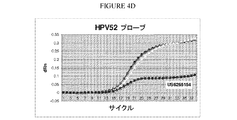
JP5795259B2 - 試験サンプル中のヒトパピローマウイルスおよびヒトベータグロビン配列を検出するためのプライマーおよびプローブ - Google Patents
試験サンプル中のヒトパピローマウイルスおよびヒトベータグロビン配列を検出するためのプライマーおよびプローブ Download PDFInfo
- Publication number
- JP5795259B2 JP5795259B2 JP2011530166A JP2011530166A JP5795259B2 JP 5795259 B2 JP5795259 B2 JP 5795259B2 JP 2011530166 A JP2011530166 A JP 2011530166A JP 2011530166 A JP2011530166 A JP 2011530166A JP 5795259 B2 JP5795259 B2 JP 5795259B2
- Authority
- JP
- Japan
- Prior art keywords
- seq
- probe
- primer
- sequence
- sequences
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6806—Preparing nucleic acids for analysis, e.g. for polymerase chain reaction [PCR] assay
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/70—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving virus or bacteriophage
- C12Q1/701—Specific hybridization probes
- C12Q1/708—Specific hybridization probes for papilloma
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/112—Disease subtyping, staging or classification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/16—Primer sets for multiplex assays
Landscapes
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Immunology (AREA)
- Analytical Chemistry (AREA)
- Genetics & Genomics (AREA)
- General Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Microbiology (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Biochemistry (AREA)
- Pathology (AREA)
- Virology (AREA)
- Oncology (AREA)
- Hospice & Palliative Care (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/241,119 | 2008-09-30 | ||
| US12/241,119 US9090948B2 (en) | 2008-09-30 | 2008-09-30 | Primers and probes for detecting human papillomavirus and human beta globin sequences in test samples |
| PCT/US2009/058992 WO2010039809A2 (en) | 2008-09-30 | 2009-09-30 | Primers and probes for detecting human papillomavirus and human beta globin sequences in test samples |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015158921A Division JP6177844B2 (ja) | 2008-09-30 | 2015-08-11 | 試験サンプル中のヒトパピローマウイルスおよびヒトベータグロビン配列を検出するためのプライマーおよびプローブ |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2012504414A JP2012504414A (ja) | 2012-02-23 |
| JP2012504414A5 JP2012504414A5 (enExample) | 2012-11-15 |
| JP5795259B2 true JP5795259B2 (ja) | 2015-10-14 |
Family
ID=41510739
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011530166A Active JP5795259B2 (ja) | 2008-09-30 | 2009-09-30 | 試験サンプル中のヒトパピローマウイルスおよびヒトベータグロビン配列を検出するためのプライマーおよびプローブ |
| JP2015158921A Active JP6177844B2 (ja) | 2008-09-30 | 2015-08-11 | 試験サンプル中のヒトパピローマウイルスおよびヒトベータグロビン配列を検出するためのプライマーおよびプローブ |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015158921A Active JP6177844B2 (ja) | 2008-09-30 | 2015-08-11 | 試験サンプル中のヒトパピローマウイルスおよびヒトベータグロビン配列を検出するためのプライマーおよびプローブ |
Country Status (7)
| Country | Link |
|---|---|
| US (3) | US9090948B2 (enExample) |
| EP (3) | EP3202920A1 (enExample) |
| JP (2) | JP5795259B2 (enExample) |
| CA (2) | CA2738512C (enExample) |
| ES (1) | ES2628432T3 (enExample) |
| RU (1) | RU2530550C2 (enExample) |
| WO (1) | WO2010039809A2 (enExample) |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2002508190A (ja) * | 1997-12-12 | 2002-03-19 | ダイジーン・コーポレーション | 万能収集媒質 |
| US7439016B1 (en) * | 2000-06-15 | 2008-10-21 | Digene Corporation | Detection of nucleic acids by type-specific hybrid capture method |
| US7601497B2 (en) | 2000-06-15 | 2009-10-13 | Qiagen Gaithersburg, Inc. | Detection of nucleic acids by target-specific hybrid capture method |
| WO2009129505A2 (en) * | 2008-04-17 | 2009-10-22 | Qiagen Gaithersburg, Inc. | Compositions, methods, and kits using synthetic probes for determining the presence of a target nucleic acid |
| CN102203293A (zh) | 2008-10-27 | 2011-09-28 | 奇亚根盖瑟斯堡股份有限公司 | 快速结果杂交捕获测定和系统 |
| EP2425019B1 (en) * | 2009-05-01 | 2014-03-19 | QIAGEN Gaithersburg, Inc. | A non-target amplification method for detection of rna splice-forms in a sample |
| JP5826752B2 (ja) | 2009-09-14 | 2015-12-02 | キアジェン ゲイサーズバーグ インコーポレイテッド | 細胞学用培地に固定された組織サンプルから核酸またはタンパク質を回収するための組成物および方法 |
| US9689047B2 (en) * | 2010-01-29 | 2017-06-27 | Qiagen Gaithersburg Inc. | Methods and compositions for sequence-specific purification and multiplex analysis of nucleic acids |
| WO2011094528A2 (en) | 2010-01-29 | 2011-08-04 | Qiagen Gaithersburg, Inc. | Method of determining and confirming the presence of an hpv in a sample |
| EP2572001A2 (en) | 2010-05-19 | 2013-03-27 | QIAGEN Gaithersburg, Inc. | Methods and compositions for sequence-specific purification and multiplex analysis of nucleic acids |
| WO2011149897A1 (en) | 2010-05-25 | 2011-12-01 | Qiagen Gaithersburg, Inc. | Fast results hybrid capture assay and associated strategically-truncated probes |
| US9885092B2 (en) | 2011-02-24 | 2018-02-06 | Qiagen Gaithersburg Inc. | Materials and methods for detection of HPV nucleic acids |
| CN103409560A (zh) * | 2013-08-19 | 2013-11-27 | 潘晓静 | 一种广谱、高效、经济的高危人乳头状瘤病毒的pcr检测方法 |
| KR101951172B1 (ko) * | 2014-11-07 | 2019-02-22 | 주식회사 파나진 | 인유두종바이러스의 유전자형 검출을 위한 ρνα 프로브, 키트 및 방법 |
| CN105400782B (zh) * | 2015-12-09 | 2018-09-07 | 中国农业科学院兰州兽医研究所 | 同时鉴定三种猪流行性腹泻病毒毒株的引物组合和通用型半套式rt-pcr方法 |
| CN107130058B (zh) * | 2017-06-28 | 2018-08-03 | 广州市宝创生物技术有限公司 | 一种新型的9种人乳头瘤病毒亚型分型试剂盒和方法 |
| CN107130059B (zh) * | 2017-06-28 | 2018-07-20 | 广州市宝创生物技术有限公司 | 一种新型的21种人乳头瘤病毒亚型分型试剂盒和方法 |
| CN107988432A (zh) * | 2017-12-18 | 2018-05-04 | 苏州国科闻普生物科技有限公司 | 在单管反应中同时检测多个高危型人乳头瘤病毒的试剂盒 |
| CN107828919A (zh) * | 2017-12-18 | 2018-03-23 | 苏州国科闻普生物科技有限公司 | 适用于四荧光通道的单管检测多个高危型hpv的试剂盒 |
| CN107828920B (zh) * | 2017-12-18 | 2021-08-06 | 苏州国科闻普生物科技有限公司 | 对多种高危型人乳头瘤病毒实现分型的试剂盒 |
| WO2020132090A1 (en) | 2018-12-18 | 2020-06-25 | Abbott Molecular Inc. | Assay for detecting human papilloma virus (hpv) |
| CN110570202B (zh) * | 2019-09-02 | 2022-06-03 | 杭州趣链科技有限公司 | 一种基于分片技术的混合共识方法 |
| US20220017978A1 (en) * | 2020-07-14 | 2022-01-20 | Northwestern University | Virofind: a novel platform for detection and discovery of the entire virogenome in clinical samples |
| WO2023076639A1 (en) * | 2021-10-28 | 2023-05-04 | Jung Joo Moon | Primer, probe and controls for detection and discrimination of covid-19 and other coronaviruses diagnostic assay for the human virus causing covid-19-cov-2(covid-19) and its variants |
| CN120041610B (zh) * | 2025-02-20 | 2025-09-12 | 广州市金圻睿生物科技有限责任公司 | 一种用于检测生殖道感染相关病原体及其耐药基因的引物组合物及其应用 |
Family Cites Families (48)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4683202A (en) | 1985-03-28 | 1987-07-28 | Cetus Corporation | Process for amplifying nucleic acid sequences |
| US4683195A (en) | 1986-01-30 | 1987-07-28 | Cetus Corporation | Process for amplifying, detecting, and/or-cloning nucleic acid sequences |
| US4889818A (en) | 1986-08-22 | 1989-12-26 | Cetus Corporation | Purified thermostable enzyme |
| US5322770A (en) | 1989-12-22 | 1994-06-21 | Hoffman-Laroche Inc. | Reverse transcription with thermostable DNA polymerases - high temperature reverse transcription |
| US5310652A (en) | 1986-08-22 | 1994-05-10 | Hoffman-La Roche Inc. | Reverse transcription with thermostable DNA polymerase-high temperature reverse transcription |
| US4849332A (en) | 1987-05-26 | 1989-07-18 | Life Technologies, Inc. | Human papillomavirus 35 nucleic acid hybridization probes and methods for employing the same |
| US5585481A (en) | 1987-09-21 | 1996-12-17 | Gen-Probe Incorporated | Linking reagents for nucleotide probes |
| US5185439A (en) | 1987-10-05 | 1993-02-09 | Gen-Probe Incorporated | Acridinium ester labelling and purification of nucleotide probes |
| US5130238A (en) | 1988-06-24 | 1992-07-14 | Cangene Corporation | Enhanced nucleic acid amplification process |
| US5447839A (en) | 1988-09-09 | 1995-09-05 | Hoffmann-La Roche Inc. | Detection of human papillomavirus by the polymerase chain reaction |
| US5234809A (en) | 1989-03-23 | 1993-08-10 | Akzo N.V. | Process for isolating nucleic acid |
| CA2020958C (en) | 1989-07-11 | 2005-01-11 | Daniel L. Kacian | Nucleic acid sequence amplification methods |
| NL9000134A (nl) | 1990-01-19 | 1991-08-16 | Stichting Res Fonds Pathologie | Primers en werkwijze voor het detecteren van humaan papilloma virus genotypen m.b.v. pcr. |
| US5210015A (en) | 1990-08-06 | 1993-05-11 | Hoffman-La Roche Inc. | Homogeneous assay system using the nuclease activity of a nucleic acid polymerase |
| CA2052413C (en) * | 1990-09-28 | 2004-07-13 | Jeffrey L. Joseph | Nucleotides sequences useful as type-specific probes, pcr primers and lcr probes for the amplifications and detection of human papilloma virus, and related kits and methods |
| US5455166A (en) | 1991-01-31 | 1995-10-03 | Becton, Dickinson And Company | Strand displacement amplification |
| AU8997991A (en) | 1991-01-31 | 1992-08-06 | Becton Dickinson & Company | Exonuclease mediated strand displacement amplification |
| US5279309A (en) | 1991-06-13 | 1994-01-18 | International Business Machines Corporation | Signaling device and method for monitoring positions in a surgical operation |
| US5411875A (en) | 1991-11-01 | 1995-05-02 | University Of Iowa Research Foundation | Method for retrieval of unknown flanking DNA sequence |
| US5270184A (en) | 1991-11-19 | 1993-12-14 | Becton, Dickinson And Company | Nucleic acid target generation |
| US5344166A (en) | 1993-09-30 | 1994-09-06 | Microcentric Corporation | Jaw assembly for chucks |
| US5925517A (en) | 1993-11-12 | 1999-07-20 | The Public Health Research Institute Of The City Of New York, Inc. | Detectably labeled dual conformation oligonucleotide probes, assays and kits |
| WO1995022626A1 (en) | 1994-02-21 | 1995-08-24 | Stichting Researchfonds Pathologie | Human papilloma virus detection in a nucleic acid amplification process using general primers |
| US5487792A (en) | 1994-06-13 | 1996-01-30 | Midwest Research Institute | Molecular assemblies as protective barriers and adhesion promotion interlayer |
| US6265154B1 (en) | 1996-10-25 | 2001-07-24 | Abbott Laboratories | Nucleic acid primers and probes for detecting oncogenic human papillomaviruses |
| US20030129589A1 (en) * | 1996-11-06 | 2003-07-10 | Hubert Koster | Dna diagnostics based on mass spectrometry |
| US5846726A (en) | 1997-05-13 | 1998-12-08 | Becton, Dickinson And Company | Detection of nucleic acids by fluorescence quenching |
| ES2180207T3 (es) * | 1997-09-16 | 2003-02-01 | Innogenetics Nv | Deteccion e identificacion del papilomavirus humano por medio de pcr y de hibridacion inversa de tipo especifico. |
| US6045993A (en) | 1998-05-30 | 2000-04-04 | Visible Genetics Inc. | Method, reagent and kit for genotyping of human papillomavirus |
| GB9826247D0 (en) * | 1998-11-30 | 1999-01-20 | Nycomed Pharma As | Method |
| US6136599A (en) | 1998-12-10 | 2000-10-24 | Bayer Corporation | Human hybrid host cell for mammalian gene expression |
| US6235504B1 (en) | 1999-01-11 | 2001-05-22 | The Rockefeller University | Methods for identifying genomic equivalent markers and their use in quantitating cells and polynucleotide sequences therein |
| US6277581B1 (en) | 1999-03-01 | 2001-08-21 | Lankenau Medical Research Center | ODC allelic analysis method for assessing carcinogenic susceptibility |
| DE60141205D1 (de) * | 2000-04-03 | 2010-03-18 | Cytyc Corp | Nachweis und typisierung des papillomavirus mittels pna-sonden |
| US7019129B1 (en) | 2000-05-09 | 2006-03-28 | Biosearch Technologies, Inc. | Dark quenchers for donor-acceptor energy transfer |
| CA2417798A1 (en) | 2000-08-23 | 2003-01-30 | Takara Bio Inc. | Method of amplifying nucleic acid |
| US7070995B2 (en) * | 2001-04-11 | 2006-07-04 | City Of Hope | CE7-specific redirected immune cells |
| EP1302550A1 (en) | 2001-10-10 | 2003-04-16 | King Car Food Industrial Co., Ltd. | Method and detector for identifying subtypes of human papilloma viruses |
| DE60231901D1 (de) * | 2001-11-14 | 2009-05-20 | Toyo Boseki | Dna-synthesepromotoren, mit dna-polymerase assoziierte faktoren und nutzung davon |
| ES2282599T3 (es) * | 2002-01-07 | 2007-10-16 | Norchip A/S | Metodo para detectar arnm del virus del papiloma humano. |
| HUP0200981A3 (en) | 2002-03-14 | 2004-06-28 | Genoid Kft | Pcr reactions with hybridizing probes using primers with broad genotype-specificity for detection of human papilloma-viruses and typing amplificates by using specifically hybridizing oligonucleotides |
| JP4076165B2 (ja) * | 2004-02-05 | 2008-04-16 | インターナショナル・ビジネス・マシーンズ・コーポレーション | 管理装置、収納カセット、現金自動預け払い機、情報処理システム、管理方法、及び制御方法 |
| PL1712639T3 (pl) | 2005-04-06 | 2009-02-27 | Maurice Stroun | Sposób diagnozowania nowotworu przez wykrywanie krążącego DNA i RNA |
| GR1005451B (el) | 2005-09-20 | 2007-02-21 | Medicon Hellas A.E. | Μεθοδος εντοπισμου διαφορων ενος νουκλεοτιδιου σεμια αλληλουχια νουκλεοτιδιων με τη χρηση ξηρων/λυοφιλοποιημενων αντιδραστηριων |
| KR100702415B1 (ko) | 2006-03-03 | 2007-04-09 | 안웅식 | 올리고 핵산 탐침 비드 어레이를 이용한 인유두종바이러스검출 키트 및 방법 |
| US20080113358A1 (en) | 2006-07-28 | 2008-05-15 | Ravi Kapur | Selection of cells using biomarkers |
| US20100167280A1 (en) | 2006-08-11 | 2010-07-01 | Ivan Brukner | Oligonucleotides for discriminating related nucleic acid sequences |
| EP2034032A1 (en) * | 2007-08-28 | 2009-03-11 | DKFZ Deutsches Krebsforschungszentrum, Stiftung des Öffentlichen Rechts | Composition comprising an oligonucleotide mixture for improved detection of human papillomavirus genotypes |
-
2008
- 2008-09-30 US US12/241,119 patent/US9090948B2/en active Active
-
2009
- 2009-09-30 EP EP17160497.8A patent/EP3202920A1/en not_active Withdrawn
- 2009-09-30 EP EP09737497A patent/EP2352843A2/en not_active Ceased
- 2009-09-30 ES ES12177093.7T patent/ES2628432T3/es active Active
- 2009-09-30 JP JP2011530166A patent/JP5795259B2/ja active Active
- 2009-09-30 EP EP12177093.7A patent/EP2565280B1/en active Active
- 2009-09-30 CA CA2738512A patent/CA2738512C/en active Active
- 2009-09-30 WO PCT/US2009/058992 patent/WO2010039809A2/en not_active Ceased
- 2009-09-30 RU RU2011117297/10A patent/RU2530550C2/ru active
- 2009-09-30 CA CA2947959A patent/CA2947959C/en active Active
-
2015
- 2015-06-19 US US14/744,115 patent/US9803232B2/en not_active Expired - Fee Related
- 2015-08-11 JP JP2015158921A patent/JP6177844B2/ja active Active
-
2017
- 2017-10-25 US US15/793,365 patent/US10689685B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010039809A3 (en) | 2010-08-05 |
| JP2016028574A (ja) | 2016-03-03 |
| EP2565280A2 (en) | 2013-03-06 |
| RU2011117297A (ru) | 2012-11-10 |
| US10689685B2 (en) | 2020-06-23 |
| ES2628432T3 (es) | 2017-08-02 |
| CA2738512C (en) | 2017-08-08 |
| EP2352843A2 (en) | 2011-08-10 |
| US20180044717A1 (en) | 2018-02-15 |
| WO2010039809A2 (en) | 2010-04-08 |
| CA2947959C (en) | 2018-04-17 |
| EP2565280A3 (en) | 2013-07-03 |
| US9803232B2 (en) | 2017-10-31 |
| CA2738512A1 (en) | 2010-04-08 |
| JP6177844B2 (ja) | 2017-08-09 |
| CA2947959A1 (en) | 2017-01-13 |
| JP2012504414A (ja) | 2012-02-23 |
| US20100081124A1 (en) | 2010-04-01 |
| US9090948B2 (en) | 2015-07-28 |
| EP2565280B1 (en) | 2017-03-15 |
| US20150361482A1 (en) | 2015-12-17 |
| RU2530550C2 (ru) | 2014-10-10 |
| EP3202920A1 (en) | 2017-08-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6177844B2 (ja) | 試験サンプル中のヒトパピローマウイルスおよびヒトベータグロビン配列を検出するためのプライマーおよびプローブ | |
| JP2005500856A (ja) | 多重フルオロフォアを使用する蛍光多重hpvpcrアッセイ | |
| CN113508182B (zh) | 用于检测人乳头状瘤病毒(hpv)的测定 | |
| US7790386B2 (en) | Neisseria gonorrhoeae specific oligonucleotide sequences | |
| US20100261154A1 (en) | Primers and probes for detecting hepatitis c virus | |
| JP2018007694A (ja) | ターゲット核酸検出のための二重プローブアッセイ | |
| US20250340957A1 (en) | Assays for the Detection of SARS-CoV2 Mutants | |
| EP4118232A1 (en) | Molecular fingerprinting methods to detect and genotype different rna targets through reverse transcription polymerase chain reaction in a single reaction | |
| JP2009515545A (ja) | 方法 | |
| US20100003665A1 (en) | Real-time HPV PCR Assays | |
| JP7582674B2 (ja) | 牛白血病ウイルスを検出するためのキット | |
| WO2024054925A1 (en) | Compositions, kits and methods for detection of viral variant sequences |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120928 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120928 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20140114 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140401 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140606 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140613 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140930 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20150317 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150616 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150714 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150812 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5795259 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |