KR20130111932A - 조합 방식을 이용하여 안구 조직을 치료하는 방법 및 장치 - Google Patents
조합 방식을 이용하여 안구 조직을 치료하는 방법 및 장치 Download PDFInfo
- Publication number
- KR20130111932A KR20130111932A KR1020127031420A KR20127031420A KR20130111932A KR 20130111932 A KR20130111932 A KR 20130111932A KR 1020127031420 A KR1020127031420 A KR 1020127031420A KR 20127031420 A KR20127031420 A KR 20127031420A KR 20130111932 A KR20130111932 A KR 20130111932A
- Authority
- KR
- South Korea
- Prior art keywords
- tissue
- ocular
- thermal
- eye
- laser
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 238000000034 method Methods 0.000 title claims abstract description 91
- 238000011282 treatment Methods 0.000 title claims description 64
- 230000005855 radiation Effects 0.000 claims abstract description 70
- 238000004132 cross linking Methods 0.000 claims abstract description 53
- AUNGANRZJHBGPY-SCRDCRAPSA-N Riboflavin Chemical compound OC[C@@H](O)[C@@H](O)[C@@H](O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-SCRDCRAPSA-N 0.000 claims abstract description 51
- 102000008186 Collagen Human genes 0.000 claims abstract description 44
- 108010035532 Collagen Proteins 0.000 claims abstract description 44
- 229920001436 collagen Polymers 0.000 claims abstract description 44
- 238000001816 cooling Methods 0.000 claims abstract description 30
- AUNGANRZJHBGPY-UHFFFAOYSA-N D-Lyxoflavin Natural products OCC(O)C(O)C(O)CN1C=2C=C(C)C(C)=CC=2N=C2C1=NC(=O)NC2=O AUNGANRZJHBGPY-UHFFFAOYSA-N 0.000 claims abstract description 25
- 229960002477 riboflavin Drugs 0.000 claims abstract description 25
- 235000019192 riboflavin Nutrition 0.000 claims abstract description 25
- 239000002151 riboflavin Substances 0.000 claims abstract description 25
- 239000000835 fiber Substances 0.000 claims abstract description 23
- 238000010521 absorption reaction Methods 0.000 claims abstract description 13
- XLYOFNOQVPJJNP-ZSJDYOACSA-N Heavy water Chemical compound [2H]O[2H] XLYOFNOQVPJJNP-ZSJDYOACSA-N 0.000 claims abstract description 6
- 210000001519 tissue Anatomy 0.000 claims description 114
- 210000001508 eye Anatomy 0.000 claims description 89
- 230000003902 lesion Effects 0.000 claims description 72
- 229910052594 sapphire Inorganic materials 0.000 claims description 48
- 239000010980 sapphire Substances 0.000 claims description 48
- 238000012014 optical coherence tomography Methods 0.000 claims description 40
- 206010015037 epilepsy Diseases 0.000 claims description 25
- 238000007634 remodeling Methods 0.000 claims description 18
- 230000008602 contraction Effects 0.000 claims description 17
- 230000007246 mechanism Effects 0.000 claims description 10
- 210000000981 epithelium Anatomy 0.000 claims description 7
- 210000004379 membrane Anatomy 0.000 claims description 4
- 239000012528 membrane Substances 0.000 claims description 4
- 239000004971 Cross linker Substances 0.000 claims description 2
- 210000005252 bulbus oculi Anatomy 0.000 claims description 2
- 239000004020 conductor Substances 0.000 claims description 2
- 238000002834 transmittance Methods 0.000 claims description 2
- 230000001939 inductive effect Effects 0.000 claims 3
- 239000002826 coolant Substances 0.000 claims 1
- 239000003431 cross linking reagent Substances 0.000 claims 1
- 210000004954 endothelial membrane Anatomy 0.000 claims 1
- 208000014674 injury Diseases 0.000 claims 1
- 230000000149 penetrating effect Effects 0.000 claims 1
- 230000008733 trauma Effects 0.000 claims 1
- 238000005259 measurement Methods 0.000 abstract description 13
- 238000011277 treatment modality Methods 0.000 abstract description 8
- 230000001225 therapeutic effect Effects 0.000 abstract description 4
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 abstract description 4
- 230000006872 improvement Effects 0.000 abstract description 2
- 238000005507 spraying Methods 0.000 abstract 1
- 230000008685 targeting Effects 0.000 abstract 1
- 238000002560 therapeutic procedure Methods 0.000 abstract 1
- 238000000015 thermotherapy Methods 0.000 abstract 1
- 210000004087 cornea Anatomy 0.000 description 36
- 238000010438 heat treatment Methods 0.000 description 21
- 230000003287 optical effect Effects 0.000 description 17
- 238000012546 transfer Methods 0.000 description 15
- 230000000694 effects Effects 0.000 description 14
- 239000010410 layer Substances 0.000 description 12
- 239000001301 oxygen Substances 0.000 description 12
- 229910052760 oxygen Inorganic materials 0.000 description 12
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 11
- 230000006870 function Effects 0.000 description 11
- 238000001356 surgical procedure Methods 0.000 description 10
- 210000004045 bowman membrane Anatomy 0.000 description 9
- 230000035515 penetration Effects 0.000 description 9
- 230000008901 benefit Effects 0.000 description 8
- 238000012937 correction Methods 0.000 description 8
- 210000004955 epithelial membrane Anatomy 0.000 description 8
- 238000009472 formulation Methods 0.000 description 8
- 239000000203 mixture Substances 0.000 description 8
- 208000001491 myopia Diseases 0.000 description 8
- 230000004379 myopia Effects 0.000 description 8
- 230000008569 process Effects 0.000 description 7
- 239000003642 reactive oxygen metabolite Substances 0.000 description 7
- 210000001747 pupil Anatomy 0.000 description 6
- 238000003325 tomography Methods 0.000 description 6
- 201000009310 astigmatism Diseases 0.000 description 5
- 230000015572 biosynthetic process Effects 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 238000003384 imaging method Methods 0.000 description 5
- 238000012544 monitoring process Methods 0.000 description 5
- 238000012876 topography Methods 0.000 description 5
- 206010021143 Hypoxia Diseases 0.000 description 4
- 238000013459 approach Methods 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 230000007850 degeneration Effects 0.000 description 4
- 230000007954 hypoxia Effects 0.000 description 4
- 238000007654 immersion Methods 0.000 description 4
- 230000005499 meniscus Effects 0.000 description 4
- 238000012545 processing Methods 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 230000000087 stabilizing effect Effects 0.000 description 4
- 238000007669 thermal treatment Methods 0.000 description 4
- 238000011269 treatment regimen Methods 0.000 description 4
- MYMOFIZGZYHOMD-UHFFFAOYSA-N Dioxygen Chemical compound O=O MYMOFIZGZYHOMD-UHFFFAOYSA-N 0.000 description 3
- 208000010412 Glaucoma Diseases 0.000 description 3
- 201000002287 Keratoconus Diseases 0.000 description 3
- 238000001994 activation Methods 0.000 description 3
- 230000004075 alteration Effects 0.000 description 3
- 238000003491 array Methods 0.000 description 3
- 238000006243 chemical reaction Methods 0.000 description 3
- 238000011284 combination treatment Methods 0.000 description 3
- 210000000795 conjunctiva Anatomy 0.000 description 3
- 210000003683 corneal stroma Anatomy 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 229910001882 dioxygen Inorganic materials 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 230000003511 endothelial effect Effects 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 210000003560 epithelium corneal Anatomy 0.000 description 3
- 210000000744 eyelid Anatomy 0.000 description 3
- 230000004438 eyesight Effects 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- 239000003504 photosensitizing agent Substances 0.000 description 3
- 239000002344 surface layer Substances 0.000 description 3
- 230000004913 activation Effects 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- 230000006907 apoptotic process Effects 0.000 description 2
- 239000000607 artificial tear Substances 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- 229960000686 benzalkonium chloride Drugs 0.000 description 2
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 2
- 208000021921 corneal disease Diseases 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 238000010586 diagram Methods 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 210000003038 endothelium Anatomy 0.000 description 2
- 238000000605 extraction Methods 0.000 description 2
- 238000005286 illumination Methods 0.000 description 2
- 208000015181 infectious disease Diseases 0.000 description 2
- 230000008595 infiltration Effects 0.000 description 2
- 238000001764 infiltration Methods 0.000 description 2
- 230000004410 intraocular pressure Effects 0.000 description 2
- 230000001788 irregular Effects 0.000 description 2
- 239000003589 local anesthetic agent Substances 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 230000000144 pharmacologic effect Effects 0.000 description 2
- 230000000649 photocoagulation Effects 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 201000010041 presbyopia Diseases 0.000 description 2
- 238000003825 pressing Methods 0.000 description 2
- 230000002265 prevention Effects 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- 238000011084 recovery Methods 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 238000007665 sagging Methods 0.000 description 2
- 230000037390 scarring Effects 0.000 description 2
- 230000011218 segmentation Effects 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- GKCBAIGFKIBETG-UHFFFAOYSA-N tetracaine Chemical compound CCCCNC1=CC=C(C(=O)OCCN(C)C)C=C1 GKCBAIGFKIBETG-UHFFFAOYSA-N 0.000 description 2
- 229960002372 tetracaine Drugs 0.000 description 2
- 230000000007 visual effect Effects 0.000 description 2
- 230000029663 wound healing Effects 0.000 description 2
- 208000035143 Bacterial infection Diseases 0.000 description 1
- 241001518005 Callisto Species 0.000 description 1
- 235000014653 Carica parviflora Nutrition 0.000 description 1
- 241000243321 Cnidaria Species 0.000 description 1
- 208000006069 Corneal Opacity Diseases 0.000 description 1
- 206010010996 Corneal degeneration Diseases 0.000 description 1
- 206010011033 Corneal oedema Diseases 0.000 description 1
- 229920002307 Dextran Polymers 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 206010020675 Hypermetropia Diseases 0.000 description 1
- 102000011782 Keratins Human genes 0.000 description 1
- 108010076876 Keratins Proteins 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- KCLANYCVBBTKTO-UHFFFAOYSA-N Proparacaine Chemical compound CCCOC1=CC=C(C(=O)OCCN(CC)CC)C=C1N KCLANYCVBBTKTO-UHFFFAOYSA-N 0.000 description 1
- 241000282898 Sus scrofa Species 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 206010047513 Vision blurred Diseases 0.000 description 1
- 206010047531 Visual acuity reduced Diseases 0.000 description 1
- 206010052428 Wound Diseases 0.000 description 1
- 208000027418 Wounds and injury Diseases 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 230000003444 anaesthetic effect Effects 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 230000002146 bilateral effect Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 201000004781 bullous keratopathy Diseases 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 230000005779 cell damage Effects 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 238000005056 compaction Methods 0.000 description 1
- 239000002131 composite material Substances 0.000 description 1
- 238000005094 computer simulation Methods 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 239000000498 cooling water Substances 0.000 description 1
- 201000004778 corneal edema Diseases 0.000 description 1
- 201000007717 corneal ulcer Diseases 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 229910003460 diamond Inorganic materials 0.000 description 1
- 239000010432 diamond Substances 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000006185 dispersion Substances 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 210000000871 endothelium corneal Anatomy 0.000 description 1
- 238000010336 energy treatment Methods 0.000 description 1
- 210000005081 epithelial layer Anatomy 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 239000003889 eye drop Substances 0.000 description 1
- 229940012356 eye drops Drugs 0.000 description 1
- 230000008713 feedback mechanism Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 201000006318 hyperopia Diseases 0.000 description 1
- 230000004305 hyperopia Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 201000000766 irregular astigmatism Diseases 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- 238000011068 loading method Methods 0.000 description 1
- 238000013507 mapping Methods 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 208000015122 neurodegenerative disease Diseases 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000000399 orthopedic effect Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 239000000546 pharmaceutical excipient Substances 0.000 description 1
- 238000002428 photodynamic therapy Methods 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 229960003981 proparacaine Drugs 0.000 description 1
- 239000011241 protective layer Substances 0.000 description 1
- 230000006950 reactive oxygen species formation Effects 0.000 description 1
- 238000002407 reforming Methods 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 150000003287 riboflavins Chemical class 0.000 description 1
- 230000001711 saccadic effect Effects 0.000 description 1
- 210000003786 sclera Anatomy 0.000 description 1
- 239000004065 semiconductor Substances 0.000 description 1
- 238000007493 shaping process Methods 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 238000007711 solidification Methods 0.000 description 1
- 230000008023 solidification Effects 0.000 description 1
- 230000003595 spectral effect Effects 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000000153 supplemental effect Effects 0.000 description 1
- 238000004381 surface treatment Methods 0.000 description 1
- 230000001360 synchronised effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000000699 topical effect Effects 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 238000009827 uniform distribution Methods 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
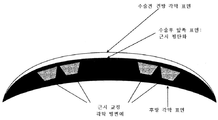
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/008—Methods or devices for eye surgery using laser
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/008—Methods or devices for eye surgery using laser
- A61F9/00821—Methods or devices for eye surgery using laser for coagulation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F7/00—Heating or cooling appliances for medical or therapeutic treatment of the human body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N5/0613—Apparatus adapted for a specific treatment
- A61N5/062—Photodynamic therapy, i.e. excitation of an agent
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/008—Methods or devices for eye surgery using laser
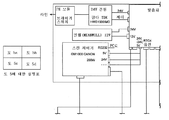
- A61F2009/00844—Feedback systems
- A61F2009/00851—Optical coherence topography [OCT]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/008—Methods or devices for eye surgery using laser
- A61F2009/00853—Laser thermal keratoplasty or radial keratotomy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/008—Methods or devices for eye surgery using laser
- A61F2009/00861—Methods or devices for eye surgery using laser adapted for treatment at a particular location
- A61F2009/00872—Cornea
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F9/00—Methods or devices for treatment of the eyes; Devices for putting in contact-lenses; Devices to correct squinting; Apparatus to guide the blind; Protective devices for the eyes, carried on the body or in the hand
- A61F9/007—Methods or devices for eye surgery
- A61F9/0079—Methods or devices for eye surgery using non-laser electromagnetic radiation, e.g. non-coherent light or microwaves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N5/00—Radiation therapy
- A61N5/06—Radiation therapy using light
- A61N2005/0658—Radiation therapy using light characterised by the wavelength of light used
- A61N2005/0659—Radiation therapy using light characterised by the wavelength of light used infrared
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Ophthalmology & Optometry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Vascular Medicine (AREA)
- Heart & Thoracic Surgery (AREA)
- Pathology (AREA)
- Optics & Photonics (AREA)
- Physics & Mathematics (AREA)
- Surgery (AREA)
- Radiology & Medical Imaging (AREA)
- Biophysics (AREA)
- Laser Surgery Devices (AREA)
- Radiation-Therapy Devices (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US33016810P | 2010-04-30 | 2010-04-30 | |
| US61/330,168 | 2010-04-30 | ||
| PCT/US2011/034823 WO2011137449A2 (en) | 2010-04-30 | 2011-05-02 | Method and apparatus for treatment of ocular tissue using combined modalities |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20130111932A true KR20130111932A (ko) | 2013-10-11 |
Family
ID=44862167
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020127031420A Withdrawn KR20130111932A (ko) | 2010-04-30 | 2011-05-02 | 조합 방식을 이용하여 안구 조직을 치료하는 방법 및 장치 |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US8945101B2 (enExample) |
| EP (1) | EP2563303A4 (enExample) |
| JP (1) | JP2013525014A (enExample) |
| KR (1) | KR20130111932A (enExample) |
| CN (1) | CN102917676A (enExample) |
| WO (1) | WO2011137449A2 (enExample) |
Families Citing this family (54)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101605504A (zh) | 2006-11-10 | 2009-12-16 | 眼科医疗公司 | 用于在眼科光医学中确定剂量测定的系统和方法 |
| KR20130111932A (ko) | 2010-04-30 | 2013-10-11 | 세로스 메디컬, 엘엘씨 | 조합 방식을 이용하여 안구 조직을 치료하는 방법 및 장치 |
| US9622911B2 (en) | 2010-09-30 | 2017-04-18 | Cxl Ophthalmics, Llc | Ophthalmic treatment device, system, and method of use |
| US10456209B2 (en) | 2010-10-13 | 2019-10-29 | Gholam A. Peyman | Remote laser treatment system with dynamic imaging |
| US11309081B2 (en) | 2010-10-13 | 2022-04-19 | Gholam A. Peyman | Telemedicine system with dynamic imaging |
| US9931171B1 (en) * | 2010-10-13 | 2018-04-03 | Gholam A. Peyman | Laser treatment of an eye structure or a body surface from a remote location |
| US20140066835A1 (en) * | 2011-05-24 | 2014-03-06 | Avedro, Inc. | Systems and methods for corneal cross-linking with pulsed light |
| DE102011052002B4 (de) * | 2011-07-20 | 2013-04-11 | Telesto GmbH | Lasertherapiesystem mit UVA- und IR-Laser-Licht zur gerichteten Erzeugung einer dermalen Kollagen-Matrix |
| US9023092B2 (en) | 2011-08-23 | 2015-05-05 | Anthony Natale | Endoscopes enhanced with pathogenic treatment |
| US8668727B2 (en) | 2011-08-23 | 2014-03-11 | Anthony Natale | Systems and methods for treating pathogenic infection |
| EP2802302A4 (en) * | 2012-01-10 | 2015-09-23 | Avedro Inc | APPLYING ENERGY FOR MEDICAL TREATMENTS |
| CN106726125B (zh) * | 2012-01-18 | 2019-07-05 | 视乐有限公司 | 根据光密度对激光能量进行调节的外科手术设备 |
| EP2830637B1 (en) | 2012-03-29 | 2024-08-21 | Epion Therapeutics, Inc. | Compositions and methods for treating or preventing diseases associated with oxidative stress |
| EP2830627B1 (en) | 2012-03-29 | 2024-05-01 | Epion Therapeutics, Inc. | Ocular treatment solutions, delivery devices and delivery augmentation methods |
| WO2013148895A1 (en) | 2012-03-29 | 2013-10-03 | Cxl Ophthalmics, Llc | Ocular cross-linking system and method for sealing corneal wounds |
| US20130310728A1 (en) * | 2012-05-16 | 2013-11-21 | Theo Seiler | Device for dissecting an eye for the introduction of photosensitizer and method of refractive surgery |
| US9677869B2 (en) | 2012-12-05 | 2017-06-13 | Perimeter Medical Imaging, Inc. | System and method for generating a wide-field OCT image of a portion of a sample |
| KR102002634B1 (ko) * | 2013-01-28 | 2019-07-22 | 노바르티스 아게 | 각막 교차 결합을 위한 장치 |
| EP2967710A4 (en) * | 2013-03-15 | 2016-11-02 | Aleyegn Inc | METHODS AND APPARATUS FOR ELASTO MODULATION OF SCLERAL TRANSLOCATION |
| US20140276678A1 (en) * | 2013-03-15 | 2014-09-18 | Michael Berry | Systems and devices for shaping human cornea and methods of use thereof |
| CN108378984B (zh) | 2013-06-25 | 2020-06-26 | Tec晶体有限责任公司 | 用于眼睛的光线疗法的装置 |
| US9877868B2 (en) * | 2013-10-09 | 2018-01-30 | Novartis Ag | Apparatus for dissecting an eye for the introduction of a photosensitizer |
| JP6293463B2 (ja) * | 2013-11-27 | 2018-03-14 | 株式会社トプコン | レーザ治療システム |
| WO2015130944A1 (en) * | 2014-02-28 | 2015-09-03 | Massachusetts Eye & Ear Infirmary | Methods for cross-linking corneal collagen with verteporfin for the treatment of disorders of the eye |
| US20150265470A1 (en) * | 2014-03-21 | 2015-09-24 | Carl Zeiss Meditec Ag | Methods and Systems for Performing a Capsulotomy |
| US9421199B2 (en) | 2014-06-24 | 2016-08-23 | Sydnexis, Inc. | Ophthalmic composition |
| WO2016172712A2 (en) | 2015-04-23 | 2016-10-27 | Sydnexis, Inc. | Ophthalmic composition |
| CN106572919B (zh) | 2014-06-27 | 2020-06-02 | Tec晶体有限责任公司 | 用于角膜胶原交联的实时声学剂量测定 |
| DE102014012675A1 (de) * | 2014-08-26 | 2016-03-03 | Wavelight Gmbh | Vernetzung von Augengewebe |
| WO2016069628A1 (en) | 2014-10-27 | 2016-05-06 | Avedro, Inc. | Systems and methods for cross-linking treatments of an eye |
| US11052094B2 (en) | 2015-05-29 | 2021-07-06 | Sydnexis, Inc. | D2O stabilized pharmaceutical formulations |
| CN116832158A (zh) | 2015-07-21 | 2023-10-03 | 艾维德洛公司 | 用光敏剂治疗眼睛的系统和方法 |
| US20170100285A1 (en) * | 2015-10-12 | 2017-04-13 | Novartis Ag | Photocoagulation with closed-loop control |
| EP3417472A4 (en) * | 2016-02-18 | 2019-08-28 | Convergent R.N.R Ltd | METHOD FOR EVALUATING A DOSE AS A FUNCTION OF THE DEPTH FOR INCORRECT X-RAY RAYS |
| US11364392B2 (en) | 2016-02-18 | 2022-06-21 | Convergent R.N.R Ltd. | Method of evaluating a dose as function of depth for nonuniform X-ray beams |
| US10864111B2 (en) * | 2016-03-22 | 2020-12-15 | Purdue Research Foundation | Technique for therapeutic contact lens systems |
| EP3442481B1 (en) | 2016-04-13 | 2023-06-28 | Avedro, Inc. | Systems for delivering drugs to an eye |
| US10231968B2 (en) | 2016-08-01 | 2019-03-19 | David R. Hardten | Medicinal solution to be continuously or pulse-delivered to the eye for treating ophthalmological conditions/maladies |
| WO2018132621A1 (en) * | 2017-01-11 | 2018-07-19 | University Of Miami | Method and system for three-dimensional thickness mapping of corneal micro-layers and corneal diagnoses |
| EP3655748B1 (en) | 2017-07-18 | 2023-08-09 | Perimeter Medical Imaging, Inc. | Sample container for stabilizing and aligning excised biological tissue samples for ex vivo analysis |
| US11890229B2 (en) | 2017-10-25 | 2024-02-06 | Lutronic Vision Inc. | Laser dosage determination by temperature monitoring |
| WO2019173759A1 (en) * | 2018-03-08 | 2019-09-12 | Aleyegn Technologies Llc | Glaucoma treatment by dilation of collector channels and ostia |
| DE102018203695A1 (de) * | 2018-03-12 | 2019-09-12 | Geuder Ag | Ophthalmologisches Operationsset sowie eine Kontaktlinse |
| WO2020077015A1 (en) * | 2018-10-09 | 2020-04-16 | Avedro, Inc. | Photoactivation systems and methods for corneal cross-linking treatments |
| EP3749264B1 (en) | 2018-11-02 | 2023-06-07 | ALeyeGN Technologies LLC | Laser therapy for treatment and prevention of eye diseases |
| US11890233B2 (en) * | 2019-04-04 | 2024-02-06 | California Institute Of Technology | Systems and methods for drug delivery |
| DE102019213737A1 (de) * | 2019-09-10 | 2021-03-11 | Carl Zeiss Meditec Ag | Augenchirurgische Behandlungsvorrichtung |
| WO2021101883A1 (en) * | 2019-11-18 | 2021-05-27 | Pamel Gregory J | Chitosan containing compositions and methods relating to same |
| TW202245810A (zh) * | 2021-02-01 | 2022-12-01 | 美商艾維娜傳送系統公司 | 散光之治療 |
| CN113520318B (zh) * | 2021-07-08 | 2022-03-08 | 哈尔滨医科大学 | 一种集成oct成像和pdt的导管设计 |
| CN114488892B (zh) * | 2022-01-18 | 2023-03-28 | 北京工业大学 | 激光照射控制系统、激光照射控制方法、设备及介质 |
| CN117258157B (zh) * | 2023-11-14 | 2024-02-09 | 超目科技(北京)有限公司 | 一种角膜交联辅助装置及角膜交联系统 |
| CN118319598B (zh) * | 2024-06-15 | 2024-10-18 | 艾视雅健康科技(苏州)有限公司 | 角膜交联仪器和方法 |
| CN120095375B (zh) * | 2025-05-08 | 2025-07-22 | 成都圣雨兰制衣有限公司 | 一种服饰面料的激光切割装置及切割方法 |
Family Cites Families (69)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4565197A (en) * | 1983-11-22 | 1986-01-21 | Lasers For Medicine | Laser ophthalmic surgical system |
| US5263951A (en) | 1989-04-21 | 1993-11-23 | Kerus Medical Systems | Correction of the optical focusing system of the eye using laser thermal keratoplasty |
| US5779696A (en) * | 1990-07-23 | 1998-07-14 | Sunrise Technologies International, Inc. | Method and apparatus for performing corneal reshaping to correct ocular refractive errors |
| US5360425A (en) * | 1990-08-17 | 1994-11-01 | Candela Laser Corporation | Sclerostomy method and apparatus |
| US5354331A (en) | 1992-07-15 | 1994-10-11 | Schachar Ronald A | Treatment of presbyopia and other eye disorders |
| CA2177580A1 (en) * | 1993-12-02 | 1995-06-08 | Michael J. Berry | Laser system for reshaping the cornea |
| US6544193B2 (en) | 1996-09-04 | 2003-04-08 | Marcio Marc Abreu | Noninvasive measurement of chemical substances |
| US5997529A (en) | 1996-10-28 | 1999-12-07 | Lasersight Technologies, Inc. | Compound astigmatic myopia or hyperopia correction by laser ablation |
| US6099521A (en) * | 1998-05-26 | 2000-08-08 | Shadduck; John H. | Semiconductor contact lens cooling system and technique for light-mediated eye therapies |
| CA2510389A1 (en) | 1998-06-10 | 1999-12-16 | Mark R. Prausnitz | Microneedle devices and methods of manufacture and use thereof |
| AU2004200303B2 (en) | 1998-06-10 | 2007-10-18 | Georgia Tech Research Corporation | Microneedle devices and methods of manufacture and use thereof |
| US7344499B1 (en) | 1998-06-10 | 2008-03-18 | Georgia Tech Research Corporation | Microneedle device for extraction and sensing of bodily fluids |
| WO1999064580A1 (en) | 1998-06-10 | 1999-12-16 | Georgia Tech Research Corporation | Microneedle devices and methods of manufacture and use thereof |
| US6503231B1 (en) | 1998-06-10 | 2003-01-07 | Georgia Tech Research Corporation | Microneedle device for transport of molecules across tissue |
| US6745775B2 (en) | 1998-11-10 | 2004-06-08 | Surgilight, Inc. | Methods and apparatus for presbyopia treatment using a scanning laser system |
| AU5461300A (en) | 1999-06-04 | 2000-12-28 | Georgia Tech Research Corporation | Devices and methods for enhanced microneedle penetration of biological barriers |
| US6611707B1 (en) | 1999-06-04 | 2003-08-26 | Georgia Tech Research Corporation | Microneedle drug delivery device |
| US6743211B1 (en) | 1999-11-23 | 2004-06-01 | Georgia Tech Research Corporation | Devices and methods for enhanced microneedle penetration of biological barriers |
| US9603741B2 (en) * | 2000-05-19 | 2017-03-28 | Michael S. Berlin | Delivery system and method of use for the eye |
| US8679089B2 (en) | 2001-05-21 | 2014-03-25 | Michael S. Berlin | Glaucoma surgery methods and systems |
| JP2002200181A (ja) | 2000-10-31 | 2002-07-16 | Shigehiro Kubota | レーザ治療装置 |
| US6824540B1 (en) | 2000-11-06 | 2004-11-30 | Surgilight, Inc. | Apparatus and methods for the treatment of presbyopia using fiber-coupled-lasers |
| US6679855B2 (en) | 2000-11-07 | 2004-01-20 | Gerald Horn | Method and apparatus for the correction of presbyopia using high intensity focused ultrasound |
| WO2002064193A2 (en) | 2000-12-14 | 2002-08-22 | Georgia Tech Research Corporation | Microneedle devices and production thereof |
| US20020099363A1 (en) * | 2001-01-23 | 2002-07-25 | Woodward Benjamin W. | Radiation treatment system and method of using same |
| ES2271220T3 (es) | 2001-02-23 | 2007-04-16 | Refocus Ocular, Inc. | Sistema para hacer incisiones para implantes oculares en la esclerotica. |
| US20020173777A1 (en) | 2001-03-30 | 2002-11-21 | Sand Bruce J. | Treatment of collagen |
| US7044945B2 (en) * | 2001-03-30 | 2006-05-16 | Sand Bruce J | Prevention of regression in thermal ciliary muscle tendinoplasty |
| US20080039769A1 (en) * | 2001-11-07 | 2008-02-14 | Minu Llc | Method of medical treatment using controlled heat delivery |
| US9681942B2 (en) * | 2001-11-07 | 2017-06-20 | Gholam A. Peyman | Method for prevention of rejection and sever encapsulation of a supportive or functioning implant |
| US9155652B2 (en) * | 2001-11-07 | 2015-10-13 | Gholam A. Peyman | Method for laser correction of refractive errors of an eye with a thin cornea |
| US7133137B2 (en) | 2002-06-27 | 2006-11-07 | Visx, Incorporated | Integrated scanning and ocular tomography system and method |
| US20050043722A1 (en) * | 2003-08-22 | 2005-02-24 | Lin J. T. | Methods and apparatus for treatment of eye disorders using articulated-arm-coupled ultraviolet lasers |
| US20100063174A1 (en) | 2004-02-04 | 2010-03-11 | Ruberti Jeffrey W | Systems and methods for controlling and forming polymer gels |
| US20060007965A1 (en) | 2004-07-12 | 2006-01-12 | Nikolai Tankovich | Passive Q-switch modulated fiber laser |
| US20060129141A1 (en) * | 2004-12-10 | 2006-06-15 | Lin J T | Treatment of eye disorders using articulated-arm coupled ultraviolet lasers |
| US20060224146A1 (en) | 2005-03-30 | 2006-10-05 | Lin J T | Method and system for non-invasive treatment of hyperopia, presbyopia and glaucoma |
| US7878204B2 (en) | 2005-04-22 | 2011-02-01 | Biolase Technology, Inc. | Methods for treating hyperopia and presbyopia via laser tunneling |
| AU2006239308C1 (en) | 2005-04-26 | 2010-06-03 | Biolase, Inc. | Methods for treating eye conditions |
| US20070083190A1 (en) * | 2005-10-11 | 2007-04-12 | Yacov Domankevitz | Compression device for a laser handpiece |
| DE102005056958A1 (de) * | 2005-11-29 | 2007-06-06 | Rowiak Gmbh | Verfahren und Vorrichtung zum Bearbeiten eines Werkstücks |
| US20070203478A1 (en) | 2006-02-21 | 2007-08-30 | Herekar Satish V | Method and system for elasto-modulation of ocular tissue |
| US8197435B2 (en) | 2006-05-02 | 2012-06-12 | Emory University | Methods and devices for drug delivery to ocular tissue using microneedle |
| US7918814B2 (en) | 2006-05-02 | 2011-04-05 | Georgia Tech Research Corporation | Method for drug delivery to ocular tissue using microneedle |
| US20080015660A1 (en) * | 2006-07-13 | 2008-01-17 | Priavision, Inc. | Method And Apparatus For Photo-Chemical Oculoplasty/Keratoplasty |
| US20090182306A1 (en) | 2006-07-21 | 2009-07-16 | Georgia Tech Research Corporation | Microneedle Devices and Methods of Drug Delivery or Fluid Withdrawal |
| US8043235B2 (en) | 2006-08-22 | 2011-10-25 | Schwartz Donald N | Ultrasonic treatment of glaucoma |
| US8102734B2 (en) | 2007-02-08 | 2012-01-24 | St. Jude Medical, Atrial Fibrillation Division, Inc. | High intensity focused ultrasound transducer with acoustic lens |
| JP4378400B2 (ja) | 2007-08-28 | 2009-12-02 | 日立コンピュータ機器株式会社 | 双方向dc−dcコンバータ及び双方向dc−dcコンバータの制御方法 |
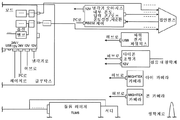
| US10398599B2 (en) * | 2007-10-05 | 2019-09-03 | Topcon Medical Laser Systems Inc. | Semi-automated ophthalmic photocoagulation method and apparatus |
| WO2009073213A1 (en) * | 2007-12-05 | 2009-06-11 | Avedro, Inc. | Eye therapy system |
| US20100057060A1 (en) | 2007-12-07 | 2010-03-04 | Seros Medical, Llc | In Situ UV/Riboflavin Ocular Treatment System |
| US20090149923A1 (en) * | 2007-12-07 | 2009-06-11 | 21X Corporation Dba Priavision, Inc. | Method for equi-dosed time fractionated pulsed uva irradiation of collagen/riboflavin mixtures for ocular structural augmentation |
| US8230866B2 (en) | 2007-12-13 | 2012-07-31 | Carl Zeiss Meditec Ag | Systems and methods for treating glaucoma and systems and methods for imaging a portion of an eye |
| WO2009094394A1 (en) | 2008-01-23 | 2009-07-30 | Georgia Tech Research Corporation | Microneedle devices and methods of drug delivery or fluid withdrawal |
| EP2092916A1 (en) | 2008-02-19 | 2009-08-26 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | A method of treating an ocular pathology by applying high intensity focused ultrasound and device thereof |
| EP2464387A4 (en) | 2009-08-12 | 2013-05-15 | Seros Medical Llc | DEUTERATED WATER SOLUTION AND RIBOFLAVIN FOR EXTENDED OXYGEN SINGULET LIFETIME IN THE TREATMENT OF OCULAR TISSUE AND METHOD OF USE |
| KR20130111932A (ko) | 2010-04-30 | 2013-10-11 | 세로스 메디컬, 엘엘씨 | 조합 방식을 이용하여 안구 조직을 치료하는 방법 및 장치 |
| US9161857B2 (en) | 2010-07-29 | 2015-10-20 | Eos Holdings, Llc | Presbyopic vision correction with controlled 3-D patterned mechanical weakening of scleral tissue |
| US9005099B2 (en) | 2011-02-15 | 2015-04-14 | Seros Medical, Llc | Method and apparatus for the delivery of photochemical (cross-linking) treatment to scleral tissue |
| US20120283804A1 (en) | 2011-05-04 | 2012-11-08 | The Johns Hopkins University | Mid-infrared laser therapy device and system |
| DE102011052002B4 (de) * | 2011-07-20 | 2013-04-11 | Telesto GmbH | Lasertherapiesystem mit UVA- und IR-Laser-Licht zur gerichteten Erzeugung einer dermalen Kollagen-Matrix |
| US9066784B2 (en) | 2011-12-19 | 2015-06-30 | Alcon Lensx, Inc. | Intra-surgical optical coherence tomographic imaging of cataract procedures |
| US9498377B2 (en) | 2012-09-07 | 2016-11-22 | Bausch & Lomb Incorporated | Vibrating surgical device for removal of vitreous and other tissue |
| US20140114296A1 (en) | 2012-10-24 | 2014-04-24 | Optimedica Corporation | Graphical user interface for laser eye surgery system |
| EP2967710A4 (en) | 2013-03-15 | 2016-11-02 | Aleyegn Inc | METHODS AND APPARATUS FOR ELASTO MODULATION OF SCLERAL TRANSLOCATION |
| CN106413642B (zh) | 2014-04-23 | 2019-03-08 | 塞罗斯医学有限责任公司 | 真空辅助药物递送装置 |
| US11351347B2 (en) | 2014-04-23 | 2022-06-07 | Vacu-Site Medical, Inc. | Vacuum-assisted drug delivery device and method |
| KR102338593B1 (ko) | 2017-03-24 | 2021-12-14 | 서울대학교산학협력단 | 기능성 콘택트 렌즈 및 이의 제조방법 |
-
2011
- 2011-05-02 KR KR1020127031420A patent/KR20130111932A/ko not_active Withdrawn
- 2011-05-02 US US13/068,126 patent/US8945101B2/en active Active
- 2011-05-02 JP JP2013508090A patent/JP2013525014A/ja active Pending
- 2011-05-02 EP EP11775711.2A patent/EP2563303A4/en not_active Withdrawn
- 2011-05-02 CN CN2011800272142A patent/CN102917676A/zh active Pending
- 2011-05-02 WO PCT/US2011/034823 patent/WO2011137449A2/en not_active Ceased
-
2014
- 2014-12-29 US US14/584,371 patent/US20150209181A1/en not_active Abandoned
-
2019
- 2019-03-21 US US16/360,792 patent/US11259963B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| US20150209181A1 (en) | 2015-07-30 |
| EP2563303A4 (en) | 2013-11-06 |
| WO2011137449A3 (en) | 2012-03-15 |
| US20200000638A1 (en) | 2020-01-02 |
| US8945101B2 (en) | 2015-02-03 |
| WO2011137449A2 (en) | 2011-11-03 |
| JP2013525014A (ja) | 2013-06-20 |
| US11259963B2 (en) | 2022-03-01 |
| US20110282333A1 (en) | 2011-11-17 |
| CN102917676A (zh) | 2013-02-06 |
| EP2563303A2 (en) | 2013-03-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11259963B2 (en) | Method and apparatus for treatment of ocular tissue using combined modalities | |
| US20220395398A1 (en) | Scleral translocation elasto-modulation methods and apparatus | |
| JP6832319B2 (ja) | 網膜光線療法用のシステム及び方法 | |
| US6099521A (en) | Semiconductor contact lens cooling system and technique for light-mediated eye therapies | |
| KR100294858B1 (ko) | 콜라겐 치료 장치 | |
| JP5184888B2 (ja) | 老視の1つ又は複数の症状を防止又は遅らせるレーザ装置 | |
| US6342053B1 (en) | Apparatus for cornea reshaping | |
| US20180207029A1 (en) | Glaucoma treatment methods and apparatus | |
| US9526656B2 (en) | Corneal vitrification, methods and devices to produce corneal vitrification and methods of use thereof | |
| US7691099B2 (en) | Deuterated ocular solutions for LTK and other surgical eye procedures | |
| US20190099291A1 (en) | Effective ocular lens positioning methods and apparatus | |
| US20080015660A1 (en) | Method And Apparatus For Photo-Chemical Oculoplasty/Keratoplasty | |
| US8991401B2 (en) | Processes and apparatus for preventing, delaying or ameliorating one or more symptoms of presbyopia | |
| US20070203478A1 (en) | Method and system for elasto-modulation of ocular tissue | |
| JPH06501633A (ja) | 眼球の屈折誤差を修正するために角膜を整形する改良された方法および装置 | |
| Birngruber | Therapeutic applications: ophthalmology | |
| Barrett et al. | Laser ophthalmology |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20121130 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |