JP7530692B2 - 身体構造の塞栓形成のためのシステムおよび方法 - Google Patents
身体構造の塞栓形成のためのシステムおよび方法 Download PDFInfo
- Publication number
- JP7530692B2 JP7530692B2 JP2020500782A JP2020500782A JP7530692B2 JP 7530692 B2 JP7530692 B2 JP 7530692B2 JP 2020500782 A JP2020500782 A JP 2020500782A JP 2020500782 A JP2020500782 A JP 2020500782A JP 7530692 B2 JP7530692 B2 JP 7530692B2
- Authority
- JP
- Japan
- Prior art keywords
- braided structure
- curvature
- radius
- longitudinal axis
- section
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title description 25
- 230000010102 embolization Effects 0.000 title description 2
- 230000007547 defect Effects 0.000 claims description 86
- 238000011282 treatment Methods 0.000 claims description 57
- 210000005166 vasculature Anatomy 0.000 claims description 19
- 239000008280 blood Substances 0.000 claims description 17
- 210000004369 blood Anatomy 0.000 claims description 17
- 206010002329 Aneurysm Diseases 0.000 description 36
- 239000000463 material Substances 0.000 description 13
- 230000002792 vascular Effects 0.000 description 11
- 201000008450 Intracranial aneurysm Diseases 0.000 description 10
- 230000006835 compression Effects 0.000 description 9
- 238000007906 compression Methods 0.000 description 9
- 238000010276 construction Methods 0.000 description 8
- 210000004556 brain Anatomy 0.000 description 7
- 230000017531 blood circulation Effects 0.000 description 5
- 210000004204 blood vessel Anatomy 0.000 description 5
- 238000001356 surgical procedure Methods 0.000 description 5
- 210000001367 artery Anatomy 0.000 description 4
- 230000036772 blood pressure Effects 0.000 description 4
- UQMRAFJOBWOFNS-UHFFFAOYSA-N butyl 2-(2,4-dichlorophenoxy)acetate Chemical compound CCCCOC(=O)COC1=CC=C(Cl)C=C1Cl UQMRAFJOBWOFNS-UHFFFAOYSA-N 0.000 description 4
- 230000001351 cycling effect Effects 0.000 description 4
- 210000005069 ears Anatomy 0.000 description 4
- 208000005189 Embolism Diseases 0.000 description 3
- 229910045601 alloy Inorganic materials 0.000 description 3
- 239000000956 alloy Substances 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 238000005056 compaction Methods 0.000 description 3
- 210000001105 femoral artery Anatomy 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 238000003856 thermoforming Methods 0.000 description 3
- 230000007556 vascular defect Effects 0.000 description 3
- 208000007536 Thrombosis Diseases 0.000 description 2
- 206010053648 Vascular occlusion Diseases 0.000 description 2
- 210000000709 aorta Anatomy 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 230000002490 cerebral effect Effects 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 230000035876 healing Effects 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 238000007373 indentation Methods 0.000 description 2
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 2
- 210000002321 radial artery Anatomy 0.000 description 2
- 238000007634 remodeling Methods 0.000 description 2
- 210000003625 skull Anatomy 0.000 description 2
- 208000021331 vascular occlusion disease Diseases 0.000 description 2
- 206010002091 Anaesthesia Diseases 0.000 description 1
- 241000946357 Atheta Species 0.000 description 1
- 239000004593 Epoxy Substances 0.000 description 1
- 208000002847 Surgical Wound Diseases 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 239000000853 adhesive Substances 0.000 description 1
- 238000004026 adhesive bonding Methods 0.000 description 1
- 230000001070 adhesive effect Effects 0.000 description 1
- 230000037005 anaesthesia Effects 0.000 description 1
- 238000004873 anchoring Methods 0.000 description 1
- 210000002376 aorta thoracic Anatomy 0.000 description 1
- 208000007474 aortic aneurysm Diseases 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000000740 bleeding effect Effects 0.000 description 1
- 238000009954 braiding Methods 0.000 description 1
- 238000005219 brazing Methods 0.000 description 1
- 230000000747 cardiac effect Effects 0.000 description 1
- 210000001715 carotid artery Anatomy 0.000 description 1
- 238000007428 craniotomy Methods 0.000 description 1
- 238000002788 crimping Methods 0.000 description 1
- 238000010586 diagram Methods 0.000 description 1
- 230000010339 dilation Effects 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 230000003073 embolic effect Effects 0.000 description 1
- 239000000945 filler Substances 0.000 description 1
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 1
- 229910052737 gold Inorganic materials 0.000 description 1
- 239000010931 gold Substances 0.000 description 1
- 230000023597 hemostasis Effects 0.000 description 1
- 239000007943 implant Substances 0.000 description 1
- 238000002513 implantation Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000012977 invasive surgical procedure Methods 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 210000005248 left atrial appendage Anatomy 0.000 description 1
- 238000004519 manufacturing process Methods 0.000 description 1
- 239000011159 matrix material Substances 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000000465 moulding Methods 0.000 description 1
- 229910001000 nickel titanium Inorganic materials 0.000 description 1
- 238000012856 packing Methods 0.000 description 1
- 229910052697 platinum Inorganic materials 0.000 description 1
- HWLDNSXPUQTBOD-UHFFFAOYSA-N platinum-iridium alloy Chemical compound [Ir].[Pt] HWLDNSXPUQTBOD-UHFFFAOYSA-N 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 239000012858 resilient material Substances 0.000 description 1
- 238000005476 soldering Methods 0.000 description 1
- 239000004753 textile Substances 0.000 description 1
- 238000003466 welding Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/12—Surgical instruments, devices or methods, e.g. tourniquets for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels, umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12131—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device
- A61B17/12168—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device having a mesh structure
- A61B17/12172—Occluding by internal devices, e.g. balloons or releasable wires characterised by the type of occluding device having a mesh structure having a pre-set deployed three-dimensional shape
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/12—Surgical instruments, devices or methods, e.g. tourniquets for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels, umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B17/12099—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder
- A61B17/12109—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel
- A61B17/12113—Occluding by internal devices, e.g. balloons or releasable wires characterised by the location of the occluder in a blood vessel within an aneurysm
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B2017/00831—Material properties
- A61B2017/00867—Material properties shape memory effect
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/12—Surgical instruments, devices or methods, e.g. tourniquets for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels, umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B2017/1205—Introduction devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B17/00—Surgical instruments, devices or methods, e.g. tourniquets
- A61B17/12—Surgical instruments, devices or methods, e.g. tourniquets for ligaturing or otherwise compressing tubular parts of the body, e.g. blood vessels, umbilical cord
- A61B17/12022—Occluding by internal devices, e.g. balloons or releasable wires
- A61B2017/1205—Introduction devices
- A61B2017/12054—Details concerning the detachment of the occluding device from the introduction device
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B90/00—Instruments, implements or accessories specially adapted for surgery or diagnosis and not covered by any of the groups A61B1/00 - A61B50/00, e.g. for luxation treatment or for protecting wound edges
- A61B90/39—Markers, e.g. radio-opaque or breast lesions markers
- A61B2090/3966—Radiopaque markers visible in an X-ray image
Landscapes
- Health & Medical Sciences (AREA)
- Surgery (AREA)
- Life Sciences & Earth Sciences (AREA)
- Heart & Thoracic Surgery (AREA)
- Molecular Biology (AREA)
- Vascular Medicine (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Reproductive Health (AREA)
- Medical Informatics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Neurosurgery (AREA)
- Surgical Instruments (AREA)
Description
Claims (12)
- 患者の血管系内の血管内欠陥の治療のためのデバイスであって、
近位端、遠位端、および縦軸を有し、前記縦軸方向に垂直な径方向に自己拡張性である血液透過性編組み構造を含み、前記編組み構造は、複数の弾力性の細長いフィラメントが編組まれて成り、前記フィラメントは、前記血液透過性編組み構造の近位端または遠位端のうち少なくとも1つで留め付けられており、前記血液透過性編組み構造は、マイクロカテーテル内でデリバリされる際には、前記径方向に拘束された細長くされた形態を有し、前記マイクロカテーテルが取り除かれた際には、前記径方向に拘束された形態に比べて前記縦軸方向に短縮されて前記径方向に拡張された形態を有し、
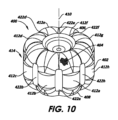
前記血液透過性編組み構造の前記拡張された形態は、前記血液透過性編組み構造の前記近位端と前記遠位端との間に延びた複数の突起部、および前記複数の突起部のそれぞれの間に配置され、前記血液透過性編組み構造の前記近位端と前記遠位端との間に延びる複数の溝部を備え、前記拡張された形態の前記径方向の断面において、前記縦軸方向に亘って、波形状の外周を形成し;
前記複数の突起部の各々の前記径方向の断面の外周は、前記波形状の山部を形成し、該山部は前記径方向の断面において、それぞれ、第1の曲率半径(r1)を有する突起部の第1の側面と、第2の曲率半径(r2)を有する前記突起部の第2の側面と、前記突起部の前記第1の側面と前記突起部の前記第2の側面との間に第3の曲率半径(r3)を有する前記突起部の中心部分とを備え、前記縦軸方向に亘って、前記径方向の断面において前記第3の曲率半径は、前記第1の曲率半径および前記第2の曲率半径よりも大きい、
デバイス。 - 前記複数の突起部は、前記血液透過性編組み構造の前記縦軸の周りに均等に配置される、請求項1に記載のデバイス。
- 前記血液透過性編組み構造の前記複数の突起部は、2個~16個の突起部を含む、請求項1に記載のデバイス。
- 前記血液透過性編組み構造は、前記溝部のそれぞれに沿ってひだを付けるように構成される、請求項3に記載のデバイス。
- 前記複数の弾力性の細長いフィラメントのそれぞれは、第1の末端、中心区画、および第2の末端を有し、前記複数のフィラメントの第1および第2の末端は、前記血液透過性編組み構造の近位端で留め付けられており、前記複数のフィラメントのそれぞれの中心区画は、前記血液透過性編組み構造の遠位領域を通過する、請求項1に記載のデバイス。
- 前記拡張された形態の前記径方向の断面は、2ミリメートルと14ミリメートルとの間の、対向する前記山部の間の距離である大径Dを有する、請求項1に記載のデバイス。
- 前記拡張された形態の前記径方向の断面は、対向する前記溝部間の距離である、小径dを有し、前記大径Dと前記小径dとの比は、約1.05と約1.35との間である、請求項6に記載のデバイス。
- 前記血液透過性編組み構造は、前記血液透過性編組み構造の前記縦軸方向に垂直な断面の外周の少なくとも一部分に、前記外周方向に延在する少なくとも1つの溝部をさらに含む、請求項1に記載のデバイス。
- 前記少なくとも1つの溝部は、へこみまたは陥凹を含む、請求項8に記載のデバイス。
- 前記少なくとも1つの溝部は、半円形の断面または三角形の断面を有する、請求項9に記載のデバイス。
- 患者の血管系内の血管内欠陥の治療のためのデバイスであって、
複数のフィラメントから形成されたメッシュ構造を備える編組み構造を備え、前記編組み構造は、近位端、遠位端、および前記近位端と前記遠位端を通して延びる縦軸を有し、前記編組み構造は、マイクロカテーテル内でデリバリされる際には、前記縦軸方向に垂直な径方向に拘束されて細長くされた形態を、および前記拘束が解かれた際には、前記径方向に拘束された形態に比べて前記縦軸方向に短縮された、前記径方向に拡張された形態を有し;
前記径方向に拡張された形態では、前記メッシュ構造が、前記編組み構造の前記近位端と前記遠位端の間にそれぞれが延びた複数の突起部と、前記複数の突起部のそれぞれの間に配置され、前記編組み構造の前記近位端と前記遠位端との間に延びる複数の溝部を備え、前記拡張された形態の前記径方向の断面において、前記縦軸方向に亘って、波形状の外周を形成し;
前記複数の突起部の各々の前記径方向の断面の外周は、前記波形状の山部を形成し、該山部は前記径方向の断面において、それぞれ、第1の曲率半径(r1)を有する突起部の第1の側面と、第2の曲率半径(r2)を有する前記突起部の第2の側面と、前記突起部の前記第1の側面と前記突起部の前記第2の側面との間に第3の曲率半径(r3)を有する前記突起部の中心部分とを備え、前記縦軸方向に亘って、前記径方向の断面において第3の曲率半径は、前記第1の曲率半径および前記第2の曲率半径よりも大きい、
デバイス。 - さらに、前記縦軸方向に垂直な断面の外周方向に延在する溝部を含む、請求項11に記載のデバイス。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201762476104P | 2017-03-24 | 2017-03-24 | |
| US62/476,104 | 2017-03-24 | ||
| PCT/US2018/022806 WO2018175221A1 (en) | 2017-03-24 | 2018-03-16 | Systems and methods for embolization of body structures |
Publications (4)
| Publication Number | Publication Date |
|---|---|
| JP2020509922A JP2020509922A (ja) | 2020-04-02 |
| JP2020509922A5 JP2020509922A5 (ja) | 2021-04-30 |
| JPWO2018175221A5 JPWO2018175221A5 (ja) | 2022-06-09 |
| JP7530692B2 true JP7530692B2 (ja) | 2024-08-08 |
Family
ID=63582004
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2020500782A Active JP7530692B2 (ja) | 2017-03-24 | 2018-03-16 | 身体構造の塞栓形成のためのシステムおよび方法 |
Country Status (5)
| Country | Link |
|---|---|
| US (3) | US10881413B2 (ja) |
| EP (1) | EP3600068B1 (ja) |
| JP (1) | JP7530692B2 (ja) |
| CN (1) | CN110944587A (ja) |
| WO (1) | WO2018175221A1 (ja) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11583289B2 (en) | 2008-05-01 | 2023-02-21 | Aneuclose Llc | Aneurysm-occluding mesh ribbon with a series of loops or segments having distal-to-proximal variation in size, shape, and/or orientation |
| US11471164B2 (en) | 2008-05-01 | 2022-10-18 | Aneuclose Llc | Methods of occluding a cerebral aneurysm by inserting embolic members or material into an intrasacular implant |
| US11357511B2 (en) | 2008-05-01 | 2022-06-14 | Aneuclose Llc | Intrasacular aneurysm occlusion device with globular first configuration and bowl-shaped second configuration |
| US11471163B2 (en) | 2008-05-01 | 2022-10-18 | Aneuclose Llc | Intrasaccular aneurysm occlusion device with net or mesh expanded by string-of-pearls embolies |
| US11464518B2 (en) | 2008-05-01 | 2022-10-11 | Aneuclose Llc | Proximal concave neck bridge with central lumen and distal net for occluding cerebral aneurysms |
| US11484322B2 (en) | 2018-01-03 | 2022-11-01 | Aneuclose Llc | Aneurysm neck bridge with a closeable opening or lumen through which embolic material is inserted into the aneurysm sac |
| CA2722672C (en) | 2008-05-02 | 2019-10-22 | Sequent Medical Inc. | Filamentary devices for treatment of vascular defects |
| US9955976B2 (en) | 2013-08-16 | 2018-05-01 | Sequent Medical, Inc. | Filamentary devices for treatment of vascular defects |
| EP3510945B1 (en) | 2014-04-30 | 2021-11-10 | Cerus Endovascular Limited | Occlusion device |
| EP4011303B1 (en) | 2015-12-07 | 2024-06-12 | Cerus Endovascular Limited | Occlusion device |
| CN109069220B (zh) | 2016-03-11 | 2021-05-25 | Cerus血管内设备有限公司 | 封堵装置 |
| EP3672499B1 (en) | 2017-08-21 | 2024-01-17 | Cerus Endovascular Limited | Occlusion device |
| US11185335B2 (en) | 2018-01-19 | 2021-11-30 | Galaxy Therapeutics Inc. | System for and method of treating aneurysms |
| WO2020190620A1 (en) | 2019-03-15 | 2020-09-24 | Sequent Medical, Inc. | Filamentary devices for treatment of vascular defects |
| US12102327B2 (en) | 2019-05-25 | 2024-10-01 | Galaxy Therapeutics, Inc. | Systems and methods for treating aneurysms |
| US11406404B2 (en) | 2020-02-20 | 2022-08-09 | Cerus Endovascular Limited | Clot removal distal protection methods |
| US12023034B2 (en) | 2020-03-11 | 2024-07-02 | Microvention, Inc. | Devices for treatment of vascular defects |
| US12070220B2 (en) | 2020-03-11 | 2024-08-27 | Microvention, Inc. | Devices having multiple permeable shells for treatment of vascular defects |
| US12108946B2 (en) * | 2020-07-07 | 2024-10-08 | St. Jude Medical, Cardiology Division, Inc. | Devices and methods for occlusion of vascular system abnormalities |
| EP4284263A4 (en) * | 2021-01-27 | 2024-06-26 | Galaxy Therapeutics, Inc. | SYSTEMS AND METHODS FOR TREATING ANEURYSMS |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001513354A (ja) | 1997-08-05 | 2001-09-04 | ボストン サイエンティフィック リミテッド | 取り外し可能な動脈瘤ネックブリッジ(ii) |
| US6368338B1 (en) | 1999-03-05 | 2002-04-09 | Board Of Regents, The University Of Texas | Occlusion method and apparatus |
| WO2002069783A2 (en) | 2000-10-24 | 2002-09-12 | Concentric Medical, Inc. | Device and methods for treating vascular malformations |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060155303A1 (en) * | 2002-04-09 | 2006-07-13 | Andras Konya | Occlusion method and apparatus |
| ES2856081T3 (es) * | 2007-04-16 | 2021-09-27 | Occlutech Holding Ag | Oclusor para la oclusión de una orejuela auricular y procedimiento de producción del mismo |
| US9259225B2 (en) * | 2008-02-19 | 2016-02-16 | St. Jude Medical, Cardiology Division, Inc. | Medical devices for treating a target site and associated method |
| CA2722672C (en) * | 2008-05-02 | 2019-10-22 | Sequent Medical Inc. | Filamentary devices for treatment of vascular defects |
| CA2778639A1 (en) * | 2009-11-05 | 2011-05-12 | Sequent Medical Inc. | Multiple layer filamentary devices or treatment of vascular defects |
| WO2011106426A1 (en) * | 2010-02-23 | 2011-09-01 | Maria Aboytes | Devices and methods for vascular recanalization |
| US9770232B2 (en) * | 2011-08-12 | 2017-09-26 | W. L. Gore & Associates, Inc. | Heart occlusion devices |
| EP2572644A1 (en) * | 2011-09-22 | 2013-03-27 | Occlutech Holding AG | Medical implantable occlusion device |
| JP6133307B2 (ja) | 2011-10-17 | 2017-05-24 | シークエント メディカル インコーポレイテッド | 編組機及び使用方法 |
| CA2865192A1 (en) | 2012-02-29 | 2013-10-17 | Occlutech Holding Ag | A device for occluding an opening in a body and associated methods |
| US20150133989A1 (en) * | 2012-04-20 | 2015-05-14 | Inceptus Medical, Llc | Expandable occlusion devices and methods of use |
| JP6133983B2 (ja) * | 2012-07-13 | 2017-05-24 | ボストン サイエンティフィック サイムド,インコーポレイテッドBoston Scientific Scimed,Inc. | 心耳用閉塞装置およびその製造方法 |
| US8597323B1 (en) | 2012-11-16 | 2013-12-03 | Sequent Medical, Inc. | Delivery and detachment systems and methods for vascular implants |
| US9078658B2 (en) | 2013-08-16 | 2015-07-14 | Sequent Medical, Inc. | Filamentary devices for treatment of vascular defects |
| US9808230B2 (en) * | 2014-06-06 | 2017-11-07 | W. L. Gore & Associates, Inc. | Sealing device and delivery system |
| CN112869920A (zh) * | 2015-02-25 | 2021-06-01 | 盖乐西医疗公司 | 治疗动脉瘤的系统和方法 |
-
2018
- 2018-03-16 CN CN201880032247.8A patent/CN110944587A/zh active Pending
- 2018-03-16 US US15/923,266 patent/US10881413B2/en active Active
- 2018-03-16 WO PCT/US2018/022806 patent/WO2018175221A1/en active Application Filing
- 2018-03-16 EP EP18770792.2A patent/EP3600068B1/en active Active
- 2018-03-16 JP JP2020500782A patent/JP7530692B2/ja active Active
-
2020
- 2020-12-02 US US17/110,212 patent/US11806020B2/en active Active
-
2023
- 2023-11-06 US US18/503,105 patent/US20240065701A1/en active Pending
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2001513354A (ja) | 1997-08-05 | 2001-09-04 | ボストン サイエンティフィック リミテッド | 取り外し可能な動脈瘤ネックブリッジ(ii) |
| US6368338B1 (en) | 1999-03-05 | 2002-04-09 | Board Of Regents, The University Of Texas | Occlusion method and apparatus |
| WO2002069783A2 (en) | 2000-10-24 | 2002-09-12 | Concentric Medical, Inc. | Device and methods for treating vascular malformations |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2020509922A (ja) | 2020-04-02 |
| EP3600068A1 (en) | 2020-02-05 |
| CN110944587A (zh) | 2020-03-31 |
| EP3600068B1 (en) | 2024-01-24 |
| US20240065701A1 (en) | 2024-02-29 |
| US10881413B2 (en) | 2021-01-05 |
| WO2018175221A1 (en) | 2018-09-27 |
| US20180271540A1 (en) | 2018-09-27 |
| US11806020B2 (en) | 2023-11-07 |
| EP3600068A4 (en) | 2020-09-30 |
| US20210169499A1 (en) | 2021-06-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7530692B2 (ja) | 身体構造の塞栓形成のためのシステムおよび方法 | |
| US12082819B2 (en) | Filamentary devices for treatment of vascular defects | |
| CN113573650B (zh) | 用于治疗血管缺陷的具有柔性连接部的丝装置 | |
| JP4913062B2 (ja) | 動脈瘤の再造形器具 | |
| JP6110427B2 (ja) | 解剖学的開口部を密閉するシステム及び方法 | |
| US20210007754A1 (en) | Filamentary devices for treatment of vascular defects | |
| EP1750619B1 (en) | Flexible vascular occluding device | |
| WO2020190639A1 (en) | Filamentary devices for treatment of vascular defects | |
| US20220257260A1 (en) | Filamentary devices for treatment of vascular defects | |
| TW202023483A (zh) | 囊內裝置定位及展開系統 | |
| US12070220B2 (en) | Devices having multiple permeable shells for treatment of vascular defects | |
| CN109745094B (zh) | 封堵装置 | |
| WO2022132778A1 (en) | Occluding medical devices and methods of use | |
| JP2024538262A (ja) | 血管障害の治療器具 | |
| WO2023215225A1 (en) | Devices for treatment of vascular defects | |
| AU2013201605B2 (en) | Flexible vascular occluding device | |
| AU2010289240B2 (en) | Systems and methods for enclosing an anatomical opening | |
| BR102015024009A2 (pt) | stent com balão acoplado para aneurisma cerebral |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20191125 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210316 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20210316 |
|
| A871 | Explanation of circumstances concerning accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A871 Effective date: 20210316 |
|
| A975 | Report on accelerated examination |
Free format text: JAPANESE INTERMEDIATE CODE: A971005 Effective date: 20210407 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20210420 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210720 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20211019 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20220221 |
|
| C60 | Trial request (containing other claim documents, opposition documents) |
Free format text: JAPANESE INTERMEDIATE CODE: C60 Effective date: 20220221 |
|
| C11 | Written invitation by the commissioner to file amendments |
Free format text: JAPANESE INTERMEDIATE CODE: C11 Effective date: 20220308 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20220531 |
|
| C21 | Notice of transfer of a case for reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C21 Effective date: 20220607 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20220715 |
|
| C211 | Notice of termination of reconsideration by examiners before appeal proceedings |
Free format text: JAPANESE INTERMEDIATE CODE: C211 Effective date: 20220726 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20220823 |
|
| C13 | Notice of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: C13 Effective date: 20230404 |
|
| C22 | Notice of designation (change) of administrative judge |
Free format text: JAPANESE INTERMEDIATE CODE: C22 Effective date: 20230418 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20230630 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20230803 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20230831 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20240311 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20240422 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20240627 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20240627 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20240724 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 7530692 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |