RU2535340C2 - Новые растворимые полипептиды cd83, композиции и способы их применения - Google Patents
Новые растворимые полипептиды cd83, композиции и способы их применения Download PDFInfo
- Publication number
- RU2535340C2 RU2535340C2 RU2010152553/10A RU2010152553A RU2535340C2 RU 2535340 C2 RU2535340 C2 RU 2535340C2 RU 2010152553/10 A RU2010152553/10 A RU 2010152553/10A RU 2010152553 A RU2010152553 A RU 2010152553A RU 2535340 C2 RU2535340 C2 RU 2535340C2
- Authority
- RU
- Russia
- Prior art keywords
- seq
- amino acid
- hcd83ext
- scd83
- sequence
- Prior art date
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 119
- 229920001184 polypeptide Polymers 0.000 title claims abstract description 106
- 102000004196 processed proteins & peptides Human genes 0.000 title claims abstract description 106
- 238000000034 method Methods 0.000 title claims abstract description 66
- 239000000203 mixture Substances 0.000 title description 52
- 125000000539 amino acid group Chemical group 0.000 claims abstract description 78
- 235000001014 amino acid Nutrition 0.000 claims abstract description 64
- 150000001413 amino acids Chemical class 0.000 claims abstract description 57
- 238000011282 treatment Methods 0.000 claims abstract description 42
- 235000018417 cysteine Nutrition 0.000 claims abstract description 37
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 claims abstract description 33
- 206010052779 Transplant rejections Diseases 0.000 claims abstract description 23
- 208000023275 Autoimmune disease Diseases 0.000 claims abstract description 20
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 claims abstract description 15
- 230000002265 prevention Effects 0.000 claims abstract description 10
- 239000003814 drug Substances 0.000 claims abstract description 8
- 201000006417 multiple sclerosis Diseases 0.000 claims abstract description 8
- 206010009900 Colitis ulcerative Diseases 0.000 claims abstract description 6
- 208000011231 Crohn disease Diseases 0.000 claims abstract description 6
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 claims abstract description 6
- 201000006704 Ulcerative Colitis Diseases 0.000 claims abstract description 6
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 claims description 52
- 108010036949 Cyclosporine Proteins 0.000 claims description 52
- 229960001265 ciclosporin Drugs 0.000 claims description 50
- 229930105110 Cyclosporin A Natural products 0.000 claims description 47
- 108091033319 polynucleotide Proteins 0.000 claims description 42
- 102000040430 polynucleotide Human genes 0.000 claims description 42
- 239000002157 polynucleotide Substances 0.000 claims description 42
- 230000001684 chronic effect Effects 0.000 claims description 16
- 239000003018 immunosuppressive agent Substances 0.000 claims description 13
- RTGDFNSFWBGLEC-SYZQJQIISA-N mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1C\C=C(/C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-SYZQJQIISA-N 0.000 claims description 11
- 229960004866 mycophenolate mofetil Drugs 0.000 claims description 11
- 241000124008 Mammalia Species 0.000 claims description 10
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 claims description 8
- 229960003444 immunosuppressant agent Drugs 0.000 claims description 8
- 238000004519 manufacturing process Methods 0.000 claims description 8
- QFJCIRLUMZQUOT-HPLJOQBZSA-N sirolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 QFJCIRLUMZQUOT-HPLJOQBZSA-N 0.000 claims description 8
- ZAHRKKWIAAJSAO-UHFFFAOYSA-N rapamycin Natural products COCC(O)C(=C/C(C)C(=O)CC(OC(=O)C1CCCCN1C(=O)C(=O)C2(O)OC(CC(OC)C(=CC=CC=CC(C)CC(C)C(=O)C)C)CCC2C)C(C)CC3CCC(O)C(C3)OC)C ZAHRKKWIAAJSAO-UHFFFAOYSA-N 0.000 claims description 7
- 229960002930 sirolimus Drugs 0.000 claims description 7
- 208000022559 Inflammatory bowel disease Diseases 0.000 claims description 5
- 230000001861 immunosuppressant effect Effects 0.000 claims description 3
- 108091081024 Start codon Proteins 0.000 claims description 2
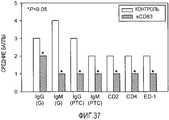
- 102100035793 CD83 antigen Human genes 0.000 abstract description 96
- 101000946856 Homo sapiens CD83 antigen Proteins 0.000 abstract description 92
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical group SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 abstract description 87
- 150000007523 nucleic acids Chemical class 0.000 abstract description 67
- 102000039446 nucleic acids Human genes 0.000 abstract description 57
- 108020004707 nucleic acids Proteins 0.000 abstract description 57
- 125000003275 alpha amino acid group Chemical group 0.000 abstract description 42
- 230000028993 immune response Effects 0.000 abstract description 31
- 230000000694 effects Effects 0.000 abstract description 23
- 238000002360 preparation method Methods 0.000 abstract description 21
- 239000000126 substance Substances 0.000 abstract description 10
- 230000004071 biological effect Effects 0.000 abstract description 7
- 210000004027 cell Anatomy 0.000 description 102
- 108090000623 proteins and genes Proteins 0.000 description 84
- 102000004169 proteins and genes Human genes 0.000 description 69
- 235000018102 proteins Nutrition 0.000 description 66
- 229940024606 amino acid Drugs 0.000 description 53
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 40
- 239000013598 vector Substances 0.000 description 38
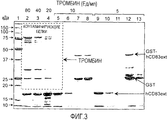
- 108090000190 Thrombin Proteins 0.000 description 34
- 229960004072 thrombin Drugs 0.000 description 34
- 210000004443 dendritic cell Anatomy 0.000 description 33
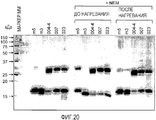
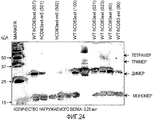
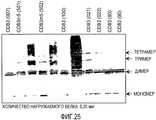
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 32
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 28
- 108010004073 cysteinylcysteine Proteins 0.000 description 27
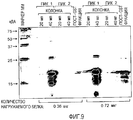
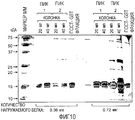
- 238000003776 cleavage reaction Methods 0.000 description 26
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 26
- 230000014509 gene expression Effects 0.000 description 26
- 230000007017 scission Effects 0.000 description 26
- 238000004458 analytical method Methods 0.000 description 25
- 201000010099 disease Diseases 0.000 description 25
- 108020004414 DNA Proteins 0.000 description 24
- OABOXRPGTFRBFZ-IMJSIDKUSA-N Cys-Cys Chemical compound SC[C@H](N)C(=O)N[C@@H](CS)C(O)=O OABOXRPGTFRBFZ-IMJSIDKUSA-N 0.000 description 23
- 108010052382 CD83 antigen Proteins 0.000 description 22
- 208000024891 symptom Diseases 0.000 description 22
- 210000001519 tissue Anatomy 0.000 description 21
- 150000001875 compounds Chemical class 0.000 description 19
- 230000004083 survival effect Effects 0.000 description 19
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 18
- 241000699670 Mus sp. Species 0.000 description 18
- 239000000178 monomer Substances 0.000 description 18
- 241001465754 Metazoa Species 0.000 description 17
- 239000012634 fragment Substances 0.000 description 17
- 210000003734 kidney Anatomy 0.000 description 17
- 239000013612 plasmid Substances 0.000 description 17
- 238000000746 purification Methods 0.000 description 17
- 241000282414 Homo sapiens Species 0.000 description 16
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 16
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 16
- 230000001506 immunosuppresive effect Effects 0.000 description 16
- 238000000338 in vitro Methods 0.000 description 16
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 15
- 239000000539 dimer Substances 0.000 description 15
- 208000035475 disorder Diseases 0.000 description 15
- 239000002158 endotoxin Substances 0.000 description 15
- 210000002216 heart Anatomy 0.000 description 15
- 238000001727 in vivo Methods 0.000 description 15
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 14
- SMYXEYRYCLIPIL-ZLUOBGJFSA-N Cys-Cys-Cys Chemical compound SC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CS)C(O)=O SMYXEYRYCLIPIL-ZLUOBGJFSA-N 0.000 description 14
- 241000699666 Mus <mouse, genus> Species 0.000 description 14
- 230000008859 change Effects 0.000 description 14
- 239000013604 expression vector Substances 0.000 description 14
- 230000006870 function Effects 0.000 description 14
- 238000009097 single-agent therapy Methods 0.000 description 14
- 230000001225 therapeutic effect Effects 0.000 description 14
- -1 amino groups form amine hydrochlorides Chemical group 0.000 description 13
- 239000000427 antigen Substances 0.000 description 13
- 108091007433 antigens Proteins 0.000 description 13
- 102000036639 antigens Human genes 0.000 description 13
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 13
- 230000004064 dysfunction Effects 0.000 description 13
- 108020001507 fusion proteins Proteins 0.000 description 13
- 102000037865 fusion proteins Human genes 0.000 description 13
- 239000002953 phosphate buffered saline Substances 0.000 description 13
- 238000002054 transplantation Methods 0.000 description 13
- 210000001744 T-lymphocyte Anatomy 0.000 description 12
- 229920006008 lipopolysaccharide Polymers 0.000 description 12
- 230000035772 mutation Effects 0.000 description 12
- 230000002829 reductive effect Effects 0.000 description 12
- 235000004400 serine Nutrition 0.000 description 12
- 241000894007 species Species 0.000 description 12
- 108020004705 Codon Proteins 0.000 description 11
- 241000700159 Rattus Species 0.000 description 11
- 241000700605 Viruses Species 0.000 description 11
- 230000015572 biosynthetic process Effects 0.000 description 11
- 239000000523 sample Substances 0.000 description 11
- 108091028043 Nucleic acid sequence Proteins 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 10
- 235000011187 glycerol Nutrition 0.000 description 10
- 210000001616 monocyte Anatomy 0.000 description 10
- 125000003729 nucleotide group Chemical group 0.000 description 10
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 10
- 238000006467 substitution reaction Methods 0.000 description 10
- 230000006433 tumor necrosis factor production Effects 0.000 description 10
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 9
- 206010062016 Immunosuppression Diseases 0.000 description 9
- 108010029485 Protein Isoforms Proteins 0.000 description 9
- 102000001708 Protein Isoforms Human genes 0.000 description 9
- 210000003719 b-lymphocyte Anatomy 0.000 description 9
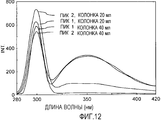
- 238000002983 circular dichroism Methods 0.000 description 9
- 230000001976 improved effect Effects 0.000 description 9
- 239000011780 sodium chloride Substances 0.000 description 9
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 8
- 206010020751 Hypersensitivity Diseases 0.000 description 8
- 101100386053 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) cys-3 gene Proteins 0.000 description 8
- 230000007815 allergy Effects 0.000 description 8
- 238000005571 anion exchange chromatography Methods 0.000 description 8
- 230000000747 cardiac effect Effects 0.000 description 8
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 8
- 238000001990 intravenous administration Methods 0.000 description 8
- 108020004999 messenger RNA Proteins 0.000 description 8
- 239000002773 nucleotide Substances 0.000 description 8
- 238000003860 storage Methods 0.000 description 8
- 238000012546 transfer Methods 0.000 description 8
- 239000013603 viral vector Substances 0.000 description 8
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 7
- HDFGOPSGAURCEO-UHFFFAOYSA-N N-ethylmaleimide Chemical compound CCN1C(=O)C=CC1=O HDFGOPSGAURCEO-UHFFFAOYSA-N 0.000 description 7
- 239000007983 Tris buffer Substances 0.000 description 7
- 239000002299 complementary DNA Substances 0.000 description 7
- 238000002591 computed tomography Methods 0.000 description 7
- 238000006471 dimerization reaction Methods 0.000 description 7
- 238000010438 heat treatment Methods 0.000 description 7
- 230000006698 induction Effects 0.000 description 7
- 230000001404 mediated effect Effects 0.000 description 7
- 239000002609 medium Substances 0.000 description 7
- 230000001177 retroviral effect Effects 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 7
- 230000003612 virological effect Effects 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 239000004475 Arginine Substances 0.000 description 6
- 241000894006 Bacteria Species 0.000 description 6
- 241000282567 Macaca fascicularis Species 0.000 description 6
- 108010076504 Protein Sorting Signals Proteins 0.000 description 6
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 6
- 230000002776 aggregation Effects 0.000 description 6
- 238000004220 aggregation Methods 0.000 description 6
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 6
- 235000009697 arginine Nutrition 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- 230000015556 catabolic process Effects 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 238000006731 degradation reaction Methods 0.000 description 6
- 238000001476 gene delivery Methods 0.000 description 6
- 238000009396 hybridization Methods 0.000 description 6
- 230000005764 inhibitory process Effects 0.000 description 6
- 230000007774 longterm Effects 0.000 description 6
- 238000010172 mouse model Methods 0.000 description 6
- 230000003647 oxidation Effects 0.000 description 6
- 238000007254 oxidation reaction Methods 0.000 description 6
- 239000008194 pharmaceutical composition Substances 0.000 description 6
- 238000005316 response function Methods 0.000 description 6
- 238000003998 size exclusion chromatography high performance liquid chromatography Methods 0.000 description 6
- 238000013518 transcription Methods 0.000 description 6
- 230000035897 transcription Effects 0.000 description 6
- 238000001262 western blot Methods 0.000 description 6
- 241000282472 Canis lupus familiaris Species 0.000 description 5
- 241000588724 Escherichia coli Species 0.000 description 5
- 241000287828 Gallus gallus Species 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 230000002378 acidificating effect Effects 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 238000010828 elution Methods 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 230000004927 fusion Effects 0.000 description 5
- 230000013595 glycosylation Effects 0.000 description 5
- 238000006206 glycosylation reaction Methods 0.000 description 5
- 229940125721 immunosuppressive agent Drugs 0.000 description 5
- 239000000047 product Substances 0.000 description 5
- 238000004611 spectroscopical analysis Methods 0.000 description 5
- 238000013519 translation Methods 0.000 description 5
- 241000701161 unidentified adenovirus Species 0.000 description 5
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 4
- 206010002556 Ankylosing Spondylitis Diseases 0.000 description 4
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 4
- 206010003694 Atrophy Diseases 0.000 description 4
- 108010024636 Glutathione Proteins 0.000 description 4
- 239000004471 Glycine Substances 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 4
- 102100037850 Interferon gamma Human genes 0.000 description 4
- 108010074328 Interferon-gamma Proteins 0.000 description 4
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 4
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 4
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 4
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 4
- 239000004472 Lysine Substances 0.000 description 4
- GHAZCVNUKKZTLG-UHFFFAOYSA-N N-ethyl-succinimide Natural products CCN1C(=O)CCC1=O GHAZCVNUKKZTLG-UHFFFAOYSA-N 0.000 description 4
- 241000288906 Primates Species 0.000 description 4
- 201000004681 Psoriasis Diseases 0.000 description 4
- 230000006052 T cell proliferation Effects 0.000 description 4
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 4
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 4
- 230000001154 acute effect Effects 0.000 description 4
- 239000002671 adjuvant Substances 0.000 description 4
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 4
- 230000037444 atrophy Effects 0.000 description 4
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 4
- 238000012512 characterization method Methods 0.000 description 4
- 239000000306 component Substances 0.000 description 4
- DDRJAANPRJIHGJ-UHFFFAOYSA-N creatinine Chemical compound CN1CC(=O)NC1=N DDRJAANPRJIHGJ-UHFFFAOYSA-N 0.000 description 4
- 229930182912 cyclosporin Natural products 0.000 description 4
- 150000001945 cysteines Chemical class 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 239000012467 final product Substances 0.000 description 4
- 239000000499 gel Substances 0.000 description 4
- 229960003180 glutathione Drugs 0.000 description 4
- 230000003308 immunostimulating effect Effects 0.000 description 4
- 230000001939 inductive effect Effects 0.000 description 4
- 208000015181 infectious disease Diseases 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 238000001155 isoelectric focusing Methods 0.000 description 4
- 210000004698 lymphocyte Anatomy 0.000 description 4
- 235000018977 lysine Nutrition 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- 239000011159 matrix material Substances 0.000 description 4
- 229930182817 methionine Natural products 0.000 description 4
- 235000006109 methionine Nutrition 0.000 description 4
- SQGYOTSLMSWVJD-UHFFFAOYSA-N silver(1+) nitrate Chemical compound [Ag+].[O-]N(=O)=O SQGYOTSLMSWVJD-UHFFFAOYSA-N 0.000 description 4
- 238000001542 size-exclusion chromatography Methods 0.000 description 4
- 239000011550 stock solution Substances 0.000 description 4
- 238000001356 surgical procedure Methods 0.000 description 4
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 4
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 4
- 239000013638 trimer Substances 0.000 description 4
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 3
- 208000030507 AIDS Diseases 0.000 description 3
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 3
- 206010016654 Fibrosis Diseases 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 102000003814 Interleukin-10 Human genes 0.000 description 3
- 108090000174 Interleukin-10 Proteins 0.000 description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 3
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 3
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 3
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 3
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 3
- 239000004473 Threonine Substances 0.000 description 3
- 239000008351 acetate buffer Substances 0.000 description 3
- 238000001042 affinity chromatography Methods 0.000 description 3
- 235000009582 asparagine Nutrition 0.000 description 3
- 229960001230 asparagine Drugs 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 230000001363 autoimmune Effects 0.000 description 3
- LMEKQMALGUDUQG-UHFFFAOYSA-N azathioprine Chemical compound CN1C=NC([N+]([O-])=O)=C1SC1=NC=NC2=C1NC=N2 LMEKQMALGUDUQG-UHFFFAOYSA-N 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 244000309464 bull Species 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 230000001086 cytosolic effect Effects 0.000 description 3
- 238000012217 deletion Methods 0.000 description 3
- 230000037430 deletion Effects 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 210000003527 eukaryotic cell Anatomy 0.000 description 3
- 230000004761 fibrosis Effects 0.000 description 3
- 238000012494 forced degradation Methods 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 3
- 235000004554 glutamine Nutrition 0.000 description 3
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 3
- 239000000710 homodimer Substances 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 230000002163 immunogen Effects 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 229940076144 interleukin-10 Drugs 0.000 description 3
- 238000007912 intraperitoneal administration Methods 0.000 description 3
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 3
- 239000002502 liposome Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 210000004962 mammalian cell Anatomy 0.000 description 3
- 230000035800 maturation Effects 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 238000010369 molecular cloning Methods 0.000 description 3
- 238000002703 mutagenesis Methods 0.000 description 3
- 231100000350 mutagenesis Toxicity 0.000 description 3
- 206010028417 myasthenia gravis Diseases 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 230000001575 pathological effect Effects 0.000 description 3
- 239000008188 pellet Substances 0.000 description 3
- 150000002978 peroxides Chemical class 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 238000011321 prophylaxis Methods 0.000 description 3
- 230000008707 rearrangement Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 description 3
- 150000003839 salts Chemical class 0.000 description 3
- 229920006395 saturated elastomer Polymers 0.000 description 3
- 206010040400 serum sickness Diseases 0.000 description 3
- 238000002741 site-directed mutagenesis Methods 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 230000002195 synergetic effect Effects 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- 235000008521 threonine Nutrition 0.000 description 3
- 241001430294 unidentified retrovirus Species 0.000 description 3
- 239000003981 vehicle Substances 0.000 description 3
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- YAOAMZOGXBMLFQ-UHFFFAOYSA-N 2-(7-aminoheptyl)guanidine Chemical compound NCCCCCCCN=C(N)N YAOAMZOGXBMLFQ-UHFFFAOYSA-N 0.000 description 2
- KJDSORYAHBAGPP-UHFFFAOYSA-N 4-(3,4-diaminophenyl)benzene-1,2-diamine;hydron;tetrachloride Chemical compound Cl.Cl.Cl.Cl.C1=C(N)C(N)=CC=C1C1=CC=C(N)C(N)=C1 KJDSORYAHBAGPP-UHFFFAOYSA-N 0.000 description 2
- 241000972773 Aulopiformes Species 0.000 description 2
- 208000030767 Autoimmune encephalitis Diseases 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 108090000994 Catalytic RNA Proteins 0.000 description 2
- 102000053642 Catalytic RNA Human genes 0.000 description 2
- 241000282693 Cercopithecidae Species 0.000 description 2
- 208000006545 Chronic Obstructive Pulmonary Disease Diseases 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 238000011537 Coomassie blue staining Methods 0.000 description 2
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 2
- 101100364969 Dictyostelium discoideum scai gene Proteins 0.000 description 2
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- HKVAMNSJSFKALM-GKUWKFKPSA-N Everolimus Chemical compound C1C[C@@H](OCCO)[C@H](OC)C[C@@H]1C[C@@H](C)[C@H]1OC(=O)[C@@H]2CCCCN2C(=O)C(=O)[C@](O)(O2)[C@H](C)CC[C@H]2C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C(=O)[C@H](OC)[C@H](O)/C(C)=C/[C@@H](C)C(=O)C1 HKVAMNSJSFKALM-GKUWKFKPSA-N 0.000 description 2
- 108700024394 Exon Proteins 0.000 description 2
- 229920001917 Ficoll Polymers 0.000 description 2
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 2
- 208000001204 Hashimoto Disease Diseases 0.000 description 2
- 208000030836 Hashimoto thyroiditis Diseases 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- SIKJAQJRHWYJAI-UHFFFAOYSA-N Indole Chemical compound C1=CC=C2NC=CC2=C1 SIKJAQJRHWYJAI-UHFFFAOYSA-N 0.000 description 2
- 108091092195 Intron Proteins 0.000 description 2
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 2
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 2
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- 101100364971 Mus musculus Scai gene Proteins 0.000 description 2
- 108700026244 Open Reading Frames Proteins 0.000 description 2
- 102000052812 Ornithine decarboxylases Human genes 0.000 description 2
- 108700005126 Ornithine decarboxylases Proteins 0.000 description 2
- 241000282577 Pan troglodytes Species 0.000 description 2
- 241000721454 Pemphigus Species 0.000 description 2
- 208000004880 Polyuria Diseases 0.000 description 2
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 2
- 241000700157 Rattus norvegicus Species 0.000 description 2
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 2
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 2
- 108020005091 Replication Origin Proteins 0.000 description 2
- 241000723873 Tobacco mosaic virus Species 0.000 description 2
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 2
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 206010047115 Vasculitis Diseases 0.000 description 2
- 230000004913 activation Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 235000004279 alanine Nutrition 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 230000000735 allogeneic effect Effects 0.000 description 2
- 150000003862 amino acid derivatives Chemical class 0.000 description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 2
- 210000004102 animal cell Anatomy 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 238000005349 anion exchange Methods 0.000 description 2
- 239000002518 antifoaming agent Substances 0.000 description 2
- 125000003118 aryl group Chemical group 0.000 description 2
- 235000010323 ascorbic acid Nutrition 0.000 description 2
- 229960005070 ascorbic acid Drugs 0.000 description 2
- 239000011668 ascorbic acid Substances 0.000 description 2
- 229940009098 aspartate Drugs 0.000 description 2
- 208000006673 asthma Diseases 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 238000004140 cleaning Methods 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 229940109239 creatinine Drugs 0.000 description 2
- 230000006240 deamidation Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 230000035619 diuresis Effects 0.000 description 2
- 239000012149 elution buffer Substances 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 229960005167 everolimus Drugs 0.000 description 2
- 230000007717 exclusion Effects 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 238000000855 fermentation Methods 0.000 description 2
- 230000004151 fermentation Effects 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 229930195712 glutamate Natural products 0.000 description 2
- 235000013922 glutamic acid Nutrition 0.000 description 2
- 239000004220 glutamic acid Substances 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 229910052739 hydrogen Inorganic materials 0.000 description 2
- 239000001257 hydrogen Substances 0.000 description 2
- 230000007062 hydrolysis Effects 0.000 description 2
- 238000006460 hydrolysis reaction Methods 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 230000002055 immunohistochemical effect Effects 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 230000010354 integration Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- HPNSFSBZBAHARI-UHFFFAOYSA-N micophenolic acid Natural products OC1=C(CC=C(C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-UHFFFAOYSA-N 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000000816 peptidomimetic Substances 0.000 description 2
- 229920000768 polyamine Polymers 0.000 description 2
- 210000001236 prokaryotic cell Anatomy 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 230000017854 proteolysis Effects 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 108091092562 ribozyme Proteins 0.000 description 2
- 235000019515 salmon Nutrition 0.000 description 2
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 2
- 229910001961 silver nitrate Inorganic materials 0.000 description 2
- 230000000087 stabilizing effect Effects 0.000 description 2
- 230000010473 stable expression Effects 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 230000001131 transforming effect Effects 0.000 description 2
- 241000701447 unidentified baculovirus Species 0.000 description 2
- 241001515965 unidentified phage Species 0.000 description 2
- 239000004474 valine Substances 0.000 description 2
- 235000014393 valine Nutrition 0.000 description 2
- 238000005406 washing Methods 0.000 description 2
- FDKWRPBBCBCIGA-REOHCLBHSA-N (2r)-2-azaniumyl-3-$l^{1}-selanylpropanoate Chemical compound [Se]C[C@H](N)C(O)=O FDKWRPBBCBCIGA-REOHCLBHSA-N 0.000 description 1
- UKAUYVFTDYCKQA-UHFFFAOYSA-N -2-Amino-4-hydroxybutanoic acid Natural products OC(=O)C(N)CCO UKAUYVFTDYCKQA-UHFFFAOYSA-N 0.000 description 1
- LDGWQMRUWMSZIU-LQDDAWAPSA-M 2,3-bis[(z)-octadec-9-enoxy]propyl-trimethylazanium;chloride Chemical compound [Cl-].CCCCCCCC\C=C/CCCCCCCCOCC(C[N+](C)(C)C)OCCCCCCCC\C=C/CCCCCCCC LDGWQMRUWMSZIU-LQDDAWAPSA-M 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- 101150094949 APRT gene Proteins 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 108010024223 Adenine phosphoribosyltransferase Proteins 0.000 description 1
- 208000010266 Aggressive Periodontitis Diseases 0.000 description 1
- 241000710929 Alphavirus Species 0.000 description 1
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 1
- 101150012469 CYS2 gene Proteins 0.000 description 1
- 101150067142 CYS3 gene Proteins 0.000 description 1
- 101150006642 CYS4 gene Proteins 0.000 description 1
- 229940122739 Calcineurin inhibitor Drugs 0.000 description 1
- 101710192106 Calcineurin-binding protein cabin-1 Proteins 0.000 description 1
- 102100024123 Calcineurin-binding protein cabin-1 Human genes 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 241000701489 Cauliflower mosaic virus Species 0.000 description 1
- 206010068051 Chimerism Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- FDKWRPBBCBCIGA-UWTATZPHSA-N D-Selenocysteine Natural products [Se]C[C@@H](N)C(O)=O FDKWRPBBCBCIGA-UWTATZPHSA-N 0.000 description 1
- 101150074155 DHFR gene Proteins 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 241000702421 Dependoparvovirus Species 0.000 description 1
- BWGNESOTFCXPMA-UHFFFAOYSA-N Dihydrogen disulfide Chemical compound SS BWGNESOTFCXPMA-UHFFFAOYSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- YQYJSBFKSSDGFO-UHFFFAOYSA-N Epihygromycin Natural products OC1C(O)C(C(=O)C)OC1OC(C(=C1)O)=CC=C1C=C(C)C(=O)NC1C(O)C(O)C2OCOC2C1O YQYJSBFKSSDGFO-UHFFFAOYSA-N 0.000 description 1
- 241000672609 Escherichia coli BL21 Species 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- 241000251152 Ginglymostoma cirratum Species 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 108010070675 Glutathione transferase Proteins 0.000 description 1
- 208000009329 Graft vs Host Disease Diseases 0.000 description 1
- 208000003807 Graves Disease Diseases 0.000 description 1
- 208000015023 Graves' disease Diseases 0.000 description 1
- 206010019315 Heart transplant rejection Diseases 0.000 description 1
- 102100029100 Hematopoietic prostaglandin D synthase Human genes 0.000 description 1
- 101000599940 Homo sapiens Interferon gamma Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- 241000700588 Human alphaherpesvirus 1 Species 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 108010091358 Hypoxanthine Phosphoribosyltransferase Proteins 0.000 description 1
- 102000010789 Interleukin-2 Receptors Human genes 0.000 description 1
- 108010038453 Interleukin-2 Receptors Proteins 0.000 description 1
- AHLPHDHHMVZTML-BYPYZUCNSA-N L-Ornithine Chemical compound NCCC[C@H](N)C(O)=O AHLPHDHHMVZTML-BYPYZUCNSA-N 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- UKAUYVFTDYCKQA-VKHMYHEASA-N L-homoserine Chemical compound OC(=O)[C@@H](N)CCO UKAUYVFTDYCKQA-VKHMYHEASA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 108010031801 Lipopolysaccharide Receptors Proteins 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 208000019693 Lung disease Diseases 0.000 description 1
- 241000282560 Macaca mulatta Species 0.000 description 1
- 241000283923 Marmota monax Species 0.000 description 1
- 241000699673 Mesocricetus auratus Species 0.000 description 1
- 101100261636 Methanothermobacter marburgensis (strain ATCC BAA-927 / DSM 2133 / JCM 14651 / NBRC 100331 / OCM 82 / Marburg) trpB2 gene Proteins 0.000 description 1
- 102100035877 Monocyte differentiation antigen CD14 Human genes 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 101100202339 Mus musculus Slc6a13 gene Proteins 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 108020004711 Nucleic Acid Probes Proteins 0.000 description 1
- 108091005461 Nucleic proteins Proteins 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 241000277275 Oncorhynchus mykiss Species 0.000 description 1
- AHLPHDHHMVZTML-UHFFFAOYSA-N Orn-delta-NH2 Natural products NCCCC(N)C(O)=O AHLPHDHHMVZTML-UHFFFAOYSA-N 0.000 description 1
- UTJLXEIPEHZYQJ-UHFFFAOYSA-N Ornithine Natural products OC(=O)C(C)CCCN UTJLXEIPEHZYQJ-UHFFFAOYSA-N 0.000 description 1
- 229940122060 Ornithine decarboxylase inhibitor Drugs 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 206010033557 Palpitations Diseases 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 206010033799 Paralysis Diseases 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 101100124346 Photorhabdus laumondii subsp. laumondii (strain DSM 15139 / CIP 105565 / TT01) hisCD gene Proteins 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 208000021738 Plummer disease Diseases 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 241001506137 Rapa Species 0.000 description 1
- 101100202330 Rattus norvegicus Slc6a11 gene Proteins 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 241000277289 Salmo salar Species 0.000 description 1
- 101100222745 Schizosaccharomyces pombe (strain 972 / ATCC 24843) met17 gene Proteins 0.000 description 1
- 208000034189 Sclerosis Diseases 0.000 description 1
- 241000710961 Semliki Forest virus Species 0.000 description 1
- 206010040070 Septic Shock Diseases 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 241000710960 Sindbis virus Species 0.000 description 1
- 241000269809 Sparus aurata Species 0.000 description 1
- 208000032005 Spinocerebellar ataxia with axonal neuropathy type 2 Diseases 0.000 description 1
- 201000002661 Spondylitis Diseases 0.000 description 1
- 230000006044 T cell activation Effects 0.000 description 1
- 230000020385 T cell costimulation Effects 0.000 description 1
- 108091036066 Three prime untranslated region Proteins 0.000 description 1
- 108020004440 Thymidine kinase Proteins 0.000 description 1
- 206010044248 Toxic shock syndrome Diseases 0.000 description 1
- 231100000650 Toxic shock syndrome Toxicity 0.000 description 1
- 108020004566 Transfer RNA Proteins 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 206010072810 Vascular wall hypertrophy Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 108020005202 Viral DNA Proteins 0.000 description 1
- 108010067390 Viral Proteins Proteins 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 230000003187 abdominal effect Effects 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000007488 abnormal function Effects 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000004480 active ingredient Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 239000000443 aerosol Substances 0.000 description 1
- 101150099105 alien gene Proteins 0.000 description 1
- 230000002152 alkylating effect Effects 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000001399 anti-metabolic effect Effects 0.000 description 1
- 239000003429 antifungal agent Substances 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 230000003078 antioxidant effect Effects 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 230000036528 appetite Effects 0.000 description 1
- 235000019789 appetite Nutrition 0.000 description 1
- 238000000376 autoradiography Methods 0.000 description 1
- 208000033361 autosomal recessive with axonal neuropathy 2 spinocerebellar ataxia Diseases 0.000 description 1
- 229940022777 azasan Drugs 0.000 description 1
- 229960002170 azathioprine Drugs 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 125000001584 benzyloxycarbonyl group Chemical group C(=O)(OCC1=CC=CC=C1)* 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 239000012148 binding buffer Substances 0.000 description 1
- 229920000249 biocompatible polymer Polymers 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 239000012503 blood component Substances 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- KQNZDYYTLMIZCT-KQPMLPITSA-N brefeldin A Chemical compound O[C@@H]1\C=C\C(=O)O[C@@H](C)CCC\C=C\[C@@H]2C[C@H](O)C[C@H]21 KQNZDYYTLMIZCT-KQPMLPITSA-N 0.000 description 1
- JUMGSHROWPPKFX-UHFFFAOYSA-N brefeldin-A Natural products CC1CCCC=CC2(C)CC(O)CC2(C)C(O)C=CC(=O)O1 JUMGSHROWPPKFX-UHFFFAOYSA-N 0.000 description 1
- 239000007975 buffered saline Substances 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- 229940041514 candida albicans extract Drugs 0.000 description 1
- 230000005189 cardiac health Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 229940107810 cellcept Drugs 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- 238000004737 colorimetric analysis Methods 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 230000008602 contraction Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000001054 cortical effect Effects 0.000 description 1
- 239000003246 corticosteroid Substances 0.000 description 1
- 229960001334 corticosteroids Drugs 0.000 description 1
- 238000009295 crossflow filtration Methods 0.000 description 1
- 239000002577 cryoprotective agent Substances 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 229960002806 daclizumab Drugs 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000009615 deamination Effects 0.000 description 1
- 238000006481 deamination reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000007857 degradation product Substances 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000000779 depleting effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 150000004985 diamines Chemical class 0.000 description 1
- 125000001028 difluoromethyl group Chemical group [H]C(F)(F)* 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 239000002612 dispersion medium Substances 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229940126534 drug product Drugs 0.000 description 1
- 230000001516 effect on protein Effects 0.000 description 1
- VLCYCQAOQCDTCN-UHFFFAOYSA-N eflornithine Chemical compound NCCCC(N)(C(F)F)C(O)=O VLCYCQAOQCDTCN-UHFFFAOYSA-N 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 125000004494 ethyl ester group Chemical group 0.000 description 1
- 238000010228 ex vivo assay Methods 0.000 description 1
- 208000012997 experimental autoimmune encephalomyelitis Diseases 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 239000000284 extract Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 238000012495 forced degradation study Methods 0.000 description 1
- 230000022244 formylation Effects 0.000 description 1
- 238000006170 formylation reaction Methods 0.000 description 1
- 238000013467 fragmentation Methods 0.000 description 1
- 238000006062 fragmentation reaction Methods 0.000 description 1
- 230000002538 fungal effect Effects 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 238000007429 general method Methods 0.000 description 1
- 230000001434 glomerular Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 101150106093 gpt gene Proteins 0.000 description 1
- 208000024908 graft versus host disease Diseases 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 101150113423 hisD gene Proteins 0.000 description 1
- 238000000265 homogenisation Methods 0.000 description 1
- 239000003906 humectant Substances 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 208000013403 hyperactivity Diseases 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000003053 immunization Effects 0.000 description 1
- 238000002649 immunization Methods 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 229960001438 immunostimulant agent Drugs 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 229940073062 imuran Drugs 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 239000005414 inactive ingredient Substances 0.000 description 1
- PZOUSPYUWWUPPK-UHFFFAOYSA-N indole Natural products CC1=CC=CC2=C1C=CN2 PZOUSPYUWWUPPK-UHFFFAOYSA-N 0.000 description 1
- RKJUIXBNRJVNHR-UHFFFAOYSA-N indolenine Natural products C1=CC=C2CC=NC2=C1 RKJUIXBNRJVNHR-UHFFFAOYSA-N 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000002054 inoculum Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 210000004153 islets of langerhan Anatomy 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 238000009533 lab test Methods 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 238000011694 lewis rat Methods 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000007791 liquid phase Substances 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000012160 loading buffer Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000011866 long-term treatment Methods 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 238000010339 medical test Methods 0.000 description 1
- 239000013028 medium composition Substances 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 229960004452 methionine Drugs 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 238000002406 microsurgery Methods 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000002438 mitochondrial effect Effects 0.000 description 1
- 210000005087 mononuclear cell Anatomy 0.000 description 1
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 229960000951 mycophenolic acid Drugs 0.000 description 1
- HPNSFSBZBAHARI-RUDMXATFSA-N mycophenolic acid Chemical compound OC1=C(C\C=C(/C)CCC(O)=O)C(OC)=C(C)C2=C1C(=O)OC2 HPNSFSBZBAHARI-RUDMXATFSA-N 0.000 description 1
- 210000001167 myeloblast Anatomy 0.000 description 1
- 210000003643 myeloid progenitor cell Anatomy 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 229940063121 neoral Drugs 0.000 description 1
- 239000002853 nucleic acid probe Substances 0.000 description 1
- 230000000414 obstructive effect Effects 0.000 description 1
- 101150093139 ompT gene Proteins 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000013386 optimize process Methods 0.000 description 1
- 229960003104 ornithine Drugs 0.000 description 1
- 239000002818 ornithine decarboxylase inhibitor Substances 0.000 description 1
- 230000001590 oxidative effect Effects 0.000 description 1
- 239000006174 pH buffer Substances 0.000 description 1
- 230000000242 pagocytic effect Effects 0.000 description 1
- 238000002559 palpation Methods 0.000 description 1
- 244000052769 pathogen Species 0.000 description 1
- 231100000915 pathological change Toxicity 0.000 description 1
- 230000036285 pathological change Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 230000003239 periodontal effect Effects 0.000 description 1
- 201000006727 periodontosis Diseases 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000002572 peristaltic effect Effects 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 210000001539 phagocyte Anatomy 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 238000002264 polyacrylamide gel electrophoresis Methods 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 230000002980 postoperative effect Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 229940072288 prograf Drugs 0.000 description 1
- 230000020978 protein processing Effects 0.000 description 1
- 238000001742 protein purification Methods 0.000 description 1
- 238000001273 protein sequence alignment Methods 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 230000004144 purine metabolism Effects 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 229940099538 rapamune Drugs 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 210000003289 regulatory T cell Anatomy 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 229940063122 sandimmune Drugs 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 235000016491 selenocysteine Nutrition 0.000 description 1
- ZKZBPNGNEQAJSX-UHFFFAOYSA-N selenocysteine Natural products [SeH]CC(N)C(O)=O ZKZBPNGNEQAJSX-UHFFFAOYSA-N 0.000 description 1
- 229940055619 selenocysteine Drugs 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 230000037432 silent mutation Effects 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 230000000392 somatic effect Effects 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 238000002798 spectrophotometry method Methods 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000005846 sugar alcohols Polymers 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 229960001967 tacrolimus Drugs 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 125000004149 thio group Chemical group *S* 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 230000003614 tolerogenic effect Effects 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000003151 transfection method Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 101150081616 trpB gene Proteins 0.000 description 1
- 101150111232 trpB-1 gene Proteins 0.000 description 1
- 239000012137 tryptone Substances 0.000 description 1
- 210000004026 tunica intima Anatomy 0.000 description 1
- 208000035408 type 1 diabetes mellitus 1 Diseases 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 239000004034 viscosity adjusting agent Substances 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
- 239000012138 yeast extract Substances 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/38—Drugs for disorders of the endocrine system of the suprarenal hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/14—Vasoprotectives; Antihaemorrhoidals; Drugs for varicose therapy; Capillary stabilisers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Immunology (AREA)
- Diabetes (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Endocrinology (AREA)
- Physical Education & Sports Medicine (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Neurology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Cell Biology (AREA)
- Toxicology (AREA)
- Zoology (AREA)
- Gastroenterology & Hepatology (AREA)
- Obesity (AREA)
- Dermatology (AREA)
- Vascular Medicine (AREA)
- Hematology (AREA)
- Emergency Medicine (AREA)
- Transplantation (AREA)
- Pain & Pain Management (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12870908P | 2008-05-23 | 2008-05-23 | |
| US61/128,709 | 2008-05-23 | ||
| PCT/US2009/003174 WO2009142759A1 (en) | 2008-05-23 | 2009-05-22 | Novel soluble cd83 polypeptides, formulations and methods of use |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2010152553A RU2010152553A (ru) | 2012-06-27 |
| RU2535340C2 true RU2535340C2 (ru) | 2014-12-10 |
Family
ID=41340432
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2010152553/10A RU2535340C2 (ru) | 2008-05-23 | 2009-05-22 | Новые растворимые полипептиды cd83, композиции и способы их применения |
Country Status (12)
| Country | Link |
|---|---|
| US (1) | US20110182903A1 (enExample) |
| EP (1) | EP2288387B1 (enExample) |
| JP (1) | JP2011520959A (enExample) |
| KR (2) | KR101679084B1 (enExample) |
| CN (1) | CN102036690B (enExample) |
| AU (1) | AU2009249540B9 (enExample) |
| BR (1) | BRPI0913577B1 (enExample) |
| CA (1) | CA2725198A1 (enExample) |
| IL (1) | IL209207A (enExample) |
| MX (1) | MX2010012576A (enExample) |
| RU (1) | RU2535340C2 (enExample) |
| WO (1) | WO2009142759A1 (enExample) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP1422241A1 (en) | 2002-11-19 | 2004-05-26 | Alexander Steinkasserer | Use of soluble forms of CD83 and nucleic acids encoding them for the treatment or prevention of diseases |
| US9102726B2 (en) * | 2002-12-04 | 2015-08-11 | Argos Therapeutics, Inc. | Nucleic acid of recombination expression vector encoding soluble forms of CD83, host cells transformed/transfected therewith and pharmaceutical compositions containing same |
| US7169898B2 (en) | 2002-12-04 | 2007-01-30 | Alexander Steinkasserer | Soluble CD83 proteins and use thereof for the treatment or prevention of a disease or medical condition caused by dysfunction or undesired function of a cellular immune response involving T cells |
| CN103243119B (zh) * | 2013-05-20 | 2016-03-30 | 中国科学技术大学 | 可溶性cd83的表达和制备方法 |
| US20190315878A1 (en) | 2016-11-07 | 2019-10-17 | Argos Therapeutics Inc. | Bispecific antibodies that modulate tlr-4 signaling and uses thereof |
| ES2954311T3 (es) | 2017-04-01 | 2023-11-21 | Avm Biotechnology Llc | Reemplazo de un preacondicionamiento citotóxico antes de una inmunoterapia celular |
| KR102282341B1 (ko) | 2018-09-12 | 2021-07-27 | 아주대학교산학협력단 | Cd83 억제제를 유효성분으로 포함하는 베체트병의 예방 또는 치료용 조성물 |
| EP3895723A1 (en) * | 2020-04-16 | 2021-10-20 | Friedrich-Alexander-Universität Erlangen-Nürnberg | Scd83 for wound healing, hair growth, and skin and hair care |
| DE102024110866A1 (de) | 2024-04-18 | 2025-10-23 | Christian Arnold | Pharmazeutische Zusammensetzung zur Heilung einer Wunde |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2232773C2 (ru) * | 1995-09-06 | 2004-07-20 | Айдек Фармасьютикалз Корпорейшн | Рекомбинантные антитела против cd4 для терапии человека |
| US20070167607A1 (en) * | 2002-11-19 | 2007-07-19 | Alexander Steinkasserer | Use of soluble forms of cd83 and nucleic acids encoding them for the treatment or prevention of diseases |
Family Cites Families (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5316920A (en) * | 1992-04-17 | 1994-05-31 | Dana-Faber Cancer Institute, Inc. | Lymphocyte activation antigen HB15, a member of the immunoglobulin superfamily |
| US5710262A (en) * | 1992-04-17 | 1998-01-20 | Dana-Faber Cancer Institute, Inc. | Nucleic acid encoding HB15 polypeptides |
| KR20010023325A (ko) * | 1997-08-26 | 2001-03-26 | 리스 데브라 케이. | 함지방세포-특이적 단백질 상동체 |
| PT1223971E (pt) | 1999-10-27 | 2005-10-31 | Alexandra Lucas | Composicoes e metodos para prevencao e tratamento da rejeicao de transplante |
| US20020014242A1 (en) * | 2000-07-31 | 2002-02-07 | Abraham Scaria | Use of rapamycin to inhibit immune response and induce tolerance to gene therapy vector and encoded transgene products |
| US20040185040A1 (en) * | 2001-11-21 | 2004-09-23 | Celltech R & D Limited | Modulating immune responses |
| US20030219436A1 (en) * | 2002-03-15 | 2003-11-27 | Ledbetter Jeffrey A. | Compositions and methods to regulate an immune response using CD83 gene expressed in tumors and using soluble CD83-Ig fusion protein |
| EP1578366A4 (en) * | 2002-10-09 | 2007-12-19 | Tolerrx Inc | MOLECULES PREFERABLY ASSOCIATED WITH EFFECTOR T CELLS OR REGULATORY T CELLS AND METHODS OF USE THEREOF |
| US9102726B2 (en) | 2002-12-04 | 2015-08-11 | Argos Therapeutics, Inc. | Nucleic acid of recombination expression vector encoding soluble forms of CD83, host cells transformed/transfected therewith and pharmaceutical compositions containing same |
| US7169898B2 (en) * | 2002-12-04 | 2007-01-30 | Alexander Steinkasserer | Soluble CD83 proteins and use thereof for the treatment or prevention of a disease or medical condition caused by dysfunction or undesired function of a cellular immune response involving T cells |
| EP2789625B1 (en) * | 2006-08-18 | 2018-06-06 | Argos Therapeutics, Inc. | Use of soluble CD83 for perfusing a tissue for transplantation |
-
2009
- 2009-05-22 AU AU2009249540A patent/AU2009249540B9/en not_active Ceased
- 2009-05-22 KR KR1020107028713A patent/KR101679084B1/ko not_active Expired - Fee Related
- 2009-05-22 CN CN2009801186344A patent/CN102036690B/zh not_active Expired - Fee Related
- 2009-05-22 KR KR1020167023212A patent/KR20160104101A/ko not_active Ceased
- 2009-05-22 RU RU2010152553/10A patent/RU2535340C2/ru active
- 2009-05-22 MX MX2010012576A patent/MX2010012576A/es active IP Right Grant
- 2009-05-22 CA CA2725198A patent/CA2725198A1/en not_active Abandoned
- 2009-05-22 US US12/736,887 patent/US20110182903A1/en not_active Abandoned
- 2009-05-22 JP JP2011510515A patent/JP2011520959A/ja active Pending
- 2009-05-22 WO PCT/US2009/003174 patent/WO2009142759A1/en not_active Ceased
- 2009-05-22 EP EP09750990A patent/EP2288387B1/en not_active Not-in-force
- 2009-05-22 BR BRPI0913577-4A patent/BRPI0913577B1/pt not_active IP Right Cessation
-
2010
- 2010-11-09 IL IL209207A patent/IL209207A/en active IP Right Grant
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| RU2232773C2 (ru) * | 1995-09-06 | 2004-07-20 | Айдек Фармасьютикалз Корпорейшн | Рекомбинантные антитела против cd4 для терапии человека |
| US20070167607A1 (en) * | 2002-11-19 | 2007-07-19 | Alexander Steinkasserer | Use of soluble forms of cd83 and nucleic acids encoding them for the treatment or prevention of diseases |
Non-Patent Citations (1)
| Title |
|---|
| OHTA Y. et al., Homologs of CD83 from elasmobranch and teleost fish, J. Immunol., 2004, vol.173 no.7, pp.4553-4560. ZINGER E. et al., Determination of the inhibitory activity and biological half-life of soluble CD83: Comparison of wild type and mutant isoforms, Immunobiology, 2006, vol.211, pp.449-453. * |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2009249540B9 (en) | 2014-09-18 |
| EP2288387A4 (en) | 2011-04-27 |
| CN102036690B (zh) | 2013-11-06 |
| KR20110018381A (ko) | 2011-02-23 |
| CA2725198A1 (en) | 2009-11-26 |
| RU2010152553A (ru) | 2012-06-27 |
| AU2009249540A8 (en) | 2011-01-20 |
| BRPI0913577B1 (pt) | 2022-05-31 |
| KR20160104101A (ko) | 2016-09-02 |
| HK1155066A1 (en) | 2012-05-11 |
| EP2288387B1 (en) | 2013-02-13 |
| AU2009249540A1 (en) | 2009-11-26 |
| US20110182903A1 (en) | 2011-07-28 |
| EP2288387A1 (en) | 2011-03-02 |
| BRPI0913577A2 (pt) | 2020-08-18 |
| MX2010012576A (es) | 2011-04-05 |
| IL209207A0 (en) | 2011-01-31 |
| IL209207A (en) | 2014-05-28 |
| AU2009249540B2 (en) | 2014-07-03 |
| WO2009142759A1 (en) | 2009-11-26 |
| KR101679084B1 (ko) | 2016-11-24 |
| CN102036690A (zh) | 2011-04-27 |
| JP2011520959A (ja) | 2011-07-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2535340C2 (ru) | Новые растворимые полипептиды cd83, композиции и способы их применения | |
| US20230158109A1 (en) | Use of cd83 in combination therapies | |
| EP1397153A2 (en) | Methods for protecting allogeneic islet transplant using soluble ctla4 mutant molecules | |
| HK1155066B (en) | Novel soluble cd83 polypeptides, formulations and methods of use | |
| KR20230097094A (ko) | 질환의 치료를 위한 융합 단백질 | |
| AU2013237703B2 (en) | Use of cd83 in combination therapies | |
| HK1130263B (en) | Use of cd83 in combination therapies |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PC41 | Official registration of the transfer of exclusive right |
Effective date: 20200127 |