JP5717650B2 - トシラート前駆体からの18f−放射性標識スチリルピリジンおよびその安定性医薬組成物の合成 - Google Patents
トシラート前駆体からの18f−放射性標識スチリルピリジンおよびその安定性医薬組成物の合成 Download PDFInfo
- Publication number
- JP5717650B2 JP5717650B2 JP2011543721A JP2011543721A JP5717650B2 JP 5717650 B2 JP5717650 B2 JP 5717650B2 JP 2011543721 A JP2011543721 A JP 2011543721A JP 2011543721 A JP2011543721 A JP 2011543721A JP 5717650 B2 JP5717650 B2 JP 5717650B2
- Authority
- JP
- Japan
- Prior art keywords
- ethoxy
- radiopharmaceutical composition
- radiopharmaceutical
- pet
- synthesis
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/02—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by the carrier, i.e. characterised by the agent or material covalently linked or complexing the radioactive nucleus
- A61K51/04—Organic compounds
- A61K51/041—Heterocyclic compounds
- A61K51/044—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins
- A61K51/0455—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine, rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/365—Lactones
- A61K31/375—Ascorbic acid, i.e. vitamin C; Salts thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M37/00—Other apparatus for introducing media into the body; Percutany, i.e. introducing medicines into the body by diffusion through the skin
- A61M37/0069—Devices for implanting pellets, e.g. markers or solid medicaments
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Veterinary Medicine (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Epidemiology (AREA)
- Neurosurgery (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Neurology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Medical Informatics (AREA)
- Psychology (AREA)
- Dermatology (AREA)
- Hospice & Palliative Care (AREA)
- Anesthesiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Psychiatry (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Pyridine Compounds (AREA)
- Medicinal Preparation (AREA)
- Enzymes And Modification Thereof (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
- Nitrogen Condensed Heterocyclic Rings (AREA)
- Steroid Compounds (AREA)
Description
本出願は、合衆国法典第35巻第119(e)条に基づき、2008年12月31日に出願された米国仮特許出願番号第61/141,885号の優先権を主張し、その開示内容はすべて、参照により本明細書に組み込まれる。
本発明は、一般には18F-放射性標識化された脳造影剤の合成法、さらに具体的には18F-放射性標識化されたスチリルピリジンおよびそのトシラート前駆体を合成する方法、および18F-放射性標識化された脳造影剤を含む安定した医薬組成物に関する。
本発明の実施形態は、有効量の18F-放射性標識化合物、約1.0%から約20%(v/v)のエチルアルコール、および少なくとも約0.1%(w/v)のアスコルビン酸またはその塩を有する脳の神経変性疾患の陽電子放出断層撮影法(PET)用の放射性医薬組成物を対象とする。様々な実施形態において、18F-放射性標識化合物は患者の脳内の病的標的に結合できる。病的標的は、自然にまたは病理学的に変化したタンパク質、ペプチド、またはオリゴヌクレオチド、β-アミロイド、α-シヌクレイン、または水疱性モノアミン輸送体2(VMAT2)の異常集中を含み得る。
説明されている特定の組成物または方法論は異なり得るため、本発明はこれらに限られない。さらに、本説明中で使用されている用語は特定のバージョンまたは実施形態を説明するものであり、本発明の範囲を限定することを意図していない。別途定義されていない限り、本明細書中で使用されているすべての技術用語および科学用語は本分野において当業者により一般的に理解されるものと同じ意味を持つ。不一致がある場合、定義を含め、本特許出願明細書が優先される。
本明細書中で開示される発明をより効率的に理解するために、以下の実施例が提供されている。これらの実施例は例証のみを目的とし、如何なる方法によっても本発明を限定するものとは解釈すべきではない。
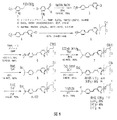
実施例1.0. AV-105トシラート前駆体の18F-AV-45への合成
実施例1.1. tert-ブチル4-ビニルフェニルカルバミン酸塩
実施例1.2. tert-ブチルメチル(4-ビニルフェニル)カルバミン酸塩
実施例1.3. 2-(2-(2-(5-ヨードピリジン-2-イルオキシ)エトキシ)エトキシ)エタノール
実施例1.4. (E)-tert-ブチル4-(2-(6-(2-(2-(2-ヒドロキシエトキシ)エトキシ)エトキシ)ピリジン-3-イル)ビニル)フェニル(メチル)カルバミン酸塩
実施例1.5. (E)-2-(2-(2-(5-(4-(tert-ブトキシカルボニル(メチル)アミノ)スチリル)ピリジン-2-イルオキシ)エトキシ)エトキシ)エチル4-メチルベンゼンスルホナート(AV-105)
実施例2.0. AV-105からの18F-AV-45の放射性合成
実施例3.0. 19F-AV-45を可溶化および18F-AV-45を安定化させるための製剤
表1. 合成終了(EOS)時の18F-AV-45注射の放射化学純度率分析
表2. 18F-AV-45注射における19F-AV-45の合成終了(EOS)時およびEOSから2時間後のUV純度分析*
表3. アスコルビン酸ナトリウム0.5%で製造および製剤された18F-AV-45注射の長期安定性
Claims (7)
- 脳の神経変性疾患の陽電子放出断層撮影(PET)を造影するための放射性医薬組成物であって、
有効量の((E)-4-(2-(6-(2-(2-(2-[18F]フルオロエトキシ)エトキシ)エトキシ)ピリジン-3-イル)ビニル)-N-メチルベンゼンアミン)(18F-AV-45)、
全組成物の少なくとも10%(v/v)のエチルアルコール、および
全組成物の90%v/vの0.9%食塩溶液、ここで、該食塩溶液は0.5%w/vのアスコルビン酸ナトリウムを含む、を含む放射性医薬組成物であって、((E)-4-(2-(6-(2-(2-(2-[ 18 F]フルオロエトキシ)エトキシ)エトキシ)ピリジン-3-イル)ビニル)-N-メチルベンゼンアミン)( 18 F-AV-45)が8〜10時間にわたり放射線分解に対して安定である、放射性医薬組成物。 - ((E)-4-(2-(6-(2-(2-(2-[18F]フルオロエトキシ)エトキシ)エトキシ)ピリジン-3-イル)ビニル)-N-メチルベンゼンアミン)がトシラート前駆体から生成される請求項1記載の放射性医薬組成物。
- 放射性医薬組成物のpHが4.5から8.0までの範囲内であるところの、請求項1または2に記載の放射性医薬組成物。
- 放射性医薬組成物が、合成終了から少なくとも12時間後の測定時に全組成物の90%またはそれ以上の18F-放射性標識化合物を含む請求項1に記載の放射性医薬組成物。
- 認知症、認知障害、アルツハイマー病、パーキンソン病、レビー小体型認知症、および血管性認知症の少なくとも一つの神経変性疾患の診断で使用するための請求項1記載の放射性医薬組成物。
- 患者の神経変性疾患を診断するための方法において使用される放射性医薬組成物であって、
放射性医薬組成物を投与する手順、
標的が位置すると予期される領域を含む患者の脳の少なくとも一部を造影する手順、および
標的を検出する手順を含むことを特徴とする、請求項1〜5のいずれかに記載の放射性医薬組成物。 - 造影手順が、陽電子放出断層撮影法(PET)、コンピュータ断層撮影と同時のPET(PET/CT)、磁気共鳴画像法と同時のPET(PET/MRI)、またはその組み合わせを含む、請求項6記載の放射性医薬組成物。
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14188508P | 2008-12-31 | 2008-12-31 | |
| US61/141,885 | 2008-12-31 | ||
| PCT/US2009/069741 WO2010078370A1 (en) | 2008-12-31 | 2009-12-29 | Synthesis of 18f-radiolabeled styrylpyridines from tosylate precursors and stable pharmaceutical compositions thereof |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2012514007A JP2012514007A (ja) | 2012-06-21 |
| JP2012514007A5 JP2012514007A5 (ja) | 2013-06-06 |
| JP5717650B2 true JP5717650B2 (ja) | 2015-05-13 |
Family
ID=42310197
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011543721A Active JP5717650B2 (ja) | 2008-12-31 | 2009-12-29 | トシラート前駆体からの18f−放射性標識スチリルピリジンおよびその安定性医薬組成物の合成 |
Country Status (35)
| Country | Link |
|---|---|
| US (1) | US20100172836A1 (ja) |
| EP (1) | EP2381967B1 (ja) |
| JP (1) | JP5717650B2 (ja) |
| KR (1) | KR20110119670A (ja) |
| CN (1) | CN102271716B (ja) |
| AU (1) | AU2009335049B2 (ja) |
| BR (1) | BRPI0923890A2 (ja) |
| CA (1) | CA2748705C (ja) |
| CO (1) | CO6341578A2 (ja) |
| CR (1) | CR20110410A (ja) |
| CY (1) | CY1118658T1 (ja) |
| DK (1) | DK2381967T3 (ja) |
| DO (1) | DOP2011000210A (ja) |
| EA (1) | EA023014B1 (ja) |
| EC (1) | ECSP11011242A (ja) |
| ES (1) | ES2620402T3 (ja) |
| HN (1) | HN2011001810A (ja) |
| HR (1) | HRP20170453T1 (ja) |
| HU (1) | HUE033649T2 (ja) |
| IL (1) | IL213674A (ja) |
| LT (1) | LT2381967T (ja) |
| MA (1) | MA33004B1 (ja) |
| MX (1) | MX2011007063A (ja) |
| MY (1) | MY162312A (ja) |
| NZ (1) | NZ593848A (ja) |
| PE (1) | PE20120007A1 (ja) |
| PL (1) | PL2381967T3 (ja) |
| PT (1) | PT2381967T (ja) |
| RS (1) | RS55674B1 (ja) |
| SG (1) | SG172439A1 (ja) |
| SI (1) | SI2381967T1 (ja) |
| TN (1) | TN2011000318A1 (ja) |
| UA (1) | UA105914C2 (ja) |
| WO (1) | WO2010078370A1 (ja) |
| ZA (1) | ZA201104824B (ja) |
Families Citing this family (30)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010039815A1 (en) * | 2008-09-30 | 2010-04-08 | Case Western Reserve University | Molecular probes for imaging of myelin |
| BR112012030935B1 (pt) | 2010-04-06 | 2020-11-17 | Life Molecular Imaging Sa | método para a produção de ligantes de beta-amiloide marcados com f-18 |
| TWI504414B (zh) | 2010-06-04 | 2015-10-21 | Bayer Schering Pharma Ag | 生產F-18標記之Aβ配位體之方法 |
| US20130217920A1 (en) * | 2010-06-04 | 2013-08-22 | Piramal Imaging Sa | Method for production of f-18 labeled amyloid beta ligands |
| EP3345545B1 (en) | 2011-04-21 | 2021-10-20 | The Regents of The University of California | Functionalized magnetic nanoparticles and use in imaging amyloid deposits and neurofibrillary tangles |
| HRP20181081T1 (hr) * | 2011-06-21 | 2018-09-21 | Piramal Imaging Sa | Formulacije fluoriranog stilbena pogodne za pet vizualizaciju |
| WO2013057199A1 (en) | 2011-10-19 | 2013-04-25 | Piramal Imaging Sa | IMPROVED METHOD FOR PRODUCTION OF F-18 LABELED Aß LIGANDS |
| CN102432529B (zh) * | 2011-11-14 | 2014-03-26 | 江苏省原子医学研究所 | 一种苯乙烯基吡啶二硫二氮衍生物的制备方法 |
| EP2791113A1 (en) * | 2011-12-15 | 2014-10-22 | GE Healthcare UK Limited | Heterocyclic compounds as imaging probes of tau pathology |
| CN102526765B (zh) * | 2011-12-31 | 2014-07-30 | 郑州泰基鸿诺药物科技有限公司 | 一种Aβ斑块显像剂及其制备方法 |
| CN103965105A (zh) * | 2013-01-29 | 2014-08-06 | 中山大学 | 苯乙烯基喹啉衍生物及其在治疗阿尔茨海默病症中的应用 |
| CN103645254B (zh) * | 2013-11-28 | 2015-01-07 | 江苏省原子医学研究所 | 一种Aβ斑块显像剂前体AV45的含量分析方法 |
| WO2017014599A1 (ko) * | 2015-07-22 | 2017-01-26 | 주식회사 씨코헬스케어 | [18f]플루오로-도파의 방사화학적 순도를 안정화시키는 조성물 및 이의 제조방법 |
| AU2016351609B2 (en) * | 2015-11-13 | 2019-08-08 | Eli Lilly And Company | Azetidine derivatives for tau imaging |
| WO2017156303A1 (en) | 2016-03-09 | 2017-09-14 | Case Western Reserve University | Radioligands for myelin |
| CN107198780A (zh) * | 2016-03-18 | 2017-09-26 | 南京江原安迪科正电子研究发展有限公司 | 放射性药物组合物及其制备方法、应用 |
| CN106018575B (zh) * | 2016-05-05 | 2018-10-19 | 江苏省原子医学研究所 | 一种PET显像剂前体TsOP-(+)-DTBZ及其光学异构体的分离测定方法 |
| US10695450B2 (en) * | 2016-07-26 | 2020-06-30 | Laboratoires Cyclopharma | Synthesis of a radioactive agent composition |
| EP3538161B1 (en) * | 2016-11-08 | 2022-07-27 | The Regents of The University of California | Methods for multi-dose synthesis of [f-18]fddnp for clinical settings |
| TW201906818A (zh) * | 2017-05-31 | 2019-02-16 | 美商511製藥公司 | 新穎氘取代之正子發射斷層掃描(pet)顯影劑及其藥理應用 |
| CA3088232A1 (en) * | 2018-01-24 | 2019-08-01 | Ac Immune Sa | Diagnostic compositions for pet imaging, a method for manufacturing the diagnostic composition and its use in diagnostics |
| CN111499544B (zh) * | 2019-01-31 | 2022-12-02 | 中国医学科学院放射医学研究所 | 一种一锅法制备N-Boc-N-甲基-4-氨基苯乙烯的合成方法 |
| CN111499570B (zh) * | 2019-01-31 | 2023-05-16 | 中国医学科学院放射医学研究所 | 一种av-45中间体的合成方法 |
| WO2020202831A1 (ja) * | 2019-03-29 | 2020-10-08 | 国立研究開発法人量子科学技術研究開発機構 | 放射性医薬の製造方法及び放射性医薬 |
| CN115403434A (zh) * | 2021-05-26 | 2022-11-29 | 汉中汉核医疗科技有限公司 | 一种放射性药物的合成方法 |
| CN115400232A (zh) * | 2021-05-26 | 2022-11-29 | 汉中汉核医疗科技有限公司 | 一种放射性药物合成方法 |
| CN113582885B (zh) * | 2021-08-30 | 2023-04-21 | 南京克米斯璀新能源科技有限公司 | 一种烷基磺酸钠的生产方法 |
| CN114805190A (zh) * | 2022-06-08 | 2022-07-29 | 吉林大学第一医院 | 一种甲基苯胺类Aβ蛋白显像剂的双柱合成方法 |
| WO2024107620A1 (en) * | 2022-11-14 | 2024-05-23 | Eli Lilly And Company | Polymorphic forms of florbetapir precursor av-105 |
| CN119080714A (zh) * | 2024-09-12 | 2024-12-06 | 四川大学 | 一种偶氮苯磺酸酯化合物及其制备方法和应用、放射性探针的制备方法 |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2007525211A (ja) * | 2004-01-19 | 2007-09-06 | テクニオン リサーチ アンド ディベロップメント ファウンデーション リミテッド | パーキンソン病の診断検査 |
| MX2007007380A (es) * | 2004-12-17 | 2007-10-11 | Univ Pennsylvania | Derivados de estilbeno y su uso para estudios de union y por imagenes de placas amiloides. |
| JP5290954B2 (ja) * | 2006-03-30 | 2013-09-18 | ザ・トラスティーズ・オブ・ザ・ユニバーシティ・オブ・ペンシルバニア | スチリルピリジン誘導体及びアミロイド斑を結合させ画像化するためのその使用 |
-
2009
- 2009-12-29 KR KR1020117017636A patent/KR20110119670A/ko not_active Ceased
- 2009-12-29 CA CA2748705A patent/CA2748705C/en active Active
- 2009-12-29 WO PCT/US2009/069741 patent/WO2010078370A1/en not_active Ceased
- 2009-12-29 ES ES09837135.4T patent/ES2620402T3/es active Active
- 2009-12-29 EA EA201170911A patent/EA023014B1/ru not_active IP Right Cessation
- 2009-12-29 SI SI200931600A patent/SI2381967T1/sl unknown
- 2009-12-29 BR BRPI0923890-5A patent/BRPI0923890A2/pt active Search and Examination
- 2009-12-29 SG SG2011048055A patent/SG172439A1/en unknown
- 2009-12-29 UA UAA201109307A patent/UA105914C2/uk unknown
- 2009-12-29 AU AU2009335049A patent/AU2009335049B2/en active Active
- 2009-12-29 MY MYPI2011003056A patent/MY162312A/en unknown
- 2009-12-29 MX MX2011007063A patent/MX2011007063A/es active IP Right Grant
- 2009-12-29 HR HRP20170453TT patent/HRP20170453T1/hr unknown
- 2009-12-29 DK DK09837135.4T patent/DK2381967T3/en active
- 2009-12-29 PT PT98371354T patent/PT2381967T/pt unknown
- 2009-12-29 US US12/648,869 patent/US20100172836A1/en not_active Abandoned
- 2009-12-29 PE PE2011001294A patent/PE20120007A1/es not_active Application Discontinuation
- 2009-12-29 HU HUE09837135A patent/HUE033649T2/en unknown
- 2009-12-29 RS RS20170117A patent/RS55674B1/sr unknown
- 2009-12-29 JP JP2011543721A patent/JP5717650B2/ja active Active
- 2009-12-29 NZ NZ593848A patent/NZ593848A/xx unknown
- 2009-12-29 LT LTEP09837135.4T patent/LT2381967T/lt unknown
- 2009-12-29 CN CN200980153414.5A patent/CN102271716B/zh active Active
- 2009-12-29 EP EP09837135.4A patent/EP2381967B1/en active Active
- 2009-12-29 MA MA34065A patent/MA33004B1/fr unknown
- 2009-12-29 PL PL09837135T patent/PL2381967T3/pl unknown
-
2011
- 2011-06-20 IL IL213674A patent/IL213674A/en active IP Right Grant
- 2011-06-24 TN TN2011000318A patent/TN2011000318A1/fr unknown
- 2011-06-29 HN HN2011001810A patent/HN2011001810A/es unknown
- 2011-06-29 ZA ZA2011/04824A patent/ZA201104824B/en unknown
- 2011-06-29 DO DO2011000210A patent/DOP2011000210A/es unknown
- 2011-07-11 CO CO11086470A patent/CO6341578A2/es active IP Right Grant
- 2011-07-29 CR CR20110410A patent/CR20110410A/es unknown
- 2011-07-29 EC EC2011011242A patent/ECSP11011242A/es unknown
-
2017
- 2017-02-24 CY CY20171100253T patent/CY1118658T1/el unknown
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5717650B2 (ja) | トシラート前駆体からの18f−放射性標識スチリルピリジンおよびその安定性医薬組成物の合成 | |
| US20200222562A1 (en) | Stabilization of radiopharmaceutical compositions using ascorbic acid | |
| ES2867814T3 (es) | Compuestos heterocíclicos deuterados y su uso como agentes de formación de imágenes | |
| US20080267879A1 (en) | Novel Imaging Tracers for Early Detection and Treatment of Amyloid Plaques Caused by Alzheimer's Disease and Related Disorders | |
| CN103298789A (zh) | 作为τ蛋白病理成像探针的杂环化合物 | |
| US20080219922A1 (en) | Alzheimer's Disease Imaging Agents | |
| Nan et al. | 6-Methoxy-indanone derivatives as potential probes for β-amyloid plaques in Alzheimer's disease | |
| WO2014052454A1 (en) | Imaging agents | |
| Neumaier et al. | Synthesis and evaluation of 18F-fluoroethylated benzothiazole derivatives for in vivo imaging of amyloid plaques in Alzheimer's disease | |
| EP3835293B1 (en) | Monoamine oxidase b imaging probe | |
| HK1162951B (en) | Synthesis of 18f-radiolabeled styrylpyridines from tosylate precursors and stable pharmaceutical compositions thereof | |
| WO2010135493A2 (en) | Alzheimer's disease imaging agents | |
| WO2014184682A1 (en) | Radiolabeled gnrh antagonists as pet imaging agents | |
| JP2021102593A (ja) | タウを画像化する新規化合物 | |
| HK1170672B (en) | Stabilization of radiopharmaceutical compositions using ascorbic acid | |
| JP2012207995A (ja) | 脳内ノルエピネフリン・トランスポータを標的とする放射性臭素標識pet分子イメージングプローブ | |
| HK1170672A (en) | Stabilization of radiopharmaceutical compositions using ascorbic acid | |
| HK1245645B (zh) | 使用抗坏血酸稳定化放射性药物组合物 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20121220 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20121220 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130418 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140311 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140603 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140610 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140709 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140716 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140808 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140815 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140910 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20150224 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150317 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5717650 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R153 | Grant of patent term extension |
Free format text: JAPANESE INTERMEDIATE CODE: R153 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |