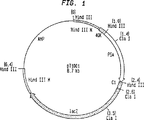
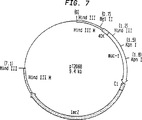
JP4475683B2 - 腫瘍関連抗原に対する免疫のための組換えポックス・ウイルス - Google Patents
腫瘍関連抗原に対する免疫のための組換えポックス・ウイルス Download PDFInfo
- Publication number
- JP4475683B2 JP4475683B2 JP50884498A JP50884498A JP4475683B2 JP 4475683 B2 JP4475683 B2 JP 4475683B2 JP 50884498 A JP50884498 A JP 50884498A JP 50884498 A JP50884498 A JP 50884498A JP 4475683 B2 JP4475683 B2 JP 4475683B2
- Authority
- JP
- Japan
- Prior art keywords
- virus
- recombinant
- gene
- vector
- cells
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Lifetime
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70503—Immunoglobulin superfamily
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2710/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA dsDNA viruses
- C12N2710/00011—Details
- C12N2710/24011—Poxviridae
- C12N2710/24041—Use of virus, viral particle or viral elements as a vector
- C12N2710/24043—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Genetics & Genomics (AREA)
- Organic Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Zoology (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Medicinal Chemistry (AREA)
- Wood Science & Technology (AREA)
- Molecular Biology (AREA)
- General Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Biochemistry (AREA)
- Biotechnology (AREA)
- Biophysics (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Virology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Plant Pathology (AREA)
- Microbiology (AREA)
- Pharmacology & Pharmacy (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Physics & Mathematics (AREA)
- Cell Biology (AREA)
- Toxicology (AREA)
- Gastroenterology & Hepatology (AREA)
- Urology & Nephrology (AREA)
- Medicines Containing Antibodies Or Antigens For Use As Internal Diagnostic Agents (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Peptides Or Proteins (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US68628096A | 1996-07-25 | 1996-07-25 | |
| US08/686,280 | 1996-07-25 | ||
| PCT/US1997/012203 WO1998004727A1 (en) | 1996-07-25 | 1997-07-15 | Recombinant pox virus for immunization against tumor-associated antigens |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009171254A Division JP2009278997A (ja) | 1996-07-25 | 2009-07-22 | 腫瘍関連抗原に対する免疫のための組換えポックス・ウイルス |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2000516459A JP2000516459A (ja) | 2000-12-12 |
| JP2000516459A5 JP2000516459A5 (enExample) | 2005-03-10 |
| JP4475683B2 true JP4475683B2 (ja) | 2010-06-09 |
Family
ID=24755674
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP50884498A Expired - Lifetime JP4475683B2 (ja) | 1996-07-25 | 1997-07-15 | 腫瘍関連抗原に対する免疫のための組換えポックス・ウイルス |
| JP2009171254A Pending JP2009278997A (ja) | 1996-07-25 | 2009-07-22 | 腫瘍関連抗原に対する免疫のための組換えポックス・ウイルス |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009171254A Pending JP2009278997A (ja) | 1996-07-25 | 2009-07-22 | 腫瘍関連抗原に対する免疫のための組換えポックス・ウイルス |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US7410644B2 (enExample) |
| EP (2) | EP2112225A1 (enExample) |
| JP (2) | JP4475683B2 (enExample) |
| AU (1) | AU731860B2 (enExample) |
| CA (1) | CA2261989C (enExample) |
| WO (1) | WO1998004727A1 (enExample) |
Families Citing this family (44)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH09504000A (ja) * | 1993-08-11 | 1997-04-22 | ジェナー テクノロジーズ | 前立腺癌ワクチン |
| US20010036928A1 (en) * | 1996-04-22 | 2001-11-01 | Chamberlain Ronald S. | Heterologous boosting immunizations |
| US6969609B1 (en) | 1998-12-09 | 2005-11-29 | The United States Of America As Represented By The Department Of Health And Human Serivces | Recombinant vector expressing multiple costimulatory molecules and uses thereof |
| US7148035B1 (en) | 1998-11-18 | 2006-12-12 | Oxford Biomedica (Uk) Limited | Polypeptide |
| EP1152060B1 (en) * | 1998-11-18 | 2015-04-15 | Oxford Biomedica (UK) Limited | 5T4 tumour-associated antigen for use in tumour immunotherapy |
| ATE361988T1 (de) * | 1998-12-09 | 2007-06-15 | Us Gov Health & Human Serv | Recombinanter vector, welcher mehrere kostimulatorische moleküle exprimiert und dessen verwenden |
| EP1865065A1 (en) * | 1998-12-09 | 2007-12-12 | The Government of the United States of America, as represented by the Secretary, Department of Health and Human Services | A recombinant vector expressing multiple costimulatory molecules and uses thereof |
| ATE369895T1 (de) * | 2002-04-09 | 2007-09-15 | Sanofi Pasteur Ltd | Modifizierte cea nucleinsäure und expressionsvektoren |
| JP2007502602A (ja) * | 2003-08-21 | 2007-02-15 | バイラックス・ディベロップメント・プロプライエタリー・リミテッド | 前立腺癌の治療用の前立腺特異的抗原をコードするポックスウィルスベクター |
| US8562970B2 (en) * | 2003-10-08 | 2013-10-22 | Sanofi Pasteur Limited | Modified CEA/B7 vector |
| AU2004289368B2 (en) | 2003-11-12 | 2010-08-26 | The Government Of The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Custom vectors for treating and preventing pancreatic cancer |
| ES2476990T3 (es) * | 2003-11-12 | 2014-07-15 | The Government Of The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Sistema para tratar y prevenir cáncer de mama |
| CN102281892A (zh) | 2009-01-13 | 2011-12-14 | 特朗斯吉有限公司 | 酿酒酵母线粒体核酸级分用于免疫刺激的用途 |
| EP2382474B1 (en) | 2009-01-20 | 2015-03-04 | Transgene SA | Soluble icam-1 as biomarker for prediction of therapeutic response |
| NZ595290A (en) | 2009-03-24 | 2012-09-28 | Transgene Sa | Biomarker for monitoring patients |
| WO2010119003A1 (en) | 2009-04-17 | 2010-10-21 | Transgene Sa | Biomarker for monitoring patients |
| CA2767458A1 (en) | 2009-07-10 | 2011-01-13 | Bruce Acres | Biomarker for selecting patients and related methods |
| AU2011285534B2 (en) | 2010-08-06 | 2015-12-17 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Biomarkers for prostate cancer and methods for their detection |
| US9725494B2 (en) * | 2013-05-17 | 2017-08-08 | United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Soluble CD27 (sCD27) and the use thereof |
| CA2928199C (en) * | 2013-11-05 | 2025-06-17 | Bavarian Nordic A/S | POLYTHERAPY FOR CANCER TREATMENT USING A POXVIRUS EXPRESSING A TUMOR ANTIGEN AND AN ANTAGONIST AND/OR AGONIST OF AN IMMUNE CHECKPOINT INHIBITOR |
| DK3142689T3 (da) | 2014-05-13 | 2021-02-15 | Bavarian Nordic As | Kombinationsbehandling til behandling af cancer med en koppevirus, som udtrykker et tumorantigen, og et monoklonalt antistof mod tim-3 |
| CN106999577B (zh) | 2014-07-16 | 2021-12-21 | 特兰斯吉恩股份有限公司 | 溶瘤病毒和免疫检查点调节因子组合 |
| WO2016128542A1 (en) | 2015-02-13 | 2016-08-18 | Transgene Sa | Immunotherapeutic vaccine and antibody combination therapy |
| ES2901468T3 (es) | 2015-07-31 | 2022-03-22 | Bavarian Nordic As | Promotores para mejorar la expresión en poxvirus |
| US20190183870A1 (en) | 2016-01-05 | 2019-06-20 | The United States Of America, As Represented By The Secretary, Dept. Of Health And Human Services | Combination of histone deacetylase inhibitor and immunotherapy |
| WO2017129769A1 (en) | 2016-01-28 | 2017-08-03 | INSERM (Institut National de la Santé et de la Recherche Médicale) | Methods for enhancing the potency of the immune checkpoint inhibitors |
| WO2017136342A1 (en) | 2016-02-02 | 2017-08-10 | The United States Of America, As Represented By The Secretary, Department Of Health And Human Services | Fulvestrant for inducing immune-mediated cytotoxic lysis of cancer cells |
| US20190134190A1 (en) | 2016-05-04 | 2019-05-09 | Transgene Sa | Combination therapy with cpg tlr9 ligand |
| US11052139B2 (en) | 2016-09-28 | 2021-07-06 | Bavarian Nordic A/S | Compositions and methods for enhancing the stability of transgenes in poxviruses |
| EP3522920A2 (en) | 2016-10-10 | 2019-08-14 | Transgene SA | Immunotherapeutic product and mdsc modulator combination therapy |
| WO2018091680A1 (en) | 2016-11-18 | 2018-05-24 | Transgene Sa | Cowpox-based oncolytic vectors |
| EP3562946B1 (en) | 2016-12-28 | 2021-11-24 | Transgene SA | Oncolytic viruses and therapeutic molecules |
| WO2018189382A1 (en) | 2017-04-14 | 2018-10-18 | Solstice Biologics, Ltd. | Immunomodulating polynucleotides, antibody conjugates thereof, and methods of their use |
| KR20200003921A (ko) | 2017-05-15 | 2020-01-10 | 얀센 백신스 앤드 프리벤션 비.브이. | 안정한 바이러스-함유 조성물 |
| KR102658198B1 (ko) | 2017-05-15 | 2024-04-16 | 얀센 백신스 앤드 프리벤션 비.브이. | 안정한 바이러스 함유 조성물 |
| WO2019020543A1 (en) | 2017-07-28 | 2019-01-31 | Transgene Sa | ONCOLYTIC VIRUSES EXPRESSING AGENTS TARGETING METABOLIC IMMUNE MODULATORS |
| KR102754876B1 (ko) | 2017-08-24 | 2025-01-13 | 버베리안 노딕 에이/에스 | 재조합 mva 및 항체의 정맥내 투여에 의한 암 치료를 위한 병용 요법 |
| WO2019170820A1 (en) | 2018-03-07 | 2019-09-12 | Transgene | Parapoxvirus vectors |
| WO2020011754A1 (en) | 2018-07-09 | 2020-01-16 | Transgene | Chimeric vaccinia viruses |
| US12150988B2 (en) | 2018-09-06 | 2024-11-26 | Bavarian Nordic A/S | Storage improved poxvirus compositions |
| US20220233607A1 (en) | 2019-05-29 | 2022-07-28 | Université De Tours | Toxoplasma platform for treating cancer |
| CN115427458A (zh) | 2020-02-28 | 2022-12-02 | 塔拉克治疗公司 | 转谷氨酰胺酶介导的缀合 |
| US20230114464A1 (en) | 2020-03-12 | 2023-04-13 | Bavarian Nordic A/S | Conditions Improving Poxvirus Stability |
| TW202413636A (zh) | 2022-08-18 | 2024-04-01 | 法商傳斯堅公司 | 嵌合痘病毒 |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4603112A (en) | 1981-12-24 | 1986-07-29 | Health Research, Incorporated | Modified vaccinia virus |
| US5174993A (en) | 1981-12-24 | 1992-12-29 | Health Research Inc. | Recombinant avipox virus and immunological use thereof |
| US5833975A (en) * | 1989-03-08 | 1998-11-10 | Virogenetics Corporation | Canarypox virus expressing cytokine and/or tumor-associated antigen DNA sequence |
| DK354487A (da) * | 1986-07-11 | 1988-01-12 | Noboru Yanaihara | Oncogen-relaterede peptider |
| US5266313A (en) | 1987-02-03 | 1993-11-30 | The United States Of America As Represented By The Department Of Health And Human Services | Raccoon poxvirus as a gene expression and vaccine vector for genes of rabies virus and other organisms |
| DE10399031I1 (de) | 1987-08-28 | 2004-01-29 | Health Research Inc | Rekombinante Viren. |
| CA1340203C (en) | 1987-09-16 | 1998-12-15 | Noboru Yanagida | Recombinant apivoxvirus |
| US5093258A (en) | 1988-08-26 | 1992-03-03 | Therion Biologics Corporation | Recombinant fowlpox virus and recombination vector |
| WO1991019803A1 (en) | 1990-06-19 | 1991-12-26 | Applied Biotechnology, Incorporated | Self assembled, defective, nonself-propagating viral particles |
| AU674492B2 (en) * | 1991-05-06 | 1997-01-02 | United States of America, as represented by the Department of Health and Human Services, The | Recombinant virus expressing carcinoembryonic antigen and methods of use thereof |
| US5445953A (en) | 1991-08-26 | 1995-08-29 | Immuno Aktiengesellschaft | Direct molecular cloning of a modified poxvirus genome |
| US5382425A (en) | 1992-01-13 | 1995-01-17 | Syntro Corporation | Recombinant swinepox virus |
| US5374149A (en) | 1993-01-13 | 1994-12-20 | Computower Technologies, Corp. | Vertical storage conveyor with symmetrical motor drive system |
| EP0784483B1 (en) * | 1994-10-03 | 2000-11-29 | THE GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES | Composition comprising a recombinant virus expressing an antigen and a recombinant virus expressing an immunostimulatory molecule |
| EP0789774A2 (en) * | 1994-10-03 | 1997-08-20 | THE GOVERNMENT OF THE UNITED STATES OF AMERICA, as represented by THE SECRETARY, DEPARTMENT OF HEALTH AND HUMAN SERVICES | Enhanced immune response by introduction of cytokine gene and/or costimulatory molecule b7 gene in a recombinant virus expressing system |
| US6001349A (en) * | 1995-02-22 | 1999-12-14 | Therion Biologics Corporation | Generation of human cytotoxic T-cells specific for carcinoma self-associated antigens and uses thereof |
| US6165460A (en) * | 1995-07-10 | 2000-12-26 | Therion Biologics Corporation | Generation of immune responses to prostate-specific antigen (PSA) |
-
1997
- 1997-07-15 AU AU37268/97A patent/AU731860B2/en not_active Ceased
- 1997-07-15 JP JP50884498A patent/JP4475683B2/ja not_active Expired - Lifetime
- 1997-07-15 WO PCT/US1997/012203 patent/WO1998004727A1/en not_active Ceased
- 1997-07-15 EP EP09010415A patent/EP2112225A1/en not_active Ceased
- 1997-07-15 EP EP97934142A patent/EP0954593A1/en not_active Withdrawn
- 1997-07-15 CA CA002261989A patent/CA2261989C/en not_active Expired - Fee Related
-
2002
- 2002-07-17 US US10/197,127 patent/US7410644B2/en not_active Expired - Fee Related
-
2009
- 2009-07-22 JP JP2009171254A patent/JP2009278997A/ja active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| JP2009278997A (ja) | 2009-12-03 |
| CA2261989A1 (en) | 1998-02-05 |
| US7410644B2 (en) | 2008-08-12 |
| EP0954593A1 (en) | 1999-11-10 |
| WO1998004727A1 (en) | 1998-02-05 |
| CA2261989C (en) | 2008-09-30 |
| AU3726897A (en) | 1998-02-20 |
| JP2000516459A (ja) | 2000-12-12 |
| AU731860B2 (en) | 2001-04-05 |
| EP2112225A1 (en) | 2009-10-28 |
| US20030003079A1 (en) | 2003-01-02 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4475683B2 (ja) | 腫瘍関連抗原に対する免疫のための組換えポックス・ウイルス | |
| US7118738B2 (en) | Recombinant pox virus for immunization against MUC1 tumor-associated antigen | |
| JP4409097B2 (ja) | 複数の共刺激分子を発現する組換えベクターおよびその利用 | |
| AU712714B2 (en) | Enhanced immune response by introduction of cytokine gene and/or costimulatory molecule B7 gene in a recombinant virus expressing system | |
| US6969609B1 (en) | Recombinant vector expressing multiple costimulatory molecules and uses thereof | |
| JP4078319B2 (ja) | 抗原を発現する組み換えウイルスと免疫刺激分子を発現する組み換えウイルスとを含む組成物 | |
| US7598225B1 (en) | Generation of immune response to prostate-specific antigen (PSA) | |
| AU718945B2 (en) | Recombinant pox virus for immunization against tumor-associated antigens | |
| CA2252406A1 (en) | Heterologous boosting immunizations | |
| US20050100558A1 (en) | Heterologous boosting immunizations | |
| AU767562B2 (en) | Recombinant pox virus for immunization against tumor-associated antigens | |
| AU2003266471A1 (en) | Recombinant pox virus for immunization against tumor-associated antigens |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20040625 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20040625 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20061003 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20061225 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20070219 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20070403 |
|
| A711 | Notification of change in applicant |
Free format text: JAPANESE INTERMEDIATE CODE: A711 Effective date: 20070724 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20080805 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20081029 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20081208 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090204 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20090324 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20090722 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20091008 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20100223 |
|
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20100309 |
|
| R150 | Certificate of patent or registration of utility model |
Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130319 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130319 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140319 Year of fee payment: 4 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| EXPY | Cancellation because of completion of term |