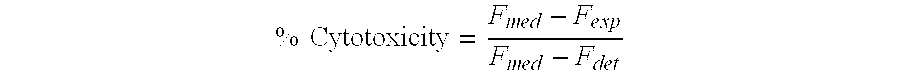US20030175884A1 - Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity - Google Patents
Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity Download PDFInfo
- Publication number
- US20030175884A1 US20030175884A1 US10/211,554 US21155402A US2003175884A1 US 20030175884 A1 US20030175884 A1 US 20030175884A1 US 21155402 A US21155402 A US 21155402A US 2003175884 A1 US2003175884 A1 US 2003175884A1
- Authority
- US
- United States
- Prior art keywords
- antibody
- human
- host cell
- cell
- region
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/10—Cells modified by introduction of foreign genetic material
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2887—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against CD20
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/06—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations
- A61K49/08—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by the carrier
- A61K49/10—Organic compounds
- A61K49/14—Peptides, e.g. proteins
- A61K49/16—Antibodies; Immunoglobulins; Fragments thereof
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3038—Kidney, bladder
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/10—Transferases (2.)
- C12N9/1048—Glycosyltransferases (2.4)
- C12N9/1051—Hexosyltransferases (2.4.1)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12P—FERMENTATION OR ENZYME-USING PROCESSES TO SYNTHESISE A DESIRED CHEMICAL COMPOUND OR COMPOSITION OR TO SEPARATE OPTICAL ISOMERS FROM A RACEMIC MIXTURE
- C12P21/00—Preparation of peptides or proteins
- C12P21/005—Glycopeptides, glycoproteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/10—Immunoglobulins specific features characterized by their source of isolation or production
- C07K2317/14—Specific host cells or culture conditions, e.g. components, pH or temperature
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/40—Immunoglobulins specific features characterized by post-translational modification
- C07K2317/41—Glycosylation, sialylation, or fucosylation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02P—CLIMATE CHANGE MITIGATION TECHNOLOGIES IN THE PRODUCTION OR PROCESSING OF GOODS
- Y02P20/00—Technologies relating to chemical industry
- Y02P20/50—Improvements relating to the production of bulk chemicals
- Y02P20/52—Improvements relating to the production of bulk chemicals using catalysts, e.g. selective catalysts
Definitions
- the present invention relates to the field of glycosylation engineering of proteins. More particularly, the present invention relates to glycosylation engineering to generate proteins with improved therapeutic properties, including antibodies with increased antibody-dependent cellular cytotoxicity.
- Glycoproteins mediate many essential functions in human beings, other eukaryotic organisms, and some prokaryotes, including catalysis, signaling, cell-cell communication, and molecular recognition and association. They make up the majority of non-cytosolic proteins in eukaryotic organisms. (Lis et al., Eur. J. Biochem. 218:1-27 (1993)). Many glycoproteins have been exploited for therapeutic purposes, and during the last two decades, recombinant versions of naturally-occurring, secreted glycoproteins have been a major product of the biotechnology industry.
- EPO erythropoietin
- therapeutic mAbs therapeutic monoclonal antibodies
- tPA tissue plasminogen activator
- IFN- ⁇ interferon- ⁇
- GM-CSF granulocyte-macrophage colony stimulating factor
- hCG human chorionic gonadotrophin
- the oligosaccharide component can significantly affect properties relevant to the efficacy of a therapeutic glycoprotein, including physical stability, resistance to protease attack, interactions with the immune system, pharmacokinetics, and specific biological activity. Such properties may depend not only on the presence or absence, but also on the specific structures, of oligosaccharides. Some generalizations between oligosaccharide structure and glycoprotein function can be made. For example, certain oligosaccharide structures mediate rapid clearance of the glycoprotein from the bloodstream through interactions with specific carbohydrate binding proteins, while others can be bound by antibodies and trigger undesired immune reactions. (Jenkins et al., Nature Biotechnol. 14:975-81 (1996)).
- Mammalian cells are the preferred hosts for production of therapeutic glycoproteins, due to their capability to glycosylate proteins in the most compatible form for human application. (Cumming et al., Glycobiology 1:115-30 (1991); Jenkins et al., Nature Biotechnol. 14:975-81 (1996)). Bacteria very rarely glycosylate proteins, and like other types of common hosts, such as yeasts, filamentous fungi, insect and plant cells, yield glycosylation patterns associated with rapid clearance from the bloodstream, undesirable immune interactions, and in some specific cases, reduced biological activity. Among mammalian cells, Chinese hamster ovary (CHO) cells have been most commonly used during the last two decades.
- these cells allow consistent generation of genetically stable, highly productive clonal cell lines. They can be cultured to high densities in simple bioreactors using serum-free media, and permit the development of safe and reproducible bioprocesses.
- Other commonly used animal cells include baby hamster kidney (BHK) cells, NS0- and SP2/0-mouse myeloma cells. More recently, production from transgenic animals has also been tested. (Jenkins et al., Nature Biotechnol. 14:975-81 (1996)).
- All antibodies contain carbohydrate structures at conserved positions in the heavy chain constant regions, with each isotype possessing a distinct array of N-linked carbohydrate structures, which variably affect protein assembly, secretion or functional activity.
- N-linked carbohydrate structures which variably affect protein assembly, secretion or functional activity.
- the structure of the attached N-linked carbohydrate varies considerably, depending on the degree of processing, and can include high-mannose, multiply-branched as well as biantennary complex oligosaccharides. (Wright, A., and Morrison, S. L., Trends Biotech. 15:26-32 (1997)).
- Unconjugated monoclonal antibodies can be useful medicines for the treatment of cancer, as demonstrated by the U.S. Food and Drug Administration's approval of Rituximab (RituxanTM; IDEC Pharmaceuticals, San Diego, Calif., and Genentech Inc., San Francisco, Calif.), for the treatment of CD20 positive B-cell, low-grade or follicular Non-Hodgkin's lymphoma, and Trastuzumab (HerceptinTM; Genentech Inc,) for the treatment of advanced breast cancer (Grillo-Lopez, A. -J., et al., Semin. Oncol. 26:66-73 (1999); Goldenberg, M. M., Clin. Ther.
- IgGl type antibodies the most commonly used antibodies in cancer immunotherapy, are glycoproteins that have a conserved N-linked glycosylation site at Asn297 in each CH2 domain.
- ADCC antibody dependent cellular cytotoxicity
- the present inventors showed previously that over expression in Chinese hamster ovary (CHO) cells of ⁇ (1,4)-N-acetylglucosaminyltransferase III (GnTIII), a glycosyltransferase catalyzing the formation of bisected oligosaccharides, significantly increases the in vitro ADCC activity of an anti-neuroblastoma chimeric monoclonal antibody (chCE7) produced by the engineered CHO cells.
- GnTIII Chinese hamster ovary
- the antibody chCE7 belongs to a large class of unconjugated mAbs which have high tumor affinity and specificity, but have too little potency to be clinically useful when produced in standard industrial cell lines lacking the GnTIII enzyme (Umana, P., et al., Nature Biotechnol. 17:176-180 (1999)). That study was the first to show that large increases of maximal in vitro ADCC activity could be obtained by increasing the proportion of constant region (Fc)-associated, bisected oligosaccharides above the levels found in naturally occurring antibodies.
- Fc constant region
- the present inventors have applied this technology to Rituximab, the anti-CD20, IDEC-C2B8 chimeric antibody.
- the present inventors have likewise applied the technology to the unconjugated anti-cancer mAb chG250.
- the present inventors have now generated new glycosylation variants of the anti-CD20 monoclonal antibody (mAb) IDEC-C2B8 (Rituximab) and the anti-cancer mAb chG250 using genetically engineered mAb-producing cell lines that overexpress N-acetylglucosaminyltransferase III (GnTIII; EC 2.1.4.144) in a tetracycline regulated fashion.
- GnTIII is required for the synthesis of bisected oligosaccharides, which are found at low to intermediate levels in naturally-occurring human antibodies but are missing in mAbs produced in standard industrial cell lines.
- MabtheraTM the version of Rixtuximab marketed in Europe
- ADCC mouse-myeloma derived chG250 in biological activity.
- the variant carrying the highest levels of bisected oligosaccharides mediated significant ADCC activity at a 125-fold lower concentration than that required to detect even low ADCC activity by the unmodified control chG250.
- the claimed invention is directed to a host cell engineered to produce a polypeptide having increased Fc-mediated cellular cytotoxicity by expression of at least one nucleic acid encoding ⁇ (1,4)-N-acetylglucosaminyltransferase III (GnT III), wherein the polypeptide produced by the host cell is selected from the group consisting of a whole antibody molecule, an antibody fragment, and a fusion protein which includes a region equivalent to the Fc region of an immunoglobulin, and wherein the GnT III is expressed in an amount sufficient to increase the proportion of said polypeptide carrying bisected hybrid oligosaccharides or galactosylated complex oligosaccharides or mixtures thereof in the Fc region relative to polypeptides carrying bisected complex oligosaccharides in the Fc region.
- GnT III ⁇ (1,4)-N-acetylglucosaminyltransferase III
- the polypeptide is IgG or a fragment thereof, most preferably, IgG1 or a fragment thereof.
- the polypeptide is a fusion protein that includes a region equivalent to the Fc region of a human IgG.
- a nucleic acid molecule comprising at least one gene encoding GnTIII has been introduced into the host cell.
- at least one gene encoding GnTIII has been introduced into the host cell chromosome.
- the host cell has been engineered such that an endogenous GnT III gene is activated, for example, by insertion of a DNA element which increases gene expression into the host chromosome.
- the endogenous GnTIII has been activated by insertion of a promoter, an enhancer, a transcription factor binding site, a transposon, or a retroviral element or combinations thereof into the host cell chromosome.
- the host cell has been selected to carry a mutation triggering expression of an endogenous GnTIII.
- the host cell is the CHO cell mutant lec 10.
- the at least one nucleic acid encoding a GnTIII is operably linked to a constitutive promoter element.
- the host cell is a CHO cell, a BHK cell, a NSO cell, a SP2/0 cell, or a hybridoma cell, a YO myeloma cell, a P3X63 mouse myeloma cell, a PER cell or a PER.C6 cell and said polypeptide is an anti-CD20 antibody.
- the host cell is a SP2/0 cell and the polypeptide is the monoclonal antibody chG250.
- the claimed invention is directed to a host cell that further comprises at least one transfected nucleic acid encoding an antibody molecule, an antibody fragment, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin.
- the host cell comprises at least one transfected nucleic acid encoding an anti-CD20 antibody, the chimeric anti-human neuroblastoma monoclonal antibody chCE7, the chimeric anti-human renal cell carcinoma monoclonal antibody chG250, the chimeric anti-human colon, lung, and breast carcinoma monoclonal antibody ING-1, the humanized anti-human 17-1A antigen monoclonal antibody 3622W94, the humanized anti-human colorectal tumor antibody A33, the anti-human melanoma antibody directed against GD3 ganglioside R24, or the chimeric anti-human squamous-cell carcinoma monoclonal antibody SF-25, an anti-human EGFR antibody, an anti-human EGFRvIII antibody, an anti-human PSMA antibody, and anti-human PSCA antibody, an anti-human CD22 antibody, an anti-human CD30 antibody, an anti-human CD33 antibody, an anti-human CD38 antibody, an anti-human CD40 antibody,
- the claimed invention is directed to a method for producing a polypeptide in a host cell comprising culturing any of the above-described the host cells under conditions which permit the production of said polypeptide having increased Fc-mediated cellular cytotoxicity.
- the method further comprises isolating said polypeptide having increased Fc-mediated cellular cytotoxicity.
- the host cell comprises at least one nucleic acid encoding a fusion protein comprising a region equivalent to a glycosylated Fc region of an immunoglobulin.
- the proportion of bisected oligosaccharides in the Fc region of said polypeptides is greater than 50%, more preferably, greater than 70%.
- the proportion of bisected hybrid oligosaccharides or galactosylated complex oligosaccharides or mixtures thereof in the Fc region is greater than the proportion of bisected complex oligosaccharides in the Fc region of said polypeptide.
- the polypeptide is an anti-CD20 antibody and the anti-CD20 antibodies produced by said host cell have a glycosylation profile, as analyzed by MALDI/TOF-MS, that is substantially equivalent to that shown in FIG. 2E.
- the polypeptide is the chG250 monoclonal antibody and the chG250 antibodies produced by said host cell have a glycosylaton profile, as analyzed by MALDI/TOF-MS, that is substantially equivalent to that shown in FIG. 7D.
- the claimed invention is directed to an antibody having increased antibody dependent cellular cytotoxicity (ADCC) produced by any of the methods described above.
- ADCC antibody dependent cellular cytotoxicity
- the antibody is selected from the group consisting of an anti-CD20 antibody, chCE7, ch-G250, a humanized anti-HER2 monoclonal antibody, ING-1, 3622W94, SF-25, A33, and R24.
- the polypeptide can be an antibody fragment that includes a region equivalent to the Fc region of an immunoglobulin, having increased Fc-mediated cellular cytotoxicity produced by any of the methods described above.
- the claimed invention is directed to a fusion protein that includes a region equivalent to the Fe region of an immunoglobulin and having increased Fc-mediated cellular cytotoxicity produced by any of the methods described above.
- the claimed invention is directed to a pharmaceutical composition
- a pharmaceutical composition comprising the antibody, antibody fragment, or fusion protein of the invention and a pharmaceutically acceptable carrier.
- the claimed invention is directed to a method for the treatment of cancer comprising administering a therapeutically effective amount of said pharmaceutical composition to a patient in need thereof.
- the invention is directed to an improved method for treating an autoimmune disease produced in whole or in part by pathogenic autoantibodies based on B-cell depletion comprising administering a therapeutically effective amount of immunologically active antibody to a human subject in need thereof, the improvement comprising administering a therapeutically effective amount of an antibody having increased ADCC prepared as described above.
- the antibody is an anti-CD20 antibody.
- autoimmune diseases or disorders include, but are not limited to, immune-mediated thrombocytopenias, such as acute idiopathic thrombocytopenic purpurea and chronic idiopathic thrombocytopenic purpurea, dermatomyositis, Sydenham's chorea, lupus nephritis, rheumatic fever, polyglandular syndromes, Henoch-Schonlein purpura, post-streptococcal nephritis, erythema nodosum, Takayasu's arteritis, Addison's disease, erythema multiform, polyarteritis nodosa, ankylosing spondylitis, Goodpasture's syndrome, thromboangitis ubiterans, primary biliary cirrhosis, Hashimoto's thyroiditis, thyrotoxicosis, chronic active hepatitis, polymyositis/der
- atopic dermatitis atopic dermatitis
- systemic scleroderma and sclerosis responses associated with inflammatory bowel disease (such as Crohn's disease and ulcerative colitis); respiratory distress syndrome (including adult respiratory distress syndrome; ARDS); dermatitis; meningitis; encephalitis; uveitis; colitis; glomerulonephritis; allergic conditions such as eczema and asthma and other conditions involving infiltration of T cells and chronic inflammatory responses; atherosclerosis; leukocyte adhesion deficiency; rheumatoidarthritis; systemic lupus erythematosus (SLE); diabetes mellitus (e.g.
- FIG. 1 Indirect immunofluorescence assay showing the reactivity of the antibody preparation C2B8-25t to CD20 positive SB cells. Negative controls, including the HSB CD20 negative cell line and cells treated only with the secondary FITC-conjugated anti-human Fc polyclonal antibody are not shown.
- FIG. 2A-2E MALDI/TOF-MS spectra of the oligosaccharides derived from MabtheraTM (FIG. 2A), C2B8-nt (FIG. 2B), C2B8-2000t (FIG. 2C), C2B8-50t (FIG. 2D), and C2B8-25t (FIG. 2E) antibody samples. Oligosaccharides appear as [M+Na + ] and [M+K + ] ions.
- FIG. 3A and 3B Illustration of a typical human IgG Fc-associated oligosaccharide structure (A) and partial N-linked glycosylation pathway (B).
- FIG. 3A The core of the oligosaccharide is composed of three mannose (M) and two N-acetylglucosamine (Gn) monosaccharide residues attached to Asn 297 .
- Galactose (G), fucose (F), and bisecting N-acetylglucosamine (Gn, boxed) can be present or absent. Terminal N-acetylneuraminic acid may be also present but it is not included in the figure.
- FIG. 3B Partial N-linked glycosylation pathway leading to the formation of the major oligosaccharide classes (dotted frames). Bisecting N-acetylglucosamine is denoted as Gn b . Subscript numbers indicate how many monosaccharide residues are present in each oligosaccharide. Each structure appears together with its sodium-associated [M+Na + ] mass. The mass of those structures that contain fucose (f) are also included.
- FIG. 4A and 4B ADCC activities of Rituximab glycosylation variants. The percentage of cytotoxicity was measured via lysis of 51 Cr labeled CD20-positive SB cells by human lymphocytes (E:T ratio of 100:1) mediated by different mAb concentrations.
- FIG. 4A Activity of C2B8 samples derived from a single cell line but produced at increasing GnTIII expression levels (i.e., decreasing tetracycline concentrations). The samples are C2B8-2000t, C2B8-50t, C2B8-25t, and C2B8-nt (control mAb derived from a clone that does not express GnTIII
- FIG. 4B ADCC activity of C2B8-50t and C2B8-25t compared to MabtheraTM.
- FIG. 5 Western blot analysis of the seven GnTIII expressing clones and the wild type. 30 ⁇ g of each sample were loaded on a 8.75% SDS gel, transferred to a PVDF membrane and probed with the anti-c-myc monoclonal antibody (9E10). WT refers to wt-chG250-SP2/0 cells.
- FIG. 6 SDS polyacrylamide gel electrophoresis of resolved purified antibody samples.
- FIG. 7A- 7 D MALDI/TOF-MS spectra of neutral oligosaccharide mixtures from chG250 mAb samples produced by clones expressing different GnTIII levels and wt-chG250-SP2/0 cells: WT (FIG. 7A), 2F1 (FIG. 7B), 3D3 (FIG. 7C), 4E6 (FIG. 7D).
- FIG. 8A- 8 D MALDI/TOF-MS spectra of neutral oligosaccharide mixtures from chG250 mAb samples produced by clones expressing different GnTIII levels: 4E8, (FIG. 8A); 5G2, (FIG. 8B); 4G3, (FIG. 8C); 5H12, (FIG. 8D).
- FIG. 9 In vitro ADCC assay of antibody samples derived from control wt-chG250-SP2/-cells and GnTIII transected clones 3D3 and 5H12.
- antibody is intended to include whole antibody molecules, antibody fragments, or fusion proteins that include a region equivalent to the Fc region of an immunoglobulin.
- region equivalent to the Fc region of an immunoglobulin is intended to include naturally occurring allelic variants of the Fc region of an immunoglobulin as well as variants having alterations which produce substitutions, additions, or deletions but which do not decrease substantially the ability of the immunoglobulin to mediate antibody dependent cellular cytotoxicity.
- one or more amino acids can be deleted from the N-terminus or C-terminus of the Fc region of an immunoglobulin without substantial loss of biological function.
- variants can be selected according to general rules known in the art so as to have minimal effect on activity. (See, e.g., Bowie, J. U. et al., Science 247:1306-10 (1990).
- glycoprotein-modifying glycosyl transferase refers to ⁇ (1,4)-N-acetylglucosaminyltransferase III (GnTIII).
- glycosylation engineering is considered to include any manipulation of the glycosylation pattern of a naturally occurring polypeptide or fragment thereof.
- Glycosylation engineering includes metabolic engineering of the glycosylation machinery of a cell, including genetic manipulations of the oligosaccharide synthesis pathways to achieve altered glycosylation of glycoproteins expressed in cells.
- glycosylation engineering includes the effects of mutations and cell environment on glycosylation.
- the term host cell covers any kind of cellular system which can be engineered to generate modified glycoforms of proteins, protein fragments, or peptides of interest, including antibodies and antibody fragments.
- the host cells have been manipulated to express optimized levels of GnT III.
- Host cells include cultured cells, e.g., mammalian cultured cells, such as CHO cells, BHK cells, NSO cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER. C6 cells or hybridoma cells, yeast cells, and insect cells, to name only a few, but also cells comprised within a transgenic animal or cultured tissue.
- Fc-mediated cellular cytotoxicity includes antibody-dependent cellular cytotoxicity and cellular cytotoxicity mediated by a soluble Fc-fusion protein containing a human Fc-region. It is an immune mechanism leading to the lysis of“antibody-targeted cells” by “human immune effector cells”, wherein:
- the “human immune effector cells” are a population of leukocytes that display Fc receptors on their surface through which they bind to the Fc-region of antibodies or of Fc-fusion proteins and perform effector functions. Such a population may include, but is not limited to, peripheral blood mononuclear cells (PBMC) and/or natural killer (NK) cells.
- PBMC peripheral blood mononuclear cells
- NK natural killer
- the “antibody-targeted cells” are cells bound by the antibodies or Fc-fusion proteins.
- the antibodies or Fc fusion-proteins bind to target cells via the protein part N-terminal to the Fc region.
- the term increased Fc-mediated cellular cytotoxicity is defined as either an increase in the number of “antibody-targeted cells” that are lysed in a given time, at a given concentration of antibody, or of Fc-fusion protein, in the medium surrounding the target cells, by the mechanism of Fc-mediated cellular cytotoxicity defined above, and/or a reduction in the concentration of antibody, or of Fc-fusion protein, in the medium surrounding the target cells, required to achieve the lysis of a given number of “antibody-targeted cells”, in a given time, by the mechanism of Fc -mediated cellular cytotoxicity.
- Fc-mediated cellular cytotoxicity is relative to the cellular cytotoxicity mediated by the same antibody, or Fc-fusion protein, produced by the same type of host cells, using the same standard production, purification, formulation and storage methods, which are known to those skilled in the art, but that has not been produced by host cells engineered to express the glycosyltransferase GnTIII by the methods described herein.
- ADCC antibody dependent cellular cytotoxicity
- the assay uses target cells that are known to express the target antigen recognized by the antigen-binding region of the antibody;
- the assay uses human peripheral blood mononuclear cells (PBMCs), isolated from blood of a randomly chosen healthy donor, as effector cells;
- PBMCs peripheral blood mononuclear cells
- the PBMCs are isolated using standard density centriftigation procedures and are suspended at 5 ⁇ 10 6 cells/ml in RPMI cell culture medium;
- the target cells are grown by standard tissue culture methods, harvested from the exponential growth phase with a viability higher than 90%, washed in RPMI cell culture medium, labelled with 100 micro-Curies of 51 Cr, washed twice with cell culture medium, and resuspended in cell culture medium at a density of 10 5 cells/ml;
- the antibody is serially-diluted from 4000 ng/ml to 0.04 ng/ml in cell culture medium and 50 microliters of the resulting antibody solutions are added to the target cells in the 96-well microtiter plate, testing in triplicate various antibody concentrations covering the whole concentration range above;
- x) the percentage of specific lysis is calculated for each antibody concentration according to the formula (ER-MR)/(MR-SR) ⁇ 100, where ER is the average radioactivity quantified (see point ix above) for that antibody concentration, MR is the average radioactivity quantified (see point ix above) for the MR controls (see point v above), and SR is the average radioactivity quantified (see point ix above) for the SR controls (see point vi above);
- “increased ADCC” is defined as either an increase in the maximum percentage of specific lysis observed within the antibody concentration range tested above, and/or a reduction in the concentration of antibody required to achieve one half of the maximum percentage of specific lysis observed within the antibody concentration range tested above.
- the increase in ADCC is relative to the ADCC, measured with the above assay, mediated by the same antibody, produced by the same type of host cells, using the same standard production, purification, formulation and storage methods, which are known to those skilled in the art, but that has not been produced by host cells engineered to overexpress the glycosyltransferase GnTIII.
- anti-CD20 antibody is intended to mean an antibody which specifically recognizes a cell surface non-glycosylated phosphoprotein of 35,000 Daltons, typically designated as the human B lymphocyte restricted differentiation antigen Bp35, commonly referred to as CD20.
- the present invention provides methods for the generation and use of host cell systems for the production of glycoforms of antibodies or antibody fragments or fusion proteins which include antibody fragments with increased antibody-dependent cellular cytotoxicity. Identification of target epitopes and generation of antibodies having potential therapeutic value, for which modification of the glycosylation pattern is desired, and isolation of their respective coding nucleic acid sequence is within the scope of the invention.
- antibodies to target epitopes of interest include but are not limited to polyclonal, monoclonal, chimeric, single chain, Fab fragments and fragments produced by an Fab expression library. Such antibodies may be useful, e.g., as diagnostic or therapeutic agents. As therapeutic agents, neutralizing antibodies, i.e., those which compete for binding with a ligand, substrate or adapter molecule, are of especially preferred interest.
- various host animals are immunized by injection with the target protein of interest including, but not limited to, rabbits, mice, rats, etc.
- Various adjuvants may be used to increase the immunological response, depending on the host species, including but not limited to Freund's (complete and incomplete), mineral gels such as aluminum hydroxide, surface active substances such as lysolecithin, pluronic polyols, polyanions, peptides, saponin, oil emulsions, keyhole limpet hemocyanin, dinitrophenol, and potentially useful human adjuvants such as BCG (bacille Calmette-Guerin) and Corynebacterium parvum.
- BCG Bacille Calmette-Guerin
- Monoclonal antibodies to the target of interest may be prepared using any technique which provides for the production of antibody molecules by continuous cell lines in culture. These include, but are not limited to, the hybridoma technique originally described by Kohler and Milstein, Nature 256:495-97 (1975), the human B-cell hybridoma technique (Kosbor et al., Immunology Today 4:72 (1983); Cote et al., Proc. Natl. Acad. Sci. U.S.A. 80:2026-30 (1983 ) and the EBV-hybridoma technique (Cole et al., Monoclonal Antibodies and Cancer Therapy 77-96 (Alan R. Liss, Inc., 1985)).
- Antibody fragments which contain specific binding sites of the target protein of interest may be generated by known techniques.
- fragments include, but are not limited to, F(ab′) 2 fragments which can be produced by pepsin digestion of the antibody molecule and the Fab fragments which can be generated by reducing the disulfide bridges of the F(ab′) 2 fragments.
- Fab expression libraries may be constructed (Huse et al., Science 246:1275-81 (1989) to allow rapid and easy identification of monoclonal Fab fragments with the desired specificity to the target protein of interest.
- the present invention provides host cell expression systems for the generation of proteins having modified glycosylation patterns.
- the present invention provides host cell systems for the generation of glycoforms of proteins having an improved therapeutic value. Therefore, the invention provides host cell expression systems selected or engineered to increase the expression of a glycoprotein-modifying glycosyltransferase, namely ⁇ (1,4)-N-acetylglucosaminyltransferase III (GnTIID).
- GnTIID glycoprotein-modifying glycosyltransferase
- such host cell expression systems may be engineered to comprise a recombinant nucleic acid molecule encoding GnTIII, operatively linked to a constitutive or regulated promoter system.
- host cell expression systems may be employed that naturally produce, are induced to produce, and/or are selected to produce GnTIII.
- the present invention provides a host cell that has been engineered to express at least one nucleic acid encoding GnTIII.
- the host cell is transformed or transfected with a nucleic acid molecule comprising at least one gene encoding GnTIII.
- the host cell has been engineered and/or selected in such way that endogenous GnTIII is activated.
- the host cell may be selected to carry a mutation triggering expression of endogenous GnTIII.
- the host cell is a CHO lec 10 mutant.
- the host cell may be engineered such that endogenous GnTIII is activated.
- the host cell is engineered such that endogenous GnTIII has been activated by insertion of a constitutive promoter element, a transposon, or a retroviral element into the host cell chromosome.
- any type of cultured cell line can be used as a background to engineer the host cell lines of the present invention.
- CHO cells, BHK cells, NS0 cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER.C6 cells or hybridoma cells, yeast cells, or insect cells are used as the background cell line to generate the engineered host cells of the invention.
- the invention is contemplated to encompass any engineered host cells expressing GnTIII as defined herein.
- One or several nucleic acids encoding GnTIII may be expressed under the control of a constitutive promoter or, alternately, a regulated expression system.
- Suitable regulated expression systems include, but are not limited to, a tetracycline-regulated expression system, an ecdysone-inducible expression system, a lac-switch expression system, a glucocorticoid-inducible expression system, a temperature-inducible promoter system, and a metallothionein metal-inducible expression system.
- nucleic acids encoding GnTIII are comprised within the host cell system, some of them may be expressed under the control of a constitutive promoter, while others are expressed under the control of a regulated promoter.
- the maximal expression level is considered to be the highest possible level of stable GnTIII expression that does not have a significant adverse effect on cell growth rate, and will be determined using routine experimentation
- Expression levels are determined by methods generally known in the art, including Western blot analysis using a GnTIII specific antibody, Northern blot analysis using a GnTIII specific nucleic acid probe, or measurement of enzymatic activity.
- a lectin may be employed which binds to biosynthetic products of the GnTIII, for example, E 4 -PHA lectin.
- the nucleic acid may be operatively linked to a reporter gene; the expression levels of the GnTIII are determined by measuring a signal correlated with the expression level of the reporter gene.
- the reporter gene may transcribed together with the nucleic acid(s) encoding said GnTIII as a single mRNA molecule; their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE).
- the reporter gene may be translated together with at least one nucleic acid encoding said GnTIII such that a single polypeptide chain is formed.
- the nucleic acid encoding the GnTIII may be operatively linked to the reporter gene under the control of a single promoter, such that the nucleic acid encoding the GnTIII and the reporter gene are transcribed into an RNA molecule which is alternatively spliced into two separate messenger RNA (mRNA) molecules; one of the resulting mRNAs is translated into said reporter protein, and the other is translated into said GnTIII.
- mRNA messenger RNA
- nucleic acids encoding GnTIII may be arranged in such way that they are transcribed as one or as several mRNA molecules. If they are transcribed as a single mRNA molecule, their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE). They may be transcribed from a single promoter into an RNA molecule which is alternatively spliced into several separate messenger RNA (mRNA) molecules, which then are each translated into their respective encoded GnTIII.
- IRS internal ribosome entry site
- CITE cap-independent translation enhancer
- the present invention provides host cell expression systems for the generation of therapeutic antibodies, having an increased antibody-dependent cellular cytotoxicity, and cells which display the IgG Fc region on the surface to promote Fc-mediated cytotoxicity.
- the host cell expression systems have been engineered and/or selected to express nucleic acids encoding the antibody for which the production of altered glycoforms is desired, along with at least one nucleic acid encoding GnTIII.
- the host cell system is transfected with at least one gene encoding GnTIII.
- the transfected cells are selected to identify and isolate clones that stably express the GnTIII.
- the host cell has been selected for expression of endogenous GnTIII.
- cells may be selected carrying mutations which trigger expression of otherwise silent GnTIII.
- CHO cells are known to carry a silent GnT III gene that is active in certain mutants, e.g., in the mutant Lec10.
- methods known in the art may be used to activate silent GnTIII, including the insertion of a regulated or constitutive promoter, the use of transposons, retroviral elements, etc.
- gene knockout technologies or the use of ribozyme methods may be used to tailor the host cell's GnTIII expression level, and is therefore within the scope of the invention.
- any type of cultured cell line can be used as background to engineer the host cell lines of the present invention.
- CHO cells, BHK cells, NS0 cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER.C6 cells or hybridoma cells, yeast cell, or insect cells may be used.
- such cell lines are engineered to further comprise at least one transfected nucleic acid encoding a whole antibody molecule, an antibody fragment, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin.
- a hybridoma cell line expressing a particular antibody of interest is used as background cell line to generate the engineered host cells of the invention.
- At least one nucleic acid in the host cell system encodes GnT III.
- One or several nucleic acids encoding GnTIII may be expressed under the control of a constitutive promoter, or alternately, a regulated expression system
- Suitable regulated expression systems include, but are not limited to, a tetracycline-regulated expression system, an ecdysone-inducible expression system, a lac-switch expression system, a glucocorticoid-inducible expression system, a temperature-inducible promoter system, and a metallothionein metal-inducible expression system.
- nucleic acids encoding GnTIII are comprised within the host cell system, some of them may be expressed under the control of a constitutive promoter, while others are expressed under the control of a regulated promoter.
- the maximal expression level is considered to be the highest possible level of stable GnTIII expression that does not have a significant adverse effect on cell growth rate, and will be determined using routine experimentation. Expression levels are determined by methods generally known in the art, including Western blot analysis using a GnTIII specific antibody, Northern blot analysis using a GnTIII specific nucleic acid probe, or measurement of GnTIII enzymatic activity.
- a lectin may be employed which binds to biosynthetic products of GnTIII, for example, E 4 -PHA lectin.
- the nucleic acid may be operatively linked to a reporter gene; the expression levels of the glycoprotein-modifying glycosyl transferase are determined by measuring a signal correlated with the expression level of the reporter gene.
- the reporter gene may transcribed together with the nucleic acid(s) encoding said glycoprotein-modifying glycosyl transferase as a single mRNA molecule; their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE).
- the reporter gene may be translated together with at least one nucleic acid encoding GnTIII such that a single polypeptide chain is formed.
- the nucleic acid encoding the GnTIII may be operatively linked to the reporter gene under the control of a single promoter, such that the nucleic acid encoding the GnTIII and the reporter gene are transcribed into an RNA molecule which is alternatively spliced into two separate messenger RNA (mRNA) molecules; one of the resulting mRNAs is translated into said reporter protein, and the other is translated into said GnTIII.
- mRNA messenger RNA
- nucleic acids encoding a GnTIII may be arranged in such way that they are transcribed as one or as several mRNA molecules. If they are transcribed as single mRNA molecule, their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE). They may be transcribed from a single promoter into an RNA molecule which is alternatively spliced into several separate messenger RNA (mRNA) molecules, which then are each translated into their respective encoded GnTIII.
- IRS internal ribosome entry site
- CITE cap-independent translation enhancer
- Methods which are well known to those skilled in the art can be used to construct expression vectors containing the coding sequence of the protein of interest and the coding sequence of the GnTIII and appropriate transcriptional/translational control signals. These methods include in vitro recombinant DNA techniques, synthetic techniques and in vivo recombination/genetic recombination. See, for example, the techniques described in Maniatis et al., Molecular Cloning A Laboratory Manual, Cold Spring Harbor Laboratory, N.Y. (1989) and Ausubel et al., Current Protocols in Molecular Biology, Greene Publishing Associates and Wiley Interscience, N.Y (1989).
- a variety of host-expression vector systems maybe utilized to express the coding sequence of the protein of interest and the coding sequence of the GnTIII.
- mammalian cells are used as host cell systems transfected with recombinant plasmid DNA or cosmid DNA expression vectors containing the coding sequence of the protein of interest and the coding sequence of the GnTIII.
- CHO cells, ByIK cells, NS0 cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER. C6 cells or hybridoma cells, yeast cells, or insect cells are used as host cell system.
- yeast cells transformed with recombinant yeast expression vectors containing the coding sequence of the protein of interest and the coding sequence of the GnTIII include, yeast cells transformed with recombinant yeast expression vectors containing the coding sequence of the protein of interest and the coding sequence of the GnTIII; insect cell systems infected with recombinant virus expression vectors (e.g., baculovirus) containing the coding sequence of the protein of interest and the coding sequence of the GnTIII; plant cell systems infected with recombinant virus expression vectors (e.g., cauliflower mosaic virus, CaMV; tobacco mosaic virus, TMV) or transformed with recombinant plasmid expression vectors (e.g., Ti plasmid) containing the coding sequence of the protein of interest and the coding sequence of the GnTIII; or animal cell systems infected with recombinant virus expression vectors (e.g., adenovirus, vaccinia virus) including cell lines engineered to contain multiple copies
- stable expression is generally preferred to transient expression because it typically achieves more reproducible results and also is more amenable to large scale production.
- host cells can be transformed with the respective coding nucleic acids controlled by appropriate expression control elements (e.g., promoter, enhancer, sequences, transcription terminators, polyadenylation sites, etc.), and a selectable marker.
- appropriate expression control elements e.g., promoter, enhancer, sequences, transcription terminators, polyadenylation sites, etc.
- engineered cells may be allowed to grow for 1-2 days in an enriched media, and then are switched to a selective media.
- the selectable marker in the recombinant plasmid confers resistance to the selection and allows selection of cells which have stably integrated the plasmid into their chromosomes and grow to form foci which in turn can be cloned and expanded into cell lines.
- a number of selection systems may be used, including, but not limited to, the herpes simplex virus thymidine kinase (Wigler et al., Cell 11:223 (1977)), hypoxanthine-guaninephosphoribosyltransferase (Szybalska & Szybalski, Proc. Natl. Acad. Sci.
- trpB which allows cells to utilize indole in place of tryptophan
- hisD which allows cells to utilize histinol in place of histidine
- ODC ornithine decarboxylase
- DFMO 2-(difluoromethyl)-DL- ornithine
- the host cells which contain the coding sequence and which express the biologically active gene products may be identified by at least four general approaches; (a) DNA-DNA or DNA-RNA hybridization; (b) the presence or absence of “marker” gene functions; (c) assessing the level of transcription as measured by the expression of the respective mRNA transcripts in the host cell; and (d) detection of the gene product as measured by immunoassay or by its biological activity.
- the presence of the coding sequence of the protein of interest and the coding sequence of the GnTIII inserted in the expression vector can be detected by DNA-DNA or DNA-RNA hybridization using probes comprising nucleotide sequences that are homologous to the respective coding sequences, respectively, or portions or derivatives thereof
- the recombinant expression vector/host system can be identified and selected based upon the presence or absence of certain “marker” gene functions (e.g., thymidine kinase activity, resistance to antibiotics, resistance to methotrexate, transformation phenotype, occlusion body formation in baculovirus, etc.).
- certain “marker” gene functions e.g., thymidine kinase activity, resistance to antibiotics, resistance to methotrexate, transformation phenotype, occlusion body formation in baculovirus, etc.
- a marker gene can be placed in tandem with the coding sequences under the control of the same or different promoter used to control the expression of the coding sequences. Expression of the marker in response to induction or selection indicates expression of the coding sequence of the protein of interest and the coding sequence of the GnTIII.
- transcriptional activity for the coding region of the protein of interest and the coding sequence of the GnTIII can be assessed by hybridization assays.
- RNA can be isolated and analyzed by Northern blot using a probe homologous to the coding sequences of the protein of interest and the coding sequence of the GnTIII or particular portions thereof
- total nucleic acids of the host cell may be extracted and assayed for hybridization to such probes.
- the expression of the protein products of the protein of interest and the coding sequence of the GnTIII can be assessed immunologically, for example by Western blots, immunoassays such as radioimmuno-precipitation, enzyme-linked immunoassays and the like.
- the ultimate test of the success of the expression system involves the detection of the biologically active gene products.
- the present invention provides glycoforms of antibodies and antibody fragments having increased antibody-dependent cellular cytotoxicity.
- ADCC a lytic attack on antibody-targeted cells, is triggered upon binding of leukocyte receptors to the constant region (Fc) of antibodies. Deo et al., Immunology Today 18:127 (1997)
- Fc ⁇ Rs lymphocyte receptors
- Protein engineering studies have shown that Fc ⁇ Rs interact with the lower hinge region of the IgG CH2 domain. Lund et al., J. Immunol. 157:4963-69 (1996). However, Fc ⁇ R binding also requires the presence of oligosaccharides covalently attached at the conserved Asn 297 in the CH2 region. Lund et al., J. Immunol. 157:4963-69 (1996); Wright and Morrison, Trends Biotech.
- An IgG molecule carries two N-linked oligosaccharides in its Fc region, one on each heavy chain.
- an antibody is produced as a population of glycoforms which share the same polypeptide backbone but have different oligosaccharides attached to the glycosylation sites.
- the oligosaccharides normally found in the Fc region of serum IgG are of complex bi-antennary type (Wormald et al., Biochemistry 36:130-38 (1997), with low level of terminal sialic acid and bisecting N-acetylglucosamine (GIcNAc), and a variable degree of terminal galactosylation and core fucosylation.
- the mouse- or hamster-derived cell lines used in industry and academia for production of unconjugated therapeutic mAbs normally attach the required oligosaccharide determinants to Fc sites.
- IgGs expressed in these cell lines lack, however, the bisecting GIcNAc found in low amounts in serum IgGs. Lifely et al., Glycobiology 318:813-22 (1995).
- CAMPATH-1H humanized IgG1
- the rat cell-derived antibody reached a similar in vitro ADCC activity as CAMPATH-1H antibodies produced in standard cell lines, but at significantly lower antibody concentrations.
- the CAMPATH antigen is normally present at high levels on lymphoma cells, and this chimeric mAb has high ADCC activity in the absence of a bisecting GlcNAc. Lifely et al., Glycobiology 318:813-22 (1995). In the N-linked glycosylation pathway, a bisecting GlcNAc is added by the enzyme ⁇ (1,4)-N-acetylglucosaminyltransferase III (GnT III). Schachter, Biochem. Cell Biol. 64:163-81 (1986).
- the present inventors used a single antibody-producing CHO cell line, that was previously engineered to express, in an externally-regulated fashion, different levels of a cloned GnT III gene. This approach established for the first time a rigorous correlation between expression of GnTIII and the ADCC activity of the modified antibody.
- C2B8 antibody modified according to the disclosed method had an about sixteen-fold higher ADCC activity than the standard, unmodified C2B8 antibody produced under identical cell culture and purification conditions.
- a C2B8 antibody sample expressed in CHO-tTA-C2B8 cells that do not have GnTIII expression showed a cytotoxic activity of about 31% (at 1 ⁇ g/ml antibody concentration), measured as in vitro lysis of SB cells (CD20+) by human lymphocytes.
- C2B8 antibody derived from a CHO cell culture expressing GnT III at a basal, largely repressed level showed at 1 ⁇ g/ml antibody concentration a 33% increase in ADCC activity against the control at the same antibody concentration.
- increasing the expression of GnT III produced a large increase of almost 80% in the maximal ADCC activity (at 1 ⁇ g/ml antibody concentration) compared to the control at the same antibody concentration.
- antibodies of the invention having increased antibody-dependent cellular cytotoxicity include, but are not limited to, anti-human neuroblastoma monoclonal antibody (chCE7) produced by the methods of the invention, a chimeric anti-human renal cell carcinoma monoclonal antibody (ch-G250) produced by the methods of the invention, a humanized anti-HER2 monoclonal antibody (e.g., Trastuzumab (HERCEPTIN)) produced by the methods of the invention, a chimeric anti-human colon, lung, and breast carcinoma monoclonal antibody (ING-1) produced by the methods of the invention, a humanized anti-human 17-1A antigen monoclonal antibody (3622W94) produced by the methods of the invention, a humanized anti-human colorectal tumor antibody (A33) produced by the methods of the invention, an anti-human melanoma antibody (R24) directed against GD3 ganglioside produced by the methods of the invention, and a chimeric anti-human squam
- the present invention relates to a method for increasing the ADCC activity of therapeutic antibodies. This is achieved by engineering the glycosylation pattern of the Fc region of such antibodies, in particular by maximizing the proportion of antibody molecules carrying bisected complex oligosaccharides and bisected hybrid oligosaccharides N-linked to the conserved glycosylation sites in their Fc regions.
- This strategy can be applied to increase Fc-mediated cellular cytotoxicity against undesirable cells mediated by any molecule carrying a region that is an equivalent to the Fc region of an immunoglobulin, not only by therapeutic antibodies, since the changes introduced by the engineering of glycosylation affect only the Fc region and therefore its interactions with the Fc receptors on the surface of effector cells involved in the ADCC mechanism.
- Fc-containing molecules to which the presently disclosed methods can be applied include, but are not limited to, (a) soluble fusion proteins made of a targeting protein domain fused to the N-terminus of an Fc-region (Chamov and Ashkenazi, Trends Biotech. 14: 52 (1996) and (b) plasma membrane-anchored fusion proteins made of a type II transmembrane domain that localizes to the plasma membrane fused to the N-terminus of an Fc region (Stumble, P. F., Nature Biotech. 16: 1357 (1998)).
- the targeting domain directs binding of the fusion protein to undesirable cells such as cancer cells, i.e., in an analogous fashion to therapeutic antibodies.
- undesirable cells such as cancer cells
- the application of presently disclosed method to enhance the Fc-mediated cellular cytotoxic activity mediated by these molecules would therefore be identical to the method applied to therapeutic antibodies.
- the undesirable cells in the body have to express the gene encoding the fusion protein.
- This can be achieved either by gene therapy approaches, i.e., by transfecting the cells in vivo with a plasmid or viral vector that directs expression of the fusion protein-encoding gene to undesirable cells, or by implantation in the body of cells genetically engineered to express the fusion protein on their surface.
- the later cells would normally be implanted in the body inside a polymer capsule (encapsulated cell therapy) where they cannot be destroyed by an Fc-mediated cellular cytotoxicity mechanism. However should the capsule device fail and the escaping cells become undesirable, then they can be eliminated by Fc-mediated cellular cytotoxicity.
- the presently disclosed method would be applied either by incorporating into the gene therapy vector an additional gene expression cassette directing adequate or maximal expression levels of GnT III or by engineering the cells to be implanted to express adequate or maximal levels of GnT III.
- the aim of the disclosed method is to increase or maximize the proportion of surface-displayed Fc regions carrying bisected complex oligosaccharides and/or bisected hybrid oligosaccharides.
- VL and VH cDNA fragments were subcloned into pBluescriptIIKS(+), sequenced and directly joined by ligation to the human constant light (Ig ⁇ ) and heavy (IgG1) chain cDNAs, respectively, using unique restriction sites introduced at the variable and constant region junctions without altering the original amino acid residue sequence (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999); Reff, M. E., et al., Blood 83:435-445 (1994)).
- Each cell line was cotranfected with vectors pC2B8L, pC2B8H, and pZeoSV2(+) (for Zeocin resistance; Invitrogen, Leek, The Netherlands) using a calcium phosphate method.
- Zeocin resistant clones were transferred to a 96-well plate and assayed for IDEC-C2B8 production using an ELISA assay specific for the human constant region (4).
- Three IDEC-C2B8 samples were obtained from parallel cultures of a selected clone (CHO-tet-GnTIII-C2B8), differing only in the tetracycline concentration added to the medium (25, 50 and 2000 ng/mL respectively).
- CD20-positive cells SB cells; ATCC deposit no. ATCC CCL120
- HSB cells CD20-negative cells
- HBSSB bovine serum albumin fraction V
- Oligosaccharide profiling by MALDI/TOF-MS were derived from C2B8 antibody samples, MabTheraTM (European counterpart of Rituximab; kind gift from R. Stahel, Universit ⁇ dot over (a) ⁇ tspital, Switzerland), C2B8-25t, C2B8-50t, C2B8-2000t, and C2B8-nt, (100 ⁇ g each) as previously described (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999)).
- the antibody samples were first treated with Arthrobacter ureafaciens sialidase (Oxford Glycosciences, Abingson, UK) to remove any sialic acid monosaccharide residues.
- Neutral N-linked oligosaccharides were then released from the desialylated antibody samples using peptide-N-glycosidase F (Oxford Glycosciences), purified using micro-columns, and analyzed by MALDI/TOF-MS in an Elite Voyager 400 spectrometer (Perseptive Biosystems, Farmingham, Mass.).
- PBMC Peripheral blood mononuclear cells
- a C2B8-producing, control cell line that does not express GnTIII was also established and cultured under the same conditions as for the three parallel cultures of CHO-tet-GnTIII. After Protein A-affinity chromatography, mAb purity was estimated to be higher than 95% by SDS-PAGE and Coomassie-blue staining.
- Sample C2B8-25t showed specific antigen binding by indirect immunofluorescence using CD20-positive and CD20-negative cells (FIG. 1), indicating that the synthesized VL and VH gene fragments were functionally correct.
- Oligosaccharide profiling with MALDI/TOF-MS The glycosylation profile of each antibody sample was analyzed by MALDI/TOF-MS of the released, neutral oligosaccharide mix. In this technique, oligosaccharides of different mass appear as separate peaks in the spectrum and their proportions are quantitatively reflected by the relative peak heights (Harvey, D. J., Rapid Common Mass Spectrom. 7:614-619 (1993); Harvey, D. J., et al., Glycoconj J. 15:333-338(1998)).
- Oligosaccharide structures were assigned to different peaks based on their expected molecular masses, previous structural data for oligosaccharides derived from IgGI mAbs produced in the same host, and information on the N-linked oligosaccharide biosynthetic pathway.
- GnTIII expression levels i.e., tetracycline concentration
- the amount of bisected oligosaccharides derived from the different antibody samples did not carry bisected oligosaccharides (FIGS. 2A and 2B).
- bisected structures amounted up to approximately 35% of the oligosaccharides pool in sample C2B8-2000t, i.e, at a basal level of GnTIII expression.
- ADCC activity of IDEC-C2B8 glycosylated variants Different C2B8 mAb glycosylation variants were compared for ADCC activity, measured as in vitro lysis of CD20-positive SB cells.
- sample C2B8-50t carried approximately equal levels of bisected and non-bisected oligosaccharides, but did not mediate significantly higher target-cell lysis.
- sample C2B8-25t which contained up to 80% of bisected oligosaccharide structures, was significantly more active than the rest of the samples in the whole antibody concentration range. It reached the maximal level of ADCC activity of sample C2B8-nt at a 10-fold lower antibody concentration (FIG. 4A).
- Sample C2B8-25t also showed a significant increase in the maximal ADCC activity with respect to the control (50% vs. 30% lysis).
- Sample C2B8-50t showed a slight increase in activity whereas sample C2B8-25t clearly out performed MabtheraTM at all antibody concentrations.
- SP2/0 mouse myeloma cells producing chG250 chimeric mAb were grown in standard cell culture medium supplemented with 1:100 (v/v) penicillin/streptomycin/antimycotic solution (SIGMA, Buchs, Switzerland). Cells were cultured at 37° C. in a 5% CO 2 humidified atmosphere in Tissue Culture Flasks. Medium was changed each 3-4 days. Cells were frozen in culture medium containing 10% DMSO.
- wt-chG250-SP2/0 myeloma cells were transfected by electroporation with a vector for constitutive expression of GnTIII operatively linked via an IRES to a puromycin resistance gene. 24 hours before electroporation culture medium was changed and cells were seeded at 5 ⁇ 10 5 cells/ml. Seven million cells were centrifuged for 4 min at 1300 rpm at 4° C. Cells were washed with 3 mL new medium and centrifuged again. Cells were resuspended in a volume of 0.3-0.5 ml of reaction mix, containing 1.25% (v/v) DMSO and 20-30 ⁇ g DNA in culture medium.
- the electroporation mix was then transferred to a 0.4 cm cuvette and pulsed at low voltage (250-300 V) and high capacitance (960 ⁇ F) using Gene Pulser from Bio Rad. After electroporation cells were quickly transferred to 6 mL 1.25% (v/v) DMSO culture medium in a T25 culture flask and incubated at 37° C. Stable integrants were selected by applying 2 ⁇ g/mL puromycin to the medium two days after electroporation. After 2-3 weeks a stable, puromycin-resitant mixed population was obtained. Single-cell derived clones were obtained via FACS and were subsequently expanded and maintained under puromycin selection.
- Clones 2F1, 3D3, 4E6, 4E8, 4G3, 5G2, 5H12 and the wild type were seeded at 3 ⁇ 10 5 cells/mL in a total volume of 130 ml culture medium, and cultivated in single Triple-flasks. Cells used for seeding were all in full exponential growth phase, therefore cells were considered to be at the same growth state when the production batches started. Cells were cultivated for 4 days. Supernatants containing the antibody were collected in the late exponential growth phase to ensure reproducibility . The chG250 monoclonal antibody was purified in two chromatographic steps.
- Culture supernatants containing the chG250 monoclonal antibody derived from each batch were first purified using a HiTrap Protein A affinity chromatography. Protein A is highly specific for the human IgG F c region. Pooled samples from the protein A eluate were buffer exchanged to PBS by cation-exchange chromatography on a Resource S 1 ml column (Amersham Pharmacia Biotech). Final purity was judged to be higher than 95% from SDS-staining and Coomassie blue staining (FIG. 6). The concentration of each sample was determined with a standard calibration curve using wild type antibody with known concentration.
- Oligosaccharide profiles were obtained by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI/TOF-MS), which accurately provides the molecular masses of the different oligosaccharide structures.
- MALDI/TOF-MS matrix-assisted laser desorption/ionization time of flight mass spectrometry
- This technique allows a quantitative analysis of proportions between different oligosacchaiide structures within a mixture.
- Neutral oligosaccharides appeared predominantly as [M+Na + ] ions, however sometimes they were accompanied by smaller [M+K + ] ions, leading to an increase in mass of m/z of 16.
- the percentage of the structure appearing as potassium ion adducts depends on the content of the matrix and may thus vary between samples.
- a mixture of neutral N-linked oligosaccharides derived from each antibody preparation was analyzed using a 2,5-dehydrobenzoic acid (2,5-DHB) as matrix.
- 2,5-DHB 2,5-dehydrobenzoic acid
- Some of the peaks in the spectra were unequivocally assigned to specific oligosaccharide structures, because of known monosaccharide composition and unique mass. However, sometimes multiple structures could be assigned to a particular mass.
- MALDI enables the determination of the mass and cannot distinguish between isomers.
- Knowledge of the biosynthetic pathway and previous structural data enable, in most cases, the assignment of an oligosaccharide structure to a peak in the spectrum.
- GCTIII generated bisected Fc-associated oligosaccharide structures of two types: complex or hybrid.
- Complex bisected oligosaccharides were unequivocally assigned to peaks at m/z 1543, 1689, 1705, 1851 and 1867 ([M+K + ] adduct).
- the increase in bisected oligosaccharides was accompanied by a concomitant reduction of peaks m/z 1486 and 1648, that correspond to nonbisected complex oligosaccharides.
- the main substrate of GnTIII m/z 1486) decreased dramatically.
- the percentage of the nonbisected complex oligosaccharide type assigned to peak at m/z 1648, had the lowest values for the clones expressing the highest GnTIII levels (clones 4E6, 4E8, 5G2 and 5H12). These two peaks decreased in favor of the accumulation of bisected complex and bisected hybrid type oligosaccharides (FIGS. 7 A- 7 D and 8 A- 8 D). The percentage of bisected complex oligosaccharides was higher for the samples derived from the clones expressing lower amounts of GnTIII. This is consistent with the fact that a higher GnTIII expression level probably shifts the biosynthetic flux to bisected hybrid structures, thereby decreasing the relative proportions of complex and complex bisected compound.
- Peaks m/z 1664, 1680, 1810 and 1826 can be assigned to either bisected hybrid type, to galactosylated complex oligosaccharides, or a mixture of them. Due to the fact that the wt-antibody preparation had a relatively low percentage of peak 1664, it was assumed that this peak, appearing in significant amounts in the antibody samples derived from the different clones, corresponded entirely to bisected hybrid structures (FIGS. 7 A- 7 D and 8 A- 8 D).
- the Calcein-AM retention method of measuring cytotoxicity measures the dye fluorescence remaining in the cells after incubation with the antibody.
- Four million G250 antigen-positive cells (target) were labelled with 10 ⁇ M Calcein-AM (Molecular Probes, Eugene, Oreg.) in 1.8 mL RPMI-1640 cell culture medium (GIBCO BRL, Basel, Switzerland) supplemented with 10% fetal calf serum for 30 min at 37° C. in a 5% CO 2 humidified atmosphere.
- the cells were washed twice in culture medium and resuspended in 12 mL AIMV serum free medium (GIBCO BRL, Basel, Switzerland).
- PBMC Peripheral blood mononuclear cells
- 96-well plate was centrifuged at 700 ⁇ g for 5 min and the supernatants were discarded.
- the cell pellets were washed twice with Hank's balanced salt solution (HBSS) and lysed in 200 ⁇ L 0.05M sodium borate, pH 9, 0.1% Triton X-100. Retention of the fluorescent dye in the target cells was measured with a FLUO star microplate reader (BMG Lab Technologies, Offenburg, Germany).
- the specific lysis was calculated relative to a total lysis control, resulting from exposure of the target cells to saponin (200 ⁇ g/mL in AIMV; SIGMA, Buchs, Switzerland) instead of exposure to antibody.
- F med represents the fluorescence of target cells treated with medium alone and considers unspecific lysis by PMBCs
- F exp represents the fluorescence of cells treated with antibody
- F det represents the fluorescence of cells treated with saponin instead of antibody.
- Unmodified chG250 antibody did not mediate significant ADCC activity over the entire concentration range used in the assay (the activity was not significantly different from background).
- Augmented ADCC activity (close to 20%, see FIG. 9) at 2 ⁇ g/mL was observed with the antibody sample derived from clone 3D3, which expressed intermediate GnTIII levels.
- the cytotoxic activity of this antibody samples did not grow at higher antibody concentrations.
- the antibody preparation derived from clone 5H12 showed a striking increase over samples 3D3 and unmodified antibody in its ability to mediate ADCC against target cells.
- the maximal ADCC activity of this antibody preparation was around 50% andwas remarkable in mediating significant ADCC activity at 125-fold less concentrated when comparing with the unmodified control sample.
- Immune-mediated, acquired pure red cell aplasia is a rare disorder frequently associated with other autoimmune phenomena.
- an anti-CD20 chimeric monoclonal antibody prepared by the methods of the present invention and having increased ADCC is administered to the subject as described in Zecca, M. et al., Blood 12:3995-97 (1997) (the entire contents of which are hereby incorporated by reference).
- a subject with PRCA and autoimmune hemolytic anemia is given two doses of antibody, 375 mg/m 2 , per week.
- substitutive treatment with intravenous immunoglobulin is initiated. This treatment produces a marked depletion of B cells and a significant rise in reticulocyte count accompanied by increased hemoglobin levels.
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Microbiology (AREA)
- General Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Urology & Nephrology (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Radiology & Medical Imaging (AREA)
- Epidemiology (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Abstract
Description
- 1. Field of the Invention
- The present invention relates to the field of glycosylation engineering of proteins. More particularly, the present invention relates to glycosylation engineering to generate proteins with improved therapeutic properties, including antibodies with increased antibody-dependent cellular cytotoxicity.
- 2. Background Art
- Glycoproteins mediate many essential functions in human beings, other eukaryotic organisms, and some prokaryotes, including catalysis, signaling, cell-cell communication, and molecular recognition and association. They make up the majority of non-cytosolic proteins in eukaryotic organisms. (Lis et al., Eur. J. Biochem. 218:1-27 (1993)). Many glycoproteins have been exploited for therapeutic purposes, and during the last two decades, recombinant versions of naturally-occurring, secreted glycoproteins have been a major product of the biotechnology industry. Examples include erythropoietin (EPO), therapeutic monoclonal antibodies (therapeutic mAbs), tissue plasminogen activator (tPA), interferon-β, (IFN-β), granulocyte-macrophage colony stimulating factor (GM-CSF), and human chorionic gonadotrophin (hCG). (Cumming et al., Glycobiology 1:115-130 (1991)).
- The oligosaccharide component can significantly affect properties relevant to the efficacy of a therapeutic glycoprotein, including physical stability, resistance to protease attack, interactions with the immune system, pharmacokinetics, and specific biological activity. Such properties may depend not only on the presence or absence, but also on the specific structures, of oligosaccharides. Some generalizations between oligosaccharide structure and glycoprotein function can be made. For example, certain oligosaccharide structures mediate rapid clearance of the glycoprotein from the bloodstream through interactions with specific carbohydrate binding proteins, while others can be bound by antibodies and trigger undesired immune reactions. (Jenkins et al., Nature Biotechnol. 14:975-81 (1996)).
- Mammalian cells are the preferred hosts for production of therapeutic glycoproteins, due to their capability to glycosylate proteins in the most compatible form for human application. (Cumming et al., Glycobiology 1:115-30 (1991); Jenkins et al., Nature Biotechnol. 14:975-81 (1996)). Bacteria very rarely glycosylate proteins, and like other types of common hosts, such as yeasts, filamentous fungi, insect and plant cells, yield glycosylation patterns associated with rapid clearance from the bloodstream, undesirable immune interactions, and in some specific cases, reduced biological activity. Among mammalian cells, Chinese hamster ovary (CHO) cells have been most commonly used during the last two decades. In addition to giving suitable glycosylation patterns, these cells allow consistent generation of genetically stable, highly productive clonal cell lines. They can be cultured to high densities in simple bioreactors using serum-free media, and permit the development of safe and reproducible bioprocesses. Other commonly used animal cells include baby hamster kidney (BHK) cells, NS0- and SP2/0-mouse myeloma cells. More recently, production from transgenic animals has also been tested. (Jenkins et al., Nature Biotechnol. 14:975-81 (1996)).
- All antibodies contain carbohydrate structures at conserved positions in the heavy chain constant regions, with each isotype possessing a distinct array of N-linked carbohydrate structures, which variably affect protein assembly, secretion or functional activity. (Wright, A., and Morrison, S. L., Trends Biotech. 15:26-32 (1997)). The structure of the attached N-linked carbohydrate varies considerably, depending on the degree of processing, and can include high-mannose, multiply-branched as well as biantennary complex oligosaccharides. (Wright, A., and Morrison, S. L., Trends Biotech. 15:26-32 (1997)). Typically, there is heterogeneous processing of the core oligosaccharide structures attached at a particular glycosylation site such that even monoclonal antibodies exist as multiple glycoforms. Likewise, it has been shown that major differences in antibody glycosylation occur between cell lines, and even minor differences are seen for a given cell line grown under different culture conditions. (Lifely, M. R. et al., Glycobiology 5(8):813-22 (1995)).
- Unconjugated monoclonal antibodies (mAbs) can be useful medicines for the treatment of cancer, as demonstrated by the U.S. Food and Drug Administration's approval of Rituximab (Rituxan™; IDEC Pharmaceuticals, San Diego, Calif., and Genentech Inc., San Francisco, Calif.), for the treatment of CD20 positive B-cell, low-grade or follicular Non-Hodgkin's lymphoma, and Trastuzumab (Herceptin™; Genentech Inc,) for the treatment of advanced breast cancer (Grillo-Lopez, A. -J., et al., Semin. Oncol. 26:66-73 (1999); Goldenberg, M. M., Clin. Ther. 21:309-18 (1999)). The success of these products relies not only on their efficacy but also on their outstanding safety profiles (Grillo-Lopez, A. -J., et al., Semin. Oncol. 26:66-73 (1999); Goldenberg, M. M., Clin. Ther. 21:309-18 (1999)). In spite of the achievements of these two drugs, there is currently a large interest in obtaining higher specific antibody activity than what is typically afforded by unconjugated mAb therapy.
- One way to obtain large increases in potency, while maintaining a simple production process and potentially avoiding significant, undesirable side effects, is to enhance the natural, cell-mediated effector functions of mabs by engineering their oligosaccharide component (Umaña, P. et al., Nature Biotechnol. 17:176-180 (1999)). IgGl type antibodies, the most commonly used antibodies in cancer immunotherapy, are glycoproteins that have a conserved N-linked glycosylation site at Asn297 in each CH2 domain. The two complex bi-antennary oligosaccharides attached to Asn297 are buried between the CH2 domains, forming extensive contacts with the polypeptide backbone, and their presence is essential for the antibody to mediate effector functions such as antibody dependent cellular cytotoxicity (ADCC) (Lifely, M. R., et al., Glycobiology 5:813-822 (1995); Jefferis, R., et al., Immunol Rev. 163:59-76 (1998); Wright, A. and Morrison, S. L., Trends Biotechnol. 15:26-32 (1997)).
- The present inventors showed previously that over expression in Chinese hamster ovary (CHO) cells of β(1,4)-N-acetylglucosaminyltransferase III (GnTIII), a glycosyltransferase catalyzing the formation of bisected oligosaccharides, significantly increases the in vitro ADCC activity of an anti-neuroblastoma chimeric monoclonal antibody (chCE7) produced by the engineered CHO cells. (See Umaña, P. et al., Nature Biotechnol. 17:176-180 (1999), International Publication No. WO 99/54342, the entire contents of each of which are hereby incorporated by reference in their entirety). The antibody chCE7 belongs to a large class of unconjugated mAbs which have high tumor affinity and specificity, but have too little potency to be clinically useful when produced in standard industrial cell lines lacking the GnTIII enzyme (Umana, P., et al., Nature Biotechnol. 17:176-180 (1999)). That study was the first to show that large increases of maximal in vitro ADCC activity could be obtained by increasing the proportion of constant region (Fc)-associated, bisected oligosaccharides above the levels found in naturally occurring antibodies. To determine if this fading could be extrapolated to an unconjugated mAb, which already has significant ADCC activity in the absence of bisected oligosaccharides, the present inventors have applied this technology to Rituximab, the anti-CD20, IDEC-C2B8 chimeric antibody. The present inventors have likewise applied the technology to the unconjugated anti-cancer mAb chG250.
- The present inventors have now generated new glycosylation variants of the anti-CD20 monoclonal antibody (mAb) IDEC-C2B8 (Rituximab) and the anti-cancer mAb chG250 using genetically engineered mAb-producing cell lines that overexpress N-acetylglucosaminyltransferase III (GnTIII; EC 2.1.4.144) in a tetracycline regulated fashion. GnTIII is required for the synthesis of bisected oligosaccharides, which are found at low to intermediate levels in naturally-occurring human antibodies but are missing in mAbs produced in standard industrial cell lines. The new glycosylated versions out performed Mabthera™ (the version of Rixtuximab marketed in Europe) and mouse-myeloma derived chG250 in biological (ADCC) activity. For example, a ten-fold lower amount of the variant carrying the highest levels of bisected oligosaccharides was required to reach the maximal ADCC activity as Mabthera™. For chG250, the variant carrying the highest levels of bisected oligosaccharides mediated significant ADCC activity at a 125-fold lower concentration than that required to detect even low ADCC activity by the unmodified control chG250. A clear correlation was found between the level of GnTIII expression and ADCC activity.
- Accordingly, in one aspect the claimed invention is directed to a host cell engineered to produce a polypeptide having increased Fc-mediated cellular cytotoxicity by expression of at least one nucleic acid encoding β(1,4)-N-acetylglucosaminyltransferase III (GnT III), wherein the polypeptide produced by the host cell is selected from the group consisting of a whole antibody molecule, an antibody fragment, and a fusion protein which includes a region equivalent to the Fc region of an immunoglobulin, and wherein the GnT III is expressed in an amount sufficient to increase the proportion of said polypeptide carrying bisected hybrid oligosaccharides or galactosylated complex oligosaccharides or mixtures thereof in the Fc region relative to polypeptides carrying bisected complex oligosaccharides in the Fc region.
- In a preferred embodiment, the polypeptide is IgG or a fragment thereof, most preferably, IgG1 or a fragment thereof. In a further preferred embodiment, the polypeptide is a fusion protein that includes a region equivalent to the Fc region of a human IgG.
- In another aspect of the claimed invention, a nucleic acid molecule comprising at least one gene encoding GnTIII has been introduced into the host cell. In a preferred embodiment, at least one gene encoding GnTIII has been introduced into the host cell chromosome.
- Alternatively, the host cell has been engineered such that an endogenous GnT III gene is activated, for example, by insertion of a DNA element which increases gene expression into the host chromosome. In a preferred embodiment, the endogenous GnTIII has been activated by insertion of a promoter, an enhancer, a transcription factor binding site, a transposon, or a retroviral element or combinations thereof into the host cell chromosome. In another aspect, the host cell has been selected to carry a mutation triggering expression of an endogenous GnTIII. Preferably, the host cell is the CHO cell mutant lec 10.
- In a further preferred embodiment of the claimed invention, the at least one nucleic acid encoding a GnTIII is operably linked to a constitutive promoter element.
- In a further preferred embodiment, the host cell is a CHO cell, a BHK cell, a NSO cell, a SP2/0 cell, or a hybridoma cell, a YO myeloma cell, a P3X63 mouse myeloma cell, a PER cell or a PER.C6 cell and said polypeptide is an anti-CD20 antibody. In another preferred embodiment, the host cell is a SP2/0 cell and the polypeptide is the monoclonal antibody chG250.
- In another aspect, the claimed invention is directed to a host cell that further comprises at least one transfected nucleic acid encoding an antibody molecule, an antibody fragment, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin. In a preferred embodiment, the host cell comprises at least one transfected nucleic acid encoding an anti-CD20 antibody, the chimeric anti-human neuroblastoma monoclonal antibody chCE7, the chimeric anti-human renal cell carcinoma monoclonal antibody chG250, the chimeric anti-human colon, lung, and breast carcinoma monoclonal antibody ING-1, the humanized anti-human 17-1A antigen monoclonal antibody 3622W94, the humanized anti-human colorectal tumor antibody A33, the anti-human melanoma antibody directed against GD3 ganglioside R24, or the chimeric anti-human squamous-cell carcinoma monoclonal antibody SF-25, an anti-human EGFR antibody, an anti-human EGFRvIII antibody, an anti-human PSMA antibody, and anti-human PSCA antibody, an anti-human CD22 antibody, an anti-human CD30 antibody, an anti-human CD33 antibody, an anti-human CD38 antibody, an anti-human CD40 antibody, an anti-human CD45 antibody, an anti-human CD52 antibody, an anti-human CD138 antibody, an anti-human HLA-DR variant antibody, an anti-human EpCAM antibody, an anti-human CEA antibody, an anti-human MUC1 antibody, an anti-human MUC1 core protein antibody, an anti-human aberrantly glycosylated MUCI antibody, an antibody against human fibronectin variants containing the ED-B domain, and an anti-human HER2/neu antibody.
- In another aspect, the claimed invention is directed to a method for producing a polypeptide in a host cell comprising culturing any of the above-described the host cells under conditions which permit the production of said polypeptide having increased Fc-mediated cellular cytotoxicity. In a preferred embodiment, the method further comprises isolating said polypeptide having increased Fc-mediated cellular cytotoxicity.
- In a further preferred embodiment, the host cell comprises at least one nucleic acid encoding a fusion protein comprising a region equivalent to a glycosylated Fc region of an immunoglobulin.
- In a preferred embodiment, the proportion of bisected oligosaccharides in the Fc region of said polypeptides is greater than 50%, more preferably, greater than 70%. In another embodiment, the proportion of bisected hybrid oligosaccharides or galactosylated complex oligosaccharides or mixtures thereof in the Fc region is greater than the proportion of bisected complex oligosaccharides in the Fc region of said polypeptide.
- In a preferred aspect of the claimed method, the polypeptide is an anti-CD20 antibody and the anti-CD20 antibodies produced by said host cell have a glycosylation profile, as analyzed by MALDI/TOF-MS, that is substantially equivalent to that shown in FIG. 2E.
- In another preferred aspect of the claimed method, the polypeptide is the chG250 monoclonal antibody and the chG250 antibodies produced by said host cell have a glycosylaton profile, as analyzed by MALDI/TOF-MS, that is substantially equivalent to that shown in FIG. 7D.
- In a further aspect, the claimed invention is directed to an antibody having increased antibody dependent cellular cytotoxicity (ADCC) produced by any of the methods described above. In preferred embodiments, the antibody is selected from the group consisting of an anti-CD20 antibody, chCE7, ch-G250, a humanized anti-HER2 monoclonal antibody, ING-1, 3622W94, SF-25, A33, and R24. Alternatively, the polypeptide can be an antibody fragment that includes a region equivalent to the Fc region of an immunoglobulin, having increased Fc-mediated cellular cytotoxicity produced by any of the methods described above.
- In a further aspect, the claimed invention is directed to a fusion protein that includes a region equivalent to the Fe region of an immunoglobulin and having increased Fc-mediated cellular cytotoxicity produced by any of the methods described above.
- In a further aspect, the claimed invention is directed to a pharmaceutical composition comprising the antibody, antibody fragment, or fusion protein of the invention and a pharmaceutically acceptable carrier.
- In a further aspect, the claimed invention is directed to a method for the treatment of cancer comprising administering a therapeutically effective amount of said pharmaceutical composition to a patient in need thereof.
- In a further aspect, the invention is directed to an improved method for treating an autoimmune disease produced in whole or in part by pathogenic autoantibodies based on B-cell depletion comprising administering a therapeutically effective amount of immunologically active antibody to a human subject in need thereof, the improvement comprising administering a therapeutically effective amount of an antibody having increased ADCC prepared as described above. In a preferred embodiment, the antibody is an anti-CD20 antibody. Examples of autoimmune diseases or disorders include, but are not limited to, immune-mediated thrombocytopenias, such as acute idiopathic thrombocytopenic purpurea and chronic idiopathic thrombocytopenic purpurea, dermatomyositis, Sydenham's chorea, lupus nephritis, rheumatic fever, polyglandular syndromes, Henoch-Schonlein purpura, post-streptococcal nephritis, erythema nodosum, Takayasu's arteritis, Addison's disease, erythema multiform, polyarteritis nodosa, ankylosing spondylitis, Goodpasture's syndrome, thromboangitis ubiterans, primary biliary cirrhosis, Hashimoto's thyroiditis, thyrotoxicosis, chronic active hepatitis, polymyositis/dermnatomyositis, polychondritis, pamphigus vulgaris, Wegener's granulomatosis, membranous nephropathy, amyotrophic lateral sclerosis, tabes dorsalis, polymyaglia, pernicious anemia, rapidly progressive glomerulonephritis and fibrosing alveolitis, inflammatory responses such as inflammatory skin diseases including psoriasis and dermatitis (e.g. atopic dermatitis); systemic scleroderma and sclerosis; responses associated with inflammatory bowel disease (such as Crohn's disease and ulcerative colitis); respiratory distress syndrome (including adult respiratory distress syndrome; ARDS); dermatitis; meningitis; encephalitis; uveitis; colitis; glomerulonephritis; allergic conditions such as eczema and asthma and other conditions involving infiltration of T cells and chronic inflammatory responses; atherosclerosis; leukocyte adhesion deficiency; rheumatoidarthritis; systemic lupus erythematosus (SLE); diabetes mellitus (e.g. Type 1 diabetes mellitus or insulin dependent diabetes mellitus); multiple sclerosis; Reynaud's syndrome; autoimmune thyroiditis; allergic encephalomyelitis; Sjorgen's syndrome; juvenile onset diabetes; and immune responses associated with acute and delayed hypersensitivity mediated by cytokines and T-lymphocytes typically found in tuberculosis, sarcoidosis, polymyositis, granulomatosis and vasculitis; pernicious amenia (Addison's disease); diseases involving leukocyte diapedesis; central nervous system (CNS) inflammatory disorder, multiple organ injury syndrome; hemolytic anemia (including, but not limited to cryoglobinemia or Coombs positive anemia); myastheniagravis; antigen-antibody complex mediated diseases; anti-glomerular basement membrane disease; antiphospholipid syndrome; allergic neuritis; Graves' disease; Lambert-Eaton myasthenic syndrome; pemphigoid bullous; pemphigus; autoimmune polyendocrinopathies; Reiter's disease; stiff-man syndrome; Behcet disease; giant cell arteritis; immune complex nephritis; IgA nephropathy; IgM polyneuropathies; immune thrombocytopenic purpura (ITP) or autoimmune thrombocytopenia etc. In this aspect of the invention, the antibodies of the invention are used to deplete the blood of normal B-cells for an extended period.
- FIG. 1. Indirect immunofluorescence assay showing the reactivity of the antibody preparation C2B8-25t to CD20 positive SB cells. Negative controls, including the HSB CD20 negative cell line and cells treated only with the secondary FITC-conjugated anti-human Fc polyclonal antibody are not shown.
- FIG. 2A-2E. MALDI/TOF-MS spectra of the oligosaccharides derived from Mabthera™ (FIG. 2A), C2B8-nt (FIG. 2B), C2B8-2000t (FIG. 2C), C2B8-50t (FIG. 2D), and C2B8-25t (FIG. 2E) antibody samples. Oligosaccharides appear as [M+Na +] and [M+K+] ions. Oligosaccharide appearing in the first two spectra were derived from cell cultures that do not express GnTIII, whereas oligosaccharides in C, D, and E were derived from a single cell line expressing GnTIII at different levels (i.e. tetracycline concentrations). FIG. 3A and 3B. Illustration of a typical human IgG Fc-associated oligosaccharide structure (A) and partial N-linked glycosylation pathway (B). FIG. 3A) The core of the oligosaccharide is composed of three mannose (M) and two N-acetylglucosamine (Gn) monosaccharide residues attached to Asn297. Galactose (G), fucose (F), and bisecting N-acetylglucosamine (Gn, boxed) can be present or absent. Terminal N-acetylneuraminic acid may be also present but it is not included in the figure. (FIG. 3B) Partial N-linked glycosylation pathway leading to the formation of the major oligosaccharide classes (dotted frames). Bisecting N-acetylglucosamine is denoted as Gnb. Subscript numbers indicate how many monosaccharide residues are present in each oligosaccharide. Each structure appears together with its sodium-associated [M+Na+] mass. The mass of those structures that contain fucose (f) are also included.
- FIG. 4A and 4B. ADCC activities of Rituximab glycosylation variants. The percentage of cytotoxicity was measured via lysis of 51Cr labeled CD20-positive SB cells by human lymphocytes (E:T ratio of 100:1) mediated by different mAb concentrations. (FIG. 4A) Activity of C2B8 samples derived from a single cell line but produced at increasing GnTIII expression levels (i.e., decreasing tetracycline concentrations). The samples are C2B8-2000t, C2B8-50t, C2B8-25t, and C2B8-nt (control mAb derived from a clone that does not express GnTIII (FIG. 4B) ADCC activity of C2B8-50t and C2B8-25t compared to Mabthera™.
- FIG. 5. Western blot analysis of the seven GnTIII expressing clones and the wild type. 30 μg of each sample were loaded on a 8.75% SDS gel, transferred to a PVDF membrane and probed with the anti-c-myc monoclonal antibody (9E10). WT refers to wt-chG250-SP2/0 cells.
- FIG. 6. SDS polyacrylamide gel electrophoresis of resolved purified antibody samples.
- FIG. 7A- 7D. MALDI/TOF-MS spectra of neutral oligosaccharide mixtures from chG250 mAb samples produced by clones expressing different GnTIII levels and wt-chG250-SP2/0 cells: WT (FIG. 7A), 2F1 (FIG. 7B), 3D3 (FIG. 7C), 4E6 (FIG. 7D).
- FIG. 8A- 8D. MALDI/TOF-MS spectra of neutral oligosaccharide mixtures from chG250 mAb samples produced by clones expressing different GnTIII levels: 4E8, (FIG. 8A); 5G2, (FIG. 8B); 4G3, (FIG. 8C); 5H12, (FIG. 8D).
- FIG. 9. In vitro ADCC assay of antibody samples derived from control wt-chG250-SP2/-cells and GnTIII transected clones 3D3 and 5H12.
- Terms are used herein as generally used in the art, unless otherwise defined as follows:
- As used herein, the term antibody is intended to include whole antibody molecules, antibody fragments, or fusion proteins that include a region equivalent to the Fc region of an immunoglobulin.
- As used herein, the term region equivalent to the Fc region of an immunoglobulin is intended to include naturally occurring allelic variants of the Fc region of an immunoglobulin as well as variants having alterations which produce substitutions, additions, or deletions but which do not decrease substantially the ability of the immunoglobulin to mediate antibody dependent cellular cytotoxicity. For example, one or more amino acids can be deleted from the N-terminus or C-terminus of the Fc region of an immunoglobulin without substantial loss of biological function. Such variants can be selected according to general rules known in the art so as to have minimal effect on activity. (See, e.g., Bowie, J. U. et al., Science 247:1306-10 (1990).
- As used herein, the term glycoprotein-modifying glycosyl transferase refers to β(1,4)-N-acetylglucosaminyltransferase III (GnTIII).
- As used herein, the terms engineer, engineered, engineering and glycosylation engineering are considered to include any manipulation of the glycosylation pattern of a naturally occurring polypeptide or fragment thereof. Glycosylation engineering includes metabolic engineering of the glycosylation machinery of a cell, including genetic manipulations of the oligosaccharide synthesis pathways to achieve altered glycosylation of glycoproteins expressed in cells. Furthermore, glycosylation engineering includes the effects of mutations and cell environment on glycosylation.
- As used herein, the term host cell covers any kind of cellular system which can be engineered to generate modified glycoforms of proteins, protein fragments, or peptides of interest, including antibodies and antibody fragments. Typically, the host cells have been manipulated to express optimized levels of GnT III. Host cells include cultured cells, e.g., mammalian cultured cells, such as CHO cells, BHK cells, NSO cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER. C6 cells or hybridoma cells, yeast cells, and insect cells, to name only a few, but also cells comprised within a transgenic animal or cultured tissue.
- As used herein, the term Fc-mediated cellular cytotoxicity includes antibody-dependent cellular cytotoxicity and cellular cytotoxicity mediated by a soluble Fc-fusion protein containing a human Fc-region. It is an immune mechanism leading to the lysis of“antibody-targeted cells” by “human immune effector cells”, wherein:
- The “human immune effector cells” are a population of leukocytes that display Fc receptors on their surface through which they bind to the Fc-region of antibodies or of Fc-fusion proteins and perform effector functions. Such a population may include, but is not limited to, peripheral blood mononuclear cells (PBMC) and/or natural killer (NK) cells.
- The “antibody-targeted cells” are cells bound by the antibodies or Fc-fusion proteins. The antibodies or Fc fusion-proteins bind to target cells via the protein part N-terminal to the Fc region.
- As used herein, the term increased Fc-mediated cellular cytotoxicity is defined as either an increase in the number of “antibody-targeted cells” that are lysed in a given time, at a given concentration of antibody, or of Fc-fusion protein, in the medium surrounding the target cells, by the mechanism of Fc-mediated cellular cytotoxicity defined above, and/or a reduction in the concentration of antibody, or of Fc-fusion protein, in the medium surrounding the target cells, required to achieve the lysis of a given number of “antibody-targeted cells”, in a given time, by the mechanism of Fc -mediated cellular cytotoxicity. The increase in Fc-mediated cellular cytotoxicity is relative to the cellular cytotoxicity mediated by the same antibody, or Fc-fusion protein, produced by the same type of host cells, using the same standard production, purification, formulation and storage methods, which are known to those skilled in the art, but that has not been produced by host cells engineered to express the glycosyltransferase GnTIII by the methods described herein.
- By antibody having increased antibody dependent cellular cytotoxicity (ADCC) is meant an antibody having increased ADCC as determined by any suitable method known to those of ordinary skill in the art. One accepted in vitro ADCC assay is as follows:
- 1) the assay uses target cells that are known to express the target antigen recognized by the antigen-binding region of the antibody;
- 2) the assay uses human peripheral blood mononuclear cells (PBMCs), isolated from blood of a randomly chosen healthy donor, as effector cells;
- 3) the assay is carried out according to following protocol:
- i) the PBMCs are isolated using standard density centriftigation procedures and are suspended at 5×10 6 cells/ml in RPMI cell culture medium;
- ii) the target cells are grown by standard tissue culture methods, harvested from the exponential growth phase with a viability higher than 90%, washed in RPMI cell culture medium, labelled with 100 micro-Curies of 51Cr, washed twice with cell culture medium, and resuspended in cell culture medium at a density of 105 cells/ml;
- iii) 100 microliters of the final target cell suspension above are transferred to each well of a 96-well microtiter plate;
- iv) the antibody is serially-diluted from 4000 ng/ml to 0.04 ng/ml in cell culture medium and 50 microliters of the resulting antibody solutions are added to the target cells in the 96-well microtiter plate, testing in triplicate various antibody concentrations covering the whole concentration range above;
- v) for the maximum release(MR) controls, 3 additional wells in the plate containing the labelled target cells, receive 50 microliters of a 2% (V/V) aqueous solution of non-ionic detergent (Nonidet, Sigma, St. Louis), instead of the antibody solution (point iv above);
- vi) for the spontaneous release (SR) controls, 3 additional wells in the plate containing the labelled target cells, receive 50 microliters of RPMI cell culture medium instead of the antibody solution (point iv above);
- vii) the 96-well microtiter plate is then centrifuged at 50×g for 1 minute and incubated for 1 hour at 4° C.;
- viii) 50 microliters of the PBMC suspension (point i above) are added to each well to yield an effector: target cell ratio of 25:1 and the plates are placed in an incubator under 5% CO 2 atmosphere at 37° C. for 4 hours;
- ix) the cell-free supernatant from each well is harvested and the experimentally released radioactivity (ER) is quantified using a gamma counter;
- x) the percentage of specific lysis is calculated for each antibody concentration according to the formula (ER-MR)/(MR-SR)×100, where ER is the average radioactivity quantified (see point ix above) for that antibody concentration, MR is the average radioactivity quantified (see point ix above) for the MR controls (see point v above), and SR is the average radioactivity quantified (see point ix above) for the SR controls (see point vi above);
- 4) “increased ADCC” is defined as either an increase in the maximum percentage of specific lysis observed within the antibody concentration range tested above, and/or a reduction in the concentration of antibody required to achieve one half of the maximum percentage of specific lysis observed within the antibody concentration range tested above. The increase in ADCC is relative to the ADCC, measured with the above assay, mediated by the same antibody, produced by the same type of host cells, using the same standard production, purification, formulation and storage methods, which are known to those skilled in the art, but that has not been produced by host cells engineered to overexpress the glycosyltransferase GnTIII.
- As used herein, the term anti-CD20 antibody is intended to mean an antibody which specifically recognizes a cell surface non-glycosylated phosphoprotein of 35,000 Daltons, typically designated as the human B lymphocyte restricted differentiation antigen Bp35, commonly referred to as CD20.
- Identification and Generation of Nucleic Acids Encoding A Protein for which Modification of the Glycosylation Pattern is Desired
- The present invention provides methods for the generation and use of host cell systems for the production of glycoforms of antibodies or antibody fragments or fusion proteins which include antibody fragments with increased antibody-dependent cellular cytotoxicity. Identification of target epitopes and generation of antibodies having potential therapeutic value, for which modification of the glycosylation pattern is desired, and isolation of their respective coding nucleic acid sequence is within the scope of the invention.
- Various procedures known in the art may be used for the production of antibodies to target epitopes of interest. Such antibodies include but are not limited to polyclonal, monoclonal, chimeric, single chain, Fab fragments and fragments produced by an Fab expression library. Such antibodies may be useful, e.g., as diagnostic or therapeutic agents. As therapeutic agents, neutralizing antibodies, i.e., those which compete for binding with a ligand, substrate or adapter molecule, are of especially preferred interest.
- For the production of antibodies, various host animals are immunized by injection with the target protein of interest including, but not limited to, rabbits, mice, rats, etc. Various adjuvants may be used to increase the immunological response, depending on the host species, including but not limited to Freund's (complete and incomplete), mineral gels such as aluminum hydroxide, surface active substances such as lysolecithin, pluronic polyols, polyanions, peptides, saponin, oil emulsions, keyhole limpet hemocyanin, dinitrophenol, and potentially useful human adjuvants such as BCG (bacille Calmette-Guerin) and Corynebacterium parvum.
- Monoclonal antibodies to the target of interest may be prepared using any technique which provides for the production of antibody molecules by continuous cell lines in culture. These include, but are not limited to, the hybridoma technique originally described by Kohler and Milstein, Nature 256:495-97 (1975), the human B-cell hybridoma technique (Kosbor et al., Immunology Today 4:72 (1983); Cote et al., Proc. Natl. Acad. Sci. U.S.A. 80:2026-30 (1983 ) and the EBV-hybridoma technique (Cole et al., Monoclonal Antibodies and Cancer Therapy 77-96 (Alan R. Liss, Inc., 1985)). In addition, techniques developed for the production of “chimeric antibodies” (Morrison et al., Proc. Natl. Acad. Sci. U.S.A. 81:6851-55 (1984); Neuberger et al., Nature 312:604-08 (1984); Takeda et al., Nature 314:452-54 (1985) by splicing the genes from a mouse antibody molecule of appropriate antigen specificity together with genes from a human antibody molecule of appropriate biological activity can be used. Alternatively, techniques described for the production of single chain antibodies (U.S. Pat. No. 4,946,778) can be adapted to produce single chain antibodies having a desired specificity.
- Antibody fragments which contain specific binding sites of the target protein of interest may be generated by known techniques. For example, such fragments include, but are not limited to, F(ab′) 2 fragments which can be produced by pepsin digestion of the antibody molecule and the Fab fragments which can be generated by reducing the disulfide bridges of the F(ab′)2 fragments. Alternatively, Fab expression libraries may be constructed (Huse et al., Science 246:1275-81 (1989) to allow rapid and easy identification of monoclonal Fab fragments with the desired specificity to the target protein of interest.
- Once an antibody or antibody fragment has been identified for which modification in the glycosylation pattern are desired, the coding nucleic acid sequence is identified and isolated using techniques well known in the art.
- a. Generation of Cell Lines for the Production of Proteins with Altered Glycosylation Pattern
- The present invention provides host cell expression systems for the generation of proteins having modified glycosylation patterns. In particular, the present invention provides host cell systems for the generation of glycoforms of proteins having an improved therapeutic value. Therefore, the invention provides host cell expression systems selected or engineered to increase the expression of a glycoprotein-modifying glycosyltransferase, namely β(1,4)-N-acetylglucosaminyltransferase III (GnTIID). Specifically, such host cell expression systems may be engineered to comprise a recombinant nucleic acid molecule encoding GnTIII, operatively linked to a constitutive or regulated promoter system. Alternatively, host cell expression systems may be employed that naturally produce, are induced to produce, and/or are selected to produce GnTIII.
- In one specific embodiment, the present invention provides a host cell that has been engineered to express at least one nucleic acid encoding GnTIII. In one aspect, the host cell is transformed or transfected with a nucleic acid molecule comprising at least one gene encoding GnTIII. In an alternate aspect, the host cell has been engineered and/or selected in such way that endogenous GnTIII is activated. For example, the host cell may be selected to carry a mutation triggering expression of endogenous GnTIII. In one specific embodiment, the host cell is a CHO lec 10 mutant. Alternatively, the host cell may be engineered such that endogenous GnTIII is activated. In again another alternative, the host cell is engineered such that endogenous GnTIII has been activated by insertion of a constitutive promoter element, a transposon, or a retroviral element into the host cell chromosome.
- Generally, any type of cultured cell line can be used as a background to engineer the host cell lines of the present invention. In a preferred embodiment, CHO cells, BHK cells, NS0 cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER.C6 cells or hybridoma cells, yeast cells, or insect cells are used as the background cell line to generate the engineered host cells of the invention.
- The invention is contemplated to encompass any engineered host cells expressing GnTIII as defined herein.
- One or several nucleic acids encoding GnTIII may be expressed under the control of a constitutive promoter or, alternately, a regulated expression system. Suitable regulated expression systems include, but are not limited to, a tetracycline-regulated expression system, an ecdysone-inducible expression system, a lac-switch expression system, a glucocorticoid-inducible expression system, a temperature-inducible promoter system, and a metallothionein metal-inducible expression system. If several different nucleic acids encoding GnTIII are comprised within the host cell system, some of them may be expressed under the control of a constitutive promoter, while others are expressed under the control of a regulated promoter. The maximal expression level is considered to be the highest possible level of stable GnTIII expression that does not have a significant adverse effect on cell growth rate, and will be determined using routine experimentation Expression levels are determined by methods generally known in the art, including Western blot analysis using a GnTIII specific antibody, Northern blot analysis using a GnTIII specific nucleic acid probe, or measurement of enzymatic activity. Alternatively, a lectin may be employed which binds to biosynthetic products of the GnTIII, for example, E 4-PHA lectin. In a further alternative, the nucleic acid may be operatively linked to a reporter gene; the expression levels of the GnTIII are determined by measuring a signal correlated with the expression level of the reporter gene. The reporter gene may transcribed together with the nucleic acid(s) encoding said GnTIII as a single mRNA molecule; their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE). The reporter gene may be translated together with at least one nucleic acid encoding said GnTIII such that a single polypeptide chain is formed. The nucleic acid encoding the GnTIII may be operatively linked to the reporter gene under the control of a single promoter, such that the nucleic acid encoding the GnTIII and the reporter gene are transcribed into an RNA molecule which is alternatively spliced into two separate messenger RNA (mRNA) molecules; one of the resulting mRNAs is translated into said reporter protein, and the other is translated into said GnTIII.
- If several different nucleic acids encoding GnTIII are expressed, they may be arranged in such way that they are transcribed as one or as several mRNA molecules. If they are transcribed as a single mRNA molecule, their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE). They may be transcribed from a single promoter into an RNA molecule which is alternatively spliced into several separate messenger RNA (mRNA) molecules, which then are each translated into their respective encoded GnTIII.
- In other embodiments, the present invention provides host cell expression systems for the generation of therapeutic antibodies, having an increased antibody-dependent cellular cytotoxicity, and cells which display the IgG Fc region on the surface to promote Fc-mediated cytotoxicity. Generally, the host cell expression systems have been engineered and/or selected to express nucleic acids encoding the antibody for which the production of altered glycoforms is desired, along with at least one nucleic acid encoding GnTIII. In one embodiment, the host cell system is transfected with at least one gene encoding GnTIII. Typically, the transfected cells are selected to identify and isolate clones that stably express the GnTIII. In another embodiment, the host cell has been selected for expression of endogenous GnTIII. For example, cells may be selected carrying mutations which trigger expression of otherwise silent GnTIII. For example, CHO cells are known to carry a silent GnT III gene that is active in certain mutants, e.g., in the mutant Lec10. Furthermore, methods known in the art may be used to activate silent GnTIII, including the insertion of a regulated or constitutive promoter, the use of transposons, retroviral elements, etc. Also the use of gene knockout technologies or the use of ribozyme methods may be used to tailor the host cell's GnTIII expression level, and is therefore within the scope of the invention.
- Any type of cultured cell line can be used as background to engineer the host cell lines of the present invention. In a preferred embodiment, CHO cells, BHK cells, NS0 cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER.C6 cells or hybridoma cells, yeast cell, or insect cells may be used. Typically, such cell lines are engineered to further comprise at least one transfected nucleic acid encoding a whole antibody molecule, an antibody fragment, or a fusion protein that includes a region equivalent to the Fc region of an immunoglobulin. In an alternative embodiment, a hybridoma cell line expressing a particular antibody of interest is used as background cell line to generate the engineered host cells of the invention.
- Typically, at least one nucleic acid in the host cell system encodes GnT III.
- One or several nucleic acids encoding GnTIII may be expressed under the control of a constitutive promoter, or alternately, a regulated expression system Suitable regulated expression systems include, but are not limited to, a tetracycline-regulated expression system, an ecdysone-inducible expression system, a lac-switch expression system, a glucocorticoid-inducible expression system, a temperature-inducible promoter system, and a metallothionein metal-inducible expression system. If several different nucleic acids encoding GnTIII are comprised within the host cell system, some of them may be expressed under the control of a constitutive promoter, while others are expressed under the control of a regulated promoter. The maximal expression level is considered to be the highest possible level of stable GnTIII expression that does not have a significant adverse effect on cell growth rate, and will be determined using routine experimentation. Expression levels are determined by methods generally known in the art, including Western blot analysis using a GnTIII specific antibody, Northern blot analysis using a GnTIII specific nucleic acid probe, or measurement of GnTIII enzymatic activity. Alternatively, a lectin may be employed which binds to biosynthetic products of GnTIII, for example, E 4-PHA lectin. In a further alternative, the nucleic acid may be operatively linked to a reporter gene; the expression levels of the glycoprotein-modifying glycosyl transferase are determined by measuring a signal correlated with the expression level of the reporter gene. The reporter gene may transcribed together with the nucleic acid(s) encoding said glycoprotein-modifying glycosyl transferase as a single mRNA molecule; their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE). The reporter gene may be translated together with at least one nucleic acid encoding GnTIII such that a single polypeptide chain is formed. The nucleic acid encoding the GnTIII may be operatively linked to the reporter gene under the control of a single promoter, such that the nucleic acid encoding the GnTIII and the reporter gene are transcribed into an RNA molecule which is alternatively spliced into two separate messenger RNA (mRNA) molecules; one of the resulting mRNAs is translated into said reporter protein, and the other is translated into said GnTIII.
- If several different nucleic acids encoding a GnTIII are expressed, they may be arranged in such way that they are transcribed as one or as several mRNA molecules. If they are transcribed as single mRNA molecule, their respective coding sequences may be linked either by an internal ribosome entry site (IRES) or by a cap-independent translation enhancer (CITE). They may be transcribed from a single promoter into an RNA molecule which is alternatively spliced into several separate messenger RNA (mRNA) molecules, which then are each translated into their respective encoded GnTIII.
- i. Expression Systems
- Methods which are well known to those skilled in the art can be used to construct expression vectors containing the coding sequence of the protein of interest and the coding sequence of the GnTIII and appropriate transcriptional/translational control signals. These methods include in vitro recombinant DNA techniques, synthetic techniques and in vivo recombination/genetic recombination. See, for example, the techniques described in Maniatis et al., Molecular Cloning A Laboratory Manual, Cold Spring Harbor Laboratory, N.Y. (1989) and Ausubel et al., Current Protocols in Molecular Biology, Greene Publishing Associates and Wiley Interscience, N.Y (1989).
- A variety of host-expression vector systems maybe utilized to express the coding sequence of the protein of interest and the coding sequence of the GnTIII. Preferably, mammalian cells are used as host cell systems transfected with recombinant plasmid DNA or cosmid DNA expression vectors containing the coding sequence of the protein of interest and the coding sequence of the GnTIII. Most preferably, CHO cells, ByIK cells, NS0 cells, SP2/0 cells, YO myeloma cells, P3X63 mouse myeloma cells, PER cells, PER. C6 cells or hybridoma cells, yeast cells, or insect cells are used as host cell system. In alternate embodiments, other eukaryotic host cell systems may be contemplated, including, yeast cells transformed with recombinant yeast expression vectors containing the coding sequence of the protein of interest and the coding sequence of the GnTIII; insect cell systems infected with recombinant virus expression vectors (e.g., baculovirus) containing the coding sequence of the protein of interest and the coding sequence of the GnTIII; plant cell systems infected with recombinant virus expression vectors (e.g., cauliflower mosaic virus, CaMV; tobacco mosaic virus, TMV) or transformed with recombinant plasmid expression vectors (e.g., Ti plasmid) containing the coding sequence of the protein of interest and the coding sequence of the GnTIII; or animal cell systems infected with recombinant virus expression vectors (e.g., adenovirus, vaccinia virus) including cell lines engineered to contain multiple copies of the DNA encoding the protein of interest and the coding sequence of the GnTIII either stably amplified (CHO/dhfr) or unstably amplified in double-minute chromosomes (e.g., murine cell lines).
- For the methods of this invention, stable expression is generally preferred to transient expression because it typically achieves more reproducible results and also is more amenable to large scale production. Rather than using expression vectors which contain viral origins of replication, host cells can be transformed with the respective coding nucleic acids controlled by appropriate expression control elements (e.g., promoter, enhancer, sequences, transcription terminators, polyadenylation sites, etc.), and a selectable marker. Following the introduction of foreign DNA, engineered cells may be allowed to grow for 1-2 days in an enriched media, and then are switched to a selective media. The selectable marker in the recombinant plasmid confers resistance to the selection and allows selection of cells which have stably integrated the plasmid into their chromosomes and grow to form foci which in turn can be cloned and expanded into cell lines.
- A number of selection systems may be used, including, but not limited to, the herpes simplex virus thymidine kinase (Wigler et al., Cell 11:223 (1977)), hypoxanthine-guaninephosphoribosyltransferase (Szybalska & Szybalski, Proc. Natl. Acad. Sci. USA 48:2026 (1962)), and adenine phosphoribosyltransferase (Lowy et al., Cell 22:817 (1980)) genes, which can be employed in tk−, hgprt− or aprt− cells, respectively Also, antimetabolite resistance can be used as the basis of selection for dhfr, which confers resistance to methotrexate (Wigler et al., Natl. Acad. Sci. USA 77:3567 (1989); O'Hare et al., Proc. Natl. Acad. Sci. USA 78:1527 (1981)); gpt, which confers resistance to mycophenolic acid (Mulligan & Berg, Proc. Natl. Acad. Sci. USA 78:2072 (1981)); neo, which confers resistance to the aminoglycoside G-418 (Colberre-Garapin et al., J Mol. Biol. 150:1 (1981)); and hygro, which confers resistance to hygromycin (Santerre et al., Gene 30:147 (1984) genes. Recently, additional selectable genes have been described, namely trpB, which allows cells to utilize indole in place of tryptophan; hisD, which allows cells to utilize histinol in place of histidine (Hartman & Mulligan, Proc. Natl. Acad. Sci. USA 85:8047 (1988)); the glutamine synthase system; and ODC (ornithine decarboxylase) which confers resistance to the ornithine decarboxylase inhibitor, 2-(difluoromethyl)-DL- ornithine, DFMO (McConlogue, in: Current Communications in Molecular Biology, Cold Spring Harbor Laboratory ed. (1987)).
- ii. Identification of Transfectants or Transformants that Express the Protein having a Modified Glycosylation Pattern
- The host cells which contain the coding sequence and which express the biologically active gene products may be identified by at least four general approaches; (a) DNA-DNA or DNA-RNA hybridization; (b) the presence or absence of “marker” gene functions; (c) assessing the level of transcription as measured by the expression of the respective mRNA transcripts in the host cell; and (d) detection of the gene product as measured by immunoassay or by its biological activity.
- In the first approach, the presence of the coding sequence of the protein of interest and the coding sequence of the GnTIII inserted in the expression vector can be detected by DNA-DNA or DNA-RNA hybridization using probes comprising nucleotide sequences that are homologous to the respective coding sequences, respectively, or portions or derivatives thereof
- In the second approach, the recombinant expression vector/host system can be identified and selected based upon the presence or absence of certain “marker” gene functions (e.g., thymidine kinase activity, resistance to antibiotics, resistance to methotrexate, transformation phenotype, occlusion body formation in baculovirus, etc.). For example, if the coding sequence of the protein of interest and the coding sequence of the GnTIII are inserted within a marker gene sequence of the vector, recombinants containing the respective coding sequences can be identified by the absence of the marker gene function. Alternatively, a marker gene can be placed in tandem with the coding sequences under the control of the same or different promoter used to control the expression of the coding sequences. Expression of the marker in response to induction or selection indicates expression of the coding sequence of the protein of interest and the coding sequence of the GnTIII.
- In the third approach, transcriptional activity for the coding region of the protein of interest and the coding sequence of the GnTIII can be assessed by hybridization assays. For example, RNA can be isolated and analyzed by Northern blot using a probe homologous to the coding sequences of the protein of interest and the coding sequence of the GnTIII or particular portions thereof Alternatively, total nucleic acids of the host cell may be extracted and assayed for hybridization to such probes.
- In the fourth approach, the expression of the protein products of the protein of interest and the coding sequence of the GnTIII can be assessed immunologically, for example by Western blots, immunoassays such as radioimmuno-precipitation, enzyme-linked immunoassays and the like. The ultimate test of the success of the expression system, however, involves the detection of the biologically active gene products.
- b. Generation and Use of Proteins and Protein Fragments having Altered Glycosylation Patterns
- I. Generation and Use of Antibodies having Increased Antibody-Dependent Cellular Cytotoxicity
- In preferred embodiments, the present invention provides glycoforms of antibodies and antibody fragments having increased antibody-dependent cellular cytotoxicity.
- Clinical trials of unconjugated monoclonal antibodies (mAbs) for the treatment of some types of cancer have recently yielded encouraging results. Dillman, Cancer Biother. & Radiopharm. 12:223-25 (1997); Deo et al., Immunology Today 18:127 (1997). A chimeric, unconjugated IgG1 has been approved for low-grade or follicular B-cell non-Hodgkin's lymphoma Dillman, Cancer Biother. & Radiopharm. 12:223-25 (1997), while another unconjugated mAb, a humanized IgG1 targeting solid breast tumors, has also been showing promising results in phase III clinical trials. Deo et al., Immunology Today 18:127 (1997). The antigens of these two mAbs are highly expressed in their respective tumor cells, and the antibodies mediate potent tumor destruction by effector cells in vitro and in vivo. In contrast, many other unconjugated mAbs with fine tumor specificities cannot trigger effector functions of sufficient potency to be clinically useful. Frost et al., Cancer 80:317-33 (1997), Surfus et al., J. Immunother. 19:184-91 (1996). For some of these weaker mAbs, adjunct cytokine therapy is currently being tested. Addition of cytokines can stimulate antibody-dependent cellular cytotoxicity (ADCC) by increasing the activity and number of circulating lymphocytes. Frost et al., Cancer 80:317-33 (1997); Surfus et al., J. Immunother. 19:184-91 (1996). ADCC, a lytic attack on antibody-targeted cells, is triggered upon binding of leukocyte receptors to the constant region (Fc) of antibodies. Deo et al., Immunology Today 18:127 (1997)
- A different, but complementary, approach to increase ADCC activity of unconjugated IgG1s is to engineer the Fc region of the antibody to increase its affinity for the lymphocyte receptors (FcγRs). Protein engineering studies have shown that FcγRs interact with the lower hinge region of the IgG CH2 domain. Lund et al., J. Immunol. 157:4963-69 (1996). However, FcγR binding also requires the presence of oligosaccharides covalently attached at the conserved Asn 297 in the CH2 region. Lund et al., J. Immunol. 157:4963-69 (1996); Wright and Morrison, Trends Biotech. 15:26-31 (1997), suggesting that either oligosaccharide and polypeptide both directly contribute to the interaction site or that the oligosaccharide is required to maintain an active CH2 polypeptide conformation. Modification of the oligosaccharide structure can therefore be explored as a means to increase the affinity of the interaction.
- An IgG molecule carries two N-linked oligosaccharides in its Fc region, one on each heavy chain. As any glycoprotein, an antibody is produced as a population of glycoforms which share the same polypeptide backbone but have different oligosaccharides attached to the glycosylation sites. The oligosaccharides normally found in the Fc region of serum IgG are of complex bi-antennary type (Wormald et al., Biochemistry 36:130-38 (1997), with low level of terminal sialic acid and bisecting N-acetylglucosamine (GIcNAc), and a variable degree of terminal galactosylation and core fucosylation. Some studies suggest that the minimal carbohydrate structure required for FcγR binding lies within the oligosaccharide core. Lund et al., J. Immunol. 157:4963-69 (1996) The removal of terminal galactoses results in approximately a two-fold reduction in ADCC activity, indicating a role for these residues in FcγR receptor binding. Lund et al., J. Immunol. 157:4963-69 (1996)
- The mouse- or hamster-derived cell lines used in industry and academia for production of unconjugated therapeutic mAbs normally attach the required oligosaccharide determinants to Fc sites. IgGs expressed in these cell lines lack, however, the bisecting GIcNAc found in low amounts in serum IgGs. Lifely et al., Glycobiology 318:813-22 (1995). In contrast, it was recently observed that a rat myeloma-produced, humanized IgG1 (CAMPATH-1H) carried a bisecting GlcNAc in some of its glycoforms. Lifely et al., Glycobiology 318:813-22 (1995). The rat cell-derived antibody reached a similar in vitro ADCC activity as CAMPATH-1H antibodies produced in standard cell lines, but at significantly lower antibody concentrations.
- The CAMPATH antigen is normally present at high levels on lymphoma cells, and this chimeric mAb has high ADCC activity in the absence of a bisecting GlcNAc. Lifely et al., Glycobiology 318:813-22 (1995). In the N-linked glycosylation pathway, a bisecting GlcNAc is added by the enzyme β(1,4)-N-acetylglucosaminyltransferase III (GnT III). Schachter, Biochem. Cell Biol. 64:163-81 (1986).
- The present inventors used a single antibody-producing CHO cell line, that was previously engineered to express, in an externally-regulated fashion, different levels of a cloned GnT III gene. This approach established for the first time a rigorous correlation between expression of GnTIII and the ADCC activity of the modified antibody.
- The present inventors previously showed that C2B8 antibody modified according to the disclosed method had an about sixteen-fold higher ADCC activity than the standard, unmodified C2B8 antibody produced under identical cell culture and purification conditions. Briefly, a C2B8 antibody sample expressed in CHO-tTA-C2B8 cells that do not have GnTIII expression showed a cytotoxic activity of about 31% (at 1 μg/ml antibody concentration), measured as in vitro lysis of SB cells (CD20+) by human lymphocytes. In contrast, C2B8 antibody derived from a CHO cell culture expressing GnT III at a basal, largely repressed level showed at 1 μg/ml antibody concentration a 33% increase in ADCC activity against the control at the same antibody concentration. Moreover, increasing the expression of GnT III produced a large increase of almost 80% in the maximal ADCC activity (at 1 μg/ml antibody concentration) compared to the control at the same antibody concentration. (See International Publication No. WO 99/54342, the entire contents of which are hereby incorporated by reference.)
- Further antibodies of the invention having increased antibody-dependent cellular cytotoxicity include, but are not limited to, anti-human neuroblastoma monoclonal antibody (chCE7) produced by the methods of the invention, a chimeric anti-human renal cell carcinoma monoclonal antibody (ch-G250) produced by the methods of the invention, a humanized anti-HER2 monoclonal antibody (e.g., Trastuzumab (HERCEPTIN)) produced by the methods of the invention, a chimeric anti-human colon, lung, and breast carcinoma monoclonal antibody (ING-1) produced by the methods of the invention, a humanized anti-human 17-1A antigen monoclonal antibody (3622W94) produced by the methods of the invention, a humanized anti-human colorectal tumor antibody (A33) produced by the methods of the invention, an anti-human melanoma antibody (R24) directed against GD3 ganglioside produced by the methods of the invention, and a chimeric anti-human squamous-cell carcinoma monoclonal antibody (SF-25) produced by the methods of the invention, an anti-human small cell lung carcinoma monoclonal antibody (BEC2, ImClone Systems, Merck KgaA) produced by the methods of the invention, an anti-human non-Hodgkin's lymphoma monoclonal antibody (Bexxar (tositumomab, Coulter Pharmaceuticals), Oncolym (Techniclone, Alpha Therapeutic)) produced by the methods of the invention, an anti-human squamous cell head and neck carcinoma monoclonal antibody (C225, ImClone Systems) prepared by the methods of the invention, an anti-human rectal and colon carcinoma monoclonal antibody (Panorex (edrecolomab), Centocor, Glaxo Wellcome) prepared by the methods of the invention, an anti-human ovarian carcinoma monoclonal antibody (Theragyn, Antisoma) produced by the methods of the invention, an anti-human acute myelogenous leukemia carcinoma monoclonal antibody (SmartM195, Protein Design Labs, Kanebo) produced by the methods of the invention, an anti-human malignant glioma monoclonal antibody (Cotara, Techniclone, Cambridge Antibody Technology) produced by the methods of the invention, an anti-human B cell non-Hodgkins lymphoma monoclonal antibody (IDEC-Y2B8, IDEC Pharmaceuticals) produced by the methods of the invention, an anti-human solid tumors monoclonal antibody (CEA-Cide, Immunomedics) produced by the methods of the invention, an anti-human colorectal carcinoma monoclonal antibody (Iodine 131-MN-14, Immunomedics) produced by the methods of the invention, an anti-human ovary, kidney, breast, and prostate carcinoma monoclonal antibody (MDX-210, Medarex, Novartis) produced by the methods of the invention, an anti-human colorectal and pancreas carcinoma monoclonal antibody (TTMA, Pharmacie & Upjohn) produced by the methods of the invention, an anti-human TAG-72 expressing carcinoma monoclonal antibody (MDX-220, Medarex) produced by the methods of the invention, an anti-human EGFr-expressing carcinoma monoclonal antibody (MDX-447) produced by the methods of the invention, Anti-VEGF monoclonal antibody (Genentech) produced by the methods of the invention, an anti-human breast, lung, prostate and pancreas carcinoma and malignant melanoma monoclonal antibody (BrevaRex, AltaRex) produced by the methods of the invention, and an anti-human acute myelogenous leukemia monoclonal antibody (Monoclonal Antibody Conjugate, Immunex) produced by the methods of the invention. In addition, the invention is directed to antibody fragment and fusion proteins comprising a region that is equivalent to the Fc region of immunoglobulins.
- ii. Generation and Use of Fusion Proteins Comprising a Region Equivalent to an Fc Region of an Immunoglobulin that Promote Fc-Mediated Cytotoxicity
- As discussed above, the present invention relates to a method for increasing the ADCC activity of therapeutic antibodies. This is achieved by engineering the glycosylation pattern of the Fc region of such antibodies, in particular by maximizing the proportion of antibody molecules carrying bisected complex oligosaccharides and bisected hybrid oligosaccharides N-linked to the conserved glycosylation sites in their Fc regions. This strategy can be applied to increase Fc-mediated cellular cytotoxicity against undesirable cells mediated by any molecule carrying a region that is an equivalent to the Fc region of an immunoglobulin, not only by therapeutic antibodies, since the changes introduced by the engineering of glycosylation affect only the Fc region and therefore its interactions with the Fc receptors on the surface of effector cells involved in the ADCC mechanism. Fc-containing molecules to which the presently disclosed methods can be applied include, but are not limited to, (a) soluble fusion proteins made of a targeting protein domain fused to the N-terminus of an Fc-region (Chamov and Ashkenazi, Trends Biotech. 14: 52 (1996) and (b) plasma membrane-anchored fusion proteins made of a type II transmembrane domain that localizes to the plasma membrane fused to the N-terminus of an Fc region (Stabila, P. F., Nature Biotech. 16: 1357 (1998)).
- In the case of soluble fusion proteins (a) the targeting domain directs binding of the fusion protein to undesirable cells such as cancer cells, i.e., in an analogous fashion to therapeutic antibodies. The application of presently disclosed method to enhance the Fc-mediated cellular cytotoxic activity mediated by these molecules would therefore be identical to the method applied to therapeutic antibodies.
- In the case of membrane-anchored fusion proteins (b) the undesirable cells in the body have to express the gene encoding the fusion protein. This can be achieved either by gene therapy approaches, i.e., by transfecting the cells in vivo with a plasmid or viral vector that directs expression of the fusion protein-encoding gene to undesirable cells, or by implantation in the body of cells genetically engineered to express the fusion protein on their surface. The later cells would normally be implanted in the body inside a polymer capsule (encapsulated cell therapy) where they cannot be destroyed by an Fc-mediated cellular cytotoxicity mechanism. However should the capsule device fail and the escaping cells become undesirable, then they can be eliminated by Fc-mediated cellular cytotoxicity. Stabila et al., Nature Biotech. 16: 1357 (1998). In this case, the presently disclosed method would be applied either by incorporating into the gene therapy vector an additional gene expression cassette directing adequate or maximal expression levels of GnT III or by engineering the cells to be implanted to express adequate or maximal levels of GnT III. In both cases, the aim of the disclosed method is to increase or maximize the proportion of surface-displayed Fc regions carrying bisected complex oligosaccharides and/or bisected hybrid oligosaccharides.
- The examples below explain the invention in more detail. The following preparations and examples are given to enable those skilled in the art to more clearly understand and to practice the present invention. The present invention, however, is not limited in scope by the exemplified embodiments, which are intended as illustrations of single aspects of the invention only, and methods which are functionally equivalent are within the scope of the invention. Indeed, various modifications of the invention in addition to those described herein will become apparent to those skilled in the art from the foregoing description and accompanying drawings. Such modifications are intended to fall within the scope of the appended claims.
- New Versions of the Chimeric Anti-CD20 Antibody IDEC-C2B8 Having Enhanced Antibody-Dependent Cellular Cytotoxicity Obtained by Glycosylation Engineering of an IDEC-CEB8 Producing Cell Line
- Synthesis of VHand VL coding regions of IDEC-C2B8 and construction of mammalian expression vectors. cDNAs encoding the VH and VL regions of IDEC-C2B8 antibody were assembled from a set of overlapping single-stranded oligonucleotides in a one-step process using PCR (Kobayashi, N., et al., Biotechniques 23:500-503 (1997)). The original sequence data coding for IDEC-C2B8 VL and VH were obtained from a published international patent application (International Publication Number: WO 94/11026). Assembled VL and VH cDNA fragments were subcloned into pBluescriptIIKS(+), sequenced and directly joined by ligation to the human constant light (Igκ) and heavy (IgG1) chain cDNAs, respectively, using unique restriction sites introduced at the variable and constant region junctions without altering the original amino acid residue sequence (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999); Reff, M. E., et al., Blood 83:435-445 (1994)). Each full-length cDNA was separately subcloned into pcDNA3.1(+) (Invitrogen, Leek, The Netherlands) yielding mammalian expression vectors for chimeric C2B8 light (pC2B8L) and heavy (pC2B8H) chains.
- Production of IDEC-C2B8 in CHO cells expressing different levels of GnTIII. Establishment of two CHO cell lines, CHO-tet-GnTIII expressing different levels of GnTIII depending on the tetracycline concentration in the culture medium; and CHO-tTA, the parental cell line that does not express GnTIII has been described previously (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999); Umana, P., et al., Biotechnol Bioeng. 65:542-549 (1999)). Each cell line was cotranfected with vectors pC2B8L, pC2B8H, and pZeoSV2(+) (for Zeocin resistance; Invitrogen, Leek, The Netherlands) using a calcium phosphate method. Zeocin resistant clones were transferred to a 96-well plate and assayed for IDEC-C2B8 production using an ELISA assay specific for the human constant region (4). Three IDEC-C2B8 samples were obtained from parallel cultures of a selected clone (CHO-tet-GnTIII-C2B8), differing only in the tetracycline concentration added to the medium (25, 50 and 2000 ng/mL respectively). Culture supernatants were harvested in the late exponential phase. An additional antibody sample was obtained from a CHO-tTA-derived clone, CHO-tTA-C2B8, cultured under identical conditions but without adding tetracycline to the medium. Antibody samples were purified from culture medium by protein A affinity chromatography and buffer exchanged to PBS on a cation exchange column as previously described (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999)). Antibody concentration was measured using a fluorescence-based kit from Molecular Probes (Leiden, The Netherlands) with Rituximab used as standard.
- Indirect immunofluorescence. CD20-positive cells (SB cells; ATCC deposit no. ATCC CCL120) and CD20-negative cells (HSB cells; ATCC deposit no. ATCC CCL120.1) were each incubated for 1h with 2.5 μg/ml of CHO-tet-GnTIII-derived IDEC-C2B8 antibody in Hank's balanced salt solution (GibcoBRL, Basel, Switzerland) and 2% bovine serum albumin fraction V (Roche Diagnostics, Rotkreuz, Switzerland) (HBSSB). As a negative control HBSSB was used instead of C2B8 antibody. A FITC-conjugated, anti-human Fc polyclonal antibody was used as a secondary antibody (SIGMA, St. Louis) for all samples. Cells were examined using a Leica fluorescence microscope (Wetzlar, Germany).
- Oligosaccharide profiling by MALDI/TOF-MS. Neutral, N-linked oligosaccharides were derived from C2B8 antibody samples, MabThera™ (European counterpart of Rituximab; kind gift from R. Stahel, Universit{dot over (a)}tspital, Switzerland), C2B8-25t, C2B8-50t, C2B8-2000t, and C2B8-nt, (100 μg each) as previously described (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999)). Briefly, the antibody samples were first treated with Arthrobacter ureafaciens sialidase (Oxford Glycosciences, Abingson, UK) to remove any sialic acid monosaccharide residues. Neutral N-linked oligosaccharides were then released from the desialylated antibody samples using peptide-N-glycosidase F (Oxford Glycosciences), purified using micro-columns, and analyzed by MALDI/TOF-MS in an Elite Voyager 400 spectrometer (Perseptive Biosystems, Farmingham, Mass.).
- ADCC Activity Assay. Peripheral blood mononuclear cells (PBMC) were separated from heparinated fresh human blood (in all experiments obtained from the same healthy donor) by centrifugation over a Ficoll-Paque (Pharmacia Biotech, Dübendorf, Switzerland) gradient. PBMC (effector) were depleted of monocytes by plastic adherence. CD20-positive SB (target) cells, were labeled for 90 min with 100 μCi 51Cr (Amersham, Dübendorf, Switzerland) at 37° C., washed twice in RPMI (GibcoBRL, Basel, Switzerland) and resuspended at a concentration of 105 cells/ml. Fifty microliters of C2B8 mAb diluted in RPMI medium was added to 100 μl SB cells (10,000 cells/well) in a 96-well round bottom microtiter plate (Greiner, Langenthal, Switzerland), centrifuged at 50×g for 1 min, and incubated for 1 h at 4° C. Subsequently, 50 μl of effector cell (suspended at 2×107 cells/ml in RPMI medium) were added to each 96-well yielding a final E:T ratio of 100. Plates were incubated for 4 h at 37° C. and 5% CO2, supernatant was harvested with a Skatron harvesting system (Skatron Instruments, Sterling, Va.) and counted (ER, experimental release) in a Cobra 05005 γ counter (Canberra Packard, Meriden, Conn.). Maximum (MR) and spontaneous (SR) releases were obtained by adding, instead of C2B8 mAb, 100 μl of 1% Nonidet (Sigma, St. Louis) or 100 μl of RPMI medium, respectively, to 100 μl labeled target cells. All data points were performed in triplicate. Specific lysis (%) was calculated with the following formula: (ER-SR)/(Mk-SR)×100.
- Results and Discussion
- Production of IDEC-C2B8 and verification of specific antigen binding. CHO-tet-GnTIII cells, with stable, tetracycline-regulated expression of GnTIII and stable, constitutive expression of IDEC-C2B8, were established and scaled-up for production of a set of antibody samples. During scale-up, parallel cultures from the same clone were grown under three different tetracycline concentrations, 25, 50 and 2000 ng/ml. These levels of tetracycline had previously been shown to result in different levels of GnTIII and bisected oligosaccharides (Umana, P., et al., Nat Biotechnol. 17:176-180(1999); Umana, P., et al., Biotechnol Bioeng. 65:542-549 (1999)). A C2B8-producing, control cell line that does not express GnTIII was also established and cultured under the same conditions as for the three parallel cultures of CHO-tet-GnTIII. After Protein A-affinity chromatography, mAb purity was estimated to be higher than 95% by SDS-PAGE and Coomassie-blue staining. The samples were named according to the tetracycline concentration added to the culture medium for their production: C2B8-25t, C2B 8-50t, C2B8-2000t and C2B8-nt (i.e., no tetracycline for the non-bisected control). Sample C2B8-25t showed specific antigen binding by indirect immunofluorescence using CD20-positive and CD20-negative cells (FIG. 1), indicating that the synthesized VL and VH gene fragments were functionally correct.
- Oligosaccharide profiling with MALDI/TOF-MS. The glycosylation profile of each antibody sample was analyzed by MALDI/TOF-MS of the released, neutral oligosaccharide mix. In this technique, oligosaccharides of different mass appear as separate peaks in the spectrum and their proportions are quantitatively reflected by the relative peak heights (Harvey, D. J., Rapid Common Mass Spectrom. 7:614-619 (1993); Harvey, D. J., et al., Glycoconj J. 15:333-338(1998)). Oligosaccharide structures were assigned to different peaks based on their expected molecular masses, previous structural data for oligosaccharides derived from IgGI mAbs produced in the same host, and information on the N-linked oligosaccharide biosynthetic pathway.
- A clear correlation was found between GnTIII expression levels (i.e., tetracycline concentration) and the amount of bisected oligosaccharides derived from the different antibody samples. As expected, MabThera™ and C2B8-nt, which are derived from hosts that do not express GnTIII, did not carry bisected oligosaccharides (FIGS. 2A and 2B). In contrast, bisected structures amounted up to approximately 35% of the oligosaccharides pool in sample C2B8-2000t, i.e, at a basal level of GnTIII expression. In this case, the main bisected oligosaccharide peaks were of complex type, unequivocally assigned to peaks at m/
z 1689 and m/z 1851 (FIG. 2C). The next higher GnTIII expression level, sample C2B8-50t, led to an increase in these peaks (including their associated potassium aducts at m/z 1705 and 1861) of around 20%. This increase was accompanied by a concomitant reduction of their non-bisected counterparts at m/z z 1486, decreased to almost base-line level, while complex bisected structures (m/z 1689 and 1851) decreased in favor of increases in peaks at mn/z - ADCC activity of IDEC-C2B8 glycosylated variants. Different C2B8 mAb glycosylation variants were compared for ADCC activity, measured as in vitro lysis of CD20-positive SB cells. An additional mAb sample, C2B8-nt, derived from the parental cell line lacking GnTIII, was also studied. Sample C2B8-2000t produced at the basal GnTIII expression level and carrying low levels of bisected oligosaccharides was slightly more active than C2B8-nt (FIG. 4A). At the next higher level of GnTIII -expression, sample C2B8-50t carried approximately equal levels of bisected and non-bisected oligosaccharides, but did not mediate significantly higher target-cell lysis. However, at the lowest tetracycline concentration, sample C2B8-25t, which contained up to 80% of bisected oligosaccharide structures, was significantly more active than the rest of the samples in the whole antibody concentration range. It reached the maximal level of ADCC activity of sample C2B8-nt at a 10-fold lower antibody concentration (FIG. 4A). Sample C2B8-25t also showed a significant increase in the maximal ADCC activity with respect to the control (50% vs. 30% lysis).
- Samples C2B8-50t and C2B8-25t, bearing the highest proportions of bisected oligosaccharides, were further compared in ADCC activity to Mabthera™, the version of Rituxan™ currently marketed in Europe (FIG. 4B). Sample C2B8-50t showed a slight increase in activity whereas sample C2B8-25t clearly out performed Mabthera™ at all antibody concentrations. Approximately a five to ten-fold lower concentration of C2B8-25t was required to reach the maximal ADCC activity of Mabthera™, and the maximal activity of C2B8-25t was about 25% higher than that of Mabthera™.
- These results show that, in general, the in vitro ADCC activity of the C2B8 antibody correlates with the proportion of molecules carrying bisected oligosaccharides in the Fc region. We had previously reported that in the case of chCE7, an antibody with a low baseline level of ADCC activity, significant increases of activity could be obtained by increasing the fraction of bisected oligosaccharides above the levels found in naturally-occurring antibodies (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999)). The same is true for the C2B8 mAb, which already has high ADCC activity in the absence of bisected oligosaccharides. In the case of chCE7, however, very large increases of ADCC activity were observed at a level of GnTIII expression where bisected oligosaccharides were predominantly of complex type (Umana, P., et al., Nat Biotechnol. 17:176-180 (1999)). For the potent C2B8 mAb, such a large boost in activity was only observed at the highest levels of GnTIII expression studied, where bisected oligosaccharides had shifted mainly to the hybrid type (FIG. 2). For both mAbs, the samples with the highest activities had considerably higher levels of bisected than non-bisected oligosaccharides. Together, these observations indicate that probably both complex and hybrid bisected oligosaccharides are important for ADCC activity.
- In both complex and hybrid oligosaccharides, a bisecting GlcNAc leads to a large change in oligosaccharide conformations (Balaji, P. V., et al., Int. J. Biol. Macromol. 18:101-114 (1996)). The change occurs in a part of the oligosaccharide that interacts extensively with the polypeptide in the CH2 domain (Jefferis, R., et al., Immunol Rev. 163:59-76 (1998)). Since the polypetide is relatively flexible at this location (Jefferis, R., et al., Immunol Rev. 163:59-76 (1998)), it is possible that the bisecting N-acetylglucosamine is mediating its biological effects through a conformational change in the Fc region. The potentially altered conformations would already exist in nature, as all serum IgGs carry bisected oligosaccharides. The main difference between the engineered and natural antibodies would be the proportion of molecules displaying the more active conformations.
- Various approaches for increasing the activity of unconjugated mAbs are currently under clinical evaluation, including radio-immunotherapy, antibody-dependent enzyme/prodrug therapy, immunotoxins and adjuvant therapy with cytokines (Hjelm Skog, A., et al., Cancer Immunol Immunother. 48:463-470 (1999); Blakey, D. C., et al., Cell Biophys. 25:175-183 (1994); Wiseman, G. A., et al., Clin Cancer Res. 5:3281s-3296s (1999); Hank, J. A., et al., Cancer Res. 50:5234-5239 (1990)). These technologies can give large increases in activity, but they can also lead to significantly higher side effects, elevated production costs and complex logistics from production to administration to patients when compared to unconjugated mAbs. The technology presented here offers an alternative way to obtain increases in potency while maintaining a simple production process, and should be applicable to many unconjugated mAbs.
- New Versions of the Anti-Renal Cell Carcinoma Antibody chG250 Having Enhanced Antibody-Dependent Cellular Cytotoxicity Obtained by Glycosylation Engineering of a chG250 Producing Cell Line
- 1. Cell Culture
- SP2/0 mouse myeloma cells producing chG250 chimeric mAb (wt-chG250-SP2/0 cells) were grown in standard cell culture medium supplemented with 1:100 (v/v) penicillin/streptomycin/antimycotic solution (SIGMA, Buchs, Switzerland). Cells were cultured at 37° C. in a 5% CO 2 humidified atmosphere in Tissue Culture Flasks. Medium was changed each 3-4 days. Cells were frozen in culture medium containing 10% DMSO.
- 2. Generation of SP2/0 Cells with pGnTIII-Puro Expression
- wt-chG250-SP2/0 myeloma cells were transfected by electroporation with a vector for constitutive expression of GnTIII operatively linked via an IRES to a puromycin resistance gene. 24 hours before electroporation culture medium was changed and cells were seeded at 5×10 5 cells/ml. Seven million cells were centrifuged for 4 min at 1300 rpm at 4° C. Cells were washed with 3 mL new medium and centrifuged again. Cells were resuspended in a volume of 0.3-0.5 ml of reaction mix, containing 1.25% (v/v) DMSO and 20-30 μg DNA in culture medium. The electroporation mix was then transferred to a 0.4 cm cuvette and pulsed at low voltage (250-300 V) and high capacitance (960 μF) using Gene Pulser from Bio Rad. After electroporation cells were quickly transferred to 6 mL 1.25% (v/v) DMSO culture medium in a T25 culture flask and incubated at 37° C. Stable integrants were selected by applying 2 μg/mL puromycin to the medium two days after electroporation. After 2-3 weeks a stable, puromycin-resitant mixed population was obtained. Single-cell derived clones were obtained via FACS and were subsequently expanded and maintained under puromycin selection.
- 3. Western Blot
- Puromycin-resistant clones were screened for GnTIII expression by Western blotting. The Western blots clearly showed that clones 5H12, 4E6 and 4E8 were expressing the highest levels of GnTIII. 5G2 also showed a GnTIII band of middle intensity, whereas 2F1, 3D3 and 4G3 had the lowest band intensities, therefore expressing lower amounts of GnTIII (FIG. 5).
- 4. Production and Purification of chG250 Monoclonal Antibody from Seven GnTIII-Expressing Clones Including Wild Type
- Clones 2F1, 3D3, 4E6, 4E8, 4G3, 5G2, 5H12 and the wild type (wt-chG250-SP2/0 cells) were seeded at 3×10 5 cells/mL in a total volume of 130 ml culture medium, and cultivated in single Triple-flasks. Cells used for seeding were all in full exponential growth phase, therefore cells were considered to be at the same growth state when the production batches started. Cells were cultivated for 4 days. Supernatants containing the antibody were collected in the late exponential growth phase to ensure reproducibility . The chG250 monoclonal antibody was purified in two chromatographic steps. Culture supernatants containing the chG250 monoclonal antibody derived from each batch were first purified using a HiTrap Protein A affinity chromatography. Protein A is highly specific for the human IgG Fc region. Pooled samples from the protein A eluate were buffer exchanged to PBS by cation-exchange chromatography on a
Resource S 1 ml column (Amersham Pharmacia Biotech). Final purity was judged to be higher than 95% from SDS-staining and Coomassie blue staining (FIG. 6). The concentration of each sample was determined with a standard calibration curve using wild type antibody with known concentration. - 5. Oligosaccharide Profiling of mAb Preparations Derived from the Seven Clones Expressing Different GnTIII Levels
- Oligosaccharide profiles were obtained by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI/TOF-MS), which accurately provides the molecular masses of the different oligosaccharide structures. This technique allows a quantitative analysis of proportions between different oligosacchaiide structures within a mixture. Neutral oligosaccharides appeared predominantly as [M+Na +] ions, however sometimes they were accompanied by smaller [M+K+] ions, leading to an increase in mass of m/z of 16. The percentage of the structure appearing as potassium ion adducts depends on the content of the matrix and may thus vary between samples. A mixture of neutral N-linked oligosaccharides derived from each antibody preparation was analyzed using a 2,5-dehydrobenzoic acid (2,5-DHB) as matrix. Some of the peaks in the spectra were unequivocally assigned to specific oligosaccharide structures, because of known monosaccharide composition and unique mass. However, sometimes multiple structures could be assigned to a particular mass. MALDI enables the determination of the mass and cannot distinguish between isomers. Knowledge of the biosynthetic pathway and previous structural data enable, in most cases, the assignment of an oligosaccharide structure to a peak in the spectrum.
- Oligosaccharides derived from the mAb sample produced in wt-chG250-SP2/0 cell line, that does not express GnTIII, contained nonbisected biantennary complex (m/z 1486) and mono- or di-galactosylated nonbisected biantennary complex structures (FIG. 7A), both α(1,6)-fucosylated in the core region (peaks m/
z - Expression of GCTIII generated bisected Fc-associated oligosaccharide structures of two types: complex or hybrid. Complex bisected oligosaccharides were unequivocally assigned to peaks at m/
z z z 1648, had the lowest values for the clones expressing the highest GnTIII levels (clones 4E6, 4E8, 5G2 and 5H12). These two peaks decreased in favor of the accumulation of bisected complex and bisected hybrid type oligosaccharides (FIGS. 7A-7D and 8A-8D). The percentage of bisected complex oligosaccharides was higher for the samples derived from the clones expressing lower amounts of GnTIII. This is consistent with the fact that a higher GnTIII expression level probably shifts the biosynthetic flux to bisected hybrid structures, thereby decreasing the relative proportions of complex and complex bisected compound. For bisected hybrid structures, two possible structures could sometimes be assigned to a single peak. Therefore, some assumptions were made in order to approximate the percentage of these structures over the total oligosaccharide pool. Peaks m/z z - 6. Measurement of Antibody Mediated Cytotoxic Activity by Calcein-AM Retention
- The Calcein-AM retention method of measuring cytotoxicity measures the dye fluorescence remaining in the cells after incubation with the antibody. Four million G250 antigen-positive cells (target) were labelled with 10 μM Calcein-AM (Molecular Probes, Eugene, Oreg.) in 1.8 mL RPMI-1640 cell culture medium (GIBCO BRL, Basel, Switzerland) supplemented with 10% fetal calf serum for 30 min at 37° C. in a 5% CO 2 humidified atmosphere. The cells were washed twice in culture medium and resuspended in 12 mL AIMV serum free medium (GIBCO BRL, Basel, Switzerland). Labelled cells were then transferred to U-bottom 96-wells (30,000 cells/well) and incubated in triplicate with different concentrations of antibody for 1 hour at 4° C. Peripheral blood mononuclear cells (PBMC) were separated from heparinated fresh human blood (in all experiments obtained from the same healthy donor) by centrifugation over a Ficoll-Paque (Pharmacia Biotech, Dübendorf, Switzerland) gradient PBMCs were added in triplicate wells in a 50 μL volume, yielding an effector to target ratio (E:T ratio) of 25:1 and a final volume of 200 μL. The 96-well plate was then incubated for 4 hours at 37° C. in a 5% CO2 atmosphere. Thereafter the 96-well plate was centrifuged at 700×g for 5 min and the supernatants were discarded. The cell pellets were washed twice with Hank's balanced salt solution (HBSS) and lysed in 200 μL 0.05M sodium borate, pH 9, 0.1% Triton X-100. Retention of the fluorescent dye in the target cells was measured with a FLUO star microplate reader (BMG Lab Technologies, Offenburg, Germany). The specific lysis was calculated relative to a total lysis control, resulting from exposure of the target cells to saponin (200 μg/mL in AIMV; SIGMA, Buchs, Switzerland) instead of exposure to antibody. Specific lysis (%) was calculated with the following formula:
- where F med represents the fluorescence of target cells treated with medium alone and considers unspecific lysis by PMBCs, Fexp represents the fluorescence of cells treated with antibody and Fdet represents the fluorescence of cells treated with saponin instead of antibody.
- To determine the effect of modified glycosylation variants of chG250 on the in vitro ADCC activity, G250 antigen-positive target cells were cultured with PBMCs with and without chG250 antibody samples at different concentrations. The cytotoxicity of unmodified chG250 antibody derived from the wild type cell line was compared with two antibody preparations derived from two cell lines (3D3, 5H12) expressing intermediate and high GnTIII levels, respectively (see FIG. 5).
- Unmodified chG250 antibody did not mediate significant ADCC activity over the entire concentration range used in the assay (the activity was not significantly different from background). Augmented ADCC activity (close to 20%, see FIG. 9) at 2 μg/mL was observed with the antibody sample derived from clone 3D3, which expressed intermediate GnTIII levels. The cytotoxic activity of this antibody samples did not grow at higher antibody concentrations. As expected the antibody preparation derived from clone 5H12 showed a striking increase over samples 3D3 and unmodified antibody in its ability to mediate ADCC against target cells. The maximal ADCC activity of this antibody preparation was around 50% andwas remarkable in mediating significant ADCC activity at 125-fold less concentrated when comparing with the unmodified control sample.
- Treatment of Immune-Mediated Thrombocytopenia in a Patient with Chronic Graft-Versus-Host Disease
- Autoimmune thrombocytopenia in chronic graft-versus-host disease represents an instance of B-cell dysregulation leading to clinical disease. To treat immune-mediated thrombocytopenia in a subject with chronic graft-versus-host disease, an anti-CD20 chimeric monoclonal antibody prepared by the methods of the present invention and having increased ADCC is administered to the subject as described in Ratanatharathom, V. et al., Ann. Intern. Med. 133(4):275-79 (2000) (the entire contents of which is hereby incorporated by reference). Specifically, a weekly infusion of the antibody, 375 mg/2 is administered to the subject for 4 weeks. The antibody therapy produces a marked depletion of B cells in the peripheral blood and decreased levels of platelet-associated antibody.
- Treatment of Severe, Immune-Mediated, Pure Red Cell Aplasia and Hemolytic Anemia
- Immune-mediated, acquired pure red cell aplasia (PRCA) is a rare disorder frequently associated with other autoimmune phenomena. To treat immune-mediated, acquired pure red cell aplasia in a subject, an anti-CD20 chimeric monoclonal antibody prepared by the methods of the present invention and having increased ADCC is administered to the subject as described in Zecca, M. et al., Blood 12:3995-97 (1997) (the entire contents of which are hereby incorporated by reference). Specifically, a subject with PRCA and autoimmune hemolytic anemia is given two doses of antibody, 375 mg/m2, per week. After antibody therapy, substitutive treatment with intravenous immunoglobulin is initiated. This treatment produces a marked depletion of B cells and a significant rise in reticulocyte count accompanied by increased hemoglobin levels.
- It will be clear that the invention may be practiced otherwise than as particularly described in the foregoing description and examples. Numerous modifications and variations of the present invention are possible in light of the above teachings and, therefore, are within the scope of the appended claims.
- The entire disclosure of all publications (including patents, patent applications, journal articles, laboratory manuals, books, or other documents) cited herein are hereby incorporated by reference.
Claims (38)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/211,554 US20030175884A1 (en) | 2001-08-03 | 2002-08-05 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US11/199,232 US8021856B2 (en) | 1998-04-20 | 2005-08-09 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US13/196,724 US8999324B2 (en) | 1998-04-20 | 2011-08-02 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US14/665,191 US9321843B2 (en) | 1998-04-20 | 2015-03-23 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US15/080,020 US9631023B2 (en) | 1998-04-20 | 2016-03-24 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US30951601P | 2001-08-03 | 2001-08-03 | |
| US10/211,554 US20030175884A1 (en) | 2001-08-03 | 2002-08-05 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/633,697 Continuation-In-Part US7517670B2 (en) | 1998-04-20 | 2003-08-05 | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/199,232 Continuation US8021856B2 (en) | 1998-04-20 | 2005-08-09 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030175884A1 true US20030175884A1 (en) | 2003-09-18 |
Family
ID=23198536
Family Applications (5)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/211,554 Abandoned US20030175884A1 (en) | 1998-04-20 | 2002-08-05 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US11/199,232 Expired - Fee Related US8021856B2 (en) | 1998-04-20 | 2005-08-09 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US13/196,724 Expired - Fee Related US8999324B2 (en) | 1998-04-20 | 2011-08-02 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US14/665,191 Expired - Fee Related US9321843B2 (en) | 1998-04-20 | 2015-03-23 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US15/080,020 Expired - Fee Related US9631023B2 (en) | 1998-04-20 | 2016-03-24 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
Family Applications After (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/199,232 Expired - Fee Related US8021856B2 (en) | 1998-04-20 | 2005-08-09 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US13/196,724 Expired - Fee Related US8999324B2 (en) | 1998-04-20 | 2011-08-02 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US14/665,191 Expired - Fee Related US9321843B2 (en) | 1998-04-20 | 2015-03-23 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US15/080,020 Expired - Fee Related US9631023B2 (en) | 1998-04-20 | 2016-03-24 | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
Country Status (15)
| Country | Link |
|---|---|
| US (5) | US20030175884A1 (en) |
| EP (2) | EP1423510A4 (en) |
| JP (2) | JP2005524379A (en) |
| KR (2) | KR20040054669A (en) |
| CN (1) | CN1555411A (en) |
| AU (1) | AU2002339845B2 (en) |
| CA (2) | CA2455365C (en) |
| HU (1) | HUP0700103A3 (en) |
| IL (2) | IL160170A0 (en) |
| MX (1) | MXPA04001072A (en) |
| NO (1) | NO332457B1 (en) |
| NZ (5) | NZ531219A (en) |
| PL (1) | PL217751B1 (en) |
| RU (1) | RU2321630C2 (en) |
| WO (1) | WO2003011878A2 (en) |
Cited By (150)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20030003097A1 (en) * | 2001-04-02 | 2003-01-02 | Idec Pharmaceutical Corporation | Recombinant antibodies coexpressed with GnTIII |
| US20040072290A1 (en) * | 1998-04-20 | 2004-04-15 | Glycart Biotechnology Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US20040132101A1 (en) * | 2002-09-27 | 2004-07-08 | Xencor | Optimized Fc variants and methods for their generation |
| US20050208617A1 (en) * | 2000-06-28 | 2005-09-22 | Piotr Bobrowicz | N-acetylglucosamintransferase III expression in lower eukaryotes |
| US20050244403A1 (en) * | 2004-03-24 | 2005-11-03 | Xencor, Inc. | Immunoglobulin variants outside the Fc region |
| US20050249723A1 (en) * | 2003-12-22 | 2005-11-10 | Xencor, Inc. | Fc polypeptides with novel Fc ligand binding sites |
| US20050272128A1 (en) * | 1998-04-20 | 2005-12-08 | Glycart Biotechnology Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US20060002930A1 (en) * | 2004-04-16 | 2006-01-05 | Genentech, Inc. | Treatment of disorders |
| US20060074225A1 (en) * | 2004-09-14 | 2006-04-06 | Xencor, Inc. | Monomeric immunoglobulin Fc domains |
| US20060173170A1 (en) * | 2004-11-12 | 2006-08-03 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US20060177898A1 (en) * | 2000-06-28 | 2006-08-10 | Glycofi, Inc. | Methods for producing modified glycoproteins |
| US20060210555A1 (en) * | 2001-12-21 | 2006-09-21 | Antigenics, Inc. | Compositions comprising immunoreactive reagents and saponins, and methods of use thereof |
| US20060223096A1 (en) * | 2005-03-25 | 2006-10-05 | Glycart Biotechnology Ag | Antigen binding molecules directed to MCSP and having increased Fc receptor binding affinity and effector function |
| US20060235208A1 (en) * | 2002-09-27 | 2006-10-19 | Xencor, Inc. | Fc variants with optimized properties |
| US20060269545A1 (en) * | 2005-02-07 | 2006-11-30 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20060275282A1 (en) * | 2005-01-12 | 2006-12-07 | Xencor, Inc. | Antibodies and Fc fusion proteins with altered immunogenicity |
| US20070003546A1 (en) * | 2002-03-01 | 2007-01-04 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US20070037248A1 (en) * | 2000-06-28 | 2007-02-15 | Piotr Bobrowicz | Production of modified glycoproteins having multiple antennary structures |
| US20070071745A1 (en) * | 2005-08-26 | 2007-03-29 | Pablo Umana | Modified antigen binding molecules with altered cell signaling activity |
| US20070087005A1 (en) * | 2005-10-14 | 2007-04-19 | Lazar Gregory A | Anti-glypican-3 antibody |
| US20070111281A1 (en) * | 2005-05-09 | 2007-05-17 | Glycart Biotechnology Ag | Antigen binding molecules having modified Fc regions and altered binding to Fc receptors |
| US20070148171A1 (en) * | 2002-09-27 | 2007-06-28 | Xencor, Inc. | Optimized anti-CD30 antibodies |
| US20070219133A1 (en) * | 2002-03-01 | 2007-09-20 | Xencor, Inc. | CD52 OPTIMIZED Fc VARIANTS AND METHODS FOR THEIR GENERATION |
| US20070237766A1 (en) * | 2003-03-03 | 2007-10-11 | Xencor, Inc. | Fc Variants Having Increased Affinity for FcyRllla |
| US20070275460A1 (en) * | 2003-03-03 | 2007-11-29 | Xencor.Inc. | Fc Variants With Optimized Fc Receptor Binding Properties |
| US20080057056A1 (en) * | 2003-03-03 | 2008-03-06 | Xencor, Inc. | Fc Variants with Increased Affinity for FcyRIIC |
| US20080095770A1 (en) * | 2006-08-09 | 2008-04-24 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20080206867A1 (en) * | 2005-10-03 | 2008-08-28 | Desjarlais John R | Fc variants with optimized Fc receptor binding properties |
| US20080254027A1 (en) * | 2002-03-01 | 2008-10-16 | Bernett Matthew J | Optimized CD5 antibodies and methods of using the same |
| US20080260731A1 (en) * | 2002-03-01 | 2008-10-23 | Bernett Matthew J | Optimized antibodies that target cd19 |
| US20080267976A1 (en) * | 2005-10-06 | 2008-10-30 | Gregory Alan Lazar | Optimized Anti-Cd30 Antibodies |
| WO2008070569A3 (en) * | 2006-12-01 | 2008-11-20 | Medarex Inc | Human antibodies that bind cd22 and uses thereof |
| US20080313379A1 (en) * | 2007-06-15 | 2008-12-18 | United Memories, Inc. | Multiple bus charge sharing |
| US20090169550A1 (en) * | 2007-12-21 | 2009-07-02 | Genentech, Inc. | Therapy of rituximab-refractory rheumatoid arthritis patients |
| US20090209024A1 (en) * | 2000-06-28 | 2009-08-20 | Gerngross Tillman U | Combinatorial DNA library for producing modified N-glycans in lower eukaryotes |
| US20090208500A1 (en) * | 2005-06-03 | 2009-08-20 | Genentech, Inc. | Method of producing antibodies with improved function |
| US20090263318A1 (en) * | 2003-01-24 | 2009-10-22 | Agensys, Inc. | Nucleic acids and corresponding proteins entitled 254p1d6b useful in treatment and detection of cancer |
| US20090269339A1 (en) * | 2008-04-29 | 2009-10-29 | Genentech, Inc. | Responses to immunizations in rheumatoid arthritis patients treated with a cd20 antibody |
| US20100028951A1 (en) * | 2007-03-07 | 2010-02-04 | Stephen Hamilton | Production of glycoproteins with modified fucosylation |
| US20100104557A1 (en) * | 2006-09-18 | 2010-04-29 | Xencor, Inc. | Optimized Antibodies that Target HM1.24 |
| US20100135987A1 (en) * | 2008-10-20 | 2010-06-03 | Hickman Robert K | Isolation and purification of antibodies using protein a affinity chromatography |
| WO2010075249A2 (en) | 2008-12-22 | 2010-07-01 | Genentech, Inc. | A method for treating rheumatoid arthritis with b-cell antagonists |
| US7776343B1 (en) | 1999-02-17 | 2010-08-17 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US20100248359A1 (en) * | 2004-07-09 | 2010-09-30 | Chugai Seiyaku Kabushiki Kaisha | Anti-Glypican 3 Antibody |
| US20100272723A1 (en) * | 2006-08-14 | 2010-10-28 | Xencor, Inc. | Optimized Antibodies that Target CD19 |
| WO2010146059A2 (en) | 2009-06-16 | 2010-12-23 | F. Hoffmann-La Roche Ag | Biomarkers for igf-1r inhibitor therapy |
| US20110033452A1 (en) * | 2004-10-26 | 2011-02-10 | Chugai Seiyaku Kabushiki Kaisha | Anti-Glypican 3 Antibody Having Modified Sugar Chain |
| WO2011019619A1 (en) | 2009-08-11 | 2011-02-17 | Genentech, Inc. | Production of proteins in glutamine-free cell culture media |
| WO2011023787A1 (en) | 2009-08-31 | 2011-03-03 | Roche Glycart Ag | Affinity-matured humanized anti cea monoclonal antibodies |
| US7923011B2 (en) | 2006-10-12 | 2011-04-12 | Genentech, Inc. | Antibodies to lymphotoxin-alpha |
| WO2011100403A1 (en) | 2010-02-10 | 2011-08-18 | Immunogen, Inc | Cd20 antibodies and uses thereof |
| WO2011101328A2 (en) | 2010-02-18 | 2011-08-25 | Roche Glycart Ag | Treatment with a humanized igg class anti egfr antibody and an antibody against insulin like growth factor 1 receptor |
| US8084582B2 (en) | 2003-03-03 | 2011-12-27 | Xencor, Inc. | Optimized anti-CD20 monoclonal antibodies having Fc variants |
| US8101720B2 (en) | 2004-10-21 | 2012-01-24 | Xencor, Inc. | Immunoglobulin insertions, deletions and substitutions |
| WO2012020006A2 (en) | 2010-08-13 | 2012-02-16 | Roche Glycart Ag | Anti-fap antibodies and methods of use |
| WO2012020038A1 (en) | 2010-08-13 | 2012-02-16 | Roche Glycart Ag | Anti-tenascin-c a2 antibodies and methods of use |
| US8188231B2 (en) | 2002-09-27 | 2012-05-29 | Xencor, Inc. | Optimized FC variants |
| WO2012107416A2 (en) | 2011-02-10 | 2012-08-16 | Roche Glycart Ag | Improved immunotherapy |
| US20120251533A1 (en) * | 2005-02-18 | 2012-10-04 | Medarex, Inc. | Monoclonal antibodies against cd30 lacking in fucosyl residues |
| WO2012146628A1 (en) | 2011-04-29 | 2012-11-01 | Roche Glycart Ag | Novel immunoconjugates |
| US8318907B2 (en) | 2004-11-12 | 2012-11-27 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| WO2013026832A1 (en) | 2011-08-23 | 2013-02-28 | Roche Glycart Ag | Anti-mcsp antibodies |
| WO2013026831A1 (en) | 2011-08-23 | 2013-02-28 | Roche Glycart Ag | Bispecific antigen binding molecules |
| EP2586788A1 (en) | 2007-07-09 | 2013-05-01 | Genentech, Inc. | Prevention of disulfide bond reduction during recombinant production of polypeptides |
| WO2013113641A1 (en) | 2012-01-31 | 2013-08-08 | Roche Glycart Ag | Use of nkp46 as a predictive biomarker for cancer treatment with adcc- enhanced antibodies |
| WO2013127465A1 (en) | 2012-03-02 | 2013-09-06 | Roche Glycart Ag | Predicitive biomarker for cancer treatment with adcc enhanced antibodies |
| US8546543B2 (en) | 2004-11-12 | 2013-10-01 | Xencor, Inc. | Fc variants that extend antibody half-life |
| US20130310273A1 (en) * | 2011-01-27 | 2013-11-21 | Torsten Witte | Methods and Means for Diagnosing Vasculitis |
| US8642742B2 (en) | 2011-03-02 | 2014-02-04 | Roche Glycart Ag | Anti-CEA antibodies |
| WO2014023679A1 (en) | 2012-08-07 | 2014-02-13 | Roche Glycart Ag | Composition comprising two antibodies engineered to have reduced and increased effector function |
| WO2014114595A1 (en) | 2013-01-23 | 2014-07-31 | Roche Glycart Ag | Predictive biomarker for cancer treatment with adcc-enhanced antibodies |
| US8802820B2 (en) | 2004-11-12 | 2014-08-12 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| WO2014131715A1 (en) | 2013-02-26 | 2014-09-04 | Roche Glycart Ag | Anti-mcsp antibodies |
| US8883980B2 (en) | 2003-11-05 | 2014-11-11 | Roche Glycart Ag | Antigen binding molecules with increased Fc receptor binding affinity and effector function |
| US8906646B2 (en) | 2006-09-13 | 2014-12-09 | Abbvie Inc. | Fed-batch method of making human anti-TNF-alpha antibody |
| US8911964B2 (en) | 2006-09-13 | 2014-12-16 | Abbvie Inc. | Fed-batch method of making human anti-TNF-alpha antibody |
| US8921526B2 (en) | 2013-03-14 | 2014-12-30 | Abbvie, Inc. | Mutated anti-TNFα antibodies and methods of their use |
| US8946395B1 (en) | 2013-10-18 | 2015-02-03 | Abbvie Inc. | Purification of proteins using hydrophobic interaction chromatography |
| US8986949B2 (en) | 2003-02-20 | 2015-03-24 | Glycofi, Inc. | Endomannosidases in the modification of glycoproteins in eukaryotes |
| US9017687B1 (en) | 2013-10-18 | 2015-04-28 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same using displacement chromatography |
| US9051373B2 (en) | 2003-05-02 | 2015-06-09 | Xencor, Inc. | Optimized Fc variants |
| US9062106B2 (en) | 2011-04-27 | 2015-06-23 | Abbvie Inc. | Methods for controlling the galactosylation profile of recombinantly-expressed proteins |
| US9067990B2 (en) | 2013-03-14 | 2015-06-30 | Abbvie, Inc. | Protein purification using displacement chromatography |
| US9085618B2 (en) | 2013-10-18 | 2015-07-21 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same |
| US9109010B2 (en) | 2008-10-20 | 2015-08-18 | Abbvie Inc. | Viral inactivation during purification of antibodies cross reference to related applications |
| US9150645B2 (en) | 2012-04-20 | 2015-10-06 | Abbvie, Inc. | Cell culture methods to reduce acidic species |
| US9181572B2 (en) | 2012-04-20 | 2015-11-10 | Abbvie, Inc. | Methods to modulate lysine variant distribution |
| US9181337B2 (en) | 2013-10-18 | 2015-11-10 | Abbvie, Inc. | Modulated lysine variant species compositions and methods for producing and using the same |
| US9193787B2 (en) | 2012-04-20 | 2015-11-24 | Abbvie Inc. | Human antibodies that bind human TNF-alpha and methods of preparing the same |
| US9206390B2 (en) | 2012-09-02 | 2015-12-08 | Abbvie, Inc. | Methods to control protein heterogeneity |
| US9234033B2 (en) | 2012-09-02 | 2016-01-12 | Abbvie, Inc. | Methods to control protein heterogeneity |
| US9249182B2 (en) | 2012-05-24 | 2016-02-02 | Abbvie, Inc. | Purification of antibodies using hydrophobic interaction chromatography |
| US9266938B2 (en) | 2011-02-10 | 2016-02-23 | Roche Glycart Ag | Mutant interleukin-2 polypeptides |
| US9403855B2 (en) | 2010-05-10 | 2016-08-02 | Academia Sinica | Zanamivir phosphonate congeners with anti-influenza activity and determining oseltamivir susceptibility of influenza viruses |
| US9475881B2 (en) | 2010-01-19 | 2016-10-25 | Xencor, Inc. | Antibody variants with enhanced complement activity |
| US9499614B2 (en) | 2013-03-14 | 2016-11-22 | Abbvie Inc. | Methods for modulating protein glycosylation profiles of recombinant protein therapeutics using monosaccharides and oligosaccharides |
| EP3095463A2 (en) | 2008-09-16 | 2016-11-23 | F. Hoffmann-La Roche AG | Methods for treating progressive multiple sclerosis |
| US9547009B2 (en) | 2012-08-21 | 2017-01-17 | Academia Sinica | Benzocyclooctyne compounds and uses thereof |
| US9550826B2 (en) | 2013-11-15 | 2017-01-24 | Abbvie Inc. | Glycoengineered binding protein compositions |
| US9598667B2 (en) | 2013-10-04 | 2017-03-21 | Abbvie Inc. | Use of metal ions for modulation of protein glycosylation profiles of recombinant proteins |
| WO2017053906A1 (en) | 2015-09-24 | 2017-03-30 | Abvitro Llc | Hiv antibody compositions and methods of use |
| US9695454B2 (en) | 2012-05-23 | 2017-07-04 | Glykos Finland Oy | Production of fucosylated glycoproteins |
| US9714282B2 (en) | 2003-09-26 | 2017-07-25 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US9759726B2 (en) | 2014-03-27 | 2017-09-12 | Academia Sinica | Reactive labelling compounds and uses thereof |
| US9782476B2 (en) | 2013-09-06 | 2017-10-10 | Academia Sinica | Human iNKT cell activation using glycolipids with altered glycosyl groups |
| US9816981B2 (en) | 2007-03-23 | 2017-11-14 | Academia Sinica | Alkynyl sugar analogs for labeling and visualization of glycoconjugates in cells |
| WO2017194441A1 (en) | 2016-05-11 | 2017-11-16 | F. Hoffmann-La Roche Ag | Modified anti-tenascin antibodies and methods of use |
| EP3252078A1 (en) | 2016-06-02 | 2017-12-06 | F. Hoffmann-La Roche AG | Type ii anti-cd20 antibody and anti-cd20/cd3 bispecific antibody for treatment of cancer |
| US9879042B2 (en) | 2014-09-08 | 2018-01-30 | Academia Sinica | Human iNKT cell activation using glycolipids |
| US9914956B2 (en) | 2012-08-18 | 2018-03-13 | Academia Sinica | Cell-permeable probes for identification and imaging of sialidases |
| US9975966B2 (en) | 2014-09-26 | 2018-05-22 | Chugai Seiyaku Kabushiki Kaisha | Cytotoxicity-inducing theraputic agent |
| US9975965B2 (en) | 2015-01-16 | 2018-05-22 | Academia Sinica | Compositions and methods for treatment and detection of cancers |
| US9982041B2 (en) | 2014-01-16 | 2018-05-29 | Academia Sinica | Compositions and methods for treatment and detection of cancers |
| US9981030B2 (en) | 2013-06-27 | 2018-05-29 | Academia Sinica | Glycan conjugates and use thereof |
| US10005847B2 (en) | 2014-05-27 | 2018-06-26 | Academia Sinica | Anti-HER2 glycoantibodies and uses thereof |
| US10023892B2 (en) | 2014-05-27 | 2018-07-17 | Academia Sinica | Compositions and methods relating to universal glycoforms for enhanced antibody efficacy |
| US10034921B2 (en) | 2013-02-13 | 2018-07-31 | Laboratoire Français Du Fractionnement Et Des Biotechnologies | Proteins with modified glycosylation and methods of production thereof |
| US10087236B2 (en) | 2009-12-02 | 2018-10-02 | Academia Sinica | Methods for modifying human antibodies by glycan engineering |
| US10086054B2 (en) | 2013-06-26 | 2018-10-02 | Academia Sinica | RM2 antigens and use thereof |
| WO2018201096A1 (en) | 2017-04-27 | 2018-11-01 | Tesaro, Inc. | Antibody agents directed against lymphocyte activation gene-3 (lag-3) and uses thereof |
| US10118969B2 (en) | 2014-05-27 | 2018-11-06 | Academia Sinica | Compositions and methods relating to universal glycoforms for enhanced antibody efficacy |
| US10130714B2 (en) | 2012-04-14 | 2018-11-20 | Academia Sinica | Enhanced anti-influenza agents conjugated with anti-inflammatory activity |
| WO2018220099A1 (en) | 2017-06-02 | 2018-12-06 | F. Hoffmann-La Roche Ag | Type ii anti-cd20 antibody and anti-cd20/cd3 bispecific antibody for treatment of cancer |
| US10150818B2 (en) | 2014-01-16 | 2018-12-11 | Academia Sinica | Compositions and methods for treatment and detection of cancers |
| US10174110B2 (en) | 2013-02-13 | 2019-01-08 | Laboratoire Français Du Fractionnement Et Des Biotechnologies | Highly galactosylated anti-TNF-α antibodies and uses thereof |
| US10274488B2 (en) | 2008-07-15 | 2019-04-30 | Academia Sinica | Glycan arrays on PTFE-like aluminum coated glass slides and related methods |
| US10336784B2 (en) | 2016-03-08 | 2019-07-02 | Academia Sinica | Methods for modular synthesis of N-glycans and arrays thereof |
| US10338069B2 (en) | 2010-04-12 | 2019-07-02 | Academia Sinica | Glycan arrays for high throughput screening of viruses |
| US10342858B2 (en) | 2015-01-24 | 2019-07-09 | Academia Sinica | Glycan conjugates and methods of use thereof |
| US10495645B2 (en) | 2015-01-16 | 2019-12-03 | Academia Sinica | Cancer markers and methods of use thereof |
| WO2019234576A1 (en) | 2018-06-03 | 2019-12-12 | Lamkap Bio Beta Ltd. | Bispecific antibodies against ceacam5 and cd47 |
| US10513724B2 (en) | 2014-07-21 | 2019-12-24 | Glykos Finland Oy | Production of glycoproteins with mammalian-like N-glycans in filamentous fungi |
| US10525137B2 (en) | 2015-12-30 | 2020-01-07 | Genentech, Inc. | Formulations with reduced degradation of polysorbate |
| US10538592B2 (en) | 2016-08-22 | 2020-01-21 | Cho Pharma, Inc. | Antibodies, binding fragments, and methods of use |
| EP3831849A1 (en) | 2019-12-02 | 2021-06-09 | LamKap Bio beta AG | Bispecific antibodies against ceacam5 and cd47 |
| US11332523B2 (en) | 2014-05-28 | 2022-05-17 | Academia Sinica | Anti-TNF-alpha glycoantibodies and uses thereof |
| US11377485B2 (en) | 2009-12-02 | 2022-07-05 | Academia Sinica | Methods for modifying human antibodies by glycan engineering |
| EP4026848A1 (en) | 2015-12-09 | 2022-07-13 | F. Hoffmann-La Roche AG | Type ii anti-cd20 antibody for reducing the cytokine release syndrome |
| US11401348B2 (en) | 2009-09-02 | 2022-08-02 | Xencor, Inc. | Heterodimeric Fc variants |
| US11820830B2 (en) | 2004-07-20 | 2023-11-21 | Xencor, Inc. | Optimized Fc variants |
| US11884739B2 (en) | 2014-05-27 | 2024-01-30 | Academia Sinica | Anti-CD20 glycoantibodies and uses thereof |
| US11932685B2 (en) | 2007-10-31 | 2024-03-19 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US12152073B2 (en) | 2018-03-14 | 2024-11-26 | Marengo Therapeutics, Inc. | Multifunctional molecules that bind to calreticulin and uses thereof |
| US12247076B2 (en) | 2015-07-06 | 2025-03-11 | Laboratoire Français Du Fractionnement Et Des Biotechnologies | Use of modified Fc fragments in immunotherapy |
| US12247060B2 (en) | 2018-01-09 | 2025-03-11 | Marengo Therapeutics, Inc. | Calreticulin binding constructs and engineered T cells for the treatment of diseases |
| US12286477B2 (en) | 2018-07-03 | 2025-04-29 | Marengo Therapeutics, Inc. | Anti-TCR antibody molecules and uses thereof |
| US12358982B2 (en) | 2019-02-21 | 2025-07-15 | Marengo Therapeutics, Inc. | Multifunctional molecules that bind to T cell related cancer cells and uses thereof |
| US12384842B2 (en) | 2019-02-21 | 2025-08-12 | Marengo Therapeutics, Inc. | Antibody molecules that bind to NKP30 and uses thereof |
| US12486326B2 (en) | 2020-01-03 | 2025-12-02 | Marengo Therapeutics, Inc. | Anti-TCR antibody molecules and uses thereof |
| US12492253B1 (en) | 2008-02-25 | 2025-12-09 | Xencor, Inc. | Anti-human C5 antibodies |
Families Citing this family (917)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6136311A (en) | 1996-05-06 | 2000-10-24 | Cornell Research Foundation, Inc. | Treatment and diagnosis of cancer |
| DE69942636D1 (en) | 1998-12-09 | 2010-09-09 | Phyton Holdings Llc | PROCESS FOR PREPARING GLYCOPROTEIN WITH HUMAN GLYCOSYLATION PICTURE |
| NZ518532A (en) | 1999-10-26 | 2004-02-27 | Plant Res Internat B | Transgenic plant expressing mammalian beta-1,4- galactosyltransferase |
| JP2003531149A (en) * | 2000-04-13 | 2003-10-21 | ザ・ロツクフエラー・ユニバーシテイ | Enhancement of antibody-derived immune response |
| BRPI0206546B1 (en) | 2001-01-19 | 2015-07-21 | Phyton Holdings Llc | Method for secretory production of human-type sugar chain glycoprotein using plant cell |
| EP1485486B1 (en) | 2002-03-19 | 2012-11-21 | Stichting Dienst Landbouwkundig Onderzoek | Optimizing glycan processing in plants |
| AU2003219402B2 (en) | 2002-03-19 | 2008-05-08 | Stichting Dienst Landbouwkundig Onderzoek | GnTIII (UDP-n-acethylglucosamine:beta-D mannoside beta (1,4)-N-acethylglucosaminy ltransferase III) expression in plants |
| AR042145A1 (en) | 2002-11-27 | 2005-06-08 | Dow Agrociences Llc | IMMUNOGLOBULIN PRODUCTION IN PLANTS WITH A REDUCED FUCOCILATION |
| AR044388A1 (en) | 2003-05-20 | 2005-09-07 | Applied Molecular Evolution | CD20 UNION MOLECULES |
| WO2005080432A2 (en) | 2004-02-19 | 2005-09-01 | Genentech, Inc. | Cdr-repaired antibodies |
| AR048098A1 (en) * | 2004-03-15 | 2006-03-29 | Wyeth Corp | CALIQUEAMYCIN CONJUGATES |
| EP1753455A2 (en) | 2004-06-04 | 2007-02-21 | Genentech, Inc. | Method for treating multiple sclerosis |
| CA2573505C (en) | 2004-07-14 | 2013-06-25 | Igeneon Krebs-Immuntherapie Forschungs-Und Entwicklungs-Ag | N-glycosylated antibody |
| KR20070100228A (en) | 2004-10-05 | 2007-10-10 | 제넨테크, 인크. | How to treat vasculitis |
| JO3000B1 (en) | 2004-10-20 | 2016-09-05 | Genentech Inc | Antibody Formulations. |
| UA94899C2 (en) | 2005-01-21 | 2011-06-25 | Дженентек, Инк. | Fixed dosing of her antibodies |
| EP1850874B1 (en) | 2005-02-23 | 2013-10-16 | Genentech, Inc. | Extending time to disease progression or survival in ovarian cancer patients using pertuzumab |
| PA8672101A1 (en) | 2005-04-29 | 2006-12-07 | Centocor Inc | ANTI-IL-6 ANTIBODIES, COMPOSITIONS, METHODS AND USES |
| CN101282993A (en) * | 2005-06-02 | 2008-10-08 | 阿斯利康公司 | Antibodies against CD20 and uses thereof |
| EP1896073B1 (en) | 2005-06-30 | 2013-03-06 | Janssen Biotech, Inc. | Anti-il-23 antibodies, compositions, methods and uses |
| US9580506B2 (en) | 2005-07-21 | 2017-02-28 | Genmab A/S | Potency assays for antibody drug substance binding to an Fc receptor |
| AU2006283560B2 (en) * | 2005-08-19 | 2011-12-08 | Centocor, Inc. | Proteolysis resistant antibody preparations |
| MY149159A (en) | 2005-11-15 | 2013-07-31 | Hoffmann La Roche | Method for treating joint damage |
| EP3006466B1 (en) | 2005-12-02 | 2018-08-01 | Genentech, Inc. | Compositions and methods for the treatment of diseases and disorders associated with cytokine signaling involving antibodies that bind to il-22 and il-22r |
| PT2548577T (en) | 2005-12-29 | 2017-05-29 | Janssen Biotech Inc | Human anti-il-23 antibodies, compositions, methods and uses |
| EP3156418A1 (en) | 2006-01-05 | 2017-04-19 | Genentech, Inc. | Anti-ephb4 antibodies and methods using same |
| US20070166306A1 (en) * | 2006-01-17 | 2007-07-19 | Fey Georg H M | Anti-CD19 antibody composition and method |
| US7884264B2 (en) | 2006-01-17 | 2011-02-08 | Biolex Therapeutics, Inc. | Compositions and methods for inhibition of fucosyltransferase and xylosyltransferase expression in duckweed plants |
| WO2008029281A2 (en) | 2006-02-10 | 2008-03-13 | Invitrogen Corporation | Labeling and detection of post translationally modified proteins |
| AR059851A1 (en) | 2006-03-16 | 2008-04-30 | Genentech Inc | ANTIBODIES OF EGFL7 AND METHODS OF USE |
| JP2009531324A (en) | 2006-03-20 | 2009-09-03 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | Engineered anti-prostatic stem cell antigen (PSCA) antibody for cancer targeting |
| RS54163B1 (en) | 2006-05-30 | 2015-12-31 | Genentech Inc. | ANTI-CD22 ANTIBODIES, THEIR IMMUNCONJUGATES AND THEIR USE |
| WO2008060705A2 (en) | 2006-06-06 | 2008-05-22 | Genentech, Inc. | Anti-dll4 antibodies and methods using same |
| AR062065A1 (en) | 2006-07-14 | 2008-10-15 | Ac Immune Sa | HUMANIZED ANTIBODY |
| TR201802814T4 (en) | 2006-07-14 | 2018-03-21 | Ac Immune Sa | Humanized antibody against amyloid beta. |
| JP2009543579A (en) | 2006-07-19 | 2009-12-10 | ザ・トラスティーズ・オブ・ザ・ユニバーシティ・オブ・ペンシルバニア | WSX-1 / p28 as a target for anti-inflammatory response |
| LT2845866T (en) | 2006-10-27 | 2017-07-10 | Genentech, Inc. | Antibodies and immunoconjugates and uses therefor |
| AU2008216495A1 (en) | 2007-02-09 | 2008-08-21 | Genentech, Inc. | Anti-Robo4 antibodies and uses therefor |
| EP2132573B1 (en) | 2007-03-02 | 2014-04-23 | Genentech, Inc. | Predicting response to a her dimerisation inhbitor based on low her3 expression |
| DK2147108T3 (en) | 2007-04-17 | 2014-06-16 | Stichting Dienst Landbouwkundi | MAMMALIA TYPE OF GLYCOSYLATION IN PLANTS BY EXPRESSION OF NON-MAMMALIA-GLYCOSYL TRANSFER |
| CA2685222C (en) | 2007-05-14 | 2017-12-19 | Medimmune, Llc | Methods of reducing eosinophil levels |
| DK2171090T3 (en) | 2007-06-08 | 2013-06-10 | Genentech Inc | Gene expression markers for tumor resistance to HER2 inhibitor therapy |
| SG182192A1 (en) | 2007-06-12 | 2012-07-30 | Ac Immune Sa | Humanized antibodies to amyloid beta |
| PT2155880T (en) * | 2007-06-15 | 2016-11-18 | Medicago Inc | Modifying glycoprotein production in plants |
| JP6126773B2 (en) | 2007-09-04 | 2017-05-10 | ザ リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | High affinity anti-prostatic stem cell antigen (PSCA) antibody for cancer targeting and detection |
| ES2566957T3 (en) | 2007-09-26 | 2016-04-18 | Chugai Seiyaku Kabushiki Kaisha | Constant region of modified antibody |
| SG178809A1 (en) | 2007-10-05 | 2012-03-29 | Genentech Inc | Use of anti-amyloid beta antibody in ocular diseases |
| CN103142999A (en) | 2007-11-07 | 2013-06-12 | 健泰科生物技术公司 | Compositions and methods for treatment of microbial disorders |
| AR069501A1 (en) | 2007-11-30 | 2010-01-27 | Genentech Inc | ANTI-VEGF ANTIBODIES (VASCULAR ENDOTELIAL GROWTH FACTOR) |
| ES2526433T3 (en) * | 2007-12-26 | 2015-01-12 | Biotest Ag | Immunoconjugates directed to CD138 and uses thereof |
| AR069979A1 (en) * | 2007-12-26 | 2010-03-03 | Biotest Ag | METHOD FOR DECREASING SECONDARY CYTOTOXIC EFFECTS AND IMPROVING THE EFFECTIVENESS OF IMMUNOCATE PLAYERS |
| EP2240516B1 (en) * | 2007-12-26 | 2015-07-08 | Biotest AG | Methods and agents for improving targeting of cd138 expressing tumor cells |
| EP2238168B8 (en) * | 2007-12-26 | 2014-07-23 | Biotest AG | Agents targeting cd138 and uses thereof |
| TWI472339B (en) | 2008-01-30 | 2015-02-11 | Genentech Inc | Composition comprising antibody that binds to domain ii of her2 and acidic variants thereof |
| KR101054362B1 (en) * | 2008-07-03 | 2011-08-05 | 재단법인 목암생명공학연구소 | How to reduce the fucose content of recombinant protein |
| US8080415B2 (en) * | 2008-09-26 | 2011-12-20 | Eureka Therapeutics, Inc. | Modified host cells and uses thereof |
| CN110317272A (en) | 2008-10-14 | 2019-10-11 | 霍夫曼-拉罗奇有限公司 | Immunoglobulin variants and application thereof |
| EP2752189B1 (en) | 2008-11-22 | 2016-10-26 | F. Hoffmann-La Roche AG | Use of anti-vegf antibody in combination with chemotherapy for treating breast cancer |
| AR074777A1 (en) | 2008-12-19 | 2011-02-09 | Glaxo Group Ltd | PROTEINS OF UNION TO ANTIGEN |
| US20120009182A1 (en) | 2008-12-23 | 2012-01-12 | Genentech, Inc. | Immunoglobulin variants with altered binding to protein a |
| US10517969B2 (en) | 2009-02-17 | 2019-12-31 | Cornell University | Methods and kits for diagnosis of cancer and prediction of therapeutic value |
| EP2401696B1 (en) * | 2009-02-26 | 2017-06-21 | Intrexon CEU, Inc. | Mammalian cell line models and related methods |
| ES2572728T3 (en) | 2009-03-20 | 2016-06-02 | F. Hoffmann-La Roche Ag | Bispecific anti-HER antibodies |
| CN102365297B (en) | 2009-03-25 | 2014-10-29 | 霍夫曼-拉罗奇有限公司 | Novel anti-α5β1 antibody and its application |
| DK3702371T5 (en) | 2009-03-25 | 2024-08-26 | Genentech Inc | Anti-FGFR3 antibodies and methods of using them |
| JP5616428B2 (en) | 2009-04-07 | 2014-10-29 | ロシュ グリクアート アクチェンゲゼルシャフト | Trivalent bispecific antibody |
| MX2011011684A (en) * | 2009-05-06 | 2012-01-20 | Biotest Ag | Uses of immunoconjugates targeting cd138. |
| EP2435476A4 (en) | 2009-05-27 | 2013-04-17 | Synageva Biopharma Corp | Avian derived antibodies |
| US9676845B2 (en) | 2009-06-16 | 2017-06-13 | Hoffmann-La Roche, Inc. | Bispecific antigen binding proteins |
| WO2011014457A1 (en) | 2009-07-27 | 2011-02-03 | Genentech, Inc. | Combination treatments |
| WO2011014750A1 (en) | 2009-07-31 | 2011-02-03 | Genentech, Inc. | Inhibition of tumor metastasis using bv8- or g-csf-antagonists |
| AU2010284446A1 (en) | 2009-08-15 | 2012-03-08 | Genentech, Inc. | Anti-angiogenesis therapy for the treatment of previously treated breast cancer |
| TWI412375B (en) * | 2009-08-28 | 2013-10-21 | Roche Glycart Ag | Humanized anti-cdcp1 antibodies |
| ES2599076T3 (en) | 2009-09-02 | 2017-01-31 | Genentech, Inc. | Smoothened mutant and methods of use thereof |
| TW201121566A (en) | 2009-10-22 | 2011-07-01 | Genentech Inc | Anti-hepsin antibodies and methods using same |
| WO2011056502A1 (en) | 2009-10-26 | 2011-05-12 | Genentech, Inc. | Bone morphogenetic protein receptor type ii compositions and methods of use |
| WO2011056494A1 (en) | 2009-10-26 | 2011-05-12 | Genentech, Inc. | Activin receptor-like kinase-1 antagonist and vegfr3 antagonist combinations |
| WO2011056497A1 (en) | 2009-10-26 | 2011-05-12 | Genentech, Inc. | Activin receptor type iib compositions and methods of use |
| JP6007420B2 (en) | 2009-11-04 | 2016-10-12 | ファブラス エルエルシー | Antibody optimization method based on affinity maturation |
| WO2011057120A1 (en) | 2009-11-05 | 2011-05-12 | Genentech, Inc. | Methods and composition for secretion of heterologous polypeptides |
| JP6251477B2 (en) | 2009-12-02 | 2017-12-20 | イマジナブ・インコーポレーテッド | J591 minibody and CYS diabody targeting human prostate specific membrane antigen (PSMA) and methods for using them |
| AR078377A1 (en) | 2009-12-11 | 2011-11-02 | Genentech Inc | ANTI-VEGF-C ANTIBODIES (ISOLATED ANTI-VASCULAR ENDOTELIAL GROWTH FACTOR C) AND ITS METHODS OF USE |
| EP2516465B1 (en) | 2009-12-23 | 2016-05-18 | F.Hoffmann-La Roche Ag | Anti-bv8 antibodies and uses thereof |
| EP2528947A4 (en) | 2010-01-28 | 2013-09-18 | Glaxo Group Ltd | CD127 BINDING PROTEINS |
| EA201290630A1 (en) | 2010-02-09 | 2013-03-29 | Глаксо Груп Лимитед | TREATMENT OF DISORDERS OF EXCHANGE OF SUBSTANCES |
| CN102892779B (en) | 2010-02-18 | 2016-12-21 | 基因泰克公司 | Neuregulin antagonist and the purposes in treatment cancer thereof |
| RU2012140447A (en) | 2010-02-23 | 2014-03-27 | Дженентек, Инк. | ANTIANGIOGENIC THERAPY FOR TREATMENT OF OVARIAN CANCER |
| UA108227C2 (en) | 2010-03-03 | 2015-04-10 | ANTIGENCY PROTEIN | |
| EP2550295A1 (en) | 2010-03-24 | 2013-01-30 | F. Hoffmann-La Roche AG | Anti-lrp6 antibodies |
| EP2374816B1 (en) | 2010-04-07 | 2016-09-28 | Agency For Science, Technology And Research | Binding molecules against Chikungunya virus and uses thereof |
| US9441032B2 (en) | 2010-04-07 | 2016-09-13 | Agency For Science, Technology And Research | Binding molecules against Chikungunya virus and uses thereof |
| WO2011146568A1 (en) | 2010-05-19 | 2011-11-24 | Genentech, Inc. | Predicting response to a her inhibitor |
| WO2011147834A1 (en) | 2010-05-26 | 2011-12-01 | Roche Glycart Ag | Antibodies against cd19 and uses thereof |
| WO2011153243A2 (en) | 2010-06-02 | 2011-12-08 | Genentech, Inc. | Anti-angiogenesis therapy for treating gastric cancer |
| UY33421A (en) | 2010-06-03 | 2011-12-30 | Glaxo Wellcome House | HUMANIZED ANTIGEN UNION PROTEINS |
| MX336109B (en) | 2010-06-03 | 2016-01-08 | Genentech Inc | FORMATION OF IMMUNE-TEP IMAGES OF ANTIBODIES AND IMMUNOCUSES AND USES OF THE SAME. |
| BR112012027995A2 (en) | 2010-06-18 | 2017-01-10 | Genentech Inc | antibody and isolated nucleic acid, host cell, method of producing an antibody, immunoconjugate, pharmaceutical formulation, use of the antibody, method of treating an individual with cancer, an individual having an immune disorder, inhibiting angiogenesis and inhibiting the constitutive activation of axl |
| WO2011161119A1 (en) | 2010-06-22 | 2011-12-29 | F. Hoffmann-La Roche Ag | Antibodies against insulin-like growth factor i receptor and uses thereof |
| WO2011161189A1 (en) | 2010-06-24 | 2011-12-29 | F. Hoffmann-La Roche Ag | Anti-hepsin antibodies and methods of use |
| JP2013539962A (en) | 2010-07-09 | 2013-10-31 | ジェネンテック, インコーポレイテッド | Anti-neuropilin antibodies and methods of use |
| EP2409712A1 (en) | 2010-07-19 | 2012-01-25 | International-Drug-Development-Biotech | Anti-CD19 antibody having ADCC and CDC functions and improved glycosylation profile |
| EP2409989A1 (en) | 2010-07-19 | 2012-01-25 | International-Drug-Development-Biotech | Method to improve glycosylation profile for antibody |
| EP2409993A1 (en) | 2010-07-19 | 2012-01-25 | International-Drug-Development-Biotech | Anti-CD19 antibody having ADCC function with improved glycosylation profile |
| WO2012010582A1 (en) | 2010-07-21 | 2012-01-26 | Roche Glycart Ag | Anti-cxcr5 antibodies and methods of use |
| SG187592A1 (en) | 2010-07-23 | 2013-03-28 | Univ Boston | Anti-despr inhibitors as therapeutics for inhibition of pathological angiogenesis and tumor cell invasiveness and for molecular imaging and targeted delivery |
| RU2013106216A (en) | 2010-08-03 | 2014-09-10 | Ф. Хоффманн-Ля Рош Аг | BIOMARKERS OF CHRONIC Lymphocytic Leukemia |
| EP2600898A1 (en) | 2010-08-05 | 2013-06-12 | F.Hoffmann-La Roche Ag | Anti-mhc antibody anti-viral cytokine fusion protein |
| CN103080132B (en) | 2010-08-25 | 2016-06-08 | 弗·哈夫曼-拉罗切有限公司 | Antibodies against IL-18R1 and uses thereof |
| RU2013114360A (en) | 2010-08-31 | 2014-10-10 | Дженентек, Инк. | BIOMARKERS AND TREATMENT METHODS |
| DK2625197T3 (en) | 2010-10-05 | 2016-10-03 | Genentech Inc | Smoothened MUTANT AND METHODS OF USING THE SAME |
| EP4029881A1 (en) | 2010-11-08 | 2022-07-20 | F. Hoffmann-La Roche AG | Subcutaneously administered anti-il-6 receptor antibody |
| EP3176184B1 (en) | 2010-11-10 | 2020-02-19 | F. Hoffmann-La Roche AG | Anti-bace1 antibodies for neural disease immunotherapy |
| JO3455B1 (en) | 2010-11-23 | 2020-07-05 | Glaxo Group Ltd | Oncostatin M (OSM) antigen binding proteins |
| AU2011333738A1 (en) | 2010-11-24 | 2013-07-11 | Glaxo Group Limited | Multispecific antigen binding proteins targeting HGF |
| EA034333B1 (en) | 2010-11-30 | 2020-01-29 | Дженентек, Инк. | Variants of an antibody for transporting a compound across the blood-brain barrier |
| TWI732259B (en) | 2010-12-16 | 2021-07-01 | 美商建南德克公司 | Diagnosis and treatments relating to th2 inhibition |
| WO2012087962A2 (en) | 2010-12-20 | 2012-06-28 | Genentech, Inc. | Anti-mesothelin antibodies and immunoconjugates |
| SG191219A1 (en) | 2010-12-22 | 2013-07-31 | Genentech Inc | Anti-pcsk9 antibodies and methods of use |
| WO2012092539A2 (en) | 2010-12-31 | 2012-07-05 | Takeda Pharmaceutical Company Limited | Antibodies to dll4 and uses thereof |
| WO2012116927A1 (en) | 2011-02-28 | 2012-09-07 | F. Hoffmann-La Roche Ag | Monovalent antigen binding proteins |
| MX341921B (en) | 2011-02-28 | 2016-09-07 | Hoffmann La Roche | Antigen binding proteins. |
| MX342240B (en) | 2011-04-07 | 2016-09-21 | Genentech Inc | Anti-fgfr4 antibodies and methods of use. |
| WO2012146630A1 (en) | 2011-04-29 | 2012-11-01 | F. Hoffmann-La Roche Ag | N-terminal acylated polypeptides, methods for their production and uses thereof |
| ES2567276T3 (en) | 2011-05-12 | 2016-04-21 | Genentech, Inc. | LC-MS / MS method of monitoring multiple reactions to detect therapeutic antibodies in animal samples using frame-changing peptides |
| BR112013026266A2 (en) | 2011-05-16 | 2020-11-10 | Genentech, Inc | treatment method, isolated and anti-fgfr1 antibodies, isolated nucleic acid, host cell, method of producing an antibody, pharmaceutical formulation, use of the antibody and method of treating diabetes in an individual |
| HUE063461T2 (en) | 2011-05-27 | 2024-01-28 | Glaxo Group Ltd | BCMA (CD269/TNFRSF17)-binding proteins |
| WO2012171996A1 (en) | 2011-06-15 | 2012-12-20 | F. Hoffmann-La Roche Ag | Anti-human epo receptor antibodies and methods of use |
| PL2723773T3 (en) | 2011-06-22 | 2018-07-31 | F.Hoffmann-La Roche Ag | Removal of target cells by circulating virus-specific cytotoxic t-cells using mhc class i comprising complexes |
| KR20140045440A (en) | 2011-06-30 | 2014-04-16 | 제넨테크, 인크. | Anti-c-met antibody formulations |
| US20130022551A1 (en) | 2011-07-22 | 2013-01-24 | Trustees Of Boston University | DEspR ANTAGONISTS AND AGONISTS AS THERAPEUTICS |
| EP2736925A2 (en) | 2011-07-27 | 2014-06-04 | Glaxo Group Limited | Anti-vegf single variable domains fused to fc domains |
| MX2014001766A (en) | 2011-08-17 | 2014-05-01 | Genentech Inc | Neuregulin antibodies and uses thereof. |
| EP2744825A1 (en) | 2011-08-17 | 2014-06-25 | F.Hoffmann-La Roche Ag | Inhibition of angiogenesis in refractory tumors |
| WO2013040433A1 (en) | 2011-09-15 | 2013-03-21 | Genentech, Inc. | Methods of promoting differentiation |
| JP2014534949A (en) | 2011-09-19 | 2014-12-25 | ジェネンテック, インコーポレイテッド | Combination treatment comprising C-MET antagonist and B-RAF antagonist |
| WO2013052155A1 (en) | 2011-10-05 | 2013-04-11 | Genentech, Inc. | Methods of treating liver conditions using notch2 antagonists |
| HUE039133T2 (en) | 2011-10-14 | 2018-12-28 | Hoffmann La Roche | ANTI-HtrA1 ANTIBODIES AND METHODS OF USE |
| RU2650873C2 (en) | 2011-10-19 | 2018-04-17 | Рош Гликарт Аг | Separation method for fucosylated antibodies |
| WO2013059531A1 (en) | 2011-10-20 | 2013-04-25 | Genentech, Inc. | Anti-gcgr antibodies and uses thereof |
| WO2013059740A1 (en) | 2011-10-21 | 2013-04-25 | Foundation Medicine, Inc. | Novel alk and ntrk1 fusion molecules and uses thereof |
| AU2012328980A1 (en) | 2011-10-28 | 2014-04-24 | Genentech, Inc. | Therapeutic combinations and methods of treating melanoma |
| AU2012340826A1 (en) | 2011-11-21 | 2014-05-29 | Genentech, Inc. | Purification of anti-c-met antibodies |
| EP2788024A1 (en) | 2011-12-06 | 2014-10-15 | F.Hoffmann-La Roche Ag | Antibody formulation |
| RU2632108C2 (en) | 2011-12-08 | 2017-10-02 | Биотест Аг | Applications of immunoconjugates, the target of which is cd138 |
| WO2013092743A2 (en) | 2011-12-22 | 2013-06-27 | F. Hoffmann-La Roche Ag | Expression vector element combinations, novel production cell generation methods and their use for the recombinant production of polypeptides |
| EP2794662A1 (en) | 2011-12-22 | 2014-10-29 | F.Hoffmann-La Roche Ag | Full length antibody display system for eukaryotic cells and its use |
| HRP20200744T1 (en) | 2011-12-22 | 2020-07-24 | F. Hoffmann - La Roche Ag | ORGANIZATION OF EXPRESSION VECTOR, NEW PROCEDURES FOR GENERATION OF CELL PRODUCTION AND THEIR USE FOR RECOMBINANT POLYPEPTIDE PRODUCTION |
| WO2013096791A1 (en) | 2011-12-23 | 2013-06-27 | Genentech, Inc. | Process for making high concentration protein formulations |
| WO2013101771A2 (en) | 2011-12-30 | 2013-07-04 | Genentech, Inc. | Compositions and method for treating autoimmune diseases |
| CA2862979A1 (en) | 2012-01-09 | 2013-07-18 | The Scripps Research Institute | Humanized antibodies with ultralong cdr3s |
| US20140050720A1 (en) | 2012-01-09 | 2014-02-20 | The Scripps Research Institute | Ultralong complementarity determining regions and uses thereof |
| EP2804629A1 (en) | 2012-01-18 | 2014-11-26 | Genentech, Inc. | Anti-lrp5 antibodies and methods of use |
| US20130183294A1 (en) | 2012-01-18 | 2013-07-18 | Genentech, Inc. | Methods of using fgf19 modulators |
| RU2014133069A (en) | 2012-02-11 | 2016-04-10 | Дженентек, Инк. | R-SPONDIN TRANSLOCATIONS AND WAYS WITH THEIR USE |
| CN104125852B9 (en) | 2012-02-15 | 2017-05-17 | 弗·哈夫曼-拉罗切有限公司 | Fc-receptor based affinity chromatography |
| JP2015514710A (en) | 2012-03-27 | 2015-05-21 | ジェネンテック, インコーポレイテッド | Diagnosis and treatment of HER3 inhibitors |
| AR090549A1 (en) | 2012-03-30 | 2014-11-19 | Genentech Inc | ANTI-LGR5 AND IMMUNOCATE PLAYERS |
| AU2013256596A1 (en) | 2012-05-01 | 2014-10-09 | Genentech, Inc. | Anti-PMEL17 antibodies and immunoconjugates |
| WO2013170191A1 (en) | 2012-05-11 | 2013-11-14 | Genentech, Inc. | Methods of using antagonists of nad biosynthesis from nicotinamide |
| MX389346B (en) | 2012-05-18 | 2025-03-20 | Genentech Inc | HIGH CONCENTRATION MONOCLONAL ANTIBODY FORMULATIONS. |
| EP3594239B1 (en) | 2012-05-21 | 2024-10-30 | F. Hoffmann-La Roche AG | Methods for improving safety of blood-brain barrier transport |
| WO2013177470A1 (en) | 2012-05-23 | 2013-11-28 | Genentech, Inc. | Selection method for therapeutic agents |
| CN103463633B (en) * | 2012-06-07 | 2016-03-30 | 复旦大学 | Chimeric hepatitis B virus core antigen therapeutic vaccine of a kind of targeting and uses thereof |
| WO2013188855A1 (en) | 2012-06-15 | 2013-12-19 | Genentech, Inc. | Anti-pcsk9 antibodies, formulations, dosing, and methods of use |
| US20140004121A1 (en) | 2012-06-27 | 2014-01-02 | Amgen Inc. | Anti-mesothelin binding proteins |
| RU2630664C2 (en) | 2012-07-04 | 2017-09-11 | Ф. Хоффманн-Ля Рош Аг | Theophylline antibodies and methods for their application |
| ES2604012T3 (en) | 2012-07-04 | 2017-03-02 | F. Hoffmann-La Roche Ag | Covalently bound antigen-antibody conjugates |
| WO2014006123A1 (en) | 2012-07-04 | 2014-01-09 | F. Hoffmann-La Roche Ag | Anti-biotin antibodies and methods of use |
| CA3188124A1 (en) | 2012-07-05 | 2014-01-09 | Genentech, Inc. | Expression and secretion system |
| US20140030282A1 (en) | 2012-07-09 | 2014-01-30 | Genentech, Inc. | Anti-cd79b antibodies and immunoconjugates |
| KR20150027829A (en) | 2012-07-09 | 2015-03-12 | 제넨테크, 인크. | Immunoconjucates comprising anti-CD22 antibodies |
| SG11201500093TA (en) | 2012-07-09 | 2015-02-27 | Genentech Inc | Immunoconjugates comprising anti-cd79b antibodies |
| TW201408696A (en) | 2012-07-09 | 2014-03-01 | Genentech Inc | Anti-CD22 antibodies and immunoconjugates |
| SMT202100621T1 (en) | 2012-07-13 | 2022-01-10 | Roche Glycart Ag | Bispecific anti-vegf/anti-ang-2 antibodies and their use in the treatment of ocular vascular diseases |
| TWI596113B (en) | 2012-07-25 | 2017-08-21 | 塞爾德克斯醫療公司 | anti-KIT antibody and use thereof |
| EP2888279A1 (en) | 2012-08-22 | 2015-07-01 | Glaxo Group Limited | Anti lrp6 antibodies |
| HK1205521A1 (en) | 2012-08-29 | 2015-12-18 | 霍夫曼-拉罗奇有限公司 | Blood brain barrier shuttle |
| CN111481552A (en) | 2012-09-07 | 2020-08-04 | 吉宁特有限公司 | Combination therapy of type II anti-CD 20 antibodies with selective Bcl-2 inhibitors |
| JP5993093B2 (en) | 2012-10-12 | 2016-09-14 | メドイミューン・リミテッドMedImmune Limited | Pyrrolobenzodiazepines and their complexes |
| US10100102B2 (en) | 2012-10-29 | 2018-10-16 | The University Of North Carolina At Chapel Hill | Compositions and methods for inhibiting pathogen infection |
| AU2013337277B2 (en) | 2012-11-05 | 2018-03-08 | Foundation Medicine, Inc. | Novel NTRK1 fusion molecules and uses thereof |
| ES2949394T3 (en) | 2012-11-05 | 2023-09-28 | Found Medicine Inc | Novel fusion molecules and their uses |
| CN104755500B (en) | 2012-11-08 | 2020-10-02 | 霍夫曼-拉罗奇有限公司 | HER3 antigen binding proteins that bind to the HER3 beta-hairpin |
| MA38176A1 (en) | 2012-11-13 | 2017-06-30 | Genentech Inc | Novel anti-haemagglutinin antibody, useful for the treatment, inhibition or prevention of viral influenza infection |
| EP2935328B1 (en) | 2012-12-21 | 2018-10-10 | F.Hoffmann-La Roche Ag | Disulfide-linked multivalent mhc class i comprising multi-function proteins |
| CA3150658A1 (en) | 2013-01-18 | 2014-07-24 | Foundation Medicine, Inc. | Methods of treating cholangiocarcinoma |
| WO2014116749A1 (en) | 2013-01-23 | 2014-07-31 | Genentech, Inc. | Anti-hcv antibodies and methods of using thereof |
| JP2016509045A (en) | 2013-02-22 | 2016-03-24 | エフ・ホフマン−ラ・ロシュ・アクチェンゲゼルシャフト | How to treat cancer and prevent drug resistance |
| WO2014138364A2 (en) | 2013-03-06 | 2014-09-12 | Genentech, Inc. | Methods of treating and preventing cancer drug resistance |
| AU2014230735B2 (en) | 2013-03-13 | 2018-03-15 | Medimmune Limited | Pyrrolobenzodiazepines and conjugates thereof |
| US9562099B2 (en) | 2013-03-14 | 2017-02-07 | Genentech, Inc. | Anti-B7-H4 antibodies and immunoconjugates |
| EP3299391B1 (en) | 2013-03-14 | 2019-12-04 | Genentech, Inc. | Anti-b7-h4 antibodies and immunoconjugates |
| WO2014153030A2 (en) | 2013-03-14 | 2014-09-25 | Genentech, Inc. | Methods of treating cancer and preventing cancer drug resistance |
| CA2903480A1 (en) | 2013-03-14 | 2014-09-25 | Genentech, Inc. | Combinations of a mek inhibitor compound with an her3/egfr inhibitor compound and methods of use |
| ES2808654T3 (en) | 2013-03-15 | 2021-03-01 | Glaxosmithkline Ip Dev Ltd | Anti-LAG-3 binding proteins |
| AU2014236815B2 (en) | 2013-03-15 | 2019-04-04 | Genentech, Inc. | Compositions and methods for diagnosis and treatment of hepatic cancers |
| AU2014235453A1 (en) | 2013-03-15 | 2015-10-08 | Genentech, Inc. | Biomarkers and methods of treating PD-1 and PD-L1 related conditions |
| MX2015011899A (en) | 2013-03-15 | 2016-05-05 | Genentech Inc | Methods of treating cancer and preventing cancer drug resistance. |
| WO2014150877A2 (en) | 2013-03-15 | 2014-09-25 | Ac Immune S.A. | Anti-tau antibodies and methods of use |
| JP2016517441A (en) | 2013-03-15 | 2016-06-16 | ジェネンテック, インコーポレイテッド | Anti-CRTh2 antibody and method of use |
| AU2014233494B2 (en) | 2013-03-15 | 2018-10-04 | Genentech, Inc. | IL-22 polypeptides and IL-22 Fc fusion proteins and methods of use |
| UA118028C2 (en) | 2013-04-03 | 2018-11-12 | Рош Глікарт Аг | Bispecific antibodies specific for fap and dr5, antibodies specific for dr5 and methods of use |
| HK1213578A1 (en) | 2013-04-29 | 2016-07-08 | 豪夫迈‧罗氏有限公司 | Human fcrn-binding modified antibodies and methods of use |
| CN118561989A (en) | 2013-04-29 | 2024-08-30 | 豪夫迈·罗氏有限公司 | FC-receptor binding modified asymmetric antibodies and methods of use |
| TWI653243B (en) | 2013-04-29 | 2019-03-11 | 赫孚孟拉羅股份公司 | Anti-IGF-1R antibody against FcRn binding and use thereof for treating vascular eye diseases |
| PL3594240T3 (en) | 2013-05-20 | 2024-04-02 | F. Hoffmann-La Roche Ag | Anti-transferrin receptor antibodies and methods of use |
| WO2015017146A2 (en) | 2013-07-18 | 2015-02-05 | Fabrus, Inc. | Antibodies with ultralong complementarity determining regions |
| CN111518199A (en) | 2013-07-18 | 2020-08-11 | 图鲁斯生物科学有限责任公司 | Humanized antibodies with ultralong complementarity determining regions |
| NZ715201A (en) | 2013-08-01 | 2021-12-24 | Five Prime Therapeutics Inc | Afucosylated anti-fgfr2iiib antibodies |
| CR20160132A (en) | 2013-08-12 | 2016-08-25 | Genentech Inc | COMPOSITIONS AND METHOD TO TREAT CONDITIONS ASSOCIATED WITH THE COMPLEMENT |
| JP2016537399A (en) | 2013-09-17 | 2016-12-01 | ジェネンテック, インコーポレイテッド | Method using anti-LGR5 antibody |
| EP3055328A1 (en) | 2013-10-11 | 2016-08-17 | F. Hoffmann-La Roche AG | Nsp4 inhibitors and methods of use |
| KR20160044060A (en) | 2013-10-11 | 2016-04-22 | 에프. 호프만-라 로슈 아게 | Multispecific domain exchanged common variable light chain antibodies |
| ES2960807T3 (en) | 2013-10-11 | 2024-03-06 | Us Health | Antibodies against TEM8 and their use |
| CA2925598A1 (en) | 2013-10-18 | 2015-04-23 | Genentech, Inc. | Anti-rspo antibodies and methods of use |
| SG11201603127WA (en) | 2013-10-23 | 2016-05-30 | Genentech Inc | Methods of diagnosing and treating eosinophilic disorders |
| SI3071597T1 (en) | 2013-11-21 | 2020-11-30 | F. Hoffmann-La Roche Ag | Anti-alpha-synuclein antibodies and methods of use |
| NZ720769A (en) | 2013-12-09 | 2022-10-28 | Allakos Inc | Anti-siglec-8 antibodies and methods of use thereof |
| SG11201604784XA (en) | 2013-12-13 | 2016-07-28 | Genentech Inc | Anti-cd33 antibodies and immunoconjugates |
| CN106030310B (en) | 2013-12-13 | 2019-01-04 | 通用医疗公司 | Soluble high-molecular amount (HMW) TAU type and its application |
| KR20240017102A (en) | 2013-12-17 | 2024-02-06 | 제넨테크, 인크. | Methods of treating cancers using pd-1 axis binding antagonists and taxanes |
| KR20160089532A (en) | 2013-12-17 | 2016-07-27 | 제넨테크, 인크. | Methods of treating cancer using pd-1 axis binding antagonists and an anti-cd20 antibody |
| BR112016013963A2 (en) | 2013-12-17 | 2017-10-10 | Genentech Inc | combination therapy comprising ox40 binding agonists and pd-1 axis binding antagonists |
| EP3192812B1 (en) | 2013-12-17 | 2020-05-27 | Genentech, Inc. | Anti-cd3 antibodies and methods of use |
| TWI670283B (en) | 2013-12-23 | 2019-09-01 | 美商建南德克公司 | Antibodies and methods of use |
| KR20220152546A (en) | 2013-12-24 | 2022-11-16 | 더 보드 오브 리젠츠 오브 더 유니버시티 오브 텍사스 시스템 | Fcrn antagonists and methods of use |
| JP6521464B2 (en) | 2014-01-03 | 2019-05-29 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | Covalently linked polypeptide toxin-antibody conjugates |
| WO2015103549A1 (en) | 2014-01-03 | 2015-07-09 | The United States Of America, As Represented By The Secretary Department Of Health And Human Services | Neutralizing antibodies to hiv-1 env and their use |
| KR102278979B1 (en) | 2014-01-03 | 2021-07-19 | 에프. 호프만-라 로슈 아게 | Covalently linked helicar-anti-helicar antibody conjugates and uses thereof |
| US10561737B2 (en) | 2014-01-03 | 2020-02-18 | Hoffmann-La Roche Inc. | Bispecific anti-hapten/anti-blood brain barrier receptor antibodies, complexes thereof and their use as blood brain barrier shuttles |
| RU2694659C2 (en) | 2014-01-06 | 2019-07-16 | Ф. Хоффманн-Ля Рош Аг | Monovalent carrier modules across blood-brain barrier |
| CA2931986A1 (en) | 2014-01-15 | 2015-07-23 | F. Hoffmann-La Roche Ag | Fc-region variants with modified fcrn- and maintained protein a-binding properties |
| KR20160111469A (en) | 2014-01-24 | 2016-09-26 | 제넨테크, 인크. | Methods of using anti-STEAP1 antibodies and immunoconjugates |
| CA2937539A1 (en) | 2014-02-04 | 2015-08-13 | Genentech, Inc. | Mutant smoothened and methods of using the same |
| CN106456729A (en) | 2014-02-08 | 2017-02-22 | 豪夫迈·罗氏有限公司 | Ways to treat Alzheimer's disease |
| BR112016018170A2 (en) | 2014-02-08 | 2018-02-20 | Genentech, Inc. | Methods To Treat Alzheimer's Disease |
| EA201691610A8 (en) | 2014-02-12 | 2018-05-31 | Дженентек, Инк. | ANTI-JAGGED1 ANTIBODIES AND METHODS OF APPLICATION |
| CR20160379A (en) | 2014-02-21 | 2016-10-07 | Genentech Inc | BISPECIFIC ANTIBODIES ANTI-IL 13 / IL-17 AND ITS USES |
| EP3110446B1 (en) | 2014-02-28 | 2021-12-01 | Allakos Inc. | Methods and compositions for treating siglec-8 associated diseases |
| AU2015229035B2 (en) | 2014-03-14 | 2021-08-05 | Genentech, Inc. | Methods and compositions for secretion of heterologous polypeptides |
| WO2015140591A1 (en) | 2014-03-21 | 2015-09-24 | Nordlandssykehuset Hf | Anti-cd14 antibodies and uses thereof |
| CN106103478B (en) | 2014-03-21 | 2020-04-03 | 豪夫迈·罗氏有限公司 | In vitro prediction of antibody half-life in vivo |
| WO2015148531A1 (en) | 2014-03-24 | 2015-10-01 | Genentech, Inc. | Cancer treatment with c-met antagonists and correlation of the latter with hgf expression |
| KR20160146747A (en) | 2014-03-31 | 2016-12-21 | 제넨테크, 인크. | Combination therapy comprising anti-angiogenesis agents and ox40 binding agonists |
| EP3126394B1 (en) | 2014-03-31 | 2019-10-30 | F.Hoffmann-La Roche Ag | Anti-ox40 antibodies and methods of use |
| WO2015164615A1 (en) | 2014-04-24 | 2015-10-29 | University Of Oslo | Anti-gluten antibodies and uses thereof |
| WO2015179658A2 (en) | 2014-05-22 | 2015-11-26 | Genentech, Inc. | Anti-gpc3 antibodies and immunoconjugates |
| CN106661622B (en) | 2014-05-23 | 2020-08-21 | 豪夫迈·罗氏有限公司 | MIT biomarkers and how to use them |
| CA2949982A1 (en) | 2014-06-11 | 2015-12-17 | Genentech, Inc. | Anti-lgr5 antibodies and uses thereof |
| US20230190750A1 (en) | 2014-06-13 | 2023-06-22 | Genentech, Inc. | Methods of treating and preventing cancer drug resistance |
| MX384037B (en) | 2014-06-26 | 2025-03-14 | Hoffmann La Roche | ANTI-BROMODEOXYURIDINE (BRDU) ANTIBODIES AND METHODS OF USE. |
| RU2715038C2 (en) | 2014-07-11 | 2020-02-21 | Дженентек, Инк. | Anti-pd-l1 antibodies and methods for their diagnostic use |
| RU2017103289A (en) | 2014-07-11 | 2018-08-14 | Дженентек, Инк. | INHIBITING THE NOTCH WAY |
| SG11201701161QA (en) | 2014-08-19 | 2017-03-30 | Merck Sharp & Dohme | Anti-tigit antibodies |
| TWI751102B (en) | 2014-08-28 | 2022-01-01 | 美商奇諾治療有限公司 | Antibodies and chimeric antigen receptors specific for cd19 |
| MX2017002605A (en) | 2014-08-28 | 2017-05-19 | Bioatla Llc | Conditionally active chimeric antigen receptors for modified t-cells. |
| CN106687141A (en) | 2014-09-10 | 2017-05-17 | 麦迪穆有限责任公司 | Pyrrolobenzodiazepines and conjugates thereof |
| PE20170935A1 (en) | 2014-09-12 | 2017-07-13 | Genentech Inc | ANTI-HER2 AND IMMUNOCONJUGATED ANTIBODIES |
| CN113698485A (en) | 2014-09-12 | 2021-11-26 | 基因泰克公司 | anti-B7-H4 antibodies and immunoconjugates |
| BR112017004802A2 (en) | 2014-09-12 | 2017-12-12 | Genentech Inc | anti-cll-1 and immunoconjugate antibodies |
| BR112017004953A2 (en) | 2014-09-17 | 2017-12-05 | Genentech Inc | immunoconjugate, pharmaceutical formulation, method of treatment and method of inhibiting cell proliferation |
| AU2015320678B2 (en) | 2014-09-23 | 2021-07-22 | Genentech, Inc. | Method of using anti-CD79b immunoconjugates |
| WO2016059602A2 (en) | 2014-10-16 | 2016-04-21 | Glaxo Group Limited | Methods of treating cancer and related compositions |
| WO2016061389A2 (en) | 2014-10-16 | 2016-04-21 | Genentech, Inc. | Anti-alpha-synuclein antibodies and methods of use |
| US10626176B2 (en) | 2014-10-31 | 2020-04-21 | Jounce Therapeutics, Inc. | Methods of treating conditions with antibodies that bind B7-H4 |
| MX2017005750A (en) | 2014-11-03 | 2017-12-15 | Genentech Inc | Assays for detecting t cell immune subsets and methods of use thereof. |
| EP3215637B1 (en) | 2014-11-03 | 2019-07-03 | F. Hoffmann-La Roche AG | Methods and biomarkers for predicting efficacy and valuation of an ox40 agonist treatment |
| KR20170080675A (en) | 2014-11-05 | 2017-07-10 | 제넨테크, 인크. | Anti-fgfr2/3 antibodies and methods using same |
| RU2714116C2 (en) | 2014-11-06 | 2020-02-11 | Ф. Хоффманн-Ля Рош Аг | VARIANTS OF Fc-DOMAIN WITH MODIFIED FcRn BINDING AND METHODS OF APPLICATION THEREOF |
| AR102522A1 (en) | 2014-11-06 | 2017-03-08 | Hoffmann La Roche | FC REGION VARIATIONS WITH MODIFIED PROPERTIES OF UNION TO FCRN AND PROTEIN A |
| WO2016073282A1 (en) | 2014-11-06 | 2016-05-12 | Genentech, Inc. | Combination therapy comprising ox40 binding agonists and tigit inhibitors |
| WO2016077369A1 (en) | 2014-11-10 | 2016-05-19 | Genentech, Inc. | Animal model for nephropathy and agents for treating the same |
| BR112017009728A2 (en) | 2014-11-10 | 2018-02-06 | Genentech, Inc. | isolated antibodies, isolated nucleic acid, vector, host cell, method for producing antibody, composition, uses of an antibody and methods of treating a disorder |
| US10160795B2 (en) | 2014-11-14 | 2018-12-25 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Neutralizing antibodies to Ebola virus glycoprotein and their use |
| DK3489256T3 (en) | 2014-11-14 | 2021-05-25 | Hoffmann La Roche | Antigen-binding molecules comprising a TNF family ligand trimer |
| SG11201703605QA (en) | 2014-11-17 | 2017-06-29 | Genentech Inc | Combination therapy comprising ox40 binding agonists and pd-1 axis binding antagonists |
| US11008403B2 (en) | 2014-11-19 | 2021-05-18 | Genentech, Inc. | Anti-transferrin receptor / anti-BACE1 multispecific antibodies and methods of use |
| US10882920B2 (en) | 2014-11-19 | 2021-01-05 | Genentech, Inc. | Antibodies against BACE1 and use thereof for neural disease immunotherapy |
| EP3221362B1 (en) | 2014-11-19 | 2019-07-24 | F.Hoffmann-La Roche Ag | Anti-transferrin receptor antibodies and methods of use |
| SG11201703237VA (en) | 2014-11-19 | 2017-06-29 | Axon Neuroscience Se | Humanized tau antibodies in alzheimer's disease |
| LT3789402T (en) | 2014-11-20 | 2022-09-26 | F. Hoffmann-La Roche Ag | Combination therapy of t cell activating bispecific antigen binding molecules and pd-1 axis binding antagonists |
| JP6721590B2 (en) | 2014-12-03 | 2020-07-15 | エフ.ホフマン−ラ ロシュ アーゲーF. Hoffmann−La Roche Aktiengesellschaft | Multispecific antibody |
| PT3227336T (en) | 2014-12-05 | 2019-09-09 | Hoffmann La Roche | Anti-cd79b antibodies and methods of use |
| CN107207591A (en) | 2014-12-10 | 2017-09-26 | 豪夫迈·罗氏有限公司 | Blood-brain barrier receptor antibody and application method |
| US9765135B2 (en) | 2014-12-19 | 2017-09-19 | Chugai Seiyaku Kabushiki Kaisha | Anti-C5 antibodies |
| JP6227191B1 (en) | 2014-12-19 | 2017-11-08 | 中外製薬株式会社 | Anti-myostatin antibody, polypeptide comprising mutant Fc region, and method of use |
| WO2016111947A2 (en) | 2015-01-05 | 2016-07-14 | Jounce Therapeutics, Inc. | Antibodies that inhibit tim-3:lilrb2 interactions and uses thereof |
| CA2973964A1 (en) | 2015-01-16 | 2016-07-21 | Juno Therapeutics, Inc. | Antibodies and chimeric antigen receptors specific for ror1 |
| EP3247723A1 (en) | 2015-01-22 | 2017-11-29 | Chugai Seiyaku Kabushiki Kaisha | A combination of two or more anti-c5 antibodies and methods of use |
| CN107407677B (en) | 2015-01-28 | 2020-07-17 | 豪夫迈·罗氏有限公司 | Gene expression markers and treatment of multiple sclerosis |
| EP3253784B1 (en) | 2015-02-04 | 2020-05-06 | Genentech, Inc. | Mutant smoothened and methods of using the same |
| CN114773470A (en) | 2015-02-05 | 2022-07-22 | 中外制药株式会社 | Antibodies comprising an ion concentration-dependent antigen-binding domain, FC region variants, IL-8-binding antibodies and uses thereof |
| WO2016138160A1 (en) | 2015-02-24 | 2016-09-01 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Middle east respiratory syndrome coronavirus immunogens, antibodies, and their use |
| HUE056775T2 (en) | 2015-03-09 | 2022-03-28 | Argenx Bvba | Methods of reducing serum levels of fc-containing agents using fcrn antagonsits |
| MX2017011486A (en) | 2015-03-16 | 2018-06-15 | Genentech Inc | Methods of detecting and quantifying il-13 and uses in diagnosing and treating th2-associated diseases. |
| WO2016146833A1 (en) | 2015-03-19 | 2016-09-22 | F. Hoffmann-La Roche Ag | Biomarkers for nad(+)-diphthamide adp ribosyltransferase resistance |
| DK3271389T3 (en) | 2015-03-20 | 2020-04-27 | Us Health | NEUTRALIZING ANTIBODIES AGAINST GP120 AND USE THEREOF |
| EP3735986A1 (en) | 2015-03-23 | 2020-11-11 | Jounce Therapeutics, Inc. | Antibodies to icos |
| PE20171790A1 (en) | 2015-03-23 | 2017-12-28 | Bayer Pharma AG | ANTI-CEACAM6 ANTIBODIES AND THEIR USES |
| WO2016161390A1 (en) | 2015-04-03 | 2016-10-06 | Eureka Therapeutics, Inc. | Constructs targeting afp peptide/mhc complexes and uses thereof |
| SI3286315T1 (en) | 2015-04-24 | 2021-09-30 | F. Hoffmann-La Roche Ag | Methods of identifying bacteria comprising binding polypeptides |
| HK1252158A1 (en) | 2015-05-01 | 2019-05-17 | Genentech, Inc. | Masked anti-cd3 antibodies and methods of use |
| WO2016179194A1 (en) | 2015-05-04 | 2016-11-10 | Jounce Therapeutics, Inc. | Lilra3 and method of using the same |
| CN115109158A (en) | 2015-05-07 | 2022-09-27 | 阿吉纳斯公司 | anti-OX 40 antibodies and methods of use thereof |
| EP3294771A1 (en) | 2015-05-11 | 2018-03-21 | H. Hoffnabb-La Roche Ag | Compositions and methods of treating lupus nephritis |
| HRP20201900T4 (en) | 2015-05-12 | 2024-06-07 | F. Hoffmann - La Roche Ag | THERAPEUTIC AND DIAGNOSTIC PROCEDURES IN CANCER |
| HK1250723A1 (en) | 2015-05-29 | 2019-01-11 | F. Hoffmann-La Roche Ag | Humanized anti-ebola virus glycoprotein antibodies and methods of use |
| IL294138A (en) | 2015-05-29 | 2022-08-01 | Genentech Inc | Therapeutic and diagnostic methods for cancer |
| HK1255200A1 (en) | 2015-05-29 | 2019-08-09 | F. Hoffmann-La Roche Ag | Pd-l1 promoter methylation in cancer |
| EP3302552A1 (en) | 2015-06-02 | 2018-04-11 | H. Hoffnabb-La Roche Ag | Compositions and methods for using anti-il-34 antibodies to treat neurological diseases |
| WO2016196975A1 (en) | 2015-06-03 | 2016-12-08 | The United States Of America, As Represented By The Secretary Department Of Health & Human Services | Neutralizing antibodies to hiv-1 env and their use |
| CA2988011C (en) | 2015-06-04 | 2021-03-23 | Ospedale San Raffaele Srl | Inhibitor of igfbp3/tmem219 axis and diabetes |
| ES2834823T3 (en) | 2015-06-04 | 2021-06-18 | Ospedale San Raffaele Srl | IGFBP3 and its uses |
| TWI827405B (en) | 2015-06-05 | 2023-12-21 | 美商建南德克公司 | Anti-tau antibodies and methods of use |
| CA2985483A1 (en) | 2015-06-08 | 2016-12-15 | Genentech, Inc. | Methods of treating cancer using anti-ox40 antibodies |
| US20170000885A1 (en) | 2015-06-08 | 2017-01-05 | Genentech, Inc. | Methods of treating cancer using anti-ox40 antibodies and pd-1 axis binding antagonists |
| JP2018524295A (en) | 2015-06-15 | 2018-08-30 | ジェネンテック, インコーポレイテッド | Antibodies and immune complexes |
| EP3310378B1 (en) | 2015-06-16 | 2024-01-24 | F. Hoffmann-La Roche AG | Anti-cll-1 antibodies and methods of use |
| HUE063270T2 (en) | 2015-06-16 | 2024-01-28 | Hoffmann La Roche | Humanized and affinity matured antibodies to fcrh5 and methods of use |
| EP3310811B1 (en) | 2015-06-16 | 2021-06-16 | Genentech, Inc. | Anti-cd3 antibodies and methods of use |
| EP3310385A4 (en) | 2015-06-17 | 2018-12-19 | Allakos Inc. | Methods and compositions for treating fibrotic diseases |
| IL256080B2 (en) | 2015-06-17 | 2025-06-01 | Genentech Inc | Methods for treating locally advanced or metastatic breast cancer using PD-1 axis-binding antagonists and taxanes |
| AU2016280159A1 (en) | 2015-06-17 | 2017-12-07 | Genentech, Inc. | Anti-HER2 antibodies and methods of use |
| DK3313879T3 (en) | 2015-06-24 | 2022-03-14 | Hoffmann La Roche | Anti-transferrin receptor antibodies with adapted affinity |
| WO2016207091A1 (en) | 2015-06-24 | 2016-12-29 | F. Hoffmann-La Roche Ag | Trispecific antibodies specific for her2 and a blood brain barrier receptor and methods of use |
| US20170029520A1 (en) | 2015-06-29 | 2017-02-02 | Genentech, Inc. | Compositions and methods for use in organ transplantation |
| ES2878316T3 (en) | 2015-06-29 | 2021-11-18 | Ventana Med Syst Inc | Materials and Procedures for Histochemical Assays for Human Pro-epiregulin and Ampheregulin |
| RU2611685C2 (en) * | 2015-07-20 | 2017-02-28 | Илья Владимирович Духовлинов | Humanized monoclonal antibody specific to syndecan-1 |
| JP7026613B2 (en) | 2015-08-07 | 2022-02-28 | イマジナブ・インコーポレーテッド | Antigen binding construct for target molecule |
| CN105384825B (en) | 2015-08-11 | 2018-06-01 | 南京传奇生物科技有限公司 | A kind of bispecific chimeric antigen receptor and its application based on single domain antibody |
| EP3341415B1 (en) | 2015-08-28 | 2021-03-24 | H. Hoffnabb-La Roche Ag | Anti-hypusine antibodies and uses thereof |
| MX2018002610A (en) | 2015-09-02 | 2018-09-27 | Immutep Sas | ANTI-LAG-3 ANTIBODIES. |
| EP3353206A1 (en) | 2015-09-22 | 2018-08-01 | Spring Bioscience Corporation | Anti-ox40 antibodies and diagnostic uses thereof |
| CA2992602A1 (en) | 2015-09-23 | 2017-03-30 | Genentech, Inc. | Optimized variants of anti-vegf antibodies |
| NZ739750A (en) | 2015-09-25 | 2019-11-29 | Genentech Inc | Anti-tigit antibodies and methods of use |
| JP7628764B2 (en) | 2015-09-29 | 2025-02-12 | アムジエン・インコーポレーテツド | mod folkpon edge cargogo search carrier Signalsure procedure always Subaturch curl carrieresert match experiencesch suffer with Japan missed crgoc car |
| AR106188A1 (en) | 2015-10-01 | 2017-12-20 | Hoffmann La Roche | ANTI-CD19 HUMANIZED HUMAN ANTIBODIES AND METHODS OF USE |
| AR106189A1 (en) | 2015-10-02 | 2017-12-20 | Hoffmann La Roche | BIESPECTIFIC ANTIBODIES AGAINST HUMAN A-b AND THE HUMAN TRANSFERRINE RECEIVER AND METHODS OF USE |
| EP3356406A1 (en) | 2015-10-02 | 2018-08-08 | H. Hoffnabb-La Roche Ag | Bispecific anti-human cd20/human transferrin receptor antibodies and methods of use |
| MA43345A (en) | 2015-10-02 | 2018-08-08 | Hoffmann La Roche | PYRROLOBENZODIAZEPINE ANTIBODY-DRUG CONJUGATES AND METHODS OF USE |
| EP3356403A2 (en) | 2015-10-02 | 2018-08-08 | H. Hoffnabb-La Roche Ag | Bispecific antibodies specific for a costimulatory tnf receptor |
| EP3150636A1 (en) | 2015-10-02 | 2017-04-05 | F. Hoffmann-La Roche AG | Tetravalent multispecific antibodies |
| MY192202A (en) | 2015-10-02 | 2022-08-06 | Hoffmann La Roche | Bispecific antibodies specific for pd1 and tim3 |
| LT3359572T (en) | 2015-10-06 | 2025-02-10 | F. Hoffmann-La Roche Ag | TREATMENT METHOD FOR MULTIPLE SCLEROSIS |
| MY193013A (en) | 2015-10-07 | 2022-09-22 | Hoffmann La Roche | Bispecific antibodies with tetravalency for a costimulatory tnf receptor |
| EP3359570A1 (en) | 2015-10-07 | 2018-08-15 | The U.S.A. As Represented By The Secretary, Department Of Health And Human Services | Il-7r-alpha specific antibodies for treating acute lymphoblastic leukemia |
| US10604577B2 (en) | 2015-10-22 | 2020-03-31 | Allakos Inc. | Methods and compositions for treating systemic mastocytosis |
| KR20180066236A (en) | 2015-10-22 | 2018-06-18 | 조운스 테라퓨틱스, 인크. | Gene traits for measuring ICOS expression |
| EP3368568B1 (en) | 2015-10-29 | 2022-04-06 | F. Hoffmann-La Roche AG | Anti-variant fc-region antibodies and methods of use |
| JO3555B1 (en) | 2015-10-29 | 2020-07-05 | Merck Sharp & Dohme | An antibody that inactivates the human pneumonia virus |
| EP3184547A1 (en) | 2015-10-29 | 2017-06-28 | F. Hoffmann-La Roche AG | Anti-tpbg antibodies and methods of use |
| WO2017075173A2 (en) | 2015-10-30 | 2017-05-04 | Genentech, Inc. | Anti-factor d antibodies and conjugates |
| EP3368578B1 (en) | 2015-10-30 | 2021-03-17 | H. Hoffnabb-La Roche Ag | Anti-htra1 antibodies and methods of use thereof |
| AU2016349392B2 (en) | 2015-11-03 | 2023-07-13 | The Trustees Of Columbia University In The City Of New York | Neutralizing antibodies to HIV-1 gp41 and their use |
| WO2017079768A1 (en) | 2015-11-08 | 2017-05-11 | Genentech, Inc. | Methods of screening for multispecific antibodies |
| EP3380523A1 (en) | 2015-11-23 | 2018-10-03 | Five Prime Therapeutics, Inc. | Fgfr2 inhibitors alone or in combination with immune stimulating agents in cancer treatment |
| SG10201707267RA (en) | 2015-12-18 | 2017-10-30 | Chugai Pharmaceutical Co Ltd | Anti-myostatin antibodies, polypeptides containing variant fc regions, and methods of use |
| SI3390442T1 (en) | 2015-12-18 | 2024-01-31 | Chugai Seiyaku Kabushiki Kaisha | Anti-c5 antibodies and methods of use |
| EP3400246B1 (en) | 2016-01-08 | 2020-10-21 | H. Hoffnabb-La Roche Ag | Methods of treating cea-positive cancers using pd-1 axis binding antagonists and anti-cea/anti-cd3 bispecific antibodies |
| BR112018014762A2 (en) | 2016-01-20 | 2018-12-26 | Genentech Inc | method of treating (early) alzheimer's disease |
| BR112018015259A2 (en) | 2016-01-27 | 2018-12-18 | Medimmune Llc | Methods for preparing antibodies with a defined glycosylation standard |
| WO2017136558A1 (en) | 2016-02-04 | 2017-08-10 | Curis, Inc. | Mutant smoothened and methods of using the same |
| US10654930B2 (en) | 2016-02-25 | 2020-05-19 | B. G. Negev Technologies And Applications Ltd., At Ben-Gurion University | Composition and method for treating amyotrophic lateral sclerosis |
| KR20180119632A (en) | 2016-02-29 | 2018-11-02 | 제넨테크, 인크. | Treatment and Diagnosis Methods for Cancer |
| WO2017159699A1 (en) | 2016-03-15 | 2017-09-21 | Chugai Seiyaku Kabushiki Kaisha | Methods of treating cancers using pd-1 axis binding antagonists and anti-gpc3 antibodies |
| CN121431692A (en) | 2016-03-25 | 2026-01-30 | 豪夫迈·罗氏有限公司 | Multiplex total antibodies and antibody conjugated drug quantification assays |
| CA3019164A1 (en) | 2016-03-29 | 2017-10-05 | Janssen Biotech, Inc. | Method of treating psoriasis with increased interval dosing of anti-il12/23 antibody |
| KR20180131557A (en) | 2016-04-05 | 2018-12-10 | 글락소스미스클라인 인털렉츄얼 프로퍼티 디벨로프먼트 리미티드 | Inhibition of TGF beta in immunotherapy |
| EP3865511A1 (en) | 2016-04-14 | 2021-08-18 | F. Hoffmann-La Roche AG | Anti-rspo3 antibodies and methods of use |
| CN109154613A (en) | 2016-04-15 | 2019-01-04 | 豪夫迈·罗氏有限公司 | For monitoring and the method for the treatment of cancer |
| EP3443120A2 (en) | 2016-04-15 | 2019-02-20 | H. Hoffnabb-La Roche Ag | Methods for monitoring and treating cancer |
| MX395074B (en) | 2016-04-15 | 2025-03-24 | Bioatla Llc | Anti-AXL antibodies, antibody fragments and their immunoconjugates and their uses |
| WO2017192589A1 (en) | 2016-05-02 | 2017-11-09 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Neutralizing antibodies to influenza ha and their use and identification |
| AU2017259869A1 (en) | 2016-05-02 | 2018-09-27 | F. Hoffmann-La Roche Ag | The contorsbody - a single chain target binder |
| EP3243836A1 (en) | 2016-05-11 | 2017-11-15 | F. Hoffmann-La Roche AG | C-terminally fused tnf family ligand trimer-containing antigen binding molecules |
| CN109071652B (en) | 2016-05-11 | 2022-09-23 | 豪夫迈·罗氏有限公司 | Antigen binding molecules comprising TNF family ligand trimers and tenascin binding modules |
| EP3243832A1 (en) | 2016-05-13 | 2017-11-15 | F. Hoffmann-La Roche AG | Antigen binding molecules comprising a tnf family ligand trimer and pd1 binding moiety |
| PL3455261T3 (en) | 2016-05-13 | 2022-12-12 | Bioatla, Inc. | ANTI-ROR2 ANTIBODY, ANTIBODY FRAGMENTS, THEIR IMMUNOCJUGATES AND THEIR APPLICATIONS |
| JP2019522633A (en) | 2016-05-20 | 2019-08-15 | ジェネンテック, インコーポレイテッド | PROTAC antibody conjugates and methods of use |
| JP7022080B2 (en) | 2016-05-27 | 2022-02-17 | ジェネンテック, インコーポレイテッド | Biochemical analytical methods for the characterization of site-specific antibody-drug conjugates |
| MX2018014375A (en) | 2016-06-17 | 2019-04-22 | Chugai Pharmaceutical Co Ltd | Anti-myostatin antibodies and methods of use. |
| CN109563160B (en) | 2016-06-24 | 2023-02-28 | 豪夫迈·罗氏有限公司 | Anti-polyubiquitin multispecific antibody |
| EP3478717B1 (en) | 2016-07-04 | 2022-01-05 | F. Hoffmann-La Roche AG | Novel antibody format |
| WO2018014260A1 (en) | 2016-07-20 | 2018-01-25 | Nanjing Legend Biotech Co., Ltd. | Multispecific antigen binding proteins and methods of use thereof |
| JP7050677B2 (en) | 2016-07-29 | 2022-04-08 | 中外製薬株式会社 | Bispecific antibody with enhanced FVIII cofactor function substitution activity |
| BR112019001327A2 (en) | 2016-07-29 | 2019-04-30 | Juno Therapeutics Inc | anti-idiotypic antibodies and related methods |
| NL2017270B1 (en) | 2016-08-02 | 2018-02-09 | Aduro Biotech Holdings Europe B V | New anti-hCTLA-4 antibodies |
| IL264626B (en) | 2016-08-05 | 2022-07-01 | Chugai Pharmaceutical Co Ltd | A preparation for the prevention or treatment of diseases related to il-8 |
| WO2018029124A1 (en) | 2016-08-08 | 2018-02-15 | F. Hoffmann-La Roche Ag | Therapeutic and diagnostic methods for cancer |
| CA3035081A1 (en) | 2016-09-02 | 2018-03-08 | Dana-Farber Cancer Institute, Inc. | Composition and methods of treating b cell disorders |
| WO2018049083A1 (en) | 2016-09-07 | 2018-03-15 | The Regents Of The University Of California | Antibodies to oxidation-specific epitopes |
| SG10201607778XA (en) | 2016-09-16 | 2018-04-27 | Chugai Pharmaceutical Co Ltd | Anti-Dengue Virus Antibodies, Polypeptides Containing Variant Fc Regions, And Methods Of Use |
| CN109689682B (en) | 2016-09-19 | 2022-11-29 | 豪夫迈·罗氏有限公司 | Complement factor-based affinity chromatography |
| ES2958593T3 (en) | 2016-09-23 | 2024-02-12 | Hoffmann La Roche | Uses of IL-13 antagonists for the treatment of atopic dermatitis |
| JOP20190055A1 (en) | 2016-09-26 | 2019-03-24 | Merck Sharp & Dohme | Anti-cd27 antibodies |
| SMT202500497T1 (en) | 2016-09-30 | 2026-01-12 | Janssen Biotech Inc | Safe and effective method of treating psoriasis with anti-il23 specific antibody |
| EP3519433A1 (en) | 2016-10-03 | 2019-08-07 | Juno Therapeutics, Inc. | Hpv-specific binding molecules |
| JP7050770B2 (en) | 2016-10-05 | 2022-04-08 | エフ・ホフマン-ラ・ロシュ・アクチェンゲゼルシャフト | Method for preparing antibody drug conjugate |
| JP7579056B2 (en) | 2016-10-06 | 2024-11-07 | ジェネンテック, インコーポレイテッド | Therapeutic and diagnostic methods for cancer |
| WO2018068201A1 (en) | 2016-10-11 | 2018-04-19 | Nanjing Legend Biotech Co., Ltd. | Single-domain antibodies and variants thereof against ctla-4 |
| US20200023072A1 (en) | 2016-10-11 | 2020-01-23 | Medimmune Limited | Antibody-drug conjugates with immune-mediated therapy agents |
| CN110267678A (en) | 2016-10-29 | 2019-09-20 | 霍夫曼-拉罗奇有限公司 | Anti-MIC antibodies and methods of use |
| HUE057559T2 (en) | 2016-11-02 | 2022-06-28 | Jounce Therapeutics Inc | Antibodies against PD-1 and their applications |
| CA3042435A1 (en) | 2016-11-15 | 2018-05-24 | Genentech, Inc. | Dosing for treatment with anti-cd20/anti-cd3 bispecific antibodies |
| KR20190078648A (en) | 2016-11-16 | 2019-07-04 | 얀센 바이오테크 인코포레이티드 | Methods for treating psoriasis with anti-IL23 specific antibodies |
| JOP20190100A1 (en) | 2016-11-19 | 2019-05-01 | Potenza Therapeutics Inc | Anti-gitr antigen-binding proteins and methods of use thereof |
| JP7313684B2 (en) | 2016-11-21 | 2023-07-25 | キュレアブ ゲーエムベーハー | Anti-GP73 antibodies and immunoconjugates |
| WO2018102746A1 (en) | 2016-12-02 | 2018-06-07 | Rigel Pharmaceuticals, Inc. | Antigen binding molecules to tigit |
| EP3551220B1 (en) | 2016-12-07 | 2025-01-29 | Genentech, Inc. | Anti-tau antibodies and methods of use |
| KR102645073B1 (en) | 2016-12-07 | 2024-03-11 | 제넨테크, 인크. | Anti-tau antibodies and methods of using the same |
| MA50948A (en) | 2016-12-07 | 2020-10-14 | Agenus Inc | ANTIBODIES AND METHODS OF USING THE SAME |
| JP2020511408A (en) | 2016-12-12 | 2020-04-16 | ジェネンテック, インコーポレイテッド | Method of treating cancer using anti-PD-L1 antibody and anti-androgen drug |
| CN117752798A (en) | 2016-12-19 | 2024-03-26 | 豪夫迈·罗氏有限公司 | Combination therapy with targeted 4-1BB (CD137) agonists |
| EP3559034B1 (en) | 2016-12-20 | 2020-12-02 | H. Hoffnabb-La Roche Ag | Combination therapy of anti-cd20/anti-cd3 bispecific antibodies and 4-1bb (cd137) agonists |
| JOP20190134A1 (en) | 2016-12-23 | 2019-06-02 | Potenza Therapeutics Inc | Anti-neuropilin antigen-binding proteins and methods of use thereof |
| MA47200A (en) | 2017-01-03 | 2019-11-13 | Hoffmann La Roche | BISPECIFIC ANTIGEN BINDING MOLECULES INCLUDING A 20H4.9 ANTI-4-1BB CLONE |
| TW201825515A (en) | 2017-01-04 | 2018-07-16 | 美商伊繆諾金公司 | MET antibody and immunoconjugate and use thereof |
| US11274157B2 (en) | 2017-01-12 | 2022-03-15 | Eureka Therapeutics, Inc. | Constructs targeting histone H3 peptide/MHC complexes and uses thereof |
| WO2018147960A1 (en) | 2017-02-08 | 2018-08-16 | Imaginab, Inc. | Extension sequences for diabodies |
| US10738131B2 (en) | 2017-02-10 | 2020-08-11 | Genentech, Inc. | Anti-tryptase antibodies, compositions thereof, and uses thereof |
| US11021535B2 (en) | 2017-02-10 | 2021-06-01 | The United States Of America As Represented By The Secretary, Department Of Health And Human Services | Neutralizing antibodies to plasmodium falciparum circumsporozoite protein and their use |
| EP3589754B1 (en) | 2017-03-01 | 2023-06-28 | F. Hoffmann-La Roche AG | Diagnostic and therapeutic methods for cancer |
| MA49279A (en) | 2017-03-22 | 2020-02-05 | Hoffmann La Roche | ANTIBODY COMPOSITIONS OPTIMIZED FOR THE TREATMENT OF EYE DISORDERS |
| EP3601337A1 (en) | 2017-03-28 | 2020-02-05 | Genentech, Inc. | Methods of treating neurodegenerative diseases |
| EP3601345A1 (en) | 2017-03-29 | 2020-02-05 | H. Hoffnabb-La Roche Ag | Bispecific antigen binding molecule for a costimulatory tnf receptor |
| CN110382542B (en) | 2017-03-29 | 2023-06-09 | 豪夫迈·罗氏有限公司 | Bispecific antigen-binding molecules targeting co-stimulatory TNF receptors |
| JOP20190203A1 (en) | 2017-03-30 | 2019-09-03 | Potenza Therapeutics Inc | Anti-tigit antigen-binding proteins and methods of use thereof |
| WO2018184966A1 (en) | 2017-04-03 | 2018-10-11 | F. Hoffmann-La Roche Ag | Antibodies binding to steap-1 |
| EP3606956B1 (en) | 2017-04-04 | 2024-07-31 | F. Hoffmann-La Roche AG | Novel bispecific antigen binding molecules capable of specific binding to cd40 and to fap |
| EP3606955B1 (en) | 2017-04-05 | 2024-11-06 | F. Hoffmann-La Roche AG | Bispecific antibodies specifically binding to pd1 and lag3 |
| EP4112644A1 (en) | 2017-04-05 | 2023-01-04 | F. Hoffmann-La Roche AG | Anti-lag3 antibodies |
| KR20200005540A (en) | 2017-04-14 | 2020-01-15 | 제넨테크, 인크. | How to diagnose and treat cancer |
| US20190078160A1 (en) | 2017-04-21 | 2019-03-14 | Genentech, Inc. | Use of klk5 antagonists for treatment of a disease |
| SG10201913582XA (en) | 2017-04-26 | 2020-02-27 | Eureka Therapeutics Inc | Constructs specifically recognizing glypican 3 and uses thereof |
| AU2018261887A1 (en) | 2017-05-05 | 2019-12-05 | Allakos Inc. | Methods and compositions for treating allergic ocular diseases |
| EP4650004A2 (en) | 2017-05-16 | 2025-11-19 | Five Prime Therapeutics, Inc. | Anti-fgfr2 antibodies in combination with chemotherapy agents in cancer treatment |
| CN110945028B (en) | 2017-07-10 | 2023-09-08 | 国际药物发展生物技术公司 | Treatment of B-cell malignancies with nonfucosylated pro-apoptotic anti-CD19 antibodies in combination with anti-CD20 antibodies or chemotherapeutic agents |
| CA3069469A1 (en) | 2017-07-21 | 2019-01-24 | Genentech, Inc. | Therapeutic and diagnostic methods for cancer |
| KR102670957B1 (en) | 2017-07-26 | 2024-05-31 | 포티 세븐, 인코포레이티드 | Anti-SIRP-alpha antibodies and related methods |
| MA49950A (en) | 2017-08-25 | 2020-07-01 | Five Prime Therapeutics Inc | ANTI-B7-H4 ANTIBODIES AND PROCEDURES FOR USE |
| JP7382922B2 (en) | 2017-09-20 | 2023-11-17 | 中外製薬株式会社 | Dosing regimen for combination therapy using PD-1 system binding antagonists and GPC3 targeting agents |
| TW201922780A (en) | 2017-09-25 | 2019-06-16 | 美商健生生物科技公司 | Safe and effective method for treating lupus with anti-IL12/IL23 antibody |
| EP3692063A1 (en) | 2017-10-03 | 2020-08-12 | Juno Therapeutics, Inc. | Hpv-specific binding molecules |
| US11230601B2 (en) | 2017-10-10 | 2022-01-25 | Tilos Therapeutics, Inc. | Methods of using anti-lap antibodies |
| AU2018347521A1 (en) | 2017-10-12 | 2020-05-07 | Immunowake Inc. | VEGFR-antibody light chain fusion protein |
| CN111511370A (en) | 2017-11-01 | 2020-08-07 | 朱诺治疗学股份有限公司 | Antibodies and chimeric antigen receptors specific for B cell maturation antigens |
| MA50505A (en) | 2017-11-01 | 2020-09-09 | Hoffmann La Roche | 2 + 1 BISPECIFIC ANTIBODIES (CONTORSBODIES) |
| MX2020004573A (en) | 2017-11-01 | 2020-09-25 | Hoffmann La Roche | Combination therapy with targeted ox40 agonists. |
| CN111246884A (en) | 2017-11-01 | 2020-06-05 | 豪夫迈·罗氏有限公司 | Novel Antigen Binding Molecules Containing Trimers of TNF Family Ligands |
| KR20200076731A (en) | 2017-11-06 | 2020-06-29 | 얀센 바이오테크 인코포레이티드 | Safe and effective way to treat psoriatic arthritis with anti-IL23 specific antibodies |
| ES2984919T3 (en) | 2017-11-06 | 2024-10-31 | Hoffmann La Roche | Diagnostic and therapeutic procedures for cancer |
| CN111556895B (en) | 2017-11-14 | 2024-09-13 | 中外制药株式会社 | Anti-C1S antibodies and methods of use |
| EP3717516A1 (en) | 2017-12-01 | 2020-10-07 | Pfizer Inc | Anti-cxcr5 antibodies and compositions and uses thereof |
| MX2020005981A (en) | 2017-12-08 | 2020-08-24 | Argenx Bvba | USE OF CRFN ANTAGONISTS FOR THE TREATMENT OF GENERALIZED MYASTENIA GRAVIS. |
| CN112204048A (en) | 2017-12-15 | 2021-01-08 | 朱诺治疗学股份有限公司 | Anti-CCT5 binding molecules and methods of use |
| CN111527107B (en) | 2017-12-21 | 2024-10-01 | 豪夫迈·罗氏有限公司 | Antibodies that bind to HLA-A2/WT1 |
| EP3502140A1 (en) | 2017-12-21 | 2019-06-26 | F. Hoffmann-La Roche AG | Combination therapy of tumor targeted icos agonists with t-cell bispecific molecules |
| EP4219559A3 (en) | 2017-12-22 | 2023-10-18 | Jounce Therapeutics, Inc. | Antibodies for lilrb2 |
| WO2019126472A1 (en) | 2017-12-22 | 2019-06-27 | Genentech, Inc. | Use of pilra binding agents for treatment of a disease |
| SG11202004158QA (en) | 2017-12-28 | 2020-06-29 | Nanjing Legend Biotech Co Ltd | Single-domain antibodies and variants thereof against tigit |
| AU2018396964C1 (en) | 2017-12-28 | 2024-10-03 | Nanjing Legend Biotech Co., Ltd. | Antibodies and variants thereof against PD-L1 |
| WO2019129677A1 (en) | 2017-12-29 | 2019-07-04 | F. Hoffmann-La Roche Ag | Anti-vegf antibodies and methods of use |
| WO2019136029A1 (en) | 2018-01-02 | 2019-07-11 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Neutralizing antibodies to ebola virus glycoprotein and their use |
| MX2020007077A (en) | 2018-01-04 | 2020-10-28 | Iconic Therapeutics Inc | ANTI-TISSUE FACTOR ANTIBODIES, ANTIBODY-DRUG CONJUGATES AND RELATED METHODS. |
| JP7426724B2 (en) | 2018-01-05 | 2024-02-02 | エイシー イミューン ソシエテ アノニム | Misfolded TDP-43 binding molecule |
| EP3508499A1 (en) | 2018-01-08 | 2019-07-10 | iOmx Therapeutics AG | Antibodies targeting, and other modulators of, an immunoglobulin gene associated with resistance against anti-tumour immune responses, and uses thereof |
| CN111699200B (en) | 2018-01-15 | 2023-05-26 | 南京传奇生物科技有限公司 | Single domain antibodies against PD-1 and variants thereof |
| EP3740505A1 (en) | 2018-01-16 | 2020-11-25 | Lakepharma Inc. | Bispecific antibody that binds cd3 and another target |
| LT3743088T (en) | 2018-01-26 | 2022-12-27 | F. Hoffmann-La Roche Ag | Il-22 fc compositions and methods of use |
| AU2019212709A1 (en) | 2018-01-26 | 2020-08-13 | Genentech, Inc. | IL-22 Fc fusion proteins and methods of use |
| TWI860665B (en) | 2018-02-01 | 2024-11-01 | 大陸商信達生物製藥(蘇州)有限公司 | Full-human anti-b cell mature antigen (bcma) single chain antibody and use thereof |
| CR20200391A (en) | 2018-02-08 | 2020-10-19 | Genentech Inc | Bispecific antigen-binding molecules and methods of use |
| JP7418337B2 (en) | 2018-02-09 | 2024-01-19 | ジェネンテック, インコーポレイテッド | Treatment and diagnosis of mast cell-mediated inflammatory diseases |
| TWI829667B (en) | 2018-02-09 | 2024-01-21 | 瑞士商赫孚孟拉羅股份公司 | Antibodies binding to gprc5d |
| CA3091646A1 (en) | 2018-02-14 | 2019-08-22 | Abba Therapeutics Ag | Anti-human pd-l2 antibodies |
| SG11202007820QA (en) | 2018-02-21 | 2020-09-29 | Five Prime Therapeutics Inc | B7-h4 antibody dosing regimens |
| SG11202007821WA (en) | 2018-02-21 | 2020-09-29 | Five Prime Therapeutics Inc | B7-h4 antibody formulations |
| EP3755364A1 (en) | 2018-02-21 | 2020-12-30 | F. Hoffmann-La Roche AG | Dosing for treatment with il-22 fc fusion proteins |
| RU2020130795A (en) | 2018-02-21 | 2022-03-21 | Дзе Юнайтед Стэйтс Оф Америка, Эс Репрезентед Бай Дзе Секретэри, Департмент Оф Хелт Энд Хьюман Сервисиз | NEUTRALIZING ANTIBODIES TO ENV HIV-1 AND THEIR USE |
| KR20200135313A (en) | 2018-02-26 | 2020-12-02 | 제넨테크, 인크. | Dosing for treatment with anti-TIGIT and anti-PD-L1 antagonist antibodies |
| JP2021516051A (en) | 2018-03-02 | 2021-07-01 | ファイブ プライム セラピューティクス, インコーポレイテッド | B7-H4 antibody and how to use it |
| EP3762015B1 (en) | 2018-03-05 | 2025-12-24 | Janssen Biotech, Inc. | Guselkumab for use in the treatment of crohn's disease with a dosage regimen |
| TWI841551B (en) | 2018-03-13 | 2024-05-11 | 瑞士商赫孚孟拉羅股份公司 | Combination therapy with targeted 4-1bb (cd137) agonists |
| AU2019236372B2 (en) | 2018-03-13 | 2024-06-20 | F. Hoffmann-La Roche Ag | Therapeutic combination of 4-1 BB agonists with anti-CD20 antibodies |
| US20200040103A1 (en) | 2018-03-14 | 2020-02-06 | Genentech, Inc. | Anti-klk5 antibodies and methods of use |
| SG11202006217YA (en) | 2018-03-14 | 2020-07-29 | Beijing Xuanyi Pharmasciences Co Ltd | Anti-claudin 18.2 antibodies |
| CN112119090B (en) | 2018-03-15 | 2023-01-13 | 中外制药株式会社 | Anti-dengue virus antibodies cross-reactive to Zika virus and methods of use |
| CA3092589A1 (en) | 2018-03-21 | 2019-09-26 | Five Prime Therapeutics, Inc. | Antibodies binding to vista at acidic ph |
| EP3775184B1 (en) | 2018-03-29 | 2025-12-10 | F. Hoffmann-La Roche AG | Modulating lactogenic activity in mammalian cells |
| EP3774917B1 (en) | 2018-03-30 | 2025-10-29 | Nanjing Legend Biotech Co., Ltd. | Single-domain antibodies against lag-3 and uses thereof |
| TW202011029A (en) | 2018-04-04 | 2020-03-16 | 美商建南德克公司 | Methods for detecting and quantifying FGF21 |
| MX2020010460A (en) | 2018-04-05 | 2021-01-29 | Juno Therapeutics Inc | T-CELL RECEPTORS, AND ENGINEERED CELLS EXPRESSING THEM. |
| SG11202007961QA (en) | 2018-04-13 | 2020-09-29 | Hoffmann La Roche | Her2-targeting antigen binding molecules comprising 4-1bbl |
| US20190345245A1 (en) | 2018-05-11 | 2019-11-14 | Janssen Biotech, Inc. | Methods of Treating Crohn's Disease with Anti-IL23 Specific Antibody |
| WO2019222294A1 (en) | 2018-05-14 | 2019-11-21 | Werewolf Therapeutics, Inc. | Activatable cytokine polypeptides and methods of use thereof |
| CN113840832A (en) | 2018-05-14 | 2021-12-24 | 狼人治疗公司 | Activatable interleukin-2 polypeptides and methods of using the same |
| WO2019224275A1 (en) | 2018-05-23 | 2019-11-28 | Adc Therapeutics Sa | Molecular adjuvant |
| WO2019227490A1 (en) | 2018-06-01 | 2019-12-05 | Tayu Huaxia Biotech Medical Group Co., Ltd. | Compositions and methods for imaging |
| CN117442717A (en) | 2018-06-01 | 2024-01-26 | 大有华夏生物医药集团有限公司 | Compositions for treating diseases or conditions and uses thereof |
| WO2019235426A1 (en) | 2018-06-04 | 2019-12-12 | 中外製薬株式会社 | Antigen-binding molecule showing changed half-life in cytoplasm |
| TWI851577B (en) | 2018-06-07 | 2024-08-11 | 美商思進公司 | Camptothecin conjugates |
| CN112513073A (en) | 2018-06-08 | 2021-03-16 | 阿根思公司 | Compositions and methods for treating immune thrombocytopenia |
| CN112469440B (en) | 2018-06-18 | 2024-09-06 | 优瑞科生物技术公司 | Constructs targeting prostate-specific membrane antigen (PSMA) and uses thereof |
| SG11202012446UA (en) | 2018-06-23 | 2021-01-28 | Genentech Inc | Methods of treating lung cancer with a pd-1 axis binding antagonist, a platinum agent, and a topoisomerase ii inhibitor |
| WO2020007817A1 (en) | 2018-07-04 | 2020-01-09 | F. Hoffmann-La Roche Ag | Novel bispecific agonistic 4-1bb antigen binding molecules |
| SG11202100096XA (en) | 2018-07-09 | 2021-02-25 | Five Prime Therapeutics Inc | Antibodies binding to ilt4 |
| WO2020014306A1 (en) | 2018-07-10 | 2020-01-16 | Immunogen, Inc. | Met antibodies and immunoconjugates and uses thereof |
| MX2021000213A (en) | 2018-07-11 | 2021-03-25 | Five Prime Therapeutics Inc | Antibodies binding to vista at acidic ph. |
| EP3823611A1 (en) | 2018-07-18 | 2021-05-26 | Genentech, Inc. | Methods of treating lung cancer with a pd-1 axis binding antagonist, an antimetabolite, and a platinum agent |
| US20200025776A1 (en) | 2018-07-18 | 2020-01-23 | Janssen Biotech, Inc. | Sustained Response Predictors After Treatment With Anti-IL23 Specific Antibody |
| US11214619B2 (en) | 2018-07-20 | 2022-01-04 | Surface Oncology, Inc. | Anti-CD112R compositions and methods |
| MX2021000827A (en) | 2018-08-03 | 2021-03-25 | Chugai Pharmaceutical Co Ltd | ANTIGEN-BINDING MOLECULE CONTAINING TWO ANTIGEN-BINDING DOMAINS THAT ARE LINKED TO EACH OTHER. |
| MA50586A (en) | 2018-08-09 | 2020-09-16 | Regeneron Pharma | METHODS FOR EVALUATING THE BINDING AFFINITY OF AN ANTIBODY VARIANT TO THE NEONATAL FC RECEPTOR |
| EP3835321A4 (en) | 2018-08-10 | 2022-11-02 | Chugai Seiyaku Kabushiki Kaisha | ANTI-CD137 ANTIGEN BINDING MOLECULE AND USE THEREOF |
| CN112584863B (en) | 2018-08-17 | 2025-10-28 | Ab工作室有限公司 | Catalytic antibodies and methods of use thereof |
| AU2019324170A1 (en) | 2018-08-23 | 2021-02-18 | Seagen, Inc. | Anti-TIGIT antibodies |
| GB201814281D0 (en) | 2018-09-03 | 2018-10-17 | Femtogenix Ltd | Cytotoxic agents |
| TWI841595B (en) | 2018-09-10 | 2024-05-11 | 大陸商南京傳奇生物科技有限公司 | Single-domain antibodies against cd33 and constructs thereof |
| CA3111401A1 (en) | 2018-09-19 | 2020-03-26 | Genentech, Inc. | Therapeutic and diagnostic methods for bladder cancer |
| AU2019342133B8 (en) | 2018-09-21 | 2025-08-07 | Genentech, Inc. | Diagnostic methods for triple-negative breast cancer |
| CA3113059A1 (en) | 2018-09-21 | 2020-03-26 | The University Of North Carolina At Chapel Hill | Synthetic binding agents for limiting permeation through mucus |
| HRP20230886T1 (en) | 2018-09-24 | 2023-11-24 | Janssen Biotech, Inc. | Safe and effective method of treating ulcerative colitis with anti-il12/il23 antibody |
| EP3856764A4 (en) | 2018-09-27 | 2022-11-02 | Xilio Development, Inc. | MASKED CYTOKINE POLYPEPTIDES |
| CN112654641A (en) | 2018-10-01 | 2021-04-13 | 豪夫迈·罗氏有限公司 | Bispecific antigen binding molecules with trivalent binding to CD40 |
| MX2021003548A (en) | 2018-10-01 | 2021-05-27 | Hoffmann La Roche | Bispecific antigen binding molecules comprising anti-fap clone 212. |
| EP3632929A1 (en) | 2018-10-02 | 2020-04-08 | Ospedale San Raffaele S.r.l. | Antibodies and uses thereof |
| AU2019355995A1 (en) | 2018-10-05 | 2021-04-08 | Five Prime Therapeutics, Inc. | Anti-FGFR2 antibody formulations |
| TW202035445A (en) | 2018-10-10 | 2020-10-01 | 美商帝洛斯療法股份有限公司 | Anti-lap antibody variants and uses thereof |
| MA53911A (en) | 2018-10-15 | 2022-01-19 | Five Prime Therapeutics Inc | POLYTHERAPY AGAINST CANCER |
| WO2020081493A1 (en) | 2018-10-16 | 2020-04-23 | Molecular Templates, Inc. | Pd-l1 binding proteins |
| AU2019361983A1 (en) | 2018-10-18 | 2021-05-20 | Genentech, Inc. | Diagnostic and therapeutic methods for sarcomatoid kidney cancer |
| MX2021004774A (en) | 2018-10-29 | 2021-08-24 | Hoffmann La Roche | Antibody formulation. |
| EP3873944A1 (en) | 2018-10-31 | 2021-09-08 | Bayer Aktiengesellschaft | Reversal agents for neutralizing the therapeutic activity of anti-fxia antibodies |
| KR20210101235A (en) | 2018-11-16 | 2021-08-18 | 메모리얼 슬로안 케터링 캔서 센터 | Antibodies to mucin-16 and methods of use thereof |
| EP3883607A4 (en) | 2018-11-20 | 2022-08-17 | Janssen Biotech, Inc. | SAFE AND EFFECTIVE PROCESS FOR TREATING PSORIASIS WITH A SPECIFIC ANTI-IL-23 ANTIBODY |
| SG11202105403XA (en) | 2018-11-27 | 2021-06-29 | Staidson Beijing Biopharmaceuticals Co Ltd | Antibodies specifically recognizing granulocyte-macrophage colony stimulating factor receptor alpha and uses thereof |
| KR20210100656A (en) | 2018-12-05 | 2021-08-17 | 제넨테크, 인크. | Diagnostic methods and compositions for cancer immunotherapy |
| US12234289B2 (en) | 2018-12-07 | 2025-02-25 | Ono Pharmaceutical Co., Ltd. | Immunosuppresive agent |
| JP2022513198A (en) | 2018-12-10 | 2022-02-07 | ジェネンテック, インコーポレイテッド | Photocrosslinkable peptide for site-specific conjugation to Fc-containing proteins |
| MA54562A (en) | 2018-12-18 | 2021-10-27 | Janssen Biotech Inc | SAFE AND EFFECTIVE METHOD OF TREATING LUPUS WITH AN ANTI-IL12/IL23 ANTIBODY |
| EP3883609A2 (en) | 2018-12-20 | 2021-09-29 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Ebola virus glycoprotein-specific monoclonal antibodies and uses thereof |
| AR117453A1 (en) | 2018-12-20 | 2021-08-04 | Genentech Inc | CF OF MODIFIED ANTIBODIES AND METHODS TO USE THEM |
| CN113621062B (en) | 2018-12-21 | 2024-07-02 | 豪夫迈·罗氏有限公司 | Antibodies that bind to CD3 |
| KR20240042566A (en) | 2018-12-21 | 2024-04-02 | 에프. 호프만-라 로슈 아게 | Antibody that binds to vegf and il-1beta and methods of use |
| EP3898683A1 (en) | 2018-12-21 | 2021-10-27 | F. Hoffmann-La Roche AG | Tumor-targeted superagonistic cd28 antigen binding molecules |
| US11608376B2 (en) | 2018-12-21 | 2023-03-21 | Hoffmann-La Roche Inc. | Tumor-targeted agonistic CD28 antigen binding molecules |
| BR112021012631A2 (en) | 2018-12-26 | 2021-12-14 | Xilio Dev Inc | Anti-ctla4 antibodies and methods of using them |
| EP3902830A1 (en) | 2018-12-30 | 2021-11-03 | F. Hoffmann-La Roche AG | Anti-rabbit cd19 antibodies and methods of use |
| CN113710702A (en) | 2019-01-14 | 2021-11-26 | 健泰科生物技术公司 | Methods of treating cancer with PD-1 axis binding antagonists and RNA vaccines |
| CA3126778A1 (en) | 2019-01-17 | 2020-07-23 | Bayer Aktiengesellschaft | Methods to determine whether a subject is suitable of being treated with an agonist of soluble guanylyl cyclase (sgc) |
| CN113795511B (en) | 2019-01-23 | 2024-07-23 | 大有华夏生物医药集团有限公司 | Anti-PD-L1 double antibody and its use |
| US20220089770A1 (en) | 2019-01-24 | 2022-03-24 | Chugai Seiyaku Kabushiki Kaisha | Novel cancer antigens and antibodies of said antigens |
| GB201901197D0 (en) | 2019-01-29 | 2019-03-20 | Femtogenix Ltd | G-A Crosslinking cytotoxic agents |
| MX2021009087A (en) | 2019-01-29 | 2021-09-08 | Juno Therapeutics Inc | Antibodies and chimeric antigen receptors specific for receptor tyrosine kinase like orphan receptor 1 (ror1). |
| CA3130695A1 (en) | 2019-02-27 | 2020-09-03 | Genentech, Inc. | Dosing for treatment with anti-tigit and anti-cd20 or anti-cd38 antibodies |
| KR20250143873A (en) | 2019-03-08 | 2025-10-02 | 제넨테크, 인크. | Methods for detecting and quantifying membrane-associated proteins on extracellular vesicles |
| EP3938403A1 (en) | 2019-03-14 | 2022-01-19 | F. Hoffmann-La Roche AG | Treatment of cancer with her2xcd3 bispecific antibodies in combination with anti-her2 mab |
| CA3133395A1 (en) | 2019-03-14 | 2020-09-17 | Janssen Biotech, Inc. | Manufacturing methods for producing anti-il12/il23 antibody compositions |
| CA3134079A1 (en) | 2019-03-18 | 2020-09-24 | Janssen Biotech, Inc. | Method of treating psoriasis in pediatric subjects with anti-il12/il23 antibody |
| GB2589049C (en) | 2019-04-11 | 2024-02-21 | argenx BV | Anti-IgE antibodies |
| CN113677403B (en) | 2019-04-12 | 2024-12-27 | 豪夫迈·罗氏有限公司 | Bispecific antigen-binding molecules comprising lipocalin muteins |
| CA3137397A1 (en) | 2019-04-19 | 2020-10-22 | Chugai Seiyaku Kabushiki Kaisha | Chimeric receptor that recognizes engineered site in antibody |
| MX2021012692A (en) | 2019-04-19 | 2021-11-12 | Genentech Inc | Anti-mertk antibodies and their methods of use. |
| US12269872B2 (en) | 2019-05-03 | 2025-04-08 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Neutralizing antibodies to Plasmodium falciparum circumsporozoite protein and their use |
| MX2021013222A (en) | 2019-05-03 | 2022-01-06 | Genentech Inc | Methods of treating cancer with an anti-pd-l1 antibody. |
| SG11202112541RA (en) | 2019-05-14 | 2021-12-30 | Werewolf Therapeutics Inc | Separation moieties and methods and use thereof |
| TW202108178A (en) | 2019-05-14 | 2021-03-01 | 美商建南德克公司 | METHODS OF USING ANTI-CD79b IMMUNOCONJUGATES TO TREAT FOLLICULAR LYMPHOMA |
| US20230085439A1 (en) | 2019-05-21 | 2023-03-16 | University Of Georgia Research Foundation, Inc. | Antibodies that bind human metapneumovirus fusion protein and their use |
| JP7805788B2 (en) | 2019-05-23 | 2026-01-26 | ヤンセン バイオテツク,インコーポレーテツド | Method for treating inflammatory bowel disease with combined therapy of antibodies against IL-23 and TNF alpha |
| KR20220012270A (en) | 2019-05-23 | 2022-02-03 | 에이씨 이뮨 에스.에이. | Anti-TDP-43 binding molecules and uses thereof |
| MX2021014953A (en) | 2019-06-04 | 2022-01-24 | Janssen Biotech Inc | Safe and effective method of treating psoriatic arthritis with anti-il23 specific antibody. |
| JP7565951B2 (en) | 2019-06-07 | 2024-10-11 | アルジェニクス ビーブイ | Pharmaceutical formulations of FcRn inhibitors suitable for subcutaneous administration |
| KR20220025848A (en) | 2019-06-26 | 2022-03-03 | 에프. 호프만-라 로슈 아게 | Fusion of antibodies that bind CEA and 4-1BBL |
| CN114531878A (en) | 2019-06-27 | 2022-05-24 | 豪夫迈·罗氏有限公司 | Novel ICOS antibodies and tumor-targeted antigen-binding molecules comprising same |
| EP3994173A1 (en) | 2019-07-02 | 2022-05-11 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Monoclonal antibodies that bind egfrviii and their use |
| AU2020311579A1 (en) | 2019-07-05 | 2022-02-03 | Iomx Therapeutics Ag | Antibodies binding IgC2 of IGSF11 (VSIG3) and uses thereof |
| AU2020311511B2 (en) | 2019-07-09 | 2024-08-29 | Beijing Solobio Genetechnology Co., Ltd. | Antibodies specifically recognizing Pseudomonas PcrV and uses thereof |
| WO2021010326A1 (en) | 2019-07-12 | 2021-01-21 | 中外製薬株式会社 | Anti-mutation type fgfr3 antibody and use therefor |
| AR119382A1 (en) | 2019-07-12 | 2021-12-15 | Hoffmann La Roche | PRE-TARGETING ANTIBODIES AND METHODS OF USE |
| AR119393A1 (en) | 2019-07-15 | 2021-12-15 | Hoffmann La Roche | ANTIBODIES THAT BIND NKG2D |
| KR20220038415A (en) | 2019-07-26 | 2022-03-28 | 벤더르빌트 유니버시티 | Human monoclonal antibody to enterovirus D68 |
| JP2022543553A (en) | 2019-07-31 | 2022-10-13 | エフ・ホフマン-ラ・ロシュ・アクチェンゲゼルシャフト | Antibody that binds to GPRC5D |
| EP4004045A1 (en) | 2019-07-31 | 2022-06-01 | F. Hoffmann-La Roche AG | Antibodies binding to gprc5d |
| TWI780464B (en) | 2019-08-06 | 2022-10-11 | 香港商新旭生技股份有限公司 | Antibodies that bind to pathological tau species and uses thereof |
| WO2021050645A1 (en) | 2019-09-12 | 2021-03-18 | Genentech, Inc. | Compositions and methods of treating lupus nephritis |
| TW202126698A (en) | 2019-09-18 | 2021-07-16 | 美商建南德克公司 | Anti-klk7 antibodies, anti-klk5 antibodies, multispecific anti-klk5/klk7 antibodies, and methods of use |
| BR112022004831A2 (en) | 2019-09-19 | 2022-06-07 | Bristol Myers Squibb Co | Antibodies that bind to seen at acidic pH |
| JP2022549218A (en) | 2019-09-20 | 2022-11-24 | ジェネンテック, インコーポレイテッド | Anti-tryptase antibody medication |
| CR20220127A (en) | 2019-09-27 | 2022-05-27 | Genentech Inc | DOSE ADMINISTRATION FOR TREATMENT WITH ANTI-TIGIT AND ANTI-PD-L1 ANTAGONIST ANTIBODIES |
| CN118557750A (en) | 2019-10-18 | 2024-08-30 | 基因泰克公司 | Methods of treating diffuse large B-cell lymphoma using anti-CD79b immunoconjugates |
| IL292458A (en) | 2019-11-06 | 2022-06-01 | Genentech Inc | Diagnostic and therapeutic methods for treatment of hematologic cancers |
| EP4058593A4 (en) | 2019-11-12 | 2023-11-15 | Foundation Medicine, Inc. | METHOD FOR DETECTING A FUSION GENE CODING A NEOANTIGEN |
| JP2023503258A (en) | 2019-11-14 | 2023-01-27 | ウェアウルフ セラピューティクス, インコーポレイテッド | Activatable cytokine polypeptides and methods of use thereof |
| BR112022008629A2 (en) | 2019-11-15 | 2022-07-19 | Enthera S R L | ISOLATED ANTIBODY OR ANTIGEN BINDING FRAGMENT THEREOF, ISOLATED POLYNUCLEOTIDE, VECTOR, ISOLATED CELL, PHARMACEUTICAL COMPOSITION, USE THEREOF AND METHOD FOR INHIBITING IGFBP3 BINDING TO THE TMEM219 RECEPTOR |
| EP3822288A1 (en) | 2019-11-18 | 2021-05-19 | Deutsches Krebsforschungszentrum, Stiftung des öffentlichen Rechts | Antibodies targeting, and other modulators of, the cd276 antigen, and uses thereof |
| EP4061839A1 (en) | 2019-11-21 | 2022-09-28 | Enthera S.R.L. | Igfbp3 antibodies and therapeutic uses thereof |
| JP2023504740A (en) | 2019-12-06 | 2023-02-06 | ジュノー セラピューティクス インコーポレイテッド | Anti-idiotypic antibodies against BCMA target binding domains and related compositions and methods |
| US20230192869A1 (en) | 2019-12-06 | 2023-06-22 | Juno Therapeutics, Inc. | Anti-idiotypic antibodies to gprc5d-targeted binding domains and related compositions and methods |
| EP4072584B1 (en) | 2019-12-13 | 2026-01-28 | Genentech, Inc. | Anti-ly6g6d antibodies and methods of use |
| CR20220256A (en) | 2019-12-18 | 2022-08-31 | Hoffmann La Roche | ANTIBODIES THAT BIND HLA-A2/MAGE-A4 |
| CN113045655A (en) | 2019-12-27 | 2021-06-29 | 高诚生物医药(香港)有限公司 | anti-OX 40 antibodies and uses thereof |
| MY202559A (en) | 2019-12-27 | 2024-05-08 | Chugai Pharmaceutical Co Ltd | Anti-ctla-4 antibody and use thereof |
| US12403175B2 (en) | 2020-01-08 | 2025-09-02 | argenx BV | Methods for treating pemphigus disorders |
| CN114929734A (en) | 2020-01-09 | 2022-08-19 | 豪夫迈·罗氏有限公司 | Novel antigen binding molecules comprising 4-1BBL trimers |
| CN110818795B (en) | 2020-01-10 | 2020-04-24 | 上海复宏汉霖生物技术股份有限公司 | anti-TIGIT antibodies and methods of use |
| WO2021194481A1 (en) | 2020-03-24 | 2021-09-30 | Genentech, Inc. | Dosing for treatment with anti-tigit and anti-pd-l1 antagonist antibodies |
| WO2022050954A1 (en) | 2020-09-04 | 2022-03-10 | Genentech, Inc. | Dosing for treatment with anti-tigit and anti-pd-l1 antagonist antibodies |
| AU2021214795A1 (en) | 2020-01-31 | 2022-08-18 | The Cleveland Clinic Foundation | Anti-Müllerian Hormone Receptor 2 antibodies and methods of use |
| CN115397459A (en) | 2020-01-31 | 2022-11-25 | 基因泰克公司 | Method for inducing new epitope-specific T cells using PD-1 axis binding antagonists and RNA vaccines |
| JP2023519105A (en) | 2020-02-11 | 2023-05-10 | ヴァンダービルト ユニバーシティ | Human monoclonal antibody against severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) |
| TWI895351B (en) | 2020-02-12 | 2025-09-01 | 日商中外製藥股份有限公司 | Anti-CD137 antigen binding molecules for the treatment of cancer |
| PH12022552122A1 (en) | 2020-02-14 | 2024-01-29 | Jounce Therapeutics Inc | Antibodies and fusion proteins that bind to ccr8 and uses thereof |
| EP4093762A1 (en) | 2020-02-20 | 2022-11-30 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Epstein-barr virus monoclonal antibodies and uses thereof |
| EP3868396A1 (en) | 2020-02-20 | 2021-08-25 | Enthera S.R.L. | Inhibitors and uses thereof |
| CN115151573A (en) | 2020-02-28 | 2022-10-04 | 上海复宏汉霖生物技术股份有限公司 | anti-CD 137 constructs, multispecific antibodies, and uses thereof |
| WO2021170067A1 (en) | 2020-02-28 | 2021-09-02 | 上海复宏汉霖生物技术股份有限公司 | Anti-cd137 construct and use thereof |
| JP2023516080A (en) | 2020-03-06 | 2023-04-17 | ジーオー セラピューティクス,インコーポレイテッド | Anti-glyco CD44 antibodies and uses thereof |
| PE20230252A1 (en) | 2020-03-13 | 2023-02-07 | Genentech Inc | ANTI-INTERLEUKIN-33 ANTIBODIES AND ITS USES FOR THEM |
| KR20250074688A (en) | 2020-03-19 | 2025-05-27 | 제넨테크, 인크. | Isoform-selective anti-tgf-beta antibodies and methods of use |
| EP4107186A1 (en) | 2020-03-23 | 2022-12-28 | Genentech, Inc. | Tocilizumab and remdesivir combination therapy for covid-19 pneumonia |
| JP2023518815A (en) | 2020-03-23 | 2023-05-08 | ジェネンテック, インコーポレイテッド | Methods of treating pneumonia, including COVID-19 pneumonia, with an IL6 antagonist |
| CN115867577A (en) | 2020-03-23 | 2023-03-28 | 基因泰克公司 | Biomarkers for predicting response to IL-6 antagonists in COVID-19 pneumonia |
| MX2022011752A (en) | 2020-03-24 | 2022-10-18 | Genentech Inc | Tie2-binding agents and methods of use. |
| JP2023518849A (en) | 2020-03-26 | 2023-05-08 | バンダービルト・ユニバーシティ | Human monoclonal antibody against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) |
| WO2021195385A1 (en) | 2020-03-26 | 2021-09-30 | Vanderbilt University | HUMAN MONOCLONAL ANTIBODIES TO SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-GoV-2) |
| US12098365B2 (en) | 2020-03-26 | 2024-09-24 | Genentech, Inc. | Modified mammalian cells |
| AR121706A1 (en) | 2020-04-01 | 2022-06-29 | Hoffmann La Roche | OX40 AND FAP-TARGETED BSPECIFIC ANTIGEN-BINDING MOLECULES |
| JP2023520515A (en) | 2020-04-03 | 2023-05-17 | ジェネンテック, インコーポレイテッド | Therapeutic and diagnostic methods for cancer |
| WO2021207449A1 (en) | 2020-04-09 | 2021-10-14 | Merck Sharp & Dohme Corp. | Affinity matured anti-lap antibodies and uses thereof |
| WO2021207662A1 (en) | 2020-04-10 | 2021-10-14 | Genentech, Inc. | Use of il-22fc for the treatment or prevention of pneumonia, acute respiratory distress syndrome, or cytokine release syndrome |
| AU2021259861A1 (en) | 2020-04-24 | 2022-11-17 | Genentech, Inc. | Methods of using anti-CD79b immunoconjugates |
| CA3172880A1 (en) | 2020-04-27 | 2021-11-04 | Sotirios Tsimikas | Isoform-independent antibodies to lipoprotein(a) |
| JP2023523450A (en) | 2020-04-28 | 2023-06-05 | ジェネンテック, インコーポレイテッド | Methods and compositions for non-small cell lung cancer immunotherapy |
| WO2021225892A1 (en) | 2020-05-03 | 2021-11-11 | Levena (Suzhou) Biopharma Co., Ltd. | Antibody-drug conjugates (adcs) comprising an anti-trop-2 antibody, compositions comprising such adcs, as well as methods of making and using the same |
| US20210347880A1 (en) | 2020-05-05 | 2021-11-11 | Janssen Biotech, Inc. | Methods of Treating Crohn's Disease with Anti-IL23 Specific Antibody |
| CA3182150A1 (en) | 2020-05-17 | 2021-11-25 | Astrazeneca Uk Limited | Sars-cov-2 antibodies and methods of selecting and using the same |
| MX2022014430A (en) | 2020-05-21 | 2023-02-22 | Janssen Biotech Inc | Method of treating inflammatory bowel disease with a combination therapy of antibodies to il-23 and tnf alpha. |
| CN113993900B (en) | 2020-05-27 | 2023-08-04 | 舒泰神(北京)生物制药股份有限公司 | Antibodies specifically recognizing nerve growth factor and uses thereof |
| CN116529260A (en) | 2020-06-02 | 2023-08-01 | 当康生物技术有限责任公司 | anti-CD 93 constructs and uses thereof |
| JP2023529842A (en) | 2020-06-02 | 2023-07-12 | ダイナミキュア バイオテクノロジー エルエルシー | Anti-CD93 constructs and uses thereof |
| MX2022015206A (en) | 2020-06-08 | 2023-01-05 | Hoffmann La Roche | ANTI-HBV ANTIBODIES AND METHODS OF USE. |
| WO2021252977A1 (en) | 2020-06-12 | 2021-12-16 | Genentech, Inc. | Methods and compositions for cancer immunotherapy |
| KR20230025691A (en) | 2020-06-16 | 2023-02-22 | 제넨테크, 인크. | Methods and compositions for treating triple negative breast cancer |
| AU2021293507A1 (en) | 2020-06-18 | 2023-02-02 | F. Hoffmann-La Roche Ag | Treatment with anti-TIGIT antibodies and PD-1 axis binding antagonists |
| CA3185513A1 (en) | 2020-06-19 | 2021-12-23 | F. Hoffmann-La Roche Ag | Antibodies binding to cd3 and folr1 |
| PH12022500027A1 (en) | 2020-06-19 | 2024-03-25 | Hoffmann La Roche | Antibodies binding to cd3 |
| IL298402A (en) | 2020-06-19 | 2023-01-01 | Hoffmann La Roche | Antibodies binding to cd3 and cd19 |
| WO2021255146A1 (en) | 2020-06-19 | 2021-12-23 | F. Hoffmann-La Roche Ag | Antibodies binding to cd3 and cea |
| JP7678005B2 (en) | 2020-06-23 | 2025-05-15 | エフ・ホフマン-ラ・ロシュ・アクチェンゲゼルシャフト | Agonistic CD28 antigen-binding molecules targeting Her2 |
| MX2022016453A (en) | 2020-06-24 | 2023-02-01 | Genentech Inc | Apoptosis resistant cell lines. |
| WO2021260064A1 (en) | 2020-06-25 | 2021-12-30 | F. Hoffmann-La Roche Ag | Anti-cd3/anti-cd28 bispecific antigen binding molecules |
| EP4175668A1 (en) | 2020-07-06 | 2023-05-10 | iOmx Therapeutics AG | Antibodies binding igv of igsf11 (vsig3) and uses thereof |
| KR20230037578A (en) | 2020-07-10 | 2023-03-16 | 에프. 호프만-라 로슈 아게 | Antibodies that bind to cancer cells and target radionuclides to those cells |
| US20220025035A1 (en) | 2020-07-13 | 2022-01-27 | Janssen Biotech, Inc. | Safe and Effective Method of Treating Psoriatic Arthritis with Anti-IL23 Specific Antibody |
| US20230303682A1 (en) | 2020-07-17 | 2023-09-28 | Genentech, Inc. | Anti-Notch2 Antibodies and Methods of Use |
| GB2597532A (en) | 2020-07-28 | 2022-02-02 | Femtogenix Ltd | Cytotoxic compounds |
| EP4188550A1 (en) | 2020-07-29 | 2023-06-07 | Dynamicure Biotechnology LLC | Anti-cd93 constructs and uses thereof |
| EP4188443A4 (en) | 2020-07-30 | 2024-09-18 | Janssen Biotech, Inc. | METHOD OF TREATMENT OF PSORIASIS IN PEDIATRIC SUBJECTS WITH ANTI-IL12/IL23 ANTIBODY |
| EP4189121A1 (en) | 2020-08-03 | 2023-06-07 | Genentech, Inc. | Diagnostic and therapeutic methods for lymphoma |
| EP4192868A1 (en) | 2020-08-05 | 2023-06-14 | Juno Therapeutics, Inc. | Anti-idiotypic antibodies to ror1-targeted binding domains and related compositions and methods |
| CA3188426A1 (en) | 2020-08-07 | 2022-02-10 | Yichin Liu | Flt3 ligand fusion proteins and methods of use |
| IL300257A (en) | 2020-08-10 | 2023-03-01 | Astrazeneca Uk Ltd | Sars-cov-2 antibodies for treatment and prevention of covid-19 |
| AR123254A1 (en) | 2020-08-14 | 2022-11-16 | Ac Immune Sa | HUMANIZED ANTI-TDP-43 BINDING MOLECULES AND THEIR USES |
| EP4204448A2 (en) | 2020-08-27 | 2023-07-05 | cureab GmbH | Anti-golph2 antibodies for macrophage and dendritic cell differentiation |
| TW202227625A (en) | 2020-08-28 | 2022-07-16 | 美商建南德克公司 | Crispr/cas9 multiplex knockout of host cell proteins |
| KR20230061458A (en) | 2020-09-04 | 2023-05-08 | 에프. 호프만-라 로슈 아게 | Antibodies that bind to VEGF-A and ANG2 and methods of use |
| KR20230069171A (en) | 2020-09-15 | 2023-05-18 | 바이엘 악티엔게젤샤프트 | Novel anti-A2AP antibodies and uses thereof |
| CN116685351A (en) | 2020-09-17 | 2023-09-01 | 基因泰克公司 | Results from EMPACTA: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of Tocilizumab in Hospitalized Patients with COVID-19 Pneumonia |
| CN118146382A (en) | 2020-09-28 | 2024-06-07 | 安济盛生物医药有限公司 | Anti-sclerostin constructs and uses thereof |
| CA3193952A1 (en) | 2020-10-05 | 2022-04-14 | Bernard Martin Fine | Dosing for treatment with anti-fcrh5/anti-cd3 bispecific antibodies |
| WO2022079211A1 (en) | 2020-10-16 | 2022-04-21 | Adc Therapeutics Sa | Glycoconjugates |
| WO2022086957A1 (en) | 2020-10-20 | 2022-04-28 | Genentech, Inc. | Peg-conjugated anti-mertk antibodies and methods of use |
| KR20230091871A (en) | 2020-10-20 | 2023-06-23 | 에프. 호프만-라 로슈 아게 | Combination therapy of a PD-1 axis binding antagonist and a LRRK2 inhibitor |
| EP4232475A1 (en) | 2020-10-20 | 2023-08-30 | Kantonsspital St. Gallen | Antibodies or antigen-binding fragments specifically binding to gremlin-1 and uses thereof |
| WO2022093981A1 (en) | 2020-10-28 | 2022-05-05 | Genentech, Inc. | Combination therapy comprising ptpn22 inhibitors and pd-l1 binding antagonists |
| US20230406961A1 (en) | 2020-11-03 | 2023-12-21 | Deutsches Krebsforschungszentrum Stiftung des öffentlichen Rechts | Target-cell restricted, costimulatory, bispecific and bivalent anti-cd28 antibodies |
| CA3196539A1 (en) | 2020-11-04 | 2022-05-12 | Chi-Chung Li | Dosing for treatment with anti-cd20/anti-cd3 bispecific antibodies |
| JP7716473B2 (en) | 2020-11-04 | 2025-07-31 | ジェネンテック, インコーポレイテッド | Subcutaneous administration of anti-CD20/anti-CD3 bispecific antibodies |
| US12516118B2 (en) | 2020-11-04 | 2026-01-06 | Genentech, Inc. | Dosing for treatment with anti-CD20/anti-CD3 bispecific antibodies and anti-CD79b antibody drug conjugates |
| CN117916261A (en) | 2020-11-16 | 2024-04-19 | 豪夫迈·罗氏有限公司 | Combination therapy with FAP-targeted CD40 agonists |
| JP2023553323A (en) | 2020-11-25 | 2023-12-21 | エクシリオ デベロップメント, インコーポレイテッド | Tumor-specific cleavable linker |
| CN116723861A (en) | 2020-12-02 | 2023-09-08 | 上海复宏汉霖生物技术股份有限公司 | Anti-GARP/TGFβ antibodies and how to use them |
| IL303134A (en) | 2020-12-02 | 2023-07-01 | Glaxosmithkline Ip Dev Ltd | Il-7 binding proteins and their use in medical therapy |
| TW202237638A (en) | 2020-12-09 | 2022-10-01 | 日商武田藥品工業股份有限公司 | Compositions of guanylyl cyclase c (gcc) antigen binding agents and methods of use thereof |
| TW202237639A (en) | 2020-12-09 | 2022-10-01 | 日商武田藥品工業股份有限公司 | Compositions of guanylyl cyclase c (gcc) antigen binding agents and methods of use thereof |
| WO2022132904A1 (en) | 2020-12-17 | 2022-06-23 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Human monoclonal antibodies targeting sars-cov-2 |
| MX2023007846A (en) | 2021-01-06 | 2023-07-07 | Hoffmann La Roche | CO-TREATMENT USING A BISPECIFIC ANTIBODY AGAINST PD1-LAG3 AND A BISPECIFIC ANTIBODY TO CD20 T LYMPHOCYTES. |
| US20240082437A1 (en) | 2021-01-12 | 2024-03-14 | Hoffmann-La Roche Inc. | Split antibodies which bind to cancer cells and target radionuclides to said cells |
| US20240173442A1 (en) | 2021-01-13 | 2024-05-30 | Hoffmann-La Roche Inc. | Combination therapy |
| WO2022162587A1 (en) | 2021-01-27 | 2022-08-04 | Centre Hospitalier Universitaire Vaudois (C.H.U.V.) | Anti-sars-cov-2 antibodies and use thereof in the treatment of sars-cov-2 infection |
| AU2022212599A1 (en) | 2021-01-28 | 2023-08-17 | Universität Ulm | Method and means for modulating b-cell mediated immune responses |
| CN117120084A (en) | 2021-01-28 | 2023-11-24 | 维肯芬特有限责任公司 | Methods and means for modulating B cell-mediated immune responses |
| WO2022162203A1 (en) | 2021-01-28 | 2022-08-04 | Vaccinvent Gmbh | Method and means for modulating b-cell mediated immune responses |
| JP2024509695A (en) | 2021-02-03 | 2024-03-05 | ジェネンテック, インコーポレイテッド | Multispecific binding proteolysis platform and methods of use |
| CA3209136A1 (en) | 2021-02-09 | 2022-08-18 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Antibodies targeting the spike protein of coronaviruses |
| CA3210753A1 (en) | 2021-02-09 | 2022-08-18 | University Of Georgia Research Foundation, Inc. | Human monoclonal antibodies against pneumococcal antigens |
| MX2023009497A (en) | 2021-02-15 | 2023-08-23 | Takeda Pharmaceuticals Co | COMPOSITIONS AND METHODS OF CELLULAR THERAPY TO MODULATE THE SIGNALING OF TRANSFORMING GROWTH FACTOR-BETA (TGF-BETA). |
| GB202102396D0 (en) | 2021-02-19 | 2021-04-07 | Adc Therapeutics Sa | Molecular adjuvant |
| CA3211686A1 (en) | 2021-02-26 | 2022-09-01 | Bayer Aktiengesellschaft | Inhibitors of il-11 or il-11ra for use in the treatment of abnormal uterine bleeding |
| JP2024508488A (en) | 2021-03-01 | 2024-02-27 | エクシリオ デベロップメント, インコーポレイテッド | Combination of masked CTLA4 and PD1/PD-L1 antibodies to treat cancer |
| WO2022187270A1 (en) | 2021-03-01 | 2022-09-09 | Xilio Development, Inc. | Combination of ctla4 and pd1/pdl1 antibodies for treating cancer |
| CN117440832A (en) | 2021-03-03 | 2024-01-23 | 索伦托药业有限公司 | Antibody-drug conjugates comprising anti-BCMA antibodies |
| CN117715933A (en) | 2021-03-05 | 2024-03-15 | 当康生物技术有限责任公司 | Anti-VISTA constructs and their uses |
| EP4301782A1 (en) | 2021-03-05 | 2024-01-10 | Go Therapeutics, Inc. | Anti-glyco-cd44 antibodies and their uses |
| EP4304732A1 (en) | 2021-03-12 | 2024-01-17 | Genentech, Inc. | Anti-klk7 antibodies, anti-klk5 antibodies, multispecific anti-klk5/klk7 antibodies, and methods of use |
| CN117751138A (en) | 2021-03-12 | 2024-03-22 | 詹森生物科技公司 | Safe and effective methods for treating psoriatic arthritis with anti-IL 23 specific antibodies |
| WO2022190034A1 (en) | 2021-03-12 | 2022-09-15 | Janssen Biotech, Inc. | Method of treating psoriatic arthritis patients with inadequate response to tnf therapy with anti-il23 specific antibody |
| KR20230156373A (en) | 2021-03-15 | 2023-11-14 | 제넨테크, 인크. | Therapeutic compositions and methods of treating lupus nephritis |
| WO2022197877A1 (en) | 2021-03-19 | 2022-09-22 | Genentech, Inc. | Methods and compositions for time delayed bio-orthogonal release of cytotoxic agents |
| CN117616041A (en) | 2021-03-25 | 2024-02-27 | 当康生物技术有限责任公司 | Anti-IGFBP7 constructs and their uses |
| US12384843B2 (en) | 2021-03-30 | 2025-08-12 | Bayer Aktiengesellschaft | Anti-semaphorin 3A antibodies |
| AR125344A1 (en) | 2021-04-15 | 2023-07-05 | Chugai Pharmaceutical Co Ltd | ANTI-C1S ANTIBODY |
| IL307501A (en) | 2021-04-19 | 2023-12-01 | Hoffmann La Roche | Modified mammalian cells |
| CN117222412A (en) | 2021-04-23 | 2023-12-12 | 豪夫迈·罗氏有限公司 | Prevention or reduction of adverse reactions associated with NK cell engagers |
| CA3213632A1 (en) | 2021-04-30 | 2022-11-03 | F. Hoffmann-La Roche Ag | Dosing for combination treatment with anti-cd20/anti-cd3 bispecific antibody and anti-cd79b antibody drug conjugate |
| TW202310876A (en) | 2021-05-12 | 2023-03-16 | 美商建南德克公司 | Methods of using anti-cd79b immunoconjugates to treat diffuse large b-cell lymphoma |
| AR125855A1 (en) | 2021-05-14 | 2023-08-16 | Genentech Inc | TREM2 AGONISTS |
| BR112023023775A2 (en) | 2021-05-14 | 2024-02-20 | Genentech Inc | METHODS FOR TREATING AN INDIVIDUAL WITH A CD20-POSITIVE CELL PROLIFERATIVE DISORDER AND FOR TREATING A POPULATION OF INDIVIDUALS WITH A CD20-POSITIVE CELL PROLIFERATIVE DISORDER |
| WO2022243261A1 (en) | 2021-05-19 | 2022-11-24 | F. Hoffmann-La Roche Ag | Agonistic cd40 antigen binding molecules targeting cea |
| WO2022246259A1 (en) | 2021-05-21 | 2022-11-24 | Genentech, Inc. | Modified cells for the production of a recombinant product of interest |
| WO2022248870A1 (en) | 2021-05-28 | 2022-12-01 | Glaxosmithkline Intellectual Property Development Limited | Combination therapies for treating cancer |
| AR126009A1 (en) | 2021-06-02 | 2023-08-30 | Hoffmann La Roche | CD28 ANTIGEN-BINDING AGONIST MOLECULES THAT TARGET EPCAM |
| WO2022255440A1 (en) | 2021-06-04 | 2022-12-08 | Chugai Seiyaku Kabushiki Kaisha | Anti-ddr2 antibodies and uses thereof |
| MX2023014563A (en) | 2021-06-09 | 2024-02-08 | Hoffmann La Roche | COMBINATION OF A PARTICULAR BRAF INHIBITOR (PARADOX SWITCH) AND A PD-1 AXIS BINDING ANTAGONIST FOR USE IN THE TREATMENT OF CANCER. |
| BR112023025863A2 (en) | 2021-06-11 | 2024-03-05 | Genentech Inc | METHODS TO TREAT CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD), TO REDUCE THE FREQUENCY OF MODERATE TO SEVERE EXACERBATIONS IN A PATIENT WITH COPD, TO TREAT OR PREVENT COPD, TO MAINTAIN AND/OR IMPROVE LUNG FUNCTION, TO IMPROVE BASELINE COUNT OF EOSINOPHILS IN THE BLOOD, KIT, ST2 ANTAGONISTS AND ANTI-ST2 ANTIBODY |
| US12227574B2 (en) | 2021-06-17 | 2025-02-18 | Amberstone Biosciences, Inc. | Anti-CD3 constructs and uses thereof |
| WO2022263638A1 (en) | 2021-06-17 | 2022-12-22 | Centre Hospitalier Universitaire Vaudois (C.H.U.V.) | Anti-sars-cov-2 antibodies and use thereof in the treatment of sars-cov-2 infection |
| AU2022297107B2 (en) | 2021-06-25 | 2024-08-01 | Chugai Seiyaku Kabushiki Kaisha | Use of anti-ctla-4 antibody |
| CR20240026A (en) | 2021-06-25 | 2024-03-14 | Chugai Pharmaceutical Co Ltd | Anti–ctla-4 antibody |
| WO2023283611A1 (en) | 2021-07-08 | 2023-01-12 | Staidson Biopharma Inc. | Antibodies specifically recognizing tnfr2 and uses thereof |
| JP2024527581A (en) | 2021-07-09 | 2024-07-25 | ヤンセン バイオテツク,インコーポレーテツド | Method for producing anti-IL12/IL23 antibody composition |
| JP2024530402A (en) | 2021-07-12 | 2024-08-21 | ジェネンテック, インコーポレイテッド | Structures for reducing antibody-lipase binding |
| CR20240074A (en) | 2021-07-14 | 2024-03-08 | Genentech Inc | Anti-c-c motif chemokine receptor 8 (ccr8) antibodies and methods of use |
| KR20240058075A (en) | 2021-07-14 | 2024-05-03 | 스테이드슨 (베이징) 바이오팔마슈티칼스 캄퍼니 리미티드 | Antibodies specifically identifying CD40 and their applications |
| EP4373859A1 (en) | 2021-07-22 | 2024-05-29 | F. Hoffmann-La Roche AG | Heterodimeric fc domain antibodies |
| JP2024526880A (en) | 2021-07-22 | 2024-07-19 | ジェネンテック, インコーポレイテッド | Brain targeting compositions and methods of use thereof |
| JP2024528217A (en) | 2021-08-03 | 2024-07-26 | エフ・ホフマン-ラ・ロシュ・アクチェンゲゼルシャフト | Bispecific antibodies and methods of use |
| WO2023014863A1 (en) | 2021-08-05 | 2023-02-09 | Go Therapeutics, Inc. | Anti-glyco-muc4 antibodies and their uses |
| US20240336697A1 (en) | 2021-08-07 | 2024-10-10 | Genentech, Inc. | Methods of using anti-cd79b immunoconjugates to treat diffuse large b-cell lymphoma |
| IL310698A (en) | 2021-08-13 | 2024-04-01 | Glaxosmithkline Ip Dev Ltd | Cytotoxic targeting chimeras for CCR2-expressing cells |
| CN117897409A (en) | 2021-08-13 | 2024-04-16 | 基因泰克公司 | Administration of anti-tryptase antibodies |
| AU2022327859A1 (en) | 2021-08-13 | 2024-02-22 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras |
| WO2023021055A1 (en) | 2021-08-19 | 2023-02-23 | F. Hoffmann-La Roche Ag | Multivalent anti-variant fc-region antibodies and methods of use |
| CA3229448A1 (en) | 2021-08-23 | 2023-03-02 | Immunitas Therapeutics, Inc. | Anti-cd161 antibodies and uses thereof |
| WO2023028591A1 (en) | 2021-08-27 | 2023-03-02 | Genentech, Inc. | Methods of treating tau pathologies |
| JP2024534853A (en) | 2021-08-30 | 2024-09-26 | ジェネンテック, インコーポレイテッド | Anti-polybiquitin multispecific antibody |
| JP2024536722A (en) | 2021-09-03 | 2024-10-08 | ジーオー セラピューティクス,インコーポレイテッド | Anti-glyco-CMET antibodies and uses thereof |
| WO2023034571A1 (en) | 2021-09-03 | 2023-03-09 | Go Therapeutics, Inc. | Anti-glyco-lamp1 antibodies and their uses |
| EP4402165A1 (en) | 2021-09-17 | 2024-07-24 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Synthetic humanized llama nanobody library and use thereof to identify sars-cov-2 neutralizing antibodies |
| TW202321308A (en) | 2021-09-30 | 2023-06-01 | 美商建南德克公司 | Methods for treatment of hematologic cancers using anti-tigit antibodies, anti-cd38 antibodies, and pd-1 axis binding antagonists |
| CA3233953A1 (en) | 2021-10-05 | 2023-04-13 | Matthew Bruce | Combination therapies for treating cancer |
| JPWO2023058723A1 (en) | 2021-10-08 | 2023-04-13 | ||
| MX2024005197A (en) | 2021-10-29 | 2024-07-24 | Janssen Biotech Inc | Methods of treating crohn's disease with anti-il23 specific antibody. |
| AU2021362997A1 (en) | 2021-11-03 | 2024-05-16 | Hangzhou Dac Biotech Co., Ltd. | Specific conjugation of an antibody |
| WO2023078279A1 (en) * | 2021-11-04 | 2023-05-11 | 澄交生物科技股份有限公司 | Immunogenic composition and use thereof |
| TW202342095A (en) | 2021-11-05 | 2023-11-01 | 英商阿斯特捷利康英國股份有限公司 | Composition for treatment and prevention of covid-19 |
| WO2023081818A1 (en) | 2021-11-05 | 2023-05-11 | American Diagnostics & Therapy, Llc (Adxrx) | Monoclonal antibodies against carcinoembryonic antigens, and their uses |
| EP4430072A1 (en) | 2021-11-10 | 2024-09-18 | Genentech, Inc. | Anti-interleukin-33 antibodies and uses thereof |
| WO2023084488A1 (en) | 2021-11-15 | 2023-05-19 | Janssen Biotech, Inc. | Methods of treating crohn's disease with anti-il23 specific antibody |
| IL312692A (en) | 2021-11-16 | 2024-07-01 | Genentech Inc | Methods and compositions for treating systemic lupus erythematosus (sle) with mosunetuzumab |
| US20250320284A1 (en) | 2021-11-16 | 2025-10-16 | Ac Immune Sa | Novel Molecules for Therapy and Diagnosis |
| IL313021A (en) | 2021-11-23 | 2024-07-01 | Janssen Biotech Inc | A method for treating ulcerative colitis with a specific anti-IL23 antibody |
| CN118284625A (en) | 2021-11-26 | 2024-07-02 | 豪夫迈·罗氏有限公司 | Combination therapy of anti-TYRP1/anti-CD3 bispecific antibody and TYRP1-specific antibody |
| EP4445911A4 (en) | 2021-12-06 | 2025-10-22 | Beijing Solobio Genetechnology Co Ltd | BISPECIFIC ANTIBODY WITH SPECIFIC BINDING TO KLEBSIELLA PNEUMONIAE O2 AND O1 ANTIGENS AND COMPOSITION |
| AR127887A1 (en) | 2021-12-10 | 2024-03-06 | Hoffmann La Roche | ANTIBODIES THAT BIND CD3 AND PLAP |
| WO2023109901A1 (en) | 2021-12-17 | 2023-06-22 | Shanghai Henlius Biotech, Inc. | Anti-ox40 antibodies and methods of use |
| JP2024547020A (en) | 2021-12-17 | 2024-12-26 | シャンハイ・ヘンリウス・バイオテック・インコーポレイテッド | Anti-OX40 antibodies, multispecific antibodies and methods of use thereof |
| CR20240246A (en) | 2021-12-20 | 2024-07-19 | Hoffmann La Roche | AGONIST ANTI-LTBR ANTIBODIES AND BISPECIFIC ANTIBODIES THAT INCLUDE THEM |
| UY40097A (en) | 2022-01-07 | 2023-07-14 | Johnson & Johnson Entpr Innovation Inc | MATERIALS AND METHODS FOR IL-1B BINDING PROTEINS |
| WO2023141445A1 (en) | 2022-01-19 | 2023-07-27 | Genentech, Inc. | Anti-notch2 antibodies and conjugates and methods of use |
| EP4476251A1 (en) | 2022-02-10 | 2024-12-18 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Human monoclonal antibodies that broadly target coronaviruses |
| KR20240150774A (en) | 2022-02-16 | 2024-10-16 | 에이씨 이뮨 에스에이 | Humanized anti-TDP-43 binding molecules and uses thereof |
| MX2024010003A (en) | 2022-02-18 | 2024-09-30 | Rakuten Medical Inc | ANTI-PROGRAMMED DEATH LIGAND 1 (PD-L1) ANTIBODY MOLECULES, POLYNUCLEOTIDES ENCODING THEM, AND METHODS OF USE. |
| TW202342510A (en) | 2022-02-18 | 2023-11-01 | 英商Rq生物科技有限公司 | Antibodies |
| WO2023161879A1 (en) | 2022-02-25 | 2023-08-31 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras for fibroblast activation protein-expressing cells |
| WO2023161877A1 (en) | 2022-02-25 | 2023-08-31 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras for integrin avb6-expressing cells |
| WO2023161876A1 (en) | 2022-02-25 | 2023-08-31 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras for cxcr3-expressing cells |
| WO2023161881A1 (en) | 2022-02-25 | 2023-08-31 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras for ccr2-expressing cells |
| CN119136836A (en) | 2022-02-25 | 2024-12-13 | 葛兰素史克知识产权发展有限公司 | Cytotoxic targeting chimera for C-C chemokine receptor 2 expressing cells |
| WO2023161875A1 (en) | 2022-02-25 | 2023-08-31 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras for prostate specific membrane antigen-expressing cells |
| WO2023161878A1 (en) | 2022-02-25 | 2023-08-31 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras for folate receptor-expressing cells |
| US20250205350A1 (en) | 2022-03-10 | 2025-06-26 | Vivasor, Inc. | Antibody-Drug Conjugates and Uses Thereof |
| US20230414750A1 (en) | 2022-03-23 | 2023-12-28 | Hoffmann-La Roche Inc. | Combination treatment of an anti-cd20/anti-cd3 bispecific antibody and chemotherapy |
| CN118974096A (en) | 2022-03-25 | 2024-11-15 | 上海复宏汉霖生物技术股份有限公司 | Anti-MSLN antibodies and methods of use |
| WO2023192827A1 (en) | 2022-03-26 | 2023-10-05 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Bispecific antibodies to hiv-1 env and their use |
| WO2023192881A1 (en) | 2022-03-28 | 2023-10-05 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Neutralizing antibodies to hiv-1 env and their use |
| WO2023186756A1 (en) | 2022-03-28 | 2023-10-05 | F. Hoffmann-La Roche Ag | Interferon gamma variants and antigen binding molecules comprising these |
| WO2023187707A1 (en) | 2022-03-30 | 2023-10-05 | Janssen Biotech, Inc. | Method of treating mild to moderate psoriasis with il-23 specific antibody |
| GB202204813D0 (en) | 2022-04-01 | 2022-05-18 | Bradcode Ltd | Human monoclonal antibodies and methods of use thereof |
| IL315770A (en) | 2022-04-01 | 2024-11-01 | Genentech Inc | Dosing for treatment with anti-fcrh5/anti-cd3 bispecific antibodies |
| EP4504775A1 (en) | 2022-04-08 | 2025-02-12 | AC Immune SA | Anti-tdp-43 binding molecules |
| CN119013300A (en) | 2022-04-13 | 2024-11-22 | 豪夫迈·罗氏有限公司 | Pharmaceutical compositions of anti-CD 20/anti-CD 3 bispecific antibodies and methods of use |
| AR129062A1 (en) | 2022-04-13 | 2024-07-10 | Genentech Inc | PHARMACEUTICAL COMPOSITIONS OF THERAPEUTIC PROTEINS AND METHODS OF USE |
| AU2023258146A1 (en) | 2022-04-20 | 2024-09-05 | Kantonsspital St. Gallen | Antibodies or antigen-binding fragments pan-specifically binding to gremlin-1 and gremlin-2 and uses thereof |
| US12060426B2 (en) | 2022-04-29 | 2024-08-13 | 23Andme, Inc. | Anti-ULBP6 antibodies |
| JP2025514858A (en) | 2022-04-29 | 2025-05-09 | アストラゼネカ・ユーケイ・リミテッド | SARS-CoV-2 antibodies and methods of use thereof |
| CN119137157A (en) | 2022-05-03 | 2024-12-13 | 基因泰克公司 | Anti-Ly6E antibodies, immunoconjugates and uses thereof |
| US20250326828A1 (en) | 2022-05-09 | 2025-10-23 | Staidson (Beijing) Biopharmaceuticals Co., Ltd. | Antibodies Specifically Recognizing Gdf15 and Uses Thereof |
| AU2022458320A1 (en) | 2022-05-11 | 2024-11-28 | Genentech, Inc. | Dosing for treatment with anti-fcrh5/anti-cd3 bispecific antibodies |
| AR129268A1 (en) | 2022-05-11 | 2024-08-07 | Hoffmann La Roche | ANTIBODY THAT BINDS TO VEGF-A AND IL6 AND METHODS OF USE |
| JP2025521118A (en) | 2022-05-18 | 2025-07-08 | ヤンセン バイオテツク,インコーポレーテツド | Methods for assessing and treating psoriatic arthritis using IL23 antibodies |
| AR129445A1 (en) | 2022-05-27 | 2024-08-28 | Glaxosmithkline Ip Dev Ltd | USE OF TNF-a BINDING PROTEINS AND IL-7 BINDING PROTEINS IN MEDICAL TREATMENT |
| WO2023235699A1 (en) | 2022-05-31 | 2023-12-07 | Jounce Therapeutics, Inc. | Antibodies to lilrb4 and uses thereof |
| IL317449A (en) | 2022-06-07 | 2025-02-01 | Genentech Inc | Method for determining the efficacy of a lung cancer treatment comprising an anti-pd-l1 antagonist and an anti-tigit antagonist antibody |
| EP4536701A2 (en) | 2022-06-08 | 2025-04-16 | Institute for Research in Biomedicine (IRB) | Cross-specific antibodies, uses and methods for discovery thereof |
| WO2023242372A1 (en) | 2022-06-15 | 2023-12-21 | argenx BV | Fcrn/hsa binding molecules and methods of use |
| JP2025523020A (en) | 2022-07-13 | 2025-07-17 | ジェネンテック, インコーポレイテッド | Administration for Treatment with Anti-FcRH5/Anti-CD3 Bispecific Antibody |
| IL318252A (en) | 2022-07-19 | 2025-03-01 | Genentech Inc | Dosing for treatment with anti-fcrh5/anti-cd3 bispecific antibodies |
| TW202417503A (en) | 2022-07-19 | 2024-05-01 | 美商舒泰神(加州)生物科技有限公司 | Antibodies specifically recognizing b- and t-lymphocyte attenuator (btla) and uses thereof |
| AR129991A1 (en) | 2022-07-22 | 2024-10-23 | Bristol Myers Squibb Co | ANTIBODIES THAT BIND HUMAN PAD4 AND THEIR USES |
| AR129995A1 (en) | 2022-07-22 | 2024-10-23 | Genentech Inc | ANTI-STEAP1 ANTIGEN BINDING MOLECULES AND THEIR USES |
| EP4565329A1 (en) | 2022-08-01 | 2025-06-11 | The United States of America, as represented by the Secretary, Department of Health and Human Services | Monoclonal antibodies that bind to the underside of influenza viral neuraminidase |
| CA3263779A1 (en) | 2022-08-05 | 2024-02-08 | Janssen Biotech, Inc. | Cd98 binding constructs for treating brain tumors |
| EP4565330A1 (en) | 2022-08-05 | 2025-06-11 | Janssen Biotech, Inc. | Transferrin receptor binding proteins for treating brain tumors |
| JP2025529896A (en) | 2022-08-22 | 2025-09-09 | アブデラ セラピューティクス インク. | DLL3-binding molecules and uses thereof |
| WO2024042112A1 (en) | 2022-08-25 | 2024-02-29 | Glaxosmithkline Intellectual Property Development Limited | Antigen binding proteins and uses thereof |
| AU2023329484A1 (en) | 2022-08-26 | 2025-02-20 | Juno Therapeutics, Inc. | Antibodies and chimeric antigen receptors specific for delta-like ligand 3 (dll3) |
| CN120153254A (en) | 2022-09-01 | 2025-06-13 | 基因泰克公司 | Bladder cancer treatment and diagnosis |
| WO2024054822A1 (en) | 2022-09-07 | 2024-03-14 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Engineered sars-cov-2 antibodies with increased neutralization breadth |
| JP2025531788A (en) | 2022-09-07 | 2025-09-25 | ダイナミキュア バイオテクノロジー エルエルシー | Anti-VISTA constructs and uses thereof |
| WO2024064826A1 (en) | 2022-09-22 | 2024-03-28 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Neutralizing antibodies to plasmodium falciparum circumsporozoite protein and their use |
| EP4596580A4 (en) | 2022-09-27 | 2026-01-21 | Staidson Beijing Biopharmaceuticals Co Ltd | ANTIBODIES FOR SPECIFIC LIGHT DETECTION AND USE THEM |
| WO2024068996A1 (en) | 2022-09-30 | 2024-04-04 | Centre Hospitalier Universitaire Vaudois (C.H.U.V.) | Anti-sars-cov-2 antibodies and use thereof in the treatment of sars-cov-2 infection |
| JP2025533849A (en) | 2022-10-07 | 2025-10-09 | ジェネンテック, インコーポレイテッド | Methods of treating cancer with anti-C-C motif chemokine receptor 8 (CCR8) antibodies |
| CN120693395A (en) | 2022-10-20 | 2025-09-23 | 北京三诺佳邑生物技术有限责任公司 | Antibody combinations and bispecific antibodies that specifically bind to TRAIL or FasL |
| WO2024091991A1 (en) | 2022-10-25 | 2024-05-02 | Genentech, Inc. | Therapeutic and diagnostic methods for multiple myeloma |
| JP2025537137A (en) | 2022-11-04 | 2025-11-14 | ギリアード サイエンシーズ, インコーポレイテッド | Anti-cancer therapy using a combination of anti-CCR8 antibodies, chemotherapy and immunotherapy |
| JP2025537197A (en) | 2022-11-08 | 2025-11-14 | ジェネンテック, インコーポレイテッド | Compositions and methods for treating childhood-onset idiopathic nephrotic syndrome |
| EP4615580A1 (en) | 2022-11-09 | 2025-09-17 | CIS Biopharma AG | Anti-l1-cam antibodies and their uses for diagnostic and therapeutic applications |
| WO2024100170A1 (en) | 2022-11-11 | 2024-05-16 | F. Hoffmann-La Roche Ag | Antibodies binding to hla-a*02/foxp3 |
| WO2024108053A1 (en) | 2022-11-17 | 2024-05-23 | Sanofi | Ceacam5 antibody-drug conjugates and methods of use thereof |
| EP4623001A1 (en) | 2022-11-22 | 2025-10-01 | Janssen Biotech, Inc. | Method of treating ulcerative colitis with anti-il23 specific antibody |
| CN120265651A (en) | 2022-11-25 | 2025-07-04 | 中外制药株式会社 | Methods for producing proteins |
| EP4631974A1 (en) | 2022-12-08 | 2025-10-15 | Nanjing Vazyme Biotech Co., Ltd. | Antibody specifically binding to rsv |
| EP4638491A1 (en) | 2022-12-19 | 2025-10-29 | The United States of America, as represented by The Secretary, Department of Health and Human Services | Monoclonal antibodies for treating sars-cov-2 infection |
| EP4491230A1 (en) | 2023-07-14 | 2025-01-15 | iOmx Therapeutics AG | Cross-specific antigen binding proteins (abp) targeting leukocyte immunoglobulin-like receptor subfamily b1 (lilrb1) and lilrb2, combinations and uses thereof |
| KR20250129687A (en) | 2022-12-23 | 2025-08-29 | 아이오엠엑스 테라퓨틱스 아게 | Cross-specific antigen binding proteins (ABPs) targeting leukocyte immunoglobulin-like receptor subfamily B1 (LILRB1) and LILRB2, combinations thereof, and uses thereof |
| IL321951A (en) | 2023-01-18 | 2025-09-01 | Genentech Inc | Multispecific antibodies and uses thereof |
| WO2024156672A1 (en) | 2023-01-25 | 2024-08-02 | F. Hoffmann-La Roche Ag | Antibodies binding to csf1r and cd3 |
| WO2024167898A1 (en) | 2023-02-07 | 2024-08-15 | Go Therapeutics, Inc. | ANTIBODY FUSION PROTEINS COMPRISING ANTI-GLYCO-MUC4 ANTIBODIES AND MIC PROTEIN α1-α2 DOMAINS, AND THEIR USES |
| CN120917043A (en) | 2023-03-08 | 2025-11-07 | Ac免疫有限公司 | Anti-TDP-43 binding molecules and uses thereof |
| EP4428159A1 (en) | 2023-03-10 | 2024-09-11 | Istituto Romagnolo per lo Studio dei Tumori "Dino Amadori" - IRST S.r.l. | Melanoma targeting human antibodies and therapeutic uses thereof |
| EP4428158A1 (en) | 2023-03-10 | 2024-09-11 | Istituto Romagnolo per lo Studio dei Tumori "Dino Amadori" - IRST S.r.l. | Lung cancer targeting human antibodies and therapeutic uses thereof |
| CN120858109A (en) | 2023-03-10 | 2025-10-28 | 基因泰克公司 | Fusions with proteases and uses thereof |
| WO2024188965A1 (en) | 2023-03-13 | 2024-09-19 | F. Hoffmann-La Roche Ag | Combination therapy employing a pd1-lag3 bispecific antibody and an hla-g t cell bispecific antibody |
| TW202446789A (en) | 2023-03-31 | 2024-12-01 | 美商建南德克公司 | Anti-alpha v beta 8 integrin antibodies and methods of use |
| WO2024211236A2 (en) | 2023-04-05 | 2024-10-10 | Sorrento Therapeutics, Inc. | Antibody-drug conjugates and uses thereof |
| WO2024211235A1 (en) | 2023-04-05 | 2024-10-10 | Sorrento Therapeutics, Inc. | Antibody-drug conjugates and uses thereof |
| EP4687996A1 (en) | 2023-04-05 | 2026-02-11 | Sorrento Therapeutics, Inc. | Antibody-drug conjugates and uses thereof |
| WO2024212827A1 (en) | 2023-04-12 | 2024-10-17 | Shanghai Kangabio Co., Limited | Multifunctional molecules comprising masked interleukin 12 and methods of use |
| CN121263210A (en) | 2023-04-17 | 2026-01-02 | 沛科生物公司 | Antibodies and antibody-drug conjugates, as well as their usage, synthesis processes, and intermediates |
| WO2024218361A1 (en) | 2023-04-21 | 2024-10-24 | Glaxosmithkline Intellectual Property Development Limited | Bispecific cytotoxicity targeting chimeras |
| WO2024218345A1 (en) | 2023-04-21 | 2024-10-24 | Glaxosmithkline Intellectual Property Development Limited | Cytotoxicity targeting chimeras for antibody-drug conjugates and bispecific antibodies |
| TW202448949A (en) | 2023-05-05 | 2024-12-16 | 美商建南德克公司 | Dosing for treatment with anti-fcrh5/anti-cd3 bispecific antibodies |
| AU2024269754A1 (en) | 2023-05-08 | 2025-10-23 | F. Hoffmann-La Roche Ag | Targeted interferon alpha fusion proteins and methods of use |
| WO2024233646A1 (en) | 2023-05-10 | 2024-11-14 | Genentech, Inc. | Methods and compositions for treating cancer |
| WO2024238537A1 (en) | 2023-05-16 | 2024-11-21 | F. Hoffmann-La Roche Ag | Pd-1 -regulated il-2 immunocytokine and uses thereof |
| WO2024243355A1 (en) | 2023-05-24 | 2024-11-28 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Human monoclonal antibodies that target the rh5 complex of blood-stage plasmodium falciparum |
| WO2024246083A1 (en) | 2023-06-01 | 2024-12-05 | F. Hoffmann-La Roche Ag | Bispecific antibodies targeting bcma and cd28 |
| AR132805A1 (en) | 2023-06-01 | 2025-07-30 | Hoffmann La Roche | Immunostimulatory antigen-binding molecules that specifically bind to BCMA |
| WO2024254455A1 (en) | 2023-06-08 | 2024-12-12 | Genentech, Inc. | Macrophage signatures for diagnostic and therapeutic methods for lymphoma |
| CN121311247A (en) | 2023-06-09 | 2026-01-09 | 舒泰神(北京)生物制药股份有限公司 | Antibodies that specifically bind MASP3 and multispecific antibodies that specifically bind MASP3 and MASP2 |
| WO2024255794A1 (en) | 2023-06-16 | 2024-12-19 | 舒泰神(北京)生物制药股份有限公司 | Antibody specifically recognizing factor xiia and use thereof |
| AU2024311339A1 (en) | 2023-06-21 | 2026-01-08 | F. Hoffmann-La Roche Ag | Combination therapy with fap-targeted lymphotoxin beta receptor agonists |
| WO2024263761A1 (en) | 2023-06-22 | 2024-12-26 | Genentech, Inc. | Antibodies and uses thereof |
| CN121443309A (en) | 2023-06-22 | 2026-01-30 | 基因泰克公司 | Treatment of multiple myeloma |
| AU2024296940A1 (en) | 2023-07-07 | 2026-01-29 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Humanized 40h3 antibody |
| WO2025024265A1 (en) | 2023-07-21 | 2025-01-30 | Bristol-Myers Squibb Company | Methods of assessing citrullination and activity of pad4 modulators |
| WO2025024233A1 (en) | 2023-07-21 | 2025-01-30 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Bispecific antibodies that broadly target coronaviruses |
| WO2025021790A2 (en) | 2023-07-24 | 2025-01-30 | F. Hoffmann-La Roche Ag | Multispecific antibodies |
| WO2025021838A1 (en) | 2023-07-26 | 2025-01-30 | F. Hoffmann-La Roche Ag | Antibodies binding to cd3 |
| WO2025034806A1 (en) | 2023-08-08 | 2025-02-13 | Wisconsin Alumni Research Foundation | Single-domain antibodies and variants thereof against fibroblast activation protein |
| AU2024323186A1 (en) | 2023-08-09 | 2026-01-15 | F. Hoffmann-La Roche Ag | Mono and multispecific anti-trem2 antibodies, methods and uses thereof |
| AU2024322991A1 (en) | 2023-08-09 | 2026-01-08 | F. Hoffmann-La Roche Ag | Anti-a-beta protein antibodies, methods and uses thereof |
| WO2025032071A1 (en) | 2023-08-09 | 2025-02-13 | F. Hoffmann-La Roche Ag | Mono and multispecific anti-trem2 antibodies, methods and uses thereof |
| WO2025038492A1 (en) | 2023-08-11 | 2025-02-20 | Abalytics Oncology, Inc. | Anti-ctla-4 antibodies and related binding molecules and methods and uses thereof |
| WO2025045251A2 (en) | 2023-09-03 | 2025-03-06 | Kira Pharmaceuticals (Us) Llc | Multispecific constructs comprising anti-factor d moiety |
| WO2025064539A1 (en) | 2023-09-19 | 2025-03-27 | The United States Of America, As Represented By The Secretary, Dept. Of Health And Human Services | Herv-e antibodies and methods of their use |
| AR133909A1 (en) | 2023-09-25 | 2025-11-12 | Hoffmann La Roche | ANTIBODY THAT BINDS TO C3bBb |
| EP4537907A1 (en) | 2023-10-10 | 2025-04-16 | Enthera S.r.l. | Cd248 inhibitors and uses thereof |
| WO2025085489A1 (en) | 2023-10-17 | 2025-04-24 | Bristol-Myers Squibb Company | Gspt1-degrading compounds, anti-cd33 antibodies and antibody-drug conjugates and uses thereof |
| TW202517674A (en) | 2023-10-19 | 2025-05-01 | 德商拜耳廠股份有限公司 | Anti-gpc3 antibodies and radioconjugates thereof |
| WO2025099120A1 (en) | 2023-11-09 | 2025-05-15 | F. Hoffmann-La Roche Ag | Multispecific antibodies with conditional activity |
| WO2025106427A1 (en) | 2023-11-14 | 2025-05-22 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Neutralizing and protective monoclonal antibodies against respiratory syncytial virus (rsv) |
| WO2025106474A1 (en) | 2023-11-14 | 2025-05-22 | Genentech, Inc. | Therapeutic and diagnostic methods for treating cancer with anti-fcrh5/anti-cd3 bispecific antibodies |
| WO2025111402A1 (en) | 2023-11-21 | 2025-05-30 | Board Of Regents Of The University Of Nebraska | Anti-amyloid beta antibodies and related compositions and methods thereof |
| WO2025117384A1 (en) | 2023-12-01 | 2025-06-05 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Broadly neutralizing influenza hemagglutinin stem-directed antibodies |
| WO2025125118A1 (en) | 2023-12-11 | 2025-06-19 | F. Hoffmann-La Roche Ag | Protease activatable fc domain binding molecules |
| WO2025125386A1 (en) | 2023-12-14 | 2025-06-19 | F. Hoffmann-La Roche Ag | Antibodies that bind to folr1 and methods of use |
| GB202319605D0 (en) | 2023-12-20 | 2024-01-31 | argenx BV | Monovalent binding molecules and methods of use |
| US20250230251A1 (en) | 2023-12-20 | 2025-07-17 | Bristol-Myers Squibb Company | Antibodies targeting il-18 receptor beta (il-18rb) and related methods |
| WO2025132503A1 (en) | 2023-12-20 | 2025-06-26 | F. Hoffmann-La Roche Ag | Antibodies binding to ceacam5 |
| WO2025133290A1 (en) | 2023-12-21 | 2025-06-26 | Temper Bio | Protein for immune regulation |
| WO2025137284A2 (en) | 2023-12-21 | 2025-06-26 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Broadly neutralizing antibodies against sars-cov-2 and sars-cov variants |
| TW202542177A (en) | 2023-12-22 | 2025-11-01 | 瑞士商赫孚孟拉羅股份公司 | Activatable fusion proteins and methods of use |
| WO2025149633A1 (en) | 2024-01-12 | 2025-07-17 | Laigo Bio B.V. | Bispecific antigen binding proteins |
| WO2025174974A1 (en) | 2024-02-14 | 2025-08-21 | Bristol-Myers Squibb Company | Anti-cd33 antibodies and uses thereof |
| WO2025179281A1 (en) | 2024-02-23 | 2025-08-28 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Treatment of cardiovascular disease with antxr1 antibodies |
| WO2025184416A1 (en) | 2024-02-27 | 2025-09-04 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Single-domain antibodies and bispecific antibodies against hiv-1 and their use |
| WO2025184421A1 (en) | 2024-02-28 | 2025-09-04 | Juno Therapeutics, Inc. | Chimeric antigen receptors and antibodies specific for delta-like ligand 3 (dll3) and related methods |
| WO2025181189A1 (en) | 2024-03-01 | 2025-09-04 | F. Hoffmann-La Roche Ag | Antibodies binding to cd3 |
| WO2025199352A2 (en) | 2024-03-20 | 2025-09-25 | Juno Therapeutics, Inc. | Antibodies specific for solute carrier family 34 member 2 (slc34a2) |
| CA3249015A1 (en) | 2024-03-20 | 2025-10-31 | Janssen Biotech, Inc. | Methods of treating crohn’s disease with anti-il23 specific antibody |
| WO2025196639A1 (en) | 2024-03-21 | 2025-09-25 | Seagen Inc. | Cd25 antibodies, antibody-drug conjugates, and uses thereof |
| WO2025215060A1 (en) | 2024-04-11 | 2025-10-16 | F. Hoffmann-La Roche Ag | Antibodies that specifically bind modified oligonucleotides |
| WO2025226808A1 (en) | 2024-04-24 | 2025-10-30 | Genentech, Inc. | Compositions and methods of treating lupus nephritis |
| WO2025229206A1 (en) | 2024-05-03 | 2025-11-06 | argenx BV | Monovalent anti-iga binding molecules and methods of use |
| WO2025240670A2 (en) | 2024-05-15 | 2025-11-20 | Abalytics Oncology, Inc. | Anti-pd-1 antibodies and related binding molecules and methods and uses thereof |
| WO2025238187A1 (en) | 2024-05-15 | 2025-11-20 | Cis Biopharma Ag | Immunoconjugates targeting l1-cam |
| WO2025242909A1 (en) | 2024-05-24 | 2025-11-27 | Paul Scherrer Institut | CD30-targeting antibody-radioligand conjugates and their therapeutic use |
| WO2025250969A1 (en) | 2024-05-31 | 2025-12-04 | Vertex Pharmaceuticals Incorporated | Anti-cd74 antibodies, conjugates and uses thereof |
| WO2025262564A1 (en) | 2024-06-17 | 2025-12-26 | Pfizer Inc. | Use of anti-cxcr5 antibodies |
| WO2025262604A1 (en) | 2024-06-17 | 2025-12-26 | Janssen Biotech, Inc. | Methods of treating crohn's disease with anti-il23 specific antibody |
| WO2026003224A2 (en) | 2024-06-26 | 2026-01-02 | Iomx Therapeutics Ag | Bispecific antigen binding proteins (abp) targeting immune checkpoint molecules and both leukocyte immunoglobulin-like receptor subfamily b1 (lilrb1) and lilrb2; combinations and uses thereof |
| WO2026003761A1 (en) | 2024-06-27 | 2026-01-02 | Janssen Biotech, Inc. | Methods of treating ulcerative colits with anti-il23 specific antibody |
| WO2026013218A1 (en) | 2024-07-10 | 2026-01-15 | Ac Immune Sa | Anti-tdp-43 vectors, binding molecules and uses thereof |
| EP4684803A1 (en) | 2024-07-25 | 2026-01-28 | CeMM - Forschungszentrum für Molekulare Medizin GmbH | Antibody conjugated chemical inducers of degradation of rbm39 and therapeutic uses thereof |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6602684B1 (en) * | 1998-04-20 | 2003-08-05 | Glycart Biotechnology Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
Family Cites Families (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4215051A (en) * | 1979-08-29 | 1980-07-29 | Standard Oil Company (Indiana) | Formation, purification and recovery of phthalic anhydride |
| KR850004274A (en) * | 1983-12-13 | 1985-07-11 | 원본미기재 | Method for preparing erythropoietin |
| US4946778A (en) | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US4978745A (en) * | 1987-11-23 | 1990-12-18 | Centocor, Inc. | Immunoreactive heterochain antibodies |
| US5047335A (en) * | 1988-12-21 | 1991-09-10 | The Regents Of The University Of Calif. | Process for controlling intracellular glycosylation of proteins |
| ZA901852B (en) * | 1989-03-10 | 1990-12-28 | Snow Brand Milk Products Co Ltd | A human-derived glycoprotein and physiologically active factor |
| DE69032855T2 (en) * | 1989-05-25 | 1999-08-12 | Sloan-Kettering Institute for Cancer Research, New York, N.Y. | ANTIIDIOTYPICAL ANTIBODY INDUCING AN IMMUNE RESPONSE TO A GLYCOSPHINGOLIPID AND ITS USE |
| DE4028800A1 (en) | 1990-09-11 | 1992-03-12 | Behringwerke Ag | GENETIC SIALYLATION OF GLYCOPROTEINS |
| WO1994004574A1 (en) | 1991-08-22 | 1994-03-03 | Nissin Shokuhin Kabushiki Kaisha | Hiv immunotherapeutics |
| US5753229A (en) * | 1991-09-25 | 1998-05-19 | Mordoh; Jose | Monoclonal antibodies reactive with tumor proliferating cells |
| US5958403A (en) * | 1992-02-28 | 1999-09-28 | Beth Israel Hospital Association | Methods and compounds for prevention of graft rejection |
| ATE139900T1 (en) | 1992-11-13 | 1996-07-15 | Idec Pharma Corp | THERAPEUTIC USE OF CHIMERIC AND LABELED ANTIBODIES AGAINST HUMAN B LYMPHOCYTE RESTRICTED DIFFERENTIATION ANTIGEN FOR THE TREATMENT OF B CELL LYMPHOMA |
| US5736137A (en) * | 1992-11-13 | 1998-04-07 | Idec Pharmaceuticals Corporation | Therapeutic application of chimeric and radiolabeled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma |
| DK0678122T3 (en) | 1993-01-12 | 2000-03-06 | Biogen Inc | Recombinant anti-VLA4 antibody molecules |
| WO1995024494A1 (en) | 1994-03-09 | 1995-09-14 | Abbott Laboratories | Humanized milk |
| US5811524A (en) * | 1995-06-07 | 1998-09-22 | Idec Pharmaceuticals Corporation | Neutralizing high affinity human monoclonal antibodies specific to RSV F-protein and methods for their manufacture and therapeutic use thereof |
| JP3606536B2 (en) * | 1995-11-17 | 2005-01-05 | タカラバイオ株式会社 | Viral replication inhibitor |
| GB9603256D0 (en) * | 1996-02-16 | 1996-04-17 | Wellcome Found | Antibodies |
| WO1998006835A2 (en) | 1996-08-16 | 1998-02-19 | The Texas A & M University System | Modifying insect cell gylcosylation pathways with baculovirus expression vectors |
| US6306393B1 (en) | 1997-03-24 | 2001-10-23 | Immunomedics, Inc. | Immunotherapy of B-cell malignancies using anti-CD22 antibodies |
| US6183744B1 (en) * | 1997-03-24 | 2001-02-06 | Immunomedics, Inc. | Immunotherapy of B-cell malignancies using anti-CD22 antibodies |
| US5952203A (en) * | 1997-04-11 | 1999-09-14 | The University Of British Columbia | Oligosaccharide synthesis using activated glycoside derivative, glycosyl transferase and catalytic amount of nucleotide phosphate |
| RU2136695C1 (en) * | 1998-03-18 | 1999-09-10 | ЗАОПП "Эндо-Фарм-А" | Serum glycoprotein showing biological activity at superlow doses |
| US6946292B2 (en) | 2000-10-06 | 2005-09-20 | Kyowa Hakko Kogyo Co., Ltd. | Cells producing antibody compositions with increased antibody dependent cytotoxic activity |
| JP2005500018A (en) * | 2001-04-02 | 2005-01-06 | アイデック ファーマスーティカルズ コーポレイション | Recombinant antibody coexpressed with GnTIII |
| EP1423510A4 (en) * | 2001-08-03 | 2005-06-01 | Glycart Biotechnology Ag | ANTIBODY GLYCOSYLATION VARIANTS WITH INCREASED CELL CYTOTOXICITY DEPENDENT OF ANTIBODIES |
| NZ582315A (en) | 2003-01-22 | 2011-01-28 | Glycart Biotechnology Ag | Fusion constructs and use of same to produce antibodies with increased Fc receptor binding affinity and effector function |
| DK2380910T3 (en) | 2003-11-05 | 2015-10-19 | Roche Glycart Ag | Antigen binding molecules with increased Fc receptor binding affinity and effector function |
| RU2488597C2 (en) | 2005-02-07 | 2013-07-27 | Гликарт Биотехнологи Аг | Antigen-binding molecules, which bind egfr, their coding vectors and their usage |
| AR055137A1 (en) | 2005-08-26 | 2007-08-08 | Glycart Biotechnology Ag | MODIFIED ANTIGEN UNION MOLECULES WITH ALTERED CELL SIGNALING ACTIVITY |
| AR062223A1 (en) | 2006-08-09 | 2008-10-22 | Glycart Biotechnology Ag | MOLECULES OF ADHESION TO THE ANTIGEN THAT ADHER TO EGFR, VECTORS THAT CODE THEM, AND THEIR USES OF THESE |
| CA2770174A1 (en) | 2009-08-31 | 2011-03-03 | Roche Glycart Ag | Affinity-matured humanized anti cea monoclonal antibodies |
-
2002
- 2002-08-05 EP EP02778191A patent/EP1423510A4/en not_active Withdrawn
- 2002-08-05 CA CA2455365A patent/CA2455365C/en not_active Expired - Lifetime
- 2002-08-05 EP EP20100000043 patent/EP2180044A1/en not_active Ceased
- 2002-08-05 NZ NZ531219A patent/NZ531219A/en not_active IP Right Cessation
- 2002-08-05 US US10/211,554 patent/US20030175884A1/en not_active Abandoned
- 2002-08-05 KR KR10-2004-7001617A patent/KR20040054669A/en not_active Withdrawn
- 2002-08-05 NZ NZ592087A patent/NZ592087A/en not_active IP Right Cessation
- 2002-08-05 KR KR1020107000746A patent/KR20100018071A/en not_active Ceased
- 2002-08-05 NZ NZ603111A patent/NZ603111A/en not_active IP Right Cessation
- 2002-08-05 CA CA2838062A patent/CA2838062C/en not_active Expired - Lifetime
- 2002-08-05 JP JP2003517069A patent/JP2005524379A/en active Pending
- 2002-08-05 NZ NZ571596A patent/NZ571596A/en not_active IP Right Cessation
- 2002-08-05 HU HU0700103A patent/HUP0700103A3/en not_active Application Discontinuation
- 2002-08-05 AU AU2002339845A patent/AU2002339845B2/en not_active Expired
- 2002-08-05 NZ NZ581474A patent/NZ581474A/en not_active IP Right Cessation
- 2002-08-05 IL IL16017002A patent/IL160170A0/en unknown
- 2002-08-05 CN CNA028181735A patent/CN1555411A/en active Pending
- 2002-08-05 MX MXPA04001072A patent/MXPA04001072A/en active IP Right Grant
- 2002-08-05 RU RU2004106559/13A patent/RU2321630C2/en active
- 2002-08-05 PL PL374178A patent/PL217751B1/en unknown
- 2002-08-05 WO PCT/US2002/024739 patent/WO2003011878A2/en not_active Ceased
-
2004
- 2004-02-02 NO NO20040453A patent/NO332457B1/en not_active IP Right Cessation
- 2004-02-02 IL IL160170A patent/IL160170A/en unknown
-
2005
- 2005-08-09 US US11/199,232 patent/US8021856B2/en not_active Expired - Fee Related
-
2008
- 2008-12-25 JP JP2008331038A patent/JP2009114201A/en active Pending
-
2011
- 2011-08-02 US US13/196,724 patent/US8999324B2/en not_active Expired - Fee Related
-
2015
- 2015-03-23 US US14/665,191 patent/US9321843B2/en not_active Expired - Fee Related
-
2016
- 2016-03-24 US US15/080,020 patent/US9631023B2/en not_active Expired - Fee Related
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6602684B1 (en) * | 1998-04-20 | 2003-08-05 | Glycart Biotechnology Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
Cited By (347)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8021856B2 (en) | 1998-04-20 | 2011-09-20 | Roche Glycart Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US20040072290A1 (en) * | 1998-04-20 | 2004-04-15 | Glycart Biotechnology Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US9631023B2 (en) | 1998-04-20 | 2017-04-25 | Roche Glycart Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US20050079605A1 (en) * | 1998-04-20 | 2005-04-14 | Glycart Biotechnology Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US20090304690A1 (en) * | 1998-04-20 | 2009-12-10 | Glycart Biotechnology Ag | Glycosylation Engineering of Antibodies for Improving Antibody-Dependent Cellular Cytotoxicity |
| US20080280322A9 (en) * | 1998-04-20 | 2008-11-13 | Glycart Biotechnology Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US7517670B2 (en) | 1998-04-20 | 2009-04-14 | Glycart Biotechnology Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US20050272128A1 (en) * | 1998-04-20 | 2005-12-08 | Glycart Biotechnology Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US9321843B2 (en) | 1998-04-20 | 2016-04-26 | Roche Glycart Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US7906329B2 (en) | 1998-04-20 | 2011-03-15 | Glycart Biotechnology Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US9068005B2 (en) | 1998-04-20 | 2015-06-30 | Roche Glycart Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US9139654B2 (en) | 1998-04-20 | 2015-09-22 | Roche GlyeArt AG | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US9718885B2 (en) | 1998-04-20 | 2017-08-01 | Roche Glycart Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US8623644B2 (en) | 1998-04-20 | 2014-01-07 | Roche Glycart Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| EP1071700B1 (en) * | 1998-04-20 | 2010-02-17 | GlycArt Biotechnology AG | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US8629248B2 (en) | 1998-04-20 | 2014-01-14 | Roche Glycart Ag | Glycosylation engineering of antibodies for improving antibody-dependent cellular cytotoxicity |
| US20110142825A1 (en) * | 1998-04-20 | 2011-06-16 | Roche Glycart Ag | Glycosylation Engineering of Antibodies for Improving Antibody-Dependent Cellular Cytotoxicity |
| US8999324B2 (en) | 1998-04-20 | 2015-04-07 | Roche Glycart Ag | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity |
| US8173141B2 (en) | 1999-02-17 | 2012-05-08 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US7776343B1 (en) | 1999-02-17 | 2010-08-17 | Csl Limited | Immunogenic complexes and methods relating thereto |
| US20100016555A1 (en) * | 2000-06-28 | 2010-01-21 | Glycofi, Inc. | N-Acetylglucosaminyltransferase III Expression in Lower Eukaryotes |
| US8067551B2 (en) | 2000-06-28 | 2011-11-29 | Glycofi, Inc. | Combinatorial DNA library for producing modified N-glycans in lower eukaryotes |
| US8697394B2 (en) | 2000-06-28 | 2014-04-15 | Glycofi, Inc. | Production of modified glycoproteins having multiple antennary structures |
| US7981660B2 (en) | 2000-06-28 | 2011-07-19 | Glycofi, Inc. | Methods for producing modified glycoproteins |
| US7598055B2 (en) | 2000-06-28 | 2009-10-06 | Glycofi, Inc. | N-acetylglucosaminyltransferase III expression in lower eukaryotes |
| US20100016561A1 (en) * | 2000-06-28 | 2010-01-21 | Glycofi, Inc. | N-Acetylglucosaminyltransferase III Expression in Lower Eukaryotes |
| US20100021991A1 (en) * | 2000-06-28 | 2010-01-28 | Glycofi, Inc. | Methods for Producing Modified Glycoproteins |
| US7935513B2 (en) | 2000-06-28 | 2011-05-03 | Glycofi, Inc. | Combinatorial DNA library for producing modified N-glycans in lower eukaryotes |
| US7923430B2 (en) | 2000-06-28 | 2011-04-12 | Glycofi, Inc. | Methods for producing modified glycoproteins |
| US20080274498A1 (en) * | 2000-06-28 | 2008-11-06 | Glycofi, Inc. | Methods for producing modified glycoproteins |
| US20060177898A1 (en) * | 2000-06-28 | 2006-08-10 | Glycofi, Inc. | Methods for producing modified glycoproteins |
| US8211691B2 (en) | 2000-06-28 | 2012-07-03 | Glycofi, Inc. | Methods for producing modified glycoproteins |
| US20090209024A1 (en) * | 2000-06-28 | 2009-08-20 | Gerngross Tillman U | Combinatorial DNA library for producing modified N-glycans in lower eukaryotes |
| US8445227B2 (en) | 2000-06-28 | 2013-05-21 | Merck Sharp & Dohme | N-acetylglucosaminyltransferase III expression in lower eukaryotes |
| US20050208617A1 (en) * | 2000-06-28 | 2005-09-22 | Piotr Bobrowicz | N-acetylglucosamintransferase III expression in lower eukaryotes |
| US8877462B2 (en) | 2000-06-28 | 2014-11-04 | Glycofi, Inc. | Combinatorial DNA library for producing modified N-glycans in lower eukaryotes |
| US20070037248A1 (en) * | 2000-06-28 | 2007-02-15 | Piotr Bobrowicz | Production of modified glycoproteins having multiple antennary structures |
| US8883483B2 (en) | 2000-06-28 | 2014-11-11 | Glycofi, Inc. | Combinatorial DNA library for producing modified N-glycans in lower eukaryotes |
| US20030003097A1 (en) * | 2001-04-02 | 2003-01-02 | Idec Pharmaceutical Corporation | Recombinant antibodies coexpressed with GnTIII |
| US20110064749A1 (en) * | 2001-12-21 | 2011-03-17 | Csl Limited | Compositions comprising immunoreactive reagents and saponins, and methods of use thereof |
| US8808692B2 (en) | 2001-12-21 | 2014-08-19 | Csl Limited | Compositions comprising immunoreactive reagents and saponins, and methods of use thereof |
| US20060210555A1 (en) * | 2001-12-21 | 2006-09-21 | Antigenics, Inc. | Compositions comprising immunoreactive reagents and saponins, and methods of use thereof |
| US20080181890A1 (en) * | 2002-03-01 | 2008-07-31 | Xencor, Inc. | Optimized Fc Variants and Methods for Their Generation |
| US20070224189A1 (en) * | 2002-03-01 | 2007-09-27 | Xencor, Inc. | CD20 OPTIMIZED Fc VARIANTS AND METHODS FOR THEIR GENERATION |
| US20080260731A1 (en) * | 2002-03-01 | 2008-10-23 | Bernett Matthew J | Optimized antibodies that target cd19 |
| US20080254027A1 (en) * | 2002-03-01 | 2008-10-16 | Bernett Matthew J | Optimized CD5 antibodies and methods of using the same |
| US8124731B2 (en) | 2002-03-01 | 2012-02-28 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US7662925B2 (en) | 2002-03-01 | 2010-02-16 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US20070003546A1 (en) * | 2002-03-01 | 2007-01-04 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8093357B2 (en) | 2002-03-01 | 2012-01-10 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8734791B2 (en) | 2002-03-01 | 2014-05-27 | Xencor, Inc. | Optimized fc variants and methods for their generation |
| US20090068175A1 (en) * | 2002-03-01 | 2009-03-12 | Xencor, Inc. | Optimized FC Variants and Methods for Their Generation |
| US20070219133A1 (en) * | 2002-03-01 | 2007-09-20 | Xencor, Inc. | CD52 OPTIMIZED Fc VARIANTS AND METHODS FOR THEIR GENERATION |
| US20090142340A1 (en) * | 2002-03-01 | 2009-06-04 | Xencor, Inc. | Optimized Fc Variants and Methods for Their Generation |
| US7317091B2 (en) | 2002-03-01 | 2008-01-08 | Xencor, Inc. | Optimized Fc variants |
| US8753628B2 (en) | 2002-09-27 | 2014-06-17 | Xencor, Inc. | Optimized Fc variants |
| US8802823B2 (en) | 2002-09-27 | 2014-08-12 | Xencor, Inc. | Optimized Fc variants |
| US10183999B2 (en) | 2002-09-27 | 2019-01-22 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US9193798B2 (en) | 2002-09-27 | 2015-11-24 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8188231B2 (en) | 2002-09-27 | 2012-05-29 | Xencor, Inc. | Optimized FC variants |
| US20090081208A1 (en) * | 2002-09-27 | 2009-03-26 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US20040132101A1 (en) * | 2002-09-27 | 2004-07-08 | Xencor | Optimized Fc variants and methods for their generation |
| US20070148171A1 (en) * | 2002-09-27 | 2007-06-28 | Xencor, Inc. | Optimized anti-CD30 antibodies |
| US8383109B2 (en) | 2002-09-27 | 2013-02-26 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8753629B2 (en) | 2002-09-27 | 2014-06-17 | Xencor, Inc. | Optimized Fc variants |
| US8039592B2 (en) | 2002-09-27 | 2011-10-18 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8093359B2 (en) | 2002-09-27 | 2012-01-10 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8735547B2 (en) | 2002-09-27 | 2014-05-27 | Xencor, Inc. | Optimized Fc Variants |
| US10184000B2 (en) | 2002-09-27 | 2019-01-22 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US9353187B2 (en) | 2002-09-27 | 2016-05-31 | Xencor, Inc. | Optimized FC variants and methods for their generation |
| US20060235208A1 (en) * | 2002-09-27 | 2006-10-19 | Xencor, Inc. | Fc variants with optimized properties |
| US20090092599A1 (en) * | 2002-09-27 | 2009-04-09 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8809503B2 (en) | 2002-09-27 | 2014-08-19 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US8858937B2 (en) | 2002-09-27 | 2014-10-14 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US20090263318A1 (en) * | 2003-01-24 | 2009-10-22 | Agensys, Inc. | Nucleic acids and corresponding proteins entitled 254p1d6b useful in treatment and detection of cancer |
| US8460881B2 (en) * | 2003-01-24 | 2013-06-11 | Agensys, Inc. | Nucleic acids and corresponding proteins entitled 254P1D6B useful in treatment and detection of cancer |
| US8986949B2 (en) | 2003-02-20 | 2015-03-24 | Glycofi, Inc. | Endomannosidases in the modification of glycoproteins in eukaryotes |
| US20110021755A1 (en) * | 2003-03-03 | 2011-01-27 | Xencor, Inc. | Optimized Fc Variants |
| US20070237766A1 (en) * | 2003-03-03 | 2007-10-11 | Xencor, Inc. | Fc Variants Having Increased Affinity for FcyRllla |
| US9663582B2 (en) | 2003-03-03 | 2017-05-30 | Xencor, Inc. | Optimized Fc variants |
| US9657106B2 (en) | 2003-03-03 | 2017-05-23 | Xencor, Inc. | Optimized Fc variants |
| US20080057056A1 (en) * | 2003-03-03 | 2008-03-06 | Xencor, Inc. | Fc Variants with Increased Affinity for FcyRIIC |
| US20070275460A1 (en) * | 2003-03-03 | 2007-11-29 | Xencor.Inc. | Fc Variants With Optimized Fc Receptor Binding Properties |
| US8388955B2 (en) | 2003-03-03 | 2013-03-05 | Xencor, Inc. | Fc variants |
| US10584176B2 (en) | 2003-03-03 | 2020-03-10 | Xencor, Inc. | Fc variants with increased affinity for FcγRIIc |
| US8084582B2 (en) | 2003-03-03 | 2011-12-27 | Xencor, Inc. | Optimized anti-CD20 monoclonal antibodies having Fc variants |
| US20070248602A1 (en) * | 2003-03-03 | 2007-10-25 | Xencor, Inc. | Fc Variants Having Increased Affinity for FcyRllc |
| US20070248603A1 (en) * | 2003-03-03 | 2007-10-25 | Xencor, Inc. | Fc Variants with Increased Affinity for FcyRlla |
| US20070243188A1 (en) * | 2003-03-03 | 2007-10-18 | Xencor, Inc. | Fc Variants Having Decreased Affinity for FcyRlla |
| US8735545B2 (en) | 2003-03-03 | 2014-05-27 | Xencor, Inc. | Fc variants having increased affinity for fcyrllc |
| US20070237765A1 (en) * | 2003-03-03 | 2007-10-11 | Xencor, Inc. | Fc Variants Having Increased Affinity for FcyRl |
| US20070238665A1 (en) * | 2003-03-03 | 2007-10-11 | Xencor, Inc. | Fc Variants Having Decreased Affinity for FcyRIIc |
| US10113001B2 (en) | 2003-03-03 | 2018-10-30 | Xencor, Inc. | Fc variants with increased affinity for FcyRIIc |
| US20070237767A1 (en) * | 2003-03-03 | 2007-10-11 | Xencor, Inc. | Fc Variants Having Decreased Affinity for FcyRllla |
| US9051373B2 (en) | 2003-05-02 | 2015-06-09 | Xencor, Inc. | Optimized Fc variants |
| US9714282B2 (en) | 2003-09-26 | 2017-07-25 | Xencor, Inc. | Optimized Fc variants and methods for their generation |
| US9296820B2 (en) | 2003-11-05 | 2016-03-29 | Roche Glycart Ag | Polynucleotides encoding anti-CD20 antigen binding molecules with increased Fc receptor binding affinity and effector function |
| US8883980B2 (en) | 2003-11-05 | 2014-11-11 | Roche Glycart Ag | Antigen binding molecules with increased Fc receptor binding affinity and effector function |
| EA036531B1 (en) * | 2003-11-05 | 2020-11-19 | Роше Гликарт Аг | Type ii anti-cd20 humanized antibody (variants), pharmaceutical composition comprising these antibody variants, and use thereof |
| US20050249723A1 (en) * | 2003-12-22 | 2005-11-10 | Xencor, Inc. | Fc polypeptides with novel Fc ligand binding sites |
| US20100093979A1 (en) * | 2003-12-22 | 2010-04-15 | Gregory Alan Lazar | Fc Polypeptides With Novel Fc Ligand Binding Sites |
| US20050244403A1 (en) * | 2004-03-24 | 2005-11-03 | Xencor, Inc. | Immunoglobulin variants outside the Fc region |
| US7276585B2 (en) | 2004-03-24 | 2007-10-02 | Xencor, Inc. | Immunoglobulin variants outside the Fc region |
| US20110064727A9 (en) * | 2004-03-24 | 2011-03-17 | Xencor, Inc. | Immunoglobulin Variants Outside the Fc Region |
| US20080248028A1 (en) * | 2004-03-24 | 2008-10-09 | Xencor, Inc. | Immunoglobulin Variants Outside the Fc Region |
| US20070031331A1 (en) * | 2004-04-16 | 2007-02-08 | Genentech, Inc. | Treatment of Disorders |
| US20060002930A1 (en) * | 2004-04-16 | 2006-01-05 | Genentech, Inc. | Treatment of disorders |
| US20100248359A1 (en) * | 2004-07-09 | 2010-09-30 | Chugai Seiyaku Kabushiki Kaisha | Anti-Glypican 3 Antibody |
| US11820830B2 (en) | 2004-07-20 | 2023-11-21 | Xencor, Inc. | Optimized Fc variants |
| US20060074225A1 (en) * | 2004-09-14 | 2006-04-06 | Xencor, Inc. | Monomeric immunoglobulin Fc domains |
| US8101720B2 (en) | 2004-10-21 | 2012-01-24 | Xencor, Inc. | Immunoglobulin insertions, deletions and substitutions |
| US20110033452A1 (en) * | 2004-10-26 | 2011-02-10 | Chugai Seiyaku Kabushiki Kaisha | Anti-Glypican 3 Antibody Having Modified Sugar Chain |
| TWI468514B (en) * | 2004-10-26 | 2015-01-11 | Chugai Pharmaceutical Co Ltd | Sugar chain modified phosphatidylinositol glyphosyl 3 antibody |
| US9803023B2 (en) | 2004-11-12 | 2017-10-31 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8338574B2 (en) | 2004-11-12 | 2012-12-25 | Xencor, Inc. | FC variants with altered binding to FCRN |
| US11198739B2 (en) | 2004-11-12 | 2021-12-14 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US10336818B2 (en) | 2004-11-12 | 2019-07-02 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8883973B2 (en) | 2004-11-12 | 2014-11-11 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US9200079B2 (en) | 2004-11-12 | 2015-12-01 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8852586B2 (en) | 2004-11-12 | 2014-10-07 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US20060173170A1 (en) * | 2004-11-12 | 2006-08-03 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8802820B2 (en) | 2004-11-12 | 2014-08-12 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US12215165B2 (en) | 2004-11-12 | 2025-02-04 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8367805B2 (en) | 2004-11-12 | 2013-02-05 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8546543B2 (en) | 2004-11-12 | 2013-10-01 | Xencor, Inc. | Fc variants that extend antibody half-life |
| US8324351B2 (en) | 2004-11-12 | 2012-12-04 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8318907B2 (en) | 2004-11-12 | 2012-11-27 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US20060275282A1 (en) * | 2005-01-12 | 2006-12-07 | Xencor, Inc. | Antibodies and Fc fusion proteins with altered immunogenicity |
| US7846432B2 (en) | 2005-02-07 | 2010-12-07 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20080279858A9 (en) * | 2005-02-07 | 2008-11-13 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US8614065B2 (en) | 2005-02-07 | 2013-12-24 | Roche Glycart Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| EP2402374A1 (en) | 2005-02-07 | 2012-01-04 | GlycArt Biotechnology AG | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US9309317B2 (en) | 2005-02-07 | 2016-04-12 | Roche Glycart Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US8097436B2 (en) | 2005-02-07 | 2012-01-17 | Roche Glycart Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20060269545A1 (en) * | 2005-02-07 | 2006-11-30 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US9957326B2 (en) | 2005-02-07 | 2018-05-01 | Roche Glycart Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| EP3660049A1 (en) | 2005-02-07 | 2020-06-03 | Roche Glycart AG | Antigen binding molecules that bind egfr, vectors encoding same, and uses thereof |
| EP2404937A1 (en) | 2005-02-07 | 2012-01-11 | GlycArt Biotechnology AG | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US7722867B2 (en) | 2005-02-07 | 2010-05-25 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US8491898B2 (en) * | 2005-02-18 | 2013-07-23 | Medarex, L.L.C. | Monoclonal antibodies against CD30 lacking in fucosyl residues |
| US20120251533A1 (en) * | 2005-02-18 | 2012-10-04 | Medarex, Inc. | Monoclonal antibodies against cd30 lacking in fucosyl residues |
| US20060223096A1 (en) * | 2005-03-25 | 2006-10-05 | Glycart Biotechnology Ag | Antigen binding molecules directed to MCSP and having increased Fc receptor binding affinity and effector function |
| US20070111281A1 (en) * | 2005-05-09 | 2007-05-17 | Glycart Biotechnology Ag | Antigen binding molecules having modified Fc regions and altered binding to Fc receptors |
| US20090208500A1 (en) * | 2005-06-03 | 2009-08-20 | Genentech, Inc. | Method of producing antibodies with improved function |
| US20070071745A1 (en) * | 2005-08-26 | 2007-03-29 | Pablo Umana | Modified antigen binding molecules with altered cell signaling activity |
| US20080206867A1 (en) * | 2005-10-03 | 2008-08-28 | Desjarlais John R | Fc variants with optimized Fc receptor binding properties |
| US9040041B2 (en) | 2005-10-03 | 2015-05-26 | Xencor, Inc. | Modified FC molecules |
| US20100249382A1 (en) * | 2005-10-03 | 2010-09-30 | Xencor, Inc. | MODIFIED Fc MOLECULES |
| US20080267976A1 (en) * | 2005-10-06 | 2008-10-30 | Gregory Alan Lazar | Optimized Anti-Cd30 Antibodies |
| US7973136B2 (en) | 2005-10-06 | 2011-07-05 | Xencor, Inc. | Optimized anti-CD30 antibodies |
| US9574006B2 (en) | 2005-10-06 | 2017-02-21 | Xencor, Inc. | Optimized anti-CD30 antibodies |
| US10118959B2 (en) | 2005-10-14 | 2018-11-06 | Chugai Seiyaku Kabushiki Kaisha | Anti-glypican-3 antibody |
| US9102739B2 (en) | 2005-10-14 | 2015-08-11 | Chugai Seiyaku Kabushiki Kaisha | Anti-glypican-3 antibody |
| US20080267979A1 (en) * | 2005-10-14 | 2008-10-30 | Gregory Alan Lazar | Anti-Glypican-3 Antibody |
| US20070087005A1 (en) * | 2005-10-14 | 2007-04-19 | Lazar Gregory A | Anti-glypican-3 antibody |
| US9074008B2 (en) | 2006-08-09 | 2015-07-07 | Roche Glycart Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20080095770A1 (en) * | 2006-08-09 | 2008-04-24 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US8088380B2 (en) | 2006-08-09 | 2012-01-03 | Roche Glycart Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US8273328B2 (en) | 2006-08-09 | 2012-09-25 | Roche Glycart Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20090232817A9 (en) * | 2006-08-09 | 2009-09-17 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US7662377B2 (en) | 2006-08-09 | 2010-02-16 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20080286277A1 (en) * | 2006-08-09 | 2008-11-20 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US7727741B2 (en) | 2006-08-09 | 2010-06-01 | Glycart Biotechnology Ag | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| EP2444422A2 (en) | 2006-08-09 | 2012-04-25 | GlycArt Biotechnology AG | Antigen binding molecules that bind EGFR, vectors encoding same, and uses thereof |
| US20100233080A1 (en) * | 2006-08-09 | 2010-09-16 | Umana Pablo | Antigen Binding Molecules that Bind EGFR, Vectors Encoding Same, and Uses Thereof |
| US8524867B2 (en) | 2006-08-14 | 2013-09-03 | Xencor, Inc. | Optimized antibodies that target CD19 |
| US20100272723A1 (en) * | 2006-08-14 | 2010-10-28 | Xencor, Inc. | Optimized Antibodies that Target CD19 |
| US10626182B2 (en) | 2006-08-14 | 2020-04-21 | Xencor, Inc. | Optimized antibodies that target CD19 |
| US11618788B2 (en) | 2006-08-14 | 2023-04-04 | Xencor, Inc. | Optimized antibodies that target CD19 |
| US9803020B2 (en) | 2006-08-14 | 2017-10-31 | Xencor, Inc. | Optimized antibodies that target CD19 |
| US8911964B2 (en) | 2006-09-13 | 2014-12-16 | Abbvie Inc. | Fed-batch method of making human anti-TNF-alpha antibody |
| US8906646B2 (en) | 2006-09-13 | 2014-12-09 | Abbvie Inc. | Fed-batch method of making human anti-TNF-alpha antibody |
| US9284371B2 (en) | 2006-09-13 | 2016-03-15 | Abbvie Inc. | Methods of producing adalimumab |
| US9073988B2 (en) | 2006-09-13 | 2015-07-07 | Abbvie Inc. | Fed batch method of making anti-TNF-alpha antibodies |
| US9090867B2 (en) | 2006-09-13 | 2015-07-28 | Abbvie Inc. | Fed-batch method of making anti-TNF-alpha antibody |
| US10119118B2 (en) | 2006-09-13 | 2018-11-06 | Abbvie Inc. | Modified serum-free cell culture medium |
| US9234032B2 (en) | 2006-09-13 | 2016-01-12 | Abbvie Inc. | Fed-batch methods for producing adalimumab |
| US9040042B2 (en) | 2006-09-18 | 2015-05-26 | Xencor, Inc. | Optimized antibodies that target HM1.24 |
| US20100104557A1 (en) * | 2006-09-18 | 2010-04-29 | Xencor, Inc. | Optimized Antibodies that Target HM1.24 |
| US8394374B2 (en) | 2006-09-18 | 2013-03-12 | Xencor, Inc. | Optimized antibodies that target HM1.24 |
| US7923011B2 (en) | 2006-10-12 | 2011-04-12 | Genentech, Inc. | Antibodies to lymphotoxin-alpha |
| US8642740B2 (en) | 2006-10-12 | 2014-02-04 | Genentech, Inc. | Antibodies to lymphotoxin-alpha |
| US8216807B2 (en) | 2006-10-12 | 2012-07-10 | Genentech, Inc. | Antibodies to lymphotoxin-α |
| US8541552B2 (en) | 2006-10-12 | 2013-09-24 | Genetech, Inc. | Antibodies to lymphotoxin-α |
| US20110208673A1 (en) * | 2006-10-12 | 2011-08-25 | Genentech, Inc. | Antibodies to lymphotoxin-alpha |
| US20110150865A1 (en) * | 2006-10-12 | 2011-06-23 | Genentech, Inc. | Antibodies to lymphotoxin-alpha |
| US20100143368A1 (en) * | 2006-12-01 | 2010-06-10 | David John King | Human Antibodies That Bind Cd22 And Uses Thereof |
| WO2008070569A3 (en) * | 2006-12-01 | 2008-11-20 | Medarex Inc | Human antibodies that bind cd22 and uses thereof |
| US8481683B2 (en) | 2006-12-01 | 2013-07-09 | Medarex, Inc. | Human antibodies that bind CD22 and uses thereof |
| US9499632B2 (en) | 2006-12-01 | 2016-11-22 | E.R. Squibb & Sons, L.L.C. | Human antibodies that bind CD22 and uses thereof |
| US20100028951A1 (en) * | 2007-03-07 | 2010-02-04 | Stephen Hamilton | Production of glycoproteins with modified fucosylation |
| US10317393B2 (en) | 2007-03-23 | 2019-06-11 | Academia Sinica | Alkynyl sugar analogs for labeling and visualization of glycoconjugates in cells |
| US9816981B2 (en) | 2007-03-23 | 2017-11-14 | Academia Sinica | Alkynyl sugar analogs for labeling and visualization of glycoconjugates in cells |
| US20080313379A1 (en) * | 2007-06-15 | 2008-12-18 | United Memories, Inc. | Multiple bus charge sharing |
| EP4245766A2 (en) | 2007-07-09 | 2023-09-20 | Genentech, Inc. | Prevention of disulfide bond reduction during recombinant production of polypeptides |
| EP4335863A2 (en) | 2007-07-09 | 2024-03-13 | Genentech, Inc. | Prevention of disulfide bond reduction during recombinant production of polypeptides |
| EP4365189A2 (en) | 2007-07-09 | 2024-05-08 | Genentech, Inc. | Prevention of disulfide bond reduction during recombinant production of polypeptides |
| EP3327026A1 (en) | 2007-07-09 | 2018-05-30 | Genentech, Inc. | Prevention of disulfide bond reduction during recombinant production of polypeptides |
| EP2586788A1 (en) | 2007-07-09 | 2013-05-01 | Genentech, Inc. | Prevention of disulfide bond reduction during recombinant production of polypeptides |
| EP4219522A2 (en) | 2007-07-09 | 2023-08-02 | Genentech, Inc. | Prevention of disulfide bond reduction during recombinant production of polypeptides |
| US11932685B2 (en) | 2007-10-31 | 2024-03-19 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US20090169550A1 (en) * | 2007-12-21 | 2009-07-02 | Genentech, Inc. | Therapy of rituximab-refractory rheumatoid arthritis patients |
| US12492253B1 (en) | 2008-02-25 | 2025-12-09 | Xencor, Inc. | Anti-human C5 antibodies |
| US20090269339A1 (en) * | 2008-04-29 | 2009-10-29 | Genentech, Inc. | Responses to immunizations in rheumatoid arthritis patients treated with a cd20 antibody |
| US10274488B2 (en) | 2008-07-15 | 2019-04-30 | Academia Sinica | Glycan arrays on PTFE-like aluminum coated glass slides and related methods |
| US9683047B2 (en) | 2008-09-16 | 2017-06-20 | Genentech, Inc. | Methods for treating progressive multiple sclerosis |
| US9994642B2 (en) | 2008-09-16 | 2018-06-12 | Genentech, Inc. | Methods for treating progressive multiple sclerosis |
| EP3095463A2 (en) | 2008-09-16 | 2016-11-23 | F. Hoffmann-La Roche AG | Methods for treating progressive multiple sclerosis |
| EP3747464A1 (en) | 2008-09-16 | 2020-12-09 | F. Hoffmann-La Roche AG | Methods for treating progessive multiple sclerosis using an anti-cd20 antibody |
| EP4364800A2 (en) | 2008-09-16 | 2024-05-08 | F. Hoffmann-La Roche AG | Methods for treating progressive multiple sclerosis |
| US20100135987A1 (en) * | 2008-10-20 | 2010-06-03 | Hickman Robert K | Isolation and purification of antibodies using protein a affinity chromatography |
| US9018361B2 (en) | 2008-10-20 | 2015-04-28 | Abbvie Inc. | Isolation and purification of antibodies using protein a affinity chromatography |
| US8895709B2 (en) | 2008-10-20 | 2014-11-25 | Abbvie Inc. | Isolation and purification of antibodies using protein A affinity chromatography |
| US9109010B2 (en) | 2008-10-20 | 2015-08-18 | Abbvie Inc. | Viral inactivation during purification of antibodies cross reference to related applications |
| WO2010075249A2 (en) | 2008-12-22 | 2010-07-01 | Genentech, Inc. | A method for treating rheumatoid arthritis with b-cell antagonists |
| WO2010146059A2 (en) | 2009-06-16 | 2010-12-23 | F. Hoffmann-La Roche Ag | Biomarkers for igf-1r inhibitor therapy |
| EP3760712A1 (en) | 2009-08-11 | 2021-01-06 | F. Hoffmann-La Roche AG | Production of proteins in glutamine-free cell culture media |
| WO2011019619A1 (en) | 2009-08-11 | 2011-02-17 | Genentech, Inc. | Production of proteins in glutamine-free cell culture media |
| US9068008B2 (en) | 2009-08-31 | 2015-06-30 | Roche Glycart Ag | Antibodies to carcinoembryonic antigen (CEA), methods of making same, and uses thereof |
| US20110104148A1 (en) * | 2009-08-31 | 2011-05-05 | Roche Glycart Ag | Antibodies to Carcinoembryonic Antigen (CEA), Methods of Making Same, and Uses Thereof |
| WO2011023787A1 (en) | 2009-08-31 | 2011-03-03 | Roche Glycart Ag | Affinity-matured humanized anti cea monoclonal antibodies |
| US11401348B2 (en) | 2009-09-02 | 2022-08-02 | Xencor, Inc. | Heterodimeric Fc variants |
| US10087236B2 (en) | 2009-12-02 | 2018-10-02 | Academia Sinica | Methods for modifying human antibodies by glycan engineering |
| US11267870B2 (en) | 2009-12-02 | 2022-03-08 | Academia Sinica | Methods for modifying human antibodies by glycan engineering |
| US11377485B2 (en) | 2009-12-02 | 2022-07-05 | Academia Sinica | Methods for modifying human antibodies by glycan engineering |
| US9475881B2 (en) | 2010-01-19 | 2016-10-25 | Xencor, Inc. | Antibody variants with enhanced complement activity |
| WO2011100403A1 (en) | 2010-02-10 | 2011-08-18 | Immunogen, Inc | Cd20 antibodies and uses thereof |
| WO2011101328A2 (en) | 2010-02-18 | 2011-08-25 | Roche Glycart Ag | Treatment with a humanized igg class anti egfr antibody and an antibody against insulin like growth factor 1 receptor |
| US10338069B2 (en) | 2010-04-12 | 2019-07-02 | Academia Sinica | Glycan arrays for high throughput screening of viruses |
| US9874562B2 (en) | 2010-05-10 | 2018-01-23 | Academia Sinica | Zanamivir phosphonate congeners with anti-influenza activity and determining oseltamivir susceptibility of influenza viruses |
| US9403855B2 (en) | 2010-05-10 | 2016-08-02 | Academia Sinica | Zanamivir phosphonate congeners with anti-influenza activity and determining oseltamivir susceptibility of influenza viruses |
| EP3333194A1 (en) | 2010-08-13 | 2018-06-13 | Roche Glycart AG | Anti-fap antibodies and methods of use |
| WO2012020038A1 (en) | 2010-08-13 | 2012-02-16 | Roche Glycart Ag | Anti-tenascin-c a2 antibodies and methods of use |
| WO2012020006A2 (en) | 2010-08-13 | 2012-02-16 | Roche Glycart Ag | Anti-fap antibodies and methods of use |
| US9915667B2 (en) * | 2011-01-27 | 2018-03-13 | Medizinische Hochschule Hannover | Methods and means for diagnosing vasculitis |
| US20130310273A1 (en) * | 2011-01-27 | 2013-11-21 | Torsten Witte | Methods and Means for Diagnosing Vasculitis |
| US10184009B2 (en) | 2011-02-10 | 2019-01-22 | Roche Glycart Ag | Mutant interleukin-2 polypeptides |
| US10323098B2 (en) | 2011-02-10 | 2019-06-18 | Roche Glycart Ag | Mutant interleukin-2 polypeptides |
| WO2012107416A2 (en) | 2011-02-10 | 2012-08-16 | Roche Glycart Ag | Improved immunotherapy |
| US9266938B2 (en) | 2011-02-10 | 2016-02-23 | Roche Glycart Ag | Mutant interleukin-2 polypeptides |
| US11111312B2 (en) | 2011-02-10 | 2021-09-07 | Roche Glycart Ag | Mutant interleukin-2 polypeptides |
| US8642742B2 (en) | 2011-03-02 | 2014-02-04 | Roche Glycart Ag | Anti-CEA antibodies |
| US9206260B2 (en) | 2011-03-02 | 2015-12-08 | Roche Glycart Ag | Anti-CEA antibodies |
| US9062106B2 (en) | 2011-04-27 | 2015-06-23 | Abbvie Inc. | Methods for controlling the galactosylation profile of recombinantly-expressed proteins |
| US9090688B2 (en) | 2011-04-27 | 2015-07-28 | Abbvie Inc. | Methods for controlling the galactosylation profile of recombinantly-expressed proteins |
| US9365645B1 (en) | 2011-04-27 | 2016-06-14 | Abbvie, Inc. | Methods for controlling the galactosylation profile of recombinantly-expressed proteins |
| US9505834B2 (en) | 2011-04-27 | 2016-11-29 | Abbvie Inc. | Methods for controlling the galactosylation profile of recombinantly-expressed proteins |
| US9255143B2 (en) | 2011-04-27 | 2016-02-09 | Abbvie Inc. | Methods for controlling the galactosylation profile of recombinantly-expressed proteins |
| US10316104B2 (en) | 2011-04-29 | 2019-06-11 | Roche Glycart Ag | Immunoconjugates |
| US9447159B2 (en) | 2011-04-29 | 2016-09-20 | Roche Glycart Ag | Immunoconjugates |
| US11130822B2 (en) | 2011-04-29 | 2021-09-28 | Roche Glycart Ag | Immunoconjugates |
| WO2012146628A1 (en) | 2011-04-29 | 2012-11-01 | Roche Glycart Ag | Novel immunoconjugates |
| US10202464B2 (en) | 2011-04-29 | 2019-02-12 | Roche Glycart Ag | Immunoconjugates |
| WO2013026832A1 (en) | 2011-08-23 | 2013-02-28 | Roche Glycart Ag | Anti-mcsp antibodies |
| WO2013026831A1 (en) | 2011-08-23 | 2013-02-28 | Roche Glycart Ag | Bispecific antigen binding molecules |
| WO2013113641A1 (en) | 2012-01-31 | 2013-08-08 | Roche Glycart Ag | Use of nkp46 as a predictive biomarker for cancer treatment with adcc- enhanced antibodies |
| WO2013127465A1 (en) | 2012-03-02 | 2013-09-06 | Roche Glycart Ag | Predicitive biomarker for cancer treatment with adcc enhanced antibodies |
| US10130714B2 (en) | 2012-04-14 | 2018-11-20 | Academia Sinica | Enhanced anti-influenza agents conjugated with anti-inflammatory activity |
| US9346879B2 (en) | 2012-04-20 | 2016-05-24 | Abbvie Inc. | Protein purification methods to reduce acidic species |
| US9334319B2 (en) | 2012-04-20 | 2016-05-10 | Abbvie Inc. | Low acidic species compositions |
| US9708400B2 (en) | 2012-04-20 | 2017-07-18 | Abbvie, Inc. | Methods to modulate lysine variant distribution |
| US9957318B2 (en) | 2012-04-20 | 2018-05-01 | Abbvie Inc. | Protein purification methods to reduce acidic species |
| US9193787B2 (en) | 2012-04-20 | 2015-11-24 | Abbvie Inc. | Human antibodies that bind human TNF-alpha and methods of preparing the same |
| US9181572B2 (en) | 2012-04-20 | 2015-11-10 | Abbvie, Inc. | Methods to modulate lysine variant distribution |
| US9150645B2 (en) | 2012-04-20 | 2015-10-06 | Abbvie, Inc. | Cell culture methods to reduce acidic species |
| US9683033B2 (en) | 2012-04-20 | 2017-06-20 | Abbvie, Inc. | Cell culture methods to reduce acidic species |
| US9359434B2 (en) | 2012-04-20 | 2016-06-07 | Abbvie, Inc. | Cell culture methods to reduce acidic species |
| US9505833B2 (en) | 2012-04-20 | 2016-11-29 | Abbvie Inc. | Human antibodies that bind human TNF-alpha and methods of preparing the same |
| US9695454B2 (en) | 2012-05-23 | 2017-07-04 | Glykos Finland Oy | Production of fucosylated glycoproteins |
| US9249182B2 (en) | 2012-05-24 | 2016-02-02 | Abbvie, Inc. | Purification of antibodies using hydrophobic interaction chromatography |
| WO2014023679A1 (en) | 2012-08-07 | 2014-02-13 | Roche Glycart Ag | Composition comprising two antibodies engineered to have reduced and increased effector function |
| EP3434695A1 (en) | 2012-08-07 | 2019-01-30 | Roche Glycart AG | Improved immunotherapy |
| US9914956B2 (en) | 2012-08-18 | 2018-03-13 | Academia Sinica | Cell-permeable probes for identification and imaging of sialidases |
| US10214765B2 (en) | 2012-08-18 | 2019-02-26 | Academia Sinica | Cell-permeable probes for identification and imaging of sialidases |
| US9547009B2 (en) | 2012-08-21 | 2017-01-17 | Academia Sinica | Benzocyclooctyne compounds and uses thereof |
| US9290568B2 (en) | 2012-09-02 | 2016-03-22 | Abbvie, Inc. | Methods to control protein heterogeneity |
| US9234033B2 (en) | 2012-09-02 | 2016-01-12 | Abbvie, Inc. | Methods to control protein heterogeneity |
| US9206390B2 (en) | 2012-09-02 | 2015-12-08 | Abbvie, Inc. | Methods to control protein heterogeneity |
| US9512214B2 (en) | 2012-09-02 | 2016-12-06 | Abbvie, Inc. | Methods to control protein heterogeneity |
| WO2014114595A1 (en) | 2013-01-23 | 2014-07-31 | Roche Glycart Ag | Predictive biomarker for cancer treatment with adcc-enhanced antibodies |
| US10174110B2 (en) | 2013-02-13 | 2019-01-08 | Laboratoire Français Du Fractionnement Et Des Biotechnologies | Highly galactosylated anti-TNF-α antibodies and uses thereof |
| US10034921B2 (en) | 2013-02-13 | 2018-07-31 | Laboratoire Français Du Fractionnement Et Des Biotechnologies | Proteins with modified glycosylation and methods of production thereof |
| WO2014131715A1 (en) | 2013-02-26 | 2014-09-04 | Roche Glycart Ag | Anti-mcsp antibodies |
| US8921526B2 (en) | 2013-03-14 | 2014-12-30 | Abbvie, Inc. | Mutated anti-TNFα antibodies and methods of their use |
| US9067990B2 (en) | 2013-03-14 | 2015-06-30 | Abbvie, Inc. | Protein purification using displacement chromatography |
| US9708399B2 (en) | 2013-03-14 | 2017-07-18 | Abbvie, Inc. | Protein purification using displacement chromatography |
| US9499614B2 (en) | 2013-03-14 | 2016-11-22 | Abbvie Inc. | Methods for modulating protein glycosylation profiles of recombinant protein therapeutics using monosaccharides and oligosaccharides |
| US10086054B2 (en) | 2013-06-26 | 2018-10-02 | Academia Sinica | RM2 antigens and use thereof |
| US9981030B2 (en) | 2013-06-27 | 2018-05-29 | Academia Sinica | Glycan conjugates and use thereof |
| US10918714B2 (en) | 2013-09-06 | 2021-02-16 | Academia Sinica | Human iNKT cell activation using glycolipids with altered glycosyl groups |
| US9782476B2 (en) | 2013-09-06 | 2017-10-10 | Academia Sinica | Human iNKT cell activation using glycolipids with altered glycosyl groups |
| US10111951B2 (en) | 2013-09-06 | 2018-10-30 | Academia Sinica | Human iNKT cell activation using glycolipids with altered glycosyl groups |
| US9598667B2 (en) | 2013-10-04 | 2017-03-21 | Abbvie Inc. | Use of metal ions for modulation of protein glycosylation profiles of recombinant proteins |
| US9499616B2 (en) | 2013-10-18 | 2016-11-22 | Abbvie Inc. | Modulated lysine variant species compositions and methods for producing and using the same |
| US8946395B1 (en) | 2013-10-18 | 2015-02-03 | Abbvie Inc. | Purification of proteins using hydrophobic interaction chromatography |
| US9017687B1 (en) | 2013-10-18 | 2015-04-28 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same using displacement chromatography |
| US9266949B2 (en) | 2013-10-18 | 2016-02-23 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same |
| US9315574B2 (en) | 2013-10-18 | 2016-04-19 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same |
| US9522953B2 (en) | 2013-10-18 | 2016-12-20 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same |
| US9181337B2 (en) | 2013-10-18 | 2015-11-10 | Abbvie, Inc. | Modulated lysine variant species compositions and methods for producing and using the same |
| US9688752B2 (en) | 2013-10-18 | 2017-06-27 | Abbvie Inc. | Low acidic species compositions and methods for producing and using the same using displacement chromatography |
| US9200069B2 (en) | 2013-10-18 | 2015-12-01 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same |
| US9085618B2 (en) | 2013-10-18 | 2015-07-21 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same |
| US9200070B2 (en) | 2013-10-18 | 2015-12-01 | Abbvie, Inc. | Low acidic species compositions and methods for producing and using the same |
| US9550826B2 (en) | 2013-11-15 | 2017-01-24 | Abbvie Inc. | Glycoengineered binding protein compositions |
| US10150818B2 (en) | 2014-01-16 | 2018-12-11 | Academia Sinica | Compositions and methods for treatment and detection of cancers |
| US9982041B2 (en) | 2014-01-16 | 2018-05-29 | Academia Sinica | Compositions and methods for treatment and detection of cancers |
| US9759726B2 (en) | 2014-03-27 | 2017-09-12 | Academia Sinica | Reactive labelling compounds and uses thereof |
| US10119972B2 (en) | 2014-03-27 | 2018-11-06 | Academia Sinica | Reactive labelling compounds and uses thereof |
| US11319567B2 (en) | 2014-05-27 | 2022-05-03 | Academia Sinica | Fucosidase from bacteroides and methods using the same |
| US10618973B2 (en) | 2014-05-27 | 2020-04-14 | Academia Sinica | Anti-HER2 glycoantibodies and uses thereof |
| US10005847B2 (en) | 2014-05-27 | 2018-06-26 | Academia Sinica | Anti-HER2 glycoantibodies and uses thereof |
| US11884739B2 (en) | 2014-05-27 | 2024-01-30 | Academia Sinica | Anti-CD20 glycoantibodies and uses thereof |
| US10118969B2 (en) | 2014-05-27 | 2018-11-06 | Academia Sinica | Compositions and methods relating to universal glycoforms for enhanced antibody efficacy |
| US10023892B2 (en) | 2014-05-27 | 2018-07-17 | Academia Sinica | Compositions and methods relating to universal glycoforms for enhanced antibody efficacy |
| US11332523B2 (en) | 2014-05-28 | 2022-05-17 | Academia Sinica | Anti-TNF-alpha glycoantibodies and uses thereof |
| US10513724B2 (en) | 2014-07-21 | 2019-12-24 | Glykos Finland Oy | Production of glycoproteins with mammalian-like N-glycans in filamentous fungi |
| US10533034B2 (en) | 2014-09-08 | 2020-01-14 | Academia Sinica | Human iNKT cell activation using glycolipids |
| US9879042B2 (en) | 2014-09-08 | 2018-01-30 | Academia Sinica | Human iNKT cell activation using glycolipids |
| US11001643B2 (en) | 2014-09-26 | 2021-05-11 | Chugai Seiyaku Kabushiki Kaisha | Cytotoxicity-inducing therapeutic agent |
| US9975966B2 (en) | 2014-09-26 | 2018-05-22 | Chugai Seiyaku Kabushiki Kaisha | Cytotoxicity-inducing theraputic agent |
| US10495645B2 (en) | 2015-01-16 | 2019-12-03 | Academia Sinica | Cancer markers and methods of use thereof |
| US9975965B2 (en) | 2015-01-16 | 2018-05-22 | Academia Sinica | Compositions and methods for treatment and detection of cancers |
| US10342858B2 (en) | 2015-01-24 | 2019-07-09 | Academia Sinica | Glycan conjugates and methods of use thereof |
| US12247076B2 (en) | 2015-07-06 | 2025-03-11 | Laboratoire Français Du Fractionnement Et Des Biotechnologies | Use of modified Fc fragments in immunotherapy |
| EP3662930A1 (en) | 2015-09-24 | 2020-06-10 | AbVitro LLC | Hiv antibody compositions and methods of use |
| WO2017053906A1 (en) | 2015-09-24 | 2017-03-30 | Abvitro Llc | Hiv antibody compositions and methods of use |
| EP4026848A1 (en) | 2015-12-09 | 2022-07-13 | F. Hoffmann-La Roche AG | Type ii anti-cd20 antibody for reducing the cytokine release syndrome |
| US10525137B2 (en) | 2015-12-30 | 2020-01-07 | Genentech, Inc. | Formulations with reduced degradation of polysorbate |
| US10933141B2 (en) | 2015-12-30 | 2021-03-02 | Genentech, Inc. | Formulations with reduced degradation of polysorbate |
| US10336784B2 (en) | 2016-03-08 | 2019-07-02 | Academia Sinica | Methods for modular synthesis of N-glycans and arrays thereof |
| WO2017194441A1 (en) | 2016-05-11 | 2017-11-16 | F. Hoffmann-La Roche Ag | Modified anti-tenascin antibodies and methods of use |
| EP3252078A1 (en) | 2016-06-02 | 2017-12-06 | F. Hoffmann-La Roche AG | Type ii anti-cd20 antibody and anti-cd20/cd3 bispecific antibody for treatment of cancer |
| US10538592B2 (en) | 2016-08-22 | 2020-01-21 | Cho Pharma, Inc. | Antibodies, binding fragments, and methods of use |
| WO2018201096A1 (en) | 2017-04-27 | 2018-11-01 | Tesaro, Inc. | Antibody agents directed against lymphocyte activation gene-3 (lag-3) and uses thereof |
| WO2018220099A1 (en) | 2017-06-02 | 2018-12-06 | F. Hoffmann-La Roche Ag | Type ii anti-cd20 antibody and anti-cd20/cd3 bispecific antibody for treatment of cancer |
| US12247060B2 (en) | 2018-01-09 | 2025-03-11 | Marengo Therapeutics, Inc. | Calreticulin binding constructs and engineered T cells for the treatment of diseases |
| US12152073B2 (en) | 2018-03-14 | 2024-11-26 | Marengo Therapeutics, Inc. | Multifunctional molecules that bind to calreticulin and uses thereof |
| US11555071B2 (en) | 2018-06-03 | 2023-01-17 | Lamkap Bio Beta Ltd. | Bispecific antibodies against CEACAM5 and CD47 |
| WO2019234576A1 (en) | 2018-06-03 | 2019-12-12 | Lamkap Bio Beta Ltd. | Bispecific antibodies against ceacam5 and cd47 |
| US12286477B2 (en) | 2018-07-03 | 2025-04-29 | Marengo Therapeutics, Inc. | Anti-TCR antibody molecules and uses thereof |
| US12351632B2 (en) | 2018-07-03 | 2025-07-08 | Marengo Therapeutics, Inc. | Anti-TCR antibody molecules and uses thereof |
| US12358982B2 (en) | 2019-02-21 | 2025-07-15 | Marengo Therapeutics, Inc. | Multifunctional molecules that bind to T cell related cancer cells and uses thereof |
| US12384842B2 (en) | 2019-02-21 | 2025-08-12 | Marengo Therapeutics, Inc. | Antibody molecules that bind to NKP30 and uses thereof |
| WO2021110647A1 (en) | 2019-12-02 | 2021-06-10 | Lamkap Bio Beta Ag | Bispecific antibodies against ceacam5 and cd47 |
| EP3831849A1 (en) | 2019-12-02 | 2021-06-09 | LamKap Bio beta AG | Bispecific antibodies against ceacam5 and cd47 |
| US12486326B2 (en) | 2020-01-03 | 2025-12-02 | Marengo Therapeutics, Inc. | Anti-TCR antibody molecules and uses thereof |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9631023B2 (en) | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity | |
| AU2002339845A1 (en) | Antibody glycosylation variants having increased antibody-dependent cellular cytotoxicity | |
| EP2248892B1 (en) | Fusion constructs and use of same to produce antibodies with increased FC receptor binding affinity and effector function | |
| CN1761746B (en) | Fusion constructs and use thereof to produce antibodies with improved Fc receptor binding affinity and effector function |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: GLYCART BIOTECHNOLOGY AG, SWITZERLAND Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:UMANA, PABLO;JEAN-MAIRET, JOEL;BAILEY, JAMES E. (DECEASED)-BY HIS LEGAL REPRESENTATIVE;REEL/FRAME:013893/0119 Effective date: 20030131 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |
|
| AS | Assignment |
Owner name: ROCHE GLYCART AG, SWITZERLAND Free format text: CHANGE OF NAME;ASSIGNOR:GLYCART BIOTECHNOLOGY AG;REEL/FRAME:026883/0831 Effective date: 20100628 |