KR20110081861A - 체중 관리를 위한 유전자 마커 및 이의 사용 방법 - Google Patents
체중 관리를 위한 유전자 마커 및 이의 사용 방법 Download PDFInfo
- Publication number
- KR20110081861A KR20110081861A KR1020117011584A KR20117011584A KR20110081861A KR 20110081861 A KR20110081861 A KR 20110081861A KR 1020117011584 A KR1020117011584 A KR 1020117011584A KR 20117011584 A KR20117011584 A KR 20117011584A KR 20110081861 A KR20110081861 A KR 20110081861A
- Authority
- KR
- South Korea
- Prior art keywords
- subject
- allele
- snp
- diet
- response
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 230000002068 genetic effect Effects 0.000 title abstract description 41
- 230000037221 weight management Effects 0.000 title description 29
- 235000005911 diet Nutrition 0.000 claims abstract description 217
- 238000000034 method Methods 0.000 claims abstract description 179
- 230000004580 weight loss Effects 0.000 claims abstract description 159
- 230000037213 diet Effects 0.000 claims abstract description 129
- 230000004044 response Effects 0.000 claims abstract description 121
- 230000000378 dietary effect Effects 0.000 claims abstract description 88
- 108700028369 Alleles Proteins 0.000 claims description 387
- 101001033249 Homo sapiens Interleukin-1 beta Proteins 0.000 claims description 282
- 102100039065 Interleukin-1 beta Human genes 0.000 claims description 280
- 235000020845 low-calorie diet Nutrition 0.000 claims description 139
- 102000054766 genetic haplotypes Human genes 0.000 claims description 120
- 235000020888 liquid diet Nutrition 0.000 claims description 117
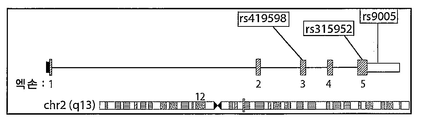
- 102100026018 Interleukin-1 receptor antagonist protein Human genes 0.000 claims description 101
- 101710144554 Interleukin-1 receptor antagonist protein Proteins 0.000 claims description 101
- 239000003153 chemical reaction reagent Substances 0.000 claims description 76
- 102220134786 rs315952 Human genes 0.000 claims description 76
- 101000959437 Homo sapiens Beta-2 adrenergic receptor Proteins 0.000 claims description 72
- 102000017919 ADRB2 Human genes 0.000 claims description 71
- 102220006123 rs1042713 Human genes 0.000 claims description 64
- 238000003205 genotyping method Methods 0.000 claims description 58
- 235000020827 calorie restriction Nutrition 0.000 claims description 55
- 101001002634 Homo sapiens Interleukin-1 alpha Proteins 0.000 claims description 49
- 102100020881 Interleukin-1 alpha Human genes 0.000 claims description 48
- 102210011187 rs12970134 Human genes 0.000 claims description 47
- 102220134620 rs9005 Human genes 0.000 claims description 41
- 102220609134 Interleukin-1 alpha_A114S_mutation Human genes 0.000 claims description 34
- 108060003355 ADRB3 Proteins 0.000 claims description 33
- 239000000872 buffer Substances 0.000 claims description 33
- 150000003839 salts Chemical class 0.000 claims description 32
- 102000017918 ADRB3 Human genes 0.000 claims description 31
- 102200094959 rs2229616 Human genes 0.000 claims description 31
- 102220133450 rs419598 Human genes 0.000 claims description 30
- 210000004369 blood Anatomy 0.000 claims description 29
- 239000008280 blood Substances 0.000 claims description 29
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 claims description 28
- 239000003550 marker Substances 0.000 claims description 27
- 102220006124 rs1042714 Human genes 0.000 claims description 25
- 102210003140 rs1143633 Human genes 0.000 claims description 17
- 230000002641 glycemic effect Effects 0.000 claims description 9
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 claims 1
- 229910052698 phosphorus Inorganic materials 0.000 claims 1
- 239000011574 phosphorus Substances 0.000 claims 1
- 108090000623 proteins and genes Proteins 0.000 abstract description 96
- 230000002503 metabolic effect Effects 0.000 abstract description 53
- 230000000694 effects Effects 0.000 abstract description 23
- 230000004060 metabolic process Effects 0.000 abstract description 17
- 238000003556 assay Methods 0.000 abstract description 13
- 230000008901 benefit Effects 0.000 abstract description 13
- 238000012423 maintenance Methods 0.000 abstract 1
- 108010021436 Type 4 Melanocortin Receptor Proteins 0.000 description 75
- 102000053602 DNA Human genes 0.000 description 71
- 108020004414 DNA Proteins 0.000 description 71
- 102100023724 Melanocortin receptor 4 Human genes 0.000 description 63
- 150000001720 carbohydrates Chemical class 0.000 description 63
- 235000014633 carbohydrates Nutrition 0.000 description 61
- 210000000577 adipose tissue Anatomy 0.000 description 45
- 239000003925 fat Substances 0.000 description 44
- 235000019197 fats Nutrition 0.000 description 44
- 201000010099 disease Diseases 0.000 description 42
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 42
- 239000013615 primer Substances 0.000 description 41
- 239000000523 sample Substances 0.000 description 37
- 108010010234 HDL Lipoproteins Proteins 0.000 description 34
- 102000015779 HDL Lipoproteins Human genes 0.000 description 34
- 108091034117 Oligonucleotide Proteins 0.000 description 33
- 150000007523 nucleic acids Chemical class 0.000 description 29
- 235000021236 calorie-restricted diet Nutrition 0.000 description 27
- 238000001514 detection method Methods 0.000 description 27
- 230000035772 mutation Effects 0.000 description 26
- 102000039446 nucleic acids Human genes 0.000 description 26
- 108020004707 nucleic acids Proteins 0.000 description 26
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 24
- 125000003729 nucleotide group Chemical group 0.000 description 23
- 238000012216 screening Methods 0.000 description 23
- 230000003321 amplification Effects 0.000 description 22
- 238000003199 nucleic acid amplification method Methods 0.000 description 22
- 238000012360 testing method Methods 0.000 description 22
- 235000020855 low-carbohydrate diet Nutrition 0.000 description 21
- 238000003752 polymerase chain reaction Methods 0.000 description 21
- 235000000346 sugar Nutrition 0.000 description 21
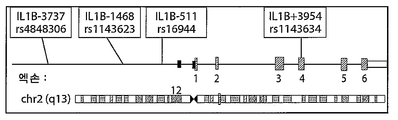
- 108010002352 Interleukin-1 Proteins 0.000 description 20
- 235000015263 low fat diet Nutrition 0.000 description 20
- 239000002773 nucleotide Substances 0.000 description 20
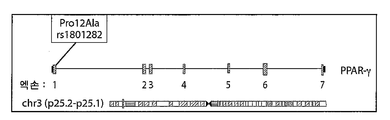
- 101000741790 Homo sapiens Peroxisome proliferator-activated receptor gamma Proteins 0.000 description 19
- 102000000589 Interleukin-1 Human genes 0.000 description 19
- 108010007622 LDL Lipoproteins Proteins 0.000 description 19
- 102000007330 LDL Lipoproteins Human genes 0.000 description 19
- 102100038825 Peroxisome proliferator-activated receptor gamma Human genes 0.000 description 18
- 235000021073 macronutrients Nutrition 0.000 description 17
- 238000009396 hybridization Methods 0.000 description 16
- 235000016709 nutrition Nutrition 0.000 description 16
- 235000018102 proteins Nutrition 0.000 description 16
- 102000004169 proteins and genes Human genes 0.000 description 16
- 229920002477 rna polymer Polymers 0.000 description 16
- 208000008589 Obesity Diseases 0.000 description 15
- 235000004251 balanced diet Nutrition 0.000 description 15
- 238000002474 experimental method Methods 0.000 description 15
- 108091008053 gene clusters Proteins 0.000 description 15
- 235000020824 obesity Nutrition 0.000 description 15
- 208000021017 Weight Gain Diseases 0.000 description 14
- 102000054765 polymorphisms of proteins Human genes 0.000 description 14
- 230000004584 weight gain Effects 0.000 description 14
- 235000019786 weight gain Nutrition 0.000 description 14
- 238000004458 analytical method Methods 0.000 description 13
- 239000000047 product Substances 0.000 description 13
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 12
- 230000004043 responsiveness Effects 0.000 description 12
- 206010033307 Overweight Diseases 0.000 description 11
- 239000013068 control sample Substances 0.000 description 11
- 230000035764 nutrition Effects 0.000 description 11
- 235000021003 saturated fats Nutrition 0.000 description 11
- 238000001356 surgical procedure Methods 0.000 description 11
- 150000003626 triacylglycerols Chemical class 0.000 description 11
- 230000037396 body weight Effects 0.000 description 10
- 235000021074 carbohydrate intake Nutrition 0.000 description 10
- 230000008859 change Effects 0.000 description 10
- 239000012634 fragment Substances 0.000 description 10
- 230000036541 health Effects 0.000 description 10
- 230000000670 limiting effect Effects 0.000 description 10
- 239000000463 material Substances 0.000 description 10
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 9
- 101150012417 IL1B gene Proteins 0.000 description 9
- 230000002159 abnormal effect Effects 0.000 description 9
- 230000002411 adverse Effects 0.000 description 9
- 239000008103 glucose Substances 0.000 description 9
- 230000002218 hypoglycaemic effect Effects 0.000 description 9
- 239000000203 mixture Substances 0.000 description 9
- 235000021084 monounsaturated fats Nutrition 0.000 description 9
- 101150033809 ADRB2 gene Proteins 0.000 description 8
- 210000004027 cell Anatomy 0.000 description 8
- 238000006243 chemical reaction Methods 0.000 description 8
- 150000002632 lipids Chemical class 0.000 description 8
- 235000004213 low-fat Nutrition 0.000 description 8
- 239000002987 primer (paints) Substances 0.000 description 8
- 230000009467 reduction Effects 0.000 description 8
- 208000032928 Dyslipidaemia Diseases 0.000 description 7
- 102000004190 Enzymes Human genes 0.000 description 7
- 108090000790 Enzymes Proteins 0.000 description 7
- 208000017170 Lipid metabolism disease Diseases 0.000 description 7
- 238000012408 PCR amplification Methods 0.000 description 7
- 230000002759 chromosomal effect Effects 0.000 description 7
- 238000003776 cleavage reaction Methods 0.000 description 7
- 235000013367 dietary fats Nutrition 0.000 description 7
- 229940088598 enzyme Drugs 0.000 description 7
- 235000015097 nutrients Nutrition 0.000 description 7
- 230000006798 recombination Effects 0.000 description 7
- 238000005215 recombination Methods 0.000 description 7
- 230000002829 reductive effect Effects 0.000 description 7
- 102220005721 rs1799883 Human genes 0.000 description 7
- 238000012163 sequencing technique Methods 0.000 description 7
- 238000002560 therapeutic procedure Methods 0.000 description 7
- 230000007963 carbohydrate restriction Effects 0.000 description 6
- 238000011161 development Methods 0.000 description 6
- 230000003050 macronutrient Effects 0.000 description 6
- 230000009257 reactivity Effects 0.000 description 6
- 108091008146 restriction endonucleases Proteins 0.000 description 6
- 230000007017 scission Effects 0.000 description 6
- 108060002716 Exonuclease Proteins 0.000 description 5
- 101150074243 Il1rn gene Proteins 0.000 description 5
- 239000000090 biomarker Substances 0.000 description 5
- 206010012601 diabetes mellitus Diseases 0.000 description 5
- 235000001434 dietary modification Nutrition 0.000 description 5
- 102000013165 exonuclease Human genes 0.000 description 5
- 239000013642 negative control Substances 0.000 description 5
- 230000037361 pathway Effects 0.000 description 5
- 230000037081 physical activity Effects 0.000 description 5
- 239000013641 positive control Substances 0.000 description 5
- 230000024977 response to activity Effects 0.000 description 5
- 210000001519 tissue Anatomy 0.000 description 5
- 235000021076 total caloric intake Nutrition 0.000 description 5
- 230000014616 translation Effects 0.000 description 5
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 4
- 108091028043 Nucleic acid sequence Proteins 0.000 description 4
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 4
- 230000004888 barrier function Effects 0.000 description 4
- 230000015556 catabolic process Effects 0.000 description 4
- 235000012000 cholesterol Nutrition 0.000 description 4
- 238000010367 cloning Methods 0.000 description 4
- 230000000295 complement effect Effects 0.000 description 4
- 230000000875 corresponding effect Effects 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 239000003814 drug Substances 0.000 description 4
- 238000007834 ligase chain reaction Methods 0.000 description 4
- 238000007477 logistic regression Methods 0.000 description 4
- 239000012071 phase Substances 0.000 description 4
- 108090000765 processed proteins & peptides Proteins 0.000 description 4
- 102200010892 rs1805192 Human genes 0.000 description 4
- 229920006395 saturated elastomer Polymers 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- 238000013519 translation Methods 0.000 description 4
- 102100026748 Fatty acid-binding protein, intestinal Human genes 0.000 description 3
- 108050008832 Fatty acid-binding protein, intestinal Proteins 0.000 description 3
- 241000287828 Gallus gallus Species 0.000 description 3
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 3
- 206010071602 Genetic polymorphism Diseases 0.000 description 3
- 108091027305 Heteroduplex Proteins 0.000 description 3
- -1 IL1RN Proteins 0.000 description 3
- 208000001145 Metabolic Syndrome Diseases 0.000 description 3
- 108091093037 Peptide nucleic acid Proteins 0.000 description 3
- 201000000690 abdominal obesity-metabolic syndrome Diseases 0.000 description 3
- 239000002253 acid Substances 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 239000003795 chemical substances by application Substances 0.000 description 3
- 210000000349 chromosome Anatomy 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 230000001276 controlling effect Effects 0.000 description 3
- 235000013305 food Nutrition 0.000 description 3
- 238000001502 gel electrophoresis Methods 0.000 description 3
- 230000007614 genetic variation Effects 0.000 description 3
- 230000037219 healthy weight Effects 0.000 description 3
- 238000012417 linear regression Methods 0.000 description 3
- 239000007788 liquid Substances 0.000 description 3
- 230000014759 maintenance of location Effects 0.000 description 3
- 238000002844 melting Methods 0.000 description 3
- 230000008018 melting Effects 0.000 description 3
- 230000037323 metabolic rate Effects 0.000 description 3
- 239000002417 nutraceutical Substances 0.000 description 3
- 235000021436 nutraceutical agent Nutrition 0.000 description 3
- 229920001184 polypeptide Polymers 0.000 description 3
- 230000002265 prevention Effects 0.000 description 3
- 102000004196 processed proteins & peptides Human genes 0.000 description 3
- 238000003757 reverse transcription PCR Methods 0.000 description 3
- 238000005070 sampling Methods 0.000 description 3
- 208000019553 vascular disease Diseases 0.000 description 3
- 201000001320 Atherosclerosis Diseases 0.000 description 2
- 108090001008 Avidin Proteins 0.000 description 2
- 108091026890 Coding region Proteins 0.000 description 2
- 229920000742 Cotton Polymers 0.000 description 2
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 description 2
- 208000028782 Hereditary disease Diseases 0.000 description 2
- 206010020772 Hypertension Diseases 0.000 description 2
- 240000008415 Lactuca sativa Species 0.000 description 2
- 240000004322 Lens culinaris Species 0.000 description 2
- 235000014647 Lens culinaris subsp culinaris Nutrition 0.000 description 2
- 235000007688 Lycopersicon esculentum Nutrition 0.000 description 2
- 208000024556 Mendelian disease Diseases 0.000 description 2
- 108010021466 Mutant Proteins Proteins 0.000 description 2
- 102000008300 Mutant Proteins Human genes 0.000 description 2
- 101710163270 Nuclease Proteins 0.000 description 2
- 240000007594 Oryza sativa Species 0.000 description 2
- 235000007164 Oryza sativa Nutrition 0.000 description 2
- 235000010627 Phaseolus vulgaris Nutrition 0.000 description 2
- 244000046052 Phaseolus vulgaris Species 0.000 description 2
- 240000003768 Solanum lycopersicum Species 0.000 description 2
- 238000000692 Student's t-test Methods 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- 235000021307 Triticum Nutrition 0.000 description 2
- 244000098338 Triticum aestivum Species 0.000 description 2
- 239000000654 additive Substances 0.000 description 2
- 230000000996 additive effect Effects 0.000 description 2
- 230000000692 anti-sense effect Effects 0.000 description 2
- 239000000935 antidepressant agent Substances 0.000 description 2
- 229940005513 antidepressants Drugs 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 235000020958 biotin Nutrition 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 210000000988 bone and bone Anatomy 0.000 description 2
- 238000004364 calculation method Methods 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 235000013339 cereals Nutrition 0.000 description 2
- 208000037976 chronic inflammation Diseases 0.000 description 2
- 230000006020 chronic inflammation Effects 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000004925 denaturation Methods 0.000 description 2
- 230000036425 denaturation Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- 235000014113 dietary fatty acids Nutrition 0.000 description 2
- 235000020979 dietary recommendations Nutrition 0.000 description 2
- 235000015872 dietary supplement Nutrition 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 238000009547 dual-energy X-ray absorptiometry Methods 0.000 description 2
- 238000005265 energy consumption Methods 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 239000000194 fatty acid Substances 0.000 description 2
- 229930195729 fatty acid Natural products 0.000 description 2
- 150000004665 fatty acids Chemical class 0.000 description 2
- 239000000499 gel Substances 0.000 description 2
- 238000012252 genetic analysis Methods 0.000 description 2
- 235000019680 high-energy food Nutrition 0.000 description 2
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 229910052500 inorganic mineral Inorganic materials 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 238000011835 investigation Methods 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- 230000033001 locomotion Effects 0.000 description 2
- 230000007774 longterm Effects 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 239000011707 mineral Substances 0.000 description 2
- 230000004048 modification Effects 0.000 description 2
- 238000012986 modification Methods 0.000 description 2
- 238000007857 nested PCR Methods 0.000 description 2
- 208000008338 non-alcoholic fatty liver disease Diseases 0.000 description 2
- 206010053219 non-alcoholic steatohepatitis Diseases 0.000 description 2
- 201000008482 osteoarthritis Diseases 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 102000040430 polynucleotide Human genes 0.000 description 2
- 108091033319 polynucleotide Proteins 0.000 description 2
- 239000002157 polynucleotide Substances 0.000 description 2
- 230000007026 protein scission Effects 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 230000000284 resting effect Effects 0.000 description 2
- 235000009566 rice Nutrition 0.000 description 2
- 235000012045 salad Nutrition 0.000 description 2
- 235000015067 sauces Nutrition 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 235000020183 skimmed milk Nutrition 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000006467 substitution reaction Methods 0.000 description 2
- 238000011477 surgical intervention Methods 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000012353 t test Methods 0.000 description 2
- 238000013518 transcription Methods 0.000 description 2
- 230000035897 transcription Effects 0.000 description 2
- 230000002103 transcriptional effect Effects 0.000 description 2
- 235000013618 yogurt Nutrition 0.000 description 2
- MJYQFWSXKFLTAY-OVEQLNGDSA-N (2r,3r)-2,3-bis[(4-hydroxy-3-methoxyphenyl)methyl]butane-1,4-diol;(2r,3r,4s,5s,6r)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O.C1=C(O)C(OC)=CC(C[C@@H](CO)[C@H](CO)CC=2C=C(OC)C(O)=CC=2)=C1 MJYQFWSXKFLTAY-OVEQLNGDSA-N 0.000 description 1
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 description 1
- 108020005065 3' Flanking Region Proteins 0.000 description 1
- 101150090657 ADRB3 gene Proteins 0.000 description 1
- 208000004611 Abdominal Obesity Diseases 0.000 description 1
- 241000251468 Actinopterygii Species 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 244000099147 Ananas comosus Species 0.000 description 1
- 235000007119 Ananas comosus Nutrition 0.000 description 1
- 108090000672 Annexin A5 Proteins 0.000 description 1
- 235000016068 Berberis vulgaris Nutrition 0.000 description 1
- 241000335053 Beta vulgaris Species 0.000 description 1
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 1
- 208000020925 Bipolar disease Diseases 0.000 description 1
- 208000031648 Body Weight Changes Diseases 0.000 description 1
- 235000011299 Brassica oleracea var botrytis Nutrition 0.000 description 1
- 235000017647 Brassica oleracea var italica Nutrition 0.000 description 1
- 244000308180 Brassica oleracea var. italica Species 0.000 description 1
- 208000005623 Carcinogenesis Diseases 0.000 description 1
- 206010065941 Central obesity Diseases 0.000 description 1
- 108010004103 Chylomicrons Proteins 0.000 description 1
- 108020004635 Complementary DNA Proteins 0.000 description 1
- 241000557626 Corvus corax Species 0.000 description 1
- 240000008067 Cucumis sativus Species 0.000 description 1
- 235000010799 Cucumis sativus var sativus Nutrition 0.000 description 1
- 108020001738 DNA Glycosylase Proteins 0.000 description 1
- 108020003215 DNA Probes Proteins 0.000 description 1
- 230000004544 DNA amplification Effects 0.000 description 1
- 102000028381 DNA glycosylase Human genes 0.000 description 1
- 239000003155 DNA primer Substances 0.000 description 1
- 239000003298 DNA probe Substances 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 1
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 1
- 244000000626 Daucus carota Species 0.000 description 1
- 235000002767 Daucus carota Nutrition 0.000 description 1
- 208000020401 Depressive disease Diseases 0.000 description 1
- 206010014486 Elevated triglycerides Diseases 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 102000004533 Endonucleases Human genes 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 1
- 238000000729 Fisher's exact test Methods 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 101000911337 Homo sapiens Fatty acid-binding protein, intestinal Proteins 0.000 description 1
- 101001076407 Homo sapiens Interleukin-1 receptor antagonist protein Proteins 0.000 description 1
- 240000005979 Hordeum vulgare Species 0.000 description 1
- 235000007340 Hordeum vulgare Nutrition 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- AVXURJPOCDRRFD-UHFFFAOYSA-N Hydroxylamine Chemical compound ON AVXURJPOCDRRFD-UHFFFAOYSA-N 0.000 description 1
- 208000035150 Hypercholesterolemia Diseases 0.000 description 1
- 101150097648 Il1a gene Proteins 0.000 description 1
- 208000026350 Inborn Genetic disease Diseases 0.000 description 1
- 102000003777 Interleukin-1 beta Human genes 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 244000017020 Ipomoea batatas Species 0.000 description 1
- 235000002678 Ipomoea batatas Nutrition 0.000 description 1
- 235000010254 Jasminum officinale Nutrition 0.000 description 1
- 240000005385 Jasminum sambac Species 0.000 description 1
- 241001397173 Kali <angiosperm> Species 0.000 description 1
- 238000008214 LDL Cholesterol Methods 0.000 description 1
- 108090000364 Ligases Proteins 0.000 description 1
- 102000003960 Ligases Human genes 0.000 description 1
- 102000057248 Lipoprotein(a) Human genes 0.000 description 1
- 108010033266 Lipoprotein(a) Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 235000019681 Low Energy Density Foods Nutrition 0.000 description 1
- CSNNHWWHGAXBCP-UHFFFAOYSA-L Magnesium sulfate Chemical compound [Mg+2].[O-][S+2]([O-])([O-])[O-] CSNNHWWHGAXBCP-UHFFFAOYSA-L 0.000 description 1
- 244000070406 Malus silvestris Species 0.000 description 1
- 235000001412 Mediterranean diet Nutrition 0.000 description 1
- 208000036626 Mental retardation Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 240000005561 Musa balbisiana Species 0.000 description 1
- 239000012807 PCR reagent Substances 0.000 description 1
- 101150023417 PPARG gene Proteins 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- 108010010677 Phosphodiesterase I Proteins 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- 240000005809 Prunus persica Species 0.000 description 1
- 235000006040 Prunus persica var persica Nutrition 0.000 description 1
- 241000220324 Pyrus Species 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 1
- 241000225041 Roestes Species 0.000 description 1
- 108020004682 Single-Stranded DNA Proteins 0.000 description 1
- 108010090804 Streptavidin Proteins 0.000 description 1
- 240000001717 Vaccinium macrocarpon Species 0.000 description 1
- 235000012545 Vaccinium macrocarpon Nutrition 0.000 description 1
- 235000002118 Vaccinium oxycoccus Nutrition 0.000 description 1
- 241000219094 Vitaceae Species 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 150000001413 amino acids Chemical class 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 230000001430 anti-depressive effect Effects 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 235000021016 apples Nutrition 0.000 description 1
- 238000003491 array Methods 0.000 description 1
- 238000003149 assay kit Methods 0.000 description 1
- 208000006673 asthma Diseases 0.000 description 1
- 235000020856 atkins diet Nutrition 0.000 description 1
- 235000012791 bagels Nutrition 0.000 description 1
- 235000021015 bananas Nutrition 0.000 description 1
- 238000007681 bariatric surgery Methods 0.000 description 1
- 108010079452 beta Adrenergic Receptors Proteins 0.000 description 1
- 102000012740 beta Adrenergic Receptors Human genes 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 1
- 238000010256 biochemical assay Methods 0.000 description 1
- 238000010170 biological method Methods 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 208000028683 bipolar I disease Diseases 0.000 description 1
- 210000001124 body fluid Anatomy 0.000 description 1
- 239000010839 body fluid Substances 0.000 description 1
- 230000004579 body weight change Effects 0.000 description 1
- 235000008429 bread Nutrition 0.000 description 1
- 238000004422 calculation algorithm Methods 0.000 description 1
- 235000019577 caloric intake Nutrition 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 231100000504 carcinogenesis Toxicity 0.000 description 1
- 230000005189 cardiac health Effects 0.000 description 1
- 230000001364 causal effect Effects 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 235000013351 cheese Nutrition 0.000 description 1
- 239000012707 chemical precursor Substances 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003086 colorant Substances 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 235000014510 cooky Nutrition 0.000 description 1
- 230000002596 correlated effect Effects 0.000 description 1
- 238000009223 counseling Methods 0.000 description 1
- 235000011894 couscous Nutrition 0.000 description 1
- 235000004634 cranberry Nutrition 0.000 description 1
- 235000021438 curry Nutrition 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 239000003398 denaturant Substances 0.000 description 1
- 229960000633 dextran sulfate Drugs 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 235000013325 dietary fiber Nutrition 0.000 description 1
- 238000006073 displacement reaction Methods 0.000 description 1
- 239000002934 diuretic Substances 0.000 description 1
- 229940030606 diuretics Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 230000037149 energy metabolism Effects 0.000 description 1
- 235000021035 energy-restricted diet Nutrition 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 208000028299 esophageal disease Diseases 0.000 description 1
- 235000004626 essential fatty acids Nutrition 0.000 description 1
- 238000009207 exercise therapy Methods 0.000 description 1
- 238000013401 experimental design Methods 0.000 description 1
- 235000013861 fat-free Nutrition 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 235000004426 flaxseed Nutrition 0.000 description 1
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 1
- 235000020509 fortified beverage Nutrition 0.000 description 1
- 238000005194 fractionation Methods 0.000 description 1
- 235000012055 fruits and vegetables Nutrition 0.000 description 1
- 235000013376 functional food Nutrition 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000011544 gradient gel Substances 0.000 description 1
- 235000021021 grapes Nutrition 0.000 description 1
- 235000012028 green salad Nutrition 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 235000008216 herbs Nutrition 0.000 description 1
- 235000021414 high glycemic load diet Nutrition 0.000 description 1
- 102000047019 human FABP2 Human genes 0.000 description 1
- 210000003917 human chromosome Anatomy 0.000 description 1
- 150000002443 hydroxylamines Chemical class 0.000 description 1
- 230000003345 hyperglycaemic effect Effects 0.000 description 1
- 238000007901 in situ hybridization Methods 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 208000027866 inflammatory disease Diseases 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 235000015110 jellies Nutrition 0.000 description 1
- 239000008274 jelly Substances 0.000 description 1
- 229940085718 lactaid Drugs 0.000 description 1
- 238000011173 large scale experimental method Methods 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 238000007443 liposuction Methods 0.000 description 1
- 238000001459 lithography Methods 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 239000012139 lysis buffer Substances 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 235000013575 mashed potatoes Nutrition 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 108020004999 messenger RNA Proteins 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- XZWYZXLIPXDOLR-UHFFFAOYSA-N metformin Chemical compound CN(C)C(=N)NC(N)=N XZWYZXLIPXDOLR-UHFFFAOYSA-N 0.000 description 1
- 229960003105 metformin Drugs 0.000 description 1
- 230000033607 mismatch repair Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 101150029137 mutY gene Proteins 0.000 description 1
- 230000000869 mutational effect Effects 0.000 description 1
- 238000001821 nucleic acid purification Methods 0.000 description 1
- 238000011330 nucleic acid test Methods 0.000 description 1
- 235000015816 nutrient absorption Nutrition 0.000 description 1
- 235000006286 nutrient intake Nutrition 0.000 description 1
- 238000005457 optimization Methods 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229910052762 osmium Inorganic materials 0.000 description 1
- SYQBFIAQOQZEGI-UHFFFAOYSA-N osmium atom Chemical compound [Os] SYQBFIAQOQZEGI-UHFFFAOYSA-N 0.000 description 1
- 229910000489 osmium tetroxide Inorganic materials 0.000 description 1
- 239000012285 osmium tetroxide Substances 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 235000021017 pears Nutrition 0.000 description 1
- 239000008177 pharmaceutical agent Substances 0.000 description 1
- PTMHPRAIXMAOOB-UHFFFAOYSA-L phosphoramidate Chemical compound NP([O-])([O-])=O PTMHPRAIXMAOOB-UHFFFAOYSA-L 0.000 description 1
- 235000013550 pizza Nutrition 0.000 description 1
- 235000021018 plums Nutrition 0.000 description 1
- 230000003234 polygenic effect Effects 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 238000004393 prognosis Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 238000002331 protein detection Methods 0.000 description 1
- 238000012175 pyrosequencing Methods 0.000 description 1
- 239000011535 reaction buffer Substances 0.000 description 1
- 229940044551 receptor antagonist Drugs 0.000 description 1
- 239000002464 receptor antagonist Substances 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 102200150805 rs1057519492 Human genes 0.000 description 1
- 102200095042 rs56379106 Human genes 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 235000019627 satiety Nutrition 0.000 description 1
- 230000036186 satiety Effects 0.000 description 1
- 208000007056 sickle cell anemia Diseases 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 239000007787 solid Substances 0.000 description 1
- 235000020920 sonoma diet Nutrition 0.000 description 1
- 235000020860 south beach diet Nutrition 0.000 description 1
- 206010041569 spinal fracture Diseases 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000007619 statistical method Methods 0.000 description 1
- 235000013547 stew Nutrition 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 235000012094 sugar confectionery Nutrition 0.000 description 1
- 150000008163 sugars Chemical class 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 229940104230 thymidine Drugs 0.000 description 1
- 238000003325 tomography Methods 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 235000021081 unsaturated fats Nutrition 0.000 description 1
- 238000012795 verification Methods 0.000 description 1
- 108700026220 vif Genes Proteins 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 235000012773 waffles Nutrition 0.000 description 1
- 235000020851 weight watchers diet Nutrition 0.000 description 1
- 235000020923 zone diet Nutrition 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6881—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for tissue or cell typing, e.g. human leukocyte antigen [HLA] probes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/118—Prognosis of disease development
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/156—Polymorphic or mutational markers
Landscapes
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Analytical Chemistry (AREA)
- Wood Science & Technology (AREA)
- Immunology (AREA)
- Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Biochemistry (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Medical Treatment And Welfare Office Work (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10745808P | 2008-10-22 | 2008-10-22 | |
| US61/107,458 | 2008-10-22 | ||
| US12/466,602 | 2009-05-15 | ||
| US12/466,602 US20100112570A1 (en) | 2008-10-22 | 2009-05-15 | Genetic Markers for Weight Management and Methods of Use Thereof |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110081861A true KR20110081861A (ko) | 2011-07-14 |
Family
ID=41665094
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020117011584A Withdrawn KR20110081861A (ko) | 2008-10-22 | 2009-10-22 | 체중 관리를 위한 유전자 마커 및 이의 사용 방법 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US20100112570A1 (enExample) |
| EP (1) | EP2350312A2 (enExample) |
| JP (1) | JP2012506256A (enExample) |
| KR (1) | KR20110081861A (enExample) |
| CN (1) | CN102439170A (enExample) |
| AU (1) | AU2009308406A1 (enExample) |
| CA (1) | CA2741331A1 (enExample) |
| WO (1) | WO2010048378A2 (enExample) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102044419B1 (ko) | 2019-08-16 | 2019-11-14 | 주식회사 클리노믹스 | 유전자 검사를 통한 사용자 맞춤형 음료추천 시스템 및 그 구동방법 |
| KR102141479B1 (ko) | 2020-01-07 | 2020-08-06 | 주식회사 클리노믹스 | 유전자 검사에 기반한 사용자 맞춤형 주류 및 안주 추천정보 제공 시스템 및 방법 |
| KR20210073494A (ko) | 2019-12-10 | 2021-06-18 | 주식회사 클리노믹스 | 게놈 자판기, 이를 활용한 o2o 전자상거래시스템 및 방법 |
| KR20220086968A (ko) | 2020-12-17 | 2022-06-24 | 다윈그룹(주) | 인공지능 기반 예측 의료정보서비스 시스템 및 그 방법 |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CA2723247A1 (en) * | 2008-05-02 | 2009-11-05 | Interleukin Genetics, Inc. | Detecting genetic predisposition to osteoarthritis associated conditions |
| US20130151270A1 (en) * | 2011-12-12 | 2013-06-13 | Pathway Genomics | Genetic Based Health Management Systems for Weight and Nutrition Control |
| US20120295256A1 (en) * | 2011-05-18 | 2012-11-22 | Genovive Llc | Weight management genetic test systems and methods |
| US10242756B2 (en) | 2012-09-21 | 2019-03-26 | Ethicon Endo-Surgery, Inc. | Systems and methods for predicting metabolic and bariatric surgery outcomes |
| EP2898101A4 (en) | 2012-09-21 | 2016-08-03 | Ethicon Endo Surgery Inc | CLINICAL PREDICTORS FOR WEIGHT LOSS |
| WO2014047388A1 (en) | 2012-09-21 | 2014-03-27 | Ethicon Endo-Surgery, Inc. | Systems and methods for predicting metabolic and bariatric surgery outcomes |
| WO2016005793A1 (en) * | 2014-07-09 | 2016-01-14 | Suisse Life Science S.A. | Cosmetic method. |
| JP2019503176A (ja) | 2016-01-12 | 2019-02-07 | インターロイキン ジェネティクス, インコーポレイテッド | 処置に対する応答を予測するための方法 |
| JP2017211886A (ja) * | 2016-05-26 | 2017-11-30 | 株式会社ブラケアジェネティクス | 女性の健康増進に有用なカスタマイズ情報の提供システム |
| CN106222289A (zh) * | 2016-08-15 | 2016-12-14 | 中国人民解放军军事医学科学院微生物流行病研究所 | rs1143634多态性在筛查发热伴血小板减少综合征患者中的应用 |
| US20180144820A1 (en) | 2016-10-24 | 2018-05-24 | Habit, Llc | System and method for implementing meal selection based on vitals, genotype and phenotype |
| US10329620B2 (en) | 2017-01-12 | 2019-06-25 | Cardioforecast Ltd. | Methods and kits for treating cardiovascular disease |
| CN108330194A (zh) * | 2017-01-20 | 2018-07-27 | 上海弥健生物科技有限公司 | 一种确定体型的方法及其试剂盒 |
| CA3142662A1 (en) | 2019-06-06 | 2020-12-10 | Sitokine Limited | Compositions and methods for treating lung, colorectal and breast cancer |
| WO2021028469A1 (en) | 2019-08-12 | 2021-02-18 | Sitokine Limited | Compositions and methods for treating cytokine release syndrome and neurotoxicity |
| WO2021205013A1 (en) | 2020-04-09 | 2021-10-14 | Sitokine Limited | Compositions and methods for treating covid-19 |
Family Cites Families (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB8311018D0 (en) * | 1983-04-22 | 1983-05-25 | Amersham Int Plc | Detecting mutations in dna |
| US4998617A (en) * | 1986-09-15 | 1991-03-12 | Laura Lupton Inc | Facial cosmetic liquid make up kit |
| US5459039A (en) * | 1989-05-12 | 1995-10-17 | Duke University | Methods for mapping genetic mutations |
| US6210877B1 (en) * | 1997-03-10 | 2001-04-03 | Interleukin Genetics, Inc. | Prediction of coronary artery disease |
| JP2005500032A (ja) * | 2001-06-15 | 2005-01-06 | インターリューキン ジェネティックス インコーポレイテッド | 老化関連症状の早期発現を検出および治療する方法 |
| CN1863928A (zh) * | 2003-08-08 | 2006-11-15 | 英特利金遗传学有限公司 | 骨质疏松的诊断和治疗 |
| US20050191678A1 (en) * | 2004-02-12 | 2005-09-01 | Geneob Usa Inc. | Genetic predictability for acquiring a disease or condition |
| US20060278241A1 (en) * | 2004-12-14 | 2006-12-14 | Gualberto Ruano | Physiogenomic method for predicting clinical outcomes of treatments in patients |
| US20060252050A1 (en) * | 2005-05-06 | 2006-11-09 | Ordovas Jose M | Genetic marker for weight regulation |
| US20070196841A1 (en) * | 2006-01-20 | 2007-08-23 | Gualberto Ruano | Physiogenomic method for predicting response to diet |
| US20080070247A1 (en) * | 2006-09-15 | 2008-03-20 | Gualberto Ruano | Physiogenomic method for predicting effects of exercise |
| CA2723247A1 (en) * | 2008-05-02 | 2009-11-05 | Interleukin Genetics, Inc. | Detecting genetic predisposition to osteoarthritis associated conditions |
-
2009
- 2009-05-15 US US12/466,602 patent/US20100112570A1/en not_active Abandoned
- 2009-10-22 KR KR1020117011584A patent/KR20110081861A/ko not_active Withdrawn
- 2009-10-22 CA CA2741331A patent/CA2741331A1/en not_active Abandoned
- 2009-10-22 CN CN2009801521234A patent/CN102439170A/zh active Pending
- 2009-10-22 WO PCT/US2009/061629 patent/WO2010048378A2/en not_active Ceased
- 2009-10-22 AU AU2009308406A patent/AU2009308406A1/en not_active Abandoned
- 2009-10-22 EP EP09740825A patent/EP2350312A2/en not_active Withdrawn
- 2009-10-22 JP JP2011533323A patent/JP2012506256A/ja active Pending
Cited By (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR102044419B1 (ko) | 2019-08-16 | 2019-11-14 | 주식회사 클리노믹스 | 유전자 검사를 통한 사용자 맞춤형 음료추천 시스템 및 그 구동방법 |
| WO2021033817A1 (ko) | 2019-08-16 | 2021-02-25 | 주식회사 클리노믹스 | 유전자 검사를 통한 사용자 맞춤형 음료추천 시스템 및 그 구동방법 |
| US12008627B2 (en) | 2019-08-16 | 2024-06-11 | Clinomics Inc. | System for recommending user-customized beverage through genetic test, and method for driving same |
| KR20210073494A (ko) | 2019-12-10 | 2021-06-18 | 주식회사 클리노믹스 | 게놈 자판기, 이를 활용한 o2o 전자상거래시스템 및 방법 |
| KR102141479B1 (ko) | 2020-01-07 | 2020-08-06 | 주식회사 클리노믹스 | 유전자 검사에 기반한 사용자 맞춤형 주류 및 안주 추천정보 제공 시스템 및 방법 |
| KR20220086968A (ko) | 2020-12-17 | 2022-06-24 | 다윈그룹(주) | 인공지능 기반 예측 의료정보서비스 시스템 및 그 방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010048378A3 (en) | 2010-07-29 |
| US20100112570A1 (en) | 2010-05-06 |
| CA2741331A1 (en) | 2010-04-29 |
| AU2009308406A1 (en) | 2010-04-29 |
| JP2012506256A (ja) | 2012-03-15 |
| WO2010048378A2 (en) | 2010-04-29 |
| CN102439170A (zh) | 2012-05-02 |
| EP2350312A2 (en) | 2011-08-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20110081861A (ko) | 체중 관리를 위한 유전자 마커 및 이의 사용 방법 | |
| KR101668122B1 (ko) | 체중 관리를 위한 유전자 마커 및 이의 사용 방법 | |
| US20130011841A1 (en) | Genetic Association of Polymorphisms in Perilipin (PLIN) Gene With Resistance to Weight Loss | |
| JP5864431B2 (ja) | 体重管理のための遺伝子マーカーおよびその使用法 | |
| da Fonseca et al. | The association of the fat mass and obesity-associated gene (FTO) rs9939609 polymorphism and the severe obesity in a Brazilian population | |
| US20130079612A1 (en) | Methods for Creating Recommended Dietary Regime | |
| Tang et al. | Identification of a novel 5–base pair deletion in calcineurin B (PPP3R1) promoter region and its association with left ventricular hypertrophy | |
| HK1218937B (zh) | 用於体重管理的遗传标记物及其使用方法 | |
| Proença et al. | The association of the fat mass and obesity-associated gene (FTO) rs9939609 polymorphism and the severe obesity in a Brazilian population | |
| Org | ScreeningCodon 233, 234 and 276 Polymorphisms in Exon 3 ofInsulin Receptor Gene in Type 2 Diabetes Mellitus Patients ofKashmir Valley | |
| HK1158703B (en) | Genetic markers for weight management and methods of use thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20110520 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| PC1203 | Withdrawal of no request for examination | ||
| WITN | Application deemed withdrawn, e.g. because no request for examination was filed or no examination fee was paid |