KR20210061995A - Adam9를 표적으로 하는 면역콘쥬게이트 및 이를 이용하는 방법들 - Google Patents
Adam9를 표적으로 하는 면역콘쥬게이트 및 이를 이용하는 방법들 Download PDFInfo
- Publication number
- KR20210061995A KR20210061995A KR1020217002564A KR20217002564A KR20210061995A KR 20210061995 A KR20210061995 A KR 20210061995A KR 1020217002564 A KR1020217002564 A KR 1020217002564A KR 20217002564 A KR20217002564 A KR 20217002564A KR 20210061995 A KR20210061995 A KR 20210061995A
- Authority
- KR
- South Korea
- Prior art keywords
- seq
- ala
- adam9
- domain
- immunoconjugate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6871—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting an enzyme
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5365—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/22—Heterocyclic compounds, e.g. ascorbic acid, tocopherol or pyrrolidones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/68033—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a maytansine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
- A61K47/6817—Toxins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6889—Conjugates wherein the antibody being the modifying agent and wherein the linker, binder or spacer confers particular properties to the conjugates, e.g. peptidic enzyme-labile linkers or acid-labile linkers, providing for an acid-labile immuno conjugate wherein the drug may be released from its antibody conjugated part in an acidic, e.g. tumoural or environment
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/40—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against enzymes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/52—Constant or Fc region; Isotype
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/77—Internalization into the cell
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Oil, Petroleum & Natural Gas (AREA)
- Toxicology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201862690052P | 2018-06-26 | 2018-06-26 | |
| US62/690,052 | 2018-06-26 | ||
| US201862691342P | 2018-06-28 | 2018-06-28 | |
| US62/691,342 | 2018-06-28 | ||
| US201962810703P | 2019-02-26 | 2019-02-26 | |
| US62/810,703 | 2019-02-26 | ||
| PCT/US2019/038992 WO2020005945A1 (en) | 2018-06-26 | 2019-06-25 | Immunoconjugates targeting adam9 and methods of use thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20210061995A true KR20210061995A (ko) | 2021-05-28 |
Family
ID=67513726
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020217002564A Ceased KR20210061995A (ko) | 2018-06-26 | 2019-06-25 | Adam9를 표적으로 하는 면역콘쥬게이트 및 이를 이용하는 방법들 |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US20210275685A1 (enExample) |
| EP (1) | EP3814378A1 (enExample) |
| JP (1) | JP2021528471A (enExample) |
| KR (1) | KR20210061995A (enExample) |
| CN (1) | CN112543770A (enExample) |
| AU (1) | AU2019294510A1 (enExample) |
| BR (1) | BR112020025346A2 (enExample) |
| CA (1) | CA3104511A1 (enExample) |
| IL (1) | IL279633A (enExample) |
| MX (1) | MX2020013466A (enExample) |
| SG (1) | SG11202012257VA (enExample) |
| TW (1) | TWI831797B (enExample) |
| WO (1) | WO2020005945A1 (enExample) |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2020191306A1 (en) * | 2019-03-21 | 2020-09-24 | Immunogen, Inc. | Methods of preparing cell-binding agent-drug conjugates |
| WO2022192134A1 (en) | 2021-03-08 | 2022-09-15 | Immunogen, Inc. | Methods for increasing efficacy of immunoconjugates targeting adam9 for the treatment of cancer |
| WO2024059263A1 (en) * | 2022-09-15 | 2024-03-21 | Immunogen, Inc. | Methods for preparing maytansinoid derivatives with self-immolative peptide linkers |
| TWI876557B (zh) * | 2022-09-30 | 2025-03-11 | 臺北醫學大學 | 用於前列腺癌診斷和早期發病的單株抗體 |
| KR20250164740A (ko) * | 2023-03-24 | 2025-11-25 | 듀얼리티 바이올로직스 (상하이) 컴퍼니 리미티드 | Adam9를 표적으로 하는 인간화 항체, 이의 항체-약물 접합체 및 이의 용도 |
| WO2025094146A1 (en) | 2023-11-02 | 2025-05-08 | Immunogen Switzerland Gmbh | Vasconstrictors for reducing ocular toxicity of antibody-maytansinoid conjugates |
| CN120058950A (zh) * | 2023-11-28 | 2025-05-30 | 昆山新蕴达生物科技有限公司 | 抗adam9的抗体及其用途 |
| WO2025124443A1 (zh) * | 2023-12-14 | 2025-06-19 | 四川科伦博泰生物医药股份有限公司 | Adam9结合蛋白及其用途 |
Family Cites Families (62)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5807715A (en) | 1984-08-27 | 1998-09-15 | The Board Of Trustees Of The Leland Stanford Junior University | Methods and transformed mammalian lymphocyte cells for producing functional antigen-binding protein including chimeric immunoglobulin |
| US5427908A (en) | 1990-05-01 | 1995-06-27 | Affymax Technologies N.V. | Recombinant library screening methods |
| DE69233482T2 (de) | 1991-05-17 | 2006-01-12 | Merck & Co., Inc. | Verfahren zur Verminderung der Immunogenität der variablen Antikörperdomänen |
| WO1994004679A1 (en) | 1991-06-14 | 1994-03-03 | Genentech, Inc. | Method for making humanized antibodies |
| GB9115364D0 (en) | 1991-07-16 | 1991-08-28 | Wellcome Found | Antibody |
| AU669124B2 (en) | 1991-09-18 | 1996-05-30 | Kyowa Hakko Kirin Co., Ltd. | Process for producing humanized chimera antibody |
| US5565332A (en) | 1991-09-23 | 1996-10-15 | Medical Research Council | Production of chimeric antibodies - a combinatorial approach |
| US5733743A (en) | 1992-03-24 | 1998-03-31 | Cambridge Antibody Technology Limited | Methods for producing members of specific binding pairs |
| US6180377B1 (en) | 1993-06-16 | 2001-01-30 | Celltech Therapeutics Limited | Humanized antibodies |
| US6265150B1 (en) | 1995-06-07 | 2001-07-24 | Becton Dickinson & Company | Phage antibodies |
| WO1998023289A1 (en) | 1996-11-27 | 1998-06-04 | The General Hospital Corporation | MODULATION OF IgG BINDING TO FcRn |
| US6277375B1 (en) | 1997-03-03 | 2001-08-21 | Board Of Regents, The University Of Texas System | Immunoglobulin-like domains with increased half-lives |
| ATE336514T1 (de) | 2000-02-11 | 2006-09-15 | Merck Patent Gmbh | Steigerung der zirkulierenden halbwertzeit von auf antikörpern basierenden fusionsproteinen |
| US20030091568A1 (en) | 2000-04-07 | 2003-05-15 | Jurgen Frey | Inhibitors for the formation of soluble human CD23 |
| EP1142910A1 (en) | 2000-04-07 | 2001-10-10 | Jürgen Prof. Dr. Frey | Inhibitors for the formation of soluble human CD23 |
| PT1355919E (pt) | 2000-12-12 | 2011-03-02 | Medimmune Llc | Moléculas com semivida longa, composições que as contêm e suas utilizações |
| CA2470763A1 (en) | 2001-12-18 | 2003-06-26 | Mondobiotech Laboratories Anstalt | Novel pharmaceutical composition of interferon gamma or pirfenidone with molecular diagnostics for the improved treatment of interstitial lung diseases |
| US6716821B2 (en) | 2001-12-21 | 2004-04-06 | Immunogen Inc. | Cytotoxic agents bearing a reactive polyethylene glycol moiety, cytotoxic conjugates comprising polyethylene glycol linking groups, and methods of making and using the same |
| US20040092466A1 (en) | 2002-11-11 | 2004-05-13 | Isis Pharmaceuticals Inc. | Modulation of ADAM9 expression |
| JP2006500364A (ja) | 2002-08-16 | 2006-01-05 | イムノージェン インコーポレーテッド | 高い反応性と溶解度を有する架橋剤および小分子薬物の標的化送達用コンジュゲートの調製におけるそれらの使用 |
| US7217797B2 (en) | 2002-10-15 | 2007-05-15 | Pdl Biopharma, Inc. | Alteration of FcRn binding affinities or serum half-lives of antibodies by mutagenesis |
| US8088387B2 (en) | 2003-10-10 | 2012-01-03 | Immunogen Inc. | Method of targeting specific cell populations using cell-binding agent maytansinoid conjugates linked via a non-cleavable linker, said conjugates, and methods of making said conjugates |
| DE10337368A1 (de) * | 2003-08-08 | 2005-03-03 | Technische Universität Dresden | Verfahren und Mittel zur Diagnose und Behandlung von Pankreastumoren |
| CA2545603A1 (en) | 2003-11-12 | 2005-05-26 | Biogen Idec Ma Inc. | Neonatal fc receptor (fcrn)-binding polypeptide variants, dimeric fc binding proteins and methods related thereto |
| WO2005091823A2 (en) | 2004-03-01 | 2005-10-06 | The Regents Of The University Of California | The use of lunasin peptide as a transcriptional activator to prevent cancer and related methods for treatment, monitoring and prognosis |
| US8802820B2 (en) | 2004-11-12 | 2014-08-12 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| US8367805B2 (en) | 2004-11-12 | 2013-02-05 | Xencor, Inc. | Fc variants with altered binding to FcRn |
| JP5328155B2 (ja) | 2005-02-02 | 2013-10-30 | マクロジェニックス ウエスト, インコーポレイテッド | Adam−9モジュレータ |
| US8445198B2 (en) | 2005-12-01 | 2013-05-21 | Medical Prognosis Institute | Methods, kits and devices for identifying biomarkers of treatment response and use thereof to predict treatment efficacy |
| HRP20140172T1 (hr) | 2006-05-30 | 2014-03-28 | Genentech, Inc. | Protutijela i imunokonjugati kao i njihove uporabe |
| WO2007143752A2 (en) | 2006-06-09 | 2007-12-13 | The Regents Of The University Of California | Targets in breast cancer for prognosis or therapy |
| KR101443214B1 (ko) | 2007-01-09 | 2014-09-24 | 삼성전자주식회사 | 폐암 환자 또는 폐암 치료를 받은 폐암 환자의 폐암 재발 위험을 진단하기 위한 조성물, 키트 및 마이크로어레이 |
| GB2453589A (en) | 2007-10-12 | 2009-04-15 | King S College London | Protease inhibition |
| GB0724357D0 (en) * | 2007-12-14 | 2008-01-23 | Glaxosmithkline Biolog Sa | Method for preparing protein conjugates |
| WO2009114532A2 (en) | 2008-03-10 | 2009-09-17 | National Jewish Health | Markers for diagnosis of pulmonary inflammation and methods related thereto |
| WO2009126616A2 (en) | 2008-04-07 | 2009-10-15 | Zymogenetics, Inc. | Thrombin activator compostions and methods of making and using the same |
| US20090285840A1 (en) * | 2008-04-29 | 2009-11-19 | New York Society For The Ruptured And Crippled Maintaining The Hospital For Special Surgery | Methods for treating pathological neovascularization |
| BRPI0911442A2 (pt) | 2008-04-30 | 2019-03-12 | Immunogen, Inc. | conjugados potentes e ligantes hidrofílicos |
| CN102076717B (zh) | 2008-04-30 | 2016-02-03 | 伊缪诺金公司 | 交联剂和它们的用途 |
| ES2675730T3 (es) | 2008-06-04 | 2018-07-12 | Macrogenics, Inc. | Anticuerpos con unión alterada a FcRn y métodos de uso de los mismos |
| WO2010014990A2 (en) | 2008-08-01 | 2010-02-04 | The Board Of Trustees Of The University Of Illinois | Method of promoting neurogenesis by modulating secretase activities |
| US20100112713A1 (en) | 2008-11-05 | 2010-05-06 | The Texas A&M University System | Methods For Detecting Colorectal Diseases And Disorders |
| SG171812A1 (en) | 2008-12-04 | 2011-07-28 | Abbott Lab | Dual variable domain immunoglobulins and uses thereof |
| JP2013506709A (ja) * | 2009-10-06 | 2013-02-28 | イミュノジェン・インコーポレーテッド | 有効なコンジュゲートおよび親水性リンカー |
| MX2012004406A (es) * | 2009-10-21 | 2012-05-08 | Immunogen Inc | Nuevo regimen de dosificacion y metodo de tratamiento. |
| US20130045244A1 (en) | 2010-02-11 | 2013-02-21 | University Of Southern California | Modified adam disintegrin domain polypeptides and uses thereof |
| US9150656B2 (en) | 2010-03-04 | 2015-10-06 | Macrogenics, Inc. | Antibodies reactive with B7-H3, immunologically active fragments thereof and uses thereof |
| US20130058947A1 (en) | 2011-09-02 | 2013-03-07 | Stem Centrx, Inc | Novel Modulators and Methods of Use |
| WO2013049704A2 (en) | 2011-09-28 | 2013-04-04 | Armune Biosciences, Inc. | Method and system of particle-phage epitope complex |
| US20140342946A1 (en) | 2011-12-31 | 2014-11-20 | Moni Abraham Kuriakose | Diagnostic tests for predicting prognosis, recurrence, resistance or sensitivity to therapy and metastatic status in cancer |
| US9534058B2 (en) | 2012-02-08 | 2017-01-03 | Abbvie Stemcentrx Llc | Anti-CD324 monoclonal antibodies and uses thereof |
| WO2014108480A1 (en) | 2013-01-09 | 2014-07-17 | Friedrich-Alexander-Universitaet Erlangen-Nuernberg | Method for in vitro detection and monitoring of a disease by measuring disease-associated protease activity in extracellular vesicles |
| US10047163B2 (en) | 2013-02-08 | 2018-08-14 | Abbvie Stemcentrx Llc | Multispecific constructs |
| KR102306490B1 (ko) * | 2013-03-15 | 2021-09-28 | 리제너론 파아마슈티컬스, 인크. | 생물학적 활성 분자, 그의 접합체 및 치료 용도 |
| WO2014168154A1 (ja) | 2013-04-08 | 2014-10-16 | 三菱レイヨン株式会社 | 眼疾患を評価するためのマイクロアレイ及び眼疾患の評価方法 |
| CA2912542A1 (en) | 2013-05-13 | 2014-11-20 | Tufts University | Methods and compositions for prognosis, diagnosis and treatment of adam8-expressing cancer |
| EP3011050B1 (en) | 2013-06-19 | 2017-09-06 | Memorial Sloan Kettering Cancer Center | Methods and compositions for the diagnosis, prognosis and treatment of brain metastasis |
| US9567641B2 (en) | 2013-07-03 | 2017-02-14 | Samsung Electronics Co., Ltd. | Combination therapy for the treatment of cancer using an anti-C-met antibody |
| EA201790871A1 (ru) | 2014-11-11 | 2017-11-30 | Амуникс Оперэйтинг Инк. | Нацеленные конъюгатные композиции xten и способы их получения |
| RU2018130108A (ru) * | 2016-02-05 | 2020-03-06 | Иммуноджен, Инк. | Эффективный способ получения конъюгатов связывающийся с клеткой агент цитотоксический агент |
| DK3558391T3 (da) * | 2016-12-23 | 2022-05-09 | Immunogen Inc | Immunkonjugater med adam9 som mål og fremgangsmåder til anvendelse deraf |
-
2019
- 2019-06-25 BR BR112020025346-4A patent/BR112020025346A2/pt unknown
- 2019-06-25 US US17/255,064 patent/US20210275685A1/en not_active Abandoned
- 2019-06-25 TW TW108122055A patent/TWI831797B/zh not_active IP Right Cessation
- 2019-06-25 MX MX2020013466A patent/MX2020013466A/es unknown
- 2019-06-25 JP JP2020573007A patent/JP2021528471A/ja active Pending
- 2019-06-25 EP EP19748615.2A patent/EP3814378A1/en not_active Withdrawn
- 2019-06-25 AU AU2019294510A patent/AU2019294510A1/en active Pending
- 2019-06-25 CA CA3104511A patent/CA3104511A1/en active Pending
- 2019-06-25 CN CN201980049672.2A patent/CN112543770A/zh active Pending
- 2019-06-25 SG SG11202012257VA patent/SG11202012257VA/en unknown
- 2019-06-25 KR KR1020217002564A patent/KR20210061995A/ko not_active Ceased
- 2019-06-25 WO PCT/US2019/038992 patent/WO2020005945A1/en not_active Ceased
-
2020
- 2020-12-21 IL IL279633A patent/IL279633A/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| WO2020005945A1 (en) | 2020-01-02 |
| AU2019294510A8 (en) | 2021-04-22 |
| MX2020013466A (es) | 2021-04-19 |
| TW202019960A (zh) | 2020-06-01 |
| AU2019294510A1 (en) | 2021-01-21 |
| CN112543770A (zh) | 2021-03-23 |
| JP2021528471A (ja) | 2021-10-21 |
| CA3104511A1 (en) | 2020-01-02 |
| EP3814378A1 (en) | 2021-05-05 |
| TWI831797B (zh) | 2024-02-11 |
| SG11202012257VA (en) | 2021-01-28 |
| US20210275685A1 (en) | 2021-09-09 |
| IL279633A (en) | 2021-03-01 |
| BR112020025346A2 (pt) | 2021-05-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102434626B1 (ko) | 항-b7-h3 항체 및 항체 약물 콘쥬게이트 | |
| KR20210061995A (ko) | Adam9를 표적으로 하는 면역콘쥬게이트 및 이를 이용하는 방법들 | |
| TWI783957B (zh) | 靶向adam9之免疫共軛物及其使用方法 | |
| AU2018279105A1 (en) | Antibody conjugates of immune-modulatory compounds and uses thereof | |
| CA3049791A1 (en) | Tumor targeting conjugates and methods of use thereof | |
| KR20180130574A (ko) | 신규 b7-h3-결합 분자, 그것의 항체 약물 콘쥬게이트 및 그것의 사용 방법 | |
| CA3137373A1 (en) | Amatoxin antibody-drug conjugates and uses thereof | |
| CA3049842A1 (en) | Construct-peptide compositions and methods of use thereof | |
| CA3138272A1 (en) | Biparatopic fr-alpha antibodies and immunoconjugates | |
| CA3231039A1 (en) | Linkers for use in antibody drug conjugates | |
| TW202043291A (zh) | 抗mertk抗體及使用方法 | |
| WO2022006340A1 (en) | Alk5 inhibitors, conjugates, and uses thereof | |
| JPWO2020219770A5 (enExample) | ||
| JPWO2020219748A5 (enExample) | ||
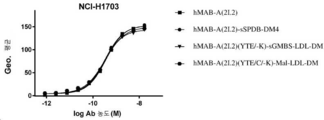
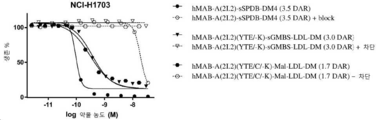
| HK40015128A (en) | Immunoconjugates targeting adam9 and methods of use thereof | |
| HK40015128B (en) | Immunoconjugates targeting adam9 and methods of use thereof | |
| JP2024528217A (ja) | 二重特異性抗体および使用方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| T11-X000 | Administrative time limit extension requested |
St.27 status event code: U-3-3-T10-T11-oth-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| PE0601 | Decision on rejection of patent |
St.27 status event code: N-2-6-B10-B15-exm-PE0601 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-3-3-R10-R18-oth-X000 |