KR20190084264A - Cd160 막 횡단 동형체에 결합하는 단클론 항체 - Google Patents
Cd160 막 횡단 동형체에 결합하는 단클론 항체 Download PDFInfo
- Publication number
- KR20190084264A KR20190084264A KR1020197014634A KR20197014634A KR20190084264A KR 20190084264 A KR20190084264 A KR 20190084264A KR 1020197014634 A KR1020197014634 A KR 1020197014634A KR 20197014634 A KR20197014634 A KR 20197014634A KR 20190084264 A KR20190084264 A KR 20190084264A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- seq
- monoclonal antibody
- cells
- cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/06—Antianaemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6811—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being a protein or peptide, e.g. transferrin or bleomycin
- A61K47/6817—Toxins
- A61K47/6819—Plant toxins
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/21—Immunoglobulins specific features characterized by taxonomic origin from primates, e.g. man
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/33—Crossreactivity, e.g. for species or epitope, or lack of said crossreactivity
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/55—Fab or Fab'
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/732—Antibody-dependent cellular cytotoxicity [ADCC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
- C07K2317/734—Complement-dependent cytotoxicity [CDC]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/76—Antagonist effect on antigen, e.g. neutralization or inhibition of binding
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Medicinal Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Hematology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Diabetes (AREA)
- Oncology (AREA)
- Transplantation (AREA)
- Communicable Diseases (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP16306392.8 | 2016-10-25 | ||
| EP16306392 | 2016-10-25 | ||
| PCT/EP2017/077261 WO2018077926A1 (en) | 2016-10-25 | 2017-10-25 | Monoclonal antibodies binding to the cd160 transmembrane isoform |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190084264A true KR20190084264A (ko) | 2019-07-16 |
Family
ID=57570549
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197014634A Ceased KR20190084264A (ko) | 2016-10-25 | 2017-10-25 | Cd160 막 횡단 동형체에 결합하는 단클론 항체 |
Country Status (9)
| Country | Link |
|---|---|
| US (1) | US11186635B2 (OSRAM) |
| EP (1) | EP3532502A1 (OSRAM) |
| JP (1) | JP2019535306A (OSRAM) |
| KR (1) | KR20190084264A (OSRAM) |
| CN (1) | CN110121508A (OSRAM) |
| AU (1) | AU2017351764A1 (OSRAM) |
| BR (1) | BR112019008345A8 (OSRAM) |
| CA (1) | CA3080270A1 (OSRAM) |
| WO (1) | WO2018077926A1 (OSRAM) |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113614109A (zh) | 2018-12-21 | 2021-11-05 | Ose免疫疗法公司 | 双功能抗pd-1/il-7分子 |
| WO2020165374A1 (en) | 2019-02-14 | 2020-08-20 | Ose Immunotherapeutics | Bifunctional molecule comprising il-15ra |
| EP3812008A1 (en) * | 2019-10-23 | 2021-04-28 | Gamamabs Pharma | Amh-competitive antagonist antibody |
| WO2021122866A1 (en) | 2019-12-17 | 2021-06-24 | Ose Immunotherapeutics | Bifunctional molecules comprising an il-7 variant |
| GB202103706D0 (en) * | 2021-03-17 | 2021-04-28 | Ucl Business Ltd | CD160 binding domain |
| EP4320156A1 (en) | 2021-04-09 | 2024-02-14 | Ose Immunotherapeutics | Scaffold for bifunctioanl molecules comprising pd-1 or cd28 and sirp binding domains |
| WO2022214653A1 (en) | 2021-04-09 | 2022-10-13 | Ose Immunotherapeutics | New scaffold for bifunctional molecules with improved properties |
| EP4490188A1 (en) * | 2022-03-09 | 2025-01-15 | Alderaan Biotechnology | Anti-cd160 transmembrane isoform antibodies |
| WO2024028386A1 (en) | 2022-08-02 | 2024-02-08 | Ose Immunotherapeutics | Multifunctional molecule directed against cd28 |
Family Cites Families (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4861719A (en) | 1986-04-25 | 1989-08-29 | Fred Hutchinson Cancer Research Center | DNA constructs for retrovirus packaging cell lines |
| IL85035A0 (en) | 1987-01-08 | 1988-06-30 | Int Genetic Eng | Polynucleotide molecule,a chimeric antibody with specificity for human b cell surface antigen,a process for the preparation and methods utilizing the same |
| US5094941A (en) * | 1987-12-31 | 1992-03-10 | Zymogenetics, Inc. | Monoclonal antibodies to PDGF |
| US5278056A (en) | 1988-02-05 | 1994-01-11 | The Trustees Of Columbia University In The City Of New York | Retroviral packaging cell lines and process of using same |
| DE68921982D1 (de) | 1988-06-14 | 1995-05-04 | Cetus Oncology Corp | Kupplungsmittel und sterisch gehinderte, mit disulfid gebundene konjugate daraus. |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| US5670488A (en) | 1992-12-03 | 1997-09-23 | Genzyme Corporation | Adenovirus vector for gene therapy |
| EP0940468A1 (en) | 1991-06-14 | 1999-09-08 | Genentech, Inc. | Humanized antibody variable domain |
| EP0617706B1 (en) | 1991-11-25 | 2001-10-17 | Enzon, Inc. | Multivalent antigen-binding proteins |
| US5714350A (en) | 1992-03-09 | 1998-02-03 | Protein Design Labs, Inc. | Increasing antibody affinity by altering glycosylation in the immunoglobulin variable region |
| US5635483A (en) | 1992-12-03 | 1997-06-03 | Arizona Board Of Regents Acting On Behalf Of Arizona State University | Tumor inhibiting tetrapeptide bearing modified phenethyl amides |
| US5780588A (en) | 1993-01-26 | 1998-07-14 | Arizona Board Of Regents | Elucidation and synthesis of selected pentapeptides |
| DE69434860T2 (de) | 1993-02-22 | 2007-03-15 | The Rockefeller University | Herstellung von helfer-freien retroviren mit hohem titer mittels transienter transfektion |
| US6214345B1 (en) | 1993-05-14 | 2001-04-10 | Bristol-Myers Squibb Co. | Lysosomal enzyme-cleavable antitumor drug conjugates |
| FR2712812B1 (fr) | 1993-11-23 | 1996-02-09 | Centre Nat Rech Scient | Composition pour la production de produits thérapeutiques in vivo. |
| US5663149A (en) | 1994-12-13 | 1997-09-02 | Arizona Board Of Regents Acting On Behalf Of Arizona State University | Human cancer inhibitory pentapeptide heterocyclic and halophenyl amides |
| IL116816A (en) | 1995-01-20 | 2003-05-29 | Rhone Poulenc Rorer Sa | Cell for the production of a defective recombinant adenovirus or an adeno-associated virus and the various uses thereof |
| US6013516A (en) | 1995-10-06 | 2000-01-11 | The Salk Institute For Biological Studies | Vector and method of use for nucleic acid delivery to non-dividing cells |
| ATE319745T1 (de) | 1997-05-21 | 2006-03-15 | Biovation Ltd | Verfahren zur herstellung von nicht-immunogenen proteinen |
| EP0985039B1 (en) | 1997-06-12 | 2008-02-20 | Novartis International Pharmaceutical Ltd. | Artificial antibody polypeptides |
| US6194551B1 (en) | 1998-04-02 | 2001-02-27 | Genentech, Inc. | Polypeptide variants |
| DK1071700T3 (da) | 1998-04-20 | 2010-06-07 | Glycart Biotechnology Ag | Glykosylerings-modifikation af antistoffer til forbedring af antistofafhængig cellulær cytotoksicitet |
| AU3672800A (en) | 1999-04-09 | 2000-11-14 | Kyowa Hakko Kogyo Co. Ltd. | Method for controlling the activity of immunologically functional molecule |
| US7029872B2 (en) | 2000-06-28 | 2006-04-18 | Glycofi, Inc | Methods for producing modified glycoproteins |
| US6884869B2 (en) | 2001-04-30 | 2005-04-26 | Seattle Genetics, Inc. | Pentapeptide compounds and uses related thereto |
| WO2003026577A2 (en) | 2001-09-24 | 2003-04-03 | Seattle Genetics, Inc. | P-amidobenzylethers in drug delivery agents |
| ATE430580T1 (de) | 2001-10-25 | 2009-05-15 | Genentech Inc | Glycoprotein-zusammensetzungen |
| ATE395413T1 (de) | 2001-12-03 | 2008-05-15 | Amgen Fremont Inc | Antikörperkategorisierung auf der grundlage von bindungseigenschaften |
| SI2357006T1 (sl) | 2002-07-31 | 2016-01-29 | Seattle Genetics, Inc. | Konjugati zdravil in njihova uporaba za zdravljenje raka, avtoimunske bolezni ali infekcijske bolezni |
| BR122018071808B8 (pt) | 2003-11-06 | 2020-06-30 | Seattle Genetics Inc | conjugado |
| AU2005216251B2 (en) | 2004-02-23 | 2011-03-10 | Genentech, Inc. | Heterocyclic self-immolative linkers and conjugates |
| AU2005218642B2 (en) | 2004-03-02 | 2011-04-28 | Seagen Inc. | Partially loaded antibodies and methods of their conjugation |
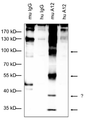
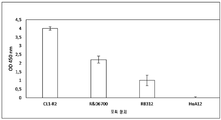
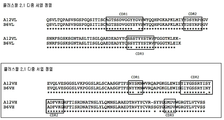
| EP1626059A1 (en) | 2004-08-09 | 2006-02-15 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Angiogenic and immunologic applications of anti-CD160 specific compounds obtainable from mAb CL1-R2 |
| EP1817336B1 (en) | 2004-11-12 | 2019-01-09 | Seattle Genetics, Inc. | Auristatins having an aminobenzoic acid unit at the n terminus |
| JP4658125B2 (ja) | 2005-06-28 | 2011-03-23 | パイオニア株式会社 | 放送受信装置、妨害検出装置および妨害検出方法 |
| SI3248613T1 (sl) | 2005-07-18 | 2022-04-29 | Seagen Inc. | Konjugati beta-glukuronidni linker-zdravilo |
| EP1880729A1 (en) | 2006-07-20 | 2008-01-23 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Use of soluble CD160 to suppress immunity |
| EA200970477A1 (ru) * | 2006-11-15 | 2009-12-30 | Медарекс, Инк. | Человеческие моноклональные антитела к btla и способы применения |
| EP2006299A1 (en) | 2007-06-18 | 2008-12-24 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Identification of new isoforms of the MHC-class I specific receptor CD160 and uses thereof |
| EP2210903A1 (en) * | 2009-01-21 | 2010-07-28 | Monoclonal Antibodies Therapeutics | Anti-CD160 monoclonal antibodies and uses thereof |
| CA2793424A1 (en) * | 2010-03-18 | 2011-09-22 | Cornell University | Engineering correctly folded antibodies using inner membrane display of twin-arginine translocation intermediates |
| KR101847572B1 (ko) * | 2010-05-28 | 2018-05-24 | 인쎄름 (엥스띠뛰 나씨오날 드 라 쌍떼 에 드 라 흐쉐르슈 메디깔) | 신혈관신생에 기초한 안 질환 치료용 항-cd160 특이적 항체 |
| US9676871B2 (en) | 2010-11-05 | 2017-06-13 | Pfizer Inc. | Engineered polypeptide conjugates and methods for making thereof using transglutaminase |
| WO2013072714A1 (en) * | 2011-11-15 | 2013-05-23 | Centre National De La Recherche Scientifique (Cnrs) | Antibodies for molecular imaging of vulnerable plaques in atherosclerosis |
| WO2016113351A1 (en) * | 2015-01-16 | 2016-07-21 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Human monoclonal antibodies against orexin receptor type 1 |
-
2017
- 2017-10-25 AU AU2017351764A patent/AU2017351764A1/en not_active Abandoned
- 2017-10-25 EP EP17797084.5A patent/EP3532502A1/en active Pending
- 2017-10-25 CN CN201780080063.4A patent/CN110121508A/zh active Pending
- 2017-10-25 BR BR112019008345A patent/BR112019008345A8/pt not_active Application Discontinuation
- 2017-10-25 US US16/343,889 patent/US11186635B2/en active Active
- 2017-10-25 KR KR1020197014634A patent/KR20190084264A/ko not_active Ceased
- 2017-10-25 CA CA3080270A patent/CA3080270A1/en not_active Abandoned
- 2017-10-25 WO PCT/EP2017/077261 patent/WO2018077926A1/en not_active Ceased
- 2017-10-25 JP JP2019542804A patent/JP2019535306A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP2019535306A (ja) | 2019-12-12 |
| CN110121508A (zh) | 2019-08-13 |
| BR112019008345A8 (pt) | 2023-03-07 |
| BR112019008345A2 (pt) | 2019-10-01 |
| EP3532502A1 (en) | 2019-09-04 |
| US20190256595A1 (en) | 2019-08-22 |
| CA3080270A1 (en) | 2018-05-03 |
| WO2018077926A1 (en) | 2018-05-03 |
| US11186635B2 (en) | 2021-11-30 |
| AU2017351764A1 (en) | 2019-06-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR102365972B1 (ko) | 항-pd-1 항체 및 이의 용도 | |
| KR20190084264A (ko) | Cd160 막 횡단 동형체에 결합하는 단클론 항체 | |
| JP6847037B2 (ja) | 抗cd73抗体とa2a受容体阻害薬とを含む併用治療薬及びその使用 | |
| JP2024164038A (ja) | 抗pd-l1抗体およびその使用 | |
| JP6141639B2 (ja) | 抗体 | |
| TWI751397B (zh) | 抗 lag-3 抗體及其用途 | |
| CN112424231B (zh) | 抗pd-1抗体及其剂量和用途 | |
| JP2022516140A (ja) | 白血球免疫グロブリン様受容体2中和抗体 | |
| US20240228649A9 (en) | Crtam antibodies and methods of treating cancer | |
| WO2019129137A1 (zh) | 抗lag-3抗体及其用途 | |
| US11492403B2 (en) | Anti-phosphotyrosinylated programmed death 1 (PD-1) monoclonal antibodies, methods of making and methods of using thereof | |
| CN114401992A (zh) | 结合igsf11(vsig3)的igc2的抗体及其用途 | |
| WO2019129136A1 (zh) | 抗pd-l1抗体及其用途 | |
| JP2022523145A (ja) | 抗trem1抗体及び関連方法 | |
| US20250179175A1 (en) | Anti-cd160 transmembrane isoform antibodies | |
| HK40016537A (en) | Anti-pd-1 antibodies and uses thereof | |
| HK40047725B (en) | Anti-pd-1 antibodies, dosages and uses thereof | |
| HK40047725A (en) | Anti-pd-1 antibodies, dosages and uses thereof | |
| TW202411252A (zh) | 抗pd-1抗體及其用途 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| R17-X000 | Change to representative recorded |
St.27 status event code: A-3-3-R10-R17-oth-X000 |
|
| A201 | Request for examination | ||
| E13-X000 | Pre-grant limitation requested |
St.27 status event code: A-2-3-E10-E13-lim-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| PN2301 | Change of applicant |
St.27 status event code: A-3-3-R10-R13-asn-PN2301 St.27 status event code: A-3-3-R10-R11-asn-PN2301 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
St.27 status event code: N-2-6-B10-B15-exm-PE0601 |
|
| R18-X000 | Changes to party contact information recorded |
St.27 status event code: A-3-3-R10-R18-oth-X000 |