US6979414B2 - Organic electroluminescence element - Google Patents
Organic electroluminescence element Download PDFInfo
- Publication number
- US6979414B2 US6979414B2 US10/683,435 US68343503A US6979414B2 US 6979414 B2 US6979414 B2 US 6979414B2 US 68343503 A US68343503 A US 68343503A US 6979414 B2 US6979414 B2 US 6979414B2
- Authority
- US
- United States
- Prior art keywords
- organic
- group
- luminescence
- carbazole derivative
- carbazole
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 0 CN(C)[Y]N1C2=CC=CC=C2C2=C1C=CC=C2.[1*]C.[2*]C.[3*]C.[4*]C.[5*]C.[6*]C.[7*]C.[8*]C Chemical compound CN(C)[Y]N1C2=CC=CC=C2C2=C1C=CC=C2.[1*]C.[2*]C.[3*]C.[4*]C.[5*]C.[6*]C.[7*]C.[8*]C 0.000 description 6
- JCDVQTOTSKZXBN-UHFFFAOYSA-N C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1/C=C\C=C/3)=CC=C2C1=C2C=CC=CC2=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(C2=C(C3=CC=CC=C3)C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C(C3=CC=CC=C3)C(C3=CC=CC=C3)=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC(C3=CC4=C(C=C3)C3=C(C=CC=C3)C4C3=CC=CC=C3)=C(C3=CC=CC=C3)C=C2C2=CC=C(C3=CC=C4C(=C3)N(C3=CC=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=CC=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C2=C1/C=C(N(C1=CC=CC=C1)C1=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C1)\C=C/2 Chemical compound C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1/C=C\C=C/3)=CC=C2C1=C2C=CC=CC2=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC=C(C2=C(C3=CC=CC=C3)C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C(C3=CC=CC=C3)C(C3=CC=CC=C3)=C2C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC=C(C2=CC(C3=CC4=C(C=C3)C3=C(C=CC=C3)C4C3=CC=CC=C3)=C(C3=CC=CC=C3)C=C2C2=CC=C(C3=CC=C4C(=C3)N(C3=CC=CC=C3)C3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=C(C3=CC=C(N(C4=CC=CC=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.CC1(C)C2=C(C=CC(N(C3=CC=CC=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C2=C1/C=C(N(C1=CC=CC=C1)C1=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C1)\C=C/2 JCDVQTOTSKZXBN-UHFFFAOYSA-N 0.000 description 2
- VFUDMQLBKNMONU-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1 VFUDMQLBKNMONU-UHFFFAOYSA-N 0.000 description 2
- NNPPMTNAJDCUHE-UHFFFAOYSA-N CC(C)C Chemical compound CC(C)C NNPPMTNAJDCUHE-UHFFFAOYSA-N 0.000 description 2
- KGVBVOYQIKFIGA-UHFFFAOYSA-N CN(C)C.CN(C)C Chemical compound CN(C)C.CN(C)C KGVBVOYQIKFIGA-UHFFFAOYSA-N 0.000 description 2
- KCKGWAKCZSRFJI-UHFFFAOYSA-N CN(C)C.CN(C)C.CN1c2ccccc2CCc2ccccc21 Chemical compound CN(C)C.CN(C)C.CN1c2ccccc2CCc2ccccc21 KCKGWAKCZSRFJI-UHFFFAOYSA-N 0.000 description 2
- OUQQOMUDRBBMTM-UHFFFAOYSA-N CN(CC1=CC=C(N(C)C)C=C1)CC1=CC=C(N(C)C)C=C1 Chemical compound CN(CC1=CC=C(N(C)C)C=C1)CC1=CC=C(N(C)C)C=C1 OUQQOMUDRBBMTM-UHFFFAOYSA-N 0.000 description 2
- IQAVHPRAHFHCOQ-UHFFFAOYSA-N C.C.C.CN(c1ccccc1)c1cccc(-c2ccccc2)c1.CN(c1ccccc1)c1ccccc1.CN1c2ccccc2-c2cc3ccccc3cc21.CN1c2ccccc2CCc2ccccc21.CN1c2ccccc2Oc2ccccc21 Chemical compound C.C.C.CN(c1ccccc1)c1cccc(-c2ccccc2)c1.CN(c1ccccc1)c1ccccc1.CN1c2ccccc2-c2cc3ccccc3cc21.CN1c2ccccc2CCc2ccccc21.CN1c2ccccc2Oc2ccccc21 IQAVHPRAHFHCOQ-UHFFFAOYSA-N 0.000 description 1
- WCBJQBXODRIAKV-UHFFFAOYSA-N C.C.C1=CC2=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C2C=C1.C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1/C=C\C=C/3)=CC=C2C1=C2C=CC=CC2=C(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)C=C5)C=C4)C=C3)C=C1)C1=C2C=CC=C1.CC1(C)C2=C(C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C2=C1/C=C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)\C=C/2.CCC(C)N1C2=CC=CC=C2C2=C1C=CC=C2 Chemical compound C.C.C1=CC2=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C2C=C1.C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1/C=C\C=C/3)=CC=C2C1=C2C=CC=CC2=C(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)C=C5)C=C4)C=C3)C=C1)C1=C2C=CC=C1.CC1(C)C2=C(C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C2=C1/C=C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)\C=C/2.CCC(C)N1C2=CC=CC=C2C2=C1C=CC=C2 WCBJQBXODRIAKV-UHFFFAOYSA-N 0.000 description 1
- YHNZIVLINLUXKP-UHFFFAOYSA-N C.Cc1ccc(N2c3ccccc3CCc3ccccc32)cc1 Chemical compound C.Cc1ccc(N2c3ccccc3CCc3ccccc32)cc1 YHNZIVLINLUXKP-UHFFFAOYSA-N 0.000 description 1
- SBNKHMAYAYHLKO-UHFFFAOYSA-N C1=CC2=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C2C=C1.C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1/C=C\C=C/3)=CC=C2C1=C2C=CC=CC2=C(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)C=C5)C=C4)C=C3)C=C1)C1=C2C=CC=C1.CC1(C)C2=C(C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C2=C1/C=C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)\C=C/2 Chemical compound C1=CC2=C(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)C=C3)=C2C=C1.C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1/C=C\C=C/3)=CC=C2C1=C2C=CC=CC2=C(C2=C3C=CC=CC3=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6/C=C\C=C/7)C=C5)C=C4)C=C3)C=C1)C1=C2C=CC=C1.CC1(C)C2=C(C=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C2=C1/C=C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)\C=C/2 SBNKHMAYAYHLKO-UHFFFAOYSA-N 0.000 description 1
- OXTBPPVPEFVMRP-UHFFFAOYSA-N C1=CC2=C(C=C1)C(N(C1=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C1)C1=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C1)=CC=C2.C1=CC=C(C2=CC=C(N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC3=C4C=CC=C3)C=C2)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N2C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3OC3=C2C=CC(N2C4=C(C=CC=C4)C4=C2C=CC=C4)=C3)C=C1 Chemical compound C1=CC2=C(C=C1)C(N(C1=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C1)C1=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C1)=CC=C2.C1=CC=C(C2=CC=C(N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C2)C=C1.C1=CC=C(N(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC3=C4C=CC=C3)C=C2)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC3=C4C=CC=C3)C=C2)C=C1.C1=CC=C(N2C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3OC3=C2C=CC(N2C4=C(C=CC=C4)C4=C2C=CC=C4)=C3)C=C1 OXTBPPVPEFVMRP-UHFFFAOYSA-N 0.000 description 1
- JLZCJVULNRWNFW-UHFFFAOYSA-N C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)=CC=C2N(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=C2C=CC=CC2=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N(C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=CC3=C(C=C2C=C1)N(C1=CC=C(N(C2=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C2=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C=C1)C1=C3C=CC=C1 Chemical compound C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)=CC=C2N(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=C2C=CC=CC2=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N(C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=CC3=C(C=C2C=C1)N(C1=CC=C(N(C2=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C2=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C2)C=C1)C1=C3C=CC=C1 JLZCJVULNRWNFW-UHFFFAOYSA-N 0.000 description 1
- BHTLZFIRHVWFQE-UHFFFAOYSA-N C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)=CC=C2N(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)=C3)C=C1)C1=C2C=CC=C1 Chemical compound C1=CC2=C(C=C1)C(N1C3=C(C=CC=C3)C3=C1C=CC=C3)=CC=C2N(C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1)C1=CC=C(N2C3=C(C=CC=C3)C3=C2C=CC=C3)C=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=CC(N4C5=C(C=CC=C5)C5=C4/C=C\C=C/5)=C3)C=C1)C1=C2C=CC=C1 BHTLZFIRHVWFQE-UHFFFAOYSA-N 0.000 description 1
- CHSXMVLWFCPLFR-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C(N(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C1)C1=C2C=CC=C1.C1=CC2=C(C=C1)N(C1=CC=C(N(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1 CHSXMVLWFCPLFR-UHFFFAOYSA-N 0.000 description 1
- OGCMDYFCBYBSCF-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)=C2)C=C1.CC1=CC(C)=CC(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(N4C5=C(C=CC=C5)C5=C4C=CC=C5)=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)=C2)C=C1.CC1=CC(C)=CC(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=C2)=C1 OGCMDYFCBYBSCF-UHFFFAOYSA-N 0.000 description 1
- RIUFXGJAIMYVRZ-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C(C=CC=C1)CC2.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)OC6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C(C=CC=C1)O2.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=C4C=CC=CC4=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=C4C=CC=CC4=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC(C5=CC=CC=C5)=C4)C=C3)=C2)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C(C=CC=C1)CC2.C1=CC2=C(C=C1)N(C1=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)OC6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=C3)C=C1)C1=C(C=CC=C1)O2.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=C4C=CC=CC4=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=C4C=CC=CC4=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC(C5=CC=CC=C5)=C4)C=C3)=C2)C=C1 RIUFXGJAIMYVRZ-UHFFFAOYSA-N 0.000 description 1
- BJQYSHXSUSHSFT-UHFFFAOYSA-N C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C4C(=C3)C3=CC(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=CC=C3N4C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC=C(N4C5=CC=C(C6=CC=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)C=C5C5=CC(C6=CC=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)=CC=C54)C=C3)=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=C(C=CC(N(C4=CC=CC=C4)C4=CC=CC=C4)=C2)N3C2=CC=CC=C2)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)OC6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)OC6=C5C=CC=C6)C=C4)=CC=C32)C=C1 Chemical compound C1=CC2=C(C=C1)N(C1=CC=C(C3=CC=C4C(=C3)C3=CC(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=CC=C3N4C3=CC=C(N4C5=C(C=CC=C5)C5=C4C=CC=C5)C=C3)C=C1)C1=C2C=CC=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(C3=CC=C(N4C5=CC=C(C6=CC=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)C=C5C5=CC(C6=CC=C(N7C8=C(C=CC=C8)C8=C7C=CC=C8)C=C6)=CC=C54)C=C3)=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C3C(=C2)C2=C(C=CC(N(C4=CC=CC=C4)C4=CC=CC=C4)=C2)N3C2=CC=CC=C2)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)OC6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)OC6=C5C=CC=C6)C=C4)=CC=C32)C=C1 BJQYSHXSUSHSFT-UHFFFAOYSA-N 0.000 description 1
- QKBWDYLFYVXTGE-UHFFFAOYSA-N C1=CC2=C(C=C1)[Ir-3]13(C4=C(C=CC=C4)C4=[N+]1C=CC=C4)(C1=C(C=CC=C1)C1=[N+]3C=CC=C1)[N+]1=C2C=CC=C1 Chemical compound C1=CC2=C(C=C1)[Ir-3]13(C4=C(C=CC=C4)C4=[N+]1C=CC=C4)(C1=C(C=CC=C1)C1=[N+]3C=CC=C1)[N+]1=C2C=CC=C1 QKBWDYLFYVXTGE-UHFFFAOYSA-N 0.000 description 1
- HDPLZALCHILFHR-UHFFFAOYSA-N C1=CC2=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=C3)C=C2C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)CCC5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)CCC5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)CCC5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)OC5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)OC5=C4C=CC=C5)C=C3)=C2)C=C1 Chemical compound C1=CC2=CC=C(C3=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=C3)C=C2C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)CCC5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N(C4=CC=CC=C4)C4=CC=CC=C4)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)CCC5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)CCC5=C4C=CC=C5)C=C3)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=C(N4C5=C(C=CC=C5)OC5=C4C=CC=C5)C=C3)=CC(C3=CC=C(N4C5=C(C=CC=C5)OC5=C4C=CC=C5)C=C3)=C2)C=C1 HDPLZALCHILFHR-UHFFFAOYSA-N 0.000 description 1
- DRKNQHXCHBPUHY-UHFFFAOYSA-N C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(N3C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4C4=C3C=CC(N3C5=C(C=CC=C5)CCC5=C3C=CC=C5)=C4)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(N3C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC=C6)C=C4C4=C3C=CC(N3C5=CC=CC=C5C5=C3C=CC=C5)=C4)=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4C4=C3C=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(N3C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4C4=C3C=CC(N3C5=C(C=CC=C5)CCC5=C3C=CC=C5)=C4)=C2)C=C1.C1=CC=C(C2=CC(C3=CC=CC=C3)=CC(N3C4=CC=C(N5C6=CC=CC=C6C6=C5C=CC=C6)C=C4C4=C3C=CC(N3C5=CC=CC=C5C5=C3C=CC=C5)=C4)=C2)C=C1.C1=CC=C(C2=CC=C(N3C4=CC=C(N(C5=CC=CC=C5)C5=CC=CC=C5)C=C4C4=C3C=CC(N(C3=CC=CC=C3)C3=CC=CC=C3)=C4)C=C2)C=C1.C1=CC=C(N(C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C2=CC=C(N3C4=C(C=CC=C4)C4=C3C=CC=C4)C=C2)C=C1 DRKNQHXCHBPUHY-UHFFFAOYSA-N 0.000 description 1
- UBGIWNIVSWYYTN-UHFFFAOYSA-N C1=CC=C(C2=CC(N3C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4C4=CC(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=CC=C43)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=CC(C5=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)=CC=C3N4C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC=C32)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC=C32)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=CC=C32)C=C1 Chemical compound C1=CC=C(C2=CC(N3C4=CC=C(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)C=C4C4=CC(C5=CC=C(N6C7=C(C=CC=C7)C7=C6C=CC=C7)C=C5)=CC=C43)=CC=C2)C=C1.C1=CC=C(N(C2=CC=CC=C2)C2=CC=C(C3=CC=C4C(=C3)C3=CC(C5=CC=C(N(C6=CC=CC=C6)C6=CC=CC=C6)C=C5)=CC=C3N4C3=CC=CC=C3)C=C2)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC=C32)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)=CC=C32)C=C1.C1=CC=C(N2C3=CC=C(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)C=C3C3=CC(C4=CC=C(N5C6=C(C=CC=C6)CCC6=C5C=CC=C6)C=C4)=CC=C32)C=C1 UBGIWNIVSWYYTN-UHFFFAOYSA-N 0.000 description 1
- QAXFOPVQSZQKMY-UHFFFAOYSA-N C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1 Chemical compound C1=CC=C(C2=CC=C(C3=CC=C(N(C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C4=CC=C(N5C6=C(C=CC=C6)C6=C5C=CC=C6)C=C4)C=C3)C=C2)C=C1 QAXFOPVQSZQKMY-UHFFFAOYSA-N 0.000 description 1
- DJJLMNYWBHRZCR-UHFFFAOYSA-N C1=CC=C(C2=CC=CC(N(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(N(C8=CC=CC(C9=CC=CC=C9)=C8)C8=CC(C9=CC=CC=C9)=CC=C8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)C3=CC=CC(C4=CC=CC=C4)=C3)=C2)C=C1.CC(C)C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=CC=C(N(C)C)C=C7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1.CN(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(N(C)C)C=C4)C4=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(N(C)C)C=C6)C6=CC=CC=C67)C=C45)C=C3C3=C2C=CC=C3)C=C1.CN(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(N(C)C)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1 Chemical compound C1=CC=C(C2=CC=CC(N(C3=CC=C(N4C5=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(N(C8=CC=CC(C9=CC=CC=C9)=C8)C8=CC(C9=CC=CC=C9)=CC=C8)C=C6)C6=CC=CC=C67)C=C5C5=C4C=CC=C5)C=C3)C3=CC=CC(C4=CC=CC=C4)=C3)=C2)C=C1.CC(C)C1=CC=C(C2=CC=C(N3C4=CC=C(C5=CC6=C(C=C5)N(C5=CC=C(C7=CC=C(N(C)C)C=C7)C=C5)C5=CC=CC=C56)C=C4C4=C3C=CC=C4)C=C2)C=C1.CN(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(N(C)C)C=C4)C4=CC=C(C6=CC7=C(C=C6)N(C6=CC=C(N(C)C)C=C6)C6=CC=CC=C67)C=C45)C=C3C3=C2C=CC=C3)C=C1.CN(C)C1=CC=C(N2C3=CC=C(C4=CC5=C(C=C4)N(C4=CC=C(N(C)C)C=C4)C4=CC=CC=C45)C=C3C3=C2C=CC=C3)C=C1 DJJLMNYWBHRZCR-UHFFFAOYSA-N 0.000 description 1
- QUEYUECGCVJUQJ-UHFFFAOYSA-N CC1=CC=CC(N(C2=CC=CC=C2)C2=CC=C(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C(N(C5=CC=CC=C5)C5=CC=C(N(C6=CC=CC=C6)C6=CC=CC(C)=C6)C=C5)C=C3)=C4)C=C2)=C1 Chemical compound CC1=CC=CC(N(C2=CC=CC=C2)C2=CC=C(N3C4=CC=CC=C4C4=C3C=CC(C3=CC=C(N(C5=CC=CC=C5)C5=CC=C(N(C6=CC=CC=C6)C6=CC=CC(C)=C6)C=C5)C=C3)=C4)C=C2)=C1 QUEYUECGCVJUQJ-UHFFFAOYSA-N 0.000 description 1
- FROCWBDLKUTNPG-UHFFFAOYSA-N CCN(C)CC.Cc1ccc2c(c1)-c1cc(C)ccc1N2C.Cc1ccc2c(c1)Oc1cc(C)ccc1N2C Chemical compound CCN(C)CC.Cc1ccc2c(c1)-c1cc(C)ccc1N2C.Cc1ccc2c(c1)Oc1cc(C)ccc1N2C FROCWBDLKUTNPG-UHFFFAOYSA-N 0.000 description 1
- UASUWESVLPQLMM-UHFFFAOYSA-N Cc1ccc(N2c3ccccc3-c3cc4ccccc4cc32)cc1 Chemical compound Cc1ccc(N2c3ccccc3-c3cc4ccccc4cc32)cc1 UASUWESVLPQLMM-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/02—Use of particular materials as binders, particle coatings or suspension media therefor
- C09K11/025—Use of particular materials as binders, particle coatings or suspension media therefor non-luminescent particle coatings or suspension media
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
- H10K50/125—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers specially adapted for multicolour light emission, e.g. for emitting white light
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/10—Organic polymers or oligomers
- H10K85/141—Organic polymers or oligomers comprising aliphatic or olefinic chains, e.g. poly N-vinylcarbazol, PVC or PTFE
- H10K85/146—Organic polymers or oligomers comprising aliphatic or olefinic chains, e.g. poly N-vinylcarbazol, PVC or PTFE poly N-vinylcarbazol; Derivatives thereof
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/341—Transition metal complexes, e.g. Ru(II)polypyridine complexes
- H10K85/342—Transition metal complexes, e.g. Ru(II)polypyridine complexes comprising iridium
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/626—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing more than one polycyclic condensed aromatic rings, e.g. bis-anthracene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/636—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising heteroaromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1022—Heterocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
- C09K2211/1033—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom with oxygen
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/18—Metal complexes
- C09K2211/185—Metal complexes of the platinum group, i.e. Os, Ir, Pt, Ru, Rh or Pd
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2101/00—Properties of the organic materials covered by group H10K85/00
- H10K2101/10—Triplet emission
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2102/00—Constructional details relating to the organic devices covered by this subclass
- H10K2102/10—Transparent electrodes, e.g. using graphene
- H10K2102/101—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO]
- H10K2102/102—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO] comprising tin oxides, e.g. fluorine-doped SnO2
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K2102/00—Constructional details relating to the organic devices covered by this subclass
- H10K2102/10—Transparent electrodes, e.g. using graphene
- H10K2102/101—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO]
- H10K2102/103—Transparent electrodes, e.g. using graphene comprising transparent conductive oxides [TCO] comprising indium oxides, e.g. ITO
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/17—Carrier injection layers
- H10K50/171—Electron injection layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/80—Constructional details
- H10K50/805—Electrodes
- H10K50/82—Cathodes
- H10K50/828—Transparent cathodes, e.g. comprising thin metal layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K71/00—Manufacture or treatment specially adapted for the organic devices covered by this subclass
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K71/00—Manufacture or treatment specially adapted for the organic devices covered by this subclass
- H10K71/30—Doping active layers, e.g. electron transporting layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K71/00—Manufacture or treatment specially adapted for the organic devices covered by this subclass
- H10K71/60—Forming conductive regions or layers, e.g. electrodes
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/30—Coordination compounds
- H10K85/321—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3]
- H10K85/324—Metal complexes comprising a group IIIA element, e.g. Tris (8-hydroxyquinoline) gallium [Gaq3] comprising aluminium, e.g. Alq3
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24942—Structurally defined web or sheet [e.g., overall dimension, etc.] including components having same physical characteristic in differing degree
Definitions
- the present invention relates to an organic electroluminescence element (which may be referred to as an organic EL element hereinafter). More specifically, the present invention relates to an organic EL element using a triplet exciton of an organic luminescence material (host material).
- organic EL elements wherein an organic luminescence layer is arranged between electrodes have been eagerly researched and developed for the following reasons and the like.
- the luminescence mechanism of such organic EL elements generally makes use of a luminescence phenomenon, which is energy conversion phenomenon caused when a fluorescent molecule in a singlet excited state (which may be referred to a S 1 state) in an organic luminescence medium is transited to a ground state radially.
- a luminescence phenomenon which is energy conversion phenomenon caused when a fluorescent molecule in a singlet excited state (which may be referred to a S 1 state) in an organic luminescence medium is transited to a ground state radially.
- a fluorescent molecule in a triplet excited state (which may be referred to a T 1 state) in an organic luminescence medium can be supposed.
- radiative transition to a ground state is forbidden; therefore, such a molecule is gradually transited from the triplet excited state to some other state by non-radiative transition. As a result, no fluorescence is emitted but thermal energy is radiated.
- singlet and triplet mean multiplicity of energy decided by combination of total spin angular momentum and total orbital angular momentum of a fluorescent molecule.
- a singlet excited state is defined as an energy state in the case that a single electron is transited from a ground state, where no unpaired electrons are present, to a higher energy level without changing the spin state of the electron.
- a triplet excited state is defined as an energy state in the case that a single electron is transited to a higher energy level while the spin state of the electron is made reverse.
- luminescence in a triplet excited state defined as above can be observed if the luminescence is caused at a very low temperature, for example, at a liquefaction temperature of liquid nitrogen ( ⁇ 196° C.). However, this temperature is not a practical temperature, and the amount of the luminescence is only a little.
- the coefficient (0.25) of ⁇ rad in the equation is decided from the matter that the probability that singlet excitons are generated is regarded as 1 ⁇ 4. Therefore, even if recombination and radiative attenuation of excitons are caused with a probability coefficient of 1, the theoretical upper limit of luminescence efficiency of the organic EL element is 25%.
- literature 1 Jpn. J. Appl. Phys., 38 (1999) L1502 discloses that even at room temperature, triplet excitons (triplet excited state) of an organic luminescence material (host material) are used to transfer energy from the triplet excitons to a phosphorescent dopant, so as to generate a fluorescent luminescence phenomenon. More specifically, the literature 1 reports that a fluorescent luminescence phenomenon is caused in an organic EL element comprising an organic luminescence layer composed of 4,4-N,N-dicarbazolylbiphenyl represented by the following formula (6) and an Ir complex, which is a phosphorescent dopant.
- the half-life of the organic EL element described in the literature 1 is below 150 hours, and the usefulness of the organic EL element is insufficient.
- the inventor made eager investigations. As a result, the following has been found: the glass-transition temperature of 4,4-N,N-dicarbazolylbiphenyl is as low as less than 110° C.; therefore, if the biphenyl is combined with an Ir complex, crystallization is easily caused in the organic luminescence layer comprising the combination to make the life of an organic EL element short.
- an object of the present invention is to provide an organic EL element which makes it possible to use triplet excitons of an organic luminescence material (host material) even at room temperature to emit fluorescence (including phosphorescence); has a practical life span; and has a superior heat-resistance.
- an organic EL element comprising:
- This organic EL element makes it possible to use the triplet exciton state of the organic luminescence material even at room temperature. Moreover, this element has a practical life, for example, a half-time of 300 hours or more, and has superior heat-resistance. Thus, this element can be sufficiently used as an organic EL element for car.
- the carbazole derivative is at least one of compounds represented by the following general formulae (1) to (4):
- the organic EL element wherein this carbazole derivative is used as a host material in the organic luminescence layer makes it possible to use the triplet exciton state more effectively, and has a practical life span.
- the carbazole derivative has at least two carbazole skeletons.
- This carbazole derivative has a large triplet energy to make it possible to use the triplet exciton state more effectively even at room temperature (20° C.), and has a practical life span.
- This structure makes it possible to transfer the triplet energy of the carbazole derivative surely to the phosphorescent dopant, and to emit fluorescence using the triplet energy even at room temperature (20° C.).
- the triplet energy (E1) of the carbazole derivative is a value of 21,000 cm ⁇ 1 or more.
- a triplet energy of 21,000 cm ⁇ 1 corresponds to a light wavelength of 488 nm.
- various phosphorescent dopants generally have a triplet energy which is equal to or less than the energy which 488 nm light has. Therefore, by using the carbazole derivative having such a large triplet energy as above, various phosphorescent dopants can be used.
- the carbazole derivative has a cyclic structure whose triplet energy is a value of 21,000 cm ⁇ 1 or more, and the cyclic structure contains an aromatic ring, a hetero ring, or combination thereof.
- This carbazole derivative makes it possible to transfer the triplet energy of the carbazole derivative more effectively to the phosphorescent dopant. Specifically, if the carbazole derivative has a cyclic structure having a triplet energy of less than 21,000 cm ⁇ 1 , the triplet energy is transferred to this cyclic structure so that the triplet energy transferred to the phosphorescent dopant may be reduced.
- the phosphorescent dopant is a metal complex comprising at least one metal selected from the group consisting of Ir (iridium), Ru (ruthenium), Pd (palladium), Pt (platinum), Os (osmium) and Re (rhenium).
- This structure makes it possible to transfer energy effectively from the triplet exciton of the carbazole derivative as a host material to the metal complex as the phosphorescent dopant.
- At least one ligand of the metal complex has at least one skeleton selected from the group consisting of phenylpyridine, bipyridyl and phenanthroline skeletons.
- the bulky and electron-withdrawing skeleton(s) contained in the molecule makes it possible to transfer energy effectively from the triplet exciton of the carbazole derivative to the metal complex.
- a blend amount of the phosphorescent dopant is 0.1 to 30 parts by weight per 100 parts of the carbazole derivative.
- This structure makes it possible to mix the phosphorescent dopant with the carbazole derivative uniformly, and transfer energy effectively from the triplet exciton of the carbazole derivative to the phosphorescent dopant.
- a hole barrier layer, an electron injection layer, or combination thereof is arranged between the anode layer and the cathode layer, and the hole barrier layer and the electron injection layer comprise an alkali metal.
- This structure makes it possible to drive the organic EL element at a lower voltage, and make the life span of the element longer.
- FIG. 1 is a view illustrating a basic structure of an organic EL element.
- FIG. 1 is a sectional view of an organic EL element 102 , and illustrates a structure wherein an anode layer 10 , an organic luminescence layer 14 and a cathode layer 16 are successively deposited on a substrate 18 .
- An embodiment of the present invention is characterized in that in the organic luminescence layer, a carbazole derivative with a glass-transition temperature of 110° C. or higher is used as a host material.
- the host material is a carbazole derivative
- the triplet exciton state of the carbazole derivative can be effectively used even at room temperature (20° C.) by combining the derivative with a phosphorescent dopant that will be described later.
- a luminescence phenomenon can be caused by transferring energy effectively from the triplet state in the carbazole derivative to the phosphorescent dopant.
- Such a carbazole derivative is preferably a carbazole derivative having at least two carbazole skeletons. This is because the glass-transition temperature and triplet energy that will be described later can easily be adjusted and the derivative can easily be mixed with the phosphorescent dopant.
- the carbazole derivative having a glass-transition temperature of 110° C. or higher is used is as follows. If the glass-transition temperature is below 110° C., any combination thereof with the phosphorescent dopant is very easily crystallized so that the life becomes short. If the glass-transition temperature is below 110° C., a short circuit is easily caused in a short time when an electric current is passed through the derivative at a high temperature. Thus, the environment where the organic element EL is used is excessively restricted.
- the glass-transition temperature of the carbazole derivative is more preferably from 115 to 170° C., and still more preferably from 120 to 150° C.
- the reason why the glass-transition temperature of the carbazole derivative is more preferably 170° C. or lower is that the kinds of carbazole derivatives having a glass-transition over 170° C. are excessively restricted and the handling of the derivatives becomes difficult because of a drop in their deposition ability.
- a Differential Scanning Calorimeter is used to make it possible to obtain the glass-transition temperature (Tg) of the carbazole derivative as a temperature of a change in the specific heat obtained when the derivative is heated at a temperature-rising rate of, for example, 10° C./minute in a nitrogen-circulating state.
- E1>E2 is satisfied in which E1 represents the triplet energy of the carbazole derivative in the organic luminescence layer and E2 represents the triplet energy of the phosphorescent dopant therein.
- the triplet exciton state of the carbazole derivative can surely be used even at room temperature. Specifically other words, a luminescence phenomenon can be caused by transferring energy certainly from the triplet state in the carbazole derivative to the phosphorescent dopant.
- the triplet energy (E1) of the carbazole derivative is set to a value of 21,000 cm ⁇ 1 or more.
- the triplet energy, 21,000 cm ⁇ 1 corresponds to a light wavelength of 488 nm.
- various phosphorescent dopants have a triplet energy which is equal to or less than the energy which 488 nm light has. Therefore, one or more selected from various phosphorescent dopants can be combined with the carbazole derivative.
- the carbazole derivative has a cyclic structure whose triplet energy is a value of 21,000 cm ⁇ 1 or more and the cyclic structure contains an aromatic ring, a hetero ring, or combination one thereof.
- the triplet exciton state of the carbazole derivative can be effectively used even at room temperature by combining the carbazole derivative with the phosphorescent dopant. That is, by causing the carbazole derivative to have, for example, a cyclic structure wherein 9-arylcarbazole is connected to a bivalent or trivalent group consisting an aromatic ring, the triplet energy can be set to 22,500 cm ⁇ 1 or less. Therefore, if the carbazole derivative has such a cyclic structure, the frequency that the triplet energy of 21,000 cm, originating from the carbazole group, is transferred in the molecule becomes small. Thus, the triplet energy which is transferred to the phosphorescent dopant becomes relatively large.
- carbazole derivatives represented by the general formulae (1) to (4) alone or in combination of two or more.
- examples of preferred aryl groups having 5 to 50 nucleus atoms include phenyl, naphthyl, anthranyl, phenanthryl, pyrenyl, coronyl, biphenyl, terphenyl, pyrrolyl, furanyl, thiophenyl, benzothiophenyl, oxadiazolyl, diphenylanthranyl, indolyl, carbazolyl, pyridyl, and benzoquinolyl, and the like.
- Examples of preferred arylene groups having 5 to 50 nucleus atoms include phenylene, naphthylene, anthranylene, phenanthrylene, pyrenylene, coronylene, biphenylene, terphenylene, pyrrolylene, furanylene, thiophenylene, benzothiophenylene, oxadiazolylene, diphenylanthranylene, indolylene, carbazolylene, pyridylene, and benzoquinolylene, and the like.
- the aromatic group having 6 to 50 carbon atoms may be substituted with one or more substituents.
- substituents include alkyl groups having 1 to 6 carbon atoms (such as methyl, ethyl, i-propyl, n-propyl, s-butyl, t-butyl, pentyl, hexyl, cyclopentyl and cyclohexyl groups); alkoxy groups having 1–6 carbon atoms (such as methoxy, ethoxy, i-propoxy, n-propoxy, s-butoxy, t-butoxy, pentoxy, hexyloxy, cyclopentoxy and cyclohexyloxy groups); aryl groups having 5 to 50 nucleus atoms; amino groups substituted with an aryl group having 5 to 50 nucleus atoms; ester groups having an aryl group having 5 to 50 nucleus atoms; ester groups having an alkyl group having 1 to 6 carbon
- the carbazole in the present invention is interpreted as a moiety formed by connecting at least two aryl groups, each of which is connected to a nitrogen atom, to each other through a single bond or a connecting groups.
- preferred examples of the connecting groups include O, S, and substituted or non-substituted alkylene and silylene, and the like.
- carbazole derivative represented by the formula (1) include a group of compounds illustrated as the following chemical formulae (7) to (24).
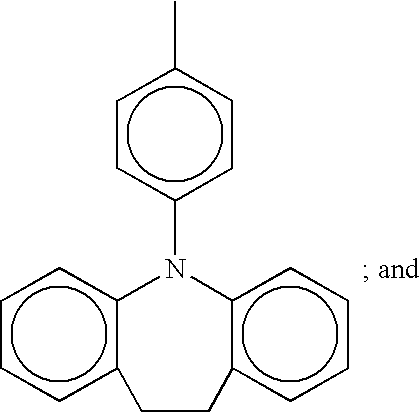
- Preferred specific examples of the carbazole derivative represented by the formula (2) include a group of compounds illustrated as the following chemical formulae (25) to (29). In the formula, a methyl group may be abbreviated to Me.
- Preferred specific examples of the carbazole derivative represented by the formula (3) include a group of compounds illustrated as the following chemical formulae (30) to (41).
- Preferred specific examples of the carbazole derivative represented by the formula (4) include a group of compounds illustrated as the following chemical formulae (42) to (49).
- carbazole derivative having a structure other than the structures represented by the general formulae (1) to (4) include a group of compounds illustrated as the following chemical formulae (50) to (59).
- the phosphorescent dopant is preferably a metal complex comprising at least one metal selected from the group consisting of Ir, Ru, Pd, Pt, Os and Re.
- the phosphorescent dopant are metal complexes such as tris(2-phenylpyridine) iridium, tris(2-phenylpyridine) ruthenium, tris(2-phenylpyridine) palladium, bis(2-phenylpyridine) platinum, tris(2-phenylpyridine) osmium, tris(2-phenylpyridine) rhenium, octaethyl platinum porphyrin, octaphenyl platinum porphyrin, octaethyl palladium porphyrin, and octaphenyl palladium porphyrin.
- metal complexes such as tris(2-phenylpyridine) iridium, tris(2-phenylpyridine) ruthenium, tris(2-phenylpyridine) palladium, bis(2-phenylpyridine) platinum, tris(2-phenylpyridine) osmium, tris(2-phenylpyridine)
- metal complexes comprising Ir, for example, tris(2-phenylpyridine) iridium represented by the following formula (60): ⁇ circle around (2) ⁇ Ligand of the Metal Complex
- At least one ligand of the metal complex preferably has at least one skeleton selected from the group consisting of phenylpyridine, bipyridyl and phenanthroline skeletons.
- the ligand in the phosphorescent dopant, preferably has a phenylpyridine skeleton among these skeletons, such as tris(2-phenylpyridine) iridium.
- a blend amount of the phosphorescent dopant is preferably 0.1 to 30 parts by weight per 100 parts by weight of the blended carbazole derivative (host material).
- the reasons for this are as follows. If the blend amount of the phosphorescent dopant is below 0.1 part by weight, the effect based on the blend is not exhibited so that energy may not be effectively transferred from triplet excitons of the carbazole derivative. On the other hand, if the blend amount of the phosphorescent dopant is over 30 parts by weight, the phosphorescent dopant is not easily blended with the carbazole derivative uniformly so that luminescence brightness may be scattered.
- the blend amount of the phosphorescent dopant is more preferably 0.5 to 20 parts by weight, and is still more preferably 1 to 15 parts by weight.
- a hole injection layer having a thickness of 5 nm to 5 ⁇ m.
- the deposition of such a hole injection layer makes it possible to inject holes satisfactorily into the organic luminescence layer, give a high luminescence brightness, and attain driving at a low voltage.
- a compound having a hole mobility of 1 ⁇ 10 ⁇ 6 cm 2 /V ⁇ second or more and an ionization energy of 5.5 eV or less is also preferred.
- the hole mobility is measured when a voltage of 1 ⁇ 10 4 to 1 ⁇ 10 6 V/cm is applied to the hole injection layer.
- constituent material of the hole injection layer are organic compounds such as porphyrin compounds, aromatic tertiary amine compounds, styrylamine compounds, aromatic dimethylidyne compounds, and condensed aromatic ring compounds, for example, 4,4′-bis[N-(1-naphthyl)-N-phenylamino]biphenyl (abbreviated to NPD) and 4,4′,4′′-tris[N-(3-methylphenyl)-N-phenylamino]triphenylamine (abbreviated to MTDATA).
- NPD 4,4′,4′′-tris[N-(3-methylphenyl)-N-phenylamino]triphenylamine
- an inorganic compound such as p-type Si or p-type SiC is preferably used.
- an organic semiconductor layer having an electric conductivity of 1 ⁇ 10 ⁇ 10 S/cm or more between the hole injection layer and the anode layer, or between the hole injection layer and the organic luminescence layer.
- the arrangement of the organic semiconductor layer makes the injection of holes into the organic luminescence layer more satisfactory.
- an electron injection layer having a thickness of 5 nm to 5 ⁇ m.
- the deposition of such an electron injection layer makes it possible to inject electrons satisfactorily into the organic luminescence layer, give a high luminescence brightness, and attain driving at a low voltage.
- the electron mobility is measured when a voltage of 1 ⁇ 10 4 to 1 ⁇ 10 6 V/cm is applied to the electron injection layer.
- constituent material of the electron injection layer are metal complexes of 8-hydroxyquinoline (Al chelate: Alq), derivatives thereof, and oxadiazole derivatives.
- the organic EL element can be driven at a notably low voltage and the life thereof can be made longer.
- a hole barrier layer having a thickness of 5 nm to 5 ⁇ m between the organic luminescence layer and the cathode.
- the arrangement of the hole barrier layer makes it possible to improve capability of confining holes in the organic luminescence layer, give a high luminescence brightness, and attain driving at a low voltage.
- Examples of the constituent material of the hole barrier layer include 2,9-diemthyl-4,7-diphenyl-1,10-phenanthroline and 2,9-diethyl-4,7-diphenyl-1,10-phenanthroline, and the like. More preferably, an alkali metal such as Li or Cs is further added thereto.
- the combination of the alkali metal with the hole barrier layer constituting material in the hole barrier layer makes it possible to drive the organic EL element at a notably low voltage and make the life thereof longer.
- the amount thereof is preferably 0.01 to 30% by weight. (if the total amount of the hole barrier layer is 100% by weight).
- the amount of the alkali metal is below 0.01% by weight, the effect of the addition thereof may be not exhibited. On the other hand, if the amount is over 30% by weight, the dispersion of the alkali metal becomes uneven so that luminescence brightness may be scattered.
- the amount of the alkali metal is more preferably 0.05 to 20% by weight, and more still preferably 0.1 to 15% by weight.
- the anode layer corresponds to a lower electrode or an opposite electrode, dependently on the structure of the organic EL display device.
- the anode layer is preferably made of a metal, an alloy or an electrically conductive compound having a large work function (for example, 4.0 eV or more), or a mixture thereof. Specifically, it is preferred to use one or a combination of two or more electrode materials selected from indium tin oxide (ITO), indium zinc oxide (IZO), copper iodide (CuI), tin oxide (SnO 2 ), zinc oxide (ZnO), gold, platinum, palladium and the like.
- ITO indium tin oxide
- IZO indium zinc oxide
- CuI copper iodide
- SnO 2 tin oxide
- ZnO zinc oxide
- the anode layer having a uniform thickness can be made using a method making deposition in a dry state possible, such as vacuum evaporation, sputtering, ion plating, electron beam evaporation, CVD (Chemical Vapor Deposition), MOCVD (Metal Oxide Chemical Vapor Deposition), or plasma CVD.
- a method making deposition in a dry state possible such as vacuum evaporation, sputtering, ion plating, electron beam evaporation, CVD (Chemical Vapor Deposition), MOCVD (Metal Oxide Chemical Vapor Deposition), or plasma CVD.
- an electrically conductive material such as ITO, IZO, CuI, SnO 2 or ZnO to set the transmissivity of EL luminescence to a value of 70% or more.
- the thickness of the anode layer is not particularly limited.
- the thickness is preferably a value of 10 to 1,000 nm, and more preferably a value of 10 to 200 nm.
- the thickness of the anode layer is set to a value within such a range, uniform thickness distribution can be obtained and the transmissivity of EL luminescence can be made to 70% or more.
- the sheet resistivity of the anode layer can be made to a value of 1000 ⁇ / ⁇ or less, and preferably 100 ⁇ / ⁇ or less.
- light is emitted from an arbitrary pixel in the luminescence face by depositing the anode layer (lower electrode), the organic luminescence medium, and the cathode layer (opposite electrode) successively and making the lower electrode and the opposite electrode into an XY matrix pattern.
- the cathode layer also corresponds to a lower electrode or an opposite electrode, dependently on the structure of the organic EL display device.
- the cathode layer is preferably made of a metal, an alloy or an electrically conductive compound having a small work function (for example, less than 4.0 eV), or a mixture thereof.
- any one or a combination of two or more electrode materials selected from sodium, sodium-potassium alloy, cesium, magnesium, lithium, magnesium-silver alloy, aluminum, aluminum oxide, aluminum-lithium alloy, indium, a rare earth metal, a mixture of an organic luminescence medium material and these metals, a mixture of an electron injection layer material and these metals, and the like.
- the thickness of the cathode layer is not particularly limited.
- the thickness is preferably a value of 10 to 1,000 nm, and more preferably a value of 10 to 200 nm.
- the transmissivity of EL luminescence when EL luminescence is taken out from the cathode layer, it is necessary to make the cathode layer to a transparent electrode. In this case, it is preferred to set the transmissivity of EL luminescence to a value of 70% or more.
- the cathode layer is preferably formed by a method making deposition in a dry state possible, such as vacuum evaporation or sputtering, in the same way as for the anode layer.
- the supporting substrate in the organic EL element is preferably a substrate which has superior mechanical strength and small permeability of moisture or oxygen.
- Specific examples thereof include glass plates, metal plates, ceramic plates and plastic plates (such as polycarbonate resin, acrylic resin, vinyl chloride resin, polyethylene terephthalate resin, polymide resin, polyester resin, epoxy resin, phenol resin, silicone resin, and fluorine resin plates) and the like.
- the water content in the supporting substrate and the gas transmissivity thereof small. Specifically, it is preferred to set the water content in the supporting substrate to 0.0001% or less by weight and set the gas transmissivity thereof to 1 ⁇ 10 ⁇ 13 cc ⁇ cm/cm 2 ⁇ sec. cmHg or less.
- a glass substrate made by Geomatic company 25 mm in width, 75 mm in length and 1.1 mm in thickness, with ITO transparent electrodes, was subjected to ultrasonic washing in isopropyl alcohol for 5 minutes and subjected to UV ozone washing for 30 minutes.
- the washed glass substrate with the ITO transparent electrodes was set to a substrate holder in a vacuum evaporation device, and then N,N-bis(N,N-diphenyl-4-aminophenyl)-N,N-diphenyl-4,4′-diamino-1,1′-biphenyl (abbreviated to TPD232 hereinafter) was vapor-deposited on the substrate at a vacuum degree of 665 ⁇ 10 ⁇ 7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form a first hole injection layer (which also had a function as a hole transport layer) having a thickness of 60 nm.
- TPD232 N,N-bis(N,N-diphenyl-4-aminophenyl)-N,N-diphenyl-4,4′-diamino-1,1′-biphenyl
- NPD 4,4-Bis[N-(1-naphtyl)-N-phenylamino]biphenyl
- the same vacuum evaporation device was used to vapor-deposit a carbazole compound (Tg: 110° C. or higher) represented by the formula (9) on the NPD film formed in the previous step at a vacuum degree of 665 ⁇ 10 ⁇ 7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form an organic luminescence layer having a thickness of 30 nm.
- a carbazole compound Tg: 110° C. or higher
- tris(2-phenylpyridine) iridium was vapor-deposited as a phosphorescent dopant.
- the vapor-deposition rate of the phosphorescent dopant was adjusted in the manner that the ratio of the amount of the blended phosphorescent dopant to the total amount of the organic luminescence layer would be 7% by weight. (if the total amount of the organic luminescence layer is 100% by weight).
- the same vacuum evaporation device was used to vapor-deposit 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (abbreviated to BCP hereinafter) on the organic luminescence layer formed in the previous step at a vacuum degree of 665 ⁇ 10 ⁇ 7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form an organic luminescence layer having a thickness of 10 nm.
- BCP 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline
- the same vacuum evaporation device was used to deposit a tris(8-quinolinol) aluminum film (abbreviated to an Alq film hereinafter) on the hole barrier layer formed in the previous step at a vacuum degree of 665 ⁇ 10 ⁇ 7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form an electron injection layer.
- a tris(8-quinolinol) aluminum film abbreviated to an Alq film hereinafter
- Li Li source made by Saesu getter company
- Alq Alq
- the same vacuum evaporation device was used to vapor-deposit metal Al on the electron injection layer formed in the previous step at a vacuum degree of 665 ⁇ 10 ⁇ 7 Pa and a vapor-deposition rate of 0.5 to 1.0 nm/sec., so as to form a cathode having a thickness of 150 nm.
- the resultant organic EL element was put into a dry box into which nitrogen was introduced. Furthermore, its luminescence face was coated with blue glass and the periphery thereof was treated with a cation-setting adhesive TB3102 (made by Three Bond Co., Ltd.) to perform sealing.
- a cation-setting adhesive TB3102 made by Three Bond Co., Ltd.
- a DC voltage of 6 V was applied between the anode and the cathode in the resultant organic EL element, so that green luminescence having a luminescence brightness of 1,200 cd/m 2 and a luminescence efficiency of 40 cd/A was obtained.
- the organic EL element was driven at a low voltage.
- the initial brightness thereof was set to 500 cd/m 2 .
- a life span test was performed.
- the half-time which is a driving time until the initial brightness becomes half, was 500 hours. This half-time is practical.
- a current-sending test as a heat-resistance test was performed at 85° C. Even after current was sent for 200 hours, green luminescence having sufficient luminescence brightness was obtained.
- An organic EL element was produced and evaluated in the same way as in Example 1 except that at the time of vapor-depositing BCP for the hole barrier layer in Example 1, metal Li, which is an alkali metal, and BCP were subjected to vapor co-deposition at a molar ratio of 1:1.
- the organic EL element was driven at a low voltage.
- the initial brightness thereof was set to 500 cd/m 2 . In this way, a life span test was performed, so that the half-time was 700 hours.
- a current-sending test as a heat-resistance test was performed at 85° C. Even after current was sent for 200 hours, green luminescence having sufficient luminescence brightness was obtained.
- An organic EL element was produced and evaluated in the same way as in Example 1 except that at the time of vapor-depositing BCP for the hole barrier layer in Example 1, metal Cs, which is an alkali metal, and BCP were subjected to vapor co-deposition at a molar ratio of 1:1.
- the organic EL element was driven at a low voltage.
- the initial brightness thereof was set to 500 cd/m 2 . In this way, a life span test was performed, so that the half-time was 800 hours.
- a current-sending test as a heat-resistance test was performed at 85° C. Even after current was sent for 200 hours, green luminescence having sufficient luminescence brightness was obtained.
- An organic EL element was produced and evaluated in the same way as in Example 1 except that instead of the carbazole compound represented by the formula (9), a carbazole compound (Tg: less than 110° C.) represented by the formula (6) was used in the organic luminescence layer.
- a carbazole compound (Tg: less than 110° C.) represented by the formula (6) was used in the organic luminescence layer.
- the organic EL element was driven at a low voltage.
- the initial brightness thereof was set to 500 cd/m 2 . In this way, a life span test was performed. However, the half-time was as short as 150 hours. This half-time is not practically allowable.
- a current-sending test as a heat-resistance test was performed at a high temperature of 85° C. A short circuit was caused after 100 hours. The organic EL element was unable to be lighted.
- its organic luminescence medium comprises a carbazole derivative with a glass-transition temperature of 110° C. or higher, and a phosphorescent dopant, so that the triplet exciton state of the carbazole derivative can be used even at room temperature and this element has a practical life and superior heat-resistance.
Landscapes
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Organic Chemistry (AREA)
- Optics & Photonics (AREA)
- Crystallography & Structural Chemistry (AREA)
- Inorganic Chemistry (AREA)
- Electroluminescent Light Sources (AREA)
Abstract
An organic electroluminescence element comprising: an anode layer, a cathode layer, and an organic luminescence layer therebetween, the organic luminescence layer having a carbazole derivative with a glass-transition temperature of 110° C. or higher, and a phosphorescent dopant. This structure makes it possible to provide an organic electroluminescence element which can make use of the triplet exciton state of the carbazole derivative even at room temperature and which has a practical life and superior heat-resistance.
Description
1. Field of the Invention
The present invention relates to an organic electroluminescence element (which may be referred to as an organic EL element hereinafter). More specifically, the present invention relates to an organic EL element using a triplet exciton of an organic luminescence material (host material).
2. Description of the Related Art
Hitherto, organic EL elements wherein an organic luminescence layer is arranged between electrodes have been eagerly researched and developed for the following reasons and the like.
-
- (1) Since these elements are completely solid, they are easy to handle and produce.
- (2) Since they can emit light by themselves, no light emitting members are necessary.
- (3) Since they can be clearly watched, they are suitable for display.
- (4) They permit full color display easily.
The luminescence mechanism of such organic EL elements generally makes use of a luminescence phenomenon, which is energy conversion phenomenon caused when a fluorescent molecule in a singlet excited state (which may be referred to a S1 state) in an organic luminescence medium is transited to a ground state radially.
A fluorescent molecule in a triplet excited state (which may be referred to a T1 state) in an organic luminescence medium can be supposed. However, radiative transition to a ground state is forbidden; therefore, such a molecule is gradually transited from the triplet excited state to some other state by non-radiative transition. As a result, no fluorescence is emitted but thermal energy is radiated.
Here, singlet and triplet mean multiplicity of energy decided by combination of total spin angular momentum and total orbital angular momentum of a fluorescent molecule. Specifically, a singlet excited state is defined as an energy state in the case that a single electron is transited from a ground state, where no unpaired electrons are present, to a higher energy level without changing the spin state of the electron. A triplet excited state is defined as an energy state in the case that a single electron is transited to a higher energy level while the spin state of the electron is made reverse.
Needless to say, luminescence in a triplet excited state defined as above can be observed if the luminescence is caused at a very low temperature, for example, at a liquefaction temperature of liquid nitrogen (−196° C.). However, this temperature is not a practical temperature, and the amount of the luminescence is only a little.
By the way, the total efficiency of luminescence from any conventional organic EL element is related to recombination efficiency (φrec) of injected charged carriers (electrons and holes), and the probability (φrad) that generated excitons cause radiative transition. Therefore, the total efficiency (φel) of luminescence from the organic EL element can be represented by the following equation:
φel=φrec×0.25φrad
φel=φrec×0.25φrad
The coefficient (0.25) of φrad in the equation is decided from the matter that the probability that singlet excitons are generated is regarded as ¼. Therefore, even if recombination and radiative attenuation of excitons are caused with a probability coefficient of 1, the theoretical upper limit of luminescence efficiency of the organic EL element is 25%.
As described above, in any conventional organic EL element, triplet excitons cannot be substantially used and only singlet excitons cause radiative transition. Thus, a problem that the upper limit of the luminescence efficiency is low arises.
Thus, literature 1 “Jpn. J. Appl. Phys., 38 (1999) L1502” discloses that even at room temperature, triplet excitons (triplet excited state) of an organic luminescence material (host material) are used to transfer energy from the triplet excitons to a phosphorescent dopant, so as to generate a fluorescent luminescence phenomenon. More specifically, the literature 1 reports that a fluorescent luminescence phenomenon is caused in an organic EL element comprising an organic luminescence layer composed of 4,4-N,N-dicarbazolylbiphenyl represented by the following formula (6) and an Ir complex, which is a phosphorescent dopant.
However, the half-life of the organic EL element described in the literature 1 is below 150 hours, and the usefulness of the organic EL element is insufficient.
Thus, the inventor made eager investigations. As a result, the following has been found: the glass-transition temperature of 4,4-N,N-dicarbazolylbiphenyl is as low as less than 110° C.; therefore, if the biphenyl is combined with an Ir complex, crystallization is easily caused in the organic luminescence layer comprising the combination to make the life of an organic EL element short.
Incidentally, in the present situation, a demand that the heat-resistance of organic EL elements for cars should be made higher has been increasing in light of environment inside cars in summer.
Thus, an object of the present invention is to provide an organic EL element which makes it possible to use triplet excitons of an organic luminescence material (host material) even at room temperature to emit fluorescence (including phosphorescence); has a practical life span; and has a superior heat-resistance.
[1] According to the present invention, provided is an organic EL element comprising:
-
- an anode layer,
- a cathode layer, and
- an organic luminescence layer therebetween, the organic luminescence layer having a carbazole derivative with a glass-transition temperature of 110° C. or higher, and a phosphorescent dopant. Thus, the above-mentioned problems can be solved.
This organic EL element makes it possible to use the triplet exciton state of the organic luminescence material even at room temperature. Moreover, this element has a practical life, for example, a half-time of 300 hours or more, and has superior heat-resistance. Thus, this element can be sufficiently used as an organic EL element for car.
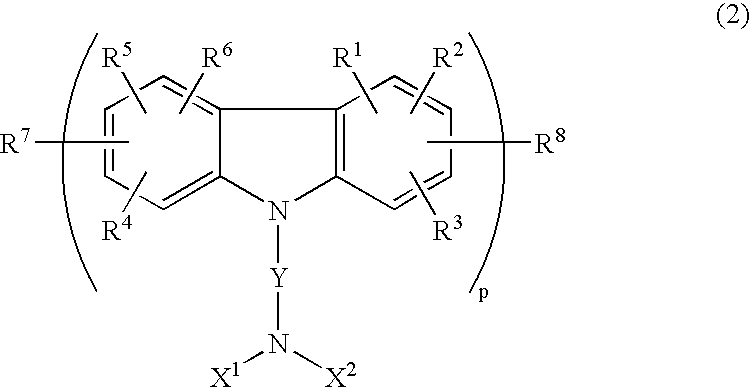
[2] In the organic EL element of the present invention, it is preferred that the carbazole derivative is at least one of compounds represented by the following general formulae (1) to (4):
-
- wherein Ar1 is a substituted or non-substituted aryl group having 6 to 50 nucleus carbon atoms; Ar2 to Ar7 are each independently a substituted or non-substituted aryl or arylene group having 6 to 50 nucleus carbon atoms; Ar2 and Ar3, Ar4 and Ar5, or Ar6 and Ar7 may be connected to each other through a single bond or through O, S or substituted or non-substituted alkylene as a connecting group; and each of repetition numbers m and n is an integer of 0 to 3,
- wherein R1 to R6 are each independently a hydrogen or halogen atom, an alkyl, aralkyl, aryl, cycloalkyl, fluoroalkyl, amino, nitro, cyano, hydroxy, or alkoxy group; R7 and R8 are each independently a hydrogen atom, an alkyl, aralkyl, aryl, or cycloalkyl group; X1 and X2 are each independently a hydrogen atom, an alkyl, aralkyl, aryl, or cycloalkyl group; Y is a single bond, an alkyl, alkylene, cycloalkyl, aryl, or aralkyl chain; a repetition number p is an integer of 1 to 3.
- wherein Ar8 to Ar11 are each independently an aryl group having 6 to 50 nucleus carbon atoms which may be substituted with an alkyl, alkoxy or aryl group; Ar8 and Ar9, or Ar10 and Ar11 may be connected to each other through a single bond or through O, S or substituted or non-substituted alkylene as a connecting group; and R9 is an alkyl or alkoxy group, or a substituted or non-substituted aryl group having 6 to 18 nucleus carbon atoms.
- wherein Z is a trivalent nitrogen atom or an aromatic group; Ar12 to Ar14 are each independently a group represented by the following general formula (5) or an aryl group having 6 to 50 nucleus carbon atoms; and at least two of Ar12 to Ar14 are groups represented by the following general formula (5):
- wherein R10 to R21 are each independently an aryl group having 6 to 50 nucleus carbon atoms which may be substituted with an alkyl, alkoxy group having 1 to 6 carbon atoms, or a phenyl group; and groups adjacent to each other may form a cyclic structure; and a repetition number q is an integer of 0 to 3.
- wherein Ar1 is a substituted or non-substituted aryl group having 6 to 50 nucleus carbon atoms; Ar2 to Ar7 are each independently a substituted or non-substituted aryl or arylene group having 6 to 50 nucleus carbon atoms; Ar2 and Ar3, Ar4 and Ar5, or Ar6 and Ar7 may be connected to each other through a single bond or through O, S or substituted or non-substituted alkylene as a connecting group; and each of repetition numbers m and n is an integer of 0 to 3,
The organic EL element wherein this carbazole derivative is used as a host material in the organic luminescence layer makes it possible to use the triplet exciton state more effectively, and has a practical life span.
[3] In the organic EL element of the present invention, it is preferred that the carbazole derivative has at least two carbazole skeletons.
This carbazole derivative has a large triplet energy to make it possible to use the triplet exciton state more effectively even at room temperature (20° C.), and has a practical life span.
[4] In the organic EL element of the present invention, it is preferred that the relationship of E1>E2 is satisfied in which E1 represents the triplet energy of the carbazole derivative and E2 represents the triplet energy of the phosphorescent dopant.
This structure makes it possible to transfer the triplet energy of the carbazole derivative surely to the phosphorescent dopant, and to emit fluorescence using the triplet energy even at room temperature (20° C.).
[5] In the organic EL element of the present invention, it is preferred that the triplet energy (E1) of the carbazole derivative is a value of 21,000 cm−1 or more.
A triplet energy of 21,000 cm−1 corresponds to a light wavelength of 488 nm. On the contrary, various phosphorescent dopants generally have a triplet energy which is equal to or less than the energy which 488 nm light has. Therefore, by using the carbazole derivative having such a large triplet energy as above, various phosphorescent dopants can be used.
Thus, by selecting an appropriate kind of the phosphorescent dopant for the carbazole derivative having such a large triplet energy as above, luminescence in green, yellow, orange, vermilion, red and the like can easily be obtained.
[6] In the organic EL element of the present invention, it is preferred that the carbazole derivative has a cyclic structure whose triplet energy is a value of 21,000 cm−1 or more, and the cyclic structure contains an aromatic ring, a hetero ring, or combination thereof.
This carbazole derivative makes it possible to transfer the triplet energy of the carbazole derivative more effectively to the phosphorescent dopant. Specifically, if the carbazole derivative has a cyclic structure having a triplet energy of less than 21,000 cm−1, the triplet energy is transferred to this cyclic structure so that the triplet energy transferred to the phosphorescent dopant may be reduced.
[7] In the organic EL element of the present invention, it is preferred that the phosphorescent dopant is a metal complex comprising at least one metal selected from the group consisting of Ir (iridium), Ru (ruthenium), Pd (palladium), Pt (platinum), Os (osmium) and Re (rhenium).
This structure makes it possible to transfer energy effectively from the triplet exciton of the carbazole derivative as a host material to the metal complex as the phosphorescent dopant.
[8] In the organic EL element of the present invention, it is preferred that at least one ligand of the metal complex has at least one skeleton selected from the group consisting of phenylpyridine, bipyridyl and phenanthroline skeletons.
The bulky and electron-withdrawing skeleton(s) contained in the molecule makes it possible to transfer energy effectively from the triplet exciton of the carbazole derivative to the metal complex.
[9] In the organic EL element of the present invention, it is preferred that a blend amount of the phosphorescent dopant is 0.1 to 30 parts by weight per 100 parts of the carbazole derivative.
This structure makes it possible to mix the phosphorescent dopant with the carbazole derivative uniformly, and transfer energy effectively from the triplet exciton of the carbazole derivative to the phosphorescent dopant.
[10] In the organic EL element of the present invention, it is preferred that a hole barrier layer, an electron injection layer, or combination thereof is arranged between the anode layer and the cathode layer, and the hole barrier layer and the electron injection layer comprise an alkali metal.
This structure makes it possible to drive the organic EL element at a lower voltage, and make the life span of the element longer.
Referring to FIG. 1 , embodiments of the organic EL elements of the present invention will be described. FIG. 1 is a sectional view of an organic EL element 102, and illustrates a structure wherein an anode layer 10, an organic luminescence layer 14 and a cathode layer 16 are successively deposited on a substrate 18.
The following will chiefly describe a carbazole derivative (host material) and a phosphorescent dopant that constitute the organic luminescence layer 14, which is a characteristic element in the present embodiment. Thus, the structure and the production process of the other elements, for example, the anode layer 10 and the cathode layer 16 will briefly be described. For elements which are not referred to, structures and production processes which are generally known in the field of organic EL elements can be adopted.
1. Carbazole Derivative
(1) Kind 1
An embodiment of the present invention is characterized in that in the organic luminescence layer, a carbazole derivative with a glass-transition temperature of 110° C. or higher is used as a host material.
This is because if the host material is a carbazole derivative, the triplet exciton state of the carbazole derivative can be effectively used even at room temperature (20° C.) by combining the derivative with a phosphorescent dopant that will be described later. Specifically, a luminescence phenomenon can be caused by transferring energy effectively from the triplet state in the carbazole derivative to the phosphorescent dopant.
Such a carbazole derivative is preferably a carbazole derivative having at least two carbazole skeletons. This is because the glass-transition temperature and triplet energy that will be described later can easily be adjusted and the derivative can easily be mixed with the phosphorescent dopant.
The reason why the carbazole derivative having a glass-transition temperature of 110° C. or higher is used is as follows. If the glass-transition temperature is below 110° C., any combination thereof with the phosphorescent dopant is very easily crystallized so that the life becomes short. If the glass-transition temperature is below 110° C., a short circuit is easily caused in a short time when an electric current is passed through the derivative at a high temperature. Thus, the environment where the organic element EL is used is excessively restricted.
Therefore, the glass-transition temperature of the carbazole derivative is more preferably from 115 to 170° C., and still more preferably from 120 to 150° C.
The reason why the glass-transition temperature of the carbazole derivative is more preferably 170° C. or lower is that the kinds of carbazole derivatives having a glass-transition over 170° C. are excessively restricted and the handling of the derivatives becomes difficult because of a drop in their deposition ability.
A Differential Scanning Calorimeter (DSC) is used to make it possible to obtain the glass-transition temperature (Tg) of the carbazole derivative as a temperature of a change in the specific heat obtained when the derivative is heated at a temperature-rising rate of, for example, 10° C./minute in a nitrogen-circulating state.
(2) Kind 2
In the embodiment of the present invention, it is preferred that the relationship of E1>E2 is satisfied in which E1 represents the triplet energy of the carbazole derivative in the organic luminescence layer and E2 represents the triplet energy of the phosphorescent dopant therein.
By combining the carbazole derivative with the phosphorescent dopant with this relationship, the triplet exciton state of the carbazole derivative can surely be used even at room temperature. Specifically other words, a luminescence phenomenon can be caused by transferring energy certainly from the triplet state in the carbazole derivative to the phosphorescent dopant.
It is also preferred that the triplet energy (E1) of the carbazole derivative is set to a value of 21,000 cm−1 or more.
Specifically, the triplet energy, 21,000 cm−1 corresponds to a light wavelength of 488 nm. In general, various phosphorescent dopants have a triplet energy which is equal to or less than the energy which 488 nm light has. Therefore, one or more selected from various phosphorescent dopants can be combined with the carbazole derivative.
Thus, by selecting the kind of the phosphorescent dopant appropriately, luminescence in green, yellow, orange, vermilion, red and the like can be obtained.
Moreover, by setting the triplet energy (E1) of the carbazole derivative to a value of 22,500 cm−1 or more, luminescence in blue also can be obtained easily.
Preferably, the carbazole derivative has a cyclic structure whose triplet energy is a value of 21,000 cm−1 or more and the cyclic structure contains an aromatic ring, a hetero ring, or combination one thereof.
If the carbazole derivative has such a cyclic structure, the triplet exciton state of the carbazole derivative can be effectively used even at room temperature by combining the carbazole derivative with the phosphorescent dopant. That is, by causing the carbazole derivative to have, for example, a cyclic structure wherein 9-arylcarbazole is connected to a bivalent or trivalent group consisting an aromatic ring, the triplet energy can be set to 22,500 cm−1 or less. Therefore, if the carbazole derivative has such a cyclic structure, the frequency that the triplet energy of 21,000 cm, originating from the carbazole group, is transferred in the molecule becomes small. Thus, the triplet energy which is transferred to the phosphorescent dopant becomes relatively large.
(3) Kind 3
It is preferred to use, as the above-mentioned carbazole derivative, carbazole derivatives represented by the general formulae (1) to (4) alone or in combination of two or more.
In the general formulae (1) to (4) representing preferred carbazole derivatives, examples of preferred aryl groups having 5 to 50 nucleus atoms include phenyl, naphthyl, anthranyl, phenanthryl, pyrenyl, coronyl, biphenyl, terphenyl, pyrrolyl, furanyl, thiophenyl, benzothiophenyl, oxadiazolyl, diphenylanthranyl, indolyl, carbazolyl, pyridyl, and benzoquinolyl, and the like.
Examples of preferred arylene groups having 5 to 50 nucleus atoms include phenylene, naphthylene, anthranylene, phenanthrylene, pyrenylene, coronylene, biphenylene, terphenylene, pyrrolylene, furanylene, thiophenylene, benzothiophenylene, oxadiazolylene, diphenylanthranylene, indolylene, carbazolylene, pyridylene, and benzoquinolylene, and the like.
The aromatic group having 6 to 50 carbon atoms may be substituted with one or more substituents. Preferred examples of the substituent include alkyl groups having 1 to 6 carbon atoms (such as methyl, ethyl, i-propyl, n-propyl, s-butyl, t-butyl, pentyl, hexyl, cyclopentyl and cyclohexyl groups); alkoxy groups having 1–6 carbon atoms (such as methoxy, ethoxy, i-propoxy, n-propoxy, s-butoxy, t-butoxy, pentoxy, hexyloxy, cyclopentoxy and cyclohexyloxy groups); aryl groups having 5 to 50 nucleus atoms; amino groups substituted with an aryl group having 5 to 50 nucleus atoms; ester groups having an aryl group having 5 to 50 nucleus atoms; ester groups having an alkyl group having 1 to 6 carbon atoms; a cyano group; a nitro group; halogen atoms. The above-mentioned substituent may be substituted with a carbazolyl group.
Moreover, as shown in the general formulae (1) and (3) described below, the carbazole in the present invention is interpreted as a moiety formed by connecting at least two aryl groups, each of which is connected to a nitrogen atom, to each other through a single bond or a connecting groups. In this case, preferred examples of the connecting groups include O, S, and substituted or non-substituted alkylene and silylene, and the like.
Here, preferred specific examples of the carbazole derivative represented by the formula (1) include a group of compounds illustrated as the following chemical formulae (7) to (24).
Preferred specific examples of the carbazole derivative represented by the formula (2) include a group of compounds illustrated as the following chemical formulae (25) to (29). In the formula, a methyl group may be abbreviated to Me.
Preferred specific examples of the carbazole derivative represented by the formula (3) include a group of compounds illustrated as the following chemical formulae (30) to (41).
Preferred specific examples of the carbazole derivative represented by the formula (4) include a group of compounds illustrated as the following chemical formulae (42) to (49).
Furthermore, specific examples of the carbazole derivative having a structure other than the structures represented by the general formulae (1) to (4) include a group of compounds illustrated as the following chemical formulae (50) to (59).
-
- wherein a repetition number is an integer of 3 to 20.
2. Phosphorescent Dopant
(1) Kind
{circle around (1)} Metal Complex
- wherein a repetition number is an integer of 3 to 20.
The phosphorescent dopant is preferably a metal complex comprising at least one metal selected from the group consisting of Ir, Ru, Pd, Pt, Os and Re.
This is because if the phosphorescent dopant is any one of these metal complexes, energy can be effectively transferred from triplet excitons of the carbazole derivative as a host material to the phosphorescent dopant.
More specific examples of the phosphorescent dopant are metal complexes such as tris(2-phenylpyridine) iridium, tris(2-phenylpyridine) ruthenium, tris(2-phenylpyridine) palladium, bis(2-phenylpyridine) platinum, tris(2-phenylpyridine) osmium, tris(2-phenylpyridine) rhenium, octaethyl platinum porphyrin, octaphenyl platinum porphyrin, octaethyl palladium porphyrin, and octaphenyl palladium porphyrin. In order to transfer energy more effectively to emit fluorescence, more preferred are metal complexes comprising Ir, for example, tris(2-phenylpyridine) iridium represented by the following formula (60):
{circle around (2)} Ligand of the Metal Complex
{circle around (2)} Ligand of the Metal Complex
At least one ligand of the metal complex preferably has at least one skeleton selected from the group consisting of phenylpyridine, bipyridyl and phenanthroline skeletons.
This is because by at least one of these electron withdrawing skeletons contained in the molecule, energy can be effectively transferred from the triplet excitons of the carbazole derivative to the metal complex.
Particularly, in the phosphorescent dopant, the ligand preferably has a phenylpyridine skeleton among these skeletons, such as tris(2-phenylpyridine) iridium.
(2) Added Amount
A blend amount of the phosphorescent dopant is preferably 0.1 to 30 parts by weight per 100 parts by weight of the blended carbazole derivative (host material).
The reasons for this are as follows. If the blend amount of the phosphorescent dopant is below 0.1 part by weight, the effect based on the blend is not exhibited so that energy may not be effectively transferred from triplet excitons of the carbazole derivative. On the other hand, if the blend amount of the phosphorescent dopant is over 30 parts by weight, the phosphorescent dopant is not easily blended with the carbazole derivative uniformly so that luminescence brightness may be scattered.
Therefore, the blend amount of the phosphorescent dopant is more preferably 0.5 to 20 parts by weight, and is still more preferably 1 to 15 parts by weight.
3. Other Organic Layers in the Organic Luminescence Medium
(1) Hole Injection Layer
It is preferred to deposit a hole injection layer having a thickness of 5 nm to 5 μm. The deposition of such a hole injection layer makes it possible to inject holes satisfactorily into the organic luminescence layer, give a high luminescence brightness, and attain driving at a low voltage.
It is also preferred to use, in the hole injection layer in the organic luminescence medium, a compound having a hole mobility of 1×10−6 cm2/V·second or more and an ionization energy of 5.5 eV or less. The hole mobility is measured when a voltage of 1×104 to 1×106 V/cm is applied to the hole injection layer.
Specific examples of the constituent material of the hole injection layer are organic compounds such as porphyrin compounds, aromatic tertiary amine compounds, styrylamine compounds, aromatic dimethylidyne compounds, and condensed aromatic ring compounds, for example, 4,4′-bis[N-(1-naphthyl)-N-phenylamino]biphenyl (abbreviated to NPD) and 4,4′,4″-tris[N-(3-methylphenyl)-N-phenylamino]triphenylamine (abbreviated to MTDATA).
As the constituent material of the hole injection layer, an inorganic compound such as p-type Si or p-type SiC is preferably used.
It is also preferred to arrange an organic semiconductor layer having an electric conductivity of 1×10−10 S/cm or more between the hole injection layer and the anode layer, or between the hole injection layer and the organic luminescence layer. The arrangement of the organic semiconductor layer makes the injection of holes into the organic luminescence layer more satisfactory.
(2) Electron Injection Layer
It is preferred to deposit an electron injection layer having a thickness of 5 nm to 5 μm. The deposition of such an electron injection layer makes it possible to inject electrons satisfactorily into the organic luminescence layer, give a high luminescence brightness, and attain driving at a low voltage.
It is also preferred to use, in the electron injection layer, a compound having an electron mobility of 1×10−6 cm2/V·second or more and an ionization energy over 5.5 eV. The electron mobility is measured when a voltage of 1×104 to 1×106 V/cm is applied to the electron injection layer.
Specific examples of the constituent material of the electron injection layer are metal complexes of 8-hydroxyquinoline (Al chelate: Alq), derivatives thereof, and oxadiazole derivatives.
If an alkali metal is incorporated into the electron injection layer in the same way as into a hole barrier layer that will be described later, the organic EL element can be driven at a notably low voltage and the life thereof can be made longer.
(3) Hole Barrier Layer
It is preferred to arrange a hole barrier layer having a thickness of 5 nm to 5 μm between the organic luminescence layer and the cathode. The arrangement of the hole barrier layer makes it possible to improve capability of confining holes in the organic luminescence layer, give a high luminescence brightness, and attain driving at a low voltage.
Examples of the constituent material of the hole barrier layer include 2,9-diemthyl-4,7-diphenyl-1,10-phenanthroline and 2,9-diethyl-4,7-diphenyl-1,10-phenanthroline, and the like. More preferably, an alkali metal such as Li or Cs is further added thereto.
The combination of the alkali metal with the hole barrier layer constituting material in the hole barrier layer makes it possible to drive the organic EL element at a notably low voltage and make the life thereof longer.
When the alkali metal is incorporated, the amount thereof is preferably 0.01 to 30% by weight. (if the total amount of the hole barrier layer is 100% by weight).
If the amount of the alkali metal is below 0.01% by weight, the effect of the addition thereof may be not exhibited. On the other hand, if the amount is over 30% by weight, the dispersion of the alkali metal becomes uneven so that luminescence brightness may be scattered.
Therefore, the amount of the alkali metal is more preferably 0.05 to 20% by weight, and more still preferably 0.1 to 15% by weight.
4. Electrode
(1) Anode Layer
The anode layer corresponds to a lower electrode or an opposite electrode, dependently on the structure of the organic EL display device. The anode layer is preferably made of a metal, an alloy or an electrically conductive compound having a large work function (for example, 4.0 eV or more), or a mixture thereof. Specifically, it is preferred to use one or a combination of two or more electrode materials selected from indium tin oxide (ITO), indium zinc oxide (IZO), copper iodide (CuI), tin oxide (SnO2), zinc oxide (ZnO), gold, platinum, palladium and the like.
By using these electrode materials, the anode layer having a uniform thickness can be made using a method making deposition in a dry state possible, such as vacuum evaporation, sputtering, ion plating, electron beam evaporation, CVD (Chemical Vapor Deposition), MOCVD (Metal Oxide Chemical Vapor Deposition), or plasma CVD.
When EL luminescence is taken out from the anode layer, it is necessary to make the anode layer to a transparent electrode. In this case, it is preferred to use an electrically conductive material such as ITO, IZO, CuI, SnO2 or ZnO to set the transmissivity of EL luminescence to a value of 70% or more.
The thickness of the anode layer is not particularly limited. The thickness is preferably a value of 10 to 1,000 nm, and more preferably a value of 10 to 200 nm.
If the thickness of the anode layer is set to a value within such a range, uniform thickness distribution can be obtained and the transmissivity of EL luminescence can be made to 70% or more. Moreover, the sheet resistivity of the anode layer can be made to a value of 1000 Ω/□ or less, and preferably 100 Ω/□ or less.
It is also preferred that light is emitted from an arbitrary pixel in the luminescence face by depositing the anode layer (lower electrode), the organic luminescence medium, and the cathode layer (opposite electrode) successively and making the lower electrode and the opposite electrode into an XY matrix pattern. By making the anode and so on into this manner, various data can easily be displayed in the organic EL element.
(2) Cathode Layer
The cathode layer also corresponds to a lower electrode or an opposite electrode, dependently on the structure of the organic EL display device. The cathode layer is preferably made of a metal, an alloy or an electrically conductive compound having a small work function (for example, less than 4.0 eV), or a mixture thereof.
Specifically, it is preferred to use any one or a combination of two or more electrode materials selected from sodium, sodium-potassium alloy, cesium, magnesium, lithium, magnesium-silver alloy, aluminum, aluminum oxide, aluminum-lithium alloy, indium, a rare earth metal, a mixture of an organic luminescence medium material and these metals, a mixture of an electron injection layer material and these metals, and the like.
The thickness of the cathode layer is not particularly limited. The thickness is preferably a value of 10 to 1,000 nm, and more preferably a value of 10 to 200 nm.
Furthermore, when EL luminescence is taken out from the cathode layer, it is necessary to make the cathode layer to a transparent electrode. In this case, it is preferred to set the transmissivity of EL luminescence to a value of 70% or more.
The cathode layer is preferably formed by a method making deposition in a dry state possible, such as vacuum evaporation or sputtering, in the same way as for the anode layer.
5. Supporting Substrate
The supporting substrate in the organic EL element is preferably a substrate which has superior mechanical strength and small permeability of moisture or oxygen. Specific examples thereof include glass plates, metal plates, ceramic plates and plastic plates (such as polycarbonate resin, acrylic resin, vinyl chloride resin, polyethylene terephthalate resin, polymide resin, polyester resin, epoxy resin, phenol resin, silicone resin, and fluorine resin plates) and the like.
To avoid invasion of moisture into the organic EL element, it is preferred to form an inorganic film or apply a fluorine resin onto the supporting substrate made of such a material as above to conduct moisture-proof treatment or hydrophobic treatment.
Particularly to avoid invasion of moisture into the organic luminescence medium, it is preferred to make the water content in the supporting substrate and the gas transmissivity thereof small. Specifically, it is preferred to set the water content in the supporting substrate to 0.0001% or less by weight and set the gas transmissivity thereof to 1×10−13 cc·cm/cm2 ·sec. cmHg or less.
(Production of an Organic EL Element)
{circle around (1)} Washing
A glass substrate (made by Geomatic company) 25 mm in width, 75 mm in length and 1.1 mm in thickness, with ITO transparent electrodes, was subjected to ultrasonic washing in isopropyl alcohol for 5 minutes and subjected to UV ozone washing for 30 minutes.
{circle around (2)} Formation of a Hole Injection Layer
The washed glass substrate with the ITO transparent electrodes was set to a substrate holder in a vacuum evaporation device, and then N,N-bis(N,N-diphenyl-4-aminophenyl)-N,N-diphenyl-4,4′-diamino-1,1′-biphenyl (abbreviated to TPD232 hereinafter) was vapor-deposited on the substrate at a vacuum degree of 665×10−7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form a first hole injection layer (which also had a function as a hole transport layer) having a thickness of 60 nm.
4,4-Bis[N-(1-naphtyl)-N-phenylamino]biphenyl (abbreviated to NPD hereinafter) was vapor-deposited on the TPD232 film at a vacuum degree of 665×10−7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form a second hole injection layer (which also had a function as a hole transport layer) having a thickness of 20 nm.
{circle around (3)} Formation of an Organic Luminescence Layer
Next, the same vacuum evaporation device was used to vapor-deposit a carbazole compound (Tg: 110° C. or higher) represented by the formula (9) on the NPD film formed in the previous step at a vacuum degree of 665×10−7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form an organic luminescence layer having a thickness of 30 nm.
In this case, at the same time of the vapor deposition of the carbazole derivative compound represented by the formula (9), tris(2-phenylpyridine) iridium was vapor-deposited as a phosphorescent dopant. In this vapor co-deposition, the vapor-deposition rate of the phosphorescent dopant was adjusted in the manner that the ratio of the amount of the blended phosphorescent dopant to the total amount of the organic luminescence layer would be 7% by weight. (if the total amount of the organic luminescence layer is 100% by weight).
{circle around (4)} Formation of a Hole Barrier Layer
Next, the same vacuum evaporation device was used to vapor-deposit 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (abbreviated to BCP hereinafter) on the organic luminescence layer formed in the previous step at a vacuum degree of 665×10−7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form an organic luminescence layer having a thickness of 10 nm.
{circle around (5)} Formation of an Electron Injection Layer
Next, the same vacuum evaporation device was used to deposit a tris(8-quinolinol) aluminum film (abbreviated to an Alq film hereinafter) on the hole barrier layer formed in the previous step at a vacuum degree of 665×10−7 Pa and a vapor-deposition rate of 0.1 to 0.3 nm/sec., so as to form an electron injection layer.
At this time, Li (Li source made by Saesu getter company) and Alq were subjected to vapor co-deposition in the manner that the molar ratio between them would be 1:1, so that the electron injection layer was made to an Alq/Li film having a thickness of 20 nm.
{circle around (6)} Formation of a Cathode
Next, the same vacuum evaporation device was used to vapor-deposit metal Al on the electron injection layer formed in the previous step at a vacuum degree of 665×10−7 Pa and a vapor-deposition rate of 0.5 to 1.0 nm/sec., so as to form a cathode having a thickness of 150 nm.
{circle around (7)} Sealing Step
The resultant organic EL element was put into a dry box into which nitrogen was introduced. Furthermore, its luminescence face was coated with blue glass and the periphery thereof was treated with a cation-setting adhesive TB3102 (made by Three Bond Co., Ltd.) to perform sealing.
In this way, the organic EL element of Example 1 was prepared.
(Evaluation of the Organic EL Element)
A DC voltage of 6 V was applied between the anode and the cathode in the resultant organic EL element, so that green luminescence having a luminescence brightness of 1,200 cd/m2 and a luminescence efficiency of 40 cd/A was obtained.
The organic EL element was driven at a low voltage. The initial brightness thereof was set to 500 cd/m2. In this way, a life span test was performed. As a result, the half-time, which is a driving time until the initial brightness becomes half, was 500 hours. This half-time is practical.
A current-sending test as a heat-resistance test was performed at 85° C. Even after current was sent for 200 hours, green luminescence having sufficient luminescence brightness was obtained.
The obtained results are shown in Table 1.
An organic EL element was produced and evaluated in the same way as in Example 1 except that at the time of vapor-depositing BCP for the hole barrier layer in Example 1, metal Li, which is an alkali metal, and BCP were subjected to vapor co-deposition at a molar ratio of 1:1.
As a result, even by application of a DC voltage of 5 V, green luminescence having a luminescence brightness of 1,300 cd/m2 and a luminescence efficiency of 37 cd/A was obtained.
The organic EL element was driven at a low voltage. The initial brightness thereof was set to 500 cd/m2. In this way, a life span test was performed, so that the half-time was 700 hours.
A current-sending test as a heat-resistance test was performed at 85° C. Even after current was sent for 200 hours, green luminescence having sufficient luminescence brightness was obtained.
An organic EL element was produced and evaluated in the same way as in Example 1 except that at the time of vapor-depositing BCP for the hole barrier layer in Example 1, metal Cs, which is an alkali metal, and BCP were subjected to vapor co-deposition at a molar ratio of 1:1.
As a result, even by application of a DC voltage of 4.5 V, green luminescence having a luminescence brightness of 1,200 cd/m2 and a luminescence efficiency of 40 cd/A was obtained.
The organic EL element was driven at a low voltage. The initial brightness thereof was set to 500 cd/m2. In this way, a life span test was performed, so that the half-time was 800 hours.
A current-sending test as a heat-resistance test was performed at 85° C. Even after current was sent for 200 hours, green luminescence having sufficient luminescence brightness was obtained.
An organic EL element was produced and evaluated in the same way as in Example 1 except that instead of the carbazole compound represented by the formula (9), a carbazole compound (Tg: less than 110° C.) represented by the formula (6) was used in the organic luminescence layer.
As a result, by application of a DC voltage of 6 V, green luminescence having a luminescence brightness of 1,100 cd/m2 and a luminescence efficiency of 38 cd/A was obtained.
The organic EL element was driven at a low voltage. The initial brightness thereof was set to 500 cd/m2. In this way, a life span test was performed. However, the half-time was as short as 150 hours. This half-time is not practically allowable.
A current-sending test as a heat-resistance test was performed at a high temperature of 85° C. A short circuit was caused after 100 hours. The organic EL element was unable to be lighted.
This demonstrated that the organic EL element of Comparative Example 1 had poor heat-resistance and was unable to be used for car.
| TABLE 1 | |||||||||
| 85° C. | |||||||||
| Organic | current- | ||||||||
| luminescence | Hole | Luminescence | Luminescence | sending | |||||
| layer | barrier | Voltage | brightness | efficiency | Luminescence | Half-time | test | ||
| Tg (° C.) | layer | (V) | (nit) | (cd/A) | color | (hours) | (hours) | ||
| Example 1 | Formula (9) | Only | 6 | 1200 | 40 | Green | 500 | >200 |
| >110 | BCP | |||||||
| Example 2 | Formula (9) | BCP/Li = | 5 | 1300 | 37 | Green | 700 | >200 |
| >110 | 1/1 | |||||||
| Example 3 | Formula (9) | BCP/Cs = | 4.5 | 1200 | 40 | Green | 800 | >200 |
| >110 | 1/1 | |||||||
| Comparative | Formula (6) | Only | 6 | 1100 | 38 | Green | 150 | <200 |
| Example 1 | >110 | BCP | ||||||
An organic EL element was produced and evaluated in the same way as in Example 1 except that instead of the compound represented by the formula (9) of Example 1, each of compounds represented by the formulae (10), (19), (26), (30), (43) and (55) was used as a carbazole compound. The obtained results are shown in Table 2.
| TABLE 2 | |||||||||
| 85° C. | |||||||||
| Organic | current- | ||||||||
| luminescence | Hole | Luminescence | Luminescence | sending | |||||
| layer | barrier | Voltage | brightness | efficiency | Luminescence | Half-time | test | ||
| Tg (° C.) | layer | (V) | (nit) | (cd/A) | color | (hours) | (hours) | ||
| Example 4 | Formula (10) | Only | 6 | 1050 | 35 | Green | 450 | >200 |
| >120 | BCP | |||||||
| Example 5 | Formula (19) | Only | 6 | 600 | 18 | Green | 350 | >200 |
| >110 | BCP | |||||||
| Example 6 | Formula (26) | Only | 6 | 450 | 10 | Green | 300 | >200 |
| >110 | BCP | |||||||
| Example 7 | Formula (30) | Only | 6 | 890 | 28 | Green | 400 | >200 |
| >110 | BCP | |||||||
| Example 8 | Formula (43) | Only | 6 | 920 | 30 | Green | 550 | >200 |
| >140 | BCP | |||||||
| Example 9 | Formula (55) | Only | 6 | 350 | 10 | Green | 400 | >200 |
| >110 | BCP | |||||||
According to the organic EL element of the present invention, its organic luminescence medium comprises a carbazole derivative with a glass-transition temperature of 110° C. or higher, and a phosphorescent dopant, so that the triplet exciton state of the carbazole derivative can be used even at room temperature and this element has a practical life and superior heat-resistance.
Claims (9)
1. An organic host material for an organic electroluminescence element, the organic host material being at least one of the carbazole derivatives
wherein
m and n is 0 or 1;
at least one of Ar1 contains a carbazole group; and m and n is 0 or 1;
wherein R1 to R8 are each independently a hydrogen or alkyl group; X1 and X2 are each independently an alkyl, or alkyl-substituted or non-substituted phenyl or biphenyl group;
Y is phenylene or biphenylene; and
R9 is an alkyl-substituted or non-substituted phenyl, biphenyl, terphenyl, naphthyl, carbazolyl group or
Ar12 to Ar14 are each independently a carbazolyl, carbazolylphenyl, carbazolylbiphenyl, carbazolylnaphthyl group or
provided that all Ar12 to Ar14 are not the same group.
2. The organic host material according to claim 1 ,
wherein the carbazole derivative has at least two carbazole skeletons.
3. The organic host material according to claim 1 ,
wherein the triplet energy of the carbazole derivative is at least 21,000 cm−1.
4. The organic host material according to claim 1 ,
wherein the carbazole derivative has a cyclic structure, the triplet energy of the cyclic structure being at least 21,000 cm−1, and the cyclic structure contains an aromatic ring, a hetero ring, or a combination thereof.
5. An organic luminescence layer for an organic electroluminescence element, comprising the organic host material according to claim 1 and a phosphorescent dopant.
6. The organic luminescence layer according to claim 5 ,
wherein a relationship of E1>E2 is satisfied in which E1 represents the triplet energy of the carbazole derivative and E2 represents the triplet energy of the phosphorescent dopant.
7. The organic luminescence layer according to claim 5 ,
wherein the phosphorescent dopant is a metal complex comprising at least one metal selected from the group consisting of Ir, Ru, Pd, Pt, Os and Re.
8. The organic luminescence layer according to claim 7 ,
wherein at least one ligand of the metal complex has at least one skeleton selected from the group consisting of phenylpyridine, bipyridyl and phenanthroline skeletons.
9. The organic luminescence layer according to claim 5 ,
wherein the phosphorescent dopant is present in an amount of 0.1 to 30 parts by weight per 100 parts of the carbazole derivative.
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/683,435 US6979414B2 (en) | 2000-03-27 | 2003-10-14 | Organic electroluminescence element |
| US11/245,092 US7226546B2 (en) | 2000-03-27 | 2005-10-07 | Organic electroluminescence element |
| US11/806,597 US20070248841A1 (en) | 2000-03-27 | 2007-06-01 | Organic electroluminescence element |
| US12/805,316 US8753757B2 (en) | 2000-03-27 | 2010-07-26 | Organic electroluminescence element |
| US14/268,485 US9564607B2 (en) | 2000-03-27 | 2014-05-02 | Organic electroluminescence element |
| US15/378,759 US20170098785A1 (en) | 2000-03-27 | 2016-12-14 | Organic electroluminescence element |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2000087622 | 2000-03-27 | ||
| JP2000-087622 | 2000-03-27 | ||
| US09/816,415 US6660410B2 (en) | 2000-03-27 | 2001-03-26 | Organic electroluminescence element |
| US10/683,435 US6979414B2 (en) | 2000-03-27 | 2003-10-14 | Organic electroluminescence element |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/816,415 Continuation US6660410B2 (en) | 2000-03-27 | 2001-03-26 | Organic electroluminescence element |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/245,092 Continuation US7226546B2 (en) | 2000-03-27 | 2005-10-07 | Organic electroluminescence element |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| US20050222429A1 US20050222429A1 (en) | 2005-10-06 |
| US6979414B2 true US6979414B2 (en) | 2005-12-27 |
Family
ID=18603600
Family Applications (7)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/816,415 Expired - Lifetime US6660410B2 (en) | 2000-03-27 | 2001-03-26 | Organic electroluminescence element |
| US10/683,435 Expired - Lifetime US6979414B2 (en) | 2000-03-27 | 2003-10-14 | Organic electroluminescence element |
| US11/245,092 Expired - Lifetime US7226546B2 (en) | 2000-03-27 | 2005-10-07 | Organic electroluminescence element |
| US11/806,597 Abandoned US20070248841A1 (en) | 2000-03-27 | 2007-06-01 | Organic electroluminescence element |
| US12/805,316 Expired - Fee Related US8753757B2 (en) | 2000-03-27 | 2010-07-26 | Organic electroluminescence element |
| US14/268,485 Expired - Fee Related US9564607B2 (en) | 2000-03-27 | 2014-05-02 | Organic electroluminescence element |
| US15/378,759 Abandoned US20170098785A1 (en) | 2000-03-27 | 2016-12-14 | Organic electroluminescence element |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/816,415 Expired - Lifetime US6660410B2 (en) | 2000-03-27 | 2001-03-26 | Organic electroluminescence element |
Family Applications After (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/245,092 Expired - Lifetime US7226546B2 (en) | 2000-03-27 | 2005-10-07 | Organic electroluminescence element |
| US11/806,597 Abandoned US20070248841A1 (en) | 2000-03-27 | 2007-06-01 | Organic electroluminescence element |
| US12/805,316 Expired - Fee Related US8753757B2 (en) | 2000-03-27 | 2010-07-26 | Organic electroluminescence element |
| US14/268,485 Expired - Fee Related US9564607B2 (en) | 2000-03-27 | 2014-05-02 | Organic electroluminescence element |
| US15/378,759 Abandoned US20170098785A1 (en) | 2000-03-27 | 2016-12-14 | Organic electroluminescence element |
Country Status (8)
| Country | Link |
|---|---|
| US (7) | US6660410B2 (en) |
| EP (1) | EP1205527B2 (en) |
| JP (1) | JP4916078B2 (en) |
| KR (1) | KR100802757B1 (en) |
| CN (2) | CN1694591A (en) |
| DE (1) | DE60133797T3 (en) |
| TW (1) | TW532048B (en) |
| WO (1) | WO2001072927A1 (en) |
Cited By (45)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040213308A1 (en) * | 2003-04-23 | 2004-10-28 | Hiroko Abe | Laser oscillator |
| US20060046098A1 (en) * | 2000-03-27 | 2006-03-02 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US20070193624A1 (en) * | 2006-02-23 | 2007-08-23 | Guardian Industries Corp. | Indium zinc oxide based front contact for photovoltaic device and method of making same |
| US20070215889A1 (en) * | 2006-03-20 | 2007-09-20 | Semiconductor Energy Laboratory Co., Ltd. | Aromatic amine compound, and light-emitting element, light-emitting device, and electronic appliance using the aromatic amine compound |
| US20080105298A1 (en) * | 2006-11-02 | 2008-05-08 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US20080143250A1 (en) * | 2006-12-14 | 2008-06-19 | Novaled Ag | Organisches Leuchtbauelement |
| US20080163929A1 (en) * | 2007-01-08 | 2008-07-10 | Guardian Industries Corp. | Zinc oxide based front electrode doped with yttrium for use in photovoltaic device or the like |
| US20080220152A1 (en) * | 2007-03-09 | 2008-09-11 | Guardian Industries Corp. | Method of making a photovoltaic device with scratch-resistant coating and resulting product |
| US20080254318A1 (en) * | 2004-12-28 | 2008-10-16 | Semiconductor Energy Laboratory Co., Ltd. | Carbazole Derivative, and Light-Emitting Element and Light-Emitting Device Using the Carbzole Derivative |
| US20080271782A1 (en) * | 2007-05-01 | 2008-11-06 | Guardian Industries Corp. | Method of making a photovoltaic device or front substrate for use in same with scratch-resistant coating and resulting product |
| US20080295884A1 (en) * | 2007-05-29 | 2008-12-04 | Sharma Pramod K | Method of making a photovoltaic device or front substrate with barrier layer for use in same and resulting product |
| US20080308145A1 (en) * | 2007-06-12 | 2008-12-18 | Guardian Industries Corp | Front electrode including transparent conductive coating on etched glass substrate for use in photovoltaic device and method of making same |
| US20090009072A1 (en) * | 2005-12-23 | 2009-01-08 | Philipp Wellmann | Organic Light Emitting Device With a Plurality of Organic Electroluminescent Units Stacked Upon Each Other |
| US20090176107A1 (en) * | 2008-01-08 | 2009-07-09 | Guardian Industries Corp. | Method of making a temperable antiglare coating, and resulting products containing the same |
| US20100051923A1 (en) * | 2008-08-04 | 2010-03-04 | Novaled Ag | Organischer Feldeffekt Transistor |
| US20100084645A1 (en) * | 2005-03-23 | 2010-04-08 | Semiconductor Energy Laboratory Co., Ltd. | Composite material, and light emitting element and light emitting device using the composite material |
| US20100133992A1 (en) * | 2008-11-28 | 2010-06-03 | Samsung Electronics Co., Ltd. | Compound comprising phosphorescence unit, emitting polymer and organic emitting device comprising the emitting polymer |
| US7830089B2 (en) | 2005-12-23 | 2010-11-09 | Novaled Ag | Electronic device with a layer structure of organic layers |
| US7888594B2 (en) | 2007-11-20 | 2011-02-15 | Guardian Industries Corp. | Photovoltaic device including front electrode having titanium oxide inclusive layer with high refractive index |
| US7911129B2 (en) | 2005-04-13 | 2011-03-22 | Novaled Ag | Arrangement for an organic pin-type light-emitting diode and method for manufacturing |
| US7964788B2 (en) | 2006-11-02 | 2011-06-21 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US7986090B2 (en) | 2005-03-15 | 2011-07-26 | Novaled Ag | Light-emitting component |
| US8012317B2 (en) | 2006-11-02 | 2011-09-06 | Guardian Industries Corp. | Front electrode including transparent conductive coating on patterned glass substrate for use in photovoltaic device and method of making same |
| US8022291B2 (en) | 2008-10-15 | 2011-09-20 | Guardian Industries Corp. | Method of making front electrode of photovoltaic device having etched surface and corresponding photovoltaic device |
| US8071976B2 (en) | 2008-08-04 | 2011-12-06 | Novaled Ag | Organic field-effect transistor and circuit |
| US8076571B2 (en) | 2006-11-02 | 2011-12-13 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US8203073B2 (en) | 2006-11-02 | 2012-06-19 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US8254165B2 (en) | 2007-04-17 | 2012-08-28 | Novaled Ag | Organic electronic memory component, memory component arrangement and method for operating an organic electronic memory component |
| US8502200B2 (en) | 2006-01-11 | 2013-08-06 | Novaled Ag | Electroluminescent light-emitting device comprising an arrangement of organic layers, and method for its production |
| US8530063B2 (en) | 2009-10-21 | 2013-09-10 | Cheil Industries, Inc. | Compound for organic photoelectric device and organic photoelectric device including the same |
| US8569743B2 (en) | 2006-04-19 | 2013-10-29 | Novaled Ag | Light-emitting component |
| US8653537B2 (en) | 2004-08-13 | 2014-02-18 | Novaled Ag | Layer assembly for a light-emitting component |
| US9112175B2 (en) | 2005-12-21 | 2015-08-18 | Novaled Ag | Organic component |
| US9246109B2 (en) | 2012-10-19 | 2016-01-26 | Samsung Display Co., Ltd. | Compound and organic light emitting device including the same |
| US9252370B2 (en) | 2012-11-05 | 2016-02-02 | Samsung Display Co., Ltd. | Heterocyclic compounds and organic light emitting devices including the same |
| KR20160019745A (en) | 2014-08-12 | 2016-02-22 | (주)아이씨비 | Fluorene-carbazole compoound for hole transfer layer and organic electronic device comprising the same |
| KR20160019744A (en) | 2014-08-12 | 2016-02-22 | (주)아이씨비 | A process of preparing fluorene-carbazole compoound for hole transfer layer of organic electronic device |
| US9385325B2 (en) | 2012-11-05 | 2016-07-05 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light emitting device including the same |
| US9391280B2 (en) | 2013-01-30 | 2016-07-12 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US9608211B2 (en) | 2013-08-21 | 2017-03-28 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting diode including the same |
| US9773987B2 (en) | 2013-01-28 | 2017-09-26 | Samsung Display Co., Ltd. | Silicon-based compound and organic light-emitting diode comprising the same |
| US9960359B2 (en) | 2013-06-03 | 2018-05-01 | Samsung Display Co., Ltd. | Arylamine-based compound and organic light emitting diode comprising the same |
| US10340462B2 (en) | 2015-09-11 | 2019-07-02 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including same |
| US11114622B2 (en) | 2015-10-22 | 2021-09-07 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US12289994B2 (en) | 2019-11-06 | 2025-04-29 | Samsung Electronics Co., Ltd. | Photoelectric conversion device and sensor and electronic device |
Families Citing this family (595)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4514841B2 (en) * | 1998-02-17 | 2010-07-28 | 淳二 城戸 | Organic electroluminescent device |
| TW536836B (en) * | 2000-05-22 | 2003-06-11 | Semiconductor Energy Lab | Light emitting device and electrical appliance |
| US6893743B2 (en) * | 2000-10-04 | 2005-05-17 | Mitsubishi Chemical Corporation | Organic electroluminescent device |
| JP2002184581A (en) * | 2000-12-13 | 2002-06-28 | Sanyo Electric Co Ltd | Organic luminescent element |
| JP4438042B2 (en) | 2001-03-08 | 2010-03-24 | キヤノン株式会社 | Metal coordination compound, electroluminescent element and display device |
| US6723828B2 (en) | 2001-05-23 | 2004-04-20 | Sri International | Conjugated electroluminescent polymers and associated methods of preparation and use |
| US6855438B2 (en) * | 2001-06-15 | 2005-02-15 | Konica Corporation | Organic electroluminescent element and full color display |
| JP4003824B2 (en) * | 2001-07-11 | 2007-11-07 | 富士フイルム株式会社 | Light emitting element |
| JP2003133075A (en) * | 2001-07-25 | 2003-05-09 | Toray Ind Inc | Luminescent element |
| JP2003109768A (en) * | 2001-07-25 | 2003-04-11 | Toray Ind Inc | Light emitting element |
| US7037598B2 (en) * | 2001-08-07 | 2006-05-02 | Fuji Photo Film Co., Ltd. | Light-emitting element and novel iridium complexes |
| US7034339B2 (en) | 2001-08-09 | 2006-04-25 | Idemitsu Kosan Co., Ltd. | Organic EL display device having host compound and phosphorescent luminous compound, and method of driving same |
| US6734457B2 (en) * | 2001-11-27 | 2004-05-11 | Semiconductor Energy Laboratory Co., Ltd. | Light emitting device |
| US6863997B2 (en) * | 2001-12-28 | 2005-03-08 | The Trustees Of Princeton University | White light emitting OLEDs from combined monomer and aggregate emission |
| US6989273B2 (en) * | 2002-02-08 | 2006-01-24 | Canon Kabushiki Kaisha | Light emissive iridium (III) complexes |
| EP2770036B1 (en) | 2002-03-15 | 2017-12-20 | Idemitsu Kosan Co., Ltd | Material for organic electroluminescent devices and organic electroluminescent devices made by using the same |
| EP2169028B1 (en) * | 2002-03-22 | 2018-11-21 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescent devices and organic electroluminescent devices made by using the same |
| CN100366703C (en) * | 2002-03-22 | 2008-02-06 | 出光兴产株式会社 | Material for organic electroluminescent device and organic electroluminescent device using the same |
| KR20040094866A (en) * | 2002-03-25 | 2004-11-10 | 이데미쓰 고산 가부시키가이샤 | Material for organic electroluminescent element and organic electroluminescent element employing the same |
| US20030205696A1 (en) * | 2002-04-25 | 2003-11-06 | Canon Kabushiki Kaisha | Carbazole-based materials for guest-host electroluminescent systems |
| US7332739B2 (en) * | 2002-06-20 | 2008-02-19 | Samsung Sdi Co., Ltd. | Organic electroluminescent device using mixture of phosphorescent material as light-emitting substance |
| KR100483986B1 (en) * | 2002-06-20 | 2005-04-15 | 삼성에스디아이 주식회사 | Organic polymer electroluminescent display device using phosphresecnce mixture as emiting material |
| US7265378B2 (en) * | 2002-07-10 | 2007-09-04 | E. I. Du Pont De Nemours And Company | Electronic devices made with electron transport and/or anti-quenching layers |
| JP4103493B2 (en) * | 2002-08-13 | 2008-06-18 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element and display device |
| CN100449818C (en) * | 2002-09-20 | 2009-01-07 | 出光兴产株式会社 | organic electroluminescent element |
| JP4210093B2 (en) * | 2002-10-03 | 2009-01-14 | 大日本印刷株式会社 | Organic electroluminescent image display device |
| JPWO2004034751A1 (en) * | 2002-10-09 | 2006-02-09 | 出光興産株式会社 | Organic electroluminescence device |
| KR20050070071A (en) * | 2002-10-21 | 2005-07-05 | 이데미쓰 고산 가부시키가이샤 | Material for organic electroluminescence element, and organic electroluminescence element using the same |
| EP2762546B1 (en) * | 2002-11-26 | 2018-06-06 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, and display and illuminator |
| JP4254211B2 (en) * | 2002-11-26 | 2009-04-15 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element and display device |
| KR100515516B1 (en) * | 2002-11-30 | 2005-09-16 | 신동명 | Organic light emitting materials and Organic electroluminescence devices using the same |
| US6872475B2 (en) * | 2002-12-03 | 2005-03-29 | Canon Kabushiki Kaisha | Binaphthalene derivatives for organic electro-luminescent devices |
| KR101035780B1 (en) * | 2002-12-12 | 2011-05-20 | 이데미쓰 고산 가부시키가이샤 | Material for organic electroluminescent device and organic electroluminescent device using same |
| JP2006510212A (en) * | 2002-12-13 | 2006-03-23 | コーニンクレッカ フィリップス エレクトロニクス エヌ ヴィ | Organic electroluminescent constructs with triplet emitter complexes |
| CN100479628C (en) * | 2002-12-27 | 2009-04-15 | 富士胶片株式会社 | Organic electroluminescent device |
| JP4580342B2 (en) * | 2003-01-24 | 2010-11-10 | 出光興産株式会社 | Organic electroluminescence device |
| KR20050100673A (en) * | 2003-02-12 | 2005-10-19 | 코닌클리케 필립스 일렉트로닉스 엔.브이. | Carbazole compounds and use of such compounds in organic electroluminescent devices |
| US6781149B1 (en) * | 2003-02-14 | 2004-08-24 | Eastman Kodak Company | OLED device with a performance-enhancing layer |
| US6824950B2 (en) * | 2003-02-14 | 2004-11-30 | Eastman Kodak Company | Forming an oled device with a performance-inhancing layer |
| EP1595933A4 (en) * | 2003-02-20 | 2009-04-08 | Idemitsu Kosan Co | MATERIAL FOR ORGANIC ELECTROLUMINESCENT DEVICES, AND ORGANIC ELECTROLUMINESCENCE DEVICE COMPRISING SUCH MATERIAL |
| JP4158562B2 (en) * | 2003-03-12 | 2008-10-01 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element and display device |
| JP4401665B2 (en) * | 2003-03-20 | 2010-01-20 | 株式会社半導体エネルギー研究所 | Electroluminescent device |
| US20040252488A1 (en) * | 2003-04-01 | 2004-12-16 | Innovalight | Light-emitting ceiling tile |
| US7279832B2 (en) * | 2003-04-01 | 2007-10-09 | Innovalight, Inc. | Phosphor materials and illumination devices made therefrom |
| US6703180B1 (en) * | 2003-04-16 | 2004-03-09 | Eastman Kodak Company | Forming an improved stability emissive layer from a donor element in an OLED device |
| JP4635869B2 (en) * | 2003-04-23 | 2011-02-23 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element, lighting device, display device |
| US7014925B2 (en) | 2003-04-29 | 2006-03-21 | Canon Kabushiki Kaisha | Heterogeneous spiro compounds in organic light emitting device elements |
| US20040247933A1 (en) * | 2003-06-03 | 2004-12-09 | Canon Kabushiki Kaisha | Bipolar asymmetric carbazole-based host materials for electrophosphorescent guest-host OLED systems |
| JP2007501507A (en) * | 2003-08-01 | 2007-01-25 | シーディーティー オックスフォード リミテッド | Electroluminescent device |
| CN100335462C (en) | 2003-09-05 | 2007-09-05 | 清华大学 | Carbazole derivative and its application in electroluminescent device |
| US7745988B2 (en) * | 2003-09-05 | 2010-06-29 | Ricoh Company, Limited | 3, 6-diphenylcarbazole compound and organic electroluminescent device |
| JP4886975B2 (en) * | 2003-10-31 | 2012-02-29 | 株式会社リコー | Electroluminescent device |
| JP4762514B2 (en) * | 2003-09-05 | 2011-08-31 | 株式会社リコー | 3,6-diphenylcarbazole derivative |
| US20050072937A1 (en) * | 2003-10-02 | 2005-04-07 | Konica Minolta Medical & Graphic, Inc. | Radiation image conversion panel and preparation method thereof |
| JP4683829B2 (en) * | 2003-10-17 | 2011-05-18 | 淳二 城戸 | Organic electroluminescent device and manufacturing method thereof |
| KR100537620B1 (en) * | 2003-10-29 | 2005-12-19 | 삼성에스디아이 주식회사 | Carbazole containing compound and organic electroluminescent display device |
| US20060251918A1 (en) * | 2003-12-11 | 2006-11-09 | Toshihiro Iwakuma | Organic electroluminescent device material and organic electroluminescent device using same |
| WO2005057987A1 (en) * | 2003-12-15 | 2005-06-23 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescent device and organic electroluminescent device using same |
| EP1698679B1 (en) * | 2003-12-26 | 2011-04-06 | Idemitsu Kosan Co., Ltd. | Material for organic electroluminescent device and organic electroluminescent device using same |
| JPWO2005072017A1 (en) * | 2004-01-21 | 2007-12-27 | 出光興産株式会社 | HOST MATERIAL FOR ORGANIC ELECTROLUMINESCENT DEVICE AND ORGANIC ELECTROLUMINESCENT DEVICE |
| DE102004010954A1 (en) | 2004-03-03 | 2005-10-06 | Novaled Gmbh | Use of a metal complex as an n-dopant for an organic semiconductive matrix material, organic semiconductor material and electronic component |
| JP4351935B2 (en) * | 2004-03-10 | 2009-10-28 | 富士フイルム株式会社 | Organic electroluminescence device |
| US6921691B1 (en) * | 2004-03-18 | 2005-07-26 | Infineon Technologies Ag | Transistor with dopant-bearing metal in source and drain |
| KR100664390B1 (en) * | 2004-03-19 | 2007-01-02 | 주식회사 엘지화학 | New hole injection or transport material and organic light emitting device using the same |
| KR100565666B1 (en) * | 2004-03-22 | 2006-03-29 | 엘지전자 주식회사 | Organic light emitting diode |
| KR100669716B1 (en) * | 2004-07-14 | 2007-01-16 | 삼성에스디아이 주식회사 | Phenylcarbazole compound and organic electroluminescent device using same |
| KR100556422B1 (en) * | 2004-04-19 | 2006-03-03 | 엘지전자 주식회사 | Blue phosphorescent organic EL device |
| KR20070015574A (en) * | 2004-05-14 | 2007-02-05 | 이데미쓰 고산 가부시키가이샤 | Organic electroluminescent device |
| KR100751316B1 (en) * | 2004-06-25 | 2007-08-22 | 삼성에스디아이 주식회사 | Organic electroluminescent element |
| WO2006000390A2 (en) * | 2004-06-26 | 2006-01-05 | Merck Patent Gmbh | Compounds for organic electronic devices |
| JP4925569B2 (en) * | 2004-07-08 | 2012-04-25 | ローム株式会社 | Organic electroluminescent device |
| US7750352B2 (en) | 2004-08-10 | 2010-07-06 | Pinion Technologies, Inc. | Light strips for lighting and backlighting applications |
| US8852755B2 (en) * | 2004-08-13 | 2014-10-07 | Merck Patent Gmbh | Oxadiazole metallic complexes and their electronic and opto-electronic applications |
| JP4910279B2 (en) * | 2004-10-20 | 2012-04-04 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element, lighting device and display device |
| US20060088728A1 (en) * | 2004-10-22 | 2006-04-27 | Raymond Kwong | Arylcarbazoles as hosts in PHOLEDs |
| US7666524B2 (en) * | 2004-10-29 | 2010-02-23 | Semiconductor Energy Laboratory Co., Ltd. | Oligonaphthalene derivatives, and light-emitting element and light-emitting device using oligonaphthalene derivatives |
| CN1300140C (en) * | 2004-12-10 | 2007-02-14 | 吉林大学 | Compound of quinacridones-carbazole group and application in organic electroluminescence device |
| TWI242999B (en) * | 2004-12-22 | 2005-11-01 | Ind Tech Res Inst | Organometallic compound and organic electroluminescent device including the same |
| KR101420608B1 (en) | 2004-12-24 | 2014-07-18 | 미쓰비시 가가꾸 가부시키가이샤 | Organic compound, charge-transporting material, and organic electroluminescent element |
| TWI305798B (en) * | 2005-02-05 | 2009-02-01 | Au Optronics Corp | Compound and organic light emitting diode and display comprising the compound |
| CN100427467C (en) * | 2005-03-03 | 2008-10-22 | 友达光电股份有限公司 | Compound, organic light emitting diode comprising compound and display |
| CN1300280C (en) * | 2005-05-12 | 2007-02-14 | 苏州大学 | Metallic organic complex with tribo-luminescent performance and its preparation process |
| JPWO2006132012A1 (en) * | 2005-06-09 | 2009-01-08 | コニカミノルタホールディングス株式会社 | Organic electroluminescence element, lighting device and display device |
| GB2471606B (en) * | 2005-06-09 | 2011-07-13 | Konica Minolta Holdings Inc | Organic electroluminescent element,illuminator and display |
| CN100357271C (en) * | 2005-06-22 | 2007-12-26 | 中国科学院长春应用化学研究所 | Hole transport materials with 9-phenyl carbazole as core and process for making same |
| EP1910289A4 (en) * | 2005-08-04 | 2010-06-09 | Semiconductor Energy Lab | Carbazole derivative, light-emitting element material obtained by using carbazole derivative, light-emitting element, and electronic device |
| KR101193179B1 (en) * | 2005-08-26 | 2012-10-19 | 삼성디스플레이 주식회사 | Organosiloxane compound and organic electroluminescence device comprsing the same |
| EP2463352B1 (en) * | 2005-09-08 | 2018-05-02 | Toray Industries, Inc. | Light emitting device material and light emitting device |
| KR101193180B1 (en) * | 2005-11-14 | 2012-10-19 | 삼성디스플레이 주식회사 | A conducting polymer composition and an electronic device employing the layer obtained from the conducting polymer composition |
| KR101243917B1 (en) * | 2005-12-19 | 2013-03-14 | 삼성디스플레이 주식회사 | A conducting polymer composition and an electrical device employing the layer obtained from the conducting polymer composition |
| EP1998387B1 (en) | 2006-03-17 | 2015-04-22 | Konica Minolta Holdings, Inc. | Organic electroluminescent device, display and illuminating device |
| JP5683784B2 (en) | 2006-03-23 | 2015-03-11 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| JP2007269735A (en) | 2006-03-31 | 2007-10-18 | Canon Inc | Metal complex, light emitting element and display device |
| JP5055818B2 (en) | 2006-04-19 | 2012-10-24 | コニカミノルタホールディングス株式会社 | ORGANIC ELECTROLUMINESCENT ELEMENT MATERIAL, ORGANIC ELECTROLUMINESCENT ELEMENT, DISPLAY DEVICE AND LIGHTING DEVICE |
| WO2007148660A1 (en) | 2006-06-22 | 2007-12-27 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent device employing heterocycle-containing arylamine derivative |
| JP2008120769A (en) * | 2006-11-15 | 2008-05-29 | Bando Chem Ind Ltd | Novel aromatic tertiary amines and their use |
| KR20080047209A (en) * | 2006-11-24 | 2008-05-28 | 삼성전자주식회사 | Organic light emitting compound and organic light emitting device having the same |
| US8518559B2 (en) * | 2006-12-08 | 2013-08-27 | Agency For Science, Technology And Research | Arylamine compounds and electronic devices |
| EP2437326A3 (en) | 2006-12-13 | 2013-11-13 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, display device and lighting device |
| US8866377B2 (en) * | 2006-12-28 | 2014-10-21 | Universal Display Corporation | Long lifetime phosphorescent organic light emitting device (OLED) structures |
| KR100795817B1 (en) | 2007-02-20 | 2008-01-21 | 삼성에스디아이 주식회사 | Organic light emitting device, method for manufacturing same and method for forming organic layer |
| EP2129739B1 (en) * | 2007-03-28 | 2011-06-08 | FUJIFILM Corporation | Organic electroluminescent device |
| WO2008140114A1 (en) * | 2007-05-16 | 2008-11-20 | Konica Minolta Holdings, Inc. | Organic electroluminescence element, display device and illuminating device |
| US20100141125A1 (en) | 2007-05-16 | 2010-06-10 | Konica Minolta Holdings, Inc. | Organic electroluminescent element, display device and lighting device |
| WO2008156105A1 (en) * | 2007-06-21 | 2008-12-24 | Konica Minolta Holdings, Inc. | Organic electroluminescence element material, organic electroluminescence element, display device and illuminating device |
| DE102007031261A1 (en) | 2007-07-05 | 2009-01-08 | Universtität Regensburg | Luminescent metal complexes with bulky auxiliary ligands |
| JP5444594B2 (en) * | 2007-07-09 | 2014-03-19 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| US20090078933A1 (en) * | 2007-09-21 | 2009-03-26 | Lg Electronics Inc. | Organic light emitting device |
| KR20090048299A (en) * | 2007-11-08 | 2009-05-13 | 주식회사 엘지화학 | New organic light emitting device material and organic light emitting device using same |
| WO2009066779A1 (en) * | 2007-11-22 | 2009-05-28 | Idemitsu Kosan Co., Ltd. | Organic el element |
| US8221905B2 (en) | 2007-12-28 | 2012-07-17 | Universal Display Corporation | Carbazole-containing materials in phosphorescent light emitting diodes |
| US8040053B2 (en) | 2008-02-09 | 2011-10-18 | Universal Display Corporation | Organic light emitting device architecture for reducing the number of organic materials |
| DE102008013691A1 (en) | 2008-03-11 | 2009-09-17 | Merck Patent Gmbh | Use of compositions of neutral transition metal complexes in opto-electronic devices |
| US8128394B2 (en) * | 2008-03-26 | 2012-03-06 | Delta Engineered Plastics | Interchangeable mold tooling |
| JP2008219033A (en) * | 2008-04-14 | 2008-09-18 | Denso Corp | Organic el element |
| DE102008027005A1 (en) | 2008-06-05 | 2009-12-10 | Merck Patent Gmbh | Organic electronic device containing metal complexes |
| DE102008033563A1 (en) | 2008-07-17 | 2010-01-21 | Merck Patent Gmbh | Complexes with Small Singlet-Triplet Energy Intervals for Use in Opto-Electronic Devices (Singlet Harvesting Effect) |
| AU2009276744A1 (en) | 2008-07-28 | 2010-02-04 | Polymedix, Inc. | Anti-malarial compounds |
| DE102008036247A1 (en) | 2008-08-04 | 2010-02-11 | Merck Patent Gmbh | Electronic devices containing metal complexes |
| DE102008036982A1 (en) | 2008-08-08 | 2010-02-11 | Merck Patent Gmbh | Organic electroluminescent device |
| JP5698135B2 (en) * | 2008-09-04 | 2015-04-08 | ユニバーサル ディスプレイ コーポレイション | White phosphorescent organic light emitting device |
| DE102008048336A1 (en) | 2008-09-22 | 2010-03-25 | Merck Patent Gmbh | Mononuclear neutral copper (I) complexes and their use for the production of optoelectronic devices |
| DE102008050841B4 (en) | 2008-10-08 | 2019-08-01 | Merck Patent Gmbh | New materials for organic electroluminescent devices |
| DE102009022858A1 (en) | 2009-05-27 | 2011-12-15 | Merck Patent Gmbh | Organic electroluminescent devices |
| US8865321B2 (en) | 2008-11-11 | 2014-10-21 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102008057050B4 (en) | 2008-11-13 | 2021-06-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR101765511B1 (en) | 2008-11-17 | 2017-08-08 | 에스에프씨 주식회사 | Carbazole derivatives and organoelectroluminescent device using the same |
| DE102008063470A1 (en) | 2008-12-17 | 2010-07-01 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102008063490B4 (en) | 2008-12-17 | 2023-06-15 | Merck Patent Gmbh | Organic electroluminescent device and method for adjusting the color locus of a white-emitting electroluminescent device |
| DE102009007038A1 (en) | 2009-02-02 | 2010-08-05 | Merck Patent Gmbh | metal complexes |
| DE102009009277B4 (en) | 2009-02-17 | 2023-12-07 | Merck Patent Gmbh | Organic electronic device, process for its production and use of compounds |
| DE102009011223A1 (en) | 2009-03-02 | 2010-09-23 | Merck Patent Gmbh | metal complexes |
| DE102009012346B4 (en) | 2009-03-09 | 2024-02-15 | Merck Patent Gmbh | Organic electroluminescent device and method for producing the same |
| DE102009013041A1 (en) | 2009-03-13 | 2010-09-16 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009017064A1 (en) | 2009-04-09 | 2010-10-14 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102009023154A1 (en) | 2009-05-29 | 2011-06-16 | Merck Patent Gmbh | A composition comprising at least one emitter compound and at least one polymer having conjugation-interrupting units |
| DE102009023155A1 (en) | 2009-05-29 | 2010-12-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2010149259A2 (en) | 2009-06-22 | 2010-12-29 | Merck Patent Gmbh | Conducting formulation |
| DE102009031021A1 (en) | 2009-06-30 | 2011-01-05 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009032922B4 (en) | 2009-07-14 | 2024-04-25 | Merck Patent Gmbh | Materials for organic electroluminescent devices, processes for their preparation, their use and electronic device |
| US8581262B2 (en) | 2009-08-04 | 2013-11-12 | Merck Patent Gmbh | Electronic devices comprising multi cyclic hydrocarbons |
| KR101853143B1 (en) * | 2009-08-28 | 2018-04-27 | 호도가야 가가쿠 고교 가부시키가이샤 | Organic electroluminescent element and compound having carbazole ring structure |
| DE102009053645A1 (en) | 2009-11-17 | 2011-05-19 | Merck Patent Gmbh | Materials for organic electroluminescent device |
| DE102009041414A1 (en) | 2009-09-16 | 2011-03-17 | Merck Patent Gmbh | metal complexes |
| DE102009053644B4 (en) | 2009-11-17 | 2019-07-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009041289A1 (en) | 2009-09-16 | 2011-03-17 | Merck Patent Gmbh | Organic electroluminescent device |
| US9666806B2 (en) | 2009-09-16 | 2017-05-30 | Merck Patent Gmbh | Formulations for the production of electronic devices |
| DE102009042693A1 (en) | 2009-09-23 | 2011-03-24 | Merck Patent Gmbh | Materials for electronic devices |
| DE102009048791A1 (en) | 2009-10-08 | 2011-04-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009049587A1 (en) | 2009-10-16 | 2011-04-21 | Merck Patent Gmbh | metal complexes |
| KR101506999B1 (en) | 2009-11-03 | 2015-03-31 | 제일모직 주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| US8828561B2 (en) | 2009-11-03 | 2014-09-09 | Cheil Industries, Inc. | Compound for organic photoelectric device and organic photoelectric device including the same |
| DE102009053382A1 (en) | 2009-11-14 | 2011-05-19 | Merck Patent Gmbh | Materials for electronic devices |
| DE102009053836A1 (en) | 2009-11-18 | 2011-05-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102009057167A1 (en) | 2009-12-05 | 2011-06-09 | Merck Patent Gmbh | Electronic device containing metal complexes |
| WO2011076314A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Electroluminescent formulations |
| WO2011076326A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Electroluminescent functional surfactants |
| WO2011076323A1 (en) | 2009-12-22 | 2011-06-30 | Merck Patent Gmbh | Formulations comprising phase-separated functional materials |
| CN102668151B (en) | 2009-12-23 | 2015-06-17 | 默克专利有限公司 | Compositions comprising organic semiconducting compounds |
| JP2013516053A (en) | 2009-12-23 | 2013-05-09 | メルク パテント ゲーエムベーハー | Composition comprising a polymeric binder |
| KR101324788B1 (en) * | 2009-12-31 | 2013-10-31 | (주)씨에스엘쏠라 | Organic light device and organic light compound for the same |
| DE102010004803A1 (en) | 2010-01-16 | 2011-07-21 | Merck Patent GmbH, 64293 | Materials for organic electroluminescent devices |
| US8288187B2 (en) * | 2010-01-20 | 2012-10-16 | Universal Display Corporation | Electroluminescent devices for lighting applications |
| DE102010005697A1 (en) | 2010-01-25 | 2011-07-28 | Merck Patent GmbH, 64293 | Connections for electronic devices |
| DE102010009193B4 (en) | 2010-02-24 | 2022-05-19 | MERCK Patent Gesellschaft mit beschränkter Haftung | Composition containing fluorine-fluorine associates, processes for their production, their use and organic electronic devices containing them |
| DE102010009903A1 (en) | 2010-03-02 | 2011-09-08 | Merck Patent Gmbh | Connections for electronic devices |
| DE102010010481A1 (en) | 2010-03-06 | 2011-09-08 | Merck Patent Gmbh | Organic electroluminescent device |
| EP2544765A1 (en) | 2010-03-11 | 2013-01-16 | Merck Patent GmbH | Fibers in therapy and cosmetics |
| WO2011110275A2 (en) | 2010-03-11 | 2011-09-15 | Merck Patent Gmbh | Radiative fibers |
| EP2550691A1 (en) | 2010-03-23 | 2013-01-30 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| DE102010012738A1 (en) | 2010-03-25 | 2011-09-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010013068A1 (en) | 2010-03-26 | 2011-09-29 | Merck Patent Gmbh | Connections for electronic devices |
| US9379323B2 (en) | 2010-04-12 | 2016-06-28 | Merck Patent Gmbh | Composition having improved performance |
| WO2011128035A1 (en) | 2010-04-12 | 2011-10-20 | Merck Patent Gmbh | Composition and method for preparation of organic electronic devices |
| DE102010014933A1 (en) | 2010-04-14 | 2011-10-20 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010018321A1 (en) | 2010-04-27 | 2011-10-27 | Merck Patent Gmbh | Organic electroluminescent device |
| KR20110122051A (en) * | 2010-05-03 | 2011-11-09 | 제일모직주식회사 | Compound for organic photoelectric device and organic photoelectric device comprising same |
| CN105949177B (en) | 2010-05-03 | 2019-02-01 | 默克专利有限公司 | Preparation and electronic device |
| DE102010019306B4 (en) | 2010-05-04 | 2021-05-20 | Merck Patent Gmbh | Organic electroluminescent devices |
| DE102010020044A1 (en) | 2010-05-11 | 2011-11-17 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010020567A1 (en) | 2010-05-14 | 2011-11-17 | Merck Patent Gmbh | metal complexes |
| SG185680A1 (en) | 2010-05-27 | 2012-12-28 | Merck Patent Gmbh | Formulation and method for preparation of organic electronic devices |
| WO2011147522A1 (en) | 2010-05-27 | 2011-12-01 | Merck Patent Gmbh | Compositions comprising quantum dots |
| JP6054290B2 (en) | 2010-06-15 | 2016-12-27 | メルク パテント ゲーエムベーハー | Metal complex |
| DE102010024335A1 (en) | 2010-06-18 | 2011-12-22 | Merck Patent Gmbh | Connections for electronic devices |
| DE102010024542A1 (en) | 2010-06-22 | 2011-12-22 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010024897A1 (en) | 2010-06-24 | 2011-12-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010027218A1 (en) * | 2010-07-15 | 2012-01-19 | Merck Patent Gmbh | Organic complexes containing metals |
| DE102010027319A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | metal complexes |
| DE102010027317A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | metal complexes |
| DE102010027316A1 (en) | 2010-07-16 | 2012-01-19 | Merck Patent Gmbh | metal complexes |
| US20130226268A1 (en) | 2010-07-26 | 2013-08-29 | Merck Patent Gmbh | Nanocrystals in devices |
| EP2599141B1 (en) | 2010-07-26 | 2019-12-11 | Merck Patent GmbH | Quantum dots and hosts |
| KR101877582B1 (en) | 2010-07-30 | 2018-07-12 | 메르크 파텐트 게엠베하 | Organic electroluminescent device |
| KR101432599B1 (en) * | 2010-08-04 | 2014-08-21 | 제일모직주식회사 | Compound for organic photoelectric device and organic photoelectric device including the same |
| DE102010033548A1 (en) | 2010-08-05 | 2012-02-09 | Merck Patent Gmbh | Materials for electronic devices |
| CN102372669B (en) * | 2010-08-20 | 2014-02-26 | 清华大学 | A kind of phenylenediamine compound and its application |
| KR20120104086A (en) * | 2010-08-31 | 2012-09-20 | 이데미쓰 고산 가부시키가이샤 | Nitrogen-containing aromatic heterocyclic derivative and organic electroluminescent element using the same |
| JP2012077293A (en) | 2010-09-09 | 2012-04-19 | Ricoh Co Ltd | New carbazole polymer, and method for producing the same |
| DE102010045405A1 (en) | 2010-09-15 | 2012-03-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010046412B4 (en) | 2010-09-23 | 2022-01-13 | Merck Patent Gmbh | metal-ligand coordination compounds |
| DE102010046512A1 (en) | 2010-09-24 | 2012-03-29 | Merck Patent Gmbh | Phosphorus-containing metal complexes |
| DE102010048074A1 (en) | 2010-10-09 | 2012-04-12 | Merck Patent Gmbh | Materials for electronic devices |
| DE102010048608A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102010048607A1 (en) | 2010-10-15 | 2012-04-19 | Merck Patent Gmbh | Connections for electronic devices |
| CN103228647B (en) | 2010-11-24 | 2017-02-15 | 默克专利有限公司 | Materials for organic electroluminescent devices |
| WO2012077902A2 (en) | 2010-12-08 | 2012-06-14 | 제일모직 주식회사 | Compound for an organic optoelectronic device, organic light-emitting diode including the compound, and display device including the organic light-emitting diode |
| DE102010054316A1 (en) | 2010-12-13 | 2012-06-14 | Merck Patent Gmbh | Substituted tetraarylbenzenes |
| DE102010054525A1 (en) | 2010-12-15 | 2012-04-26 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102011106849A1 (en) | 2010-12-15 | 2012-06-21 | Merck Patent Gmbh | Process for the synthesis of N-N linked and around the N-N bond of rotation-inhibited bis-N-heterocyclic carbenes and their use as ligands for metal complexes |
| KR101478000B1 (en) * | 2010-12-21 | 2015-01-05 | 롬엔드하스전자재료코리아유한회사 | Novel organic electroluminescent compounds and organic electroluminescent device using the same |
| DE102010055902A1 (en) | 2010-12-23 | 2012-06-28 | Merck Patent Gmbh | Organic electroluminescent device |
| DE102010055901A1 (en) | 2010-12-23 | 2012-06-28 | Merck Patent Gmbh | Organic electroluminescent device |
| DE112011104715A5 (en) | 2011-01-13 | 2014-02-06 | Merck Patent Gmbh | Compounds for organic electroluminescent devices |
| DE102012000064A1 (en) | 2011-01-21 | 2012-07-26 | Merck Patent Gmbh | New heterocyclic compound excluding 9,10-dioxa-4b-aza-9a-phospha-indeno(1,2-a)indene and 9,10-dioxa-4b-aza-indeno(1,2-a)indene, useful e.g. as matrix material for fluorescent or phosphorescent emitters of electronic devices |
| US8751777B2 (en) | 2011-01-28 | 2014-06-10 | Honeywell International Inc. | Methods and reconfigurable systems to optimize the performance of a condition based health maintenance system |
| DE102011010841A1 (en) | 2011-02-10 | 2012-08-16 | Merck Patent Gmbh | (1,3) -dioxane-5-one compounds |
| DE102011011104A1 (en) | 2011-02-12 | 2012-08-16 | Merck Patent Gmbh | Substituted dibenzonaphthacenes |
| WO2012110178A1 (en) | 2011-02-14 | 2012-08-23 | Merck Patent Gmbh | Device and method for treatment of cells and cell tissue |
| DE102011011539A1 (en) | 2011-02-17 | 2012-08-23 | Merck Patent Gmbh | Connections for electronic devices |
| US9287512B2 (en) | 2011-03-08 | 2016-03-15 | Rohm And Haas Electronic Materials Korea Ltd. | Organic electroluminescent compounds, layers and organic electroluminescent device using the same |
| WO2012126566A1 (en) | 2011-03-24 | 2012-09-27 | Merck Patent Gmbh | Organic ionic functional materials |
| US9469667B2 (en) | 2011-04-04 | 2016-10-18 | Merck Patent Gmbh | Metal complexes |
| JP6038879B2 (en) | 2011-04-05 | 2016-12-07 | メルク パテント ゲーエムベーハー | Organic electroluminescent device |
| WO2012139693A1 (en) | 2011-04-13 | 2012-10-18 | Merck Patent Gmbh | Compounds for electronic devices |
| JP6271417B2 (en) | 2011-04-13 | 2018-01-31 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| KR101929602B1 (en) | 2011-04-18 | 2018-12-14 | 메르크 파텐트 게엠베하 | Compounds for electronic devices |
| CN103492383B (en) | 2011-04-18 | 2017-05-10 | 默克专利有限公司 | Materials for organic electroluminescent devices |
| WO2012149992A1 (en) | 2011-05-04 | 2012-11-08 | Merck Patent Gmbh | Device for preserving fresh goods |
| CN103503188B (en) | 2011-05-05 | 2016-08-31 | 默克专利有限公司 | compound for electronic device |
| JP6193215B2 (en) | 2011-05-05 | 2017-09-06 | メルク パテント ゲーエムベーハー | Compounds for electronic devices |
| EP2707911B1 (en) | 2011-05-12 | 2017-07-05 | Merck Patent GmbH | Compositions and electronic devices |
| DE102012007810A1 (en) | 2011-05-16 | 2012-11-22 | Merck Patent Gmbh | Electronic device, preferably organic electroluminescent device comprises a transition metal compound exhibiting a transition metal-tin bond |
| DE102011102586A1 (en) * | 2011-05-27 | 2012-11-29 | Merck Patent Gmbh | Organic electronic device |
| JP6092195B2 (en) | 2011-06-03 | 2017-03-08 | メルク パテント ゲーエムベーハー | Organic electroluminescence device |
| KR101972184B1 (en) | 2011-06-03 | 2019-04-24 | 메르크 파텐트 게엠베하 | Metal complexes |
| US9673402B2 (en) | 2011-06-28 | 2017-06-06 | Merck Patent Gmbh | Platinum metal complexes with divalent groups bridging two ligands |
| CN103718316B (en) | 2011-07-29 | 2017-11-10 | 默克专利有限公司 | Compounds for Electronic Devices |
| EP2740165B1 (en) | 2011-08-03 | 2018-11-07 | Merck Patent GmbH | Materials for electronic devices |
| JP6054394B2 (en) | 2011-08-10 | 2016-12-27 | メルク パテント ゲーエムベーハー | Metal complex |
| DE102012016192A1 (en) | 2011-08-19 | 2013-02-21 | Merck Patent Gmbh | New compounds capable of forming hydrogen bonds are useful in electronic device, e.g. organic electroluminescent device, organic light-emitting transistor and organic light-emitting electrochemical cell |
| CN103765623B (en) | 2011-08-22 | 2016-06-01 | 默克专利有限公司 | Organic electroluminescence device |
| EP2758372B1 (en) | 2011-09-21 | 2017-05-17 | Merck Patent GmbH | Carbazole derivatives for organic electroluminescent devices |
| US20130103123A1 (en) * | 2011-10-14 | 2013-04-25 | Sazzadur Rahman Khan | Light-Emitting Devices for Wound Healing |
| DE102011116165A1 (en) | 2011-10-14 | 2013-04-18 | Merck Patent Gmbh | Benzodioxepin-3-one compounds |
| WO2013056776A1 (en) | 2011-10-20 | 2013-04-25 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US9812643B2 (en) | 2011-10-27 | 2017-11-07 | Merck Patent Gmbh | Materials for electronic devices |
| DE102011117422A1 (en) | 2011-10-28 | 2013-05-02 | Merck Patent Gmbh | Hyperbranched polymers, process for their preparation and their use in electronic devices |
| EP2773721B1 (en) | 2011-11-01 | 2015-11-25 | Merck Patent GmbH | Organic electroluminescent device |
| WO2013083216A1 (en) | 2011-11-17 | 2013-06-13 | Merck Patent Gmbh | Spiro dihydroacridine derivatives and the use thereof as materials for organic electroluminescence devices |
| WO2013087142A1 (en) | 2011-12-12 | 2013-06-20 | Merck Patent Gmbh | Compounds for electronic devices |
| DE102012022880B4 (en) | 2011-12-22 | 2024-12-24 | Merck Patent Gmbh | Electronic devices containing organic layers |
| KR20140117440A (en) | 2011-12-27 | 2014-10-07 | 메르크 파텐트 게엠베하 | Metal complexes comprising 1,2,3-triazoles |
| CN106986858B (en) | 2012-01-16 | 2019-08-27 | 默克专利有限公司 | Metal-organic complex |
| JP6271442B2 (en) | 2012-01-30 | 2018-01-31 | メルク パテント ゲーエムベーハー | Nanocrystals on fiber |
| KR102045198B1 (en) | 2012-02-14 | 2019-11-15 | 메르크 파텐트 게엠베하 | Spirobifluorene compounds for organic electroluminescent devices |
| CN104205390B (en) | 2012-03-15 | 2017-08-25 | 默克专利有限公司 | Electronic device |
| CN104203955B (en) | 2012-03-23 | 2017-11-17 | 默克专利有限公司 | 9,9 for electroluminescent device,Spiral shell dioxoanthracene derivative |
| KR102082111B1 (en) | 2012-05-24 | 2020-02-27 | 메르크 파텐트 게엠베하 | Metal complexes comprising condensed heteroaromatic rings |
| DE102012011335A1 (en) | 2012-06-06 | 2013-12-12 | Merck Patent Gmbh | Connections for Organic Electronic Devices |
| JP6060530B2 (en) * | 2012-06-12 | 2017-01-18 | ソニー株式会社 | Organic electroluminescent device and display device |
| JP6262226B2 (en) | 2012-07-10 | 2018-01-17 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| KR102076481B1 (en) | 2012-07-13 | 2020-02-12 | 메르크 파텐트 게엠베하 | Metal complexes |
| CN104603111A (en) | 2012-07-23 | 2015-05-06 | 默克专利有限公司 | Fluorene and electronic devices containing said fluorene |
| CN104507932B (en) | 2012-07-23 | 2016-12-07 | 默克专利有限公司 | Material for organic electroluminescence device |
| CN108054293B (en) | 2012-07-23 | 2020-05-22 | 默克专利有限公司 | Derivatives of 2-diarylaminofluorene and organic electronic complexes containing said 2-diarylaminofluorene derivatives |
| US9595681B2 (en) | 2012-07-23 | 2017-03-14 | Merck Patent Gmbh | Compounds and organic electroluminescent devices |
| WO2014021571A1 (en) * | 2012-07-31 | 2014-02-06 | Sk Chemicals Co., Ltd. | Carbazole-based compound for organic electroluminescent device and organic electroluminescent device including the same |
| KR20140017428A (en) * | 2012-07-31 | 2014-02-11 | 에스케이케미칼주식회사 | Carbazole based compound for organic electroluminescent device and organic electroluminescent device including the same |
| CN104520308B (en) | 2012-08-07 | 2018-09-28 | 默克专利有限公司 | Metal complex |
| KR102265997B1 (en) | 2012-08-10 | 2021-06-16 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescence devices |
| KR101497138B1 (en) | 2012-08-21 | 2015-02-27 | 제일모직 주식회사 | Organic optoelectronic device and display including the same |
| EP2898042B1 (en) | 2012-09-18 | 2016-07-06 | Merck Patent GmbH | Materials for electronic devices |
| WO2014044347A1 (en) | 2012-09-20 | 2014-03-27 | Merck Patent Gmbh | Metal complexes |
| JP6250684B2 (en) | 2012-10-11 | 2017-12-20 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| DE102012020167A1 (en) | 2012-10-13 | 2014-04-17 | Eberhard Karls Universität Tübingen | metal complexes |
| JP6469579B2 (en) | 2012-10-31 | 2019-02-13 | メルク パテント ゲーエムベーハー | Electronic element |
| DE102012021650A1 (en) | 2012-11-03 | 2014-05-08 | Eberhard Karls Universität Tübingen | metal complexes |
| JP6306033B2 (en) | 2012-11-12 | 2018-04-04 | メルク パテント ゲーエムベーハー | Materials for electronic devices |
| EP2923391A1 (en) | 2012-11-20 | 2015-09-30 | Merck Patent GmbH | Formulation in high-purity solvent for producing electronic devices |
| US10065959B2 (en) | 2012-11-30 | 2018-09-04 | Merck Patent Gmbh | Electronic device |
| US20150329772A1 (en) | 2013-01-03 | 2015-11-19 | Merck Patent Gmbh | Materials for Electronic Devices |
| US20150340627A1 (en) | 2013-01-03 | 2015-11-26 | Merck Patent Gmbh | Materials for electronic devices |
| KR102232333B1 (en) * | 2013-03-22 | 2021-03-25 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| DE102013008189A1 (en) | 2013-05-14 | 2014-12-04 | Eberhard Karls Universität Tübingen | metal complexes |
| US20160163987A1 (en) | 2013-07-29 | 2016-06-09 | Merck Patent Gmbh | Electro-optical device and the use thereof |
| KR20250140117A (en) | 2013-07-30 | 2025-09-24 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| EP3027707B1 (en) | 2013-07-30 | 2019-12-11 | Merck Patent GmbH | Materials for electronic devices |
| CN105612164A (en) | 2013-10-02 | 2016-05-25 | 默克专利有限公司 | Boron-containing compounds for use in OLEDs |
| KR20160094430A (en) | 2013-12-06 | 2016-08-09 | 메르크 파텐트 게엠베하 | Compositions containing a polymeric binder which comprises acrylic and/or methacrylic acid ester units |
| JP6896422B2 (en) | 2013-12-06 | 2021-06-30 | メルク パテント ゲーエムベーハー | Compounds and organic electronic devices |
| CN105793246B (en) | 2013-12-06 | 2019-07-05 | 默克专利有限公司 | Substituted oxepin |
| EP3080229B1 (en) | 2013-12-12 | 2018-01-17 | Merck Patent GmbH | Materials for electronic devices |
| CN112851613A (en) | 2013-12-19 | 2021-05-28 | 默克专利有限公司 | Heterocyclic spiro compounds |
| CN103911145A (en) * | 2014-02-28 | 2014-07-09 | 烟台万润精细化工股份有限公司 | Novel OLED electron transport material and application thereof |
| US10208026B2 (en) | 2014-03-18 | 2019-02-19 | Universal Display Corporation | Organic electroluminescent materials and devices |
| US9768397B2 (en) | 2014-03-31 | 2017-09-19 | Commonwealth Scientific And Industrial Research Organisation | Phenylenediamine compounds for phosphorescent diazaborole metal complexes |
| AU2015201630A1 (en) | 2014-03-31 | 2015-10-15 | Commonwealth Scientific And Industrial Research Organisation | Diamine compounds for phosphorescent diazaborole metal complexes and electroluminescent devices |
| CN106255687B (en) | 2014-04-30 | 2020-06-05 | 默克专利有限公司 | Material for electronic devices |
| US10622565B2 (en) | 2014-05-05 | 2020-04-14 | Merck Patent Gmbh | Materials for organic light emitting devices |
| US10461260B2 (en) * | 2014-06-03 | 2019-10-29 | Universal Display Corporation | Organic electroluminescent materials and devices |
| DE102015006708A1 (en) | 2014-06-12 | 2015-12-17 | Merck Patent Gmbh | metal complexes |
| KR102300023B1 (en) * | 2014-06-13 | 2021-09-09 | 삼성디스플레이 주식회사 | Organic light-emitting devices and method for manufacturing the same |
| DE102014008722B4 (en) | 2014-06-18 | 2024-08-22 | Merck Patent Gmbh | Compositions for electronic devices, formulation containing them, use of the composition, use of the formulation and organic electronic device containing the composition |
| US10644246B2 (en) | 2014-06-25 | 2020-05-05 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US10749118B2 (en) | 2014-06-26 | 2020-08-18 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| EP2960315A1 (en) | 2014-06-27 | 2015-12-30 | cynora GmbH | Organic electroluminescent device |
| EP2963044A1 (en) | 2014-06-30 | 2016-01-06 | cynora GmbH | Binuclear metal (I) complexes with tetradentate ligands for optoelectronic applications |
| KR102313358B1 (en) | 2014-07-10 | 2021-10-18 | 삼성디스플레이 주식회사 | Organic light emitting device |
| JP6629291B2 (en) | 2014-07-21 | 2020-01-15 | メルク、パテント、ゲゼルシャフト、ミット、ベシュレンクテル、ハフツングMerck Patent GmbH | Materials for electronic devices |
| FR3024144B1 (en) * | 2014-07-28 | 2017-05-19 | Univ De Tours Francois-Rabelais | NOVEL SYNTHONS FOR THE PRODUCTION OF ORGANIC SEMICONDUCTORS |
| DE102014012818A1 (en) | 2014-08-28 | 2016-03-03 | Eberhard Karls Universität Tübingen | metal complexes |
| KR20160029187A (en) * | 2014-09-04 | 2016-03-15 | 삼성디스플레이 주식회사 | Organic light emitting diode and organic light emitting display device including the same |
| JP6695863B2 (en) | 2014-09-05 | 2020-05-20 | メルク パテント ゲーエムベーハー | Formulations and electronics |
| EP3204463B1 (en) | 2014-10-08 | 2019-04-17 | cynora GmbH | Metal complexes with tridentate ligands for optoelectronic applications |
| KR102496045B1 (en) | 2014-11-11 | 2023-02-03 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP3221422B1 (en) | 2014-11-18 | 2018-06-13 | cynora GmbH | Copper(i) complexes for optoelectronic applications |
| KR102493553B1 (en) | 2014-12-12 | 2023-01-30 | 메르크 파텐트 게엠베하 | Organic compounds with soluble groups |
| WO2016107663A1 (en) | 2014-12-30 | 2016-07-07 | Merck Patent Gmbh | Formulations and electronic devices |
| EP3247772B8 (en) * | 2015-01-20 | 2019-12-18 | cynora GmbH | Symmetrical organic molecules for use in optoelectronic components |
| DE102015108002A1 (en) * | 2015-01-20 | 2016-07-21 | Cynora Gmbh | Composition for use in optoelectronic devices |
| KR102610947B1 (en) | 2015-01-30 | 2023-12-06 | 메르크 파텐트 게엠베하 | Formulations with a low particle content |
| KR102626977B1 (en) | 2015-01-30 | 2024-01-19 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| EP3254317B1 (en) | 2015-02-03 | 2019-07-31 | Merck Patent GmbH | Metal complexes |
| CN104916790B (en) * | 2015-03-05 | 2017-05-03 | 上海道亦化工科技有限公司 | Organic electroluminescent device |
| JP6800879B2 (en) | 2015-03-30 | 2020-12-16 | メルク パテント ゲーエムベーハー | Formulations of organic functional materials containing siloxane solvents |
| US9478758B1 (en) * | 2015-05-08 | 2016-10-25 | Universal Display Corporation | Organic electroluminescent materials and devices |
| JP2018525331A (en) | 2015-06-10 | 2018-09-06 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescent devices |
| EP3581633B1 (en) | 2015-06-12 | 2021-01-27 | Merck Patent GmbH | Esters containing non-aromatic cycles as solvents for oled formulations |
| US20180212166A1 (en) | 2015-07-15 | 2018-07-26 | Merck Patent Gmbh | Composition comprising organic semiconducting compounds |
| US20180219156A1 (en) | 2015-07-22 | 2018-08-02 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| GB201513037D0 (en) | 2015-07-23 | 2015-09-09 | Merck Patent Gmbh | Phenyl-derived compound for use in organic electronic devices |
| CN113248392B (en) | 2015-07-29 | 2024-04-16 | 默克专利有限公司 | Materials for organic electroluminescent devices |
| EP4301110A3 (en) | 2015-07-30 | 2024-04-10 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| JP6862420B2 (en) | 2015-08-13 | 2021-04-21 | メルク パテント ゲーエムベーハー | Hexamethyl indane |
| JP6786591B2 (en) | 2015-08-14 | 2020-11-18 | メルク、パテント、ゲゼルシャフト、ミット、ベシュレンクテル、ハフツングMerck Patent GmbH | Phenoxazine derivatives for organic electroluminescence devices |
| WO2017036573A1 (en) | 2015-08-28 | 2017-03-09 | Merck Patent Gmbh | Compounds for electronic devices |
| EP3341981B1 (en) | 2015-08-28 | 2020-08-19 | Merck Patent GmbH | Formulation of an organic functional material comprising an epoxy group containing solvent |
| JP6112166B2 (en) * | 2015-09-14 | 2017-04-12 | コニカミノルタ株式会社 | Organic electroluminescence element, display device and lighting device |
| DE102015013381A1 (en) | 2015-10-14 | 2017-04-20 | Eberhard Karls Universität Tübingen | metal complexes |
| JP6974315B2 (en) | 2015-10-27 | 2021-12-01 | メルク パテント ゲーエムベーハー | Materials for organic electroluminescence devices |
| WO2017097391A1 (en) | 2015-12-10 | 2017-06-15 | Merck Patent Gmbh | Formulations containing ketones comprising non-aromatic cycles |
| DE102015016016A1 (en) | 2015-12-10 | 2017-06-14 | Eberhard Karls Universität Tübingen | metal complexes |
| US11171294B2 (en) | 2015-12-15 | 2021-11-09 | Merck Patent Gmbh | Esters containing aromatic groups as solvents for organic electronic formulations |
| CN108431143A (en) | 2015-12-16 | 2018-08-21 | 默克专利有限公司 | Formulations containing solid solvents |
| EP3390550B1 (en) | 2015-12-16 | 2022-09-28 | Merck Patent GmbH | Formulations containing a mixture of at least two different solvents |
| KR101940169B1 (en) | 2016-02-04 | 2019-01-18 | 삼성에스디아이 주식회사 | Organic compound for optoelectric device and organic optoelectric device and display device |
| CN108603107B (en) | 2016-02-05 | 2022-08-26 | 默克专利有限公司 | Material for electronic devices |
| US10840448B2 (en) | 2016-02-17 | 2020-11-17 | Merck Patent Gmbh | Formulation of an organic functional material |
| WO2017148564A1 (en) | 2016-03-03 | 2017-09-08 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| DE102016003104A1 (en) | 2016-03-15 | 2017-09-21 | Merck Patent Gmbh | Container comprising a formulation containing at least one organic semiconductor |
| TWI821807B (en) | 2016-03-17 | 2023-11-11 | 德商麥克專利有限公司 | Compounds having spirobifluorene structures |
| KR102740104B1 (en) | 2016-04-11 | 2024-12-06 | 메르크 파텐트 게엠베하 | Heterocyclic compounds comprising dibenzofuran and/or dibenzothiopene structures |
| KR102385482B1 (en) | 2016-04-29 | 2022-04-12 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| WO2017204594A1 (en) * | 2016-05-27 | 2017-11-30 | 주식회사 엘지화학 | Organic light emitting element |
| CN116283863A (en) | 2016-06-03 | 2023-06-23 | 默克专利有限公司 | Materials for Organic Electroluminescent Devices |
| JP2019523997A (en) | 2016-06-16 | 2019-08-29 | メルク パテント ゲーエムベーハー | Formulation of organic functional materials |
| EP3472249B1 (en) | 2016-06-17 | 2022-02-02 | Merck Patent GmbH | Formulation of an organic functional material |
| TW201815998A (en) | 2016-06-28 | 2018-05-01 | 德商麥克專利有限公司 | Organic functional material formulation |
| CN109563115B (en) | 2016-06-30 | 2021-10-01 | 默克专利有限公司 | Method for separating enantiomeric mixtures of metal complexes |
| KR102599160B1 (en) | 2016-07-08 | 2023-11-07 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| KR102432968B1 (en) | 2016-07-14 | 2022-08-16 | 메르크 파텐트 게엠베하 | metal complex |
| JP7030781B2 (en) | 2016-07-25 | 2022-03-07 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Use of metal complexes as illuminants in organic electroluminescence devices |
| CN109496216A (en) | 2016-07-25 | 2019-03-19 | 默克专利有限公司 | Double-core comprising two tooth Asia ligand of tripodia and few core metal complex and its purposes in electronic device |
| JP6980757B2 (en) | 2016-08-04 | 2021-12-15 | メルク パテント ゲーエムベーハー | Formulation of organic functional materials |
| WO2018041769A1 (en) | 2016-08-30 | 2018-03-08 | Merck Patent Gmbh | Binuclear and trinuclear metal complexes composed of two inter-linked tripodal hexadentate ligands for use in electroluminescent devices |
| US11447464B2 (en) | 2016-09-14 | 2022-09-20 | Merck Patent Gmbh | Compounds with spirobifluorene-structures |
| EP3512848B1 (en) | 2016-09-14 | 2020-11-18 | Merck Patent GmbH | Compounds with carbazole structures |
| WO2018054798A1 (en) | 2016-09-21 | 2018-03-29 | Merck Patent Gmbh | Binuclear metal complexes for use as emitters in organic electroluminescent devices |
| WO2018060218A1 (en) | 2016-09-30 | 2018-04-05 | Merck Patent Gmbh | Carbazoles with diazadibenzofurane or diazadibenzothiophene structures |
| TWI766884B (en) | 2016-09-30 | 2022-06-11 | 德商麥克專利有限公司 | Compounds having diazadibenzofuran or diazadibenzothiophene structures, process for preparing the same and use thereof |
| TWI764942B (en) | 2016-10-10 | 2022-05-21 | 德商麥克專利有限公司 | Electronic device |
| EP3526227B1 (en) | 2016-10-12 | 2020-06-03 | Merck Patent GmbH | Binuclear metal complexes and electronic devices, in particular organic electroluminescent devices containing said metal complexes |
| KR102472248B1 (en) | 2016-10-12 | 2022-11-29 | 메르크 파텐트 게엠베하 | metal complex |
| CN109790192A (en) | 2016-10-13 | 2019-05-21 | 默克专利有限公司 | Metal complex |
| DE102017008794A1 (en) | 2016-10-17 | 2018-04-19 | Merck Patent Gmbh | Materials for use in electronic devices |
| CN109863157A (en) | 2016-10-25 | 2019-06-07 | 默克专利有限公司 | metal complex |
| JP7013459B2 (en) | 2016-10-31 | 2022-01-31 | メルク パテント ゲーエムベーハー | Formulation of organic functional materials |
| WO2018077660A1 (en) | 2016-10-31 | 2018-05-03 | Merck Patent Gmbh | Formulation of an organic functional material |
| TWI745467B (en) | 2016-11-02 | 2021-11-11 | 德商麥克專利有限公司 | Materials for electronic devices |
| CN110088112A (en) | 2016-11-08 | 2019-08-02 | 默克专利有限公司 | Compound for electronic device |
| KR102474328B1 (en) | 2016-11-09 | 2022-12-06 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| TWI756292B (en) | 2016-11-14 | 2022-03-01 | 德商麥克專利有限公司 | Compounds having an acceptor group and a donor group |
| EP3541890B1 (en) | 2016-11-17 | 2020-10-28 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW201833118A (en) | 2016-11-22 | 2018-09-16 | 德商麥克專利有限公司 | Materials for electronic devices |
| TW201829385A (en) | 2016-11-30 | 2018-08-16 | 德商麥克專利有限公司 | a compound having a valeramine structure |
| TW201831468A (en) | 2016-12-05 | 2018-09-01 | 德商麥克專利有限公司 | Nitrogen-containing heterocyclic compound |
| WO2018104193A1 (en) | 2016-12-05 | 2018-06-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| EP3548486B1 (en) | 2016-12-05 | 2021-10-27 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US10892414B2 (en) | 2016-12-06 | 2021-01-12 | Merck Patent Gmbh | Process for making electronic device |
| CN110168047B (en) | 2016-12-13 | 2023-08-08 | 默克专利有限公司 | Preparation of organic functional material |
| WO2018114744A1 (en) | 2016-12-20 | 2018-06-28 | Merck Patent Gmbh | A white light emitting solid state light source |
| EP3559078B1 (en) | 2016-12-22 | 2023-10-25 | Merck Patent GmbH | Materials for electronic devices |
| CN110088925A (en) | 2016-12-22 | 2019-08-02 | 默克专利有限公司 | Mixture comprising at least two organic functions chemical combination objects |
| US11495751B2 (en) | 2017-01-04 | 2022-11-08 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR102625926B1 (en) | 2017-01-25 | 2024-01-17 | 메르크 파텐트 게엠베하 | Carbazole derivatives |
| TWI763772B (en) | 2017-01-30 | 2022-05-11 | 德商麥克專利有限公司 | Method for forming an organic element of an electronic device |
| TWI791481B (en) | 2017-01-30 | 2023-02-11 | 德商麥克專利有限公司 | Method for forming an organic electroluminescence (el) element |
| EP3573985B1 (en) | 2017-01-30 | 2023-01-18 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| EP3577101B1 (en) | 2017-02-02 | 2021-03-03 | Merck Patent GmbH | Materials for electronic devices |
| TW201835075A (en) | 2017-02-14 | 2018-10-01 | 德商麥克專利有限公司 | Material for organic electroluminescent device |
| EP3590141B1 (en) | 2017-03-01 | 2025-04-30 | Merck Patent GmbH | Organic electroluminescent device |
| WO2018157981A1 (en) | 2017-03-02 | 2018-09-07 | Merck Patent Gmbh | Materials for organic electronic devices |
| TW201843143A (en) | 2017-03-13 | 2018-12-16 | 德商麥克專利有限公司 | Compound containing an arylamine structure |
| KR102539248B1 (en) | 2017-03-15 | 2023-06-02 | 메르크 파텐트 게엠베하 | Materials for Organic Electroluminescent Devices |
| WO2018177981A1 (en) | 2017-03-29 | 2018-10-04 | Merck Patent Gmbh | Aromatic compounds |
| KR20190131554A (en) | 2017-03-31 | 2019-11-26 | 메르크 파텐트 게엠베하 | Printing method for organic light emitting diodes (OLED) |
| CN110494514A (en) | 2017-04-10 | 2019-11-22 | 默克专利有限公司 | The preparation of organic functional material |
| US11778907B2 (en) | 2017-04-13 | 2023-10-03 | Merck Patent Gmbh | Composition for organic electronic devices |
| KR102585423B1 (en) | 2017-04-25 | 2023-10-05 | 메르크 파텐트 게엠베하 | Compounds for electronic devices |
| JP7330898B2 (en) | 2017-05-03 | 2023-08-22 | メルク パテント ゲーエムベーハー | Formulation of organic functional material |
| WO2018206526A1 (en) | 2017-05-11 | 2018-11-15 | Merck Patent Gmbh | Organoboron complexes for organic electroluminescent devices |
| KR102592390B1 (en) | 2017-05-11 | 2023-10-20 | 메르크 파텐트 게엠베하 | Carbazole-based bodypiece for organic electroluminescent devices |
| CN110637017A (en) | 2017-05-22 | 2019-12-31 | 默克专利有限公司 | Hexacyclo heteroaromatic compounds for electronic devices |
| TW201920343A (en) | 2017-06-21 | 2019-06-01 | 德商麥克專利有限公司 | Materials for electronic devices |
| WO2018234346A1 (en) | 2017-06-23 | 2018-12-27 | Merck Patent Gmbh | MATERIALS FOR ORGANIC ELECTROLUMINESCENT DEVICES |
| KR20200022010A (en) | 2017-06-26 | 2020-03-02 | 메르크 파텐트 게엠베하 | Homogeneous mixture |
| WO2019002190A1 (en) | 2017-06-28 | 2019-01-03 | Merck Patent Gmbh | Materials for electronic devices |
| TWI813576B (en) | 2017-07-03 | 2023-09-01 | 德商麥克專利有限公司 | Formulations with a low content of phenol type impurities |
| CN110785415A (en) | 2017-07-05 | 2020-02-11 | 默克专利有限公司 | Composition for organic electronic device |
| KR102653984B1 (en) | 2017-07-05 | 2024-04-02 | 메르크 파텐트 게엠베하 | Compositions for organic electronic devices |
| CN110892543B (en) | 2017-07-18 | 2023-07-28 | 默克专利有限公司 | Formulation of organic functional materials |
| TWI776926B (en) | 2017-07-25 | 2022-09-11 | 德商麥克專利有限公司 | Metal complexes |
| EP3658541A1 (en) | 2017-07-28 | 2020-06-03 | Merck Patent GmbH | Spirobifluorene derivatives for use in electronic devices |
| WO2019030604A1 (en) * | 2017-08-10 | 2019-02-14 | 株式会社半導体エネルギー研究所 | Organic compound, light-emitting element, light-emitting device, electronic device, and lighting apparatus |
| CN118405982A (en) | 2017-09-08 | 2024-07-30 | 默克专利有限公司 | Materials for electronic devices |
| US11370965B2 (en) | 2017-09-12 | 2022-06-28 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN111164086A (en) | 2017-10-06 | 2020-05-15 | 默克专利有限公司 | Materials for organic electroluminescent devices |
| CN108675975A (en) | 2017-10-17 | 2018-10-19 | 默克专利有限公司 | Material for organic electroluminescence device |
| CN111225919A (en) | 2017-10-24 | 2020-06-02 | 默克专利有限公司 | Materials for organic electroluminescent devices |
| TWI785142B (en) | 2017-11-14 | 2022-12-01 | 德商麥克專利有限公司 | Composition for organic electronic devices |
| KR102777515B1 (en) | 2017-11-23 | 2025-03-07 | 메르크 파텐트 게엠베하 | Materials for electronic devices |
| TWI838352B (en) | 2017-11-24 | 2024-04-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI820057B (en) | 2017-11-24 | 2023-11-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI791701B (en) | 2017-12-13 | 2023-02-11 | 德商麥克專利有限公司 | Metal complexes |
| US20200308129A1 (en) | 2017-12-15 | 2020-10-01 | Merck Patent Gmbh | Substituted aromatic amines for use in organic electroluminescent devices |
| JP7293229B2 (en) | 2017-12-15 | 2023-06-19 | メルク パテント ゲーエムベーハー | Formulation of organic functional material |
| TW201938562A (en) | 2017-12-19 | 2019-10-01 | 德商麥克專利有限公司 | Heterocyclic compounds |
| JP7402800B2 (en) | 2017-12-20 | 2023-12-21 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | heteroaromatic compounds |
| TWI811290B (en) | 2018-01-25 | 2023-08-11 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI820084B (en) | 2018-02-13 | 2023-11-01 | 愛爾蘭商Udc愛爾蘭責任有限公司 | Metal complexes, process for preparation of the same, use thereof, and electronic devices comprising the same |
| JP7247231B2 (en) | 2018-02-26 | 2023-03-28 | メルク パテント ゲーエムベーハー | Formulation of organic functional material |
| TWI802656B (en) | 2018-03-06 | 2023-05-21 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TW201938761A (en) | 2018-03-06 | 2019-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20200132912A (en) | 2018-03-16 | 2020-11-25 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| TWI828664B (en) | 2018-03-19 | 2024-01-11 | 愛爾蘭商Udc愛爾蘭責任有限公司 | Metal complexes |
| TWI713848B (en) * | 2018-05-18 | 2020-12-21 | 國立交通大學 | Organic light-emitting device |
| CN112166112A (en) | 2018-05-30 | 2021-01-01 | 默克专利有限公司 | Compositions for organic electronic devices |
| KR20210018438A (en) | 2018-06-07 | 2021-02-17 | 메르크 파텐트 게엠베하 | Organic electroluminescent device |
| CN112236488A (en) | 2018-06-15 | 2021-01-15 | 默克专利有限公司 | Formulation of organic functional materials |
| WO2020011686A1 (en) | 2018-07-09 | 2020-01-16 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR102789335B1 (en) | 2018-07-20 | 2025-03-31 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| EP3844243B1 (en) | 2018-08-28 | 2022-06-22 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TWI823993B (en) | 2018-08-28 | 2023-12-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TWI837167B (en) | 2018-08-28 | 2024-04-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| EP3850055A1 (en) | 2018-09-12 | 2021-07-21 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| TW202030902A (en) | 2018-09-12 | 2020-08-16 | 德商麥克專利有限公司 | Electroluminescent devices |
| TWI826522B (en) | 2018-09-12 | 2023-12-21 | 德商麥克專利有限公司 | Electroluminescent devices |
| JP2022502829A (en) | 2018-09-24 | 2022-01-11 | メルク パテント ゲーエムベーハー | Methods for Producing Granular Materials |
| EP4601449A3 (en) | 2018-09-27 | 2025-09-17 | Merck Patent GmbH | Compounds usable as active compounds in an organic electronic device |
| KR102799882B1 (en) | 2018-09-27 | 2025-04-23 | 메르크 파텐트 게엠베하 | Method for producing sterically hindered nitrogen heteroaromatic compounds |
| CN112930343A (en) | 2018-10-31 | 2021-06-08 | 默克专利有限公司 | Material for organic electroluminescent device |
| JP2022506382A (en) | 2018-11-05 | 2022-01-17 | メルク パテント ゲーエムベーハー | Compounds that can be used in organic electronic devices |
| EP3877373B1 (en) | 2018-11-06 | 2023-01-11 | Merck Patent GmbH | 5,6-diphenyl-5,6-dihydro-dibenz[c,e][1,2]azaphosphorine and 6-phenyl-6h-dibenzo[c,e][1,2]thiazine-5,5-dioxide derivatives and related compounds as organic electroluminescent materials for oleds |
| KR20210083347A (en) | 2018-11-06 | 2021-07-06 | 메르크 파텐트 게엠베하 | Method of Forming Organic Elements of Electronic Devices |
| WO2020099349A1 (en) | 2018-11-14 | 2020-05-22 | Merck Patent Gmbh | Compounds that can be used for producing an organic electronic device |
| US12035625B2 (en) | 2018-11-15 | 2024-07-09 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202039493A (en) | 2018-12-19 | 2020-11-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR102702683B1 (en) | 2018-12-28 | 2024-09-03 | 엘지디스플레이 주식회사 | Organic light emitting diode and organic light emitting device including the same |
| WO2020148243A1 (en) | 2019-01-16 | 2020-07-23 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TWI850329B (en) | 2019-02-11 | 2024-08-01 | 愛爾蘭商Udc愛爾蘭責任有限公司 | Metal complexes |
| EP3928360A1 (en) | 2019-02-18 | 2021-12-29 | Merck Patent GmbH | Composition for organic electronic devices |
| US20220127286A1 (en) | 2019-03-04 | 2022-04-28 | Merck Patent Gmbh | Ligands for nano-sized materials |
| EP3938367A1 (en) | 2019-03-12 | 2022-01-19 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| KR20210141593A (en) | 2019-03-20 | 2021-11-23 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| WO2020193447A1 (en) | 2019-03-25 | 2020-10-01 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR102769410B1 (en) | 2019-04-11 | 2025-02-17 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| KR20210151905A (en) | 2019-04-15 | 2021-12-14 | 메르크 파텐트 게엠베하 | metal complex |
| WO2021037401A1 (en) | 2019-08-26 | 2021-03-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US12108665B2 (en) | 2019-09-02 | 2024-10-01 | Merck Kgaa | Materials for organic electroluminescent devices |
| TW202122558A (en) | 2019-09-03 | 2021-06-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| CN114450286A (en) | 2019-09-16 | 2022-05-06 | 默克专利有限公司 | Material of organic electroluminescent device |
| CN114342103A (en) | 2019-09-19 | 2022-04-12 | 默克专利有限公司 | Mixture of two host materials and organic electroluminescent device comprising the same |
| EP4031549A1 (en) | 2019-09-20 | 2022-07-27 | Merck Patent GmbH | Peri-condensed heterocyclic compounds as materials for electronic devices |
| EP4049325A1 (en) | 2019-10-22 | 2022-08-31 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| EP4048675A1 (en) | 2019-10-25 | 2022-08-31 | Merck Patent GmbH | Compounds that can be used in an organic electronic device |
| WO2021089450A1 (en) | 2019-11-04 | 2021-05-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202134252A (en) | 2019-11-12 | 2021-09-16 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20220110534A (en) | 2019-12-04 | 2022-08-08 | 메르크 파텐트 게엠베하 | metal complex |
| TW202136181A (en) | 2019-12-04 | 2021-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| TW202136471A (en) | 2019-12-17 | 2021-10-01 | 德商麥克專利有限公司 | Materials for organic electroluminescent devices |
| KR20220116013A (en) | 2019-12-18 | 2022-08-19 | 메르크 파텐트 게엠베하 | Aromatic compounds for organic electroluminescent devices |
| US20230104248A1 (en) | 2019-12-19 | 2023-04-06 | Merck Patent Gmbh | Polycyclic compounds for organic electroluminescent devices |
| US20230139809A1 (en) | 2020-01-29 | 2023-05-04 | Merck Patent Gmbh | Benzimidazole derivatives |
| WO2021170522A1 (en) | 2020-02-25 | 2021-09-02 | Merck Patent Gmbh | Use of heterocyclic compounds in an organic electronic device |
| WO2021175706A1 (en) | 2020-03-02 | 2021-09-10 | Merck Patent Gmbh | Use of sulfone compounds in an organic electronic device |
| WO2021185829A1 (en) | 2020-03-17 | 2021-09-23 | Merck Patent Gmbh | Heterocyclic compounds for organic electroluminescent devices |
| CN115298187A (en) | 2020-03-17 | 2022-11-04 | 默克专利有限公司 | Heteroaromatic compounds for organic electroluminescent devices |
| KR20220157456A (en) | 2020-03-23 | 2022-11-29 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| KR20220158017A (en) | 2020-03-24 | 2022-11-29 | 메르크 파텐트 게엠베하 | Materials for Electronic Devices |
| EP4126880A1 (en) | 2020-03-26 | 2023-02-08 | Merck Patent GmbH | Cyclic compounds for organic electroluminescent devices |
| US20230151026A1 (en) | 2020-04-02 | 2023-05-18 | Merck Patent Gmbh | Multi-layer body for diffuse transillumination |
| WO2021204646A1 (en) | 2020-04-06 | 2021-10-14 | Merck Patent Gmbh | Polycyclic compounds for organic electroluminescent devices |
| KR20230002655A (en) | 2020-04-21 | 2023-01-05 | 메르크 파텐트 게엠베하 | Formulation of Organic Functional Materials |
| CN115700058A (en) | 2020-04-21 | 2023-02-03 | 默克专利有限公司 | Emulsions containing organic functional materials |
| TW202208594A (en) | 2020-05-27 | 2022-03-01 | 德商麥克專利有限公司 | Materials for electronic devices |
| CN115916767A (en) | 2020-06-18 | 2023-04-04 | 默克专利有限公司 | Indenonazanaphthalenes |
| WO2021259824A1 (en) | 2020-06-23 | 2021-12-30 | Merck Patent Gmbh | Method for producing a mixture |
| JP2023531470A (en) | 2020-06-29 | 2023-07-24 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Heteroaromatic compounds for organic electroluminescent devices |
| CN115916794A (en) | 2020-06-29 | 2023-04-04 | 默克专利有限公司 | Heterocyclic compounds for organic electroluminescent devices |
| WO2022017997A1 (en) | 2020-07-22 | 2022-01-27 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| US20230292596A1 (en) | 2020-08-06 | 2023-09-14 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202233641A (en) | 2020-08-13 | 2022-09-01 | 德商麥克專利有限公司 | Metal complexes |
| KR20230053629A (en) | 2020-08-18 | 2023-04-21 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| KR20230053633A (en) | 2020-08-19 | 2023-04-21 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| JP2023542915A (en) | 2020-09-18 | 2023-10-12 | 三星ディスプレイ株式會社 | organic electroluminescent device |
| EP4222155B1 (en) | 2020-09-29 | 2025-06-11 | UDC Ireland Limited | Mononuclear tripodal hexadentate iridium complexes for use in oleds |
| TW202229215A (en) | 2020-09-30 | 2022-08-01 | 德商麥克專利有限公司 | Compounds for structuring of functional layers of organic electroluminescent devices |
| TW202222748A (en) | 2020-09-30 | 2022-06-16 | 德商麥克專利有限公司 | Compounds usable for structuring of functional layers of organic electroluminescent devices |
| EP4229064A1 (en) | 2020-10-16 | 2023-08-23 | Merck Patent GmbH | Heterocyclic compounds for organic electroluminescent devices |
| CN116406414A (en) | 2020-10-16 | 2023-07-07 | 默克专利有限公司 | Compounds containing heteroatoms for organic electroluminescent devices |
| WO2022101171A1 (en) | 2020-11-10 | 2022-05-19 | Merck Patent Gmbh | Sulfurous compounds for organic electroluminescent devices |
| US20230416264A1 (en) | 2020-12-02 | 2023-12-28 | Merck Patent Gmbh | Heterocyclic compounds for organic electroluminescent devices |
| WO2022122607A1 (en) | 2020-12-08 | 2022-06-16 | Merck Patent Gmbh | An ink system and a method for inkjet printing |
| US20240057479A1 (en) | 2020-12-10 | 2024-02-15 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| CN116649013A (en) | 2020-12-18 | 2023-08-25 | 默克专利有限公司 | Nitrogen-containing heteroaromatic compounds for organic electroluminescent devices |
| US20240124769A1 (en) | 2020-12-18 | 2024-04-18 | Merck Patent Gmbh | Nitrogenous compounds for organic electroluminescent devices |
| CN116583522A (en) | 2020-12-18 | 2023-08-11 | 默克专利有限公司 | Indolo[3.2.1-JK]carbazole-6-carbonitrile derivatives as blue fluorescent emitters in OLEDs |
| EP4274827A1 (en) | 2021-01-05 | 2023-11-15 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| CN116710454A (en) | 2021-01-25 | 2023-09-05 | 默克专利有限公司 | Nitrogen-containing compounds for organic electroluminescent devices |
| KR20230154439A (en) | 2021-03-02 | 2023-11-08 | 메르크 파텐트 게엠베하 | Compounds for organic electroluminescent devices |
| KR20230158657A (en) | 2021-03-18 | 2023-11-21 | 메르크 파텐트 게엠베하 | Heteroaromatic compounds for organic electroluminescent devices |
| EP4079742A1 (en) | 2021-04-14 | 2022-10-26 | Merck Patent GmbH | Metal complexes |
| KR20240000559A (en) | 2021-04-23 | 2024-01-02 | 메르크 파텐트 게엠베하 | Formulation of organic functional materials |
| CN117203191A (en) | 2021-04-29 | 2023-12-08 | 默克专利有限公司 | Material for organic electroluminescent device |
| EP4330240B1 (en) | 2021-04-29 | 2025-10-15 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| US20240246983A1 (en) | 2021-04-30 | 2024-07-25 | Merck Patent Gmbh | Nitrogenous heterocyclic compounds for organic electroluminescent devices |
| WO2022243403A1 (en) | 2021-05-21 | 2022-11-24 | Merck Patent Gmbh | Method for the continuous purification of at least one functional material and device for the continuous purification of at least one functional material |
| WO2022200638A1 (en) | 2021-07-06 | 2022-09-29 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| KR20240045247A (en) | 2021-08-02 | 2024-04-05 | 메르크 파텐트 게엠베하 | Printing method by combining inks |
| WO2023041454A1 (en) | 2021-09-14 | 2023-03-23 | Merck Patent Gmbh | Boronic heterocyclic compounds for organic electroluminescent devices |
| EP4410074B1 (en) | 2021-09-28 | 2025-05-21 | Merck Patent GmbH | Materials for electronic devices |
| EP4410071A1 (en) | 2021-09-28 | 2024-08-07 | Merck Patent GmbH | Materials for electronic devices |
| WO2023052272A1 (en) | 2021-09-28 | 2023-04-06 | Merck Patent Gmbh | Materials for electronic devices |
| CN118056486A (en) | 2021-09-28 | 2024-05-17 | 默克专利有限公司 | Material for electronic devices |
| TW202349760A (en) | 2021-10-05 | 2023-12-16 | 德商麥克專利有限公司 | Method for forming an organic element of an electronic device |
| EP4423209B1 (en) | 2021-10-27 | 2025-05-07 | Merck Patent GmbH | Boronic and nitrogenous heterocyclic compounds for organic electroluminescent devices |
| WO2023094412A1 (en) | 2021-11-25 | 2023-06-01 | Merck Patent Gmbh | Materials for electronic devices |
| WO2023099543A1 (en) | 2021-11-30 | 2023-06-08 | Merck Patent Gmbh | Compounds having fluorene structures |
| KR20240122858A (en) | 2021-12-13 | 2024-08-13 | 메르크 파텐트 게엠베하 | Materials for organic electroluminescent devices |
| CN118354991A (en) | 2021-12-21 | 2024-07-16 | 默克专利有限公司 | Method for preparing deuterated organic compounds |
| EP4453128A1 (en) | 2021-12-21 | 2024-10-30 | Merck Patent GmbH | Electronic devices |
| EP4453129A1 (en) | 2021-12-21 | 2024-10-30 | Merck Patent GmbH | Electronic devices |
| EP4476215A1 (en) | 2022-02-09 | 2024-12-18 | Merck Patent GmbH | Materials for organic electroluminescent devices |
| CN118647604A (en) | 2022-02-14 | 2024-09-13 | 默克专利有限公司 | Materials for electronic devices |
| WO2023161168A1 (en) | 2022-02-23 | 2023-08-31 | Merck Patent Gmbh | Aromatic hetreocycles for organic electroluminescent devices |
| JP2025508763A (en) | 2022-02-23 | 2025-04-10 | メルク パテント ゲゼルシャフト ミット ベシュレンクテル ハフツング | Nitrogen-containing heterocycles for organic electroluminescent devices. |
| WO2023213837A1 (en) | 2022-05-06 | 2023-11-09 | Merck Patent Gmbh | Cyclic compounds for organic electroluminescent devices |
| KR20250011205A (en) | 2022-05-18 | 2025-01-21 | 메르크 파텐트 게엠베하 | Method for the preparation of deuterated organic compounds |
| TW202411366A (en) | 2022-06-07 | 2024-03-16 | 德商麥克專利有限公司 | Method of printing a functional layer of an electronic device by combining inks |
| KR20250025717A (en) | 2022-06-20 | 2025-02-24 | 메르크 파텐트 게엠베하 | Organic heterocycles for photovoltaic devices |
| KR20250025718A (en) | 2022-06-20 | 2025-02-24 | 메르크 파텐트 게엠베하 | Complex ring for photovoltaic devices |
| WO2024013004A1 (en) | 2022-07-11 | 2024-01-18 | Merck Patent Gmbh | Materials for electronic devices |
| EP4311849B1 (en) | 2022-07-27 | 2025-01-29 | UDC Ireland Limited | Metal complexes |
| KR20250075627A (en) | 2022-09-22 | 2025-05-28 | 메르크 파텐트 게엠베하 | Nitrogen-containing compounds for organic electroluminescent devices |
| WO2024061948A1 (en) | 2022-09-22 | 2024-03-28 | Merck Patent Gmbh | Nitrogen-containing hetreocycles for organic electroluminescent devices |
| CN120152954A (en) | 2022-11-01 | 2025-06-13 | 默克专利有限公司 | Nitrogen-containing heterocyclic compounds for organic electroluminescent devices |
| WO2024105066A1 (en) | 2022-11-17 | 2024-05-23 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| TW202440819A (en) | 2022-12-16 | 2024-10-16 | 德商麥克專利有限公司 | Formulation of an organic functional material |
| WO2024132993A1 (en) | 2022-12-19 | 2024-06-27 | Merck Patent Gmbh | Materials for electronic devices |
| KR20250126107A (en) | 2022-12-20 | 2025-08-22 | 메르크 파텐트 게엠베하 | Method for producing deuterated aromatic compounds |
| CN120500491A (en) | 2023-01-10 | 2025-08-15 | 默克专利有限公司 | Nitrogen-containing heterocyclic compound for organic electroluminescent device |
| CN120569385A (en) | 2023-01-17 | 2025-08-29 | 默克专利有限公司 | Heterocyclic compounds for organic electroluminescent devices |
| WO2024170605A1 (en) | 2023-02-17 | 2024-08-22 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024184050A1 (en) | 2023-03-07 | 2024-09-12 | Merck Patent Gmbh | Cyclic nitrogen compounds for organic electroluminescent devices |
| WO2024194264A1 (en) | 2023-03-20 | 2024-09-26 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2024218109A1 (en) | 2023-04-20 | 2024-10-24 | Merck Patent Gmbh | Materials for electronic devices |
| WO2024240725A1 (en) | 2023-05-25 | 2024-11-28 | Merck Patent Gmbh | Tris[1,2,4]triazolo[1,5-a:1',5'-c:1'',5''-e][1,3,5]triazine derivatives for use in organic electroluminescent devices |
| WO2024256357A1 (en) | 2023-06-12 | 2024-12-19 | Merck Patent Gmbh | Organic heterocycles for photoelectric devices |
| WO2025003084A1 (en) | 2023-06-28 | 2025-01-02 | Merck Patent Gmbh | Dicyanoaryl compounds for organic electroluminescent devices |
| EP4486099A1 (en) | 2023-06-30 | 2025-01-01 | Merck Patent GmbH | Compounds for organic electroluminescent devices |
| WO2025012253A1 (en) | 2023-07-12 | 2025-01-16 | Merck Patent Gmbh | Materials for electronic devices |
| WO2025021855A1 (en) | 2023-07-27 | 2025-01-30 | Merck Patent Gmbh | Materials for organic light-emitting devices and organic sensors |
| WO2025032039A1 (en) | 2023-08-07 | 2025-02-13 | Merck Patent Gmbh | Process for the preparation of an electronic device |
| WO2025045851A1 (en) | 2023-08-30 | 2025-03-06 | Merck Patent Gmbh | Materials for organic light-emitting devices |
| WO2025045842A1 (en) | 2023-08-30 | 2025-03-06 | Merck Patent Gmbh | Materials for organic light-emitting devices |
| WO2025045843A1 (en) | 2023-08-30 | 2025-03-06 | Merck Patent Gmbh | Materials for organic light-emitting devices |
| WO2025045935A1 (en) | 2023-08-31 | 2025-03-06 | Merck Patent Gmbh | Cyano group-containing aromatic compounds for organic electroluminescent devices |
| WO2025109056A1 (en) | 2023-11-24 | 2025-05-30 | Merck Patent Gmbh | Oxygen-containing heterocycles for organic electroluminescent devices |
| WO2025132547A1 (en) | 2023-12-21 | 2025-06-26 | Merck Patent Gmbh | Mechanochemical method for deuterating organic compounds |
| WO2025132551A1 (en) | 2023-12-22 | 2025-06-26 | Merck Patent Gmbh | Materials for electronic devices |
| WO2025181044A1 (en) | 2024-02-29 | 2025-09-04 | Merck Patent Gmbh | Nitrogen-containing compounds for organic electroluminescent devices |
| WO2025181097A1 (en) | 2024-02-29 | 2025-09-04 | Merck Patent Gmbh | Nitrogen-containing hetreocycles for organic electroluminescent devices |
| WO2025181124A1 (en) | 2024-03-01 | 2025-09-04 | Merck Patent Gmbh | Materials for organic electroluminescent devices |
| WO2025196145A1 (en) | 2024-03-22 | 2025-09-25 | Merck Patent Gmbh | Materials for organic light emitting devices |
Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03289090A (en) | 1990-04-03 | 1991-12-19 | Mitsui Toatsu Chem Inc | Organic complex light emitting device |
| EP0517542A1 (en) | 1991-06-05 | 1992-12-09 | Sumitomo Chemical Company, Limited | Organic electroluminescence devices |
| JPH083547A (en) | 1994-03-31 | 1996-01-09 | Toray Ind Inc | Luminous element |
| US5487953A (en) | 1993-09-22 | 1996-01-30 | Pioneer Electronic Corporation | Organic electroluminescent device |
| JPH09298088A (en) | 1996-05-01 | 1997-11-18 | Mitsubishi Chem Corp | Organic electroluminescent device and method of manufacturing the same |
| JPH09310066A (en) | 1996-05-23 | 1997-12-02 | Idemitsu Kosan Co Ltd | Organic electroluminescent device |
| JPH10226785A (en) * | 1997-02-14 | 1998-08-25 | Idemitsu Kosan Co Ltd | Organic electroluminescent device |
| JPH10316658A (en) | 1997-05-21 | 1998-12-02 | Mitsubishi Chem Corp | 4,4'-bis (N-carbazolyl) diphenylamine derivative and organic electroluminescent device using the same |
| US5891587A (en) | 1997-02-27 | 1999-04-06 | Xerox Corporation | Electroluminescent devices |
| JPH11144866A (en) * | 1997-11-05 | 1999-05-28 | Toray Ind Inc | Electroluminescent element |
| EP0936844A2 (en) | 1998-02-17 | 1999-08-18 | Junji Kido | Organic electroluminescent devices |
| JPH11329737A (en) | 1998-03-13 | 1999-11-30 | Taiho Ind Co Ltd | Organic multilayer electroluminescence device and method for synthesizing structure for organic multilayer electroluminescence device |
| JP2000021572A (en) | 1998-07-06 | 2000-01-21 | Mitsubishi Chemicals Corp | Organic electroluminescent device |
| JP2000044519A (en) | 1998-07-27 | 2000-02-15 | Chisso Corp | Trinaphthylbenzene derivative and organic electroluminescent device using the same |
| JP2000063335A (en) | 1998-08-17 | 2000-02-29 | Minolta Co Ltd | New-amino compound, its production and use |
| US6310360B1 (en) | 1999-07-21 | 2001-10-30 | The Trustees Of Princeton University | Intersystem crossing agents for efficient utilization of excitons in organic light emitting devices |
| US20020034656A1 (en) | 1998-09-14 | 2002-03-21 | Thompson Mark E. | Organometallic complexes as phosphorescent emitters in organic LEDs |
| US6660410B2 (en) * | 2000-03-27 | 2003-12-09 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
Family Cites Families (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03210481A (en) | 1990-01-12 | 1991-09-13 | Olympus Optical Co Ltd | Movable probe type multipoint contact apparatus for inspecting printed circuit board |
| JPH03232856A (en) * | 1990-02-07 | 1991-10-16 | Bando Chem Ind Ltd | 4,4’,4”-tricarbazolyltriphenylamine |
| JP3069139B2 (en) * | 1990-03-16 | 2000-07-24 | 旭化成工業株式会社 | Dispersion type electroluminescent device |
| JPH05263073A (en) * | 1992-03-17 | 1993-10-12 | Sumitomo Chem Co Ltd | Organic electroluminescent device |
| CA2091692C (en) | 1991-07-16 | 2000-10-17 | James Richardson | Enhanced injection site |
| JP3210481B2 (en) † | 1993-04-30 | 2001-09-17 | バンドー化学株式会社 | 1,3,5-tris [4- (N-carbazolyl) phenyl] benzene derivative |
| EP0666298A3 (en) * | 1994-02-08 | 1995-11-15 | Tdk Corp | Organic EL element and compound used therein. |
| JP3529543B2 (en) * | 1995-04-27 | 2004-05-24 | パイオニア株式会社 | Organic electroluminescence device |
| JP3674133B2 (en) * | 1996-03-18 | 2005-07-20 | 東レ株式会社 | Light emitting element |
| JP4111406B2 (en) * | 1997-02-27 | 2008-07-02 | エルジー.フィリップス エルシーデー カンパニー,リミテッド | EL device and photoconductive imaging member |
| US6242115B1 (en) * | 1997-09-08 | 2001-06-05 | The University Of Southern California | OLEDs containing thermally stable asymmetric charge carrier materials |
| US6069442A (en) * | 1997-09-18 | 2000-05-30 | Eastman Kodak Company | Organic electroluminescent device with inorganic electron transporting layer |
| JP3856546B2 (en) * | 1997-11-11 | 2006-12-13 | 三井化学株式会社 | Organic electroluminescence device |
| JP3792027B2 (en) | 1997-11-20 | 2006-06-28 | 三井化学株式会社 | Organic electroluminescence device |
| JP3792029B2 (en) | 1997-11-25 | 2006-06-28 | 三井化学株式会社 | Organic electroluminescence device |
| JP3856550B2 (en) | 1997-12-05 | 2006-12-13 | 三井化学株式会社 | Organic electroluminescence device |
| JP3792031B2 (en) | 1997-12-10 | 2006-06-28 | 三井化学株式会社 | Organic electroluminescence device |
| JP3821562B2 (en) | 1997-12-18 | 2006-09-13 | 三井化学株式会社 | Organic electroluminescence device |
| JP3877419B2 (en) | 1998-02-03 | 2007-02-07 | 三井化学株式会社 | Organic electroluminescence device |
| JP3835917B2 (en) | 1998-02-04 | 2006-10-18 | 三井化学株式会社 | Organic electroluminescence device |
| JP3835918B2 (en) | 1998-02-19 | 2006-10-18 | 三井化学株式会社 | Organic electroluminescence device |
| JP3884557B2 (en) | 1998-04-01 | 2007-02-21 | 三井化学株式会社 | Organic electroluminescence device |
| WO2000003565A1 (en) † | 1998-07-10 | 2000-01-20 | Fed Corporation | Amorphous molecular materials for optoelectronic devices and process for producing the same |
| US20030186144A1 (en) | 1998-07-31 | 2003-10-02 | Mitsuhiro Kunieda | Electrophotographic photosensitive member, process cartridge and electrophotographic apparatus |
| EP1449238B1 (en) † | 1999-05-13 | 2006-11-02 | The Trustees Of Princeton University | Very high efficiency organic light emitting devices based on electrophosphorescence |
| JP3924648B2 (en) * | 1999-11-02 | 2007-06-06 | ソニー株式会社 | Organic electroluminescence device |
| US6639357B1 (en) * | 2000-02-28 | 2003-10-28 | The Trustees Of Princeton University | High efficiency transparent organic light emitting devices |
| EP2192632B1 (en) * | 2002-04-12 | 2014-09-03 | Konica Corporation | Organic Electroluminescence Element |
| KR101140500B1 (en) * | 2003-11-21 | 2012-04-30 | 반도 카가쿠 가부시키가이샤 | Organo-electronic functional material and use thereof |
| KR101384785B1 (en) * | 2006-06-01 | 2014-04-14 | 가부시키가이샤 한도오따이 에네루기 켄큐쇼 | Light-emitting element, light-emitting device and an electronic device |
-
2001
- 2001-03-26 TW TW090107093A patent/TW532048B/en not_active IP Right Cessation
- 2001-03-26 US US09/816,415 patent/US6660410B2/en not_active Expired - Lifetime
- 2001-03-27 WO PCT/JP2001/002454 patent/WO2001072927A1/en active IP Right Grant
- 2001-03-27 CN CNA2005100659787A patent/CN1694591A/en active Pending
- 2001-03-27 EP EP01915817.9A patent/EP1205527B2/en not_active Expired - Lifetime
- 2001-03-27 JP JP2001571844A patent/JP4916078B2/en not_active Expired - Fee Related
- 2001-03-27 KR KR1020017015089A patent/KR100802757B1/en not_active Expired - Fee Related
- 2001-03-27 DE DE60133797.2T patent/DE60133797T3/en not_active Expired - Lifetime
- 2001-03-27 CN CNB018006892A patent/CN1206311C/en not_active Expired - Fee Related
-
2003
- 2003-10-14 US US10/683,435 patent/US6979414B2/en not_active Expired - Lifetime
-
2005
- 2005-10-07 US US11/245,092 patent/US7226546B2/en not_active Expired - Lifetime
-
2007
- 2007-06-01 US US11/806,597 patent/US20070248841A1/en not_active Abandoned
-
2010
- 2010-07-26 US US12/805,316 patent/US8753757B2/en not_active Expired - Fee Related
-
2014
- 2014-05-02 US US14/268,485 patent/US9564607B2/en not_active Expired - Fee Related
-
2016
- 2016-12-14 US US15/378,759 patent/US20170098785A1/en not_active Abandoned
Patent Citations (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03289090A (en) | 1990-04-03 | 1991-12-19 | Mitsui Toatsu Chem Inc | Organic complex light emitting device |
| EP0517542A1 (en) | 1991-06-05 | 1992-12-09 | Sumitomo Chemical Company, Limited | Organic electroluminescence devices |
| US5487953A (en) | 1993-09-22 | 1996-01-30 | Pioneer Electronic Corporation | Organic electroluminescent device |
| JPH083547A (en) | 1994-03-31 | 1996-01-09 | Toray Ind Inc | Luminous element |
| JPH09298088A (en) | 1996-05-01 | 1997-11-18 | Mitsubishi Chem Corp | Organic electroluminescent device and method of manufacturing the same |
| JPH09310066A (en) | 1996-05-23 | 1997-12-02 | Idemitsu Kosan Co Ltd | Organic electroluminescent device |
| JPH10226785A (en) * | 1997-02-14 | 1998-08-25 | Idemitsu Kosan Co Ltd | Organic electroluminescent device |
| US5891587A (en) | 1997-02-27 | 1999-04-06 | Xerox Corporation | Electroluminescent devices |
| JPH10316658A (en) | 1997-05-21 | 1998-12-02 | Mitsubishi Chem Corp | 4,4'-bis (N-carbazolyl) diphenylamine derivative and organic electroluminescent device using the same |
| JPH11144866A (en) * | 1997-11-05 | 1999-05-28 | Toray Ind Inc | Electroluminescent element |
| EP0936844A2 (en) | 1998-02-17 | 1999-08-18 | Junji Kido | Organic electroluminescent devices |
| JPH11329737A (en) | 1998-03-13 | 1999-11-30 | Taiho Ind Co Ltd | Organic multilayer electroluminescence device and method for synthesizing structure for organic multilayer electroluminescence device |
| JP2000021572A (en) | 1998-07-06 | 2000-01-21 | Mitsubishi Chemicals Corp | Organic electroluminescent device |
| JP2000044519A (en) | 1998-07-27 | 2000-02-15 | Chisso Corp | Trinaphthylbenzene derivative and organic electroluminescent device using the same |
| JP2000063335A (en) | 1998-08-17 | 2000-02-29 | Minolta Co Ltd | New-amino compound, its production and use |
| US20020034656A1 (en) | 1998-09-14 | 2002-03-21 | Thompson Mark E. | Organometallic complexes as phosphorescent emitters in organic LEDs |
| US6310360B1 (en) | 1999-07-21 | 2001-10-30 | The Trustees Of Princeton University | Intersystem crossing agents for efficient utilization of excitons in organic light emitting devices |
| US6660410B2 (en) * | 2000-03-27 | 2003-12-09 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
Non-Patent Citations (5)
| Title |
|---|
| Baldo et al., Applied Physics Letters, "Very high-efficiency in Organic light-emitting devices based on electrophosphorescence," 75 (1), pp. 4-6, Jul. 1999. |
| Machine-assisted translation of JP 10-226785 A (Aug. 1998). * |
| Machine-assisted translation of JP 10-316658 (Dec. 1998). * |
| Machine-assisted translation of JP 11-144866 A (May 1999). * |
| Tetsuo Tsutsui et al., "High Quantum Efficiency in Organic Light-Emitting Devices with Iridium-Complex as a Triplet Emissive Center" Jpn J. Appl. Phys. vol. 38 (1999) pp. L1502-L 1504 Part 2, No. 12B, Dec. 15, 1999, 1999 Publication Board, Japanese Journal of Applied Physics. |
Cited By (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20110163660A1 (en) * | 2000-03-27 | 2011-07-07 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US20060046098A1 (en) * | 2000-03-27 | 2006-03-02 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US7226546B2 (en) * | 2000-03-27 | 2007-06-05 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US9564607B2 (en) * | 2000-03-27 | 2017-02-07 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US20070248841A1 (en) * | 2000-03-27 | 2007-10-25 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US20140239282A1 (en) * | 2000-03-27 | 2014-08-28 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US8753757B2 (en) | 2000-03-27 | 2014-06-17 | Idemitsu Kosan Co., Ltd. | Organic electroluminescence element |
| US7505487B2 (en) | 2003-04-23 | 2009-03-17 | Semiconductor Energy Laboratory Co., Ltd. | Laser oscillator including phosphorescent material |
| US20040213308A1 (en) * | 2003-04-23 | 2004-10-28 | Hiroko Abe | Laser oscillator |
| US8653537B2 (en) | 2004-08-13 | 2014-02-18 | Novaled Ag | Layer assembly for a light-emitting component |
| US20080254318A1 (en) * | 2004-12-28 | 2008-10-16 | Semiconductor Energy Laboratory Co., Ltd. | Carbazole Derivative, and Light-Emitting Element and Light-Emitting Device Using the Carbzole Derivative |
| US7986090B2 (en) | 2005-03-15 | 2011-07-26 | Novaled Ag | Light-emitting component |
| US8405070B2 (en) | 2005-03-23 | 2013-03-26 | Semiconductor Energy Laboratory Co., Ltd. | Composite material, and light emitting element and light emitting device using the composite material |
| US20100084645A1 (en) * | 2005-03-23 | 2010-04-08 | Semiconductor Energy Laboratory Co., Ltd. | Composite material, and light emitting element and light emitting device using the composite material |
| US7911129B2 (en) | 2005-04-13 | 2011-03-22 | Novaled Ag | Arrangement for an organic pin-type light-emitting diode and method for manufacturing |
| US9112175B2 (en) | 2005-12-21 | 2015-08-18 | Novaled Ag | Organic component |
| US20090009072A1 (en) * | 2005-12-23 | 2009-01-08 | Philipp Wellmann | Organic Light Emitting Device With a Plurality of Organic Electroluminescent Units Stacked Upon Each Other |
| US7830089B2 (en) | 2005-12-23 | 2010-11-09 | Novaled Ag | Electronic device with a layer structure of organic layers |
| US8502200B2 (en) | 2006-01-11 | 2013-08-06 | Novaled Ag | Electroluminescent light-emitting device comprising an arrangement of organic layers, and method for its production |
| US20070193624A1 (en) * | 2006-02-23 | 2007-08-23 | Guardian Industries Corp. | Indium zinc oxide based front contact for photovoltaic device and method of making same |
| US7919773B2 (en) | 2006-03-20 | 2011-04-05 | Semiconductor Energy Laboratory Co., Ltd. | Aromatic amine compound, and light-emitting element, light-emitting device, and electronic appliance using the aromatic amine compound |
| US20070215889A1 (en) * | 2006-03-20 | 2007-09-20 | Semiconductor Energy Laboratory Co., Ltd. | Aromatic amine compound, and light-emitting element, light-emitting device, and electronic appliance using the aromatic amine compound |
| US20100001638A1 (en) * | 2006-03-20 | 2010-01-07 | Semiconductor Energy Laboratory Co., Ltd. | Aromatic amine compound, and light-emitting element, light-emitting device, and electronic appliance using the aromatic amine compound |
| US8569743B2 (en) | 2006-04-19 | 2013-10-29 | Novaled Ag | Light-emitting component |
| US8076571B2 (en) | 2006-11-02 | 2011-12-13 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US7964788B2 (en) | 2006-11-02 | 2011-06-21 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US20080105298A1 (en) * | 2006-11-02 | 2008-05-08 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US8012317B2 (en) | 2006-11-02 | 2011-09-06 | Guardian Industries Corp. | Front electrode including transparent conductive coating on patterned glass substrate for use in photovoltaic device and method of making same |
| US8203073B2 (en) | 2006-11-02 | 2012-06-19 | Guardian Industries Corp. | Front electrode for use in photovoltaic device and method of making same |
| US20080143250A1 (en) * | 2006-12-14 | 2008-06-19 | Novaled Ag | Organisches Leuchtbauelement |
| US20080163929A1 (en) * | 2007-01-08 | 2008-07-10 | Guardian Industries Corp. | Zinc oxide based front electrode doped with yttrium for use in photovoltaic device or the like |
| US8936842B2 (en) | 2007-01-08 | 2015-01-20 | Guardian Industris Corp. | Low-E coating having zinc aluminum oxide based layer doped with yttrium |
| US8334452B2 (en) | 2007-01-08 | 2012-12-18 | Guardian Industries Corp. | Zinc oxide based front electrode doped with yttrium for use in photovoltaic device or the like |
| US7767253B2 (en) | 2007-03-09 | 2010-08-03 | Guardian Industries Corp. | Method of making a photovoltaic device with antireflective coating |
| US8450600B2 (en) | 2007-03-09 | 2013-05-28 | Guardian Industries Corp. | Photovoltaic device with scratch-resistant coating |
| US20080220152A1 (en) * | 2007-03-09 | 2008-09-11 | Guardian Industries Corp. | Method of making a photovoltaic device with scratch-resistant coating and resulting product |
| US8254165B2 (en) | 2007-04-17 | 2012-08-28 | Novaled Ag | Organic electronic memory component, memory component arrangement and method for operating an organic electronic memory component |
| US20080271782A1 (en) * | 2007-05-01 | 2008-11-06 | Guardian Industries Corp. | Method of making a photovoltaic device or front substrate for use in same with scratch-resistant coating and resulting product |
| US8237047B2 (en) | 2007-05-01 | 2012-08-07 | Guardian Industries Corp. | Method of making a photovoltaic device or front substrate for use in same with scratch-resistant coating and resulting product |
| US20080295884A1 (en) * | 2007-05-29 | 2008-12-04 | Sharma Pramod K | Method of making a photovoltaic device or front substrate with barrier layer for use in same and resulting product |
| US20080308145A1 (en) * | 2007-06-12 | 2008-12-18 | Guardian Industries Corp | Front electrode including transparent conductive coating on etched glass substrate for use in photovoltaic device and method of making same |
| US7888594B2 (en) | 2007-11-20 | 2011-02-15 | Guardian Industries Corp. | Photovoltaic device including front electrode having titanium oxide inclusive layer with high refractive index |
| US8114472B2 (en) | 2008-01-08 | 2012-02-14 | Guardian Industries Corp. | Method of making a temperable antiglare coating, and resulting products containing the same |
| US20090176107A1 (en) * | 2008-01-08 | 2009-07-09 | Guardian Industries Corp. | Method of making a temperable antiglare coating, and resulting products containing the same |
| US20100051923A1 (en) * | 2008-08-04 | 2010-03-04 | Novaled Ag | Organischer Feldeffekt Transistor |
| US8071976B2 (en) | 2008-08-04 | 2011-12-06 | Novaled Ag | Organic field-effect transistor and circuit |
| US8212241B2 (en) | 2008-08-04 | 2012-07-03 | Novaled Ag | Organic field-effect transistor |
| US8022291B2 (en) | 2008-10-15 | 2011-09-20 | Guardian Industries Corp. | Method of making front electrode of photovoltaic device having etched surface and corresponding photovoltaic device |
| US20100133992A1 (en) * | 2008-11-28 | 2010-06-03 | Samsung Electronics Co., Ltd. | Compound comprising phosphorescence unit, emitting polymer and organic emitting device comprising the emitting polymer |
| US9206170B2 (en) * | 2008-11-28 | 2015-12-08 | Samsung Electronics Co., Ltd. | Compound comprising phosphorescence unit, emitting polymer and organic emitting device comprising the emitting polymer |
| US8530063B2 (en) | 2009-10-21 | 2013-09-10 | Cheil Industries, Inc. | Compound for organic photoelectric device and organic photoelectric device including the same |
| US9246109B2 (en) | 2012-10-19 | 2016-01-26 | Samsung Display Co., Ltd. | Compound and organic light emitting device including the same |
| US9252370B2 (en) | 2012-11-05 | 2016-02-02 | Samsung Display Co., Ltd. | Heterocyclic compounds and organic light emitting devices including the same |
| US9385325B2 (en) | 2012-11-05 | 2016-07-05 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light emitting device including the same |
| US9773987B2 (en) | 2013-01-28 | 2017-09-26 | Samsung Display Co., Ltd. | Silicon-based compound and organic light-emitting diode comprising the same |
| US9391280B2 (en) | 2013-01-30 | 2016-07-12 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting device including the same |
| US9960359B2 (en) | 2013-06-03 | 2018-05-01 | Samsung Display Co., Ltd. | Arylamine-based compound and organic light emitting diode comprising the same |
| US9608211B2 (en) | 2013-08-21 | 2017-03-28 | Samsung Display Co., Ltd. | Heterocyclic compound and organic light-emitting diode including the same |
| KR20160019744A (en) | 2014-08-12 | 2016-02-22 | (주)아이씨비 | A process of preparing fluorene-carbazole compoound for hole transfer layer of organic electronic device |
| KR20160019745A (en) | 2014-08-12 | 2016-02-22 | (주)아이씨비 | Fluorene-carbazole compoound for hole transfer layer and organic electronic device comprising the same |
| US10340462B2 (en) | 2015-09-11 | 2019-07-02 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including same |
| US11114622B2 (en) | 2015-10-22 | 2021-09-07 | Samsung Display Co., Ltd. | Compound and organic light-emitting device including the same |
| US12289994B2 (en) | 2019-11-06 | 2025-04-29 | Samsung Electronics Co., Ltd. | Photoelectric conversion device and sensor and electronic device |
Also Published As
| Publication number | Publication date |
|---|---|
| KR100802757B1 (en) | 2008-02-12 |
| EP1205527B2 (en) | 2020-10-07 |
| CN1206311C (en) | 2005-06-15 |
| US8753757B2 (en) | 2014-06-17 |
| CN1694591A (en) | 2005-11-09 |
| JP4916078B2 (en) | 2012-04-11 |
| DE60133797T3 (en) | 2021-03-25 |
| WO2001072927A1 (en) | 2001-10-04 |
| TW532048B (en) | 2003-05-11 |
| US20050222429A1 (en) | 2005-10-06 |
| EP1205527B1 (en) | 2008-04-30 |
| US6660410B2 (en) | 2003-12-09 |
| DE60133797D1 (en) | 2008-06-12 |
| US7226546B2 (en) | 2007-06-05 |
| EP1205527A4 (en) | 2007-04-25 |
| US20110163660A1 (en) | 2011-07-07 |
| US9564607B2 (en) | 2017-02-07 |
| DE60133797T2 (en) | 2009-06-25 |
| CN1365381A (en) | 2002-08-21 |
| US20060046098A1 (en) | 2006-03-02 |
| US20070248841A1 (en) | 2007-10-25 |
| US20140239282A1 (en) | 2014-08-28 |
| US20020045061A1 (en) | 2002-04-18 |
| US20170098785A1 (en) | 2017-04-06 |
| EP1205527A1 (en) | 2002-05-15 |
| KR20020026866A (en) | 2002-04-12 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6979414B2 (en) | Organic electroluminescence element | |
| JP4037033B2 (en) | Organic electroluminescence device | |
| EP1486550B1 (en) | Material for organic electroluminescent devices and organic electroluminescent devices made by using the same | |
| CN100407448C (en) | Ultra-efficient organic light-emitting devices based on electrophosphorescence | |
| JPH1092578A (en) | Blue-color organic electroluminescent element | |
| KR20100071726A (en) | Amine derivatives and organic light emitting diode device comprising the same | |
| US20070116982A1 (en) | Host material for organic electroluminescent element and organic electroluminescent element | |
| KR20090072152A (en) | Pyrene Derivatives and Organic Electroluminescent Devices Using the Same | |
| JP2004026732A (en) | Asymmetric 1,4-phenylenediamine derivative and organic electroluminescent device using the same | |
| US8941099B2 (en) | Organic light emitting device and materials for use in same | |
| JP5109236B2 (en) | Hole blocking material and organic electroluminescent device | |
| JPH11302639A (en) | Organic electroluminescent device | |
| JPH11312587A (en) | Organic electroluminescent device | |
| JPH11312586A (en) | Organic electroluminescent device | |
| KR100799223B1 (en) | Hole injection layer material and organic electroluminescent device comprising same | |
| KR100611852B1 (en) | Red phosphorescent iridium complex and organic electroluminescent device comprising same | |
| KR20070016299A (en) | Organic EL material and organic EL element using same | |
| KR20070016298A (en) | Organic host material and organic electroluminescent device using same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCF | Information on status: patent grant |
Free format text: PATENTED CASE |
|
| FEPP | Fee payment procedure |
Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| FPAY | Fee payment |
Year of fee payment: 12 |