JP5504690B2 - 分析チップ - Google Patents
分析チップ Download PDFInfo
- Publication number
- JP5504690B2 JP5504690B2 JP2009118440A JP2009118440A JP5504690B2 JP 5504690 B2 JP5504690 B2 JP 5504690B2 JP 2009118440 A JP2009118440 A JP 2009118440A JP 2009118440 A JP2009118440 A JP 2009118440A JP 5504690 B2 JP5504690 B2 JP 5504690B2
- Authority
- JP
- Japan
- Prior art keywords
- holding tank
- liquid
- analysis chip
- flow path
- reagent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 238000004458 analytical method Methods 0.000 title claims description 563
- 239000007788 liquid Substances 0.000 claims description 1304
- 239000003153 chemical reaction reagent Substances 0.000 claims description 516
- 238000000926 separation method Methods 0.000 claims description 373
- 238000003860 storage Methods 0.000 claims description 337
- 238000006243 chemical reaction Methods 0.000 claims description 335
- 230000002093 peripheral effect Effects 0.000 claims description 267
- 239000000725 suspension Substances 0.000 claims description 225
- 230000005484 gravity Effects 0.000 claims description 173
- 230000009471 action Effects 0.000 claims description 61
- 239000000126 substance Substances 0.000 claims description 40
- 239000000427 antigen Substances 0.000 claims description 35
- 102000036639 antigens Human genes 0.000 claims description 35
- 108091007433 antigens Proteins 0.000 claims description 35
- 238000012360 testing method Methods 0.000 claims description 30
- 210000004369 blood Anatomy 0.000 claims description 29
- 239000008280 blood Substances 0.000 claims description 29
- 210000000601 blood cell Anatomy 0.000 claims description 29
- 210000002381 plasma Anatomy 0.000 claims description 22
- 238000011045 prefiltration Methods 0.000 claims description 16
- 210000002966 serum Anatomy 0.000 claims description 9
- 238000007599 discharging Methods 0.000 claims description 8
- 230000001900 immune effect Effects 0.000 claims description 3
- 238000005194 fractionation Methods 0.000 claims description 2
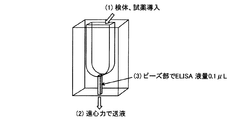
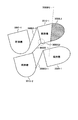
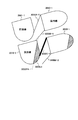
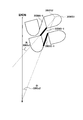
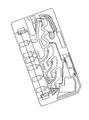
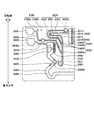
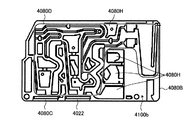
- 238000010586 diagram Methods 0.000 description 81
- 239000000523 sample Substances 0.000 description 70
- 238000003018 immunoassay Methods 0.000 description 45
- 239000000243 solution Substances 0.000 description 42
- 239000000758 substrate Substances 0.000 description 38
- 238000000034 method Methods 0.000 description 36
- 239000002699 waste material Substances 0.000 description 33
- 239000011347 resin Substances 0.000 description 27
- 229920005989 resin Polymers 0.000 description 27
- 238000004140 cleaning Methods 0.000 description 26
- 239000000463 material Substances 0.000 description 25
- 238000005259 measurement Methods 0.000 description 19
- 238000002156 mixing Methods 0.000 description 19
- 239000000203 mixture Substances 0.000 description 18
- -1 specimen Substances 0.000 description 18
- 238000002834 transmittance Methods 0.000 description 15
- 230000000694 effects Effects 0.000 description 14
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 14
- 230000036961 partial effect Effects 0.000 description 13
- 230000001965 increasing effect Effects 0.000 description 12
- 102000004190 Enzymes Human genes 0.000 description 11
- 108090000790 Enzymes Proteins 0.000 description 11
- 239000007789 gas Substances 0.000 description 11
- 239000011521 glass Substances 0.000 description 10
- 239000002245 particle Substances 0.000 description 10
- 239000013076 target substance Substances 0.000 description 10
- 238000012546 transfer Methods 0.000 description 10
- 238000005406 washing Methods 0.000 description 10
- 239000004743 Polypropylene Substances 0.000 description 9
- 229920001155 polypropylene Polymers 0.000 description 9
- 230000002829 reductive effect Effects 0.000 description 9
- 102000004127 Cytokines Human genes 0.000 description 8
- 108090000695 Cytokines Proteins 0.000 description 8
- 239000004793 Polystyrene Substances 0.000 description 8
- 238000010521 absorption reaction Methods 0.000 description 7
- 239000007853 buffer solution Substances 0.000 description 7
- 230000005661 hydrophobic surface Effects 0.000 description 7
- 102000039446 nucleic acids Human genes 0.000 description 7
- 108020004707 nucleic acids Proteins 0.000 description 7
- 150000007523 nucleic acids Chemical class 0.000 description 7
- 239000007787 solid Substances 0.000 description 7
- 210000002700 urine Anatomy 0.000 description 7
- 230000027455 binding Effects 0.000 description 6
- 238000009739 binding Methods 0.000 description 6
- 239000000969 carrier Substances 0.000 description 6
- 239000002131 composite material Substances 0.000 description 6
- 230000007423 decrease Effects 0.000 description 6
- 229920002223 polystyrene Polymers 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 238000001514 detection method Methods 0.000 description 5
- 208000015181 infectious disease Diseases 0.000 description 5
- 238000004519 manufacturing process Methods 0.000 description 5
- 239000011259 mixed solution Substances 0.000 description 5
- 229920003229 poly(methyl methacrylate) Polymers 0.000 description 5
- 239000004926 polymethyl methacrylate Substances 0.000 description 5
- 238000001179 sorption measurement Methods 0.000 description 5
- 102000004890 Interleukin-8 Human genes 0.000 description 4
- 108090001007 Interleukin-8 Proteins 0.000 description 4
- 239000004698 Polyethylene Substances 0.000 description 4
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 4
- 239000011324 bead Substances 0.000 description 4
- 210000004027 cell Anatomy 0.000 description 4
- 239000000919 ceramic Substances 0.000 description 4
- 238000006911 enzymatic reaction Methods 0.000 description 4
- 235000013305 food Nutrition 0.000 description 4
- 238000002347 injection Methods 0.000 description 4
- 239000007924 injection Substances 0.000 description 4
- XKTZWUACRZHVAN-VADRZIEHSA-N interleukin-8 Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@@H](NC(C)=O)CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1[C@H](CCC1)C(=O)N1[C@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC(O)=CC=1)C(=O)N[C@H](CO)C(=O)N1[C@H](CCC1)C(N)=O)C1=CC=CC=C1 XKTZWUACRZHVAN-VADRZIEHSA-N 0.000 description 4
- 229940096397 interleukin-8 Drugs 0.000 description 4
- 238000000465 moulding Methods 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 229920000573 polyethylene Polymers 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 230000000717 retained effect Effects 0.000 description 4
- 239000004925 Acrylic resin Substances 0.000 description 3
- 229920000178 Acrylic resin Polymers 0.000 description 3
- 238000002965 ELISA Methods 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 239000004372 Polyvinyl alcohol Substances 0.000 description 3
- 208000007536 Thrombosis Diseases 0.000 description 3
- LEHOTFFKMJEONL-UHFFFAOYSA-N Uric Acid Chemical compound N1C(=O)NC(=O)C2=C1NC(=O)N2 LEHOTFFKMJEONL-UHFFFAOYSA-N 0.000 description 3
- TVWHNULVHGKJHS-UHFFFAOYSA-N Uric acid Natural products N1C(=O)NC(=O)C2NC(=O)NC21 TVWHNULVHGKJHS-UHFFFAOYSA-N 0.000 description 3
- 239000012472 biological sample Substances 0.000 description 3
- 238000005119 centrifugation Methods 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- 238000004891 communication Methods 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- 238000011049 filling Methods 0.000 description 3
- 239000012530 fluid Substances 0.000 description 3
- 229920001477 hydrophilic polymer Polymers 0.000 description 3
- 230000005660 hydrophilic surface Effects 0.000 description 3
- 230000008105 immune reaction Effects 0.000 description 3
- 230000001771 impaired effect Effects 0.000 description 3
- 238000004020 luminiscence type Methods 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 229910052751 metal Inorganic materials 0.000 description 3
- 239000002184 metal Substances 0.000 description 3
- 229920000306 polymethylpentene Polymers 0.000 description 3
- 239000011116 polymethylpentene Substances 0.000 description 3
- 239000004810 polytetrafluoroethylene Substances 0.000 description 3
- 229920001343 polytetrafluoroethylene Polymers 0.000 description 3
- 229920002451 polyvinyl alcohol Polymers 0.000 description 3
- 102000004169 proteins and genes Human genes 0.000 description 3
- 108090000623 proteins and genes Proteins 0.000 description 3
- 238000004080 punching Methods 0.000 description 3
- 238000011002 quantification Methods 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 230000001629 suppression Effects 0.000 description 3
- 239000004094 surface-active agent Substances 0.000 description 3
- 239000012780 transparent material Substances 0.000 description 3
- 229940116269 uric acid Drugs 0.000 description 3
- RZVAJINKPMORJF-UHFFFAOYSA-N Acetaminophen Chemical compound CC(=O)NC1=CC=C(O)C=C1 RZVAJINKPMORJF-UHFFFAOYSA-N 0.000 description 2
- 229920000936 Agarose Polymers 0.000 description 2
- 108090001008 Avidin Proteins 0.000 description 2
- MHAJPDPJQMAIIY-UHFFFAOYSA-N Hydrogen peroxide Chemical compound OO MHAJPDPJQMAIIY-UHFFFAOYSA-N 0.000 description 2
- VVQNEPGJFQJSBK-UHFFFAOYSA-N Methyl methacrylate Chemical compound COC(=O)C(C)=C VVQNEPGJFQJSBK-UHFFFAOYSA-N 0.000 description 2
- 239000004677 Nylon Substances 0.000 description 2
- CDBYLPFSWZWCQE-UHFFFAOYSA-L Sodium Carbonate Chemical compound [Na+].[Na+].[O-]C([O-])=O CDBYLPFSWZWCQE-UHFFFAOYSA-L 0.000 description 2
- 108010090804 Streptavidin Proteins 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 230000000903 blocking effect Effects 0.000 description 2
- 239000005385 borate glass Substances 0.000 description 2
- 239000005388 borosilicate glass Substances 0.000 description 2
- 238000011088 calibration curve Methods 0.000 description 2
- 239000006285 cell suspension Substances 0.000 description 2
- 210000003850 cellular structure Anatomy 0.000 description 2
- 150000001875 compounds Chemical class 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 229920001577 copolymer Polymers 0.000 description 2
- 239000013078 crystal Substances 0.000 description 2
- 230000003111 delayed effect Effects 0.000 description 2
- 239000003398 denaturant Substances 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 238000005553 drilling Methods 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 230000007613 environmental effect Effects 0.000 description 2
- 238000001914 filtration Methods 0.000 description 2
- 238000001917 fluorescence detection Methods 0.000 description 2
- 239000007850 fluorescent dye Substances 0.000 description 2
- 239000012634 fragment Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 230000002209 hydrophobic effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 238000001746 injection moulding Methods 0.000 description 2
- 229910010272 inorganic material Inorganic materials 0.000 description 2
- 239000011147 inorganic material Substances 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 235000013372 meat Nutrition 0.000 description 2
- 239000011325 microbead Substances 0.000 description 2
- 244000005700 microbiome Species 0.000 description 2
- 229920001778 nylon Polymers 0.000 description 2
- 239000011368 organic material Substances 0.000 description 2
- 229910002077 partially stabilized zirconia Inorganic materials 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 229920000515 polycarbonate Polymers 0.000 description 2
- 239000004417 polycarbonate Substances 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 229920005553 polystyrene-acrylate Polymers 0.000 description 2
- 238000002360 preparation method Methods 0.000 description 2
- 238000012545 processing Methods 0.000 description 2
- 239000005297 pyrex Substances 0.000 description 2
- 239000000941 radioactive substance Substances 0.000 description 2
- 230000000630 rising effect Effects 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000007790 solid phase Substances 0.000 description 2
- 229910001220 stainless steel Inorganic materials 0.000 description 2
- 239000010935 stainless steel Substances 0.000 description 2
- 239000006228 supernatant Substances 0.000 description 2
- 239000000375 suspending agent Substances 0.000 description 2
- 238000009423 ventilation Methods 0.000 description 2
- 229910052727 yttrium Inorganic materials 0.000 description 2
- VWQVUPCCIRVNHF-UHFFFAOYSA-N yttrium atom Chemical compound [Y] VWQVUPCCIRVNHF-UHFFFAOYSA-N 0.000 description 2
- HVCOBJNICQPDBP-UHFFFAOYSA-N 3-[3-[3,5-dihydroxy-6-methyl-4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyoxan-2-yl]oxydecanoyloxy]decanoic acid;hydrate Chemical compound O.OC1C(OC(CC(=O)OC(CCCCCCC)CC(O)=O)CCCCCCC)OC(C)C(O)C1OC1C(O)C(O)C(O)C(C)O1 HVCOBJNICQPDBP-UHFFFAOYSA-N 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 206010018910 Haemolysis Diseases 0.000 description 1
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 1
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 102000001706 Immunoglobulin Fab Fragments Human genes 0.000 description 1
- 108010054477 Immunoglobulin Fab Fragments Proteins 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 229920002873 Polyethylenimine Polymers 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 229920000122 acrylonitrile butadiene styrene Polymers 0.000 description 1
- 238000005273 aeration Methods 0.000 description 1
- 239000013566 allergen Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 238000005452 bending Methods 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 238000000071 blow moulding Methods 0.000 description 1
- 239000000872 buffer Substances 0.000 description 1
- 239000002775 capsule Substances 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 239000006229 carbon black Substances 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 229920002301 cellulose acetate Polymers 0.000 description 1
- 210000001175 cerebrospinal fluid Anatomy 0.000 description 1
- 239000003593 chromogenic compound Substances 0.000 description 1
- 238000003759 clinical diagnosis Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 238000011109 contamination Methods 0.000 description 1
- 239000003431 cross linking reagent Substances 0.000 description 1
- 238000000354 decomposition reaction Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000003745 diagnosis Methods 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 239000004205 dimethyl polysiloxane Substances 0.000 description 1
- KPUWHANPEXNPJT-UHFFFAOYSA-N disiloxane Chemical class [SiH3]O[SiH3] KPUWHANPEXNPJT-UHFFFAOYSA-N 0.000 description 1
- 238000004049 embossing Methods 0.000 description 1
- 238000003891 environmental analysis Methods 0.000 description 1
- 231100000584 environmental toxicity Toxicity 0.000 description 1
- 238000001704 evaporation Methods 0.000 description 1
- 230000008020 evaporation Effects 0.000 description 1
- 239000004744 fabric Substances 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- NBVXSUQYWXRMNV-UHFFFAOYSA-N fluoromethane Chemical compound FC NBVXSUQYWXRMNV-UHFFFAOYSA-N 0.000 description 1
- 230000008588 hemolysis Effects 0.000 description 1
- 238000009396 hybridization Methods 0.000 description 1
- 230000028993 immune response Effects 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 239000003547 immunosorbent Substances 0.000 description 1
- 230000008676 import Effects 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 238000007689 inspection Methods 0.000 description 1
- 238000009434 installation Methods 0.000 description 1
- 239000011810 insulating material Substances 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 230000001678 irradiating effect Effects 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 230000005499 meniscus Effects 0.000 description 1
- 150000002739 metals Chemical class 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000010979 pH adjustment Methods 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 210000004180 plasmocyte Anatomy 0.000 description 1
- 229920000435 poly(dimethylsiloxane) Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 239000002861 polymer material Substances 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 239000013615 primer Substances 0.000 description 1
- 239000002987 primer (paints) Substances 0.000 description 1
- 239000012460 protein solution Substances 0.000 description 1
- 239000012429 reaction media Substances 0.000 description 1
- 230000035484 reaction time Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 230000002940 repellent Effects 0.000 description 1
- 239000005871 repellent Substances 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 210000003296 saliva Anatomy 0.000 description 1
- 238000004062 sedimentation Methods 0.000 description 1
- 239000005368 silicate glass Substances 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- 229920002050 silicone resin Polymers 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 239000002689 soil Substances 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 239000003381 stabilizer Substances 0.000 description 1
- 238000003756 stirring Methods 0.000 description 1
- 239000012089 stop solution Substances 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
Images
Landscapes
- Automatic Analysis And Handling Materials Therefor (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2009118440A JP5504690B2 (ja) | 2008-05-15 | 2009-05-15 | 分析チップ |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2008128723 | 2008-05-15 | ||
| JP2008128723 | 2008-05-15 | ||
| JP2009118440A JP5504690B2 (ja) | 2008-05-15 | 2009-05-15 | 分析チップ |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009300433A JP2009300433A (ja) | 2009-12-24 |
| JP2009300433A5 JP2009300433A5 (enExample) | 2012-06-28 |
| JP5504690B2 true JP5504690B2 (ja) | 2014-05-28 |
Family
ID=41547460
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009118440A Active JP5504690B2 (ja) | 2008-05-15 | 2009-05-15 | 分析チップ |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP5504690B2 (enExample) |
Families Citing this family (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5862006B2 (ja) * | 2010-11-17 | 2016-02-16 | セイコーエプソン株式会社 | 熱サイクル装置及び熱サイクル方法 |
| JP5951219B2 (ja) * | 2011-10-24 | 2016-07-13 | ローム株式会社 | 液体試薬内蔵型マイクロチップ |
| US10197480B2 (en) | 2012-11-07 | 2019-02-05 | Sandstone Diagnostics, Inc. | Methods and devices for processing samples and counting cells |
| JP6590697B2 (ja) | 2012-11-07 | 2019-10-16 | サンドストーン ダイアグノスティックス インコーポレイテッドSandstone Diagnostics,Inc. | サンプル処理及び細胞計数のための方法及びデバイス |
| AU2014214886B2 (en) * | 2013-02-07 | 2017-09-07 | Laboratory Corporation Of America Holdings | Automated sample processing, fluid distribution, and sedimentation assay |
| JP6028624B2 (ja) * | 2013-02-28 | 2016-11-16 | ブラザー工業株式会社 | 検査チップ及び検査システム |
| JP6244589B2 (ja) * | 2013-05-21 | 2017-12-13 | 国立大学法人名古屋大学 | 微粒子分離用マイクロ流路チップ、移流集積ユニット、微粒子分離用システム及び微粒子分離方法 |
| JP2017122600A (ja) * | 2016-01-05 | 2017-07-13 | 富士電機株式会社 | マイクロチップ及びマイクロチップの製造方法 |
| JP6759841B2 (ja) | 2016-08-15 | 2020-09-23 | 住友ゴム工業株式会社 | マイクロ流路チップ |
| EP3521830B1 (en) | 2016-09-29 | 2025-04-23 | Green Cross Medical Science | Separable cassette for measuring glycated hemoglobin |
| JP6799308B2 (ja) * | 2016-09-30 | 2020-12-16 | 株式会社アイビー | 液体試料搬送方法および試薬チップ |
| KR102398283B1 (ko) * | 2016-11-04 | 2022-05-17 | 주식회사 녹십자엠에스 | 당화혈색소 비율의 측정 방법 |
| CN108872081B (zh) * | 2018-09-04 | 2023-06-23 | 重庆科技学院 | 一种检测重金属离子的多层微流控芯片 |
| CN108704684B (zh) * | 2018-09-04 | 2023-06-06 | 重庆科技学院 | 一种检测用多层微流控芯片的使用方法 |
| CN109669037B (zh) * | 2019-02-28 | 2024-03-15 | 深圳市易瑞生物技术股份有限公司 | 一种分离式多通道层析装置 |
| CN115516311A (zh) * | 2020-12-01 | 2022-12-23 | 王锦弘 | 离心式反应装置和离心式反应方法 |
| CN117129715B (zh) * | 2023-06-30 | 2024-05-10 | 盛吉盛智能装备(江苏)有限公司 | 用于芯片测试的浮动装置 |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3134560A1 (de) * | 1981-09-01 | 1983-03-17 | Boehringer Mannheim Gmbh, 6800 Mannheim | Vorrichtung und verfahren zum steuern und mischen einer der zentrifugalkraft ausgesetzten fluessigkeitsstroemung |
| US5639428A (en) * | 1994-07-19 | 1997-06-17 | Becton Dickinson And Company | Method and apparatus for fully automated nucleic acid amplification, nucleic acid assay and immunoassay |
| GB9620278D0 (en) * | 1996-09-28 | 1996-11-13 | Central Research Lab Ltd | Apparatus for chemical analysis |
| WO2003083491A2 (en) * | 2002-03-25 | 2003-10-09 | Centrifluidics, Inc. | Method and apparatus for controlling fluid movement in a microfluidic system |
| JP2006105638A (ja) * | 2004-10-01 | 2006-04-20 | Hitachi High-Technologies Corp | 化学分析装置 |
-
2009
- 2009-05-15 JP JP2009118440A patent/JP5504690B2/ja active Active
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009300433A (ja) | 2009-12-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5504690B2 (ja) | 分析チップ | |
| JP5636629B2 (ja) | 送液チップおよび分析方法 | |
| CN102472739B (zh) | 离心式微流装置和用于检测来自液体样本的分析物的方法 | |
| CN101437616B (zh) | 用于化学、生物化学、生物和物理分析、反应、测定等的装置和方法 | |
| CN105353159B (zh) | 微流体检验装置及其运作方法 | |
| CN1864058B (zh) | 芯片的使用方法及检查芯片 | |
| EP1946830B1 (en) | Microreactor | |
| AU2004202662B2 (en) | Reducing working fluid dilution in liquid systems | |
| US8858897B2 (en) | Microfluidic chip for analysis for fluid sample | |
| EP2285491A1 (en) | Flow control in microfluidic systems | |
| JP5716276B2 (ja) | 分離チップおよび分離方法 | |
| CN105015200A (zh) | 基于纳米印章固定单抗修饰层的光学微流控芯片 | |
| CN119608256B (zh) | 微流控芯片的检测方法及血浆分离导流一体式微流控芯片 | |
| CN108716938B (zh) | 一种液体定量装置及其应用 | |
| JP5125680B2 (ja) | 分離チップおよび分離方法 | |
| JP2019113460A (ja) | 検査デバイス | |
| JP5740650B2 (ja) | 円盤型マイクロ流体チップ及びそれを用いた測定システム | |
| JP5407150B2 (ja) | 免疫分析方法 | |
| RU200301U1 (ru) | Микрофлюидный чип для проведения многопараметрического иммуноанализа | |
| JP4935424B2 (ja) | 免疫分析チップ | |
| JP2008268194A (ja) | 分析方法 | |
| Nelson | Design principles for microfluidic biomedical diagnostics in space |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120511 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20120511 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20130321 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20131203 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140128 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20140218 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20140303 |
|
| R151 | Written notification of patent or utility model registration |
Ref document number: 5504690 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R151 |