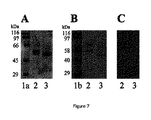
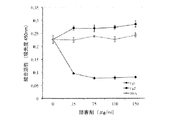
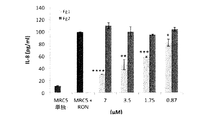
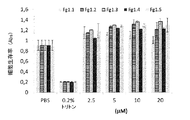
JP6840086B2 - 薬物として、特に皮膚炎症性疾患において使用するためのフィブリノゲン由来の単離されたペプチドおよびそれらのフラグメント - Google Patents
薬物として、特に皮膚炎症性疾患において使用するためのフィブリノゲン由来の単離されたペプチドおよびそれらのフラグメント Download PDFInfo
- Publication number
- JP6840086B2 JP6840086B2 JP2017549280A JP2017549280A JP6840086B2 JP 6840086 B2 JP6840086 B2 JP 6840086B2 JP 2017549280 A JP2017549280 A JP 2017549280A JP 2017549280 A JP2017549280 A JP 2017549280A JP 6840086 B2 JP6840086 B2 JP 6840086B2
- Authority
- JP
- Japan
- Prior art keywords
- acnes
- seq
- fibrinogen
- cells
- protein
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/745—Blood coagulation or fibrinolysis factors
- C07K14/75—Fibrinogen
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0019—Injectable compositions; Intramuscular, intravenous, arterial, subcutaneous administration; Compositions to be administered through the skin in an invasive manner
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/10—Anti-acne agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Dermatology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Zoology (AREA)
- Genetics & Genomics (AREA)
- Biochemistry (AREA)
- Wood Science & Technology (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Gastroenterology & Hepatology (AREA)
- Toxicology (AREA)
- Pain & Pain Management (AREA)
- Rheumatology (AREA)
- Hematology (AREA)
- Biomedical Technology (AREA)
- Virology (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Immunology (AREA)
- General Engineering & Computer Science (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP15305414.3 | 2015-03-20 | ||
| EP15305414 | 2015-03-20 | ||
| PCT/EP2016/056179 WO2016150926A1 (en) | 2015-03-20 | 2016-03-21 | Isolated peptides and fragments thereof from fibrinogen for use as drugs, particularly in skin inflammatory diseases |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2018512844A JP2018512844A (ja) | 2018-05-24 |
| JP2018512844A5 JP2018512844A5 (enExample) | 2019-04-25 |
| JP6840086B2 true JP6840086B2 (ja) | 2021-03-10 |
Family
ID=52807757
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017549280A Active JP6840086B2 (ja) | 2015-03-20 | 2016-03-21 | 薬物として、特に皮膚炎症性疾患において使用するためのフィブリノゲン由来の単離されたペプチドおよびそれらのフラグメント |
Country Status (10)
| Country | Link |
|---|---|
| US (1) | US10323078B2 (enExample) |
| EP (1) | EP3270950B1 (enExample) |
| JP (1) | JP6840086B2 (enExample) |
| CN (1) | CN107636011B (enExample) |
| AU (1) | AU2016236297B2 (enExample) |
| BR (1) | BR112017020008A8 (enExample) |
| CA (1) | CA2979812C (enExample) |
| DK (1) | DK3270950T3 (enExample) |
| ES (1) | ES2880795T3 (enExample) |
| WO (1) | WO2016150926A1 (enExample) |
Families Citing this family (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN121285384A (zh) | 2023-05-05 | 2026-01-06 | 赛诺菲巴斯德有限公司 | 用于在痤疮的治疗中使用的组合物 |
| CN117304303B (zh) * | 2023-07-14 | 2024-07-05 | 杭州恩和生物科技有限公司 | 纤连蛋白截短片段、组合物及用途 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1990012866A1 (en) * | 1989-04-10 | 1990-11-01 | Louisiana State University And Agricultural And Mechanical College | Lytic peptides, use for growth, infection and cancer |
| US5639940A (en) * | 1994-03-03 | 1997-06-17 | Pharmaceutical Proteins Ltd. | Production of fibrinogen in transgenic animals |
| US6783961B1 (en) * | 1999-02-26 | 2004-08-31 | Genset S.A. | Expressed sequence tags and encoded human proteins |
| CA2296792A1 (en) * | 1999-02-26 | 2000-08-26 | Genset S.A. | Expressed sequence tags and encoded human proteins |
| JP2003503425A (ja) * | 1999-06-30 | 2003-01-28 | リージェンツ オブ ザ ユニバーシティ オブ カリフォルニア | 血栓性又は血栓塞栓性疾患のための診断的試験 |
| WO2003003988A2 (en) * | 2001-07-06 | 2003-01-16 | Oregon Health & Science University | Peptides which modulate blood coagulation and methods of use thereof |
| US20040142325A1 (en) * | 2001-09-14 | 2004-07-22 | Liat Mintz | Methods and systems for annotating biomolecular sequences |
| US20070172471A1 (en) * | 2003-04-23 | 2007-07-26 | Hansa Medical Ab | Method for identifying an anti- streptococcal agent and its use for treating streptococcal infections |
| CA2624893C (en) * | 2005-10-21 | 2015-03-17 | Erhard Kopetzki | Method for the recombinant expression of a polypeptide |
| US20130280820A1 (en) * | 2005-12-16 | 2013-10-24 | Schering Corporation | Biomarkers for psoriasis |
| ES2389813T3 (es) * | 2006-08-04 | 2012-11-02 | Stb Lifesaving Technologies, Inc. | Procedimientos para la producción de apósito sólido para tratar tejido lesionado |
| WO2008116468A2 (en) * | 2007-03-26 | 2008-10-02 | Dako Denmark A/S | Mhc peptide complexes and uses thereof in infectious diseases |
| CN101618211A (zh) * | 2008-07-03 | 2010-01-06 | 珠海联邦制药股份有限公司 | 一种乙肝多肽疫苗及其应用 |
| JP5819725B2 (ja) * | 2008-07-09 | 2015-11-24 | プロフィブリックス ビーブイ | 組換えフィブリノゲン |
| EP2352998A4 (en) * | 2008-11-07 | 2011-09-21 | Centocor Ortho Biotech Inc | MARKERS AND METHOD FOR THE STUDY AND TREATMENT OF LIGHT-SENSITIVELY LUPUS PATIENTS |
| US10706955B2 (en) * | 2010-03-23 | 2020-07-07 | Iogenetics, Llc | Bioinformatic processes for determination of peptide binding |
| CA2825765C (en) * | 2011-02-02 | 2020-03-10 | Academisch Ziekenhuis Leiden H.O.D.N. Lumc | Anti-carbamylated protein antibodies and the risk for arthritis |
-
2016
- 2016-03-21 ES ES16713796T patent/ES2880795T3/es active Active
- 2016-03-21 WO PCT/EP2016/056179 patent/WO2016150926A1/en not_active Ceased
- 2016-03-21 JP JP2017549280A patent/JP6840086B2/ja active Active
- 2016-03-21 DK DK16713796.7T patent/DK3270950T3/da active
- 2016-03-21 BR BR112017020008A patent/BR112017020008A8/pt not_active IP Right Cessation
- 2016-03-21 AU AU2016236297A patent/AU2016236297B2/en active Active
- 2016-03-21 CN CN201680026130.XA patent/CN107636011B/zh active Active
- 2016-03-21 EP EP16713796.7A patent/EP3270950B1/en active Active
- 2016-03-21 CA CA2979812A patent/CA2979812C/en active Active
- 2016-03-21 US US15/559,984 patent/US10323078B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| ES2880795T3 (es) | 2021-11-25 |
| CN107636011B (zh) | 2021-07-02 |
| EP3270950B1 (en) | 2021-04-21 |
| DK3270950T3 (da) | 2021-06-07 |
| US20180105574A1 (en) | 2018-04-19 |
| WO2016150926A1 (en) | 2016-09-29 |
| US10323078B2 (en) | 2019-06-18 |
| CA2979812A1 (en) | 2016-09-29 |
| AU2016236297A1 (en) | 2017-09-28 |
| JP2018512844A (ja) | 2018-05-24 |
| BR112017020008A2 (pt) | 2018-06-19 |
| CA2979812C (en) | 2023-08-29 |
| AU2016236297B2 (en) | 2020-07-23 |
| CN107636011A (zh) | 2018-01-26 |
| EP3270950A1 (en) | 2018-01-24 |
| BR112017020008A8 (pt) | 2023-05-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6981878B2 (ja) | 抗菌療法 | |
| E. Greber et al. | Antimicrobial peptides under clinical trials | |
| Li et al. | OM‐LV20, a novel peptide from odorous frog skin, accelerates wound healing in vitro and in vivo | |
| Lacroix-Lamandé et al. | Downregulation of the Na/K-ATPase pump by leptospiral glycolipoprotein activates the NLRP3 inflammasome | |
| EP1151009B1 (en) | Antimicrobial/endotoxin neutralizing polypeptide | |
| Gebrim et al. | Antitumor effects of snake venom chemically modified Lys49 phospholipase A2-like BthTX-I and a synthetic peptide derived from its C-terminal region | |
| DE69133485T2 (de) | Verwendung von Staphylococcus Enterotoxin Homologe für Krebs-Therapie | |
| Farfán et al. | The immunomodulatory potential of phage therapy to treat acne: a review on bacterial lysis and immunomodulation | |
| Lim et al. | Antimicrobial efficacy of granulysin‐derived synthetic peptides in acne vulgaris | |
| Rotllant et al. | Generation, purification and functional characterization of three C3a anaphylatoxins in rainbow trout: role in leukocyte chemotaxis and respiratory burst | |
| Fernandes et al. | Lactoferrin-derived peptide lactofungin is potently synergistic with amphotericin B | |
| JP6840086B2 (ja) | 薬物として、特に皮膚炎症性疾患において使用するためのフィブリノゲン由来の単離されたペプチドおよびそれらのフラグメント | |
| Wu et al. | Inhibitory and anti‐inflammatory effects of two antimicrobial peptides moronecidin and temporin‐1Dra against Propionibacterium acnes in vitro and in vivo | |
| Kim et al. | Antimicrobial activity of antimicrobial peptide LPcin-YK3 derived from bovine lactophoricin | |
| Lesiak et al. | Significance of host antimicrobial peptides in the pathogenesis and treatment of acne vulgaris | |
| Popov et al. | Host-defense peptides AC12, DK16 and RC11 with immunomodulatory activity isolated from Hypsiboas raniceps skin secretion | |
| CN101607992A (zh) | 奶牛血液中分离出的抗菌肽及其编码序列和用途 | |
| CA3035770A1 (en) | Nnif and nnif-related peptides and related methods | |
| Fu et al. | The anti-myotoxic effects and mechanisms of Sinonatrix annularis serum and a novel plasma metalloproteinase inhibitor towards Deinagkistrodon acutus envenomation | |
| Li et al. | Discovery of novel caeridins from the skin secretion of the Australian white’s tree frog, Litoria caerulea | |
| JP5101280B2 (ja) | ヘルペスウイルス感染の治療のためのペプチド | |
| KR20240078664A (ko) | 락토페린 조성물 및 사용 방법 | |
| BR112022021750A2 (pt) | Anticorpo monoclonal anti-apc, material biológico relacionado ao anticorpo monoclonal anti-apc, produto de preparação e uso do anticorpo monoclonal anti-apc ou do material biológico | |
| Lee et al. | PEGylated lysozymes with anti-septic effects in human endothelial cells and in mice | |
| JP2018512844A5 (enExample) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20190318 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20190318 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200407 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20200707 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20200907 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20200929 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20201225 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20210126 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20210216 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6840086 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313111 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313115 |
|
| R370 | Written measure of declining of transfer procedure |
Free format text: JAPANESE INTERMEDIATE CODE: R370 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313115 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313115 |
|
| R370 | Written measure of declining of transfer procedure |
Free format text: JAPANESE INTERMEDIATE CODE: R370 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313115 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| S111 | Request for change of ownership or part of ownership |
Free format text: JAPANESE INTERMEDIATE CODE: R313115 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |