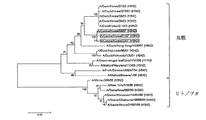
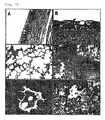
JP5404636B2 - 新規のイヌインフルエンザウイルス及びこのワクチン - Google Patents
新規のイヌインフルエンザウイルス及びこのワクチン Download PDFInfo
- Publication number
- JP5404636B2 JP5404636B2 JP2010530910A JP2010530910A JP5404636B2 JP 5404636 B2 JP5404636 B2 JP 5404636B2 JP 2010530910 A JP2010530910 A JP 2010530910A JP 2010530910 A JP2010530910 A JP 2010530910A JP 5404636 B2 JP5404636 B2 JP 5404636B2
- Authority
- JP
- Japan
- Prior art keywords
- influenza virus
- seq
- canine
- amino acid
- represented
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
- A61K39/145—Orthomyxoviridae, e.g. influenza virus
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/12—Viral antigens
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/12—Antidiarrhoeals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/16—Otologicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/005—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from viruses
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/14—Hydrolases (3)
- C12N9/24—Hydrolases (3) acting on glycosyl compounds (3.2)
- C12N9/2402—Hydrolases (3) acting on glycosyl compounds (3.2) hydrolysing O- and S- glycosyl compounds (3.2.1)
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y302/00—Hydrolases acting on glycosyl compounds, i.e. glycosylases (3.2)
- C12Y302/01—Glycosidases, i.e. enzymes hydrolysing O- and S-glycosyl compounds (3.2.1)
- C12Y302/01018—Exo-alpha-sialidase (3.2.1.18), i.e. trans-sialidase
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/51—Medicinal preparations containing antigens or antibodies comprising whole cells, viruses or DNA/RNA
- A61K2039/525—Virus
- A61K2039/5252—Virus inactivated (killed)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/55—Medicinal preparations containing antigens or antibodies characterised by the host/recipient, e.g. newborn with maternal antibodies
- A61K2039/552—Veterinary vaccine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/555—Medicinal preparations containing antigens or antibodies characterised by a specific combination antigen/adjuvant
- A61K2039/55505—Inorganic adjuvants
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/16011—Orthomyxoviridae
- C12N2760/16111—Influenzavirus A, i.e. influenza A virus
- C12N2760/16121—Viruses as such, e.g. new isolates, mutants or their genomic sequences
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/16011—Orthomyxoviridae
- C12N2760/16111—Influenzavirus A, i.e. influenza A virus
- C12N2760/16122—New viral proteins or individual genes, new structural or functional aspects of known viral proteins or genes
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2760/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssRNA viruses negative-sense
- C12N2760/00011—Details
- C12N2760/16011—Orthomyxoviridae
- C12N2760/16111—Influenzavirus A, i.e. influenza A virus
- C12N2760/16134—Use of virus or viral component as vaccine, e.g. live-attenuated or inactivated virus, VLP, viral protein
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Health & Medical Sciences (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Medicinal Chemistry (AREA)
- Virology (AREA)
- General Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Microbiology (AREA)
- Biomedical Technology (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Biotechnology (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Pulmonology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Mycology (AREA)
- Biophysics (AREA)
- Epidemiology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Gastroenterology & Hepatology (AREA)
- Physics & Mathematics (AREA)
- Plant Pathology (AREA)
- Communicable Diseases (AREA)
- Oncology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| KR10-2007-0109535 | 2007-10-30 | ||
| KR1020070109535A KR100850545B1 (ko) | 2007-10-30 | 2007-10-30 | 신규한 개 인플루엔자 바이러스 및 이의 백신 |
| PCT/KR2007/005789 WO2009057843A1 (en) | 2007-10-30 | 2007-11-16 | A novel canine influenza virus and vaccine therefore |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2011501950A JP2011501950A (ja) | 2011-01-20 |
| JP2011501950A5 JP2011501950A5 (enExample) | 2013-06-20 |
| JP5404636B2 true JP5404636B2 (ja) | 2014-02-05 |
Family
ID=39881230
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2010530910A Expired - Fee Related JP5404636B2 (ja) | 2007-10-30 | 2007-11-16 | 新規のイヌインフルエンザウイルス及びこのワクチン |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US8246962B2 (enExample) |
| EP (1) | EP2078083B1 (enExample) |
| JP (1) | JP5404636B2 (enExample) |
| KR (1) | KR100850545B1 (enExample) |
| CN (1) | CN101605898B (enExample) |
| AT (1) | ATE554789T1 (enExample) |
| ES (1) | ES2383158T3 (enExample) |
| WO (1) | WO2009057843A1 (enExample) |
Families Citing this family (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN101838708B (zh) * | 2010-03-30 | 2012-07-18 | 华南农业大学 | 一种检测犬流感的方法 |
| CA2829226C (en) | 2011-03-14 | 2023-01-03 | Boehringer Ingelheim Vetmedica, Inc. | Equine rhinitis vaccine |
| CN102220293B (zh) * | 2011-05-26 | 2012-12-19 | 中国农业科学院上海兽医研究所 | 犬流感重组病毒及其制备方法和应用 |
| KR101390554B1 (ko) | 2012-05-22 | 2014-05-21 | 충남대학교산학협력단 | 신규한 개 인플루엔자 바이러스(h3n2), 이를 포함하는 백신 조성물 및 h3n2 바이러스 검정 키트 |
| KR101484588B1 (ko) * | 2012-10-24 | 2015-01-20 | 대한민국 | 개 인플루엔자 vlp 백신의 제조방법 |
| CN103223162B (zh) * | 2013-04-03 | 2014-07-30 | 华南农业大学 | 一株h3n2犬流感病毒株cgd1的应用 |
| KR101511619B1 (ko) * | 2013-04-30 | 2015-04-13 | 한국생명공학연구원 | 개 인플루엔자 바이러스 h3n2의 ha1 단백질을 항원으로 포함하는 개 인플루엔자 바이러스 h3n2에 특이적인 혈청 진단용 키트 |
| KR101627623B1 (ko) | 2013-08-29 | 2016-06-08 | 한국생명공학연구원 | 이종 인플루엔자 바이러스에 대한 백신 조성물 |
| KR101768600B1 (ko) * | 2015-05-18 | 2017-08-17 | 한국생명공학연구원 | 범용성 개 인플루엔자 바이러스 백신 조성물 |
| MX382530B (es) * | 2015-06-26 | 2025-03-13 | Boehringer Ingelheim Animal Health Usa Inc | Vacunas inactivadas contra la influenza canina, metodos para elaborarlas y sus usos. |
| WO2017031401A2 (en) * | 2015-08-20 | 2017-02-23 | University Of Rochester | Ns1 truncated virus for the development of canine influenza vaccines |
| WO2017031404A1 (en) | 2015-08-20 | 2017-02-23 | University Of Rochester | Live-attenuated vaccine having mutations in viral polymerase for the treatment and prevention of canine influenza virus |
| CN105463021A (zh) * | 2015-12-25 | 2016-04-06 | 华南农业大学 | 表达h3n2犬流感ha蛋白的重组犬腺病毒疫苗载体的构建方法 |
| CN106248937A (zh) * | 2016-09-09 | 2016-12-21 | 华中农业大学 | 一种检测犬副流感病毒抗体的间接elisa试剂盒 |
| MX2020008927A (es) | 2018-02-27 | 2021-01-08 | Univ Rochester | Vacuna contra la influenza atenuada viva multivalente para la prevencion y el control del virus de la influenza equina (eiv) en caballos. |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0733113B1 (en) * | 1994-01-11 | 2007-05-02 | Vlaams Interuniversitair Instituut voor Biotechnologie | Influenza vaccine |
| AT407958B (de) * | 1999-02-11 | 2001-07-25 | Immuno Ag | Inaktivierte influenza-virus-vakzine zur nasalen oder oralen applikation |
| CA2621320C (en) * | 2005-10-07 | 2016-05-24 | Pfizer Products Inc. | Vaccines and methods to treat canine influenza |
-
2007
- 2007-10-30 KR KR1020070109535A patent/KR100850545B1/ko active Active
- 2007-11-16 AT AT07834095T patent/ATE554789T1/de active
- 2007-11-16 CN CN2007800299717A patent/CN101605898B/zh not_active Expired - Fee Related
- 2007-11-16 ES ES07834095T patent/ES2383158T3/es active Active
- 2007-11-16 JP JP2010530910A patent/JP5404636B2/ja not_active Expired - Fee Related
- 2007-11-16 US US12/375,924 patent/US8246962B2/en active Active
- 2007-11-16 EP EP07834095A patent/EP2078083B1/en active Active
- 2007-11-16 WO PCT/KR2007/005789 patent/WO2009057843A1/en not_active Ceased
Also Published As
| Publication number | Publication date |
|---|---|
| EP2078083A1 (en) | 2009-07-15 |
| KR100850545B1 (ko) | 2008-08-05 |
| EP2078083B1 (en) | 2012-04-25 |
| CN101605898A (zh) | 2009-12-16 |
| EP2078083A4 (en) | 2009-10-28 |
| JP2011501950A (ja) | 2011-01-20 |
| ATE554789T1 (de) | 2012-05-15 |
| US8246962B2 (en) | 2012-08-21 |
| CN101605898B (zh) | 2012-05-23 |
| WO2009057843A1 (en) | 2009-05-07 |
| US20100285063A1 (en) | 2010-11-11 |
| ES2383158T3 (es) | 2012-06-18 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5404636B2 (ja) | 新規のイヌインフルエンザウイルス及びこのワクチン | |
| US11160859B2 (en) | Materials and methods for respiratory disease control in canines | |
| RU2711807C2 (ru) | Вирус гриппа, способный инфицировать собачьих, и его применение | |
| JP6301855B2 (ja) | イヌにおける呼吸器疾患管理のための材料および方法 | |
| KR101390554B1 (ko) | 신규한 개 인플루엔자 바이러스(h3n2), 이를 포함하는 백신 조성물 및 h3n2 바이러스 검정 키트 | |
| AU2018203186A1 (en) | Influenza viruses able to infect canids, uses thereof | |
| KR101626798B1 (ko) | 신규한 h3n2 혈청형의 인플루엔자 바이러스 및 이를 포함하는 백신 조성물 | |
| KR101627623B1 (ko) | 이종 인플루엔자 바이러스에 대한 백신 조성물 | |
| US11865172B2 (en) | Materials and methods for respiratory disease control in canines | |
| KR20250042901A (ko) | 유전자가 재배열된 h3n1 혈청형의 개 인플루엔자 바이러스 및 이를 포함하는 백신 조성물 | |
| HK1167660B (en) | Materials for respiratory disease control in canines |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20121113 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130131 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130207 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130312 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130319 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20130412 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20130419 |
|
| A524 | Written submission of copy of amendment under article 19 pct |
Free format text: JAPANESE INTERMEDIATE CODE: A524 Effective date: 20130430 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20130723 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130829 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20131001 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20131029 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5404636 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |