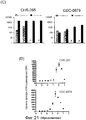
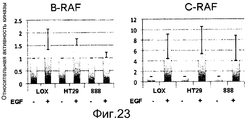
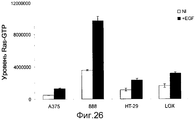
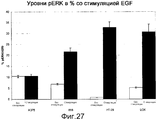
RU2553379C2 - ОПРЕДЕЛЕНИЕ ЧУВСТВИТЕЛЬНОСТИ КЛЕТОК К ОБРАБОТКЕ ИНГИБИТОРОМ B-Raf ПУТЕМ ДЕТЕКЦИИ МУТАЦИИ K-ras И УРОВНЕЙ ЭКСПРЕССИИ RTK - Google Patents
ОПРЕДЕЛЕНИЕ ЧУВСТВИТЕЛЬНОСТИ КЛЕТОК К ОБРАБОТКЕ ИНГИБИТОРОМ B-Raf ПУТЕМ ДЕТЕКЦИИ МУТАЦИИ K-ras И УРОВНЕЙ ЭКСПРЕССИИ RTK Download PDFInfo
- Publication number
- RU2553379C2 RU2553379C2 RU2012111231/15A RU2012111231A RU2553379C2 RU 2553379 C2 RU2553379 C2 RU 2553379C2 RU 2012111231/15 A RU2012111231/15 A RU 2012111231/15A RU 2012111231 A RU2012111231 A RU 2012111231A RU 2553379 C2 RU2553379 C2 RU 2553379C2
- Authority
- RU
- Russia
- Prior art keywords
- ras
- raf
- mutant
- inhibitor
- cancer
- Prior art date
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/517—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with carbocyclic ring systems, e.g. quinazoline, perimidine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57415—Specifically defined cancers of breast
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57419—Specifically defined cancers of colon
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57423—Specifically defined cancers of lung
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/5743—Specifically defined cancers of skin, e.g. melanoma
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57438—Specifically defined cancers of liver, pancreas or kidney
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57449—Specifically defined cancers of ovaries
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57492—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds localized on the membrane of tumor or cancer cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57496—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving intracellular compounds
-
- G01N33/5751—
-
- G01N33/57515—
-
- G01N33/5752—
-
- G01N33/57525—
-
- G01N33/57535—
-
- G01N33/57545—
-
- G01N33/57557—
-
- G01N33/5759—
-
- G01N33/57595—
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/112—Disease subtyping, staging or classification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/118—Prognosis of disease development
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/156—Polymorphic or mutational markers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/91—Transferases (2.)
- G01N2333/912—Transferases (2.) transferring phosphorus containing groups, e.g. kinases (2.7)
- G01N2333/91205—Phosphotransferases in general
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/914—Hydrolases (3)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Immunology (AREA)
- Molecular Biology (AREA)
- Urology & Nephrology (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- General Health & Medical Sciences (AREA)
- Analytical Chemistry (AREA)
- Pathology (AREA)
- Oncology (AREA)
- Cell Biology (AREA)
- Medicinal Chemistry (AREA)
- Hospice & Palliative Care (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Organic Chemistry (AREA)
- Food Science & Technology (AREA)
- General Physics & Mathematics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Zoology (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Gastroenterology & Hepatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US23646609P | 2009-08-24 | 2009-08-24 | |
| US61/236,466 | 2009-08-24 | ||
| US30114910P | 2010-02-03 | 2010-02-03 | |
| US61/301,149 | 2010-02-03 | ||
| PCT/US2010/046520 WO2011028540A1 (en) | 2009-08-24 | 2010-08-24 | Determining sensitivity of cells to b-raf inhibitor treatment by detecting kras mutation and rtk expression levels |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015104058/15A Division RU2015104058A (ru) | 2009-08-24 | 2010-08-24 | ОПРЕДЕЛЕНИЕ ЧУВСТВИТЕЛЬНОСТИ КЛЕТОК К ОБРАБОТКЕ ИНГИБИТОРОМ B-Raf ПУТЕМ ДЕТЕКЦИИ МУТАЦИИ KRAS И УРОВНЕЙ ЭКСПРЕССИИ RTK |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2012111231A RU2012111231A (ru) | 2013-10-10 |
| RU2553379C2 true RU2553379C2 (ru) | 2015-06-10 |
Family
ID=43649583
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2012111231/15A RU2553379C2 (ru) | 2009-08-24 | 2010-08-24 | ОПРЕДЕЛЕНИЕ ЧУВСТВИТЕЛЬНОСТИ КЛЕТОК К ОБРАБОТКЕ ИНГИБИТОРОМ B-Raf ПУТЕМ ДЕТЕКЦИИ МУТАЦИИ K-ras И УРОВНЕЙ ЭКСПРЕССИИ RTK |
| RU2015104058/15A RU2015104058A (ru) | 2009-08-24 | 2010-08-24 | ОПРЕДЕЛЕНИЕ ЧУВСТВИТЕЛЬНОСТИ КЛЕТОК К ОБРАБОТКЕ ИНГИБИТОРОМ B-Raf ПУТЕМ ДЕТЕКЦИИ МУТАЦИИ KRAS И УРОВНЕЙ ЭКСПРЕССИИ RTK |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2015104058/15A RU2015104058A (ru) | 2009-08-24 | 2010-08-24 | ОПРЕДЕЛЕНИЕ ЧУВСТВИТЕЛЬНОСТИ КЛЕТОК К ОБРАБОТКЕ ИНГИБИТОРОМ B-Raf ПУТЕМ ДЕТЕКЦИИ МУТАЦИИ KRAS И УРОВНЕЙ ЭКСПРЕССИИ RTK |
Country Status (15)
| Country | Link |
|---|---|
| US (2) | US20120214828A1 (enExample) |
| EP (1) | EP2470898A4 (enExample) |
| JP (1) | JP2013502236A (enExample) |
| KR (1) | KR20120050493A (enExample) |
| CN (1) | CN102859355B (enExample) |
| AU (1) | AU2010289794B2 (enExample) |
| BR (1) | BR112012003926A2 (enExample) |
| CA (1) | CA2771369A1 (enExample) |
| IL (2) | IL218099A0 (enExample) |
| IN (1) | IN2012DN01403A (enExample) |
| MX (1) | MX338856B (enExample) |
| MY (1) | MY165154A (enExample) |
| RU (2) | RU2553379C2 (enExample) |
| SG (2) | SG10201402917XA (enExample) |
| WO (1) | WO2011028540A1 (enExample) |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2008337286B2 (en) | 2007-12-19 | 2014-08-07 | Cancer Research Technology Limited | Pyrido[2,3-b]pyrazine-8-substituted compounds and their use |
| PH12012501361A1 (en) | 2009-12-31 | 2012-10-22 | Centro Nac De Investigaciones Oncologicas Cnio | Tricyclic compounds for use as kinase inhibitors |
| US8815896B2 (en) | 2010-02-01 | 2014-08-26 | The Institute Of Cancer Research: Royal Cancer Hospital | 1-(5-tert-butyl-2-phenyl-2H-pyrazol-3-yl)-3-[2-fluoro-4-(1-methyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-B]pyridin-7-yloxy)-phenyl]-urea and related compounds and their use in therapy |
| US8709419B2 (en) | 2010-08-17 | 2014-04-29 | Hoffmann-La Roche, Inc. | Combination therapy |
| US9295669B2 (en) | 2010-12-14 | 2016-03-29 | Hoffman La-Roche Inc. | Combination therapy for proliferative disorders |
| WO2012098387A1 (en) | 2011-01-18 | 2012-07-26 | Centro Nacional De Investigaciones Oncológicas (Cnio) | 6, 7-ring-fused triazolo [4, 3 - b] pyridazine derivatives as pim inhibitors |
| RU2014114617A (ru) * | 2011-09-19 | 2015-10-27 | Дженентек, Инк. | Комбинированные виды лечения, содержащие антагонисты с-мет и антагонисты b-raf |
| US9408885B2 (en) | 2011-12-01 | 2016-08-09 | Vib Vzw | Combinations of therapeutic agents for treating melanoma |
| US20150017261A1 (en) * | 2012-01-11 | 2015-01-15 | Duke University | Methods of Treating and Preventing Cancer by Disrupting the Binding of Copper in the Map Kinase Pathway |
| US9216170B2 (en) | 2012-03-19 | 2015-12-22 | Hoffmann-La Roche Inc. | Combination therapy for proliferative disorders |
| RS58734B1 (sr) * | 2012-08-07 | 2019-06-28 | Novartis Ag | Farmaceutske kombinacije koje obuhvataju b-raf inhibitor, egfr inhibitor i opciono pi3k-alfa inhibitor |
| CA2879252C (en) | 2012-08-17 | 2017-10-10 | F. Hoffmann-La Roche Ag | Combination therapies for melanoma comprising administering cobimetinib and vemurafinib |
| CA2889530A1 (en) * | 2012-10-25 | 2014-05-01 | Glaxosmithkline Llc | Combination |
| EP2786764B1 (en) | 2013-04-01 | 2017-03-08 | Samsung Electronics Co., Ltd. | Combination therapy using anti-c-met antibody and sorafenib |
| US9532987B2 (en) | 2013-09-05 | 2017-01-03 | Genentech, Inc. | Use of a combination of a MEK inhibitor and an ERK inhibitor for treatment of hyperproliferative diseases |
| GB201320729D0 (en) | 2013-11-25 | 2014-01-08 | Cancer Rec Tech Ltd | Therapeutic compounds and their use |
| GB201320732D0 (en) | 2013-11-25 | 2014-01-08 | Cancer Rec Tech Ltd | Methods of chemical synthesis |
| EP3143163B1 (en) | 2014-05-13 | 2020-11-25 | Board of Regents, The University of Texas System | Gene mutations and copy number alterations of egfr, kras and met |
| CA2972189A1 (en) * | 2014-12-23 | 2016-06-30 | Millennium Pharmaceuticals, Inc. | Combination of raf inhibitors and taxanes |
| WO2017066664A1 (en) * | 2015-10-16 | 2017-04-20 | Millennium Pharmaceuticals, Inc. | Combination therapy including a raf inhibitor for the treatment of colorectal cancer |
| WO2017187937A1 (ja) * | 2016-04-28 | 2017-11-02 | デンカ株式会社 | 上皮性細胞成長因子受容体阻害剤に対する癌細胞の耐性を判定する方法 |
| JP7341060B2 (ja) | 2017-02-10 | 2023-09-08 | アンスティチュ ナショナル ドゥ ラ サンテ エ ドゥ ラ ルシェルシュ メディカル | Mapk経路の活性化に関連付けられる癌の処置のための方法及び医薬組成物 |
| WO2019133810A1 (en) | 2017-12-28 | 2019-07-04 | Tract Pharmaceuticals, Inc. | Stem cell culture systems for columnar epithelial stem cells, and uses related thereto |
| TW202015719A (zh) | 2018-06-19 | 2020-05-01 | 美商尼恩醫療公司 | 新抗原及其用途 |
| MX2022013430A (es) * | 2020-04-27 | 2022-11-14 | Verastem Inc | Metodos para tratar el crecimiento celular anormal. |
| US11873296B2 (en) | 2022-06-07 | 2024-01-16 | Verastem, Inc. | Solid forms of a dual RAF/MEK inhibitor |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2564127T5 (es) * | 2004-06-04 | 2020-03-26 | Genentech Inc | Mutaciones en EGFR |
| WO2006116423A2 (en) * | 2005-04-26 | 2006-11-02 | Eisai Co., Ltd | Compositions and methods for cancer immunotherapy |
| JP2008546421A (ja) * | 2005-06-28 | 2008-12-25 | ジェネンテック・インコーポレーテッド | Egfrおよびkras変異 |
| JP2009505658A (ja) * | 2005-08-24 | 2009-02-12 | ブリストル−マイヤーズ スクイブ カンパニー | 上皮増殖因子受容体モデュレーターに対する感受性を決定するためのバイオマーカーおよび方法 |
| EP2118322A2 (en) * | 2007-03-13 | 2009-11-18 | Amgen Inc. | K-ras and b-raf mutations and anti-egfr antibody therapy |
-
2010
- 2010-08-24 EP EP10814253A patent/EP2470898A4/en not_active Withdrawn
- 2010-08-24 SG SG10201402917XA patent/SG10201402917XA/en unknown
- 2010-08-24 JP JP2012526924A patent/JP2013502236A/ja active Pending
- 2010-08-24 US US13/392,405 patent/US20120214828A1/en not_active Abandoned
- 2010-08-24 MX MX2012002292A patent/MX338856B/es active IP Right Grant
- 2010-08-24 RU RU2012111231/15A patent/RU2553379C2/ru not_active IP Right Cessation
- 2010-08-24 MY MYPI2012000821A patent/MY165154A/en unknown
- 2010-08-24 CN CN201080048007.0A patent/CN102859355B/zh not_active Expired - Fee Related
- 2010-08-24 CA CA2771369A patent/CA2771369A1/en not_active Abandoned
- 2010-08-24 AU AU2010289794A patent/AU2010289794B2/en not_active Ceased
- 2010-08-24 WO PCT/US2010/046520 patent/WO2011028540A1/en not_active Ceased
- 2010-08-24 SG SG2012012860A patent/SG178866A1/en unknown
- 2010-08-24 KR KR1020127007529A patent/KR20120050493A/ko not_active Ceased
- 2010-08-24 BR BR112012003926-1A patent/BR112012003926A2/pt not_active Application Discontinuation
- 2010-08-24 IN IN1403DEN2012 patent/IN2012DN01403A/en unknown
- 2010-08-24 RU RU2015104058/15A patent/RU2015104058A/ru not_active Application Discontinuation
-
2012
- 2012-02-14 IL IL218099A patent/IL218099A0/en unknown
-
2014
- 2014-05-21 US US14/284,027 patent/US20150164895A1/en not_active Abandoned
- 2014-10-30 IL IL235398A patent/IL235398A0/en unknown
Non-Patent Citations (1)
| Title |
|---|
| ТЮРЯЕВА И.И. Опухолевые антигены. Цитология. 2008; 50(3): 189-209 [Найдено 08.08.2013] [он-лайн], Найдено из Интернет: URL: http://www.tsitologiya.cytspb.rssi.ru/50_3/tyuryaeva.pdf * |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20120050493A (ko) | 2012-05-18 |
| IN2012DN01403A (enExample) | 2015-06-05 |
| AU2010289794B2 (en) | 2014-10-02 |
| SG10201402917XA (en) | 2014-08-28 |
| CA2771369A1 (en) | 2011-03-10 |
| AU2010289794A1 (en) | 2012-04-05 |
| IL235398A0 (en) | 2014-12-31 |
| EP2470898A1 (en) | 2012-07-04 |
| WO2011028540A1 (en) | 2011-03-10 |
| MX2012002292A (es) | 2012-03-19 |
| HK1175248A1 (zh) | 2013-06-28 |
| CN102859355A (zh) | 2013-01-02 |
| US20150164895A1 (en) | 2015-06-18 |
| IL218099A0 (en) | 2012-04-30 |
| MX338856B (es) | 2016-05-03 |
| EP2470898A4 (en) | 2013-03-13 |
| BR112012003926A2 (pt) | 2020-08-11 |
| RU2015104058A (ru) | 2015-06-10 |
| US20120214828A1 (en) | 2012-08-23 |
| SG178866A1 (en) | 2012-04-27 |
| MY165154A (en) | 2018-02-28 |
| JP2013502236A (ja) | 2013-01-24 |
| CN102859355B (zh) | 2015-10-07 |
| RU2012111231A (ru) | 2013-10-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2553379C2 (ru) | ОПРЕДЕЛЕНИЕ ЧУВСТВИТЕЛЬНОСТИ КЛЕТОК К ОБРАБОТКЕ ИНГИБИТОРОМ B-Raf ПУТЕМ ДЕТЕКЦИИ МУТАЦИИ K-ras И УРОВНЕЙ ЭКСПРЕССИИ RTK | |
| US20130230511A1 (en) | Biomarkers for response to tyrosine kinase pathway inhibitors in cancer | |
| US20160158235A1 (en) | Biological markers predictive of anti-cancer response to epidermal growth factor receptor kinase inhibitors | |
| Ariyasu et al. | High ratio of T790M to EGFR activating mutations correlate with the osimertinib response in non-small-cell lung cancer | |
| JP6149034B2 (ja) | 上皮成長因子受容体遺伝子における変異 | |
| EP2519826A2 (en) | Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors | |
| HK1175248B (en) | Determining sensitivity of cells to b-raf inhibitor treatment by detecting kras mutation and rtk expression levels | |
| US20110269139A1 (en) | Biomarkers and methods for determining sensitivity to epidermal growth factor receptor modulators | |
| Ozretić et al. | The role of molecular diagnostics in cancer diagnosis and treatment | |
| AU2014203196A1 (en) | Determining sensitivity of cells to B-Raf inhibitor treatment by detecting Kras mutation and RTK expression levels | |
| WO2010131080A1 (en) | A method for predicting the therapeutic responsiveness of a patient to a medical treatment with an egfr inhibitor | |
| EP2702409B1 (en) | CXCR1 as a predictor of response to treatment with epidermal growth factor receptor targeted therapeutic | |
| Goetsch et al. | Selection criteria for c-Met-targeted therapies: emerging evidence for biomarkers | |
| CN104364655A (zh) | 贝伐单抗组合疗法用于治疗乳腺癌的血浆生物标志物 | |
| JP2025528800A (ja) | Egfrキナーゼ活性の阻害剤に対する応答を予測するための方法 | |
| Ma et al. | Combination Therapy of Trastuzumab and Lapatinib in Chemorefractory HER2-Positive Metastatic Colorectal Cancer: An Efficacy and Safety Analysis |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20160825 |