JP5425618B2 - 成熟樹状細胞組成物およびそれを培養するための方法 - Google Patents
成熟樹状細胞組成物およびそれを培養するための方法 Download PDFInfo
- Publication number
- JP5425618B2 JP5425618B2 JP2009504346A JP2009504346A JP5425618B2 JP 5425618 B2 JP5425618 B2 JP 5425618B2 JP 2009504346 A JP2009504346 A JP 2009504346A JP 2009504346 A JP2009504346 A JP 2009504346A JP 5425618 B2 JP5425618 B2 JP 5425618B2
- Authority
- JP
- Japan
- Prior art keywords
- cells
- cd40l
- ifn
- rna
- cell
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 210000004443 dendritic cell Anatomy 0.000 title claims abstract description 564
- 238000000034 method Methods 0.000 title claims abstract description 209
- 239000000203 mixture Substances 0.000 title description 35
- 238000012258 culturing Methods 0.000 title description 10
- 108010029697 CD40 Ligand Proteins 0.000 claims abstract description 370
- 102100032937 CD40 ligand Human genes 0.000 claims abstract description 370
- 210000004027 cell Anatomy 0.000 claims abstract description 321
- 239000000556 agonist Substances 0.000 claims abstract description 57
- 230000011664 signaling Effects 0.000 claims abstract description 49
- 102100036301 C-C chemokine receptor type 7 Human genes 0.000 claims abstract description 43
- 101000716065 Homo sapiens C-C chemokine receptor type 7 Proteins 0.000 claims abstract description 43
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 claims abstract description 41
- 108060008682 Tumor Necrosis Factor Proteins 0.000 claims abstract description 40
- 108010085650 interferon gamma receptor Proteins 0.000 claims abstract description 39
- 108091032973 (ribonucleotides)n+m Proteins 0.000 claims description 177
- 108091007433 antigens Proteins 0.000 claims description 161
- 102000036639 antigens Human genes 0.000 claims description 161
- 239000000427 antigen Substances 0.000 claims description 160
- 108010074328 Interferon-gamma Proteins 0.000 claims description 121
- 102100037850 Interferon gamma Human genes 0.000 claims description 120
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 93
- 238000000338 in vitro Methods 0.000 claims description 61
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 claims description 40
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 claims description 36
- 102100039620 Granulocyte-macrophage colony-stimulating factor Human genes 0.000 claims description 36
- 125000000539 amino acid group Chemical group 0.000 claims description 36
- VQFKFAKEUMHBLV-BYSUZVQFSA-N 1-O-(alpha-D-galactosyl)-N-hexacosanoylphytosphingosine Chemical group CCCCCCCCCCCCCCCCCCCCCCCCCC(=O)N[C@H]([C@H](O)[C@H](O)CCCCCCCCCCCCCC)CO[C@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O VQFKFAKEUMHBLV-BYSUZVQFSA-N 0.000 claims description 33
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 claims description 31
- 108090000978 Interleukin-4 Proteins 0.000 claims description 30
- 108020004705 Codon Proteins 0.000 claims description 26
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 claims description 23
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 claims description 23
- 239000002299 complementary DNA Substances 0.000 claims description 22
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 claims description 17
- 229930182817 methionine Natural products 0.000 claims description 16
- 150000001875 compounds Chemical class 0.000 claims description 13
- 229960005486 vaccine Drugs 0.000 claims description 11
- 210000003162 effector t lymphocyte Anatomy 0.000 claims description 10
- 210000003071 memory t lymphocyte Anatomy 0.000 claims description 7
- DDOVBCWVTOHGCU-QMXMISKISA-N n-[(e,2s,3r)-3-hydroxy-1-[(2r,3r,4s,5r,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxynonadec-4-en-2-yl]octadecanamide Chemical compound CCCCCCCCCCCCCCCCCC(=O)N[C@H]([C@H](O)\C=C\CCCCCCCCCCCCCC)CO[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O DDOVBCWVTOHGCU-QMXMISKISA-N 0.000 claims description 7
- 102000003816 Interleukin-13 Human genes 0.000 claims description 6
- 108090000176 Interleukin-13 Proteins 0.000 claims description 6
- MYYARUCLXARAEE-ZATZPJRKSA-N alpha-C-GalCer Chemical compound CCCCCCCCCCCCCCCCCCCCCCCCCCC(=O)N[C@@H](CC[C@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O)[C@H](O)[C@H](O)CCCCCCCCCCCCCCCCC MYYARUCLXARAEE-ZATZPJRKSA-N 0.000 claims description 3
- HOMYIYLRRDTKAA-UHFFFAOYSA-N 2-hydroxy-N-[3-hydroxy-1-[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoctadeca-4,8-dien-2-yl]hexadecanamide Chemical compound CCCCCCCCCCCCCCC(O)C(=O)NC(C(O)C=CCCC=CCCCCCCCCC)COC1OC(CO)C(O)C(O)C1O HOMYIYLRRDTKAA-UHFFFAOYSA-N 0.000 claims description 2
- 241001385733 Aesculus indica Species 0.000 claims 1
- 108020004999 messenger RNA Proteins 0.000 abstract description 172
- 108090000765 processed proteins & peptides Proteins 0.000 abstract description 142
- 102000004196 processed proteins & peptides Human genes 0.000 abstract description 96
- 229920001184 polypeptide Polymers 0.000 abstract description 76
- 102000040430 polynucleotide Human genes 0.000 abstract description 72
- 108091033319 polynucleotide Proteins 0.000 abstract description 72
- 239000002157 polynucleotide Substances 0.000 abstract description 72
- 230000008569 process Effects 0.000 abstract description 57
- 102000013462 Interleukin-12 Human genes 0.000 abstract description 48
- 108010065805 Interleukin-12 Proteins 0.000 abstract description 48
- 229940123189 CD40 agonist Drugs 0.000 abstract description 35
- 230000028993 immune response Effects 0.000 abstract description 35
- 102000003814 Interleukin-10 Human genes 0.000 abstract description 33
- 108090000174 Interleukin-10 Proteins 0.000 abstract description 33
- 230000028327 secretion Effects 0.000 abstract description 33
- 101150013553 CD40 gene Proteins 0.000 abstract description 32
- 102000005962 receptors Human genes 0.000 abstract description 24
- 108020003175 receptors Proteins 0.000 abstract description 24
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 abstract description 23
- 230000002163 immunogen Effects 0.000 abstract description 19
- 230000001965 increasing effect Effects 0.000 abstract description 15
- 230000001052 transient effect Effects 0.000 abstract description 10
- 230000002708 enhancing effect Effects 0.000 abstract description 3
- 230000003308 immunostimulating effect Effects 0.000 abstract description 3
- 230000001270 agonistic effect Effects 0.000 abstract 1
- 230000003247 decreasing effect Effects 0.000 abstract 1
- 108090000623 proteins and genes Proteins 0.000 description 131
- 230000014509 gene expression Effects 0.000 description 90
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 82
- 108090000695 Cytokines Proteins 0.000 description 74
- 102000004127 Cytokines Human genes 0.000 description 73
- 102000004169 proteins and genes Human genes 0.000 description 67
- 235000018102 proteins Nutrition 0.000 description 64
- 150000007523 nucleic acids Chemical class 0.000 description 52
- 238000013519 translation Methods 0.000 description 50
- 102000039446 nucleic acids Human genes 0.000 description 48
- 108020004707 nucleic acids Proteins 0.000 description 48
- 238000001890 transfection Methods 0.000 description 47
- 239000013598 vector Substances 0.000 description 46
- 108020005345 3' Untranslated Regions Proteins 0.000 description 43
- 229940117681 interleukin-12 Drugs 0.000 description 43
- 125000003729 nucleotide group Chemical group 0.000 description 42
- 230000035800 maturation Effects 0.000 description 41
- 239000002609 medium Substances 0.000 description 37
- 235000001014 amino acid Nutrition 0.000 description 35
- 239000012634 fragment Substances 0.000 description 35
- 239000002773 nucleotide Substances 0.000 description 35
- 229940024606 amino acid Drugs 0.000 description 34
- 150000001413 amino acids Chemical class 0.000 description 34
- 101000578784 Homo sapiens Melanoma antigen recognized by T-cells 1 Proteins 0.000 description 33
- 102100028389 Melanoma antigen recognized by T-cells 1 Human genes 0.000 description 33
- 206010028980 Neoplasm Diseases 0.000 description 32
- 239000013612 plasmid Substances 0.000 description 32
- 108010002350 Interleukin-2 Proteins 0.000 description 31
- 102000000588 Interleukin-2 Human genes 0.000 description 31
- 230000004044 response Effects 0.000 description 31
- 108020004414 DNA Proteins 0.000 description 30
- 238000001727 in vivo Methods 0.000 description 30
- 102000004388 Interleukin-4 Human genes 0.000 description 29
- 238000004520 electroporation Methods 0.000 description 28
- 102100034922 T-cell surface glycoprotein CD8 alpha chain Human genes 0.000 description 26
- 230000000694 effects Effects 0.000 description 26
- 229940028885 interleukin-4 Drugs 0.000 description 25
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 24
- 238000013518 transcription Methods 0.000 description 24
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 23
- 230000006870 function Effects 0.000 description 22
- 230000006698 induction Effects 0.000 description 22
- 238000004519 manufacturing process Methods 0.000 description 22
- 210000000130 stem cell Anatomy 0.000 description 21
- 210000000612 antigen-presenting cell Anatomy 0.000 description 20
- 230000000139 costimulatory effect Effects 0.000 description 20
- 230000035897 transcription Effects 0.000 description 20
- 238000003556 assay Methods 0.000 description 19
- 238000009396 hybridization Methods 0.000 description 19
- 239000003446 ligand Substances 0.000 description 19
- 238000010186 staining Methods 0.000 description 19
- 239000000126 substance Substances 0.000 description 19
- 108020003589 5' Untranslated Regions Proteins 0.000 description 18
- 238000004458 analytical method Methods 0.000 description 18
- 201000011510 cancer Diseases 0.000 description 18
- 238000006243 chemical reaction Methods 0.000 description 18
- 239000012636 effector Substances 0.000 description 18
- 230000003834 intracellular effect Effects 0.000 description 18
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 18
- 239000000047 product Substances 0.000 description 18
- 239000000523 sample Substances 0.000 description 18
- 238000007792 addition Methods 0.000 description 17
- 125000003275 alpha amino acid group Chemical group 0.000 description 17
- 239000000872 buffer Substances 0.000 description 17
- 102000004190 Enzymes Human genes 0.000 description 16
- 108090000790 Enzymes Proteins 0.000 description 16
- 229940088598 enzyme Drugs 0.000 description 16
- 210000001616 monocyte Anatomy 0.000 description 16
- 210000000581 natural killer T-cell Anatomy 0.000 description 16
- 238000002965 ELISA Methods 0.000 description 15
- 102100022087 Granzyme M Human genes 0.000 description 15
- 101000900697 Homo sapiens Granzyme M Proteins 0.000 description 15
- 108090001005 Interleukin-6 Proteins 0.000 description 15
- 102000004889 Interleukin-6 Human genes 0.000 description 15
- 108091081024 Start codon Proteins 0.000 description 15
- 210000004369 blood Anatomy 0.000 description 15
- 239000008280 blood Substances 0.000 description 15
- 238000003501 co-culture Methods 0.000 description 15
- 230000000875 corresponding effect Effects 0.000 description 15
- 239000012642 immune effector Substances 0.000 description 15
- 229940121354 immunomodulator Drugs 0.000 description 15
- 229940100601 interleukin-6 Drugs 0.000 description 15
- 230000036039 immunity Effects 0.000 description 14
- 210000001519 tissue Anatomy 0.000 description 14
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 13
- 241000725303 Human immunodeficiency virus Species 0.000 description 13
- -1 antibody Substances 0.000 description 13
- 230000032258 transport Effects 0.000 description 13
- 108091028043 Nucleic acid sequence Proteins 0.000 description 12
- 241000702670 Rotavirus Species 0.000 description 12
- 241000700605 Viruses Species 0.000 description 12
- 206010022000 influenza Diseases 0.000 description 12
- 241000699666 Mus <mouse, genus> Species 0.000 description 11
- 208000006265 Renal cell carcinoma Diseases 0.000 description 11
- 230000001939 inductive effect Effects 0.000 description 11
- 239000000243 solution Substances 0.000 description 11
- 101150003479 Parg gene Proteins 0.000 description 10
- 239000006228 supernatant Substances 0.000 description 10
- 108091026890 Coding region Proteins 0.000 description 9
- 108010029485 Protein Isoforms Proteins 0.000 description 9
- 102000001708 Protein Isoforms Human genes 0.000 description 9
- 108010092262 T-Cell Antigen Receptors Proteins 0.000 description 9
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 description 9
- 230000000295 complement effect Effects 0.000 description 9
- 238000012217 deletion Methods 0.000 description 9
- 230000037430 deletion Effects 0.000 description 9
- 239000012091 fetal bovine serum Substances 0.000 description 9
- 238000003018 immunoassay Methods 0.000 description 9
- 238000011534 incubation Methods 0.000 description 9
- 230000003993 interaction Effects 0.000 description 9
- 238000002955 isolation Methods 0.000 description 9
- 238000002360 preparation method Methods 0.000 description 9
- 230000000638 stimulation Effects 0.000 description 9
- 102100031573 Hematopoietic progenitor cell antigen CD34 Human genes 0.000 description 8
- 101000777663 Homo sapiens Hematopoietic progenitor cell antigen CD34 Proteins 0.000 description 8
- 230000003321 amplification Effects 0.000 description 8
- 210000003719 b-lymphocyte Anatomy 0.000 description 8
- 230000001413 cellular effect Effects 0.000 description 8
- 238000002474 experimental method Methods 0.000 description 8
- 210000002443 helper t lymphocyte Anatomy 0.000 description 8
- 239000012528 membrane Substances 0.000 description 8
- 230000004048 modification Effects 0.000 description 8
- 238000012986 modification Methods 0.000 description 8
- 239000013642 negative control Substances 0.000 description 8
- 238000003199 nucleic acid amplification method Methods 0.000 description 8
- 244000052769 pathogen Species 0.000 description 8
- 239000000018 receptor agonist Substances 0.000 description 8
- 229940044601 receptor agonist Drugs 0.000 description 8
- 238000006467 substitution reaction Methods 0.000 description 8
- 238000012546 transfer Methods 0.000 description 8
- 239000003981 vehicle Substances 0.000 description 8
- 230000003612 virological effect Effects 0.000 description 8
- 102000025850 HLA-A2 Antigen Human genes 0.000 description 7
- 108010074032 HLA-A2 Antigen Proteins 0.000 description 7
- 101100059511 Homo sapiens CD40LG gene Proteins 0.000 description 7
- 101000914484 Homo sapiens T-lymphocyte activation antigen CD80 Proteins 0.000 description 7
- 241000124008 Mammalia Species 0.000 description 7
- 241001529936 Murinae Species 0.000 description 7
- 102100027222 T-lymphocyte activation antigen CD80 Human genes 0.000 description 7
- 230000004913 activation Effects 0.000 description 7
- 238000001514 detection method Methods 0.000 description 7
- 230000004069 differentiation Effects 0.000 description 7
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 7
- 208000015181 infectious disease Diseases 0.000 description 7
- 239000003550 marker Substances 0.000 description 7
- 230000035772 mutation Effects 0.000 description 7
- 238000011282 treatment Methods 0.000 description 7
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 6
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 6
- 102000004269 Granulocyte Colony-Stimulating Factor Human genes 0.000 description 6
- 101000713106 Homo sapiens C-C motif chemokine 19 Proteins 0.000 description 6
- 101000599940 Homo sapiens Interferon gamma Proteins 0.000 description 6
- 101000654734 Homo sapiens Septin-4 Proteins 0.000 description 6
- 102100032743 Septin-4 Human genes 0.000 description 6
- 230000000903 blocking effect Effects 0.000 description 6
- 230000010261 cell growth Effects 0.000 description 6
- 238000005119 centrifugation Methods 0.000 description 6
- 239000003153 chemical reaction reagent Substances 0.000 description 6
- 239000003937 drug carrier Substances 0.000 description 6
- 239000003623 enhancer Substances 0.000 description 6
- 238000000684 flow cytometry Methods 0.000 description 6
- 230000012010 growth Effects 0.000 description 6
- 230000005847 immunogenicity Effects 0.000 description 6
- 239000002502 liposome Substances 0.000 description 6
- 230000005012 migration Effects 0.000 description 6
- 238000013508 migration Methods 0.000 description 6
- 230000001717 pathogenic effect Effects 0.000 description 6
- 210000005259 peripheral blood Anatomy 0.000 description 6
- 239000011886 peripheral blood Substances 0.000 description 6
- 230000008488 polyadenylation Effects 0.000 description 6
- 230000035755 proliferation Effects 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 6
- 238000011830 transgenic mouse model Methods 0.000 description 6
- 238000001262 western blot Methods 0.000 description 6
- 241000180579 Arca Species 0.000 description 5
- 238000011510 Elispot assay Methods 0.000 description 5
- 241001465754 Metazoa Species 0.000 description 5
- 241000699660 Mus musculus Species 0.000 description 5
- 108010004729 Phycoerythrin Proteins 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- 230000000890 antigenic effect Effects 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 230000004071 biological effect Effects 0.000 description 5
- 238000004113 cell culture Methods 0.000 description 5
- 230000008859 change Effects 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 239000012228 culture supernatant Substances 0.000 description 5
- 201000010099 disease Diseases 0.000 description 5
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 5
- 238000005516 engineering process Methods 0.000 description 5
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 5
- 239000013604 expression vector Substances 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 5
- 230000000977 initiatory effect Effects 0.000 description 5
- 230000007774 longterm Effects 0.000 description 5
- 210000001165 lymph node Anatomy 0.000 description 5
- 210000002540 macrophage Anatomy 0.000 description 5
- 210000004962 mammalian cell Anatomy 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 230000001404 mediated effect Effects 0.000 description 5
- 229930014626 natural product Natural products 0.000 description 5
- 102000002574 p38 Mitogen-Activated Protein Kinases Human genes 0.000 description 5
- 108010068338 p38 Mitogen-Activated Protein Kinases Proteins 0.000 description 5
- 238000010188 recombinant method Methods 0.000 description 5
- 230000014621 translational initiation Effects 0.000 description 5
- 241000701161 unidentified adenovirus Species 0.000 description 5
- 241001430294 unidentified retrovirus Species 0.000 description 5
- 239000013603 viral vector Substances 0.000 description 5
- DGVVWUTYPXICAM-UHFFFAOYSA-N β‐Mercaptoethanol Chemical compound OCCS DGVVWUTYPXICAM-UHFFFAOYSA-N 0.000 description 5
- 102000007469 Actins Human genes 0.000 description 4
- 108010085238 Actins Proteins 0.000 description 4
- 101000605172 Aspergillus niger (strain CBS 513.88 / FGSC A1513) Probable endopolygalacturonase E Proteins 0.000 description 4
- 101000605171 Aspergillus niger Endopolygalacturonase E Proteins 0.000 description 4
- 101000941281 Bos taurus Gastric triacylglycerol lipase Proteins 0.000 description 4
- 241000282693 Cercopithecidae Species 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 102000008949 Histocompatibility Antigens Class I Human genes 0.000 description 4
- 241000282412 Homo Species 0.000 description 4
- 101000756632 Homo sapiens Actin, cytoplasmic 1 Proteins 0.000 description 4
- 101000899111 Homo sapiens Hemoglobin subunit beta Proteins 0.000 description 4
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 4
- 102000000440 Melanoma-associated antigen Human genes 0.000 description 4
- 108050008953 Melanoma-associated antigen Proteins 0.000 description 4
- 241000713896 Spleen necrosis virus Species 0.000 description 4
- 230000001464 adherent effect Effects 0.000 description 4
- 239000002671 adjuvant Substances 0.000 description 4
- 230000030741 antigen processing and presentation Effects 0.000 description 4
- 229910002091 carbon monoxide Inorganic materials 0.000 description 4
- 210000000349 chromosome Anatomy 0.000 description 4
- 238000010924 continuous production Methods 0.000 description 4
- 230000002596 correlated effect Effects 0.000 description 4
- 231100000433 cytotoxic Toxicity 0.000 description 4
- 230000001472 cytotoxic effect Effects 0.000 description 4
- 239000005547 deoxyribonucleotide Substances 0.000 description 4
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 4
- 230000001419 dependent effect Effects 0.000 description 4
- 238000004090 dissolution Methods 0.000 description 4
- 239000012894 fetal calf serum Substances 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 238000003780 insertion Methods 0.000 description 4
- 230000037431 insertion Effects 0.000 description 4
- 229940076144 interleukin-10 Drugs 0.000 description 4
- 238000010212 intracellular staining Methods 0.000 description 4
- 238000001990 intravenous administration Methods 0.000 description 4
- 210000000265 leukocyte Anatomy 0.000 description 4
- 230000002101 lytic effect Effects 0.000 description 4
- 238000010232 migration assay Methods 0.000 description 4
- 230000026731 phosphorylation Effects 0.000 description 4
- 238000006366 phosphorylation reaction Methods 0.000 description 4
- 239000002243 precursor Substances 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 230000002829 reductive effect Effects 0.000 description 4
- 230000010076 replication Effects 0.000 description 4
- 108091008146 restriction endonucleases Proteins 0.000 description 4
- 230000001177 retroviral effect Effects 0.000 description 4
- 238000012552 review Methods 0.000 description 4
- 230000003248 secreting effect Effects 0.000 description 4
- 210000002966 serum Anatomy 0.000 description 4
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 238000010257 thawing Methods 0.000 description 4
- 238000002560 therapeutic procedure Methods 0.000 description 4
- 238000005406 washing Methods 0.000 description 4
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 3
- 102100021663 Baculoviral IAP repeat-containing protein 5 Human genes 0.000 description 3
- 241000283690 Bos taurus Species 0.000 description 3
- 241000282472 Canis lupus familiaris Species 0.000 description 3
- 102100024423 Carbonic anhydrase 9 Human genes 0.000 description 3
- 201000009030 Carcinoma Diseases 0.000 description 3
- 150000008574 D-amino acids Chemical class 0.000 description 3
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 3
- 241000702421 Dependoparvovirus Species 0.000 description 3
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 3
- 108010088652 Histocompatibility Antigens Class I Proteins 0.000 description 3
- 101100165850 Homo sapiens CA9 gene Proteins 0.000 description 3
- 101100220044 Homo sapiens CD34 gene Proteins 0.000 description 3
- 101100099884 Homo sapiens CD40 gene Proteins 0.000 description 3
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 3
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 3
- 108020005350 Initiator Codon Proteins 0.000 description 3
- 102000014154 Interleukin-12 Subunit p35 Human genes 0.000 description 3
- 108010011301 Interleukin-12 Subunit p35 Proteins 0.000 description 3
- 108010002386 Interleukin-3 Proteins 0.000 description 3
- 102000000646 Interleukin-3 Human genes 0.000 description 3
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 3
- 101000808007 Mus musculus Vascular endothelial growth factor A Proteins 0.000 description 3
- 241000699670 Mus sp. Species 0.000 description 3
- 108020004711 Nucleic Acid Probes Proteins 0.000 description 3
- 108700026244 Open Reading Frames Proteins 0.000 description 3
- 229930040373 Paraformaldehyde Natural products 0.000 description 3
- 241001494479 Pecora Species 0.000 description 3
- 102000035195 Peptidases Human genes 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- 102000003992 Peroxidases Human genes 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 239000012980 RPMI-1640 medium Substances 0.000 description 3
- 108091028664 Ribonucleotide Proteins 0.000 description 3
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 3
- 108010044012 STAT1 Transcription Factor Proteins 0.000 description 3
- 102000006381 STAT1 Transcription Factor Human genes 0.000 description 3
- 238000012300 Sequence Analysis Methods 0.000 description 3
- 241000702677 Simian rotavirus Species 0.000 description 3
- 108010002687 Survivin Proteins 0.000 description 3
- 230000006044 T cell activation Effects 0.000 description 3
- 230000005867 T cell response Effects 0.000 description 3
- 241000723792 Tobacco etch virus Species 0.000 description 3
- 230000000735 allogeneic effect Effects 0.000 description 3
- 210000004102 animal cell Anatomy 0.000 description 3
- 230000001580 bacterial effect Effects 0.000 description 3
- 238000002659 cell therapy Methods 0.000 description 3
- 238000010367 cloning Methods 0.000 description 3
- 230000016396 cytokine production Effects 0.000 description 3
- 230000009089 cytolysis Effects 0.000 description 3
- 231100000599 cytotoxic agent Toxicity 0.000 description 3
- 210000003527 eukaryotic cell Anatomy 0.000 description 3
- 108020001507 fusion proteins Proteins 0.000 description 3
- 102000037865 fusion proteins Human genes 0.000 description 3
- 238000001476 gene delivery Methods 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 238000004128 high performance liquid chromatography Methods 0.000 description 3
- 102000057041 human TNF Human genes 0.000 description 3
- 210000005260 human cell Anatomy 0.000 description 3
- 239000001257 hydrogen Substances 0.000 description 3
- 229910052739 hydrogen Inorganic materials 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 238000009169 immunotherapy Methods 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 239000000411 inducer Substances 0.000 description 3
- 238000001802 infusion Methods 0.000 description 3
- 230000010354 integration Effects 0.000 description 3
- 229940076264 interleukin-3 Drugs 0.000 description 3
- 238000011068 loading method Methods 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 201000001441 melanoma Diseases 0.000 description 3
- 210000005170 neoplastic cell Anatomy 0.000 description 3
- 239000002853 nucleic acid probe Substances 0.000 description 3
- 229920002866 paraformaldehyde Polymers 0.000 description 3
- 239000002245 particle Substances 0.000 description 3
- 239000000816 peptidomimetic Substances 0.000 description 3
- 108040007629 peroxidase activity proteins Proteins 0.000 description 3
- 239000008363 phosphate buffer Substances 0.000 description 3
- 239000013641 positive control Substances 0.000 description 3
- 230000003389 potentiating effect Effects 0.000 description 3
- 239000003755 preservative agent Substances 0.000 description 3
- 230000006337 proteolytic cleavage Effects 0.000 description 3
- 239000001397 quillaja saponaria molina bark Substances 0.000 description 3
- 230000002285 radioactive effect Effects 0.000 description 3
- 238000003127 radioimmunoassay Methods 0.000 description 3
- 238000011084 recovery Methods 0.000 description 3
- 239000002336 ribonucleotide Substances 0.000 description 3
- 125000002652 ribonucleotide group Chemical group 0.000 description 3
- 229930182490 saponin Natural products 0.000 description 3
- 150000007949 saponins Chemical class 0.000 description 3
- 238000000926 separation method Methods 0.000 description 3
- 238000002741 site-directed mutagenesis Methods 0.000 description 3
- 239000011734 sodium Substances 0.000 description 3
- 239000003381 stabilizer Substances 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 239000000758 substrate Substances 0.000 description 3
- 230000001225 therapeutic effect Effects 0.000 description 3
- 230000005026 transcription initiation Effects 0.000 description 3
- 238000010361 transduction Methods 0.000 description 3
- 230000026683 transduction Effects 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- 101800001779 2'-O-methyltransferase Proteins 0.000 description 2
- KDCGOANMDULRCW-UHFFFAOYSA-N 7H-purine Chemical compound N1=CNC2=NC=NC2=C1 KDCGOANMDULRCW-UHFFFAOYSA-N 0.000 description 2
- 241000710929 Alphavirus Species 0.000 description 2
- 108091023037 Aptamer Proteins 0.000 description 2
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 2
- 241000972773 Aulopiformes Species 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 102100036846 C-C motif chemokine 21 Human genes 0.000 description 2
- 108010032795 CD8 receptor Proteins 0.000 description 2
- 101100507655 Canis lupus familiaris HSPA1 gene Proteins 0.000 description 2
- 108090000994 Catalytic RNA Proteins 0.000 description 2
- 102000053642 Catalytic RNA Human genes 0.000 description 2
- 108010012236 Chemokines Proteins 0.000 description 2
- 102000019034 Chemokines Human genes 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 108090000317 Chymotrypsin Proteins 0.000 description 2
- 241000699800 Cricetinae Species 0.000 description 2
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 2
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 2
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- 229920001917 Ficoll Polymers 0.000 description 2
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 2
- 241000287828 Gallus gallus Species 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 102000016355 Granulocyte-Macrophage Colony-Stimulating Factor Receptors Human genes 0.000 description 2
- 108010092372 Granulocyte-Macrophage Colony-Stimulating Factor Receptors Proteins 0.000 description 2
- 102000006354 HLA-DR Antigens Human genes 0.000 description 2
- 108010058597 HLA-DR Antigens Proteins 0.000 description 2
- 101000713085 Homo sapiens C-C motif chemokine 21 Proteins 0.000 description 2
- 101000746373 Homo sapiens Granulocyte-macrophage colony-stimulating factor Proteins 0.000 description 2
- 101001009007 Homo sapiens Hemoglobin subunit alpha Proteins 0.000 description 2
- 101000599852 Homo sapiens Intercellular adhesion molecule 1 Proteins 0.000 description 2
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 2
- 102100037877 Intercellular adhesion molecule 1 Human genes 0.000 description 2
- 102100035678 Interferon gamma receptor 1 Human genes 0.000 description 2
- 108020004684 Internal Ribosome Entry Sites Proteins 0.000 description 2
- 150000008575 L-amino acids Chemical class 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- 102000004058 Leukemia inhibitory factor Human genes 0.000 description 2
- 108090000581 Leukemia inhibitory factor Proteins 0.000 description 2
- 108091054437 MHC class I family Proteins 0.000 description 2
- 241000282560 Macaca mulatta Species 0.000 description 2
- 238000000636 Northern blotting Methods 0.000 description 2
- 101710163270 Nuclease Proteins 0.000 description 2
- 108091005461 Nucleic proteins Proteins 0.000 description 2
- 108091034117 Oligonucleotide Proteins 0.000 description 2
- 108010038807 Oligopeptides Proteins 0.000 description 2
- 102000015636 Oligopeptides Human genes 0.000 description 2
- 241000283973 Oryctolagus cuniculus Species 0.000 description 2
- 241000282579 Pan Species 0.000 description 2
- 102000057297 Pepsin A Human genes 0.000 description 2
- 108090000284 Pepsin A Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 206010035226 Plasma cell myeloma Diseases 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- 108010072866 Prostate-Specific Antigen Proteins 0.000 description 2
- 102100038358 Prostate-specific antigen Human genes 0.000 description 2
- 101000884281 Rattus norvegicus Signal transducer CD24 Proteins 0.000 description 2
- MEFKEPWMEQBLKI-AIRLBKTGSA-N S-adenosyl-L-methioninate Chemical compound O[C@@H]1[C@H](O)[C@@H](C[S+](CC[C@H](N)C([O-])=O)C)O[C@H]1N1C2=NC=NC(N)=C2N=C1 MEFKEPWMEQBLKI-AIRLBKTGSA-N 0.000 description 2
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 2
- 230000006052 T cell proliferation Effects 0.000 description 2
- 102000036693 Thrombopoietin Human genes 0.000 description 2
- 108010041111 Thrombopoietin Proteins 0.000 description 2
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 2
- GLNADSQYFUSGOU-GPTZEZBUSA-J Trypan blue Chemical compound [Na+].[Na+].[Na+].[Na+].C1=C(S([O-])(=O)=O)C=C2C=C(S([O-])(=O)=O)C(/N=N/C3=CC=C(C=C3C)C=3C=C(C(=CC=3)\N=N\C=3C(=CC4=CC(=CC(N)=C4C=3O)S([O-])(=O)=O)S([O-])(=O)=O)C)=C(O)C2=C1N GLNADSQYFUSGOU-GPTZEZBUSA-J 0.000 description 2
- 108090000631 Trypsin Proteins 0.000 description 2
- 102000004142 Trypsin Human genes 0.000 description 2
- 108700042768 University of Wisconsin-lactobionate solution Proteins 0.000 description 2
- 108091023045 Untranslated Region Proteins 0.000 description 2
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 2
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 2
- 230000002159 abnormal effect Effects 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- 239000002253 acid Substances 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 229960001570 ademetionine Drugs 0.000 description 2
- 229960000723 ampicillin Drugs 0.000 description 2
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 2
- 230000000259 anti-tumor effect Effects 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000015572 biosynthetic process Effects 0.000 description 2
- 201000000053 blastoma Diseases 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 210000002798 bone marrow cell Anatomy 0.000 description 2
- KQNZDYYTLMIZCT-KQPMLPITSA-N brefeldin A Chemical compound O[C@@H]1\C=C\C(=O)O[C@@H](C)CCC\C=C\[C@@H]2C[C@H](O)C[C@H]21 KQNZDYYTLMIZCT-KQPMLPITSA-N 0.000 description 2
- JUMGSHROWPPKFX-UHFFFAOYSA-N brefeldin-A Natural products CC1CCCC=CC2(C)CC(O)CC2(C)C(O)C=CC(=O)O1 JUMGSHROWPPKFX-UHFFFAOYSA-N 0.000 description 2
- 238000005251 capillar electrophoresis Methods 0.000 description 2
- 229960002376 chymotrypsin Drugs 0.000 description 2
- ATDGTVJJHBUTRL-UHFFFAOYSA-N cyanogen bromide Chemical compound BrC#N ATDGTVJJHBUTRL-UHFFFAOYSA-N 0.000 description 2
- 238000004163 cytometry Methods 0.000 description 2
- 239000002254 cytotoxic agent Substances 0.000 description 2
- 229940127089 cytotoxic agent Drugs 0.000 description 2
- 230000007423 decrease Effects 0.000 description 2
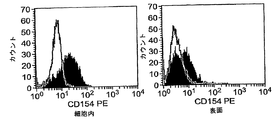
- 230000004041 dendritic cell maturation Effects 0.000 description 2
- 238000011161 development Methods 0.000 description 2
- 230000018109 developmental process Effects 0.000 description 2
- 238000003745 diagnosis Methods 0.000 description 2
- 230000029087 digestion Effects 0.000 description 2
- 108010067396 dornase alfa Proteins 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 201000008184 embryoma Diseases 0.000 description 2
- 239000000839 emulsion Substances 0.000 description 2
- 210000002889 endothelial cell Anatomy 0.000 description 2
- 150000002148 esters Chemical class 0.000 description 2
- 239000000284 extract Substances 0.000 description 2
- 210000004700 fetal blood Anatomy 0.000 description 2
- 230000005714 functional activity Effects 0.000 description 2
- 230000002538 fungal effect Effects 0.000 description 2
- 230000013595 glycosylation Effects 0.000 description 2
- 238000006206 glycosylation reaction Methods 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 239000010931 gold Substances 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 239000003102 growth factor Substances 0.000 description 2
- 230000003394 haemopoietic effect Effects 0.000 description 2
- 238000003306 harvesting Methods 0.000 description 2
- 230000036541 health Effects 0.000 description 2
- 102000046157 human CSF2 Human genes 0.000 description 2
- JYGXADMDTFJGBT-VWUMJDOOSA-N hydrocortisone Chemical compound O=C1CC[C@]2(C)[C@H]3[C@@H](O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 JYGXADMDTFJGBT-VWUMJDOOSA-N 0.000 description 2
- 238000010166 immunofluorescence Methods 0.000 description 2
- 239000000367 immunologic factor Substances 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 238000000099 in vitro assay Methods 0.000 description 2
- 230000002757 inflammatory effect Effects 0.000 description 2
- 230000002401 inhibitory effect Effects 0.000 description 2
- 238000002347 injection Methods 0.000 description 2
- 239000007924 injection Substances 0.000 description 2
- 229960003130 interferon gamma Drugs 0.000 description 2
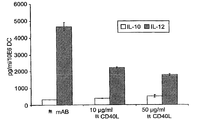
- 230000019734 interleukin-12 production Effects 0.000 description 2
- 230000004068 intracellular signaling Effects 0.000 description 2
- 230000002147 killing effect Effects 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 238000011005 laboratory method Methods 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 2
- 210000000663 muscle cell Anatomy 0.000 description 2
- 210000000822 natural killer cell Anatomy 0.000 description 2
- 229910052757 nitrogen Inorganic materials 0.000 description 2
- 108091027963 non-coding RNA Proteins 0.000 description 2
- 102000042567 non-coding RNA Human genes 0.000 description 2
- 238000005457 optimization Methods 0.000 description 2
- 125000004430 oxygen atom Chemical group O* 0.000 description 2
- 238000004091 panning Methods 0.000 description 2
- 230000037361 pathway Effects 0.000 description 2
- 229940111202 pepsin Drugs 0.000 description 2
- 210000001539 phagocyte Anatomy 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 238000001556 precipitation Methods 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 230000037452 priming Effects 0.000 description 2
- 239000011535 reaction buffer Substances 0.000 description 2
- 239000011541 reaction mixture Substances 0.000 description 2
- 230000007115 recruitment Effects 0.000 description 2
- 230000003362 replicative effect Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 239000011347 resin Substances 0.000 description 2
- 229920005989 resin Polymers 0.000 description 2
- 230000002441 reversible effect Effects 0.000 description 2
- 108091092562 ribozyme Proteins 0.000 description 2
- 235000019515 salmon Nutrition 0.000 description 2
- 238000005070 sampling Methods 0.000 description 2
- 238000002864 sequence alignment Methods 0.000 description 2
- 238000003196 serial analysis of gene expression Methods 0.000 description 2
- DAEPDZWVDSPTHF-UHFFFAOYSA-M sodium pyruvate Chemical compound [Na+].CC(=O)C([O-])=O DAEPDZWVDSPTHF-UHFFFAOYSA-M 0.000 description 2
- 230000002269 spontaneous effect Effects 0.000 description 2
- 229960005322 streptomycin Drugs 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000004809 thin layer chromatography Methods 0.000 description 2
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- 230000002103 transcriptional effect Effects 0.000 description 2
- 230000010474 transient expression Effects 0.000 description 2
- 230000007704 transition Effects 0.000 description 2
- 239000013638 trimer Substances 0.000 description 2
- 239000012588 trypsin Substances 0.000 description 2
- 241000701447 unidentified baculovirus Species 0.000 description 2
- 241001515965 unidentified phage Species 0.000 description 2
- 238000011144 upstream manufacturing Methods 0.000 description 2
- 108010027510 vaccinia virus capping enzyme Proteins 0.000 description 2
- 230000035899 viability Effects 0.000 description 2
- RDEIXVOBVLKYNT-VQBXQJRRSA-N (2r,3r,4r,5r)-2-[(1s,2s,3r,4s,6r)-4,6-diamino-3-[(2r,3r,6s)-3-amino-6-(1-aminoethyl)oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol;(2r,3r,4r,5r)-2-[(1s,2s,3r,4s,6r)-4,6-diamino-3-[(2r,3r,6s)-3-amino-6-(aminomethyl)oxan-2-yl]o Chemical compound OS(O)(=O)=O.O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H](CC[C@@H](CN)O2)N)[C@@H](N)C[C@H]1N.O1C[C@@](O)(C)[C@H](NC)[C@@H](O)[C@H]1O[C@@H]1[C@@H](O)[C@H](O[C@@H]2[C@@H](CC[C@H](O2)C(C)N)N)[C@@H](N)C[C@H]1N.O1[C@H](C(C)NC)CC[C@@H](N)[C@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](NC)[C@@](C)(O)CO2)O)[C@H](N)C[C@@H]1N RDEIXVOBVLKYNT-VQBXQJRRSA-N 0.000 description 1
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- KZMAWJRXKGLWGS-UHFFFAOYSA-N 2-chloro-n-[4-(4-methoxyphenyl)-1,3-thiazol-2-yl]-n-(3-methoxypropyl)acetamide Chemical compound S1C(N(C(=O)CCl)CCCOC)=NC(C=2C=CC(OC)=CC=2)=C1 KZMAWJRXKGLWGS-UHFFFAOYSA-N 0.000 description 1
- 238000010600 3H thymidine incorporation assay Methods 0.000 description 1
- SQDAZGGFXASXDW-UHFFFAOYSA-N 5-bromo-2-(trifluoromethoxy)pyridine Chemical compound FC(F)(F)OC1=CC=C(Br)C=N1 SQDAZGGFXASXDW-UHFFFAOYSA-N 0.000 description 1
- 230000005730 ADP ribosylation Effects 0.000 description 1
- 101150060590 ANAPC5 gene Proteins 0.000 description 1
- 108091006112 ATPases Proteins 0.000 description 1
- 241001556567 Acanthamoeba polyphaga mimivirus Species 0.000 description 1
- LPMXVESGRSUGHW-UHFFFAOYSA-N Acolongiflorosid K Natural products OC1C(O)C(O)C(C)OC1OC1CC2(O)CCC3C4(O)CCC(C=5COC(=O)C=5)C4(C)CC(O)C3C2(CO)C(O)C1 LPMXVESGRSUGHW-UHFFFAOYSA-N 0.000 description 1
- 208000003200 Adenoma Diseases 0.000 description 1
- 206010001233 Adenoma benign Diseases 0.000 description 1
- 102000057290 Adenosine Triphosphatases Human genes 0.000 description 1
- 229940122450 Altered peptide ligand Drugs 0.000 description 1
- APKFDSVGJQXUKY-KKGHZKTASA-N Amphotericin-B Natural products O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1C=CC=CC=CC=CC=CC=CC=C[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-KKGHZKTASA-N 0.000 description 1
- 102000052588 Anaphase-Promoting Complex-Cyclosome Apc5 Subunit Human genes 0.000 description 1
- 108700004604 Anaphase-Promoting Complex-Cyclosome Apc5 Subunit Proteins 0.000 description 1
- 241001156002 Anthonomus pomorum Species 0.000 description 1
- 244000105975 Antidesma platyphyllum Species 0.000 description 1
- 102000006306 Antigen Receptors Human genes 0.000 description 1
- 108010083359 Antigen Receptors Proteins 0.000 description 1
- 108020005544 Antisense RNA Proteins 0.000 description 1
- 241000282709 Aotus trivirgatus Species 0.000 description 1
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 1
- 241001422755 Atys Species 0.000 description 1
- 241000714235 Avian retrovirus Species 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 1
- 230000003844 B-cell-activation Effects 0.000 description 1
- 206010005003 Bladder cancer Diseases 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- 102100036842 C-C motif chemokine 19 Human genes 0.000 description 1
- 101150077194 CAP1 gene Proteins 0.000 description 1
- 102000013925 CD34 antigen Human genes 0.000 description 1
- 108050003733 CD34 antigen Proteins 0.000 description 1
- 210000001239 CD8-positive, alpha-beta cytotoxic T lymphocyte Anatomy 0.000 description 1
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 1
- 229940045513 CTLA4 antagonist Drugs 0.000 description 1
- 241000288950 Callithrix jacchus Species 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 206010008342 Cervix carcinoma Diseases 0.000 description 1
- 229920001287 Chondroitin sulfate Polymers 0.000 description 1
- 108091062157 Cis-regulatory element Proteins 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 108091035707 Consensus sequence Proteins 0.000 description 1
- 108010069514 Cyclic Peptides Proteins 0.000 description 1
- 102000001189 Cyclic Peptides Human genes 0.000 description 1
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 1
- 102000012410 DNA Ligases Human genes 0.000 description 1
- 108010061982 DNA Ligases Proteins 0.000 description 1
- 102000004594 DNA Polymerase I Human genes 0.000 description 1
- 108010017826 DNA Polymerase I Proteins 0.000 description 1
- 102100027834 DNA repair protein XRCC1 Human genes 0.000 description 1
- 101000876610 Dictyostelium discoideum Extracellular signal-regulated kinase 2 Proteins 0.000 description 1
- 108010016626 Dipeptides Proteins 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- 102100025137 Early activation antigen CD69 Human genes 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 108700024394 Exon Proteins 0.000 description 1
- 102100029880 Glycodelin Human genes 0.000 description 1
- 108060003393 Granulin Proteins 0.000 description 1
- 102000001398 Granzyme Human genes 0.000 description 1
- 108060005986 Granzyme Proteins 0.000 description 1
- 239000007995 HEPES buffer Substances 0.000 description 1
- 102000011786 HLA-A Antigens Human genes 0.000 description 1
- 108010075704 HLA-A Antigens Proteins 0.000 description 1
- 102000015789 HLA-DP Antigens Human genes 0.000 description 1
- 108010010378 HLA-DP Antigens Proteins 0.000 description 1
- 101150031823 HSP70 gene Proteins 0.000 description 1
- 206010066476 Haematological malignancy Diseases 0.000 description 1
- 101100028493 Haloferax volcanii (strain ATCC 29605 / DSM 3757 / JCM 8879 / NBRC 14742 / NCIMB 2012 / VKM B-1768 / DS2) pan2 gene Proteins 0.000 description 1
- 102100040352 Heat shock 70 kDa protein 1A Human genes 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- 108091005902 Hemoglobin subunit alpha Proteins 0.000 description 1
- 102100027685 Hemoglobin subunit alpha Human genes 0.000 description 1
- 108091005904 Hemoglobin subunit beta Proteins 0.000 description 1
- 102100021519 Hemoglobin subunit beta Human genes 0.000 description 1
- 241001272567 Hominoidea Species 0.000 description 1
- 101000868215 Homo sapiens CD40 ligand Proteins 0.000 description 1
- 101000649341 Homo sapiens DNA repair protein XRCC1 Proteins 0.000 description 1
- 101000934374 Homo sapiens Early activation antigen CD69 Proteins 0.000 description 1
- 101000585553 Homo sapiens Glycodelin Proteins 0.000 description 1
- 101001037759 Homo sapiens Heat shock 70 kDa protein 1A Proteins 0.000 description 1
- 101000935040 Homo sapiens Integrin beta-2 Proteins 0.000 description 1
- 101001001420 Homo sapiens Interferon gamma receptor 1 Proteins 0.000 description 1
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 1
- 101001052493 Homo sapiens Mitogen-activated protein kinase 1 Proteins 0.000 description 1
- 101000738771 Homo sapiens Receptor-type tyrosine-protein phosphatase C Proteins 0.000 description 1
- 101000997835 Homo sapiens Tyrosine-protein kinase JAK1 Proteins 0.000 description 1
- 101000997832 Homo sapiens Tyrosine-protein kinase JAK2 Proteins 0.000 description 1
- 108090000144 Human Proteins Proteins 0.000 description 1
- 102000003839 Human Proteins Human genes 0.000 description 1
- 108060003951 Immunoglobulin Proteins 0.000 description 1
- 206010061218 Inflammation Diseases 0.000 description 1
- 102100034343 Integrase Human genes 0.000 description 1
- 102100025390 Integrin beta-2 Human genes 0.000 description 1
- 101710174028 Interferon gamma receptor 1 Proteins 0.000 description 1
- 102100036157 Interferon gamma receptor 2 Human genes 0.000 description 1
- 102000003777 Interleukin-1 beta Human genes 0.000 description 1
- 108090000193 Interleukin-1 beta Proteins 0.000 description 1
- 102100030698 Interleukin-12 subunit alpha Human genes 0.000 description 1
- 102000004125 Interleukin-1alpha Human genes 0.000 description 1
- 108010082786 Interleukin-1alpha Proteins 0.000 description 1
- 102000015696 Interleukins Human genes 0.000 description 1
- 108010063738 Interleukins Proteins 0.000 description 1
- 108091092195 Intron Proteins 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 102100020880 Kit ligand Human genes 0.000 description 1
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 1
- 108090000364 Ligases Proteins 0.000 description 1
- 102000003960 Ligases Human genes 0.000 description 1
- 102000005482 Lipopolysaccharide Receptors Human genes 0.000 description 1
- 108010031801 Lipopolysaccharide Receptors Proteins 0.000 description 1
- 102000004895 Lipoproteins Human genes 0.000 description 1
- 108090001030 Lipoproteins Proteins 0.000 description 1
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 1
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 1
- 206010025323 Lymphomas Diseases 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 102000043131 MHC class II family Human genes 0.000 description 1
- 108091054438 MHC class II family Proteins 0.000 description 1
- 241000282561 Macaca nemestrina Species 0.000 description 1
- 108700018351 Major Histocompatibility Complex Proteins 0.000 description 1
- 241000556720 Manga Species 0.000 description 1
- 108091027974 Mature messenger RNA Proteins 0.000 description 1
- 102100022430 Melanocyte protein PMEL Human genes 0.000 description 1
- 102000018697 Membrane Proteins Human genes 0.000 description 1
- 108010052285 Membrane Proteins Proteins 0.000 description 1
- 102000004232 Mitogen-Activated Protein Kinase Kinases Human genes 0.000 description 1
- 108090000744 Mitogen-Activated Protein Kinase Kinases Proteins 0.000 description 1
- 102100024193 Mitogen-activated protein kinase 1 Human genes 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 101100335081 Mus musculus Flt3 gene Proteins 0.000 description 1
- 101100245221 Mus musculus Prss8 gene Proteins 0.000 description 1
- 108091007491 NSP3 Papain-like protease domains Proteins 0.000 description 1
- 229930193140 Neomycin Natural products 0.000 description 1
- 239000004677 Nylon Substances 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- LPMXVESGRSUGHW-GHYGWZAOSA-N Ouabain Natural products O([C@@H]1[C@@H](O)[C@@H](O)[C@@H](O)[C@H](C)O1)[C@H]1C[C@@H](O)[C@@]2(CO)[C@@](O)(C1)CC[C@H]1[C@]3(O)[C@@](C)([C@H](C4=CC(=O)OC4)CC3)C[C@@H](O)[C@H]21 LPMXVESGRSUGHW-GHYGWZAOSA-N 0.000 description 1
- 101710160107 Outer membrane protein A Proteins 0.000 description 1
- 206010033128 Ovarian cancer Diseases 0.000 description 1
- 206010061535 Ovarian neoplasm Diseases 0.000 description 1
- 239000002033 PVDF binder Substances 0.000 description 1
- 241000282577 Pan troglodytes Species 0.000 description 1
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 1
- 229930182555 Penicillin Natural products 0.000 description 1
- JGSARLDLIJGVTE-MBNYWOFBSA-N Penicillin G Chemical compound N([C@H]1[C@H]2SC([C@@H](N2C1=O)C(O)=O)(C)C)C(=O)CC1=CC=CC=C1 JGSARLDLIJGVTE-MBNYWOFBSA-N 0.000 description 1
- 206010035664 Pneumonia Diseases 0.000 description 1
- 101710124239 Poly(A) polymerase Proteins 0.000 description 1
- 241000288906 Primates Species 0.000 description 1
- 206010060862 Prostate cancer Diseases 0.000 description 1
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 1
- 102000007327 Protamines Human genes 0.000 description 1
- 108010007568 Protamines Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 101900161471 Pseudomonas aeruginosa Exotoxin A Proteins 0.000 description 1
- 102100033479 RAF proto-oncogene serine/threonine-protein kinase Human genes 0.000 description 1
- 101710141955 RAF proto-oncogene serine/threonine-protein kinase Proteins 0.000 description 1
- 108091034057 RNA (poly(A)) Proteins 0.000 description 1
- 102000009572 RNA Polymerase II Human genes 0.000 description 1
- 108010009460 RNA Polymerase II Proteins 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 239000012979 RPMI medium Substances 0.000 description 1
- 102100037422 Receptor-type tyrosine-protein phosphatase C Human genes 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- 201000000582 Retinoblastoma Diseases 0.000 description 1
- 238000011579 SCID mouse model Methods 0.000 description 1
- 206010039491 Sarcoma Diseases 0.000 description 1
- 241000710961 Semliki Forest virus Species 0.000 description 1
- 241000710960 Sindbis virus Species 0.000 description 1
- FKNQFGJONOIPTF-UHFFFAOYSA-N Sodium cation Chemical compound [Na+] FKNQFGJONOIPTF-UHFFFAOYSA-N 0.000 description 1
- 108010039445 Stem Cell Factor Proteins 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 244000166550 Strophanthus gratus Species 0.000 description 1
- 101800001271 Surface protein Proteins 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 210000000173 T-lymphoid precursor cell Anatomy 0.000 description 1
- 208000024313 Testicular Neoplasms Diseases 0.000 description 1
- 206010057644 Testis cancer Diseases 0.000 description 1
- 108091036066 Three prime untranslated region Proteins 0.000 description 1
- 108020004566 Transfer RNA Proteins 0.000 description 1
- 108700019146 Transgenes Proteins 0.000 description 1
- 206010052779 Transplant rejections Diseases 0.000 description 1
- 239000013504 Triton X-100 Substances 0.000 description 1
- 229920004890 Triton X-100 Polymers 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 102100033444 Tyrosine-protein kinase JAK2 Human genes 0.000 description 1
- 229910052770 Uranium Inorganic materials 0.000 description 1
- 108010046334 Urease Proteins 0.000 description 1
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 1
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 1
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 description 1
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 108010067390 Viral Proteins Proteins 0.000 description 1
- 208000036142 Viral infection Diseases 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 230000003044 adaptive effect Effects 0.000 description 1
- 230000004721 adaptive immunity Effects 0.000 description 1
- 238000012382 advanced drug delivery Methods 0.000 description 1
- 238000000246 agarose gel electrophoresis Methods 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 1
- 230000000172 allergic effect Effects 0.000 description 1
- 125000003550 alpha-D-galactosyl group Chemical group C1([C@H](O)[C@@H](O)[C@@H](O)[C@H](O1)CO)* 0.000 description 1
- 230000006229 amino acid addition Effects 0.000 description 1
- APKFDSVGJQXUKY-INPOYWNPSA-N amphotericin B Chemical compound O[C@H]1[C@@H](N)[C@H](O)[C@@H](C)O[C@H]1O[C@H]1/C=C/C=C/C=C/C=C/C=C/C=C/C=C/[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@](O)(C[C@H](O)[C@H]2C(O)=O)O[C@H]2C1 APKFDSVGJQXUKY-INPOYWNPSA-N 0.000 description 1
- 229960003942 amphotericin b Drugs 0.000 description 1
- 238000010171 animal model Methods 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 230000002547 anomalous effect Effects 0.000 description 1
- 230000003092 anti-cytokine Effects 0.000 description 1
- 230000005809 anti-tumor immunity Effects 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 101150082657 apc5 gene Proteins 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 229960001230 asparagine Drugs 0.000 description 1
- 235000009582 asparagine Nutrition 0.000 description 1
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 1
- 208000010668 atopic eczema Diseases 0.000 description 1
- 238000000376 autoradiography Methods 0.000 description 1
- 230000003385 bacteriostatic effect Effects 0.000 description 1
- 230000037429 base substitution Effects 0.000 description 1
- 210000003651 basophil Anatomy 0.000 description 1
- 238000002306 biochemical method Methods 0.000 description 1
- 229920000249 biocompatible polymer Polymers 0.000 description 1
- 238000005460 biophysical method Methods 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000000481 breast Anatomy 0.000 description 1
- 210000004899 c-terminal region Anatomy 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 150000001720 carbohydrates Chemical class 0.000 description 1
- 235000014633 carbohydrates Nutrition 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 125000004432 carbon atom Chemical group C* 0.000 description 1
- 239000000969 carrier Substances 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 239000006143 cell culture medium Substances 0.000 description 1
- 239000012578 cell culture reagent Substances 0.000 description 1
- 230000011712 cell development Effects 0.000 description 1
- 230000024245 cell differentiation Effects 0.000 description 1
- 239000013592 cell lysate Substances 0.000 description 1
- 230000004663 cell proliferation Effects 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000005859 cell recognition Effects 0.000 description 1
- 230000006041 cell recruitment Effects 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 229940106189 ceramide Drugs 0.000 description 1
- 150000001783 ceramides Chemical class 0.000 description 1
- 201000010881 cervical cancer Diseases 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 230000035605 chemotaxis Effects 0.000 description 1
- 229940059329 chondroitin sulfate Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 239000003593 chromogenic compound Substances 0.000 description 1
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 238000009643 clonogenic assay Methods 0.000 description 1
- 231100000096 clonogenic assay Toxicity 0.000 description 1
- 238000012761 co-transfection Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 210000001072 colon Anatomy 0.000 description 1
- 239000003283 colorimetric indicator Substances 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000010835 comparative analysis Methods 0.000 description 1
- 239000003184 complementary RNA Substances 0.000 description 1
- 238000002591 computed tomography Methods 0.000 description 1
- 239000003636 conditioned culture medium Substances 0.000 description 1
- 238000012790 confirmation Methods 0.000 description 1
- 239000000356 contaminant Substances 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 238000011443 conventional therapy Methods 0.000 description 1
- IDLFZVILOHSSID-OVLDLUHVSA-N corticotropin Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)NC(=O)[C@@H](N)CO)C1=CC=C(O)C=C1 IDLFZVILOHSSID-OVLDLUHVSA-N 0.000 description 1
- 230000004940 costimulation Effects 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 230000009260 cross reactivity Effects 0.000 description 1
- 230000002338 cryopreservative effect Effects 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 210000005220 cytoplasmic tail Anatomy 0.000 description 1
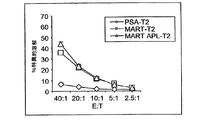
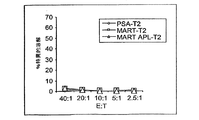
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 238000002784 cytotoxicity assay Methods 0.000 description 1
- 231100000263 cytotoxicity test Toxicity 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 230000002939 deleterious effect Effects 0.000 description 1
- 238000000432 density-gradient centrifugation Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- ZZVUWRFHKOJYTH-UHFFFAOYSA-N diphenhydramine Chemical compound C=1C=CC=CC=1C(OCCN(C)C)C1=CC=CC=C1 ZZVUWRFHKOJYTH-UHFFFAOYSA-N 0.000 description 1
- 229960000520 diphenhydramine Drugs 0.000 description 1
- PXEDJBXQKAGXNJ-QTNFYWBSSA-L disodium L-glutamate Chemical compound [Na+].[Na+].[O-]C(=O)[C@@H](N)CCC([O-])=O PXEDJBXQKAGXNJ-QTNFYWBSSA-L 0.000 description 1
- 238000010494 dissociation reaction Methods 0.000 description 1
- 230000005593 dissociations Effects 0.000 description 1
- 238000009826 distribution Methods 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000005538 encapsulation Methods 0.000 description 1
- 239000002158 endotoxin Substances 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 210000003979 eosinophil Anatomy 0.000 description 1
- 230000010502 episomal replication Effects 0.000 description 1
- 210000002919 epithelial cell Anatomy 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 230000000925 erythroid effect Effects 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 239000003797 essential amino acid Substances 0.000 description 1
- 235000020776 essential amino acid Nutrition 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- 150000002170 ethers Chemical class 0.000 description 1
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 description 1
- 229960005542 ethidium bromide Drugs 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 150000004665 fatty acids Chemical group 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000012467 final product Substances 0.000 description 1
- 108700014844 flt3 ligand Proteins 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- MHMNJMPURVTYEJ-UHFFFAOYSA-N fluorescein-5-isothiocyanate Chemical compound O1C(=O)C2=CC(N=C=S)=CC=C2C21C1=CC=C(O)C=C1OC1=CC(O)=CC=C21 MHMNJMPURVTYEJ-UHFFFAOYSA-N 0.000 description 1
- 238000012921 fluorescence analysis Methods 0.000 description 1
- 235000012041 food component Nutrition 0.000 description 1
- 239000005417 food ingredient Substances 0.000 description 1
- 239000012737 fresh medium Substances 0.000 description 1
- 238000010230 functional analysis Methods 0.000 description 1
- 210000000609 ganglia Anatomy 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 210000001035 gastrointestinal tract Anatomy 0.000 description 1
- 239000000499 gel Substances 0.000 description 1
- 230000002068 genetic effect Effects 0.000 description 1
- 238000010353 genetic engineering Methods 0.000 description 1
- 208000005017 glioblastoma Diseases 0.000 description 1
- 108060003196 globin Proteins 0.000 description 1
- 102000018146 globin Human genes 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 210000003714 granulocyte Anatomy 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 235000009424 haa Nutrition 0.000 description 1
- 230000011132 hemopoiesis Effects 0.000 description 1
- 210000004408 hybridoma Anatomy 0.000 description 1
- 229960000890 hydrocortisone Drugs 0.000 description 1
- 230000007062 hydrolysis Effects 0.000 description 1
- 238000006460 hydrolysis reaction Methods 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000006058 immune tolerance Effects 0.000 description 1
- 238000003119 immunoblot Methods 0.000 description 1
- 230000000951 immunodiffusion Effects 0.000 description 1
- 238000000760 immunoelectrophoresis Methods 0.000 description 1
- 102000018358 immunoglobulin Human genes 0.000 description 1
- 238000010324 immunological assay Methods 0.000 description 1
- 238000001114 immunoprecipitation Methods 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 238000003017 in situ immunoassay Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000004054 inflammatory process Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 238000011221 initial treatment Methods 0.000 description 1
- 230000008611 intercellular interaction Effects 0.000 description 1
- 230000031261 interleukin-10 production Effects 0.000 description 1
- 230000004073 interleukin-2 production Effects 0.000 description 1
- 230000000968 intestinal effect Effects 0.000 description 1
- 238000007918 intramuscular administration Methods 0.000 description 1
- 238000007912 intraperitoneal administration Methods 0.000 description 1
- 108010028930 invariant chain Proteins 0.000 description 1
- 230000002427 irreversible effect Effects 0.000 description 1
- 230000007794 irritation Effects 0.000 description 1
- 210000002510 keratinocyte Anatomy 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 210000003292 kidney cell Anatomy 0.000 description 1
- 238000000021 kinase assay Methods 0.000 description 1
- 238000011031 large-scale manufacturing process Methods 0.000 description 1
- 230000000670 limiting effect Effects 0.000 description 1
- 229920006008 lipopolysaccharide Polymers 0.000 description 1
- 201000007270 liver cancer Diseases 0.000 description 1
- 208000014018 liver neoplasm Diseases 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 201000005202 lung cancer Diseases 0.000 description 1
- 208000020816 lung neoplasm Diseases 0.000 description 1
- 210000002751 lymph Anatomy 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 210000003563 lymphoid tissue Anatomy 0.000 description 1
- 238000002595 magnetic resonance imaging Methods 0.000 description 1
- 230000003211 malignant effect Effects 0.000 description 1
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 1
- 241001515942 marmosets Species 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 210000004779 membrane envelope Anatomy 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 239000002923 metal particle Substances 0.000 description 1
- 208000037819 metastatic cancer Diseases 0.000 description 1
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 1
- MYWUZJCMWCOHBA-VIFPVBQESA-N methamphetamine Chemical compound CN[C@@H](C)CC1=CC=CC=C1 MYWUZJCMWCOHBA-VIFPVBQESA-N 0.000 description 1
- 238000012737 microarray-based gene expression Methods 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 230000002906 microbiologic effect Effects 0.000 description 1
- 238000000520 microinjection Methods 0.000 description 1
- 230000002438 mitochondrial effect Effects 0.000 description 1
- ZAHQPTJLOCWVPG-UHFFFAOYSA-N mitoxantrone dihydrochloride Chemical compound Cl.Cl.O=C1C2=C(O)C=CC(O)=C2C(=O)C2=C1C(NCCNCCO)=CC=C2NCCNCCO ZAHQPTJLOCWVPG-UHFFFAOYSA-N 0.000 description 1
- 102000035118 modified proteins Human genes 0.000 description 1
- 108091005573 modified proteins Proteins 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 235000013923 monosodium glutamate Nutrition 0.000 description 1
- 238000012243 multiplex automated genomic engineering Methods 0.000 description 1
- 238000002703 mutagenesis Methods 0.000 description 1
- 231100000350 mutagenesis Toxicity 0.000 description 1
- 210000000066 myeloid cell Anatomy 0.000 description 1
- 201000000050 myeloid neoplasm Diseases 0.000 description 1
- 229920005615 natural polymer Polymers 0.000 description 1
- 229960004927 neomycin Drugs 0.000 description 1
- 210000005036 nerve Anatomy 0.000 description 1
- 210000000440 neutrophil Anatomy 0.000 description 1
- 108091008104 nucleic acid aptamers Proteins 0.000 description 1
- 210000004940 nucleus Anatomy 0.000 description 1
- 229920001778 nylon Polymers 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 150000002482 oligosaccharides Chemical class 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- LPMXVESGRSUGHW-HBYQJFLCSA-N ouabain Chemical compound O[C@@H]1[C@H](O)[C@@H](O)[C@H](C)O[C@H]1O[C@@H]1C[C@@]2(O)CC[C@H]3[C@@]4(O)CC[C@H](C=5COC(=O)C=5)[C@@]4(C)C[C@@H](O)[C@@H]3[C@@]2(CO)[C@H](O)C1 LPMXVESGRSUGHW-HBYQJFLCSA-N 0.000 description 1
- 229960003343 ouabain Drugs 0.000 description 1
- 210000001672 ovary Anatomy 0.000 description 1
- 239000001301 oxygen Substances 0.000 description 1
- 229910052760 oxygen Inorganic materials 0.000 description 1
- 238000002559 palpation Methods 0.000 description 1
- 101150081585 panB gene Proteins 0.000 description 1
- 210000000496 pancreas Anatomy 0.000 description 1
- 201000002528 pancreatic cancer Diseases 0.000 description 1
- 208000008443 pancreatic carcinoma Diseases 0.000 description 1
- 238000007911 parenteral administration Methods 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 229940049954 penicillin Drugs 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 210000004976 peripheral blood cell Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 238000002823 phage display Methods 0.000 description 1
- 239000012071 phase Substances 0.000 description 1
- 230000004962 physiological condition Effects 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 239000004417 polycarbonate Substances 0.000 description 1
- 229920000515 polycarbonate Polymers 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920000642 polymer Polymers 0.000 description 1
- 238000003752 polymerase chain reaction Methods 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920002981 polyvinylidene fluoride Polymers 0.000 description 1
- 239000011148 porous material Substances 0.000 description 1
- 230000004481 post-translational protein modification Effects 0.000 description 1
- 230000001124 posttranscriptional effect Effects 0.000 description 1
- 238000011533 pre-incubation Methods 0.000 description 1
- 238000002203 pretreatment Methods 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 210000004986 primary T-cell Anatomy 0.000 description 1
- 238000011809 primate model Methods 0.000 description 1
- 230000001566 pro-viral effect Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000000770 proinflammatory effect Effects 0.000 description 1
- 210000001236 prokaryotic cell Anatomy 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 210000005267 prostate cell Anatomy 0.000 description 1
- 229950008679 protamine sulfate Drugs 0.000 description 1
- 239000012474 protein marker Substances 0.000 description 1
- 238000002106 pulse oximetry Methods 0.000 description 1
- 238000004451 qualitative analysis Methods 0.000 description 1
- 238000003908 quality control method Methods 0.000 description 1
- 238000004445 quantitative analysis Methods 0.000 description 1
- 230000005855 radiation Effects 0.000 description 1
- 239000000700 radioactive tracer Substances 0.000 description 1
- 238000002601 radiography Methods 0.000 description 1
- 230000009257 reactivity Effects 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 1
- 210000003289 regulatory T cell Anatomy 0.000 description 1
- 238000012827 research and development Methods 0.000 description 1
- 230000000284 resting effect Effects 0.000 description 1
- 210000001995 reticulocyte Anatomy 0.000 description 1
- 238000003757 reverse transcription PCR Methods 0.000 description 1
- 238000003345 scintillation counting Methods 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 238000007790 scraping Methods 0.000 description 1
- 238000012216 screening Methods 0.000 description 1
- 125000002914 sec-butyl group Chemical group [H]C([H])([H])C([H])([H])C([H])(*)C([H])([H])[H] 0.000 description 1
- 210000005212 secondary lymphoid organ Anatomy 0.000 description 1
- 238000012163 sequencing technique Methods 0.000 description 1
- 230000007781 signaling event Effects 0.000 description 1
- 210000003491 skin Anatomy 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229940073490 sodium glutamate Drugs 0.000 description 1
- 229910001415 sodium ion Inorganic materials 0.000 description 1
- 229940054269 sodium pyruvate Drugs 0.000 description 1
- 239000007790 solid phase Substances 0.000 description 1
- 230000003381 solubilizing effect Effects 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 238000002798 spectrophotometry method Methods 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 239000012089 stop solution Substances 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- 125000001424 substituent group Chemical group 0.000 description 1
- 239000013595 supernatant sample Substances 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000375 suspending agent Substances 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 238000002636 symptomatic treatment Methods 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 201000003120 testicular cancer Diseases 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 230000008719 thickening Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 229940113082 thymine Drugs 0.000 description 1
- 230000036962 time dependent Effects 0.000 description 1
- 230000001988 toxicity Effects 0.000 description 1
- 231100000419 toxicity Toxicity 0.000 description 1
- 235000013619 trace mineral Nutrition 0.000 description 1
- 239000011573 trace mineral Substances 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 238000003151 transfection method Methods 0.000 description 1
- 230000001131 transforming effect Effects 0.000 description 1
- 238000011820 transgenic animal model Methods 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 238000002604 ultrasonography Methods 0.000 description 1
- 229940035893 uracil Drugs 0.000 description 1
- 210000003932 urinary bladder Anatomy 0.000 description 1
- 201000005112 urinary bladder cancer Diseases 0.000 description 1
- 238000002255 vaccination Methods 0.000 description 1
- 230000029812 viral genome replication Effects 0.000 description 1
- 230000009385 viral infection Effects 0.000 description 1
- 230000000007 visual effect Effects 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 239000000080 wetting agent Substances 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
- C12N5/0634—Cells from the blood or the immune system
- C12N5/0639—Dendritic cells, e.g. Langherhans cells in the epidermis
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/46—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates
- C07K14/47—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans from vertebrates from mammals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/10—Cellular immunotherapy characterised by the cell type used
- A61K40/19—Dendritic cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/20—Cellular immunotherapy characterised by the effect or the function of the cells
- A61K40/24—Antigen-presenting cells [APC]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4238—Regulators of development
- A61K40/424—Apoptosis related proteins, e.g. survivin or livin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4244—Enzymes
- A61K40/4246—Telomerase or [telomerase reverse transcriptase [TERT]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K40/00—Cellular immunotherapy
- A61K40/40—Cellular immunotherapy characterised by antigens that are targeted or presented by cells of the immune system
- A61K40/41—Vertebrate antigens
- A61K40/42—Cancer antigens
- A61K40/4271—Melanoma antigens
- A61K40/4272—Melan-A/MART
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/87—Introduction of foreign genetic material using processes not otherwise provided for, e.g. co-transformation
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N5/00—Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor
- C12N5/06—Animal cells or tissues; Human cells or tissues
- C12N5/0602—Vertebrate cells
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2239/00—Indexing codes associated with cellular immunotherapy of group A61K40/00
- A61K2239/46—Indexing codes associated with cellular immunotherapy of group A61K40/00 characterised by the cancer treated
- A61K2239/56—Kidney
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/20—Cytokines; Chemokines
- C12N2501/24—Interferons [IFN]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2501/00—Active agents used in cell culture processes, e.g. differentation
- C12N2501/50—Cell markers; Cell surface determinants
- C12N2501/52—CD40, CD40-ligand (CD154)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2510/00—Genetically modified cells
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Epidemiology (AREA)
- Biomedical Technology (AREA)
- Genetics & Genomics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Immunology (AREA)
- Zoology (AREA)
- Biotechnology (AREA)
- Wood Science & Technology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Microbiology (AREA)
- Cell Biology (AREA)
- Hematology (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Biophysics (AREA)
- Toxicology (AREA)
- Gastroenterology & Hepatology (AREA)
- Oncology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/400,774 US8513008B2 (en) | 2004-10-07 | 2006-04-07 | Mature dendritic cell compositions and methods for culturing same |
| US11/400,774 | 2006-04-07 | ||
| PCT/US2007/008734 WO2007117682A2 (en) | 2006-04-07 | 2007-04-06 | Mature dendritic cell compositions and methods for culturing same |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2009533025A JP2009533025A (ja) | 2009-09-17 |
| JP2009533025A5 JP2009533025A5 (enExample) | 2010-05-20 |
| JP5425618B2 true JP5425618B2 (ja) | 2014-02-26 |
Family
ID=38581685
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009504346A Expired - Fee Related JP5425618B2 (ja) | 2006-04-07 | 2007-04-06 | 成熟樹状細胞組成物およびそれを培養するための方法 |
Country Status (10)
| Country | Link |
|---|---|
| US (4) | US8513008B2 (enExample) |
| EP (1) | EP2003969B1 (enExample) |
| JP (1) | JP5425618B2 (enExample) |
| KR (1) | KR101425686B1 (enExample) |
| CN (1) | CN101460054B (enExample) |
| AT (1) | ATE510451T1 (enExample) |
| AU (1) | AU2007235227B2 (enExample) |
| CA (1) | CA2648675C (enExample) |
| DK (1) | DK2003969T3 (enExample) |
| WO (1) | WO2007117682A2 (enExample) |
Families Citing this family (27)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8513008B2 (en) | 2004-10-07 | 2013-08-20 | Argos Therapeutics, Inc. | Mature dendritic cell compositions and methods for culturing same |
| KR101243577B1 (ko) | 2004-10-07 | 2013-03-20 | 아고스 쎄라퓨틱스, 인코포레이티드 | 성숙 수지상 세포 조성물 및 그의 배양 방법 |
| JP5020935B2 (ja) | 2005-04-08 | 2012-09-05 | アルゴス セラピューティクス,インコーポレイティド | 樹状細胞組成物および方法 |
| ES2520026T3 (es) | 2006-09-18 | 2014-11-11 | The Board Of Trustees Of The University Of Arkansas | Composiciones y métodos para potenciar respuestas inmunitarias |
| WO2009039198A2 (en) * | 2007-09-17 | 2009-03-26 | The Trustees Of The University Of Pennsylvania | Generation of hyperstable mrnas |
| BRPI0818736A2 (pt) * | 2007-10-30 | 2017-06-13 | Univ Arkansas | composições e métodos para intensificar imunorrepostas à bactéria flagelada |
| CN101969990B (zh) | 2007-11-01 | 2014-07-09 | 阿肯色大学评议会 | 增强针对艾美球虫属的免疫反应的组合物和方法 |
| EP2072617A1 (en) * | 2007-12-12 | 2009-06-24 | Trimed Biotech GmbH | Method for producing dendritic cells |
| EP2254998B1 (en) * | 2008-02-28 | 2015-12-23 | Argos Therapeutics, Inc. | Transient expression of immunomodulatory polypeptides for the prevention and treatment of autoimmune disease, allergy and transplant rejection |
| CN102811734B (zh) | 2010-01-21 | 2016-02-10 | 阿肯色大学评议会 | 增强免疫应答的疫苗载体和方法 |
| CN102971008B (zh) | 2010-06-09 | 2015-11-25 | 阿肯色大学评议会 | 降低弯曲菌属感染的疫苗和方法 |
| WO2012041635A1 (en) * | 2010-09-29 | 2012-04-05 | Universite De Liege | Combination of an agonistic anti-cd40 monoclonal antibody or a cd40 ligand and inactivated or attenuated bacteria for use in the treatment and/or prevention of mastitis |
| CN109536446B (zh) * | 2012-02-10 | 2022-08-02 | 医疗法人社团博心厚生会 | 单核细胞增殖剂、单核细胞增殖用培养基、及单核细胞的制造方法 |
| US9603915B2 (en) | 2013-02-14 | 2017-03-28 | The Board of Trustees of the University of Akansas | Compositions and methods of enhancing immune responses to Eimeria or limiting Eimeria infection |
| BR112015023024B1 (pt) | 2013-03-15 | 2022-04-19 | The Board Of Trustees Of The University Of Arkansas | Vetor de vacina e composições farmacêuticas compreendendo o mesmo |
| AU2015218865B2 (en) * | 2014-02-21 | 2021-06-10 | Coimmune, Inc. | TSCM cells and methods for use |
| EP3316897A4 (en) | 2015-07-02 | 2019-03-13 | Primevax Immuno-Oncology, Inc. | COMPOSITIONS AND METHODS FOR COMBINATION THERAPY WITH DENGUE VIRUS AND DENDRITIC CELLS |
| JP2018535191A (ja) * | 2015-09-26 | 2018-11-29 | プライムヴァックス イミュノ−オンコロジー,インク. | 樹状細胞を産生するための組成物及び方法 |
| WO2017102010A1 (en) * | 2015-12-17 | 2017-06-22 | Biontech Rna Pharmaceuticals Gmbh | Novel cytokine fusion proteins |
| WO2017123242A1 (en) * | 2016-01-15 | 2017-07-20 | Ding Enyu | A universal nucleic acid drug vector enhancing mrna stability and translatability |
| KR102767638B1 (ko) | 2016-05-03 | 2025-02-20 | 더 보드 오브 트러스티스 오브 더 유니버시티 오브 아칸소 | 면역자극 및 항원 폴리펩티드를 포함하는 효모 백신 벡터, 및 그를 이용하는 방법 |
| JP2020500847A (ja) | 2016-11-16 | 2020-01-16 | プライムヴァックス イミュノ−オンコロジー,インク. | 癌の処置のための併用免疫療法 |
| CA3049225A1 (en) | 2017-01-04 | 2018-07-12 | PrimeVax Immuno-Oncology, Inc. | Compositions and methods for therapy with dengue virus |
| US11779599B2 (en) | 2017-11-07 | 2023-10-10 | Coimmune, Inc. | Methods and uses for dendritic cell therapy |
| CN111166876B (zh) * | 2018-11-13 | 2022-10-11 | 北京启辰生生物科技有限公司 | 一种免疫增强剂组合和编码核酸及其应用 |
| KR102698871B1 (ko) * | 2021-09-10 | 2024-08-28 | 이종균 | 수지상 세포, 자연 살해 세포 및 세포 독성 t 세포를 포함하는 암의 예방 또는 치료용 생물학적 조성물 |
| WO2024231621A1 (fr) * | 2023-05-05 | 2024-11-14 | Centre National De La Recherche Scientifique | Acide nucleique synthetique et ses utilisations therapeutiques |
Family Cites Families (46)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0411228B1 (en) | 1989-07-31 | 1995-11-08 | International Business Machines Corporation | Controlled fiber-optic switch |
| US5936076A (en) | 1991-08-29 | 1999-08-10 | Kirin Beer Kabushiki Kaisha | αgalactosylceramide derivatives |
| US7405270B2 (en) | 1991-10-25 | 2008-07-29 | Immunex Corporation | CD40-Ligand lacking native-pattern glycosylation |
| US5962406A (en) | 1991-10-25 | 1999-10-05 | Immunex Corporation | Recombinant soluble CD40 ligand polypeptide and pharmaceutical composition containing the same |
| US5981724A (en) | 1991-10-25 | 1999-11-09 | Immunex Corporation | DNA encoding CD40 ligand, a cytokine that binds CD40 |
| CA2121798C (en) | 1991-10-25 | 2007-07-24 | Richard J. Armitage | Novel cytokine |
| TW261533B (enExample) | 1992-07-16 | 1995-11-01 | Kirin Brewery | |
| KR100281264B1 (ko) | 1992-10-22 | 2001-02-01 | 마나배 게이사꾸 | 신규한 스핀고당 지질 및 그의 사용 |
| CN1045302C (zh) | 1993-04-15 | 1999-09-29 | 麒麟麦酒株式会社 | 新型(神经)鞘糖脂、含有它们的药物组合物及其用途 |
| PL181496B1 (pl) | 1996-03-20 | 2001-07-31 | Politechnika Slaska Im Wincent | Sposób wytwarzania ß-naftolu PL |
| AU705647B2 (en) | 1996-07-10 | 1999-05-27 | Immunex Corporation | Method of activating dendritic cells |
| US6017527A (en) | 1996-07-10 | 2000-01-25 | Immunex Corporation | Activated dendritic cells and methods for their activation |
| TW555562B (en) | 1996-12-27 | 2003-10-01 | Kirin Brewery | Method for activation of human antigen-presenting cells, activated human antigen-presenting cells and use thereof |
| ES2235324T3 (es) | 1997-04-10 | 2005-07-01 | Kirin Beer Kabushiki Kaisha | Empleo de a-glicosilceramidas para la obtencion de un agente terapeutico para enfermedades autoinmunes. |
| TW575420B (en) | 1997-09-22 | 2004-02-11 | Kirin Brewery | Composition for enhancing cellular immunogenicity comprising alpha-glycosylceramides |
| ATE428769T1 (de) | 1997-10-27 | 2009-05-15 | Univ Rockefeller | Methode und zusammensetzung zur herstellung von reifen dendritischen zellen |
| CA2328604A1 (en) | 1998-05-26 | 1999-12-02 | Andrew Segal | Compositions and methods of modulating an immune response to an antigen |
| EP1185627A2 (en) | 1999-06-01 | 2002-03-13 | Cornell Research Foundation, Inc. | Activation of dendritic cells to enhance immunity |
| US20030077263A1 (en) | 1999-10-29 | 2003-04-24 | Immunex Corporation | Method of activating dendritic cells |
| US20030059427A1 (en) | 2000-04-28 | 2003-03-27 | Force Walker R. | Isolation and characterization of highly active anti-CD40 antibody |
| US7063845B2 (en) | 2000-04-28 | 2006-06-20 | Gemini Science, Inc. | Human anti-CD40 antibodies |
| ES2389645T3 (es) | 2000-06-19 | 2012-10-30 | Beth Israel Deaconess Medical Center | Composiciones y métodos de anticuerpos monoclonales y policlonales específicos para subpoblaciones de linfocitos T |
| AU2001268564A1 (en) | 2000-06-22 | 2002-01-02 | The Brigham And Women's Hospital, Inc. | Alpha-glycosylceramides for treating bacterial and fungal infections |
| DE10041515A1 (de) | 2000-08-24 | 2002-03-14 | Gerold Schuler | Verfahren zur Herstellung gebrauchsfertiger, Antigen-beladener oder -unbeladener, kryokonservierter reifer dendritischer Zellen |
| US20040247563A1 (en) | 2000-11-02 | 2004-12-09 | Lynch David H. | Method of enhancing lymphocyte-mediated immune responses |
| AU2002224851A1 (en) | 2000-11-14 | 2002-05-27 | Universite Libre De Bruxelles | Generation and use of dendritic cells |
| JP2004520033A (ja) | 2001-01-15 | 2004-07-08 | イ・デ・エム・イミュノ−デジネ・モレキュル | 分化拘束された成熟樹状細胞の調製用補助組成物 |
| WO2002074345A2 (en) | 2001-03-16 | 2002-09-26 | Johns Hopkins University School Of Medicine | Immune modulation by transduced hematopoietic stem cells expressing antigens and antigen-presenting cell regulatory molecules |
| EP1270732A1 (en) | 2001-06-21 | 2003-01-02 | Schuler, Gerold | Improved transfection of eukaryontic cells with linear polynucleotides by electroporation |
| WO2003010301A1 (en) | 2001-07-25 | 2003-02-06 | I.D.M. Immuno-Designed Molecules | New isolated dendritic cells, a process for preparing the same and their use in pharmaceutical compositions |
| CA2453880A1 (en) | 2001-07-25 | 2003-02-06 | New York University | Use of glycosylceramides as adjuvants for vaccines against infections and cancer |
| DK1437358T3 (da) | 2001-08-16 | 2007-02-05 | Daiichi Asubio Pharma Co Ltd | Hidtil ukendt glycolipid og medikament til autoimmune sygdomme indeholdende dette som det aktive stof |
| EP1444330A1 (en) | 2001-11-07 | 2004-08-11 | Kirin Beer Kabushiki Kaisha | Expansion of t cells in vitro and expanded t cell populations |
| CA2493690C (en) | 2002-06-13 | 2011-11-08 | New York University | Synthetic c-glycolipid and its use for treating cancer, infectious diseases and autoimmune diseases |
| EP1572228A4 (en) | 2002-09-19 | 2009-03-04 | Centocor Inc | METHOD FOR THE INTRODUCTION OF THE TREATMENT OF DENDRITIC CELLS AND ITS USES |
| WO2004026318A1 (ja) | 2002-09-20 | 2004-04-01 | Kirin Beer Kabushiki Kaisha | α-グリコシルセラミドを有効成分とするC型肝炎ウイルス抑制剤 |
| US20040146948A1 (en) * | 2002-10-18 | 2004-07-29 | Centenary Institute Of Cancer Medicine And Cell Biology | Compositions and methods for targeting antigen-presenting cells with antibody single-chain variable region fragments |
| WO2004053095A2 (en) * | 2002-12-10 | 2004-06-24 | Merix Bioscience, Inc. | In situ maturation of dendritic cells |
| SE0301109D0 (sv) | 2003-04-14 | 2003-04-14 | Mallen Huang | Nucleotide vaccine composition |
| US20050003533A1 (en) | 2003-05-08 | 2005-01-06 | Pawel Kalinski | Mature type-1 polarized dendritic cells with enhanced IL-12 production and methods of serum-free production and use |
| AU2004277376B2 (en) | 2003-07-18 | 2011-06-02 | Eastern Virginia Medical School | Apparatus for generating electrical pulses and methods of using the same |
| CN1882603A (zh) | 2003-11-25 | 2006-12-20 | 阿哥斯医疗公司 | mRNA转染的抗原呈递细胞 |
| US20050222048A1 (en) | 2004-03-31 | 2005-10-06 | The Research Foundation Of The City University Of New York | Novel synthetic C-glycolipids, their synthesis and use to treat infections, cancer and autoimmune diseases |
| KR101243577B1 (ko) | 2004-10-07 | 2013-03-20 | 아고스 쎄라퓨틱스, 인코포레이티드 | 성숙 수지상 세포 조성물 및 그의 배양 방법 |
| US8513008B2 (en) | 2004-10-07 | 2013-08-20 | Argos Therapeutics, Inc. | Mature dendritic cell compositions and methods for culturing same |
| JP5020935B2 (ja) * | 2005-04-08 | 2012-09-05 | アルゴス セラピューティクス,インコーポレイティド | 樹状細胞組成物および方法 |
-
2006
- 2006-04-07 US US11/400,774 patent/US8513008B2/en not_active Expired - Fee Related
-
2007
- 2007-04-06 KR KR1020087024226A patent/KR101425686B1/ko not_active Expired - Fee Related
- 2007-04-06 WO PCT/US2007/008734 patent/WO2007117682A2/en not_active Ceased
- 2007-04-06 US US12/226,092 patent/US20120269832A1/en not_active Abandoned
- 2007-04-06 JP JP2009504346A patent/JP5425618B2/ja not_active Expired - Fee Related
- 2007-04-06 AT AT07755113T patent/ATE510451T1/de active
- 2007-04-06 DK DK07755113.3T patent/DK2003969T3/da active
- 2007-04-06 AU AU2007235227A patent/AU2007235227B2/en not_active Ceased
- 2007-04-06 CA CA2648675A patent/CA2648675C/en active Active
- 2007-04-06 CN CN200780020901.5A patent/CN101460054B/zh not_active Expired - Fee Related
- 2007-04-06 EP EP07755113A patent/EP2003969B1/en active Active
-
2013
- 2013-07-15 US US13/941,627 patent/US9523077B2/en not_active Expired - Fee Related
-
2016
- 2016-12-19 US US15/383,574 patent/US10184108B2/en not_active Expired - Lifetime
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009533025A (ja) | 2009-09-17 |
| EP2003969A4 (en) | 2009-05-13 |
| CN101460054A (zh) | 2009-06-17 |
| CN101460054B (zh) | 2014-03-12 |
| KR20090006078A (ko) | 2009-01-14 |
| EP2003969A2 (en) | 2008-12-24 |
| ATE510451T1 (de) | 2011-06-15 |
| WO2007117682A2 (en) | 2007-10-18 |
| AU2007235227B2 (en) | 2012-10-04 |
| US20120269832A1 (en) | 2012-10-25 |
| CA2648675C (en) | 2015-03-10 |
| US20170096639A1 (en) | 2017-04-06 |
| US10184108B2 (en) | 2019-01-22 |
| US9523077B2 (en) | 2016-12-20 |
| DK2003969T3 (da) | 2011-09-12 |
| AU2007235227A1 (en) | 2007-10-18 |
| US20140017788A1 (en) | 2014-01-16 |
| WO2007117682A3 (en) | 2008-10-16 |
| KR101425686B1 (ko) | 2014-08-01 |
| US8513008B2 (en) | 2013-08-20 |
| CA2648675A1 (en) | 2007-10-18 |
| US20070082400A1 (en) | 2007-04-12 |
| EP2003969B1 (en) | 2011-05-25 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5425618B2 (ja) | 成熟樹状細胞組成物およびそれを培養するための方法 | |
| CN101080487B (zh) | 成熟树突细胞组合物及其培养方法 | |
| EP3107996B1 (en) | Tscm cells and methods for use | |
| HK1105997B (en) | Mature dendritic cell compositions and methods for culturing same | |
| HK1144588A (en) | Mature dendritic cell compositions and methods for culturing same | |
| HK1144588B (en) | Mature dendritic cell compositions and methods for culturing same |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100401 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100406 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A712 Effective date: 20100413 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100929 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20111011 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120803 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20121031 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20121107 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20121203 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20121210 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130104 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130111 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130204 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130726 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20131011 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20131101 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20131127 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 Ref document number: 5425618 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |