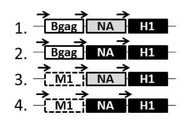
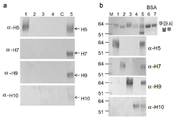
KR20180032586A - 소 면역결핍 바이러스 gag 단백질을 사용하는 재조합 바이러스 유사 입자 - Google Patents
소 면역결핍 바이러스 gag 단백질을 사용하는 재조합 바이러스 유사 입자 Download PDFInfo
- Publication number
- KR20180032586A KR20180032586A KR1020187003292A KR20187003292A KR20180032586A KR 20180032586 A KR20180032586 A KR 20180032586A KR 1020187003292 A KR1020187003292 A KR 1020187003292A KR 20187003292 A KR20187003292 A KR 20187003292A KR 20180032586 A KR20180032586 A KR 20180032586A
- Authority
- KR
- South Korea
- Prior art keywords
- bgag
- vlp
- vlps
- influenza
- virus
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/21—Retroviridae, e.g. equine infectious anemia virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/145—Orthomyxoviridae, e.g. influenza virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
- C12N15/866—Baculoviral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
- C12N7/04—Inactivation or attenuation; Producing viral sub-units
- C12N7/045—Pseudoviral particles; Non infectious pseudovirions, e.g. genetically engineered
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
- A61K2039/5258—Virus-like particles
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/14011—Deltaretrovirus, e.g. bovine leukeamia virus
- C12N2740/14034—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/15011—Lentivirus, not HIV, e.g. FIV, SIV
- C12N2740/15023—Virus like particles [VLP]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/15011—Lentivirus, not HIV, e.g. FIV, SIV
- C12N2740/15051—Methods of production or purification of viral material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2740/00—Reverse transcribing RNA viruses
- C12N2740/00011—Details
- C12N2740/10011—Retroviridae
- C12N2740/16011—Human Immunodeficiency Virus, HIV
- C12N2740/16023—Virus like particles [VLP]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/16011—Orthomyxoviridae
- C12N2760/16111—Influenzavirus A, i.e. influenza A virus
- C12N2760/16134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Virology (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Immunology (AREA)
- Microbiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Pharmacology & Pharmacy (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Molecular Biology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Communicable Diseases (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Biophysics (AREA)
- Pulmonology (AREA)
- Physics & Mathematics (AREA)
- Urology & Nephrology (AREA)
- Gastroenterology & Hepatology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Pathology (AREA)
- Cell Biology (AREA)
- Food Science & Technology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562188084P | 2015-07-02 | 2015-07-02 | |
| US62/188,084 | 2015-07-02 | ||
| PCT/US2016/040838 WO2017004586A1 (en) | 2015-07-02 | 2016-07-01 | Recombinant virus like particles using bovine immunodeficiency virus gag protein |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20180032586A true KR20180032586A (ko) | 2018-03-30 |
Family
ID=57609369
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020187003292A Ceased KR20180032586A (ko) | 2015-07-02 | 2016-07-01 | 소 면역결핍 바이러스 gag 단백질을 사용하는 재조합 바이러스 유사 입자 |
Country Status (13)
| Country | Link |
|---|---|
| US (2) | US11576965B2 (enExample) |
| EP (1) | EP3316906A4 (enExample) |
| JP (2) | JP6978079B2 (enExample) |
| KR (1) | KR20180032586A (enExample) |
| CN (2) | CN108348596B (enExample) |
| BR (1) | BR112018000037A2 (enExample) |
| CA (1) | CA2991213A1 (enExample) |
| EA (1) | EA201890187A1 (enExample) |
| MA (1) | MA42312A (enExample) |
| MX (1) | MX390927B (enExample) |
| PE (1) | PE20180771A1 (enExample) |
| RU (2) | RU2757723C2 (enExample) |
| WO (1) | WO2017004586A1 (enExample) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| MA42312A (fr) * | 2015-07-02 | 2018-05-09 | Medigen Inc | Particules de type virus recombinantes utilisant la protéine gag du virus de l'immunodéficience bovine |
| CN112279900A (zh) * | 2020-12-30 | 2021-01-29 | 乾元浩生物股份有限公司 | H9n2亚型禽流感病毒基因工程亚单位疫苗及其制备方法与应用 |
| CN113403343A (zh) * | 2021-04-30 | 2021-09-17 | 吉林大学 | 一种h3n2与h9n2亚型禽流感二价嵌合型病毒样颗粒的制备 |
| CN113398259A (zh) * | 2021-04-30 | 2021-09-17 | 吉林大学 | 一种h9n2亚型禽流感新型标记疫苗的制备方法及其应用 |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6864085B2 (en) | 1999-12-14 | 2005-03-08 | Novartis Ag | Bovine immunodeficiency virus (BIV) based vectors |
| EP1576143B1 (en) | 2002-05-17 | 2010-11-24 | Emory University | Virus-like particles, methods of preparation, and immonogenic compositions |
| WO2004042001A2 (en) * | 2002-05-17 | 2004-05-21 | Emory University | Virus-like particles, methods of preparation, and immonogenic compositions |
| US8506967B2 (en) * | 2003-07-11 | 2013-08-13 | Novavax, Inc. | Functional influenza virus like particles (VLPs) |
| WO2008005777A2 (en) | 2006-06-30 | 2008-01-10 | Novavax, Inc. | Methods of enhancing protein incorporation into virus like particles |
| WO2008094200A2 (en) | 2006-07-27 | 2008-08-07 | Ligocyte Pharmaceuticals, Inc | Chimeric virus-like particles |
| US20100247574A1 (en) | 2007-02-21 | 2010-09-30 | Kutub Mahmood | CHIMERIC NEWCASTLE DISEASE VIRUS VLPs |
| SG10201903161XA (en) | 2007-05-29 | 2019-05-30 | Christopher Reid | Methods for production and uses of multipotent cell populations |
| AU2008275895A1 (en) * | 2007-07-19 | 2009-01-22 | Novavax, Inc. | Varicella Zoster Virus-virus like particles (VLPS) and antigens |
| WO2011087839A1 (en) * | 2009-12-22 | 2011-07-21 | Baxter International Inc. | Vaccine to influenza a virus |
| US20110293700A1 (en) * | 2010-05-26 | 2011-12-01 | Selecta Biosciences, Inc. | Nanocarrier compositions with uncoupled adjuvant |
| US20140004146A1 (en) * | 2011-03-17 | 2014-01-02 | Institut Pasteur Of Shanghai, Chinese Academy Of Sciences | Method for producing virus-like particle by using drosophila cell and applications thereof |
| WO2015066715A1 (en) | 2013-11-04 | 2015-05-07 | Viracell Advanced Products, Llc | Virus-like particles and methods related thereto |
| MA42312A (fr) * | 2015-07-02 | 2018-05-09 | Medigen Inc | Particules de type virus recombinantes utilisant la protéine gag du virus de l'immunodéficience bovine |
-
2016
- 2016-07-01 MA MA042312A patent/MA42312A/fr unknown
- 2016-07-01 JP JP2018520386A patent/JP6978079B2/ja active Active
- 2016-07-01 RU RU2020125098A patent/RU2757723C2/ru active
- 2016-07-01 KR KR1020187003292A patent/KR20180032586A/ko not_active Ceased
- 2016-07-01 CN CN201680050161.9A patent/CN108348596B/zh active Active
- 2016-07-01 EP EP16818931.4A patent/EP3316906A4/en active Pending
- 2016-07-01 WO PCT/US2016/040838 patent/WO2017004586A1/en not_active Ceased
- 2016-07-01 MX MX2018000023A patent/MX390927B/es unknown
- 2016-07-01 PE PE2018000003A patent/PE20180771A1/es not_active Application Discontinuation
- 2016-07-01 BR BR112018000037A patent/BR112018000037A2/pt active Search and Examination
- 2016-07-01 RU RU2018103757A patent/RU2734118C2/ru active
- 2016-07-01 CN CN202210931466.8A patent/CN115927212A/zh active Pending
- 2016-07-01 US US15/741,443 patent/US11576965B2/en active Active
- 2016-07-01 CA CA2991213A patent/CA2991213A1/en active Pending
- 2016-07-01 EA EA201890187A patent/EA201890187A1/ru unknown
-
2020
- 2020-09-14 JP JP2020153664A patent/JP2020198893A/ja not_active Withdrawn
-
2023
- 2023-01-12 US US18/153,667 patent/US12303560B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CN108348596A (zh) | 2018-07-31 |
| RU2757723C2 (ru) | 2021-10-21 |
| JP2020198893A (ja) | 2020-12-17 |
| US20230310579A1 (en) | 2023-10-05 |
| US12303560B2 (en) | 2025-05-20 |
| MA42312A (fr) | 2018-05-09 |
| WO2017004586A1 (en) | 2017-01-05 |
| MX2018000023A (es) | 2018-08-15 |
| RU2020125098A3 (enExample) | 2020-12-11 |
| BR112018000037A2 (pt) | 2018-09-04 |
| CN108348596B (zh) | 2022-08-23 |
| CA2991213A1 (en) | 2017-01-05 |
| CN115927212A (zh) | 2023-04-07 |
| RU2018103757A (ru) | 2019-08-05 |
| JP2018525028A (ja) | 2018-09-06 |
| EA201890187A1 (ru) | 2018-06-29 |
| RU2020125098A (ru) | 2020-09-04 |
| RU2734118C2 (ru) | 2020-10-13 |
| US20180369364A1 (en) | 2018-12-27 |
| EP3316906A1 (en) | 2018-05-09 |
| RU2018103757A3 (enExample) | 2019-12-09 |
| EP3316906A4 (en) | 2019-01-02 |
| PE20180771A1 (es) | 2018-05-07 |
| HK1259067A1 (zh) | 2019-11-22 |
| JP6978079B2 (ja) | 2021-12-08 |
| US11576965B2 (en) | 2023-02-14 |
| MX390927B (es) | 2025-03-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Chen et al. | Advances in development and application of influenza vaccines | |
| US12303560B2 (en) | Recombinant bovine immunodeficiency virus-like particles comprising an influenza HA transmembrane domain and C-terminus | |
| Kapczynski et al. | Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses | |
| JP2018134107A (ja) | インフルエンザウイルスワクチン及びその使用 | |
| EA024250B1 (ru) | Дозированная форма иммуногенного препарата против гриппа для применения у человека и содержащая её вакцина, способы их приготовления и их применение для лечения или предупреждения заболеваний, вызываемых вирусом гриппа у людей | |
| JP2021536228A (ja) | ヘマグルチニン(ha)タンパク質内の非ドミナントエピトープに対する免疫応答を誘起するためのベクター | |
| KR20070100882A (ko) | 결손 인플루엔자 바이러스 입자 | |
| WO2017040203A1 (en) | Generation of infectious influenza viruses from virus-like particles | |
| EP2744514B1 (en) | Influenza h5 vaccines | |
| EP2958586B1 (en) | H5 proteins of h5n1 influenza virus for use as a medicament | |
| KR101908905B1 (ko) | 인플루엔자 바이러스의 h9 및 h5의 다중 아형에 대한 교차 면역반응을 형성하는 신규한 재조합 인플루엔자 바이러스 및 이를 포함하는 백신 | |
| HK40090606A (zh) | 用牛免疫缺陷病毒gag蛋白的重组病毒样颗粒 | |
| TWI494430B (zh) | 製備與真實流感病毒粒子高度相似的流感類病毒顆粒之轉殖基因哺乳動物細胞表現系統 | |
| HK1259067B (en) | Recombinant virus like particles using bovine immunodeficiency virus gag protein | |
| RU2681482C1 (ru) | MDCK клетка-продуцент белков вируса гриппа (варианты) | |
| RU2680537C1 (ru) | Лентивирусная плазмида (варианты), способ ее получения (варианты), набор праймеров для получения лентивирусного плазмидного вектора (варианты) | |
| RU2680703C1 (ru) | Кассета, предназначенная для получения плазмидных векторов, используемых для создания клеток-продуцентов вирусоподобных частиц (ВПЧ) вируса гриппа | |
| EA042635B1 (ru) | Рекомбинантные вирусоподобные частицы (vlp) с использованием протеина группового антигена (gag) вируса бычьего иммунодефицита | |
| Sadler | Evaluation of a single cycle influenza virus as a candidate vaccine | |
| Li | Self-Adjuvanted Nanoparticle Based Vaccines for Poultry Viral Respiratory Diseases | |
| Pfeiffer | Antigenic and biological characterization of H5 avian influenza viruses | |
| Shehata | Truncated Sequences of Influenza Subtype H5 Haemagglutinin for Vaccination and Diagnostic Purposes: Avian influenza, Yeast expression, Peptide vaccination, recombinant Elisa | |
| Baker | The Packaging Signals of Influenza A and B Viruses Contribute to Their Speciation and Can Be Manipulated for Novel Vaccine Development | |
| Yang | A novel method to produce an avian influenza vaccine bearing membrane-bound cytokines |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
St.27 status event code: A-0-1-A10-A15-nap-PA0105 |
|
| AMND | Amendment | ||
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PG1501 | Laying open of application |
St.27 status event code: A-1-1-Q10-Q12-nap-PG1501 |
|
| PA0201 | Request for examination |
St.27 status event code: A-1-2-D10-D11-exm-PA0201 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| AMND | Amendment | ||
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
St.27 status event code: N-2-6-B10-B15-exm-PE0601 |
|
| AMND | Amendment | ||
| P11-X000 | Amendment of application requested |
St.27 status event code: A-2-2-P10-P11-nap-X000 |
|
| P13-X000 | Application amended |
St.27 status event code: A-2-2-P10-P13-nap-X000 |
|
| PX0901 | Re-examination |
St.27 status event code: A-2-3-E10-E12-rex-PX0901 |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
St.27 status event code: A-1-2-D10-D21-exm-PE0902 |
|
| PX0601 | Decision of rejection after re-examination |
St.27 status event code: N-2-6-B10-B17-rex-PX0601 |
|
| X601 | Decision of rejection after re-examination |