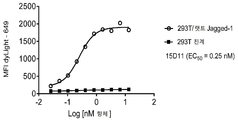
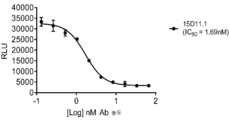
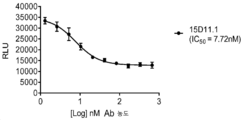
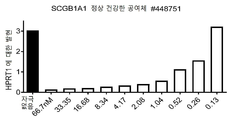
KR20200012848A - 항-jagged1 항원 결합 단백질 - Google Patents
항-jagged1 항원 결합 단백질 Download PDFInfo
- Publication number
- KR20200012848A KR20200012848A KR1020197032633A KR20197032633A KR20200012848A KR 20200012848 A KR20200012848 A KR 20200012848A KR 1020197032633 A KR1020197032633 A KR 1020197032633A KR 20197032633 A KR20197032633 A KR 20197032633A KR 20200012848 A KR20200012848 A KR 20200012848A
- Authority
- KR
- South Korea
- Prior art keywords
- seq
- variable region
- chain variable
- antibody
- antigen binding
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 102000025171 antigen binding proteins Human genes 0.000 title claims abstract 22
- 108091000831 antigen binding proteins Proteins 0.000 title claims abstract 22
- 238000000034 method Methods 0.000 claims abstract 21
- 102000049556 Jagged-1 Human genes 0.000 claims abstract 3
- 208000019693 Lung disease Diseases 0.000 claims abstract 2
- 239000012634 fragment Substances 0.000 claims 14
- 102000039446 nucleic acids Human genes 0.000 claims 4
- 108020004707 nucleic acids Proteins 0.000 claims 4
- 150000007523 nucleic acids Chemical class 0.000 claims 4
- 125000003275 alpha amino acid group Chemical group 0.000 claims 3
- 101000994437 Homo sapiens Protein jagged-1 Proteins 0.000 claims 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 claims 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 claims 2
- 102000056036 human JAG1 Human genes 0.000 claims 2
- 101000994439 Danio rerio Protein jagged-1a Proteins 0.000 claims 1
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 claims 1
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 claims 1
- 241000124008 Mammalia Species 0.000 claims 1
- 239000000427 antigen Substances 0.000 claims 1
- 102000036639 antigens Human genes 0.000 claims 1
- 108091007433 antigens Proteins 0.000 claims 1
- 238000002372 labelling Methods 0.000 claims 1
- 239000008194 pharmaceutical composition Substances 0.000 claims 1
- 239000000546 pharmaceutical excipient Substances 0.000 claims 1
- 102000004169 proteins and genes Human genes 0.000 claims 1
- 108090000623 proteins and genes Proteins 0.000 claims 1
- 230000003248 secreting effect Effects 0.000 claims 1
- 238000005507 spraying Methods 0.000 claims 1
- 238000010254 subcutaneous injection Methods 0.000 claims 1
- 239000007929 subcutaneous injection Substances 0.000 claims 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/3955—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against proteinaceous materials, e.g. enzymes, hormones, lymphokines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/22—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against growth factors ; against growth regulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2896—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against molecules with a "CD"-designation, not provided for elsewhere
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/545—Medicinal preparations containing antigens or antibodies characterised by the dose, timing or administration schedule
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39558—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against tumor tissues, cells, antigens
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/54—F(ab')2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/20—Fusion polypeptide containing a tag with affinity for a non-protein ligand
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Endocrinology (AREA)
- Microbiology (AREA)
- Mycology (AREA)
- Epidemiology (AREA)
- Pulmonology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762512805P | 2017-05-31 | 2017-05-31 | |
| US62/512,805 | 2017-05-31 | ||
| PCT/US2018/035209 WO2018222770A1 (en) | 2017-05-31 | 2018-05-30 | Anti-jagged1 antigen binding proteins |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20200012848A true KR20200012848A (ko) | 2020-02-05 |
Family
ID=62683483
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197032633A Ceased KR20200012848A (ko) | 2017-05-31 | 2018-05-30 | 항-jagged1 항원 결합 단백질 |
Country Status (23)
| Country | Link |
|---|---|
| US (3) | US11028180B2 (enExample) |
| EP (1) | EP3630820A1 (enExample) |
| JP (1) | JP7222614B2 (enExample) |
| KR (1) | KR20200012848A (enExample) |
| CN (1) | CN110621695B (enExample) |
| AR (1) | AR111767A1 (enExample) |
| AU (1) | AU2018275231B2 (enExample) |
| BR (1) | BR112019023118A2 (enExample) |
| CA (1) | CA3059975A1 (enExample) |
| CL (1) | CL2019003167A1 (enExample) |
| CO (1) | CO2019012204A2 (enExample) |
| CR (1) | CR20190513A (enExample) |
| EA (1) | EA201992348A1 (enExample) |
| JO (1) | JOP20190259A1 (enExample) |
| MA (1) | MA48777A (enExample) |
| MX (1) | MX2019013211A (enExample) |
| PE (1) | PE20200487A1 (enExample) |
| PH (1) | PH12019502449A1 (enExample) |
| SG (1) | SG11201909529XA (enExample) |
| TW (1) | TW201908339A (enExample) |
| UY (1) | UY37752A (enExample) |
| WO (1) | WO2018222770A1 (enExample) |
| ZA (1) | ZA201906821B (enExample) |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2936565C (en) * | 2014-02-12 | 2020-08-11 | Genentech, Inc. | Anti-jagged1 antibodies and methods of use |
| JOP20190259A1 (ar) * | 2017-05-31 | 2019-10-31 | Amgen Inc | بروتينات ربط مولد ضد مضادة لـ jagged1 |
| CN111471643B (zh) * | 2020-04-09 | 2020-12-29 | 创芯国际生物科技(广州)有限公司 | 一种通用型上呼吸道粘膜类器官的培养基及培养方法 |
Family Cites Families (76)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3773919A (en) | 1969-10-23 | 1973-11-20 | Du Pont | Polylactide-drug mixtures |
| US4263428A (en) | 1978-03-24 | 1981-04-21 | The Regents Of The University Of California | Bis-anthracycline nucleic acid function inhibitors and improved method for administering the same |
| US4399216A (en) | 1980-02-25 | 1983-08-16 | The Trustees Of Columbia University | Processes for inserting DNA into eucaryotic cells and for producing proteinaceous materials |
| IE52535B1 (en) | 1981-02-16 | 1987-12-09 | Ici Plc | Continuous release pharmaceutical compositions |
| EP0088046B1 (de) | 1982-02-17 | 1987-12-09 | Ciba-Geigy Ag | Lipide in wässriger Phase |
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| HUT35524A (en) | 1983-08-02 | 1985-07-29 | Hoechst Ag | Process for preparing pharmaceutical compositions containing regulatory /regulative/ peptides providing for the retarded release of the active substance |
| US4615885A (en) | 1983-11-01 | 1986-10-07 | Terumo Kabushiki Kaisha | Pharmaceutical composition containing urokinase |
| US4740461A (en) | 1983-12-27 | 1988-04-26 | Genetics Institute, Inc. | Vectors and methods for transformation of eucaryotic cells |
| US4959455A (en) | 1986-07-14 | 1990-09-25 | Genetics Institute, Inc. | Primate hematopoietic growth factors IL-3 and pharmaceutical compositions |
| DE3785186T2 (de) | 1986-09-02 | 1993-07-15 | Enzon Lab Inc | Bindungsmolekuele mit einzelpolypeptidkette. |
| US4946778A (en) | 1987-09-21 | 1990-08-07 | Genex Corporation | Single polypeptide chain binding molecules |
| US5260203A (en) | 1986-09-02 | 1993-11-09 | Enzon, Inc. | Single polypeptide chain binding molecules |
| US4912040A (en) | 1986-11-14 | 1990-03-27 | Genetics Institute, Inc. | Eucaryotic expression system |
| US5011912A (en) | 1986-12-19 | 1991-04-30 | Immunex Corporation | Hybridoma and monoclonal antibody for use in an immunoaffinity purification system |
| US4965195A (en) | 1987-10-26 | 1990-10-23 | Immunex Corp. | Interleukin-7 |
| US4968607A (en) | 1987-11-25 | 1990-11-06 | Immunex Corporation | Interleukin-1 receptors |
| GB8823869D0 (en) | 1988-10-12 | 1988-11-16 | Medical Res Council | Production of antibodies |
| AU643427B2 (en) | 1988-10-31 | 1993-11-18 | Immunex Corporation | Interleukin-4 receptors |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5683888A (en) | 1989-07-22 | 1997-11-04 | University Of Wales College Of Medicine | Modified bioluminescent proteins and their use |
| US5292658A (en) | 1989-12-29 | 1994-03-08 | University Of Georgia Research Foundation, Inc. Boyd Graduate Studies Research Center | Cloning and expressions of Renilla luciferase |
| EP1690935A3 (en) | 1990-01-12 | 2008-07-30 | Abgenix, Inc. | Generation of xenogeneic antibodies |
| US6713610B1 (en) | 1990-01-12 | 2004-03-30 | Raju Kucherlapati | Human antibodies derived from immunized xenomice |
| US6673986B1 (en) | 1990-01-12 | 2004-01-06 | Abgenix, Inc. | Generation of xenogeneic antibodies |
| AU651596B2 (en) | 1990-06-05 | 1994-07-28 | Immunex Corporation | Type II interleukin-1 receptors |
| US5789650A (en) | 1990-08-29 | 1998-08-04 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5874299A (en) | 1990-08-29 | 1999-02-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5814318A (en) | 1990-08-29 | 1998-09-29 | Genpharm International Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5633425A (en) | 1990-08-29 | 1997-05-27 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US6255458B1 (en) | 1990-08-29 | 2001-07-03 | Genpharm International | High affinity human antibodies and human antibodies against digoxin |
| US6300129B1 (en) | 1990-08-29 | 2001-10-09 | Genpharm International | Transgenic non-human animals for producing heterologous antibodies |
| JP2938569B2 (ja) | 1990-08-29 | 1999-08-23 | ジェンファーム インターナショナル,インコーポレイティド | 異種免疫グロブリンを作る方法及びトランスジェニックマウス |
| US5625126A (en) | 1990-08-29 | 1997-04-29 | Genpharm International, Inc. | Transgenic non-human animals for producing heterologous antibodies |
| US5770429A (en) | 1990-08-29 | 1998-06-23 | Genpharm International, Inc. | Transgenic non-human animals capable of producing heterologous antibodies |
| US5545806A (en) | 1990-08-29 | 1996-08-13 | Genpharm International, Inc. | Ransgenic non-human animals for producing heterologous antibodies |
| US5661016A (en) | 1990-08-29 | 1997-08-26 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| US5877397A (en) | 1990-08-29 | 1999-03-02 | Genpharm International Inc. | Transgenic non-human animals capable of producing heterologous antibodies of various isotypes |
| EP0575319B1 (en) | 1991-03-11 | 1999-11-10 | The University Of Georgia Research Foundation, Inc. | Cloning and expression of renilla luciferase |
| US6565841B1 (en) | 1991-03-15 | 2003-05-20 | Amgen, Inc. | Pulmonary administration of granulocyte colony stimulating factor |
| AU2249592A (en) | 1991-06-14 | 1993-01-12 | Dnx Corporation | Production of human hemoglobin in transgenic pigs |
| DE69224906T2 (de) | 1991-07-08 | 1998-10-29 | Univ Massachusetts | Thermotropes flüssig-kristallines segment-blockcopolymer |
| US6004924A (en) | 1991-12-11 | 1999-12-21 | Imperial Cancer Research Technology, Ltd. | Protein sequences of serrate gene products |
| WO1993015722A1 (en) | 1992-02-07 | 1993-08-19 | Syntex (Usa) Inc. | Controlled delivery of pharmaceuticals from preformed porous microparticles |
| SG48760A1 (en) | 1992-07-24 | 2003-03-18 | Abgenix Inc | Generation of xenogenetic antibodies |
| ATE400651T1 (de) | 1993-09-10 | 2008-07-15 | Univ Columbia | Verwendung von grünem fluoreszenzprotein |
| WO1995021191A1 (en) | 1994-02-04 | 1995-08-10 | William Ward | Bioluminescent indicator based upon the expression of a gene for a modified green-fluorescent protein |
| US5777079A (en) | 1994-11-10 | 1998-07-07 | The Regents Of The University Of California | Modified green fluorescent proteins |
| DE69637481T2 (de) | 1995-04-27 | 2009-04-09 | Amgen Fremont Inc. | Aus immunisierten Xenomäusen stammende menschliche Antikörper gegen IL-8 |
| US5874304A (en) | 1996-01-18 | 1999-02-23 | University Of Florida Research Foundation, Inc. | Humanized green fluorescent protein genes and methods |
| US5804387A (en) | 1996-02-01 | 1998-09-08 | The Board Of Trustees Of The Leland Stanford Junior University | FACS-optimized mutants of the green fluorescent protein (GFP) |
| US5876995A (en) | 1996-02-06 | 1999-03-02 | Bryan; Bruce | Bioluminescent novelty items |
| US5925558A (en) | 1996-07-16 | 1999-07-20 | The Regents Of The University Of California | Assays for protein kinases using fluorescent protein substrates |
| US5976796A (en) | 1996-10-04 | 1999-11-02 | Loma Linda University | Construction and expression of renilla luciferase and green fluorescent protein fusion genes |
| ES2301183T3 (es) | 1996-12-03 | 2008-06-16 | Amgen Fremont Inc. | Anticuerpo completamente humano que se une al receptor del egfr. |
| EP1015872B1 (en) | 1996-12-12 | 2005-03-02 | Prolume, Ltd. | Apparatus and method for detecting and identifying infectious agents |
| CA2196496A1 (en) | 1997-01-31 | 1998-07-31 | Stephen William Watson Michnick | Protein fragment complementation assay for the detection of protein-protein interactions |
| US6136952A (en) * | 1997-06-25 | 2000-10-24 | University Of Washington | Human jagged polypeptide, encoding nucleic acids and methods of use |
| US6342220B1 (en) | 1997-08-25 | 2002-01-29 | Genentech, Inc. | Agonist antibodies |
| DE69938293T2 (de) | 1998-03-27 | 2009-03-12 | Bruce J. Beverly Hills Bryan | Luciferase, gfp fluoreszenzproteine, kodierende nukleinsaüre und ihre verwendung in der diagnose |
| US20050232927A1 (en) * | 2004-02-03 | 2005-10-20 | The Regents Of The University Of Michigan | Compositions and methods for characterizing, regulating, diagnosing, and treating cancer |
| US8802103B2 (en) | 2007-05-15 | 2014-08-12 | Oncomed Pharmaceuticals, Inc. | Compositions and methods for diagnosing and treating cancer |
| US8557965B2 (en) | 2008-04-07 | 2013-10-15 | Ablynx N.V. | Single variable domains against notch pathway members |
| WO2011063237A2 (en) | 2009-11-19 | 2011-05-26 | Oncomed Pharmaceuticals, Inc. | Jagged-binding agents and uses thereof |
| WO2012106529A1 (en) * | 2011-02-02 | 2012-08-09 | The Trustees Of Princeton University | Jagged1 as a marker and therapeutic target for breast cancer bone metastasis |
| JP5782185B2 (ja) | 2012-06-01 | 2015-09-24 | 日本電信電話株式会社 | パケット転送処理方法およびパケット転送処理装置 |
| BR112014031689A2 (pt) * | 2012-06-22 | 2017-07-25 | Cytomx Therapeutics Inc | anticorpos reativos cruzados anti-jagged 1/jagged 2, anticorpos anti-jagged ativáveis e métodos de uso deles |
| MX357675B (es) * | 2012-08-13 | 2018-07-18 | Genentech Inc | Anticuerpos anti-jagged y métodos de uso. |
| WO2014110506A2 (en) * | 2013-01-14 | 2014-07-17 | The Trustees Of Columbia University In The City Of New York | Methods of treating, preventing and diagnosing leukemia and other blood diseases and disorders |
| GB201300706D0 (en) | 2013-01-15 | 2013-02-27 | Cancer Rec Tech Ltd | Antibody |
| MX2015011444A (es) * | 2013-03-15 | 2015-12-16 | Genentech Inc | Composiciones y metodos para el diagnostico y tratamiento del cancer hepatico. |
| CA2936565C (en) | 2014-02-12 | 2020-08-11 | Genentech, Inc. | Anti-jagged1 antibodies and methods of use |
| US9300829B2 (en) | 2014-04-04 | 2016-03-29 | Canon Kabushiki Kaisha | Image reading apparatus and correction method thereof |
| KR101599370B1 (ko) * | 2014-04-24 | 2016-03-03 | 한국생명공학연구원 | Jagged 1에 대한 인간 단일클론항체 J1J22 |
| US10435476B2 (en) * | 2015-03-06 | 2019-10-08 | Sorrento Therapeutics, Inc. | Antibody therapeutics that bind JAG1 |
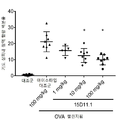
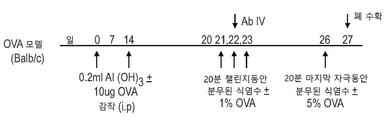
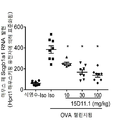
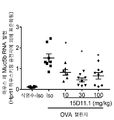
| JOP20190259A1 (ar) * | 2017-05-31 | 2019-10-31 | Amgen Inc | بروتينات ربط مولد ضد مضادة لـ jagged1 |
-
2017
- 2017-06-16 JO JOP/2019/0259A patent/JOP20190259A1/ar unknown
-
2018
- 2018-05-30 EA EA201992348A patent/EA201992348A1/ru unknown
- 2018-05-30 EP EP18732590.7A patent/EP3630820A1/en active Pending
- 2018-05-30 SG SG11201909529X patent/SG11201909529XA/en unknown
- 2018-05-30 CR CR20190513A patent/CR20190513A/es unknown
- 2018-05-30 KR KR1020197032633A patent/KR20200012848A/ko not_active Ceased
- 2018-05-30 US US15/993,448 patent/US11028180B2/en active Active
- 2018-05-30 CN CN201880029832.2A patent/CN110621695B/zh active Active
- 2018-05-30 BR BR112019023118-8A patent/BR112019023118A2/pt not_active Application Discontinuation
- 2018-05-30 MA MA048777A patent/MA48777A/fr unknown
- 2018-05-30 MX MX2019013211A patent/MX2019013211A/es unknown
- 2018-05-30 CA CA3059975A patent/CA3059975A1/en active Pending
- 2018-05-30 PE PE2019002278A patent/PE20200487A1/es unknown
- 2018-05-30 WO PCT/US2018/035209 patent/WO2018222770A1/en not_active Ceased
- 2018-05-30 AU AU2018275231A patent/AU2018275231B2/en active Active
- 2018-05-31 AR ARP180101447A patent/AR111767A1/es unknown
- 2018-05-31 JP JP2018104357A patent/JP7222614B2/ja active Active
- 2018-05-31 UY UY0001037752A patent/UY37752A/es not_active Application Discontinuation
- 2018-05-31 TW TW107118769A patent/TW201908339A/zh unknown
-
2019
- 2019-10-16 ZA ZA2019/06821A patent/ZA201906821B/en unknown
- 2019-10-29 PH PH12019502449A patent/PH12019502449A1/en unknown
- 2019-10-30 CO CONC2019/0012204A patent/CO2019012204A2/es unknown
- 2019-11-04 CL CL2019003167A patent/CL2019003167A1/es unknown
-
2021
- 2021-05-13 US US17/320,128 patent/US11897963B2/en active Active
-
2022
- 2022-02-16 US US17/672,889 patent/US11897965B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| PE20200487A1 (es) | 2020-03-03 |
| AU2018275231B2 (en) | 2024-09-12 |
| CO2019012204A2 (es) | 2020-02-07 |
| WO2018222770A1 (en) | 2018-12-06 |
| CL2019003167A1 (es) | 2020-03-20 |
| EP3630820A1 (en) | 2020-04-08 |
| CN110621695A (zh) | 2019-12-27 |
| US20210340270A1 (en) | 2021-11-04 |
| US11897963B2 (en) | 2024-02-13 |
| ZA201906821B (en) | 2021-06-30 |
| JOP20190259A1 (ar) | 2019-10-31 |
| US20220169744A1 (en) | 2022-06-02 |
| JP7222614B2 (ja) | 2023-02-15 |
| PH12019502449A1 (en) | 2020-07-20 |
| TW201908339A (zh) | 2019-03-01 |
| EA201992348A1 (ru) | 2020-03-24 |
| CR20190513A (es) | 2020-01-17 |
| US20190023802A1 (en) | 2019-01-24 |
| JP2018203735A (ja) | 2018-12-27 |
| US11028180B2 (en) | 2021-06-08 |
| AR111767A1 (es) | 2019-08-14 |
| AU2018275231A1 (en) | 2019-10-31 |
| MX2019013211A (es) | 2020-07-27 |
| MA48777A (fr) | 2020-04-08 |
| UY37752A (es) | 2019-01-02 |
| CN110621695B (zh) | 2023-10-17 |
| CA3059975A1 (en) | 2018-12-06 |
| BR112019023118A2 (pt) | 2020-10-20 |
| SG11201909529XA (en) | 2019-11-28 |
| US11897965B2 (en) | 2024-02-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| AU2014312184B2 (en) | GITR antigen binding proteins | |
| DK2711375T3 (en) | Human antigen-binding proteins that bind Beta-Klotho, FGF receptors and complexes thereof | |
| KR101017301B1 (ko) | 앤지오포이에틴-2에 대한 항체 및 그의 용도 | |
| TWI688403B (zh) | 人類cgrp受體結合蛋白 | |
| KR101516569B1 (ko) | 사람 델타 유사 리간드 4에 대한 사람 항체 | |
| KR101531422B1 (ko) | Pdgfr-알파에 대한 표적화된 결합제 및 그의 용도 | |
| KR20110057244A (ko) | Dll4에 대한 항체 및 이의 용도 | |
| JP2017002084A (ja) | T細胞の免疫グロブリンドメインおよびムチンドメイン1(tim−1)抗原に対する抗体を用いて卵巣癌および腎臓癌を処置する方法 | |
| AU2022202539A1 (en) | Antibodies, uses & methods | |
| KR20080113278A (ko) | uPAR에 대한 표적화 결합제 및 이의 용도 | |
| JP2012502967A (ja) | ソニック・ヘッジホッグホモログに対する抗体およびその使用 | |
| AU2014235430A1 (en) | Human antigen binding proteins that bind to proprotein convertase subtilisin kexin type 9 (PCSK9) | |
| KR20080031001A (ko) | Cd20에 대한 항체 및 그의 용도 | |
| US11897965B2 (en) | Anti-Jagged1 antigen binding proteins | |
| KR20180081465A (ko) | 항 α-syn 항체 및 그 용도 | |
| KR20200128125A (ko) | 신체 조성을 변경하기 위한 방법 | |
| KR20210104064A (ko) | 항-il-27 항체 및 그의 용도 | |
| EA045562B1 (ru) | Антигенсвязывающие белки, взаимодействующие с jagged1 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20191104 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210525 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230717 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240201 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230717 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |