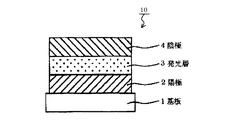
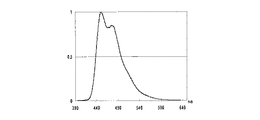
JP5064787B2 - ベンゾフルオランテン化合物及びこれを使用した有機発光素子 - Google Patents
ベンゾフルオランテン化合物及びこれを使用した有機発光素子 Download PDFInfo
- Publication number
- JP5064787B2 JP5064787B2 JP2006349579A JP2006349579A JP5064787B2 JP 5064787 B2 JP5064787 B2 JP 5064787B2 JP 2006349579 A JP2006349579 A JP 2006349579A JP 2006349579 A JP2006349579 A JP 2006349579A JP 5064787 B2 JP5064787 B2 JP 5064787B2
- Authority
- JP
- Japan
- Prior art keywords
- group
- compound
- light emitting
- organic light
- general formula
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- BFHOMIVZJFOJHR-UHFFFAOYSA-N CC(C)(C)C(C=C(C12)C=C3)=CC1C=Cc1c2c3ccc1-c(cc1)ccc1-c(cc1C2(C)C)ccc1-c(cc1)c2cc1-c(cc1)cc(C2(C)C)c1-c1c2cccc1 Chemical compound CC(C)(C)C(C=C(C12)C=C3)=CC1C=Cc1c2c3ccc1-c(cc1)ccc1-c(cc1C2(C)C)ccc1-c(cc1)c2cc1-c(cc1)cc(C2(C)C)c1-c1c2cccc1 BFHOMIVZJFOJHR-UHFFFAOYSA-N 0.000 description 1
- YSQNYMMPXMBVPK-UHFFFAOYSA-N CC1(C)c2ccc(C(C3=CC=CC4C33)=CC=C3c3c4c(-c4ccccc4)c(cccc4)c4c3-c3ccccc3)nc2-c2ccccc12 Chemical compound CC1(C)c2ccc(C(C3=CC=CC4C33)=CC=C3c3c4c(-c4ccccc4)c(cccc4)c4c3-c3ccccc3)nc2-c2ccccc12 YSQNYMMPXMBVPK-UHFFFAOYSA-N 0.000 description 1
- LPXKWZADBGGRSH-UHFFFAOYSA-N Cc(c(C=N)c1)cc2c1c(-c(c1c3c-4ccc1)ccc3-c1c-4c(-c3ccccc3)c(cccc3)c3c1-c1ccccc1)c(cccc1)c1n2 Chemical compound Cc(c(C=N)c1)cc2c1c(-c(c1c3c-4ccc1)ccc3-c1c-4c(-c3ccccc3)c(cccc3)c3c1-c1ccccc1)c(cccc1)c1n2 LPXKWZADBGGRSH-UHFFFAOYSA-N 0.000 description 1
- ZPXZKCMEVFTYFD-UHFFFAOYSA-N Cc(cc1)cc2c1c(-c(c1c3c-4ccc1)ccc3-c1c-4c(-c3ccccc3)c(cccc3)c3c1-c1ccccc1)c(cccc1)c1n2 Chemical compound Cc(cc1)cc2c1c(-c(c1c3c-4ccc1)ccc3-c1c-4c(-c3ccccc3)c(cccc3)c3c1-c1ccccc1)c(cccc1)c1n2 ZPXZKCMEVFTYFD-UHFFFAOYSA-N 0.000 description 1
- DIGQYYJUDLZESM-UHFFFAOYSA-N Cc1c(-c2ccccc2)c(cccc2)c2c(-c(cc2)c(cccc3-4)c3c2-c2c-4c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)n1 Chemical compound Cc1c(-c2ccccc2)c(cccc2)c2c(-c(cc2)c(cccc3-4)c3c2-c2c-4c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)n1 DIGQYYJUDLZESM-UHFFFAOYSA-N 0.000 description 1
- AGNWODQHLHKTNH-UHFFFAOYSA-N Cc1c(C)nc(-c(cc2)c(cccc3-4)c3c2-c2c-4c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)c2c1cccc2 Chemical compound Cc1c(C)nc(-c(cc2)c(cccc3-4)c3c2-c2c-4c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)c2c1cccc2 AGNWODQHLHKTNH-UHFFFAOYSA-N 0.000 description 1
- QZCGFBHVAIQLQB-UHFFFAOYSA-N Cc1cc(-c(ccc(-c(cc2)c(cccc3-4)c3c2-c2c-4c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)c2)c2-c2ccc3)c2c3n1 Chemical compound Cc1cc(-c(ccc(-c(cc2)c(cccc3-4)c3c2-c2c-4c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)c2)c2-c2ccc3)c2c3n1 QZCGFBHVAIQLQB-UHFFFAOYSA-N 0.000 description 1
- XOALGLRJINPWCU-UHFFFAOYSA-N Cc1cc(-c2cc3nc(cccc4)c4c(-c(cc4)c5c6c4-c4c(-c7ccccc7)c(cccc7)c7c(-c7ccccc7)c4-c6ccc5)c3cc2)cc(N)c1 Chemical compound Cc1cc(-c2cc3nc(cccc4)c4c(-c(cc4)c5c6c4-c4c(-c7ccccc7)c(cccc7)c7c(-c7ccccc7)c4-c6ccc5)c3cc2)cc(N)c1 XOALGLRJINPWCU-UHFFFAOYSA-N 0.000 description 1
- TWTWWQKTSOFGRY-UHFFFAOYSA-N Cc1nc(cccc2-c3cccc(-c(cc4)c(cccc5-6)c5c4-c4c-6c(-c5ccccc5)c(cccc5)c5c4-c4ccccc4)c3-3)c2c-3c1 Chemical compound Cc1nc(cccc2-c3cccc(-c(cc4)c(cccc5-6)c5c4-c4c-6c(-c5ccccc5)c(cccc5)c5c4-c4ccccc4)c3-3)c2c-3c1 TWTWWQKTSOFGRY-UHFFFAOYSA-N 0.000 description 1
- YUVXHAMUDVHMLU-UHFFFAOYSA-N Cc1nc2cccc(-c(cc3)c-4cc3-c(c3c5c-6ccc3)ccc5-c3c-6c(-c5ccccc5)c(cccc5)c5c3-c3ccccc3)c2c-4c1 Chemical compound Cc1nc2cccc(-c(cc3)c-4cc3-c(c3c5c-6ccc3)ccc5-c3c-6c(-c5ccccc5)c(cccc5)c5c3-c3ccccc3)c2c-4c1 YUVXHAMUDVHMLU-UHFFFAOYSA-N 0.000 description 1
- KMRQSHXWYSRJJC-UHFFFAOYSA-N FC(c(cc1)cc2c1c(-c(c1c3c-4ccc1)ccc3-c1c-4c(-c3ccccc3)c(cccc3)c3c1-c1ccccc1)c(cccc1)c1n2)(F)F Chemical compound FC(c(cc1)cc2c1c(-c(c1c3c-4ccc1)ccc3-c1c-4c(-c3ccccc3)c(cccc3)c3c1-c1ccccc1)c(cccc1)c1n2)(F)F KMRQSHXWYSRJJC-UHFFFAOYSA-N 0.000 description 1
- APCLABGYLZEKQY-UHFFFAOYSA-N FC(c(cc1)ccc1-c1cc(cccc2)c2c(C(C2=CC=CC3C22)=CC=C2c2c3c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)n1)(F)F Chemical compound FC(c(cc1)ccc1-c1cc(cccc2)c2c(C(C2=CC=CC3C22)=CC=C2c2c3c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)n1)(F)F APCLABGYLZEKQY-UHFFFAOYSA-N 0.000 description 1
- VEEOYJABGKFNKU-UHFFFAOYSA-N Fc(cc1)ccc1-c1cc(cccc2)c2c(C(C2=CC=CC3C22)=CC=C2c2c3c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)n1 Chemical compound Fc(cc1)ccc1-c1cc(cccc2)c2c(C(C2=CC=CC3C22)=CC=C2c2c3c(-c3ccccc3)c(cccc3)c3c2-c2ccccc2)n1 VEEOYJABGKFNKU-UHFFFAOYSA-N 0.000 description 1
- YKBHSHUXQBKEQS-UHFFFAOYSA-N Fc(cc1)ccc1-c1cccc2c1ccnc2C(C1=CC=CC2C11)=CC=C1c1c2c(-c2ccccc2)c(cccc2)c2c1-c1ccccc1 Chemical compound Fc(cc1)ccc1-c1cccc2c1ccnc2C(C1=CC=CC2C11)=CC=C1c1c2c(-c2ccccc2)c(cccc2)c2c1-c1ccccc1 YKBHSHUXQBKEQS-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/04—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, directly attached to the ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D221/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00
- C07D221/02—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00 condensed with carbocyclic rings or ring systems
- C07D221/04—Ortho- or peri-condensed ring systems
- C07D221/06—Ring systems of three rings
- C07D221/16—Ring systems of three rings containing carbocyclic rings other than six-membered
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D221/00—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00
- C07D221/02—Heterocyclic compounds containing six-membered rings having one nitrogen atom as the only ring hetero atom, not provided for by groups C07D211/00 - C07D219/00 condensed with carbocyclic rings or ring systems
- C07D221/04—Ortho- or peri-condensed ring systems
- C07D221/18—Ring systems of four or more rings
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K11/00—Luminescent, e.g. electroluminescent, chemiluminescent materials
- C09K11/06—Luminescent, e.g. electroluminescent, chemiluminescent materials containing organic luminescent materials
-
- H—ELECTRICITY
- H05—ELECTRIC TECHNIQUES NOT OTHERWISE PROVIDED FOR
- H05B—ELECTRIC HEATING; ELECTRIC LIGHT SOURCES NOT OTHERWISE PROVIDED FOR; CIRCUIT ARRANGEMENTS FOR ELECTRIC LIGHT SOURCES, IN GENERAL
- H05B33/00—Electroluminescent light sources
- H05B33/12—Light sources with substantially two-dimensional radiating surfaces
- H05B33/14—Light sources with substantially two-dimensional radiating surfaces characterised by the chemical or physical composition or the arrangement of the electroluminescent material, or by the simultaneous addition of the electroluminescent material in or onto the light source
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K50/00—Organic light-emitting devices
- H10K50/10—OLEDs or polymer light-emitting diodes [PLED]
- H10K50/11—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers
- H10K50/12—OLEDs or polymer light-emitting diodes [PLED] characterised by the electroluminescent [EL] layers comprising dopants
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/615—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene
- H10K85/623—Polycyclic condensed aromatic hydrocarbons, e.g. anthracene containing five rings, e.g. pentacene
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/631—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine
- H10K85/633—Amine compounds having at least two aryl rest on at least one amine-nitrogen atom, e.g. triphenylamine comprising polycyclic condensed aromatic hydrocarbons as substituents on the nitrogen atom
-
- H—ELECTRICITY
- H10—SEMICONDUCTOR DEVICES; ELECTRIC SOLID-STATE DEVICES NOT OTHERWISE PROVIDED FOR
- H10K—ORGANIC ELECTRIC SOLID-STATE DEVICES
- H10K85/00—Organic materials used in the body or electrodes of devices covered by this subclass
- H10K85/60—Organic compounds having low molecular weight
- H10K85/649—Aromatic compounds comprising a hetero atom
- H10K85/657—Polycyclic condensed heteroaromatic hydrocarbons
- H10K85/6572—Polycyclic condensed heteroaromatic hydrocarbons comprising only nitrogen in the heteroaromatic polycondensed ring system, e.g. phenanthroline or carbazole
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1007—Non-condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1011—Condensed systems
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1003—Carbocyclic compounds
- C09K2211/1014—Carbocyclic compounds bridged by heteroatoms, e.g. N, P, Si or B
-
- C—CHEMISTRY; METALLURGY
- C09—DYES; PAINTS; POLISHES; NATURAL RESINS; ADHESIVES; COMPOSITIONS NOT OTHERWISE PROVIDED FOR; APPLICATIONS OF MATERIALS NOT OTHERWISE PROVIDED FOR
- C09K—MATERIALS FOR MISCELLANEOUS APPLICATIONS, NOT PROVIDED FOR ELSEWHERE
- C09K2211/00—Chemical nature of organic luminescent or tenebrescent compounds
- C09K2211/10—Non-macromolecular compounds
- C09K2211/1018—Heterocyclic compounds
- C09K2211/1025—Heterocyclic compounds characterised by ligands
- C09K2211/1029—Heterocyclic compounds characterised by ligands containing one nitrogen atom as the heteroatom
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/917—Electroluminescent
Landscapes
- Chemical & Material Sciences (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Materials Engineering (AREA)
- Physics & Mathematics (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Optics & Photonics (AREA)
- Electroluminescent Light Sources (AREA)
- Other In-Based Heterocyclic Compounds (AREA)
Priority Applications (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2006349579A JP5064787B2 (ja) | 2006-12-26 | 2006-12-26 | ベンゾフルオランテン化合物及びこれを使用した有機発光素子 |
| US12/296,058 US8034944B2 (en) | 2006-12-26 | 2007-12-12 | Benzofluoranthene compound and organic light-emitting device using the compound |
| KR1020097015427A KR101115156B1 (ko) | 2006-12-26 | 2007-12-20 | 벤조플루오란텐 화합물 및 이 화합물을 사용한 유기 발광소자 |
| CN2007800195506A CN101454288B (zh) | 2006-12-26 | 2007-12-20 | 苯并荧蒽化合物和使用该化合物的有机发光器件 |
| PCT/JP2007/075231 WO2008078824A1 (en) | 2006-12-26 | 2007-12-20 | Benzofluoranthene compound and organic light-emitting device using the compound |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2006349579A JP5064787B2 (ja) | 2006-12-26 | 2006-12-26 | ベンゾフルオランテン化合物及びこれを使用した有機発光素子 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008156315A JP2008156315A (ja) | 2008-07-10 |
| JP2008156315A5 JP2008156315A5 (enExample) | 2011-04-28 |
| JP5064787B2 true JP5064787B2 (ja) | 2012-10-31 |
Family
ID=39562614
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2006349579A Expired - Fee Related JP5064787B2 (ja) | 2006-12-26 | 2006-12-26 | ベンゾフルオランテン化合物及びこれを使用した有機発光素子 |
Country Status (5)
| Country | Link |
|---|---|
| US (1) | US8034944B2 (enExample) |
| JP (1) | JP5064787B2 (enExample) |
| KR (1) | KR101115156B1 (enExample) |
| CN (1) | CN101454288B (enExample) |
| WO (1) | WO2008078824A1 (enExample) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP5074754B2 (ja) | 2006-03-31 | 2012-11-14 | キヤノン株式会社 | 新規化合物及びそれを用いた有機発光素子 |
| JP5317414B2 (ja) | 2006-04-20 | 2013-10-16 | キヤノン株式会社 | ジベンゾアントラセン化合物およびそれを有する有機発光素子 |
| JP5142589B2 (ja) * | 2007-05-28 | 2013-02-13 | キヤノン株式会社 | インデノクリセン誘導体及びそれを用いた有機発光素子 |
| JP5335284B2 (ja) * | 2008-05-22 | 2013-11-06 | キヤノン株式会社 | 縮合多環化合物およびそれを有する有機発光素子 |
| JP2010121036A (ja) * | 2008-11-19 | 2010-06-03 | Canon Inc | 発光素子及び画像表示装置および新規有機化合物 |
| US8922109B2 (en) * | 2010-01-29 | 2014-12-30 | Sumitomo Chemical Company, Limited | Light emitting material, ink composition, thin film, light emitting device and method for manufacturing light emitting device |
| WO2012046839A1 (ja) * | 2010-10-08 | 2012-04-12 | 出光興産株式会社 | ベンゾ[k]フルオランテン誘導体及びそれを含んでなる有機エレクトロルミネッセンス素子 |
| JP5777408B2 (ja) | 2011-05-30 | 2015-09-09 | キヤノン株式会社 | 縮合多環化合物及びこれを用いた有機発光素子 |
| JP6269060B2 (ja) * | 2012-06-12 | 2018-01-31 | 東レ株式会社 | 発光素子材料および発光素子 |
| KR20150026114A (ko) * | 2013-08-30 | 2015-03-11 | 삼성디스플레이 주식회사 | 인데노피리딘계 화합물 및 이를 포함한 유기 발광 소자 |
| JP6298608B2 (ja) * | 2013-10-03 | 2018-03-20 | 出光興産株式会社 | フルオランテン誘導体、有機エレクトロルミネッセンス素子及び電子機器 |
| US10629821B2 (en) | 2015-04-08 | 2020-04-21 | Idemitsu Kosan Co., Ltd. | Compound, material for organic electroluminescent elements using same, and organic electroluminescent element and electronic device each using same |
| KR102424977B1 (ko) | 2015-04-14 | 2022-07-26 | 삼성디스플레이 주식회사 | 축합환 화합물 및 이를 포함한 유기 발광 소자 |
| WO2016204150A1 (ja) * | 2015-06-16 | 2016-12-22 | 出光興産株式会社 | 化合物、有機エレクトロルミネッセンス素子用材料、有機エレクトロルミネッセンス素子及び電子機器 |
| US20180226601A1 (en) * | 2015-09-11 | 2018-08-09 | Idemitsu Kosan Co., Ltd. | Organic electroluminescent element, illumination device, display device, and mixed material |
| CN111205234A (zh) * | 2015-09-30 | 2020-05-29 | 北京鼎材科技有限公司 | 一种喹喔啉基团的稠环芳烃衍生物及其应用 |
| KR102630644B1 (ko) | 2015-12-17 | 2024-01-30 | 삼성디스플레이 주식회사 | 유기 발광 소자 |
| JP7630932B2 (ja) * | 2019-11-12 | 2025-02-18 | キヤノン株式会社 | 有機化合物及び有機発光素子 |
| KR102136791B1 (ko) * | 2020-04-14 | 2020-07-23 | 삼성디스플레이 주식회사 | 인데노피리딘계 화합물 및 이를 포함한 유기 발광 소자 |
| CN114621752B (zh) * | 2021-12-03 | 2022-10-28 | 昆明理工大学 | 一种室温磷光水性聚合物防伪材料及其制备方法和应用 |
| CN114456167A (zh) * | 2022-02-14 | 2022-05-10 | 上海八亿时空先进材料有限公司 | 一种氮杂荧蒽衍生物及其应用 |
| CN114456033B (zh) * | 2022-02-16 | 2023-11-24 | 复旦大学 | 一种荧蒽衍生物的制备方法 |
Family Cites Families (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP3853042B2 (ja) | 1996-11-07 | 2006-12-06 | 三井化学株式会社 | 有機電界発光素子 |
| JP3807018B2 (ja) | 1997-04-21 | 2006-08-09 | 三菱化学株式会社 | 有機電界発光素子及び蛍光材料 |
| JP2000311786A (ja) | 1999-04-27 | 2000-11-07 | Toyota Central Res & Dev Lab Inc | 有機電界発光素子 |
| JP2001160489A (ja) | 1999-12-01 | 2001-06-12 | Toyota Central Res & Dev Lab Inc | 有機電界発光素子 |
| JP4562884B2 (ja) * | 2000-08-25 | 2010-10-13 | 出光興産株式会社 | 有機エレクトロルミネッセンス素子 |
| JP4224252B2 (ja) | 2001-04-19 | 2009-02-12 | Tdk株式会社 | 有機el素子用化合物、有機el素子 |
| DE60233080D1 (de) | 2001-04-19 | 2009-09-03 | Tdk Corp | Fluoranthen-Derivate zur Verwendung in organischen Elektrolumineszenz-Vorrichtungen |
| JP4322457B2 (ja) | 2002-01-22 | 2009-09-02 | 財団法人石油産業活性化センター | アミノ基を有する新規アザ芳香族化合物及びそれを利用した有機エレクトロルミネッセンス素子 |
| JP4059822B2 (ja) * | 2003-08-26 | 2008-03-12 | 三井化学株式会社 | ベンゾフルオランテン化合物、および該ベンゾフルオランテン化合物を含有する有機電界発光素子 |
| JP2005068367A (ja) | 2003-08-27 | 2005-03-17 | Toyo Ink Mfg Co Ltd | 有機エレクトロルミネッセンス素子用材料および有機エレクトロルミネッセンス素子 |
| JP4616587B2 (ja) | 2004-07-05 | 2011-01-19 | ケミプロ化成株式会社 | 1,8−ナフチリジン誘導体を少なくとも一種含む層を、一対の電極間に有する有機電界発光素子、その製法および該有機電界発電素子を利用した部品 |
| JP5089947B2 (ja) * | 2005-09-23 | 2012-12-05 | 三星モバイルディスプレイ株式會社 | 有機発光化合物及びこれを備えた有機発光素子 |
| JP2007314511A (ja) | 2006-04-24 | 2007-12-06 | Canon Inc | 化合物および有機発光素子 |
| JP4164514B2 (ja) | 2006-04-28 | 2008-10-15 | キヤノン株式会社 | 有機化合物および有機発光素子 |
| JP4227628B2 (ja) | 2006-04-25 | 2009-02-18 | キヤノン株式会社 | 化合物および有機発光素子 |
| JP5089235B2 (ja) | 2006-08-04 | 2012-12-05 | キヤノン株式会社 | 縮合複素環化合物および有機発光素子 |
-
2006
- 2006-12-26 JP JP2006349579A patent/JP5064787B2/ja not_active Expired - Fee Related
-
2007
- 2007-12-12 US US12/296,058 patent/US8034944B2/en not_active Expired - Fee Related
- 2007-12-20 CN CN2007800195506A patent/CN101454288B/zh not_active Expired - Fee Related
- 2007-12-20 WO PCT/JP2007/075231 patent/WO2008078824A1/en not_active Ceased
- 2007-12-20 KR KR1020097015427A patent/KR101115156B1/ko not_active Expired - Fee Related
Also Published As
| Publication number | Publication date |
|---|---|
| CN101454288A (zh) | 2009-06-10 |
| JP2008156315A (ja) | 2008-07-10 |
| CN101454288B (zh) | 2012-12-12 |
| US8034944B2 (en) | 2011-10-11 |
| KR101115156B1 (ko) | 2012-03-14 |
| KR20090094159A (ko) | 2009-09-03 |
| US20090278118A1 (en) | 2009-11-12 |
| WO2008078824A1 (en) | 2008-07-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| KR101115156B1 (ko) | 벤조플루오란텐 화합물 및 이 화합물을 사용한 유기 발광소자 | |
| JP5089235B2 (ja) | 縮合複素環化合物および有機発光素子 | |
| KR101124749B1 (ko) | 인데노크리센 유도체 및 그것을 이용한 유기 발광소자 | |
| US8765270B2 (en) | Triazine compound and organic light emitting device using the same | |
| JP5366505B2 (ja) | インデノピレン化合物及びこれを用いた有機発光素子 | |
| JP5159164B2 (ja) | ベンゾ[ghi]フルオランテン誘導体及びこれを用いた有機発光素子 | |
| JP5202051B2 (ja) | 有機電界発光素子 | |
| JP2006151844A (ja) | アミノアントリル誘導基置換化合物および有機発光素子 | |
| CN101548408A (zh) | 有机电致发光元件用材料及有机电致发光元件 | |
| JP2007230951A (ja) | シリル化合物、発光材料およびそれを用いた有機発光素子 | |
| US8173275B2 (en) | Azaindenochrysene derivative and organic light-emitting device | |
| WO2011040631A1 (en) | Diindenopicene compound and organic light emitting device using the same | |
| JP2010263065A (ja) | 有機発光素子 | |
| JP4583470B2 (ja) | 芳香族アミン化合物を用いた有機電界発光素子 | |
| JP2006032638A (ja) | 発光素子 | |
| TWI706943B (zh) | 胺基氧芴化合物及其有機電激發光元件 | |
| JP3968978B2 (ja) | 有機電界発光素子 | |
| JP2004161892A (ja) | 新規化合物およびこれを用いた有機電界発光素子 | |
| JP2004155665A (ja) | 新規化合物およびこれを用いた有機電界発光素子 | |
| CN112745285A (zh) | 胺基氧芴化合物及其有机电致发光器件 | |
| JP2004043581A (ja) | 化合物およびこれを用いた有機電界発光素子 | |
| JP2007297303A (ja) | フルオレン化合物及び有機発光素子 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20091218 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110315 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20120807 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20120809 |
|
| R151 | Written notification of patent or utility model registration |
Ref document number: 5064787 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R151 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20150817 Year of fee payment: 3 |
|
| LAPS | Cancellation because of no payment of annual fees |