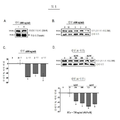
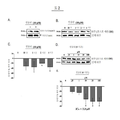
KR20110028457A - 신경섬유 매듭과 연관된 신경변성 장애를 치료하는 방법 - Google Patents
신경섬유 매듭과 연관된 신경변성 장애를 치료하는 방법 Download PDFInfo
- Publication number
- KR20110028457A KR20110028457A KR1020107028636A KR20107028636A KR20110028457A KR 20110028457 A KR20110028457 A KR 20110028457A KR 1020107028636 A KR1020107028636 A KR 1020107028636A KR 20107028636 A KR20107028636 A KR 20107028636A KR 20110028457 A KR20110028457 A KR 20110028457A
- Authority
- KR
- South Korea
- Prior art keywords
- leptin
- glu
- val
- ala
- leu
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Ceased
Links
- 210000004126 nerve fiber Anatomy 0.000 title description 3
- 208000015122 neurodegenerative disease Diseases 0.000 title 1
- 238000000034 method Methods 0.000 claims abstract description 84
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 67
- 201000010099 disease Diseases 0.000 claims abstract description 49
- 230000000750 progressive effect Effects 0.000 claims abstract description 27
- NRYBAZVQPHGZNS-ZSOCWYAHSA-N leptin Chemical compound O=C([C@H](CO)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](CCC(N)=O)NC(=O)[C@@H](N)CC(C)C)CCSC)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](CS)C(O)=O NRYBAZVQPHGZNS-ZSOCWYAHSA-N 0.000 claims description 272
- 108010092277 Leptin Proteins 0.000 claims description 250
- 102000016267 Leptin Human genes 0.000 claims description 247
- 229940039781 leptin Drugs 0.000 claims description 247
- 239000000203 mixture Substances 0.000 claims description 212
- 239000003814 drug Substances 0.000 claims description 101
- 238000006366 phosphorylation reaction Methods 0.000 claims description 81
- 230000026731 phosphorylation Effects 0.000 claims description 80
- 230000037396 body weight Effects 0.000 claims description 79
- 150000003839 salts Chemical class 0.000 claims description 69
- 229940124597 therapeutic agent Drugs 0.000 claims description 60
- 238000009825 accumulation Methods 0.000 claims description 58
- 238000011282 treatment Methods 0.000 claims description 31
- 208000010877 cognitive disease Diseases 0.000 claims description 26
- 208000024827 Alzheimer disease Diseases 0.000 claims description 24
- 206010012289 Dementia Diseases 0.000 claims description 17
- 102000001253 Protein Kinase Human genes 0.000 claims description 17
- 229940088597 hormone Drugs 0.000 claims description 17
- 239000005556 hormone Substances 0.000 claims description 17
- 108060006633 protein kinase Proteins 0.000 claims description 17
- 239000000556 agonist Substances 0.000 claims description 16
- 230000003920 cognitive function Effects 0.000 claims description 16
- 208000028698 Cognitive impairment Diseases 0.000 claims description 15
- 230000001419 dependent effect Effects 0.000 claims description 15
- 210000004556 brain Anatomy 0.000 claims description 14
- 239000003937 drug carrier Substances 0.000 claims description 14
- 239000003963 antioxidant agent Substances 0.000 claims description 13
- 210000002682 neurofibrillary tangle Anatomy 0.000 claims description 13
- 230000003278 mimic effect Effects 0.000 claims description 12
- 229940121375 antifungal agent Drugs 0.000 claims description 11
- 239000000739 antihistaminic agent Substances 0.000 claims description 11
- 210000001175 cerebrospinal fluid Anatomy 0.000 claims description 11
- 229940088594 vitamin Drugs 0.000 claims description 11
- 229930003231 vitamin Natural products 0.000 claims description 11
- 235000013343 vitamin Nutrition 0.000 claims description 11
- 239000011782 vitamin Substances 0.000 claims description 11
- 230000003115 biocidal effect Effects 0.000 claims description 10
- 239000002246 antineoplastic agent Substances 0.000 claims description 9
- 229940127089 cytotoxic agent Drugs 0.000 claims description 9
- GUGOEEXESWIERI-UHFFFAOYSA-N Terfenadine Chemical compound C1=CC(C(C)(C)C)=CC=C1C(O)CCCN1CCC(C(O)(C=2C=CC=CC=2)C=2C=CC=CC=2)CC1 GUGOEEXESWIERI-UHFFFAOYSA-N 0.000 claims description 7
- 239000012190 activator Substances 0.000 claims description 7
- 230000001387 anti-histamine Effects 0.000 claims description 7
- 230000003110 anti-inflammatory effect Effects 0.000 claims description 7
- 230000003078 antioxidant effect Effects 0.000 claims description 7
- 230000002360 prefrontal effect Effects 0.000 claims description 7
- 150000003722 vitamin derivatives Chemical class 0.000 claims description 7
- 208000020406 Creutzfeldt Jacob disease Diseases 0.000 claims description 5
- 208000003407 Creutzfeldt-Jakob Syndrome Diseases 0.000 claims description 5
- 208000010859 Creutzfeldt-Jakob disease Diseases 0.000 claims description 5
- 239000005557 antagonist Substances 0.000 claims description 5
- 230000000890 antigenic effect Effects 0.000 claims description 5
- 210000000349 chromosome Anatomy 0.000 claims description 5
- 230000007850 degeneration Effects 0.000 claims description 4
- 208000017004 dementia pugilistica Diseases 0.000 claims description 4
- 239000003112 inhibitor Substances 0.000 claims description 4
- 230000003637 steroidlike Effects 0.000 claims description 4
- 230000002123 temporal effect Effects 0.000 claims description 4
- 206010033799 Paralysis Diseases 0.000 claims description 2
- 208000021090 palsy Diseases 0.000 claims description 2
- 230000000843 anti-fungal effect Effects 0.000 claims 2
- 230000000840 anti-viral effect Effects 0.000 claims 2
- 230000000973 chemotherapeutic effect Effects 0.000 claims 1
- 239000006041 probiotic Substances 0.000 claims 1
- 230000000529 probiotic effect Effects 0.000 claims 1
- 235000018291 probiotics Nutrition 0.000 claims 1
- 208000035475 disorder Diseases 0.000 abstract description 17
- 230000001149 cognitive effect Effects 0.000 abstract description 3
- 238000011084 recovery Methods 0.000 abstract description 3
- 108010026424 tau Proteins Proteins 0.000 description 133
- 102000013498 tau Proteins Human genes 0.000 description 133
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 109
- 210000004027 cell Anatomy 0.000 description 90
- -1 Chitoconazole Chemical compound 0.000 description 56
- 229940125396 insulin Drugs 0.000 description 55
- 102000004877 Insulin Human genes 0.000 description 54
- 108090001061 Insulin Proteins 0.000 description 54
- 108090000765 processed proteins & peptides Proteins 0.000 description 41
- 230000000694 effects Effects 0.000 description 37
- 229940079593 drug Drugs 0.000 description 34
- 229940068196 placebo Drugs 0.000 description 32
- 239000000902 placebo Substances 0.000 description 32
- RTRQQBHATOEIAF-UUOKFMHZSA-N acadesine Chemical compound NC1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 RTRQQBHATOEIAF-UUOKFMHZSA-N 0.000 description 31
- 229960003000 acadesine Drugs 0.000 description 31
- 239000000725 suspension Substances 0.000 description 28
- 210000002569 neuron Anatomy 0.000 description 27
- 239000000843 powder Substances 0.000 description 25
- 239000000126 substance Substances 0.000 description 25
- 241000282414 Homo sapiens Species 0.000 description 24
- 150000001875 compounds Chemical class 0.000 description 24
- 239000002245 particle Substances 0.000 description 24
- 102000004196 processed proteins & peptides Human genes 0.000 description 23
- 108090000623 proteins and genes Proteins 0.000 description 22
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 21
- 238000009472 formulation Methods 0.000 description 20
- 229920000642 polymer Polymers 0.000 description 20
- 239000004480 active ingredient Substances 0.000 description 18
- SHGAZHPCJJPHSC-YCNIQYBTSA-N all-trans-retinoic acid Chemical compound OC(=O)\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-YCNIQYBTSA-N 0.000 description 18
- 239000000463 material Substances 0.000 description 18
- 239000000969 carrier Substances 0.000 description 17
- 239000003795 chemical substances by application Substances 0.000 description 17
- 238000002474 experimental method Methods 0.000 description 17
- 102000004169 proteins and genes Human genes 0.000 description 17
- 239000000243 solution Substances 0.000 description 17
- 229960001727 tretinoin Drugs 0.000 description 17
- SITLTJHOQZFJGG-UHFFFAOYSA-N N-L-alpha-glutamyl-L-valine Natural products CC(C)C(C(O)=O)NC(=O)C(N)CCC(O)=O SITLTJHOQZFJGG-UHFFFAOYSA-N 0.000 description 16
- 238000002347 injection Methods 0.000 description 16
- 239000007924 injection Substances 0.000 description 16
- 235000018102 proteins Nutrition 0.000 description 16
- 229930002330 retinoic acid Natural products 0.000 description 16
- 230000001225 therapeutic effect Effects 0.000 description 16
- KOSRFJWDECSPRO-UHFFFAOYSA-N alpha-L-glutamyl-L-glutamic acid Natural products OC(=O)CCC(N)C(=O)NC(CCC(O)=O)C(O)=O KOSRFJWDECSPRO-UHFFFAOYSA-N 0.000 description 15
- 108010090849 Amyloid beta-Peptides Proteins 0.000 description 14
- 102000013455 Amyloid beta-Peptides Human genes 0.000 description 14
- 239000002253 acid Substances 0.000 description 14
- 239000003242 anti bacterial agent Substances 0.000 description 14
- 239000000284 extract Substances 0.000 description 14
- UDMBCSSLTHHNCD-KQYNXXCUSA-N adenosine 5'-monophosphate Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O UDMBCSSLTHHNCD-KQYNXXCUSA-N 0.000 description 13
- 239000000839 emulsion Substances 0.000 description 13
- 239000012528 membrane Substances 0.000 description 13
- 210000004379 membrane Anatomy 0.000 description 13
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 12
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 12
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 12
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 12
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 12
- 238000001262 western blot Methods 0.000 description 12
- 150000001413 amino acids Chemical class 0.000 description 11
- 235000006708 antioxidants Nutrition 0.000 description 11
- 239000011230 binding agent Substances 0.000 description 11
- 210000001519 tissue Anatomy 0.000 description 11
- 235000001014 amino acid Nutrition 0.000 description 10
- 230000007423 decrease Effects 0.000 description 10
- 230000003111 delayed effect Effects 0.000 description 10
- 235000014113 dietary fatty acids Nutrition 0.000 description 10
- 150000002148 esters Chemical class 0.000 description 10
- 229930195729 fatty acid Natural products 0.000 description 10
- 239000000194 fatty acid Substances 0.000 description 10
- 239000002609 medium Substances 0.000 description 10
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 10
- 102000005962 receptors Human genes 0.000 description 10
- 108020003175 receptors Proteins 0.000 description 10
- 239000007787 solid Substances 0.000 description 10
- 239000002904 solvent Substances 0.000 description 10
- 208000024891 symptom Diseases 0.000 description 10
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 9
- 102100031775 Leptin receptor Human genes 0.000 description 9
- 239000013543 active substance Substances 0.000 description 9
- 229940088710 antibiotic agent Drugs 0.000 description 9
- 239000003443 antiviral agent Substances 0.000 description 9
- 239000008194 pharmaceutical composition Substances 0.000 description 9
- 239000006187 pill Substances 0.000 description 9
- 239000000080 wetting agent Substances 0.000 description 9
- 208000037259 Amyloid Plaque Diseases 0.000 description 8
- 101001071234 Arabidopsis thaliana SEC12-like protein 1 Proteins 0.000 description 8
- 108010010803 Gelatin Proteins 0.000 description 8
- 241001465754 Metazoa Species 0.000 description 8
- XMBSYZWANAQXEV-UHFFFAOYSA-N N-alpha-L-glutamyl-L-phenylalanine Natural products OC(=O)CCC(N)C(=O)NC(C(O)=O)CC1=CC=CC=C1 XMBSYZWANAQXEV-UHFFFAOYSA-N 0.000 description 8
- 102000001708 Protein Isoforms Human genes 0.000 description 8
- 108010029485 Protein Isoforms Proteins 0.000 description 8
- YFOCMOVJBQDBCE-NRPADANISA-N Val-Ala-Glu Chemical compound C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N YFOCMOVJBQDBCE-NRPADANISA-N 0.000 description 8
- 238000010521 absorption reaction Methods 0.000 description 8
- 230000002490 cerebral effect Effects 0.000 description 8
- 230000008859 change Effects 0.000 description 8
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 8
- 239000008273 gelatin Substances 0.000 description 8
- 229920000159 gelatin Polymers 0.000 description 8
- 235000019322 gelatine Nutrition 0.000 description 8
- 235000011852 gelatine desserts Nutrition 0.000 description 8
- 239000007788 liquid Substances 0.000 description 8
- 230000001537 neural effect Effects 0.000 description 8
- 230000007170 pathology Effects 0.000 description 8
- 108010073025 phenylalanylphenylalanine Proteins 0.000 description 8
- 239000002953 phosphate buffered saline Substances 0.000 description 8
- 238000002360 preparation method Methods 0.000 description 8
- 239000003755 preservative agent Substances 0.000 description 8
- 230000008569 process Effects 0.000 description 8
- 238000012360 testing method Methods 0.000 description 8
- 101710137189 Amyloid-beta A4 protein Proteins 0.000 description 7
- 102100022704 Amyloid-beta precursor protein Human genes 0.000 description 7
- 101710151993 Amyloid-beta precursor protein Proteins 0.000 description 7
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 7
- 201000011240 Frontotemporal dementia Diseases 0.000 description 7
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 7
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 7
- DZHSAHHDTRWUTF-SIQRNXPUSA-N amyloid-beta polypeptide 42 Chemical compound C([C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(=O)NCC(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)NCC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](C)C(O)=O)[C@@H](C)CC)C(C)C)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(C)C)C1=CC=CC=C1 DZHSAHHDTRWUTF-SIQRNXPUSA-N 0.000 description 7
- 239000003429 antifungal agent Substances 0.000 description 7
- 230000001276 controlling effect Effects 0.000 description 7
- 230000006378 damage Effects 0.000 description 7
- 150000004665 fatty acids Chemical class 0.000 description 7
- 235000003599 food sweetener Nutrition 0.000 description 7
- 108010055341 glutamyl-glutamic acid Proteins 0.000 description 7
- 108010019813 leptin receptors Proteins 0.000 description 7
- 230000003902 lesion Effects 0.000 description 7
- 230000036961 partial effect Effects 0.000 description 7
- 229920001223 polyethylene glycol Polymers 0.000 description 7
- 239000011780 sodium chloride Substances 0.000 description 7
- 239000002294 steroidal antiinflammatory agent Substances 0.000 description 7
- 238000013268 sustained release Methods 0.000 description 7
- 239000012730 sustained-release form Substances 0.000 description 7
- 239000003765 sweetening agent Substances 0.000 description 7
- 238000011200 topical administration Methods 0.000 description 7
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 6
- CCDFBRZVTDDJNM-GUBZILKMSA-N Ala-Leu-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O CCDFBRZVTDDJNM-GUBZILKMSA-N 0.000 description 6
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 6
- ULGZDMOVFRHVEP-RWJQBGPGSA-N Erythromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ULGZDMOVFRHVEP-RWJQBGPGSA-N 0.000 description 6
- CQQGCWPXDHTTNF-GUBZILKMSA-N Leu-Ala-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCC(O)=O CQQGCWPXDHTTNF-GUBZILKMSA-N 0.000 description 6
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 6
- COYHRQWNJDJCNA-NUJDXYNKSA-N Thr-Thr-Thr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O COYHRQWNJDJCNA-NUJDXYNKSA-N 0.000 description 6
- 108010008685 alanyl-glutamyl-aspartic acid Proteins 0.000 description 6
- 108010064397 amyloid beta-protein (1-40) Proteins 0.000 description 6
- 108010064539 amyloid beta-protein (1-42) Proteins 0.000 description 6
- 239000000872 buffer Substances 0.000 description 6
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 6
- 239000002270 dispersing agent Substances 0.000 description 6
- 239000000499 gel Substances 0.000 description 6
- 230000000670 limiting effect Effects 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 231100000252 nontoxic Toxicity 0.000 description 6
- 230000003000 nontoxic effect Effects 0.000 description 6
- 208000020016 psychiatric disease Diseases 0.000 description 6
- 230000009467 reduction Effects 0.000 description 6
- 239000000375 suspending agent Substances 0.000 description 6
- 102000014156 AMP-Activated Protein Kinases Human genes 0.000 description 5
- 108010011376 AMP-Activated Protein Kinases Proteins 0.000 description 5
- MFMDKJIPHSWSBM-GUBZILKMSA-N Ala-Lys-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O MFMDKJIPHSWSBM-GUBZILKMSA-N 0.000 description 5
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 5
- DDXZHOHEABQXSE-NKIYYHGXSA-N Glu-Thr-His Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O DDXZHOHEABQXSE-NKIYYHGXSA-N 0.000 description 5
- OLPPXYMMIARYAL-QMMMGPOBSA-N Gly-Gly-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)CNC(=O)CN OLPPXYMMIARYAL-QMMMGPOBSA-N 0.000 description 5
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 5
- 102100040243 Microtubule-associated protein tau Human genes 0.000 description 5
- 239000002202 Polyethylene glycol Substances 0.000 description 5
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 5
- 239000000443 aerosol Substances 0.000 description 5
- 210000000170 cell membrane Anatomy 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 239000003623 enhancer Substances 0.000 description 5
- 239000012091 fetal bovine serum Substances 0.000 description 5
- 230000006870 function Effects 0.000 description 5
- 235000011187 glycerol Nutrition 0.000 description 5
- 108010078326 glycyl-glycyl-valine Proteins 0.000 description 5
- 108010092114 histidylphenylalanine Proteins 0.000 description 5
- 238000000338 in vitro Methods 0.000 description 5
- 239000002502 liposome Substances 0.000 description 5
- 239000000314 lubricant Substances 0.000 description 5
- 230000004770 neurodegeneration Effects 0.000 description 5
- 235000008390 olive oil Nutrition 0.000 description 5
- 239000004006 olive oil Substances 0.000 description 5
- 230000035515 penetration Effects 0.000 description 5
- 230000000069 prophylactic effect Effects 0.000 description 5
- 239000003909 protein kinase inhibitor Substances 0.000 description 5
- 230000004044 response Effects 0.000 description 5
- 239000007921 spray Substances 0.000 description 5
- 210000003478 temporal lobe Anatomy 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- WHNFPRLDDSXQCL-UAZQEYIDSA-N α-msh Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(N)=O)NC(=O)[C@H](CO)NC(C)=O)C1=CC=C(O)C=C1 WHNFPRLDDSXQCL-UAZQEYIDSA-N 0.000 description 5
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 4
- QGZKDVFQNNGYKY-UHFFFAOYSA-N Ammonia Chemical compound N QGZKDVFQNNGYKY-UHFFFAOYSA-N 0.000 description 4
- XEDQMTWEYFBOIK-ACZMJKKPSA-N Asp-Ala-Glu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O XEDQMTWEYFBOIK-ACZMJKKPSA-N 0.000 description 4
- PBVLJOIPOGUQQP-CIUDSAMLSA-N Asp-Ala-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O PBVLJOIPOGUQQP-CIUDSAMLSA-N 0.000 description 4
- YDJVIBMKAMQPPP-LAEOZQHASA-N Asp-Glu-Val Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O YDJVIBMKAMQPPP-LAEOZQHASA-N 0.000 description 4
- QWOJMRHUQHTCJG-UHFFFAOYSA-N CC([CH2-])=O Chemical compound CC([CH2-])=O QWOJMRHUQHTCJG-UHFFFAOYSA-N 0.000 description 4
- IAYPIBMASNFSPL-UHFFFAOYSA-N Ethylene oxide Chemical compound C1CO1 IAYPIBMASNFSPL-UHFFFAOYSA-N 0.000 description 4
- LKDIBBOKUAASNP-FXQIFTODSA-N Glu-Ala-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O LKDIBBOKUAASNP-FXQIFTODSA-N 0.000 description 4
- MWMJCGBSIORNCD-AVGNSLFASA-N Glu-Leu-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O MWMJCGBSIORNCD-AVGNSLFASA-N 0.000 description 4
- RLFSBAPJTYKSLG-WHFBIAKZSA-N Gly-Ala-Asp Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(O)=O RLFSBAPJTYKSLG-WHFBIAKZSA-N 0.000 description 4
- IDQNVIWPPWAFSY-AVGNSLFASA-N His-His-Gln Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCC(N)=O)C(O)=O IDQNVIWPPWAFSY-AVGNSLFASA-N 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 4
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 4
- PMGDADKJMCOXHX-UHFFFAOYSA-N L-Arginyl-L-glutamin-acetat Natural products NC(=N)NCCCC(N)C(=O)NC(CCC(N)=O)C(O)=O PMGDADKJMCOXHX-UHFFFAOYSA-N 0.000 description 4
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 4
- NJMXCOOEFLMZSR-AVGNSLFASA-N Leu-Met-Val Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](C(C)C)C(O)=O NJMXCOOEFLMZSR-AVGNSLFASA-N 0.000 description 4
- 241000699670 Mus sp. Species 0.000 description 4
- YBAFDPFAUTYYRW-UHFFFAOYSA-N N-L-alpha-glutamyl-L-leucine Natural products CC(C)CC(C(O)=O)NC(=O)C(N)CCC(O)=O YBAFDPFAUTYYRW-UHFFFAOYSA-N 0.000 description 4
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 4
- 208000018737 Parkinson disease Diseases 0.000 description 4
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 4
- 229920002472 Starch Polymers 0.000 description 4
- UDQBCBUXAQIZAK-GLLZPBPUSA-N Thr-Glu-Glu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O UDQBCBUXAQIZAK-GLLZPBPUSA-N 0.000 description 4
- CPGJELLYDQEDRK-NAKRPEOUSA-N Val-Ile-Ala Chemical compound CC[C@H](C)[C@H](NC(=O)[C@@H](N)C(C)C)C(=O)N[C@@H](C)C(O)=O CPGJELLYDQEDRK-NAKRPEOUSA-N 0.000 description 4
- MIKHIIQMRFYVOR-RCWTZXSCSA-N Val-Pro-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@@H]1CCCN1C(=O)[C@H](C(C)C)N)O MIKHIIQMRFYVOR-RCWTZXSCSA-N 0.000 description 4
- NLNCNKIVJPEFBC-DLOVCJGASA-N Val-Val-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CCC(O)=O NLNCNKIVJPEFBC-DLOVCJGASA-N 0.000 description 4
- 208000027418 Wounds and injury Diseases 0.000 description 4
- 230000004913 activation Effects 0.000 description 4
- 239000002671 adjuvant Substances 0.000 description 4
- 108010005233 alanylglutamic acid Proteins 0.000 description 4
- 235000010443 alginic acid Nutrition 0.000 description 4
- 229920000615 alginic acid Polymers 0.000 description 4
- 229940125715 antihistaminic agent Drugs 0.000 description 4
- 108010008355 arginyl-glutamine Proteins 0.000 description 4
- 229960005070 ascorbic acid Drugs 0.000 description 4
- 108010040443 aspartyl-aspartic acid Proteins 0.000 description 4
- 239000002585 base Substances 0.000 description 4
- 230000015572 biosynthetic process Effects 0.000 description 4
- 210000004369 blood Anatomy 0.000 description 4
- 239000008280 blood Substances 0.000 description 4
- 238000004113 cell culture Methods 0.000 description 4
- 238000006243 chemical reaction Methods 0.000 description 4
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 4
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 4
- 239000003086 colorant Substances 0.000 description 4
- 238000013270 controlled release Methods 0.000 description 4
- GVJHHUAWPYXKBD-UHFFFAOYSA-N d-alpha-tocopherol Natural products OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 4
- 238000011161 development Methods 0.000 description 4
- 230000018109 developmental process Effects 0.000 description 4
- 230000004069 differentiation Effects 0.000 description 4
- 239000003925 fat Substances 0.000 description 4
- 235000019197 fats Nutrition 0.000 description 4
- 239000000835 fiber Substances 0.000 description 4
- 239000000945 filler Substances 0.000 description 4
- XBGGUPMXALFZOT-UHFFFAOYSA-N glycyl-L-tyrosine hemihydrate Natural products NCC(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 XBGGUPMXALFZOT-UHFFFAOYSA-N 0.000 description 4
- 108010087823 glycyltyrosine Proteins 0.000 description 4
- 239000008187 granular material Substances 0.000 description 4
- 108010028295 histidylhistidine Proteins 0.000 description 4
- 230000006951 hyperphosphorylation Effects 0.000 description 4
- 210000003016 hypothalamus Anatomy 0.000 description 4
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 4
- 230000006698 induction Effects 0.000 description 4
- 230000002401 inhibitory effect Effects 0.000 description 4
- 208000014674 injury Diseases 0.000 description 4
- 229940043355 kinase inhibitor Drugs 0.000 description 4
- 239000008101 lactose Substances 0.000 description 4
- 102000005861 leptin receptors Human genes 0.000 description 4
- 108010057821 leucylproline Proteins 0.000 description 4
- 230000007774 longterm Effects 0.000 description 4
- 210000004072 lung Anatomy 0.000 description 4
- HQKMJHAJHXVSDF-UHFFFAOYSA-L magnesium stearate Chemical compound [Mg+2].CCCCCCCCCCCCCCCCCC([O-])=O.CCCCCCCCCCCCCCCCCC([O-])=O HQKMJHAJHXVSDF-UHFFFAOYSA-L 0.000 description 4
- 230000035772 mutation Effects 0.000 description 4
- 239000000346 nonvolatile oil Substances 0.000 description 4
- 239000003921 oil Substances 0.000 description 4
- 235000019198 oils Nutrition 0.000 description 4
- 210000000056 organ Anatomy 0.000 description 4
- 239000000546 pharmaceutical excipient Substances 0.000 description 4
- 229920003023 plastic Polymers 0.000 description 4
- 239000004033 plastic Substances 0.000 description 4
- 229920001184 polypeptide Polymers 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 229960004063 propylene glycol Drugs 0.000 description 4
- 235000013772 propylene glycol Nutrition 0.000 description 4
- YGSDEFSMJLZEOE-UHFFFAOYSA-N salicylic acid Chemical compound OC(=O)C1=CC=CC=C1O YGSDEFSMJLZEOE-UHFFFAOYSA-N 0.000 description 4
- 230000028327 secretion Effects 0.000 description 4
- 229910052708 sodium Inorganic materials 0.000 description 4
- 239000011734 sodium Substances 0.000 description 4
- 239000003381 stabilizer Substances 0.000 description 4
- 235000019698 starch Nutrition 0.000 description 4
- 235000000346 sugar Nutrition 0.000 description 4
- 150000008163 sugars Chemical class 0.000 description 4
- 239000006188 syrup Substances 0.000 description 4
- 235000020357 syrup Nutrition 0.000 description 4
- 230000001988 toxicity Effects 0.000 description 4
- 231100000419 toxicity Toxicity 0.000 description 4
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 4
- 239000003981 vehicle Substances 0.000 description 4
- PQFMROVJTOPVDF-JBDRJPRFSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-amino-3-carboxypropanoyl]amino]-3-carboxypropanoyl]amino]-4-carboxybutanoyl]amino]butanedioic acid Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O PQFMROVJTOPVDF-JBDRJPRFSA-N 0.000 description 3
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 3
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 3
- KQFRUSHJPKXBMB-BHDSKKPTSA-N Ala-Ala-Trp Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](C)NC(=O)[C@@H](N)C)C(O)=O)=CNC2=C1 KQFRUSHJPKXBMB-BHDSKKPTSA-N 0.000 description 3
- NHCPCLJZRSIDHS-ZLUOBGJFSA-N Ala-Asp-Ala Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(O)=O NHCPCLJZRSIDHS-ZLUOBGJFSA-N 0.000 description 3
- KIUYPHAMDKDICO-WHFBIAKZSA-N Ala-Asp-Gly Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O KIUYPHAMDKDICO-WHFBIAKZSA-N 0.000 description 3
- KXEVYGKATAMXJJ-ACZMJKKPSA-N Ala-Glu-Asp Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O KXEVYGKATAMXJJ-ACZMJKKPSA-N 0.000 description 3
- PCIFXPRIFWKWLK-YUMQZZPRSA-N Ala-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@H](C)N PCIFXPRIFWKWLK-YUMQZZPRSA-N 0.000 description 3
- KUFVXLQLDHJVOG-SHGPDSBTSA-N Ala-Thr-Thr Chemical compound C[C@H]([C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](C)N)O KUFVXLQLDHJVOG-SHGPDSBTSA-N 0.000 description 3
- OMSKGWFGWCQFBD-KZVJFYERSA-N Ala-Val-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O OMSKGWFGWCQFBD-KZVJFYERSA-N 0.000 description 3
- 102000002659 Amyloid Precursor Protein Secretases Human genes 0.000 description 3
- 108010043324 Amyloid Precursor Protein Secretases Proteins 0.000 description 3
- IGULQRCJLQQPSM-DCAQKATOSA-N Arg-Cys-Leu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(C)C)C(O)=O IGULQRCJLQQPSM-DCAQKATOSA-N 0.000 description 3
- HQIZDMIGUJOSNI-IUCAKERBSA-N Arg-Gly-Arg Chemical compound N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(O)=O HQIZDMIGUJOSNI-IUCAKERBSA-N 0.000 description 3
- NKNILFJYKKHBKE-WPRPVWTQSA-N Arg-Gly-Val Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O NKNILFJYKKHBKE-WPRPVWTQSA-N 0.000 description 3
- OLVIPTLKNSAYRJ-YUMQZZPRSA-N Asn-Gly-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)CNC(=O)[C@H](CC(=O)N)N OLVIPTLKNSAYRJ-YUMQZZPRSA-N 0.000 description 3
- MYCSPQIARXTUTP-SRVKXCTJSA-N Asn-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(=O)N)N MYCSPQIARXTUTP-SRVKXCTJSA-N 0.000 description 3
- VOKWBBBXJONREA-DCAQKATOSA-N Asn-Met-His Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC(=O)N)N VOKWBBBXJONREA-DCAQKATOSA-N 0.000 description 3
- WQAOZCVOOYUWKG-LSJOCFKGSA-N Asn-Val-Val Chemical compound CC(C)[C@@H](C(=O)N[C@@H](C(C)C)C(=O)O)NC(=O)[C@H](CC(=O)N)N WQAOZCVOOYUWKG-LSJOCFKGSA-N 0.000 description 3
- JUWZKMBALYLZCK-WHFBIAKZSA-N Asp-Gly-Asn Chemical compound OC(=O)C[C@H](N)C(=O)NCC(=O)N[C@@H](CC(N)=O)C(O)=O JUWZKMBALYLZCK-WHFBIAKZSA-N 0.000 description 3
- YWLDTBBUHZJQHW-KKUMJFAQSA-N Asp-Lys-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(=O)O)N YWLDTBBUHZJQHW-KKUMJFAQSA-N 0.000 description 3
- FAUPLTGRUBTXNU-FXQIFTODSA-N Asp-Pro-Ser Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O FAUPLTGRUBTXNU-FXQIFTODSA-N 0.000 description 3
- CUQDCPXNZPDYFQ-ZLUOBGJFSA-N Asp-Ser-Asp Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O CUQDCPXNZPDYFQ-ZLUOBGJFSA-N 0.000 description 3
- KGHLGJAXYSVNJP-WHFBIAKZSA-N Asp-Ser-Gly Chemical compound OC(=O)C[C@H](N)C(=O)N[C@@H](CO)C(=O)NCC(O)=O KGHLGJAXYSVNJP-WHFBIAKZSA-N 0.000 description 3
- AWPWHMVCSISSQK-QWRGUYRKSA-N Asp-Tyr-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)NCC(O)=O AWPWHMVCSISSQK-QWRGUYRKSA-N 0.000 description 3
- XWKPSMRPIKKDDU-RCOVLWMOSA-N Asp-Val-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O XWKPSMRPIKKDDU-RCOVLWMOSA-N 0.000 description 3
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 3
- CPELXLSAUQHCOX-UHFFFAOYSA-M Bromide Chemical compound [Br-] CPELXLSAUQHCOX-UHFFFAOYSA-M 0.000 description 3
- 101100512078 Caenorhabditis elegans lys-1 gene Proteins 0.000 description 3
- 229920002261 Corn starch Polymers 0.000 description 3
- BMHBJCVEXUBGFI-BIIVOSGPSA-N Cys-Cys-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CS)NC(=O)[C@H](CS)N)C(=O)O BMHBJCVEXUBGFI-BIIVOSGPSA-N 0.000 description 3
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 3
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 3
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 3
- BLOXULLYFRGYKZ-GUBZILKMSA-N Gln-Glu-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O BLOXULLYFRGYKZ-GUBZILKMSA-N 0.000 description 3
- MAGNEQBFSBREJL-DCAQKATOSA-N Gln-Glu-Lys Chemical compound C(CCN)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N MAGNEQBFSBREJL-DCAQKATOSA-N 0.000 description 3
- HDUDGCZEOZEFOA-KBIXCLLPSA-N Gln-Ile-Ala Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)O)NC(=O)[C@H](CCC(=O)N)N HDUDGCZEOZEFOA-KBIXCLLPSA-N 0.000 description 3
- ZNTDJIMJKNNSLR-RWRJDSDZSA-N Gln-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](CCC(=O)N)N ZNTDJIMJKNNSLR-RWRJDSDZSA-N 0.000 description 3
- YPFFHGRJCUBXPX-NHCYSSNCSA-N Gln-Pro-Val Chemical compound CC(C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)CCC(N)=O)C(O)=O YPFFHGRJCUBXPX-NHCYSSNCSA-N 0.000 description 3
- YLABFXCRQQMMHS-AVGNSLFASA-N Gln-Tyr-Cys Chemical compound C1=CC(=CC=C1C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)N)N)O YLABFXCRQQMMHS-AVGNSLFASA-N 0.000 description 3
- SZXSSXUNOALWCH-ACZMJKKPSA-N Glu-Ala-Asn Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O SZXSSXUNOALWCH-ACZMJKKPSA-N 0.000 description 3
- WZZSKAJIHTUUSG-ACZMJKKPSA-N Glu-Ala-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O WZZSKAJIHTUUSG-ACZMJKKPSA-N 0.000 description 3
- YYOBUPFZLKQUAX-FXQIFTODSA-N Glu-Asn-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O YYOBUPFZLKQUAX-FXQIFTODSA-N 0.000 description 3
- DSPQRJXOIXHOHK-WDSKDSINSA-N Glu-Asp-Gly Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(O)=O DSPQRJXOIXHOHK-WDSKDSINSA-N 0.000 description 3
- CGOHAEBMDSEKFB-FXQIFTODSA-N Glu-Glu-Ala Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O CGOHAEBMDSEKFB-FXQIFTODSA-N 0.000 description 3
- NKLRYVLERDYDBI-FXQIFTODSA-N Glu-Glu-Asp Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O NKLRYVLERDYDBI-FXQIFTODSA-N 0.000 description 3
- BUZMZDDKFCSKOT-CIUDSAMLSA-N Glu-Glu-Glu Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O BUZMZDDKFCSKOT-CIUDSAMLSA-N 0.000 description 3
- QJCKNLPMTPXXEM-AUTRQRHGSA-N Glu-Glu-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O QJCKNLPMTPXXEM-AUTRQRHGSA-N 0.000 description 3
- KXTAGESXNQEZKB-DZKIICNBSA-N Glu-Phe-Val Chemical compound OC(=O)CC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](C(C)C)C(O)=O)CC1=CC=CC=C1 KXTAGESXNQEZKB-DZKIICNBSA-N 0.000 description 3
- ARIORLIIMJACKZ-KKUMJFAQSA-N Glu-Pro-Tyr Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O ARIORLIIMJACKZ-KKUMJFAQSA-N 0.000 description 3
- DMYACXMQUABZIQ-NRPADANISA-N Glu-Ser-Val Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C(C)C)C(O)=O DMYACXMQUABZIQ-NRPADANISA-N 0.000 description 3
- BRFJMRSRMOMIMU-WHFBIAKZSA-N Gly-Ala-Asn Chemical compound NCC(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O BRFJMRSRMOMIMU-WHFBIAKZSA-N 0.000 description 3
- OVSKVOOUFAKODB-UWVGGRQHSA-N Gly-Arg-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@@H](NC(=O)CN)CCCN=C(N)N OVSKVOOUFAKODB-UWVGGRQHSA-N 0.000 description 3
- UESJMAMHDLEHGM-NHCYSSNCSA-N Gly-Ile-Leu Chemical compound NCC(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(C)C)C(O)=O UESJMAMHDLEHGM-NHCYSSNCSA-N 0.000 description 3
- PAWIVEIWWYGBAM-YUMQZZPRSA-N Gly-Leu-Ala Chemical compound NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(O)=O PAWIVEIWWYGBAM-YUMQZZPRSA-N 0.000 description 3
- LHYJCVCQPWRMKZ-WEDXCCLWSA-N Gly-Leu-Thr Chemical compound [H]NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O LHYJCVCQPWRMKZ-WEDXCCLWSA-N 0.000 description 3
- XHVONGZZVUUORG-WEDXCCLWSA-N Gly-Thr-Lys Chemical compound NCC(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CCCCN XHVONGZZVUUORG-WEDXCCLWSA-N 0.000 description 3
- UVTSZKIATYSKIR-RYUDHWBXSA-N Gly-Tyr-Glu Chemical compound [H]NCC(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O UVTSZKIATYSKIR-RYUDHWBXSA-N 0.000 description 3
- 239000004471 Glycine Substances 0.000 description 3
- AEMRFAOFKBGASW-UHFFFAOYSA-N Glycolic acid Chemical compound OCC(O)=O AEMRFAOFKBGASW-UHFFFAOYSA-N 0.000 description 3
- RVKIPWVMZANZLI-UHFFFAOYSA-N H-Lys-Trp-OH Natural products C1=CC=C2C(CC(NC(=O)C(N)CCCCN)C(O)=O)=CNC2=C1 RVKIPWVMZANZLI-UHFFFAOYSA-N 0.000 description 3
- VXZZUXWAOMWWJH-QTKMDUPCSA-N His-Thr-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C(C)C)C(O)=O VXZZUXWAOMWWJH-QTKMDUPCSA-N 0.000 description 3
- XOZOSAUOGRPCES-STECZYCISA-N Ile-Pro-Tyr Chemical compound CC[C@H](C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 XOZOSAUOGRPCES-STECZYCISA-N 0.000 description 3
- ZNOBVZFCHNHKHA-KBIXCLLPSA-N Ile-Ser-Glu Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N ZNOBVZFCHNHKHA-KBIXCLLPSA-N 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 102000003746 Insulin Receptor Human genes 0.000 description 3
- 108010001127 Insulin Receptor Proteins 0.000 description 3
- LKXANTUNFMVCNF-IHPCNDPISA-N Leu-His-Trp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O LKXANTUNFMVCNF-IHPCNDPISA-N 0.000 description 3
- XVZCXCTYGHPNEM-UHFFFAOYSA-N Leu-Leu-Pro Natural products CC(C)CC(N)C(=O)NC(CC(C)C)C(=O)N1CCCC1C(O)=O XVZCXCTYGHPNEM-UHFFFAOYSA-N 0.000 description 3
- XOEDPXDZJHBQIX-ULQDDVLXSA-N Leu-Val-Phe Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 XOEDPXDZJHBQIX-ULQDDVLXSA-N 0.000 description 3
- MWVUEPNEPWMFBD-SRVKXCTJSA-N Lys-Cys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@H](C(O)=O)CCCCN MWVUEPNEPWMFBD-SRVKXCTJSA-N 0.000 description 3
- GUYHHBZCBQZLFW-GUBZILKMSA-N Lys-Gln-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CS)C(=O)O)N GUYHHBZCBQZLFW-GUBZILKMSA-N 0.000 description 3
- MIFFFXHMAHFACR-KATARQTJSA-N Lys-Ser-Thr Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CCCCN MIFFFXHMAHFACR-KATARQTJSA-N 0.000 description 3
- IEVXCWPVBYCJRZ-IXOXFDKPSA-N Lys-Thr-His Chemical compound NCCCC[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@H](C(O)=O)CC1=CN=CN1 IEVXCWPVBYCJRZ-IXOXFDKPSA-N 0.000 description 3
- 102400000740 Melanocyte-stimulating hormone alpha Human genes 0.000 description 3
- 101710151321 Melanostatin Proteins 0.000 description 3
- 101710200814 Melanotropin alpha Proteins 0.000 description 3
- OHMKUHXCDSCOMT-QXEWZRGKSA-N Met-Asn-Val Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O OHMKUHXCDSCOMT-QXEWZRGKSA-N 0.000 description 3
- FVKRQMQQFGBXHV-QXEWZRGKSA-N Met-Asp-Val Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O FVKRQMQQFGBXHV-QXEWZRGKSA-N 0.000 description 3
- SODXFJOPSCXOHE-IHRRRGAJSA-N Met-Leu-Leu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O SODXFJOPSCXOHE-IHRRRGAJSA-N 0.000 description 3
- AWGBEIYZPAXXSX-RWMBFGLXSA-N Met-Leu-Pro Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCSC)N AWGBEIYZPAXXSX-RWMBFGLXSA-N 0.000 description 3
- GFDBWMDLBKCLQH-IHRRRGAJSA-N Met-Phe-Cys Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CS)C(=O)O)N GFDBWMDLBKCLQH-IHRRRGAJSA-N 0.000 description 3
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 3
- 206010029260 Neuroblastoma Diseases 0.000 description 3
- 102400000064 Neuropeptide Y Human genes 0.000 description 3
- 241000283973 Oryctolagus cuniculus Species 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 101150096038 PTH1R gene Proteins 0.000 description 3
- 235000019483 Peanut oil Nutrition 0.000 description 3
- CYZBFPYMSJGBRL-DRZSPHRISA-N Phe-Ala-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(O)=O CYZBFPYMSJGBRL-DRZSPHRISA-N 0.000 description 3
- MQWISMJKHOUEMW-ULQDDVLXSA-N Phe-Arg-His Chemical compound C([C@H](N)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1NC=NC=1)C(O)=O)C1=CC=CC=C1 MQWISMJKHOUEMW-ULQDDVLXSA-N 0.000 description 3
- METZZBCMDXHFMK-BZSNNMDCSA-N Phe-Leu-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CC2=CC=CC=C2)N METZZBCMDXHFMK-BZSNNMDCSA-N 0.000 description 3
- IEIFEYBAYFSRBQ-IHRRRGAJSA-N Phe-Val-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CO)C(=O)O)NC(=O)[C@H](CC1=CC=CC=C1)N IEIFEYBAYFSRBQ-IHRRRGAJSA-N 0.000 description 3
- 229920003171 Poly (ethylene oxide) Polymers 0.000 description 3
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 3
- SZZBUDVXWZZPDH-BQBZGAKWSA-N Pro-Cys-Gly Chemical compound OC(=O)CNC(=O)[C@H](CS)NC(=O)[C@@H]1CCCN1 SZZBUDVXWZZPDH-BQBZGAKWSA-N 0.000 description 3
- FRKBNXCFJBPJOL-GUBZILKMSA-N Pro-Glu-Glu Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O FRKBNXCFJBPJOL-GUBZILKMSA-N 0.000 description 3
- XQHGISDMVBTGAL-ULQDDVLXSA-N Pro-His-Phe Chemical compound C([C@@H](C(=O)[O-])NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H]1[NH2+]CCC1)C1=CC=CC=C1 XQHGISDMVBTGAL-ULQDDVLXSA-N 0.000 description 3
- VGNYHOBZJKWRGI-CIUDSAMLSA-N Ser-Asn-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CO VGNYHOBZJKWRGI-CIUDSAMLSA-N 0.000 description 3
- OHKLFYXEOGGGCK-ZLUOBGJFSA-N Ser-Asp-Asn Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O OHKLFYXEOGGGCK-ZLUOBGJFSA-N 0.000 description 3
- HVKMTOIAYDOJPL-NRPADANISA-N Ser-Gln-Val Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O HVKMTOIAYDOJPL-NRPADANISA-N 0.000 description 3
- HJEBZBMOTCQYDN-ACZMJKKPSA-N Ser-Glu-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O HJEBZBMOTCQYDN-ACZMJKKPSA-N 0.000 description 3
- 206010048327 Supranuclear palsy Diseases 0.000 description 3
- MQCPGOZXFSYJPS-KZVJFYERSA-N Thr-Ala-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O MQCPGOZXFSYJPS-KZVJFYERSA-N 0.000 description 3
- DCLBXIWHLVEPMQ-JRQIVUDYSA-N Thr-Asp-Tyr Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 DCLBXIWHLVEPMQ-JRQIVUDYSA-N 0.000 description 3
- ZLNWJMRLHLGKFX-SVSWQMSJSA-N Thr-Cys-Ile Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O ZLNWJMRLHLGKFX-SVSWQMSJSA-N 0.000 description 3
- MMTOHPRBJKEZHT-BWBBJGPYSA-N Thr-Cys-Ser Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CO)C(O)=O MMTOHPRBJKEZHT-BWBBJGPYSA-N 0.000 description 3
- FHDLKMFZKRUQCE-HJGDQZAQSA-N Thr-Glu-Arg Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O FHDLKMFZKRUQCE-HJGDQZAQSA-N 0.000 description 3
- ZBKDBZUTTXINIX-RWRJDSDZSA-N Thr-Ile-Gln Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O ZBKDBZUTTXINIX-RWRJDSDZSA-N 0.000 description 3
- SCSVNSNWUTYSFO-WDCWCFNPSA-N Thr-Lys-Glu Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O SCSVNSNWUTYSFO-WDCWCFNPSA-N 0.000 description 3
- ZMYCLHFLHRVOEA-HEIBUPTGSA-N Thr-Thr-Ser Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O ZMYCLHFLHRVOEA-HEIBUPTGSA-N 0.000 description 3
- 229920001615 Tragacanth Polymers 0.000 description 3
- ZMANZCXQSJIPKH-UHFFFAOYSA-N Triethylamine Chemical compound CCN(CC)CC ZMANZCXQSJIPKH-UHFFFAOYSA-N 0.000 description 3
- LTLBNCDNXQCOLB-UBHSHLNASA-N Trp-Asp-Ser Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O)=CNC2=C1 LTLBNCDNXQCOLB-UBHSHLNASA-N 0.000 description 3
- XEHGAHOCTDKOKP-XIRDDKMYSA-N Trp-Cys-Lys Chemical compound C1=CC=C2C(=C1)C(=CN2)C[C@@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CCCCN)C(=O)O)N XEHGAHOCTDKOKP-XIRDDKMYSA-N 0.000 description 3
- WNGMGTMSUBARLB-RXVVDRJESA-N Trp-Trp-Gly Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CC=3C4=CC=CC=C4NC=3)N)C(=O)NCC(O)=O)=CNC2=C1 WNGMGTMSUBARLB-RXVVDRJESA-N 0.000 description 3
- UNUZEBFXGWVAOP-DZKIICNBSA-N Tyr-Glu-Val Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UNUZEBFXGWVAOP-DZKIICNBSA-N 0.000 description 3
- QKXAEWMHAAVVGS-KKUMJFAQSA-N Tyr-Pro-Glu Chemical compound N[C@@H](Cc1ccc(O)cc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCC(O)=O)C(O)=O QKXAEWMHAAVVGS-KKUMJFAQSA-N 0.000 description 3
- YODDULVCGFQRFZ-ZKWXMUAHSA-N Val-Asp-Ser Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O YODDULVCGFQRFZ-ZKWXMUAHSA-N 0.000 description 3
- SZTTYWIUCGSURQ-AUTRQRHGSA-N Val-Glu-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O SZTTYWIUCGSURQ-AUTRQRHGSA-N 0.000 description 3
- OQWNEUXPKHIEJO-NRPADANISA-N Val-Glu-Ser Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CO)C(=O)O)N OQWNEUXPKHIEJO-NRPADANISA-N 0.000 description 3
- WFENBJPLZMPVAX-XVKPBYJWSA-N Val-Gly-Glu Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CCC(O)=O WFENBJPLZMPVAX-XVKPBYJWSA-N 0.000 description 3
- LAYSXAOGWHKNED-XPUUQOCRSA-N Val-Gly-Ser Chemical compound CC(C)[C@H](N)C(=O)NCC(=O)N[C@@H](CO)C(O)=O LAYSXAOGWHKNED-XPUUQOCRSA-N 0.000 description 3
- XBJKAZATRJBDCU-GUBZILKMSA-N Val-Pro-Ala Chemical compound CC(C)[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C)C(O)=O XBJKAZATRJBDCU-GUBZILKMSA-N 0.000 description 3
- ZXYPHBKIZLAQTL-QXEWZRGKSA-N Val-Pro-Asp Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(=O)O)C(=O)O)N ZXYPHBKIZLAQTL-QXEWZRGKSA-N 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 239000000654 additive Substances 0.000 description 3
- 238000005273 aeration Methods 0.000 description 3
- 230000032683 aging Effects 0.000 description 3
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 3
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 3
- 150000001412 amines Chemical class 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 108010062796 arginyllysine Proteins 0.000 description 3
- 235000010323 ascorbic acid Nutrition 0.000 description 3
- 239000011668 ascorbic acid Substances 0.000 description 3
- 108010021908 aspartyl-aspartyl-glutamyl-aspartic acid Proteins 0.000 description 3
- 108010092854 aspartyllysine Proteins 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000004071 biological effect Effects 0.000 description 3
- 229910000019 calcium carbonate Inorganic materials 0.000 description 3
- 239000001506 calcium phosphate Substances 0.000 description 3
- 229910000389 calcium phosphate Inorganic materials 0.000 description 3
- 235000011010 calcium phosphates Nutrition 0.000 description 3
- 239000002775 capsule Substances 0.000 description 3
- 150000001720 carbohydrates Chemical class 0.000 description 3
- 235000014633 carbohydrates Nutrition 0.000 description 3
- 230000005754 cellular signaling Effects 0.000 description 3
- 210000003169 central nervous system Anatomy 0.000 description 3
- 210000004720 cerebrum Anatomy 0.000 description 3
- 238000000576 coating method Methods 0.000 description 3
- 239000008120 corn starch Substances 0.000 description 3
- 108010016616 cysteinylglycine Proteins 0.000 description 3
- 239000003085 diluting agent Substances 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000002552 dosage form Substances 0.000 description 3
- 231100000673 dose–response relationship Toxicity 0.000 description 3
- 239000006196 drop Substances 0.000 description 3
- 238000012377 drug delivery Methods 0.000 description 3
- 229920001971 elastomer Polymers 0.000 description 3
- 239000003995 emulsifying agent Substances 0.000 description 3
- 229960003276 erythromycin Drugs 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 239000006260 foam Substances 0.000 description 3
- 230000037406 food intake Effects 0.000 description 3
- 235000012631 food intake Nutrition 0.000 description 3
- 239000007789 gas Substances 0.000 description 3
- 108010049041 glutamylalanine Proteins 0.000 description 3
- VPZXBVLAVMBEQI-UHFFFAOYSA-N glycyl-DL-alpha-alanine Natural products OC(=O)C(C)NC(=O)CN VPZXBVLAVMBEQI-UHFFFAOYSA-N 0.000 description 3
- 108010025306 histidylleucine Proteins 0.000 description 3
- 108010085325 histidylproline Proteins 0.000 description 3
- XMBWDFGMSWQBCA-UHFFFAOYSA-N hydrogen iodide Chemical compound I XMBWDFGMSWQBCA-UHFFFAOYSA-N 0.000 description 3
- 229960001680 ibuprofen Drugs 0.000 description 3
- 239000012729 immediate-release (IR) formulation Substances 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 230000003834 intracellular effect Effects 0.000 description 3
- 238000010255 intramuscular injection Methods 0.000 description 3
- 239000007927 intramuscular injection Substances 0.000 description 3
- 235000010445 lecithin Nutrition 0.000 description 3
- 239000000787 lecithin Substances 0.000 description 3
- 229940067606 lecithin Drugs 0.000 description 3
- 150000002632 lipids Chemical class 0.000 description 3
- 229940057995 liquid paraffin Drugs 0.000 description 3
- 108010064235 lysylglycine Proteins 0.000 description 3
- 108010038320 lysylphenylalanine Proteins 0.000 description 3
- 108010017391 lysylvaline Proteins 0.000 description 3
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 3
- 238000002156 mixing Methods 0.000 description 3
- 210000000214 mouth Anatomy 0.000 description 3
- 210000005036 nerve Anatomy 0.000 description 3
- URPYMXQQVHTUDU-OFGSCBOVSA-N nucleopeptide y Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(N)=O)NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 URPYMXQQVHTUDU-OFGSCBOVSA-N 0.000 description 3
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 3
- 239000000312 peanut oil Substances 0.000 description 3
- 230000000144 pharmacologic effect Effects 0.000 description 3
- 235000021317 phosphate Nutrition 0.000 description 3
- 150000003904 phospholipids Chemical class 0.000 description 3
- 229920002643 polyglutamic acid Polymers 0.000 description 3
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 3
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 3
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 3
- 229910052700 potassium Inorganic materials 0.000 description 3
- 239000011591 potassium Substances 0.000 description 3
- NLKNQRATVPKPDG-UHFFFAOYSA-M potassium iodide Chemical compound [K+].[I-] NLKNQRATVPKPDG-UHFFFAOYSA-M 0.000 description 3
- 239000000047 product Substances 0.000 description 3
- 108010090894 prolylleucine Proteins 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 239000005060 rubber Substances 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- 239000000600 sorbitol Substances 0.000 description 3
- 238000001694 spray drying Methods 0.000 description 3
- 238000007920 subcutaneous administration Methods 0.000 description 3
- 238000010254 subcutaneous injection Methods 0.000 description 3
- 239000007929 subcutaneous injection Substances 0.000 description 3
- KDYFGRWQOYBRFD-UHFFFAOYSA-N succinic acid Chemical compound OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 3
- 239000000454 talc Substances 0.000 description 3
- 229910052623 talc Inorganic materials 0.000 description 3
- 235000012222 talc Nutrition 0.000 description 3
- 108010033670 threonyl-aspartyl-tyrosine Proteins 0.000 description 3
- 108010031491 threonyl-lysyl-glutamic acid Proteins 0.000 description 3
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 3
- 230000000699 topical effect Effects 0.000 description 3
- 238000002054 transplantation Methods 0.000 description 3
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 3
- 108010029384 tryptophyl-histidine Proteins 0.000 description 3
- IBIDRSSEHFLGSD-UHFFFAOYSA-N valinyl-arginine Natural products CC(C)C(N)C(=O)NC(C(O)=O)CCCN=C(N)N IBIDRSSEHFLGSD-UHFFFAOYSA-N 0.000 description 3
- 235000015112 vegetable and seed oil Nutrition 0.000 description 3
- 239000008158 vegetable oil Substances 0.000 description 3
- PUPZLCDOIYMWBV-UHFFFAOYSA-N (+/-)-1,3-Butanediol Chemical compound CC(O)CCO PUPZLCDOIYMWBV-UHFFFAOYSA-N 0.000 description 2
- WSWCOQWTEOXDQX-MQQKCMAXSA-M (E,E)-sorbate Chemical compound C\C=C\C=C\C([O-])=O WSWCOQWTEOXDQX-MQQKCMAXSA-M 0.000 description 2
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 description 2
- ZORQXIQZAOLNGE-UHFFFAOYSA-N 1,1-difluorocyclohexane Chemical compound FC1(F)CCCCC1 ZORQXIQZAOLNGE-UHFFFAOYSA-N 0.000 description 2
- IXPNQXFRVYWDDI-UHFFFAOYSA-N 1-methyl-2,4-dioxo-1,3-diazinane-5-carboximidamide Chemical compound CN1CC(C(N)=N)C(=O)NC1=O IXPNQXFRVYWDDI-UHFFFAOYSA-N 0.000 description 2
- VBICKXHEKHSIBG-UHFFFAOYSA-N 1-monostearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)CO VBICKXHEKHSIBG-UHFFFAOYSA-N 0.000 description 2
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N 13-cis retinol Natural products OCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-UHFFFAOYSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- IZHVBANLECCAGF-UHFFFAOYSA-N 2-hydroxy-3-(octadecanoyloxy)propyl octadecanoate Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCCCC IZHVBANLECCAGF-UHFFFAOYSA-N 0.000 description 2
- FZIPCQLKPTZZIM-UHFFFAOYSA-N 2-oxidanylpropane-1,2,3-tricarboxylic acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O.OC(=O)CC(O)(C(O)=O)CC(O)=O FZIPCQLKPTZZIM-UHFFFAOYSA-N 0.000 description 2
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 2
- 244000215068 Acacia senegal Species 0.000 description 2
- 229920001817 Agar Polymers 0.000 description 2
- UCIYCBSJBQGDGM-LPEHRKFASA-N Ala-Arg-Pro Chemical compound C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)O)N UCIYCBSJBQGDGM-LPEHRKFASA-N 0.000 description 2
- GORKKVHIBWAQHM-GCJQMDKQSA-N Ala-Asn-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GORKKVHIBWAQHM-GCJQMDKQSA-N 0.000 description 2
- PBAMJJXWDQXOJA-FXQIFTODSA-N Ala-Asp-Arg Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PBAMJJXWDQXOJA-FXQIFTODSA-N 0.000 description 2
- LSLIRHLIUDVNBN-CIUDSAMLSA-N Ala-Asp-Lys Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](C(O)=O)CCCCN LSLIRHLIUDVNBN-CIUDSAMLSA-N 0.000 description 2
- BGNLUHXLSAQYRQ-FXQIFTODSA-N Ala-Glu-Gln Chemical compound C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O BGNLUHXLSAQYRQ-FXQIFTODSA-N 0.000 description 2
- XYTNPQNAZREREP-XQXXSGGOSA-N Ala-Glu-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O XYTNPQNAZREREP-XQXXSGGOSA-N 0.000 description 2
- AJBVYEYZVYPFCF-CIUDSAMLSA-N Ala-Lys-Asn Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(N)=O)C(O)=O AJBVYEYZVYPFCF-CIUDSAMLSA-N 0.000 description 2
- GMGWOTQMUKYZIE-UBHSHLNASA-N Ala-Pro-Phe Chemical compound C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(O)=O)CC1=CC=CC=C1 GMGWOTQMUKYZIE-UBHSHLNASA-N 0.000 description 2
- VYSRNGOMGHOJCK-GUBZILKMSA-N Arg-Ala-Met Chemical compound C[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N VYSRNGOMGHOJCK-GUBZILKMSA-N 0.000 description 2
- BMNVSPMWMICFRV-DCAQKATOSA-N Arg-His-Asp Chemical compound NC(N)=NCCC[C@H](N)C(=O)N[C@H](C(=O)N[C@@H](CC(O)=O)C(O)=O)CC1=CN=CN1 BMNVSPMWMICFRV-DCAQKATOSA-N 0.000 description 2
- NVCIXQYNWYTLDO-IHRRRGAJSA-N Arg-His-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCCN=C(N)N)N NVCIXQYNWYTLDO-IHRRRGAJSA-N 0.000 description 2
- OTZMRMHZCMZOJZ-SRVKXCTJSA-N Arg-Leu-Glu Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O OTZMRMHZCMZOJZ-SRVKXCTJSA-N 0.000 description 2
- ZEBDYGZVMMKZNB-SRVKXCTJSA-N Arg-Met-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCCN=C(N)N)N ZEBDYGZVMMKZNB-SRVKXCTJSA-N 0.000 description 2
- FMYQECOAIFGQGU-CYDGBPFRSA-N Arg-Val-Ile Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O FMYQECOAIFGQGU-CYDGBPFRSA-N 0.000 description 2
- XHFXZQHTLJVZBN-FXQIFTODSA-N Asn-Arg-Asn Chemical compound C(C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CC(=O)N)N)CN=C(N)N XHFXZQHTLJVZBN-FXQIFTODSA-N 0.000 description 2
- ACKNRKFVYUVWAC-ZPFDUUQYSA-N Asn-Ile-Lys Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CC(=O)N)N ACKNRKFVYUVWAC-ZPFDUUQYSA-N 0.000 description 2
- NYGILGUOUOXGMJ-YUMQZZPRSA-N Asn-Lys-Gly Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)NCC(O)=O NYGILGUOUOXGMJ-YUMQZZPRSA-N 0.000 description 2
- LSJQOMAZIKQMTJ-SRVKXCTJSA-N Asn-Phe-Asp Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(O)=O)C(O)=O LSJQOMAZIKQMTJ-SRVKXCTJSA-N 0.000 description 2
- IDUUACUJKUXKKD-VEVYYDQMSA-N Asn-Pro-Thr Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(O)=O IDUUACUJKUXKKD-VEVYYDQMSA-N 0.000 description 2
- BEHQTVDBCLSCBY-CFMVVWHZSA-N Asn-Tyr-Ile Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O BEHQTVDBCLSCBY-CFMVVWHZSA-N 0.000 description 2
- UAXIKORUDGGIGA-DCAQKATOSA-N Asp-Pro-Lys Chemical compound C1C[C@H](N(C1)C(=O)[C@H](CC(=O)O)N)C(=O)N[C@@H](CCCCN)C(=O)O UAXIKORUDGGIGA-DCAQKATOSA-N 0.000 description 2
- 241000416162 Astragalus gummifer Species 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- BVKZGUZCCUSVTD-UHFFFAOYSA-M Bicarbonate Chemical class OC([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-M 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- 201000009030 Carcinoma Diseases 0.000 description 2
- 239000000055 Corticotropin-Releasing Hormone Substances 0.000 description 2
- 108010022152 Corticotropin-Releasing Hormone Proteins 0.000 description 2
- 102000012289 Corticotropin-Releasing Hormone Human genes 0.000 description 2
- DZLQXIFVQFTFJY-BYPYZUCNSA-N Cys-Gly-Gly Chemical compound SC[C@H](N)C(=O)NCC(=O)NCC(O)=O DZLQXIFVQFTFJY-BYPYZUCNSA-N 0.000 description 2
- AFYGNOJUTMXQIG-FXQIFTODSA-N Cys-Met-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)N AFYGNOJUTMXQIG-FXQIFTODSA-N 0.000 description 2
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 2
- 229920002307 Dextran Polymers 0.000 description 2
- ROSDSFDQCJNGOL-UHFFFAOYSA-N Dimethylamine Chemical compound CNC ROSDSFDQCJNGOL-UHFFFAOYSA-N 0.000 description 2
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 2
- LVGKNOAMLMIIKO-UHFFFAOYSA-N Elaidinsaeure-aethylester Natural products CCCCCCCCC=CCCCCCCCC(=O)OCC LVGKNOAMLMIIKO-UHFFFAOYSA-N 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- QUSNBJAOOMFDIB-UHFFFAOYSA-N Ethylamine Chemical compound CCN QUSNBJAOOMFDIB-UHFFFAOYSA-N 0.000 description 2
- JSYULGSPLTZDHM-NRPADANISA-N Gln-Ala-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O JSYULGSPLTZDHM-NRPADANISA-N 0.000 description 2
- MGJMFSBEMSNYJL-AVGNSLFASA-N Gln-Asn-Tyr Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O MGJMFSBEMSNYJL-AVGNSLFASA-N 0.000 description 2
- CITDWMLWXNUQKD-FXQIFTODSA-N Gln-Gln-Asn Chemical compound C(CC(=O)N)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC(=O)N)C(=O)O)N CITDWMLWXNUQKD-FXQIFTODSA-N 0.000 description 2
- XJKAKYXMFHUIHT-AUTRQRHGSA-N Gln-Glu-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CCC(=O)N)N XJKAKYXMFHUIHT-AUTRQRHGSA-N 0.000 description 2
- LKVCNGLNTAPMSZ-JYJNAYRXSA-N Gln-His-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CC2=CN=CN2)NC(=O)[C@H](CCC(=O)N)N LKVCNGLNTAPMSZ-JYJNAYRXSA-N 0.000 description 2
- GXMBDEGTXHQBAO-NKIYYHGXSA-N Gln-His-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)N)N)O GXMBDEGTXHQBAO-NKIYYHGXSA-N 0.000 description 2
- TWTWUBHEWQPMQW-ZPFDUUQYSA-N Gln-Ile-Arg Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O TWTWUBHEWQPMQW-ZPFDUUQYSA-N 0.000 description 2
- IOFDDSNZJDIGPB-GVXVVHGQSA-N Gln-Leu-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O IOFDDSNZJDIGPB-GVXVVHGQSA-N 0.000 description 2
- HPCOBEHVEHWREJ-DCAQKATOSA-N Gln-Lys-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(O)=O HPCOBEHVEHWREJ-DCAQKATOSA-N 0.000 description 2
- UQKVUFGUSVYJMQ-IRIUXVKKSA-N Gln-Tyr-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CC1=CC=C(C=C1)O)NC(=O)[C@H](CCC(=O)N)N)O UQKVUFGUSVYJMQ-IRIUXVKKSA-N 0.000 description 2
- GJLXZITZLUUXMJ-NHCYSSNCSA-N Gln-Val-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCSC)C(=O)O)NC(=O)[C@H](CCC(=O)N)N GJLXZITZLUUXMJ-NHCYSSNCSA-N 0.000 description 2
- RUFHOVYUYSNDNY-ACZMJKKPSA-N Glu-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCC(O)=O RUFHOVYUYSNDNY-ACZMJKKPSA-N 0.000 description 2
- RCCDHXSRMWCOOY-GUBZILKMSA-N Glu-Arg-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(O)=O RCCDHXSRMWCOOY-GUBZILKMSA-N 0.000 description 2
- KEBACWCLVOXFNC-DCAQKATOSA-N Glu-Arg-Met Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCSC)C(O)=O KEBACWCLVOXFNC-DCAQKATOSA-N 0.000 description 2
- AUTNXSQEVVHSJK-YVNDNENWSA-N Glu-Glu-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CCC(O)=O AUTNXSQEVVHSJK-YVNDNENWSA-N 0.000 description 2
- WVYJNPCWJYBHJG-YVNDNENWSA-N Glu-Ile-Gln Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(O)=O WVYJNPCWJYBHJG-YVNDNENWSA-N 0.000 description 2
- FGSGPLRPQCZBSQ-AVGNSLFASA-N Glu-Phe-Ser Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(O)=O FGSGPLRPQCZBSQ-AVGNSLFASA-N 0.000 description 2
- TWYFJOHWGCCRIR-DCAQKATOSA-N Glu-Pro-Arg Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O TWYFJOHWGCCRIR-DCAQKATOSA-N 0.000 description 2
- DTLLNDVORUEOTM-WDCWCFNPSA-N Glu-Thr-Lys Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(O)=O DTLLNDVORUEOTM-WDCWCFNPSA-N 0.000 description 2
- YPHPEHMXOYTEQG-LAEOZQHASA-N Glu-Val-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCC(O)=O YPHPEHMXOYTEQG-LAEOZQHASA-N 0.000 description 2
- KCCNSVHJSMMGFS-NRPADANISA-N Glu-Val-Cys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)[C@H](CCC(=O)O)N KCCNSVHJSMMGFS-NRPADANISA-N 0.000 description 2
- ZALGPUWUVHOGAE-GVXVVHGQSA-N Glu-Val-His Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZALGPUWUVHOGAE-GVXVVHGQSA-N 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- AIJAPFVDBFYNKN-WHFBIAKZSA-N Gly-Asn-Asp Chemical compound C([C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)O)NC(=O)CN)C(=O)N AIJAPFVDBFYNKN-WHFBIAKZSA-N 0.000 description 2
- VLIJYPMATZSOLL-YUMQZZPRSA-N Gly-Lys-Cys Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CS)C(=O)O)NC(=O)CN VLIJYPMATZSOLL-YUMQZZPRSA-N 0.000 description 2
- QSQXZZCGPXQBPP-BQBZGAKWSA-N Gly-Pro-Cys Chemical compound C1C[C@H](N(C1)C(=O)CN)C(=O)N[C@@H](CS)C(=O)O QSQXZZCGPXQBPP-BQBZGAKWSA-N 0.000 description 2
- 102000019058 Glycogen Synthase Kinase 3 beta Human genes 0.000 description 2
- 108010051975 Glycogen Synthase Kinase 3 beta Proteins 0.000 description 2
- 239000000579 Gonadotropin-Releasing Hormone Substances 0.000 description 2
- 229920000084 Gum arabic Polymers 0.000 description 2
- IPIVXQQRZXEUGW-UWJYBYFXSA-N His-Ala-His Chemical compound C([C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1NC=NC=1)C(O)=O)C1=CN=CN1 IPIVXQQRZXEUGW-UWJYBYFXSA-N 0.000 description 2
- NWGXCPUKPVISSJ-AVGNSLFASA-N His-Gln-Lys Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O)N NWGXCPUKPVISSJ-AVGNSLFASA-N 0.000 description 2
- NTXIJPDAHXSHNL-ONGXEEELSA-N His-Gly-Val Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)NCC(=O)N[C@@H](C(C)C)C(O)=O NTXIJPDAHXSHNL-ONGXEEELSA-N 0.000 description 2
- NTYJJOPFIAHURM-UHFFFAOYSA-N Histamine Chemical compound NCCC1=CN=CN1 NTYJJOPFIAHURM-UHFFFAOYSA-N 0.000 description 2
- 108091006905 Human Serum Albumin Proteins 0.000 description 2
- 102000008100 Human Serum Albumin Human genes 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- 206010020751 Hypersensitivity Diseases 0.000 description 2
- WLRJHVNFGAOYPS-HJPIBITLSA-N Ile-Ser-Tyr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC1=CC=C(C=C1)O)C(=O)O)N WLRJHVNFGAOYPS-HJPIBITLSA-N 0.000 description 2
- ZDXPYRJPNDTMRX-VKHMYHEASA-N L-glutamine Chemical compound OC(=O)[C@@H](N)CCC(N)=O ZDXPYRJPNDTMRX-VKHMYHEASA-N 0.000 description 2
- 229930182816 L-glutamine Natural products 0.000 description 2
- XNSAINXGIQZQOO-UHFFFAOYSA-N L-pyroglutamyl-L-histidyl-L-proline amide Natural products NC(=O)C1CCCN1C(=O)C(NC(=O)C1NC(=O)CC1)CC1=CN=CN1 XNSAINXGIQZQOO-UHFFFAOYSA-N 0.000 description 2
- TYYLDKGBCJGJGW-UHFFFAOYSA-N L-tryptophan-L-tyrosine Natural products C=1NC2=CC=CC=C2C=1CC(N)C(=O)NC(C(O)=O)CC1=CC=C(O)C=C1 TYYLDKGBCJGJGW-UHFFFAOYSA-N 0.000 description 2
- CZCSUZMIRKFFFA-CIUDSAMLSA-N Leu-Ala-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(O)=O CZCSUZMIRKFFFA-CIUDSAMLSA-N 0.000 description 2
- DLFAACQHIRSQGG-CIUDSAMLSA-N Leu-Asp-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O DLFAACQHIRSQGG-CIUDSAMLSA-N 0.000 description 2
- CQGSYZCULZMEDE-SRVKXCTJSA-N Leu-Gln-Pro Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N1CCC[C@H]1C(O)=O CQGSYZCULZMEDE-SRVKXCTJSA-N 0.000 description 2
- CQGSYZCULZMEDE-UHFFFAOYSA-N Leu-Gln-Pro Natural products CC(C)CC(N)C(=O)NC(CCC(N)=O)C(=O)N1CCCC1C(O)=O CQGSYZCULZMEDE-UHFFFAOYSA-N 0.000 description 2
- KVMULWOHPPMHHE-DCAQKATOSA-N Leu-Glu-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KVMULWOHPPMHHE-DCAQKATOSA-N 0.000 description 2
- REPBGZHJKYWFMJ-KKUMJFAQSA-N Leu-Lys-His Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N REPBGZHJKYWFMJ-KKUMJFAQSA-N 0.000 description 2
- GNRPTBRHRRZCMA-RWMBFGLXSA-N Leu-Met-Pro Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@@H]1C(=O)O)N GNRPTBRHRRZCMA-RWMBFGLXSA-N 0.000 description 2
- YRRCOJOXAJNSAX-IHRRRGAJSA-N Leu-Pro-Lys Chemical compound CC(C)C[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)O)N YRRCOJOXAJNSAX-IHRRRGAJSA-N 0.000 description 2
- WUHBLPVELFTPQK-KKUMJFAQSA-N Leu-Tyr-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(N)=O)C(O)=O WUHBLPVELFTPQK-KKUMJFAQSA-N 0.000 description 2
- 240000007472 Leucaena leucocephala Species 0.000 description 2
- 235000010643 Leucaena leucocephala Nutrition 0.000 description 2
- WHXSMMKQMYFTQS-UHFFFAOYSA-N Lithium Chemical compound [Li] WHXSMMKQMYFTQS-UHFFFAOYSA-N 0.000 description 2
- FZIJIFCXUCZHOL-CIUDSAMLSA-N Lys-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN FZIJIFCXUCZHOL-CIUDSAMLSA-N 0.000 description 2
- IRNSXVOWSXSULE-DCAQKATOSA-N Lys-Ala-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCCN IRNSXVOWSXSULE-DCAQKATOSA-N 0.000 description 2
- FLCMXEFCTLXBTL-DCAQKATOSA-N Lys-Asp-Arg Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)N FLCMXEFCTLXBTL-DCAQKATOSA-N 0.000 description 2
- WBSCNDJQPKSPII-KKUMJFAQSA-N Lys-Lys-Lys Chemical compound NCCCC[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O WBSCNDJQPKSPII-KKUMJFAQSA-N 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- XUMBMVFBXHLACL-UHFFFAOYSA-N Melanin Chemical compound O=C1C(=O)C(C2=CNC3=C(C(C(=O)C4=C32)=O)C)=C2C4=CNC2=C1C XUMBMVFBXHLACL-UHFFFAOYSA-N 0.000 description 2
- 101800001751 Melanocyte-stimulating hormone alpha Proteins 0.000 description 2
- MVQGZYIOMXAFQG-GUBZILKMSA-N Met-Ala-Arg Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@H](C(O)=O)CCCNC(N)=N MVQGZYIOMXAFQG-GUBZILKMSA-N 0.000 description 2
- CTVJSFRHUOSCQQ-DCAQKATOSA-N Met-Arg-Glu Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O CTVJSFRHUOSCQQ-DCAQKATOSA-N 0.000 description 2
- NSGXXVIHCIAISP-CIUDSAMLSA-N Met-Asn-Gln Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O NSGXXVIHCIAISP-CIUDSAMLSA-N 0.000 description 2
- FWAHLGXNBLWIKB-NAKRPEOUSA-N Met-Ile-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@@H](N)CCSC FWAHLGXNBLWIKB-NAKRPEOUSA-N 0.000 description 2
- KMSMNUFBNCHMII-IHRRRGAJSA-N Met-Leu-Lys Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](C(O)=O)CCCCN KMSMNUFBNCHMII-IHRRRGAJSA-N 0.000 description 2
- CERQOIWHTDAKMF-UHFFFAOYSA-M Methacrylate Chemical compound CC(=C)C([O-])=O CERQOIWHTDAKMF-UHFFFAOYSA-M 0.000 description 2
- BAVYZALUXZFZLV-UHFFFAOYSA-N Methylamine Chemical compound NC BAVYZALUXZFZLV-UHFFFAOYSA-N 0.000 description 2
- 229920000168 Microcrystalline cellulose Polymers 0.000 description 2
- 102000029749 Microtubule Human genes 0.000 description 2
- 108091022875 Microtubule Proteins 0.000 description 2
- 101710115937 Microtubule-associated protein tau Proteins 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- PVNIIMVLHYAWGP-UHFFFAOYSA-N Niacin Chemical compound OC(=O)C1=CC=CN=C1 PVNIIMVLHYAWGP-UHFFFAOYSA-N 0.000 description 2
- 102000002512 Orexin Human genes 0.000 description 2
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 2
- WYPVCIACUMJRIB-JYJNAYRXSA-N Phe-Gln-Lys Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCCCN)C(=O)O)N WYPVCIACUMJRIB-JYJNAYRXSA-N 0.000 description 2
- CDQCFGOQNYOICK-IHRRRGAJSA-N Phe-Glu-Gln Chemical compound NC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=CC=C1 CDQCFGOQNYOICK-IHRRRGAJSA-N 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 208000000609 Pick Disease of the Brain Diseases 0.000 description 2
- GLUUGHFHXGJENI-UHFFFAOYSA-N Piperazine Chemical compound C1CNCCN1 GLUUGHFHXGJENI-UHFFFAOYSA-N 0.000 description 2
- NQRYJNQNLNOLGT-UHFFFAOYSA-N Piperidine Chemical compound C1CCNCC1 NQRYJNQNLNOLGT-UHFFFAOYSA-N 0.000 description 2
- 229920002732 Polyanhydride Polymers 0.000 description 2
- 102100022033 Presenilin-1 Human genes 0.000 description 2
- UTAUEDINXUMHLG-FXQIFTODSA-N Pro-Asp-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@@H]1CCCN1 UTAUEDINXUMHLG-FXQIFTODSA-N 0.000 description 2
- GZNYIXWOIUFLGO-ZJDVBMNYSA-N Pro-Thr-Thr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GZNYIXWOIUFLGO-ZJDVBMNYSA-N 0.000 description 2
- 102100027467 Pro-opiomelanocortin Human genes 0.000 description 2
- VYGQUTWHTHXGQB-FFHKNEKCSA-N Retinol Palmitate Chemical compound CCCCCCCCCCCCCCCC(=O)OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C VYGQUTWHTHXGQB-FFHKNEKCSA-N 0.000 description 2
- HZWAHWQZPSXNCB-BPUTZDHNSA-N Ser-Arg-Trp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC1=CNC2=C1C=CC=C2)C(O)=O HZWAHWQZPSXNCB-BPUTZDHNSA-N 0.000 description 2
- MESDJCNHLZBMEP-ZLUOBGJFSA-N Ser-Asp-Asp Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O MESDJCNHLZBMEP-ZLUOBGJFSA-N 0.000 description 2
- YQQKYAZABFEYAF-FXQIFTODSA-N Ser-Glu-Gln Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O YQQKYAZABFEYAF-FXQIFTODSA-N 0.000 description 2
- OQPNSDWGAMFJNU-QWRGUYRKSA-N Ser-Gly-Tyr Chemical compound OC[C@H](N)C(=O)NCC(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 OQPNSDWGAMFJNU-QWRGUYRKSA-N 0.000 description 2
- BEAFYHFQTOTVFS-VGDYDELISA-N Ser-Ile-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)NC(=O)[C@H](CO)N BEAFYHFQTOTVFS-VGDYDELISA-N 0.000 description 2
- MUJQWSAWLLRJCE-KATARQTJSA-N Ser-Leu-Thr Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(O)=O MUJQWSAWLLRJCE-KATARQTJSA-N 0.000 description 2
- OCWWJBZQXGYQCA-DCAQKATOSA-N Ser-Lys-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(O)=O OCWWJBZQXGYQCA-DCAQKATOSA-N 0.000 description 2
- ANOQEBQWIAYIMV-AEJSXWLSSA-N Ser-Val-Pro Chemical compound CC(C)[C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N ANOQEBQWIAYIMV-AEJSXWLSSA-N 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 2
- 229920002125 Sokalan® Polymers 0.000 description 2
- 101000857870 Squalus acanthias Gonadoliberin Proteins 0.000 description 2
- 235000021355 Stearic acid Nutrition 0.000 description 2
- 102000009822 Sterol Regulatory Element Binding Proteins Human genes 0.000 description 2
- 108010020396 Sterol Regulatory Element Binding Proteins Proteins 0.000 description 2
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 2
- 229930006000 Sucrose Natural products 0.000 description 2
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 2
- 239000004098 Tetracycline Substances 0.000 description 2
- BSNZTJXVDOINSR-JXUBOQSCSA-N Thr-Ala-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(O)=O BSNZTJXVDOINSR-JXUBOQSCSA-N 0.000 description 2
- GZYNMZQXFRWDFH-YTWAJWBKSA-N Thr-Arg-Pro Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)N1CCC[C@@H]1C(=O)O)N)O GZYNMZQXFRWDFH-YTWAJWBKSA-N 0.000 description 2
- AYCQVUUPIJHJTA-IXOXFDKPSA-N Thr-His-Leu Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(O)=O AYCQVUUPIJHJTA-IXOXFDKPSA-N 0.000 description 2
- MXDOAJQRJBMGMO-FJXKBIBVSA-N Thr-Pro-Gly Chemical compound C[C@@H](O)[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O MXDOAJQRJBMGMO-FJXKBIBVSA-N 0.000 description 2
- LECUEEHKUFYOOV-ZJDVBMNYSA-N Thr-Thr-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)O)NC(=O)[C@@H](N)[C@@H](C)O LECUEEHKUFYOOV-ZJDVBMNYSA-N 0.000 description 2
- ILUOMMDDGREELW-OSUNSFLBSA-N Thr-Val-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)[C@@H](C)O ILUOMMDDGREELW-OSUNSFLBSA-N 0.000 description 2
- 239000000627 Thyrotropin-Releasing Hormone Substances 0.000 description 2
- 101800004623 Thyrotropin-releasing hormone Proteins 0.000 description 2
- 102400000336 Thyrotropin-releasing hormone Human genes 0.000 description 2
- CZWIHKFGHICAJX-BPUTZDHNSA-N Trp-Glu-Glu Chemical compound C1=CC=C2C(C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1 CZWIHKFGHICAJX-BPUTZDHNSA-N 0.000 description 2
- UPOGHWJJZAZNSW-XIRDDKMYSA-N Trp-His-Ser Chemical compound [H]N[C@@H](CC1=CNC2=C1C=CC=C2)C(=O)N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CO)C(O)=O UPOGHWJJZAZNSW-XIRDDKMYSA-N 0.000 description 2
- 102000004243 Tubulin Human genes 0.000 description 2
- 108090000704 Tubulin Proteins 0.000 description 2
- XQYHLZNPOTXRMQ-KKUMJFAQSA-N Tyr-Glu-Arg Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O XQYHLZNPOTXRMQ-KKUMJFAQSA-N 0.000 description 2
- HIINQLBHPIQYHN-JTQLQIEISA-N Tyr-Gly-Gly Chemical compound OC(=O)CNC(=O)CNC(=O)[C@@H](N)CC1=CC=C(O)C=C1 HIINQLBHPIQYHN-JTQLQIEISA-N 0.000 description 2
- KSCVLGXNQXKUAR-JYJNAYRXSA-N Tyr-Leu-Glu Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O KSCVLGXNQXKUAR-JYJNAYRXSA-N 0.000 description 2
- CWVHKVVKAQIJKY-ACRUOGEOSA-N Tyr-Lys-Phe Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC2=CC=C(C=C2)O)N CWVHKVVKAQIJKY-ACRUOGEOSA-N 0.000 description 2
- LMKKMCGTDANZTR-BZSNNMDCSA-N Tyr-Phe-Asp Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CC(O)=O)C(O)=O)C1=CC=C(O)C=C1 LMKKMCGTDANZTR-BZSNNMDCSA-N 0.000 description 2
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 2
- ISERLACIZUGCDX-ZKWXMUAHSA-N Val-Asp-Ala Chemical compound C[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)O)NC(=O)[C@H](C(C)C)N ISERLACIZUGCDX-ZKWXMUAHSA-N 0.000 description 2
- BMGOFDMKDVVGJG-NHCYSSNCSA-N Val-Asp-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)O)N BMGOFDMKDVVGJG-NHCYSSNCSA-N 0.000 description 2
- FPCIBLUVDNXPJO-XPUUQOCRSA-N Val-Cys-Gly Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CS)C(=O)NCC(O)=O FPCIBLUVDNXPJO-XPUUQOCRSA-N 0.000 description 2
- XGJLNBNZNMVJRS-NRPADANISA-N Val-Glu-Ala Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O XGJLNBNZNMVJRS-NRPADANISA-N 0.000 description 2
- WDIGUPHXPBMODF-UMNHJUIQSA-N Val-Glu-Pro Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N1CCC[C@@H]1C(=O)O)N WDIGUPHXPBMODF-UMNHJUIQSA-N 0.000 description 2
- APQIVBCUIUDSMB-OSUNSFLBSA-N Val-Ile-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H]([C@@H](C)O)C(=O)O)NC(=O)[C@H](C(C)C)N APQIVBCUIUDSMB-OSUNSFLBSA-N 0.000 description 2
- IEBGHUMBJXIXHM-AVGNSLFASA-N Val-Lys-Met Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCSC)C(=O)O)N IEBGHUMBJXIXHM-AVGNSLFASA-N 0.000 description 2
- UVHFONIHVHLDDQ-IFFSRLJSSA-N Val-Thr-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](C(C)C)N)O UVHFONIHVHLDDQ-IFFSRLJSSA-N 0.000 description 2
- 229930003268 Vitamin C Natural products 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 235000010489 acacia gum Nutrition 0.000 description 2
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 2
- RJURFGZVJUQBHK-UHFFFAOYSA-N actinomycin D Natural products CC1OC(=O)C(C(C)C)N(C)C(=O)CN(C)C(=O)C2CCCN2C(=O)C(C(C)C)NC(=O)C1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)NC4C(=O)NC(C(N5CCCC5C(=O)N(C)CC(=O)N(C)C(C(C)C)C(=O)OC4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-UHFFFAOYSA-N 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000000853 adhesive Substances 0.000 description 2
- 230000001070 adhesive effect Effects 0.000 description 2
- 210000000577 adipose tissue Anatomy 0.000 description 2
- 238000012387 aerosolization Methods 0.000 description 2
- 239000008272 agar Substances 0.000 description 2
- 235000010419 agar Nutrition 0.000 description 2
- 230000002776 aggregation Effects 0.000 description 2
- 238000004220 aggregation Methods 0.000 description 2
- 108010044940 alanylglutamine Proteins 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 150000001342 alkaline earth metals Chemical class 0.000 description 2
- 125000005907 alkyl ester group Chemical group 0.000 description 2
- 229910052782 aluminium Inorganic materials 0.000 description 2
- XAGFODPZIPBFFR-UHFFFAOYSA-N aluminium Chemical compound [Al] XAGFODPZIPBFFR-UHFFFAOYSA-N 0.000 description 2
- 229910021529 ammonia Inorganic materials 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 239000008365 aqueous carrier Substances 0.000 description 2
- 239000008346 aqueous phase Substances 0.000 description 2
- 108010001271 arginyl-glutamyl-arginine Proteins 0.000 description 2
- WZPBZJONDBGPKJ-VEHQQRBSSA-N aztreonam Chemical compound O=C1N(S([O-])(=O)=O)[C@@H](C)[C@@H]1NC(=O)C(=N/OC(C)(C)C(O)=O)\C1=CSC([NH3+])=N1 WZPBZJONDBGPKJ-VEHQQRBSSA-N 0.000 description 2
- 230000004888 barrier function Effects 0.000 description 2
- SRSXLGNVWSONIS-UHFFFAOYSA-N benzenesulfonic acid Chemical compound OS(=O)(=O)C1=CC=CC=C1 SRSXLGNVWSONIS-UHFFFAOYSA-N 0.000 description 2
- ISAOCJYIOMOJEB-UHFFFAOYSA-N benzoin Chemical compound C=1C=CC=CC=1C(O)C(=O)C1=CC=CC=C1 ISAOCJYIOMOJEB-UHFFFAOYSA-N 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- 210000005013 brain tissue Anatomy 0.000 description 2
- 125000000484 butyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- OSGAYBCDTDRGGQ-UHFFFAOYSA-L calcium sulfate Chemical compound [Ca+2].[O-]S([O-])(=O)=O OSGAYBCDTDRGGQ-UHFFFAOYSA-L 0.000 description 2
- 239000001768 carboxy methyl cellulose Substances 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 229960004926 chlorobutanol Drugs 0.000 description 2
- 235000012000 cholesterol Nutrition 0.000 description 2
- MYSWGUAQZAJSOK-UHFFFAOYSA-N ciprofloxacin Chemical compound C12=CC(N3CCNCC3)=C(F)C=C2C(=O)C(C(=O)O)=CN1C1CC1 MYSWGUAQZAJSOK-UHFFFAOYSA-N 0.000 description 2
- 239000011248 coating agent Substances 0.000 description 2
- 239000005515 coenzyme Substances 0.000 description 2
- 238000002591 computed tomography Methods 0.000 description 2
- 229940041967 corticotropin-releasing hormone Drugs 0.000 description 2
- KLVRDXBAMSPYKH-RKYZNNDCSA-N corticotropin-releasing hormone (human) Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(N)=O)[C@@H](C)CC)NC(=O)[C@H](C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](C)NC(=O)[C@H](CCSC)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CC=1C=CC=CC=1)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)[C@H]1N(CCC1)C(=O)[C@H]1N(CCC1)C(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CO)[C@@H](C)CC)C(C)C)C(C)C)C1=CNC=N1 KLVRDXBAMSPYKH-RKYZNNDCSA-N 0.000 description 2
- 239000006071 cream Substances 0.000 description 2
- VFLDPWHFBUODDF-FCXRPNKRSA-N curcumin Chemical compound C1=C(O)C(OC)=CC(\C=C\C(=O)CC(=O)\C=C\C=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-FCXRPNKRSA-N 0.000 description 2
- 108010038764 cytoplasmic linker protein 170 Proteins 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 229960003957 dexamethasone Drugs 0.000 description 2
- 206010012601 diabetes mellitus Diseases 0.000 description 2
- XBDQKXXYIPTUBI-UHFFFAOYSA-N dimethylselenoniopropionate Natural products CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 2
- 239000013583 drug formulation Substances 0.000 description 2
- 239000012636 effector Substances 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 230000019439 energy homeostasis Effects 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 229940088598 enzyme Drugs 0.000 description 2
- 230000003628 erosive effect Effects 0.000 description 2
- BEFDCLMNVWHSGT-UHFFFAOYSA-N ethenylcyclopentane Chemical compound C=CC1CCCC1 BEFDCLMNVWHSGT-UHFFFAOYSA-N 0.000 description 2
- 229940093471 ethyl oleate Drugs 0.000 description 2
- 230000035558 fertility Effects 0.000 description 2
- 239000000796 flavoring agent Substances 0.000 description 2
- LPEPZBJOKDYZAD-UHFFFAOYSA-N flufenamic acid Chemical compound OC(=O)C1=CC=CC=C1NC1=CC=CC(C(F)(F)F)=C1 LPEPZBJOKDYZAD-UHFFFAOYSA-N 0.000 description 2
- SYTBZMRGLBWNTM-UHFFFAOYSA-N flurbiprofen Chemical compound FC1=CC(C(C(O)=O)C)=CC=C1C1=CC=CC=C1 SYTBZMRGLBWNTM-UHFFFAOYSA-N 0.000 description 2
- 239000003205 fragrance Substances 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 239000007903 gelatin capsule Substances 0.000 description 2
- RWSXRVCMGQZWBV-WDSKDSINSA-N glutathione Chemical compound OC(=O)[C@@H](N)CCC(=O)N[C@@H](CS)C(=O)NCC(O)=O RWSXRVCMGQZWBV-WDSKDSINSA-N 0.000 description 2
- 108010050848 glycylleucine Proteins 0.000 description 2
- XLXSAKCOAKORKW-AQJXLSMYSA-N gonadorelin Chemical compound C([C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)NCC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H]1NC(=O)CC1)C1=CC=C(O)C=C1 XLXSAKCOAKORKW-AQJXLSMYSA-N 0.000 description 2
- 229940035638 gonadotropin-releasing hormone Drugs 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- 238000003306 harvesting Methods 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- BXWNKGSJHAJOGX-UHFFFAOYSA-N hexadecan-1-ol Chemical compound CCCCCCCCCCCCCCCCO BXWNKGSJHAJOGX-UHFFFAOYSA-N 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 239000000017 hydrogel Substances 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 235000010979 hydroxypropyl methyl cellulose Nutrition 0.000 description 2
- 239000001866 hydroxypropyl methyl cellulose Substances 0.000 description 2
- 229920003088 hydroxypropyl methyl cellulose Polymers 0.000 description 2
- UFVKGYZPFZQRLF-UHFFFAOYSA-N hydroxypropyl methyl cellulose Chemical compound OC1C(O)C(OC)OC(CO)C1OC1C(O)C(O)C(OC2C(C(O)C(OC3C(C(O)C(O)C(CO)O3)O)C(CO)O2)O)C(CO)O1 UFVKGYZPFZQRLF-UHFFFAOYSA-N 0.000 description 2
- 230000001771 impaired effect Effects 0.000 description 2
- 238000001727 in vivo Methods 0.000 description 2
- 229960000905 indomethacin Drugs 0.000 description 2
- 239000004615 ingredient Substances 0.000 description 2
- 230000003933 intellectual function Effects 0.000 description 2
- 238000001361 intraarterial administration Methods 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 108010044374 isoleucyl-tyrosine Proteins 0.000 description 2
- 108010078274 isoleucylvaline Proteins 0.000 description 2
- FZWBNHMXJMCXLU-BLAUPYHCSA-N isomaltotriose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C=O)O1 FZWBNHMXJMCXLU-BLAUPYHCSA-N 0.000 description 2
- 238000010902 jet-milling Methods 0.000 description 2
- JVTAAEKCZFNVCJ-UHFFFAOYSA-N lactic acid Chemical compound CC(O)C(O)=O JVTAAEKCZFNVCJ-UHFFFAOYSA-N 0.000 description 2
- 108010034529 leucyl-lysine Proteins 0.000 description 2
- 229910052744 lithium Inorganic materials 0.000 description 2
- 239000007937 lozenge Substances 0.000 description 2
- 238000009593 lumbar puncture Methods 0.000 description 2
- 108010054155 lysyllysine Proteins 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 235000019359 magnesium stearate Nutrition 0.000 description 2
- 239000011976 maleic acid Substances 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 239000011159 matrix material Substances 0.000 description 2
- 230000003340 mental effect Effects 0.000 description 2
- 229910052751 metal Inorganic materials 0.000 description 2
- 239000002184 metal Substances 0.000 description 2
- BDAGIHXWWSANSR-UHFFFAOYSA-N methanoic acid Natural products OC=O BDAGIHXWWSANSR-UHFFFAOYSA-N 0.000 description 2
- 229920000609 methyl cellulose Polymers 0.000 description 2
- 239000001923 methylcellulose Substances 0.000 description 2
- 235000010981 methylcellulose Nutrition 0.000 description 2
- 235000019813 microcrystalline cellulose Nutrition 0.000 description 2
- 229940016286 microcrystalline cellulose Drugs 0.000 description 2
- 239000008108 microcrystalline cellulose Substances 0.000 description 2
- 210000004688 microtubule Anatomy 0.000 description 2
- 239000002480 mineral oil Substances 0.000 description 2
- 239000007758 minimum essential medium Substances 0.000 description 2
- 239000001788 mono and diglycerides of fatty acids Substances 0.000 description 2
- 229940126619 mouse monoclonal antibody Drugs 0.000 description 2
- 210000004400 mucous membrane Anatomy 0.000 description 2
- CMWTZPSULFXXJA-VIFPVBQESA-N naproxen Chemical compound C1=C([C@H](C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-VIFPVBQESA-N 0.000 description 2
- 229920005615 natural polymer Polymers 0.000 description 2
- 230000003557 neuropsychological effect Effects 0.000 description 2
- NQDJXKOVJZTUJA-UHFFFAOYSA-N nevirapine Chemical compound C12=NC=CC=C2C(=O)NC=2C(C)=CC=NC=2N1C1CC1 NQDJXKOVJZTUJA-UHFFFAOYSA-N 0.000 description 2
- DFPAKSUCGFBDDF-UHFFFAOYSA-N nicotinic acid amide Natural products NC(=O)C1=CC=CN=C1 DFPAKSUCGFBDDF-UHFFFAOYSA-N 0.000 description 2
- 108020004707 nucleic acids Proteins 0.000 description 2
- 102000039446 nucleic acids Human genes 0.000 description 2
- 150000007523 nucleic acids Chemical class 0.000 description 2
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 2
- 108060005714 orexin Proteins 0.000 description 2
- 150000007524 organic acids Chemical class 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- FJKROLUGYXJWQN-UHFFFAOYSA-N papa-hydroxy-benzoic acid Natural products OC(=O)C1=CC=C(O)C=C1 FJKROLUGYXJWQN-UHFFFAOYSA-N 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 230000008447 perception Effects 0.000 description 2
- 230000002093 peripheral effect Effects 0.000 description 2
- 230000004526 pharmaceutical effect Effects 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- IVBHGBMCVLDMKU-GXNBUGAJSA-N piperacillin Chemical compound O=C1C(=O)N(CC)CCN1C(=O)N[C@H](C=1C=CC=CC=1)C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 IVBHGBMCVLDMKU-GXNBUGAJSA-N 0.000 description 2
- 229960002292 piperacillin Drugs 0.000 description 2
- 229950010765 pivalate Drugs 0.000 description 2
- IUGYQRQAERSCNH-UHFFFAOYSA-N pivalic acid Chemical compound CC(C)(C)C(O)=O IUGYQRQAERSCNH-UHFFFAOYSA-N 0.000 description 2
- 229920001282 polysaccharide Polymers 0.000 description 2
- 239000005017 polysaccharide Substances 0.000 description 2
- 150000004804 polysaccharides Chemical class 0.000 description 2
- 229920000136 polysorbate Polymers 0.000 description 2
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 2
- 230000002035 prolonged effect Effects 0.000 description 2
- 108010053725 prolylvaline Proteins 0.000 description 2
- 239000003380 propellant Substances 0.000 description 2
- 125000001436 propyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])[H] 0.000 description 2
- XNSAINXGIQZQOO-SRVKXCTJSA-N protirelin Chemical compound NC(=O)[C@@H]1CCCN1C(=O)[C@@H](NC(=O)[C@H]1NC(=O)CC1)CC1=CN=CN1 XNSAINXGIQZQOO-SRVKXCTJSA-N 0.000 description 2
- 230000002685 pulmonary effect Effects 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- WKSAUQYGYAYLPV-UHFFFAOYSA-N pyrimethamine Chemical compound CCC1=NC(N)=NC(N)=C1C1=CC=C(Cl)C=C1 WKSAUQYGYAYLPV-UHFFFAOYSA-N 0.000 description 2
- LOUPRKONTZGTKE-LHHVKLHASA-N quinidine Chemical compound C([C@H]([C@H](C1)C=C)C2)C[N@@]1[C@H]2[C@@H](O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-LHHVKLHASA-N 0.000 description 2
- 210000000664 rectum Anatomy 0.000 description 2
- 230000001105 regulatory effect Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 229960004889 salicylic acid Drugs 0.000 description 2
- 238000000926 separation method Methods 0.000 description 2
- 210000002966 serum Anatomy 0.000 description 2
- 239000008159 sesame oil Substances 0.000 description 2
- 235000011803 sesame oil Nutrition 0.000 description 2
- 235000010413 sodium alginate Nutrition 0.000 description 2
- 239000000661 sodium alginate Substances 0.000 description 2
- 229940005550 sodium alginate Drugs 0.000 description 2
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 2
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 2
- 235000019333 sodium laurylsulphate Nutrition 0.000 description 2
- 229940075554 sorbate Drugs 0.000 description 2
- 235000010199 sorbic acid Nutrition 0.000 description 2
- 239000004334 sorbic acid Substances 0.000 description 2
- 229940075582 sorbic acid Drugs 0.000 description 2
- 235000011069 sorbitan monooleate Nutrition 0.000 description 2
- 239000001593 sorbitan monooleate Substances 0.000 description 2
- 229940035049 sorbitan monooleate Drugs 0.000 description 2
- 238000010561 standard procedure Methods 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 239000008117 stearic acid Substances 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 125000001424 substituent group Chemical group 0.000 description 2
- 239000005720 sucrose Substances 0.000 description 2
- 239000000829 suppository Substances 0.000 description 2
- 239000004094 surface-active agent Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 230000002459 sustained effect Effects 0.000 description 2
- 210000000225 synapse Anatomy 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- 229920001059 synthetic polymer Polymers 0.000 description 2
- 230000009885 systemic effect Effects 0.000 description 2
- 235000002906 tartaric acid Nutrition 0.000 description 2
- 239000011975 tartaric acid Substances 0.000 description 2
- 235000019364 tetracycline Nutrition 0.000 description 2
- 150000003522 tetracyclines Chemical class 0.000 description 2
- 238000011287 therapeutic dose Methods 0.000 description 2
- 238000011285 therapeutic regimen Methods 0.000 description 2
- 239000002562 thickening agent Substances 0.000 description 2
- 108010061238 threonyl-glycine Proteins 0.000 description 2
- 229940034199 thyrotropin-releasing hormone Drugs 0.000 description 2
- 229930003799 tocopherol Natural products 0.000 description 2
- 235000010384 tocopherol Nutrition 0.000 description 2
- 229960001295 tocopherol Drugs 0.000 description 2
- 239000011732 tocopherol Substances 0.000 description 2
- 235000010487 tragacanth Nutrition 0.000 description 2
- 239000000196 tragacanth Substances 0.000 description 2
- 229940116362 tragacanth Drugs 0.000 description 2
- 230000032258 transport Effects 0.000 description 2
- URAYPUMNDPQOKB-UHFFFAOYSA-N triacetin Chemical compound CC(=O)OCC(OC(C)=O)COC(C)=O URAYPUMNDPQOKB-UHFFFAOYSA-N 0.000 description 2
- GETQZCLCWQTVFV-UHFFFAOYSA-N trimethylamine Chemical compound CN(C)C GETQZCLCWQTVFV-UHFFFAOYSA-N 0.000 description 2
- 239000003656 tris buffered saline Substances 0.000 description 2
- 108010044292 tryptophyltyrosine Proteins 0.000 description 2
- 108010017949 tyrosyl-glycyl-glycine Proteins 0.000 description 2
- 238000009827 uniform distribution Methods 0.000 description 2
- 229940070710 valerate Drugs 0.000 description 2
- NQPDZGIKBAWPEJ-UHFFFAOYSA-N valeric acid Chemical compound CCCCC(O)=O NQPDZGIKBAWPEJ-UHFFFAOYSA-N 0.000 description 2
- 235000019154 vitamin C Nutrition 0.000 description 2
- 239000011718 vitamin C Substances 0.000 description 2
- GVJHHUAWPYXKBD-IEOSBIPESA-N α-tocopherol Chemical compound OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-IEOSBIPESA-N 0.000 description 2
- HDTRYLNUVZCQOY-UHFFFAOYSA-N α-D-glucopyranosyl-α-D-glucopyranoside Natural products OC1C(O)C(O)C(CO)OC1OC1C(O)C(O)C(O)C(CO)O1 HDTRYLNUVZCQOY-UHFFFAOYSA-N 0.000 description 1
- LSPHULWDVZXLIL-UHFFFAOYSA-N (+/-)-Camphoric acid Chemical class CC1(C)C(C(O)=O)CCC1(C)C(O)=O LSPHULWDVZXLIL-UHFFFAOYSA-N 0.000 description 1
- SNICXCGAKADSCV-JTQLQIEISA-N (-)-Nicotine Chemical compound CN1CCC[C@H]1C1=CC=CN=C1 SNICXCGAKADSCV-JTQLQIEISA-N 0.000 description 1
- YKSVGLFNJPQDJE-YDMQLZBCSA-N (19E,21E,23E,25E,27E,29E,31E)-33-[(2R,3S,4R,5S,6R)-4-amino-3,5-dihydroxy-6-methyloxan-2-yl]oxy-17-[7-(4-aminophenyl)-5-hydroxy-4-methyl-7-oxoheptan-2-yl]-1,3,5,7,37-pentahydroxy-18-methyl-9,13,15-trioxo-16,39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid Chemical compound CC(CC(C)C1OC(=O)CC(=O)CCCC(=O)CC(O)CC(O)CC(O)CC2(O)CC(O)C(C(CC(O[C@@H]3O[C@H](C)[C@@H](O)[C@@H](N)[C@@H]3O)\C=C\C=C\C=C\C=C\C=C\C=C\C=C\C1C)O2)C(O)=O)C(O)CC(=O)C1=CC=C(N)C=C1 YKSVGLFNJPQDJE-YDMQLZBCSA-N 0.000 description 1
- YLJXZSWHZFXCDY-RQJHMYQMSA-N (1r,3s)-3-amino-n-(3-amino-3-iminopropyl)cyclopentane-1-carboxamide Chemical compound N[C@H]1CC[C@@H](C(=O)NCCC(N)=N)C1 YLJXZSWHZFXCDY-RQJHMYQMSA-N 0.000 description 1
- MNULEGDCPYONBU-WMBHJXFZSA-N (1r,4s,5e,5'r,6'r,7e,10s,11r,12s,14r,15s,16s,18r,19s,20r,21e,25s,26r,27s,29s)-4-ethyl-11,12,15,19-tetrahydroxy-6'-[(2s)-2-hydroxypropyl]-5',10,12,14,16,18,20,26,29-nonamethylspiro[24,28-dioxabicyclo[23.3.1]nonacosa-5,7,21-triene-27,2'-oxane]-13,17,23-trio Polymers O([C@@H]1CC[C@@H](/C=C/C=C/C[C@H](C)[C@@H](O)[C@](C)(O)C(=O)[C@H](C)[C@@H](O)[C@H](C)C(=O)[C@H](C)[C@@H](O)[C@H](C)/C=C/C(=O)O[C@H]([C@H]2C)[C@H]1C)CC)[C@]12CC[C@@H](C)[C@@H](C[C@H](C)O)O1 MNULEGDCPYONBU-WMBHJXFZSA-N 0.000 description 1
- MNULEGDCPYONBU-DJRUDOHVSA-N (1s,4r,5z,5'r,6'r,7e,10s,11r,12s,14r,15s,18r,19r,20s,21e,26r,27s)-4-ethyl-11,12,15,19-tetrahydroxy-6'-(2-hydroxypropyl)-5',10,12,14,16,18,20,26,29-nonamethylspiro[24,28-dioxabicyclo[23.3.1]nonacosa-5,7,21-triene-27,2'-oxane]-13,17,23-trione Polymers O([C@H]1CC[C@H](\C=C/C=C/C[C@H](C)[C@@H](O)[C@](C)(O)C(=O)[C@H](C)[C@@H](O)C(C)C(=O)[C@H](C)[C@H](O)[C@@H](C)/C=C/C(=O)OC([C@H]2C)C1C)CC)[C@]12CC[C@@H](C)[C@@H](CC(C)O)O1 MNULEGDCPYONBU-DJRUDOHVSA-N 0.000 description 1
- BLSQLHNBWJLIBQ-OZXSUGGESA-N (2R,4S)-terconazole Chemical compound C1CN(C(C)C)CCN1C(C=C1)=CC=C1OC[C@@H]1O[C@@](CN2N=CN=C2)(C=2C(=CC(Cl)=CC=2)Cl)OC1 BLSQLHNBWJLIBQ-OZXSUGGESA-N 0.000 description 1
- RJMIEHBSYVWVIN-LLVKDONJSA-N (2r)-2-[4-(3-oxo-1h-isoindol-2-yl)phenyl]propanoic acid Chemical compound C1=CC([C@H](C(O)=O)C)=CC=C1N1C(=O)C2=CC=CC=C2C1 RJMIEHBSYVWVIN-LLVKDONJSA-N 0.000 description 1
- JNYAEWCLZODPBN-JGWLITMVSA-N (2r,3r,4s)-2-[(1r)-1,2-dihydroxyethyl]oxolane-3,4-diol Chemical class OC[C@@H](O)[C@H]1OC[C@H](O)[C@H]1O JNYAEWCLZODPBN-JGWLITMVSA-N 0.000 description 1
- LNAZSHAWQACDHT-XIYTZBAFSA-N (2r,3r,4s,5r,6s)-4,5-dimethoxy-2-(methoxymethyl)-3-[(2s,3r,4s,5r,6r)-3,4,5-trimethoxy-6-(methoxymethyl)oxan-2-yl]oxy-6-[(2r,3r,4s,5r,6r)-4,5,6-trimethoxy-2-(methoxymethyl)oxan-3-yl]oxyoxane Chemical compound CO[C@@H]1[C@@H](OC)[C@H](OC)[C@@H](COC)O[C@H]1O[C@H]1[C@H](OC)[C@@H](OC)[C@H](O[C@H]2[C@@H]([C@@H](OC)[C@H](OC)O[C@@H]2COC)OC)O[C@@H]1COC LNAZSHAWQACDHT-XIYTZBAFSA-N 0.000 description 1
- SICITCLFXRGKJW-IIZANFQQSA-N (2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2-[[(2s)-2,6-diaminohexanoyl]amino]-4-methylpentanoyl]amino]-3-methylbutanoyl]amino]-3-phenylpropanoyl]amino]-3-phenylpropanoic acid Chemical compound C([C@H](NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)[C@@H](N)CCCCN)CC(C)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CC=CC=C1 SICITCLFXRGKJW-IIZANFQQSA-N 0.000 description 1
- NNRXCKZMQLFUPL-WBMZRJHASA-N (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione;(2r,3 Chemical compound OC(=O)[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O.O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 NNRXCKZMQLFUPL-WBMZRJHASA-N 0.000 description 1
- ZXBDZLHAHGPXIG-VTXLJDRKSA-N (3r,4s,5s,6r,7r,9r,11r,12r,13s,14r)-6-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-14-ethyl-7,12,13-trihydroxy-4-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione;(2r,3 Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O)C(O)=O.O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 ZXBDZLHAHGPXIG-VTXLJDRKSA-N 0.000 description 1
- MNULEGDCPYONBU-YNZHUHFTSA-N (4Z,18Z,20Z)-22-ethyl-7,11,14,15-tetrahydroxy-6'-(2-hydroxypropyl)-5',6,8,10,12,14,16,28,29-nonamethylspiro[2,26-dioxabicyclo[23.3.1]nonacosa-4,18,20-triene-27,2'-oxane]-3,9,13-trione Polymers CC1C(C2C)OC(=O)\C=C/C(C)C(O)C(C)C(=O)C(C)C(O)C(C)C(=O)C(C)(O)C(O)C(C)C\C=C/C=C\C(CC)CCC2OC21CCC(C)C(CC(C)O)O2 MNULEGDCPYONBU-YNZHUHFTSA-N 0.000 description 1
- SGKRLCUYIXIAHR-AKNGSSGZSA-N (4s,4ar,5s,5ar,6r,12ar)-4-(dimethylamino)-1,5,10,11,12a-pentahydroxy-6-methyl-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1=CC=C2[C@H](C)[C@@H]([C@H](O)[C@@H]3[C@](C(O)=C(C(N)=O)C(=O)[C@H]3N(C)C)(O)C3=O)C3=C(O)C2=C1O SGKRLCUYIXIAHR-AKNGSSGZSA-N 0.000 description 1
- FFTVPQUHLQBXQZ-KVUCHLLUSA-N (4s,4as,5ar,12ar)-4,7-bis(dimethylamino)-1,10,11,12a-tetrahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide Chemical compound C1C2=C(N(C)C)C=CC(O)=C2C(O)=C2[C@@H]1C[C@H]1[C@H](N(C)C)C(=O)C(C(N)=O)=C(O)[C@@]1(O)C2=O FFTVPQUHLQBXQZ-KVUCHLLUSA-N 0.000 description 1
- MNULEGDCPYONBU-VVXVDZGXSA-N (5e,5'r,7e,10s,11r,12s,14s,15r,16r,18r,19s,20r,21e,26r,29s)-4-ethyl-11,12,15,19-tetrahydroxy-6'-[(2s)-2-hydroxypropyl]-5',10,12,14,16,18,20,26,29-nonamethylspiro[24,28-dioxabicyclo[23.3.1]nonacosa-5,7,21-triene-27,2'-oxane]-13,17,23-trione Polymers C([C@H](C)[C@@H](O)[C@](C)(O)C(=O)[C@@H](C)[C@H](O)[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@H](C)/C=C/C(=O)OC([C@H]1C)[C@H]2C)\C=C\C=C\C(CC)CCC2OC21CC[C@@H](C)C(C[C@H](C)O)O2 MNULEGDCPYONBU-VVXVDZGXSA-N 0.000 description 1
- GPYKKBAAPVOCIW-HSASPSRMSA-N (6r,7s)-7-[[(2r)-2-amino-2-phenylacetyl]amino]-3-chloro-8-oxo-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid;hydrate Chemical compound O.C1([C@H](C(=O)N[C@@H]2C(N3C(=C(Cl)CC[C@@H]32)C(O)=O)=O)N)=CC=CC=C1 GPYKKBAAPVOCIW-HSASPSRMSA-N 0.000 description 1
- WRIDQFICGBMAFQ-UHFFFAOYSA-N (E)-8-Octadecenoic acid Natural products CCCCCCCCCC=CCCCCCCC(O)=O WRIDQFICGBMAFQ-UHFFFAOYSA-N 0.000 description 1
- MPIPASJGOJYODL-SFHVURJKSA-N (R)-isoconazole Chemical compound ClC1=CC(Cl)=CC=C1[C@@H](OCC=1C(=CC=CC=1Cl)Cl)CN1C=NC=C1 MPIPASJGOJYODL-SFHVURJKSA-N 0.000 description 1
- AGNGYMCLFWQVGX-AGFFZDDWSA-N (e)-1-[(2s)-2-amino-2-carboxyethoxy]-2-diazonioethenolate Chemical compound OC(=O)[C@@H](N)CO\C([O-])=C\[N+]#N AGNGYMCLFWQVGX-AGFFZDDWSA-N 0.000 description 1
- DTOUUUZOYKYHEP-UHFFFAOYSA-N 1,3-bis(2-ethylhexyl)-5-methyl-1,3-diazinan-5-amine Chemical compound CCCCC(CC)CN1CN(CC(CC)CCCC)CC(C)(N)C1 DTOUUUZOYKYHEP-UHFFFAOYSA-N 0.000 description 1
- UBCHPRBFMUDMNC-UHFFFAOYSA-N 1-(1-adamantyl)ethanamine Chemical compound C1C(C2)CC3CC2CC1(C(N)C)C3 UBCHPRBFMUDMNC-UHFFFAOYSA-N 0.000 description 1
- AFNXATANNDIXLG-SFHVURJKSA-N 1-[(2r)-2-[(4-chlorophenyl)methylsulfanyl]-2-(2,4-dichlorophenyl)ethyl]imidazole Chemical compound C1=CC(Cl)=CC=C1CS[C@H](C=1C(=CC(Cl)=CC=1)Cl)CN1C=NC=C1 AFNXATANNDIXLG-SFHVURJKSA-N 0.000 description 1
- KPQFKCWYCKXXIP-XLPZGREQSA-N 1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-(methylamino)pyrimidine-2,4-dione Chemical compound O=C1NC(=O)C(NC)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 KPQFKCWYCKXXIP-XLPZGREQSA-N 0.000 description 1
- VFWCMGCRMGJXDK-UHFFFAOYSA-N 1-chlorobutane Chemical compound CCCCCl VFWCMGCRMGJXDK-UHFFFAOYSA-N 0.000 description 1
- VUQPJRPDRDVQMN-UHFFFAOYSA-N 1-chlorooctadecane Chemical compound CCCCCCCCCCCCCCCCCCCl VUQPJRPDRDVQMN-UHFFFAOYSA-N 0.000 description 1
- LEZWWPYKPKIXLL-UHFFFAOYSA-N 1-{2-(4-chlorobenzyloxy)-2-(2,4-dichlorophenyl)ethyl}imidazole Chemical compound C1=CC(Cl)=CC=C1COC(C=1C(=CC(Cl)=CC=1)Cl)CN1C=NC=C1 LEZWWPYKPKIXLL-UHFFFAOYSA-N 0.000 description 1
- DGHHQBMTXTWTJV-BQAIUKQQSA-N 119413-54-6 Chemical compound Cl.C1=C(O)C(CN(C)C)=C2C=C(CN3C4=CC5=C(C3=O)COC(=O)[C@]5(O)CC)C4=NC2=C1 DGHHQBMTXTWTJV-BQAIUKQQSA-N 0.000 description 1
- VOXZDWNPVJITMN-ZBRFXRBCSA-N 17β-estradiol Chemical compound OC1=CC=C2[C@H]3CC[C@](C)([C@H](CC4)O)[C@@H]4[C@@H]3CCC2=C1 VOXZDWNPVJITMN-ZBRFXRBCSA-N 0.000 description 1
- SGTNSNPWRIOYBX-UHFFFAOYSA-N 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile Chemical compound C1=C(OC)C(OC)=CC=C1CCN(C)CCCC(C#N)(C(C)C)C1=CC=C(OC)C(OC)=C1 SGTNSNPWRIOYBX-UHFFFAOYSA-N 0.000 description 1
- VHVPQPYKVGDNFY-DFMJLFEVSA-N 2-[(2r)-butan-2-yl]-4-[4-[4-[4-[[(2r,4s)-2-(2,4-dichlorophenyl)-2-(1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]piperazin-1-yl]phenyl]-1,2,4-triazol-3-one Chemical compound O=C1N([C@H](C)CC)N=CN1C1=CC=C(N2CCN(CC2)C=2C=CC(OC[C@@H]3O[C@](CN4N=CN=C4)(OC3)C=3C(=CC(Cl)=CC=3)Cl)=CC=2)C=C1 VHVPQPYKVGDNFY-DFMJLFEVSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- VIQKNUMVXOVXAX-UHFFFAOYSA-N 2-hydroxyethenesulfonic acid Chemical compound OC=CS(O)(=O)=O VIQKNUMVXOVXAX-UHFFFAOYSA-N 0.000 description 1
- 229940080296 2-naphthalenesulfonate Drugs 0.000 description 1
- LQJBNNIYVWPHFW-UHFFFAOYSA-N 20:1omega9c fatty acid Natural products CCCCCCCCCCC=CCCCCCCCC(O)=O LQJBNNIYVWPHFW-UHFFFAOYSA-N 0.000 description 1
- XMIIGOLPHOKFCH-UHFFFAOYSA-M 3-phenylpropionate Chemical compound [O-]C(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-M 0.000 description 1
- AOJJSUZBOXZQNB-VTZDEGQISA-N 4'-epidoxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-VTZDEGQISA-N 0.000 description 1
- OSWFIVFLDKOXQC-UHFFFAOYSA-N 4-(3-methoxyphenyl)aniline Chemical compound COC1=CC=CC(C=2C=CC(N)=CC=2)=C1 OSWFIVFLDKOXQC-UHFFFAOYSA-N 0.000 description 1
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 1
- MNULEGDCPYONBU-UHFFFAOYSA-N 4-ethyl-11,12,15,19-tetrahydroxy-6'-(2-hydroxypropyl)-5',10,12,14,16,18,20,26,29-nonamethylspiro[24,28-dioxabicyclo[23.3.1]nonacosa-5,7,21-triene-27,2'-oxane]-13,17,23-trione Polymers CC1C(C2C)OC(=O)C=CC(C)C(O)C(C)C(=O)C(C)C(O)C(C)C(=O)C(C)(O)C(O)C(C)CC=CC=CC(CC)CCC2OC21CCC(C)C(CC(C)O)O2 MNULEGDCPYONBU-UHFFFAOYSA-N 0.000 description 1
- SYCHUQUJURZQMO-UHFFFAOYSA-N 4-hydroxy-2-methyl-1,1-dioxo-n-(1,3-thiazol-2-yl)-1$l^{6},2-benzothiazine-3-carboxamide Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC1=NC=CS1 SYCHUQUJURZQMO-UHFFFAOYSA-N 0.000 description 1
- FHVDTGUDJYJELY-UHFFFAOYSA-N 6-{[2-carboxy-4,5-dihydroxy-6-(phosphanyloxy)oxan-3-yl]oxy}-4,5-dihydroxy-3-phosphanyloxane-2-carboxylic acid Chemical compound O1C(C(O)=O)C(P)C(O)C(O)C1OC1C(C(O)=O)OC(OP)C(O)C1O FHVDTGUDJYJELY-UHFFFAOYSA-N 0.000 description 1
- MYYIMZRZXIQBGI-HVIRSNARSA-N 6alpha-Fluoroprednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](F)C2=C1 MYYIMZRZXIQBGI-HVIRSNARSA-N 0.000 description 1
- QSBYPNXLFMSGKH-UHFFFAOYSA-N 9-Heptadecensaeure Natural products CCCCCCCC=CCCCCCCCC(O)=O QSBYPNXLFMSGKH-UHFFFAOYSA-N 0.000 description 1
- 229930000680 A04AD01 - Scopolamine Natural products 0.000 description 1
- 101150037123 APOE gene Proteins 0.000 description 1
- 235000006491 Acacia senegal Nutrition 0.000 description 1
- IKHGUXGNUITLKF-UHFFFAOYSA-N Acetaldehyde Chemical compound CC=O IKHGUXGNUITLKF-UHFFFAOYSA-N 0.000 description 1
- NIXOWILDQLNWCW-UHFFFAOYSA-N Acrylic acid Chemical compound OC(=O)C=C NIXOWILDQLNWCW-UHFFFAOYSA-N 0.000 description 1
- 108700021677 Agouti-Related Proteins 0.000 description 1
- YYSWCHMLFJLLBJ-ZLUOBGJFSA-N Ala-Ala-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](CO)C(O)=O YYSWCHMLFJLLBJ-ZLUOBGJFSA-N 0.000 description 1
- PXKLCFFSVLKOJM-ACZMJKKPSA-N Ala-Asn-Glu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O PXKLCFFSVLKOJM-ACZMJKKPSA-N 0.000 description 1
- JYEBJTDTPNKQJG-FXQIFTODSA-N Ala-Asn-Met Chemical compound C[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)N[C@@H](CCSC)C(=O)O)N JYEBJTDTPNKQJG-FXQIFTODSA-N 0.000 description 1
- BUDNAJYVCUHLSV-ZLUOBGJFSA-N Ala-Asp-Ser Chemical compound C[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O BUDNAJYVCUHLSV-ZLUOBGJFSA-N 0.000 description 1
- FVSOUJZKYWEFOB-KBIXCLLPSA-N Ala-Gln-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](C)N FVSOUJZKYWEFOB-KBIXCLLPSA-N 0.000 description 1
- NWVVKQZOVSTDBQ-CIUDSAMLSA-N Ala-Glu-Arg Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O NWVVKQZOVSTDBQ-CIUDSAMLSA-N 0.000 description 1
- FBHOPGDGELNWRH-DRZSPHRISA-N Ala-Glu-Phe Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O FBHOPGDGELNWRH-DRZSPHRISA-N 0.000 description 1
- FAJIYNONGXEXAI-CQDKDKBSSA-N Ala-His-Phe Chemical compound C([C@H](NC(=O)[C@@H](N)C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)C1=CNC=N1 FAJIYNONGXEXAI-CQDKDKBSSA-N 0.000 description 1
- CFPQUJZTLUQUTJ-HTFCKZLJSA-N Ala-Ile-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H]([C@@H](C)CC)NC(=O)[C@H](C)N CFPQUJZTLUQUTJ-HTFCKZLJSA-N 0.000 description 1
- SUMYEVXWCAYLLJ-GUBZILKMSA-N Ala-Leu-Gln Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O SUMYEVXWCAYLLJ-GUBZILKMSA-N 0.000 description 1
- OQWQTGBOFPJOIF-DLOVCJGASA-N Ala-Lys-His Chemical compound C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N OQWQTGBOFPJOIF-DLOVCJGASA-N 0.000 description 1
- XSTZMVAYYCJTNR-DCAQKATOSA-N Ala-Met-Leu Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O XSTZMVAYYCJTNR-DCAQKATOSA-N 0.000 description 1
- WQKAQKZRDIZYNV-VZFHVOOUSA-N Ala-Ser-Thr Chemical compound [H]N[C@@H](C)C(=O)N[C@@H](CO)C(=O)N[C@@H]([C@@H](C)O)C(O)=O WQKAQKZRDIZYNV-VZFHVOOUSA-N 0.000 description 1
- ANNKVZSFQJGVDY-XUXIUFHCSA-N Ala-Val-Pro-Pro Chemical compound C[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N1[C@H](C(O)=O)CCC1 ANNKVZSFQJGVDY-XUXIUFHCSA-N 0.000 description 1
- 208000035285 Allergic Seasonal Rhinitis Diseases 0.000 description 1
- 239000005995 Aluminium silicate Substances 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- KGNQFVOROXFGAU-RSSWFWKPSA-N Amorphigenol O-vicianoside Chemical compound O([C@H](C([C@@H](O)[C@@H]1O)O)OC(C)(CO)C2CC3=C4OC5COC=6C=C(C(=CC=6C5C(=O)C4=CC=C3O2)OC)OC)C1CO[C@@H]1OC[C@H](O)C(O)C1O KGNQFVOROXFGAU-RSSWFWKPSA-N 0.000 description 1
- UTJHAADBJGQRKW-UHFFFAOYSA-N Amorphol Natural products COc1cc2OCC3Oc4c5CC(Oc5ccc4C(=O)C3c2cc1OC)C(C)COC6OC(COC7OCC(O)C(O)C7O)C(O)C(O)C6O UTJHAADBJGQRKW-UHFFFAOYSA-N 0.000 description 1
- APKFDSVGJQXUKY-KKGHZKTASA-N Amphotericin-B Natural products O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1C=CC=CC=CC=CC=CC=CC=C[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-KKGHZKTASA-N 0.000 description 1
- 229920000856 Amylose Polymers 0.000 description 1
- 206010002383 Angina Pectoris Diseases 0.000 description 1
- 208000000103 Anorexia Nervosa Diseases 0.000 description 1
- WZPBZJONDBGPKJ-UHFFFAOYSA-N Antibiotic SQ 26917 Natural products O=C1N(S(O)(=O)=O)C(C)C1NC(=O)C(=NOC(C)(C)C(O)=O)C1=CSC(N)=N1 WZPBZJONDBGPKJ-UHFFFAOYSA-N 0.000 description 1
- 102100029470 Apolipoprotein E Human genes 0.000 description 1
- 101710095339 Apolipoprotein E Proteins 0.000 description 1
- VKKYFICVTYKFIO-CIUDSAMLSA-N Arg-Ala-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)[C@@H](N)CCCN=C(N)N VKKYFICVTYKFIO-CIUDSAMLSA-N 0.000 description 1
- MFAMTAVAFBPXDC-LPEHRKFASA-N Arg-Asp-Pro Chemical compound C1C[C@@H](N(C1)C(=O)[C@H](CC(=O)O)NC(=O)[C@H](CCCN=C(N)N)N)C(=O)O MFAMTAVAFBPXDC-LPEHRKFASA-N 0.000 description 1
- FEZJJKXNPSEYEV-CIUDSAMLSA-N Arg-Gln-Ala Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C)C(O)=O FEZJJKXNPSEYEV-CIUDSAMLSA-N 0.000 description 1
- KBBKCNHWCDJPGN-GUBZILKMSA-N Arg-Gln-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O KBBKCNHWCDJPGN-GUBZILKMSA-N 0.000 description 1
- RYRQZJVFDVWURI-SRVKXCTJSA-N Arg-Gln-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)N)NC(=O)[C@H](CCCN=C(N)N)N RYRQZJVFDVWURI-SRVKXCTJSA-N 0.000 description 1
- DGFXIWKPTDKBLF-AVGNSLFASA-N Arg-His-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCCN=C(N)N)N DGFXIWKPTDKBLF-AVGNSLFASA-N 0.000 description 1
- PYZPXCZNQSEHDT-GUBZILKMSA-N Arg-Met-Asn Chemical compound CSCC[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)O)NC(=O)[C@H](CCCN=C(N)N)N PYZPXCZNQSEHDT-GUBZILKMSA-N 0.000 description 1
- JCROZIFVIYMXHM-GUBZILKMSA-N Arg-Met-Ser Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CCCN=C(N)N JCROZIFVIYMXHM-GUBZILKMSA-N 0.000 description 1
- WKPXXXUSUHAXDE-SRVKXCTJSA-N Arg-Pro-Arg Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCN=C(N)N)C(O)=O WKPXXXUSUHAXDE-SRVKXCTJSA-N 0.000 description 1
- HGKHPCFTRQDHCU-IUCAKERBSA-N Arg-Pro-Gly Chemical compound NC(N)=NCCC[C@H](N)C(=O)N1CCC[C@H]1C(=O)NCC(O)=O HGKHPCFTRQDHCU-IUCAKERBSA-N 0.000 description 1
- VRTWYUYCJGNFES-CIUDSAMLSA-N Arg-Ser-Gln Chemical compound [H]N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCC(N)=O)C(O)=O VRTWYUYCJGNFES-CIUDSAMLSA-N 0.000 description 1
- ULBHWNVWSCJLCO-NHCYSSNCSA-N Arg-Val-Glu Chemical compound OC(=O)CC[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](N)CCCN=C(N)N ULBHWNVWSCJLCO-NHCYSSNCSA-N 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- WVCJSDCHTUTONA-FXQIFTODSA-N Asn-Asp-Arg Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O WVCJSDCHTUTONA-FXQIFTODSA-N 0.000 description 1
- PPMTUXJSQDNUDE-CIUDSAMLSA-N Asn-Glu-Arg Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@H](C(O)=O)CCCN=C(N)N PPMTUXJSQDNUDE-CIUDSAMLSA-N 0.000 description 1
- OGMDXNFGPOPZTK-GUBZILKMSA-N Asn-Glu-His Chemical compound C1=C(NC=N1)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](CC(=O)N)N OGMDXNFGPOPZTK-GUBZILKMSA-N 0.000 description 1
- OPEPUCYIGFEGSW-WDSKDSINSA-N Asn-Gly-Glu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O OPEPUCYIGFEGSW-WDSKDSINSA-N 0.000 description 1
- AEZCCDMZZJOGII-DCAQKATOSA-N Asn-Met-Leu Chemical compound [H]N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O AEZCCDMZZJOGII-DCAQKATOSA-N 0.000 description 1
- ZUFPUBYQYWCMDB-NUMRIWBASA-N Asn-Thr-Glu Chemical compound NC(=O)C[C@H](N)C(=O)N[C@@H]([C@H](O)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O ZUFPUBYQYWCMDB-NUMRIWBASA-N 0.000 description 1
- RGKKALNPOYURGE-ZKWXMUAHSA-N Asp-Ala-Val Chemical compound N[C@@H](CC(=O)O)C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)O RGKKALNPOYURGE-ZKWXMUAHSA-N 0.000 description 1
- AXXCUABIFZPKPM-BQBZGAKWSA-N Asp-Arg-Gly Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCC(O)=O AXXCUABIFZPKPM-BQBZGAKWSA-N 0.000 description 1
- SBHUBSDEZQFJHJ-CIUDSAMLSA-N Asp-Asp-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)CC(O)=O SBHUBSDEZQFJHJ-CIUDSAMLSA-N 0.000 description 1
- XJQRWGXKUSDEFI-ACZMJKKPSA-N Asp-Glu-Asn Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O XJQRWGXKUSDEFI-ACZMJKKPSA-N 0.000 description 1
- RXBGWGRSWXOBGK-KKUMJFAQSA-N Asp-Lys-Tyr Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O RXBGWGRSWXOBGK-KKUMJFAQSA-N 0.000 description 1
- GIKOVDMXBAFXDF-NHCYSSNCSA-N Asp-Val-Leu Chemical compound [H]N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)C(O)=O GIKOVDMXBAFXDF-NHCYSSNCSA-N 0.000 description 1
- 108010011485 Aspartame Proteins 0.000 description 1
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 1
- 206010003694 Atrophy Diseases 0.000 description 1
- 239000012583 B-27 Supplement Substances 0.000 description 1
- KUVIULQEHSCUHY-XYWKZLDCSA-N Beclometasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)COC(=O)CC)(OC(=O)CC)[C@@]1(C)C[C@@H]2O KUVIULQEHSCUHY-XYWKZLDCSA-N 0.000 description 1
- 102100021257 Beta-secretase 1 Human genes 0.000 description 1
- 208000014644 Brain disease Diseases 0.000 description 1
- 102100028737 CAP-Gly domain-containing linker protein 1 Human genes 0.000 description 1
- BCZXFFBUYPCTSJ-UHFFFAOYSA-L Calcium propionate Chemical compound [Ca+2].CCC([O-])=O.CCC([O-])=O BCZXFFBUYPCTSJ-UHFFFAOYSA-L 0.000 description 1
- 239000004215 Carbon black (E152) Substances 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 229930186147 Cephalosporin Natural products 0.000 description 1
- 229920001661 Chitosan Polymers 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- GJSURZIOUXUGAL-UHFFFAOYSA-N Clonidine Chemical compound ClC1=CC=CC(Cl)=C1NC1=NCCN1 GJSURZIOUXUGAL-UHFFFAOYSA-N 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 208000011990 Corticobasal Degeneration Diseases 0.000 description 1
- WHPAGCJNPTUGGD-UHFFFAOYSA-N Croconazole Chemical compound ClC1=CC=CC(COC=2C(=CC=CC=2)C(=C)N2C=NC=C2)=C1 WHPAGCJNPTUGGD-UHFFFAOYSA-N 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- PMATZTZNYRCHOR-CGLBZJNRSA-N Cyclosporin A Chemical compound CC[C@@H]1NC(=O)[C@H]([C@H](O)[C@H](C)C\C=C\C)N(C)C(=O)[C@H](C(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)CN(C)C1=O PMATZTZNYRCHOR-CGLBZJNRSA-N 0.000 description 1
- 108010036949 Cyclosporine Proteins 0.000 description 1
- UHDGCWIWMRVCDJ-CCXZUQQUSA-N Cytarabine Chemical compound O=C1N=C(N)C=CN1[C@H]1[C@@H](O)[C@H](O)[C@@H](CO)O1 UHDGCWIWMRVCDJ-CCXZUQQUSA-N 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- ZAKOWWREFLAJOT-CEFNRUSXSA-N D-alpha-tocopherylacetate Chemical compound CC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C ZAKOWWREFLAJOT-CEFNRUSXSA-N 0.000 description 1
- CKLJMWTZIZZHCS-UWTATZPHSA-N D-aspartic acid Chemical compound OC(=O)[C@H](N)CC(O)=O CKLJMWTZIZZHCS-UWTATZPHSA-N 0.000 description 1
- KDXKERNSBIXSRK-RXMQYKEDSA-N D-lysine Chemical compound NCCCC[C@@H](N)C(O)=O KDXKERNSBIXSRK-RXMQYKEDSA-N 0.000 description 1
- WQZGKKKJIJFFOK-QTVWNMPRSA-N D-mannopyranose Chemical compound OC[C@H]1OC(O)[C@@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-QTVWNMPRSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 108010092160 Dactinomycin Proteins 0.000 description 1
- 101100216294 Danio rerio apoeb gene Proteins 0.000 description 1
- 239000004375 Dextrin Substances 0.000 description 1
- 229920001353 Dextrin Polymers 0.000 description 1
- RWSOTUBLDIXVET-UHFFFAOYSA-N Dihydrogen sulfide Chemical compound S RWSOTUBLDIXVET-UHFFFAOYSA-N 0.000 description 1
- IIUZTXTZRGLYTI-UHFFFAOYSA-N Dihydrogriseofulvin Natural products COC1CC(=O)CC(C)C11C(=O)C(C(OC)=CC(OC)=C2Cl)=C2O1 IIUZTXTZRGLYTI-UHFFFAOYSA-N 0.000 description 1
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 1
- 238000002965 ELISA Methods 0.000 description 1
- 201000009051 Embryonal Carcinoma Diseases 0.000 description 1
- HTIJFSOGRVMCQR-UHFFFAOYSA-N Epirubicin Natural products COc1cccc2C(=O)c3c(O)c4CC(O)(CC(OC5CC(N)C(=O)C(C)O5)c4c(O)c3C(=O)c12)C(=O)CO HTIJFSOGRVMCQR-UHFFFAOYSA-N 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 101710089384 Extracellular protease Proteins 0.000 description 1
- WJOHZNCJWYWUJD-IUGZLZTKSA-N Fluocinonide Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)COC(=O)C)[C@@]2(C)C[C@@H]1O WJOHZNCJWYWUJD-IUGZLZTKSA-N 0.000 description 1
- YCKRFDGAMUMZLT-UHFFFAOYSA-N Fluorine atom Chemical compound [F] YCKRFDGAMUMZLT-UHFFFAOYSA-N 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 208000002339 Frontotemporal Lobar Degeneration Diseases 0.000 description 1
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- CEAZRRDELHUEMR-URQXQFDESA-N Gentamicin Chemical compound O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N CEAZRRDELHUEMR-URQXQFDESA-N 0.000 description 1
- 229930182566 Gentamicin Natural products 0.000 description 1
- DXJZITDUDUPINW-WHFBIAKZSA-N Gln-Asn Chemical compound NC(=O)CC[C@H](N)C(=O)N[C@@H](CC(N)=O)C(O)=O DXJZITDUDUPINW-WHFBIAKZSA-N 0.000 description 1
- ZPDVKYLJTOFQJV-WDSKDSINSA-N Gln-Asn-Gly Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(O)=O ZPDVKYLJTOFQJV-WDSKDSINSA-N 0.000 description 1
- IKDOHQHEFPPGJG-FXQIFTODSA-N Gln-Asp-Glu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O IKDOHQHEFPPGJG-FXQIFTODSA-N 0.000 description 1
- UFNSPPFJOHNXRE-AUTRQRHGSA-N Gln-Gln-Val Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](C(C)C)C(O)=O UFNSPPFJOHNXRE-AUTRQRHGSA-N 0.000 description 1
- MCAVASRGVBVPMX-FXQIFTODSA-N Gln-Glu-Ala Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(O)=O MCAVASRGVBVPMX-FXQIFTODSA-N 0.000 description 1
- IHSGESFHTMFHRB-GUBZILKMSA-N Gln-Lys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCC(N)=O IHSGESFHTMFHRB-GUBZILKMSA-N 0.000 description 1
- TWIAMTNJOMRDAK-GUBZILKMSA-N Gln-Lys-Asp Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(O)=O)C(O)=O TWIAMTNJOMRDAK-GUBZILKMSA-N 0.000 description 1
- NMYFPKCIGUJMIK-GUBZILKMSA-N Gln-Met-Gln Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCC(=O)N)N NMYFPKCIGUJMIK-GUBZILKMSA-N 0.000 description 1
- LPIKVBWNNVFHCQ-GUBZILKMSA-N Gln-Ser-Leu Chemical compound [H]N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O LPIKVBWNNVFHCQ-GUBZILKMSA-N 0.000 description 1
- JJKKWYQVHRUSDG-GUBZILKMSA-N Glu-Ala-Lys Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CCCCN)C(O)=O JJKKWYQVHRUSDG-GUBZILKMSA-N 0.000 description 1
- KKCUFHUTMKQQCF-SRVKXCTJSA-N Glu-Arg-Leu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(O)=O KKCUFHUTMKQQCF-SRVKXCTJSA-N 0.000 description 1
- ZPASCJBSSCRWMC-GVXVVHGQSA-N Glu-His-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC1=CN=CN1)NC(=O)[C@H](CCC(=O)O)N ZPASCJBSSCRWMC-GVXVVHGQSA-N 0.000 description 1
- KJBGAZSLZAQDPV-KKUMJFAQSA-N Glu-Phe-Arg Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CCC(=O)O)N KJBGAZSLZAQDPV-KKUMJFAQSA-N 0.000 description 1
- UMZHHILWZBFPGL-LOKLDPHHSA-N Glu-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CCC(=O)O)N)O UMZHHILWZBFPGL-LOKLDPHHSA-N 0.000 description 1
- BPCLDCNZBUYGOD-BPUTZDHNSA-N Glu-Trp-Glu Chemical compound C1=CC=C2C(C[C@H](NC(=O)[C@H](CCC(O)=O)N)C(=O)N[C@@H](CCC(O)=O)C(O)=O)=CNC2=C1 BPCLDCNZBUYGOD-BPUTZDHNSA-N 0.000 description 1
- LZEUDRYSAZAJIO-AUTRQRHGSA-N Glu-Val-Glu Chemical compound [H]N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O LZEUDRYSAZAJIO-AUTRQRHGSA-N 0.000 description 1
- ZYRXTRTUCAVNBQ-GVXVVHGQSA-N Glu-Val-Lys Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)O)NC(=O)[C@H](CCC(=O)O)N ZYRXTRTUCAVNBQ-GVXVVHGQSA-N 0.000 description 1
- 108010024636 Glutathione Proteins 0.000 description 1
- YMUFWNJHVPQNQD-ZKWXMUAHSA-N Gly-Ala-Ile Chemical compound CC[C@H](C)[C@@H](C(O)=O)NC(=O)[C@H](C)NC(=O)CN YMUFWNJHVPQNQD-ZKWXMUAHSA-N 0.000 description 1
- QSTLUOIOYLYLLF-WDSKDSINSA-N Gly-Asp-Glu Chemical compound [H]NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O QSTLUOIOYLYLLF-WDSKDSINSA-N 0.000 description 1
- YSDLIYZLOTZZNP-UWVGGRQHSA-N Gly-Leu-Met Chemical compound CSCC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)CN YSDLIYZLOTZZNP-UWVGGRQHSA-N 0.000 description 1
- SOEGEPHNZOISMT-BYPYZUCNSA-N Gly-Ser-Gly Chemical compound NCC(=O)N[C@@H](CO)C(=O)NCC(O)=O SOEGEPHNZOISMT-BYPYZUCNSA-N 0.000 description 1
- KSOBNUBCYHGUKH-UWVGGRQHSA-N Gly-Val-Val Chemical compound CC(C)[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)CN KSOBNUBCYHGUKH-UWVGGRQHSA-N 0.000 description 1
- 244000068988 Glycine max Species 0.000 description 1
- 235000010469 Glycine max Nutrition 0.000 description 1
- 108010007979 Glycocholic Acid Proteins 0.000 description 1
- 108010015899 Glycopeptides Proteins 0.000 description 1
- 102000002068 Glycopeptides Human genes 0.000 description 1
- UXWOXTQWVMFRSE-UHFFFAOYSA-N Griseoviridin Natural products O=C1OC(C)CC=C(C(NCC=CC=CC(O)CC(O)C2)=O)SCC1NC(=O)C1=COC2=N1 UXWOXTQWVMFRSE-UHFFFAOYSA-N 0.000 description 1
- 101710088172 HTH-type transcriptional regulator RipA Proteins 0.000 description 1
- SQUHHTBVTRBESD-UHFFFAOYSA-N Hexa-Ac-myo-Inositol Natural products CC(=O)OC1C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O SQUHHTBVTRBESD-UHFFFAOYSA-N 0.000 description 1
- YPLYIXGKCRQZGW-SRVKXCTJSA-N His-Arg-Glu Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(O)=O)C(O)=O YPLYIXGKCRQZGW-SRVKXCTJSA-N 0.000 description 1
- ZZLWLWSUIBSMNP-CIUDSAMLSA-N His-Asp-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CO)C(O)=O ZZLWLWSUIBSMNP-CIUDSAMLSA-N 0.000 description 1
- JIUYRPFQJJRSJB-QWRGUYRKSA-N His-His-Gly Chemical compound C([C@H](N)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)NCC(O)=O)C1=CN=CN1 JIUYRPFQJJRSJB-QWRGUYRKSA-N 0.000 description 1
- SKYULSWNBYAQMG-IHRRRGAJSA-N His-Leu-Arg Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O SKYULSWNBYAQMG-IHRRRGAJSA-N 0.000 description 1
- LVXFNTIIGOQBMD-SRVKXCTJSA-N His-Leu-Ser Chemical compound [H]N[C@@H](CC1=CNC=N1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O LVXFNTIIGOQBMD-SRVKXCTJSA-N 0.000 description 1
- YAEKRYQASVCDLK-JYJNAYRXSA-N His-Phe-Glu Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)O)NC(=O)[C@H](CC2=CN=CN2)N YAEKRYQASVCDLK-JYJNAYRXSA-N 0.000 description 1
- WYKXJGWSJUULSL-AVGNSLFASA-N His-Val-Arg Chemical compound CC(C)[C@H](NC(=O)[C@@H](N)Cc1cnc[nH]1)C(=O)N[C@@H](CCCNC(=N)N)C(=O)O WYKXJGWSJUULSL-AVGNSLFASA-N 0.000 description 1
- GGXUJBKENKVYNV-ULQDDVLXSA-N His-Val-Phe Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O)NC(=O)[C@H](CC2=CN=CN2)N GGXUJBKENKVYNV-ULQDDVLXSA-N 0.000 description 1
- 101000894895 Homo sapiens Beta-secretase 1 Proteins 0.000 description 1
- 101100005713 Homo sapiens CD4 gene Proteins 0.000 description 1
- DLVOSEUFIRPIRM-KAQKJVHQSA-N Hydrocortisone cypionate Chemical compound O=C([C@@]1(O)CC[C@H]2[C@H]3[C@@H]([C@]4(CCC(=O)C=C4CC3)C)[C@@H](O)C[C@@]21C)COC(=O)CCC1CCCC1 DLVOSEUFIRPIRM-KAQKJVHQSA-N 0.000 description 1
- CPELXLSAUQHCOX-UHFFFAOYSA-N Hydrogen bromide Chemical compound Br CPELXLSAUQHCOX-UHFFFAOYSA-N 0.000 description 1
- 239000004354 Hydroxyethyl cellulose Substances 0.000 description 1
- 229920000663 Hydroxyethyl cellulose Polymers 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- STECJAGHUSJQJN-GAUPFVANSA-N Hyoscine Natural products C1([C@H](CO)C(=O)OC2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-GAUPFVANSA-N 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 1
- CYHYBSGMHMHKOA-CIQUZCHMSA-N Ile-Ala-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N CYHYBSGMHMHKOA-CIQUZCHMSA-N 0.000 description 1
- ZGGWRNBSBOHIGH-HVTMNAMFSA-N Ile-Gln-His Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CC1=CN=CN1)C(=O)O)N ZGGWRNBSBOHIGH-HVTMNAMFSA-N 0.000 description 1
- NYEYYMLUABXDMC-NHCYSSNCSA-N Ile-Gly-Leu Chemical compound CC[C@H](C)[C@@H](C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)O)N NYEYYMLUABXDMC-NHCYSSNCSA-N 0.000 description 1
- CKRFDMPBSWYOBT-PPCPHDFISA-N Ile-Lys-Thr Chemical compound CC[C@H](C)[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)O)N CKRFDMPBSWYOBT-PPCPHDFISA-N 0.000 description 1
- DLEBSGAVWRPTIX-PEDHHIEDSA-N Ile-Val-Ile Chemical compound CC[C@H](C)[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)[C@@H](C)CC DLEBSGAVWRPTIX-PEDHHIEDSA-N 0.000 description 1
- 229930010555 Inosine Natural products 0.000 description 1
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 1
- IMQLKJBTEOYOSI-GPIVLXJGSA-N Inositol-hexakisphosphate Chemical compound OP(O)(=O)O[C@H]1[C@H](OP(O)(O)=O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@@H]1OP(O)(O)=O IMQLKJBTEOYOSI-GPIVLXJGSA-N 0.000 description 1
- 206010022489 Insulin Resistance Diseases 0.000 description 1
- 102000006992 Interferon-alpha Human genes 0.000 description 1
- 108010047761 Interferon-alpha Proteins 0.000 description 1
- 102000014150 Interferons Human genes 0.000 description 1
- 108010050904 Interferons Proteins 0.000 description 1
- PWWVAXIEGOYWEE-UHFFFAOYSA-N Isophenergan Chemical compound C1=CC=C2N(CC(C)N(C)C)C3=CC=CC=C3SC2=C1 PWWVAXIEGOYWEE-UHFFFAOYSA-N 0.000 description 1
- SHGAZHPCJJPHSC-NUEINMDLSA-N Isotretinoin Chemical compound OC(=O)C=C(C)/C=C/C=C(C)C=CC1=C(C)CCCC1(C)C SHGAZHPCJJPHSC-NUEINMDLSA-N 0.000 description 1
- ONIBWKKTOPOVIA-BYPYZUCNSA-N L-Proline Chemical compound OC(=O)[C@@H]1CCCN1 ONIBWKKTOPOVIA-BYPYZUCNSA-N 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- SITWEMZOJNKJCH-UHFFFAOYSA-N L-alanine-L-arginine Natural products CC(N)C(=O)NC(C(O)=O)CCCNC(N)=N SITWEMZOJNKJCH-UHFFFAOYSA-N 0.000 description 1
- 239000002211 L-ascorbic acid Substances 0.000 description 1
- 235000000069 L-ascorbic acid Nutrition 0.000 description 1
- 150000000996 L-ascorbic acids Chemical class 0.000 description 1
- WHUUTDBJXJRKMK-VKHMYHEASA-N L-glutamic acid Chemical compound OC(=O)[C@@H](N)CCC(O)=O WHUUTDBJXJRKMK-VKHMYHEASA-N 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- KDXKERNSBIXSRK-YFKPBYRVSA-N L-lysine Chemical compound NCCCC[C@H](N)C(O)=O KDXKERNSBIXSRK-YFKPBYRVSA-N 0.000 description 1
- COLNVLDHVKWLRT-QMMMGPOBSA-N L-phenylalanine Chemical compound OC(=O)[C@@H](N)CC1=CC=CC=C1 COLNVLDHVKWLRT-QMMMGPOBSA-N 0.000 description 1
- 125000002842 L-seryl group Chemical group O=C([*])[C@](N([H])[H])([H])C([H])([H])O[H] 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 1
- JVTAAEKCZFNVCJ-UHFFFAOYSA-M Lactate Chemical compound CC(O)C([O-])=O JVTAAEKCZFNVCJ-UHFFFAOYSA-M 0.000 description 1
- ZRTQSJFIDWNVJW-WYMLVPIESA-N Lanoconazole Chemical compound ClC1=CC=CC=C1C(CS\1)SC/1=C(\C#N)N1C=NC=C1 ZRTQSJFIDWNVJW-WYMLVPIESA-N 0.000 description 1
- 229920002884 Laureth 4 Polymers 0.000 description 1
- 241000880493 Leptailurus serval Species 0.000 description 1
- VCSBGUACOYUIGD-CIUDSAMLSA-N Leu-Asn-Asp Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O VCSBGUACOYUIGD-CIUDSAMLSA-N 0.000 description 1
- JNDYEOUZBLOVOF-AVGNSLFASA-N Leu-Leu-Gln Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(O)=O JNDYEOUZBLOVOF-AVGNSLFASA-N 0.000 description 1
- LXKNSJLSGPNHSK-KKUMJFAQSA-N Leu-Leu-Lys Chemical compound CC(C)C[C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N LXKNSJLSGPNHSK-KKUMJFAQSA-N 0.000 description 1
- KPYAOIVPJKPIOU-KKUMJFAQSA-N Leu-Lys-Lys Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(O)=O KPYAOIVPJKPIOU-KKUMJFAQSA-N 0.000 description 1
- JDBQSGMJBMPNFT-AVGNSLFASA-N Leu-Pro-Val Chemical compound CC(C)C[C@H](N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(O)=O JDBQSGMJBMPNFT-AVGNSLFASA-N 0.000 description 1
- XOWMDXHFSBCAKQ-SRVKXCTJSA-N Leu-Ser-Leu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H](CO)C(=O)N[C@H](C(O)=O)CC(C)C XOWMDXHFSBCAKQ-SRVKXCTJSA-N 0.000 description 1
- AEDWWMMHUGYIFD-HJGDQZAQSA-N Leu-Thr-Asn Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(N)=O)C(O)=O AEDWWMMHUGYIFD-HJGDQZAQSA-N 0.000 description 1
- LFSQWRSVPNKJGP-WDCWCFNPSA-N Leu-Thr-Glu Chemical compound CC(C)C[C@H](N)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@H](C(O)=O)CCC(O)=O LFSQWRSVPNKJGP-WDCWCFNPSA-N 0.000 description 1
- GZRABTMNWJXFMH-UVOCVTCTSA-N Leu-Thr-Thr Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H]([C@@H](C)O)C(O)=O GZRABTMNWJXFMH-UVOCVTCTSA-N 0.000 description 1
- AIMGJYMCTAABEN-GVXVVHGQSA-N Leu-Val-Glu Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCC(O)=O)C(O)=O AIMGJYMCTAABEN-GVXVVHGQSA-N 0.000 description 1
- QQXJROOJCMIHIV-AVGNSLFASA-N Leu-Val-Met Chemical compound [H]N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCSC)C(O)=O QQXJROOJCMIHIV-AVGNSLFASA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 102000011965 Lipoprotein Receptors Human genes 0.000 description 1
- 108010061306 Lipoprotein Receptors Proteins 0.000 description 1
- FACUGMGEFUEBTI-SRVKXCTJSA-N Lys-Asn-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](N)CCCCN FACUGMGEFUEBTI-SRVKXCTJSA-N 0.000 description 1
- GRADYHMSAUIKPS-DCAQKATOSA-N Lys-Glu-Gln Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CCC(N)=O)C(O)=O GRADYHMSAUIKPS-DCAQKATOSA-N 0.000 description 1
- KNKJPYAZQUFLQK-IHRRRGAJSA-N Lys-His-Arg Chemical compound C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCCN=C(N)N)C(=O)O)NC(=O)[C@H](CCCCN)N KNKJPYAZQUFLQK-IHRRRGAJSA-N 0.000 description 1
- XOQMURBBIXRRCR-SRVKXCTJSA-N Lys-Lys-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)CCCCN XOQMURBBIXRRCR-SRVKXCTJSA-N 0.000 description 1
- PYFNONMJYNJENN-AVGNSLFASA-N Lys-Lys-Gln Chemical compound C(CCN)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)N)C(=O)O)N PYFNONMJYNJENN-AVGNSLFASA-N 0.000 description 1
- BXPHMHQHYHILBB-BZSNNMDCSA-N Lys-Lys-Tyr Chemical compound [H]N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O BXPHMHQHYHILBB-BZSNNMDCSA-N 0.000 description 1
- WKUXWMWQTOYTFI-SRVKXCTJSA-N Lys-Met-Gln Chemical compound CSCC[C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)O)NC(=O)[C@H](CCCCN)N WKUXWMWQTOYTFI-SRVKXCTJSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- 108091077621 MAPRE family Proteins 0.000 description 1
- 239000005913 Maltodextrin Substances 0.000 description 1
- 229920002774 Maltodextrin Polymers 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- SBDNJUWAMKYJOX-UHFFFAOYSA-N Meclofenamic Acid Chemical compound CC1=CC=C(Cl)C(NC=2C(=CC=CC=2)C(O)=O)=C1Cl SBDNJUWAMKYJOX-UHFFFAOYSA-N 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 206010027304 Menopausal symptoms Diseases 0.000 description 1
- UZVWDRPUTHXQAM-FXQIFTODSA-N Met-Asp-Ala Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C)C(O)=O UZVWDRPUTHXQAM-FXQIFTODSA-N 0.000 description 1
- MYKLINMAGAIRPJ-CIUDSAMLSA-N Met-Gln-Asn Chemical compound [H]N[C@@H](CCSC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(N)=O)C(O)=O MYKLINMAGAIRPJ-CIUDSAMLSA-N 0.000 description 1
- QZPXMHVKPHJNTR-DCAQKATOSA-N Met-Leu-Asn Chemical compound CSCC[C@H](N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O QZPXMHVKPHJNTR-DCAQKATOSA-N 0.000 description 1
- NHXXGBXJTLRGJI-GUBZILKMSA-N Met-Pro-Ser Chemical compound [H]N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CO)C(O)=O NHXXGBXJTLRGJI-GUBZILKMSA-N 0.000 description 1
- OVTOTTGZBWXLFU-QXEWZRGKSA-N Met-Val-Asp Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](C(O)=O)CC(O)=O OVTOTTGZBWXLFU-QXEWZRGKSA-N 0.000 description 1
- CQRGINSEMFBACV-WPRPVWTQSA-N Met-Val-Gly Chemical compound CSCC[C@H](N)C(=O)N[C@@H](C(C)C)C(=O)NCC(O)=O CQRGINSEMFBACV-WPRPVWTQSA-N 0.000 description 1
- RJQXTJLFIWVMTO-TYNCELHUSA-N Methicillin Chemical compound COC1=CC=CC(OC)=C1C(=O)N[C@@H]1C(=O)N2[C@@H](C(O)=O)C(C)(C)S[C@@H]21 RJQXTJLFIWVMTO-TYNCELHUSA-N 0.000 description 1
- FQISKWAFAHGMGT-SGJOWKDISA-M Methylprednisolone sodium succinate Chemical compound [Na+].C([C@@]12C)=CC(=O)C=C1[C@@H](C)C[C@@H]1[C@@H]2[C@@H](O)C[C@]2(C)[C@@](O)(C(=O)COC(=O)CCC([O-])=O)CC[C@H]21 FQISKWAFAHGMGT-SGJOWKDISA-M 0.000 description 1
- 102000009664 Microtubule-Associated Proteins Human genes 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 206010049816 Muscle tightness Diseases 0.000 description 1
- YLJXZSWHZFXCDY-UHFFFAOYSA-N Myxoviromycin Natural products NC1CCC(C(=O)NCCC(N)=N)C1 YLJXZSWHZFXCDY-UHFFFAOYSA-N 0.000 description 1
- KJHOZAZQWVKILO-UHFFFAOYSA-N N-(diaminomethylidene)-4-morpholinecarboximidamide Chemical compound NC(N)=NC(=N)N1CCOCC1 KJHOZAZQWVKILO-UHFFFAOYSA-N 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- STECJAGHUSJQJN-UHFFFAOYSA-N N-Methyl-scopolamin Natural products C1C(C2C3O2)N(C)C3CC1OC(=O)C(CO)C1=CC=CC=C1 STECJAGHUSJQJN-UHFFFAOYSA-N 0.000 description 1
- WXNXCEHXYPACJF-ZETCQYMHSA-N N-acetyl-L-leucine Chemical compound CC(C)C[C@@H](C(O)=O)NC(C)=O WXNXCEHXYPACJF-ZETCQYMHSA-N 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- 108010079364 N-glycylalanine Proteins 0.000 description 1
- 108010002311 N-glycylglutamic acid Proteins 0.000 description 1
- SEBFKMXJBCUCAI-UHFFFAOYSA-N NSC 227190 Natural products C1=C(O)C(OC)=CC(C2C(OC3=CC=C(C=C3O2)C2C(C(=O)C3=C(O)C=C(O)C=C3O2)O)CO)=C1 SEBFKMXJBCUCAI-UHFFFAOYSA-N 0.000 description 1
- CMWTZPSULFXXJA-UHFFFAOYSA-N Naproxen Natural products C1=C(C(C)C(O)=O)C=CC2=CC(OC)=CC=C21 CMWTZPSULFXXJA-UHFFFAOYSA-N 0.000 description 1
- DDUHZTYCFQRHIY-UHFFFAOYSA-N Negwer: 6874 Natural products COC1=CC(=O)CC(C)C11C(=O)C(C(OC)=CC(OC)=C2Cl)=C2O1 DDUHZTYCFQRHIY-UHFFFAOYSA-N 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 102100028782 Neprilysin Human genes 0.000 description 1
- 108090000028 Neprilysin Proteins 0.000 description 1
- 208000012902 Nervous system disease Diseases 0.000 description 1
- 108010065395 Neuropep-1 Proteins 0.000 description 1
- JZFPYUNJRRFVQU-UHFFFAOYSA-N Niflumic acid Chemical compound OC(=O)C1=CC=CN=C1NC1=CC=CC(C(F)(F)F)=C1 JZFPYUNJRRFVQU-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- SNIOPGDIGTZGOP-UHFFFAOYSA-N Nitroglycerin Chemical compound [O-][N+](=O)OCC(O[N+]([O-])=O)CO[N+]([O-])=O SNIOPGDIGTZGOP-UHFFFAOYSA-N 0.000 description 1
- 239000000006 Nitroglycerin Substances 0.000 description 1
- 208000008589 Obesity Diseases 0.000 description 1
- REYJJPSVUYRZGE-UHFFFAOYSA-N Octadecylamine Chemical class CCCCCCCCCCCCCCCCCCN REYJJPSVUYRZGE-UHFFFAOYSA-N 0.000 description 1
- 239000005642 Oleic acid Substances 0.000 description 1
- ZQPPMHVWECSIRJ-UHFFFAOYSA-N Oleic acid Natural products CCCCCCCCC=CCCCCCCCC(O)=O ZQPPMHVWECSIRJ-UHFFFAOYSA-N 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 229930012538 Paclitaxel Natural products 0.000 description 1
- MKPDWECBUAZOHP-AFYJWTTESA-N Paramethasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O MKPDWECBUAZOHP-AFYJWTTESA-N 0.000 description 1
- 101000579647 Penaeus vannamei Penaeidin-2a Proteins 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- 102000035195 Peptidases Human genes 0.000 description 1
- 108091005804 Peptidases Proteins 0.000 description 1
- LNIIRLODKOWQIY-IHRRRGAJSA-N Phe-Asn-Met Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CCSC)C(O)=O LNIIRLODKOWQIY-IHRRRGAJSA-N 0.000 description 1
- AKJAKCBHLJGRBU-JYJNAYRXSA-N Phe-Glu-His Chemical compound C1=CC=C(C=C1)C[C@@H](C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O)N AKJAKCBHLJGRBU-JYJNAYRXSA-N 0.000 description 1
- JJHVFCUWLSKADD-ONGXEEELSA-N Phe-Gly-Ala Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)NCC(=O)N[C@@H](C)C(O)=O JJHVFCUWLSKADD-ONGXEEELSA-N 0.000 description 1
- RYQWALWYQWBUKN-FHWLQOOXSA-N Phe-Phe-Glu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CCC(O)=O)C(O)=O RYQWALWYQWBUKN-FHWLQOOXSA-N 0.000 description 1
- UNBFGVQVQGXXCK-KKUMJFAQSA-N Phe-Ser-Leu Chemical compound [H]N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(O)=O UNBFGVQVQGXXCK-KKUMJFAQSA-N 0.000 description 1
- 244000304393 Phlox paniculata Species 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 229920002319 Poly(methyl acrylate) Polymers 0.000 description 1
- 239000004698 Polyethylene Substances 0.000 description 1
- 108010020346 Polyglutamic Acid Proteins 0.000 description 1
- 229920000954 Polyglycolide Polymers 0.000 description 1
- TVQZAMVBTVNYLA-UHFFFAOYSA-N Pranoprofen Chemical compound C1=CC=C2CC3=CC(C(C(O)=O)C)=CC=C3OC2=N1 TVQZAMVBTVNYLA-UHFFFAOYSA-N 0.000 description 1
- 102000012412 Presenilin-1 Human genes 0.000 description 1
- 108010036933 Presenilin-1 Proteins 0.000 description 1
- 102000015499 Presenilins Human genes 0.000 description 1
- 108010050254 Presenilins Proteins 0.000 description 1
- DZZCICYRSZASNF-FXQIFTODSA-N Pro-Ala-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@@H]1CCCN1 DZZCICYRSZASNF-FXQIFTODSA-N 0.000 description 1
- OOLOTUZJUBOMAX-GUBZILKMSA-N Pro-Ala-Val Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C)C(=O)N[C@@H](C(C)C)C(O)=O OOLOTUZJUBOMAX-GUBZILKMSA-N 0.000 description 1
- QBFONMUYNSNKIX-AVGNSLFASA-N Pro-Arg-His Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC2=CN=CN2)C(=O)O QBFONMUYNSNKIX-AVGNSLFASA-N 0.000 description 1
- GRIRJQGZZJVANI-CYDGBPFRSA-N Pro-Arg-Ile Chemical compound CC[C@H](C)[C@@H](C(=O)O)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H]1CCCN1 GRIRJQGZZJVANI-CYDGBPFRSA-N 0.000 description 1
- ICTZKEXYDDZZFP-SRVKXCTJSA-N Pro-Arg-Pro Chemical compound N([C@@H](CCCN=C(N)N)C(=O)N1[C@@H](CCC1)C(O)=O)C(=O)[C@@H]1CCCN1 ICTZKEXYDDZZFP-SRVKXCTJSA-N 0.000 description 1
- FMLRRBDLBJLJIK-DCAQKATOSA-N Pro-Leu-Ala Chemical compound OC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]1CCCN1 FMLRRBDLBJLJIK-DCAQKATOSA-N 0.000 description 1
- OFGUOWQVEGTVNU-DCAQKATOSA-N Pro-Lys-Ala Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C)C(O)=O OFGUOWQVEGTVNU-DCAQKATOSA-N 0.000 description 1
- DWGFLKQSGRUQTI-IHRRRGAJSA-N Pro-Lys-Lys Chemical compound NCCCC[C@@H](C(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1 DWGFLKQSGRUQTI-IHRRRGAJSA-N 0.000 description 1
- JLMZKEQFMVORMA-SRVKXCTJSA-N Pro-Pro-Arg Chemical compound NC(N)=NCCC[C@@H](C(O)=O)NC(=O)[C@@H]1CCCN1C(=O)[C@H]1NCCC1 JLMZKEQFMVORMA-SRVKXCTJSA-N 0.000 description 1
- VVAWNPIOYXAMAL-KJEVXHAQSA-N Pro-Thr-Tyr Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1=CC=C(O)C=C1)C(O)=O VVAWNPIOYXAMAL-KJEVXHAQSA-N 0.000 description 1
- RJTUIDFUUHPJMP-FHWLQOOXSA-N Pro-Trp-His Chemical compound C1C[C@H](NC1)C(=O)N[C@@H](CC2=CNC3=CC=CC=C32)C(=O)N[C@@H](CC4=CN=CN4)C(=O)O RJTUIDFUUHPJMP-FHWLQOOXSA-N 0.000 description 1
- ZAUHSLVPDLNTRZ-QXEWZRGKSA-N Pro-Val-Asn Chemical compound [H]N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(N)=O)C(O)=O ZAUHSLVPDLNTRZ-QXEWZRGKSA-N 0.000 description 1
- OOZJHTXCLJUODH-QXEWZRGKSA-N Pro-Val-Asp Chemical compound OC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H]1CCCN1 OOZJHTXCLJUODH-QXEWZRGKSA-N 0.000 description 1
- 102000003946 Prolactin Human genes 0.000 description 1
- 108010057464 Prolactin Proteins 0.000 description 1
- ONIBWKKTOPOVIA-UHFFFAOYSA-N Proline Natural products OC(=O)C1CCCN1 ONIBWKKTOPOVIA-UHFFFAOYSA-N 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-M Propionate Chemical compound CCC([O-])=O XBDQKXXYIPTUBI-UHFFFAOYSA-M 0.000 description 1
- 239000004365 Protease Substances 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- IWUCXVSUMQZMFG-AFCXAGJDSA-N Ribavirin Chemical compound N1=C(C(=O)N)N=CN1[C@H]1[C@H](O)[C@H](O)[C@@H](CO)O1 IWUCXVSUMQZMFG-AFCXAGJDSA-N 0.000 description 1
- WTWGOQRNRFHFQD-JBDRJPRFSA-N Ser-Ala-Ile Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H]([C@@H](C)CC)C(O)=O WTWGOQRNRFHFQD-JBDRJPRFSA-N 0.000 description 1
- IYCBDVBJWDXQRR-FXQIFTODSA-N Ser-Ala-Met Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCSC)C(O)=O IYCBDVBJWDXQRR-FXQIFTODSA-N 0.000 description 1
- FMDHKPRACUXATF-ACZMJKKPSA-N Ser-Gln-Ser Chemical compound OC[C@H](N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CO)C(O)=O FMDHKPRACUXATF-ACZMJKKPSA-N 0.000 description 1
- GZFAWAQTEYDKII-YUMQZZPRSA-N Ser-Gly-Leu Chemical compound CC(C)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CO GZFAWAQTEYDKII-YUMQZZPRSA-N 0.000 description 1
- VMLONWHIORGALA-SRVKXCTJSA-N Ser-Leu-Leu Chemical compound CC(C)C[C@@H](C([O-])=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H]([NH3+])CO VMLONWHIORGALA-SRVKXCTJSA-N 0.000 description 1
- YUJLIIRMIAGMCQ-CIUDSAMLSA-N Ser-Leu-Ser Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CO)C(O)=O YUJLIIRMIAGMCQ-CIUDSAMLSA-N 0.000 description 1
- XKFJENWJGHMDLI-QWRGUYRKSA-N Ser-Phe-Gly Chemical compound [H]N[C@@H](CO)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)NCC(O)=O XKFJENWJGHMDLI-QWRGUYRKSA-N 0.000 description 1
- ZSDXEKUKQAKZFE-XAVMHZPKSA-N Ser-Thr-Pro Chemical compound C[C@H]([C@@H](C(=O)N1CCC[C@@H]1C(=O)O)NC(=O)[C@H](CO)N)O ZSDXEKUKQAKZFE-XAVMHZPKSA-N 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- 244000028419 Styrax benzoin Species 0.000 description 1
- 235000000126 Styrax benzoin Nutrition 0.000 description 1
- 208000032851 Subarachnoid Hemorrhage Diseases 0.000 description 1
- 235000008411 Sumatra benzointree Nutrition 0.000 description 1
- 102000019197 Superoxide Dismutase Human genes 0.000 description 1
- 108010012715 Superoxide dismutase Proteins 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 108010053950 Teicoplanin Proteins 0.000 description 1
- 244000269722 Thea sinensis Species 0.000 description 1
- WKDDRNSBRWANNC-UHFFFAOYSA-N Thienamycin Natural products C1C(SCCN)=C(C(O)=O)N2C(=O)C(C(O)C)C21 WKDDRNSBRWANNC-UHFFFAOYSA-N 0.000 description 1
- IGROJMCBGRFRGI-YTLHQDLWSA-N Thr-Ala-Ala Chemical compound C[C@@H](O)[C@H](N)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(O)=O IGROJMCBGRFRGI-YTLHQDLWSA-N 0.000 description 1
- VUVCRYXYUUPGSB-GLLZPBPUSA-N Thr-Gln-Glu Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=O)O)C(=O)O)N)O VUVCRYXYUUPGSB-GLLZPBPUSA-N 0.000 description 1
- KLCCPYZXGXHAGS-QTKMDUPCSA-N Thr-His-Met Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC1=CN=CN1)C(=O)N[C@@H](CCSC)C(=O)O)N)O KLCCPYZXGXHAGS-QTKMDUPCSA-N 0.000 description 1
- MECLEFZMPPOEAC-VOAKCMCISA-N Thr-Leu-Lys Chemical compound C[C@H]([C@@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)O)N)O MECLEFZMPPOEAC-VOAKCMCISA-N 0.000 description 1
- KZSYAEWQMJEGRZ-RHYQMDGZSA-N Thr-Leu-Val Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(O)=O KZSYAEWQMJEGRZ-RHYQMDGZSA-N 0.000 description 1
- JWQNAFHCXKVZKZ-UVOCVTCTSA-N Thr-Lys-Thr Chemical compound [H]N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(O)=O JWQNAFHCXKVZKZ-UVOCVTCTSA-N 0.000 description 1
- HDTRYLNUVZCQOY-WSWWMNSNSA-N Trehalose Natural products O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-WSWWMNSNSA-N 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- JRXKIVGWMMIIOF-YDHLFZDLSA-N Tyr-Asn-Val Chemical compound CC(C)[C@@H](C(=O)O)NC(=O)[C@H](CC(=O)N)NC(=O)[C@H](CC1=CC=C(C=C1)O)N JRXKIVGWMMIIOF-YDHLFZDLSA-N 0.000 description 1
- LOOCQRRBKZTPKO-AVGNSLFASA-N Tyr-Glu-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](N)CC1=CC=C(O)C=C1 LOOCQRRBKZTPKO-AVGNSLFASA-N 0.000 description 1
- CDHQEOXPWBDFPL-QWRGUYRKSA-N Tyr-Gly-Asn Chemical compound NC(=O)C[C@@H](C(O)=O)NC(=O)CNC(=O)[C@@H](N)CC1=CC=C(O)C=C1 CDHQEOXPWBDFPL-QWRGUYRKSA-N 0.000 description 1
- BXPOOVDVGWEXDU-WZLNRYEVSA-N Tyr-Ile-Thr Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(O)=O BXPOOVDVGWEXDU-WZLNRYEVSA-N 0.000 description 1
- GQVZBMROTPEPIF-SRVKXCTJSA-N Tyr-Ser-Asp Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(O)=O)C(O)=O GQVZBMROTPEPIF-SRVKXCTJSA-N 0.000 description 1
- WQOHKVRQDLNDIL-YJRXYDGGSA-N Tyr-Thr-Ser Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CO)C(O)=O WQOHKVRQDLNDIL-YJRXYDGGSA-N 0.000 description 1
- MJUTYRIMFIICKL-JYJNAYRXSA-N Tyr-Val-Arg Chemical compound [H]N[C@@H](CC1=CC=C(O)C=C1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O MJUTYRIMFIICKL-JYJNAYRXSA-N 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 description 1
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 description 1
- UDNYEPLJTRDMEJ-RCOVLWMOSA-N Val-Asn-Gly Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC(=O)N)C(=O)NCC(=O)O)N UDNYEPLJTRDMEJ-RCOVLWMOSA-N 0.000 description 1
- VLOYGOZDPGYWFO-LAEOZQHASA-N Val-Asp-Glu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(O)=O VLOYGOZDPGYWFO-LAEOZQHASA-N 0.000 description 1
- ROLGIBMFNMZANA-GVXVVHGQSA-N Val-Glu-Leu Chemical compound CC(C)C[C@@H](C(=O)O)NC(=O)[C@H](CCC(=O)O)NC(=O)[C@H](C(C)C)N ROLGIBMFNMZANA-GVXVVHGQSA-N 0.000 description 1
- UEHRGZCNLSWGHK-DLOVCJGASA-N Val-Glu-Val Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C(C)C)C(O)=O UEHRGZCNLSWGHK-DLOVCJGASA-N 0.000 description 1
- BZWUSZGQOILYEU-STECZYCISA-N Val-Ile-Tyr Chemical compound CC(C)[C@H](N)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](C(O)=O)CC1=CC=C(O)C=C1 BZWUSZGQOILYEU-STECZYCISA-N 0.000 description 1
- ZRSZTKTVPNSUNA-IHRRRGAJSA-N Val-Lys-Leu Chemical compound CC(C)C[C@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](N)C(C)C)C(O)=O ZRSZTKTVPNSUNA-IHRRRGAJSA-N 0.000 description 1
- RQOMPQGUGBILAG-AVGNSLFASA-N Val-Met-Leu Chemical compound CC(C)[C@H](N)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(C)C)C(O)=O RQOMPQGUGBILAG-AVGNSLFASA-N 0.000 description 1
- QPPZEDOTPZOSEC-RCWTZXSCSA-N Val-Met-Thr Chemical compound C[C@H]([C@@H](C(=O)O)NC(=O)[C@H](CCSC)NC(=O)[C@H](C(C)C)N)O QPPZEDOTPZOSEC-RCWTZXSCSA-N 0.000 description 1
- NZGOVKLVQNOEKP-YDHLFZDLSA-N Val-Phe-Asn Chemical compound CC(C)[C@@H](C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC(=O)N)C(=O)O)N NZGOVKLVQNOEKP-YDHLFZDLSA-N 0.000 description 1
- BCBFMJYTNKDALA-UFYCRDLUSA-N Val-Phe-Phe Chemical compound N[C@@H](C(C)C)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)O BCBFMJYTNKDALA-UFYCRDLUSA-N 0.000 description 1
- 108010059993 Vancomycin Proteins 0.000 description 1
- 206010047163 Vasospasm Diseases 0.000 description 1
- 208000012886 Vertigo Diseases 0.000 description 1
- OIRDTQYFTABQOQ-UHTZMRCNSA-N Vidarabine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@@H]1O OIRDTQYFTABQOQ-UHTZMRCNSA-N 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- FPIPGXGPPPQFEQ-BOOMUCAASA-N Vitamin A Natural products OC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-BOOMUCAASA-N 0.000 description 1
- 229930003537 Vitamin B3 Natural products 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- 235000009754 Vitis X bourquina Nutrition 0.000 description 1
- 235000012333 Vitis X labruscana Nutrition 0.000 description 1
- 240000006365 Vitis vinifera Species 0.000 description 1
- 235000014787 Vitis vinifera Nutrition 0.000 description 1
- WERKSKAQRVDLDW-ANOHMWSOSA-N [(2s,3r,4r,5r)-2,3,4,5,6-pentahydroxyhexyl] (z)-octadec-9-enoate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO WERKSKAQRVDLDW-ANOHMWSOSA-N 0.000 description 1
- DLGSOJOOYHWROO-WQLSENKSSA-N [(z)-(1-methyl-2-oxoindol-3-ylidene)amino]thiourea Chemical compound C1=CC=C2N(C)C(=O)\C(=N/NC(N)=S)C2=C1 DLGSOJOOYHWROO-WQLSENKSSA-N 0.000 description 1
- 239000000205 acacia gum Substances 0.000 description 1
- 229940022663 acetate Drugs 0.000 description 1
- 150000001242 acetic acid derivatives Chemical class 0.000 description 1
- 229960000669 acetylleucine Drugs 0.000 description 1
- 229960001138 acetylsalicylic acid Drugs 0.000 description 1
- RJURFGZVJUQBHK-IIXSONLDSA-N actinomycin D Chemical compound C[C@H]1OC(=O)[C@H](C(C)C)N(C)C(=O)CN(C)C(=O)[C@@H]2CCCN2C(=O)[C@@H](C(C)C)NC(=O)[C@H]1NC(=O)C1=C(N)C(=O)C(C)=C2OC(C(C)=CC=C3C(=O)N[C@@H]4C(=O)N[C@@H](C(N5CCC[C@H]5C(=O)N(C)CC(=O)N(C)[C@@H](C(C)C)C(=O)O[C@@H]4C)=O)C(C)C)=C3N=C21 RJURFGZVJUQBHK-IIXSONLDSA-N 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 230000006978 adaptation Effects 0.000 description 1
- 230000004721 adaptive immunity Effects 0.000 description 1
- WNLRTRBMVRJNCN-UHFFFAOYSA-N adipic acid Chemical class OC(=O)CCCCC(O)=O WNLRTRBMVRJNCN-UHFFFAOYSA-N 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 229940013181 advil Drugs 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- BNPSSFBOAGDEEL-UHFFFAOYSA-N albuterol sulfate Chemical compound OS(O)(=O)=O.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1.CC(C)(C)NCC(O)C1=CC=C(O)C(CO)=C1 BNPSSFBOAGDEEL-UHFFFAOYSA-N 0.000 description 1
- 150000001299 aldehydes Chemical class 0.000 description 1
- 229940060515 aleve Drugs 0.000 description 1
- 229940072056 alginate Drugs 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 229910052783 alkali metal Inorganic materials 0.000 description 1
- 150000001350 alkyl halides Chemical class 0.000 description 1
- 125000002947 alkylene group Chemical group 0.000 description 1
- 229930002945 all-trans-retinaldehyde Natural products 0.000 description 1
- 229940061720 alpha hydroxy acid Drugs 0.000 description 1
- 150000001280 alpha hydroxy acids Chemical class 0.000 description 1
- HDTRYLNUVZCQOY-LIZSDCNHSA-N alpha,alpha-trehalose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 HDTRYLNUVZCQOY-LIZSDCNHSA-N 0.000 description 1
- WQZGKKKJIJFFOK-PHYPRBDBSA-N alpha-D-galactose Chemical compound OC[C@H]1O[C@H](O)[C@H](O)[C@@H](O)[C@H]1O WQZGKKKJIJFFOK-PHYPRBDBSA-N 0.000 description 1
- AZDRQVAHHNSJOQ-UHFFFAOYSA-N alumane Chemical class [AlH3] AZDRQVAHHNSJOQ-UHFFFAOYSA-N 0.000 description 1
- 235000012211 aluminium silicate Nutrition 0.000 description 1
- DKNWSYNQZKUICI-UHFFFAOYSA-N amantadine Chemical compound C1C(C2)CC3CC2CC1(N)C3 DKNWSYNQZKUICI-UHFFFAOYSA-N 0.000 description 1
- 229960003805 amantadine Drugs 0.000 description 1
- 229960004821 amikacin Drugs 0.000 description 1
- LKCWBDHBTVXHDL-RMDFUYIESA-N amikacin Chemical compound O([C@@H]1[C@@H](N)C[C@H]([C@@H]([C@H]1O)O[C@@H]1[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O1)O)NC(=O)[C@@H](O)CCN)[C@H]1O[C@H](CN)[C@@H](O)[C@H](O)[C@H]1O LKCWBDHBTVXHDL-RMDFUYIESA-N 0.000 description 1
- 229940126575 aminoglycoside Drugs 0.000 description 1
- HAMNKKUPIHEESI-UHFFFAOYSA-N aminoguanidine Chemical compound NNC(N)=N HAMNKKUPIHEESI-UHFFFAOYSA-N 0.000 description 1
- 229960003022 amoxicillin Drugs 0.000 description 1
- LSQZJLSUYDQPKJ-NJBDSQKTSA-N amoxicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=C(O)C=C1 LSQZJLSUYDQPKJ-NJBDSQKTSA-N 0.000 description 1
- 229940009444 amphotericin Drugs 0.000 description 1
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 1
- 229960003942 amphotericin b Drugs 0.000 description 1
- 229960000723 ampicillin Drugs 0.000 description 1
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 1
- VJZITPJGSQKZMX-XDPRQOKASA-N amrubicin Chemical compound O([C@H]1C[C@](CC2=C(O)C=3C(=O)C4=CC=CC=C4C(=O)C=3C(O)=C21)(N)C(=O)C)[C@H]1C[C@H](O)[C@H](O)CO1 VJZITPJGSQKZMX-XDPRQOKASA-N 0.000 description 1
- 229960002550 amrubicin Drugs 0.000 description 1
- 206010002022 amyloidosis Diseases 0.000 description 1
- 239000003263 anabolic agent Substances 0.000 description 1
- 229940070021 anabolic steroids Drugs 0.000 description 1
- 230000000202 analgesic effect Effects 0.000 description 1
- 229940035676 analgesics Drugs 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 210000003484 anatomy Anatomy 0.000 description 1
- 235000019728 animal nutrition Nutrition 0.000 description 1
- 125000000129 anionic group Chemical group 0.000 description 1
- 239000003945 anionic surfactant Substances 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 239000000730 antalgic agent Substances 0.000 description 1
- 230000000844 anti-bacterial effect Effects 0.000 description 1
- 229940124599 anti-inflammatory drug Drugs 0.000 description 1
- 229940111131 antiinflammatory and antirheumatic product propionic acid derivative Drugs 0.000 description 1
- 229940045988 antineoplastic drug protein kinase inhibitors Drugs 0.000 description 1
- 239000003904 antiprotozoal agent Substances 0.000 description 1
- 101150089041 aph-1 gene Proteins 0.000 description 1
- 235000019789 appetite Nutrition 0.000 description 1
- 230000036528 appetite Effects 0.000 description 1
- 235000021407 appetite control Nutrition 0.000 description 1
- 239000007900 aqueous suspension Substances 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 108010060035 arginylproline Proteins 0.000 description 1
- 125000003289 ascorbyl group Chemical group [H]O[C@@]([H])(C([H])([H])O*)[C@@]1([H])OC(=O)C(O*)=C1O* 0.000 description 1
- 108010077245 asparaginyl-proline Proteins 0.000 description 1
- 239000000605 aspartame Substances 0.000 description 1
- IAOZJIPTCAWIRG-QWRGUYRKSA-N aspartame Chemical compound OC(=O)C[C@H](N)C(=O)N[C@H](C(=O)OC)CC1=CC=CC=C1 IAOZJIPTCAWIRG-QWRGUYRKSA-N 0.000 description 1
- 235000010357 aspartame Nutrition 0.000 description 1
- 229960003438 aspartame Drugs 0.000 description 1
- CKLJMWTZIZZHCS-REOHCLBHSA-L aspartate group Chemical class N[C@@H](CC(=O)[O-])C(=O)[O-] CKLJMWTZIZZHCS-REOHCLBHSA-L 0.000 description 1
- 108010093581 aspartyl-proline Proteins 0.000 description 1
- 108010038633 aspartylglutamate Proteins 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 239000000305 astragalus gummifer gum Substances 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 230000037444 atrophy Effects 0.000 description 1
- 229960001671 azapropazone Drugs 0.000 description 1
- WOIIIUDZSOLAIW-NSHDSACASA-N azapropazone Chemical compound C1=C(C)C=C2N3C(=O)[C@H](CC=C)C(=O)N3C(N(C)C)=NC2=C1 WOIIIUDZSOLAIW-NSHDSACASA-N 0.000 description 1
- 229950011321 azaserine Drugs 0.000 description 1
- 229960004099 azithromycin Drugs 0.000 description 1
- MQTOSJVFKKJCRP-BICOPXKESA-N azithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)N(C)C[C@H](C)C[C@@](C)(O)[C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 MQTOSJVFKKJCRP-BICOPXKESA-N 0.000 description 1
- 229960003644 aztreonam Drugs 0.000 description 1
- 239000007640 basal medium Substances 0.000 description 1
- 150000007514 bases Chemical class 0.000 description 1
- OGBUMNBNEWYMNJ-UHFFFAOYSA-N batilol Chemical class CCCCCCCCCCCCCCCCCCOCC(O)CO OGBUMNBNEWYMNJ-UHFFFAOYSA-N 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 229950000210 beclometasone dipropionate Drugs 0.000 description 1
- 235000013871 bee wax Nutrition 0.000 description 1
- 239000012166 beeswax Substances 0.000 description 1
- 230000003542 behavioural effect Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 239000000440 bentonite Substances 0.000 description 1
- 229910000278 bentonite Inorganic materials 0.000 description 1
- SVPXDRXYRYOSEX-UHFFFAOYSA-N bentoquatam Chemical compound O.O=[Si]=O.O=[Al]O[Al]=O SVPXDRXYRYOSEX-UHFFFAOYSA-N 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 229940092714 benzenesulfonic acid Drugs 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- 229960002130 benzoin Drugs 0.000 description 1
- 235000019445 benzyl alcohol Nutrition 0.000 description 1
- 125000001797 benzyl group Chemical group [H]C1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])* 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- 150000001277 beta hydroxy acids Chemical class 0.000 description 1
- DRTQHJPVMGBUCF-PSQAKQOGSA-N beta-L-uridine Natural products O[C@H]1[C@@H](O)[C@H](CO)O[C@@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-PSQAKQOGSA-N 0.000 description 1
- XMIIGOLPHOKFCH-UHFFFAOYSA-N beta-phenylpropanoic acid Natural products OC(=O)CCC1=CC=CC=C1 XMIIGOLPHOKFCH-UHFFFAOYSA-N 0.000 description 1
- 229960002537 betamethasone Drugs 0.000 description 1
- UREBDLICKHMUKA-DVTGEIKXSA-N betamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-DVTGEIKXSA-N 0.000 description 1
- 239000003833 bile salt Substances 0.000 description 1
- 229940093761 bile salts Drugs 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 229940093797 bioflavonoids Drugs 0.000 description 1
- 239000003124 biologic agent Substances 0.000 description 1
- 230000008827 biological function Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- QRZAKQDHEVVFRX-UHFFFAOYSA-N biphenyl-4-ylacetic acid Chemical compound C1=CC(CC(=O)O)=CC=C1C1=CC=CC=C1 QRZAKQDHEVVFRX-UHFFFAOYSA-N 0.000 description 1
- 230000008499 blood brain barrier function Effects 0.000 description 1
- 230000036765 blood level Effects 0.000 description 1
- 210000004204 blood vessel Anatomy 0.000 description 1
- 210000001218 blood-brain barrier Anatomy 0.000 description 1
- KGBXLFKZBHKPEV-UHFFFAOYSA-N boric acid Chemical compound OB(O)O KGBXLFKZBHKPEV-UHFFFAOYSA-N 0.000 description 1
- 239000004327 boric acid Substances 0.000 description 1
- 210000004958 brain cell Anatomy 0.000 description 1
- 230000006931 brain damage Effects 0.000 description 1
- 231100000874 brain damage Toxicity 0.000 description 1
- 208000029028 brain injury Diseases 0.000 description 1
- QBSGXIBYUQJHMJ-UHFFFAOYSA-N bromochlorosalicylanilide Chemical compound OC1=CC=C(Br)C=C1C(=O)NC1=CC=C(Cl)C=C1 QBSGXIBYUQJHMJ-UHFFFAOYSA-N 0.000 description 1
- 229960000712 bromochlorosalicylanilide Drugs 0.000 description 1
- 230000001680 brushing effect Effects 0.000 description 1
- ZGJHIFYEQJEUKA-UHFFFAOYSA-N buclosamide Chemical compound CCCCNC(=O)C1=CC=C(Cl)C=C1O ZGJHIFYEQJEUKA-UHFFFAOYSA-N 0.000 description 1
- 229950000430 buclosamide Drugs 0.000 description 1
- 239000006172 buffering agent Substances 0.000 description 1
- 230000003139 buffering effect Effects 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 150000004648 butanoic acid derivatives Chemical class 0.000 description 1
- 210000004900 c-terminal fragment Anatomy 0.000 description 1
- 235000010216 calcium carbonate Nutrition 0.000 description 1
- FUFJGUQYACFECW-UHFFFAOYSA-L calcium hydrogenphosphate Chemical compound [Ca+2].OP([O-])([O-])=O FUFJGUQYACFECW-UHFFFAOYSA-L 0.000 description 1
- 239000004330 calcium propionate Substances 0.000 description 1
- 235000010331 calcium propionate Nutrition 0.000 description 1
- 159000000007 calcium salts Chemical class 0.000 description 1
- 235000011132 calcium sulphate Nutrition 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- MIOPJNTWMNEORI-UHFFFAOYSA-N camphorsulfonic acid Chemical class C1CC2(CS(O)(=O)=O)C(=O)CC1C2(C)C MIOPJNTWMNEORI-UHFFFAOYSA-N 0.000 description 1
- 229960004348 candicidin Drugs 0.000 description 1
- 229940041011 carbapenems Drugs 0.000 description 1
- 229960003669 carbenicillin Drugs 0.000 description 1
- FPPNZSSZRUTDAP-UWFZAAFLSA-N carbenicillin Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)C(C(O)=O)C1=CC=CC=C1 FPPNZSSZRUTDAP-UWFZAAFLSA-N 0.000 description 1
- 229960001631 carbomer Drugs 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 150000004649 carbonic acid derivatives Chemical class 0.000 description 1
- 125000002843 carboxylic acid group Chemical class 0.000 description 1
- 150000001735 carboxylic acids Chemical class 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 239000003093 cationic surfactant Substances 0.000 description 1
- XIURVHNZVLADCM-IUODEOHRSA-N cefalotin Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC(=O)C)C(O)=O)C(=O)CC1=CC=CS1 XIURVHNZVLADCM-IUODEOHRSA-N 0.000 description 1
- 229960000603 cefalotin Drugs 0.000 description 1
- SNBUBQHDYVFSQF-HIFRSBDPSA-N cefmetazole Chemical compound S([C@@H]1[C@@](C(N1C=1C(O)=O)=O)(NC(=O)CSCC#N)OC)CC=1CSC1=NN=NN1C SNBUBQHDYVFSQF-HIFRSBDPSA-N 0.000 description 1
- 229960003585 cefmetazole Drugs 0.000 description 1
- WYUSVOMTXWRGEK-HBWVYFAYSA-N cefpodoxime Chemical compound N([C@H]1[C@@H]2N(C1=O)C(=C(CS2)COC)C(O)=O)C(=O)C(=N/OC)\C1=CSC(N)=N1 WYUSVOMTXWRGEK-HBWVYFAYSA-N 0.000 description 1
- 229960005090 cefpodoxime Drugs 0.000 description 1
- 229960000484 ceftazidime Drugs 0.000 description 1
- NMVPEQXCMGEDNH-TZVUEUGBSA-N ceftazidime pentahydrate Chemical compound O.O.O.O.O.S([C@@H]1[C@@H](C(N1C=1C([O-])=O)=O)NC(=O)\C(=N/OC(C)(C)C(O)=O)C=2N=C(N)SC=2)CC=1C[N+]1=CC=CC=C1 NMVPEQXCMGEDNH-TZVUEUGBSA-N 0.000 description 1
- 229960004755 ceftriaxone Drugs 0.000 description 1
- VAAUVRVFOQPIGI-SPQHTLEESA-N ceftriaxone Chemical compound S([C@@H]1[C@@H](C(N1C=1C(O)=O)=O)NC(=O)\C(=N/OC)C=2N=C(N)SC=2)CC=1CSC1=NC(=O)C(=O)NN1C VAAUVRVFOQPIGI-SPQHTLEESA-N 0.000 description 1
- 229960001668 cefuroxime Drugs 0.000 description 1
- JFPVXVDWJQMJEE-IZRZKJBUSA-N cefuroxime Chemical compound N([C@@H]1C(N2C(=C(COC(N)=O)CS[C@@H]21)C(O)=O)=O)C(=O)\C(=N/OC)C1=CC=CO1 JFPVXVDWJQMJEE-IZRZKJBUSA-N 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 229920002678 cellulose Chemical class 0.000 description 1
- 239000001913 cellulose Chemical class 0.000 description 1
- 235000010980 cellulose Nutrition 0.000 description 1
- 229940124587 cephalosporin Drugs 0.000 description 1
- 150000001780 cephalosporins Chemical class 0.000 description 1
- 229960000541 cetyl alcohol Drugs 0.000 description 1
- 239000013522 chelant Substances 0.000 description 1
- JQXXHWHPUNPDRT-BQVAUQFYSA-N chembl1523493 Chemical compound O([C@](C1=O)(C)O\C=C/[C@@H]([C@H]([C@@H](OC(C)=O)[C@H](C)[C@H](O)[C@H](C)[C@@H](O)[C@@H](C)/C=C\C=C(C)/C(=O)NC=2C(O)=C3C(O)=C4C)C)OC)C4=C1C3=C(O)C=2C=NN1CCN(C)CC1 JQXXHWHPUNPDRT-BQVAUQFYSA-N 0.000 description 1
- DDTDNCYHLGRFBM-YZEKDTGTSA-N chembl2367892 Chemical compound CC(=O)N[C@H]1[C@@H](O)[C@H](O)[C@H](CO)O[C@H]1O[C@@H]([C@H]1C(N[C@@H](C2=CC(O)=CC(O[C@@H]3[C@H]([C@H](O)[C@H](O)[C@@H](CO)O3)O)=C2C=2C(O)=CC=C(C=2)[C@@H](NC(=O)[C@@H]2NC(=O)[C@@H]3C=4C=C(O)C=C(C=4)OC=4C(O)=CC=C(C=4)[C@@H](N)C(=O)N[C@H](CC=4C=C(Cl)C(O5)=CC=4)C(=O)N3)C(=O)N1)C(O)=O)=O)C(C=C1Cl)=CC=C1OC1=C(O[C@H]3[C@H]([C@@H](O)[C@H](O)[C@H](CO)O3)NC(C)=O)C5=CC2=C1 DDTDNCYHLGRFBM-YZEKDTGTSA-N 0.000 description 1
- 239000012829 chemotherapy agent Substances 0.000 description 1
- 229960005091 chloramphenicol Drugs 0.000 description 1
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 1
- 229960000849 chlormidazole Drugs 0.000 description 1
- WNAQOLSMVPFGTE-UHFFFAOYSA-N chlormidazole Chemical compound CC1=NC2=CC=CC=C2N1CC1=CC=C(Cl)C=C1 WNAQOLSMVPFGTE-UHFFFAOYSA-N 0.000 description 1
- NPSLCOWKFFNQKK-ZPSUVKRCSA-N chloroprednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3C[C@H](Cl)C2=C1 NPSLCOWKFFNQKK-ZPSUVKRCSA-N 0.000 description 1
- 229950006229 chloroprednisone Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 229960001265 ciclosporin Drugs 0.000 description 1
- LOUPRKONTZGTKE-UHFFFAOYSA-N cinchonine Natural products C1C(C(C2)C=C)CCN2C1C(O)C1=CC=NC2=CC=C(OC)C=C21 LOUPRKONTZGTKE-UHFFFAOYSA-N 0.000 description 1
- 229960003405 ciprofloxacin Drugs 0.000 description 1
- 150000001860 citric acid derivatives Chemical class 0.000 description 1
- 229960002626 clarithromycin Drugs 0.000 description 1
- AGOYDEPGAOXOCK-KCBOHYOISA-N clarithromycin Chemical compound O([C@@H]1[C@@H](C)C(=O)O[C@@H]([C@@]([C@H](O)[C@@H](C)C(=O)[C@H](C)C[C@](C)([C@H](O[C@H]2[C@@H]([C@H](C[C@@H](C)O2)N(C)C)O)[C@H]1C)OC)(C)O)CC)[C@H]1C[C@@](C)(OC)[C@@H](O)[C@H](C)O1 AGOYDEPGAOXOCK-KCBOHYOISA-N 0.000 description 1
- 238000004140 cleaning Methods 0.000 description 1
- YNNUSGIPVFPVBX-NHCUHLMSSA-N clemastine Chemical compound CN1CCC[C@@H]1CCO[C@@](C)(C=1C=CC(Cl)=CC=1)C1=CC=CC=C1 YNNUSGIPVFPVBX-NHCUHLMSSA-N 0.000 description 1
- 229960002881 clemastine Drugs 0.000 description 1
- 229960002227 clindamycin Drugs 0.000 description 1
- KDLRVYVGXIQJDK-AWPVFWJPSA-N clindamycin Chemical compound CN1C[C@H](CCC)C[C@H]1C(=O)N[C@H]([C@H](C)Cl)[C@@H]1[C@H](O)[C@H](O)[C@@H](O)[C@@H](SC)O1 KDLRVYVGXIQJDK-AWPVFWJPSA-N 0.000 description 1
- 229960002842 clobetasol Drugs 0.000 description 1
- CBGUOGMQLZIXBE-XGQKBEPLSA-N clobetasol propionate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@H](C)[C@@](C(=O)CCl)(OC(=O)CC)[C@@]1(C)C[C@@H]2O CBGUOGMQLZIXBE-XGQKBEPLSA-N 0.000 description 1
- 229960002896 clonidine Drugs 0.000 description 1
- 229960004022 clotrimazole Drugs 0.000 description 1
- VNFPBHJOKIVQEB-UHFFFAOYSA-N clotrimazole Chemical compound ClC1=CC=CC=C1C(N1C=NC=C1)(C=1C=CC=CC=1)C1=CC=CC=C1 VNFPBHJOKIVQEB-UHFFFAOYSA-N 0.000 description 1
- 229960003326 cloxacillin Drugs 0.000 description 1
- LQOLIRLGBULYKD-JKIFEVAISA-N cloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1Cl LQOLIRLGBULYKD-JKIFEVAISA-N 0.000 description 1
- 229940110456 cocoa butter Drugs 0.000 description 1
- 235000019868 cocoa butter Nutrition 0.000 description 1
- 239000003240 coconut oil Substances 0.000 description 1
- 235000019864 coconut oil Nutrition 0.000 description 1
- 229960001338 colchicine Drugs 0.000 description 1
- 229940075614 colloidal silicon dioxide Drugs 0.000 description 1
- 239000000084 colloidal system Substances 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 239000000470 constituent Substances 0.000 description 1
- 208000013044 corticobasal degeneration disease Diseases 0.000 description 1
- BMCQMVFGOVHVNG-TUFAYURCSA-N cortisol 17-butyrate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)CO)(OC(=O)CCC)[C@@]1(C)C[C@@H]2O BMCQMVFGOVHVNG-TUFAYURCSA-N 0.000 description 1
- FZCHYNWYXKICIO-FZNHGJLXSA-N cortisol 17-valerate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)CO)(OC(=O)CCCC)[C@@]1(C)C[C@@H]2O FZCHYNWYXKICIO-FZNHGJLXSA-N 0.000 description 1
- ALEXXDVDDISNDU-JZYPGELDSA-N cortisol 21-acetate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2O ALEXXDVDDISNDU-JZYPGELDSA-N 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 239000012531 culture fluid Substances 0.000 description 1
- 239000004148 curcumin Substances 0.000 description 1
- 229940109262 curcumin Drugs 0.000 description 1
- 235000012754 curcumin Nutrition 0.000 description 1
- 150000001913 cyanates Chemical class 0.000 description 1
- 229940097362 cyclodextrins Drugs 0.000 description 1
- 229930182912 cyclosporin Natural products 0.000 description 1
- 229960000684 cytarabine Drugs 0.000 description 1
- 102000003675 cytokine receptors Human genes 0.000 description 1
- 108010057085 cytokine receptors Proteins 0.000 description 1
- 229960000640 dactinomycin Drugs 0.000 description 1
- 229940099385 daraprim Drugs 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 125000002704 decyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000003412 degenerative effect Effects 0.000 description 1
- 230000000593 degrading effect Effects 0.000 description 1
- 238000010217 densitometric analysis Methods 0.000 description 1
- KXGVEGMKQFWNSR-LLQZFEROSA-N deoxycholic acid Chemical compound C([C@H]1CC2)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 KXGVEGMKQFWNSR-LLQZFEROSA-N 0.000 description 1
- 229960003964 deoxycholic acid Drugs 0.000 description 1
- WBGKWQHBNHJJPZ-LECWWXJVSA-N desonide Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@@H]2[C@@H]1[C@@H]1C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]1(C)C[C@@H]2O WBGKWQHBNHJJPZ-LECWWXJVSA-N 0.000 description 1
- 229960003662 desonide Drugs 0.000 description 1
- 229960002593 desoximetasone Drugs 0.000 description 1
- VWVSBHGCDBMOOT-IIEHVVJPSA-N desoximetasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@H](C(=O)CO)[C@@]1(C)C[C@@H]2O VWVSBHGCDBMOOT-IIEHVVJPSA-N 0.000 description 1
- 238000001514 detection method Methods 0.000 description 1
- 239000003599 detergent Substances 0.000 description 1
- UREBDLICKHMUKA-CXSFZGCWSA-N dexamethasone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]1(C)C[C@@H]2O UREBDLICKHMUKA-CXSFZGCWSA-N 0.000 description 1
- 229960004833 dexamethasone phosphate Drugs 0.000 description 1
- VQODGRNSFPNSQE-CXSFZGCWSA-N dexamethasone phosphate Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1C[C@@H](C)[C@@](C(=O)COP(O)(O)=O)(O)[C@@]1(C)C[C@@H]2O VQODGRNSFPNSQE-CXSFZGCWSA-N 0.000 description 1
- 235000019425 dextrin Nutrition 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000002059 diagnostic imaging Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 150000008050 dialkyl sulfates Chemical class 0.000 description 1
- 235000019700 dicalcium phosphate Nutrition 0.000 description 1
- 229950009888 dichlorisone Drugs 0.000 description 1
- YNNURTVKPVJVEI-GSLJADNHSA-N dichlorisone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(Cl)[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)C)(O)[C@@]1(C)C[C@@H]2Cl YNNURTVKPVJVEI-GSLJADNHSA-N 0.000 description 1
- 229960001259 diclofenac Drugs 0.000 description 1
- DCOPUUMXTXDBNB-UHFFFAOYSA-N diclofenac Chemical compound OC(=O)CC1=CC=CC=C1NC1=C(Cl)C=CC=C1Cl DCOPUUMXTXDBNB-UHFFFAOYSA-N 0.000 description 1
- 229960001585 dicloxacillin Drugs 0.000 description 1
- YFAGHNZHGGCZAX-JKIFEVAISA-N dicloxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=C(Cl)C=CC=C1Cl YFAGHNZHGGCZAX-JKIFEVAISA-N 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 125000004177 diethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- HPNMFZURTQLUMO-UHFFFAOYSA-N diethylamine Chemical compound CCNCC HPNMFZURTQLUMO-UHFFFAOYSA-N 0.000 description 1
- VFLDPWHFBUODDF-UHFFFAOYSA-N diferuloylmethane Natural products C1=C(O)C(OC)=CC(C=CC(=O)CC(=O)C=CC=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-UHFFFAOYSA-N 0.000 description 1
- 229960002124 diflorasone diacetate Drugs 0.000 description 1
- BOBLHFUVNSFZPJ-JOYXJVLSSA-N diflorasone diacetate Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@H](C)[C@@](C(=O)COC(C)=O)(OC(C)=O)[C@@]2(C)C[C@@H]1O BOBLHFUVNSFZPJ-JOYXJVLSSA-N 0.000 description 1
- HUPFGZXOMWLGNK-UHFFFAOYSA-N diflunisal Chemical compound C1=C(O)C(C(=O)O)=CC(C=2C(=CC(F)=CC=2)F)=C1 HUPFGZXOMWLGNK-UHFFFAOYSA-N 0.000 description 1
- 229960000616 diflunisal Drugs 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- UGMCXQCYOVCMTB-UHFFFAOYSA-K dihydroxy(stearato)aluminium Chemical compound CCCCCCCCCCCCCCCCCC(=O)O[Al](O)O UGMCXQCYOVCMTB-UHFFFAOYSA-K 0.000 description 1
- BZCOSCNPHJNQBP-OWOJBTEDSA-N dihydroxyfumaric acid Chemical compound OC(=O)C(\O)=C(/O)C(O)=O BZCOSCNPHJNQBP-OWOJBTEDSA-N 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 125000000118 dimethyl group Chemical group [H]C([H])([H])* 0.000 description 1
- GAFRWLVTHPVQGK-UHFFFAOYSA-N dipentyl sulfate Chemical class CCCCCOS(=O)(=O)OCCCCC GAFRWLVTHPVQGK-UHFFFAOYSA-N 0.000 description 1
- 150000002016 disaccharides Chemical class 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 125000003438 dodecyl group Chemical group [H]C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])* 0.000 description 1
- 229960003638 dopamine Drugs 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 229960003722 doxycycline Drugs 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 230000004064 dysfunction Effects 0.000 description 1
- 235000006694 eating habits Nutrition 0.000 description 1
- 229960003913 econazole Drugs 0.000 description 1
- 230000012202 endocytosis Effects 0.000 description 1
- 238000005265 energy consumption Methods 0.000 description 1
- IDYZIJYBMGIQMJ-UHFFFAOYSA-N enoxacin Chemical compound N1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 IDYZIJYBMGIQMJ-UHFFFAOYSA-N 0.000 description 1
- 229960002549 enoxacin Drugs 0.000 description 1
- 229960001904 epirubicin Drugs 0.000 description 1
- NSYZCCDSJNWWJL-YXOIYICCSA-N erythromycin ethylsuccinate Chemical compound O1[C@H](C)C[C@H](N(C)C)[C@@H](OC(=O)CCC(=O)OCC)[C@@H]1O[C@H]1[C@@](O)(C)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@](C)(O)[C@@H](CC)OC(=O)[C@H](C)[C@@H](O[C@@H]2O[C@@H](C)[C@H](O)[C@](C)(OC)C2)[C@@H]1C NSYZCCDSJNWWJL-YXOIYICCSA-N 0.000 description 1
- 229960000741 erythromycin ethylsuccinate Drugs 0.000 description 1
- 229960004213 erythromycin lactobionate Drugs 0.000 description 1
- 229930182833 estradiol Natural products 0.000 description 1
- 229960005309 estradiol Drugs 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 125000001495 ethyl group Chemical group [H]C([H])([H])C([H])([H])* 0.000 description 1
- LVGKNOAMLMIIKO-QXMHVHEDSA-N ethyl oleate Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC LVGKNOAMLMIIKO-QXMHVHEDSA-N 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 230000005713 exacerbation Effects 0.000 description 1
- 238000013265 extended release Methods 0.000 description 1
- 239000011536 extraction buffer Substances 0.000 description 1
- 229960000192 felbinac Drugs 0.000 description 1
- 229960001395 fenbufen Drugs 0.000 description 1
- ZPAKPRAICRBAOD-UHFFFAOYSA-N fenbufen Chemical compound C1=CC(C(=O)CCC(=O)O)=CC=C1C1=CC=CC=C1 ZPAKPRAICRBAOD-UHFFFAOYSA-N 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 235000019634 flavors Nutrition 0.000 description 1
- 238000007667 floating Methods 0.000 description 1
- 229960004413 flucytosine Drugs 0.000 description 1
- XRECTZIEBJDKEO-UHFFFAOYSA-N flucytosine Chemical compound NC1=NC(=O)NC=C1F XRECTZIEBJDKEO-UHFFFAOYSA-N 0.000 description 1
- AAXVEMMRQDVLJB-BULBTXNYSA-N fludrocortisone Chemical compound O=C1CC[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 AAXVEMMRQDVLJB-BULBTXNYSA-N 0.000 description 1
- 229960002011 fludrocortisone Drugs 0.000 description 1
- 229960004369 flufenamic acid Drugs 0.000 description 1
- 229960003469 flumetasone Drugs 0.000 description 1
- WXURHACBFYSXBI-GQKYHHCASA-N flumethasone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@]1(F)[C@@H]2[C@@H]2C[C@@H](C)[C@@](C(=O)CO)(O)[C@@]2(C)C[C@@H]1O WXURHACBFYSXBI-GQKYHHCASA-N 0.000 description 1
- 229960000676 flunisolide Drugs 0.000 description 1
- 229960000785 fluocinonide Drugs 0.000 description 1
- GAKMQHDJQHZUTJ-ULHLPKEOSA-N fluocortolone Chemical compound C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@@H](C)[C@H](C(=O)CO)[C@@]2(C)C[C@@H]1O GAKMQHDJQHZUTJ-ULHLPKEOSA-N 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 229910052731 fluorine Inorganic materials 0.000 description 1
- 239000011737 fluorine Substances 0.000 description 1
- 229940060037 fluorine Drugs 0.000 description 1
- 229940124307 fluoroquinolone Drugs 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 229960003590 fluperolone Drugs 0.000 description 1
- HHPZZKDXAFJLOH-QZIXMDIESA-N fluperolone Chemical compound C1CC2=CC(=O)C=C[C@]2(C)[C@]2(F)[C@@H]1[C@@H]1CC[C@@](C(=O)[C@@H](OC(C)=O)C)(O)[C@@]1(C)C[C@@H]2O HHPZZKDXAFJLOH-QZIXMDIESA-N 0.000 description 1
- 229960003238 fluprednidene Drugs 0.000 description 1
- YVHXHNGGPURVOS-SBTDHBFYSA-N fluprednidene Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@](C(=C)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 YVHXHNGGPURVOS-SBTDHBFYSA-N 0.000 description 1
- 229960000618 fluprednisolone Drugs 0.000 description 1
- 229960000690 flutrimazole Drugs 0.000 description 1
- QHMWCHQXCUNUAK-UHFFFAOYSA-N flutrimazole Chemical compound C1=CC(F)=CC=C1C(N1C=NC=C1)(C=1C(=CC=CC=1)F)C1=CC=CC=C1 QHMWCHQXCUNUAK-UHFFFAOYSA-N 0.000 description 1
- 239000011888 foil Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 235000019253 formic acid Nutrition 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000012458 free base Substances 0.000 description 1
- 230000000855 fungicidal effect Effects 0.000 description 1
- 239000000417 fungicide Substances 0.000 description 1
- 229930182830 galactose Natural products 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 229940083124 ganglion-blocking antiadrenergic secondary and tertiary amines Drugs 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 1
- 229960005277 gemcitabine Drugs 0.000 description 1
- 239000011521 glass Substances 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 239000003292 glue Substances 0.000 description 1
- 229930195712 glutamate Natural products 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- 229960003180 glutathione Drugs 0.000 description 1
- SYUXAJSOZXEFPP-UHFFFAOYSA-N glutin Natural products COc1c(O)cc2OC(=CC(=O)c2c1O)c3ccccc3OC4OC(CO)C(O)C(O)C4O SYUXAJSOZXEFPP-UHFFFAOYSA-N 0.000 description 1
- 150000002315 glycerophosphates Chemical class 0.000 description 1
- 229940074045 glyceryl distearate Drugs 0.000 description 1
- 229940075507 glyceryl monostearate Drugs 0.000 description 1
- 239000001087 glyceryl triacetate Substances 0.000 description 1
- 235000013773 glyceryl triacetate Nutrition 0.000 description 1
- 229960003711 glyceryl trinitrate Drugs 0.000 description 1
- RFDAIACWWDREDC-FRVQLJSFSA-N glycocholic acid Chemical compound C([C@H]1C[C@H]2O)[C@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@@H](CCC(=O)NCC(O)=O)C)[C@@]2(C)[C@@H](O)C1 RFDAIACWWDREDC-FRVQLJSFSA-N 0.000 description 1
- 150000002334 glycols Chemical class 0.000 description 1
- 229930182470 glycoside Natural products 0.000 description 1
- 150000002338 glycosides Chemical class 0.000 description 1
- 150000002339 glycosphingolipids Chemical class 0.000 description 1
- 108010090037 glycyl-alanyl-isoleucine Proteins 0.000 description 1
- 108010037850 glycylvaline Proteins 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 229960002867 griseofulvin Drugs 0.000 description 1
- DDUHZTYCFQRHIY-RBHXEPJQSA-N griseofulvin Chemical compound COC1=CC(=O)C[C@@H](C)[C@@]11C(=O)C(C(OC)=CC(OC)=C2Cl)=C2O1 DDUHZTYCFQRHIY-RBHXEPJQSA-N 0.000 description 1
- 235000019382 gum benzoic Nutrition 0.000 description 1
- 229960003372 hachimycin Drugs 0.000 description 1
- IDWJWYPAJJDASX-GKXBZDASSA-N hachimycin Chemical compound O1C(=O)CC(=O)CC(O)CC(O)CCCC(O)CC(O)CC(O2)(O)CC(O)C(C(O)=O)C2CC(OC2C(C(N)C(O)C(C)O2)O)\C=C/C=C\C=C\C=C/C=C\C=C\C=C\C(C)C1C(C)CCC(O)CC(=O)C1=CC=C(N)C=C1 IDWJWYPAJJDASX-GKXBZDASSA-N 0.000 description 1
- 229950005233 haletazole Drugs 0.000 description 1
- TXOKWXJQVFUUDD-UHFFFAOYSA-N haletazole Chemical compound C1=CC(OCCN(CC)CC)=CC=C1C1=NC2=CC(Cl)=CC=C2S1 TXOKWXJQVFUUDD-UHFFFAOYSA-N 0.000 description 1
- 150000002366 halogen compounds Chemical class 0.000 description 1
- MNWFXJYAOYHMED-UHFFFAOYSA-N heptanoic acid Chemical compound CCCCCCC(O)=O MNWFXJYAOYHMED-UHFFFAOYSA-N 0.000 description 1
- FBPFZTCFMRRESA-UHFFFAOYSA-N hexane-1,2,3,4,5,6-hexol Chemical compound OCC(O)C(O)C(O)C(O)CO FBPFZTCFMRRESA-UHFFFAOYSA-N 0.000 description 1
- FUZZWVXGSFPDMH-UHFFFAOYSA-N hexanoic acid Chemical compound CCCCCC(O)=O FUZZWVXGSFPDMH-UHFFFAOYSA-N 0.000 description 1
- 229960004867 hexetidine Drugs 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 229960001340 histamine Drugs 0.000 description 1
- 108010040030 histidinoalanine Proteins 0.000 description 1
- 235000003642 hunger Nutrition 0.000 description 1
- 239000010903 husk Substances 0.000 description 1
- 229930195733 hydrocarbon Natural products 0.000 description 1
- 150000002430 hydrocarbons Chemical class 0.000 description 1
- 229950000208 hydrocortamate Drugs 0.000 description 1
- FWFVLWGEFDIZMJ-FOMYWIRZSA-N hydrocortamate Chemical compound C1CC2=CC(=O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@@](C(=O)COC(=O)CN(CC)CC)(O)[C@@]1(C)C[C@@H]2O FWFVLWGEFDIZMJ-FOMYWIRZSA-N 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 229960001067 hydrocortisone acetate Drugs 0.000 description 1
- 229960001524 hydrocortisone butyrate Drugs 0.000 description 1
- 229960003331 hydrocortisone cypionate Drugs 0.000 description 1
- 229960000631 hydrocortisone valerate Drugs 0.000 description 1
- 239000008172 hydrogenated vegetable oil Substances 0.000 description 1
- 150000004679 hydroxides Chemical class 0.000 description 1
- 150000005165 hydroxybenzoic acids Chemical class 0.000 description 1
- 235000019447 hydroxyethyl cellulose Nutrition 0.000 description 1
- 229920003063 hydroxymethyl cellulose Polymers 0.000 description 1
- 229940031574 hydroxymethyl cellulose Drugs 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 229960004716 idoxuridine Drugs 0.000 description 1
- 229960002182 imipenem Drugs 0.000 description 1
- ZSKVGTPCRGIANV-ZXFLCMHBSA-N imipenem Chemical compound C1C(SCC\N=C\N)=C(C(O)=O)N2C(=O)[C@H]([C@H](O)C)[C@H]21 ZSKVGTPCRGIANV-ZXFLCMHBSA-N 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 238000011534 incubation Methods 0.000 description 1
- 229960004187 indoprofen Drugs 0.000 description 1
- 239000003701 inert diluent Substances 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000007972 injectable composition Substances 0.000 description 1
- 229960003786 inosine Drugs 0.000 description 1
- 229960000367 inositol Drugs 0.000 description 1
- CDAISMWEOUEBRE-GPIVLXJGSA-N inositol Chemical compound O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@H](O)[C@@H]1O CDAISMWEOUEBRE-GPIVLXJGSA-N 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229940047124 interferons Drugs 0.000 description 1
- 230000031891 intestinal absorption Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 239000003456 ion exchange resin Substances 0.000 description 1
- 229920003303 ion-exchange polymer Polymers 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- GURKHSYORGJETM-WAQYZQTGSA-N irinotecan hydrochloride (anhydrous) Chemical compound Cl.C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 GURKHSYORGJETM-WAQYZQTGSA-N 0.000 description 1
- 230000000622 irritating effect Effects 0.000 description 1
- 229960004849 isoconazole Drugs 0.000 description 1
- QXJSBBXBKPUZAA-UHFFFAOYSA-N isooleic acid Natural products CCCCCCCC=CCCCCCCCCC(O)=O QXJSBBXBKPUZAA-UHFFFAOYSA-N 0.000 description 1
- 239000000644 isotonic solution Substances 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 229960005280 isotretinoin Drugs 0.000 description 1
- YYUAYBYLJSNDCX-UHFFFAOYSA-N isoxicam Chemical compound OC=1C2=CC=CC=C2S(=O)(=O)N(C)C=1C(=O)NC=1C=C(C)ON=1 YYUAYBYLJSNDCX-UHFFFAOYSA-N 0.000 description 1
- 229950002252 isoxicam Drugs 0.000 description 1
- 229960004130 itraconazole Drugs 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- 229930027917 kanamycin Natural products 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- NLYAJNPCOHFWQQ-UHFFFAOYSA-N kaolin Chemical compound O.O.O=[Al]O[Si](=O)O[Si](=O)O[Al]=O NLYAJNPCOHFWQQ-UHFFFAOYSA-N 0.000 description 1
- 235000014655 lactic acid Nutrition 0.000 description 1
- 239000004310 lactic acid Substances 0.000 description 1
- 229950010163 lanoconazole Drugs 0.000 description 1
- 229940062711 laureth-9 Drugs 0.000 description 1
- 239000003446 ligand Substances 0.000 description 1
- 125000005647 linker group Chemical group 0.000 description 1
- 230000004132 lipogenesis Effects 0.000 description 1
- 235000019136 lipoic acid Nutrition 0.000 description 1
- AGBQKNBQESQNJD-UHFFFAOYSA-N lipoic acid Chemical compound OC(=O)CCCCC1CCSS1 AGBQKNBQESQNJD-UHFFFAOYSA-N 0.000 description 1
- 230000004130 lipolysis Effects 0.000 description 1
- 239000006194 liquid suspension Substances 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 229960001977 loracarbef Drugs 0.000 description 1
- 229960003088 loratadine Drugs 0.000 description 1
- JCCNYMKQOSZNPW-UHFFFAOYSA-N loratadine Chemical compound C1CN(C(=O)OCC)CCC1=C1C2=NC=CC=C2CCC2=CC(Cl)=CC=C21 JCCNYMKQOSZNPW-UHFFFAOYSA-N 0.000 description 1
- 239000006210 lotion Substances 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 229960003646 lysine Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000003120 macrolide antibiotic agent Substances 0.000 description 1
- 229940041033 macrolides Drugs 0.000 description 1
- 229920002521 macromolecule Polymers 0.000 description 1
- 150000002680 magnesium Chemical class 0.000 description 1
- 229940078752 magnesium ascorbyl phosphate Drugs 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 229940035034 maltodextrin Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 231100000682 maximum tolerated dose Toxicity 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 229960003803 meclofenamic acid Drugs 0.000 description 1
- HYYBABOKPJLUIN-UHFFFAOYSA-N mefenamic acid Chemical compound CC1=CC=CC(NC=2C(=CC=CC=2)C(O)=O)=C1C HYYBABOKPJLUIN-UHFFFAOYSA-N 0.000 description 1
- 229960003464 mefenamic acid Drugs 0.000 description 1
- 230000005906 menstruation Effects 0.000 description 1
- 230000003924 mental process Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 235000010270 methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 229960004584 methylprednisolone Drugs 0.000 description 1
- 229960003085 meticillin Drugs 0.000 description 1
- 229960003152 metisazone Drugs 0.000 description 1
- 229960000198 mezlocillin Drugs 0.000 description 1
- YPBATNHYBCGSSN-VWPFQQQWSA-N mezlocillin Chemical compound N([C@@H](C(=O)N[C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C=1C=CC=CC=1)C(=O)N1CCN(S(C)(=O)=O)C1=O YPBATNHYBCGSSN-VWPFQQQWSA-N 0.000 description 1
- 239000004530 micro-emulsion Substances 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 239000003094 microcapsule Substances 0.000 description 1
- 239000011859 microparticle Substances 0.000 description 1
- 238000000386 microscopy Methods 0.000 description 1
- 239000004005 microsphere Substances 0.000 description 1
- 102000021160 microtubule binding proteins Human genes 0.000 description 1
- 108091011150 microtubule binding proteins Proteins 0.000 description 1
- 235000013336 milk Nutrition 0.000 description 1
- 239000008267 milk Substances 0.000 description 1
- 210000004080 milk Anatomy 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 150000007522 mineralic acids Chemical class 0.000 description 1
- 229960004023 minocycline Drugs 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- KKZJGLLVHKMTCM-UHFFFAOYSA-N mitoxantrone Chemical compound O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO KKZJGLLVHKMTCM-UHFFFAOYSA-N 0.000 description 1
- 229960001156 mitoxantrone Drugs 0.000 description 1
- 229940041009 monobactams Drugs 0.000 description 1
- 150000002772 monosaccharides Chemical class 0.000 description 1
- 229960005389 moroxydine Drugs 0.000 description 1
- 201000003152 motion sickness Diseases 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- 238000011201 multiple comparisons test Methods 0.000 description 1
- 125000001421 myristyl group Chemical group [H]C([*])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])C([H])([H])[H] 0.000 description 1
- OSFCMRGOZNQUSW-UHFFFAOYSA-N n-[4-[2-(6,7-dimethoxy-3,4-dihydro-1h-isoquinolin-2-yl)ethyl]phenyl]-5-methoxy-9-oxo-10h-acridine-4-carboxamide Chemical compound N1C2=C(OC)C=CC=C2C(=O)C2=C1C(C(=O)NC1=CC=C(C=C1)CCN1CCC=3C=C(C(=CC=3C1)OC)OC)=CC=C2 OSFCMRGOZNQUSW-UHFFFAOYSA-N 0.000 description 1
- OHDXDNUPVVYWOV-UHFFFAOYSA-N n-methyl-1-(2-naphthalen-1-ylsulfanylphenyl)methanamine Chemical compound CNCC1=CC=CC=C1SC1=CC=CC2=CC=CC=C12 OHDXDNUPVVYWOV-UHFFFAOYSA-N 0.000 description 1
- GPXLMGHLHQJAGZ-JTDSTZFVSA-N nafcillin Chemical compound C1=CC=CC2=C(C(=O)N[C@@H]3C(N4[C@H](C(C)(C)S[C@@H]43)C(O)=O)=O)C(OCC)=CC=C21 GPXLMGHLHQJAGZ-JTDSTZFVSA-N 0.000 description 1
- 229960000515 nafcillin Drugs 0.000 description 1
- 239000002105 nanoparticle Substances 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-M naphthalene-2-sulfonate Chemical compound C1=CC=CC2=CC(S(=O)(=O)[O-])=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-M 0.000 description 1
- KVBGVZZKJNLNJU-UHFFFAOYSA-N naphthalene-2-sulfonic acid Chemical compound C1=CC=CC2=CC(S(=O)(=O)O)=CC=C21 KVBGVZZKJNLNJU-UHFFFAOYSA-N 0.000 description 1
- 229960002009 naproxen Drugs 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 229960003255 natamycin Drugs 0.000 description 1
- 239000004311 natamycin Substances 0.000 description 1
- 235000010298 natamycin Nutrition 0.000 description 1
- NCXMLFZGDNKEPB-FFPOYIOWSA-N natamycin Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C[C@@H](C)OC(=O)/C=C/[C@H]2O[C@@H]2C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 NCXMLFZGDNKEPB-FFPOYIOWSA-N 0.000 description 1
- 239000005445 natural material Substances 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 230000000926 neurological effect Effects 0.000 description 1
- 230000016273 neuron death Effects 0.000 description 1
- 230000004031 neuronal differentiation Effects 0.000 description 1
- BPGXUIVWLQTVLZ-OFGSCBOVSA-N neuropeptide y(npy) Chemical compound C([C@@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCN=C(N)N)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(O)=O)NC(=O)[C@H](CC=1N=CNC=1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@H](CO)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CCCCN)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@@H](N)CC=1C=CC(O)=CC=1)C1=CC=C(O)C=C1 BPGXUIVWLQTVLZ-OFGSCBOVSA-N 0.000 description 1
- 238000010855 neuropsychological testing Methods 0.000 description 1
- 210000001719 neurosecretory cell Anatomy 0.000 description 1
- 231100000189 neurotoxic Toxicity 0.000 description 1
- 230000002887 neurotoxic effect Effects 0.000 description 1
- 229960000689 nevirapine Drugs 0.000 description 1
- 102000046701 nicastrin Human genes 0.000 description 1
- 108700022821 nicastrin Proteins 0.000 description 1
- 235000005152 nicotinamide Nutrition 0.000 description 1
- 239000011570 nicotinamide Substances 0.000 description 1
- 229960003966 nicotinamide Drugs 0.000 description 1
- 229960002715 nicotine Drugs 0.000 description 1
- SNICXCGAKADSCV-UHFFFAOYSA-N nicotine Natural products CN1CCCC1C1=CC=CN=C1 SNICXCGAKADSCV-UHFFFAOYSA-N 0.000 description 1
- 235000001968 nicotinic acid Nutrition 0.000 description 1
- 229960003512 nicotinic acid Drugs 0.000 description 1
- 239000011664 nicotinic acid Substances 0.000 description 1
- 229960000916 niflumic acid Drugs 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 229960000564 nitrofurantoin Drugs 0.000 description 1
- NXFQHRVNIOXGAQ-YCRREMRBSA-N nitrofurantoin Chemical compound O1C([N+](=O)[O-])=CC=C1\C=N\N1C(=O)NC(=O)C1 NXFQHRVNIOXGAQ-YCRREMRBSA-N 0.000 description 1
- QJGQUHMNIGDVPM-UHFFFAOYSA-N nitrogen group Chemical group [N] QJGQUHMNIGDVPM-UHFFFAOYSA-N 0.000 description 1
- 231100000344 non-irritating Toxicity 0.000 description 1
- 239000012457 nonaqueous media Substances 0.000 description 1
- OGJPXUAPXNRGGI-UHFFFAOYSA-N norfloxacin Chemical compound C1=C2N(CC)C=C(C(O)=O)C(=O)C2=CC(F)=C1N1CCNCC1 OGJPXUAPXNRGGI-UHFFFAOYSA-N 0.000 description 1
- 229960001180 norfloxacin Drugs 0.000 description 1
- 229960000988 nystatin Drugs 0.000 description 1
- VQOXZBDYSJBXMA-NQTDYLQESA-N nystatin A1 Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/CC/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 VQOXZBDYSJBXMA-NQTDYLQESA-N 0.000 description 1
- 235000020824 obesity Nutrition 0.000 description 1
- 239000002674 ointment Substances 0.000 description 1
- ZQPPMHVWECSIRJ-KTKRTIGZSA-N oleic acid Chemical compound CCCCCCCC\C=C/CCCCCCCC(O)=O ZQPPMHVWECSIRJ-KTKRTIGZSA-N 0.000 description 1
- 238000006384 oligomerization reaction Methods 0.000 description 1
- 229930191479 oligomycin Natural products 0.000 description 1
- MNULEGDCPYONBU-AWJDAWNUSA-N oligomycin A Polymers O([C@H]1CC[C@H](/C=C/C=C/C[C@@H](C)[C@H](O)[C@@](C)(O)C(=O)[C@@H](C)[C@H](O)[C@@H](C)C(=O)[C@@H](C)[C@H](O)[C@@H](C)/C=C/C(=O)O[C@@H]([C@@H]2C)[C@@H]1C)CC)[C@@]12CC[C@H](C)[C@H](C[C@@H](C)O)O1 MNULEGDCPYONBU-AWJDAWNUSA-N 0.000 description 1
- 229960004031 omoconazole Drugs 0.000 description 1
- JMFOSJNGKJCTMJ-ZHZULCJRSA-N omoconazole Chemical compound C1=CN=CN1C(/C)=C(C=1C(=CC(Cl)=CC=1)Cl)\OCCOC1=CC=C(Cl)C=C1 JMFOSJNGKJCTMJ-ZHZULCJRSA-N 0.000 description 1
- 150000002894 organic compounds Chemical class 0.000 description 1
- 230000003204 osmotic effect Effects 0.000 description 1
- 229960001019 oxacillin Drugs 0.000 description 1
- UWYHMGVUTGAWSP-JKIFEVAISA-N oxacillin Chemical compound N([C@@H]1C(N2[C@H](C(C)(C)S[C@@H]21)C(O)=O)=O)C(=O)C1=C(C)ON=C1C1=CC=CC=C1 UWYHMGVUTGAWSP-JKIFEVAISA-N 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 229960002739 oxaprozin Drugs 0.000 description 1
- OFPXSFXSNFPTHF-UHFFFAOYSA-N oxaprozin Chemical compound O1C(CCC(=O)O)=NC(C=2C=CC=CC=2)=C1C1=CC=CC=C1 OFPXSFXSNFPTHF-UHFFFAOYSA-N 0.000 description 1
- 229950005708 oxepinac Drugs 0.000 description 1
- 229960003483 oxiconazole Drugs 0.000 description 1
- QRJJEGAJXVEBNE-MOHJPFBDSA-N oxiconazole Chemical compound ClC1=CC(Cl)=CC=C1CO\N=C(C=1C(=CC(Cl)=CC=1)Cl)\CN1C=NC=C1 QRJJEGAJXVEBNE-MOHJPFBDSA-N 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 229960000649 oxyphenbutazone Drugs 0.000 description 1
- HFHZKZSRXITVMK-UHFFFAOYSA-N oxyphenbutazone Chemical compound O=C1C(CCCC)C(=O)N(C=2C=CC=CC=2)N1C1=CC=C(O)C=C1 HFHZKZSRXITVMK-UHFFFAOYSA-N 0.000 description 1
- LSQZJLSUYDQPKJ-UHFFFAOYSA-N p-Hydroxyampicillin Natural products O=C1N2C(C(O)=O)C(C)(C)SC2C1NC(=O)C(N)C1=CC=C(O)C=C1 LSQZJLSUYDQPKJ-UHFFFAOYSA-N 0.000 description 1
- 229960001592 paclitaxel Drugs 0.000 description 1
- 229960005489 paracetamol Drugs 0.000 description 1
- 229960002858 paramethasone Drugs 0.000 description 1
- 210000001152 parietal lobe Anatomy 0.000 description 1
- 230000009745 pathological pathway Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 235000010987 pectin Nutrition 0.000 description 1
- 239000001814 pectin Substances 0.000 description 1
- 229920001277 pectin Polymers 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 229940056360 penicillin g Drugs 0.000 description 1
- 150000002960 penicillins Chemical class 0.000 description 1
- 239000002304 perfume Substances 0.000 description 1
- 229940021222 peritoneal dialysis isotonic solution Drugs 0.000 description 1
- JRKICGRDRMAZLK-UHFFFAOYSA-L peroxydisulfate Chemical compound [O-]S(=O)(=O)OOS([O-])(=O)=O JRKICGRDRMAZLK-UHFFFAOYSA-L 0.000 description 1
- FHHJDRFHHWUPDG-UHFFFAOYSA-L peroxysulfate(2-) Chemical compound [O-]OS([O-])(=O)=O FHHJDRFHHWUPDG-UHFFFAOYSA-L 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 229960003742 phenol Drugs 0.000 description 1
- COLNVLDHVKWLRT-UHFFFAOYSA-N phenylalanine Natural products OC(=O)C(N)CC1=CC=CC=C1 COLNVLDHVKWLRT-UHFFFAOYSA-N 0.000 description 1
- 229960002895 phenylbutazone Drugs 0.000 description 1
- VYMDGNCVAMGZFE-UHFFFAOYSA-N phenylbutazonum Chemical compound O=C1C(CCCC)C(=O)N(C=2C=CC=CC=2)N1C1=CC=CC=C1 VYMDGNCVAMGZFE-UHFFFAOYSA-N 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 230000037081 physical activity Effects 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 239000002504 physiological saline solution Substances 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 150000004885 piperazines Chemical class 0.000 description 1
- 150000003053 piperidines Chemical class 0.000 description 1
- 230000036470 plasma concentration Effects 0.000 description 1
- 108010049148 plastin Proteins 0.000 description 1
- 229930192033 plastin Natural products 0.000 description 1
- ONJQDTZCDSESIW-UHFFFAOYSA-N polidocanol Chemical compound CCCCCCCCCCCCOCCOCCOCCOCCOCCOCCOCCOCCOCCO ONJQDTZCDSESIW-UHFFFAOYSA-N 0.000 description 1
- 229920001483 poly(ethyl methacrylate) polymer Polymers 0.000 description 1
- 229920000212 poly(isobutyl acrylate) Polymers 0.000 description 1
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 1
- 229920000058 polyacrylate Polymers 0.000 description 1
- 239000004584 polyacrylic acid Substances 0.000 description 1
- 229920000573 polyethylene Polymers 0.000 description 1
- 229920000197 polyisopropyl acrylate Polymers 0.000 description 1
- 239000004926 polymethyl methacrylate Substances 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 235000010482 polyoxyethylene sorbitan monooleate Nutrition 0.000 description 1
- 239000000244 polyoxyethylene sorbitan monooleate Substances 0.000 description 1
- 229920000182 polyphenyl methacrylate Polymers 0.000 description 1
- 229920000053 polysorbate 80 Polymers 0.000 description 1
- 230000020775 positive regulation of microtubule depolymerization Effects 0.000 description 1
- 238000002600 positron emission tomography Methods 0.000 description 1
- 229960003101 pranoprofen Drugs 0.000 description 1
- 229960005205 prednisolone Drugs 0.000 description 1
- OIGNJSKKLXVSLS-VWUMJDOOSA-N prednisolone Chemical compound O=C1C=C[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 OIGNJSKKLXVSLS-VWUMJDOOSA-N 0.000 description 1
- 229960004618 prednisone Drugs 0.000 description 1
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 1
- 210000002442 prefrontal cortex Anatomy 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 150000003141 primary amines Chemical class 0.000 description 1
- 210000002243 primary neuron Anatomy 0.000 description 1
- 229940002612 prodrug Drugs 0.000 description 1
- 239000000651 prodrug Substances 0.000 description 1
- 230000007425 progressive decline Effects 0.000 description 1
- 208000037821 progressive disease Diseases 0.000 description 1
- 229940097325 prolactin Drugs 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 108010031719 prolyl-serine Proteins 0.000 description 1
- 108010004914 prolylarginine Proteins 0.000 description 1
- 108010029020 prolylglycine Proteins 0.000 description 1
- 229960003910 promethazine Drugs 0.000 description 1
- AMLFJZRZIOZGPW-UHFFFAOYSA-N prop-1-en-1-amine Chemical compound CC=CN AMLFJZRZIOZGPW-UHFFFAOYSA-N 0.000 description 1
- 238000011321 prophylaxis Methods 0.000 description 1
- 235000019260 propionic acid Nutrition 0.000 description 1
- 150000005599 propionic acid derivatives Chemical class 0.000 description 1
- 239000003223 protective agent Substances 0.000 description 1
- 238000002731 protein assay Methods 0.000 description 1
- 238000000751 protein extraction Methods 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 229960000611 pyrimethamine Drugs 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- MIXMJCQRHVAJIO-TZHJZOAOSA-N qk4dys664x Chemical compound O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O.C1([C@@H](F)C2)=CC(=O)C=C[C@]1(C)[C@@H]1[C@@H]2[C@@H]2C[C@H]3OC(C)(C)O[C@@]3(C(=O)CO)[C@@]2(C)C[C@@H]1O MIXMJCQRHVAJIO-TZHJZOAOSA-N 0.000 description 1
- IUVKMZGDUIUOCP-BTNSXGMBSA-N quinbolone Chemical compound O([C@H]1CC[C@H]2[C@H]3[C@@H]([C@]4(C=CC(=O)C=C4CC3)C)CC[C@@]21C)C1=CCCC1 IUVKMZGDUIUOCP-BTNSXGMBSA-N 0.000 description 1
- 229960001404 quinidine Drugs 0.000 description 1
- 150000007660 quinolones Chemical class 0.000 description 1
- 238000010814 radioimmunoprecipitation assay Methods 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 230000036647 reaction Effects 0.000 description 1
- 230000018406 regulation of metabolic process Effects 0.000 description 1
- 235000020945 retinal Nutrition 0.000 description 1
- 239000011604 retinal Substances 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- NCYCYZXNIZJOKI-OVSJKPMPSA-N retinal group Chemical group C\C(=C/C=O)\C=C\C=C(\C=C\C1=C(CCCC1(C)C)C)/C NCYCYZXNIZJOKI-OVSJKPMPSA-N 0.000 description 1
- 229960003471 retinol Drugs 0.000 description 1
- 235000020944 retinol Nutrition 0.000 description 1
- 239000011607 retinol Substances 0.000 description 1
- 229940108325 retinyl palmitate Drugs 0.000 description 1
- 235000019172 retinyl palmitate Nutrition 0.000 description 1
- 239000011769 retinyl palmitate Substances 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 229960000329 ribavirin Drugs 0.000 description 1
- HZCAHMRRMINHDJ-DBRKOABJSA-N ribavirin Natural products O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1N=CN=C1 HZCAHMRRMINHDJ-DBRKOABJSA-N 0.000 description 1
- 229960001225 rifampicin Drugs 0.000 description 1
- 229960000888 rimantadine Drugs 0.000 description 1
- CVHZOJJKTDOEJC-UHFFFAOYSA-N saccharin Chemical compound C1=CC=C2C(=O)NS(=O)(=O)C2=C1 CVHZOJJKTDOEJC-UHFFFAOYSA-N 0.000 description 1
- 235000019204 saccharin Nutrition 0.000 description 1
- 229940081974 saccharin Drugs 0.000 description 1
- 239000000901 saccharin and its Na,K and Ca salt Substances 0.000 description 1
- WKEDVNSFRWHDNR-UHFFFAOYSA-N salicylanilide Chemical compound OC1=CC=CC=C1C(=O)NC1=CC=CC=C1 WKEDVNSFRWHDNR-UHFFFAOYSA-N 0.000 description 1
- 230000036186 satiety Effects 0.000 description 1
- 235000019627 satiety Nutrition 0.000 description 1
- 102000014452 scavenger receptors Human genes 0.000 description 1
- 108010078070 scavenger receptors Proteins 0.000 description 1
- STECJAGHUSJQJN-FWXGHANASA-N scopolamine Chemical compound C1([C@@H](CO)C(=O)O[C@H]2C[C@@H]3N([C@H](C2)[C@@H]2[C@H]3O2)C)=CC=CC=C1 STECJAGHUSJQJN-FWXGHANASA-N 0.000 description 1
- 229960002646 scopolamine Drugs 0.000 description 1
- CDAISMWEOUEBRE-UHFFFAOYSA-N scyllo-inosotol Natural products OC1C(O)C(O)C(O)C(O)C1O CDAISMWEOUEBRE-UHFFFAOYSA-N 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000009919 sequestration Effects 0.000 description 1
- 239000012679 serum free medium Substances 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- SEBFKMXJBCUCAI-HKTJVKLFSA-N silibinin Chemical compound C1=C(O)C(OC)=CC([C@@H]2[C@H](OC3=CC=C(C=C3O2)[C@@H]2[C@H](C(=O)C3=C(O)C=C(O)C=C3O2)O)CO)=C1 SEBFKMXJBCUCAI-HKTJVKLFSA-N 0.000 description 1
- RMAQACBXLXPBSY-UHFFFAOYSA-N silicic acid Chemical compound O[Si](O)(O)O RMAQACBXLXPBSY-UHFFFAOYSA-N 0.000 description 1
- 239000010703 silicon Substances 0.000 description 1
- 229910052710 silicon Inorganic materials 0.000 description 1
- 235000012239 silicon dioxide Nutrition 0.000 description 1
- 229960004245 silymarin Drugs 0.000 description 1
- 235000017700 silymarin Nutrition 0.000 description 1
- 238000002603 single-photon emission computed tomography Methods 0.000 description 1
- 239000002002 slurry Substances 0.000 description 1
- 210000000813 small intestine Anatomy 0.000 description 1
- 230000005586 smoking cessation Effects 0.000 description 1
- YRWWOAFMPXPHEJ-OFBPEYICSA-K sodium L-ascorbic acid 2-phosphate Chemical compound [Na+].[Na+].[Na+].OC[C@H](O)[C@H]1OC(=O)C(OP([O-])([O-])=O)=C1[O-] YRWWOAFMPXPHEJ-OFBPEYICSA-K 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229940048058 sodium ascorbyl phosphate Drugs 0.000 description 1
- WXMKPNITSTVMEF-UHFFFAOYSA-M sodium benzoate Chemical compound [Na+].[O-]C(=O)C1=CC=CC=C1 WXMKPNITSTVMEF-UHFFFAOYSA-M 0.000 description 1
- 239000004299 sodium benzoate Substances 0.000 description 1
- 235000010234 sodium benzoate Nutrition 0.000 description 1
- 229910001467 sodium calcium phosphate Inorganic materials 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 239000001488 sodium phosphate Substances 0.000 description 1
- JXKPEJDQGNYQSM-UHFFFAOYSA-M sodium propionate Chemical compound [Na+].CCC([O-])=O JXKPEJDQGNYQSM-UHFFFAOYSA-M 0.000 description 1
- 239000004324 sodium propionate Substances 0.000 description 1
- 235000010334 sodium propionate Nutrition 0.000 description 1
- 229960003212 sodium propionate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 239000008247 solid mixture Substances 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 244000148755 species properties Species 0.000 description 1
- 210000000278 spinal cord Anatomy 0.000 description 1
- 238000009987 spinning Methods 0.000 description 1
- 230000037351 starvation Effects 0.000 description 1
- 239000008223 sterile water Substances 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 210000002784 stomach Anatomy 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 230000035882 stress Effects 0.000 description 1
- 230000002739 subcortical effect Effects 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-L succinate(2-) Chemical compound [O-]C(=O)CCC([O-])=O KDYFGRWQOYBRFD-UHFFFAOYSA-L 0.000 description 1
- 239000001384 succinic acid Substances 0.000 description 1
- 229950005175 sudoxicam Drugs 0.000 description 1
- 229960002607 sulconazole Drugs 0.000 description 1
- SEEPANYCNGTZFQ-UHFFFAOYSA-N sulfadiazine Chemical compound C1=CC(N)=CC=C1S(=O)(=O)NC1=NC=CC=N1 SEEPANYCNGTZFQ-UHFFFAOYSA-N 0.000 description 1
- 229960004306 sulfadiazine Drugs 0.000 description 1
- 229960005404 sulfamethoxazole Drugs 0.000 description 1
- 125000000446 sulfanediyl group Chemical group *S* 0.000 description 1
- JLKIGFTWXXRPMT-UHFFFAOYSA-N sulphamethoxazole Chemical compound O1C(C)=CC(NS(=O)(=O)C=2C=CC(N)=CC=2)=N1 JLKIGFTWXXRPMT-UHFFFAOYSA-N 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 239000003826 tablet Substances 0.000 description 1
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 1
- 229960003865 tazobactam Drugs 0.000 description 1
- LPQZKKCYTLCDGQ-WEDXCCLWSA-N tazobactam Chemical compound C([C@]1(C)S([C@H]2N(C(C2)=O)[C@H]1C(O)=O)(=O)=O)N1C=CN=N1 LPQZKKCYTLCDGQ-WEDXCCLWSA-N 0.000 description 1
- 229960001608 teicoplanin Drugs 0.000 description 1
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 description 1
- 229960001278 teniposide Drugs 0.000 description 1
- DOMXUEMWDBAQBQ-WEVVVXLNSA-N terbinafine Chemical compound C1=CC=C2C(CN(C\C=C\C#CC(C)(C)C)C)=CC=CC2=C1 DOMXUEMWDBAQBQ-WEVVVXLNSA-N 0.000 description 1
- 229960002722 terbinafine Drugs 0.000 description 1
- 229960000580 terconazole Drugs 0.000 description 1
- RLNWRDKVJSXXPP-UHFFFAOYSA-N tert-butyl 2-[(2-bromoanilino)methyl]piperidine-1-carboxylate Chemical compound CC(C)(C)OC(=O)N1CCCCC1CNC1=CC=CC=C1Br RLNWRDKVJSXXPP-UHFFFAOYSA-N 0.000 description 1
- 229960002180 tetracycline Drugs 0.000 description 1
- 229930101283 tetracycline Natural products 0.000 description 1
- 229940040944 tetracyclines Drugs 0.000 description 1
- CBXCPBUEXACCNR-UHFFFAOYSA-N tetraethylammonium Chemical compound CC[N+](CC)(CC)CC CBXCPBUEXACCNR-UHFFFAOYSA-N 0.000 description 1
- RTKIYNMVFMVABJ-UHFFFAOYSA-L thimerosal Chemical compound [Na+].CC[Hg]SC1=CC=CC=C1C([O-])=O RTKIYNMVFMVABJ-UHFFFAOYSA-L 0.000 description 1
- 229940033663 thimerosal Drugs 0.000 description 1
- 229960002663 thioctic acid Drugs 0.000 description 1
- 125000003396 thiol group Chemical group [H]S* 0.000 description 1
- 229960004659 ticarcillin Drugs 0.000 description 1
- OHKOGUYZJXTSFX-KZFFXBSXSA-N ticarcillin Chemical compound C=1([C@@H](C(O)=O)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)C=CSC=1 OHKOGUYZJXTSFX-KZFFXBSXSA-N 0.000 description 1
- 229960000707 tobramycin Drugs 0.000 description 1
- NLVFBUXFDBBNBW-PBSUHMDJSA-N tobramycin Chemical compound N[C@@H]1C[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N NLVFBUXFDBBNBW-PBSUHMDJSA-N 0.000 description 1
- 229940042585 tocopherol acetate Drugs 0.000 description 1
- YEZNLOUZAIOMLT-UHFFFAOYSA-N tolfenamic acid Chemical compound CC1=C(Cl)C=CC=C1NC1=CC=CC=C1C(O)=O YEZNLOUZAIOMLT-UHFFFAOYSA-N 0.000 description 1
- 229960002905 tolfenamic acid Drugs 0.000 description 1
- 229950007633 tolindate Drugs 0.000 description 1
- ANJNOJFLVNXCHT-UHFFFAOYSA-N tolindate Chemical compound C=1C=C2CCCC2=CC=1OC(=S)N(C)C1=CC=CC(C)=C1 ANJNOJFLVNXCHT-UHFFFAOYSA-N 0.000 description 1
- 229960001017 tolmetin Drugs 0.000 description 1
- UPSPUYADGBWSHF-UHFFFAOYSA-N tolmetin Chemical compound C1=CC(C)=CC=C1C(=O)C1=CC=C(CC(O)=O)N1C UPSPUYADGBWSHF-UHFFFAOYSA-N 0.000 description 1
- 229960004880 tolnaftate Drugs 0.000 description 1
- FUSNMLFNXJSCDI-UHFFFAOYSA-N tolnaftate Chemical compound C=1C=C2C=CC=CC2=CC=1OC(=S)N(C)C1=CC=CC(C)=C1 FUSNMLFNXJSCDI-UHFFFAOYSA-N 0.000 description 1
- 229960000303 topotecan Drugs 0.000 description 1
- 231100000331 toxic Toxicity 0.000 description 1
- 230000002588 toxic effect Effects 0.000 description 1
- 230000037317 transdermal delivery Effects 0.000 description 1
- 238000012546 transfer Methods 0.000 description 1
- 238000012301 transgenic model Methods 0.000 description 1
- ODLHGICHYURWBS-LKONHMLTSA-N trappsol cyclo Chemical compound CC(O)COC[C@H]([C@H]([C@@H]([C@H]1O)O)O[C@H]2O[C@@H]([C@@H](O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O[C@H]3O[C@H](COCC(C)O)[C@H]([C@@H]([C@H]3O)O)O3)[C@H](O)[C@H]2O)COCC(O)C)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O)[C@@H]3O[C@@H]1COCC(C)O ODLHGICHYURWBS-LKONHMLTSA-N 0.000 description 1
- 229960002622 triacetin Drugs 0.000 description 1
- 150000003626 triacylglycerols Chemical class 0.000 description 1
- 229960005294 triamcinolone Drugs 0.000 description 1
- GFNANZIMVAIWHM-OBYCQNJPSA-N triamcinolone Chemical compound O=C1C=C[C@]2(C)[C@@]3(F)[C@@H](O)C[C@](C)([C@@]([C@H](O)C4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 GFNANZIMVAIWHM-OBYCQNJPSA-N 0.000 description 1
- OFVFGKQCUDMLLP-UHFFFAOYSA-N tribuzone Chemical compound O=C1C(CCC(=O)C(C)(C)C)C(=O)N(C=2C=CC=CC=2)N1C1=CC=CC=C1 OFVFGKQCUDMLLP-UHFFFAOYSA-N 0.000 description 1
- 229950000919 tribuzone Drugs 0.000 description 1
- HTJNEBVCZXHBNJ-XCTPRCOBSA-H trimagnesium;(2r)-2-[(1s)-1,2-dihydroxyethyl]-3,4-dihydroxy-2h-furan-5-one;diphosphate Chemical compound [Mg+2].[Mg+2].[Mg+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O.OC[C@H](O)[C@H]1OC(=O)C(O)=C1O HTJNEBVCZXHBNJ-XCTPRCOBSA-H 0.000 description 1
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 1
- 229960000832 tromantadine Drugs 0.000 description 1
- UXQDWARBDDDTKG-UHFFFAOYSA-N tromantadine Chemical compound C1C(C2)CC3CC2CC1(NC(=O)COCCN(C)C)C3 UXQDWARBDDDTKG-UHFFFAOYSA-N 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 108010051110 tyrosyl-lysine Proteins 0.000 description 1
- ZDPHROOEEOARMN-UHFFFAOYSA-N undecanoic acid Chemical class CCCCCCCCCCC(O)=O ZDPHROOEEOARMN-UHFFFAOYSA-N 0.000 description 1
- DRTQHJPVMGBUCF-UHFFFAOYSA-N uracil arabinoside Natural products OC1C(O)C(CO)OC1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-UHFFFAOYSA-N 0.000 description 1
- 229940116269 uric acid Drugs 0.000 description 1
- 229940045145 uridine Drugs 0.000 description 1
- 108010015385 valyl-prolyl-proline Proteins 0.000 description 1
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 1
- 229960003165 vancomycin Drugs 0.000 description 1
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 1
- 210000003462 vein Anatomy 0.000 description 1
- 229940070384 ventolin Drugs 0.000 description 1
- 229960001722 verapamil Drugs 0.000 description 1
- 229960003636 vidarabine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 235000019155 vitamin A Nutrition 0.000 description 1
- 239000011719 vitamin A Substances 0.000 description 1
- NCYCYZXNIZJOKI-UHFFFAOYSA-N vitamin A aldehyde Natural products O=CC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-UHFFFAOYSA-N 0.000 description 1
- 235000019160 vitamin B3 Nutrition 0.000 description 1
- 239000011708 vitamin B3 Substances 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 229940045997 vitamin a Drugs 0.000 description 1
- 239000008215 water for injection Substances 0.000 description 1
- 239000012130 whole-cell lysate Substances 0.000 description 1
- XDWXRAYGALQIFG-UHFFFAOYSA-L zinc;propanoate Chemical compound [Zn+2].CCC([O-])=O.CCC([O-])=O XDWXRAYGALQIFG-UHFFFAOYSA-L 0.000 description 1
- IHOVFYSQUDPMCN-QKUIIBHLSA-N zosuquidar Chemical compound C([C@H](COC=1C2=CC=CN=C2C=CC=1)O)N(CC1)CCN1C1C2=CC=CC=C2C2C(F)(F)C2C2=CC=CC=C12 IHOVFYSQUDPMCN-QKUIIBHLSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/2264—Obesity-gene products, e.g. leptin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/33—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans derived from pro-opiomelanocortin, pro-enkephalin or pro-dynorphin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/17—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- A61K38/22—Hormones
- A61K38/28—Insulins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Epidemiology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Endocrinology (AREA)
- Biomedical Technology (AREA)
- Neurology (AREA)
- Neurosurgery (AREA)
- Obesity (AREA)
- Genetics & Genomics (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Diabetes (AREA)
- Hospice & Palliative Care (AREA)
- Psychiatry (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Acyclic And Carbocyclic Compounds In Medicinal Compositions (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US5500908P | 2008-05-21 | 2008-05-21 | |
| US61/055,009 | 2008-05-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| KR20110028457A true KR20110028457A (ko) | 2011-03-18 |
Family
ID=41340901
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| KR1020107028636A Ceased KR20110028457A (ko) | 2008-05-21 | 2009-05-21 | 신경섬유 매듭과 연관된 신경변성 장애를 치료하는 방법 |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US8642543B2 (enExample) |
| EP (1) | EP2326339A4 (enExample) |
| JP (1) | JP5622720B2 (enExample) |
| KR (1) | KR20110028457A (enExample) |
| CN (1) | CN102099048A (enExample) |
| AU (1) | AU2009248914A1 (enExample) |
| CA (1) | CA2725143A1 (enExample) |
| WO (1) | WO2009143380A2 (enExample) |
| ZA (1) | ZA201008303B (enExample) |
Families Citing this family (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8481043B2 (en) * | 2001-06-22 | 2013-07-09 | Cpex Pharmaceuticals, Inc. | Nasal immunization |
| WO2009143380A2 (en) * | 2008-05-21 | 2009-11-26 | Neurotez, Inc. | Methods for treating progressive cognitive disorders related to neurofibrillary tangles |
| AU2009313562B2 (en) * | 2008-11-04 | 2012-11-15 | Neurotez, Inc. | Leptin compositions and methods for treating progressive cognitive function disorders resulting from accumulation of neurofibrillary tangles and amlyoid beta |
| EP3620154A1 (en) * | 2009-02-06 | 2020-03-11 | University Of Southern California | Therapeutic compositions comprising monoterpenes |
| EP2542082B8 (en) | 2010-03-03 | 2020-06-17 | Neonc Technologies Inc. | PHARMACEUTICAL COMPOSITIONS COMPRISING (S)-perillyl alcohol |
| CN105078973B (zh) | 2010-08-27 | 2020-10-09 | 尼昂克技术公司 | 包含poh衍生物的药物组合物 |
| US20160038600A1 (en) | 2012-08-03 | 2016-02-11 | Neonc Technologies Inc. | Pharmaceutical compositions comprising poh derivatives |
| EP2651864B1 (en) | 2010-12-17 | 2016-07-13 | Neonc Technologies Inc. | Methods and devices for using isoperillyl alcohol |
| US20140088000A1 (en) * | 2012-09-27 | 2014-03-27 | Neurotez, Inc. | Leptin Rescues Neurons from Alzheimer's Disease Related Pathways Triggered by Lipid Burden |
| WO2014165043A1 (en) * | 2013-03-13 | 2014-10-09 | Neurotez, Inc. | Fragments, mutants and chimeric fusion proteins of leptin for treating alzheimer's disease |
| JP6674957B2 (ja) | 2015-02-12 | 2020-04-01 | ネオンク テクノロジーズ インク. | ペリリルアルコール誘導体を含む医薬組成物 |
| WO2018102412A1 (en) | 2016-11-30 | 2018-06-07 | Neonc Technologies, Inc. | A perillyl alcohol-3 bromopyruvate conjugate and methods of treating cancer |
| WO2019157195A1 (en) | 2018-02-08 | 2019-08-15 | Neonc Technologies, Inc | Methods of permeabilizing the blood brain barrier |
| CN113613665A (zh) | 2019-03-14 | 2021-11-05 | Om药物公司 | 治疗和/或预防哮喘、哮喘恶化、过敏性哮喘和/或与呼吸系统病症相关的微生物群相关的病症的方法 |
| WO2021151100A1 (en) | 2020-01-24 | 2021-07-29 | Aim Immunotech Inc. | Methods, compositions, and vaccines for treating a virus infection |
| CN114958760B (zh) * | 2021-02-23 | 2024-04-26 | 南京启真基因工程有限公司 | 一种构建阿尔兹海默症模型猪的基因编辑技术及其应用 |
Family Cites Families (93)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB1521000A (en) * | 1975-06-13 | 1978-08-09 | Syntex Puerto Rico Inc | Inhalation device |
| US4192309A (en) * | 1978-09-05 | 1980-03-11 | Syntex Puerto Rico, Inc. | Inhalation device with capsule opener |
| US4227522A (en) * | 1978-09-05 | 1980-10-14 | Syntex Puerto Rico, Inc. | Inhalation device |
| IT1203660B (it) * | 1982-10-08 | 1989-02-15 | Glaxo Group Ltd | Dispositivi per somministrare farmaci a pazienti e confezione di blister per essi |
| US4778054A (en) * | 1982-10-08 | 1988-10-18 | Glaxo Group Limited | Pack for administering medicaments to patients |
| GB2178965B (en) * | 1985-07-30 | 1988-08-03 | Glaxo Group Ltd | Devices for administering medicaments to patients |
| JPH05963A (ja) | 1990-04-13 | 1993-01-08 | Toray Ind Inc | ポリペプチド類組成物 |
| US6582728B1 (en) * | 1992-07-08 | 2003-06-24 | Inhale Therapeutic Systems, Inc. | Spray drying of macromolecules to produce inhaleable dry powders |
| US5837672A (en) * | 1992-07-10 | 1998-11-17 | Athena Neurosciences, Inc. | Methods and compositions for the detection of soluble β-amyloid peptide |
| US6471956B1 (en) * | 1994-08-17 | 2002-10-29 | The Rockefeller University | Ob polypeptides, modified forms and compositions thereto |
| US6048837A (en) * | 1994-08-17 | 2000-04-11 | The Rockefeller University | OB polypeptides as modulators of body weight |
| US20020107211A1 (en) * | 1995-06-07 | 2002-08-08 | The Rockefeller University | Modulators of body weight, corresponding nucleic acids and proteins, and diagnostic and therapeutic uses thereof |
| US6001968A (en) * | 1994-08-17 | 1999-12-14 | The Rockefeller University | OB polypeptides, modified forms and compositions |
| US6309853B1 (en) * | 1994-08-17 | 2001-10-30 | The Rockfeller University | Modulators of body weight, corresponding nucleic acids and proteins, and diagnostic and therapeutic uses thereof |
| US6429290B1 (en) * | 1994-08-17 | 2002-08-06 | The Rockefeller University | OB polypeptides, modified forms and derivatives |
| JPH11501297A (ja) | 1995-01-31 | 1999-02-02 | イーライ・リリー・アンド・カンパニー | 抗肥満症タンパク質 |
| US5605886A (en) | 1995-01-31 | 1997-02-25 | Eli Lilly And Company | Anti-obesity proteins |
| US5552522A (en) * | 1995-01-31 | 1996-09-03 | Eli Lilly And Company | Anti-obesity proteins |
| US5521283A (en) * | 1995-01-31 | 1996-05-28 | Eli Lilly And Company | Anti-obesity proteins |
| US5559208A (en) | 1995-01-31 | 1996-09-24 | Eli Lilly And Company | Anti-obesity proteins |
| US5552523A (en) * | 1995-01-31 | 1996-09-03 | Eli Lilly And Company | Anti-obesity proteins |
| US5554727A (en) | 1995-01-31 | 1996-09-10 | Eli Lilly And Company | Anti-obesity proteins |
| US5552524A (en) * | 1995-01-31 | 1996-09-03 | Eli Lilly And Company | Anti-obesity proteins |
| JPH10513450A (ja) | 1995-01-31 | 1998-12-22 | イーライ・リリー・アンド・カンパニー | 抗肥満症タンパク質 |
| WO1996029405A2 (en) | 1995-03-20 | 1996-09-26 | Ligand Pharmaceuticals Incorporated | MODULATORS OF ob GENE AND SCREENING METHODS THEREFOR |
| EP0741187A2 (en) * | 1995-05-05 | 1996-11-06 | F. Hoffmann-La Roche Ag | Recombinant obese (Ob) proteins |
| EA199800104A1 (ru) * | 1995-06-30 | 1998-10-29 | Эли Лилли Энд Компани | Способ лечения диабета |
| US5698389A (en) * | 1995-11-16 | 1997-12-16 | Tularik, Inc. | Transcriptional promoter of the murine obesity gene |
| US6936439B2 (en) * | 1995-11-22 | 2005-08-30 | Amgen Inc. | OB fusion protein compositions and methods |
| BR9612359A (pt) | 1995-12-27 | 1999-07-13 | Genentech Inc | Derivado covalente de uma proteína ob composição polipeptídeo quimérico sequência de ácido nucleico vetor de express o replicável célula hospedeira e métodos associados aos mesmos |
| US7074397B1 (en) * | 1996-01-08 | 2006-07-11 | Genentech, Inc. | Method for enhancing proliferation or differentiation of a cell using ob protein |
| US6025324A (en) * | 1996-05-15 | 2000-02-15 | Hoffmann-La Roche Inc. | Pegylated obese (ob) protein compositions |
| US5830450A (en) * | 1996-06-19 | 1998-11-03 | Lallone; Roger L. | Compositions of leptin bound to an apolipoprotein |
| US6630346B1 (en) * | 1996-06-20 | 2003-10-07 | Merck & Co., Inc. | Gene therapy for obesity |
| US20060205660A1 (en) * | 1996-06-20 | 2006-09-14 | Sauvage Frederic D | OB protein-immunoglobulin chimeras |
| EP0921820A4 (en) | 1996-06-20 | 2003-01-15 | Merck & Co Inc | GENETERAPY FOR OBESITY |
| US6001816A (en) * | 1996-06-20 | 1999-12-14 | Merck & Co., Inc. | Gene therapy for leptin deficiency |
| WO1997048806A1 (en) | 1996-06-20 | 1997-12-24 | Merck & Co., Inc. | Gene therapy for obesity |
| WO1998024896A2 (en) | 1996-12-06 | 1998-06-11 | F. Hoffmann-La Roche Ag | Muteins of obese protein |
| ES2183351T3 (es) | 1997-04-17 | 2003-03-16 | Amgen Inc | Composicones que comprenden conjugados de proteina ob humana activa estable con una cadena fc de inmunoglobulinas y metodos. |
| US20020019352A1 (en) * | 1997-04-17 | 2002-02-14 | David N. Brems | Stable, active, human ob protein compositions and methods |
| US6020004A (en) * | 1997-04-17 | 2000-02-01 | Amgen Inc. | Biodegradable microparticles for the sustained delivery of therapeutic drugs |
| IL120733A0 (en) * | 1997-04-29 | 1997-08-14 | Yeda Res & Dev | Leptin as an inhibitor of cell proliferation |
| EP0950417A3 (en) * | 1998-02-23 | 2000-02-23 | Pfizer Products Inc. | Treatment of skeletal disorders |
| JP4215857B2 (ja) * | 1998-03-26 | 2009-01-28 | 裕 大村 | 学習・記憶能力改善剤 |
| GB9807062D0 (en) | 1998-04-02 | 1998-06-03 | Imp College Innovations Ltd | Immune response |
| DE69920921T2 (de) * | 1998-07-28 | 2006-03-09 | Vlaams Interuniversitair Instituut Voor Biotechnologie Vzw. | Leptin vermittelte geninduktion |
| AU5347099A (en) | 1998-08-10 | 2000-03-06 | Amgen, Inc. | Dextran-leptin conjugates, pharmaceutical compositions and related methods |
| US6777388B1 (en) | 1998-08-21 | 2004-08-17 | Clf Medical Technology Acceleration Program, Inc. | Leptin-related peptides |
| US7208572B2 (en) * | 1998-08-21 | 2007-04-24 | Albany Medical College | Leptin-related peptides |
| AU6396999A (en) | 1998-10-02 | 2000-04-26 | Amgen, Inc. | Method to determine a predisposition to leptin treatment |
| US6716810B1 (en) * | 1998-12-09 | 2004-04-06 | Eleanor Roosevelt Institute | Composition and method for regulation of body weight and associated conditions |
| MXPA01005818A (es) | 1998-12-09 | 2003-07-21 | Eleanor Rooseveltl Inst | Composicion y metodo para la regulacion del peso del cuerpo y condiciones asociadas. |
| SK9432001A3 (en) | 1999-01-07 | 2003-02-04 | Lexigen Pharm Corp | Expression and export of anti-obesity proteins as Fc fusion proteins |
| ATE323766T1 (de) | 1999-02-12 | 2006-05-15 | Amgen Inc | Glykosylierte leptinzusammensetzungen und zugehörige verfahren |
| US20030215423A1 (en) * | 1999-04-01 | 2003-11-20 | Merck & Co., Inc. | Gene therapy for obesity |
| US6475984B2 (en) * | 1999-04-29 | 2002-11-05 | The Nemours Foundation | Administration of leptin |
| CA2376933A1 (en) | 1999-06-11 | 2000-12-21 | Baylor College Of Medicine | Methods and compositions for control of bone formation via modulation of leptin activity |
| AU767068B2 (en) * | 1999-06-11 | 2003-10-30 | Baylor College Of Medicine | Methods and compositions for control of bone formation via modulation of leptin activity |
| JP2003520199A (ja) * | 1999-08-10 | 2003-07-02 | ユーエイビー リサーチ ファンデーション | 非ステロイド性抗炎症薬及び天然産コノトキシンを使用する外傷性脳及び脊髄損傷、並びに他の神経原性症状の治療方法 |
| CA2382666A1 (en) | 1999-08-23 | 2001-03-01 | The Administrators Of The Tulane Educational Fund | Modulation of the blood-brain barrier transporter for leptin |
| JP5247963B2 (ja) * | 2000-01-24 | 2013-07-24 | インノジェネティクス・エヌ・ブイ | タウオパチーの診断 |
| US7582292B2 (en) * | 2000-02-26 | 2009-09-01 | Artecel, Inc. | Adipose tissue derived stromal cells for the treatment of neurological disorders |
| US20030049255A1 (en) * | 2001-08-07 | 2003-03-13 | Sims John E. | Interleukin-1 receptors in the treatment of diseases |
| WO2003020303A1 (en) | 2001-08-29 | 2003-03-13 | The University Of Buckingham | Use of leptin for infant with low birth weight for prevention of obesity |
| PL214862B1 (pl) * | 2001-10-22 | 2013-09-30 | Amgen | Sposób in vitro do okreslania predyspozycji pacjenta ludzkiego z lipoatrofia do reagowania na leczenie bialkiem leptyny, kompozycje farmaceutyczne do zastosowania do leczenia lipoatrofii i kompozycje farmaceutyczne zawierajace bialko leptyny |
| WO2003049673A2 (en) * | 2001-12-05 | 2003-06-19 | Baylor College Of Medicine | Methods and compositions for control of bone formation via modulation of sympathetic tone |
| US20050154046A1 (en) * | 2004-01-12 | 2005-07-14 | Longgui Wang | Methods of treating an inflammatory-related disease |
| US20030220374A1 (en) * | 2002-01-14 | 2003-11-27 | Pharmacia Corporation | Compositions and methods of treatment involving peroxisome proliferator-activated receptor-gamma agonists and cyclooxygenase-2 selective inhibitors |
| US7642281B2 (en) * | 2002-08-07 | 2010-01-05 | Helicon Therapeutics, Inc. | Indolone compounds useful to treat cognitive impairment |
| GB0323039D0 (en) * | 2003-10-01 | 2003-11-05 | Danisco | Method |
| DE10353593A1 (de) * | 2003-11-17 | 2005-06-23 | Klinikum der Universität München Großhadern-Innenstadt | Leptinantagonist und Verfahren zur quantitativen Messung von Leptin |
| US7407929B2 (en) * | 2004-05-07 | 2008-08-05 | Boston Biomedical Research Institute | Leptin peptide antagonists |
| US20090088367A1 (en) | 2004-05-10 | 2009-04-02 | Burnham Institute For Medical Research | Treatment of Insulin Resistance/Metabolic Syndrome to Alleviate the Risks of Dementia |
| US20060281699A1 (en) * | 2004-05-12 | 2006-12-14 | Merchiers Pascal G | Methods, compositions and compound assays for inhibiting amyloid-beta protein production |
| US8043619B2 (en) * | 2004-10-08 | 2011-10-25 | Yaron Ilan | Methods and uses of leptin in immune modulation |
| US7863240B2 (en) * | 2004-10-08 | 2011-01-04 | Enzo Therapeutics, Inc. | Methods and uses of leptin in hepatocellular carcinoma |
| US7307142B2 (en) * | 2004-11-26 | 2007-12-11 | Yissum Research And Development Company Of The Hebrew University Of Jerusalem | Leptin antagonists |
| US7629315B2 (en) * | 2005-03-09 | 2009-12-08 | University Of Pittsburgh Of The Commonwealth System Of Higher Education | Compositions for blocking the inhibitory effect of human CRP on human leptin |
| US8227408B2 (en) * | 2005-09-07 | 2012-07-24 | Neurotez, Inc. | Leptin as an anti-amyloidogenic biologic and methods for delaying the onset and reducing Alzheimer's disease-like pathology |
| US9333240B2 (en) * | 2005-09-16 | 2016-05-10 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Compounds for improving nutritional status, cognition and survival |
| EP1795895A1 (en) * | 2005-12-08 | 2007-06-13 | KeyNeurotek AG | A tissue-based assay system for Alzheimer-specific degeneration and pathology |
| US20070218504A1 (en) * | 2006-03-09 | 2007-09-20 | University Of Pittsburgh | Human leptin-derived polypeptides and uses thereof |
| WO2007139818A2 (en) * | 2006-05-22 | 2007-12-06 | The Board Of Trustees Of The Leland Stanford Junior University | Pharmacological treatment of cognitive impairment |
| WO2008048691A2 (en) | 2006-10-18 | 2008-04-24 | Amylin Pharmaceuticals, Inc. | Use of leptin for treating post-lipectomy ectopic fat deposition and other post-lipectomy associated disorders |
| WO2008115880A2 (en) | 2007-03-21 | 2008-09-25 | The Board Of Regents Of The University Of Texas System | Use of leptin for the treatment or prevention of parkinson's disease |
| US20100267630A1 (en) | 2007-06-20 | 2010-10-21 | Universitat De Les Illes Balears | Use of leptin in the prevention of unhealthy food habits and cardiovascular diseases |
| US8889622B2 (en) * | 2007-07-25 | 2014-11-18 | Washington University | Methods of inhibiting seizure in a subject |
| US20090031434A1 (en) * | 2007-07-26 | 2009-01-29 | Weiping Han | Animal models for obesity and neurodegenerative diseases |
| WO2009108340A2 (en) | 2008-02-27 | 2009-09-03 | Temple University - Of The Commonwealth System Of Higher Education | Leptin agonist and methods of use |
| WO2009138762A2 (en) | 2008-05-15 | 2009-11-19 | Regen Therapeutics Plc | Therapeutic use of peptides |
| WO2009143380A2 (en) | 2008-05-21 | 2009-11-26 | Neurotez, Inc. | Methods for treating progressive cognitive disorders related to neurofibrillary tangles |
| AU2009313562B2 (en) * | 2008-11-04 | 2012-11-15 | Neurotez, Inc. | Leptin compositions and methods for treating progressive cognitive function disorders resulting from accumulation of neurofibrillary tangles and amlyoid beta |
-
2009
- 2009-05-21 WO PCT/US2009/044907 patent/WO2009143380A2/en not_active Ceased
- 2009-05-21 CN CN200980128037XA patent/CN102099048A/zh active Pending
- 2009-05-21 JP JP2011510712A patent/JP5622720B2/ja active Active
- 2009-05-21 CA CA2725143A patent/CA2725143A1/en not_active Abandoned
- 2009-05-21 EP EP09751608A patent/EP2326339A4/en not_active Withdrawn
- 2009-05-21 KR KR1020107028636A patent/KR20110028457A/ko not_active Ceased
- 2009-05-21 AU AU2009248914A patent/AU2009248914A1/en not_active Abandoned
- 2009-05-21 US US12/470,427 patent/US8642543B2/en active Active
-
2010
- 2010-11-19 ZA ZA2010/08303A patent/ZA201008303B/en unknown
-
2013
- 2013-09-18 US US14/030,661 patent/US20140121157A1/en not_active Abandoned
Also Published As
| Publication number | Publication date |
|---|---|
| US20120010132A9 (en) | 2012-01-12 |
| JP5622720B2 (ja) | 2014-11-12 |
| JP2011520981A (ja) | 2011-07-21 |
| WO2009143380A2 (en) | 2009-11-26 |
| US20140121157A1 (en) | 2014-05-01 |
| CA2725143A1 (en) | 2009-11-26 |
| CN102099048A (zh) | 2011-06-15 |
| WO2009143380A3 (en) | 2010-01-14 |
| ZA201008303B (en) | 2012-04-25 |
| EP2326339A4 (en) | 2012-06-20 |
| US20090291894A1 (en) | 2009-11-26 |
| AU2009248914A1 (en) | 2009-11-26 |
| EP2326339A2 (en) | 2011-06-01 |
| US8642543B2 (en) | 2014-02-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR20110028457A (ko) | 신경섬유 매듭과 연관된 신경변성 장애를 치료하는 방법 | |
| CN102300581B (zh) | 用于治疗因神经原纤维缠结和β淀粉状蛋白的积聚引起的进行性认知功能障碍的瘦蛋白组合物和方法 | |
| KR102231074B1 (ko) | 인슐린 글라진/릭시세나티드 고정비 제형 | |
| JP2021512051A (ja) | ペプチドyy薬学的調剤物、組成物、および方法 | |
| US20240000896A1 (en) | Elevated intracranial pressure treatment | |
| EP3197482A1 (en) | Methods of treating traumatic brain injury | |
| WO2008040260A1 (es) | Uso del factor de crecimiento epidérmico para la restauración morfofuncional de nervios periféricos en la neuropatía diabética | |
| JP2021130704A (ja) | At2r活性を調節するための組成物および方法 | |
| HK40076546B (en) | Incretin for elevated intracranial pressure treatment | |
| HK40076546A (en) | Incretin for elevated intracranial pressure treatment | |
| WO2008062420A2 (en) | Use of glucagon and insulin in methods and compositions for the treatment of acute brain injury and neurodegenerative disorders | |
| TW202535919A (zh) | 類升糖素肽2(glp-2)類似物之調配物及其用途 | |
| WO2025160184A1 (en) | Methods for the prophylaxis and treatment of obesity and related conditions and disorders | |
| CA2915413C (en) | Insulin glargine/lixisenatide fixed ratio formulation | |
| WO2022125548A1 (en) | Growth and differentiation factor 15 for treatment of proliferative vitreoretinopathy therapy |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PA0105 | International application |
Patent event date: 20101220 Patent event code: PA01051R01D Comment text: International Patent Application |
|
| PG1501 | Laying open of application | ||
| A201 | Request for examination | ||
| PA0201 | Request for examination |
Patent event code: PA02012R01D Patent event date: 20140521 Comment text: Request for Examination of Application |
|
| E902 | Notification of reason for refusal | ||
| PE0902 | Notice of grounds for rejection |
Comment text: Notification of reason for refusal Patent event date: 20150909 Patent event code: PE09021S01D |
|
| E601 | Decision to refuse application | ||
| PE0601 | Decision on rejection of patent |
Patent event date: 20160501 Comment text: Decision to Refuse Application Patent event code: PE06012S01D Patent event date: 20150909 Comment text: Notification of reason for refusal Patent event code: PE06011S01I |
|
| J201 | Request for trial against refusal decision | ||
| PJ0201 | Trial against decision of rejection |
Patent event date: 20160601 Comment text: Request for Trial against Decision on Refusal Patent event code: PJ02012R01D Patent event date: 20160501 Comment text: Decision to Refuse Application Patent event code: PJ02011S01I Appeal kind category: Appeal against decision to decline refusal Appeal identifier: 2016101003297 Request date: 20160601 |
|
| PJ0501 | Disposition of invalidation of trial |
Comment text: Request for Amendment Patent event date: 20151117 Patent event code: PJ05011S01I Appeal kind category: Appeal against decision to decline refusal Request date: 20160601 Appeal identifier: 2016101003297 |