RU2420268C2 - Способ программируемой плавучей доставки - Google Patents
Способ программируемой плавучей доставки Download PDFInfo
- Publication number
- RU2420268C2 RU2420268C2 RU2009112396/15A RU2009112396A RU2420268C2 RU 2420268 C2 RU2420268 C2 RU 2420268C2 RU 2009112396/15 A RU2009112396/15 A RU 2009112396/15A RU 2009112396 A RU2009112396 A RU 2009112396A RU 2420268 C2 RU2420268 C2 RU 2420268C2
- Authority
- RU
- Russia
- Prior art keywords
- core
- active agent
- pharmaceutically active
- agents
- polymer layer
- Prior art date
Links
- 238000000034 method Methods 0.000 title claims abstract description 32
- 238000007667 floating Methods 0.000 title description 15
- 239000013543 active substance Substances 0.000 claims abstract description 145
- 229920000642 polymer Polymers 0.000 claims abstract description 145
- 230000002496 gastric effect Effects 0.000 claims abstract description 47
- 239000003814 drug Substances 0.000 claims abstract description 12
- 239000000126 substance Substances 0.000 claims abstract description 12
- 230000002035 prolonged effect Effects 0.000 claims abstract description 4
- 239000003826 tablet Substances 0.000 claims description 64
- 239000000463 material Substances 0.000 claims description 48
- 238000000576 coating method Methods 0.000 claims description 45
- 239000011248 coating agent Substances 0.000 claims description 44
- 235000010980 cellulose Nutrition 0.000 claims description 34
- 229920002678 cellulose Polymers 0.000 claims description 34
- 229920000036 polyvinylpyrrolidone Polymers 0.000 claims description 32
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 claims description 32
- 239000003795 chemical substances by application Substances 0.000 claims description 31
- 229920001577 copolymer Polymers 0.000 claims description 29
- 239000001913 cellulose Substances 0.000 claims description 28
- -1 polyoxyethylene Polymers 0.000 claims description 28
- 239000001267 polyvinylpyrrolidone Substances 0.000 claims description 27
- 210000002784 stomach Anatomy 0.000 claims description 27
- 239000012530 fluid Substances 0.000 claims description 26
- 239000000203 mixture Substances 0.000 claims description 22
- 239000001856 Ethyl cellulose Substances 0.000 claims description 15
- 235000019325 ethyl cellulose Nutrition 0.000 claims description 15
- 229920001249 ethyl cellulose Polymers 0.000 claims description 15
- 210000001035 gastrointestinal tract Anatomy 0.000 claims description 15
- 230000000968 intestinal effect Effects 0.000 claims description 15
- 210000000936 intestine Anatomy 0.000 claims description 15
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 15
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims description 14
- 238000010521 absorption reaction Methods 0.000 claims description 14
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 claims description 13
- HYWYRSMBCFDLJT-UHFFFAOYSA-N nimesulide Chemical compound CS(=O)(=O)NC1=CC=C([N+]([O-])=O)C=C1OC1=CC=CC=C1 HYWYRSMBCFDLJT-UHFFFAOYSA-N 0.000 claims description 13
- 229960000965 nimesulide Drugs 0.000 claims description 11
- 239000002904 solvent Substances 0.000 claims description 11
- 229940079593 drug Drugs 0.000 claims description 10
- 239000004014 plasticizer Substances 0.000 claims description 10
- 235000019333 sodium laurylsulphate Nutrition 0.000 claims description 10
- 229920002554 vinyl polymer Polymers 0.000 claims description 10
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 claims description 9
- 230000015572 biosynthetic process Effects 0.000 claims description 9
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 9
- 239000000194 fatty acid Substances 0.000 claims description 9
- 229930195729 fatty acid Natural products 0.000 claims description 9
- KPYSYYIEGFHWSV-UHFFFAOYSA-N Baclofen Chemical compound OC(=O)CC(CN)C1=CC=C(Cl)C=C1 KPYSYYIEGFHWSV-UHFFFAOYSA-N 0.000 claims description 8
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 claims description 8
- 229940081735 acetylcellulose Drugs 0.000 claims description 8
- 229960000794 baclofen Drugs 0.000 claims description 8
- 229920002301 cellulose acetate Polymers 0.000 claims description 8
- 239000011159 matrix material Substances 0.000 claims description 8
- 150000003839 salts Chemical class 0.000 claims description 8
- 239000004094 surface-active agent Substances 0.000 claims description 8
- 229960001967 tacrolimus Drugs 0.000 claims description 8
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 claims description 8
- 238000011282 treatment Methods 0.000 claims description 8
- SMZOUWXMTYCWNB-UHFFFAOYSA-N 2-(2-methoxy-5-methylphenyl)ethanamine Chemical compound COC1=CC=C(C)C=C1CCN SMZOUWXMTYCWNB-UHFFFAOYSA-N 0.000 claims description 7
- 239000002775 capsule Substances 0.000 claims description 7
- 239000003623 enhancer Substances 0.000 claims description 7
- 150000004665 fatty acids Chemical class 0.000 claims description 7
- 239000007789 gas Substances 0.000 claims description 7
- 239000012729 immediate-release (IR) formulation Substances 0.000 claims description 7
- 239000003112 inhibitor Substances 0.000 claims description 7
- LKJPYSCBVHEWIU-KRWDZBQOSA-N (R)-bicalutamide Chemical compound C([C@@](O)(C)C(=O)NC=1C=C(C(C#N)=CC=1)C(F)(F)F)S(=O)(=O)C1=CC=C(F)C=C1 LKJPYSCBVHEWIU-KRWDZBQOSA-N 0.000 claims description 6
- NIXOWILDQLNWCW-UHFFFAOYSA-N 2-Propenoic acid Natural products OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 claims description 6
- 108090000790 Enzymes Proteins 0.000 claims description 6
- 102000004190 Enzymes Human genes 0.000 claims description 6
- 108010000817 Leuprolide Proteins 0.000 claims description 6
- 229960000997 bicalutamide Drugs 0.000 claims description 6
- 239000011230 binding agent Substances 0.000 claims description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 claims description 6
- YMTINGFKWWXKFG-UHFFFAOYSA-N fenofibrate Chemical compound C1=CC(OC(C)(C)C(=O)OC(C)C)=CC=C1C(=O)C1=CC=C(Cl)C=C1 YMTINGFKWWXKFG-UHFFFAOYSA-N 0.000 claims description 6
- 229960002297 fenofibrate Drugs 0.000 claims description 6
- 229910052500 inorganic mineral Inorganic materials 0.000 claims description 6
- 210000002429 large intestine Anatomy 0.000 claims description 6
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 claims description 6
- 229960004338 leuprorelin Drugs 0.000 claims description 6
- JCCNYMKQOSZNPW-UHFFFAOYSA-N loratadine Chemical compound C1CN(C(=O)OCC)CCC1=C1C2=NC=CC=C2CCC2=CC(Cl)=CC=C21 JCCNYMKQOSZNPW-UHFFFAOYSA-N 0.000 claims description 6
- 229960003088 loratadine Drugs 0.000 claims description 6
- XZWYZXLIPXDOLR-UHFFFAOYSA-N metformin Chemical compound CN(C)C(=N)NC(N)=N XZWYZXLIPXDOLR-UHFFFAOYSA-N 0.000 claims description 6
- 229920002845 Poly(methacrylic acid) Polymers 0.000 claims description 5
- 239000003146 anticoagulant agent Substances 0.000 claims description 5
- 239000007767 bonding agent Substances 0.000 claims description 5
- 125000004181 carboxyalkyl group Chemical class 0.000 claims description 5
- 229960004195 carvedilol Drugs 0.000 claims description 5
- NPAKNKYSJIDKMW-UHFFFAOYSA-N carvedilol Chemical compound COC1=CC=CC=C1OCCNCC(O)COC1=CC=CC2=NC3=CC=C[CH]C3=C12 NPAKNKYSJIDKMW-UHFFFAOYSA-N 0.000 claims description 5
- 239000000314 lubricant Substances 0.000 claims description 5
- 229920005615 natural polymer Polymers 0.000 claims description 5
- 229920001282 polysaccharide Polymers 0.000 claims description 5
- 239000005017 polysaccharide Substances 0.000 claims description 5
- CAVQBDOACNULDN-NRCOEFLKSA-N (1s,2s)-2-(methylamino)-1-phenylpropan-1-ol;sulfuric acid Chemical compound OS(O)(=O)=O.CN[C@@H](C)[C@@H](O)C1=CC=CC=C1.CN[C@@H](C)[C@@H](O)C1=CC=CC=C1 CAVQBDOACNULDN-NRCOEFLKSA-N 0.000 claims description 4
- 229920001661 Chitosan Polymers 0.000 claims description 4
- 241001465754 Metazoa Species 0.000 claims description 4
- 229920002125 Sokalan® Polymers 0.000 claims description 4
- 229910052783 alkali metal Inorganic materials 0.000 claims description 4
- 229920013820 alkyl cellulose Polymers 0.000 claims description 4
- 239000000812 cholinergic antagonist Substances 0.000 claims description 4
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 claims description 4
- 238000001816 cooling Methods 0.000 claims description 4
- GHVNFZFCNZKVNT-UHFFFAOYSA-N decanoic acid Chemical compound CCCCCCCCCC(O)=O GHVNFZFCNZKVNT-UHFFFAOYSA-N 0.000 claims description 4
- POULHZVOKOAJMA-UHFFFAOYSA-N dodecanoic acid Chemical compound CCCCCCCCCCCC(O)=O POULHZVOKOAJMA-UHFFFAOYSA-N 0.000 claims description 4
- 230000002209 hydrophobic effect Effects 0.000 claims description 4
- 239000011707 mineral Substances 0.000 claims description 4
- 239000006187 pill Substances 0.000 claims description 4
- 229920000193 polymethacrylate Polymers 0.000 claims description 4
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 4
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 4
- 102000004169 proteins and genes Human genes 0.000 claims description 4
- 108090000623 proteins and genes Proteins 0.000 claims description 4
- 229960004159 pseudoephedrine sulfate Drugs 0.000 claims description 4
- 230000003637 steroidlike Effects 0.000 claims description 4
- 229920001059 synthetic polymer Polymers 0.000 claims description 4
- 235000015112 vegetable and seed oil Nutrition 0.000 claims description 4
- 239000008158 vegetable oil Substances 0.000 claims description 4
- 229920000623 Cellulose acetate phthalate Polymers 0.000 claims description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 claims description 3
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 claims description 3
- 239000003699 antiulcer agent Substances 0.000 claims description 3
- 239000003833 bile salt Substances 0.000 claims description 3
- 229940093761 bile salts Drugs 0.000 claims description 3
- 229940081734 cellulose acetate phthalate Drugs 0.000 claims description 3
- 239000002738 chelating agent Substances 0.000 claims description 3
- 229920006237 degradable polymer Polymers 0.000 claims description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 3
- 150000002191 fatty alcohols Chemical class 0.000 claims description 3
- 229930195733 hydrocarbon Natural products 0.000 claims description 3
- 150000002430 hydrocarbons Chemical class 0.000 claims description 3
- 229920013821 hydroxy alkyl cellulose Polymers 0.000 claims description 3
- 229920003132 hydroxypropyl methylcellulose phthalate Polymers 0.000 claims description 3
- 229940031704 hydroxypropyl methylcellulose phthalate Drugs 0.000 claims description 3
- 229920002521 macromolecule Polymers 0.000 claims description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 claims description 3
- 239000011976 maleic acid Substances 0.000 claims description 3
- 229960003105 metformin Drugs 0.000 claims description 3
- 230000003232 mucoadhesive effect Effects 0.000 claims description 3
- 230000036961 partial effect Effects 0.000 claims description 3
- 229920000233 poly(alkylene oxides) Polymers 0.000 claims description 3
- 230000000717 retained effect Effects 0.000 claims description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 claims description 3
- NOOLISFMXDJSKH-UTLUCORTSA-N (+)-Neomenthol Chemical compound CC(C)[C@@H]1CC[C@@H](C)C[C@@H]1O NOOLISFMXDJSKH-UTLUCORTSA-N 0.000 claims description 2
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 claims description 2
- TVYLLZQTGLZFBW-ZBFHGGJFSA-N (R,R)-tramadol Chemical compound COC1=CC=CC([C@]2(O)[C@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-ZBFHGGJFSA-N 0.000 claims description 2
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 claims description 2
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 claims description 2
- 206010000599 Acromegaly Diseases 0.000 claims description 2
- 208000024827 Alzheimer disease Diseases 0.000 claims description 2
- 108010039627 Aprotinin Proteins 0.000 claims description 2
- XUKUURHRXDUEBC-KAYWLYCHSA-N Atorvastatin Chemical compound C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CC[C@@H](O)C[C@@H](O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-KAYWLYCHSA-N 0.000 claims description 2
- XUKUURHRXDUEBC-UHFFFAOYSA-N Atorvastatin Natural products C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CCC(O)CC(O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-UHFFFAOYSA-N 0.000 claims description 2
- 239000005632 Capric acid (CAS 334-48-5) Substances 0.000 claims description 2
- 229920000858 Cyclodextrin Polymers 0.000 claims description 2
- 201000003883 Cystic fibrosis Diseases 0.000 claims description 2
- NOOLISFMXDJSKH-UHFFFAOYSA-N DL-menthol Natural products CC(C)C1CCC(C)CC1O NOOLISFMXDJSKH-UHFFFAOYSA-N 0.000 claims description 2
- ZBNZXTGUTAYRHI-UHFFFAOYSA-N Dasatinib Chemical compound C=1C(N2CCN(CCO)CC2)=NC(C)=NC=1NC(S1)=NC=C1C(=O)NC1=C(C)C=CC=C1Cl ZBNZXTGUTAYRHI-UHFFFAOYSA-N 0.000 claims description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 claims description 2
- 108010061435 Enalapril Proteins 0.000 claims description 2
- 201000009273 Endometriosis Diseases 0.000 claims description 2
- 239000002067 L01XE06 - Dasatinib Substances 0.000 claims description 2
- 239000005639 Lauric acid Substances 0.000 claims description 2
- CERQOIWHTDAKMF-UHFFFAOYSA-N Methacrylic acid Chemical compound CC(=C)C(O)=O CERQOIWHTDAKMF-UHFFFAOYSA-N 0.000 claims description 2
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 claims description 2
- 229940121948 Muscarinic receptor antagonist Drugs 0.000 claims description 2
- 239000005642 Oleic acid Substances 0.000 claims description 2
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 claims description 2
- 208000001132 Osteoporosis Diseases 0.000 claims description 2
- TUZYXOIXSAXUGO-UHFFFAOYSA-N Pravastatin Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(O)C=C21 TUZYXOIXSAXUGO-UHFFFAOYSA-N 0.000 claims description 2
- JUJWROOIHBZHMG-UHFFFAOYSA-N Pyridine Chemical compound C1=CC=NC=C1 JUJWROOIHBZHMG-UHFFFAOYSA-N 0.000 claims description 2
- RYMZZMVNJRMUDD-UHFFFAOYSA-N SJ000286063 Natural products C12C(OC(=O)C(C)(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 RYMZZMVNJRMUDD-UHFFFAOYSA-N 0.000 claims description 2
- 229920001800 Shellac Polymers 0.000 claims description 2
- ABBQHOQBGMUPJH-UHFFFAOYSA-M Sodium salicylate Chemical compound [Na+].OC1=CC=CC=C1C([O-])=O ABBQHOQBGMUPJH-UHFFFAOYSA-M 0.000 claims description 2
- 229920002494 Zein Polymers 0.000 claims description 2
- 229960004150 aciclovir Drugs 0.000 claims description 2
- MKUXAQIIEYXACX-UHFFFAOYSA-N aciclovir Chemical compound N1C(N)=NC(=O)C2=C1N(COCCO)C=N2 MKUXAQIIEYXACX-UHFFFAOYSA-N 0.000 claims description 2
- 229940035676 analgesics Drugs 0.000 claims description 2
- 239000000730 antalgic agent Substances 0.000 claims description 2
- 239000003242 anti bacterial agent Substances 0.000 claims description 2
- 230000002744 anti-aggregatory effect Effects 0.000 claims description 2
- 230000001088 anti-asthma Effects 0.000 claims description 2
- 230000003474 anti-emetic effect Effects 0.000 claims description 2
- 230000001384 anti-glaucoma Effects 0.000 claims description 2
- 230000000648 anti-parkinson Effects 0.000 claims description 2
- 230000002921 anti-spasmodic effect Effects 0.000 claims description 2
- 239000000924 antiasthmatic agent Substances 0.000 claims description 2
- 229940088710 antibiotic agent Drugs 0.000 claims description 2
- 239000003529 anticholesteremic agent Substances 0.000 claims description 2
- 229940127090 anticoagulant agent Drugs 0.000 claims description 2
- 229940125681 anticonvulsant agent Drugs 0.000 claims description 2
- 239000001961 anticonvulsive agent Substances 0.000 claims description 2
- 229940125708 antidiabetic agent Drugs 0.000 claims description 2
- 239000003472 antidiabetic agent Substances 0.000 claims description 2
- 229940125683 antiemetic agent Drugs 0.000 claims description 2
- 239000002111 antiemetic agent Substances 0.000 claims description 2
- 239000000427 antigen Substances 0.000 claims description 2
- 108091007433 antigens Proteins 0.000 claims description 2
- 102000036639 antigens Human genes 0.000 claims description 2
- 229940006133 antiglaucoma drug and miotics carbonic anhydrase inhibitors Drugs 0.000 claims description 2
- 239000000739 antihistaminic agent Substances 0.000 claims description 2
- 229940124433 antimigraine drug Drugs 0.000 claims description 2
- 239000000939 antiparkinson agent Substances 0.000 claims description 2
- 239000003435 antirheumatic agent Substances 0.000 claims description 2
- 229940124575 antispasmodic agent Drugs 0.000 claims description 2
- 229960004676 antithrombotic agent Drugs 0.000 claims description 2
- 239000003434 antitussive agent Substances 0.000 claims description 2
- 229940124584 antitussives Drugs 0.000 claims description 2
- 239000003443 antiviral agent Substances 0.000 claims description 2
- 229960004405 aprotinin Drugs 0.000 claims description 2
- 229960005370 atorvastatin Drugs 0.000 claims description 2
- 229960000686 benzalkonium chloride Drugs 0.000 claims description 2
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 claims description 2
- FAKRSMQSSFJEIM-RQJHMYQMSA-N captopril Chemical compound SC[C@@H](C)C(=O)N1CCC[C@H]1C(O)=O FAKRSMQSSFJEIM-RQJHMYQMSA-N 0.000 claims description 2
- 229960000830 captopril Drugs 0.000 claims description 2
- 239000003489 carbonate dehydratase inhibitor Substances 0.000 claims description 2
- 210000002421 cell wall Anatomy 0.000 claims description 2
- 229920003086 cellulose ether Polymers 0.000 claims description 2
- 229940045110 chitosan Drugs 0.000 claims description 2
- 229960003405 ciprofloxacin Drugs 0.000 claims description 2
- 229940097362 cyclodextrins Drugs 0.000 claims description 2
- 229960002448 dasatinib Drugs 0.000 claims description 2
- 229960003964 deoxycholic acid Drugs 0.000 claims description 2
- 239000000645 desinfectant Substances 0.000 claims description 2
- 239000003599 detergent Substances 0.000 claims description 2
- 229960000633 dextran sulfate Drugs 0.000 claims description 2
- 229960004166 diltiazem Drugs 0.000 claims description 2
- HSUGRBWQSSZJOP-RTWAWAEBSA-N diltiazem Chemical compound C1=CC(OC)=CC=C1[C@H]1[C@@H](OC(C)=O)C(=O)N(CCN(C)C)C2=CC=CC=C2S1 HSUGRBWQSSZJOP-RTWAWAEBSA-N 0.000 claims description 2
- 208000035475 disorder Diseases 0.000 claims description 2
- 239000003136 dopamine receptor stimulating agent Substances 0.000 claims description 2
- GBXSMTUPTTWBMN-XIRDDKMYSA-N enalapril Chemical compound C([C@@H](C(=O)OCC)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(O)=O)CC1=CC=CC=C1 GBXSMTUPTTWBMN-XIRDDKMYSA-N 0.000 claims description 2
- 229960000873 enalapril Drugs 0.000 claims description 2
- 239000002871 fertility agent Substances 0.000 claims description 2
- 235000013305 food Nutrition 0.000 claims description 2
- 229940125695 gastrointestinal agent Drugs 0.000 claims description 2
- 239000004083 gastrointestinal agent Substances 0.000 claims description 2
- WIGIZIANZCJQQY-RUCARUNLSA-N glimepiride Chemical compound O=C1C(CC)=C(C)CN1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)N[C@@H]2CC[C@@H](C)CC2)C=C1 WIGIZIANZCJQQY-RUCARUNLSA-N 0.000 claims description 2
- 229960004346 glimepiride Drugs 0.000 claims description 2
- 229960001381 glipizide Drugs 0.000 claims description 2
- ZJJXGWJIGJFDTL-UHFFFAOYSA-N glipizide Chemical compound C1=NC(C)=CN=C1C(=O)NCCC1=CC=C(S(=O)(=O)NC(=O)NC2CCCCC2)C=C1 ZJJXGWJIGJFDTL-UHFFFAOYSA-N 0.000 claims description 2
- 229930182470 glycoside Natural products 0.000 claims description 2
- 229940088597 hormone Drugs 0.000 claims description 2
- 239000005556 hormone Substances 0.000 claims description 2
- 239000003326 hypnotic agent Substances 0.000 claims description 2
- 230000000147 hypnotic effect Effects 0.000 claims description 2
- 239000002955 immunomodulating agent Substances 0.000 claims description 2
- 229940121354 immunomodulator Drugs 0.000 claims description 2
- 229960003444 immunosuppressant agent Drugs 0.000 claims description 2
- 239000003018 immunosuppressive agent Substances 0.000 claims description 2
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 claims description 2
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 claims description 2
- 229960004844 lovastatin Drugs 0.000 claims description 2
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 claims description 2
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 claims description 2
- 239000003695 memory enhancer Substances 0.000 claims description 2
- 229940041616 menthol Drugs 0.000 claims description 2
- 229960003793 midazolam Drugs 0.000 claims description 2
- DDLIGBOFAVUZHB-UHFFFAOYSA-N midazolam Chemical compound C12=CC(Cl)=CC=C2N2C(C)=NC=C2CN=C1C1=CC=CC=C1F DDLIGBOFAVUZHB-UHFFFAOYSA-N 0.000 claims description 2
- 239000003149 muscarinic antagonist Substances 0.000 claims description 2
- 229940035363 muscle relaxants Drugs 0.000 claims description 2
- 239000003158 myorelaxant agent Substances 0.000 claims description 2
- 229960001800 nefazodone Drugs 0.000 claims description 2
- VRBKIVRKKCLPHA-UHFFFAOYSA-N nefazodone Chemical compound O=C1N(CCOC=2C=CC=CC=2)C(CC)=NN1CCCN(CC1)CCN1C1=CC=CC(Cl)=C1 VRBKIVRKKCLPHA-UHFFFAOYSA-N 0.000 claims description 2
- 229960001597 nifedipine Drugs 0.000 claims description 2
- HYIMSNHJOBLJNT-UHFFFAOYSA-N nifedipine Chemical compound COC(=O)C1=C(C)NC(C)=C(C(=O)OC)C1C1=CC=CC=C1[N+]([O-])=O HYIMSNHJOBLJNT-UHFFFAOYSA-N 0.000 claims description 2
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 claims description 2
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 claims description 2
- 229940127073 nucleoside analogue Drugs 0.000 claims description 2
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 claims description 2
- 229940094443 oxytocics prostaglandins Drugs 0.000 claims description 2
- 239000000734 parasympathomimetic agent Substances 0.000 claims description 2
- 230000007170 pathology Effects 0.000 claims description 2
- 229920001184 polypeptide Polymers 0.000 claims description 2
- 229920000136 polysorbate Polymers 0.000 claims description 2
- 229940068965 polysorbates Drugs 0.000 claims description 2
- 229960002965 pravastatin Drugs 0.000 claims description 2
- TUZYXOIXSAXUGO-PZAWKZKUSA-N pravastatin Chemical compound C1=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC(O)=O)[C@H]2[C@@H](OC(=O)[C@@H](C)CC)C[C@H](O)C=C21 TUZYXOIXSAXUGO-PZAWKZKUSA-N 0.000 claims description 2
- 150000003180 prostaglandins Chemical class 0.000 claims description 2
- 239000000932 sedative agent Substances 0.000 claims description 2
- 229940125723 sedative agent Drugs 0.000 claims description 2
- 229960003946 selegiline Drugs 0.000 claims description 2
- MEZLKOACVSPNER-GFCCVEGCSA-N selegiline Chemical compound C#CCN(C)[C@H](C)CC1=CC=CC=C1 MEZLKOACVSPNER-GFCCVEGCSA-N 0.000 claims description 2
- 239000004208 shellac Substances 0.000 claims description 2
- 229940113147 shellac Drugs 0.000 claims description 2
- ZLGIYFNHBLSMPS-ATJNOEHPSA-N shellac Chemical compound OCCCCCC(O)C(O)CCCCCCCC(O)=O.C1C23[C@H](C(O)=O)CCC2[C@](C)(CO)[C@@H]1C(C(O)=O)=C[C@@H]3O ZLGIYFNHBLSMPS-ATJNOEHPSA-N 0.000 claims description 2
- 235000013874 shellac Nutrition 0.000 claims description 2
- 229960002855 simvastatin Drugs 0.000 claims description 2
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 claims description 2
- 208000017520 skin disease Diseases 0.000 claims description 2
- FHHPUSMSKHSNKW-SMOYURAASA-M sodium deoxycholate Chemical compound [Na+].C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 FHHPUSMSKHSNKW-SMOYURAASA-M 0.000 claims description 2
- OABYVIYXWMZFFJ-ZUHYDKSRSA-M sodium glycocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCC([O-])=O)C)[C@@]2(C)[C@@H](O)C1 OABYVIYXWMZFFJ-ZUHYDKSRSA-M 0.000 claims description 2
- 229960004025 sodium salicylate Drugs 0.000 claims description 2
- JAJWGJBVLPIOOH-IZYKLYLVSA-M sodium taurocholate Chemical compound [Na+].C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCCS([O-])(=O)=O)C)[C@@]2(C)[C@@H](O)C1 JAJWGJBVLPIOOH-IZYKLYLVSA-M 0.000 claims description 2
- 239000002294 steroidal antiinflammatory agent Substances 0.000 claims description 2
- 150000003431 steroids Chemical class 0.000 claims description 2
- 229960004380 tramadol Drugs 0.000 claims description 2
- TVYLLZQTGLZFBW-GOEBONIOSA-N tramadol Natural products COC1=CC=CC([C@@]2(O)[C@@H](CCCC2)CN(C)C)=C1 TVYLLZQTGLZFBW-GOEBONIOSA-N 0.000 claims description 2
- 239000003204 tranquilizing agent Substances 0.000 claims description 2
- 230000002936 tranquilizing effect Effects 0.000 claims description 2
- 210000001635 urinary tract Anatomy 0.000 claims description 2
- 229960005486 vaccine Drugs 0.000 claims description 2
- 229920006163 vinyl copolymer Polymers 0.000 claims description 2
- 235000013343 vitamin Nutrition 0.000 claims description 2
- 229940088594 vitamin Drugs 0.000 claims description 2
- 239000011782 vitamin Substances 0.000 claims description 2
- 229930003231 vitamin Natural products 0.000 claims description 2
- 239000005019 zein Substances 0.000 claims description 2
- 229940093612 zein Drugs 0.000 claims description 2
- 150000004676 glycans Chemical class 0.000 claims 2
- AQHHHDLHHXJYJD-UHFFFAOYSA-N propranolol Chemical compound C1=CC=C2C(OCC(O)CNC(C)C)=CC=CC2=C1 AQHHHDLHHXJYJD-UHFFFAOYSA-N 0.000 claims 2
- SGTNSNPWRIOYBX-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCCC(C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-UHFFFAOYSA-N 0.000 claims 1
- 208000007848 Alcoholism Diseases 0.000 claims 1
- 208000010228 Erectile Dysfunction Diseases 0.000 claims 1
- GUGOEEXESWIERI-UHFFFAOYSA-N Terfenadine Chemical compound C1=CC(C(C)(C)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 GUGOEEXESWIERI-UHFFFAOYSA-N 0.000 claims 1
- 206010001584 alcohol abuse Diseases 0.000 claims 1
- 208000025746 alcohol use disease Diseases 0.000 claims 1
- 230000002075 anti-alcohol Effects 0.000 claims 1
- 230000001387 anti-histamine Effects 0.000 claims 1
- 230000002924 anti-infective effect Effects 0.000 claims 1
- 239000002246 antineoplastic agent Substances 0.000 claims 1
- 239000006196 drop Substances 0.000 claims 1
- 230000002526 effect on cardiovascular system Effects 0.000 claims 1
- 229960001596 famotidine Drugs 0.000 claims 1
- XUFQPHANEAPEMJ-UHFFFAOYSA-N famotidine Chemical compound NC(N)=NC1=NC(CSCCC(N)=NS(N)(=O)=O)=CS1 XUFQPHANEAPEMJ-UHFFFAOYSA-N 0.000 claims 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 claims 1
- 201000001881 impotence Diseases 0.000 claims 1
- 239000012678 infectious agent Substances 0.000 claims 1
- 229940063559 methacrylic acid Drugs 0.000 claims 1
- 229940124641 pain reliever Drugs 0.000 claims 1
- 229960003712 propranolol Drugs 0.000 claims 1
- 230000000541 pulsatile effect Effects 0.000 claims 1
- 230000005855 radiation Effects 0.000 claims 1
- 230000029058 respiratory gaseous exchange Effects 0.000 claims 1
- 239000012178 vegetable wax Substances 0.000 claims 1
- 229960001722 verapamil Drugs 0.000 claims 1
- 239000002994 raw material Substances 0.000 abstract description 4
- 230000000694 effects Effects 0.000 abstract description 3
- 231100000252 nontoxic Toxicity 0.000 abstract description 3
- 230000003000 nontoxic effect Effects 0.000 abstract description 3
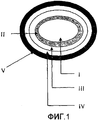
- 239000010410 layer Substances 0.000 description 148
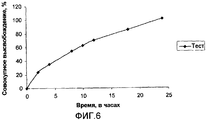
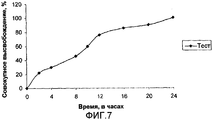
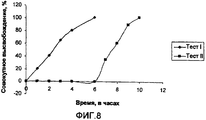
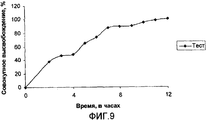
- 238000012360 testing method Methods 0.000 description 31
- 239000000243 solution Substances 0.000 description 23
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 21
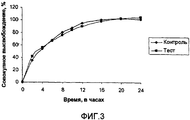
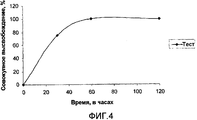
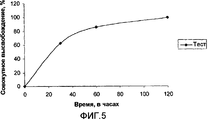
- 238000004090 dissolution Methods 0.000 description 20
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 17
- 239000008213 purified water Substances 0.000 description 15
- 239000004615 ingredient Substances 0.000 description 14
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 14
- 239000002202 Polyethylene glycol Substances 0.000 description 13
- 229920001223 polyethylene glycol Polymers 0.000 description 13
- YMWUJEATGCHHMB-UHFFFAOYSA-N Dichloromethane Chemical compound ClCCl YMWUJEATGCHHMB-UHFFFAOYSA-N 0.000 description 12
- 239000000047 product Substances 0.000 description 12
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 11
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 11
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 11
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 11
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 11
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 9
- 230000014759 maintenance of location Effects 0.000 description 9
- 229920002472 Starch Polymers 0.000 description 7
- 239000004480 active ingredient Substances 0.000 description 7
- 239000012467 final product Substances 0.000 description 7
- 239000008187 granular material Substances 0.000 description 7
- 235000019359 magnesium stearate Nutrition 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 235000019698 starch Nutrition 0.000 description 7
- 238000013268 sustained release Methods 0.000 description 7
- 239000012730 sustained-release form Substances 0.000 description 7
- 229920002785 Croscarmellose sodium Polymers 0.000 description 6
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 6
- ZFOZVQLOBQUTQQ-UHFFFAOYSA-N Tributyl citrate Chemical compound CCCCOC(=O)CC(O)(C(=O)OCCCC)CC(=O)OCCCC ZFOZVQLOBQUTQQ-UHFFFAOYSA-N 0.000 description 6
- 239000001768 carboxy methyl cellulose Substances 0.000 description 6
- 239000002552 dosage form Substances 0.000 description 6
- 238000011156 evaluation Methods 0.000 description 6
- 238000000338 in vitro Methods 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 6
- 239000008108 microcrystalline cellulose Substances 0.000 description 6
- 229940016286 microcrystalline cellulose Drugs 0.000 description 6
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 5
- WHNWPMSKXPGLAX-UHFFFAOYSA-N N-Vinyl-2-pyrrolidone Chemical compound C=CN1CCCC1=O WHNWPMSKXPGLAX-UHFFFAOYSA-N 0.000 description 5
- 239000004372 Polyvinyl alcohol Substances 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 210000001072 colon Anatomy 0.000 description 5
- 238000007906 compression Methods 0.000 description 5
- 230000006835 compression Effects 0.000 description 5
- 229960001681 croscarmellose sodium Drugs 0.000 description 5
- 235000010947 crosslinked sodium carboxy methyl cellulose Nutrition 0.000 description 5
- 230000001419 dependent effect Effects 0.000 description 5
- 238000012377 drug delivery Methods 0.000 description 5
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 5
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 5
- OETHQSJEHLVLGH-UHFFFAOYSA-N metformin hydrochloride Chemical compound Cl.CN(C)C(=N)N=C(N)N OETHQSJEHLVLGH-UHFFFAOYSA-N 0.000 description 5
- 239000002245 particle Substances 0.000 description 5
- 230000036470 plasma concentration Effects 0.000 description 5
- 229920002451 polyvinyl alcohol Polymers 0.000 description 5
- 239000008107 starch Substances 0.000 description 5
- 229940032147 starch Drugs 0.000 description 5
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- DOOTYTYQINUNNV-UHFFFAOYSA-N Triethyl citrate Chemical compound CCOC(=O)CC(O)(C(=O)OCC)CC(=O)OCC DOOTYTYQINUNNV-UHFFFAOYSA-N 0.000 description 4
- 239000012752 auxiliary agent Substances 0.000 description 4
- 239000000306 component Substances 0.000 description 4
- 238000013270 controlled release Methods 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- 229960001375 lactose Drugs 0.000 description 4
- 229920000609 methyl cellulose Polymers 0.000 description 4
- 235000010981 methylcellulose Nutrition 0.000 description 4
- 239000001923 methylcellulose Substances 0.000 description 4
- 235000010755 mineral Nutrition 0.000 description 4
- 238000012986 modification Methods 0.000 description 4
- 230000004048 modification Effects 0.000 description 4
- 238000000465 moulding Methods 0.000 description 4
- 229940069328 povidone Drugs 0.000 description 4
- 239000000843 powder Substances 0.000 description 4
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 4
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 4
- 239000001069 triethyl citrate Substances 0.000 description 4
- VMYFZRTXGLUXMZ-UHFFFAOYSA-N triethyl citrate Natural products CCOC(=O)C(O)(C(=O)OCC)C(=O)OCC VMYFZRTXGLUXMZ-UHFFFAOYSA-N 0.000 description 4
- 235000013769 triethyl citrate Nutrition 0.000 description 4
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 235000010443 alginic acid Nutrition 0.000 description 3
- 229920000615 alginic acid Polymers 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 239000003125 aqueous solvent Substances 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- KWGRBVOPPLSCSI-UHFFFAOYSA-N d-ephedrine Natural products CNC(C)C(O)C1=CC=CC=C1 KWGRBVOPPLSCSI-UHFFFAOYSA-N 0.000 description 3
- SWXVUIWOUIDPGS-UHFFFAOYSA-N diacetone alcohol Natural products CC(=O)CC(C)(C)O SWXVUIWOUIDPGS-UHFFFAOYSA-N 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000007884 disintegrant Substances 0.000 description 3
- 239000000975 dye Substances 0.000 description 3
- 230000006870 function Effects 0.000 description 3
- 238000005469 granulation Methods 0.000 description 3
- 230000003179 granulation Effects 0.000 description 3
- 238000011031 large-scale manufacturing process Methods 0.000 description 3
- 230000000670 limiting effect Effects 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 229960004329 metformin hydrochloride Drugs 0.000 description 3
- 239000012229 microporous material Substances 0.000 description 3
- 150000004804 polysaccharides Chemical class 0.000 description 3
- 230000008569 process Effects 0.000 description 3
- 238000012545 processing Methods 0.000 description 3
- 229960003908 pseudoephedrine Drugs 0.000 description 3
- KWGRBVOPPLSCSI-WCBMZHEXSA-N pseudoephedrine Chemical compound CN[C@@H](C)[C@@H](O)C1=CC=CC=C1 KWGRBVOPPLSCSI-WCBMZHEXSA-N 0.000 description 3
- 238000005507 spraying Methods 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 238000007619 statistical method Methods 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 230000002123 temporal effect Effects 0.000 description 3
- 239000001993 wax Substances 0.000 description 3
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- WSVLPVUVIUVCRA-KPKNDVKVSA-N Alpha-lactose monohydrate Chemical compound O.O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O WSVLPVUVIUVCRA-KPKNDVKVSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 2
- PYGXAGIECVVIOZ-UHFFFAOYSA-N Dibutyl decanedioate Chemical compound CCCCOC(=O)CCCCCCCCC(=O)OCCCC PYGXAGIECVVIOZ-UHFFFAOYSA-N 0.000 description 2
- 229920003134 Eudragit® polymer Polymers 0.000 description 2
- 229920002907 Guar gum Polymers 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 2
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 2
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- GWEVSGVZZGPLCZ-UHFFFAOYSA-N Titan oxide Chemical compound O=[Ti]=O GWEVSGVZZGPLCZ-UHFFFAOYSA-N 0.000 description 2
- XTXRWKRVRITETP-UHFFFAOYSA-N Vinyl acetate Chemical compound CC(=O)OC=C XTXRWKRVRITETP-UHFFFAOYSA-N 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 150000001252 acrylic acid derivatives Chemical class 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 229940072056 alginate Drugs 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000003254 anti-foaming effect Effects 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 2
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 2
- 239000008199 coating composition Substances 0.000 description 2
- 238000000354 decomposition reaction Methods 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 238000013461 design Methods 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- DOIRQSBPFJWKBE-UHFFFAOYSA-N dibutyl phthalate Chemical compound CCCCOC(=O)C1=CC=CC=C1C(=O)OCCCC DOIRQSBPFJWKBE-UHFFFAOYSA-N 0.000 description 2
- FLKPEMZONWLCSK-UHFFFAOYSA-N diethyl phthalate Chemical compound CCOC(=O)C1=CC=CC=C1C(=O)OCC FLKPEMZONWLCSK-UHFFFAOYSA-N 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- OVBPIULPVIDEAO-LBPRGKRZSA-N folic acid Chemical compound C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-LBPRGKRZSA-N 0.000 description 2
- 229940093617 glumetza Drugs 0.000 description 2
- 235000010417 guar gum Nutrition 0.000 description 2
- 239000000665 guar gum Substances 0.000 description 2
- 229960002154 guar gum Drugs 0.000 description 2
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 2
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 2
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 2
- 229960004768 irinotecan Drugs 0.000 description 2
- 229960001021 lactose monohydrate Drugs 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 229960001571 loperamide Drugs 0.000 description 2
- RDOIQAHITMMDAJ-UHFFFAOYSA-N loperamide Chemical compound C=1C=CC=CC=1C(C=1C=CC=CC=1)(C(=O)N(C)C)CCN(CC1)CCC1(O)C1=CC=C(Cl)C=C1 RDOIQAHITMMDAJ-UHFFFAOYSA-N 0.000 description 2
- 210000003750 lower gastrointestinal tract Anatomy 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 210000004379 membrane Anatomy 0.000 description 2
- 239000003094 microcapsule Substances 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- XNGIFLGASWRNHJ-UHFFFAOYSA-L phthalate(2-) Chemical compound [O-]C(=O)C1=CC=CC=C1C([O-])=O XNGIFLGASWRNHJ-UHFFFAOYSA-L 0.000 description 2
- 229940068196 placebo Drugs 0.000 description 2
- 239000000902 placebo Substances 0.000 description 2
- 229920003023 plastic Polymers 0.000 description 2
- 239000004033 plastic Substances 0.000 description 2
- 229920001451 polypropylene glycol Polymers 0.000 description 2
- 239000011148 porous material Substances 0.000 description 2
- 210000001187 pylorus Anatomy 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 235000010413 sodium alginate Nutrition 0.000 description 2
- 239000000661 sodium alginate Substances 0.000 description 2
- 229940005550 sodium alginate Drugs 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 2
- 230000004584 weight gain Effects 0.000 description 2
- 235000019786 weight gain Nutrition 0.000 description 2
- 238000005550 wet granulation Methods 0.000 description 2
- 229920001285 xanthan gum Polymers 0.000 description 2
- 235000010493 xanthan gum Nutrition 0.000 description 2
- 239000000230 xanthan gum Substances 0.000 description 2
- 229940082509 xanthan gum Drugs 0.000 description 2
- PYMYPHUHKUWMLA-UHFFFAOYSA-N 2,3,4,5-tetrahydroxypentanal Chemical compound OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 1
- AFGJFZRBBVSQJT-UHFFFAOYSA-N 2-(3-oxobutanoyloxycarbonyl)benzoic acid Chemical compound CC(=O)CC(=O)OC(=O)C1=CC=CC=C1C(O)=O AFGJFZRBBVSQJT-UHFFFAOYSA-N 0.000 description 1
- GVIRAXRGZUXHCI-UHFFFAOYSA-N 2-acetyloxycarbonylbenzoic acid Chemical compound CC(=O)OC(=O)C1=CC=CC=C1C(O)=O GVIRAXRGZUXHCI-UHFFFAOYSA-N 0.000 description 1
- PYSRRFNXTXNWCD-UHFFFAOYSA-N 3-(2-phenylethenyl)furan-2,5-dione Chemical compound O=C1OC(=O)C(C=CC=2C=CC=CC=2)=C1 PYSRRFNXTXNWCD-UHFFFAOYSA-N 0.000 description 1
- GSDSWSVVBLHKDQ-UHFFFAOYSA-N 9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-oxo-2,3-dihydro-7H-[1,4]oxazino[2,3,4-ij]quinoline-6-carboxylic acid Chemical compound FC1=CC(C(C(C(O)=O)=C2)=O)=C3N2C(C)COC3=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-UHFFFAOYSA-N 0.000 description 1
- 239000005541 ACE inhibitor Substances 0.000 description 1
- 244000215068 Acacia senegal Species 0.000 description 1
- 229920002126 Acrylic acid copolymer Polymers 0.000 description 1
- 239000004382 Amylase Substances 0.000 description 1
- 102000013142 Amylases Human genes 0.000 description 1
- 108010065511 Amylases Proteins 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 241000416162 Astragalus gummifer Species 0.000 description 1
- PTHCMJGKKRQCBF-UHFFFAOYSA-N Cellulose, microcrystalline Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC)C(CO)O1 PTHCMJGKKRQCBF-UHFFFAOYSA-N 0.000 description 1
- 229920002101 Chitin Polymers 0.000 description 1
- JZUFKLXOESDKRF-UHFFFAOYSA-N Chlorothiazide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NCNS2(=O)=O JZUFKLXOESDKRF-UHFFFAOYSA-N 0.000 description 1
- 229920001268 Cholestyramine Polymers 0.000 description 1
- 244000303965 Cyamopsis psoralioides Species 0.000 description 1
- 102000002004 Cytochrome P-450 Enzyme System Human genes 0.000 description 1
- 108010015742 Cytochrome P-450 Enzyme System Proteins 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 235000019739 Dicalciumphosphate Nutrition 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 229920003139 Eudragit® L 100 Polymers 0.000 description 1
- 229920003135 Eudragit® L 100-55 Polymers 0.000 description 1
- 229920003141 Eudragit® S 100 Polymers 0.000 description 1
- 229920003137 Eudragit® S polymer Polymers 0.000 description 1
- 229920000926 Galactomannan Polymers 0.000 description 1
- 241000206672 Gelidium Species 0.000 description 1
- 229920000084 Gum arabic Polymers 0.000 description 1
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 1
- UFHFLCQGNIYNRP-UHFFFAOYSA-N Hydrogen Chemical compound [H][H] UFHFLCQGNIYNRP-UHFFFAOYSA-N 0.000 description 1
- 229920001479 Hydroxyethyl methyl cellulose Polymers 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229920001202 Inulin Polymers 0.000 description 1
- 108010007859 Lisinopril Proteins 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- OVBPIULPVIDEAO-UHFFFAOYSA-N N-Pteroyl-L-glutaminsaeure Natural products C=1N=C2NC(N)=NC(=O)C2=NC=1CNC1=CC=C(C(=O)NC(CCC(O)=O)C(O)=O)C=C1 OVBPIULPVIDEAO-UHFFFAOYSA-N 0.000 description 1
- 206010028813 Nausea Diseases 0.000 description 1
- WXOMTJVVIMOXJL-BOBFKVMVSA-A O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)OS(=O)(=O)OC[C@H]1O[C@@H](O[C@]2(COS(=O)(=O)O[Al](O)O)O[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]2OS(=O)(=O)O[Al](O)O)[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]1OS(=O)(=O)O[Al](O)O Chemical compound O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)O.O[Al](O)OS(=O)(=O)OC[C@H]1O[C@@H](O[C@]2(COS(=O)(=O)O[Al](O)O)O[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]2OS(=O)(=O)O[Al](O)O)[C@H](OS(=O)(=O)O[Al](O)O)[C@@H](OS(=O)(=O)O[Al](O)O)[C@@H]1OS(=O)(=O)O[Al](O)O WXOMTJVVIMOXJL-BOBFKVMVSA-A 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 229920000148 Polycarbophil calcium Polymers 0.000 description 1
- GOOHAUXETOMSMM-UHFFFAOYSA-N Propylene oxide Chemical compound CC1CO1 GOOHAUXETOMSMM-UHFFFAOYSA-N 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 229920002385 Sodium hyaluronate Polymers 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 229920000147 Styrene maleic anhydride Polymers 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- 229920002807 Thiomer Polymers 0.000 description 1
- 229920001615 Tragacanth Polymers 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- 241000482268 Zea mays subsp. mays Species 0.000 description 1
- 210000001015 abdomen Anatomy 0.000 description 1
- 235000010489 acacia gum Nutrition 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- IYKJEILNJZQJPU-UHFFFAOYSA-N acetic acid;butanedioic acid Chemical compound CC(O)=O.OC(=O)CCC(O)=O IYKJEILNJZQJPU-UHFFFAOYSA-N 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 150000001253 acrylic acids Chemical class 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 239000004964 aerogel Substances 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001340 alkali metals Chemical class 0.000 description 1
- 150000003863 ammonium salts Chemical class 0.000 description 1
- 235000019418 amylase Nutrition 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 229940044094 angiotensin-converting-enzyme inhibitor Drugs 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 229940069428 antacid Drugs 0.000 description 1
- 239000003159 antacid agent Substances 0.000 description 1
- 239000002518 antifoaming agent Substances 0.000 description 1
- 229940125715 antihistaminic agent Drugs 0.000 description 1
- 229960005475 antiinfective agent Drugs 0.000 description 1
- 230000009876 antimalignant effect Effects 0.000 description 1
- 239000004599 antimicrobial Substances 0.000 description 1
- 239000003212 astringent agent Substances 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- 230000035587 bioadhesion Effects 0.000 description 1
- 239000008280 blood Substances 0.000 description 1
- 210000004369 blood Anatomy 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 238000009835 boiling Methods 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- VHEMBTYWURNBQQ-UHFFFAOYSA-N butanoic acid;phthalic acid Chemical compound CCCC(O)=O.OC(=O)C1=CC=CC=C1C(O)=O VHEMBTYWURNBQQ-UHFFFAOYSA-N 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 229940095672 calcium sulfate Drugs 0.000 description 1
- 235000011132 calcium sulphate Nutrition 0.000 description 1
- 239000001569 carbon dioxide Substances 0.000 description 1
- 229910002092 carbon dioxide Inorganic materials 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 239000002327 cardiovascular agent Substances 0.000 description 1
- 229940125692 cardiovascular agent Drugs 0.000 description 1
- 229920001525 carrageenan Polymers 0.000 description 1
- 235000010418 carrageenan Nutrition 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 229920006184 cellulose methylcellulose Polymers 0.000 description 1
- 229910010293 ceramic material Inorganic materials 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 229920001688 coating polymer Polymers 0.000 description 1
- 238000005056 compaction Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- 239000000599 controlled substance Substances 0.000 description 1
- 229920006037 cross link polymer Polymers 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical compound [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 229940038472 dicalcium phosphate Drugs 0.000 description 1
- 229910000390 dicalcium phosphate Inorganic materials 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 238000007907 direct compression Methods 0.000 description 1
- 201000010099 disease Diseases 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- 238000007908 dry granulation Methods 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 229940088598 enzyme Drugs 0.000 description 1
- 230000001856 erectile effect Effects 0.000 description 1
- MVPICKVDHDWCJQ-UHFFFAOYSA-N ethyl 3-pyrrolidin-1-ylpropanoate Chemical compound CCOC(=O)CCN1CCCC1 MVPICKVDHDWCJQ-UHFFFAOYSA-N 0.000 description 1
- 229960004667 ethyl cellulose Drugs 0.000 description 1
- GDCRSXZBSIRSFR-UHFFFAOYSA-N ethyl prop-2-enoate;2-methylprop-2-enoic acid Chemical compound CC(=C)C(O)=O.CCOC(=O)C=C GDCRSXZBSIRSFR-UHFFFAOYSA-N 0.000 description 1
- 238000013265 extended release Methods 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000006260 foam Substances 0.000 description 1
- 229960000304 folic acid Drugs 0.000 description 1
- 235000019152 folic acid Nutrition 0.000 description 1
- 239000011724 folic acid Substances 0.000 description 1
- XAOWEWOKLXWUHI-UHFFFAOYSA-N furan-2,5-dione;methyl prop-2-enoate;prop-2-enenitrile Chemical compound C=CC#N.COC(=O)C=C.O=C1OC(=O)C=C1 XAOWEWOKLXWUHI-UHFFFAOYSA-N 0.000 description 1
- ZZUFCTLCJUWOSV-UHFFFAOYSA-N furosemide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC(C(O)=O)=C1NCC1=CC=CO1 ZZUFCTLCJUWOSV-UHFFFAOYSA-N 0.000 description 1
- 229960003883 furosemide Drugs 0.000 description 1
- 230000004927 fusion Effects 0.000 description 1
- 210000004211 gastric acid Anatomy 0.000 description 1
- 210000004051 gastric juice Anatomy 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 239000007903 gelatin capsule Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 125000005908 glyceryl ester group Chemical group 0.000 description 1
- 239000001087 glyceryl triacetate Substances 0.000 description 1
- 235000013773 glyceryl triacetate Nutrition 0.000 description 1
- 230000005484 gravity Effects 0.000 description 1
- 239000012943 hotmelt Substances 0.000 description 1
- 150000004677 hydrates Chemical class 0.000 description 1
- 229960002003 hydrochlorothiazide Drugs 0.000 description 1
- 239000000416 hydrocolloid Substances 0.000 description 1
- 229920001477 hydrophilic polymer Polymers 0.000 description 1
- 229920001600 hydrophobic polymer Polymers 0.000 description 1
- 125000002768 hydroxyalkyl group Chemical group 0.000 description 1
- 229940071676 hydroxypropylcellulose Drugs 0.000 description 1
- 229920000639 hydroxypropylmethylcellulose acetate succinate Polymers 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 150000002484 inorganic compounds Chemical class 0.000 description 1
- 229910010272 inorganic material Inorganic materials 0.000 description 1
- 229940029339 inulin Drugs 0.000 description 1
- JYJIGFIDKWBXDU-MNNPPOADSA-N inulin Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)OC[C@]1(OC[C@]2(OC[C@]3(OC[C@]4(OC[C@]5(OC[C@]6(OC[C@]7(OC[C@]8(OC[C@]9(OC[C@]%10(OC[C@]%11(OC[C@]%12(OC[C@]%13(OC[C@]%14(OC[C@]%15(OC[C@]%16(OC[C@]%17(OC[C@]%18(OC[C@]%19(OC[C@]%20(OC[C@]%21(OC[C@]%22(OC[C@]%23(OC[C@]%24(OC[C@]%25(OC[C@]%26(OC[C@]%27(OC[C@]%28(OC[C@]%29(OC[C@]%30(OC[C@]%31(OC[C@]%32(OC[C@]%33(OC[C@]%34(OC[C@]%35(OC[C@]%36(O[C@@H]%37[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O%37)O)[C@H]([C@H](O)[C@@H](CO)O%36)O)[C@H]([C@H](O)[C@@H](CO)O%35)O)[C@H]([C@H](O)[C@@H](CO)O%34)O)[C@H]([C@H](O)[C@@H](CO)O%33)O)[C@H]([C@H](O)[C@@H](CO)O%32)O)[C@H]([C@H](O)[C@@H](CO)O%31)O)[C@H]([C@H](O)[C@@H](CO)O%30)O)[C@H]([C@H](O)[C@@H](CO)O%29)O)[C@H]([C@H](O)[C@@H](CO)O%28)O)[C@H]([C@H](O)[C@@H](CO)O%27)O)[C@H]([C@H](O)[C@@H](CO)O%26)O)[C@H]([C@H](O)[C@@H](CO)O%25)O)[C@H]([C@H](O)[C@@H](CO)O%24)O)[C@H]([C@H](O)[C@@H](CO)O%23)O)[C@H]([C@H](O)[C@@H](CO)O%22)O)[C@H]([C@H](O)[C@@H](CO)O%21)O)[C@H]([C@H](O)[C@@H](CO)O%20)O)[C@H]([C@H](O)[C@@H](CO)O%19)O)[C@H]([C@H](O)[C@@H](CO)O%18)O)[C@H]([C@H](O)[C@@H](CO)O%17)O)[C@H]([C@H](O)[C@@H](CO)O%16)O)[C@H]([C@H](O)[C@@H](CO)O%15)O)[C@H]([C@H](O)[C@@H](CO)O%14)O)[C@H]([C@H](O)[C@@H](CO)O%13)O)[C@H]([C@H](O)[C@@H](CO)O%12)O)[C@H]([C@H](O)[C@@H](CO)O%11)O)[C@H]([C@H](O)[C@@H](CO)O%10)O)[C@H]([C@H](O)[C@@H](CO)O9)O)[C@H]([C@H](O)[C@@H](CO)O8)O)[C@H]([C@H](O)[C@@H](CO)O7)O)[C@H]([C@H](O)[C@@H](CO)O6)O)[C@H]([C@H](O)[C@@H](CO)O5)O)[C@H]([C@H](O)[C@@H](CO)O4)O)[C@H]([C@H](O)[C@@H](CO)O3)O)[C@H]([C@H](O)[C@@H](CO)O2)O)[C@@H](O)[C@H](O)[C@@H](CO)O1 JYJIGFIDKWBXDU-MNNPPOADSA-N 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 238000002386 leaching Methods 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 229960002394 lisinopril Drugs 0.000 description 1
- CZRQXSDBMCMPNJ-ZUIPZQNBSA-N lisinopril dihydrate Chemical compound O.O.C([C@H](N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(O)=O)C(O)=O)CC1=CC=CC=C1 CZRQXSDBMCMPNJ-ZUIPZQNBSA-N 0.000 description 1
- 239000004620 low density foam Substances 0.000 description 1
- 238000005461 lubrication Methods 0.000 description 1
- 229940037627 magnesium lauryl sulfate Drugs 0.000 description 1
- 229940057948 magnesium stearate Drugs 0.000 description 1
- HBNDBUATLJAUQM-UHFFFAOYSA-L magnesium;dodecyl sulfate Chemical compound [Mg+2].CCCCCCCCCCCCOS([O-])(=O)=O.CCCCCCCCCCCCOS([O-])(=O)=O HBNDBUATLJAUQM-UHFFFAOYSA-L 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 229960001855 mannitol Drugs 0.000 description 1
- 239000008204 material by function Substances 0.000 description 1
- 238000007909 melt granulation Methods 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 229920003145 methacrylic acid copolymer Polymers 0.000 description 1
- IQSHMXAZFHORGY-UHFFFAOYSA-N methyl prop-2-enoate;2-methylprop-2-enoic acid Chemical compound COC(=O)C=C.CC(=C)C(O)=O IQSHMXAZFHORGY-UHFFFAOYSA-N 0.000 description 1
- MYSWGNHLJGOCPT-UHFFFAOYSA-N methyl prop-2-enoate;prop-2-enoic acid Chemical compound OC(=O)C=C.COC(=O)C=C MYSWGNHLJGOCPT-UHFFFAOYSA-N 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 210000004400 mucous membrane Anatomy 0.000 description 1
- 230000008693 nausea Effects 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 229960001699 ofloxacin Drugs 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 239000003960 organic solvent Substances 0.000 description 1
- 238000004806 packaging method and process Methods 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 238000005453 pelletization Methods 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 229950005134 polycarbophil Drugs 0.000 description 1
- 229920005606 polypropylene copolymer Polymers 0.000 description 1
- 229920003124 powdered cellulose Polymers 0.000 description 1
- 235000019814 powdered cellulose Nutrition 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 229960001520 ranitidine hydrochloride Drugs 0.000 description 1
- GGWBHVILAJZWKJ-KJEVSKRMSA-N ranitidine hydrochloride Chemical compound [H+].[Cl-].[O-][N+](=O)\C=C(/NC)NCCSCC1=CC=C(CN(C)C)O1 GGWBHVILAJZWKJ-KJEVSKRMSA-N 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 239000011347 resin Substances 0.000 description 1
- 229920005989 resin Polymers 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 239000002356 single layer Substances 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229940010747 sodium hyaluronate Drugs 0.000 description 1
- RYYKJJJTJZKILX-UHFFFAOYSA-M sodium octadecanoate Chemical compound [Na+].CCCCCCCCCCCCCCCCCC([O-])=O RYYKJJJTJZKILX-UHFFFAOYSA-M 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229940045902 sodium stearyl fumarate Drugs 0.000 description 1
- YWIVKILSMZOHHF-QJZPQSOGSA-N sodium;(2s,3s,4s,5r,6r)-6-[(2s,3r,4r,5s,6r)-3-acetamido-2-[(2s,3s,4r,5r,6r)-6-[(2r,3r,4r,5s,6r)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2- Chemical compound [Na+].CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 YWIVKILSMZOHHF-QJZPQSOGSA-N 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 239000002195 soluble material Substances 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 229960002920 sorbitol Drugs 0.000 description 1
- 235000010356 sorbitol Nutrition 0.000 description 1
- 238000005563 spheronization Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 229960004291 sucralfate Drugs 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 229960004793 sucrose Drugs 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 239000007916 tablet composition Substances 0.000 description 1
- IMCGHZIGRANKHV-AJNGGQMLSA-N tert-butyl (3s,5s)-2-oxo-5-[(2s,4s)-5-oxo-4-propan-2-yloxolan-2-yl]-3-propan-2-ylpyrrolidine-1-carboxylate Chemical compound O1C(=O)[C@H](C(C)C)C[C@H]1[C@H]1N(C(=O)OC(C)(C)C)C(=O)[C@H](C(C)C)C1 IMCGHZIGRANKHV-AJNGGQMLSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 229920001169 thermoplastic Polymers 0.000 description 1
- 239000010409 thin film Substances 0.000 description 1
- 239000004408 titanium dioxide Substances 0.000 description 1
- 229960005196 titanium dioxide Drugs 0.000 description 1
- 235000010215 titanium dioxide Nutrition 0.000 description 1
- 235000010487 tragacanth Nutrition 0.000 description 1
- 239000000196 tragacanth Substances 0.000 description 1
- 229940116362 tragacanth Drugs 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 229960002622 triacetin Drugs 0.000 description 1
- WEAPVABOECTMGR-UHFFFAOYSA-N triethyl 2-acetyloxypropane-1,2,3-tricarboxylate Chemical compound CCOC(=O)CC(C(=O)OCC)(OC(C)=O)CC(=O)OCC WEAPVABOECTMGR-UHFFFAOYSA-N 0.000 description 1
- 210000002438 upper gastrointestinal tract Anatomy 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 235000013311 vegetables Nutrition 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0053—Mouth and digestive tract, i.e. intraoral and peroral administration
- A61K9/0065—Forms with gastric retention, e.g. floating on gastric juice, adhering to gastric mucosa, expanding to prevent passage through the pylorus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/34—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyesters, polyamino acids, polysiloxanes, polyphosphazines, copolymers of polyalkylene glycol or poloxamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/06—Anti-spasmodics, e.g. drugs for colics, esophagic dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/08—Antiallergic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2072—Pills, tablets, discs, rods characterised by shape, structure or size; Tablets with holes, special break lines or identification marks; Partially coated tablets; Disintegrating flat shaped forms
- A61K9/2086—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat
- A61K9/209—Layered tablets, e.g. bilayer tablets; Tablets of the type inert core-active coat containing drug in at least two layers or in the core and in at least one outer layer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2095—Tabletting processes; Dosage units made by direct compression of powders or specially processed granules, by eliminating solvents, by melt-extrusion, by injection molding, by 3D printing
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/28—Dragees; Coated pills or tablets, e.g. with film or compression coating
- A61K9/2886—Dragees; Coated pills or tablets, e.g. with film or compression coating having two or more different drug-free coatings; Tablets of the type inert core-drug layer-inactive layer
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Public Health (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Diabetes (AREA)
- Physiology (AREA)
- Nutrition Science (AREA)
- Inorganic Chemistry (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Endocrinology (AREA)
- Emergency Medicine (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pulmonology (AREA)
- Immunology (AREA)
- Urology & Nephrology (AREA)
- Vascular Medicine (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Medicinal Preparation (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN1411MU2006 | 2006-09-04 | ||
| IN1411/MUM/2006 | 2006-09-04 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| RU2009112396A RU2009112396A (ru) | 2010-10-20 |
| RU2420268C2 true RU2420268C2 (ru) | 2011-06-10 |
Family
ID=39430167
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| RU2009112396/15A RU2420268C2 (ru) | 2006-09-04 | 2007-09-03 | Способ программируемой плавучей доставки |
Country Status (16)
| Country | Link |
|---|---|
| US (1) | US8277843B2 (enExample) |
| EP (1) | EP2068844A4 (enExample) |
| JP (1) | JP2010502591A (enExample) |
| KR (1) | KR20090065524A (enExample) |
| CN (1) | CN101511346B (enExample) |
| AR (1) | AR062644A1 (enExample) |
| AU (1) | AU2007323018B2 (enExample) |
| BR (1) | BRPI0716481A2 (enExample) |
| CA (1) | CA2661172A1 (enExample) |
| CL (1) | CL2007002562A1 (enExample) |
| IL (1) | IL197121A0 (enExample) |
| MX (1) | MX2009002371A (enExample) |
| NO (1) | NO20090807A (enExample) |
| RU (1) | RU2420268C2 (enExample) |
| WO (1) | WO2008062440A2 (enExample) |
| ZA (1) | ZA200901162B (enExample) |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7201920B2 (en) | 2003-11-26 | 2007-04-10 | Acura Pharmaceuticals, Inc. | Methods and compositions for deterring abuse of opioid containing dosage forms |
| WO2009153635A1 (en) * | 2008-06-19 | 2009-12-23 | University Of Witwatersrand, Johannesburg | A chronotherapeutic pharmaceutical dosage form |
| ZA200903858B (en) * | 2008-06-19 | 2013-10-30 | Univ Of Witwatesrand Johannesburg | Pharmaceutical dosage form |
| CA2638240C (en) * | 2008-08-29 | 2010-02-02 | Alexander Macgregor | Method of treating dysglycemia and glucose excursions |
| CA2767143A1 (en) * | 2009-07-06 | 2011-01-13 | Kyorin Pharmaceutical Co., Ltd. | Tablet having hollow structure |
| US8901113B2 (en) | 2009-09-30 | 2014-12-02 | Acura Pharmaceuticals, Inc. | Methods and compositions for deterring abuse |
| US20110177231A1 (en) * | 2010-01-16 | 2011-07-21 | Alexander Grinberg | Nano-, Micro-, Macro- Encapsulation And Release Of Materials |
| US20110200671A1 (en) * | 2010-02-17 | 2011-08-18 | Sun Pharma Advanced Research Company Ltd. | Method of treating a disease condition susceptible to baclofen therapy |
| EP2415460A1 (de) * | 2010-08-03 | 2012-02-08 | ratiopharm GmbH | Orale Darreichungsform von Pregabalin |
| GB201102148D0 (en) | 2011-02-08 | 2011-03-23 | Ucl Business Plc | Layered bodies, compositions containing them and processes for producing them |
| BR112014000773A8 (pt) * | 2011-07-14 | 2017-10-03 | Apet Holding B V | Composições de nicotinamida e uso terapêutico das mesmas |
| ES2691982T3 (es) | 2012-11-30 | 2018-11-29 | Acura Pharmaceuticals, Inc. | Liberación autorregulada de un principio activo farmacéutico |
| US8859005B2 (en) | 2012-12-03 | 2014-10-14 | Intercontinental Great Brands Llc | Enteric delivery of functional ingredients suitable for hot comestible applications |
| KR102241487B1 (ko) | 2013-02-20 | 2021-04-16 | 주식회사 종근당 | 제어방출 펠릿으로 된 약제학적 조성물 |
| US20160242632A1 (en) * | 2013-10-22 | 2016-08-25 | Ganyu Lu | System and Method for Capsule Device with Multiple Phases of Density |
| WO2015145461A1 (en) | 2014-03-26 | 2015-10-01 | Sun Pharma Advanced Research Company Ltd. | Abuse deterrent immediate release biphasic matrix solid dosage form |
| CN106714893B (zh) | 2014-06-11 | 2021-01-01 | 麻省理工学院 | 自组装的驻留装置及相关方法 |
| US20170266112A1 (en) | 2014-06-11 | 2017-09-21 | Massachusetts Institute Of Technology | Residence structures and related methods |
| WO2017040607A1 (en) | 2015-08-31 | 2017-03-09 | Acura Pharmaceuticals, Inc. | Methods and compositions for self-regulated release of active pharmaceutical ingredient |
| HK1258050A1 (zh) | 2015-10-23 | 2019-11-01 | Lyndra Therapeutics, Inc. | 用於治疗剂缓释的胃驻留系统及其使用方法 |
| WO2017100367A1 (en) | 2015-12-08 | 2017-06-15 | Lyndra, Inc. | Geometric configurations for gastric residence systems |
| AU2017268840B2 (en) | 2016-05-27 | 2022-02-10 | Lyndra Therapeutics, Inc. | Materials architecture for gastric residence systems |
| WO2018064630A1 (en) | 2016-09-30 | 2018-04-05 | Lyndra, Inc. | Gastric residence systems for sustained delivery of adamantane-class drugs |
| US11534404B2 (en) * | 2016-10-06 | 2022-12-27 | Sucampo Ag | Multilayer beads for pharmaceutical use |
| CA3066658A1 (en) | 2017-06-09 | 2018-12-13 | Lyndra, Inc. | Gastric residence systems with release rate-modulating films |
| WO2019025869A1 (en) * | 2017-07-31 | 2019-02-07 | Teva Pharmaceutical Industries Limited | GALENIC FORMS WITH CONTROLLED RELEASE MANUFACTURED IN ADDITIVE MANNER |
| CA3086153A1 (en) | 2017-12-18 | 2019-06-27 | Tris Pharma, Inc. | Modified release drug powder composition comprising gastro-retentive raft forming systems having trigger pulse drug release |
| US11337920B2 (en) | 2017-12-18 | 2022-05-24 | Tris Pharma, Inc. | Pharmaceutical composition comprising GHB gastro-retentive raft forming systems having trigger pulse drug release |
| CA3085941A1 (en) | 2017-12-18 | 2019-06-27 | Tris Pharma, Inc. | Ghb pharmaceutical compositions comprising a floating interpenetrating polymer network forming system |
| JP7000148B2 (ja) * | 2017-12-27 | 2022-01-19 | 日本化薬株式会社 | ダサチニブを有効成分とする医薬組成物 |
| EP4013401A4 (en) | 2019-08-12 | 2023-07-12 | Massachusetts Institute of Technology | Articles and methods for administration of therapeutic agents |
| TWI763036B (zh) * | 2020-09-14 | 2022-05-01 | 王子賓 | 可膨脹微膠囊 |
| BR112023018524A2 (pt) | 2021-03-15 | 2023-11-21 | Epitomee Medical Ltd | Dispositivos expansíveis para entrega de agentes ativos aos tecidos |
| KR20250112750A (ko) | 2022-09-21 | 2025-07-24 | 이피토미 메디칼 엘티디 | 제어 가능하게 붕해될 수 있는 자체-확장가능한 섭취형 디바이스 |
| NL2033549B1 (en) * | 2022-11-17 | 2024-05-28 | Taiwan Mercury Medical Corp | Metformin tablet for relieving pain and reducing inflammation and manufacturing method thereof |
| AU2024265092A1 (en) * | 2023-05-03 | 2025-11-20 | Dark Canyon Laboratories, Llc | A floating drug delivery system for solid forms of salts used in bowel cleansing products |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999001112A1 (en) * | 1997-07-01 | 1999-01-14 | Astra Aktiebolag (Publ) | Multiple unit effervescent dosage form |
| WO2001058424A1 (en) * | 2000-02-09 | 2001-08-16 | West Pharmaceutical Services Drug Delivery & Clinical Research Centre Limited | Floating drug delivery composition |
| EP1574534A1 (en) * | 2004-03-11 | 2005-09-14 | Rohm And Haas Company | Polymer carriers and process |
Family Cites Families (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5512411B2 (enExample) * | 1974-03-12 | 1980-04-02 | ||
| JPS5134879A (en) * | 1974-09-19 | 1976-03-24 | Eisai Co Ltd | Bishochukuryushinoseizoho |
| US5002772A (en) * | 1988-05-31 | 1991-03-26 | Pfizer Inc. | Gastric retention system for controlled drug release |
| NZ233403A (en) * | 1989-04-28 | 1992-09-25 | Mcneil Ppc Inc | Simulated capsule-like medicament |
| JPH03101615A (ja) * | 1989-09-14 | 1991-04-26 | Sumitomo Pharmaceut Co Ltd | 胃内滞留型持続性製剤 |
| IT1246382B (it) * | 1990-04-17 | 1994-11-18 | Eurand Int | Metodo per la cessione mirata e controllata di farmaci nell'intestino e particolarmente nel colon |
| US5314696A (en) * | 1991-06-27 | 1994-05-24 | Paulos Manley A | Methods for making and administering a blinded oral dosage form and blinded oral dosage form therefor |
| UA60318C2 (uk) * | 1997-03-26 | 2003-10-15 | Янссен Фармацевтика Н.В. | Пілюля, яка має ядро, покрите протигрибковим агентом і полімером, та спосіб її виготовлення |
| US5895663A (en) * | 1997-07-31 | 1999-04-20 | L. Perrigo Company | Pseudoephedrine hydrochloride extended-release tablets |
| US6050796A (en) | 1998-05-18 | 2000-04-18 | General Motors Corporation | Vane pump |
| JP2002370970A (ja) * | 2001-06-12 | 2002-12-24 | Sanwa Kagaku Kenkyusho Co Ltd | 胃内浮遊型固形製剤及びその製造方法 |
| JP4426286B2 (ja) * | 2001-07-10 | 2010-03-03 | テバ ファーマシューティカル インダストリーズ リミティド | 0次、0次−二相性、上昇型又は下降型薬物放出のための薬物送達システム |
| US7763663B2 (en) * | 2001-12-19 | 2010-07-27 | University Of Massachusetts | Polysaccharide-containing block copolymer particles and uses thereof |
-
2007
- 2007-09-03 CN CN200780032818XA patent/CN101511346B/zh not_active Expired - Fee Related
- 2007-09-03 ZA ZA200901162A patent/ZA200901162B/xx unknown
- 2007-09-03 US US12/439,775 patent/US8277843B2/en not_active Expired - Fee Related
- 2007-09-03 RU RU2009112396/15A patent/RU2420268C2/ru not_active IP Right Cessation
- 2007-09-03 EP EP07866696A patent/EP2068844A4/en not_active Withdrawn
- 2007-09-03 BR BRPI0716481-5A2A patent/BRPI0716481A2/pt not_active IP Right Cessation
- 2007-09-03 KR KR1020097006969A patent/KR20090065524A/ko not_active Ceased
- 2007-09-03 CA CA002661172A patent/CA2661172A1/en not_active Abandoned
- 2007-09-03 WO PCT/IN2007/000392 patent/WO2008062440A2/en not_active Ceased
- 2007-09-03 JP JP2009526263A patent/JP2010502591A/ja active Pending
- 2007-09-03 AU AU2007323018A patent/AU2007323018B2/en not_active Ceased
- 2007-09-03 MX MX2009002371A patent/MX2009002371A/es unknown
- 2007-09-04 CL CL200702562A patent/CL2007002562A1/es unknown
- 2007-09-04 AR ARP070103903A patent/AR062644A1/es not_active Application Discontinuation
-
2009
- 2009-02-19 IL IL197121A patent/IL197121A0/en unknown
- 2009-02-19 NO NO20090807A patent/NO20090807A/no not_active Application Discontinuation
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1999001112A1 (en) * | 1997-07-01 | 1999-01-14 | Astra Aktiebolag (Publ) | Multiple unit effervescent dosage form |
| WO2001058424A1 (en) * | 2000-02-09 | 2001-08-16 | West Pharmaceutical Services Drug Delivery & Clinical Research Centre Limited | Floating drug delivery composition |
| EP1574534A1 (en) * | 2004-03-11 | 2005-09-14 | Rohm And Haas Company | Polymer carriers and process |
Also Published As
| Publication number | Publication date |
|---|---|
| BRPI0716481A2 (pt) | 2014-03-18 |
| WO2008062440A3 (en) | 2008-07-31 |
| RU2009112396A (ru) | 2010-10-20 |
| CL2007002562A1 (es) | 2008-02-08 |
| KR20090065524A (ko) | 2009-06-22 |
| NO20090807A (no) | 2009-06-04 |
| CN101511346A (zh) | 2009-08-19 |
| MX2009002371A (es) | 2009-03-12 |
| AU2007323018B2 (en) | 2011-02-03 |
| CA2661172A1 (en) | 2008-05-29 |
| AU2007323018A1 (en) | 2008-05-29 |
| CN101511346B (zh) | 2012-07-04 |
| EP2068844A4 (en) | 2013-01-23 |
| WO2008062440A2 (en) | 2008-05-29 |
| JP2010502591A (ja) | 2010-01-28 |
| IL197121A0 (en) | 2009-11-18 |
| AR062644A1 (es) | 2008-11-19 |
| EP2068844A2 (en) | 2009-06-17 |
| US20100015224A1 (en) | 2010-01-21 |
| US8277843B2 (en) | 2012-10-02 |
| ZA200901162B (en) | 2010-06-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2420268C2 (ru) | Способ программируемой плавучей доставки | |
| CA2879282C (en) | Gastro-retentive drug delivery system | |
| Dehghan et al. | Gastroretentive drug delivery systems: A patent perspective | |
| CN101987081B (zh) | 一种控释制剂 | |
| US20080286344A1 (en) | Solid form | |
| US20080311162A1 (en) | Solid form | |
| KR20190049543A (ko) | 붕해가 개선된 경구용 고형 제제 조성물 및 이의 제조 방법 | |
| US20180078503A1 (en) | Gastro-retentive drug delivery system | |
| JP2022104916A (ja) | 自己調節性の浸透性胃保持性薬物送達システム | |
| WO2008140461A1 (en) | Solid form | |
| JP2018531960A (ja) | 最適化高用量メサラジン含有錠剤 | |
| Thakur et al. | Floating Drug Delivery System. | |
| Chordiya et al. | Technologies, optimization and analytical parameters in gastroretentive drug delivery systems | |
| DK2679216T3 (en) | Pharmaceutical form for modified release of betahistine | |
| Pahwa et al. | Recent advances in gastric floating drug delivery technology: a review | |
| Janhavi et al. | A Comprehensive Review on Gastro-Retentive Floating Drug Delivery Systems | |
| US20240299304A1 (en) | A method for the production of gastroretentive compact matrices for the controlled release of active substances and compact matrices thus obtained | |
| CN102238944A (zh) | 用于制备生物粘附压制基质的方法 | |
| Rahim | Preparation and Characterisation of Floating Tablets to Target the Stomach | |
| Liu | Update on polymers for oral drug delivery | |
| Renthlei et al. | Floating Drug Delivery System: An Outlook | |
| Thakur | Gastroretentive Drug Delivery System (GRDDS): A Novel Approach to Improve Therapeutic Efficacy | |
| Gangurde et al. | Gastroretentive Dosage Forms: An Overview |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| MM4A | The patent is invalid due to non-payment of fees |
Effective date: 20130904 |