KR20230016175A - Angptl3의 염기 편집 및 질환 치료를 위한 이의 사용 방법 - Google Patents
Angptl3의 염기 편집 및 질환 치료를 위한 이의 사용 방법 Download PDFInfo
- Publication number
- KR20230016175A KR20230016175A KR1020227038926A KR20227038926A KR20230016175A KR 20230016175 A KR20230016175 A KR 20230016175A KR 1020227038926 A KR1020227038926 A KR 1020227038926A KR 20227038926 A KR20227038926 A KR 20227038926A KR 20230016175 A KR20230016175 A KR 20230016175A
- Authority
- KR
- South Korea
- Prior art keywords
- composition
- editing
- guide rna
- administered
- angptl3
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 228
- 101000693085 Homo sapiens Angiopoietin-related protein 3 Proteins 0.000 title claims description 330
- 102100025668 Angiopoietin-related protein 3 Human genes 0.000 title claims description 138
- 238000011282 treatment Methods 0.000 title claims description 61
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 title description 52
- 201000010099 disease Diseases 0.000 title description 42
- 239000000203 mixture Substances 0.000 claims abstract description 418
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 79
- 210000004185 liver Anatomy 0.000 claims abstract description 71
- 241000282567 Macaca fascicularis Species 0.000 claims description 418
- 108020005004 Guide RNA Proteins 0.000 claims description 394
- 108020004999 messenger RNA Proteins 0.000 claims description 327
- 238000001802 infusion Methods 0.000 claims description 171
- 238000001990 intravenous administration Methods 0.000 claims description 170
- 230000037396 body weight Effects 0.000 claims description 164
- 108020001507 fusion proteins Proteins 0.000 claims description 163
- 102000037865 fusion proteins Human genes 0.000 claims description 163
- 230000004075 alteration Effects 0.000 claims description 120
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 claims description 96
- 125000003729 nucleotide group Chemical group 0.000 claims description 95
- 239000002773 nucleotide Substances 0.000 claims description 93
- 229960000643 adenine Drugs 0.000 claims description 81
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 claims description 80
- 229930024421 Adenine Natural products 0.000 claims description 80
- 241000282693 Cercopithecidae Species 0.000 claims description 78
- 108091033409 CRISPR Proteins 0.000 claims description 72
- 125000006850 spacer group Chemical group 0.000 claims description 69
- 230000009467 reduction Effects 0.000 claims description 61
- 108020004414 DNA Proteins 0.000 claims description 60
- 210000004369 blood Anatomy 0.000 claims description 59
- 239000008280 blood Substances 0.000 claims description 59
- 102000004169 proteins and genes Human genes 0.000 claims description 59
- 238000007481 next generation sequencing Methods 0.000 claims description 40
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 39
- 238000007480 sanger sequencing Methods 0.000 claims description 39
- 108091032973 (ribonucleotides)n+m Proteins 0.000 claims description 37
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims description 35
- 230000000295 complement effect Effects 0.000 claims description 34
- 241000282414 Homo sapiens Species 0.000 claims description 32
- 210000005229 liver cell Anatomy 0.000 claims description 31
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 claims description 28
- 230000004568 DNA-binding Effects 0.000 claims description 26
- 230000027455 binding Effects 0.000 claims description 26
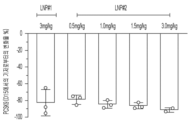
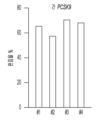
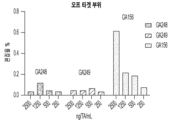
- 101150094724 PCSK9 gene Proteins 0.000 claims description 24
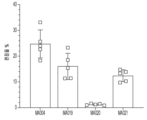
- 201000001320 Atherosclerosis Diseases 0.000 claims description 21
- 108010033266 Lipoprotein(a) Proteins 0.000 claims description 19
- 206010003210 Arteriosclerosis Diseases 0.000 claims description 18
- 230000001939 inductive effect Effects 0.000 claims description 18
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 claims description 18
- 101710163270 Nuclease Proteins 0.000 claims description 17
- 235000012000 cholesterol Nutrition 0.000 claims description 17
- 150000002632 lipids Chemical class 0.000 claims description 16
- 108020005067 RNA Splice Sites Proteins 0.000 claims description 15
- 238000001727 in vivo Methods 0.000 claims description 15
- 238000004159 blood analysis Methods 0.000 claims description 14
- 238000010241 blood sampling Methods 0.000 claims description 14
- 229940035893 uracil Drugs 0.000 claims description 14
- 108010008532 Deoxyribonuclease I Proteins 0.000 claims description 13
- 102000007260 Deoxyribonuclease I Human genes 0.000 claims description 13
- 230000007423 decrease Effects 0.000 claims description 13
- 239000002105 nanoparticle Substances 0.000 claims description 13
- 230000008859 change Effects 0.000 claims description 12
- 230000000737 periodic effect Effects 0.000 claims description 12
- 238000012360 testing method Methods 0.000 claims description 12
- 238000012317 liver biopsy Methods 0.000 claims description 8
- 230000002459 sustained effect Effects 0.000 claims description 8
- 229940113082 thymine Drugs 0.000 claims description 8
- 238000012217 deletion Methods 0.000 claims description 7
- 239000002245 particle Substances 0.000 claims description 7
- 238000004458 analytical method Methods 0.000 claims description 6
- 230000003143 atherosclerotic effect Effects 0.000 claims description 6
- 239000008194 pharmaceutical composition Substances 0.000 claims description 6
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 6
- 230000001225 therapeutic effect Effects 0.000 claims description 6
- 210000001519 tissue Anatomy 0.000 claims description 6
- 208000019553 vascular disease Diseases 0.000 claims description 6
- 108010052875 Adenine deaminase Proteins 0.000 claims description 5
- 102000004127 Cytokines Human genes 0.000 claims description 5
- 108090000695 Cytokines Proteins 0.000 claims description 5
- 108020004485 Nonsense Codon Proteins 0.000 claims description 5
- 230000000692 anti-sense effect Effects 0.000 claims description 5
- 230000037430 deletion Effects 0.000 claims description 5
- 230000037433 frameshift Effects 0.000 claims description 5
- 238000003780 insertion Methods 0.000 claims description 5
- 230000037431 insertion Effects 0.000 claims description 5
- 230000030648 nucleus localization Effects 0.000 claims description 5
- 230000001052 transient effect Effects 0.000 claims description 5
- 230000002159 abnormal effect Effects 0.000 claims description 4
- 230000004913 activation Effects 0.000 claims description 4
- 239000003937 drug carrier Substances 0.000 claims description 4
- 239000000654 additive Substances 0.000 claims description 3
- 230000000996 additive effect Effects 0.000 claims description 3
- 230000028993 immune response Effects 0.000 claims description 3
- 238000000338 in vitro Methods 0.000 claims description 3
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 claims description 2
- 102000004506 Blood Proteins Human genes 0.000 claims description 2
- 108010017384 Blood Proteins Proteins 0.000 claims description 2
- 238000011887 Necropsy Methods 0.000 claims description 2
- 108091081021 Sense strand Proteins 0.000 claims description 2
- 230000001919 adrenal effect Effects 0.000 claims description 2
- 238000002296 dynamic light scattering Methods 0.000 claims description 2
- 210000000952 spleen Anatomy 0.000 claims description 2
- 102000057248 Lipoprotein(a) Human genes 0.000 claims 9
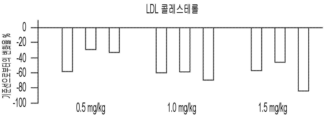
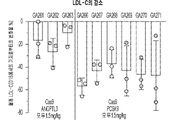
- 108010028554 LDL Cholesterol Proteins 0.000 abstract description 44
- 208000024172 Cardiovascular disease Diseases 0.000 abstract description 9
- 238000012239 gene modification Methods 0.000 abstract description 4
- 230000005017 genetic modification Effects 0.000 abstract description 4
- 235000013617 genetically modified food Nutrition 0.000 abstract description 4
- 239000002585 base Substances 0.000 description 359
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 214
- 102000055025 Adenosine deaminases Human genes 0.000 description 214
- 230000035772 mutation Effects 0.000 description 146
- 150000007523 nucleic acids Chemical class 0.000 description 100
- 102000039446 nucleic acids Human genes 0.000 description 95
- 108020004707 nucleic acids Proteins 0.000 description 95
- 102000040430 polynucleotide Human genes 0.000 description 89
- 108091033319 polynucleotide Proteins 0.000 description 89
- 239000002157 polynucleotide Substances 0.000 description 89
- 102100038955 Proprotein convertase subtilisin/kexin type 9 Human genes 0.000 description 82
- 101710180553 Proprotein convertase subtilisin/kexin type 9 Proteins 0.000 description 82
- 230000000875 corresponding effect Effects 0.000 description 78
- 102000053602 DNA Human genes 0.000 description 58
- 235000001014 amino acid Nutrition 0.000 description 56
- 229940024606 amino acid Drugs 0.000 description 55
- 150000001413 amino acids Chemical class 0.000 description 54
- 125000005647 linker group Chemical group 0.000 description 50
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 49
- 235000018102 proteins Nutrition 0.000 description 48
- 210000003494 hepatocyte Anatomy 0.000 description 32
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 30
- 241001465754 Metazoa Species 0.000 description 28
- 210000004027 cell Anatomy 0.000 description 26
- 101000910035 Streptococcus pyogenes serotype M1 CRISPR-associated endonuclease Cas9/Csn1 Proteins 0.000 description 24
- 229940104302 cytosine Drugs 0.000 description 24
- 230000002829 reductive effect Effects 0.000 description 23
- 238000012986 modification Methods 0.000 description 21
- 238000002965 ELISA Methods 0.000 description 20
- 230000004048 modification Effects 0.000 description 20
- 238000001294 liquid chromatography-tandem mass spectrometry Methods 0.000 description 18
- 102000052510 DNA-Binding Proteins Human genes 0.000 description 17
- 101710096438 DNA-binding protein Proteins 0.000 description 17
- 238000007385 chemical modification Methods 0.000 description 17
- 235000000346 sugar Nutrition 0.000 description 17
- 238000001262 western blot Methods 0.000 description 17
- 230000000694 effects Effects 0.000 description 16
- 238000010362 genome editing Methods 0.000 description 15
- 108090000765 processed proteins & peptides Proteins 0.000 description 15
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 14
- 241000699670 Mus sp. Species 0.000 description 14
- 229960005305 adenosine Drugs 0.000 description 14
- 239000003795 chemical substances by application Substances 0.000 description 14
- 150000003626 triacylglycerols Chemical class 0.000 description 14
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 13
- 239000003814 drug Substances 0.000 description 12
- 230000008685 targeting Effects 0.000 description 12
- 102100040214 Apolipoprotein(a) Human genes 0.000 description 11
- 229930010555 Inosine Natural products 0.000 description 11
- 229960003786 inosine Drugs 0.000 description 11
- 208000024891 symptom Diseases 0.000 description 11
- 241000699666 Mus <mouse, genus> Species 0.000 description 10
- 102000004389 Ribonucleoproteins Human genes 0.000 description 10
- 108010081734 Ribonucleoproteins Proteins 0.000 description 10
- 206010012601 diabetes mellitus Diseases 0.000 description 10
- 238000006467 substitution reaction Methods 0.000 description 10
- 102100026846 Cytidine deaminase Human genes 0.000 description 9
- 108010031325 Cytidine deaminase Proteins 0.000 description 9
- 102000004190 Enzymes Human genes 0.000 description 9
- 108090000790 Enzymes Proteins 0.000 description 9
- -1 but not limited to Chemical class 0.000 description 9
- 238000011161 development Methods 0.000 description 9
- 230000018109 developmental process Effects 0.000 description 9
- 208000035475 disorder Diseases 0.000 description 9
- 229940088598 enzyme Drugs 0.000 description 9
- 229920001184 polypeptide Polymers 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 102000004196 processed proteins & peptides Human genes 0.000 description 9
- 102000000311 Cytosine Deaminase Human genes 0.000 description 8
- 108010080611 Cytosine Deaminase Proteins 0.000 description 8
- 108091034117 Oligonucleotide Proteins 0.000 description 8
- 238000006481 deamination reaction Methods 0.000 description 8
- 230000001976 improved effect Effects 0.000 description 8
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 8
- 230000002265 prevention Effects 0.000 description 8
- 150000003839 salts Chemical class 0.000 description 8
- 108020004705 Codon Proteins 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 230000009615 deamination Effects 0.000 description 7
- 238000013461 design Methods 0.000 description 7
- 239000003112 inhibitor Substances 0.000 description 7
- 125000000956 methoxy group Chemical group [H]C([H])([H])O* 0.000 description 7
- 229940124597 therapeutic agent Drugs 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- 108010023302 HDL Cholesterol Proteins 0.000 description 6
- 108091028043 Nucleic acid sequence Proteins 0.000 description 6
- 108091028113 Trans-activating crRNA Proteins 0.000 description 6
- 239000002253 acid Substances 0.000 description 6
- 125000000539 amino acid group Chemical group 0.000 description 6
- 230000005782 double-strand break Effects 0.000 description 6
- 238000009472 formulation Methods 0.000 description 6
- 230000014509 gene expression Effects 0.000 description 6
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 6
- 208000006575 hypertriglyceridemia Diseases 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 229920000642 polymer Polymers 0.000 description 6
- 239000013598 vector Substances 0.000 description 6
- 102100036475 Alanine aminotransferase 1 Human genes 0.000 description 5
- 108010082126 Alanine transaminase Proteins 0.000 description 5
- 108010003415 Aspartate Aminotransferases Proteins 0.000 description 5
- 102000004625 Aspartate Aminotransferases Human genes 0.000 description 5
- 241000588724 Escherichia coli Species 0.000 description 5
- 102000015779 HDL Lipoproteins Human genes 0.000 description 5
- 108010010234 HDL Lipoproteins Proteins 0.000 description 5
- 102000007330 LDL Lipoproteins Human genes 0.000 description 5
- 108010007622 LDL Lipoproteins Proteins 0.000 description 5
- 102000004895 Lipoproteins Human genes 0.000 description 5
- 108090001030 Lipoproteins Proteins 0.000 description 5
- 229910019142 PO4 Inorganic materials 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- 230000033590 base-excision repair Effects 0.000 description 5
- 238000010253 intravenous injection Methods 0.000 description 5
- 239000010452 phosphate Substances 0.000 description 5
- 230000008439 repair process Effects 0.000 description 5
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 5
- UHDGCWIWMRVCDJ-UHFFFAOYSA-N 1-beta-D-Xylofuranosyl-NH-Cytosine Natural products O=C1N=C(N)C=CN1C1C(O)C(O)C(CO)O1 UHDGCWIWMRVCDJ-UHFFFAOYSA-N 0.000 description 4
- 241000282472 Canis lupus familiaris Species 0.000 description 4
- UHDGCWIWMRVCDJ-PSQAKQOGSA-N Cytidine Natural products O=C1N=C(N)C=CN1[C@@H]1[C@@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-PSQAKQOGSA-N 0.000 description 4
- 230000007018 DNA scission Effects 0.000 description 4
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 4
- PYMYPHUHKUWMLA-LMVFSUKVSA-N Ribose Natural products OC[C@@H](O)[C@@H](O)[C@@H](O)C=O PYMYPHUHKUWMLA-LMVFSUKVSA-N 0.000 description 4
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 4
- HMFHBZSHGGEWLO-UHFFFAOYSA-N alpha-D-Furanose-Ribose Natural products OCC1OC(O)C(O)C1O HMFHBZSHGGEWLO-UHFFFAOYSA-N 0.000 description 4
- 238000010171 animal model Methods 0.000 description 4
- 230000004071 biological effect Effects 0.000 description 4
- 230000003197 catalytic effect Effects 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 239000013078 crystal Substances 0.000 description 4
- 230000001186 cumulative effect Effects 0.000 description 4
- UHDGCWIWMRVCDJ-ZAKLUEHWSA-N cytidine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O1 UHDGCWIWMRVCDJ-ZAKLUEHWSA-N 0.000 description 4
- 239000003085 diluting agent Substances 0.000 description 4
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 4
- 239000012634 fragment Substances 0.000 description 4
- 230000006870 function Effects 0.000 description 4
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 4
- 239000003550 marker Substances 0.000 description 4
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 239000000047 product Substances 0.000 description 4
- 239000000523 sample Substances 0.000 description 4
- 239000000126 substance Substances 0.000 description 4
- ASJSAQIRZKANQN-CRCLSJGQSA-N 2-deoxy-D-ribose Chemical compound OC[C@@H](O)[C@@H](O)CC=O ASJSAQIRZKANQN-CRCLSJGQSA-N 0.000 description 3
- 108010079649 APOBEC-1 Deaminase Proteins 0.000 description 3
- 102100040397 C->U-editing enzyme APOBEC-1 Human genes 0.000 description 3
- HMFHBZSHGGEWLO-SOOFDHNKSA-N D-ribofuranose Chemical compound OC[C@H]1OC(O)[C@H](O)[C@@H]1O HMFHBZSHGGEWLO-SOOFDHNKSA-N 0.000 description 3
- 241000702421 Dependoparvovirus Species 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 229940113491 Glycosylase inhibitor Drugs 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 241000725303 Human immunodeficiency virus Species 0.000 description 3
- 208000035150 Hypercholesterolemia Diseases 0.000 description 3
- 108091093037 Peptide nucleic acid Proteins 0.000 description 3
- 238000010357 RNA editing Methods 0.000 description 3
- 230000026279 RNA modification Effects 0.000 description 3
- 241000700605 Viruses Species 0.000 description 3
- 239000008186 active pharmaceutical agent Substances 0.000 description 3
- 238000003556 assay Methods 0.000 description 3
- 238000001574 biopsy Methods 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 230000002596 correlated effect Effects 0.000 description 3
- 230000001419 dependent effect Effects 0.000 description 3
- 229940079593 drug Drugs 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 230000036541 health Effects 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 230000007774 longterm Effects 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 239000000178 monomer Substances 0.000 description 3
- 238000002703 mutagenesis Methods 0.000 description 3
- 231100000350 mutagenesis Toxicity 0.000 description 3
- 231100000252 nontoxic Toxicity 0.000 description 3
- 230000003000 nontoxic effect Effects 0.000 description 3
- 230000008707 rearrangement Effects 0.000 description 3
- 230000001105 regulatory effect Effects 0.000 description 3
- 102220089709 rs869320709 Human genes 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 150000008163 sugars Chemical class 0.000 description 3
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 3
- 238000011269 treatment regimen Methods 0.000 description 3
- 208000001072 type 2 diabetes mellitus Diseases 0.000 description 3
- RFLVMTUMFYRZCB-UHFFFAOYSA-N 1-methylguanine Chemical compound O=C1N(C)C(N)=NC2=C1N=CN2 RFLVMTUMFYRZCB-UHFFFAOYSA-N 0.000 description 2
- VGONTNSXDCQUGY-RRKCRQDMSA-N 2'-deoxyinosine Chemical group C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC2=O)=C2N=C1 VGONTNSXDCQUGY-RRKCRQDMSA-N 0.000 description 2
- FZWGECJQACGGTI-UHFFFAOYSA-N 2-amino-7-methyl-1,7-dihydro-6H-purin-6-one Chemical compound NC1=NC(O)=C2N(C)C=NC2=N1 FZWGECJQACGGTI-UHFFFAOYSA-N 0.000 description 2
- HVBSAKJJOYLTQU-UHFFFAOYSA-N 4-aminobenzenesulfonic acid Chemical compound NC1=CC=C(S(O)(=O)=O)C=C1 HVBSAKJJOYLTQU-UHFFFAOYSA-N 0.000 description 2
- OVONXEQGWXGFJD-UHFFFAOYSA-N 4-sulfanylidene-1h-pyrimidin-2-one Chemical compound SC=1C=CNC(=O)N=1 OVONXEQGWXGFJD-UHFFFAOYSA-N 0.000 description 2
- OIVLITBTBDPEFK-UHFFFAOYSA-N 5,6-dihydrouracil Chemical compound O=C1CCNC(=O)N1 OIVLITBTBDPEFK-UHFFFAOYSA-N 0.000 description 2
- ZLAQATDNGLKIEV-UHFFFAOYSA-N 5-methyl-2-sulfanylidene-1h-pyrimidin-4-one Chemical compound CC1=CNC(=S)NC1=O ZLAQATDNGLKIEV-UHFFFAOYSA-N 0.000 description 2
- LRSASMSXMSNRBT-UHFFFAOYSA-N 5-methylcytosine Chemical compound CC1=CNC(=O)N=C1N LRSASMSXMSNRBT-UHFFFAOYSA-N 0.000 description 2
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 2
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 2
- 101710095342 Apolipoprotein B Proteins 0.000 description 2
- 102100040202 Apolipoprotein B-100 Human genes 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 241000283690 Bos taurus Species 0.000 description 2
- 102100021943 C-C motif chemokine 2 Human genes 0.000 description 2
- 101710155857 C-C motif chemokine 2 Proteins 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 230000008265 DNA repair mechanism Effects 0.000 description 2
- 108091092584 GDNA Proteins 0.000 description 2
- 102000005720 Glutathione transferase Human genes 0.000 description 2
- 108010070675 Glutathione transferase Proteins 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 2
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 2
- ZRALSGWEFCBTJO-UHFFFAOYSA-N Guanidine Chemical compound NC(N)=N ZRALSGWEFCBTJO-UHFFFAOYSA-N 0.000 description 2
- 241000606768 Haemophilus influenzae Species 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- 108090001061 Insulin Proteins 0.000 description 2
- 102000004877 Insulin Human genes 0.000 description 2
- 206010022489 Insulin Resistance Diseases 0.000 description 2
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- HYVABZIGRDEKCD-UHFFFAOYSA-N N(6)-dimethylallyladenine Chemical compound CC(C)=CCNC1=NC=NC2=C1N=CN2 HYVABZIGRDEKCD-UHFFFAOYSA-N 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 238000012408 PCR amplification Methods 0.000 description 2
- 108010011964 Phosphatidylcholine-sterol O-acyltransferase Proteins 0.000 description 2
- 102100031538 Phosphatidylcholine-sterol acyltransferase Human genes 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 230000004570 RNA-binding Effects 0.000 description 2
- 108091028664 Ribonucleotide Proteins 0.000 description 2
- 206010039491 Sarcoma Diseases 0.000 description 2
- 241000863432 Shewanella putrefaciens Species 0.000 description 2
- 102100022433 Single-stranded DNA cytosine deaminase Human genes 0.000 description 2
- 101710143275 Single-stranded DNA cytosine deaminase Proteins 0.000 description 2
- 208000006011 Stroke Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 238000008050 Total Bilirubin Reagent Methods 0.000 description 2
- 101710172430 Uracil-DNA glycosylase inhibitor Proteins 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- 108010062497 VLDL Lipoproteins Proteins 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 235000011054 acetic acid Nutrition 0.000 description 2
- 229960000583 acetic acid Drugs 0.000 description 2
- 230000002378 acidificating effect Effects 0.000 description 2
- 150000007513 acids Chemical class 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 238000013308 adult mouse model Methods 0.000 description 2
- 239000003513 alkali Substances 0.000 description 2
- 150000001412 amines Chemical class 0.000 description 2
- 239000012491 analyte Substances 0.000 description 2
- 239000000427 antigen Substances 0.000 description 2
- 108091007433 antigens Proteins 0.000 description 2
- 102000036639 antigens Human genes 0.000 description 2
- 210000001367 artery Anatomy 0.000 description 2
- 125000004429 atom Chemical group 0.000 description 2
- 238000011888 autopsy Methods 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 108700023293 biotin carboxyl carrier Proteins 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 239000007795 chemical reaction product Substances 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 210000004351 coronary vessel Anatomy 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- 230000001627 detrimental effect Effects 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 229940088679 drug related substance Drugs 0.000 description 2
- 230000008030 elimination Effects 0.000 description 2
- 238000003379 elimination reaction Methods 0.000 description 2
- 210000002257 embryonic structure Anatomy 0.000 description 2
- 238000006911 enzymatic reaction Methods 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- 239000003925 fat Substances 0.000 description 2
- 235000019197 fats Nutrition 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 239000001530 fumaric acid Substances 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 239000005090 green fluorescent protein Substances 0.000 description 2
- 210000005260 human cell Anatomy 0.000 description 2
- 125000001183 hydrocarbyl group Chemical group 0.000 description 2
- 230000003301 hydrolyzing effect Effects 0.000 description 2
- 208000003532 hypothyroidism Diseases 0.000 description 2
- 230000002989 hypothyroidism Effects 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 230000001965 increasing effect Effects 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- 230000000977 initiatory effect Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 229940125396 insulin Drugs 0.000 description 2
- SUMDYPCJJOFFON-UHFFFAOYSA-N isethionic acid Chemical compound OCCS(O)(=O)=O SUMDYPCJJOFFON-UHFFFAOYSA-N 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 230000006780 non-homologous end joining Effects 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000001575 pathological effect Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 150000003904 phospholipids Chemical class 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 108020001580 protein domains Proteins 0.000 description 2
- 150000003212 purines Chemical class 0.000 description 2
- 150000003230 pyrimidines Chemical class 0.000 description 2
- 239000002336 ribonucleotide Substances 0.000 description 2
- 102220294979 rs140094683 Human genes 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 150000003573 thiols Chemical class 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 238000011816 wild-type C57Bl6 mouse Methods 0.000 description 2
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- UWYVPFMHMJIBHE-OWOJBTEDSA-N (e)-2-hydroxybut-2-enedioic acid Chemical compound OC(=O)\C=C(\O)C(O)=O UWYVPFMHMJIBHE-OWOJBTEDSA-N 0.000 description 1
- WJNGQIYEQLPJMN-IOSLPCCCSA-N 1-methylinosine Chemical compound C1=NC=2C(=O)N(C)C=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O WJNGQIYEQLPJMN-IOSLPCCCSA-N 0.000 description 1
- MXHRCPNRJAMMIM-SHYZEUOFSA-N 2'-deoxyuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 MXHRCPNRJAMMIM-SHYZEUOFSA-N 0.000 description 1
- CKTSBUTUHBMZGZ-SHYZEUOFSA-N 2'‐deoxycytidine Chemical compound O=C1N=C(N)C=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 CKTSBUTUHBMZGZ-SHYZEUOFSA-N 0.000 description 1
- HLYBTPMYFWWNJN-UHFFFAOYSA-N 2-(2,4-dioxo-1h-pyrimidin-5-yl)-2-hydroxyacetic acid Chemical compound OC(=O)C(O)C1=CNC(=O)NC1=O HLYBTPMYFWWNJN-UHFFFAOYSA-N 0.000 description 1
- PIINGYXNCHTJTF-UHFFFAOYSA-N 2-(2-azaniumylethylamino)acetate Chemical group NCCNCC(O)=O PIINGYXNCHTJTF-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- SGAKLDIYNFXTCK-UHFFFAOYSA-N 2-[(2,4-dioxo-1h-pyrimidin-5-yl)methylamino]acetic acid Chemical compound OC(=O)CNCC1=CNC(=O)NC1=O SGAKLDIYNFXTCK-UHFFFAOYSA-N 0.000 description 1
- YSAJFXWTVFGPAX-UHFFFAOYSA-N 2-[(2,4-dioxo-1h-pyrimidin-5-yl)oxy]acetic acid Chemical compound OC(=O)COC1=CNC(=O)NC1=O YSAJFXWTVFGPAX-UHFFFAOYSA-N 0.000 description 1
- XMSMHKMPBNTBOD-UHFFFAOYSA-N 2-dimethylamino-6-hydroxypurine Chemical compound N1C(N(C)C)=NC(=O)C2=C1N=CN2 XMSMHKMPBNTBOD-UHFFFAOYSA-N 0.000 description 1
- SMADWRYCYBUIKH-UHFFFAOYSA-N 2-methyl-7h-purin-6-amine Chemical compound CC1=NC(N)=C2NC=NC2=N1 SMADWRYCYBUIKH-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- KOLPWZCZXAMXKS-UHFFFAOYSA-N 3-methylcytosine Chemical compound CN1C(N)=CC=NC1=O KOLPWZCZXAMXKS-UHFFFAOYSA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- GJAKJCICANKRFD-UHFFFAOYSA-N 4-acetyl-4-amino-1,3-dihydropyrimidin-2-one Chemical compound CC(=O)C1(N)NC(=O)NC=C1 GJAKJCICANKRFD-UHFFFAOYSA-N 0.000 description 1
- WPYRHVXCOQLYLY-UHFFFAOYSA-N 5-[(methoxyamino)methyl]-2-sulfanylidene-1h-pyrimidin-4-one Chemical compound CONCC1=CNC(=S)NC1=O WPYRHVXCOQLYLY-UHFFFAOYSA-N 0.000 description 1
- LQLQRFGHAALLLE-UHFFFAOYSA-N 5-bromouracil Chemical compound BrC1=CNC(=O)NC1=O LQLQRFGHAALLLE-UHFFFAOYSA-N 0.000 description 1
- VKLFQTYNHLDMDP-PNHWDRBUSA-N 5-carboxymethylaminomethyl-2-thiouridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=S)NC(=O)C(CNCC(O)=O)=C1 VKLFQTYNHLDMDP-PNHWDRBUSA-N 0.000 description 1
- ZFTBZKVVGZNMJR-UHFFFAOYSA-N 5-chlorouracil Chemical compound ClC1=CNC(=O)NC1=O ZFTBZKVVGZNMJR-UHFFFAOYSA-N 0.000 description 1
- KSNXJLQDQOIRIP-UHFFFAOYSA-N 5-iodouracil Chemical compound IC1=CNC(=O)NC1=O KSNXJLQDQOIRIP-UHFFFAOYSA-N 0.000 description 1
- KELXHQACBIUYSE-UHFFFAOYSA-N 5-methoxy-1h-pyrimidine-2,4-dione Chemical compound COC1=CNC(=O)NC1=O KELXHQACBIUYSE-UHFFFAOYSA-N 0.000 description 1
- BYPCVKNBGDKXLB-UHFFFAOYSA-N 6-(aminomethyl)-5-methyl-1h-pyrimidine-2,4-dione Chemical compound CC1=C(CN)NC(=O)NC1=O BYPCVKNBGDKXLB-UHFFFAOYSA-N 0.000 description 1
- DCPSTSVLRXOYGS-UHFFFAOYSA-N 6-amino-1h-pyrimidine-2-thione Chemical compound NC1=CC=NC(S)=N1 DCPSTSVLRXOYGS-UHFFFAOYSA-N 0.000 description 1
- VKKXEIQIGGPMHT-UHFFFAOYSA-N 7h-purine-2,8-diamine Chemical compound NC1=NC=C2NC(N)=NC2=N1 VKKXEIQIGGPMHT-UHFFFAOYSA-N 0.000 description 1
- MSSXOMSJDRHRMC-UHFFFAOYSA-N 9H-purine-2,6-diamine Chemical compound NC1=NC(N)=C2NC=NC2=N1 MSSXOMSJDRHRMC-UHFFFAOYSA-N 0.000 description 1
- 108010004483 APOBEC-3G Deaminase Proteins 0.000 description 1
- 108010011170 Ala-Trp-Arg-His-Pro-Gln-Phe-Gly-Gly Proteins 0.000 description 1
- 108010088751 Albumins Proteins 0.000 description 1
- 102000009027 Albumins Human genes 0.000 description 1
- 108700028369 Alleles Proteins 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 241000272814 Anser sp. Species 0.000 description 1
- 102100030970 Apolipoprotein C-III Human genes 0.000 description 1
- 108010056301 Apolipoprotein C-III Proteins 0.000 description 1
- 108010012927 Apoprotein(a) Proteins 0.000 description 1
- 241000039087 Apostates Species 0.000 description 1
- 102000008682 Argonaute Proteins Human genes 0.000 description 1
- 108010088141 Argonaute Proteins Proteins 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 208000037260 Atherosclerotic Plaque Diseases 0.000 description 1
- 241000714230 Avian leukemia virus Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 102000015735 Beta-catenin Human genes 0.000 description 1
- 108060000903 Beta-catenin Proteins 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 1
- QCMYYKRYFNMIEC-UHFFFAOYSA-N COP(O)=O Chemical class COP(O)=O QCMYYKRYFNMIEC-UHFFFAOYSA-N 0.000 description 1
- 108091079001 CRISPR RNA Proteins 0.000 description 1
- 238000010356 CRISPR-Cas9 genome editing Methods 0.000 description 1
- 238000010453 CRISPR/Cas method Methods 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 206010049993 Cardiac death Diseases 0.000 description 1
- 208000031229 Cardiomyopathies Diseases 0.000 description 1
- 108700004991 Cas12a Proteins 0.000 description 1
- 241000010804 Caulobacter vibrioides Species 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- JZUFKLXOESDKRF-UHFFFAOYSA-N Chlorothiazide Chemical compound C1=C(Cl)C(S(=O)(=O)N)=CC2=C1NCNS2(=O)=O JZUFKLXOESDKRF-UHFFFAOYSA-N 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- 102220605874 Cytosolic arginine sensor for mTORC1 subunit 2_D10A_mutation Human genes 0.000 description 1
- 102220546508 DNA (cytosine-5)-methyltransferase 1_T17S_mutation Human genes 0.000 description 1
- 108091028709 DNA adenine Proteins 0.000 description 1
- 102100038076 DNA dC->dU-editing enzyme APOBEC-3G Human genes 0.000 description 1
- 230000005778 DNA damage Effects 0.000 description 1
- 231100000277 DNA damage Toxicity 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 206010011906 Death Diseases 0.000 description 1
- CKTSBUTUHBMZGZ-UHFFFAOYSA-N Deoxycytidine Natural products O=C1N=C(N)C=CN1C1OC(CO)C(O)C1 CKTSBUTUHBMZGZ-UHFFFAOYSA-N 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 206010013801 Duchenne Muscular Dystrophy Diseases 0.000 description 1
- 108700034637 EC 3.2.-.- Proteins 0.000 description 1
- 102000004533 Endonucleases Human genes 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 241000991587 Enterovirus C Species 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000702189 Escherichia virus Mu Species 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 201000001376 Familial Combined Hyperlipidemia Diseases 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 230000010337 G2 phase Effects 0.000 description 1
- 108020004206 Gamma-glutamyltransferase Proteins 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- 208000031448 Genomic Instability Diseases 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 208000032003 Glycogen storage disease due to glucose-6-phosphatase deficiency Diseases 0.000 description 1
- 241000282575 Gorilla Species 0.000 description 1
- HVLSXIKZNLPZJJ-TXZCQADKSA-N HA peptide Chemical compound C([C@@H](C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](C)C(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 HVLSXIKZNLPZJJ-TXZCQADKSA-N 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 1
- 208000001021 Hyperlipoproteinemia Type I Diseases 0.000 description 1
- 206010020710 Hyperphagia Diseases 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- SHGAZHPCJJPHSC-NUEINMDLSA-N Isotretinoin Chemical compound OC(=O)C=C(C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-NUEINMDLSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- 241000713666 Lentivirus Species 0.000 description 1
- 108090000364 Ligases Proteins 0.000 description 1
- 102000003960 Ligases Human genes 0.000 description 1
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 1
- 208000003221 Lysosomal acid lipase deficiency Diseases 0.000 description 1
- 241000829100 Macaca mulatta polyomavirus 1 Species 0.000 description 1
- YNAVUWVOSKDBBP-UHFFFAOYSA-N Morpholine Chemical group C1COCCN1 YNAVUWVOSKDBBP-UHFFFAOYSA-N 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000714177 Murine leukemia virus Species 0.000 description 1
- 101100001705 Mus musculus Angptl3 gene Proteins 0.000 description 1
- 101100135848 Mus musculus Pcsk9 gene Proteins 0.000 description 1
- 241000713883 Myeloproliferative sarcoma virus Species 0.000 description 1
- SGSSKEDGVONRGC-UHFFFAOYSA-N N(2)-methylguanine Chemical compound O=C1NC(NC)=NC2=C1N=CN2 SGSSKEDGVONRGC-UHFFFAOYSA-N 0.000 description 1
- CHJJGSNFBQVOTG-UHFFFAOYSA-N N-methyl-guanidine Natural products CNC(N)=N CHJJGSNFBQVOTG-UHFFFAOYSA-N 0.000 description 1
- 125000001429 N-terminal alpha-amino-acid group Chemical group 0.000 description 1
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 1
- 206010028980 Neoplasm Diseases 0.000 description 1
- 206010029164 Nephrotic syndrome Diseases 0.000 description 1
- 208000009869 Neu-Laxova syndrome Diseases 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 108010077850 Nuclear Localization Signals Proteins 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- 240000007594 Oryza sativa Species 0.000 description 1
- 235000007164 Oryza sativa Nutrition 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 206010033645 Pancreatitis Diseases 0.000 description 1
- 206010033647 Pancreatitis acute Diseases 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 201000011252 Phenylketonuria Diseases 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 230000009948 RNA mutation Effects 0.000 description 1
- 238000003559 RNA-seq method Methods 0.000 description 1
- 102220473730 Ras-related protein Rab-5A_A56E_mutation Human genes 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 230000018199 S phase Effects 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 235000014680 Saccharomyces cerevisiae Nutrition 0.000 description 1
- 241000293871 Salmonella enterica subsp. enterica serovar Typhi Species 0.000 description 1
- 101000833181 Schizosaccharomyces pombe (strain 972 / ATCC 24843) Glycerol dehydrogenase 1 Proteins 0.000 description 1
- 241000700584 Simplexvirus Species 0.000 description 1
- 102220582735 Solute carrier family 2, facilitated glucose transporter member 1_R51H_mutation Human genes 0.000 description 1
- 241000713896 Spleen necrosis virus Species 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 1
- COYHRQWNJDJCNA-NUJDXYNKSA-N Thr-Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O COYHRQWNJDJCNA-NUJDXYNKSA-N 0.000 description 1
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 1
- 206010045254 Type II hyperlipidaemia Diseases 0.000 description 1
- 102220505382 Uncharacterized protein C1orf141_E85G_mutation Human genes 0.000 description 1
- 108010069201 VLDL Cholesterol Proteins 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 238000011481 absorbance measurement Methods 0.000 description 1
- 239000000370 acceptor Substances 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 201000003229 acute pancreatitis Diseases 0.000 description 1
- 210000005006 adaptive immune system Anatomy 0.000 description 1
- 108010039040 adenine glycosylase Proteins 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 125000000217 alkyl group Chemical group 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 208000030961 allergic reaction Diseases 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 1
- 229910052782 aluminium Inorganic materials 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000001363 autoimmune Effects 0.000 description 1
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- 125000002619 bicyclic group Chemical class 0.000 description 1
- 239000012620 biological material Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N biotin Natural products N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 150000004657 carbamic acid derivatives Chemical class 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 150000007942 carboxylates Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 125000002091 cationic group Chemical group 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 210000003855 cell nucleus Anatomy 0.000 description 1
- 108091092328 cellular RNA Proteins 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000004700 cellular uptake Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000012707 chemical precursor Substances 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 239000013611 chromosomal DNA Substances 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 238000011970 concomitant therapy Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 230000008094 contradictory effect Effects 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000007711 cytoplasmic localization Effects 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- VGONTNSXDCQUGY-UHFFFAOYSA-N desoxyinosine Natural products C1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 VGONTNSXDCQUGY-UHFFFAOYSA-N 0.000 description 1
- MXHRCPNRJAMMIM-UHFFFAOYSA-N desoxyuridine Natural products C1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 MXHRCPNRJAMMIM-UHFFFAOYSA-N 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- ANCLJVISBRWUTR-UHFFFAOYSA-N diaminophosphinic acid Chemical compound NP(N)(O)=O ANCLJVISBRWUTR-UHFFFAOYSA-N 0.000 description 1
- PGUYAANYCROBRT-UHFFFAOYSA-N dihydroxy-selanyl-selanylidene-lambda5-phosphane Chemical class OP(O)([SeH])=[Se] PGUYAANYCROBRT-UHFFFAOYSA-N 0.000 description 1
- 238000006471 dimerization reaction Methods 0.000 description 1
- SWSQBOPZIKWTGO-UHFFFAOYSA-N dimethylaminoamidine Natural products CN(C)C(N)=N SWSQBOPZIKWTGO-UHFFFAOYSA-N 0.000 description 1
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- NAGJZTKCGNOGPW-UHFFFAOYSA-N dithiophosphoric acid Chemical class OP(O)(S)=S NAGJZTKCGNOGPW-UHFFFAOYSA-N 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 230000034431 double-strand break repair via homologous recombination Effects 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-N ethanedisulfonic acid Chemical compound OS(=O)(=O)CCS(O)(=O)=O AFAXGSQYZLGZPG-UHFFFAOYSA-N 0.000 description 1
- 201000000542 familial hypobetalipoproteinemia 2 Diseases 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 102000006640 gamma-Glutamyltransferase Human genes 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 208000004104 gestational diabetes Diseases 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 125000000291 glutamic acid group Chemical group N[C@@H](CCC(O)=O)C(=O)* 0.000 description 1
- 201000004541 glycogen storage disease I Diseases 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 210000002768 hair cell Anatomy 0.000 description 1
- 230000035876 healing Effects 0.000 description 1
- 210000005161 hepatic lobe Anatomy 0.000 description 1
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 229960002003 hydrochlorothiazide Drugs 0.000 description 1
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 1
- 229940071870 hydroiodic acid Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000005847 immunogenicity Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 238000001361 intraarterial administration Methods 0.000 description 1
- 230000008863 intramolecular interaction Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 238000007919 intrasynovial administration Methods 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 229960005280 isotretinoin Drugs 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 229910052744 lithium Inorganic materials 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000004777 loss-of-function mutation Effects 0.000 description 1
- 230000017156 mRNA modification Effects 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 238000007726 management method Methods 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 208000030159 metabolic disease Diseases 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 1
- IZAGSTRIDUNNOY-UHFFFAOYSA-N methyl 2-[(2,4-dioxo-1h-pyrimidin-5-yl)oxy]acetate Chemical compound COC(=O)COC1=CNC(=O)NC1=O IZAGSTRIDUNNOY-UHFFFAOYSA-N 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 230000033607 mismatch repair Effects 0.000 description 1
- 230000011278 mitosis Effects 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- LNOPIUAQISRISI-UHFFFAOYSA-N n'-hydroxy-2-propan-2-ylsulfonylethanimidamide Chemical compound CC(C)S(=O)(=O)CC(N)=NO LNOPIUAQISRISI-UHFFFAOYSA-N 0.000 description 1
- XJVXMWNLQRTRGH-UHFFFAOYSA-N n-(3-methylbut-3-enyl)-2-methylsulfanyl-7h-purin-6-amine Chemical compound CSC1=NC(NCCC(C)=C)=C2NC=NC2=N1 XJVXMWNLQRTRGH-UHFFFAOYSA-N 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 210000003061 neural cell Anatomy 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 230000037434 nonsense mutation Effects 0.000 description 1
- 230000030147 nuclear export Effects 0.000 description 1
- 230000000269 nucleophilic effect Effects 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 150000003833 nucleoside derivatives Chemical class 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 230000009437 off-target effect Effects 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 235000020830 overeating Nutrition 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 101150036331 pah gene Proteins 0.000 description 1
- WLJNZVDCPSBLRP-UHFFFAOYSA-N pamoic acid Chemical compound C1=CC=C2C(CC=3C4=CC=CC=C4C=C(C=3O)C(=O)O)=C(O)C(C(O)=O)=CC2=C1 WLJNZVDCPSBLRP-UHFFFAOYSA-N 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 150000002972 pentoses Chemical class 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 208000030613 peripheral artery disease Diseases 0.000 description 1
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- XUYJLQHKOGNDPB-UHFFFAOYSA-N phosphonoacetic acid Chemical compound OC(=O)CP(O)(O)=O XUYJLQHKOGNDPB-UHFFFAOYSA-N 0.000 description 1
- PTMHPRAIXMAOOB-UHFFFAOYSA-L phosphoramidate Chemical compound NP([O-])([O-])=O PTMHPRAIXMAOOB-UHFFFAOYSA-L 0.000 description 1
- 150000008298 phosphoramidates Chemical class 0.000 description 1
- 150000008299 phosphorodiamidates Chemical class 0.000 description 1
- 229910052698 phosphorus Inorganic materials 0.000 description 1
- 239000011574 phosphorus Substances 0.000 description 1
- 239000006187 pill Substances 0.000 description 1
- 239000013612 plasmid Substances 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920002704 polyhistidine Polymers 0.000 description 1
- 102000054765 polymorphisms of proteins Human genes 0.000 description 1
- 235000020777 polyunsaturated fatty acids Nutrition 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- OLBCVFGFOZPWHH-UHFFFAOYSA-N propofol Chemical compound CC(C)C1=CC=CC(C(C)C)=C1O OLBCVFGFOZPWHH-UHFFFAOYSA-N 0.000 description 1
- 229960004134 propofol Drugs 0.000 description 1
- 230000004853 protein function Effects 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 239000000700 radioactive tracer Substances 0.000 description 1
- 238000003127 radioimmunoassay Methods 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000008263 repair mechanism Effects 0.000 description 1
- 230000000979 retarding effect Effects 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 125000000548 ribosyl group Chemical group C1([C@H](O)[C@H](O)[C@H](O1)CO)* 0.000 description 1
- 235000009566 rice Nutrition 0.000 description 1
- 102200001961 rs104894324 Human genes 0.000 description 1
- 102220257864 rs1050971384 Human genes 0.000 description 1
- 102220192253 rs1057515879 Human genes 0.000 description 1
- 102220319450 rs1554378297 Human genes 0.000 description 1
- 102220340881 rs1554949196 Human genes 0.000 description 1
- 102200075749 rs397514044 Human genes 0.000 description 1
- 102220253616 rs746666691 Human genes 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 150000004671 saturated fatty acids Chemical class 0.000 description 1
- JRPHGDYSKGJTKZ-UHFFFAOYSA-N selenophosphoric acid Chemical class OP(O)([SeH])=O JRPHGDYSKGJTKZ-UHFFFAOYSA-N 0.000 description 1
- 230000001953 sensory effect Effects 0.000 description 1
- 238000002864 sequence alignment Methods 0.000 description 1
- 229940126586 small molecule drug Drugs 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 238000005063 solubilization Methods 0.000 description 1
- 230000007928 solubilization Effects 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 239000007921 spray Substances 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 150000003431 steroids Chemical group 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 108010018381 streptavidin-binding peptide Proteins 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 229950000244 sulfanilic acid Drugs 0.000 description 1
- 229910052717 sulfur Inorganic materials 0.000 description 1
- 125000004434 sulfur atom Chemical group 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 230000001360 synchronised effect Effects 0.000 description 1
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000011285 therapeutic regimen Methods 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 150000005691 triesters Chemical class 0.000 description 1
- 125000002264 triphosphate group Chemical group [H]OP(=O)(O[H])OP(=O)(O[H])OP(=O)(O[H])O* 0.000 description 1
- 201000011296 tyrosinemia Diseases 0.000 description 1
- 241000701161 unidentified adenovirus Species 0.000 description 1
- 235000021122 unsaturated fatty acids Nutrition 0.000 description 1
- 150000004670 unsaturated fatty acids Chemical class 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
- UGZADUVQMDAIAO-UHFFFAOYSA-L zinc hydroxide Chemical compound [OH-].[OH-].[Zn+2] UGZADUVQMDAIAO-UHFFFAOYSA-L 0.000 description 1
- 229940007718 zinc hydroxide Drugs 0.000 description 1
- 229910021511 zinc hydroxide Inorganic materials 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/111—General methods applicable to biologically active non-coding nucleic acids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7088—Compounds having three or more nucleosides or nucleotides
- A61K31/7105—Natural ribonucleic acids, i.e. containing only riboses attached to adenine, guanine, cytosine or uracil and having 3'-5' phosphodiester links
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/465—Hydrolases (3) acting on ester bonds (3.1), e.g. lipases, ribonucleases
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
- A61K48/0066—Manipulation of the nucleic acid to modify its expression pattern, e.g. enhance its duration of expression, achieved by the presence of particular introns in the delivered nucleic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/127—Synthetic bilayered vehicles, e.g. liposomes or liposomes with cholesterol as the only non-phosphatidyl surfactant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/475—Growth factors; Growth regulators
- C07K14/515—Angiogenesic factors; Angiogenin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
- C12N15/90—Stable introduction of foreign DNA into chromosome
- C12N15/902—Stable introduction of foreign DNA into chromosome using homologous recombination
- C12N15/907—Stable introduction of foreign DNA into chromosome using homologous recombination in mammalian cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/16—Hydrolases (3) acting on ester bonds (3.1)
- C12N9/22—Ribonucleases [RNase]; Deoxyribonucleases [DNase]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/48—Hydrolases (3) acting on peptide bonds (3.4)
- C12N9/50—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25)
- C12N9/64—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue
- C12N9/6421—Proteinases, e.g. Endopeptidases (3.4.21-3.4.25) derived from animal tissue from mammals
- C12N9/6424—Serine endopeptidases (3.4.21)
- C12N9/6454—Dibasic site splicing serine proteases, e.g. kexin (3.4.21.61); furin (3.4.21.75) and other proprotein convertases
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/78—Hydrolases (3) acting on carbon to nitrogen bonds other than peptide bonds (3.5)
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/09—Fusion polypeptide containing a localisation/targetting motif containing a nuclear localisation signal
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/80—Fusion polypeptide containing a DNA binding domain, e.g. Lacl or Tet-repressor
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/20—Type of nucleic acid involving clustered regularly interspaced short palindromic repeats [CRISPR]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/31—Chemical structure of the backbone
- C12N2310/315—Phosphorothioates
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/32—Chemical structure of the sugar
- C12N2310/321—2'-O-R Modification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
- C12N2310/331—Universal or degenerate base
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/33—Chemical structure of the base
- C12N2310/335—Modified T or U
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/34—Spatial arrangement of the modifications
- C12N2310/344—Position-specific modifications, e.g. on every purine, at the 3'-end
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/30—Chemical structure
- C12N2310/35—Nature of the modification
- C12N2310/352—Nature of the modification linked to the nucleic acid via a carbon atom
- C12N2310/3521—Methyl
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/80—Vectors containing sites for inducing double-stranded breaks, e.g. meganuclease restriction sites
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y304/00—Hydrolases acting on peptide bonds, i.e. peptidases (3.4)
- C12Y304/21—Serine endopeptidases (3.4.21)
- C12Y304/21061—Kexin (3.4.21.61), i.e. proprotein convertase subtilisin/kexin type 9
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biomedical Technology (AREA)
- Molecular Biology (AREA)
- Wood Science & Technology (AREA)
- General Health & Medical Sciences (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Medicinal Chemistry (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Vascular Medicine (AREA)
- Toxicology (AREA)
- Cell Biology (AREA)
- Mycology (AREA)
- Immunology (AREA)
- Dispersion Chemistry (AREA)
- Urology & Nephrology (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (11)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US202063007803P | 2020-04-09 | 2020-04-09 | |
| US202063007797P | 2020-04-09 | 2020-04-09 | |
| US63/007,803 | 2020-04-09 | ||
| US63/007,797 | 2020-04-09 | ||
| US202063045033P | 2020-06-26 | 2020-06-26 | |
| US202063045032P | 2020-06-26 | 2020-06-26 | |
| US63/045,032 | 2020-06-26 | ||
| US63/045,033 | 2020-06-26 | ||
| US202163136087P | 2021-01-11 | 2021-01-11 | |
| US63/136,087 | 2021-01-11 | ||
| PCT/US2021/026729 WO2021207710A2 (en) | 2020-04-09 | 2021-04-09 | Base editing of angptl3 and methods of using same for treatment of disease |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20230016175A true KR20230016175A (ko) | 2023-02-01 |
Family
ID=78022701
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227038926A Pending KR20230016175A (ko) | 2020-04-09 | 2021-04-09 | Angptl3의 염기 편집 및 질환 치료를 위한 이의 사용 방법 |
| KR1020227038947A Pending KR20230016176A (ko) | 2020-04-09 | 2021-04-09 | Pcsk9의 염기 편집 및 질환 치료를 위한 이의 사용 방법 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227038947A Pending KR20230016176A (ko) | 2020-04-09 | 2021-04-09 | Pcsk9의 염기 편집 및 질환 치료를 위한 이의 사용 방법 |
Country Status (13)
| Country | Link |
|---|---|
| US (5) | US12029795B2 (enExample) |
| EP (3) | EP4133071A4 (enExample) |
| JP (4) | JP7457832B2 (enExample) |
| KR (2) | KR20230016175A (enExample) |
| CN (2) | CN116096429A (enExample) |
| AU (4) | AU2021252991B2 (enExample) |
| BR (2) | BR112022020407A2 (enExample) |
| CA (2) | CA3179863A1 (enExample) |
| GB (8) | GB2632564B (enExample) |
| IL (2) | IL297193A (enExample) |
| MX (1) | MX2022012683A (enExample) |
| PH (2) | PH12022553015A1 (enExample) |
| WO (3) | WO2021207710A2 (enExample) |
Families Citing this family (23)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN119351474A (zh) | 2017-02-22 | 2025-01-24 | 克里斯珀医疗股份公司 | 用于基因编辑的组合物和方法 |
| GB2632564B (en) | 2020-04-09 | 2025-06-18 | Verve Therapeutics Inc | Base editing of angptl3 and methods of using same for treatment of disease |
| IL303195A (en) | 2020-11-25 | 2023-07-01 | Akagera Medicines Inc | Lipid nanoparticles for delivery of nucleic acids and related methods of use |
| US20240424138A1 (en) * | 2021-10-21 | 2024-12-26 | Prime Medicine, Inc. | Genome editing compositions and method for treatment of retinitis pigmentosa |
| US12037616B2 (en) * | 2022-03-01 | 2024-07-16 | Crispr Therapeutics Ag | Methods and compositions for treating angiopoietin-like 3 (ANGPTL3) related conditions |
| WO2023180904A1 (en) * | 2022-03-21 | 2023-09-28 | Crispr Therapeutics Ag | Methods and compositions for treating lipoprotein-related diseases |
| JP2025522311A (ja) | 2022-05-25 | 2025-07-15 | アカゲラ・メディスンズ,インコーポレイテッド | 核酸を送達するための脂質ナノ粒子およびその使用方法 |
| CN119948162A (zh) * | 2022-09-22 | 2025-05-06 | 锐正基因(苏州)有限公司 | 用于治疗高胆固醇血症和/或心血管疾病的组合物和方法 |
| KR20250124819A (ko) * | 2022-12-21 | 2025-08-20 | 인텔리아 테라퓨틱스, 인크. | 프로단백질 전환효소 서브틸리신 켁신 9(pcsk9) 편집을 위한 조성물 및 방법 |
| WO2024152058A1 (en) * | 2023-01-13 | 2024-07-18 | The Trustees Of The University Of Pennsylvania | Compositions and methods for the treatment of hereditary tyrosinemia type 1 |
| WO2024175550A1 (en) * | 2023-02-20 | 2024-08-29 | Proqr Therapeutics Ii B.V. | Antisense oligonucleotides for the treatment of atherosclerotic cardiovascular disease |
| WO2024197065A2 (en) * | 2023-03-20 | 2024-09-26 | Verve Therapeutics, Inc. | In vivo nickase-based editing of the lpa gene for treatment of cardiovascular disease |
| CN118813611B (zh) * | 2023-04-19 | 2025-09-16 | 清华大学 | 一种基于rna核酶的dear核酸操纵系统及其应用 |
| WO2025006782A2 (en) * | 2023-06-30 | 2025-01-02 | Chroma Medicine, Inc. | Guide rna compositions |
| WO2025045247A1 (en) * | 2023-08-31 | 2025-03-06 | Geneditbio Limited | Nucleic acids encoding crispr-associated proteins and uses thereof |
| WO2025054363A1 (en) * | 2023-09-05 | 2025-03-13 | Cornell University | Minigene cassettes, recombinant polynucleotides, and methods of their use |
| WO2025064656A1 (en) * | 2023-09-19 | 2025-03-27 | Beam Therapeutics Inc. | Compositions and methods for altering a nucleobase in a lipoprotein(a) polynucleotide |
| CN117587013B (zh) * | 2023-11-27 | 2024-06-14 | 湖南师范大学 | 一种血管发育异常的斑马鱼模型的构建方法 |
| CN117568313B (zh) * | 2024-01-15 | 2024-04-26 | 上海贝斯昂科生物科技有限公司 | 基因编辑组合物及其用途 |
| CN118956837B (zh) * | 2024-02-26 | 2025-09-23 | 深圳信立泰药业股份有限公司 | 用于pcsk9基因修饰或编辑的组合物及其使用方法 |
| WO2025184520A1 (en) * | 2024-02-29 | 2025-09-04 | Intellia Therapeutics, Inc. | Compositions and methods for angiopoietin like 3 (angptl3) editing |
| JP2025139413A (ja) * | 2024-03-12 | 2025-09-26 | サミー株式会社 | 遊技機 |
| CN120130446A (zh) * | 2025-03-10 | 2025-06-13 | 苏州系统医学研究所 | 一种制备疾病动物模型的方法及其应用 |
Family Cites Families (73)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001034768A2 (en) | 1999-11-09 | 2001-05-17 | Human Genome Sciences, Inc. | 15 human secreted proteins |
| EP1027033B1 (en) | 1997-05-14 | 2009-07-22 | The University Of British Columbia | High efficiency encapsulation of nucleic acids in lipid vesicles |
| EP1067182A3 (en) | 1999-07-08 | 2001-11-21 | Helix Research Institute | Secretory protein or membrane protein |
| US20030119038A1 (en) | 1999-09-09 | 2003-06-26 | Bingham Brendan William | NARC1, novel subtilase-like homologs |
| US7029895B2 (en) | 1999-09-27 | 2006-04-18 | Millennium Pharmaceuticals, Inc. | 27411, a novel human PGP synthase |
| CA2388617A1 (en) | 1999-10-22 | 2001-05-03 | Millennium Pharmaceuticals, Inc. | Nucleic acid molecules derived from rat brain and programmed cell death models |
| AU785007B2 (en) | 1999-11-24 | 2006-08-24 | Mcs Micro Carrier Systems Gmbh | Polypeptides comprising multimers of nuclear localization signals or of protein transduction domains and their use for transferring molecules into cells |
| WO2001057081A2 (en) | 2000-02-07 | 2001-08-09 | Millennium Pharmaceuticals, Inc. | Narc-1, subtilase-like homologs |
| AU2001261024A1 (en) | 2000-04-12 | 2001-10-30 | Delta Biotechnology Limited | Albumin fusion proteins |
| JP2004515217A (ja) | 2000-06-16 | 2004-05-27 | インサイト・ゲノミックス・インコーポレイテッド | プロテアーゼ |
| AU2001280471A1 (en) | 2000-08-11 | 2002-02-25 | Eli Lilly And Company | Novel secreted proteins and their uses |
| AU2002227280A1 (en) | 2000-12-08 | 2002-06-18 | Incyte Genomics, Inc. | Protein modification and maintenance molecules |
| DE10109897A1 (de) | 2001-02-21 | 2002-11-07 | Novosom Ag | Fakultativ kationische Liposomen und Verwendung dieser |
| EP1423134A2 (en) | 2001-03-21 | 2004-06-02 | Human Genome Sciences | Human secreted proteins |
| US7858117B2 (en) | 2002-02-21 | 2010-12-28 | Novosom Ag | Amphoteric liposomes and their use |
| EP1440981A3 (en) | 2003-01-21 | 2005-11-23 | Research Association for Biotechnology | Full-length human cdna |
| EP1471152A1 (en) | 2003-04-25 | 2004-10-27 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Mutations in the human PCSK9 gene associated to hypercholesterolemia |
| DE102004054730A1 (de) | 2004-03-28 | 2006-05-11 | Novosom Ag | Serumstabile amphotere Liposomen |
| WO2006007712A1 (en) | 2004-07-19 | 2006-01-26 | Protiva Biotherapeutics, Inc. | Methods comprising polyethylene glycol-lipid conjugates for delivery of therapeutic agents |
| US20070087045A1 (en) | 2005-10-14 | 2007-04-19 | Industrial Technology Research Institute | Lipid carrier and method of preparing the same |
| US20100040610A1 (en) | 2006-11-07 | 2010-02-18 | Ayesha Sitlani | Antagonists of pcsk9 |
| TWI445716B (zh) * | 2008-09-12 | 2014-07-21 | Rinat Neuroscience Corp | Pcsk9拮抗劑類 |
| WO2013143555A1 (en) | 2012-03-26 | 2013-10-03 | Biontech Ag | Rna formulation for immunotherapy |
| US9283287B2 (en) | 2012-04-02 | 2016-03-15 | Moderna Therapeutics, Inc. | Modified polynucleotides for the production of nuclear proteins |
| KR102271292B1 (ko) * | 2013-03-15 | 2021-07-02 | 더 제너럴 하스피탈 코포레이션 | Rna-안내 게놈 편집의 특이성을 증가시키기 위한 rna-안내 foki 뉴클레아제(rfn)의 용도 |
| US20160194625A1 (en) | 2013-09-03 | 2016-07-07 | Moderna Therapeutics, Inc. | Chimeric polynucleotides |
| US9228207B2 (en) | 2013-09-06 | 2016-01-05 | President And Fellows Of Harvard College | Switchable gRNAs comprising aptamers |
| US9737604B2 (en) | 2013-09-06 | 2017-08-22 | President And Fellows Of Harvard College | Use of cationic lipids to deliver CAS9 |
| FR3012370B1 (fr) | 2013-10-30 | 2017-04-14 | Michelin & Cie | Armature de sommet pour pneumatique d'avion |
| ES2774968T3 (es) | 2013-12-19 | 2020-07-23 | Novartis Ag | Lípidos y composiciones lipídicas para la administración de agentes activos |
| KR102298476B1 (ko) * | 2013-12-24 | 2021-09-03 | 아이오니스 파마수티컬즈, 인코포레이티드 | 안지오포이에틴-유사 3 발현의 조절 |
| PT3766916T (pt) | 2014-06-25 | 2022-11-28 | Acuitas Therapeutics Inc | Novos lípidos e formulações de nanopartículas lipídicas para distribuição de ácidos nucleicos |
| CA2964796C (en) * | 2014-10-17 | 2022-01-11 | The Penn State Research Foundation | Methods and compositions for multiplex rna guided genome editing and other rna technologies |
| MA41123A (fr) * | 2014-12-02 | 2017-10-10 | Oncomed Pharm Inc | Polythérapie pour le traitement du cancer |
| WO2017004279A2 (en) * | 2015-06-29 | 2017-01-05 | Massachusetts Institute Of Technology | Compositions comprising nucleic acids and methods of using the same |
| AU2016285852B2 (en) | 2015-06-29 | 2020-12-17 | Acuitas Therapeutics Inc. | Lipids and lipid nanoparticle formulations for delivery of nucleic acids |
| CA2989884A1 (en) | 2015-07-09 | 2017-01-12 | Insmed Incorporated | Compositions and methods for treating lung diseases and lung injury |
| KR102405695B1 (ko) * | 2015-08-31 | 2022-06-03 | 엘지디스플레이 주식회사 | 유기 발광 표시 장치 및 그 제조 방법 |
| LT3368507T (lt) | 2015-10-28 | 2023-03-10 | Acuitas Therapeutics Inc. | Nauji lipidai ir lipidų nanodalelių kompozicijos, skirtos nukleorūgščių tiekimui |
| EP3373979A1 (en) * | 2015-11-12 | 2018-09-19 | Pfizer Inc | Tissue-specific genome engineering using crispr-cas9 |
| CA3006618A1 (en) * | 2015-12-01 | 2017-06-08 | Crispr Therapeutics Ag | Materials and methods for treatment of alpha-1 antitrypsin deficiency |
| JP7080172B2 (ja) | 2015-12-10 | 2022-06-03 | モデルナティエックス インコーポレイテッド | 治療薬の送達のための組成物及び方法 |
| WO2017136794A1 (en) * | 2016-02-03 | 2017-08-10 | Massachusetts Institute Of Technology | Structure-guided chemical modification of guide rna and its applications |
| BR112018069795A2 (pt) | 2016-03-30 | 2019-01-29 | Intellia Therapeutics, Inc. | formulações de nanopartículas lipídicas para componentes de crispr/cas |
| US10767175B2 (en) * | 2016-06-08 | 2020-09-08 | Agilent Technologies, Inc. | High specificity genome editing using chemically modified guide RNAs |
| US10927383B2 (en) * | 2016-06-30 | 2021-02-23 | Ethris Gmbh | Cas9 mRNAs |
| WO2018119514A1 (en) | 2016-12-28 | 2018-07-05 | Precision Nanosystems Inc. | Compositions for transfecting resistant cell types |
| GB2573062A (en) * | 2016-10-14 | 2019-10-23 | Harvard College | AAV delivery of nucleobase editors |
| SG10202106058WA (en) * | 2016-12-08 | 2021-07-29 | Intellia Therapeutics Inc | Modified guide rnas |
| KR102569848B1 (ko) * | 2016-12-23 | 2023-08-25 | 프레지던트 앤드 펠로우즈 오브 하바드 칼리지 | Pcsk9의 유전자 편집 |
| EA201991804A1 (ru) * | 2017-02-01 | 2020-02-07 | МОДЕРНАТиЭкс, ИНК. | ИММУНОМОДУЛИРУЮЩИЕ ТЕРАПЕВТИЧЕСКИЕ КОМПОЗИЦИИ мРНК, КОДИРУЮЩЕЙ ПЕПТИДЫ С АКТИВИРУЮЩЕЙ ОНКОГЕННОЙ МУТАЦИЕЙ |
| TW201839136A (zh) * | 2017-02-06 | 2018-11-01 | 瑞士商諾華公司 | 治療血色素異常症之組合物及方法 |
| JP7277052B2 (ja) | 2017-02-22 | 2023-05-18 | クリスパー セラピューティクス アーゲー | プロタンパク質転換酵素サブチリシン/ケキシン9型(pcsk9)関連障害の処置のための組成物および方法 |
| CN119351474A (zh) * | 2017-02-22 | 2025-01-24 | 克里斯珀医疗股份公司 | 用于基因编辑的组合物和方法 |
| WO2018183808A1 (en) * | 2017-03-31 | 2018-10-04 | Agenovir Corporation | Antiviral therapeutic |
| WO2018191719A1 (en) | 2017-04-13 | 2018-10-18 | Acuitas Therapeutics, Inc. | Lipid delivery of therapeutic agents to adipose tissue |
| EP3645054A4 (en) | 2017-06-26 | 2021-03-31 | The Broad Institute, Inc. | CRISPR / CAS-ADENINE DESAMINASE COMPOSITIONS, TARGETED NUCLEIC ACID EDITING SYSTEMS AND METHODS |
| JP2020534795A (ja) * | 2017-07-28 | 2020-12-03 | プレジデント アンド フェローズ オブ ハーバード カレッジ | ファージによって支援される連続的進化(pace)を用いて塩基編集因子を進化させるための方法および組成物 |
| EP3437650A1 (en) | 2017-07-31 | 2019-02-06 | Accanis Biotech F&E GmbH & Co KG | Treatment of local skin hypotrophy conditions |
| US11542225B2 (en) | 2017-08-17 | 2023-01-03 | Acuitas Therapeutics, Inc. | Lipids for use in lipid nanoparticle formulations |
| AR113154A1 (es) | 2017-09-29 | 2020-01-29 | Intellia Therapeutics Inc | Polinucleótidos, composiciones y métodos para edición del genoma |
| KR20250042147A (ko) | 2017-09-29 | 2025-03-26 | 인텔리아 테라퓨틱스, 인크. | 제제 |
| KR102832035B1 (ko) | 2017-09-29 | 2025-07-09 | 인텔리아 테라퓨틱스, 인크. | 지질 나노파티클을 이용한 mRNA 전달의 체외 방법 |
| WO2019213183A1 (en) | 2018-04-30 | 2019-11-07 | The Trustees Of The University Of Pennsylvania | In utero crispr-mediated therapeutic editing of genes |
| CN112601816B (zh) | 2018-05-11 | 2024-12-17 | 比姆医疗股份有限公司 | 使用可编程碱基编辑器系统遏止病原性突变的方法 |
| US12157760B2 (en) * | 2018-05-23 | 2024-12-03 | The Broad Institute, Inc. | Base editors and uses thereof |
| US20210301274A1 (en) * | 2018-09-07 | 2021-09-30 | Beam Therapeutics Inc. | Compositions and Methods for Delivering a Nucleobase Editing System |
| IL322436A (en) | 2018-09-21 | 2025-09-01 | Acuitas Therapeutics Inc | Systems and methods for producing lipid nanoparticles and liposomes |
| JP7543259B2 (ja) | 2018-10-18 | 2024-09-02 | アクイタス セラピューティクス インコーポレイテッド | 活性剤の脂質ナノ粒子送達のための脂質 |
| WO2021056302A1 (en) * | 2019-09-26 | 2021-04-01 | Syngenta Crop Protection Ag | Methods and compositions for dna base editing |
| IL296133A (en) * | 2020-03-04 | 2022-11-01 | Verve Therapeutics Inc | Preparations and methods for administering targeted RNA |
| GB2632564B (en) | 2020-04-09 | 2025-06-18 | Verve Therapeutics Inc | Base editing of angptl3 and methods of using same for treatment of disease |
| WO2022060871A1 (en) * | 2020-09-15 | 2022-03-24 | Verve Therapeutics, Inc. | Lipid formulations for gene editing |
-
2021
- 2021-04-09 GB GB2413231.8A patent/GB2632564B/en active Active
- 2021-04-09 AU AU2021252991A patent/AU2021252991B2/en active Active
- 2021-04-09 GB GB2413218.5A patent/GB2632563B/en active Active
- 2021-04-09 WO PCT/US2021/026729 patent/WO2021207710A2/en not_active Ceased
- 2021-04-09 WO PCT/US2021/026732 patent/WO2021207712A2/en not_active Ceased
- 2021-04-09 AU AU2021253959A patent/AU2021253959B2/en active Active
- 2021-04-09 IL IL297193A patent/IL297193A/en unknown
- 2021-04-09 CA CA3179863A patent/CA3179863A1/en active Pending
- 2021-04-09 US US17/918,057 patent/US12029795B2/en active Active
- 2021-04-09 GB GB2413232.6A patent/GB2632565B/en active Active
- 2021-04-09 US US17/918,092 patent/US12115230B2/en active Active
- 2021-04-09 CN CN202180043135.4A patent/CN116096429A/zh active Pending
- 2021-04-09 BR BR112022020407A patent/BR112022020407A2/pt unknown
- 2021-04-09 KR KR1020227038926A patent/KR20230016175A/ko active Pending
- 2021-04-09 MX MX2022012683A patent/MX2022012683A/es unknown
- 2021-04-09 PH PH1/2022/553015A patent/PH12022553015A1/en unknown
- 2021-04-09 JP JP2022562027A patent/JP7457832B2/ja active Active
- 2021-04-09 EP EP21784686.4A patent/EP4133071A4/en active Pending
- 2021-04-09 BR BR112022020428A patent/BR112022020428A2/pt unknown
- 2021-04-09 WO PCT/US2021/026731 patent/WO2021207711A2/en not_active Ceased
- 2021-04-09 GB GB2317179.6A patent/GB2625902B/en active Active
- 2021-04-09 EP EP21785068.4A patent/EP4133072A4/en active Pending
- 2021-04-09 CA CA3179862A patent/CA3179862A1/en active Pending
- 2021-04-09 IL IL297194A patent/IL297194A/en unknown
- 2021-04-09 KR KR1020227038947A patent/KR20230016176A/ko active Pending
- 2021-04-09 CN CN202180041917.4A patent/CN116096883A/zh active Pending
- 2021-04-09 GB GB2216687.0A patent/GB2612452C/en active Active
- 2021-04-09 EP EP21783997.6A patent/EP4132591A4/en active Pending
- 2021-04-09 PH PH1/2022/553033A patent/PH12022553033A1/en unknown
- 2021-04-09 GB GB2413235.9A patent/GB2632566B/en active Active
- 2021-04-09 GB GB2316054.2A patent/GB2623891B/en active Active
- 2021-04-09 US US17/918,069 patent/US20230158174A1/en active Pending
- 2021-04-09 GB GB2216693.8A patent/GB2613457B/en active Active
- 2021-04-09 JP JP2022562030A patent/JP7457833B2/ja active Active
-
2024
- 2024-01-11 JP JP2024002316A patent/JP2024038326A/ja active Pending
- 2024-01-11 JP JP2024002319A patent/JP2024038327A/ja active Pending
- 2024-02-20 US US18/582,406 patent/US20240390523A1/en active Pending
- 2024-10-02 US US18/904,423 patent/US20250152746A1/en active Pending
-
2025
- 2025-06-03 AU AU2025204143A patent/AU2025204143A1/en active Pending
- 2025-06-04 AU AU2025204175A patent/AU2025204175A1/en active Pending
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20230016175A (ko) | Angptl3의 염기 편집 및 질환 치료를 위한 이의 사용 방법 | |
| CN111163633A (zh) | 包含人源化ttr基因座的非人类动物及其使用方法 | |
| US20240390520A1 (en) | Gene editing of pcsk9 or angptl3 and compositions and methods of using same for treatment of disease | |
| HK40119708A (en) | Base editing of pcsk9 and methods of using same for treatment of disease | |
| CN118284692A (zh) | Pcsk9或angptl3的基因编辑和使用其治疗疾病的组合物和方法 | |
| HK40121610A (en) | Base editing of pcsk9 and methods of using same for treatment of disease | |
| KR20240010720A (ko) | 트랜스티레틴 유전자의 염기 편집 | |
| HK40122389A (en) | Base editing of angptl3 and methods of using same for treatment of disease | |
| HK40123032A (en) | Base editing of angptl3 and methods of using same for treatment of disease |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20221107 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20240409 Comment text: Request for Examination of Application |