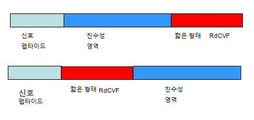
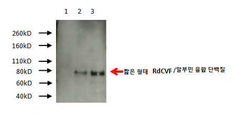
KR20190058388A - 짧은 형태의 간상체 유래 원추체 생존능 인자와 친수성 펩타이드 사이의 융합 단백질 - Google Patents
짧은 형태의 간상체 유래 원추체 생존능 인자와 친수성 펩타이드 사이의 융합 단백질 Download PDFInfo
- Publication number
- KR20190058388A KR20190058388A KR1020187037891A KR20187037891A KR20190058388A KR 20190058388 A KR20190058388 A KR 20190058388A KR 1020187037891 A KR1020187037891 A KR 1020187037891A KR 20187037891 A KR20187037891 A KR 20187037891A KR 20190058388 A KR20190058388 A KR 20190058388A
- Authority
- KR
- South Korea
- Prior art keywords
- peptide sequence
- fusion protein
- sequence
- leu
- rdcvf
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/42—Proteins; Polypeptides; Degradation products thereof; Derivatives thereof, e.g. albumin, gelatin or zein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/643—Albumins, e.g. HSA, BSA, ovalbumin or a Keyhole Limpet Hemocyanin [KHL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0048—Eye, e.g. artificial tears
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/76—Albumins
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/111—General methods applicable to biologically active non-coding nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/0004—Oxidoreductases (1.)
- C12N9/0012—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.6, 1.7)
- C12N9/0036—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.6, 1.7) acting on NADH or NADPH (1.6)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y108/00—Oxidoreductases acting on sulfur groups as donors (1.8)
- C12Y108/01—Oxidoreductases acting on sulfur groups as donors (1.8) with NAD+ or NADP+ as acceptor (1.8.1)
- C12Y108/01008—Protein-disulfide reductase (1.8.1.8), i.e. thioredoxin
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/02—Fusion polypeptide containing a localisation/targetting motif containing a signal sequence
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/31—Fusion polypeptide fusions, other than Fc, for prolonged plasma life, e.g. albumin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
- C12N2750/14143—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/22—Vectors comprising a coding region that has been codon optimised for expression in a respective host
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Microbiology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Virology (AREA)
- Immunology (AREA)
- Psychology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Marine Sciences & Fisheries (AREA)
- Ophthalmology & Optometry (AREA)
- Inorganic Chemistry (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR1020237037858A KR20230156440A (ko) | 2016-10-11 | 2017-10-11 | 짧은 형태의 간상체 유래 원추체 생존능 인자와 친수성 펩타이드 사이의 융합 단백질 |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662406552P | 2016-10-11 | 2016-10-11 | |
| US62/406,552 | 2016-10-11 | ||
| PCT/US2017/056030 WO2018071465A1 (en) | 2016-10-11 | 2017-10-11 | Fusion protein between short form rod-derived cone viability factor and a hydrophilic peptide |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020237037858A Division KR20230156440A (ko) | 2016-10-11 | 2017-10-11 | 짧은 형태의 간상체 유래 원추체 생존능 인자와 친수성 펩타이드 사이의 융합 단백질 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190058388A true KR20190058388A (ko) | 2019-05-29 |
Family
ID=61906325
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187037891A Ceased KR20190058388A (ko) | 2016-10-11 | 2017-10-11 | 짧은 형태의 간상체 유래 원추체 생존능 인자와 친수성 펩타이드 사이의 융합 단백질 |
| KR1020237037858A Pending KR20230156440A (ko) | 2016-10-11 | 2017-10-11 | 짧은 형태의 간상체 유래 원추체 생존능 인자와 친수성 펩타이드 사이의 융합 단백질 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020237037858A Pending KR20230156440A (ko) | 2016-10-11 | 2017-10-11 | 짧은 형태의 간상체 유래 원추체 생존능 인자와 친수성 펩타이드 사이의 융합 단백질 |
Country Status (13)
| Country | Link |
|---|---|
| US (3) | US10946063B2 (cg-RX-API-DMAC7.html) |
| EP (1) | EP3526238A4 (cg-RX-API-DMAC7.html) |
| JP (1) | JP7028802B2 (cg-RX-API-DMAC7.html) |
| KR (2) | KR20190058388A (cg-RX-API-DMAC7.html) |
| CN (1) | CN109415423A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2017344059B2 (cg-RX-API-DMAC7.html) |
| BR (1) | BR112018076674A2 (cg-RX-API-DMAC7.html) |
| CA (1) | CA3025977A1 (cg-RX-API-DMAC7.html) |
| IL (1) | IL263990A (cg-RX-API-DMAC7.html) |
| MX (1) | MX2018015596A (cg-RX-API-DMAC7.html) |
| NZ (1) | NZ748634A (cg-RX-API-DMAC7.html) |
| WO (1) | WO2018071465A1 (cg-RX-API-DMAC7.html) |
| ZA (1) | ZA201808041B (cg-RX-API-DMAC7.html) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113584059B (zh) * | 2018-08-07 | 2023-02-10 | 康码(上海)生物科技有限公司 | 信号肽相关序列及其在蛋白质合成中的应用 |
| EP4074828A4 (en) * | 2019-12-09 | 2023-12-27 | Chigenovo Co., Ltd. | Uses of cyp4v2 and rdcvf in preparation of drugs |
| CN111733174B (zh) * | 2020-08-07 | 2021-02-09 | 北京大学第三医院(北京大学第三临床医学院) | 一种分离的核酸分子及其用途 |
| WO2024206928A1 (en) * | 2023-03-30 | 2024-10-03 | Pharma Cinq, Llc | VECTOR ENCODING ROD-DERIVED CONE VIABILITY FACTOR AND HUMAN IgK SIGNAL SEQUENCE |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07241196A (ja) * | 1994-03-04 | 1995-09-19 | Teruhiko Beppu | アルブミンおよびヒトアポリポプロテインe様遺伝子を有する融合遺伝子、それを挿入したプラスミド並びにその用途 |
| EP0943003B1 (en) | 1996-12-02 | 2004-11-17 | Valentis Inc. | Insulin-like growth factor i (igf-i) expression system and methods of use |
| FR2823221B1 (fr) | 2001-04-06 | 2004-04-02 | Univ Pasteur | Sequences associees a la degenerescence retinienne et applications |
| AU2002364587A1 (en) | 2001-12-21 | 2003-07-30 | Human Genome Sciences, Inc. | Albumin fusion proteins |
| CN1241946C (zh) * | 2002-07-01 | 2006-02-15 | 美国福源集团 | 对多种细胞具刺激增生作用的人血清白蛋白重组融合蛋白 |
| DE10260805A1 (de) | 2002-12-23 | 2004-07-22 | Geneart Gmbh | Verfahren und Vorrichtung zum Optimieren einer Nucleotidsequenz zur Expression eines Proteins |
| FR2870241B1 (fr) | 2004-05-13 | 2015-02-27 | Novartis Ag | Facteur de viabilite des cones derive des batonnets ou rdcvf et applications |
| EP2027889A1 (en) | 2007-06-05 | 2009-02-25 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | New neuronal viability factor and use thereof |
| EP2281047B1 (en) | 2008-04-15 | 2020-04-08 | Genzyme Corporation | Methods to produce rod-derived cone viability factor (rdcvf) |
| US8779093B2 (en) | 2008-09-10 | 2014-07-15 | Inserm (Institut National De La Sante Et De La Recherche Medicale) | Neuronal viability factor and use thereof |
| US20120088819A1 (en) | 2008-10-31 | 2012-04-12 | Makoto Inoue | Method for enhancing expression of recombinant protein |
| CA2833176C (en) * | 2011-04-12 | 2023-05-16 | C.B. Appaiah | Chimeric antibacterial polypeptides |
| BR112014010091B1 (pt) * | 2011-10-27 | 2022-11-08 | Wellstat Ophthalmics Corporation | Ácido nucleico, vetor viral, preparação farmacêutica, uso destes, método para produção de uma proteína rdcvf, e método in vitro ou ex vivo de secreção de uma proteína rdcvf a partir de uma célula |
| WO2014012082A2 (en) | 2012-07-13 | 2014-01-16 | Zymeworks Inc. | Multivalent heteromultimer scaffold design an constructs |
| WO2015082690A1 (en) | 2013-12-06 | 2015-06-11 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods and pharmaceutical compositions for expressing a polynucleotide of interest in the retinal pigment epithelium of a subject |
| US10857240B2 (en) | 2016-01-05 | 2020-12-08 | The Trustees Of The University Of Pennsylvania | Methods and compositions for treatment of ocular disorders and blinding diseases |
-
2017
- 2017-10-11 US US16/301,764 patent/US10946063B2/en active Active
- 2017-10-11 AU AU2017344059A patent/AU2017344059B2/en active Active
- 2017-10-11 NZ NZ748634A patent/NZ748634A/en unknown
- 2017-10-11 CA CA3025977A patent/CA3025977A1/en active Pending
- 2017-10-11 MX MX2018015596A patent/MX2018015596A/es unknown
- 2017-10-11 EP EP17860101.9A patent/EP3526238A4/en active Pending
- 2017-10-11 KR KR1020187037891A patent/KR20190058388A/ko not_active Ceased
- 2017-10-11 KR KR1020237037858A patent/KR20230156440A/ko active Pending
- 2017-10-11 BR BR112018076674-7A patent/BR112018076674A2/pt unknown
- 2017-10-11 WO PCT/US2017/056030 patent/WO2018071465A1/en not_active Ceased
- 2017-10-11 CN CN201780040158.3A patent/CN109415423A/zh active Pending
- 2017-10-11 JP JP2018563544A patent/JP7028802B2/ja active Active
-
2018
- 2018-11-28 ZA ZA2018/08041A patent/ZA201808041B/en unknown
- 2018-12-27 IL IL263990A patent/IL263990A/en unknown
-
2021
- 2021-02-10 US US17/172,202 patent/US12076367B2/en active Active
-
2024
- 2024-07-26 US US18/785,213 patent/US20240374683A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| KR20230156440A (ko) | 2023-11-14 |
| MX2018015596A (es) | 2019-03-14 |
| CN109415423A (zh) | 2019-03-01 |
| US20240374683A1 (en) | 2024-11-14 |
| US20210162005A1 (en) | 2021-06-03 |
| WO2018071465A1 (en) | 2018-04-19 |
| EP3526238A4 (en) | 2020-04-29 |
| US20190151410A1 (en) | 2019-05-23 |
| JP7028802B2 (ja) | 2022-03-02 |
| IL263990A (en) | 2019-01-31 |
| JP2019532616A (ja) | 2019-11-14 |
| NZ748634A (en) | 2025-11-28 |
| CA3025977A1 (en) | 2018-04-19 |
| BR112018076674A2 (pt) | 2019-04-02 |
| US12076367B2 (en) | 2024-09-03 |
| AU2017344059A1 (en) | 2018-12-13 |
| US10946063B2 (en) | 2021-03-16 |
| AU2017344059B2 (en) | 2021-12-23 |
| ZA201808041B (en) | 2019-09-25 |
| RU2018144780A (ru) | 2020-11-17 |
| EP3526238A1 (en) | 2019-08-21 |
| RU2018144780A3 (cg-RX-API-DMAC7.html) | 2021-02-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20240374683A1 (en) | Fusion protein between short form rod-derived cone viability factor and a hydrophilic peptide | |
| JP6293664B2 (ja) | 桿体由来錐体生存因子をコードするベクター | |
| CA2876293C (en) | Combination for treating an inflammatory disorder | |
| AU2016320551C1 (en) | Treatment of retinitis pigmentosa | |
| CN113631182B (zh) | 二硫键稳定的多肽组合物和使用方法 | |
| RU2639521C2 (ru) | Аналоги фактора комплемента в и их применения | |
| KR20160048103A (ko) | Vegf-c 및 ccbe1의 치료적 용도 | |
| CN111742044A (zh) | 用遗传修饰的β细胞治疗糖尿病 | |
| CN112567053A (zh) | 重组核酸构建体 | |
| KR20240023126A (ko) | 망막 장애 | |
| CA3061656A1 (en) | Gene therapy for tuberous sclerosis | |
| RU2773368C2 (ru) | Слитый белок короткой формы фактора жизнеспособности колбочек, полученного из палочек, и гидрофильного пептида | |
| US20240342313A1 (en) | Vector encoding rod-derived cone viability factor and human igk signal sequence | |
| WO2024140281A1 (en) | Phase-separation-reversing polypeptide variants and uses thereof | |
| CN121285568A (zh) | 编码视杆细胞源性视锥细胞活力因子和人IgK信号序列的载体 | |
| RU2557385C1 (ru) | Кодон-оптимизированные последовательности и фармацевтическая композиция для восстановления кровеносных сосудов | |
| JP2023549620A (ja) | 組換え補体タンパク質を産生させる方法、そのベクターおよび治療的使用 | |
| JP2021520204A (ja) | 酸化ストレスに対する遺伝子療法 | |
| US20160122713A1 (en) | Genetically-modified micro-organ secreting a therapeutic peptide and methods of use thereof | |
| NZ616479B2 (en) | Complement factor b analogs and their uses |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20181227 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20201007 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20220816 Patent event code: PE09021S01D |
|
| E90F | Notification of reason for final refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Final Notice of Reason for Refusal Patent event date: 20230503 Patent event code: PE09021S02D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20231113 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20230503 Comment text: Final Notice of Reason for Refusal Patent event code: PE06011S02I Patent event date: 20220816 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |