JP4827357B2 - ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 - Google Patents
ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 Download PDFInfo
- Publication number
- JP4827357B2 JP4827357B2 JP2001541031A JP2001541031A JP4827357B2 JP 4827357 B2 JP4827357 B2 JP 4827357B2 JP 2001541031 A JP2001541031 A JP 2001541031A JP 2001541031 A JP2001541031 A JP 2001541031A JP 4827357 B2 JP4827357 B2 JP 4827357B2
- Authority
- JP
- Japan
- Prior art keywords
- steap
- protein
- cells
- expression
- cancer
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
- 208000000236 Prostatic Neoplasms Diseases 0.000 title claims abstract description 147
- 206010060862 Prostate cancer Diseases 0.000 title claims abstract description 145
- 239000000427 antigen Substances 0.000 title claims abstract description 27
- 108091007433 antigens Proteins 0.000 title claims abstract description 27
- 102000036639 antigens Human genes 0.000 title claims abstract description 27
- 238000000034 method Methods 0.000 title claims description 113
- 210000004027 cell Anatomy 0.000 claims abstract description 320
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 282
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 188
- 206010028980 Neoplasm Diseases 0.000 claims abstract description 184
- 150000001413 amino acids Chemical class 0.000 claims abstract description 136
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 136
- 201000011510 cancer Diseases 0.000 claims description 98
- 239000002299 complementary DNA Substances 0.000 claims description 97
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 95
- 102000040430 polynucleotide Human genes 0.000 claims description 89
- 108091033319 polynucleotide Proteins 0.000 claims description 89
- 239000002157 polynucleotide Substances 0.000 claims description 89
- 125000003275 alpha amino acid group Chemical group 0.000 claims description 81
- 229920001184 polypeptide Polymers 0.000 claims description 72
- 108020004999 messenger RNA Proteins 0.000 claims description 71
- 239000000523 sample Substances 0.000 claims description 69
- 239000012634 fragment Substances 0.000 claims description 54
- 239000013598 vector Substances 0.000 claims description 50
- 230000027455 binding Effects 0.000 claims description 49
- 238000003556 assay Methods 0.000 claims description 45
- 241001465754 Metazoa Species 0.000 claims description 25
- 239000013612 plasmid Substances 0.000 claims description 23
- 239000012472 biological sample Substances 0.000 claims description 21
- 238000009396 hybridization Methods 0.000 claims description 21
- 230000000692 anti-sense effect Effects 0.000 claims description 20
- 230000002401 inhibitory effect Effects 0.000 claims description 19
- 230000000295 complement effect Effects 0.000 claims description 17
- 108091026890 Coding region Proteins 0.000 claims description 15
- 239000003814 drug Substances 0.000 claims description 15
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 claims description 14
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 claims description 14
- 238000004519 manufacturing process Methods 0.000 claims description 14
- 239000003550 marker Substances 0.000 claims description 12
- 239000013604 expression vector Substances 0.000 claims description 10
- 229940124597 therapeutic agent Drugs 0.000 claims description 10
- 230000009261 transgenic effect Effects 0.000 claims description 9
- 150000001875 compounds Chemical class 0.000 claims description 8
- 102000004190 Enzymes Human genes 0.000 claims description 7
- 108090000790 Enzymes Proteins 0.000 claims description 7
- 230000010261 cell growth Effects 0.000 claims description 7
- 239000003053 toxin Substances 0.000 claims description 5
- 231100000765 toxin Toxicity 0.000 claims description 5
- 108700012359 toxins Proteins 0.000 claims description 5
- IAKHMKGGTNLKSZ-INIZCTEOSA-N (S)-colchicine Chemical compound C1([C@@H](NC(C)=O)CC2)=CC(=O)C(OC)=CC=C1C1=C2C=C(OC)C(OC)=C1OC IAKHMKGGTNLKSZ-INIZCTEOSA-N 0.000 claims description 4
- VPFUWHKTPYPNGT-UHFFFAOYSA-N 3-(3,4-dihydroxyphenyl)-1-(5-hydroxy-2,2-dimethylchromen-6-yl)propan-1-one Chemical compound OC1=C2C=CC(C)(C)OC2=CC=C1C(=O)CCC1=CC=C(O)C(O)=C1 VPFUWHKTPYPNGT-UHFFFAOYSA-N 0.000 claims description 4
- 108010066676 Abrin Proteins 0.000 claims description 4
- AOJJSUZBOXZQNB-TZSSRYMLSA-N Doxorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(=O)CO)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 AOJJSUZBOXZQNB-TZSSRYMLSA-N 0.000 claims description 4
- 108010039491 Ricin Proteins 0.000 claims description 4
- ZMMJGEGLRURXTF-UHFFFAOYSA-N ethidium bromide Chemical compound [Br-].C12=CC(N)=CC=C2C2=CC=C(N)C=C2[N+](CC)=C1C1=CC=CC=C1 ZMMJGEGLRURXTF-UHFFFAOYSA-N 0.000 claims description 4
- 229960005542 ethidium bromide Drugs 0.000 claims description 4
- 210000004408 hybridoma Anatomy 0.000 claims description 4
- 229930012538 Paclitaxel Natural products 0.000 claims description 3
- 239000002738 chelating agent Substances 0.000 claims description 3
- 239000007850 fluorescent dye Substances 0.000 claims description 3
- 239000003112 inhibitor Substances 0.000 claims description 3
- 229910052751 metal Inorganic materials 0.000 claims description 3
- 239000002184 metal Substances 0.000 claims description 3
- 229960001592 paclitaxel Drugs 0.000 claims description 3
- 230000008569 process Effects 0.000 claims description 3
- 238000003259 recombinant expression Methods 0.000 claims description 3
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 claims description 3
- FBUTXZSKZCQABC-UHFFFAOYSA-N 2-amino-1-methyl-7h-purine-6-thione Chemical compound S=C1N(C)C(N)=NC2=C1NC=N2 FBUTXZSKZCQABC-UHFFFAOYSA-N 0.000 claims description 2
- STQGQHZAVUOBTE-UHFFFAOYSA-N 7-Cyan-hept-2t-en-4,6-diinsaeure Natural products C1=2C(O)=C3C(=O)C=4C(OC)=CC=CC=4C(=O)C3=C(O)C=2CC(O)(C(C)=O)CC1OC1CC(N)C(O)C(C)O1 STQGQHZAVUOBTE-UHFFFAOYSA-N 0.000 claims description 2
- 108700032819 Croton tiglium crotin II Proteins 0.000 claims description 2
- 108010092160 Dactinomycin Proteins 0.000 claims description 2
- 102000016607 Diphtheria Toxin Human genes 0.000 claims description 2
- 108010053187 Diphtheria Toxin Proteins 0.000 claims description 2
- 108700004714 Gelonium multiflorum GEL Proteins 0.000 claims description 2
- 229930192392 Mitomycin Natural products 0.000 claims description 2
- NWIBSHFKIJFRCO-WUDYKRTCSA-N Mytomycin Chemical compound C1N2C(C(C(C)=C(N)C3=O)=O)=C3[C@@H](COC(N)=O)[C@@]2(OC)[C@@H]2[C@H]1N2 NWIBSHFKIJFRCO-WUDYKRTCSA-N 0.000 claims description 2
- JXLYSJRDGCGARV-WWYNWVTFSA-N Vinblastine Natural products O=C(O[C@H]1[C@](O)(C(=O)OC)[C@@H]2N(C)c3c(cc(c(OC)c3)[C@]3(C(=O)OC)c4[nH]c5c(c4CCN4C[C@](O)(CC)C[C@H](C3)C4)cccc5)[C@@]32[C@H]2[C@@]1(CC)C=CCN2CC3)C JXLYSJRDGCGARV-WWYNWVTFSA-N 0.000 claims description 2
- 229930183665 actinomycin Natural products 0.000 claims description 2
- 108010001818 alpha-sarcin Proteins 0.000 claims description 2
- 229930195731 calicheamicin Natural products 0.000 claims description 2
- HXCHCVDVKSCDHU-LULTVBGHSA-N calicheamicin Chemical compound C1[C@H](OC)[C@@H](NCC)CO[C@H]1O[C@H]1[C@H](O[C@@H]2C\3=C(NC(=O)OC)C(=O)C[C@](C/3=C/CSSSC)(O)C#C\C=C/C#C2)O[C@H](C)[C@@H](NO[C@@H]2O[C@H](C)[C@@H](SC(=O)C=3C(=C(OC)C(O[C@H]4[C@@H]([C@H](OC)[C@@H](O)[C@H](C)O4)O)=C(I)C=3C)OC)[C@@H](O)C2)[C@@H]1O HXCHCVDVKSCDHU-LULTVBGHSA-N 0.000 claims description 2
- 229960001338 colchicine Drugs 0.000 claims description 2
- STQGQHZAVUOBTE-VGBVRHCVSA-N daunorubicin Chemical compound O([C@H]1C[C@@](O)(CC=2C(O)=C3C(=O)C=4C=CC=C(C=4C(=O)C3=C(O)C=21)OC)C(C)=O)[C@H]1C[C@H](N)[C@H](O)[C@H](C)O1 STQGQHZAVUOBTE-VGBVRHCVSA-N 0.000 claims description 2
- 229960000975 daunorubicin Drugs 0.000 claims description 2
- 229960004679 doxorubicin Drugs 0.000 claims description 2
- 108010028531 enomycin Proteins 0.000 claims description 2
- VJJPUSNTGOMMGY-MRVIYFEKSA-N etoposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@H](C)OC[C@H]4O3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 VJJPUSNTGOMMGY-MRVIYFEKSA-N 0.000 claims description 2
- 229960005420 etoposide Drugs 0.000 claims description 2
- 239000003862 glucocorticoid Substances 0.000 claims description 2
- 229960004857 mitomycin Drugs 0.000 claims description 2
- 108010076042 phenomycin Proteins 0.000 claims description 2
- NRUKOCRGYNPUPR-QBPJDGROSA-N teniposide Chemical compound COC1=C(O)C(OC)=CC([C@@H]2C3=CC=4OCOC=4C=C3[C@@H](O[C@H]3[C@@H]([C@@H](O)[C@@H]4O[C@@H](OC[C@H]4O3)C=3SC=CC=3)O)[C@@H]3[C@@H]2C(OC3)=O)=C1 NRUKOCRGYNPUPR-QBPJDGROSA-N 0.000 claims description 2
- 229960001278 teniposide Drugs 0.000 claims description 2
- 229960003048 vinblastine Drugs 0.000 claims description 2
- JXLYSJRDGCGARV-XQKSVPLYSA-N vincaleukoblastine Chemical compound C([C@@H](C[C@]1(C(=O)OC)C=2C(=CC3=C([C@]45[C@H]([C@@]([C@H](OC(C)=O)[C@]6(CC)C=CCN([C@H]56)CC4)(O)C(=O)OC)N3C)C=2)OC)C[C@@](C2)(O)CC)N2CCC2=C1NC1=CC=CC=C21 JXLYSJRDGCGARV-XQKSVPLYSA-N 0.000 claims description 2
- 229960004528 vincristine Drugs 0.000 claims description 2
- OGWKCGZFUXNPDA-XQKSVPLYSA-N vincristine Chemical compound C([N@]1C[C@@H](C[C@]2(C(=O)OC)C=3C(=CC4=C([C@]56[C@H]([C@@]([C@H](OC(C)=O)[C@]7(CC)C=CCN([C@H]67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)C[C@@](C1)(O)CC)CC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-XQKSVPLYSA-N 0.000 claims description 2
- OGWKCGZFUXNPDA-UHFFFAOYSA-N vincristine Natural products C1C(CC)(O)CC(CC2(C(=O)OC)C=3C(=CC4=C(C56C(C(C(OC(C)=O)C7(CC)C=CCN(C67)CC5)(O)C(=O)OC)N4C=O)C=3)OC)CN1CCC1=C2NC2=CC=CC=C12 OGWKCGZFUXNPDA-UHFFFAOYSA-N 0.000 claims description 2
- 101000762949 Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1) Exotoxin A Proteins 0.000 claims 2
- 238000012258 culturing Methods 0.000 claims 1
- 238000010839 reverse transcription Methods 0.000 claims 1
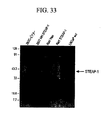
- 101000628547 Homo sapiens Metalloreductase STEAP1 Proteins 0.000 abstract description 661
- 102100026712 Metalloreductase STEAP1 Human genes 0.000 abstract description 583
- 230000014509 gene expression Effects 0.000 abstract description 223
- 210000001519 tissue Anatomy 0.000 abstract description 125
- 210000002307 prostate Anatomy 0.000 abstract description 102
- 230000003834 intracellular effect Effects 0.000 abstract description 20
- 102000054725 human STEAP1 Human genes 0.000 abstract description 19
- 102000042326 STEAP family Human genes 0.000 abstract description 18
- 108091077778 STEAP family Proteins 0.000 abstract description 18
- 210000005267 prostate cell Anatomy 0.000 abstract description 9
- 102000018697 Membrane Proteins Human genes 0.000 abstract description 8
- 108010052285 Membrane Proteins Proteins 0.000 abstract description 8
- WYTGDNHDOZPMIW-RCBQFDQVSA-N alstonine Natural products C1=CC2=C3C=CC=CC3=NC2=C2N1C[C@H]1[C@H](C)OC=C(C(=O)OC)[C@H]1C2 WYTGDNHDOZPMIW-RCBQFDQVSA-N 0.000 abstract description 5
- 102000003839 Human Proteins Human genes 0.000 abstract description 4
- 108090000144 Human Proteins Proteins 0.000 abstract description 4
- 235000018102 proteins Nutrition 0.000 description 158
- 235000001014 amino acid Nutrition 0.000 description 141
- 229940024606 amino acid Drugs 0.000 description 137
- 230000001225 therapeutic effect Effects 0.000 description 46
- 108020004414 DNA Proteins 0.000 description 41
- 238000004458 analytical method Methods 0.000 description 41
- 239000000203 mixture Substances 0.000 description 41
- 239000002773 nucleotide Substances 0.000 description 37
- 125000003729 nucleotide group Chemical group 0.000 description 37
- 238000011282 treatment Methods 0.000 description 34
- 239000000047 product Substances 0.000 description 32
- 238000013459 approach Methods 0.000 description 30
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 29
- 230000006870 function Effects 0.000 description 29
- 241000699666 Mus <mouse, genus> Species 0.000 description 24
- 230000004913 activation Effects 0.000 description 24
- 201000010099 disease Diseases 0.000 description 24
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 24
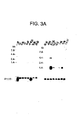
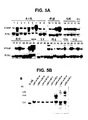
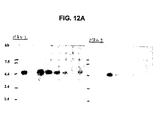
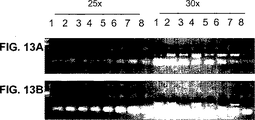
- 238000001262 western blot Methods 0.000 description 24
- 206010027476 Metastases Diseases 0.000 description 23
- 206010009944 Colon cancer Diseases 0.000 description 22
- 238000000636 Northern blotting Methods 0.000 description 22
- 125000000539 amino acid group Chemical group 0.000 description 22
- 210000004962 mammalian cell Anatomy 0.000 description 22
- 230000000694 effects Effects 0.000 description 21
- 230000002163 immunogen Effects 0.000 description 21
- 241000588724 Escherichia coli Species 0.000 description 20
- 241000699670 Mus sp. Species 0.000 description 20
- 108700026244 Open Reading Frames Proteins 0.000 description 20
- 208000029742 colonic neoplasm Diseases 0.000 description 20
- 150000007523 nucleic acids Chemical group 0.000 description 20
- 206010005003 Bladder cancer Diseases 0.000 description 19
- 208000007097 Urinary Bladder Neoplasms Diseases 0.000 description 19
- 230000026731 phosphorylation Effects 0.000 description 19
- 238000006366 phosphorylation reaction Methods 0.000 description 19
- 238000001514 detection method Methods 0.000 description 18
- 102000037865 fusion proteins Human genes 0.000 description 18
- 108020001507 fusion proteins Proteins 0.000 description 18
- 102000039446 nucleic acids Human genes 0.000 description 18
- 108020004707 nucleic acids Proteins 0.000 description 18
- 238000003757 reverse transcription PCR Methods 0.000 description 18
- 201000005112 urinary bladder cancer Diseases 0.000 description 18
- 238000006467 substitution reaction Methods 0.000 description 17
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 16
- 239000003098 androgen Substances 0.000 description 16
- 230000009401 metastasis Effects 0.000 description 16
- 230000004048 modification Effects 0.000 description 16
- 238000012986 modification Methods 0.000 description 16
- 238000010186 staining Methods 0.000 description 16
- 230000035897 transcription Effects 0.000 description 16
- 238000013518 transcription Methods 0.000 description 16
- 102000005720 Glutathione transferase Human genes 0.000 description 15
- 108010070675 Glutathione transferase Proteins 0.000 description 15
- 102000025850 HLA-A2 Antigen Human genes 0.000 description 15
- 108010074032 HLA-A2 Antigen Proteins 0.000 description 15
- 238000010367 cloning Methods 0.000 description 15
- 230000004927 fusion Effects 0.000 description 15
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 15
- 108091003079 Bovine Serum Albumin Proteins 0.000 description 14
- 108091034117 Oligonucleotide Proteins 0.000 description 14
- 238000012360 testing method Methods 0.000 description 14
- 108091060211 Expressed sequence tag Proteins 0.000 description 13
- 102100024193 Mitogen-activated protein kinase 1 Human genes 0.000 description 13
- 238000006243 chemical reaction Methods 0.000 description 13
- 239000003153 chemical reaction reagent Substances 0.000 description 13
- 239000003446 ligand Substances 0.000 description 13
- 210000004072 lung Anatomy 0.000 description 13
- 230000001404 mediated effect Effects 0.000 description 13
- 230000037361 pathway Effects 0.000 description 13
- 102000007469 Actins Human genes 0.000 description 12
- 108010085238 Actins Proteins 0.000 description 12
- ZHNUHDYFZUAESO-UHFFFAOYSA-N Formamide Chemical compound NC=O ZHNUHDYFZUAESO-UHFFFAOYSA-N 0.000 description 12
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 12
- 229940022399 cancer vaccine Drugs 0.000 description 12
- 238000009566 cancer vaccine Methods 0.000 description 12
- 210000001072 colon Anatomy 0.000 description 12
- 239000012091 fetal bovine serum Substances 0.000 description 12
- 238000002991 immunohistochemical analysis Methods 0.000 description 12
- 201000005202 lung cancer Diseases 0.000 description 12
- 208000020816 lung neoplasm Diseases 0.000 description 12
- 230000001105 regulatory effect Effects 0.000 description 12
- 230000019491 signal transduction Effects 0.000 description 12
- 239000000126 substance Substances 0.000 description 12
- 210000003932 urinary bladder Anatomy 0.000 description 12
- 229960000723 ampicillin Drugs 0.000 description 11
- AVKUERGKIZMTKX-NJBDSQKTSA-N ampicillin Chemical compound C1([C@@H](N)C(=O)N[C@H]2[C@H]3SC([C@@H](N3C2=O)C(O)=O)(C)C)=CC=CC=C1 AVKUERGKIZMTKX-NJBDSQKTSA-N 0.000 description 11
- 230000003321 amplification Effects 0.000 description 11
- 210000000349 chromosome Anatomy 0.000 description 11
- 230000003053 immunization Effects 0.000 description 11
- 238000000338 in vitro Methods 0.000 description 11
- 238000012423 maintenance Methods 0.000 description 11
- 239000012528 membrane Substances 0.000 description 11
- 238000003199 nucleic acid amplification method Methods 0.000 description 11
- 238000002560 therapeutic procedure Methods 0.000 description 11
- 241000701022 Cytomegalovirus Species 0.000 description 10
- 241001494479 Pecora Species 0.000 description 10
- 238000002474 experimental method Methods 0.000 description 10
- 238000002649 immunization Methods 0.000 description 10
- 230000005764 inhibitory process Effects 0.000 description 10
- 150000003384 small molecules Chemical class 0.000 description 10
- 210000004881 tumor cell Anatomy 0.000 description 10
- 206010004446 Benign prostatic hyperplasia Diseases 0.000 description 9
- 102000053642 Catalytic RNA Human genes 0.000 description 9
- 108090000994 Catalytic RNA Proteins 0.000 description 9
- 238000002965 ELISA Methods 0.000 description 9
- 206010061535 Ovarian neoplasm Diseases 0.000 description 9
- 208000004403 Prostatic Hyperplasia Diseases 0.000 description 9
- 230000015572 biosynthetic process Effects 0.000 description 9
- 210000004443 dendritic cell Anatomy 0.000 description 9
- 230000001419 dependent effect Effects 0.000 description 9
- 235000014304 histidine Nutrition 0.000 description 9
- 238000002955 isolation Methods 0.000 description 9
- 210000004185 liver Anatomy 0.000 description 9
- 102000002574 p38 Mitogen-Activated Protein Kinases Human genes 0.000 description 9
- 108010068338 p38 Mitogen-Activated Protein Kinases Proteins 0.000 description 9
- 108091092562 ribozyme Proteins 0.000 description 9
- 230000014616 translation Effects 0.000 description 9
- 108020004705 Codon Proteins 0.000 description 8
- 102000043136 MAP kinase family Human genes 0.000 description 8
- 108091054455 MAP kinase family Proteins 0.000 description 8
- 206010033128 Ovarian cancer Diseases 0.000 description 8
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 8
- 238000011579 SCID mouse model Methods 0.000 description 8
- 235000004279 alanine Nutrition 0.000 description 8
- 108010006025 bovine growth hormone Proteins 0.000 description 8
- 210000004556 brain Anatomy 0.000 description 8
- 230000001413 cellular effect Effects 0.000 description 8
- 238000002512 chemotherapy Methods 0.000 description 8
- 230000034994 death Effects 0.000 description 8
- 231100000517 death Toxicity 0.000 description 8
- 238000012217 deletion Methods 0.000 description 8
- 230000037430 deletion Effects 0.000 description 8
- 238000011161 development Methods 0.000 description 8
- 230000018109 developmental process Effects 0.000 description 8
- 238000010195 expression analysis Methods 0.000 description 8
- 238000003384 imaging method Methods 0.000 description 8
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 8
- 208000010658 metastatic prostate carcinoma Diseases 0.000 description 8
- -1 nucleoside phosphorothioate Chemical class 0.000 description 8
- 201000002528 pancreatic cancer Diseases 0.000 description 8
- 208000008443 pancreatic carcinoma Diseases 0.000 description 8
- 230000035755 proliferation Effects 0.000 description 8
- 102000005962 receptors Human genes 0.000 description 8
- 108020003175 receptors Proteins 0.000 description 8
- 238000012216 screening Methods 0.000 description 8
- 210000002966 serum Anatomy 0.000 description 8
- 239000011780 sodium chloride Substances 0.000 description 8
- 238000013519 translation Methods 0.000 description 8
- 108060003951 Immunoglobulin Proteins 0.000 description 7
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 7
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 7
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 7
- 238000010240 RT-PCR analysis Methods 0.000 description 7
- 108020004511 Recombinant DNA Proteins 0.000 description 7
- 108010090804 Streptavidin Proteins 0.000 description 7
- 239000002671 adjuvant Substances 0.000 description 7
- 238000009175 antibody therapy Methods 0.000 description 7
- 230000006907 apoptotic process Effects 0.000 description 7
- 238000001574 biopsy Methods 0.000 description 7
- 210000004899 c-terminal region Anatomy 0.000 description 7
- 210000000170 cell membrane Anatomy 0.000 description 7
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 7
- 239000003205 fragrance Substances 0.000 description 7
- HNDVDQJCIGZPNO-UHFFFAOYSA-N histidine Natural products OC(=O)C(N)CC1=CN=CN1 HNDVDQJCIGZPNO-UHFFFAOYSA-N 0.000 description 7
- 102000018358 immunoglobulin Human genes 0.000 description 7
- 238000003780 insertion Methods 0.000 description 7
- 230000037431 insertion Effects 0.000 description 7
- 230000003993 interaction Effects 0.000 description 7
- 229960004452 methionine Drugs 0.000 description 7
- 239000013642 negative control Substances 0.000 description 7
- 230000002018 overexpression Effects 0.000 description 7
- 210000000496 pancreas Anatomy 0.000 description 7
- 238000002360 preparation method Methods 0.000 description 7
- 238000002864 sequence alignment Methods 0.000 description 7
- 239000000243 solution Substances 0.000 description 7
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 7
- 241001430294 unidentified retrovirus Species 0.000 description 7
- 238000005406 washing Methods 0.000 description 7
- WVDDGKGOMKODPV-UHFFFAOYSA-N Benzyl alcohol Chemical compound OCC1=CC=CC=C1 WVDDGKGOMKODPV-UHFFFAOYSA-N 0.000 description 6
- 208000006168 Ewing Sarcoma Diseases 0.000 description 6
- 102100041003 Glutamate carboxypeptidase 2 Human genes 0.000 description 6
- 108090000862 Ion Channels Proteins 0.000 description 6
- 102000004310 Ion Channels Human genes 0.000 description 6
- ROHFNLRQFUQHCH-UHFFFAOYSA-N Leucine Natural products CC(C)CC(N)C(O)=O ROHFNLRQFUQHCH-UHFFFAOYSA-N 0.000 description 6
- 108700019146 Transgenes Proteins 0.000 description 6
- JLCPHMBAVCMARE-UHFFFAOYSA-N [3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[3-[[3-[[3-[[3-[[3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-[[5-(2-amino-6-oxo-1H-purin-9-yl)-3-hydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(6-aminopurin-9-yl)oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-5-(4-amino-2-oxopyrimidin-1-yl)oxolan-2-yl]methyl [5-(6-aminopurin-9-yl)-2-(hydroxymethyl)oxolan-3-yl] hydrogen phosphate Polymers Cc1cn(C2CC(OP(O)(=O)OCC3OC(CC3OP(O)(=O)OCC3OC(CC3O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c3nc(N)[nH]c4=O)C(COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3COP(O)(=O)OC3CC(OC3CO)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3ccc(N)nc3=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cc(C)c(=O)[nH]c3=O)n3cc(C)c(=O)[nH]c3=O)n3ccc(N)nc3=O)n3cc(C)c(=O)[nH]c3=O)n3cnc4c3nc(N)[nH]c4=O)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)n3cnc4c(N)ncnc34)O2)c(=O)[nH]c1=O JLCPHMBAVCMARE-UHFFFAOYSA-N 0.000 description 6
- 239000002246 antineoplastic agent Substances 0.000 description 6
- 239000000074 antisense oligonucleotide Substances 0.000 description 6
- 238000012230 antisense oligonucleotides Methods 0.000 description 6
- 230000003385 bacteriostatic effect Effects 0.000 description 6
- 210000000988 bone and bone Anatomy 0.000 description 6
- 230000004663 cell proliferation Effects 0.000 description 6
- 229940127089 cytotoxic agent Drugs 0.000 description 6
- 238000005516 engineering process Methods 0.000 description 6
- 230000013595 glycosylation Effects 0.000 description 6
- 238000006206 glycosylation reaction Methods 0.000 description 6
- 238000000099 in vitro assay Methods 0.000 description 6
- 238000001727 in vivo Methods 0.000 description 6
- 230000000977 initiatory effect Effects 0.000 description 6
- 238000002347 injection Methods 0.000 description 6
- 239000007924 injection Substances 0.000 description 6
- 238000001990 intravenous administration Methods 0.000 description 6
- 108010045069 keyhole-limpet hemocyanin Proteins 0.000 description 6
- 210000003734 kidney Anatomy 0.000 description 6
- 229930182817 methionine Natural products 0.000 description 6
- 230000011987 methylation Effects 0.000 description 6
- 238000007069 methylation reaction Methods 0.000 description 6
- 239000013641 positive control Substances 0.000 description 6
- 208000023958 prostate neoplasm Diseases 0.000 description 6
- 210000000952 spleen Anatomy 0.000 description 6
- 229960005486 vaccine Drugs 0.000 description 6
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 5
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 5
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 5
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 5
- KZSNJWFQEVHDMF-BYPYZUCNSA-N L-valine Chemical compound CC(C)[C@H](N)C(O)=O KZSNJWFQEVHDMF-BYPYZUCNSA-N 0.000 description 5
- 208000003788 Neoplasm Micrometastasis Diseases 0.000 description 5
- 241000283973 Oryctolagus cuniculus Species 0.000 description 5
- 241000700159 Rattus Species 0.000 description 5
- DBMJMQXJHONAFJ-UHFFFAOYSA-M Sodium laurylsulphate Chemical compound [Na+].CCCCCCCCCCCCOS([O-])(=O)=O DBMJMQXJHONAFJ-UHFFFAOYSA-M 0.000 description 5
- 238000002105 Southern blotting Methods 0.000 description 5
- KZSNJWFQEVHDMF-UHFFFAOYSA-N Valine Natural products CC(C)C(N)C(O)=O KZSNJWFQEVHDMF-UHFFFAOYSA-N 0.000 description 5
- 239000002253 acid Substances 0.000 description 5
- 230000003302 anti-idiotype Effects 0.000 description 5
- 230000000259 anti-tumor effect Effects 0.000 description 5
- 230000001580 bacterial effect Effects 0.000 description 5
- 239000013592 cell lysate Substances 0.000 description 5
- 238000003776 cleavage reaction Methods 0.000 description 5
- 238000003745 diagnosis Methods 0.000 description 5
- 238000010586 diagram Methods 0.000 description 5
- 230000001605 fetal effect Effects 0.000 description 5
- 239000000499 gel Substances 0.000 description 5
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 5
- 239000005090 green fluorescent protein Substances 0.000 description 5
- 230000012010 growth Effects 0.000 description 5
- 230000028993 immune response Effects 0.000 description 5
- 238000001114 immunoprecipitation Methods 0.000 description 5
- 238000007901 in situ hybridization Methods 0.000 description 5
- 230000001965 increasing effect Effects 0.000 description 5
- 210000001165 lymph node Anatomy 0.000 description 5
- 230000036210 malignancy Effects 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 238000010369 molecular cloning Methods 0.000 description 5
- 238000012544 monitoring process Methods 0.000 description 5
- 238000010606 normalization Methods 0.000 description 5
- 210000001672 ovary Anatomy 0.000 description 5
- 230000036961 partial effect Effects 0.000 description 5
- 230000001575 pathological effect Effects 0.000 description 5
- 210000002826 placenta Anatomy 0.000 description 5
- 230000005855 radiation Effects 0.000 description 5
- 238000003753 real-time PCR Methods 0.000 description 5
- 230000007017 scission Effects 0.000 description 5
- 210000000813 small intestine Anatomy 0.000 description 5
- 210000001550 testis Anatomy 0.000 description 5
- 238000001890 transfection Methods 0.000 description 5
- 230000014621 translational initiation Effects 0.000 description 5
- 239000004474 valine Substances 0.000 description 5
- UXFSPRAGHGMRSQ-UHFFFAOYSA-N 3-isobutyl-2-methoxypyrazine Chemical compound COC1=NC=CN=C1CC(C)C UXFSPRAGHGMRSQ-UHFFFAOYSA-N 0.000 description 4
- 108020000948 Antisense Oligonucleotides Proteins 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Chemical compound NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 description 4
- 101000950669 Homo sapiens Mitogen-activated protein kinase 9 Proteins 0.000 description 4
- ROHFNLRQFUQHCH-YFKPBYRVSA-N L-leucine Chemical compound CC(C)C[C@H](N)C(O)=O ROHFNLRQFUQHCH-YFKPBYRVSA-N 0.000 description 4
- 101710175625 Maltose/maltodextrin-binding periplasmic protein Proteins 0.000 description 4
- 102100037809 Mitogen-activated protein kinase 9 Human genes 0.000 description 4
- 229930193140 Neomycin Natural products 0.000 description 4
- PXHVJJICTQNCMI-UHFFFAOYSA-N Nickel Chemical compound [Ni] PXHVJJICTQNCMI-UHFFFAOYSA-N 0.000 description 4
- 102100036735 Prostate stem cell antigen Human genes 0.000 description 4
- 101710120463 Prostate stem cell antigen Proteins 0.000 description 4
- 239000013614 RNA sample Substances 0.000 description 4
- 108700008625 Reporter Genes Proteins 0.000 description 4
- 101100289792 Squirrel monkey polyomavirus large T gene Proteins 0.000 description 4
- 108091023040 Transcription factor Proteins 0.000 description 4
- 102000040945 Transcription factor Human genes 0.000 description 4
- 230000010056 antibody-dependent cellular cytotoxicity Effects 0.000 description 4
- 230000004071 biological effect Effects 0.000 description 4
- 238000007413 biotinylation Methods 0.000 description 4
- 230000006287 biotinylation Effects 0.000 description 4
- 229940079593 drug Drugs 0.000 description 4
- 230000008482 dysregulation Effects 0.000 description 4
- CBOQJANXLMLOSS-UHFFFAOYSA-N ethyl vanillin Chemical compound CCOC1=CC(C=O)=CC=C1O CBOQJANXLMLOSS-UHFFFAOYSA-N 0.000 description 4
- 239000000284 extract Substances 0.000 description 4
- 238000000684 flow cytometry Methods 0.000 description 4
- 238000009472 formulation Methods 0.000 description 4
- 210000002216 heart Anatomy 0.000 description 4
- 210000000987 immune system Anatomy 0.000 description 4
- 230000005847 immunogenicity Effects 0.000 description 4
- 238000011532 immunohistochemical staining Methods 0.000 description 4
- 238000005462 in vivo assay Methods 0.000 description 4
- 238000011534 incubation Methods 0.000 description 4
- 230000010354 integration Effects 0.000 description 4
- 238000007726 management method Methods 0.000 description 4
- 238000013507 mapping Methods 0.000 description 4
- 239000000463 material Substances 0.000 description 4
- 239000011159 matrix material Substances 0.000 description 4
- 239000002609 medium Substances 0.000 description 4
- 206010061289 metastatic neoplasm Diseases 0.000 description 4
- 229960004927 neomycin Drugs 0.000 description 4
- 238000006386 neutralization reaction Methods 0.000 description 4
- 210000004940 nucleus Anatomy 0.000 description 4
- 239000002953 phosphate buffered saline Substances 0.000 description 4
- 230000008488 polyadenylation Effects 0.000 description 4
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 4
- 238000000746 purification Methods 0.000 description 4
- 238000011002 quantification Methods 0.000 description 4
- 230000010076 replication Effects 0.000 description 4
- 239000001509 sodium citrate Substances 0.000 description 4
- 238000010561 standard procedure Methods 0.000 description 4
- 210000001541 thymus gland Anatomy 0.000 description 4
- 210000002303 tibia Anatomy 0.000 description 4
- 230000005030 transcription termination Effects 0.000 description 4
- 102000035160 transmembrane proteins Human genes 0.000 description 4
- 108091005703 transmembrane proteins Proteins 0.000 description 4
- 230000005740 tumor formation Effects 0.000 description 4
- 230000004614 tumor growth Effects 0.000 description 4
- 102000010637 Aquaporins Human genes 0.000 description 3
- 206010006187 Breast cancer Diseases 0.000 description 3
- 208000026310 Breast neoplasm Diseases 0.000 description 3
- OBMZMSLWNNWEJA-XNCRXQDQSA-N C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 Chemical compound C1=CC=2C(C[C@@H]3NC(=O)[C@@H](NC(=O)[C@H](NC(=O)N(CC#CCN(CCCC[C@H](NC(=O)[C@@H](CC4=CC=CC=C4)NC3=O)C(=O)N)CC=C)NC(=O)[C@@H](N)C)CC3=CNC4=C3C=CC=C4)C)=CNC=2C=C1 OBMZMSLWNNWEJA-XNCRXQDQSA-N 0.000 description 3
- 108010078791 Carrier Proteins Proteins 0.000 description 3
- 102000010970 Connexin Human genes 0.000 description 3
- 108050001175 Connexin Proteins 0.000 description 3
- 102000053602 DNA Human genes 0.000 description 3
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 101000892862 Homo sapiens Glutamate carboxypeptidase 2 Proteins 0.000 description 3
- 108060001084 Luciferase Proteins 0.000 description 3
- 239000005089 Luciferase Substances 0.000 description 3
- 208000007433 Lymphatic Metastasis Diseases 0.000 description 3
- 206010027459 Metastases to lymph nodes Diseases 0.000 description 3
- 241000699660 Mus musculus Species 0.000 description 3
- 108091028043 Nucleic acid sequence Proteins 0.000 description 3
- 108700020796 Oncogene Proteins 0.000 description 3
- 238000012408 PCR amplification Methods 0.000 description 3
- 229910019142 PO4 Inorganic materials 0.000 description 3
- 101710176384 Peptide 1 Proteins 0.000 description 3
- 241000364051 Pima Species 0.000 description 3
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 3
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 3
- 102000018674 Sodium Channels Human genes 0.000 description 3
- 108010052164 Sodium Channels Proteins 0.000 description 3
- 210000001744 T-lymphocyte Anatomy 0.000 description 3
- 208000024313 Testicular Neoplasms Diseases 0.000 description 3
- 206010057644 Testis cancer Diseases 0.000 description 3
- 102000007238 Transferrin Receptors Human genes 0.000 description 3
- 108010033576 Transferrin Receptors Proteins 0.000 description 3
- 230000002159 abnormal effect Effects 0.000 description 3
- 150000007513 acids Chemical class 0.000 description 3
- 238000000246 agarose gel electrophoresis Methods 0.000 description 3
- 230000004075 alteration Effects 0.000 description 3
- 230000001640 apoptogenic effect Effects 0.000 description 3
- 210000003719 b-lymphocyte Anatomy 0.000 description 3
- 210000000270 basal cell Anatomy 0.000 description 3
- 230000008901 benefit Effects 0.000 description 3
- 230000033228 biological regulation Effects 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 210000001185 bone marrow Anatomy 0.000 description 3
- 239000000872 buffer Substances 0.000 description 3
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 3
- 239000000969 carrier Substances 0.000 description 3
- 238000012761 co-transfection Methods 0.000 description 3
- 230000021615 conjugation Effects 0.000 description 3
- 230000001086 cytosolic effect Effects 0.000 description 3
- 231100000433 cytotoxic Toxicity 0.000 description 3
- 230000001472 cytotoxic effect Effects 0.000 description 3
- 230000002950 deficient Effects 0.000 description 3
- 230000004069 differentiation Effects 0.000 description 3
- LOKCTEFSRHRXRJ-UHFFFAOYSA-I dipotassium trisodium dihydrogen phosphate hydrogen phosphate dichloride Chemical compound P(=O)(O)(O)[O-].[K+].P(=O)(O)([O-])[O-].[Na+].[Na+].[Cl-].[K+].[Cl-].[Na+] LOKCTEFSRHRXRJ-UHFFFAOYSA-I 0.000 description 3
- 239000012636 effector Substances 0.000 description 3
- 210000002472 endoplasmic reticulum Anatomy 0.000 description 3
- 239000003623 enhancer Substances 0.000 description 3
- 210000000981 epithelium Anatomy 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 238000002744 homologous recombination Methods 0.000 description 3
- 230000006801 homologous recombination Effects 0.000 description 3
- 230000002209 hydrophobic effect Effects 0.000 description 3
- 230000001900 immune effect Effects 0.000 description 3
- 238000003119 immunoblot Methods 0.000 description 3
- 230000002055 immunohistochemical effect Effects 0.000 description 3
- 238000010253 intravenous injection Methods 0.000 description 3
- 210000000265 leukocyte Anatomy 0.000 description 3
- 238000011068 loading method Methods 0.000 description 3
- 230000004807 localization Effects 0.000 description 3
- 239000006166 lysate Substances 0.000 description 3
- 238000013508 migration Methods 0.000 description 3
- 230000003278 mimic effect Effects 0.000 description 3
- 239000013610 patient sample Substances 0.000 description 3
- 238000002823 phage display Methods 0.000 description 3
- 239000010452 phosphate Substances 0.000 description 3
- 238000004393 prognosis Methods 0.000 description 3
- 230000000069 prophylactic effect Effects 0.000 description 3
- 208000021046 prostate intraepithelial neoplasia Diseases 0.000 description 3
- 230000009257 reactivity Effects 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 238000011160 research Methods 0.000 description 3
- 230000001177 retroviral effect Effects 0.000 description 3
- 238000010845 search algorithm Methods 0.000 description 3
- 230000011664 signaling Effects 0.000 description 3
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 3
- 210000002784 stomach Anatomy 0.000 description 3
- 230000008685 targeting Effects 0.000 description 3
- 201000003120 testicular cancer Diseases 0.000 description 3
- 230000001988 toxicity Effects 0.000 description 3
- 231100000419 toxicity Toxicity 0.000 description 3
- 238000012546 transfer Methods 0.000 description 3
- 230000005748 tumor development Effects 0.000 description 3
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N (+)-Biotin Chemical compound N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 2
- HLCSDJLATUNSSI-JXMROGBWSA-N (2e)-3,7-dimethylocta-2,6-dienenitrile Chemical compound CC(C)=CCC\C(C)=C\C#N HLCSDJLATUNSSI-JXMROGBWSA-N 0.000 description 2
- 108020003589 5' Untranslated Regions Proteins 0.000 description 2
- 108700028369 Alleles Proteins 0.000 description 2
- DCXYFEDJOCDNAF-UHFFFAOYSA-N Asparagine Natural products OC(=O)C(N)CC(N)=O DCXYFEDJOCDNAF-UHFFFAOYSA-N 0.000 description 2
- 241000972773 Aulopiformes Species 0.000 description 2
- 102000014914 Carrier Proteins Human genes 0.000 description 2
- 108010001857 Cell Surface Receptors Proteins 0.000 description 2
- 102000000844 Cell Surface Receptors Human genes 0.000 description 2
- 206010008342 Cervix carcinoma Diseases 0.000 description 2
- 108091006146 Channels Proteins 0.000 description 2
- HEDRZPFGACZZDS-UHFFFAOYSA-N Chloroform Chemical compound ClC(Cl)Cl HEDRZPFGACZZDS-UHFFFAOYSA-N 0.000 description 2
- 108020004635 Complementary DNA Proteins 0.000 description 2
- 108091035707 Consensus sequence Proteins 0.000 description 2
- 108091029523 CpG island Proteins 0.000 description 2
- 108091029430 CpG site Proteins 0.000 description 2
- 230000004544 DNA amplification Effects 0.000 description 2
- 241000702421 Dependoparvovirus Species 0.000 description 2
- 108700024394 Exon Proteins 0.000 description 2
- 239000004471 Glycine Substances 0.000 description 2
- 102000004457 Granulocyte-Macrophage Colony-Stimulating Factor Human genes 0.000 description 2
- 108010017213 Granulocyte-Macrophage Colony-Stimulating Factor Proteins 0.000 description 2
- 241000238631 Hexapoda Species 0.000 description 2
- 108010001336 Horseradish Peroxidase Proteins 0.000 description 2
- 206010061598 Immunodeficiency Diseases 0.000 description 2
- 108010021625 Immunoglobulin Fragments Proteins 0.000 description 2
- 102000008394 Immunoglobulin Fragments Human genes 0.000 description 2
- 108091092195 Intron Proteins 0.000 description 2
- 208000008839 Kidney Neoplasms Diseases 0.000 description 2
- DCXYFEDJOCDNAF-REOHCLBHSA-N L-asparagine Chemical compound OC(=O)[C@@H](N)CC(N)=O DCXYFEDJOCDNAF-REOHCLBHSA-N 0.000 description 2
- AGPKZVBTJJNPAG-WHFBIAKZSA-N L-isoleucine Chemical compound CC[C@H](C)[C@H](N)C(O)=O AGPKZVBTJJNPAG-WHFBIAKZSA-N 0.000 description 2
- 101710128836 Large T antigen Proteins 0.000 description 2
- 241000713666 Lentivirus Species 0.000 description 2
- 206010027452 Metastases to bone Diseases 0.000 description 2
- 241001529936 Murinae Species 0.000 description 2
- 241001045988 Neogene Species 0.000 description 2
- 101150102573 PCR1 gene Proteins 0.000 description 2
- 108010067902 Peptide Library Proteins 0.000 description 2
- 108091093037 Peptide nucleic acid Proteins 0.000 description 2
- ISWSIDIOOBJBQZ-UHFFFAOYSA-N Phenol Chemical compound OC1=CC=CC=C1 ISWSIDIOOBJBQZ-UHFFFAOYSA-N 0.000 description 2
- 239000004698 Polyethylene Substances 0.000 description 2
- 239000002202 Polyethylene glycol Substances 0.000 description 2
- 101710182846 Polyhedrin Proteins 0.000 description 2
- 108010059712 Pronase Proteins 0.000 description 2
- 102000001253 Protein Kinase Human genes 0.000 description 2
- 108010076504 Protein Sorting Signals Proteins 0.000 description 2
- 206010038389 Renal cancer Diseases 0.000 description 2
- 208000034189 Sclerosis Diseases 0.000 description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 2
- PXIPVTKHYLBLMZ-UHFFFAOYSA-N Sodium azide Chemical compound [Na+].[N-]=[N+]=[N-] PXIPVTKHYLBLMZ-UHFFFAOYSA-N 0.000 description 2
- 230000024932 T cell mediated immunity Effects 0.000 description 2
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 description 2
- 208000006105 Uterine Cervical Neoplasms Diseases 0.000 description 2
- 108700005077 Viral Genes Proteins 0.000 description 2
- 108010084455 Zeocin Proteins 0.000 description 2
- 230000003213 activating effect Effects 0.000 description 2
- 238000001042 affinity chromatography Methods 0.000 description 2
- 239000002870 angiogenesis inducing agent Substances 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 238000011319 anticancer therapy Methods 0.000 description 2
- 230000030741 antigen processing and presentation Effects 0.000 description 2
- 235000009582 asparagine Nutrition 0.000 description 2
- 229960001230 asparagine Drugs 0.000 description 2
- 235000019445 benzyl alcohol Nutrition 0.000 description 2
- 238000003766 bioinformatics method Methods 0.000 description 2
- 230000037396 body weight Effects 0.000 description 2
- 210000000481 breast Anatomy 0.000 description 2
- 239000012830 cancer therapeutic Substances 0.000 description 2
- 239000002775 capsule Substances 0.000 description 2
- 125000000837 carbohydrate group Chemical group 0.000 description 2
- 231100000504 carcinogenesis Toxicity 0.000 description 2
- 230000024245 cell differentiation Effects 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 230000006037 cell lysis Effects 0.000 description 2
- 230000012292 cell migration Effects 0.000 description 2
- 230000036755 cellular response Effects 0.000 description 2
- 230000005754 cellular signaling Effects 0.000 description 2
- 201000010881 cervical cancer Diseases 0.000 description 2
- 239000003795 chemical substances by application Substances 0.000 description 2
- 239000003636 conditioned culture medium Substances 0.000 description 2
- 230000002380 cytological effect Effects 0.000 description 2
- 230000009089 cytolysis Effects 0.000 description 2
- 238000012303 cytoplasmic staining Methods 0.000 description 2
- 210000000172 cytosol Anatomy 0.000 description 2
- 231100000599 cytotoxic agent Toxicity 0.000 description 2
- 238000002716 delivery method Methods 0.000 description 2
- 230000017858 demethylation Effects 0.000 description 2
- 238000010520 demethylation reaction Methods 0.000 description 2
- 230000030609 dephosphorylation Effects 0.000 description 2
- 238000006209 dephosphorylation reaction Methods 0.000 description 2
- 229960000633 dextran sulfate Drugs 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 238000010494 dissociation reaction Methods 0.000 description 2
- 230000005593 dissociations Effects 0.000 description 2
- 230000007783 downstream signaling Effects 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 210000001671 embryonic stem cell Anatomy 0.000 description 2
- 210000002919 epithelial cell Anatomy 0.000 description 2
- 229940073505 ethyl vanillin Drugs 0.000 description 2
- 230000001747 exhibiting effect Effects 0.000 description 2
- 238000001476 gene delivery Methods 0.000 description 2
- 230000002068 genetic effect Effects 0.000 description 2
- 210000004602 germ cell Anatomy 0.000 description 2
- 229940022353 herceptin Drugs 0.000 description 2
- 150000002411 histidines Chemical class 0.000 description 2
- 239000005556 hormone Substances 0.000 description 2
- 229940088597 hormone Drugs 0.000 description 2
- 230000004727 humoral immunity Effects 0.000 description 2
- 238000003018 immunoassay Methods 0.000 description 2
- 229940127121 immunoconjugate Drugs 0.000 description 2
- 238000010324 immunological assay Methods 0.000 description 2
- 229940121354 immunomodulator Drugs 0.000 description 2
- 238000009169 immunotherapy Methods 0.000 description 2
- 238000011065 in-situ storage Methods 0.000 description 2
- 230000006882 induction of apoptosis Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 238000001802 infusion Methods 0.000 description 2
- 230000002452 interceptive effect Effects 0.000 description 2
- 230000031146 intracellular signal transduction Effects 0.000 description 2
- 238000007918 intramuscular administration Methods 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 230000002601 intratumoral effect Effects 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 229960000310 isoleucine Drugs 0.000 description 2
- AGPKZVBTJJNPAG-UHFFFAOYSA-N isoleucine Natural products CCC(C)C(N)C(O)=O AGPKZVBTJJNPAG-UHFFFAOYSA-N 0.000 description 2
- 201000010982 kidney cancer Diseases 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 230000003902 lesion Effects 0.000 description 2
- 125000001909 leucine group Chemical group [H]N(*)C(C(*)=O)C([H])([H])C(C([H])([H])[H])C([H])([H])[H] 0.000 description 2
- 150000002632 lipids Chemical group 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 210000001161 mammalian embryo Anatomy 0.000 description 2
- 108010082117 matrigel Proteins 0.000 description 2
- 238000007855 methylation-specific PCR Methods 0.000 description 2
- 230000005012 migration Effects 0.000 description 2
- 238000007479 molecular analysis Methods 0.000 description 2
- 125000004573 morpholin-4-yl group Chemical group N1(CCOCC1)* 0.000 description 2
- 238000002703 mutagenesis Methods 0.000 description 2
- 231100000350 mutagenesis Toxicity 0.000 description 2
- 230000035772 mutation Effects 0.000 description 2
- 210000004897 n-terminal region Anatomy 0.000 description 2
- 101150091879 neo gene Proteins 0.000 description 2
- 229910052759 nickel Inorganic materials 0.000 description 2
- 238000011580 nude mouse model Methods 0.000 description 2
- 210000000287 oocyte Anatomy 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- 230000001582 osteoblastic effect Effects 0.000 description 2
- 238000004806 packaging method and process Methods 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 239000008188 pellet Substances 0.000 description 2
- 210000005259 peripheral blood Anatomy 0.000 description 2
- 239000011886 peripheral blood Substances 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- CWCMIVBLVUHDHK-ZSNHEYEWSA-N phleomycin D1 Chemical compound N([C@H](C(=O)N[C@H](C)[C@@H](O)[C@H](C)C(=O)N[C@@H]([C@H](O)C)C(=O)NCCC=1SC[C@@H](N=1)C=1SC=C(N=1)C(=O)NCCCCNC(N)=N)[C@@H](O[C@H]1[C@H]([C@@H](O)[C@H](O)[C@H](CO)O1)O[C@@H]1[C@H]([C@@H](OC(N)=O)[C@H](O)[C@@H](CO)O1)O)C=1N=CNC=1)C(=O)C1=NC([C@H](CC(N)=O)NC[C@H](N)C(N)=O)=NC(N)=C1C CWCMIVBLVUHDHK-ZSNHEYEWSA-N 0.000 description 2
- 150000003013 phosphoric acid derivatives Chemical class 0.000 description 2
- 229920000573 polyethylene Polymers 0.000 description 2
- 229920001223 polyethylene glycol Polymers 0.000 description 2
- 229920000642 polymer Polymers 0.000 description 2
- 102000054765 polymorphisms of proteins Human genes 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 229940002612 prodrug Drugs 0.000 description 2
- 239000000651 prodrug Substances 0.000 description 2
- 210000000064 prostate epithelial cell Anatomy 0.000 description 2
- 238000011471 prostatectomy Methods 0.000 description 2
- 238000002331 protein detection Methods 0.000 description 2
- 230000004853 protein function Effects 0.000 description 2
- 108060006633 protein kinase Proteins 0.000 description 2
- 230000012743 protein tagging Effects 0.000 description 2
- 230000004850 protein–protein interaction Effects 0.000 description 2
- 238000003127 radioimmunoassay Methods 0.000 description 2
- 238000001959 radiotherapy Methods 0.000 description 2
- 230000009467 reduction Effects 0.000 description 2
- 108091008146 restriction endonucleases Proteins 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 235000019515 salmon Nutrition 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 238000012163 sequencing technique Methods 0.000 description 2
- 125000003607 serino group Chemical group [H]N([H])[C@]([H])(C(=O)[*])C(O[H])([H])[H] 0.000 description 2
- 238000002741 site-directed mutagenesis Methods 0.000 description 2
- 210000002027 skeletal muscle Anatomy 0.000 description 2
- 210000003491 skin Anatomy 0.000 description 2
- 238000002415 sodium dodecyl sulfate polyacrylamide gel electrophoresis Methods 0.000 description 2
- 239000001488 sodium phosphate Substances 0.000 description 2
- 229910000162 sodium phosphate Inorganic materials 0.000 description 2
- 239000007787 solid Substances 0.000 description 2
- 241000894007 species Species 0.000 description 2
- 239000008223 sterile water Substances 0.000 description 2
- 230000000638 stimulation Effects 0.000 description 2
- 239000000758 substrate Substances 0.000 description 2
- 238000001356 surgical procedure Methods 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- 238000007910 systemic administration Methods 0.000 description 2
- 231100000331 toxic Toxicity 0.000 description 2
- 230000002588 toxic effect Effects 0.000 description 2
- 239000003440 toxic substance Substances 0.000 description 2
- 230000002103 transcriptional effect Effects 0.000 description 2
- 238000011277 treatment modality Methods 0.000 description 2
- RYFMWSXOAZQYPI-UHFFFAOYSA-K trisodium phosphate Chemical compound [Na+].[Na+].[Na+].[O-]P([O-])([O-])=O RYFMWSXOAZQYPI-UHFFFAOYSA-K 0.000 description 2
- 241000701161 unidentified adenovirus Species 0.000 description 2
- 241000701447 unidentified baculovirus Species 0.000 description 2
- 241001515965 unidentified phage Species 0.000 description 2
- 210000004291 uterus Anatomy 0.000 description 2
- MTCFGRXMJLQNBG-REOHCLBHSA-N (2S)-2-Amino-3-hydroxypropansäure Chemical compound OC[C@H](N)C(O)=O MTCFGRXMJLQNBG-REOHCLBHSA-N 0.000 description 1
- JUDOLRSMWHVKGX-UHFFFAOYSA-N 1,1-dioxo-1$l^{6},2-benzodithiol-3-one Chemical compound C1=CC=C2C(=O)SS(=O)(=O)C2=C1 JUDOLRSMWHVKGX-UHFFFAOYSA-N 0.000 description 1
- FDSYTWVNUJTPMA-UHFFFAOYSA-N 2-[3,9-bis(carboxymethyl)-3,6,9,15-tetrazabicyclo[9.3.1]pentadeca-1(15),11,13-trien-6-yl]acetic acid Chemical compound C1N(CC(O)=O)CCN(CC(=O)O)CCN(CC(O)=O)CC2=CC=CC1=N2 FDSYTWVNUJTPMA-UHFFFAOYSA-N 0.000 description 1
- JKMHFZQWWAIEOD-UHFFFAOYSA-N 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid Chemical compound OCC[NH+]1CCN(CCS([O-])(=O)=O)CC1 JKMHFZQWWAIEOD-UHFFFAOYSA-N 0.000 description 1
- OTLLEIBWKHEHGU-UHFFFAOYSA-N 2-[5-[[5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy]-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3,5-dihydroxy-4-phosphonooxyhexanedioic acid Chemical compound C1=NC=2C(N)=NC=NC=2N1C(C(C1O)O)OC1COC1C(CO)OC(OC(C(O)C(OP(O)(O)=O)C(O)C(O)=O)C(O)=O)C(O)C1O OTLLEIBWKHEHGU-UHFFFAOYSA-N 0.000 description 1
- QKNYBSVHEMOAJP-UHFFFAOYSA-N 2-amino-2-(hydroxymethyl)propane-1,3-diol;hydron;chloride Chemical compound Cl.OCC(N)(CO)CO QKNYBSVHEMOAJP-UHFFFAOYSA-N 0.000 description 1
- QRXMUCSWCMTJGU-UHFFFAOYSA-N 5-bromo-4-chloro-3-indolyl phosphate Chemical compound C1=C(Br)C(Cl)=C2C(OP(O)(=O)O)=CNC2=C1 QRXMUCSWCMTJGU-UHFFFAOYSA-N 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 208000014697 Acute lymphocytic leukaemia Diseases 0.000 description 1
- 229920001817 Agar Polymers 0.000 description 1
- 229920000936 Agarose Polymers 0.000 description 1
- 102100032187 Androgen receptor Human genes 0.000 description 1
- 108020004491 Antisense DNA Proteins 0.000 description 1
- 208000019901 Anxiety disease Diseases 0.000 description 1
- 108010063290 Aquaporins Proteins 0.000 description 1
- 101100490659 Arabidopsis thaliana AGP17 gene Proteins 0.000 description 1
- 101100519158 Arabidopsis thaliana PCR2 gene Proteins 0.000 description 1
- 239000004475 Arginine Substances 0.000 description 1
- 208000002109 Argyria Diseases 0.000 description 1
- 206010003445 Ascites Diseases 0.000 description 1
- 101000669426 Aspergillus restrictus Ribonuclease mitogillin Proteins 0.000 description 1
- 241000201370 Autographa californica nucleopolyhedrovirus Species 0.000 description 1
- 108090001008 Avidin Proteins 0.000 description 1
- 208000003950 B-cell lymphoma Diseases 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 208000018084 Bone neoplasm Diseases 0.000 description 1
- 206010055113 Breast cancer metastatic Diseases 0.000 description 1
- 125000001433 C-terminal amino-acid group Chemical group 0.000 description 1
- 241000178270 Canarypox virus Species 0.000 description 1
- 101710132601 Capsid protein Proteins 0.000 description 1
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 1
- 208000005623 Carcinogenesis Diseases 0.000 description 1
- 201000009030 Carcinoma Diseases 0.000 description 1
- 206010057248 Cell death Diseases 0.000 description 1
- 102000034573 Channels Human genes 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- 101710094648 Coat protein Proteins 0.000 description 1
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 1
- 206010010099 Combined immunodeficiency Diseases 0.000 description 1
- 102000000989 Complement System Proteins Human genes 0.000 description 1
- 108010069112 Complement System Proteins Proteins 0.000 description 1
- 108010047041 Complementarity Determining Regions Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 229920001076 Cutan Polymers 0.000 description 1
- 102000005636 Cyclic AMP Response Element-Binding Protein Human genes 0.000 description 1
- 108010045171 Cyclic AMP Response Element-Binding Protein Proteins 0.000 description 1
- 102100023033 Cyclic AMP-dependent transcription factor ATF-2 Human genes 0.000 description 1
- 101710112752 Cytotoxin Proteins 0.000 description 1
- IGXWBGJHJZYPQS-SSDOTTSWSA-N D-Luciferin Chemical compound OC(=O)[C@H]1CSC(C=2SC3=CC=C(O)C=C3N=2)=N1 IGXWBGJHJZYPQS-SSDOTTSWSA-N 0.000 description 1
- 102000012410 DNA Ligases Human genes 0.000 description 1
- 108010061982 DNA Ligases Proteins 0.000 description 1
- 239000003298 DNA probe Substances 0.000 description 1
- 238000001712 DNA sequencing Methods 0.000 description 1
- 229940021995 DNA vaccine Drugs 0.000 description 1
- 108010014303 DNA-directed DNA polymerase Proteins 0.000 description 1
- 102000016928 DNA-directed DNA polymerase Human genes 0.000 description 1
- 102000000541 Defensins Human genes 0.000 description 1
- 108010002069 Defensins Proteins 0.000 description 1
- CYCGRDQQIOGCKX-UHFFFAOYSA-N Dehydro-luciferin Natural products OC(=O)C1=CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 CYCGRDQQIOGCKX-UHFFFAOYSA-N 0.000 description 1
- 206010061818 Disease progression Diseases 0.000 description 1
- 239000006144 Dulbecco’s modified Eagle's medium Substances 0.000 description 1
- UPEZCKBFRMILAV-JNEQICEOSA-N Ecdysone Natural products O=C1[C@H]2[C@@](C)([C@@H]3C([C@@]4(O)[C@@](C)([C@H]([C@H]([C@@H](O)CCC(O)(C)C)C)CC4)CC3)=C1)C[C@H](O)[C@H](O)C2 UPEZCKBFRMILAV-JNEQICEOSA-N 0.000 description 1
- 241000196324 Embryophyta Species 0.000 description 1
- 108010013369 Enteropeptidase Proteins 0.000 description 1
- 102100029727 Enteropeptidase Human genes 0.000 description 1
- 241000991587 Enterovirus C Species 0.000 description 1
- 241000701959 Escherichia virus Lambda Species 0.000 description 1
- 108050001049 Extracellular proteins Proteins 0.000 description 1
- 108091006020 Fc-tagged proteins Proteins 0.000 description 1
- BJGNCJDXODQBOB-UHFFFAOYSA-N Fivefly Luciferin Natural products OC(=O)C1CSC(C=2SC3=CC(O)=CC=C3N=2)=N1 BJGNCJDXODQBOB-UHFFFAOYSA-N 0.000 description 1
- 238000012413 Fluorescence activated cell sorting analysis Methods 0.000 description 1
- 241000700662 Fowlpox virus Species 0.000 description 1
- 102100039554 Galectin-8 Human genes 0.000 description 1
- 108700028146 Genetic Enhancer Elements Proteins 0.000 description 1
- WHUUTDBJXJRKMK-UHFFFAOYSA-N Glutamic acid Natural products OC(=O)C(N)CCC(O)=O WHUUTDBJXJRKMK-UHFFFAOYSA-N 0.000 description 1
- 102100021181 Golgi phosphoprotein 3 Human genes 0.000 description 1
- 101000974934 Homo sapiens Cyclic AMP-dependent transcription factor ATF-2 Proteins 0.000 description 1
- 101000608769 Homo sapiens Galectin-8 Proteins 0.000 description 1
- 101000997829 Homo sapiens Glial cell line-derived neurotrophic factor Proteins 0.000 description 1
- 101000628535 Homo sapiens Metalloreductase STEAP2 Proteins 0.000 description 1
- 101000880398 Homo sapiens Metalloreductase STEAP3 Proteins 0.000 description 1
- 101000880402 Homo sapiens Metalloreductase STEAP4 Proteins 0.000 description 1
- 108010002231 IgA-specific serine endopeptidase Proteins 0.000 description 1
- DGAQECJNVWCQMB-PUAWFVPOSA-M Ilexoside XXIX Chemical compound C[C@@H]1CC[C@@]2(CC[C@@]3(C(=CC[C@H]4[C@]3(CC[C@@H]5[C@@]4(CC[C@@H](C5(C)C)OS(=O)(=O)[O-])C)C)[C@@H]2[C@]1(C)O)C)C(=O)O[C@H]6[C@@H]([C@H]([C@@H]([C@H](O6)CO)O)O)O.[Na+] DGAQECJNVWCQMB-PUAWFVPOSA-M 0.000 description 1
- 108700005091 Immunoglobulin Genes Proteins 0.000 description 1
- 102100034343 Integrase Human genes 0.000 description 1
- 108010002350 Interleukin-2 Proteins 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- DDWFXDSYGUXRAY-UHFFFAOYSA-N Luciferin Natural products CCc1c(C)c(CC2NC(=O)C(=C2C=C)C)[nH]c1Cc3[nH]c4C(=C5/NC(CC(=O)O)C(C)C5CC(=O)O)CC(=O)c4c3C DDWFXDSYGUXRAY-UHFFFAOYSA-N 0.000 description 1
- 239000004472 Lysine Substances 0.000 description 1
- KDXKERNSBIXSRK-UHFFFAOYSA-N Lysine Natural products NCCCCC(N)C(O)=O KDXKERNSBIXSRK-UHFFFAOYSA-N 0.000 description 1
- 102000043129 MHC class I family Human genes 0.000 description 1
- 108091054437 MHC class I family Proteins 0.000 description 1
- 101710125418 Major capsid protein Proteins 0.000 description 1
- 206010027458 Metastases to lung Diseases 0.000 description 1
- 208000034578 Multiple myelomas Diseases 0.000 description 1
- 125000000729 N-terminal amino-acid group Chemical group 0.000 description 1
- 108091061960 Naked DNA Proteins 0.000 description 1
- 108700019961 Neoplasm Genes Proteins 0.000 description 1
- 102000048850 Neoplasm Genes Human genes 0.000 description 1
- 101100049938 Neurospora crassa (strain ATCC 24698 / 74-OR23-1A / CBS 708.71 / DSM 1257 / FGSC 987) exr-1 gene Proteins 0.000 description 1
- 239000000020 Nitrocellulose Substances 0.000 description 1
- 108010077850 Nuclear Localization Signals Proteins 0.000 description 1
- 101710141454 Nucleoprotein Proteins 0.000 description 1
- 206010035226 Plasma cell myeloma Diseases 0.000 description 1
- 241000276498 Pollachius virens Species 0.000 description 1
- 102000004257 Potassium Channel Human genes 0.000 description 1
- 102100025067 Potassium voltage-gated channel subfamily H member 4 Human genes 0.000 description 1
- 101710163352 Potassium voltage-gated channel subfamily H member 4 Proteins 0.000 description 1
- 208000006664 Precursor Cell Lymphoblastic Leukemia-Lymphoma Diseases 0.000 description 1
- 101710083689 Probable capsid protein Proteins 0.000 description 1
- 108010029485 Protein Isoforms Proteins 0.000 description 1
- 102000001708 Protein Isoforms Human genes 0.000 description 1
- 108091034057 RNA (poly(A)) Proteins 0.000 description 1
- 238000002123 RNA extraction Methods 0.000 description 1
- 108010092799 RNA-directed DNA polymerase Proteins 0.000 description 1
- 208000001647 Renal Insufficiency Diseases 0.000 description 1
- 206010061481 Renal injury Diseases 0.000 description 1
- 108010073443 Ribi adjuvant Proteins 0.000 description 1
- 108091028664 Ribonucleotide Proteins 0.000 description 1
- 241000283984 Rodentia Species 0.000 description 1
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 1
- 229920002684 Sepharose Polymers 0.000 description 1
- 241000710960 Sindbis virus Species 0.000 description 1
- 206010061363 Skeletal injury Diseases 0.000 description 1
- DWAQJAXMDSEUJJ-UHFFFAOYSA-M Sodium bisulfite Chemical compound [Na+].OS([O-])=O DWAQJAXMDSEUJJ-UHFFFAOYSA-M 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- 230000005867 T cell response Effects 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-N Thiophosphoric acid Chemical class OP(O)(S)=O RYYWUUFWQRZTIU-UHFFFAOYSA-N 0.000 description 1
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 1
- 239000004473 Threonine Substances 0.000 description 1
- 102000000591 Tight Junction Proteins Human genes 0.000 description 1
- 108010002321 Tight Junction Proteins Proteins 0.000 description 1
- 206010066901 Treatment failure Diseases 0.000 description 1
- 108091023045 Untranslated Region Proteins 0.000 description 1
- 241000700618 Vaccinia virus Species 0.000 description 1
- 241000269370 Xenopus <genus> Species 0.000 description 1
- 238000002679 ablation Methods 0.000 description 1
- 238000010317 ablation therapy Methods 0.000 description 1
- 239000012190 activator Substances 0.000 description 1
- 210000004100 adrenal gland Anatomy 0.000 description 1
- 239000008272 agar Substances 0.000 description 1
- 230000002776 aggregation Effects 0.000 description 1
- 238000004220 aggregation Methods 0.000 description 1
- 125000003295 alanine group Chemical group N[C@@H](C)C(=O)* 0.000 description 1
- 238000012867 alanine scanning Methods 0.000 description 1
- UPEZCKBFRMILAV-UHFFFAOYSA-N alpha-Ecdysone Natural products C1C(O)C(O)CC2(C)C(CCC3(C(C(C(O)CCC(C)(C)O)C)CCC33O)C)C3=CC(=O)C21 UPEZCKBFRMILAV-UHFFFAOYSA-N 0.000 description 1
- 108010080146 androgen receptors Proteins 0.000 description 1
- 210000004102 animal cell Anatomy 0.000 description 1
- 238000000137 annealing Methods 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000001093 anti-cancer Effects 0.000 description 1
- 230000006023 anti-tumor response Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 238000011230 antibody-based therapy Methods 0.000 description 1
- 210000000612 antigen-presenting cell Anatomy 0.000 description 1
- 229940041181 antineoplastic drug Drugs 0.000 description 1
- 239000003816 antisense DNA Substances 0.000 description 1
- 230000036506 anxiety Effects 0.000 description 1
- 210000000709 aorta Anatomy 0.000 description 1
- ODKSFYDXXFIFQN-UHFFFAOYSA-N arginine Natural products OC(=O)C(N)CCCNC(N)=N ODKSFYDXXFIFQN-UHFFFAOYSA-N 0.000 description 1
- 210000004436 artificial bacterial chromosome Anatomy 0.000 description 1
- 210000001106 artificial yeast chromosome Anatomy 0.000 description 1
- 238000002820 assay format Methods 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 229960002756 azacitidine Drugs 0.000 description 1
- 238000012742 biochemical analysis Methods 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 239000000090 biomarker Substances 0.000 description 1
- 229960002685 biotin Drugs 0.000 description 1
- 235000020958 biotin Nutrition 0.000 description 1
- 239000011616 biotin Substances 0.000 description 1
- 108700021042 biotin binding protein Proteins 0.000 description 1
- 102000043871 biotin binding protein Human genes 0.000 description 1
- 201000001531 bladder carcinoma Diseases 0.000 description 1
- 210000002459 blastocyst Anatomy 0.000 description 1
- 238000006664 bond formation reaction Methods 0.000 description 1
- 229940098773 bovine serum albumin Drugs 0.000 description 1
- 238000010804 cDNA synthesis Methods 0.000 description 1
- BQRGNLJZBFXNCZ-UHFFFAOYSA-N calcein am Chemical compound O1C(=O)C2=CC=CC=C2C21C1=CC(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(C)=O)=C(OC(C)=O)C=C1OC1=C2C=C(CN(CC(=O)OCOC(C)=O)CC(=O)OCOC(=O)C)C(OC(C)=O)=C1 BQRGNLJZBFXNCZ-UHFFFAOYSA-N 0.000 description 1
- 230000003185 calcium uptake Effects 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 230000036952 cancer formation Effects 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 238000002619 cancer immunotherapy Methods 0.000 description 1
- 230000000711 cancerogenic effect Effects 0.000 description 1
- 150000001718 carbodiimides Chemical class 0.000 description 1
- 229910052799 carbon Inorganic materials 0.000 description 1
- 231100000315 carcinogenic Toxicity 0.000 description 1
- 238000012219 cassette mutagenesis Methods 0.000 description 1
- 210000001159 caudate nucleus Anatomy 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000022534 cell killing Effects 0.000 description 1
- 238000001516 cell proliferation assay Methods 0.000 description 1
- 230000005859 cell recognition Effects 0.000 description 1
- 230000005889 cellular cytotoxicity Effects 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 210000001638 cerebellum Anatomy 0.000 description 1
- 210000003710 cerebral cortex Anatomy 0.000 description 1
- 210000003679 cervix uteri Anatomy 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002975 chemoattractant Substances 0.000 description 1
- 230000001889 chemoattractive effect Effects 0.000 description 1
- 210000000038 chest Anatomy 0.000 description 1
- 210000004978 chinese hamster ovary cell Anatomy 0.000 description 1
- 230000002759 chromosomal effect Effects 0.000 description 1
- 239000012568 clinical material Substances 0.000 description 1
- 238000012411 cloning technique Methods 0.000 description 1
- 238000011260 co-administration Methods 0.000 description 1
- 230000005757 colony formation Effects 0.000 description 1
- 238000003271 compound fluorescence assay Methods 0.000 description 1
- 230000001143 conditioned effect Effects 0.000 description 1
- 108091036078 conserved sequence Proteins 0.000 description 1
- 238000007796 conventional method Methods 0.000 description 1
- 208000029078 coronary artery disease Diseases 0.000 description 1
- 238000004132 cross linking Methods 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 125000000151 cysteine group Chemical group N[C@@H](CS)C(=O)* 0.000 description 1
- OPTASPLRGRRNAP-UHFFFAOYSA-N cytosine Chemical class NC=1C=CNC(=O)N=1 OPTASPLRGRRNAP-UHFFFAOYSA-N 0.000 description 1
- 239000002254 cytotoxic agent Substances 0.000 description 1
- 230000003013 cytotoxicity Effects 0.000 description 1
- 231100000135 cytotoxicity Toxicity 0.000 description 1
- 239000002619 cytotoxin Substances 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 239000003398 denaturant Substances 0.000 description 1
- 238000004925 denaturation Methods 0.000 description 1
- 230000036425 denaturation Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000002059 diagnostic imaging Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 230000029087 digestion Effects 0.000 description 1
- 238000007865 diluting Methods 0.000 description 1
- 230000005750 disease progression Effects 0.000 description 1
- 238000009509 drug development Methods 0.000 description 1
- UPEZCKBFRMILAV-JMZLNJERSA-N ecdysone Chemical compound C1[C@@H](O)[C@@H](O)C[C@]2(C)[C@@H](CC[C@@]3([C@@H]([C@@H]([C@H](O)CCC(C)(C)O)C)CC[C@]33O)C)C3=CC(=O)[C@@H]21 UPEZCKBFRMILAV-JMZLNJERSA-N 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000004520 electroporation Methods 0.000 description 1
- 210000002308 embryonic cell Anatomy 0.000 description 1
- 230000002255 enzymatic effect Effects 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 231100000776 exotoxin Toxicity 0.000 description 1
- 239000002095 exotoxin Substances 0.000 description 1
- 230000002349 favourable effect Effects 0.000 description 1
- 239000012894 fetal calf serum Substances 0.000 description 1
- 210000003754 fetus Anatomy 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 239000000706 filtrate Substances 0.000 description 1
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 1
- 230000037433 frameshift Effects 0.000 description 1
- 210000001652 frontal lobe Anatomy 0.000 description 1
- 238000002825 functional assay Methods 0.000 description 1
- 210000003976 gap junction Anatomy 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 238000001502 gel electrophoresis Methods 0.000 description 1
- 230000004545 gene duplication Effects 0.000 description 1
- 238000003209 gene knockout Methods 0.000 description 1
- 238000003208 gene overexpression Methods 0.000 description 1
- 238000013412 genome amplification Methods 0.000 description 1
- 238000010362 genome editing Methods 0.000 description 1
- 210000004907 gland Anatomy 0.000 description 1
- 230000000762 glandular Effects 0.000 description 1
- 210000001703 glandular epithelial cell Anatomy 0.000 description 1
- 235000013922 glutamic acid Nutrition 0.000 description 1
- 239000004220 glutamic acid Substances 0.000 description 1
- ZDXPYRJPNDTMRX-UHFFFAOYSA-N glutamine Natural products OC(=O)C(N)CCC(N)=O ZDXPYRJPNDTMRX-UHFFFAOYSA-N 0.000 description 1
- 230000003394 haemopoietic effect Effects 0.000 description 1
- 210000003128 head Anatomy 0.000 description 1
- 208000019622 heart disease Diseases 0.000 description 1
- 238000004128 high performance liquid chromatography Methods 0.000 description 1
- 238000012203 high throughput assay Methods 0.000 description 1
- 210000001320 hippocampus Anatomy 0.000 description 1
- 230000003054 hormonal effect Effects 0.000 description 1
- 102000053231 human STEAP2 Human genes 0.000 description 1
- 102000048286 human STEAP3 Human genes 0.000 description 1
- 102000048782 human STEAP4 Human genes 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 210000003917 human chromosome Anatomy 0.000 description 1
- 210000002758 humerus Anatomy 0.000 description 1
- 230000028996 humoral immune response Effects 0.000 description 1
- 230000006607 hypermethylation Effects 0.000 description 1
- 230000014200 hypermethylation of CpG island Effects 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- 239000012642 immune effector Substances 0.000 description 1
- 229940124452 immunizing agent Drugs 0.000 description 1
- 238000010166 immunofluorescence Methods 0.000 description 1
- 238000012151 immunohistochemical method Methods 0.000 description 1
- 238000003364 immunohistochemistry Methods 0.000 description 1
- 239000002955 immunomodulating agent Substances 0.000 description 1
- 230000002584 immunomodulator Effects 0.000 description 1
- 239000012133 immunoprecipitate Substances 0.000 description 1
- 230000002637 immunotoxin Effects 0.000 description 1
- 239000002596 immunotoxin Substances 0.000 description 1
- 229940051026 immunotoxin Drugs 0.000 description 1
- 231100000608 immunotoxin Toxicity 0.000 description 1
- 230000001976 improved effect Effects 0.000 description 1
- 239000012535 impurity Substances 0.000 description 1
- 238000012613 in situ experiment Methods 0.000 description 1
- 238000011503 in vivo imaging Methods 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 210000005061 intracellular organelle Anatomy 0.000 description 1
- 230000010189 intracellular transport Effects 0.000 description 1
- 150000002500 ions Chemical class 0.000 description 1
- 201000006370 kidney failure Diseases 0.000 description 1
- 208000037806 kidney injury Diseases 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 208000032839 leukemia Diseases 0.000 description 1
- 150000002617 leukotrienes Chemical class 0.000 description 1
- 108020001756 ligand binding domains Proteins 0.000 description 1
- 238000009630 liquid culture Methods 0.000 description 1
- 210000005228 liver tissue Anatomy 0.000 description 1
- 238000004020 luminiscence type Methods 0.000 description 1
- 230000014759 maintenance of location Effects 0.000 description 1
- 210000005075 mammary gland Anatomy 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 238000002844 melting Methods 0.000 description 1
- 230000008018 melting Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 208000037819 metastatic cancer Diseases 0.000 description 1
- 208000011575 metastatic malignant neoplasm Diseases 0.000 description 1
- 208000029691 metastatic malignant neoplasm in the lymph nodes Diseases 0.000 description 1
- 238000012737 microarray-based gene expression Methods 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 208000024191 minimally invasive lung adenocarcinoma Diseases 0.000 description 1
- 230000002297 mitogenic effect Effects 0.000 description 1
- 108010010621 modeccin Proteins 0.000 description 1
- 239000003068 molecular probe Substances 0.000 description 1
- 230000000877 morphologic effect Effects 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 238000012243 multiplex automated genomic engineering Methods 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 210000000663 muscle cell Anatomy 0.000 description 1
- 210000003739 neck Anatomy 0.000 description 1
- 230000035407 negative regulation of cell proliferation Effects 0.000 description 1
- 210000002569 neuron Anatomy 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 229920001220 nitrocellulos Polymers 0.000 description 1
- 230000009871 nonspecific binding Effects 0.000 description 1
- 238000007899 nucleic acid hybridization Methods 0.000 description 1
- 239000002777 nucleoside Substances 0.000 description 1
- 210000000869 occipital lobe Anatomy 0.000 description 1
- 229940046166 oligodeoxynucleotide Drugs 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 230000011164 ossification Effects 0.000 description 1
- 230000000010 osteolytic effect Effects 0.000 description 1
- 210000003101 oviduct Anatomy 0.000 description 1
- 230000003647 oxidation Effects 0.000 description 1
- 238000007254 oxidation reaction Methods 0.000 description 1
- 125000004430 oxygen atom Chemical group O* 0.000 description 1
- 210000002741 palatine tonsil Anatomy 0.000 description 1
- 239000002245 particle Substances 0.000 description 1
- 210000004197 pelvis Anatomy 0.000 description 1
- 230000000149 penetrating effect Effects 0.000 description 1
- 238000010647 peptide synthesis reaction Methods 0.000 description 1
- 230000008447 perception Effects 0.000 description 1
- 230000000737 periodic effect Effects 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 238000009520 phase I clinical trial Methods 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 125000002467 phosphate group Chemical group [H]OP(=O)(O[H])O[*] 0.000 description 1
- 108091005981 phosphorylated proteins Proteins 0.000 description 1
- 238000003566 phosphorylation assay Methods 0.000 description 1
- 230000035790 physiological processes and functions Effects 0.000 description 1
- 230000001817 pituitary effect Effects 0.000 description 1
- 238000007747 plating Methods 0.000 description 1
- 229920002704 polyhistidine Polymers 0.000 description 1
- 229920001451 polypropylene glycol Polymers 0.000 description 1
- 239000004800 polyvinyl chloride Substances 0.000 description 1
- 229920000915 polyvinyl chloride Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 230000029279 positive regulation of transcription, DNA-dependent Effects 0.000 description 1
- 108020001213 potassium channel Proteins 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 239000002244 precipitate Substances 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 230000002335 preservative effect Effects 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 239000013615 primer Substances 0.000 description 1
- 239000002987 primer (paints) Substances 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 210000001948 pro-b lymphocyte Anatomy 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 201000001514 prostate carcinoma Diseases 0.000 description 1
- 238000000159 protein binding assay Methods 0.000 description 1
- 230000000722 protumoral effect Effects 0.000 description 1
- 210000002637 putamen Anatomy 0.000 description 1
- JFINOWIINSTUNY-UHFFFAOYSA-N pyrrolidin-3-ylmethanesulfonamide Chemical compound NS(=O)(=O)CC1CCNC1 JFINOWIINSTUNY-UHFFFAOYSA-N 0.000 description 1
- 238000002601 radiography Methods 0.000 description 1
- 101150101384 rat1 gene Proteins 0.000 description 1
- 239000011535 reaction buffer Substances 0.000 description 1
- 239000011541 reaction mixture Substances 0.000 description 1
- 238000010188 recombinant method Methods 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 239000013074 reference sample Substances 0.000 description 1
- 230000009703 regulation of cell differentiation Effects 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 230000000717 retained effect Effects 0.000 description 1
- 230000002441 reversible effect Effects 0.000 description 1
- 239000002336 ribonucleotide Substances 0.000 description 1
- 125000002652 ribonucleotide group Chemical group 0.000 description 1
- 210000003079 salivary gland Anatomy 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 238000007423 screening assay Methods 0.000 description 1
- 230000003248 secreting effect Effects 0.000 description 1
- 230000028327 secretion Effects 0.000 description 1
- 210000000582 semen Anatomy 0.000 description 1
- 210000001625 seminal vesicle Anatomy 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 238000004904 shortening Methods 0.000 description 1
- 108091006024 signal transducing proteins Proteins 0.000 description 1
- 102000034285 signal transducing proteins Human genes 0.000 description 1
- 210000004927 skin cell Anatomy 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 239000011734 sodium Substances 0.000 description 1
- 229910052708 sodium Inorganic materials 0.000 description 1
- FQENQNTWSFEDLI-UHFFFAOYSA-J sodium diphosphate Chemical compound [Na+].[Na+].[Na+].[Na+].[O-]P([O-])(=O)OP([O-])([O-])=O FQENQNTWSFEDLI-UHFFFAOYSA-J 0.000 description 1
- 235000010267 sodium hydrogen sulphite Nutrition 0.000 description 1
- 239000012064 sodium phosphate buffer Substances 0.000 description 1
- 229940048086 sodium pyrophosphate Drugs 0.000 description 1
- 238000002798 spectrophotometry method Methods 0.000 description 1
- 210000000278 spinal cord Anatomy 0.000 description 1
- 208000021550 spleen neoplasm Diseases 0.000 description 1
- 230000006641 stabilisation Effects 0.000 description 1
- 238000011105 stabilization Methods 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000012916 structural analysis Methods 0.000 description 1
- 238000007920 subcutaneous administration Methods 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-N sulfuric acid group Chemical group S(O)(O)(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-N 0.000 description 1
- 239000006228 supernatant Substances 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000002194 synthesizing effect Effects 0.000 description 1
- 102000055501 telomere Human genes 0.000 description 1
- 108091035539 telomere Proteins 0.000 description 1
- 210000003411 telomere Anatomy 0.000 description 1
- 208000001608 teratocarcinoma Diseases 0.000 description 1
- 230000002381 testicular Effects 0.000 description 1
- 235000019818 tetrasodium diphosphate Nutrition 0.000 description 1
- 239000001577 tetrasodium phosphonato phosphate Substances 0.000 description 1
- RYYWUUFWQRZTIU-UHFFFAOYSA-K thiophosphate Chemical class [O-]P([O-])([O-])=S RYYWUUFWQRZTIU-UHFFFAOYSA-K 0.000 description 1
- 210000001578 tight junction Anatomy 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 108091006106 transcriptional activators Proteins 0.000 description 1
- 230000009466 transformation Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- 230000007704 transition Effects 0.000 description 1
- 238000011269 treatment regimen Methods 0.000 description 1
- HRXKRNGNAMMEHJ-UHFFFAOYSA-K trisodium citrate Chemical compound [Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O HRXKRNGNAMMEHJ-UHFFFAOYSA-K 0.000 description 1
- 229940038773 trisodium citrate Drugs 0.000 description 1
- 230000005747 tumor angiogenesis Effects 0.000 description 1
- 230000004565 tumor cell growth Effects 0.000 description 1
- 238000000539 two dimensional gel electrophoresis Methods 0.000 description 1
- 238000003160 two-hybrid assay Methods 0.000 description 1
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 241000712461 unidentified influenza virus Species 0.000 description 1
- 210000000689 upper leg Anatomy 0.000 description 1
- 230000003827 upregulation Effects 0.000 description 1
- 229940035893 uracil Drugs 0.000 description 1
- 208000010570 urinary bladder carcinoma Diseases 0.000 description 1
- 210000002700 urine Anatomy 0.000 description 1
- 210000003741 urothelium Anatomy 0.000 description 1
- 201000010653 vesiculitis Diseases 0.000 description 1
- 229920002554 vinyl polymer Polymers 0.000 description 1
- 239000013603 viral vector Substances 0.000 description 1
- 230000003612 virological effect Effects 0.000 description 1
- 238000011179 visual inspection Methods 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
- 210000005253 yeast cell Anatomy 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/30—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants from tumour cells
- C07K16/3069—Reproductive system, e.g. ovaria, uterus, testes, prostate
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
- C12Q1/6886—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material for cancer
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/30—Immunoglobulins specific features characterized by aspects of specificity or valency
- C07K2317/34—Identification of a linear epitope shorter than 20 amino acid residues or of a conformational epitope defined by amino acid residues
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
- C07K2319/30—Non-immunoglobulin-derived peptide or protein having an immunoglobulin constant or Fc region, or a fragment thereof, attached thereto
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/112—Disease subtyping, staging or classification
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/136—Screening for pharmacological compounds
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/154—Methylation markers
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/158—Expression markers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2500/00—Screening for compounds of potential therapeutic value
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Immunology (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Engineering & Computer Science (AREA)
- Biochemistry (AREA)
- Genetics & Genomics (AREA)
- Biophysics (AREA)
- Cell Biology (AREA)
- Veterinary Medicine (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Public Health (AREA)
- Pathology (AREA)
- Zoology (AREA)
- Analytical Chemistry (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Urology & Nephrology (AREA)
- Hospice & Palliative Care (AREA)
- Oncology (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Hematology (AREA)
- Microbiology (AREA)
- Wood Science & Technology (AREA)
- Physics & Mathematics (AREA)
- Reproductive Health (AREA)
- General Physics & Mathematics (AREA)
- Toxicology (AREA)
- Gynecology & Obstetrics (AREA)
- Pregnancy & Childbirth (AREA)
- Gastroenterology & Hepatology (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/455,486 US6833438B1 (en) | 1999-06-01 | 1999-12-06 | Serpentine transmembrane antigens expressed in human cancers and uses thereof |
| US09/455,486 | 1999-12-06 | ||
| PCT/US2000/033040 WO2001040276A2 (en) | 1999-12-06 | 2000-12-06 | Serpentine transmembrane antigens expressed in human prostate cancers and uses thereof |
Related Child Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2005365540A Division JP2006204290A (ja) | 1999-12-06 | 2005-12-19 | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 |
| JP2010212334A Division JP2011062201A (ja) | 1999-12-06 | 2010-09-22 | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2003517306A JP2003517306A (ja) | 2003-05-27 |
| JP4827357B2 true JP4827357B2 (ja) | 2011-11-30 |
Family
ID=23809010
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2001541031A Expired - Lifetime JP4827357B2 (ja) | 1999-12-06 | 2000-12-06 | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 |
| JP2005365540A Withdrawn JP2006204290A (ja) | 1999-12-06 | 2005-12-19 | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 |
| JP2010212334A Pending JP2011062201A (ja) | 1999-12-06 | 2010-09-22 | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2005365540A Withdrawn JP2006204290A (ja) | 1999-12-06 | 2005-12-19 | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 |
| JP2010212334A Pending JP2011062201A (ja) | 1999-12-06 | 2010-09-22 | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 |
Country Status (12)
| Country | Link |
|---|---|
| EP (2) | EP1642909A3 (enExample) |
| JP (3) | JP4827357B2 (enExample) |
| AT (1) | ATE456578T1 (enExample) |
| AU (2) | AU775366B2 (enExample) |
| CA (1) | CA2395053C (enExample) |
| CY (1) | CY1111034T1 (enExample) |
| DE (1) | DE60043784D1 (enExample) |
| DK (1) | DK1244705T3 (enExample) |
| ES (1) | ES2340249T3 (enExample) |
| IL (3) | IL150018A0 (enExample) |
| PT (1) | PT1244705E (enExample) |
| WO (1) | WO2001040276A2 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010500568A (ja) * | 2006-08-09 | 2010-01-07 | ホームステッド クリニカル コーポレイション | 器官特異的蛋白質およびその使用方法 |
Families Citing this family (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040141975A1 (en) | 1998-06-01 | 2004-07-22 | Raitano Arthur B. | Nucleic acid and corresponding protein entitled 98P4B6 useful in treatment and detection of cancer |
| US6833438B1 (en) | 1999-06-01 | 2004-12-21 | Agensys, Inc. | Serpentine transmembrane antigens expressed in human cancers and uses thereof |
| US20030149531A1 (en) | 2000-12-06 | 2003-08-07 | Hubert Rene S. | Serpentine transmembrane antigens expressed in human cancers and uses thereof |
| US6329503B1 (en) | 1998-06-01 | 2001-12-11 | Agensys, Inc. | Serpentine transmembrane antigens expressed in human cancers and uses thereof |
| US20060052321A1 (en) | 2002-04-05 | 2006-03-09 | Raitano Arthur B | Nucleic acid and corresponding protein entitled 98P4B6 useful in treatment and detection of cancer |
| US7037667B1 (en) | 1998-06-01 | 2006-05-02 | Agensys, Inc. | Tumor antigen useful in diagnosis and therapy of prostate and colon cancer |
| US20060073150A1 (en) * | 2001-09-06 | 2006-04-06 | Mary Faris | Nucleic acid and corresponding protein entitled STEAP-1 useful in treatment and detection of cancer |
| US7189565B2 (en) | 2001-03-23 | 2007-03-13 | Fahri Saatcioglu | Prostate-specific or testis-specific nucleic acid molecules, polypeptides, and diagnostic and therapeutic methods |
| US7611892B2 (en) | 2000-03-24 | 2009-11-03 | President And Fellows Of Harvard College | Prostate-specific or testis-specific nucleic acid molecules, polypeptides, and diagnostic and therapeutic methods |
| AU2001270118A1 (en) * | 2000-08-24 | 2002-03-04 | Genentech, Inc. | Compositions and methods for the diagnosis and treatment of tumor |
| US7494646B2 (en) | 2001-09-06 | 2009-02-24 | Agensys, Inc. | Antibodies and molecules derived therefrom that bind to STEAP-1 proteins |
| WO2003087306A2 (en) * | 2002-04-05 | 2003-10-23 | Agensys, Inc. | Nucleic acid and corresponding protein entitled 98p4b6 useful in treatment and detection of cancer |
| US7906620B2 (en) * | 2002-08-16 | 2011-03-15 | Yeda Research And Development Co. Ltd. | Tumor associated antigen, peptides thereof, and use of same as anti-tumor vaccines |
| JP5840351B2 (ja) | 2002-09-06 | 2016-01-06 | アジェンシス,インコーポレイテッド | 癌の処置および検出において有用な98p4b6と称される、核酸および対応タンパク質 |
| EP1605893A4 (en) * | 2003-03-05 | 2008-08-13 | Innexus Biotechnology Inc | TRANS-MEMBRANE ANTIBODY-INDUCED APOPTOSIS INHIBITION |
| NZ583292A (en) | 2003-11-06 | 2012-03-30 | Seattle Genetics Inc | Monomethylvaline compounds capable of conjugation to ligands |
| CA2557053A1 (en) * | 2004-02-19 | 2005-12-01 | Biomedisinsk Innovasjon As | Stamp2 nucleic acid molecules, polypeptides, and diagnostic and therapeutic methods |
| PT1742966E (pt) | 2004-04-22 | 2014-02-05 | Agensys Inc | Anticorpos e moléculas derivadas daí que se ligam às proteínas steap-1 |
| JP4191189B2 (ja) | 2005-12-08 | 2008-12-03 | 康生 梅津 | がん関連遺伝子活性化能を有するペプチド |
| MX2009003938A (es) | 2006-10-27 | 2009-04-24 | Genentech Inc | Anticuerpos e inmunoconjugados y sus usos. |
| JP5394246B2 (ja) * | 2007-03-30 | 2014-01-22 | ジェネンテック, インコーポレイテッド | 抗体及びイムノコンジュゲートとこれらの使用方法 |
| MY150531A (en) | 2007-07-16 | 2014-01-30 | Genentech Inc | Anti-cd79b antibodies and immunoconjugates and methods of use |
| RU2557319C2 (ru) | 2007-07-16 | 2015-07-20 | Дженентек, Инк. | ГУМАНИЗИРОВАННЫЕ АНТИТЕЛА ПРОТИВ CD79b И ИММУНОКОНЪЮГАТЫ И СПОСОБЫ ПРИМЕНЕНИЯ |
| WO2009046739A1 (en) | 2007-10-09 | 2009-04-16 | Curevac Gmbh | Composition for treating prostate cancer (pca) |
| DK2657253T3 (en) | 2008-01-31 | 2017-10-09 | Genentech Inc | Anti-CD79b antibodies and immune conjugates and methods of use |
| WO2010037408A1 (en) | 2008-09-30 | 2010-04-08 | Curevac Gmbh | Composition comprising a complexed (m)rna and a naked mrna for providing or enhancing an immunostimulatory response in a mammal and uses thereof |
| WO2014165818A2 (en) | 2013-04-05 | 2014-10-09 | T Cell Therapeutics, Inc. | Compositions and methods for preventing and treating prostate cancer |
| DK3262071T3 (da) | 2014-09-23 | 2020-06-15 | Hoffmann La Roche | Fremgangsmåde til anvendelse af anti-CD79b-immunkonjugater |
| US9879087B2 (en) | 2014-11-12 | 2018-01-30 | Siamab Therapeutics, Inc. | Glycan-interacting compounds and methods of use |
| IL278574B2 (en) | 2014-11-12 | 2024-11-01 | Seagen Inc | Glycan-interacting compounds and methods of use |
| WO2017083582A1 (en) | 2015-11-12 | 2017-05-18 | Siamab Therapeutics, Inc. | Glycan-interacting compounds and methods of use |
| AU2017331361B2 (en) * | 2016-09-23 | 2024-07-18 | Regeneron Pharmaceuticals, Inc. | Anti-STEAP2 antibodies, antibody-drug conjugates, and bispecific antigen-binding molecules that bind STEAP2 and CD3, and uses thereof |
| WO2018094143A1 (en) | 2016-11-17 | 2018-05-24 | Siamab Therapeutics, Inc. | Glycan-interacting compounds and methods of use |
| KR20240044544A (ko) | 2017-03-03 | 2024-04-04 | 씨젠 인크. | 글리칸-상호작용 화합물 및 사용 방법 |
| BR112019018767A2 (pt) | 2017-04-03 | 2020-05-05 | Hoffmann La Roche | anticorpos, molécula de ligação ao antígeno biespecífica, um ou mais polinucleotídeos isolados, um ou mais vetores, célula hospedeira, método para produzir um anticorpo, composição farmacêutica, usos, método para tratar uma doença em um indivíduo e invenção |
| WO2019125184A1 (en) | 2017-12-19 | 2019-06-27 | Auckland Uniservices Limited | Use of biomarker in cancer therapy |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994009150A1 (en) * | 1992-10-08 | 1994-04-28 | The Government Of The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Monoclonal antibodies to prostate cells |
| WO1998018489A1 (en) * | 1996-10-30 | 1998-05-07 | The Uab Research Foundation | Enhancement of tumor cell chemosensitivity and radiosensitivity using single chain intracellular antibodies |
| WO1999061469A2 (en) * | 1998-05-22 | 1999-12-02 | Incyte Pharmaceuticals, Inc. | Prostate growth-associated membrane proteins |
| WO1999062941A2 (en) * | 1998-06-01 | 1999-12-09 | Urogenesys, Inc. | Serpentine transmembrane antigens expressed in human cancers and uses thereof |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5919652A (en) | 1994-11-09 | 1999-07-06 | The Regents Of The University Of California | Nucleic acid molecules comprising the prostate specific antigen (PSA) promoter and uses thereof |
| WO2000035937A1 (en) * | 1998-12-17 | 2000-06-22 | Human Genome Sciences, Inc. | 47 human secreted proteins |
| US20020015950A1 (en) * | 1999-07-07 | 2002-02-07 | Karen Anne Jones | Atherosclerosis-associated genes |
| AU6906800A (en) * | 1999-08-17 | 2001-03-13 | Incyte Genomics, Inc. | Membrane associated proteins |
-
2000
- 2000-12-06 EP EP05014435A patent/EP1642909A3/en not_active Withdrawn
- 2000-12-06 EP EP00983938A patent/EP1244705B1/en not_active Expired - Lifetime
- 2000-12-06 WO PCT/US2000/033040 patent/WO2001040276A2/en not_active Ceased
- 2000-12-06 IL IL15001800A patent/IL150018A0/xx unknown
- 2000-12-06 JP JP2001541031A patent/JP4827357B2/ja not_active Expired - Lifetime
- 2000-12-06 AT AT00983938T patent/ATE456578T1/de active
- 2000-12-06 AU AU20629/01A patent/AU775366B2/en not_active Expired
- 2000-12-06 PT PT00983938T patent/PT1244705E/pt unknown
- 2000-12-06 ES ES00983938T patent/ES2340249T3/es not_active Expired - Lifetime
- 2000-12-06 DK DK00983938.2T patent/DK1244705T3/da active
- 2000-12-06 DE DE60043784T patent/DE60043784D1/de not_active Expired - Lifetime
- 2000-12-06 CA CA2395053A patent/CA2395053C/en not_active Expired - Lifetime
-
2002
- 2002-06-04 IL IL150018A patent/IL150018A/en active IP Right Grant
-
2004
- 2004-10-29 AU AU2004224964A patent/AU2004224964B2/en not_active Expired
-
2005
- 2005-12-19 JP JP2005365540A patent/JP2006204290A/ja not_active Withdrawn
-
2009
- 2009-06-10 IL IL199275A patent/IL199275A0/en active IP Right Grant
-
2010
- 2010-04-26 CY CY20101100373T patent/CY1111034T1/el unknown
- 2010-09-22 JP JP2010212334A patent/JP2011062201A/ja active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1994009150A1 (en) * | 1992-10-08 | 1994-04-28 | The Government Of The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Monoclonal antibodies to prostate cells |
| WO1998018489A1 (en) * | 1996-10-30 | 1998-05-07 | The Uab Research Foundation | Enhancement of tumor cell chemosensitivity and radiosensitivity using single chain intracellular antibodies |
| WO1999061469A2 (en) * | 1998-05-22 | 1999-12-02 | Incyte Pharmaceuticals, Inc. | Prostate growth-associated membrane proteins |
| WO1999062941A2 (en) * | 1998-06-01 | 1999-12-09 | Urogenesys, Inc. | Serpentine transmembrane antigens expressed in human cancers and uses thereof |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010500568A (ja) * | 2006-08-09 | 2010-01-07 | ホームステッド クリニカル コーポレイション | 器官特異的蛋白質およびその使用方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| DE60043784D1 (de) | 2010-03-18 |
| EP1244705A2 (en) | 2002-10-02 |
| CA2395053A1 (en) | 2001-06-07 |
| IL150018A0 (en) | 2002-12-01 |
| IL150018A (en) | 2011-04-28 |
| CA2395053C (en) | 2012-05-29 |
| EP1642909A2 (en) | 2006-04-05 |
| WO2001040276A2 (en) | 2001-06-07 |
| AU775366B2 (en) | 2004-07-29 |
| AU2004224964B2 (en) | 2008-10-02 |
| ES2340249T3 (es) | 2010-06-01 |
| CY1111034T1 (el) | 2015-08-05 |
| JP2006204290A (ja) | 2006-08-10 |
| EP1244705B1 (en) | 2010-01-27 |
| IL199275A0 (en) | 2011-07-31 |
| ATE456578T1 (de) | 2010-02-15 |
| EP1642909A3 (en) | 2006-04-12 |
| AU2062901A (en) | 2001-06-12 |
| JP2011062201A (ja) | 2011-03-31 |
| JP2003517306A (ja) | 2003-05-27 |
| AU2004224964A1 (en) | 2004-11-25 |
| DK1244705T3 (da) | 2010-05-31 |
| WO2001040276A3 (en) | 2002-01-10 |
| PT1244705E (pt) | 2010-05-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4827357B2 (ja) | ヒト前立腺癌において発現されるヘビ状膜貫通抗原およびその使用方法 | |
| AU2008264161B2 (en) | Serpentine transmembrane antigens expressed in human cancers and uses thereof | |
| JP4373637B2 (ja) | ヒト前立腺癌において発現されるc型レクチン膜貫通抗原およびその使用 | |
| US7834156B2 (en) | 125P5C8: tissue specific protein highly expressed in various cancers | |
| US7172898B2 (en) | 103P2D6: tissue specific protein highly expressed in various cancers | |
| US7947459B2 (en) | Serpentine transmembrane antigens expressed in human cancers and uses thereof | |
| JP4875671B2 (ja) | 前立腺ガンにおいてアップレギュレートされるgタンパク質結合レセプターおよびその使用 | |
| US20020055478A1 (en) | GTP-binding protein useful in treatment and detection of cancer | |
| JP4325943B2 (ja) | Sgp28関連分子を使用する、癌の診断および治療 | |
| JP3946044B2 (ja) | 84p2a9:前立腺癌において高度に発現される前立腺および精巣特異的タンパク質 | |
| US6924358B2 (en) | 121P1F1: a tissue specific protein highly expressed in various cancers | |
| AU2005202361A1 (en) | Serpentine transmembrane antigens expressed in human cancers and uses thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20071203 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20090319 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20091005 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20091225 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100107 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100128 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20100526 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20100824 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20100831 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100922 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20110207 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110531 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20110608 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20110808 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110809 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110831 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110913 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140922 Year of fee payment: 3 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 4827357 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |