JP2015508780A - 免疫不全患者のインフルエンザ及びパラインフルエンザウイルスを処置するための方法、化合物、及び組成物 - Google Patents
免疫不全患者のインフルエンザ及びパラインフルエンザウイルスを処置するための方法、化合物、及び組成物 Download PDFInfo
- Publication number
- JP2015508780A JP2015508780A JP2014557870A JP2014557870A JP2015508780A JP 2015508780 A JP2015508780 A JP 2015508780A JP 2014557870 A JP2014557870 A JP 2014557870A JP 2014557870 A JP2014557870 A JP 2014557870A JP 2015508780 A JP2015508780 A JP 2015508780A
- Authority
- JP
- Japan
- Prior art keywords
- patient
- das181
- virus
- infection
- sialidase
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 238000000034 method Methods 0.000 title claims abstract description 72
- 239000000203 mixture Substances 0.000 title claims abstract description 51
- 208000002606 Paramyxoviridae Infections Diseases 0.000 title claims abstract description 17
- 206010061598 Immunodeficiency Diseases 0.000 title claims abstract description 14
- 241000700605 Viruses Species 0.000 title claims description 174
- 150000001875 compounds Chemical class 0.000 title claims description 11
- 206010022000 influenza Diseases 0.000 title description 3
- 108010006232 Neuraminidase Proteins 0.000 claims abstract description 53
- 102000005348 Neuraminidase Human genes 0.000 claims abstract description 52
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 35
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 35
- 230000009385 viral infection Effects 0.000 claims abstract description 29
- 230000000694 effects Effects 0.000 claims abstract description 22
- 241000712461 unidentified influenza virus Species 0.000 claims abstract description 15
- 210000002345 respiratory system Anatomy 0.000 claims abstract description 12
- 108700013356 oplunofusp Proteins 0.000 claims description 186
- 208000015181 infectious disease Diseases 0.000 claims description 116
- 230000003197 catalytic effect Effects 0.000 claims description 22
- 229920002683 Glycosaminoglycan Polymers 0.000 claims description 20
- 230000000241 respiratory effect Effects 0.000 claims description 20
- 239000006199 nebulizer Substances 0.000 claims description 17
- 239000000843 powder Substances 0.000 claims description 15
- 239000007788 liquid Substances 0.000 claims description 14
- 229940112141 dry powder inhaler Drugs 0.000 claims description 13
- 108090000765 processed proteins & peptides Proteins 0.000 claims description 12
- 230000002829 reductive effect Effects 0.000 claims description 11
- 229920001184 polypeptide Polymers 0.000 claims description 8
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 8
- 241000186044 Actinomyces viscosus Species 0.000 claims description 5
- 239000000443 aerosol Substances 0.000 claims description 5
- 238000004873 anchoring Methods 0.000 claims description 5
- 239000007921 spray Substances 0.000 claims description 5
- 239000007922 nasal spray Substances 0.000 claims description 4
- 229940097496 nasal spray Drugs 0.000 claims description 4
- 101000809450 Homo sapiens Amphiregulin Proteins 0.000 claims description 3
- -1 endotracheal tube Substances 0.000 claims description 3
- 102000043494 human AREG Human genes 0.000 claims description 3
- 238000002650 immunosuppressive therapy Methods 0.000 claims description 3
- 229940071648 metered dose inhaler Drugs 0.000 claims description 3
- 239000002245 particle Substances 0.000 claims description 3
- 108010083590 Apoproteins Proteins 0.000 claims description 2
- 102000006410 Apoproteins Human genes 0.000 claims description 2
- 101000583741 Clostridium perfringens Sialidase Proteins 0.000 claims description 2
- 101000757319 Homo sapiens Antithrombin-III Proteins 0.000 claims description 2
- 101001055222 Homo sapiens Interleukin-8 Proteins 0.000 claims description 2
- 101000582950 Homo sapiens Platelet factor 4 Proteins 0.000 claims description 2
- 241000187708 Micromonospora Species 0.000 claims description 2
- 208000031951 Primary immunodeficiency Diseases 0.000 claims description 2
- 206010054979 Secondary immunodeficiency Diseases 0.000 claims description 2
- 239000002246 antineoplastic agent Substances 0.000 claims description 2
- 229940127089 cytotoxic agent Drugs 0.000 claims description 2
- 102000052624 human CXCL8 Human genes 0.000 claims description 2
- 102000052196 human PF4 Human genes 0.000 claims description 2
- 102000052834 human SERPINC1 Human genes 0.000 claims description 2
- 229960004336 human antithrombin iii Drugs 0.000 claims description 2
- 230000009325 pulmonary function Effects 0.000 claims description 2
- 125000003275 alpha amino acid group Chemical group 0.000 claims 2
- 230000004202 respiratory function Effects 0.000 claims 2
- 239000006200 vaporizer Substances 0.000 claims 2
- BLUGYPPOFIHFJS-UUFHNPECSA-N (2s)-n-[(2s)-1-[[(3r,4s,5s)-3-methoxy-1-[(2s)-2-[(1r,2r)-1-methoxy-2-methyl-3-oxo-3-[[(1s)-2-phenyl-1-(1,3-thiazol-2-yl)ethyl]amino]propyl]pyrrolidin-1-yl]-5-methyl-1-oxoheptan-4-yl]-methylamino]-3-methyl-1-oxobutan-2-yl]-3-methyl-2-(methylamino)butanamid Chemical compound CN[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N(C)[C@@H]([C@@H](C)CC)[C@H](OC)CC(=O)N1CCC[C@H]1[C@H](OC)[C@@H](C)C(=O)N[C@H](C=1SC=CN=1)CC1=CC=CC=C1 BLUGYPPOFIHFJS-UUFHNPECSA-N 0.000 claims 1
- 208000007934 ACTH-independent macronodular adrenal hyperplasia Diseases 0.000 claims 1
- 241001524178 Paenarthrobacter ureafaciens Species 0.000 claims 1
- 210000004204 blood vessel Anatomy 0.000 claims 1
- 230000005012 migration Effects 0.000 claims 1
- 238000013508 migration Methods 0.000 claims 1
- 210000004027 cell Anatomy 0.000 description 139
- 238000011282 treatment Methods 0.000 description 77
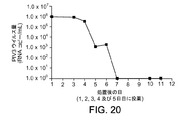
- 230000003612 virological effect Effects 0.000 description 71
- 238000003556 assay Methods 0.000 description 45
- 239000003814 drug Substances 0.000 description 31
- 229940079593 drug Drugs 0.000 description 30
- 238000002962 plaque-reduction assay Methods 0.000 description 28
- 238000004458 analytical method Methods 0.000 description 27
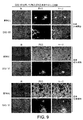
- 230000000120 cytopathologic effect Effects 0.000 description 26
- 239000006228 supernatant Substances 0.000 description 25
- 208000036142 Viral infection Diseases 0.000 description 24
- 239000000523 sample Substances 0.000 description 24
- 150000001413 amino acids Chemical group 0.000 description 22
- 239000003153 chemical reaction reagent Substances 0.000 description 20
- 230000030833 cell death Effects 0.000 description 18
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 16
- 238000002474 experimental method Methods 0.000 description 16
- 229910052760 oxygen Inorganic materials 0.000 description 16
- 239000001301 oxygen Substances 0.000 description 16
- 230000012010 growth Effects 0.000 description 15
- 210000004072 lung Anatomy 0.000 description 15
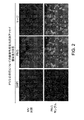
- 238000010186 staining Methods 0.000 description 15
- 230000006872 improvement Effects 0.000 description 14
- 239000002609 medium Substances 0.000 description 14
- 229920000936 Agarose Polymers 0.000 description 13
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 12
- 238000009472 formulation Methods 0.000 description 12
- 238000011081 inoculation Methods 0.000 description 12
- 238000012360 testing method Methods 0.000 description 11
- 206010011224 Cough Diseases 0.000 description 10
- 230000003321 amplification Effects 0.000 description 10
- 239000000427 antigen Substances 0.000 description 10
- 108091007433 antigens Proteins 0.000 description 10
- 102000036639 antigens Human genes 0.000 description 10
- 230000005764 inhibitory process Effects 0.000 description 10
- 238000003199 nucleic acid amplification method Methods 0.000 description 10
- 208000000059 Dyspnea Diseases 0.000 description 9
- 206010013975 Dyspnoeas Diseases 0.000 description 9
- 239000012228 culture supernatant Substances 0.000 description 9
- 210000001519 tissue Anatomy 0.000 description 9
- 206010035664 Pneumonia Diseases 0.000 description 8
- 230000002411 adverse Effects 0.000 description 8
- 210000000038 chest Anatomy 0.000 description 8
- 238000010790 dilution Methods 0.000 description 8
- 239000012895 dilution Substances 0.000 description 8
- 108020001507 fusion proteins Proteins 0.000 description 8
- 102000037865 fusion proteins Human genes 0.000 description 8
- 230000009467 reduction Effects 0.000 description 8
- 239000000243 solution Substances 0.000 description 8
- 230000001629 suppression Effects 0.000 description 8
- 208000009329 Graft vs Host Disease Diseases 0.000 description 7
- SQVRNKJHWKZAKO-UHFFFAOYSA-N beta-N-Acetyl-D-neuraminic acid Natural products CC(=O)NC1C(O)CC(O)(C(O)=O)OC1C(O)C(O)CO SQVRNKJHWKZAKO-UHFFFAOYSA-N 0.000 description 7
- 238000004113 cell culture Methods 0.000 description 7
- 208000024908 graft versus host disease Diseases 0.000 description 7
- 230000001965 increasing effect Effects 0.000 description 7
- 239000007758 minimum essential medium Substances 0.000 description 7
- 239000000758 substrate Substances 0.000 description 7
- SXRSQZLOMIGNAQ-UHFFFAOYSA-N Glutaraldehyde Chemical compound O=CCCCC=O SXRSQZLOMIGNAQ-UHFFFAOYSA-N 0.000 description 6
- 238000012790 confirmation Methods 0.000 description 6
- 239000011521 glass Substances 0.000 description 6
- 239000001963 growth medium Substances 0.000 description 6
- 230000002458 infectious effect Effects 0.000 description 6
- 230000007505 plaque formation Effects 0.000 description 6
- 230000004044 response Effects 0.000 description 6
- 241000701161 unidentified adenovirus Species 0.000 description 6
- 238000011529 RT qPCR Methods 0.000 description 5
- 239000013553 cell monolayer Substances 0.000 description 5
- 230000003013 cytotoxicity Effects 0.000 description 5
- 231100000135 cytotoxicity Toxicity 0.000 description 5
- 230000003247 decreasing effect Effects 0.000 description 5
- 238000012423 maintenance Methods 0.000 description 5
- 239000000463 material Substances 0.000 description 5
- 125000001360 methionine group Chemical group N[C@@H](CCSC)C(=O)* 0.000 description 5
- 210000000214 mouth Anatomy 0.000 description 5
- 210000001331 nose Anatomy 0.000 description 5
- 238000013207 serial dilution Methods 0.000 description 5
- 150000003431 steroids Chemical class 0.000 description 5
- 230000009469 supplementation Effects 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 238000012549 training Methods 0.000 description 5
- HJCMDXDYPOUFDY-WHFBIAKZSA-N Ala-Gln Chemical compound C[C@H](N)C(=O)N[C@H](C(O)=O)CCC(N)=O HJCMDXDYPOUFDY-WHFBIAKZSA-N 0.000 description 4
- 208000037656 Respiratory Sounds Diseases 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 239000002775 capsule Substances 0.000 description 4
- 239000003795 chemical substances by application Substances 0.000 description 4
- 230000001684 chronic effect Effects 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 239000002552 dosage form Substances 0.000 description 4
- 239000012530 fluid Substances 0.000 description 4
- 230000008014 freezing Effects 0.000 description 4
- 238000007710 freezing Methods 0.000 description 4
- 239000013610 patient sample Substances 0.000 description 4
- 239000008194 pharmaceutical composition Substances 0.000 description 4
- 210000003800 pharynx Anatomy 0.000 description 4
- 238000009613 pulmonary function test Methods 0.000 description 4
- SQVRNKJHWKZAKO-OQPLDHBCSA-N sialic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)OC1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-OQPLDHBCSA-N 0.000 description 4
- 125000005629 sialic acid group Chemical group 0.000 description 4
- 238000006467 substitution reaction Methods 0.000 description 4
- QRXMUCSWCMTJGU-UHFFFAOYSA-N 5-bromo-4-chloro-3-indolyl phosphate Chemical compound C1=C(Br)C(Cl)=C2C(OP(O)(=O)O)=CNC2=C1 QRXMUCSWCMTJGU-UHFFFAOYSA-N 0.000 description 3
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 3
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 3
- 206010006448 Bronchiolitis Diseases 0.000 description 3
- IAZDPXIOMUYVGZ-UHFFFAOYSA-N Dimethylsulphoxide Chemical compound CS(C)=O IAZDPXIOMUYVGZ-UHFFFAOYSA-N 0.000 description 3
- 241000588724 Escherichia coli Species 0.000 description 3
- SQVRNKJHWKZAKO-PFQGKNLYSA-N N-acetyl-beta-neuraminic acid Chemical compound CC(=O)N[C@@H]1[C@@H](O)C[C@@](O)(C(O)=O)O[C@H]1[C@H](O)[C@H](O)CO SQVRNKJHWKZAKO-PFQGKNLYSA-N 0.000 description 3
- 206010067482 No adverse event Diseases 0.000 description 3
- 206010037660 Pyrexia Diseases 0.000 description 3
- 206010057190 Respiratory tract infections Diseases 0.000 description 3
- QJJXYPPXXYFBGM-LFZNUXCKSA-N Tacrolimus Chemical compound C1C[C@@H](O)[C@H](OC)C[C@@H]1\C=C(/C)[C@@H]1[C@H](C)[C@@H](O)CC(=O)[C@H](CC=C)/C=C(C)/C[C@H](C)C[C@H](OC)[C@H]([C@H](C[C@H]2C)OC)O[C@@]2(O)C(=O)C(=O)N2CCCC[C@H]2C(=O)O1 QJJXYPPXXYFBGM-LFZNUXCKSA-N 0.000 description 3
- 108020000999 Viral RNA Proteins 0.000 description 3
- 238000002820 assay format Methods 0.000 description 3
- 210000004369 blood Anatomy 0.000 description 3
- 239000008280 blood Substances 0.000 description 3
- 238000013276 bronchoscopy Methods 0.000 description 3
- 230000007423 decrease Effects 0.000 description 3
- 201000010099 disease Diseases 0.000 description 3
- 238000002618 extracorporeal membrane oxygenation Methods 0.000 description 3
- 238000012921 fluorescence analysis Methods 0.000 description 3
- 230000002401 inhibitory effect Effects 0.000 description 3
- 239000010410 layer Substances 0.000 description 3
- 239000012669 liquid formulation Substances 0.000 description 3
- 238000012986 modification Methods 0.000 description 3
- 230000004048 modification Effects 0.000 description 3
- RTGDFNSFWBGLEC-SYZQJQIISA-N mycophenolate mofetil Chemical compound COC1=C(C)C=2COC(=O)C=2C(O)=C1C\C=C(/C)CCC(=O)OCCN1CCOCC1 RTGDFNSFWBGLEC-SYZQJQIISA-N 0.000 description 3
- 244000052769 pathogen Species 0.000 description 3
- 239000000546 pharmaceutical excipient Substances 0.000 description 3
- XOFYZVNMUHMLCC-ZPOLXVRWSA-N prednisone Chemical compound O=C1C=C[C@]2(C)[C@H]3C(=O)C[C@](C)([C@@](CC4)(O)C(=O)CO)[C@@H]4[C@@H]3CCC2=C1 XOFYZVNMUHMLCC-ZPOLXVRWSA-N 0.000 description 3
- 229960004618 prednisone Drugs 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000001681 protective effect Effects 0.000 description 3
- 230000029058 respiratory gaseous exchange Effects 0.000 description 3
- 238000003757 reverse transcription PCR Methods 0.000 description 3
- 238000012216 screening Methods 0.000 description 3
- 208000013220 shortness of breath Diseases 0.000 description 3
- 239000002356 single layer Substances 0.000 description 3
- 239000012128 staining reagent Substances 0.000 description 3
- 239000011550 stock solution Substances 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 229960001967 tacrolimus Drugs 0.000 description 3
- QJJXYPPXXYFBGM-SHYZHZOCSA-N tacrolimus Natural products CO[C@H]1C[C@H](CC[C@@H]1O)C=C(C)[C@H]2OC(=O)[C@H]3CCCCN3C(=O)C(=O)[C@@]4(O)O[C@@H]([C@H](C[C@H]4C)OC)[C@@H](C[C@H](C)CC(=C[C@@H](CC=C)C(=O)C[C@H](O)[C@H]2C)C)OC QJJXYPPXXYFBGM-SHYZHZOCSA-N 0.000 description 3
- WRMNZCZEMHIOCP-UHFFFAOYSA-N 2-phenylethanol Chemical compound OCCC1=CC=CC=C1 WRMNZCZEMHIOCP-UHFFFAOYSA-N 0.000 description 2
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 2
- VWFCHDSQECPREK-LURJTMIESA-N Cidofovir Chemical compound NC=1C=CN(C[C@@H](CO)OCP(O)(O)=O)C(=O)N=1 VWFCHDSQECPREK-LURJTMIESA-N 0.000 description 2
- 206010011376 Crepitations Diseases 0.000 description 2
- 208000011231 Crohn disease Diseases 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 2
- 102000003886 Glycoproteins Human genes 0.000 description 2
- 108090000288 Glycoproteins Proteins 0.000 description 2
- 206010021143 Hypoxia Diseases 0.000 description 2
- 201000009794 Idiopathic Pulmonary Fibrosis Diseases 0.000 description 2
- 208000029523 Interstitial Lung disease Diseases 0.000 description 2
- GSDSWSVVBLHKDQ-JTQLQIEISA-N Levofloxacin Chemical compound C([C@@H](N1C2=C(C(C(C(O)=O)=C1)=O)C=C1F)C)OC2=C1N1CCN(C)CC1 GSDSWSVVBLHKDQ-JTQLQIEISA-N 0.000 description 2
- 229920001213 Polysorbate 20 Polymers 0.000 description 2
- 239000004793 Polystyrene Substances 0.000 description 2
- 238000002123 RNA extraction Methods 0.000 description 2
- 208000004756 Respiratory Insufficiency Diseases 0.000 description 2
- 102000004142 Trypsin Human genes 0.000 description 2
- 108090000631 Trypsin Proteins 0.000 description 2
- 108010059993 Vancomycin Proteins 0.000 description 2
- 206010047924 Wheezing Diseases 0.000 description 2
- 125000001931 aliphatic group Chemical group 0.000 description 2
- 230000000735 allogeneic effect Effects 0.000 description 2
- 239000004599 antimicrobial Substances 0.000 description 2
- 230000009286 beneficial effect Effects 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- 230000003115 biocidal effect Effects 0.000 description 2
- 238000004820 blood count Methods 0.000 description 2
- 210000001185 bone marrow Anatomy 0.000 description 2
- 210000000621 bronchi Anatomy 0.000 description 2
- 239000000872 buffer Substances 0.000 description 2
- 229910002091 carbon monoxide Inorganic materials 0.000 description 2
- 229960000484 ceftazidime Drugs 0.000 description 2
- NMVPEQXCMGEDNH-TZVUEUGBSA-N ceftazidime pentahydrate Chemical compound O.O.O.O.O.S([C@@H]1[C@@H](C(N1C=1C([O-])=O)=O)NC(=O)\C(=N/OC(C)(C)C(O)=O)C=2N=C(N)SC=2)CC=1C[N+]1=CC=CC=C1 NMVPEQXCMGEDNH-TZVUEUGBSA-N 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- OSASVXMJTNOKOY-UHFFFAOYSA-N chlorobutanol Chemical compound CC(C)(O)C(Cl)(Cl)Cl OSASVXMJTNOKOY-UHFFFAOYSA-N 0.000 description 2
- 229960000724 cidofovir Drugs 0.000 description 2
- 238000002591 computed tomography Methods 0.000 description 2
- 238000002716 delivery method Methods 0.000 description 2
- 230000008021 deposition Effects 0.000 description 2
- 238000009792 diffusion process Methods 0.000 description 2
- 238000005516 engineering process Methods 0.000 description 2
- 238000011156 evaluation Methods 0.000 description 2
- 238000013401 experimental design Methods 0.000 description 2
- 239000012737 fresh medium Substances 0.000 description 2
- 238000011134 hematopoietic stem cell transplantation Methods 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 208000037797 influenza A Diseases 0.000 description 2
- 208000037798 influenza B Diseases 0.000 description 2
- 239000002054 inoculum Substances 0.000 description 2
- 208000036971 interstitial lung disease 2 Diseases 0.000 description 2
- 238000002955 isolation Methods 0.000 description 2
- BPHPUYQFMNQIOC-NXRLNHOXSA-N isopropyl beta-D-thiogalactopyranoside Chemical compound CC(C)S[C@@H]1O[C@H](CO)[C@H](O)[C@H](O)[C@H]1O BPHPUYQFMNQIOC-NXRLNHOXSA-N 0.000 description 2
- 238000002372 labelling Methods 0.000 description 2
- 208000032839 leukemia Diseases 0.000 description 2
- 229960003376 levofloxacin Drugs 0.000 description 2
- 230000004199 lung function Effects 0.000 description 2
- 238000004519 manufacturing process Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 229960004866 mycophenolate mofetil Drugs 0.000 description 2
- 210000003928 nasal cavity Anatomy 0.000 description 2
- 238000010899 nucleation Methods 0.000 description 2
- 230000000414 obstructive effect Effects 0.000 description 2
- 150000002482 oligosaccharides Polymers 0.000 description 2
- 230000008520 organization Effects 0.000 description 2
- 238000006213 oxygenation reaction Methods 0.000 description 2
- 244000144985 peep Species 0.000 description 2
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 2
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 2
- 229920002223 polystyrene Polymers 0.000 description 2
- 239000003755 preservative agent Substances 0.000 description 2
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000007859 qualitative PCR Methods 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 238000003753 real-time PCR Methods 0.000 description 2
- 108020003175 receptors Proteins 0.000 description 2
- 201000004193 respiratory failure Diseases 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 239000003381 stabilizer Substances 0.000 description 2
- 230000000153 supplemental effect Effects 0.000 description 2
- 230000017960 syncytium formation Effects 0.000 description 2
- 208000011580 syndromic disease Diseases 0.000 description 2
- 230000001225 therapeutic effect Effects 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- 231100000027 toxicology Toxicity 0.000 description 2
- 239000012588 trypsin Substances 0.000 description 2
- 229960003165 vancomycin Drugs 0.000 description 2
- MYPYJXKWCTUITO-LYRMYLQWSA-N vancomycin Chemical compound O([C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]1OC1=C2C=C3C=C1OC1=CC=C(C=C1Cl)[C@@H](O)[C@H](C(N[C@@H](CC(N)=O)C(=O)N[C@H]3C(=O)N[C@H]1C(=O)N[C@H](C(N[C@@H](C3=CC(O)=CC(O)=C3C=3C(O)=CC=C1C=3)C(O)=O)=O)[C@H](O)C1=CC=C(C(=C1)Cl)O2)=O)NC(=O)[C@@H](CC(C)C)NC)[C@H]1C[C@](C)(N)[C@H](O)[C@H](C)O1 MYPYJXKWCTUITO-LYRMYLQWSA-N 0.000 description 2
- MYPYJXKWCTUITO-UHFFFAOYSA-N vancomycin Natural products O1C(C(=C2)Cl)=CC=C2C(O)C(C(NC(C2=CC(O)=CC(O)=C2C=2C(O)=CC=C3C=2)C(O)=O)=O)NC(=O)C3NC(=O)C2NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(CC(C)C)NC)C(O)C(C=C3Cl)=CC=C3OC3=CC2=CC1=C3OC1OC(CO)C(O)C(O)C1OC1CC(C)(N)C(O)C(C)O1 MYPYJXKWCTUITO-UHFFFAOYSA-N 0.000 description 2
- 230000029812 viral genome replication Effects 0.000 description 2
- 239000005723 virus inoculator Substances 0.000 description 2
- 208000030507 AIDS Diseases 0.000 description 1
- 208000029483 Acquired immunodeficiency Diseases 0.000 description 1
- 208000024893 Acute lymphoblastic leukemia Diseases 0.000 description 1
- 206010001052 Acute respiratory distress syndrome Diseases 0.000 description 1
- 102000007299 Amphiregulin Human genes 0.000 description 1
- 108010033760 Amphiregulin Proteins 0.000 description 1
- 241000186063 Arthrobacter Species 0.000 description 1
- 241000271566 Aves Species 0.000 description 1
- 208000035143 Bacterial infection Diseases 0.000 description 1
- LSNNMFCWUKXFEE-UHFFFAOYSA-M Bisulfite Chemical compound OS([O-])=O LSNNMFCWUKXFEE-UHFFFAOYSA-M 0.000 description 1
- 206010006187 Breast cancer Diseases 0.000 description 1
- 208000026310 Breast neoplasm Diseases 0.000 description 1
- CURLTUGMZLYLDI-UHFFFAOYSA-N Carbon dioxide Chemical compound O=C=O CURLTUGMZLYLDI-UHFFFAOYSA-N 0.000 description 1
- 206010008479 Chest Pain Diseases 0.000 description 1
- 208000029147 Collagen-vascular disease Diseases 0.000 description 1
- 208000035473 Communicable disease Diseases 0.000 description 1
- 206010011225 Cough decreased Diseases 0.000 description 1
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 1
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 1
- 208000017850 Diffuse alveolar hemorrhage Diseases 0.000 description 1
- ZGTMUACCHSMWAC-UHFFFAOYSA-L EDTA disodium salt (anhydrous) Chemical compound [Na+].[Na+].OC(=O)CN(CC([O-])=O)CCN(CC(O)=O)CC([O-])=O ZGTMUACCHSMWAC-UHFFFAOYSA-L 0.000 description 1
- 239000006145 Eagle's minimal essential medium Substances 0.000 description 1
- 101710204837 Envelope small membrane protein Proteins 0.000 description 1
- 241000283074 Equus asinus Species 0.000 description 1
- 241000283073 Equus caballus Species 0.000 description 1
- 206010017533 Fungal infection Diseases 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- 102100031181 Glyceraldehyde-3-phosphate dehydrogenase Human genes 0.000 description 1
- 229930186217 Glycolipid Natural products 0.000 description 1
- 206010019233 Headaches Diseases 0.000 description 1
- 208000000616 Hemoptysis Diseases 0.000 description 1
- HTTJABKRGRZYRN-UHFFFAOYSA-N Heparin Chemical compound OC1C(NC(=O)C)C(O)OC(COS(O)(=O)=O)C1OC1C(OS(O)(=O)=O)C(O)C(OC2C(C(OS(O)(=O)=O)C(OC3C(C(O)C(O)C(O3)C(O)=O)OS(O)(=O)=O)C(CO)O2)NS(O)(=O)=O)C(C(O)=O)O1 HTTJABKRGRZYRN-UHFFFAOYSA-N 0.000 description 1
- 208000017604 Hodgkin disease Diseases 0.000 description 1
- 208000010747 Hodgkins lymphoma Diseases 0.000 description 1
- 241001559187 Human rubulavirus 2 Species 0.000 description 1
- 206010020751 Hypersensitivity Diseases 0.000 description 1
- 206010021450 Immunodeficiency congenital Diseases 0.000 description 1
- 206010062016 Immunosuppression Diseases 0.000 description 1
- 238000012404 In vitro experiment Methods 0.000 description 1
- 201000008225 Klebsiella pneumonia Diseases 0.000 description 1
- 241000588747 Klebsiella pneumoniae Species 0.000 description 1
- 125000000570 L-alpha-aspartyl group Chemical group [H]OC(=O)C([H])([H])[C@]([H])(N([H])[H])C(*)=O 0.000 description 1
- 125000002059 L-arginyl group Chemical group O=C([*])[C@](N([H])[H])([H])C([H])([H])C([H])([H])C([H])([H])N([H])C(=N[H])N([H])[H] 0.000 description 1
- 125000002066 L-histidyl group Chemical group [H]N1C([H])=NC(C([H])([H])[C@](C(=O)[*])([H])N([H])[H])=C1[H] 0.000 description 1
- 125000002061 L-isoleucyl group Chemical group [H]N([H])[C@]([H])(C(=O)[*])[C@](C([H])([H])[H])([H])C(C([H])([H])[H])([H])[H] 0.000 description 1
- 125000003440 L-leucyl group Chemical group O=C([*])[C@](N([H])[H])([H])C([H])([H])C(C([H])([H])[H])([H])C([H])([H])[H] 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 208000000112 Myalgia Diseases 0.000 description 1
- 208000031888 Mycoses Diseases 0.000 description 1
- 206010029164 Nephrotic syndrome Diseases 0.000 description 1
- 206010068319 Oropharyngeal pain Diseases 0.000 description 1
- 201000007100 Pharyngitis Diseases 0.000 description 1
- 206010035717 Pneumonia klebsiella Diseases 0.000 description 1
- 239000002202 Polyethylene glycol Substances 0.000 description 1
- 239000004372 Polyvinyl alcohol Substances 0.000 description 1
- 206010036790 Productive cough Diseases 0.000 description 1
- XBDQKXXYIPTUBI-UHFFFAOYSA-N Propionic acid Chemical class CCC(O)=O XBDQKXXYIPTUBI-UHFFFAOYSA-N 0.000 description 1
- 238000010802 RNA extraction kit Methods 0.000 description 1
- 108010008281 Recombinant Fusion Proteins Proteins 0.000 description 1
- 102000007056 Recombinant Fusion Proteins Human genes 0.000 description 1
- 101710088839 Replication initiation protein Proteins 0.000 description 1
- 102100028755 Sialidase-2 Human genes 0.000 description 1
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 1
- 206010044314 Tracheobronchitis Diseases 0.000 description 1
- 206010052779 Transplant rejections Diseases 0.000 description 1
- 206010046306 Upper respiratory tract infection Diseases 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 229940124532 absorption promoter Drugs 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 239000002253 acid Substances 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000001154 acute effect Effects 0.000 description 1
- 229960002964 adalimumab Drugs 0.000 description 1
- 230000002730 additional effect Effects 0.000 description 1
- 208000026935 allergic disease Diseases 0.000 description 1
- 150000004716 alpha keto acids Chemical class 0.000 description 1
- 150000001408 amides Chemical class 0.000 description 1
- 239000003242 anti bacterial agent Substances 0.000 description 1
- 230000000845 anti-microbial effect Effects 0.000 description 1
- 230000001857 anti-mycotic effect Effects 0.000 description 1
- 230000000840 anti-viral effect Effects 0.000 description 1
- 229940088710 antibiotic agent Drugs 0.000 description 1
- 239000002543 antimycotic Substances 0.000 description 1
- 239000003963 antioxidant agent Substances 0.000 description 1
- 235000006708 antioxidants Nutrition 0.000 description 1
- 239000007864 aqueous solution Substances 0.000 description 1
- 125000003118 aryl group Chemical group 0.000 description 1
- 229940072107 ascorbate Drugs 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 208000022362 bacterial infectious disease Diseases 0.000 description 1
- 229960000686 benzalkonium chloride Drugs 0.000 description 1
- 150000001558 benzoic acid derivatives Chemical class 0.000 description 1
- CADWTSSKOVRVJC-UHFFFAOYSA-N benzyl(dimethyl)azanium;chloride Chemical compound [Cl-].C[NH+](C)CC1=CC=CC=C1 CADWTSSKOVRVJC-UHFFFAOYSA-N 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- 230000031018 biological processes and functions Effects 0.000 description 1
- 230000008512 biological response Effects 0.000 description 1
- 230000001851 biosynthetic effect Effects 0.000 description 1
- 238000010322 bone marrow transplantation Methods 0.000 description 1
- 238000004364 calculation method Methods 0.000 description 1
- 201000011510 cancer Diseases 0.000 description 1
- 150000001720 carbohydrates Chemical group 0.000 description 1
- 235000011089 carbon dioxide Nutrition 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 230000006037 cell lysis Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 238000005119 centrifugation Methods 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 239000002738 chelating agent Substances 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 229960004926 chlorobutanol Drugs 0.000 description 1
- 208000017760 chronic graft versus host disease Diseases 0.000 description 1
- 230000008094 contradictory effect Effects 0.000 description 1
- IDLFZVILOHSSID-OVLDLUHVSA-N corticotropin Chemical compound C([C@@H](C(=O)N[C@@H](CO)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1NC=NC=1)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)NCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(N)=O)C(=O)NCC(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CO)C(=O)N[C@@H](C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N1[C@@H](CCC1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(O)=O)NC(=O)[C@@H](N)CO)C1=CC=C(O)C=C1 IDLFZVILOHSSID-OVLDLUHVSA-N 0.000 description 1
- 231100000433 cytotoxic Toxicity 0.000 description 1
- 230000001472 cytotoxic effect Effects 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000001419 dependent effect Effects 0.000 description 1
- 238000013461 design Methods 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 206010012601 diabetes mellitus Diseases 0.000 description 1
- 238000011026 diafiltration Methods 0.000 description 1
- 238000002405 diagnostic procedure Methods 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 239000002270 dispersing agent Substances 0.000 description 1
- 238000004090 dissolution Methods 0.000 description 1
- 231100000673 dose–response relationship Toxicity 0.000 description 1
- 239000006196 drop Substances 0.000 description 1
- 238000001647 drug administration Methods 0.000 description 1
- 238000012377 drug delivery Methods 0.000 description 1
- 208000017574 dry cough Diseases 0.000 description 1
- 229920001971 elastomer Polymers 0.000 description 1
- 239000003995 emulsifying agent Substances 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 150000002270 gangliosides Chemical class 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 108020004445 glyceraldehyde-3-phosphate dehydrogenase Proteins 0.000 description 1
- 235000011187 glycerol Nutrition 0.000 description 1
- 231100000869 headache Toxicity 0.000 description 1
- 229920000669 heparin Polymers 0.000 description 1
- 229960002897 heparin Drugs 0.000 description 1
- 208000021760 high fever Diseases 0.000 description 1
- 239000012510 hollow fiber Substances 0.000 description 1
- 230000002209 hydrophobic effect Effects 0.000 description 1
- 230000009610 hypersensitivity Effects 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- 230000001146 hypoxic effect Effects 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 230000008595 infiltration Effects 0.000 description 1
- 238000001764 infiltration Methods 0.000 description 1
- 230000002757 inflammatory effect Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 239000007951 isotonicity adjuster Substances 0.000 description 1
- 210000000867 larynx Anatomy 0.000 description 1
- 238000007449 liver function test Methods 0.000 description 1
- 238000000464 low-speed centrifugation Methods 0.000 description 1
- 238000013123 lung function test Methods 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 206010025482 malaise Diseases 0.000 description 1
- 238000005399 mechanical ventilation Methods 0.000 description 1
- QSHDDOUJBYECFT-UHFFFAOYSA-N mercury Chemical compound [Hg] QSHDDOUJBYECFT-UHFFFAOYSA-N 0.000 description 1
- 229910052753 mercury Inorganic materials 0.000 description 1
- 229940041669 mercury Drugs 0.000 description 1
- 229910052751 metal Inorganic materials 0.000 description 1
- 239000002184 metal Substances 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 210000003097 mucus Anatomy 0.000 description 1
- 208000013465 muscle pain Diseases 0.000 description 1
- 238000002663 nebulization Methods 0.000 description 1
- 229920001542 oligosaccharide Polymers 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 210000003300 oropharynx Anatomy 0.000 description 1
- 238000002640 oxygen therapy Methods 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 210000005259 peripheral blood Anatomy 0.000 description 1
- 239000011886 peripheral blood Substances 0.000 description 1
- 230000002572 peristaltic effect Effects 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 239000003186 pharmaceutical solution Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 229940067107 phenylethyl alcohol Drugs 0.000 description 1
- 238000002616 plasmapheresis Methods 0.000 description 1
- 239000013600 plasmid vector Substances 0.000 description 1
- 239000004033 plastic Substances 0.000 description 1
- 229920003023 plastic Polymers 0.000 description 1
- 201000000317 pneumocystosis Diseases 0.000 description 1
- 229920001223 polyethylene glycol Polymers 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 229920002451 polyvinyl alcohol Polymers 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002062 proliferating effect Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000000069 prophylactic effect Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 230000000306 recurrent effect Effects 0.000 description 1
- 230000017610 release of virus from host Effects 0.000 description 1
- 210000001533 respiratory mucosa Anatomy 0.000 description 1
- 208000023504 respiratory system disease Diseases 0.000 description 1
- 208000020029 respiratory tract infectious disease Diseases 0.000 description 1
- 238000010839 reverse transcription Methods 0.000 description 1
- 206010039083 rhinitis Diseases 0.000 description 1
- 239000005060 rubber Substances 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000011780 sodium chloride Substances 0.000 description 1
- 229940037001 sodium edetate Drugs 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 239000002904 solvent Substances 0.000 description 1
- 239000000600 sorbitol Substances 0.000 description 1
- 241000894007 species Species 0.000 description 1
- 208000024794 sputum Diseases 0.000 description 1
- 210000003802 sputum Anatomy 0.000 description 1
- 238000012289 standard assay Methods 0.000 description 1
- 239000007858 starting material Substances 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 230000004083 survival effect Effects 0.000 description 1
- 239000000725 suspension Substances 0.000 description 1
- 230000008961 swelling Effects 0.000 description 1
- 230000002195 synergetic effect Effects 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 239000002562 thickening agent Substances 0.000 description 1
- 238000004448 titration Methods 0.000 description 1
- 210000003437 trachea Anatomy 0.000 description 1
- 239000006163 transport media Substances 0.000 description 1
- 238000009423 ventilation Methods 0.000 description 1
- 230000007485 viral shedding Effects 0.000 description 1
- 210000002845 virion Anatomy 0.000 description 1
- 238000012800 visualization Methods 0.000 description 1
- 210000001260 vocal cord Anatomy 0.000 description 1
- 238000005406 washing Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K38/00—Medicinal preparations containing peptides
- A61K38/16—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- A61K38/43—Enzymes; Proenzymes; Derivatives thereof
- A61K38/46—Hydrolases (3)
- A61K38/47—Hydrolases (3) acting on glycosyl compounds (3.2), e.g. cellulases, lactases
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/007—Pulmonary tract; Aromatherapy
- A61K9/0073—Sprays or powders for inhalation; Aerolised or nebulised preparations generated by other means than thermal energy
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/12—Mucolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
- A61P31/14—Antivirals for RNA viruses
- A61P31/16—Antivirals for RNA viruses for influenza or rhinoviruses
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Y—ENZYMES
- C12Y302/00—Hydrolases acting on glycosyl compounds, i.e. glycosylases (3.2)
- C12Y302/01—Glycosidases, i.e. enzymes hydrolysing O- and S-glycosyl compounds (3.2.1)
- C12Y302/01018—Exo-alpha-sialidase (3.2.1.18), i.e. trans-sialidase
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Virology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pulmonology (AREA)
- Epidemiology (AREA)
- Molecular Biology (AREA)
- Oncology (AREA)
- Communicable Diseases (AREA)
- Gastroenterology & Hepatology (AREA)
- Immunology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Zoology (AREA)
- General Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Wood Science & Technology (AREA)
- Otolaryngology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Medicinal Preparation (AREA)
- Peptides Or Proteins (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Applications Claiming Priority (5)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201261600545P | 2012-02-17 | 2012-02-17 | |
| US61/600,545 | 2012-02-17 | ||
| US201261727627P | 2012-11-16 | 2012-11-16 | |
| US61/727,627 | 2012-11-16 | ||
| PCT/US2013/026754 WO2013123521A1 (en) | 2012-02-17 | 2013-02-19 | Methods, Compounds and Compositions for Treatment of Parainfluenza Virus in Immunocompromised Patients |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2015508780A true JP2015508780A (ja) | 2015-03-23 |
| JP2015508780A5 JP2015508780A5 (enExample) | 2016-04-21 |
Family
ID=48984833
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2014557870A Pending JP2015508780A (ja) | 2012-02-17 | 2013-02-19 | 免疫不全患者のインフルエンザ及びパラインフルエンザウイルスを処置するための方法、化合物、及び組成物 |
Country Status (9)
| Country | Link |
|---|---|
| US (5) | US20130280332A1 (enExample) |
| EP (1) | EP2841092B1 (enExample) |
| JP (1) | JP2015508780A (enExample) |
| CN (2) | CN109529026A (enExample) |
| AU (3) | AU2013221230A1 (enExample) |
| CA (1) | CA2864746A1 (enExample) |
| ES (1) | ES2970401T3 (enExample) |
| IL (2) | IL234116B (enExample) |
| WO (1) | WO2013123521A1 (enExample) |
Families Citing this family (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| PL2571503T3 (pl) | 2010-05-14 | 2015-06-30 | Dana Farber Cancer Inst Inc | Kompozycje i sposoby leczenia nowotworu, choroby zapalnej i innych zaburzeń |
| CA2799403C (en) | 2010-05-14 | 2020-01-21 | Dana-Farber Cancer Institute, Inc. | Compositions and methods for treating leukemia |
| EP2571875A4 (en) | 2010-05-14 | 2013-10-30 | Dana Farber Cancer Inst Inc | CONTRACEPTIVE COMPOSITIONS FOR MEN AND METHODS OF USE THEREOF |
| WO2015013635A2 (en) | 2013-07-25 | 2015-01-29 | Dana-Farber Cancer Institute, Inc. | Inhibitors of transcription factors and uses thereof |
| WO2015070020A2 (en) | 2013-11-08 | 2015-05-14 | Dana-Farber Cancer Institute, Inc. | Combination therapy for cancer using bromodomain and extra-terminal (bet) protein inhibitors |
| KR20160115953A (ko) | 2014-01-31 | 2016-10-06 | 다나-파버 캔서 인스티튜트 인크. | 디아미노피리미딘 벤젠술폰 유도체 및 그의 용도 |
| WO2015117087A1 (en) | 2014-01-31 | 2015-08-06 | Dana-Farber Cancer Institute, Inc. | Uses of diazepane derivatives |
| CN106456653A (zh) | 2014-02-28 | 2017-02-22 | 腾沙治疗公司 | 高胰岛素血症相关病症的治疗 |
| WO2016022902A1 (en) | 2014-08-08 | 2016-02-11 | Dana-Farber Cancer Institute, Inc. | Diazepane derivatives and uses thereof |
| CA2955077A1 (en) | 2014-08-08 | 2016-02-11 | Dana-Farber Cancer Institute, Inc. | Dihydropteridinone derivatives and uses thereof |
| MX376748B (es) | 2014-10-27 | 2025-03-07 | Tensha Therapeutics Inc | Inhibidores del bromodominio. |
| CN107249593A (zh) * | 2014-11-06 | 2017-10-13 | 达纳-法伯癌症研究所股份有限公司 | 调节染色质结构的组合物用于移植物抗宿主疾病(gvhd)的用途 |
| KR20170081213A (ko) | 2014-11-06 | 2017-07-11 | 다나-파버 캔서 인스티튜트 인크. | Ezh2 억제제 및 그의 용도 |
| WO2016200916A1 (en) * | 2015-06-08 | 2016-12-15 | Ansun Biopharma, Inc. | Treatment of human metapneumovirus |
| WO2016201370A1 (en) | 2015-06-12 | 2016-12-15 | Dana-Farber Cancer Institute, Inc. | Combination therapy of transcription inhibitors and kinase inhibitors |
| JP2018526424A (ja) | 2015-09-11 | 2018-09-13 | ダナ−ファーバー キャンサー インスティテュート, インコーポレイテッド | アセトアミドチエノトリアゾロジアゼピンおよびこれらの使用 |
| KR20180049058A (ko) | 2015-09-11 | 2018-05-10 | 다나-파버 캔서 인스티튜트 인크. | 시아노 티에노트리아졸로디아제핀 및 그의 용도 |
| CN114957291A (zh) | 2015-11-25 | 2022-08-30 | 达纳-法伯癌症研究所股份有限公司 | 二价溴结构域抑制剂及其用途 |
| JP7493304B2 (ja) | 2016-04-22 | 2024-05-31 | ダナ-ファーバー キャンサー インスティテュート, インコーポレイテッド | Ezh2インヒビターおよびそれらの使用 |
| US20230330140A1 (en) * | 2019-11-25 | 2023-10-19 | Ansun Biopharma, Inc. | Immune cell delivery of sialidase cancer cells, immune cells and the tumor microenvironment |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009524667A (ja) * | 2006-01-24 | 2009-07-02 | ネクスバイオ,インク. | 高分子マイクロスフェアの調製技術 |
| WO2011057081A1 (en) * | 2009-11-06 | 2011-05-12 | Nexbio, Inc. | Methods, compounds and compositions for treatment and prophylaxis in the respiratory tract |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7334580B2 (en) * | 2002-05-07 | 2008-02-26 | Smaldone Gerald C | Methods, devices and formulations for targeted endobronchial therapy |
| CA2497845C (en) * | 2002-09-06 | 2012-08-14 | Chrysalis Technologies Incorporated | Liquid aerosol formulations and aerosol generating devices and methods for generating aerosols |
| CA2506526C (en) | 2002-11-22 | 2017-04-18 | Mang Yu | Broad spectrum anti-viral therapeutics and prophylaxis |
| US7807174B2 (en) * | 2002-11-22 | 2010-10-05 | Nexbio, Inc. | Class of therapeutic protein based molecules |
-
2013
- 2013-02-19 ES ES13749329T patent/ES2970401T3/es active Active
- 2013-02-19 AU AU2013221230A patent/AU2013221230A1/en not_active Abandoned
- 2013-02-19 WO PCT/US2013/026754 patent/WO2013123521A1/en not_active Ceased
- 2013-02-19 US US13/770,991 patent/US20130280332A1/en not_active Abandoned
- 2013-02-19 CN CN201811368987.7A patent/CN109529026A/zh active Pending
- 2013-02-19 EP EP13749329.2A patent/EP2841092B1/en active Active
- 2013-02-19 JP JP2014557870A patent/JP2015508780A/ja active Pending
- 2013-02-19 CN CN201380009763.6A patent/CN104203267A/zh active Pending
- 2013-02-19 CA CA2864746A patent/CA2864746A1/en not_active Abandoned
-
2014
- 2014-08-13 IL IL234116A patent/IL234116B/en active IP Right Grant
-
2015
- 2015-01-26 US US14/605,572 patent/US20150132274A1/en not_active Abandoned
-
2017
- 2017-02-10 US US15/430,288 patent/US20170151315A1/en not_active Abandoned
- 2017-11-10 AU AU2017258955A patent/AU2017258955A1/en not_active Abandoned
-
2019
- 2019-09-05 AU AU2019226190A patent/AU2019226190B2/en not_active Ceased
-
2020
- 2020-03-10 IL IL273194A patent/IL273194B2/en unknown
- 2020-07-31 US US16/945,776 patent/US20210046165A1/en not_active Abandoned
-
2025
- 2025-01-21 US US19/033,237 patent/US20250387457A1/en active Pending
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009524667A (ja) * | 2006-01-24 | 2009-07-02 | ネクスバイオ,インク. | 高分子マイクロスフェアの調製技術 |
| WO2011057081A1 (en) * | 2009-11-06 | 2011-05-12 | Nexbio, Inc. | Methods, compounds and compositions for treatment and prophylaxis in the respiratory tract |
Non-Patent Citations (2)
| Title |
|---|
| CLINICAL INFECTIOUS DISEASES, vol. 53, no. 7, JPN6016046059, 2011, pages e77-e80 * |
| JOURNAL OF INFECTIOUS DISEASES, vol. 202, no. 2, JPN6016046060, 2010, pages 234 - 241 * |
Also Published As
| Publication number | Publication date |
|---|---|
| EP2841092C0 (en) | 2023-12-06 |
| IL273194A (en) | 2020-04-30 |
| US20210046165A1 (en) | 2021-02-18 |
| WO2013123521A1 (en) | 2013-08-22 |
| CA2864746A1 (en) | 2013-08-22 |
| AU2013221230A1 (en) | 2014-10-02 |
| HK1207975A1 (en) | 2016-02-19 |
| US20150132274A1 (en) | 2015-05-14 |
| AU2019226190B2 (en) | 2021-10-07 |
| CN104203267A (zh) | 2014-12-10 |
| US20130280332A1 (en) | 2013-10-24 |
| AU2019226190A1 (en) | 2019-09-26 |
| EP2841092A4 (en) | 2015-09-16 |
| CN109529026A (zh) | 2019-03-29 |
| US20250387457A1 (en) | 2025-12-25 |
| IL234116B (en) | 2020-03-31 |
| ES2970401T3 (es) | 2024-05-28 |
| AU2017258955A1 (en) | 2017-12-14 |
| US20170151315A1 (en) | 2017-06-01 |
| IL273194B (en) | 2022-10-01 |
| EP2841092B1 (en) | 2023-12-06 |
| EP2841092A1 (en) | 2015-03-04 |
| IL273194B2 (en) | 2023-02-01 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20250387457A1 (en) | Methods, Compounds and Compositions for Treatment of Influenza and Parainfluenza Patients | |
| Parray et al. | Inhalation monoclonal antibody therapy: a new way to treat and manage respiratory infections | |
| Rajnik et al. | Features, evaluation, and treatment of coronavirus (COVID-19) | |
| US11364227B2 (en) | Sphingosine kinase 2 inhibitor for treating coronavirus infection | |
| US11413298B2 (en) | Medicament for prevention of treatment of rhinovirus infection | |
| JP5970465B2 (ja) | ペプチドおよびウイルス・ノイラミニダーゼ阻害剤を含んでなる組成物 | |
| WO2022119535A1 (en) | New dosage regimen for inhaled vasoactive intestinal polypeptide | |
| EP2544705B1 (en) | Interferon beta for use in the treatment of lower respiratory tract illness caused by influenza | |
| JP2023526200A (ja) | SARS-CoV-2感染症の予防または治療のための適合溶質 | |
| US20220025019A1 (en) | Methods and compositions for preventing or treating acute exacerbations with polyclonal immunoglobulin | |
| US12083135B2 (en) | Use of a nitrogen-containing bisphosphonate in combination with a glucocorticoid in preventing or treating viral pneumonia | |
| HK40004532A (en) | Methods, compounds and compositions for treating influenza and parainfluenza patients | |
| CN108926707A (zh) | Pf4的抗rsv应用 | |
| EP4248986A1 (en) | Use of polypeptide having superoxide dismutase activity and extracellular vesicles for treatment or prevention of respiratory viral infection | |
| HK1207975B (en) | Compounds and compositions for use in a method of treatment of parainfluenza virus | |
| US11471448B2 (en) | Sphingosine kinase 2 inhibitor for treating coronavirus infection in moderately severe patients with pneumonia | |
| US20250222075A1 (en) | Novel application of human airway surfactant, bpifa1, as prophylactic antiviral against respiratory syncytial virus (rsv) | |
| WO2023198757A1 (en) | Alpha-1-antitrypsin for treating paramyxoviridae or orthomyxoviridae infections | |
| KR20230124613A (ko) | 바이러스 유래의 호흡기 감염을 치료하기 위한 펩타이드 | |
| Qiu et al. | Analysis on Diagnosis of Family Clustering Infection of SARS-CoV-2 | |
| WO2024005563A1 (ko) | 편도유래줄기세포를 유효성분으로 포함하는 감염성 폐 질환의 예방 또는 치료용 조성물 | |
| CN115461356A (zh) | 用于预防或治疗covid-19的肽 | |
| HK40029346A (en) | Use of a small molecule inhibitor for treatment of respiratory viral pneumonia | |
| WO2021195883A1 (zh) | TFF2蛋白和IFN-κ蛋白联用在治疗新型冠状病毒感染中的应用 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20160219 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20160219 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20161128 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20161206 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170306 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20170508 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20170606 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20171107 |