RU2490024C2 - Расширение спектра т клеток для включения субдоминантных эпитопов путем вакцинации с антигенами, доставленными как белковые фрагменты или пептидные коктейли - Google Patents
Расширение спектра т клеток для включения субдоминантных эпитопов путем вакцинации с антигенами, доставленными как белковые фрагменты или пептидные коктейли Download PDFInfo
- Publication number
- RU2490024C2 RU2490024C2 RU2009102389/15A RU2009102389A RU2490024C2 RU 2490024 C2 RU2490024 C2 RU 2490024C2 RU 2009102389/15 A RU2009102389/15 A RU 2009102389/15A RU 2009102389 A RU2009102389 A RU 2009102389A RU 2490024 C2 RU2490024 C2 RU 2490024C2
- Authority
- RU
- Russia
- Prior art keywords
- epitopes
- peptides
- protein
- peptide
- vaccine
- Prior art date
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims description 164
- 102000004169 proteins and genes Human genes 0.000 title claims description 49
- 108090000623 proteins and genes Proteins 0.000 title claims description 49
- 238000002255 vaccination Methods 0.000 title abstract description 53
- 210000001744 T-lymphocyte Anatomy 0.000 title abstract description 25
- 102000036639 antigens Human genes 0.000 title abstract description 25
- 108091007433 antigens Proteins 0.000 title abstract description 25
- 239000000427 antigen Substances 0.000 title abstract description 23
- 239000012634 fragment Substances 0.000 title abstract description 13
- 238000001228 spectrum Methods 0.000 title abstract description 4
- 229960005486 vaccine Drugs 0.000 claims abstract description 60
- 208000017667 Chronic Disease Diseases 0.000 claims abstract description 26
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 98
- 239000000203 mixture Substances 0.000 claims description 84
- 150000001413 amino acids Chemical class 0.000 claims description 35
- 239000002502 liposome Substances 0.000 claims description 31
- 239000002671 adjuvant Substances 0.000 claims description 21
- 238000000034 method Methods 0.000 claims description 21
- 230000001580 bacterial effect Effects 0.000 claims description 19
- 201000010099 disease Diseases 0.000 claims description 19
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 19
- 150000002632 lipids Chemical class 0.000 claims description 18
- 241000894006 Bacteria Species 0.000 claims description 12
- 241001465754 Metazoa Species 0.000 claims description 11
- 241000700605 Viruses Species 0.000 claims description 10
- 230000001684 chronic effect Effects 0.000 claims description 9
- 201000008827 tuberculosis Diseases 0.000 claims description 9
- 208000035143 Bacterial infection Diseases 0.000 claims description 7
- 230000006337 proteolytic cleavage Effects 0.000 claims description 7
- 208000036142 Viral infection Diseases 0.000 claims description 6
- 208000022362 bacterial infectious disease Diseases 0.000 claims description 6
- 239000003795 chemical substances by application Substances 0.000 claims description 6
- 238000011282 treatment Methods 0.000 claims description 6
- 241000606153 Chlamydia trachomatis Species 0.000 claims description 5
- 108091005804 Peptidases Proteins 0.000 claims description 5
- 229940038705 chlamydia trachomatis Drugs 0.000 claims description 5
- 244000045947 parasite Species 0.000 claims description 5
- 108010051815 Glutamyl endopeptidase Proteins 0.000 claims description 4
- 102000035195 Peptidases Human genes 0.000 claims description 4
- 239000000556 agonist Substances 0.000 claims description 4
- 150000001875 compounds Chemical class 0.000 claims description 4
- 208000006454 hepatitis Diseases 0.000 claims description 4
- 231100000283 hepatitis Toxicity 0.000 claims description 4
- 208000002672 hepatitis B Diseases 0.000 claims description 4
- 229940024999 proteolytic enzymes for treatment of wounds and ulcers Drugs 0.000 claims description 4
- 108090000317 Chymotrypsin Proteins 0.000 claims description 3
- 101710088335 Diacylglycerol acyltransferase/mycolyltransferase Ag85A Proteins 0.000 claims description 3
- 101710088334 Diacylglycerol acyltransferase/mycolyltransferase Ag85B Proteins 0.000 claims description 3
- 208000005176 Hepatitis C Diseases 0.000 claims description 3
- 241000222722 Leishmania <genus> Species 0.000 claims description 3
- 241000186359 Mycobacterium Species 0.000 claims description 3
- 241000187479 Mycobacterium tuberculosis Species 0.000 claims description 3
- 108090000631 Trypsin Proteins 0.000 claims description 3
- 102000004142 Trypsin Human genes 0.000 claims description 3
- 230000015572 biosynthetic process Effects 0.000 claims description 3
- 125000002091 cationic group Chemical group 0.000 claims description 3
- 229960002376 chymotrypsin Drugs 0.000 claims description 3
- 230000021615 conjugation Effects 0.000 claims description 3
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 claims description 3
- NQGIJDNPUZEBRU-UHFFFAOYSA-N dodecanoyl chloride Chemical compound CCCCCCCCCCCC(Cl)=O NQGIJDNPUZEBRU-UHFFFAOYSA-N 0.000 claims description 3
- 230000013595 glycosylation Effects 0.000 claims description 3
- 238000006206 glycosylation reaction Methods 0.000 claims description 3
- 238000002372 labelling Methods 0.000 claims description 3
- 230000021633 leukocyte mediated immunity Effects 0.000 claims description 3
- 238000004519 manufacturing process Methods 0.000 claims description 3
- 230000002265 prevention Effects 0.000 claims description 3
- 239000012588 trypsin Substances 0.000 claims description 3
- 241001467553 Mycobacterium africanum Species 0.000 claims description 2
- 241000186366 Mycobacterium bovis Species 0.000 claims description 2
- 241000186362 Mycobacterium leprae Species 0.000 claims description 2
- 241000224016 Plasmodium Species 0.000 claims description 2
- 230000009385 viral infection Effects 0.000 claims 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 1
- 239000013043 chemical agent Substances 0.000 claims 1
- 230000004044 response Effects 0.000 abstract description 35
- 230000005867 T cell response Effects 0.000 abstract description 12
- 238000002649 immunization Methods 0.000 abstract description 11
- 230000006698 induction Effects 0.000 abstract description 8
- 239000000126 substance Substances 0.000 abstract description 7
- 230000000694 effects Effects 0.000 abstract description 6
- 230000001939 inductive effect Effects 0.000 abstract description 5
- 208000037581 Persistent Infection Diseases 0.000 abstract description 4
- 230000004807 localization Effects 0.000 abstract description 2
- 239000003814 drug Substances 0.000 abstract 1
- 235000018102 proteins Nutrition 0.000 description 43
- 241000699670 Mus sp. Species 0.000 description 37
- 208000015181 infectious disease Diseases 0.000 description 27
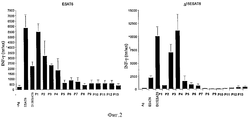
- 101710166488 6 kDa early secretory antigenic target Proteins 0.000 description 25
- 210000004027 cell Anatomy 0.000 description 23
- 230000028993 immune response Effects 0.000 description 21
- 235000001014 amino acid Nutrition 0.000 description 20
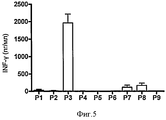
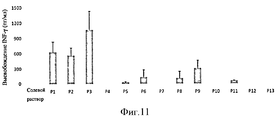
- 102100037850 Interferon gamma Human genes 0.000 description 19
- 108010074328 Interferon-gamma Proteins 0.000 description 19
- 229920001184 polypeptide Polymers 0.000 description 15
- 230000002163 immunogen Effects 0.000 description 12
- 238000002965 ELISA Methods 0.000 description 11
- 206010028980 Neoplasm Diseases 0.000 description 10
- 125000000539 amino acid group Chemical group 0.000 description 10
- 201000011510 cancer Diseases 0.000 description 10
- 239000010410 layer Substances 0.000 description 10
- YIOCIFXUGBYCJR-UHFFFAOYSA-N bis(4-chlorophenyl)acetic acid Chemical compound C=1C=C(Cl)C=CC=1C(C(=O)O)C1=CC=C(Cl)C=C1 YIOCIFXUGBYCJR-UHFFFAOYSA-N 0.000 description 9
- 238000000338 in vitro Methods 0.000 description 9
- 210000004072 lung Anatomy 0.000 description 9
- 239000012071 phase Substances 0.000 description 9
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 8
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 8
- 239000008280 blood Substances 0.000 description 8
- 230000003053 immunization Effects 0.000 description 8
- 230000003612 virological effect Effects 0.000 description 8
- 108090000695 Cytokines Proteins 0.000 description 7
- 102000004127 Cytokines Human genes 0.000 description 7
- 108020004414 DNA Proteins 0.000 description 7
- 230000028327 secretion Effects 0.000 description 7
- 230000036755 cellular response Effects 0.000 description 6
- 230000001681 protective effect Effects 0.000 description 6
- 241000725303 Human immunodeficiency virus Species 0.000 description 5
- 238000007792 addition Methods 0.000 description 5
- 230000036039 immunity Effects 0.000 description 5
- 230000003993 interaction Effects 0.000 description 5
- 239000013612 plasmid Substances 0.000 description 5
- 230000000638 stimulation Effects 0.000 description 5
- -1 ESAT6 Proteins 0.000 description 4
- 208000030852 Parasitic disease Diseases 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 4
- 102000002689 Toll-like receptor Human genes 0.000 description 4
- 108020000411 Toll-like receptor Proteins 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 238000003776 cleavage reaction Methods 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 230000007017 scission Effects 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 102000043129 MHC class I family Human genes 0.000 description 3
- 108091054437 MHC class I family Proteins 0.000 description 3
- 238000010171 animal model Methods 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 210000000601 blood cell Anatomy 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 230000008348 humoral response Effects 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 230000001717 pathogenic effect Effects 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- 230000037452 priming Effects 0.000 description 3
- 239000002356 single layer Substances 0.000 description 3
- 238000006467 substitution reaction Methods 0.000 description 3
- 239000000829 suppository Substances 0.000 description 3
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 2
- 108010042708 Acetylmuramyl-Alanyl-Isoglutamine Proteins 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 2
- 101100038180 Caenorhabditis briggsae rpb-1 gene Proteins 0.000 description 2
- 238000011238 DNA vaccination Methods 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 108010065805 Interleukin-12 Proteins 0.000 description 2
- 108010002350 Interleukin-2 Proteins 0.000 description 2
- 102000043131 MHC class II family Human genes 0.000 description 2
- 108091054438 MHC class II family Proteins 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 108091036414 Polyinosinic:polycytidylic acid Proteins 0.000 description 2
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 2
- WNROFYMDJYEPJX-UHFFFAOYSA-K aluminium hydroxide Chemical compound [OH-].[OH-].[OH-].[Al+3] WNROFYMDJYEPJX-UHFFFAOYSA-K 0.000 description 2
- 238000000540 analysis of variance Methods 0.000 description 2
- 230000005875 antibody response Effects 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 239000008346 aqueous phase Substances 0.000 description 2
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 238000004587 chromatography analysis Methods 0.000 description 2
- XVOYSCVBGLVSOL-UHFFFAOYSA-N cysteic acid Chemical compound OC(=O)C(N)CS(O)(=O)=O XVOYSCVBGLVSOL-UHFFFAOYSA-N 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 239000002158 endotoxin Substances 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 208000000509 infertility Diseases 0.000 description 2
- 230000036512 infertility Effects 0.000 description 2
- 231100000535 infertility Toxicity 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 229940035032 monophosphoryl lipid a Drugs 0.000 description 2
- BSOQXXWZTUDTEL-ZUYCGGNHSA-N muramyl dipeptide Chemical compound OC(=O)CC[C@H](C(N)=O)NC(=O)[C@H](C)NC(=O)[C@@H](C)O[C@H]1[C@H](O)[C@@H](CO)O[C@@H](O)[C@@H]1NC(C)=O BSOQXXWZTUDTEL-ZUYCGGNHSA-N 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 230000003449 preventive effect Effects 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 238000011321 prophylaxis Methods 0.000 description 2
- 230000000306 recurrent effect Effects 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 210000000952 spleen Anatomy 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 229940021747 therapeutic vaccine Drugs 0.000 description 2
- 229940104230 thymidine Drugs 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 230000004580 weight loss Effects 0.000 description 2
- 238000001262 western blot Methods 0.000 description 2
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- QZCJOXAIQXPLNS-UHFFFAOYSA-N 1,1,2,2,3,3,4,4,4a,5,5,6,6,7,7,8,8,8a-octadecafluoronaphthalene 4-(2-aminoethyl)benzene-1,2-diol Chemical compound NCCc1ccc(O)c(O)c1.FC1(F)C(F)(F)C(F)(F)C2(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C2(F)C1(F)F QZCJOXAIQXPLNS-UHFFFAOYSA-N 0.000 description 1
- QRXMUCSWCMTJGU-UHFFFAOYSA-N 5-bromo-4-chloro-3-indolyl phosphate Chemical compound C1=C(Br)C(Cl)=C2C(OP(O)(=O)O)=CNC2=C1 QRXMUCSWCMTJGU-UHFFFAOYSA-N 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 1
- ATRRKUHOCOJYRX-UHFFFAOYSA-N Ammonium bicarbonate Chemical compound [NH4+].OC([O-])=O ATRRKUHOCOJYRX-UHFFFAOYSA-N 0.000 description 1
- 229910000013 Ammonium bicarbonate Inorganic materials 0.000 description 1
- OBMZMSLWNNWEJA-XNCRXQDQSA-N C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 Chemical compound C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 OBMZMSLWNNWEJA-XNCRXQDQSA-N 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 241001495184 Chlamydia sp. Species 0.000 description 1
- 241000037164 Collema parvum Species 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 102100036242 HLA class II histocompatibility antigen, DQ alpha 2 chain Human genes 0.000 description 1
- 101000930801 Homo sapiens HLA class II histocompatibility antigen, DQ alpha 2 chain Proteins 0.000 description 1
- 101000658138 Homo sapiens Thymosin beta-10 Proteins 0.000 description 1
- 108090000174 Interleukin-10 Proteins 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 1
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 1
- 208000032420 Latent Infection Diseases 0.000 description 1
- 239000000232 Lipid Bilayer Substances 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 101710085938 Matrix protein Proteins 0.000 description 1
- 101710127721 Membrane protein Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 108091028043 Nucleic acid sequence Proteins 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
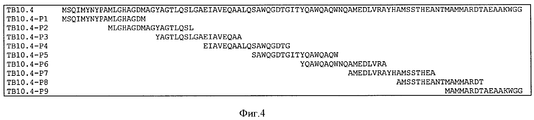
- 101710176384 Peptide 1 Proteins 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 241000223960 Plasmodium falciparum Species 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 229920002472 Starch Polymers 0.000 description 1
- 108091008874 T cell receptors Proteins 0.000 description 1
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 1
- 102100034998 Thymosin beta-10 Human genes 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- UZQJVUCHXGYFLQ-AYDHOLPZSA-N [(2s,3r,4s,5r,6r)-4-[(2s,3r,4s,5r,6r)-4-[(2r,3r,4s,5r,6r)-4-[(2s,3r,4s,5r,6r)-3,5-dihydroxy-6-(hydroxymethyl)-4-[(2s,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,5-dihydroxy-6-(hy Chemical compound O([C@H]1[C@H](O)[C@@H](CO)O[C@H]([C@@H]1O)O[C@H]1[C@H](O)[C@@H](CO)O[C@H]([C@@H]1O)O[C@H]1CC[C@]2(C)[C@H]3CC=C4[C@@]([C@@]3(CC[C@H]2[C@@]1(C=O)C)C)(C)CC(O)[C@]1(CCC(CC14)(C)C)C(=O)O[C@H]1[C@@H]([C@@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O[C@H]4[C@@H]([C@@H](O[C@H]5[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O5)O)[C@H](O)[C@@H](CO)O4)O)[C@H](O)[C@@H](CO)O3)O)[C@H](O)[C@@H](CO)O2)O)[C@H](O)[C@@H](CO)O1)O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O UZQJVUCHXGYFLQ-AYDHOLPZSA-N 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- 229940037003 alum Drugs 0.000 description 1
- 235000012538 ammonium bicarbonate Nutrition 0.000 description 1
- 239000001099 ammonium carbonate Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 235000003704 aspartic acid Nutrition 0.000 description 1
- 239000012752 auxiliary agent Substances 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 238000010382 chemical cross-linking Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 239000003085 diluting agent Substances 0.000 description 1
- PSLWZOIUBRXAQW-UHFFFAOYSA-M dimethyl(dioctadecyl)azanium;bromide Chemical group [Br-].CCCCCCCCCCCCCCCCCC[N+](C)(C)CCCCCCCCCCCCCCCCCC PSLWZOIUBRXAQW-UHFFFAOYSA-M 0.000 description 1
- 208000037771 disease arising from reactivation of latent virus Diseases 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 238000011013 endotoxin removal Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 238000002523 gelfiltration Methods 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 230000004727 humoral immunity Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000008073 immune recognition Effects 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 238000010324 immunological assay Methods 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000011081 inoculation Methods 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 239000008101 lactose Substances 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 150000002634 lipophilic molecules Chemical class 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 210000002540 macrophage Anatomy 0.000 description 1
- ZLNQQNXFFQJAID-UHFFFAOYSA-L magnesium carbonate Chemical compound [Mg+2].[O-]C([O-])=O ZLNQQNXFFQJAID-UHFFFAOYSA-L 0.000 description 1
- 239000001095 magnesium carbonate Substances 0.000 description 1
- 229910000021 magnesium carbonate Inorganic materials 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 238000004949 mass spectrometry Methods 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 210000001616 monocyte Anatomy 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 229910052757 nitrogen Inorganic materials 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108700018460 nysyl-leucyl-lysyl-leucyl-leucyl-leucyl-leucyl-leucyl-lysyl-leucyl-lysinamide Proteins 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 229920000724 poly(L-arginine) polymer Polymers 0.000 description 1
- 229920001515 polyalkylene glycol Polymers 0.000 description 1
- 108010011110 polyarginine Proteins 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000000843 powder Substances 0.000 description 1
- 238000009021 pre-vaccination Methods 0.000 description 1
- 208000037920 primary disease Diseases 0.000 description 1
- 229940023867 prime-boost vaccine Drugs 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 229940021993 prophylactic vaccine Drugs 0.000 description 1
- 230000004845 protein aggregation Effects 0.000 description 1
- 230000007065 protein hydrolysis Effects 0.000 description 1
- 229940023143 protein vaccine Drugs 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 230000007420 reactivation Effects 0.000 description 1
- 108020003175 receptors Proteins 0.000 description 1
- 102000005962 receptors Human genes 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 230000037390 scarring Effects 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- ZFRKQXVRDFCRJG-UHFFFAOYSA-N skatole Natural products C1=CC=C2C(C)=CNC2=C1 ZFRKQXVRDFCRJG-UHFFFAOYSA-N 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 238000010532 solid phase synthesis reaction Methods 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 239000008107 starch Substances 0.000 description 1
- 235000019698 starch Nutrition 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229960002317 succinimide Drugs 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 238000010189 synthetic method Methods 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- TXEYQDLBPFQVAA-UHFFFAOYSA-N tetrafluoromethane Chemical compound FC(F)(F)F TXEYQDLBPFQVAA-UHFFFAOYSA-N 0.000 description 1
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/04—Mycobacterium, e.g. Mycobacterium tuberculosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/29—Hepatitis virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
- A61P31/06—Antibacterial agents for tuberculosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/18—Antivirals for RNA viruses for HIV
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
- A61P33/06—Antimalarials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55511—Organic adjuvants
- A61K2039/5555—Muramyl dipeptides
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Immunology (AREA)
- Communicable Diseases (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Microbiology (AREA)
- Tropical Medicine & Parasitology (AREA)
- Virology (AREA)
- Oncology (AREA)
- Pulmonology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Diabetes (AREA)
- Engineering & Computer Science (AREA)
- Endocrinology (AREA)
- Emergency Medicine (AREA)
- Obesity (AREA)
- Hematology (AREA)
- AIDS & HIV (AREA)
- Molecular Biology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| DKPA200600861 | 2006-06-28 | ||
| DKPA200600861 | 2006-06-28 | ||
| PCT/DK2007/000312 WO2008000261A2 (en) | 2006-06-28 | 2007-06-26 | Expanding the t cell repertoire to include subdominant epitopes by vaccination with antigens delivered as protein fragments or peptide cocktails |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2009102389A RU2009102389A (ru) | 2010-08-10 |
| RU2490024C2 true RU2490024C2 (ru) | 2013-08-20 |
Family
ID=38845996
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2009102389/15A RU2490024C2 (ru) | 2006-06-28 | 2007-06-26 | Расширение спектра т клеток для включения субдоминантных эпитопов путем вакцинации с антигенами, доставленными как белковые фрагменты или пептидные коктейли |
Country Status (11)
| Country | Link |
|---|---|
| US (2) | US8105614B2 (enExample) |
| EP (3) | EP2402024A1 (enExample) |
| JP (1) | JP5165681B2 (enExample) |
| KR (1) | KR20090024292A (enExample) |
| AU (1) | AU2007264205B2 (enExample) |
| BR (1) | BRPI0713978A2 (enExample) |
| CA (1) | CA2654271A1 (enExample) |
| DK (1) | DK2040745T3 (enExample) |
| ES (1) | ES2400531T3 (enExample) |
| RU (1) | RU2490024C2 (enExample) |
| WO (1) | WO2008000261A2 (enExample) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2348475A1 (en) * | 1998-11-04 | 2000-05-11 | Isis Innovation Limited | Tuberculosis diagnostic test |
| EP1523331B1 (en) * | 2002-07-13 | 2013-02-27 | Statens Serum Institut | Therapeutic tb vaccine |
| MX2009013008A (es) * | 2007-05-31 | 2010-06-09 | Vacunas para la influenza. | |
| CA2691840C (en) * | 2007-06-29 | 2016-08-09 | Statens Serum Institut | The use of monomycolyl glycerol (mmg) as an adjuvant |
| AU2008303023B2 (en) | 2007-09-27 | 2014-01-09 | Immunovaccine Technologies Inc. | Use of liposomes in a carrier comprising a continuous hydrophobic phase for delivery of polynucleotides in vivo |
| CN102056622B (zh) * | 2008-06-05 | 2016-04-06 | 免疫疫苗技术有限公司 | 包含脂质体、抗原、多核苷酸和含疏水物质的连续相的载体的组合物 |
| US20100015171A1 (en) | 2008-07-15 | 2010-01-21 | Statens Serum Institute | Vaccines comprising tb 10.4 |
| HUE030520T2 (en) * | 2008-11-28 | 2017-05-29 | Statens Seruminstitut | Optimized influenza vaccines |
| US9074001B2 (en) * | 2009-04-24 | 2015-07-07 | Statens Serum Institut | Tuberculosis TB vaccine to prevent reactivation |
| JP5600170B2 (ja) * | 2009-11-05 | 2014-10-01 | アメリカ合衆国 | Plasmodiumfalciparumスポロゾイト及び肝臓段階抗原 |
| EP2501396B1 (en) * | 2009-11-18 | 2018-04-25 | Auburn University | Low antigen-dose immunization for maximizing t-helper cell 1 (th1) immunity against disease |
| US20120244178A1 (en) * | 2011-03-25 | 2012-09-27 | Denise Doolan | Plasmodium falciparum antigens |
| DK3079716T3 (da) * | 2013-12-13 | 2019-08-19 | Us Health | Multiepitop-tarp-peptidvaccine og anvendelser deraf. |
| EP3134116B1 (en) | 2014-04-24 | 2019-04-03 | Statens Serum Institut | New m.tuberculosis vaccines |
| CN117064854A (zh) * | 2023-08-14 | 2023-11-17 | 广州谦毅生物医药有限公司 | 一种卡瓦脂质体的制备技术 |
Family Cites Families (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4599231A (en) | 1984-03-09 | 1986-07-08 | Scripps Clinic And Research Foundation | Synthetic hepatitis B virus vaccine including both T cell and B cell determinants |
| US4599230A (en) | 1984-03-09 | 1986-07-08 | Scripps Clinic And Research Foundation | Synthetic hepatitis B virus vaccine including both T cell and B cell determinants |
| US4608251A (en) | 1984-11-09 | 1986-08-26 | Pitman-Moore, Inc. | LHRH analogues useful in stimulating anti-LHRH antibodies and vaccines containing such analogues |
| US4601903A (en) | 1985-05-01 | 1986-07-22 | The United States Of America As Represented By The Department Of Health And Human Services | Vaccine against Neisseria meningitidis Group B serotype 2 invasive disease |
| US4975282A (en) | 1985-06-26 | 1990-12-04 | The Liposome Company, Inc. | Multilamellar liposomes having improved trapping efficiencies |
| US6641814B1 (en) * | 1997-04-02 | 2003-11-04 | Statens Serum Institut | Nucleic acids fragments and polypeptide fragments derived from M. tuberculosis |
| ES2197170T3 (es) * | 1993-11-04 | 2004-01-01 | Innogenetics N.V. | Epitopos de celulas t humanas inmunologicamente dominantes del virus de la hepatitis c. |
| KR100692227B1 (ko) * | 1998-04-07 | 2007-03-09 | 코릭사 코포레이션 | 마이코박테리움 튜베르쿨로시스 항원의 융합 단백질 및이의 용도 |
| US6649170B1 (en) | 1999-05-12 | 2003-11-18 | Statens Serum Institut | Adjuvant combinations for immunization composition and vaccines |
| SE9903031D0 (sv) * | 1999-08-27 | 1999-08-27 | Eurodiagnostica Ab | Peptide mixture and vaccine against a chronic viral infection |
| JP2003510370A (ja) * | 1999-10-07 | 2003-03-18 | コリクサ コーポレイション | Mycobacteriumtuberculosisの融合タンパク質 |
| AT410173B (de) | 2000-06-08 | 2003-02-25 | Cistem Biotechnologies Gmbh | Antigene zusammensetzung |
| DE60100814T2 (de) | 2000-06-08 | 2004-07-01 | Intercell Biomedizinische Forschungs- Und Entwicklungs Gmbh | Immunstimulierende oligodeoxynukleotide |
| AT410635B (de) | 2000-10-18 | 2003-06-25 | Cistem Biotechnologies Gmbh | Vakzin-zusammensetzung |
| GB0118532D0 (en) | 2001-07-30 | 2001-09-19 | Isis Innovation | Materials and methods relating to improved vaccination strategies |
| AU2003245729A1 (en) * | 2002-06-27 | 2004-01-19 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for modulating a cytotoxic t lymphocyte immune response |
| DE602005009535D1 (de) | 2004-07-07 | 2008-10-16 | Statens Seruminstitut | Von adjuvans-formulierungen auf lipid-basis unter verwendung von glykolipiden |
| US7749520B2 (en) | 2004-07-07 | 2010-07-06 | Statens Serum Institut | Compositions and methods for stabilizing lipid based adjuvant formulations using glycolipids |
| FR2873467A1 (fr) | 2004-07-26 | 2006-01-27 | Proton World Internatinal Nv | Enregistrement d'une cle dans un circuit integre |
| PL1991266T3 (pl) * | 2006-01-26 | 2013-11-29 | Zoetis Services Llc | Nowe kompozycje adiuwantów glikolipidowych |
| GB0608368D0 (en) * | 2006-04-28 | 2006-06-07 | Isis Innovation | Process for making Oligopeptides |
-
2007
- 2007-06-26 KR KR1020097001606A patent/KR20090024292A/ko not_active Ceased
- 2007-06-26 JP JP2009516901A patent/JP5165681B2/ja not_active Expired - Fee Related
- 2007-06-26 EP EP11182885A patent/EP2402024A1/en not_active Withdrawn
- 2007-06-26 EP EP07764444A patent/EP2040745B1/en not_active Not-in-force
- 2007-06-26 RU RU2009102389/15A patent/RU2490024C2/ru not_active IP Right Cessation
- 2007-06-26 EP EP11182884A patent/EP2402023A1/en not_active Withdrawn
- 2007-06-26 DK DK07764444.1T patent/DK2040745T3/da active
- 2007-06-26 BR BRPI0713978-0A patent/BRPI0713978A2/pt not_active IP Right Cessation
- 2007-06-26 ES ES07764444T patent/ES2400531T3/es active Active
- 2007-06-26 CA CA002654271A patent/CA2654271A1/en not_active Abandoned
- 2007-06-26 AU AU2007264205A patent/AU2007264205B2/en not_active Expired - Fee Related
- 2007-06-26 WO PCT/DK2007/000312 patent/WO2008000261A2/en not_active Ceased
- 2007-06-27 US US11/823,402 patent/US8105614B2/en not_active Expired - Fee Related
-
2011
- 2011-12-16 US US13/328,319 patent/US20120156282A1/en not_active Abandoned
Non-Patent Citations (1)
| Title |
|---|
| JESPER DAVIDSEN et al. Characterization of cationic liposomes based on dimethyldioctadecyl ammonium and synthetic cord factor from М. tuberculosis (trehalose 6,6'-dibehenate) - A novel adjuvant inducing both strong CMI and antibody responses, Biochimica et Biophysica Acta, 2005 v.1718, pp.22-31. KIROKOHCHI K. et al., Use of recombinant protein to identify a motif-negative human cytotoxic T-cell epitope presented by HLA-A2 in hepatitis С virus NS3 region, J. Virol., v. 70, №1, pp.232-240. * |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20090024292A (ko) | 2009-03-06 |
| CA2654271A1 (en) | 2008-01-03 |
| AU2007264205B2 (en) | 2013-04-18 |
| WO2008000261A2 (en) | 2008-01-03 |
| EP2402023A1 (en) | 2012-01-04 |
| ES2400531T3 (es) | 2013-04-10 |
| EP2040745A2 (en) | 2009-04-01 |
| JP2009541373A (ja) | 2009-11-26 |
| EP2040745B1 (en) | 2012-12-05 |
| WO2008000261A3 (en) | 2008-03-20 |
| EP2402024A1 (en) | 2012-01-04 |
| US20080008724A1 (en) | 2008-01-10 |
| US20120156282A1 (en) | 2012-06-21 |
| US8105614B2 (en) | 2012-01-31 |
| DK2040745T3 (da) | 2013-03-18 |
| JP5165681B2 (ja) | 2013-03-21 |
| AU2007264205A1 (en) | 2008-01-03 |
| BRPI0713978A2 (pt) | 2012-11-27 |
| RU2009102389A (ru) | 2010-08-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2490024C2 (ru) | Расширение спектра т клеток для включения субдоминантных эпитопов путем вакцинации с антигенами, доставленными как белковые фрагменты или пептидные коктейли | |
| US10519202B2 (en) | Tuberculosis TB vaccine to prevent reactivation | |
| KR101188045B1 (ko) | 감염 잠복기 동안 발현되는 항원을 포함하는 결핵 백신 | |
| RU2723046C2 (ru) | Вакцины против Chlamydia sp. | |
| AU772617B2 (en) | Vaccine compositions | |
| US10004793B2 (en) | M.tuberculosis vaccines | |
| Golovliov et al. | Adjuvanticity of ISCOMs incorporating a T cell-reactive lipoprotein of the facultative intracellular pathogen Francisella tularensis | |
| JP2001517069A (ja) | 結核の免疫治療および診断のための化合物および方法 | |
| WO2014063704A2 (en) | M. tuberculosis vaccines | |
| WO2002004018A2 (en) | Mid-life vaccine and methods for boosting anti-mycobacterial immunity | |
| US20060286128A1 (en) | Adjuvant combinations of liposomes and mycobacteriaial lipids for immunization compositions and vaccines | |
| WO2005061534A2 (en) | Improved tuberculosis vaccines | |
| CN102488898B (zh) | 龋齿疫苗及制备方法 | |
| MX2012005522A (es) | Uso de una fuente de l3 y/o l5 como vacuna o como diagnostico para una enfermedad parasitaria. | |
| EP2370100B1 (en) | Peptide adjuvants | |
| KR20110081824A (ko) | 백신 보조제 | |
| KR20010033132A (ko) | 미코박테리움 바카이에서 유도한 조성물 및 이의 이용방법 | |
| Baldwin | Novel vaccine approaches against aerogenic infection with Mycobacterium tuberculosis | |
| HK1226632A1 (en) | New m.tuberculosis vaccines | |
| CZ20002321A3 (cs) | Kompozice získané z Mycobacterium vaccae a způsoby jejich použití |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20140627 |