-12-05 | 2004-06-17 | Novozymes A/S | Beer mashing process |
| WO2004053039A2 (en) | 2002-12-11 | 2004-06-24 | Novozymes A/S | Detergent composition comprising endo-glucanase |
| EP2311941A1 (en) | 2002-12-11 | 2011-04-20 | Novozymes A/S | Detergent composition comprising endo-glucanase |
| US7189552B2 (en) | 2002-12-17 | 2007-03-13 | Novozymes A/S | Thermostable alpha-amylases |
| US9938512B2 (en) | 2002-12-17 | 2018-04-10 | Novozymes A/S | Thermostable alpha-amylases |
| US9394533B2 (en) | 2002-12-17 | 2016-07-19 | Novozymes A/S | Thermostable alpha-amylases |
| US8039241B2 (en) | 2002-12-17 | 2011-10-18 | I Novozymes A/S | Thermostable alpha-amylases |
| EP1923455A2 (en) | 2003-02-18 | 2008-05-21 | Novozymes A/S | Detergent compositions |
| WO2005005646A2 (en) | 2003-06-10 | 2005-01-20 | Novozymes North America, Inc. | Fermentation processes and compositions |
| WO2004111221A1 (en) | 2003-06-19 | 2004-12-23 | Novozymes A/S | Proteases |
| WO2004113551A1 (en) | 2003-06-25 | 2004-12-29 | Novozymes A/S | Process for the hydrolysis of starch |
| EP2284259A2 (en) | 2003-10-10 | 2011-02-16 | Novozymes A/S | Protease variants |
| EP2308966A1 (en) | 2003-10-10 | 2011-04-13 | Novozymes A/S | Protease variants |
| EP2213732A1 (en) | 2003-10-28 | 2010-08-04 | Novozymes North America, Inc. | Hybrid glucoamylases |
| EP2258838A1 (en) | 2004-06-21 | 2010-12-08 | Novozymes A/S | Nocardiopsis proteases |
| EP2258839A1 (en) | 2004-06-21 | 2010-12-08 | Novozymes A/S | Nocardiopsis proteases |
| WO2005123911A2 (en) | 2004-06-21 | 2005-12-29 | Novozymes A/S | Proteases |
| EP3620523A2 (en) | 2004-07-05 | 2020-03-11 | Novozymes A/S | Alpha-amylase variants with altered properties |
| WO2006002643A2 (en) | 2004-07-05 | 2006-01-12 | Novozymes A/S | Alpha-amylase variants with altered properties |
| EP4269684A2 (en) | 2004-07-05 | 2023-11-01 | Novozymes A/S | Alpha-amylase variants with altered properties |
| EP2258837A1 (en) | 2004-09-10 | 2010-12-08 | Novozymes North America, Inc. | Methods for preventing, removing, reducing, or disrupting biofilm |
| EP2258836A1 (en) | 2004-09-10 | 2010-12-08 | Novozymes North America, Inc. | Methods for preventing, removing, reducing, or disrupting biofilm |
| US7659101B2 (en) | 2004-12-15 | 2010-02-09 | Novozymes A/S | Alkaline Bacillus amylase |
| WO2006063594A1 (en) * | 2004-12-15 | 2006-06-22 | Novozymes A/S | Alkaline bacillus amylase |
| US7794997B2 (en) | 2004-12-15 | 2010-09-14 | Novozymes A/S | Alkaline Bacillus amylase |
| WO2006066596A2 (en) | 2004-12-22 | 2006-06-29 | Novozymes A/S | Hybrid enzymes consisting of an endo-amylase first amino acid sequence and a carbohydrate -binding module as second amino acid sequence |
| WO2007035730A2 (en) | 2005-09-20 | 2007-03-29 | Novozymes North America, Inc. | Process of producing a fermentation product |
| EP2495316A2 (en) | 2006-06-21 | 2012-09-05 | Novozymes North America, Inc. | Desizing and scouring process of starch |
| WO2008021761A2 (en) | 2006-08-11 | 2008-02-21 | Novozymes Biologicals, Inc. | Bacteria cultures and compositions comprising bacteria cultures |
| EP3339412A1 (en) | 2007-02-15 | 2018-06-27 | Ecolab Usa Inc. | Fast dissolving solid detergent |
| US11261406B2 (en) | 2007-02-15 | 2022-03-01 | Ecolab Usa Inc. | Fast dissolving solid detergent |
| EP2617804A1 (en) | 2007-02-15 | 2013-07-24 | Ecolab Inc. | Fast dissolving solid detergent |
| US9267097B2 (en) | 2007-02-15 | 2016-02-23 | Ecolab Usa Inc. | Fast dissolving solid detergent |
| US10005986B2 (en) | 2007-02-15 | 2018-06-26 | Ecolab Usa Inc. | Fast dissolving solid detergent |
| US10577565B2 (en) | 2007-02-15 | 2020-03-03 | Ecolab Usa Inc. | Fast dissolving solid detergent |
| EP2500325A1 (en) | 2007-03-23 | 2012-09-19 | Novozymes Biologicals, Inc. | Preventing and Reducing Biofilm Formation and Planktonic Proliferation |
| US8809031B2 (en) | 2007-03-23 | 2014-08-19 | Danisco Us Inc. | Enhanced amylase production by N-terminal addition to mature amylase protein |
| WO2008118749A2 (en) | 2007-03-23 | 2008-10-02 | Novozymes Biologicals, Inc. | Preventing and reducing biofilm formation and planktonic proliferation |
| EP2677023A2 (en) | 2007-10-18 | 2013-12-25 | Ecolab Inc. | Pressed, waxy, solid cleaning compositions and methods of making them |
| WO2009050684A2 (en) | 2007-10-18 | 2009-04-23 | Ecolab Inc. | Pressed, waxy, solid cleaning compositions and methods of making them |
| EP3438235A1 (en) | 2007-10-18 | 2019-02-06 | Ecolab USA Inc. | Pressed, waxy, solid cleaning compositions and methods of making them |
| US8153412B2 (en) | 2007-11-05 | 2012-04-10 | Danisco Us Inc. | Variants of Bacillus sp. TS-23 alpha-amylase with altered properties |
| US8206966B2 (en) | 2007-11-05 | 2012-06-26 | Danisco Us Inc. | Alpha-amylase variants with altered properties |
| US8962283B2 (en) | 2008-02-04 | 2015-02-24 | Danisco Us Inc. | TS-23 alpha-amylase variants with altered properties |
| US8236545B2 (en) | 2008-02-04 | 2012-08-07 | Danisco Us Inc., Genencor Division | TS23 alpha-amylase variants with altered properties |
| US8507244B2 (en) | 2008-02-04 | 2013-08-13 | Danisco Us Inc. | Variants of bacillus sp. TS-23 alpha-amylase with altered properties |
| US8460916B2 (en) | 2008-02-04 | 2013-06-11 | Danisco Us Inc. | TS-23 alpha-amylase variants with altered properties |
| US8323945B2 (en) | 2008-06-06 | 2012-12-04 | Danisco Us Inc. | Variant alpha-amylases from Bacillus subtilis and methods of uses, thereof |
| US9040278B2 (en) | 2008-06-06 | 2015-05-26 | Danisco Us Inc. | Production of glucose from starch using alpha-amylases from Bacillus subtilis |
| US9040279B2 (en) | 2008-06-06 | 2015-05-26 | Danisco Us Inc. | Saccharification enzyme composition and method of saccharification thereof |
| EP2447361A2 (en) | 2008-06-06 | 2012-05-02 | Danisco US Inc. | Geobacillus stearothermophilus alpha-amylase (AMYS) variants with improved properties |
| EP2623591A2 (en) | 2008-06-06 | 2013-08-07 | Danisco US Inc. | Geobacillus stearothermophilus alpha-amylase (AMYS) variants with improved properties |
| US8975056B2 (en) | 2008-06-06 | 2015-03-10 | Danisco Us Inc. | Variant alpha-amylases from Bacillus subtilis and methods of uses, thereof |
| US9090887B2 (en) | 2008-06-06 | 2015-07-28 | Danisco Us Inc. | Variant alpha-amylases from Bacillus subtilis and methods of use, thereof |
| US8084240B2 (en) | 2008-06-06 | 2011-12-27 | Danisco Us Inc. | Geobacillus stearothermophilus α-amylase (AmyS) variants with improved properties |
| EP2135934A1 (en) | 2008-06-16 | 2009-12-23 | Unilever PLC | Use of a laundry detergent composition |
| EP2135931A1 (en) | 2008-06-16 | 2009-12-23 | The Procter and Gamble Company | Use of soil release polymer in fabric treatment compositions |
| EP2149786A1 (en) | 2008-08-01 | 2010-02-03 | Unilever PLC | Improvements relating to detergent analysis |
| DE212009000119U1 (en) | 2008-09-12 | 2011-12-30 | Unilever N.V. | Dispenser and pretreatment agent for viscous liquids |
| US8507243B2 (en) | 2008-09-25 | 2013-08-13 | Danisco Us Inc. | Alpha-amylase blends and methods for using said blends |
| EP2202290A1 (en) | 2008-12-23 | 2010-06-30 | Unilever PLC | A flowable laundry composition and packaging therefor |
| WO2010108002A1 (en) | 2009-03-18 | 2010-09-23 | The Procter & Gamble Company | Structured fluid detergent compositions comprising dibenzylidene sorbitol acetal derivatives |
| WO2010108000A1 (en) | 2009-03-18 | 2010-09-23 | The Procter & Gamble Company | Structured fluid detergent compositions comprising dibenzylidene polyol acetal derivatives and detersive enzymes |
| WO2010127919A1 (en) | 2009-05-05 | 2010-11-11 | Unilever Plc | Shading composition |
| WO2011042372A1 (en) | 2009-10-08 | 2011-04-14 | Unilever Plc | Shading composition |
| WO2011045195A1 (en) | 2009-10-13 | 2011-04-21 | Unilever Plc | Dye polymers |
| WO2011049945A2 (en) | 2009-10-23 | 2011-04-28 | Danisco Us Inc. | Methods for reducing blue saccharide |
| WO2011047987A1 (en) | 2009-10-23 | 2011-04-28 | Unilever Plc | Dye polymers |
| WO2011080267A2 (en) | 2009-12-29 | 2011-07-07 | Novozymes A/S | Polypetides having detergency enhancing effect |
| US9394532B2 (en) | 2010-01-04 | 2016-07-19 | Novozymes A/S | Stabilization of alpha-amylases towards calcium depletion and acidic pH |
| US8591969B2 (en) | 2010-01-04 | 2013-11-26 | Novozymes A/S | Alpha-amylases |
| US9695405B2 (en) | 2010-01-04 | 2017-07-04 | Novozymes A/S | Stabilization of alpha-amylases towards calcium depletion and acidic pH |
| US9273297B2 (en) | 2010-01-04 | 2016-03-01 | Novozymes A/S | Stabilization of alpha-amylases towards calcium depletion and acidic pH |
| WO2011082429A1 (en) | 2010-01-04 | 2011-07-07 | Novozymes A/S | Alpha-amylases |
| US8435577B2 (en) | 2010-01-04 | 2013-05-07 | Novozymes A/S | Alpha-amylases |
| WO2011080352A1 (en) | 2010-01-04 | 2011-07-07 | Novozymes A/S | Alpha-amylases |
| US8900848B2 (en) | 2010-01-04 | 2014-12-02 | Novozymes A/S | Stabilization of alpha-amylases towards calcium depletion and acidic PH |
| WO2011080353A1 (en) | 2010-01-04 | 2011-07-07 | Novozymes A/S | Stabilization of alpha-amylases towards calcium depletion and acidic ph |
| US9809806B2 (en) | 2010-01-04 | 2017-11-07 | Novozymes A/S | Alpha-amylases |
| US9139822B2 (en) | 2010-01-04 | 2015-09-22 | Novozymes A/S | Alpha-amylases |
| WO2011080354A1 (en) | 2010-01-04 | 2011-07-07 | Novozymes A/S | Alpha-amylases |
| US9506047B2 (en) | 2010-01-04 | 2016-11-29 | Novozymes A/S | Alpha-amylases |
| WO2011082889A1 (en) | 2010-01-07 | 2011-07-14 | Unilever Plc | Natural shading agents |
| WO2011098355A1 (en) | 2010-02-09 | 2011-08-18 | Unilever Plc | Dye polymers |
| WO2011100410A2 (en) | 2010-02-10 | 2011-08-18 | The Procter & Gamble Company | Cleaning composition comprising amylase variants with high stability in the presence of a chelating agent |
| US11959107B2 (en) | 2010-02-10 | 2024-04-16 | Novozymes A/S | Variants and compositions comprising variants with high stability in presence of a chelating agent |
| EP3428260A2 (en) | 2010-02-10 | 2019-01-16 | The Procter & Gamble Company | Cleaning composition comprising amylase variants with high stability in the presence of a chelating agent |
| EP3730595A2 (en) | 2010-02-10 | 2020-10-28 | The Procter & Gamble Company | Cleaning composition comprising amylase variants with high stability in the presence of a chelating agent |
| EP3404087A1 (en) | 2010-02-10 | 2018-11-21 | Novozymes A/S | Alpha-amylase variants with high stability in presence of a chelating agent |
| US10655116B2 (en) | 2010-02-10 | 2020-05-19 | Novozymes A/S | Variants and compositions comprising variants with high stability in presence of a chelating agent |
| US10240135B2 (en) | 2010-02-10 | 2019-03-26 | Novozymes A/S | Variants and compositions comprising variants with high stability in presence of a chelating agent |
| US9896673B2 (en) | 2010-02-10 | 2018-02-20 | Novozymes A/S | Compositions of high stability alpha amylase variants |
| EP3892709A2 (en) | 2010-02-10 | 2021-10-13 | Novozymes A/S | Variants and compositions comprising variants with high stability in presence of a chelating agent |
| EP2357220A1 (en) | 2010-02-10 | 2011-08-17 | The Procter & Gamble Company | Cleaning composition comprising amylase variants with high stability in the presence of a chelating agent |
| WO2011098531A1 (en) | 2010-02-10 | 2011-08-18 | Novozymes A/S | Variants and compositions comprising variants with high stability in presence of a chelating agent |
| US11453868B2 (en) | 2010-02-10 | 2022-09-27 | Novozymes A/S | Variants and compositions comprising variants with high stability in presence of a chelating agent |
| WO2011098356A1 (en) | 2010-02-12 | 2011-08-18 | Unilever Plc | Laundry treatment composition comprising bis-azo shading dyes |
| WO2011104339A1 (en) | 2010-02-25 | 2011-09-01 | Novozymes A/S | Variants of a lysozyme and polynucleotides encoding same |
| EP2840134A1 (en) | 2010-04-26 | 2015-02-25 | Novozymes A/S | Enzyme granules |
| WO2011134809A1 (en) | 2010-04-26 | 2011-11-03 | Novozymes A/S | Enzyme granules |
| WO2011134685A1 (en) | 2010-04-29 | 2011-11-03 | Unilever Plc | Bis-heterocyclic azo dyes |
| WO2011161135A1 (en) | 2010-06-22 | 2011-12-29 | Novozymes A/S | Enzyme dehairing of skins and hides |
| WO2012019159A1 (en) | 2010-08-06 | 2012-02-09 | Danisco Us Inc. | Neutral ph saccharification and fermentation |
| WO2012019169A1 (en) | 2010-08-06 | 2012-02-09 | Danisco Us Inc. | Production of isoprene under neutral ph conditions |
| WO2012035103A1 (en) | 2010-09-16 | 2012-03-22 | Novozymes A/S | Lysozymes |
| WO2012038144A1 (en) | 2010-09-20 | 2012-03-29 | Unilever Plc | Fabric treatment compositions comprising target benefit agents |
| WO2012048910A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaged particulate detergent composition |
| WO2012049178A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| EP2441822A1 (en) | 2010-10-14 | 2012-04-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Laundry detergent particles |
| EP2441825A1 (en) | 2010-10-14 | 2012-04-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Process for preparing laundry detergent particles |
| EP2441820A1 (en) | 2010-10-14 | 2012-04-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Laundry detergent particles |
| WO2012049053A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Package comprising a laundry composition, dispenser for said package and method for washing using said dispenser and said package |
| WO2012048951A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012049032A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Refill and refillable packages of concentrated particulate detergent compositions |
| WO2012049034A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaging and dispensing of detergent compositions |
| WO2012048950A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012048955A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaging and dispensing of detergent compositions |
| WO2012049033A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Top-loading laundry vessel method |
| WO2012048948A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012048956A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaged concentrated particulate detergent composition |
| WO2012048945A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Particulate detergent compositions comprising fluorescer |
| WO2012049055A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Transparent packaging of detergent compositions |
| WO2012048909A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Packaged particulate detergent composition |
| WO2012048947A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particles |
| WO2012048949A1 (en) | 2010-10-14 | 2012-04-19 | Unilever Plc | Laundry detergent particle |
| WO2012052306A1 (en) | 2010-10-22 | 2012-04-26 | Unilever Plc | Externally structured aqueous detergent liquid |
| WO2012098046A1 (en) | 2011-01-17 | 2012-07-26 | Unilever Plc | Dye polymer for laundry treatment |
| WO2012104159A1 (en) | 2011-01-31 | 2012-08-09 | Unilever Plc | Alkaline liquid detergent compositions |
| WO2012112718A1 (en) | 2011-02-15 | 2012-08-23 | Novozymes Biologicals, Inc. | Mitigation of odor in cleaning machines and cleaning processes |
| EP3431581A2 (en) | 2011-02-15 | 2019-01-23 | Novozymes Biologicals, Inc. | Mitigation of odor in cleaning machines and cleaning processes |
| WO2012119859A1 (en) | 2011-03-10 | 2012-09-13 | Unilever Plc | Dye polymer |
| WO2012130492A1 (en) | 2011-03-25 | 2012-10-04 | Unilever Plc | Dye polymer |
| EP2476743A1 (en) | 2011-04-04 | 2012-07-18 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Method of laundering fabric |
| WO2012136427A1 (en) | 2011-04-04 | 2012-10-11 | Unilever Plc | Method of laundering fabric |
| WO2012149288A1 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Single ph process for starch liquefaction and saccharification for high-density glucose syrups |
| US8785165B2 (en) | 2011-04-29 | 2014-07-22 | Danisco Us Inc. | Single pH process for starch liquefaction and saccharification for high-density glucose syrups |
| WO2012149275A1 (en) | 2011-04-29 | 2012-11-01 | Danisco Us Inc. | Use of cellulase and glucoamylase to improve ethanol yields from fermentation |
| EP2522715A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| EP2522714A1 (en) | 2011-05-13 | 2012-11-14 | Unilever Plc, A Company Registered In England And Wales under company no. 41424 of Unilever House | Aqueous concentrated laundry detergent compositions |
| WO2012156250A1 (en) | 2011-05-13 | 2012-11-22 | Unilever Plc | Aqueous concentrated laundry detergent compositions |
| WO2012160498A2 (en) | 2011-05-20 | 2012-11-29 | Ecolab Usa Inc. | Acid formulations for use in a system for warewashing |
| EP2902471A1 (en) | 2011-05-20 | 2015-08-05 | Ecolab USA Inc. | Non-phosphate detergents and non-phosphoric acids in an alternating alkali/acid system for warewashing |
| EP2792737A1 (en) | 2011-05-20 | 2014-10-22 | Ecolab USA Inc. | Non-phosphate detergents and non-phosphoric acids in an alternating alkali/acid system for warewashing |
| WO2012159778A1 (en) | 2011-05-26 | 2012-11-29 | Unilever Plc | Liquid laundry composition |
| EP3354792A1 (en) | 2011-06-01 | 2018-08-01 | Unilever PLC, a company registered in England and Wales under company no. 41424 of | Liquid detergent composition containing dye polymer |
| EP4134424A1 (en) | 2011-06-01 | 2023-02-15 | Unilever IP Holdings B.V. | Liquid detergent composition containing dye polymer |
| EP2537918A1 (en) | 2011-06-20 | 2012-12-26 | The Procter & Gamble Company | Consumer products with lipase comprising coated particles |
| WO2012175401A2 (en) | 2011-06-20 | 2012-12-27 | Novozymes A/S | Particulate composition |
| WO2013003025A1 (en) | 2011-06-20 | 2013-01-03 | The Procter & Gamble Company | Consumer products with lipase comprising coated particles |
| EP4026901A2 (en) | 2011-06-30 | 2022-07-13 | Novozymes A/S | Method for screening alpha-amylases |
| EP2540824A1 (en) | 2011-06-30 | 2013-01-02 | The Procter & Gamble Company | Cleaning compositions comprising amylase variants reference to a sequence listing |
| EP2540825A2 (en) | 2011-06-30 | 2013-01-02 | The Procter & Gamble Company | Cleaning compositions comprising amylase variants reference to a sequence listing |
| US9434932B2 (en) | 2011-06-30 | 2016-09-06 | Novozymes A/S | Alpha-amylase variants |
| US10752889B2 (en) | 2011-06-30 | 2020-08-25 | Novozymes A/S | Alpha-amylase variants |
| US12012623B2 (en) | 2011-06-30 | 2024-06-18 | Novozymes A/S | Alpha-amylase variants |
| EP3121270A2 (en) | 2011-06-30 | 2017-01-25 | The Procter & Gamble Company | Cleaning compositions comprising amylase variants reference to a sequence listing |
| WO2013001087A2 (en) | 2011-06-30 | 2013-01-03 | Novozymes A/S | Method for screening alpha-amylases |
| WO2013003659A1 (en) | 2011-06-30 | 2013-01-03 | The Procter & Gamble Company | Cleaning compositions comprising amylase variants reference to a sequence listing |
| EP3543333A2 (en) | 2011-06-30 | 2019-09-25 | Novozymes A/S | Method for screening alpha-amylases |
| US11091748B2 (en) | 2011-06-30 | 2021-08-17 | Novozymes A/S | Alpha-amylase variants |
| US10167458B2 (en) | 2011-06-30 | 2019-01-01 | Novozymes A/S | Alpha-amylase variants |
| US9765316B2 (en) | 2011-07-06 | 2017-09-19 | Novozymes A/S | Alpha amylase variants and polynucleotides encoding same |
| WO2013006756A2 (en) | 2011-07-06 | 2013-01-10 | Novozymes A/S | Alpha amylase variants and polynucleotides encoding same |
| US9267124B2 (en) | 2011-07-06 | 2016-02-23 | Novozymes A/S | Alpha amylase variants and polynucleotides encoding same |
| WO2013011071A1 (en) | 2011-07-21 | 2013-01-24 | Unilever Plc | Liquid laundry composition |
| WO2013022799A1 (en) | 2011-08-05 | 2013-02-14 | Danisco Us Inc. | PRODUCTION OF ISOPRENOIDS UNDER NEUTRAL pH CONDITIONS |
| US8951764B2 (en) | 2011-08-05 | 2015-02-10 | Danisco Us Inc. | Production of isoprenoids under neutral pH conditions |
| WO2013023938A1 (en) | 2011-08-12 | 2013-02-21 | Novozymes A/S | Reduction of culture viscosity by manganese addition |
| WO2013026796A1 (en) | 2011-08-19 | 2013-02-28 | Novozymes A/S | Polypeptides having protease activity |
| WO2013057141A2 (en) | 2011-10-17 | 2013-04-25 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| EP4253534A2 (en) | 2011-10-17 | 2023-10-04 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| EP3495479A1 (en) | 2011-10-17 | 2019-06-12 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2013057143A2 (en) | 2011-10-17 | 2013-04-25 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| EP3282004A1 (en) | 2011-12-13 | 2018-02-14 | Ecolab USA Inc. | Warewashing method |
| WO2013092052A1 (en) | 2011-12-20 | 2013-06-27 | Unilever Plc | Isotropic liquid detergents comprising soil release polymer |
| WO2013098185A1 (en) | 2011-12-28 | 2013-07-04 | Novozymes A/S | Polypeptides having protease activity |
| EP2639291A1 (en) | 2012-03-13 | 2013-09-18 | Unilever PLC | Packaged particulate detergent composition |
| WO2013139702A1 (en) | 2012-03-21 | 2013-09-26 | Unilever Plc | Laundry detergent particles |
| WO2013149755A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particles |
| WO2013149753A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particles |
| WO2013149752A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particles |
| WO2013149754A1 (en) | 2012-04-03 | 2013-10-10 | Unilever Plc | Laundry detergent particle |
| WO2013160025A1 (en) | 2012-04-23 | 2013-10-31 | Unilever Plc | Structured aqueous liquid detergent |
| WO2014006040A1 (en) | 2012-07-06 | 2014-01-09 | Novozymes A/S | Inactivation of a production strain using a fatty acid |
| US9540669B2 (en) | 2012-08-14 | 2017-01-10 | Danisco Us Inc. | Trichoderma reesei glucoamylase variants resistant to oxidation-related activity loss and the use thereof |
| EP3470516A1 (en) | 2012-09-10 | 2019-04-17 | Ecolab USA Inc. | Stable liquid manual dishwashing compositions containing enzymes |
| WO2014048857A1 (en) | 2012-09-25 | 2014-04-03 | Unilever Plc | Laundry detergent particles |
| WO2014068083A1 (en) | 2012-11-01 | 2014-05-08 | Novozymes A/S | Method for removal of dna |
| WO2014086659A2 (en) | 2012-12-06 | 2014-06-12 | Ahmedabad Textile Industry's Research Association | Method for enzymatical preparation of textiles |
| EP3882346A1 (en) | 2013-05-29 | 2021-09-22 | Danisco US Inc. | Novel metalloproteases |
| WO2014194032A1 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| EP3260538A1 (en) | 2013-05-29 | 2017-12-27 | Danisco US Inc. | Novel metalloproteases |
| WO2014194034A2 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| WO2014194117A2 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| EP4159854A1 (en) | 2013-05-29 | 2023-04-05 | Danisco US Inc | Novel metalloproteases |
| WO2014194054A1 (en) | 2013-05-29 | 2014-12-04 | Danisco Us Inc. | Novel metalloproteases |
| EP3636662A1 (en) | 2013-05-29 | 2020-04-15 | Danisco US Inc. | Novel metalloproteases |
| WO2014191170A1 (en) | 2013-05-30 | 2014-12-04 | Novozymes A/S | Particulate enzyme composition |
| WO2014198840A1 (en) | 2013-06-12 | 2014-12-18 | Earth Alive Clean Technologies Inc. | Dust suppressant |
| US9839894B2 (en) | 2013-08-05 | 2017-12-12 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US10583415B2 (en) | 2013-08-05 | 2020-03-10 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US11185837B2 (en) | 2013-08-05 | 2021-11-30 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US10272410B2 (en) | 2013-08-05 | 2019-04-30 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US10639609B2 (en) | 2013-08-05 | 2020-05-05 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US10632445B2 (en) | 2013-08-05 | 2020-04-28 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US10773232B2 (en) | 2013-08-05 | 2020-09-15 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US9833761B2 (en) | 2013-08-05 | 2017-12-05 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US10618024B2 (en) | 2013-08-05 | 2020-04-14 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US11452980B2 (en) | 2013-08-05 | 2022-09-27 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US11559778B2 (en) | 2013-08-05 | 2023-01-24 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| US10384188B2 (en) | 2013-08-05 | 2019-08-20 | Twist Bioscience Corporation | De novo synthesized gene libraries |
| WO2015057517A1 (en) | 2013-10-17 | 2015-04-23 | Danisco Us Inc. | Use of hemicellulases to improve ethanol production |
| EP3514230A1 (en) | 2013-12-13 | 2019-07-24 | Danisco US Inc. | Serine proteases of bacillus species |
| WO2015089447A1 (en) | 2013-12-13 | 2015-06-18 | Danisco Us Inc. | Serine proteases of the bacillus gibsonii-clade |
| EP3910057A1 (en) | 2013-12-13 | 2021-11-17 | Danisco US Inc. | Serine proteases of the bacillus gibsonii-clade |
| EP3553173A1 (en) | 2013-12-13 | 2019-10-16 | Danisco US Inc. | Serine proteases of the bacillus gibsonii-clade |
| WO2015089441A1 (en) | 2013-12-13 | 2015-06-18 | Danisco Us Inc. | Serine proteases of bacillus species |
| EP4163305A1 (en) | 2013-12-16 | 2023-04-12 | Nutrition & Biosciences USA 4, Inc. | Use of poly alpha-1,3-glucan ethers as viscosity modifiers |
| EP3789407A1 (en) | 2013-12-18 | 2021-03-10 | Nutrition & Biosciences USA 4, Inc. | Cationic poly alpha-1,3-glucan ethers |
| WO2015095358A1 (en) | 2013-12-18 | 2015-06-25 | E. I. Du Pont De Nemours And Company | Cationic poly alpha-1,3-glucan ethers |
| WO2015123323A1 (en) | 2014-02-14 | 2015-08-20 | E. I. Du Pont De Nemours And Company | Poly-alpha-1,3-1,6-glucans for viscosity modification |
| WO2015138283A1 (en) | 2014-03-11 | 2015-09-17 | E. I. Du Pont De Nemours And Company | Oxidized poly alpha-1,3-glucan as detergent builder |
| EP4155398A1 (en) | 2014-03-21 | 2023-03-29 | Danisco US Inc. | Serine proteases of bacillus species |
| EP3587569A1 (en) | 2014-03-21 | 2020-01-01 | Danisco US Inc. | Serine proteases of bacillus species |
| EP3550015A1 (en) | 2014-04-10 | 2019-10-09 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| EP2940116B1 (en) | 2014-04-30 | 2018-10-17 | The Procter and Gamble Company | Detergent |
| US10428321B2 (en) | 2014-06-12 | 2019-10-01 | Novozymes A/S | Alpha-amylase variants |
| EP3919599A1 (en) | 2014-06-19 | 2021-12-08 | Nutrition & Biosciences USA 4, Inc. | Compositions containing one or more poly alpha-1,3-glucan ether compounds |
| WO2015195777A1 (en) | 2014-06-19 | 2015-12-23 | E. I. Du Pont De Nemours And Company | Compositions containing one or more poly alpha-1,3-glucan ether compounds |
| WO2015195960A1 (en) | 2014-06-19 | 2015-12-23 | E. I. Du Pont De Nemours And Company | Compositions containing one or more poly alpha-1,3-glucan ether compounds |
| WO2016041676A1 (en) | 2014-09-18 | 2016-03-24 | Unilever Plc | Whitening composition |
| WO2016061438A1 (en) | 2014-10-17 | 2016-04-21 | Danisco Us Inc. | Serine proteases of bacillus species |
| EP3550017A1 (en) | 2014-10-27 | 2019-10-09 | Danisco US Inc. | Serine proteases |
| WO2016069569A2 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| WO2016069552A1 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| WO2016069548A2 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| EP4403631A2 (en) | 2014-10-27 | 2024-07-24 | Danisco US Inc. | Serine proteases |
| WO2016069557A1 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases of bacillus species |
| WO2016069544A1 (en) | 2014-10-27 | 2016-05-06 | Danisco Us Inc. | Serine proteases |
| US10287562B2 (en) | 2014-11-20 | 2019-05-14 | Novoszymes A/S | Alicyclobacillus variants and polynucleotides encoding same |
| WO2016079305A1 (en) | 2014-11-20 | 2016-05-26 | Novozymes A/S | Alicyclobacillus variants and polynucleotides encoding same |
| WO2016106011A1 (en) | 2014-12-23 | 2016-06-30 | E. I. Du Pont De Nemours And Company | Enzymatically produced cellulose |
| WO2016110378A1 (en) | 2015-01-09 | 2016-07-14 | Unilever Plc | Laundry treatment composition comprising a dye |
| US11697668B2 (en) | 2015-02-04 | 2023-07-11 | Twist Bioscience Corporation | Methods and devices for de novo oligonucleic acid assembly |
| US10669304B2 (en) | 2015-02-04 | 2020-06-02 | Twist Bioscience Corporation | Methods and devices for de novo oligonucleic acid assembly |
| WO2016128466A1 (en) | 2015-02-13 | 2016-08-18 | Unilever Plc | Laundry liquid composition |
| WO2016155993A1 (en) | 2015-04-02 | 2016-10-06 | Unilever Plc | Composition |
| US9981239B2 (en) | 2015-04-21 | 2018-05-29 | Twist Bioscience Corporation | Devices and methods for oligonucleic acid library synthesis |
| US11691118B2 (en) | 2015-04-21 | 2023-07-04 | Twist Bioscience Corporation | Devices and methods for oligonucleic acid library synthesis |
| US10744477B2 (en) | 2015-04-21 | 2020-08-18 | Twist Bioscience Corporation | Devices and methods for oligonucleic acid library synthesis |
| US10316275B2 (en) | 2015-05-08 | 2019-06-11 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| US12018237B2 (en) | 2015-05-08 | 2024-06-25 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2016180748A1 (en) | 2015-05-08 | 2016-11-17 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| EP4212609A1 (en) | 2015-05-08 | 2023-07-19 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| WO2016180792A1 (en) | 2015-05-08 | 2016-11-17 | Novozymes A/S | Alpha-amylase variants having improved performance and stability |
| US11319509B2 (en) | 2015-05-08 | 2022-05-03 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| US11365375B2 (en) | 2015-05-08 | 2022-06-21 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| US10647946B2 (en) | 2015-05-08 | 2020-05-12 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| US11952557B2 (en) | 2015-05-08 | 2024-04-09 | Novozymes A/S | Alpha-amylase variants and polynucleotides encoding same |
| EP3872174A1 (en) | 2015-05-13 | 2021-09-01 | Danisco US Inc. | Aprl-clade protease variants and uses thereof |
| EP4219704A2 (en) | 2015-05-13 | 2023-08-02 | Danisco US Inc | Aprl-clade protease variants and uses thereof |
| WO2016201040A1 (en) | 2015-06-09 | 2016-12-15 | Danisco Us Inc. | Water-triggered enzyme suspension |
| WO2016201044A1 (en) | 2015-06-09 | 2016-12-15 | Danisco Us Inc | Osmotic burst encapsulates |
| WO2016201069A1 (en) | 2015-06-09 | 2016-12-15 | Danisco Us Inc | Low-density enzyme-containing particles |
| WO2016205755A1 (en) | 2015-06-17 | 2016-12-22 | Danisco Us Inc. | Bacillus gibsonii-clade serine proteases |
| EP4234693A2 (en) | 2015-06-17 | 2023-08-30 | Danisco US Inc | Bacillus gibsonii-clade serine proteases |
| WO2017036916A1 (en) | 2015-08-28 | 2017-03-09 | Unilever N.V. | Process to manufacture cross-linked enzyme aggregates |
| WO2017036917A1 (en) | 2015-08-28 | 2017-03-09 | Unilever N.V. | Liquid detergency composition comprising lipase and protease |
| WO2017036915A1 (en) | 2015-08-28 | 2017-03-09 | Unilever N.V. | Liquid detergency composition comprising protease and non-protease enzyme |
| US10844373B2 (en) | 2015-09-18 | 2020-11-24 | Twist Bioscience Corporation | Oligonucleic acid variant libraries and synthesis thereof |
| US11807956B2 (en) | 2015-09-18 | 2023-11-07 | Twist Bioscience Corporation | Oligonucleic acid variant libraries and synthesis thereof |
| US11512347B2 (en) | 2015-09-22 | 2022-11-29 | Twist Bioscience Corporation | Flexible substrates for nucleic acid synthesis |
| WO2017079756A1 (en) | 2015-11-05 | 2017-05-11 | Danisco Us Inc | Paenibacillus and bacillus spp. mannanases |
| WO2017079751A1 (en) | 2015-11-05 | 2017-05-11 | Danisco Us Inc | Paenibacillus sp. mannanases |
| EP4141113A1 (en) | 2015-11-05 | 2023-03-01 | Danisco US Inc | Paenibacillus sp. mannanases |
| WO2017083229A1 (en) | 2015-11-13 | 2017-05-18 | E. I. Du Pont De Nemours And Company | Glucan fiber compositions for use in laundry care and fabric care |
| US10822574B2 (en) | 2015-11-13 | 2020-11-03 | Dupont Industrial Biosciences Usa, Llc | Glucan fiber compositions for use in laundry care and fabric care |
| WO2017083226A1 (en) | 2015-11-13 | 2017-05-18 | E. I. Du Pont De Nemours And Company | Glucan fiber compositions for use in laundry care and fabric care |
| WO2017083228A1 (en) | 2015-11-13 | 2017-05-18 | E. I. Du Pont De Nemours And Company | Glucan fiber compositions for use in laundry care and fabric care |
| US10876074B2 (en) | 2015-11-13 | 2020-12-29 | Dupont Industrial Biosciences Usa, Llc | Glucan fiber compositions for use in laundry care and fabric care |
| US10844324B2 (en) | 2015-11-13 | 2020-11-24 | Dupont Industrial Biosciences Usa, Llc | Glucan fiber compositions for use in laundry care and fabric care |
| US9895673B2 (en) | 2015-12-01 | 2018-02-20 | Twist Bioscience Corporation | Functionalized surfaces and preparation thereof |
| US10987648B2 (en) | 2015-12-01 | 2021-04-27 | Twist Bioscience Corporation | Functionalized surfaces and preparation thereof |
| US10384189B2 (en) | 2015-12-01 | 2019-08-20 | Twist Bioscience Corporation | Functionalized surfaces and preparation thereof |
| US11920170B2 (en) | 2015-12-09 | 2024-03-05 | Danisco Us Inc. | Alpha-amylase combinatorial variants |
| WO2017106676A1 (en) | 2015-12-18 | 2017-06-22 | Danisco Us Inc | Polypeptides with endoglucanase activity and uses thereof |
| EP3190167A1 (en) | 2016-01-07 | 2017-07-12 | Unilever PLC | Bitter pill |
| WO2017121714A1 (en) | 2016-01-15 | 2017-07-20 | Unilever Plc | Dye |
| WO2017133879A1 (en) | 2016-02-04 | 2017-08-10 | Unilever Plc | Detergent liquid |
| WO2017140391A1 (en) | 2016-02-17 | 2017-08-24 | Unilever Plc | Whitening composition |
| WO2017140392A1 (en) | 2016-02-17 | 2017-08-24 | Unilever Plc | Whitening composition |
| WO2017162378A1 (en) | 2016-03-21 | 2017-09-28 | Unilever Plc | Laundry detergent composition |
| WO2017173324A2 (en) | 2016-04-01 | 2017-10-05 | Danisco Us Inc. | Alpha-amylases, compositions & methods |
| WO2017173190A2 (en) | 2016-04-01 | 2017-10-05 | Danisco Us Inc. | Alpha-amylases, compositions & methods |
| WO2017174251A1 (en) | 2016-04-08 | 2017-10-12 | Unilever Plc | Laundry detergent composition |
| WO2017192692A1 (en) | 2016-05-03 | 2017-11-09 | Danisco Us Inc | Protease variants and uses thereof |
| WO2017192300A1 (en) | 2016-05-05 | 2017-11-09 | Danisco Us Inc | Protease variants and uses thereof |
| EP3845642A1 (en) | 2016-05-05 | 2021-07-07 | Danisco US Inc. | Protease variants and uses thereof |
| WO2017210295A1 (en) | 2016-05-31 | 2017-12-07 | Danisco Us Inc. | Protease variants and uses thereof |
| WO2017211697A1 (en) | 2016-06-09 | 2017-12-14 | Unilever Plc | Laundry products |
| EP4151726A1 (en) | 2016-06-17 | 2023-03-22 | Danisco US Inc | Protease variants and uses thereof |
| WO2017219011A1 (en) | 2016-06-17 | 2017-12-21 | Danisco Us Inc | Protease variants and uses thereof |
| US10975372B2 (en) | 2016-08-22 | 2021-04-13 | Twist Bioscience Corporation | De novo synthesized nucleic acid libraries |
| US10053688B2 (en) | 2016-08-22 | 2018-08-21 | Twist Bioscience Corporation | De novo synthesized nucleic acid libraries |
| US10754994B2 (en) | 2016-09-21 | 2020-08-25 | Twist Bioscience Corporation | Nucleic acid based data storage |
| US12056264B2 (en) | 2016-09-21 | 2024-08-06 | Twist Bioscience Corporation | Nucleic acid based data storage |
| US11562103B2 (en) | 2016-09-21 | 2023-01-24 | Twist Bioscience Corporation | Nucleic acid based data storage |
| US11263354B2 (en) | 2016-09-21 | 2022-03-01 | Twist Bioscience Corporation | Nucleic acid based data storage |
| US10417457B2 (en) | 2016-09-21 | 2019-09-17 | Twist Bioscience Corporation | Nucleic acid based data storage |
| WO2018072979A1 (en) | 2016-10-18 | 2018-04-26 | Unilever Plc | Whitening composition |
| WO2018085524A2 (en) | 2016-11-07 | 2018-05-11 | Danisco Us Inc | Laundry detergent composition |
| WO2018112123A1 (en) | 2016-12-15 | 2018-06-21 | Danisco Us Inc. | Polypeptides with endoglucanase activity and uses thereof |
| WO2018108382A1 (en) | 2016-12-15 | 2018-06-21 | Unilever Plc | Laundry detergent composition |
| US10907274B2 (en) | 2016-12-16 | 2021-02-02 | Twist Bioscience Corporation | Variant libraries of the immunological synapse and synthesis thereof |
| EP4424805A2 (en) | 2016-12-21 | 2024-09-04 | Danisco Us Inc | Bacillus gibsonii-clade serine proteases |
| EP4212622A2 (en) | 2016-12-21 | 2023-07-19 | Danisco US Inc. | Bacillus gibsonii-clade serine proteases |
| WO2018118917A1 (en) | 2016-12-21 | 2018-06-28 | Danisco Us Inc. | Protease variants and uses thereof |
| WO2018118950A1 (en) | 2016-12-21 | 2018-06-28 | Danisco Us Inc. | Bacillus gibsonii-clade serine proteases |
| US11550939B2 (en) | 2017-02-22 | 2023-01-10 | Twist Bioscience Corporation | Nucleic acid based data storage using enzymatic bioencryption |
| WO2018169750A1 (en) | 2017-03-15 | 2018-09-20 | Danisco Us Inc | Trypsin-like serine proteases and uses thereof |
| US10894959B2 (en) | 2017-03-15 | 2021-01-19 | Twist Bioscience Corporation | Variant libraries of the immunological synapse and synthesis thereof |
| WO2018183662A1 (en) | 2017-03-31 | 2018-10-04 | Danisco Us Inc | Delayed release enzyme formulations for bleach-containing detergents |
| WO2018184004A1 (en) | 2017-03-31 | 2018-10-04 | Danisco Us Inc | Alpha-amylase combinatorial variants |
| WO2018206202A1 (en) | 2017-05-10 | 2018-11-15 | Unilever Plc | Laundry detergent composition |
| WO2018206197A1 (en) | 2017-05-10 | 2018-11-15 | Unilever Plc | Laundry detergent composition |
| US11377676B2 (en) | 2017-06-12 | 2022-07-05 | Twist Bioscience Corporation | Methods for seamless nucleic acid assembly |
| US10696965B2 (en) | 2017-06-12 | 2020-06-30 | Twist Bioscience Corporation | Methods for seamless nucleic acid assembly |
| US11332740B2 (en) | 2017-06-12 | 2022-05-17 | Twist Bioscience Corporation | Methods for seamless nucleic acid assembly |
| WO2018234056A1 (en) | 2017-06-20 | 2018-12-27 | Unilever N.V. | Particulate detergent composition comprising perfume |
| WO2018234003A1 (en) | 2017-06-21 | 2018-12-27 | Unilever Plc | Packaging and dispensing of detergent compositions |
| WO2019006077A1 (en) | 2017-06-30 | 2019-01-03 | Danisco Us Inc | Low-agglomeration, enzyme-containing particles |
| WO2019008036A1 (en) | 2017-07-07 | 2019-01-10 | Unilever Plc | Whitening composition |
| WO2019008035A1 (en) | 2017-07-07 | 2019-01-10 | Unilever Plc | Laundry cleaning composition |
| WO2019036721A2 (en) | 2017-08-18 | 2019-02-21 | Danisco Us Inc | Alpha-amylase variants |
| CN111212906B (en) * | 2017-08-18 | 2024-02-02 | 丹尼斯科美国公司 | Alpha-amylase variants |
| WO2019036721A3 (en) * | 2017-08-18 | 2019-03-28 | Danisco Us Inc | Alpha-amylase variants |
| US12084694B2 (en) | 2017-08-18 | 2024-09-10 | Danisco Us Inc. | Alpha-amylase variants |
| CN111212906A (en) * | 2017-08-18 | 2020-05-29 | 丹尼斯科美国公司 | α -amylase variants |
| WO2019038187A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| WO2019038186A1 (en) | 2017-08-24 | 2019-02-28 | Unilever Plc | Improvements relating to fabric cleaning |
| US11407837B2 (en) | 2017-09-11 | 2022-08-09 | Twist Bioscience Corporation | GPCR binding proteins and synthesis thereof |
| US10894242B2 (en) | 2017-10-20 | 2021-01-19 | Twist Bioscience Corporation | Heated nanowells for polynucleotide synthesis |
| US11745159B2 (en) | 2017-10-20 | 2023-09-05 | Twist Bioscience Corporation | Heated nanowells for polynucleotide synthesis |
| WO2019108599A1 (en) | 2017-11-29 | 2019-06-06 | Danisco Us Inc | Subtilisin variants having improved stability |
| WO2019105675A1 (en) | 2017-11-30 | 2019-06-06 | Unilever Plc | Detergent composition comprising protease |
| WO2019125683A1 (en) | 2017-12-21 | 2019-06-27 | Danisco Us Inc | Enzyme-containing, hot-melt granules comprising a thermotolerant desiccant |
| US10936953B2 (en) | 2018-01-04 | 2021-03-02 | Twist Bioscience Corporation | DNA-based digital information storage with sidewall electrodes |
| US12086722B2 (en) | 2018-01-04 | 2024-09-10 | Twist Bioscience Corporation | DNA-based digital information storage with sidewall electrodes |
| WO2019156670A1 (en) | 2018-02-08 | 2019-08-15 | Danisco Us Inc. | Thermally-resistant wax matrix particles for enzyme encapsulation |
| WO2019168650A1 (en) * | 2018-02-28 | 2019-09-06 | The Procter & Gamble Company | Methods of cleaning |
| EP3533858A1 (en) * | 2018-02-28 | 2019-09-04 | The Procter & Gamble Company | Cleaning composition comprising a glycogen-debranching enzyme and methods of cleaning |
| WO2019219302A1 (en) | 2018-05-17 | 2019-11-21 | Unilever Plc | Cleaning composition comprising rhamnolipid and alkyl ether carboxylate surfactants |
| WO2019219531A1 (en) | 2018-05-17 | 2019-11-21 | Unilever Plc | Cleaning composition |
| US11732294B2 (en) | 2018-05-18 | 2023-08-22 | Twist Bioscience Corporation | Polynucleotides, reagents, and methods for nucleic acid hybridization |
| US11492665B2 (en) | 2018-05-18 | 2022-11-08 | Twist Bioscience Corporation | Polynucleotides, reagents, and methods for nucleic acid hybridization |
| WO2019238423A1 (en) | 2018-06-12 | 2019-12-19 | Novozymes A/S | Less added sugar in baked products |
| WO2019245704A1 (en) | 2018-06-19 | 2019-12-26 | Danisco Us Inc | Subtilisin variants |
| WO2019245705A1 (en) | 2018-06-19 | 2019-12-26 | Danisco Us Inc | Subtilisin variants |
| WO2020016097A1 (en) | 2018-07-17 | 2020-01-23 | Unilever Plc | Use of a rhamnolipid in a surfactant system |
| WO2020020703A1 (en) | 2018-07-27 | 2020-01-30 | Unilever N.V. | Laundry detergent |
| WO2020028443A1 (en) | 2018-07-31 | 2020-02-06 | Danisco Us Inc | Variant alpha-amylases having amino acid substitutions that lower the pka of the general acid |
| WO2020047215A1 (en) | 2018-08-30 | 2020-03-05 | Danisco Us Inc | Enzyme-containing granules |
| WO2020058024A1 (en) | 2018-09-17 | 2020-03-26 | Unilever Plc | Detergent composition |
| WO2020068486A1 (en) | 2018-09-27 | 2020-04-02 | Danisco Us Inc | Compositions for medical instrument cleaning |
| WO2020077331A2 (en) | 2018-10-12 | 2020-04-16 | Danisco Us Inc | Alpha-amylases with mutations that improve stability in the presence of chelants |
| WO2020104155A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104156A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104157A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104158A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020104159A1 (en) | 2018-11-20 | 2020-05-28 | Unilever Plc | Detergent composition |
| WO2020112599A1 (en) | 2018-11-28 | 2020-06-04 | Danisco Us Inc | Subtilisin variants having improved stability |
| WO2020114965A1 (en) | 2018-12-03 | 2020-06-11 | Novozymes A/S | LOW pH POWDER DETERGENT COMPOSITION |
| WO2020151959A1 (en) | 2019-01-22 | 2020-07-30 | Unilever N.V. | Laundry detergent |
| WO2020151992A1 (en) | 2019-01-22 | 2020-07-30 | Unilever N.V. | Laundry detergent |
| US11492727B2 (en) | 2019-02-26 | 2022-11-08 | Twist Bioscience Corporation | Variant nucleic acid libraries for GLP1 receptor |
| US11492728B2 (en) | 2019-02-26 | 2022-11-08 | Twist Bioscience Corporation | Variant nucleic acid libraries for antibody optimization |
| WO2020229535A1 (en) | 2019-05-16 | 2020-11-19 | Unilever Plc | Laundry composition |
| WO2020242858A1 (en) | 2019-05-24 | 2020-12-03 | Danisco Us Inc | Subtilisin variants and methods of use |
| WO2020247582A1 (en) | 2019-06-06 | 2020-12-10 | Danisco Us Inc | Methods and compositions for cleaning |
| WO2020249546A1 (en) | 2019-06-13 | 2020-12-17 | Basf Se | Method of recovering a protein from fermentation broth using a divalent cation |
| US11332738B2 (en) | 2019-06-21 | 2022-05-17 | Twist Bioscience Corporation | Barcode-based nucleic acid sequence assembly |
| WO2020264552A1 (en) | 2019-06-24 | 2020-12-30 | The Procter & Gamble Company | Cleaning compositions comprising amylase variants |
| WO2020260223A1 (en) | 2019-06-24 | 2020-12-30 | Novozymes A/S | Alpha-amylase variants |
| WO2020260006A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent compositions |
| WO2020260038A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020260040A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259949A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259948A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2020259947A1 (en) | 2019-06-28 | 2020-12-30 | Unilever Plc | Detergent composition |
| WO2021004830A1 (en) | 2019-07-05 | 2021-01-14 | Basf Se | Industrial fermentation process for microbial cells using a fed-batch pre-culture |
| WO2021022045A1 (en) | 2019-07-31 | 2021-02-04 | Ecolab Usa Inc. | Personal protective equipment free delimer compositions |
| WO2021043764A1 (en) | 2019-09-02 | 2021-03-11 | Unilever Global Ip Limited | Detergent composition |
| DE112020004477T5 (en) | 2019-09-19 | 2022-06-30 | Unilever Global Ip Limited | DETERGENT COMPOSITIONS |
| WO2021053122A1 (en) | 2019-09-19 | 2021-03-25 | Unilever Ip Holdings B.V. | Detergent compositions |
| US12091777B2 (en) | 2019-09-23 | 2024-09-17 | Twist Bioscience Corporation | Variant nucleic acid libraries for CRTH2 |
| WO2021069516A1 (en) | 2019-10-07 | 2021-04-15 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021080948A2 (en) | 2019-10-24 | 2021-04-29 | Danisco Us Inc | Variant maltopentaose/maltohexaose-forming alpha-amylases |
| WO2021151536A1 (en) | 2020-01-29 | 2021-08-05 | Unilever Ip Holdings B.V. | Laundry detergent product |
| WO2021185956A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2021185870A1 (en) | 2020-03-19 | 2021-09-23 | Unilever Ip Holdings B.V. | Detergent composition |
| DE102020205400A1 (en) | 2020-04-29 | 2021-11-04 | Henkel Ag & Co. Kgaa | Highly alkaline laundry detergent with protease |
| DE102020205381A1 (en) | 2020-04-29 | 2021-11-04 | Henkel Ag & Co. Kgaa | Highly alkaline laundry detergent with protease |
| WO2021219296A1 (en) | 2020-04-29 | 2021-11-04 | Henkel Ag & Co. Kgaa | Highly alkaline textile washing agent comprising protease |
| WO2021219297A1 (en) | 2020-04-29 | 2021-11-04 | Henkel Ag & Co. Kgaa | Highly alkaline textile detergent containing protease |
| WO2021249927A1 (en) | 2020-06-08 | 2021-12-16 | Unilever Ip Holdings B.V. | Method of improving protease activity |
| WO2022047149A1 (en) | 2020-08-27 | 2022-03-03 | Danisco Us Inc | Enzymes and enzyme compositions for cleaning |
| WO2022042989A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022043138A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Surfactant and detergent composition |
| WO2022043042A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022042977A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022043045A1 (en) | 2020-08-28 | 2022-03-03 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2022074037A2 (en) | 2020-10-07 | 2022-04-14 | Novozymes A/S | Alpha-amylase variants |
| WO2022090562A1 (en) | 2020-11-02 | 2022-05-05 | Novozymes A/S | Baked and par-baked products with thermostable amg variants from penicillium |
| WO2022122480A1 (en) | 2020-12-07 | 2022-06-16 | Unilever Ip Holdings B.V. | Detergent compositions |
| WO2022122481A1 (en) | 2020-12-07 | 2022-06-16 | Unilever Ip Holdings B.V. | Detergent compositions |
| EP4011256A1 (en) | 2020-12-14 | 2022-06-15 | Henkel AG & Co. KGaA | Method for cleaning an electric motorised kitchen appliance |
| EP4012011A1 (en) | 2020-12-14 | 2022-06-15 | Henkel AG & Co. KGaA | Cleaning agent, particularly for a kitchen appliance |
| WO2022128620A1 (en) | 2020-12-14 | 2022-06-23 | Henkel Ag & Co. Kgaa | Method for cleaning a food processor that is driven by an electric motor |
| WO2022128786A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Use and cleaning composition |
| WO2022128781A1 (en) | 2020-12-17 | 2022-06-23 | Unilever Ip Holdings B.V. | Cleaning composition |
| WO2022165107A1 (en) | 2021-01-29 | 2022-08-04 | Danisco Us Inc | Compositions for cleaning and methods related thereto |
| WO2022171780A2 (en) | 2021-02-12 | 2022-08-18 | Novozymes A/S | Alpha-amylase variants |
| CN112844816A (en) * | 2021-03-18 | 2021-05-28 | 河南金源黄金矿业有限责任公司 | Gravity separation regrinding process and equipment for treating Nielsen products |
| CN112844816B (en) * | 2021-03-18 | 2024-01-19 | 河南金源黄金矿业有限责任公司 | Gravity dressing regrinding process and equipment for treating Nelson products |
| WO2022199418A1 (en) | 2021-03-26 | 2022-09-29 | Novozymes A/S | Detergent composition with reduced polymer content |
| WO2022268885A1 (en) | 2021-06-23 | 2022-12-29 | Novozymes A/S | Alpha-amylase polypeptides |
| WO2023278297A1 (en) | 2021-06-30 | 2023-01-05 | Danisco Us Inc | Variant lipases and uses thereof |
| WO2023034486A2 (en) | 2021-09-03 | 2023-03-09 | Danisco Us Inc. | Laundry compositions for cleaning |
| WO2023039270A2 (en) | 2021-09-13 | 2023-03-16 | Danisco Us Inc. | Bioactive-containing granules |
| WO2023041694A1 (en) | 2021-09-20 | 2023-03-23 | Unilever Ip Holdings B.V. | Detergent composition |
| WO2023057367A1 (en) | 2021-10-08 | 2023-04-13 | Unilever Ip Holdings B.V. | Laundry composition |
| DE102021213462A1 (en) | 2021-11-30 | 2023-06-01 | Henkel Ag & Co. Kgaa | Method for cleaning a food processor operated by an electric motor |
| WO2023114988A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Variant maltopentaose/maltohexaose-forming alpha-amylases |
| WO2023114932A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2023114936A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2023114939A2 (en) | 2021-12-16 | 2023-06-22 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2023168234A1 (en) | 2022-03-01 | 2023-09-07 | Danisco Us Inc. | Enzymes and enzyme compositions for cleaning |
| WO2023213424A1 (en) | 2022-05-04 | 2023-11-09 | Novozymes A/S | Brewing with thermostable amg variants |
| WO2023225459A2 (en) | 2022-05-14 | 2023-11-23 | Novozymes A/S | Compositions and methods for preventing, treating, supressing and/or eliminating phytopathogenic infestations and infections |
| WO2023227335A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Liquid composition comprising linear alkyl benzene sulphonate, methyl ester ethoxylate and alkoxylated zwitterionic polyamine polymer |
| WO2023227375A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Laundry liquid composition comprising a surfactant, an aminocarboxylate, an organic acid and a fragrance |
| WO2023227331A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Composition comprising a specific methyl ester ethoxylate surfactant and a lipase |
| WO2023227356A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Composition containing enzyme |
| WO2023227421A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Laundry liquid composition comprising a surfactant, an alkoxylated zwitterionic polyamine polymer, and a fragrance |
| WO2023227332A1 (en) | 2022-05-27 | 2023-11-30 | Unilever Ip Holdings B.V. | Laundry liquid composition comprising a surfactant, an alkoxylated zwitterionic polyamine polymer and a protease |
| DE102022205594A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | PERFORMANCE-IMPROVED AND STORAGE-STABLE PROTEASE VARIANTS |
| DE102022205591A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | DETERGENT AND CLEANING AGENTS WITH IMPROVED ENZYME STABILITY |
| WO2023232193A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | Detergents and cleaning agents with an improved enzyme stability |
| WO2023232192A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | Detergent and cleaning agent with improved enzyme stability |
| DE102022205593A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | DETERGENT AND CLEANING AGENTS WITH IMPROVED ENZYME STABILITY |
| DE102022205588A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | DETERGENT AND CLEANING AGENTS WITH IMPROVED ENZYME STABILITY |
| WO2023232194A1 (en) | 2022-06-01 | 2023-12-07 | Henkel Ag & Co. Kgaa | Detergents and cleaning agents with an improved enzyme stability |
| WO2023233028A1 (en) | 2022-06-03 | 2023-12-07 | Unilever Ip Holdings B.V. | Laundry detergent product |
| WO2023250301A1 (en) | 2022-06-21 | 2023-12-28 | Danisco Us Inc. | Methods and compositions for cleaning comprising a polypeptide having thermolysin activity |
| WO2024028161A1 (en) | 2022-08-04 | 2024-02-08 | Unilever Ip Holdings B.V. | Packaged homecare product |
| WO2024028159A1 (en) | 2022-08-04 | 2024-02-08 | Unilever Ip Holdings B.V. | Packaged homecare product |
| WO2024028160A1 (en) | 2022-08-04 | 2024-02-08 | Unilever Ip Holdings B.V. | Packaged homecare product |
| EP4324900A1 (en) | 2022-08-17 | 2024-02-21 | Henkel AG & Co. KGaA | Detergent composition comprising enzymes |
| WO2024046595A1 (en) | 2022-09-01 | 2024-03-07 | Novozymes A/S | Baking with thermostable amyloglucosidase (amg) variants (ec 3.2.1.3) and low added sugar |
| WO2024050339A1 (en) | 2022-09-02 | 2024-03-07 | Danisco Us Inc. | Mannanase variants and methods of use |
| WO2024050343A1 (en) | 2022-09-02 | 2024-03-07 | Danisco Us Inc. | Subtilisin variants and methods related thereto |
| WO2024050346A1 (en) | 2022-09-02 | 2024-03-07 | Danisco Us Inc. | Detergent compositions and methods related thereto |
| WO2024056331A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| WO2024056278A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| WO2024056333A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| WO2024056334A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| WO2024056332A1 (en) | 2022-09-13 | 2024-03-21 | Unilever Ip Holdings B.V. | Washing machine and washing method |
| EP4349942A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349947A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349948A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349943A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349946A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Unit dose fabric treatment product |
| EP4349945A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| EP4349944A1 (en) | 2022-10-05 | 2024-04-10 | Unilever IP Holdings B.V. | Laundry liquid composition |
| WO2024088550A1 (en) | 2022-10-24 | 2024-05-02 | Novozymes A/S | Baking method for pulse protein fortified bread employing thermostable amyloglucosidase variante (ec 3.2.1.3) |
| WO2024088549A1 (en) | 2022-10-24 | 2024-05-02 | Novozymes A/S | Baking method with thermostable amg variant and alpha-amylase |
| WO2024088716A1 (en) | 2022-10-25 | 2024-05-02 | Unilever Ip Holdings B.V. | Composition |
| EP4361239A1 (en) | 2022-10-25 | 2024-05-01 | Unilever IP Holdings B.V. | Laundry liquid composition |
| WO2024088706A1 (en) | 2022-10-25 | 2024-05-02 | Unilever Ip Holdings B.V. | Composition |
| WO2024102698A1 (en) | 2022-11-09 | 2024-05-16 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2024115106A1 (en) | 2022-11-29 | 2024-06-06 | Unilever Ip Holdings B.V. | Composition |
| DE102022131732A1 (en) | 2022-11-30 | 2024-06-06 | Henkel Ag & Co. Kgaa | Improved washing performance through the use of a protease fused with a special adhesion promoter peptide |
| WO2024118096A1 (en) | 2022-11-30 | 2024-06-06 | Novozymes A/S | Baking at low-ph with thermostable glucoamylase variants |
| WO2024115082A1 (en) | 2022-11-30 | 2024-06-06 | Henkel Ag & Co. Kgaa | Improved washing performance through the use of a protease fused with a special adhesion promoter peptide |
| WO2024163584A1 (en) | 2023-02-01 | 2024-08-08 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2024186819A1 (en) | 2023-03-06 | 2024-09-12 | Danisco Us Inc. | Subtilisin variants and methods of use |
| WO2024191711A1 (en) | 2023-03-16 | 2024-09-19 | Nutrition & Biosciences USA 4, Inc. | Brevibacillus fermentate extracts for cleaning and malodor control and use thereof |
| WO2024194190A1 (en) | 2023-03-17 | 2024-09-26 | Unilever Ip Holdings B.V. | Composition |
| WO2024193937A1 (en) | 2023-03-17 | 2024-09-26 | Unilever Ip Holdings B.V. | Machine dishwash filter cleaner |
| WO2024194098A1 (en) | 2023-03-21 | 2024-09-26 | Unilever Ip Holdings B.V. | Detergent unit dose |
Also Published As
| Publication number | Publication date |
|---|---|
| US5824531A (en) | 1998-10-20 |
| ATE510010T1 (en) | 2011-06-15 |
| MX196038B (en) | 2000-04-14 |
| FI119693B (en) | 2009-02-13 |
| FI20080645A (en) | 2008-12-02 |
| AU2067795A (en) | 1995-10-17 |
| FI963861A0 (en) | 1996-09-27 |
| EP1637596B1 (en) | 2011-05-18 |
| MX9604313A (en) | 1997-06-28 |
| JP4057613B2 (en) | 2008-03-05 |
| JPH09510617A (en) | 1997-10-28 |
| FI120910B (en) | 2010-04-30 |
| EP0753057A1 (en) | 1997-01-15 |
| JP2006187285A (en) | 2006-07-20 |
| ATE305031T1 (en) | 2005-10-15 |
| CA2186592C (en) | 2008-02-19 |
| CN1326994C (en) | 2007-07-18 |
| US5856164A (en) | 1999-01-05 |
| ES2250969T3 (en) | 2006-04-16 |
| CN1144538A (en) | 1997-03-05 |
| DK0753057T3 (en) | 2006-01-30 |
| DE69534464T2 (en) | 2006-09-28 |
| FI963861A (en) | 1996-09-27 |
| DE69534464D1 (en) | 2006-02-02 |
| EP1637596A1 (en) | 2006-03-22 |
| CA2186592A1 (en) | 1995-10-05 |
| EP0753057B1 (en) | 2005-09-21 |
| BR9507229A (en) | 1997-09-16 |
| KR970702363A (en) | 1997-05-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5856164A (en) | Alkaline bacillus amylase | |
| EP1212409B1 (en) | Alkaline bacillus amylase | |
| MXPA96004313A (en) | Amilasa de bacillus alcal | |
| CA2821537C (en) | Amylase variants | |
| US5753460A (en) | Amylase variants | |
| US6093562A (en) | Amylase variants | |
| CA2320759A1 (en) | Alkaline bacillus amylase | |
| US5830837A (en) | Amylase variants | |
| US7078213B1 (en) | Alkaline Bacillus amylase | |
| EP2199386A1 (en) | Amylase variants |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 95192312.9 Country of ref document: CN |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 08446803 Country of ref document: US |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AM AT AU BB BG BR BY CA CH CN CZ DE DK EE ES FI GB GE HU IS JP KE KG KP KR KZ LK LR LT LU LV MD MG MN MW MX NL NO NZ PL PT RO RU SD SE SG SI SK TJ TM TT UA UG US UZ VN |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): KE MW SD SZ UG AT BE CH DE DK ES FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 1995913062 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/1996/004313 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2186592 Country of ref document: CA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 963861 Country of ref document: FI |
|
| WWP | Wipo information: published in national office |
Ref document number: 1995913062 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWG | Wipo information: grant in national office |
Ref document number: 1995913062 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 20080645 Country of ref document: FI |