JP2020525215A - Artificial heart valve device - Google Patents
Artificial heart valve device Download PDFInfo
- Publication number
- JP2020525215A JP2020525215A JP2019572460A JP2019572460A JP2020525215A JP 2020525215 A JP2020525215 A JP 2020525215A JP 2019572460 A JP2019572460 A JP 2019572460A JP 2019572460 A JP2019572460 A JP 2019572460A JP 2020525215 A JP2020525215 A JP 2020525215A
- Authority
- JP
- Japan
- Prior art keywords
- valve
- inner core
- heart valve
- outer material
- recesses
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 0 *C1(CCC2C3)C2C3C*1 Chemical compound *C1(CCC2C3)C2C3C*1 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2412—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body with soft flexible valve members, e.g. tissue valves shaped like natural valves
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2409—Support rings therefor, e.g. for connecting valves to tissue
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2/00—Filters implantable into blood vessels; Prostheses, i.e. artificial substitutes or replacements for parts of the body; Appliances for connecting them with the body; Devices providing patency to, or preventing collapsing of, tubular structures of the body, e.g. stents
- A61F2/02—Prostheses implantable into the body
- A61F2/24—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body
- A61F2/2412—Heart valves ; Vascular valves, e.g. venous valves; Heart implants, e.g. passive devices for improving the function of the native valve or the heart muscle; Transmyocardial revascularisation [TMR] devices; Valves implantable in the body with soft flexible valve members, e.g. tissue valves shaped like natural valves
- A61F2/2418—Scaffolds therefor, e.g. support stents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2210/00—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2210/0014—Particular material properties of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof using shape memory or superelastic materials, e.g. nitinol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2220/00—Fixations or connections for prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2220/0008—Fixation appliances for connecting prostheses to the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/0067—Means for introducing or releasing pharmaceutical products into the body
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/0067—Means for introducing or releasing pharmaceutical products into the body
- A61F2250/0068—Means for introducing or releasing pharmaceutical products into the body the pharmaceutical product being in a reservoir
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61F—FILTERS IMPLANTABLE INTO BLOOD VESSELS; PROSTHESES; DEVICES PROVIDING PATENCY TO, OR PREVENTING COLLAPSING OF, TUBULAR STRUCTURES OF THE BODY, e.g. STENTS; ORTHOPAEDIC, NURSING OR CONTRACEPTIVE DEVICES; FOMENTATION; TREATMENT OR PROTECTION OF EYES OR EARS; BANDAGES, DRESSINGS OR ABSORBENT PADS; FIRST-AID KITS
- A61F2250/00—Special features of prostheses classified in groups A61F2/00 - A61F2/26 or A61F2/82 or A61F9/00 or A61F11/00 or subgroups thereof
- A61F2250/0058—Additional features; Implant or prostheses properties not otherwise provided for
- A61F2250/0096—Markers and sensors for detecting a position or changes of a position of an implant, e.g. RF sensors, ultrasound markers
- A61F2250/0098—Markers and sensors for detecting a position or changes of a position of an implant, e.g. RF sensors, ultrasound markers radio-opaque, e.g. radio-opaque markers
Landscapes
- Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Cardiology (AREA)
- Oral & Maxillofacial Surgery (AREA)
- Transplantation (AREA)
- Heart & Thoracic Surgery (AREA)
- Vascular Medicine (AREA)
- Life Sciences & Earth Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Prostheses (AREA)
Abstract
本技術は、天然弁輪および天然弁尖を有する人間の心臓の天然弁を治療するための、人工心臓弁デバイス、ならびに関連システムおよび方法である。一実施形態は、弁支持体と、弁支持体内の人工弁アセンブリと、上流部および下流部を有する固着部材と、備える。本デバイスは、固定フレームに連結され、かつそこから半径方向外向きに延在する、拡張部材をさらに含む。拡張部材は、複数のワイヤを含み、それらのワイヤの少なくとも一部分は、外側材料によって包囲された内核を含む。ワイヤは、外側材料の厚さの少なくとも一部分を通して延在する複数の凹部、および人工心臓弁デバイスが天然弁輪に位置付けられたときに、生体構造に送達するための、凹部内にある治療剤を含む。【選択図】図7The present technology is an artificial heart valve device, as well as related systems and methods, for treating the natural valve of the human heart with natural annulus and leaflet. One embodiment comprises a valve support, an artificial valve assembly within the valve support, and a fastening member having upstream and downstream portions. The device further includes an extension member that is connected to and extends radially outward from the fixed frame. The expansion member comprises a plurality of wires, at least a portion of those wires including an inner core surrounded by an outer material. The wire contains multiple recesses that extend through at least a portion of the thickness of the outer material, and a therapeutic agent within the recesses for delivery to the anatomy when the artificial heart valve device is positioned on the natural annulus. Including. [Selection diagram] Fig. 7
Description
関連出願の相互参照
本出願は、参照によりその全体が本明細書に組み込まれる、2017年7月6日に出願された米国特許出願番号第15/642,834号に対する優先権を主張するものである。
CROSS REFERENCE TO RELATED APPLICATIONS This application claims priority to US patent application Ser. No. 15/642,834 filed July 6, 2017, which is hereby incorporated by reference in its entirety. is there.
本出願は、(1)2014年3月14日に出願された国際特許出願第PCT/US2014/029549号、(2)2012年10月19日に出願された国際特許出願第PCT/US2012/061219号、(3)2012年10月19日に出願された国際特許出願第PCT/US2012/061215号、(4)2012年6月21日に出願された国際特許出願第PCT/US2012/043636号の主題を組み込む。本出願はまた、2017年4月18日に出願された米国特許出願番号第15/490,047号、およびこれと共に同時に出願された米国特許出願番号第15/643,011号(弁理士整理番号C00013493.USU2)の主題を組み込む。 This application is (1) International Patent Application No. PCT/US2014/029549 filed on March 14, 2014, (2) International Patent Application No. PCT/US2012/061219 filed on October 19, 2012. (3) International Patent Application No. PCT/US2012/061215 filed on October 19, 2012, (4) International Patent Application No. PCT/US2012/043636 filed on June 21, 2012 Incorporate the subject. This application is also filed on April 18, 2017, US Patent Application No. 15/490,047, and concurrently filed with it, US Patent Application No. 15/643,011 (Patent Attorney Docket No. C00013493.USU2) incorporated.
本技術は、概して、人工心臓弁デバイスに関する。具体的には、いくつかの実施形態は、天然僧帽弁の経皮的修復および/または置換のための人工僧帽弁およびデバイス、ならびに関連システムおよび方法を対象とする。 The present technology relates generally to prosthetic heart valve devices. Specifically, some embodiments are directed to prosthetic mitral valves and devices for percutaneous repair and/or replacement of native mitral valves, and related systems and methods.
心臓弁は、いくつかの条件に影響を受けることができる。例えば、僧帽弁は、僧帽弁逆流、僧帽弁脱出、および僧帽弁狭窄症に影響を受けることができる。僧帽弁逆流は、僧帽弁尖がピーク収縮圧力で並列に接合できない、心臓の障害に起因する、左室から左心房の中への血液の異常漏出である。心臓病が、心筋の拡張をしばしば引き起こし、次に、弁尖が収縮期中に接合しない程度にまで、天然僧帽弁輪を拡大するので、僧帽弁尖は、十分に接合しなくてもよい。異常逆流はまた、乳頭筋が虚血または他の条件に起因して機能的に損なわれたときに起こり得る。より具体的には、左心室が収縮期中に収縮すると、罹患乳頭筋が、弁尖の適正な閉鎖を達成するほど十分には収縮しない。 Heart valves can be affected by several conditions. For example, the mitral valve can be affected by mitral regurgitation, mitral valve prolapse, and mitral stenosis. Mitral regurgitation is an abnormal leakage of blood from the left ventricle into the left atrium due to heart failure, where the mitral leaflets cannot join in parallel at peak systolic pressure. The mitral leaflets do not need to be well joined because heart disease often causes dilation of the myocardium, which then enlarges the natural mitral annulus to the extent that the leaflets do not join during systole. .. Abnormal reflux can also occur when the papillary muscles are functionally impaired due to ischemia or other conditions. More specifically, when the left ventricle contracts during systole, the affected papillary muscles do not contract sufficiently to achieve proper closure of the leaflets.
僧帽弁脱出は、僧帽弁尖が左心房の中まで異常に突出するときの症状である。これは、僧帽弁の不規則的な挙動を引き起こし、僧帽弁逆流につながり得る。乳頭筋を僧帽弁尖(腱索)の下側に接続する腱が、伸張または裂傷し得るので、弁尖は、逸脱して、接合できない場合がある。僧帽弁狭窄症は、拡張期の左心室の充填を阻止する僧帽弁口の狭小である。 Mitral valve prolapse is a symptom when the mitral valve leaflets project abnormally into the left atrium. This causes irregular behavior of the mitral valve, which can lead to mitral regurgitation. The leaflets may deviate and fail to join because the tendons that connect the papillary muscles to the underside of the mitral valve leaflets (chordae tendineae) may stretch or tear. Mitral stenosis is the narrowing of the mitral valve orifice that prevents diastolic left ventricular filling.
僧帽弁逆流は、左心房の中へ戻って流れる血液の量を低減させるように、多くの場合、利尿剤および/または血管拡張剤を使用して治療される。弁の修復または置換のいずれかのための外科的アプローチ(開胸および血管内)はまた、僧帽弁逆流を治療するために使用されてきた。例えば、典型的な修復技法は、拡張した弁輪の部分を締めること、または切除することを伴う。締めることは、例えば、弁輪または周辺組織に略固定される、環状または略環状リングを埋め込むことを含む。他の修復手技はまた、相互に、部分的に並列に弁尖を縫合すること、または締める。 Mitral regurgitation is often treated with diuretics and/or vasodilators to reduce the amount of blood flowing back into the left atrium. Surgical approaches (thoracotomy and endovascular) for either valve repair or replacement have also been used to treat mitral regurgitation. For example, typical repair techniques involve tightening or excising portions of the expanded annulus. Tightening includes, for example, implanting an annular or generally annular ring that is generally secured to the annulus or surrounding tissue. Other repair procedures also suture or tighten the leaflets in parallel with each other, partially in parallel.
代替として、より侵襲的な手技は、機械弁または生物組織を天然僧帽弁の代わりに心臓に埋め込むことによって、弁全体自体を置換する。これらの侵襲手技は、従来、大規模な開胸術を必要とし、したがって、非常に苦痛を伴い、有意な罹患率を有し、長い回復期間を必要とする。その上、多くの修復および置換手技では、デバイスの耐久性、あるいは弁輪形成リングまたは置換弁の不適切なサイズ決定が、患者にとって付加的な問題をもたらし得る。修復手技はまた、不良または不正確に配置され縫合糸が手技の成功に影響を及ぼし得るので、高度な技術を持つ心臓外科医を必要とする。 Alternatively, a more invasive procedure replaces the entire valve by implanting a mechanical valve or biological tissue in the heart instead of the native mitral valve. These invasive procedures traditionally require extensive thoracotomy and are therefore very painful, have significant morbidity, and require a long recovery period. Moreover, in many repair and replacement procedures, device durability or improper sizing of the annuloplasty ring or replacement valve can present additional problems to the patient. Restorative procedures also require a highly skilled cardiac surgeon, as poorly or incorrectly placed sutures can affect the success of the procedure.
近年、大動脈弁置換への低侵襲アプローチが実装されている。事前に組み立てられた経皮的人工弁の実施例は、例えば、Medtronic/Corevalve Inc.(Irvine,CA,USA)からのCoreValve Revalving(登録商標)System、およびEdwards Lifesciences(Irvine,CA,USA)からのEdwards−Sapien(登録商標)Valveを含む。両方の弁システムは、拡大可能なフレームと、拡大可能なフレームに取り付けられた三葉生体弁と、を含む。大動脈弁は、実質的に対照で円形であり、筋性の円環を有する。天然生体構造に一致するために、また、三葉人工弁が、人工弁尖の適正な接合のために円形の対称性を必要とするので、大動脈用途における拡大可能なフレームは、大動脈弁輪における対称な円形形状を有する。したがって、大動脈弁生体構造が実質的に均一、対称、かつ極めて筋性であるため、大動脈弁生体構造は、置換弁を収納する拡大可能なフレームに適する。しかしながら、他の心臓弁生体構造は、均一ではなく、対称ではなく、または十分に筋性ではなく、これにより、大動脈弁置換遺贈は、他の種類の心臓弁に適切でなくてもよい。 Recently, minimally invasive approaches to aortic valve replacement have been implemented. Examples of pre-assembled percutaneous prosthetic valves are described, for example, in Medtronic/Corevalve Inc. CoreValve Reviving® Systems from (Irvine, CA, USA), and Edwards-Sapien® Valve from Edwards Lifesciences (Irvine, CA, USA). Both valve systems include an expandable frame and a trilobal biovalve attached to the expandable frame. The aortic valve is substantially symmetrical, circular and has a muscular torus. In order to match the natural anatomy, and because the trilobal prosthesis requires circular symmetry for proper coaptation of the prosthetic leaflets, an expandable frame in aortic applications has been found in the aortic annulus. It has a symmetrical circular shape. Thus, the aortic valve anatomy is suitable for an expandable frame that houses a replacement valve, as the aortic valve anatomy is substantially uniform, symmetrical, and highly muscular. However, other heart valve anatomy is not uniform, not symmetrical, or not sufficiently muscular, so that aortic valve replacement bequest may not be appropriate for other types of heart valves.
心臓の右側の三尖弁は通常3つの弁尖を有するものの、僧帽弁のように、低侵襲治療に対して類似の課題を提起する。それ故に、三尖弁疾患を治療するためのより良い補綴に対する必要性もまた存在する。 The tricuspid valve on the right side of the heart usually has three leaflets, but like the mitral valve, it presents similar challenges for minimally invasive treatment. Therefore, there is also a need for better prostheses to treat tricuspid valve disease.
現在の手技と関連付けられる困難を考慮すると、機能不全の心臓弁を治療するための単純で効果的な低侵襲デバイスおよび方法の必要性が残っている。 Given the difficulties associated with current procedures, there remains a need for simple and effective minimally invasive devices and methods for treating dysfunctional heart valves.
概説
本技術のいくつかの実施形態は、天然僧帽弁を経皮的に交換する独自の難題に対処し、かつ生体構造に治療剤を送達するのに適切である僧帽弁置換デバイスを対象とする。大動脈弁を置換することと比較して、経皮的僧帽弁置換は、経皮的僧帽弁置換を大動脈弁置換よりも有意に困難にする独特の解剖学的障害物に直面する。第1に、比較的対称かつ均一な大動脈弁と異なり、僧帽弁輪は、しばしば対称性が欠けている非平面的な鞍のような幾何学形状を伴う、非円形のD字形または腎臓のような形状を有する。僧帽弁の複雑かつ高度に可変の生体構造は、特定の患者の天然僧帽弁輪にうまく適合する僧帽弁補綴を設計することを困難にする。結果として、補綴は、血液の逆流が起こることを可能にする隙間を残すことができる、天然弁尖および/または弁輪とうまく嵌合しなくてもよい。例えば、天然僧帽弁内への円筒形弁補綴の配置は、弁周囲漏出が起こってもよい天然弁の交連領域中に間隙を残してもよい。
Overview Some embodiments of the present technology are directed to mitral valve replacement devices that address the unique challenges of percutaneously replacing the natural mitral valve and are suitable for delivering therapeutic agents to anatomy. And Compared to replacing the aortic valve, percutaneous mitral valve replacement faces unique anatomical obstacles that make percutaneous mitral valve replacement significantly more difficult than aortic valve replacement. First, unlike the relatively symmetric and uniform aortic valve, the mitral annulus is a non-circular D-shaped or renal, often with a non-planar saddle-like geometry that lacks symmetry. It has such a shape. The complex and highly variable anatomy of the mitral valve makes it difficult to design a mitral valve prosthesis that fits well into the natural mitral annulus of a particular patient. As a result, the prosthesis may not fit well with the native leaflets and/or annulus, which may leave gaps that allow blood regurgitation to occur. For example, placement of a cylindrical valve prosthesis within the native mitral valve may leave a gap in the commissural area of the native valve where perivalvular leakage may occur.
以下の図面を参照して、本開示の多くの態様をより良く理解することができる。図面中の構成要素は、必ずしも一定の縮尺で描かれておらず、代わりに、本開示の原理を明確に例証することに重点が置かれている。さらに、構成要素は、図示された構成要素が必然的に透明であることを示すためではなく、例証を明確にするためだけに、ある図中で透明として示される場合がある。参照しやすいように、本開示の全体を通して、同様または類似の構成要素または特徴を識別するために、同一の参照番号および/または文字が使用されるが、同一参照番号の使用は、部品が同一であると解釈されるべきであることを暗示しない。実際、本明細書に記載される多くの例において、同一の番号が付けられた構成要素は、構造および/または機能が別個の、異なる実施形態を指す。本明細書で提供される見出しは、便宜のためにすぎない。 Many aspects of the present disclosure can be better understood with reference to the following drawings. The components in the drawings are not necessarily drawn to scale, and instead focused on clearly illustrating the principles of the present disclosure. Furthermore, components may be shown as transparent in some figures for clarity of illustration only, not to indicate that the illustrated components are necessarily transparent. For ease of reference, the same reference numbers and/or letters are used throughout the disclosure to identify similar or similar components or features, but the use of the same reference numbers indicates that the parts are the same. Does not imply that it should be interpreted as. In fact, in many of the examples described herein, identically numbered components refer to different embodiments having separate structures and/or functions. The headings provided herein are for convenience only.
本技術のいくつかの実施形態の具体的詳細が、図1〜10を参照して以下で説明される。実施形態の多くは、天然僧帽弁の経皮的置換のための人工弁デバイス、システム、および方法に関して以下で説明されるが、本明細書で説明されるものに加えて、他の用途および他の実施形態も本技術の範囲内である。加えて、本技術のいくつかの他の実施形態は、本明細書で説明されるものとは異なる構成、構成要素、または手技を有することができる。したがって、当業者であれば、本技術が、付加的な要素を伴う他の実施形態を有することができ、または本技術が、図1〜10を参照して以下で示され、説明される特徴のうちのいくつかを伴わない他の実施形態を有することができることを理解するであろう。 Specific details of some embodiments of the present technology are described below with reference to FIGS. Many of the embodiments are described below with respect to prosthetic valve devices, systems, and methods for percutaneous replacement of a native mitral valve, although in addition to those described herein, other applications and Other embodiments are within the scope of the present technology. In addition, some other embodiments of the present technology may have different configurations, components, or procedures than those described herein. Thus, one of ordinary skill in the art may have other embodiments where the technology is with additional elements, or the technology is shown and described below with reference to FIGS. It will be appreciated that other embodiments may be provided without some of the.
本説明内の「遠位」および「近位」という用語に関して、特に指定されない限り、該用語は、オペレータおよび/または血管系または心臓内の場所を参照して、人工弁デバイスおよび/または関連送達デバイスの部分の相対位置を指すことができる。例えば、本明細書で説明される種々の人工弁デバイスを送達して位置付けるために好適な送達カテーテルを指す際に、「近位」は、デバイスのオペレータまたは血管系の中への切開により近い位置を指すことができ、「遠位」は、デバイスのオペレータからより遠位にあるか、または血管系に沿った切開からさらに遠い位置(例えば、カテーテルの端部)を指すことができる。人工心臓弁デバイスに関して、「近位」および「遠位」という用語は、血流の方向に対するデバイスの部分の場所を指すことができる。例えば、近位は、上流位置、または血液がデバイス内に流入する位置(例えば、流入領域)を指すことができ、遠位は、下流位置、または血液がデバイスから流出する位置(例えば、流出領域)を指すことができる。 With respect to the terms "distal" and "proximal" within this description, unless otherwise specified, the term refers to a location within the operator and/or vasculature or heart to the prosthetic valve device and/or associated delivery. It can refer to the relative position of parts of the device. For example, when referring to a delivery catheter suitable for delivering and positioning the various prosthetic valve devices described herein, “proximal” means a position closer to the incision of the device into the operator or vasculature. Can be referred to as “distal” and can refer to a location that is more distal from the device operator or further away from the incision along the vasculature (eg, the end of the catheter). With respect to prosthetic heart valve devices, the terms "proximal" and "distal" can refer to the location of portions of the device with respect to the direction of blood flow. For example, proximal can refer to an upstream location, or a location where blood flows into the device (eg, inflow area), and distal can refer to a downstream location, or where blood exits the device (eg, outflow area). ) Can be pointed out.
経皮的大動脈弁置換のために開発された現在の人工弁は、僧帽弁における使用に不適切である。第1に、これらのデバイスの多くは、弁輪および/または弁尖に接触するステント状構造と、人工弁との間の直接構造接続を必要とする。いくつかのデバイスでは、人工弁を支持するステント柱もまた、弁輪または他の周辺組織に接触する。これらの種類のデバイスは、弁支持体および人工弁尖に、心臓が収縮するにつれて組織および血液によって及ぼされる力を直接的に移送し、その力は、次に、その所望の円筒形状から弁支持体を歪曲する。これは、大抵の心臓置換デバイスが、生涯の何年にもわたって3つの弁尖の適正な開放および閉鎖のために人工弁の周囲で実質的に対称の円筒形支持体を必要とする、三葉弁を使用するので、懸念されている。結果として、これらのデバイスが弁輪および他の周辺組織からの運動および力を受けているとき、補綴は、圧縮および/または歪曲され、人工弁尖を機能不全にさせ得る。また、罹患僧帽弁輪は、任意の利用可能な人工大動脈弁よりもはるかに大きい。弁のサイズが増加するに伴い、弁尖への力は劇的に増加するため、人工大動脈のサイズを拡張した僧帽弁輪のサイズに単に増加させることは、劇的により厚く、より長い弁尖を必要とし、実現可能ではないであろう。 Current prosthetic valves developed for percutaneous aortic valve replacement are unsuitable for use in mitral valves. First, many of these devices require a direct structural connection between the stent-like structure that contacts the annulus and/or leaflets and the prosthetic valve. In some devices, the stent posts that support the prosthetic valve also contact the annulus or other surrounding tissue. These types of devices directly transfer the force exerted by the tissue and blood as the heart contracts to the valve support and prosthetic leaflets, which in turn, from its desired cylindrical shape. Distort the body. This is because most heart replacement devices require a substantially symmetrical cylindrical support around the prosthetic valve for proper opening and closing of the three leaflets over the years of life, It is a concern because it uses a tri-leaflet valve. As a result, when these devices are subject to motion and forces from the annulus and other surrounding tissue, the prosthesis can be compressed and/or distorted, rendering the artificial valve leaflets dysfunctional. Also, the diseased mitral valve annulus is much larger than any available prosthetic aortic valve. As the size of the valve increases dramatically, the force on the leaflets increases dramatically, so simply increasing the size of the artificial aorta to the size of the dilated mitral annulus is dramatically thicker and longer. It requires cusps and will not be feasible.
各拍動の最中にサイズを変える、その不規則で複雑な形状に加えて、僧帽弁輪は、周辺組織からの有意量の半径方向支持を欠く。人工弁を固着するのに十分な支持を提供する線維弾性組織によって完全に包囲される大動脈弁と比較して、僧帽弁は、外壁上の筋組織のみによって結合される。僧帽弁生体構造の内壁は、大動脈流出路の下部分から僧帽弁輪を分離する、薄い血管壁によって結合される。結果として、ステント補綴を拡大することによって付与される力などの僧帽弁輪への有意な半径方向力は、大動脈路の下部分の虚脱につながり得る。その上、より大きな補綴は、より多くの力を及ぼして、より大きな寸法に拡大し、それにより、僧帽弁置換用途についてこの問題を悪化させる。 In addition to its irregular and complex shape, which changes size during each beat, the mitral annulus lacks a significant amount of radial support from surrounding tissue. Compared to the aortic valve, which is completely surrounded by fibroelastic tissue, which provides sufficient support to anchor the prosthetic valve, the mitral valve is bound only by muscle tissue on the outer wall. The inner walls of the mitral valve anatomy are joined by a thin vessel wall that separates the mitral annulus from the lower portion of the aortic outflow tract. As a result, significant radial forces on the mitral valve annulus, such as those exerted by expanding the stent prosthesis, can lead to collapse of the lower portion of the aortic tract. Moreover, larger prostheses exert more force and expand to larger dimensions, thereby exacerbating this problem for mitral valve replacement applications.
左心室の腱索もまた、人工僧帽弁を展開することにおいて障害を提示し得る。大動脈弁と違って、僧帽弁は、埋め込みの間の展開カテーテルおよび交換デバイスの移動および位置を制限する左心室における弁尖の下に、迷路のような腱索を有する。結果として、天然僧帽弁輪の心室側に弁置換デバイスを展開し、位置付けし、固着することは、複雑となる。 The chordae chordae of the left ventricle may also present an obstacle in deploying a prosthetic mitral valve. Unlike the aortic valve, the mitral valve has a labyrinthine chordae below the leaflets in the left ventricle that limit the movement and position of the deployment catheter and exchange device during implantation. As a result, deploying, positioning and anchoring the valve replacement device on the ventricular side of the native mitral valve annulus is complicated.
本技術の実施形態は、僧帽弁の生体構造に関連付けられた課題に対処し、かつ局所的生体構造への治療剤の送達を提供する、僧帽弁などの身体の心臓弁を治療するためのシステム、方法、および装置を提供する。本装置および方法は、静脈または動脈を通して心臓の中へ、または心臓壁を通して挿入されたカニューレを通して、血管内送達されるカテーテルを使用する、経皮的アプローチを可能にする。例えば、装置および方法は、経中隔アプローチに特に適切であるが、心臓内の標的位置への人工置換弁の経心尖、経心房、および直接大動脈送達であり得る。加えて、本明細書で説明されるようなデバイスおよび方法の実施形態は、順行性または逆行性アプローチおよびそれらの組み合わせで心臓の弁(例えば、僧帽弁または三尖弁)にアクセスする既知の方法などの、多くの既知の手術および手技と組み合わせることができる。 Embodiments of the present technology address the challenges associated with mitral valve anatomy and provide for the delivery of therapeutic agents to local anatomy to treat a body heart valve, such as the mitral valve. Systems, methods, and devices. The present devices and methods allow a percutaneous approach using a catheter that is delivered intravascularly through a vein or artery into the heart or through a cannula inserted through the heart wall. For example, the devices and methods are particularly suitable for transseptal approaches, but may be transapical, transatrial, and direct aortic delivery of artificial replacement valves to target locations within the heart. In addition, embodiments of devices and methods as described herein are known for accessing cardiac valves (eg, mitral or tricuspid valves) with antegrade or retrograde approaches and combinations thereof. Can be combined with many known surgeries and procedures, such as
僧帽弁へのアクセス
本技術による弁置換デバイスの構造および動作をよりよく理解するために、最初にデバイスを埋め込むためのアプローチを理解してみると役に立つ。僧帽弁または他の種類の房室弁は、経皮的な様式で患者の血管系を通してアクセスすることができる。経皮的とは、典型的には、例えば、Seldinger技法を通した針アクセスの使用などの外科的切開手技または最小侵襲手技を使用して、心臓から遠隔にある血管系の場所が皮膚を通してアクセスされることを意味する。遠隔血管系に経皮的にアクセスする能力は、周知であり、特許および医学文献で説明されている。血管アクセスの点に応じて、僧帽弁へのアクセスは、順行性であり得、心房中隔を横断すること(例えば、経中隔アプローチ)による左心房の中への進入に依存し得る。代替として、僧帽弁へのアクセスは、大動脈弁を通して左心室に進入する、逆行性であり得る。僧帽弁へのアクセスは、経心尖アプローチを介してカニューレを使用して達成されてもよい。アプローチに応じて、介入ツールおよび支持カテーテル(複数可)が、本明細書で説明されるように種々の様式で、血管内で心臓まで前進させられ、標的心臓弁に隣接して位置付けられてもよい。
Access to the Mitral Valve To better understand the structure and operation of valve replacement devices according to the present technology, it is helpful to first understand the approach for implanting the device. The mitral valve or other type of atrioventricular valve can be accessed through the patient's vasculature in a percutaneous manner. Percutaneous is typically accessed through the skin at a location in the vasculature that is remote from the heart, using, for example, surgical incision procedures or minimally invasive procedures, such as the use of needle access through the Seldinger technique. Means to be done. The ability to percutaneously access remote vascular systems is well known and described in the patent and medical literature. Depending on the point of vascular access, access to the mitral valve may be antegrade and may depend on entry into the left atrium by crossing the atrial septum (eg, transseptal approach). .. Alternatively, access to the mitral valve may be retrograde, entering the left ventricle through the aortic valve. Access to the mitral valve may be achieved using a cannula via a transapical approach. Depending on the approach, the interventional tool and supporting catheter(s) may be advanced endovascularly to the heart and positioned adjacent the target heart valve in a variety of manners as described herein. Good.
図1は、弁置換デバイスを埋め込むための経中隔アプローチの段階を示す。経中隔アプローチにおいて、下大静脈IVCまたは上大静脈SVCを介して、右心房RAを通り、心房中隔IASを横断して、僧帽弁MVより上側の左心房LAの中へ、アクセスする。図1に示されるように、針2を有するカテーテル1が、下大静脈IVCから右心房RAの中へ移動する。いったんカテーテル1が心房中隔IASの前側に到達すると、針2は、例えば、左心房LAの中への卵円窩FOまたは卵円孔において、中隔を貫通するように前進する。この時点で、ガイドワイヤが針2と交換され、カテーテル1が引き出される。 FIG. 1 shows the stages of a transseptal approach for implanting a valve replacement device. In the transseptal approach, through the inferior vena cava IVC or superior vena cava SVC, through the right atrium RA, across the atrial septum IAS, and into the left atrium LA above the mitral valve MV. .. As shown in FIG. 1, a catheter 1 with a needle 2 is moved from the inferior vena cava IVC into the right atrium RA. Once the catheter 1 reaches the anterior side of the atrial septum IAS, the needle 2 is advanced through the septum, for example at the fossa ovalis or foramen ovale into the left atrium LA. At this point the guide wire is replaced with the needle 2 and the catheter 1 is withdrawn.
図2は、ガイドワイヤ6およびガイドカテーテル4が心房中隔IASを通過する、経中隔アプローチの後の段階を示す。ガイドカテーテル4は、本技術による弁置換デバイスを埋め込むための僧帽弁へのアクセスを提供する。
FIG. 2 shows the latter stage of the transseptal approach, where the guidewire 6 and guide
代替的な順行性アプローチ(図示せず)では、好ましくは肋骨を除去することなく、肋間切開を通して、外科的アクセスが得られてもよく、小さい穿孔または切開が左心房壁に加えられてもよい。次いで、ガイドカテーテルが、この穿孔または切開を通過して左心房の中へ直接入り、巾着縫合糸によって密閉される。 An alternative antegrade approach (not shown) may provide surgical access through an intercostal incision, preferably without removing the ribs, even if a small perforation or incision is made in the left atrial wall. Good. The guide catheter is then passed through this perforation or incision directly into the left atrium and sealed with a purse string suture.
上で説明されるような僧帽弁への順行性または経中隔アプローチは、多くの点で有利であり得る。例えば、順行性アプローチは、通常、ガイドカテーテルおよび/または人工弁デバイスのより正確かつ効果的なセンタリングおよび安定化を可能にするであろう。順行性アプローチはまた、カテーテルおよび他の介入ツールを用いて腱索または他の弁下構造を損傷するリスクを低減させ得る。加えて、順行性アプローチは、逆行性アプローチの場合のような大動脈弁の横断と関連付けられるリスクを減少させ得る。これは、全くまたは実質的な損傷のリスクを伴わずには横断することができない、人工大動脈弁を持つ患者に特に関係があり得る。 An antegrade or transseptal approach to the mitral valve as described above can be advantageous in many ways. For example, the antegrade approach will typically allow more accurate and effective centering and stabilization of the guide catheter and/or prosthetic valve device. The antegrade approach may also reduce the risk of damaging chordae chordae or other subvalvular structures with catheters and other interventional tools. In addition, the antegrade approach may reduce the risk associated with traversing the aortic valve as in the retrograde approach. This may be particularly relevant for patients with prosthetic aortic valves that cannot be traversed without any or substantial risk of injury.
図3および4は、僧帽弁にアクセスする逆行性アプローチの例を示す。僧帽弁MVへのアクセスは、大動脈弓AAから、大動脈弁AVを横断して、僧帽弁MVより下側の左心室LVの中へ達成されてもよい。大動脈弓AAは、従来の大腿動脈アクセス経路を通して、または上腕動脈、腋窩動脈、橈骨動脈、もしくは頸動脈を介したより直接的なアプローチを通してアクセスされてもよい。そのようなアクセスは、ガイドワイヤ6の使用により達成されてもよい。いったん定位置になると、ガイドカテーテル4は、ガイドワイヤ6上で追跡されてもよい。代替として、好ましくは、肋骨を除去することなく肋間で、大動脈自体の穿孔を通してガイドカテーテルを配置し、胸部の切開を通して、外科的アプローチがとられてもよい。ガイドカテーテル4は、本明細書でさらに詳細に説明されるように、人工弁デバイスの配置を可能にするように後続のアクセスを提供する。逆行性アプローチは、有利なことに経中隔穿孔を必要としない。心臓専門医はまた、より一般的には、逆行性アプローチを使用しており、したがって、逆行性アプローチは、よく知られている。
3 and 4 show an example of a retrograde approach to access the mitral valve. Access to the mitral valve MV may be achieved from the aortic arch AA, across the aortic valve AV, and into the left ventricle LV below the mitral valve MV. The aortic arch AA may be accessed through the conventional femoral artery access route or through a more direct approach through the brachial, axillary, radial, or carotid arteries. Such access may be achieved through the use of guidewire 6. Once in place, the
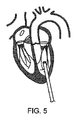
図5は、経心尖穿孔を介して経心尖アプローチを示す。このアプローチでは、心臓へのアクセスは、従来の開胸術または胸骨切開術、あるいはより小さい肋間または剣状突起下切開または穿孔であり得る、胸部切開を介する。次いで、アクセスカニューレが、心尖における、またはその付近の左心室の壁の中の、穿孔を通して配置される。次いで、本発明のカテーテルおよび人工装具は、このアクセスカニューレを通して左心室に導入されてもよい。経心尖アプローチは、僧帽または大動脈弁へのより短く真っ直ぐな直線経路を提供する。さらに、それが血管内アクセスを伴わないため、経心尖アプローチは、他の経皮的アプローチで必要とされるカテーテル法を行うために介入心臓学の訓練を必要としない。 FIG. 5 shows a transapical approach via transapical perforation. In this approach, access to the heart is through a thoracotomy, which may be a conventional thoracotomy or sternotomy, or a smaller intercostal or subxiphoid incision or perforation. An access cannula is then placed through the perforation in the wall of the left ventricle at or near the apex of the heart. The catheter and prosthesis of the present invention may then be introduced into the left ventricle through this access cannula. The transapical approach provides a shorter, straighter straight path to the mitral or aortic valve. Furthermore, because it does not involve intravascular access, the transapical approach does not require interventional cardiology training to perform the catheterization required by other percutaneous approaches.
人工心臓弁デバイスおよび方法の選択された実施形態
本技術の実施形態は、心臓の弁のうちの1つ以上を治療することができ、特にいくつかの実施形態は、有利なことに僧帽弁を治療する。本技術の人工弁デバイスはまた、患者の心臓内の他の弁(例えば、二尖または三尖弁)の置換に好適であり得る。本技術の実施形態による、人工心臓弁デバイスの実施例が、図6A〜10を参照してこの節で説明される。図6A〜10を参照して説明される実施形態の特定の要素、下部構造、利点、用途、および/または他の特徴は、相互と好適に入れ替え、置換し、または別様に構成できる。さらに、図6A〜10を参照して説明される実施形態の好適な要素は、独立型および/または内蔵型デバイスとして使用することができる。
Selected Embodiments of Prosthetic Heart Valve Devices and Methods Embodiments of the present technology may treat one or more of the valves of the heart, and in particular, some embodiments may advantageously be a mitral valve. To treat. The prosthetic valve device of the present technology may also be suitable for replacement of other valves (eg, bicuspid or tricuspid valves) in the patient's heart. Examples of prosthetic heart valve devices according to embodiments of the present technology are described in this section with reference to FIGS. 6A-10. Certain elements, substructures, advantages, applications, and/or other features of the embodiments described with reference to FIGS. 6A-10 may be suitably interchanged, substituted, or otherwise configured with each other. Moreover, suitable elements of the embodiments described with reference to Figures 6A-10 can be used as stand-alone and/or self-contained devices.
図6Aは、本技術の実施形態による人工心臓弁デバイス(「デバイス」)100の、側面断面図であり、図6Bは、平面図である。デバイス100は、弁支持体110と、弁支持体110に取り付けられた固着部材120と、弁支持体110内の人工弁アセンブリ150と、を含む。図6Aを参照すると、弁支持体110は、流入領域112および流出領域114を有する。人工弁アセンブリ150は、弁支持体110の中に配列されて、血液が流入領域112から流出領域114(矢印BF)まで流れることを可能にするが、血液が流出領域114から流入領域112までの方向に流れることを防止する。
6A is a side cross-sectional view and FIG. 6B is a plan view of a prosthetic heart valve device (“device”) 100 according to an embodiment of the present technology. The
図6Aに示される実施形態において、固着部材120は、弁支持体110の流出領域114に取り付けられた基部122と、基部122から外向きに横方向に突出する複数の腕部124と、を含む。固着部材120はまた、腕部124から延在する固定構造130を含む。固定構造130は、第1の部分132および第2の部分134を含むことができる。固定構造130の第1の部分132は、例えば、固定構造130の上流領域であり得、固定構造130は、図6Aに示されるような展開構成において、間隙Gによって弁支持体110の流入領域112から外向きに横方向に離間される。固定構造130の第2の部分134は、固定構造130の最下流部であり得る。固定構造130は、円筒形リング(例えば、直線状円筒、または円錐形)であり得、固定構造130の外面は、天然弁輪に対して外向きに押し付けるように構成されている環状係合表面を画定することができる。固定構造130は、外向きに半径方向に突出し、かつ上流方向に向かって傾斜する、複数の固定要素136をさらに含むことができる。固定要素136は、例えば、鉤、フック、または上流方向(例えば、デバイス100の下流部から離れて延在する方向)にのみ傾斜している他の要素であり得る。
In the embodiment shown in FIG. 6A, the anchoring
さらに図6Aを参照すると、固着部材120は、腕部124と固定構造130との間の滑らかな屈曲140を有する。例えば、固定構造130の第2の部分134は、滑らかな屈曲140で腕部124から延在する。滑らかな屈曲140が連続的支柱の屈曲部分であるように、腕部124および固定構造130は、連続的支柱または支持要素から一体的に形成され得る。他の実施形態において、滑らかな屈曲140は、腕部124または固定構造130のどちらかに関する別個の構成要素であり得る。例えば、滑らかな屈曲140は、滑らかな接続を形成する溶接、接着剤、または他の技術を使用して腕部124および/または固定構造130に取り付けられ得る。デバイス100が少なくとも部分的に展開されたあと、デバイス100がカプセルまたは他の容器内で再捕捉され得るように、滑らかな屈曲140は構成されている。
Still referring to FIG. 6A, the anchoring
デバイス100は、弁支持体110上の第1の封止部材162と、固着部材120上の第2の封止部材164と、をさらに含むことができる。第1の封止部材162および第2の封止部材164は、Dacron(登録商標)または別の種類の高分子材料などの可撓性材料から作製することができる。第1の封止部材162は、弁支持体110の内部表面および/または外部表面を被覆することができる。図6Aに示される実施形態において、第1の封止部材162は、弁支持体110の内部表面に取り付けられ、人工弁アセンブリ150は、第1の封止部材162、および弁支持体110の交連部分に取り付けられる。第2の封止部材164は、固着部材120の内部表面に取り付けられる。結果として、固定構造130の外側環状係合表面が、天然弁輪の組織に直接的に接触するように、固定構造130の外側環状係合表面は、第2の封止部材164によって被覆されない。
The
デバイス100は、拡張部材170をさらに含むことができる。拡張部材170は、第2の封止部材164の拡張部分である得るか、またはそれは、固定構造130の第2の封止部材164および/または第1の部分132に取り付けられた別個の構成要素であり得る。拡張部材170は、図6Aに示される展開状態において、固定構造130の第1の部分132に対して屈曲する可撓性部材であり得る。動作中、デバイスが所望の高度に位置し、天然弁輪に対して中心に置かれるように、拡張部材170は、埋め込みの間、デバイス100を滑動する。下記のように、拡張部材170は、埋め込みの間、視覚化され得、および/または治療剤を送達するように構成されている、金属ワイヤまたは他の構造などの支持部材を含むことができる。
The
図7Aは、デバイス100の例の頂部等角図である。この実施形態において、弁支持体110は、第1のフレーム(例えば、内側フレーム)を画定し、固着部材120の固定構造130は、各々が複数の構造要素を含む第2のフレーム(例えば、外側フレーム)を画定する。固定構造130は、より具体的には、図7Aに示されるように自由にかつ完全に拡大されるとき、少なくとも実質的に円筒形のリングを一緒に形成する、ダイヤモンド形セル138内に配列された構造要素137を含む。構造要素137は、自己拡大できる、またはバルーンもしくは他の種類の機械的拡大器によって拡大できる、金属、ポリマー、または他の好適な材料から形成される支柱または他の構造特徴であり得る。
FIG. 7A is a top isometric view of an example of
固定構造130のいくつかの実施形態は、外向き係合表面を有する概ね円筒形の固定リングであり得る。例えば、図7Aに示される実施形態において、構造要素137の外表面は、展開状態の天然弁輪に対して外向きに押し付けるように構成されている環状係合表面を画定する。何らの制限もない完全拡大状態において、固定構造130は、弁支持体110と実質的に少なくとも平行である。しかしながら、固定構造130は、それが心臓弁の天然弁輪の内部表面に対して半径方向に外向きに押し付けるとき、展開状態に内向き(矢印I)に屈曲することができる。
Some embodiments of the locking
図7Aに示されるデバイス100の実施形態は、弁支持体110の内部表面に沿って並ぶ第1の封止部材162と、固定構造130の内部表面に沿った第2の封止部材164と、を含む。拡張部材170は、可撓性ウェブ172(例えば、織物)と、可撓性ウェブ172に取り付けられた複数の撚り線180(例えば、ワイヤ、支柱、ケーブル、リボン、繊維など)を備える支持部材174と、を有する。可撓性ウェブ172は、固定構造130と支持部材174との金属同士の接続なしで、第2の封止部材164から延在することができる。例えば、拡張部材170は、第2の封止部材164の材料の延長であり得る。拡張部材170のいくつかの実施形態は、これにより、固定構造130に関して容易に屈曲することができる柔らかい構造である。
The embodiment of the
図7Bは、図7Aに示される支持部材174の撚り線180のうちの1つの一部分の拡大図であり、図7Cは、7C−7C線に沿って切り取った、図7Bに示される撚り線180の断面図である。図7Bおよび7Cを一緒に参照すると、撚り線180の少なくとも一部分は、厚さtを有する外側材料184によって包囲された内核186を含むことができる。外側材料184は、第1の材料であり得、内核186は、第1の材料とは異なる第2の材料であり得る。例えば、いくつかの実施形態において、撚り線180の少なくとも一部分は、撚り線180が、異種材料の各々の所望の物理的および機械的特質を含むような、少なくとも2つの異種材料から形成される引き抜き充填管(「DFTのもの」)である。図7A〜7Cに示される実施形態において、外側材料184は、撚り線180に構造支持体を提供する超弾性合金(例えば、ニチノール、コバルト・クロム、MP35N(登録商標)など)であり、内核186は、支持部材174の可視化を改善し、および/または撚り線180に追加の構造支持体を提供する、X線不透過性材料(例えば、プラチナ、タンタル、タングステン、パラジウム、金、銀など)である。
7B is an enlarged view of a portion of one of the
ある特定の実施形態において、撚り線180は、外側材料184と内核186との間に位置付けられた任意の中間材料185(図7C、破線で示される)をさらに含む。そのような実施形態において、内核材料および外側材料184は同一であってもよく、中間材料185は異なる材料であってもよい。例えば、外側材料184および内核材料は第1の材料であってもよく、中間材料185は。第1の材料とは異なる第2の材料であってもよい。例えば、外側材料184および内核材料186は、超弾性合金(例えば、ニチノール、コバルト・クロムなど)であってもよく、中間材料185は、X線不透過性材料(例えば、プラチナ、タンタル、パラジウム、金など)であってもよい。他の実施形態において、外側材料184、内核材料、および中間材料185は、異なる材料である。さらに他の実施形態において、撚り線180は、材料の4つ以上の層を含んでもよい。
In certain embodiments, the stranded
図7Bおよび7Cに示されるように、撚り線180の少なくとも一部分は、外側材料184の厚さtの少なくとも一部分を通して延在する複数の凹部182と、凹部182内の1つ以上の治療剤188と、を含む。いくつかの実施形態において、内核186の上面が凹部182を通して露出されるように、凹部182のうちの1つ以上は、外側材料184の全体厚さtを通して延在する深度を有してもよい。ある特定の実施形態において、凹部182が、内核186内の深度で終端するように、凹部182のうちの1つ以上は、外側材料184の全体厚さおよび内核186の厚さの一部分を通して延在してもよい。前述の実施形態のどちらにおいても、治療剤188は、内核186上に位置付けられてもよく、あるいは、内核186に治療剤188を含浸してもよい。他の実施形態において、内核186が露出されず、かつ凹部182が、外側材料184内の中間深度で終端するように、凹部182は、外側材料184の厚さtの一部分のみを通して延在する。そのような実施形態において、治療剤188は、外側材料184の露出した内部表面上に位置付けられてもよい。追加として、撚り線180は、外側材料184および/または内核186内の異なる深さに延在する凹部を含んでもよい。その上、中間材料185を含むそれらの実施形態において、撚り線180は、外層を通して延在し、かつ中間材料185を露出する1つ以上の第1の凹部と、外側材料184および中間材料185を通して延在し、かつ内核186を露出する1つ以上の第2の凹部と、を含んでもよい。いくつかの実施形態において、凹部182は、撚り線180の完全な断面寸法(例えば、直径)を通過する貫通孔である。前述の実施形態のいずれにおいてでも、治療剤188は、凹部182の全部または一部分を占めてもよい。
As shown in FIGS. 7B and 7C, at least a portion of the stranded
図7A〜7Cに示される凹部182が円形断面形状を有するが、他の実施形態において、凹部のうちの1つ以上は、他の好適な断面形状(例えば、正方形、卵形、長方形など)を有してもよい。同様に、凹部182のうちの1つ以上は、同じ断面積または異なる断面積(異なる深度の凹部において)を有することができる。例えば、凹部182のうちの1つ以上は、凹部の底部表面に向かって漸減する断面積を有してもよい。また、凹部182のうちの少なくともいくつかは、撚り線に沿って同じ周囲位置に位置付けられてもよく、および/または、凹部182のうちの少なくともいくつかは、異なる周囲位置を有してもよい。その上、いくつかの実施形態において、複数の凹部は、撚り線180に沿って同じ軸方向位置に位置付けられてもよい。他の実施形態において、凹部182の全ては、撚り線に沿って異なる軸方向位置に位置付けられる。
Although the
好適な治療剤188は、弁輪において炎症を低減させ、かつ拡張部材170と弁尖および/または心房床のうちの1つ以上との間の内方成長を促進する、1つ以上の薬および/または生物活性剤を含んでもよい。よって、1つ以上の治療剤188の組み込みは、人工心臓弁デバイスの耐性および組み込みを増加し、ならびに/または弁傍の漏出を低減することができる。好適な抗炎症薬の例としては、パクリタキセル、シロリムスなどが挙げられる。生物活性剤の例としては、血小板由来成長因子(「PDGF」)、線維芽細胞成長因子(「FGF」)、変異成長因子(「TGF」)、ならびに内皮細胞、平滑筋細胞、および線維芽細胞の成長を刺激する他の好適な分裂促進因子などの1つ以上の成長因子が挙げられる。
A suitable
いくつかの実施形態において、治療剤188は、所望の時間にわたって、制御された送達速度を有するように構成されてもよい。例えば、治療剤188は、送達ビヒクル(例えば、治療剤188の徐放性のための生体再吸収性ポリマーブレンドなど)と関連付けられ(例えば、送達ビヒクルによって封入され、送達ビヒクルに結合され)てもよい。ある特定の実施形態において、ウェブに、生物活性剤または生物活性剤および送達ビヒクルを含浸して、生物活性剤の解放の速度を制御してもよい。
In some embodiments,
図7Aに図示される実施形態が、十字の蛇状パターン(例えば、ジグザグパターン、菱形パターン)を形成する、撚り線180の略同軸配列を図示する一方で、1つ以上の撚り線180の他の配列およびパターン(例えば、蛇状、波状、半径方向アーム、正方形など)もまた、拡張部材170に所望の剛性を提供するように形成することができる。その上、図7Aに示される支持部材174が固定構造130から分離しているが、他の実施形態において、支持部材174は固定構造130と一体である。加えて、支持部材174、弁支持体110、および/または固着部材120は、上述のように、材料、合金、支柱、ワイヤ、撚り線の種類、および/または撚り線の寸法の混合物を含んでもよい。例えば、いくつかの実施形態において、構造、支柱、または撚り線の一部分は、DFTのものでもよく、構造、支柱、または撚り線の別の部分は、ポリマー繊維および/または金属ワイヤでできていてもよい。特定の実施形態において、拡張部材は、織物のヘム内に、または環状ウェブの周囲に垂直なストリップとして、環状織物ウェブおよび編組超弾性材料(例えば、ニチノール)を含む。本明細書に開示される実施形態のいずれにおいても、拡張部材は、プラチナ含浸織物を含み、および/または織物上に堆積された薄膜超弾性材料(例えば、ニチノール)を含んでもよい。加えて、本明細書に開示される構造部材のいずれも、1つ以上のレーザ切断ニチノールストリップ(例えば、直線状ストリップ、曲線ストリップなど)を含んでもよい。いくつかの実施形態において、構造部材、支持部材174、弁支持体110、および/または固着部材120のうちの1つ以上の部分は、X線不透過性のマーカーを用いてリベットで留められた1つ以上の孔を有してもよい。
The embodiment illustrated in FIG. 7A illustrates a generally coaxial arrangement of
図8Aは、展開構成の人工心臓弁デバイス200(「デバイス200」)の別の実施形態の概略上面図であり、図8Bは、送達カテーテルC内腔内に位置付けられた送達構成のデバイス200の概略端面図である。図8C〜8Eは、デバイス200が展開している(図示のしやすさのために図8C〜8Eに示されない送達カテーテルC)とき、8C−8E線(図8B)に沿って切り取られた、デバイス200の断面図である。デバイス200は、図6A〜7Cにおいて、デバイス100に構造および機能が概ね類似するいくつかの構成要素を含んでもよい。例えば、デバイス200は、弁支持体110(図8A)、固着部材120(図8A)、人工弁アセンブリ150(図8A)、および第2の封止部材164(図8A〜8Eにおいて視認可能ではない)を含むことができ、それらの全ては、図6A〜7Cを参照して上述のしたものに概ね類似している。よって、共通の動作および構造ならびに/または下部構造は、同じ照合番号によって識別され、動作および構造における有意な差のみを後述する。
8A is a schematic top view of another embodiment of a prosthetic heart valve device 200 (“
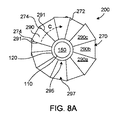
図8Aを参照すると、デバイス200は、その周囲に複数の個別織物セグメント290を有する(1つのセグメント290のみに図示のしやすさのために図8にラベル付けした)拡張部材270を含み、各個別織物セグメント290は、固着部材120における第1の端部295と、固着部材120と離間している第2の端部297と、対向する縁部291の間に延在する幅と、を有する。いくつかの実施形態において、縁部291が互いに対して自由に摺動するように、隣接したセグメント290は、可撓性部分292(図9Aおよび9Bを参照)によってそれらのそれぞれの縁部291に沿って連結されてもよい。
Referring to FIG. 8A,
図9Aおよび9Bは、それぞれ重複および延長構成に示される、3つの隣接した絶縁セグメント(それぞれ、「第1〜3のセグメント290a〜c」と称される)の端面図である。重複構成(図9A)において、第1のセグメント290aの縁部291aおよび第3のセグメント290cの縁部291cは、第2のセグメント290bの隣接した縁部291bに重複する。包囲する生体構造によって拡張部材270に及ぼされる1つ以上の力に応答して、第1〜第3のセグメント290a〜cの一部分は、互いから離れて円周方向に反対側の方向(図9Aにおいて、矢印A1によって指示される)に移動してもよく、これによって、縁部291aおよび291cは、円周方向Cに縁部291bに対して摺動する。及ぼされた力に応じて、縁部291aおよび291cは、対応する隣接した縁部291bを越えて摺動してもよく、これによって、第1〜第3のセグメント290a〜cは、縁部291aおよび291cが、拡張部材270の周囲Cに沿って、対応する隣接した縁部291bから離間されている、延長構成(図9B)をしている。いくつかの実施形態において、縁部291aおよび291cの各々は、可撓性部分292の長さ以下だけ、対応する隣接した縁部291bから離間されてもよい。第1〜第3のセグメント290a〜cが、延長構成をしていて、1つ以上の力が、包囲する生体構造によって拡張部材270に及ぼされるとき、第1〜第3のセグメント290a〜cのうちの少なくとも一部分は、互いに円周方向に反対側の方向(図9B中の矢A2によって指示される)に移動してもよい。
9A and 9B are end views of three adjacent insulating segments (referred to as "first to
いくつかの実施形態において、拡張部材270および/またはセグメント290は、他の構成を有することができる。例えば、図9Cおよび9Dは、それぞれ重複および延長構成に示される、本技術の別の実施形態によって構成されている拡張部材270’の3つの絶縁セグメント290a〜290c(集合的に「セグメント290」)の端面図である。同様に、図9Eおよび9Fは、それぞれ重複および延長構成に示される、本技術によって構成されている別の拡張部材270’’の3つの絶縁セグメント290a〜290c(集合的に「セグメント290」)の端面図である。図9C〜9Fに示されるようないくつかの実施形態において、セグメント290は、可撓性部分292によってそれらのそれぞれの縁部291に沿って連結されない。デバイス200が弁輪の中に位置付けられるとき、複数のセグメント290は、互いに対して移動して、弁輪の自然幾何形状に適合し、および/または、必要に応じてそれらの相対位置を調節してもよい一方で、拡張部材270は、局所的心臓生体構造で封止する。
In some embodiments,
いくつかの実施形態において、個々のセグメント290は、それらの個々の縁部のうちの少なくとも一部分に沿って延在し、かつ/またはその一部分を画定する支持部材274(例えば、金属またはポリマー撚り線)を含み、可撓性ウェブ272(例えば、織物)は、各セグメント290の支持部材274の間に延在してもよい。そのような実施形態において、可撓性部分292は、隣接したセグメント290の縁部291の間に延在する別個の可撓性ウェブを備えてもよい。他の実施形態において、拡張部材270および/または個々のセグメント290は、他の好適な構成を有してもよい。例えば、いくつかの実施形態において、個々のセグメント290は、固定構造130から外向きに半径方向に延在する支持部材274(例えば、金属またはポリマー撚り線)を含み、個々のセグメント290は、各支持部材274から延在する可撓性ウェブ272(例えば、織物)によって被覆された少なくとも1つの支持部材を有する。いくつかの実施形態において、個々の各セグメント290の可撓性ウェブ272の少なくとも一部分は、隣接した個々のセグメント部材290の可撓性ウェブ272の少なくとも一部分に重複する。いくつかの実施形態において、可撓性ウェブ272は、各セグメント290の支持部材274の間に延在する。いくつかの実施形態において、拡張部材270は、固定構造130の周りで連続的である可撓性ウェブ272を備える。例えば、一実施形態において、可撓性ウェブ272は、個々の支持部材290を1つ以上のポケットの中で自由に移動または摺動することができる1つ以上のポケットを備えてもよい。一実施形態において、可撓性ウェブポケットの数は、個々の支持部材の数に等しい。代替的実施形態において、ポケットの数は、個々の支持部材の数よりも少ない。例えば、一実施形態において、個々の支持部材の全ては、単一のポケットの中に存在する。
In some embodiments,
図10は、本技術の別の実施形態による、人工心臓弁デバイス300の概略等角図である。デバイス300は、図6A〜7Cにおいて、デバイス100に構造および機能が概ね類似するいくつかの構成要素を含んでもよい。例えば、デバイス300は、弁支持体110、固着部材120、人工弁アセンブリ150、および第2の封止部材164を含むことができ、それらの全ては、図6A〜7Cを参照して上述のしたものに概ね類似している。よって、共通の動作および構造ならびに/または下部構造は、同じ照合番号によって識別され、動作および構造における有意な差のみを後述する。
FIG. 10 is a schematic isometric view of a prosthetic
図10に示される実施形態において、拡張部材370は、固着部材120から半径方向外向きに延在する第1の環状部分370aと、第1の環状部分370aから半径方向外向きに延在する第2の環状部分370bと、を含む。第1の環状部分370aは、図8〜9Bに示される拡張部材270のセグメント290および可撓性部分292と同様に、複数の個別セグメント390aおよび可撓性部分392(各々のうちの1つのみをラベル付けした)を有してもよい。第2の環状部分370bはまた、図8〜9Bに示される拡張部材270のセグメント290および可撓性部分292と同様に、複数の個別セグメント390bおよび可撓性部分392b(各々のうちの1つのみをラベル付けした)を有してもよい。図10に示されるように、いくつかの実施形態において、第2の環状部分370bのセグメント390bは、第1の環状部分370aのセグメント390bと円周方向に整合され、第2の環状部分370bの可撓性部分392bは、第1の環状部分370aの可撓性部分392aと円周方向に整合される。
In the embodiment shown in FIG. 10, the
以下の実施例は、本技術のいくつかの実施形態を例証する。
1.人工心臓弁デバイスであって、
上流部および下流部を有する環状固定フレームを有する固着部材と、
前記固定フレームの前記下流部に連結した第1の部分と、前記固定フレームの前記上流部から半径方向に内向きに離間した第2の部分と、を有する管状弁支持体と、
前記弁支持体に連結され、かつ前記弁支持体を通る血流が遮断される閉鎖位置と、下流方向への前記弁支持体を通る血流が許容される開放位置とから移動可能である少なくとも1つの弁尖を有する、弁アセンブリと、
前記固定フレームに連結され、かつそこから半径方向に外向きに延在する拡張部材であって、前記拡張部材が、複数のワイヤを含み、前記ワイヤの少なくとも一部分が、外側材料によって包囲された内核を含み、(a)前記内核が、第1の材料であり、(b)前記外側材料が、前記第1の材料とは異なる第2の材料であり、かつある厚さを有し、(c)前記ワイヤが、前記外側材料の前記厚さの少なくとも一部分を通して延在する複数の凹部を含み、(d)前記人工心臓弁デバイスが、天然弁輪に位置付けられるとき、治療剤が、前記生体構造への送達のための前記凹部内にある、拡張部材と、を備える、人工心臓弁デバイス。
2.前記固定フレームの前記上流部が、前記管状弁支持体の前記第2の部分を実質的に変形させることなく、半径方向に変形可能である、実施例1に記載のデバイス。
3.前記拡張部材が、その長さの少なくとも一部分に沿って延在する封止部材をさらに含み、前記ワイヤが、前記封止部材に連結されている、実施例1または実施例2に記載のデバイス。
4.前記ワイヤが、前記固定フレームの前記上流部と一体である、実施例1〜3のいずれか1つに記載のデバイス。
5.前記ワイヤが、前記固定フレームから機械的に絶縁される、実施例1〜3のいずれか1つに記載のデバイス。
6.前記第1の材料が、X線不透過性であり、前記第2の材料が、超弾性合金である、実施例1〜5のいずれか1つに記載のデバイス。
7.前記治療剤が、抗炎症薬および生物活性剤のうちの少なくとも1つを含む、実施例1〜6のいずれか1つに記載のデバイス。
8.前記複数の凹部のうちの少なくともいくつかが、前記外側材料の全体厚さを通して延在し、それによって、前記内核の少なくとも一部分を露出する、実施例1〜7のいずれか1つに記載のデバイス。
9.前記複数の凹部のうちの少なくともいくつかが、前記外側材料の全体厚さ、および前記内核の厚さの少なくとも一部分を通して延在し、これによって、前記複数の凹部のうちの前記少なくともいくつかが、前記内核内の中間の深さで終端する、実施例1〜8のいずれか1つに記載のデバイス。
10.前記拡張部材が、前記内核と前記外側材料との間に位置付けられた中間材料をさらに含む、実施例1〜9のいずれか1つに記載のデバイス。
11.前記凹部のうちの少なくともいくつかが、前記外側材料の全体厚さを通して延在し、それによって、前記中間材料の少なくとも一部分を露出する、実施例10に記載のデバイス。
12.前記凹部のうちの少なくともいくつかが、前記外側材料の全体厚さ、および前記中間材料の全体厚さを通して延在し、それによって、前記内核の少なくとも一部分を露出する、実施例10に記載のデバイス。
13.人工心臓弁デバイスであって、
上流部および下流部を有する環状固定フレームを有する固着部材と、
前記固着部材の前記下流部に連結した第1の部分と、前記固着部材の前記上流部から半径方向に内向きに離間した第2の部分と、を有する管状弁支持体と、
前記弁支持体に連結され、かつ前記弁支持体を通る血流が遮断される閉鎖位置と、下流方向への前記弁支持体を通る血流が許容される開放位置とから移動可能である少なくとも1つの弁尖を有する、弁アセンブリと、
前記固定フレームに連結され、かつそこから半径方向に外向きに延在する拡張部材であって、前記拡張部材が、複数のワイヤを含み、前記ワイヤの少なくとも一部分が、外側材料によって囲まれる内核を含み、(a)前記内核が、第1の材料であり、(b)前記外側材料が、前記第1の材料とは異なる第2の材料であり、かつある厚さを有し、(c)前記ワイヤが、前記外側材料の前記厚さの少なくとも一部分を通して延在する複数の凹部を含み、(d)X線不透過性材料が、凹部内にある、拡張部材と、を備える、人工心臓弁デバイス。
14.前記固定フレームの前記上流部が、前記管状弁支持体の前記第2の部分を実質的に変形させることなく、半径方向に変形可能である、実施例13に記載のデバイス。
15.前記拡張部材が、その長さの少なくとも一部分に沿って延在する封止部材をさらに含み、前記ワイヤが、前記封止部材に連結されている、実施例13または実施例14に記載のデバイス。
16.前記ワイヤが、前記固定フレームの前記上流部と一体である、実施例13〜15のいずれか1つに記載のデバイス。
17.前記ワイヤが、前記固定フレームから機械的に絶縁される、実施例13〜15のいずれか1つに記載のデバイス。
18.前記第1の材料が、X線不透過性であり、前記第2の材料が、超弾性合金である、実施例13〜17のいずれか1つに記載のデバイス。
19.前記治療剤が、抗炎症薬および生物活性剤のうちの少なくとも1つを含む、実施例13〜18のいずれか1つにデバイス。
20.前記複数の凹部のうちの少なくともいくつかが、前記外側材料の全体厚さを通して延在し、それによって、前記内核の少なくとも一部分を露出する、実施例13〜19のいずれか1つに記載のデバイス。
21.前記複数の凹部のうちの少なくともいくつかが、前記外側材料の全体厚さ、および前記内核の厚さの少なくとも一部分を通して延在し、これによって、前記複数の凹部のうちの前記少なくともいくつかが、前記内核内の中間の深さで終端する、実施例13〜20のいずれか1つに記載のデバイス。
22.前記拡張部材が、前記内核と前記外側材料との間に位置付けられた中間材料をさらに含む、実施例13〜21のいずれか1つに記載のデバイス。
23.前記凹部のうちの少なくともいくつかが、前記外側材料の全体厚さを通して延在し、それによって、前記中間材料の少なくとも一部分を露出する、実施例22に記載のデバイス。
24.前記凹部のうちの少なくともいくつかが、前記外側材料の全体厚さ、および前記中間材料の全体厚さを通して延在し、それによって、前記内核の少なくとも一部分を露出する、実施例22に記載のデバイス。
27.人工心臓弁デバイスであって、
内部を伴い、上流部および下流部を有する半径方向に拡大可能なフレームを有する固着部材であって、前記上流部が、対象中の心臓弁の天然弁輪に、および/またはその下流に位置する組織に対して外向きに押し付けるように構成され、かつ前記組織の形状に一致するように少なくとも部分的に変形可能であるように構成される組織固定部分を含む、固着部材と、
前記固着部材に対して位置付けられ、かつ前記内部を通る血流が遮断される閉鎖位置と、前記上流部から前記下流部へ向かう流れの方向で前記内部を通る血流が許容される開放位置とから移動可能である少なくとも1つの弁尖を有する、弁であって、前記弁が、前記組織固定部分が前記組織の前記形状に一致するように変形されるとき、前記弁が有能なままであるように、前記固着部材の前記組織固定部分から内向きに離間される、弁と、
展開構成の前記固着部材の前記上流部から離れて横方向に延在する拡張アセンブリであって、前記拡張部材が、前記固着部材における第1の端部と、前記固着部材から離間される第2の端部と、を有する複数の個別織物区分を備え、前記織物区分が、前記拡張部材の周りに並んで配列されており、隣接した織物区分が、可撓性連結手段によって一緒にゆるく連結し、これによって、隣接した織物区分の前記第2の端部が、前記展開状態において互いに独立して構成され得る、拡張アセンブリと、を備える、人工心臓弁デバイス。
28.前記セグメントが、構造部材を含まない、実施例27に記載の人工心臓弁デバイス。
29.前記拡張部材が、前記展開構成の前記拡大可能なフレームに対して変形するように構成されている、実施例27または実施例28に記載の人工心臓弁デバイス。
30.前記連結手段が、弾性である、実施例27〜29のいずれか1つに記載の人工心臓弁デバイス。
31.前記連結手段が、縫合糸である、実施例27〜29のいずれか1つに記載の人工心臓弁デバイス。
32.前記セグメントの前記遠位終端が、前記拡張部材の前記第2の端部と一致する、実施例27〜31のいずれか1つに記載の人工心臓弁デバイス。
33.前記セグメントが前記固着部材に間接的にのみ連結されるように、前記セグメントが前記拡大可能なフレームから離間される、実施例27〜32のいずれか1つに記載の人工心臓弁デバイス。
34.前記セグメントが、前記拡大可能なフレームに直接的に接続される、実施例27〜32のいずれか1つに記載の人工心臓弁デバイス。
The following examples illustrate some embodiments of the present technology.
1. An artificial heart valve device,
A fixing member having an annular fixing frame having an upstream portion and a downstream portion,
A tubular valve support having a first portion connected to the downstream portion of the stationary frame and a second portion radially inwardly spaced from the upstream portion of the stationary frame;
At least movable from a closed position connected to the valve support and blocking blood flow through the valve support, and an open position allowing blood flow through the valve support in the downstream direction. A valve assembly having one leaflet;
An expansion member coupled to the stationary frame and extending radially outwardly therefrom, the expansion member including a plurality of wires, at least a portion of the wires being surrounded by an outer material. (A) the inner core is a first material, (b) the outer material is a second material different from the first material, and has a thickness; ) The wire includes a plurality of recesses extending through at least a portion of the thickness of the outer material; A prosthetic heart valve device within the recess for delivery to the prosthetic heart valve device.
2. The device of example 1 wherein the upstream portion of the stationary frame is radially deformable without substantially deforming the second portion of the tubular valve support.
3. The device of example 1 or example 2, wherein the expansion member further comprises a sealing member extending along at least a portion of its length, and the wire is coupled to the sealing member.
4. The device of any one of Examples 1-3, wherein the wire is integral with the upstream portion of the stationary frame.
5. The device of any one of Examples 1-3, wherein the wire is mechanically isolated from the fixed frame.
6. The device of any one of Examples 1-5, wherein the first material is radiopaque and the second material is a superelastic alloy.
7. The device of any one of Examples 1-6, wherein the therapeutic agent comprises at least one of an anti-inflammatory agent and a bioactive agent.
8. The device of any one of Examples 1-7, wherein at least some of the plurality of recesses extend through the entire thickness of the outer material, thereby exposing at least a portion of the inner core. ..
9. At least some of the plurality of recesses extend through the overall thickness of the outer material and at least a portion of the thickness of the inner core, such that at least some of the plurality of recesses include: The device of any one of Examples 1-8, terminating at an intermediate depth within the inner core.
10. 10. The device of any one of Examples 1-9, wherein the expansion member further comprises an intermediate material positioned between the inner core and the outer material.
11. The device of example 10, wherein at least some of the recesses extend through the entire thickness of the outer material, thereby exposing at least a portion of the intermediate material.
12. The device of Example 10, wherein at least some of the recesses extend through the entire thickness of the outer material and the intermediate material, thereby exposing at least a portion of the inner core. ..
13. An artificial heart valve device,
A fixing member having an annular fixing frame having an upstream portion and a downstream portion,
A tubular valve support having a first portion connected to the downstream portion of the anchoring member and a second portion radially inwardly spaced from the upstream portion of the anchoring member;
At least movable from a closed position connected to the valve support and blocking blood flow through the valve support, and an open position allowing blood flow through the valve support in the downstream direction. A valve assembly having one leaflet;
An expansion member coupled to the stationary frame and extending radially outwardly therefrom, the expansion member comprising a plurality of wires, at least a portion of the wires defining an inner core surrounded by an outer material. Including (a) the inner core is a first material, (b) the outer material is a second material different from the first material, and has a thickness; (c) The wire includes a plurality of recesses extending through at least a portion of the thickness of the outer material, and (d) a radiopaque material in the recesses; and an expansion member. device.
14. 14. The device of example 13, wherein the upstream portion of the stationary frame is radially deformable without substantially deforming the second portion of the tubular valve support.
15. 15. The device of example 13 or example 14, wherein the expansion member further comprises a sealing member extending along at least a portion of its length and the wire is coupled to the sealing member.
16. 16. The device of any one of Examples 13-15, wherein the wire is integral with the upstream portion of the stationary frame.
17. 16. The device according to any one of Examples 13-15, wherein the wire is mechanically insulated from the fixed frame.
18. 18. The device according to any one of Examples 13-17, wherein the first material is radiopaque and the second material is a superelastic alloy.
19. The device of any one of Examples 13-18, wherein the therapeutic agent comprises at least one of an anti-inflammatory agent and a bioactive agent.
20. The device of any one of Examples 13-19, wherein at least some of the plurality of recesses extend through the entire thickness of the outer material, thereby exposing at least a portion of the inner core. ..
21. At least some of the plurality of recesses extend through the overall thickness of the outer material and at least a portion of the thickness of the inner core, such that at least some of the plurality of recesses include: 21. The device according to any one of Examples 13-20, terminating at an intermediate depth within the inner core.
22. 22. The device of any one of Examples 13-21, wherein the expansion member further comprises an intermediate material positioned between the inner core and the outer material.
23. 23. The device of example 22, wherein at least some of the recesses extend through the entire thickness of the outer material, thereby exposing at least a portion of the intermediate material.
24. 23. The device of example 22, wherein at least some of the recesses extend through the total thickness of the outer material and the intermediate material, thereby exposing at least a portion of the inner core. ..
27. An artificial heart valve device,
An anchoring member having a radially expandable frame with an interior and an upstream portion and a downstream portion, the upstream portion being located at and/or downstream of a natural annulus of a heart valve of interest. An anchoring member comprising a tissue anchoring portion configured to be pressed outwardly against tissue and configured to be at least partially deformable to conform to the shape of said tissue;
A closed position that is positioned with respect to the fixing member and that blocks blood flow through the interior, and an open position that allows blood flow through the interior in the direction of flow from the upstream portion to the downstream portion A valve having at least one leaflet movable from the valve, the valve remaining capable when the tissue anchoring portion is deformed to conform to the shape of the tissue. A valve inwardly spaced from the tissue fixation portion of the fixation member;
A expansion assembly extending laterally away from the upstream portion of the securing member in a deployed configuration, the expansion member being spaced apart from the first end of the securing member and the securing member. A plurality of individual fabric sections having an end of, the fabric sections being arranged side by side around the expansion member, and adjacent fabric sections being loosely coupled together by a flexible coupling means. A dilation assembly whereby the second ends of adjacent textile sections may be configured independently of each other in the deployed state.
28. The prosthetic heart valve device of Example 27, wherein the segment does not include a structural member.
29. 29. The prosthetic heart valve device of example 27 or example 28, wherein the expansion member is configured to deform with respect to the expandable frame in the deployed configuration.
30. The prosthetic heart valve device of any one of Examples 27-29, wherein the connecting means is elastic.
31. The artificial heart valve device according to any one of Examples 27 to 29, wherein the connecting means is a suture.
32. The prosthetic heart valve device of any one of Examples 27-31, wherein the distal end of the segment coincides with the second end of the expansion member.
33. 33. The prosthetic heart valve device of any one of Examples 27-32, wherein the segment is spaced from the expandable frame such that the segment is only indirectly connected to the anchoring member.
34. The prosthetic heart valve device of any one of Examples 27-32, wherein the segment is directly connected to the expandable frame.
以上により、本発明の具体的実施形態が、例証の目的で本明細書において説明されているが、本発明の範囲から逸脱することなく、種々の修正が行われてもよいことが理解されるであろう。例えば、いくつかの個々の構成要素は、異なる実施形態において、互いと入れ替えることができる。したがって、本発明は、添付の特許請求の範囲による場合を除いて限定されない。 While the above describes specific embodiments of the present invention for purposes of illustration, it is understood that various modifications may be made without departing from the scope of the present invention. Will. For example, some individual components may be interchangeable with each other in different embodiments. Therefore, the present invention is not limited except as by the appended claims.
Claims (34)
上流部および下流部を有する環状固定フレームを有する固着部材と、
前記固定フレームの前記下流部に連結した第1の部分と、前記固定フレームの前記上流部から半径方向に内向きに離間した第2の部分と、を有する管状弁支持体と、
前記弁支持体に連結され、かつ前記弁支持体を通る血流が遮断される閉鎖位置と、下流方向への前記弁支持体を通る血流が許容される開放位置とから移動可能である少なくとも1つの弁尖を有する、弁アセンブリと、
前記固定フレームに連結され、かつそこから半径方向に外向きに延在する拡張部材であって、前記拡張部材が、複数のワイヤを含み、前記ワイヤの少なくとも一部分が、外側材料によって包囲された内核を含み、(a)前記内核が、第1の材料であり、(b)前記外側材料が、前記第1の材料とは異なる第2の材料であり、かつある厚さを有し、(c)前記ワイヤが、前記外側材料の前記厚さの少なくとも一部分を通して延在する複数の凹部を含み、(d)前記人工心臓弁デバイスが、天然弁輪に位置付けられるとき、生体構造への送達のための前記凹部内にある治療剤、拡張部材と、を備える、人工心臓弁デバイス。 An artificial heart valve device,
A fixing member having an annular fixing frame having an upstream portion and a downstream portion,
A tubular valve support having a first portion connected to the downstream portion of the stationary frame and a second portion radially inwardly spaced from the upstream portion of the stationary frame;
At least movable from a closed position connected to the valve support and blocking blood flow through the valve support, and an open position allowing blood flow through the valve support in the downstream direction. A valve assembly having one leaflet;
An expander member coupled to the stationary frame and extending radially outwardly therefrom, the expander member comprising a plurality of wires, at least a portion of the wires being surrounded by an outer material. (A) the inner core is a first material, (b) the outer material is a second material different from the first material, and has a thickness; ) The wire includes a plurality of recesses extending through at least a portion of the thickness of the outer material, and (d) for delivery to an anatomy when the prosthetic heart valve device is positioned in a native annulus. A prosthetic heart valve device comprising: a therapeutic agent in the recess of the.
前記第2の材料が、超弾性合金であり、
前記治療剤が、抗炎症薬および生物活性剤のうちの少なくとも1つを含む、請求項1に記載のデバイス。 The first material is radiopaque,
The second material is a superelastic alloy,
The device of claim 1, wherein the therapeutic agent comprises at least one of an anti-inflammatory agent and a bioactive agent.
上流部および下流部を有する環状固定フレームを有する固着部材と、
前記固着部材の前記下流部に連結した第1の部分と、前記固着部材の前記上流部から半径方向に内向きに離間した第2の部分と、を有する管状弁支持体と、
前記弁支持体に連結され、かつ前記弁支持体を通る血流が遮断される閉鎖位置と、下流方向への前記弁支持体を通る血流が許容される開放位置とから移動可能である少なくとも1つの弁尖を有する、弁アセンブリと、
前記固定フレームに連結され、かつそこから半径方向に外向きに延在する拡張部材であって、前記拡張部材が、複数のワイヤを含み、前記ワイヤの少なくとも一部分が、外側材料によって包囲された内核を含み、(a)前記内核が、第1の材料であり、(b)前記外側材料が、前記第1の材料とは異なる第2の材料であり、かつある厚さを有し、(c)前記ワイヤが、前記外側材料の前記厚さの少なくとも一部分を通して延在する複数の凹部を含み、(d)X線不透過性材料が、凹部内にある、拡張部材と、を備える、人工心臓弁デバイス。 An artificial heart valve device,
A fixing member having an annular fixing frame having an upstream portion and a downstream portion,
A tubular valve support having a first portion connected to the downstream portion of the anchoring member and a second portion radially inwardly spaced from the upstream portion of the anchoring member;
At least movable from a closed position connected to the valve support and blocking blood flow through the valve support, and an open position allowing blood flow through the valve support in the downstream direction. A valve assembly having one leaflet;
An expander member coupled to the stationary frame and extending radially outwardly therefrom, the expander member comprising a plurality of wires, at least a portion of the wires being surrounded by an outer material. (A) the inner core is a first material, (b) the outer material is a second material different from the first material, and has a thickness; ) The wire comprises a plurality of recesses extending through at least a portion of the thickness of the outer material, and (d) a radiopaque material in the recesses; and an expansion member. Valve device.
前記第2の材料が、超弾性合金であり、
前記治療剤が、抗炎症薬および生物活性剤のうちの少なくとも1つを含む、請求項14に記載のデバイス。 The first material is radiopaque,
The second material is a superelastic alloy,
15. The device of claim 14, wherein the therapeutic agent comprises at least one of an anti-inflammatory drug and a bioactive agent.
内部を伴い、上流部および下流部を有する半径方向に拡大可能なフレームを有する固着部材であって、前記上流部が、対象中の心臓弁の天然弁輪に、および/またはその下流に位置する組織に対して外向きに押し付けるように構成され、かつ前記組織の形状に一致するように少なくとも部分的に変形可能であるように構成される組織固定部分を含む、固着部材と、
前記固着部材に対して位置付けられ、かつ前記内部を通る血流が遮断される閉鎖位置と、前記上流部から前記下流部へ向かう流れの方向で前記内部を通る血流が許容される開放位置とから移動可能である少なくとも1つの弁尖を有する、弁であって、前記弁が、前記組織固定部分が前記組織の前記形状に一致するように変形されるとき、前記弁が有能なままであるように、前記固着部材の前記組織固定部分から内向きに離間される、弁と、
展開構成の前記固着部材の前記上流部から離れて横方向に延在する拡張アセンブリであって、前記拡張部材が、前記固着部材における第1の端部と、前記固着部材から離間される第2の端部と、を有する複数の個別織物セグメントを備え、前記織物セグメントが、前記拡張部材の周りに並んで配列されており、隣接した織物セグメントが、可撓性連結手段によって一緒にゆるく連結し、これによって、隣接した織物セグメントの前記第2の端部が、前記展開状態において互いに独立して構成され得る、拡張アセンブリと、を備える、人工心臓弁デバイス。 An artificial heart valve device,
An anchoring member having a radially expandable frame with an interior and an upstream portion and a downstream portion, the upstream portion being located at and/or downstream of a natural annulus of a heart valve of interest. An anchoring member comprising a tissue anchoring portion configured to be pressed outwardly against tissue and configured to be at least partially deformable to conform to the shape of said tissue;
A closed position that is positioned with respect to the fixing member and that blocks blood flow through the interior, and an open position that allows blood flow through the interior in the direction of flow from the upstream portion to the downstream portion. A valve having at least one leaflet movable from the valve, the valve remaining capable when the tissue anchoring portion is deformed to conform to the shape of the tissue. A valve inwardly spaced from the tissue fixation portion of the fixation member;
A expansion assembly extending laterally away from the upstream portion of the securing member in a deployed configuration, the expansion member being spaced apart from the first end of the securing member and the securing member. A plurality of individual fabric segments having an end of, the fabric segments being arranged side by side around the expansion member, and adjacent fabric segments being loosely coupled together by a flexible coupling means. A dilatation assembly whereby the second ends of adjacent fabric segments may be configured independently of each other in the deployed state.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US15/642,834 | 2017-07-06 | ||
| US15/642,834 US10729541B2 (en) | 2017-07-06 | 2017-07-06 | Prosthetic heart valve devices and associated systems and methods |
| PCT/US2018/038841 WO2019010010A1 (en) | 2017-07-06 | 2018-06-21 | Prosthetic heart valve devices |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2020525215A true JP2020525215A (en) | 2020-08-27 |
| JP2020525215A5 JP2020525215A5 (en) | 2021-07-29 |
Family
ID=62981328
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2019572460A Pending JP2020525215A (en) | 2017-07-06 | 2018-06-21 | Artificial heart valve device |
Country Status (6)
| Country | Link |
|---|---|
| US (3) | US10729541B2 (en) |
| EP (2) | EP3648707B1 (en) |
| JP (1) | JP2020525215A (en) |
| CN (2) | CN115444619A (en) |
| AU (1) | AU2018297210A1 (en) |
| WO (1) | WO2019010010A1 (en) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20090276040A1 (en) * | 2008-05-01 | 2009-11-05 | Edwards Lifesciences Corporation | Device and method for replacing mitral valve |
| US8444689B2 (en) | 2009-03-30 | 2013-05-21 | Causper Medical Inc. | Valve prosthesis with movably attached claspers with apex |
| US11406497B2 (en) | 2013-03-14 | 2022-08-09 | Jc Medical, Inc. | Heart valve prosthesis |
| US11259923B2 (en) | 2013-03-14 | 2022-03-01 | Jc Medical, Inc. | Methods and devices for delivery of a prosthetic valve |
| JP2016512077A (en) | 2013-03-14 | 2016-04-25 | カーディオヴァンテージ・メディカル・インク | Embolization protection device and method of use |
| WO2015148241A1 (en) * | 2014-03-26 | 2015-10-01 | St. Jude Medical, Cardiology Division, Inc. | Transcatheter mitral valve stent frames |
| US11833034B2 (en) | 2016-01-13 | 2023-12-05 | Shifamed Holdings, Llc | Prosthetic cardiac valve devices, systems, and methods |
| US10786352B2 (en) | 2017-07-06 | 2020-09-29 | Twelve, Inc. | Prosthetic heart valve devices and associated systems and methods |
| JP6990315B2 (en) | 2018-01-07 | 2022-01-12 | ジェイシー メディカル、インコーポレイテッド | Artificial heart valve delivery system |
| CN210673509U (en) | 2018-01-07 | 2020-06-05 | 苏州杰成医疗科技有限公司 | Valve prosthesis delivery device |
| AU2019353156A1 (en) | 2018-10-05 | 2021-05-13 | Shifamed Holdings, Llc | Prosthetic cardiac valve devices, systems, and methods |
| WO2020191216A1 (en) | 2019-03-19 | 2020-09-24 | Shifamed Holdings, Llc | Prosthetic cardiac valve devices, systems, and methods |
| AU2021217945A1 (en) | 2020-02-06 | 2022-08-25 | Laplace Interventional Inc. | Transcatheter heart valve prosthesis assembled inside heart chambers or blood vessels |
| US11318013B2 (en) | 2020-04-21 | 2022-05-03 | Medtronic, Inc. | Compact prosthetic heart valve device |
| WO2021257722A1 (en) * | 2020-06-16 | 2021-12-23 | Shifamed Holdings, Llc | Minimal frame prosthetic cardiac valve delivery devices, systems, and methods |
| WO2022012010A1 (en) * | 2020-07-15 | 2022-01-20 | 上海臻亿医疗科技有限公司 | Heart valve prosthesis |
| WO2022047393A1 (en) | 2020-08-31 | 2022-03-03 | Shifamed Holdings, Llc | Prosthetic delivery system |
| US11850371B2 (en) | 2021-07-13 | 2023-12-26 | Medtronic, Inc. | Prosthetic delivery device trays, packaging systems and methods |
| CN113679512A (en) * | 2021-08-11 | 2021-11-23 | 上海傲流医疗科技有限公司 | Repair device for treating tricuspid valve regurgitation |
| WO2023161766A1 (en) * | 2022-02-25 | 2023-08-31 | Medtronic, Inc. | Prosthetic heart valve |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010519957A (en) * | 2007-03-01 | 2010-06-10 | ボストン サイエンティフィック リミテッド | Coated medical device for delivering a drug outside the lumen |
| US20120067103A1 (en) * | 2010-09-17 | 2012-03-22 | Medtronic Vascular, Inc. | Method of Forming a Drug-Eluting Medical Device |
| JP2015510415A (en) * | 2012-01-27 | 2015-04-09 | メドトロニック ヴァスキュラー インコーポレイテッド | Drug-filled hollow stent and method for forming a drug-filled hollow stent |
| JP2016512753A (en) * | 2013-03-15 | 2016-05-09 | トゥエルヴ, インコーポレイテッド | Prosthetic heart valve device, prosthetic mitral valve, and related systems and methods |
Family Cites Families (779)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3526219A (en) | 1967-07-21 | 1970-09-01 | Ultrasonic Systems | Method and apparatus for ultrasonically removing tissue from a biological organism |
| NL145136C (en) | 1967-07-25 | 1900-01-01 | ||
| US3565062A (en) | 1968-06-13 | 1971-02-23 | Ultrasonic Systems | Ultrasonic method and apparatus for removing cholesterol and other deposits from blood vessels and the like |
| US3667474A (en) | 1970-01-05 | 1972-06-06 | Konstantin Vasilievich Lapkin | Dilator for performing mitral and tricuspidal commissurotomy per atrium cordis |
| US3739402A (en) * | 1970-10-15 | 1973-06-19 | Cutter Lab | Bicuspid fascia lata valve |
| DE2219790C3 (en) | 1972-04-22 | 1974-11-07 | R Pohlman | Device for generating brittle fractures in hard stones |
| US3861391A (en) | 1972-07-02 | 1975-01-21 | Blackstone Corp | Apparatus for disintegration of urinary calculi |
| DE2242863A1 (en) | 1972-08-31 | 1974-03-14 | Karl Storz | SURGICAL ELEMENT FOR CRUSHING STONES IN THE HUMAN BODY BY ULTRASOUND |
| US4188952A (en) | 1973-12-28 | 1980-02-19 | Loschilov Vladimir I | Surgical instrument for ultrasonic separation of biological tissue |
| US4042979A (en) | 1976-07-12 | 1977-08-23 | Angell William W | Valvuloplasty ring and prosthetic method |
| US4388735A (en) | 1980-11-03 | 1983-06-21 | Shiley Inc. | Low profile prosthetic xenograft heart valve |
| IT1144379B (en) | 1981-07-14 | 1986-10-29 | Sorin Biomedica Spa | CARDIAC VALVE PROSTHESIS |
| US4431006A (en) | 1982-01-07 | 1984-02-14 | Technicare Corporation | Passive ultrasound needle probe locator |
| ATE21330T1 (en) | 1982-01-20 | 1986-08-15 | Martin Morris Black | ARTIFICIAL HEART VALVES. |
| US4445509A (en) | 1982-02-04 | 1984-05-01 | Auth David C | Method and apparatus for removal of enclosed abnormal deposits |
| JPS58173931U (en) | 1982-05-14 | 1983-11-21 | アルパイン株式会社 | car radio receiver |
| US4484579A (en) | 1982-07-19 | 1984-11-27 | University Of Pittsburgh | Commissurotomy catheter apparatus and method |
| DE3230858C2 (en) | 1982-08-19 | 1985-01-24 | Ahmadi, Ali, Dr. med., 7809 Denzlingen | Ring prosthesis |
| EP0139753B1 (en) | 1983-04-04 | 1988-11-09 | Sumitomo Bakelite Company Limited | Ultrasonic oscillator |
| IT1159433B (en) | 1983-07-25 | 1987-02-25 | Sorin Biomedica Spa | PROCEDURE AND EQUIPMENT FOR THE MANUFACTURE OF VALVE FLAPS FOR CARDIAC VALVE PROSTHESIS AND CARDIAC VALVE PROSTHESIS PROVIDED WITH SUCH FLAPS |
| US5387247A (en) | 1983-10-25 | 1995-02-07 | Sorin Biomedia S.P.A. | Prosthetic device having a biocompatible carbon film thereon and a method of and apparatus for forming such device |
| US4629459A (en) | 1983-12-28 | 1986-12-16 | Shiley Inc. | Alternate stent covering for tissue valves |
| IT1208326B (en) | 1984-03-16 | 1989-06-12 | Sorin Biomedica Spa | CARDIAC VALVE PROSTHESIS PROVIDED WITH VALVES OF ORGANIC FABRIC |
| CA1237482A (en) | 1984-03-09 | 1988-05-31 | Frank B. Stiles | Catheter for effecting removal of obstructions from a biological duct |
| US4646736A (en) | 1984-09-10 | 1987-03-03 | E. R. Squibb & Sons, Inc. | Transluminal thrombectomy apparatus |
| US4960411A (en) | 1984-09-18 | 1990-10-02 | Medtronic Versaflex, Inc. | Low profile sterrable soft-tip catheter |
| US4589419A (en) | 1984-11-01 | 1986-05-20 | University Of Iowa Research Foundation | Catheter for treating arterial occlusion |
| DE3583431D1 (en) | 1984-12-20 | 1991-08-14 | Matsushita Electric Ind Co Ltd | MICROWAVE OVEN. |
| US4750902A (en) | 1985-08-28 | 1988-06-14 | Sonomed Technology, Inc. | Endoscopic ultrasonic aspirators |
| DE3685873T2 (en) | 1985-11-26 | 1992-12-17 | Sorin Biomedica Spa | PRODUCTION OF A PROSTHESIS DEVICE. |
| US5084151A (en) | 1985-11-26 | 1992-01-28 | Sorin Biomedica S.P.A. | Method and apparatus for forming prosthetic device having a biocompatible carbon film thereon |
| CH668192A5 (en) | 1985-11-29 | 1988-12-15 | Schneider Medintag Ag | CATHETER FOR TREATING NARROW BODIES, FOR EXAMPLE IN A BLOOD VESSEL. |
| CA1293663C (en) | 1986-01-06 | 1991-12-31 | David Christopher Auth | Transluminal microdissection device |
| US4653577A (en) | 1986-01-23 | 1987-03-31 | Shiley, Inc. | Unitary heat exchanger and debubbler for a liquid |
| US4679556A (en) | 1986-04-16 | 1987-07-14 | Shiley Inc. | Releasable holder and method of use |
| US4777951A (en) | 1986-09-19 | 1988-10-18 | Mansfield Scientific, Inc. | Procedure and catheter instrument for treating patients for aortic stenosis |
| US4747821A (en) | 1986-10-22 | 1988-05-31 | Intravascular Surgical Instruments, Inc. | Catheter with high speed moving working head |
| US5314407A (en) | 1986-11-14 | 1994-05-24 | Heart Technology, Inc. | Clinically practical rotational angioplasty system |
| US4808153A (en) | 1986-11-17 | 1989-02-28 | Ultramed Corporation | Device for removing plaque from arteries |
| DE3689793T2 (en) | 1986-11-27 | 1994-09-22 | Sumitomo Bakelite Co | SURGICAL ULTRASONIC DEVICE. |
| IT1196836B (en) | 1986-12-12 | 1988-11-25 | Sorin Biomedica Spa | Polymeric or metal alloy prosthesis with biocompatible carbon coating |
| US4878495A (en) | 1987-05-15 | 1989-11-07 | Joseph Grayzel | Valvuloplasty device with satellite expansion means |
| US4841977A (en) | 1987-05-26 | 1989-06-27 | Inter Therapy, Inc. | Ultra-thin acoustic transducer and balloon catheter using same in imaging array subassembly |
| US4796629A (en) | 1987-06-03 | 1989-01-10 | Joseph Grayzel | Stiffened dilation balloon catheter device |
| US4898575A (en) | 1987-08-31 | 1990-02-06 | Medinnovations, Inc. | Guide wire following tunneling catheter system and method for transluminal arterial atherectomy |
| US4819751A (en) | 1987-10-16 | 1989-04-11 | Baxter Travenol Laboratories, Inc. | Valvuloplasty catheter and method |
| US4870953A (en) | 1987-11-13 | 1989-10-03 | Donmicheal T Anthony | Intravascular ultrasonic catheter/probe and method for treating intravascular blockage |
| IT1218947B (en) | 1988-01-12 | 1990-04-24 | Sorin Biomedica Spa | CARDIAC VALVE PROSTHESIS |
| US4892540A (en) | 1988-04-21 | 1990-01-09 | Sorin Biomedica S.P.A. | Two-leaflet prosthetic heart valve |
| US4909252A (en) | 1988-05-26 | 1990-03-20 | The Regents Of The Univ. Of California | Perfusion balloon catheter |
| JPH024516A (en) | 1988-06-23 | 1990-01-09 | Seiko Epson Corp | Ink jet head |
| US4920954A (en) | 1988-08-05 | 1990-05-01 | Sonic Needle Corporation | Ultrasonic device for applying cavitation forces |
| US4919133A (en) | 1988-08-18 | 1990-04-24 | Chiang Tien Hon | Catheter apparatus employing shape memory alloy structures |
| US5904679A (en) | 1989-01-18 | 1999-05-18 | Applied Medical Resources Corporation | Catheter with electrosurgical cutter |
| US4936281A (en) | 1989-04-13 | 1990-06-26 | Everest Medical Corporation | Ultrasonically enhanced RF ablation catheter |
| US4986830A (en) | 1989-09-22 | 1991-01-22 | Schneider (U.S.A.) Inc. | Valvuloplasty catheter with balloon which remains stable during inflation |
| US5076276A (en) | 1989-11-01 | 1991-12-31 | Olympus Optical Co., Ltd. | Ultrasound type treatment apparatus |
| US5069664A (en) | 1990-01-25 | 1991-12-03 | Inter Therapy, Inc. | Intravascular ultrasonic angioplasty probe |
| IT1240110B (en) | 1990-02-21 | 1993-11-27 | Sorin Biomedica Spa | CARDIAC VALVE PROSTHESIS |
| US5344426A (en) | 1990-04-25 | 1994-09-06 | Advanced Cardiovascular Systems, Inc. | Method and system for stent delivery |
| US5411552A (en) | 1990-05-18 | 1995-05-02 | Andersen; Henning R. | Valve prothesis for implantation in the body and a catheter for implanting such valve prothesis |
| DK124690D0 (en) | 1990-05-18 | 1990-05-18 | Henning Rud Andersen | FAT PROTECTION FOR IMPLEMENTATION IN THE BODY FOR REPLACEMENT OF NATURAL FLEET AND CATS FOR USE IN IMPLEMENTING A SUCH FAT PROTECTION |
| US5106302A (en) | 1990-09-26 | 1992-04-21 | Ormco Corporation | Method of fracturing interfaces with an ultrasonic tool |
| US5269291A (en) | 1990-12-10 | 1993-12-14 | Coraje, Inc. | Miniature ultrasonic transducer for plaque ablation |
| US5248296A (en) | 1990-12-24 | 1993-09-28 | Sonic Needle Corporation | Ultrasonic device having wire sheath |
| US5304115A (en) | 1991-01-11 | 1994-04-19 | Baxter International Inc. | Ultrasonic angioplasty device incorporating improved transmission member and ablation probe |
| US5957882A (en) | 1991-01-11 | 1999-09-28 | Advanced Cardiovascular Systems, Inc. | Ultrasound devices for ablating and removing obstructive matter from anatomical passageways and blood vessels |
| US5916192A (en) | 1991-01-11 | 1999-06-29 | Advanced Cardiovascular Systems, Inc. | Ultrasonic angioplasty-atherectomy catheter and method of use |
| US5267954A (en) | 1991-01-11 | 1993-12-07 | Baxter International Inc. | Ultra-sound catheter for removing obstructions from tubular anatomical structures such as blood vessels |
| DE69215722T3 (en) | 1991-03-22 | 2001-03-08 | Katsuro Tachibana | Amplifiers for ultrasound therapy of diseases and liquid pharmaceutical compositions containing them |
| US5295958A (en) | 1991-04-04 | 1994-03-22 | Shturman Cardiology Systems, Inc. | Method and apparatus for in vivo heart valve decalcification |
| IT1245750B (en) | 1991-05-24 | 1994-10-14 | Sorin Biomedica Emodialisi S R | CARDIAC VALVE PROSTHESIS, PARTICULARLY FOR REPLACING THE AORTIC VALVE |
| US5489297A (en) | 1992-01-27 | 1996-02-06 | Duran; Carlos M. G. | Bioprosthetic heart valve with absorbable stent |
| US5332402A (en) | 1992-05-12 | 1994-07-26 | Teitelbaum George P | Percutaneously-inserted cardiac valve |
| US5382261A (en) | 1992-09-01 | 1995-01-17 | Expandable Grafts Partnership | Method and apparatus for occluding vessels |
| US5318014A (en) | 1992-09-14 | 1994-06-07 | Coraje, Inc. | Ultrasonic ablation/dissolution transducer |
| US5356418A (en) | 1992-10-28 | 1994-10-18 | Shturman Cardiology Systems, Inc. | Apparatus and method for rotational atherectomy |
| US5397293A (en) | 1992-11-25 | 1995-03-14 | Misonix, Inc. | Ultrasonic device with sheath and transverse motion damping |
| US5352199A (en) | 1993-05-28 | 1994-10-04 | Numed, Inc. | Balloon catheter |
| US5989280A (en) | 1993-10-22 | 1999-11-23 | Scimed Lifesystems, Inc | Stent delivery apparatus and method |
| AU1301095A (en) | 1993-12-13 | 1995-07-03 | Brigham And Women's Hospital | Aortic valve supporting device |
| US5449373A (en) | 1994-03-17 | 1995-09-12 | Medinol Ltd. | Articulated stent |
| US5609151A (en) | 1994-09-08 | 1997-03-11 | Medtronic, Inc. | Method for R-F ablation |
| WO1996010366A1 (en) | 1994-10-03 | 1996-04-11 | Heart Technology, Inc. | Transluminal thrombectomy apparatus |
| IT1268777B1 (en) | 1994-10-05 | 1997-03-06 | Fogazzi Di Venturelli Andrea & | HYDRAULIC STENT INTRODUCER |
| US6689086B1 (en) | 1994-10-27 | 2004-02-10 | Advanced Cardiovascular Systems, Inc. | Method of using a catheter for delivery of ultrasonic energy and medicament |
| US5827229A (en) | 1995-05-24 | 1998-10-27 | Boston Scientific Corporation Northwest Technology Center, Inc. | Percutaneous aspiration thrombectomy catheter system |
| US6774278B1 (en) * | 1995-06-07 | 2004-08-10 | Cook Incorporated | Coated implantable medical device |
| US5681336A (en) | 1995-09-07 | 1997-10-28 | Boston Scientific Corporation | Therapeutic device for treating vien graft lesions |
| US5725494A (en) | 1995-11-30 | 1998-03-10 | Pharmasonics, Inc. | Apparatus and methods for ultrasonically enhanced intraluminal therapy |
| DE19605042A1 (en) | 1996-02-12 | 1998-01-15 | Figulla Hans Reiner Prof Dr Me | Vessel implant for bridging vascular weaknesses |
| US8865788B2 (en) | 1996-02-13 | 2014-10-21 | The General Hospital Corporation | Radiation and melt treated ultra high molecular weight polyethylene prosthetic devices |
| AU721034B2 (en) | 1996-02-15 | 2000-06-22 | Biosense, Inc. | Catheter based surgery |
| US5972004A (en) | 1996-02-23 | 1999-10-26 | Cardiovascular Technologies, Llc. | Wire fasteners for use in minimally invasive surgery and apparatus and methods for handling those fasteners |
| IT1285308B1 (en) | 1996-03-12 | 1998-06-03 | Sorin Biomedica Cardio Spa | PROCEDURE FOR THE PREPARATION OF BIOLOGICAL MATERIAL FOR PLANT |
| US5853422A (en) | 1996-03-22 | 1998-12-29 | Scimed Life Systems, Inc. | Apparatus and method for closing a septal defect |
| US5855601A (en) | 1996-06-21 | 1999-01-05 | The Trustees Of Columbia University In The City Of New York | Artificial heart valve and method and device for implanting the same |
| US5897566A (en) | 1996-07-15 | 1999-04-27 | Shturman Cardiology Systems, Inc. | Rotational atherectomy device |
| US5662671A (en) | 1996-07-17 | 1997-09-02 | Embol-X, Inc. | Atherectomy device having trapping and excising means for removal of plaque from the aorta and other arteries |
| US6080170A (en) | 1996-07-26 | 2000-06-27 | Kensey Nash Corporation | System and method of use for revascularizing stenotic bypass grafts and other occluded blood vessels |
| US5782931A (en) | 1996-07-30 | 1998-07-21 | Baxter International Inc. | Methods for mitigating calcification and improving durability in glutaraldehyde-fixed bioprostheses and articles manufactured by such methods |
| CA2213948C (en) | 1996-09-19 | 2006-06-06 | United States Surgical Corporation | Ultrasonic dissector |
| US5868781A (en) | 1996-10-22 | 1999-02-09 | Scimed Life Systems, Inc. | Locking stent |
| US6217595B1 (en) | 1996-11-18 | 2001-04-17 | Shturman Cardiology Systems, Inc. | Rotational atherectomy device |
| EP0850607A1 (en) | 1996-12-31 | 1998-07-01 | Cordis Corporation | Valve prosthesis for implantation in body channels |
| US6183411B1 (en) | 1998-09-21 | 2001-02-06 | Myocor, Inc. | External stress reduction device and method |
| US5873811A (en) | 1997-01-10 | 1999-02-23 | Sci-Med Life Systems | Composition containing a radioactive component for treatment of vessel wall |
| US6129734A (en) | 1997-01-21 | 2000-10-10 | Shturman Cardiology Systems, Inc. | Rotational atherectomy device with radially expandable prime mover coupling |
| US5827321A (en) | 1997-02-07 | 1998-10-27 | Cornerstone Devices, Inc. | Non-Foreshortening intraluminal prosthesis |
| KR100469779B1 (en) | 1997-02-10 | 2005-02-02 | 스미카 다케다 노야쿠 가부시키가이샤 | Aqueous suspension of agrochemical |
| US5817101A (en) | 1997-03-13 | 1998-10-06 | Schneider (Usa) Inc | Fluid actuated stent delivery system |
| US5989208A (en) | 1997-05-16 | 1999-11-23 | Nita; Henry | Therapeutic ultrasound system |
| US6269819B1 (en) | 1997-06-27 | 2001-08-07 | The Trustees Of Columbia University In The City Of New York | Method and apparatus for circulatory valve repair |
| WO1999004730A1 (en) | 1997-07-22 | 1999-02-04 | Baxter International Inc. | Expandable annuloplasty ring |
| US6494890B1 (en) | 1997-08-14 | 2002-12-17 | Shturman Cardiology Systems, Inc. | Eccentric rotational atherectomy device |
| US6132444A (en) | 1997-08-14 | 2000-10-17 | Shturman Cardiology Systems, Inc. | Eccentric drive shaft for atherectomy device and method for manufacture |
| FR2768324B1 (en) | 1997-09-12 | 1999-12-10 | Jacques Seguin | SURGICAL INSTRUMENT FOR PERCUTANEOUSLY FIXING TWO AREAS OF SOFT TISSUE, NORMALLY MUTUALLY REMOTE, TO ONE ANOTHER |
| US6221006B1 (en) | 1998-02-10 | 2001-04-24 | Artemis Medical Inc. | Entrapping apparatus and method for use |
| EP2258312B9 (en) | 1997-12-29 | 2012-09-19 | The Cleveland Clinic Foundation | Deployable surgical platform and system for the removal and delivery of a medical device comprising such deployable surgical platform |
| US6530952B2 (en) | 1997-12-29 | 2003-03-11 | The Cleveland Clinic Foundation | Bioprosthetic cardiovascular valve system |
| US6254635B1 (en) | 1998-02-02 | 2001-07-03 | St. Jude Medical, Inc. | Calcification-resistant medical articles |
| US6423032B2 (en) | 1998-03-13 | 2002-07-23 | Arteria Medical Science, Inc. | Apparatus and methods for reducing embolization during treatment of carotid artery disease |
| US6047700A (en) | 1998-03-30 | 2000-04-11 | Arthrocare Corporation | Systems and methods for electrosurgical removal of calcified deposits |
| CA2331386A1 (en) | 1998-06-26 | 2000-01-06 | Incyte Pharmaceuticals, Inc. | Human signal peptide-containing proteins |
| US6085754A (en) | 1998-07-13 | 2000-07-11 | Acorn Cardiovascular, Inc. | Cardiac disease treatment method |
| US6113608A (en) | 1998-11-20 | 2000-09-05 | Scimed Life Systems, Inc. | Stent delivery device |
| US6425916B1 (en) | 1999-02-10 | 2002-07-30 | Michi E. Garrison | Methods and devices for implanting cardiac valves |
| US6855123B2 (en) | 2002-08-02 | 2005-02-15 | Flow Cardia, Inc. | Therapeutic ultrasound system |
| US20040044350A1 (en) | 1999-04-09 | 2004-03-04 | Evalve, Inc. | Steerable access sheath and methods of use |
| US7226467B2 (en) | 1999-04-09 | 2007-06-05 | Evalve, Inc. | Fixation device delivery catheter, systems and methods of use |
| US6752813B2 (en) | 1999-04-09 | 2004-06-22 | Evalve, Inc. | Methods and devices for capturing and fixing leaflets in valve repair |
| WO2000060995A2 (en) | 1999-04-09 | 2000-10-19 | Evalve, Inc. | Methods and apparatus for cardiac valve repair |
| GB2349824B (en) | 1999-05-04 | 2003-03-12 | Medtronic Inc | Apparatus for inhibiting or minimizing calcification of aortic valves |
| US6648854B1 (en) | 1999-05-14 | 2003-11-18 | Scimed Life Systems, Inc. | Single lumen balloon-tipped micro catheter with reinforced shaft |
| US7628803B2 (en) | 2001-02-05 | 2009-12-08 | Cook Incorporated | Implantable vascular device |
| US6626899B2 (en) | 1999-06-25 | 2003-09-30 | Nidus Medical, Llc | Apparatus and methods for treating tissue |
| US6997951B2 (en) | 1999-06-30 | 2006-02-14 | Edwards Lifesciences Ag | Method and device for treatment of mitral insufficiency |
| US6168579B1 (en) | 1999-08-04 | 2001-01-02 | Scimed Life Systems, Inc. | Filter flush system and methods of use |
| US9694121B2 (en) | 1999-08-09 | 2017-07-04 | Cardiokinetix, Inc. | Systems and methods for improving cardiac function |
| US6231561B1 (en) | 1999-09-20 | 2001-05-15 | Appriva Medical, Inc. | Method and apparatus for closing a body lumen |
| IT1307268B1 (en) | 1999-09-30 | 2001-10-30 | Sorin Biomedica Cardio Spa | DEVICE FOR HEART VALVE REPAIR OR REPLACEMENT. |
| US6626930B1 (en) | 1999-10-21 | 2003-09-30 | Edwards Lifesciences Corporation | Minimally invasive mitral valve repair method and apparatus |
| US6440164B1 (en) | 1999-10-21 | 2002-08-27 | Scimed Life Systems, Inc. | Implantable prosthetic valve |
| US7018406B2 (en) | 1999-11-17 | 2006-03-28 | Corevalve Sa | Prosthetic valve for transluminal delivery |
| US8579966B2 (en) | 1999-11-17 | 2013-11-12 | Medtronic Corevalve Llc | Prosthetic valve for transluminal delivery |
| US6458153B1 (en) | 1999-12-31 | 2002-10-01 | Abps Venture One, Ltd. | Endoluminal cardiac and venous valve prostheses and methods of manufacture and delivery thereof |
| US6494891B1 (en) | 1999-12-30 | 2002-12-17 | Advanced Cardiovascular Systems, Inc. | Ultrasonic angioplasty transmission member |
| US7507252B2 (en) | 2000-01-31 | 2009-03-24 | Edwards Lifesciences Ag | Adjustable transluminal annuloplasty system |
| US6402781B1 (en) | 2000-01-31 | 2002-06-11 | Mitralife | Percutaneous mitral annuloplasty and cardiac reinforcement |
| WO2001056512A1 (en) | 2000-02-02 | 2001-08-09 | Snyders Robert V | Artificial heart valve |
| JP2003526448A (en) | 2000-03-10 | 2003-09-09 | パラコー サージカル インコーポレイテッド | Inflatable cardiac harness for treating congestive heart failure |
| US10092313B2 (en) | 2000-04-05 | 2018-10-09 | Boston Scientific Limited | Medical sealed tubular structures |
| CA2403925C (en) | 2000-04-05 | 2008-09-16 | Stx Medical, Inc. | Intralumenal material removal systems and methods |
| US8475484B2 (en) | 2000-04-05 | 2013-07-02 | Medrad, Inc. | Liquid seal assembly for a rotating torque tube |
| US6565588B1 (en) | 2000-04-05 | 2003-05-20 | Pathway Medical Technologies, Inc. | Intralumenal material removal using an expandable cutting device |
| US7344546B2 (en) | 2000-04-05 | 2008-03-18 | Pathway Medical Technologies | Intralumenal material removal using a cutting device for differential cutting |
| US20040243162A1 (en) | 2000-04-05 | 2004-12-02 | Pathway Medical Technologies, Inc. | Interventional catheter assemblies and control systems |
| US6454799B1 (en) | 2000-04-06 | 2002-09-24 | Edwards Lifesciences Corporation | Minimally-invasive heart valves and methods of use |
| US6616689B1 (en) | 2000-05-03 | 2003-09-09 | Advanced Cardiovascular Systems, Inc. | Intravascular stent |
| EP1512383B1 (en) | 2000-06-26 | 2013-02-20 | Rex Medical, L.P. | A vascular system for valve leaflet apposition |
| US6419696B1 (en) | 2000-07-06 | 2002-07-16 | Paul A. Spence | Annuloplasty devices and related heart valve repair methods |
| US7077861B2 (en) | 2000-07-06 | 2006-07-18 | Medtentia Ab | Annuloplasty instrument |
| US8956407B2 (en) | 2000-09-20 | 2015-02-17 | Mvrx, Inc. | Methods for reshaping a heart valve annulus using a tensioning implant |
| WO2004030569A2 (en) | 2002-10-01 | 2004-04-15 | Ample Medical, Inc. | Devices, systems, and methods for reshaping a heart valve annulus |
| US6893459B1 (en) | 2000-09-20 | 2005-05-17 | Ample Medical, Inc. | Heart valve annulus device and method of using same |
| US20090287179A1 (en) | 2003-10-01 | 2009-11-19 | Ample Medical, Inc. | Devices, systems, and methods for reshaping a heart valve annulus, including the use of magnetic tools |
| US20080091264A1 (en) | 2002-11-26 | 2008-04-17 | Ample Medical, Inc. | Devices, systems, and methods for reshaping a heart valve annulus, including the use of magnetic tools |
| US8784482B2 (en) | 2000-09-20 | 2014-07-22 | Mvrx, Inc. | Method of reshaping a heart valve annulus using an intravascular device |
| US6461382B1 (en) | 2000-09-22 | 2002-10-08 | Edwards Lifesciences Corporation | Flexible heart valve having moveable commissures |
| US6669722B2 (en) | 2000-09-22 | 2003-12-30 | Cordis Corporation | Stent with optimal strength and radiopacity characteristics |
| WO2002026168A2 (en) | 2000-09-29 | 2002-04-04 | Tricardia, Llc | Venous valvuloplasty device |
| WO2002028421A1 (en) | 2000-10-06 | 2002-04-11 | University Of Washington | Methods of inhibition of stenosis and/or sclerosis of the aortic valve |
| US7608704B2 (en) | 2000-11-08 | 2009-10-27 | Incyte Corporation | Secreted proteins |
| WO2002039908A2 (en) | 2000-11-16 | 2002-05-23 | Hill J Donald | Automatic suture fixation apparatus and method |
| US6974476B2 (en) | 2003-05-05 | 2005-12-13 | Rex Medical, L.P. | Percutaneous aortic valve |
| US6579308B1 (en) | 2000-11-28 | 2003-06-17 | Scimed Life Systems, Inc. | Stent devices with detachable distal or proximal wires |
| US6623452B2 (en) | 2000-12-19 | 2003-09-23 | Scimed Life Systems, Inc. | Drug delivery catheter having a highly compliant balloon with infusion holes |
| US6893456B2 (en) | 2000-12-22 | 2005-05-17 | Advanced Cardiovascular Systems, Inc. | Catheter and method for making the same |
| US6733525B2 (en) | 2001-03-23 | 2004-05-11 | Edwards Lifesciences Corporation | Rolled minimally-invasive heart valves and methods of use |
| US7186264B2 (en) | 2001-03-29 | 2007-03-06 | Viacor, Inc. | Method and apparatus for improving mitral valve function |
| US8202315B2 (en) | 2001-04-24 | 2012-06-19 | Mitralign, Inc. | Catheter-based annuloplasty using ventricularly positioned catheter |
| US7935145B2 (en) | 2001-05-17 | 2011-05-03 | Edwards Lifesciences Corporation | Annuloplasty ring for ischemic mitral valve insuffuciency |
| US6599314B2 (en) | 2001-06-08 | 2003-07-29 | Cordis Corporation | Apparatus and method for stenting a vessel using balloon-actuated stent with interlocking elements |
| ES2230262T3 (en) | 2001-06-11 | 2005-05-01 | Sorin Biomedica Cardio S.R.L. | PROTECTION OF ANULOPLASTY AND METHOD FOR THEIR PRODUCTION. |
| US8623077B2 (en) | 2001-06-29 | 2014-01-07 | Medtronic, Inc. | Apparatus for replacing a cardiac valve |
| FR2826863B1 (en) | 2001-07-04 | 2003-09-26 | Jacques Seguin | ASSEMBLY FOR PLACING A PROSTHETIC VALVE IN A BODY CONDUIT |
| EP1423066B1 (en) | 2001-09-07 | 2008-07-16 | Mardil, Inc. | Method and apparatus for external heart stabilization |
| US20030064095A1 (en) | 2001-09-14 | 2003-04-03 | Imedd, Inc. | Microfabricated nanopore device for sustained release of therapeutic agent |
| US20040057955A1 (en) | 2001-10-05 | 2004-03-25 | O'brien Kevin D. | Methods of inhibition of stenosis and/or sclerosis of the aortic valve |
| US6893460B2 (en) | 2001-10-11 | 2005-05-17 | Percutaneous Valve Technologies Inc. | Implantable prosthetic valve |
| US6852118B2 (en) | 2001-10-19 | 2005-02-08 | Shturman Cardiology Systems, Inc. | Self-indexing coupling for rotational angioplasty device |
| US7052487B2 (en) | 2001-10-26 | 2006-05-30 | Cohn William E | Method and apparatus for reducing mitral regurgitation |
| GB0125925D0 (en) | 2001-10-29 | 2001-12-19 | Univ Glasgow | Mitral valve prosthesis |
| WO2003043685A2 (en) | 2001-11-19 | 2003-05-30 | Cardiovascular Systems, Inc | High torque, low profile intravascular guidewire system |
| US20080249504A1 (en) | 2007-04-06 | 2008-10-09 | Lattouf Omar M | Instrument port |
| US6811801B2 (en) | 2001-12-12 | 2004-11-02 | Abbott Laboratories | Methods and compositions for brightening the color of thermally processed nutritionals |
| US20030120340A1 (en) | 2001-12-26 | 2003-06-26 | Jan Liska | Mitral and tricuspid valve repair |
| WO2003105670A2 (en) | 2002-01-10 | 2003-12-24 | Guided Delivery Systems, Inc. | Devices and methods for heart valve repair |
| US7018404B2 (en) | 2002-01-24 | 2006-03-28 | St. Jude Medical, Inc. | Conduit for aorta or pulmonary artery replacement |
| US7125420B2 (en) | 2002-02-05 | 2006-10-24 | Viacor, Inc. | Method and apparatus for improving mitral valve function |
| ATE518501T1 (en) | 2002-03-27 | 2011-08-15 | Sorin Biomedica Cardio Srl | ANNULOPLASTY PROSTHESIS WITH PERFORATED ELEMENT |
| US7588582B2 (en) | 2002-06-13 | 2009-09-15 | Guided Delivery Systems Inc. | Methods for remodeling cardiac tissue |
| US7753922B2 (en) | 2003-09-04 | 2010-07-13 | Guided Delivery Systems, Inc. | Devices and methods for cardiac annulus stabilization and treatment |
| US8641727B2 (en) | 2002-06-13 | 2014-02-04 | Guided Delivery Systems, Inc. | Devices and methods for heart valve repair |
| US8348963B2 (en) | 2002-07-03 | 2013-01-08 | Hlt, Inc. | Leaflet reinforcement for regurgitant valves |
| US6686820B1 (en) | 2002-07-11 | 2004-02-03 | Intel Corporation | Microelectromechanical (MEMS) switching apparatus |
| WO2004008996A1 (en) | 2002-07-22 | 2004-01-29 | Timur Paul Sarac | Percutaneous endovascular apparatus for repair of aneurysms and arterial blockages |
| EP1545371B1 (en) | 2002-08-01 | 2016-04-13 | Robert A. Levine | Cardiac devices and methods for minimally invasive repair of ischemic mitral regurgitation |
| US8172856B2 (en) | 2002-08-02 | 2012-05-08 | Cedars-Sinai Medical Center | Methods and apparatus for atrioventricular valve repair |
| US6702748B1 (en) | 2002-09-20 | 2004-03-09 | Flowcardia, Inc. | Connector for securing ultrasound catheter to transducer |
| EP1402826B1 (en) | 2002-08-20 | 2013-06-12 | Nipro Corporation | Thrombus capture catheter |
| CA2827984A1 (en) | 2002-08-28 | 2004-03-11 | Heart Leaflet Technologies, Inc. | Method and device for treating diseased valve |
| ATE464028T1 (en) | 2002-08-29 | 2010-04-15 | St Jude Medical Cardiology Div | IMPLANTABLE DEVICES FOR CONTROLLING THE INNER DIAMETER OF AN OPENING IN THE BODY |
| CO5500017A1 (en) | 2002-09-23 | 2005-03-31 | 3F Therapeutics Inc | MITRAL PROTESTIC VALVE |
| US8512252B2 (en) | 2002-10-07 | 2013-08-20 | Integrated Sensing Systems Inc. | Delivery method and system for monitoring cardiovascular pressures |
| US8979923B2 (en) | 2002-10-21 | 2015-03-17 | Mitralign, Inc. | Tissue fastening systems and methods utilizing magnetic guidance |
| US20040082910A1 (en) | 2002-10-29 | 2004-04-29 | Constantz Brent R. | Devices and methods for treating aortic valve stenosis |
| US7404824B1 (en) | 2002-11-15 | 2008-07-29 | Advanced Cardiovascular Systems, Inc. | Valve aptation assist device |
| US6746463B1 (en) | 2003-01-27 | 2004-06-08 | Scimed Life Systems, Inc | Device for percutaneous cutting and dilating a stenosis of the aortic valve |
| US7381210B2 (en) | 2003-03-14 | 2008-06-03 | Edwards Lifesciences Corporation | Mitral valve repair system and method for use |
| SE0300854D0 (en) | 2003-03-26 | 2003-03-26 | Oeyvind Reitan | Device for the treatment of a heart valve insufficiency |
| US8083707B2 (en) | 2003-04-17 | 2011-12-27 | Tosaya Carol A | Non-contact damage-free ultrasonic cleaning of implanted or natural structures having moving parts and located in a living body |
| US7175656B2 (en) | 2003-04-18 | 2007-02-13 | Alexander Khairkhahan | Percutaneous transcatheter heart valve replacement |
| US7279002B2 (en) | 2003-04-25 | 2007-10-09 | Boston Scientific Scimed, Inc. | Cutting stent and balloon |
| US20040260394A1 (en) | 2003-06-20 | 2004-12-23 | Medtronic Vascular, Inc. | Cardiac valve annulus compressor system |
| JP4942031B2 (en) | 2003-07-08 | 2012-05-30 | メドトロニック ベンター テクノロジーズ リミティド | In particular, an implantable prosthetic device suitable for transarterial delivery in the treatment of aortic stenosis, and a method of implanting the prosthetic device |
| US7201772B2 (en) | 2003-07-08 | 2007-04-10 | Ventor Technologies, Ltd. | Fluid flow prosthetic device |
| US7744620B2 (en) | 2003-07-18 | 2010-06-29 | Intervalve, Inc. | Valvuloplasty catheter |
| WO2005018507A2 (en) | 2003-07-18 | 2005-03-03 | Ev3 Santa Rosa, Inc. | Remotely activated mitral annuloplasty system and methods |
| ATE442107T1 (en) | 2003-07-21 | 2009-09-15 | Univ Pennsylvania | PERCUTANE HEART VALVE |
| JP4002293B2 (en) | 2003-07-22 | 2007-10-31 | コラゾン テクノロジーズ インコーポレーティッド | Apparatus and method for treating aortic valve stenosis |
| DE10334868B4 (en) | 2003-07-29 | 2013-10-17 | Pfm Medical Ag | Implantable device as a replacement organ valve, its manufacturing process and basic body and membrane element for it |
| US7044966B2 (en) | 2003-10-06 | 2006-05-16 | 3F Therapeutics, Inc. | Minimally invasive valve replacement system |
| US10219899B2 (en) | 2004-04-23 | 2019-03-05 | Medtronic 3F Therapeutics, Inc. | Cardiac valve replacement systems |
| US7261732B2 (en) | 2003-12-22 | 2007-08-28 | Henri Justino | Stent mounted valve |
| US20050137691A1 (en) | 2003-12-23 | 2005-06-23 | Sadra Medical | Two piece heart valve and anchor |
| US8828078B2 (en) | 2003-12-23 | 2014-09-09 | Sadra Medical, Inc. | Methods and apparatus for endovascular heart valve replacement comprising tissue grasping elements |
| US7445631B2 (en) | 2003-12-23 | 2008-11-04 | Sadra Medical, Inc. | Methods and apparatus for endovascularly replacing a patient's heart valve |
| US7748389B2 (en) | 2003-12-23 | 2010-07-06 | Sadra Medical, Inc. | Leaflet engagement elements and methods for use thereof |
| US8343213B2 (en) | 2003-12-23 | 2013-01-01 | Sadra Medical, Inc. | Leaflet engagement elements and methods for use thereof |
| US7381219B2 (en) | 2003-12-23 | 2008-06-03 | Sadra Medical, Inc. | Low profile heart valve and delivery system |
| US7431726B2 (en) | 2003-12-23 | 2008-10-07 | Mitralign, Inc. | Tissue fastening systems and methods utilizing magnetic guidance |
| US8182528B2 (en) | 2003-12-23 | 2012-05-22 | Sadra Medical, Inc. | Locking heart valve anchor |
| US7780725B2 (en) | 2004-06-16 | 2010-08-24 | Sadra Medical, Inc. | Everting heart valve |
| US8864822B2 (en) | 2003-12-23 | 2014-10-21 | Mitralign, Inc. | Devices and methods for introducing elements into tissue |
| AU2004308508B2 (en) | 2003-12-23 | 2011-03-10 | Sadra Medical, Inc. | Repositionable heart valve |
| US7871435B2 (en) | 2004-01-23 | 2011-01-18 | Edwards Lifesciences Corporation | Anatomically approximate prosthetic mitral heart valve |
| WO2005092203A1 (en) | 2004-03-03 | 2005-10-06 | Nmt Medical, Inc. | Delivery/recovery system for septal occluder |
| ITTO20040135A1 (en) | 2004-03-03 | 2004-06-03 | Sorin Biomedica Cardio Spa | CARDIAC VALVE PROSTHESIS |
| EP1734903B2 (en) | 2004-03-11 | 2022-01-19 | Percutaneous Cardiovascular Solutions Pty Limited | Percutaneous heart valve prosthesis |
| JP4503331B2 (en) | 2004-03-30 | 2010-07-14 | 西松建設株式会社 | Granular filling device and filling method |
| US20050228477A1 (en) | 2004-04-09 | 2005-10-13 | Xtent, Inc. | Topographic coatings and coating methods for medical devices |
| DE602005015446D1 (en) | 2004-06-29 | 2009-08-27 | Fertin Pharma As | TOBACCO ALCOHOL RELEASING CHEWING GUM |
| EP1796597B1 (en) | 2004-09-14 | 2013-01-09 | Edwards Lifesciences AG | Device for treatment of heart valve regurgitation |
| US6951571B1 (en) | 2004-09-30 | 2005-10-04 | Rohit Srivastava | Valve implanting device |
| WO2006041877A2 (en) | 2004-10-05 | 2006-04-20 | Ample Medical, Inc. | Atrioventricular valve annulus repair systems and methods including retro-chordal anchors |
| JP5219518B2 (en) | 2004-12-09 | 2013-06-26 | ザ ファウンドリー, エルエルシー | Aortic valve repair |
| JP2008528117A (en) | 2005-01-21 | 2008-07-31 | イノビア,リミティド ライアビリティー カンパニー | Stent valve and placement catheter for use therewith |
| US20100298929A1 (en) | 2005-02-07 | 2010-11-25 | Thornton Troy L | Methods, systems and devices for cardiac valve repair |
| WO2011034628A1 (en) | 2005-02-07 | 2011-03-24 | Evalve, Inc. | Methods, systems and devices for cardiac valve repair |
| EP3967269A3 (en) | 2005-02-07 | 2022-07-13 | Evalve, Inc. | Systems and devices for cardiac valve repair |
| ITTO20050074A1 (en) | 2005-02-10 | 2006-08-11 | Sorin Biomedica Cardio Srl | CARDIAC VALVE PROSTHESIS |
| WO2006089236A1 (en) | 2005-02-18 | 2006-08-24 | The Cleveland Clinic Foundation | Apparatus and methods for replacing a cardiac valve |
| DE102006039823A1 (en) | 2005-02-25 | 2008-03-20 | Strecker, Ernst Peter, Prof. Dr. med. | Catheter-stent-device for treatment of e.g. body hollow organ, has catheter surrounding stent, where catheter and stent are movable by difference of pressure developed by pressuring medium consisting of liquid or gas for implanting stent |
| WO2006097931A2 (en) | 2005-03-17 | 2006-09-21 | Valtech Cardio, Ltd. | Mitral valve treatment techniques |
| EP1868663B1 (en) | 2005-03-23 | 2011-11-16 | Abbott Laboratories | Delivery of highly lipophilic agents via medical devices |
| US8062359B2 (en) | 2005-04-06 | 2011-11-22 | Edwards Lifesciences Corporation | Highly flexible heart valve connecting band |
| SE531468C2 (en) | 2005-04-21 | 2009-04-14 | Edwards Lifesciences Ag | An apparatus for controlling blood flow |
| ATE534346T1 (en) | 2005-05-06 | 2011-12-15 | Sorin Biomedica Cardio Srl | ANNULOPLASTY PROSTHESIS |
| ES2425779T3 (en) | 2005-05-06 | 2013-10-17 | Vasonova, Inc. | Apparatus for guiding and positioning an endovascular device |
| WO2006127756A2 (en) | 2005-05-24 | 2006-11-30 | Edwards Lifesciences Corporation | Rapid deployment prosthetic heart valve |
| US8951285B2 (en) | 2005-07-05 | 2015-02-10 | Mitralign, Inc. | Tissue anchor, anchoring system and methods of using the same |
| US7682391B2 (en) | 2005-07-13 | 2010-03-23 | Edwards Lifesciences Corporation | Methods of implanting a prosthetic mitral heart valve having a contoured sewing ring |
| US7530253B2 (en) | 2005-09-09 | 2009-05-12 | Edwards Lifesciences Corporation | Prosthetic valve crimping device |
| WO2007030823A2 (en) | 2005-09-09 | 2007-03-15 | Edwards Lifesciences Corporation | Device and method for reshaping mitral valve annulus |
| US20070073391A1 (en) | 2005-09-28 | 2007-03-29 | Henry Bourang | System and method for delivering a mitral valve repair device |
| US8167932B2 (en) | 2005-10-18 | 2012-05-01 | Edwards Lifesciences Corporation | Heart valve delivery system with valve catheter |
| US7785366B2 (en) | 2005-10-26 | 2010-08-31 | Maurer Christopher W | Mitral spacer |
| US8449606B2 (en) | 2005-10-26 | 2013-05-28 | Cardiosolutions, Inc. | Balloon mitral spacer |
| DE102005052628B4 (en) | 2005-11-04 | 2014-06-05 | Jenavalve Technology Inc. | Self-expanding, flexible wire mesh with integrated valvular prosthesis for the transvascular heart valve replacement and a system with such a device and a delivery catheter |
| EP3167847B1 (en) | 2005-11-10 | 2020-10-14 | Edwards Lifesciences CardiAQ LLC | Heart valve prosthesis |
| US7632308B2 (en) | 2005-11-23 | 2009-12-15 | Didier Loulmet | Methods, devices, and kits for treating mitral valve prolapse |
| WO2007067820A2 (en) | 2005-12-09 | 2007-06-14 | Edwards Lifesciences Corporation | Improved anchoring system for medical implant |
| WO2007100408A2 (en) | 2005-12-15 | 2007-09-07 | Georgia Tech Research Corporation | Papillary muscle position control devices, systems & methods |
| WO2007100410A2 (en) | 2005-12-15 | 2007-09-07 | Georgia Tech Research Corporation | Systems and methods for enabling heart valve replacement |
| US20070213813A1 (en) | 2005-12-22 | 2007-09-13 | Symetis Sa | Stent-valves for valve replacement and associated methods and systems for surgery |
| EP1803420B1 (en) | 2005-12-28 | 2009-07-01 | Sorin Biomedica Cardio S.R.L. | Annuloplasty prosthesis with an auxetic structure |
| WO2008029296A2 (en) | 2006-02-16 | 2008-03-13 | Endocor Pte Ltd. | Minimally invasive heart valve replacement |
| US7749266B2 (en) | 2006-02-27 | 2010-07-06 | Aortx, Inc. | Methods and devices for delivery of prosthetic heart valves and other prosthetics |
| US7806926B2 (en) | 2006-04-14 | 2010-10-05 | Edwards Lifesciences Corporation | Holders for prosthetic aortic heart valves |
| US8551161B2 (en) | 2006-04-25 | 2013-10-08 | Medtronic Vascular, Inc. | Cardiac valve annulus restraining device |
| DE102006020035A1 (en) | 2006-04-26 | 2007-10-31 | B. Braun Melsungen Ag | Preparation and use of poly (hydroxyethyl starch) chitin and poly (carboxymethyl starch) chitin compounds |
| EP1849440A1 (en) | 2006-04-28 | 2007-10-31 | Younes Boudjemline | Vascular stents with varying diameter |
| US20140276782A1 (en) | 2013-03-15 | 2014-09-18 | Larry D. Paskar | Catheter system |
| US8932348B2 (en) | 2006-05-18 | 2015-01-13 | Edwards Lifesciences Corporation | Device and method for improving heart valve function |
| CN102283721B (en) | 2006-06-01 | 2015-08-26 | 爱德华兹生命科学公司 | For improving the prosthetic insert of heart valve function |
| US8449605B2 (en) | 2006-06-28 | 2013-05-28 | Kardium Inc. | Method for anchoring a mitral valve |
| US8920411B2 (en) | 2006-06-28 | 2014-12-30 | Kardium Inc. | Apparatus and method for intra-cardiac mapping and ablation |
| WO2008013915A2 (en) | 2006-07-28 | 2008-01-31 | Arshad Quadri | Percutaneous valve prosthesis and system and method for implanting same |
| EP2056750A2 (en) | 2006-08-14 | 2009-05-13 | BUCH, Wally S. | Methods and apparatus for mitral valve repair |
| US8876895B2 (en) | 2006-09-19 | 2014-11-04 | Medtronic Ventor Technologies Ltd. | Valve fixation member having engagement arms |
| FR2906454B1 (en) | 2006-09-28 | 2009-04-10 | Perouse Soc Par Actions Simpli | IMPLANT INTENDED TO BE PLACED IN A BLOOD CIRCULATION CONDUIT. |
| US8068920B2 (en) | 2006-10-03 | 2011-11-29 | Vincent A Gaudiani | Transcoronary sinus pacing system, LV summit pacing, early mitral closure pacing, and methods therefor |
| US8029556B2 (en) | 2006-10-04 | 2011-10-04 | Edwards Lifesciences Corporation | Method and apparatus for reshaping a ventricle |
| FR2906998B1 (en) | 2006-10-16 | 2009-04-10 | Perouse Soc Par Actions Simpli | IMPLANT INTENDED TO BE PLACED IN A BLOOD CIRCULATION CONDUIT. |
| DE102006052564B3 (en) | 2006-11-06 | 2007-12-13 | Georg Lutter | Mitral valve stent for surgical implantation and fixation of heart valve prosthesis to heart, has stent clips arranged distally, where one of stent clips forms section that is externally rolled in unfolded condition of stent |
| WO2008060553A1 (en) | 2006-11-14 | 2008-05-22 | The Government Of The United States Of America As Represented By The Secretary Of The Department Of Health And Human Services | Transcatheter coronary sinus mitral valve annuloplasty procedure and coronary artery and myocardial protection device |
| AU2007329243B2 (en) | 2006-12-06 | 2014-04-03 | Medtronic CV Luxembourg S.a.r.l | System and method for transapical delivery of an annulus anchored self-expanding valve |
| US8470024B2 (en) | 2006-12-19 | 2013-06-25 | Sorin Group Italia S.R.L. | Device for in situ positioning of cardiac valve prosthesis |
| EP1967164A3 (en) | 2006-12-19 | 2009-01-28 | Sorin Biomedica Cardio S.R.L. | Instrument for in situ deployment of cardiac valve prostheses |
| US8070799B2 (en) | 2006-12-19 | 2011-12-06 | Sorin Biomedica Cardio S.R.L. | Instrument and method for in situ deployment of cardiac valve prostheses |
| FR2910269B1 (en) | 2006-12-22 | 2009-02-27 | Corevalve Inc | TREATMENT EQUIPMENT FOR A CARDIAC VALVE, IN PARTICULAR A MITRAL VALVE |
| US9107750B2 (en) | 2007-01-03 | 2015-08-18 | St. Jude Medical, Cardiology Division, Inc. | Implantable devices for controlling the size and shape of an anatomical structure or lumen |
| US9192471B2 (en) | 2007-01-08 | 2015-11-24 | Millipede, Inc. | Device for translumenal reshaping of a mitral valve annulus |
| WO2008091515A2 (en) | 2007-01-19 | 2008-07-31 | Medtronic, Inc. | Stented heart valve devices and methods for atrioventricular valve replacement |
| WO2008091614A1 (en) | 2007-01-23 | 2008-07-31 | Cvdevices, Llc | Systems and methods for valve annulus remodeling |
| WO2008103280A2 (en) | 2007-02-16 | 2008-08-28 | Medtronic, Inc. | Delivery systems and methods of implantation for replacement prosthetic heart valves |
| US7753949B2 (en) | 2007-02-23 | 2010-07-13 | The Trustees Of The University Of Pennsylvania | Valve prosthesis systems and methods |
| US8070802B2 (en) | 2007-02-23 | 2011-12-06 | The Trustees Of The University Of Pennsylvania | Mitral valve system |
| US8845723B2 (en) | 2007-03-13 | 2014-09-30 | Mitralign, Inc. | Systems and methods for introducing elements into tissue |
| US7896915B2 (en) | 2007-04-13 | 2011-03-01 | Jenavalve Technology, Inc. | Medical device for treating a heart valve insufficiency |
| FR2915087B1 (en) | 2007-04-20 | 2021-11-26 | Corevalve Inc | IMPLANT FOR TREATMENT OF A HEART VALVE, IN PARTICULAR OF A MITRAL VALVE, EQUIPMENT INCLUDING THIS IMPLANT AND MATERIAL FOR PLACING THIS IMPLANT. |
| US20080262603A1 (en) | 2007-04-23 | 2008-10-23 | Sorin Biomedica Cardio | Prosthetic heart valve holder |
| FR2915678B1 (en) | 2007-05-02 | 2010-04-16 | Lapeyre Ind Llc | MECHANICAL PROTHETIC CARDIAC VALVE |
| EP2157916A2 (en) | 2007-06-04 | 2010-03-03 | Mor Research Applications Ltd. | Cardiac valve leaflet augmentation |
| US20100235981A1 (en) | 2007-06-05 | 2010-09-23 | Vivi Aakjaer Jensen | Container unit for storage and disinfection |
| JP5660890B2 (en) | 2007-06-26 | 2015-01-28 | バソノバ・インコーポレイテッドVasonova, Inc. | Vascular access and guidance system |
| EP2014257B1 (en) | 2007-07-12 | 2010-09-08 | Sorin Biomedica Cardio S.R.L. | Expandable prosthetic valve crimping device |
| US8006535B2 (en) | 2007-07-12 | 2011-08-30 | Sorin Biomedica Cardio S.R.L. | Expandable prosthetic valve crimping device |
| EP2026280B1 (en) | 2007-07-23 | 2013-10-23 | Esaote S.p.A. | Method and corresponding apparatus for quantitative measurements on sequences of images, particularly ultrasonic images |
| US9814611B2 (en) | 2007-07-31 | 2017-11-14 | Edwards Lifesciences Cardiaq Llc | Actively controllable stent, stent graft, heart valve and method of controlling same |
| US9566178B2 (en) | 2010-06-24 | 2017-02-14 | Edwards Lifesciences Cardiaq Llc | Actively controllable stent, stent graft, heart valve and method of controlling same |
| FR2919798B1 (en) | 2007-08-09 | 2010-08-27 | Univ Haute Alsace | VALVULAR ENDOPROTHESIS |
| US8114154B2 (en) | 2007-09-07 | 2012-02-14 | Sorin Biomedica Cardio S.R.L. | Fluid-filled delivery system for in situ deployment of cardiac valve prostheses |
| DE602007013225D1 (en) | 2007-09-07 | 2011-04-28 | Mayo Foundation | Fluid-filled delivery system for in situ use of heart valve prostheses |
| EP2399527A1 (en) | 2007-09-07 | 2011-12-28 | Sorin Biomedica Cardio S.r.l. | Prosthetic valve delivery system including retrograde/antegrade approach |
| US8808367B2 (en) | 2007-09-07 | 2014-08-19 | Sorin Group Italia S.R.L. | Prosthetic valve delivery system including retrograde/antegrade approach |
| ES2396738T3 (en) | 2007-09-07 | 2013-02-25 | Sorin Biomedica Cardio S.R.L. | Microprocessor-controlled delivery system of a heart valve prosthesis |
| DE102007043830A1 (en) | 2007-09-13 | 2009-04-02 | Lozonschi, Lucian, Madison | Heart valve stent |
| US8425593B2 (en) | 2007-09-26 | 2013-04-23 | St. Jude Medical, Inc. | Collapsible prosthetic heart valves |
| WO2009045334A1 (en) | 2007-09-28 | 2009-04-09 | St. Jude Medical, Inc. | Collapsible/expandable prosthetic heart valves with native calcified leaflet retention features |
| US8454686B2 (en) | 2007-09-28 | 2013-06-04 | St. Jude Medical, Inc. | Two-stage collapsible/expandable prosthetic heart valves and anchoring systems |
| US9532868B2 (en) | 2007-09-28 | 2017-01-03 | St. Jude Medical, Inc. | Collapsible-expandable prosthetic heart valves with structures for clamping native tissue |
| EP2194888B1 (en) | 2007-10-03 | 2021-04-28 | Bioventrix, Inc. | System for treating dysfunctional cardiac tissue |
| US9848981B2 (en) | 2007-10-12 | 2017-12-26 | Mayo Foundation For Medical Education And Research | Expandable valve prosthesis with sealing mechanism |
| US7981151B2 (en) | 2007-10-15 | 2011-07-19 | Edwards Lifesciences Corporation | Transcatheter heart valve with micro-anchors |
| US8197464B2 (en) | 2007-10-19 | 2012-06-12 | Cordis Corporation | Deflecting guide catheter for use in a minimally invasive medical procedure for the treatment of mitral valve regurgitation |
| US8597347B2 (en) | 2007-11-15 | 2013-12-03 | Cardiosolutions, Inc. | Heart regurgitation method and apparatus |
| US8679176B2 (en) | 2007-12-18 | 2014-03-25 | Cormatrix Cardiovascular, Inc | Prosthetic tissue valve |
| US8257434B2 (en) | 2007-12-18 | 2012-09-04 | Cormatrix Cardiovascular, Inc. | Prosthetic tissue valve |
| US9131928B2 (en) | 2007-12-20 | 2015-09-15 | Mor Research Applications Ltd. | Elongated body for deployment in a heart |
| EP2240121B1 (en) | 2008-01-16 | 2019-05-22 | St. Jude Medical, Inc. | Delivery and retrieval systems for collapsible/expandable prosthetic heart valves |
| WO2009094197A1 (en) | 2008-01-24 | 2009-07-30 | Medtronic, Inc. | Stents for prosthetic heart valves |
| US7972378B2 (en) | 2008-01-24 | 2011-07-05 | Medtronic, Inc. | Stents for prosthetic heart valves |
| US8317858B2 (en) | 2008-02-26 | 2012-11-27 | Jenavalve Technology, Inc. | Stent for the positioning and anchoring of a valvular prosthesis in an implantation site in the heart of a patient |
| US8398704B2 (en) | 2008-02-26 | 2013-03-19 | Jenavalve Technology, Inc. | Stent for the positioning and anchoring of a valvular prosthesis in an implantation site in the heart of a patient |
| US8968393B2 (en) | 2008-02-28 | 2015-03-03 | Medtronic, Inc. | System and method for percutaneous mitral valve repair |
| US9241792B2 (en) | 2008-02-29 | 2016-01-26 | Edwards Lifesciences Corporation | Two-step heart valve implantation |
| US8313525B2 (en) | 2008-03-18 | 2012-11-20 | Medtronic Ventor Technologies, Ltd. | Valve suturing and implantation procedures |
| US8430927B2 (en) | 2008-04-08 | 2013-04-30 | Medtronic, Inc. | Multiple orifice implantable heart valve and methods of implantation |
| US20100121435A1 (en) | 2008-04-16 | 2010-05-13 | Cardiovascular Technologies, Llc | Percutaneous transvalvular intrannular band for mitral valve repair |
| FR2930137B1 (en) | 2008-04-18 | 2010-04-23 | Corevalve Inc | TREATMENT EQUIPMENT FOR A CARDIAC VALVE, IN PARTICULAR A MITRAL VALVE. |
| EP3141219A1 (en) | 2008-04-23 | 2017-03-15 | Medtronic, Inc. | Stented heart valve devices |
| US20090276040A1 (en) | 2008-05-01 | 2009-11-05 | Edwards Lifesciences Corporation | Device and method for replacing mitral valve |
| US20090287304A1 (en) | 2008-05-13 | 2009-11-19 | Kardium Inc. | Medical Device for Constricting Tissue or a Bodily Orifice, for example a mitral valve |
| ES2386239T3 (en) | 2008-05-16 | 2012-08-14 | Sorin Biomedica Cardio S.R.L. | Atraumatic cardiovalvular prosthesis |
| WO2009155088A1 (en) | 2008-05-30 | 2009-12-23 | University Of Cincinnati | Use of zinc chelators to inhibit biofilm formation |
| CA2726807C (en) | 2008-06-05 | 2016-05-31 | Medtronic, Inc. | Connection systems for two piece prosthetic heart valve assemblies and methods for making and using them |
| US8591460B2 (en) | 2008-06-13 | 2013-11-26 | Cardiosolutions, Inc. | Steerable catheter and dilator and system and method for implanting a heart implant |
| US8323335B2 (en) | 2008-06-20 | 2012-12-04 | Edwards Lifesciences Corporation | Retaining mechanisms for prosthetic valves and methods for using |
| US8647254B2 (en) | 2008-07-01 | 2014-02-11 | Maquet Cardiovascular Llc | Epicardial clip |
| EP4176845A1 (en) | 2008-07-15 | 2023-05-10 | St. Jude Medical, LLC | Collapsible and re-expandable prosthetic heart valve cuff designs |
| US9226820B2 (en) | 2008-07-15 | 2016-01-05 | St. Jude Medical, Inc. | Axially anchoring collapsible and re-expandable prosthetic heart valves for various disease states |
| ES2421333T3 (en) | 2008-07-17 | 2013-08-30 | Nvt Ag | Cardiac valve prosthesis system |
| US7951193B2 (en) | 2008-07-23 | 2011-05-31 | Boston Scientific Scimed, Inc. | Drug-eluting stent |
| US20100030330A1 (en) | 2008-08-01 | 2010-02-04 | Edwards Lifesciences Corporation | Device and method for mitral valve repair |
| US8778016B2 (en) | 2008-08-14 | 2014-07-15 | Edwards Lifesciences Corporation | Method and apparatus for repairing or replacing chordae tendinae |
| US8652202B2 (en) | 2008-08-22 | 2014-02-18 | Edwards Lifesciences Corporation | Prosthetic heart valve and delivery apparatus |
| WO2010031060A1 (en) | 2008-09-15 | 2010-03-18 | Medtronic Ventor Technologies Ltd. | Prosthetic heart valve having identifiers for aiding in radiographic positioning |
| US9314335B2 (en) | 2008-09-19 | 2016-04-19 | Edwards Lifesciences Corporation | Prosthetic heart valve configured to receive a percutaneous prosthetic heart valve implantation |
| US20100076376A1 (en) | 2008-09-19 | 2010-03-25 | Sorin Biomedica Cardio S. r. l. | Surgical tool for vascular exposure and access |
| EP2165651B1 (en) | 2008-09-19 | 2011-08-17 | Sorin Biomedica Cardio S.R.L. | Surgical tool for vascular exposure and access |
| AU2009295960A1 (en) | 2008-09-29 | 2010-04-01 | Cardiaq Valve Technologies, Inc. | Heart valve |
| US8137398B2 (en) | 2008-10-13 | 2012-03-20 | Medtronic Ventor Technologies Ltd | Prosthetic valve having tapered tip when compressed for delivery |
| EP2358297B1 (en) | 2008-11-21 | 2019-09-11 | Percutaneous Cardiovascular Solutions Pty Limited | Heart valve prosthesis |
| US8308798B2 (en) | 2008-12-19 | 2012-11-13 | Edwards Lifesciences Corporation | Quick-connect prosthetic heart valve and methods |
| US8688234B2 (en) | 2008-12-19 | 2014-04-01 | Cardiac Pacemakers, Inc. | Devices, methods, and systems including cardiac pacing |
| ES2551694T3 (en) | 2008-12-23 | 2015-11-23 | Sorin Group Italia S.R.L. | Expandable prosthetic valve with anchoring appendages |
| EP2376011B1 (en) | 2009-01-09 | 2019-07-03 | ReCor Medical, Inc. | Apparatus for treatment of mitral valve insufficiency |
| US9402720B2 (en) | 2009-01-12 | 2016-08-02 | Valve Medical Ltd. | Modular percutaneous valve structure and delivery method |
| US20100217382A1 (en) | 2009-02-25 | 2010-08-26 | Edwards Lifesciences | Mitral valve replacement with atrial anchoring |
| US8808366B2 (en) | 2009-02-27 | 2014-08-19 | St. Jude Medical, Inc. | Stent features for collapsible prosthetic heart valves |
| US8715207B2 (en) | 2009-03-19 | 2014-05-06 | Sorin Group Italia S.R.L. | Universal valve annulus sizing device |
| ES2543460T3 (en) | 2009-03-26 | 2015-08-19 | Sorin Group Usa, Inc. | Annuloplasty sizing devices for minimally invasive interventions |
| US8444689B2 (en) | 2009-03-30 | 2013-05-21 | Causper Medical Inc. | Valve prosthesis with movably attached claspers with apex |
| CA2758156A1 (en) | 2009-04-10 | 2010-10-14 | Lon Sutherland Annest | An implantable scaffolding containing an orifice for use with a prosthetic or bio-prosthetic valve |
| US9011522B2 (en) | 2009-04-10 | 2015-04-21 | Lon Sutherland ANNEST | Device and method for temporary or permanent suspension of an implantable scaffolding containing an orifice for placement of a prosthetic or bio-prosthetic valve |
| US8414644B2 (en) | 2009-04-15 | 2013-04-09 | Cardiaq Valve Technologies, Inc. | Vascular implant and delivery system |
| US8512397B2 (en) | 2009-04-27 | 2013-08-20 | Sorin Group Italia S.R.L. | Prosthetic vascular conduit |
| EP4035623A1 (en) | 2009-04-29 | 2022-08-03 | Edwards Lifesciences Corporation | Apparatus and method for replacing a diseased cardiac valve |
| US8353953B2 (en) | 2009-05-13 | 2013-01-15 | Sorin Biomedica Cardio, S.R.L. | Device for the in situ delivery of heart valves |
| EP2250975B1 (en) | 2009-05-13 | 2013-02-27 | Sorin Biomedica Cardio S.r.l. | Device for the in situ delivery of heart valves |
| US9168105B2 (en) | 2009-05-13 | 2015-10-27 | Sorin Group Italia S.R.L. | Device for surgical interventions |
| EP2250976B1 (en) | 2009-05-13 | 2015-08-26 | Sorin Group Italia S.r.l. | Device for the in situ delivery of heart valves |
| EP2263747B1 (en) | 2009-06-15 | 2015-08-26 | Sorin CRM SAS | Implantable cardiac prosthesis comprising means for analysing patient's tolerance of stimulation mode aiming at spontaneous atrio-ventricular conduction |
| AU2010266210B2 (en) | 2009-07-02 | 2015-01-22 | The Cleveland Clinic Foundation | Apparatus and method for replacing a diseased cardiac valve |
| US8845722B2 (en) | 2009-08-03 | 2014-09-30 | Shlomo Gabbay | Heart valve prosthesis and method of implantation thereof |
| WO2011025945A1 (en) | 2009-08-27 | 2011-03-03 | Medtronic Inc. | Transcatheter valve delivery systems and methods |
| WO2011025981A1 (en) | 2009-08-28 | 2011-03-03 | 3F Therapeutics, Inc. | Transapical delivery device and method of use |
| US8652203B2 (en) | 2010-09-23 | 2014-02-18 | Cardiaq Valve Technologies, Inc. | Replacement heart valves, delivery devices and methods |
| WO2011041571A2 (en) | 2009-10-01 | 2011-04-07 | Kardium Inc. | Medical device, kit and method for constricting tissue or a bodily orifice, for example, a mitral valve |
| CA2777067A1 (en) | 2009-10-14 | 2011-04-21 | Cardiovascular Technologies, Llc | Percutaneous transvalvular intraannular band for mitral valve repair |
| WO2011051043A1 (en) | 2009-11-02 | 2011-05-05 | Symetis Sa | Aortic bioprosthesis and systems for delivery thereof |
| WO2011057087A1 (en) | 2009-11-05 | 2011-05-12 | The Trustees University Of Pennsylvania | Valve prosthesis |
| EP2327366B1 (en) | 2009-11-30 | 2012-03-14 | Sorin CRM SAS | Kit for piercing the cardiac septum and implanting a transseptal probe, in particular a probe for detection/stimulation of a left cavity of the heart |
| US20130190861A1 (en) | 2012-01-23 | 2013-07-25 | Tendyne Holdings, Inc. | Prosthetic Valve for Replacing Mitral Valve |
| US8449599B2 (en) | 2009-12-04 | 2013-05-28 | Edwards Lifesciences Corporation | Prosthetic valve for replacing mitral valve |
| US8870950B2 (en) | 2009-12-08 | 2014-10-28 | Mitral Tech Ltd. | Rotation-based anchoring of an implant |
| EP3649985B8 (en) | 2009-12-08 | 2021-04-21 | Avalon Medical Ltd. | Device and system for transcatheter mitral valve replacement |
| US9504562B2 (en) | 2010-01-12 | 2016-11-29 | Valve Medical Ltd. | Self-assembling modular percutaneous valve and methods of folding, assembly and delivery |
| CN102905626A (en) * | 2010-01-29 | 2013-01-30 | Dc设备公司 | Devices and systems for treating heart failure |
| US8812431B2 (en) | 2010-02-03 | 2014-08-19 | Siemens Aktiengesellschaft | Method and system for medical decision support using organ models and learning based discriminative distance functions |
| US20110208293A1 (en) | 2010-02-23 | 2011-08-25 | Medtronic, Inc. | Catheter-Based Heart Valve Therapy System with Sizing Balloon |
| US9072603B2 (en) | 2010-02-24 | 2015-07-07 | Medtronic Ventor Technologies, Ltd. | Mitral prosthesis and methods for implantation |
| US9522062B2 (en) | 2010-02-24 | 2016-12-20 | Medtronic Ventor Technologies, Ltd. | Mitral prosthesis and methods for implantation |
| US8579788B2 (en) | 2010-03-08 | 2013-11-12 | Wilmo Orejola | Auto-regulated R-wave synchronized intraventricular balloon pump heart assist device |
| US20110224785A1 (en) | 2010-03-10 | 2011-09-15 | Hacohen Gil | Prosthetic mitral valve with tissue anchors |
| SE535690C2 (en) | 2010-03-25 | 2012-11-13 | Jan Otto Solem | An implantable device and cardiac support kit, comprising means for generating longitudinal movement of the mitral valve |
| SE535140C2 (en) | 2010-03-25 | 2012-04-24 | Jan Otto Solem | An implantable device, kit and system for improving cardiac function, including means for generating longitudinal movement of the mitral valve |
| US8652204B2 (en) | 2010-04-01 | 2014-02-18 | Medtronic, Inc. | Transcatheter valve with torsion spring fixation and related systems and methods |
| JP6085553B2 (en) | 2010-04-13 | 2017-02-22 | センターハート・インコーポレイテッドSentreHEART, Inc. | Devices and methods for accessing and delivering devices to the heart |
| EP3384879B1 (en) | 2010-04-23 | 2020-09-30 | Medtronic, Inc. | Delivery systems for prosthetic heart valves |
| EP2563278B1 (en) | 2010-04-27 | 2018-07-11 | Medtronic, Inc. | Transcatheter prosthetic heart valve delivery device with biased release features |
| US8579964B2 (en) | 2010-05-05 | 2013-11-12 | Neovasc Inc. | Transcatheter mitral valve prosthesis |
| IT1400327B1 (en) | 2010-05-21 | 2013-05-24 | Sorin Biomedica Cardio Srl | SUPPORT DEVICE FOR VALVULAR PROSTHESIS AND CORRESPONDING CORRESPONDENT. |
| IT1400544B1 (en) | 2010-06-09 | 2013-06-11 | Sorin Biomedica Cardio Srl | PROCESS OF DETOXIFICATION OF BIOLOGICAL FABRIC. |
| WO2011156714A2 (en) | 2010-06-10 | 2011-12-15 | Chambers Dr Jeffrey W | Systems and methods for preventing formation of blood clots in the left atrium |
| WO2011159779A2 (en) | 2010-06-15 | 2011-12-22 | Mayo Foundation For Medical Education And Research | Percutaneously deliverable valves |
| US8496671B1 (en) | 2010-06-16 | 2013-07-30 | Cardica, Inc. | Mitral valve treatment |
| EP4018966A1 (en) | 2010-06-21 | 2022-06-29 | Edwards Lifesciences CardiAQ LLC | Replacement heart valve |
| US8657872B2 (en) | 2010-07-19 | 2014-02-25 | Jacques Seguin | Cardiac valve repair system and methods of use |
| IT1401060B1 (en) | 2010-07-20 | 2013-07-12 | Global Engineering Const S R L | WATER WASTE SYSTEM OF THE SEA WAVES IN TUBS TO EXPLOIT THE ENERGY OF THE WILD MOTORCYCLE TO PRODUCE ELECTRICITY BY MEANS OF HYDRAULIC TURBINES AND CURRENT GENERATORS. |
| US8992604B2 (en) | 2010-07-21 | 2015-03-31 | Mitraltech Ltd. | Techniques for percutaneous mitral valve replacement and sealing |
| WO2012012660A2 (en) | 2010-07-21 | 2012-01-26 | Accola Kevin D | Prosthetic heart valves and devices, systems, and methods for deploying prosthetic heart valves |
| US9763657B2 (en) | 2010-07-21 | 2017-09-19 | Mitraltech Ltd. | Techniques for percutaneous mitral valve replacement and sealing |
| US9132009B2 (en) | 2010-07-21 | 2015-09-15 | Mitraltech Ltd. | Guide wires with commissural anchors to advance a prosthetic valve |
| WO2012019052A2 (en) | 2010-08-04 | 2012-02-09 | Micardia Corporation | Percutaneous transcatheter repair of heart valves |
| WO2012023978A2 (en) | 2010-08-17 | 2012-02-23 | St. Jude Medical, Inc. | Delivery system for collapsible heart valve |
| US9039759B2 (en) | 2010-08-24 | 2015-05-26 | St. Jude Medical, Cardiology Division, Inc. | Repositioning of prosthetic heart valve and deployment |
| US20120053680A1 (en) | 2010-08-24 | 2012-03-01 | Bolling Steven F | Reconfiguring Heart Features |
| US10105224B2 (en) * | 2010-09-01 | 2018-10-23 | Mvalve Technologies Ltd. | Cardiac valve support structure |
| AU2011296361B2 (en) | 2010-09-01 | 2015-05-28 | Medtronic Vascular Galway | Prosthetic valve support structure |
| JP5970458B2 (en) | 2010-09-01 | 2016-08-17 | ムバルブ・テクノロジーズ・リミテッド | Heart valve support structure |
| FR2964855B1 (en) | 2010-09-17 | 2013-10-18 | Ct Hospitalier Regional Universitaire D Amiens | IMPLANT INTENDED TO BE PLACED IN AURICULO-VENTRICULAR BLOOD PASSAGE |
| US10321998B2 (en) | 2010-09-23 | 2019-06-18 | Transmural Systems Llc | Methods and systems for delivering prostheses using rail techniques |
| US20120078360A1 (en) | 2010-09-23 | 2012-03-29 | Nasser Rafiee | Prosthetic devices, systems and methods for replacing heart valves |
| US9579193B2 (en) | 2010-09-23 | 2017-02-28 | Transmural Systems Llc | Methods and systems for delivering prostheses using rail techniques |
| EP3111889B1 (en) | 2010-09-24 | 2019-11-13 | Symetis SA | A transcatheter aortic valve implantation system |
| US8845720B2 (en) | 2010-09-27 | 2014-09-30 | Edwards Lifesciences Corporation | Prosthetic heart valve frame with flexible commissures |
| US8712133B2 (en) | 2010-09-29 | 2014-04-29 | Siemens Aktiengesellschaft | Cardiac chamber volume computation from contours and base plane in cardiac MR Cine images |
| US8792699B2 (en) | 2010-09-29 | 2014-07-29 | Siemens Aktiengesellschaft | Motion tracking for clinical parameter derivation and adaptive flow acquisition in magnetic resonance imaging |
| WO2012043898A1 (en) | 2010-09-29 | 2012-04-05 | Kim June-Hong | Tissue protective device for coronary sinus and tricuspid valve, knot delivery device, and device for mitral valve cerclage, containing same |
| US8532352B2 (en) | 2010-10-06 | 2013-09-10 | Siemens Aktiengesellschaft | Method and system for intraoperative guidance using physiological image fusion |
| EP2629699B1 (en) | 2010-10-21 | 2017-01-04 | Medtronic, Inc. | Mitral bioprosthesis with low ventricular profile |
| GB201017921D0 (en) | 2010-10-22 | 2010-12-01 | Ucl Business Plc | Prothesis delivery system |
| EP2633457B1 (en) | 2010-10-26 | 2021-10-06 | Oslo Universitetssykehus HF | Method for myocardial segment work analysis |
| CA2817319A1 (en) | 2010-11-09 | 2012-05-18 | Ironwood Pharmaceuticals, Inc. | Triazole derivatives as sgc stimulators |
| JP6010545B2 (en) | 2010-12-23 | 2016-10-19 | トゥエルヴ, インコーポレイテッド | System for mitral valve repair and replacement |
| EP2474287A1 (en) | 2011-01-11 | 2012-07-11 | Symetis Sa | Delivery catheter for stent-valve, and sub-assembly therefor |
| EP2663257B1 (en) | 2011-01-11 | 2016-10-26 | Hans Reiner Figulla | Valve prosthesis for replacing an atrioventricular valve of the heart |
| EP2478868A1 (en) | 2011-01-25 | 2012-07-25 | The Provost, Fellows, Foundation Scholars, and the other Members of Board, of the College of the Holy and Undivided Trinity of Queen Elizabeth | Implant device |
| US8845717B2 (en) | 2011-01-28 | 2014-09-30 | Middle Park Medical, Inc. | Coaptation enhancement implant, system, and method |
| US8888843B2 (en) | 2011-01-28 | 2014-11-18 | Middle Peak Medical, Inc. | Device, system, and method for transcatheter treatment of valve regurgitation |
| WO2012106602A2 (en) | 2011-02-04 | 2012-08-09 | Annest Lon Sutherland | A device and method for temporaty or permanent suspension of an implantable scaffolding containing an orifice for placement of a prosthetic or bio-prosthetic valve |
| EP2486894B1 (en) | 2011-02-14 | 2021-06-09 | Sorin Group Italia S.r.l. | Sutureless anchoring device for cardiac valve prostheses |
| ES2641902T3 (en) | 2011-02-14 | 2017-11-14 | Sorin Group Italia S.R.L. | Sutureless anchoring device for cardiac valve prostheses |
| US9445898B2 (en) | 2011-03-01 | 2016-09-20 | Medtronic Ventor Technologies Ltd. | Mitral valve repair |
| US8454656B2 (en) | 2011-03-01 | 2013-06-04 | Medtronic Ventor Technologies Ltd. | Self-suturing anchors |
| US20120226340A1 (en) | 2011-03-03 | 2012-09-06 | Empire Technology Development, Llc | Temporary perfusion channel for percutaneous delivery of balloon-expandable stents |
| EP2688516B1 (en) | 2011-03-21 | 2022-08-17 | Cephea Valve Technologies, Inc. | Disk-based valve apparatus |
| US9072511B2 (en) | 2011-03-25 | 2015-07-07 | Kardium Inc. | Medical kit for constricting tissue or a bodily orifice, for example, a mitral valve |
| EP3644194B1 (en) | 2011-04-15 | 2022-12-07 | Heartstitch, Inc. | Suturing devices for suturing an anatomic valve |
| US9554897B2 (en) | 2011-04-28 | 2017-01-31 | Neovasc Tiara Inc. | Methods and apparatus for engaging a valve prosthesis with tissue |
| US9308087B2 (en) | 2011-04-28 | 2016-04-12 | Neovasc Tiara Inc. | Sequentially deployed transcatheter mitral valve prosthesis |
| EP2522307B1 (en) | 2011-05-08 | 2020-09-30 | ITSO Medical AB | Device for delivery of medical devices to a cardiac valve |
| CN102883662B (en) | 2011-05-11 | 2015-04-08 | 株式会社东芝 | Medical image processing device and method for same |
| US20120303048A1 (en) | 2011-05-24 | 2012-11-29 | Sorin Biomedica Cardio S.R.I. | Transapical valve replacement |
| AU2012262538B2 (en) | 2011-05-27 | 2015-09-17 | Cormatrix Cardiovascular, Inc. | Sterilized, acellular extracellular matrix compositions and methods of making thereof |
| US9011523B2 (en) | 2011-06-20 | 2015-04-21 | Jacques Seguin | Prosthetic leaflet assembly for repairing a defective cardiac valve and methods of using the same |
| CA2840084C (en) | 2011-06-21 | 2019-11-05 | Foundry Newco Xii, Inc. | Prosthetic heart valve devices and associated systems and methods |
| EP2723272A4 (en) | 2011-06-24 | 2015-01-28 | Inceptus Medical LLC | Percutaneously implantable artificial heart valve system and associated methods and devices |
| EP2723277B1 (en) | 2011-06-27 | 2018-05-30 | University of Maryland, Baltimore | Transapical mitral valve repair device |
| WO2013017359A1 (en) | 2011-08-03 | 2013-02-07 | Aeeg Ab | Delivery device for medical implant and medical procedure |
| US20140324164A1 (en) | 2011-08-05 | 2014-10-30 | Mitraltech Ltd. | Techniques for percutaneous mitral valve replacement and sealing |
| WO2013021374A2 (en) | 2011-08-05 | 2013-02-14 | Mitraltech Ltd. | Techniques for percutaneous mitral valve replacement and sealing |
| US8852272B2 (en) | 2011-08-05 | 2014-10-07 | Mitraltech Ltd. | Techniques for percutaneous mitral valve replacement and sealing |
| EP3417813B1 (en) | 2011-08-05 | 2020-05-13 | Cardiovalve Ltd | Percutaneous mitral valve replacement |
| US9480559B2 (en) * | 2011-08-11 | 2016-11-01 | Tendyne Holdings, Inc. | Prosthetic valves and related inventions |
| DE102014102725A1 (en) | 2014-02-28 | 2015-09-17 | Highlife Sas | Transcatheter valve prosthesis |
| DE102014102653A1 (en) | 2014-02-28 | 2015-09-03 | Highlife Sas | Transcatheter valve prosthesis |
| ES2616030T3 (en) | 2011-09-12 | 2017-06-09 | Highlife Sas | Treatment catheter system |
| DE102014102721A1 (en) | 2014-02-28 | 2015-09-03 | Highlife Sas | Transcatheter valve prosthesis |
| CN103917194B (en) | 2011-09-12 | 2017-02-15 | 高品质生活简化股份公司 | Transcatheter valve prosthesis |
| US10080651B2 (en) | 2011-09-12 | 2018-09-25 | Highlife Sas | Transcatheter valve prosthesis |
| US9387075B2 (en) | 2011-09-12 | 2016-07-12 | Highlife Sas | Transcatheter valve prosthesis |
| WO2015020971A1 (en) | 2013-08-04 | 2015-02-12 | Mehr Medical Llc | Devices, systems and methods for repairing lumenal systems |
| US9549817B2 (en) | 2011-09-22 | 2017-01-24 | Transmural Systems Llc | Devices, systems and methods for repairing lumenal systems |
| US8900295B2 (en) | 2011-09-26 | 2014-12-02 | Edwards Lifesciences Corporation | Prosthetic valve with ventricular tethers |
| US9039757B2 (en) * | 2011-10-19 | 2015-05-26 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| US9655722B2 (en) | 2011-10-19 | 2017-05-23 | Twelve, Inc. | Prosthetic heart valve devices, prosthetic mitral valves and associated systems and methods |
| CN107028685B (en) | 2011-10-19 | 2019-11-15 | 托尔福公司 | Artificial heart valve film device, artificial mitral valve and related systems and methods |
| WO2013059743A1 (en) | 2011-10-19 | 2013-04-25 | Foundry Newco Xii, Inc. | Devices, systems and methods for heart valve replacement |
| EP2586492B1 (en) | 2011-10-24 | 2014-06-18 | St. Jude Medical AB | Pacing sequence optimization |
| CN104023646A (en) | 2011-11-23 | 2014-09-03 | 奥特鲁泰克控股有限公司 | Medical occlusion device |
| US20140350669A1 (en) | 2011-12-01 | 2014-11-27 | The Trustees if The University of Pennsylvania | Percutaneous valve replacement devices |
| EP2790609B1 (en) | 2011-12-12 | 2015-09-09 | David Alon | Heart valve repair device |
| US9827092B2 (en) | 2011-12-16 | 2017-11-28 | Tendyne Holdings, Inc. | Tethers for prosthetic mitral valve |
| US10321988B2 (en) | 2011-12-21 | 2019-06-18 | The Trustees Of The University Of Pennsylvania | Platforms for mitral valve replacement |
| EP2609893B1 (en) | 2011-12-29 | 2014-09-03 | Sorin Group Italia S.r.l. | A kit for implanting prosthetic vascular conduits |
| WO2013103612A1 (en) | 2012-01-04 | 2013-07-11 | Tendyne Holdings, Inc. | Improved multi-component cuff designs for transcatheter mitral valve replacement, subvalvular sealing apparatus for transcatheter mitral valves and wire framed leaflet assembly |
| FR2986149B1 (en) | 2012-01-26 | 2014-12-26 | Ct Hospitalier Universitaire De Clermont Fd | DEVICE FOR REPLACING AT LEAST ONE CORDAGE OF THE MITRAL VALVE AND KIT COMPRISING AT LEAST TWO DEVICES |
| US20130197354A1 (en) | 2012-01-30 | 2013-08-01 | Siemens Aktiengesellschaft | Minimally invasive treatment of mitral regurgitation |
| EP2809263B1 (en) | 2012-01-31 | 2017-08-23 | Mitral Valve Technologies Sàrl | Mitral valve docking devices, systems |
| AU2013221670A1 (en) | 2012-02-13 | 2014-10-02 | Mitraspan, Inc | Method and apparatus for repairing a mitral valve |
| CA3097321A1 (en) | 2012-02-22 | 2013-08-29 | Edwards Lifesciences Cardiaq Llc | Actively controllable stent, stent graft, heart valve and method of controlling same |
| US20130304197A1 (en) | 2012-02-28 | 2013-11-14 | Mvalve Technologies Ltd. | Cardiac valve modification device |
| AU2013227235B2 (en) | 2012-02-28 | 2017-10-19 | Mvalve Technologies Ltd. | Single-ring cardiac valve support |
| US20150094802A1 (en) | 2012-02-28 | 2015-04-02 | Mvalve Technologies Ltd. | Single-ring cardiac valve support |
| WO2013128432A1 (en) | 2012-02-28 | 2013-09-06 | Mvalve Technologies Ltd. | Cardiac valve support structure |
| US9180008B2 (en) | 2012-02-29 | 2015-11-10 | Valcare, Inc. | Methods, devices, and systems for percutaneously anchoring annuloplasty rings |
| EP3542758B1 (en) | 2012-02-29 | 2022-12-14 | Valcare, Inc. | Percutaneous annuloplasty system with anterior-posterior adjustment |
| US9579198B2 (en) | 2012-03-01 | 2017-02-28 | Twelve, Inc. | Hydraulic delivery systems for prosthetic heart valve devices and associated methods |
| AU2013229583B2 (en) | 2012-03-06 | 2017-04-13 | Highlife Sas | Treatment catheter member with encircling function |
| GB2500432A (en) | 2012-03-22 | 2013-09-25 | Stephen Brecker | Replacement heart valve with resiliently deformable securing means |
| ES2675936T3 (en) | 2012-03-23 | 2018-07-13 | Sorin Group Italia S.R.L. | Folding valve prosthesis |
| US9295547B2 (en) | 2012-03-28 | 2016-03-29 | Medtronic Vascular Galway | Prosthesis for transcatheter valve implantation |
| EP2644158A1 (en) | 2012-03-28 | 2013-10-02 | Sorin Group Italia S.r.l. | A kit for the manipulation of implantable medical devices |
| US8926694B2 (en) | 2012-03-28 | 2015-01-06 | Medtronic Vascular Galway Limited | Dual valve prosthesis for transcatheter valve implantation |
| US9023098B2 (en) | 2012-03-28 | 2015-05-05 | Medtronic, Inc. | Dual valve prosthesis for transcatheter valve implantation |
| US9066800B2 (en) | 2012-03-28 | 2015-06-30 | Medtronic, Inc. | Dual valve prosthesis for transcatheter valve implantation |
| EP2647354B1 (en) | 2012-04-04 | 2015-10-07 | Sorin Group Italia S.r.l. | A support device for heart valve prostheses |
| WO2013152161A1 (en) | 2012-04-04 | 2013-10-10 | The Trustees Of The University Of Pennsylvania | Treatment of mitral regurgitation |
| WO2013150512A1 (en) | 2012-04-05 | 2013-10-10 | Mvalve Technologies Ltd. | Cardiac valve support structure |
| US9259261B2 (en) | 2012-04-12 | 2016-02-16 | Vanderbilt University | Ablation catheter having temperature-controlled anchor and related methods |
| US9427315B2 (en) | 2012-04-19 | 2016-08-30 | Caisson Interventional, LLC | Valve replacement systems and methods |
| US9011515B2 (en) | 2012-04-19 | 2015-04-21 | Caisson Interventional, LLC | Heart valve assembly systems and methods |
| US20130289699A1 (en) | 2012-04-30 | 2013-10-31 | St. Jude Medical, Cardiology Division, Inc. | Aortic valve holder with stent protection and/or ability to decrease valve profile |
| WO2013163762A1 (en) | 2012-05-02 | 2013-11-07 | The Royal Institution For The Advancement Of Learning/Mcgill University | Device for soft tissue support and method for anchoring |
| US20130304180A1 (en) | 2012-05-09 | 2013-11-14 | Michael L. Green | Catheter having dual balloon hydraulic actuator |
| US9271855B2 (en) | 2012-05-09 | 2016-03-01 | Abbott Cardiovascular Systems Inc. | Catheter having hydraulic actuator with tandem chambers |
| EP2849681B1 (en) | 2012-05-16 | 2017-08-02 | Edwards Lifesciences Corporation | Devices for reducing cardiac valve regurgitation |
| EP2873011A1 (en) | 2012-05-16 | 2015-05-20 | Feops BVBA | Pre -operative simulation of trans - catheter valve implantation |
| EP2849680B1 (en) | 2012-05-16 | 2019-01-09 | Edwards Lifesciences Corporation | Coaptation element for reducing cardiac valve regurgitation |
| US9474605B2 (en) | 2012-05-16 | 2016-10-25 | Edwards Lifesciences Corporation | Devices and methods for reducing cardiac valve regurgitation |
| US20130310436A1 (en) | 2012-05-18 | 2013-11-21 | The Regents Of The University Of Colorado, A Body Corporate | Methods for predicting response to beta-blocker therapy in non-ischemic heart failure patients |
| AU2013264730B2 (en) | 2012-05-20 | 2018-02-01 | Tel Hashomer Medical Research Infrastructure And Services Ltd. | Prosthetic mitral valve |
| RU2508918C2 (en) | 2012-05-24 | 2014-03-10 | Закрытое Акционерное Общество Научно-Производственное Предприятие "Мединж" | Flexible atrioventricular valve prosthesis |
| US9526610B2 (en) | 2012-06-12 | 2016-12-27 | Medtronic, Inc. | Method and device for percutaneous valve annuloplasty |
| US9883941B2 (en) | 2012-06-19 | 2018-02-06 | Boston Scientific Scimed, Inc. | Replacement heart valve |
| EP2863844A4 (en) | 2012-06-22 | 2015-12-09 | Middle Peak Medical Inc | Device, system, and method for transcatheter treatment of valve regurgitation |
| JP5892657B2 (en) | 2012-07-17 | 2016-03-23 | 富士通株式会社 | Display processing program, display processing method, and display processing apparatus |
| US20140046436A1 (en) | 2012-07-27 | 2014-02-13 | The Regents Of The University Of California | Implantable prosthetic valves and methods |
| WO2014022124A1 (en) | 2012-07-28 | 2014-02-06 | Tendyne Holdings, Inc. | Improved multi-component designs for heart valve retrieval device, sealing structures and stent assembly |
| US9675454B2 (en) | 2012-07-30 | 2017-06-13 | Tendyne Holdings, Inc. | Delivery systems and methods for transcatheter prosthetic valves |
| US9693862B2 (en) | 2012-07-31 | 2017-07-04 | Edwards Lifesciences Corporation | Holders for prosthetic heart valves |
| ES2735536T3 (en) | 2012-08-10 | 2019-12-19 | Sorin Group Italia Srl | A valve prosthesis and a kit |
| US9468525B2 (en) | 2012-08-13 | 2016-10-18 | Medtronic, Inc. | Heart valve prosthesis |
| CN102805676B (en) | 2012-08-14 | 2015-06-17 | 杭州启明医疗器械有限公司 | Compression device for artificial valve replacement device |
| US9445899B2 (en) | 2012-08-22 | 2016-09-20 | Joseph M. Arcidi | Method and apparatus for mitral valve annuloplasty |
| US20140066895A1 (en) | 2012-08-29 | 2014-03-06 | Robert Kipperman | Anatomic device delivery and positioning system and method of use |
| US20140067048A1 (en) | 2012-09-06 | 2014-03-06 | Edwards Lifesciences Corporation | Heart Valve Sealing Devices |
| WO2014043527A2 (en) | 2012-09-14 | 2014-03-20 | Millepede, Llc. | Mitral valve inversion prostheses |
| US9309235B2 (en) | 2012-09-18 | 2016-04-12 | Ironwood Pharmaceuticals, Inc. | SGC stimulators |
| CA2885645A1 (en) | 2012-09-19 | 2014-03-27 | Ironwood Pharmaceuticals, Inc. | Sgc stimulators |
| US10512482B2 (en) | 2012-10-05 | 2019-12-24 | Board Of Regents Of The University Of Texas System | System and method for scoring the left ventricular endocardium to increase left ventricular compliance |
| EP2906151B1 (en) | 2012-10-12 | 2023-06-21 | Mardil, Inc. | Cardiac treatment system |
| US20140257473A1 (en) | 2012-10-22 | 2014-09-11 | Nalini Marie Rajamannan | Methods for inhibiting stenosis, obstruction, or calcification of a stented heart valve or bioprosthesis |
| US20140114407A1 (en) | 2012-10-22 | 2014-04-24 | ConcieValve LLC | Methods for inhibiting stenosis, obstruction, or calcification of a stented heart valve |
| WO2014064694A2 (en) | 2012-10-23 | 2014-05-01 | Valtech Cardio, Ltd. | Controlled steering functionality for implant-delivery tool |
| US9023099B2 (en) | 2012-10-31 | 2015-05-05 | Medtronic Vascular Galway Limited | Prosthetic mitral valve and delivery method |
| EP2928538B1 (en) | 2012-12-07 | 2018-11-21 | Valcare, Inc. | Devices for percutaneously anchoring annuloplasty rings |
| US20140163652A1 (en) | 2012-12-10 | 2014-06-12 | Thomas Witzel | Method for treating and repairing mitral valve annulus |
| CH707319A1 (en) | 2012-12-11 | 2014-06-13 | Carag Ag | Stent applicator. |
| WO2014093861A1 (en) | 2012-12-14 | 2014-06-19 | Mayo Foundation For Medical Education And Research | Mitral valve repair devices |
| CN103908729B (en) | 2012-12-28 | 2016-12-28 | 米特拉利根公司 | Energy aid in tissue sting device and using method thereof |
| US20140200662A1 (en) | 2013-01-16 | 2014-07-17 | Mvalve Technologies Ltd. | Anchoring elements for intracardiac devices |
| EP3417827B1 (en) | 2013-01-22 | 2022-08-31 | Frederick Guy | Kit for tooth and bone restoration via plasma deposition |
| WO2014116502A1 (en) | 2013-01-23 | 2014-07-31 | Rox Medical, Inc. | Methods, systems and devices for treating cardiac arrhythmias |
| US9681952B2 (en) | 2013-01-24 | 2017-06-20 | Mitraltech Ltd. | Anchoring of prosthetic valve supports |
| WO2014114794A2 (en) | 2013-01-25 | 2014-07-31 | Medtentia International Ltd Oy | A medical device and method for facilitating selection of an annuloplasty implant |
| ES2965612T3 (en) | 2013-01-25 | 2024-04-16 | Hvr Cardio Oy | A medical system with a device for collecting cords and/or leaflets |
| PL2948102T3 (en) | 2013-01-25 | 2019-08-30 | Medtentia International Ltd Oy | A valve for short time replacement, for taking over the function of and/or for temporary or partial support of a native valve in a heart |
| RU2672804C2 (en) | 2013-01-25 | 2018-11-19 | Медтентиа Интернэшнл Лтд Ой | System for cardiac valve repair |
| US20140222040A1 (en) | 2013-02-01 | 2014-08-07 | Jin S. Park | Method and Device for Connecting a Conduit to a Hollow Organ |
| JP6280932B2 (en) | 2013-02-04 | 2018-02-14 | トゥエルヴ, インコーポレイテッド | Hydraulic delivery system and related methods for prosthetic heart valve devices |
| US9439763B2 (en) | 2013-02-04 | 2016-09-13 | Edwards Lifesciences Corporation | Prosthetic valve for replacing mitral valve |
| US20160000564A1 (en) | 2013-02-20 | 2016-01-07 | Mvalve Technologies Ltd. | Delivery systems for cardiac valve support devices |
| US9326850B2 (en) | 2013-02-25 | 2016-05-03 | St. Jude Medical, Cardiology Division, Inc. | Sutureless prosthetic device |
| US20140243954A1 (en) | 2013-02-27 | 2014-08-28 | Donald Shannon | Transcatheter mitral valve prosthesis |
| US20160008636A1 (en) | 2013-02-28 | 2016-01-14 | Guided Interventions, Inc. | Ultrasound imaging sheath and associated method for guided percutaneous trans-catheter therapy |
| US10034667B2 (en) | 2013-02-28 | 2018-07-31 | St. Jude Medical, Cardiology Division, Inc. | Apparatus and method for heart valve repair |
| US9844435B2 (en) | 2013-03-01 | 2017-12-19 | St. Jude Medical, Cardiology Division, Inc. | Transapical mitral valve replacement |
| WO2014138194A1 (en) | 2013-03-07 | 2014-09-12 | Medtronic Vascular Galway | Prosthesis for transcatheter valve implantation |
| WO2014138284A1 (en) | 2013-03-07 | 2014-09-12 | Cedars-Sinai Medical Center | Catheter based apical approach heart prostheses delivery system |
| WO2014138482A1 (en) | 2013-03-07 | 2014-09-12 | Cedars-Sinai Medical Center | Method and apparatus for percutaneous delivery and deployment of a cardiovascular prosthesis |
| US10583002B2 (en) | 2013-03-11 | 2020-03-10 | Neovasc Tiara Inc. | Prosthetic valve with anti-pivoting mechanism |
| US9119713B2 (en) | 2013-03-11 | 2015-09-01 | St. Jude Medical, Cardiology Division, Inc. | Transcatheter valve replacement |
| US20140277408A1 (en) | 2013-03-12 | 2014-09-18 | Boston Scientific Scimed, Inc. | Prosthetic Heart Valve System |
| US9333043B2 (en) | 2013-03-12 | 2016-05-10 | Fetal Care Consultants, LLC | Fetal intervention using magnetically-guided navigation |
| EP2967870A4 (en) * | 2013-03-13 | 2016-11-16 | Aortic Innovations Llc | Dual frame stent and valve devices and implantation |
| US9539094B2 (en) | 2013-03-13 | 2017-01-10 | St. Jude Medical, Cardiology Division, Inc. | Simulated environment for transcatheter heart valve repair |
| US9999425B2 (en) | 2013-03-13 | 2018-06-19 | St. Jude Medical, Cardiology Division, Inc. | Mitral valve leaflet clip |
| US9730791B2 (en) | 2013-03-14 | 2017-08-15 | Edwards Lifesciences Cardiaq Llc | Prosthesis for atraumatically grasping intralumenal tissue and methods of delivery |
| US9681951B2 (en) | 2013-03-14 | 2017-06-20 | Edwards Lifesciences Cardiaq Llc | Prosthesis with outer skirt and anchors |
| EP2973126A2 (en) | 2013-03-14 | 2016-01-20 | Cardio Art Technologies Ltd. | System and method for personalized hemodynamics modeling and monitoring |
| US9687346B2 (en) | 2013-03-14 | 2017-06-27 | Edwards Lifesciences Corporation | Multi-stranded heat set annuloplasty rings |
| EP2777616B1 (en) | 2013-03-14 | 2020-08-19 | Edwards Lifesciences CardiAQ LLC | Prosthesis for atraumatically grasping intralumenal tissue |
| KR102362835B1 (en) | 2013-03-15 | 2022-02-14 | 사이클리온 테라퓨틱스, 인크. | sGC STIMULATORS |
| EP2777617B1 (en) | 2013-03-15 | 2022-09-14 | Edwards Lifesciences CardiAQ LLC | Prosthesis with outer skirt |
| US9724195B2 (en) | 2013-03-15 | 2017-08-08 | Mitralign, Inc. | Translation catheters and systems |
| US9289297B2 (en) | 2013-03-15 | 2016-03-22 | Cardiosolutions, Inc. | Mitral valve spacer and system and method for implanting the same |
| US9232998B2 (en) | 2013-03-15 | 2016-01-12 | Cardiosolutions Inc. | Trans-apical implant systems, implants and methods |
| CN110393608B (en) | 2013-03-15 | 2023-02-17 | 心脏结构导航公司 | Catheter-guided valve replacement devices and methods |
| FR2998167B1 (en) | 2013-03-20 | 2015-01-09 | Marco Vola | DEVICE FOR PERFORMING AN ANNULOPLASTY BY THE TRANSAPICAL PATH OF THE MITRAL VALVE |
| US9089414B2 (en) | 2013-03-22 | 2015-07-28 | Edwards Lifesciences Corporation | Device and method for increasing flow through the left atrial appendage |
| US20140296969A1 (en) | 2013-04-02 | 2014-10-02 | Tendyne Holdlings, Inc. | Anterior Leaflet Clip Device for Prosthetic Mitral Valve |
| US9486306B2 (en) | 2013-04-02 | 2016-11-08 | Tendyne Holdings, Inc. | Inflatable annular sealing device for prosthetic mitral valve |
| WO2014162306A2 (en) | 2013-04-02 | 2014-10-09 | Tendyne Holdings, Inc. | Improved devices and methods for transcatheter prosthetic heart valves |
| US20140296970A1 (en) | 2013-04-02 | 2014-10-02 | Tendyne Holdings, Inc. | Positioning Tool for Transcatheter Valve Delivery |
| US20140296971A1 (en) | 2013-04-02 | 2014-10-02 | Tendyne Holdings | Alignment Device for Asymmetric Transcatheter Valve |
| US10463489B2 (en) | 2013-04-02 | 2019-11-05 | Tendyne Holdings, Inc. | Prosthetic heart valve and systems and methods for delivering the same |
| DK2786817T3 (en) | 2013-04-05 | 2019-06-03 | Lillbacka Powerco Oy | Shrinking machine system |
| FR3004336A1 (en) | 2013-04-12 | 2014-10-17 | St George Medical Inc | MITRAL HEART VALVE PROSTHESIS AND RELIEF CATHETER |
| DE102013208038B4 (en) | 2013-05-02 | 2016-09-08 | Michael Siegenthaler | Catheter-based cardiac assist system |
| EP2994072B1 (en) | 2013-05-09 | 2022-01-19 | Mitrassist Medical Ltd. | Heart valve assistive prosthesis |
| EP2999435B1 (en) | 2013-05-20 | 2022-12-21 | Twelve, Inc. | Implantable heart valve devices, mitral valve repair devices and associated systems |
| ES2908132T3 (en) | 2013-05-20 | 2022-04-27 | Edwards Lifesciences Corp | Prosthetic Heart Valve Delivery Apparatus |
| US10813751B2 (en) | 2013-05-22 | 2020-10-27 | Valcare, Inc. | Transcatheter prosthetic valve for mitral or tricuspid valve replacement |
| EP3533417A1 (en) | 2013-05-22 | 2019-09-04 | ValCare, Inc. | Transcatheter prosthetic valve for mitral or tricuspid valve replacement |
| WO2014190329A1 (en) | 2013-05-24 | 2014-11-27 | Valcare, Inc. | Heart and peripheral vascular valve replacement in conjunction with a support ring |
| CN105578991B (en) | 2013-05-29 | 2017-11-14 | M阀门技术有限公司 | It is equipped with valve leaflets heart valve support device |
| US9610159B2 (en) | 2013-05-30 | 2017-04-04 | Tendyne Holdings, Inc. | Structural members for prosthetic mitral valves |
| US20140358224A1 (en) | 2013-05-30 | 2014-12-04 | Tendyne Holdlings, Inc. | Six cell inner stent device for prosthetic mitral valves |
| WO2014197924A1 (en) | 2013-06-14 | 2014-12-18 | Perfusion Solutions Pty Ltd | Cardiac function evaluation system |
| US9968445B2 (en) | 2013-06-14 | 2018-05-15 | The Regents Of The University Of California | Transcatheter mitral valve |
| WO2014201384A1 (en) | 2013-06-14 | 2014-12-18 | The Regents Of The University Of California | Transcatheter mitral valve |
| KR20160041040A (en) | 2013-06-14 | 2016-04-15 | 카디오솔루션즈, 인코포레이티드 | Mitral valve spacer and system and method for implanting the same |
| US20140371844A1 (en) | 2013-06-18 | 2014-12-18 | St. Jude Medical, Cardiology Division, Inc. | Transcatheter mitral valve and delivery system |
| WO2014210124A1 (en) | 2013-06-25 | 2014-12-31 | Mark Christianson | Thrombus management and structural compliance features for prosthetic heart valves |
| US20140379076A1 (en) | 2013-06-25 | 2014-12-25 | Tendyne Holdings, Inc. | Halo Wire Fluid Seal Device for Prosthetic Mitral Valves |
| EP3013262B1 (en) | 2013-06-26 | 2020-04-22 | Strait Access Technologies Holdings (PTY) LTD | Orientation device for use in mitral valve repair |
| US20150005874A1 (en) | 2013-06-27 | 2015-01-01 | Tendyne Holdings, Inc. | Atrial Thrombogenic Sealing Pockets for Prosthetic Mitral Valves |
| WO2014210299A1 (en) | 2013-06-27 | 2014-12-31 | Bridges Charles R | Device, system, and method for implanting a prosthetic heart valve |
| US9561103B2 (en) | 2013-07-17 | 2017-02-07 | Cephea Valve Technologies, Inc. | System and method for cardiac valve repair and replacement |
| US9895219B2 (en) | 2013-07-31 | 2018-02-20 | Medtronic Vascular Galway | Mitral valve prosthesis for transcatheter valve implantation |
| JP6465883B2 (en) | 2013-08-01 | 2019-02-06 | テンダイン ホールディングス,インコーポレイテッド | Epicardial anchor device and method |
| EP2835112B1 (en) | 2013-08-08 | 2021-01-27 | Sorin Group Italia S.r.l. | Heart valve prosthesis |
| CN105451686B (en) | 2013-08-14 | 2018-03-20 | 索林集团意大利有限责任公司 | Apparatus and method for chordae tendineae displacement |
| LT3545906T (en) | 2013-08-14 | 2021-03-10 | Mitral Valve Technologies Sarl | Replacement heart valve apparatus |
| EP2839815A1 (en) | 2013-08-20 | 2015-02-25 | Biotronik AG | Catheter system with movable sleeve |
| WO2015028986A1 (en) | 2013-08-29 | 2015-03-05 | June-Hong Kim | Rvot wire capturing (rwc) system in mitral valve cerclage annuloplasty |
| US20150094803A1 (en) | 2013-09-30 | 2015-04-02 | The Cleveland Clinic Foundation | Apparatus and method for treating a regurgitant heart valve |
| US9393111B2 (en) | 2014-01-15 | 2016-07-19 | Sino Medical Sciences Technology Inc. | Device and method for mitral valve regurgitation treatment |
| WO2015057407A1 (en) | 2013-10-05 | 2015-04-23 | Sino Medical Sciences Technology, Inc. | Device and method for mitral valve regurgitation method |
| US9839511B2 (en) | 2013-10-05 | 2017-12-12 | Sino Medical Sciences Technology Inc. | Device and method for mitral valve regurgitation treatment |
| US20150100116A1 (en) | 2013-10-07 | 2015-04-09 | Medizinische Universitat Wien | Implant and method for improving coaptation of an atrioventricular valve |
| EP3054895A4 (en) | 2013-10-08 | 2017-07-12 | The Medical Research, Infrastructure, And Health Services Fund Of The Tel Aviv Medical Center | Cardiac prostheses and their deployment |
| WO2015051430A1 (en) | 2013-10-10 | 2015-04-16 | Neves Filho Antonio Francisco | Arrangement applied to supporting element for heart-valve replacement or plasty |
| US10226333B2 (en) | 2013-10-15 | 2019-03-12 | Cedars-Sinai Medical Center | Anatomically-orientated and self-positioning transcatheter mitral valve |
| WO2015057995A2 (en) | 2013-10-16 | 2015-04-23 | Cedars-Sinai Medical Center | Modular dis-assembly of transcatheter valve replacement devices and uses thereof |
| WO2015058039A1 (en) | 2013-10-17 | 2015-04-23 | Robert Vidlund | Apparatus and methods for alignment and deployment of intracardiac devices |
| US20160279297A1 (en) | 2013-10-22 | 2016-09-29 | ConcieValve LLC | Methods for inhibiting stenosis, obstruction, or calcification of a stented heart valve or bioprosthesis |
| US9050188B2 (en) | 2013-10-23 | 2015-06-09 | Caisson Interventional, LLC | Methods and systems for heart valve therapy |
| US9662202B2 (en) | 2013-10-24 | 2017-05-30 | Medtronic, Inc. | Heart valve prosthesis |
| US10166098B2 (en) | 2013-10-25 | 2019-01-01 | Middle Peak Medical, Inc. | Systems and methods for transcatheter treatment of valve regurgitation |
| US9526611B2 (en) | 2013-10-29 | 2016-12-27 | Tendyne Holdings, Inc. | Apparatus and methods for delivery of transcatheter prosthetic valves |
| EP3572047A1 (en) | 2013-11-06 | 2019-11-27 | St. Jude Medical, Cardiology Division, Inc. | Reduced profile prosthetic heart valve |
| CN111419472B (en) | 2013-11-11 | 2023-01-10 | 爱德华兹生命科学卡迪尔克有限责任公司 | System and method for manufacturing stent frames |
| ES2637544T3 (en) | 2013-11-12 | 2017-10-13 | St. Jude Medical, Cardiology Division, Inc. | Delivery device for transfemoral mitral valve repair |
| US9848880B2 (en) | 2013-11-20 | 2017-12-26 | James E. Coleman | Adjustable heart valve implant |
| US20150173898A1 (en) | 2013-12-03 | 2015-06-25 | William Joseph Drasler | Transcatheter Mitral Valve Replacement Apparatus |
| WO2015092554A2 (en) | 2013-12-03 | 2015-06-25 | Mark Lynn Jenson | Transcatheter mitral valve replacement apparatus |
| WO2015081775A1 (en) | 2013-12-05 | 2015-06-11 | 中国科学院过程工程研究所 | Method for comprehensively using high-chromium-content vanadium-titanium magnetite concentrate |
| CN105764447A (en) | 2013-12-11 | 2016-07-13 | 雪松-西奈医学中心 | Methods, devices and systems for transcatheter mitral valve replacement in a double-orifice mitral valve |
| US9901444B2 (en) | 2013-12-17 | 2018-02-27 | Edwards Lifesciences Corporation | Inverted valve structure |
| KR102577607B1 (en) | 2014-02-04 | 2023-09-11 | 이노브하르트 에세.에레.엘레. | Prosthetic device for a heart valve |
| WO2015120122A2 (en) | 2014-02-05 | 2015-08-13 | Robert Vidlund | Apparatus and methods for transfemoral delivery of prosthetic mitral valve |
| US9986993B2 (en) | 2014-02-11 | 2018-06-05 | Tendyne Holdings, Inc. | Adjustable tether and epicardial pad system for prosthetic heart valve |
| EP2907479A1 (en) | 2014-02-18 | 2015-08-19 | Medtentia International Ltd Oy | A system and a method for delivery of an annuloplasty implant |
| CN115089349A (en) | 2014-02-20 | 2022-09-23 | 米特拉尔维尔福科技有限责任公司 | Convoluted anchor for supporting a prosthetic heart valve, prosthetic heart valve and deployment device |
| CR20160366A (en) | 2014-02-21 | 2016-11-15 | Mitral Valve Tecnhnologies Sarl | DEVICES, SYSTEMS AND METHODS OF SUPPLY OF PROSTHETIC MITRAL VALVE AND ANCHORAGE DEVICE |
| CN106170269B (en) | 2014-02-21 | 2019-01-11 | 爱德华兹生命科学卡迪尔克有限责任公司 | The delivery apparatus of controlled deployment for valve substitutes |
| US10064719B2 (en) | 2014-03-11 | 2018-09-04 | Highlife Sas | Transcatheter valve prosthesis |
| US9763779B2 (en) | 2014-03-11 | 2017-09-19 | Highlife Sas | Transcatheter valve prosthesis |
| WO2015128747A2 (en) | 2014-02-28 | 2015-09-03 | Highlife Sas | Transcatheter valve prosthesis |
| JP6625060B2 (en) | 2014-02-28 | 2019-12-25 | ハイライフ エスエーエス | Transcatheter type artificial valve |
| US20150250590A1 (en) | 2014-03-10 | 2015-09-10 | St. Jude Medical, Cardiology Division, Inc. | Transapical mitral chordae replacement |
| US9687343B2 (en) | 2014-03-11 | 2017-06-27 | Highlife Sas | Transcatheter valve prosthesis |
| BR102014006114B1 (en) | 2014-03-14 | 2022-05-10 | Antônio Francisco Neves Filho | Mechanical or biological heart valve stent for minimally invasive valve replacement procedure and stent delivery device |
| US10390943B2 (en) | 2014-03-17 | 2019-08-27 | Evalve, Inc. | Double orifice device for transcatheter mitral valve replacement |
| AU2015231788B2 (en) | 2014-03-18 | 2019-05-16 | St. Jude Medical, Cardiology Division, Inc. | Mitral valve replacement toggle cell securement |
| WO2015148241A1 (en) | 2014-03-26 | 2015-10-01 | St. Jude Medical, Cardiology Division, Inc. | Transcatheter mitral valve stent frames |
| WO2015171743A2 (en) | 2014-05-07 | 2015-11-12 | Baylor College Of Medicine | Artificial, flexible valves and methods of fabricating and serially expanding the same |
| CN112891028B (en) | 2014-05-09 | 2024-09-17 | 福达斯公司 | Replacement heart valve and methods of use and manufacture thereof |
| EP3142603B1 (en) | 2014-05-14 | 2018-03-07 | Sorin Group Italia S.r.l. | Implant device and implantation kit |
| US20150328000A1 (en) | 2014-05-19 | 2015-11-19 | Cardiaq Valve Technologies, Inc. | Replacement mitral valve with annular flap |
| US9757232B2 (en) | 2014-05-22 | 2017-09-12 | Edwards Lifesciences Corporation | Crimping apparatus for crimping prosthetic valve with protruding anchors |
| US9532870B2 (en) * | 2014-06-06 | 2017-01-03 | Edwards Lifesciences Corporation | Prosthetic valve for replacing a mitral valve |
| WO2015191604A1 (en) | 2014-06-10 | 2015-12-17 | Hlozek Edward | Direct view optical cardiac catheter |
| US9662203B2 (en) | 2014-06-11 | 2017-05-30 | Medtronic Vascular, Inc. | Prosthetic valve with vortice-inducing baffle |
| US10111749B2 (en) | 2014-06-11 | 2018-10-30 | Medtronic Vascular, Inc. | Prosthetic valve with flow director |
| US9974647B2 (en) | 2014-06-12 | 2018-05-22 | Caisson Interventional, LLC | Two stage anchor and mitral valve assembly |
| ES2908178T3 (en) | 2014-06-18 | 2022-04-28 | Polares Medical Inc | Mitral valve implants for the treatment of valvular regurgitation |
| US9345824B2 (en) | 2014-07-07 | 2016-05-24 | Assistocor Gmbh & Co Kg | Ventricular assist device |
| FR3023703B1 (en) | 2014-07-17 | 2021-01-29 | Cormove | BLOOD CIRCULATION DUCT TREATMENT DEVICE |
| US9180005B1 (en) | 2014-07-17 | 2015-11-10 | Millipede, Inc. | Adjustable endolumenal mitral valve ring |
| EP3174503A1 (en) | 2014-08-03 | 2017-06-07 | Mvalve Technologies Ltd. | Sealing elements for intracardiac devices |
| EP2982336A1 (en) | 2014-08-04 | 2016-02-10 | Alvimedica Tibb Ürünler San. Ve Dis Tic. A.S. | Mitral valve prosthesis, particularly suitable for transcatheter implantation |
| US20160038283A1 (en) | 2014-08-06 | 2016-02-11 | The University Of Iowa Research Foundation | Systems and methods utilizing expandable transcatheter valve |
| US10881461B2 (en) | 2014-08-07 | 2021-01-05 | Henry Ford Health System | Method of analyzing hollow anatomical structures for percutaneous implantation |
| US20170273782A1 (en) | 2014-08-21 | 2017-09-28 | Mvalve Technologies Ltd. | Cardiac valve support devices having improved compatibility with transcatheter prosthetic valves |
| US10016272B2 (en) | 2014-09-12 | 2018-07-10 | Mitral Valve Technologies Sarl | Mitral repair and replacement devices and methods |
| EP3213718B1 (en) | 2014-09-24 | 2019-06-26 | Sorin Group Italia S.r.l. | A holder for heart valve prostheses, corresponding storage arrangement, delivery instrument and kit |
| FR3027212A1 (en) | 2014-10-16 | 2016-04-22 | Seguin Jacques | INTERVALVULAR IMPLANT FOR MITRAL VALVE |
| US9750607B2 (en) | 2014-10-23 | 2017-09-05 | Caisson Interventional, LLC | Systems and methods for heart valve therapy |
| US9750605B2 (en) | 2014-10-23 | 2017-09-05 | Caisson Interventional, LLC | Systems and methods for heart valve therapy |
| JP6504516B2 (en) | 2014-10-24 | 2019-04-24 | Toto株式会社 | Hot water storage type electric water heater |
| US20160120643A1 (en) | 2014-11-05 | 2016-05-05 | Tara Kupumbati | Transcatheter cardiac valve prosthetic |
| EP4410245A3 (en) | 2014-11-26 | 2024-10-16 | Edwards Lifesciences Corporation | Transcatheter prosthetic heart valve and delivery system |
| US9492273B2 (en) | 2014-12-09 | 2016-11-15 | Cephea Valve Technologies, Inc. | Replacement cardiac valves and methods of use and manufacture |
| EP3037064B1 (en) | 2014-12-23 | 2018-03-14 | Venus MedTech (HangZhou), Inc. | Minimally invasive mitral valve replacement with brim |
| JP2017538540A (en) | 2014-12-19 | 2017-12-28 | 杭州啓明医療器械有限公司Venus Medtech (Hangzhou), Inc. | Minimally invasive brachial mitral valve replacement |
| WO2016108181A1 (en) | 2014-12-30 | 2016-07-07 | Tel Hashomer Medical Research, Infrastructure And Services Ltd. | Cardiac prostheses |
| US9861477B2 (en) | 2015-01-26 | 2018-01-09 | Boston Scientific Scimed Inc. | Prosthetic heart valve square leaflet-leaflet stitch |
| WO2016130991A1 (en) | 2015-02-13 | 2016-08-18 | Millipede, Inc. | Valve replacement using rotational anchors |
| WO2016133950A1 (en) | 2015-02-17 | 2016-08-25 | Medtronic Vascular Inc. | Methods for anchoring a heart valve prosthesis in a transcatheter valve implantation procedure |
| US10595992B2 (en) | 2015-02-20 | 2020-03-24 | 4C Medical Technologies, Inc. | Devices, systems and methods for cardiac treatment |
| CN107613908B (en) | 2015-03-19 | 2020-03-10 | 凯森因特万逊奈尔有限公司 | Systems and methods for heart valve therapy |
| EP3270825B1 (en) | 2015-03-20 | 2020-04-22 | JenaValve Technology, Inc. | Heart valve prosthesis delivery system |
| EP3273910A2 (en) | 2015-03-24 | 2018-01-31 | St. Jude Medical, Cardiology Division, Inc. | Mitral heart valve replacement |
| AU2016262564B2 (en) | 2015-05-14 | 2020-11-05 | Cephea Valve Technologies, Inc. | Replacement mitral valves |
| WO2016201024A1 (en) | 2015-06-12 | 2016-12-15 | St. Jude Medical, Cardiology Division, Inc. | Heart valve repair and replacement |
| US10226335B2 (en) | 2015-06-22 | 2019-03-12 | Edwards Lifesciences Cardiaq Llc | Actively controllable heart valve implant and method of controlling same |
| US9974650B2 (en) | 2015-07-14 | 2018-05-22 | Edwards Lifesciences Corporation | Prosthetic heart valve |
| ITUB20152409A1 (en) | 2015-07-22 | 2017-01-22 | Sorin Group Italia Srl | VALVE SLEEVE FOR VALVULAR PROSTHESIS AND CORRESPONDING DEVICE |
| WO2017030940A1 (en) | 2015-08-14 | 2017-02-23 | Caisson Interventional Llc | Systems and methods for heart valve therapy |
| US10327894B2 (en) | 2015-09-18 | 2019-06-25 | Tendyne Holdings, Inc. | Methods for delivery of prosthetic mitral valves |
| US10238495B2 (en) | 2015-10-09 | 2019-03-26 | Evalve, Inc. | Delivery catheter handle and methods of use |
| US11259920B2 (en) | 2015-11-03 | 2022-03-01 | Edwards Lifesciences Corporation | Adapter for prosthesis delivery device and methods of use |
| CN108992209B (en) | 2015-11-06 | 2022-03-04 | 麦克尔有限公司 | Mitral valve prosthesis |
| US10376364B2 (en) | 2015-11-10 | 2019-08-13 | Edwards Lifesciences Corporation | Implant delivery capsule |
| JP2018535754A (en) | 2015-12-03 | 2018-12-06 | テンダイン ホールディングス,インコーポレイテッド | Frame features for artificial mitral valves |
| CN105581858B (en) | 2015-12-15 | 2018-04-10 | 先健科技(深圳)有限公司 | Prosthetic heart valve holder and heart valve prosthesis |
| JP7002451B2 (en) | 2015-12-15 | 2022-01-20 | ニオバスク ティアラ インコーポレイテッド | Transseptal delivery system |
| EP3184081B1 (en) | 2015-12-22 | 2021-03-24 | Medira Ag | Prosthetic mitral valve coaptation enhancement device |
| US10265166B2 (en) | 2015-12-30 | 2019-04-23 | Caisson Interventional, LLC | Systems and methods for heart valve therapy |
| WO2017127939A1 (en) | 2016-01-29 | 2017-08-03 | Neovasc Tiara Inc. | Prosthetic valve for avoiding obstruction of outflow |
| CN108601655B (en) | 2016-02-04 | 2020-06-09 | 波士顿科学国际有限公司 | Mitral valve transition prosthesis |
| US10667904B2 (en) | 2016-03-08 | 2020-06-02 | Edwards Lifesciences Corporation | Valve implant with integrated sensor and transmitter |
| DE102016204345A1 (en) | 2016-03-16 | 2017-09-21 | Bayerische Motoren Werke Aktiengesellschaft | Remote laser processing system and method for operating a remote laser processing system |
| JP2019513510A (en) | 2016-04-01 | 2019-05-30 | リヴァノヴァ ユーエスエイ インコーポレイテッド | How to choose vagal nerve stimulation patients |
| DE102016106575A1 (en) | 2016-04-11 | 2017-10-12 | Biotronik Ag | Heart valve prosthesis |
| US10470877B2 (en) | 2016-05-03 | 2019-11-12 | Tendyne Holdings, Inc. | Apparatus and methods for anterior valve leaflet management |
| US10172710B2 (en) | 2016-05-10 | 2019-01-08 | William Joseph Drasler | Two component mitral valve |
| WO2017196909A1 (en) | 2016-05-12 | 2017-11-16 | St. Jude Medical, Cardiology Division, Inc. | Mitral heart valve replacement |
| EP3439582A4 (en) | 2016-05-13 | 2019-11-27 | Cardiosolutions, Inc. | Heart valve implant and methods for delivering and implanting same |
| EP3454788B1 (en) | 2016-05-13 | 2020-02-05 | St. Jude Medical, Cardiology Division, Inc. | Mitral valve delivery device |
| US11173026B2 (en) | 2016-06-15 | 2021-11-16 | Corcym S.R.L. | Two-part mitral valve and implant method |
| US20170360558A1 (en) | 2016-06-16 | 2017-12-21 | Jianlu Ma | Method and design for a mitral regurgitation treatment device |
| US10350062B2 (en) * | 2016-07-21 | 2019-07-16 | Edwards Lifesciences Corporation | Replacement heart valve prosthesis |
| EP3848003A1 (en) | 2016-08-10 | 2021-07-14 | Cardiovalve Ltd. | Prosthetic valve with concentric frames |
| US10688308B2 (en) | 2016-12-12 | 2020-06-23 | Sorin Crm Sas | System and method for extra cardiac defibrillation |
| EP3576670A4 (en) | 2017-02-06 | 2020-12-30 | Caisson Interventional LLC | Systems and methods for heart valve therapy |
| WO2018167536A1 (en) | 2017-03-15 | 2018-09-20 | Sorin Group Italia S.R.L. | Implantable material and method for preserving |
| US10702378B2 (en) | 2017-04-18 | 2020-07-07 | Twelve, Inc. | Prosthetic heart valve device and associated systems and methods |
| WO2018217338A1 (en) | 2017-05-26 | 2018-11-29 | Caisson Interventional, LLC | Systems and methods for heart valve therapy |
| US10786352B2 (en) | 2017-07-06 | 2020-09-29 | Twelve, Inc. | Prosthetic heart valve devices and associated systems and methods |
| US12083013B2 (en) | 2017-10-07 | 2024-09-10 | Corcym S.R.L. | Bendable cardiac surgery instruments |
| WO2019209927A1 (en) | 2018-04-24 | 2019-10-31 | Caisson Interventional, LLC | Systems and methods for heart valve therapy |
-
2017
- 2017-07-06 US US15/642,834 patent/US10729541B2/en active Active
-
2018
- 2018-06-21 CN CN202210554877.XA patent/CN115444619A/en active Pending
- 2018-06-21 CN CN201880045252.2A patent/CN110891525B/en active Active
- 2018-06-21 EP EP18743631.6A patent/EP3648707B1/en active Active
- 2018-06-21 JP JP2019572460A patent/JP2020525215A/en active Pending
- 2018-06-21 WO PCT/US2018/038841 patent/WO2019010010A1/en unknown
- 2018-06-21 EP EP20192122.8A patent/EP3763332A1/en active Pending
- 2018-06-21 AU AU2018297210A patent/AU2018297210A1/en not_active Abandoned
-
2020
- 2020-07-31 US US16/944,957 patent/US11877926B2/en active Active
-
2023
- 2023-12-15 US US18/541,105 patent/US20240108466A1/en active Pending
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2010519957A (en) * | 2007-03-01 | 2010-06-10 | ボストン サイエンティフィック リミテッド | Coated medical device for delivering a drug outside the lumen |
| US20120067103A1 (en) * | 2010-09-17 | 2012-03-22 | Medtronic Vascular, Inc. | Method of Forming a Drug-Eluting Medical Device |
| JP2015510415A (en) * | 2012-01-27 | 2015-04-09 | メドトロニック ヴァスキュラー インコーポレイテッド | Drug-filled hollow stent and method for forming a drug-filled hollow stent |
| JP2016512753A (en) * | 2013-03-15 | 2016-05-09 | トゥエルヴ, インコーポレイテッド | Prosthetic heart valve device, prosthetic mitral valve, and related systems and methods |
Also Published As
| Publication number | Publication date |
|---|---|
| EP3648707B1 (en) | 2024-03-27 |
| US20240108466A1 (en) | 2024-04-04 |
| EP3648707A1 (en) | 2020-05-13 |
| US11877926B2 (en) | 2024-01-23 |
| CN115444619A (en) | 2022-12-09 |
| WO2019010010A1 (en) | 2019-01-10 |
| US20190008635A1 (en) | 2019-01-10 |
| CN110891525A (en) | 2020-03-17 |
| US10729541B2 (en) | 2020-08-04 |
| US20200360137A1 (en) | 2020-11-19 |
| AU2018297210A1 (en) | 2020-02-06 |
| CN110891525B (en) | 2022-07-08 |
| EP3763332A1 (en) | 2021-01-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US11877926B2 (en) | Prosthetic heart valve devices and associated systems and methods | |
| US12016772B2 (en) | Prosthetic heart valve devices and associated systems and methods | |
| US11654021B2 (en) | Prosthetic heart valve device and associated systems and methods | |
| JP6736637B2 (en) | Devices, systems and methods for heart valve replacement | |
| US20190000619A1 (en) | Catheter-guided replacement valves apparatus and methods | |
| US9763780B2 (en) | Devices, systems and methods for heart valve replacement | |
| US9775704B2 (en) | Implantable valve prosthesis | |
| TW202245716A (en) | Expandable prosthetic heart valve with flattened apices |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20210618 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20210618 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20220624 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20220628 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20220927 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20221125 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20221226 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20230323 |