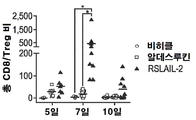
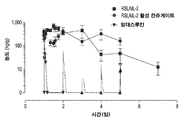
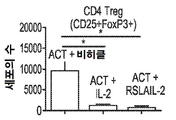
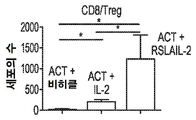
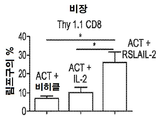
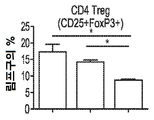
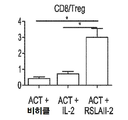
KR20190121773A - 입양 세포 이식 요법과 조합된 인터루킨-2 수용체 알파, 베타 선택성 효현제를 이용한 면역 요법적 종양 치료 방법 - Google Patents
입양 세포 이식 요법과 조합된 인터루킨-2 수용체 알파, 베타 선택성 효현제를 이용한 면역 요법적 종양 치료 방법 Download PDFInfo
- Publication number
- KR20190121773A KR20190121773A KR1020197024673A KR20197024673A KR20190121773A KR 20190121773 A KR20190121773 A KR 20190121773A KR 1020197024673 A KR1020197024673 A KR 1020197024673A KR 20197024673 A KR20197024673 A KR 20197024673A KR 20190121773 A KR20190121773 A KR 20190121773A
- Authority
- KR
- South Korea
- Prior art keywords
- cells
- cancer
- biasing
- tumor
- agonist
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 230
- 239000000556 agonist Substances 0.000 title claims abstract description 122
- 238000002054 transplantation Methods 0.000 title abstract description 56
- 238000002560 therapeutic procedure Methods 0.000 title abstract description 22
- 108010032774 Interleukin-2 Receptor alpha Subunit Proteins 0.000 title description 2
- 102000007351 Interleukin-2 Receptor alpha Subunit Human genes 0.000 title description 2
- 238000009169 immunotherapy Methods 0.000 title description 2
- 238000011282 treatment Methods 0.000 claims abstract description 113
- 238000000034 method Methods 0.000 claims abstract description 86
- 201000011510 cancer Diseases 0.000 claims abstract description 75
- 108010002350 Interleukin-2 Proteins 0.000 claims description 147
- 102000000588 Interleukin-2 Human genes 0.000 claims description 147
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 87
- 210000003289 regulatory T cell Anatomy 0.000 claims description 53
- 229920001223 polyethylene glycol Polymers 0.000 claims description 47
- 108700025316 aldesleukin Proteins 0.000 claims description 30
- 229960005310 aldesleukin Drugs 0.000 claims description 30
- 239000002202 Polyethylene glycol Substances 0.000 claims description 21
- 238000000338 in vitro Methods 0.000 claims description 19
- 230000004044 response Effects 0.000 claims description 19
- 230000002459 sustained effect Effects 0.000 claims description 19
- 230000004913 activation Effects 0.000 claims description 18
- 238000001727 in vivo Methods 0.000 claims description 15
- 210000003162 effector t lymphocyte Anatomy 0.000 claims description 13
- 150000003839 salts Chemical class 0.000 claims description 13
- 241000282414 Homo sapiens Species 0.000 claims description 11
- 239000007787 solid Substances 0.000 claims description 11
- 150000001875 compounds Chemical class 0.000 claims description 9
- 238000001802 infusion Methods 0.000 claims description 9
- 230000000717 retained effect Effects 0.000 claims description 7
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 claims description 6
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 claims description 6
- 102100026878 Interleukin-2 receptor subunit alpha Human genes 0.000 claims description 6
- 238000009825 accumulation Methods 0.000 claims description 6
- 125000003983 fluorenyl group Chemical group C1(=CC=CC=2C3=CC=CC=C3CC12)* 0.000 claims description 6
- 239000007788 liquid Substances 0.000 claims description 6
- 238000002347 injection Methods 0.000 claims description 5
- 239000007924 injection Substances 0.000 claims description 5
- 230000002401 inhibitory effect Effects 0.000 claims description 4
- 210000003171 tumor-infiltrating lymphocyte Anatomy 0.000 claims description 4
- 238000010171 animal model Methods 0.000 claims description 3
- 230000001394 metastastic effect Effects 0.000 claims description 2
- 206010061289 metastatic neoplasm Diseases 0.000 claims description 2
- 125000003827 glycol group Chemical group 0.000 claims 2
- 230000009286 beneficial effect Effects 0.000 claims 1
- 230000005764 inhibitory process Effects 0.000 claims 1
- 230000001568 sexual effect Effects 0.000 claims 1
- 239000000203 mixture Substances 0.000 abstract description 22
- 210000004027 cell Anatomy 0.000 description 147
- 101000946843 Homo sapiens T-cell surface glycoprotein CD8 alpha chain Proteins 0.000 description 60
- 239000003981 vehicle Substances 0.000 description 37
- 210000004698 lymphocyte Anatomy 0.000 description 36
- 229920000642 polymer Polymers 0.000 description 29
- 201000001441 melanoma Diseases 0.000 description 24
- 210000000952 spleen Anatomy 0.000 description 23
- 230000001965 increasing effect Effects 0.000 description 21
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 19
- 241000699670 Mus sp. Species 0.000 description 17
- 238000011467 adoptive cell therapy Methods 0.000 description 17
- 230000000694 effects Effects 0.000 description 16
- 230000000259 anti-tumor effect Effects 0.000 description 15
- 230000004614 tumor growth Effects 0.000 description 14
- 241001529936 Murinae Species 0.000 description 13
- 108091008874 T cell receptors Proteins 0.000 description 12
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 12
- 239000000427 antigen Substances 0.000 description 11
- 102000036639 antigens Human genes 0.000 description 11
- 108091007433 antigens Proteins 0.000 description 11
- 238000002648 combination therapy Methods 0.000 description 11
- 239000012636 effector Substances 0.000 description 11
- 210000002865 immune cell Anatomy 0.000 description 11
- 238000013459 approach Methods 0.000 description 10
- 238000003364 immunohistochemistry Methods 0.000 description 10
- 230000002688 persistence Effects 0.000 description 10
- 102000005962 receptors Human genes 0.000 description 10
- 108020003175 receptors Proteins 0.000 description 10
- 210000001519 tissue Anatomy 0.000 description 10
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 9
- 150000001413 amino acids Chemical class 0.000 description 9
- 238000004458 analytical method Methods 0.000 description 9
- 230000001225 therapeutic effect Effects 0.000 description 9
- 206010006187 Breast cancer Diseases 0.000 description 8
- 208000026310 Breast neoplasm Diseases 0.000 description 8
- 238000002619 cancer immunotherapy Methods 0.000 description 8
- 238000000684 flow cytometry Methods 0.000 description 8
- 210000000822 natural killer cell Anatomy 0.000 description 8
- 108090000765 processed proteins & peptides Proteins 0.000 description 8
- 238000010186 staining Methods 0.000 description 8
- 230000000638 stimulation Effects 0.000 description 8
- 206010009944 Colon cancer Diseases 0.000 description 7
- 208000012766 Growth delay Diseases 0.000 description 7
- 108090000172 Interleukin-15 Proteins 0.000 description 7
- 102000003812 Interleukin-15 Human genes 0.000 description 7
- 108010038453 Interleukin-2 Receptors Proteins 0.000 description 7
- 102000010789 Interleukin-2 Receptors Human genes 0.000 description 7
- 241001465754 Metazoa Species 0.000 description 7
- 241000699666 Mus <mouse, genus> Species 0.000 description 7
- 206010005003 Bladder cancer Diseases 0.000 description 6
- 108010002586 Interleukin-7 Proteins 0.000 description 6
- 102000000704 Interleukin-7 Human genes 0.000 description 6
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 6
- 230000000735 allogeneic effect Effects 0.000 description 6
- 238000006243 chemical reaction Methods 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 239000003085 diluting agent Substances 0.000 description 6
- 238000003384 imaging method Methods 0.000 description 6
- 230000002085 persistent effect Effects 0.000 description 6
- 239000000546 pharmaceutical excipient Substances 0.000 description 6
- 108090000623 proteins and genes Proteins 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 238000012360 testing method Methods 0.000 description 6
- 201000005112 urinary bladder cancer Diseases 0.000 description 6
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 5
- 208000008839 Kidney Neoplasms Diseases 0.000 description 5
- 206010038389 Renal cancer Diseases 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 230000029918 bioluminescence Effects 0.000 description 5
- 238000005415 bioluminescence Methods 0.000 description 5
- 230000010261 cell growth Effects 0.000 description 5
- 238000002659 cell therapy Methods 0.000 description 5
- 208000029742 colonic neoplasm Diseases 0.000 description 5
- 238000001990 intravenous administration Methods 0.000 description 5
- 201000010982 kidney cancer Diseases 0.000 description 5
- 239000000178 monomer Substances 0.000 description 5
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 5
- 238000002360 preparation method Methods 0.000 description 5
- 102000004196 processed proteins & peptides Human genes 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- 210000004881 tumor cell Anatomy 0.000 description 5
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 5
- 208000003174 Brain Neoplasms Diseases 0.000 description 4
- 102000004127 Cytokines Human genes 0.000 description 4
- 108090000695 Cytokines Proteins 0.000 description 4
- 102000013462 Interleukin-12 Human genes 0.000 description 4
- 108010065805 Interleukin-12 Proteins 0.000 description 4
- 102000015696 Interleukins Human genes 0.000 description 4
- 108010063738 Interleukins Proteins 0.000 description 4
- 108060001084 Luciferase Proteins 0.000 description 4
- 239000005089 Luciferase Substances 0.000 description 4
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 4
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 4
- 230000005867 T cell response Effects 0.000 description 4
- 208000003721 Triple Negative Breast Neoplasms Diseases 0.000 description 4
- 230000003213 activating effect Effects 0.000 description 4
- 125000003275 alpha amino acid group Chemical group 0.000 description 4
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 4
- 230000006023 anti-tumor response Effects 0.000 description 4
- 210000004970 cd4 cell Anatomy 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical class OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 4
- 230000021615 conjugation Effects 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 4
- 230000012010 growth Effects 0.000 description 4
- 230000007062 hydrolysis Effects 0.000 description 4
- 238000006460 hydrolysis reaction Methods 0.000 description 4
- 238000011532 immunohistochemical staining Methods 0.000 description 4
- 230000001976 improved effect Effects 0.000 description 4
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 4
- 108010034529 leucyl-lysine Proteins 0.000 description 4
- 230000004962 physiological condition Effects 0.000 description 4
- 235000018102 proteins Nutrition 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 230000001105 regulatory effect Effects 0.000 description 4
- 208000022679 triple-negative breast carcinoma Diseases 0.000 description 4
- BKZFBJYIVSBXCO-KKUMJFAQSA-N Asn-Phe-His Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CNC=N1)C(O)=O BKZFBJYIVSBXCO-KKUMJFAQSA-N 0.000 description 3
- QNNBHTFDFFFHGC-KKUMJFAQSA-N Asn-Tyr-Lys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N)O QNNBHTFDFFFHGC-KKUMJFAQSA-N 0.000 description 3
- 206010008342 Cervix carcinoma Diseases 0.000 description 3
- 108010019670 Chimeric Antigen Receptors Proteins 0.000 description 3
- HHABWQIFXZPZCK-ACZMJKKPSA-N Cys-Gln-Ser Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CS)N HHABWQIFXZPZCK-ACZMJKKPSA-N 0.000 description 3
- QKCZZAZNMMVICF-DCAQKATOSA-N Gln-Leu-Glu Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O QKCZZAZNMMVICF-DCAQKATOSA-N 0.000 description 3
- MUSGDMDGNGXULI-DCAQKATOSA-N Glu-Glu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O MUSGDMDGNGXULI-DCAQKATOSA-N 0.000 description 3
- IVGJYOOGJLFKQE-AVGNSLFASA-N Glu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N IVGJYOOGJLFKQE-AVGNSLFASA-N 0.000 description 3
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 3
- DGKBSGNCMCLDSL-BYULHYEWSA-N Gly-Ile-Asn Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)CN DGKBSGNCMCLDSL-BYULHYEWSA-N 0.000 description 3
- TWYOYAKMLHWMOJ-ZPFDUUQYSA-N Ile-Leu-Asn Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O TWYOYAKMLHWMOJ-ZPFDUUQYSA-N 0.000 description 3
- AXZGZMGRBDQTEY-SRVKXCTJSA-N Leu-Gln-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O AXZGZMGRBDQTEY-SRVKXCTJSA-N 0.000 description 3
- PDQDCFBVYXEFSD-SRVKXCTJSA-N Leu-Leu-Asp Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O PDQDCFBVYXEFSD-SRVKXCTJSA-N 0.000 description 3
- 206010025323 Lymphomas Diseases 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical class CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 206010033128 Ovarian cancer Diseases 0.000 description 3
- 206010061535 Ovarian neoplasm Diseases 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 3
- 206010060862 Prostate cancer Diseases 0.000 description 3
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 3
- 102000001712 STAT5 Transcription Factor Human genes 0.000 description 3
- 108010029477 STAT5 Transcription Factor Proteins 0.000 description 3
- 206010041067 Small cell lung cancer Diseases 0.000 description 3
- GYBVHTWOQJMYAM-HRCADAONSA-N Tyr-Met-Pro Chemical compound CSCC[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CC2=CC=C(C=C2)O)N GYBVHTWOQJMYAM-HRCADAONSA-N 0.000 description 3
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 3
- LYERIXUFCYVFFX-GVXVVHGQSA-N Val-Leu-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N LYERIXUFCYVFFX-GVXVVHGQSA-N 0.000 description 3
- 238000007792 addition Methods 0.000 description 3
- 235000001014 amino acid Nutrition 0.000 description 3
- 125000003277 amino group Chemical group 0.000 description 3
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 3
- 201000010881 cervical cancer Diseases 0.000 description 3
- 230000008602 contraction Effects 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 230000002708 enhancing effect Effects 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 238000009472 formulation Methods 0.000 description 3
- 230000013595 glycosylation Effects 0.000 description 3
- 238000006206 glycosylation reaction Methods 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 201000010536 head and neck cancer Diseases 0.000 description 3
- 208000014829 head and neck neoplasm Diseases 0.000 description 3
- 102000055277 human IL2 Human genes 0.000 description 3
- 230000028993 immune response Effects 0.000 description 3
- 210000000987 immune system Anatomy 0.000 description 3
- 230000001506 immunosuppresive effect Effects 0.000 description 3
- 230000001024 immunotherapeutic effect Effects 0.000 description 3
- 238000002955 isolation Methods 0.000 description 3
- 208000032839 leukemia Diseases 0.000 description 3
- 210000000265 leukocyte Anatomy 0.000 description 3
- 230000001926 lymphatic effect Effects 0.000 description 3
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 3
- 239000002609 medium Substances 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 238000010172 mouse model Methods 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 201000002528 pancreatic cancer Diseases 0.000 description 3
- 208000008443 pancreatic carcinoma Diseases 0.000 description 3
- 239000012188 paraffin wax Substances 0.000 description 3
- 230000006320 pegylation Effects 0.000 description 3
- 125000001151 peptidyl group Chemical group 0.000 description 3
- 230000002093 peripheral effect Effects 0.000 description 3
- 239000002953 phosphate buffered saline Substances 0.000 description 3
- 230000004983 pleiotropic effect Effects 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 208000000587 small cell lung carcinoma Diseases 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 239000006228 supernatant Substances 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 230000009261 transgenic effect Effects 0.000 description 3
- 230000005909 tumor killing Effects 0.000 description 3
- FFZJHQODAYHGPO-KZVJFYERSA-N Ala-Pro-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C)N FFZJHQODAYHGPO-KZVJFYERSA-N 0.000 description 2
- WKPXXXUSUHAXDE-SRVKXCTJSA-N Arg-Pro-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O WKPXXXUSUHAXDE-SRVKXCTJSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- CQMQJWRCRQSBAF-BPUTZDHNSA-N Asn-Arg-Trp Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(=O)N)N CQMQJWRCRQSBAF-BPUTZDHNSA-N 0.000 description 2
- PNHQRQTVBRDIEF-CIUDSAMLSA-N Asn-Leu-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(=O)N)N PNHQRQTVBRDIEF-CIUDSAMLSA-N 0.000 description 2
- AWXDRZJQCVHCIT-DCAQKATOSA-N Asn-Pro-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CC(N)=O AWXDRZJQCVHCIT-DCAQKATOSA-N 0.000 description 2
- JNCRAQVYJZGIOW-QSFUFRPTSA-N Asn-Val-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O JNCRAQVYJZGIOW-QSFUFRPTSA-N 0.000 description 2
- XDGBFDYXZCMYEX-NUMRIWBASA-N Asp-Glu-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(=O)O)N)O XDGBFDYXZCMYEX-NUMRIWBASA-N 0.000 description 2
- RQHLMGCXCZUOGT-ZPFDUUQYSA-N Asp-Leu-Ile Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O RQHLMGCXCZUOGT-ZPFDUUQYSA-N 0.000 description 2
- 206010005949 Bone cancer Diseases 0.000 description 2
- 208000018084 Bone neoplasm Diseases 0.000 description 2
- 210000005236 CD8+ effector T cell Anatomy 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- XLLSMEFANRROJE-GUBZILKMSA-N Cys-Leu-Glu Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CS)N XLLSMEFANRROJE-GUBZILKMSA-N 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 2
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 208000032612 Glial tumor Diseases 0.000 description 2
- 206010018338 Glioma Diseases 0.000 description 2
- KPNWAJMEMRCLAL-GUBZILKMSA-N Gln-Ser-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N KPNWAJMEMRCLAL-GUBZILKMSA-N 0.000 description 2
- QNJNPKSWAHPYGI-JYJNAYRXSA-N Glu-Phe-Leu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(O)=O)CC1=CC=CC=C1 QNJNPKSWAHPYGI-JYJNAYRXSA-N 0.000 description 2
- HZISRJBYZAODRV-XQXXSGGOSA-N Glu-Thr-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(O)=O HZISRJBYZAODRV-XQXXSGGOSA-N 0.000 description 2
- CAQXJMUDOLSBPF-SUSMZKCASA-N Glu-Thr-Thr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O CAQXJMUDOLSBPF-SUSMZKCASA-N 0.000 description 2
- VIPDPMHGICREIS-GVXVVHGQSA-N Glu-Val-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O VIPDPMHGICREIS-GVXVVHGQSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 2
- UROVZOUMHNXPLZ-AVGNSLFASA-N His-Leu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 UROVZOUMHNXPLZ-AVGNSLFASA-N 0.000 description 2
- 208000017604 Hodgkin disease Diseases 0.000 description 2
- 208000021519 Hodgkin lymphoma Diseases 0.000 description 2
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 2
- 241000282412 Homo Species 0.000 description 2
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 description 2
- RQJUKVXWAKJDBW-SVSWQMSJSA-N Ile-Ser-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N RQJUKVXWAKJDBW-SVSWQMSJSA-N 0.000 description 2
- WXLYNEHOGRYNFU-URLPEUOOSA-N Ile-Thr-Phe Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N WXLYNEHOGRYNFU-URLPEUOOSA-N 0.000 description 2
- 102100030703 Interleukin-22 Human genes 0.000 description 2
- WIDZHJTYKYBLSR-DCAQKATOSA-N Leu-Glu-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O WIDZHJTYKYBLSR-DCAQKATOSA-N 0.000 description 2
- ZDJQVSIPFLMNOX-RHYQMDGZSA-N Leu-Thr-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N ZDJQVSIPFLMNOX-RHYQMDGZSA-N 0.000 description 2
- KNKHAVVBVXKOGX-JXUBOQSCSA-N Lys-Ala-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O KNKHAVVBVXKOGX-JXUBOQSCSA-N 0.000 description 2
- FHIAJWBDZVHLAH-YUMQZZPRSA-N Lys-Gly-Ser Chemical compound NCCCC[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O FHIAJWBDZVHLAH-YUMQZZPRSA-N 0.000 description 2
- 208000005450 Maxillary Sinus Neoplasms Diseases 0.000 description 2
- XDGFFEZAZHRZFR-RHYQMDGZSA-N Met-Leu-Thr Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XDGFFEZAZHRZFR-RHYQMDGZSA-N 0.000 description 2
- 206010027480 Metastatic malignant melanoma Diseases 0.000 description 2
- 208000015914 Non-Hodgkin lymphomas Diseases 0.000 description 2
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- KLXQWABNAWDRAY-ACRUOGEOSA-N Phe-Lys-Phe Chemical compound C([C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 KLXQWABNAWDRAY-ACRUOGEOSA-N 0.000 description 2
- VHDNDCPMHQMXIR-IHRRRGAJSA-N Phe-Met-Cys Chemical compound SC[C@@H](C(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC1=CC=CC=C1 VHDNDCPMHQMXIR-IHRRRGAJSA-N 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- HFNPOYOKIPGAEI-SRVKXCTJSA-N Pro-Leu-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 HFNPOYOKIPGAEI-SRVKXCTJSA-N 0.000 description 2
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 description 2
- 206010039491 Sarcoma Diseases 0.000 description 2
- XQJCEKXQUJQNNK-ZLUOBGJFSA-N Ser-Ser-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O XQJCEKXQUJQNNK-ZLUOBGJFSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 208000005718 Stomach Neoplasms Diseases 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- RKDFEMGVMMYYNG-WDCWCFNPSA-N Thr-Gln-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O RKDFEMGVMMYYNG-WDCWCFNPSA-N 0.000 description 2
- XYFISNXATOERFZ-OSUNSFLBSA-N Thr-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H]([C@@H](C)O)N XYFISNXATOERFZ-OSUNSFLBSA-N 0.000 description 2
- MGJLBZFUXUGMML-VOAKCMCISA-N Thr-Lys-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)O)N)O MGJLBZFUXUGMML-VOAKCMCISA-N 0.000 description 2
- 208000024770 Thyroid neoplasm Diseases 0.000 description 2
- BURPTJBFWIOHEY-UWJYBYFXSA-N Tyr-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 BURPTJBFWIOHEY-UWJYBYFXSA-N 0.000 description 2
- 230000001919 adrenal effect Effects 0.000 description 2
- 230000001270 agonistic effect Effects 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 150000001299 aldehydes Chemical class 0.000 description 2
- 150000001408 amides Chemical class 0.000 description 2
- 210000000612 antigen-presenting cell Anatomy 0.000 description 2
- 108010038633 aspartylglutamate Proteins 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 125000004429 atom Chemical group 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 210000004369 blood Anatomy 0.000 description 2
- 239000008280 blood Substances 0.000 description 2
- 229910021538 borax Inorganic materials 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 238000009172 cell transfer therapy Methods 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 238000002512 chemotherapy Methods 0.000 description 2
- 238000002591 computed tomography Methods 0.000 description 2
- 230000003750 conditioning effect Effects 0.000 description 2
- 230000001054 cortical effect Effects 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 208000035250 cutaneous malignant susceptibility to 1 melanoma Diseases 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- FSXRLASFHBWESK-UHFFFAOYSA-N dipeptide phenylalanyl-tyrosine Natural products C=1C=C(O)C=CC=1CC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FSXRLASFHBWESK-UHFFFAOYSA-N 0.000 description 2
- 230000008034 disappearance Effects 0.000 description 2
- 239000002552 dosage form Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 230000003511 endothelial effect Effects 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 201000004101 esophageal cancer Diseases 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 206010017758 gastric cancer Diseases 0.000 description 2
- 125000001475 halogen functional group Chemical group 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 2
- 108010025306 histidylleucine Proteins 0.000 description 2
- 229920001519 homopolymer Polymers 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000008595 infiltration Effects 0.000 description 2
- 238000001764 infiltration Methods 0.000 description 2
- 108010074108 interleukin-21 Proteins 0.000 description 2
- 238000001361 intraarterial administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 230000005865 ionizing radiation Effects 0.000 description 2
- 108010078274 isoleucylvaline Proteins 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 239000004310 lactic acid Substances 0.000 description 2
- 235000014655 lactic acid Nutrition 0.000 description 2
- 108010090333 leucyl-lysyl-proline Proteins 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 125000005647 linker group Chemical group 0.000 description 2
- 210000004185 liver Anatomy 0.000 description 2
- 201000007270 liver cancer Diseases 0.000 description 2
- 208000014018 liver neoplasm Diseases 0.000 description 2
- 230000005923 long-lasting effect Effects 0.000 description 2
- 210000003810 lymphokine-activated killer cell Anatomy 0.000 description 2
- 108010054155 lysyllysine Proteins 0.000 description 2
- 230000014759 maintenance of location Effects 0.000 description 2
- 201000004488 maxillary sinus cancer Diseases 0.000 description 2
- 208000019303 maxillary sinus carcinoma Diseases 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 210000003071 memory t lymphocyte Anatomy 0.000 description 2
- 208000021039 metastatic melanoma Diseases 0.000 description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 2
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 230000002062 proliferating effect Effects 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 235000010339 sodium tetraborate Nutrition 0.000 description 2
- 230000003393 splenic effect Effects 0.000 description 2
- 201000011549 stomach cancer Diseases 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000004083 survival effect Effects 0.000 description 2
- 201000002510 thyroid cancer Diseases 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 238000010361 transduction Methods 0.000 description 2
- 230000026683 transduction Effects 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- BSVBQGMMJUBVOD-UHFFFAOYSA-N trisodium borate Chemical compound [Na+].[Na+].[Na+].[O-]B([O-])[O-] BSVBQGMMJUBVOD-UHFFFAOYSA-N 0.000 description 2
- 238000011870 unpaired t-test Methods 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- CNKBMTKICGGSCQ-ACRUOGEOSA-N (2S)-2-[[(2S)-2-[[(2S)-2,6-diamino-1-oxohexyl]amino]-1-oxo-3-phenylpropyl]amino]-3-(4-hydroxyphenyl)propanoic acid Chemical compound C([C@H](NC(=O)[C@@H](N)CCCCN)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)C1=CC=CC=C1 CNKBMTKICGGSCQ-ACRUOGEOSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- UWYVPFMHMJIBHE-OWOJBTEDSA-N (e)-2-hydroxybut-2-enedioic acid Chemical compound OC(=O)\C=C(\O)C(O)=O UWYVPFMHMJIBHE-OWOJBTEDSA-N 0.000 description 1
- LDMOEFOXLIZJOW-UHFFFAOYSA-N 1-dodecanesulfonic acid Chemical class CCCCCCCCCCCCS(O)(=O)=O LDMOEFOXLIZJOW-UHFFFAOYSA-N 0.000 description 1
- LBLYYCQCTBFVLH-UHFFFAOYSA-N 2-Methylbenzenesulfonic acid Chemical compound CC1=CC=CC=C1S(O)(=O)=O LBLYYCQCTBFVLH-UHFFFAOYSA-N 0.000 description 1
- WLJVXDMOQOGPHL-PPJXEINESA-N 2-phenylacetic acid Chemical compound O[14C](=O)CC1=CC=CC=C1 WLJVXDMOQOGPHL-PPJXEINESA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- MVBWLRJESQOQTM-ACZMJKKPSA-N Ala-Gln-Ser Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O MVBWLRJESQOQTM-ACZMJKKPSA-N 0.000 description 1
- LSMDIAAALJJLRO-XQXXSGGOSA-N Ala-Thr-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(O)=O LSMDIAAALJJLRO-XQXXSGGOSA-N 0.000 description 1
- VNFSAYFQLXPHPY-CIQUZCHMSA-N Ala-Thr-Ile Chemical compound [H]N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O VNFSAYFQLXPHPY-CIQUZCHMSA-N 0.000 description 1
- 201000003076 Angiosarcoma Diseases 0.000 description 1
- OTCJMMRQBVDQRK-DCAQKATOSA-N Arg-Asp-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O OTCJMMRQBVDQRK-DCAQKATOSA-N 0.000 description 1
- OISWSORSLQOGFV-AVGNSLFASA-N Arg-Met-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CCCN=C(N)N OISWSORSLQOGFV-AVGNSLFASA-N 0.000 description 1
- POZKLUIXMHIULG-FDARSICLSA-N Arg-Trp-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)NC(=O)[C@H](CCCN=C(N)N)N POZKLUIXMHIULG-FDARSICLSA-N 0.000 description 1
- BVLIJXXSXBUGEC-SRVKXCTJSA-N Asn-Asn-Tyr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BVLIJXXSXBUGEC-SRVKXCTJSA-N 0.000 description 1
- PTSDPWIHOYMRGR-UGYAYLCHSA-N Asn-Ile-Asn Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(O)=O PTSDPWIHOYMRGR-UGYAYLCHSA-N 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 206010003571 Astrocytoma Diseases 0.000 description 1
- 206010004146 Basal cell carcinoma Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- 208000031872 Body Remains Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 1
- 102100024217 CAMPATH-1 antigen Human genes 0.000 description 1
- 108010005327 CD19-specific chimeric antigen receptor Proteins 0.000 description 1
- 108010065524 CD52 Antigen Proteins 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 201000005488 Capillary Leak Syndrome Diseases 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- 241000282693 Cercopithecidae Species 0.000 description 1
- 208000005243 Chondrosarcoma Diseases 0.000 description 1
- 201000009047 Chordoma Diseases 0.000 description 1
- 102000029816 Collagenase Human genes 0.000 description 1
- 108060005980 Collagenase Proteins 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- CMSMOCZEIVJLDB-UHFFFAOYSA-N Cyclophosphamide Chemical compound ClCCN(CCCl)P1(=O)NCCCO1 CMSMOCZEIVJLDB-UHFFFAOYSA-N 0.000 description 1
- AOZBJZBKFHOYHL-AVGNSLFASA-N Cys-Glu-Tyr Chemical compound [H]N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O AOZBJZBKFHOYHL-AVGNSLFASA-N 0.000 description 1
- 230000004544 DNA amplification Effects 0.000 description 1
- 102000016911 Deoxyribonucleases Human genes 0.000 description 1
- 108010053770 Deoxyribonucleases Proteins 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- LYCAIKOWRPUZTN-UHFFFAOYSA-N Ethylene glycol Chemical group OCCO LYCAIKOWRPUZTN-UHFFFAOYSA-N 0.000 description 1
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical class C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 1
- 208000006168 Ewing Sarcoma Diseases 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 201000008808 Fibrosarcoma Diseases 0.000 description 1
- UVAOVENCIONMJP-GUBZILKMSA-N Gln-Cys-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O UVAOVENCIONMJP-GUBZILKMSA-N 0.000 description 1
- LGIKBBLQVSWUGK-DCAQKATOSA-N Gln-Leu-Gln Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O LGIKBBLQVSWUGK-DCAQKATOSA-N 0.000 description 1
- DOMHVQBSRJNNKD-ZPFDUUQYSA-N Gln-Met-Ile Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O DOMHVQBSRJNNKD-ZPFDUUQYSA-N 0.000 description 1
- SXFPZRRVWSUYII-KBIXCLLPSA-N Gln-Ser-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(=O)N)N SXFPZRRVWSUYII-KBIXCLLPSA-N 0.000 description 1
- QJCKNLPMTPXXEM-AUTRQRHGSA-N Glu-Glu-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O QJCKNLPMTPXXEM-AUTRQRHGSA-N 0.000 description 1
- HVKAAUOFFTUSAA-XDTLVQLUSA-N Glu-Tyr-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C)C(O)=O HVKAAUOFFTUSAA-XDTLVQLUSA-N 0.000 description 1
- FGPLUIQCSKGLTI-WDSKDSINSA-N Gly-Ser-Glu Chemical compound NCC(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CCC(O)=O FGPLUIQCSKGLTI-WDSKDSINSA-N 0.000 description 1
- 208000001258 Hemangiosarcoma Diseases 0.000 description 1
- YAALVYQFVJNXIV-KKUMJFAQSA-N His-Leu-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](N)CC1=CN=CN1 YAALVYQFVJNXIV-KKUMJFAQSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 208000001953 Hypotension Diseases 0.000 description 1
- 101150083678 IL2 gene Proteins 0.000 description 1
- VAXBXNPRXPHGHG-BJDJZHNGSA-N Ile-Ala-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)O)N VAXBXNPRXPHGHG-BJDJZHNGSA-N 0.000 description 1
- QIHJTGSVGIPHIW-QSFUFRPTSA-N Ile-Asn-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](C(C)C)C(=O)O)N QIHJTGSVGIPHIW-QSFUFRPTSA-N 0.000 description 1
- PFPUFNLHBXKPHY-HTFCKZLJSA-N Ile-Ile-Ser Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)O)N PFPUFNLHBXKPHY-HTFCKZLJSA-N 0.000 description 1
- JODPUDMBQBIWCK-GHCJXIJMSA-N Ile-Ser-Asn Chemical compound [H]N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(O)=O JODPUDMBQBIWCK-GHCJXIJMSA-N 0.000 description 1
- YWCJXQKATPNPOE-UKJIMTQDSA-N Ile-Val-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N YWCJXQKATPNPOE-UKJIMTQDSA-N 0.000 description 1
- 229940076838 Immune checkpoint inhibitor Drugs 0.000 description 1
- 102000008070 Interferon-gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 108010066719 Interleukin Receptor Common gamma Subunit Proteins 0.000 description 1
- 102000018682 Interleukin Receptor Common gamma Subunit Human genes 0.000 description 1
- 108010002352 Interleukin-1 Proteins 0.000 description 1
- PWWVAXIEGOYWEE-UHFFFAOYSA-N Isophenergan Chemical compound C1=CC=C2N(CC(C)N(C)C)C3=CC=CC=C3SC2=C1 PWWVAXIEGOYWEE-UHFFFAOYSA-N 0.000 description 1
- IBMVEYRWAWIOTN-UHFFFAOYSA-N L-Leucyl-L-Arginyl-L-Proline Natural products CC(C)CC(N)C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(O)=O IBMVEYRWAWIOTN-UHFFFAOYSA-N 0.000 description 1
- FADYJNXDPBKVCA-UHFFFAOYSA-N L-Phenylalanyl-L-lysin Natural products NCCCCC(C(O)=O)NC(=O)C(N)CC1=CC=CC=C1 FADYJNXDPBKVCA-UHFFFAOYSA-N 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- 208000018142 Leiomyosarcoma Diseases 0.000 description 1
- MJOZZTKJZQFKDK-GUBZILKMSA-N Leu-Ala-Gln Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(N)=O MJOZZTKJZQFKDK-GUBZILKMSA-N 0.000 description 1
- IBMVEYRWAWIOTN-RWMBFGLXSA-N Leu-Arg-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(O)=O IBMVEYRWAWIOTN-RWMBFGLXSA-N 0.000 description 1
- IGUOAYLTQJLPPD-DCAQKATOSA-N Leu-Asn-Arg Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N IGUOAYLTQJLPPD-DCAQKATOSA-N 0.000 description 1
- OIARJGNVARWKFP-YUMQZZPRSA-N Leu-Asn-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O OIARJGNVARWKFP-YUMQZZPRSA-N 0.000 description 1
- JKGHDYGZRDWHGA-SRVKXCTJSA-N Leu-Asn-Leu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(O)=O JKGHDYGZRDWHGA-SRVKXCTJSA-N 0.000 description 1
- DLCOFDAHNMMQPP-SRVKXCTJSA-N Leu-Asp-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O DLCOFDAHNMMQPP-SRVKXCTJSA-N 0.000 description 1
- DXYBNWJZJVSZAE-GUBZILKMSA-N Leu-Gln-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N DXYBNWJZJVSZAE-GUBZILKMSA-N 0.000 description 1
- IWTBYNQNAPECCS-AVGNSLFASA-N Leu-Glu-His Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IWTBYNQNAPECCS-AVGNSLFASA-N 0.000 description 1
- QVFGXCVIXXBFHO-AVGNSLFASA-N Leu-Glu-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O QVFGXCVIXXBFHO-AVGNSLFASA-N 0.000 description 1
- HRTRLSRYZZKPCO-BJDJZHNGSA-N Leu-Ile-Ser Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CO)C(O)=O HRTRLSRYZZKPCO-BJDJZHNGSA-N 0.000 description 1
- LVTJJOJKDCVZGP-QWRGUYRKSA-N Leu-Lys-Gly Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O LVTJJOJKDCVZGP-QWRGUYRKSA-N 0.000 description 1
- REPBGZHJKYWFMJ-KKUMJFAQSA-N Leu-Lys-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N REPBGZHJKYWFMJ-KKUMJFAQSA-N 0.000 description 1
- KIZIOFNVSOSKJI-CIUDSAMLSA-N Leu-Ser-Cys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CS)C(=O)O)N KIZIOFNVSOSKJI-CIUDSAMLSA-N 0.000 description 1
- ODRREERHVHMIPT-OEAJRASXSA-N Leu-Thr-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 ODRREERHVHMIPT-OEAJRASXSA-N 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- NCTDKZKNBDZDOL-GARJFASQSA-N Lys-Asn-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)N)NC(=O)[C@H](CCCCN)N)C(=O)O NCTDKZKNBDZDOL-GARJFASQSA-N 0.000 description 1
- OWRUUFUVXFREBD-KKUMJFAQSA-N Lys-His-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O OWRUUFUVXFREBD-KKUMJFAQSA-N 0.000 description 1
- OIQSIMFSVLLWBX-VOAKCMCISA-N Lys-Leu-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OIQSIMFSVLLWBX-VOAKCMCISA-N 0.000 description 1
- XOQMURBBIXRRCR-SRVKXCTJSA-N Lys-Lys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN XOQMURBBIXRRCR-SRVKXCTJSA-N 0.000 description 1
- PLDJDCJLRCYPJB-VOAKCMCISA-N Lys-Lys-Thr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PLDJDCJLRCYPJB-VOAKCMCISA-N 0.000 description 1
- HYSVGEAWTGPMOA-IHRRRGAJSA-N Lys-Pro-Leu Chemical compound [H]N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(O)=O HYSVGEAWTGPMOA-IHRRRGAJSA-N 0.000 description 1
- UWHCKWNPWKTMBM-WDCWCFNPSA-N Lys-Thr-Gln Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(N)=O)C(O)=O UWHCKWNPWKTMBM-WDCWCFNPSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 241000124008 Mammalia Species 0.000 description 1
- 208000000172 Medulloblastoma Diseases 0.000 description 1
- CNUPMMXDISGXMU-CIUDSAMLSA-N Met-Cys-Glu Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(O)=O)C(O)=O CNUPMMXDISGXMU-CIUDSAMLSA-N 0.000 description 1
- VOOINLQYUZOREH-SRVKXCTJSA-N Met-Gln-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCSC)N VOOINLQYUZOREH-SRVKXCTJSA-N 0.000 description 1
- WXXNVZMWHOLNRJ-AVGNSLFASA-N Met-Pro-Lys Chemical compound CSCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(O)=O WXXNVZMWHOLNRJ-AVGNSLFASA-N 0.000 description 1
- VOAKKHOIAFKOQZ-JYJNAYRXSA-N Met-Tyr-Arg Chemical compound NC(=N)NCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)CCSC)CC1=CC=C(O)C=C1 VOAKKHOIAFKOQZ-JYJNAYRXSA-N 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- 101100342977 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) leu-1 gene Proteins 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 101710163270 Nuclease Proteins 0.000 description 1
- 201000010133 Oligodendroglioma Diseases 0.000 description 1
- 108091034117 Oligonucleotide Proteins 0.000 description 1
- 235000021314 Palmitic acid Nutrition 0.000 description 1
- VADLTGVIOIOKGM-BZSNNMDCSA-N Phe-His-Leu Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)C(O)=O)NC(=O)[C@@H](N)CC=1C=CC=CC=1)C1=CN=CN1 VADLTGVIOIOKGM-BZSNNMDCSA-N 0.000 description 1
- KBVJZCVLQWCJQN-KKUMJFAQSA-N Phe-Leu-Asn Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O KBVJZCVLQWCJQN-KKUMJFAQSA-N 0.000 description 1
- 208000007641 Pinealoma Diseases 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- SSSFPISOZOLQNP-GUBZILKMSA-N Pro-Arg-Asp Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(O)=O SSSFPISOZOLQNP-GUBZILKMSA-N 0.000 description 1
- RMODQFBNDDENCP-IHRRRGAJSA-N Pro-Lys-Leu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(O)=O RMODQFBNDDENCP-IHRRRGAJSA-N 0.000 description 1
- RMJZWERKFFNNNS-XGEHTFHBSA-N Pro-Thr-Ser Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O RMJZWERKFFNNNS-XGEHTFHBSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 206010037368 Pulmonary congestion Diseases 0.000 description 1
- 239000012980 RPMI-1640 medium Substances 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- BRKHVZNDAOMAHX-BIIVOSGPSA-N Ser-Ala-Pro Chemical compound C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N BRKHVZNDAOMAHX-BIIVOSGPSA-N 0.000 description 1
- KYKKKSWGEPFUMR-NAKRPEOUSA-N Ser-Arg-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O KYKKKSWGEPFUMR-NAKRPEOUSA-N 0.000 description 1
- YMEXHZTVKDAKIY-GHCJXIJMSA-N Ser-Asn-Ile Chemical compound CC[C@H](C)[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO)C(O)=O YMEXHZTVKDAKIY-GHCJXIJMSA-N 0.000 description 1
- GZBKRJVCRMZAST-XKBZYTNZSA-N Ser-Glu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GZBKRJVCRMZAST-XKBZYTNZSA-N 0.000 description 1
- FUMGHWDRRFCKEP-CIUDSAMLSA-N Ser-Leu-Ala Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O FUMGHWDRRFCKEP-CIUDSAMLSA-N 0.000 description 1
- HDBOEVPDIDDEPC-CIUDSAMLSA-N Ser-Lys-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O HDBOEVPDIDDEPC-CIUDSAMLSA-N 0.000 description 1
- PYTKULIABVRXSC-BWBBJGPYSA-N Ser-Ser-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O PYTKULIABVRXSC-BWBBJGPYSA-N 0.000 description 1
- NADLKBTYNKUJEP-KATARQTJSA-N Ser-Thr-Leu Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(O)=O NADLKBTYNKUJEP-KATARQTJSA-N 0.000 description 1
- PCJLFYBAQZQOFE-KATARQTJSA-N Ser-Thr-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CO)N)O PCJLFYBAQZQOFE-KATARQTJSA-N 0.000 description 1
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 208000031932 Systemic capillary leak syndrome Diseases 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 210000000662 T-lymphocyte subset Anatomy 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- XVNZSJIKGJLQLH-RCWTZXSCSA-N Thr-Arg-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCSC)C(=O)O)N)O XVNZSJIKGJLQLH-RCWTZXSCSA-N 0.000 description 1
- LHEZGZQRLDBSRR-WDCWCFNPSA-N Thr-Glu-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O LHEZGZQRLDBSRR-WDCWCFNPSA-N 0.000 description 1
- VRUFCJZQDACGLH-UVOCVTCTSA-N Thr-Leu-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O VRUFCJZQDACGLH-UVOCVTCTSA-N 0.000 description 1
- VGYVVSQFSSKZRJ-OEAJRASXSA-N Thr-Phe-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](N)[C@H](O)C)CC1=CC=CC=C1 VGYVVSQFSSKZRJ-OEAJRASXSA-N 0.000 description 1
- BDYBHQWMHYDRKJ-UNQGMJICSA-N Thr-Phe-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCSC)C(=O)O)N)O BDYBHQWMHYDRKJ-UNQGMJICSA-N 0.000 description 1
- ABWNZPOIUJMNKT-IXOXFDKPSA-N Thr-Phe-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O ABWNZPOIUJMNKT-IXOXFDKPSA-N 0.000 description 1
- WPSKTVVMQCXPRO-BWBBJGPYSA-N Thr-Ser-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CO)C(O)=O WPSKTVVMQCXPRO-BWBBJGPYSA-N 0.000 description 1
- QJIODPFLAASXJC-JHYOHUSXSA-N Thr-Thr-Phe Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N)O QJIODPFLAASXJC-JHYOHUSXSA-N 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- YDTKYBHPRULROG-LTHWPDAASA-N Trp-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](CC1=CNC2=CC=CC=C21)N YDTKYBHPRULROG-LTHWPDAASA-N 0.000 description 1
- FOADDSDHGRFUOC-DZKIICNBSA-N Val-Glu-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)N FOADDSDHGRFUOC-DZKIICNBSA-N 0.000 description 1
- DJQIUOKSNRBTSV-CYDGBPFRSA-N Val-Ile-Val Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](C(C)C)N DJQIUOKSNRBTSV-CYDGBPFRSA-N 0.000 description 1
- FEXILLGKGGTLRI-NHCYSSNCSA-N Val-Leu-Asn Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](C(C)C)N FEXILLGKGGTLRI-NHCYSSNCSA-N 0.000 description 1
- PQSNETRGCRUOGP-KKHAAJSZSA-N Val-Thr-Asn Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CC(N)=O PQSNETRGCRUOGP-KKHAAJSZSA-N 0.000 description 1
- 208000008383 Wilms tumor Diseases 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- 229940022663 acetate Drugs 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 208000009956 adenocarcinoma Diseases 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 1
- 125000001931 aliphatic group Chemical group 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- 102000006707 alpha-beta T-Cell Antigen Receptors Human genes 0.000 description 1
- 108010087408 alpha-beta T-Cell Antigen Receptors Proteins 0.000 description 1
- BJEPYKJPYRNKOW-UHFFFAOYSA-N alpha-hydroxysuccinic acid Natural products OC(=O)C(O)CC(O)=O BJEPYKJPYRNKOW-UHFFFAOYSA-N 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001412 amines Chemical class 0.000 description 1
- 125000000539 amino acid group Chemical group 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical group [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 239000008228 bacteriostatic water for injection Substances 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960004365 benzoic acid Drugs 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 208000026900 bile duct neoplasm Diseases 0.000 description 1
- 201000009036 biliary tract cancer Diseases 0.000 description 1
- 208000020790 biliary tract neoplasm Diseases 0.000 description 1
- 239000011230 binding agent Substances 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 239000011203 carbon fibre reinforced carbon Substances 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 150000001733 carboxylic acid esters Chemical class 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000020411 cell activation Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 208000006990 cholangiocarcinoma Diseases 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 229960002424 collagenase Drugs 0.000 description 1
- 238000011220 combination immunotherapy Methods 0.000 description 1
- 238000011284 combination treatment Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 229920001577 copolymer Polymers 0.000 description 1
- 238000011461 current therapy Methods 0.000 description 1
- 229960004397 cyclophosphamide Drugs 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 230000007850 degeneration Effects 0.000 description 1
- 239000008367 deionised water Substances 0.000 description 1
- 229910021641 deionized water Inorganic materials 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000012217 deletion Methods 0.000 description 1
- 230000037430 deletion Effects 0.000 description 1
- 230000006323 depegylation Effects 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000002059 diagnostic imaging Methods 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- POULHZVOKOAJMA-UHFFFAOYSA-M dodecanoate Chemical compound CCCCCCCCCCCC([O-])=O POULHZVOKOAJMA-UHFFFAOYSA-M 0.000 description 1
- 239000003937 drug carrier Substances 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 230000000235 effect on cancer Effects 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 102000015694 estrogen receptors Human genes 0.000 description 1
- 108010038795 estrogen receptors Proteins 0.000 description 1
- AFAXGSQYZLGZPG-UHFFFAOYSA-N ethanedisulfonic acid Chemical compound OS(=O)(=O)CCS(O)(=O)=O AFAXGSQYZLGZPG-UHFFFAOYSA-N 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- 229940117927 ethylene oxide Drugs 0.000 description 1
- 239000012091 fetal bovine serum Substances 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 239000012537 formulation buffer Substances 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-L fumarate(2-) Chemical class [O-]C(=O)\C=C\C([O-])=O VZCYOOQTPOCHFL-OWOJBTEDSA-L 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 125000000524 functional group Chemical group 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 238000012239 gene modification Methods 0.000 description 1
- 230000005017 genetic modification Effects 0.000 description 1
- 235000013617 genetically modified food Nutrition 0.000 description 1
- 238000010362 genome editing Methods 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 229960002989 glutamic acid Drugs 0.000 description 1
- 229940093915 gynecological organic acid Drugs 0.000 description 1
- 210000002443 helper t lymphocyte Anatomy 0.000 description 1
- 201000002222 hemangioblastoma Diseases 0.000 description 1
- 201000005787 hematologic cancer Diseases 0.000 description 1
- 208000024200 hematopoietic and lymphoid system neoplasm Diseases 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 description 1
- 230000003284 homeostatic effect Effects 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-M hydrogensulfate Chemical compound OS([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-M 0.000 description 1
- 230000036543 hypotension Effects 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 150000002466 imines Chemical class 0.000 description 1
- 230000005934 immune activation Effects 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 230000008629 immune suppression Effects 0.000 description 1
- 239000012274 immune-checkpoint protein inhibitor Substances 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 229960001438 immunostimulant agent Drugs 0.000 description 1
- 230000003308 immunostimulating effect Effects 0.000 description 1
- 238000010874 in vitro model Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 229960003130 interferon gamma Drugs 0.000 description 1
- 108040006849 interleukin-2 receptor activity proteins Proteins 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000007913 intrathecal administration Methods 0.000 description 1
- 230000002601 intratumoral effect Effects 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 210000003292 kidney cell Anatomy 0.000 description 1
- 230000002045 lasting effect Effects 0.000 description 1
- 229940070765 laurate Drugs 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 206010024627 liposarcoma Diseases 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000001165 lymph node Anatomy 0.000 description 1
- 208000012804 lymphangiosarcoma Diseases 0.000 description 1
- 108010038320 lysylphenylalanine Proteins 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 150000002688 maleic acid derivatives Chemical class 0.000 description 1
- 239000001630 malic acid Substances 0.000 description 1
- 235000011090 malic acid Nutrition 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 208000006178 malignant mesothelioma Diseases 0.000 description 1
- 230000000873 masking effect Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 206010027191 meningioma Diseases 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 208000037819 metastatic cancer Diseases 0.000 description 1
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 125000001570 methylene group Chemical group [H]C([H])([*:1])[*:2] 0.000 description 1
- 239000011325 microbead Substances 0.000 description 1
- 230000005012 migration Effects 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- LNOPIUAQISRISI-UHFFFAOYSA-N n'-hydroxy-2-propan-2-ylsulfonylethanimidamide Chemical compound CC(C)S(=O)(=O)CC(N)=NO LNOPIUAQISRISI-UHFFFAOYSA-N 0.000 description 1
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 1
- 125000005487 naphthalate group Chemical group 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-M oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC([O-])=O ZQPPMHVWECSIRJ-KTKRTIGZSA-M 0.000 description 1
- 229940049964 oleate Drugs 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 230000000174 oncolytic effect Effects 0.000 description 1
- 235000005985 organic acids Nutrition 0.000 description 1
- 150000002905 orthoesters Chemical class 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 1
- 208000004019 papillary adenocarcinoma Diseases 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 229940124531 pharmaceutical excipient Drugs 0.000 description 1
- 150000003014 phosphoric acid esters Chemical class 0.000 description 1
- 238000003566 phosphorylation assay Methods 0.000 description 1
- 230000006461 physiological response Effects 0.000 description 1
- 208000024724 pineal body neoplasm Diseases 0.000 description 1
- 201000004123 pineal gland cancer Diseases 0.000 description 1
- 229940068917 polyethylene glycols Drugs 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 102000003998 progesterone receptors Human genes 0.000 description 1
- 108090000468 progesterone receptors Proteins 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 238000000163 radioactive labelling Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 230000001177 retroviral effect Effects 0.000 description 1
- 201000009410 rhabdomyosarcoma Diseases 0.000 description 1
- 229960004889 salicylic acid Drugs 0.000 description 1
- 238000003118 sandwich ELISA Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 208000018964 sebaceous gland cancer Diseases 0.000 description 1
- 238000013207 serial dilution Methods 0.000 description 1
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 239000004017 serum-free culture medium Substances 0.000 description 1
- 230000011664 signaling Effects 0.000 description 1
- 238000002741 site-directed mutagenesis Methods 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 210000004989 spleen cell Anatomy 0.000 description 1
- 210000004988 splenocyte Anatomy 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 239000000021 stimulant Substances 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 150000003900 succinic acid esters Chemical class 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 1
- 238000013268 sustained release Methods 0.000 description 1
- 239000012730 sustained-release form Substances 0.000 description 1
- 201000008759 sweat gland cancer Diseases 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000009044 synergistic interaction Effects 0.000 description 1
- 230000005737 synergistic response Effects 0.000 description 1
- 206010042863 synovial sarcoma Diseases 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 150000007970 thio esters Chemical class 0.000 description 1
- 239000003104 tissue culture media Substances 0.000 description 1
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 1
- 230000002110 toxicologic effect Effects 0.000 description 1
- 231100000027 toxicology Toxicity 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- 230000001960 triggered effect Effects 0.000 description 1
- 108010051110 tyrosyl-lysine Proteins 0.000 description 1
- 241001430294 unidentified retrovirus Species 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 229940070710 valerate Drugs 0.000 description 1
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 230000002861 ventricular Effects 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 229920003169 water-soluble polymer Polymers 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/715—Receptors; Cell surface antigens; Cell surface determinants for cytokines; for lymphokines; for interferons
- C07K14/7155—Receptors; Cell surface antigens; Cell surface determinants for cytokines; for lymphokines; for interferons for interleukins [IL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K35/00—Medicinal preparations containing materials or reaction products thereof with undetermined constitution
- A61K35/12—Materials from mammals; Compositions comprising non-specified tissues or cells; Compositions comprising non-embryonic stem cells; Genetically modified cells
- A61K35/14—Blood; Artificial blood
- A61K35/17—Lymphocytes; B-cells; T-cells; Natural killer cells; Interferon-activated or cytokine-activated lymphocytes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/19—Cytokines; Lymphokines; Interferons
- A61K38/20—Interleukins [IL]
- A61K38/2013—IL-2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/11—T-cells, e.g. tumour infiltrating lymphocytes [TIL] or regulatory T [Treg] cells; Lymphokine-activated killer [LAK] cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4271—Melanoma antigens
- A61K40/4273—Glycoprotein 100 [Gp100]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/56—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule
- A61K47/59—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes
- A61K47/60—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an organic macromolecular compound, e.g. an oligomeric, polymeric or dendrimeric molecule obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyureas or polyurethanes the organic macromolecular compound being a polyoxyalkylene oligomer, polymer or dendrimer, e.g. PEG, PPG, PEO or polyglycerol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/04—Antineoplastic agents specific for metastasis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/52—Cytokines; Lymphokines; Interferons
- C07K14/54—Interleukins [IL]
- C07K14/55—IL-2
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/57—Skin; melanoma
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Cell Biology (AREA)
- Oncology (AREA)
- Toxicology (AREA)
- Molecular Biology (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Developmental Biology & Embryology (AREA)
- Virology (AREA)
- Dermatology (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762465506P | 2017-03-01 | 2017-03-01 | |
| US62/465,506 | 2017-03-01 | ||
| US201762480971P | 2017-04-03 | 2017-04-03 | |
| US62/480,971 | 2017-04-03 | ||
| PCT/US2018/020514 WO2018160877A1 (en) | 2017-03-01 | 2018-03-01 | Immunotherapeutic tumor treatment method using an interleukin-2 receptor alpha, beta-selective agonist in combination with adoptive cell transfer therapy |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190121773A true KR20190121773A (ko) | 2019-10-28 |
Family
ID=63371348
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197024673A Ceased KR20190121773A (ko) | 2017-03-01 | 2018-03-01 | 입양 세포 이식 요법과 조합된 인터루킨-2 수용체 알파, 베타 선택성 효현제를 이용한 면역 요법적 종양 치료 방법 |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US11318164B2 (enExample) |
| EP (1) | EP3589303A4 (enExample) |
| JP (1) | JP2020509018A (enExample) |
| KR (1) | KR20190121773A (enExample) |
| CN (1) | CN110366421A (enExample) |
| AU (1) | AU2018227810A1 (enExample) |
| CA (1) | CA3055040A1 (enExample) |
| IL (1) | IL269015A (enExample) |
| MX (1) | MX2019010382A (enExample) |
| WO (1) | WO2018160877A1 (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2018234810B2 (en) | 2017-03-15 | 2023-05-11 | Pandion Operations, Inc. | Targeted immunotolerance |
| US10676516B2 (en) | 2017-05-24 | 2020-06-09 | Pandion Therapeutics, Inc. | Targeted immunotolerance |
| US10946068B2 (en) | 2017-12-06 | 2021-03-16 | Pandion Operations, Inc. | IL-2 muteins and uses thereof |
| USRE50550E1 (en) | 2017-12-06 | 2025-08-26 | Pandion Operations, Inc. | IL-2 muteins and uses thereof |
| US10174092B1 (en) | 2017-12-06 | 2019-01-08 | Pandion Therapeutics, Inc. | IL-2 muteins |
| JP7497340B2 (ja) | 2018-05-14 | 2024-06-10 | ウェアウルフ セラピューティクス, インコーポレイテッド | 活性化可能なインターロイキン12ポリペプチド及びその使用方法 |
| FI3794024T3 (fi) | 2018-05-14 | 2023-08-10 | Werewolf Therapeutics Inc | Aktivoitavia interleukiini-2-polypeptidejä ja niiden käyttömenetelmiä |
| EP3969035A4 (en) | 2019-05-14 | 2023-06-21 | Werewolf Therapeutics, Inc. | SEPARATION UNITS AND METHODS AND THEIR USE |
| WO2020236875A1 (en) | 2019-05-20 | 2020-11-26 | Pandion Therapeutics, Inc. | Madcam targeted immunotolerance |
| MX2022005666A (es) | 2019-11-14 | 2022-10-07 | Werewolf Therapeutics Inc | Polipeptidos de citocina activables y metodos de uso de los mismos. |
| CN113121670B (zh) * | 2020-01-15 | 2022-11-22 | 天津键凯科技有限公司 | 二取代peg化白细胞介素2及其制备方法、应用 |
| US11981715B2 (en) | 2020-02-21 | 2024-05-14 | Pandion Operations, Inc. | Tissue targeted immunotolerance with a CD39 effector |
| WO2022260916A1 (en) * | 2021-06-08 | 2022-12-15 | Merck Sharp & Dohme Llc | Functional cell-based potency assay for measuring biological activity of interleukin 2 (il-2) analogs |
| CN116036243A (zh) * | 2022-04-20 | 2023-05-02 | 北京大学宁波海洋药物研究院 | 受体偏向的peg化il-2变体组合及其应用 |
Family Cites Families (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5126132A (en) * | 1989-08-21 | 1992-06-30 | The United States Of America As Represented By The Department Of Health And Human Services | Tumor infiltrating lymphocytes as a treatment modality for human cancer |
| BRPI0611872B8 (pt) * | 2005-06-16 | 2021-05-25 | Nektar Therapeutics | reagente polimérico, conjugado, método para preparação de um conjugado e composição farmacêutica |
| KR20200070407A (ko) | 2010-11-12 | 2020-06-17 | 넥타르 테라퓨틱스 | Il-2 부분 및 중합체의 접합체 |
| SI3134123T1 (sl) * | 2014-02-21 | 2021-08-31 | Nektar Therapeutics (India) Pvt. Ltd. | IL-2Rbeta-selektivni agonisti v kombinaciji s protitelesom proti- CTLA-4 ali protitelesom proti-PD-1 |
| CA2943389C (en) | 2014-03-20 | 2023-10-31 | H. Lee Moffitt Cancer Center And Research Institute, Inc. | Tumor-infiltrating lymphocytes for adoptive cell therapy |
| EP3034092A1 (en) * | 2014-12-17 | 2016-06-22 | Université de Lausanne | Adoptive immunotherapy for treating cancer |
| EP3565888A1 (en) * | 2017-01-06 | 2019-11-13 | Iovance Biotherapeutics, Inc. | Expansion of tumor infiltrating lymphocytes (tils) with tumor necrosis factor receptor superfamily (tnfrsf) agonists and therapeutic combinations of tils and tnfrsf agonists |
-
2018
- 2018-03-01 WO PCT/US2018/020514 patent/WO2018160877A1/en not_active Ceased
- 2018-03-01 CA CA3055040A patent/CA3055040A1/en active Pending
- 2018-03-01 US US16/490,443 patent/US11318164B2/en active Active
- 2018-03-01 AU AU2018227810A patent/AU2018227810A1/en not_active Abandoned
- 2018-03-01 JP JP2019547077A patent/JP2020509018A/ja active Pending
- 2018-03-01 EP EP18760525.8A patent/EP3589303A4/en not_active Withdrawn
- 2018-03-01 KR KR1020197024673A patent/KR20190121773A/ko not_active Ceased
- 2018-03-01 MX MX2019010382A patent/MX2019010382A/es unknown
- 2018-03-01 CN CN201880014971.8A patent/CN110366421A/zh active Pending
-
2019
- 2019-08-29 IL IL26901519A patent/IL269015A/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| EP3589303A4 (en) | 2020-11-25 |
| EP3589303A1 (en) | 2020-01-08 |
| CA3055040A1 (en) | 2018-09-07 |
| WO2018160877A1 (en) | 2018-09-07 |
| US11318164B2 (en) | 2022-05-03 |
| JP2020509018A (ja) | 2020-03-26 |
| CN110366421A (zh) | 2019-10-22 |
| IL269015A (en) | 2019-10-31 |
| US20200016206A1 (en) | 2020-01-16 |
| MX2019010382A (es) | 2019-10-22 |
| AU2018227810A1 (en) | 2019-08-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11318164B2 (en) | Immunotherapeutic treatment method using an interleukin-2 receptor beta-selective agonist in combination with adoptive cell transfer therapy | |
| ES2872848T3 (es) | Agonistas selectivos de IL-2Rbeta en combinación con un anticuerpo anti-CTLA-4 o un anticuerpo anti-PD-1 | |
| KR20190105568A (ko) | 종양의 면역 요법적 치료 방법 | |
| JP2022521792A (ja) | 癌を治療するための免疫療法的併用 | |
| JP7672342B2 (ja) | 細胞免疫療法を増強するための方法 | |
| US20210154277A1 (en) | Immunotherapeutic combination for treating cancer | |
| KR101294290B1 (ko) | 예방 또는 치료를 위해 제i류 주조직 적합성복합체〔mhc〕-제한 에피토프에 대한 면역 반응을 유발,향상 및 지속하는 방법 | |
| JP2023099731A (ja) | IL-2Rβ選択的作動薬と長時間作用型IL-15作動薬との併用 | |
| EP3795583A1 (en) | Il10/fc fusion proteins useful as enhancers of immunotherapies | |
| CA3198822A1 (en) | Multi-functional and multi-valent interleukin-tgf-beta receptor fusion polypeptides | |
| CA3000211C (en) | Combination of an il-2rbeta-selective agonist and a long-acting il-15 agonist |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190822 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20210215 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230601 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20230821 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230601 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |