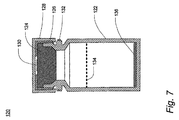
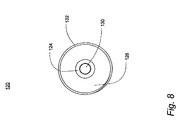
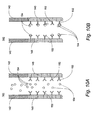
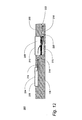
JP4804464B2 - 呼気凝縮液中のリポ多糖体を利用するグラム陰性菌性肺炎の診断 - Google Patents
呼気凝縮液中のリポ多糖体を利用するグラム陰性菌性肺炎の診断 Download PDFInfo
- Publication number
- JP4804464B2 JP4804464B2 JP2007527565A JP2007527565A JP4804464B2 JP 4804464 B2 JP4804464 B2 JP 4804464B2 JP 2007527565 A JP2007527565 A JP 2007527565A JP 2007527565 A JP2007527565 A JP 2007527565A JP 4804464 B2 JP4804464 B2 JP 4804464B2
- Authority
- JP
- Japan
- Prior art keywords
- central chamber
- condensate
- gram
- assembly
- breath condensate
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/0813—Measurement of pulmonary parameters by tracers, e.g. radioactive tracers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/083—Measuring rate of metabolism by using breath test, e.g. measuring rate of oxygen consumption
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/08—Measuring devices for evaluating the respiratory organs
- A61B5/097—Devices for facilitating collection of breath or for directing breath into or through measuring devices
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61B—DIAGNOSIS; SURGERY; IDENTIFICATION
- A61B5/00—Measuring for diagnostic purposes; Identification of persons
- A61B5/41—Detecting, measuring or recording for evaluating the immune or lymphatic systems
- A61B5/412—Detecting or monitoring sepsis
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/02—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving viable microorganisms
- C12Q1/04—Determining presence or kind of microorganism; Use of selective media for testing antibiotics or bacteriocides; Compositions containing a chemical indicator therefor
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/579—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving limulus lysate
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/92—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving lipids, e.g. cholesterol, lipoproteins, or their receptors
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2400/00—Assays, e.g. immunoassays or enzyme assays, involving carbohydrates
- G01N2400/10—Polysaccharides, i.e. having more than five saccharide radicals attached to each other by glycosidic linkages; Derivatives thereof, e.g. ethers, esters
- G01N2400/50—Lipopolysaccharides; LPS
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- General Health & Medical Sciences (AREA)
- Immunology (AREA)
- Pathology (AREA)
- Biophysics (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Animal Behavior & Ethology (AREA)
- Medical Informatics (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Pulmonology (AREA)
- Urology & Nephrology (AREA)
- Surgery (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Physiology (AREA)
- Microbiology (AREA)
- Organic Chemistry (AREA)
- Analytical Chemistry (AREA)
- Wood Science & Technology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Food Science & Technology (AREA)
- Cell Biology (AREA)
- General Physics & Mathematics (AREA)
- Zoology (AREA)
- Medicinal Chemistry (AREA)
- Obesity (AREA)
- Toxicology (AREA)
- Vascular Medicine (AREA)
- Emergency Medicine (AREA)
- Endocrinology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Engineering & Computer Science (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US57764104P | 2004-06-07 | 2004-06-07 | |
| US60/577,641 | 2004-06-07 | ||
| PCT/US2005/018232 WO2006007180A2 (en) | 2004-06-07 | 2005-05-24 | Utilizing lipopolysaccharide in exhaled breath condensate to diagnose gram negative pneumonia |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009118170A Division JP4852121B2 (ja) | 2004-06-07 | 2009-05-15 | 呼気凝縮液中のリポ多糖体を利用するグラム陰性菌性肺炎の診断 |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2008501976A JP2008501976A (ja) | 2008-01-24 |
| JP2008501976A5 JP2008501976A5 (enExample) | 2008-07-10 |
| JP4804464B2 true JP4804464B2 (ja) | 2011-11-02 |
Family
ID=35784291
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2007527565A Expired - Fee Related JP4804464B2 (ja) | 2004-06-07 | 2005-05-24 | 呼気凝縮液中のリポ多糖体を利用するグラム陰性菌性肺炎の診断 |
| JP2009118170A Expired - Fee Related JP4852121B2 (ja) | 2004-06-07 | 2009-05-15 | 呼気凝縮液中のリポ多糖体を利用するグラム陰性菌性肺炎の診断 |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009118170A Expired - Fee Related JP4852121B2 (ja) | 2004-06-07 | 2009-05-15 | 呼気凝縮液中のリポ多糖体を利用するグラム陰性菌性肺炎の診断 |
Country Status (8)
| Country | Link |
|---|---|
| EP (1) | EP1761166A4 (enExample) |
| JP (2) | JP4804464B2 (enExample) |
| AU (1) | AU2005262701B2 (enExample) |
| BR (1) | BRPI0511846A (enExample) |
| CA (1) | CA2566804A1 (enExample) |
| IL (5) | IL179137A (enExample) |
| MX (1) | MXPA06014248A (enExample) |
| WO (1) | WO2006007180A2 (enExample) |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
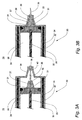
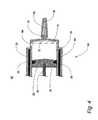
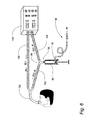
| US7547285B2 (en) | 2003-02-14 | 2009-06-16 | The Charlotte-Mecklenburg Hospital Authority | Device and method for collection of exhaled alveolar breath condensate |
| US7828741B2 (en) | 2002-12-20 | 2010-11-09 | The Charlotte-Mecklenburg Hospital Authority | Utilizing lipopolysaccharide in exhaled breath condensate to diagnose gram negative pneumonia |
| EP1571985B1 (en) | 2002-12-20 | 2009-04-01 | The Charlotte-Mecklenburg Hospital Authority | Disposable hand-held device for collection of exhaled breath condensate |
| KR20100084528A (ko) * | 2007-10-02 | 2010-07-26 | 안나-카린 올린 | 호기 입자의 수집 및 측정 |
| RU2010146772A (ru) * | 2008-06-04 | 2012-07-20 | КейСиАй Лайсензинг, Инк. (US) | Обнаружение инфекции при терапии ран пониженным давлением |
| GB201312635D0 (en) * | 2013-07-15 | 2013-08-28 | Univ Plymouth | Water testing |
| US10413216B2 (en) * | 2016-02-03 | 2019-09-17 | Quintron Instrument Company, Inc. | Breath testing apparatus |
| CN106018362B (zh) * | 2016-05-16 | 2018-06-08 | 江苏国恒检测有限公司 | 一种检测苦味酸的测定器 |
| CN108871908B9 (zh) * | 2018-09-06 | 2024-07-26 | 杭州优思达生物技术股份有限公司 | 生物样本处理装置 |
| EP4013302A1 (en) * | 2019-08-13 | 2022-06-22 | Respiration Scan Ltd | System and method for determining onset and disease progression |
| CN114449957B (zh) * | 2019-08-26 | 2023-11-24 | 泽特奥科技公司 | 利用呼出气诊断结核病和其他疾病 |
| WO2023038582A2 (en) * | 2021-09-10 | 2023-03-16 | National University Of Singapore | Exhaled breath condensate collection kit |
| EP4404848A1 (en) * | 2021-09-20 | 2024-07-31 | Vosbio, Inc. | Devices, methods and kits for biological sample capture and processing |
| AU2024283662A1 (en) * | 2023-06-05 | 2025-12-04 | Charles River Laboratories, Inc. | Recombinant amebocyte clotting factors and uses thereof |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| IL59383A (en) * | 1980-02-14 | 1983-03-31 | Technion Res & Dev Foundation | Micromethod for the determination of endotoxins with limulus amebocyte lysate |
| US4349626A (en) * | 1980-10-28 | 1982-09-14 | The Monell Chemical Senses Center | Method of detecting Pseudomonas aeruginosa infections utilizing selected ketone and/or sulfur metabolites |
| US5541057A (en) * | 1989-09-18 | 1996-07-30 | Biostar, Inc. | Methods for detection of an analyte |
| SE9704329D0 (sv) * | 1997-11-25 | 1997-11-25 | Siemens Elema Ab | Gasmätare |
| EP1139870A4 (en) * | 1998-12-18 | 2004-07-28 | Univ Virginia | DEVICE AND METHOD FOR MONITORING ASTHMA |
| EP1169640B1 (en) * | 1999-03-09 | 2005-06-15 | Osmetech PLC | Method for detecting conditions by analysis of aqueous condensate from respiratory gases |
| AU2001261091A1 (en) * | 2000-05-01 | 2001-11-12 | Respiratory Research, Inc. | Method and device for collecting and analyzing exhaled breath |
| US20040234971A1 (en) * | 2001-02-01 | 2004-11-25 | Joany Jackman | Diagnosis of pathogen infections using mass spectral analysis of immune system modulators in post-exposure biological samples |
-
2005
- 2005-05-24 AU AU2005262701A patent/AU2005262701B2/en not_active Ceased
- 2005-05-24 BR BRPI0511846-8A patent/BRPI0511846A/pt not_active IP Right Cessation
- 2005-05-24 WO PCT/US2005/018232 patent/WO2006007180A2/en not_active Ceased
- 2005-05-24 EP EP05753778A patent/EP1761166A4/en not_active Withdrawn
- 2005-05-24 CA CA002566804A patent/CA2566804A1/en not_active Abandoned
- 2005-05-24 MX MXPA06014248A patent/MXPA06014248A/es active IP Right Grant
- 2005-05-24 JP JP2007527565A patent/JP4804464B2/ja not_active Expired - Fee Related
-
2006
- 2006-11-09 IL IL179137A patent/IL179137A/en not_active IP Right Cessation
-
2009
- 2009-05-15 JP JP2009118170A patent/JP4852121B2/ja not_active Expired - Fee Related
-
2012
- 2012-02-19 IL IL218191A patent/IL218191A0/en unknown
- 2012-02-19 IL IL218189A patent/IL218189A0/en unknown
- 2012-02-19 IL IL218188A patent/IL218188A0/en unknown
- 2012-02-19 IL IL218190A patent/IL218190A0/en unknown
Also Published As
| Publication number | Publication date |
|---|---|
| IL179137A (en) | 2012-07-31 |
| BRPI0511846A (pt) | 2008-01-15 |
| WO2006007180A3 (en) | 2006-11-30 |
| IL218188A0 (en) | 2012-04-30 |
| MXPA06014248A (es) | 2007-06-25 |
| AU2005262701B2 (en) | 2011-12-01 |
| IL218191A0 (en) | 2012-04-30 |
| WO2006007180A2 (en) | 2006-01-19 |
| EP1761166A2 (en) | 2007-03-14 |
| IL179137A0 (en) | 2007-03-08 |
| CA2566804A1 (en) | 2006-01-19 |
| JP2009186487A (ja) | 2009-08-20 |
| AU2005262701A1 (en) | 2006-01-19 |
| JP4852121B2 (ja) | 2012-01-11 |
| IL218190A0 (en) | 2012-04-30 |
| IL218189A0 (en) | 2012-04-30 |
| EP1761166A4 (en) | 2009-11-11 |
| JP2008501976A (ja) | 2008-01-24 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4852121B2 (ja) | 呼気凝縮液中のリポ多糖体を利用するグラム陰性菌性肺炎の診断 | |
| US7828741B2 (en) | Utilizing lipopolysaccharide in exhaled breath condensate to diagnose gram negative pneumonia | |
| Rossi et al. | Sinus aspirates and radiographic abnormalities in severe attacks of asthma | |
| US20130190639A1 (en) | Utilizing lipopolysaccharide in exhaled breath condensate to diagnose gram negative pneumonia | |
| O’Neal et al. | Subglottic secretion viscosity and evacuation efficiency | |
| SE414410B (sv) | Medel for pavisande av neisseria gonorrheae genom dess oxidasaktivitet | |
| Smith | What diagnostic tests are needed for community-acquired pneumonia? | |
| Tobin | Diagnosis of pneumonia: techniques and problems | |
| AU2012201117A1 (en) | Utilizing lipopolysaccharide in exhaled breath condensate to diagnose gram negative pneumonia | |
| Grattan-Smith et al. | Viral supraglottitis | |
| US20090275015A1 (en) | Non-invasive respiratory rapid diagnosis method | |
| JP4711846B2 (ja) | 甚急性乳房炎の判定方法 | |
| RU2305838C1 (ru) | Способ прогнозирования возникновения повторных респираторных заболеваний у детей раннего возраста, часто и длительно болеющих орз | |
| Solijon o‘g‘li | TESTING FOR TRACHEAL INFECTIONS IN CHILDREN | |
| Carvalho et al. | Unusual case of a giant lung abscess initially misdiagnosed and treated as an empyema | |
| RU2823997C1 (ru) | Способ дифференциальной диагностики пиелонефрита и мочекаменной болезни | |
| RU2826767C1 (ru) | Способ отбора проб бронхоальвеолярного лаважа для прижизненной диагностики бронхопневмонии у телят | |
| Forsah et al. | Bilateral empyema with beta hemolytic group C streptococcus and Streptococcus constellatus co-infection resulting from an esophageal perforation and associated with septic shock, diffuse ST elevation, and new-onset atrial fibrillation | |
| Abdullaev et al. | ETIOLOGICAL, DIAGNOSTIC AND MORPHOLOGICAL FEATURES OF SEVERE PNEUMONIA (LITERATURE REVIEW) | |
| Hulsebosch et al. | Diagnostic Testing for Infectious Respiratory Tract Disease | |
| Özpınar et al. | A rare agent of empyema: Gemella morbillorum. | |
| SU1575116A1 (ru) | Способ дифференциальной диагностики саркоидоза и туберкулеза органов дыхани | |
| Ridley | Miscellaneous Laboratory Analyses/Gastric Fluid | |
| Danasu et al. | A Comparative Study to assess the efficacy of CPI Score Vs APACHE Score to predict ventilator associated Pneumonia among patients admitted in ICU at SMVMCH, Puducherry | |
| RU2535026C1 (ru) | Способ оценки активности воспалительного процесса |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080519 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20080519 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20101111 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20101119 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20110131 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20110207 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20110510 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20110722 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20110809 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140819 Year of fee payment: 3 |
|
| LAPS | Cancellation because of no payment of annual fees |