KR20190057305A - 자궁내막증에 대한 바이오마커로서의 microRNAs - Google Patents
자궁내막증에 대한 바이오마커로서의 microRNAs Download PDFInfo
- Publication number
- KR20190057305A KR20190057305A KR1020197009115A KR20197009115A KR20190057305A KR 20190057305 A KR20190057305 A KR 20190057305A KR 1020197009115 A KR1020197009115 A KR 1020197009115A KR 20197009115 A KR20197009115 A KR 20197009115A KR 20190057305 A KR20190057305 A KR 20190057305A
- Authority
- KR
- South Korea
- Prior art keywords
- mir
- mirna
- endometriosis
- subject
- sample
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 239000002679 microRNA Substances 0.000 title claims abstract description 478
- 201000009273 Endometriosis Diseases 0.000 title claims abstract description 421
- 108700011259 MicroRNAs Proteins 0.000 title claims description 131
- 239000000090 biomarker Substances 0.000 title description 100
- 108091070501 miRNA Proteins 0.000 claims abstract description 362
- 238000000034 method Methods 0.000 claims abstract description 321
- 230000014509 gene expression Effects 0.000 claims abstract description 89
- 239000000523 sample Substances 0.000 claims description 206
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 111
- 150000007523 nucleic acids Chemical class 0.000 claims description 109
- 108091065175 miR-3613 stem-loop Proteins 0.000 claims description 104
- 102000039446 nucleic acids Human genes 0.000 claims description 93
- 238000011282 treatment Methods 0.000 claims description 93
- 108020004707 nucleic acids Proteins 0.000 claims description 92
- 108091084619 miR-125b-1 stem-loop Proteins 0.000 claims description 89
- 108091063409 miR-125b-2 stem-loop Proteins 0.000 claims description 89
- 108091050014 miR-125b-3 stem-loop Proteins 0.000 claims description 88
- 201000010099 disease Diseases 0.000 claims description 86
- 108091055145 miR-342 stem-loop Proteins 0.000 claims description 85
- 239000012472 biological sample Substances 0.000 claims description 60
- 108091049400 miR-6755 stem-loop Proteins 0.000 claims description 52
- 210000002966 serum Anatomy 0.000 claims description 51
- 241000282414 Homo sapiens Species 0.000 claims description 50
- 108091046841 MiR-150 Proteins 0.000 claims description 50
- 239000002299 complementary DNA Substances 0.000 claims description 46
- 108091090860 miR-150 stem-loop Proteins 0.000 claims description 46
- 108091038400 miR-553 stem-loop Proteins 0.000 claims description 44
- 230000001105 regulatory effect Effects 0.000 claims description 43
- 108091064282 miR-125 stem-loop Proteins 0.000 claims description 42
- 108091037066 miR-125-1 stem-loop Proteins 0.000 claims description 42
- 108091062107 miR-125-2 stem-loop Proteins 0.000 claims description 42
- 108091079767 miR-125-3 stem-loop Proteins 0.000 claims description 42
- -1 miR-451 Proteins 0.000 claims description 41
- 210000003296 saliva Anatomy 0.000 claims description 41
- 108091062221 miR-18a stem-loop Proteins 0.000 claims description 39
- 238000003752 polymerase chain reaction Methods 0.000 claims description 37
- 108091007423 let-7b Proteins 0.000 claims description 36
- 108091038080 miR-18a-1 stem-loop Proteins 0.000 claims description 36
- 108091032770 miR-451 stem-loop Proteins 0.000 claims description 36
- 108091030646 miR-451a stem-loop Proteins 0.000 claims description 36
- 108091028684 Mir-145 Proteins 0.000 claims description 35
- 210000001124 body fluid Anatomy 0.000 claims description 35
- 239000010839 body fluid Substances 0.000 claims description 34
- 238000006243 chemical reaction Methods 0.000 claims description 33
- 108091073532 miR-143 stem-loop Proteins 0.000 claims description 33
- 238000012163 sequencing technique Methods 0.000 claims description 33
- 108091055732 miR-4668 stem-loop Proteins 0.000 claims description 31
- 230000003321 amplification Effects 0.000 claims description 30
- 238000003199 nucleic acid amplification method Methods 0.000 claims description 30
- 210000002381 plasma Anatomy 0.000 claims description 29
- 230000035945 sensitivity Effects 0.000 claims description 28
- 108091028066 Mir-126 Proteins 0.000 claims description 26
- 108091027766 Mir-143 Proteins 0.000 claims description 26
- 108091053592 miR-145-1 stem-loop Proteins 0.000 claims description 26
- 108091056559 miR-145-2 stem-loop Proteins 0.000 claims description 26
- 238000003556 assay Methods 0.000 claims description 25
- 210000004369 blood Anatomy 0.000 claims description 25
- 239000008280 blood Substances 0.000 claims description 25
- 150000001875 compounds Chemical class 0.000 claims description 25
- 108091043157 miR-500a stem-loop Proteins 0.000 claims description 25
- 210000002700 urine Anatomy 0.000 claims description 25
- 108091087529 miR-500 stem-loop Proteins 0.000 claims description 24
- 108091036496 miR-500-2 stem-loop Proteins 0.000 claims description 24
- 108091026807 MiR-214 Proteins 0.000 claims description 22
- 208000024891 symptom Diseases 0.000 claims description 19
- 206010036790 Productive cough Diseases 0.000 claims description 17
- 210000003802 sputum Anatomy 0.000 claims description 17
- 208000024794 sputum Diseases 0.000 claims description 17
- 238000012544 monitoring process Methods 0.000 claims description 16
- 210000001175 cerebrospinal fluid Anatomy 0.000 claims description 15
- 239000003814 drug Substances 0.000 claims description 15
- 239000012530 fluid Substances 0.000 claims description 15
- 210000001179 synovial fluid Anatomy 0.000 claims description 15
- 210000002751 lymph Anatomy 0.000 claims description 14
- 108091027698 miR-18-1 stem-loop Proteins 0.000 claims description 14
- 108091090961 miR-18-2 stem-loop Proteins 0.000 claims description 14
- 210000003097 mucus Anatomy 0.000 claims description 14
- 238000003753 real-time PCR Methods 0.000 claims description 14
- 239000003153 chemical reaction reagent Substances 0.000 claims description 13
- 210000003608 fece Anatomy 0.000 claims description 13
- 230000002829 reductive effect Effects 0.000 claims description 13
- 238000010839 reverse transcription Methods 0.000 claims description 13
- 238000002493 microarray Methods 0.000 claims description 12
- 230000027455 binding Effects 0.000 claims description 11
- 230000008859 change Effects 0.000 claims description 11
- 238000001794 hormone therapy Methods 0.000 claims description 11
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 claims description 10
- 101000857870 Squalus acanthias Gonadoliberin Proteins 0.000 claims description 10
- 239000000556 agonist Substances 0.000 claims description 10
- XLXSAKCOAKORKW-AQJXLSMYSA-N gonadorelin Chemical group C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 XLXSAKCOAKORKW-AQJXLSMYSA-N 0.000 claims description 10
- 229940035638 gonadotropin-releasing hormone Drugs 0.000 claims description 10
- 108091023663 let-7 stem-loop Proteins 0.000 claims description 10
- 108091063478 let-7-1 stem-loop Proteins 0.000 claims description 10
- 108091049777 let-7-2 stem-loop Proteins 0.000 claims description 10
- 108091091807 let-7a stem-loop Proteins 0.000 claims description 10
- 108091057746 let-7a-4 stem-loop Proteins 0.000 claims description 10
- 108091028376 let-7a-5 stem-loop Proteins 0.000 claims description 10
- 108091024393 let-7a-6 stem-loop Proteins 0.000 claims description 10
- 108091091174 let-7a-7 stem-loop Proteins 0.000 claims description 10
- 229940079593 drug Drugs 0.000 claims description 8
- 230000002441 reversible effect Effects 0.000 claims description 8
- 108700028369 Alleles Proteins 0.000 claims description 7
- 238000012165 high-throughput sequencing Methods 0.000 claims description 7
- 208000002193 Pain Diseases 0.000 claims description 6
- 239000003433 contraceptive agent Substances 0.000 claims description 6
- 238000009830 intercalation Methods 0.000 claims description 6
- 108091091360 miR-125b stem-loop Proteins 0.000 claims description 6
- 230000036407 pain Effects 0.000 claims description 6
- 239000002474 gonadorelin antagonist Substances 0.000 claims description 5
- 108091058688 miR-141 stem-loop Proteins 0.000 claims description 5
- 108091089775 miR-200b stem-loop Proteins 0.000 claims description 5
- 108091059199 miR-200a stem-loop Proteins 0.000 claims description 4
- 108091090583 miR-34c stem-loop Proteins 0.000 claims description 4
- 108091082133 miR-34c-1 stem-loop Proteins 0.000 claims description 4
- 239000013642 negative control Substances 0.000 claims description 4
- 239000013641 positive control Substances 0.000 claims description 4
- 230000004044 response Effects 0.000 claims description 4
- 229910052500 inorganic mineral Inorganic materials 0.000 claims description 3
- 108091037327 miR-449 stem-loop Proteins 0.000 claims description 3
- 108091040525 miR-449a stem-loop Proteins 0.000 claims description 3
- 239000011707 mineral Substances 0.000 claims description 3
- 239000005557 antagonist Substances 0.000 claims description 2
- 229940124558 contraceptive agent Drugs 0.000 claims description 2
- 108091041042 miR-18 stem-loop Proteins 0.000 claims description 2
- 108091056935 miR-145b stem-loop Proteins 0.000 claims 2
- 108091029500 miR-183 stem-loop Proteins 0.000 claims 2
- 230000003054 hormonal effect Effects 0.000 claims 1
- 108091043994 let-7g stem-loop Proteins 0.000 claims 1
- 108091053972 let-7g-1 stem-loop Proteins 0.000 claims 1
- 108091024867 let-7g-2 stem-loop Proteins 0.000 claims 1
- 239000000203 mixture Substances 0.000 abstract description 30
- 239000013615 primer Substances 0.000 description 58
- 210000004027 cell Anatomy 0.000 description 44
- 238000003745 diagnosis Methods 0.000 description 30
- 239000002773 nucleotide Substances 0.000 description 29
- 125000003729 nucleotide group Chemical group 0.000 description 29
- 238000012360 testing method Methods 0.000 description 28
- 108090000623 proteins and genes Proteins 0.000 description 27
- 238000009396 hybridization Methods 0.000 description 26
- 208000035475 disorder Diseases 0.000 description 24
- 108020004414 DNA Proteins 0.000 description 21
- 239000003550 marker Substances 0.000 description 20
- 108020004711 Nucleic Acid Probes Proteins 0.000 description 18
- 230000000295 complement effect Effects 0.000 description 18
- 238000001514 detection method Methods 0.000 description 18
- 239000002853 nucleic acid probe Substances 0.000 description 18
- 108090000765 processed proteins & peptides Proteins 0.000 description 18
- 241001465754 Metazoa Species 0.000 description 17
- 108091028043 Nucleic acid sequence Proteins 0.000 description 17
- 239000000463 material Substances 0.000 description 17
- 239000000047 product Substances 0.000 description 17
- 210000001519 tissue Anatomy 0.000 description 17
- 230000000694 effects Effects 0.000 description 16
- 108091026331 miR-214 stem-loop Proteins 0.000 description 15
- 108091048888 miR-214-1 stem-loop Proteins 0.000 description 15
- 108091078347 miR-214-2 stem-loop Proteins 0.000 description 15
- 108091035552 miR-214-3 stem-loop Proteins 0.000 description 15
- 238000001356 surgical procedure Methods 0.000 description 15
- 238000005259 measurement Methods 0.000 description 14
- 108091023084 miR-126 stem-loop Proteins 0.000 description 14
- 108091065272 miR-126-1 stem-loop Proteins 0.000 description 14
- 108091081187 miR-126-2 stem-loop Proteins 0.000 description 14
- 108091030790 miR-126-3 stem-loop Proteins 0.000 description 14
- 108091092317 miR-126-4 stem-loop Proteins 0.000 description 14
- 102000004169 proteins and genes Human genes 0.000 description 13
- 238000004458 analytical method Methods 0.000 description 11
- 102000004196 processed proteins & peptides Human genes 0.000 description 11
- 238000002560 therapeutic procedure Methods 0.000 description 11
- 108091050724 let-7b stem-loop Proteins 0.000 description 10
- 230000001225 therapeutic effect Effects 0.000 description 10
- 239000003795 chemical substances by application Substances 0.000 description 9
- 108091007772 MIRLET7C Proteins 0.000 description 8
- 230000000692 anti-sense effect Effects 0.000 description 8
- 238000005119 centrifugation Methods 0.000 description 8
- 239000000975 dye Substances 0.000 description 8
- 238000011156 evaluation Methods 0.000 description 8
- 108020004999 messenger RNA Proteins 0.000 description 8
- 239000002987 primer (paints) Substances 0.000 description 8
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 7
- 238000012300 Sequence Analysis Methods 0.000 description 7
- 230000001413 cellular effect Effects 0.000 description 7
- 230000002357 endometrial effect Effects 0.000 description 7
- 239000003112 inhibitor Substances 0.000 description 7
- 230000003902 lesion Effects 0.000 description 7
- 108091024449 let-7e stem-loop Proteins 0.000 description 7
- 108091044227 let-7e-1 stem-loop Proteins 0.000 description 7
- 108091071181 let-7e-2 stem-loop Proteins 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 239000011541 reaction mixture Substances 0.000 description 7
- 238000013518 transcription Methods 0.000 description 7
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 7
- 102000004190 Enzymes Human genes 0.000 description 6
- 108090000790 Enzymes Proteins 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 6
- 238000011529 RT qPCR Methods 0.000 description 6
- 239000012190 activator Substances 0.000 description 6
- 230000008901 benefit Effects 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- 239000012634 fragment Substances 0.000 description 6
- 108091033319 polynucleotide Proteins 0.000 description 6
- 102000040430 polynucleotide Human genes 0.000 description 6
- 239000002157 polynucleotide Substances 0.000 description 6
- 229920001184 polypeptide Polymers 0.000 description 6
- 230000004083 survival effect Effects 0.000 description 6
- 230000035897 transcription Effects 0.000 description 6
- 108020005187 Oligonucleotide Probes Proteins 0.000 description 5
- 208000000450 Pelvic Pain Diseases 0.000 description 5
- 108091093037 Peptide nucleic acid Proteins 0.000 description 5
- 230000004913 activation Effects 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 238000012512 characterization method Methods 0.000 description 5
- 238000007796 conventional method Methods 0.000 description 5
- 230000000875 corresponding effect Effects 0.000 description 5
- 238000001727 in vivo Methods 0.000 description 5
- 208000000509 infertility Diseases 0.000 description 5
- 230000036512 infertility Effects 0.000 description 5
- 231100000535 infertility Toxicity 0.000 description 5
- 230000000977 initiatory effect Effects 0.000 description 5
- 108091033753 let-7d stem-loop Proteins 0.000 description 5
- 108091007427 let-7g Proteins 0.000 description 5
- 230000035772 mutation Effects 0.000 description 5
- 238000002966 oligonucleotide array Methods 0.000 description 5
- 239000002751 oligonucleotide probe Substances 0.000 description 5
- 230000002285 radioactive effect Effects 0.000 description 5
- 238000007619 statistical method Methods 0.000 description 5
- 238000006467 substitution reaction Methods 0.000 description 5
- 238000003786 synthesis reaction Methods 0.000 description 5
- 238000013519 translation Methods 0.000 description 5
- 102000053602 DNA Human genes 0.000 description 4
- 241000124008 Mammalia Species 0.000 description 4
- 238000000585 Mann–Whitney U test Methods 0.000 description 4
- 108091005461 Nucleic proteins Proteins 0.000 description 4
- 230000002159 abnormal effect Effects 0.000 description 4
- 239000000730 antalgic agent Substances 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 238000002512 chemotherapy Methods 0.000 description 4
- 238000013211 curve analysis Methods 0.000 description 4
- 238000012217 deletion Methods 0.000 description 4
- 230000037430 deletion Effects 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 230000003828 downregulation Effects 0.000 description 4
- 230000036541 health Effects 0.000 description 4
- 230000001976 improved effect Effects 0.000 description 4
- 238000002357 laparoscopic surgery Methods 0.000 description 4
- 108091063986 let-7f stem-loop Proteins 0.000 description 4
- 230000000670 limiting effect Effects 0.000 description 4
- 230000002018 overexpression Effects 0.000 description 4
- 230000007170 pathology Effects 0.000 description 4
- 238000002360 preparation method Methods 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 238000003757 reverse transcription PCR Methods 0.000 description 4
- 229940124597 therapeutic agent Drugs 0.000 description 4
- 238000011285 therapeutic regimen Methods 0.000 description 4
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 3
- 108091030146 MiRBase Proteins 0.000 description 3
- 108091034117 Oligonucleotide Proteins 0.000 description 3
- 241000282520 Papio Species 0.000 description 3
- 238000007792 addition Methods 0.000 description 3
- 150000001413 amino acids Chemical group 0.000 description 3
- 229940035676 analgesics Drugs 0.000 description 3
- 238000000137 annealing Methods 0.000 description 3
- 238000003491 array Methods 0.000 description 3
- 239000007795 chemical reaction product Substances 0.000 description 3
- 238000012790 confirmation Methods 0.000 description 3
- 239000013068 control sample Substances 0.000 description 3
- 230000002559 cytogenic effect Effects 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 230000007812 deficiency Effects 0.000 description 3
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 3
- 238000013399 early diagnosis Methods 0.000 description 3
- 238000005516 engineering process Methods 0.000 description 3
- 231100000502 fertility decrease Toxicity 0.000 description 3
- 239000007850 fluorescent dye Substances 0.000 description 3
- 239000000499 gel Substances 0.000 description 3
- 230000009368 gene silencing by RNA Effects 0.000 description 3
- 230000007062 hydrolysis Effects 0.000 description 3
- 238000006460 hydrolysis reaction Methods 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 238000000338 in vitro Methods 0.000 description 3
- 238000000099 in vitro assay Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 230000005764 inhibitory process Effects 0.000 description 3
- 238000002372 labelling Methods 0.000 description 3
- 238000002844 melting Methods 0.000 description 3
- 230000008018 melting Effects 0.000 description 3
- 239000008194 pharmaceutical composition Substances 0.000 description 3
- 229920000642 polymer Polymers 0.000 description 3
- 238000011002 quantification Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 229940126585 therapeutic drug Drugs 0.000 description 3
- 238000012800 visualization Methods 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- 229930024421 Adenine Natural products 0.000 description 2
- GFFGJBXGBJISGV-UHFFFAOYSA-N Adenine Chemical compound NC1=NC=NC2=C1N=CN2 GFFGJBXGBJISGV-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 208000001154 Dermoid Cyst Diseases 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 108091062170 Mir-22 Proteins 0.000 description 2
- 238000000636 Northern blotting Methods 0.000 description 2
- RJKFOVLPORLFTN-LEKSSAKUSA-N Progesterone Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 RJKFOVLPORLFTN-LEKSSAKUSA-N 0.000 description 2
- 238000001190 Q-PCR Methods 0.000 description 2
- 238000002123 RNA extraction Methods 0.000 description 2
- 108091030071 RNAI Proteins 0.000 description 2
- 108020004459 Small interfering RNA Proteins 0.000 description 2
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 2
- 229960000643 adenine Drugs 0.000 description 2
- 230000001668 ameliorated effect Effects 0.000 description 2
- 239000008346 aqueous phase Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 239000012620 biological material Substances 0.000 description 2
- 238000001574 biopsy Methods 0.000 description 2
- 238000009534 blood test Methods 0.000 description 2
- 238000010805 cDNA synthesis kit Methods 0.000 description 2
- 230000001684 chronic effect Effects 0.000 description 2
- 230000004087 circulation Effects 0.000 description 2
- 238000003776 cleavage reaction Methods 0.000 description 2
- 238000004590 computer program Methods 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- 238000002405 diagnostic procedure Methods 0.000 description 2
- 238000011984 electrochemiluminescence immunoassay Methods 0.000 description 2
- 210000005168 endometrial cell Anatomy 0.000 description 2
- 201000002595 endometriosis of ovary Diseases 0.000 description 2
- 210000004696 endometrium Anatomy 0.000 description 2
- 230000008029 eradication Effects 0.000 description 2
- 238000002474 experimental method Methods 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 230000002349 favourable effect Effects 0.000 description 2
- 230000035558 fertility Effects 0.000 description 2
- 238000007672 fourth generation sequencing Methods 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- UYTPUPDQBNUYGX-UHFFFAOYSA-N guanine Chemical compound O=C1NC(N)=NC2=C1N=CN2 UYTPUPDQBNUYGX-UHFFFAOYSA-N 0.000 description 2
- 231100001261 hazardous Toxicity 0.000 description 2
- 230000013632 homeostatic process Effects 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 238000003384 imaging method Methods 0.000 description 2
- 238000012309 immunohistochemistry technique Methods 0.000 description 2
- 238000009169 immunotherapy Methods 0.000 description 2
- 238000005462 in vivo assay Methods 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 238000002350 laparotomy Methods 0.000 description 2
- 108091030917 let-7b-1 stem-loop Proteins 0.000 description 2
- 108091082924 let-7b-2 stem-loop Proteins 0.000 description 2
- 239000003446 ligand Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000000891 luminescent agent Substances 0.000 description 2
- 238000007726 management method Methods 0.000 description 2
- 108091044988 miR-125a stem-loop Proteins 0.000 description 2
- 108091045872 miR-135 stem-loop Proteins 0.000 description 2
- 108091043249 miR-135-1 stem-loop Proteins 0.000 description 2
- 108091064876 miR-135-2 stem-loop Proteins 0.000 description 2
- 108091026523 miR-135a stem-loop Proteins 0.000 description 2
- 108091046933 miR-18b stem-loop Proteins 0.000 description 2
- 108091050398 miR-500b stem-loop Proteins 0.000 description 2
- 108091024443 miRa-135-1 stem-loop Proteins 0.000 description 2
- 238000010208 microarray analysis Methods 0.000 description 2
- BQJCRHHNABKAKU-KBQPJGBKSA-N morphine Chemical compound O([C@H]1[C@H](C=C[C@H]23)O)C4=C5[C@@]12CCN(C)[C@@H]3CC5=CC=C4O BQJCRHHNABKAKU-KBQPJGBKSA-N 0.000 description 2
- 230000009871 nonspecific binding Effects 0.000 description 2
- 210000004940 nucleus Anatomy 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 210000003463 organelle Anatomy 0.000 description 2
- 208000030747 ovarian endometriosis Diseases 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 230000001575 pathological effect Effects 0.000 description 2
- PTMHPRAIXMAOOB-UHFFFAOYSA-L phosphoramidate Chemical compound NP([O-])([O-])=O PTMHPRAIXMAOOB-UHFFFAOYSA-L 0.000 description 2
- 230000001323 posttranslational effect Effects 0.000 description 2
- 230000002062 proliferating effect Effects 0.000 description 2
- 230000000069 prophylactic effect Effects 0.000 description 2
- 230000001850 reproductive effect Effects 0.000 description 2
- 150000003839 salts Chemical class 0.000 description 2
- 230000007017 scission Effects 0.000 description 2
- 230000003248 secreting effect Effects 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 239000002924 silencing RNA Substances 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 238000001308 synthesis method Methods 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 230000008685 targeting Effects 0.000 description 2
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 2
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 2
- 230000003827 upregulation Effects 0.000 description 2
- 210000004291 uterus Anatomy 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- WWYNJERNGUHSAO-XUDSTZEESA-N (+)-Norgestrel Chemical compound O=C1CC[C@@H]2[C@H]3CC[C@](CC)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1 WWYNJERNGUHSAO-XUDSTZEESA-N 0.000 description 1
- ZGGHKIMDNBDHJB-NRFPMOEYSA-M (3R,5S)-fluvastatin sodium Chemical compound [Na+].C12=CC=CC=C2N(C(C)C)C(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)=C1C1=CC=C(F)C=C1 ZGGHKIMDNBDHJB-NRFPMOEYSA-M 0.000 description 1
- PIINGYXNCHTJTF-UHFFFAOYSA-N 2-(2-azaniumylethylamino)acetate Chemical group NCCNCC(O)=O PIINGYXNCHTJTF-UHFFFAOYSA-N 0.000 description 1
- 206010000060 Abdominal distension Diseases 0.000 description 1
- DLFVBJFMPXGRIB-UHFFFAOYSA-N Acetamide Chemical compound CC(N)=O DLFVBJFMPXGRIB-UHFFFAOYSA-N 0.000 description 1
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 206010001233 Adenoma benign Diseases 0.000 description 1
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 1
- 108020005544 Antisense RNA Proteins 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- XUKUURHRXDUEBC-KAYWLYCHSA-N Atorvastatin Chemical compound C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CC[C@@H](O)C[C@@H](O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-KAYWLYCHSA-N 0.000 description 1
- XUKUURHRXDUEBC-UHFFFAOYSA-N Atorvastatin Natural products C=1C=CC=CC=1C1=C(C=2C=CC(F)=CC=2)N(CCC(O)CC(O)CC(O)=O)C(C(C)C)=C1C(=O)NC1=CC=CC=C1 XUKUURHRXDUEBC-UHFFFAOYSA-N 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 238000009010 Bradford assay Methods 0.000 description 1
- 108010037003 Buserelin Proteins 0.000 description 1
- KXDHJXZQYSOELW-UHFFFAOYSA-M Carbamate Chemical compound NC([O-])=O KXDHJXZQYSOELW-UHFFFAOYSA-M 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- 208000005623 Carcinogenesis Diseases 0.000 description 1
- 208000024172 Cardiovascular disease Diseases 0.000 description 1
- 108090000994 Catalytic RNA Proteins 0.000 description 1
- 102000053642 Catalytic RNA Human genes 0.000 description 1
- 108091033380 Coding strand Proteins 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 206010011732 Cyst Diseases 0.000 description 1
- 201000005171 Cystadenoma Diseases 0.000 description 1
- 102000004127 Cytokines Human genes 0.000 description 1
- 108090000695 Cytokines Proteins 0.000 description 1
- 102000012410 DNA Ligases Human genes 0.000 description 1
- 108010061982 DNA Ligases Proteins 0.000 description 1
- 108020003215 DNA Probes Proteins 0.000 description 1
- 239000003155 DNA primer Substances 0.000 description 1
- 239000003298 DNA probe Substances 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- GJKXGJCSJWBJEZ-XRSSZCMZSA-N Deslorelin Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CNC2=CC=CC=C12 GJKXGJCSJWBJEZ-XRSSZCMZSA-N 0.000 description 1
- SHIBSTMRCDJXLN-UHFFFAOYSA-N Digoxigenin Natural products C1CC(C2C(C3(C)CCC(O)CC3CC2)CC2O)(O)C2(C)C1C1=CC(=O)OC1 SHIBSTMRCDJXLN-UHFFFAOYSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 206010061819 Disease recurrence Diseases 0.000 description 1
- 206010014733 Endometrial cancer Diseases 0.000 description 1
- 206010014759 Endometrial neoplasm Diseases 0.000 description 1
- 206010016654 Fibrosis Diseases 0.000 description 1
- 108010069236 Goserelin Proteins 0.000 description 1
- BLCLNMBMMGCOAS-URPVMXJPSA-N Goserelin Chemical compound C([C@@H](C(=O)N[C@H](COC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NNC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 BLCLNMBMMGCOAS-URPVMXJPSA-N 0.000 description 1
- 101000829171 Hypocrea virens (strain Gv29-8 / FGSC 10586) Effector TSP1 Proteins 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102100034343 Integrase Human genes 0.000 description 1
- 108010000817 Leuprolide Proteins 0.000 description 1
- 239000005089 Luciferase Substances 0.000 description 1
- 108060001084 Luciferase Proteins 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 108060004795 Methyltransferase Proteins 0.000 description 1
- 108091007780 MiR-122 Proteins 0.000 description 1
- 108091080995 Mir-9/mir-79 microRNA precursor family Proteins 0.000 description 1
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 description 1
- 108010085220 Multiprotein Complexes Proteins 0.000 description 1
- 102000007474 Multiprotein Complexes Human genes 0.000 description 1
- 108010021717 Nafarelin Proteins 0.000 description 1
- 206010029260 Neuroblastoma Diseases 0.000 description 1
- TTZMPOZCBFTTPR-UHFFFAOYSA-N O=P1OCO1 Chemical compound O=P1OCO1 TTZMPOZCBFTTPR-UHFFFAOYSA-N 0.000 description 1
- BRUQQQPBMZOVGD-XFKAJCMBSA-N Oxycodone Chemical compound O=C([C@@H]1O2)CC[C@@]3(O)[C@H]4CC5=CC=C(OC)C2=C5[C@@]13CCN4C BRUQQQPBMZOVGD-XFKAJCMBSA-N 0.000 description 1
- 206010058674 Pelvic Infection Diseases 0.000 description 1
- 208000012896 Peritoneal disease Diseases 0.000 description 1
- 229920002594 Polyethylene Glycol 8000 Polymers 0.000 description 1
- TUZYXOIXSAXUGO-UHFFFAOYSA-N Pravastatin Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(O)C=C21 TUZYXOIXSAXUGO-UHFFFAOYSA-N 0.000 description 1
- 108091034057 RNA (poly(A)) Proteins 0.000 description 1
- 108020004518 RNA Probes Proteins 0.000 description 1
- 238000010802 RNA extraction kit Methods 0.000 description 1
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 1
- 101710086015 RNA ligase Proteins 0.000 description 1
- 239000003391 RNA probe Substances 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 102000006382 Ribonucleases Human genes 0.000 description 1
- 108010083644 Ribonucleases Proteins 0.000 description 1
- RYMZZMVNJRMUDD-UHFFFAOYSA-N SJ000286063 Natural products C12C(OC(=O)C(C)(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 RYMZZMVNJRMUDD-UHFFFAOYSA-N 0.000 description 1
- AJLFOPYRIVGYMJ-UHFFFAOYSA-N SJ000287055 Natural products C12C(OC(=O)C(C)CC)CCC=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 AJLFOPYRIVGYMJ-UHFFFAOYSA-N 0.000 description 1
- 108091081021 Sense strand Proteins 0.000 description 1
- 102000039471 Small Nuclear RNA Human genes 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 206010042618 Surgical procedure repeated Diseases 0.000 description 1
- 108700005078 Synthetic Genes Proteins 0.000 description 1
- 108010050144 Triptorelin Pamoate Proteins 0.000 description 1
- 102000006943 Uracil-DNA Glycosidase Human genes 0.000 description 1
- 108010072685 Uracil-DNA Glycosidase Proteins 0.000 description 1
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 1
- DUDJTRNGXIUJEB-UHFFFAOYSA-N [N].NCC(O)=O Chemical compound [N].NCC(O)=O DUDJTRNGXIUJEB-UHFFFAOYSA-N 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- 230000003213 activating effect Effects 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 239000000654 additive Substances 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 230000032683 aging Effects 0.000 description 1
- 238000007844 allele-specific PCR Methods 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 239000003098 androgen Substances 0.000 description 1
- 229940030486 androgens Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 230000003466 anti-cipated effect Effects 0.000 description 1
- 239000000074 antisense oligonucleotide Substances 0.000 description 1
- 238000012230 antisense oligonucleotides Methods 0.000 description 1
- 239000003886 aromatase inhibitor Substances 0.000 description 1
- 229940046844 aromatase inhibitors Drugs 0.000 description 1
- 238000007846 asymmetric PCR Methods 0.000 description 1
- 229960005370 atorvastatin Drugs 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 239000005082 bioluminescent agent Substances 0.000 description 1
- 239000000091 biomarker candidate Substances 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 208000024330 bloating Diseases 0.000 description 1
- 210000000601 blood cell Anatomy 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 238000004820 blood count Methods 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 229960002719 buserelin Drugs 0.000 description 1
- CUWODFFVMXJOKD-UVLQAERKSA-N buserelin Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](COC(C)(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 CUWODFFVMXJOKD-UVLQAERKSA-N 0.000 description 1
- 238000010804 cDNA synthesis Methods 0.000 description 1
- VTYYLEPIZMXCLO-UHFFFAOYSA-L calcium carbonate Substances [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 231100000504 carcinogenesis Toxicity 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 230000004709 cell invasion Effects 0.000 description 1
- 230000010001 cellular homeostasis Effects 0.000 description 1
- 229960005110 cerivastatin Drugs 0.000 description 1
- SEERZIQQUAZTOL-ANMDKAQQSA-N cerivastatin Chemical compound COCC1=C(C(C)C)N=C(C(C)C)C(\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)=C1C1=CC=C(F)C=C1 SEERZIQQUAZTOL-ANMDKAQQSA-N 0.000 description 1
- 239000005081 chemiluminescent agent Substances 0.000 description 1
- 238000000546 chi-square test Methods 0.000 description 1
- 230000035606 childbirth Effects 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000000052 comparative effect Effects 0.000 description 1
- 239000003184 complementary RNA Substances 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 229940111134 coxibs Drugs 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 125000004122 cyclic group Chemical group 0.000 description 1
- 230000001351 cycling effect Effects 0.000 description 1
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 description 1
- 208000031513 cyst Diseases 0.000 description 1
- 230000009089 cytolysis Effects 0.000 description 1
- 229940104302 cytosine Drugs 0.000 description 1
- POZRVZJJTULAOH-LHZXLZLDSA-N danazol Chemical compound C1[C@]2(C)[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=CC2=C1C=NO2 POZRVZJJTULAOH-LHZXLZLDSA-N 0.000 description 1
- 229960000766 danazol Drugs 0.000 description 1
- 230000009849 deactivation Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000003935 denaturing gradient gel electrophoresis Methods 0.000 description 1
- 239000005549 deoxyribonucleoside Substances 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000008021 deposition Effects 0.000 description 1
- 238000000586 desensitisation Methods 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 229960005408 deslorelin Drugs 0.000 description 1
- 108700025485 deslorelin Proteins 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 239000000104 diagnostic biomarker Substances 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 235000005911 diet Nutrition 0.000 description 1
- 230000037213 diet Effects 0.000 description 1
- 235000015872 dietary supplement Nutrition 0.000 description 1
- 230000004069 differentiation Effects 0.000 description 1
- 238000007847 digital PCR Methods 0.000 description 1
- QONQRTHLHBTMGP-UHFFFAOYSA-N digitoxigenin Natural products CC12CCC(C3(CCC(O)CC3CC3)C)C3C11OC1CC2C1=CC(=O)OC1 QONQRTHLHBTMGP-UHFFFAOYSA-N 0.000 description 1
- SHIBSTMRCDJXLN-KCZCNTNESA-N digoxigenin Chemical compound C1([C@@H]2[C@@]3([C@@](CC2)(O)[C@H]2[C@@H]([C@@]4(C)CC[C@H](O)C[C@H]4CC2)C[C@H]3O)C)=CC(=O)OC1 SHIBSTMRCDJXLN-KCZCNTNESA-N 0.000 description 1
- NAGJZTKCGNOGPW-UHFFFAOYSA-K dioxido-sulfanylidene-sulfido-$l^{5}-phosphane Chemical compound [O-]P([O-])([S-])=S NAGJZTKCGNOGPW-UHFFFAOYSA-K 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 238000011304 droplet digital PCR Methods 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- JGMOKGBVKVMRFX-HQZYFCCVSA-N dydrogesterone Chemical compound C1=CC2=CC(=O)CC[C@@]2(C)[C@H]2[C@@H]1[C@@H]1CC[C@H](C(=O)C)[C@@]1(C)CC2 JGMOKGBVKVMRFX-HQZYFCCVSA-N 0.000 description 1
- 229960004913 dydrogesterone Drugs 0.000 description 1
- 201000006828 endometrial hyperplasia Diseases 0.000 description 1
- 208000016018 endometrial polyp Diseases 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 230000003628 erosive effect Effects 0.000 description 1
- 230000007717 exclusion Effects 0.000 description 1
- 210000001808 exosome Anatomy 0.000 description 1
- 238000010195 expression analysis Methods 0.000 description 1
- 210000001723 extracellular space Anatomy 0.000 description 1
- 230000004761 fibrosis Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 229960003765 fluvastatin Drugs 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 238000002695 general anesthesia Methods 0.000 description 1
- 229960002913 goserelin Drugs 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 201000000079 gynecomastia Diseases 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 206010073071 hepatocellular carcinoma Diseases 0.000 description 1
- 231100000844 hepatocellular carcinoma Toxicity 0.000 description 1
- 230000002962 histologic effect Effects 0.000 description 1
- 108700020746 histrelin Proteins 0.000 description 1
- 229960002193 histrelin Drugs 0.000 description 1
- HHXHVIJIIXKSOE-QILQGKCVSA-N histrelin Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC(N=C1)=CN1CC1=CC=CC=C1 HHXHVIJIIXKSOE-QILQGKCVSA-N 0.000 description 1
- 238000007849 hot-start PCR Methods 0.000 description 1
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 1
- 238000009802 hysterectomy Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- 238000007901 in situ hybridization Methods 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 208000021267 infertility disease Diseases 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- GFIJNRVAKGFPGQ-LIJARHBVSA-N leuprolide Chemical compound CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)CC1=CC=C(O)C=C1 GFIJNRVAKGFPGQ-LIJARHBVSA-N 0.000 description 1
- 229960004338 leuprorelin Drugs 0.000 description 1
- 229960004400 levonorgestrel Drugs 0.000 description 1
- 208000019423 liver disease Diseases 0.000 description 1
- 238000001325 log-rank test Methods 0.000 description 1
- 238000007477 logistic regression Methods 0.000 description 1
- 229960004844 lovastatin Drugs 0.000 description 1
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 description 1
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 description 1
- 235000019689 luncheon sausage Nutrition 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000004880 lymph fluid Anatomy 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 238000007403 mPCR Methods 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 210000004962 mammalian cell Anatomy 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000001404 mediated effect Effects 0.000 description 1
- 238000013160 medical therapy Methods 0.000 description 1
- 229960002985 medroxyprogesterone acetate Drugs 0.000 description 1
- PSGAAPLEWMOORI-PEINSRQWSA-N medroxyprogesterone acetate Chemical compound C([C@@]12C)CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2CC[C@]2(C)[C@@](OC(C)=O)(C(C)=O)CC[C@H]21 PSGAAPLEWMOORI-PEINSRQWSA-N 0.000 description 1
- 239000000155 melt Substances 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 230000006894 menstrual cycle phase Effects 0.000 description 1
- 125000000325 methylidene group Chemical group [H]C([H])=* 0.000 description 1
- YACKEPLHDIMKIO-UHFFFAOYSA-N methylphosphonic acid Chemical compound CP(O)(O)=O YACKEPLHDIMKIO-UHFFFAOYSA-N 0.000 description 1
- 229950009116 mevastatin Drugs 0.000 description 1
- AJLFOPYRIVGYMJ-INTXDZFKSA-N mevastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=CCC[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 AJLFOPYRIVGYMJ-INTXDZFKSA-N 0.000 description 1
- BOZILQFLQYBIIY-UHFFFAOYSA-N mevastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CCC=C21 BOZILQFLQYBIIY-UHFFFAOYSA-N 0.000 description 1
- 108091051828 miR-122 stem-loop Proteins 0.000 description 1
- 108091026375 miR-135b stem-loop Proteins 0.000 description 1
- 108091086065 miR-135b-2 stem-loop Proteins 0.000 description 1
- 108091091751 miR-17 stem-loop Proteins 0.000 description 1
- 108091044046 miR-17-1 stem-loop Proteins 0.000 description 1
- 108091065423 miR-17-3 stem-loop Proteins 0.000 description 1
- 108091025686 miR-199a stem-loop Proteins 0.000 description 1
- 108091029956 miR-200a-1 stem-loop Proteins 0.000 description 1
- 108091088721 miR-200a-2 stem-loop Proteins 0.000 description 1
- 108091070312 miR-200a-3 stem-loop Proteins 0.000 description 1
- 108091056309 miR-200b-1 stem-loop Proteins 0.000 description 1
- 108091026985 miR-200b-2 stem-loop Proteins 0.000 description 1
- 108091049679 miR-20a stem-loop Proteins 0.000 description 1
- 108091083237 miR-451b stem-loop Proteins 0.000 description 1
- 108091060293 miR-453 stem-loop Proteins 0.000 description 1
- 108091025542 miR-668 stem-loop Proteins 0.000 description 1
- 108091047084 miR-9 stem-loop Proteins 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 230000000897 modulatory effect Effects 0.000 description 1
- 239000000178 monomer Substances 0.000 description 1
- 229960005181 morphine Drugs 0.000 description 1
- 210000000214 mouth Anatomy 0.000 description 1
- 208000004707 mucinous cystadenoma Diseases 0.000 description 1
- 238000010202 multivariate logistic regression analysis Methods 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- RWHUEXWOYVBUCI-ITQXDASVSA-N nafarelin Chemical compound C([C@@H](C(=O)N[C@H](CC=1C=C2C=CC=CC2=CC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 RWHUEXWOYVBUCI-ITQXDASVSA-N 0.000 description 1
- 229960002333 nafarelin Drugs 0.000 description 1
- 239000011807 nanoball Substances 0.000 description 1
- 230000003533 narcotic effect Effects 0.000 description 1
- 238000007857 nested PCR Methods 0.000 description 1
- 238000007481 next generation sequencing Methods 0.000 description 1
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 1
- 229940053934 norethindrone Drugs 0.000 description 1
- VIKNJXKGJWUCNN-XGXHKTLJSA-N norethisterone Chemical compound O=C1CC[C@@H]2[C@H]3CC[C@](C)([C@](CC4)(O)C#C)[C@@H]4[C@@H]3CCC2=C1 VIKNJXKGJWUCNN-XGXHKTLJSA-N 0.000 description 1
- 238000002515 oligonucleotide synthesis Methods 0.000 description 1
- 238000009806 oophorectomy Methods 0.000 description 1
- 229940127234 oral contraceptive Drugs 0.000 description 1
- 239000003539 oral contraceptive agent Substances 0.000 description 1
- 150000007530 organic bases Chemical class 0.000 description 1
- 230000009818 osteogenic differentiation Effects 0.000 description 1
- 201000008968 osteosarcoma Diseases 0.000 description 1
- 208000025661 ovarian cyst Diseases 0.000 description 1
- 201000010302 ovarian serous cystadenocarcinoma Diseases 0.000 description 1
- 230000027758 ovulation cycle Effects 0.000 description 1
- 229960002085 oxycodone Drugs 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 239000013610 patient sample Substances 0.000 description 1
- 206010034260 pelvic mass Diseases 0.000 description 1
- 210000003200 peritoneal cavity Anatomy 0.000 description 1
- 239000012466 permeate Substances 0.000 description 1
- 150000004713 phosphodiesters Chemical class 0.000 description 1
- 150000008300 phosphoramidites Chemical class 0.000 description 1
- 239000005080 phosphorescent agent Substances 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 229960002797 pitavastatin Drugs 0.000 description 1
- VGYFMXBACGZSIL-MCBHFWOFSA-N pitavastatin Chemical compound OC(=O)C[C@H](O)C[C@H](O)\C=C\C1=C(C2CC2)N=C2C=CC=CC2=C1C1=CC=C(F)C=C1 VGYFMXBACGZSIL-MCBHFWOFSA-N 0.000 description 1
- 210000004910 pleural fluid Anatomy 0.000 description 1
- 229920002401 polyacrylamide Polymers 0.000 description 1
- 230000008488 polyadenylation Effects 0.000 description 1
- 230000001124 posttranscriptional effect Effects 0.000 description 1
- 229960002965 pravastatin Drugs 0.000 description 1
- TUZYXOIXSAXUGO-PZAWKZKUSA-N pravastatin Chemical compound C1=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC(O)=O)[C@H]2[C@@H](OC(=O)[C@@H](C)CC)C[C@H](O)C=C21 TUZYXOIXSAXUGO-PZAWKZKUSA-N 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000035935 pregnancy Effects 0.000 description 1
- 238000004321 preservation Methods 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 239000000186 progesterone Substances 0.000 description 1
- 229960003387 progesterone Drugs 0.000 description 1
- 239000000583 progesterone congener Substances 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000035752 proliferative phase Effects 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 239000013074 reference sample Substances 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 239000002342 ribonucleoside Substances 0.000 description 1
- 108091092562 ribozyme Proteins 0.000 description 1
- 238000005096 rolling process Methods 0.000 description 1
- 229960000672 rosuvastatin Drugs 0.000 description 1
- BPRHUIZQVSMCRT-VEUZHWNKSA-N rosuvastatin Chemical compound CC(C)C1=NC(N(C)S(C)(=O)=O)=NC(C=2C=CC(F)=CC=2)=C1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O BPRHUIZQVSMCRT-VEUZHWNKSA-N 0.000 description 1
- 238000007480 sanger sequencing Methods 0.000 description 1
- 238000007423 screening assay Methods 0.000 description 1
- 238000007789 sealing Methods 0.000 description 1
- 239000004065 semiconductor Substances 0.000 description 1
- 238000007841 sequencing by ligation Methods 0.000 description 1
- 208000005893 serous cystadenoma Diseases 0.000 description 1
- 230000007727 signaling mechanism Effects 0.000 description 1
- 229960002855 simvastatin Drugs 0.000 description 1
- RYMZZMVNJRMUDD-HGQWONQESA-N simvastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)C(C)(C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 RYMZZMVNJRMUDD-HGQWONQESA-N 0.000 description 1
- 238000007860 single-cell PCR Methods 0.000 description 1
- 108091029842 small nuclear ribonucleic acid Proteins 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 238000011895 specific detection Methods 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 150000003457 sulfones Chemical class 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 238000005382 thermal cycling Methods 0.000 description 1
- 150000003568 thioethers Chemical class 0.000 description 1
- 229940113082 thymine Drugs 0.000 description 1
- 230000002103 transcriptional effect Effects 0.000 description 1
- 230000001052 transient effect Effects 0.000 description 1
- 229960004824 triptorelin Drugs 0.000 description 1
- VXKHXGOKWPXYNA-PGBVPBMZSA-N triptorelin Chemical compound C([C@@H](C(=O)N[C@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 VXKHXGOKWPXYNA-PGBVPBMZSA-N 0.000 description 1
- 230000005760 tumorsuppression Effects 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 238000012285 ultrasound imaging Methods 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 229940035893 uracil Drugs 0.000 description 1
- 206010046811 uterine polyp Diseases 0.000 description 1
- 230000035899 viability Effects 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 239000011782 vitamin Substances 0.000 description 1
- 235000013343 vitamin Nutrition 0.000 description 1
- 229940088594 vitamin Drugs 0.000 description 1
- 229930003231 vitamin Natural products 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 230000005186 women's health Effects 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/689—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids related to pregnancy or the gonads
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/5308—Immunoassay; Biospecific binding assay; Materials therefor for analytes not provided for elsewhere, e.g. nucleic acids, uric acid, worms, mites
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/106—Pharmacogenomics, i.e. genetic variability in individual responses to drugs and drug metabolism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/112—Disease subtyping, staging or classification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/158—Expression markers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/178—Oligonucleotides characterized by their use miRNA, siRNA or ncRNA
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/36—Gynecology or obstetrics
- G01N2800/364—Endometriosis, i.e. non-malignant disorder in which functioning endometrial tissue is present outside the uterine cavity
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Analytical Chemistry (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pathology (AREA)
- Biotechnology (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Medicinal Chemistry (AREA)
- Food Science & Technology (AREA)
- General Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Reproductive Health (AREA)
- Gynecology & Obstetrics (AREA)
- General Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Tropical Medicine & Parasitology (AREA)
- Pregnancy & Childbirth (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Endocrinology (AREA)
- General Chemical & Material Sciences (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662381130P | 2016-08-30 | 2016-08-30 | |
| US62/381,130 | 2016-08-30 | ||
| PCT/US2017/049284 WO2018044979A1 (en) | 2016-08-30 | 2017-08-30 | Micrornas as biomarkers for endometriosis |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20190057305A true KR20190057305A (ko) | 2019-05-28 |
Family
ID=61301726
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020197009115A Ceased KR20190057305A (ko) | 2016-08-30 | 2017-08-30 | 자궁내막증에 대한 바이오마커로서의 microRNAs |
Country Status (11)
| Country | Link |
|---|---|
| US (3) | US10982282B2 (enExample) |
| EP (1) | EP3506912B1 (enExample) |
| JP (2) | JP2019528081A (enExample) |
| KR (1) | KR20190057305A (enExample) |
| CN (1) | CN109890394A (enExample) |
| AU (1) | AU2017319325A1 (enExample) |
| BR (1) | BR112019004075A2 (enExample) |
| CA (1) | CA3035429A1 (enExample) |
| MX (1) | MX2019002425A (enExample) |
| SG (2) | SG10202102509TA (enExample) |
| WO (1) | WO2018044979A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102242639B1 (ko) * | 2020-01-28 | 2021-04-21 | 고려대학교 산학협력단 | 간 섬유화 진단용 바이오마커 miRNA4668-5p |
| WO2024123061A1 (ko) * | 2022-12-06 | 2024-06-13 | 연세대학교 산학협력단 | 자궁내막증 진단용 조성물 |
Families Citing this family (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
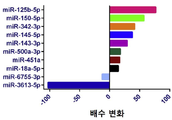
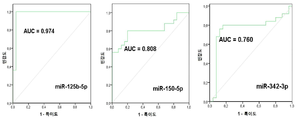
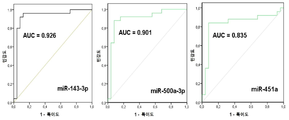
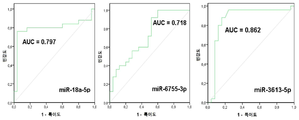
| EP3122905B1 (en) | 2014-03-27 | 2020-09-09 | Yale University | Circulating micrornas as biomarkers for endometriosis |
| BR112019004075A2 (pt) | 2016-08-30 | 2019-05-28 | Univ Yale | métodos para diagnosticar um indivíduo com suspeita de endometriose, para detectar mirna, para diagnosticar um indivíduo que apresenta sintomas de endometriose ou com suspeita de endometriose, para tratar um indivíduo com endometriose, para diagnosticar e tratar um indivíduo com suspeita de endometriose, para diagnosticar endometriose em um indivíduo, para diagnosticar ou prover um prognóstico para endometriose em um indivíduo e para monitorar uma resposta a um tratamento de endometriose em um indivíduo, e, kit. |
| CA3073829A1 (en) * | 2017-08-30 | 2019-03-07 | Dot Laboratories, Inc. | Methods and compositions for detecting and treating endometriosis |
| EP3820483A4 (en) * | 2018-07-13 | 2023-03-01 | Yale University | COMPOSITIONS AND METHODS FOR THE TREATMENT OF ENDOMETRIOSIS |
| KR102089559B1 (ko) | 2018-08-31 | 2020-03-16 | 차의과학대학교 산학협력단 | 난소의 fsh에 대한 반응성의 진단 또는 예측용 바이오마커 및 이의 용도 |
| CA3118133A1 (en) * | 2018-10-31 | 2020-05-07 | Yale University | A quantitative algorithm for endometriosis |
| US11315660B2 (en) | 2018-10-31 | 2022-04-26 | Dot Laboratories, Inc. | Method of detecting and treating endometriosis in a female subject |
| WO2020146544A1 (en) * | 2019-01-09 | 2020-07-16 | Research Institute At Nationwide Children's Hospital | A novel method for monitoring endometriosis and associated disorders |
| WO2020179895A1 (ja) | 2019-03-07 | 2020-09-10 | 東レ株式会社 | 子宮平滑筋肉腫の検出のためのキット、デバイス及び方法 |
| ES2787725A1 (es) * | 2019-04-15 | 2020-10-16 | Univ Granada | Metodo de obtencion de datos utiles para diagnosticar telangiectasia hemorragica hereditaria |
| CN110144394A (zh) * | 2019-04-18 | 2019-08-20 | 辽宁省肿瘤医院 | 一种子宫内膜异位症诊断、预后标志物及其应用 |
| CN114144674A (zh) * | 2019-07-22 | 2022-03-04 | 豪夫迈·罗氏有限公司 | P物质作为用于子宫内膜异位症的非侵入性诊断的血液生物标志物 |
| KR20220016210A (ko) * | 2019-07-22 | 2022-02-08 | 에프. 호프만-라 로슈 아게 | 자궁내막증의 비-침습적 진단용 혈액 생물표지자로써 s100a9 |
| WO2021013784A1 (en) * | 2019-07-22 | 2021-01-28 | F. Hoffmann-La Roche Ag | S100a8 as blood biomarker for the non-invasive diagnosis of endometriosis |
| US11965879B2 (en) | 2019-08-23 | 2024-04-23 | Mcmaster University | Method for diagnosing and assessing endometriosis |
| KR102316507B1 (ko) * | 2020-02-28 | 2021-10-22 | 차의과학대학교 산학협력단 | 난소 예비력 바이오마커 및 이의 용도 |
| CN111909997B (zh) | 2020-09-09 | 2021-09-28 | 上海交通大学 | miRNA标志物在制备奥氮平治疗精神分裂症疗效评估方面的产品中的应用及试剂盒 |
| JP2022063860A (ja) * | 2020-10-12 | 2022-04-22 | 学校法人近畿大学 | ホウ素化合物およびその製造方法 |
Family Cites Families (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009143181A2 (en) | 2008-05-19 | 2009-11-26 | The Regents Of The University Of California | Micro-rna profile in human saliva and its use for detection of oral cancer |
| CN102301002A (zh) | 2008-11-12 | 2011-12-28 | 卡里斯生命科学卢森堡控股有限责任公司 | 使用外来体来确定表现型的方法和系统 |
| WO2010118979A1 (en) | 2009-04-07 | 2010-10-21 | Velin-Pharma A/S | Method and device for treatment of conditions associated with inflammation or undesirable activation of the immune system |
| CA2791905A1 (en) * | 2010-03-01 | 2011-09-09 | Caris Life Sciences Luxembourg Holdings, S.A.R.L. | Biomarkers for theranostics |
| WO2012103355A2 (en) | 2011-01-26 | 2012-08-02 | Cepheid | Methods of detecting lung cancer |
| US20140024590A1 (en) * | 2011-02-18 | 2014-01-23 | Yale University | KRAS-Variant And Endometriosis |
| FR2984358A1 (fr) | 2011-12-16 | 2013-06-21 | Genethon | Methodes pour le diagnostic de dystrophies musculaires |
| WO2013148151A1 (en) | 2012-03-29 | 2013-10-03 | University Of Pittsburgh - Of The Commonwealth System Of Higher Education | Plasma microribonucleic acids as biomarkers for endometriosis and endometriosis-associated ovarian cancer |
| US10927412B2 (en) | 2013-10-01 | 2021-02-23 | The Regents Of The University Of California | Endometriosis classifier |
| ES2813699T3 (es) * | 2013-11-18 | 2021-03-24 | Diamir Llc | Métodos de uso de miARN de fluidos corporales para la detección y monitoreo de la enfermedad de Parkinson (PD) |
| GB201403489D0 (en) * | 2014-02-27 | 2014-04-16 | Univ London Queen Mary | Biomarkers for endometriosis |
| US9650676B2 (en) | 2014-03-19 | 2017-05-16 | University Of South Carolina | Leukocyte MicroRNAS for use in diagnosis and treatment of endometriosis |
| EP3122905B1 (en) | 2014-03-27 | 2020-09-09 | Yale University | Circulating micrornas as biomarkers for endometriosis |
| EP2924126B1 (en) * | 2014-03-28 | 2018-04-18 | Tervisetehnoloogiate Arenduskeskus AS | Method for using microRNA (miRNA) for detection of endometriosis |
| GB201603967D0 (en) * | 2016-03-08 | 2016-04-20 | Univ Birmingham | Biomarkers of traumatic brain injury |
| BR112019004075A2 (pt) | 2016-08-30 | 2019-05-28 | Univ Yale | métodos para diagnosticar um indivíduo com suspeita de endometriose, para detectar mirna, para diagnosticar um indivíduo que apresenta sintomas de endometriose ou com suspeita de endometriose, para tratar um indivíduo com endometriose, para diagnosticar e tratar um indivíduo com suspeita de endometriose, para diagnosticar endometriose em um indivíduo, para diagnosticar ou prover um prognóstico para endometriose em um indivíduo e para monitorar uma resposta a um tratamento de endometriose em um indivíduo, e, kit. |
| CA3073829A1 (en) | 2017-08-30 | 2019-03-07 | Dot Laboratories, Inc. | Methods and compositions for detecting and treating endometriosis |
| CA3118133A1 (en) | 2018-10-31 | 2020-05-07 | Yale University | A quantitative algorithm for endometriosis |
| US11315660B2 (en) | 2018-10-31 | 2022-04-26 | Dot Laboratories, Inc. | Method of detecting and treating endometriosis in a female subject |
| BR112021021866A2 (pt) | 2019-04-29 | 2021-12-21 | Dot Laboraties Inc | Classificadores para a detecção de endometriose |
-
2017
- 2017-08-30 BR BR112019004075A patent/BR112019004075A2/pt unknown
- 2017-08-30 CA CA3035429A patent/CA3035429A1/en active Pending
- 2017-08-30 KR KR1020197009115A patent/KR20190057305A/ko not_active Ceased
- 2017-08-30 CN CN201780066779.9A patent/CN109890394A/zh active Pending
- 2017-08-30 SG SG10202102509TA patent/SG10202102509TA/en unknown
- 2017-08-30 WO PCT/US2017/049284 patent/WO2018044979A1/en not_active Ceased
- 2017-08-30 MX MX2019002425A patent/MX2019002425A/es unknown
- 2017-08-30 JP JP2019511953A patent/JP2019528081A/ja active Pending
- 2017-08-30 US US16/329,436 patent/US10982282B2/en active Active
- 2017-08-30 EP EP17847431.8A patent/EP3506912B1/en active Active
- 2017-08-30 AU AU2017319325A patent/AU2017319325A1/en not_active Abandoned
- 2017-08-30 SG SG11201901834WA patent/SG11201901834WA/en unknown
-
2021
- 2021-02-25 US US17/184,894 patent/US11220713B2/en active Active
- 2021-11-02 US US17/517,014 patent/US12077803B2/en active Active
-
2022
- 2022-09-16 JP JP2022147738A patent/JP2022188086A/ja active Pending
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102242639B1 (ko) * | 2020-01-28 | 2021-04-21 | 고려대학교 산학협력단 | 간 섬유화 진단용 바이오마커 miRNA4668-5p |
| WO2024123061A1 (ko) * | 2022-12-06 | 2024-06-13 | 연세대학교 산학협력단 | 자궁내막증 진단용 조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| US12077803B2 (en) | 2024-09-03 |
| MX2019002425A (es) | 2019-08-16 |
| JP2019528081A (ja) | 2019-10-10 |
| EP3506912A4 (en) | 2020-08-19 |
| CA3035429A1 (en) | 2018-03-08 |
| EP3506912A1 (en) | 2019-07-10 |
| US20190276893A1 (en) | 2019-09-12 |
| US20210180134A1 (en) | 2021-06-17 |
| US10982282B2 (en) | 2021-04-20 |
| SG11201901834WA (en) | 2019-03-28 |
| WO2018044979A1 (en) | 2018-03-08 |
| SG10202102509TA (en) | 2021-04-29 |
| JP2022188086A (ja) | 2022-12-20 |
| BR112019004075A2 (pt) | 2019-05-28 |
| US11220713B2 (en) | 2022-01-11 |
| EP3506912B1 (en) | 2024-04-03 |
| US20220049312A1 (en) | 2022-02-17 |
| AU2017319325A1 (en) | 2019-03-21 |
| CN109890394A (zh) | 2019-06-14 |
Similar Documents
| Publication | Publication Date | Title |
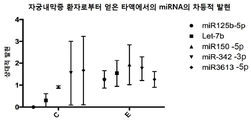
|---|---|---|
| US12077803B2 (en) | MicroRNAs as biomarkers for endometriosis | |
| JP5690588B2 (ja) | 肝細胞癌のサブタイプを決定し、肝癌幹細胞を検出するための方法 | |
| AU2010321555B2 (en) | Materials and methods useful for affecting tumor cell growth, migration and invasion | |
| EP3122905B1 (en) | Circulating micrornas as biomarkers for endometriosis | |
| AU2016300175A1 (en) | FGFR expression and susceptibility to an FGFR inhibitor | |
| WO2020092672A2 (en) | A quantitative algorithm for endometriosis | |
| US20190316207A1 (en) | Mir-320e and colorectal cancer | |
| WO2015085164A2 (en) | Uveal melanoma prognosis | |
| WO2018095933A1 (en) | Method of prognosticating, or for determining the efficiency of a compound for treating cancer | |
| HK40049419A (en) | Circulating micrornas as biomarkers for endometriosis | |
| HK40011172A (en) | Micrornas as biomarkers for endometriosis | |
| HK40011172B (en) | Micrornas as biomarkers for endometriosis | |
| US20250263793A1 (en) | Methods of detecting and treating gynecological diseases | |
| WO2020234072A1 (en) | Pcos diagnosis | |
| Hiew | The prognostic potential of the long non-coding RNA MALAT1 and LDH related metabolism in prostate cancer | |
| Zhou | tRNA Fragments: Expression and Function in Ovarian Cancer | |
| WO2015006543A1 (en) | Method for predicting and detecting tumor metastasis in kidney cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20190329 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20200828 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20220224 Patent event code: PE09021S01D |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20221222 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20240507 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20221222 Comment text: Notification of reason for refusal Patent event code: PE06011S01I Patent event date: 20220224 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |