US20140206753A1 - Lipid nanoparticle compositions and methods for mrna delivery - Google Patents
Lipid nanoparticle compositions and methods for mrna delivery Download PDFInfo
- Publication number
- US20140206753A1 US20140206753A1 US14/124,608 US201214124608A US2014206753A1 US 20140206753 A1 US20140206753 A1 US 20140206753A1 US 201214124608 A US201214124608 A US 201214124608A US 2014206753 A1 US2014206753 A1 US 2014206753A1
- Authority
- US
- United States
- Prior art keywords
- mrna
- protein
- lipid
- compositions
- hgla
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- KYVNOZVWIVNANS-UHFFFAOYSA-N CC(C)CCCC(C)C1CCC2C3CC=C4CC(OC(=O)CCC5=CN=CC5)CCC4(C)C3CCC12C Chemical compound CC(C)CCCC(C)C1CCC2C3CC=C4CC(OC(=O)CCC5=CN=CC5)CCC4(C)C3CCC12C KYVNOZVWIVNANS-UHFFFAOYSA-N 0.000 description 1
- DETCDJMMODGQRP-KWXKLSQISA-N CCCCC/C=C\C/C=C\CCCCCCCCOCC(CSSCCN(C)C)OCCCCCCCC/C=C\C/C=C\CCCCC Chemical compound CCCCC/C=C\C/C=C\CCCCCCCCOCC(CSSCCN(C)C)OCCCCCCCC/C=C\C/C=C\CCCCC DETCDJMMODGQRP-KWXKLSQISA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0008—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/713—Double-stranded nucleic acids or oligonucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
- A61K38/1816—Erythropoietin [EPO]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/47—Hydrolases (3) acting on glycosyl compounds (3.2), e.g. cellulases, lactases
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/48—Hydrolases (3) acting on peptide bonds (3.4)
- A61K38/482—Serine endopeptidases (3.4.21)
- A61K38/4846—Factor VII (3.4.21.21); Factor IX (3.4.21.22); Factor Xa (3.4.21.6); Factor XI (3.4.21.27); Factor XII (3.4.21.38)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/55—Protease inhibitors
- A61K38/57—Protease inhibitors from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0008—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition
- A61K48/0025—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition wherein the non-active part clearly interacts with the delivered nucleic acid
- A61K48/0041—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'non-active' part of the composition delivered, e.g. wherein such 'non-active' part is not delivered simultaneously with the 'active' part of the composition wherein the non-active part clearly interacts with the delivered nucleic acid the non-active part being polymeric
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0075—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the delivery route, e.g. oral, subcutaneous
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0083—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the administration regime
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/0091—Purification or manufacturing processes for gene therapy compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
- A61K9/1271—Non-conventional liposomes, e.g. PEGylated liposomes or liposomes coated or grafted with polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
- A61K9/1271—Non-conventional liposomes, e.g. PEGylated liposomes or liposomes coated or grafted with polymers
- A61K9/1272—Non-conventional liposomes, e.g. PEGylated liposomes or liposomes coated or grafted with polymers comprising non-phosphatidyl surfactants as bilayer-forming substances, e.g. cationic lipids or non-phosphatidyl liposomes coated or grafted with polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/5123—Organic compounds, e.g. fats, sugars
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
- C07K14/505—Erythropoietin [EPO]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/81—Protease inhibitors
- C07K14/8107—Endopeptidase (E.C. 3.4.21-99) inhibitors
- C07K14/811—Serine protease (E.C. 3.4.21) inhibitors
- C07K14/8121—Serpins
- C07K14/8125—Alpha-1-antitrypsin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/52—Genes encoding for enzymes or proenzymes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/88—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation using microencapsulation, e.g. using amphiphile liposome vesicle
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
- C12N9/2402—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1)
- C12N9/2465—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1) acting on alpha-galactose-glycoside bonds, e.g. alpha-galactosidase (3.2.1.22)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/644—Coagulation factor IXa (3.4.21.22)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y302/00—Hydrolases acting on glycosyl compounds, i.e. glycosylases (3.2)
- C12Y302/01—Glycosidases, i.e. enzymes hydrolysing O- and S-glycosyl compounds (3.2.1)
- C12Y302/01022—Alpha-galactosidase (3.2.1.22)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21022—Coagulation factor IXa (3.4.21.22)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
Definitions
- lysosomal storage diseases are a group of approximately 50 rare inherited metabolic disorders that result from defects in lysosomal function, usually due to a deficiency of an enzyme required for metabolism.
- Fabry disease is a lysosomal storage disease that results from a deficiency of the enzyme alpha galactosidase (GLA), which causes a glycolipid known as globotriaosylceramide to accumulate in blood vessels and other tissues, leading to various painful manifestations.
- GLA alpha galactosidase
- Fabry disease there is a need for replacement of a protein or enzyme that is normally secreted by cells into the blood stream.
- Therapies, such as gene therapy that increase the level or production of an affected protein or enzyme could provide a treatment or even a cure for such disorders.
- conventional gene therapy for this purpose.
- gene therapy with DNA may result in the impairment of a vital genetic function in the treated host, such as e.g., elimination or deleteriously reduced production of an essential enzyme or interruption of a gene critical for the regulation of cell growth, resulting in unregulated or cancerous cell proliferation.
- a vital genetic function such as e.g., elimination or deleteriously reduced production of an essential enzyme or interruption of a gene critical for the regulation of cell growth, resulting in unregulated or cancerous cell proliferation.
- it is necessary for effective expression of the desired gene product to include a strong promoter sequence which again may lead to undesirable changes in the regulation of normal gene expression in the cell.
- the DNA based genetic material will result in the induction of undesired anti-DNA antibodies, which in turn, may trigger a possibly fatal immune response.
- Gene therapy approaches using viral vectors can also result in an adverse immune response. In some circumstances, the viral vector may even integrate into the host genome.
- RNA does not involve the risk of being stably integrated into the genome of the transfected cell, thus eliminating the concern that the introduced genetic material will disrupt the normal functioning of an essential gene, or cause a mutation that results in deleterious or oncogenic effects; (2) extraneous promoter sequences are not required for effective translation of the encoded protein, again avoiding possible deleterious side effects; (3) in contrast to plasmid DNA (pDNA), messenger RNA (mRNA) is devoid of immunogenic CpG motifs so that anti-RNA antibodies are not generated; and (4) any deleterious effects that do result from mRNA based on gene therapy would be of limited duration due to the relatively short half-life of RNA. In addition, it is not necessary for mRNA to enter the nucleus to perform its function, while DNA must overcome this major barrier.
- mRNA based gene therapy has not been used more in the past is that mRNA is far less stable than DNA, especially when it reaches the cytoplasm of a cell and is exposed to degrading enzymes.
- the presence of a hydroxyl group on the second carbon of the sugar moiety in mRNA causes steric hinderance that prevents the mRNA from forming the more stable double helix structure of DNA and thus makes the mRNA more prone to hydrolytic degradation.
- mRNA was too labile to withstand transfection protocols.
- Advances in RNA stabilizing modifications have sparked more interest in the use of mRNA in place of plasmid DNA in gene therapy.
- Certain delivery vehicles such as cationic lipid or polymer delivery vehicles may also help protect the transfected mRNA from endogenous RNases. Yet, in spite of increased stability of modified mRNA, delivery of mRNA to cells in vivo in a manner allowing for therapeutic levels of protein production is still a challenge, particularly for mRNA encoding full length proteins. While delivery of mRNA encoding secreted proteins has been contemplated (US2009/0286852), the levels of a full length secreted protein that would actually be produced via in vivo mRNA delivery are not known and there is not a reason to expect the levels would exceed those observed with DNA based gene therapy.
- the invention provides methods for delivery of mRNA gene therapeutic agents that lead to the production of therapeutically effective levels of secreted proteins via a “depot effect.”
- mRNA encoding a secreted protein is loaded in lipid nanoparticles and delivered to target cells in vivo. Target cells then act as a depot source for production of soluble, secreted protein into the circulatory system at therapeutic levels.
- the levels of secreted protein produced are above normal physiological levels.
- the invention provides compositions and methods for intracellular delivery of mRNA in a liposomal transfer vehicle to one or more target cells for production of therapeutic levels of secreted functional protein.
- compositions and methods of the invention are useful in the management and treatment of a large number of diseases, in particular diseases which result from protein and/or enzyme deficiencies, wherein the protein or enzyme is normally secreted.
- Individuals suffering from such diseases may have underlying genetic defects that lead to the compromised expression of a protein or enzyme, including, for example, the non-synthesis of the secreted protein, the reduced synthesis of the secreted protein, or synthesis of a secreted protein lacking or having diminished biological activity.
- the methods and compositions of the invention are useful for the treatment of lysosomal storage disorders and/or the urea cycle metabolic disorders that occur as a result of one or more defects in the biosynthesis of secreted enzymes involved in the urea cycle.
- compositions of the invention comprise an mRNA, a transfer vehicle and, optionally, an agent to facilitate contact with, and subsequent transfection of a target cell.
- the mRNA can encode a clinically useful secreted protein.
- the mRNA may encode a functional secreted urea cycle enzyme or a secreted enzyme implicated in lysosomal storage disorders.
- the mRNA can encode, for example, erythropoietin (e.g., human EPO) or ⁇ -galactosidase (e.g., human ⁇ -galactosidase (human GLA).
- the mRNA can comprise one or more modifications that confer stability to the mRNA (e.g., compared to a wild-type or native version of the mRNA) and may also comprise one or more modifications relative to the wild-type which correct a defect implicated in the associated aberrant expression of the protein.
- the nucleic acids of the present invention may comprise modifications to one or both of the 5′ and 3′ untranslated regions. Such modifications may include, but are not limited to, the inclusion of a partial sequence of a cytomegalovirus (CMV) immediate-early 1 (IE1) gene, a poly A tail, a Cap1 structure or a sequence encoding human growth hormone (hGH)).
- CMV cytomegalovirus
- IE1 immediate-early 1
- hGH human growth hormone
- the mRNA is modified to decrease mRNA immunogenicity.
- Methods of treating a subject comprising administering a composition of the invention, are also contemplated.
- methods of treating or preventing conditions in which production of a particular secreted protein and/or utilization of a particular secreted protein is inadequate or compromised are provided.
- the methods provided herein can be used to treat a subject having a deficiency in one or more urea cycle enzymes or in one or more enzymes deficient in a lysosomal storage disorder.
- the mRNA in the compositions of the invention is formulated in a liposomal transfer vehicle to facilitate delivery to the target cell.
- Contemplated transfer vehicles may comprise one or more cationic lipids, non-cationic lipids, and/or PEG-modified lipids.
- the transfer vehicle may comprise at least one of the following cationic lipids: C12-200, DLin-KC2-DMA, DODAP, HGT4003, ICE, HGT5000, or HGT5001.
- the transfer vehicle comprises cholesterol (chol) and/or a PEG-modified lipid.
- the transfer vehicles comprises DMG-PEG2K.
- the tranfer vehicle comprises one of the following lipid formulations: C12-200, DOPE, chol, DMG-PEG2K; DODAP, DOPE, cholesterol, DMG-PEG2K; HGT5000, DOPE, chol, DMG-PEG2K, HGT5001, DOPE, chol, DMG-PEG2K.
- the invention also provides compositions and methods useful for facilitating the transfection and delivery of one or more mRNA molecules to target cells capable of exhibiting the “depot effect.”
- the compositions and methods of the present invention contemplate the use of targeting ligands capable of enhancing the affinity of the composition to one or more target cells.
- the targeting ligand is apolipoprotein-B or apolipoprotein-E and corresponding target cells express low-density lipoprotein receptors, thereby facilitating recognition of the targeting ligand.
- the methods and compositions of the present invention may be used to preferentially target a vast number of target cells.
- contemplated target cells include, but are not limited to, hepatocytes, epithelial cells, hematopoietic cells, epithelial cells, endothelial cells, lung cells, bone cells, stem cells, mesenchymal cells, neural cells, cardiac cells, adipocytes, vascular smooth muscle cells, cardiomyocytes, skeletal muscle cells, beta cells, pituitary cells, synovial lining cells, ovarian cells, testicular cells, fibroblasts, B cells, T cells, reticulocytes, leukocytes, granulocytes and tumor cells.
- the secreted protein is produced by the target cell for sustained amounts of time.
- the secreted protein may be producted for more than one hour, more than four, more than six, more than 12, more than 24, more than 48 hours, or more than 72 hours after administration.
- the polypeptide is expressed at a peak level about six hours after administration.
- the expression of the polypeptide is sustained at least at a therapeutic level.
- the polypeptide is expressed at at least a therapeutic level for more than one, more than four, more than six, more than 12, more than 24, more than 48 hours, or more than 72 hours after administration.
- the polypeptide is detectable at the level in patient serum or tissue (e.g., liver, or lung).
- the level of detectable polypeptide is from continuous expression from the mRNA composition over periods of time of more than one, more than four, more than six, more than 12, more than 24, more than 48 hours, or more than 72 hours after administration.
- the secreted protein is produced at levels above normal physiological levels.
- the level of secreted protein may be increased as compared to a control.
- control is the baseline physiological level of the polypeptide in a normal individual or in a population of normal individuals. In other embodiments the control is the baseline physiological level of the polypeptide in an individual having a deficiency in the relevant protein or polypeptide or in a population of individuals having a deficiency in the relevant protein or polypeptide. In some embodiments the control can be the normal level of the relevant protein or polypeptide in the individual to whom the composition is administered. In other embodiments the control is the expression level of the polypeptide upon other therapeutic intervention, e.g., upon direct injection of the corresponding polypeptide, at one or more comparable time points.
- the polypeptide is expressed by the target cell at a level which is at least 1.5-fold, at least 2-fold, at least 5-fold, at least 10-fold, at least 20-fold, 30-fold, at least 100-fold, at least 500-fold, at least 5000-fold, at least 50,000-fold or at least 100,000-fold greater than a control.
- the fold increase of expression greater than control is sustained for more than one, more than four, more than six, more than 12, more than 24, or more than 48 hours, or more than 72 hours after administration.
- the levels of secreted protein are detected in the serum at least 1.5-fold, at least 2-fold, at least 5-fold, at least 10-fold, at least 20-fold, 30-fold, at least 100-fold, at least 500-fold, at least 5000-fold, at least 50,000-fold or at least 100,000-fold greater than a control for at least 48 hours or 2 days.
- the levels of secreted protein are detectable at 3 days, 4 days, 5 days, or 1 week or more after administration. Increased levels of secreted protein may be observed in the serum and/or in a tissue (e.g. liver, lung).
- the method yields a sustained circulation half-life of the desired secreted protein.
- the secreted protein may be detected for hours or days longer than the half-life observed via subcutaneous injection of the secreted protein.
- the half-life of the secreted protein is sustained for more than 1 day, 2 days, 3 days, 4 days, 5 days, or 1 week or more.
- administration comprises a single or repeated doses.
- the dose is administered intravenously, or by pulmonary delivery.
- the polypeptide can be, for example, one or more of erythropoietin, ⁇ -galactosidase, LDL receptor, Factor VIII, Factor IX, ⁇ -L-iduronidase (for MPS I), iduronate sulfatase (for MPS II), heparin-N-sulfatase (for MPS IIIA), ⁇ -N-acetylglucosaminidase (for MPS IIIB), galactose 6-sultatase (for MPS IVA), lysosomal acid lipase, arylsulfatase-A.
- compositions and methods that provide to a cell or subject mRNA, at least a part of which encodes a functional protein, in an amount that is substantially less that the amount of corresponding functional protein generated from that mRNA.
- the mRNA delivered to the cell can produce an amount of protein that is substantially greater than the amount of mRNA delivered to the cell.
- the amount of corresponding protein generated by that mRNA can be at least 1.5, 2, 3, 5, 10, 15, 20, 25, 50, 100, 150, 200, 250, 300, 400, 500, or more times greater that the amount of mRNA actually administered to the cell or subject.
- This can be measured on a mass-by-mass basis, on a mole-by-mole basis, and/or on a molecule-by-molecule basis.
- the protein can be measured in various ways. For example, for a cell, the measured protein can be measured as intracellular protein, extracellular protein, or a combination of the two.
- the measured protein can be protein measured in serum; in a specific tissue or tissues such as the liver, kidney, heart, or brain; in a specific cell type such as one of the various cell types of the liver or brain; or in any combination of serum, tissue, and/or cell type.
- a baseline amount of endogenous protein can be measured in the cell or subject prior to administration of the mRNA and then subtracted from the protein measured after administration of the mRNA to yield the amount of corresponding protein generated from the mRNA.
- the mRNA can provide a reservoir or depot source of a large amount of therapeutic material to the cell or subject, for example, as compared to amount of mRNA delivered to the cell or subject.
- the depot source can act as a continuous source for polypeptide expression from the mRNA over sustained periods of time.
- FIG. 1 shows the nucleotide sequence of a 5′ CMV sequence (SEQ ID NO:1), wherein X, if present is GGA.
- FIG. 2 shows the nucleotide sequence of a 3′ hGH sequence (SEQ ID NO:2).
- FIG. 3 shows the nucleotide sequence of human erythropoietic (EPO) mRNA (SEQ ID NO:3). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2.
- FIG. 4 shows the nucleotide sequence of human alpha-galactosidase (GLA) mRNA (SEQ ID NO:4). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2.
- FIG. 5 shows the nucleotide sequence of human alpha-1 antitrypsin (A1AT) mRNA (SEQ ID NO:5). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2.
- FIG. 6 shows the nucleotide sequence of human factor IX (FIX) mRNA (SEQ ID NO:6). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2.
- FIG. 7 shows quantification of secreted hEPO protein levels as measured via ELISA.
- the protein detected is a result of its production from hEPO mRNA delivered intravenously via a single dose of various lipid nanoparticle formulations.
- the formulations C12-200 (30 ug), HGT4003 (150 ug), ICE (100 ug), DODAP (200 ug) are represented as the cationic/ionizable lipid component of each test article (Formulations 1-4). Values are based on blood sample four hours post-administration.
- FIG. 8 shows the hematocrit measurement of mice treated with a single IV dose of human EPO mRNA-loaded lipid nanoparticles (Formulations 1-4).
- Whole blood samples were taken at 4 hr (Day 1), 24 hr (Day 2), 4 days, 7 days, and 10 days post-administration.
- FIG. 9 shows hematocrit measurements of mice treated with human EPO-mRNA-loaded lipid nanoparticles with either a single IV dose or three injections (day 1, day 3, day 5).
- Whole blood samples were taken prior to injection (day ⁇ 4), day 7, and day 15.
- Formulation 1 was administered: (30 ug, single dose) or (3 ⁇ 10 ug, dose day 1, day 3, day 5);
- Formulation 2 was administered: (3 ⁇ 50 ug, dose day 1, day 3, day 5).
- FIG. 10 shows quantification of secreted human ⁇ -galactosidase (hGLA) protein levels as measured via ELISA.
- the protein detected is a result of the production from hGLA mRNA delivered via lipid nanoparticles (Formulation 1; 30 ug single intravenous dose, based on encapsulated mRNA).
- hGLA protein is detected through 48 hours.
- FIG. 11 shows hGLA activity in serum. hGLA activity was measured using substrate 4-methylumbelliferyl- ⁇ -D-galactopyranoside (4-MU- ⁇ -gal) at 37° C. Data are average of 6 to 9 individual measurements.
- FIG. 12 shows quantification of hGLA protein levels in serum as measured via ELISA.
- Protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (C12-200:DOPE:Chol:DMGPEG2K, 40:30:25:5 (Formulation 1); 30 ug mRNA based on encapsulated mRNA, single IV dose).
- hGLA protein is monitored through 72 hours. per single intravenous dose, based on encapsulated mRNA).
- hGLA protein is monitored through 72 hours.
- FIG. 13 shows quantification of hGLA protein levels in liver, kidney, and spleen as measured via ELISA.
- Protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (Formulation 1; 30 ug mRNA based on encapsulated mRNA, single IV dose).
- hGLA protein is monitored through 72 hours.
- FIG. 15 shows the pharmacokinetic profiles of ERT-based Alpha-galactosidase in athymjic nude mice (40 ug/kg dose) and hGLA protein produced from MRT (Formulation 1; 1.0 mg/kg mRNA dose).
- FIG. 16 shows the quantification of secreted hGLA protein levels in MRT-treated Fabry mice as measured using ELISA.
- hGLA protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (Formulation 1; 10 ug mRNA per single intravenous dose, based on encapsulated mRNA). Serum is monitored through 72 hours.
- FIG. 17 shows the quantification of hGLA protein levels in liver, kidney, spleen, and heart of MRT-treated Fabry KO mice as measured via ELISA.
- Protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (Formulation 1; 30 ug mRNA based on encapsulated mRNA, single IV dose).
- hGLA protein is monitored through 72 hours.
- Literature values representing normal physiological levels are graphed as dashed lines.
- FIG. 18 shows the quantification of secreted hGLA protein levels in MRT and Alpha-galactosidase-treated Fabry mice as measured using ELISA. Both therapies were dosed as a single 1.0 mg/kg intravenous dose.
- FIG. 19 shows the quantification of hGLA protein levels in liver, kidney, spleen, and heart of MRT and ERT (Alpha-galactosidase)-treated Fabry KO mice as measured via ELISA.
- Protein produced from hGLA mRNA delivered via lipid nanoparticles (Formulation 1; 1.0 mg/kg mRNA based on encapsulated mRNA, single IV dose).
- FIG. 20 shows the relative quantification of globotrioasylceramide (Gb3) and lyso-Gb3 in the kidneys of treated and untreated mice.
- Male Fabry KO mice were treated with a single dose either GLA mRNA-loaded lipid nanoparticles or Alpha-galactosidase at 1.0 mg/kg. Amounts reflect quantity of Gb3/lyso-Gb3 one week post-administration.
- FIG. 21 shows the relative quantification of globotrioasylceramide (Gb3) and lyso-Gb3 in the heart of treated and untreated mice.
- Male Fabry KO mice were treated with a single dose either GLA mRNA-loaded lipid nanoparticles or Alpha-galactosidase at 1.0 mg/kg. Amounts reflect quantity of Gb3/lyso-Gb3 one week post-administration.
- FIG. 24 shows the quantification of secreted human Factor IX protein levels measured using ELISA (mean ng/mL ⁇ standard deviation).
- FIX protein is produced from FIX mRNA delivered via C12-200-based lipid nanoparticles (C12-200:DOPE:Chol:DMGPEG2K, 40:30:25:5 (Formulation 1); 30 ug mRNA per single intravenous dose, based on encapsulated mRNA).
- FIG. 25 shows the quantification of secreted human ⁇ -1-antitrypsin (A1AT) protein levels measured using ELISA.
- A1AT protein is produced from A1AT mRNA delivered via C12-200-based lipid nanoparticles (C12-200:DOPE:Chol:DMGPEG2K, 40:30:25:5 (Formulation 1); 30 ug mRNA per single intravenous dose, based on encapsulated mRNA).
- A1AT protein is monitored through 24 hours.
- the invention provides compositions and methods for intracellular delivery of mRNA in a liposomal transfer vehicle to one or more target cells for production of therapeutic levels of secreted functional protein.
- mRNA compositions of the invention are useful for the treatment of a various metabolic or genetic disorders, and in particular those genetic or metabolic disorders which involve the non-expression, misexpression or deficiency of a protein or enzyme.
- therapeutic levels refers to levels of protein detected in the blood or tissues that are above control levels, wherein the control may be normal physiological levels, or the levels in the subject prior to administration of the mRNA composition.
- secreted refers to protein that is detected outside the target cell, in extracellular space.
- the term “produced” is used in its broadest sense to refer the translation of at least one mRNA into a protein or enzyme.
- the compositions include a transfer vehicle.
- the term “transfer vehicle” includes any of the standard pharmaceutical carriers, diluents, excipients and the like which are generally intended for use in connection with the administration of biologically active agents, including nucleic acids.
- the compositions and in particular the transfer vehicles described herein are capable of delivering mRNA to the target cell.
- the transfer vehicle is a lipid nanoparticle.
- the mRNA in the compositions of the invention may encode, for example, a secreted hormone, enzyme, receptor, polypeptide, peptide or other protein of interest that is normally secreted.
- the mRNA may optionally have chemical or biological modifications which, for example, improve the stability and/or half-life of such mRNA or which improve or otherwise facilitate protein production.
- the methods of the invention provide for optional co-delivery of one or more unique mRNA to target cells, for example, by combining two unique mRNAs into a single transfer vehicle.
- a therapeutic first mRNA, and a therapeutic second mRNA may be formulated in a single transfer vehicle and administered.
- the present invention also contemplates co-delivery and/or co-administration of a therapeutic first mRNA and a second nucleic acid to facilitate and/or enhance the function or delivery of the therapeutic first mRNA.
- such a second nucleic acid may encode a membrane transporter protein that upon expression (e.g., translation of the exogenous or synthetic mRNA) facilitates the delivery or enhances the biological activity of the first mRNA.
- the therapeutic first mRNA may be administered with a second nucleic acid that functions as a “chaperone” for example, to direct the folding of either the therapeutic first mRNA.
- compositions of the present invention may comprise a therapeutic first mRNA which, for example, is administered to correct an endogenous protein or enzyme deficiency, and which is accompanied by a second nucleic acid, which is administered to deactivate or “knock-down” a malfunctioning endogenous nucleic acid and its protein or enzyme product.
- second nucleic acids may encode, for example mRNA or siRNA.
- a natural mRNA in the compositions of the invention may decay with a half-life of between 30 minutes and several days.
- the mRNA in the compositions of the invention preferably retain at least some ability to be translated, thereby producing a functional secreted protein or enzyme.
- the invention provides compositions comprising and methods of administering a stabilized mRNA.
- the activity of the mRNA is prolonged over an extended period of time.
- the activity of the mRNA may be prolonged such that the compositions of the present invention are administered to a subject on a semi-weekly or bi-weekly basis, or more preferably on a monthly, bi-monthly, quarterly or an annual basis.
- the extended or prolonged activity of the mRNA of the present invention is directly related to the quantity of secreted functional protein or enzyme produced from such mRNA.
- the activity of the compositions of the present invention may be further extended or prolonged by modifications made to improve or enhance translation of the mRNA.
- the quantity of functional protein or enzyme produced by the target cell is a function of the quantity of mRNA delivered to the target cells and the stability of such mRNA. To the extent that the stability of the mRNA of the present invention may be improved or enhanced, the half-life, the activity of the produced secreted protein or enzyme and the dosing frequency of the composition may be further extended.
- the mRNA in the compositions of the invention comprise at least one modification which confers increased or enhanced stability to the nucleic acid, including, for example, improved resistance to nuclease digestion in vivo.
- modification and “modified” as such terms relate to the nucleic acids provided herein, include at least one alteration which preferably enhances stability and renders the mRNA more stable (e.g., resistant to nuclease digestion) than the wild-type or naturally occurring version of the mRNA.
- stable and “stability” as such terms relate to the nucleic acids of the present invention, and particularly with respect to the mRNA, refer to increased or enhanced resistance to degradation by, for example nucleases (i.e., endonucleases or exonucleases) which are normally capable of degrading such mRNA.
- Increased stability can include, for example, less sensitivity to hydrolysis or other destruction by endogenous enzymes (e.g., endonucleases or exonucleases) or conditions within the target cell or tissue, thereby increasing or enhancing the residence of such mRNA in the target cell, tissue, subject and/or cytoplasm.
- the stabilized mRNA molecules provided herein demonstrate longer half-lives relative to their naturally occurring, unmodified counterparts (e.g. the wild-type version of the mRNA).
- modified and “modified” as such terms related to the mRNA of the present invention are alterations which improve or enhance translation of mRNA nucleic acids, including for example, the inclusion of sequences which function in the initiation of protein translation (e.g., the Kozac consensus sequence). (Kozak, M., Nucleic Acids Res 15 (20): 8125-48 (1987)).
- the mRNA of the invention have undergone a chemical or biological modification to render them more stable.
- exemplary modifications to an mRNA include the depletion of a base (e.g., by deletion or by the substitution of one nucleotide for another) or modification of a base, for example, the chemical modification of a base.
- the phrase “chemical modifications” as used herein, includes modifications which introduce chemistries which differ from those seen in naturally occurring mRNA, for example, covalent modifications such as the introduction of modified nucleotides, (e.g., nucleotide analogs, or the inclusion of pendant groups which are not naturally found in such mRNA molecules).
- suitable modifications include alterations in one or more nucleotides of a codon such that the codon encodes the same amino acid but is more stable than the codon found in the wild-type version of the mRNA.
- C's cytidines
- U's uridines
- RNA devoid of C and U residues have been found to be stable to most RNases (Heidenreich, et al. J Biol Chem 269, 2131-8 (1994)).
- the number of C and/or U residues in an mRNA sequence is reduced.
- the number of C and/or U residues is reduced by substitution of one codon encoding a particular amino acid for another codon encoding the same or a related amino acid.
- Contemplated modifications to the mRNA nucleic acids of the present invention also include the incorporatation of pseudouridines.
- the incorporation of pseudouridines into the mRNA nucleic acids of the present invention may enhance stability and translational capacity, as well as diminishing immunogenicity in vivo. See, e.g., Karikó, K., et al., Molecular Therapy 16 (11): 1833-1840 (2008).
- Substitutions and modifications to the mRNA of the present invention may be performed by methods readily known to one or ordinary skill in the art.
- the constraints on reducing the number of C and U residues in a sequence will likely be greater within the coding region of an mRNA, compared to an untranslated region, (i.e., it will likely not be possible to eliminate all of the C and U residues present in the message while still retaining the ability of the message to encode the desired amino acid sequence).
- the degeneracy of the genetic code presents an opportunity to allow the number of C and/or U residues that are present in the sequence to be reduced, while maintaining the same coding capacity (i.e., depending on which amino acid is encoded by a codon, several different possibilities for modification of RNA sequences may be possible).
- the codons for Gly can be altered to GGA or GGG instead of GGU or GGC.
- modification also includes, for example, the incorporation of non-nucleotide linkages or modified nucleotides into the mRNA sequences of the present invention (e.g., modifications to one or both the 3′ and 5′ ends of an mRNA molecule encoding a functional secreted protein or enzyme).
- modifications include the addition of bases to an mRNA sequence (e.g., the inclusion of a poly A tail or a longer poly A tail), the alteration of the 3′ UTR or the 5′ UTR, complexing the mRNA with an agent (e.g., a protein or a complementary nucleic acid molecule), and inclusion of elements which change the structure of an mRNA molecule (e.g., which form secondary structures).
- the poly A tail is thought to stabilize natural messengers. Therefore, in one embodiment a long poly A tail can be added to an mRNA molecule thus rendering the mRNA more stable.
- Poly A tails can be added using a variety of art-recognized techniques. For example, long poly A tails can be added to synthetic or in vitro transcribed mRNA using poly A polymerase (Yokoe, et al. Nature Biotechnology. 1996; 14: 1252-1256).
- a transcription vector can also encode long poly A tails.
- poly A tails can be added by transcription directly from PCR products. In one embodiment, the length of the poly A tail is at least about 90, 200, 300, 400 at least 500 nucleotides.
- the length of the poly A tail is adjusted to control the stability of a modified mRNA molecule of the invention and, thus, the transcription of protein.
- the length of the poly A tail can influence the half-life of an mRNA molecule, the length of the poly A tail can be adjusted to modify the level of resistance of the mRNA to nucleases and thereby control the time course of protein expression in a cell.
- the stabilized mRNA molecules are sufficiently resistant to in vivo degradation (e.g., by nucleases), such that they may be delivered to the target cell without a transfer vehicle.
- an mRNA can be modified by the incorporation 3′ and/or 5′ untranslated (UTR) sequences which are not naturally found in the wild-type mRNA.
- 3′ and/or 5′ flanking sequence which naturally flanks an mRNA and encodes a second, unrelated protein can be incorporated into the nucleotide sequence of an mRNA molecule encoding a therapeutic or functional protein in order to modify it.
- 3′ or 5′ sequences from mRNA molecules which are stable can be incorporated into the 3′ and/or 5′ region of a sense mRNA nucleic acid molecule to increase the stability of the sense mRNA molecule.
- stable e.g., globin, actin, GAPDH, tubulin, histone, or citric acid cycle enzymes
- the mRNA in the compositions of the invention include modification of the 5′ end of the mRNA to include a partial sequence of a CMV immediate-early 1 (IE1) gene, or a fragment thereof (e.g., SEQ ID NO:1) to improve the nuclease resistance and/or improve the half-life of the mRNA.
- IE1 CMV immediate-early 1
- a human growth hormone (hGH) gene sequence or a fragment thereof (e.g., SEQ ID NO:2) to the 3′ ends of the nucleic acid (e.g., mRNA) to further stabilize the mRNA.
- preferred modifications improve the stability and/or pharmacokinetic properties (e.g., half-life) of the mRNA relative to their unmodified counterparts, and include, for example modifications made to improve such mRNA's resistance to in vivo nuclease digestion.
- Variants may have greater than 90%, greater than 95%, greater than 98%, or greater than 99% sequence identity to SEQ ID NO:1 or SEQ ID NO:2.
- the composition can comprise a stabilizing reagent.
- the compositions can include one or more formulation reagents that bind directly or indirectly to, and stabilize the mRNA, thereby enhancing residence time in the target cell.
- Such reagents preferably lead to an improved half-life of the mRNA in the target cells.
- the stability of an mRNA and efficiency of translation may be increased by the incorporation of “stabilizing reagents” that form complexes with the mRNA that naturally occur within a cell (see e.g., U.S. Pat. No. 5,677,124).
- a stabilizing reagent can be accomplished for example, by combining the poly A and a protein with the mRNA to be stabilized in vitro before loading or encapsulating the mRNA within a transfer vehicle.
- exemplary stabilizing reagents include one or more proteins, peptides, aptamers, translational accessory protein, mRNA binding proteins, and/or translation initiation factors.
- Stabilization of the compositions may also be improved by the use of opsonization-inhibiting moieties, which are typically large hydrophilic polymers that are chemically or physically bound to the transfer vehicle (e.g., by the intercalation of a lipid-soluble anchor into the membrane itself, or by binding directly to active groups of membrane lipids).
- opsonization-inhibiting hydrophilic polymers form a protective surface layer which significantly decreases the uptake of the liposomes by the macrophage-monocyte system and reticulo-endothelial system (e.g., as described in U.S. Pat. No. 4,920,016, the entire disclosure of which is herein incorporated by reference).
- Transfer vehicles modified with opsonization-inhibition moieties thus remain in the circulation much longer than their unmodified counterparts.
- RNA When RNA is hybridized to a complementary nucleic acid molecule (e.g., DNA or RNA) it may be protected from nucleases. (Krieg, et al. Melton. Methods in Enzymology. 1987; 155, 397-415). The stability of hybridized mRNA is likely due to the inherent single strand specificity of most RNases.
- the stabilizing reagent selected to complex a mRNA is a eukaryotic protein, (e.g., a mammalian protein).
- the mRNA can be modified by hybridization to a second nucleic acid molecule. If an entire mRNA molecule were hybridized to a complementary nucleic acid molecule translation initiation may be reduced.
- the 5′ untranslated region and the AUG start region of the mRNA molecule may optionally be left unhybridized. Following translation initiation, the unwinding activity of the ribosome complex can function even on high affinity duplexes so that translation can proceed. (Liebhaber. J. Mol. Biol. 1992; 226: 2-13; Monia, et al. J Biol. Chem. 1993; 268: 14514-22).
- any of the above described methods for enhancing the stability of mRNA may be used either alone or in combination with one or more of any of the other above-described methods and/or compositions.
- the mRNA of the present invention may be optionally combined with a reporter gene (e.g., upstream or downstream of the coding region of the mRNA) which, for example, facilitates the determination of mRNA delivery to the target cells or tissues.
- a reporter gene e.g., upstream or downstream of the coding region of the mRNA
- Suitable reporter genes may include, for example, Green Fluorescent Protein mRNA (GFP mRNA), Renilla Luciferase mRNA (Luciferase mRNA), Firefly Luciferase mRNA, or any combinations thereof.
- GFP mRNA may be fused with a mRNA encoding a secretable protein to facilitate confirmation of mRNA localization in the target cells that will act as a depot for protein production.
- transfect or “transfection” mean the intracellular introduction of a mRNA into a cell, or preferably into a target cell.
- the introduced mRNA may be stably or transiently maintained in the target cell.
- transfection efficiency refers to the relative amount of mRNA taken up by the target cell which is subject to transfection. In practice, transfection efficiency is estimated by the amount of a reporter nucleic acid product expressed by the target cells following transfection.
- Preferred embodiments include compositions with high transfection efficacies and in particular those compositions that minimize adverse effects which are mediated by transfection of non-target cells.
- the transfer vehicles of the present invention are capable of delivering large mRNA sequences (e.g., mRNA of at least 1 kDa, 1.5 kDa, 2 kDa, 2.5 kDa, 5 kDa, 10 kDa, 12 kDa, 15 kDa, 20 kDa, 25 kDa, 30 kDa, or more).
- the mRNA can be formulated with one or more acceptable reagents, which provide a vehicle for delivering such mRNA to target cells.
- Appropriate reagents are generally selected with regard to a number of factors, which include, among other things, the biological or chemical properties of the mRNA, the intended route of administration, the anticipated biological environment to which such mRNA will be exposed and the specific properties of the intended target cells.
- transfer vehicles such as liposomes, encapsulate the mRNA without compromising biological activity.
- the transfer vehicle demonstrates preferential and/or substantial binding to a target cell relative to non-target cells.
- the transfer vehicle delivers its contents to the target cell such that the mRNA are delivered to the appropriate subcellular compartment, such as the cytoplasm.
- the transfer vehicle in the compositions of the invention is a liposomal transfer vehicle, e.g. a lipid nanoparticle.
- the transfer vehicle may be selected and/or prepared to optimize delivery of the mRNA to a target cell. For example, if the target cell is a hepatocyte the properties of the transfer vehicle (e.g., size, charge and/or pH) may be optimized to effectively deliver such transfer vehicle to the target cell, reduce immune clearance and/or promote retention in that target cell.
- the target cell is the central nervous system (e.g., mRNA administered for the treatment of neurodegenerative diseases may specifically target brain or spinal tissue)
- selection and preparation of the transfer vehicle must consider penetration of, and retention within the blood brain barrier and/or the use of alternate means of directly delivering such transfer vehicle to such target cell.
- the compositions of the present invention may be combined with agents that facilitate the transfer of exogenous mRNA (e.g., agents which disrupt or improve the permeability of the blood brain barrier and thereby enhance the transfer of exogenous mRNA to the target cells).
- Liposomes e.g., liposomal lipid nanoparticles
- Liposomes are generally useful in a variety of applications in research, industry, and medicine, particularly for their use as transfer vehicles of diagnostic or therapeutic compounds in vivo (Lasic, Trends Biotechnol., 16: 307-321, 1998; Drummond et al., Pharmacol. Rev., 51: 691-743, 1999) and are usually characterized as microscopic vesicles having an interior aqua space sequestered from an outer medium by a membrane of one or more bilayers.
- Bilayer membranes of liposomes are typically formed by amphiphilic molecules, such as lipids of synthetic or natural origin that comprise spatially separated hydrophilic and hydrophobic domains (Lasic, Trends Biotechnol., 16: 307-321, 1998). Bilayer membranes of the liposomes can also be formed by amphiphilic polymers and surfactants (e.g., polymerosomes, niosomes, etc.).
- a liposomal transfer vehicle typically serves to transport the mRNA to the target cell.
- the liposomal transfer vehicles are prepared to contain the desired nucleic acids.
- the process of incorporation of a desired entity (e.g., a nucleic acid) into a liposome is often referred to as “loading” (Lasic, et al., FEBS Lett., 312: 255-258, 1992).
- the liposome-incorporated nucleic acids may be completely or partially located in the interior space of the liposome, within the bilayer membrane of the liposome, or associated with the exterior surface of the liposome membrane.
- nucleic acid into liposomes is also referred to herein as “encapsulation” wherein the nucleic acid is entirely contained within the interior space of the liposome.
- encapsulation wherein the nucleic acid is entirely contained within the interior space of the liposome.
- the purpose of incorporating a mRNA into a transfer vehicle, such as a liposome, is often to protect the nucleic acid from an environment which may contain enzymes or chemicals that degrade nucleic acids and/or systems or receptors that cause the rapid excretion of the nucleic acids. Accordingly, in a preferred embodiment of the present invention, the selected transfer vehicle is capable of enhancing the stability of the mRNA contained therein.
- the liposome can allow the encapsulated mRNA to reach the target cell and/or may preferentially allow the encapsulated mRNA to reach the target cell, or alternatively limit the delivery of such mRNA to other sites or cells where the presence of the administered mRNA may be useless or undesirable. Furthermore, incorporating the mRNA into a transfer vehicle, such as for example, a cationic liposome, also facilitates the delivery of such mRNA into a target cell.
- a transfer vehicle such as for example, a cationic liposome
- liposomal transfer vehicles are prepared to encapsulate one or more desired mRNA such that the compositions demonstrate a high transfection efficiency and enhanced stability. While liposomes can facilitate introduction of nucleic acids into target cells, the addition of polycations (e.g., poly L-lysine and protamine), as a copolymer can facilitate, and in some instances markedly enhance the transfection efficiency of several types of cationic liposomes by 2-28 fold in a number of cell lines both in vitro and in vivo. (See N. J. Caplen, et al., Gene Ther. 1995; 2: 603; S. Li, et al., Gene Ther. 1997; 4, 891).
- polycations e.g., poly L-lysine and protamine
- the transfer vehicle is formulated as a lipid nanoparticle.
- lipid nanoparticle refers to a transfer vehicle comprising one or more lipids (e.g., cationic lipids, non-cationic lipids, and PEG-modified lipids).
- the lipid nanoparticles are formulated to deliver one or more mRNA to one or more target cells.
- lipids include, for example, the phosphatidyl compounds (e.g., phosphatidylglycerol, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, sphingolipids, cerebrosides, and gangliosides). Also contemplated is the use of polymers as transfer vehicles, whether alone or in combination with other transfer vehicles.
- phosphatidyl compounds e.g., phosphatidylglycerol, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, sphingolipids, cerebrosides, and gangliosides.
- polymers as transfer vehicles, whether alone or in combination with other transfer vehicles.
- Suitable polymers may include, for example, polyacrylates, polyalkycyanoacrylates, polylactide, polylactide-polyglycolide copolymers, polycaprolactones, dextran, albumin, gelatin, alginate, collagen, chitosan, cyclodextrins, dendrimers and polyethylenimine.
- the transfer vehicle is selected based upon its ability to facilitate the transfection of a mRNA to a target cell.
- lipid nanoparticles as transfer vehicles comprising a cationic lipid to encapsulate and/or enhance the delivery of mRNA into the target cell that will act as a depot for protein production.
- cationic lipid refers to any of a number of lipid species that carry a net positive charge at a selected pH, such as physiological pH.
- the contemplated lipid nanoparticles may be prepared by including multi-component lipid mixtures of varying ratios employing one or more cationic lipids, non-cationic lipids and PEG-modified lipids.
- Several cationic lipids have been described in the literature, many of which are commercially available.
- compositions and methods of the invention include those described in international patent publication WO 2010/053572, incorporated herein by reference, and most particularly, C12-200 described at paragraph [00225] of WO 2010/053572.
- the compositions and methods of the invention employ a lipid nanoparticles comprising an ionizable cationic lipid described in U.S. provisional patent application 61/617,468, filed Mar.
- the cationic lipid N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride or “DOTMA” is used.
- DOTMA can be formulated alone or can be combined with the neutral lipid, dioleoylphosphatidyl-ethanolamine or “DOPE” or other cationic or non-cationic lipids into a liposomal transfer vehicle or a lipid nanoparticle, and such liposomes can be used to enhance the delivery of nucleic acids into target cells.
- Suitable cationic lipids include, for example, 5-carboxyspermylglycinedioctadecylamide or “DOGS,” 2,3-dioleyloxy-N-[2(spermine-carboxamido)ethyl]-N,N-dimethyl-1-propanaminium or “DOSPA” (Behr et al. Proc. Nat'l Acad. Sci. 86, 6982 (1989); U.S. Pat. No. 5,171,678; U.S. Pat. No.
- Contemplated cationic lipids also include 1,2-distearyloxy-N,N-dimethyl-3-aminopropane or “DSDMA”, 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane or “DODMA”, 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane or “DLinDMA”, 1,2-dilinolenyloxy-N,N-dimethyl-3-aminopropane or “DLenDMA”, N-dioleyl-N,N-dimethylammonium chloride or “DODAC”, N,N-distearyl-N,N-dimethylammonium bromide or “DDAB”, N-(1,2-dim
- cholesterol-based cationic lipids are also contemplated by the present invention.
- Such cholesterol-based cationic lipids can be used, either alone or in combination with other cationic or non-cationic lipids.
- Suitable cholesterol-based cationic lipids include, for example, DC-Chol (N,N-dimethyl-N-ethylcarboxamidocholesterol), 1,4-bis(3-N-oleylamino-propyl)piperazine (Gao, et al. Biochem. Biophys. Res. Comm. 179, 280 (1991); Wolf et al. BioTechniques 23, 139 (1997); U.S. Pat. No. 5,744,335), or ICE.
- LIPOFECTIN DOTMA:DOPE
- DOSPA:DOPE LIPOFECTAMINE
- LIPOFECTAMINE2000. Invitrogen
- FUGENE FUGENE
- TRANSFECTAM DOGS
- EFFECTENE EFFECTENE
- cationic lipids such as the dialkylamino-based, imidazole-based, and guanidinium-based lipids.
- certain embodiments are directed to a composition comprising one or more imidazole-based cationic lipids, for example, the imidazole cholesterol ester or “ICE” lipid (3S,10R,13R,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl 3-(1H-imidazol-4-yl)propanoate, as represented by structure (I) below.
- imidazole cholesterol ester or “ICE” lipid 3S,10R,13R,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,
- a transfer vehicle for delivery of mRNA may comprise one or more imidazole-based cationic lipids, for example, the imidazole cholesterol ester or “ICE” lipid (3S,10R,13R,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl 3-(1H-imidazol-4-yl)propanoate, as represented by structure (I).
- imidazole cholesterol ester or “ICE” lipid 3S,10R,13R,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl
- the fusogenicity of the imidazole-based cationic lipid ICE is related to the endosomal disruption which is facilitated by the imidazole group, which has a lower pKa relative to traditional cationic lipids.
- the endosomal disruption in turn promotes osmotic swelling and the disruption of the liposomal membrane, followed by the transfection or intracellular release of the nucleic acid(s) contents loaded therein into the target cell.
- the imidazole-based cationic lipids are also characterized by their reduced toxicity relative to other cationic lipids.
- the imidazole-based cationic lipids e.g., ICE
- the imidazole-based cationic lipids may be used as the sole cationic lipid in the lipid nanoparticle, or alternatively may be combined with traditional cationic lipids, non-cationic lipids, and PEG-modified lipids.
- the cationic lipid may comprise a molar ratio of about 1% to about 90%, about 2% to about 70%, about 5% to about 50%, about 10% to about 40% of the total lipid present in the transfer vehicle, or preferably about 20% to about 70% of the total lipid present in the transfer vehicle.
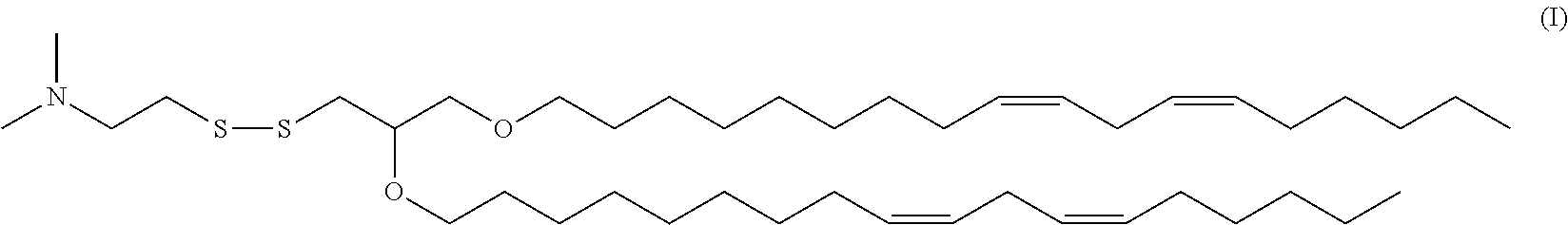
- certain embodiments are directed to lipid nanoparticles comprising the HGT4003 cationic lipid 2-((2,3-Bis((9Z,12Z)-octadeca-9,12-dien-1-yloxy)propyl)disulfanyl)-N,N-dimethylethanamine, as represented by structure (II) below, and as further described in U.S. Provisional Application No:61/494,745, filed Jun. 8, 2011, the entire teachings of which are incorporated herein by reference in their entirety:
- compositions and methods described herein are directed to lipid nanoparticles comprising one or more cleavable lipids, such as, for example, one or more cationic lipids or compounds that comprise a cleavable disulfide (S—S) functional group (e.g., HGT4001, HGT4002, HGT4003, HGT4004 and HGT4005), as further described in U.S. Provisional Application No. 61/494,745, the entire teachings of which are incorporated herein by reference in their entirety.
- S—S cleavable disulfide
- PEG polyethylene glycol
- PEG-CER derivatized ceramides
- C8 PEG-2000 ceramide N-Octanoyl-Sphingosine-1-[Succinyl(Methoxy Polyethylene Glycol)-2000]
- Contemplated PEG-modified lipids include, but is not limited to, a polyethylene glycol chain of up to 5 kDa in length covalently attached to a lipid with alkyl chain(s) of C 6 -C 20 length.
- the addition of such components may prevent complex aggregation and may also provide a means for increasing circulation lifetime and increasing the delivery of the lipid-nucleic acid composition to the target cell, (Klibanov et al. (1990) FEBS Letters, 268 (1): 235-237), or they may be selected to rapidly exchange out of the formulation in vivo (see U.S. Pat. No. 5,885,613).
- Particularly useful exchangeable lipids are PEG-ceramides having shorter acyl chains (e.g., C14 or C18).
- the PEG-modified phospholipid and derivitized lipids of the present invention may comprise a molar ratio from about 0% to about 20%, about 0.5% to about 20%, about 1% to about 15%, about 4% to about 10%, or about 2% of the total lipid present in the liposomal transfer vehicle.
- non-cationic lipid refers to any neutral, zwitterionic or anionic lipid.
- anionic lipid refers to any of a number of lipid species that carry a net negative charge at a selected pH, such as physiological pH.
- Non-cationic lipids include, but are not limited to, distearoylphosphatidylcholine (DSPC), dioleoylphosphatidylcholine (DOPC), dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylglycerol (DOPG), dipalmitoylphosphatidylglycerol (DPPG), dioleoylphosphatidylethanolamine (DOPE), palmitoyloleoylphosphatidylcholine (POPC), palmitoyloleoyl-phosphatidylethanolamine (POPE), dioleoyl-phosphatidylethanolamine 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (DOPE-mal), dipalmitoyl phosphatidyl ethanolamine (DPPE), dimyristoylphosphoethanolamine (DMPE), distearoyl-phosphatidyl-ethanolamine (DSPE
- non-cationic lipids may be used alone, but are preferably used in combination with other excipients, for example, cationic lipids.
- the non-cationic lipid may comprise a molar ratio of 5% to about 90%, or preferably about 10% to about 70% of the total lipid present in the transfer vehicle.
- the transfer vehicle (e.g., a lipid nanoparticle) is prepared by combining multiple lipid and/or polymer components.
- a transfer vehicle may be prepared using C12-200, DOPE, chol, DMG-PEG2K at a molar ratio of 40:30:25:5, or DODAP, DOPE, cholesterol, DMG-PEG2K at a molar ratio of 18:56:20:6, or HGT5000, DOPE, chol, DMG-PEG2K at a molar ratio of 40:20:35:5, or HGT5001, DOPE, chol, DMG-PEG2K at a molar ratio of 40:20:35:5.
- cationic lipids non-cationic lipids and/or PEG-modified lipids which comprise the lipid nanoparticle, as well as the relative molar ratio of such lipids to each other, is based upon the characteristics of the selected lipid(s), the nature of the intended target cells, the characteristics of the mRNA to be delivered. Additional considerations include, for example, the saturation of the alkyl chain, as well as the size, charge, pH, pKa, fusogenicity and toxicity of the selected lipid(s). Thus the molar ratios may be adjusted accordingly.
- the percentage of cationic lipid in the lipid nanoparticle may be greater than 10%, greater than 20%, greater than 30%, greater than 40%, greater than 50%, greater than 60%, or greater than 70%.
- the percentage of non-cationic lipid in the lipid nanoparticle may be greater than 5%, greater than 10%, greater than 20%, greater than 30%, or greater than 40%.
- the percentage of cholesterol in the lipid nanoparticle may be greater than 10%, greater than 20%, greater than 30%, or greater than 40%.
- the percentage of PEG-modified lipid in the lipid nanoparticle may be greater than 1%, greater than 2%, greater than 5%, greater than 10%, or greater than 20%.
- the lipid nanoparticles of the invention comprise at least one of the following cationic lipids: C12-200, DLin-KC2-DMA, DODAP, HGT4003, ICE, HGT5000, or HGT5001.
- the transfer vehicle comprises cholesterol and/or a PEG-modified lipid.
- the transfer vehicles comprises DMG-PEG2K.
- the tranfer vehicle comprises one of the following lipid formulations: C12-200, DOPE, chol, DMG-PEG2K; DODAP, DOPE, cholesterol, DMG-PEG2K; HGT5000, DOPE, chol, DMG-PEG2K, HGT5001, DOPE, chol, DMG-PEG2K.
- the liposomal transfer vehicles for use in the compositions of the invention can be prepared by various techniques which are presently known in the art.
- Multi-lamellar vesicles may be prepared conventional techniques, for example, by depositing a selected lipid on the inside wall of a suitable container or vessel by dissolving the lipid in an appropriate solvent, and then evaporating the solvent to leave a thin film on the inside of the vessel or by spray drying. An aqueous phase may then added to the vessel with a vortexing motion which results in the formation of MLVs.
- Uni-lamellar vesicles (ULV) can then be formed by homogenization, sonication or extrusion of the multi-lamellar vesicles.
- unilamellar vesicles can be formed by detergent removal techniques.
- compositions of the present invention comprise a transfer vehicle wherein the mRNA is associated on both the surface of the transfer vehicle and encapsulated within the same transfer vehicle.
- cationic liposomal transfer vehicles may associate with the mRNA through electrostatic interactions.
- compositions of the invention may be loaded with diagnostic radionuclide, fluorescent materials or other materials that are detectable in both in vitro and in vivo applications.
- suitable diagnostic materials for use in the present invention may include Rhodamine-dioleoylphosphatidylethanolamine (Rh-PE), Green Fluorescent Protein mRNA (GFP mRNA), Renilla Luciferase mRNA and Firefly Luciferase mRNA.
- a liposomal transfer vehicle may be sized such that its dimensions are smaller than the fenestrations of the endothelial layer lining hepatic sinusoids in the liver; accordingly the liposomal transfer vehicle can readily penetrate such endothelial fenestrations to reach the target hepatocytes.
- a liposomal transfer vehicle may be sized such that the dimensions of the liposome are of a sufficient diameter to limit or expressly avoid distribution into certain cells or tissues.
- a liposomal transfer vehicle may be sized such that its dimensions are larger than the fenestrations of the endothelial layer lining hepatic sinusoids to thereby limit distribution of the liposomal transfer vehicle to hepatocytes.
- the size of the transfer vehicle is within the range of about 25 to 250 nm, preferably less than about 250 nm, 175 nm, 150 nm, 125 nm, 100 nm, 75 nm, 50 nm, 25 nm or 10 nm.
- the size of the liposomal vesicles may be determined by quasi-electric light scattering (QELS) as described in Bloomfield, Ann. Rev. Biophys. Bioeng., 10:421-450 (1981), incorporated herein by reference. Average liposome diameter may be reduced by sonication of formed liposomes. Intermittent sonication cycles may be alternated with QELS assessment to guide efficient liposome synthesis.
- QELS quasi-electric light scattering
- target cell refers to a cell or tissue to which a composition of the invention is to be directed or targeted.
- the target cells are deficient in a protein or enzyme of interest.
- the hepatocyte represents the target cell.
- the compositions of the invention transfect the target cells on a discriminatory basis (i.e., do not transfect non-target cells).
- compositions of the invention may also be prepared to preferentially target a variety of target cells, which include, but are not limited to, hepatocytes, epithelial cells, hematopoietic cells, epithelial cells, endothelial cells, lung cells, bone cells, stem cells, mesenchymal cells, neural cells (e.g., meninges, astrocytes, motor neurons, cells of the dorsal root ganglia and anterior horn motor neurons), photoreceptor cells (e.g., rods and cones), retinal pigmented epithelial cells, secretory cells, cardiac cells, adipocytes, vascular smooth muscle cells, cardiomyocytes, skeletal muscle cells, beta cells, pituitary cells, synovial lining cells, ovarian cells, testicular cells, fibroblasts, B cells, T cells, reticulocytes, leukocytes, granulocytes and tumor cells.
- target cells include, but are not limited to, hepatocytes, epi
- compositions of the invention may be prepared to preferentially distribute to target cells such as in the heart, lungs, kidneys, liver, and spleen.
- the compositions of the invention distribute into the cells of the liver to facilitate the delivery and the subsequent expression of the mRNA comprised therein by the cells of the liver (e.g., hepatocytes).
- the targeted hepatocytes may function as a biological “reservoir” or “depot” capable of producing, and systemically excreting a functional protein or enzyme.
- the liposomal transfer vehicle may target hepatocytes and/or preferentially distribute to the cells of the liver upon delivery.
- the mRNA loaded in the liposomal vehicle are translated and a functional protein product is produced, excreted and systemically distributed.
- cells other than hepatocytes e.g., lung, spleen, heart, ocular, or cells of the central nervous system
- the compositions of the invention facilitate a subject's endogenous production of one or more functional proteins and/or enzymes, and in particular the production of proteins and/or enzymes which demonstrate less immunogenicity relative to their recombinantly-prepared counterparts.
- the transfer vehicles comprise mRNA which encode a deficient protein or enzyme.
- the exogenous mRNA loaded into the liposomal transfer vehicle e.g., a lipid nanoparticle
- the exogenous mRNA loaded into the liposomal transfer vehicle may be translated in vivo to produce a functional protein or enzyme encoded by the exogenously administered mRNA (e.g., a protein or enzyme in which the subject is deficient).
- compositions of the present invention exploit a subject's ability to translate exogenously- or recombinantly-prepared mRNA to produce an endogenously-translated protein or enzyme, and thereby produce (and where applicable excrete) a functional protein or enzyme.
- the expressed or translated proteins or enzymes may also be characterized by the in vivo inclusion of native post-translational modifications which may often be absent in recombinantly-prepared proteins or enzymes, thereby further reducing the immunogenicity of the translated protein or enzyme.
- mRNA encoding a deficient protein or enzyme avoids the need to deliver the nucleic acids to specific organelles within a target cell (e.g., mitochondria). Rather, upon transfection of a target cell and delivery of the nucleic acids to the cytoplasm of the target cell, the mRNA contents of a transfer vehicle may be translated and a functional protein or enzyme expressed.
- the present invention also contemplates the discriminatory targeting of target cells and tissues by both passive and active targeting means.
- the phenomenon of passive targeting exploits the natural distributions patterns of a transfer vehicle in vivo without relying upon the use of additional excipients or means to enhance recognition of the transfer vehicle by target cells.
- transfer vehicles which are subject to phagocytosis by the cells of the reticulo-endothelial system are likely to accumulate in the liver or spleen, and accordingly may provide means to passively direct the delivery of the compositions to such target cells.
- the present invention contemplates active targeting, which involves the use of additional excipients, referred to herein as “targeting ligands” that may be bound (either covalently or non-covalently) to the transfer vehicle to encourage localization of such transfer vehicle at certain target cells or target tissues.
- targeting may be mediated by the inclusion of one or more endogenous targeting ligands (e.g., apolipoprotein E) in or on the transfer vehicle to encourage distribution to the target cells or tissues.
- endogenous targeting ligands e.g., apolipoprotein E
- the composition can comprise a ligand capable of enhancing affinity of the composition to the target cell.
- Targeting ligands may be linked to the outer bilayer of the lipid particle during formulation or post-formulation.
- lipid particle formulations may employ fusogenic polymers such as PEAA, hemagluttinin, other lipopeptides (see U.S. patent application Ser. No. 08/835,281, and U.S. patent application Ser. No. 60/083,294, which are incorporated herein by reference) and other features useful for in vivo and/or intracellular delivery.
- fusogenic polymers such as PEAA, hemagluttinin, other lipopeptides (see U.S. patent application Ser. No. 08/835,281, and U.S. patent application Ser. No. 60/083,294, which are incorporated herein by reference) and other features useful for in vivo and/or intracellular delivery.
- the compositions of the present invention demonstrate improved transfection efficacies, and/or demonstrate enhanced selectivity towards target cells or tissues of interest.
- compositions which comprise one or more ligands (e.g., peptides, aptamers, oligonucleotides, a vitamin or other molecules) that are capable of enhancing the affinity of the compositions and their nucleic acid contents for the target cells or tissues.
- ligands may optionally be bound or linked to the surface of the transfer vehicle.
- the targeting ligand may span the surface of a transfer vehicle or be encapsulated within the transfer vehicle.
- Suitable ligands and are selected based upon their physical, chemical or biological properties (e.g., selective affinity and/or recognition of target cell surface markers or features.) Cell-specific target sites and their corresponding targeting ligand can vary widely.
- compositions of the invention may include surface markers (e.g., apolipoprotein-B or apolipoprotein-E) that selectively enhance recognition of, or affinity to hepatocytes (e.g., by receptor-mediated recognition of and binding to such surface markers).
- surface markers e.g., apolipoprotein-B or apolipoprotein-E
- the use of galactose as a targeting ligand would be expected to direct the compositions of the present invention to parenchymal hepatocytes, or alternatively the use of mannose containing sugar residues as a targeting ligand would be expected to direct the compositions of the present invention to liver endothelial cells (e.g., mannose containing sugar residues that may bind preferentially to the asialoglycoprotein receptor present in hepatocytes). (See Hillery A M, et al.
- targeting ligands that have been conjugated to moieties present in the transfer vehicle (e.g., a lipid nanoparticle) therefore facilitate recognition and uptake of the compositions of the present invention in target cells and tissues.
- suitable targeting ligands include one or more peptides, proteins, aptamers, vitamins and oligonucleotides.
- the term “subject” refers to any animal (e.g., a mammal), including, but not limited to, humans, non-human primates, rodents, and the like, to which the compositions and methods of the present invention are administered.
- the terms “subject” and “patient” are used interchangeably herein in reference to a human subject.
- compositions and methods of the invention provide for the delivery of mRNA to treat a number of disorders.
- the compositions and methods of the present invention are suitable for the treatment of diseases or disorders relating to the deficiency of proteins and/or enzymes that are excreted or secreted by the target cell into the surrounding extracellular fluid (e.g., mRNA encoding hormones and neurotransmitters).
- the disease may involve a defect or deficiency in a secreted protein (e.g. Fabry disease, or ALS).
- the disease may not be caused by a defect or deficit in a secreted protein, but may benefit from providing a secreted protein.
- the symptoms of a disease may be improved by providing the compositions of the invention (e.g. cystic fibrosis).
- disorders for which the present invention are useful include, but are not limited to, disorders such as Huntington's Disease; Parkinson's Disease; muscular dystrophies (such as, e.g.
- hemophelia diseases such as, e.g., hemophilioa B (FIX), hemophilia A (FVIII); SMN1-related spinal muscular atrophy (SMA); amyotrophic lateral sclerosis (ALS); GALT-related galactosemia; Cystic Fibrosis (CF); SLC3A1-related disorders including cystinuria; COL4A5-related disorders including Alport syndrome; galactocerebrosidase deficiencies; X-linked adrenoleukodystrophy and adrenomyeloneuropathy; Friedreich's ataxia; Pelizaeus-Merzbacher disease; TSC1 and TSC2-related tuberous sclerosis; Sanfilippo B syndrome (MPS IIIB); CTNS-related cystinosis; the FMR1-related disorders which include Fragile X syndrome, Fragile X-Associated Tremor/Ataxia Syndrome and Fragile X Premature Ovarian Failure Syndrome
- the nucleic acids, and in particular mRNA, of the invention may encode functional proteins or enzymes that are secreted into extracellular space.
- the secreted proteins include clotting factors, components of the complement pathway, cytokines, chemokines, chemoattractants, protein hormones (e.g. EGF, PDF), protein components of serum, antibodies, secretable toll-like receptors, and others.
- the compositions of the present invention may include mRNA encoding erythropoietin, ⁇ 1-antitrypsin, carboxypeptidase N or human growth hormone.
- the invention encodes a secreted protein that is made up of subunits that are encoded by more than one gene.
- the secreted protein may be a heterodimer, wherein each chain or subunit of the is encoded by a separate gene. It is possible that more than one mRNA molecule is delivered in the transfer vehicle and the mRNA encodes separate subunit of the secreted protein.
- a single mRNA may be engineered to encode more than one subunit (e.g. in the case of a single-chain Fv antibody).
- separate mRNA molecules encoding the individual subunits may be administered in separate transfer vehicles.
- the mRNA may encode full length antibodies (both heavy and light chains of the variable and constant regions) or fragments of antibodies (e.g. Fab, Fv, or a single chain Fv (scFv) to confer immunity to a subject.
- Fab, Fv, or a single chain Fv (scFv) to confer immunity to a subject.
- scFv single chain Fv
- the compositions of the present invention encode antibodies that may be used to transiently or chronically effect a functional response in subjects.
- the mRNA of the present invention may encode a functional monoclonal or polyclonal antibody, which upon translation and secretion from target cell may be useful for targeting and/or inactivating a biological target (e.g., a stimulatory cytokine such as tumor necrosis factor).
- a biological target e.g., a stimulatory cytokine such as tumor necrosis factor
- the mRNA nucleic acids of the present invention may encode, for example, functional anti-nephritic factor antibodies useful for the treatment of membranoproliferative glomerulonephritis type II or acute hemolytic uremic syndrome, or alternatively may encode anti-vascular endothelial growth factor (VEGF) antibodies useful for the treatment of VEGF-mediated diseases, such as cancer.
- the secreted protein is a cytokine or other secreted protein comprised of more than one subunit (e.g. IL-12, or IL-23).
- compositions of the invention can be administered to a subject.
- the composition is formulated in combination with one or more additional nucleic acids, carriers, targeting ligands or stabilizing reagents, or in pharmacological compositions where it is mixed with suitable excipients.
- the compositions of the invention may be prepared to deliver mRNA encoding two or more distinct proteins or enzymes. Techniques for formulation and administration of drugs may be found in “Remington's Pharmaceutical Sciences,” Mack Publishing Co., Easton, Pa., latest edition.
- a wide range of molecules that can exert pharmaceutical or therapeutic effects can be delivered into target cells using compositions and methods of the invention.
- the molecules can be organic or inorganic.
- Organic molecules can be peptides, proteins, carbohydrates, lipids, sterols, nucleic acids (including peptide nucleic acids), or any combination thereof.
- a formulation for delivery into target cells can comprise more than one type of molecule, for example, two different nucleotide sequences, or a protein, an enzyme or a steroid.
- compositions of the present invention may be administered and dosed in accordance with current medical practice, taking into account the clinical condition of the subject, the site and method of administration, the scheduling of administration, the subject's age, sex, body weight and other factors relevant to clinicians of ordinary skill in the art.
- the “effective amount” for the purposes herein may be determined by such relevant considerations as are known to those of ordinary skill in experimental clinical research, pharmacological, clinical and medical arts.
- the amount administered is effective to achieve at least some stabilization, improvement or elimination of symptoms and other indicators as are selected as appropriate measures of disease progress, regression or improvement by those of skill in the art.
- a suitable amount and dosing regimen is one that causes at least transient protein production.
- Suitable routes of administration include, for example, oral, rectal, vaginal, transmucosal, pulmonary including intratracheal or inhaled, or intestinal administration; parenteral delivery, including intramuscular, subcutaneous, intramedullary injections, as well as intrathecal, direct intraventricular, intravenous, intraperitoneal, intranasal, or intraocular injections.
- compositions of the invention may be administered in a local rather than systemic manner, for example, via injection of the pharmaceutical composition directly into a targeted tissue, preferably in a sustained release formulation.
- Local delivery can be affected in various ways, depending on the tissue to be targeted.
- compositions of the present invention can be inhaled (for nasal, tracheal, or bronchial delivery); compositions of the present invention can be injected into the site of injury, disease manifestation, or pain, for example; compositions can be provided in lozenges for oral, tracheal, or esophageal application; can be supplied in liquid, tablet or capsule form for administration to the stomach or intestines, can be supplied in suppository form for rectal or vaginal application; or can even be delivered to the eye by use of creams, drops, or even injection.
- Formulations containing compositions of the present invention complexed with therapeutic molecules or ligands can even be surgically administered, for example in association with a polymer or other structure or substance that can allow the compositions to diffuse from the site of implantation to surrounding cells. Alternatively, they can be applied surgically without the use of polymers or supports.
- compositions of the invention are formulated such that they are suitable for extended-release of the mRNA contained therein.
- extended-release compositions may be conveniently administered to a subject at extended dosing intervals.
- the compositions of the present invention are administered to a subject twice day, daily or every other day.
- the compositions of the present invention are administered to a subject twice a week, once a week, every ten days, every two weeks, every three weeks, or more preferably every four weeks, once a month, every six weeks, every eight weeks, every other month, every three months, every four months, every six months, every eight months, every nine months or annually.
- compositions and liposomal vehicles which are formulated for depot administration (e.g., intramuscularly, subcutaneously, intravitreally) to either deliver or release a mRNA over extended periods of time.
- depot administration e.g., intramuscularly, subcutaneously, intravitreally
- the extended-release means employed are combined with modifications made to the mRNA to enhance stability.
- lyophilized pharmaceutical compositions comprising one or more of the liposomal nanoparticles disclosed herein and related methods for the use of such lyophilized compositions as disclosed for example, in U.S. Provisional Application No. 61/494,882, filed Jun. 8, 2011, the teachings of which are incorporated herein by reference in their entirety.
- lyophilized pharmaceutical compositions according to the invention may be reconstituted prior to administration or can be reconstituted in vivo.
- a lyophilized pharmaceutical composition can be formulated in an appropriate dosage form (e.g., an intradermal dosage form such as a disk, rod or membrane) and administered such that the dosage form is rehydrated over time in vivo by the individual's bodily fluids.
- EPO Human erythropoietin
- GLA human alpha-galactosidase
- A1AT human alpha-1 antitrypsin
- FIX human factor IX
- Formulation 1 Aliquots of 50 mg/mL ethanolic solutions of C12-200, DOPE, Chol and DMG-PEG2K (40:30:25:5) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1 ⁇ PBS (pH 7.4), concentrated and stored at 2-8° C.
- aqueous buffered solution 10 mM citrate/150 mM NaCl, pH 4.5
- Formulation 2 Aliquots of 50 mg/mL ethanolic solutions of DODAP, DOPE, cholesterol and DMG-PEG2K (18:56:20:6) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of EPO mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1 ⁇ PBS (pH 7.4), concentrated and stored at 2-8° C.
- aqueous buffered solution 10 mM citrate/150 mM NaCl, pH 4.5
- lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol.
- the resulting nanoparticle suspension was filtered, diafiltrated with 1 ⁇ PBS (pH 7.4), concentrated and stored at 2-8° C.
- Formulation 4 Aliquots of 50 mg/mL ethanolic solutions of ICE, DOPE and DMG-PEG2K (70:25:5) were mixed and diluted with ethanol to 3 mL final volume.
- an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of mRNA was prepared from a 1 mg/mL stock.
- lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol.
- the resulting nanoparticle suspension was filtered, diafiltrated with 1 ⁇ PBS (pH 7.4), concentrated and stored at 2-8° C.
- Formulation 5 Aliquots of 50 mg/mL ethanolic solutions of HGT5000, DOPE, cholesterol and DMG-PEG2K (40:20:35:5) were mixed and diluted with ethanol to 3 mL final volume.
- an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of EPO mRNA was prepared from a 1 mg/mL stock.
- an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of EPO mRNA was prepared from a 1 mg/mL stock.
- the lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol.
- the resulting nanoparticle suspension was filtered, diafiltrated with 1 ⁇ PBS (pH 7.4), concentrated and stored at 2-8° C.
- liver and spleen of each mouse was harvested, apportioned into three parts, and stored in either 10% neutral buffered formalin or snap-frozen and stored at ⁇ 80° C. for analysis.
- EPO protein Quantification of EPO protein was performed following procedures reported for human EPO ELISA kit (Quantikine IVD, R&D Systems, Catalog #Dep-00). Positive controls employed consisted of ultrapure and tissue culture grade recombinant human erythropoietin protein (R&D Systems, Catalog #286-EP and 287-TC, respectively). Detection was monitored via absorption (450 nm) on a Molecular Device Flex Station instrument.
- the work described in this example demonstrates the use of mRNA-encapsulated lipid nanoparticles as a depot source for the production of protein.
- a depot effect can be achieved in multiple sites within the body (i.e., liver, kidney, spleen, and muscle).
- Measurement of the desired exogenous-based protein derived from messenger RNA delivered via liposomal nanoparticles was achieved and quantified, and the secretion of protein from a depot using human erythropoietin (hEPO), human alpha-galactosidase (hGLA), human alpha-1 antitrypsin (hA1AT), and human Factor IX (hFIX) mRNA was demonstrated.
- hEPO human erythropoietin
- hGLA human alpha-galactosidase
- hA1AT human alpha-1 antitrypsin
- hFIX human Factor IX
- hEPO protein was demonstrated with various lipid nanoparticle formulations.
- C12-200-based lipid nanoparticles produced the highest quantity of hEPO protein after four hours post intravenous administration as measured by ELISA ( FIG. 7 ).
- This formulation (Formulation 1) resulted in 18.3 ug/mL hEPO protein secreted into the bloodstream.
- Normal hEPO protein levels in serum for human are 3.3-16.6 mIU/mL (NCCLS Document C28-P; Vol. 12, No. 2).
- NCCLS Document C28-P Vol. 12, No. 2
- a single 30 ug dose of a C12-200-based cationic lipid formulation encapsulating hEPO mRNA yielded an increase in respective protein of over 100,000-fold physiological levels.
- the DODAP-based lipid nanoparticle formulation was the least effective.
- the observed quantity of human EPO protein derived from delivery via a DODAP-based lipid nanoparticle encapsulating EPO mRNA was 4.1 ng/mL, which is still greater than 30-fold over normal physiological levels of EPO protein (Table 1).
- hematocrit changes were monitored over a ten day period for five different lipid nanoparticle formulations ( FIG. 8 , Table 1) to evaluate protein activity. During this time period, two of the five formulations demonstrated an increase in hematocrit ( ⁇ 15%), which is indicative of active hEPO protein being produced from such systems.
- hematocrit changes were monitored over a 15-day period ( FIG. 9 , Table 2).
- the lipid nanoparticle formulation (Formulation 1) was administered either as a single 30 ⁇ g dose, or as three smaller 10 ⁇ g doses injected on day 1, day 3 and day 5.
- Formulation 2 was administered as 3 doses of 50 ⁇ g on day 1, day 3, and day 5.
- C12-200 produced a significant increase in hematocrit.
- Overall an increase of up to ⁇ 25% change was observed, which is indicative of active human EPO protein being produced from such systems.
- hGLA protein Measurable levels of hGLA protein were observed throughout the time course of the experiment with a maximum level of 2.0 ug/mL hGLA protein at six hours ( FIG. 10 ).
- Table 3 lists the specific quantities of hGLA found in the serum. Normal activity in healthy human males has been reported to be approximately 3.05 nanomol/hr/mL. The activity for Alpha-galactosidase, a recombinant human alpha-galactosidase protein, 3.56 ⁇ 10 6 nanomol/hr/mg. Analysis of these values yields a quantity of approximately 856 pg/mL of hGLA protein in normal healthy male individuals.
- the quantity of 2.0 ug/mL hGLA protein observed after six hours when dosing a hGLA mRNA-loaded lipid nanoparticle is over 2300-fold greater than normal physiological levels. Further, after 48 hours, one can still detect appreciable levels of hGLA protein (86.2 ng/mL). This level is representative of almost 100-fold greater quantities of hGLA protein over physiological amounts still present at 48 hours.
- the half-life of Alpha-galactosidase when administered at 0.2 mg/kg is approximately 108 minutes.
- Production of GLA protein via the “depot effect” when administering GLA mRNA-loaded lipid nanoparticles shows a substantial increase in blood residence time when compared to direct injection of the naked recombinant protein. As described above, significant quantities of protein are present after 48 hours.
- the activity profile of the ⁇ -galactosidase protein produced from GLA mRNA-loaded lipid nanoparticles was measured as a function of 4-methylumbelliferyl- ⁇ -D-galactopyranoside (4-MU- ⁇ -gal) metabolism. As shown in FIG. 11 , the protein produced from these nanoparticle systems is quite active and reflective of the levels of protein available ( FIG. 12 , Table 3). AUC comparisons of mRNA therapy-based hGLA production versus enzyme replacement therapy (ERT) in mice and humans show a 182-fold and 30-fold increase, respectively (Table 4).
- FIG. 12 shows again that robust protein production is observed upon dosing wild type (CD-1) mice with a single 30 ug dose of hGLA mRNA-loaded in C12-200-based lipid nanoparticles (Formulation 1).
- CD-1 wild type mice
- hGLA levels were evaluated over a 72 hour period.
- a maximum average of 4.0 ug human hGLA protein/mL serum is detected six hours post-administration.
- hGLA MRT Based on a value of ⁇ 1 ng/mL hGLA protein for normal physiological levels, hGLA MRT provides roughly 4000-fold higher protein levels. As before, hGLA protein could be detected out to 48 hr post-administration ( FIG. 12 ).
- FIG. 13 An analysis of tissues isolated from this same experiment provided insight into the distribution of hGLA protein in hGLA MRT-treated mice ( FIG. 13 ). Supraphysiological levels of hGLA protein were detected in the liver, spleen and kidneys of all mice treated with a maximum observed between 12 and 24 hour post-administration. Detectable levels of MRT-derived protein could be observed three days after a single injection of hGLA-loaded lipid nanoparticles.
- lipid nanoparticle-mediated mRNA replacement therapy would be the pharmacokinetic profile of the respective protein produced.
- ERT-based treatment of mice employing Alpha-galactosidase results in a plasma half-life of approximately 100 minutes.
- MRT-derived alpha-galactosidase has a blood residence time of approximately 72 hrs with a peak time of 6 hours. This allows for much greater exposure for organs to participate in possible continuous uptake of the desired protein.
- a comparison of PK profiles is shown in FIG. 15 and demonstrates the stark difference in clearance rates and ultimately a major shift in area under the curve (AUC) can be achieved via MRT-based treatment.
- hGLA MRT was applied to a mouse disease model, hGLA KO mice (Fabry mice).
- a 0.33 mg/kg dose of hGLA mRNA-loaded C12-200-based lipid nanoparticles (Formulation 1) was administered to female KO mice as a single, intravenous injection.
- Substantial quantities of MRT-derived hGLA protein were produced with a peak at 6 hr ( ⁇ 560 ng/mL serum) which is approximately 600-fold higher than normal physiological levels. Further, hGLA protein was still detectable 72 hr post-administration ( FIG. 16 ).
- MRT-derived GLA protein in vital organs demonstrated substantial accumulation as shown in FIG. 17 .
- a comparison of observed MRT-derived hGLA protein to reported normal physiological levels that are found in key organs is plotted (normal levels plotted as dashed lines). While levels of protein at 24 hours are higher than at 72 hours post-administration, the levels of hGLA protein detected in the liver, kidney, spleen and hearts of the treated Fabry mice are equivalent to wild type levels. For example, 3.1 ng hGLA protein/mg tissue were found in the kidneys of treated mice 3 days after a single MRT treatment.
- ERT-based Alpha-galactosidase treatment versus hGLA MRT-based treatment of male Fabry KO mice was conducted.
- a single, intravenous dose of 1.0 mg/kg was given for each therapy and the mice were sacrificed one week post-administration.
- Serum levels of hGLA protein were monitored at 6 hr and 1 week post-injection.
- Liver, kidney, spleen, and heart were analyzed for hGLA protein accumulation one week post-administration.
- a measure of efficacy was determined via measurement of globotrioasylceramide (Gb3) and lyso-Gb3 reductions in the kidney and heart.
- FIG. 18 shows the serum levels of hGLA protein after treatment of either Alpha-galactosidase or GLA mRNA loaded lipid nanoparticles (Formulation 1) in male Fabry mice. Serum samples were analyzed at 6 hr and 1 week post-administration. A robust signal was detected for MRT-treated mice after 6 hours, with hGLA protein serum levels of ⁇ 4.0 ug/mL. In contrast, there was no detectable Alpha-galactosidase remaining in the bloodstream at this time.
- FIG. 19 shows a comparison of human GLA protein found in each respective organ after either hGLA MRT or Alpha-galactosidase ERT treatment. Levels correspond to hGLA present one week post-administration. hGLA protein was detected in all organs analyzed. For example, MRT-treated mice resulted in hGLA protein accumulation in the kidney of 2.42 ng hGLA protein/mg protein, while Alpha-galactosidase-treated mice had only residual levels (0.37 ng/mg protein).
- Gb3 globotrioasylceramide
- lyso-Gb3 levels in key organs.
- a direct comparison of Gb3 reduction after a single, intravenous 1.0 mg/kg GLA MRT treatment as compared to a Alpha-galactosidase ERT-based therapy of an equivalent dose yielded a sizeable difference in levels of Gb3 in the kidneys as well as heart.
- Gb3 levels for GLA MRT versus Alpha-galactosidase yielded reductions of 60.2% vs 26.8%, respectively ( FIG. 20 ).
- Gb3 levels in the heart were reduced by 92.1% vs 66.9% for MRT and Alpha-galactosidase, respectively ( FIG. 21 ).
- a second relevant biomarker for measurement of efficacy is lyso-Gb3.
- GLA MRT reduced lyso-Gb3 more efficiently than Alpha-galactosidase as well in the kidneys and heart ( FIG. 20 and FIG. 21 , respectively).
- MRT-treated Fabry mice demonstrated reductions of lyso-Gb3 of 86.1% and 87.9% in the kidneys and heart as compared to Alpha-galactosidase-treated mice yielding a decrease of 47.8% and 61.3%, respectively.
- hGLA mRNA loaded into HGT4003 (Formulation 3) or HGT5000-based (Formulation 5) lipid nanoparticles administered as a single dose IV result in production of hGLA at 24 hours post administration ( FIG. 22 ).
- the production of hGLA exhibited a dose response.
- hGLA production was observed at 6 hours and 24 hours after administration of hGLA mRNA loaded into HGT5001-based (Formulation 6) lipid nanoparticles administered as a single dose IV.
- hGLA production was observed in the serum ( FIG. 23A ), as well as in organs ( FIG. 23B ).
- mRNA replacement therapy applied as a depot for protein production produces large quantities of active, functionally therapeutic protein at supraphysiological levels. This method has been demonstrated to yield a sustained circulation half-life of the desired protein and this MRT-derived protein is highly efficacious for therapy as demonstrated with alpha-galactosidase enzyme in Fabry mice.
- FIX Factor IX
- A1AT alpha-1-antitrypsin
- detectable levels of human A1AT protein derived from A1AT MRT could be observed over a 24 hour time period post-administration.
- a maximum serum level of ⁇ 48 ug A1AT protein/mL serum was detected 12 hours after injection.
- mice All studies were performed using female CD-1 or BALB/C mice of approximately 7-10 weeks of age at the beginning of each experiment. Test articles were introduced via a single intratracheal aerosolized administration. Mice were sacrificed and perfused with saline at the designated time points. The lungs of each mouse were harvested, apportioned into two parts, and stored in either 10% neutral buffered formalin or snap-frozen and stored at ⁇ 80° C. for analysis. Serum was isolated as described in Example 1. EPO ELISA as described in Example 1.
- the depot effect can be achieved via pulmonary delivery (e.g. intranasal, intratracheal, nebulization). Measurement of the desired exogenous-based protein derived from messenger RNA delivered via nanoparticle systems was achieved and quantified.
- pulmonary delivery e.g. intranasal, intratracheal, nebulization.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Zoology (AREA)
- Molecular Biology (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- Biomedical Technology (AREA)
- General Engineering & Computer Science (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Microbiology (AREA)
- Immunology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Physics & Mathematics (AREA)
- Dispersion Chemistry (AREA)
- Plant Pathology (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Dermatology (AREA)
- Toxicology (AREA)
- Otolaryngology (AREA)
- Pulmonology (AREA)
- Nanotechnology (AREA)
- Optics & Photonics (AREA)
Abstract
Description
- Novel approaches and therapies are still needed for the treatment of protein and enzyme deficiencies. For example, lysosomal storage diseases are a group of approximately 50 rare inherited metabolic disorders that result from defects in lysosomal function, usually due to a deficiency of an enzyme required for metabolism. Fabry disease is a lysosomal storage disease that results from a deficiency of the enzyme alpha galactosidase (GLA), which causes a glycolipid known as globotriaosylceramide to accumulate in blood vessels and other tissues, leading to various painful manifestations. For certain diseases, like Fabry disease, there is a need for replacement of a protein or enzyme that is normally secreted by cells into the blood stream. Therapies, such as gene therapy, that increase the level or production of an affected protein or enzyme could provide a treatment or even a cure for such disorders. However, there have been several limitations to using conventional gene therapy for this purpose.
- Conventional gene therapy involves the use of DNA for insertion of desired genetic information into host cells. The DNA introduced into the cell is usually integrated to a certain extent into the genome of one or more transfected cells, allowing for long-lasting action of the introduced genetic material in the host. While there may be substantial benefits to such sustained action, integration of exogenous DNA into a host genome may also have many deleterious effects. For example, it is possible that the introduced DNA will be inserted into an intact gene, resulting in a mutation which impedes or even totally eliminates the function of the endogenous gene. Thus, gene therapy with DNA may result in the impairment of a vital genetic function in the treated host, such as e.g., elimination or deleteriously reduced production of an essential enzyme or interruption of a gene critical for the regulation of cell growth, resulting in unregulated or cancerous cell proliferation. In addition, with conventional DNA based gene therapy it is necessary for effective expression of the desired gene product to include a strong promoter sequence, which again may lead to undesirable changes in the regulation of normal gene expression in the cell. It is also possible that the DNA based genetic material will result in the induction of undesired anti-DNA antibodies, which in turn, may trigger a possibly fatal immune response. Gene therapy approaches using viral vectors can also result in an adverse immune response. In some circumstances, the viral vector may even integrate into the host genome. In addition, production of clinical grade viral vectors is also expensive and time consuming. Targeting delivery of the introduced genetic material using viral vectors can also be difficult to control. Thus, while DNA based gene therapy has been evaluated for delivery of secreted proteins using viral vectors (U.S. Pat. No. 6,066,626; US2004/0110709), these approaches may be limited for these various reasons.
- Another obstacle apparent in these prior approaches at delivery of nucleic acids encoding secreted proteins, is in the levels of protein that are ultimately produced. It is difficult to achieve significant levels of the desired protein in the blood, and the amounts are not sustained over time. For example, the amount of protein produced by nucleic acid delivery does not reach normal physiological levels. See e.g., US2004/0110709.
- In contrast to DNA, the use of RNA as a gene therapy agent is substantially safer because (1) RNA does not involve the risk of being stably integrated into the genome of the transfected cell, thus eliminating the concern that the introduced genetic material will disrupt the normal functioning of an essential gene, or cause a mutation that results in deleterious or oncogenic effects; (2) extraneous promoter sequences are not required for effective translation of the encoded protein, again avoiding possible deleterious side effects; (3) in contrast to plasmid DNA (pDNA), messenger RNA (mRNA) is devoid of immunogenic CpG motifs so that anti-RNA antibodies are not generated; and (4) any deleterious effects that do result from mRNA based on gene therapy would be of limited duration due to the relatively short half-life of RNA. In addition, it is not necessary for mRNA to enter the nucleus to perform its function, while DNA must overcome this major barrier.
- One reason that mRNA based gene therapy has not been used more in the past is that mRNA is far less stable than DNA, especially when it reaches the cytoplasm of a cell and is exposed to degrading enzymes. The presence of a hydroxyl group on the second carbon of the sugar moiety in mRNA causes steric hinderance that prevents the mRNA from forming the more stable double helix structure of DNA and thus makes the mRNA more prone to hydrolytic degradation. As a result, until recently, it was widely believed that mRNA was too labile to withstand transfection protocols. Advances in RNA stabilizing modifications have sparked more interest in the use of mRNA in place of plasmid DNA in gene therapy. Certain delivery vehicles, such as cationic lipid or polymer delivery vehicles may also help protect the transfected mRNA from endogenous RNases. Yet, in spite of increased stability of modified mRNA, delivery of mRNA to cells in vivo in a manner allowing for therapeutic levels of protein production is still a challenge, particularly for mRNA encoding full length proteins. While delivery of mRNA encoding secreted proteins has been contemplated (US2009/0286852), the levels of a full length secreted protein that would actually be produced via in vivo mRNA delivery are not known and there is not a reason to expect the levels would exceed those observed with DNA based gene therapy.
- To date, significant progress using mRNA gene therapy has only been made in applications for which low levels of translation has not been a limiting factor, such as immunization with mRNA encoding antigens. Clinical trials involving vaccination against tumor antigens by intradermal injection of naked or protamine-complexed mRNA have demonstrated feasibility, lack of toxicity, and promising results. X. Su et al., Mol. Pharmaceutics. 8:774-787 (2011). Unfortunately, low levels of translation has greatly restricted the exploitation of mRNA based gene therapy in other applications which require higher levels of sustained expression of the mRNA encoded protein to exert a biological or therapeutic effect.
- The invention provides methods for delivery of mRNA gene therapeutic agents that lead to the production of therapeutically effective levels of secreted proteins via a “depot effect.” In embodiments of the invention, mRNA encoding a secreted protein is loaded in lipid nanoparticles and delivered to target cells in vivo. Target cells then act as a depot source for production of soluble, secreted protein into the circulatory system at therapeutic levels. In some embodiments, the levels of secreted protein produced are above normal physiological levels.
- The invention provides compositions and methods for intracellular delivery of mRNA in a liposomal transfer vehicle to one or more target cells for production of therapeutic levels of secreted functional protein.
- The compositions and methods of the invention are useful in the management and treatment of a large number of diseases, in particular diseases which result from protein and/or enzyme deficiencies, wherein the protein or enzyme is normally secreted. Individuals suffering from such diseases may have underlying genetic defects that lead to the compromised expression of a protein or enzyme, including, for example, the non-synthesis of the secreted protein, the reduced synthesis of the secreted protein, or synthesis of a secreted protein lacking or having diminished biological activity. In particular, the methods and compositions of the invention are useful for the treatment of lysosomal storage disorders and/or the urea cycle metabolic disorders that occur as a result of one or more defects in the biosynthesis of secreted enzymes involved in the urea cycle.
- The compositions of the invention comprise an mRNA, a transfer vehicle and, optionally, an agent to facilitate contact with, and subsequent transfection of a target cell. The mRNA can encode a clinically useful secreted protein. For example, the mRNA may encode a functional secreted urea cycle enzyme or a secreted enzyme implicated in lysosomal storage disorders. The mRNA can encode, for example, erythropoietin (e.g., human EPO) or α-galactosidase (e.g., human α-galactosidase (human GLA).
- In some embodiments the mRNA can comprise one or more modifications that confer stability to the mRNA (e.g., compared to a wild-type or native version of the mRNA) and may also comprise one or more modifications relative to the wild-type which correct a defect implicated in the associated aberrant expression of the protein. For example, the nucleic acids of the present invention may comprise modifications to one or both of the 5′ and 3′ untranslated regions. Such modifications may include, but are not limited to, the inclusion of a partial sequence of a cytomegalovirus (CMV) immediate-early 1 (IE1) gene, a poly A tail, a Cap1 structure or a sequence encoding human growth hormone (hGH)). In some embodiments, the mRNA is modified to decrease mRNA immunogenicity.
- Methods of treating a subject comprising administering a composition of the invention, are also contemplated. For example, methods of treating or preventing conditions in which production of a particular secreted protein and/or utilization of a particular secreted protein is inadequate or compromised are provided. In one embodiment, the methods provided herein can be used to treat a subject having a deficiency in one or more urea cycle enzymes or in one or more enzymes deficient in a lysosomal storage disorder.
- In a preferred embodiment, the mRNA in the compositions of the invention is formulated in a liposomal transfer vehicle to facilitate delivery to the target cell. Contemplated transfer vehicles may comprise one or more cationic lipids, non-cationic lipids, and/or PEG-modified lipids. For example, the transfer vehicle may comprise at least one of the following cationic lipids: C12-200, DLin-KC2-DMA, DODAP, HGT4003, ICE, HGT5000, or HGT5001. In embodiments, the transfer vehicle comprises cholesterol (chol) and/or a PEG-modified lipid. In some embodiments, the transfer vehicles comprises DMG-PEG2K. In certain embodiments, the tranfer vehicle comprises one of the following lipid formulations: C12-200, DOPE, chol, DMG-PEG2K; DODAP, DOPE, cholesterol, DMG-PEG2K; HGT5000, DOPE, chol, DMG-PEG2K, HGT5001, DOPE, chol, DMG-PEG2K.
- The invention also provides compositions and methods useful for facilitating the transfection and delivery of one or more mRNA molecules to target cells capable of exhibiting the “depot effect.” For example, the compositions and methods of the present invention contemplate the use of targeting ligands capable of enhancing the affinity of the composition to one or more target cells. In one embodiment, the targeting ligand is apolipoprotein-B or apolipoprotein-E and corresponding target cells express low-density lipoprotein receptors, thereby facilitating recognition of the targeting ligand. The methods and compositions of the present invention may be used to preferentially target a vast number of target cells. For example, contemplated target cells include, but are not limited to, hepatocytes, epithelial cells, hematopoietic cells, epithelial cells, endothelial cells, lung cells, bone cells, stem cells, mesenchymal cells, neural cells, cardiac cells, adipocytes, vascular smooth muscle cells, cardiomyocytes, skeletal muscle cells, beta cells, pituitary cells, synovial lining cells, ovarian cells, testicular cells, fibroblasts, B cells, T cells, reticulocytes, leukocytes, granulocytes and tumor cells.
- In embodiments, the secreted protein is produced by the target cell for sustained amounts of time. For example, the secreted protein may be producted for more than one hour, more than four, more than six, more than 12, more than 24, more than 48 hours, or more than 72 hours after administration. In some embodiments the polypeptide is expressed at a peak level about six hours after administration. In some embodiments the expression of the polypeptide is sustained at least at a therapeutic level. In some embodiments the polypeptide is expressed at at least a therapeutic level for more than one, more than four, more than six, more than 12, more than 24, more than 48 hours, or more than 72 hours after administration. In some embodiments the polypeptide is detectable at the level in patient serum or tissue (e.g., liver, or lung). In some embodiments, the level of detectable polypeptide is from continuous expression from the mRNA composition over periods of time of more than one, more than four, more than six, more than 12, more than 24, more than 48 hours, or more than 72 hours after administration.
- In certain embodiments, the secreted protein is produced at levels above normal physiological levels. The level of secreted protein may be increased as compared to a control.
- In some embodiments the control is the baseline physiological level of the polypeptide in a normal individual or in a population of normal individuals. In other embodiments the control is the baseline physiological level of the polypeptide in an individual having a deficiency in the relevant protein or polypeptide or in a population of individuals having a deficiency in the relevant protein or polypeptide. In some embodiments the control can be the normal level of the relevant protein or polypeptide in the individual to whom the composition is administered. In other embodiments the control is the expression level of the polypeptide upon other therapeutic intervention, e.g., upon direct injection of the corresponding polypeptide, at one or more comparable time points.
- In certain embodiments the polypeptide is expressed by the target cell at a level which is at least 1.5-fold, at least 2-fold, at least 5-fold, at least 10-fold, at least 20-fold, 30-fold, at least 100-fold, at least 500-fold, at least 5000-fold, at least 50,000-fold or at least 100,000-fold greater than a control. In some embodiments, the fold increase of expression greater than control is sustained for more than one, more than four, more than six, more than 12, more than 24, or more than 48 hours, or more than 72 hours after administration. For example, in one embodiment, the levels of secreted protein are detected in the serum at least 1.5-fold, at least 2-fold, at least 5-fold, at least 10-fold, at least 20-fold, 30-fold, at least 100-fold, at least 500-fold, at least 5000-fold, at least 50,000-fold or at least 100,000-fold greater than a control for at least 48 hours or 2 days. In certain embodiments, the levels of secreted protein are detectable at 3 days, 4 days, 5 days, or 1 week or more after administration. Increased levels of secreted protein may be observed in the serum and/or in a tissue (e.g. liver, lung).
- In some embodiments, the method yields a sustained circulation half-life of the desired secreted protein. For example, the secreted protein may be detected for hours or days longer than the half-life observed via subcutaneous injection of the secreted protein. In embodiments, the half-life of the secreted protein is sustained for more than 1 day, 2 days, 3 days, 4 days, 5 days, or 1 week or more.
- In some embodiments administration comprises a single or repeated doses. In certain embodiments, the dose is administered intravenously, or by pulmonary delivery.
- The polypeptide can be, for example, one or more of erythropoietin, α-galactosidase, LDL receptor, Factor VIII, Factor IX, α-L-iduronidase (for MPS I), iduronate sulfatase (for MPS II), heparin-N-sulfatase (for MPS IIIA), α-N-acetylglucosaminidase (for MPS IIIB), galactose 6-sultatase (for MPS IVA), lysosomal acid lipase, arylsulfatase-A.
- Certain embodiments relate to compositions and methods that provide to a cell or subject mRNA, at least a part of which encodes a functional protein, in an amount that is substantially less that the amount of corresponding functional protein generated from that mRNA. Put another way, in certain embodiments the mRNA delivered to the cell can produce an amount of protein that is substantially greater than the amount of mRNA delivered to the cell. For example, in a given amount of time, for example 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 15, 20, or 24 hours from administration of the mRNA to a cell or subject, the amount of corresponding protein generated by that mRNA can be at least 1.5, 2, 3, 5, 10, 15, 20, 25, 50, 100, 150, 200, 250, 300, 400, 500, or more times greater that the amount of mRNA actually administered to the cell or subject. This can be measured on a mass-by-mass basis, on a mole-by-mole basis, and/or on a molecule-by-molecule basis. The protein can be measured in various ways. For example, for a cell, the measured protein can be measured as intracellular protein, extracellular protein, or a combination of the two. For a subject, the measured protein can be protein measured in serum; in a specific tissue or tissues such as the liver, kidney, heart, or brain; in a specific cell type such as one of the various cell types of the liver or brain; or in any combination of serum, tissue, and/or cell type. Moreover, a baseline amount of endogenous protein can be measured in the cell or subject prior to administration of the mRNA and then subtracted from the protein measured after administration of the mRNA to yield the amount of corresponding protein generated from the mRNA. In this way, the mRNA can provide a reservoir or depot source of a large amount of therapeutic material to the cell or subject, for example, as compared to amount of mRNA delivered to the cell or subject. The depot source can act as a continuous source for polypeptide expression from the mRNA over sustained periods of time.
- The above discussed and many other features and attendant advantages of the present invention will become better understood by reference to the following detailed description of the invention when taken in conjunction with the accompanying examples. The various embodiments described herein are complimentary and can be combined or used together in a manner understood by the skilled person in view of the teachings contained herein.
-
FIG. 1 shows the nucleotide sequence of a 5′ CMV sequence (SEQ ID NO:1), wherein X, if present is GGA. -
FIG. 2 shows the nucleotide sequence of a 3′ hGH sequence (SEQ ID NO:2). -
FIG. 3 shows the nucleotide sequence of human erythropoietic (EPO) mRNA (SEQ ID NO:3). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2. -
FIG. 4 shows the nucleotide sequence of human alpha-galactosidase (GLA) mRNA (SEQ ID NO:4). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2. -
FIG. 5 shows the nucleotide sequence of human alpha-1 antitrypsin (A1AT) mRNA (SEQ ID NO:5). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2. -
FIG. 6 shows the nucleotide sequence of human factor IX (FIX) mRNA (SEQ ID NO:6). This sequence can be flanked on the 5′ end with SEQ ID NO:1 and on the 3′ end with SEQ ID NO:2. -
FIG. 7 shows quantification of secreted hEPO protein levels as measured via ELISA. The protein detected is a result of its production from hEPO mRNA delivered intravenously via a single dose of various lipid nanoparticle formulations. The formulations C12-200 (30 ug), HGT4003 (150 ug), ICE (100 ug), DODAP (200 ug) are represented as the cationic/ionizable lipid component of each test article (Formulations 1-4). Values are based on blood sample four hours post-administration. -
FIG. 8 shows the hematocrit measurement of mice treated with a single IV dose of human EPO mRNA-loaded lipid nanoparticles (Formulations 1-4). Whole blood samples were taken at 4 hr (Day 1), 24 hr (Day 2), 4 days, 7 days, and 10 days post-administration. -
FIG. 9 shows hematocrit measurements of mice treated with human EPO-mRNA-loaded lipid nanoparticles with either a single IV dose or three injections (day 1,day 3, day 5). Whole blood samples were taken prior to injection (day −4),day 7, andday 15.Formulation 1 was administered: (30 ug, single dose) or (3×10 ug, doseday 1,day 3, day 5);Formulation 2 was administered: (3×50 ug, doseday 1,day 3, day 5). -
FIG. 10 shows quantification of secreted human α-galactosidase (hGLA) protein levels as measured via ELISA. The protein detected is a result of the production from hGLA mRNA delivered via lipid nanoparticles (Formulation 1; 30 ug single intravenous dose, based on encapsulated mRNA). hGLA protein is detected through 48 hours. -
FIG. 11 shows hGLA activity in serum. hGLA activity was measured using substrate 4-methylumbelliferyl-α-D-galactopyranoside (4-MU-α-gal) at 37° C. Data are average of 6 to 9 individual measurements. -
FIG. 12 shows quantification of hGLA protein levels in serum as measured via ELISA. Protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (C12-200:DOPE:Chol:DMGPEG2K, 40:30:25:5 (Formulation 1); 30 ug mRNA based on encapsulated mRNA, single IV dose). hGLA protein is monitored through 72 hours. per single intravenous dose, based on encapsulated mRNA). hGLA protein is monitored through 72 hours. -
FIG. 13 shows quantification of hGLA protein levels in liver, kidney, and spleen as measured via ELISA. Protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (Formulation 1; 30 ug mRNA based on encapsulated mRNA, single IV dose). hGLA protein is monitored through 72 hours. -
FIG. 14 shows a dose response study monitoring protein production of hGLA as secreted MRT-derived human GLA protein in serum (A) and liver (B). Samples were measured 24 hours post-administration (Formulation 1; single dose, IV, N=4 mice/group) and quantified via ELISA. -
FIG. 15 shows the pharmacokinetic profiles of ERT-based Alpha-galactosidase in athymjic nude mice (40 ug/kg dose) and hGLA protein produced from MRT (Formulation 1; 1.0 mg/kg mRNA dose). -
FIG. 16 shows the quantification of secreted hGLA protein levels in MRT-treated Fabry mice as measured using ELISA. hGLA protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (Formulation 1; 10 ug mRNA per single intravenous dose, based on encapsulated mRNA). Serum is monitored through 72 hours. -
FIG. 17 shows the quantification of hGLA protein levels in liver, kidney, spleen, and heart of MRT-treated Fabry KO mice as measured via ELISA. Protein is produced from hGLA mRNA delivered via C12-200-based lipid nanoparticles (Formulation 1; 30 ug mRNA based on encapsulated mRNA, single IV dose). hGLA protein is monitored through 72 hours. Literature values representing normal physiological levels are graphed as dashed lines. -
FIG. 18 shows the quantification of secreted hGLA protein levels in MRT and Alpha-galactosidase-treated Fabry mice as measured using ELISA. Both therapies were dosed as a single 1.0 mg/kg intravenous dose. -
FIG. 19 shows the quantification of hGLA protein levels in liver, kidney, spleen, and heart of MRT and ERT (Alpha-galactosidase)-treated Fabry KO mice as measured via ELISA. Protein produced from hGLA mRNA delivered via lipid nanoparticles (Formulation 1; 1.0 mg/kg mRNA based on encapsulated mRNA, single IV dose). -
FIG. 20 shows the relative quantification of globotrioasylceramide (Gb3) and lyso-Gb3 in the kidneys of treated and untreated mice. Male Fabry KO mice were treated with a single dose either GLA mRNA-loaded lipid nanoparticles or Alpha-galactosidase at 1.0 mg/kg. Amounts reflect quantity of Gb3/lyso-Gb3 one week post-administration. -
FIG. 21 shows the relative quantification of globotrioasylceramide (Gb3) and lyso-Gb3 in the heart of treated and untreated mice. Male Fabry KO mice were treated with a single dose either GLA mRNA-loaded lipid nanoparticles or Alpha-galactosidase at 1.0 mg/kg. Amounts reflect quantity of Gb3/lyso-Gb3 one week post-administration. -
FIG. 22 shows a dose response study monitoring protein production of GLA as secreted MRT-derived human GLA protein in serum. Samples were measured 24 hours post-administration (single dose, IV, N=4 mice/group) of either HGT4003 (Formulation 3) or HGT5000-based lipid nanoparticles (Formulation 5) and quantified via ELISA. -
FIG. 23 shows hGLA protein production as measured in serum (A) or in liver, kidney, and spleen (B). Samples were measured 6 hours and 24 hours post-administration (single dose, IV, N=4 mice/group) of HGT5001-based lipid nanoparticles (Formulation 6) and quantified via ELISA. -
FIG. 24 shows the quantification of secreted human Factor IX protein levels measured using ELISA (mean ng/mL±standard deviation). FIX protein is produced from FIX mRNA delivered via C12-200-based lipid nanoparticles (C12-200:DOPE:Chol:DMGPEG2K, 40:30:25:5 (Formulation 1); 30 ug mRNA per single intravenous dose, based on encapsulated mRNA). FIX protein is monitored through 72 hours. (n=24 mice) -
FIG. 25 shows the quantification of secreted human α-1-antitrypsin (A1AT) protein levels measured using ELISA. A1AT protein is produced from A1AT mRNA delivered via C12-200-based lipid nanoparticles (C12-200:DOPE:Chol:DMGPEG2K, 40:30:25:5 (Formulation 1); 30 ug mRNA per single intravenous dose, based on encapsulated mRNA). A1AT protein is monitored through 24 hours. -
FIG. 26 shows an ELISA-based quantification of hEPO protein detected in the lungs and serum of treated mice after intratracheal administration of hEPO mRNA-loaded nanoparticles (measured mIU) (C12-200, HGT5000, or HGT5001-based lipid nanoparticles;Formulations - The invention provides compositions and methods for intracellular delivery of mRNA in a liposomal transfer vehicle to one or more target cells for production of therapeutic levels of secreted functional protein.
- The term “functional,” as used herein to qualify a protein or enzyme, means that the protein or enzyme has biological activity, or alternatively is able to perform the same, or a similar function as the native or normally-functioning protein or enzyme. The mRNA compositions of the invention are useful for the treatment of a various metabolic or genetic disorders, and in particular those genetic or metabolic disorders which involve the non-expression, misexpression or deficiency of a protein or enzyme. The term “therapeutic levels” refers to levels of protein detected in the blood or tissues that are above control levels, wherein the control may be normal physiological levels, or the levels in the subject prior to administration of the mRNA composition. The term “secreted” refers to protein that is detected outside the target cell, in extracellular space. The protein may be detected in the blood or in tissues. In the context of the present invention the term “produced” is used in its broadest sense to refer the translation of at least one mRNA into a protein or enzyme. As provided herein, the compositions include a transfer vehicle. As used herein, the term “transfer vehicle” includes any of the standard pharmaceutical carriers, diluents, excipients and the like which are generally intended for use in connection with the administration of biologically active agents, including nucleic acids. The compositions and in particular the transfer vehicles described herein are capable of delivering mRNA to the target cell. In embodiments, the transfer vehicle is a lipid nanoparticle.
- mRNA
- The mRNA in the compositions of the invention may encode, for example, a secreted hormone, enzyme, receptor, polypeptide, peptide or other protein of interest that is normally secreted. In one embodiment of the invention, the mRNA may optionally have chemical or biological modifications which, for example, improve the stability and/or half-life of such mRNA or which improve or otherwise facilitate protein production.
- The methods of the invention provide for optional co-delivery of one or more unique mRNA to target cells, for example, by combining two unique mRNAs into a single transfer vehicle. In one embodiment of the present invention, a therapeutic first mRNA, and a therapeutic second mRNA, may be formulated in a single transfer vehicle and administered. The present invention also contemplates co-delivery and/or co-administration of a therapeutic first mRNA and a second nucleic acid to facilitate and/or enhance the function or delivery of the therapeutic first mRNA. For example, such a second nucleic acid (e.g., exogenous or synthetic mRNA) may encode a membrane transporter protein that upon expression (e.g., translation of the exogenous or synthetic mRNA) facilitates the delivery or enhances the biological activity of the first mRNA. Alternatively, the therapeutic first mRNA may be administered with a second nucleic acid that functions as a “chaperone” for example, to direct the folding of either the therapeutic first mRNA.
- The methods of the invention also provide for the delivery of one or more therapeutic nucleic acids to treat a single disorder or deficiency, wherein each such therapeutic nucleic acid functions by a different mechanism of action. For example, the compositions of the present invention may comprise a therapeutic first mRNA which, for example, is administered to correct an endogenous protein or enzyme deficiency, and which is accompanied by a second nucleic acid, which is administered to deactivate or “knock-down” a malfunctioning endogenous nucleic acid and its protein or enzyme product. Such “second” nucleic acids may encode, for example mRNA or siRNA.
- Upon transfection, a natural mRNA in the compositions of the invention may decay with a half-life of between 30 minutes and several days. The mRNA in the compositions of the invention preferably retain at least some ability to be translated, thereby producing a functional secreted protein or enzyme. Accordingly, the invention provides compositions comprising and methods of administering a stabilized mRNA. In some embodiments of the invention, the activity of the mRNA is prolonged over an extended period of time. For example, the activity of the mRNA may be prolonged such that the compositions of the present invention are administered to a subject on a semi-weekly or bi-weekly basis, or more preferably on a monthly, bi-monthly, quarterly or an annual basis. The extended or prolonged activity of the mRNA of the present invention, is directly related to the quantity of secreted functional protein or enzyme produced from such mRNA. Similarly, the activity of the compositions of the present invention may be further extended or prolonged by modifications made to improve or enhance translation of the mRNA. Furthermore, the quantity of functional protein or enzyme produced by the target cell is a function of the quantity of mRNA delivered to the target cells and the stability of such mRNA. To the extent that the stability of the mRNA of the present invention may be improved or enhanced, the half-life, the activity of the produced secreted protein or enzyme and the dosing frequency of the composition may be further extended.
- Accordingly, in some embodiments, the mRNA in the compositions of the invention comprise at least one modification which confers increased or enhanced stability to the nucleic acid, including, for example, improved resistance to nuclease digestion in vivo. As used herein, the terms “modification” and “modified” as such terms relate to the nucleic acids provided herein, include at least one alteration which preferably enhances stability and renders the mRNA more stable (e.g., resistant to nuclease digestion) than the wild-type or naturally occurring version of the mRNA. As used herein, the terms “stable” and “stability” as such terms relate to the nucleic acids of the present invention, and particularly with respect to the mRNA, refer to increased or enhanced resistance to degradation by, for example nucleases (i.e., endonucleases or exonucleases) which are normally capable of degrading such mRNA. Increased stability can include, for example, less sensitivity to hydrolysis or other destruction by endogenous enzymes (e.g., endonucleases or exonucleases) or conditions within the target cell or tissue, thereby increasing or enhancing the residence of such mRNA in the target cell, tissue, subject and/or cytoplasm. The stabilized mRNA molecules provided herein demonstrate longer half-lives relative to their naturally occurring, unmodified counterparts (e.g. the wild-type version of the mRNA). Also contemplated by the terms “modification” and “modified” as such terms related to the mRNA of the present invention are alterations which improve or enhance translation of mRNA nucleic acids, including for example, the inclusion of sequences which function in the initiation of protein translation (e.g., the Kozac consensus sequence). (Kozak, M., Nucleic Acids Res 15 (20): 8125-48 (1987)).
- In some embodiments, the mRNA of the invention have undergone a chemical or biological modification to render them more stable. Exemplary modifications to an mRNA include the depletion of a base (e.g., by deletion or by the substitution of one nucleotide for another) or modification of a base, for example, the chemical modification of a base. The phrase “chemical modifications” as used herein, includes modifications which introduce chemistries which differ from those seen in naturally occurring mRNA, for example, covalent modifications such as the introduction of modified nucleotides, (e.g., nucleotide analogs, or the inclusion of pendant groups which are not naturally found in such mRNA molecules).
- In addition, suitable modifications include alterations in one or more nucleotides of a codon such that the codon encodes the same amino acid but is more stable than the codon found in the wild-type version of the mRNA. For example, an inverse relationship between the stability of RNA and a higher number cytidines (C's) and/or uridines (U's) residues has been demonstrated, and RNA devoid of C and U residues have been found to be stable to most RNases (Heidenreich, et al. J Biol Chem 269, 2131-8 (1994)). In some embodiments, the number of C and/or U residues in an mRNA sequence is reduced. In a another embodiment, the number of C and/or U residues is reduced by substitution of one codon encoding a particular amino acid for another codon encoding the same or a related amino acid. Contemplated modifications to the mRNA nucleic acids of the present invention also include the incorporatation of pseudouridines. The incorporation of pseudouridines into the mRNA nucleic acids of the present invention may enhance stability and translational capacity, as well as diminishing immunogenicity in vivo. See, e.g., Karikó, K., et al., Molecular Therapy 16 (11): 1833-1840 (2008). Substitutions and modifications to the mRNA of the present invention may be performed by methods readily known to one or ordinary skill in the art.
- The constraints on reducing the number of C and U residues in a sequence will likely be greater within the coding region of an mRNA, compared to an untranslated region, (i.e., it will likely not be possible to eliminate all of the C and U residues present in the message while still retaining the ability of the message to encode the desired amino acid sequence). The degeneracy of the genetic code, however presents an opportunity to allow the number of C and/or U residues that are present in the sequence to be reduced, while maintaining the same coding capacity (i.e., depending on which amino acid is encoded by a codon, several different possibilities for modification of RNA sequences may be possible). For example, the codons for Gly can be altered to GGA or GGG instead of GGU or GGC.
- The term modification also includes, for example, the incorporation of non-nucleotide linkages or modified nucleotides into the mRNA sequences of the present invention (e.g., modifications to one or both the 3′ and 5′ ends of an mRNA molecule encoding a functional secreted protein or enzyme). Such modifications include the addition of bases to an mRNA sequence (e.g., the inclusion of a poly A tail or a longer poly A tail), the alteration of the 3′ UTR or the 5′ UTR, complexing the mRNA with an agent (e.g., a protein or a complementary nucleic acid molecule), and inclusion of elements which change the structure of an mRNA molecule (e.g., which form secondary structures).
- The poly A tail is thought to stabilize natural messengers. Therefore, in one embodiment a long poly A tail can be added to an mRNA molecule thus rendering the mRNA more stable. Poly A tails can be added using a variety of art-recognized techniques. For example, long poly A tails can be added to synthetic or in vitro transcribed mRNA using poly A polymerase (Yokoe, et al. Nature Biotechnology. 1996; 14: 1252-1256). A transcription vector can also encode long poly A tails. In addition, poly A tails can be added by transcription directly from PCR products. In one embodiment, the length of the poly A tail is at least about 90, 200, 300, 400 at least 500 nucleotides. In one embodiment, the length of the poly A tail is adjusted to control the stability of a modified mRNA molecule of the invention and, thus, the transcription of protein. For example, since the length of the poly A tail can influence the half-life of an mRNA molecule, the length of the poly A tail can be adjusted to modify the level of resistance of the mRNA to nucleases and thereby control the time course of protein expression in a cell. In one embodiment, the stabilized mRNA molecules are sufficiently resistant to in vivo degradation (e.g., by nucleases), such that they may be delivered to the target cell without a transfer vehicle.
- In one embodiment, an mRNA can be modified by the
incorporation 3′ and/or 5′ untranslated (UTR) sequences which are not naturally found in the wild-type mRNA. In one embodiment, 3′ and/or 5′ flanking sequence which naturally flanks an mRNA and encodes a second, unrelated protein can be incorporated into the nucleotide sequence of an mRNA molecule encoding a therapeutic or functional protein in order to modify it. For example, 3′ or 5′ sequences from mRNA molecules which are stable (e.g., globin, actin, GAPDH, tubulin, histone, or citric acid cycle enzymes) can be incorporated into the 3′ and/or 5′ region of a sense mRNA nucleic acid molecule to increase the stability of the sense mRNA molecule. See, e.g., US2003/0083272. - In some embodiments, the mRNA in the compositions of the invention include modification of the 5′ end of the mRNA to include a partial sequence of a CMV immediate-early 1 (IE1) gene, or a fragment thereof (e.g., SEQ ID NO:1) to improve the nuclease resistance and/or improve the half-life of the mRNA. In addition to increasing the stability of the mRNA nucleic acid sequence, it has been surprisingly discovered the inclusion of a partial sequence of a CMV immediate-early 1 (IE1) gene enhances the translation of the mRNA and the expression of the functional protein or enzyme. Also contemplated is the inclusion of a human growth hormone (hGH) gene sequence, or a fragment thereof (e.g., SEQ ID NO:2) to the 3′ ends of the nucleic acid (e.g., mRNA) to further stabilize the mRNA. Generally, preferred modifications improve the stability and/or pharmacokinetic properties (e.g., half-life) of the mRNA relative to their unmodified counterparts, and include, for example modifications made to improve such mRNA's resistance to in vivo nuclease digestion.
- Further contemplated are variants of the nucelic acid sequence of SEQ ID NO:1 and/or SEQ ID NO:2, wherein the variants maintain the functional properties of the nucleic acids including stabilization of the mRNA and/or pharmacokinetic properties (e.g., half-life). Variants may have greater than 90%, greater than 95%, greater than 98%, or greater than 99% sequence identity to SEQ ID NO:1 or SEQ ID NO:2.
- In some embodiments, the composition can comprise a stabilizing reagent. The compositions can include one or more formulation reagents that bind directly or indirectly to, and stabilize the mRNA, thereby enhancing residence time in the target cell. Such reagents preferably lead to an improved half-life of the mRNA in the target cells. For example, the stability of an mRNA and efficiency of translation may be increased by the incorporation of “stabilizing reagents” that form complexes with the mRNA that naturally occur within a cell (see e.g., U.S. Pat. No. 5,677,124). Incorporation of a stabilizing reagent can be accomplished for example, by combining the poly A and a protein with the mRNA to be stabilized in vitro before loading or encapsulating the mRNA within a transfer vehicle. Exemplary stabilizing reagents include one or more proteins, peptides, aptamers, translational accessory protein, mRNA binding proteins, and/or translation initiation factors.
- Stabilization of the compositions may also be improved by the use of opsonization-inhibiting moieties, which are typically large hydrophilic polymers that are chemically or physically bound to the transfer vehicle (e.g., by the intercalation of a lipid-soluble anchor into the membrane itself, or by binding directly to active groups of membrane lipids). These opsonization-inhibiting hydrophilic polymers form a protective surface layer which significantly decreases the uptake of the liposomes by the macrophage-monocyte system and reticulo-endothelial system (e.g., as described in U.S. Pat. No. 4,920,016, the entire disclosure of which is herein incorporated by reference). Transfer vehicles modified with opsonization-inhibition moieties thus remain in the circulation much longer than their unmodified counterparts.
- When RNA is hybridized to a complementary nucleic acid molecule (e.g., DNA or RNA) it may be protected from nucleases. (Krieg, et al. Melton. Methods in Enzymology. 1987; 155, 397-415). The stability of hybridized mRNA is likely due to the inherent single strand specificity of most RNases. In some embodiments, the stabilizing reagent selected to complex a mRNA is a eukaryotic protein, (e.g., a mammalian protein). In yet another embodiment, the mRNA can be modified by hybridization to a second nucleic acid molecule. If an entire mRNA molecule were hybridized to a complementary nucleic acid molecule translation initiation may be reduced. In some embodiments the 5′ untranslated region and the AUG start region of the mRNA molecule may optionally be left unhybridized. Following translation initiation, the unwinding activity of the ribosome complex can function even on high affinity duplexes so that translation can proceed. (Liebhaber. J. Mol. Biol. 1992; 226: 2-13; Monia, et al. J Biol. Chem. 1993; 268: 14514-22).
- It will be understood that any of the above described methods for enhancing the stability of mRNA may be used either alone or in combination with one or more of any of the other above-described methods and/or compositions.
- The mRNA of the present invention may be optionally combined with a reporter gene (e.g., upstream or downstream of the coding region of the mRNA) which, for example, facilitates the determination of mRNA delivery to the target cells or tissues. Suitable reporter genes may include, for example, Green Fluorescent Protein mRNA (GFP mRNA), Renilla Luciferase mRNA (Luciferase mRNA), Firefly Luciferase mRNA, or any combinations thereof. For example, GFP mRNA may be fused with a mRNA encoding a secretable protein to facilitate confirmation of mRNA localization in the target cells that will act as a depot for protein production.
- As used herein, the terms “transfect” or “transfection” mean the intracellular introduction of a mRNA into a cell, or preferably into a target cell. The introduced mRNA may be stably or transiently maintained in the target cell. The term “transfection efficiency” refers to the relative amount of mRNA taken up by the target cell which is subject to transfection. In practice, transfection efficiency is estimated by the amount of a reporter nucleic acid product expressed by the target cells following transfection. Preferred embodiments include compositions with high transfection efficacies and in particular those compositions that minimize adverse effects which are mediated by transfection of non-target cells. The compositions of the present invention that demonstrate high transfection efficacies improve the likelihood that appropriate dosages of the mRNA will be delivered to the target cell, while minimizing potential systemic adverse effects. In one embodiment of the present invention, the transfer vehicles of the present invention are capable of delivering large mRNA sequences (e.g., mRNA of at least 1 kDa, 1.5 kDa, 2 kDa, 2.5 kDa, 5 kDa, 10 kDa, 12 kDa, 15 kDa, 20 kDa, 25 kDa, 30 kDa, or more). The mRNA can be formulated with one or more acceptable reagents, which provide a vehicle for delivering such mRNA to target cells. Appropriate reagents are generally selected with regard to a number of factors, which include, among other things, the biological or chemical properties of the mRNA, the intended route of administration, the anticipated biological environment to which such mRNA will be exposed and the specific properties of the intended target cells. In some embodiments, transfer vehicles, such as liposomes, encapsulate the mRNA without compromising biological activity. In some embodiments, the transfer vehicle demonstrates preferential and/or substantial binding to a target cell relative to non-target cells. In a preferred embodiment, the transfer vehicle delivers its contents to the target cell such that the mRNA are delivered to the appropriate subcellular compartment, such as the cytoplasm.
- Transfer Vehicle
- In embodiments, the transfer vehicle in the compositions of the invention is a liposomal transfer vehicle, e.g. a lipid nanoparticle. In one embodiment, the transfer vehicle may be selected and/or prepared to optimize delivery of the mRNA to a target cell. For example, if the target cell is a hepatocyte the properties of the transfer vehicle (e.g., size, charge and/or pH) may be optimized to effectively deliver such transfer vehicle to the target cell, reduce immune clearance and/or promote retention in that target cell. Alternatively, if the target cell is the central nervous system (e.g., mRNA administered for the treatment of neurodegenerative diseases may specifically target brain or spinal tissue), selection and preparation of the transfer vehicle must consider penetration of, and retention within the blood brain barrier and/or the use of alternate means of directly delivering such transfer vehicle to such target cell. In one embodiment, the compositions of the present invention may be combined with agents that facilitate the transfer of exogenous mRNA (e.g., agents which disrupt or improve the permeability of the blood brain barrier and thereby enhance the transfer of exogenous mRNA to the target cells).
- The use of liposomal transfer vehicles to facilitate the delivery of nucleic acids to target cells is contemplated by the present invention. Liposomes (e.g., liposomal lipid nanoparticles) are generally useful in a variety of applications in research, industry, and medicine, particularly for their use as transfer vehicles of diagnostic or therapeutic compounds in vivo (Lasic, Trends Biotechnol., 16: 307-321, 1998; Drummond et al., Pharmacol. Rev., 51: 691-743, 1999) and are usually characterized as microscopic vesicles having an interior aqua space sequestered from an outer medium by a membrane of one or more bilayers. Bilayer membranes of liposomes are typically formed by amphiphilic molecules, such as lipids of synthetic or natural origin that comprise spatially separated hydrophilic and hydrophobic domains (Lasic, Trends Biotechnol., 16: 307-321, 1998). Bilayer membranes of the liposomes can also be formed by amphiphilic polymers and surfactants (e.g., polymerosomes, niosomes, etc.).
- In the context of the present invention, a liposomal transfer vehicle typically serves to transport the mRNA to the target cell. For the purposes of the present invention, the liposomal transfer vehicles are prepared to contain the desired nucleic acids. The process of incorporation of a desired entity (e.g., a nucleic acid) into a liposome is often referred to as “loading” (Lasic, et al., FEBS Lett., 312: 255-258, 1992). The liposome-incorporated nucleic acids may be completely or partially located in the interior space of the liposome, within the bilayer membrane of the liposome, or associated with the exterior surface of the liposome membrane. The incorporation of a nucleic acid into liposomes is also referred to herein as “encapsulation” wherein the nucleic acid is entirely contained within the interior space of the liposome. The purpose of incorporating a mRNA into a transfer vehicle, such as a liposome, is often to protect the nucleic acid from an environment which may contain enzymes or chemicals that degrade nucleic acids and/or systems or receptors that cause the rapid excretion of the nucleic acids. Accordingly, in a preferred embodiment of the present invention, the selected transfer vehicle is capable of enhancing the stability of the mRNA contained therein. The liposome can allow the encapsulated mRNA to reach the target cell and/or may preferentially allow the encapsulated mRNA to reach the target cell, or alternatively limit the delivery of such mRNA to other sites or cells where the presence of the administered mRNA may be useless or undesirable. Furthermore, incorporating the mRNA into a transfer vehicle, such as for example, a cationic liposome, also facilitates the delivery of such mRNA into a target cell.
- Ideally, liposomal transfer vehicles are prepared to encapsulate one or more desired mRNA such that the compositions demonstrate a high transfection efficiency and enhanced stability. While liposomes can facilitate introduction of nucleic acids into target cells, the addition of polycations (e.g., poly L-lysine and protamine), as a copolymer can facilitate, and in some instances markedly enhance the transfection efficiency of several types of cationic liposomes by 2-28 fold in a number of cell lines both in vitro and in vivo. (See N. J. Caplen, et al., Gene Ther. 1995; 2: 603; S. Li, et al., Gene Ther. 1997; 4, 891).
- Lipid Nanoparticles
- In a preferred embodiment of the present invention, the transfer vehicle is formulated as a lipid nanoparticle. As used herein, the phrase “lipid nanoparticle” refers to a transfer vehicle comprising one or more lipids (e.g., cationic lipids, non-cationic lipids, and PEG-modified lipids). Preferably, the lipid nanoparticles are formulated to deliver one or more mRNA to one or more target cells. Examples of suitable lipids include, for example, the phosphatidyl compounds (e.g., phosphatidylglycerol, phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, sphingolipids, cerebrosides, and gangliosides). Also contemplated is the use of polymers as transfer vehicles, whether alone or in combination with other transfer vehicles. Suitable polymers may include, for example, polyacrylates, polyalkycyanoacrylates, polylactide, polylactide-polyglycolide copolymers, polycaprolactones, dextran, albumin, gelatin, alginate, collagen, chitosan, cyclodextrins, dendrimers and polyethylenimine. In one embodiment, the transfer vehicle is selected based upon its ability to facilitate the transfection of a mRNA to a target cell.
- The invention contemplates the use of lipid nanoparticles as transfer vehicles comprising a cationic lipid to encapsulate and/or enhance the delivery of mRNA into the target cell that will act as a depot for protein production. As used herein, the phrase “cationic lipid” refers to any of a number of lipid species that carry a net positive charge at a selected pH, such as physiological pH. The contemplated lipid nanoparticles may be prepared by including multi-component lipid mixtures of varying ratios employing one or more cationic lipids, non-cationic lipids and PEG-modified lipids. Several cationic lipids have been described in the literature, many of which are commercially available.
- Particularly suitable cationic lipids for use in the compositions and methods of the invention include those described in international patent publication WO 2010/053572, incorporated herein by reference, and most particularly, C12-200 described at paragraph [00225] of WO 2010/053572. In certain embodiments, the compositions and methods of the invention employ a lipid nanoparticles comprising an ionizable cationic lipid described in U.S. provisional patent application 61/617,468, filed Mar. 29, 2012 (incorporated herein by reference), such as, e.g, (15Z,18Z)-N,N-dimethyl-6-(9Z,12Z)-octadeca-9,12-dien-1-yl)tetracosa-15,18-dien-1-amine (HGT5000), (15Z,18Z)-N,N-dimethyl-6-((9Z,12Z)-octadeca-9,12-dien-1-yl)tetracosa-4,15,18-trien-1-amine (HGT5001), and (15Z,18Z)-N,N-dimethyl-6-((9Z,12Z)-octadeca-9,12-dien-1-yl)tetracosa-5,15,18-trien-1-amine (HGT5002).
- In some embodiments, the cationic lipid N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride or “DOTMA” is used. (Feigner et al. (Proc. Nat'l Acad. Sci. 84, 7413 (1987); U.S. Pat. No. 4,897,355). DOTMA can be formulated alone or can be combined with the neutral lipid, dioleoylphosphatidyl-ethanolamine or “DOPE” or other cationic or non-cationic lipids into a liposomal transfer vehicle or a lipid nanoparticle, and such liposomes can be used to enhance the delivery of nucleic acids into target cells. Other suitable cationic lipids include, for example, 5-carboxyspermylglycinedioctadecylamide or “DOGS,” 2,3-dioleyloxy-N-[2(spermine-carboxamido)ethyl]-N,N-dimethyl-1-propanaminium or “DOSPA” (Behr et al. Proc. Nat'l Acad. Sci. 86, 6982 (1989); U.S. Pat. No. 5,171,678; U.S. Pat. No. 5,334,761), 1,2-Dioleoyl-3-Dimethylammonium-Propane or “DODAP”, 1,2-Dioleoyl-3-Trimethylammonium-Propane or “DOTAP”. Contemplated cationic lipids also include 1,2-distearyloxy-N,N-dimethyl-3-aminopropane or “DSDMA”, 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane or “DODMA”, 1,2-dilinoleyloxy-N,N-dimethyl-3-aminopropane or “DLinDMA”, 1,2-dilinolenyloxy-N,N-dimethyl-3-aminopropane or “DLenDMA”, N-dioleyl-N,N-dimethylammonium chloride or “DODAC”, N,N-distearyl-N,N-dimethylammonium bromide or “DDAB”, N-(1,2-dimyristyloxyprop-3-yl)-N,N-dimethyl-N-hydroxyethyl ammonium bromide or “DMRIE”, 3-dimethylamino-2-(cholest-5-en-3-beta-oxybutan-4-oxy)-1-(cis,cis-9,12-octadecadienoxy)propane or “CLinDMA”, 2-[5′-(cholest-5-en-3-beta-oxy)-3′-oxapentoxy)-3-dimethy 1-1-(cis,cis-9′,1-2′-octadecadienoxy)propane or “CpLinDMA”, N,N-dimethyl-3,4-dioleyloxybenzylamine or “DMOBA”, 1,2-N,N′-dioleylcarbamyl-3-dimethylaminopropane or “DOcarbDAP”, 2,3-Dilinoleoyloxy-N,N-dimethylpropylamine or “DLinDAP”, 1,2-N,N′-Dilinoleylcarbamyl-3-dimethylaminopropane or “DLincarbDAP”, 1,2-Dilinoleoylcarbamyl-3-dimethylaminopropane or “DLinCDAP”, 2,2-dilinoleyl-4-dimethylaminomethyl-[1,3]-dioxolane or “DLin-K-DMA”, 2,2-dilinoleyl-4-dimethylaminoethyl-[1,3]-dioxolane or “DLin-K-XTC2-DMA”, and 2-(2,2-di((9Z,12Z)-octadeca-9,12-dien-1-yl)-1,3-dioxolan-4-yl)-N,N-dimethylethanamine (DLin-KC2-DMA)) (See, WO 2010/042877; Semple et al., Nature Biotech. 28:172-176 (2010)), or mixtures thereof. (Heyes, J., et al., J Controlled Release 107: 276-287 (2005); Morrissey, D V., et al., Nat. Biotechnol. 23(8): 1003-1007 (2005); PCT Publication WO2005/121348A1).
- The use of cholesterol-based cationic lipids is also contemplated by the present invention. Such cholesterol-based cationic lipids can be used, either alone or in combination with other cationic or non-cationic lipids. Suitable cholesterol-based cationic lipids include, for example, DC-Chol (N,N-dimethyl-N-ethylcarboxamidocholesterol), 1,4-bis(3-N-oleylamino-propyl)piperazine (Gao, et al. Biochem. Biophys. Res. Comm. 179, 280 (1991); Wolf et al. BioTechniques 23, 139 (1997); U.S. Pat. No. 5,744,335), or ICE.
- In addition, several reagents are commercially available to enhance transfection efficacy. Suitable examples include LIPOFECTIN (DOTMA:DOPE) (Invitrogen, Carlsbad, Calif.), LIPOFECTAMINE (DOSPA:DOPE) (Invitrogen), LIPOFECTAMINE2000. (Invitrogen), FUGENE, TRANSFECTAM (DOGS), and EFFECTENE.
- Also contemplated are cationic lipids such as the dialkylamino-based, imidazole-based, and guanidinium-based lipids. For example, certain embodiments are directed to a composition comprising one or more imidazole-based cationic lipids, for example, the imidazole cholesterol ester or “ICE” lipid (3S,10R,13R,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl 3-(1H-imidazol-4-yl)propanoate, as represented by structure (I) below. In a preferred embodiment, a transfer vehicle for delivery of mRNA may comprise one or more imidazole-based cationic lipids, for example, the imidazole cholesterol ester or “ICE” lipid (3S,10R,13R,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl 3-(1H-imidazol-4-yl)propanoate, as represented by structure (I).
- Without wishing to be bound by a particular theory, it is believed that the fusogenicity of the imidazole-based cationic lipid ICE is related to the endosomal disruption which is facilitated by the imidazole group, which has a lower pKa relative to traditional cationic lipids. The endosomal disruption in turn promotes osmotic swelling and the disruption of the liposomal membrane, followed by the transfection or intracellular release of the nucleic acid(s) contents loaded therein into the target cell.
- The imidazole-based cationic lipids are also characterized by their reduced toxicity relative to other cationic lipids. The imidazole-based cationic lipids (e.g., ICE) may be used as the sole cationic lipid in the lipid nanoparticle, or alternatively may be combined with traditional cationic lipids, non-cationic lipids, and PEG-modified lipids. The cationic lipid may comprise a molar ratio of about 1% to about 90%, about 2% to about 70%, about 5% to about 50%, about 10% to about 40% of the total lipid present in the transfer vehicle, or preferably about 20% to about 70% of the total lipid present in the transfer vehicle.
- Similarly, certain embodiments are directed to lipid nanoparticles comprising the HGT4003 cationic lipid 2-((2,3-Bis((9Z,12Z)-octadeca-9,12-dien-1-yloxy)propyl)disulfanyl)-N,N-dimethylethanamine, as represented by structure (II) below, and as further described in U.S. Provisional Application No:61/494,745, filed Jun. 8, 2011, the entire teachings of which are incorporated herein by reference in their entirety:
- In other embodiments the compositions and methods described herein are directed to lipid nanoparticles comprising one or more cleavable lipids, such as, for example, one or more cationic lipids or compounds that comprise a cleavable disulfide (S—S) functional group (e.g., HGT4001, HGT4002, HGT4003, HGT4004 and HGT4005), as further described in U.S. Provisional Application No. 61/494,745, the entire teachings of which are incorporated herein by reference in their entirety.
- The use of polyethylene glycol (PEG)-modified phospholipids and derivatized lipids such as derivatized ceramides (PEG-CER), including N-Octanoyl-Sphingosine-1-[Succinyl(Methoxy Polyethylene Glycol)-2000] (C8 PEG-2000 ceramide) is also contemplated by the present invention, either alone or preferably in combination with other lipids together which comprise the transfer vehicle (e.g., a lipid nanoparticle). Contemplated PEG-modified lipids include, but is not limited to, a polyethylene glycol chain of up to 5 kDa in length covalently attached to a lipid with alkyl chain(s) of C6-C20 length. The addition of such components may prevent complex aggregation and may also provide a means for increasing circulation lifetime and increasing the delivery of the lipid-nucleic acid composition to the target cell, (Klibanov et al. (1990) FEBS Letters, 268 (1): 235-237), or they may be selected to rapidly exchange out of the formulation in vivo (see U.S. Pat. No. 5,885,613). Particularly useful exchangeable lipids are PEG-ceramides having shorter acyl chains (e.g., C14 or C18). The PEG-modified phospholipid and derivitized lipids of the present invention may comprise a molar ratio from about 0% to about 20%, about 0.5% to about 20%, about 1% to about 15%, about 4% to about 10%, or about 2% of the total lipid present in the liposomal transfer vehicle.
- The present invention also contemplates the use of non-cationic lipids. As used herein, the phrase “non-cationic lipid” refers to any neutral, zwitterionic or anionic lipid. As used herein, the phrase “anionic lipid” refers to any of a number of lipid species that carry a net negative charge at a selected pH, such as physiological pH. Non-cationic lipids include, but are not limited to, distearoylphosphatidylcholine (DSPC), dioleoylphosphatidylcholine (DOPC), dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylglycerol (DOPG), dipalmitoylphosphatidylglycerol (DPPG), dioleoylphosphatidylethanolamine (DOPE), palmitoyloleoylphosphatidylcholine (POPC), palmitoyloleoyl-phosphatidylethanolamine (POPE), dioleoyl-phosphatidylethanolamine 4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (DOPE-mal), dipalmitoyl phosphatidyl ethanolamine (DPPE), dimyristoylphosphoethanolamine (DMPE), distearoyl-phosphatidyl-ethanolamine (DSPE), 16-O-monomethyl PE, 16-O-dimethyl PE, 18-1-trans PE, 1-stearoyl-2-oleoyl-phosphatidyethanolamine (SOPE), cholesterol, or a mixture thereof. Such non-cationic lipids may be used alone, but are preferably used in combination with other excipients, for example, cationic lipids. When used in combination with a cationic lipid, the non-cationic lipid may comprise a molar ratio of 5% to about 90%, or preferably about 10% to about 70% of the total lipid present in the transfer vehicle.
- Preferably, the transfer vehicle (e.g., a lipid nanoparticle) is prepared by combining multiple lipid and/or polymer components. For example, a transfer vehicle may be prepared using C12-200, DOPE, chol, DMG-PEG2K at a molar ratio of 40:30:25:5, or DODAP, DOPE, cholesterol, DMG-PEG2K at a molar ratio of 18:56:20:6, or HGT5000, DOPE, chol, DMG-PEG2K at a molar ratio of 40:20:35:5, or HGT5001, DOPE, chol, DMG-PEG2K at a molar ratio of 40:20:35:5. The selection of cationic lipids, non-cationic lipids and/or PEG-modified lipids which comprise the lipid nanoparticle, as well as the relative molar ratio of such lipids to each other, is based upon the characteristics of the selected lipid(s), the nature of the intended target cells, the characteristics of the mRNA to be delivered. Additional considerations include, for example, the saturation of the alkyl chain, as well as the size, charge, pH, pKa, fusogenicity and toxicity of the selected lipid(s). Thus the molar ratios may be adjusted accordingly. For example, in embodiments, the percentage of cationic lipid in the lipid nanoparticle may be greater than 10%, greater than 20%, greater than 30%, greater than 40%, greater than 50%, greater than 60%, or greater than 70%. The percentage of non-cationic lipid in the lipid nanoparticle may be greater than 5%, greater than 10%, greater than 20%, greater than 30%, or greater than 40%. The percentage of cholesterol in the lipid nanoparticle may be greater than 10%, greater than 20%, greater than 30%, or greater than 40%. The percentage of PEG-modified lipid in the lipid nanoparticle may be greater than 1%, greater than 2%, greater than 5%, greater than 10%, or greater than 20%.
- In certain preferred embodiments, the lipid nanoparticles of the invention comprise at least one of the following cationic lipids: C12-200, DLin-KC2-DMA, DODAP, HGT4003, ICE, HGT5000, or HGT5001. In embodiments, the transfer vehicle comprises cholesterol and/or a PEG-modified lipid. In some embodiments, the transfer vehicles comprises DMG-PEG2K. In certain embodiments, the tranfer vehicle comprises one of the following lipid formulations: C12-200, DOPE, chol, DMG-PEG2K; DODAP, DOPE, cholesterol, DMG-PEG2K; HGT5000, DOPE, chol, DMG-PEG2K, HGT5001, DOPE, chol, DMG-PEG2K.
- The liposomal transfer vehicles for use in the compositions of the invention can be prepared by various techniques which are presently known in the art. Multi-lamellar vesicles (MLV) may be prepared conventional techniques, for example, by depositing a selected lipid on the inside wall of a suitable container or vessel by dissolving the lipid in an appropriate solvent, and then evaporating the solvent to leave a thin film on the inside of the vessel or by spray drying. An aqueous phase may then added to the vessel with a vortexing motion which results in the formation of MLVs. Uni-lamellar vesicles (ULV) can then be formed by homogenization, sonication or extrusion of the multi-lamellar vesicles. In addition, unilamellar vesicles can be formed by detergent removal techniques.
- In certain embodiments of this invention, the compositions of the present invention comprise a transfer vehicle wherein the mRNA is associated on both the surface of the transfer vehicle and encapsulated within the same transfer vehicle. For example, during preparation of the compositions of the present invention, cationic liposomal transfer vehicles may associate with the mRNA through electrostatic interactions.
- In certain embodiments, the compositions of the invention may be loaded with diagnostic radionuclide, fluorescent materials or other materials that are detectable in both in vitro and in vivo applications. For example, suitable diagnostic materials for use in the present invention may include Rhodamine-dioleoylphosphatidylethanolamine (Rh-PE), Green Fluorescent Protein mRNA (GFP mRNA), Renilla Luciferase mRNA and Firefly Luciferase mRNA.
- Selection of the appropriate size of a liposomal transfer vehicle must take into consideration the site of the target cell or tissue and to some extent the application for which the liposome is being made. In some embodiments, it may be desirable to limit transfection of the mRNA to certain cells or tissues. For example, to target hepatocytes a liposomal transfer vehicle may be sized such that its dimensions are smaller than the fenestrations of the endothelial layer lining hepatic sinusoids in the liver; accordingly the liposomal transfer vehicle can readily penetrate such endothelial fenestrations to reach the target hepatocytes. Alternatively, a liposomal transfer vehicle may be sized such that the dimensions of the liposome are of a sufficient diameter to limit or expressly avoid distribution into certain cells or tissues. For example, a liposomal transfer vehicle may be sized such that its dimensions are larger than the fenestrations of the endothelial layer lining hepatic sinusoids to thereby limit distribution of the liposomal transfer vehicle to hepatocytes. Generally, the size of the transfer vehicle is within the range of about 25 to 250 nm, preferably less than about 250 nm, 175 nm, 150 nm, 125 nm, 100 nm, 75 nm, 50 nm, 25 nm or 10 nm.
- A variety of alternative methods known in the art are available for sizing of a population of liposomal transfer vehicles. One such sizing method is described in U.S. Pat. No. 4,737,323, incorporated herein by reference. Sonicating a liposome suspension either by bath or probe sonication produces a progressive size reduction down to small ULV less than about 0.05 microns in diameter. Homogenization is another method that relies on shearing energy to fragment large liposomes into smaller ones. In a typical homogenization procedure, MLV are recirculated through a standard emulsion homogenizer until selected liposome sizes, typically between about 0.1 and 0.5 microns, are observed. The size of the liposomal vesicles may be determined by quasi-electric light scattering (QELS) as described in Bloomfield, Ann. Rev. Biophys. Bioeng., 10:421-450 (1981), incorporated herein by reference. Average liposome diameter may be reduced by sonication of formed liposomes. Intermittent sonication cycles may be alternated with QELS assessment to guide efficient liposome synthesis.
- Target Cells
- As used herein, the term “target cell” refers to a cell or tissue to which a composition of the invention is to be directed or targeted. In some embodiments, the target cells are deficient in a protein or enzyme of interest. For example, where it is desired to deliver a nucleic acid to a hepatocyte, the hepatocyte represents the target cell. In some embodiments, the compositions of the invention transfect the target cells on a discriminatory basis (i.e., do not transfect non-target cells). The compositions of the invention may also be prepared to preferentially target a variety of target cells, which include, but are not limited to, hepatocytes, epithelial cells, hematopoietic cells, epithelial cells, endothelial cells, lung cells, bone cells, stem cells, mesenchymal cells, neural cells (e.g., meninges, astrocytes, motor neurons, cells of the dorsal root ganglia and anterior horn motor neurons), photoreceptor cells (e.g., rods and cones), retinal pigmented epithelial cells, secretory cells, cardiac cells, adipocytes, vascular smooth muscle cells, cardiomyocytes, skeletal muscle cells, beta cells, pituitary cells, synovial lining cells, ovarian cells, testicular cells, fibroblasts, B cells, T cells, reticulocytes, leukocytes, granulocytes and tumor cells.
- The compositions of the invention may be prepared to preferentially distribute to target cells such as in the heart, lungs, kidneys, liver, and spleen. In some embodiments, the compositions of the invention distribute into the cells of the liver to facilitate the delivery and the subsequent expression of the mRNA comprised therein by the cells of the liver (e.g., hepatocytes). The targeted hepatocytes may function as a biological “reservoir” or “depot” capable of producing, and systemically excreting a functional protein or enzyme. Accordingly, in one embodiment of the invention the liposomal transfer vehicle may target hepatocytes and/or preferentially distribute to the cells of the liver upon delivery. Following transfection of the target hepatocytes, the mRNA loaded in the liposomal vehicle are translated and a functional protein product is produced, excreted and systemically distributed. In other embodiments, cells other than hepatocytes (e.g., lung, spleen, heart, ocular, or cells of the central nervous system) can serve as a depot location for protein production.
- In one embodiment, the compositions of the invention facilitate a subject's endogenous production of one or more functional proteins and/or enzymes, and in particular the production of proteins and/or enzymes which demonstrate less immunogenicity relative to their recombinantly-prepared counterparts. In a preferred embodiment of the present invention, the transfer vehicles comprise mRNA which encode a deficient protein or enzyme. Upon distribution of such compositions to the target tissues and the subsequent transfection of such target cells, the exogenous mRNA loaded into the liposomal transfer vehicle (e.g., a lipid nanoparticle) may be translated in vivo to produce a functional protein or enzyme encoded by the exogenously administered mRNA (e.g., a protein or enzyme in which the subject is deficient). Accordingly, the compositions of the present invention exploit a subject's ability to translate exogenously- or recombinantly-prepared mRNA to produce an endogenously-translated protein or enzyme, and thereby produce (and where applicable excrete) a functional protein or enzyme. The expressed or translated proteins or enzymes may also be characterized by the in vivo inclusion of native post-translational modifications which may often be absent in recombinantly-prepared proteins or enzymes, thereby further reducing the immunogenicity of the translated protein or enzyme.
- The administration of mRNA encoding a deficient protein or enzyme avoids the need to deliver the nucleic acids to specific organelles within a target cell (e.g., mitochondria). Rather, upon transfection of a target cell and delivery of the nucleic acids to the cytoplasm of the target cell, the mRNA contents of a transfer vehicle may be translated and a functional protein or enzyme expressed.
- The present invention also contemplates the discriminatory targeting of target cells and tissues by both passive and active targeting means. The phenomenon of passive targeting exploits the natural distributions patterns of a transfer vehicle in vivo without relying upon the use of additional excipients or means to enhance recognition of the transfer vehicle by target cells. For example, transfer vehicles which are subject to phagocytosis by the cells of the reticulo-endothelial system are likely to accumulate in the liver or spleen, and accordingly may provide means to passively direct the delivery of the compositions to such target cells.
- Alternatively, the present invention contemplates active targeting, which involves the use of additional excipients, referred to herein as “targeting ligands” that may be bound (either covalently or non-covalently) to the transfer vehicle to encourage localization of such transfer vehicle at certain target cells or target tissues. For example, targeting may be mediated by the inclusion of one or more endogenous targeting ligands (e.g., apolipoprotein E) in or on the transfer vehicle to encourage distribution to the target cells or tissues. Recognition of the targeting ligand by the target tissues actively facilitates tissue distribution and cellular uptake of the transfer vehicle and/or its contents in the target cells and tissues (e.g., the inclusion of an apolipoprotein-E targeting ligand in or on the transfer vehicle encourages recognition and binding of the transfer vehicle to endogenous low density lipoprotein receptors expressed by hepatocytes). As provided herein, the composition can comprise a ligand capable of enhancing affinity of the composition to the target cell. Targeting ligands may be linked to the outer bilayer of the lipid particle during formulation or post-formulation. These methods are well known in the art. In addition, some lipid particle formulations may employ fusogenic polymers such as PEAA, hemagluttinin, other lipopeptides (see U.S. patent application Ser. No. 08/835,281, and U.S. patent application Ser. No. 60/083,294, which are incorporated herein by reference) and other features useful for in vivo and/or intracellular delivery. In other some embodiments, the compositions of the present invention demonstrate improved transfection efficacies, and/or demonstrate enhanced selectivity towards target cells or tissues of interest. Contemplated therefore are compositions which comprise one or more ligands (e.g., peptides, aptamers, oligonucleotides, a vitamin or other molecules) that are capable of enhancing the affinity of the compositions and their nucleic acid contents for the target cells or tissues. Suitable ligands may optionally be bound or linked to the surface of the transfer vehicle. In some embodiments, the targeting ligand may span the surface of a transfer vehicle or be encapsulated within the transfer vehicle. Suitable ligands and are selected based upon their physical, chemical or biological properties (e.g., selective affinity and/or recognition of target cell surface markers or features.) Cell-specific target sites and their corresponding targeting ligand can vary widely. Suitable targeting ligands are selected such that the unique characteristics of a target cell are exploited, thus allowing the composition to discriminate between target and non-target cells. For example, compositions of the invention may include surface markers (e.g., apolipoprotein-B or apolipoprotein-E) that selectively enhance recognition of, or affinity to hepatocytes (e.g., by receptor-mediated recognition of and binding to such surface markers). Additionally, the use of galactose as a targeting ligand would be expected to direct the compositions of the present invention to parenchymal hepatocytes, or alternatively the use of mannose containing sugar residues as a targeting ligand would be expected to direct the compositions of the present invention to liver endothelial cells (e.g., mannose containing sugar residues that may bind preferentially to the asialoglycoprotein receptor present in hepatocytes). (See Hillery A M, et al. “Drug Delivery and Targeting: For Pharmacists and Pharmaceutical Scientists” (2002) Taylor & Francis, Inc.) The presentation of such targeting ligands that have been conjugated to moieties present in the transfer vehicle (e.g., a lipid nanoparticle) therefore facilitate recognition and uptake of the compositions of the present invention in target cells and tissues. Examples of suitable targeting ligands include one or more peptides, proteins, aptamers, vitamins and oligonucleotides.
- Application and Administration
- As used herein, the term “subject” refers to any animal (e.g., a mammal), including, but not limited to, humans, non-human primates, rodents, and the like, to which the compositions and methods of the present invention are administered. Typically, the terms “subject” and “patient” are used interchangeably herein in reference to a human subject.
- The compositions and methods of the invention provide for the delivery of mRNA to treat a number of disorders. In particular, the compositions and methods of the present invention are suitable for the treatment of diseases or disorders relating to the deficiency of proteins and/or enzymes that are excreted or secreted by the target cell into the surrounding extracellular fluid (e.g., mRNA encoding hormones and neurotransmitters). In embodiments the disease may involve a defect or deficiency in a secreted protein (e.g. Fabry disease, or ALS). In certain embodiments, the disease may not be caused by a defect or deficit in a secreted protein, but may benefit from providing a secreted protein. For example, the symptoms of a disease may be improved by providing the compositions of the invention (e.g. cystic fibrosis). Disorders for which the present invention are useful include, but are not limited to, disorders such as Huntington's Disease; Parkinson's Disease; muscular dystrophies (such as, e.g. Duchenne and Becker); hemophelia diseases (such as, e.g., hemophilioa B (FIX), hemophilia A (FVIII); SMN1-related spinal muscular atrophy (SMA); amyotrophic lateral sclerosis (ALS); GALT-related galactosemia; Cystic Fibrosis (CF); SLC3A1-related disorders including cystinuria; COL4A5-related disorders including Alport syndrome; galactocerebrosidase deficiencies; X-linked adrenoleukodystrophy and adrenomyeloneuropathy; Friedreich's ataxia; Pelizaeus-Merzbacher disease; TSC1 and TSC2-related tuberous sclerosis; Sanfilippo B syndrome (MPS IIIB); CTNS-related cystinosis; the FMR1-related disorders which include Fragile X syndrome, Fragile X-Associated Tremor/Ataxia Syndrome and Fragile X Premature Ovarian Failure Syndrome; Prader-Willi syndrome; hereditary hemorrhagic telangiectasia (AT); Niemann-Pick disease Type C1; the neuronal ceroid lipofuscinoses-related diseases including Juvenile Neuronal Ceroid Lipofuscinosis (JNCL), Juvenile Batten disease, Santavuori-Haltia disease, Jansky-Bielschowsky disease, and PTT-1 and TPP1 deficiencies; EIF2B1, EIF2B2, EIF2B3, EIF2B4 and EIF2B5-related childhood ataxia with central nervous system hypomyelination/vanishing white matter; CACNA1A and CACNB4-related Episodic Ataxia Type 2; the MECP2-related disorders including Classic Rett Syndrome, MECP2-related Severe Neonatal Encephalopathy and PPM-X Syndrome; CDKL5-related Atypical Rett Syndrome; Kennedy's disease (SBMA); Notch-3 related cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL); SCN1A and SCN1B-related seizure disorders; the Polymerase G-related disorders which include Alpers-Huttenlocher syndrome, POLG-related sensory ataxic neuropathy, dysarthria, and ophthalmoparesis, and autosomal dominant and recessive progressive external ophthalmoplegia with mitochondrial DNA deletions; X-Linked adrenal hypoplasia; X-linked agammaglobulinemia; Wilson's disease; and Fabry Disease. In one embodiment, the nucleic acids, and in particular mRNA, of the invention may encode functional proteins or enzymes that are secreted into extracellular space. For example, the secreted proteins include clotting factors, components of the complement pathway, cytokines, chemokines, chemoattractants, protein hormones (e.g. EGF, PDF), protein components of serum, antibodies, secretable toll-like receptors, and others. In some embodiments, the compositions of the present invention may include mRNA encoding erythropoietin, α1-antitrypsin, carboxypeptidase N or human growth hormone.
- In embodiments, the invention encodes a secreted protein that is made up of subunits that are encoded by more than one gene. For example, the secreted protein may be a heterodimer, wherein each chain or subunit of the is encoded by a separate gene. It is possible that more than one mRNA molecule is delivered in the transfer vehicle and the mRNA encodes separate subunit of the secreted protein. Alternatively, a single mRNA may be engineered to encode more than one subunit (e.g. in the case of a single-chain Fv antibody). In certain embodiments, separate mRNA molecules encoding the individual subunits may be administered in separate transfer vehicles. In one embodiment, the mRNA may encode full length antibodies (both heavy and light chains of the variable and constant regions) or fragments of antibodies (e.g. Fab, Fv, or a single chain Fv (scFv) to confer immunity to a subject. While one embodiment of the present invention relates to methods and compositions useful for conferring immunity to a subject (e.g., via the translation of mRNA encoding functional antibodies), the inventions disclosed herein and contemplated hereby are broadly applicable. In an alternative embodiment the compositions of the present invention encode antibodies that may be used to transiently or chronically effect a functional response in subjects. For example, the mRNA of the present invention may encode a functional monoclonal or polyclonal antibody, which upon translation and secretion from target cell may be useful for targeting and/or inactivating a biological target (e.g., a stimulatory cytokine such as tumor necrosis factor). Similarly, the mRNA nucleic acids of the present invention may encode, for example, functional anti-nephritic factor antibodies useful for the treatment of membranoproliferative glomerulonephritis type II or acute hemolytic uremic syndrome, or alternatively may encode anti-vascular endothelial growth factor (VEGF) antibodies useful for the treatment of VEGF-mediated diseases, such as cancer. In other embodiments, the secreted protein is a cytokine or other secreted protein comprised of more than one subunit (e.g. IL-12, or IL-23).
- The compositions of the invention can be administered to a subject. In some embodiments, the composition is formulated in combination with one or more additional nucleic acids, carriers, targeting ligands or stabilizing reagents, or in pharmacological compositions where it is mixed with suitable excipients. For example, in one embodiment, the compositions of the invention may be prepared to deliver mRNA encoding two or more distinct proteins or enzymes. Techniques for formulation and administration of drugs may be found in “Remington's Pharmaceutical Sciences,” Mack Publishing Co., Easton, Pa., latest edition.
- A wide range of molecules that can exert pharmaceutical or therapeutic effects can be delivered into target cells using compositions and methods of the invention. The molecules can be organic or inorganic. Organic molecules can be peptides, proteins, carbohydrates, lipids, sterols, nucleic acids (including peptide nucleic acids), or any combination thereof. A formulation for delivery into target cells can comprise more than one type of molecule, for example, two different nucleotide sequences, or a protein, an enzyme or a steroid.
- The compositions of the present invention may be administered and dosed in accordance with current medical practice, taking into account the clinical condition of the subject, the site and method of administration, the scheduling of administration, the subject's age, sex, body weight and other factors relevant to clinicians of ordinary skill in the art. The “effective amount” for the purposes herein may be determined by such relevant considerations as are known to those of ordinary skill in experimental clinical research, pharmacological, clinical and medical arts. In some embodiments, the amount administered is effective to achieve at least some stabilization, improvement or elimination of symptoms and other indicators as are selected as appropriate measures of disease progress, regression or improvement by those of skill in the art. For example, a suitable amount and dosing regimen is one that causes at least transient protein production.
- Suitable routes of administration include, for example, oral, rectal, vaginal, transmucosal, pulmonary including intratracheal or inhaled, or intestinal administration; parenteral delivery, including intramuscular, subcutaneous, intramedullary injections, as well as intrathecal, direct intraventricular, intravenous, intraperitoneal, intranasal, or intraocular injections.
- Alternately, the compositions of the invention may be administered in a local rather than systemic manner, for example, via injection of the pharmaceutical composition directly into a targeted tissue, preferably in a sustained release formulation. Local delivery can be affected in various ways, depending on the tissue to be targeted. For example, aerosols containing compositions of the present invention can be inhaled (for nasal, tracheal, or bronchial delivery); compositions of the present invention can be injected into the site of injury, disease manifestation, or pain, for example; compositions can be provided in lozenges for oral, tracheal, or esophageal application; can be supplied in liquid, tablet or capsule form for administration to the stomach or intestines, can be supplied in suppository form for rectal or vaginal application; or can even be delivered to the eye by use of creams, drops, or even injection. Formulations containing compositions of the present invention complexed with therapeutic molecules or ligands can even be surgically administered, for example in association with a polymer or other structure or substance that can allow the compositions to diffuse from the site of implantation to surrounding cells. Alternatively, they can be applied surgically without the use of polymers or supports.
- In one embodiment, the compositions of the invention are formulated such that they are suitable for extended-release of the mRNA contained therein. Such extended-release compositions may be conveniently administered to a subject at extended dosing intervals. For example, in one embodiment, the compositions of the present invention are administered to a subject twice day, daily or every other day. In a preferred embodiment, the compositions of the present invention are administered to a subject twice a week, once a week, every ten days, every two weeks, every three weeks, or more preferably every four weeks, once a month, every six weeks, every eight weeks, every other month, every three months, every four months, every six months, every eight months, every nine months or annually. Also contemplated are compositions and liposomal vehicles which are formulated for depot administration (e.g., intramuscularly, subcutaneously, intravitreally) to either deliver or release a mRNA over extended periods of time. Preferably, the extended-release means employed are combined with modifications made to the mRNA to enhance stability.
- Also contemplated herein are lyophilized pharmaceutical compositions comprising one or more of the liposomal nanoparticles disclosed herein and related methods for the use of such lyophilized compositions as disclosed for example, in U.S. Provisional Application No. 61/494,882, filed Jun. 8, 2011, the teachings of which are incorporated herein by reference in their entirety. For example, lyophilized pharmaceutical compositions according to the invention may be reconstituted prior to administration or can be reconstituted in vivo. For example, a lyophilized pharmaceutical composition can be formulated in an appropriate dosage form (e.g., an intradermal dosage form such as a disk, rod or membrane) and administered such that the dosage form is rehydrated over time in vivo by the individual's bodily fluids.
- While certain compounds, compositions and methods of the present invention have been described with specificity in accordance with certain embodiments, the following examples serve only to illustrate the compounds of the invention and are not intended to limit the same. Each of the publications, reference materials, accession numbers and the like referenced herein to describe the background of the invention and to provide additional detail regarding its practice are hereby incorporated by reference in their entirety.
- The articles “a” and “an” as used herein in the specification and in the claims, unless clearly indicated to the contrary, should be understood to include the plural referents. Claims or descriptions that include “or” between one or more members of a group are considered satisfied if one, more than one, or all of the group members are present in, employed in, or otherwise relevant to a given product or process unless indicated to the contrary or otherwise evident from the context. The invention includes embodiments in which exactly one member of the group is present in, employed in, or otherwise relevant to a given product or process. The invention also includes embodiments in which more than one, or the entire group members are present in, employed in, or otherwise relevant to a given product or process. Furthermore, it is to be understood that the invention encompasses all variations, combinations, and permutations in which one or more limitations, elements, clauses, descriptive terms, etc., from one or more of the listed claims is introduced into another claim dependent on the same base claim (or, as relevant, any other claim) unless otherwise indicated or unless it would be evident to one of ordinary skill in the art that a contradiction or inconsistency would arise. Where elements are presented as lists, (e.g., in Markush group or similar format) it is to be understood that each subgroup of the elements is also disclosed, and any element(s) can be removed from the group. It should be understood that, in general, where the invention, or aspects of the invention, is/are referred to as comprising particular elements, features, etc., certain embodiments of the invention or aspects of the invention consist, or consist essentially of, such elements, features, etc. For purposes of simplicity those embodiments have not in every case been specifically set forth in so many words herein. It should also be understood that any embodiment or aspect of the invention can be explicitly excluded from the claims, regardless of whether the specific exclusion is recited in the specification. The publications and other reference materials referenced herein to describe the background of the invention and to provide additional detail regarding its practice are hereby incorporated by reference.
- Human erythropoietin (EPO) (SEQ ID NO: 3;
FIG. 3 ), human alpha-galactosidase (GLA) (SEQ ID NO: 4;FIG. 4 ), human alpha-1 antitrypsin (A1AT) (SEQ ID NO: 5;FIG. 5 ), and human factor IX (FIX) (SEQ ID NO: 6;FIG. 6 ) were synthesized by in vitro transcription from a plasmid DNA template encoding the gene, which was followed by the addition of a 5′ cap structure (Cap1) (Fechter & Brownlee, J. Gen. Virology 86:1239-1249 (2005)) and a 3′ poly(A) tail of approximately 200 nucleotides in length as determined by gel electrophoresis. 5′ and 3′ untranslated regions were present in each mRNA product in the following examples and are defined by SEQ ID NOs: 1 and 2 (FIG. 1 andFIG. 2 ) respectively. - Formulation 1: Aliquots of 50 mg/mL ethanolic solutions of C12-200, DOPE, Chol and DMG-PEG2K (40:30:25:5) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1×PBS (pH 7.4), concentrated and stored at 2-8° C.
Formulation 2: Aliquots of 50 mg/mL ethanolic solutions of DODAP, DOPE, cholesterol and DMG-PEG2K (18:56:20:6) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of EPO mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1×PBS (pH 7.4), concentrated and stored at 2-8° C. Final concentration=1.35 mg/mL EPO mRNA (encapsulated). Zave=75.9 nm (Dv(50)=57.3 nm; Dv(90)=92.1 nm).
Formulation 3: Aliquots of 50 mg/mL ethanolic solutions of HGT4003, DOPE, cholesterol and DMG-PEG2K (50:25:20:5) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1×PBS (pH 7.4), concentrated and stored at 2-8° C.
Formulation 4: Aliquots of 50 mg/mL ethanolic solutions of ICE, DOPE and DMG-PEG2K (70:25:5) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1×PBS (pH 7.4), concentrated and stored at 2-8° C.
Formulation 5: Aliquots of 50 mg/mL ethanolic solutions of HGT5000, DOPE, cholesterol and DMG-PEG2K (40:20:35:5) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of EPO mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1×PBS (pH 7.4), concentrated and stored at 2-8° C. Final concentration=1.82 mg/mL EPO mRNA (encapsulated). Zave=105.6 nm (Dv(50)=53.7 nm; Dv(90)=157 nm).
Formulation 6: Aliquots of 50 mg/mL ethanolic solutions of HGT5001, DOPE, cholesterol and DMG-PEG2K (40:20:35:5) were mixed and diluted with ethanol to 3 mL final volume. Separately, an aqueous buffered solution (10 mM citrate/150 mM NaCl, pH 4.5) of EPO mRNA was prepared from a 1 mg/mL stock. The lipid solution was injected rapidly into the aqueous mRNA solution and shaken to yield a final suspension in 20% ethanol. The resulting nanoparticle suspension was filtered, diafiltrated with 1×PBS (pH 7.4), concentrated and stored at 2-8° C.
Analysis of Protein Produced Via Intravenously Delivered mRNA-Loaded Nanoparticles Injection Protocol - Studies were performed using male CD-1 mice of approximately 6-8 weeks of age at the beginning of each experiment, unless otherwise indicated. Samples were introduced by a single bolus tail-vein injection of an equivalent total dose of 30-200 micrograms of encapsulated mRNA. Mice were sacrificed and perfused with saline at the designated time points.
- The liver and spleen of each mouse was harvested, apportioned into three parts, and stored in either 10% neutral buffered formalin or snap-frozen and stored at −80° C. for analysis.
- All animals were euthanized by CO2 asphyxiation 48 hours post dose administration (±5%) followed by thoracotomy and terminal cardiac blood collection. Whole blood (maximal obtainable volume) was collected via cardiac puncture on euthanized animals into serum separator tubes, allowed to clot at room temperature for at least 30 minutes, centrifuged at 22° C.±5° C. at 9300 g for 10 minutes, and the serum extracted. For interim blood collections, approximately 40-50 μL of whole blood was collected via facial vein puncture or tail snip. Samples collected from non treatment animals were used as a baseline for comparison to study animals.
- Quantification of EPO protein was performed following procedures reported for human EPO ELISA kit (Quantikine IVD, R&D Systems, Catalog #Dep-00). Positive controls employed consisted of ultrapure and tissue culture grade recombinant human erythropoietin protein (R&D Systems, Catalog #286-EP and 287-TC, respectively). Detection was monitored via absorption (450 nm) on a Molecular Device Flex Station instrument.
- Standard ELISA procedures were followed employing sheep anti-Alpha-galactosidase G-188 IgG as the capture antibody with rabbit anti-Alpha-galactosidase TK-88 IgG as the secondary (detection) antibody (Shire Human Genetic Therapies). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG was used for activation of the 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution. The reaction was quenched using 2N H2SO4 after 20 minutes. Detection was monitored via absorption (450 nm) on a Molecular Device Flex Station instrument. Untreated mouse scrum and human Alpha-galactosidase protein were used as negative and positive controls, respectively.
- Quantification of FIX protein was performed following procedures reported for human FIX ELISA kit (AssayMax, Assay Pro, Catalog #EF1009-1).
- Quantification of A1AT protein was performed following procedures reported for human A1AT ELISA kit (Innovative Research, Catalog #IRAPKT015).
- Western blot analyses were performed using an anti-hEPO antibody (R&D Systems #MAB2871) and ultrapure human EPO protein (R&D Systems #286-EP) as the control.
- The work described in this example demonstrates the use of mRNA-encapsulated lipid nanoparticles as a depot source for the production of protein. Such a depot effect can be achieved in multiple sites within the body (i.e., liver, kidney, spleen, and muscle). Measurement of the desired exogenous-based protein derived from messenger RNA delivered via liposomal nanoparticles was achieved and quantified, and the secretion of protein from a depot using human erythropoietin (hEPO), human alpha-galactosidase (hGLA), human alpha-1 antitrypsin (hA1AT), and human Factor IX (hFIX) mRNA was demonstrated.
- The production of hEPO protein was demonstrated with various lipid nanoparticle formulations. Of four different cationic lipid systems, C12-200-based lipid nanoparticles produced the highest quantity of hEPO protein after four hours post intravenous administration as measured by ELISA (
FIG. 7 ). This formulation (Formulation 1) resulted in 18.3 ug/mL hEPO protein secreted into the bloodstream. Normal hEPO protein levels in serum for human are 3.3-16.6 mIU/mL (NCCLS Document C28-P; Vol. 12, No. 2). Based on a specific activity of 120,000 IU/mg of EPO protein, that yields a quantity of 27.5-138 pg/mL hEPO protein in normal human individuals. Therefore, a single 30 ug dose of a C12-200-based cationic lipid formulation encapsulating hEPO mRNA yielded an increase in respective protein of over 100,000-fold physiological levels. - Of the lipid systems tested, the DODAP-based lipid nanoparticle formulation was the least effective. However, the observed quantity of human EPO protein derived from delivery via a DODAP-based lipid nanoparticle encapsulating EPO mRNA was 4.1 ng/mL, which is still greater than 30-fold over normal physiological levels of EPO protein (Table 1).
-
TABLE 1 Raw values of secreted hEPO protein for various cationic lipid-based nanoparticle systems as measured via ELISA analysis (as depicted in FIG. 8). Doses are based on encapsulated hEPO mRNA. Values of protein are depicted as nanogram of human EPO protein per milliliter of serum. Hematocrit changes are based on comparison of pre-bleed (Day −1) and Day 10.Secreted Dose of Human Increase in Cationic/Ionizable Lipid Encapsulated EPO Protein Hematocrit Component mRNA (ug) (ng/mL) (%) C12-200 30 18,306 15.0 HGT4003 150 164 0.0 ICE 100 56.2 0.0 DODAP 200 4.1 0.0 - In addition, the resulting protein was tested to determine if it was active and functioned properly. In the case of mRNA replacement therapy (MRT) employing hEPO mRNA, hematocrit changes were monitored over a ten day period for five different lipid nanoparticle formulations (
FIG. 8 , Table 1) to evaluate protein activity. During this time period, two of the five formulations demonstrated an increase in hematocrit (≧15%), which is indicative of active hEPO protein being produced from such systems. - In another experiment, hematocrit changes were monitored over a 15-day period (
FIG. 9 , Table 2). The lipid nanoparticle formulation (Formulation 1) was administered either as a single 30 μg dose, or as three smaller 10 μg doses injected onday 1,day 3 andday 5. Similarly,Formulation 2 was administered as 3 doses of 50 μg onday 1,day 3, andday 5. C12-200 produced a significant increase in hematocrit. Overall an increase of up to ˜25% change was observed, which is indicative of active human EPO protein being produced from such systems. -
TABLE 2 Hematocrit levels of each group over a 15 day observation period (FIG. 9). Mice were either dosed as a single injection, or three injections, every other day. N = 4 mice per group. Dose (μg/ Hct Levels Mean (%) ± SEM Test Article animal) Day −4 Day 7Day 10Day 15aC12-200 30 (single 50.8 ± 1.8 58.3 ± 3.3 62.8 ± 1.3 59.9 ± 3.3 dose) C12-200 30 (over 3 52.2 ± 0.5 55.3 ± 2.3 63.3 ± 1.6 62.3 ± 1.9 doses) DODAP 150 (over 54.8 ± 1.7 53.5 ± 1.6 54.2 ± 3.3 54.0 ± 0.3 3 doses) Hct = hematocrit; SEM = standard error of the mean. aBlood samples were collected into non-heparinized hematocrit tubes. - A second exogenous-based protein system was explored to demonstrate the “depot effect” when employing mRNA-loaded lipid nanoparticles. Animals were injected intravenously with a single 30 microgram dose of encapsulated human alpha-galactosidase (hGLA) mRNA using a C12-200-based lipid nanoparticle system and sacrificed after six hours (Formulation 1). Quantification of secreted hGLA protein was performed via ELISA. Untreated mouse serum and human Alpha-galactosidase protein were used as controls. Detection of human alpha-galactosidase protein was monitored over a 48 hour period.
- Measurable levels of hGLA protein were observed throughout the time course of the experiment with a maximum level of 2.0 ug/mL hGLA protein at six hours (
FIG. 10 ). Table 3 lists the specific quantities of hGLA found in the serum. Normal activity in healthy human males has been reported to be approximately 3.05 nanomol/hr/mL. The activity for Alpha-galactosidase, a recombinant human alpha-galactosidase protein, 3.56×106 nanomol/hr/mg. Analysis of these values yields a quantity of approximately 856 pg/mL of hGLA protein in normal healthy male individuals. The quantity of 2.0 ug/mL hGLA protein observed after six hours when dosing a hGLA mRNA-loaded lipid nanoparticle is over 2300-fold greater than normal physiological levels. Further, after 48 hours, one can still detect appreciable levels of hGLA protein (86.2 ng/mL). This level is representative of almost 100-fold greater quantities of hGLA protein over physiological amounts still present at 48 hours. -
TABLE 3 Raw values of secreted hGLA protein over time as measured via ELISA analysis (as depicted in FIG. 10). Values are depicted as nanogram of hGLA protein per milliliter of serum. N = 4 mice per group. Time Secreted Human Post-Administration (hr) GLA Protein (ng/mL) 6 2,038 12 1,815 24 414 48 86.2 - In addition, the half-life of Alpha-galactosidase when administered at 0.2 mg/kg is approximately 108 minutes. Production of GLA protein via the “depot effect” when administering GLA mRNA-loaded lipid nanoparticles shows a substantial increase in blood residence time when compared to direct injection of the naked recombinant protein. As described above, significant quantities of protein are present after 48 hours.
- The activity profile of the α-galactosidase protein produced from GLA mRNA-loaded lipid nanoparticles was measured as a function of 4-methylumbelliferyl-α-D-galactopyranoside (4-MU-α-gal) metabolism. As shown in
FIG. 11 , the protein produced from these nanoparticle systems is quite active and reflective of the levels of protein available (FIG. 12 , Table 3). AUC comparisons of mRNA therapy-based hGLA production versus enzyme replacement therapy (ERT) in mice and humans show a 182-fold and 30-fold increase, respectively (Table 4). -
TABLE 4 Comparison of Cmax and AUCinf values in Fabry patients post-IV dosing 0.2 mg/kg of Alpha-galactosidase (pharmacological dose) with those in mice post- IV dosing Alpha-galactosidase and GLA mRNA. AUCinf Test Dose Cmax (hr · Article Descrption (mg/kg) (U/mL) U/mL) n Fabrya α-GAL Transplant 0.2 3478 3683 11 Patient Protein Dialysis 0.2 3887 3600 6 Non-ESRDb 0.2 3710 4283 18 Mouse α-GAL Athymic nude 0.04 3807 797 3 Protein (MM1) α-GAL Athymic nude 0.04 3705 602 3 Protein (MM2) Mouse α-GAL mouse 0.95 5885 109428 6 mRNA (Cat 6 hr)c aData were from a published paper (Gregory M. Pastores et al. Safety and Pharmacokinetics of hGLA in patients with Fabry disease and end-stage renal disease. Nephrol Dial Transplant (2007) 22:1920-1925. bnon-end-stage renal disease. cα-Galactosidase activity at 6 hours after dosing (the earliest time point tested in the study). - The ability of mRNA encapsulated lipid nanoparticles to target organs which can act as a depot for the production of a desired protein has been demonstrated. The levels of secreted protein observed have been several orders of magnitude above normal physiological levels. This “depot effect” is repeatable.
FIG. 12 shows again that robust protein production is observed upon dosing wild type (CD-1) mice with a single 30 ug dose of hGLA mRNA-loaded in C12-200-based lipid nanoparticles (Formulation 1). In this experiment, hGLA levels were evaluated over a 72 hour period. A maximum average of 4.0 ug human hGLA protein/mL serum is detected six hours post-administration. Based on a value of ˜1 ng/mL hGLA protein for normal physiological levels, hGLA MRT provides roughly 4000-fold higher protein levels. As before, hGLA protein could be detected out to 48 hr post-administration (FIG. 12 ). - An analysis of tissues isolated from this same experiment provided insight into the distribution of hGLA protein in hGLA MRT-treated mice (
FIG. 13 ). Supraphysiological levels of hGLA protein were detected in the liver, spleen and kidneys of all mice treated with a maximum observed between 12 and 24 hour post-administration. Detectable levels of MRT-derived protein could be observed three days after a single injection of hGLA-loaded lipid nanoparticles. - In addition, the production of hGLA upon administration of hGLA mRNA loaded C12-200 nanoparticles was shown to exhibit a dose a response in the serum (
FIG. 14A ), as well as in the liver (FIG. 14B ). - One inherent characteristic of lipid nanoparticle-mediated mRNA replacement therapy would be the pharmacokinetic profile of the respective protein produced. For example, ERT-based treatment of mice employing Alpha-galactosidase results in a plasma half-life of approximately 100 minutes. In contrast, MRT-derived alpha-galactosidase has a blood residence time of approximately 72 hrs with a peak time of 6 hours. This allows for much greater exposure for organs to participate in possible continuous uptake of the desired protein. A comparison of PK profiles is shown in
FIG. 15 and demonstrates the stark difference in clearance rates and ultimately a major shift in area under the curve (AUC) can be achieved via MRT-based treatment. - In a separate experiment, hGLA MRT was applied to a mouse disease model, hGLA KO mice (Fabry mice). A 0.33 mg/kg dose of hGLA mRNA-loaded C12-200-based lipid nanoparticles (Formulation 1) was administered to female KO mice as a single, intravenous injection. Substantial quantities of MRT-derived hGLA protein were produced with a peak at 6 hr (˜560 ng/mL serum) which is approximately 600-fold higher than normal physiological levels. Further, hGLA protein was still detectable 72 hr post-administration (
FIG. 16 ). - Quantification of MRT-derived GLA protein in vital organs demonstrated substantial accumulation as shown in
FIG. 17 . A comparison of observed MRT-derived hGLA protein to reported normal physiological levels that are found in key organs is plotted (normal levels plotted as dashed lines). While levels of protein at 24 hours are higher than at 72 hours post-administration, the levels of hGLA protein detected in the liver, kidney, spleen and hearts of the treated Fabry mice are equivalent to wild type levels. For example, 3.1 ng hGLA protein/mg tissue were found in the kidneys of treatedmice 3 days after a single MRT treatment. - In a subsequent experiment, a comparison of ERT-based Alpha-galactosidase treatment versus hGLA MRT-based treatment of male Fabry KO mice was conducted. A single, intravenous dose of 1.0 mg/kg was given for each therapy and the mice were sacrificed one week post-administration. Serum levels of hGLA protein were monitored at 6 hr and 1 week post-injection. Liver, kidney, spleen, and heart were analyzed for hGLA protein accumulation one week post-administration. In addition to the biodistribution analyses, a measure of efficacy was determined via measurement of globotrioasylceramide (Gb3) and lyso-Gb3 reductions in the kidney and heart.
FIG. 18 shows the serum levels of hGLA protein after treatment of either Alpha-galactosidase or GLA mRNA loaded lipid nanoparticles (Formulation 1) in male Fabry mice. Serum samples were analyzed at 6 hr and 1 week post-administration. A robust signal was detected for MRT-treated mice after 6 hours, with hGLA protein serum levels of ˜4.0 ug/mL. In contrast, there was no detectable Alpha-galactosidase remaining in the bloodstream at this time. - The Fabry mice in this experiment were sacrificed one week after the initial injection and the organs were harvested and analyzed (liver, kidney, spleen, heart).
FIG. 19 shows a comparison of human GLA protein found in each respective organ after either hGLA MRT or Alpha-galactosidase ERT treatment. Levels correspond to hGLA present one week post-administration. hGLA protein was detected in all organs analyzed. For example, MRT-treated mice resulted in hGLA protein accumulation in the kidney of 2.42 ng hGLA protein/mg protein, while Alpha-galactosidase-treated mice had only residual levels (0.37 ng/mg protein). This corresponds to a ˜6.5-fold higher level of hGLA protein when treated via hGLA MRT. Upon analysis of the heart, 11.5 ng hGLA protein/mg protein was found for the MRT-treated cohort as compared to only 1.0 ng/mg protein Alpha-galactosidase. This corresponds to an ˜11-fold higher accumulation in the heart for hGLA MRT-treated mice over ERT-based therapies. - In addition to the biodistribution analyses conducted, evaluations of efficacy were determined via measurement of globotrioasylceramide (Gb3) and lyso-Gb3 levels in key organs. A direct comparison of Gb3 reduction after a single, intravenous 1.0 mg/kg GLA MRT treatment as compared to a Alpha-galactosidase ERT-based therapy of an equivalent dose yielded a sizeable difference in levels of Gb3 in the kidneys as well as heart. For example, Gb3 levels for GLA MRT versus Alpha-galactosidase yielded reductions of 60.2% vs 26.8%, respectively (
FIG. 20 ). Further, Gb3 levels in the heart were reduced by 92.1% vs 66.9% for MRT and Alpha-galactosidase, respectively (FIG. 21 ). - A second relevant biomarker for measurement of efficacy is lyso-Gb3. GLA MRT reduced lyso-Gb3 more efficiently than Alpha-galactosidase as well in the kidneys and heart (
FIG. 20 andFIG. 21 , respectively). In particular, MRT-treated Fabry mice demonstrated reductions of lyso-Gb3 of 86.1% and 87.9% in the kidneys and heart as compared to Alpha-galactosidase-treated mice yielding a decrease of 47.8% and 61.3%, respectively. - The results with for hGLA in C12-200 based lipid nanoparticles extend to other lipid nanoparticle formulations. For example, hGLA mRNA loaded into HGT4003 (Formulation 3) or HGT5000-based (Formulation 5) lipid nanoparticles administered as a single dose IV result in production of hGLA at 24 hours post administration (
FIG. 22 ). The production of hGLA exhibited a dose response. Similarly, hGLA production was observed at 6 hours and 24 hours after administration of hGLA mRNA loaded into HGT5001-based (Formulation 6) lipid nanoparticles administered as a single dose IV. hGLA production was observed in the serum (FIG. 23A ), as well as in organs (FIG. 23B ). - Overall, mRNA replacement therapy applied as a depot for protein production produces large quantities of active, functionally therapeutic protein at supraphysiological levels. This method has been demonstrated to yield a sustained circulation half-life of the desired protein and this MRT-derived protein is highly efficacious for therapy as demonstrated with alpha-galactosidase enzyme in Fabry mice.
- Studies were performed administering Factor IX (FIX) mRNA-loaded lipid nanoparticles in wild type mice (CD-1) and determining FIX protein that is secreted into the bloodstream. Upon intravenous injection of a single dose of 30 ug C12-200-based (C12-200:DOPE:Chol:PEG at a ratio of 40:30:25:5) FIX mRNA-loaded lipid nanoparticles (dose based on encapsulated mRNA) (Formulation 1), a robust protein production was observed (
FIG. 24 ). - A pharmacokinetic analysis over 72 hours showed MRT-derived FIX protein could be detected at all timepoints tested (
FIG. 24 ). The peak serum concentration was observed at 24 hr post-injection with a value of ˜3 ug (2995+738 ng/mL) FIX protein/mL serum. This represents another successful example of the depot effect. - Studies were performed administering alpha-1-antitrypsin (A1AT) mRNA-loaded lipid nanoparticles in wild type mice (CD-1) and determining A1AT protein that is secreted into the bloodstream. Upon intravenous injection of a single dose of 30 ug C12-200-based A1AT mRNA-loaded lipid nanoparticles (dose based on encapsulated mRNA) (Formulation 1), a robust protein production was observed (
FIG. 25 ). - As depicted in
FIG. 25 , detectable levels of human A1AT protein derived from A1AT MRT could be observed over a 24 hour time period post-administration. A maximum serum level of ˜48 ug A1AT protein/mL serum was detected 12 hours after injection. - All studies were performed using female CD-1 or BALB/C mice of approximately 7-10 weeks of age at the beginning of each experiment. Test articles were introduced via a single intratracheal aerosolized administration. Mice were sacrificed and perfused with saline at the designated time points. The lungs of each mouse were harvested, apportioned into two parts, and stored in either 10% neutral buffered formalin or snap-frozen and stored at −80° C. for analysis. Serum was isolated as described in Example 1. EPO ELISA as described in Example 1.
- The depot effect can be achieved via pulmonary delivery (e.g. intranasal, intratracheal, nebulization). Measurement of the desired exogenous-based protein derived from messenger RNA delivered via nanoparticle systems was achieved and quantified.
- The production of human EPO protein via hEPO mRNA-loaded lipid nanoparticles was tested in CD-1 mice via a single intratracheal administration (MicroSprayer®). Several formulations were tested using various cationic lipids (
Formulations - Human EPO protein was detected at the site of administration (lungs) upon treatment via aerosol delivery. Analysis of the serum six hours post-administration showed detectable amounts of hEPO protein in circulation. These data (shown in
FIG. 26 ) demonstrate the ability of the lung to act as a “depot” for the production (and secretion) of hEPO protein.
Claims (6)
Priority Applications (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14/124,608 US20140206753A1 (en) | 2011-06-08 | 2012-06-08 | Lipid nanoparticle compositions and methods for mrna delivery |
| US14/308,546 US9308281B2 (en) | 2011-06-08 | 2014-06-18 | MRNA therapy for Fabry disease |
| US14/308,554 US9597413B2 (en) | 2011-06-08 | 2014-06-18 | Pulmonary delivery of mRNA |
| US15/482,117 US10238754B2 (en) | 2011-06-08 | 2017-04-07 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US16/233,031 US10413618B2 (en) | 2011-06-08 | 2018-12-26 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US16/286,400 US10350303B1 (en) | 2011-06-08 | 2019-02-26 | Lipid nanoparticle compositions and methods for mRNA delivery |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161494881P | 2011-06-08 | 2011-06-08 | |
| US14/124,608 US20140206753A1 (en) | 2011-06-08 | 2012-06-08 | Lipid nanoparticle compositions and methods for mrna delivery |
| PCT/US2012/041724 WO2012170930A1 (en) | 2011-06-08 | 2012-06-08 | Lipid nanoparticle compositions and methods for mrna delivery |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2012/041724 A-371-Of-International WO2012170930A1 (en) | 2011-06-08 | 2012-06-08 | Lipid nanoparticle compositions and methods for mrna delivery |
| USPCT/US2012/141724 A-371-Of-International | 2012-06-08 |
Related Child Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/308,546 Continuation US9308281B2 (en) | 2011-06-08 | 2014-06-18 | MRNA therapy for Fabry disease |
| US14/308,554 Continuation US9597413B2 (en) | 2011-06-08 | 2014-06-18 | Pulmonary delivery of mRNA |
| US15/482,117 Division US10238754B2 (en) | 2011-06-08 | 2017-04-07 | Lipid nanoparticle compositions and methods for MRNA delivery |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20140206753A1 true US20140206753A1 (en) | 2014-07-24 |
Family
ID=46317537
Family Applications (24)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/124,608 Abandoned US20140206753A1 (en) | 2011-06-08 | 2012-06-08 | Lipid nanoparticle compositions and methods for mrna delivery |
| US14/308,554 Active US9597413B2 (en) | 2011-06-08 | 2014-06-18 | Pulmonary delivery of mRNA |
| US14/308,546 Active US9308281B2 (en) | 2011-06-08 | 2014-06-18 | MRNA therapy for Fabry disease |
| US15/482,117 Active US10238754B2 (en) | 2011-06-08 | 2017-04-07 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US16/233,031 Active US10413618B2 (en) | 2011-06-08 | 2018-12-26 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US16/286,400 Active US10350303B1 (en) | 2011-06-08 | 2019-02-26 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US16/502,672 Active US10507249B2 (en) | 2011-06-08 | 2019-07-03 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US16/681,565 Abandoned US20200085973A1 (en) | 2011-06-08 | 2019-11-12 | Lipid nanoparticle compositions and methods for mrna delivery |
| US16/931,231 Active US10888626B2 (en) | 2011-06-08 | 2020-07-16 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/099,462 Active US11052159B2 (en) | 2011-06-08 | 2020-11-16 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/341,016 Active US11185595B2 (en) | 2011-06-08 | 2021-06-07 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/509,674 Active US11291734B2 (en) | 2011-06-08 | 2021-10-25 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/556,978 Abandoned US20220111071A1 (en) | 2011-06-08 | 2021-12-20 | Lipid nanoparticle compositions and methods for mrna delivery |
| US17/556,573 Active US11547764B2 (en) | 2011-06-08 | 2021-12-20 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US17/556,974 Active US11338044B2 (en) | 2011-06-08 | 2021-12-20 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/558,310 Active US11730825B2 (en) | 2011-06-08 | 2021-12-21 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/832,196 Active US12121592B2 (en) | 2011-06-08 | 2022-06-03 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/832,202 Abandoned US20220362406A1 (en) | 2011-06-08 | 2022-06-03 | Lipid nanoparticle compositions and methods for mrna delivery |
| US18/295,152 Active US11951180B2 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US18/295,139 Abandoned US20230293723A1 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for mrna delivery |
| US18/295,159 Active US11951181B2 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US18/295,150 Active US11951179B2 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US18/806,139 Pending US20250108126A1 (en) | 2011-06-08 | 2024-08-15 | Lipid Nanoparticle Compositions and Methods for mRNA Delivery |
| US19/027,351 Pending US20250152735A1 (en) | 2011-06-08 | 2025-01-17 | Lipid Nanoparticle Compositions and Methods for mRNA Delivery |
Family Applications After (23)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US14/308,554 Active US9597413B2 (en) | 2011-06-08 | 2014-06-18 | Pulmonary delivery of mRNA |
| US14/308,546 Active US9308281B2 (en) | 2011-06-08 | 2014-06-18 | MRNA therapy for Fabry disease |
| US15/482,117 Active US10238754B2 (en) | 2011-06-08 | 2017-04-07 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US16/233,031 Active US10413618B2 (en) | 2011-06-08 | 2018-12-26 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US16/286,400 Active US10350303B1 (en) | 2011-06-08 | 2019-02-26 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US16/502,672 Active US10507249B2 (en) | 2011-06-08 | 2019-07-03 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US16/681,565 Abandoned US20200085973A1 (en) | 2011-06-08 | 2019-11-12 | Lipid nanoparticle compositions and methods for mrna delivery |
| US16/931,231 Active US10888626B2 (en) | 2011-06-08 | 2020-07-16 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/099,462 Active US11052159B2 (en) | 2011-06-08 | 2020-11-16 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/341,016 Active US11185595B2 (en) | 2011-06-08 | 2021-06-07 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/509,674 Active US11291734B2 (en) | 2011-06-08 | 2021-10-25 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/556,978 Abandoned US20220111071A1 (en) | 2011-06-08 | 2021-12-20 | Lipid nanoparticle compositions and methods for mrna delivery |
| US17/556,573 Active US11547764B2 (en) | 2011-06-08 | 2021-12-20 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US17/556,974 Active US11338044B2 (en) | 2011-06-08 | 2021-12-20 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/558,310 Active US11730825B2 (en) | 2011-06-08 | 2021-12-21 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/832,196 Active US12121592B2 (en) | 2011-06-08 | 2022-06-03 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US17/832,202 Abandoned US20220362406A1 (en) | 2011-06-08 | 2022-06-03 | Lipid nanoparticle compositions and methods for mrna delivery |
| US18/295,152 Active US11951180B2 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US18/295,139 Abandoned US20230293723A1 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for mrna delivery |
| US18/295,159 Active US11951181B2 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for mRNA delivery |
| US18/295,150 Active US11951179B2 (en) | 2011-06-08 | 2023-04-03 | Lipid nanoparticle compositions and methods for MRNA delivery |
| US18/806,139 Pending US20250108126A1 (en) | 2011-06-08 | 2024-08-15 | Lipid Nanoparticle Compositions and Methods for mRNA Delivery |
| US19/027,351 Pending US20250152735A1 (en) | 2011-06-08 | 2025-01-17 | Lipid Nanoparticle Compositions and Methods for mRNA Delivery |
Country Status (29)
| Country | Link |
|---|---|
| US (24) | US20140206753A1 (en) |
| EP (4) | EP4043025A1 (en) |
| JP (6) | JP6184945B2 (en) |
| KR (4) | KR20220025112A (en) |
| CN (3) | CN111671919A (en) |
| AU (5) | AU2012267531B2 (en) |
| BR (1) | BR112013031553A2 (en) |
| CA (2) | CA3107288A1 (en) |
| CL (1) | CL2013003478A1 (en) |
| CY (1) | CY1122123T1 (en) |
| DK (2) | DK2717893T3 (en) |
| ES (2) | ES2740248T3 (en) |
| HK (1) | HK1199206A1 (en) |
| HR (1) | HRP20191338T1 (en) |
| HU (1) | HUE044277T2 (en) |
| IL (3) | IL307511A (en) |
| LT (1) | LT2717893T (en) |
| ME (1) | ME03491B (en) |
| MX (2) | MX367605B (en) |
| NZ (1) | NZ719345A (en) |
| PE (1) | PE20140797A1 (en) |
| PL (2) | PL3586861T3 (en) |
| PT (2) | PT3586861T (en) |
| RS (1) | RS59037B1 (en) |
| RU (1) | RU2013154295A (en) |
| SI (1) | SI2717893T1 (en) |
| SM (1) | SMT201900445T1 (en) |
| TR (1) | TR201910686T4 (en) |
| WO (1) | WO2012170930A1 (en) |
Cited By (153)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20140199233A1 (en) * | 2011-05-11 | 2014-07-17 | The Regents Of The University Of California | Enhanced Growth Inhibition of Osteosarcoma by Cytotoxic Polymerized Liposomal Nanoparticles Targeting the Alcam Cell Surface Receptor |
| US20140294938A1 (en) * | 2011-06-08 | 2014-10-02 | Shire Human Genetic Therapies, Inc. | Mrna therapy for fabry disease |
| US9061021B2 (en) | 2010-11-30 | 2015-06-23 | Shire Human Genetic Therapies, Inc. | mRNA for use in treatment of human genetic diseases |
| US9095552B2 (en) | 2012-04-02 | 2015-08-04 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding copper metabolism (MURR1) domain containing 1 |
| US9107886B2 (en) | 2012-04-02 | 2015-08-18 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding basic helix-loop-helix family member E41 |
| US9181319B2 (en) | 2010-08-06 | 2015-11-10 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| US9181321B2 (en) | 2013-03-14 | 2015-11-10 | Shire Human Genetic Therapies, Inc. | CFTR mRNA compositions and related methods and uses |
| US9186372B2 (en) | 2011-12-16 | 2015-11-17 | Moderna Therapeutics, Inc. | Split dose administration |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| US9512456B2 (en) | 2012-08-14 | 2016-12-06 | Modernatx, Inc. | Enzymes and polymerases for the synthesis of RNA |
| US9522176B2 (en) | 2013-10-22 | 2016-12-20 | Shire Human Genetic Therapies, Inc. | MRNA therapy for phenylketonuria |
| US9533047B2 (en) | 2011-03-31 | 2017-01-03 | Modernatx, Inc. | Delivery and formulation of engineered nucleic acids |
| US9572897B2 (en) | 2012-04-02 | 2017-02-21 | Modernatx, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| US9597380B2 (en) | 2012-11-26 | 2017-03-21 | Modernatx, Inc. | Terminally modified RNA |
| US9629804B2 (en) | 2013-10-22 | 2017-04-25 | Shire Human Genetic Therapies, Inc. | Lipid formulations for delivery of messenger RNA |
| US9668980B2 (en) | 2014-07-02 | 2017-06-06 | Rana Therapeutics, Inc. | Encapsulation of messenger RNA |
| WO2017099823A1 (en) * | 2015-12-10 | 2017-06-15 | Modernatx, Inc. | Compositions and methods for delivery of therapeutic agents |
| US9701965B2 (en) | 2010-10-01 | 2017-07-11 | Modernatx, Inc. | Engineered nucleic acids and methods of use thereof |
| WO2017127750A1 (en) | 2016-01-22 | 2017-07-27 | Modernatx, Inc. | Messenger ribonucleic acids for the production of intracellular binding polypeptides and methods of use thereof |
| WO2017180917A2 (en) | 2016-04-13 | 2017-10-19 | Modernatx, Inc. | Lipid compositions and their uses for intratumoral polynucleotide delivery |
| WO2017201350A1 (en) | 2016-05-18 | 2017-11-23 | Modernatx, Inc. | Polynucleotides encoding interleukin-12 (il12) and uses thereof |
| US9850269B2 (en) | 2014-04-25 | 2017-12-26 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US9943595B2 (en) | 2014-12-05 | 2018-04-17 | Translate Bio, Inc. | Messenger RNA therapy for treatment of articular disease |
| US9957499B2 (en) | 2013-03-14 | 2018-05-01 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US20180153822A1 (en) * | 2016-11-10 | 2018-06-07 | Translate Bio, Inc. | Process of Preparing mRNA-Loaded Lipid Nanoparticles |
| WO2018129544A1 (en) | 2017-01-09 | 2018-07-12 | Whitehead Institute For Biomedical Research | Methods of altering gene expression by perturbing transcription factor multimers that structure regulatory loops |
| US10022455B2 (en) | 2014-05-30 | 2018-07-17 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US10023626B2 (en) | 2013-09-30 | 2018-07-17 | Modernatx, Inc. | Polynucleotides encoding immune modulating polypeptides |
| US10064935B2 (en) | 2015-10-22 | 2018-09-04 | Modernatx, Inc. | Human cytomegalovirus RNA vaccines |
| US10087247B2 (en) | 2013-03-14 | 2018-10-02 | Translate Bio, Inc. | Methods and compositions for delivering mRNA coded antibodies |
| US10124055B2 (en) | 2015-10-22 | 2018-11-13 | Modernatx, Inc. | Zika virus RNA vaccines |
| US10130649B2 (en) | 2013-03-15 | 2018-11-20 | Translate Bio, Inc. | Synergistic enhancement of the delivery of nucleic acids via blended formulations |
| US10138213B2 (en) | 2014-06-24 | 2018-11-27 | Translate Bio, Inc. | Stereochemically enriched compositions for delivery of nucleic acids |
| US10144942B2 (en) | 2015-10-14 | 2018-12-04 | Translate Bio, Inc. | Modification of RNA-related enzymes for enhanced production |
| US10143758B2 (en) | 2009-12-01 | 2018-12-04 | Translate Bio, Inc. | Liver specific delivery of messenger RNA |
| WO2018231990A2 (en) | 2017-06-14 | 2018-12-20 | Modernatx, Inc. | Polynucleotides encoding methylmalonyl-coa mutase |
| US10172924B2 (en) | 2015-03-19 | 2019-01-08 | Translate Bio, Inc. | MRNA therapy for pompe disease |
| US10245229B2 (en) | 2012-06-08 | 2019-04-02 | Translate Bio, Inc. | Pulmonary delivery of mRNA to non-lung target cells |
| WO2019067999A1 (en) * | 2017-09-29 | 2019-04-04 | Intellia Therapeutics, Inc. | In vitro method of mrna delivery using lipid nanoparticles |
| US10258698B2 (en) | 2013-03-14 | 2019-04-16 | Modernatx, Inc. | Formulation and delivery of modified nucleoside, nucleotide, and nucleic acid compositions |
| US10266843B2 (en) | 2016-04-08 | 2019-04-23 | Translate Bio, Inc. | Multimeric coding nucleic acid and uses thereof |
| US10273269B2 (en) | 2017-02-16 | 2019-04-30 | Modernatx, Inc. | High potency immunogenic zika virus compositions |
| US10323076B2 (en) | 2013-10-03 | 2019-06-18 | Modernatx, Inc. | Polynucleotides encoding low density lipoprotein receptor |
| WO2019148101A1 (en) | 2018-01-29 | 2019-08-01 | Modernatx, Inc. | Rsv rna vaccines |
| US10449244B2 (en) | 2015-07-21 | 2019-10-22 | Modernatx, Inc. | Zika RNA vaccines |
| US10507183B2 (en) | 2011-06-08 | 2019-12-17 | Translate Bio, Inc. | Cleavable lipids |
| US20200038494A1 (en) * | 2018-08-02 | 2020-02-06 | Grifols Worldwide Operations Limited | Composition comprising highly-concentrated alpha1 proteinase inhibitor and method for obtaining thereof |
| US10653767B2 (en) | 2017-09-14 | 2020-05-19 | Modernatx, Inc. | Zika virus MRNA vaccines |
| US10695419B2 (en) | 2016-10-21 | 2020-06-30 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| US10709779B2 (en) | 2014-04-23 | 2020-07-14 | Modernatx, Inc. | Nucleic acid vaccines |
| US10780052B2 (en) | 2013-10-22 | 2020-09-22 | Translate Bio, Inc. | CNS delivery of MRNA and uses thereof |
| US10835583B2 (en) | 2016-06-13 | 2020-11-17 | Translate Bio, Inc. | Messenger RNA therapy for the treatment of ornithine transcarbamylase deficiency |
| US10849920B2 (en) | 2015-10-05 | 2020-12-01 | Modernatx, Inc. | Methods for therapeutic administration of messenger ribonucleic acid drugs |
| WO2021154763A1 (en) | 2020-01-28 | 2021-08-05 | Modernatx, Inc. | Coronavirus rna vaccines |
| WO2021159040A2 (en) | 2020-02-07 | 2021-08-12 | Modernatx, Inc. | Sars-cov-2 mrna domain vaccines |
| WO2021159130A2 (en) | 2020-05-15 | 2021-08-12 | Modernatx, Inc. | Coronavirus rna vaccines and methods of use |
| US11103596B2 (en) * | 2015-05-11 | 2021-08-31 | Ucl Business Plc | Fabry disease gene therapy |
| WO2021222304A1 (en) | 2020-04-27 | 2021-11-04 | Modernatx, Inc. | Sars-cov-2 rna vaccines |
| US11167043B2 (en) | 2017-12-20 | 2021-11-09 | Translate Bio, Inc. | Composition and methods for treatment of ornithine transcarbamylase deficiency |
| US20210346293A1 (en) * | 2019-01-25 | 2021-11-11 | Hoffmann-La Roche Inc. | Lipid Vesicle for Oral Drug Delivery |
| US11174500B2 (en) | 2018-08-24 | 2021-11-16 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US11173190B2 (en) | 2017-05-16 | 2021-11-16 | Translate Bio, Inc. | Treatment of cystic fibrosis by delivery of codon-optimized mRNA encoding CFTR |
| US20220001023A1 (en) * | 2017-08-31 | 2022-01-06 | Life Technologies Corporation | Cationic lipid compositions for tissue-specific delivery |
| US11219634B2 (en) | 2015-01-21 | 2022-01-11 | Genevant Sciences Gmbh | Methods, compositions, and systems for delivering therapeutic and diagnostic agents into cells |
| US11224642B2 (en) | 2013-10-22 | 2022-01-18 | Translate Bio, Inc. | MRNA therapy for argininosuccinate synthetase deficiency |
| US20220047517A1 (en) * | 2020-03-19 | 2022-02-17 | Shinshu University | Composition, lipid particle manufacturing kit, substance delivery method, and detection method |
| US11254936B2 (en) | 2012-06-08 | 2022-02-22 | Translate Bio, Inc. | Nuclease resistant polynucleotides and uses thereof |
| US11253605B2 (en) | 2017-02-27 | 2022-02-22 | Translate Bio, Inc. | Codon-optimized CFTR MRNA |
| WO2022067010A1 (en) | 2020-09-25 | 2022-03-31 | Modernatx, Inc. | Multi-proline-substituted coronavirus spike protein vaccines |
| WO2022099003A1 (en) | 2020-11-06 | 2022-05-12 | Sanofi | Lipid nanoparticles for delivering mrna vaccines |
| US11357726B2 (en) | 2018-08-29 | 2022-06-14 | Translate Bio, Inc. | Process of preparing mRNA-loaded lipid nanoparticles |
| US11364292B2 (en) | 2015-07-21 | 2022-06-21 | Modernatx, Inc. | CHIKV RNA vaccines |
| EP3846822A4 (en) * | 2018-09-04 | 2022-07-06 | The Board Of Regents Of The University Of Texas System | COMPOSITIONS AND METHODS FOR ORGAN SPECIFIC DELIVERY OF NUCLEIC ACIDS |
| WO2022150717A1 (en) | 2021-01-11 | 2022-07-14 | Modernatx, Inc. | Seasonal rna influenza virus vaccines |
| WO2022155530A1 (en) | 2021-01-15 | 2022-07-21 | Modernatx, Inc. | Variant strain-based coronavirus vaccines |
| WO2022155524A1 (en) | 2021-01-15 | 2022-07-21 | Modernatx, Inc. | Variant strain-based coronavirus vaccines |
| US11406703B2 (en) | 2020-08-25 | 2022-08-09 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| WO2022197624A1 (en) | 2021-03-15 | 2022-09-22 | Modernatx, Inc. | Therapeutic use of sars-cov-2 mrna domain vaccines |
| US20220323606A1 (en) * | 2021-04-06 | 2022-10-13 | Animatus Biosciences, Inc. | Targeting Technology to Selectively Express mRNAs in Cardiomyocytes While Avoiding Stimulation of Cardiac Fibroblasts |
| WO2022221335A1 (en) | 2021-04-13 | 2022-10-20 | Modernatx, Inc. | Respiratory virus combination vaccines |
| WO2022221440A1 (en) | 2021-04-14 | 2022-10-20 | Modernatx, Inc. | Influenza-coronavirus combination vaccines |
| US11492611B2 (en) | 2020-08-31 | 2022-11-08 | The Board Of Trustees Of The Leland Stanford Junior University | Systems and methods for producing RNA constructs with increased translation and stability |
| WO2022245888A1 (en) | 2021-05-19 | 2022-11-24 | Modernatx, Inc. | Seasonal flu rna vaccines and methods of use |
| US11524023B2 (en) | 2021-02-19 | 2022-12-13 | Modernatx, Inc. | Lipid nanoparticle compositions and methods of formulating the same |
| WO2022264109A1 (en) | 2021-06-18 | 2022-12-22 | Sanofi | Multivalent influenza vaccines |
| US11564893B2 (en) | 2015-08-17 | 2023-01-31 | Modernatx, Inc. | Methods for preparing particles and related compositions |
| WO2023025404A1 (en) | 2021-08-24 | 2023-03-02 | BioNTech SE | In vitro transcription technologies |
| US11603399B2 (en) | 2013-03-13 | 2023-03-14 | Modernatx, Inc. | Long-lived polynucleotide molecules |
| WO2023069498A1 (en) | 2021-10-22 | 2023-04-27 | Senda Biosciences, Inc. | Mrna vaccine composition |
| WO2023079507A1 (en) | 2021-11-05 | 2023-05-11 | Sanofi | Respiratory syncytial virus rna vaccine |
| US11649461B2 (en) * | 2016-05-18 | 2023-05-16 | Modernatx, Inc. | Polynucleotides encoding α-galactosidase A for the treatment of Fabry disease |
| WO2023092069A1 (en) | 2021-11-18 | 2023-05-25 | Modernatx, Inc. | Sars-cov-2 mrna domain vaccines and methods of use |
| WO2023096858A1 (en) | 2021-11-23 | 2023-06-01 | Senda Biosciences, Inc. | A bacteria-derived lipid composition and use thereof |
| WO2023102373A1 (en) | 2021-11-30 | 2023-06-08 | Sanofi Pasteur Inc. | Human metapneumovirus vaccines |
| WO2023107999A2 (en) | 2021-12-08 | 2023-06-15 | Modernatx, Inc. | Herpes simplex virus mrna vaccines |
| WO2023111262A1 (en) | 2021-12-17 | 2023-06-22 | Sanofi | Lyme disease rna vaccine |
| WO2023122080A1 (en) | 2021-12-20 | 2023-06-29 | Senda Biosciences, Inc. | Compositions comprising mrna and lipid reconstructed plant messenger packs |
| US11739317B2 (en) | 2020-07-13 | 2023-08-29 | The Board Of Trustees Of The Leland Stanford Junior University | Systems and methods to assess RNA stability |
| US11744801B2 (en) | 2017-08-31 | 2023-09-05 | Modernatx, Inc. | Methods of making lipid nanoparticles |
| US11766408B2 (en) | 2018-09-04 | 2023-09-26 | The Board Of Regents Of The University Of Texas System | Compositions and methods for organ specific delivery of nucleic acids |
| WO2023196914A1 (en) | 2022-04-08 | 2023-10-12 | Modernatx, Inc. | Influenza nucleic acid compositions and uses thereof |
| US11786607B2 (en) | 2017-06-15 | 2023-10-17 | Modernatx, Inc. | RNA formulations |
| WO2023214082A2 (en) | 2022-05-06 | 2023-11-09 | Sanofi | Signal sequences for nucleic acid vaccines |
| WO2023230481A1 (en) | 2022-05-24 | 2023-11-30 | Modernatx, Inc. | Orthopoxvirus vaccines |
| WO2024015890A1 (en) | 2022-07-13 | 2024-01-18 | Modernatx, Inc. | Norovirus mrna vaccines |
| WO2024044108A1 (en) | 2022-08-22 | 2024-02-29 | The Henry M. Jackson Foundation For The Advancement Of Military Medicine, Inc. | Vaccines against coronaviruses |
| WO2024050483A1 (en) | 2022-08-31 | 2024-03-07 | Modernatx, Inc. | Variant strain-based coronavirus vaccines and uses thereof |
| WO2024084089A1 (en) | 2022-10-21 | 2024-04-25 | BioNTech SE | Methods and uses associated with liquid compositions |
| US11969480B2 (en) | 2020-02-25 | 2024-04-30 | Translate Bio, Inc. | Processes of preparing mRNA-loaded lipid nanoparticles |
| WO2024094881A1 (en) | 2022-11-04 | 2024-05-10 | Sanofi | Respiratory syncytial virus rna vaccination |
| WO2024094876A1 (en) | 2022-11-04 | 2024-05-10 | Sanofi | Methods for messenger rna tailing |
| WO2024102434A1 (en) | 2022-11-10 | 2024-05-16 | Senda Biosciences, Inc. | Rna compositions comprising lipid nanoparticles or lipid reconstructed natural messenger packs |
| WO2024126847A1 (en) | 2022-12-15 | 2024-06-20 | Sanofi | Mrna recombinant capping enzymes |
| WO2024126809A1 (en) | 2022-12-15 | 2024-06-20 | Sanofi | Mrna encoding influenza virus-like particle |
| WO2024133884A2 (en) | 2022-12-23 | 2024-06-27 | Sanofi | Optimized tailing of messenger rna |
| WO2024159172A1 (en) | 2023-01-27 | 2024-08-02 | Senda Biosciences, Inc. | A modified lipid composition and uses thereof |
| US12053551B2 (en) | 2019-12-20 | 2024-08-06 | Translate Bio, Inc. | Process of preparing mRNA-loaded lipid nanoparticles |
| WO2024163465A1 (en) | 2023-01-30 | 2024-08-08 | Modernatx, Inc. | Epstein-barr virus mrna vaccines |
| US12070495B2 (en) | 2019-03-15 | 2024-08-27 | Modernatx, Inc. | HIV RNA vaccines |
| WO2024180262A1 (en) | 2023-03-02 | 2024-09-06 | Sanofi | Compositions for use in treatment of chlamydia |
| WO2024184489A1 (en) | 2023-03-07 | 2024-09-12 | Sanofi | Manufacture of messenger rna with kp34 polymerase |
| US12090235B2 (en) | 2018-09-20 | 2024-09-17 | Modernatx, Inc. | Preparation of lipid nanoparticles and methods of administration thereof |
| WO2024192277A2 (en) | 2023-03-15 | 2024-09-19 | Renagade Therapeutics Management Inc. | Lipid nanoparticles comprising coding rna molecules for use in gene editing and as vaccines and therapeutic agents |
| WO2024192291A1 (en) | 2023-03-15 | 2024-09-19 | Renagade Therapeutics Management Inc. | Delivery of gene editing systems and methods of use thereof |
| WO2024209404A1 (en) * | 2023-04-05 | 2024-10-10 | Seqirus Inc. | Rna delivery vehicle |
| WO2024215721A1 (en) | 2023-04-10 | 2024-10-17 | Modernatx, Inc. | Lyme disease vaccines |
| WO2024220712A2 (en) | 2023-04-19 | 2024-10-24 | Sail Biomedicines, Inc. | Vaccine compositions |
| WO2024231727A1 (en) | 2023-05-05 | 2024-11-14 | Sanofi | Compositions for use in treatment of acne |
| WO2024231565A1 (en) | 2023-05-10 | 2024-11-14 | Sanofi | Combination respiratory mrna vaccines |
| US12151029B2 (en) | 2018-09-19 | 2024-11-26 | Modernatx, Inc. | PEG lipids and uses thereof |
| WO2024254552A1 (en) | 2023-06-08 | 2024-12-12 | Modernatx, Inc. | Stabilized flavivirus vaccines |
| WO2024263826A1 (en) | 2023-06-22 | 2024-12-26 | Modernatx, Inc. | Sars-cov-2 t cell vaccines |
| WO2025003760A1 (en) | 2023-06-28 | 2025-01-02 | Sanofi | Sterol analogs in lipid nanoparticle formulations |
| US12195505B2 (en) | 2018-11-21 | 2025-01-14 | Translate Bio, Inc. | Treatment of cystic fibrosis by delivery of nebulized mRNA encoding CFTR |
| WO2025017151A1 (en) | 2023-07-18 | 2025-01-23 | Sanofi | Stable poly(a)-encoding messenger rna templates |
| WO2025017202A2 (en) | 2023-07-19 | 2025-01-23 | Sanofi | Porphyromonas gingivalis antigenic constructs |
| WO2025019352A2 (en) | 2023-07-14 | 2025-01-23 | Modernatx, Inc. | Mers-cov mrna vaccines |
| WO2025029700A1 (en) | 2023-07-28 | 2025-02-06 | Modernatx, Inc. | Vlp enteroviral vaccines |
| WO2025034612A1 (en) | 2023-08-04 | 2025-02-13 | Modernatx, Inc. | Varicella-zoster virus mrna vaccine |
| WO2025049959A2 (en) | 2023-09-01 | 2025-03-06 | Renagade Therapeutics Management Inc. | Gene editing systems, compositions, and methods for treatment of vexas syndrome |
| WO2025051975A1 (en) | 2023-09-06 | 2025-03-13 | Sanofi | Modified influenza b hemagglutinin polypeptides and nucleic acids and uses thereof |
| WO2025081042A1 (en) | 2023-10-12 | 2025-04-17 | Renagade Therapeutics Management Inc. | Nickase-retron template-based precision editing system and methods of use |
| WO2025128871A2 (en) | 2023-12-13 | 2025-06-19 | Renagade Therapeutics Management Inc. | Lipid nanoparticles comprising coding rna molecules for use in gene editing and as vaccines and therapeutic agents |
| WO2025134071A1 (en) | 2023-12-22 | 2025-06-26 | Sanofi | Malic and glutaric acid based ionizable lipids |
| WO2025141521A1 (en) | 2023-12-29 | 2025-07-03 | Sanofi | Lipids having dendritic moieties |
| US12357580B2 (en) | 2018-06-19 | 2025-07-15 | The Board Of Regents Of The University Of Texas System | Lipid nanoparticle compositions for delivery of mRNA and long nucleic acids |
| WO2025155753A2 (en) | 2024-01-17 | 2025-07-24 | Renagade Therapeutics Management Inc. | Improved gene editing system, guides, and methods |
| US12383508B2 (en) | 2018-09-19 | 2025-08-12 | Modernatx, Inc. | High-purity peg lipids and uses thereof |
| WO2025174765A1 (en) | 2024-02-12 | 2025-08-21 | Renagade Therapeutics Management Inc. | Lipid nanoparticles comprising coding rna molecules for use in gene editing and as vaccines and therapeutic agents |
| WO2025210592A1 (en) | 2024-04-05 | 2025-10-09 | Sanofi Pasteur Inc. | Muscle cell lnp-mrna quality control assays |
| WO2025226656A1 (en) | 2024-04-23 | 2025-10-30 | Modernatx, Inc. | Hepatitis b virus mrna vaccines |
| US12458604B2 (en) | 2020-10-14 | 2025-11-04 | The Trustees Of The University Of Pennsylvania | Methods of lipid nanoparticle manufacture and compositions derived therefrom |
| WO2026008743A1 (en) | 2024-07-02 | 2026-01-08 | Sanofi Pasteur Inc. | Water-soluble polyanionic polymer as adjuvant for carrier-formulated nucleic acid |
Families Citing this family (344)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MX353900B (en) | 2008-11-07 | 2018-02-01 | Massachusetts Inst Technology | Aminoalcohol lipidoids and uses thereof. |
| US9193827B2 (en) | 2010-08-26 | 2015-11-24 | Massachusetts Institute Of Technology | Poly(beta-amino alcohols), their preparation, and uses thereof |
| US9238716B2 (en) | 2011-03-28 | 2016-01-19 | Massachusetts Institute Of Technology | Conjugated lipomers and uses thereof |
| EP3332802A1 (en) | 2011-07-06 | 2018-06-13 | GlaxoSmithKline Biologicals SA | Immunogenic combination compositions and uses thereof |
| CA2853829C (en) | 2011-07-22 | 2023-09-26 | President And Fellows Of Harvard College | Evaluation and improvement of nuclease cleavage specificity |
| US9464124B2 (en) | 2011-09-12 | 2016-10-11 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| DK3682905T3 (en) | 2011-10-03 | 2022-02-28 | Modernatx Inc | MODIFIED NUCLEOSIDES, NUCLEOTIDES AND NUCLEIC ACIDS AND USES THEREOF |
| PE20181541A1 (en) | 2011-10-27 | 2018-09-26 | Massachusetts Inst Technology | DERIVATIVES OF AMINO ACIDS FUNCTIONALIZED IN THE N TERMINAL, CAPABLE OF FORMING DRUG ENCAPSULATING MICROSPHERES |
| CA2868030C (en) | 2012-03-29 | 2021-05-25 | Shire Human Genetic Therapies, Inc. | Lipid-derived neutral nanoparticles |
| CN108949772A (en) | 2012-04-02 | 2018-12-07 | 现代泰克斯公司 | For generating the modification polynucleotides of biological agent relevant to human diseases and protein |
| US10501513B2 (en) | 2012-04-02 | 2019-12-10 | Modernatx, Inc. | Modified polynucleotides for the production of oncology-related proteins and peptides |
| EP2882706A1 (en) | 2012-08-13 | 2015-06-17 | Massachusetts Institute of Technology | Amine-containing lipidoids and uses thereof |
| ES2968649T3 (en) * | 2012-12-07 | 2024-05-13 | Translate Bio Inc | Lipid nanoparticles for mRNA delivery to the lungs |
| HK1219955A1 (en) | 2013-03-14 | 2017-04-21 | Shire Human Genetic Therapies, Inc. | Ribonucleic acids with 4'-thio-modified nucleotides and related methods |
| US8980864B2 (en) | 2013-03-15 | 2015-03-17 | Moderna Therapeutics, Inc. | Compositions and methods of altering cholesterol levels |
| WO2014179562A1 (en) | 2013-05-01 | 2014-11-06 | Massachusetts Institute Of Technology | 1,3,5-triazinane-2,4,6-trione derivatives and uses thereof |
| EP3677567A1 (en) * | 2013-07-23 | 2020-07-08 | Arbutus Biopharma Corporation | Compositions and methods for delivering messenger rna |
| US9163284B2 (en) | 2013-08-09 | 2015-10-20 | President And Fellows Of Harvard College | Methods for identifying a target site of a Cas9 nuclease |
| EP3041938A1 (en) | 2013-09-03 | 2016-07-13 | Moderna Therapeutics, Inc. | Circular polynucleotides |
| JP2016530294A (en) | 2013-09-03 | 2016-09-29 | モデルナ セラピューティクス インコーポレイテッドModerna Therapeutics,Inc. | Chimeric polynucleotide |
| US9340800B2 (en) | 2013-09-06 | 2016-05-17 | President And Fellows Of Harvard College | Extended DNA-sensing GRNAS |
| EP3985118A1 (en) | 2013-11-22 | 2022-04-20 | MiNA Therapeutics Limited | C/ebp alpha short activating rna compositions and methods of use |
| US9840699B2 (en) | 2013-12-12 | 2017-12-12 | President And Fellows Of Harvard College | Methods for nucleic acid editing |
| PT3116900T (en) | 2014-03-09 | 2020-10-08 | Univ Pennsylvania | Compositions useful in treatment of ornithine transcarbamylase (otc) deficiency |
| DK3122878T3 (en) | 2014-03-24 | 2019-02-04 | Translate Bio Inc | MRNA THERAPY FOR TREATMENT OF EYE DISEASES |
| JP6868394B2 (en) | 2014-05-16 | 2021-05-12 | ファイザー・インク | Bispecific antibody |
| EP3521456B1 (en) | 2014-06-10 | 2023-01-04 | CureVac Manufacturing GmbH | Methods and means for enhancing rna production |
| EP3164379A1 (en) | 2014-07-02 | 2017-05-10 | Massachusetts Institute of Technology | Polyamine-fatty acid derived lipidoids and uses thereof |
| CA2955250A1 (en) | 2014-07-16 | 2016-01-21 | Moderna Therapeutics, Inc. | Chimeric polynucleotides |
| WO2016014846A1 (en) | 2014-07-23 | 2016-01-28 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of intrabodies |
| WO2016022363A2 (en) | 2014-07-30 | 2016-02-11 | President And Fellows Of Harvard College | Cas9 proteins including ligand-dependent inteins |
| GB201420139D0 (en) | 2014-11-12 | 2014-12-24 | Ucl Business Plc | Factor IX gene therapy |
| ES2944889T3 (en) | 2014-12-22 | 2023-06-26 | Codexis Inc | Human alpha-galactosidase variants |
| WO2016130600A2 (en) | 2015-02-09 | 2016-08-18 | Duke University | Compositions and methods for epigenome editing |
| US20170151281A1 (en) | 2015-02-19 | 2017-06-01 | Batu Biologics, Inc. | Chimeric antigen receptor dendritic cell (car-dc) for treatment of cancer |
| US10653768B2 (en) | 2015-04-13 | 2020-05-19 | Curevac Real Estate Gmbh | Method for producing RNA compositions |
| WO2016176191A1 (en) | 2015-04-27 | 2016-11-03 | The Trustees Of The University Of Pennsylvania | Dual aav vector system for crispr/cas9 mediated correction of human disease |
| CA2990172A1 (en) | 2015-06-19 | 2016-12-22 | Massachusetts Institute Of Technology | Alkenyl substituted 2,5-piperazinediones and their use in compositions for delivering an agent to a subject or cell |
| WO2017015637A1 (en) | 2015-07-22 | 2017-01-26 | Duke University | High-throughput screening of regulatory element function with epigenome editing technologies |
| JP6905755B2 (en) | 2015-08-25 | 2021-07-21 | デューク ユニバーシティ | Compositions and Methods to Improve Specificity in Genomic Engineering Using RNA-Guided Endonucleases |
| ES2969082T3 (en) | 2015-09-17 | 2024-05-16 | Modernatx Inc | Compounds and compositions for intracellular administration of therapeutic agents |
| WO2017066497A2 (en) | 2015-10-13 | 2017-04-20 | Duke University | Genome engineering with type i crispr systems in eukaryotic cells |
| KR20180096591A (en) * | 2015-10-22 | 2018-08-29 | 모더나티엑스, 인크. | A broad-spectrum influenza virus vaccine |
| IL294014B2 (en) | 2015-10-23 | 2024-07-01 | Harvard College | Nucleobase editors and their uses |
| US10799463B2 (en) | 2015-12-22 | 2020-10-13 | Modernatx, Inc. | Compounds and compositions for intracellular delivery of agents |
| PT3394093T (en) | 2015-12-23 | 2022-05-30 | Modernatx Inc | Methods of using ox40 ligand encoding polynucleotides |
| US20190241658A1 (en) | 2016-01-10 | 2019-08-08 | Modernatx, Inc. | Therapeutic mRNAs encoding anti CTLA-4 antibodies |
| RU2759737C2 (en) | 2016-03-31 | 2021-11-17 | Этрис Гмбх | New minimum utr sequences |
| WO2017180915A2 (en) | 2016-04-13 | 2017-10-19 | Duke University | Crispr/cas9-based repressors for silencing gene targets in vivo and methods of use |
| US20180126003A1 (en) * | 2016-05-04 | 2018-05-10 | Curevac Ag | New targets for rna therapeutics |
| EP3458108A4 (en) | 2016-05-18 | 2020-04-22 | ModernaTX, Inc. | POLYNUCLEOTIDES FOR CODING THE TRANSMEMBRANE CONDUCTIVE REGULATOR OF CYSTIC FIBROSE FOR TREATING CYSTIC FIBROSE |
| KR20230074598A (en) | 2016-05-18 | 2023-05-30 | 모더나티엑스, 인크. | Polynucleotides encoding relaxin |
| AU2017286980B2 (en) | 2016-06-30 | 2023-10-26 | Arbutus Biopharma Corporation | Compositions and methods for delivering messenger RNA |
| WO2018017754A1 (en) | 2016-07-19 | 2018-01-25 | Duke University | Therapeutic applications of cpf1-based genome editing |
| AU2017306676B2 (en) | 2016-08-03 | 2024-02-22 | President And Fellows Of Harvard College | Adenosine nucleobase editors and uses thereof |
| EP3497214B1 (en) | 2016-08-09 | 2023-06-28 | President and Fellows of Harvard College | Programmable cas9-recombinase fusion proteins and uses thereof |
| US10907156B2 (en) | 2016-08-19 | 2021-02-02 | Fundación Para La Investigación Biomédica Del Hospital Univ. Ramón Y Cajal | MiR-127 agents for use in the treatment of renal fibrosis |
| US11542509B2 (en) | 2016-08-24 | 2023-01-03 | President And Fellows Of Harvard College | Incorporation of unnatural amino acids into proteins using base editing |
| WO2018071868A1 (en) | 2016-10-14 | 2018-04-19 | President And Fellows Of Harvard College | Aav delivery of nucleobase editors |
| WO2018089540A1 (en) | 2016-11-08 | 2018-05-17 | Modernatx, Inc. | Stabilized formulations of lipid nanoparticles |
| EP3538136A1 (en) | 2016-11-10 | 2019-09-18 | Translate Bio, Inc. | Subcutaneous delivery of messenger rna |
| IL310050A (en) * | 2016-11-10 | 2024-03-01 | Translate Bio Inc | Improved ice-based lipid nanoparticle formulation for delivery of mrna |
| WO2018089851A2 (en) | 2016-11-11 | 2018-05-17 | Modernatx, Inc. | Influenza vaccine |
| EP3973955B1 (en) * | 2016-11-23 | 2025-05-21 | Mayo Foundation for Medical Education and Research | Particle-mediated delivery of inhibitory rna |
| WO2018119359A1 (en) | 2016-12-23 | 2018-06-28 | President And Fellows Of Harvard College | Editing of ccr5 receptor gene to protect against hiv infection |
| US11104887B2 (en) | 2017-01-03 | 2021-08-31 | Ethris Gmbh | Ornithine transcarbamylase coding polyribonucleotides and formulations thereof |
| AU2018224843B2 (en) | 2017-02-27 | 2024-08-15 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| MA47607A (en) * | 2017-02-27 | 2020-01-01 | Translate Bio Inc | LARGE-SCALE SYNTHESIS OF ARN MESSENGER |
| DK3585892T3 (en) * | 2017-02-27 | 2022-08-22 | Translate Bio Inc | METHODS FOR PURIFICATION OF MESSENGER RNA |
| JOP20190200A1 (en) | 2017-02-28 | 2019-08-27 | Univ Pennsylvania | Compositions useful in treatment of spinal muscular atrophy |
| SMT202400080T1 (en) | 2017-02-28 | 2024-05-14 | Univ Pennsylvania | Adeno-associated virus (aav) clade f vector and uses therefor |
| AU2018228881B2 (en) | 2017-03-01 | 2024-01-25 | The Trustees Of The University Of Pennsylvania | Gene therapy for ocular disorders |
| EP3592728A1 (en) | 2017-03-07 | 2020-01-15 | Translate Bio, Inc. | Polyanionic delivery of nucleic acids |
| EP3592853A1 (en) | 2017-03-09 | 2020-01-15 | President and Fellows of Harvard College | Suppression of pain by gene editing |
| WO2018165631A1 (en) | 2017-03-09 | 2018-09-13 | President And Fellows Of Harvard College | Cancer vaccine |
| WO2018165629A1 (en) | 2017-03-10 | 2018-09-13 | President And Fellows Of Harvard College | Cytosine to guanine base editor |
| EP3609534A4 (en) | 2017-03-15 | 2021-01-13 | ModernaTX, Inc. | BROAD SPECTRUM VACCINE AGAINST THE INFLUENZA VIRUS |
| AU2018234814B2 (en) | 2017-03-15 | 2022-06-30 | Modernatx, Inc. | Crystal forms of amino lipids |
| EP3595713A4 (en) | 2017-03-15 | 2021-01-13 | ModernaTX, Inc. | RESPIRATORY SYNCYTIAL VIRUS VACCINE |
| ES3020935T3 (en) | 2017-03-15 | 2025-05-23 | Modernatx Inc | Lipid nanoparticle formulation |
| ES2940259T3 (en) | 2017-03-15 | 2023-05-04 | Modernatx Inc | Compound and compositions for intracellular delivery of therapeutic agents |
| KR20240116572A (en) | 2017-03-23 | 2024-07-29 | 프레지던트 앤드 펠로우즈 오브 하바드 칼리지 | Nucleobase editors comprising nucleic acid programmable dna binding proteins |
| US11905525B2 (en) | 2017-04-05 | 2024-02-20 | Modernatx, Inc. | Reduction of elimination of immune responses to non-intravenous, e.g., subcutaneously administered therapeutic proteins |
| AU2018251187B2 (en) | 2017-04-14 | 2024-03-28 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for transient gene therapy with enhanced stability |
| WO2018200542A1 (en) | 2017-04-24 | 2018-11-01 | The Trustees Of The University Of Pennsylvania | Gene therapy for ocular disorders |
| WO2018209205A1 (en) | 2017-05-11 | 2018-11-15 | The Trustees Of The University Of Pennsylvania | Gene therapy for neuronal ceroid lipofuscinoses |
| US11560566B2 (en) | 2017-05-12 | 2023-01-24 | President And Fellows Of Harvard College | Aptazyme-embedded guide RNAs for use with CRISPR-Cas9 in genome editing and transcriptional activation |
| EP4253544A3 (en) | 2017-05-18 | 2023-12-20 | ModernaTX, Inc. | Modified messenger rna comprising functional rna elements |
| JP7285220B2 (en) | 2017-05-18 | 2023-06-01 | モデルナティエックス インコーポレイテッド | Lipid nanoparticles comprising linked interleukin-12 (IL12) polypeptide-encoding polynucleotides |
| KR102636537B1 (en) * | 2017-05-31 | 2024-02-15 | 울트라제닉스 파마수티컬 인코포레이티드 | Treatment for Glycogen Storage Disease Type III |
| WO2018218359A1 (en) | 2017-05-31 | 2018-12-06 | The Trustees Of The University Of Pennsylvania | Gene therapy for treating peroxisomal disorders |
| EP3638678B1 (en) | 2017-06-14 | 2025-12-03 | ModernaTX, Inc. | Compounds and compositions for intracellular delivery of agents |
| MA49395A (en) | 2017-06-14 | 2020-04-22 | Modernatx Inc | POLYNUCLEOTIDES COAGULATION FACTOR VIII CODING |
| US10034951B1 (en) | 2017-06-21 | 2018-07-31 | New England Biolabs, Inc. | Use of thermostable RNA polymerases to produce RNAs having reduced immunogenicity |
| CN111801345A (en) | 2017-07-28 | 2020-10-20 | 哈佛大学的校长及成员们 | Methods and compositions for evolutionary base editors using phage-assisted sequential evolution (PACE) |
| US11319532B2 (en) | 2017-08-30 | 2022-05-03 | President And Fellows Of Harvard College | High efficiency base editors comprising Gam |
| WO2019048645A1 (en) | 2017-09-08 | 2019-03-14 | Mina Therapeutics Limited | Stabilized cebpa sarna compositions and methods of use |
| US11162099B2 (en) | 2017-09-08 | 2021-11-02 | Mina Therapeutics Limited | HNF4A saRNA compositions and methods of use |
| WO2019079347A1 (en) | 2017-10-16 | 2019-04-25 | The Broad Institute, Inc. | Uses of adenosine base editors |
| EP3714047A2 (en) | 2017-11-22 | 2020-09-30 | ModernaTX, Inc. | Polynucleotides encoding phenylalanine hydroxylase for the treatment of phenylketonuria |
| JP7424976B2 (en) | 2017-11-22 | 2024-01-30 | モダーナティエックス・インコーポレイテッド | Polynucleotide encoding propionyl-CoA carboxylase alpha and beta subunits for the treatment of propionic acidemia |
| MA50803A (en) | 2017-11-22 | 2020-09-30 | Modernatx Inc | POLYNUCLEOTIDES CODING ORNITHINE TRANSCARBAMYLASE FOR THE TREATMENT OF UREA CYCLE DISORDERS |
| EP3717652A4 (en) | 2017-11-30 | 2021-11-24 | The Trustees of the University of Pennsylvania | GENE THERAPY FOR MUCOPOLY SACCHARIDOSIS IIIB |
| BR112020010735A2 (en) | 2017-11-30 | 2020-11-10 | The Trustees Of The University Of Pennsylvania | gene therapy for mucopolysaccharidosis iii a |
| US12406749B2 (en) | 2017-12-15 | 2025-09-02 | The Broad Institute, Inc. | Systems and methods for predicting repair outcomes in genetic engineering |
| MA51523A (en) | 2018-01-05 | 2020-11-11 | Modernatx Inc | POLYNUCLEOTIDES CODING ANTI-BODY ANTI-CHIKUNGUNYA VIRUS |
| CN111971066B (en) * | 2018-01-18 | 2025-03-11 | 伊泽阿恩埃免疫疗法股份有限公司 | Lipid Nanoparticles |
| WO2019152802A1 (en) | 2018-02-02 | 2019-08-08 | Translate Bio, Inc. | Cationic polymers |
| EP3773745A1 (en) | 2018-04-11 | 2021-02-17 | ModernaTX, Inc. | Messenger rna comprising functional rna elements |
| WO2019197845A1 (en) | 2018-04-12 | 2019-10-17 | Mina Therapeutics Limited | Sirt1-sarna compositions and methods of use |
| US11603543B2 (en) * | 2018-04-18 | 2023-03-14 | Oisin Biotechnologies, Inc. | Fusogenic lipid nanoparticles for target cell-specific production of a therapeutic protein |
| JP7448488B2 (en) * | 2018-05-15 | 2024-03-12 | トランスレイト バイオ, インコーポレイテッド | Subcutaneous delivery of messenger RNA |
| JP7384832B2 (en) | 2018-05-16 | 2023-11-21 | トランスレイト バイオ, インコーポレイテッド | Ribose cationic lipid |
| WO2019226650A1 (en) | 2018-05-23 | 2019-11-28 | Modernatx, Inc. | Delivery of dna |
| EP3797160A1 (en) | 2018-05-23 | 2021-03-31 | The Broad Institute Inc. | Base editors and uses thereof |
| KR20210056953A (en) * | 2018-05-30 | 2021-05-20 | 트랜슬레이트 바이오 인코포레이티드 | Messenger RNA vaccine and uses thereof |
| WO2020014261A1 (en) | 2018-07-09 | 2020-01-16 | The Broad Institute, Inc. | Rna programmable epigenetic rna modifiers and uses thereof |
| US20220184185A1 (en) | 2018-07-25 | 2022-06-16 | Modernatx, Inc. | Mrna based enzyme replacement therapy combined with a pharmacological chaperone for the treatment of lysosomal storage disorders |
| US10842885B2 (en) | 2018-08-20 | 2020-11-24 | Ucl Business Ltd | Factor IX encoding nucleotides |
| GB201813528D0 (en) | 2018-08-20 | 2018-10-03 | Ucl Business Plc | Factor IX encoding nucleotides |
| MX2021001980A (en) * | 2018-08-24 | 2021-05-27 | Flagship Pioneering Innovations Vi Llc | MODIFIED VEGETABLE MESSENGER PACKAGES AND THEIR USES. |
| MA53545A (en) | 2018-09-02 | 2021-07-14 | Modernatx Inc | POLYNUCLEOTIDES ENCODED FOR VERY LONG CHAIN ACYL-COA DEHYDROGENASE FOR THE TREATMENT OF VERY LONG CHAIN ACYL-COA DEHYDROGENASE DEFICIENCY |
| US20230009009A1 (en) | 2018-09-13 | 2023-01-12 | Modernatx, Inc. | Polynucleotides encoding glucose-6-phosphatase for the treatment of glycogen storage disease |
| WO2020056155A2 (en) | 2018-09-13 | 2020-03-19 | Modernatx, Inc. | Polynucleotides encoding branched-chain alpha-ketoacid dehydrogenase complex e1-alpha, e1-beta, and e2 subunits for the treatment of maple syrup urine disease |
| EP3850102A1 (en) | 2018-09-14 | 2021-07-21 | ModernaTX, Inc. | Polynucleotides encoding uridine diphosphate glycosyltransferase 1 family, polypeptide a1 for the treatment of crigler-najjar syndrome |
| JP7410135B2 (en) | 2018-09-19 | 2024-01-09 | モデルナティエックス インコーポレイテッド | Compounds and compositions for intracellular delivery of therapeutic agents |
| US20220152225A1 (en) | 2018-09-27 | 2022-05-19 | Modernatx, Inc. | Polynucleotides encoding arginase 1 for the treatment of arginase deficiency |
| SG11202102164PA (en) | 2018-09-28 | 2021-04-29 | Nutcracker Therapeutics Inc | Tertiary amino lipidated cationic peptides for nucleic acid delivery |
| WO2020072914A1 (en) | 2018-10-04 | 2020-04-09 | New England Biolabs, Inc. | Methods and compositions for increasing capping efficiency of transcribed rna |
| US11072808B2 (en) | 2018-10-04 | 2021-07-27 | New England Biolabs, Inc. | Methods and compositions for increasing capping efficiency of transcribed RNA |
| EA202190688A1 (en) * | 2018-10-09 | 2021-06-07 | Дзе Юниверсити Оф Бритиш Коламбиа | COMPOSITIONS AND SYSTEMS CONTAINING TRANSFECTIONALLY COMPETENT VESICULES, NOT CONTAINING ORGANIC SOLVENTS AND DETERGENTS, AND RELATED METHODS |
| WO2020092453A1 (en) | 2018-10-29 | 2020-05-07 | The Broad Institute, Inc. | Nucleobase editors comprising geocas9 and uses thereof |
| KR20210089648A (en) | 2018-11-08 | 2021-07-16 | 트랜슬레이트 바이오 인코포레이티드 | Methods and compositions for messenger RNA purification |
| WO2020097409A2 (en) | 2018-11-08 | 2020-05-14 | Modernatx, Inc. | Use of mrna encoding ox40l to treat cancer in human patients |
| WO2020102172A2 (en) | 2018-11-12 | 2020-05-22 | Translate Bio, Inc. | Methods for inducing immune tolerance |
| WO2020118115A1 (en) | 2018-12-06 | 2020-06-11 | Arcturus Therapeutics, Inc. | Compositions and methods for treating ornithine transcarbamylase deficiency |
| WO2020132252A2 (en) | 2018-12-20 | 2020-06-25 | Codexis, Inc. | Human alpha-galactosidase variants |
| BR112021009422A2 (en) | 2018-12-21 | 2021-10-26 | Curevac Ag | RNA FOR VACCINES AGAINST MALARIA |
| WO2020154500A1 (en) | 2019-01-23 | 2020-07-30 | The Broad Institute, Inc. | Supernegatively charged proteins and uses thereof |
| EP4491229A3 (en) | 2019-02-08 | 2025-05-14 | CureVac SE | Coding rna administered into the suprachoroidal space in the treatment of ophtalmic diseases |
| BR112021018607A2 (en) | 2019-03-19 | 2021-11-23 | Massachusetts Inst Technology | Methods and compositions for editing nucleotide sequences |
| WO2020208361A1 (en) | 2019-04-12 | 2020-10-15 | Mina Therapeutics Limited | Sirt1-sarna compositions and methods of use |
| EP3956349A1 (en) | 2019-04-17 | 2022-02-23 | The Broad Institute, Inc. | Adenine base editors with reduced off-target effects |
| EP3965797A1 (en) | 2019-05-08 | 2022-03-16 | AstraZeneca AB | Compositions for skin and wounds and methods of use thereof |
| AU2020274758B2 (en) | 2019-05-14 | 2025-05-01 | Translate Bio, Inc. | Improved process of preparing mRNA-loaded lipid nanoparticles |
| IL310266B2 (en) | 2019-05-22 | 2025-11-01 | Massachusetts Inst Technology | Circular RNA Preparations and Methods |
| EP3986452A1 (en) | 2019-06-18 | 2022-04-27 | CureVac AG | Rotavirus mrna vaccine |
| JP2022537052A (en) * | 2019-06-21 | 2022-08-23 | カーナル バイオロジクス, インコーポレイテッド | Engineered tumor-selective protein expression |
| MA56539A (en) | 2019-06-24 | 2022-04-27 | Modernatx Inc | ENDONUCLEASE RESISTANT MESSENGER RNA AND USES THEREOF |
| JP7589998B2 (en) * | 2019-06-24 | 2024-11-26 | 国立大学法人北海道大学 | Lipid Nanoparticles |
| US20220387628A1 (en) | 2019-06-24 | 2022-12-08 | Modernatx, Inc. | Messenger rna comprising functional rna elements and uses thereof |
| EP3999097B1 (en) | 2019-07-18 | 2024-05-29 | Institut National de la Santé et de la Recherche Médicale (INSERM) | Rac2 mutant for use in ablating hematopoiesis and in the treatment of hematopoietic malignancies |
| US20220287969A1 (en) * | 2019-07-19 | 2022-09-15 | University Of Florida Research Foundation, Inc. | Multilamellar rna nanoparticles |
| IL290078B2 (en) | 2019-07-30 | 2025-09-01 | Translate Bio Inc | Treatment of cystic fibrosis by delivery of nebulized mrna encoding cftr |
| AU2020328855A1 (en) | 2019-08-14 | 2022-03-03 | CureVac SE | RNA combinations and compositions with decreased immunostimulatory properties |
| LT4013385T (en) | 2019-08-14 | 2024-09-25 | Acuitas Therapeutics, Inc. | Improved lipid nanoparticles for delivery of nucleic acids |
| CA3154618A1 (en) | 2019-09-19 | 2021-03-25 | Modernatx, Inc. | Branched tail lipid compounds and compositions for intracellular delivery of therapeutic agents |
| WO2021072172A1 (en) | 2019-10-09 | 2021-04-15 | Translate Bio, Inc. | Compositions, methods and uses of messenger rna |
| US12435330B2 (en) | 2019-10-10 | 2025-10-07 | The Broad Institute, Inc. | Methods and compositions for prime editing RNA |
| US12378560B2 (en) | 2019-10-29 | 2025-08-05 | Northwestern University | Sequence multiplicity within spherical nucleic acids |
| ES2961245T3 (en) | 2019-12-04 | 2024-03-11 | Orna Therapeutics Inc | Circular RNA compositions and methods |
| US10980836B1 (en) | 2019-12-11 | 2021-04-20 | Myeloid Therapeutics, Inc. | Therapeutic cell compositions and methods of manufacturing and use thereof |
| IL294073A (en) * | 2019-12-20 | 2022-08-01 | Translate Bio Inc | Rectal delivery of messenger rna |
| JP2023519078A (en) * | 2020-01-10 | 2023-05-10 | アンドラニック アンドリュー アプリキャン, | Nanoparticles for modulation of expression of genes of interest and/or signaling pathways |
| MX2022009460A (en) | 2020-02-04 | 2022-12-16 | Curevac Ag | Coronavirus vaccine. |
| EP4114470A4 (en) | 2020-03-03 | 2024-04-17 | Arcturus Therapeutics, Inc. | COMPOSITIONS AND METHODS FOR TREATING ORNITHINE TRANSCARBAMYLASE DEFICIENCY |
| AU2021261471B2 (en) | 2020-04-22 | 2024-10-24 | BioNTech SE | Coronavirus vaccine |
| AU2021267940A1 (en) | 2020-05-08 | 2022-12-08 | President And Fellows Of Harvard College | Methods and compositions for simultaneous editing of both strands of a target double-stranded nucleotide sequence |
| IL298138A (en) | 2020-05-12 | 2023-01-01 | Univ Pennsylvania | Compositions for drg-specific reduction of transgene expression |
| EP4149556B1 (en) * | 2020-05-14 | 2026-01-07 | Translate Bio, Inc. | Peg lipidoid compounds |
| MX2022014660A (en) | 2020-05-19 | 2023-02-16 | Orna Therapeutics Inc | COMPOSITIONS AND METHODS OF CIRCULAR RNA. |
| MX2022015132A (en) | 2020-05-29 | 2023-03-08 | CureVac SE | Nucleic acid based combination vaccines. |
| AU2021285812A1 (en) | 2020-06-01 | 2023-01-05 | Modernatx, Inc. | Phenylalanine hydroxylase variants and uses thereof |
| CN116133668A (en) | 2020-06-12 | 2023-05-16 | 罗切斯特大学 | Coding and expression of ACE-tRNA |
| AU2021292200A1 (en) | 2020-06-17 | 2023-02-02 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treatment of gene therapy patients |
| WO2025056938A1 (en) | 2023-09-11 | 2025-03-20 | BioNTech SE | Rna compositions for delivery of incretin agents |
| CN116438311A (en) | 2020-07-13 | 2023-07-14 | 宾夕法尼亚州大学信托人 | Compositions useful in the treatment of Charcot-Marie-Tooth disease |
| US20230272052A1 (en) | 2020-07-31 | 2023-08-31 | CureVac SE | Nucleic acid encoded antibody mixtures |
| CA3170743A1 (en) | 2020-08-31 | 2022-03-03 | Susanne RAUCH | Multivalent nucleic acid based coronavirus vaccines |
| AU2021358781A1 (en) | 2020-10-07 | 2023-05-18 | Tern Therapeutics, Llc | Gene therapy for ocular manifestations of cln2 disease |
| US20230365955A1 (en) | 2020-10-09 | 2023-11-16 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treatment of fabry disease |
| MX2023005201A (en) * | 2020-11-04 | 2023-06-28 | Myeloid Therapeutics Inc | Engineered chimeric fusion protein compositions and methods of use thereof. |
| WO2022104131A1 (en) | 2020-11-13 | 2022-05-19 | Modernatx, Inc. | Polynucleotides encoding cystic fibrosis transmembrane conductance regulator for the treatment of cystic fibrosis |
| IL303236A (en) | 2020-12-01 | 2023-07-01 | Univ Pennsylvania | New compounds with specific tissue-targeting motifs and preparations containing them |
| AU2021392642A1 (en) | 2020-12-01 | 2023-06-22 | The Trustees Of The University Of Pennsylvania | Compositions and uses thereof for treatment of angelman syndrome |
| US20240050378A1 (en) * | 2020-12-02 | 2024-02-15 | Merck Sharp & Dohme Llc | Lipid nanoparticle compositions containing monoester cationic lipids |
| GB2603454A (en) | 2020-12-09 | 2022-08-10 | Ucl Business Ltd | Novel therapeutics for the treatment of neurodegenerative disorders |
| WO2022137133A1 (en) | 2020-12-22 | 2022-06-30 | Curevac Ag | Rna vaccine against sars-cov-2 variants |
| WO2022135993A2 (en) | 2020-12-22 | 2022-06-30 | Curevac Ag | Pharmaceutical composition comprising lipid-based carriers encapsulating rna for multidose administration |
| WO2022155404A1 (en) * | 2021-01-14 | 2022-07-21 | Translate Bio, Inc. | Methods and compositions for delivering mrna coded antibodies |
| WO2022162027A2 (en) | 2021-01-27 | 2022-08-04 | Curevac Ag | Method of reducing the immunostimulatory properties of in vitro transcribed rna |
| US20240091380A1 (en) | 2021-02-01 | 2024-03-21 | Regenxbio Inc. | Gene therapy for neuronal ceroid lipofuscinoses |
| GB2623191A (en) | 2021-03-17 | 2024-04-10 | Myeloid Therapeutics Inc | Engineered chimeric fusion protein compositions and methods of use thereof |
| KR20240005692A (en) | 2021-03-22 | 2024-01-12 | 리코드 테라퓨틱스, 인크. | Compositions and methods for targeted delivery to cells |
| EP4313001A4 (en) * | 2021-03-22 | 2025-11-26 | Univ Texas | COMPOSITIONS AND METHOD FOR TARGETED DELIVERY TO CELLS |
| EP4314290A4 (en) | 2021-03-23 | 2025-01-15 | Recode Therapeutics, Inc. | Polynucleotide compositions, related formulations, and methods of use thereof |
| WO2022204371A1 (en) | 2021-03-24 | 2022-09-29 | Modernatx, Inc. | Lipid nanoparticles containing polynucleotides encoding glucose-6-phosphatase and uses thereof |
| US20240207444A1 (en) | 2021-03-24 | 2024-06-27 | Modernatx, Inc. | Lipid nanoparticles containing polynucleotides encoding phenylalanine hydroxylase and uses thereof |
| WO2022204369A1 (en) | 2021-03-24 | 2022-09-29 | Modernatx, Inc. | Polynucleotides encoding methylmalonyl-coa mutase for the treatment of methylmalonic acidemia |
| EP4314260A1 (en) | 2021-03-24 | 2024-02-07 | Modernatx, Inc. | Lipid nanoparticles and polynucleotides encoding ornithine transcarbamylase for the treatment of ornithine transcarbamylase deficiency |
| US20240216288A1 (en) | 2021-03-24 | 2024-07-04 | Modernatx, Inc. | Lipid nanoparticles containing polynucleotides encoding propionyl-coa carboxylase alpha and beta subunits and uses thereof |
| EP4314292A1 (en) | 2021-03-26 | 2024-02-07 | MiNA Therapeutics Limited | Tmem173 sarna compositions and methods of use |
| US20240181038A1 (en) | 2021-03-26 | 2024-06-06 | Glaxosmithkline Biologicals Sa | Immunogenic compositions |
| US20250345524A1 (en) | 2021-03-31 | 2025-11-13 | CureVac SE | Syringes containing pharmaceutical compositions comprising rna |
| IL307633A (en) | 2021-04-12 | 2023-12-01 | Univ Pennsylvania | Compositions useful for treating spinal and bulbar muscular atrophy (sbma) |
| CA3216004A1 (en) | 2021-04-23 | 2022-10-27 | The Trustees Of The University Of Pennsylvania | Novel compositions with brain-specific targeting motifs and compositions containing same |
| CA3171589A1 (en) | 2021-05-03 | 2022-11-03 | Moritz THRAN | Improved nucleic acid sequence for cell type specific expression |
| JP2024518100A (en) | 2021-05-11 | 2024-04-24 | マイエロイド・セラピューティクス,インコーポレーテッド | Methods and compositions for genomic integration |
| US20240384277A1 (en) | 2021-06-15 | 2024-11-21 | Modernatx, Inc. | Engineered polynucleotides for cell-type or microenvironment-specific expression |
| WO2022271776A1 (en) | 2021-06-22 | 2022-12-29 | Modernatx, Inc. | Polynucleotides encoding uridine diphosphate glycosyltransferase 1 family, polypeptide a1 for the treatment of crigler-najjar syndrome |
| WO2023002509A1 (en) | 2021-07-23 | 2023-01-26 | Institute For Stem Cell Science And Regenerative Medicine | Lipid formulation for delivery of therapeutic agents |
| JP7776176B2 (en) | 2021-08-27 | 2025-11-26 | 北京大学 | Constructs and methods for preparing circular RNA |
| CN117940158A (en) | 2021-09-03 | 2024-04-26 | 库瑞瓦格欧洲公司 | Novel lipid nanoparticles comprising phosphatidylserine for nucleic acid delivery |
| AU2022336209B2 (en) | 2021-09-03 | 2025-10-16 | CureVac SE | Novel lipid nanoparticles for delivery of nucleic acids |
| JP2024534915A (en) | 2021-09-03 | 2024-09-26 | グラクソスミスクライン バイオロジカルズ ソシエテ アノニム | Nucleotide base substitutions in self-amplifying messenger ribonucleic acid. |
| US20250017867A1 (en) | 2021-10-01 | 2025-01-16 | Modernatx, Inc. | Polynucleotides encoding relaxin for the treatment of fibrosis and/or cardiovascular disease |
| EP4415690A4 (en) * | 2021-10-14 | 2025-12-17 | Kernal Biologics Inc | Compositions and methods for delivery of agents |
| EP4422698A1 (en) | 2021-10-29 | 2024-09-04 | CureVac SE | Improved circular rna for expressing therapeutic proteins |
| AU2022375820A1 (en) | 2021-11-01 | 2024-06-13 | Tome Biosciences, Inc. | Single construct platform for simultaneous delivery of gene editing machinery and nucleic acid cargo |
| KR20240107139A (en) | 2021-11-08 | 2024-07-08 | 오나 테라퓨틱스, 인코포레이티드 | Lipid nanoparticle compositions for delivering circular polynucleotides |
| WO2023087019A2 (en) | 2021-11-15 | 2023-05-19 | The Trustees Of The University Of Pennsylvania | Compositions for drg-specific reduction of transgene expression |
| US20250235405A1 (en) | 2021-11-19 | 2025-07-24 | Branca Bunus Limited | A composition comprising a therapeutically active agent packaged within a drug delivery vehicle |
| US12186387B2 (en) | 2021-11-29 | 2025-01-07 | BioNTech SE | Coronavirus vaccine |
| WO2023099884A1 (en) | 2021-12-01 | 2023-06-08 | Mina Therapeutics Limited | Pax6 sarna compositions and methods of use |
| WO2023102517A1 (en) | 2021-12-02 | 2023-06-08 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treatment of fabry disease |
| GB202117758D0 (en) | 2021-12-09 | 2022-01-26 | Ucl Business Ltd | Therapeutics for the treatment of neurodegenerative disorders |
| AU2022420615A1 (en) | 2021-12-22 | 2024-07-04 | Tome Biosciences, Inc. | Co-delivery of a gene editor construct and a donor template |
| KR20240126870A (en) | 2021-12-22 | 2024-08-21 | 캠프4 테라퓨틱스 코포레이션 | Modulation of gene transcription using antisense oligonucleotides targeting regulatory RNAs |
| TW202340467A (en) | 2022-01-10 | 2023-10-16 | 賓州大學委員會 | Compositions and methods useful for treatment of c9orf72-mediated disorders |
| US20250084389A1 (en) | 2022-01-17 | 2025-03-13 | Institut National de la Santé et de la Recherche Médicale | Methods of inducing cell death of a population of solid tumor cells |
| EP4466029A1 (en) | 2022-01-21 | 2024-11-27 | Orna Therapeutics, Inc. | Systemic administration of circular rna polynucleotides encoding muscle proteins or protein complexes |
| US20250345416A1 (en) | 2022-01-27 | 2025-11-13 | BioNTech SE | Pharmaceutical compositions for delivery of herpes simplex virus antigens and related methods |
| US20250099614A1 (en) | 2022-01-28 | 2025-03-27 | CureVac SE | Nucleic acid encoded transcription factor inhibitors |
| US20250134919A1 (en) | 2022-02-07 | 2025-05-01 | University Of Rochester | OPTIMIZED SEQUENCES FOR ENHANCED tRNA EXPRESSION OR/AND NONSENSE MUTATION SUPPRESSION |
| JP2025508467A (en) | 2022-02-24 | 2025-03-26 | アイオー バイオテック エーピーエス | Nucleotide Delivery for Cancer Therapy |
| WO2023170435A1 (en) | 2022-03-07 | 2023-09-14 | Mina Therapeutics Limited | Il10 sarna compositions and methods of use |
| WO2023177904A1 (en) | 2022-03-18 | 2023-09-21 | Modernatx, Inc. | Sterile filtration of lipid nanoparticles and filtration analysis thereof for biological applications |
| WO2023178425A1 (en) | 2022-03-23 | 2023-09-28 | Nanovation Therapeutics Inc. | High sterol-containing lipid nanoparticles |
| WO2023183909A2 (en) | 2022-03-25 | 2023-09-28 | Modernatx, Inc. | Polynucleotides encoding fanconi anemia, complementation group proteins for the treatment of fanconi anemia |
| KR20240167063A (en) | 2022-04-01 | 2024-11-26 | 나노베이션 테라퓨틱스 인크. | mRNA delivery method and composition thereof |
| EP4507719A1 (en) | 2022-04-13 | 2025-02-19 | Universitat Autònoma de Barcelona | Treatment of neuromuscular diseases via gene therapy that expresses klotho protein |
| WO2023205744A1 (en) | 2022-04-20 | 2023-10-26 | Tome Biosciences, Inc. | Programmable gene insertion compositions |
| WO2023215831A1 (en) | 2022-05-04 | 2023-11-09 | Tome Biosciences, Inc. | Guide rna compositions for programmable gene insertion |
| WO2023225670A2 (en) | 2022-05-20 | 2023-11-23 | Tome Biosciences, Inc. | Ex vivo programmable gene insertion |
| CA3248209A1 (en) | 2022-05-25 | 2023-11-30 | CureVac SE | Nucleic acid based vaccine encoding an escherichia coli fimh antigenic polypeptide |
| US20250320519A1 (en) | 2022-05-30 | 2025-10-16 | Shanghai Circode Biomed Co., Ltd. | Synthetic circular rna compositions and methods of use thereof |
| KR20250022763A (en) | 2022-06-10 | 2025-02-17 | 캠프4 테라퓨틱스 코포레이션 | Method for modulating progranulin expression using antisense oligonucleotides targeting regulatory RNA |
| CN119604304A (en) | 2022-06-18 | 2025-03-11 | 葛兰素史克生物有限公司 | Recombinant RNA molecules comprising an untranslated region or a segment encoding a spike protein from severe acute respiratory coronavirus 2 Omicron strain |
| US12297285B2 (en) | 2022-06-24 | 2025-05-13 | Orna Therapeutics, Inc. | Circular RNA encoding chimeric antigen receptors targeting BCMA |
| IL317874A (en) | 2022-06-24 | 2025-02-01 | Tune Therapeutics Inc | Compositions, systems, and methods for reducing low-density lipoprotein through targeted gene repression |
| WO2024002985A1 (en) | 2022-06-26 | 2024-01-04 | BioNTech SE | Coronavirus vaccine |
| EP4560021A1 (en) * | 2022-07-19 | 2025-05-28 | Shenzhen Shenxin Biotechnology Co., Ltd. | Mrna for sars-cov-2 s protein and use thereof |
| WO2024020587A2 (en) | 2022-07-22 | 2024-01-25 | Tome Biosciences, Inc. | Pleiopluripotent stem cell programmable gene insertion |
| MA71619A (en) | 2022-07-26 | 2025-05-30 | Modernatx, Inc. | MODIFIED POLYNUCLEOTIDES FOR TEMPORAL REGULATION OF EXPRESSION |
| WO2024044147A1 (en) | 2022-08-23 | 2024-02-29 | Modernatx, Inc. | Methods for purification of ionizable lipids |
| AU2023274159A1 (en) * | 2022-09-07 | 2024-03-21 | Eyegene Inc. | COMPOSITION FOR IN-VIVO DELIVERING mRNA CONTAINING MODIFIED NUCLEOTIDE |
| WO2024063788A1 (en) | 2022-09-23 | 2024-03-28 | BioNTech SE | Compositions for delivery of malaria antigens and related methods |
| CN120225219A (en) | 2022-09-23 | 2025-06-27 | 生物技术欧洲股份公司 | Compositions and related methods for delivering liver stage antigens |
| JP2025533527A (en) | 2022-09-23 | 2025-10-07 | ビオンテック・ソシエタス・エウロパエア | Compositions and Related Methods for Delivery of Plasmodium CSP Antigens |
| WO2024063789A1 (en) | 2022-09-23 | 2024-03-28 | BioNTech SE | Compositions for delivery of malaria antigens and related methods |
| AU2023353931A1 (en) | 2022-09-26 | 2025-03-20 | Glaxosmithkline Biologicals Sa | Influenza virus vaccines |
| EP4594743A1 (en) * | 2022-09-30 | 2025-08-06 | Merck Sharp & Dohme LLC | An analytical method using lc-ms/ms proteomics to characterize proteins translated from mrna |
| EP4608442A1 (en) | 2022-10-28 | 2025-09-03 | GlaxoSmithKline Biologicals S.A. | Nucleic acid based vaccine |
| WO2024102762A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Lipids and lipid nanoparticle compositions for delivering polynucleotides |
| CN120390656A (en) | 2022-11-08 | 2025-07-29 | 欧纳医疗公司 | Circular RNA composition |
| WO2024102730A1 (en) | 2022-11-08 | 2024-05-16 | Orna Therapeutics, Inc. | Lipids and nanoparticle compositions for delivering polynucleotides |
| WO2024102954A1 (en) | 2022-11-10 | 2024-05-16 | Massachusetts Institute Of Technology | Activation induced clipping system (aics) |
| JP2025540101A (en) | 2022-12-01 | 2025-12-11 | キャンプ4 セラピューティクス コーポレイション | Modulation of SYNGAP1 gene transcription using antisense oligonucleotides targeting regulatory RNAs |
| WO2024119276A1 (en) * | 2022-12-07 | 2024-06-13 | The University Of British Columbia | Compositions and methods for peptide or protein delivery to the delivery to the central nervous system |
| WO2024123633A1 (en) | 2022-12-08 | 2024-06-13 | Recode Therapeutics, Inc. | Lipid nanoparticle compositions and uses thereof |
| WO2024124148A1 (en) * | 2022-12-09 | 2024-06-13 | Ohio State Innovation Foundation | Lipid compounds and methods of making and use thereof |
| WO2024121378A1 (en) | 2022-12-09 | 2024-06-13 | Institut National de la Santé et de la Recherche Médicale | Novel human antiviral genes related to the eleos and lamassu prokaryotic systems |
| EP4634388A1 (en) | 2022-12-14 | 2025-10-22 | Providence Therapeutics Holdings Inc. | Compositions and methods for infectious diseases |
| EP4634390A2 (en) | 2022-12-15 | 2025-10-22 | Orna Therapeutics, Inc. | Circular rna compositions and methods |
| TW202440939A (en) | 2022-12-17 | 2024-10-16 | 賓州大學委員會 | Recombinant aav mutant vectors with cardiac and skeletal muscle-specific targeting motifs and compositions containing same |
| WO2024130070A2 (en) | 2022-12-17 | 2024-06-20 | The Trustees Of The University Of Pennsylvania | Recombinant aav capsids with cardiac- and skeletal muscle- specific targeting motifs and uses thereof |
| WO2024133160A1 (en) | 2022-12-19 | 2024-06-27 | Glaxosmithkline Biologicals Sa | Hepatitis b compositions |
| WO2024138194A1 (en) | 2022-12-22 | 2024-06-27 | Tome Biosciences, Inc. | Platforms, compositions, and methods for in vivo programmable gene insertion |
| WO2024134199A1 (en) | 2022-12-22 | 2024-06-27 | Mina Therapeutics Limited | Chemically modified sarna compositions and methods of use |
| EP4654996A1 (en) | 2023-01-27 | 2025-12-03 | BioNTech SE | Pharmaceutical compositions for delivery of herpes simplex virus glycoprotein c, glycoprotein d, and glycoprotein e antigens and related methods |
| WO2024160936A1 (en) | 2023-02-03 | 2024-08-08 | Glaxosmithkline Biologicals Sa | Rna formulation |
| GB202302092D0 (en) | 2023-02-14 | 2023-03-29 | Glaxosmithkline Biologicals Sa | Analytical method |
| KR20250155004A (en) | 2023-02-23 | 2025-10-29 | 유니버시티 오브 로체스터 | Formulations and methods for preparing closed-ended DNA strand molecules |
| DE112024001143T5 (en) | 2023-03-08 | 2025-12-18 | CureVac SE | NEW LIPID NANOPARTICLE FORMULAS FOR NUCLEAN ACID RELEASE |
| WO2024197033A1 (en) | 2023-03-21 | 2024-09-26 | Modernatx, Inc. | Polynucleotides encoding relaxin for the treatment of heart failure |
| WO2024194484A1 (en) | 2023-03-23 | 2024-09-26 | Institut National de la Santé et de la Recherche Médicale | Modulating the expression and/or activity of gas7 for modulating viral replication |
| WO2024205657A2 (en) | 2023-03-29 | 2024-10-03 | Orna Therapeutics, Inc. | Lipids and lipid nanoparticle compositions for delivering polynucleotides |
| CN121001738A (en) | 2023-04-27 | 2025-11-21 | 葛兰素史克生物有限公司 | Influenza vaccine |
| WO2024223724A1 (en) | 2023-04-27 | 2024-10-31 | Glaxosmithkline Biologicals Sa | Influenza virus vaccines |
| AU2024265158A1 (en) | 2023-05-03 | 2025-11-06 | BioNTech SE | Optimized csp variants and related methods |
| WO2024228044A1 (en) | 2023-05-03 | 2024-11-07 | BioNTech SE | Optimized csp variants and related methods |
| AU2024265267A1 (en) | 2023-05-03 | 2025-11-20 | Modernatx, Inc. | Polynucleotides encoding cystic fibrosis transmembrane conductance regulator for the treatment of cystic fibrosis |
| AU2024269222A1 (en) | 2023-05-05 | 2025-10-09 | Orna Therapeutics, Inc. | Circular rna compositions and methods |
| WO2024234006A1 (en) | 2023-05-11 | 2024-11-14 | Tome Biosciences, Inc. | Systems, compositions, and methods for targeting liver sinusodial endothelial cells (lsecs) |
| WO2024230934A1 (en) | 2023-05-11 | 2024-11-14 | CureVac SE | Therapeutic nucleic acid for the treatment of ophthalmic diseases |
| WO2024258961A1 (en) | 2023-06-12 | 2024-12-19 | The Trustees Of The University Of Pennsylvania | Aav gene therapy for mucopolysaccharidosis iiib |
| WO2025007046A1 (en) | 2023-06-29 | 2025-01-02 | The Trustees Of The University Of Pennsylvania | Mutant aav with central nervous system targeting motifs and compositions containing same |
| WO2025011529A2 (en) | 2023-07-07 | 2025-01-16 | Shanghai Circode Biomed Co., Ltd. | Circular rna vaccines for seasonal flu and methods of uses |
| AU2024301077A1 (en) | 2023-07-21 | 2026-01-08 | BioNTech SE | Compositions for delivery of plasmodium antigens and related methods |
| WO2025024324A1 (en) | 2023-07-21 | 2025-01-30 | BioNTech SE | Compositions for delivery of plasmodium antigens and related methods |
| WO2025024337A1 (en) | 2023-07-24 | 2025-01-30 | BioNTech SE | Compositions for delivery of plasmodium antigens and related methods |
| WO2025030097A2 (en) | 2023-08-03 | 2025-02-06 | BioNTech SE | Pharmaceutical compositions for delivery of herpes simplex virus antigens and related methods |
| WO2025030154A1 (en) | 2023-08-03 | 2025-02-06 | The Trustees Of The University Of Pennsylvania | Pharmaceutical compositions for delivery of herpes simplex virus glycoprotein b antigens and related methods |
| WO2025035143A1 (en) | 2023-08-10 | 2025-02-13 | The Trustees Of The University Of Pennsylvania | Compositions and methods for treatment of spinal muscular atrophy |
| WO2025036272A1 (en) | 2023-08-11 | 2025-02-20 | Suzhou Abogen Biosciences Co., Ltd. | Capped mrna and method of preparation thereof |
| WO2025045142A1 (en) | 2023-08-29 | 2025-03-06 | Shanghai Circode Biomed Co., Ltd. | Circular rna encoding vegf polypeptides, formulations, and methods of uses |
| WO2025050069A1 (en) | 2023-09-01 | 2025-03-06 | Tome Biosciences, Inc. | Programmable gene insertion using engineered integration enzymes |
| EP4520345A1 (en) | 2023-09-06 | 2025-03-12 | Myneo Nv | Product |
| WO2025054556A1 (en) | 2023-09-07 | 2025-03-13 | BioNTech SE | Rna compositions for delivery of mpox antigens and related methods |
| WO2025057088A1 (en) | 2023-09-11 | 2025-03-20 | BioNTech SE | Rna compositions for delivery of incretin agents |
| WO2025072482A1 (en) | 2023-09-27 | 2025-04-03 | Modernatx, Inc. | Immunoglobulin a protease polypeptides, polynucleotides, and uses thereof |
| WO2025083211A1 (en) | 2023-10-20 | 2025-04-24 | Institut National de la Santé et de la Recherche Médicale | Use of factor h for the treatment of dementia |
| WO2025087266A1 (en) | 2023-10-23 | 2025-05-01 | Abogen Biosciences (Shanghai) Co., Ltd. | Immunomodulatory mrna cassettes, and uses thereof |
| WO2025101501A1 (en) | 2023-11-07 | 2025-05-15 | Orna Therapeutics, Inc. | Circular rna compositions |
| WO2025101862A1 (en) | 2023-11-08 | 2025-05-15 | Axelyf ehf. | Single domain antibody binders of myc |
| WO2025101685A1 (en) | 2023-11-09 | 2025-05-15 | University Of Rochester | Suppression of nonsense mutations using anticodon engineered (ace)-trnas |
| WO2025102034A1 (en) | 2023-11-10 | 2025-05-15 | The Trustees Of The University Of Pennsylvania | Gene therapy for barth syndrome |
| WO2025106661A1 (en) | 2023-11-14 | 2025-05-22 | The Trustees Of The University Of Pennsylvania | Compositions with cardiac and skeletal musclespecific targeting motifs and uses thereof |
| TW202523695A (en) | 2023-11-22 | 2025-06-16 | 美商旗艦先鋒創新有限責任(Vii)公司 | Methods and compositions for treating non-alcoholic fatty liver disease |
| US12364773B2 (en) | 2023-12-01 | 2025-07-22 | Recode Therapeutics, Inc. | Lipid nanoparticle compositions and uses thereof |
| WO2025132839A1 (en) | 2023-12-21 | 2025-06-26 | Glaxosmithkline Biologicals Sa | Influenza virus vaccines |
| WO2025130965A1 (en) * | 2023-12-22 | 2025-06-26 | Beijing Jitai Pharmaceutical Technology Co., Ltd. | Uses of lipid nanoparticle compositions |
| WO2025184172A1 (en) | 2024-02-27 | 2025-09-04 | Overt Bio, Inc. | Cancer reactive t cell receptors |
| WO2025184170A1 (en) | 2024-02-27 | 2025-09-04 | Overt Bio, Inc. | Methods and compositions for improving t cell function in an immunosuppressive environment |
| WO2025186788A1 (en) | 2024-03-08 | 2025-09-12 | Waters Technologies Corporation | Methods for chromatographic characterization of lipid nanoparticle compositions |
| WO2025194019A1 (en) | 2024-03-14 | 2025-09-18 | Flagship Pioneering Innovations Vii, Llc | Methods for treating liver fibrosis and non-alcoholic fatty liver disease |
| WO2025196732A1 (en) | 2024-03-22 | 2025-09-25 | Waters Technologies Corporation | Methods for characterizing lipid nanoparticle compositions |
| WO2025202937A1 (en) | 2024-03-26 | 2025-10-02 | BioNTech SE | Cancer vaccines |
| GB202404607D0 (en) | 2024-03-29 | 2024-05-15 | Glaxosmithkline Biologicals Sa | RNA formulation |
| WO2025213131A1 (en) | 2024-04-05 | 2025-10-09 | BioNTech SE | Rna compositions for delivery of orthopox antigens and related methods |
| WO2025215072A1 (en) | 2024-04-10 | 2025-10-16 | Institut National de la Santé et de la Recherche Médicale | Class i lanthipeptides with anti-viral function |
| WO2025219970A1 (en) | 2024-04-19 | 2025-10-23 | Waters Technologies Corporation | Dextran improves the sizing analysis of lipid nanoparticles during size exclusion chromatography analysis |
| EP4677108A1 (en) | 2024-04-22 | 2026-01-14 | Basecamp Research Ltd | Method and compositions for detecting off-target editing |
| WO2025224182A2 (en) | 2024-04-23 | 2025-10-30 | Basecamp Research Ltd | Single construct platform for simultaneous delivery of gene editing machinery and nucleic acid cargo |
| WO2025229572A1 (en) | 2024-05-01 | 2025-11-06 | Glaxosmithkline Biologicals Sa | Epstein-barr virus antigen-encoding messenger ribonucleic acid and antigen protein vaccines |
| WO2025231403A1 (en) * | 2024-05-03 | 2025-11-06 | Revivity Pharma Corporation | Methods of administering and manufacturing toxic compounds |
| WO2025235862A1 (en) | 2024-05-10 | 2025-11-13 | Inndura Therapeutics Inc. | A modified immune cell receptor comprising a target-binding domain and the extracellular domain of cd16a |
| WO2025245188A2 (en) | 2024-05-21 | 2025-11-27 | Flagship Pioneering Innovations Vii, Llc | Methods of treating liver steatosis and non-alcoholic fatty liver disease |
| WO2025248096A1 (en) | 2024-05-31 | 2025-12-04 | Institut National de la Santé et de la Recherche Médicale | Antimicrobial peptides having liquid-liquid phase separation activities |
| WO2025250751A1 (en) | 2024-05-31 | 2025-12-04 | Orna Therapeutics, Inc. | Circular rna compositions and methods |
| WO2025255388A1 (en) | 2024-06-05 | 2025-12-11 | Camp4 Therapeutics Corporation | Modulation of syngap1 gene transcription using antisense oligonucleotides targeting regulatory rnas |
| WO2025255199A1 (en) | 2024-06-05 | 2025-12-11 | Modernatx, Inc. | Argininosuccinate synthase 1 and argininosuccinate lyase polypeptides and polynucleotides and uses thereof |
| WO2026006151A2 (en) | 2024-06-24 | 2026-01-02 | University Of Rochester | Functionalization of ace-trna encoding synthetic linear picovectors |
| WO2026003582A2 (en) | 2024-06-27 | 2026-01-02 | Axelyf ehf. | Lipids and lipid nanoparticles |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150004217A1 (en) * | 2010-11-30 | 2015-01-01 | Shire Human Genetic Therapies, Inc. | mRNA FOR USE IN TREATMENT OF HUMAN GENETIC DISEASES |
Family Cites Families (526)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2647121A (en) | 1951-02-02 | 1953-07-28 | Ruth P Jacoby | Diamine-bis-acetamides |
| US2819718A (en) | 1953-07-16 | 1958-01-14 | Isidore H Goldman | Drainage tube |
| US2717909A (en) | 1953-09-24 | 1955-09-13 | Monsanto Chemicals | Hydroxyethyl-keryl-alkylene-ammonium compounds |
| US2844629A (en) | 1956-04-25 | 1958-07-22 | American Home Prod | Fatty acid amides and derivatives thereof |
| US3096560A (en) | 1958-11-21 | 1963-07-09 | William J Liebig | Process for synthetic vascular implants |
| GB1072118A (en) | 1962-12-01 | 1967-06-14 | Sandoz Ag | Amides of aminopropionic acid |
| FR1378382A (en) | 1962-12-01 | 1964-11-13 | Sandoz Sa | Amides of amino-propionic acid, usable in particular for the treatment of textile fibers |
| JPS5141663B1 (en) | 1966-03-12 | 1976-11-11 | ||
| JPS4822365B1 (en) | 1968-10-25 | 1973-07-05 | ||
| NL143127B (en) | 1969-02-04 | 1974-09-16 | Rhone Poulenc Sa | REINFORCEMENT DEVICE FOR A DEFECTIVE HEART VALVE. |
| JPS4822365Y1 (en) | 1969-11-28 | 1973-06-29 | ||
| US3614955A (en) | 1970-02-09 | 1971-10-26 | Medtronic Inc | Standby defibrillator and method of operation |
| US3614954A (en) | 1970-02-09 | 1971-10-26 | Medtronic Inc | Electronic standby defibrillator |
| JPS5024216B1 (en) | 1970-12-29 | 1975-08-14 | ||
| JPS5012146Y2 (en) | 1971-07-27 | 1975-04-15 | ||
| JPS5123537Y2 (en) | 1972-01-17 | 1976-06-17 | ||
| US3945052A (en) | 1972-05-01 | 1976-03-23 | Meadox Medicals, Inc. | Synthetic vascular graft and method for manufacturing the same |
| US3805301A (en) | 1972-07-28 | 1974-04-23 | Meadox Medicals Inc | Tubular grafts having indicia thereon |
| JPS5210847Y2 (en) | 1972-10-11 | 1977-03-09 | ||
| JPS49127908A (en) | 1973-04-20 | 1974-12-07 | ||
| JPS5624664B2 (en) | 1973-06-28 | 1981-06-08 | ||
| US4013507A (en) | 1973-09-18 | 1977-03-22 | California Institute Of Technology | Ionene polymers for selectively inhibiting the vitro growth of malignant cells |
| JPS5123537A (en) | 1974-04-26 | 1976-02-25 | Adeka Argus Chemical Co Ltd | KASOZAISOSEIBUTSU |
| GB1527592A (en) | 1974-08-05 | 1978-10-04 | Ici Ltd | Wound dressing |
| US3995623A (en) | 1974-12-23 | 1976-12-07 | American Hospital Supply Corporation | Multipurpose flow-directed catheter |
| JPS5813576B2 (en) | 1974-12-27 | 1983-03-14 | アデカ ア−ガスカガク カブシキガイシヤ | Stabilized synthetic polymer composition |
| JPS5524302Y2 (en) | 1975-03-31 | 1980-06-10 | ||
| DE2520814A1 (en) | 1975-05-09 | 1976-11-18 | Bayer Ag | Light stabilisation of polyurethanes - using polymeric tert. amines from aliphatic diamines and (meth)acrylic esters or amides |
| US4281669A (en) | 1975-05-09 | 1981-08-04 | Macgregor David C | Pacemaker electrode with porous system |
| JPS5210847A (en) | 1975-07-16 | 1977-01-27 | Nippon Steel Corp | Pinch roll |
| US4096860A (en) | 1975-10-08 | 1978-06-27 | Mclaughlin William F | Dual flow encatheter |
| CA1069652A (en) | 1976-01-09 | 1980-01-15 | Alain F. Carpentier | Supported bioprosthetic heart valve with compliant orifice ring |
| US4134402A (en) | 1976-02-11 | 1979-01-16 | Mahurkar Sakharam D | Double lumen hemodialysis catheter |
| US4072146A (en) | 1976-09-08 | 1978-02-07 | Howes Randolph M | Venous catheter device |
| US4335723A (en) | 1976-11-26 | 1982-06-22 | The Kendall Company | Catheter having inflatable retention means |
| US4099528A (en) | 1977-02-17 | 1978-07-11 | Sorenson Research Co., Inc. | Double lumen cannula |
| US4140126A (en) | 1977-02-18 | 1979-02-20 | Choudhury M Hasan | Method for performing aneurysm repair |
| US4265745A (en) | 1977-05-25 | 1981-05-05 | Teijin Limited | Permselective membrane |
| US4182833A (en) | 1977-12-07 | 1980-01-08 | Celanese Polymer Specialties Company | Cationic epoxide-amine reaction products |
| US4180068A (en) | 1978-04-13 | 1979-12-25 | Motion Control, Incorporated | Bi-directional flow catheter with retractable trocar/valve structure |
| DE2960875D1 (en) | 1978-04-19 | 1981-12-10 | Ici Plc | A method of preparing a tubular product by electrostatic spinning |
| US4284459A (en) | 1978-07-03 | 1981-08-18 | The Kendall Company | Method for making a molded catheter |
| US4227533A (en) | 1978-11-03 | 1980-10-14 | Bristol-Myers Company | Flushable urinary catheter |
| US4375817A (en) | 1979-07-19 | 1983-03-08 | Medtronic, Inc. | Implantable cardioverter |
| DE3010841A1 (en) | 1980-03-21 | 1981-10-08 | Ulrich Dr.med. 6936 Haag Uthmann | CATHEDER |
| US4308085A (en) | 1980-07-28 | 1981-12-29 | Jenoptik Jena Gmbh | Process for the preparation of high molecular thermoplastic epoxide-amine-polyadducts |
| US4339369A (en) | 1981-04-23 | 1982-07-13 | Celanese Corporation | Cationic epoxide-amine reaction products |
| US4406656A (en) | 1981-06-01 | 1983-09-27 | Brack Gillium Hattler | Venous catheter having collapsible multi-lumens |
| US4475972A (en) | 1981-10-01 | 1984-10-09 | Ontario Research Foundation | Implantable material |
| US4401472A (en) | 1982-02-26 | 1983-08-30 | Martin Marietta Corporation | Hydraulic cement mixes and processes for improving hydraulic cement mixes |
| US4568329A (en) | 1982-03-08 | 1986-02-04 | Mahurkar Sakharam D | Double lumen catheter |
| US4546499A (en) | 1982-12-13 | 1985-10-15 | Possis Medical, Inc. | Method of supplying blood to blood receiving vessels |
| US4530113A (en) | 1983-05-20 | 1985-07-23 | Intervascular, Inc. | Vascular grafts with cross-weave patterns |
| US4550447A (en) | 1983-08-03 | 1985-11-05 | Shiley Incorporated | Vascular graft prosthesis |
| US4647416A (en) | 1983-08-03 | 1987-03-03 | Shiley Incorporated | Method of preparing a vascular graft prosthesis |
| US5104399A (en) | 1986-12-10 | 1992-04-14 | Endovascular Technologies, Inc. | Artificial graft and implantation method |
| US4571241A (en) | 1983-12-16 | 1986-02-18 | Christopher T Graham | Urinary catheter with collapsible urethral tube |
| US4710169A (en) | 1983-12-16 | 1987-12-01 | Christopher T Graham | Urinary catheter with collapsible urethral tube |
| US4737518A (en) | 1984-04-03 | 1988-04-12 | Takeda Chemical Industries, Ltd. | Lipid derivatives, their production and use |
| US4562596A (en) | 1984-04-25 | 1986-01-07 | Elliot Kornberg | Aortic graft, device and method for performing an intraluminal abdominal aortic aneurysm repair |
| US4782836A (en) | 1984-05-24 | 1988-11-08 | Intermedics, Inc. | Rate adaptive cardiac pacemaker responsive to patient activity and temperature |
| US4897355A (en) | 1985-01-07 | 1990-01-30 | Syntex (U.S.A.) Inc. | N[ω,(ω-1)-dialkyloxy]- and N-[ω,(ω-1)-dialkenyloxy]-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| US4662382A (en) | 1985-01-16 | 1987-05-05 | Intermedics, Inc. | Pacemaker lead with enhanced sensitivity |
| US4762915A (en) | 1985-01-18 | 1988-08-09 | Liposome Technology, Inc. | Protein-liposome conjugates |
| US4860751A (en) | 1985-02-04 | 1989-08-29 | Cordis Corporation | Activity sensor for pacemaker control |
| US5223263A (en) | 1988-07-07 | 1993-06-29 | Vical, Inc. | Liponucleotide-containing liposomes |
| CA1320724C (en) | 1985-07-19 | 1993-07-27 | Koichi Kanehira | Terpene amino alcohols and medicinal uses thereof |
| US4701162A (en) | 1985-09-24 | 1987-10-20 | The Kendall Company | Foley catheter assembly |
| US4737323A (en) | 1986-02-13 | 1988-04-12 | Liposome Technology, Inc. | Liposome extrusion method |
| DE3616824A1 (en) | 1986-05-17 | 1987-11-19 | Schering Ag | USE OF CURABLE RESIN MIXTURES FOR SURFACE COATINGS AND PRINTING INKS AND METHOD FOR THE PRODUCTION THEREOF |
| DE3780374D1 (en) | 1986-07-31 | 1992-08-20 | Irnich Werner | FREQUENCY ADAPTING HEART PACEMAKER. |
| US4960409A (en) | 1986-09-11 | 1990-10-02 | Catalano Marc L | Method of using bilumen peripheral venous catheter with adapter |
| JPH0829776B2 (en) | 1986-10-29 | 1996-03-27 | 東燃化学株式会社 | Synthetic resin container and mold for manufacturing the same |
| US4720517A (en) | 1986-11-24 | 1988-01-19 | Ciba-Geigy Corporation | Compositions stabilized with N-hydroxyiminodiacetic and dipropionic acids and esters thereof |
| US4920016A (en) | 1986-12-24 | 1990-04-24 | Linear Technology, Inc. | Liposomes with enhanced circulation time |
| JPS63125144U (en) | 1987-02-09 | 1988-08-16 | ||
| JPS63154788U (en) | 1987-03-31 | 1988-10-11 | ||
| WO1988007850A1 (en) | 1987-04-16 | 1988-10-20 | The Liposome Company, Inc. | Liposome continuous size reduction method and apparatus |
| JPS63312934A (en) | 1987-06-16 | 1988-12-21 | Hitachi Cable Ltd | Lead frame material for semiconductor |
| DE3728917A1 (en) | 1987-08-29 | 1989-03-09 | Roth Hermann J | Novel lipids containing an asymmetrically substituted disulphide bridge, processes for their preparation, and their use as medicaments |
| US4946683A (en) | 1987-11-18 | 1990-08-07 | Vestar, Inc. | Multiple step entrapment/loading procedure for preparing lipophilic drug-containing liposomes |
| US5047540A (en) | 1987-12-17 | 1991-09-10 | Shionogi & Co., Ltd. | Lipid derivatives |
| US5138067A (en) | 1987-12-17 | 1992-08-11 | Shionogi & Co. Ltd. | Lipid derivatives |
| US4892540A (en) | 1988-04-21 | 1990-01-09 | Sorin Biomedica S.P.A. | Two-leaflet prosthetic heart valve |
| US5176661A (en) | 1988-09-06 | 1993-01-05 | Advanced Cardiovascular Systems, Inc. | Composite vascular catheter |
| US5024671A (en) | 1988-09-19 | 1991-06-18 | Baxter International Inc. | Microporous vascular graft |
| US5200395A (en) | 1988-10-18 | 1993-04-06 | Ajinomoto Company, Inc. | Pharmaceutical composition of BUF-5 for treating anemia |
| CA2001401A1 (en) | 1988-10-25 | 1990-04-25 | Claude Piantadosi | Quaternary amine containing ether or ester lipid derivatives and therapeutic compositions |
| US5185154A (en) | 1989-02-02 | 1993-02-09 | Liposome Technology, Inc. | Method for instant preparation of a drug containing large unilamellar vesicles |
| US6214804B1 (en) * | 1989-03-21 | 2001-04-10 | Vical Incorporated | Induction of a protective immune response in a mammal by injecting a DNA sequence |
| US5703055A (en) | 1989-03-21 | 1997-12-30 | Wisconsin Alumni Research Foundation | Generation of antibodies through lipid mediated DNA delivery |
| DK0737750T3 (en) | 1989-03-21 | 2003-09-01 | Vical Inc | Expression of exogenous polynucleotide sequences in vertebrates |
| FR2645866B1 (en) | 1989-04-17 | 1991-07-05 | Centre Nat Rech Scient | NEW LIPOPOLYAMINES, THEIR PREPARATION AND THEIR USE |
| US5194654A (en) | 1989-11-22 | 1993-03-16 | Vical, Inc. | Lipid derivatives of phosphonoacids for liposomal incorporation and method of use |
| US5279833A (en) | 1990-04-04 | 1994-01-18 | Yale University | Liposomal transfection of nucleic acids into animal cells |
| US5101824A (en) | 1990-04-16 | 1992-04-07 | Siemens-Pacesetter, Inc. | Rate-responsive pacemaker with circuitry for processing multiple sensor inputs |
| US5264618A (en) | 1990-04-19 | 1993-11-23 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| US5405379A (en) | 1990-07-26 | 1995-04-11 | Lane; Rodney J. | Self expanding vascular endoprosthesis for aneurysms |
| US5693338A (en) | 1994-09-29 | 1997-12-02 | Emisphere Technologies, Inc. | Diketopiperazine-based delivery systems |
| JPH0765267B2 (en) | 1990-08-22 | 1995-07-12 | 花王株式会社 | Softening agent |
| US5206027A (en) | 1990-09-13 | 1993-04-27 | Fuji Photo Film Co., Ltd. | Amphipathic compound and liposome comprising the same |
| AU633453B2 (en) | 1990-10-09 | 1993-01-28 | Cook Incorporated | Percutaneous stent assembly |
| EP0491082B1 (en) | 1990-12-19 | 1995-04-12 | Peter Dr. Ing. Osypka | Pacemaker lead with an inner duct and with an electrode head |
| US5116360A (en) | 1990-12-27 | 1992-05-26 | Corvita Corporation | Mesh composite graft |
| US5405363A (en) | 1991-03-15 | 1995-04-11 | Angelon Corporation | Implantable cardioverter defibrillator having a smaller displacement volume |
| US5330768A (en) | 1991-07-05 | 1994-07-19 | Massachusetts Institute Of Technology | Controlled drug delivery using polymer/pluronic blends |
| US5545449A (en) | 1991-10-02 | 1996-08-13 | Weyerhaeuser Company | Polyether-reinforced fiber-based materials |
| US5151105A (en) | 1991-10-07 | 1992-09-29 | Kwan Gett Clifford | Collapsible vessel sleeve implant |
| JPH0753535B2 (en) | 1992-02-14 | 1995-06-07 | 株式会社カワタ | A valve device in a powder material supply device |
| US5284491A (en) | 1992-02-27 | 1994-02-08 | Medtronic, Inc. | Cardiac pacemaker with hysteresis behavior |
| US5352461A (en) | 1992-03-11 | 1994-10-04 | Pharmaceutical Discovery Corporation | Self assembling diketopiperazine drug delivery system |
| SE9200951D0 (en) | 1992-03-27 | 1992-03-27 | Kabi Pharmacia Ab | PHARMACEUTICAL COMPOSITION CONTAINING A DEFINED LIPID SYSTEM |
| DE69325096T2 (en) | 1992-04-06 | 1999-09-30 | Biosite Diagnostics Inc., San Diego | MORPHINE DERIVATIVES AND THEIR CONJUGATES AND LABELS WITH PROTEINS AND POLYPEPTIDES |
| US6670178B1 (en) | 1992-07-10 | 2003-12-30 | Transkaryotic Therapies, Inc. | In Vivo production and delivery of insulinotropin for gene therapy |
| CA2141685A1 (en) | 1992-08-04 | 1994-02-17 | Koji Naito | Antiallergic composition |
| US5334761A (en) | 1992-08-28 | 1994-08-02 | Life Technologies, Inc. | Cationic lipids |
| US5461223A (en) | 1992-10-09 | 1995-10-24 | Eastman Kodak Company | Bar code detecting circuitry |
| US5300022A (en) | 1992-11-12 | 1994-04-05 | Martin Klapper | Urinary catheter and bladder irrigation system |
| US5496362A (en) | 1992-11-24 | 1996-03-05 | Cardiac Pacemakers, Inc. | Implantable conformal coil patch electrode with multiple conductive elements for cardioversion and defibrillation |
| US5552155A (en) | 1992-12-04 | 1996-09-03 | The Liposome Company, Inc. | Fusogenic lipsomes and methods for making and using same |
| US5716395A (en) | 1992-12-11 | 1998-02-10 | W.L. Gore & Associates, Inc. | Prosthetic vascular graft |
| RU2123492C1 (en) | 1993-02-19 | 1998-12-20 | Ниппон Синяку Ко., Лтд | Glycerol derivatives, an agent for delivery of physiologically active substance, pharmaceutical composition |
| US5395619A (en) | 1993-03-03 | 1995-03-07 | Liposome Technology, Inc. | Lipid-polymer conjugates and liposomes |
| US5697953A (en) | 1993-03-13 | 1997-12-16 | Angeion Corporation | Implantable cardioverter defibrillator having a smaller displacement volume |
| US5624976A (en) | 1994-03-25 | 1997-04-29 | Dentsply Gmbh | Dental filling composition and method |
| WO1994027435A1 (en) | 1993-06-01 | 1994-12-08 | Life Technologies, Inc. | Genetic immunization with cationic lipids |
| US5314430A (en) | 1993-06-24 | 1994-05-24 | Medtronic, Inc. | Atrial defibrillator employing transvenous and subcutaneous electrodes and method of use |
| DE4325848A1 (en) | 1993-07-31 | 1995-02-02 | Basf Ag | Process for the preparation of N- (2-hydroxyethyl) piperazine |
| DE69428057T2 (en) | 1993-10-06 | 2002-04-18 | Mitsubishi Jukogyo K.K., Tokio/Tokyo | Process for the separation of carbon dioxide from combustion gases |
| US5609624A (en) | 1993-10-08 | 1997-03-11 | Impra, Inc. | Reinforced vascular graft and method of making same |
| SE9303481L (en) | 1993-10-22 | 1995-04-23 | Berol Nobel Ab | hygiene composition |
| AU1091095A (en) | 1993-11-08 | 1995-05-29 | Harrison M. Lazarus | Intraluminal vascular graft and method |
| CA2176714A1 (en) | 1993-11-24 | 1995-06-01 | Timothy D. Heath | Amphiphilic derivatives of piperazine |
| US5595756A (en) | 1993-12-22 | 1997-01-21 | Inex Pharmaceuticals Corporation | Liposomal compositions for enhanced retention of bioactive agents |
| US5464924A (en) | 1994-01-07 | 1995-11-07 | The Dow Chemical Company | Flexible poly(amino ethers) for barrier packaging |
| US6077835A (en) | 1994-03-23 | 2000-06-20 | Case Western Reserve University | Delivery of compacted nucleic acid to cells |
| US5844107A (en) | 1994-03-23 | 1998-12-01 | Case Western Reserve University | Compacted nucleic acids and their delivery to cells |
| ATE234605T1 (en) | 1994-04-12 | 2003-04-15 | Liposome Co Inc | FUSOGENIC LIPOSOMES AND METHOD FOR THE PRODUCTION AND USE THEREOF |
| US5833979A (en) | 1994-07-20 | 1998-11-10 | Cytotherapeutics, Inc. | Methods and compositions of growth control for cells encapsulated within bioartificial organs |
| US5885613A (en) | 1994-09-30 | 1999-03-23 | The University Of British Columbia | Bilayer stabilizing components and their use in forming programmable fusogenic liposomes |
| US5820873A (en) | 1994-09-30 | 1998-10-13 | The University Of British Columbia | Polyethylene glycol modified ceramide lipids and liposome uses thereof |
| US5641665A (en) | 1994-11-28 | 1997-06-24 | Vical Incorporated | Plasmids suitable for IL-2 expression |
| US6071890A (en) | 1994-12-09 | 2000-06-06 | Genzyme Corporation | Organ-specific targeting of cationic amphiphile/DNA complexes for gene therapy |
| US5965434A (en) | 1994-12-29 | 1999-10-12 | Wolff; Jon A. | Amphipathic PH sensitive compounds and delivery systems for delivering biologically active compounds |
| US6485726B1 (en) | 1995-01-17 | 2002-11-26 | The Brigham And Women's Hospital, Inc. | Receptor specific transepithelial transport of therapeutics |
| US5830430A (en) | 1995-02-21 | 1998-11-03 | Imarx Pharmaceutical Corp. | Cationic lipids and the use thereof |
| EP0822835A1 (en) | 1995-04-17 | 1998-02-11 | Imarx Pharmaceutical Corp. | Hybrid magnetic resonance contrast agents |
| US5772694A (en) | 1995-05-16 | 1998-06-30 | Medical Carbon Research Institute L.L.C. | Prosthetic heart valve with improved blood flow |
| US5783383A (en) | 1995-05-23 | 1998-07-21 | The Board Of Trustees Of The Leland Stanford Junior University | Method of detecting cytomegalovirus (CMV) |
| US5981501A (en) | 1995-06-07 | 1999-11-09 | Inex Pharmaceuticals Corp. | Methods for encapsulating plasmids in lipid bilayers |
| US5609629A (en) | 1995-06-07 | 1997-03-11 | Med Institute, Inc. | Coated implantable medical device |
| ES2231819T3 (en) | 1995-06-07 | 2005-05-16 | Inex Pharmaceuticals Corp | PARTICULES OF NUCLEIC LIPIDO-ACID PREPARED THROUGH A NIPLEIC HYDROPHOBIC NIPLEIC ACID COMPLEX INTERMEDIATE AND USE TO TRANSFER GENES. |
| US5705385A (en) | 1995-06-07 | 1998-01-06 | Inex Pharmaceuticals Corporation | Lipid-nucleic acid particles prepared via a hydrophobic lipid-nucleic acid complex intermediate and use for gene transfer |
| US7422902B1 (en) | 1995-06-07 | 2008-09-09 | The University Of British Columbia | Lipid-nucleic acid particles prepared via a hydrophobic lipid-nucleic acid complex intermediate and use for gene transfer |
| US5607385A (en) | 1995-08-17 | 1997-03-04 | Medtronic, Inc. | Device and algorithm for a combined cardiomyostimulator and a cardiac pacer-carioverter-defibrillator |
| US5744335A (en) | 1995-09-19 | 1998-04-28 | Mirus Corporation | Process of transfecting a cell with a polynucleotide mixed with an amphipathic compound and a DNA-binding protein |
| FR2740978B1 (en) | 1995-11-10 | 1998-01-02 | Ela Medical Sa | IMPLANTABLE DEFIBRILLATOR / CARDIOVERVER ACTIVE MEDICAL DEVICE |
| US6183774B1 (en) | 1996-01-31 | 2001-02-06 | Collaborative Laboratories, Inc. | Stabilizing vitamin A derivatives by encapsulation in lipid vesicles formed with alkylammonium fatty acid salts |
| US5874105A (en) | 1996-01-31 | 1999-02-23 | Collaborative Laboratories, Inc. | Lipid vesicles formed with alkylammonium fatty acid salts |
| US5783566A (en) * | 1996-05-10 | 1998-07-21 | California Institute Of Technology | Method for increasing or decreasing transfection efficiency |
| CA2252055C (en) | 1996-04-11 | 2007-01-16 | The University Of British Columbia | Fusogenic liposomes |
| US5935936A (en) | 1996-06-03 | 1999-08-10 | Genzyme Corporation | Compositions comprising cationic amphiphiles and co-lipids for intracellular delivery of therapeutic molecules |
| US5913848A (en) | 1996-06-06 | 1999-06-22 | Luther Medical Products, Inc. | Hard tip over-the-needle catheter and method of manufacturing the same |
| US5677124A (en) | 1996-07-03 | 1997-10-14 | Ambion, Inc. | Ribonuclease resistant viral RNA standards |
| US5736573A (en) | 1996-07-31 | 1998-04-07 | Galat; Alexander | Lipid and water soluble derivatives of drugs |
| US7288266B2 (en) | 1996-08-19 | 2007-10-30 | United States Of America As Represented By The Secretary, Department Of Health And Human Services | Liposome complexes for increased systemic delivery |
| ATE252891T1 (en) | 1996-08-26 | 2003-11-15 | Transgene Sa | CATIONIC LIPID-NUCLIC ACID COMPLEXES |
| US6458574B1 (en) | 1996-09-12 | 2002-10-01 | Transkaryotic Therapies, Inc. | Treatment of a α-galactosidase a deficiency |
| CA2271388C (en) | 1996-09-13 | 2007-11-06 | The School Of Pharmacy, University Of London | Liposomes encapsulating polynucleotides operatively coding for immunogenic polypeptides |
| TW520297B (en) | 1996-10-11 | 2003-02-11 | Sequus Pharm Inc | Fusogenic liposome composition and method |
| AU738384B2 (en) | 1996-11-04 | 2001-09-20 | Qiagen Gmbh | Cationic reagents for transfection |
| US6887665B2 (en) | 1996-11-14 | 2005-05-03 | Affymetrix, Inc. | Methods of array synthesis |
| US5985930A (en) | 1996-11-21 | 1999-11-16 | Pasinetti; Giulio M. | Treatment of neurodegenerative conditions with nimesulide |
| US6204297B1 (en) | 1996-11-26 | 2001-03-20 | Rhodia Inc. | Nonionic gemini surfactants |
| JPH10197978A (en) | 1997-01-09 | 1998-07-31 | Mitsubishi Paper Mills Ltd | Silver halide photographic material |
| EP0853123A1 (en) | 1997-01-10 | 1998-07-15 | Roche Diagnostics GmbH | Purification of DNA by 'cross-flow-filtration' |
| FR2760193B1 (en) | 1997-02-28 | 1999-05-28 | Transgene Sa | LIPIDS AND COMPLEXES OF CATIONIC LIPIDS AND ACTIVE SUBSTANCES, IN PARTICULAR FOR THE TRANSFECTION OF CELLS |
| US5837283A (en) | 1997-03-12 | 1998-11-17 | The Regents Of The University Of California | Cationic lipid compositions targeting angiogenic endothelial cells |
| ATE486614T1 (en) * | 1997-03-14 | 2010-11-15 | Philadelphia Children Hospital | COMPOSITIONS FOR USE IN GENE THERAPY FOR THE TREATMENT OF HEMOPHILIA |
| US5945326A (en) | 1997-03-20 | 1999-08-31 | New England Biolabs, Inc. | Method for cloning and producing the Spel restriction endonuclease |
| US6060082A (en) | 1997-04-18 | 2000-05-09 | Massachusetts Institute Of Technology | Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery |
| US6835395B1 (en) | 1997-05-14 | 2004-12-28 | The University Of British Columbia | Composition containing small multilamellar oligodeoxynucleotide-containing lipid vesicles |
| US20030104044A1 (en) | 1997-05-14 | 2003-06-05 | Semple Sean C. | Compositions for stimulating cytokine secretion and inducing an immune response |
| DE69841002D1 (en) | 1997-05-14 | 2009-09-03 | Univ British Columbia | Highly effective encapsulation of nucleic acids in lipid vesicles |
| JPH115786A (en) | 1997-06-13 | 1999-01-12 | Pola Chem Ind Inc | Novel aminohydroxypropylpiperazine derivative |
| US6067471A (en) | 1998-08-07 | 2000-05-23 | Cardiac Pacemakers, Inc. | Atrial and ventricular implantable cardioverter-defibrillator and lead system |
| US6165484A (en) | 1997-08-26 | 2000-12-26 | Wake Forest University | EDTA and other chelators with or without antifungal antimicrobial agents for the prevention and treatment of fungal infections |
| US6060308A (en) | 1997-09-04 | 2000-05-09 | Connaught Laboratories Limited | RNA respiratory syncytial virus vaccines |
| JPH1180142A (en) | 1997-09-05 | 1999-03-26 | Pola Chem Ind Inc | Production of diphenylalkyl compound |
| EP1021549A2 (en) | 1997-09-19 | 2000-07-26 | Sequitur, Inc. | SENSE mRNA THERAPY |
| US6734171B1 (en) | 1997-10-10 | 2004-05-11 | Inex Pharmaceuticals Corp. | Methods for encapsulating nucleic acids in lipid bilayers |
| ATE355826T1 (en) | 1997-10-10 | 2007-03-15 | Inex Pharmaceuticals Corp | METHOD FOR ENCAPSULATING NUCLEIC ACIDS IN LIPID DOUBLE LAYERS |
| EP2147681A1 (en) | 1997-10-29 | 2010-01-27 | Genzyme Corporation | Compositions and methods for treating lysosomal storage disease |
| US6165763A (en) | 1997-10-30 | 2000-12-26 | Smithkline Beecham Corporation | Ornithine carbamoyltransferase |
| US6096075A (en) | 1998-01-22 | 2000-08-01 | Medical Carbon Research Institute, Llc | Prosthetic heart valve |
| US6617171B2 (en) | 1998-02-27 | 2003-09-09 | The General Hospital Corporation | Methods for diagnosing and treating autoimmune disease |
| US6271209B1 (en) | 1998-04-03 | 2001-08-07 | Valentis, Inc. | Cationic lipid formulation delivering nucleic acid to peritoneal tumors |
| US6176877B1 (en) | 1998-04-20 | 2001-01-23 | St. Jude Medical, Inc. | Two piece prosthetic heart valve |
| DE19822602A1 (en) | 1998-05-20 | 1999-11-25 | Goldschmidt Ag Th | Process for the preparation of polyamino acid esters by esterification of acidic polyamino acids or transesterification of polyamino acid esters |
| NO313244B1 (en) | 1998-07-08 | 2002-09-02 | Crew Dev Corp | Process for the isolation and production of magnesite or magnesium chloride |
| US6055454A (en) | 1998-07-27 | 2000-04-25 | Cardiac Pacemakers, Inc. | Cardiac pacemaker with automatic response optimization of a physiologic sensor based on a second sensor |
| JP2002521423A (en) | 1998-07-31 | 2002-07-16 | コリア インスティチュート オブ サイエンス アンド テクノロージ | Lipid emulsions and solid lipid microparticles as gene or drug carriers |
| WO2000010622A1 (en) | 1998-08-20 | 2000-03-02 | Cook Incorporated | Coated implantable medical device |
| US6210892B1 (en) | 1998-10-07 | 2001-04-03 | Isis Pharmaceuticals, Inc. | Alteration of cellular behavior by antisense modulation of mRNA processing |
| WO2000030444A1 (en) | 1998-11-25 | 2000-06-02 | Vanderbilt University | Cationic liposomes for gene transfer |
| US6248725B1 (en) | 1999-02-23 | 2001-06-19 | Amgen, Inc. | Combinations and methods for promoting in vivo liver cell proliferation and enhancing in vivo liver-directed gene transduction |
| WO2000060120A2 (en) | 1999-04-02 | 2000-10-12 | Mosaic Technologies | Methods of detecting microorganism using immobilized probes |
| US6379698B1 (en) | 1999-04-06 | 2002-04-30 | Isis Pharmaceuticals, Inc. | Fusogenic lipids and vesicles |
| US8647864B2 (en) | 1999-04-14 | 2014-02-11 | Novartis Ag | Compositions and methods for generating an immune response utilizing alphavirus-based vector systems |
| EP1173600A2 (en) | 1999-04-20 | 2002-01-23 | The University Of British Columbia | Cationic peg-lipids and methods of use |
| HUP0201425A3 (en) | 1999-04-23 | 2006-07-28 | Hadasit Med Res Service | Conjugate having a cleavable linkage for use in a liposome |
| US6169923B1 (en) | 1999-04-23 | 2001-01-02 | Pacesetter, Inc. | Implantable cardioverter-defibrillator with automatic arrhythmia detection criteria adjustment |
| MXPA01011845A (en) | 1999-05-19 | 2002-06-21 | Lexigen Pharm Corp | EXPRESSION AND EXPORT OF INTERFERON-ALPHA PROTEINS AS Fc FUSION PROTEINS. |
| US6696424B1 (en) | 1999-05-28 | 2004-02-24 | Vical Incorporated | Cytofectin dimers and methods of use thereof |
| US6346382B1 (en) | 1999-06-01 | 2002-02-12 | Vanderbilt University | Human carbamyl phosphate synthetase I polymorphism and diagnostic methods related thereto |
| US7094423B1 (en) | 1999-07-15 | 2006-08-22 | Inex Pharmaceuticals Corp. | Methods for preparation of lipid-encapsulated therapeutic agents |
| WO2001005375A1 (en) | 1999-07-16 | 2001-01-25 | Purdue Research Foundation | Vinyl ether lipids with cleavable hydrophilic headgroups |
| BR0012627A (en) | 1999-07-23 | 2002-04-09 | Genentech Inc | Process to purify the plasmatic DNA of prokaryotic cells by using tangential flow filtration |
| US6358278B1 (en) | 1999-09-24 | 2002-03-19 | St. Jude Medical, Inc. | Heart valve prosthesis with rotatable cuff |
| AU7725500A (en) | 1999-09-30 | 2001-04-30 | National Jewish Medical And Research Center | Method for inhibition of pathogenic microorganisms |
| US6371983B1 (en) | 1999-10-04 | 2002-04-16 | Ernest Lane | Bioprosthetic heart valve |
| AU7868400A (en) | 1999-10-08 | 2001-04-23 | Alza Corporation | Neutral-cationic lipid for nucleic acid and drug delivery |
| US7060291B1 (en) | 1999-11-24 | 2006-06-13 | Transave, Inc. | Modular targeted liposomal delivery system |
| CA2395636A1 (en) | 1999-12-30 | 2001-07-12 | Novartis Ag | Novel colloid synthetic vectors for gene therapy |
| WO2001060414A2 (en) | 2000-02-17 | 2001-08-23 | Genzyme Corporation | Genetic modification of the lung as a portal for gene delivery |
| US6370434B1 (en) | 2000-02-28 | 2002-04-09 | Cardiac Pacemakers, Inc. | Cardiac lead and method for lead implantation |
| US6565960B2 (en) | 2000-06-01 | 2003-05-20 | Shriners Hospital Of Children | Polymer composite compositions |
| WO2002000870A2 (en) | 2000-06-26 | 2002-01-03 | Christian Plank | Method for transfecting cells using a magnetic field |
| IL138474A0 (en) | 2000-09-14 | 2001-10-31 | Epox Ltd | Highly branched water-soluble polyamine oligomers, process for their preparation and applications thereof |
| USRE43612E1 (en) | 2000-10-10 | 2012-08-28 | Massachusetts Institute Of Technology | Biodegradable poly(β-amino esters) and uses thereof |
| US6998115B2 (en) | 2000-10-10 | 2006-02-14 | Massachusetts Institute Of Technology | Biodegradable poly(β-amino esters) and uses thereof |
| US7427394B2 (en) | 2000-10-10 | 2008-09-23 | Massachusetts Institute Of Technology | Biodegradable poly(β-amino esters) and uses thereof |
| AU2002214854A1 (en) | 2000-10-25 | 2002-05-06 | Inex Pharmaceuticals Corporation | Lipid formulations for target delivery |
| GB0028361D0 (en) | 2000-11-21 | 2001-01-03 | Glaxo Group Ltd | Method of separating extra chromosomal dna from other cellular components |
| US20020094528A1 (en) | 2000-11-29 | 2002-07-18 | Salafsky Joshua S. | Method and apparatus using a surface-selective nonlinear optical technique for detection of probe-target interations |
| JP2002167368A (en) | 2000-12-01 | 2002-06-11 | Nitto Denko Corp | Alkyl-substituted dendrimer and method for producing the same |
| US20050004058A1 (en) | 2000-12-07 | 2005-01-06 | Patrick Benoit | Sequences upstream of the carp gene, vectors containing them and uses thereof |
| DE10109897A1 (en) | 2001-02-21 | 2002-11-07 | Novosom Ag | Optional cationic liposomes and their use |
| US20020192721A1 (en) | 2001-03-28 | 2002-12-19 | Engeneos, Inc. | Modular molecular clasps and uses thereof |
| KR100823815B1 (en) | 2001-04-23 | 2008-04-21 | 신에쓰 가가꾸 고교 가부시끼가이샤 | New tertiary amine compound having ester structure and preparation method thereof |
| US6585410B1 (en) | 2001-05-03 | 2003-07-01 | The United States Of America As Represented By The Administrator Of The National Aeronautics And Space Administration | Radiant temperature nulling radiometer |
| US20030077251A1 (en) | 2001-05-23 | 2003-04-24 | Nicolas Escriou | Replicons derived from positive strand RNA virus genomes useful for the production of heterologous proteins |
| ATE464317T1 (en) | 2001-06-05 | 2010-04-15 | Curevac Gmbh | STABILIZED MRNA WITH INCREASED G/C CONTENT FOR GENE THERAPY |
| EP2390328A1 (en) | 2001-09-28 | 2011-11-30 | Max-Planck-Gesellschaft zur Förderung der Wissenschaften e.V. | MicroRNA molecules |
| US7132295B2 (en) | 2001-11-09 | 2006-11-07 | Bayer Aktiengesellschaft | Isotopically coded affinity markers 3 |
| DE10162480A1 (en) | 2001-12-19 | 2003-08-07 | Ingmar Hoerr | The application of mRNA for use as a therapeutic agent against tumor diseases |
| DE10207178A1 (en) | 2002-02-19 | 2003-09-04 | Novosom Ag | Components for the production of amphoteric liposomes |
| DE10214983A1 (en) * | 2002-04-04 | 2004-04-08 | TransMIT Gesellschaft für Technologietransfer mbH | Nebulisable liposomes and their use for pulmonary application of active substances |
| US20030215395A1 (en) | 2002-05-14 | 2003-11-20 | Lei Yu | Controllably degradable polymeric biomolecule or drug carrier and method of synthesizing said carrier |
| US7601367B2 (en) | 2002-05-28 | 2009-10-13 | Mirus Bio Llc | Compositions and processes using siRNA, amphipathic compounds and polycations |
| US7901708B2 (en) | 2002-06-28 | 2011-03-08 | Protiva Biotherapeutics, Inc. | Liposomal apparatus and manufacturing methods |
| DE10229872A1 (en) | 2002-07-03 | 2004-01-29 | Curevac Gmbh | Immune stimulation through chemically modified RNA |
| US20040028804A1 (en) | 2002-08-07 | 2004-02-12 | Anderson Daniel G. | Production of polymeric microarrays |
| EP1539936A2 (en) | 2002-08-22 | 2005-06-15 | Celltran Limited | Cell culture surface |
| EP1565512B1 (en) | 2002-11-04 | 2018-07-04 | Momentive Performance Materials GmbH | Linear polyamino and/or polyammonium polysiloxane copolymers i |
| CA2506843A1 (en) | 2002-11-22 | 2004-06-10 | Novo-Nordisk A/S | 2,5-diketopiperazines for the treatment of obesity |
| CA2508279A1 (en) | 2002-12-23 | 2004-07-22 | Vical Incorporated | Method for freeze-drying nucleic acid/block copolymer/cationic surfactant complexes |
| US7381422B2 (en) | 2002-12-23 | 2008-06-03 | Vical Incorporated | Method for producing sterile polynucleotide based medicaments |
| ATE471335T1 (en) | 2002-12-23 | 2010-07-15 | Vical Inc | VACCINES AGAINST INFECTIONS WITH THE HUMAN CYTOMEGALIVIRUS BASED ON CODONE-OPTIMIZED POLYNUCLEOTIDES |
| US7169892B2 (en) | 2003-01-10 | 2007-01-30 | Astellas Pharma Inc. | Lipid-peptide-polymer conjugates for long blood circulation and tumor specific drug delivery systems |
| US8054291B2 (en) | 2003-01-20 | 2011-11-08 | Asahi Kasei Emd Corporation | Pointing device |
| TW200427695A (en) | 2003-03-05 | 2004-12-16 | Senesco Technologies Inc | Use of antisense oligonucleotides or sirna to suppress expression of eif-5a1 |
| US20040224912A1 (en) | 2003-05-07 | 2004-11-11 | Isis Pharmaceuticals Inc. | Modulation of PAI-1 mRNA-binding protein expression |
| US7619017B2 (en) | 2003-05-19 | 2009-11-17 | Wacker Chemical Corporation | Polymer emulsions resistant to biodeterioration |
| WO2005007810A2 (en) | 2003-06-16 | 2005-01-27 | Grinstaff Mark W | Functional synthetic molecules and macromolecules for gene delivery |
| US20070003607A1 (en) | 2003-09-02 | 2007-01-04 | Vibhudutta Awasthi | Neutral liposome-encapsulated compounds and methods of making and using thereof |
| AU2004272646B2 (en) | 2003-09-15 | 2011-11-24 | Arbutus Biopharma Corporation | Polyethyleneglycol-modified lipid compounds and uses thereof |
| EP1675943A4 (en) | 2003-09-15 | 2007-12-05 | Massachusetts Inst Technology | NANOLITRE SYNTHESIS OF BIOMATERIALS IN NETWORKS AND SCREENING THEREOF |
| US20050069590A1 (en) | 2003-09-30 | 2005-03-31 | Buehler Gail K. | Stable suspensions for medicinal dosages |
| US8663657B2 (en) | 2003-10-10 | 2014-03-04 | Powderject Vaccines, Inc. | Nucleic acid constructs |
| WO2005037226A2 (en) | 2003-10-17 | 2005-04-28 | Georgia Tech Research Corporation | Genetically engineered enteroendocrine cells for treating glucose-related metabolic disorders |
| WO2005044782A1 (en) | 2003-11-10 | 2005-05-19 | Nippon Kayaku Kabushiki Kaisha | Diimonium salt compound and use thereof |
| CA2549720C (en) * | 2003-12-19 | 2013-10-15 | Chiron Corporation | Cell transfecting formulations of small interfering rna, related compositions and methods of making and use |
| US7022214B2 (en) | 2004-01-21 | 2006-04-04 | Bio-Rad Laboratories, Inc. | Carrier ampholytes of high pH range |
| US7556684B2 (en) | 2004-02-26 | 2009-07-07 | Construction Research & Technology Gmbh | Amine containing strength improvement admixture |
| US20060228404A1 (en) | 2004-03-04 | 2006-10-12 | Anderson Daniel G | Compositions and methods for treatment of hypertrophic tissues |
| US20060024670A1 (en) | 2004-05-18 | 2006-02-02 | Luke Catherine J | Influenza virus vaccine composition and methods of use |
| NZ550085A (en) | 2004-05-19 | 2009-06-26 | Clariant Finance Bvi Ltd | Monoazo dyes |
| EP1766034B1 (en) | 2004-05-21 | 2014-03-19 | Novartis Vaccines and Diagnostics, Inc. | Alphavirus vectors for influenza virus vaccines |
| US7799565B2 (en) | 2004-06-07 | 2010-09-21 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering RNA |
| EP1781593B1 (en) | 2004-06-07 | 2011-12-14 | Protiva Biotherapeutics Inc. | Cationic lipids and methods of use |
| US7670595B2 (en) | 2004-06-28 | 2010-03-02 | Merck Patent Gmbh | Fc-interferon-beta fusion proteins |
| GB0418172D0 (en) | 2004-08-13 | 2004-09-15 | Ic Vec Ltd | Vector |
| DE102004042546A1 (en) | 2004-09-02 | 2006-03-09 | Curevac Gmbh | Combination therapy for immune stimulation |
| DE102004043342A1 (en) | 2004-09-08 | 2006-03-09 | Bayer Materialscience Ag | Blocked polyurethane prepolymers as adhesives |
| EP1807050A1 (en) | 2004-11-05 | 2007-07-18 | Novosom AG | Improvements in or relating to pharmaceutical compositions comprising an oligonucleotide as an active agent |
| US20060134221A1 (en) | 2004-12-03 | 2006-06-22 | Vical Incorporated | Methods for producing block copolymer/amphiphilic particles |
| WO2006074546A1 (en) | 2005-01-13 | 2006-07-20 | Protiva Biotherapeutics, Inc. | Lipid encapsulated interfering rna |
| GB0502482D0 (en) | 2005-02-07 | 2005-03-16 | Glaxo Group Ltd | Novel compounds |
| CA2597724A1 (en) | 2005-02-14 | 2007-08-02 | Sirna Therapeutics, Inc. | Cationic lipids and formulated molecular compositions containing them |
| EP1869106B1 (en) | 2005-03-28 | 2014-06-25 | Dendritic Nanotechnologies, Inc. | Janus dendrimers and dendrons |
| AU2006259415B2 (en) | 2005-06-15 | 2012-08-30 | Massachusetts Institute Of Technology | Amine-containing lipids and uses thereof |
| EP4174179B1 (en) | 2005-08-23 | 2025-05-07 | The Trustees of the University of Pennsylvania | Rna containing modified nucleosides and methods of use thereof |
| US9012219B2 (en) | 2005-08-23 | 2015-04-21 | The Trustees Of The University Of Pennsylvania | RNA preparations comprising purified modified RNA for reprogramming cells |
| WO2007031091A2 (en) | 2005-09-15 | 2007-03-22 | Santaris Pharma A/S | Rna antagonist compounds for the modulation of p21 ras expression |
| EP1945247A1 (en) | 2005-10-18 | 2008-07-23 | Novartis Vaccines and Diagnostics, Inc. | Mucosal and systemic immunizations with alphavirus replicon particles |
| CN101346393B (en) | 2005-11-02 | 2015-07-22 | 普洛体维生物治疗公司 | Modified siRNA molecules and uses thereof |
| WO2007070705A2 (en) | 2005-12-15 | 2007-06-21 | The Trustees Of The University Of Pennsylvania | Cationic lipid-mediated vectors |
| US7238791B1 (en) | 2005-12-16 | 2007-07-03 | Roche Diagnostics Operations, Inc. | 6-monoacetylmorphine derivatives useful in immunoassay |
| US20090221684A1 (en) | 2005-12-22 | 2009-09-03 | Trustees Of Boston University | Molecules for Gene Delivery and Gene Therapy, and Methods of Use Thereof |
| CN100569877C (en) | 2005-12-30 | 2009-12-16 | 财团法人工业技术研究院 | Branched structure compound containing multiple UV crosslinking reactive groups and application thereof |
| US20070281336A1 (en) | 2006-04-14 | 2007-12-06 | Epicentre Technologies | Kits and methods for generating 5' capped RNA |
| US9085778B2 (en) | 2006-05-03 | 2015-07-21 | VL27, Inc. | Exosome transfer of nucleic acids to cells |
| US20070275923A1 (en) | 2006-05-25 | 2007-11-29 | Nastech Pharmaceutical Company Inc. | CATIONIC PEPTIDES FOR siRNA INTRACELLULAR DELIVERY |
| EP2032652A4 (en) | 2006-06-05 | 2011-08-17 | Massachusetts Inst Technology | RETICULATED AND DEGRADABLE POLYMERS AND USES THEREOF |
| US20090186805A1 (en) | 2006-07-06 | 2009-07-23 | Aaron Thomas Tabor | Compositions and Methods for Genetic Modification of Cells Having Cosmetic Function to Enhance Cosmetic Appearance |
| FR2904144A1 (en) | 2006-07-19 | 2008-01-25 | St Microelectronics Rousset | METHOD FOR MANUFACTURING A SEMICONDUCTOR WAFER COMPRISING AN INTEGRATED OPTICAL FILTER |
| ES2293834B1 (en) | 2006-07-20 | 2009-02-16 | Consejo Superior Investig. Cientificas | COMPOSED WITH INHIBITING ACTIVITY OF UBC13-UEV INTERACTIONS, PHARMACEUTICAL COMPOSITIONS THAT INCLUDE IT AND ITS THERAPEUTIC APPLICATIONS. |
| WO2008011561A2 (en) | 2006-07-21 | 2008-01-24 | Massachusetts Institute Of Technology | End-modified poly(beta-amino esters) and uses thereof |
| EP2068886B1 (en) | 2006-10-03 | 2013-09-18 | Tekmira Pharmaceuticals Corporation | Lipid containing formulations |
| JP2010506838A (en) | 2006-10-12 | 2010-03-04 | コペルニクス セラピューティクス インコーポレイテッド | Codon optimized CFTR |
| DE102006051516A1 (en) | 2006-10-31 | 2008-05-08 | Curevac Gmbh | (Base) modified RNA to increase the expression of a protein |
| EP1920765A1 (en) | 2006-11-07 | 2008-05-14 | Medigene AG | Liposome preparation by single-pass process |
| DK2104739T3 (en) | 2006-12-21 | 2013-10-07 | Novozymes Inc | Modified messenger RNA stabilization sequences for expression of genes in bacterial cells |
| DE102007001370A1 (en) | 2007-01-09 | 2008-07-10 | Curevac Gmbh | RNA-encoded antibodies |
| US7700734B2 (en) * | 2007-01-09 | 2010-04-20 | Shu-Wha Lin | Recombinant human factor IX and use thereof |
| US8859229B2 (en) | 2007-02-02 | 2014-10-14 | Yale University | Transient transfection with RNA |
| WO2008113364A2 (en) | 2007-03-20 | 2008-09-25 | Recepticon Aps | Amino derivatives to prevent nephrotoxicity and cancer |
| JP5186126B2 (en) | 2007-03-29 | 2013-04-17 | 公益財団法人地球環境産業技術研究機構 | Novel triazine derivatives, their production and their use as gas separation membranes |
| AU2008242827B2 (en) | 2007-04-18 | 2014-06-05 | Cornerstone Pharmaceuticals, Inc. | Pharmaceutical formulations containing lipoic acid derivatives |
| WO2008137470A1 (en) | 2007-05-01 | 2008-11-13 | Pgr-Solutions | Multi-chain lipophilic polyamines |
| US20090163705A1 (en) | 2007-05-21 | 2009-06-25 | Alnylam Pharmaceuticals, Inc. | Cationic lipids |
| BRPI0815725A2 (en) | 2007-08-23 | 2015-02-10 | Novartis Ag | OLIGONUCLEOTIDE DETECTION METHODS |
| WO2009030254A1 (en) | 2007-09-04 | 2009-03-12 | Curevac Gmbh | Complexes of rna and cationic peptides for transfection and for immunostimulation |
| AU2008308679B2 (en) | 2007-10-02 | 2015-01-29 | Marina Biotech, Inc. | Lipopeptides for delivery of nucleic acids |
| WO2009046739A1 (en) | 2007-10-09 | 2009-04-16 | Curevac Gmbh | Composition for treating prostate cancer (pca) |
| EP2221371B1 (en) | 2007-11-22 | 2013-11-13 | Japan Science and Technology Agency | Translation regulation system in cell or artificial cell model by using low-molecular-weight rna |
| US20090247608A1 (en) | 2007-12-04 | 2009-10-01 | Alnylam Pharmaceuticals, Inc. | Targeting Lipids |
| CA2710713C (en) | 2007-12-27 | 2017-09-19 | Protiva Biotherapeutics, Inc. | Silencing of polo-like kinase expression using interfering rna |
| WO2009120247A2 (en) | 2007-12-27 | 2009-10-01 | The Ohio State University Research Foundation | Lipid nanoparticle compositions and methods of making and using the same |
| WO2009088891A1 (en) | 2008-01-02 | 2009-07-16 | Alnylam Pharmaceuticals, Inc. | Screening method for selected amino lipid-containing compositions |
| EP2224912B1 (en) | 2008-01-02 | 2016-05-11 | TEKMIRA Pharmaceuticals Corporation | Improved compositions and methods for the delivery of nucleic acids |
| WO2009098277A1 (en) | 2008-02-08 | 2009-08-13 | Intercell Ag | Flaviviridae mutants comprising a deletion in the capsid protein for use as vaccines |
| EP3604533A1 (en) | 2008-04-11 | 2020-02-05 | Arbutus Biopharma Corporation | Site-specific delivery of nucleic acids by combining targeting ligands with endosomolytic components |
| JP5475753B2 (en) | 2008-04-15 | 2014-04-16 | プロチバ バイオセラピューティクス インコーポレイティッド | Lipid formulations for nucleic acid delivery |
| US20090263407A1 (en) | 2008-04-16 | 2009-10-22 | Abbott Laboratories | Cationic Lipids and Uses Thereof |
| WO2009127230A1 (en) | 2008-04-16 | 2009-10-22 | Curevac Gmbh | MODIFIED (m)RNA FOR SUPPRESSING OR AVOIDING AN IMMUNOSTIMULATORY RESPONSE AND IMMUNOSUPPRESSIVE COMPOSITION |
| WO2009149182A1 (en) | 2008-06-04 | 2009-12-10 | The Board Of Regents Of The University Of Texas System | Modulation of gene expression through endogenous small rna targeting of gene promoters |
| WO2010009065A2 (en) | 2008-07-15 | 2010-01-21 | Novartis Ag | Amphipathic peptide compositions |
| EP2313085A2 (en) | 2008-07-15 | 2011-04-27 | Novartis AG | Immunogenic amphipathic peptide compositions |
| JP5024216B2 (en) | 2008-07-23 | 2012-09-12 | トヨタ自動車株式会社 | Ignition timing control device and ignition timing control method for internal combustion engine |
| US20100035249A1 (en) | 2008-08-05 | 2010-02-11 | Kabushiki Kaisha Dnaform | Rna sequencing and analysis using solid support |
| JP5492207B2 (en) | 2008-08-27 | 2014-05-14 | ライフ テクノロジーズ コーポレーション | Biological sample processing apparatus and processing method |
| WO2010037408A1 (en) | 2008-09-30 | 2010-04-08 | Curevac Gmbh | Composition comprising a complexed (m)rna and a naked mrna for providing or enhancing an immunostimulatory response in a mammal and uses thereof |
| CA2740000C (en) * | 2008-10-09 | 2017-12-12 | Tekmira Pharmaceuticals Corporation | Improved amino lipids and methods for the delivery of nucleic acids |
| AU2009305639B2 (en) | 2008-10-16 | 2016-06-23 | Marina Biotech, Inc. | Processes and compositions for liposomal and efficient delivery of gene silencing therapeutics |
| US9080211B2 (en) | 2008-10-24 | 2015-07-14 | Epicentre Technologies Corporation | Transposon end compositions and methods for modifying nucleic acids |
| US20120009222A1 (en) | 2008-10-27 | 2012-01-12 | Massachusetts Institute Of Technology | Modulation of the immune response |
| MX353900B (en) | 2008-11-07 | 2018-02-01 | Massachusetts Inst Technology | Aminoalcohol lipidoids and uses thereof. |
| CA2743139C (en) | 2008-11-10 | 2019-04-02 | Alnylam Pharmaceuticals, Inc. | Novel lipids and compositions for the delivery of therapeutics |
| EP2350296A4 (en) | 2008-11-17 | 2013-04-03 | Enzon Pharmaceuticals Inc | Branched cationic lipids for nucleic acids delivery system |
| EP2405921A4 (en) | 2009-01-26 | 2013-05-22 | Protiva Biotherapeutics Inc | Compositions and methods for silencing apolipoprotein c-iii expression |
| EP3243504A1 (en) | 2009-01-29 | 2017-11-15 | Arbutus Biopharma Corporation | Improved lipid formulation |
| US20100222489A1 (en) | 2009-02-27 | 2010-09-02 | Jiang Dayue D | Copolymer composition, membrane article, and methods thereof |
| US20100267806A1 (en) | 2009-03-12 | 2010-10-21 | David Bumcrot | LIPID FORMULATED COMPOSITIONS AND METHODS FOR INHIBITING EXPRESSION OF Eg5 AND VEGF GENES |
| EP2415124B1 (en) | 2009-04-02 | 2017-02-15 | The Siemon Company | Telecommunications patch panel |
| JP5713325B2 (en) | 2009-04-17 | 2015-05-07 | アイシス イノヴェイション リミテッド | Composition for delivery of genetic material |
| US9220682B2 (en) | 2009-04-22 | 2015-12-29 | Emory University | Nanocarrier therapy for treating invasive tumors |
| KR102229618B1 (en) | 2009-05-05 | 2021-03-18 | 알닐람 파마슈티칼스 인코포레이티드 | Lipid compositions |
| NZ622843A (en) | 2009-06-10 | 2015-10-30 | Tekmira Pharmaceuticals Corp | Improved lipid formulation |
| WO2010147992A1 (en) | 2009-06-15 | 2010-12-23 | Alnylam Pharmaceuticals, Inc. | Methods for increasing efficacy of lipid formulated sirna |
| NZ597504A (en) * | 2009-06-15 | 2013-10-25 | Alnylam Pharmaceuticals Inc | Lipid formulated dsrna targeting the pcsk9 gene |
| US8569256B2 (en) | 2009-07-01 | 2013-10-29 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods for the delivery of therapeutic agents |
| CA2767129C (en) | 2009-07-01 | 2015-01-06 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing apolipoprotein b |
| US9018187B2 (en) | 2009-07-01 | 2015-04-28 | Protiva Biotherapeutics, Inc. | Cationic lipids and methods for the delivery of therapeutic agents |
| US20120100207A1 (en) | 2009-07-02 | 2012-04-26 | Konica Minolta Holdings, Inc. | Process for producing liposomes by two-step emulsification method utilizing outer aqueous phase containing specific dispersing agent, process for producing liposome dispersion or dry powder thereof using the process for producing liposomes, and liposome dispersion or dry powder thereof produced thereby |
| WO2011005799A2 (en) | 2009-07-06 | 2011-01-13 | Novartis Ag | Self replicating rna molecules and uses thereof |
| WO2011008974A2 (en) | 2009-07-15 | 2011-01-20 | Novartis Ag | Rsv f protein compositions and methods for making same |
| WO2011011447A1 (en) | 2009-07-20 | 2011-01-27 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing ebola virus gene expression |
| IN2012DN01378A (en) | 2009-07-30 | 2015-06-05 | Salvat Lab Sa | |
| EA022786B1 (en) | 2009-07-31 | 2016-03-31 | Этрис Гмбх | Rna with a combination of unmodified and modified nucleotides for protein expression |
| DE102009043342A1 (en) | 2009-09-29 | 2011-03-31 | Bayer Technology Services Gmbh | Substances for self-organized carriers for the controlled release of an active substance |
| EP2502617B1 (en) | 2009-11-20 | 2019-03-20 | Konica Minolta Holdings, Inc. | Process for production of w/o/w emulsion, process for production of liposome employing the process, and porous membrane for use in the methods |
| RS57314B1 (en) | 2009-12-01 | 2018-08-31 | Translate Bio Inc | Delivery of mrna for the augmentation of proteins and enzymes in human genetic diseases |
| CN114317612B (en) | 2009-12-07 | 2024-10-29 | 宾夕法尼亚州大学信托人 | RNA preparations comprising purified modified RNA for reprogramming cells |
| WO2011069529A1 (en) | 2009-12-09 | 2011-06-16 | Curevac Gmbh | Mannose-containing solution for lyophilization, transfection and/or injection of nucleic acids |
| WO2011075656A1 (en) | 2009-12-18 | 2011-06-23 | The University Of British Columbia | Methods and compositions for delivery of nucleic acids |
| EP2338520A1 (en) | 2009-12-21 | 2011-06-29 | Ludwig Maximilians Universität | Conjugate with targeting ligand and use of same |
| SG10201407996PA (en) | 2009-12-23 | 2015-01-29 | Novartis Ag | Lipids, lipid compositions, and methods of using them |
| AU2014259532B2 (en) | 2009-12-23 | 2016-09-08 | Novartis Ag | Lipids, lipid compositions, and methods of using them |
| EP2569276B1 (en) | 2010-05-12 | 2021-02-24 | Arbutus Biopharma Corporation | Novel cationic lipids and methods of use thereof |
| KR101198715B1 (en) * | 2010-05-14 | 2012-11-13 | 한국생명공학연구원 | Asymmetric liposomes with higher encapsulation efficiency of nucleic acids and hydrophilic anion chemicals |
| KR101967411B1 (en) | 2010-06-03 | 2019-04-10 | 알닐람 파마슈티칼스 인코포레이티드 | Biodegradable lipids for the delivery of active agents |
| CN101863544B (en) | 2010-06-29 | 2011-09-28 | 湖南科技大学 | Cyanuric acid-based heavy metal chelating flocculant and preparation method thereof |
| US9006417B2 (en) | 2010-06-30 | 2015-04-14 | Protiva Biotherapeutics, Inc. | Non-liposomal systems for nucleic acid delivery |
| SI3243526T1 (en) | 2010-07-06 | 2020-02-28 | Glaxosmithkline Biologicals S.A. | RNA delivery to trigger multiple immune pathways |
| EP3449910A1 (en) | 2010-07-06 | 2019-03-06 | GlaxoSmithKline Biologicals S.A. | Cationic oil-in-water emulsions |
| US9770463B2 (en) | 2010-07-06 | 2017-09-26 | Glaxosmithkline Biologicals Sa | Delivery of RNA to different cell types |
| HUE026646T2 (en) | 2010-07-06 | 2016-07-28 | Glaxosmithkline Biologicals Sa | Liposomes with lipids having an advantageous pka-value for rna delivery |
| EP2590676B1 (en) | 2010-07-06 | 2016-08-17 | GlaxoSmithKline Biologicals SA | Virion-like delivery particles for self-replicating rna molecules |
| PL2591114T3 (en) | 2010-07-06 | 2017-08-31 | Glaxosmithkline Biologicals Sa | Immunisation of large mammals with low doses of rna |
| WO2012016184A2 (en) | 2010-07-30 | 2012-02-02 | Alnylam Pharmaceuticals, Inc. | Methods and compositions for delivery of active agents |
| EP3578205A1 (en) | 2010-08-06 | 2019-12-11 | ModernaTX, Inc. | A pharmaceutical formulation comprising engineered nucleic acids and medical use thereof |
| WO2012019630A1 (en) | 2010-08-13 | 2012-02-16 | Curevac Gmbh | Nucleic acid comprising or coding for a histone stem-loop and a poly(a) sequence or a polyadenylation signal for increasing the expression of an encoded protein |
| US9193827B2 (en) | 2010-08-26 | 2015-11-24 | Massachusetts Institute Of Technology | Poly(beta-amino alcohols), their preparation, and uses thereof |
| RS63329B1 (en) | 2010-08-31 | 2022-07-29 | Glaxosmithkline Biologicals Sa | PEGYLATED LIPOSOMES FOR THE DELIVERY OF IMMUNOGENE-ENCODING RNA |
| AU2011295938B2 (en) | 2010-08-31 | 2016-01-14 | Glaxosmithkline Biologicals S.A. | Lipids suitable for liposomal delivery of protein-coding RNA |
| DK4008357T3 (en) | 2010-08-31 | 2023-02-20 | Glaxosmithkline Biologicals Sa | Small liposomes for delivery of immunogen-encoding RNA |
| HUE069586T2 (en) | 2010-10-01 | 2025-03-28 | Modernatx Inc | Modified nucleosides, nucleotides, and nucleic acids, and uses thereof |
| WO2012116715A1 (en) | 2011-03-02 | 2012-09-07 | Curevac Gmbh | Vaccination in newborns and infants |
| WO2012113413A1 (en) | 2011-02-21 | 2012-08-30 | Curevac Gmbh | Vaccine composition comprising complexed immunostimulatory nucleic acids and antigens packaged with disulfide-linked polyethyleneglycol/peptide conjugates |
| WO2012116714A1 (en) | 2011-03-02 | 2012-09-07 | Curevac Gmbh | Vaccination in elderly patients |
| US9238716B2 (en) | 2011-03-28 | 2016-01-19 | Massachusetts Institute Of Technology | Conjugated lipomers and uses thereof |
| WO2012133737A1 (en) | 2011-03-31 | 2012-10-04 | 公益財団法人地球環境産業技術研究機構 | Crosslinkable amine compound, polymer membrane using crosslinkable amine compound, and method for producing polymer membrane |
| JP2014511687A (en) | 2011-03-31 | 2014-05-19 | モデルナ セラピューティクス インコーポレイテッド | Engineered nucleic acid delivery and formulation |
| CA2835428A1 (en) | 2011-05-17 | 2012-11-22 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof for non-human vertebrates |
| EP2532649B1 (en) | 2011-06-07 | 2015-04-08 | Incella GmbH | Amino lipids, their synthesis and uses thereof |
| CN103748078B (en) | 2011-06-08 | 2016-11-09 | 夏尔人类遗传性治疗公司 | cleavable lipid |
| LT2717893T (en) | 2011-06-08 | 2019-08-12 | Translate Bio, Inc. | METHODS OF LIPID NAN PARTICULATE COMPOSITION AND MRNA DELIVERY |
| US9862926B2 (en) | 2011-06-27 | 2018-01-09 | Cellscript, Llc. | Inhibition of innate immune response |
| EP3456316A1 (en) | 2011-07-06 | 2019-03-20 | GlaxoSmithKline Biologicals S.A. | Cationic oil-in-water emulsions |
| EP3508219A1 (en) | 2011-07-06 | 2019-07-10 | GlaxoSmithKline Biologicals S.A. | Self-replicating rna prime - protein boost vaccines |
| EP3332802A1 (en) | 2011-07-06 | 2018-06-13 | GlaxoSmithKline Biologicals SA | Immunogenic combination compositions and uses thereof |
| JP2014520806A (en) | 2011-07-06 | 2014-08-25 | ノバルティス アーゲー | Liposomes with useful N: P ratio for delivery of RNA molecules |
| TR201802662T4 (en) | 2011-07-06 | 2018-03-21 | Glaxosmithkline Biologicals Sa | Oil-in-water emulsions containing nucleic acids. |
| HUE041800T2 (en) | 2011-08-31 | 2019-05-28 | Glaxosmithkline Biologicals Sa | Pegylated liposomes for delivery of immunogen-encoding rna |
| WO2013039857A1 (en) | 2011-09-12 | 2013-03-21 | modeRNA Therapeutics | Engineered nucleic acids and methods of use thereof |
| US9464124B2 (en) | 2011-09-12 | 2016-10-11 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| EP2755693A4 (en) | 2011-09-12 | 2015-05-20 | Moderna Therapeutics Inc | MODIFIED NUCLEIC ACIDS AND METHODS OF USE |
| DK3682905T3 (en) | 2011-10-03 | 2022-02-28 | Modernatx Inc | MODIFIED NUCLEOSIDES, NUCLEOTIDES AND NUCLEIC ACIDS AND USES THEREOF |
| KR20140097143A (en) | 2011-10-05 | 2014-08-06 | 프로티바 바이오쎄라퓨틱스, 인코포레이티드 | Compositions and methods for silencing aldehyde dehydrogenase |
| PE20181541A1 (en) | 2011-10-27 | 2018-09-26 | Massachusetts Inst Technology | DERIVATIVES OF AMINO ACIDS FUNCTIONALIZED IN THE N TERMINAL, CAPABLE OF FORMING DRUG ENCAPSULATING MICROSPHERES |
| EP2791364A4 (en) | 2011-12-14 | 2015-11-11 | Moderna Therapeutics Inc | METHODS OF RESPONSE TO A BIOLOGICAL THREAT |
| US20140343129A1 (en) | 2011-12-14 | 2014-11-20 | Moderna Therapeutics, Inc. | Modified nucleic acids, and acute care uses thereof |
| HRP20220717T1 (en) | 2011-12-16 | 2022-07-22 | Modernatx, Inc. | Modified mrna compositions |
| RU2014129863A (en) | 2011-12-21 | 2016-02-10 | Модерна Терапьютикс, Инк. | WAYS TO INCREASE VITALITY OR INCREASE THE LIFE OF A BODY OR EXPLANATE BODY |
| WO2013101690A1 (en) | 2011-12-29 | 2013-07-04 | modeRNA Therapeutics | Modified mrnas encoding cell-penetrating polypeptides |
| EP3421601B1 (en) | 2011-12-30 | 2019-12-04 | Cellscript, Llc | Making and using in vitro-synthesized ssrna for introducing into mammalian cells to induce a biological or biochemical effect |
| WO2013106496A1 (en) | 2012-01-10 | 2013-07-18 | modeRNA Therapeutics | Methods and compositions for targeting agents into and across the blood-brain barrier |
| WO2013120499A1 (en) | 2012-02-15 | 2013-08-22 | Curevac Gmbh | Nucleic acid comprising or coding for a histone stem-loop and a poly (a) sequence or a polyadenylation signal for increasing the expression of an encoded pathogenic antigen |
| WO2013120497A1 (en) | 2012-02-15 | 2013-08-22 | Curevac Gmbh | Nucleic acid comprising or coding for a histone stem-loop and a poly(a) sequence or a polyadenylation signal for increasing the expression of an encoded therapeutic protein |
| DK2817287T3 (en) | 2012-02-24 | 2019-01-02 | Arbutus Biopharma Corp | TRIALKYL CATIONIC LIPID AND METHODS FOR USING IT |
| MX2019002345A (en) | 2012-03-27 | 2022-08-24 | Curevac Ag | Artificial nucleic acid molecules for improved protein or peptide expression. |
| HK1206645A1 (en) | 2012-03-29 | 2016-01-15 | Shire Human Genetic Therapies, Inc. | Ionizable cationic lipids |
| CA2868030C (en) | 2012-03-29 | 2021-05-25 | Shire Human Genetic Therapies, Inc. | Lipid-derived neutral nanoparticles |
| JP6189415B2 (en) | 2012-04-02 | 2017-08-30 | モデルナティエックス インコーポレイテッドModernaTX,Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| US10501512B2 (en) | 2012-04-02 | 2019-12-10 | Modernatx, Inc. | Modified polynucleotides |
| US20150050354A1 (en) | 2012-04-02 | 2015-02-19 | Moderna Therapeutics, Inc. | Modified polynucleotides for the treatment of otic diseases and conditions |
| US20140275229A1 (en) | 2012-04-02 | 2014-09-18 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding udp glucuronosyltransferase 1 family, polypeptide a1 |
| CN108949772A (en) | 2012-04-02 | 2018-12-07 | 现代泰克斯公司 | For generating the modification polynucleotides of biological agent relevant to human diseases and protein |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| US9572897B2 (en) | 2012-04-02 | 2017-02-21 | Modernatx, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| US20150267192A1 (en) | 2012-06-08 | 2015-09-24 | Shire Human Genetic Therapies, Inc. | Nuclease resistant polynucleotides and uses thereof |
| HK1208618A1 (en) | 2012-06-08 | 2016-03-11 | 夏尔人类遗传性治疗公司 | Pulmonary delivery of mrna to non-lung target cells |
| EP2858677B1 (en) | 2012-06-08 | 2020-08-05 | ethris GmbH | Pulmonary delivery of messenger rna |
| RU2488732C1 (en) | 2012-07-26 | 2013-07-27 | Общество с ограниченной ответственностью "Новые композитные технологии" | Method of making combined pressure pipe |
| EP2882706A1 (en) | 2012-08-13 | 2015-06-17 | Massachusetts Institute of Technology | Amine-containing lipidoids and uses thereof |
| EP2922554B1 (en) | 2012-11-26 | 2022-02-23 | ModernaTX, Inc. | Terminally modified rna |
| US9636301B2 (en) | 2012-12-04 | 2017-05-02 | Arbutus Biopharma Corporation | In vitro release assay for liposome encapsulated vincristine |
| ES2968649T3 (en) | 2012-12-07 | 2024-05-13 | Translate Bio Inc | Lipid nanoparticles for mRNA delivery to the lungs |
| EP2931319B1 (en) | 2012-12-13 | 2019-08-21 | ModernaTX, Inc. | Modified nucleic acid molecules and uses thereof |
| WO2014093574A1 (en) | 2012-12-13 | 2014-06-19 | Moderna Therapeutics, Inc. | Modified polynucleotides for altering cell phenotype |
| JP2016504050A (en) | 2013-01-17 | 2016-02-12 | モデルナ セラピューティクス インコーポレイテッドModerna Therapeutics,Inc. | Signal sensor polynucleotide for modification of cell phenotype |
| US20160022774A1 (en) | 2013-03-12 | 2016-01-28 | Moderna Therapeutics, Inc. | Diagnosis and treatment of fibrosis |
| US20160024181A1 (en) | 2013-03-13 | 2016-01-28 | Moderna Therapeutics, Inc. | Long-lived polynucleotide molecules |
| SMT202100430T1 (en) | 2013-03-14 | 2021-09-14 | Translate Bio Inc | Methods and compositions for delivering mrna coded antibodies |
| EP3467108B1 (en) | 2013-03-14 | 2024-05-22 | Translate Bio, Inc. | Methods for purification of messenger rna |
| HK1219955A1 (en) | 2013-03-14 | 2017-04-21 | Shire Human Genetic Therapies, Inc. | Ribonucleic acids with 4'-thio-modified nucleotides and related methods |
| EP2970940B1 (en) | 2013-03-14 | 2018-07-25 | Translate Bio, Inc. | Mrna therapeutic compositions and use to treat diseases and disorders |
| WO2014152659A1 (en) | 2013-03-14 | 2014-09-25 | Shire Human Genetic Therapies, Inc. | Quantitative assessment for cap efficiency of messenger rna |
| MX393573B (en) | 2013-03-14 | 2025-03-21 | Shire Human Genetic Therapies | Cftr mrna compositions and related methods and uses |
| EP2971010B1 (en) | 2013-03-14 | 2020-06-10 | ModernaTX, Inc. | Formulation and delivery of modified nucleoside, nucleotide, and nucleic acid compositions |
| DK2971098T3 (en) | 2013-03-14 | 2019-02-18 | Translate Bio Inc | QUANTITATIVE ASSESSMENT FOR CAPE EFFECTIVENESS OF MESSENGER RNA |
| US20160032316A1 (en) | 2013-03-14 | 2016-02-04 | The Trustees Of The University Of Pennsylvania | Purification and Purity Assessment of RNA Molecules Synthesized with Modified Nucleosides |
| EP3578663A1 (en) | 2013-03-15 | 2019-12-11 | ModernaTX, Inc. | Manufacturing methods for production of rna transcripts |
| US20160032273A1 (en) | 2013-03-15 | 2016-02-04 | Moderna Therapeutics, Inc. | Characterization of mrna molecules |
| EP4614148A3 (en) | 2013-03-15 | 2025-12-17 | Translate Bio, Inc. | Synergistic enhancement of the delivery of nucleic acids via blended formulations |
| EP2971165A4 (en) | 2013-03-15 | 2016-11-23 | Moderna Therapeutics Inc | ELIMINATING DNA FRAGMENTS IN METHODS OF PRODUCING MRNA |
| EP3578652B1 (en) | 2013-03-15 | 2023-07-12 | ModernaTX, Inc. | Ribonucleic acid purification |
| US8980864B2 (en) | 2013-03-15 | 2015-03-17 | Moderna Therapeutics, Inc. | Compositions and methods of altering cholesterol levels |
| US20160017313A1 (en) | 2013-03-15 | 2016-01-21 | Moderna Therapeutics, Inc. | Analysis of mrna heterogeneity and stability |
| WO2014144767A1 (en) | 2013-03-15 | 2014-09-18 | Moderna Therapeutics, Inc. | Ion exchange purification of mrna |
| WO2014179562A1 (en) | 2013-05-01 | 2014-11-06 | Massachusetts Institute Of Technology | 1,3,5-triazinane-2,4,6-trione derivatives and uses thereof |
| WO2014210356A1 (en) | 2013-06-26 | 2014-12-31 | Massachusetts Institute Of Technology | Multi-tailed lipids and uses thereof |
| SI3019619T1 (en) | 2013-07-11 | 2021-12-31 | Modernatx, Inc. | Compositions comprising synthetic polynucleotides encoding crispr related proteins and synthetic sgrnas and methods of use |
| EP3677567A1 (en) | 2013-07-23 | 2020-07-08 | Arbutus Biopharma Corporation | Compositions and methods for delivering messenger rna |
| BR112016003361A2 (en) | 2013-08-21 | 2017-11-21 | Curevac Ag | respiratory syncytial virus vaccine (rsv) |
| JP2016530294A (en) | 2013-09-03 | 2016-09-29 | モデルナ セラピューティクス インコーポレイテッドModerna Therapeutics,Inc. | Chimeric polynucleotide |
| EP3041938A1 (en) | 2013-09-03 | 2016-07-13 | Moderna Therapeutics, Inc. | Circular polynucleotides |
| EP3052106A4 (en) | 2013-09-30 | 2017-07-19 | ModernaTX, Inc. | Polynucleotides encoding immune modulating polypeptides |
| EP3052511A4 (en) | 2013-10-02 | 2017-05-31 | Moderna Therapeutics, Inc. | Polynucleotide molecules and uses thereof |
| EP3052479A4 (en) | 2013-10-02 | 2017-10-25 | Moderna Therapeutics, Inc. | Polynucleotide molecules and uses thereof |
| US20160243221A1 (en) | 2013-10-18 | 2016-08-25 | Moderna Therapeutics, Inc. | Compositions and methods for tolerizing cellular systems |
| WO2015061461A1 (en) | 2013-10-22 | 2015-04-30 | Shire Human Genetic Therapies, Inc. | Cns delivery of mrna and uses thereof |
| EA201992208A1 (en) | 2013-10-22 | 2020-07-31 | Транслейт Био, Инк. | TREATMENT OF PHENYLKETONURIA USING mRNA |
| CN105813656B (en) * | 2013-10-22 | 2021-01-15 | 夏尔人类遗传性治疗公司 | Lipid formulations for delivery of messenger RNA |
| AU2014340092B2 (en) | 2013-10-22 | 2019-09-19 | Translate Bio, Inc. | mRNA therapy for Argininosuccinate Synthetase Deficiency |
| US20170173128A1 (en) | 2013-12-06 | 2017-06-22 | Moderna TX, Inc. | Targeted adaptive vaccines |
| US20150167017A1 (en) | 2013-12-13 | 2015-06-18 | Moderna Therapeutics, Inc. | Alternative nucleic acid molecules and uses thereof |
| CN105874072A (en) | 2013-12-30 | 2016-08-17 | 库瑞瓦格股份公司 | Artificial nucleic acid molecules |
| US20170002060A1 (en) | 2014-01-08 | 2017-01-05 | Moderna Therapeutics, Inc. | Polynucleotides for the in vivo production of antibodies |
| EP3590529A1 (en) | 2014-03-12 | 2020-01-08 | CureVac AG | Combination of vaccination and ox40 agonists |
| WO2015154188A1 (en) | 2014-04-09 | 2015-10-15 | The University Of British Columbia | Drill cover and chuck mechanism |
| HUE069802T2 (en) | 2014-04-23 | 2025-04-28 | Modernatx Inc | Nucleic acid vaccines |
| EP3521456B1 (en) | 2014-06-10 | 2023-01-04 | CureVac Manufacturing GmbH | Methods and means for enhancing rna production |
| WO2015196128A2 (en) | 2014-06-19 | 2015-12-23 | Moderna Therapeutics, Inc. | Alternative nucleic acid molecules and uses thereof |
| US20170175129A1 (en) | 2014-06-19 | 2017-06-22 | Moderna Therapeutics, Inc. | Alternative nucleic acid molecules and uses thereof |
| ES2931832T3 (en) | 2014-06-25 | 2023-01-03 | Acuitas Therapeutics Inc | Novel lipids and lipid nanoparticle formulations for nucleic acid delivery |
| WO2016005004A1 (en) | 2014-07-11 | 2016-01-14 | Biontech Rna Pharmaceuticals Gmbh | Stabilization of poly(a) sequence encoding dna sequences |
| EP3169334B1 (en) | 2014-07-16 | 2021-04-28 | Ethris GmbH | Rna for use in the treatment of ligament or tendon lesions |
| CA2963271A1 (en) | 2014-10-02 | 2016-04-07 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing hepatitis b virus gene expression |
| WO2016071857A1 (en) | 2014-11-07 | 2016-05-12 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing ebola virus expression |
| WO2016077123A1 (en) | 2014-11-10 | 2016-05-19 | Moderna Therapeutics, Inc. | Multiparametric nucleic acid optimization |
| EP3461904A1 (en) | 2014-11-10 | 2019-04-03 | ModernaTX, Inc. | Alternative nucleic acid molecules containing reduced uracil content and uses thereof |
| US20180000953A1 (en) | 2015-01-21 | 2018-01-04 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| WO2016118725A1 (en) | 2015-01-23 | 2016-07-28 | Moderna Therapeutics, Inc. | Lipid nanoparticle compositions |
| US20180245077A1 (en) | 2015-03-20 | 2018-08-30 | Protiva Biotherapeutics, Inc. | Compositions and methods for treating hypertriglyceridemia |
| WO2016164762A1 (en) | 2015-04-08 | 2016-10-13 | Moderna Therapeutics, Inc. | Polynucleotides encoding low density lipoprotein receptor egf-a and intracellular domain mutants and methods of using the same |
| WO2016183366A2 (en) | 2015-05-12 | 2016-11-17 | Protiva Biotherapeutics, Inc. | Compositions and methods for silencing expression of hepatitis d virus rna |
| WO2016197132A1 (en) | 2015-06-04 | 2016-12-08 | Protiva Biotherapeutics Inc. | Treating hepatitis b virus infection using crispr |
| WO2016197133A1 (en) | 2015-06-04 | 2016-12-08 | Protiva Biotherapeutics, Inc. | Delivering crispr therapeutics with lipid nanoparticles |
| WO2016201377A1 (en) | 2015-06-10 | 2016-12-15 | Moderna Therapeutics, Inc. | Targeted adaptive vaccines |
| PT3313829T (en) | 2015-06-29 | 2024-07-08 | Acuitas Therapeutics Inc | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| CN108350455A (en) | 2015-07-29 | 2018-07-31 | 阿布特斯生物制药公司 | Composition for making hepatitis B virus silenced gene expression and method |
| WO2017049286A1 (en) | 2015-09-17 | 2017-03-23 | Moderna Therapeutics, Inc. | Polynucleotides containing a morpholino linker |
| AU2016324463B2 (en) | 2015-09-17 | 2022-10-27 | Modernatx, Inc. | Polynucleotides containing a stabilizing tail region |
| WO2017049074A1 (en) | 2015-09-18 | 2017-03-23 | Moderna Therapeutics, Inc. | Polynucleotide formulations for use in the treatment of renal diseases |
| PT3368507T (en) | 2015-10-28 | 2023-02-07 | Acuitas Therapeutics Inc | NEW LIPIDS AND LIPID NANOPARTICLE FORMULATIONS FOR NUCLEIC ACIDS DELIVERY |
| WO2017102010A1 (en) | 2015-12-17 | 2017-06-22 | Biontech Rna Pharmaceuticals Gmbh | Novel cytokine fusion proteins |
| US20180371392A1 (en) | 2015-12-21 | 2018-12-27 | Curevac Ag | Inlay for a culture plate and corresponding method for preparing a culture plate system with such inlay |
| EP3701963A1 (en) | 2015-12-22 | 2020-09-02 | CureVac AG | Method for producing rna molecule compositions |
| US11248223B2 (en) | 2015-12-23 | 2022-02-15 | Curevac Ag | Method of RNA in vitro transcription using a buffer containing a dicarboxylic acid or tricarboxylic acid or a salt thereof |
| EP3397613A1 (en) | 2015-12-30 | 2018-11-07 | Acuitas Therapeutics Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| US9834510B2 (en) | 2015-12-30 | 2017-12-05 | Arcturus Therapeutics, Inc. | Aromatic ionizable cationic lipid |
| MX2020011166A (en) | 2018-04-25 | 2022-10-19 | Ethris Gmbh | Cryoprotective agents for particulate formulations. |
| WO2022137133A1 (en) | 2020-12-22 | 2022-06-30 | Curevac Ag | Rna vaccine against sars-cov-2 variants |
-
2012
- 2012-06-08 LT LTEP12728007.1T patent/LT2717893T/en unknown
- 2012-06-08 RS RS20190937A patent/RS59037B1/en unknown
- 2012-06-08 BR BR112013031553-9A patent/BR112013031553A2/en not_active IP Right Cessation
- 2012-06-08 ME MEP-2019-205A patent/ME03491B/en unknown
- 2012-06-08 IL IL307511A patent/IL307511A/en unknown
- 2012-06-08 KR KR1020227003899A patent/KR20220025112A/en not_active Ceased
- 2012-06-08 JP JP2014514907A patent/JP6184945B2/en active Active
- 2012-06-08 KR KR1020147000142A patent/KR102128248B1/en active Active
- 2012-06-08 CN CN202010554844.6A patent/CN111671919A/en active Pending
- 2012-06-08 CA CA3107288A patent/CA3107288A1/en active Pending
- 2012-06-08 HU HUE12728007 patent/HUE044277T2/en unknown
- 2012-06-08 TR TR2019/10686T patent/TR201910686T4/en unknown
- 2012-06-08 AU AU2012267531A patent/AU2012267531B2/en active Active
- 2012-06-08 EP EP22152288.1A patent/EP4043025A1/en active Pending
- 2012-06-08 RU RU2013154295/15A patent/RU2013154295A/en unknown
- 2012-06-08 KR KR1020247029172A patent/KR20240135871A/en active Pending
- 2012-06-08 PL PL19172992T patent/PL3586861T3/en unknown
- 2012-06-08 EP EP24197314.8A patent/EP4458350B1/en active Active
- 2012-06-08 EP EP19172992.0A patent/EP3586861B1/en active Active
- 2012-06-08 ES ES12728007T patent/ES2740248T3/en active Active
- 2012-06-08 PT PT191729920T patent/PT3586861T/en unknown
- 2012-06-08 PL PL12728007T patent/PL2717893T3/en unknown
- 2012-06-08 ES ES19172992T patent/ES2911653T3/en active Active
- 2012-06-08 HK HK14112735.9A patent/HK1199206A1/en unknown
- 2012-06-08 KR KR1020207018197A patent/KR20200084048A/en not_active Ceased
- 2012-06-08 SM SM20190445T patent/SMT201900445T1/en unknown
- 2012-06-08 DK DK12728007.1T patent/DK2717893T3/en active
- 2012-06-08 EP EP12728007.1A patent/EP2717893B1/en active Active
- 2012-06-08 PT PT12728007T patent/PT2717893T/en unknown
- 2012-06-08 NZ NZ719345A patent/NZ719345A/en unknown
- 2012-06-08 MX MX2013014419A patent/MX367605B/en active IP Right Grant
- 2012-06-08 US US14/124,608 patent/US20140206753A1/en not_active Abandoned
- 2012-06-08 CA CA2838069A patent/CA2838069C/en active Active
- 2012-06-08 WO PCT/US2012/041724 patent/WO2012170930A1/en not_active Ceased
- 2012-06-08 MX MX2019010226A patent/MX421064B/en unknown
- 2012-06-08 CN CN201280036309.5A patent/CN103906527B/en active Active
- 2012-06-08 DK DK19172992.0T patent/DK3586861T3/en active
- 2012-06-08 SI SI201231638T patent/SI2717893T1/en unknown
- 2012-06-08 CN CN202010553959.3A patent/CN111671918A/en active Pending
- 2012-06-08 IL IL280771A patent/IL280771B2/en unknown
- 2012-06-08 PE PE2013002767A patent/PE20140797A1/en not_active Application Discontinuation
- 2012-06-08 HR HRP20191338TT patent/HRP20191338T1/en unknown
-
2013
- 2013-11-28 IL IL229699A patent/IL229699B/en active IP Right Grant
- 2013-12-04 CL CL2013003478A patent/CL2013003478A1/en unknown
-
2014
- 2014-06-18 US US14/308,554 patent/US9597413B2/en active Active
- 2014-06-18 US US14/308,546 patent/US9308281B2/en active Active
-
2016
- 2016-10-12 JP JP2016200575A patent/JP6372042B2/en active Active
-
2017
- 2017-04-07 US US15/482,117 patent/US10238754B2/en active Active
- 2017-06-30 AU AU2017204509A patent/AU2017204509C1/en active Active
- 2017-08-24 JP JP2017161030A patent/JP6463810B2/en active Active
-
2018
- 2018-12-26 US US16/233,031 patent/US10413618B2/en active Active
-
2019
- 2019-01-04 JP JP2019000252A patent/JP6752299B2/en active Active
- 2019-02-26 US US16/286,400 patent/US10350303B1/en active Active
- 2019-03-20 AU AU2019201924A patent/AU2019201924B2/en active Active
- 2019-07-03 US US16/502,672 patent/US10507249B2/en active Active
- 2019-07-30 CY CY20191100812T patent/CY1122123T1/en unknown
- 2019-11-12 US US16/681,565 patent/US20200085973A1/en not_active Abandoned
-
2020
- 2020-07-16 US US16/931,231 patent/US10888626B2/en active Active
- 2020-08-18 JP JP2020137929A patent/JP7645058B2/en active Active
- 2020-11-16 US US17/099,462 patent/US11052159B2/en active Active
-
2021
- 2021-06-07 US US17/341,016 patent/US11185595B2/en active Active
- 2021-07-01 AU AU2021204597A patent/AU2021204597A1/en not_active Abandoned
- 2021-10-25 US US17/509,674 patent/US11291734B2/en active Active
- 2021-12-20 US US17/556,978 patent/US20220111071A1/en not_active Abandoned
- 2021-12-20 US US17/556,573 patent/US11547764B2/en active Active
- 2021-12-20 US US17/556,974 patent/US11338044B2/en active Active
- 2021-12-21 US US17/558,310 patent/US11730825B2/en active Active
-
2022
- 2022-06-03 US US17/832,196 patent/US12121592B2/en active Active
- 2022-06-03 US US17/832,202 patent/US20220362406A1/en not_active Abandoned
-
2023
- 2023-04-03 US US18/295,152 patent/US11951180B2/en active Active
- 2023-04-03 US US18/295,139 patent/US20230293723A1/en not_active Abandoned
- 2023-04-03 US US18/295,159 patent/US11951181B2/en active Active
- 2023-04-03 US US18/295,150 patent/US11951179B2/en active Active
- 2023-05-08 JP JP2023076542A patent/JP2023087094A/en active Pending
-
2024
- 2024-08-15 US US18/806,139 patent/US20250108126A1/en active Pending
- 2024-12-11 AU AU2024278311A patent/AU2024278311A1/en active Pending
-
2025
- 2025-01-17 US US19/027,351 patent/US20250152735A1/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150004217A1 (en) * | 2010-11-30 | 2015-01-01 | Shire Human Genetic Therapies, Inc. | mRNA FOR USE IN TREATMENT OF HUMAN GENETIC DISEASES |
Non-Patent Citations (2)
| Title |
|---|
| http://en.wikipedia.org/wiki/Blood_proteins, downloaded 5/17/15, Entitled "Blood Proteins", authors unknown, published by WikiPedia, San Francisco, CA, 2 pages long. * |
| Kariko, et al. (2008) "Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability", Molecular Therapy, 16(11): 1833-40. * |
Cited By (278)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10576166B2 (en) | 2009-12-01 | 2020-03-03 | Translate Bio, Inc. | Liver specific delivery of messenger RNA |
| US10143758B2 (en) | 2009-12-01 | 2018-12-04 | Translate Bio, Inc. | Liver specific delivery of messenger RNA |
| US9937233B2 (en) | 2010-08-06 | 2018-04-10 | Modernatx, Inc. | Engineered nucleic acids and methods of use thereof |
| US9181319B2 (en) | 2010-08-06 | 2015-11-10 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| US9447164B2 (en) | 2010-08-06 | 2016-09-20 | Moderna Therapeutics, Inc. | Engineered nucleic acids and methods of use thereof |
| US9701965B2 (en) | 2010-10-01 | 2017-07-11 | Modernatx, Inc. | Engineered nucleic acids and methods of use thereof |
| US11135274B2 (en) | 2010-11-30 | 2021-10-05 | Translate Bio, Inc. | MRNA for use in treatment of human genetic diseases |
| US9061021B2 (en) | 2010-11-30 | 2015-06-23 | Shire Human Genetic Therapies, Inc. | mRNA for use in treatment of human genetic diseases |
| US9956271B2 (en) | 2010-11-30 | 2018-05-01 | Translate Bio, Inc. | mRNA for use in treatment of human genetic diseases |
| US9533047B2 (en) | 2011-03-31 | 2017-01-03 | Modernatx, Inc. | Delivery and formulation of engineered nucleic acids |
| US9950068B2 (en) | 2011-03-31 | 2018-04-24 | Modernatx, Inc. | Delivery and formulation of engineered nucleic acids |
| US20140199233A1 (en) * | 2011-05-11 | 2014-07-17 | The Regents Of The University Of California | Enhanced Growth Inhibition of Osteosarcoma by Cytotoxic Polymerized Liposomal Nanoparticles Targeting the Alcam Cell Surface Receptor |
| US20140294938A1 (en) * | 2011-06-08 | 2014-10-02 | Shire Human Genetic Therapies, Inc. | Mrna therapy for fabry disease |
| US12121592B2 (en) | 2011-06-08 | 2024-10-22 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US10507249B2 (en) | 2011-06-08 | 2019-12-17 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US10888626B2 (en) | 2011-06-08 | 2021-01-12 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US10507183B2 (en) | 2011-06-08 | 2019-12-17 | Translate Bio, Inc. | Cleavable lipids |
| US11052159B2 (en) | 2011-06-08 | 2021-07-06 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US10238754B2 (en) | 2011-06-08 | 2019-03-26 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for MRNA delivery |
| US10350303B1 (en) | 2011-06-08 | 2019-07-16 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US11547764B2 (en) | 2011-06-08 | 2023-01-10 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for MRNA delivery |
| US11185595B2 (en) | 2011-06-08 | 2021-11-30 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US9308281B2 (en) * | 2011-06-08 | 2016-04-12 | Shire Human Genetic Therapies, Inc. | MRNA therapy for Fabry disease |
| US10702478B2 (en) | 2011-06-08 | 2020-07-07 | Translate Bio, Inc. | Cleavable lipids |
| US12102720B2 (en) | 2011-06-08 | 2024-10-01 | Translate Bio, Inc. | Cleavable lipids |
| US11951179B2 (en) | 2011-06-08 | 2024-04-09 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for MRNA delivery |
| US10413618B2 (en) | 2011-06-08 | 2019-09-17 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US11338044B2 (en) | 2011-06-08 | 2022-05-24 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US11234936B2 (en) | 2011-06-08 | 2022-02-01 | Translate Bio, Inc. | Cleavable lipids |
| US11951181B2 (en) | 2011-06-08 | 2024-04-09 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US9597413B2 (en) | 2011-06-08 | 2017-03-21 | Shire Human Genetic Therapies, Inc. | Pulmonary delivery of mRNA |
| US11951180B2 (en) | 2011-06-08 | 2024-04-09 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for MRNA delivery |
| US11730825B2 (en) | 2011-06-08 | 2023-08-22 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US11291734B2 (en) | 2011-06-08 | 2022-04-05 | Translate Bio, Inc. | Lipid nanoparticle compositions and methods for mRNA delivery |
| US9186372B2 (en) | 2011-12-16 | 2015-11-17 | Moderna Therapeutics, Inc. | Split dose administration |
| US9295689B2 (en) | 2011-12-16 | 2016-03-29 | Moderna Therapeutics, Inc. | Formulation and delivery of PLGA microspheres |
| US9572897B2 (en) | 2012-04-02 | 2017-02-21 | Modernatx, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| US9675668B2 (en) | 2012-04-02 | 2017-06-13 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding hepatitis A virus cellular receptor 2 |
| US9782462B2 (en) | 2012-04-02 | 2017-10-10 | Modernatx, Inc. | Modified polynucleotides for the production of proteins associated with human disease |
| US9587003B2 (en) | 2012-04-02 | 2017-03-07 | Modernatx, Inc. | Modified polynucleotides for the production of oncology-related proteins and peptides |
| US9814760B2 (en) | 2012-04-02 | 2017-11-14 | Modernatx, Inc. | Modified polynucleotides for the production of biologics and proteins associated with human disease |
| US9301993B2 (en) | 2012-04-02 | 2016-04-05 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding apoptosis inducing factor 1 |
| US9827332B2 (en) | 2012-04-02 | 2017-11-28 | Modernatx, Inc. | Modified polynucleotides for the production of proteins |
| US9828416B2 (en) | 2012-04-02 | 2017-11-28 | Modernatx, Inc. | Modified polynucleotides for the production of secreted proteins |
| US9303079B2 (en) | 2012-04-02 | 2016-04-05 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of cytoplasmic and cytoskeletal proteins |
| US9878056B2 (en) | 2012-04-02 | 2018-01-30 | Modernatx, Inc. | Modified polynucleotides for the production of cosmetic proteins and peptides |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| US9254311B2 (en) | 2012-04-02 | 2016-02-09 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of proteins |
| US9255129B2 (en) | 2012-04-02 | 2016-02-09 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding SIAH E3 ubiquitin protein ligase 1 |
| US9233141B2 (en) | 2012-04-02 | 2016-01-12 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of proteins associated with blood and lymphatic disorders |
| US9221891B2 (en) | 2012-04-02 | 2015-12-29 | Moderna Therapeutics, Inc. | In vivo production of proteins |
| US9220792B2 (en) | 2012-04-02 | 2015-12-29 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding aquaporin-5 |
| US9220755B2 (en) | 2012-04-02 | 2015-12-29 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of proteins associated with blood and lymphatic disorders |
| US9216205B2 (en) | 2012-04-02 | 2015-12-22 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding granulysin |
| US9192651B2 (en) | 2012-04-02 | 2015-11-24 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of secreted proteins |
| US9149506B2 (en) | 2012-04-02 | 2015-10-06 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding septin-4 |
| US9114113B2 (en) | 2012-04-02 | 2015-08-25 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding citeD4 |
| US9107886B2 (en) | 2012-04-02 | 2015-08-18 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding basic helix-loop-helix family member E41 |
| US9095552B2 (en) | 2012-04-02 | 2015-08-04 | Moderna Therapeutics, Inc. | Modified polynucleotides encoding copper metabolism (MURR1) domain containing 1 |
| US10245229B2 (en) | 2012-06-08 | 2019-04-02 | Translate Bio, Inc. | Pulmonary delivery of mRNA to non-lung target cells |
| US11254936B2 (en) | 2012-06-08 | 2022-02-22 | Translate Bio, Inc. | Nuclease resistant polynucleotides and uses thereof |
| US11090264B2 (en) | 2012-06-08 | 2021-08-17 | Translate Bio, Inc. | Pulmonary delivery of mRNA to non-lung target cells |
| US9512456B2 (en) | 2012-08-14 | 2016-12-06 | Modernatx, Inc. | Enzymes and polymerases for the synthesis of RNA |
| US9597380B2 (en) | 2012-11-26 | 2017-03-21 | Modernatx, Inc. | Terminally modified RNA |
| US11603399B2 (en) | 2013-03-13 | 2023-03-14 | Modernatx, Inc. | Long-lived polynucleotide molecules |
| US11510937B2 (en) | 2013-03-14 | 2022-11-29 | Translate Bio, Inc. | CFTR MRNA compositions and related methods and uses |
| US10258698B2 (en) | 2013-03-14 | 2019-04-16 | Modernatx, Inc. | Formulation and delivery of modified nucleoside, nucleotide, and nucleic acid compositions |
| US10899830B2 (en) | 2013-03-14 | 2021-01-26 | Translate Bio, Inc. | Methods and compositions for delivering MRNA coded antibodies |
| US12234446B2 (en) | 2013-03-14 | 2025-02-25 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US9713626B2 (en) | 2013-03-14 | 2017-07-25 | Rana Therapeutics, Inc. | CFTR mRNA compositions and related methods and uses |
| US10087247B2 (en) | 2013-03-14 | 2018-10-02 | Translate Bio, Inc. | Methods and compositions for delivering mRNA coded antibodies |
| US9957499B2 (en) | 2013-03-14 | 2018-05-01 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US10584165B2 (en) | 2013-03-14 | 2020-03-10 | Translate Bio, Inc. | Methods and compositions for delivering mRNA coded antibodies |
| US10876104B2 (en) | 2013-03-14 | 2020-12-29 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US11820977B2 (en) | 2013-03-14 | 2023-11-21 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US9181321B2 (en) | 2013-03-14 | 2015-11-10 | Shire Human Genetic Therapies, Inc. | CFTR mRNA compositions and related methods and uses |
| US11692189B2 (en) | 2013-03-14 | 2023-07-04 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US10420791B2 (en) | 2013-03-14 | 2019-09-24 | Translate Bio, Inc. | CFTR MRNA compositions and related methods and uses |
| US10646504B2 (en) | 2013-03-15 | 2020-05-12 | Translate Bio, Inc. | Synergistic enhancement of the delivery of nucleic acids via blended formulations |
| US10130649B2 (en) | 2013-03-15 | 2018-11-20 | Translate Bio, Inc. | Synergistic enhancement of the delivery of nucleic acids via blended formulations |
| US12453739B2 (en) | 2013-03-15 | 2025-10-28 | Translate Bio, Inc. | Synergistic enhancement of the delivery of nucleic acids via blended formulations |
| US10815291B2 (en) | 2013-09-30 | 2020-10-27 | Modernatx, Inc. | Polynucleotides encoding immune modulating polypeptides |
| US10023626B2 (en) | 2013-09-30 | 2018-07-17 | Modernatx, Inc. | Polynucleotides encoding immune modulating polypeptides |
| US10323076B2 (en) | 2013-10-03 | 2019-06-18 | Modernatx, Inc. | Polynucleotides encoding low density lipoprotein receptor |
| US12509666B2 (en) | 2013-10-22 | 2025-12-30 | Translate Bio, Inc. | MRNA therapy for phenylketonuria |
| US12016954B2 (en) | 2013-10-22 | 2024-06-25 | Translate Bio, Inc. | CNS delivery of mRNA and uses thereof |
| US11890377B2 (en) | 2013-10-22 | 2024-02-06 | Translate Bio, Inc. | Lipid formulations for delivery of messenger RNA |
| US10959953B2 (en) | 2013-10-22 | 2021-03-30 | Translate Bio, Inc. | Lipid formulations for delivery of messenger RNA |
| US10493031B2 (en) | 2013-10-22 | 2019-12-03 | Translate Bio, Inc. | Lipid formulations for delivery of messenger RNA |
| US11377642B2 (en) | 2013-10-22 | 2022-07-05 | Translate Bio, Inc. | mRNA therapy for phenylketonuria |
| US9522176B2 (en) | 2013-10-22 | 2016-12-20 | Shire Human Genetic Therapies, Inc. | MRNA therapy for phenylketonuria |
| US10052284B2 (en) | 2013-10-22 | 2018-08-21 | Translate Bio, Inc. | Lipid formulations for delivery of messenger RNA |
| US10208295B2 (en) | 2013-10-22 | 2019-02-19 | Translate Bio, Inc. | MRNA therapy for phenylketonuria |
| US11224642B2 (en) | 2013-10-22 | 2022-01-18 | Translate Bio, Inc. | MRNA therapy for argininosuccinate synthetase deficiency |
| US10780052B2 (en) | 2013-10-22 | 2020-09-22 | Translate Bio, Inc. | CNS delivery of MRNA and uses thereof |
| US9629804B2 (en) | 2013-10-22 | 2017-04-25 | Shire Human Genetic Therapies, Inc. | Lipid formulations for delivery of messenger RNA |
| US12274743B2 (en) | 2014-04-23 | 2025-04-15 | Modernatx, Inc. | Nucleic acid vaccines |
| US10709779B2 (en) | 2014-04-23 | 2020-07-14 | Modernatx, Inc. | Nucleic acid vaccines |
| US12329812B2 (en) | 2014-04-23 | 2025-06-17 | Modernatx, Inc. | Nucleic acid vaccines |
| US11059841B2 (en) | 2014-04-25 | 2021-07-13 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US9850269B2 (en) | 2014-04-25 | 2017-12-26 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US11884692B2 (en) | 2014-04-25 | 2024-01-30 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US12060381B2 (en) | 2014-04-25 | 2024-08-13 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US10155785B2 (en) | 2014-04-25 | 2018-12-18 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US10022455B2 (en) | 2014-05-30 | 2018-07-17 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US10286083B2 (en) | 2014-05-30 | 2019-05-14 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US10286082B2 (en) | 2014-05-30 | 2019-05-14 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US10293057B2 (en) | 2014-05-30 | 2019-05-21 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US11433144B2 (en) | 2014-05-30 | 2022-09-06 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US10912844B2 (en) | 2014-05-30 | 2021-02-09 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US10493166B2 (en) | 2014-05-30 | 2019-12-03 | Translate Bio, Inc. | Biodegradable lipids for delivery of nucleic acids |
| US11104652B2 (en) | 2014-06-24 | 2021-08-31 | Translate Bio, Inc. | Stereochemically enriched compositions for delivery of nucleic acids |
| US10138213B2 (en) | 2014-06-24 | 2018-11-27 | Translate Bio, Inc. | Stereochemically enriched compositions for delivery of nucleic acids |
| US9668980B2 (en) | 2014-07-02 | 2017-06-06 | Rana Therapeutics, Inc. | Encapsulation of messenger RNA |
| US10864267B2 (en) | 2014-12-05 | 2020-12-15 | Translate Bio, Inc. | Messenger RNA therapy for treatment of articular disease |
| US11998601B2 (en) | 2014-12-05 | 2024-06-04 | Translate Bio, Inc. | Messenger RNA therapy for treatment of articular disease |
| US9943595B2 (en) | 2014-12-05 | 2018-04-17 | Translate Bio, Inc. | Messenger RNA therapy for treatment of articular disease |
| US11219634B2 (en) | 2015-01-21 | 2022-01-11 | Genevant Sciences Gmbh | Methods, compositions, and systems for delivering therapeutic and diagnostic agents into cells |
| US11712463B2 (en) | 2015-03-19 | 2023-08-01 | Translate Bio, Inc. | MRNA therapy for pompe disease |
| US11090368B2 (en) | 2015-03-19 | 2021-08-17 | Translate Bio, Inc. | MRNA therapy for Pompe disease |
| US10172924B2 (en) | 2015-03-19 | 2019-01-08 | Translate Bio, Inc. | MRNA therapy for pompe disease |
| US11103596B2 (en) * | 2015-05-11 | 2021-08-31 | Ucl Business Plc | Fabry disease gene therapy |
| US12370268B2 (en) | 2015-05-11 | 2025-07-29 | Ucl Business Ltd | Fabry disease gene therapy |
| US11007260B2 (en) | 2015-07-21 | 2021-05-18 | Modernatx, Inc. | Infectious disease vaccines |
| US10702597B2 (en) | 2015-07-21 | 2020-07-07 | Modernatx, Inc. | CHIKV RNA vaccines |
| US10449244B2 (en) | 2015-07-21 | 2019-10-22 | Modernatx, Inc. | Zika RNA vaccines |
| US11364292B2 (en) | 2015-07-21 | 2022-06-21 | Modernatx, Inc. | CHIKV RNA vaccines |
| US11564893B2 (en) | 2015-08-17 | 2023-01-31 | Modernatx, Inc. | Methods for preparing particles and related compositions |
| US10849920B2 (en) | 2015-10-05 | 2020-12-01 | Modernatx, Inc. | Methods for therapeutic administration of messenger ribonucleic acid drugs |
| US12246030B2 (en) | 2015-10-05 | 2025-03-11 | Modernatx, Inc. | Methods for therapeutic administration of messenger ribonucleic acid drugs |
| US11590157B2 (en) | 2015-10-05 | 2023-02-28 | Modernatx, Inc. | Methods for therapeutic administration of messenger ribonucleic acid drugs |
| US10144942B2 (en) | 2015-10-14 | 2018-12-04 | Translate Bio, Inc. | Modification of RNA-related enzymes for enhanced production |
| US10995354B2 (en) | 2015-10-14 | 2021-05-04 | Translate Bio, Inc. | Modification of RNA-related enzymes for enhanced production |
| US10716846B2 (en) | 2015-10-22 | 2020-07-21 | Modernatx, Inc. | Human cytomegalovirus RNA vaccines |
| US10124055B2 (en) | 2015-10-22 | 2018-11-13 | Modernatx, Inc. | Zika virus RNA vaccines |
| US10238731B2 (en) | 2015-10-22 | 2019-03-26 | Modernatx, Inc. | Chikagunya virus RNA vaccines |
| US10517940B2 (en) | 2015-10-22 | 2019-12-31 | Modernatx, Inc. | Zika virus RNA vaccines |
| US11484590B2 (en) | 2015-10-22 | 2022-11-01 | Modernatx, Inc. | Human cytomegalovirus RNA vaccines |
| US10675342B2 (en) | 2015-10-22 | 2020-06-09 | Modernatx, Inc. | Chikungunya virus RNA vaccines |
| US11235052B2 (en) | 2015-10-22 | 2022-02-01 | Modernatx, Inc. | Chikungunya virus RNA vaccines |
| US11278611B2 (en) | 2015-10-22 | 2022-03-22 | Modernatx, Inc. | Zika virus RNA vaccines |
| US10383937B2 (en) | 2015-10-22 | 2019-08-20 | Modernatx, Inc. | Human cytomegalovirus RNA vaccines |
| US10064935B2 (en) | 2015-10-22 | 2018-09-04 | Modernatx, Inc. | Human cytomegalovirus RNA vaccines |
| US11285222B2 (en) | 2015-12-10 | 2022-03-29 | Modernatx, Inc. | Compositions and methods for delivery of agents |
| WO2017099823A1 (en) * | 2015-12-10 | 2017-06-15 | Modernatx, Inc. | Compositions and methods for delivery of therapeutic agents |
| US10485885B2 (en) | 2015-12-10 | 2019-11-26 | Modernatx, Inc. | Compositions and methods for delivery of agents |
| US10207010B2 (en) | 2015-12-10 | 2019-02-19 | Modernatx, Inc. | Compositions and methods for delivery of agents |
| US10556018B2 (en) | 2015-12-10 | 2020-02-11 | Modernatx, Inc. | Compositions and methods for delivery of agents |
| US12491260B2 (en) | 2015-12-10 | 2025-12-09 | Modernatx, Inc. | Compositions and methods for delivery of agents |
| WO2017127750A1 (en) | 2016-01-22 | 2017-07-27 | Modernatx, Inc. | Messenger ribonucleic acids for the production of intracellular binding polypeptides and methods of use thereof |
| US10266843B2 (en) | 2016-04-08 | 2019-04-23 | Translate Bio, Inc. | Multimeric coding nucleic acid and uses thereof |
| US10428349B2 (en) | 2016-04-08 | 2019-10-01 | Translate Bio, Inc. | Multimeric coding nucleic acid and uses thereof |
| US11124804B2 (en) | 2016-04-08 | 2021-09-21 | Translate Bio, Inc. | Multimeric coding nucleic acid and uses thereof |
| WO2017180917A2 (en) | 2016-04-13 | 2017-10-19 | Modernatx, Inc. | Lipid compositions and their uses for intratumoral polynucleotide delivery |
| WO2017201350A1 (en) | 2016-05-18 | 2017-11-23 | Modernatx, Inc. | Polynucleotides encoding interleukin-12 (il12) and uses thereof |
| US11649461B2 (en) * | 2016-05-18 | 2023-05-16 | Modernatx, Inc. | Polynucleotides encoding α-galactosidase A for the treatment of Fabry disease |
| US12201677B2 (en) | 2016-06-13 | 2025-01-21 | Translate Bio, Inc. | Messenger RNA therapy for the treatment of ornithine transcarbamylase deficiency |
| US10835583B2 (en) | 2016-06-13 | 2020-11-17 | Translate Bio, Inc. | Messenger RNA therapy for the treatment of ornithine transcarbamylase deficiency |
| US11541113B2 (en) | 2016-10-21 | 2023-01-03 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| US10695419B2 (en) | 2016-10-21 | 2020-06-30 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| US11197927B2 (en) | 2016-10-21 | 2021-12-14 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| US20180153822A1 (en) * | 2016-11-10 | 2018-06-07 | Translate Bio, Inc. | Process of Preparing mRNA-Loaded Lipid Nanoparticles |
| EP4249501A2 (en) | 2017-01-09 | 2023-09-27 | Whitehead Institute for Biomedical Research | Methods of altering gene expression by perturbing transcription factor multimers that structure regulatory loops |
| WO2018129544A1 (en) | 2017-01-09 | 2018-07-12 | Whitehead Institute For Biomedical Research | Methods of altering gene expression by perturbing transcription factor multimers that structure regulatory loops |
| US10273269B2 (en) | 2017-02-16 | 2019-04-30 | Modernatx, Inc. | High potency immunogenic zika virus compositions |
| US11253605B2 (en) | 2017-02-27 | 2022-02-22 | Translate Bio, Inc. | Codon-optimized CFTR MRNA |
| US11173190B2 (en) | 2017-05-16 | 2021-11-16 | Translate Bio, Inc. | Treatment of cystic fibrosis by delivery of codon-optimized mRNA encoding CFTR |
| WO2018231990A2 (en) | 2017-06-14 | 2018-12-20 | Modernatx, Inc. | Polynucleotides encoding methylmalonyl-coa mutase |
| US11786607B2 (en) | 2017-06-15 | 2023-10-17 | Modernatx, Inc. | RNA formulations |
| US12521447B2 (en) * | 2017-08-31 | 2026-01-13 | Life Technologies Corporation | Cationic lipid compositions for tissue-specific delivery |
| US20220001023A1 (en) * | 2017-08-31 | 2022-01-06 | Life Technologies Corporation | Cationic lipid compositions for tissue-specific delivery |
| US12357575B2 (en) | 2017-08-31 | 2025-07-15 | Modernatx, Inc. | Methods of making lipid nanoparticles |
| US11744801B2 (en) | 2017-08-31 | 2023-09-05 | Modernatx, Inc. | Methods of making lipid nanoparticles |
| US11207398B2 (en) | 2017-09-14 | 2021-12-28 | Modernatx, Inc. | Zika virus mRNA vaccines |
| US10653767B2 (en) | 2017-09-14 | 2020-05-19 | Modernatx, Inc. | Zika virus MRNA vaccines |
| WO2019067999A1 (en) * | 2017-09-29 | 2019-04-04 | Intellia Therapeutics, Inc. | In vitro method of mrna delivery using lipid nanoparticles |
| US11167043B2 (en) | 2017-12-20 | 2021-11-09 | Translate Bio, Inc. | Composition and methods for treatment of ornithine transcarbamylase deficiency |
| US12268754B2 (en) | 2017-12-20 | 2025-04-08 | Translate Bio, Inc. | Composition and methods for treatment of ornithine transcarbamylase deficiency |
| WO2019148101A1 (en) | 2018-01-29 | 2019-08-01 | Modernatx, Inc. | Rsv rna vaccines |
| US12357580B2 (en) | 2018-06-19 | 2025-07-15 | The Board Of Regents Of The University Of Texas System | Lipid nanoparticle compositions for delivery of mRNA and long nucleic acids |
| US12144849B2 (en) | 2018-08-02 | 2024-11-19 | Grifols Worldwide Operations Limited | Composition comprising highly-concentrated Alpha1 proteinase inhibitor and method for obtaining thereof |
| US11701412B2 (en) | 2018-08-02 | 2023-07-18 | Grifols Worldwide Operations Limited | Composition comprising highly-concentrated α1 proteinase inhibitor and method for obtaining thereof |
| US20200038494A1 (en) * | 2018-08-02 | 2020-02-06 | Grifols Worldwide Operations Limited | Composition comprising highly-concentrated alpha1 proteinase inhibitor and method for obtaining thereof |
| US11253578B2 (en) * | 2018-08-02 | 2022-02-22 | Grifols Worldwide Operations Limited | Composition comprising highly-concentrated ALPHA1 proteinase inhibitor and method for obtaining thereof |
| US11174500B2 (en) | 2018-08-24 | 2021-11-16 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US12084702B2 (en) | 2018-08-24 | 2024-09-10 | Translate Bio, Inc. | Methods for purification of messenger RNA |
| US11357726B2 (en) | 2018-08-29 | 2022-06-14 | Translate Bio, Inc. | Process of preparing mRNA-loaded lipid nanoparticles |
| EP3846822A4 (en) * | 2018-09-04 | 2022-07-06 | The Board Of Regents Of The University Of Texas System | COMPOSITIONS AND METHODS FOR ORGAN SPECIFIC DELIVERY OF NUCLEIC ACIDS |
| US11766408B2 (en) | 2018-09-04 | 2023-09-26 | The Board Of Regents Of The University Of Texas System | Compositions and methods for organ specific delivery of nucleic acids |
| US12133924B2 (en) | 2018-09-04 | 2024-11-05 | The Board Of Regents Of The University Of Texas System | Compositions and methods for organ specific delivery of nucleic acids |
| US12151029B2 (en) | 2018-09-19 | 2024-11-26 | Modernatx, Inc. | PEG lipids and uses thereof |
| US12383508B2 (en) | 2018-09-19 | 2025-08-12 | Modernatx, Inc. | High-purity peg lipids and uses thereof |
| US12090235B2 (en) | 2018-09-20 | 2024-09-17 | Modernatx, Inc. | Preparation of lipid nanoparticles and methods of administration thereof |
| US12195505B2 (en) | 2018-11-21 | 2025-01-14 | Translate Bio, Inc. | Treatment of cystic fibrosis by delivery of nebulized mRNA encoding CFTR |
| US20210346293A1 (en) * | 2019-01-25 | 2021-11-11 | Hoffmann-La Roche Inc. | Lipid Vesicle for Oral Drug Delivery |
| US12070495B2 (en) | 2019-03-15 | 2024-08-27 | Modernatx, Inc. | HIV RNA vaccines |
| US12053551B2 (en) | 2019-12-20 | 2024-08-06 | Translate Bio, Inc. | Process of preparing mRNA-loaded lipid nanoparticles |
| WO2021154763A1 (en) | 2020-01-28 | 2021-08-05 | Modernatx, Inc. | Coronavirus rna vaccines |
| WO2021159040A2 (en) | 2020-02-07 | 2021-08-12 | Modernatx, Inc. | Sars-cov-2 mrna domain vaccines |
| US11969480B2 (en) | 2020-02-25 | 2024-04-30 | Translate Bio, Inc. | Processes of preparing mRNA-loaded lipid nanoparticles |
| US20220047517A1 (en) * | 2020-03-19 | 2022-02-17 | Shinshu University | Composition, lipid particle manufacturing kit, substance delivery method, and detection method |
| WO2021222304A1 (en) | 2020-04-27 | 2021-11-04 | Modernatx, Inc. | Sars-cov-2 rna vaccines |
| WO2021159130A2 (en) | 2020-05-15 | 2021-08-12 | Modernatx, Inc. | Coronavirus rna vaccines and methods of use |
| US11739317B2 (en) | 2020-07-13 | 2023-08-29 | The Board Of Trustees Of The Leland Stanford Junior University | Systems and methods to assess RNA stability |
| US11406703B2 (en) | 2020-08-25 | 2022-08-09 | Modernatx, Inc. | Human cytomegalovirus vaccine |
| US11492611B2 (en) | 2020-08-31 | 2022-11-08 | The Board Of Trustees Of The Leland Stanford Junior University | Systems and methods for producing RNA constructs with increased translation and stability |
| WO2022067010A1 (en) | 2020-09-25 | 2022-03-31 | Modernatx, Inc. | Multi-proline-substituted coronavirus spike protein vaccines |
| US12458604B2 (en) | 2020-10-14 | 2025-11-04 | The Trustees Of The University Of Pennsylvania | Methods of lipid nanoparticle manufacture and compositions derived therefrom |
| US11771653B2 (en) | 2020-11-06 | 2023-10-03 | Sanofi | Lipid nanoparticles for delivering mRNA vaccines |
| US12239735B2 (en) | 2020-11-06 | 2025-03-04 | Sanofi | Lipid nanoparticles for delivering mRNA vaccines |
| WO2022099003A1 (en) | 2020-11-06 | 2022-05-12 | Sanofi | Lipid nanoparticles for delivering mrna vaccines |
| US11771652B2 (en) | 2020-11-06 | 2023-10-03 | Sanofi | Lipid nanoparticles for delivering mRNA vaccines |
| WO2022150717A1 (en) | 2021-01-11 | 2022-07-14 | Modernatx, Inc. | Seasonal rna influenza virus vaccines |
| WO2022155524A1 (en) | 2021-01-15 | 2022-07-21 | Modernatx, Inc. | Variant strain-based coronavirus vaccines |
| WO2022155530A1 (en) | 2021-01-15 | 2022-07-21 | Modernatx, Inc. | Variant strain-based coronavirus vaccines |
| US11524023B2 (en) | 2021-02-19 | 2022-12-13 | Modernatx, Inc. | Lipid nanoparticle compositions and methods of formulating the same |
| US11622972B2 (en) | 2021-02-19 | 2023-04-11 | Modernatx, Inc. | Lipid nanoparticle compositions and methods of formulating the same |
| US12508278B2 (en) | 2021-02-19 | 2025-12-30 | Modernatx, Inc. | Lipid nanoparticle compositions and methods of formulating the same |
| WO2022197624A1 (en) | 2021-03-15 | 2022-09-22 | Modernatx, Inc. | Therapeutic use of sars-cov-2 mrna domain vaccines |
| US11744902B2 (en) * | 2021-04-06 | 2023-09-05 | Animatus Biosciences, Inc. | Targeting technology to selectively express mRNAs in cardiomyocytes while avoiding stimulation of cardiac fibroblasts |
| US20220323606A1 (en) * | 2021-04-06 | 2022-10-13 | Animatus Biosciences, Inc. | Targeting Technology to Selectively Express mRNAs in Cardiomyocytes While Avoiding Stimulation of Cardiac Fibroblasts |
| WO2022221335A1 (en) | 2021-04-13 | 2022-10-20 | Modernatx, Inc. | Respiratory virus combination vaccines |
| WO2022221440A1 (en) | 2021-04-14 | 2022-10-20 | Modernatx, Inc. | Influenza-coronavirus combination vaccines |
| WO2022245888A1 (en) | 2021-05-19 | 2022-11-24 | Modernatx, Inc. | Seasonal flu rna vaccines and methods of use |
| WO2022264109A1 (en) | 2021-06-18 | 2022-12-22 | Sanofi | Multivalent influenza vaccines |
| WO2023025404A1 (en) | 2021-08-24 | 2023-03-02 | BioNTech SE | In vitro transcription technologies |
| WO2023069498A1 (en) | 2021-10-22 | 2023-04-27 | Senda Biosciences, Inc. | Mrna vaccine composition |
| WO2023079507A1 (en) | 2021-11-05 | 2023-05-11 | Sanofi | Respiratory syncytial virus rna vaccine |
| WO2023092069A1 (en) | 2021-11-18 | 2023-05-25 | Modernatx, Inc. | Sars-cov-2 mrna domain vaccines and methods of use |
| WO2023096858A1 (en) | 2021-11-23 | 2023-06-01 | Senda Biosciences, Inc. | A bacteria-derived lipid composition and use thereof |
| WO2023102373A1 (en) | 2021-11-30 | 2023-06-08 | Sanofi Pasteur Inc. | Human metapneumovirus vaccines |
| WO2023107999A2 (en) | 2021-12-08 | 2023-06-15 | Modernatx, Inc. | Herpes simplex virus mrna vaccines |
| WO2023111262A1 (en) | 2021-12-17 | 2023-06-22 | Sanofi | Lyme disease rna vaccine |
| WO2023122080A1 (en) | 2021-12-20 | 2023-06-29 | Senda Biosciences, Inc. | Compositions comprising mrna and lipid reconstructed plant messenger packs |
| WO2023196914A1 (en) | 2022-04-08 | 2023-10-12 | Modernatx, Inc. | Influenza nucleic acid compositions and uses thereof |
| WO2023214082A2 (en) | 2022-05-06 | 2023-11-09 | Sanofi | Signal sequences for nucleic acid vaccines |
| WO2023230481A1 (en) | 2022-05-24 | 2023-11-30 | Modernatx, Inc. | Orthopoxvirus vaccines |
| WO2024015890A1 (en) | 2022-07-13 | 2024-01-18 | Modernatx, Inc. | Norovirus mrna vaccines |
| WO2024044108A1 (en) | 2022-08-22 | 2024-02-29 | The Henry M. Jackson Foundation For The Advancement Of Military Medicine, Inc. | Vaccines against coronaviruses |
| WO2024050483A1 (en) | 2022-08-31 | 2024-03-07 | Modernatx, Inc. | Variant strain-based coronavirus vaccines and uses thereof |
| WO2024083345A1 (en) | 2022-10-21 | 2024-04-25 | BioNTech SE | Methods and uses associated with liquid compositions |
| WO2024084089A1 (en) | 2022-10-21 | 2024-04-25 | BioNTech SE | Methods and uses associated with liquid compositions |
| WO2024094881A1 (en) | 2022-11-04 | 2024-05-10 | Sanofi | Respiratory syncytial virus rna vaccination |
| WO2024094876A1 (en) | 2022-11-04 | 2024-05-10 | Sanofi | Methods for messenger rna tailing |
| WO2024102434A1 (en) | 2022-11-10 | 2024-05-16 | Senda Biosciences, Inc. | Rna compositions comprising lipid nanoparticles or lipid reconstructed natural messenger packs |
| WO2024126847A1 (en) | 2022-12-15 | 2024-06-20 | Sanofi | Mrna recombinant capping enzymes |
| WO2024126809A1 (en) | 2022-12-15 | 2024-06-20 | Sanofi | Mrna encoding influenza virus-like particle |
| WO2024133884A2 (en) | 2022-12-23 | 2024-06-27 | Sanofi | Optimized tailing of messenger rna |
| WO2024159172A1 (en) | 2023-01-27 | 2024-08-02 | Senda Biosciences, Inc. | A modified lipid composition and uses thereof |
| WO2024163465A1 (en) | 2023-01-30 | 2024-08-08 | Modernatx, Inc. | Epstein-barr virus mrna vaccines |
| WO2024180262A1 (en) | 2023-03-02 | 2024-09-06 | Sanofi | Compositions for use in treatment of chlamydia |
| WO2024184489A1 (en) | 2023-03-07 | 2024-09-12 | Sanofi | Manufacture of messenger rna with kp34 polymerase |
| WO2024192277A2 (en) | 2023-03-15 | 2024-09-19 | Renagade Therapeutics Management Inc. | Lipid nanoparticles comprising coding rna molecules for use in gene editing and as vaccines and therapeutic agents |
| WO2024192291A1 (en) | 2023-03-15 | 2024-09-19 | Renagade Therapeutics Management Inc. | Delivery of gene editing systems and methods of use thereof |
| WO2024209404A1 (en) * | 2023-04-05 | 2024-10-10 | Seqirus Inc. | Rna delivery vehicle |
| WO2024215721A1 (en) | 2023-04-10 | 2024-10-17 | Modernatx, Inc. | Lyme disease vaccines |
| WO2024220712A2 (en) | 2023-04-19 | 2024-10-24 | Sail Biomedicines, Inc. | Vaccine compositions |
| WO2024231727A1 (en) | 2023-05-05 | 2024-11-14 | Sanofi | Compositions for use in treatment of acne |
| WO2024231565A1 (en) | 2023-05-10 | 2024-11-14 | Sanofi | Combination respiratory mrna vaccines |
| WO2024254552A1 (en) | 2023-06-08 | 2024-12-12 | Modernatx, Inc. | Stabilized flavivirus vaccines |
| WO2024263826A1 (en) | 2023-06-22 | 2024-12-26 | Modernatx, Inc. | Sars-cov-2 t cell vaccines |
| WO2025003760A1 (en) | 2023-06-28 | 2025-01-02 | Sanofi | Sterol analogs in lipid nanoparticle formulations |
| WO2025019352A2 (en) | 2023-07-14 | 2025-01-23 | Modernatx, Inc. | Mers-cov mrna vaccines |
| WO2025017151A1 (en) | 2023-07-18 | 2025-01-23 | Sanofi | Stable poly(a)-encoding messenger rna templates |
| WO2025017202A2 (en) | 2023-07-19 | 2025-01-23 | Sanofi | Porphyromonas gingivalis antigenic constructs |
| WO2025029700A1 (en) | 2023-07-28 | 2025-02-06 | Modernatx, Inc. | Vlp enteroviral vaccines |
| WO2025034612A1 (en) | 2023-08-04 | 2025-02-13 | Modernatx, Inc. | Varicella-zoster virus mrna vaccine |
| WO2025049959A2 (en) | 2023-09-01 | 2025-03-06 | Renagade Therapeutics Management Inc. | Gene editing systems, compositions, and methods for treatment of vexas syndrome |
| WO2025051975A1 (en) | 2023-09-06 | 2025-03-13 | Sanofi | Modified influenza b hemagglutinin polypeptides and nucleic acids and uses thereof |
| WO2025081042A1 (en) | 2023-10-12 | 2025-04-17 | Renagade Therapeutics Management Inc. | Nickase-retron template-based precision editing system and methods of use |
| WO2025128871A2 (en) | 2023-12-13 | 2025-06-19 | Renagade Therapeutics Management Inc. | Lipid nanoparticles comprising coding rna molecules for use in gene editing and as vaccines and therapeutic agents |
| WO2025134071A1 (en) | 2023-12-22 | 2025-06-26 | Sanofi | Malic and glutaric acid based ionizable lipids |
| WO2025141521A1 (en) | 2023-12-29 | 2025-07-03 | Sanofi | Lipids having dendritic moieties |
| WO2025155753A2 (en) | 2024-01-17 | 2025-07-24 | Renagade Therapeutics Management Inc. | Improved gene editing system, guides, and methods |
| WO2025174765A1 (en) | 2024-02-12 | 2025-08-21 | Renagade Therapeutics Management Inc. | Lipid nanoparticles comprising coding rna molecules for use in gene editing and as vaccines and therapeutic agents |
| WO2025210592A1 (en) | 2024-04-05 | 2025-10-09 | Sanofi Pasteur Inc. | Muscle cell lnp-mrna quality control assays |
| WO2025226656A1 (en) | 2024-04-23 | 2025-10-30 | Modernatx, Inc. | Hepatitis b virus mrna vaccines |
| WO2026008743A1 (en) | 2024-07-02 | 2026-01-08 | Sanofi Pasteur Inc. | Water-soluble polyanionic polymer as adjuvant for carrier-formulated nucleic acid |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11951181B2 (en) | Lipid nanoparticle compositions and methods for mRNA delivery | |
| HK40120170A (en) | Lipid nanoparticle compositions and methods for mrna delivery | |
| HK40079866A (en) | Lipid nanoparticle compositions and methods for mrna delivery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: SHIRE HUMAN GENETIC THERAPIES, INC., MASSACHUSETTS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:GUILD, BRAYDON CHARLES;DEROSA, FRANK;HEARTLEIN, MICHAEL;SIGNING DATES FROM 20140124 TO 20140207;REEL/FRAME:032220/0341 |
|
| AS | Assignment |
Owner name: RANA THERAPEUTICS, INC., MASSACHUSETTS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:SHIRE HUMAN GENETIC THERAPIES, INC.;REEL/FRAME:042177/0032 Effective date: 20161216 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |
|
| AS | Assignment |
Owner name: TRANSLATE BIO, INC., MASSACHUSETTS Free format text: CHANGE OF NAME;ASSIGNOR:RANA THERAPEUTICS, INC.;REEL/FRAME:043267/0165 Effective date: 20170626 |
|
| AS | Assignment |
Owner name: TRANSLATE BIO, INC., MASSACHUSETTS Free format text: CHANGE OF NAME;ASSIGNOR:RANA THERAPEUTICS, INC.;REEL/FRAME:056695/0001 Effective date: 20170626 |