JP5101288B2 - アプタマー調節される核酸及びその利用 - Google Patents
アプタマー調節される核酸及びその利用 Download PDFInfo
- Publication number
- JP5101288B2 JP5101288B2 JP2007534921A JP2007534921A JP5101288B2 JP 5101288 B2 JP5101288 B2 JP 5101288B2 JP 2007534921 A JP2007534921 A JP 2007534921A JP 2007534921 A JP2007534921 A JP 2007534921A JP 5101288 B2 JP5101288 B2 JP 5101288B2
- Authority
- JP
- Japan
- Prior art keywords
- nucleic acid
- aptamer
- ligand
- cell
- expression
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 108091023037 Aptamer Proteins 0.000 title claims abstract description 410
- 150000007523 nucleic acids Chemical class 0.000 title claims abstract description 355
- 108020004707 nucleic acids Proteins 0.000 title claims abstract description 350
- 102000039446 nucleic acids Human genes 0.000 title claims abstract description 349
- 230000001105 regulatory effect Effects 0.000 title claims description 210
- 239000003446 ligand Substances 0.000 claims abstract description 309
- 239000012636 effector Substances 0.000 claims abstract description 150
- 210000004027 cell Anatomy 0.000 claims description 188
- 108090000623 proteins and genes Proteins 0.000 claims description 185
- 230000014509 gene expression Effects 0.000 claims description 153
- 238000000034 method Methods 0.000 claims description 112
- 229920002477 rna polymer Polymers 0.000 claims description 108
- 230000027455 binding Effects 0.000 claims description 87
- 230000000694 effects Effects 0.000 claims description 85
- 108020004999 messenger RNA Proteins 0.000 claims description 61
- 238000001727 in vivo Methods 0.000 claims description 56
- 239000000758 substrate Substances 0.000 claims description 50
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 claims description 45
- 108020004459 Small interfering RNA Proteins 0.000 claims description 45
- 230000009368 gene silencing by RNA Effects 0.000 claims description 45
- 102000004190 Enzymes Human genes 0.000 claims description 44
- 108090000790 Enzymes Proteins 0.000 claims description 44
- 125000003729 nucleotide group Chemical group 0.000 claims description 43
- 239000002773 nucleotide Substances 0.000 claims description 41
- 230000004044 response Effects 0.000 claims description 39
- 102000004169 proteins and genes Human genes 0.000 claims description 38
- 230000008859 change Effects 0.000 claims description 37
- 239000002679 microRNA Substances 0.000 claims description 37
- 238000000338 in vitro Methods 0.000 claims description 32
- 239000002207 metabolite Substances 0.000 claims description 31
- 150000003839 salts Chemical class 0.000 claims description 27
- 238000013518 transcription Methods 0.000 claims description 20
- 230000035897 transcription Effects 0.000 claims description 20
- 230000003834 intracellular effect Effects 0.000 claims description 18
- 101710163270 Nuclease Proteins 0.000 claims description 17
- 230000037353 metabolic pathway Effects 0.000 claims description 17
- 244000052769 pathogen Species 0.000 claims description 17
- 230000001717 pathogenic effect Effects 0.000 claims description 16
- 239000002243 precursor Substances 0.000 claims description 16
- 108091026890 Coding region Proteins 0.000 claims description 15
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 15
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 15
- 230000010261 cell growth Effects 0.000 claims description 14
- 108700020796 Oncogene Proteins 0.000 claims description 13
- 230000019491 signal transduction Effects 0.000 claims description 13
- 230000024245 cell differentiation Effects 0.000 claims description 12
- 230000001404 mediated effect Effects 0.000 claims description 12
- 230000004663 cell proliferation Effects 0.000 claims description 10
- 208000015181 infectious disease Diseases 0.000 claims description 10
- 230000037361 pathway Effects 0.000 claims description 10
- 150000003384 small molecules Chemical class 0.000 claims description 10
- 230000015572 biosynthetic process Effects 0.000 claims description 9
- 239000003814 drug Substances 0.000 claims description 9
- 239000000284 extract Substances 0.000 claims description 9
- 238000006243 chemical reaction Methods 0.000 claims description 8
- 230000030279 gene silencing Effects 0.000 claims description 8
- 210000000130 stem cell Anatomy 0.000 claims description 8
- 230000004083 survival effect Effects 0.000 claims description 8
- 229940079593 drug Drugs 0.000 claims description 7
- 230000030833 cell death Effects 0.000 claims description 6
- 206010020718 hyperplasia Diseases 0.000 claims description 6
- 230000002503 metabolic effect Effects 0.000 claims description 6
- 239000008194 pharmaceutical composition Substances 0.000 claims description 6
- 239000003937 drug carrier Substances 0.000 claims description 5
- 239000002777 nucleoside Substances 0.000 claims description 5
- 150000003833 nucleoside derivatives Chemical class 0.000 claims description 5
- 241000894007 species Species 0.000 claims description 5
- 231100000419 toxicity Toxicity 0.000 claims description 5
- 230000001988 toxicity Effects 0.000 claims description 5
- 230000001276 controlling effect Effects 0.000 claims description 4
- 230000004069 differentiation Effects 0.000 claims description 4
- 230000002255 enzymatic effect Effects 0.000 claims description 4
- 238000012226 gene silencing method Methods 0.000 claims description 4
- 230000035699 permeability Effects 0.000 claims description 4
- 229920001184 polypeptide Polymers 0.000 claims description 4
- 150000001720 carbohydrates Chemical class 0.000 claims description 3
- 235000014633 carbohydrates Nutrition 0.000 claims description 3
- 206010012601 diabetes mellitus Diseases 0.000 claims description 3
- 125000000524 functional group Chemical group 0.000 claims description 3
- 230000028993 immune response Effects 0.000 claims description 3
- 230000000977 initiatory effect Effects 0.000 claims description 3
- 229910021645 metal ion Inorganic materials 0.000 claims description 3
- 230000035945 sensitivity Effects 0.000 claims description 3
- 150000003431 steroids Chemical class 0.000 claims description 3
- 230000008238 biochemical pathway Effects 0.000 claims description 2
- 125000001369 canonical nucleoside group Chemical group 0.000 claims description 2
- 235000014113 dietary fatty acids Nutrition 0.000 claims description 2
- 231100000673 dose–response relationship Toxicity 0.000 claims description 2
- 230000009483 enzymatic pathway Effects 0.000 claims description 2
- 229930195729 fatty acid Natural products 0.000 claims description 2
- 239000000194 fatty acid Substances 0.000 claims description 2
- 150000004665 fatty acids Chemical class 0.000 claims description 2
- 150000002632 lipids Chemical class 0.000 claims description 2
- 238000012544 monitoring process Methods 0.000 claims description 2
- 229930014626 natural product Natural products 0.000 claims description 2
- 239000000813 peptide hormone Substances 0.000 claims description 2
- 102000035123 post-translationally modified proteins Human genes 0.000 claims description 2
- 108091005626 post-translationally modified proteins Proteins 0.000 claims description 2
- 230000002463 transducing effect Effects 0.000 claims description 2
- 108091070501 miRNA Proteins 0.000 claims 10
- 239000002924 silencing RNA Substances 0.000 claims 4
- 238000002425 crystallisation Methods 0.000 claims 2
- 230000008025 crystallization Effects 0.000 claims 2
- 210000003630 histaminocyte Anatomy 0.000 claims 2
- 230000004565 tumor cell growth Effects 0.000 claims 2
- 102000015271 Intercellular Adhesion Molecule-1 Human genes 0.000 claims 1
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 claims 1
- 230000002103 transcriptional effect Effects 0.000 claims 1
- ZFXYFBGIUFBOJW-UHFFFAOYSA-N theophylline Chemical compound O=C1N(C)C(=O)N(C)C2=C1NC=N2 ZFXYFBGIUFBOJW-UHFFFAOYSA-N 0.000 description 161
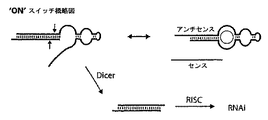
- 230000000692 anti-sense effect Effects 0.000 description 105
- 229960000278 theophylline Drugs 0.000 description 80
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 42
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 42
- 239000005090 green fluorescent protein Substances 0.000 description 42
- 108700011259 MicroRNAs Proteins 0.000 description 31
- 239000002585 base Substances 0.000 description 28
- 230000007246 mechanism Effects 0.000 description 28
- 239000004055 small Interfering RNA Substances 0.000 description 28
- 102100023387 Endoribonuclease Dicer Human genes 0.000 description 26
- 101000907904 Homo sapiens Endoribonuclease Dicer Proteins 0.000 description 26
- 230000008685 targeting Effects 0.000 description 26
- 239000013612 plasmid Substances 0.000 description 25
- 230000001419 dependent effect Effects 0.000 description 22
- 230000000295 complement effect Effects 0.000 description 20
- 239000000203 mixture Substances 0.000 description 20
- 238000013461 design Methods 0.000 description 19
- 238000009396 hybridization Methods 0.000 description 19
- RYYVLZVUVIJVGH-UHFFFAOYSA-N caffeine Chemical compound CN1C(=O)N(C)C(=O)C2=C1N=CN2C RYYVLZVUVIJVGH-UHFFFAOYSA-N 0.000 description 18
- 238000013519 translation Methods 0.000 description 18
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 17
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 17
- 238000003776 cleavage reaction Methods 0.000 description 17
- 230000007017 scission Effects 0.000 description 17
- 238000011282 treatment Methods 0.000 description 16
- 239000004098 Tetracycline Substances 0.000 description 14
- 239000002253 acid Substances 0.000 description 14
- 229960002180 tetracycline Drugs 0.000 description 14
- 229930101283 tetracycline Natural products 0.000 description 14
- 235000019364 tetracycline Nutrition 0.000 description 14
- 150000003522 tetracyclines Chemical class 0.000 description 14
- 210000001519 tissue Anatomy 0.000 description 14
- 239000013604 expression vector Substances 0.000 description 13
- 108091034117 Oligonucleotide Proteins 0.000 description 11
- 238000003556 assay Methods 0.000 description 11
- 238000005516 engineering process Methods 0.000 description 11
- 230000012010 growth Effects 0.000 description 11
- 230000005764 inhibitory process Effects 0.000 description 11
- 230000002265 prevention Effects 0.000 description 11
- YMHOBZXQZVXHBM-UHFFFAOYSA-N 2,5-dimethoxy-4-bromophenethylamine Chemical compound COC1=CC(CCN)=C(OC)C=C1Br YMHOBZXQZVXHBM-UHFFFAOYSA-N 0.000 description 10
- 241001465754 Metazoa Species 0.000 description 10
- 108091081021 Sense strand Proteins 0.000 description 10
- 108091081024 Start codon Proteins 0.000 description 10
- 241000545067 Venus Species 0.000 description 10
- 230000033228 biological regulation Effects 0.000 description 10
- 230000006870 function Effects 0.000 description 10
- 239000000543 intermediate Substances 0.000 description 10
- 108091027963 non-coding RNA Proteins 0.000 description 10
- 102000042567 non-coding RNA Human genes 0.000 description 10
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 9
- LPHGQDQBBGAPDZ-UHFFFAOYSA-N Isocaffeine Natural products CN1C(=O)N(C)C(=O)C2=C1N(C)C=N2 LPHGQDQBBGAPDZ-UHFFFAOYSA-N 0.000 description 9
- 101100144701 Mus musculus Drosha gene Proteins 0.000 description 9
- 101710135898 Myc proto-oncogene protein Proteins 0.000 description 9
- 101710150448 Transcriptional regulator Myc Proteins 0.000 description 9
- 230000004913 activation Effects 0.000 description 9
- VJEONQKOZGKCAK-UHFFFAOYSA-N caffeine Natural products CN1C(=O)N(C)C(=O)C2=C1C=CN2C VJEONQKOZGKCAK-UHFFFAOYSA-N 0.000 description 9
- 229960001948 caffeine Drugs 0.000 description 9
- 239000003795 chemical substances by application Substances 0.000 description 9
- 239000000499 gel Substances 0.000 description 9
- 230000001965 increasing effect Effects 0.000 description 9
- 241000588724 Escherichia coli Species 0.000 description 8
- 102100038895 Myc proto-oncogene protein Human genes 0.000 description 8
- 102000043276 Oncogene Human genes 0.000 description 8
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 8
- 230000003281 allosteric effect Effects 0.000 description 8
- -1 aromatic small molecules Chemical class 0.000 description 8
- 230000001413 cellular effect Effects 0.000 description 8
- 230000002401 inhibitory effect Effects 0.000 description 8
- 239000000126 substance Substances 0.000 description 8
- 108700008625 Reporter Genes Proteins 0.000 description 7
- 108091027967 Small hairpin RNA Proteins 0.000 description 7
- 201000010099 disease Diseases 0.000 description 7
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 7
- 238000009472 formulation Methods 0.000 description 7
- 230000004927 fusion Effects 0.000 description 7
- 239000002609 medium Substances 0.000 description 7
- 239000013615 primer Substances 0.000 description 7
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 108010083644 Ribonucleases Proteins 0.000 description 6
- 102000006382 Ribonucleases Human genes 0.000 description 6
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 6
- IQFYYKKMVGJFEH-XLPZGREQSA-N Thymidine Chemical compound O=C1NC(=O)C(C)=CN1[C@@H]1O[C@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-XLPZGREQSA-N 0.000 description 6
- 230000000903 blocking effect Effects 0.000 description 6
- 210000000170 cell membrane Anatomy 0.000 description 6
- 239000002299 complementary DNA Substances 0.000 description 6
- 230000007613 environmental effect Effects 0.000 description 6
- 229930182830 galactose Natural products 0.000 description 6
- 230000003993 interaction Effects 0.000 description 6
- 210000004962 mammalian cell Anatomy 0.000 description 6
- 229910052751 metal Inorganic materials 0.000 description 6
- 239000002184 metal Substances 0.000 description 6
- 150000002739 metals Chemical class 0.000 description 6
- 230000004048 modification Effects 0.000 description 6
- 238000012986 modification Methods 0.000 description 6
- 238000012545 processing Methods 0.000 description 6
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 6
- 108090000994 Catalytic RNA Proteins 0.000 description 5
- 102000053642 Catalytic RNA Human genes 0.000 description 5
- 238000011529 RT qPCR Methods 0.000 description 5
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 5
- 150000001412 amines Chemical group 0.000 description 5
- 239000012491 analyte Substances 0.000 description 5
- 239000000074 antisense oligonucleotide Substances 0.000 description 5
- 238000012230 antisense oligonucleotides Methods 0.000 description 5
- 230000006907 apoptotic process Effects 0.000 description 5
- 238000013459 approach Methods 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 238000012512 characterization method Methods 0.000 description 5
- 239000003184 complementary RNA Substances 0.000 description 5
- 230000000875 corresponding effect Effects 0.000 description 5
- 238000010586 diagram Methods 0.000 description 5
- 230000002068 genetic effect Effects 0.000 description 5
- 150000002500 ions Chemical class 0.000 description 5
- 238000013507 mapping Methods 0.000 description 5
- 230000009467 reduction Effects 0.000 description 5
- 230000002829 reductive effect Effects 0.000 description 5
- 230000010076 replication Effects 0.000 description 5
- 208000037803 restenosis Diseases 0.000 description 5
- 108091092562 ribozyme Proteins 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- 238000006467 substitution reaction Methods 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- 239000013598 vector Substances 0.000 description 5
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 4
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 206010028980 Neoplasm Diseases 0.000 description 4
- 108091093037 Peptide nucleic acid Proteins 0.000 description 4
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 4
- 108010057163 Ribonuclease III Proteins 0.000 description 4
- 102000003661 Ribonuclease III Human genes 0.000 description 4
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 4
- 241000700605 Viruses Species 0.000 description 4
- 150000007513 acids Chemical class 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 230000008860 allosteric change Effects 0.000 description 4
- 239000003242 anti bacterial agent Substances 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 238000010276 construction Methods 0.000 description 4
- 239000000975 dye Substances 0.000 description 4
- 150000002148 esters Chemical class 0.000 description 4
- 238000003384 imaging method Methods 0.000 description 4
- 230000001939 inductive effect Effects 0.000 description 4
- 230000010354 integration Effects 0.000 description 4
- 238000002955 isolation Methods 0.000 description 4
- 210000003734 kidney Anatomy 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 229920005615 natural polymer Polymers 0.000 description 4
- 229920000642 polymer Polymers 0.000 description 4
- 229920001282 polysaccharide Polymers 0.000 description 4
- 239000005017 polysaccharide Substances 0.000 description 4
- 150000004804 polysaccharides Chemical class 0.000 description 4
- 230000035755 proliferation Effects 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 239000000243 solution Substances 0.000 description 4
- 238000011105 stabilization Methods 0.000 description 4
- 229920001059 synthetic polymer Polymers 0.000 description 4
- 238000001890 transfection Methods 0.000 description 4
- 230000001052 transient effect Effects 0.000 description 4
- LRFVTYWOQMYALW-UHFFFAOYSA-N 9H-xanthine Chemical compound O=C1NC(=O)NC2=C1NC=N2 LRFVTYWOQMYALW-UHFFFAOYSA-N 0.000 description 3
- QTBSBXVTEAMEQO-UHFFFAOYSA-N Acetic acid Chemical compound CC(O)=O QTBSBXVTEAMEQO-UHFFFAOYSA-N 0.000 description 3
- 241000894006 Bacteria Species 0.000 description 3
- 108020004705 Codon Proteins 0.000 description 3
- 108020004394 Complementary RNA Proteins 0.000 description 3
- 102000005685 Cyclic AMP-Dependent Protein Kinase Type I Human genes 0.000 description 3
- 108010084920 Cyclic AMP-Dependent Protein Kinase Type I Proteins 0.000 description 3
- 102000004127 Cytokines Human genes 0.000 description 3
- 108090000695 Cytokines Proteins 0.000 description 3
- 108020004414 DNA Proteins 0.000 description 3
- 102000053602 DNA Human genes 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 108090000288 Glycoproteins Proteins 0.000 description 3
- 102000003886 Glycoproteins Human genes 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- VEXZGXHMUGYJMC-UHFFFAOYSA-N Hydrochloric acid Chemical compound Cl VEXZGXHMUGYJMC-UHFFFAOYSA-N 0.000 description 3
- 206010061218 Inflammation Diseases 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- MUBZPKHOEPUJKR-UHFFFAOYSA-N Oxalic acid Chemical compound OC(=O)C(O)=O MUBZPKHOEPUJKR-UHFFFAOYSA-N 0.000 description 3
- 108020004511 Recombinant DNA Proteins 0.000 description 3
- 108010046983 Ribonuclease T1 Proteins 0.000 description 3
- 241000251131 Sphyrna Species 0.000 description 3
- 229960000723 ampicillin Drugs 0.000 description 3
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 3
- 229940088710 antibiotic agent Drugs 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- IQFYYKKMVGJFEH-UHFFFAOYSA-N beta-L-thymidine Natural products O=C1NC(=O)C(C)=CN1C1OC(CO)C(O)C1 IQFYYKKMVGJFEH-UHFFFAOYSA-N 0.000 description 3
- 201000011510 cancer Diseases 0.000 description 3
- 230000003833 cell viability Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 238000010367 cloning Methods 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 230000002950 deficient Effects 0.000 description 3
- 238000010494 dissociation reaction Methods 0.000 description 3
- 230000005593 dissociations Effects 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 230000003828 downregulation Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000012737 fresh medium Substances 0.000 description 3
- 239000005556 hormone Substances 0.000 description 3
- 229940088597 hormone Drugs 0.000 description 3
- 238000011534 incubation Methods 0.000 description 3
- 230000004054 inflammatory process Effects 0.000 description 3
- 239000000138 intercalating agent Substances 0.000 description 3
- 239000003550 marker Substances 0.000 description 3
- YACKEPLHDIMKIO-UHFFFAOYSA-N methylphosphonic acid Chemical class CP(O)(O)=O YACKEPLHDIMKIO-UHFFFAOYSA-N 0.000 description 3
- 150000007522 mineralic acids Chemical class 0.000 description 3
- 230000011278 mitosis Effects 0.000 description 3
- 239000013642 negative control Substances 0.000 description 3
- 230000007935 neutral effect Effects 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 210000004940 nucleus Anatomy 0.000 description 3
- 150000007524 organic acids Chemical class 0.000 description 3
- 150000008298 phosphoramidates Chemical class 0.000 description 3
- 230000006641 stabilisation Effects 0.000 description 3
- 238000010561 standard procedure Methods 0.000 description 3
- 238000003786 synthesis reaction Methods 0.000 description 3
- 239000003053 toxin Substances 0.000 description 3
- 231100000765 toxin Toxicity 0.000 description 3
- 108700012359 toxins Proteins 0.000 description 3
- 230000007704 transition Effects 0.000 description 3
- 230000001960 triggered effect Effects 0.000 description 3
- 210000005253 yeast cell Anatomy 0.000 description 3
- FQVLRGLGWNWPSS-BXBUPLCLSA-N (4r,7s,10s,13s,16r)-16-acetamido-13-(1h-imidazol-5-ylmethyl)-10-methyl-6,9,12,15-tetraoxo-7-propan-2-yl-1,2-dithia-5,8,11,14-tetrazacycloheptadecane-4-carboxamide Chemical compound N1C(=O)[C@@H](NC(C)=O)CSSC[C@@H](C(N)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@H](C)NC(=O)[C@@H]1CC1=CN=CN1 FQVLRGLGWNWPSS-BXBUPLCLSA-N 0.000 description 2
- LMDZBCPBFSXMTL-UHFFFAOYSA-N 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide Chemical compound CCN=C=NCCCN(C)C LMDZBCPBFSXMTL-UHFFFAOYSA-N 0.000 description 2
- RFLVMTUMFYRZCB-UHFFFAOYSA-N 1-methylguanine Chemical compound O=C1N(C)C(N)=NC2=C1N=CN2 RFLVMTUMFYRZCB-UHFFFAOYSA-N 0.000 description 2
- YSAJFXWTVFGPAX-UHFFFAOYSA-N 2-[(2,4-dioxo-1h-pyrimidin-5-yl)oxy]acetic acid Chemical compound OC(=O)COC1=CNC(=O)NC1=O YSAJFXWTVFGPAX-UHFFFAOYSA-N 0.000 description 2
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 2
- FZWGECJQACGGTI-UHFFFAOYSA-N 2-amino-7-methyl-1,7-dihydro-6H-purin-6-one Chemical compound NC1=NC(O)=C2N(C)C=NC2=N1 FZWGECJQACGGTI-UHFFFAOYSA-N 0.000 description 2
- OVONXEQGWXGFJD-UHFFFAOYSA-N 4-sulfanylidene-1h-pyrimidin-2-one Chemical compound SC=1C=CNC(=O)N=1 OVONXEQGWXGFJD-UHFFFAOYSA-N 0.000 description 2
- OIVLITBTBDPEFK-UHFFFAOYSA-N 5,6-dihydrouracil Chemical compound O=C1CCNC(=O)N1 OIVLITBTBDPEFK-UHFFFAOYSA-N 0.000 description 2
- LQLQRFGHAALLLE-UHFFFAOYSA-N 5-bromouracil Chemical group BrC1=CNC(=O)NC1=O LQLQRFGHAALLLE-UHFFFAOYSA-N 0.000 description 2
- ZLAQATDNGLKIEV-UHFFFAOYSA-N 5-methyl-2-sulfanylidene-1h-pyrimidin-4-one Chemical compound CC1=CNC(=S)NC1=O ZLAQATDNGLKIEV-UHFFFAOYSA-N 0.000 description 2
- 102100034035 Alcohol dehydrogenase 1A Human genes 0.000 description 2
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 2
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 2
- 108020005544 Antisense RNA Proteins 0.000 description 2
- 102100021569 Apoptosis regulator Bcl-2 Human genes 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- 208000010839 B-cell chronic lymphocytic leukemia Diseases 0.000 description 2
- 108091032955 Bacterial small RNA Proteins 0.000 description 2
- 101150017888 Bcl2 gene Proteins 0.000 description 2
- DWRXFEITVBNRMK-UHFFFAOYSA-N Beta-D-1-Arabinofuranosylthymine Natural products O=C1NC(=O)C(C)=CN1C1C(O)C(O)C(CO)O1 DWRXFEITVBNRMK-UHFFFAOYSA-N 0.000 description 2
- 101100268645 Caenorhabditis elegans abl-1 gene Proteins 0.000 description 2
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 2
- ZAMOUSCENKQFHK-UHFFFAOYSA-N Chlorine atom Chemical compound [Cl] ZAMOUSCENKQFHK-UHFFFAOYSA-N 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 108091062157 Cis-regulatory element Proteins 0.000 description 2
- 108050006400 Cyclin Proteins 0.000 description 2
- 102000016736 Cyclin Human genes 0.000 description 2
- 102000003903 Cyclin-dependent kinases Human genes 0.000 description 2
- 108090000266 Cyclin-dependent kinases Proteins 0.000 description 2
- RGHNJXZEOKUKBD-SQOUGZDYSA-N D-gluconic acid Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@@H](O)C(O)=O RGHNJXZEOKUKBD-SQOUGZDYSA-N 0.000 description 2
- 101710177611 DNA polymerase II large subunit Proteins 0.000 description 2
- 101710184669 DNA polymerase II small subunit Proteins 0.000 description 2
- 108090000626 DNA-directed RNA polymerases Proteins 0.000 description 2
- 102000004163 DNA-directed RNA polymerases Human genes 0.000 description 2
- 102000012199 E3 ubiquitin-protein ligase Mdm2 Human genes 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 2
- VZCYOOQTPOCHFL-OWOJBTEDSA-N Fumaric acid Chemical compound OC(=O)\C=C\C(O)=O VZCYOOQTPOCHFL-OWOJBTEDSA-N 0.000 description 2
- 101000892220 Geobacillus thermodenitrificans (strain NG80-2) Long-chain-alcohol dehydrogenase 1 Proteins 0.000 description 2
- 108090001102 Hammerhead ribozyme Proteins 0.000 description 2
- 101000780443 Homo sapiens Alcohol dehydrogenase 1A Proteins 0.000 description 2
- XQFRJNBWHJMXHO-RRKCRQDMSA-N IDUR Chemical compound C1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C(I)=C1 XQFRJNBWHJMXHO-RRKCRQDMSA-N 0.000 description 2
- 238000012404 In vitro experiment Methods 0.000 description 2
- 102100034343 Integrase Human genes 0.000 description 2
- 108060001084 Luciferase Proteins 0.000 description 2
- 239000005089 Luciferase Substances 0.000 description 2
- 208000031422 Lymphocytic Chronic B-Cell Leukemia Diseases 0.000 description 2
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 2
- AFVFQIVMOAPDHO-UHFFFAOYSA-N Methanesulfonic acid Chemical compound CS(O)(=O)=O AFVFQIVMOAPDHO-UHFFFAOYSA-N 0.000 description 2
- 101100444898 Mus musculus Egr1 gene Proteins 0.000 description 2
- HYVABZIGRDEKCD-UHFFFAOYSA-N N(6)-dimethylallyladenine Chemical compound CC(C)=CCNC1=NC=NC2=C1N=CN2 HYVABZIGRDEKCD-UHFFFAOYSA-N 0.000 description 2
- 229910019142 PO4 Inorganic materials 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-N Phosphoric acid Chemical compound OP(O)(O)=O NBIIXXVUZAFLBC-UHFFFAOYSA-N 0.000 description 2
- 108010087776 Proto-Oncogene Proteins c-myb Proteins 0.000 description 2
- 102000009096 Proto-Oncogene Proteins c-myb Human genes 0.000 description 2
- 102000009092 Proto-Oncogene Proteins c-myc Human genes 0.000 description 2
- 108010087705 Proto-Oncogene Proteins c-myc Proteins 0.000 description 2
- 108010029869 Proto-Oncogene Proteins c-raf Proteins 0.000 description 2
- 102100033479 RAF proto-oncogene serine/threonine-protein kinase Human genes 0.000 description 2
- 239000013614 RNA sample Substances 0.000 description 2
- 102000018120 Recombinases Human genes 0.000 description 2
- 108010091086 Recombinases Proteins 0.000 description 2
- 108091028664 Ribonucleotide Proteins 0.000 description 2
- 108091028736 Riboregulator Proteins 0.000 description 2
- QAOWNCQODCNURD-UHFFFAOYSA-N Sulfuric acid Chemical compound OS(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 2
- 108010017842 Telomerase Proteins 0.000 description 2
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 2
- 108091023040 Transcription factor Proteins 0.000 description 2
- 102000040945 Transcription factor Human genes 0.000 description 2
- 238000009825 accumulation Methods 0.000 description 2
- OIRDTQYFTABQOQ-KQYNXXCUSA-N adenosine Chemical compound C1=NC=2C(N)=NC=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O OIRDTQYFTABQOQ-KQYNXXCUSA-N 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 229910052784 alkaline earth metal Inorganic materials 0.000 description 2
- 239000002168 alkylating agent Substances 0.000 description 2
- 229940100198 alkylating agent Drugs 0.000 description 2
- 150000001413 amino acids Chemical class 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 230000002155 anti-virotic effect Effects 0.000 description 2
- PYMYPHUHKUWMLA-UHFFFAOYSA-N arabinose Natural products OCC(O)C(O)C(O)C=O PYMYPHUHKUWMLA-UHFFFAOYSA-N 0.000 description 2
- 239000011324 bead Substances 0.000 description 2
- WPYMKLBDIGXBTP-UHFFFAOYSA-N benzoic acid Chemical compound OC(=O)C1=CC=CC=C1 WPYMKLBDIGXBTP-UHFFFAOYSA-N 0.000 description 2
- SRBFZHDQGSBBOR-UHFFFAOYSA-N beta-D-Pyranose-Lyxose Natural products OC1COC(O)C(O)C1O SRBFZHDQGSBBOR-UHFFFAOYSA-N 0.000 description 2
- 230000001851 biosynthetic effect Effects 0.000 description 2
- 239000011575 calcium Substances 0.000 description 2
- 229910052791 calcium Inorganic materials 0.000 description 2
- 150000001768 cations Chemical class 0.000 description 2
- 108010046616 cdc25 Phosphatases Proteins 0.000 description 2
- 102000007588 cdc25 Phosphatases Human genes 0.000 description 2
- 210000002421 cell wall Anatomy 0.000 description 2
- SMNPLAKEGAEPJD-UHFFFAOYSA-N chembl34922 Chemical compound Cl.Cl.Cl.C1CN(C)CCN1C1=CC=C(NC(=N2)C=3C=C4N=C(NC4=CC=3)C=3C=CC(O)=CC=3)C2=C1 SMNPLAKEGAEPJD-UHFFFAOYSA-N 0.000 description 2
- 229960005091 chloramphenicol Drugs 0.000 description 2
- WIIZWVCIJKGZOK-RKDXNWHRSA-N chloramphenicol Chemical compound ClC(Cl)C(=O)N[C@H](CO)[C@H](O)C1=CC=C([N+]([O-])=O)C=C1 WIIZWVCIJKGZOK-RKDXNWHRSA-N 0.000 description 2
- 239000000460 chlorine Substances 0.000 description 2
- 229910052801 chlorine Inorganic materials 0.000 description 2
- 208000032852 chronic lymphocytic leukemia Diseases 0.000 description 2
- 238000001816 cooling Methods 0.000 description 2
- 210000000805 cytoplasm Anatomy 0.000 description 2
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical compound NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 2
- 230000003247 decreasing effect Effects 0.000 description 2
- 230000008260 defense mechanism Effects 0.000 description 2
- 238000001514 detection method Methods 0.000 description 2
- VYFYYTLLBUKUHU-UHFFFAOYSA-N dopamine Chemical compound NCCC1=CC=C(O)C(O)=C1 VYFYYTLLBUKUHU-UHFFFAOYSA-N 0.000 description 2
- 230000009977 dual effect Effects 0.000 description 2
- 238000001962 electrophoresis Methods 0.000 description 2
- 229960002949 fluorouracil Drugs 0.000 description 2
- 229940093915 gynecological organic acid Drugs 0.000 description 2
- IPCSVZSSVZVIGE-UHFFFAOYSA-N hexadecanoic acid Chemical compound CCCCCCCCCCCCCCCC(O)=O IPCSVZSSVZVIGE-UHFFFAOYSA-N 0.000 description 2
- 125000002887 hydroxy group Chemical group [H]O* 0.000 description 2
- 230000002390 hyperplastic effect Effects 0.000 description 2
- FDGQSTZJBFJUBT-UHFFFAOYSA-N hypoxanthine Chemical compound O=C1NC=NC2=C1NC=N2 FDGQSTZJBFJUBT-UHFFFAOYSA-N 0.000 description 2
- 238000002513 implantation Methods 0.000 description 2
- 230000002779 inactivation Effects 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 208000027866 inflammatory disease Diseases 0.000 description 2
- 101150066555 lacZ gene Proteins 0.000 description 2
- 210000004698 lymphocyte Anatomy 0.000 description 2
- 239000011777 magnesium Substances 0.000 description 2
- 229910052749 magnesium Inorganic materials 0.000 description 2
- 238000012423 maintenance Methods 0.000 description 2
- 101150024228 mdm2 gene Proteins 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 238000002844 melting Methods 0.000 description 2
- 230000008018 melting Effects 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- GLVAUDGFNGKCSF-UHFFFAOYSA-N mercaptopurine Chemical compound S=C1NC=NC2=C1NC=N2 GLVAUDGFNGKCSF-UHFFFAOYSA-N 0.000 description 2
- 238000012269 metabolic engineering Methods 0.000 description 2
- 238000010369 molecular cloning Methods 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 235000015097 nutrients Nutrition 0.000 description 2
- 235000005985 organic acids Nutrition 0.000 description 2
- 239000010452 phosphate Substances 0.000 description 2
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 2
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 2
- 150000004713 phosphodiesters Chemical class 0.000 description 2
- 230000004962 physiological condition Effects 0.000 description 2
- 229920002401 polyacrylamide Polymers 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 102000016914 ras Proteins Human genes 0.000 description 2
- 108010014186 ras Proteins Proteins 0.000 description 2
- 230000033458 reproduction Effects 0.000 description 2
- 239000002336 ribonucleotide Substances 0.000 description 2
- 125000002652 ribonucleotide group Chemical group 0.000 description 2
- 230000011664 signaling Effects 0.000 description 2
- 210000000329 smooth muscle myocyte Anatomy 0.000 description 2
- 230000009870 specific binding Effects 0.000 description 2
- ATHGHQPFGPMSJY-UHFFFAOYSA-N spermidine Chemical compound NCCCCNCCCN ATHGHQPFGPMSJY-UHFFFAOYSA-N 0.000 description 2
- PFNFFQXMRSDOHW-UHFFFAOYSA-N spermine Chemical compound NCCCNCCCCNCCCN PFNFFQXMRSDOHW-UHFFFAOYSA-N 0.000 description 2
- 230000007480 spreading Effects 0.000 description 2
- 238000003892 spreading Methods 0.000 description 2
- UCSJYZPVAKXKNQ-HZYVHMACSA-N streptomycin Chemical compound CN[C@H]1[C@H](O)[C@@H](O)[C@H](CO)O[C@H]1O[C@@H]1[C@](C=O)(O)[C@H](C)O[C@H]1O[C@@H]1[C@@H](NC(N)=N)[C@H](O)[C@@H](NC(N)=N)[C@H](O)[C@H]1O UCSJYZPVAKXKNQ-HZYVHMACSA-N 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical compound [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 2
- 229940104230 thymidine Drugs 0.000 description 2
- RWQNBRDOKXIBIV-UHFFFAOYSA-N thymine Chemical compound CC1=CNC(=O)NC1=O RWQNBRDOKXIBIV-UHFFFAOYSA-N 0.000 description 2
- WYWHKKSPHMUBEB-UHFFFAOYSA-N tioguanine Chemical compound N1C(N)=NC(=S)C2=C1N=CN2 WYWHKKSPHMUBEB-UHFFFAOYSA-N 0.000 description 2
- JOXIMZWYDAKGHI-UHFFFAOYSA-N toluene-4-sulfonic acid Chemical compound CC1=CC=C(S(O)(=O)=O)C=C1 JOXIMZWYDAKGHI-UHFFFAOYSA-N 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- VZCYOOQTPOCHFL-UHFFFAOYSA-N trans-butenedioic acid Natural products OC(=O)C=CC(O)=O VZCYOOQTPOCHFL-UHFFFAOYSA-N 0.000 description 2
- 238000011269 treatment regimen Methods 0.000 description 2
- 210000004881 tumor cell Anatomy 0.000 description 2
- 229940035893 uracil Drugs 0.000 description 2
- 239000013603 viral vector Substances 0.000 description 2
- 230000003612 virological effect Effects 0.000 description 2
- CADQNXRGRFJSQY-UOWFLXDJSA-N (2r,3r,4r)-2-fluoro-2,3,4,5-tetrahydroxypentanal Chemical compound OC[C@@H](O)[C@@H](O)[C@@](O)(F)C=O CADQNXRGRFJSQY-UOWFLXDJSA-N 0.000 description 1
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 1
- BJEPYKJPYRNKOW-REOHCLBHSA-N (S)-malic acid Chemical compound OC(=O)[C@@H](O)CC(O)=O BJEPYKJPYRNKOW-REOHCLBHSA-N 0.000 description 1
- TUSDEZXZIZRFGC-UHFFFAOYSA-N 1-O-galloyl-3,6-(R)-HHDP-beta-D-glucose Natural products OC1C(O2)COC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)OC1C(O)C2OC(=O)C1=CC(O)=C(O)C(O)=C1 TUSDEZXZIZRFGC-UHFFFAOYSA-N 0.000 description 1
- WJNGQIYEQLPJMN-IOSLPCCCSA-N 1-methylinosine Chemical compound C1=NC=2C(=O)N(C)C=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O WJNGQIYEQLPJMN-IOSLPCCCSA-N 0.000 description 1
- VGONTNSXDCQUGY-RRKCRQDMSA-N 2'-deoxyinosine Chemical group C1[C@H](O)[C@@H](CO)O[C@H]1N1C(N=CNC2=O)=C2N=C1 VGONTNSXDCQUGY-RRKCRQDMSA-N 0.000 description 1
- IQFYYKKMVGJFEH-BIIVOSGPSA-N 2'-deoxythymidine Natural products O=C1NC(=O)C(C)=CN1[C@@H]1O[C@@H](CO)[C@@H](O)C1 IQFYYKKMVGJFEH-BIIVOSGPSA-N 0.000 description 1
- IHPYMWDTONKSCO-UHFFFAOYSA-N 2,2'-piperazine-1,4-diylbisethanesulfonic acid Chemical compound OS(=O)(=O)CCN1CCN(CCS(O)(=O)=O)CC1 IHPYMWDTONKSCO-UHFFFAOYSA-N 0.000 description 1
- CRDMKNQTGHROAB-UHFFFAOYSA-N 2-(5-methoxy-2,4-dioxo-1H-pyrimidin-6-yl)acetic acid Chemical compound COC=1C(NC(NC=1CC(=O)O)=O)=O CRDMKNQTGHROAB-UHFFFAOYSA-N 0.000 description 1
- SGAKLDIYNFXTCK-UHFFFAOYSA-N 2-[(2,4-dioxo-1h-pyrimidin-5-yl)methylamino]acetic acid Chemical compound OC(=O)CNCC1=CNC(=O)NC1=O SGAKLDIYNFXTCK-UHFFFAOYSA-N 0.000 description 1
- IOOMXAQUNPWDLL-UHFFFAOYSA-N 2-[6-(diethylamino)-3-(diethyliminiumyl)-3h-xanthen-9-yl]-5-sulfobenzene-1-sulfonate Chemical compound C=12C=CC(=[N+](CC)CC)C=C2OC2=CC(N(CC)CC)=CC=C2C=1C1=CC=C(S(O)(=O)=O)C=C1S([O-])(=O)=O IOOMXAQUNPWDLL-UHFFFAOYSA-N 0.000 description 1
- XMSMHKMPBNTBOD-UHFFFAOYSA-N 2-dimethylamino-6-hydroxypurine Chemical compound N1C(N(C)C)=NC(=O)C2=C1N=CN2 XMSMHKMPBNTBOD-UHFFFAOYSA-N 0.000 description 1
- LXBGSDVWAMZHDD-UHFFFAOYSA-N 2-methyl-1h-imidazole Chemical compound CC1=NC=CN1 LXBGSDVWAMZHDD-UHFFFAOYSA-N 0.000 description 1
- SMADWRYCYBUIKH-UHFFFAOYSA-N 2-methyl-7h-purin-6-amine Chemical compound CC1=NC(N)=C2NC=NC2=N1 SMADWRYCYBUIKH-UHFFFAOYSA-N 0.000 description 1
- BMYNFMYTOJXKLE-UHFFFAOYSA-N 3-azaniumyl-2-hydroxypropanoate Chemical compound NCC(O)C(O)=O BMYNFMYTOJXKLE-UHFFFAOYSA-N 0.000 description 1
- KOLPWZCZXAMXKS-UHFFFAOYSA-N 3-methylcytosine Chemical compound CN1C(N)=CC=NC1=O KOLPWZCZXAMXKS-UHFFFAOYSA-N 0.000 description 1
- GJAKJCICANKRFD-UHFFFAOYSA-N 4-acetyl-4-amino-1,3-dihydropyrimidin-2-one Chemical compound CC(=O)C1(N)NC(=O)NC=C1 GJAKJCICANKRFD-UHFFFAOYSA-N 0.000 description 1
- 101710169336 5'-deoxyadenosine deaminase Proteins 0.000 description 1
- MQJSSLBGAQJNER-UHFFFAOYSA-N 5-(methylaminomethyl)-1h-pyrimidine-2,4-dione Chemical compound CNCC1=CNC(=O)NC1=O MQJSSLBGAQJNER-UHFFFAOYSA-N 0.000 description 1
- WPYRHVXCOQLYLY-UHFFFAOYSA-N 5-[(methoxyamino)methyl]-2-sulfanylidene-1h-pyrimidin-4-one Chemical compound CONCC1=CNC(=S)NC1=O WPYRHVXCOQLYLY-UHFFFAOYSA-N 0.000 description 1
- VKLFQTYNHLDMDP-PNHWDRBUSA-N 5-carboxymethylaminomethyl-2-thiouridine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=S)NC(=O)C(CNCC(O)=O)=C1 VKLFQTYNHLDMDP-PNHWDRBUSA-N 0.000 description 1
- ZFTBZKVVGZNMJR-UHFFFAOYSA-N 5-chlorouracil Chemical compound ClC1=CNC(=O)NC1=O ZFTBZKVVGZNMJR-UHFFFAOYSA-N 0.000 description 1
- KSNXJLQDQOIRIP-UHFFFAOYSA-N 5-iodouracil Chemical compound IC1=CNC(=O)NC1=O KSNXJLQDQOIRIP-UHFFFAOYSA-N 0.000 description 1
- KELXHQACBIUYSE-UHFFFAOYSA-N 5-methoxy-1h-pyrimidine-2,4-dione Chemical compound COC1=CNC(=O)NC1=O KELXHQACBIUYSE-UHFFFAOYSA-N 0.000 description 1
- LRSASMSXMSNRBT-UHFFFAOYSA-N 5-methylcytosine Chemical compound CC1=CNC(=O)N=C1N LRSASMSXMSNRBT-UHFFFAOYSA-N 0.000 description 1
- LMEHJKJEPRYEEB-UHFFFAOYSA-N 5-prop-1-ynylpyrimidine Chemical compound CC#CC1=CN=CN=C1 LMEHJKJEPRYEEB-UHFFFAOYSA-N 0.000 description 1
- DCPSTSVLRXOYGS-UHFFFAOYSA-N 6-amino-1h-pyrimidine-2-thione Chemical compound NC1=CC=NC(S)=N1 DCPSTSVLRXOYGS-UHFFFAOYSA-N 0.000 description 1
- ZCYVEMRRCGMTRW-UHFFFAOYSA-N 7553-56-2 Chemical compound [I] ZCYVEMRRCGMTRW-UHFFFAOYSA-N 0.000 description 1
- MSSXOMSJDRHRMC-UHFFFAOYSA-N 9H-purine-2,6-diamine Chemical compound NC1=NC(N)=C2NC=NC2=N1 MSSXOMSJDRHRMC-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 208000009304 Acute Kidney Injury Diseases 0.000 description 1
- 102000055025 Adenosine deaminases Human genes 0.000 description 1
- QGZKDVFQNNGYKY-UHFFFAOYSA-O Ammonium Chemical compound [NH4+] QGZKDVFQNNGYKY-UHFFFAOYSA-O 0.000 description 1
- 102000008682 Argonaute Proteins Human genes 0.000 description 1
- 108010088141 Argonaute Proteins Proteins 0.000 description 1
- 208000023275 Autoimmune disease Diseases 0.000 description 1
- 239000005711 Benzoic acid Substances 0.000 description 1
- WKBOTKDWSSQWDR-UHFFFAOYSA-N Bromine atom Chemical compound [Br] WKBOTKDWSSQWDR-UHFFFAOYSA-N 0.000 description 1
- 239000002126 C01EB10 - Adenosine Substances 0.000 description 1
- 0 CC*(C(C)C)C1=CN1 Chemical compound CC*(C(C)C)C1=CN1 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 108020004635 Complementary DNA Proteins 0.000 description 1
- 239000004971 Cross linker Substances 0.000 description 1
- 102100033270 Cyclin-dependent kinase inhibitor 1 Human genes 0.000 description 1
- 229920000858 Cyclodextrin Polymers 0.000 description 1
- GUBGYTABKSRVRQ-CUHNMECISA-N D-Cellobiose Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)OC(O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-CUHNMECISA-N 0.000 description 1
- RGHNJXZEOKUKBD-UHFFFAOYSA-N D-gluconic acid Natural products OCC(O)C(O)C(O)C(O)C(O)=O RGHNJXZEOKUKBD-UHFFFAOYSA-N 0.000 description 1
- ZAQJHHRNXZUBTE-WUJLRWPWSA-N D-xylulose Chemical compound OC[C@@H](O)[C@H](O)C(=O)CO ZAQJHHRNXZUBTE-WUJLRWPWSA-N 0.000 description 1
- 102000012410 DNA Ligases Human genes 0.000 description 1
- 108010061982 DNA Ligases Proteins 0.000 description 1
- 239000003155 DNA primer Substances 0.000 description 1
- 102000016911 Deoxyribonucleases Human genes 0.000 description 1
- 108010053770 Deoxyribonucleases Proteins 0.000 description 1
- FEWJPZIEWOKRBE-JCYAYHJZSA-N Dextrotartaric acid Chemical compound OC(=O)[C@H](O)[C@@H](O)C(O)=O FEWJPZIEWOKRBE-JCYAYHJZSA-N 0.000 description 1
- XBPCUCUWBYBCDP-UHFFFAOYSA-N Dicyclohexylamine Chemical compound C1CCCCC1NC1CCCCC1 XBPCUCUWBYBCDP-UHFFFAOYSA-N 0.000 description 1
- 241000255581 Drosophila <fruit fly, genus> Species 0.000 description 1
- 241000255601 Drosophila melanogaster Species 0.000 description 1
- 108010042407 Endonucleases Proteins 0.000 description 1
- 102000004533 Endonucleases Human genes 0.000 description 1
- PIICEJLVQHRZGT-UHFFFAOYSA-N Ethylenediamine Chemical compound NCCN PIICEJLVQHRZGT-UHFFFAOYSA-N 0.000 description 1
- 241000206602 Eukaryota Species 0.000 description 1
- 108060002716 Exonuclease Proteins 0.000 description 1
- 208000007241 Experimental Diabetes Mellitus Diseases 0.000 description 1
- 239000001263 FEMA 3042 Substances 0.000 description 1
- 108700039691 Genetic Promoter Regions Proteins 0.000 description 1
- 208000034951 Genetic Translocation Diseases 0.000 description 1
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical class C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 1
- 102100031547 HLA class II histocompatibility antigen, DO alpha chain Human genes 0.000 description 1
- 101000866278 Homo sapiens HLA class II histocompatibility antigen, DO alpha chain Proteins 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- UGQMRVRMYYASKQ-UHFFFAOYSA-N Hypoxanthine nucleoside Natural products OC1C(O)C(CO)OC1N1C(NC=NC2=O)=C2N=C1 UGQMRVRMYYASKQ-UHFFFAOYSA-N 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 229930010555 Inosine Natural products 0.000 description 1
- UGQMRVRMYYASKQ-KQYNXXCUSA-N Inosine Chemical compound O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C2=NC=NC(O)=C2N=C1 UGQMRVRMYYASKQ-KQYNXXCUSA-N 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 241000764238 Isis Species 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- QIVBCDIJIAJPQS-VIFPVBQESA-N L-tryptophane Chemical compound C1=CC=C2C(C[C@H](N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-VIFPVBQESA-N 0.000 description 1
- 108091026898 Leader sequence (mRNA) Proteins 0.000 description 1
- 102000003960 Ligases Human genes 0.000 description 1
- 108090000364 Ligases Proteins 0.000 description 1
- 239000006391 Luria-Bertani Medium Substances 0.000 description 1
- 108090000157 Metallothionein Proteins 0.000 description 1
- 108060004795 Methyltransferase Proteins 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 108010021466 Mutant Proteins Proteins 0.000 description 1
- 102000008300 Mutant Proteins Human genes 0.000 description 1
- SGSSKEDGVONRGC-UHFFFAOYSA-N N(2)-methylguanine Chemical compound O=C1NC(NC)=NC2=C1N=CN2 SGSSKEDGVONRGC-UHFFFAOYSA-N 0.000 description 1
- MBBZMMPHUWSWHV-BDVNFPICSA-N N-methylglucamine Chemical compound CNC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO MBBZMMPHUWSWHV-BDVNFPICSA-N 0.000 description 1
- 229910002651 NO3 Inorganic materials 0.000 description 1
- NHNBFGGVMKEFGY-UHFFFAOYSA-N Nitrate Chemical compound [O-][N+]([O-])=O NHNBFGGVMKEFGY-UHFFFAOYSA-N 0.000 description 1
- GRYLNZFGIOXLOG-UHFFFAOYSA-N Nitric acid Chemical compound O[N+]([O-])=O GRYLNZFGIOXLOG-UHFFFAOYSA-N 0.000 description 1
- 108091092724 Noncoding DNA Proteins 0.000 description 1
- 108700026244 Open Reading Frames Proteins 0.000 description 1
- 239000007990 PIPES buffer Substances 0.000 description 1
- 235000021314 Palmitic acid Nutrition 0.000 description 1
- 229920002230 Pectic acid Polymers 0.000 description 1
- LRBQNJMCXXYXIU-PPKXGCFTSA-N Penta-digallate-beta-D-glucose Natural products OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@@H]2[C@H]([C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-PPKXGCFTSA-N 0.000 description 1
- OAICVXFJPJFONN-UHFFFAOYSA-N Phosphorus Chemical compound [P] OAICVXFJPJFONN-UHFFFAOYSA-N 0.000 description 1
- 108010020346 Polyglutamic Acid Proteins 0.000 description 1
- ZLMJMSJWJFRBEC-UHFFFAOYSA-N Potassium Chemical compound [K] ZLMJMSJWJFRBEC-UHFFFAOYSA-N 0.000 description 1
- OFOBLEOULBTSOW-UHFFFAOYSA-N Propanedioic acid Natural products OC(=O)CC(O)=O OFOBLEOULBTSOW-UHFFFAOYSA-N 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000055027 Protein Methyltransferases Human genes 0.000 description 1
- 108700040121 Protein Methyltransferases Proteins 0.000 description 1
- 108010076504 Protein Sorting Signals Proteins 0.000 description 1
- 201000004681 Psoriasis Diseases 0.000 description 1
- 102000014450 RNA Polymerase III Human genes 0.000 description 1
- 108010078067 RNA Polymerase III Proteins 0.000 description 1
- 238000013381 RNA quantification Methods 0.000 description 1
- 230000007022 RNA scission Effects 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 238000010240 RT-PCR analysis Methods 0.000 description 1
- MUPFEKGTMRGPLJ-RMMQSMQOSA-N Raffinose Natural products O(C[C@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@@H](O[C@@]2(CO)[C@H](O)[C@@H](O)[C@@H](CO)O2)O1)[C@@H]1[C@H](O)[C@@H](O)[C@@H](O)[C@@H](CO)O1 MUPFEKGTMRGPLJ-RMMQSMQOSA-N 0.000 description 1
- 208000033626 Renal failure acute Diseases 0.000 description 1
- 206010063837 Reperfusion injury Diseases 0.000 description 1
- 108020005091 Replication Origin Proteins 0.000 description 1
- 108090000621 Ribonuclease P Proteins 0.000 description 1
- 102000004167 Ribonuclease P Human genes 0.000 description 1
- 108020004422 Riboswitch Proteins 0.000 description 1
- 241000714474 Rous sarcoma virus Species 0.000 description 1
- 108091006627 SLC12A9 Proteins 0.000 description 1
- 238000012300 Sequence Analysis Methods 0.000 description 1
- VMHLLURERBWHNL-UHFFFAOYSA-M Sodium acetate Chemical compound [Na+].CC([O-])=O VMHLLURERBWHNL-UHFFFAOYSA-M 0.000 description 1
- KDYFGRWQOYBRFD-UHFFFAOYSA-N Succinic acid Natural products OC(=O)CCC(O)=O KDYFGRWQOYBRFD-UHFFFAOYSA-N 0.000 description 1
- 229930006000 Sucrose Natural products 0.000 description 1
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 1
- NINIDFKCEFEMDL-UHFFFAOYSA-N Sulfur Chemical group [S] NINIDFKCEFEMDL-UHFFFAOYSA-N 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 210000001744 T-lymphocyte Anatomy 0.000 description 1
- 108010006785 Taq Polymerase Proteins 0.000 description 1
- FEWJPZIEWOKRBE-UHFFFAOYSA-N Tartaric acid Natural products [H+].[H+].[O-]C(=O)C(O)C(O)C([O-])=O FEWJPZIEWOKRBE-UHFFFAOYSA-N 0.000 description 1
- 102000006601 Thymidine Kinase Human genes 0.000 description 1
- 108020004440 Thymidine kinase Proteins 0.000 description 1
- 102000046299 Transforming Growth Factor beta1 Human genes 0.000 description 1
- 101800002279 Transforming growth factor beta-1 Proteins 0.000 description 1
- 101800001690 Transmembrane protein gp41 Proteins 0.000 description 1
- QIVBCDIJIAJPQS-UHFFFAOYSA-N Tryptophan Natural products C1=CC=C2C(CC(N)C(O)=O)=CNC2=C1 QIVBCDIJIAJPQS-UHFFFAOYSA-N 0.000 description 1
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 1
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 1
- MUPFEKGTMRGPLJ-UHFFFAOYSA-N UNPD196149 Natural products OC1C(O)C(CO)OC1(CO)OC1C(O)C(O)C(O)C(COC2C(C(O)C(O)C(CO)O2)O)O1 MUPFEKGTMRGPLJ-UHFFFAOYSA-N 0.000 description 1
- 108020004417 Untranslated RNA Proteins 0.000 description 1
- 102000039634 Untranslated RNA Human genes 0.000 description 1
- DRTQHJPVMGBUCF-XVFCMESISA-N Uridine Chemical class O[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1N1C(=O)NC(=O)C=C1 DRTQHJPVMGBUCF-XVFCMESISA-N 0.000 description 1
- OZKXLOZHHUHGNV-UHFFFAOYSA-N Viomycin Natural products NCCCC(N)CC(=O)NC1CNC(=O)C(=CNC(=O)N)NC(=O)C(CO)NC(=O)C(CO)NC(=O)C(NC1=O)C2CC(O)NC(=N)N2 OZKXLOZHHUHGNV-UHFFFAOYSA-N 0.000 description 1
- 108010015940 Viomycin Proteins 0.000 description 1
- GKMBRSQQKZUCGD-UHFFFAOYSA-M [9-[4-(chloromethyl)phenyl]-6-(dimethylamino)xanthen-3-ylidene]-dimethylazanium;bromide Chemical compound [Br-].C=12C=CC(=[N+](C)C)C=C2OC2=CC(N(C)C)=CC=C2C=1C1=CC=C(CCl)C=C1 GKMBRSQQKZUCGD-UHFFFAOYSA-M 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000010521 absorption reaction Methods 0.000 description 1
- 150000001241 acetals Chemical class 0.000 description 1
- 235000011054 acetic acid Nutrition 0.000 description 1
- 230000013564 activation of immune response Effects 0.000 description 1
- 201000011040 acute kidney failure Diseases 0.000 description 1
- 208000012998 acute renal failure Diseases 0.000 description 1
- 229960000643 adenine Drugs 0.000 description 1
- 229960005305 adenosine Drugs 0.000 description 1
- 210000001789 adipocyte Anatomy 0.000 description 1
- 238000001261 affinity purification Methods 0.000 description 1
- 238000000246 agarose gel electrophoresis Methods 0.000 description 1
- 239000000783 alginic acid Substances 0.000 description 1
- 229920000615 alginic acid Polymers 0.000 description 1
- 229960001126 alginic acid Drugs 0.000 description 1
- 235000010443 alginic acid Nutrition 0.000 description 1
- 150000004781 alginic acids Chemical class 0.000 description 1
- 239000003513 alkali Substances 0.000 description 1
- 150000001447 alkali salts Chemical class 0.000 description 1
- 150000001342 alkaline earth metals Chemical class 0.000 description 1
- 230000008848 allosteric regulation Effects 0.000 description 1
- 229910000147 aluminium phosphate Inorganic materials 0.000 description 1
- 150000001450 anions Chemical class 0.000 description 1
- 238000011319 anticancer therapy Methods 0.000 description 1
- 239000000427 antigen Substances 0.000 description 1
- 108091007433 antigens Proteins 0.000 description 1
- 102000036639 antigens Human genes 0.000 description 1
- 239000008346 aqueous phase Substances 0.000 description 1
- PYMYPHUHKUWMLA-WDCZJNDASA-N arabinose Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)C=O PYMYPHUHKUWMLA-WDCZJNDASA-N 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 230000003305 autocrine Effects 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 235000010233 benzoic acid Nutrition 0.000 description 1
- 229960004365 benzoic acid Drugs 0.000 description 1
- 108010005774 beta-Galactosidase Proteins 0.000 description 1
- 102000005936 beta-Galactosidase Human genes 0.000 description 1
- 230000003115 biocidal effect Effects 0.000 description 1
- 230000004071 biological effect Effects 0.000 description 1
- 229920001222 biopolymer Polymers 0.000 description 1
- 230000006696 biosynthetic metabolic pathway Effects 0.000 description 1
- 230000008499 blood brain barrier function Effects 0.000 description 1
- 210000001218 blood-brain barrier Anatomy 0.000 description 1
- GDTBXPJZTBHREO-UHFFFAOYSA-N bromine Substances BrBr GDTBXPJZTBHREO-UHFFFAOYSA-N 0.000 description 1
- 229910052794 bromium Inorganic materials 0.000 description 1
- KDYFGRWQOYBRFD-NUQCWPJISA-N butanedioic acid Chemical compound O[14C](=O)CC[14C](O)=O KDYFGRWQOYBRFD-NUQCWPJISA-N 0.000 description 1
- 238000010804 cDNA synthesis Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000003197 catalytic effect Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 230000036978 cell physiology Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 210000002230 centromere Anatomy 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- VDANGULDQQJODZ-UHFFFAOYSA-N chloroprocaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1Cl VDANGULDQQJODZ-UHFFFAOYSA-N 0.000 description 1
- 229960002023 chloroprocaine Drugs 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 210000000349 chromosome Anatomy 0.000 description 1
- 230000008632 circadian clock Effects 0.000 description 1
- 230000004186 co-expression Effects 0.000 description 1
- 238000004440 column chromatography Methods 0.000 description 1
- 238000011217 control strategy Methods 0.000 description 1
- 239000005289 controlled pore glass Substances 0.000 description 1
- 230000002079 cooperative effect Effects 0.000 description 1
- 238000007887 coronary angioplasty Methods 0.000 description 1
- 239000002537 cosmetic Substances 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 238000013211 curve analysis Methods 0.000 description 1
- 229940104302 cytosine Drugs 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000002716 delivery method Methods 0.000 description 1
- 239000005547 deoxyribonucleotide Substances 0.000 description 1
- 125000002637 deoxyribonucleotide group Chemical group 0.000 description 1
- 230000002074 deregulated effect Effects 0.000 description 1
- 230000001687 destabilization Effects 0.000 description 1
- 230000000368 destabilizing effect Effects 0.000 description 1
- ANCLJVISBRWUTR-UHFFFAOYSA-N diaminophosphinic acid Chemical compound NP(N)(O)=O ANCLJVISBRWUTR-UHFFFAOYSA-N 0.000 description 1
- ZBCBWPMODOFKDW-UHFFFAOYSA-N diethanolamine Chemical compound OCCNCCO ZBCBWPMODOFKDW-UHFFFAOYSA-N 0.000 description 1
- 238000009792 diffusion process Methods 0.000 description 1
- RJBIAAZJODIFHR-UHFFFAOYSA-N dihydroxy-imino-sulfanyl-$l^{5}-phosphane Chemical compound NP(O)(O)=S RJBIAAZJODIFHR-UHFFFAOYSA-N 0.000 description 1
- 238000010790 dilution Methods 0.000 description 1
- 239000012895 dilution Substances 0.000 description 1
- NAGJZTKCGNOGPW-UHFFFAOYSA-K dioxido-sulfanylidene-sulfido-$l^{5}-phosphane Chemical compound [O-]P([O-])([S-])=S NAGJZTKCGNOGPW-UHFFFAOYSA-K 0.000 description 1
- 230000008034 disappearance Effects 0.000 description 1
- 229960003638 dopamine Drugs 0.000 description 1
- 230000008030 elimination Effects 0.000 description 1
- 238000003379 elimination reaction Methods 0.000 description 1
- 238000010828 elution Methods 0.000 description 1
- 230000003511 endothelial effect Effects 0.000 description 1
- 230000008556 epithelial cell proliferation Effects 0.000 description 1
- 238000012869 ethanol precipitation Methods 0.000 description 1
- 210000003527 eukaryotic cell Anatomy 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 102000013165 exonuclease Human genes 0.000 description 1
- 230000003631 expected effect Effects 0.000 description 1
- 239000013613 expression plasmid Substances 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 229960003704 framycetin Drugs 0.000 description 1
- PGBHMTALBVVCIT-VCIWKGPPSA-N framycetin Chemical compound N[C@@H]1[C@@H](O)[C@H](O)[C@H](CN)O[C@@H]1O[C@H]1[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](N)C[C@@H](N)[C@@H]2O)O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CN)O2)N)O[C@@H]1CO PGBHMTALBVVCIT-VCIWKGPPSA-N 0.000 description 1
- 239000001530 fumaric acid Substances 0.000 description 1
- 230000005714 functional activity Effects 0.000 description 1
- 108020001507 fusion proteins Proteins 0.000 description 1
- 102000037865 fusion proteins Human genes 0.000 description 1
- 101150045500 galK gene Proteins 0.000 description 1
- 101150041954 galU gene Proteins 0.000 description 1
- LRBQNJMCXXYXIU-QWKBTXIPSA-N gallotannic acid Chemical compound OC1=C(O)C(O)=CC(C(=O)OC=2C(=C(O)C=C(C=2)C(=O)OC[C@H]2[C@@H]([C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)[C@@H](OC(=O)C=3C=C(OC(=O)C=4C=C(O)C(O)=C(O)C=4)C(O)=C(O)C=3)O2)OC(=O)C=2C=C(OC(=O)C=3C=C(O)C(O)=C(O)C=3)C(O)=C(O)C=2)O)=C1 LRBQNJMCXXYXIU-QWKBTXIPSA-N 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 238000001415 gene therapy Methods 0.000 description 1
- 102000034356 gene-regulatory proteins Human genes 0.000 description 1
- 108091006104 gene-regulatory proteins Proteins 0.000 description 1
- 230000007614 genetic variation Effects 0.000 description 1
- 230000001434 glomerular Effects 0.000 description 1
- 239000000174 gluconic acid Substances 0.000 description 1
- 235000012208 gluconic acid Nutrition 0.000 description 1
- 239000003102 growth factor Substances 0.000 description 1
- 101150096208 gtaB gene Proteins 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 210000003494 hepatocyte Anatomy 0.000 description 1
- 150000002402 hexoses Chemical class 0.000 description 1
- 230000006801 homologous recombination Effects 0.000 description 1
- 238000002744 homologous recombination Methods 0.000 description 1
- 210000002865 immune cell Anatomy 0.000 description 1
- 230000008102 immune modulation Effects 0.000 description 1
- 229960003444 immunosuppressant agent Drugs 0.000 description 1
- 239000003018 immunosuppressive agent Substances 0.000 description 1
- 238000011065 in-situ storage Methods 0.000 description 1
- 230000006882 induction of apoptosis Effects 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 239000003112 inhibitor Substances 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 229960003786 inosine Drugs 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 230000004068 intracellular signaling Effects 0.000 description 1
- 230000008863 intramolecular interaction Effects 0.000 description 1
- 229910052740 iodine Inorganic materials 0.000 description 1
- 239000011630 iodine Substances 0.000 description 1
- 229960000318 kanamycin Drugs 0.000 description 1
- SBUJHOSQTJFQJX-NOAMYHISSA-N kanamycin Chemical compound O[C@@H]1[C@@H](O)[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N SBUJHOSQTJFQJX-NOAMYHISSA-N 0.000 description 1
- 229930182823 kanamycin A Natural products 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 239000002502 liposome Substances 0.000 description 1
- 239000007788 liquid Substances 0.000 description 1
- XIXADJRWDQXREU-UHFFFAOYSA-M lithium acetate Chemical compound [Li+].CC([O-])=O XIXADJRWDQXREU-UHFFFAOYSA-M 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- VZCYOOQTPOCHFL-UPHRSURJSA-N maleic acid Chemical compound OC(=O)\C=C/C(O)=O VZCYOOQTPOCHFL-UPHRSURJSA-N 0.000 description 1
- 239000011976 maleic acid Substances 0.000 description 1
- 210000001161 mammalian embryo Anatomy 0.000 description 1
- 239000000463 material Substances 0.000 description 1
- 229960001428 mercaptopurine Drugs 0.000 description 1
- 229940098779 methanesulfonic acid Drugs 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- IZAGSTRIDUNNOY-UHFFFAOYSA-N methyl 2-[(2,4-dioxo-1h-pyrimidin-5-yl)oxy]acetate Chemical compound COC(=O)COC1=CNC(=O)NC1=O IZAGSTRIDUNNOY-UHFFFAOYSA-N 0.000 description 1
- 125000002496 methyl group Chemical group [H]C([H])([H])* 0.000 description 1
- 230000011987 methylation Effects 0.000 description 1
- 238000007069 methylation reaction Methods 0.000 description 1
- XLSZMDLNRCVEIJ-UHFFFAOYSA-N methylimidazole Natural products CC1=CNC=N1 XLSZMDLNRCVEIJ-UHFFFAOYSA-N 0.000 description 1
- 238000013508 migration Methods 0.000 description 1
- 230000003278 mimic effect Effects 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 230000009149 molecular binding Effects 0.000 description 1
- 238000001823 molecular biology technique Methods 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 230000035772 mutation Effects 0.000 description 1
- 108700024542 myc Genes Proteins 0.000 description 1
- XJVXMWNLQRTRGH-UHFFFAOYSA-N n-(3-methylbut-3-enyl)-2-methylsulfanyl-7h-purin-6-amine Chemical compound CSC1=NC(NCCC(C)=C)=C2NC=NC2=N1 XJVXMWNLQRTRGH-UHFFFAOYSA-N 0.000 description 1
- WQEPLUUGTLDZJY-UHFFFAOYSA-N n-Pentadecanoic acid Natural products CCCCCCCCCCCCCCC(O)=O WQEPLUUGTLDZJY-UHFFFAOYSA-N 0.000 description 1
- YZMHQCWXYHARLS-UHFFFAOYSA-N naphthalene-1,2-disulfonic acid Chemical compound C1=CC=CC2=C(S(O)(=O)=O)C(S(=O)(=O)O)=CC=C21 YZMHQCWXYHARLS-UHFFFAOYSA-N 0.000 description 1
- PSZYNBSKGUBXEH-UHFFFAOYSA-N naphthalene-1-sulfonic acid Chemical compound C1=CC=C2C(S(=O)(=O)O)=CC=CC2=C1 PSZYNBSKGUBXEH-UHFFFAOYSA-N 0.000 description 1
- 230000008692 neointimal formation Effects 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- 229910017604 nitric acid Inorganic materials 0.000 description 1
- 231100000252 nontoxic Toxicity 0.000 description 1
- 230000003000 nontoxic effect Effects 0.000 description 1
- 108091008104 nucleic acid aptamers Proteins 0.000 description 1
- 238000007899 nucleic acid hybridization Methods 0.000 description 1
- 230000001293 nucleolytic effect Effects 0.000 description 1
- 101150012154 nupG gene Proteins 0.000 description 1
- 108091008819 oncoproteins Proteins 0.000 description 1
- 210000000287 oocyte Anatomy 0.000 description 1
- 230000001151 other effect Effects 0.000 description 1
- 230000002018 overexpression Effects 0.000 description 1
- 235000006408 oxalic acid Nutrition 0.000 description 1
- 238000010422 painting Methods 0.000 description 1
- 229940098695 palmitic acid Drugs 0.000 description 1
- 230000003076 paracrine Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000008188 pellet Substances 0.000 description 1
- 239000003961 penetration enhancing agent Substances 0.000 description 1
- 230000002688 persistence Effects 0.000 description 1
- 239000008363 phosphate buffer Substances 0.000 description 1
- PTMHPRAIXMAOOB-UHFFFAOYSA-L phosphoramidate Chemical compound NP([O-])([O-])=O PTMHPRAIXMAOOB-UHFFFAOYSA-L 0.000 description 1
- 125000005642 phosphothioate group Chemical group 0.000 description 1
- 230000000704 physical effect Effects 0.000 description 1
- 239000002798 polar solvent Substances 0.000 description 1
- 229920000768 polyamine Polymers 0.000 description 1
- 239000010318 polygalacturonic acid Substances 0.000 description 1
- 229920002643 polyglutamic acid Polymers 0.000 description 1
- 102000040430 polynucleotide Human genes 0.000 description 1
- 108091033319 polynucleotide Proteins 0.000 description 1
- 239000002157 polynucleotide Substances 0.000 description 1
- 230000001124 posttranscriptional effect Effects 0.000 description 1
- 230000007859 posttranscriptional regulation of gene expression Effects 0.000 description 1
- BITYAPCSNKJESK-UHFFFAOYSA-N potassiosodium Chemical compound [Na].[K] BITYAPCSNKJESK-UHFFFAOYSA-N 0.000 description 1
- 239000011591 potassium Substances 0.000 description 1
- 229910052700 potassium Inorganic materials 0.000 description 1
- 108091007428 primary miRNA Proteins 0.000 description 1
- MFDFERRIHVXMIY-UHFFFAOYSA-N procaine Chemical compound CCN(CC)CCOC(=O)C1=CC=C(N)C=C1 MFDFERRIHVXMIY-UHFFFAOYSA-N 0.000 description 1
- 229960004919 procaine Drugs 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 239000002213 purine nucleotide Substances 0.000 description 1
- 150000003212 purines Chemical class 0.000 description 1
- 239000002719 pyrimidine nucleotide Substances 0.000 description 1
- 150000003230 pyrimidines Chemical class 0.000 description 1
- 238000011002 quantification Methods 0.000 description 1
- 230000002285 radioactive effect Effects 0.000 description 1
- MUPFEKGTMRGPLJ-ZQSKZDJDSA-N raffinose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@@H]2[C@@H]([C@@H](O)[C@@H](O)[C@@H](CO)O2)O)O1 MUPFEKGTMRGPLJ-ZQSKZDJDSA-N 0.000 description 1
- 239000000376 reactant Substances 0.000 description 1
- 230000023276 regulation of development, heterochronic Effects 0.000 description 1
- 230000008844 regulatory mechanism Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004043 responsiveness Effects 0.000 description 1
- 108091008146 restriction endonucleases Proteins 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002207 retinal effect Effects 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 239000002342 ribonucleoside Substances 0.000 description 1
- YGSDEFSMJLZEOE-UHFFFAOYSA-M salicylate Chemical compound OC1=CC=CC=C1C([O-])=O YGSDEFSMJLZEOE-UHFFFAOYSA-M 0.000 description 1
- 229960001860 salicylate Drugs 0.000 description 1
- HFHDHCJBZVLPGP-UHFFFAOYSA-N schardinger α-dextrin Chemical compound O1C(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC(C(O)C2O)C(CO)OC2OC(C(C2O)O)C(CO)OC2OC2C(O)C(O)C1OC2CO HFHDHCJBZVLPGP-UHFFFAOYSA-N 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 210000002966 serum Anatomy 0.000 description 1
- 239000013605 shuttle vector Substances 0.000 description 1
- 238000001542 size-exclusion chromatography Methods 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 229940126586 small molecule drug Drugs 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- 210000001082 somatic cell Anatomy 0.000 description 1
- 238000011895 specific detection Methods 0.000 description 1
- 229940063673 spermidine Drugs 0.000 description 1
- 229940063675 spermine Drugs 0.000 description 1
- 230000000087 stabilizing effect Effects 0.000 description 1
- 229960005322 streptomycin Drugs 0.000 description 1
- 239000005720 sucrose Substances 0.000 description 1
- 230000009897 systematic effect Effects 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 229920002258 tannic acid Polymers 0.000 description 1
- 235000015523 tannic acid Nutrition 0.000 description 1
- 229940033123 tannic acid Drugs 0.000 description 1
- 238000002626 targeted therapy Methods 0.000 description 1
- 239000011975 tartaric acid Substances 0.000 description 1
- 235000002906 tartaric acid Nutrition 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 229960003087 tioguanine Drugs 0.000 description 1
- 229960000707 tobramycin Drugs 0.000 description 1
- NLVFBUXFDBBNBW-PBSUHMDJSA-N tobramycin Chemical compound N[C@@H]1C[C@H](O)[C@@H](CN)O[C@@H]1O[C@H]1[C@H](O)[C@@H](O[C@@H]2[C@@H]([C@@H](N)[C@H](O)[C@@H](CO)O2)O)[C@H](N)C[C@@H]1N NLVFBUXFDBBNBW-PBSUHMDJSA-N 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 230000005030 transcription termination Effects 0.000 description 1
- 108091006106 transcriptional activators Proteins 0.000 description 1
- 230000014616 translation Effects 0.000 description 1
- 230000014621 translational initiation Effects 0.000 description 1
- 230000005945 translocation Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 230000004614 tumor growth Effects 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 238000011144 upstream manufacturing Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 210000003556 vascular endothelial cell Anatomy 0.000 description 1
- 229950001272 viomycin Drugs 0.000 description 1
- GXFAIFRPOKBQRV-GHXCTMGLSA-N viomycin Chemical compound N1C(=O)\C(=C\NC(N)=O)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@@H](NC(=O)C[C@@H](N)CCCN)CNC(=O)[C@@H]1[C@@H]1NC(=N)N[C@@H](O)C1 GXFAIFRPOKBQRV-GHXCTMGLSA-N 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- 238000001262 western blot Methods 0.000 description 1
- 229940075420 xanthine Drugs 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6844—Nucleic acid amplification reactions
- C12Q1/686—Polymerase chain reaction [PCR]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/111—General methods applicable to biologically active non-coding nucleic acids
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/115—Aptamers, i.e. nucleic acids binding a target molecule specifically and with high affinity without hybridising therewith ; Nucleic acids binding to non-nucleic acids, e.g. aptamers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/635—Externally inducible repressor mediated regulation of gene expression, e.g. tetR inducible by tetracyline
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6897—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids involving reporter genes operably linked to promoters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/11—Antisense
- C12N2310/111—Antisense spanning the whole gene, or a large part of it
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/16—Aptamers
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- Biotechnology (AREA)
- General Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Biochemistry (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Plant Pathology (AREA)
- Pharmacology & Pharmacy (AREA)
- General Chemical & Material Sciences (AREA)
- Medicinal Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Immunology (AREA)
- Diabetes (AREA)
- Analytical Chemistry (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Hematology (AREA)
- Obesity (AREA)
- Child & Adolescent Psychology (AREA)
- Endocrinology (AREA)
- Emergency Medicine (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Measuring Or Testing Involving Enzymes Or Micro-Organisms (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US61597704P | 2004-10-05 | 2004-10-05 | |
| US60/615,977 | 2004-10-05 | ||
| US64165805P | 2005-01-06 | 2005-01-06 | |
| US60/641,658 | 2005-01-06 | ||
| PCT/US2005/036161 WO2006042112A2 (en) | 2004-10-05 | 2005-10-05 | Aptamer regulated nucleic acids and uses thereof |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012178625A Division JP5795563B2 (ja) | 2004-10-05 | 2012-08-10 | アプタマー調節される核酸及びその利用 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008515405A JP2008515405A (ja) | 2008-05-15 |
| JP2008515405A5 JP2008515405A5 (enExample) | 2008-11-27 |
| JP5101288B2 true JP5101288B2 (ja) | 2012-12-19 |
Family
ID=36075582
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007534921A Expired - Fee Related JP5101288B2 (ja) | 2004-10-05 | 2005-10-05 | アプタマー調節される核酸及びその利用 |
| JP2012178625A Expired - Fee Related JP5795563B2 (ja) | 2004-10-05 | 2012-08-10 | アプタマー調節される核酸及びその利用 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012178625A Expired - Fee Related JP5795563B2 (ja) | 2004-10-05 | 2012-08-10 | アプタマー調節される核酸及びその利用 |
Country Status (5)
| Country | Link |
|---|---|
| US (3) | US9315862B2 (enExample) |
| EP (1) | EP1799825B1 (enExample) |
| JP (2) | JP5101288B2 (enExample) |
| AT (1) | ATE514776T1 (enExample) |
| WO (1) | WO2006042112A2 (enExample) |
Families Citing this family (98)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050256071A1 (en) * | 2003-07-15 | 2005-11-17 | California Institute Of Technology | Inhibitor nucleic acids |
| ATE514776T1 (de) * | 2004-10-05 | 2011-07-15 | California Inst Of Techn | Aptamer-regulierte nukleinsäuren und verwendungen davon |
| US7727721B2 (en) | 2005-03-08 | 2010-06-01 | California Institute Of Technology | Hybridization chain reaction amplification for in situ imaging |
| ATE526975T1 (de) | 2005-10-07 | 2011-10-15 | California Inst Of Techn | Pkr-aktivierung mittels hybridisierungskettenreaktion |
| WO2007100412A2 (en) * | 2005-12-21 | 2007-09-07 | Yale University | Methods and compositions related to the modulation of riboswitches |
| US20080026947A1 (en) * | 2006-02-08 | 2008-01-31 | Gmeiner William H | Cytotoxic nucleotides for targeted therapeutics |
| US20090023221A1 (en) * | 2006-05-19 | 2009-01-22 | Exigon A/S | Oligonucleotide probes useful for detection and analysis of microrna precursors |
| WO2007136833A2 (en) * | 2006-05-19 | 2007-11-29 | Codon Devices, Inc. | Methods and compositions for aptamer production and uses thereof |
| WO2007147159A2 (en) * | 2006-06-16 | 2007-12-21 | Cornell Research Foundation, Inc. | Functional nucleic acid ligands to fluorescent proteins |
| WO2008127382A2 (en) * | 2006-10-19 | 2008-10-23 | Yale University | Computational design of ribozymes |
| WO2008058291A2 (en) | 2006-11-09 | 2008-05-15 | California Institute Of Technology | Modular aptamer-regulated ribozymes |
| EP2471925A1 (en) * | 2007-03-22 | 2012-07-04 | Yale University | Methods and compositions related to riboswitches that control alternative splicing |
| WO2008122088A1 (en) * | 2007-04-05 | 2008-10-16 | Lifeprint Australia Pty Ltd | Methods for detecting a target nucleotide sequence in a sample utilising a nuclease-aptamer complex |
| JP5223237B2 (ja) * | 2007-05-14 | 2013-06-26 | ソニー株式会社 | 検出用核酸鎖及び物質間の結合又は相互作用検出方法 |
| WO2008144562A1 (en) * | 2007-05-16 | 2008-11-27 | California Institute Of Technology | A versatile nucleic acid hairpin motif for programming biomolecular self-assembly pathways |
| WO2009011855A2 (en) * | 2007-07-16 | 2009-01-22 | California Institute Of Technology | Selection of nucleic acid-based sensor domains within nucleic acid switch platform |
| AU2008286701B2 (en) * | 2007-08-14 | 2015-02-12 | Commonwealth Scientific And Industrial Research Organisation | Improved gene silencing methods |
| US8367815B2 (en) * | 2007-08-28 | 2013-02-05 | California Institute Of Technology | Modular polynucleotides for ligand-controlled regulatory systems |
| US20120165387A1 (en) | 2007-08-28 | 2012-06-28 | Smolke Christina D | General composition framework for ligand-controlled RNA regulatory systems |
| US8865667B2 (en) | 2007-09-12 | 2014-10-21 | California Institute Of Technology | Higher-order cellular information processing devices |
| US9029524B2 (en) * | 2007-12-10 | 2015-05-12 | California Institute Of Technology | Signal activated RNA interference |
| US8329889B2 (en) * | 2008-02-15 | 2012-12-11 | Trustees Of Boston University | In vivo gene sensors |
| US8497364B2 (en) * | 2008-02-27 | 2013-07-30 | California Institute Of Technology | Triggered RNAi |
| JP5463621B2 (ja) * | 2008-02-27 | 2014-04-09 | ソニー株式会社 | 標的物質の定量方法 |
| US20100021904A1 (en) * | 2008-05-21 | 2010-01-28 | Pierce Niles A | Shielded cross-linking probes |
| US8241854B2 (en) * | 2008-05-22 | 2012-08-14 | California Institute Of Technology | Triggered RNAi |
| US20100021901A1 (en) * | 2008-05-22 | 2010-01-28 | Peng Yin | Compositions and methods for detecting analytes |
| WO2010005055A1 (ja) * | 2008-07-09 | 2010-01-14 | 公立大学法人大阪市立大学 | オリゴヌクレオチド構造体および遺伝子発現制御方法 |
| CA2730083C (en) * | 2008-07-11 | 2016-11-01 | The Administrators Of The Tulane Educational Fund | Stimulus-responsive apta-chelamers |
| US8329882B2 (en) | 2009-02-18 | 2012-12-11 | California Institute Of Technology | Genetic control of mammalian cells with synthetic RNA regulatory systems |
| US9145555B2 (en) * | 2009-04-02 | 2015-09-29 | California Institute Of Technology | Integrated—ligand-responsive microRNAs |
| US9284559B2 (en) * | 2009-04-14 | 2016-03-15 | Wake Forest University Health Sciences | Multivalent aptamer complexes |
| WO2011050000A2 (en) * | 2009-10-20 | 2011-04-28 | The Regents Of The University Of California | Single molecule nucleic acid nanoparticles |
| US8658780B2 (en) | 2010-05-18 | 2014-02-25 | California Institute Of Technology | Triggered covalent probes for imaging and silencing genetic expression |
| WO2011163526A2 (en) * | 2010-06-23 | 2011-12-29 | California Institute Of Technology | Signal activated molecular delivery |
| US9834439B2 (en) | 2010-07-20 | 2017-12-05 | California Institute Of Technology | Biomolecular self-assembly |
| US8962241B2 (en) | 2010-07-20 | 2015-02-24 | California Institute Of Technology | Triggered molecular geometry based bioimaging probes |
| US8877438B2 (en) | 2010-07-20 | 2014-11-04 | California Institute Of Technology | Self-assembled polynucleotide structure |
| WO2012056441A1 (en) * | 2010-10-28 | 2012-05-03 | Nanodoc Ltd. | Compositions and methods for specific cleavage of exogenous rna in a cell |
| US9115355B2 (en) | 2011-06-23 | 2015-08-25 | California Institute Of Technology | Exonuclease resistant polynucleotide and related duplex polynucleotides, constructs, compositions, methods and systems |
| US9725715B2 (en) | 2011-06-23 | 2017-08-08 | California Institute Of Technology | Signal activatable constructs and related components compositions methods and systems |
| CA2853829C (en) | 2011-07-22 | 2023-09-26 | President And Fellows Of Harvard College | Evaluation and improvement of nuclease cleavage specificity |
| WO2013119888A1 (en) | 2012-02-09 | 2013-08-15 | President And Fellows Of Harvard College | Selective nucleic acid amplification from nucleic acid pools |
| WO2013142735A1 (en) | 2012-03-21 | 2013-09-26 | California Institute Of Technology | Targeting domain and related signal activated molecular delivery |
| JP5731016B2 (ja) * | 2012-04-27 | 2015-06-10 | キリン株式会社 | リガンドを高感度に検出する核酸分子並びに該核酸分子のスクリーニング方法および該核酸分子の感度の最適化方法 |
| US9856472B2 (en) | 2013-07-01 | 2018-01-02 | California Institute Of Technology | Small conditional RNAs |
| CA2917627A1 (en) | 2013-07-18 | 2015-01-22 | President And Fellows Of Harvard College | Specific nucleic acid amplification with compounded selectivity |
| US9163284B2 (en) | 2013-08-09 | 2015-10-20 | President And Fellows Of Harvard College | Methods for identifying a target site of a Cas9 nuclease |
| US9359599B2 (en) | 2013-08-22 | 2016-06-07 | President And Fellows Of Harvard College | Engineered transcription activator-like effector (TALE) domains and uses thereof |
| US9526784B2 (en) | 2013-09-06 | 2016-12-27 | President And Fellows Of Harvard College | Delivery system for functional nucleases |
| US9388430B2 (en) | 2013-09-06 | 2016-07-12 | President And Fellows Of Harvard College | Cas9-recombinase fusion proteins and uses thereof |
| US9228207B2 (en) | 2013-09-06 | 2016-01-05 | President And Fellows Of Harvard College | Switchable gRNAs comprising aptamers |
| US9486533B2 (en) | 2013-09-27 | 2016-11-08 | Wake Forest University Health Sciences | Pharmaceutical compositions for high-capacity targeted delivery |
| JP6267217B2 (ja) * | 2013-09-30 | 2018-01-24 | Necソリューションイノベータ株式会社 | ターゲットの分析方法および分析キット |
| CN111218447B (zh) | 2013-11-07 | 2024-10-11 | 爱迪塔斯医药有限公司 | 使用统治型gRNA的CRISPR相关方法和组合物 |
| US20150166982A1 (en) | 2013-12-12 | 2015-06-18 | President And Fellows Of Harvard College | Methods for correcting pi3k point mutations |
| WO2015095633A1 (en) * | 2013-12-20 | 2015-06-25 | Board Of Regents, The University Of Texas System | Methods and compositions related to nucleic acid circuits and signal transducers |
| EP3674408A1 (en) * | 2014-06-16 | 2020-07-01 | The Johns Hopkins University | Compositions and methods for the expression of crispr guide rnas |
| WO2016018963A1 (en) | 2014-07-30 | 2016-02-04 | President And Fellows Of Harvard College | Probe library construction |
| EP3177718B1 (en) | 2014-07-30 | 2022-03-16 | President and Fellows of Harvard College | Cas9 proteins including ligand-dependent inteins |
| KR101719285B1 (ko) | 2014-11-04 | 2017-03-23 | 한국과학기술원 | 표적 물질에 의해 조절되는 핵산 중합효소 활성을 이용한 생체물질의 검출 및 정량 방법 |
| HUE054624T2 (hu) | 2015-02-02 | 2021-09-28 | Meiragtx Uk Ii Ltd | A génexpresszió szabályozása az alternatív splicing aptamer által közvetített modulálásával |
| US9434996B1 (en) * | 2015-03-13 | 2016-09-06 | Tracy Ann Hayden | All mini-STR multiplex with increased C.E. through-put by STR prolongation template fusion |
| US20180094309A1 (en) * | 2015-04-03 | 2018-04-05 | President And Fellows Of Harvard College | Nucleic acid retro-activated primers |
| SG11201803173VA (en) | 2015-10-23 | 2018-05-30 | Harvard College | Nucleobase editors and uses thereof |
| CA3029625A1 (en) | 2016-07-05 | 2018-01-11 | California Institute Of Technology | Fractional initiator hybridization chain reaction |
| JP7231935B2 (ja) | 2016-08-03 | 2023-03-08 | プレジデント アンド フェローズ オブ ハーバード カレッジ | アデノシン核酸塩基編集因子およびそれらの使用 |
| CA3033327A1 (en) | 2016-08-09 | 2018-02-15 | President And Fellows Of Harvard College | Programmable cas9-recombinase fusion proteins and uses thereof |
| WO2018039438A1 (en) | 2016-08-24 | 2018-03-01 | President And Fellows Of Harvard College | Incorporation of unnatural amino acids into proteins using base editing |
| US10815519B2 (en) | 2016-08-30 | 2020-10-27 | California Institute Of Technology | Immunohistochemistry via hybridization chain reaction |
| WO2018071868A1 (en) | 2016-10-14 | 2018-04-19 | President And Fellows Of Harvard College | Aav delivery of nucleobase editors |
| US10745677B2 (en) | 2016-12-23 | 2020-08-18 | President And Fellows Of Harvard College | Editing of CCR5 receptor gene to protect against HIV infection |
| EP3592853A1 (en) | 2017-03-09 | 2020-01-15 | President and Fellows of Harvard College | Suppression of pain by gene editing |
| US12390514B2 (en) | 2017-03-09 | 2025-08-19 | President And Fellows Of Harvard College | Cancer vaccine |
| EP4389894A3 (en) * | 2017-03-10 | 2024-08-28 | The Medical College of Wisconsin, Inc. | Riboswitch modulated gene therapy for retinal diseases |
| JP2020510439A (ja) | 2017-03-10 | 2020-04-09 | プレジデント アンド フェローズ オブ ハーバード カレッジ | シトシンからグアニンへの塩基編集因子 |
| KR20190140918A (ko) | 2017-03-15 | 2019-12-20 | 더 브로드 인스티튜트, 인코퍼레이티드 | 바이러스 검출을 위한 crispr 이펙터 시스템 기반 진단 |
| KR20240116572A (ko) | 2017-03-23 | 2024-07-29 | 프레지던트 앤드 펠로우즈 오브 하바드 칼리지 | 핵산 프로그램가능한 dna 결합 단백질을 포함하는 핵염기 편집제 |
| US11560566B2 (en) | 2017-05-12 | 2023-01-24 | President And Fellows Of Harvard College | Aptazyme-embedded guide RNAs for use with CRISPR-Cas9 in genome editing and transcriptional activation |
| US11788123B2 (en) | 2017-05-26 | 2023-10-17 | President And Fellows Of Harvard College | Systems and methods for high-throughput image-based screening |
| WO2018226666A1 (en) * | 2017-06-05 | 2018-12-13 | Maumita Mandal | Methods, compositions, and devices involving pseudoknot formation |
| EP3658573A1 (en) | 2017-07-28 | 2020-06-03 | President and Fellows of Harvard College | Methods and compositions for evolving base editors using phage-assisted continuous evolution (pace) |
| WO2019139645A2 (en) | 2017-08-30 | 2019-07-18 | President And Fellows Of Harvard College | High efficiency base editors comprising gam |
| AU2018352592C1 (en) | 2017-10-16 | 2025-09-25 | Beam Therapeutics, Inc. | Uses of adenosine base editors |
| EP3724214A4 (en) | 2017-12-15 | 2021-09-01 | The Broad Institute Inc. | SYSTEMS AND METHODS FOR PREDICTING REPAIR RESULTS IN GENETIC ENGINEERING |
| US12157760B2 (en) | 2018-05-23 | 2024-12-03 | The Broad Institute, Inc. | Base editors and uses thereof |
| US12281338B2 (en) | 2018-10-29 | 2025-04-22 | The Broad Institute, Inc. | Nucleobase editors comprising GeoCas9 and uses thereof |
| AU2019398322A1 (en) | 2018-12-13 | 2021-06-24 | President And Fellows Of Harvard College | Amplification methods and systems for MERFISH and other applications |
| US12351837B2 (en) | 2019-01-23 | 2025-07-08 | The Broad Institute, Inc. | Supernegatively charged proteins and uses thereof |
| GB2601617B (en) | 2019-03-19 | 2024-02-21 | Broad Inst Inc | Methods and compositions for editing nucleotide sequences |
| US12473543B2 (en) | 2019-04-17 | 2025-11-18 | The Broad Institute, Inc. | Adenine base editors with reduced off-target effects |
| US12435330B2 (en) | 2019-10-10 | 2025-10-07 | The Broad Institute, Inc. | Methods and compositions for prime editing RNA |
| JP2023517054A (ja) * | 2020-03-06 | 2023-04-21 | カリフォルニア インスティチュート オブ テクノロジー | ハイブリダイゼーション連鎖反応を介した試料内の標的分子の分析 |
| DE112021002672T5 (de) | 2020-05-08 | 2023-04-13 | President And Fellows Of Harvard College | Vefahren und zusammensetzungen zum gleichzeitigen editieren beider stränge einer doppelsträngigen nukleotid-zielsequenz |
| CN116096729A (zh) * | 2020-05-27 | 2023-05-09 | 威廉马歇莱思大学 | Dna扩增反应中的不可延伸的寡核苷酸 |
| WO2022164796A1 (en) | 2021-01-26 | 2022-08-04 | California Institute Of Technology | Allosteric conditional guide rnas for cell-selective regulation of crispr/cas |
| KR102661292B1 (ko) * | 2021-09-09 | 2024-04-30 | 포항공과대학교 산학협력단 | 금속 이온 반응성 dna 중합 효소 스위치 및 이에 의해 제어되는 등온 증폭 방법 |
| US20250302992A1 (en) * | 2021-12-15 | 2025-10-02 | Meiragtx Uk Ii Limited | Polycistronic Expression of Gut Peptides |
Family Cites Families (117)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6019A (en) * | 1849-01-09 | Cast-iron car-wheel | ||
| US1000289A (en) | 1910-09-14 | 1911-08-08 | Arthur Low Patterson | Stocking-folder. |
| US4235871A (en) * | 1978-02-24 | 1980-11-25 | Papahadjopoulos Demetrios P | Method of encapsulating biologically active materials in lipid vesicles |
| US4426330A (en) * | 1981-07-20 | 1984-01-17 | Lipid Specialties, Inc. | Synthetic phospholipid compounds |
| US4534899A (en) * | 1981-07-20 | 1985-08-13 | Lipid Specialties, Inc. | Synthetic phospholipid compounds |
| US4501728A (en) * | 1983-01-06 | 1985-02-26 | Technology Unlimited, Inc. | Masking of liposomes from RES recognition |
| US4897355A (en) * | 1985-01-07 | 1990-01-30 | Syntex (U.S.A.) Inc. | N[ω,(ω-1)-dialkyloxy]- and N-[ω,(ω-1)-dialkenyloxy]-alk-1-yl-N,N,N-tetrasubstituted ammonium lipids and uses therefor |
| US4737323A (en) * | 1986-02-13 | 1988-04-12 | Liposome Technology, Inc. | Liposome extrusion method |
| US4902505A (en) | 1986-07-30 | 1990-02-20 | Alkermes | Chimeric peptides for neuropeptide delivery through the blood-brain barrier |
| EP0260032B1 (en) * | 1986-09-08 | 1994-01-26 | Ajinomoto Co., Inc. | Compounds for the cleavage at a specific position of RNA, oligomers employed for the formation of said compounds, and starting materials for the synthesis of said oligomers |
| US5116742A (en) | 1986-12-03 | 1992-05-26 | University Patents, Inc. | RNA ribozyme restriction endoribonucleases and methods |
| US4987071A (en) | 1986-12-03 | 1991-01-22 | University Patents, Inc. | RNA ribozyme polymerases, dephosphorylases, restriction endoribonucleases and methods |
| US4837028A (en) * | 1986-12-24 | 1989-06-06 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5276019A (en) | 1987-03-25 | 1994-01-04 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US5264423A (en) * | 1987-03-25 | 1993-11-23 | The United States Of America As Represented By The Department Of Health And Human Services | Inhibitors for replication of retroviruses and for the expression of oncogene products |
| US4904582A (en) | 1987-06-11 | 1990-02-27 | Synthetic Genetics | Novel amphiphilic nucleic acid conjugates |
| US5176996A (en) * | 1988-12-20 | 1993-01-05 | Baylor College Of Medicine | Method for making synthetic oligonucleotides which bind specifically to target sites on duplex DNA molecules, by forming a colinear triplex, the synthetic oligonucleotides and methods of use |
| US5354844A (en) * | 1989-03-16 | 1994-10-11 | Boehringer Ingelheim International Gmbh | Protein-polycation conjugates |
| US5108921A (en) * | 1989-04-03 | 1992-04-28 | Purdue Research Foundation | Method for enhanced transmembrane transport of exogenous molecules |
| ZA902710B (en) | 1989-05-22 | 1991-12-24 | Univ Georgia Res Found | Enzyme luminescence assay |
| US5256775A (en) * | 1989-06-05 | 1993-10-26 | Gilead Sciences, Inc. | Exonuclease-resistant oligonucleotides |
| US5227170A (en) * | 1989-06-22 | 1993-07-13 | Vestar, Inc. | Encapsulation process |
| US5356633A (en) * | 1989-10-20 | 1994-10-18 | Liposome Technology, Inc. | Method of treatment of inflamed tissues |
| US5013556A (en) * | 1989-10-20 | 1991-05-07 | Liposome Technology, Inc. | Liposomes with enhanced circulation time |
| US5527528A (en) * | 1989-10-20 | 1996-06-18 | Sequus Pharmaceuticals, Inc. | Solid-tumor treatment method |
| US5264564A (en) * | 1989-10-24 | 1993-11-23 | Gilead Sciences | Oligonucleotide analogs with novel linkages |
| US5469854A (en) * | 1989-12-22 | 1995-11-28 | Imarx Pharmaceutical Corp. | Methods of preparing gas-filled liposomes |
| US5580575A (en) * | 1989-12-22 | 1996-12-03 | Imarx Pharmaceutical Corp. | Therapeutic drug delivery systems |
| US5264618A (en) * | 1990-04-19 | 1993-11-23 | Vical, Inc. | Cationic lipids for intracellular delivery of biologically active molecules |
| WO1991017424A1 (en) | 1990-05-03 | 1991-11-14 | Vical, Inc. | Intracellular delivery of biologically active substances by means of self-assembling lipid complexes |
| US5270163A (en) * | 1990-06-11 | 1993-12-14 | University Research Corporation | Methods for identifying nucleic acid ligands |
| DE69133511T2 (de) | 1990-08-13 | 2006-09-28 | Isis Pharmaceuticals, Inc., Carlsbad | Zuckermodifizierte oligonukleotide zum erkennen und steuern der genexpression |
| US5500357A (en) * | 1990-11-02 | 1996-03-19 | Agency Of Industrial Science & Technology, Ministry Of International Trade & Industry | RNA transcription system using novel ribozyme |
| JP3220180B2 (ja) * | 1991-05-23 | 2001-10-22 | 三菱化学株式会社 | 薬剤含有タンパク質結合リポソーム |
| US5582981A (en) * | 1991-08-14 | 1996-12-10 | Gilead Sciences, Inc. | Method for identifying an oligonucleotide aptamer specific for a target |
| US5525719A (en) * | 1991-08-30 | 1996-06-11 | Chemgenes Corporation | N-protected-2'-O-methyl-and N-protected-3'-O-methyl-ribonucleosides and their phosphoramidite derivatives |
| US5214135A (en) * | 1991-08-30 | 1993-05-25 | Chemgenes Corporation | N-protected-2'-O-methyl-ribonucleosides and N-protected 2'-O-methyl-3'-cyanoethyl-N-,N-diisopropyl phosphoramidite ribonucleosides |
| NZ244306A (en) * | 1991-09-30 | 1995-07-26 | Boehringer Ingelheim Int | Composition for introducing nucleic acid complexes into eucaryotic cells, complex containing nucleic acid and endosomolytic agent, peptide with endosomolytic domain and nucleic acid binding domain and preparation |
| US5521291A (en) * | 1991-09-30 | 1996-05-28 | Boehringer Ingelheim International, Gmbh | Conjugates for introducing nucleic acid into higher eucaryotic cells |
| TW393513B (en) * | 1991-11-26 | 2000-06-11 | Isis Pharmaceuticals Inc | Enhanced triple-helix and double-helix formation with oligomers containing modified pyrimidines |
| US5756291A (en) * | 1992-08-21 | 1998-05-26 | Gilead Sciences, Inc. | Aptamers specific for biomolecules and methods of making |
| US5583020A (en) * | 1992-11-24 | 1996-12-10 | Ribozyme Pharmaceuticals, Inc. | Permeability enhancers for negatively charged polynucleotides |
| JP3351476B2 (ja) * | 1993-01-22 | 2002-11-25 | 三菱化学株式会社 | リン脂質誘導体及びそれを含有するリポソーム |
| US5395619A (en) * | 1993-03-03 | 1995-03-07 | Liposome Technology, Inc. | Lipid-polymer conjugates and liposomes |
| US5462854A (en) * | 1993-04-19 | 1995-10-31 | Beckman Instruments, Inc. | Inverse linkage oligonucleotides for chemical and enzymatic processes |
| US5534259A (en) * | 1993-07-08 | 1996-07-09 | Liposome Technology, Inc. | Polymer compound and coated particle composition |
| US5543158A (en) * | 1993-07-23 | 1996-08-06 | Massachusetts Institute Of Technology | Biodegradable injectable nanoparticles |
| US5417978A (en) * | 1993-07-29 | 1995-05-23 | Board Of Regents, The University Of Texas System | Liposomal antisense methyl phosphonate oligonucleotides and methods for their preparation and use |
| US5595756A (en) * | 1993-12-22 | 1997-01-21 | Inex Pharmaceuticals Corporation | Liposomal compositions for enhanced retention of bioactive agents |
| US5651981A (en) * | 1994-03-29 | 1997-07-29 | Northwestern University | Cationic phospholipids for transfection |
| US5543152A (en) * | 1994-06-20 | 1996-08-06 | Inex Pharmaceuticals Corporation | Sphingosomes for enhanced drug delivery |
| US5777153A (en) * | 1994-07-08 | 1998-07-07 | Gilead Sciences, Inc. | Cationic lipids |
| US5591721A (en) * | 1994-10-25 | 1997-01-07 | Hybridon, Inc. | Method of down-regulating gene expression |
| US5512295A (en) * | 1994-11-10 | 1996-04-30 | The Board Of Trustees Of The Leland Stanford Junior University | Synthetic liposomes for enhanced uptake and delivery |
| US5767099A (en) * | 1994-12-09 | 1998-06-16 | Genzyme Corporation | Cationic amphiphiles containing amino acid or dervatized amino acid groups for intracellular delivery of therapeutic molecules |
| US5830430A (en) * | 1995-02-21 | 1998-11-03 | Imarx Pharmaceutical Corp. | Cationic lipids and the use thereof |
| US5736392A (en) * | 1995-06-07 | 1998-04-07 | Life Technologies, Inc. | Peptide-enhanced cationic lipid transfections |
| US5851548A (en) * | 1995-06-07 | 1998-12-22 | Gen-Probe Incorporated | Liposomes containing cationic lipids and vitamin D |
| AU2994997A (en) | 1996-05-07 | 1997-11-26 | T Cell Sciences, Inc. | Aptamers for complement protein c3b |
| WO1999004800A1 (en) | 1997-07-22 | 1999-02-04 | Genitrix, Llc | Nucleic acid compositions and methods of introducing nucleic acids into cells |
| US5849902A (en) | 1996-09-26 | 1998-12-15 | Oligos Etc. Inc. | Three component chimeric antisense oligonucleotides |
| WO1999027133A1 (en) | 1997-11-26 | 1999-06-03 | Medical Research Council | Improved selex procedure and an anti-cd4 aptamer |
| US6180348B1 (en) | 1998-04-20 | 2001-01-30 | Weihua Li | Method of isolating target specific oligonucleotide ligands |
| US6458559B1 (en) * | 1998-04-22 | 2002-10-01 | Cornell Research Foundation, Inc. | Multivalent RNA aptamers and their expression in multicellular organisms |
| WO1999055857A2 (en) * | 1998-04-29 | 1999-11-04 | Ribozyme Pharmaceuticals, Inc. | Nucleoside triphosphates and their incorporation into ribozymes |
| US7001733B1 (en) * | 1998-05-12 | 2006-02-21 | Rigel Pharmaceuticals, Inc. | Methods and compositions for screening for modulations of IgE synthesis, secretion and switch rearrangement |
| WO2000011218A1 (en) * | 1998-08-21 | 2000-03-02 | Affymetrix, Inc. | Expression monitoring for human cytomegalovirus (hcmv) infection |
| WO2000020040A1 (en) | 1998-10-08 | 2000-04-13 | University Of Massachusetts | Controlling gene expression in living cells |
| US20060172925A1 (en) * | 1998-10-26 | 2006-08-03 | Board Of Regents, The University Of Texas System | Thio-siRNA aptamers |
| US20060121510A1 (en) * | 1998-11-03 | 2006-06-08 | Breaker Ronald R | Multidomain polynucleotide molecular sensors |
| US6958215B1 (en) * | 1999-03-15 | 2005-10-25 | Geron Corporation | Methods and compositions for inhibition of RNA splicing |
| US6951722B2 (en) * | 1999-03-19 | 2005-10-04 | Takara Bio Inc. | Method for amplifying nucleic acid sequence |
| US20040072785A1 (en) * | 1999-11-23 | 2004-04-15 | Wolff Jon A. | Intravascular delivery of non-viral nucleic acid |
| US7098030B2 (en) * | 1999-12-31 | 2006-08-29 | Mirus Bio Corporation | Polyampholytes for delivering polyions to a cell |
| AU2001245793A1 (en) * | 2000-03-16 | 2001-09-24 | Cold Spring Harbor Laboratory | Methods and compositions for rna interference |
| AU2001259586A1 (en) * | 2000-05-05 | 2001-11-20 | Agilix Corporation | Highly multiplexed reporter carrier systems |
| US20030104520A1 (en) * | 2000-06-15 | 2003-06-05 | Ellington Andrew D. | Regulatable, catalytically active nucleic acids |
| US6706474B1 (en) * | 2000-06-27 | 2004-03-16 | Board Of Trustees Of The University Of Illinois | Nucleic acid enzyme biosensors for ions |
| IL154508A0 (en) * | 2000-08-18 | 2003-09-17 | Gencell Sa | System for regulating in vivo the expression of a transgene by conditional inhibition |
| US7115369B2 (en) * | 2001-03-15 | 2006-10-03 | Iowa State University Research Foundation, Inc. | Functional nucleic acid probes and uses thereof |
| US6951720B2 (en) * | 2001-05-10 | 2005-10-04 | San Diego State University Foundation | Use of phosphorothiolate polynucleotides in ligating nucleic acids |
| WO2002097114A2 (en) * | 2001-05-29 | 2002-12-05 | Sirna Therapeutics, Inc. | Nucleic acid treatment of diseases or conditions related to levels of ras, her2 and hiv |
| US20040063654A1 (en) * | 2001-11-02 | 2004-04-01 | Davis Mark E. | Methods and compositions for therapeutic use of RNA interference |
| US20030157030A1 (en) * | 2001-11-02 | 2003-08-21 | Insert Therapeutics, Inc. | Methods and compositions for therapeutic use of rna interference |
| EP1451588A4 (en) * | 2001-11-06 | 2005-03-09 | Agilix Corp | SENSITIVE CODED DEFINITION SYSTEMS |
| AU2002343792A1 (en) * | 2001-11-28 | 2003-06-10 | Center For Advanced Science And Technology Incubation, Ltd. | siRNA EXPRESSION SYSTEM AND PROCESS FOR PRODUCING FUNCTIONAL GENE-KNOCKDOWN CELLS AND THE LIKE USING THE SAME |
| WO2004033653A2 (en) * | 2002-10-10 | 2004-04-22 | Oxford Biomedica (Uk) Limited | Gene regulation with aptamer and modulator complexes for gene therapy |
| EP1585756B1 (en) * | 2002-11-26 | 2010-04-21 | University of Massachusetts | Delivery of sirnas |
| DE10302421A1 (de) | 2003-01-21 | 2004-07-29 | Ribopharma Ag | Doppelsträngige Ribonukleinsäure mit verbesserter Wirksamkeit |
| US20040162235A1 (en) * | 2003-02-18 | 2004-08-19 | Trubetskoy Vladimir S. | Delivery of siRNA to cells using polyampholytes |
| US20050026286A1 (en) * | 2003-03-05 | 2005-02-03 | Jen-Tsan Chi | Methods and compositions for selective RNAi mediated inhibition of gene expression in mammal cells |
| WO2005001039A2 (en) * | 2003-05-29 | 2005-01-06 | Creighton University | Ribozyme-regulated small inhibitory rna (sirna) production and methods of use thereof |
| US20060178327A1 (en) * | 2003-05-30 | 2006-08-10 | Yeung Wah Hin A | Inhibition of gene expression by delivery of specially selected double stranded or other forms of small interfering RNA precursors enabling the formation and function of small interfering RNA in vivo and in vitro |
| US20050026823A1 (en) * | 2003-06-20 | 2005-02-03 | Biomarin Pharmaceutical Inc. | Use of the chaperone receptor-associated protein (RAP) for the delivery of therapeutic compounds to the brain and other tissues |
| US7672786B2 (en) * | 2003-07-02 | 2010-03-02 | Sergey Krylov | Non-equilibrium capillary electrophoresis of equilibrium mixtures (NECEEM)—based methods for drug and diagnostic development |
| US20050256071A1 (en) * | 2003-07-15 | 2005-11-17 | California Institute Of Technology | Inhibitor nucleic acids |
| JP4951338B2 (ja) * | 2003-07-16 | 2012-06-13 | プロチバ バイオセラピューティクス インコーポレイティッド | 脂質に封入された干渉rna |
| US20050265957A1 (en) * | 2004-04-08 | 2005-12-01 | Monahan Sean D | Polymerized formamides for use in delivery of compounds to cells |
| US20060105975A1 (en) * | 2004-04-19 | 2006-05-18 | Shannon Pendergrast | Aptamer-mediated intracellular delivery of therapeutic oligonucleotides |
| AU2005252273B2 (en) * | 2004-06-07 | 2011-04-28 | Arbutus Biopharma Corporation | Lipid encapsulated interfering RNA |
| ATE514776T1 (de) | 2004-10-05 | 2011-07-15 | California Inst Of Techn | Aptamer-regulierte nukleinsäuren und verwendungen davon |
| WO2006086669A2 (en) | 2005-02-09 | 2006-08-17 | California Institute Of Technology | Aptamer regulated nucleic acids and uses thereof |
| US20060200878A1 (en) * | 2004-12-21 | 2006-09-07 | Linda Lutfiyya | Recombinant DNA constructs and methods for controlling gene expression |
| JP2009523823A (ja) * | 2006-01-23 | 2009-06-25 | アボット・ラボラトリーズ | siRNA送達のための化学的に修飾されたポリカチオンポリマー |
| EP1986609A2 (en) | 2006-01-26 | 2008-11-05 | University of Massachusetts | Rna silencing agents for use in therapy and nanotransporters for efficient delivery of same |
| ES2610703T3 (es) * | 2006-06-23 | 2017-05-03 | Engeneic Molecular Delivery Pty Ltd. | Administración dirigida de fármacos, ácidos nucleicos terapéuticos y ácidos nucleicos funcionales a células mamíferas mediante células bacterianas muertas intactas |
| KR101133799B1 (ko) * | 2006-08-18 | 2012-04-24 | 에프. 호프만-라 로슈 아게 | 폴리뉴클레오타이드의 생체내 전달을 위한 다가접합체 |
| US8252755B2 (en) | 2006-09-22 | 2012-08-28 | Dharmacon, Inc. | Duplex oligonucleotide complexes and methods for gene silencing by RNA interference |
| US8039010B2 (en) * | 2006-11-03 | 2011-10-18 | Allergan, Inc. | Sustained release intraocular drug delivery systems comprising a water soluble therapeutic agent and a release modifier |
| WO2008058291A2 (en) | 2006-11-09 | 2008-05-15 | California Institute Of Technology | Modular aptamer-regulated ribozymes |
| WO2009011855A2 (en) * | 2007-07-16 | 2009-01-22 | California Institute Of Technology | Selection of nucleic acid-based sensor domains within nucleic acid switch platform |
| US20120165387A1 (en) | 2007-08-28 | 2012-06-28 | Smolke Christina D | General composition framework for ligand-controlled RNA regulatory systems |
| US8367815B2 (en) * | 2007-08-28 | 2013-02-05 | California Institute Of Technology | Modular polynucleotides for ligand-controlled regulatory systems |
| US8865667B2 (en) * | 2007-09-12 | 2014-10-21 | California Institute Of Technology | Higher-order cellular information processing devices |
| US9029524B2 (en) * | 2007-12-10 | 2015-05-12 | California Institute Of Technology | Signal activated RNA interference |
| US8329882B2 (en) * | 2009-02-18 | 2012-12-11 | California Institute Of Technology | Genetic control of mammalian cells with synthetic RNA regulatory systems |
| US9145555B2 (en) * | 2009-04-02 | 2015-09-29 | California Institute Of Technology | Integrated—ligand-responsive microRNAs |
-
2005
- 2005-10-05 AT AT05803129T patent/ATE514776T1/de not_active IP Right Cessation
- 2005-10-05 EP EP05803129A patent/EP1799825B1/en not_active Expired - Lifetime
- 2005-10-05 WO PCT/US2005/036161 patent/WO2006042112A2/en not_active Ceased
- 2005-10-05 JP JP2007534921A patent/JP5101288B2/ja not_active Expired - Fee Related
- 2005-10-05 US US11/243,889 patent/US9315862B2/en not_active Expired - Fee Related
-
2006
- 2006-02-09 US US11/884,110 patent/US8772464B2/en active Active
-
2012
- 2012-08-10 JP JP2012178625A patent/JP5795563B2/ja not_active Expired - Fee Related
-
2014
- 2014-05-27 US US14/287,824 patent/US9309568B2/en not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| ATE514776T1 (de) | 2011-07-15 |
| JP2008515405A (ja) | 2008-05-15 |
| JP5795563B2 (ja) | 2015-10-14 |
| JP2012223201A (ja) | 2012-11-15 |
| US20060088864A1 (en) | 2006-04-27 |
| WO2006042112A9 (en) | 2006-06-29 |
| US8772464B2 (en) | 2014-07-08 |
| EP1799825B1 (en) | 2011-06-29 |
| EP1799825A2 (en) | 2007-06-27 |
| WO2006042112A2 (en) | 2006-04-20 |
| US20120035065A1 (en) | 2012-02-09 |
| US9309568B2 (en) | 2016-04-12 |
| US9315862B2 (en) | 2016-04-19 |
| US20150024954A1 (en) | 2015-01-22 |
| WO2006042112A3 (en) | 2006-12-21 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP5101288B2 (ja) | アプタマー調節される核酸及びその利用 | |
| US9040495B2 (en) | General composition framework for ligand-controlled RNA regulatory systems | |
| US8367815B2 (en) | Modular polynucleotides for ligand-controlled regulatory systems | |
| US8603996B2 (en) | Modular aptamer-regulated ribozymes | |
| Taylor et al. | A modular XNAzyme cleaves long, structured RNAs under physiological conditions and enables allele-specific gene silencing | |
| US20090082217A1 (en) | Selection of nucleic acid-based sensor domains within nucleic acid switch platform | |
| US8865667B2 (en) | Higher-order cellular information processing devices | |
| WO2020033938A1 (en) | Programmable conditional sirnas and uses thereof | |
| Scherr et al. | Detection of antisense and ribozyme accessible sites on native mRNAs: application to NCOA3 mRNA | |
| US20100292299A1 (en) | Nucleotide Motifs Providing Localization Elements and Methods of Use | |
| Bansal | MicroRNA therapeutics: Discovering novel targets and developing specific therapy | |
| Shestopalov et al. | Oligonucleotide-based tools for studying zebrafish development | |
| CN101072874A (zh) | 适体调节的核酸及其应用 | |
| US8604176B2 (en) | Protein-responsive RNA control devices and uses thereof | |
| Pérez et al. | MicroRNA interference | |
| Zhu et al. | MiRNA Locker: A Modularized DNA Assembly As miRNA Inhibitors | |
| EP3746555B1 (en) | Inhibition of a lncrna for treatment of neuroblastoma | |
| Singh et al. | Modulating populational variance of methyl-guanine methyl transferase expression through miR-181d degradation: a novel mechanism of temozolomide resistance | |
| US20120110686A1 (en) | Cand45 tRNA-Derived Expression System for Gene Modulation | |
| Yi et al. | Efficient Silencing of Gene Expression by an ASON–Bulge–DNAzyme Complex | |
| Jain et al. | Technologies, Companies & Markets | |
| Crater et al. | Antisense technologies in the studying of Toxoplasma gondii | |
| Rossi | RNA interference: application to drug discovery and challenges to pharmaceutical development | |
| Lataniotis et al. | Gene Editing and Specific MicroRNA Inhibition | |
| Litke | A Ribozyme-Mediated Mammalian Expression System and Its Utility for RNA-Based Devices and Aptamers in Live Cells |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20081006 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20081006 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110705 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20111005 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20111013 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111107 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120410 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120710 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120718 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120810 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120904 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120926 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20151005 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 5101288 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |