JP7028802B2 - 短鎖型桿体由来錐体生存因子及び親水性ペプチド間の融合タンパク質 - Google Patents
短鎖型桿体由来錐体生存因子及び親水性ペプチド間の融合タンパク質 Download PDFInfo
- Publication number
- JP7028802B2 JP7028802B2 JP2018563544A JP2018563544A JP7028802B2 JP 7028802 B2 JP7028802 B2 JP 7028802B2 JP 2018563544 A JP2018563544 A JP 2018563544A JP 2018563544 A JP2018563544 A JP 2018563544A JP 7028802 B2 JP7028802 B2 JP 7028802B2
- Authority
- JP
- Japan
- Prior art keywords
- fusion protein
- sequence
- peptide sequence
- rdcvf
- vector
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 102000037865 fusion proteins Human genes 0.000 title claims description 87
- 108020001507 fusion proteins Proteins 0.000 title claims description 87
- 108090000765 processed proteins & peptides Proteins 0.000 title claims description 79
- 230000004083 survival effect Effects 0.000 title description 9
- 102000004196 processed proteins & peptides Human genes 0.000 title description 2
- 150000001413 amino acids Chemical class 0.000 claims description 44
- 210000004027 cell Anatomy 0.000 claims description 36
- 239000013598 vector Substances 0.000 claims description 34
- 102000008100 Human Serum Albumin Human genes 0.000 claims description 32
- 108091006905 Human Serum Albumin Proteins 0.000 claims description 32
- 108010076504 Protein Sorting Signals Proteins 0.000 claims description 32
- 150000007523 nucleic acids Chemical class 0.000 claims description 24
- 108020004707 nucleic acids Proteins 0.000 claims description 23
- 102000039446 nucleic acids Human genes 0.000 claims description 23
- 239000013604 expression vector Substances 0.000 claims description 19
- 239000013612 plasmid Substances 0.000 claims description 19
- 238000002347 injection Methods 0.000 claims description 12
- 239000007924 injection Substances 0.000 claims description 12
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 10
- 230000028327 secretion Effects 0.000 claims description 10
- 239000013607 AAV vector Substances 0.000 claims description 8
- 201000010099 disease Diseases 0.000 claims description 8
- 238000000034 method Methods 0.000 claims description 8
- 238000004113 cell culture Methods 0.000 claims description 7
- 208000011580 syndromic disease Diseases 0.000 claims description 7
- 208000002780 macular degeneration Diseases 0.000 claims description 6
- 239000008194 pharmaceutical composition Substances 0.000 claims description 6
- 210000000608 photoreceptor cell Anatomy 0.000 claims description 6
- 206010064930 age-related macular degeneration Diseases 0.000 claims description 5
- 208000017532 inherited retinal dystrophy Diseases 0.000 claims description 5
- 208000032578 Inherited retinal disease Diseases 0.000 claims description 4
- 208000032430 Retinal dystrophy Diseases 0.000 claims description 4
- 208000000208 Wet Macular Degeneration Diseases 0.000 claims description 4
- 201000006321 fundus dystrophy Diseases 0.000 claims description 4
- 206010003694 Atrophy Diseases 0.000 claims description 3
- 230000037444 atrophy Effects 0.000 claims description 3
- 239000013613 expression plasmid Substances 0.000 claims description 3
- 208000030533 eye disease Diseases 0.000 claims description 3
- 208000024827 Alzheimer disease Diseases 0.000 claims description 2
- 208000037663 Best vitelliform macular dystrophy Diseases 0.000 claims description 2
- 206010010356 Congenital anomaly Diseases 0.000 claims description 2
- 208000010412 Glaucoma Diseases 0.000 claims description 2
- 208000003807 Graves Disease Diseases 0.000 claims description 2
- 208000015023 Graves' disease Diseases 0.000 claims description 2
- 206010020772 Hypertension Diseases 0.000 claims description 2
- 208000022873 Ocular disease Diseases 0.000 claims description 2
- 208000018737 Parkinson disease Diseases 0.000 claims description 2
- 206010038910 Retinitis Diseases 0.000 claims description 2
- 208000035475 disorder Diseases 0.000 claims description 2
- 239000003937 drug carrier Substances 0.000 claims description 2
- 208000038015 macular disease Diseases 0.000 claims description 2
- 238000004519 manufacturing process Methods 0.000 claims description 2
- 210000000844 retinal pigment epithelial cell Anatomy 0.000 claims description 2
- 230000001177 retroviral effect Effects 0.000 claims description 2
- 210000001685 thyroid gland Anatomy 0.000 claims description 2
- 201000007790 vitelliform macular dystrophy Diseases 0.000 claims description 2
- 208000020938 vitelliform macular dystrophy 2 Diseases 0.000 claims description 2
- 239000003795 chemical substances by application Substances 0.000 claims 3
- 239000013603 viral vector Substances 0.000 claims 3
- 239000003814 drug Substances 0.000 claims 2
- 239000003223 protective agent Substances 0.000 claims 2
- 229940124597 therapeutic agent Drugs 0.000 claims 2
- 206010008096 Cerebral atrophy Diseases 0.000 claims 1
- 206010012689 Diabetic retinopathy Diseases 0.000 claims 1
- 208000023105 Huntington disease Diseases 0.000 claims 1
- 206010061218 Inflammation Diseases 0.000 claims 1
- 230000004054 inflammatory process Effects 0.000 claims 1
- 239000004615 ingredient Substances 0.000 claims 1
- 229940024606 amino acid Drugs 0.000 description 34
- 108090000623 proteins and genes Proteins 0.000 description 30
- 235000018102 proteins Nutrition 0.000 description 26
- 102000004169 proteins and genes Human genes 0.000 description 26
- 230000002209 hydrophobic effect Effects 0.000 description 22
- 108091026890 Coding region Proteins 0.000 description 14
- 102000009027 Albumins Human genes 0.000 description 12
- 108010088751 Albumins Proteins 0.000 description 12
- 239000012528 membrane Substances 0.000 description 10
- 208000007014 Retinitis pigmentosa Diseases 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- 241000702421 Dependoparvovirus Species 0.000 description 8
- 239000005018 casein Substances 0.000 description 7
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 7
- 235000021240 caseins Nutrition 0.000 description 7
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 7
- 239000000203 mixture Substances 0.000 description 7
- 239000002953 phosphate buffered saline Substances 0.000 description 7
- 125000006850 spacer group Chemical group 0.000 description 7
- 238000003756 stirring Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 101000597428 Homo sapiens Nucleoredoxin-like protein 1 Proteins 0.000 description 5
- 241000699666 Mus <mouse, genus> Species 0.000 description 5
- 210000004899 c-terminal region Anatomy 0.000 description 5
- 230000004927 fusion Effects 0.000 description 5
- 210000001525 retina Anatomy 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- 230000003248 secreting effect Effects 0.000 description 5
- 238000001262 western blot Methods 0.000 description 5
- 241000282412 Homo Species 0.000 description 4
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- 102000057445 human NXNL1 Human genes 0.000 description 4
- 230000008488 polyadenylation Effects 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 238000011144 upstream manufacturing Methods 0.000 description 4
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical group C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 3
- 238000001712 DNA sequencing Methods 0.000 description 3
- 239000000499 gel Substances 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 210000005260 human cell Anatomy 0.000 description 3
- 238000001727 in vivo Methods 0.000 description 3
- 239000013608 rAAV vector Substances 0.000 description 3
- 230000004517 retinal physiology Effects 0.000 description 3
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 2
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008827 biological function Effects 0.000 description 2
- 239000006143 cell culture medium Substances 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 238000005119 centrifugation Methods 0.000 description 2
- 239000003153 chemical reaction reagent Substances 0.000 description 2
- 238000010367 cloning Methods 0.000 description 2
- 239000002299 complementary DNA Substances 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000007850 degeneration Effects 0.000 description 2
- 208000011325 dry age related macular degeneration Diseases 0.000 description 2
- 230000008014 freezing Effects 0.000 description 2
- 238000007710 freezing Methods 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 230000002163 immunogen Effects 0.000 description 2
- 238000000338 in vitro Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 210000004962 mammalian cell Anatomy 0.000 description 2
- 230000008569 process Effects 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 108020001580 protein domains Proteins 0.000 description 2
- 210000002763 pyramidal cell Anatomy 0.000 description 2
- 230000002207 retinal effect Effects 0.000 description 2
- 230000004243 retinal function Effects 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 229920000936 Agarose Polymers 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 201000004569 Blindness Diseases 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 102100021519 Hemoglobin subunit beta Human genes 0.000 description 1
- 108091005904 Hemoglobin subunit beta Proteins 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 108091092195 Intron Proteins 0.000 description 1
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 101000597433 Mus musculus Nucleoredoxin-like protein 1 Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 101710163270 Nuclease Proteins 0.000 description 1
- 102100036205 Nucleoredoxin-like protein 2 Human genes 0.000 description 1
- 101710106007 Nucleoredoxin-like protein 2 Proteins 0.000 description 1
- 108010038807 Oligopeptides Proteins 0.000 description 1
- 102000015636 Oligopeptides Human genes 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 206010038848 Retinal detachment Diseases 0.000 description 1
- 108010034546 Serratia marcescens nuclease Proteins 0.000 description 1
- 208000027073 Stargardt disease Diseases 0.000 description 1
- 101710159478 Thioredoxin-like protein Proteins 0.000 description 1
- 239000007983 Tris buffer Substances 0.000 description 1
- 208000014769 Usher Syndromes Diseases 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 229960003964 deoxycholic acid Drugs 0.000 description 1
- KXGVEGMKQFWNSR-LLQZFEROSA-N deoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 KXGVEGMKQFWNSR-LLQZFEROSA-N 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 230000009982 effect on human Effects 0.000 description 1
- 239000012149 elution buffer Substances 0.000 description 1
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 230000003993 interaction Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 229960000310 isoleucine Drugs 0.000 description 1
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 1
- 150000002632 lipids Chemical class 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 239000002773 nucleotide Substances 0.000 description 1
- 125000003729 nucleotide group Chemical group 0.000 description 1
- 201000005111 ocular hyperemia Diseases 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 108091008695 photoreceptors Proteins 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 230000001124 posttranscriptional effect Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 238000009163 protein therapy Methods 0.000 description 1
- 238000000746 purification Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 230000002829 reductive effect Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000004264 retinal detachment Effects 0.000 description 1
- 210000003583 retinal pigment epithelium Anatomy 0.000 description 1
- 210000000880 retinal rod photoreceptor cell Anatomy 0.000 description 1
- 239000012723 sample buffer Substances 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 238000010257 thawing Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 238000013519 translation Methods 0.000 description 1
- LENZDBCJOHFCAS-UHFFFAOYSA-N tris Chemical compound OCC(N)(CO)CO LENZDBCJOHFCAS-UHFFFAOYSA-N 0.000 description 1
- 230000001228 trophic effect Effects 0.000 description 1
- 239000004474 valine Substances 0.000 description 1
- 238000003260 vortexing Methods 0.000 description 1
- 239000011534 wash buffer Substances 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/1703—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- A61K38/1709—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/42—Proteins; Polypeptides; Degradation products thereof; Derivatives thereof, e.g. albumin, gelatin or zein
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/62—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being a protein, peptide or polyamino acid
- A61K47/64—Drug-peptide, drug-protein or drug-polyamino acid conjugates, i.e. the modifying agent being a peptide, protein or polyamino acid which is covalently bonded or complexed to a therapeutically active agent
- A61K47/643—Albumins, e.g. HSA, BSA, ovalbumin or a Keyhole Limpet Hemocyanin [KHL]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K48/00—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy
- A61K48/005—Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy characterised by an aspect of the 'active' part of the composition delivered, i.e. the nucleic acid delivered
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0048—Eye, e.g. artificial tears
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/76—Albumins
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/111—General methods applicable to biologically active non-coding nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/0004—Oxidoreductases (1.)
- C12N9/0012—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.6, 1.7)
- C12N9/0036—Oxidoreductases (1.) acting on nitrogen containing compounds as donors (1.4, 1.5, 1.6, 1.7) acting on NADH or NADPH (1.6)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y108/00—Oxidoreductases acting on sulfur groups as donors (1.8)
- C12Y108/01—Oxidoreductases acting on sulfur groups as donors (1.8) with NAD+ or NADP+ as acceptor (1.8.1)
- C12Y108/01008—Protein-disulfide reductase (1.8.1.8), i.e. thioredoxin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/01—Fusion polypeptide containing a localisation/targetting motif
- C07K2319/02—Fusion polypeptide containing a localisation/targetting motif containing a signal sequence
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/31—Fusion polypeptide fusions, other than Fc, for prolonged plasma life, e.g. albumin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
- C12N2750/14143—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2800/00—Nucleic acids vectors
- C12N2800/22—Vectors comprising a coding region that has been codon optimised for expression in a respective host
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- General Health & Medical Sciences (AREA)
- Biomedical Technology (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Medicinal Chemistry (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Pharmacology & Pharmacy (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Plant Pathology (AREA)
- Physics & Mathematics (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Virology (AREA)
- Immunology (AREA)
- Marine Sciences & Fisheries (AREA)
- Neurosurgery (AREA)
- Neurology (AREA)
- Psychology (AREA)
- Ophthalmology & Optometry (AREA)
- Inorganic Chemistry (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Description
Annex C/ST.25テキストファイルの形態で電子的に提出された配列表と、付属ファイル参照番号WOC-019PCTは、開示の一部である。
配列番号1:融合タンパク質のN末端でヒトアルブミンを有する、ヒトアルブミンと短鎖RdCVFとの融合タンパク質のアミノ酸配列(ALB-RdCVFS)。
理論に縛られることを望むものではないが、短鎖型RdCVFを発現及び分泌することの困難性は、その高い疎水性アミノ酸組成に起因する可能性がある。短鎖型及び長鎖型ヒトRdCVFタンパク質の両方のアミノ酸組成を慎重に分析することにより、短鎖型RdCVFタンパク質が極めて疎水性であることが明らかになった(図1A及び1B)。短鎖RdCVFの109のうちの42アミノ酸(38.5%)は、疎水性アミノ酸である。1ストレッチの6つの疎水性アミノ酸、1ストレッチの4つの疎水性アミノ酸、及び2ストレッチの3つの疎水性アミノ酸がそれぞれ存在する。疎水性アミノ酸組成が高い割合であると、疎水性-疎水性相互作用を介して脂質膜に付着する可能性がより高いので、短鎖RdCVFを哺乳動物細胞からインビトロ及びインビボで効率的に発現及び分泌することを非常に困難にする可能性が高い。興味深いことに、長鎖型RdCVFのN末端109アミノ酸は短鎖RdCVF全体と同一であるが、長鎖RdCVFのC末端の103アミノ酸は疎水性ではなく、わずか25%のアミノ酸が疎水性であるのみである(103のうち26)。長鎖RdCVFのこのC末端における最も長い疎水性アミノ酸のストレッチの長さはわずか4アミノ酸である。3つの疎水性アミノ酸のストレッチはない。長鎖RdCVFのC末端における比較的親水性の性質は、長鎖RdCVFの全体的な疎水性を低下させるのに重要な役割を果たし得る。
Ala: 1.800
Arg: -4.500
Asn: -3.500
Asp: -3.500
Cys: 2.500
Gln: -3.500
Glu: -3.500
Gly: -0.400
His: -3.200
Ile: 4.500
Leu: 3.800
Lys: -3.900
Met: 1.900
Phe: 2.800
Pro: -1.600
Ser: -0.800
Thr: -0.700
Trp: -0.900
Tyr: -1.300
Val: 4.200
[実施例]
プラスミドクローニング
N末端でヒトアルブミンと融合しているコドン最適化されたヒト短鎖型RdCVFタンパク質のcDNAを、GENEART(登録商標)(フィー・フォー・サービスの会社)によって合成し、アデノ随伴ウイルスベクタープラスミドpAAV-MCS(Cell Biolabs社製、San Diego、CA)にクローニングし、プラスミドpAAV-ALB-RdCVFSを作製した。ヒトアルブミン由来のシグナルペプチドもまた、RdCVF及びアルブミン融合タンパク質コード配列の上流に組み込まれた。
プラスミドpAAV-ALB-RdCVFS又はpAAV-RdCVFS-ALB、pHELPER(Cell BioLabs社製、カタログ番号340202)及びpRC2(Cell BioLabs社製、カタログ番号340201)をDH10Bコンピテントバクテリア細胞(Invitrogen社製、カタログ番号18297-010)に形質転換し、Qiagen EndoFree Plasmid Maxi Kit又はEndoFree Plasmid Mega Kitを製造者の指示に従って使用してスケールアップした。Beckman DU-600分光光度計を用いてプラスミド濃度を測定した。各々のプラスミドの同一性は、制限酵素切断及びDNA配列決定分析によって確認した。
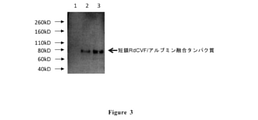
4~20%のSDS-PAGEゲルを用いたウエスタンブロット分析を用いて、標準技術を用いてRdCVF及びアルブミン融合タンパク質発現を検出した。対照として、5μL容量のMAGICMARK(商標)XPウエスタンタンパク質標準(Invitrogen社製、カタログ番号LC5602)を外側のウェルに添加した。タンパク質試料緩衝液と混合したrAAVベクター形質導入ヒト293細胞の各々からの細胞培養液30μLを各ウェルに添加した。染料がゲルの底に達するまでゲルを200Vで流した。ウエスタンブロット分析は、Vector Laboratories社のVECTASTAIN(登録商標)ABC-Ampウエスタンブロット分析キットを用いて、製造会社の修正版の使用説明書に従って行った。SDS-PAGEをトランスファーバッファーで20分間平衡化し、SDS-PAGEで分離したタンパク質をTrans Blot Semi-Dry Transfer Cellを用いて20Vで40分間、ニトロセルロース膜に転写した。転写が完了した後、室温(RT)で少なくとも2時間、又は4℃で終夜、ロッカープラットフォーム上で穏やかに撹拌しながら、200mLの1xカゼイン溶液中で膜をブロックした。4℃で終夜又は室温で1時間穏やかに撹拌しながら、1:2000に希釈したウサギ抗RdCVFタンパク質特異的抗体(一次抗体、Covance社(Denver、PA)によって生成された)を含む50mLの1xカゼイン溶液とともに、膜をインキュベートした。30mLの1xカゼイン溶液で4回、穏やかに撹拌しながら室温で各々5分間、膜を洗浄した。膜を、1:10000に希釈した30mLのビオチン化ヤギ抗ウサギIgG(二次抗体)とともに、1xカゼイン溶液中で穏やかに撹拌しながら室温で1時間インキュベートした。30mLの1xカゼイン溶液中で3回、穏やかに撹拌しながら室温で各々5分間、膜を洗浄した。100μLの試薬A及び100μLの試薬Bを含む50mLの1xカゼイン中のVectastain ABC-AmPで45分間、膜をインキュベートした。30mLの1xカゼイン溶液中で3回、室温で緩やかに撹拌しながら各々5分間、膜を洗浄した。
N末端でヒトアルブミン融合タンパク質コード配列と融合しているコドン最適化された(再コードされた)ヒト短鎖RdCVFをコードする組換えAAVベクターは、ヒト細胞内での融合タンパク質の発現及びヒト細胞からの分泌を媒介することができた。C末端でヒトアルブミン融合タンパク質と融合しているコドン最適化されたヒト短鎖RdCVFをコードする組換えAAVベクターは、ヒト細胞内での融合タンパク質の発現及びヒト細胞からの分泌を媒介することができた。
Claims (20)
- (a)第1のN末端シグナルペプチド配列、前記シグナルペプチド配列のC末端にある第2のペプチド配列、及び前記第2のペプチド配列のC末端にある第3のペプチド配列;又は(b)第2のペプチド配列及び前記第2のペプチド配列のC末端にある第3のペプチド配列;を含む融合タンパク質であって、
前記第1のペプチド配列が、Igkシグナル配列であり、前記第2のペプチド配列がRdCVF-短鎖ペプチド配列であり、前記第3のペプチド配列がヒトアルブミンであり;かつ
配列(配列番号3)又は(配列番号3)のアミノ酸22~732を有する、前記融合タンパク質。 - 請求項1に記載の融合タンパク質をコードする核酸。
- DNAである、請求項2に記載の核酸。
- 配列(配列番号3)を有する融合タンパク質をコードする、請求項2に記載の核酸。
- 配列(配列番号4)を有する、請求項4に記載の核酸。
- 制御配列に作動可能に連結された、請求項2~5のいずれかに記載の核酸を含む発現ベクター。
- 制御配列がプロモーターである、請求項6に記載の発現ベクター。
- プロモーターがCMVプロモーターである、請求項7に記載の発現ベクター。
- ベクターがプラスミドである、請求項6に記載の発現ベクター。
- ベクターがAAV発現プラスミドである、請求項9に記載の発現ベクター。
- ベクターがウイルスベクターである、請求項6に記載の発現ベクター。
- ウイルスベクターが、AAVベクター、レンチウイルスベクター、レトロウイルスベクター、アデノウイルスベクター、及び合成ウイルスベクターからなる群から選択される、請求項11に記載の発現ベクター。
- 請求項2~5のいずれかに記載の核酸又は請求項6~12のいずれかに記載の発現ベクターを含む細胞。
- (i)請求項1に記載の融合タンパク質、請求項2~5のいずれかに記載の核酸、請求項6~12のいずれかに記載の発現ベクター、及び、請求項13に記載の細胞からなる群から選択される成分;及び(ii)薬学的に許容される担体;を含む医薬組成物。
- 哺乳動物患者における状態の治療剤であって、請求項1に記載の融合タンパク質、請求項2~5のいずれかに記載の核酸、請求項6~12のいずれかに記載の発現ベクター、請求項13に記載の細胞、又は請求項14に記載の医薬組成物を含み、投与され、それにより前記患者における前記状態が治療され、
前記状態が、網膜ジストロフィー、シュタルガルト病、色素性網膜炎、萎縮型加齢性黄斑変性症(萎縮型AMD)、地図状萎縮(萎縮型AMDの進行期)、滲出型加齢性黄斑変性症(滲出型AMD)、高眼圧を伴う又は伴わない緑内障、糖尿病性網膜症、バルデ・ビードル症候群、バッセン・コーンツヴァイク症候群、ベスト病、コロイデマ、脳回転状萎縮症、先天性黒内障、レフサン症候群、アッシャー症候群、甲状腺関連眼疾患、グレーブ病、網膜色素上皮細胞に関連する疾患、前眼部疾患、水晶体疾患/白内障、眼杯障害、ブドウ膜炎、アルツハイマー病、ハンチントン病、パーキンソン病、及び嗅覚疾患からなる群から選択される、前記治療剤。 - 状態が眼の状態であり、投与が網膜下注射及び硝子体内注射からなる群から選択される、請求項15に記載の剤。
- 患者における眼の光受容器細胞の保護剤であって、請求項1に記載の融合タンパク質、請求項2~5のいずれかに記載の核酸、請求項6~12のいずれかに記載の発現ベクター、請求項13に記載の細胞、又は請求項14に記載の医薬組成物を含み、投与され、それにより前記患者における前記眼の光受容器細胞を保護する、前記保護剤。
- 投与が、網膜下注射及び硝子体内注射からなる群から選択される、請求項17に記載の剤。
- 患者がヒト患者である、請求項15~18のいずれかに記載の剤。
- 請求項1に記載の融合タンパク質を産生する方法であって、コードされた前記融合タンパク質の発現及び分泌を可能にする条件下で請求項13に記載の細胞を培養するステップであって、前記細胞が発現ベクターを含み、前記発現ベクターが配列(配列番号3)を有する融合タンパク質をコードする核酸を含み、前記核酸が制御配列に作動可能に連結されている、前記ステップ、並びに前記融合タンパク質を前記細胞培養物から単離するステップを含む、前記方法。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662406552P | 2016-10-11 | 2016-10-11 | |
| US62/406,552 | 2016-10-11 | ||
| PCT/US2017/056030 WO2018071465A1 (en) | 2016-10-11 | 2017-10-11 | Fusion protein between short form rod-derived cone viability factor and a hydrophilic peptide |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2019532616A JP2019532616A (ja) | 2019-11-14 |
| JP2019532616A5 JP2019532616A5 (ja) | 2020-11-19 |
| JP7028802B2 true JP7028802B2 (ja) | 2022-03-02 |
Family
ID=61906325
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2018563544A Active JP7028802B2 (ja) | 2016-10-11 | 2017-10-11 | 短鎖型桿体由来錐体生存因子及び親水性ペプチド間の融合タンパク質 |
Country Status (12)
| Country | Link |
|---|---|
| US (2) | US10946063B2 (ja) |
| EP (1) | EP3526238A4 (ja) |
| JP (1) | JP7028802B2 (ja) |
| KR (2) | KR20190058388A (ja) |
| CN (1) | CN109415423A (ja) |
| AU (1) | AU2017344059B2 (ja) |
| BR (1) | BR112018076674A2 (ja) |
| CA (1) | CA3025977A1 (ja) |
| IL (1) | IL263990A (ja) |
| MX (1) | MX2018015596A (ja) |
| WO (1) | WO2018071465A1 (ja) |
| ZA (1) | ZA201808041B (ja) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN113667685B (zh) * | 2018-08-07 | 2023-02-28 | 康码(上海)生物科技有限公司 | 信号肽相关序列及其在蛋白质合成中的应用 |
| EP4268895A3 (en) * | 2019-12-09 | 2024-01-10 | Chigenovo Co., Ltd. | Uses of cyp4v2 and rdcvf in preparation of drugs |
| CN111733174B (zh) * | 2020-08-07 | 2021-02-09 | 北京大学第三医院(北京大学第三临床医学院) | 一种分离的核酸分子及其用途 |
| WO2024206928A1 (en) * | 2023-03-30 | 2024-10-03 | Pharma Cinq, Llc | VECTOR ENCODING ROD-DERIVED CONE VIABILITY FACTOR AND HUMAN IgK SIGNAL SEQUENCE |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009146183A1 (en) | 2008-04-15 | 2009-12-03 | Genzyme Corporation | Methods to produce rod-derived cone viability factor (rdcvf) |
| JP2010529958A (ja) | 2007-06-05 | 2010-09-02 | アンスティテュ、ナショナル、ド、ラ、サント、エ、ド、ラ、ルシェルシュ、メディカル(アンセルム) | ニューロン生存因子およびその使用 |
| JP2014516358A (ja) | 2011-04-12 | 2014-07-10 | ガンガゲン インコーポレーティッド | キメラ抗菌ポリペプチド |
| JP2015501156A (ja) | 2011-10-27 | 2015-01-15 | ウェルスタット オフサルミクス コーポレイション | 桿体由来錐体生存因子をコードするベクター |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH07241196A (ja) * | 1994-03-04 | 1995-09-19 | Teruhiko Beppu | アルブミンおよびヒトアポリポプロテインe様遺伝子を有する融合遺伝子、それを挿入したプラスミド並びにその用途 |
| DE69731660T2 (de) | 1996-12-02 | 2005-12-22 | Valentis Inc., Burlingame | Insulinähnlicher wachstumfaktor i (igf-i) expressionssystem und methode zur verwendung |
| FR2823221B1 (fr) | 2001-04-06 | 2004-04-02 | Univ Pasteur | Sequences associees a la degenerescence retinienne et applications |
| EP1463752A4 (en) | 2001-12-21 | 2005-07-13 | Human Genome Sciences Inc | ALBUMIN FUSION PROTEINS |
| CN1241946C (zh) | 2002-07-01 | 2006-02-15 | 美国福源集团 | 对多种细胞具刺激增生作用的人血清白蛋白重组融合蛋白 |
| DE10260805A1 (de) | 2002-12-23 | 2004-07-22 | Geneart Gmbh | Verfahren und Vorrichtung zum Optimieren einer Nucleotidsequenz zur Expression eines Proteins |
| FR2870241B1 (fr) | 2004-05-13 | 2015-02-27 | Novartis Ag | Facteur de viabilite des cones derive des batonnets ou rdcvf et applications |
| ES2548980T3 (es) | 2008-09-10 | 2015-10-22 | Inserm - Institut National De La Santé Et De La Recherche Médicale | Factor de viabilidad neuronal y uso del mismo |
| WO2010050586A1 (ja) | 2008-10-31 | 2010-05-06 | ディナベック株式会社 | 組み換え蛋白質の発現を増強する方法 |
| WO2014012082A2 (en) | 2012-07-13 | 2014-01-16 | Zymeworks Inc. | Multivalent heteromultimer scaffold design an constructs |
| TN2016000220A1 (en) | 2013-12-06 | 2017-10-06 | Ct Hospitalier Universitaire Montpellier | Methods and pharmaceutical compositions for expressing a polynucleotide of interest in the retinal pigment epithelium of a subject. |
| US10857240B2 (en) | 2016-01-05 | 2020-12-08 | The Trustees Of The University Of Pennsylvania | Methods and compositions for treatment of ocular disorders and blinding diseases |
-
2017
- 2017-10-11 CA CA3025977A patent/CA3025977A1/en active Pending
- 2017-10-11 AU AU2017344059A patent/AU2017344059B2/en active Active
- 2017-10-11 US US16/301,764 patent/US10946063B2/en active Active
- 2017-10-11 WO PCT/US2017/056030 patent/WO2018071465A1/en unknown
- 2017-10-11 BR BR112018076674-7A patent/BR112018076674A2/pt unknown
- 2017-10-11 JP JP2018563544A patent/JP7028802B2/ja active Active
- 2017-10-11 KR KR1020187037891A patent/KR20190058388A/ko not_active Application Discontinuation
- 2017-10-11 CN CN201780040158.3A patent/CN109415423A/zh active Pending
- 2017-10-11 KR KR1020237037858A patent/KR20230156440A/ko active Application Filing
- 2017-10-11 EP EP17860101.9A patent/EP3526238A4/en active Pending
- 2017-10-11 MX MX2018015596A patent/MX2018015596A/es unknown
-
2018
- 2018-11-28 ZA ZA2018/08041A patent/ZA201808041B/en unknown
- 2018-12-27 IL IL263990A patent/IL263990A/en unknown
-
2021
- 2021-02-10 US US17/172,202 patent/US12076367B2/en active Active
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010529958A (ja) | 2007-06-05 | 2010-09-02 | アンスティテュ、ナショナル、ド、ラ、サント、エ、ド、ラ、ルシェルシュ、メディカル(アンセルム) | ニューロン生存因子およびその使用 |
| WO2009146183A1 (en) | 2008-04-15 | 2009-12-03 | Genzyme Corporation | Methods to produce rod-derived cone viability factor (rdcvf) |
| JP2014516358A (ja) | 2011-04-12 | 2014-07-10 | ガンガゲン インコーポレーティッド | キメラ抗菌ポリペプチド |
| JP2015501156A (ja) | 2011-10-27 | 2015-01-15 | ウェルスタット オフサルミクス コーポレイション | 桿体由来錐体生存因子をコードするベクター |
Non-Patent Citations (1)
| Title |
|---|
| J Clin Invest, 2015 Jan, vol. 125, no. 1, pp. 105-116 |
Also Published As
| Publication number | Publication date |
|---|---|
| KR20190058388A (ko) | 2019-05-29 |
| AU2017344059B2 (en) | 2021-12-23 |
| KR20230156440A (ko) | 2023-11-14 |
| CN109415423A (zh) | 2019-03-01 |
| AU2017344059A1 (en) | 2018-12-13 |
| BR112018076674A2 (pt) | 2019-04-02 |
| RU2018144780A3 (ja) | 2021-02-02 |
| EP3526238A4 (en) | 2020-04-29 |
| US12076367B2 (en) | 2024-09-03 |
| JP2019532616A (ja) | 2019-11-14 |
| ZA201808041B (en) | 2019-09-25 |
| WO2018071465A1 (en) | 2018-04-19 |
| RU2018144780A (ru) | 2020-11-17 |
| EP3526238A1 (en) | 2019-08-21 |
| US10946063B2 (en) | 2021-03-16 |
| CA3025977A1 (en) | 2018-04-19 |
| MX2018015596A (es) | 2019-03-14 |
| IL263990A (en) | 2019-01-31 |
| US20210162005A1 (en) | 2021-06-03 |
| US20190151410A1 (en) | 2019-05-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US12076367B2 (en) | Fusion protein between short form rod-derived cone viability factor and a hydrophilic peptide | |
| JP6293664B2 (ja) | 桿体由来錐体生存因子をコードするベクター | |
| JP7078620B2 (ja) | アレルギーの治療のための核酸 | |
| RU2639521C2 (ru) | Аналоги фактора комплемента в и их применения | |
| US10035829B2 (en) | Methods to produce rod-derived cone viability factor (RDCVF) | |
| KR20210104661A (ko) | 인테인 단백질 및 이의 용도 | |
| JP2023504773A (ja) | 眼遺伝子送達のためのaavベクター変異体 | |
| KR20200141435A (ko) | 망막 질환 치료를 위한 조성물 및 방법 | |
| CN112567053A (zh) | 重组核酸构建体 | |
| RU2773368C2 (ru) | Слитый белок короткой формы фактора жизнеспособности колбочек, полученного из палочек, и гидрофильного пептида | |
| CN112601454B (zh) | 用于治疗杜兴肌营养不良的组合物和方法 | |
| CN116925192A (zh) | 一种融合型腺相关病毒及其应用 | |
| US20240342313A1 (en) | Vector encoding rod-derived cone viability factor and human igk signal sequence | |
| KR20240023126A (ko) | 망막 장애 | |
| KR20240023127A (ko) | 망막 장애 | |
| JP2021520204A (ja) | 酸化ストレスに対する遺伝子療法 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201007 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20201007 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20210709 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210719 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20211014 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20220207 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20220217 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7028802 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313113 |