KR20170128256A - Cd48 항체 및 이의 접합체 - Google Patents
Cd48 항체 및 이의 접합체 Download PDFInfo
- Publication number
- KR20170128256A KR20170128256A KR1020177024226A KR20177024226A KR20170128256A KR 20170128256 A KR20170128256 A KR 20170128256A KR 1020177024226 A KR1020177024226 A KR 1020177024226A KR 20177024226 A KR20177024226 A KR 20177024226A KR 20170128256 A KR20170128256 A KR 20170128256A
- Authority
- KR
- South Korea
- Prior art keywords
- antibody
- drug
- linker
- drug conjugate
- ser
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/395—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum
- A61K39/39533—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals
- A61K39/39566—Antibodies; Immunoglobulins; Immune serum, e.g. antilymphocytic serum against materials from animals against immunoglobulins, e.g. anti-idiotypic antibodies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/68031—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug being an auristatin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6801—Drug-antibody or immunoglobulin conjugates defined by the pharmacologically or therapeutically active agent
- A61K47/6803—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates
- A61K47/6807—Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates the drug or compound being a sugar, nucleoside, nucleotide, nucleic acid, e.g. RNA antisense
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6849—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site the antibody targeting a receptor, a cell surface antigen or a cell surface determinant
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/50—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates
- A61K47/51—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent
- A61K47/68—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment
- A61K47/6835—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient the non-active ingredient being chemically bound to the active ingredient, e.g. polymer-drug conjugates the non-active ingredient being a modifying agent the modifying agent being an antibody, an immunoglobulin or a fragment thereof, e.g. an Fc-fragment the modifying agent being an antibody or an immunoglobulin bearing at least one antigen-binding site
- A61K47/6883—Polymer-drug antibody conjugates, e.g. mitomycin-dextran-Ab; DNA-polylysine-antibody complex or conjugate used for therapy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K5/00—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof
- C07K5/02—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link
- C07K5/0205—Peptides containing up to four amino acids in a fully defined sequence; Derivatives thereof containing at least one abnormal peptide link containing the structure -NH-(X)3-C(=0)-, e.g. statine or derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/505—Medicinal preparations containing antigens or antibodies comprising antibodies
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/20—Immunoglobulins specific features characterized by taxonomic origin
- C07K2317/24—Immunoglobulins specific features characterized by taxonomic origin containing regions, domains or residues from different species, e.g. chimeric, humanized or veneered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/50—Immunoglobulins specific features characterized by immunoglobulin fragments
- C07K2317/56—Immunoglobulins specific features characterized by immunoglobulin fragments variable (Fv) region, i.e. VH and/or VL
- C07K2317/565—Complementarity determining region [CDR]
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/73—Inducing cell death, e.g. apoptosis, necrosis or inhibition of cell proliferation
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/90—Immunoglobulins specific features characterized by (pharmaco)kinetic aspects or by stability of the immunoglobulin
- C07K2317/92—Affinity (KD), association rate (Ka), dissociation rate (Kd) or EC50 value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Cell Biology (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Mycology (AREA)
- Microbiology (AREA)
- Crystallography & Structural Chemistry (AREA)
- Biotechnology (AREA)
- Hematology (AREA)
- Oncology (AREA)
- Peptides Or Proteins (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Preparation Of Compounds By Using Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicinal Preparation (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562134981P | 2015-03-18 | 2015-03-18 | |
| US62/134,981 | 2015-03-18 | ||
| PCT/US2016/022943 WO2016149535A1 (en) | 2015-03-18 | 2016-03-17 | Cd48 antibodies and conjugates thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20170128256A true KR20170128256A (ko) | 2017-11-22 |
Family
ID=56919489
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020177024226A Withdrawn KR20170128256A (ko) | 2015-03-18 | 2016-03-17 | Cd48 항체 및 이의 접합체 |
Country Status (16)
| Country | Link |
|---|---|
| US (2) | US10722592B2 (enExample) |
| EP (2) | EP3270965B1 (enExample) |
| JP (2) | JP6892826B2 (enExample) |
| KR (1) | KR20170128256A (enExample) |
| CN (1) | CN107530422B (enExample) |
| AU (1) | AU2016232839B2 (enExample) |
| BR (1) | BR112017019617A2 (enExample) |
| CA (1) | CA2976740A1 (enExample) |
| DK (1) | DK3270965T3 (enExample) |
| EA (2) | EA035374B1 (enExample) |
| ES (1) | ES2795818T3 (enExample) |
| IL (1) | IL254027B (enExample) |
| MX (1) | MX382582B (enExample) |
| SG (1) | SG11201706786UA (enExample) |
| WO (1) | WO2016149535A1 (enExample) |
| ZA (1) | ZA201705935B (enExample) |
Families Citing this family (21)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11844839B2 (en) | 2016-03-25 | 2023-12-19 | Seagen Inc. | Process for the preparation of pegylated drug-linkers and intermediates thereof |
| JP7527787B2 (ja) | 2017-03-24 | 2024-08-05 | シージェン インコーポレイテッド | グルクロニド薬物-リンカーの調製のためのプロセスおよびその中間体 |
| BR112020023623A2 (pt) | 2018-05-29 | 2021-02-17 | Intocell, Inc. | derivados de benzodiazepina e seus usos |
| TWI851577B (zh) | 2018-06-07 | 2024-08-11 | 美商思進公司 | 喜樹鹼結合物 |
| AU2019370859B2 (en) | 2018-10-31 | 2023-09-21 | Intocell, Inc. | Fused heterocyclic benzodiazepine derivatives and uses thereof |
| US11617798B2 (en) * | 2019-02-05 | 2023-04-04 | Seagen Inc. | Anti-CD228 antibodies and antibody-drug conjugates |
| KR20220079606A (ko) | 2019-10-04 | 2022-06-13 | 씨젠 인크. | 캄프토테신 펩티드 접합체 |
| KR20220075351A (ko) | 2019-10-04 | 2022-06-08 | 씨젠 인크. | 항-pd-l1 항체 및 항체-약물 접합체 |
| WO2021067820A1 (en) | 2019-10-04 | 2021-04-08 | Seagen Inc. | Formulation of antibody-drug conjugate |
| US20230173093A1 (en) | 2020-04-10 | 2023-06-08 | Seagen Inc. | Charge variant linkers |
| AU2021388021A1 (en) * | 2020-11-24 | 2023-06-22 | Novartis Ag | Anti-cd48 antibodies, antibody drug conjugates, and uses thereof |
| US20240042051A1 (en) * | 2020-11-24 | 2024-02-08 | Francesca Rocchetti | Mcl-1 inhibitor antibody-drug conjugates and methods of use |
| TW202300178A (zh) | 2021-03-18 | 2023-01-01 | 美商西根公司 | 內化的生物活性化合物偶聯物選擇性釋放藥物 |
| EP4346906A1 (en) | 2021-05-28 | 2024-04-10 | Seagen Inc. | Anthracycline antibody conjugates |
| PE20242179A1 (es) | 2022-03-17 | 2024-11-07 | Seagen Inc | Conjugados de camptotecina |
| EP4634227A1 (en) | 2022-12-13 | 2025-10-22 | Seagen Inc. | Site-specific engineered cysteine antibody drug conjugates |
| CN121240890A (zh) | 2023-04-18 | 2025-12-30 | 阿斯利康(瑞典)有限公司 | 包含可裂解接头的缀合物 |
| TW202525851A (zh) | 2023-08-15 | 2025-07-01 | 瑞典商阿斯特捷利康公司 | 包含可切割連接子的軛合物 |
| WO2025090774A1 (en) | 2023-10-24 | 2025-05-01 | Seagen Inc. | Chemotherapeutic compounds and methods of use |
| WO2025149947A1 (en) | 2024-01-12 | 2025-07-17 | Seagen Inc. | Antibody-drug conjugates |
| WO2025185617A1 (en) * | 2024-03-05 | 2025-09-12 | Hutchmed Limited | Linker, antibody-drug conjugate and use thereof |
Family Cites Families (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US5225539A (en) | 1986-03-27 | 1993-07-06 | Medical Research Council | Recombinant altered antibodies and methods of making altered antibodies |
| DE3883899T3 (de) | 1987-03-18 | 1999-04-22 | Sb2, Inc., Danville, Calif. | Geänderte antikörper. |
| US5530101A (en) | 1988-12-28 | 1996-06-25 | Protein Design Labs, Inc. | Humanized immunoglobulins |
| US5859205A (en) | 1989-12-21 | 1999-01-12 | Celltech Limited | Humanised antibodies |
| AU675916B2 (en) | 1991-06-14 | 1997-02-27 | Genentech Inc. | Method for making humanized antibodies |
| WO1997035614A1 (en) * | 1996-03-27 | 1997-10-02 | Crc For Biopharmaceutical Research Pty. Ltd. | The use of antibodies against cd48 for the treatment of t and b cell lymphomas and leukemias |
| US5834597A (en) | 1996-05-20 | 1998-11-10 | Protein Design Labs, Inc. | Mutated nonactivating IgG2 domains and anti CD3 antibodies incorporating the same |
| EP2298809A3 (en) | 2001-07-12 | 2012-02-15 | FOOTE, Jefferson | Super humanized antibodies |
| SI1545613T1 (sl) | 2002-07-31 | 2011-11-30 | Seattle Genetics Inc | Avristatinski konjugati in njihova uporaba za zdravljenje raka avtoimunske bolezni ali infekcijskebolezni |
| DK1725249T3 (en) | 2003-11-06 | 2014-03-17 | Seattle Genetics Inc | Monomethylvaline compounds capable of conjugating to ligands. |
| EP1749538A4 (en) * | 2004-03-31 | 2009-11-11 | Kirin Pharma Kk | METHOD FOR DETERMINING THE DIFFERENTIATION OF REGULATORY T CELLS AND THEIR PROLIFERATION WITH GPI ANCHOR PROTEIN AGONISTS AND MEDICINAL COMPOSITION THEREFOR |
| EP1747237A4 (en) | 2004-04-16 | 2008-05-21 | Macrogenics Inc | SPECIFIC GAMMA FC ANTIBODIES AND METHODS OF USING THE SAME |
| JP5171621B2 (ja) | 2005-07-07 | 2013-03-27 | シアトル ジェネティックス, インコーポレイテッド | フェニルアラニン側鎖修飾をc末端に有するモノメチルバリン化合物 |
| WO2007008848A2 (en) | 2005-07-07 | 2007-01-18 | Seattle Genetics, Inc. | Monomethylvaline compounds having phenylalanine carboxy modifications at the c-terminus |
| AU2006269940C1 (en) * | 2005-07-18 | 2013-11-07 | Seagen Inc. | Beta-glucuronide-linker drug conjugates |
| KR101624587B1 (ko) * | 2005-12-20 | 2016-05-26 | 에스비아이 바이오테크 가부시키가이샤 | 항-ilt7 항체 |
| WO2009052431A2 (en) | 2007-10-19 | 2009-04-23 | Seattle Genetics, Inc. | Cd19 binding agents and uses thereof |
| EP2500362A3 (en) * | 2007-11-13 | 2012-10-24 | Teva Biopharmaceuticals USA, Inc. | Humanized antibodies against TL1A |
| WO2009099580A2 (en) * | 2008-02-05 | 2009-08-13 | Monsanto Technology, Llc | Isolated novel nucleic acid and protein molecules from soy and methods of using those molecules to generate transgenic plants with enhanced agronomic traits |
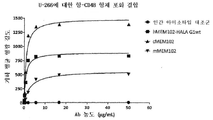
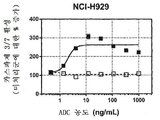
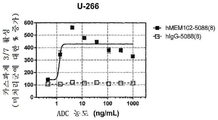
| US9097717B2 (en) | 2009-04-10 | 2015-08-04 | Osaka University | Methods of killing myeloma stem and precursor cells by administering anti-CD48 antibodies |
| EP2287197A1 (en) * | 2009-08-21 | 2011-02-23 | Pierre Fabre Medicament | Anti-cMET antibody and its use for the detection and the diagnosis of cancer |
| AR083044A1 (es) * | 2010-09-27 | 2013-01-30 | Regeneron Pharma | Anticuerpos anti-cd48 y usos de los mismos |
| KR20140029446A (ko) * | 2011-04-15 | 2014-03-10 | 컴퓨젠 엘티디. | 면역 관련 장애 및 암의 치료를 위한 폴리펩티드 및 폴리뉴클레오티드, 및 이들의 용도 |
| BR112014022228A2 (pt) * | 2012-03-08 | 2017-07-11 | Halozyme Inc | anticorpos anti-epidérmicos de receptor de fator de crescimento condicionalmente ativo e métodos de uso dos mesmos |
| JP6423340B2 (ja) | 2012-05-15 | 2018-11-14 | シアトル ジェネティクス,インコーポレイティド | 自己安定化リンカー結合体 |
| CA3187392A1 (en) * | 2013-10-15 | 2015-04-23 | Seagen Inc. | Pegylated drug-linkers for improved ligand-drug conjugate pharmacokinetics |
-
2016
- 2016-03-17 AU AU2016232839A patent/AU2016232839B2/en not_active Ceased
- 2016-03-17 CA CA2976740A patent/CA2976740A1/en not_active Abandoned
- 2016-03-17 CN CN201680013161.1A patent/CN107530422B/zh active Active
- 2016-03-17 BR BR112017019617-4A patent/BR112017019617A2/pt not_active IP Right Cessation
- 2016-03-17 SG SG11201706786UA patent/SG11201706786UA/en unknown
- 2016-03-17 ES ES16765773T patent/ES2795818T3/es active Active
- 2016-03-17 EA EA201792055A patent/EA035374B1/ru unknown
- 2016-03-17 DK DK16765773.3T patent/DK3270965T3/da active
- 2016-03-17 EA EA201992756A patent/EA201992756A3/ru unknown
- 2016-03-17 JP JP2017548953A patent/JP6892826B2/ja active Active
- 2016-03-17 KR KR1020177024226A patent/KR20170128256A/ko not_active Withdrawn
- 2016-03-17 EP EP16765773.3A patent/EP3270965B1/en active Active
- 2016-03-17 US US15/557,910 patent/US10722592B2/en active Active
- 2016-03-17 WO PCT/US2016/022943 patent/WO2016149535A1/en not_active Ceased
- 2016-03-17 MX MX2017011432A patent/MX382582B/es unknown
- 2016-03-17 EP EP19219476.9A patent/EP3662933A1/en not_active Withdrawn
-
2017
- 2017-08-16 IL IL254027A patent/IL254027B/en active IP Right Grant
- 2017-08-31 ZA ZA2017/05935A patent/ZA201705935B/en unknown
-
2020
- 2020-05-26 JP JP2020091335A patent/JP2020141700A/ja active Pending
- 2020-06-04 US US16/892,529 patent/US20200289661A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| CA2976740A1 (en) | 2016-09-22 |
| US10722592B2 (en) | 2020-07-28 |
| EA201992756A3 (ru) | 2020-06-30 |
| WO2016149535A1 (en) | 2016-09-22 |
| ES2795818T3 (es) | 2020-11-24 |
| IL254027A0 (en) | 2017-10-31 |
| IL254027B (en) | 2021-05-31 |
| JP2020141700A (ja) | 2020-09-10 |
| US20180092984A1 (en) | 2018-04-05 |
| US20200289661A1 (en) | 2020-09-17 |
| BR112017019617A2 (pt) | 2018-05-22 |
| JP6892826B2 (ja) | 2021-06-23 |
| CN107530422A (zh) | 2018-01-02 |
| JP2018509908A (ja) | 2018-04-12 |
| EP3270965A1 (en) | 2018-01-24 |
| ZA201705935B (en) | 2021-01-27 |
| MX382582B (es) | 2025-03-13 |
| DK3270965T3 (da) | 2020-06-08 |
| EP3662933A1 (en) | 2020-06-10 |
| SG11201706786UA (en) | 2017-09-28 |
| EP3270965B1 (en) | 2020-05-06 |
| EA201992756A2 (ru) | 2020-03-31 |
| AU2016232839A1 (en) | 2017-08-31 |
| EA201792055A1 (ru) | 2018-01-31 |
| CN107530422B (zh) | 2021-09-21 |
| HK1248538A1 (zh) | 2018-10-19 |
| AU2016232839B2 (en) | 2021-02-25 |
| MX2017011432A (es) | 2017-11-10 |
| EP3270965A4 (en) | 2018-09-05 |
| EA035374B1 (ru) | 2020-06-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20200289661A1 (en) | Cd48 antibodies and conjugates thereof | |
| JP7254861B2 (ja) | エリブリンをベースとする抗体-薬物コンジュゲート及び使用方法 | |
| AU2015308179B2 (en) | Stability-modulating linkers for use with antibody drug conjugates | |
| US20230173093A1 (en) | Charge variant linkers | |
| KR20230088426A (ko) | 항-ccr8 단클론성 항체 및 그것의 용도 | |
| JP2023089195A (ja) | グリピカン3抗体およびそのコンジュゲート | |
| CA3221398A1 (en) | Anthracycline antibody conjugates | |
| CN110352074B (zh) | 用于偶联的半胱氨酸突变抗体 | |
| EP4087657A1 (en) | Alk5 inhibitor conjugates and uses thereof | |
| JP2025109708A (ja) | Bリンパ球特異的アマトキシン抗体コンジュゲート | |
| HK1248538B (zh) | Cd48抗体和其缀合物 | |
| EP4479430A1 (en) | Novel methods of therapy | |
| EP4479429A1 (en) | Novel methods of therapy | |
| HK40011245A (en) | Cysteine mutated antibodies for conjugation | |
| HK1253305B (en) | Anti-cd123 antibodies and conjugates thereof | |
| HK1253305A1 (en) | Anti-cd123 antibodies and conjugates thereof | |
| CA2986796A1 (en) | Anti-ntb-a antibodies and related compositions and methods | |
| HK1237350B (zh) | 用於抗体药物缀合物的稳定性调节接头 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20170829 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20201209 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20230213 Patent event code: PE09021S01D |
|
| PC1202 | Submission of document of withdrawal before decision of registration |
Comment text: [Withdrawal of Procedure relating to Patent, etc.] Withdrawal (Abandonment) Patent event code: PC12021R01D Patent event date: 20230329 |
|
| WITB | Written withdrawal of application |