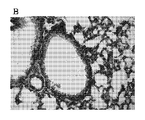
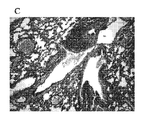
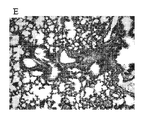
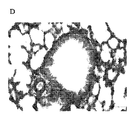
JP5678034B2 - アレルゲン駆動性気道病態の予防または治療のためのワクチン - Google Patents
アレルゲン駆動性気道病態の予防または治療のためのワクチン Download PDFInfo
- Publication number
- JP5678034B2 JP5678034B2 JP2012507703A JP2012507703A JP5678034B2 JP 5678034 B2 JP5678034 B2 JP 5678034B2 JP 2012507703 A JP2012507703 A JP 2012507703A JP 2012507703 A JP2012507703 A JP 2012507703A JP 5678034 B2 JP5678034 B2 JP 5678034B2
- Authority
- JP
- Japan
- Prior art keywords
- pertussis
- ova
- bpze1
- attenuated
- allergen
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 239000013566 allergen Substances 0.000 title claims description 47
- 229960005486 vaccine Drugs 0.000 title claims description 20
- 238000011282 treatment Methods 0.000 title claims description 8
- 230000002265 prevention Effects 0.000 title claims description 6
- 241000588832 Bordetella pertussis Species 0.000 claims description 74
- 230000002238 attenuated effect Effects 0.000 claims description 59
- 230000007170 pathology Effects 0.000 claims description 27
- 229940066827 pertussis vaccine Drugs 0.000 claims description 20
- 108010081690 Pertussis Toxin Proteins 0.000 claims description 18
- 239000003053 toxin Substances 0.000 claims description 13
- 231100000765 toxin Toxicity 0.000 claims description 13
- 206010040893 Skin necrosis Diseases 0.000 claims description 8
- 239000000427 antigen Substances 0.000 claims description 8
- 108091007433 antigens Proteins 0.000 claims description 8
- 102000036639 antigens Human genes 0.000 claims description 8
- 208000006673 asthma Diseases 0.000 claims description 8
- APUIQTDTBPKWKE-RNPGEKFLSA-N (2r,6s)-6-[[(4r)-4-[[(2s)-2-[2-[[(1r,2s,3r,4r,5r)-4-acetamido-2-[(2s,3r,4r,5s,6r)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6,8-dioxabicyclo[3.2.1]octan-3-yl]oxy]propanoylamino]propanoyl]amino]-4-carboxybutanoyl]amino]-2-amino-7-[[(2r)-2-am Chemical compound O([C@@H]1[C@H]2CO[C@H](O2)[C@H](NC(C)=O)[C@H]1OC(C)C(=O)N[C@@H](C)C(=O)N[C@H](CCC(=O)N[C@@H](CCC[C@@H](N)C(O)=O)C(=O)NC(=O)[C@H](N)C)C(O)=O)[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(C)=O APUIQTDTBPKWKE-RNPGEKFLSA-N 0.000 claims description 6
- 201000009961 allergic asthma Diseases 0.000 claims description 5
- 208000029523 Interstitial Lung disease Diseases 0.000 claims description 4
- 208000035285 Allergic Seasonal Rhinitis Diseases 0.000 claims description 2
- 241000588807 Bordetella Species 0.000 claims 1
- 230000002009 allergenic effect Effects 0.000 claims 1
- 108010058846 Ovalbumin Proteins 0.000 description 66
- 229940092253 ovalbumin Drugs 0.000 description 66
- 241000699670 Mus sp. Species 0.000 description 28
- 230000004044 response Effects 0.000 description 26
- 206010070834 Sensitisation Diseases 0.000 description 22
- 208000015181 infectious disease Diseases 0.000 description 22
- 230000008313 sensitization Effects 0.000 description 22
- 231100000331 toxic Toxicity 0.000 description 22
- 230000002588 toxic effect Effects 0.000 description 22
- 208000026935 allergic disease Diseases 0.000 description 21
- 206010020751 Hypersensitivity Diseases 0.000 description 20
- 230000007815 allergy Effects 0.000 description 19
- 238000002649 immunization Methods 0.000 description 15
- 230000003053 immunization Effects 0.000 description 15
- 102000003816 Interleukin-13 Human genes 0.000 description 14
- 108090000176 Interleukin-13 Proteins 0.000 description 14
- 210000004027 cell Anatomy 0.000 description 12
- 108700012359 toxins Proteins 0.000 description 12
- 102000000743 Interleukin-5 Human genes 0.000 description 11
- 108010002616 Interleukin-5 Proteins 0.000 description 11
- 230000000694 effects Effects 0.000 description 11
- 102000004127 Cytokines Human genes 0.000 description 9
- 108090000695 Cytokines Proteins 0.000 description 9
- 230000037452 priming Effects 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- 210000003979 eosinophil Anatomy 0.000 description 8
- 108090000623 proteins and genes Proteins 0.000 description 8
- 210000002966 serum Anatomy 0.000 description 8
- 230000000172 allergic effect Effects 0.000 description 7
- 208000010668 atopic eczema Diseases 0.000 description 7
- 230000006698 induction Effects 0.000 description 7
- 230000008595 infiltration Effects 0.000 description 7
- 238000001764 infiltration Methods 0.000 description 7
- 230000009885 systemic effect Effects 0.000 description 7
- 238000011161 development Methods 0.000 description 6
- 230000028993 immune response Effects 0.000 description 6
- 210000004072 lung Anatomy 0.000 description 6
- 210000003097 mucus Anatomy 0.000 description 6
- 206010003645 Atopy Diseases 0.000 description 5
- 206010061218 Inflammation Diseases 0.000 description 5
- 102100037850 Interferon gamma Human genes 0.000 description 5
- 108010074328 Interferon-gamma Proteins 0.000 description 5
- 201000005702 Pertussis Diseases 0.000 description 5
- 101150103308 ampG gene Proteins 0.000 description 5
- 230000006378 damage Effects 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 230000004054 inflammatory process Effects 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 230000000241 respiratory effect Effects 0.000 description 5
- 229940125575 vaccine candidate Drugs 0.000 description 5
- 241000894006 Bacteria Species 0.000 description 4
- 229940124832 acellular pertussis vaccine Drugs 0.000 description 4
- 239000002671 adjuvant Substances 0.000 description 4
- 208000037883 airway inflammation Diseases 0.000 description 4
- 238000010171 animal model Methods 0.000 description 4
- 230000002950 deficient Effects 0.000 description 4
- 230000005713 exacerbation Effects 0.000 description 4
- 239000012530 fluid Substances 0.000 description 4
- 230000036039 immunity Effects 0.000 description 4
- 210000000440 neutrophil Anatomy 0.000 description 4
- 102000004169 proteins and genes Human genes 0.000 description 4
- 210000002345 respiratory system Anatomy 0.000 description 4
- 210000004989 spleen cell Anatomy 0.000 description 4
- 238000002255 vaccination Methods 0.000 description 4
- 239000000304 virulence factor Substances 0.000 description 4
- 230000007923 virulence factor Effects 0.000 description 4
- MSFSPUZXLOGKHJ-UHFFFAOYSA-N Muraminsaeure Natural products OC(=O)C(C)OC1C(N)C(O)OC(CO)C1O MSFSPUZXLOGKHJ-UHFFFAOYSA-N 0.000 description 3
- 108010013639 Peptidoglycan Proteins 0.000 description 3
- 208000037884 allergic airway inflammation Diseases 0.000 description 3
- 238000004458 analytical method Methods 0.000 description 3
- 230000007613 environmental effect Effects 0.000 description 3
- 206010020718 hyperplasia Diseases 0.000 description 3
- 238000000034 method Methods 0.000 description 3
- 239000000203 mixture Substances 0.000 description 3
- 238000010172 mouse model Methods 0.000 description 3
- 230000035772 mutation Effects 0.000 description 3
- 239000000843 powder Substances 0.000 description 3
- 208000005069 pulmonary fibrosis Diseases 0.000 description 3
- 230000007115 recruitment Effects 0.000 description 3
- 230000009467 reduction Effects 0.000 description 3
- 239000000243 solution Substances 0.000 description 3
- 239000000725 suspension Substances 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 108700028369 Alleles Proteins 0.000 description 2
- 208000012657 Atopic disease Diseases 0.000 description 2
- 208000035143 Bacterial infection Diseases 0.000 description 2
- 238000002965 ELISA Methods 0.000 description 2
- 206010014950 Eosinophilia Diseases 0.000 description 2
- 241000588724 Escherichia coli Species 0.000 description 2
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- 102000003814 Interleukin-10 Human genes 0.000 description 2
- 108090000174 Interleukin-10 Proteins 0.000 description 2
- 241000699666 Mus <mouse, genus> Species 0.000 description 2
- 108091028043 Nucleic acid sequence Proteins 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- 210000004241 Th2 cell Anatomy 0.000 description 2
- 239000000443 aerosol Substances 0.000 description 2
- 230000001580 bacterial effect Effects 0.000 description 2
- 208000022362 bacterial infectious disease Diseases 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 210000003123 bronchiole Anatomy 0.000 description 2
- 239000002619 cytotoxin Substances 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 201000010099 disease Diseases 0.000 description 2
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 2
- 238000009472 formulation Methods 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 230000004941 influx Effects 0.000 description 2
- 230000001665 lethal effect Effects 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000007246 mechanism Effects 0.000 description 2
- 230000004660 morphological change Effects 0.000 description 2
- 230000003843 mucus production Effects 0.000 description 2
- LWGJTAZLEJHCPA-UHFFFAOYSA-N n-(2-chloroethyl)-n-nitrosomorpholine-4-carboxamide Chemical compound ClCCN(N=O)C(=O)N1CCOCC1 LWGJTAZLEJHCPA-UHFFFAOYSA-N 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 150000007523 nucleic acids Chemical group 0.000 description 2
- 230000010287 polarization Effects 0.000 description 2
- 230000035755 proliferation Effects 0.000 description 2
- 230000009696 proliferative response Effects 0.000 description 2
- 208000023504 respiratory system disease Diseases 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 238000010186 staining Methods 0.000 description 2
- 238000007619 statistical method Methods 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 230000001629 suppression Effects 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 1
- 206010027654 Allergic conditions Diseases 0.000 description 1
- 101000878581 Aplysia californica Feeding circuit activating peptides Proteins 0.000 description 1
- 208000034309 Bacterial disease carrier Diseases 0.000 description 1
- 241000588779 Bordetella bronchiseptica Species 0.000 description 1
- 108010078791 Carrier Proteins Proteins 0.000 description 1
- DBAKFASWICGISY-BTJKTKAUSA-N Chlorpheniramine maleate Chemical compound OC(=O)\C=C/C(O)=O.C=1C=CC=NC=1C(CCN(C)C)C1=CC=C(Cl)C=C1 DBAKFASWICGISY-BTJKTKAUSA-N 0.000 description 1
- 108010062580 Concanavalin A Proteins 0.000 description 1
- 206010011224 Cough Diseases 0.000 description 1
- 206010058314 Dysplasia Diseases 0.000 description 1
- 241000588921 Enterobacteriaceae Species 0.000 description 1
- 108090000790 Enzymes Proteins 0.000 description 1
- 102000004190 Enzymes Human genes 0.000 description 1
- 108010092408 Eosinophil Peroxidase Proteins 0.000 description 1
- 102000044708 Eosinophil peroxidases Human genes 0.000 description 1
- 208000034826 Genetic Predisposition to Disease Diseases 0.000 description 1
- 241000589989 Helicobacter Species 0.000 description 1
- 108090000978 Interleukin-4 Proteins 0.000 description 1
- 238000012313 Kruskal-Wallis test Methods 0.000 description 1
- 241000589248 Legionella Species 0.000 description 1
- 208000007764 Legionnaires' Disease Diseases 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000588621 Moraxella Species 0.000 description 1
- 206010073310 Occupational exposures Diseases 0.000 description 1
- 101710093543 Probable non-specific lipid-transfer protein Proteins 0.000 description 1
- 241000589516 Pseudomonas Species 0.000 description 1
- 101150104836 Ptx gene Proteins 0.000 description 1
- 208000002200 Respiratory Hypersensitivity Diseases 0.000 description 1
- 241000607142 Salmonella Species 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 241000122971 Stenotrophomonas Species 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 239000013543 active substance Substances 0.000 description 1
- 230000000240 adjuvant effect Effects 0.000 description 1
- 230000010085 airway hyperresponsiveness Effects 0.000 description 1
- 230000008369 airway response Effects 0.000 description 1
- 230000009285 allergic inflammation Effects 0.000 description 1
- 230000037446 allergic sensitization Effects 0.000 description 1
- 230000005875 antibody response Effects 0.000 description 1
- 230000003190 augmentative effect Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 210000002469 basement membrane Anatomy 0.000 description 1
- 239000011324 bead Substances 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000015572 biosynthetic process Effects 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000006555 catalytic reaction Methods 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 229940030156 cell vaccine Drugs 0.000 description 1
- 210000002421 cell wall Anatomy 0.000 description 1
- 210000000038 chest Anatomy 0.000 description 1
- 210000000254 ciliated cell Anatomy 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 239000012228 culture supernatant Substances 0.000 description 1
- 210000000172 cytosol Anatomy 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 101150099497 dnt gene Proteins 0.000 description 1
- 239000003814 drug Substances 0.000 description 1
- 239000000839 emulsion Substances 0.000 description 1
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000003090 exacerbative effect Effects 0.000 description 1
- 230000001605 fetal effect Effects 0.000 description 1
- 238000000684 flow cytometry Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 238000012239 gene modification Methods 0.000 description 1
- 230000005017 genetic modification Effects 0.000 description 1
- 235000013617 genetically modified food Nutrition 0.000 description 1
- 210000002175 goblet cell Anatomy 0.000 description 1
- 210000005256 gram-negative cell Anatomy 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000007490 hematoxylin and eosin (H&E) staining Methods 0.000 description 1
- 230000036732 histological change Effects 0.000 description 1
- 230000008088 immune pathway Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 210000004969 inflammatory cell Anatomy 0.000 description 1
- 230000002401 inhibitory effect Effects 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 239000007924 injection Substances 0.000 description 1
- 238000002347 injection Methods 0.000 description 1
- 239000007928 intraperitoneal injection Substances 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 230000021633 leukocyte mediated immunity Effects 0.000 description 1
- 229940124590 live attenuated vaccine Drugs 0.000 description 1
- 229940023012 live-attenuated vaccine Drugs 0.000 description 1
- 230000005923 long-lasting effect Effects 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 230000035800 maturation Effects 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 239000003595 mist Substances 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 239000007922 nasal spray Substances 0.000 description 1
- 229940097496 nasal spray Drugs 0.000 description 1
- 229960005030 other vaccine in atc Drugs 0.000 description 1
- 239000012188 paraffin wax Substances 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 230000001991 pathophysiological effect Effects 0.000 description 1
- 229960001412 pentobarbital Drugs 0.000 description 1
- WEXRUCMBJFQVBZ-UHFFFAOYSA-N pentobarbital Chemical compound CCCC(C)C1(CC)C(=O)NC(=O)NC1=O WEXRUCMBJFQVBZ-UHFFFAOYSA-N 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 238000002360 preparation method Methods 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 239000003223 protective agent Substances 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 201000009732 pulmonary eosinophilia Diseases 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000000638 stimulation Effects 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 229940031626 subunit vaccine Drugs 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000000451 tissue damage Effects 0.000 description 1
- 231100000827 tissue damage Toxicity 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/02—Bacterial antigens
- A61K39/099—Bordetella
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/52—Bacterial cells; Fungal cells; Protozoal cells
- A61K2039/522—Bacterial cells; Fungal cells; Protozoal cells avirulent or attenuated
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/52—Bacterial cells; Fungal cells; Protozoal cells
- A61K2039/523—Bacterial cells; Fungal cells; Protozoal cells expressing foreign proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
- A61K2039/541—Mucosal route
- A61K2039/543—Mucosal route intranasal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/54—Medicinal preparations containing antigens or antibodies characterised by the route of administration
- A61K2039/541—Mucosal route
- A61K2039/544—Mucosal route to the airways
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Medicinal Chemistry (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Immunology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Organic Chemistry (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP09305371 | 2009-04-28 | ||
| EP09305371.8 | 2009-04-28 | ||
| PCT/EP2010/055507 WO2010125014A1 (en) | 2009-04-28 | 2010-04-26 | Vaccine for prophylaxis or treatment of an allergen-driven airway pathology |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2012525346A JP2012525346A (ja) | 2012-10-22 |
| JP2012525346A5 JP2012525346A5 (enExample) | 2013-05-02 |
| JP5678034B2 true JP5678034B2 (ja) | 2015-02-25 |
Family
ID=41057387
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012507703A Active JP5678034B2 (ja) | 2009-04-28 | 2010-04-26 | アレルゲン駆動性気道病態の予防または治療のためのワクチン |
Country Status (10)
| Country | Link |
|---|---|
| US (7) | US20120177688A1 (enExample) |
| EP (3) | EP2424564B1 (enExample) |
| JP (1) | JP5678034B2 (enExample) |
| CN (1) | CN102448490B (enExample) |
| AU (1) | AU2010243708C1 (enExample) |
| CA (1) | CA2759280C (enExample) |
| DK (1) | DK2424564T3 (enExample) |
| ES (2) | ES3024937T3 (enExample) |
| SG (1) | SG175803A1 (enExample) |
| WO (1) | WO2010125014A1 (enExample) |
Families Citing this family (13)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| AU2007224734C1 (en) | 2006-03-10 | 2016-07-14 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Live attenuated Bordetella strains as a single dose vaccine against whooping cough |
| US20120177688A1 (en) | 2009-04-28 | 2012-07-12 | Institut Pasteur De Lille | Vaccine for Prophylaxis or Treatment of an Allergen-Driven Airway Pathology |
| EP2944320A1 (en) | 2009-06-15 | 2015-11-18 | National University of Singapore | Influenza vaccine, composition, and methods of use |
| WO2013066272A1 (en) | 2011-11-02 | 2013-05-10 | National University Of Singapore | Effect of an attenuated bordetella strain against allergic disease |
| EP2722338A1 (en) | 2012-10-17 | 2014-04-23 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Novel recombinant Bordetella strains |
| CN105722530B (zh) | 2013-08-16 | 2020-06-02 | 罗切斯特大学 | 用于紧密连接屏障调节的设计肽 |
| US9655959B2 (en) * | 2014-10-01 | 2017-05-23 | National University Of Singapore | Adenylate cyclase deficient bordetella strains |
| CN114767718A (zh) * | 2016-03-29 | 2022-07-22 | 里尔巴斯德研究所 | 突变型博德特氏菌属菌株及其使用方法 |
| JP6598724B2 (ja) | 2016-04-07 | 2019-10-30 | 株式会社ジェイテクト | ステアリング装置 |
| SG11202003333WA (en) | 2017-10-18 | 2020-05-28 | Pasteur Institut | Bordetella strains expressing serotype 3 fimbriae |
| US20220249364A1 (en) | 2019-06-05 | 2022-08-11 | University Of Rochester | Designed Inhibitors of Tight Junction Formation |
| EP4208472A1 (en) * | 2020-09-04 | 2023-07-12 | Intravacc B.V. | Ompa mutations enhance omv production in bordetella pertussis |
| EP3909971A1 (en) | 2020-09-28 | 2021-11-17 | Institute of Life Sciences (ILS) | Whole cell livestock vaccine for respiratory diseases |
Family Cites Families (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4883761A (en) | 1986-03-25 | 1989-11-28 | The United States Of America As Represented By The Department Of Health And Human Services | Pertussis toxin gene: cloning and expression of protective antigen |
| US5101019A (en) * | 1987-05-22 | 1992-03-31 | Takeda Chemical Industries, Ltd. | Method for removing pertussis endotoxin, a pertussis toxoid and its production |
| US6713072B1 (en) | 1987-11-02 | 2004-03-30 | Chiron S.R.L. | Immunologically active polypeptides with altered toxicity useful for the preparation of an antipertussis vaccine |
| GB9115332D0 (en) | 1991-07-16 | 1991-08-28 | Connaught Lab | Manipulation of gene copy number in bordetella |
| FR2754543B1 (fr) | 1996-10-11 | 1998-12-31 | Pasteur Institut | Souche de bordetella deficiente dans la production de toxine et exprimant une proteine hydride, liposomes comprenant de la fha et leurs utilisations comme vaccins, et utilisation de la fha pour stimuler les reponses immunitaires |
| FR2840319B1 (fr) * | 2002-05-30 | 2004-08-20 | Pasteur Institut | Souches de bordetella rendues deficientes par attenuation genetique |
| EP1617805A4 (en) * | 2003-04-11 | 2012-07-04 | Medimmune Llc | METHODS FOR PREVENTING OR TREATING RESPIRATORY CONDITIONS |
| GB0402131D0 (en) | 2004-01-30 | 2004-03-03 | Isis Innovation | Delivery method |
| AU2007224734C1 (en) * | 2006-03-10 | 2016-07-14 | Institut National De La Sante Et De La Recherche Medicale (Inserm) | Live attenuated Bordetella strains as a single dose vaccine against whooping cough |
| WO2008118592A2 (en) | 2007-02-23 | 2008-10-02 | The Penn State Research Foundation | Use of an avirulent bordetella mutant as a live vaccine vector |
| CN100537767C (zh) * | 2007-04-29 | 2009-09-09 | 中国药品生物制品检定所 | 百日咳疫苗保护性抗原的重组表达及其应用 |
| US20120177688A1 (en) | 2009-04-28 | 2012-07-12 | Institut Pasteur De Lille | Vaccine for Prophylaxis or Treatment of an Allergen-Driven Airway Pathology |
| CN101947323B (zh) * | 2010-07-26 | 2013-02-27 | 北京绿竹生物制药有限公司 | 一种革兰氏阴性细菌疫苗及其制备方法 |
| WO2013066272A1 (en) | 2011-11-02 | 2013-05-10 | National University Of Singapore | Effect of an attenuated bordetella strain against allergic disease |
| EP2722338A1 (en) | 2012-10-17 | 2014-04-23 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Novel recombinant Bordetella strains |
| US9655959B2 (en) | 2014-10-01 | 2017-05-23 | National University Of Singapore | Adenylate cyclase deficient bordetella strains |
| CN114767718A (zh) | 2016-03-29 | 2022-07-22 | 里尔巴斯德研究所 | 突变型博德特氏菌属菌株及其使用方法 |
| US10799573B2 (en) | 2016-03-30 | 2020-10-13 | Regents Of The University Of Minnesota | Pertussis vaccines and methods of making and using |
| WO2018213361A1 (en) | 2017-05-15 | 2018-11-22 | Vanderbilt University | LONG-CIRCULATING ZWITTERIONIC POLYPLEXES FOR siRNA DELIVERY |
| CA3070039A1 (en) | 2017-07-18 | 2019-01-24 | Serum Institute Of India Pvt Ltd. | An immunogenic composition having improved stability, enhanced immunogenicity and reduced reactogenicity and process for preparation thereof |
| SG11202003333WA (en) | 2017-10-18 | 2020-05-28 | Pasteur Institut | Bordetella strains expressing serotype 3 fimbriae |
| US12036405B2 (en) | 2018-03-23 | 2024-07-16 | The Board Of Regents Of The University Of Oklahoma | System and method of electric-induced acoustic tomography for electrotherapy monitoring |
-
2010
- 2010-04-26 US US13/266,561 patent/US20120177688A1/en not_active Abandoned
- 2010-04-26 ES ES19176782T patent/ES3024937T3/es active Active
- 2010-04-26 CA CA2759280A patent/CA2759280C/en active Active
- 2010-04-26 WO PCT/EP2010/055507 patent/WO2010125014A1/en not_active Ceased
- 2010-04-26 CN CN201080019636.0A patent/CN102448490B/zh active Active
- 2010-04-26 ES ES10715245.6T patent/ES2535412T3/es active Active
- 2010-04-26 EP EP10715245.6A patent/EP2424564B1/en active Active
- 2010-04-26 EP EP19176782.1A patent/EP3590532B1/en active Active
- 2010-04-26 DK DK10715245T patent/DK2424564T3/en active
- 2010-04-26 JP JP2012507703A patent/JP5678034B2/ja active Active
- 2010-04-26 SG SG2011078771A patent/SG175803A1/en unknown
- 2010-04-26 EP EP15154206.5A patent/EP2910252A1/en not_active Ceased
- 2010-04-26 AU AU2010243708A patent/AU2010243708C1/en active Active
-
2013
- 2013-03-01 US US13/782,754 patent/US8986709B2/en active Active
-
2015
- 2015-01-30 US US14/609,461 patent/US9839683B2/en active Active
-
2017
- 2017-11-01 US US15/800,921 patent/US10420827B2/en active Active
-
2019
- 2019-08-09 US US16/537,342 patent/US10653765B2/en active Active
-
2020
- 2020-04-03 US US16/840,276 patent/US11110161B2/en active Active
-
2021
- 2021-08-02 US US17/392,066 patent/US11819545B2/en active Active
Also Published As
| Publication number | Publication date |
|---|---|
| AU2010243708B2 (en) | 2014-06-12 |
| CN102448490B (zh) | 2015-05-13 |
| WO2010125014A1 (en) | 2010-11-04 |
| US20200282041A1 (en) | 2020-09-10 |
| EP3590532A1 (en) | 2020-01-08 |
| AU2010243708A1 (en) | 2011-11-03 |
| US8986709B2 (en) | 2015-03-24 |
| US10653765B2 (en) | 2020-05-19 |
| US9839683B2 (en) | 2017-12-12 |
| US11819545B2 (en) | 2023-11-21 |
| CA2759280C (en) | 2016-09-20 |
| EP2424564A1 (en) | 2012-03-07 |
| ES3024937T3 (en) | 2025-06-05 |
| EP2424564B1 (en) | 2015-02-25 |
| AU2010243708C1 (en) | 2014-10-02 |
| US20180085450A1 (en) | 2018-03-29 |
| US20120177688A1 (en) | 2012-07-12 |
| JP2012525346A (ja) | 2012-10-22 |
| EP3590532B1 (en) | 2025-03-19 |
| US20190358309A1 (en) | 2019-11-28 |
| US10420827B2 (en) | 2019-09-24 |
| CA2759280A1 (en) | 2010-11-04 |
| EP3590532C0 (en) | 2025-03-19 |
| ES2535412T3 (es) | 2015-05-11 |
| US20150182614A1 (en) | 2015-07-02 |
| US11110161B2 (en) | 2021-09-07 |
| CN102448490A (zh) | 2012-05-09 |
| DK2424564T3 (en) | 2015-04-27 |
| SG175803A1 (en) | 2011-12-29 |
| US20210361759A1 (en) | 2021-11-25 |
| EP2910252A1 (en) | 2015-08-26 |
| US20130183336A1 (en) | 2013-07-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5678034B2 (ja) | アレルゲン駆動性気道病態の予防または治療のためのワクチン | |
| US20250032600A1 (en) | Attenuated Bordetella Strains | |
| Fujihashi et al. | Mucosal immunity and tolerance in the elderly | |
| Ennis et al. | Prior Bordetella pertussis infection modulates allergen priming and the severity of airway pathology in a murine model of allergic asthma | |
| Kavanagh et al. | Attenuated Bordetella pertussis vaccine strain BPZE1 modulates allergen‐induced immunity and prevents allergic pulmonary pathology in a murine model | |
| HK40048647A (en) | Live attenuated bordetella strains as a single dose vaccine against whooping cough | |
| Protection | Nasal Immunization with | |
| HK1226777B (en) | Live attenuated bordetella strains as a single dose vaccine against whooping cough | |
| HK1226777A1 (en) | Live attenuated bordetella strains as a single dose vaccine against whooping cough | |
| HK1124090B (en) | Live attenuated bordetella strains as a single dose vaccine against whooping cough |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130311 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130311 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140401 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140618 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140625 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20140819 |
|
| RD03 | Notification of appointment of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7423 Effective date: 20140930 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20141003 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20140930 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20141215 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20150105 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5678034 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |