KR20140031227A - 지질 나노입자들 및 코르티코스테로이드 또는 비타민 d 유도체를 포함하는 조성물 - Google Patents
지질 나노입자들 및 코르티코스테로이드 또는 비타민 d 유도체를 포함하는 조성물 Download PDFInfo
- Publication number
- KR20140031227A KR20140031227A KR1020137027812A KR20137027812A KR20140031227A KR 20140031227 A KR20140031227 A KR 20140031227A KR 1020137027812 A KR1020137027812 A KR 1020137027812A KR 20137027812 A KR20137027812 A KR 20137027812A KR 20140031227 A KR20140031227 A KR 20140031227A
- Authority
- KR
- South Korea
- Prior art keywords
- lipid
- pharmaceutical composition
- skin
- oil
- weight
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 150000002632 lipids Chemical class 0.000 title claims abstract description 278
- 239000002105 nanoparticle Substances 0.000 title claims abstract description 117
- 150000003710 vitamin D derivatives Chemical class 0.000 title claims abstract description 33
- 239000003246 corticosteroid Substances 0.000 title claims abstract description 24
- 239000000203 mixture Substances 0.000 title claims description 131
- 239000004480 active ingredient Substances 0.000 claims abstract description 49
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims abstract description 32
- 235000014113 dietary fatty acids Nutrition 0.000 claims abstract description 24
- 239000000194 fatty acid Substances 0.000 claims abstract description 24
- 229930195729 fatty acid Natural products 0.000 claims abstract description 24
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 claims abstract description 23
- 150000004665 fatty acids Chemical class 0.000 claims abstract description 22
- 150000002191 fatty alcohols Chemical class 0.000 claims abstract description 21
- 150000002148 esters Chemical class 0.000 claims abstract description 17
- 235000012000 cholesterol Nutrition 0.000 claims abstract description 16
- 238000002844 melting Methods 0.000 claims abstract description 14
- 230000008018 melting Effects 0.000 claims abstract description 14
- 230000036760 body temperature Effects 0.000 claims abstract description 10
- 239000007787 solid Substances 0.000 claims abstract description 10
- 125000003976 glyceryl group Chemical group [H]C([*])([H])C(O[H])([H])C(O[H])([H])[H] 0.000 claims abstract description 7
- 239000008180 pharmaceutical surfactant Substances 0.000 claims abstract description 7
- 239000007962 solid dispersion Substances 0.000 claims abstract description 5
- 239000006104 solid solution Substances 0.000 claims abstract description 5
- 150000005691 triesters Chemical class 0.000 claims abstract description 5
- 239000008194 pharmaceutical composition Substances 0.000 claims abstract 22
- 150000005690 diesters Chemical class 0.000 claims abstract 2
- 210000003491 skin Anatomy 0.000 claims description 216
- 239000002245 particle Substances 0.000 claims description 39
- 238000011282 treatment Methods 0.000 claims description 37
- LWQQLNNNIPYSNX-UROSTWAQSA-N calcipotriol Chemical group C1([C@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-UROSTWAQSA-N 0.000 claims description 35
- 239000003814 drug Substances 0.000 claims description 35
- 238000000034 method Methods 0.000 claims description 31
- -1 behenol Chemical compound 0.000 claims description 30
- 206010012438 Dermatitis atopic Diseases 0.000 claims description 29
- 201000008937 atopic dermatitis Diseases 0.000 claims description 29
- 239000012049 topical pharmaceutical composition Substances 0.000 claims description 26
- 229960002882 calcipotriol Drugs 0.000 claims description 25
- 239000008346 aqueous phase Substances 0.000 claims description 21
- 239000012071 phase Substances 0.000 claims description 21
- 239000004094 surface-active agent Substances 0.000 claims description 20
- 208000002874 Acne Vulgaris Diseases 0.000 claims description 19
- 206010000496 acne Diseases 0.000 claims description 19
- CIWBQSYVNNPZIQ-XYWKZLDCSA-N betamethasone dipropionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CIWBQSYVNNPZIQ-XYWKZLDCSA-N 0.000 claims description 19
- 229940074979 cetyl palmitate Drugs 0.000 claims description 17
- 210000003780 hair follicle Anatomy 0.000 claims description 17
- PXDJXZJSCPSGGI-UHFFFAOYSA-N hexadecanoic acid hexadecyl ester Natural products CCCCCCCCCCCCCCCCOC(=O)CCCCCCCCCCCCCCC PXDJXZJSCPSGGI-UHFFFAOYSA-N 0.000 claims description 17
- 239000003921 oil Substances 0.000 claims description 17
- 235000019198 oils Nutrition 0.000 claims description 17
- FLPJVCMIKUWSDR-UHFFFAOYSA-N 2-(4-formylphenoxy)acetamide Chemical compound NC(=O)COC1=CC=C(C=O)C=C1 FLPJVCMIKUWSDR-UHFFFAOYSA-N 0.000 claims description 15
- 201000004624 Dermatitis Diseases 0.000 claims description 15
- 239000000839 emulsion Substances 0.000 claims description 15
- 150000003626 triacylglycerols Chemical class 0.000 claims description 15
- 239000002562 thickening agent Substances 0.000 claims description 12
- 201000004681 Psoriasis Diseases 0.000 claims description 11
- 239000003974 emollient agent Substances 0.000 claims description 11
- 230000000699 topical effect Effects 0.000 claims description 11
- RVGRUAULSDPKGF-UHFFFAOYSA-N Poloxamer Chemical compound C1CO1.CC1CO1 RVGRUAULSDPKGF-UHFFFAOYSA-N 0.000 claims description 10
- 239000002253 acid Substances 0.000 claims description 10
- PVNIQBQSYATKKL-UHFFFAOYSA-N tripalmitin Chemical compound CCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCC PVNIQBQSYATKKL-UHFFFAOYSA-N 0.000 claims description 10
- 239000001993 wax Substances 0.000 claims description 10
- 229920001983 poloxamer Polymers 0.000 claims description 9
- 230000008685 targeting Effects 0.000 claims description 9
- 239000004359 castor oil Substances 0.000 claims description 8
- 235000019438 castor oil Nutrition 0.000 claims description 8
- ZEMPKEQAKRGZGQ-XOQCFJPHSA-N glycerol triricinoleate Natural products CCCCCC[C@@H](O)CC=CCCCCCCCC(=O)OC[C@@H](COC(=O)CCCCCCCC=CC[C@@H](O)CCCCCC)OC(=O)CCCCCCCC=CC[C@H](O)CCCCCC ZEMPKEQAKRGZGQ-XOQCFJPHSA-N 0.000 claims description 8
- 229960000502 poloxamer Drugs 0.000 claims description 8
- 208000017520 skin disease Diseases 0.000 claims description 8
- 210000001732 sebaceous gland Anatomy 0.000 claims description 7
- 229920002125 Sokalan® Polymers 0.000 claims description 6
- 239000000872 buffer Substances 0.000 claims description 6
- GMRQFYUYWCNGIN-NKMMMXOESA-N calcitriol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@@H](CCCC(C)(C)O)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C GMRQFYUYWCNGIN-NKMMMXOESA-N 0.000 claims description 6
- 239000011612 calcitriol Substances 0.000 claims description 6
- 238000001816 cooling Methods 0.000 claims description 6
- 239000003995 emulsifying agent Substances 0.000 claims description 6
- WWZKQHOCKIZLMA-UHFFFAOYSA-N octanoic acid Chemical compound CCCCCCCC(O)=O WWZKQHOCKIZLMA-UHFFFAOYSA-N 0.000 claims description 6
- 201000004700 rosacea Diseases 0.000 claims description 6
- BILPUZXRUDPOOF-UHFFFAOYSA-N stearyl palmitate Chemical compound CCCCCCCCCCCCCCCCCCOC(=O)CCCCCCCCCCCCCCC BILPUZXRUDPOOF-UHFFFAOYSA-N 0.000 claims description 6
- HIQIXEFWDLTDED-UHFFFAOYSA-N 4-hydroxy-1-piperidin-4-ylpyrrolidin-2-one Chemical compound O=C1CC(O)CN1C1CCNCC1 HIQIXEFWDLTDED-UHFFFAOYSA-N 0.000 claims description 5
- 206010037888 Rash pustular Diseases 0.000 claims description 5
- 241001303601 Rosacea Species 0.000 claims description 5
- 150000001412 amines Chemical group 0.000 claims description 5
- 229960002537 betamethasone Drugs 0.000 claims description 5
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 claims description 5
- 229960005084 calcitriol Drugs 0.000 claims description 5
- 235000020964 calcitriol Nutrition 0.000 claims description 5
- 239000006185 dispersion Substances 0.000 claims description 5
- 229940057995 liquid paraffin Drugs 0.000 claims description 5
- 229940057917 medium chain triglycerides Drugs 0.000 claims description 5
- 208000029561 pustule Diseases 0.000 claims description 5
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 claims description 4
- 206010012442 Dermatitis contact Diseases 0.000 claims description 4
- 239000004354 Hydroxyethyl cellulose Substances 0.000 claims description 4
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 claims description 4
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 claims description 4
- 235000019482 Palm oil Nutrition 0.000 claims description 4
- 206010033733 Papule Diseases 0.000 claims description 4
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical class CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 claims description 4
- 239000001913 cellulose Substances 0.000 claims description 4
- 229920002678 cellulose Polymers 0.000 claims description 4
- CBGUOGMQLZIXBE-XGQKBEPLSA-N clobetasol propionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CBGUOGMQLZIXBE-XGQKBEPLSA-N 0.000 claims description 4
- 208000010247 contact dermatitis Diseases 0.000 claims description 4
- UHUSDOQQWJGJQS-UHFFFAOYSA-N glycerol 1,2-dioctadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(CO)OC(=O)CCCCCCCCCCCCCCCCC UHUSDOQQWJGJQS-UHFFFAOYSA-N 0.000 claims description 4
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 claims description 4
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 claims description 4
- 229940071826 hydroxyethyl cellulose Drugs 0.000 claims description 4
- 239000001863 hydroxypropyl cellulose Substances 0.000 claims description 4
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 claims description 4
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 claims description 4
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 claims description 4
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 claims description 4
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 claims description 4
- XUGNVMKQXJXZCD-UHFFFAOYSA-N isopropyl palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC(C)C XUGNVMKQXJXZCD-UHFFFAOYSA-N 0.000 claims description 4
- 239000002540 palm oil Substances 0.000 claims description 4
- 229920000136 polysorbate Polymers 0.000 claims description 4
- 229920002545 silicone oil Polymers 0.000 claims description 4
- DUXYWXYOBMKGIN-UHFFFAOYSA-N trimyristin Chemical compound CCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCC DUXYWXYOBMKGIN-UHFFFAOYSA-N 0.000 claims description 4
- DCXXMTOCNZCJGO-UHFFFAOYSA-N tristearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC DCXXMTOCNZCJGO-UHFFFAOYSA-N 0.000 claims description 4
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical class OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 claims description 3
- 208000003251 Pruritus Diseases 0.000 claims description 3
- 239000003963 antioxidant agent Substances 0.000 claims description 3
- 230000003078 antioxidant effect Effects 0.000 claims description 3
- 208000010668 atopic eczema Diseases 0.000 claims description 3
- 229960001631 carbomer Drugs 0.000 claims description 3
- 208000031513 cyst Diseases 0.000 claims description 3
- 230000003463 hyperproliferative effect Effects 0.000 claims description 3
- 230000002757 inflammatory effect Effects 0.000 claims description 3
- 238000004519 manufacturing process Methods 0.000 claims description 3
- GAQPWOABOQGPKA-UHFFFAOYSA-N octadecyl docosanoate Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCCCCCCCCCCCCCCCCCC GAQPWOABOQGPKA-UHFFFAOYSA-N 0.000 claims description 3
- 229950008882 polysorbate Drugs 0.000 claims description 3
- LADGBHLMCUINGV-UHFFFAOYSA-N tricaprin Chemical compound CCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCC)COC(=O)CCCCCCCCC LADGBHLMCUINGV-UHFFFAOYSA-N 0.000 claims description 3
- GNWCZBXSKIIURR-UHFFFAOYSA-N (2-docosanoyloxy-3-hydroxypropyl) docosanoate Chemical compound CCCCCCCCCCCCCCCCCCCCCC(=O)OCC(CO)OC(=O)CCCCCCCCCCCCCCCCCCCCC GNWCZBXSKIIURR-UHFFFAOYSA-N 0.000 claims description 2
- KIUKXJAPPMFGSW-DNGZLQJQSA-N (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-Acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid Chemical compound CC(=O)N[C@H]1[C@H](O)O[C@H](CO)[C@@H](O)[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](O[C@H]3[C@@H]([C@@H](O)[C@H](O)[C@H](O3)C(O)=O)O)[C@H](O)[C@@H](CO)O2)NC(C)=O)[C@@H](C(O)=O)O1 KIUKXJAPPMFGSW-DNGZLQJQSA-N 0.000 claims description 2
- FUFLCEKSBBHCMO-UHFFFAOYSA-N 11-dehydrocorticosterone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)C(=O)CO)C4C3CCC2=C1 FUFLCEKSBBHCMO-UHFFFAOYSA-N 0.000 claims description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 claims description 2
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 claims description 2
- MYYIMZRZXIQBGI-HVIRSNARSA-N 6alpha-Fluoroprednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](F)C2=C1 MYYIMZRZXIQBGI-HVIRSNARSA-N 0.000 claims description 2
- 235000019489 Almond oil Nutrition 0.000 claims description 2
- QWOJMRHUQHTCJG-UHFFFAOYSA-N CC([CH2-])=O Chemical class CC([CH2-])=O QWOJMRHUQHTCJG-UHFFFAOYSA-N 0.000 claims description 2
- MFYSYFVPBJMHGN-ZPOLXVRWSA-N Cortisone Chemical compound O=C1CC[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 MFYSYFVPBJMHGN-ZPOLXVRWSA-N 0.000 claims description 2
- MFYSYFVPBJMHGN-UHFFFAOYSA-N Cortisone Natural products O=C1CCC2(C)C3C(=O)CC(C)(C(CC4)(O)C(=O)CO)C4C3CCC2=C1 MFYSYFVPBJMHGN-UHFFFAOYSA-N 0.000 claims description 2
- 229920002307 Dextran Polymers 0.000 claims description 2
- MKPDWECBUAZOHP-AFYJWTTESA-N Paramethasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O MKPDWECBUAZOHP-AFYJWTTESA-N 0.000 claims description 2
- 235000019483 Peanut oil Nutrition 0.000 claims description 2
- 206010051246 Photodermatosis Diseases 0.000 claims description 2
- 235000019484 Rapeseed oil Nutrition 0.000 claims description 2
- 235000019485 Safflower oil Nutrition 0.000 claims description 2
- 206010039710 Scleroderma Diseases 0.000 claims description 2
- 235000021355 Stearic acid Nutrition 0.000 claims description 2
- 235000019486 Sunflower oil Nutrition 0.000 claims description 2
- GSEJCLTVZPLZKY-UHFFFAOYSA-N Triethanolamine Chemical group OCCN(CCO)CCO GSEJCLTVZPLZKY-UHFFFAOYSA-N 0.000 claims description 2
- 208000024780 Urticaria Diseases 0.000 claims description 2
- 239000004164 Wax ester Substances 0.000 claims description 2
- 208000009621 actinic keratosis Diseases 0.000 claims description 2
- 229940072056 alginate Drugs 0.000 claims description 2
- 235000010443 alginic acid Nutrition 0.000 claims description 2
- 229920000615 alginic acid Polymers 0.000 claims description 2
- 239000008168 almond oil Substances 0.000 claims description 2
- 238000004873 anchoring Methods 0.000 claims description 2
- 239000008135 aqueous vehicle Substances 0.000 claims description 2
- 235000021302 avocado oil Nutrition 0.000 claims description 2
- 239000008163 avocado oil Substances 0.000 claims description 2
- 235000013871 bee wax Nutrition 0.000 claims description 2
- 239000012166 beeswax Substances 0.000 claims description 2
- SNHRLVCMMWUAJD-SUYDQAKGSA-N betamethasone valerate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(OC(=O)CCCC)[C@@]1(C)C[C@@H]2O SNHRLVCMMWUAJD-SUYDQAKGSA-N 0.000 claims description 2
- 235000021324 borage oil Nutrition 0.000 claims description 2
- 239000010474 borage seed oil Substances 0.000 claims description 2
- 229940082500 cetostearyl alcohol Drugs 0.000 claims description 2
- 229960002842 clobetasol Drugs 0.000 claims description 2
- 235000005687 corn oil Nutrition 0.000 claims description 2
- 239000002285 corn oil Substances 0.000 claims description 2
- 229960004544 cortisone Drugs 0.000 claims description 2
- 235000012343 cottonseed oil Nutrition 0.000 claims description 2
- 239000002385 cottonseed oil Substances 0.000 claims description 2
- 229960003662 desonide Drugs 0.000 claims description 2
- WBGKWQHBNHJJPZ-LECWWXJVSA-N desonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O WBGKWQHBNHJJPZ-LECWWXJVSA-N 0.000 claims description 2
- 229960002593 desoximetasone Drugs 0.000 claims description 2
- VWVSBHGCDBMOOT-IIEHVVJPSA-N desoximetasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@H](C(=O)CO)[C@@]1(C)C[C@@H]2O VWVSBHGCDBMOOT-IIEHVVJPSA-N 0.000 claims description 2
- 229960003957 dexamethasone Drugs 0.000 claims description 2
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 claims description 2
- 229960002086 dextran Drugs 0.000 claims description 2
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 claims description 2
- 229960004091 diflucortolone Drugs 0.000 claims description 2
- OGPWIDANBSLJPC-RFPWEZLHSA-N diflucortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O OGPWIDANBSLJPC-RFPWEZLHSA-N 0.000 claims description 2
- 235000008524 evening primrose extract Nutrition 0.000 claims description 2
- 239000010475 evening primrose oil Substances 0.000 claims description 2
- 229940089020 evening primrose oil Drugs 0.000 claims description 2
- 229960003469 flumetasone Drugs 0.000 claims description 2
- WXURHACBFYSXBI-GQKYHHCASA-N flumethasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O WXURHACBFYSXBI-GQKYHHCASA-N 0.000 claims description 2
- 229960000676 flunisolide Drugs 0.000 claims description 2
- 229960001048 fluorometholone Drugs 0.000 claims description 2
- FAOZLTXFLGPHNG-KNAQIMQKSA-N fluorometholone Chemical compound C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@]2(F)[C@@H](O)C[C@]2(C)[C@@](O)(C(C)=O)CC[C@H]21 FAOZLTXFLGPHNG-KNAQIMQKSA-N 0.000 claims description 2
- 229960000618 fluprednisolone Drugs 0.000 claims description 2
- 229960002714 fluticasone Drugs 0.000 claims description 2
- MGNNYOODZCAHBA-GQKYHHCASA-N fluticasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)SCF)(O)[C@@]2(C)C[C@@H]1O MGNNYOODZCAHBA-GQKYHHCASA-N 0.000 claims description 2
- YQEMORVAKMFKLG-UHFFFAOYSA-N glycerine monostearate Natural products CCCCCCCCCCCCCCCCCC(=O)OC(CO)CO YQEMORVAKMFKLG-UHFFFAOYSA-N 0.000 claims description 2
- SVUQHVRAGMNPLW-UHFFFAOYSA-N glycerol monostearate Natural products CCCCCCCCCCCCCCCCC(=O)OCC(O)CO SVUQHVRAGMNPLW-UHFFFAOYSA-N 0.000 claims description 2
- 125000005908 glyceryl ester group Chemical group 0.000 claims description 2
- 239000008169 grapeseed oil Substances 0.000 claims description 2
- 229940115747 halobetasol Drugs 0.000 claims description 2
- 229920002674 hyaluronan Polymers 0.000 claims description 2
- 229960003160 hyaluronic acid Drugs 0.000 claims description 2
- 229960000890 hydrocortisone Drugs 0.000 claims description 2
- 229940049290 hydrogenated coco-glycerides Drugs 0.000 claims description 2
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 claims description 2
- 229940119170 jojoba wax Drugs 0.000 claims description 2
- 229960001664 mometasone Drugs 0.000 claims description 2
- QLIIKPVHVRXHRI-CXSFZGCWSA-N mometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CCl)(O)[C@@]1(C)C[C@@H]2O QLIIKPVHVRXHRI-CXSFZGCWSA-N 0.000 claims description 2
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 claims description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 claims description 2
- 235000008390 olive oil Nutrition 0.000 claims description 2
- 239000004006 olive oil Substances 0.000 claims description 2
- 239000003346 palm kernel oil Substances 0.000 claims description 2
- 235000019865 palm kernel oil Nutrition 0.000 claims description 2
- 229960002858 paramethasone Drugs 0.000 claims description 2
- 239000000312 peanut oil Substances 0.000 claims description 2
- 150000003904 phospholipids Chemical class 0.000 claims description 2
- 230000008845 photoaging Effects 0.000 claims description 2
- 229960005205 prednisolone Drugs 0.000 claims description 2
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 claims description 2
- 229960004618 prednisone Drugs 0.000 claims description 2
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 claims description 2
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 claims description 2
- 235000005713 safflower oil Nutrition 0.000 claims description 2
- 239000003813 safflower oil Substances 0.000 claims description 2
- 235000011803 sesame oil Nutrition 0.000 claims description 2
- 239000008159 sesame oil Substances 0.000 claims description 2
- 230000009759 skin aging Effects 0.000 claims description 2
- 235000012424 soybean oil Nutrition 0.000 claims description 2
- 239000003549 soybean oil Substances 0.000 claims description 2
- 239000008117 stearic acid Substances 0.000 claims description 2
- 239000002600 sunflower oil Substances 0.000 claims description 2
- BJYLYJCXYAMOFT-RSFVBTMBSA-N tacalcitol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CC[C@@H](O)C(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C BJYLYJCXYAMOFT-RSFVBTMBSA-N 0.000 claims description 2
- 229960004907 tacalcitol Drugs 0.000 claims description 2
- OULAJFUGPPVRBK-UHFFFAOYSA-N tetratriacontyl alcohol Natural products CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCO OULAJFUGPPVRBK-UHFFFAOYSA-N 0.000 claims description 2
- 229960005294 triamcinolone Drugs 0.000 claims description 2
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 claims description 2
- LEHFPXVYPMWYQD-XHIJKXOTSA-N ulobetasol Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)CCl)(O)[C@@]2(C)C[C@@H]1O LEHFPXVYPMWYQD-XHIJKXOTSA-N 0.000 claims description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 claims description 2
- 239000008158 vegetable oil Substances 0.000 claims description 2
- 235000019386 wax ester Nutrition 0.000 claims description 2
- 239000010497 wheat germ oil Substances 0.000 claims description 2
- 229960001810 meprednisone Drugs 0.000 claims 2
- PIDANAQULIKBQS-RNUIGHNZSA-N meprednisone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)CC2=O PIDANAQULIKBQS-RNUIGHNZSA-N 0.000 claims 2
- 230000002265 prevention Effects 0.000 claims 2
- 235000013162 Cocos nucifera Nutrition 0.000 claims 1
- 244000060011 Cocos nucifera Species 0.000 claims 1
- 239000013543 active substance Substances 0.000 claims 1
- 229960004154 diflorasone Drugs 0.000 claims 1
- WXURHACBFYSXBI-XHIJKXOTSA-N diflorasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O WXURHACBFYSXBI-XHIJKXOTSA-N 0.000 claims 1
- IPCSVZSSVZVIGE-UHFFFAOYSA-M hexadecanoate Chemical compound CCCCCCCCCCCCCCCC([O-])=O IPCSVZSSVZVIGE-UHFFFAOYSA-M 0.000 claims 1
- 125000001449 isopropyl group Chemical group [H]C([H])([H])C([H])(*)C([H])([H])[H] 0.000 claims 1
- GLDOVTGHNKAZLK-UHFFFAOYSA-N octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCCCO GLDOVTGHNKAZLK-UHFFFAOYSA-N 0.000 claims 1
- 229940068965 polysorbates Drugs 0.000 claims 1
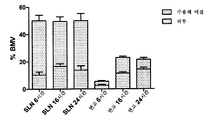
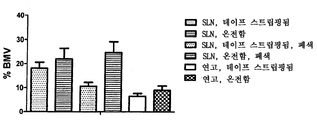
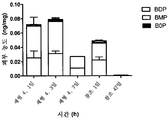
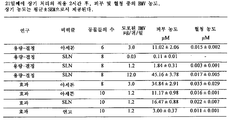
- 241000724256 Brome mosaic virus Species 0.000 description 128
- 239000002047 solid lipid nanoparticle Substances 0.000 description 111
- CSCPPACGZOOCGX-UHFFFAOYSA-N Acetone Chemical compound CC(C)=O CSCPPACGZOOCGX-UHFFFAOYSA-N 0.000 description 75
- 239000002674 ointment Substances 0.000 description 64
- 238000009472 formulation Methods 0.000 description 40
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 33
- 229940079593 drug Drugs 0.000 description 32
- 230000000694 effects Effects 0.000 description 32
- 230000035515 penetration Effects 0.000 description 31
- 229940088679 drug related substance Drugs 0.000 description 28
- 102000005962 receptors Human genes 0.000 description 28
- 108020003175 receptors Proteins 0.000 description 28
- 239000008186 active pharmaceutical agent Substances 0.000 description 27
- 241000699670 Mus sp. Species 0.000 description 23
- 210000000434 stratum corneum Anatomy 0.000 description 22
- SJHPCNCNNSSLPL-CSKARUKUSA-N (4e)-4-(ethoxymethylidene)-2-phenyl-1,3-oxazol-5-one Chemical compound O1C(=O)C(=C/OCC)\N=C1C1=CC=CC=C1 SJHPCNCNNSSLPL-CSKARUKUSA-N 0.000 description 19
- 229960001102 betamethasone dipropionate Drugs 0.000 description 18
- 239000003981 vehicle Substances 0.000 description 18
- 102000004127 Cytokines Human genes 0.000 description 16
- 108090000695 Cytokines Proteins 0.000 description 16
- 230000001965 increasing effect Effects 0.000 description 16
- 210000001519 tissue Anatomy 0.000 description 16
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 16
- 230000004888 barrier function Effects 0.000 description 15
- 210000005069 ears Anatomy 0.000 description 15
- 210000002615 epidermis Anatomy 0.000 description 14
- 239000010410 layer Substances 0.000 description 13
- 229960001334 corticosteroids Drugs 0.000 description 12
- 210000002374 sebum Anatomy 0.000 description 12
- 238000005259 measurement Methods 0.000 description 11
- 229940068196 placebo Drugs 0.000 description 11
- 239000000902 placebo Substances 0.000 description 11
- 229940107161 cholesterol Drugs 0.000 description 10
- 239000006071 cream Substances 0.000 description 10
- 210000002966 serum Anatomy 0.000 description 10
- 230000008591 skin barrier function Effects 0.000 description 10
- 239000000126 substance Substances 0.000 description 10
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- 229930003316 Vitamin D Natural products 0.000 description 9
- QYSXJUFSXHHAJI-XFEUOLMDSA-N Vitamin D3 Natural products C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C/C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-XFEUOLMDSA-N 0.000 description 9
- 150000001875 compounds Chemical class 0.000 description 9
- 235000019166 vitamin D Nutrition 0.000 description 9
- 239000011710 vitamin D Substances 0.000 description 9
- 229940046008 vitamin d Drugs 0.000 description 9
- 241000700159 Rattus Species 0.000 description 8
- 210000004207 dermis Anatomy 0.000 description 8
- 238000000338 in vitro Methods 0.000 description 8
- 238000003860 storage Methods 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 7
- 238000009792 diffusion process Methods 0.000 description 7
- 239000007788 liquid Substances 0.000 description 7
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 6
- XEKOWRVHYACXOJ-UHFFFAOYSA-N Ethyl acetate Chemical compound CCOC(C)=O XEKOWRVHYACXOJ-UHFFFAOYSA-N 0.000 description 6
- 238000004458 analytical method Methods 0.000 description 6
- 210000004027 cell Anatomy 0.000 description 6
- 230000007423 decrease Effects 0.000 description 6
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 6
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 6
- 229940068968 polysorbate 80 Drugs 0.000 description 6
- 229920000053 polysorbate 80 Polymers 0.000 description 6
- 238000002360 preparation method Methods 0.000 description 6
- 239000000243 solution Substances 0.000 description 6
- 108090001007 Interleukin-8 Proteins 0.000 description 5
- 206010040880 Skin irritation Diseases 0.000 description 5
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- 238000001574 biopsy Methods 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000013078 crystal Substances 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 5
- 238000004128 high performance liquid chromatography Methods 0.000 description 5
- 230000000622 irritating effect Effects 0.000 description 5
- 239000000546 pharmaceutical excipient Substances 0.000 description 5
- 238000004445 quantitative analysis Methods 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- 230000036556 skin irritation Effects 0.000 description 5
- 231100000475 skin irritation Toxicity 0.000 description 5
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 4
- 108010065805 Interleukin-12 Proteins 0.000 description 4
- 108090000978 Interleukin-4 Proteins 0.000 description 4
- IMNFDUFMRHMDMM-UHFFFAOYSA-N N-Heptane Chemical compound CCCCCCC IMNFDUFMRHMDMM-UHFFFAOYSA-N 0.000 description 4
- 239000000969 carrier Substances 0.000 description 4
- 238000007405 data analysis Methods 0.000 description 4
- 238000001514 detection method Methods 0.000 description 4
- 231100000673 dose–response relationship Toxicity 0.000 description 4
- 238000000265 homogenisation Methods 0.000 description 4
- 238000001727 in vivo Methods 0.000 description 4
- 230000004054 inflammatory process Effects 0.000 description 4
- 238000004949 mass spectrometry Methods 0.000 description 4
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 230000004044 response Effects 0.000 description 4
- 239000002344 surface layer Substances 0.000 description 4
- 230000001225 therapeutic effect Effects 0.000 description 4
- 102000009310 vitamin D receptors Human genes 0.000 description 4
- 108050000156 vitamin D receptors Proteins 0.000 description 4
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 229920000742 Cotton Polymers 0.000 description 3
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 108090000174 Interleukin-10 Proteins 0.000 description 3
- 240000001307 Myosotis scorpioides Species 0.000 description 3
- HEMHJVSKTPXQMS-UHFFFAOYSA-M Sodium hydroxide Chemical compound [OH-].[Na+] HEMHJVSKTPXQMS-UHFFFAOYSA-M 0.000 description 3
- 230000002378 acidificating effect Effects 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 238000012377 drug delivery Methods 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 230000003993 interaction Effects 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 239000002207 metabolite Substances 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 230000008506 pathogenesis Effects 0.000 description 3
- 239000002904 solvent Substances 0.000 description 3
- 238000003756 stirring Methods 0.000 description 3
- 238000012360 testing method Methods 0.000 description 3
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 2
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 2
- USFZMSVCRYTOJT-UHFFFAOYSA-N Ammonium acetate Chemical compound N.CC(O)=O USFZMSVCRYTOJT-UHFFFAOYSA-N 0.000 description 2
- 239000005695 Ammonium acetate Substances 0.000 description 2
- OLCWFLWEHWLBTO-HSZRJFAPSA-N BMS-214662 Chemical compound C=1C=CSC=1S(=O)(=O)N([C@@H](C1)CC=2C=CC=CC=2)CC2=CC(C#N)=CC=C2N1CC1=CN=CN1 OLCWFLWEHWLBTO-HSZRJFAPSA-N 0.000 description 2
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 2
- 229930105110 Cyclosporin A Natural products 0.000 description 2
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 2
- 108010036949 Cyclosporine Proteins 0.000 description 2
- 206010013786 Dry skin Diseases 0.000 description 2
- 206010015150 Erythema Diseases 0.000 description 2
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 2
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 2
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 2
- 238000010521 absorption reaction Methods 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- POJWUDADGALRAB-UHFFFAOYSA-N allantoin Chemical class NC(=O)NC1NC(=O)NC1=O POJWUDADGALRAB-UHFFFAOYSA-N 0.000 description 2
- 229940043376 ammonium acetate Drugs 0.000 description 2
- 235000019257 ammonium acetate Nutrition 0.000 description 2
- 230000003110 anti-inflammatory effect Effects 0.000 description 2
- 238000003556 assay Methods 0.000 description 2
- 230000006399 behavior Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000004071 biological effect Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 235000011089 carbon dioxide Nutrition 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 238000012512 characterization method Methods 0.000 description 2
- 229960001265 ciclosporin Drugs 0.000 description 2
- 229960004703 clobetasol propionate Drugs 0.000 description 2
- 239000002537 cosmetic Substances 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 238000013480 data collection Methods 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 230000001627 detrimental effect Effects 0.000 description 2
- 208000035475 disorder Diseases 0.000 description 2
- 239000003596 drug target Substances 0.000 description 2
- 230000037336 dry skin Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 231100000321 erythema Toxicity 0.000 description 2
- 238000001704 evaporation Methods 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 239000013020 final formulation Substances 0.000 description 2
- 235000019253 formic acid Nutrition 0.000 description 2
- 230000004927 fusion Effects 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 210000004209 hair Anatomy 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 230000028709 inflammatory response Effects 0.000 description 2
- 238000011081 inoculation Methods 0.000 description 2
- 229960002725 isoflurane Drugs 0.000 description 2
- 210000002510 keratinocyte Anatomy 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 2
- 239000006210 lotion Substances 0.000 description 2
- 239000012139 lysis buffer Substances 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 238000003801 milling Methods 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- BDJRBEYXGGNYIS-UHFFFAOYSA-N nonanedioic acid Chemical compound OC(=O)CCCCCCCC(O)=O BDJRBEYXGGNYIS-UHFFFAOYSA-N 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 230000000149 penetrating effect Effects 0.000 description 2
- 229920000223 polyglycerol Chemical class 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 229940068977 polysorbate 20 Drugs 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000008213 purified water Substances 0.000 description 2
- 238000007390 skin biopsy Methods 0.000 description 2
- 230000037067 skin hydration Effects 0.000 description 2
- 239000011780 sodium chloride Substances 0.000 description 2
- 239000007921 spray Substances 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 229940125379 topical corticosteroid Drugs 0.000 description 2
- 230000001052 transient effect Effects 0.000 description 2
- 239000003643 water by type Substances 0.000 description 2
- YZOUYRAONFXZSI-SBHWVFSVSA-N (1S,3R,5R,6R,8R,10R,11R,13R,15R,16R,18R,20R,21R,23R,25R,26R,28R,30R,31S,33R,35R,36R,37S,38R,39S,40R,41S,42R,43S,44R,45S,46R,47S,48R,49S)-5,10,15,20,25,30,35-heptakis(hydroxymethyl)-37,39,40,41,42,43,44,45,46,47,48,49-dodecamethoxy-2,4,7,9,12,14,17,19,22,24,27,29,32,34-tetradecaoxaoctacyclo[31.2.2.23,6.28,11.213,16.218,21.223,26.228,31]nonatetracontane-36,38-diol Chemical compound O([C@@H]([C@H]([C@@H]1OC)OC)O[C@H]2[C@@H](O)[C@@H]([C@@H](O[C@@H]3[C@@H](CO)O[C@@H]([C@H]([C@@H]3O)OC)O[C@@H]3[C@@H](CO)O[C@@H]([C@H]([C@@H]3OC)OC)O[C@@H]3[C@@H](CO)O[C@@H]([C@H]([C@@H]3OC)OC)O[C@@H]3[C@@H](CO)O[C@@H]([C@H]([C@@H]3OC)OC)O3)O[C@@H]2CO)OC)[C@H](CO)[C@H]1O[C@@H]1[C@@H](OC)[C@H](OC)[C@H]3[C@@H](CO)O1 YZOUYRAONFXZSI-SBHWVFSVSA-N 0.000 description 1
- LWQQLNNNIPYSNX-CIJZWTHJSA-N (1r,3s,5z)-5-[(2e)-2-[(1r,3as,7ar)-1-[(e,2r,5r)-5-cyclopropyl-5-hydroxypent-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1h-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol Chemical compound C1([C@@H](O)/C=C/[C@@H](C)[C@@H]2[C@]3(CCCC(/[C@@H]3CC2)=C\C=C\2C([C@@H](O)C[C@H](O)C/2)=C)C)CC1 LWQQLNNNIPYSNX-CIJZWTHJSA-N 0.000 description 1
- JESIHYIJKKUWIS-UHFFFAOYSA-N 1-(4-Methylphenyl)ethanol Chemical compound CC(O)C1=CC=C(C)C=C1 JESIHYIJKKUWIS-UHFFFAOYSA-N 0.000 description 1
- POJWUDADGALRAB-PVQJCKRUSA-N Allantoin Chemical class NC(=O)N[C@@H]1NC(=O)NC1=O POJWUDADGALRAB-PVQJCKRUSA-N 0.000 description 1
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 235000005749 Anthriscus sylvestris Nutrition 0.000 description 1
- 238000009020 BCA Protein Assay Kit Methods 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 208000034309 Bacterial disease carrier Diseases 0.000 description 1
- 239000004342 Benzoyl peroxide Substances 0.000 description 1
- OMPJBNCRMGITSC-UHFFFAOYSA-N Benzoylperoxide Chemical compound C=1C=CC=CC=1C(=O)OOC(=O)C1=CC=CC=C1 OMPJBNCRMGITSC-UHFFFAOYSA-N 0.000 description 1
- 241001340526 Chrysoclista linneella Species 0.000 description 1
- 241000186427 Cutibacterium acnes Species 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- SNPLKNRPJHDVJA-ZETCQYMHSA-N D-panthenol Chemical class OCC(C)(C)[C@@H](O)C(=O)NCCCO SNPLKNRPJHDVJA-ZETCQYMHSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 108010067770 Endopeptidase K Proteins 0.000 description 1
- 208000010201 Exanthema Diseases 0.000 description 1
- 102100028314 Filaggrin Human genes 0.000 description 1
- 101710088660 Filaggrin Proteins 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- IECPWNUMDGFDKC-UHFFFAOYSA-N Fusicsaeure Natural products C12C(O)CC3C(=C(CCC=C(C)C)C(O)=O)C(OC(C)=O)CC3(C)C1(C)CCC1C2(C)CCC(O)C1C IECPWNUMDGFDKC-UHFFFAOYSA-N 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 206010020649 Hyperkeratosis Diseases 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 208000003367 Hypopigmentation Diseases 0.000 description 1
- 101150081923 IL4 gene Proteins 0.000 description 1
- 102100037850 Interferon gamma Human genes 0.000 description 1
- 108010074328 Interferon-gamma Proteins 0.000 description 1
- 102000003777 Interleukin-1 beta Human genes 0.000 description 1
- 108090000193 Interleukin-1 beta Proteins 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- 102000000588 Interleukin-2 Human genes 0.000 description 1
- 102000004388 Interleukin-4 Human genes 0.000 description 1
- 108010002616 Interleukin-5 Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- SHGAZHPCJJPHSC-NUEINMDLSA-N Isotretinoin Chemical compound OC(=O)C=C(C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-NUEINMDLSA-N 0.000 description 1
- 239000004909 Moisturizer Substances 0.000 description 1
- 108091029480 NONCODE Proteins 0.000 description 1
- 208000012868 Overgrowth Diseases 0.000 description 1
- 208000009675 Perioral Dermatitis Diseases 0.000 description 1
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 1
- 229940124158 Protease/peptidase inhibitor Drugs 0.000 description 1
- 206010070834 Sensitisation Diseases 0.000 description 1
- BQCADISMDOOEFD-UHFFFAOYSA-N Silver Chemical compound [Ag] BQCADISMDOOEFD-UHFFFAOYSA-N 0.000 description 1
- 206010040799 Skin atrophy Diseases 0.000 description 1
- 206010040925 Skin striae Diseases 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 1
- 238000003639 Student–Newman–Keuls (SNK) method Methods 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 206010043275 Teratogenicity Diseases 0.000 description 1
- ZPVGIKNDGJGLCO-VGAMQAOUSA-N [(2s,3r,4s,5s,6r)-2-[(2s,3s,4s,5r)-3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl] hexadecanoate Chemical compound CCCCCCCCCCCCCCCC(=O)O[C@@]1([C@]2(CO)[C@H]([C@H](O)[C@@H](CO)O2)O)O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O ZPVGIKNDGJGLCO-VGAMQAOUSA-N 0.000 description 1
- SZYSLWCAWVWFLT-UTGHZIEOSA-N [(2s,3s,4s,5r)-3,4-dihydroxy-5-(hydroxymethyl)-2-[(2r,3r,4s,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxolan-2-yl]methyl octadecanoate Chemical compound O([C@@H]1[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O1)O)[C@]1(COC(=O)CCCCCCCCCCCCCCCCC)O[C@H](CO)[C@@H](O)[C@@H]1O SZYSLWCAWVWFLT-UTGHZIEOSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 239000008351 acetate buffer Substances 0.000 description 1
- 229960002916 adapalene Drugs 0.000 description 1
- LZCDAPDGXCYOEH-UHFFFAOYSA-N adapalene Chemical compound C1=C(C(O)=O)C=CC2=CC(C3=CC=C(C(=C3)C34CC5CC(CC(C5)C3)C4)OC)=CC=C21 LZCDAPDGXCYOEH-UHFFFAOYSA-N 0.000 description 1
- 210000004404 adrenal cortex Anatomy 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- OFHCOWSQAMBJIW-AVJTYSNKSA-N alfacalcidol Chemical compound C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)C[C@H](O)C1=C OFHCOWSQAMBJIW-AVJTYSNKSA-N 0.000 description 1
- 229960002535 alfacalcidol Drugs 0.000 description 1
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 1
- 229960000458 allantoin Drugs 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 230000007815 allergy Effects 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 150000008064 anhydrides Chemical class 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000947 anti-immunosuppressive effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 230000000890 antigenic effect Effects 0.000 description 1
- 230000037365 barrier function of the epidermis Effects 0.000 description 1
- 235000019400 benzoyl peroxide Nutrition 0.000 description 1
- 229960004311 betamethasone valerate Drugs 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000002051 biphasic effect Effects 0.000 description 1
- 238000010241 blood sampling Methods 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 231100000481 chemical toxicant Toxicity 0.000 description 1
- 229960002227 clindamycin Drugs 0.000 description 1
- KDLRVYVGXIQJDK-AWPVFWJPSA-N clindamycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 KDLRVYVGXIQJDK-AWPVFWJPSA-N 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 238000013270 controlled release Methods 0.000 description 1
- STORWMDPIHOSMF-UHFFFAOYSA-N decanoic acid;octanoic acid;propane-1,2,3-triol Chemical compound OCC(O)CO.CCCCCCCC(O)=O.CCCCCCCCCC(O)=O STORWMDPIHOSMF-UHFFFAOYSA-N 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 150000002009 diols Chemical class 0.000 description 1
- KDQPSPMLNJTZAL-UHFFFAOYSA-L disodium hydrogenphosphate dihydrate Chemical compound O.O.[Na+].[Na+].OP([O-])([O-])=O KDQPSPMLNJTZAL-UHFFFAOYSA-L 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 238000002296 dynamic light scattering Methods 0.000 description 1
- 230000000435 effect on ear Effects 0.000 description 1
- 230000002500 effect on skin Effects 0.000 description 1
- 239000008344 egg yolk phospholipid Substances 0.000 description 1
- 210000001513 elbow Anatomy 0.000 description 1
- 239000003792 electrolyte Substances 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 1
- 229960003276 erythromycin Drugs 0.000 description 1
- 229940011871 estrogen Drugs 0.000 description 1
- 239000000262 estrogen Substances 0.000 description 1
- RTZKZFJDLAIYFH-UHFFFAOYSA-N ether Substances CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 201000005884 exanthem Diseases 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 230000003325 follicular Effects 0.000 description 1
- 235000021588 free fatty acids Nutrition 0.000 description 1
- 230000008014 freezing Effects 0.000 description 1
- 238000007710 freezing Methods 0.000 description 1
- 229940048400 fucidin Drugs 0.000 description 1
- 238000001879 gelation Methods 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 230000000762 glandular Effects 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 229960005150 glycerol Drugs 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- UBHWBODXJBSFLH-UHFFFAOYSA-N hexadecan-1-ol;octadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO.CCCCCCCCCCCCCCCCCCO UBHWBODXJBSFLH-UHFFFAOYSA-N 0.000 description 1
- 229940088597 hormone Drugs 0.000 description 1
- 239000005556 hormone Substances 0.000 description 1
- 230000036571 hydration Effects 0.000 description 1
- 238000006703 hydration reaction Methods 0.000 description 1
- IXCSERBJSXMMFS-UHFFFAOYSA-N hydrogen chloride Substances Cl.Cl IXCSERBJSXMMFS-UHFFFAOYSA-N 0.000 description 1
- 229910000041 hydrogen chloride Inorganic materials 0.000 description 1
- 230000003425 hypopigmentation Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 239000002085 irritant Substances 0.000 description 1
- 231100000021 irritant Toxicity 0.000 description 1
- 229960005280 isotretinoin Drugs 0.000 description 1
- 230000002147 killing effect Effects 0.000 description 1
- 210000003127 knee Anatomy 0.000 description 1
- 150000002634 lipophilic molecules Chemical class 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 230000004807 localization Effects 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 235000013372 meat Nutrition 0.000 description 1
- WSFSSNUMVMOOMR-NJFSPNSNSA-N methanone Chemical compound O=[14CH2] WSFSSNUMVMOOMR-NJFSPNSNSA-N 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 244000000010 microbial pathogen Species 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000001333 moisturizer Effects 0.000 description 1
- 150000004682 monohydrates Chemical class 0.000 description 1
- 239000004570 mortar (masonry) Substances 0.000 description 1
- 239000007908 nanoemulsion Substances 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 125000001400 nonyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- 239000007764 o/w emulsion Substances 0.000 description 1
- 230000000414 obstructive effect Effects 0.000 description 1
- 238000001543 one-way ANOVA Methods 0.000 description 1
- 206010033675 panniculitis Diseases 0.000 description 1
- 229940101267 panthenol Drugs 0.000 description 1
- 239000011619 pantothenol Chemical class 0.000 description 1
- 235000020957 pantothenol Nutrition 0.000 description 1
- 238000003921 particle size analysis Methods 0.000 description 1
- 239000003961 penetration enhancing agent Substances 0.000 description 1
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 1
- 239000000825 pharmaceutical preparation Substances 0.000 description 1
- 229940127557 pharmaceutical product Drugs 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- WTJKGGKOPKCXLL-RRHRGVEJSA-N phosphatidylcholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCCCCCCC=CCCCCCCCC WTJKGGKOPKCXLL-RRHRGVEJSA-N 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920001993 poloxamer 188 Polymers 0.000 description 1
- 229940044519 poloxamer 188 Drugs 0.000 description 1
- 229920001992 poloxamer 407 Polymers 0.000 description 1
- 229940044476 poloxamer 407 Drugs 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 239000003755 preservative agent Substances 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 239000003380 propellant Substances 0.000 description 1
- 229940055019 propionibacterium acne Drugs 0.000 description 1
- 102000004169 proteins and genes Human genes 0.000 description 1
- 108090000623 proteins and genes Proteins 0.000 description 1
- 238000007388 punch biopsy Methods 0.000 description 1
- 206010037844 rash Diseases 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 238000005070 sampling Methods 0.000 description 1
- 210000004761 scalp Anatomy 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 210000003786 sclera Anatomy 0.000 description 1
- 238000006748 scratching Methods 0.000 description 1
- 230000002393 scratching effect Effects 0.000 description 1
- 210000004378 sebocyte Anatomy 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 230000008313 sensitization Effects 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 229910052709 silver Inorganic materials 0.000 description 1
- 239000004332 silver Substances 0.000 description 1
- 230000037380 skin damage Effects 0.000 description 1
- 206010040872 skin infection Diseases 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- HJHVQCXHVMGZNC-JCJNLNMISA-M sodium;(2z)-2-[(3r,4s,5s,8s,9s,10s,11r,13r,14s,16s)-16-acetyloxy-3,11-dihydroxy-4,8,10,14-tetramethyl-2,3,4,5,6,7,9,11,12,13,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17-ylidene]-6-methylhept-5-enoate Chemical compound [Na+].O[C@@H]([C@@H]12)C[C@H]3\C(=C(/CCC=C(C)C)C([O-])=O)[C@@H](OC(C)=O)C[C@]3(C)[C@@]2(C)CC[C@@H]2[C@]1(C)CC[C@@H](O)[C@H]2C HJHVQCXHVMGZNC-JCJNLNMISA-M 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 238000000527 sonication Methods 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 230000035882 stress Effects 0.000 description 1
- 210000004304 subcutaneous tissue Anatomy 0.000 description 1
- 239000013595 supernatant sample Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 231100000211 teratogenicity Toxicity 0.000 description 1
- 150000003512 tertiary amines Chemical class 0.000 description 1
- OFVLGDICTFRJMM-WESIUVDSSA-N tetracycline Chemical compound C1=CC=C2[C@](O)(C)[C@H]3C[C@H]4[C@H](N(C)C)C(O)=C(C(N)=O)C(=O)[C@@]4(O)C(O)=C3C(=O)C2=C1O OFVLGDICTFRJMM-WESIUVDSSA-N 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 230000009974 thixotropic effect Effects 0.000 description 1
- 206010043778 thyroiditis Diseases 0.000 description 1
- 239000003440 toxic substance Substances 0.000 description 1
- 229960001727 tretinoin Drugs 0.000 description 1
- VLPFTAMPNXLGLX-UHFFFAOYSA-N trioctanoin Chemical group CCCCCCCC(=O)OCC(OC(=O)CCCCCCC)COC(=O)CCCCCCC VLPFTAMPNXLGLX-UHFFFAOYSA-N 0.000 description 1
- 238000004704 ultra performance liquid chromatography Methods 0.000 description 1
- 210000001635 urinary tract Anatomy 0.000 description 1
- 229940070710 valerate Drugs 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 230000003639 vasoconstrictive effect Effects 0.000 description 1
- QYSXJUFSXHHAJI-YRZJJWOYSA-N vitamin D3 Chemical class C1(/[C@@H]2CC[C@@H]([C@]2(CCC1)C)[C@H](C)CCCC(C)C)=C\C=C1\C[C@@H](O)CCC1=C QYSXJUFSXHHAJI-YRZJJWOYSA-N 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000005303 weighing Methods 0.000 description 1
- 230000037303 wrinkles Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/59—Compounds containing 9, 10- seco- cyclopenta[a]hydrophenanthrene ring systems
- A61K31/593—9,10-Secocholestane derivatives, e.g. cholecalciferol, i.e. vitamin D3
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/57—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of two carbon atoms, e.g. pregnane or progesterone
- A61K31/573—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of two carbon atoms, e.g. pregnane or progesterone substituted in position 21, e.g. cortisone, dexamethasone, prednisone or aldosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/06—Ointments; Bases therefor; Other semi-solid forms, e.g. creams, sticks, gels
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/51—Nanocapsules; Nanoparticles
- A61K9/5107—Excipients; Inactive ingredients
- A61K9/5123—Organic compounds, e.g. fats, sugars
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/02—Drugs for dermatological disorders for treating wounds, ulcers, burns, scars, keloids, or the like
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/08—Antiseborrheics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2300/00—Mixtures or combinations of active ingredients, wherein at least one active ingredient is fully defined in groups A61K31/00 - A61K41/00
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Medicinal Chemistry (AREA)
- Life Sciences & Earth Sciences (AREA)
- Epidemiology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Dermatology (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Nanotechnology (AREA)
- Biomedical Technology (AREA)
- Obesity (AREA)
- Nutrition Science (AREA)
- Diabetes (AREA)
- Hematology (AREA)
- Medicinal Preparation (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161467192P | 2011-03-24 | 2011-03-24 | |
| US61/467,192 | 2011-03-24 | ||
| PCT/EP2012/055222 WO2012127037A2 (en) | 2011-03-24 | 2012-03-23 | A composition comprising lipid nanoparticles and a corticosteroid or vitamin d derivative |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20140031227A true KR20140031227A (ko) | 2014-03-12 |
Family
ID=45894467
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020137027812A Withdrawn KR20140031227A (ko) | 2011-03-24 | 2012-03-23 | 지질 나노입자들 및 코르티코스테로이드 또는 비타민 d 유도체를 포함하는 조성물 |
Country Status (7)
| Country | Link |
|---|---|
| US (1) | US20140079785A1 (enExample) |
| EP (1) | EP2688560A2 (enExample) |
| JP (1) | JP2014508796A (enExample) |
| KR (1) | KR20140031227A (enExample) |
| CN (1) | CN103442700B (enExample) |
| RU (1) | RU2602171C2 (enExample) |
| WO (1) | WO2012127037A2 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20180004164A (ko) * | 2015-05-08 | 2018-01-10 | 액티버스 파마 컴퍼니 리미티드 | 글루코코르티코스테로이드의 나노미립자를 함유하는 수성 현탁액제 |
| WO2022203145A1 (ko) * | 2021-03-22 | 2022-09-29 | 주식회사 앱스바이오 | 매스틱검 또는 감마오리자놀로 제조한 지질나노입자 조성물 |
| KR102725138B1 (ko) * | 2023-10-30 | 2024-11-05 | 김윤영 | 나노비타민 복합 조성물 |
Families Citing this family (31)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US10695432B2 (en) | 2010-10-29 | 2020-06-30 | Infirst Healthcare Limited | Solid solution compositions and use in severe pain |
| US9744132B2 (en) | 2010-10-29 | 2017-08-29 | Infirst Healthcare Limited | Solid solution compositions and use in chronic inflammation |
| US10695431B2 (en) | 2010-10-29 | 2020-06-30 | Infirst Healthcare Limited | Solid solution compositions and use in cardiovascular disease |
| US8895537B2 (en) | 2010-10-29 | 2014-11-25 | Infirst Healthcare Ltd. | Compositions and methods for treating cardiovascular diseases |
| US11202831B2 (en) | 2010-10-29 | 2021-12-21 | Infirst Healthcare Limited | Solid solution compositions and use in cardiovascular disease |
| US9504664B2 (en) | 2010-10-29 | 2016-11-29 | Infirst Healthcare Limited | Compositions and methods for treating severe pain |
| US9271950B2 (en) | 2010-10-29 | 2016-03-01 | Infirst Healthcare Limited | Compositions for treating chronic inflammation and inflammatory diseases |
| US9308213B2 (en) | 2010-10-29 | 2016-04-12 | Infirst Healthcare Limited | Solid solution compositions and use in chronic inflammation |
| US11730709B2 (en) | 2010-10-29 | 2023-08-22 | Infirst Healthcare Limited | Compositions and methods for treating severe pain |
| US11224659B2 (en) | 2010-10-29 | 2022-01-18 | Infirst Healthcare Limited | Solid solution compositions and use in severe pain |
| CN104968368B (zh) * | 2013-01-14 | 2018-05-04 | 因佛斯特医疗有限公司 | 固溶体组合物及其在剧痛中的用途 |
| MX2015014701A (es) | 2013-04-24 | 2016-08-08 | Salk Inst For Biological Studi | Circuito genomico del receptor de vitamina d/smad que regula la compuerta de la respuesta fibrotica. |
| CN105451818A (zh) | 2013-06-05 | 2016-03-30 | 萨克生物研究学院 | 治疗涉及cxcl12活性的疾病的维生素d受体激动剂 |
| EP2821077A1 (en) * | 2013-07-04 | 2015-01-07 | Praxis Biopharma Research Institute | Lipid nanoparticles for wound healing |
| CN103417507B (zh) * | 2013-08-23 | 2015-12-02 | 王显著 | 布地奈德药物组合物 |
| TW201636025A (zh) * | 2015-04-15 | 2016-10-16 | Maruho Kk | 皮膚用之醫藥組成物 |
| CZ307681B6 (cs) | 2016-02-29 | 2019-02-13 | Ústav makromolekulární chemie AV ČR, v. v. i. | Fotoaktivovatelná nanočástice pro fotodynamické aplikace, způsob její přípravy, farmaceutická kompozice ji obsahující a jejich použití |
| WO2017194454A1 (en) * | 2016-05-09 | 2017-11-16 | Astrazeneca Ab | Lipid nanoparticles comprising lipophilic anti-inflammatory agents and methods of use thereof |
| CN106265485A (zh) * | 2016-08-22 | 2017-01-04 | 江苏知原药业有限公司 | 一种稳定性改善的卡泊三醇组合物 |
| WO2019023149A1 (en) | 2017-07-24 | 2019-01-31 | Salk Institute For Biological Studies | USE OF BROMODOMAIN-CONTAINING PROTEIN-9 ANTAGONISTS IN ASSOCIATION WITH VITAMIN D RECEPTOR AGONISTS IN THE TREATMENT OF DIABETES |
| CA3085694A1 (en) * | 2017-12-12 | 2019-06-20 | Lead Biotherapeutics Ltd. | Solid lipid nanoparticle for intracellular release of active substances and method for production the same |
| RU2018114533A (ru) * | 2018-04-19 | 2019-10-21 | Общество С Ограниченной Ответственностью "Научно-Исследовательская Компания "Медбиофарм" | Композиция на основе твердых липидных частиц, обладающая свойством направленной доставки для лечения вирусных заболеваний (варианты) |
| CN108904445A (zh) * | 2018-08-06 | 2018-11-30 | 江苏知原药业有限公司 | 钙泊三醇纳米悬浮液 |
| SI3820439T1 (sl) * | 2018-09-11 | 2022-02-28 | Lead Biotherapeutics Ltd. | Sistem mukoadhezivne disperzije nanodelcev in postopek za njegovo izdelavo |
| WO2020231800A1 (en) * | 2019-05-10 | 2020-11-19 | Encube Ethicals Private Limited | Topical antifungal formulation |
| EP3741378A1 (de) | 2019-05-23 | 2020-11-25 | Dimitrios Tsakouridis | Zusammensetzung zur topischen behandlung und pflege der psoriatischen haut |
| CN111588696A (zh) * | 2020-04-28 | 2020-08-28 | 南通华山药业有限公司 | 一种阿法骨化醇口服脂质体药物及其制备方法与应用 |
| CN111494321A (zh) * | 2020-04-28 | 2020-08-07 | 南通华山药业有限公司 | 一种骨化三醇长循环脂质体及其制备方法 |
| CA3182478A1 (en) * | 2020-05-12 | 2021-11-18 | Chemistry Rx | Compositions for treating hair loss |
| RU2749360C1 (ru) * | 2020-10-09 | 2021-06-09 | Федеральное государственное бюджетное военное образовательное учреждение высшего образования "Военно-медицинская академия имени С.М. Кирова" Министерства обороны Российской Федерации (ВМедА) | Способ лечения ладонно-подошвенного пустулеза |
| JP7633712B2 (ja) * | 2023-05-12 | 2025-02-20 | アピ株式会社 | 外用組成物 |
Family Cites Families (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU658608B2 (en) * | 1991-03-25 | 1995-04-27 | Astellas Pharma Europe B.V. | Topical preparation containing a suspension of solid lipid particles |
| DE4131562A1 (de) | 1991-09-18 | 1993-03-25 | Medac Klinische Spezialpraep | Arzneistofftraeger aus festen lipidteilchen-feste lipidnanosphaeren (sln) |
| GB9300763D0 (en) | 1993-01-15 | 1993-03-03 | Leo Pharm Prod Ltd | Chemical compound |
| US5785976A (en) * | 1993-03-05 | 1998-07-28 | Pharmacia & Upjohn Ab | Solid lipid particles, particles of bioactive agents and methods for the manufacture and use thereof |
| DK0979654T3 (da) * | 1998-03-04 | 2006-01-02 | Teijin Ltd | Emulsionslotioner med aktivt vitamin D3 |
| US8663692B1 (en) * | 1999-05-07 | 2014-03-04 | Pharmasol Gmbh | Lipid particles on the basis of mixtures of liquid and solid lipids and method for producing same |
| TR200103188T2 (tr) * | 1999-05-07 | 2002-04-22 | Pharmasol Gmbh | Sıvı ve katı lipit karışımları temelli lipid parçacıklar. |
| US7147841B2 (en) * | 2002-06-17 | 2006-12-12 | Ciba Specialty Chemicals Corporation | Formulation of UV absorbers by incorporation in solid lipid nanoparticles |
| JP5777846B2 (ja) * | 2005-06-15 | 2015-09-09 | マサチューセッツ インスティテュート オブ テクノロジー | アミン含有脂質およびその使用 |
| FR2893847B1 (fr) * | 2005-11-30 | 2010-10-29 | Galderma Sa | Composition sous forme de spray comprenant un derive de vitamine d et une phase huileuse |
| JP2010502624A (ja) * | 2006-08-29 | 2010-01-28 | テバ ファーマシューティカル インダストリーズ リミティド | 低pH親和性を有するコルチコステロイド化合物及びビタミンD含有化合物を含む安定な薬理活性組成物 |
| FR2909284B1 (fr) * | 2006-11-30 | 2012-09-21 | Galderma Sa | Nouvelles compositions sous forme d'onguent sans vaseline comprenant un derive de vitamine d et eventuellement un anti-inflammatoire steroidien |
| US10265265B2 (en) * | 2007-03-15 | 2019-04-23 | Drug Delivery Solutions Limited | Topical composition |
| US8470304B2 (en) * | 2009-08-04 | 2013-06-25 | Avidas Pharmaceuticals Llc | Therapeutic vitamin D sun-protecting formulations and methods for their use |
-
2012
- 2012-03-23 RU RU2013147429/15A patent/RU2602171C2/ru not_active IP Right Cessation
- 2012-03-23 US US14/006,890 patent/US20140079785A1/en not_active Abandoned
- 2012-03-23 CN CN201280014897.2A patent/CN103442700B/zh not_active Expired - Fee Related
- 2012-03-23 EP EP12710939.5A patent/EP2688560A2/en not_active Withdrawn
- 2012-03-23 KR KR1020137027812A patent/KR20140031227A/ko not_active Withdrawn
- 2012-03-23 WO PCT/EP2012/055222 patent/WO2012127037A2/en not_active Ceased
- 2012-03-23 JP JP2014500411A patent/JP2014508796A/ja not_active Ceased
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR20180004164A (ko) * | 2015-05-08 | 2018-01-10 | 액티버스 파마 컴퍼니 리미티드 | 글루코코르티코스테로이드의 나노미립자를 함유하는 수성 현탁액제 |
| WO2022203145A1 (ko) * | 2021-03-22 | 2022-09-29 | 주식회사 앱스바이오 | 매스틱검 또는 감마오리자놀로 제조한 지질나노입자 조성물 |
| KR102725138B1 (ko) * | 2023-10-30 | 2024-11-05 | 김윤영 | 나노비타민 복합 조성물 |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2014508796A (ja) | 2014-04-10 |
| WO2012127037A3 (en) | 2012-12-27 |
| EP2688560A2 (en) | 2014-01-29 |
| US20140079785A1 (en) | 2014-03-20 |
| CN103442700B (zh) | 2016-04-06 |
| RU2013147429A (ru) | 2015-04-27 |
| RU2602171C2 (ru) | 2016-11-10 |
| WO2012127037A2 (en) | 2012-09-27 |
| CN103442700A (zh) | 2013-12-11 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2602171C2 (ru) | Композиция, содержащая липидные наночастицы и кортикостероид или производное витамина d | |
| Kandekar et al. | Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers | |
| KR101930246B1 (ko) | 국소용 코르티코스테로이드 조성물 | |
| Tamarkin | Foam: a unique delivery vehicle for topically applied formulations | |
| AU744712B2 (en) | Activated vitamin D3 emulsion-type lotions | |
| CN102781425B (zh) | 含有维生素d类似物以及溶剂和表面活性剂混合物的皮肤用组合物 | |
| RU2690659C2 (ru) | Композиции для местного применения, содержащие кортикостероид | |
| US12295935B2 (en) | Method for therapeutic treatment of rosacea | |
| Şenyiğit et al. | Deoxycholate hydrogels of betamethasone-17-valerate intended for topical use: in vitro and in vivo evaluation | |
| KR20230011997A (ko) | 모낭으로 생물활성제를 전달하기 위한 조성물 | |
| JP2023139134A (ja) | 皮膚疾患を治療するためのフェノルドパム局所製剤 | |
| JP7579006B2 (ja) | 製剤 | |
| HK1177899B (en) | Cutaneous composition comprising vitamin d analogue and a mixture of solvent and surfactants | |
| Raposo | Pharmaceutical topical dosage forms as carriers for glucocorticoids |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20131022 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |