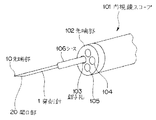
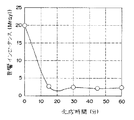
JP4074169B2 - 超音波送受信ユニット - Google Patents
超音波送受信ユニット Download PDFInfo
- Publication number
- JP4074169B2 JP4074169B2 JP2002279906A JP2002279906A JP4074169B2 JP 4074169 B2 JP4074169 B2 JP 4074169B2 JP 2002279906 A JP2002279906 A JP 2002279906A JP 2002279906 A JP2002279906 A JP 2002279906A JP 4074169 B2 JP4074169 B2 JP 4074169B2
- Authority
- JP
- Japan
- Prior art keywords
- ultrasonic
- piezoelectric
- puncture needle
- ultrasonic transducer
- needle
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 238000005452 bending Methods 0.000 claims description 33
- 230000005540 biological transmission Effects 0.000 claims description 28
- 238000006073 displacement reaction Methods 0.000 claims description 27
- 229910021426 porous silicon Inorganic materials 0.000 claims description 20
- 239000000463 material Substances 0.000 claims description 16
- 239000013078 crystal Substances 0.000 claims description 15
- 238000001514 detection method Methods 0.000 claims description 9
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 claims description 9
- 238000010276 construction Methods 0.000 claims description 3
- 239000000945 filler Substances 0.000 claims 2
- 239000010408 film Substances 0.000 description 57
- 210000001519 tissue Anatomy 0.000 description 50
- 239000011347 resin Substances 0.000 description 31
- 229920005989 resin Polymers 0.000 description 31
- 210000004027 cell Anatomy 0.000 description 22
- 238000012545 processing Methods 0.000 description 22
- 238000012986 modification Methods 0.000 description 19
- 230000004048 modification Effects 0.000 description 19
- 229910052451 lead zirconate titanate Inorganic materials 0.000 description 15
- 239000000919 ceramic Substances 0.000 description 11
- 238000000034 method Methods 0.000 description 11
- 230000015572 biosynthetic process Effects 0.000 description 10
- 230000000694 effects Effects 0.000 description 10
- 230000004044 response Effects 0.000 description 10
- 238000001228 spectrum Methods 0.000 description 8
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 7
- 201000011510 cancer Diseases 0.000 description 7
- 238000006243 chemical reaction Methods 0.000 description 7
- 229910052710 silicon Inorganic materials 0.000 description 7
- 239000010703 silicon Substances 0.000 description 7
- KRHYYFGTRYWZRS-UHFFFAOYSA-N Fluorane Chemical compound F KRHYYFGTRYWZRS-UHFFFAOYSA-N 0.000 description 6
- 238000002405 diagnostic procedure Methods 0.000 description 6
- 230000035945 sensitivity Effects 0.000 description 6
- 206010028980 Neoplasm Diseases 0.000 description 5
- 230000007423 decrease Effects 0.000 description 5
- 238000003745 diagnosis Methods 0.000 description 5
- 230000007246 mechanism Effects 0.000 description 5
- 239000000523 sample Substances 0.000 description 5
- MCMNRKCIXSYSNV-UHFFFAOYSA-N Zirconium dioxide Chemical compound O=[Zr]=O MCMNRKCIXSYSNV-UHFFFAOYSA-N 0.000 description 4
- 239000002872 contrast media Substances 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- 239000002504 physiological saline solution Substances 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- XLOMVQKBTHCTTD-UHFFFAOYSA-N Zinc monoxide Chemical compound [Zn]=O XLOMVQKBTHCTTD-UHFFFAOYSA-N 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 239000010931 gold Substances 0.000 description 3
- GQYHUHYESMUTHG-UHFFFAOYSA-N lithium niobate Chemical compound [Li+].[O-][Nb](=O)=O GQYHUHYESMUTHG-UHFFFAOYSA-N 0.000 description 3
- UKDIAJWKFXFVFG-UHFFFAOYSA-N potassium;oxido(dioxo)niobium Chemical compound [K+].[O-][Nb](=O)=O UKDIAJWKFXFVFG-UHFFFAOYSA-N 0.000 description 3
- 239000010409 thin film Substances 0.000 description 3
- 238000002604 ultrasonography Methods 0.000 description 3
- 239000004697 Polyetherimide Substances 0.000 description 2
- WVEIBSXNFJMONP-UHFFFAOYSA-N [Ta].[K] Chemical compound [Ta].[K] WVEIBSXNFJMONP-UHFFFAOYSA-N 0.000 description 2
- 239000011358 absorbing material Substances 0.000 description 2
- 230000003321 amplification Effects 0.000 description 2
- 230000017531 blood circulation Effects 0.000 description 2
- 210000004204 blood vessel Anatomy 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- 230000008878 coupling Effects 0.000 description 2
- 238000010168 coupling process Methods 0.000 description 2
- 238000005859 coupling reaction Methods 0.000 description 2
- 238000007599 discharging Methods 0.000 description 2
- 239000002961 echo contrast media Substances 0.000 description 2
- PCHJSUWPFVWCPO-UHFFFAOYSA-N gold Chemical compound [Au] PCHJSUWPFVWCPO-UHFFFAOYSA-N 0.000 description 2
- 229910052737 gold Inorganic materials 0.000 description 2
- 238000003780 insertion Methods 0.000 description 2
- 230000037431 insertion Effects 0.000 description 2
- 239000012212 insulator Substances 0.000 description 2
- 239000007788 liquid Substances 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 201000011591 microinvasive gastric cancer Diseases 0.000 description 2
- 238000003199 nucleic acid amplification method Methods 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 230000010287 polarization Effects 0.000 description 2
- 229920001601 polyetherimide Polymers 0.000 description 2
- 239000006104 solid solution Substances 0.000 description 2
- 239000000243 solution Substances 0.000 description 2
- 238000013519 translation Methods 0.000 description 2
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 238000012327 Endoscopic diagnosis Methods 0.000 description 1
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000002048 anodisation reaction Methods 0.000 description 1
- 230000002238 attenuated effect Effects 0.000 description 1
- 210000003855 cell nucleus Anatomy 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- PMHQVHHXPFUNSP-UHFFFAOYSA-M copper(1+);methylsulfanylmethane;bromide Chemical compound Br[Cu].CSC PMHQVHHXPFUNSP-UHFFFAOYSA-M 0.000 description 1
- 238000012937 correction Methods 0.000 description 1
- 238000013016 damping Methods 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 238000000151 deposition Methods 0.000 description 1
- 238000003748 differential diagnosis Methods 0.000 description 1
- 238000007598 dipping method Methods 0.000 description 1
- 238000001035 drying Methods 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 238000002674 endoscopic surgery Methods 0.000 description 1
- 230000005284 excitation Effects 0.000 description 1
- 238000011010 flushing procedure Methods 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000001027 hydrothermal synthesis Methods 0.000 description 1
- 238000005286 illumination Methods 0.000 description 1
- 230000001771 impaired effect Effects 0.000 description 1
- HFGPZNIAWCZYJU-UHFFFAOYSA-N lead zirconate titanate Chemical compound [O-2].[O-2].[O-2].[O-2].[O-2].[Ti+4].[Zr+4].[Pb+2] HFGPZNIAWCZYJU-UHFFFAOYSA-N 0.000 description 1
- 238000005259 measurement Methods 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000035515 penetration Effects 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000003825 pressing Methods 0.000 description 1
- 238000007639 printing Methods 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000002271 resection Methods 0.000 description 1
- 229910052594 sapphire Inorganic materials 0.000 description 1
- 239000010980 sapphire Substances 0.000 description 1
- 238000007650 screen-printing Methods 0.000 description 1
- 229910052814 silicon oxide Inorganic materials 0.000 description 1
- 238000003980 solgel method Methods 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 229910001220 stainless steel Inorganic materials 0.000 description 1
- 239000010935 stainless steel Substances 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000002123 temporal effect Effects 0.000 description 1
- 239000011800 void material Substances 0.000 description 1
- 239000011787 zinc oxide Substances 0.000 description 1
Images
Landscapes
- Ultra Sonic Daignosis Equipment (AREA)
- Transducers For Ultrasonic Waves (AREA)
- Apparatuses For Generation Of Mechanical Vibrations (AREA)
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002279906A JP4074169B2 (ja) | 2002-09-25 | 2002-09-25 | 超音波送受信ユニット |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2002279906A JP4074169B2 (ja) | 2002-09-25 | 2002-09-25 | 超音波送受信ユニット |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| JP2004113391A JP2004113391A (ja) | 2004-04-15 |
| JP2004113391A5 JP2004113391A5 (enExample) | 2005-11-04 |
| JP4074169B2 true JP4074169B2 (ja) | 2008-04-09 |
Family
ID=32274775
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002279906A Expired - Fee Related JP4074169B2 (ja) | 2002-09-25 | 2002-09-25 | 超音波送受信ユニット |
Country Status (1)
| Country | Link |
|---|---|
| JP (1) | JP4074169B2 (enExample) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2019006081A1 (en) * | 2017-06-30 | 2019-01-03 | Enlightenvue, Llc | ENDOSCOPY SYSTEMS AND METHODS OF USE |
| US11832798B2 (en) | 2018-09-12 | 2023-12-05 | Enlightenvue, Inc. | Direct endoluminal-and/or endovascular-illumination systems and methods of use thereof |
| US12226075B2 (en) | 2015-08-07 | 2025-02-18 | Enlightenvue, Inc. | Endoscope with variable profile tip |
Families Citing this family (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP4682360B2 (ja) * | 2005-07-05 | 2011-05-11 | 独立行政法人産業技術総合研究所 | 針一体型バイオセンサー |
| JP2014018282A (ja) * | 2012-07-13 | 2014-02-03 | Olympus Medical Systems Corp | 超音波内視鏡 |
| JP6478183B2 (ja) * | 2014-07-22 | 2019-03-06 | オムロン株式会社 | 発電装置 |
| JP7110570B2 (ja) * | 2017-09-25 | 2022-08-02 | Tdk株式会社 | 振動ユニット |
| WO2019108724A1 (en) * | 2017-11-29 | 2019-06-06 | Avent, Inc. | Needle assembly having a stylet transducer and injection capability |
| CN108456641B (zh) * | 2018-05-14 | 2023-08-15 | 苏州大学 | 卵母细胞透明带的压电超声切削系统及方法 |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS6090542A (ja) * | 1983-10-24 | 1985-05-21 | 株式会社日立製作所 | 超音波診断装置 |
| JPS6224973A (ja) * | 1985-07-19 | 1987-02-02 | オリンパス光学工業株式会社 | 微細物体操作装置 |
| AU7847487A (en) * | 1986-09-18 | 1988-02-04 | Selfridge, A.R. | Cannulation of blood vessels |
| JP3462904B2 (ja) * | 1994-07-13 | 2003-11-05 | 株式会社日立製作所 | 針状超音波探触子 |
| JP3483053B2 (ja) * | 1994-12-01 | 2004-01-06 | 株式会社日立メディコ | 針状超音波探触子およびこれを用いる超音波画像診断装置 |
| JP3490551B2 (ja) * | 1995-09-20 | 2004-01-26 | オリンパス株式会社 | 体内触診装置 |
-
2002
- 2002-09-25 JP JP2002279906A patent/JP4074169B2/ja not_active Expired - Fee Related
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US12226075B2 (en) | 2015-08-07 | 2025-02-18 | Enlightenvue, Inc. | Endoscope with variable profile tip |
| WO2019006081A1 (en) * | 2017-06-30 | 2019-01-03 | Enlightenvue, Llc | ENDOSCOPY SYSTEMS AND METHODS OF USE |
| US11812985B2 (en) | 2017-06-30 | 2023-11-14 | Enlightenvue, Inc. | Endoscopy systems and methods of use thereof |
| US11832798B2 (en) | 2018-09-12 | 2023-12-05 | Enlightenvue, Inc. | Direct endoluminal-and/or endovascular-illumination systems and methods of use thereof |
| US12262873B2 (en) | 2018-09-12 | 2025-04-01 | Enlightenvue, Inc. | Direct endoluminal- and/or endovascular-illumination systems and methods of use thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2004113391A (ja) | 2004-04-15 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5505088A (en) | Ultrasound microscope for imaging living tissues | |
| JP3594278B2 (ja) | 体腔内超音波プローブ装置 | |
| CN101011264B (zh) | 超声波探头和超声波图像取得装置 | |
| WO2006046471A1 (ja) | 静電容量型超音波振動子及びその体腔内超音波診断システム | |
| JP2010502297A (ja) | 低輪郭音響トランスデューサ組立体 | |
| CN105147332A (zh) | 基于微型压电超声传感器阵列的光声/超声双模内窥镜 | |
| CN105105791A (zh) | 一种血管内超声聚焦方法、聚焦诊断仪及聚焦换能器 | |
| JP2021191503A (ja) | 超音波診断装置、及び、超音波診断装置の作動方法 | |
| JP4074169B2 (ja) | 超音波送受信ユニット | |
| JP6885341B2 (ja) | 内視鏡下手術用手持ち器具 | |
| US8021305B2 (en) | Ultrasound probe, ultrasonograph, and ultrasonography | |
| JP5719275B2 (ja) | 超音波内視鏡システム | |
| CN109431547B (zh) | 多频面阵超声波内镜系统 | |
| JP2008073391A (ja) | 超音波診断装置 | |
| JP3844869B2 (ja) | 針状超音波探触子および超音波診断装置 | |
| JP4785102B2 (ja) | 体腔内用超音波探触子、その製造方法、及び、超音波診断システム | |
| Chen | Capacitive micromachined ultrasonic transducer arrays for minimally invasive medical ultrasound | |
| EP1698281A1 (en) | Ultrasonic probe | |
| JP4383108B2 (ja) | 超音波診断装置 | |
| JP3676453B2 (ja) | 針状超音波探触子 | |
| JP4594603B2 (ja) | 超音波診断装置 | |
| CN105167807A (zh) | 一种人体体内的超声检测方法、诊断仪及换能器 | |
| JP3929524B2 (ja) | 針状超音波探触子及びそれを用いた超音波診断装置 | |
| JP2007082629A (ja) | 超音波プローブ | |
| JP2001299748A (ja) | 超音波プローブ |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20050804 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20050804 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20071004 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20071016 |
|
| A521 | Written amendment |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20071211 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20080122 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20080124 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110201 Year of fee payment: 3 |
|
| R151 | Written notification of patent or utility model registration |
Ref document number: 4074169 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R151 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20110201 Year of fee payment: 3 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120201 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20120201 Year of fee payment: 4 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20130201 Year of fee payment: 5 |
|
| FPAY | Renewal fee payment (event date is renewal date of database) |
Free format text: PAYMENT UNTIL: 20140201 Year of fee payment: 6 |
|
| S531 | Written request for registration of change of domicile |
Free format text: JAPANESE INTERMEDIATE CODE: R313531 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| LAPS | Cancellation because of no payment of annual fees |