JP6219304B2 - 前立腺癌分析のための組成物及び方法 - Google Patents
前立腺癌分析のための組成物及び方法 Download PDFInfo
- Publication number
- JP6219304B2 JP6219304B2 JP2014544870A JP2014544870A JP6219304B2 JP 6219304 B2 JP6219304 B2 JP 6219304B2 JP 2014544870 A JP2014544870 A JP 2014544870A JP 2014544870 A JP2014544870 A JP 2014544870A JP 6219304 B2 JP6219304 B2 JP 6219304B2
- Authority
- JP
- Japan
- Prior art keywords
- antibody
- steap
- prostate
- cancer cells
- cancer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000000034 method Methods 0.000 title claims description 100
- 208000000236 Prostatic Neoplasms Diseases 0.000 title claims description 60
- 206010060862 Prostate cancer Diseases 0.000 title claims description 59
- 239000000203 mixture Substances 0.000 title claims description 31
- 238000004458 analytical method Methods 0.000 title description 8
- 210000004027 cell Anatomy 0.000 claims description 246
- 206010028980 Neoplasm Diseases 0.000 claims description 133
- 201000011510 cancer Diseases 0.000 claims description 117
- 210000002307 prostate Anatomy 0.000 claims description 86
- 239000003550 marker Substances 0.000 claims description 82
- 230000014509 gene expression Effects 0.000 claims description 72
- 210000004369 blood Anatomy 0.000 claims description 60
- 239000008280 blood Substances 0.000 claims description 60
- 239000003446 ligand Substances 0.000 claims description 42
- 239000000427 antigen Substances 0.000 claims description 38
- 108091007433 antigens Proteins 0.000 claims description 36
- 102000036639 antigens Human genes 0.000 claims description 36
- 238000011282 treatment Methods 0.000 claims description 30
- 239000006249 magnetic particle Substances 0.000 claims description 20
- 238000012360 testing method Methods 0.000 claims description 20
- 210000000265 leukocyte Anatomy 0.000 claims description 16
- 239000003153 chemical reaction reagent Substances 0.000 claims description 15
- 210000004408 hybridoma Anatomy 0.000 claims description 15
- 238000001514 detection method Methods 0.000 claims description 14
- 239000000611 antibody drug conjugate Substances 0.000 claims description 11
- 229940049595 antibody-drug conjugate Drugs 0.000 claims description 11
- 102000018651 Epithelial Cell Adhesion Molecule Human genes 0.000 claims description 10
- 108010066687 Epithelial Cell Adhesion Molecule Proteins 0.000 claims description 10
- 102000011782 Keratins Human genes 0.000 claims description 10
- 108010076876 Keratins Proteins 0.000 claims description 10
- 229940126619 mouse monoclonal antibody Drugs 0.000 claims description 10
- 229940127089 cytotoxic agent Drugs 0.000 claims description 8
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 claims description 7
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 claims description 7
- 239000003814 drug Substances 0.000 claims description 7
- 230000004044 response Effects 0.000 claims description 7
- 238000004163 cytometry Methods 0.000 claims description 6
- 239000002254 cytotoxic agent Substances 0.000 claims description 6
- 231100000599 cytotoxic agent Toxicity 0.000 claims description 6
- 229940079593 drug Drugs 0.000 claims description 6
- 230000001413 cellular effect Effects 0.000 claims description 5
- IEDXPSOJFSVCKU-HOKPPMCLSA-N [4-[[(2S)-5-(carbamoylamino)-2-[[(2S)-2-[6-(2,5-dioxopyrrolidin-1-yl)hexanoylamino]-3-methylbutanoyl]amino]pentanoyl]amino]phenyl]methyl N-[(2S)-1-[[(2S)-1-[[(3R,4S,5S)-1-[(2S)-2-[(1R,2R)-3-[[(1S,2R)-1-hydroxy-1-phenylpropan-2-yl]amino]-1-methoxy-2-methyl-3-oxopropyl]pyrrolidin-1-yl]-3-methoxy-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]-N-methylcarbamate Chemical compound CC[C@H](C)[C@@H]([C@@H](CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C)[C@@H](O)c1ccccc1)OC)N(C)C(=O)[C@@H](NC(=O)[C@H](C(C)C)N(C)C(=O)OCc1ccc(NC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@@H](NC(=O)CCCCCN2C(=O)CCC2=O)C(C)C)cc1)C(C)C IEDXPSOJFSVCKU-HOKPPMCLSA-N 0.000 claims description 4
- 239000000835 fiber Substances 0.000 claims description 4
- 238000000684 flow cytometry Methods 0.000 claims description 4
- 238000010820 immunofluorescence microscopy Methods 0.000 claims description 4
- 244000005700 microbiome Species 0.000 claims description 4
- 238000012544 monitoring process Methods 0.000 claims description 4
- 108010093470 monomethyl auristatin E Proteins 0.000 claims description 4
- 239000003053 toxin Substances 0.000 claims description 4
- 231100000765 toxin Toxicity 0.000 claims description 4
- 108700012359 toxins Proteins 0.000 claims description 4
- 239000002246 antineoplastic agent Substances 0.000 claims description 3
- FWBHETKCLVMNFS-UHFFFAOYSA-N 4',6-Diamino-2-phenylindol Chemical group C1=CC(C(=N)N)=CC=C1C1=CC2=CC=C(C(N)=N)C=C2N1 FWBHETKCLVMNFS-UHFFFAOYSA-N 0.000 claims description 2
- 239000003242 anti bacterial agent Substances 0.000 claims description 2
- 229940088710 antibiotic agent Drugs 0.000 claims description 2
- 239000002122 magnetic nanoparticle Substances 0.000 claims description 2
- 101710163270 Nuclease Proteins 0.000 claims 1
- 230000003247 decreasing effect Effects 0.000 claims 1
- 229940127121 immunoconjugate Drugs 0.000 claims 1
- 239000000523 sample Substances 0.000 description 53
- 208000005443 Circulating Neoplastic Cells Diseases 0.000 description 40
- 210000005266 circulating tumour cell Anatomy 0.000 description 34
- 241001494479 Pecora Species 0.000 description 30
- 238000009739 binding Methods 0.000 description 28
- 230000027455 binding Effects 0.000 description 27
- 150000007523 nucleic acids Chemical class 0.000 description 26
- 108020004707 nucleic acids Proteins 0.000 description 25
- 102000039446 nucleic acids Human genes 0.000 description 25
- 238000003556 assay Methods 0.000 description 20
- 238000010186 staining Methods 0.000 description 18
- 239000000975 dye Substances 0.000 description 15
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 14
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 14
- 239000013598 vector Substances 0.000 description 14
- 230000000694 effects Effects 0.000 description 13
- 241000699666 Mus <mouse, genus> Species 0.000 description 11
- 238000003364 immunohistochemistry Methods 0.000 description 11
- 241000283973 Oryctolagus cuniculus Species 0.000 description 10
- 125000003275 alpha amino acid group Chemical group 0.000 description 9
- 239000002609 medium Substances 0.000 description 9
- 108090000623 proteins and genes Proteins 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 230000000813 microbial effect Effects 0.000 description 8
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 7
- 238000003745 diagnosis Methods 0.000 description 7
- 238000010494 dissociation reaction Methods 0.000 description 7
- 230000005593 dissociations Effects 0.000 description 7
- 239000012634 fragment Substances 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 235000018102 proteins Nutrition 0.000 description 7
- 102000004169 proteins and genes Human genes 0.000 description 7
- 238000012216 screening Methods 0.000 description 7
- 238000000926 separation method Methods 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- 201000010099 disease Diseases 0.000 description 6
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 6
- 230000001225 therapeutic effect Effects 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- 239000006285 cell suspension Substances 0.000 description 5
- 238000004393 prognosis Methods 0.000 description 5
- 210000004881 tumor cell Anatomy 0.000 description 5
- 241000894006 Bacteria Species 0.000 description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 description 4
- 241000196324 Embryophyta Species 0.000 description 4
- 102000014160 PTEN Phosphohydrolase Human genes 0.000 description 4
- 108010011536 PTEN Phosphohydrolase Proteins 0.000 description 4
- 229920001213 Polysorbate 20 Polymers 0.000 description 4
- 210000002919 epithelial cell Anatomy 0.000 description 4
- 210000000981 epithelium Anatomy 0.000 description 4
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 4
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 4
- 238000012552 review Methods 0.000 description 4
- 230000004083 survival effect Effects 0.000 description 4
- 241000282693 Cercopithecidae Species 0.000 description 3
- 102000004190 Enzymes Human genes 0.000 description 3
- 108090000790 Enzymes Proteins 0.000 description 3
- 241000233866 Fungi Species 0.000 description 3
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 description 3
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 description 3
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 3
- 108060003951 Immunoglobulin Proteins 0.000 description 3
- 206010027476 Metastases Diseases 0.000 description 3
- 241001465754 Metazoa Species 0.000 description 3
- 108090000854 Oxidoreductases Proteins 0.000 description 3
- 102000004316 Oxidoreductases Human genes 0.000 description 3
- 108010004729 Phycoerythrin Proteins 0.000 description 3
- 101710120463 Prostate stem cell antigen Proteins 0.000 description 3
- 102100036735 Prostate stem cell antigen Human genes 0.000 description 3
- 108010003723 Single-Domain Antibodies Proteins 0.000 description 3
- 108010090804 Streptavidin Proteins 0.000 description 3
- 208000026487 Triploidy Diseases 0.000 description 3
- 125000000539 amino acid group Chemical group 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000010367 cloning Methods 0.000 description 3
- 229940088598 enzyme Drugs 0.000 description 3
- 239000007850 fluorescent dye Substances 0.000 description 3
- 239000011521 glass Substances 0.000 description 3
- 230000013595 glycosylation Effects 0.000 description 3
- 238000006206 glycosylation reaction Methods 0.000 description 3
- 230000005484 gravity Effects 0.000 description 3
- 102000018358 immunoglobulin Human genes 0.000 description 3
- 210000003292 kidney cell Anatomy 0.000 description 3
- 239000000463 material Substances 0.000 description 3
- 238000005259 measurement Methods 0.000 description 3
- 210000005259 peripheral blood Anatomy 0.000 description 3
- 239000011886 peripheral blood Substances 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- HZAXFHJVJLSVMW-UHFFFAOYSA-N 2-Aminoethan-1-ol Chemical compound NCCO HZAXFHJVJLSVMW-UHFFFAOYSA-N 0.000 description 2
- AUUIARVPJHGTSA-UHFFFAOYSA-N 3-(aminomethyl)chromen-2-one Chemical compound C1=CC=C2OC(=O)C(CN)=CC2=C1 AUUIARVPJHGTSA-UHFFFAOYSA-N 0.000 description 2
- FPQQSJJWHUJYPU-UHFFFAOYSA-N 3-(dimethylamino)propyliminomethylidene-ethylazanium;chloride Chemical compound Cl.CCN=C=NCCCN(C)C FPQQSJJWHUJYPU-UHFFFAOYSA-N 0.000 description 2
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- UQSXHKLRYXJYBZ-UHFFFAOYSA-N Iron oxide Chemical compound [Fe]=O UQSXHKLRYXJYBZ-UHFFFAOYSA-N 0.000 description 2
- 241000124008 Mammalia Species 0.000 description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 description 2
- 238000011529 RT qPCR Methods 0.000 description 2
- -1 SISBA Proteins 0.000 description 2
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 2
- PNDPGZBMCMUPRI-XXSWNUTMSA-N [125I][125I] Chemical compound [125I][125I] PNDPGZBMCMUPRI-XXSWNUTMSA-N 0.000 description 2
- 108010004469 allophycocyanin Proteins 0.000 description 2
- 230000000890 antigenic effect Effects 0.000 description 2
- 238000013459 approach Methods 0.000 description 2
- 229960002685 biotin Drugs 0.000 description 2
- 239000011616 biotin Substances 0.000 description 2
- 229940098773 bovine serum albumin Drugs 0.000 description 2
- 238000004113 cell culture Methods 0.000 description 2
- 238000006243 chemical reaction Methods 0.000 description 2
- 210000000349 chromosome Anatomy 0.000 description 2
- 239000010415 colloidal nanoparticle Substances 0.000 description 2
- 238000012258 culturing Methods 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000009826 distribution Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 239000013604 expression vector Substances 0.000 description 2
- GNBHRKFJIUUOQI-UHFFFAOYSA-N fluorescein Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 GNBHRKFJIUUOQI-UHFFFAOYSA-N 0.000 description 2
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 2
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 2
- 210000003494 hepatocyte Anatomy 0.000 description 2
- 230000016784 immunoglobulin production Effects 0.000 description 2
- 238000007901 in situ hybridization Methods 0.000 description 2
- 230000003993 interaction Effects 0.000 description 2
- 238000001155 isoelectric focusing Methods 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 238000007885 magnetic separation Methods 0.000 description 2
- 210000004962 mammalian cell Anatomy 0.000 description 2
- 230000009401 metastasis Effects 0.000 description 2
- 230000001394 metastastic effect Effects 0.000 description 2
- 206010061289 metastatic neoplasm Diseases 0.000 description 2
- 238000011275 oncology therapy Methods 0.000 description 2
- 210000001672 ovary Anatomy 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 239000013610 patient sample Substances 0.000 description 2
- 229940068977 polysorbate 20 Drugs 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 239000000092 prognostic biomarker Substances 0.000 description 2
- 230000002250 progressing effect Effects 0.000 description 2
- 230000000750 progressive effect Effects 0.000 description 2
- 238000000159 protein binding assay Methods 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 102000005962 receptors Human genes 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- PYWVYCXTNDRMGF-UHFFFAOYSA-N rhodamine B Chemical compound [Cl-].C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=CC=C1C(O)=O PYWVYCXTNDRMGF-UHFFFAOYSA-N 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 238000002198 surface plasmon resonance spectroscopy Methods 0.000 description 2
- 239000000725 suspension Substances 0.000 description 2
- 230000009261 transgenic effect Effects 0.000 description 2
- 238000010200 validation analysis Methods 0.000 description 2
- KGLPWQKSKUVKMJ-UHFFFAOYSA-N 2,3-dihydrophthalazine-1,4-dione Chemical compound C1=CC=C2C(=O)NNC(=O)C2=C1 KGLPWQKSKUVKMJ-UHFFFAOYSA-N 0.000 description 1
- 102100031126 6-phosphogluconolactonase Human genes 0.000 description 1
- 108010029731 6-phosphogluconolactonase Proteins 0.000 description 1
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 101710145634 Antigen 1 Proteins 0.000 description 1
- 101100519151 Arabidopsis thaliana CEP10 gene Proteins 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 108090000363 Bacterial Luciferases Proteins 0.000 description 1
- 206010006002 Bone pain Diseases 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 241000282465 Canis Species 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 102000016289 Cell Adhesion Molecules Human genes 0.000 description 1
- 108010067225 Cell Adhesion Molecules Proteins 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 241000282552 Chlorocebus aethiops Species 0.000 description 1
- 241000699802 Cricetulus griseus Species 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 108090000331 Firefly luciferases Proteins 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- 108010015133 Galactose oxidase Proteins 0.000 description 1
- 108010073178 Glucan 1,4-alpha-Glucosidase Proteins 0.000 description 1
- 102100022624 Glucoamylase Human genes 0.000 description 1
- 108010015776 Glucose oxidase Proteins 0.000 description 1
- 239000004366 Glucose oxidase Substances 0.000 description 1
- 108010018962 Glucosephosphate Dehydrogenase Proteins 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- FBOZXECLQNJBKD-ZDUSSCGKSA-N L-methotrexate Chemical compound C=1N=C2N=C(N)N=C(N)C2=NC=1CN(C)C1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 FBOZXECLQNJBKD-ZDUSSCGKSA-N 0.000 description 1
- 108010023244 Lactoperoxidase Proteins 0.000 description 1
- 102000045576 Lactoperoxidases Human genes 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- 102000016943 Muramidase Human genes 0.000 description 1
- 108010014251 Muramidase Proteins 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108010062010 N-Acetylmuramoyl-L-alanine Amidase Proteins 0.000 description 1
- NQTADLQHYWFPDB-UHFFFAOYSA-N N-Hydroxysuccinimide Chemical compound ON1C(=O)CCC1=O NQTADLQHYWFPDB-UHFFFAOYSA-N 0.000 description 1
- 108020005187 Oligonucleotide Probes Proteins 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 1
- 241000609499 Palicourea Species 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 238000003559 RNA-seq method Methods 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 241000700157 Rattus norvegicus Species 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- 241000256251 Spodoptera frugiperda Species 0.000 description 1
- 102000004142 Trypsin Human genes 0.000 description 1
- 108090000631 Trypsin Proteins 0.000 description 1
- 108010092464 Urate Oxidase Proteins 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 244000000188 Vaccinium ovalifolium Species 0.000 description 1
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 description 1
- 229940122803 Vinca alkaloid Drugs 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 229940009456 adriamycin Drugs 0.000 description 1
- 239000003146 anticoagulant agent Substances 0.000 description 1
- 229940127219 anticoagulant drug Drugs 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 238000001574 biopsy Methods 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 210000000988 bone and bone Anatomy 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 239000005018 casein Substances 0.000 description 1
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 1
- 235000021240 caseins Nutrition 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 239000002791 cell membrane marker Substances 0.000 description 1
- 210000003855 cell nucleus Anatomy 0.000 description 1
- 239000002458 cell surface marker Substances 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229940044683 chemotherapy drug Drugs 0.000 description 1
- 229960004630 chlorambucil Drugs 0.000 description 1
- JCKYGMPEJWAADB-UHFFFAOYSA-N chlorambucil Chemical compound OC(=O)CCCC1=CC=C(N(CCCl)CCCl)C=C1 JCKYGMPEJWAADB-UHFFFAOYSA-N 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 230000007012 clinical effect Effects 0.000 description 1
- 238000002648 combination therapy Methods 0.000 description 1
- 238000012875 competitive assay Methods 0.000 description 1
- 230000000295 complement effect Effects 0.000 description 1
- 230000009918 complex formation Effects 0.000 description 1
- 230000001268 conjugating effect Effects 0.000 description 1
- 230000021615 conjugation Effects 0.000 description 1
- 239000013068 control sample Substances 0.000 description 1
- 210000004292 cytoskeleton Anatomy 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 125000001295 dansyl group Chemical group [H]C1=C([H])C(N(C([H])([H])[H])C([H])([H])[H])=C2C([H])=C([H])C([H])=C(C2=C1[H])S(*)(=O)=O 0.000 description 1
- 229960000975 daunorubicin Drugs 0.000 description 1
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000000032 diagnostic agent Substances 0.000 description 1
- 229940039227 diagnostic agent Drugs 0.000 description 1
- 125000002228 disulfide group Chemical group 0.000 description 1
- NLEBIOOXCVAHBD-QKMCSOCLSA-N dodecyl beta-D-maltoside Chemical compound O[C@@H]1[C@@H](O)[C@H](OCCCCCCCCCCCC)O[C@H](CO)[C@H]1O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 NLEBIOOXCVAHBD-QKMCSOCLSA-N 0.000 description 1
- 229960004679 doxorubicin Drugs 0.000 description 1
- 239000012636 effector Substances 0.000 description 1
- 238000001493 electron microscopy Methods 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000006911 enzymatic reaction Methods 0.000 description 1
- 238000001952 enzyme assay Methods 0.000 description 1
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 description 1
- 229960005420 etoposide Drugs 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 239000010419 fine particle Substances 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 238000011223 gene expression profiling Methods 0.000 description 1
- 229940116332 glucose oxidase Drugs 0.000 description 1
- 235000019420 glucose oxidase Nutrition 0.000 description 1
- 230000012010 growth Effects 0.000 description 1
- 239000003966 growth inhibitor Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 125000000623 heterocyclic group Chemical group 0.000 description 1
- 230000001900 immune effect Effects 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 229940072221 immunoglobulins Drugs 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 238000001727 in vivo Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 239000000138 intercalating agent Substances 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 230000009878 intermolecular interaction Effects 0.000 description 1
- 229940044173 iodine-125 Drugs 0.000 description 1
- 238000004255 ion exchange chromatography Methods 0.000 description 1
- 230000000366 juvenile effect Effects 0.000 description 1
- 229940057428 lactoperoxidase Drugs 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 210000005265 lung cell Anatomy 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 239000008176 lyophilized powder Substances 0.000 description 1
- 239000006166 lysate Substances 0.000 description 1
- 229960000274 lysozyme Drugs 0.000 description 1
- 239000004325 lysozyme Substances 0.000 description 1
- 235000010335 lysozyme Nutrition 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 210000005075 mammary gland Anatomy 0.000 description 1
- 229960001924 melphalan Drugs 0.000 description 1
- SGDBTWWWUNNDEQ-LBPRGKRZSA-N melphalan Chemical compound OC(=O)[C@@H](N)CC1=CC=C(N(CCCl)CCCl)C=C1 SGDBTWWWUNNDEQ-LBPRGKRZSA-N 0.000 description 1
- 208000010658 metastatic prostate carcinoma Diseases 0.000 description 1
- 229960000485 methotrexate Drugs 0.000 description 1
- 238000010208 microarray analysis Methods 0.000 description 1
- 108010029942 microperoxidase Proteins 0.000 description 1
- 229960004857 mitomycin Drugs 0.000 description 1
- 239000003607 modifier Substances 0.000 description 1
- 230000009149 molecular binding Effects 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 230000037435 normal mutation Effects 0.000 description 1
- 230000001293 nucleolytic effect Effects 0.000 description 1
- 239000002751 oligonucleotide probe Substances 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000007170 pathology Effects 0.000 description 1
- 230000037361 pathway Effects 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 229920001481 poly(stearyl methacrylate) Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920001184 polypeptide Polymers 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000003449 preventive effect Effects 0.000 description 1
- 102000004196 processed proteins & peptides Human genes 0.000 description 1
- 108090000765 processed proteins & peptides Proteins 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000035755 proliferation Effects 0.000 description 1
- 230000001902 propagating effect Effects 0.000 description 1
- 210000005267 prostate cell Anatomy 0.000 description 1
- 208000023958 prostate neoplasm Diseases 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000005180 public health Effects 0.000 description 1
- 238000012797 qualification Methods 0.000 description 1
- 238000010791 quenching Methods 0.000 description 1
- 230000000171 quenching effect Effects 0.000 description 1
- 150000003254 radicals Chemical class 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- 229910052761 rare earth metal Inorganic materials 0.000 description 1
- 150000002910 rare earth metals Chemical class 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 210000000664 rectum Anatomy 0.000 description 1
- 230000015227 regulation of liquid surface tension Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 238000004007 reversed phase HPLC Methods 0.000 description 1
- 235000002020 sage Nutrition 0.000 description 1
- 208000011581 secondary neoplasm Diseases 0.000 description 1
- 210000000717 sertoli cell Anatomy 0.000 description 1
- 150000003384 small molecules Chemical class 0.000 description 1
- 239000001632 sodium acetate Substances 0.000 description 1
- 235000017281 sodium acetate Nutrition 0.000 description 1
- 229910000029 sodium carbonate Inorganic materials 0.000 description 1
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- MPLHNVLQVRSVEE-UHFFFAOYSA-N texas red Chemical compound [O-]S(=O)(=O)C1=CC(S(Cl)(=O)=O)=CC=C1C(C1=CC=2CCCN3CCCC(C=23)=C1O1)=C2C1=C(CCC1)C3=[N+]1CCCC3=C2 MPLHNVLQVRSVEE-UHFFFAOYSA-N 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 238000001890 transfection Methods 0.000 description 1
- 239000012588 trypsin Substances 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 241001515965 unidentified phage Species 0.000 description 1
- 229960003048 vinblastine Drugs 0.000 description 1
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 description 1
- 229960004528 vincristine Drugs 0.000 description 1
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 description 1
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57434—Specifically defined cancers of prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3069—Reproductive system, e.g. ovaria, uterus, testes, prostate
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0693—Tumour cells; Cancer cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57484—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites
- G01N33/57492—Immunoassay; Biospecific binding assay; Materials therefor for cancer involving compounds serving as markers for tumor, cancer, neoplasia, e.g. cellular determinants, receptors, heat shock/stress proteins, A-protein, oligosaccharides, metabolites involving compounds localized on the membrane of tumor or cancer cells
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y116/00—Oxidoreductases oxidizing metal ions (1.16)
- C12Y116/01—Oxidoreductases oxidizing metal ions (1.16) with NAD+ or NADP+ as acceptor (1.16.1)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y301/00—Hydrolases acting on ester bonds (3.1)
- C12Y301/03—Phosphoric monoester hydrolases (3.1.3)
- C12Y301/03048—Protein-tyrosine-phosphatase (3.1.3.48)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/46—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans from vertebrates
- G01N2333/47—Assays involving proteins of known structure or function as defined in the subgroups
- G01N2333/4701—Details
- G01N2333/4742—Keratin; Cytokeratin
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/435—Assays involving biological materials from specific organisms or of a specific nature from animals; from humans
- G01N2333/705—Assays involving receptors, cell surface antigens or cell surface determinants
- G01N2333/70596—Molecules with a "CD"-designation not provided for elsewhere in G01N2333/705
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/902—Oxidoreductases (1.)
- G01N2333/90287—Oxidoreductases (1.) oxidising metal ions (1.16)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2333/00—Assays involving biological materials from specific organisms or of a specific nature
- G01N2333/90—Enzymes; Proenzymes
- G01N2333/914—Hydrolases (3)
- G01N2333/916—Hydrolases (3) acting on ester bonds (3.1), e.g. phosphatases (3.1.3), phospholipases C or phospholipases D (3.1.4)
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/483—Physical analysis of biological material
- G01N33/487—Physical analysis of biological material of liquid biological material
- G01N33/49—Blood
- G01N33/4915—Blood using flow cells
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/577—Immunoassay; Biospecific binding assay; Materials therefor involving monoclonal antibodies binding reaction mechanisms characterised by the use of monoclonal antibodies; monoclonal antibodies per se are classified with their corresponding antigens
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/58—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances
- G01N33/582—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving labelled substances with fluorescent label
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Biomedical Technology (AREA)
- Cell Biology (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Hematology (AREA)
- Urology & Nephrology (AREA)
- Biotechnology (AREA)
- Biochemistry (AREA)
- Oncology (AREA)
- Medicinal Chemistry (AREA)
- Microbiology (AREA)
- Genetics & Genomics (AREA)
- Food Science & Technology (AREA)
- Hospice & Palliative Care (AREA)
- General Physics & Mathematics (AREA)
- Physics & Mathematics (AREA)
- Pathology (AREA)
- Analytical Chemistry (AREA)
- Wood Science & Technology (AREA)
- Zoology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biophysics (AREA)
- Reproductive Health (AREA)
- Pregnancy & Childbirth (AREA)
- Gynecology & Obstetrics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
Description
本出願は、その内容全体が本明細書において出典明示により援用される、2011年11月29日に出願された米国特許仮出願第61/629886号及び2012年9月19日に出願された米国特許仮出願第61/703099号の米国特許法第119条(e)に基く優先権を主張する。
用語「腫瘍」又は「癌」は、本明細書では互換的に使用され、腫瘍性又は悪性の細胞の成長、増殖もしくは転移によって特徴づけられるいかなる病状も意味し、また、固形癌と、白血病などの非固形癌の両方を含む。
「単離された」核酸は、その天然の環境の成分から分離された核酸分子を指す。単離された核酸は、核酸分子を通常含む細胞に含まれる核酸分子を含むが、しかし、その核酸分子は、染色体外又は染色体上の本来の位置とは異なる染色体位置に存在している。
一態様において、本発明は、被検体より採取した血液試料を用いて被検体における前立腺癌の診断又はステージングを行うための方法を提供する。特に、本発明は、CTCが一つ又はそれ以上の前立腺特異的マーカーを発現しているかどうかを判定することにより、被検体における前立腺癌の診断又はステージングを行うための方法を提供する。
別の態様において、本発明は、抗体15A5が結合するエピトープと実質的に同じエピトープに結合する抗体を提供し、この抗体15A5は微生物寄託番号PTA−12259として寄託されているハイブリドーマ細胞により産生されるものである。
抗体は、例えば米国特許第4816567号記載の組換え方法及び組成物を用いて作製され得る。一実施態様において、本明細書に記載される抗STEAP−1抗体をコードする単離された核酸が提供される。該核酸は、抗体のVLを構成するアミノ酸配列、及び/又は抗体のVHを構成するアミノ酸配列(例えば、抗体の軽鎖及び/又は重鎖)をコードし得る。更なる実施態様において、該核酸を含む一つ以上のベクター(例えば、発現ベクター)が提供される。更なる実施態様において、該核酸を含む宿主細胞が提供される。このような一実施態様において、宿主細胞(例えば、以下により形質転換された宿主細胞)は、(1)抗体のVLを構成するアミノ酸配列、及び抗体のVHを構成するアミノ酸配列をコードする核酸を含むベクター、又は(2)抗体のVLを構成するアミノ酸配列をコードする核酸を含む第1ベクター、及び抗体のVHを構成するアミノ酸配列をコードする核酸を含む第2ベクターを含む。一実施態様では、該宿主細胞は、真核生物、例えばチャイニーズハムスター卵巣(CHO)細胞又はリンパ系細胞(例えば、Y0、NS0、Sp20細胞)である。一実施態様では、抗STEAP−1抗体の作製方法が提供され、その方法は、上記のように抗体の発現に好適な条件下で、上記の抗体をコードする核酸を含む宿主細胞を培養することを含み、宿主細胞(又は宿主細胞培養培地)から任意選択的に抗体を回収することを含む。
本明細書で提供される抗体は、前立腺癌の診断試薬の製造に使用可能である。この抗体は更に、診断目的に好適で検出可能な標識に結合し、好適な形態、例えば凍結乾燥された粉末で、又は、好適な液剤の形態で提供される。
本発明の別の態様において、前立腺癌の診断又は予後診断に有用な組成物を含む検査キットが提供される。
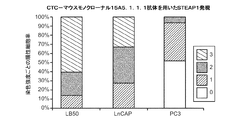
免疫組織化学(IHC)アッセイを用いて3つの前立腺癌細胞株の表面に発現したSTEAP−1を検出するために、3つの抗STEAP−1抗体が使用された。293LB50はSTEAP−1高発現細胞株として使用され、LnCAPnerはSTEAP−1中程度発現細胞株として使用され、PC3はSTEAP−1低発現-陰性細胞株として使用された。検査された抗STEAP−1抗体は、抗体37(マウスモノクローナル抗STEAP−1抗体)、ヒツジポリクローナル抗STEAP−1抗体、及びsc−25514(ウサギポリクローナル抗STEAP−1抗体)である。この3つの抗体がフルオロフォアAF−488とコンジュゲートされた。
3つの抗体(マウス抗体37、ヒツジポリクローナル抗体、及びウサギsc−25514)が、LB50細胞及びPC3細胞表面のSTEAP−1発現検出の能力について、それぞれCellSearch(登録商標)システムで検査された。
血液試料中に添加した細胞表面のSTEAP−1発現を判定するために、抗STEAP−1ヒツジポリクローナル抗体を用いた。このスパイクインアッセイは、実施例2と同様の手順で実施した。3つの細胞株、293LB50、LnCAPner及びPC3をそれぞれ血液試料に添加し、完全に混ぜた。ヒツジポリクローナル抗体を、PBSで1:50に希釈し、CellSearchの第4フィルターで血液試料に添加した。これらの試料でCellSearchを実施し、CellTracksアナライザーでCTCがスコア化された。サイトケラチン及びDAPIに対して陽性に染まるがCD45に対して陰性に染まる細胞が、CTCと判定された。CellSearchオートプレップシステム上でSTEAP−1の染色を示すCTCが選択され、更にSTEAP−1の発現レベルを示す抗STEAP−1抗体の蛍光強度が定量化された。実施例2と同じ方法でHスコアが算出された。
前立腺患者11名の血液試料をある1件の診療所から得た。これらの血液試料を、抗STEAP−1ヒツジポリクローナル抗体を用いてCellSearch(登録商標)システムで解析した。ヒツジポリクローナル抗体を、PBSで1:50に希釈し、CellSearchの第4フィルターで血液試料に添加した。これらの試料でCellSearchを実施し、CellTracksアナライザーでCTCがスコア化された。サイトケラチン及びDAPIに対して陽性に染まるがCD45に対して陰性に染まる細胞が、CTCと判定された。CellSearchオートプレップシステム上でSTEAP−1の染色を示すCTCが選択され、更にSTEAP−1の発現レベルを示す抗STEAP−1抗体の蛍光強度が定量化された。各試料のCTCの数がカウントされ、Hスコアも実施例2に記載の通り算出された。結果を図4に示す。
第I相試験で10名の前立腺患者から血液試料及び腫瘍組織試料を採取した。
マウスモノクローナル抗体15A5が、スパイクインアッセイを用いてCellSearch(登録商標)システムで検査され、ヒツジポリクローナル抗体と比較された。実施例2に記載の通り、293LB50細胞(高発現体)、LnCAPner細胞(中発現体)及びPC3細胞(低発現体)がそれぞれ血液試料に添加された。これらの血液試料が、ヒツジポリクローナル抗体及びマウスモノクローナル抗体15A5をそれぞれ利用して、CellSearch(登録商標)システムで解析された。Hスコアもまた算出された。この手順と方法は実施例2記載のものと同様である。
前立腺癌患者から血液試料を採取し、抗STEAP−1モノクローナル抗体15A5を用いてCellSearch(登録商標)システムで解析した。抗体15A5を、例えばPBSで1:50に希釈し、血液試料に添加した。この試料でCellSearchを実行し、CellTracksアナライザーでCTCが数えられる。サイトケラチン及びDAPIに対して陽性に染まるがCD45に対して陰性に染まる細胞が、CTCと判定される。CellSearchオートプレップシステム上のCTCの内STEAP−1の染色を示すCTCが選択され、更にSTEAP−1の発現レベルを示す抗STEAP−1抗体の蛍光強度が定量化される。各試料のCTCの数がカウントされ、Hスコアも実施例2に記載の通り算出される。
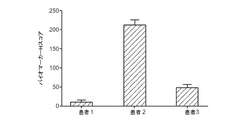
前立腺癌患者の血液を、治療開始前にペアで採取した(ベースライン試料)。試料はCellSearch(登録商標)システムで解析され、上記のようにCellTracksアナライザーでCTC計数が得られた。簡単には、サイトケラチン及びDAPIに対して陽性に染まるがCD45に対しては陰性に染まる細胞が、CTCとして数えられた。各試料ペアについてCTC数の平均値及び標準偏差値が計算され、また、誤差(+―SDEV)がヒストグラム上にプロットされた。図7Aで見られるように、これらの患者のCTC数の平均値に大きなダイナミックレンジがあり、また、試料ペア間でもカウントがほぼ一致していた(エラーバーが小さい)。これは、このシステムを使用したCTC計数の再現性が高いことを示している。
Claims (26)
- 被検体における前立腺癌細胞の存在を検出する方法であって、
a)被検体由来の血液試料から得られた、上皮起源の癌細胞を、前立腺特異的マーカーに特異的に結合する抗STEAP−1抗体と接触させることであって、前記抗STEAP−1抗体がポリクローナル抗体であるか又は微生物寄託番号PTA−12259のハイブリドーマ細胞により産生されるマウスモノクロール抗体15A5である、前記接触させること、及び
b)癌細胞のいずれかが前立腺特異的マーカーを発現するかを判定すること
を含み、
前立腺特異的マーカーを発現する癌細胞の存在が被検体における前立腺癌への罹患を予測し、前立腺特異的マーカーがSTEAP−1である、方法。 - 前立腺特異的マーカーを発現する癌細胞の量を測定することを更に含み、その量が被検体における前立腺癌のステージを予測するものである、請求項1に記載の方法。
- 癌細胞上の前立腺特異的マーカーの発現レベルを測定することを更に含む、請求項1又は2に記載の方法。
- 前立腺特異的マーカーの発現レベルに基づいて癌細胞をグレード分類し、各グレードにおける癌細胞のパーセンテージを決定することを更に含む、請求項1から3の何れか一項に記載の方法。
- 各グレードに対するグレードスコアを、該グレードにおける癌細胞のパーセンテージに、該グレードにおける前立腺特異的マーカーの発現レベルを表すグレード固有の番号を乗じることによって、算出することと、グレードスコアを全て合計することによって、被検体における前立腺癌のステージを示す指標であるHスコアを得ることを更に含む、請求項1から4の何れか一項に記載の方法。
- 癌細胞が、上皮由来の癌細胞に特異的に結合するリガンドを含む捕捉組成物を用いて血液試料から識別される、請求項1から5の何れか一項に記載の方法。
- リガンドが、癌細胞に優先的に発現される上皮抗原に特異的に結合する抗体である、請求項6に記載の方法。
- 上皮抗原が上皮細胞接着分子(EpCAM)である、請求項7に記載の方法。
- 識別された癌細胞が血液試料から分離される細胞画分に濃縮される、請求項6から8の何れか一項に記載の方法。
- 細胞画分が磁場下で分離される、請求項9に記載の方法。
- 捕捉組成物中のリガンドが磁性粒子(例えばコロイド状磁性粒子、例えばコロイド状磁性ナノ粒子)に結合する、請求項10に記載の方法。
- リガンドがEpCAM抗体を含む、請求項11に記載の方法。
- 抗STEAP−1抗体がKD値1000nM以下でSTEAP−1と結合する、請求項1から12の何れか一項に記載の方法。
- 抗STEAP−1抗体が検出可能な第1の標識とコンジュゲートされる、請求項1から13の何れか一項に記載の方法。
- 癌細胞が、上皮由来の癌細胞の検出を可能にする一つ以上の試薬を用いて識別される、請求項1から14の何れか一項に記載の方法。
- 試薬が、サイトケラチンに特異的に結合するリガンドを含み、リガンドが、検出可能な第2の標識と任意選択的にコンジュゲートされる、請求項15に記載の方法。
- 試薬が細胞を非細胞成分から識別する色素を更に含む、請求項15又は16に記載の方法。
- 色素が4',6-ジアミジノ-2-フェニルインドール(DAPI)である、請求項17に記載の方法。
- 試薬が白血球マーカーに特異的に結合するリガンドを更に含み、リガンドが検出可能な第3の標識と任意選択的にコンジュゲートされる、請求項15から18の何れか一項に記載の方法。
- 白血球マーカーに対するリガンドがCD45抗体である、請求項19に記載の方法。
- 判定が、免疫蛍光顕微鏡法、フローサイトメトリー、光ファイバースキャンサイトメトリー、又はレーザースキャンサイトメトリーに基づく方法による、請求項1から20の何れか一項に記載の方法。
- 被検体における前立腺癌治療への応答をモニターする方法であって、
a)被検体由来の第1血液試料から得られた、上皮起源の癌細胞の第1群を、前立腺特異的マーカーに特異的に結合する抗STEAP−1抗体と接触させること、
b)前立腺特異的マーカーを発現する第1群の癌細胞の量及び/又は癌細胞中の前立腺特異的マーカーの発現レベルを測定すること、
c)前立腺癌治療の試験期間後の被検体由来の第2血液試料から得られた、上皮起源の癌細胞の第2群を、前立腺特異的マーカーに特異的に結合する抗STEAP−1抗体と接触させること、
d)前立腺特異的マーカーを発現する第2群の癌細胞の量及び/又は癌細胞中の前立腺特異的マーカーの発現レベルを測定すること、及び
e)ステップb)で測定した前立腺特異的マーカーを発現する癌細胞の量及び/又は前立腺特異的マーカーの発現レベルと、ステップd)でのそれらの値を比較すること
を含み、前立腺特異的マーカーを発現する癌細胞の量の減少及び/又は癌細胞中の前立腺特異的マーカーの発現レベルの低下が、被検体における前立腺癌治療への応答を示し、前記抗STEAP−1抗体がポリクローナル抗体であるか又は微生物寄託番号PTA−12259のハイブリドーマ細胞により産生されるマウスモノクロール抗体15A5であり、前立腺特異的マーカーがSTEAP−1である、方法。 - 前立腺癌治療が前立腺特異的マーカーに結合する抗体又は抗体−薬物コンジュゲート(ADC)を含む、請求項22に記載の方法。
- ADCが、細胞傷害剤に共有結合した抗STEAP−1抗体を含む、請求項22又は23に記載の方法。
- 細胞傷害剤が、毒素、化学療法剤、薬剤の構成成分、モノメチルアウリスタチンE(MMAE)、抗生剤、放射性同位体、及び核酸分解酵素から選択される、請求項24に記載の方法。
- 請求項1から25の何れか一項に記載の方法を実施するためのキットであって、微生物寄託番号PTA−12259のハイブリドーマ細胞により産生されるマウスモノクロール抗体15A5を含む、キット。
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201161629886P | 2011-11-29 | 2011-11-29 | |
| US61/629,886 | 2011-11-29 | ||
| US201261703099P | 2012-09-19 | 2012-09-19 | |
| US61/703,099 | 2012-09-19 | ||
| PCT/US2012/066998 WO2013082249A2 (en) | 2011-11-29 | 2012-11-29 | Compositions and methods for prostate cancer analysis |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2015507175A JP2015507175A (ja) | 2015-03-05 |
| JP2015507175A5 JP2015507175A5 (ja) | 2016-01-28 |
| JP6219304B2 true JP6219304B2 (ja) | 2017-10-25 |
Family
ID=47324459
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014544870A Active JP6219304B2 (ja) | 2011-11-29 | 2012-11-29 | 前立腺癌分析のための組成物及び方法 |
Country Status (15)
| Country | Link |
|---|---|
| US (2) | US20130143237A1 (ja) |
| EP (1) | EP2786151B1 (ja) |
| JP (1) | JP6219304B2 (ja) |
| KR (1) | KR101951514B1 (ja) |
| CN (1) | CN104067127A (ja) |
| AR (1) | AR089028A1 (ja) |
| AU (1) | AU2012345926A1 (ja) |
| BR (1) | BR112014012882A2 (ja) |
| CA (1) | CA2854042A1 (ja) |
| IL (1) | IL232466A (ja) |
| MX (1) | MX350807B (ja) |
| RU (1) | RU2641968C2 (ja) |
| SG (1) | SG11201402711SA (ja) |
| WO (1) | WO2013082249A2 (ja) |
| ZA (1) | ZA201403110B (ja) |
Families Citing this family (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN105646661A (zh) * | 2014-03-24 | 2016-06-08 | 朱育盼 | 特异性结合前列腺癌的短肽pcp2及其应用 |
| EP3286565A1 (en) | 2015-04-21 | 2018-02-28 | Genentech, Inc. | Compositions and methods for prostate cancer analysis |
| WO2018184966A1 (en) | 2017-04-03 | 2018-10-11 | F. Hoffmann-La Roche Ag | Antibodies binding to steap-1 |
| CN108179134B (zh) * | 2017-12-27 | 2020-11-24 | 武汉大学 | 基于EpCAM/PSMA双抗体功能化微流控芯片及其制备方法和应用 |
| AU2019299357A1 (en) | 2018-07-02 | 2021-01-14 | Amgen Inc. | Anti-steap1 antigen-binding protein |
| KR20220057575A (ko) * | 2019-09-05 | 2022-05-09 | 메모리얼 슬로안 케터링 캔서 센터 | 항-steap1 항체 및 이의 용도 |
| BR102020013625A2 (pt) * | 2020-07-02 | 2022-01-11 | Universidade Federal de Uberlândia | Aptâmero modificado, sistema aptaimunológico, painel aptaimunológico, kit, método e uso no diagnóstico de câncer de próstata |
Family Cites Families (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4816567A (en) | 1983-04-08 | 1989-03-28 | Genentech, Inc. | Recombinant immunoglobin preparations |
| US4737456A (en) | 1985-05-09 | 1988-04-12 | Syntex (U.S.A.) Inc. | Reducing interference in ligand-receptor binding assays |
| US5597531A (en) | 1985-10-04 | 1997-01-28 | Immunivest Corporation | Resuspendable coated magnetic particles and stable magnetic particle suspensions |
| EP0368684B2 (en) | 1988-11-11 | 2004-09-29 | Medical Research Council | Cloning immunoglobulin variable domain sequences. |
| DE3920358A1 (de) | 1989-06-22 | 1991-01-17 | Behringwerke Ag | Bispezifische und oligospezifische, mono- und oligovalente antikoerperkonstrukte, ihre herstellung und verwendung |
| US5698271A (en) | 1989-08-22 | 1997-12-16 | Immunivest Corporation | Methods for the manufacture of magnetically responsive particles |
| US5959177A (en) | 1989-10-27 | 1999-09-28 | The Scripps Research Institute | Transgenic plants expressing assembled secretory antibodies |
| US5571894A (en) | 1991-02-05 | 1996-11-05 | Ciba-Geigy Corporation | Recombinant antibodies specific for a growth factor receptor |
| GB9114948D0 (en) | 1991-07-11 | 1991-08-28 | Pfizer Ltd | Process for preparing sertraline intermediates |
| US7018809B1 (en) | 1991-09-19 | 2006-03-28 | Genentech, Inc. | Expression of functional antibody fragments |
| US5587458A (en) | 1991-10-07 | 1996-12-24 | Aronex Pharmaceuticals, Inc. | Anti-erbB-2 antibodies, combinations thereof, and therapeutic and diagnostic uses thereof |
| CA2372813A1 (en) | 1992-02-06 | 1993-08-19 | L.L. Houston | Biosynthetic binding protein for cancer marker |
| US5789199A (en) | 1994-11-03 | 1998-08-04 | Genentech, Inc. | Process for bacterial production of polypeptides |
| US5840523A (en) | 1995-03-01 | 1998-11-24 | Genetech, Inc. | Methods and compositions for secretion of heterologous polypeptides |
| US5869046A (en) | 1995-04-14 | 1999-02-09 | Genentech, Inc. | Altered polypeptides with increased half-life |
| US6046048A (en) * | 1996-01-09 | 2000-04-04 | Genetech, Inc. | Apo-2 ligand |
| US6040498A (en) | 1998-08-11 | 2000-03-21 | North Caroline State University | Genetically engineered duckweed |
| WO1999041613A1 (en) | 1998-02-12 | 1999-08-19 | Immunivest | Methods and reagents for the rapid and efficient isolation of circulating cancer cells |
| US6200765B1 (en) * | 1998-05-04 | 2001-03-13 | Pacific Northwest Cancer Foundation | Non-invasive methods to detect prostate cancer |
| DE69941187D1 (de) | 1998-06-01 | 2009-09-10 | Agensys Inc | Serpentintransmembranantigene exprimiert in menschlichem krebs und deren verwendungen |
| IL129299A0 (en) * | 1999-03-31 | 2000-02-17 | Mor Research Applic Ltd | Monoclonal antibodies antigens and diagnosis of malignant diseases |
| US7125978B1 (en) | 1999-10-04 | 2006-10-24 | Medicago Inc. | Promoter for regulating expression of foreign genes |
| NZ517906A (en) | 1999-10-04 | 2003-01-31 | Medicago Inc | Cloning of genomic sequences encoding nitrite reductase (NiR) for use in regulated expression of foreign genes in host plants |
| CA2438112A1 (en) * | 2001-02-16 | 2003-08-07 | Immunivest Corporation | Methods and reagents for the rapid and efficient isolation of circulating cancer cells |
| US20030190602A1 (en) * | 2001-03-12 | 2003-10-09 | Monogen, Inc. | Cell-based detection and differentiation of disease states |
| US7219016B2 (en) | 2001-04-20 | 2007-05-15 | Yale University | Systems and methods for automated analysis of cells and tissues |
| US7494646B2 (en) | 2001-09-06 | 2009-02-24 | Agensys, Inc. | Antibodies and molecules derived therefrom that bind to STEAP-1 proteins |
| PT1742966E (pt) * | 2004-04-22 | 2014-02-05 | Agensys Inc | Anticorpos e moléculas derivadas daí que se ligam às proteínas steap-1 |
| US20100111856A1 (en) | 2004-09-23 | 2010-05-06 | Herman Gill | Zirconium-radiolabeled, cysteine engineered antibody conjugates |
| PL2845866T3 (pl) * | 2006-10-27 | 2017-10-31 | Genentech Inc | Przeciwciała i immunokoniugaty oraz ich zastosowanie |
| AU2007317306B2 (en) * | 2006-11-08 | 2012-06-14 | The Regents Of The University Of Michigan | SPINK1 as a prostate cancer marker and uses thereof |
| JP5394246B2 (ja) * | 2007-03-30 | 2014-01-22 | ジェネンテック, インコーポレイテッド | 抗体及びイムノコンジュゲートとこれらの使用方法 |
| CA2736178A1 (en) | 2008-09-05 | 2010-03-11 | Peter Kuhn | Methods for the detection of circulating tumor cells |
| JP5975983B2 (ja) * | 2010-04-16 | 2016-08-23 | ベリカム ファーマシューティカルズ, インコーポレイテッド | 固形腫瘍を処置するための方法 |
-
2012
- 2012-11-29 EP EP12798559.6A patent/EP2786151B1/en active Active
- 2012-11-29 MX MX2014006187A patent/MX350807B/es active IP Right Grant
- 2012-11-29 AR ARP120104485A patent/AR089028A1/es unknown
- 2012-11-29 WO PCT/US2012/066998 patent/WO2013082249A2/en active Application Filing
- 2012-11-29 JP JP2014544870A patent/JP6219304B2/ja active Active
- 2012-11-29 AU AU2012345926A patent/AU2012345926A1/en not_active Abandoned
- 2012-11-29 BR BR112014012882A patent/BR112014012882A2/pt not_active Application Discontinuation
- 2012-11-29 SG SG11201402711SA patent/SG11201402711SA/en unknown
- 2012-11-29 KR KR1020147017563A patent/KR101951514B1/ko active IP Right Grant
- 2012-11-29 US US13/689,407 patent/US20130143237A1/en not_active Abandoned
- 2012-11-29 RU RU2014126358A patent/RU2641968C2/ru not_active IP Right Cessation
- 2012-11-29 CA CA2854042A patent/CA2854042A1/en not_active Abandoned
- 2012-11-29 CN CN201280067510.XA patent/CN104067127A/zh active Pending
-
2014
- 2014-04-29 ZA ZA2014/03110A patent/ZA201403110B/en unknown
- 2014-05-05 IL IL232466A patent/IL232466A/en not_active IP Right Cessation
-
2015
- 2015-10-21 US US14/919,388 patent/US9632091B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| CA2854042A1 (en) | 2013-06-06 |
| BR112014012882A2 (pt) | 2017-06-13 |
| IL232466A (en) | 2017-10-31 |
| US20130143237A1 (en) | 2013-06-06 |
| RU2014126358A (ru) | 2016-01-27 |
| US20160274116A1 (en) | 2016-09-22 |
| RU2641968C2 (ru) | 2018-01-23 |
| JP2015507175A (ja) | 2015-03-05 |
| US9632091B2 (en) | 2017-04-25 |
| EP2786151A2 (en) | 2014-10-08 |
| AU2012345926A1 (en) | 2014-05-29 |
| AR089028A1 (es) | 2014-07-23 |
| KR101951514B1 (ko) | 2019-02-22 |
| EP2786151B1 (en) | 2019-07-03 |
| MX2014006187A (es) | 2014-06-23 |
| ZA201403110B (en) | 2017-06-28 |
| SG11201402711SA (en) | 2014-06-27 |
| WO2013082249A2 (en) | 2013-06-06 |
| WO2013082249A3 (en) | 2013-10-10 |
| KR20140100544A (ko) | 2014-08-14 |
| IL232466A0 (en) | 2014-06-30 |
| CN104067127A (zh) | 2014-09-24 |
| MX350807B (es) | 2017-09-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP7022191B2 (ja) | 前立腺がんの分析のための組成物及び方法 | |
| JP6219304B2 (ja) | 前立腺癌分析のための組成物及び方法 | |
| JP2014098706A (ja) | 細胞増殖性疾患の検出及び診断に有用なigf−1r特異抗体 | |
| JP6129956B2 (ja) | 抗c−Met抗体 | |
| JP6138780B2 (ja) | 癌の検出および診断のための抗体i−3859の使用 | |
| JP6977105B2 (ja) | Igf−1r抗体および癌の診断のためのその使用 | |
| TW201927820A (zh) | 抗pd-l1抗體及使用其來偵測pd-l1之方法 | |
| EP2659273A1 (en) | Monoclonal antibodies against alpha-actinin-4 antigens, and uses therefor | |
| CN107709362B (zh) | Igf-1r抗体及其用于癌症诊断的用途 | |
| US20240117042A1 (en) | Immunohistochemistry methods and kir3dl2-specific reagents | |
| WO2014079954A1 (en) | Methods of diagnosing and therapeutic agents for use in the treatment of cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20151130 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20151130 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20160914 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160920 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20161219 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170220 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170316 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20170829 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170927 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6219304 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |