KR20220075438A - 비효율적 적혈구생성 치료를 위한 방법 및 조성물 - Google Patents
비효율적 적혈구생성 치료를 위한 방법 및 조성물 Download PDFInfo
- Publication number
- KR20220075438A KR20220075438A KR1020227017193A KR20227017193A KR20220075438A KR 20220075438 A KR20220075438 A KR 20220075438A KR 1020227017193 A KR1020227017193 A KR 1020227017193A KR 20227017193 A KR20227017193 A KR 20227017193A KR 20220075438 A KR20220075438 A KR 20220075438A
- Authority
- KR
- South Korea
- Prior art keywords
- actriib
- glu
- gdf trap
- leu
- gly
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000000203 mixture Substances 0.000 title claims abstract description 32
- 238000000034 method Methods 0.000 title abstract description 54
- 230000010437 erythropoiesis Effects 0.000 title description 61
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 253
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 245
- 229920001184 polypeptide Polymers 0.000 claims description 242
- 150000001413 amino acids Chemical class 0.000 claims description 104
- 238000011282 treatment Methods 0.000 claims description 100
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 57
- 230000002378 acidificating effect Effects 0.000 claims description 13
- 239000008194 pharmaceutical composition Substances 0.000 claims description 9
- 208000031162 sideroblastic anemia Diseases 0.000 claims 1
- 210000003743 erythrocyte Anatomy 0.000 abstract description 216
- 230000001965 increasing effect Effects 0.000 abstract description 68
- 108010054147 Hemoglobins Proteins 0.000 abstract description 56
- 102000001554 Hemoglobins Human genes 0.000 abstract description 56
- 241000251539 Vertebrata <Metazoa> Species 0.000 abstract description 11
- 241000283984 Rodentia Species 0.000 abstract description 3
- 241000288906 Primates Species 0.000 abstract description 2
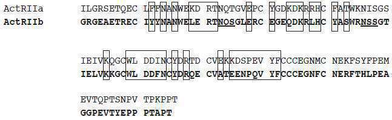
- 108010057453 activin receptor type II-B Proteins 0.000 description 331
- 102100027647 Activin receptor type-2B Human genes 0.000 description 257
- 102000003951 Erythropoietin Human genes 0.000 description 157
- 108090000394 Erythropoietin Proteins 0.000 description 157
- 229940105423 erythropoietin Drugs 0.000 description 155
- OXCMYAYHXIHQOA-UHFFFAOYSA-N potassium;[2-butyl-5-chloro-3-[[4-[2-(1,2,4-triaza-3-azanidacyclopenta-1,4-dien-5-yl)phenyl]phenyl]methyl]imidazol-4-yl]methanol Chemical compound [K+].CCCCC1=NC(Cl)=C(CO)N1CC1=CC=C(C=2C(=CC=CC=2)C2=N[N-]N=N2)C=C1 OXCMYAYHXIHQOA-UHFFFAOYSA-N 0.000 description 136
- 102220479051 CD59 glycoprotein_L79D_mutation Human genes 0.000 description 120
- 235000001014 amino acid Nutrition 0.000 description 112
- 230000000694 effects Effects 0.000 description 110
- 108090000623 proteins and genes Proteins 0.000 description 106
- 210000004027 cell Anatomy 0.000 description 104
- 229940024606 amino acid Drugs 0.000 description 103
- XEEYBQQBJWHFJM-UHFFFAOYSA-N Iron Chemical compound [Fe] XEEYBQQBJWHFJM-UHFFFAOYSA-N 0.000 description 98
- 208000007502 anemia Diseases 0.000 description 98
- 241000699670 Mus sp. Species 0.000 description 95
- 230000027455 binding Effects 0.000 description 90
- 102000004169 proteins and genes Human genes 0.000 description 83
- 235000018102 proteins Nutrition 0.000 description 80
- 239000003446 ligand Substances 0.000 description 77
- 210000004369 blood Anatomy 0.000 description 63
- 239000008280 blood Substances 0.000 description 63
- 239000003981 vehicle Substances 0.000 description 61
- 102000037865 fusion proteins Human genes 0.000 description 59
- 108020001507 fusion proteins Proteins 0.000 description 59
- 239000000488 activin Substances 0.000 description 56
- 108010059616 Activins Proteins 0.000 description 54
- 150000001875 compounds Chemical class 0.000 description 54
- 102100026818 Inhibin beta E chain Human genes 0.000 description 53
- 229910052742 iron Inorganic materials 0.000 description 49
- 102000005962 receptors Human genes 0.000 description 48
- 108020003175 receptors Proteins 0.000 description 48
- 230000014509 gene expression Effects 0.000 description 43
- 230000002829 reductive effect Effects 0.000 description 42
- 230000035772 mutation Effects 0.000 description 37
- 210000000952 spleen Anatomy 0.000 description 36
- 210000001519 tissue Anatomy 0.000 description 36
- 239000000126 substance Substances 0.000 description 34
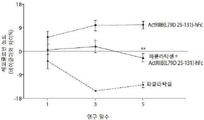
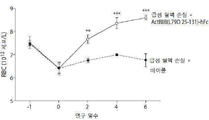
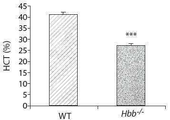
- 238000005534 hematocrit Methods 0.000 description 33
- 150000007523 nucleic acids Chemical class 0.000 description 33
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 32
- 208000005980 beta thalassemia Diseases 0.000 description 31
- 238000010172 mouse model Methods 0.000 description 31
- 210000001185 bone marrow Anatomy 0.000 description 29
- 238000004519 manufacturing process Methods 0.000 description 29
- 108091006084 receptor activators Proteins 0.000 description 29
- 230000000875 corresponding effect Effects 0.000 description 28
- 239000002773 nucleotide Substances 0.000 description 28
- 125000003729 nucleotide group Chemical group 0.000 description 28
- 230000003394 haemopoietic effect Effects 0.000 description 27
- 239000013598 vector Substances 0.000 description 26
- 102000039446 nucleic acids Human genes 0.000 description 25
- 108020004707 nucleic acids Proteins 0.000 description 25
- 206010065973 Iron Overload Diseases 0.000 description 24
- 210000003924 normoblast Anatomy 0.000 description 24
- 208000035475 disorder Diseases 0.000 description 23
- 230000004927 fusion Effects 0.000 description 23
- 108020001756 ligand binding domains Proteins 0.000 description 23
- 238000001727 in vivo Methods 0.000 description 22
- 210000000988 bone and bone Anatomy 0.000 description 21
- 238000001802 infusion Methods 0.000 description 21
- 230000004048 modification Effects 0.000 description 20
- 238000012986 modification Methods 0.000 description 20
- 241000282414 Homo sapiens Species 0.000 description 19
- 239000003814 drug Substances 0.000 description 19
- 230000001225 therapeutic effect Effects 0.000 description 19
- 238000004458 analytical method Methods 0.000 description 18
- 230000036772 blood pressure Effects 0.000 description 18
- 238000006467 substitution reaction Methods 0.000 description 18
- 102100030655 Platelet-activating factor acetylhydrolase IB subunit beta Human genes 0.000 description 17
- 208000002903 Thalassemia Diseases 0.000 description 17
- -1 activin A Chemical compound 0.000 description 17
- 238000003556 assay Methods 0.000 description 17
- 210000001995 reticulocyte Anatomy 0.000 description 17
- 108010007726 Bone Morphogenetic Proteins Proteins 0.000 description 16
- 102000007350 Bone Morphogenetic Proteins Human genes 0.000 description 16
- 108091028043 Nucleic acid sequence Proteins 0.000 description 16
- 229940112869 bone morphogenetic protein Drugs 0.000 description 16
- 230000004069 differentiation Effects 0.000 description 16
- 230000013595 glycosylation Effects 0.000 description 16
- 238000006206 glycosylation reaction Methods 0.000 description 16
- 230000011664 signaling Effects 0.000 description 16
- 208000011580 syndromic disease Diseases 0.000 description 16
- 102100040898 Growth/differentiation factor 11 Human genes 0.000 description 15
- 206010028980 Neoplasm Diseases 0.000 description 15
- 108010023082 activin A Proteins 0.000 description 15
- 230000015572 biosynthetic process Effects 0.000 description 15
- 230000007423 decrease Effects 0.000 description 15
- 101710194452 Growth/differentiation factor 11 Proteins 0.000 description 14
- 101000937269 Homo sapiens Activin receptor type-2B Proteins 0.000 description 14
- 102000003978 Tissue Plasminogen Activator Human genes 0.000 description 14
- 108090000373 Tissue Plasminogen Activator Proteins 0.000 description 14
- 102000045412 human ACVR2B Human genes 0.000 description 14
- 239000002609 medium Substances 0.000 description 14
- 229960000187 tissue plasminogen activator Drugs 0.000 description 14
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 13
- 208000020832 chronic kidney disease Diseases 0.000 description 13
- 238000000746 purification Methods 0.000 description 13
- 230000001105 regulatory effect Effects 0.000 description 13
- 210000002966 serum Anatomy 0.000 description 13
- 210000000130 stem cell Anatomy 0.000 description 13
- 238000012360 testing method Methods 0.000 description 13
- 102100039939 Growth/differentiation factor 8 Human genes 0.000 description 12
- 108010056852 Myostatin Proteins 0.000 description 12
- 206010041660 Splenomegaly Diseases 0.000 description 12
- 230000001154 acute effect Effects 0.000 description 12
- 125000000539 amino acid group Chemical group 0.000 description 12
- 229940009098 aspartate Drugs 0.000 description 12
- 201000011510 cancer Diseases 0.000 description 12
- 230000000913 erythropoietic effect Effects 0.000 description 12
- 239000003112 inhibitor Substances 0.000 description 12
- 229960001592 paclitaxel Drugs 0.000 description 12
- 230000007170 pathology Effects 0.000 description 12
- 239000013612 plasmid Substances 0.000 description 12
- 210000000689 upper leg Anatomy 0.000 description 12
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 11
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 11
- 229930012538 Paclitaxel Natural products 0.000 description 11
- 230000003247 decreasing effect Effects 0.000 description 11
- 239000012634 fragment Substances 0.000 description 11
- 108060003558 hepcidin Proteins 0.000 description 11
- 102000040430 polynucleotide Human genes 0.000 description 11
- 108091033319 polynucleotide Proteins 0.000 description 11
- 239000002157 polynucleotide Substances 0.000 description 11
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 11
- XJOTXKZIRSHZQV-RXHOOSIZSA-N (3S)-3-amino-4-[[(2S,3R)-1-[[(2S)-1-[[(2S)-1-[(2S)-2-[[(2S,3S)-1-[[(1R,6R,12R,17R,20S,23S,26R,31R,34R,39R,42S,45S,48S,51S,59S)-51-(4-aminobutyl)-31-[[(2S)-6-amino-1-[[(1S,2R)-1-carboxy-2-hydroxypropyl]amino]-1-oxohexan-2-yl]carbamoyl]-20-benzyl-23-[(2S)-butan-2-yl]-45-(3-carbamimidamidopropyl)-48-(hydroxymethyl)-42-(1H-imidazol-4-ylmethyl)-59-(2-methylsulfanylethyl)-7,10,19,22,25,33,40,43,46,49,52,54,57,60,63,64-hexadecaoxo-3,4,14,15,28,29,36,37-octathia-8,11,18,21,24,32,41,44,47,50,53,55,58,61,62,65-hexadecazatetracyclo[32.19.8.26,17.212,39]pentahexacontan-26-yl]amino]-3-methyl-1-oxopentan-2-yl]carbamoyl]pyrrolidin-1-yl]-1-oxo-3-phenylpropan-2-yl]amino]-3-(1H-imidazol-4-yl)-1-oxopropan-2-yl]amino]-3-hydroxy-1-oxobutan-2-yl]amino]-4-oxobutanoic acid Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@@H](N)CC(O)=O)[C@@H](C)O)C(=O)N[C@H]1CSSC[C@H](NC(=O)[C@@H]2CSSC[C@@H]3NC(=O)[C@@H]4CSSC[C@H](NC(=O)[C@H](Cc5ccccc5)NC(=O)[C@@H](NC1=O)[C@@H](C)CC)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1cnc[nH]1)NC3=O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N2)C(=O)NCC(=O)N4)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XJOTXKZIRSHZQV-RXHOOSIZSA-N 0.000 description 10
- 241000699666 Mus <mouse, genus> Species 0.000 description 10
- 239000000556 agonist Substances 0.000 description 10
- 210000004899 c-terminal region Anatomy 0.000 description 10
- 230000008859 change Effects 0.000 description 10
- 239000003795 chemical substances by application Substances 0.000 description 10
- 238000012217 deletion Methods 0.000 description 10
- 230000037430 deletion Effects 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 102000018511 hepcidin Human genes 0.000 description 10
- 229940066919 hepcidin Drugs 0.000 description 10
- 210000003205 muscle Anatomy 0.000 description 10
- 238000002560 therapeutic procedure Methods 0.000 description 10
- 102220542870 Presenilins-associated rhomboid-like protein, mitochondrial_L79E_mutation Human genes 0.000 description 9
- 238000007792 addition Methods 0.000 description 9
- 239000000306 component Substances 0.000 description 9
- 230000006378 damage Effects 0.000 description 9
- 201000010099 disease Diseases 0.000 description 9
- 229940079593 drug Drugs 0.000 description 9
- 210000003527 eukaryotic cell Anatomy 0.000 description 9
- 239000013604 expression vector Substances 0.000 description 9
- 238000000338 in vitro Methods 0.000 description 9
- 210000003734 kidney Anatomy 0.000 description 9
- 230000035800 maturation Effects 0.000 description 9
- 239000002243 precursor Substances 0.000 description 9
- 102220525561 Coiled-coil domain-containing protein 200_A24N_mutation Human genes 0.000 description 8
- 241000282412 Homo Species 0.000 description 8
- CKLJMWTZIZZHCS-REOHCLBHSA-N L-aspartic acid Chemical compound OC(=O)[C@@H](N)CC(O)=O CKLJMWTZIZZHCS-REOHCLBHSA-N 0.000 description 8
- 108010076504 Protein Sorting Signals Proteins 0.000 description 8
- 241000700159 Rattus Species 0.000 description 8
- 102100026144 Transferrin receptor protein 1 Human genes 0.000 description 8
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 8
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 8
- 230000004075 alteration Effects 0.000 description 8
- CKLJMWTZIZZHCS-REOHCLBHSA-L aspartate group Chemical group N[C@@H](CC(=O)[O-])C(=O)[O-] CKLJMWTZIZZHCS-REOHCLBHSA-L 0.000 description 8
- 238000004820 blood count Methods 0.000 description 8
- 238000000423 cell based assay Methods 0.000 description 8
- 230000001684 chronic effect Effects 0.000 description 8
- 239000012091 fetal bovine serum Substances 0.000 description 8
- 230000011132 hemopoiesis Effects 0.000 description 8
- 239000002502 liposome Substances 0.000 description 8
- 210000004185 liver Anatomy 0.000 description 8
- 229920000609 methyl cellulose Polymers 0.000 description 8
- 239000001923 methylcellulose Substances 0.000 description 8
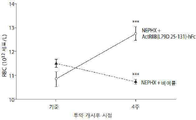
- 238000013059 nephrectomy Methods 0.000 description 8
- 230000035755 proliferation Effects 0.000 description 8
- 230000001177 retroviral effect Effects 0.000 description 8
- ZRKFYGHZFMAOKI-QMGMOQQFSA-N tgfbeta Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)CNC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CCSC)C(C)C)[C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](C)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N1[C@@H](CCC1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O)C1=CC=C(O)C=C1 ZRKFYGHZFMAOKI-QMGMOQQFSA-N 0.000 description 8
- 239000003656 tris buffered saline Substances 0.000 description 8
- 101000835093 Homo sapiens Transferrin receptor protein 1 Proteins 0.000 description 7
- 206010021143 Hypoxia Diseases 0.000 description 7
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 7
- 239000007983 Tris buffer Substances 0.000 description 7
- 235000004279 alanine Nutrition 0.000 description 7
- 230000009286 beneficial effect Effects 0.000 description 7
- 230000004071 biological effect Effects 0.000 description 7
- 238000002512 chemotherapy Methods 0.000 description 7
- 210000000078 claw Anatomy 0.000 description 7
- 238000011284 combination treatment Methods 0.000 description 7
- 230000003993 interaction Effects 0.000 description 7
- 210000000265 leukocyte Anatomy 0.000 description 7
- 210000004962 mammalian cell Anatomy 0.000 description 7
- 239000011159 matrix material Substances 0.000 description 7
- 238000005259 measurement Methods 0.000 description 7
- 230000007246 mechanism Effects 0.000 description 7
- 230000001404 mediated effect Effects 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 239000011780 sodium chloride Substances 0.000 description 7
- 235000000346 sugar Nutrition 0.000 description 7
- 230000004083 survival effect Effects 0.000 description 7
- 229940124597 therapeutic agent Drugs 0.000 description 7
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 7
- BPYKTIZUTYGOLE-IFADSCNNSA-N Bilirubin Chemical compound N1C(=O)C(C)=C(C=C)\C1=C\C1=C(C)C(CCC(O)=O)=C(CC2=C(C(C)=C(\C=C/3C(=C(C=C)C(=O)N\3)C)N2)CCC(O)=O)N1 BPYKTIZUTYGOLE-IFADSCNNSA-N 0.000 description 6
- 238000011740 C57BL/6 mouse Methods 0.000 description 6
- 102000014914 Carrier Proteins Human genes 0.000 description 6
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 6
- 102000005720 Glutathione transferase Human genes 0.000 description 6
- 108010070675 Glutathione transferase Proteins 0.000 description 6
- 239000004471 Glycine Substances 0.000 description 6
- 108091005904 Hemoglobin subunit beta Proteins 0.000 description 6
- 208000032843 Hemorrhage Diseases 0.000 description 6
- 241001529936 Murinae Species 0.000 description 6
- 230000004988 N-glycosylation Effects 0.000 description 6
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 6
- 241000700605 Viruses Species 0.000 description 6
- 230000004913 activation Effects 0.000 description 6
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 6
- 108091008324 binding proteins Proteins 0.000 description 6
- 150000001720 carbohydrates Chemical group 0.000 description 6
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 6
- 238000004587 chromatography analysis Methods 0.000 description 6
- 238000003776 cleavage reaction Methods 0.000 description 6
- 235000018417 cysteine Nutrition 0.000 description 6
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 6
- 230000001086 cytosolic effect Effects 0.000 description 6
- 238000001514 detection method Methods 0.000 description 6
- 238000002474 experimental method Methods 0.000 description 6
- 230000002209 hydrophobic effect Effects 0.000 description 6
- 150000002632 lipids Chemical group 0.000 description 6
- 239000000463 material Substances 0.000 description 6
- 229910052760 oxygen Inorganic materials 0.000 description 6
- 239000001301 oxygen Substances 0.000 description 6
- 229920001223 polyethylene glycol Polymers 0.000 description 6
- 210000001236 prokaryotic cell Anatomy 0.000 description 6
- 230000004044 response Effects 0.000 description 6
- 239000000523 sample Substances 0.000 description 6
- 230000007017 scission Effects 0.000 description 6
- 238000011269 treatment regimen Methods 0.000 description 6
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 5
- 241000282693 Cercopithecidae Species 0.000 description 5
- 108020004414 DNA Proteins 0.000 description 5
- 206010018910 Haemolysis Diseases 0.000 description 5
- 206010020772 Hypertension Diseases 0.000 description 5
- 208000015710 Iron-Deficiency Anemia Diseases 0.000 description 5
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 5
- 241000282567 Macaca fascicularis Species 0.000 description 5
- 208000009527 Refractory anemia Diseases 0.000 description 5
- 206010072684 Refractory cytopenia with unilineage dysplasia Diseases 0.000 description 5
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 5
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 5
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 5
- 239000004473 Threonine Substances 0.000 description 5
- 108010023079 activin B Proteins 0.000 description 5
- 201000006288 alpha thalassemia Diseases 0.000 description 5
- 239000005557 antagonist Substances 0.000 description 5
- 208000034158 bleeding Diseases 0.000 description 5
- 230000000740 bleeding effect Effects 0.000 description 5
- 230000001413 cellular effect Effects 0.000 description 5
- 230000000295 complement effect Effects 0.000 description 5
- 235000005911 diet Nutrition 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 238000001415 gene therapy Methods 0.000 description 5
- 229930195712 glutamate Natural products 0.000 description 5
- 230000012010 growth Effects 0.000 description 5
- 239000003102 growth factor Substances 0.000 description 5
- 230000008588 hemolysis Effects 0.000 description 5
- 230000001976 improved effect Effects 0.000 description 5
- 230000001939 inductive effect Effects 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 230000001737 promoting effect Effects 0.000 description 5
- 239000003642 reactive oxygen metabolite Substances 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 238000003860 storage Methods 0.000 description 5
- 238000010254 subcutaneous injection Methods 0.000 description 5
- 239000007929 subcutaneous injection Substances 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 5
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 4
- 239000004475 Arginine Substances 0.000 description 4
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 4
- 101150002621 EPO gene Proteins 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 108091006020 Fc-tagged proteins Proteins 0.000 description 4
- 108091005902 Hemoglobin subunit alpha Proteins 0.000 description 4
- 241000238631 Hexapoda Species 0.000 description 4
- 108060003951 Immunoglobulin Proteins 0.000 description 4
- 206010061218 Inflammation Diseases 0.000 description 4
- 206010022971 Iron Deficiencies Diseases 0.000 description 4
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 4
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 4
- AYFVYJQAPQTCCC-GBXIJSLDSA-N L-threonine Chemical compound C[C@@H](O)[C@H](N)C(O)=O AYFVYJQAPQTCCC-GBXIJSLDSA-N 0.000 description 4
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 4
- 239000002202 Polyethylene glycol Substances 0.000 description 4
- 229920002684 Sepharose Polymers 0.000 description 4
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 4
- 229930006000 Sucrose Natural products 0.000 description 4
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 4
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 4
- 238000009825 accumulation Methods 0.000 description 4
- 239000013543 active substance Substances 0.000 description 4
- 230000006907 apoptotic process Effects 0.000 description 4
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 4
- 235000009582 asparagine Nutrition 0.000 description 4
- 229960001230 asparagine Drugs 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 230000003111 delayed effect Effects 0.000 description 4
- 230000037213 diet Effects 0.000 description 4
- 238000009826 distribution Methods 0.000 description 4
- 239000000839 emulsion Substances 0.000 description 4
- 238000005516 engineering process Methods 0.000 description 4
- 230000002708 enhancing effect Effects 0.000 description 4
- 229940088598 enzyme Drugs 0.000 description 4
- 235000019441 ethanol Nutrition 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 229920000159 gelatin Polymers 0.000 description 4
- 235000019322 gelatine Nutrition 0.000 description 4
- 235000011187 glycerol Nutrition 0.000 description 4
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 description 4
- 230000013632 homeostatic process Effects 0.000 description 4
- 229940088597 hormone Drugs 0.000 description 4
- 239000005556 hormone Substances 0.000 description 4
- 229910052739 hydrogen Inorganic materials 0.000 description 4
- 230000007954 hypoxia Effects 0.000 description 4
- 102000018358 immunoglobulin Human genes 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 230000000977 initiatory effect Effects 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 229910052500 inorganic mineral Inorganic materials 0.000 description 4
- 238000002372 labelling Methods 0.000 description 4
- VDXZNPDIRNWWCW-JFTDCZMZSA-N melittin Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(N)=O)CC1=CNC2=CC=CC=C12 VDXZNPDIRNWWCW-JFTDCZMZSA-N 0.000 description 4
- 239000011707 mineral Substances 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- 210000005259 peripheral blood Anatomy 0.000 description 4
- 239000011886 peripheral blood Substances 0.000 description 4
- 238000001959 radiotherapy Methods 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 150000003839 salts Chemical class 0.000 description 4
- 238000012163 sequencing technique Methods 0.000 description 4
- 230000019491 signal transduction Effects 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 230000000087 stabilizing effect Effects 0.000 description 4
- 238000010186 staining Methods 0.000 description 4
- 230000000638 stimulation Effects 0.000 description 4
- 239000005720 sucrose Substances 0.000 description 4
- 150000008163 sugars Chemical class 0.000 description 4
- 239000000725 suspension Substances 0.000 description 4
- 208000024891 symptom Diseases 0.000 description 4
- 238000003786 synthesis reaction Methods 0.000 description 4
- 102000035160 transmembrane proteins Human genes 0.000 description 4
- 108091005703 transmembrane proteins Proteins 0.000 description 4
- 241000701447 unidentified baculovirus Species 0.000 description 4
- 239000004474 valine Substances 0.000 description 4
- 229960004295 valine Drugs 0.000 description 4
- UBWXUGDQUBIEIZ-UHFFFAOYSA-N (13-methyl-3-oxo-2,6,7,8,9,10,11,12,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-yl) 3-phenylpropanoate Chemical compound CC12CCC(C3CCC(=O)C=C3CC3)C3C1CCC2OC(=O)CCC1=CC=CC=C1 UBWXUGDQUBIEIZ-UHFFFAOYSA-N 0.000 description 3
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 3
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 3
- UZOVYGYOLBIAJR-UHFFFAOYSA-N 4-isocyanato-4'-methyldiphenylmethane Chemical compound C1=CC(C)=CC=C1CC1=CC=C(N=C=O)C=C1 UZOVYGYOLBIAJR-UHFFFAOYSA-N 0.000 description 3
- 102000005606 Activins Human genes 0.000 description 3
- 108010088751 Albumins Proteins 0.000 description 3
- 102000009027 Albumins Human genes 0.000 description 3
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 3
- 108010049870 Bone Morphogenetic Protein 7 Proteins 0.000 description 3
- 102100022544 Bone morphogenetic protein 7 Human genes 0.000 description 3
- 241000283690 Bos taurus Species 0.000 description 3
- 101100297347 Caenorhabditis elegans pgl-3 gene Proteins 0.000 description 3
- 102220578642 Chorion-specific transcription factor GCMb_K74M_mutation Human genes 0.000 description 3
- 206010010356 Congenital anomaly Diseases 0.000 description 3
- 241000699802 Cricetulus griseus Species 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 3
- 102000016970 Follistatin Human genes 0.000 description 3
- 108010014612 Follistatin Proteins 0.000 description 3
- 108010010803 Gelatin Proteins 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 3
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 3
- 101000970954 Homo sapiens Activin receptor type-2A Proteins 0.000 description 3
- 101000987586 Homo sapiens Eosinophil peroxidase Proteins 0.000 description 3
- 101000869690 Homo sapiens Protein S100-A8 Proteins 0.000 description 3
- KFZMGEQAYNKOFK-UHFFFAOYSA-N Isopropanol Chemical compound CC(C)O KFZMGEQAYNKOFK-UHFFFAOYSA-N 0.000 description 3
- ODKSFYDXXFIFQN-BYPYZUCNSA-P L-argininium(2+) Chemical compound NC(=[NH2+])NCCC[C@H]([NH3+])C(O)=O ODKSFYDXXFIFQN-BYPYZUCNSA-P 0.000 description 3
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 3
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 3
- 240000007472 Leucaena leucocephala Species 0.000 description 3
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 3
- 108060001084 Luciferase Proteins 0.000 description 3
- 239000005089 Luciferase Substances 0.000 description 3
- 102100025751 Mothers against decapentaplegic homolog 2 Human genes 0.000 description 3
- 101710143123 Mothers against decapentaplegic homolog 2 Proteins 0.000 description 3
- 102000044547 Nodal Human genes 0.000 description 3
- 108700024442 Nodal Proteins 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 102000035195 Peptidases Human genes 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 102100032442 Protein S100-A8 Human genes 0.000 description 3
- 241000714474 Rous sarcoma virus Species 0.000 description 3
- 108091081021 Sense strand Proteins 0.000 description 3
- 102000043168 TGF-beta family Human genes 0.000 description 3
- 108091085018 TGF-beta family Proteins 0.000 description 3
- 208000007536 Thrombosis Diseases 0.000 description 3
- 238000010521 absorption reaction Methods 0.000 description 3
- 230000009471 action Effects 0.000 description 3
- 238000001042 affinity chromatography Methods 0.000 description 3
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 3
- 230000000692 anti-sense effect Effects 0.000 description 3
- 235000003704 aspartic acid Nutrition 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 239000011324 bead Substances 0.000 description 3
- OQFSQFPPLPISGP-UHFFFAOYSA-N beta-carboxyaspartic acid Natural products OC(=O)C(N)C(C(O)=O)C(O)=O OQFSQFPPLPISGP-UHFFFAOYSA-N 0.000 description 3
- 210000000601 blood cell Anatomy 0.000 description 3
- 239000001506 calcium phosphate Substances 0.000 description 3
- 230000022159 cartilage development Effects 0.000 description 3
- 238000004113 cell culture Methods 0.000 description 3
- 208000022831 chronic renal failure syndrome Diseases 0.000 description 3
- 238000001246 colloidal dispersion Methods 0.000 description 3
- 102000003675 cytokine receptors Human genes 0.000 description 3
- 108010057085 cytokine receptors Proteins 0.000 description 3
- 230000007812 deficiency Effects 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000003937 drug carrier Substances 0.000 description 3
- 239000008273 gelatin Substances 0.000 description 3
- 235000011852 gelatine desserts Nutrition 0.000 description 3
- 235000013922 glutamic acid Nutrition 0.000 description 3
- 239000004220 glutamic acid Substances 0.000 description 3
- 239000005090 green fluorescent protein Substances 0.000 description 3
- 210000002216 heart Anatomy 0.000 description 3
- 208000019622 heart disease Diseases 0.000 description 3
- 125000001165 hydrophobic group Chemical group 0.000 description 3
- 229910052588 hydroxylapatite Inorganic materials 0.000 description 3
- 206010020718 hyperplasia Diseases 0.000 description 3
- 230000003463 hyperproliferative effect Effects 0.000 description 3
- 208000003532 hypothyroidism Diseases 0.000 description 3
- 230000002989 hypothyroidism Effects 0.000 description 3
- 230000001771 impaired effect Effects 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 208000015181 infectious disease Diseases 0.000 description 3
- 230000002757 inflammatory effect Effects 0.000 description 3
- 229940075525 iron chelating agent Drugs 0.000 description 3
- 239000000797 iron chelating agent Substances 0.000 description 3
- 208000017169 kidney disease Diseases 0.000 description 3
- 239000008101 lactose Substances 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 208000019423 liver disease Diseases 0.000 description 3
- 230000001926 lymphatic effect Effects 0.000 description 3
- 210000001161 mammalian embryo Anatomy 0.000 description 3
- 238000007726 management method Methods 0.000 description 3
- 210000003716 mesoderm Anatomy 0.000 description 3
- 108020004999 messenger RNA Proteins 0.000 description 3
- 230000037257 muscle growth Effects 0.000 description 3
- 230000009437 off-target effect Effects 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- XYJRXVWERLGGKC-UHFFFAOYSA-D pentacalcium;hydroxide;triphosphate Chemical compound [OH-].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O XYJRXVWERLGGKC-UHFFFAOYSA-D 0.000 description 3
- 239000000816 peptidomimetic Substances 0.000 description 3
- 230000003285 pharmacodynamic effect Effects 0.000 description 3
- 150000003904 phospholipids Chemical class 0.000 description 3
- 239000006187 pill Substances 0.000 description 3
- 229920002704 polyhistidine Polymers 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 238000000159 protein binding assay Methods 0.000 description 3
- 230000017854 proteolysis Effects 0.000 description 3
- 206010039073 rheumatoid arthritis Diseases 0.000 description 3
- 238000012216 screening Methods 0.000 description 3
- 238000007423 screening assay Methods 0.000 description 3
- 230000028327 secretion Effects 0.000 description 3
- 150000003384 small molecules Chemical class 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 230000003393 splenic effect Effects 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 239000013589 supplement Substances 0.000 description 3
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 239000003826 tablet Substances 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 230000005030 transcription termination Effects 0.000 description 3
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 3
- 238000005406 washing Methods 0.000 description 3
- 230000003442 weekly effect Effects 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical compound OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 2
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 2
- UUUHXMGGBIUAPW-UHFFFAOYSA-N 1-[1-[2-[[5-amino-2-[[1-[5-(diaminomethylideneamino)-2-[[1-[3-(1h-indol-3-yl)-2-[(5-oxopyrrolidine-2-carbonyl)amino]propanoyl]pyrrolidine-2-carbonyl]amino]pentanoyl]pyrrolidine-2-carbonyl]amino]-5-oxopentanoyl]amino]-3-methylpentanoyl]pyrrolidine-2-carbon Chemical compound C1CCC(C(=O)N2C(CCC2)C(O)=O)N1C(=O)C(C(C)CC)NC(=O)C(CCC(N)=O)NC(=O)C1CCCN1C(=O)C(CCCN=C(N)N)NC(=O)C1CCCN1C(=O)C(CC=1C2=CC=CC=C2NC=1)NC(=O)C1CCC(=O)N1 UUUHXMGGBIUAPW-UHFFFAOYSA-N 0.000 description 2
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 2
- 102000018918 Activin Receptors Human genes 0.000 description 2
- 108010052946 Activin Receptors Proteins 0.000 description 2
- 102100034134 Activin receptor type-1B Human genes 0.000 description 2
- 208000030090 Acute Disease Diseases 0.000 description 2
- 208000031261 Acute myeloid leukaemia Diseases 0.000 description 2
- 102220556625 Acyl-coenzyme A thioesterase 11_K55A_mutation Human genes 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- NLXLAEXVIDQMFP-UHFFFAOYSA-N Ammonia chloride Chemical compound [NH4+].[Cl-] NLXLAEXVIDQMFP-UHFFFAOYSA-N 0.000 description 2
- 206010002065 Anaemia megaloblastic Diseases 0.000 description 2
- 208000030760 Anaemia of chronic disease Diseases 0.000 description 2
- 102220638160 Arylamine N-acetyltransferase 1_R64K_mutation Human genes 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 206010065553 Bone marrow failure Diseases 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 208000031229 Cardiomyopathies Diseases 0.000 description 2
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- 102220578645 Chorion-specific transcription factor GCMb_N65A_mutation Human genes 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 108020004705 Codon Proteins 0.000 description 2
- 102000008186 Collagen Human genes 0.000 description 2
- 108010035532 Collagen Proteins 0.000 description 2
- 206010009944 Colon cancer Diseases 0.000 description 2
- 208000029767 Congenital, Hereditary, and Neonatal Diseases and Abnormalities Diseases 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- 101100179813 Drosophila melanogaster Actbeta gene Proteins 0.000 description 2
- 102220575187 Egl nine homolog 1_F82A_mutation Human genes 0.000 description 2
- 241000196324 Embryophyta Species 0.000 description 2
- 108010013369 Enteropeptidase Proteins 0.000 description 2
- 102100029727 Enteropeptidase Human genes 0.000 description 2
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical compound OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 2
- 102100029379 Follistatin-related protein 3 Human genes 0.000 description 2
- 241000206672 Gelidium Species 0.000 description 2
- 102000005744 Glycoside Hydrolases Human genes 0.000 description 2
- 108010031186 Glycoside Hydrolases Proteins 0.000 description 2
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 2
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 2
- 241000713858 Harvey murine sarcoma virus Species 0.000 description 2
- 101710154606 Hemagglutinin Proteins 0.000 description 2
- 102100021519 Hemoglobin subunit beta Human genes 0.000 description 2
- 101000799189 Homo sapiens Activin receptor type-1B Proteins 0.000 description 2
- 101001062529 Homo sapiens Follistatin-related protein 3 Proteins 0.000 description 2
- 101001059454 Homo sapiens Serine/threonine-protein kinase MARK2 Proteins 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- 201000002980 Hyperparathyroidism Diseases 0.000 description 2
- 102000000646 Interleukin-3 Human genes 0.000 description 2
- 108010002386 Interleukin-3 Proteins 0.000 description 2
- 102000004889 Interleukin-6 Human genes 0.000 description 2
- 108090001005 Interleukin-6 Proteins 0.000 description 2
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 2
- HNDVDQJCIGZPNO-YFKPBYRVSA-N L-histidine Chemical compound OC(=O)[C@@H](N)CC1=CN=CN1 HNDVDQJCIGZPNO-YFKPBYRVSA-N 0.000 description 2
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 2
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- 208000000682 Megaloblastic Anemia Diseases 0.000 description 2
- 208000029725 Metabolic bone disease Diseases 0.000 description 2
- 241000713869 Moloney murine leukemia virus Species 0.000 description 2
- 208000034578 Multiple myelomas Diseases 0.000 description 2
- 102000007474 Multiprotein Complexes Human genes 0.000 description 2
- 108010085220 Multiprotein Complexes Proteins 0.000 description 2
- 101100238610 Mus musculus Msh3 gene Proteins 0.000 description 2
- 208000033776 Myeloid Acute Leukemia Diseases 0.000 description 2
- UBQYURCVBFRUQT-UHFFFAOYSA-N N-benzoyl-Ferrioxamine B Chemical compound CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN UBQYURCVBFRUQT-UHFFFAOYSA-N 0.000 description 2
- 230000004989 O-glycosylation Effects 0.000 description 2
- 102220567352 Ornithine decarboxylase antizyme 1_K74F_mutation Human genes 0.000 description 2
- 208000001132 Osteoporosis Diseases 0.000 description 2
- 101710093908 Outer capsid protein VP4 Proteins 0.000 description 2
- 101710135467 Outer capsid protein sigma-1 Proteins 0.000 description 2
- 206010033661 Pancytopenia Diseases 0.000 description 2
- 102000004270 Peptidyl-Dipeptidase A Human genes 0.000 description 2
- 108090000882 Peptidyl-Dipeptidase A Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 101710176177 Protein A56 Proteins 0.000 description 2
- 102220495689 Putative uncharacterized protein FLJ43944_R40A_mutation Human genes 0.000 description 2
- 102100028904 Serine/threonine-protein kinase MARK2 Human genes 0.000 description 2
- 102000007374 Smad Proteins Human genes 0.000 description 2
- 108010007945 Smad Proteins Proteins 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 238000000692 Student's t-test Methods 0.000 description 2
- 108020005038 Terminator Codon Proteins 0.000 description 2
- 102000040945 Transcription factor Human genes 0.000 description 2
- 108091023040 Transcription factor Proteins 0.000 description 2
- 102000004338 Transferrin Human genes 0.000 description 2
- 108090000901 Transferrin Proteins 0.000 description 2
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 239000012190 activator Substances 0.000 description 2
- 239000004480 active ingredient Substances 0.000 description 2
- 239000002671 adjuvant Substances 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 235000010443 alginic acid Nutrition 0.000 description 2
- 229920000615 alginic acid Polymers 0.000 description 2
- BFNBIHQBYMNNAN-UHFFFAOYSA-N ammonium sulfate Chemical compound N.N.OS(O)(=O)=O BFNBIHQBYMNNAN-UHFFFAOYSA-N 0.000 description 2
- 229910052921 ammonium sulfate Inorganic materials 0.000 description 2
- 235000011130 ammonium sulphate Nutrition 0.000 description 2
- 208000022400 anemia due to chronic disease Diseases 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 239000000440 bentonite Substances 0.000 description 2
- 229910000278 bentonite Inorganic materials 0.000 description 2
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 2
- SESFRYSPDFLNCH-UHFFFAOYSA-N benzyl benzoate Chemical compound C=1C=CC=CC=1C(=O)OCC1=CC=CC=C1 SESFRYSPDFLNCH-UHFFFAOYSA-N 0.000 description 2
- 210000002798 bone marrow cell Anatomy 0.000 description 2
- 238000010322 bone marrow transplantation Methods 0.000 description 2
- 210000004556 brain Anatomy 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 2
- 238000012754 cardiac puncture Methods 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 230000004663 cell proliferation Effects 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 230000007248 cellular mechanism Effects 0.000 description 2
- 238000002655 chelation therapy Methods 0.000 description 2
- 238000001311 chemical methods and process Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 229920001436 collagen Polymers 0.000 description 2
- 230000001332 colony forming effect Effects 0.000 description 2
- 230000009918 complex formation Effects 0.000 description 2
- 239000003636 conditioned culture medium Substances 0.000 description 2
- 239000013068 control sample Substances 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 208000024389 cytopenia Diseases 0.000 description 2
- 229960000958 deferoxamine Drugs 0.000 description 2
- 230000022811 deglycosylation Effects 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000000502 dialysis Methods 0.000 description 2
- 239000000539 dimer Substances 0.000 description 2
- 230000004064 dysfunction Effects 0.000 description 2
- 230000013020 embryo development Effects 0.000 description 2
- 239000003995 emulsifying agent Substances 0.000 description 2
- 230000001804 emulsifying effect Effects 0.000 description 2
- 239000003623 enhancer Substances 0.000 description 2
- 230000002255 enzymatic effect Effects 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 239000000945 filler Substances 0.000 description 2
- 238000002866 fluorescence resonance energy transfer Methods 0.000 description 2
- 239000008187 granular material Substances 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 239000000185 hemagglutinin Substances 0.000 description 2
- 230000002489 hematologic effect Effects 0.000 description 2
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 2
- 238000013537 high throughput screening Methods 0.000 description 2
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 2
- 238000009396 hybridization Methods 0.000 description 2
- 230000001631 hypertensive effect Effects 0.000 description 2
- 238000005462 in vivo assay Methods 0.000 description 2
- 239000000411 inducer Substances 0.000 description 2
- 239000003701 inert diluent Substances 0.000 description 2
- 239000000893 inhibin Substances 0.000 description 2
- 230000005764 inhibitory process Effects 0.000 description 2
- ZPNFWUPYTFPOJU-LPYSRVMUSA-N iniprol Chemical compound C([C@H]1C(=O)NCC(=O)NCC(=O)N[C@H]2CSSC[C@H]3C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@H](C(N[C@H](C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC=4C=CC=CC=4)C(=O)N[C@@H](CC=4C=CC(O)=CC=4)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC=4C=CC=CC=4)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCN)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC2=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CSSC[C@H](NC(=O)[C@H](CC=2C=CC=CC=2)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]2N(CCC2)C(=O)[C@@H](N)CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N2[C@@H](CCC2)C(=O)N2[C@@H](CCC2)C(=O)N[C@@H](CC=2C=CC(O)=CC=2)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N2[C@@H](CCC2)C(=O)N3)C(=O)NCC(=O)NCC(=O)N[C@@H](C)C(O)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@H](C(=O)N[C@@H](CC=2C=CC=CC=2)C(=O)N[C@H](C(=O)N1)C(C)C)[C@@H](C)O)[C@@H](C)CC)=O)[C@@H](C)CC)C1=CC=C(O)C=C1 ZPNFWUPYTFPOJU-LPYSRVMUSA-N 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 229940076264 interleukin-3 Drugs 0.000 description 2
- 229940100601 interleukin-6 Drugs 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 229940082629 iron antianemic preparations Drugs 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 239000008297 liquid dosage form Substances 0.000 description 2
- 230000004807 localization Effects 0.000 description 2
- 210000004072 lung Anatomy 0.000 description 2
- 210000002540 macrophage Anatomy 0.000 description 2
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 238000011418 maintenance treatment Methods 0.000 description 2
- 231100001016 megaloblastic anemia Toxicity 0.000 description 2
- 239000000693 micelle Substances 0.000 description 2
- 208000010555 moderate anemia Diseases 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 231100000350 mutagenesis Toxicity 0.000 description 2
- 230000004766 neurogenesis Effects 0.000 description 2
- 210000002569 neuron Anatomy 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 239000004006 olive oil Substances 0.000 description 2
- 235000008390 olive oil Nutrition 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 238000007911 parenteral administration Methods 0.000 description 2
- 238000000059 patterning Methods 0.000 description 2
- 238000010647 peptide synthesis reaction Methods 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 239000012071 phase Substances 0.000 description 2
- 125000001997 phenyl group Chemical group [H]C1=C([H])C([H])=C(*)C([H])=C1[H] 0.000 description 2
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 235000021317 phosphate Nutrition 0.000 description 2
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 208000024335 physical disease Diseases 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 229920000747 poly(lactic acid) Polymers 0.000 description 2
- 239000004626 polylactic acid Substances 0.000 description 2
- 102000054765 polymorphisms of proteins Human genes 0.000 description 2
- 230000004481 post-translational protein modification Effects 0.000 description 2
- 230000001323 posttranslational effect Effects 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 125000001500 prolyl group Chemical group [H]N1C([H])(C(=O)[*])C([H])([H])C([H])([H])C1([H])[H] 0.000 description 2
- 230000006337 proteolytic cleavage Effects 0.000 description 2
- 238000011552 rat model Methods 0.000 description 2
- 239000000018 receptor agonist Substances 0.000 description 2
- 229940044601 receptor agonist Drugs 0.000 description 2
- 230000008844 regulatory mechanism Effects 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 230000000717 retained effect Effects 0.000 description 2
- 102200089383 rs57977969 Human genes 0.000 description 2
- 238000002864 sequence alignment Methods 0.000 description 2
- 208000007056 sickle cell anemia Diseases 0.000 description 2
- 230000022379 skeletal muscle tissue development Effects 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 210000004989 spleen cell Anatomy 0.000 description 2
- 210000004988 splenocyte Anatomy 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 229910052717 sulfur Inorganic materials 0.000 description 2
- 230000000153 supplemental effect Effects 0.000 description 2
- 239000006188 syrup Substances 0.000 description 2
- 235000020357 syrup Nutrition 0.000 description 2
- 201000000596 systemic lupus erythematosus Diseases 0.000 description 2
- 238000012353 t test Methods 0.000 description 2
- 230000000451 tissue damage Effects 0.000 description 2
- 238000001890 transfection Methods 0.000 description 2
- 239000012581 transferrin Substances 0.000 description 2
- 230000009466 transformation Effects 0.000 description 2
- 238000011830 transgenic mouse model Methods 0.000 description 2
- 230000001052 transient effect Effects 0.000 description 2
- 229940078499 tricalcium phosphate Drugs 0.000 description 2
- 229910000391 tricalcium phosphate Inorganic materials 0.000 description 2
- 235000019731 tricalcium phosphate Nutrition 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 241001430294 unidentified retrovirus Species 0.000 description 2
- 125000002987 valine group Chemical group [H]N([H])C([H])(C(*)=O)C([H])(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 229910052720 vanadium Inorganic materials 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 239000000080 wetting agent Substances 0.000 description 2
- KILNVBDSWZSGLL-KXQOOQHDSA-N 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCC KILNVBDSWZSGLL-KXQOOQHDSA-N 0.000 description 1
- NRJAVPSFFCBXDT-HUESYALOSA-N 1,2-distearoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCCCCCCCC NRJAVPSFFCBXDT-HUESYALOSA-N 0.000 description 1
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 1
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- GZCWLCBFPRFLKL-UHFFFAOYSA-N 1-prop-2-ynoxypropan-2-ol Chemical compound CC(O)COCC#C GZCWLCBFPRFLKL-UHFFFAOYSA-N 0.000 description 1
- WNWHHMBRJJOGFJ-UHFFFAOYSA-N 16-methylheptadecan-1-ol Chemical class CC(C)CCCCCCCCCCCCCCCO WNWHHMBRJJOGFJ-UHFFFAOYSA-N 0.000 description 1
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 1
- ZBMRKNMTMPPMMK-UHFFFAOYSA-N 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid;azane Chemical compound [NH4+].CP(O)(=O)CCC(N)C([O-])=O ZBMRKNMTMPPMMK-UHFFFAOYSA-N 0.000 description 1
- OQUFOZNPBIIJTN-UHFFFAOYSA-N 2-hydroxypropane-1,2,3-tricarboxylic acid;sodium Chemical compound [Na].OC(=O)CC(O)(C(O)=O)CC(O)=O OQUFOZNPBIIJTN-UHFFFAOYSA-N 0.000 description 1
- JNODDICFTDYODH-UHFFFAOYSA-N 2-hydroxytetrahydrofuran Chemical compound OC1CCCO1 JNODDICFTDYODH-UHFFFAOYSA-N 0.000 description 1
- OSJPPGNTCRNQQC-UWTATZPHSA-N 3-phospho-D-glyceric acid Chemical compound OC(=O)[C@H](O)COP(O)(O)=O OSJPPGNTCRNQQC-UWTATZPHSA-N 0.000 description 1
- XAUDJQYHKZQPEU-KVQBGUIXSA-N 5-aza-2'-deoxycytidine Chemical compound O=C1N=C(N)N=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 XAUDJQYHKZQPEU-KVQBGUIXSA-N 0.000 description 1
- WYWHKKSPHMUBEB-UHFFFAOYSA-N 6-Mercaptoguanine Natural products N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- 208000004998 Abdominal Pain Diseases 0.000 description 1
- 102000013563 Acid Phosphatase Human genes 0.000 description 1
- 108010051457 Acid Phosphatase Proteins 0.000 description 1
- 102100034111 Activin receptor type-1 Human genes 0.000 description 1
- 102100034135 Activin receptor type-1C Human genes 0.000 description 1
- 101710173005 Activin receptor type-1C Proteins 0.000 description 1
- 206010000830 Acute leukaemia Diseases 0.000 description 1
- 208000026872 Addison Disease Diseases 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 102100033312 Alpha-2-macroglobulin Human genes 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- 241000269627 Amphiuma means Species 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 102000008873 Angiotensin II receptor Human genes 0.000 description 1
- 108050000824 Angiotensin II receptor Proteins 0.000 description 1
- 241000269350 Anura Species 0.000 description 1
- 208000032467 Aplastic anaemia Diseases 0.000 description 1
- 235000017060 Arachis glabrata Nutrition 0.000 description 1
- 244000105624 Arachis hypogaea Species 0.000 description 1
- 235000010777 Arachis hypogaea Nutrition 0.000 description 1
- 235000018262 Arachis monticola Nutrition 0.000 description 1
- CKLJMWTZIZZHCS-UHFFFAOYSA-N Aspartic acid Chemical compound OC(=O)C(N)CC(O)=O CKLJMWTZIZZHCS-UHFFFAOYSA-N 0.000 description 1
- 244000186140 Asperula odorata Species 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 241000714235 Avian retrovirus Species 0.000 description 1
- 208000000412 Avitaminosis Diseases 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 102100026189 Beta-galactosidase Human genes 0.000 description 1
- 208000018240 Bone Marrow Failure disease Diseases 0.000 description 1
- 208000020084 Bone disease Diseases 0.000 description 1
- 206010006002 Bone pain Diseases 0.000 description 1
- 101000937267 Bos taurus Activin receptor type-2B Proteins 0.000 description 1
- 208000014644 Brain disease Diseases 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 229940127291 Calcium channel antagonist Drugs 0.000 description 1
- 101710132601 Capsid protein Proteins 0.000 description 1
- 229920002134 Carboxymethyl cellulose Polymers 0.000 description 1
- 206010008479 Chest Pain Diseases 0.000 description 1
- 101710094648 Coat protein Proteins 0.000 description 1
- 206010009900 Colitis ulcerative Diseases 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 201000006306 Cor pulmonale Diseases 0.000 description 1
- 102000004420 Creatine Kinase Human genes 0.000 description 1
- 108010042126 Creatine kinase Proteins 0.000 description 1
- 208000011231 Crohn disease Diseases 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 241000701022 Cytomegalovirus Species 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 230000006820 DNA synthesis Effects 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 206010058314 Dysplasia Diseases 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 102000002322 Egg Proteins Human genes 0.000 description 1
- 108010000912 Egg Proteins Proteins 0.000 description 1
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 1
- 208000005189 Embolism Diseases 0.000 description 1
- 208000017701 Endocrine disease Diseases 0.000 description 1
- 108010074604 Epoetin Alfa Proteins 0.000 description 1
- 208000031637 Erythroblastic Acute Leukemia Diseases 0.000 description 1
- 208000036566 Erythroleukaemia Diseases 0.000 description 1
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 1
- 108010075944 Erythropoietin Receptors Proteins 0.000 description 1
- 102100036509 Erythropoietin receptor Human genes 0.000 description 1
- 206010015548 Euthanasia Diseases 0.000 description 1
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 1
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 1
- 108010054218 Factor VIII Proteins 0.000 description 1
- 102000001690 Factor VIII Human genes 0.000 description 1
- 108010074864 Factor XI Proteins 0.000 description 1
- 108010074860 Factor Xa Proteins 0.000 description 1
- 102000009109 Fc receptors Human genes 0.000 description 1
- 108010087819 Fc receptors Proteins 0.000 description 1
- 101150050927 Fcgrt gene Proteins 0.000 description 1
- VTLYFUHAOXGGBS-UHFFFAOYSA-N Fe3+ Chemical compound [Fe+3] VTLYFUHAOXGGBS-UHFFFAOYSA-N 0.000 description 1
- 102000008857 Ferritin Human genes 0.000 description 1
- 108050000784 Ferritin Proteins 0.000 description 1
- 238000008416 Ferritin Methods 0.000 description 1
- 239000004606 Fillers/Extenders Substances 0.000 description 1
- 229940123414 Folate antagonist Drugs 0.000 description 1
- 206010016880 Folate deficiency Diseases 0.000 description 1
- 208000010188 Folic Acid Deficiency Diseases 0.000 description 1
- 108010012820 Follistatin-Related Proteins Proteins 0.000 description 1
- 102000019203 Follistatin-Related Proteins Human genes 0.000 description 1
- 102000011852 GATA2 Transcription Factor Human genes 0.000 description 1
- 108010075641 GATA2 Transcription Factor Proteins 0.000 description 1
- 235000008526 Galium odoratum Nutrition 0.000 description 1
- 239000001828 Gelatine Substances 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- 102000006395 Globulins Human genes 0.000 description 1
- 108010044091 Globulins Proteins 0.000 description 1
- KOSRFJWDECSPRO-WDSKDSINSA-N Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(O)=O KOSRFJWDECSPRO-WDSKDSINSA-N 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- JZNWSCPGTDBMEW-UHFFFAOYSA-N Glycerophosphorylethanolamin Natural products NCCOP(O)(=O)OCC(O)CO JZNWSCPGTDBMEW-UHFFFAOYSA-N 0.000 description 1
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 102000028180 Glycophorins Human genes 0.000 description 1
- 108091005250 Glycophorins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102100021181 Golgi phosphoprotein 3 Human genes 0.000 description 1
- 101150105462 HIS6 gene Proteins 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 208000037357 HIV infectious disease Diseases 0.000 description 1
- 206010059484 Haemodilution Diseases 0.000 description 1
- 208000036581 Haemorrhagic anaemia Diseases 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 108010068323 Hemoglobin E Proteins 0.000 description 1
- 208000012925 Hemoglobin H disease Diseases 0.000 description 1
- 102100027685 Hemoglobin subunit alpha Human genes 0.000 description 1
- 206010019668 Hepatic fibrosis Diseases 0.000 description 1
- 239000004705 High-molecular-weight polyethylene Substances 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 101000799140 Homo sapiens Activin receptor type-1 Proteins 0.000 description 1
- 101000893545 Homo sapiens Growth/differentiation factor 11 Proteins 0.000 description 1
- 101001109620 Homo sapiens Nucleolar and coiled-body phosphoprotein 1 Proteins 0.000 description 1
- 241000701044 Human gammaherpesvirus 4 Species 0.000 description 1
- 208000006031 Hydrops Fetalis Diseases 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- PMMYEEVYMWASQN-DMTCNVIQSA-N Hydroxyproline Chemical compound O[C@H]1CN[C@H](C(O)=O)C1 PMMYEEVYMWASQN-DMTCNVIQSA-N 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- 206010021137 Hypovolaemia Diseases 0.000 description 1
- 102000009490 IgG Receptors Human genes 0.000 description 1
- 108010073807 IgG Receptors Proteins 0.000 description 1
- 102000006496 Immunoglobulin Heavy Chains Human genes 0.000 description 1
- 108010019476 Immunoglobulin Heavy Chains Proteins 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 208000022559 Inflammatory bowel disease Diseases 0.000 description 1
- 206010022004 Influenza like illness Diseases 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 102000000589 Interleukin-1 Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- 102100021592 Interleukin-7 Human genes 0.000 description 1
- 108010002586 Interleukin-7 Proteins 0.000 description 1
- 239000007760 Iscove's Modified Dulbecco's Medium Substances 0.000 description 1
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 1
- 206010024305 Leukaemia monocytic Diseases 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 101710125418 Major capsid protein Proteins 0.000 description 1
- 206010025476 Malabsorption Diseases 0.000 description 1
- 208000002720 Malnutrition Diseases 0.000 description 1
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 description 1
- 240000003183 Manihot esculenta Species 0.000 description 1
- 235000016735 Manihot esculenta subsp esculenta Nutrition 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 206010026865 Mass Diseases 0.000 description 1
- 108010036176 Melitten Proteins 0.000 description 1
- 206010027439 Metal poisoning Diseases 0.000 description 1
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 1
- 208000036696 Microcytic anaemia Diseases 0.000 description 1
- 206010027540 Microcytosis Diseases 0.000 description 1
- 102100026632 Mimecan Human genes 0.000 description 1
- 102100025748 Mothers against decapentaplegic homolog 3 Human genes 0.000 description 1
- 101710143111 Mothers against decapentaplegic homolog 3 Proteins 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101000762244 Mus musculus Cadherin-17 Proteins 0.000 description 1
- 208000007101 Muscle Cramp Diseases 0.000 description 1
- 206010028289 Muscle atrophy Diseases 0.000 description 1
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 1
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 1
- 201000003793 Myelodysplastic syndrome Diseases 0.000 description 1
- OVRNDRQMDRJTHS-CBQIKETKSA-N N-Acetyl-D-Galactosamine Chemical compound CC(=O)N[C@H]1[C@@H](O)O[C@H](CO)[C@H](O)[C@@H]1O OVRNDRQMDRJTHS-CBQIKETKSA-N 0.000 description 1
- OVRNDRQMDRJTHS-UHFFFAOYSA-N N-acelyl-D-glucosamine Natural products CC(=O)NC1C(O)OC(CO)C(O)C1O OVRNDRQMDRJTHS-UHFFFAOYSA-N 0.000 description 1
- MBLBDJOUHNCFQT-UHFFFAOYSA-N N-acetyl-D-galactosamine Natural products CC(=O)NC(C=O)C(O)C(O)C(O)CO MBLBDJOUHNCFQT-UHFFFAOYSA-N 0.000 description 1
- OVRNDRQMDRJTHS-FMDGEEDCSA-N N-acetyl-beta-D-glucosamine Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O OVRNDRQMDRJTHS-FMDGEEDCSA-N 0.000 description 1
- MBLBDJOUHNCFQT-LXGUWJNJSA-N N-acetylglucosamine Natural products CC(=O)N[C@@H](C=O)[C@@H](O)[C@H](O)[C@H](O)CO MBLBDJOUHNCFQT-LXGUWJNJSA-N 0.000 description 1
- 101100395023 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) his-7 gene Proteins 0.000 description 1
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 1
- 102000002001 Non-Receptor Type 6 Protein Tyrosine Phosphatase Human genes 0.000 description 1
- 108010015793 Non-Receptor Type 6 Protein Tyrosine Phosphatase Proteins 0.000 description 1
- 102100022726 Nucleolar and coiled-body phosphoprotein 1 Human genes 0.000 description 1
- 101710141454 Nucleoprotein Proteins 0.000 description 1
- 206010061876 Obstruction Diseases 0.000 description 1
- 240000007817 Olea europaea Species 0.000 description 1
- 108700020796 Oncogene Proteins 0.000 description 1
- 239000004235 Orange GGN Substances 0.000 description 1
- 101800002327 Osteoinductive factor Proteins 0.000 description 1
- 206010031252 Osteomyelitis Diseases 0.000 description 1
- 206010049088 Osteopenia Diseases 0.000 description 1
- 101150012394 PHO5 gene Proteins 0.000 description 1
- 102220633854 PRKCA-binding protein_D80A_mutation Human genes 0.000 description 1
- 208000035467 Pancreatic insufficiency Diseases 0.000 description 1
- 102000057297 Pepsin A Human genes 0.000 description 1
- 108090000284 Pepsin A Proteins 0.000 description 1
- 108010033276 Peptide Fragments Proteins 0.000 description 1
- 102000007079 Peptide Fragments Human genes 0.000 description 1
- 108091000080 Phosphotransferase Proteins 0.000 description 1
- 102000013566 Plasminogen Human genes 0.000 description 1
- 108010051456 Plasminogen Proteins 0.000 description 1
- 108010022233 Plasminogen Activator Inhibitor 1 Proteins 0.000 description 1
- 102220509036 Platelet-activating factor acetylhydrolase IB subunit beta_L79A_mutation Human genes 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 102220577876 Poly(rC)-binding protein 1_W78A_mutation Human genes 0.000 description 1
- 229920002732 Polyanhydride Polymers 0.000 description 1
- 208000008601 Polycythemia Diseases 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 208000018525 Postpartum Hemorrhage Diseases 0.000 description 1
- 108010015078 Pregnancy-Associated alpha 2-Macroglobulins Proteins 0.000 description 1
- 101710083689 Probable capsid protein Proteins 0.000 description 1
- 102000004079 Prolyl Hydroxylases Human genes 0.000 description 1
- 108010043005 Prolyl Hydroxylases Proteins 0.000 description 1
- 229940078467 Prolyl hydroxylase inhibitor Drugs 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102220469872 Protein argonaute-3_E37A_mutation Human genes 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 108700014121 Pyruvate Kinase Deficiency of Red Cells Proteins 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 206010062237 Renal impairment Diseases 0.000 description 1
- 108700008625 Reporter Genes Proteins 0.000 description 1
- 206010038802 Reticuloendothelial system stimulated Diseases 0.000 description 1
- 208000000924 Right ventricular hypertrophy Diseases 0.000 description 1
- 108091006976 SLC40A1 Proteins 0.000 description 1
- 108010071390 Serum Albumin Proteins 0.000 description 1
- 102000007562 Serum Albumin Human genes 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 244000061456 Solanum tuberosum Species 0.000 description 1
- 235000002595 Solanum tuberosum Nutrition 0.000 description 1
- 208000005392 Spasm Diseases 0.000 description 1
- SSZBUIDZHHWXNJ-UHFFFAOYSA-N Stearinsaeure-hexadecylester Natural products CCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCC SSZBUIDZHHWXNJ-UHFFFAOYSA-N 0.000 description 1
- 101710137500 T7 RNA polymerase Proteins 0.000 description 1
- 229940123237 Taxane Drugs 0.000 description 1
- 206010043390 Thalassaemia alpha Diseases 0.000 description 1
- 206010043391 Thalassaemia beta Diseases 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- 108090001109 Thermolysin Proteins 0.000 description 1
- 102100036407 Thioredoxin Human genes 0.000 description 1
- 108090000190 Thrombin Proteins 0.000 description 1
- 101710120037 Toxin CcdB Proteins 0.000 description 1
- 108700009124 Transcription Initiation Site Proteins 0.000 description 1
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 1
- 108010033576 Transferrin Receptors Proteins 0.000 description 1
- 241000145122 Tridens <angiosperm> Species 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102100021657 Tyrosine-protein phosphatase non-receptor type 6 Human genes 0.000 description 1
- 101710128901 Tyrosine-protein phosphatase non-receptor type 6 Proteins 0.000 description 1
- 201000006704 Ulcerative Colitis Diseases 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 206010046865 Vaccinia virus infection Diseases 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 108010003533 Viral Envelope Proteins Proteins 0.000 description 1
- 108700005077 Viral Genes Proteins 0.000 description 1
- 206010047627 Vitamin deficiencies Diseases 0.000 description 1
- 102220469729 Voltage-dependent L-type calcium channel subunit beta-2_D54K_mutation Human genes 0.000 description 1
- 102220479829 Voltage-dependent L-type calcium channel subunit beta-2_R56K_mutation Human genes 0.000 description 1
- 241000269370 Xenopus <genus> Species 0.000 description 1
- 241000269368 Xenopus laevis Species 0.000 description 1
- 240000008042 Zea mays Species 0.000 description 1
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 1
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 1
- ATBOMIWRCZXYSZ-XZBBILGWSA-N [1-[2,3-dihydroxypropoxy(hydroxy)phosphoryl]oxy-3-hexadecanoyloxypropan-2-yl] (9e,12e)-octadeca-9,12-dienoate Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(COP(O)(=O)OCC(O)CO)OC(=O)CCCCCCC\C=C\C\C=C\CCCCC ATBOMIWRCZXYSZ-XZBBILGWSA-N 0.000 description 1
- 238000002835 absorbance Methods 0.000 description 1
- 239000002250 absorbent Substances 0.000 description 1
- 230000002745 absorbent Effects 0.000 description 1
- 239000003655 absorption accelerator Substances 0.000 description 1
- 229960000583 acetic acid Drugs 0.000 description 1
- 230000021736 acetylation Effects 0.000 description 1
- 238000006640 acetylation reaction Methods 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 208000021841 acute erythroid leukemia Diseases 0.000 description 1
- 208000038016 acute inflammation Diseases 0.000 description 1
- 230000006022 acute inflammation Effects 0.000 description 1
- 230000010933 acylation Effects 0.000 description 1
- 238000005917 acylation reaction Methods 0.000 description 1
- 230000011759 adipose tissue development Effects 0.000 description 1
- 230000001800 adrenalinergic effect Effects 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 229940100198 alkylating agent Drugs 0.000 description 1
- 239000002168 alkylating agent Substances 0.000 description 1
- 239000002160 alpha blocker Substances 0.000 description 1
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 1
- 229940124308 alpha-adrenoreceptor antagonist Drugs 0.000 description 1
- AWUCVROLDVIAJX-UHFFFAOYSA-N alpha-glycerophosphate Natural products OCC(O)COP(O)(O)=O AWUCVROLDVIAJX-UHFFFAOYSA-N 0.000 description 1
- 150000004645 aluminates Chemical class 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 125000003277 amino group Chemical group 0.000 description 1
- 235000019270 ammonium chloride Nutrition 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 229940045799 anthracyclines and related substance Drugs 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 230000000843 anti-fungal effect Effects 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 229940121375 antifungal agent Drugs 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 239000002246 antineoplastic agent Substances 0.000 description 1
- 239000003972 antineoplastic antibiotic Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 239000008365 aqueous carrier Substances 0.000 description 1
- 125000000637 arginyl group Chemical group N[C@@H](CCCNC(N)=N)C(=O)* 0.000 description 1
- 239000000823 artificial membrane Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 125000000613 asparagine group Chemical group N[C@@H](CC(N)=O)C(=O)* 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- 229960002903 benzyl benzoate Drugs 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 208000022809 beta-thalassemia intermedia Diseases 0.000 description 1
- 208000022806 beta-thalassemia major Diseases 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 238000004166 bioassay Methods 0.000 description 1
- 239000003462 bioceramic Substances 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 239000005312 bioglass Substances 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 210000002459 blastocyst Anatomy 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 239000012503 blood component Substances 0.000 description 1
- 210000001772 blood platelet Anatomy 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 230000037182 bone density Effects 0.000 description 1
- 230000005983 bone marrow dysfunction Effects 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 239000006227 byproduct Substances 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- 239000000480 calcium channel blocker Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- CJZGTCYPCWQAJB-UHFFFAOYSA-L calcium stearate Chemical compound [Ca+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O CJZGTCYPCWQAJB-UHFFFAOYSA-L 0.000 description 1
- 239000008116 calcium stearate Substances 0.000 description 1
- 235000013539 calcium stearate Nutrition 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 229910002091 carbon monoxide Inorganic materials 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 235000010948 carboxy methyl cellulose Nutrition 0.000 description 1
- 230000021523 carboxylation Effects 0.000 description 1
- 238000006473 carboxylation reaction Methods 0.000 description 1
- 239000008112 carboxymethyl-cellulose Substances 0.000 description 1
- 210000004413 cardiac myocyte Anatomy 0.000 description 1
- 210000000845 cartilage Anatomy 0.000 description 1
- 239000004359 castor oil Substances 0.000 description 1
- 235000019438 castor oil Nutrition 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 238000005277 cation exchange chromatography Methods 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000006369 cell cycle progression Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 210000004671 cell-free system Anatomy 0.000 description 1
- 239000000919 ceramic Substances 0.000 description 1
- 235000013339 cereals Nutrition 0.000 description 1
- 229930183167 cerebroside Natural products 0.000 description 1
- 150000001784 cerebrosides Chemical class 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- 230000009920 chelation Effects 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 238000013375 chromatographic separation Methods 0.000 description 1
- 208000037976 chronic inflammation Diseases 0.000 description 1
- 230000006020 chronic inflammation Effects 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 208000025302 chronic primary adrenal insufficiency Diseases 0.000 description 1
- 230000007882 cirrhosis Effects 0.000 description 1
- 208000019425 cirrhosis of liver Diseases 0.000 description 1
- 239000004927 clay Substances 0.000 description 1
- 238000009643 clonogenic assay Methods 0.000 description 1
- 231100000096 clonogenic assay Toxicity 0.000 description 1
- 230000015271 coagulation Effects 0.000 description 1
- 238000005345 coagulation Methods 0.000 description 1
- 229910017052 cobalt Inorganic materials 0.000 description 1
- 239000010941 cobalt Substances 0.000 description 1
- GUTLYIVDDKVIGB-UHFFFAOYSA-N cobalt atom Chemical compound [Co] GUTLYIVDDKVIGB-UHFFFAOYSA-N 0.000 description 1
- 230000003920 cognitive function Effects 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 208000029742 colonic neoplasm Diseases 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 238000004040 coloring Methods 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 230000001447 compensatory effect Effects 0.000 description 1
- 238000011970 concomitant therapy Methods 0.000 description 1
- 210000002808 connective tissue Anatomy 0.000 description 1
- 108010084052 continuous erythropoietin receptor activator Proteins 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 235000005822 corn Nutrition 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 235000012343 cottonseed oil Nutrition 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 229960003603 decitabine Drugs 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 229960001489 deferasirox Drugs 0.000 description 1
- FMSOAWSKCWYLBB-VBGLAJCLSA-N deferasirox Chemical compound C1=CC(C(=O)O)=CC=C1N(N\C(N\1)=C\2C(C=CC=C/2)=O)C/1=C\1C(=O)C=CC=C/1 FMSOAWSKCWYLBB-VBGLAJCLSA-N 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000001934 delay Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- NEFBYIFKOOEVPA-UHFFFAOYSA-K dicalcium phosphate Chemical class [Ca+2].[Ca+2].[O-]P([O-])([O-])=O NEFBYIFKOOEVPA-UHFFFAOYSA-K 0.000 description 1
- 230000000378 dietary effect Effects 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 230000000447 dimerizing effect Effects 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical group P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- PMMYEEVYMWASQN-UHFFFAOYSA-N dl-hydroxyproline Natural products OC1C[NH2+]C(C([O-])=O)C1 PMMYEEVYMWASQN-UHFFFAOYSA-N 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000008298 dragée Substances 0.000 description 1
- 238000007877 drug screening Methods 0.000 description 1
- 241001493065 dsRNA viruses Species 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 235000013345 egg yolk Nutrition 0.000 description 1
- 210000002969 egg yolk Anatomy 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000003028 elevating effect Effects 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 208000028208 end stage renal disease Diseases 0.000 description 1
- 201000000523 end stage renal failure Diseases 0.000 description 1
- 230000002124 endocrine Effects 0.000 description 1
- 210000003372 endocrine gland Anatomy 0.000 description 1
- 208000030172 endocrine system disease Diseases 0.000 description 1
- 210000001900 endoderm Anatomy 0.000 description 1
- 230000009786 epithelial differentiation Effects 0.000 description 1
- 229960003388 epoetin alfa Drugs 0.000 description 1
- 108010002601 epoetin beta Proteins 0.000 description 1
- 229960004579 epoetin beta Drugs 0.000 description 1
- 108010067416 epoetin delta Proteins 0.000 description 1
- 229950002109 epoetin delta Drugs 0.000 description 1
- 108010090921 epoetin omega Proteins 0.000 description 1
- 229950008767 epoetin omega Drugs 0.000 description 1
- 230000000925 erythroid effect Effects 0.000 description 1
- 210000003013 erythroid precursor cell Anatomy 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- 229940093471 ethyl oleate Drugs 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 230000029142 excretion Effects 0.000 description 1
- 230000003631 expected effect Effects 0.000 description 1
- 210000002744 extracellular matrix Anatomy 0.000 description 1
- 238000001125 extrusion Methods 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 230000002550 fecal effect Effects 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000000796 flavoring agent Substances 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 238000002875 fluorescence polarization Methods 0.000 description 1
- 108091006047 fluorescent proteins Proteins 0.000 description 1
- 102000034287 fluorescent proteins Human genes 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 238000001641 gel filtration chromatography Methods 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 238000001476 gene delivery Methods 0.000 description 1
- 230000007274 generation of a signal involved in cell-cell signaling Effects 0.000 description 1
- 208000016361 genetic disease Diseases 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 239000012362 glacial acetic acid Substances 0.000 description 1
- 102000018146 globin Human genes 0.000 description 1
- 108060003196 globin Proteins 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-L glutamate group Chemical group N[C@@H](CCC(=O)[O-])C(=O)[O-] WHUUTDBJXJRKMK-VKHMYHEASA-L 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 1
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 1
- 150000004676 glycans Polymers 0.000 description 1
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 description 1
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 230000002414 glycolytic effect Effects 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 150000002338 glycosides Chemical class 0.000 description 1
- 108010067216 glycyl-glycyl-glycine Proteins 0.000 description 1
- XKUKSGPZAADMRA-UHFFFAOYSA-N glycyl-glycyl-glycine Natural products NCC(=O)NCC(=O)NCC(O)=O XKUKSGPZAADMRA-UHFFFAOYSA-N 0.000 description 1
- 210000002149 gonad Anatomy 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 150000003278 haem Chemical class 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 108010013846 hematide Proteins 0.000 description 1
- 210000000777 hematopoietic system Anatomy 0.000 description 1
- 208000034737 hemoglobinopathy Diseases 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 208000009601 hereditary spherocytosis Diseases 0.000 description 1
- 239000000833 heterodimer Substances 0.000 description 1
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 1
- 238000012203 high throughput assay Methods 0.000 description 1
- 235000012907 honey Nutrition 0.000 description 1
- 102000044890 human EPO Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 208000033519 human immunodeficiency virus infectious disease Diseases 0.000 description 1
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 1
- WGCNASOHLSPBMP-UHFFFAOYSA-N hydroxyacetaldehyde Natural products OCC=O WGCNASOHLSPBMP-UHFFFAOYSA-N 0.000 description 1
- 229960002591 hydroxyproline Drugs 0.000 description 1
- 230000001146 hypoxic effect Effects 0.000 description 1
- 230000036737 immune function Effects 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 230000002163 immunogen Effects 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 230000000415 inactivating effect Effects 0.000 description 1
- 230000002779 inactivation Effects 0.000 description 1
- 210000003000 inclusion body Anatomy 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 208000018337 inherited hemoglobinopathy Diseases 0.000 description 1
- 229940102223 injectable solution Drugs 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 229940100994 interleukin-7 Drugs 0.000 description 1
- 230000031146 intracellular signal transduction Effects 0.000 description 1
- 238000005342 ion exchange Methods 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 230000010438 iron metabolism Effects 0.000 description 1
- DCYOBGZUOMKFPA-UHFFFAOYSA-N iron(2+);iron(3+);octadecacyanide Chemical compound [Fe+2].[Fe+2].[Fe+2].[Fe+3].[Fe+3].[Fe+3].[Fe+3].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-].N#[C-] DCYOBGZUOMKFPA-UHFFFAOYSA-N 0.000 description 1
- JEIPFZHSYJVQDO-UHFFFAOYSA-N iron(III) oxide Inorganic materials O=[Fe]O[Fe]=O JEIPFZHSYJVQDO-UHFFFAOYSA-N 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 210000004731 jugular vein Anatomy 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 210000001865 kupffer cell Anatomy 0.000 description 1
- 208000008127 lead poisoning Diseases 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 230000017687 left/right axis specification Effects 0.000 description 1
- 229960004942 lenalidomide Drugs 0.000 description 1
- GOTYRUGSSMKFNF-UHFFFAOYSA-N lenalidomide Chemical compound C1C=2C(N)=CC=CC=2C(=O)N1C1CCC(=O)NC1=O GOTYRUGSSMKFNF-UHFFFAOYSA-N 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 150000002614 leucines Chemical class 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 210000000982 limb bud Anatomy 0.000 description 1
- 230000006517 limb development Effects 0.000 description 1
- 230000029226 lipidation Effects 0.000 description 1
- 239000007937 lozenge Substances 0.000 description 1
- 239000000314 lubricant Substances 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 208000019420 lymphoid neoplasm Diseases 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 235000019359 magnesium stearate Nutrition 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 201000004792 malaria Diseases 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 229960004961 mechlorethamine Drugs 0.000 description 1
- HAWPXGHAZFHHAD-UHFFFAOYSA-N mechlorethamine Chemical class ClCCN(C)CCCl HAWPXGHAZFHHAD-UHFFFAOYSA-N 0.000 description 1
- 238000002483 medication Methods 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 210000004779 membrane envelope Anatomy 0.000 description 1
- 230000037323 metabolic rate Effects 0.000 description 1
- 235000020938 metabolic status Nutrition 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 229960001046 methoxy polyethylene glycol-epoetin beta Drugs 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 229940016286 microcrystalline cellulose Drugs 0.000 description 1
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 1
- 239000008108 microcrystalline cellulose Substances 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 230000029115 microtubule polymerization Effects 0.000 description 1
- 210000003470 mitochondria Anatomy 0.000 description 1
- 230000000394 mitotic effect Effects 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 238000012900 molecular simulation Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 201000006894 monocytic leukemia Diseases 0.000 description 1
- CQDGTJPVBWZJAZ-UHFFFAOYSA-N monoethyl carbonate Chemical compound CCOC(O)=O CQDGTJPVBWZJAZ-UHFFFAOYSA-N 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 230000004660 morphological change Effects 0.000 description 1
- 230000008383 multiple organ dysfunction Effects 0.000 description 1
- 201000000585 muscular atrophy Diseases 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 210000001167 myeloblast Anatomy 0.000 description 1
- 206010028537 myelofibrosis Diseases 0.000 description 1
- 210000003643 myeloid progenitor cell Anatomy 0.000 description 1
- 230000003039 myelosuppressive effect Effects 0.000 description 1
- 210000003098 myoblast Anatomy 0.000 description 1
- 229950006780 n-acetylglucosamine Drugs 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 229930014626 natural product Natural products 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000001537 neural effect Effects 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 210000003458 notochord Anatomy 0.000 description 1
- 235000018343 nutrient deficiency Nutrition 0.000 description 1
- 235000019198 oils Nutrition 0.000 description 1
- 210000001706 olfactory mucosa Anatomy 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000008816 organ damage Effects 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 150000002895 organic esters Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 230000011164 ossification Effects 0.000 description 1
- 230000002188 osteogenic effect Effects 0.000 description 1
- 230000002611 ovarian Effects 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 235000010603 pastilles Nutrition 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 235000020232 peanut Nutrition 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 229940111202 pepsin Drugs 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- WVDDGKGOMKODPV-ZQBYOMGUSA-N phenyl(114C)methanol Chemical compound O[14CH2]C1=CC=CC=C1 WVDDGKGOMKODPV-ZQBYOMGUSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000002953 phosphate buffered saline Substances 0.000 description 1
- 125000001095 phosphatidyl group Chemical group 0.000 description 1
- 150000008104 phosphatidylethanolamines Chemical class 0.000 description 1
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 1
- 102000020233 phosphotransferase Human genes 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 230000008288 physiological mechanism Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 230000019612 pigmentation Effects 0.000 description 1
- 230000001817 pituitary effect Effects 0.000 description 1
- 230000003169 placental effect Effects 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 150000004804 polysaccharides Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000008092 positive effect Effects 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 230000002028 premature Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 1
- 208000037920 primary disease Diseases 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000000644 propagated effect Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- XJMOSONTPMZWPB-UHFFFAOYSA-M propidium iodide Chemical compound [I-].[I-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CCC[N+](C)(CC)CC)=C1C1=CC=CC=C1 XJMOSONTPMZWPB-UHFFFAOYSA-M 0.000 description 1
- 235000021075 protein intake Nutrition 0.000 description 1
- 230000009145 protein modification Effects 0.000 description 1
- 229960003351 prussian blue Drugs 0.000 description 1
- 239000013225 prussian blue Substances 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 150000003856 quaternary ammonium compounds Chemical class 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 238000003259 recombinant expression Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000007261 regionalization Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 238000003571 reporter gene assay Methods 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 210000003660 reticulum Anatomy 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 210000003705 ribosome Anatomy 0.000 description 1
- 229960004641 rituximab Drugs 0.000 description 1
- 102220125997 rs141863326 Human genes 0.000 description 1
- 102220288227 rs141863326 Human genes 0.000 description 1
- 102220140258 rs146572379 Human genes 0.000 description 1
- 102200145357 rs1555341957 Human genes 0.000 description 1
- 102220036189 rs273585616 Human genes 0.000 description 1
- 102220004761 rs33990858 Human genes 0.000 description 1
- 102220005147 rs34173382 Human genes 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 150000004760 silicates Chemical class 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 238000009097 single-agent therapy Methods 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 208000017520 skin disease Diseases 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 239000007909 solid dosage form Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 239000004334 sorbic acid Substances 0.000 description 1
- 229940075582 sorbic acid Drugs 0.000 description 1
- 235000010199 sorbic acid Nutrition 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 150000003408 sphingolipids Chemical class 0.000 description 1
- 210000003594 spinal ganglia Anatomy 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 238000009120 supportive therapy Methods 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 230000002459 sustained effect Effects 0.000 description 1
- 239000003765 sweetening agent Substances 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 235000012222 talc Nutrition 0.000 description 1
- 125000005931 tert-butyloxycarbonyl group Chemical group [H]C([H])([H])C(OC(*)=O)(C([H])([H])[H])C([H])([H])[H] 0.000 description 1
- 229960003433 thalidomide Drugs 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 108060008226 thioredoxin Proteins 0.000 description 1
- 229940094937 thioredoxin Drugs 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 125000000341 threoninyl group Chemical group [H]OC([H])(C([H])([H])[H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 229960004072 thrombin Drugs 0.000 description 1
- 210000002303 tibia Anatomy 0.000 description 1
- MNRILEROXIRVNJ-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=NC=N[C]21 MNRILEROXIRVNJ-UHFFFAOYSA-N 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 230000008467 tissue growth Effects 0.000 description 1
- 230000000287 tissue oxygenation Effects 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- FGMPLJWBKKVCDB-UHFFFAOYSA-N trans-L-hydroxy-proline Natural products ON1CCCC1C(O)=O FGMPLJWBKKVCDB-UHFFFAOYSA-N 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000010474 transient expression Effects 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- 230000014621 translational initiation Effects 0.000 description 1
- 238000002054 transplantation Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- ITMCEJHCFYSIIV-UHFFFAOYSA-N triflic acid Chemical compound OS(=O)(=O)C(F)(F)F ITMCEJHCFYSIIV-UHFFFAOYSA-N 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 102000003390 tumor necrosis factor Human genes 0.000 description 1
- 238000003160 two-hybrid assay Methods 0.000 description 1
- 238000010396 two-hybrid screening Methods 0.000 description 1
- 238000000108 ultra-filtration Methods 0.000 description 1
- 241001529453 unidentified herpesvirus Species 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 230000002485 urinary effect Effects 0.000 description 1
- 238000005353 urine analysis Methods 0.000 description 1
- 208000007089 vaccinia Diseases 0.000 description 1
- 229940124549 vasodilator Drugs 0.000 description 1
- 239000003071 vasodilator agent Substances 0.000 description 1
- 235000015112 vegetable and seed oil Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- 238000011100 viral filtration Methods 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 238000009736 wetting Methods 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/18—Growth factors; Growth regulators
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/10—Transferases (2.)
- C12N9/12—Transferases (2.) transferring phosphorus containing groups, e.g. kinases (2.7)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/177—Receptors; Cell surface antigens; Cell surface determinants
- A61K38/179—Receptors; Cell surface antigens; Cell surface determinants for growth factors; for growth regulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/45—Transferases (2)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y207/00—Transferases transferring phosphorus-containing groups (2.7)
- C12Y207/11—Protein-serine/threonine kinases (2.7.11)
- C12Y207/1103—Receptor protein serine/threonine kinase (2.7.11.30)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- General Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Cell Biology (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Microbiology (AREA)
- Biotechnology (AREA)
- Biomedical Technology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Endocrinology (AREA)
- Mycology (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161547932P | 2011-10-17 | 2011-10-17 | |
| US61/547,932 | 2011-10-17 | ||
| PCT/US2012/060650 WO2013059347A1 (en) | 2011-10-17 | 2012-10-17 | Methods and compositions for treating ineffective erythropoiesis |
| KR1020207004201A KR20200019261A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207004201A Division KR20200019261A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20220075438A true KR20220075438A (ko) | 2022-06-08 |
Family
ID=48141315
Family Applications (4)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020227017193A Ceased KR20220075438A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020197025484A Active KR102079482B1 (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020147013325A Ceased KR20140084211A (ko) | 2011-10-17 | 2012-10-17 | 효과가 없는 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020207004201A Ceased KR20200019261A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197025484A Active KR102079482B1 (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020147013325A Ceased KR20140084211A (ko) | 2011-10-17 | 2012-10-17 | 효과가 없는 적혈구생성 치료를 위한 방법 및 조성물 |
| KR1020207004201A Ceased KR20200019261A (ko) | 2011-10-17 | 2012-10-17 | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 |
Country Status (13)
| Country | Link |
|---|---|
| US (4) | US20130243743A1 (enExample) |
| EP (3) | EP3520805B1 (enExample) |
| JP (4) | JP6092883B2 (enExample) |
| KR (4) | KR20220075438A (enExample) |
| CN (3) | CN107693776A (enExample) |
| AU (4) | AU2012326207B2 (enExample) |
| BR (3) | BR122019023174B1 (enExample) |
| CA (1) | CA2852683A1 (enExample) |
| DK (2) | DK3875104T3 (enExample) |
| EA (2) | EA202090053A3 (enExample) |
| ES (3) | ES2869168T3 (enExample) |
| HK (2) | HK1250341A1 (enExample) |
| WO (1) | WO2013059347A1 (enExample) |
Families Citing this family (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DK2332977T3 (en) | 2004-07-23 | 2016-02-29 | Acceleron Pharma Inc | ActRII receptor polypeptides |
| CA3045808C (en) | 2005-11-23 | 2022-08-16 | Acceleron Pharma, Inc. | Activin-actriia antagonists and uses for promoting bone growth |
| US8128933B2 (en) | 2005-11-23 | 2012-03-06 | Acceleron Pharma, Inc. | Method of promoting bone growth by an anti-activin B antibody |
| US8895016B2 (en) | 2006-12-18 | 2014-11-25 | Acceleron Pharma, Inc. | Antagonists of activin-actriia and uses for increasing red blood cell levels |
| RU2473362C2 (ru) | 2007-02-01 | 2013-01-27 | Акселерон Фарма Инк. | АНТАГОНИСТЫ АКТИВИНА-ActRIIa-Fc И ИХ ПРИМЕНЕНИЕ ДЛЯ ЛЕЧЕНИЯ ИЛИ ПРОФИЛАКТИКИ РАКА МОЛОЧНОЙ ЖЕЛЕЗЫ |
| TW201907946A (zh) | 2007-02-02 | 2019-03-01 | 美商艾瑟勒朗法瑪公司 | 衍生自ActRIIB的變體與其用途 |
| WO2008100384A2 (en) | 2007-02-09 | 2008-08-21 | Acceleron Pharma Inc. | Activin-actriia antagonists and uses for promoting bone growth in cancer patients |
| CN101861161B (zh) | 2007-09-18 | 2017-04-19 | 阿塞勒隆制药公司 | 活化素‑actriia拮抗剂和减少或抑制fsh分泌的用途 |
| TW201919685A (zh) | 2008-08-14 | 2019-06-01 | 美商艾瑟勒朗法瑪公司 | 使用gdf阱以增加紅血球水平 |
| US8216997B2 (en) | 2008-08-14 | 2012-07-10 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| CA2764890A1 (en) | 2009-06-08 | 2010-12-16 | Acceleron Pharma Inc. | Methods for increasing thermogenic adipocytes |
| CN104805105A (zh) | 2009-06-12 | 2015-07-29 | 阿塞勒隆制药公司 | 截短的actriib-fc融合蛋白 |
| CA2773494A1 (en) * | 2009-09-09 | 2011-03-17 | Acceleron Pharma Inc. | Actriib antagonists and dosing and uses thereof |
| EP2501400B1 (en) | 2009-11-17 | 2017-11-01 | Acceleron Pharma, Inc. | Actriib proteins and variants and uses therefore relating to utrophin induction for muscular dystrophy therapy |
| AU2011326586A1 (en) | 2010-11-08 | 2013-05-30 | Acceleron Pharma, Inc. | ActRIIA binding agents and uses thereof |
| US9925221B2 (en) | 2011-09-09 | 2018-03-27 | Celularity, Inc. | Treatment of amyotrophic lateral sclerosis using placental stem cells |
| CA2890217C (en) | 2012-11-02 | 2021-07-20 | Yifu FANG | Activin-actrii antagonists and uses for treating bone and other disorders |
| WO2015143403A1 (en) * | 2014-03-21 | 2015-09-24 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis by inhibiting activin b and/or gdf11 |
| AP2016009549A0 (en) * | 2014-04-18 | 2016-11-30 | Acceleron Pharma Inc | Methods for increasing red blood cell levels and treating sickle-cell disease |
| AP2017009674A0 (en) | 2014-06-13 | 2017-01-31 | Acceleron Pharma Inc | Methods and compositions for treating ulcers |
| MA41052A (fr) | 2014-10-09 | 2017-08-15 | Celgene Corp | Traitement d'une maladie cardiovasculaire à l'aide de pièges de ligands d'actrii |
| MA41119A (fr) * | 2014-12-03 | 2017-10-10 | Acceleron Pharma Inc | Méthodes de traitement de syndromes myélodysplasiques et d'anémie sidéroblastique |
| LT3227675T (lt) | 2014-12-03 | 2023-06-12 | Celgene Corporation | Aktivino actrii antagonistai ir naudojimas mielodisplastiniam sindromui gydyti |
| RU2733492C2 (ru) | 2015-04-22 | 2020-10-02 | Байоджен Ма Инк. | Новые гибридные ActRIIB белки-ловушки лигандов для лечения заболеваний, связанных с мышечной атрофией |
| JP6976859B2 (ja) * | 2015-05-13 | 2021-12-08 | セルジーン コーポレイション | ActRIIリガンドトラップを用いたβ−サラセミアの治療 |
| EP4190805A3 (en) | 2015-05-20 | 2023-08-16 | Celgene Corporation | In vitro cell culture methods for beta-thalassemia using activin type ii receptor ligand traps |
| US11123430B2 (en) * | 2015-11-04 | 2021-09-21 | Acceleron Pharma Inc. | Methods for increasing red blood cell levels and treating ineffective erythropoiesis |
| EP3380121B1 (en) | 2015-11-23 | 2023-12-20 | Acceleron Pharma Inc. | Actrii antagonist for use in treating eye disorders |
| CA3031909A1 (en) | 2016-07-27 | 2018-02-01 | Acceleron Pharma Inc. | Methods and compositions for treating myelofibrosis |
| KR102595559B1 (ko) | 2016-10-05 | 2023-10-30 | 악셀레론 파마 인코포레이티드 | 변이체 actriib 단백질 및 이의 용도 |
| JOP20190085A1 (ar) | 2016-10-20 | 2019-04-17 | Biogen Ma Inc | طرق علاج الضمور العضلي ومرض العظام باستخدام بروتينات احتجاز مركب ترابطي actriib هجين حديثة |
| CN110461349B (zh) | 2016-11-10 | 2024-08-13 | 科乐斯疗法公司 | 激活素受体iia型变体及其使用方法 |
| US20200101134A1 (en) * | 2017-06-14 | 2020-04-02 | Celgene Corporation | Methods for treating myeloproliferative neoplasm-associated myelofibrosis and anemia |
| EP3706777B1 (en) | 2017-11-09 | 2024-05-22 | Keros Therapeutics, Inc. | Activin receptor type iia variants and methods of use thereof |
| CA3087008A1 (en) | 2018-01-12 | 2019-07-18 | Keros Therapeutics, Inc. | Activin receptor type iib variants and methods of use thereof |
| JP7405772B2 (ja) | 2018-05-09 | 2023-12-26 | ケロス セラピューティクス インコーポレイテッド | アクチビンiia型受容体変異体および同変異体を含む医薬組成物 |
| JP2022549506A (ja) | 2019-09-27 | 2022-11-25 | ディスク・メディシン・インコーポレイテッド | 骨髄線維症および関連状態を処置するための方法 |
| EP4121088A4 (en) | 2020-03-20 | 2024-07-03 | Keros Therapeutics, Inc. | Methods of using activin receptor type iib variants |
| AU2021270458A1 (en) | 2020-05-13 | 2022-11-10 | AbbVie Deutschland GmbH & Co. KG | Anti-hemojuvelin (HJV) antibodies for treating myelofibrosis |
| US12186370B1 (en) | 2020-11-05 | 2025-01-07 | Celgene Corporation | ACTRIIB ligand trap compositions and uses thereof |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US1265208A (en) | 1915-09-07 | 1918-05-07 | Edward C Kahn | Liquid-fuel burner. |
| WO1987005330A1 (en) | 1986-03-07 | 1987-09-11 | Michel Louis Eugene Bergh | Method for enhancing glycoprotein stability |
| US5080891A (en) | 1987-08-03 | 1992-01-14 | Ddi Pharmaceuticals, Inc. | Conjugates of superoxide dismutase coupled to high molecular weight polyalkylene glycols |
| WO1990008822A1 (en) | 1989-02-03 | 1990-08-09 | Genetics Institute, Inc. | Erythropoietin receptor |
| US5354934A (en) * | 1993-02-04 | 1994-10-11 | Amgen Inc. | Pulmonary administration of erythropoietin |
| US5677196A (en) | 1993-05-18 | 1997-10-14 | University Of Utah Research Foundation | Apparatus and methods for multi-analyte homogeneous fluoro-immunoassays |
| US5525490A (en) | 1994-03-29 | 1996-06-11 | Onyx Pharmaceuticals, Inc. | Reverse two-hybrid method |
| US5885574A (en) | 1994-07-26 | 1999-03-23 | Amgen Inc. | Antibodies which activate an erythropoietin receptor |
| US5814565A (en) | 1995-02-23 | 1998-09-29 | University Of Utah Research Foundation | Integrated optic waveguide immunosensor |
| DE69636866D1 (en) | 1995-04-11 | 2007-03-15 | Gen Hospital Corp | Reverse "two-hybrid"-systeme |
| US5807839A (en) * | 1997-04-15 | 1998-09-15 | Advanced Viral Research Corp. | Method for stimulating red blood cell production |
| ATE322681T1 (de) | 1999-01-21 | 2006-04-15 | Metamorphix Inc | Inhibitoren für wachstumdifferenzierungsfaktor und ihre anwendungen |
| WO2004034962A2 (en) * | 2002-10-15 | 2004-04-29 | Celgene Corporation | Selective cytokine inhibitory drugs for treating myelodysplastic syndrome |
| DK2332977T3 (en) | 2004-07-23 | 2016-02-29 | Acceleron Pharma Inc | ActRII receptor polypeptides |
| US8067562B2 (en) | 2005-11-01 | 2011-11-29 | Amgen Inc. | Isolated nucleic acid molecule comprising the amino acid sequence of SEQ ID NO:1 |
| US20100028332A1 (en) * | 2006-12-18 | 2010-02-04 | Acceleron Pharma Inc. | Antagonists of actriib and uses for increasing red blood cell levels |
| TW201907946A (zh) | 2007-02-02 | 2019-03-01 | 美商艾瑟勒朗法瑪公司 | 衍生自ActRIIB的變體與其用途 |
| TW201919685A (zh) * | 2008-08-14 | 2019-06-01 | 美商艾瑟勒朗法瑪公司 | 使用gdf阱以增加紅血球水平 |
| US8216997B2 (en) * | 2008-08-14 | 2012-07-10 | Acceleron Pharma, Inc. | Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators |
| RU2642302C1 (ru) * | 2009-08-13 | 2018-01-24 | Акселерон Фарма Инк. | Комбинированное применение ловушек gdf и активаторов рецепторов эритропоэтина для повышения содержания эритроцитов |
-
2012
- 2012-10-17 EA EA202090053A patent/EA202090053A3/ru unknown
- 2012-10-17 KR KR1020227017193A patent/KR20220075438A/ko not_active Ceased
- 2012-10-17 ES ES19161424T patent/ES2869168T3/es active Active
- 2012-10-17 JP JP2014537186A patent/JP6092883B2/ja active Active
- 2012-10-17 BR BR122019023174-5A patent/BR122019023174B1/pt active IP Right Grant
- 2012-10-17 KR KR1020197025484A patent/KR102079482B1/ko active Active
- 2012-10-17 EP EP19161424.7A patent/EP3520805B1/en active Active
- 2012-10-17 EP EP12842601.2A patent/EP2797620B1/en active Active
- 2012-10-17 BR BR122019023166-4A patent/BR122019023166B1/pt active IP Right Grant
- 2012-10-17 BR BR112014009528-0A patent/BR112014009528B1/pt active IP Right Grant
- 2012-10-17 KR KR1020147013325A patent/KR20140084211A/ko not_active Ceased
- 2012-10-17 EA EA201490809A patent/EA034563B1/ru not_active IP Right Cessation
- 2012-10-17 ES ES12842601T patent/ES2741477T3/es active Active
- 2012-10-17 DK DK21153891.3T patent/DK3875104T3/da active
- 2012-10-17 CN CN201711069457.8A patent/CN107693776A/zh active Pending
- 2012-10-17 AU AU2012326207A patent/AU2012326207B2/en active Active
- 2012-10-17 WO PCT/US2012/060650 patent/WO2013059347A1/en not_active Ceased
- 2012-10-17 DK DK19161424.7T patent/DK3520805T3/da active
- 2012-10-17 EP EP21153891.3A patent/EP3875104B1/en active Active
- 2012-10-17 CN CN201711069431.3A patent/CN107837390A/zh active Pending
- 2012-10-17 ES ES21153891T patent/ES3010483T3/es active Active
- 2012-10-17 KR KR1020207004201A patent/KR20200019261A/ko not_active Ceased
- 2012-10-17 US US13/654,191 patent/US20130243743A1/en not_active Abandoned
- 2012-10-17 CN CN201280061672.2A patent/CN103987403B/zh active Active
- 2012-10-17 CA CA2852683A patent/CA2852683A1/en active Pending
-
2017
- 2017-01-23 US US15/413,184 patent/US20170137791A1/en not_active Abandoned
- 2017-02-09 JP JP2017022176A patent/JP6383821B2/ja active Active
- 2017-03-08 AU AU2017201580A patent/AU2017201580B2/en active Active
- 2017-07-28 US US15/663,361 patent/US20170327800A1/en not_active Abandoned
-
2018
- 2018-07-30 HK HK18109792.1A patent/HK1250341A1/zh unknown
- 2018-08-06 JP JP2018147507A patent/JP6796626B2/ja active Active
- 2018-09-26 HK HK18112295.7A patent/HK1252940A1/zh unknown
- 2018-10-11 AU AU2018247262A patent/AU2018247262B2/en active Active
-
2020
- 2020-08-07 US US16/987,756 patent/US20210207107A1/en active Pending
- 2020-08-24 JP JP2020140889A patent/JP2020186277A/ja active Pending
- 2020-12-09 AU AU2020286229A patent/AU2020286229A1/en not_active Abandoned
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102079482B1 (ko) | 비효율적 적혈구생성 치료를 위한 방법 및 조성물 | |
| JP7329573B2 (ja) | 赤血球レベルを高めるためのgdfトラップの使用 | |
| CN102655872B (zh) | Gdf捕获物和促红细胞生成素受体激活剂联合应用以增加红细胞水平 | |
| RU2814047C2 (ru) | Комбинированное применение ловушек gdf и активаторов рецепторов эритропоэтина для повышения содержания эритроцитов | |
| HK40057480A (en) | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels | |
| HK40057480B (zh) | Gdf捕获物和促红细胞生成素受体激活剂联合应用以增加红细胞水平 | |
| HK40053188A (en) | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels | |
| HK1224576A1 (en) | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels | |
| HK1224199B (en) | Combined use of gdf traps and erythropoietin receptor activators to increase red blood cell levels | |
| HK1223818B (zh) | Gdf捕获物和促红细胞生成素受体激活剂联合应用以增加红细胞水平 | |
| HK1224576B (zh) | Gdf捕获物和促红细胞生成素受体激活剂联合应用以增加红细胞水平 | |
| HK1203413B (en) | Compositions for treating iron overload in thalassemia |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A107 | Divisional application of patent | ||
| PA0104 | Divisional application for international application |
Comment text: Divisional Application for International Patent Patent event code: PA01041R01D Patent event date: 20220523 Application number text: 1020207004201 Filing date: 20200212 |
|
| PA0201 | Request for examination | ||
| PG1501 | Laying open of application | ||
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20221214 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20230217 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20221214 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |