KR20200110377A - 조합 용량의 독성을 최소화하기 위한 프로토콜 및 검증을 위한 조영제 - Google Patents
조합 용량의 독성을 최소화하기 위한 프로토콜 및 검증을 위한 조영제 Download PDFInfo
- Publication number
- KR20200110377A KR20200110377A KR1020207023337A KR20207023337A KR20200110377A KR 20200110377 A KR20200110377 A KR 20200110377A KR 1020207023337 A KR1020207023337 A KR 1020207023337A KR 20207023337 A KR20207023337 A KR 20207023337A KR 20200110377 A KR20200110377 A KR 20200110377A
- Authority
- KR
- South Korea
- Prior art keywords
- agent
- conjugate
- tumor
- subject
- peg
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000002872 contrast media Substances 0.000 title claims abstract description 46
- 230000001988 toxicity Effects 0.000 title claims description 15
- 231100000419 toxicity Toxicity 0.000 title claims description 15
- 238000010200 validation analysis Methods 0.000 title 1
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 188
- 239000003814 drug Substances 0.000 claims abstract description 103
- 229940079593 drug Drugs 0.000 claims abstract description 90
- 230000000694 effects Effects 0.000 claims abstract description 49
- 238000011282 treatment Methods 0.000 claims abstract description 20
- 239000002246 antineoplastic agent Substances 0.000 claims abstract description 17
- 229940127089 cytotoxic agent Drugs 0.000 claims abstract description 17
- 230000014759 maintenance of location Effects 0.000 claims abstract description 13
- 230000035699 permeability Effects 0.000 claims abstract description 12
- 230000001976 improved effect Effects 0.000 claims abstract description 9
- 229920001223 polyethylene glycol Polymers 0.000 claims description 104
- 238000000034 method Methods 0.000 claims description 79
- 239000002202 Polyethylene glycol Substances 0.000 claims description 78
- 239000003795 chemical substances by application Substances 0.000 claims description 69
- 239000003112 inhibitor Substances 0.000 claims description 24
- 239000002105 nanoparticle Substances 0.000 claims description 24
- 238000002600 positron emission tomography Methods 0.000 claims description 23
- 230000035508 accumulation Effects 0.000 claims description 21
- 238000009825 accumulation Methods 0.000 claims description 21
- 239000000203 mixture Substances 0.000 claims description 18
- 108090000623 proteins and genes Proteins 0.000 claims description 18
- 239000002738 chelating agent Substances 0.000 claims description 15
- 231100000331 toxic Toxicity 0.000 claims description 15
- 230000002588 toxic effect Effects 0.000 claims description 15
- 239000012661 PARP inhibitor Substances 0.000 claims description 14
- 229940121906 Poly ADP ribose polymerase inhibitor Drugs 0.000 claims description 14
- 229920002521 macromolecule Polymers 0.000 claims description 14
- 230000033616 DNA repair Effects 0.000 claims description 13
- 238000003384 imaging method Methods 0.000 claims description 13
- HWGQMRYQVZSGDQ-HZPDHXFCSA-N chembl3137320 Chemical compound CN1N=CN=C1[C@H]([C@H](N1)C=2C=CC(F)=CC=2)C2=NNC(=O)C3=C2C1=CC(F)=C3 HWGQMRYQVZSGDQ-HZPDHXFCSA-N 0.000 claims description 12
- 238000009826 distribution Methods 0.000 claims description 12
- 238000012544 monitoring process Methods 0.000 claims description 12
- 230000035772 mutation Effects 0.000 claims description 12
- 229940124597 therapeutic agent Drugs 0.000 claims description 12
- 230000008901 benefit Effects 0.000 claims description 10
- 230000001839 systemic circulation Effects 0.000 claims description 10
- 239000003534 dna topoisomerase inhibitor Substances 0.000 claims description 8
- 229940044693 topoisomerase inhibitor Drugs 0.000 claims description 8
- 230000002195 synergetic effect Effects 0.000 claims description 7
- 238000002648 combination therapy Methods 0.000 claims description 6
- 239000012141 concentrate Substances 0.000 claims description 6
- 230000008030 elimination Effects 0.000 claims description 6
- 238000003379 elimination reaction Methods 0.000 claims description 6
- 108700020463 BRCA1 Proteins 0.000 claims description 5
- 102000036365 BRCA1 Human genes 0.000 claims description 5
- 101150072950 BRCA1 gene Proteins 0.000 claims description 5
- 102000052609 BRCA2 Human genes 0.000 claims description 5
- 108700020462 BRCA2 Proteins 0.000 claims description 5
- 101150008921 Brca2 gene Proteins 0.000 claims description 5
- 150000002148 esters Chemical class 0.000 claims description 4
- 238000009472 formulation Methods 0.000 claims description 4
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 claims description 4
- 229960001756 oxaliplatin Drugs 0.000 claims description 4
- 102000004169 proteins and genes Human genes 0.000 claims description 4
- 230000002285 radioactive effect Effects 0.000 claims description 4
- 102000004459 Nitroreductase Human genes 0.000 claims description 3
- BPEGJWRSRHCHSN-UHFFFAOYSA-N Temozolomide Chemical compound O=C1N(C)N=NC2=C(C(N)=O)N=CN21 BPEGJWRSRHCHSN-UHFFFAOYSA-N 0.000 claims description 3
- 150000001408 amides Chemical class 0.000 claims description 3
- 125000003118 aryl group Chemical group 0.000 claims description 3
- 150000004657 carbamic acid derivatives Chemical class 0.000 claims description 3
- 150000004649 carbonic acid derivatives Chemical class 0.000 claims description 3
- 230000007062 hydrolysis Effects 0.000 claims description 3
- 238000006460 hydrolysis reaction Methods 0.000 claims description 3
- 238000002955 isolation Methods 0.000 claims description 3
- 229940124302 mTOR inhibitor Drugs 0.000 claims description 3
- 239000003628 mammalian target of rapamycin inhibitor Substances 0.000 claims description 3
- 125000000449 nitro group Chemical group [O-][N+](*)=O 0.000 claims description 3
- 108020001162 nitroreductase Proteins 0.000 claims description 3
- 229910052697 platinum Inorganic materials 0.000 claims description 3
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Substances [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 claims description 3
- 230000017854 proteolysis Effects 0.000 claims description 3
- 230000009467 reduction Effects 0.000 claims description 3
- 229960004964 temozolomide Drugs 0.000 claims description 3
- 229960004528 vincristine Drugs 0.000 claims description 3
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 claims description 3
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 claims description 3
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical group O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 claims 4
- 230000034431 double-strand break repair via homologous recombination Effects 0.000 claims 4
- XXPXYPLPSDPERN-UHFFFAOYSA-N Ecteinascidin 743 Natural products COc1cc2C(NCCc2cc1O)C(=O)OCC3N4C(O)C5Cc6cc(C)c(OC)c(O)c6C(C4C(S)c7c(OC(=O)C)c(C)c8OCOc8c37)N5C XXPXYPLPSDPERN-UHFFFAOYSA-N 0.000 claims 2
- PXGOKWXKJXAPGV-UHFFFAOYSA-N Fluorine Chemical compound FF PXGOKWXKJXAPGV-UHFFFAOYSA-N 0.000 claims 2
- 229940123237 Taxane Drugs 0.000 claims 2
- 239000000654 additive Substances 0.000 claims 2
- 230000000996 additive effect Effects 0.000 claims 2
- 229940045799 anthracyclines and related substance Drugs 0.000 claims 2
- 239000003124 biologic agent Substances 0.000 claims 2
- 229930013356 epothilone Natural products 0.000 claims 2
- 150000003883 epothilone derivatives Chemical class 0.000 claims 2
- 229910052731 fluorine Inorganic materials 0.000 claims 2
- 239000011737 fluorine Substances 0.000 claims 2
- 238000001727 in vivo Methods 0.000 claims 2
- 239000002777 nucleoside Substances 0.000 claims 2
- DKPFODGZWDEEBT-QFIAKTPHSA-N taxane Chemical class C([C@]1(C)CCC[C@@H](C)[C@H]1C1)C[C@H]2[C@H](C)CC[C@@H]1C2(C)C DKPFODGZWDEEBT-QFIAKTPHSA-N 0.000 claims 2
- PKVRCIRHQMSYJX-AIFWHQITSA-N trabectedin Chemical compound C([C@@]1(C(OC2)=O)NCCC3=C1C=C(C(=C3)O)OC)S[C@@H]1C3=C(OC(C)=O)C(C)=C4OCOC4=C3[C@H]2N2[C@@H](O)[C@H](CC=3C4=C(O)C(OC)=C(C)C=3)N(C)[C@H]4[C@@H]21 PKVRCIRHQMSYJX-AIFWHQITSA-N 0.000 claims 2
- 229960000977 trabectedin Drugs 0.000 claims 2
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 claims 2
- 239000005483 tyrosine kinase inhibitor Substances 0.000 claims 2
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 claims 2
- 229940035893 uracil Drugs 0.000 claims 2
- 102000000872 ATM Human genes 0.000 claims 1
- 108010004586 Ataxia Telangiectasia Mutated Proteins Proteins 0.000 claims 1
- 150000003833 nucleoside derivatives Chemical class 0.000 claims 1
- 125000003835 nucleoside group Chemical group 0.000 claims 1
- 241000699670 Mus sp. Species 0.000 description 52
- 210000001519 tissue Anatomy 0.000 description 32
- 239000000243 solution Substances 0.000 description 30
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 24
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 18
- 238000004128 high performance liquid chromatography Methods 0.000 description 18
- 239000011148 porous material Substances 0.000 description 14
- BZLVMXJERCGZMT-UHFFFAOYSA-N Methyl tert-butyl ether Chemical compound COC(C)(C)C BZLVMXJERCGZMT-UHFFFAOYSA-N 0.000 description 12
- 241000700159 Rattus Species 0.000 description 12
- 238000010521 absorption reaction Methods 0.000 description 12
- 210000004027 cell Anatomy 0.000 description 12
- 238000006243 chemical reaction Methods 0.000 description 12
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 12
- 239000000126 substance Substances 0.000 description 12
- 229960004562 carboplatin Drugs 0.000 description 11
- 190000008236 carboplatin Chemical compound 0.000 description 11
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 11
- 229960004316 cisplatin Drugs 0.000 description 11
- 150000001875 compounds Chemical class 0.000 description 10
- 229960004768 irinotecan Drugs 0.000 description 10
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 241000699666 Mus <mouse, genus> Species 0.000 description 9
- 239000008280 blood Substances 0.000 description 9
- 238000000105 evaporative light scattering detection Methods 0.000 description 9
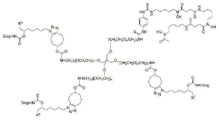
- 125000005647 linker group Chemical group 0.000 description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 9
- WWUZIQQURGPMPG-UHFFFAOYSA-N (-)-D-erythro-Sphingosine Natural products CCCCCCCCCCCCCC=CC(O)C(N)CO WWUZIQQURGPMPG-UHFFFAOYSA-N 0.000 description 8
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 8
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 8
- 238000013459 approach Methods 0.000 description 8
- 231100000433 cytotoxic Toxicity 0.000 description 8
- 230000001472 cytotoxic effect Effects 0.000 description 8
- 229960004679 doxorubicin Drugs 0.000 description 8
- 229960002949 fluorouracil Drugs 0.000 description 8
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 8
- 210000000056 organ Anatomy 0.000 description 8
- 239000007787 solid Substances 0.000 description 8
- WWUZIQQURGPMPG-KRWOKUGFSA-N sphingosine Chemical compound CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)CO WWUZIQQURGPMPG-KRWOKUGFSA-N 0.000 description 8
- 230000009885 systemic effect Effects 0.000 description 8
- 239000003981 vehicle Substances 0.000 description 8
- 210000004369 blood Anatomy 0.000 description 7
- 239000000969 carrier Substances 0.000 description 7
- 239000002245 particle Substances 0.000 description 7
- 230000002792 vascular Effects 0.000 description 7
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 7
- 229960002066 vinorelbine Drugs 0.000 description 7
- 230000020172 G2/M transition checkpoint Effects 0.000 description 6
- JGFZNNIVVJXRND-UHFFFAOYSA-N N,N-Diisopropylethylamine (DIPEA) Chemical compound CCN(C(C)C)C(C)C JGFZNNIVVJXRND-UHFFFAOYSA-N 0.000 description 6
- 238000012879 PET imaging Methods 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- ODKNJVUHOIMIIZ-RRKCRQDMSA-N floxuridine Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(F)=C1 ODKNJVUHOIMIIZ-RRKCRQDMSA-N 0.000 description 6
- 239000002502 liposome Substances 0.000 description 6
- 239000000047 product Substances 0.000 description 6
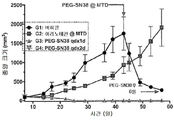
- 230000004614 tumor growth Effects 0.000 description 6
- 239000003039 volatile agent Substances 0.000 description 6
- 241001465754 Metazoa Species 0.000 description 5
- 238000013461 design Methods 0.000 description 5
- 238000002474 experimental method Methods 0.000 description 5
- 238000005259 measurement Methods 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
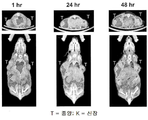
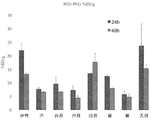
- 230000036326 tumor accumulation Effects 0.000 description 5
- 108090000323 DNA Topoisomerases Proteins 0.000 description 4
- 102000003915 DNA Topoisomerases Human genes 0.000 description 4
- 229940123780 DNA topoisomerase I inhibitor Drugs 0.000 description 4
- 102000012338 Poly(ADP-ribose) Polymerases Human genes 0.000 description 4
- 108010061844 Poly(ADP-ribose) Polymerases Proteins 0.000 description 4
- 229920000776 Poly(Adenosine diphosphate-ribose) polymerase Polymers 0.000 description 4
- 239000000365 Topoisomerase I Inhibitor Substances 0.000 description 4
- 230000000875 corresponding effect Effects 0.000 description 4
- 230000008878 coupling Effects 0.000 description 4
- 238000010168 coupling process Methods 0.000 description 4
- 238000005859 coupling reaction Methods 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 239000000032 diagnostic agent Substances 0.000 description 4
- 229940039227 diagnostic agent Drugs 0.000 description 4
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 4
- 229960005277 gemcitabine Drugs 0.000 description 4
- 230000001965 increasing effect Effects 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 238000011580 nude mouse model Methods 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 239000011541 reaction mixture Substances 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 238000003756 stirring Methods 0.000 description 4
- -1 trastuzumab Chemical compound 0.000 description 4
- QDAKCRDTMCMQDS-UHFFFAOYSA-N 6-azidohexyl (2,5-dioxopyrrolidin-1-yl) carbonate Chemical compound [N-]=[N+]=NCCCCCCOC(=O)ON1C(=O)CCC1=O QDAKCRDTMCMQDS-UHFFFAOYSA-N 0.000 description 3
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 3
- 108700028369 Alleles Proteins 0.000 description 3
- KLWPJMFMVPTNCC-UHFFFAOYSA-N Camptothecin Natural products CCC1(O)C(=O)OCC2=C1C=C3C4Nc5ccccc5C=C4CN3C2=O KLWPJMFMVPTNCC-UHFFFAOYSA-N 0.000 description 3
- 108020004414 DNA Proteins 0.000 description 3
- 229940124087 DNA topoisomerase II inhibitor Drugs 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 206010029113 Neovascularisation Diseases 0.000 description 3
- 229930012538 Paclitaxel Natural products 0.000 description 3
- 229940127361 Receptor Tyrosine Kinase Inhibitors Drugs 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 239000000317 Topoisomerase II Inhibitor Substances 0.000 description 3
- 102000007537 Type II DNA Topoisomerases Human genes 0.000 description 3
- 108010046308 Type II DNA Topoisomerases Proteins 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000006907 apoptotic process Effects 0.000 description 3
- 238000007068 beta-elimination reaction Methods 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 229940127093 camptothecin Drugs 0.000 description 3
- VSJKWCGYPAHWDS-FQEVSTJZSA-N camptothecin Chemical compound C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-FQEVSTJZSA-N 0.000 description 3
- 230000015556 catabolic process Effects 0.000 description 3
- 238000003776 cleavage reaction Methods 0.000 description 3
- 229960000975 daunorubicin Drugs 0.000 description 3
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 3
- 238000006731 degradation reaction Methods 0.000 description 3
- VSJKWCGYPAHWDS-UHFFFAOYSA-N dl-camptothecin Natural products C1=CC=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)C5(O)CC)C4=NC2=C1 VSJKWCGYPAHWDS-UHFFFAOYSA-N 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 230000002496 gastric effect Effects 0.000 description 3
- 230000001939 inductive effect Effects 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 210000004185 liver Anatomy 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 229960001592 paclitaxel Drugs 0.000 description 3
- VLTRZXGMWDSKGL-UHFFFAOYSA-M perchlorate Inorganic materials [O-]Cl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-M 0.000 description 3
- VLTRZXGMWDSKGL-UHFFFAOYSA-N perchloric acid Chemical compound OCl(=O)(=O)=O VLTRZXGMWDSKGL-UHFFFAOYSA-N 0.000 description 3
- 238000012636 positron electron tomography Methods 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 230000022983 regulation of cell cycle Effects 0.000 description 3
- HMABYWSNWIZPAG-UHFFFAOYSA-N rucaparib Chemical compound C1=CC(CNC)=CC=C1C(N1)=C2CCNC(=O)C3=C2C1=CC(F)=C3 HMABYWSNWIZPAG-UHFFFAOYSA-N 0.000 description 3
- 229950004707 rucaparib Drugs 0.000 description 3
- 230000007017 scission Effects 0.000 description 3
- 150000003384 small molecules Chemical class 0.000 description 3
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 3
- 210000004881 tumor cell Anatomy 0.000 description 3
- PVVTWNMXEHROIA-UHFFFAOYSA-N 2-(3-hydroxypropyl)-1h-quinazolin-4-one Chemical compound C1=CC=C2NC(CCCO)=NC(=O)C2=C1 PVVTWNMXEHROIA-UHFFFAOYSA-N 0.000 description 2
- 206010003591 Ataxia Diseases 0.000 description 2
- 108010006654 Bleomycin Proteins 0.000 description 2
- 230000005778 DNA damage Effects 0.000 description 2
- 231100000277 DNA damage Toxicity 0.000 description 2
- 239000012623 DNA damaging agent Substances 0.000 description 2
- VTLYFUHAOXGGBS-UHFFFAOYSA-N Fe3+ Chemical compound [Fe+3] VTLYFUHAOXGGBS-UHFFFAOYSA-N 0.000 description 2
- 230000008051 G1/S transition checkpoint Effects 0.000 description 2
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 2
- 206010027476 Metastases Diseases 0.000 description 2
- 241000699660 Mus musculus Species 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- 108091000080 Phosphotransferase Proteins 0.000 description 2
- 102000001253 Protein Kinase Human genes 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 229960001561 bleomycin Drugs 0.000 description 2
- OYVAGSVQBOHSSS-UAPAGMARSA-O bleomycin A2 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC=C(N=1)C=1SC=C(N=1)C(=O)NCCC[S+](C)C)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C OYVAGSVQBOHSSS-UAPAGMARSA-O 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 230000022131 cell cycle Effects 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 230000004087 circulation Effects 0.000 description 2
- 230000021615 conjugation Effects 0.000 description 2
- 125000004093 cyano group Chemical group *C#N 0.000 description 2
- 229960001425 deferoxamine mesylate Drugs 0.000 description 2
- IDDIJAWJANBQLJ-UHFFFAOYSA-N desferrioxamine B mesylate Chemical compound [H+].CS([O-])(=O)=O.CC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCNC(=O)CCC(=O)N(O)CCCCCN IDDIJAWJANBQLJ-UHFFFAOYSA-N 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 238000012377 drug delivery Methods 0.000 description 2
- 229960005420 etoposide Drugs 0.000 description 2
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- 229930182480 glucuronide Natural products 0.000 description 2
- 150000008134 glucuronides Chemical class 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 239000012535 impurity Substances 0.000 description 2
- 229940043355 kinase inhibitor Drugs 0.000 description 2
- 108010082117 matrigel Proteins 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 229960000485 methotrexate Drugs 0.000 description 2
- 102000020233 phosphotransferase Human genes 0.000 description 2
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 2
- 239000002244 precipitate Substances 0.000 description 2
- 108060006633 protein kinase Proteins 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000002390 rotary evaporation Methods 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 239000003277 telomerase inhibitor Substances 0.000 description 2
- 238000011287 therapeutic dose Methods 0.000 description 2
- 229960000303 topotecan Drugs 0.000 description 2
- UCFGDBYHRUNTLO-QHCPKHFHSA-N topotecan Chemical compound C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 UCFGDBYHRUNTLO-QHCPKHFHSA-N 0.000 description 2
- WMSUFWLPZLCIHP-UHFFFAOYSA-N (2,5-dioxopyrrolidin-1-yl) 9h-fluoren-9-ylmethyl carbonate Chemical compound C12=CC=CC=C2C2=CC=CC=C2C1COC(=O)ON1C(=O)CCC1=O WMSUFWLPZLCIHP-UHFFFAOYSA-N 0.000 description 1
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 1
- TZCPCKNHXULUIY-RGULYWFUSA-N 1,2-distearoyl-sn-glycero-3-phosphoserine Chemical compound CCCCCCCCCCCCCCCCCC(=O)OC[C@H](COP(O)(=O)OC[C@H](N)C(O)=O)OC(=O)CCCCCCCCCCCCCCCCC TZCPCKNHXULUIY-RGULYWFUSA-N 0.000 description 1
- VSNHCAURESNICA-NJFSPNSNSA-N 1-oxidanylurea Chemical compound N[14C](=O)NO VSNHCAURESNICA-NJFSPNSNSA-N 0.000 description 1
- QRZUPJILJVGUFF-UHFFFAOYSA-N 2,8-dibenzylcyclooctan-1-one Chemical compound C1CCCCC(CC=2C=CC=CC=2)C(=O)C1CC1=CC=CC=C1 QRZUPJILJVGUFF-UHFFFAOYSA-N 0.000 description 1
- JVKUCNQGESRUCL-UHFFFAOYSA-N 2-Hydroxyethyl 12-hydroxyoctadecanoate Chemical compound CCCCCCC(O)CCCCCCCCCCC(=O)OCCO JVKUCNQGESRUCL-UHFFFAOYSA-N 0.000 description 1
- NDMPLJNOPCLANR-UHFFFAOYSA-N 3,4-dihydroxy-15-(4-hydroxy-18-methoxycarbonyl-5,18-seco-ibogamin-18-yl)-16-methoxy-1-methyl-6,7-didehydro-aspidospermidine-3-carboxylic acid methyl ester Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 NDMPLJNOPCLANR-UHFFFAOYSA-N 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- UZOFELREXGAFOI-UHFFFAOYSA-N 4-methylpiperidine Chemical compound CC1CCNCC1 UZOFELREXGAFOI-UHFFFAOYSA-N 0.000 description 1
- PBCZSGKMGDDXIJ-HQCWYSJUSA-N 7-hydroxystaurosporine Chemical compound N([C@H](O)C1=C2C3=CC=CC=C3N3C2=C24)C(=O)C1=C2C1=CC=CC=C1N4[C@H]1C[C@@H](NC)[C@@H](OC)[C@]3(C)O1 PBCZSGKMGDDXIJ-HQCWYSJUSA-N 0.000 description 1
- PBCZSGKMGDDXIJ-UHFFFAOYSA-N 7beta-hydroxystaurosporine Natural products C12=C3N4C5=CC=CC=C5C3=C3C(O)NC(=O)C3=C2C2=CC=CC=C2N1C1CC(NC)C(OC)C4(C)O1 PBCZSGKMGDDXIJ-UHFFFAOYSA-N 0.000 description 1
- 229940125775 ATR kinase inhibitor Drugs 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 241000269586 Ambystoma 'unisexual hybrid' Species 0.000 description 1
- 241000125205 Anethum Species 0.000 description 1
- 108091007914 CDKs Proteins 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 229940123587 Cell cycle inhibitor Drugs 0.000 description 1
- 206010009944 Colon cancer Diseases 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 102000003903 Cyclin-dependent kinases Human genes 0.000 description 1
- 108090000266 Cyclin-dependent kinases Proteins 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- 108010037414 Cytoskeletal Proteins Proteins 0.000 description 1
- 102000010831 Cytoskeletal Proteins Human genes 0.000 description 1
- 230000007035 DNA breakage Effects 0.000 description 1
- 230000004543 DNA replication Effects 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 108010016626 Dipeptides Proteins 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- ZWZWYGMENQVNFU-UHFFFAOYSA-N Glycerophosphorylserin Natural products OC(=O)C(N)COP(O)(=O)OCC(O)CO ZWZWYGMENQVNFU-UHFFFAOYSA-N 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 239000007821 HATU Substances 0.000 description 1
- 108010033040 Histones Proteins 0.000 description 1
- 102000006947 Histones Human genes 0.000 description 1
- XDXDZDZNSLXDNA-TZNDIEGXSA-N Idarubicin Chemical compound C1[C@H](N)[C@H](O)[C@H](C)O[C@H]1O[C@@H]1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2C[C@@](O)(C(C)=O)C1 XDXDZDZNSLXDNA-TZNDIEGXSA-N 0.000 description 1
- XDXDZDZNSLXDNA-UHFFFAOYSA-N Idarubicin Natural products C1C(N)C(O)C(C)OC1OC1C2=C(O)C(C(=O)C3=CC=CC=C3C3=O)=C3C(O)=C2CC(O)(C(C)=O)C1 XDXDZDZNSLXDNA-UHFFFAOYSA-N 0.000 description 1
- FXHOOIRPVKKKFG-UHFFFAOYSA-N N,N-Dimethylacetamide Chemical compound CN(C)C(C)=O FXHOOIRPVKKKFG-UHFFFAOYSA-N 0.000 description 1
- BKAYIFDRRZZKNF-VIFPVBQESA-N N-acetylcarnosine Chemical compound CC(=O)NCCC(=O)N[C@H](C(O)=O)CC1=CN=CN1 BKAYIFDRRZZKNF-VIFPVBQESA-N 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 1
- 101100288143 Rattus norvegicus Klkb1 gene Proteins 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 229920001304 Solutol HS 15 Polymers 0.000 description 1
- 108010017842 Telomerase Proteins 0.000 description 1
- 229940123582 Telomerase inhibitor Drugs 0.000 description 1
- 108091023040 Transcription factor Proteins 0.000 description 1
- 102000040945 Transcription factor Human genes 0.000 description 1
- 102000044209 Tumor Suppressor Genes Human genes 0.000 description 1
- 108700025716 Tumor Suppressor Genes Proteins 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 239000004037 angiogenesis inhibitor Substances 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000000259 anti-tumor effect Effects 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 238000003782 apoptosis assay Methods 0.000 description 1
- 238000010461 azide-alkyne cycloaddition reaction Methods 0.000 description 1
- 230000001588 bifunctional effect Effects 0.000 description 1
- 230000008033 biological extinction Effects 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 230000037396 body weight Effects 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 230000012820 cell cycle checkpoint Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 229940000425 combination drug Drugs 0.000 description 1
- 238000011284 combination treatment Methods 0.000 description 1
- 238000002591 computed tomography Methods 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 229940043378 cyclin-dependent kinase inhibitor Drugs 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 231100000599 cytotoxic agent Toxicity 0.000 description 1
- XCEBOJWFQSQZKR-UHFFFAOYSA-N dbco-nhs Chemical compound C1C2=CC=CC=C2C#CC2=CC=CC=C2N1C(=O)CCC(=O)ON1C(=O)CCC1=O XCEBOJWFQSQZKR-UHFFFAOYSA-N 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 239000000890 drug combination Substances 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 231100000414 gastrointestinal toxicity Toxicity 0.000 description 1
- 229960002584 gefitinib Drugs 0.000 description 1
- 238000005227 gel permeation chromatography Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 229940045109 genistein Drugs 0.000 description 1
- TZBJGXHYKVUXJN-UHFFFAOYSA-N genistein Natural products C1=CC(O)=CC=C1C1=COC2=CC(O)=CC(O)=C2C1=O TZBJGXHYKVUXJN-UHFFFAOYSA-N 0.000 description 1
- 235000006539 genistein Nutrition 0.000 description 1
- ZCOLJUOHXJRHDI-CMWLGVBASA-N genistein 7-O-beta-D-glucoside Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=CC(O)=C2C(=O)C(C=3C=CC(O)=CC=3)=COC2=C1 ZCOLJUOHXJRHDI-CMWLGVBASA-N 0.000 description 1
- 230000009036 growth inhibition Effects 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 229910052739 hydrogen Inorganic materials 0.000 description 1
- 239000001257 hydrogen Substances 0.000 description 1
- 229960000908 idarubicin Drugs 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 239000000411 inducer Substances 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 230000026045 iodination Effects 0.000 description 1
- 238000006192 iodination reaction Methods 0.000 description 1
- 210000003734 kidney Anatomy 0.000 description 1
- 150000002605 large molecules Chemical class 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 230000001926 lymphatic effect Effects 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 231100000682 maximum tolerated dose Toxicity 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 230000009401 metastasis Effects 0.000 description 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 1
- 125000004170 methylsulfonyl group Chemical group [H]C([H])([H])S(*)(=O)=O 0.000 description 1
- 239000000693 micelle Substances 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 230000002438 mitochondrial effect Effects 0.000 description 1
- 230000008811 mitochondrial respiratory chain Effects 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 230000011278 mitosis Effects 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 238000005192 partition Methods 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 230000026731 phosphorylation Effects 0.000 description 1
- 238000006366 phosphorylation reaction Methods 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 231100000614 poison Toxicity 0.000 description 1
- 239000002574 poison Substances 0.000 description 1
- 231100001271 preclinical toxicology Toxicity 0.000 description 1
- 238000002953 preparative HPLC Methods 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 230000005522 programmed cell death Effects 0.000 description 1
- GGOZGYRTNQBSSA-UHFFFAOYSA-N pyridine-2,3-diol Chemical class OC1=CC=CN=C1O GGOZGYRTNQBSSA-UHFFFAOYSA-N 0.000 description 1
- JUJWROOIHBZHMG-UHFFFAOYSA-O pyridinium Chemical compound C1=CC=[NH+]C=C1 JUJWROOIHBZHMG-UHFFFAOYSA-O 0.000 description 1
- 230000008439 repair process Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000028617 response to DNA damage stimulus Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 230000009919 sequestration Effects 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 239000012798 spherical particle Substances 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 239000007929 subcutaneous injection Substances 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 229950004550 talazoparib Drugs 0.000 description 1
- 108091035539 telomere Proteins 0.000 description 1
- 102000055501 telomere Human genes 0.000 description 1
- 210000003411 telomere Anatomy 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 229940126585 therapeutic drug Drugs 0.000 description 1
- 238000002560 therapeutic procedure Methods 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 229960000575 trastuzumab Drugs 0.000 description 1
- 239000000439 tumor marker Substances 0.000 description 1
- 230000005760 tumorsuppression Effects 0.000 description 1
- 230000008728 vascular permeability Effects 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960004355 vindesine Drugs 0.000 description 1
- UGGWPQSBPIFKDZ-KOTLKJBCSA-N vindesine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(N)=O)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1N=C1[C]2C=CC=C1 UGGWPQSBPIFKDZ-KOTLKJBCSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/06—Macromolecular compounds, carriers being organic macromolecular compounds, i.e. organic oligomeric, polymeric, dendrimeric molecules
- A61K51/065—Macromolecular compounds, carriers being organic macromolecular compounds, i.e. organic oligomeric, polymeric, dendrimeric molecules conjugates with carriers being macromolecules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/475—Quinolines; Isoquinolines having an indole ring, e.g. yohimbine, reserpine, strychnine, vinblastine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/4995—Pyrazines or piperazines forming part of bridged ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/50—Pyridazines; Hydrogenated pyridazines
- A61K31/5025—Pyridazines; Hydrogenated pyridazines ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/513—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim having oxo groups directly attached to the heterocyclic ring, e.g. cytosine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/555—Heterocyclic compounds containing heavy metals, e.g. hemin, hematin, melarsoprol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K33/00—Medicinal preparations containing inorganic active ingredients
- A61K33/24—Heavy metals; Compounds thereof
- A61K33/243—Platinum; Compounds thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/69—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit
- A61K47/6921—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere
- A61K47/6927—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores
- A61K47/6929—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores the form being a nanoparticle, e.g. an immuno-nanoparticle
- A61K47/6931—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores the form being a nanoparticle, e.g. an immuno-nanoparticle the material constituting the nanoparticle being a polymer
- A61K47/6935—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the conjugate being characterised by physical or galenical forms, e.g. emulsion, particle, inclusion complex, stent or kit the form being a particulate, a powder, an adsorbate, a bead or a sphere the form being a solid microparticle having no hollow or gas-filled cores the form being a nanoparticle, e.g. an immuno-nanoparticle the material constituting the nanoparticle being a polymer the polymer being obtained otherwise than by reactions involving carbon to carbon unsaturated bonds, e.g. polyesters, polyamides or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Epidemiology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Nanotechnology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Inorganic Chemistry (AREA)
- Biomedical Technology (AREA)
- Immunology (AREA)
- Dermatology (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
- Polyethers (AREA)
Applications Claiming Priority (15)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201862617095P | 2018-01-12 | 2018-01-12 | |
| US62/617,095 | 2018-01-12 | ||
| US201862674483P | 2018-05-21 | 2018-05-21 | |
| US62/674,483 | 2018-05-21 | ||
| US201862700147P | 2018-07-18 | 2018-07-18 | |
| US62/700,147 | 2018-07-18 | ||
| US201862711423P | 2018-07-27 | 2018-07-27 | |
| US201862711421P | 2018-07-27 | 2018-07-27 | |
| US62/711,421 | 2018-07-27 | ||
| US62/711,423 | 2018-07-27 | ||
| US201862716788P | 2018-08-09 | 2018-08-09 | |
| US201862716796P | 2018-08-09 | 2018-08-09 | |
| US62/716,796 | 2018-08-09 | ||
| US62/716,788 | 2018-08-09 | ||
| PCT/US2019/013306 WO2019140266A1 (en) | 2018-01-12 | 2019-01-11 | Protocol for minimizing toxicity of combination dosages and imaging agent for verification |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200110377A true KR20200110377A (ko) | 2020-09-23 |
Family
ID=67218383
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207023337A Ceased KR20200110377A (ko) | 2018-01-12 | 2019-01-11 | 조합 용량의 독성을 최소화하기 위한 프로토콜 및 검증을 위한 조영제 |
| KR1020207023338A Ceased KR20200109345A (ko) | 2018-01-12 | 2019-01-11 | 상승적 암 치료 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020207023338A Ceased KR20200109345A (ko) | 2018-01-12 | 2019-01-11 | 상승적 암 치료 |
Country Status (10)
| Country | Link |
|---|---|
| US (4) | US11730836B2 (enExample) |
| EP (2) | EP3737383A4 (enExample) |
| JP (3) | JP2021510702A (enExample) |
| KR (2) | KR20200110377A (enExample) |
| CN (2) | CN111587126A (enExample) |
| AU (2) | AU2019206623A1 (enExample) |
| CA (2) | CA3087967A1 (enExample) |
| MX (3) | MX2020007306A (enExample) |
| RU (1) | RU2020126774A (enExample) |
| WO (2) | WO2019140271A2 (enExample) |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11634508B2 (en) | 2019-07-10 | 2023-04-25 | Cybrexa 2, Inc. | Peptide conjugates of cytotoxins as therapeutics |
| CN114302744B (zh) | 2019-07-10 | 2025-08-26 | 赛博克萨3公司 | 作为治疗剂的微管靶向剂的肽缀合物 |
| MX2022002340A (es) * | 2019-08-28 | 2022-04-06 | Prolynx Llc | Inhibidores conjugados de la respuesta al daño de adn. |
| EP4509552A1 (en) * | 2022-04-14 | 2025-02-19 | National Institutes for Quantum Science and Technology | Single-polymer particles, active molecular complex, method for producing single-polymer particles, method for measuring tumor size, method for measuring fine structure within tumor, method for imaging biological tissue, drug delivery system, and contrast agent kit |
| WO2024030998A2 (en) * | 2022-08-04 | 2024-02-08 | Prolynx Llc | Methods of treating cancer with long-acting topoisomerase i inhibitor |
| WO2024031005A2 (en) * | 2022-08-04 | 2024-02-08 | Prolynx Llc | Treating cancer with long-acting topoisomerase i inhibitor |
Family Cites Families (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2256133B1 (en) | 1997-01-08 | 2016-12-14 | Sigma-Aldrich Co. LLC | Bioconjugation of macromolecules |
| US7850990B2 (en) | 2001-10-03 | 2010-12-14 | Celator Pharmaceuticals, Inc. | Compositions for delivery of drug combinations |
| CA2491661A1 (en) | 2002-07-12 | 2004-01-22 | Biomarin Pharmaceutical Inc. | The use of isocyanate linkers to make hydrolyzable active agent biopolymer conjugates |
| AU2003280592B2 (en) | 2002-10-31 | 2008-12-18 | Nippon Kayaku Kabushiki Kaisha | High-molecular weight derivatives of camptothecins |
| US20040247624A1 (en) | 2003-06-05 | 2004-12-09 | Unger Evan Charles | Methods of making pharmaceutical formulations for the delivery of drugs having low aqueous solubility |
| LT1675622T (lt) | 2003-09-17 | 2017-09-11 | Nektar Therapeutics | Daugiašakio polimero provaistai |
| US7462627B2 (en) | 2006-02-09 | 2008-12-09 | Enzon Pharmaceuticals, Inc. | Multi-arm polymeric conjugates of 7-ethyl-10-hydroxycamptothecin for treatment of breast, colorectal, pancreatic, ovarian and lung cancers |
| WO2008034124A2 (en) | 2006-09-15 | 2008-03-20 | Enzon Pharmaceuticals, Inc. | Targeted polymeric prodrugs containing multifunctional linkers |
| WO2009141826A2 (en) * | 2008-05-22 | 2009-11-26 | Ramot At Tel Aviv University Ltd. | Novel conjugates of polymers having a therapeutically active agent and an angiogenesis targeting moiety attached thereto and uses thereof in the treatment of angiogenesis related diseases |
| WO2009158668A1 (en) | 2008-06-26 | 2009-12-30 | Prolynx Llc | Prodrugs and drug-macromolecule conjugates having controlled drug release rates |
| KR20110075029A (ko) | 2008-10-21 | 2011-07-05 | 엔즌 파마슈티칼스, 인코포레이티드 | 7-에틸-10-하이드록시캄토테신의 다중-암 고분자 컨쥬게이트로 신경모세포종의 치료 |
| WO2010059661A1 (en) | 2008-11-18 | 2010-05-27 | Baxter International Inc. | Methods of determining polydispersity and/or molecular weight distribution of a polyethylene glycol sample |
| CA2762877A1 (en) | 2009-05-28 | 2010-12-02 | Mersana Therapeutics, Inc. | Polyal drug conjugates comprising variable rate-releasing linkers |
| AU2010321882B2 (en) | 2009-11-18 | 2016-01-14 | Nektar Therapeutics | Salt form of a multi-arm polymer-drug conjugate |
| US8946405B2 (en) | 2010-05-05 | 2015-02-03 | Prolynx Llc | Controlled release from solid supports |
| DK2566335T3 (en) | 2010-05-05 | 2016-10-03 | Prolynx Llc | Controlled release of active ingredients from macromolecular CONJUGATES |
| EP2571496A4 (en) | 2010-05-05 | 2016-03-30 | Prolynx Llc | CONTROLLED RELEASE OF MEDICINE FROM DENIMERS |
| TW201215412A (en) | 2010-08-30 | 2012-04-16 | Sun Pharma Advanced Res Co Ltd | Stable pharmaceutical composition |
| RU2017127088A (ru) * | 2010-11-16 | 2019-02-04 | Эррэй Биофарма Инк. | Комбинация ингибиторов чекпойнт-киназы 1 и ингибиторов киназы wee 1 |
| WO2012088282A1 (en) | 2010-12-21 | 2012-06-28 | Nektar Therapeutics | Multi-arm polymeric prodrug conjugates of pemetrexed-based compounds |
| WO2013049719A1 (en) | 2011-09-30 | 2013-04-04 | Vertex Pharmaceuticals Incorporated | Compounds useful as inhibitors of atr kinase |
| IN2014MN01819A (enExample) * | 2012-03-05 | 2015-07-03 | Univ Ramot | |
| TWI486341B (zh) * | 2012-05-18 | 2015-06-01 | Univ Kaohsiung Medical | 抑制atr與fancd2激活之組成物與方法 |
| WO2014194289A1 (en) | 2013-05-31 | 2014-12-04 | Nektar Therapeutics | Method for predicting and evaluating responsiveness to cancer treatment with dna-damaging chemotherapeutic agents |
| SG11201602258WA (en) * | 2013-10-04 | 2016-04-28 | Prolynx Llc | Slow-release conjugates of sn-38 |
| WO2015066053A2 (en) | 2013-10-28 | 2015-05-07 | Synta Pharmaceuticals Corp. | Targeted therapeutics |
| WO2015118338A1 (en) * | 2014-02-07 | 2015-08-13 | Mission Therapeutics Limited | Methods for exploiting synthetic lethality and chemo-sensitisation in dna damage response (ddr) pathways |
| US20150343100A1 (en) * | 2014-05-28 | 2015-12-03 | Memorial Sloan Kettering Cancer Center | Bimodal fluorophore-labeled liposomes and associated methods and systems |
| CN106573070A (zh) * | 2014-08-13 | 2017-04-19 | 约翰霍普金斯大学 | 树枝状聚合物到脑肿瘤的选择性传递 |
| WO2016028636A1 (en) | 2014-08-16 | 2016-02-25 | Memorial Sloan Kettering Cancer Center | Helical polycarbodiimide polymers and associated imaging, diagnostic, and therapeutic methods |
| JP6378584B2 (ja) | 2014-08-29 | 2018-08-22 | キヤノン株式会社 | 通信システム、画像処理装置、画像処理装置の制御方法、及びプログラム |
| US10196412B2 (en) | 2014-12-24 | 2019-02-05 | The Board Of Trustees Of The Leland Stanford Junior University | Probe for imaging PARP-1 activity |
| EP3277681B1 (de) | 2015-04-02 | 2019-05-08 | Merck Patent GmbH | Imidazolonylchinoline und deren verwendung als atm kinase inhibitoren |
| CA2980462C (en) * | 2015-04-07 | 2023-08-01 | The Curators Of The University Of Missouri | Nanoparticle immunoconjugates |
| WO2016176462A1 (en) * | 2015-04-28 | 2016-11-03 | University Of Central Florida Foundation, Inc. | Methods and compositions for theranostic nanoparticles |
| WO2017031442A1 (en) | 2015-08-20 | 2017-02-23 | Merrimack Pharmaceuticals, Inc. | Combination therapy using liposomal irinotecan and a parp inhibitor for cancer treatment |
| WO2017105565A2 (en) | 2015-09-10 | 2017-06-22 | Memorial Sloan Kettering Cancer Center | Compositions for therapeutics, targeted pet imaging and methods of their use |
| WO2017059397A1 (en) | 2015-10-01 | 2017-04-06 | Whitehead Institute For Biomedical Research | Labeling of antibodies |
| US11154538B2 (en) | 2016-02-29 | 2021-10-26 | Synta Pharmaceuticals Corporation | Combination therapy for treatment of ovarian cancer |
| WO2017172678A1 (en) | 2016-03-30 | 2017-10-05 | Merrimack Pharmaceuticals, Inc. | Methods for treating cancer using combination therapies comprising an oligoclonal anti-egfr antibody preparation and lipsomal irinotecan |
| AU2017257254B2 (en) * | 2016-04-27 | 2022-02-24 | Immunomedics, Inc. | Efficacy of anti-Trop-2-SN-38 antibody drug conjugates for therapy of tumors relapsed/refractory to checkpoint inhibitors |
| AU2017258415B2 (en) * | 2016-04-29 | 2023-03-30 | Cornell University | Compositions and methods for targeted particle penetration, distribution, and response in malignant brain tumors |
| CN106084005B (zh) | 2016-06-15 | 2020-02-14 | 广州军区广州总医院 | 靶向生长抑素受体的Al18F-NOTA-PEG6-TATE及其制备方法和应用 |
| CN107050471A (zh) * | 2016-12-27 | 2017-08-18 | 中国药科大学 | 基于富勒烯的靶向且具有快速清除特征的显像纳米探针及其制备方法和应用 |
-
2019
- 2019-01-11 US US16/961,640 patent/US11730836B2/en active Active
- 2019-01-11 WO PCT/US2019/013314 patent/WO2019140271A2/en not_active Ceased
- 2019-01-11 WO PCT/US2019/013306 patent/WO2019140266A1/en not_active Ceased
- 2019-01-11 CN CN201980008252.XA patent/CN111587126A/zh active Pending
- 2019-01-11 JP JP2020538844A patent/JP2021510702A/ja active Pending
- 2019-01-11 US US16/961,633 patent/US20200360545A1/en not_active Abandoned
- 2019-01-11 KR KR1020207023337A patent/KR20200110377A/ko not_active Ceased
- 2019-01-11 AU AU2019206623A patent/AU2019206623A1/en not_active Abandoned
- 2019-01-11 CA CA3087967A patent/CA3087967A1/en active Pending
- 2019-01-11 EP EP19738883.8A patent/EP3737383A4/en not_active Withdrawn
- 2019-01-11 AU AU2019208024A patent/AU2019208024A1/en not_active Abandoned
- 2019-01-11 EP EP19738881.2A patent/EP3737416A4/en not_active Withdrawn
- 2019-01-11 KR KR1020207023338A patent/KR20200109345A/ko not_active Ceased
- 2019-01-11 CA CA3087628A patent/CA3087628A1/en active Pending
- 2019-01-11 RU RU2020126774A patent/RU2020126774A/ru unknown
- 2019-01-11 MX MX2020007306A patent/MX2020007306A/es unknown
- 2019-01-11 CN CN201980008227.1A patent/CN111587115A/zh active Pending
- 2019-01-11 MX MX2020007442A patent/MX2020007442A/es unknown
- 2019-01-11 JP JP2020538819A patent/JP2021510701A/ja active Pending
-
2020
- 2020-07-13 MX MX2023005786A patent/MX2023005786A/es unknown
-
2023
- 2023-03-07 US US18/118,650 patent/US20230321286A1/en not_active Abandoned
- 2023-07-11 US US18/220,742 patent/US20240082436A1/en not_active Abandoned
- 2023-10-31 JP JP2023186470A patent/JP7703001B2/ja active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US20230321286A1 (en) | 2023-10-12 |
| CN111587126A (zh) | 2020-08-25 |
| MX2020007442A (es) | 2020-09-14 |
| EP3737416A4 (en) | 2022-03-30 |
| EP3737416A1 (en) | 2020-11-18 |
| AU2019208024A1 (en) | 2020-08-13 |
| US20200397778A1 (en) | 2020-12-24 |
| US20240082436A1 (en) | 2024-03-14 |
| JP2021510701A (ja) | 2021-04-30 |
| RU2020126853A3 (enExample) | 2022-02-14 |
| US11730836B2 (en) | 2023-08-22 |
| KR20200109345A (ko) | 2020-09-22 |
| JP7703001B2 (ja) | 2025-07-04 |
| MX2020007306A (es) | 2020-09-25 |
| CA3087967A1 (en) | 2019-07-18 |
| WO2019140266A1 (en) | 2019-07-18 |
| JP2024012442A (ja) | 2024-01-30 |
| WO2019140271A2 (en) | 2019-07-18 |
| RU2020126774A (ru) | 2022-02-14 |
| EP3737383A2 (en) | 2020-11-18 |
| CN111587115A (zh) | 2020-08-25 |
| MX2023005786A (es) | 2023-05-29 |
| EP3737383A4 (en) | 2021-12-15 |
| US20200360545A1 (en) | 2020-11-19 |
| WO2019140271A3 (en) | 2019-08-22 |
| RU2020126853A (ru) | 2022-02-14 |
| JP2021510702A (ja) | 2021-04-30 |
| CA3087628A1 (en) | 2019-07-18 |
| AU2019206623A1 (en) | 2020-07-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20200110377A (ko) | 조합 용량의 독성을 최소화하기 위한 프로토콜 및 검증을 위한 조영제 | |
| US20240408251A1 (en) | Compositions and methods for targeted particle penetration, distribution, and response in malignant brain tumors | |
| Kotagiri et al. | Radionuclides transform chemotherapeutics into phototherapeutics for precise treatment of disseminated cancer | |
| Hendrikse et al. | Visualization of multidrug resistance in vivo | |
| Vasey et al. | Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl) methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents—drug-polymer conjugates | |
| KR102087854B1 (ko) | 단백질-중합체-약물 접합체 | |
| Valetti et al. | Peptide-functionalized nanoparticles for selective targeting of pancreatic tumor | |
| US20120258042A1 (en) | Combined use of tgf-b signaling inhibitor and antitumor agent | |
| JP2023504286A (ja) | 薬物送達のためのデンドリマー組成物および方法 | |
| Bellat et al. | Functional Peptide Nanofibers with Unique Tumor Targeting and Enzyme‐Induced Local Retention Properties | |
| TWI431014B (zh) | 腫瘤標靶胜肽及其於檢測及治療癌症之用途 | |
| Madajewski et al. | Molecular engineering of ultrasmall silica nanoparticle–drug conjugates as lung cancer therapeutics | |
| AU2019345320B2 (en) | Methods of treating cancer | |
| Del Vecchio et al. | Scintigraphic detection of multidrug resistance in cancer | |
| WO2013106824A1 (en) | Epherin receptor targeting agents | |
| Hendrikse | Monitoring interactions at ATP-dependent drug efflux pumps. | |
| US20210388361A1 (en) | Modified nucleic acid having improved treatment efficacy, and anticancer pharmaceutical composition containing same | |
| Liu et al. | Exploring the impact of PEGylation on pharmacokinetics: a size-dependent effect of polyethylene glycol on prostate-specific membrane antigen inhibitors | |
| US20250312498A1 (en) | Protocol for minimizing toxicity of combination dosages and imaging agent for verification | |
| Kong et al. | Multifunctional Probe Based on “Chemical Antibody–Aptamer” for Noninvasive Detection of PD-L1 Expression in Cancer | |
| US20240374763A1 (en) | Diagnosis and monitoring using extradomain-b fibronectin targeted probes | |
| EP3868774A1 (en) | Modified nucleic acid having improved treatment efficacy, and anticancer pharmaceutical composition containing same | |
| US20220118116A1 (en) | Adhesive/adsorption switch on nanoparticles to increase tumor uptake and delay tumor clearance | |
| Gardeen et al. | Use of carbonic anhydrase IX inhibitors for selective delivery of attached drugs to solid tumors | |
| TWI904072B (zh) | 治療癌症之方法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20200812 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20220111 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20240328 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20241125 Comment text: Decision to Refuse Application Patent event code: PE06012S01D |