JP2015166380A - 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalatingdosingregimen) - Google Patents
体重減少の達成および肥満症の治療のための漸増用量投与計画(escalatingdosingregimen) Download PDFInfo
- Publication number
- JP2015166380A JP2015166380A JP2015102962A JP2015102962A JP2015166380A JP 2015166380 A JP2015166380 A JP 2015166380A JP 2015102962 A JP2015102962 A JP 2015102962A JP 2015102962 A JP2015102962 A JP 2015102962A JP 2015166380 A JP2015166380 A JP 2015166380A
- Authority
- JP
- Japan
- Prior art keywords
- topiramate
- phentermine
- sympathomimetic
- composition
- day
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Granted
Links
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/357—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having two or more oxygen atoms in the same ring, e.g. crown ethers, guanadrel
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/13—Amines
- A61K31/135—Amines having aromatic rings, e.g. ketamine, nortriptyline
- A61K31/137—Arylalkylamines, e.g. amphetamine, epinephrine, salbutamol, ephedrine or methadone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7048—Compounds having saccharide radicals and heterocyclic rings having oxygen as a ring hetero atom, e.g. leucoglucosan, hesperidin, erythromycin, nystatin, digitoxin or digoxin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/36—Polysaccharides; Derivatives thereof, e.g. gums, starch, alginate, dextrin, hyaluronic acid, chitosan, inulin, agar or pectin
- A61K47/38—Cellulose; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
- A61K9/1623—Sugars or sugar alcohols, e.g. lactose; Derivatives thereof; Homeopathic globules
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1629—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/167—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction with an outer layer or coating comprising drug; with chemically bound drugs or non-active substances on their surface
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/167—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction with an outer layer or coating comprising drug; with chemically bound drugs or non-active substances on their surface
- A61K9/1676—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction with an outer layer or coating comprising drug; with chemically bound drugs or non-active substances on their surface having a drug-free core with discrete complete coating layer containing drug
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4866—Organic macromolecular compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5026—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5036—Polysaccharides, e.g. gums, alginate; Cyclodextrin
- A61K9/5042—Cellulose; Cellulose derivatives, e.g. phthalate or acetate succinate esters of hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5036—Polysaccharides, e.g. gums, alginate; Cyclodextrin
- A61K9/5042—Cellulose; Cellulose derivatives, e.g. phthalate or acetate succinate esters of hydroxypropyl methylcellulose
- A61K9/5047—Cellulose ethers containing no ester groups, e.g. hydroxypropyl methylcellulose
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5073—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5084—Mixtures of one or more drugs in different galenical forms, at least one of which being granules, microcapsules or (coated) microparticles according to A61K9/16 or A61K9/50, e.g. for obtaining a specific release pattern or for combining different drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/08—Drugs for disorders of the alimentary tract or the digestive system for nausea, cinetosis or vertigo; Antiemetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/06—Antigout agents, e.g. antihyperuricemic or uricosuric agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/06—Antimigraine agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Engineering & Computer Science (AREA)
- Epidemiology (AREA)
- Organic Chemistry (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Emergency Medicine (AREA)
- Neurology (AREA)
- Diabetes (AREA)
- Molecular Biology (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Pulmonology (AREA)
- Heart & Thoracic Surgery (AREA)
- Endocrinology (AREA)
- Pain & Pain Management (AREA)
- Cardiology (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Rheumatology (AREA)
- Inorganic Chemistry (AREA)
- Biophysics (AREA)
- Anesthesiology (AREA)
- Child & Adolescent Psychology (AREA)
- Hospice & Palliative Care (AREA)
- Otolaryngology (AREA)
- Immunology (AREA)
- Reproductive Health (AREA)
Abstract
【解決手段】トピラメートの有効量、微結晶セルロース、及びメチルセルロースを含む制御放出組成物。該トピラメート、該微結晶セルロース、及び該メチルセルロースが、エチルセルロースでコーティングされているビーズのマトリックスコアに存在し、該マトリックスコアがポリビニルピロリドンで更にコーティングされ、カプセル化されている該組成物、又は該カプセル剤が交感神経刺激薬(フェンテルミン又はブプロピオン)を更に含む該組成物、及び該組成物を用いた治療方法。
【選択図】なし
Description
本願は、2008年6月9日に出願された米国特許出願第12/135,953号の一部継続出願であって、その開示は参照することによって援用される。
小児および成人の両方における肥満症の有病率は、先進国、特に米国のみならず、中国およびインドなどの多くの発展途上国においても上昇している。膝および足首関節悪化などの身体的問題から、自尊心に関わる問題および体重が重い人々に対する社会の態度に起因する情緒的問題に至るまで、人々の生活の多くの側面が肥満症によって影響されている。肥満症に起因する医学的問題は重篤なものとなり得、しばしば生命を脅かし、糖尿病、息切れならびに喘息および肺高血圧症などの他の呼吸器疾患、胆嚢疾患、脂質異常症(例えば、高コレステロールまたは高濃度のトリグリセリド)および脂質異常症の高血圧症、骨関節炎および他の整形外科的障害、逆流性食道炎(胸やけ)、いびき、睡眠時無呼吸、月経不順、不妊症、妊娠と関連する障害、痛風、冠動脈疾患および他の心臓疾患などの心血管障害、筋ジストロフィー、ならびに低αリポタンパク血症、家族性複合型高脂血症、およびインスリン抵抗性シンドロームXを含むシンドロームXなどの代謝障害を含む。さらに、肥満症は特定の癌、特に結腸癌、直腸癌、前立腺癌、乳癌、子宮癌、および子宮頸癌の発生率上昇と関連する。
本発明は、体重減少を達成し、肥満症を治療し、かつ、過剰体重もしくは肥満症に起因し、またはこれらと関連する状態を治療するための新規なトピラメート組成物および方法を提供する。該組成物は単一活性薬剤としてトピラメートを含有してよいが、より典型的には、少なくとも1つの交感神経刺激薬と組み合わせてトピラメートを含有する。用語「交感神経刺激薬(sympathomimetic agent)」は専門用語であり、交感神経系の刺激を模倣または変化する薬剤または化合物を指す。代表的な交感神経刺激薬は、フェンテルミンおよびブプロピオンを含む。好ましくは、該トピラメートおよび該交感神経刺激薬は単一剤形に含有され、該交感神経刺激薬の即時放出、ならびに該トピラメートの制御放出、例えば、持続放出、遅延放出、または持続放出および遅延放出の両方を提供する。
定義および命名法:
本明細書および添付の特許請求の範囲にて用いられている単数形「a」、「an」および「the」は、文脈においてはっきりと別段の指示がない限り、複数の指示対象を含むことに留意しなければならない。それゆえ、例えば、「活性薬剤(an active agent)」は単一の活性薬剤のみならず、2つ以上の異なる活性薬剤の組み合わせも指し、「剤形(a dosage form)」は単一剤形のみならず剤形の組み合わせをも指す、などである。
本発明は、体重減少を達成し、かつ、肥満症、過剰体重または肥満症と関連する状態、糖尿病(肥満症と関連しているかどうかに関わらず)、ならびに以下で説明するような他の状態および障害を治療するための新規な方法および組成物を提供する。米国疾病対策センターによると、過体重(該用語は本明細書で用語「過剰体重」と同義的に用いられている)であることの臨床的定義は、25.0〜29.9kg/mの間のボディー・マス・インデックス(BMI)を有していることである;BMIは、個人の体重(キログラム)と身長(メートル)を掛け合わせることによって計算される。CDCは肥満症を30以上のBMIを有するもの定義している。1つの実施態様では、本発明は体重減少を達成し、かつ、過体重、肥満症、ならびに過剰体重および肥満症と関連する状態を治療する方法を提供し、交感神経刺激薬フェンテルミンおよび抗痙攣剤トピラメートの組み合わせの投与を含む。
本明細書で用いられている用語「トピラメート」は、2,3,4,5−ビス−(O)−(1−メチルエチリデン)−β−D−フルクトピラノーススルファメートのみならず、個々のエナンチオマー、個々のジアステレオマー、またはその混合物を包含する。本明細書で用いられている用語「トピラメート」は、トピラメート塩のみならず、多形、溶媒和物(水和物および混合溶媒和物、ならびに塩の水和物を含む)、共結晶(例えば、他の化合物または他の形態のトピラメートとの共結晶)、非晶質、ならびに式(I)の化合物の無水物形態をも包含する。該化合物がスルファミン酸誘導体であるという事実から分かるように、本発明と関連して有用なトピラメート塩は、医薬的に許容される塩基付加塩である。該塩は、式(I)の化合物のスルファミン酸基と結合する医薬的に許容されるカチオンを提供する塩基から製造される。適切な医薬的に許容されるカチオンは有機および無機カチオンの両方を含み、ナトリウム、ナトリウム、カリウム、リチウム、マグネシウム、カルシウム、アルミニウム、亜鉛、プロカイン、ベンザチン、クロロプロカイン、コリン、ジエチルアミン、エチレンジアミン、N−メチルグルカミン、ベネタミン、クレミゾール、ジエチルアミン、ピペラジン、トロメタミン、トリエチルアミン、エタノールアミン、トリエタノールアミン、アルギニン、リジン、ヒスチジン、トリブチルアミン、2−アミノ−2−ペンチルプロパノール、2−アミノ−2−メチル−1,3−プロパンジオール、トリス(ヒドロキシメチル)アミノメタン、ベンジルアミン、2−(ジメチルアミノ)エタノール、バリウムまたはビスマス対イオン(barium or bismuth counter ions)を含み、これらに限定されない。特に好ましいカチオンはナトリウム、リチウム、およびカリウムである。上記で参照した他の形態のトピラメートは、当該技術分野で周知の方法を用いて製造されてよい;例えば、米国特許第7,351,695号を参照のこと。本発明の方法は、トピラメートの単独または、より好ましくは、交感神経刺激薬と組み合わせた投与のための投与計画を含む。特定の態様では、本発明は、例えば、ブプロピオンまたはフェンテルミンと組み合わせたトピラメートを含む医薬組成物の投与のための投与計画を提供する。
本発明の有用性を見出しうる特に興味深い状態は、過体重、肥満症ならびに過剰体重および肥満症としばしば関連し、および/またはそれらに起因する状態を含む。本明細書で提供する投与計画に従って投与されるトピラメート組成物および組み合わせは、有意な治療効果および有害作用の減少を生じるため、これらの医薬組成物を非常に有効な治療法としており、特に過体重、肥満症ならびに/または、過剰体重もしくは肥満症自体と関連し、および/もしくはこれらに起因する状態を含む関連状態の治療に有効なものとしている。それゆえ、本発明の併用療法の治療計画での治療に適切な対象は、肥満症と関連する状態を患う個人を含み、該状態は下記を含み、これらに限定されない:
糖尿病、インスリン抵抗性、および耐糖能異常;
肺高血圧症、喘息、および息切れなどの呼吸器疾患;
胆嚢疾患;
脂質異常症、例えば、高コレステロール、および高濃度のトリグリセリドなど;
骨関節炎および他の整形外科的障害;
逆流性食道炎;
睡眠時無呼吸および大いびきを含む、睡眠と関連する有害状態;
月経不順、不妊症、および妊娠合併症;
痛風;
高血圧、すなわち、高血圧症;
冠動脈疾患および他の心臓疾患などの心血管障害;
筋ジストロフィー;
脳卒中、特に血栓脳卒中および深部静脈血栓症(DVT);
片頭痛;
低αリポタンパク血症、家族性複合型高脂血症、およびインスリン抵抗性シンドロームXを含むシンドロームXなどの代謝障害;ならびに
結腸癌、直腸癌、腎臓癌、食道癌、胆嚢癌、膵臓癌、前立腺癌、乳癌、子宮癌、卵巣癌、子宮内膜癌、および子宮頸癌。
本発明の方法を実施するためのパッケージ化された医薬製剤も提供される。パッケージ化された製剤は密封容器内にある本発明の組成物を含有し、典型的には、各々ブリスター・パックなどの密封ハウジング(sealed housing)内にある個々の剤形を複数含有するが、単一密封容器内にある1つ以上の剤形も含有しうる。任意に、用量漸増(dose titration)および用量増加(dose escalation)のために、一方または両方の活性薬剤の低用量を有する剤形も含まれてよい。
押出球形化プロセスを用いて制御放出トピラメートビーズを製造し、トピラメート、40.0%w/w;微結晶セルロース(アビセルPH102(Avicel(登録商標)PH102))、56.5%w/w;およびメトセルA15LV(Methocel(商標)A15LV)、3.5%w/wから成るマトリックスコアを製造する。次いで、該トピラメートコアをエチルセルロース、5.47%w/w、およびポビドンK30、2.39%w/wでコーティングした。
成分 %w/w
トピラメート 36.85
微結晶セルロース、
(Avicel(登録商標)PH102) 52.05
メチルセルロース
(Methocel(商標)A15LV) 3.22
エチルセルロース 5.47
ポリビニルピロリドン
(ポビドンK30) 2.39
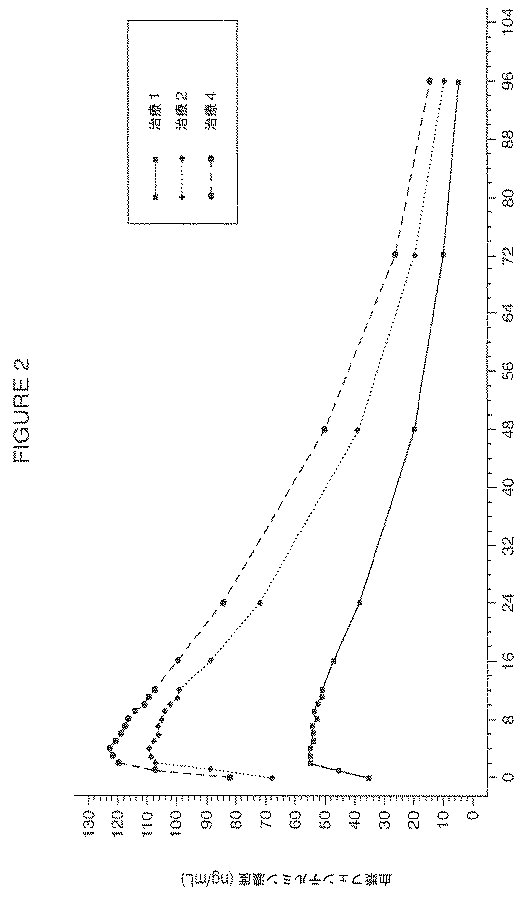
フェンテルミンと組み合わせた本発明のトピラメートの制御放出製剤と、即時放出トピラメート(トパマックス(Topamax(登録商標)))とを比較した研究において、本発明のトピラメートの制御放出製剤はフェンテルミン曝露に対して10〜15%低い効果を有した(図2)。
Claims (30)
- トピラメートの有効量;
微結晶セルロース;および
メチルセルロース、
を含む、対象において肥満症、糖尿病または関連状態を治療するための制御放出組成物。 - 該トピラメート、該微結晶セルロース、および該メチルセルロースがビーズのマトリックスコアに存在する、請求項1記載の組成物。
- 該マトリックスコアがエチルセルロースでコーティングされている、請求項2記載の組成物。
- 該マトリックスコアがポリビニルピロリドンでさらにコーティングされている、請求項3記載の組成物。
- 該ビーズがカプセル剤にカプセル化されている、請求項4記載の組成物。
- 該カプセル剤が交感神経刺激薬をさらに含む、請求項5記載の組成物。
- 該交感神経刺激薬がフェンテルミンまたはブプロピオンである、請求項6記載の組成物。
- 該フェンテルミンまたは該ブプロピオンが糖球(sugar sphere)上にコーティングされている、請求項7記載の組成物。
- 該フェンテルミンまたは該ブプロピオンが制御放出トピラメートビーズ上にコーティングされている、請求項7記載の組成物。
- 該フェンテルミンまたは該ブプロピオンが即時放出型である、請求項7記載の組成物。
- 該フェンテルミンまたは該ブプロピオンが制御放出型である、請求項7記載の組成物。
- 該トピラメートがフェンテルミンまたはブプロピオン曝露を減少させ、フェンテルミンまたはブプロピオンと関連する副作用を減少させる、請求項7記載の組成物。
- 対象において体重減少を達成する方法であって、漸増用量投与計画(escalating dosing regimen)に従ってトピラメートの有効量を該対象に投与することを含む方法であって、該トピラメートが制御放出型である方法。
- 該制御放出型が該トピラメートの持続放出、遅延放出または持続放出および遅延放出の両方を提供する、請求項13記載の方法。
- 該投与計画が、
トピラメートの初回の1日投与量を該個人に一定期間投与すること;および
様々な指定の時点で該投与量を徐々に増加すること、
を含む、請求項13記載の方法。 - 初回の1日投与量が20mg〜100mgの範囲にある、請求項15記載の方法。
- トピラメートの初回の1日投与量が23mg/日である、請求項16記載の方法。
- 交感神経刺激薬の有効量を投与することをさらに含む、請求項15記載の方法。
- 該交感神経刺激薬がフェンテルミンまたはブプロピオンである、請求項18記載の方法。
- 該交感神経刺激薬が即時放出型である、請求項18記載の方法。
- 漸増用量投与計画(escalating dosing regimen)に従ってトピラメートの有効量を対象に投与することによって、該対象において体重減少を達成する方法であって、
第一週の間、15mg/日〜30mg/日の範囲の投与量でトピラメートの初回用量を投与すること;
その後、第二週の間、35mg/日〜55mg/日のトピラメートを投与すること;
その後、第三週の間、60mg/日〜80mg/日のトピラメートを投与すること;および
その後、第四週の間、85mg/日〜125mg/日のトピラメートを投与すること、
を含む方法。 - 該漸増用量投与計画(escalating dosage regimen)を通じて交感神経刺激薬の1日投与量を投与することをさらに含む、請求項21記載の方法。
- 該交感神経刺激薬の1日投与量が毎週徐々に増加する、請求項22記載の方法。
- 該交感神経刺激薬がフェンテルミンである、請求項22または23記載の方法。
- フェンテルミンの1日投与量が2mg〜15mgの範囲にある、請求項24記載の方法。
- トピラメート、および体重減少を達成するために漸増用量投与計画(escalating dosing regimen)に従って該トピラメートを投与するための説明書を含む、パッケージ化された医薬製剤。
- 交感神経刺激薬をさらに含む、請求項26のパッケージ化された医薬製剤。
- 該トピラメートおよび該交感神経刺激薬が用量設定カード(titration card)に提供され、該カードが4週間の各薬剤の1日投与量を提供する、請求項27のパッケージ化された医薬製剤。
- 該トピラメートの1日投与量が毎週増加する、請求項28のパッケージ化された医薬製剤。
- 該交感神経刺激薬の1日投与量が毎週増加する、請求項29のパッケージ化された医薬製剤。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US12/135,953 | 2008-06-09 | ||
| US12/135,953 US20090304789A1 (en) | 2008-06-09 | 2008-06-09 | Novel topiramate compositions and an escalating dosing strategy for treating obesity and related disorders |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011513647A Division JP5752595B2 (ja) | 2008-06-09 | 2009-06-09 | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalatingdosingregimen) |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2017002448A Division JP2017105788A (ja) | 2008-06-09 | 2017-01-11 | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalating dosing regimen) |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2015166380A true JP2015166380A (ja) | 2015-09-24 |
| JP6077053B2 JP6077053B2 (ja) | 2017-02-08 |
Family
ID=40863844
Family Applications (6)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011513647A Active JP5752595B2 (ja) | 2008-06-09 | 2009-06-09 | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalatingdosingregimen) |
| JP2011513646A Pending JP2011522896A (ja) | 2008-06-09 | 2009-06-09 | 低用量トピラメート/フェンテルミン組成物およびその使用方法 |
| JP2015102962A Active JP6077053B2 (ja) | 2008-06-09 | 2015-05-20 | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalatingdosingregimen) |
| JP2015151088A Pending JP2016006085A (ja) | 2008-06-09 | 2015-07-30 | 低用量トピラメート/フェンテルミン組成物およびその使用方法 |
| JP2016246871A Active JP6214750B2 (ja) | 2008-06-09 | 2016-12-20 | 低用量トピラメート/フェンテルミン組成物およびその使用方法 |
| JP2017002448A Pending JP2017105788A (ja) | 2008-06-09 | 2017-01-11 | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalating dosing regimen) |
Family Applications Before (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2011513647A Active JP5752595B2 (ja) | 2008-06-09 | 2009-06-09 | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalatingdosingregimen) |
| JP2011513646A Pending JP2011522896A (ja) | 2008-06-09 | 2009-06-09 | 低用量トピラメート/フェンテルミン組成物およびその使用方法 |
Family Applications After (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015151088A Pending JP2016006085A (ja) | 2008-06-09 | 2015-07-30 | 低用量トピラメート/フェンテルミン組成物およびその使用方法 |
| JP2016246871A Active JP6214750B2 (ja) | 2008-06-09 | 2016-12-20 | 低用量トピラメート/フェンテルミン組成物およびその使用方法 |
| JP2017002448A Pending JP2017105788A (ja) | 2008-06-09 | 2017-01-11 | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalating dosing regimen) |
Country Status (18)
| Country | Link |
|---|---|
| US (11) | US20090304789A1 (ja) |
| EP (2) | EP2317997B1 (ja) |
| JP (6) | JP5752595B2 (ja) |
| KR (3) | KR20110042280A (ja) |
| CN (4) | CN102112126A (ja) |
| AU (2) | AU2009257572C1 (ja) |
| BR (2) | BRPI0914985B1 (ja) |
| CA (2) | CA2727313C (ja) |
| CL (2) | CL2010001366A1 (ja) |
| CY (1) | CY1118103T1 (ja) |
| DK (1) | DK2317997T3 (ja) |
| ES (1) | ES2606041T3 (ja) |
| HK (1) | HK1213489A1 (ja) |
| IL (2) | IL209874B (ja) |
| MX (2) | MX342684B (ja) |
| PT (1) | PT2317997T (ja) |
| WO (2) | WO2009152189A1 (ja) |
| ZA (2) | ZA201008840B (ja) |
Families Citing this family (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US7674776B2 (en) | 1999-06-14 | 2010-03-09 | Vivus, Inc. | Combination therapy for effecting weight loss and treating obesity |
| US20080255093A1 (en) * | 1999-06-14 | 2008-10-16 | Tam Peter Y | Compositions and methods for treating obesity and related disorders |
| US8580298B2 (en) | 2008-06-09 | 2013-11-12 | Vivus, Inc. | Low dose topiramate/phentermine composition and methods of use thereof |
| US20090304789A1 (en) | 2008-06-09 | 2009-12-10 | Thomas Najarian | Novel topiramate compositions and an escalating dosing strategy for treating obesity and related disorders |
| AU2011203970A1 (en) * | 2010-01-07 | 2012-07-12 | Vivus, Inc. | Treatment of obstructive sleep apnea syndrome with a combination of a carbonic anhydrase inhibitor and an additional active agent |
| EP2567959B1 (en) | 2011-09-12 | 2014-04-16 | Sanofi | 6-(4-hydroxy-phenyl)-3-styryl-1h-pyrazolo[3,4-b]pyridine-4-carboxylic acid amide derivatives as kinase inhibitors |
| BR102012004888A2 (pt) * | 2012-03-05 | 2013-10-22 | Phartrials Pesquisas Farmaceuticas Ltda | Associação de um derivado de sulfamato monossubstituído do monossacarídeo natural d-frutose (topiramato) com um anti-depressivo da classe das fenilcetonas (bupropiona) para tratamento de obesidade e das síndromes plurimetabólicas |
| IN2012DE00826A (ja) * | 2012-03-21 | 2015-08-21 | Ranbaxy Lab Ltd | |
| US9457008B2 (en) | 2012-03-23 | 2016-10-04 | Institute Of Pharmacology And Toxicology Academy Of Military Medical Sciences P.L.A. China | Joint product comprising synephrine and topiramate |
| CN102579367B (zh) * | 2012-03-23 | 2014-03-12 | 中国人民解放军军事医学科学院毒物药物研究所 | 托吡酯缓释药物组合物、其制备方法及用途 |
| CN103316026B (zh) * | 2012-03-23 | 2016-05-11 | 中国人民解放军军事医学科学院毒物药物研究所 | 含芬特明和托吡酯的联合产品及其制备方法 |
| BR102012026555B1 (pt) * | 2012-10-17 | 2022-10-04 | Phartrials Pesquisas Farmacêuticas Ltda | Processo de obtenção e composição de fármaco proveniente do quenodeoxicolato ligado a um derivado de sulfamato monossacarídeo natural d-frutose (topiramato) e a uma fenilcetona (bupropriona) para tratamento da obesidade e das síndromes plurimetabólicas |
| US8652527B1 (en) * | 2013-03-13 | 2014-02-18 | Upsher-Smith Laboratories, Inc | Extended-release topiramate capsules |
| US9101545B2 (en) | 2013-03-15 | 2015-08-11 | Upsher-Smith Laboratories, Inc. | Extended-release topiramate capsules |
| US20140294950A1 (en) * | 2013-03-15 | 2014-10-02 | Vivus, Inc. | Methods of treating obesity in responder and non-responder populations |
| US11992604B2 (en) | 2014-11-09 | 2024-05-28 | Sipnose Ltd. | Devices and methods for delivering a substance to a body cavity |
| US11116914B2 (en) | 2014-11-09 | 2021-09-14 | Sipnose Ltd. | Device and method for aerosolized delivering of substance to a natural orifice of the body |
| US12329902B2 (en) | 2013-08-22 | 2025-06-17 | Sipnose Ltd. | Drug delivery devices and methods for administering substances to a body cavity by heterogenous aerosolization |
| DE202013105715U1 (de) | 2013-08-22 | 2014-02-19 | Sipnose Ltd. | Vorrichtung zur Abgabe einer vorbestimmten Menge einer Substanz an eine natürliche Öffnung des Körpers |
| US11471618B2 (en) | 2014-11-09 | 2022-10-18 | Sipnose Ltd. | Adjustable dosing delivery and multi sectioned drug compartment |
| US12491325B2 (en) | 2013-08-22 | 2025-12-09 | Aptargroup, Inc. | Drug delivery devices and methods for administering substances to a body cavity by heterogenous aerosolization for treatment of binge-eating disorders and/or obesity |
| US11278682B2 (en) | 2014-11-09 | 2022-03-22 | Sipnose Ltd. | Device and method for aerosolized delivery of substance to a natural orifice of the body |
| CA2926321C (en) * | 2013-10-08 | 2023-02-28 | Vivus, Inc. | Methods of preventing progression to type 2 diabetes mellitus |
| US20150157672A1 (en) * | 2013-12-09 | 2015-06-11 | Phytology Labs, Inc. | Kits and methods for sustained weight loss |
| US10278948B1 (en) | 2015-09-03 | 2019-05-07 | Tian Xia | Method for transnasal delivery of anticonvulsant and therapeutic treatments |
| US20170071970A1 (en) * | 2015-09-15 | 2017-03-16 | Janssen Pharmaceutica Nv | Co-therapy comprising canagliflozin and phentermine for the treatment of obesity and obesity related disorders |
| US10498398B2 (en) * | 2015-10-12 | 2019-12-03 | Walmart Apollo, Llc | Data synthesis using near field communication |
| US20170319540A1 (en) * | 2016-05-06 | 2017-11-09 | Vivus, Inc. | Methods and Compositions for the Treatment of Non-Alcoholic Steatohepatitis |
| WO2020131784A1 (en) * | 2018-12-18 | 2020-06-25 | DDP Specialty Electronic Materials US, Inc. | A sustained release composition comprising a methylcellulose |
| CN109908165B (zh) * | 2019-04-26 | 2020-06-09 | 北京大学 | 一种含有雷公藤红素的组合物及其应用 |
| CN109999044B (zh) * | 2019-05-24 | 2020-06-09 | 北京大学 | 一种含有醉茄素a的组合物及其应用 |
| KR102199452B1 (ko) | 2019-10-17 | 2021-01-06 | 안지훈 | 퇴식처리장치 |
| WO2021245605A1 (en) * | 2020-06-04 | 2021-12-09 | Sipnose Ltd | Drug delivery devices and methods for administering substances to a body cavity by heterogenous aerosolization for treatment of binge-eating disorders and/or obesity |
| US12046341B2 (en) | 2021-10-05 | 2024-07-23 | Bradford Rabin | Methods and systems for electronically adjusting a dosing pattern of a patient undergoing a medical regimen |
| KR20250173977A (ko) | 2024-06-04 | 2025-12-11 | 주식회사 티에치팜 | 토피라메이트를 포함하는 약물 적재 필라멘트 및 이를 사용하여 제조된 비만 예방 또는 치료용 약학 제제 |
Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008061226A2 (en) * | 2006-11-17 | 2008-05-22 | Supernus Pharmaceuticals Inc. | Sustained-release formulations of topiramate |
Family Cites Families (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4513006A (en) * | 1983-09-26 | 1985-04-23 | Mcneil Lab., Inc. | Anticonvulsant sulfamate derivatives |
| US4895845A (en) * | 1986-09-15 | 1990-01-23 | Seed John C | Method of assisting weight loss |
| US4792569A (en) * | 1987-08-27 | 1988-12-20 | Mcneilab, Inc. | Anticonvulsant phenethyl sulfamates |
| US5273993A (en) * | 1989-06-12 | 1993-12-28 | A. H. Robins Company, Incorporated | Compounds having one or more aminosulfonyloxy radicals useful as pharmaceuticals |
| US5242391A (en) * | 1990-04-25 | 1993-09-07 | Alza Corporation | Urethral insert for treatment of erectile dysfunction |
| FR2684101B1 (fr) * | 1991-11-22 | 1993-12-31 | Adir Cie | Nouveaux derives de benzoate d'ethanolamine, leur procede de preparation et les compositions pharmaceutiques les renfermant. |
| US5242942A (en) * | 1992-04-28 | 1993-09-07 | Mcneilab, Inc. | Anticonvulsant fructopyranose cyclic sulfites and sulfates |
| US5384327A (en) * | 1992-12-22 | 1995-01-24 | Mcneilab, Inc. | Anticonvulsant sorbopyranose sulfamates |
| US5543405A (en) * | 1993-10-22 | 1996-08-06 | Keown; Wendy J. | Composition and method for weight reduction and long term management of obesity |
| US5498629A (en) * | 1993-12-23 | 1996-03-12 | Ortho Pharmaceutical Corporation | Anticonvulsant pseudofructopyranose sulfamates |
| US5527788A (en) * | 1994-01-18 | 1996-06-18 | Louisiana State Univ. Medical Center Foundation | Method and composition for treating obesity comprising dehydroepiandrosterone (DHEA), or a derivative thereof, and an anorectic agent |
| US5753693A (en) * | 1996-06-28 | 1998-05-19 | Ortho Pharmaceutical Corporation | Anticonvulsant derivatives useful in treating manic-depressive bipolar disorder |
| SK284305B6 (sk) * | 1996-06-28 | 2005-01-03 | Ortho-Mcneil Pharmaceutical, Inc. | Liečivo na liečbu obezity |
| US5753694A (en) * | 1996-06-28 | 1998-05-19 | Ortho Pharmaceutical Corporation | Anticonvulsant derivatives useful in treating amyotrophic lateral sclerosis (ALS) |
| US5900418A (en) | 1997-02-10 | 1999-05-04 | Synapse Pharmaceuticals International, Inc. | Method for treatment of obesity |
| US5795895A (en) * | 1997-06-13 | 1998-08-18 | Anchors; J. Michael | Combination anorexiant drug therapy for obesity using phentermine and an SSRI drug |
| US5990418A (en) * | 1997-07-29 | 1999-11-23 | International Business Machines Corporation | Hermetic CBGA/CCGA structure with thermal paste cooling |
| US5885616A (en) * | 1997-08-18 | 1999-03-23 | Impax Pharmaceuticals, Inc. | Sustained release drug delivery system suitable for oral administration |
| ES2228465T3 (es) * | 1999-01-19 | 2005-04-16 | Ortho-Mcneil Pharmaceutical, Inc. | Uso derivados anticonvulsionantes para el tratamiento de cefaleas en brotes. |
| WO2000050020A2 (en) | 1999-02-24 | 2000-08-31 | University Of Cincinnati | Use of sulfamate derivatives for treating impulse control disorders |
| AU775849B2 (en) * | 1999-04-08 | 2004-08-19 | Ortho-Mcneil Pharmaceutical, Inc. | Anticonvulsant derivatives useful in lowering blood pressure |
| NZ514811A (en) * | 1999-04-08 | 2005-01-28 | Ortho Mcneil Pharm Inc | Anticonvulsant derivatives useful in reducing blood glucose levels |
| US6420369B1 (en) * | 1999-05-24 | 2002-07-16 | Ortho-Mcneil Pharmaceutical, Inc. | Anticonvulsant derivatives useful in treating dementia |
| PT1187603E (pt) | 1999-06-14 | 2007-11-16 | Vivus Inc | ''terapia de associação para promover a perda de peso e o tratamento da obesidade'' |
| US7056890B2 (en) * | 1999-06-14 | 2006-06-06 | Vivus, Inc. | Combination therapy for effecting weight loss and treating obesity |
| US7659256B2 (en) * | 1999-06-14 | 2010-02-09 | Vivus, Inc. | Combination therapy for effecting weight loss and treating obesity |
| US7674776B2 (en) * | 1999-06-14 | 2010-03-09 | Vivus, Inc. | Combination therapy for effecting weight loss and treating obesity |
| US20080255093A1 (en) * | 1999-06-14 | 2008-10-16 | Tam Peter Y | Compositions and methods for treating obesity and related disorders |
| US7553818B2 (en) * | 1999-06-14 | 2009-06-30 | Vivus, Inc. | Combination therapy for effecting weight loss and treating obesity |
| US20080103179A1 (en) * | 2006-10-27 | 2008-05-01 | Tam Peter Y | Combination Therapy |
| US6759437B2 (en) * | 1999-10-04 | 2004-07-06 | Martin C. Hinz | Comprehensive pharmacologic therapy for treatment of obesity including cysteine |
| WO2002003984A2 (en) * | 2000-07-07 | 2002-01-17 | Ortho-Mcneil Pharmaceutical, Inc. | Anticonvulsant derivatives useful for treating and preventing the development of type ii diabetes mellitus and syndrome x |
| US6627653B2 (en) * | 2000-08-02 | 2003-09-30 | Ortho-Mcneil Pharmaceutical, Inc. | Anticonvulsant derivatives useful for the treatment of depression |
| US20020091114A1 (en) * | 2000-10-04 | 2002-07-11 | Odile Piot-Grosjean | Combination of a CB1 receptor antagonist and of sibutramine, the pharmaceutical compositions comprising them and their use in the treatment of obesity |
| JP2004518718A (ja) * | 2000-10-30 | 2004-06-24 | オーソ−マクニール・フアーマシユーチカル・インコーポレーテツド | 抗糖尿病薬および抗痙攣薬を含んで成る併用療法 |
| CA2437333A1 (en) * | 2001-02-02 | 2002-08-22 | Ortho-Mcneil Pharmaceutical, Inc. | Treatment of neurological dysfunction comprising fructopyranose sulfamates and erythropoietin |
| US20030072802A1 (en) * | 2001-10-11 | 2003-04-17 | R.T. Alamo Ventures, Inc. | Sustained release topiramate |
| US6559293B1 (en) * | 2002-02-15 | 2003-05-06 | Transform Pharmaceuticals, Inc. | Topiramate sodium trihydrate |
| EP2301537A1 (en) * | 2002-05-17 | 2011-03-30 | Duke University | Zonisamide for the treatment of obesity |
| US20050215552A1 (en) | 2002-05-17 | 2005-09-29 | Gadde Kishore M | Method for treating obesity |
| US20040122033A1 (en) * | 2002-12-10 | 2004-06-24 | Nargund Ravi P. | Combination therapy for the treatment of obesity |
| KR20060123493A (ko) | 2003-12-23 | 2006-12-01 | 알자 코포레이션 | 제어된 전달을 위한 약물 조성물의 용해도를 증가시키는방법 및 제형 |
| EP1734955A2 (en) * | 2004-01-13 | 2006-12-27 | Duke University | Compositions of an anticonvulsant and an antipsychotic drug for affecting weigt loss |
| MX2007001366A (es) * | 2004-08-03 | 2007-04-02 | Orexigen Therapeutics Inc | Combinacion de bupropion y un segundo compuesto para afectar la perdida de peso. |
| EP2286837A3 (en) * | 2004-11-01 | 2013-09-04 | Amylin Pharmaceuticals, LLC | Treatment of obesity and obesity related diseases |
| US7875627B2 (en) * | 2004-12-07 | 2011-01-25 | Abbott Laboratories | Thienopyridyl compounds that inhibit vanilloid receptor subtype 1 (VR1) and uses thereof |
| WO2006063078A2 (en) | 2004-12-08 | 2006-06-15 | Elan Corporation, Plc | Topiramate pharmaceuticals composition |
| EP2111859A1 (en) | 2004-12-23 | 2009-10-28 | Arena Pharmaceuticals, Inc. | 5HT2C receptor modulator compositions and methods of use |
| WO2006124506A2 (en) | 2005-05-13 | 2006-11-23 | Abbott Laboratories | Combination and use of drugs |
| WO2007089318A2 (en) * | 2005-11-23 | 2007-08-09 | Orexigen Therapeutics, Inc. | Compositions and methods for reducing food cravings |
| WO2007084290A2 (en) | 2006-01-12 | 2007-07-26 | Orexigen Therapeutics, Inc. | Compositions of an anticonvulsant and psychotherapeutic and methods of using the same for reversing weight gain |
| US9744137B2 (en) * | 2006-08-31 | 2017-08-29 | Supernus Pharmaceuticals, Inc. | Topiramate compositions and methods of enhancing its bioavailability |
| FR2907550B1 (fr) | 2006-10-18 | 2009-01-16 | Renault Sas | Ligne de prelevement de gaz circulant dans un conduit de moteur a combustion interne et banc d'essais comportant une telle ligne |
| AU2007319471B9 (en) | 2006-11-09 | 2012-10-04 | Nalpropion Pharmaceuticals Llc | Layered pharmaceutical formulations comprising an intermediate rapidly dissolving layer |
| US8071557B2 (en) | 2007-06-13 | 2011-12-06 | Vivus, Inc. | Treatment of pulmonary hypertension with carbonic anhydrase inhibitors |
| WO2009061436A1 (en) | 2007-11-06 | 2009-05-14 | University Of Florida Research Foundation | Compound for activating 5-ht2c receptors in combination with an amphetamine compound |
| US8580298B2 (en) * | 2008-06-09 | 2013-11-12 | Vivus, Inc. | Low dose topiramate/phentermine composition and methods of use thereof |
| US20090304789A1 (en) * | 2008-06-09 | 2009-12-10 | Thomas Najarian | Novel topiramate compositions and an escalating dosing strategy for treating obesity and related disorders |
| AU2011203970A1 (en) | 2010-01-07 | 2012-07-12 | Vivus, Inc. | Treatment of obstructive sleep apnea syndrome with a combination of a carbonic anhydrase inhibitor and an additional active agent |
-
2008
- 2008-06-09 US US12/135,953 patent/US20090304789A1/en not_active Abandoned
-
2009
- 2009-06-09 AU AU2009257572A patent/AU2009257572C1/en active Active
- 2009-06-09 KR KR1020117000416A patent/KR20110042280A/ko not_active Ceased
- 2009-06-09 JP JP2011513647A patent/JP5752595B2/ja active Active
- 2009-06-09 PT PT97634794T patent/PT2317997T/pt unknown
- 2009-06-09 EP EP09763479.4A patent/EP2317997B1/en active Active
- 2009-06-09 US US12/481,548 patent/US8580299B2/en active Active
- 2009-06-09 DK DK09763479.4T patent/DK2317997T3/en active
- 2009-06-09 CN CN2009801303795A patent/CN102112126A/zh active Pending
- 2009-06-09 CN CN201510977746.2A patent/CN105534921A/zh active Pending
- 2009-06-09 AU AU2009257573A patent/AU2009257573B2/en active Active
- 2009-06-09 KR KR1020117000417A patent/KR20110044847A/ko not_active Ceased
- 2009-06-09 JP JP2011513646A patent/JP2011522896A/ja active Pending
- 2009-06-09 BR BRPI0914985-6A patent/BRPI0914985B1/pt active IP Right Grant
- 2009-06-09 WO PCT/US2009/046804 patent/WO2009152189A1/en not_active Ceased
- 2009-06-09 BR BRPI0914991A patent/BRPI0914991A2/pt not_active Application Discontinuation
- 2009-06-09 MX MX2010013505A patent/MX342684B/es active IP Right Grant
- 2009-06-09 KR KR1020147027268A patent/KR20140121491A/ko not_active Ceased
- 2009-06-09 WO PCT/US2009/046805 patent/WO2009152190A1/en not_active Ceased
- 2009-06-09 MX MX2010013503A patent/MX2010013503A/es active IP Right Grant
- 2009-06-09 CA CA2727313A patent/CA2727313C/en active Active
- 2009-06-09 CA CA2727319A patent/CA2727319C/en active Active
- 2009-06-09 CN CN2009801304444A patent/CN102112127A/zh active Pending
- 2009-06-09 EP EP09763480A patent/EP2300002A1/en not_active Ceased
- 2009-06-09 CN CN201510112539.0A patent/CN104825477A/zh active Pending
- 2009-06-09 ES ES09763479.4T patent/ES2606041T3/es active Active
-
2010
- 2010-12-07 CL CL2010001366A patent/CL2010001366A1/es unknown
- 2010-12-07 CL CL2010001365A patent/CL2010001365A1/es unknown
- 2010-12-08 ZA ZA2010/08840A patent/ZA201008840B/en unknown
- 2010-12-08 ZA ZA2010/08839A patent/ZA201008839B/en unknown
- 2010-12-09 IL IL209874A patent/IL209874B/en active IP Right Grant
- 2010-12-09 IL IL209875A patent/IL209875A/en active IP Right Grant
-
2013
- 2013-10-08 US US14/048,416 patent/US8895057B2/en active Active
-
2014
- 2014-09-24 US US14/495,250 patent/US9011906B2/en active Active
-
2015
- 2015-04-06 US US14/679,754 patent/US20150209325A1/en not_active Abandoned
- 2015-05-20 JP JP2015102962A patent/JP6077053B2/ja active Active
- 2015-07-30 JP JP2015151088A patent/JP2016006085A/ja active Pending
- 2015-10-08 US US14/878,235 patent/US20160022630A1/en not_active Abandoned
-
2016
- 2016-02-12 HK HK16101537.0A patent/HK1213489A1/zh unknown
- 2016-05-10 US US15/150,907 patent/US20160250180A1/en not_active Abandoned
- 2016-10-19 CY CY20161101053T patent/CY1118103T1/el unknown
- 2016-12-20 JP JP2016246871A patent/JP6214750B2/ja active Active
-
2017
- 2017-01-11 JP JP2017002448A patent/JP2017105788A/ja active Pending
-
2018
- 2018-12-14 US US16/220,276 patent/US20190350897A1/en not_active Abandoned
-
2020
- 2020-02-20 US US16/796,194 patent/US20200188352A1/en not_active Abandoned
-
2021
- 2021-08-23 US US17/408,881 patent/US20210379013A1/en not_active Abandoned
-
2023
- 2023-03-22 US US18/124,774 patent/US20230233521A1/en active Pending
Patent Citations (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008061226A2 (en) * | 2006-11-17 | 2008-05-22 | Supernus Pharmaceuticals Inc. | Sustained-release formulations of topiramate |
Also Published As
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP6077053B2 (ja) | 体重減少の達成および肥満症の治療のための漸増用量投与計画(escalatingdosingregimen) | |
| US20210378972A1 (en) | Low dose topiramate/phentermine composition and methods of use thereof | |
| AU2016203698B2 (en) | Low Dose Topiramate/Phentermine Composition and Methods of Use Thereof | |
| AU2016203699A1 (en) | Escalating Dosing Regimen for Effecting Weight Loss and Treating Obesity | |
| AU2014202124A1 (en) | Escalating Dosing Regimen for Effecting Weight Loss and Treating Obesity |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150618 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20150618 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20160218 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20160517 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20160725 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20161116 |
|
| TRDD | Decision of grant or rejection written | ||
| A01 | Written decision to grant a patent or to grant a registration (utility model) |
Free format text: JAPANESE INTERMEDIATE CODE: A01 Effective date: 20161213 |
|
| A61 | First payment of annual fees (during grant procedure) |
Free format text: JAPANESE INTERMEDIATE CODE: A61 Effective date: 20170111 |
|
| R150 | Certificate of patent or registration of utility model |
Ref document number: 6077053 Country of ref document: JP Free format text: JAPANESE INTERMEDIATE CODE: R150 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| S533 | Written request for registration of change of name |
Free format text: JAPANESE INTERMEDIATE CODE: R313533 |
|
| R350 | Written notification of registration of transfer |
Free format text: JAPANESE INTERMEDIATE CODE: R350 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |
|
| R250 | Receipt of annual fees |
Free format text: JAPANESE INTERMEDIATE CODE: R250 |