JP2013520681A - 質量スペクトル分析を用いた、治療薬投与のための癌患者のセレクション - Google Patents
質量スペクトル分析を用いた、治療薬投与のための癌患者のセレクション Download PDFInfo
- Publication number
- JP2013520681A JP2013520681A JP2012555001A JP2012555001A JP2013520681A JP 2013520681 A JP2013520681 A JP 2013520681A JP 2012555001 A JP2012555001 A JP 2012555001A JP 2012555001 A JP2012555001 A JP 2012555001A JP 2013520681 A JP2013520681 A JP 2013520681A
- Authority
- JP
- Japan
- Prior art keywords
- patient
- combination
- egfr
- cancer
- treatment
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
- 206010028980 Neoplasm Diseases 0.000 title claims abstract description 139
- 201000011510 cancer Diseases 0.000 title claims abstract description 89
- 238000011282 treatment Methods 0.000 title claims abstract description 72
- 238000010183 spectrum analysis Methods 0.000 title description 2
- 239000003814 drug Substances 0.000 claims abstract description 94
- 230000037361 pathway Effects 0.000 claims abstract description 80
- 229940124597 therapeutic agent Drugs 0.000 claims abstract description 61
- 102000005962 receptors Human genes 0.000 claims abstract description 56
- 108020003175 receptors Proteins 0.000 claims abstract description 56
- 238000000034 method Methods 0.000 claims abstract description 53
- 102000043136 MAP kinase family Human genes 0.000 claims abstract description 47
- 108091054455 MAP kinase family Proteins 0.000 claims abstract description 47
- 108090000315 Protein Kinase C Proteins 0.000 claims abstract description 45
- 102000003923 Protein Kinase C Human genes 0.000 claims abstract description 45
- 108090000623 proteins and genes Proteins 0.000 claims abstract description 34
- 102000004169 proteins and genes Human genes 0.000 claims abstract description 27
- 239000007787 solid Substances 0.000 claims abstract description 23
- 238000007635 classification algorithm Methods 0.000 claims abstract description 18
- 238000011144 upstream manufacturing Methods 0.000 claims abstract description 16
- 230000017128 negative regulation of NF-kappaB transcription factor activity Effects 0.000 claims abstract description 15
- 239000000556 agonist Substances 0.000 claims abstract description 14
- 230000008685 targeting Effects 0.000 claims abstract description 13
- FAWLNURBQMTKEB-URDPEVQOSA-N 213546-53-3 Chemical compound N([C@@H](C)C(=O)N[C@H](C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C)C(=O)N1CCC[C@H]1C(=O)N[C@H](C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCSC)C(=O)N1[C@@H](CCC1)C(O)=O)C(C)C)C(C)C)C(=O)[C@@H]1CCCN1C(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](C)NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)N)C(C)C FAWLNURBQMTKEB-URDPEVQOSA-N 0.000 claims abstract description 10
- 101001012157 Homo sapiens Receptor tyrosine-protein kinase erbB-2 Proteins 0.000 claims abstract description 9
- 102100030086 Receptor tyrosine-protein kinase erbB-2 Human genes 0.000 claims abstract description 9
- 102000052116 epidermal growth factor receptor activity proteins Human genes 0.000 claims abstract 9
- 108700015053 epidermal growth factor receptor activity proteins Proteins 0.000 claims abstract 9
- YOHYSYJDKVYCJI-UHFFFAOYSA-N n-[3-[[6-[3-(trifluoromethyl)anilino]pyrimidin-4-yl]amino]phenyl]cyclopropanecarboxamide Chemical compound FC(F)(F)C1=CC=CC(NC=2N=CN=C(NC=3C=C(NC(=O)C4CC4)C=CC=3)C=2)=C1 YOHYSYJDKVYCJI-UHFFFAOYSA-N 0.000 claims abstract 9
- 238000001819 mass spectrum Methods 0.000 claims description 36
- 238000001228 spectrum Methods 0.000 claims description 33
- 210000004369 blood Anatomy 0.000 claims description 28
- 239000008280 blood Substances 0.000 claims description 28
- 229940111134 coxibs Drugs 0.000 claims description 28
- 239000003255 cyclooxygenase 2 inhibitor Substances 0.000 claims description 28
- 230000008901 benefit Effects 0.000 claims description 24
- 229940121647 egfr inhibitor Drugs 0.000 claims description 24
- 238000012549 training Methods 0.000 claims description 15
- 230000001225 therapeutic effect Effects 0.000 claims description 11
- 229960000590 celecoxib Drugs 0.000 claims description 10
- RZEKVGVHFLEQIL-UHFFFAOYSA-N celecoxib Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1 RZEKVGVHFLEQIL-UHFFFAOYSA-N 0.000 claims description 10
- 102000005789 Vascular Endothelial Growth Factors Human genes 0.000 claims description 8
- 108010019530 Vascular Endothelial Growth Factors Proteins 0.000 claims description 8
- 229960000371 rofecoxib Drugs 0.000 claims description 7
- RZJQGNCSTQAWON-UHFFFAOYSA-N rofecoxib Chemical compound C1=CC(S(=O)(=O)C)=CC=C1C1=C(C=2C=CC=CC=2)C(=O)OC1 RZJQGNCSTQAWON-UHFFFAOYSA-N 0.000 claims description 7
- 238000003860 storage Methods 0.000 claims description 6
- 229960000575 trastuzumab Drugs 0.000 claims description 4
- 229940124638 COX inhibitor Drugs 0.000 claims description 3
- 101000725401 Homo sapiens Cytochrome c oxidase subunit 2 Proteins 0.000 abstract description 17
- 101000605127 Homo sapiens Prostaglandin G/H synthase 2 Proteins 0.000 abstract description 17
- 238000004949 mass spectrometry Methods 0.000 abstract description 15
- 102100027456 Cytochrome c oxidase subunit 2 Human genes 0.000 abstract 1
- 102000054727 Serum Amyloid A Human genes 0.000 description 99
- 108700028909 Serum Amyloid A Proteins 0.000 description 93
- 102000001301 EGF receptor Human genes 0.000 description 60
- 108060006698 EGF receptor Proteins 0.000 description 58
- 230000004913 activation Effects 0.000 description 57
- 210000004027 cell Anatomy 0.000 description 46
- 238000011318 VeriStrat test Methods 0.000 description 32
- 208000002154 non-small cell lung carcinoma Diseases 0.000 description 32
- 239000000523 sample Substances 0.000 description 31
- 208000029729 tumor suppressor gene on chromosome 11 Diseases 0.000 description 30
- 229940079593 drug Drugs 0.000 description 29
- 102100038280 Prostaglandin G/H synthase 2 Human genes 0.000 description 26
- XGALLCVXEZPNRQ-UHFFFAOYSA-N gefitinib Chemical compound C=12C=C(OCCCN3CCOCC3)C(OC)=CC2=NC=NC=1NC1=CC=C(F)C(Cl)=C1 XGALLCVXEZPNRQ-UHFFFAOYSA-N 0.000 description 26
- 239000005411 L01XE02 - Gefitinib Substances 0.000 description 24
- 238000002512 chemotherapy Methods 0.000 description 24
- 229960002584 gefitinib Drugs 0.000 description 24
- 210000002966 serum Anatomy 0.000 description 24
- 230000014509 gene expression Effects 0.000 description 23
- 230000000694 effects Effects 0.000 description 22
- 206010058467 Lung neoplasm malignant Diseases 0.000 description 21
- 208000020816 lung neoplasm Diseases 0.000 description 21
- 102000002574 p38 Mitogen-Activated Protein Kinases Human genes 0.000 description 21
- 108010068338 p38 Mitogen-Activated Protein Kinases Proteins 0.000 description 21
- 201000005202 lung cancer Diseases 0.000 description 20
- 108091008611 Protein Kinase B Proteins 0.000 description 19
- 230000011664 signaling Effects 0.000 description 19
- 102000007665 Extracellular Signal-Regulated MAP Kinases Human genes 0.000 description 18
- 108010007457 Extracellular Signal-Regulated MAP Kinases Proteins 0.000 description 18
- 206010061218 Inflammation Diseases 0.000 description 18
- 230000004054 inflammatory process Effects 0.000 description 18
- 108010055717 JNK Mitogen-Activated Protein Kinases Proteins 0.000 description 15
- 238000012360 testing method Methods 0.000 description 15
- 230000007246 mechanism Effects 0.000 description 14
- 230000004044 response Effects 0.000 description 14
- 108010057466 NF-kappa B Proteins 0.000 description 13
- 102000003945 NF-kappa B Human genes 0.000 description 13
- 102000005622 Receptor for Advanced Glycation End Products Human genes 0.000 description 13
- 108010045108 Receptor for Advanced Glycation End Products Proteins 0.000 description 13
- 230000006907 apoptotic process Effects 0.000 description 13
- 230000001965 increasing effect Effects 0.000 description 13
- 239000003112 inhibitor Substances 0.000 description 13
- 230000003993 interaction Effects 0.000 description 13
- 230000034190 positive regulation of NF-kappaB transcription factor activity Effects 0.000 description 13
- 230000004083 survival effect Effects 0.000 description 13
- 229940121358 tyrosine kinase inhibitor Drugs 0.000 description 12
- 230000027455 binding Effects 0.000 description 11
- 230000005764 inhibitory process Effects 0.000 description 11
- 239000005483 tyrosine kinase inhibitor Substances 0.000 description 11
- 206010009944 Colon cancer Diseases 0.000 description 10
- 108010037462 Cyclooxygenase 2 Proteins 0.000 description 10
- 231100000504 carcinogenesis Toxicity 0.000 description 10
- 208000005623 Carcinogenesis Diseases 0.000 description 9
- 239000005551 L01XE03 - Erlotinib Substances 0.000 description 9
- 239000000090 biomarker Substances 0.000 description 9
- 230000036952 cancer formation Effects 0.000 description 9
- 238000002648 combination therapy Methods 0.000 description 9
- 229960001433 erlotinib Drugs 0.000 description 9
- AAKJLRGGTJKAMG-UHFFFAOYSA-N erlotinib Chemical compound C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 AAKJLRGGTJKAMG-UHFFFAOYSA-N 0.000 description 9
- 230000006870 function Effects 0.000 description 9
- 230000035772 mutation Effects 0.000 description 9
- 238000010606 normalization Methods 0.000 description 9
- 208000001333 Colorectal Neoplasms Diseases 0.000 description 8
- 208000000102 Squamous Cell Carcinoma of Head and Neck Diseases 0.000 description 8
- 102000040945 Transcription factor Human genes 0.000 description 8
- 108091023040 Transcription factor Proteins 0.000 description 8
- 229960005395 cetuximab Drugs 0.000 description 8
- 201000010099 disease Diseases 0.000 description 8
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 8
- 102000027426 receptor tyrosine kinases Human genes 0.000 description 8
- 108091008598 receptor tyrosine kinases Proteins 0.000 description 8
- 210000004881 tumor cell Anatomy 0.000 description 8
- 102000004127 Cytokines Human genes 0.000 description 7
- 108090000695 Cytokines Proteins 0.000 description 7
- 102000010834 Extracellular Matrix Proteins Human genes 0.000 description 7
- 108010037362 Extracellular Matrix Proteins Proteins 0.000 description 7
- 102100039360 Toll-like receptor 4 Human genes 0.000 description 7
- 238000011161 development Methods 0.000 description 7
- 230000018109 developmental process Effects 0.000 description 7
- XEYBRNLFEZDVAW-ARSRFYASSA-N dinoprostone Chemical compound CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O XEYBRNLFEZDVAW-ARSRFYASSA-N 0.000 description 7
- 210000002744 extracellular matrix Anatomy 0.000 description 7
- 201000000459 head and neck squamous cell carcinoma Diseases 0.000 description 7
- 239000003446 ligand Substances 0.000 description 7
- 230000008569 process Effects 0.000 description 7
- 230000019491 signal transduction Effects 0.000 description 7
- 206010006187 Breast cancer Diseases 0.000 description 6
- 101000669447 Homo sapiens Toll-like receptor 4 Proteins 0.000 description 6
- 102100021126 N-formyl peptide receptor 2 Human genes 0.000 description 6
- 101710190759 Serum amyloid A protein Proteins 0.000 description 6
- 230000004071 biological effect Effects 0.000 description 6
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 6
- 230000000875 corresponding effect Effects 0.000 description 6
- 210000002919 epithelial cell Anatomy 0.000 description 6
- 230000002757 inflammatory effect Effects 0.000 description 6
- 210000004072 lung Anatomy 0.000 description 6
- 230000001404 mediated effect Effects 0.000 description 6
- 238000010837 poor prognosis Methods 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 150000003384 small molecules Chemical class 0.000 description 6
- 238000002626 targeted therapy Methods 0.000 description 6
- 238000002560 therapeutic procedure Methods 0.000 description 6
- 208000026310 Breast neoplasm Diseases 0.000 description 5
- 201000009030 Carcinoma Diseases 0.000 description 5
- 102000004190 Enzymes Human genes 0.000 description 5
- 108090000790 Enzymes Proteins 0.000 description 5
- 102000003688 G-Protein-Coupled Receptors Human genes 0.000 description 5
- 108090000045 G-Protein-Coupled Receptors Proteins 0.000 description 5
- 102000004890 Interleukin-8 Human genes 0.000 description 5
- 108090001007 Interleukin-8 Proteins 0.000 description 5
- 102000019149 MAP kinase activity proteins Human genes 0.000 description 5
- 108040008097 MAP kinase activity proteins Proteins 0.000 description 5
- 108091007960 PI3Ks Proteins 0.000 description 5
- 108090000430 Phosphatidylinositol 3-kinases Proteins 0.000 description 5
- 102000003993 Phosphatidylinositol 3-kinases Human genes 0.000 description 5
- 102000001708 Protein Isoforms Human genes 0.000 description 5
- 108010029485 Protein Isoforms Proteins 0.000 description 5
- 102000004022 Protein-Tyrosine Kinases Human genes 0.000 description 5
- 108090000412 Protein-Tyrosine Kinases Proteins 0.000 description 5
- 102000002689 Toll-like receptor Human genes 0.000 description 5
- 108020000411 Toll-like receptor Proteins 0.000 description 5
- 208000009956 adenocarcinoma Diseases 0.000 description 5
- 230000002424 anti-apoptotic effect Effects 0.000 description 5
- 230000015556 catabolic process Effects 0.000 description 5
- 239000003795 chemical substances by application Substances 0.000 description 5
- 210000000038 chest Anatomy 0.000 description 5
- 230000002596 correlated effect Effects 0.000 description 5
- 238000006731 degradation reaction Methods 0.000 description 5
- 238000001514 detection method Methods 0.000 description 5
- 229960002986 dinoprostone Drugs 0.000 description 5
- 230000002401 inhibitory effect Effects 0.000 description 5
- 210000002540 macrophage Anatomy 0.000 description 5
- 238000007781 pre-processing Methods 0.000 description 5
- 108090000765 processed proteins & peptides Proteins 0.000 description 5
- XEYBRNLFEZDVAW-UHFFFAOYSA-N prostaglandin E2 Natural products CCCCCC(O)C=CC1C(O)CC(=O)C1CC=CCCCC(O)=O XEYBRNLFEZDVAW-UHFFFAOYSA-N 0.000 description 5
- 238000001959 radiotherapy Methods 0.000 description 5
- 230000003595 spectral effect Effects 0.000 description 5
- VBEQCZHXXJYVRD-GACYYNSASA-N uroanthelone Chemical compound C([C@@H](C(=O)N[C@H](C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@H](C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCC(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CO)C(=O)NCC(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CC=1C2=CC=CC=C2NC=1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O)C(C)C)[C@@H](C)O)NC(=O)[C@H](CO)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](NC(=O)[C@H](CC=1NC=NC=1)NC(=O)[C@H](CCSC)NC(=O)[C@H](CS)NC(=O)[C@@H](NC(=O)CNC(=O)CNC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CS)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)CNC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]1N(CCC1)C(=O)[C@H](CS)NC(=O)CNC(=O)[C@H]1N(CCC1)C(=O)[C@H](CC=1C=CC(O)=CC=1)NC(=O)[C@H](CO)NC(=O)[C@@H](N)CC(N)=O)C(C)C)[C@@H](C)CC)C1=CC=C(O)C=C1 VBEQCZHXXJYVRD-GACYYNSASA-N 0.000 description 5
- HJTAZXHBEBIQQX-UHFFFAOYSA-N 1,5-bis(chloromethyl)naphthalene Chemical compound C1=CC=C2C(CCl)=CC=CC2=C1CCl HJTAZXHBEBIQQX-UHFFFAOYSA-N 0.000 description 4
- BSYNRYMUTXBXSQ-UHFFFAOYSA-N Aspirin Chemical compound CC(=O)OC1=CC=CC=C1C(O)=O BSYNRYMUTXBXSQ-UHFFFAOYSA-N 0.000 description 4
- 102100022623 Hepatocyte growth factor receptor Human genes 0.000 description 4
- 101000818546 Homo sapiens N-formyl peptide receptor 2 Proteins 0.000 description 4
- 101000831567 Homo sapiens Toll-like receptor 2 Proteins 0.000 description 4
- 206010069755 K-ras gene mutation Diseases 0.000 description 4
- 206010027476 Metastases Diseases 0.000 description 4
- CXQHYVUVSFXTMY-UHFFFAOYSA-N N1'-[3-fluoro-4-[[6-methoxy-7-[3-(4-morpholinyl)propoxy]-4-quinolinyl]oxy]phenyl]-N1-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide Chemical compound C1=CN=C2C=C(OCCCN3CCOCC3)C(OC)=CC2=C1OC(C(=C1)F)=CC=C1NC(=O)C1(C(=O)NC=2C=CC(F)=CC=2)CC1 CXQHYVUVSFXTMY-UHFFFAOYSA-N 0.000 description 4
- 206010061902 Pancreatic neoplasm Diseases 0.000 description 4
- 108091000080 Phosphotransferase Proteins 0.000 description 4
- 102100024333 Toll-like receptor 2 Human genes 0.000 description 4
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 4
- 229960001138 acetylsalicylic acid Drugs 0.000 description 4
- 230000009471 action Effects 0.000 description 4
- 238000004458 analytical method Methods 0.000 description 4
- 230000033115 angiogenesis Effects 0.000 description 4
- 238000011319 anticancer therapy Methods 0.000 description 4
- GOLCXWYRSKYTSP-UHFFFAOYSA-N arsenic trioxide Inorganic materials O1[As]2O[As]1O2 GOLCXWYRSKYTSP-UHFFFAOYSA-N 0.000 description 4
- 229960000397 bevacizumab Drugs 0.000 description 4
- 230000004663 cell proliferation Effects 0.000 description 4
- 208000029742 colonic neoplasm Diseases 0.000 description 4
- VFLDPWHFBUODDF-FCXRPNKRSA-N curcumin Chemical compound C1=C(O)C(OC)=CC(\C=C\C(=O)CC(=O)\C=C\C=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-FCXRPNKRSA-N 0.000 description 4
- CGIGDMFJXJATDK-UHFFFAOYSA-N indomethacin Chemical compound CC1=C(CC(O)=O)C2=CC(OC)=CC=C2N1C(=O)C1=CC=C(Cl)C=C1 CGIGDMFJXJATDK-UHFFFAOYSA-N 0.000 description 4
- 208000015486 malignant pancreatic neoplasm Diseases 0.000 description 4
- 238000004519 manufacturing process Methods 0.000 description 4
- 238000005259 measurement Methods 0.000 description 4
- 210000001616 monocyte Anatomy 0.000 description 4
- 239000000041 non-steroidal anti-inflammatory agent Substances 0.000 description 4
- 229940021182 non-steroidal anti-inflammatory drug Drugs 0.000 description 4
- 201000002528 pancreatic cancer Diseases 0.000 description 4
- 208000008443 pancreatic carcinoma Diseases 0.000 description 4
- 102000020233 phosphotransferase Human genes 0.000 description 4
- 239000000902 placebo Substances 0.000 description 4
- 229940068196 placebo Drugs 0.000 description 4
- BASFCYQUMIYNBI-UHFFFAOYSA-N platinum Chemical compound [Pt] BASFCYQUMIYNBI-UHFFFAOYSA-N 0.000 description 4
- 102000016914 ras Proteins Human genes 0.000 description 4
- UEJJHQNACJXSKW-UHFFFAOYSA-N 2-(2,6-dioxopiperidin-3-yl)-1H-isoindole-1,3(2H)-dione Chemical compound O=C1C2=CC=CC=C2C(=O)N1C1CCC(=O)NC1=O UEJJHQNACJXSKW-UHFFFAOYSA-N 0.000 description 3
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 3
- 102000053028 CD36 Antigens Human genes 0.000 description 3
- 108010045374 CD36 Antigens Proteins 0.000 description 3
- 230000004543 DNA replication Effects 0.000 description 3
- -1 EMD 12104063 Chemical compound 0.000 description 3
- 101800003838 Epidermal growth factor Proteins 0.000 description 3
- 108010076288 Formyl peptide receptors Proteins 0.000 description 3
- 102000011652 Formyl peptide receptors Human genes 0.000 description 3
- 102100030708 GTPase KRas Human genes 0.000 description 3
- 108010010234 HDL Lipoproteins Proteins 0.000 description 3
- 102000015779 HDL Lipoproteins Human genes 0.000 description 3
- 101000584612 Homo sapiens GTPase KRas Proteins 0.000 description 3
- 101000972946 Homo sapiens Hepatocyte growth factor receptor Proteins 0.000 description 3
- 108010031794 IGF Type 1 Receptor Proteins 0.000 description 3
- HEFNNWSXXWATRW-UHFFFAOYSA-N Ibuprofen Chemical compound CC(C)CC1=CC=C(C(C)C(O)=O)C=C1 HEFNNWSXXWATRW-UHFFFAOYSA-N 0.000 description 3
- 102100039688 Insulin-like growth factor 1 receptor Human genes 0.000 description 3
- 102000002274 Matrix Metalloproteinases Human genes 0.000 description 3
- 108010000684 Matrix Metalloproteinases Proteins 0.000 description 3
- 102000018745 NF-KappaB Inhibitor alpha Human genes 0.000 description 3
- 108010052419 NF-KappaB Inhibitor alpha Proteins 0.000 description 3
- 102100033237 Pro-epidermal growth factor Human genes 0.000 description 3
- 102000004005 Prostaglandin-endoperoxide synthases Human genes 0.000 description 3
- 108090000459 Prostaglandin-endoperoxide synthases Proteins 0.000 description 3
- 206010060862 Prostate cancer Diseases 0.000 description 3
- 208000000236 Prostatic Neoplasms Diseases 0.000 description 3
- 101710100969 Receptor tyrosine-protein kinase erbB-3 Proteins 0.000 description 3
- 102000006747 Transforming Growth Factor alpha Human genes 0.000 description 3
- 101800004564 Transforming growth factor alpha Proteins 0.000 description 3
- 108060008682 Tumor Necrosis Factor Proteins 0.000 description 3
- 102000000852 Tumor Necrosis Factor-alpha Human genes 0.000 description 3
- 108091008605 VEGF receptors Proteins 0.000 description 3
- 102000009484 Vascular Endothelial Growth Factor Receptors Human genes 0.000 description 3
- 230000003213 activating effect Effects 0.000 description 3
- 239000002246 antineoplastic agent Substances 0.000 description 3
- 229960002594 arsenic trioxide Drugs 0.000 description 3
- 230000008827 biological function Effects 0.000 description 3
- 230000033228 biological regulation Effects 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 210000000481 breast Anatomy 0.000 description 3
- 235000012000 cholesterol Nutrition 0.000 description 3
- 229960004316 cisplatin Drugs 0.000 description 3
- DQLATGHUWYMOKM-UHFFFAOYSA-L cisplatin Chemical compound N[Pt](N)(Cl)Cl DQLATGHUWYMOKM-UHFFFAOYSA-L 0.000 description 3
- 230000000112 colonic effect Effects 0.000 description 3
- 238000006471 dimerization reaction Methods 0.000 description 3
- 229940116977 epidermal growth factor Drugs 0.000 description 3
- 229960005277 gemcitabine Drugs 0.000 description 3
- SDUQYLNIPVEERB-QPPQHZFASA-N gemcitabine Chemical compound O=C1N=C(N)C=CN1[C@H]1C(F)(F)[C@H](O)[C@@H](CO)O1 SDUQYLNIPVEERB-QPPQHZFASA-N 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 230000012010 growth Effects 0.000 description 3
- 239000003102 growth factor Substances 0.000 description 3
- 229960001680 ibuprofen Drugs 0.000 description 3
- 150000002500 ions Chemical class 0.000 description 3
- 239000011159 matrix material Substances 0.000 description 3
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 3
- 230000009401 metastasis Effects 0.000 description 3
- 210000000440 neutrophil Anatomy 0.000 description 3
- 238000004393 prognosis Methods 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 108010014186 ras Proteins Proteins 0.000 description 3
- 238000009097 single-agent therapy Methods 0.000 description 3
- 206010041823 squamous cell carcinoma Diseases 0.000 description 3
- 230000000638 stimulation Effects 0.000 description 3
- 230000001629 suppression Effects 0.000 description 3
- 229960003433 thalidomide Drugs 0.000 description 3
- 229940126585 therapeutic drug Drugs 0.000 description 3
- 230000003827 upregulation Effects 0.000 description 3
- LNPDTQAFDNKSHK-UHFFFAOYSA-N valdecoxib Chemical compound CC=1ON=C(C=2C=CC=CC=2)C=1C1=CC=C(S(N)(=O)=O)C=C1 LNPDTQAFDNKSHK-UHFFFAOYSA-N 0.000 description 3
- MZOFCQQQCNRIBI-VMXHOPILSA-N (3s)-4-[[(2s)-1-[[(2s)-1-[[(1s)-1-carboxy-2-hydroxyethyl]amino]-4-methyl-1-oxopentan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-3-[[2-[[(2s)-2,6-diaminohexanoyl]amino]acetyl]amino]-4-oxobutanoic acid Chemical compound OC[C@@H](C(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](CC(O)=O)NC(=O)CNC(=O)[C@@H](N)CCCCN MZOFCQQQCNRIBI-VMXHOPILSA-N 0.000 description 2
- 102000011767 Acute-Phase Proteins Human genes 0.000 description 2
- 108010062271 Acute-Phase Proteins Proteins 0.000 description 2
- 101100067974 Arabidopsis thaliana POP2 gene Proteins 0.000 description 2
- MLDQJTXFUGDVEO-UHFFFAOYSA-N BAY-43-9006 Chemical compound C1=NC(C(=O)NC)=CC(OC=2C=CC(NC(=O)NC=3C=C(C(Cl)=CC=3)C(F)(F)F)=CC=2)=C1 MLDQJTXFUGDVEO-UHFFFAOYSA-N 0.000 description 2
- 101100118549 Homo sapiens EGFR gene Proteins 0.000 description 2
- 101000611183 Homo sapiens Tumor necrosis factor Proteins 0.000 description 2
- 108090000723 Insulin-Like Growth Factor I Proteins 0.000 description 2
- 102000015696 Interleukins Human genes 0.000 description 2
- 108010063738 Interleukins Proteins 0.000 description 2
- 102000019145 JUN kinase activity proteins Human genes 0.000 description 2
- 239000002147 L01XE04 - Sunitinib Substances 0.000 description 2
- 239000005511 L01XE05 - Sorafenib Substances 0.000 description 2
- 239000002136 L01XE07 - Lapatinib Substances 0.000 description 2
- 239000003798 L01XE11 - Pazopanib Substances 0.000 description 2
- 102000005741 Metalloproteases Human genes 0.000 description 2
- 108010006035 Metalloproteases Proteins 0.000 description 2
- 101710091942 N-formyl peptide receptor 2 Proteins 0.000 description 2
- 108010014632 NF-kappa B kinase Proteins 0.000 description 2
- 108700020796 Oncogene Proteins 0.000 description 2
- 102100036201 Oxygen-dependent coproporphyrinogen-III oxidase, mitochondrial Human genes 0.000 description 2
- 229930012538 Paclitaxel Natural products 0.000 description 2
- 102100029986 Receptor tyrosine-protein kinase erbB-3 Human genes 0.000 description 2
- 101710100963 Receptor tyrosine-protein kinase erbB-4 Proteins 0.000 description 2
- 102100029981 Receptor tyrosine-protein kinase erbB-4 Human genes 0.000 description 2
- 239000006146 Roswell Park Memorial Institute medium Substances 0.000 description 2
- 101100123851 Saccharomyces cerevisiae (strain ATCC 204508 / S288c) HER1 gene Proteins 0.000 description 2
- 206010070834 Sensitisation Diseases 0.000 description 2
- 102000013275 Somatomedins Human genes 0.000 description 2
- 108010060804 Toll-Like Receptor 4 Proteins 0.000 description 2
- 102000008228 Toll-like receptor 2 Human genes 0.000 description 2
- 108010060888 Toll-like receptor 2 Proteins 0.000 description 2
- 102100040247 Tumor necrosis factor Human genes 0.000 description 2
- 238000002835 absorbance Methods 0.000 description 2
- 230000001154 acute effect Effects 0.000 description 2
- 206010002022 amyloidosis Diseases 0.000 description 2
- 239000012491 analyte Substances 0.000 description 2
- 238000011861 anti-inflammatory therapy Methods 0.000 description 2
- 230000001640 apoptogenic effect Effects 0.000 description 2
- 230000002238 attenuated effect Effects 0.000 description 2
- 238000004422 calculation algorithm Methods 0.000 description 2
- 230000022131 cell cycle Effects 0.000 description 2
- 230000010261 cell growth Effects 0.000 description 2
- 238000009104 chemotherapy regimen Methods 0.000 description 2
- 230000006020 chronic inflammation Effects 0.000 description 2
- 208000037976 chronic inflammation Diseases 0.000 description 2
- 229940109262 curcumin Drugs 0.000 description 2
- 235000012754 curcumin Nutrition 0.000 description 2
- 239000004148 curcumin Substances 0.000 description 2
- 230000007812 deficiency Effects 0.000 description 2
- 230000001419 dependent effect Effects 0.000 description 2
- VFLDPWHFBUODDF-UHFFFAOYSA-N diferuloylmethane Natural products C1=C(O)C(OC)=CC(C=CC(=O)CC(=O)C=CC=2C=C(OC)C(O)=CC=2)=C1 VFLDPWHFBUODDF-UHFFFAOYSA-N 0.000 description 2
- 230000007783 downstream signaling Effects 0.000 description 2
- 239000000890 drug combination Substances 0.000 description 2
- 239000006274 endogenous ligand Substances 0.000 description 2
- 238000002509 fluorescent in situ hybridization Methods 0.000 description 2
- 230000036252 glycation Effects 0.000 description 2
- 230000009036 growth inhibition Effects 0.000 description 2
- 108010064060 high density lipoprotein receptors Proteins 0.000 description 2
- 230000028993 immune response Effects 0.000 description 2
- 210000000987 immune system Anatomy 0.000 description 2
- 230000001506 immunosuppresive effect Effects 0.000 description 2
- 229960000905 indomethacin Drugs 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000001939 inductive effect Effects 0.000 description 2
- 210000004969 inflammatory cell Anatomy 0.000 description 2
- 230000037456 inflammatory mechanism Effects 0.000 description 2
- 238000007689 inspection Methods 0.000 description 2
- 230000003834 intracellular effect Effects 0.000 description 2
- 229960004891 lapatinib Drugs 0.000 description 2
- BCFGMOOMADDAQU-UHFFFAOYSA-N lapatinib Chemical compound O1C(CNCCS(=O)(=O)C)=CC=C1C1=CC=C(N=CN=C2NC=3C=C(Cl)C(OCC=4C=C(F)C=CC=4)=CC=3)C2=C1 BCFGMOOMADDAQU-UHFFFAOYSA-N 0.000 description 2
- 150000002632 lipids Chemical class 0.000 description 2
- 238000013173 literature analysis Methods 0.000 description 2
- 201000005296 lung carcinoma Diseases 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 230000010534 mechanism of action Effects 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- 230000002297 mitogenic effect Effects 0.000 description 2
- 231100000590 oncogenic Toxicity 0.000 description 2
- 230000002246 oncogenic effect Effects 0.000 description 2
- 230000002018 overexpression Effects 0.000 description 2
- 229940094443 oxytocics prostaglandins Drugs 0.000 description 2
- 229960001592 paclitaxel Drugs 0.000 description 2
- 229960001972 panitumumab Drugs 0.000 description 2
- 229960000639 pazopanib Drugs 0.000 description 2
- CUIHSIWYWATEQL-UHFFFAOYSA-N pazopanib Chemical compound C1=CC2=C(C)N(C)N=C2C=C1N(C)C(N=1)=CC=NC=1NC1=CC=C(C)C(S(N)(=O)=O)=C1 CUIHSIWYWATEQL-UHFFFAOYSA-N 0.000 description 2
- 229960005079 pemetrexed Drugs 0.000 description 2
- QOFFJEBXNKRSPX-ZDUSSCGKSA-N pemetrexed Chemical compound C1=N[C]2NC(N)=NC(=O)C2=C1CCC1=CC=C(C(=O)N[C@@H](CCC(O)=O)C(O)=O)C=C1 QOFFJEBXNKRSPX-ZDUSSCGKSA-N 0.000 description 2
- 230000026731 phosphorylation Effects 0.000 description 2
- 238000006366 phosphorylation reaction Methods 0.000 description 2
- 229910052697 platinum Inorganic materials 0.000 description 2
- 230000023603 positive regulation of transcription initiation, DNA-dependent Effects 0.000 description 2
- 230000003389 potentiating effect Effects 0.000 description 2
- 230000000861 pro-apoptotic effect Effects 0.000 description 2
- 102000004196 processed proteins & peptides Human genes 0.000 description 2
- 230000000770 proinflammatory effect Effects 0.000 description 2
- 230000001737 promoting effect Effects 0.000 description 2
- 150000003180 prostaglandins Chemical class 0.000 description 2
- 238000012552 review Methods 0.000 description 2
- 108010078070 scavenger receptors Proteins 0.000 description 2
- 102000014452 scavenger receptors Human genes 0.000 description 2
- 230000028327 secretion Effects 0.000 description 2
- 230000035945 sensitivity Effects 0.000 description 2
- 230000008313 sensitization Effects 0.000 description 2
- 229960003787 sorafenib Drugs 0.000 description 2
- 229960000894 sulindac Drugs 0.000 description 2
- MLKXDPUZXIRXEP-MFOYZWKCSA-N sulindac Chemical compound CC1=C(CC(O)=O)C2=CC(F)=CC=C2\C1=C/C1=CC=C(S(C)=O)C=C1 MLKXDPUZXIRXEP-MFOYZWKCSA-N 0.000 description 2
- 229960001796 sunitinib Drugs 0.000 description 2
- WINHZLLDWRZWRT-ATVHPVEESA-N sunitinib Chemical compound CCN(CC)CCNC(=O)C1=C(C)NC(\C=C/2C3=CC(F)=CC=C3NC\2=O)=C1C WINHZLLDWRZWRT-ATVHPVEESA-N 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- RCINICONZNJXQF-MZXODVADSA-N taxol Chemical compound O([C@@H]1[C@@]2(C[C@@H](C(C)=C(C2(C)C)[C@H](C([C@]2(C)[C@@H](O)C[C@H]3OC[C@]3([C@H]21)OC(C)=O)=O)OC(=O)C)OC(=O)[C@H](O)[C@@H](NC(=O)C=1C=CC=CC=1)C=1C=CC=CC=1)O)C(=O)C1=CC=CC=C1 RCINICONZNJXQF-MZXODVADSA-N 0.000 description 2
- 210000001519 tissue Anatomy 0.000 description 2
- 238000011269 treatment regimen Methods 0.000 description 2
- 230000001960 triggered effect Effects 0.000 description 2
- 230000009107 upstream regulation Effects 0.000 description 2
- WCWUXEGQKLTGDX-LLVKDONJSA-N (2R)-1-[[4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-5-methyl-6-pyrrolo[2,1-f][1,2,4]triazinyl]oxy]-2-propanol Chemical compound C1=C2NC(C)=CC2=C(F)C(OC2=NC=NN3C=C(C(=C32)C)OC[C@H](O)C)=C1 WCWUXEGQKLTGDX-LLVKDONJSA-N 0.000 description 1
- LOGFVTREOLYCPF-KXNHARMFSA-N (2s,3r)-2-[[(2r)-1-[(2s)-2,6-diaminohexanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxybutanoic acid Chemical compound C[C@@H](O)[C@@H](C(O)=O)NC(=O)[C@H]1CCCN1C(=O)[C@@H](N)CCCCN LOGFVTREOLYCPF-KXNHARMFSA-N 0.000 description 1
- XYPANFGXFLOTDK-CJLNJPJHSA-N 1b-de-l-phenylalanine-insulin Chemical compound C([C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O)C1=CC=C(O)C=C1.C([C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](CS)NC(=O)[C@H](C(C)C)NC(=O)[C@H](CO)NC(=O)[C@H](C)NC(=O)[C@H](CS)NC(=O)[C@H](CS)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](C(C)C)NC(=O)[C@@H](NC(=O)CN)[C@@H](C)CC)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC(O)=CC=1)C(=O)N[C@@H](CS)C(=O)N[C@@H](CC(N)=O)C(O)=O)C1=CC=C(O)C=C1 XYPANFGXFLOTDK-CJLNJPJHSA-N 0.000 description 1
- XXJWYDDUDKYVKI-UHFFFAOYSA-N 4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-[3-(1-pyrrolidinyl)propoxy]quinazoline Chemical compound COC1=CC2=C(OC=3C(=C4C=C(C)NC4=CC=3)F)N=CN=C2C=C1OCCCN1CCCC1 XXJWYDDUDKYVKI-UHFFFAOYSA-N 0.000 description 1
- CIUKPBWULKEZMF-UHFFFAOYSA-N 6-(1-methylpyrazol-4-yl)-2-[[3-[5-(2-morpholin-4-ylethoxy)pyrimidin-2-yl]phenyl]methyl]pyridazin-3-one Chemical compound C1=NN(C)C=C1C1=NN(CC=2C=C(C=CC=2)C=2N=CC(OCCN3CCOCC3)=CN=2)C(=O)C=C1 CIUKPBWULKEZMF-UHFFFAOYSA-N 0.000 description 1
- HEAIZQNMNCHNFD-UHFFFAOYSA-N AMG-208 Chemical compound C=1C=NC2=CC(OC)=CC=C2C=1OCC(N1N=2)=NN=C1C=CC=2C1=CC=CC=C1 HEAIZQNMNCHNFD-UHFFFAOYSA-N 0.000 description 1
- 206010000871 Acute monocytic leukaemia Diseases 0.000 description 1
- 230000007730 Akt signaling Effects 0.000 description 1
- 102000004145 Annexin A1 Human genes 0.000 description 1
- 108090000663 Annexin A1 Proteins 0.000 description 1
- 108010078554 Aromatase Proteins 0.000 description 1
- 102000014654 Aromatase Human genes 0.000 description 1
- 102000004506 Blood Proteins Human genes 0.000 description 1
- 108010017384 Blood Proteins Proteins 0.000 description 1
- 208000003174 Brain Neoplasms Diseases 0.000 description 1
- 102100036848 C-C motif chemokine 20 Human genes 0.000 description 1
- 101150071146 COX2 gene Proteins 0.000 description 1
- 102000000905 Cadherin Human genes 0.000 description 1
- 108050007957 Cadherin Proteins 0.000 description 1
- 101100114534 Caenorhabditis elegans ctc-2 gene Proteins 0.000 description 1
- 102100025064 Cellular tumor antigen p53 Human genes 0.000 description 1
- 101710150820 Cellular tumor antigen p53 Proteins 0.000 description 1
- 108010012236 Chemokines Proteins 0.000 description 1
- 102000019034 Chemokines Human genes 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 208000018458 Colitis-Associated Neoplasms Diseases 0.000 description 1
- 102100027907 Cytoplasmic tyrosine-protein kinase BMX Human genes 0.000 description 1
- IELOKBJPULMYRW-NJQVLOCASA-N D-alpha-Tocopheryl Acid Succinate Chemical compound OC(=O)CCC(=O)OC1=C(C)C(C)=C2O[C@@](CCC[C@H](C)CCC[C@H](C)CCCC(C)C)(C)CCC2=C1C IELOKBJPULMYRW-NJQVLOCASA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 102000009058 Death Domain Receptors Human genes 0.000 description 1
- 108010049207 Death Domain Receptors Proteins 0.000 description 1
- 101100508533 Drosophila melanogaster IKKbeta gene Proteins 0.000 description 1
- 206010059866 Drug resistance Diseases 0.000 description 1
- 101150029707 ERBB2 gene Proteins 0.000 description 1
- 241000588724 Escherichia coli Species 0.000 description 1
- 208000000461 Esophageal Neoplasms Diseases 0.000 description 1
- 206010073306 Exposure to radiation Diseases 0.000 description 1
- GHASVSINZRGABV-UHFFFAOYSA-N Fluorouracil Chemical compound FC1=CNC(=O)NC1=O GHASVSINZRGABV-UHFFFAOYSA-N 0.000 description 1
- 102100029974 GTPase HRas Human genes 0.000 description 1
- 101710091881 GTPase HRas Proteins 0.000 description 1
- 206010018338 Glioma Diseases 0.000 description 1
- 102000003886 Glycoproteins Human genes 0.000 description 1
- 108090000288 Glycoproteins Proteins 0.000 description 1
- 108010017080 Granulocyte Colony-Stimulating Factor Proteins 0.000 description 1
- 102100039619 Granulocyte colony-stimulating factor Human genes 0.000 description 1
- 102000009465 Growth Factor Receptors Human genes 0.000 description 1
- 108010009202 Growth Factor Receptors Proteins 0.000 description 1
- 208000002250 Hematologic Neoplasms Diseases 0.000 description 1
- 101000713099 Homo sapiens C-C motif chemokine 20 Proteins 0.000 description 1
- 101000919849 Homo sapiens Cytochrome c oxidase subunit 1 Proteins 0.000 description 1
- 101000935548 Homo sapiens Cytoplasmic tyrosine-protein kinase BMX Proteins 0.000 description 1
- 101000716481 Homo sapiens Lysosome membrane protein 2 Proteins 0.000 description 1
- 101000979342 Homo sapiens Nuclear factor NF-kappa-B p105 subunit Proteins 0.000 description 1
- 101000605639 Homo sapiens Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform Proteins 0.000 description 1
- 101000605122 Homo sapiens Prostaglandin G/H synthase 1 Proteins 0.000 description 1
- 101001074035 Homo sapiens Zinc finger protein GLI2 Proteins 0.000 description 1
- 101001057752 Human cytomegalovirus (strain AD169) Uncharacterized protein IRL4 Proteins 0.000 description 1
- 206010020880 Hypertrophy Diseases 0.000 description 1
- 102000003777 Interleukin-1 beta Human genes 0.000 description 1
- 108090000193 Interleukin-1 beta Proteins 0.000 description 1
- 108010065805 Interleukin-12 Proteins 0.000 description 1
- 102000013462 Interleukin-12 Human genes 0.000 description 1
- 108090001005 Interleukin-6 Proteins 0.000 description 1
- 102000004889 Interleukin-6 Human genes 0.000 description 1
- 208000005016 Intestinal Neoplasms Diseases 0.000 description 1
- 208000008839 Kidney Neoplasms Diseases 0.000 description 1
- 239000005517 L01XE01 - Imatinib Substances 0.000 description 1
- 239000005536 L01XE08 - Nilotinib Substances 0.000 description 1
- UCEQXRCJXIVODC-PMACEKPBSA-N LSM-1131 Chemical compound C1CCC2=CC=CC3=C2N1C=C3[C@@H]1C(=O)NC(=O)[C@H]1C1=CNC2=CC=CC=C12 UCEQXRCJXIVODC-PMACEKPBSA-N 0.000 description 1
- 229930184725 Lipoxin Natural products 0.000 description 1
- 102100020983 Lysosome membrane protein 2 Human genes 0.000 description 1
- 230000037364 MAPK/ERK pathway Effects 0.000 description 1
- 238000000134 MTT assay Methods 0.000 description 1
- 231100000002 MTT assay Toxicity 0.000 description 1
- 108010048043 Macrophage Migration-Inhibitory Factors Proteins 0.000 description 1
- 102100037791 Macrophage migration inhibitory factor Human genes 0.000 description 1
- 102000001776 Matrix metalloproteinase-9 Human genes 0.000 description 1
- 108010015302 Matrix metalloproteinase-9 Proteins 0.000 description 1
- 108090000744 Mitogen-Activated Protein Kinase Kinases Proteins 0.000 description 1
- 102000004232 Mitogen-Activated Protein Kinase Kinases Human genes 0.000 description 1
- 208000035489 Monocytic Acute Leukemia Diseases 0.000 description 1
- 241001529936 Murinae Species 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- ZDZOTLJHXYCWBA-VCVYQWHSSA-N N-debenzoyl-N-(tert-butoxycarbonyl)-10-deacetyltaxol Chemical compound O([C@H]1[C@H]2[C@@](C([C@H](O)C3=C(C)[C@@H](OC(=O)[C@H](O)[C@@H](NC(=O)OC(C)(C)C)C=4C=CC=CC=4)C[C@]1(O)C3(C)C)=O)(C)[C@@H](O)C[C@H]1OC[C@]12OC(=O)C)C(=O)C1=CC=CC=C1 ZDZOTLJHXYCWBA-VCVYQWHSSA-N 0.000 description 1
- 208000001894 Nasopharyngeal Neoplasms Diseases 0.000 description 1
- 206010061306 Nasopharyngeal cancer Diseases 0.000 description 1
- 206010028851 Necrosis Diseases 0.000 description 1
- 206010029379 Neutrophilia Diseases 0.000 description 1
- 102100023050 Nuclear factor NF-kappa-B p105 subunit Human genes 0.000 description 1
- 206010030155 Oesophageal carcinoma Diseases 0.000 description 1
- 101150000187 PTGS2 gene Proteins 0.000 description 1
- 208000037273 Pathologic Processes Diseases 0.000 description 1
- 102100038332 Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform Human genes 0.000 description 1
- 102100038277 Prostaglandin G/H synthase 1 Human genes 0.000 description 1
- 108010089836 Proto-Oncogene Proteins c-met Proteins 0.000 description 1
- 108010001859 Proto-Oncogene Proteins c-rel Proteins 0.000 description 1
- 102000000850 Proto-Oncogene Proteins c-rel Human genes 0.000 description 1
- 230000010799 Receptor Interactions Effects 0.000 description 1
- 102000004278 Receptor Protein-Tyrosine Kinases Human genes 0.000 description 1
- 108090000873 Receptor Protein-Tyrosine Kinases Proteins 0.000 description 1
- 208000015634 Rectal Neoplasms Diseases 0.000 description 1
- 206010038389 Renal cancer Diseases 0.000 description 1
- 208000006265 Renal cell carcinoma Diseases 0.000 description 1
- 108091079463 SAA family Proteins 0.000 description 1
- 102000041928 SAA family Human genes 0.000 description 1
- 108010017324 STAT3 Transcription Factor Proteins 0.000 description 1
- 102100024040 Signal transducer and activator of transcription 3 Human genes 0.000 description 1
- 208000005718 Stomach Neoplasms Diseases 0.000 description 1
- 101710119418 Superoxide dismutase [Mn] Proteins 0.000 description 1
- 101710202572 Superoxide dismutase [Mn], mitochondrial Proteins 0.000 description 1
- 102100032891 Superoxide dismutase [Mn], mitochondrial Human genes 0.000 description 1
- 230000024932 T cell mediated immunity Effects 0.000 description 1
- 102100036407 Thioredoxin Human genes 0.000 description 1
- 108010000499 Thromboplastin Proteins 0.000 description 1
- 102100030859 Tissue factor Human genes 0.000 description 1
- 102000008233 Toll-Like Receptor 4 Human genes 0.000 description 1
- 108090001012 Transforming Growth Factor beta Proteins 0.000 description 1
- 102000004887 Transforming Growth Factor beta Human genes 0.000 description 1
- 206010064390 Tumour invasion Diseases 0.000 description 1
- 241000251539 Vertebrata <Metazoa> Species 0.000 description 1
- 102100035558 Zinc finger protein GLI2 Human genes 0.000 description 1
- 230000002159 abnormal effect Effects 0.000 description 1
- 230000033289 adaptive immune response Effects 0.000 description 1
- 239000002671 adjuvant Substances 0.000 description 1
- 230000002152 alkylating effect Effects 0.000 description 1
- 150000001413 amino acids Chemical group 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 229940124650 anti-cancer therapies Drugs 0.000 description 1
- 230000000340 anti-metabolite Effects 0.000 description 1
- 230000001028 anti-proliverative effect Effects 0.000 description 1
- 238000009175 antibody therapy Methods 0.000 description 1
- 229940100197 antimetabolite Drugs 0.000 description 1
- 239000002256 antimetabolite Substances 0.000 description 1
- 108010062636 apomyoglobin Proteins 0.000 description 1
- 230000005735 apoptotic response Effects 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000003305 autocrine Effects 0.000 description 1
- 229960003005 axitinib Drugs 0.000 description 1
- RITAVMQDGBJQJZ-FMIVXFBMSA-N axitinib Chemical compound CNC(=O)C1=CC=CC=C1SC1=CC=C(C(\C=C\C=2N=CC=CC=2)=NN2)C2=C1 RITAVMQDGBJQJZ-FMIVXFBMSA-N 0.000 description 1
- 210000003719 b-lymphocyte Anatomy 0.000 description 1
- 230000005540 biological transmission Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 210000001185 bone marrow Anatomy 0.000 description 1
- HFCFMRYTXDINDK-WNQIDUERSA-N cabozantinib malate Chemical compound OC(=O)[C@@H](O)CC(O)=O.C=12C=C(OC)C(OC)=CC2=NC=CC=1OC(C=C1)=CC=C1NC(=O)C1(C(=O)NC=2C=CC(F)=CC=2)CC1 HFCFMRYTXDINDK-WNQIDUERSA-N 0.000 description 1
- 239000003560 cancer drug Substances 0.000 description 1
- 230000005907 cancer growth Effects 0.000 description 1
- 230000009400 cancer invasion Effects 0.000 description 1
- 229960004562 carboplatin Drugs 0.000 description 1
- 190000008236 carboplatin Chemical compound 0.000 description 1
- 150000001768 cations Chemical class 0.000 description 1
- 229960002412 cediranib Drugs 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000032823 cell division Effects 0.000 description 1
- 230000003915 cell function Effects 0.000 description 1
- 230000004709 cell invasion Effects 0.000 description 1
- 210000000170 cell membrane Anatomy 0.000 description 1
- 230000012292 cell migration Effects 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000030570 cellular localization Effects 0.000 description 1
- 230000036755 cellular response Effects 0.000 description 1
- 230000008859 change Effects 0.000 description 1
- 238000012512 characterization method Methods 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 239000007795 chemical reaction product Substances 0.000 description 1
- 239000003153 chemical reaction reagent Substances 0.000 description 1
- 230000002113 chemopreventative effect Effects 0.000 description 1
- 230000003399 chemotactic effect Effects 0.000 description 1
- 230000000973 chemotherapeutic effect Effects 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 230000008576 chronic process Effects 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 230000004186 co-expression Effects 0.000 description 1
- 206010009887 colitis Diseases 0.000 description 1
- 201000011024 colonic benign neoplasm Diseases 0.000 description 1
- 108091036078 conserved sequence Proteins 0.000 description 1
- 230000006552 constitutive activation Effects 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 229960005061 crizotinib Drugs 0.000 description 1
- 238000009109 curative therapy Methods 0.000 description 1
- 238000005520 cutting process Methods 0.000 description 1
- 229940127089 cytotoxic agent Drugs 0.000 description 1
- 238000007405 data analysis Methods 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 230000003247 decreasing effect Effects 0.000 description 1
- 230000002950 deficient Effects 0.000 description 1
- 230000003111 delayed effect Effects 0.000 description 1
- 210000004443 dendritic cell Anatomy 0.000 description 1
- 238000003795 desorption Methods 0.000 description 1
- 230000001627 detrimental effect Effects 0.000 description 1
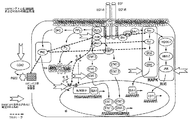
- 238000010586 diagram Methods 0.000 description 1
- 235000014113 dietary fatty acids Nutrition 0.000 description 1
- 238000002349 difference gel electrophoresis Methods 0.000 description 1
- 239000012470 diluted sample Substances 0.000 description 1
- 230000009266 disease activity Effects 0.000 description 1
- 239000003534 dna topoisomerase inhibitor Substances 0.000 description 1
- 229960003668 docetaxel Drugs 0.000 description 1
- 230000003828 downregulation Effects 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 238000001962 electrophoresis Methods 0.000 description 1
- 210000002889 endothelial cell Anatomy 0.000 description 1
- 239000003623 enhancer Substances 0.000 description 1
- 230000008971 epithelial apoptosis Effects 0.000 description 1
- 230000008556 epithelial cell proliferation Effects 0.000 description 1
- 229940082789 erbitux Drugs 0.000 description 1
- 201000004101 esophageal cancer Diseases 0.000 description 1
- 238000011156 evaluation Methods 0.000 description 1
- 230000017188 evasion or tolerance of host immune response Effects 0.000 description 1
- 238000002474 experimental method Methods 0.000 description 1
- 102100021145 fMet-Leu-Phe receptor Human genes 0.000 description 1
- 101710108492 fMet-Leu-Phe receptor Proteins 0.000 description 1
- 229930195729 fatty acid Natural products 0.000 description 1
- 239000000194 fatty acid Substances 0.000 description 1
- 150000004665 fatty acids Chemical class 0.000 description 1
- 210000002950 fibroblast Anatomy 0.000 description 1
- 229960002949 fluorouracil Drugs 0.000 description 1
- 206010017758 gastric cancer Diseases 0.000 description 1
- 230000002496 gastric effect Effects 0.000 description 1
- 230000000762 glandular Effects 0.000 description 1
- 229950007540 glesatinib Drugs 0.000 description 1
- 239000001963 growth medium Substances 0.000 description 1
- 210000003958 hematopoietic stem cell Anatomy 0.000 description 1
- 208000029824 high grade glioma Diseases 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 206010020718 hyperplasia Diseases 0.000 description 1
- KTUFNOKKBVMGRW-UHFFFAOYSA-N imatinib Chemical compound C1CN(C)CCN1CC1=CC=C(C(=O)NC=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)C=C1 KTUFNOKKBVMGRW-UHFFFAOYSA-N 0.000 description 1
- 229960002411 imatinib Drugs 0.000 description 1
- 230000036039 immunity Effects 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 208000014674 injury Diseases 0.000 description 1
- 230000015788 innate immune response Effects 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 230000002452 interceptive effect Effects 0.000 description 1
- 229940096397 interleukin-8 Drugs 0.000 description 1
- XKTZWUACRZHVAN-VADRZIEHSA-N interleukin-8 Chemical compound C([C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC=1C2=CC=CC=C2NC=1)NC(=O)[C@@H](NC(C)=O)CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC=1C=CC=CC=1)C(=O)N[C@@H]([C@@H](C)O)C(=O)NCC(=O)N[C@@H](CCSC)C(=O)N1[C@H](CCC1)C(=O)N1[C@H](CCC1)C(=O)N[C@@H](C)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CCC(O)=O)C(=O)N[C@H](CC(O)=O)C(=O)N[C@H](CC=1C=CC(O)=CC=1)C(=O)N[C@H](CO)C(=O)N1[C@H](CCC1)C(N)=O)C1=CC=CC=C1 XKTZWUACRZHVAN-VADRZIEHSA-N 0.000 description 1
- 229940047122 interleukins Drugs 0.000 description 1
- 201000002313 intestinal cancer Diseases 0.000 description 1
- 210000002490 intestinal epithelial cell Anatomy 0.000 description 1
- 210000004347 intestinal mucosa Anatomy 0.000 description 1
- 230000009545 invasion Effects 0.000 description 1
- 230000005865 ionizing radiation Effects 0.000 description 1
- 229940084651 iressa Drugs 0.000 description 1
- 229960004768 irinotecan Drugs 0.000 description 1
- UWKQSNNFCGGAFS-XIFFEERXSA-N irinotecan Chemical compound C1=C2C(CC)=C3CN(C(C4=C([C@@](C(=O)OC4)(O)CC)C=4)=O)C=4C3=NC2=CC=C1OC(=O)N(CC1)CCC1N1CCCCC1 UWKQSNNFCGGAFS-XIFFEERXSA-N 0.000 description 1
- 201000010982 kidney cancer Diseases 0.000 description 1
- 229940043355 kinase inhibitor Drugs 0.000 description 1
- 238000002372 labelling Methods 0.000 description 1
- 230000003902 lesion Effects 0.000 description 1
- MPVGZUGXCQEXTM-UHFFFAOYSA-N linifanib Chemical compound CC1=CC=C(F)C(NC(=O)NC=2C=CC(=CC=2)C=2C=3C(N)=NNC=3C=CC=2)=C1 MPVGZUGXCQEXTM-UHFFFAOYSA-N 0.000 description 1
- 150000002639 lipoxins Chemical class 0.000 description 1
- 238000004895 liquid chromatography mass spectrometry Methods 0.000 description 1
- 210000004185 liver Anatomy 0.000 description 1
- 229960000994 lumiracoxib Drugs 0.000 description 1
- KHPKQFYUPIUARC-UHFFFAOYSA-N lumiracoxib Chemical compound OC(=O)CC1=CC(C)=CC=C1NC1=C(F)C=CC=C1Cl KHPKQFYUPIUARC-UHFFFAOYSA-N 0.000 description 1
- 230000004758 lung carcinogenesis Effects 0.000 description 1
- 208000037841 lung tumor Diseases 0.000 description 1
- 210000004698 lymphocyte Anatomy 0.000 description 1
- 230000036210 malignancy Effects 0.000 description 1
- 201000011614 malignant glioma Diseases 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 210000004379 membrane Anatomy 0.000 description 1
- 239000012528 membrane Substances 0.000 description 1
- 102000006240 membrane receptors Human genes 0.000 description 1
- 108020004084 membrane receptors Proteins 0.000 description 1
- 230000002503 metabolic effect Effects 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 239000002207 metabolite Substances 0.000 description 1
- 230000001394 metastastic effect Effects 0.000 description 1
- 206010061289 metastatic neoplasm Diseases 0.000 description 1
- 239000000203 mixture Substances 0.000 description 1
- 230000004001 molecular interaction Effects 0.000 description 1
- 229950003968 motesanib Drugs 0.000 description 1
- RAHBGWKEPAQNFF-UHFFFAOYSA-N motesanib Chemical compound C=1C=C2C(C)(C)CNC2=CC=1NC(=O)C1=CC=CN=C1NCC1=CC=NC=C1 RAHBGWKEPAQNFF-UHFFFAOYSA-N 0.000 description 1
- 238000010172 mouse model Methods 0.000 description 1
- 210000004877 mucosa Anatomy 0.000 description 1
- DMFYYBFUYUTKKD-UHFFFAOYSA-N n-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine;4-[5-(4-methylphenyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide Chemical compound C1=CC(C)=CC=C1C1=CC(C(F)(F)F)=NN1C1=CC=C(S(N)(=O)=O)C=C1.C=12C=C(OCCOC)C(OCCOC)=CC2=NC=NC=1NC1=CC=CC(C#C)=C1 DMFYYBFUYUTKKD-UHFFFAOYSA-N 0.000 description 1
- YRCHYHRCBXNYNU-UHFFFAOYSA-N n-[[3-fluoro-4-[2-[5-[(2-methoxyethylamino)methyl]pyridin-2-yl]thieno[3,2-b]pyridin-7-yl]oxyphenyl]carbamothioyl]-2-(4-fluorophenyl)acetamide Chemical compound N1=CC(CNCCOC)=CC=C1C1=CC2=NC=CC(OC=3C(=CC(NC(=S)NC(=O)CC=4C=CC(F)=CC=4)=CC=3)F)=C2S1 YRCHYHRCBXNYNU-UHFFFAOYSA-N 0.000 description 1
- 230000017074 necrotic cell death Effects 0.000 description 1
- 230000006654 negative regulation of apoptotic process Effects 0.000 description 1
- 230000023578 negative regulation of cell adhesion Effects 0.000 description 1
- 230000030354 negative regulation of interleukin-12 production Effects 0.000 description 1
- 230000009826 neoplastic cell growth Effects 0.000 description 1
- 230000001613 neoplastic effect Effects 0.000 description 1
- HHZIURLSWUIHRB-UHFFFAOYSA-N nilotinib Chemical compound C1=NC(C)=CN1C1=CC(NC(=O)C=2C=C(NC=3N=C(C=CN=3)C=3C=NC=CC=3)C(C)=CC=2)=CC(C(F)(F)F)=C1 HHZIURLSWUIHRB-UHFFFAOYSA-N 0.000 description 1
- 229960001346 nilotinib Drugs 0.000 description 1
- 229960004378 nintedanib Drugs 0.000 description 1
- XZXHXSATPCNXJR-ZIADKAODSA-N nintedanib Chemical compound O=C1NC2=CC(C(=O)OC)=CC=C2\C1=C(C=1C=CC=CC=1)\NC(C=C1)=CC=C1N(C)C(=O)CN1CCN(C)CC1 XZXHXSATPCNXJR-ZIADKAODSA-N 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 238000011580 nude mouse model Methods 0.000 description 1
- 230000005959 oncogenic signaling Effects 0.000 description 1
- 238000011275 oncology therapy Methods 0.000 description 1
- 201000002740 oral squamous cell carcinoma Diseases 0.000 description 1
- 230000008520 organization Effects 0.000 description 1
- 229960001756 oxaliplatin Drugs 0.000 description 1
- DWAFYCQODLXJNR-BNTLRKBRSA-L oxaliplatin Chemical compound O1C(=O)C(=O)O[Pt]11N[C@@H]2CCCC[C@H]2N1 DWAFYCQODLXJNR-BNTLRKBRSA-L 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000008506 pathogenesis Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 230000009054 pathological process Effects 0.000 description 1
- 239000013610 patient sample Substances 0.000 description 1
- 238000003359 percent control normalization Methods 0.000 description 1
- 230000002085 persistent effect Effects 0.000 description 1
- 210000001539 phagocyte Anatomy 0.000 description 1
- 239000003757 phosphotransferase inhibitor Substances 0.000 description 1
- 230000033885 plasminogen activation Effects 0.000 description 1
- 230000030786 positive chemotaxis Effects 0.000 description 1
- 230000034803 positive regulation of interleukin-10 production Effects 0.000 description 1
- 230000029279 positive regulation of transcription, DNA-dependent Effects 0.000 description 1
- 230000001023 pro-angiogenic effect Effects 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 210000002307 prostate Anatomy 0.000 description 1
- 230000001681 protective effect Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 230000009396 radiation induced apoptosis Effects 0.000 description 1
- 238000011084 recovery Methods 0.000 description 1
- 206010038038 rectal cancer Diseases 0.000 description 1
- 201000001275 rectum cancer Diseases 0.000 description 1
- 238000007634 remodeling Methods 0.000 description 1
- 230000010076 replication Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000008261 resistance mechanism Effects 0.000 description 1
- 210000001533 respiratory mucosa Anatomy 0.000 description 1
- 206010039073 rheumatoid arthritis Diseases 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000011218 segmentation Effects 0.000 description 1
- 230000001568 sexual effect Effects 0.000 description 1
- PCMORTLOPMLEFB-ONEGZZNKSA-N sinapic acid Chemical compound COC1=CC(\C=C\C(O)=O)=CC(OC)=C1O PCMORTLOPMLEFB-ONEGZZNKSA-N 0.000 description 1
- PCMORTLOPMLEFB-UHFFFAOYSA-N sinapinic acid Natural products COC1=CC(C=CC(O)=O)=CC(OC)=C1O PCMORTLOPMLEFB-UHFFFAOYSA-N 0.000 description 1
- 230000009870 specific binding Effects 0.000 description 1
- 238000011255 standard chemotherapy Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 238000011272 standard treatment Methods 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000000365 steroidogenetic effect Effects 0.000 description 1
- 150000003431 steroids Chemical class 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 201000011549 stomach cancer Diseases 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 238000000756 surface-enhanced laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 1
- 230000004654 survival pathway Effects 0.000 description 1
- 210000002437 synoviocyte Anatomy 0.000 description 1
- 230000004797 therapeutic response Effects 0.000 description 1
- 108060008226 thioredoxin Proteins 0.000 description 1
- 229940094937 thioredoxin Drugs 0.000 description 1
- 229950005976 tivantinib Drugs 0.000 description 1
- 229940044693 topoisomerase inhibitor Drugs 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 230000026683 transduction Effects 0.000 description 1
- 238000010361 transduction Methods 0.000 description 1
- 230000032258 transport Effects 0.000 description 1
- 230000008733 trauma Effects 0.000 description 1
- 230000005747 tumor angiogenesis Effects 0.000 description 1
- 239000000107 tumor biomarker Substances 0.000 description 1
- 230000005748 tumor development Effects 0.000 description 1
- 230000005740 tumor formation Effects 0.000 description 1
- 239000000439 tumor marker Substances 0.000 description 1
- 231100000588 tumorigenic Toxicity 0.000 description 1
- 230000000381 tumorigenic effect Effects 0.000 description 1
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 1
- 150000004917 tyrosine kinase inhibitor derivatives Chemical class 0.000 description 1
- 125000001493 tyrosinyl group Chemical group [H]OC1=C([H])C([H])=C(C([H])=C1[H])C([H])([H])C([H])(N([H])[H])C(*)=O 0.000 description 1
- 229960002004 valdecoxib Drugs 0.000 description 1
- 238000010200 validation analysis Methods 0.000 description 1
- 230000002792 vascular Effects 0.000 description 1
- 230000008728 vascular permeability Effects 0.000 description 1
- 239000002525 vasculotropin inhibitor Substances 0.000 description 1
- ABDKAPXRBAPSQN-UHFFFAOYSA-N veratrole Chemical group COC1=CC=CC=C1OC ABDKAPXRBAPSQN-UHFFFAOYSA-N 0.000 description 1
- GBABOYUKABKIAF-GHYRFKGUSA-N vinorelbine Chemical compound C1N(CC=2C3=CC=CC=C3NC=22)CC(CC)=C[C@H]1C[C@]2(C(=O)OC)C1=CC([C@]23[C@H]([C@]([C@H](OC(C)=O)[C@]4(CC)C=CCN([C@H]34)CC2)(O)C(=O)OC)N2C)=C2C=C1OC GBABOYUKABKIAF-GHYRFKGUSA-N 0.000 description 1
- 229960002066 vinorelbine Drugs 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/68—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing involving proteins, peptides or amino acids
- G01N33/6803—General methods of protein analysis not limited to specific proteins or families of proteins
- G01N33/6848—Methods of protein analysis involving mass spectrometry
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N33/00—Investigating or analysing materials by specific methods not covered by groups G01N1/00 - G01N31/00
- G01N33/48—Biological material, e.g. blood, urine; Haemocytometers
- G01N33/50—Chemical analysis of biological material, e.g. blood, urine; Testing involving biospecific ligand binding methods; Immunological testing
- G01N33/53—Immunoassay; Biospecific binding assay; Materials therefor
- G01N33/574—Immunoassay; Biospecific binding assay; Materials therefor for cancer
- G01N33/57407—Specifically defined cancers
- G01N33/57415—Specifically defined cancers of breast
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16C—COMPUTATIONAL CHEMISTRY; CHEMOINFORMATICS; COMPUTATIONAL MATERIALS SCIENCE
- G16C20/00—Chemoinformatics, i.e. ICT specially adapted for the handling of physicochemical or structural data of chemical particles, elements, compounds or mixtures
- G16C20/70—Machine learning, data mining or chemometrics
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01J—ELECTRIC DISCHARGE TUBES OR DISCHARGE LAMPS
- H01J49/00—Particle spectrometers or separator tubes
- H01J49/26—Mass spectrometers or separator tubes
- H01J49/34—Dynamic spectrometers
- H01J49/40—Time-of-flight spectrometers
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N2800/00—Detection or diagnosis of diseases
- G01N2800/52—Predicting or monitoring the response to treatment, e.g. for selection of therapy based on assay results in personalised medicine; Prognosis
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16C—COMPUTATIONAL CHEMISTRY; CHEMOINFORMATICS; COMPUTATIONAL MATERIALS SCIENCE
- G16C20/00—Chemoinformatics, i.e. ICT specially adapted for the handling of physicochemical or structural data of chemical particles, elements, compounds or mixtures
- G16C20/20—Identification of molecular entities, parts thereof or of chemical compositions
-
- G—PHYSICS
- G16—INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR SPECIFIC APPLICATION FIELDS
- G16H—HEALTHCARE INFORMATICS, i.e. INFORMATION AND COMMUNICATION TECHNOLOGY [ICT] SPECIALLY ADAPTED FOR THE HANDLING OR PROCESSING OF MEDICAL OR HEALTHCARE DATA
- G16H10/00—ICT specially adapted for the handling or processing of patient-related medical or healthcare data
- G16H10/40—ICT specially adapted for the handling or processing of patient-related medical or healthcare data for data related to laboratory analysis, e.g. patient specimen analysis
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01J—ELECTRIC DISCHARGE TUBES OR DISCHARGE LAMPS
- H01J49/00—Particle spectrometers or separator tubes
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A90/00—Technologies having an indirect contribution to adaptation to climate change
- Y02A90/10—Information and communication technologies [ICT] supporting adaptation to climate change, e.g. for weather forecasting or climate simulation
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Chemical & Material Sciences (AREA)
- Molecular Biology (AREA)
- Immunology (AREA)
- Physics & Mathematics (AREA)
- Urology & Nephrology (AREA)
- Biomedical Technology (AREA)
- Hematology (AREA)
- General Health & Medical Sciences (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Bioinformatics & Computational Biology (AREA)
- Medicinal Chemistry (AREA)
- Analytical Chemistry (AREA)
- Microbiology (AREA)
- Food Science & Technology (AREA)
- Biochemistry (AREA)
- General Physics & Mathematics (AREA)
- Biotechnology (AREA)
- Cell Biology (AREA)
- Pathology (AREA)
- Spectroscopy & Molecular Physics (AREA)
- Biophysics (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Animal Behavior & Ethology (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Artificial Intelligence (AREA)
- Computer Vision & Pattern Recognition (AREA)
- Data Mining & Analysis (AREA)
- Databases & Information Systems (AREA)
- Evolutionary Computation (AREA)
- Medical Informatics (AREA)
- Software Systems (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US33893810P | 2010-02-24 | 2010-02-24 | |
| US61/338,938 | 2010-02-24 | ||
| PCT/US2011/000323 WO2011106084A1 (en) | 2010-02-24 | 2011-02-22 | Cancer patient selection for administration of therapeutic agents using mass spectral analysis |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015121648A Division JP2015222257A (ja) | 2010-02-24 | 2015-06-17 | 質量スペクトル分析を用いた、治療薬投与のための癌患者のセレクション |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2013520681A true JP2013520681A (ja) | 2013-06-06 |
| JP2013520681A5 JP2013520681A5 (enExample) | 2014-05-08 |
Family
ID=44477214
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2012555001A Withdrawn JP2013520681A (ja) | 2010-02-24 | 2011-02-22 | 質量スペクトル分析を用いた、治療薬投与のための癌患者のセレクション |
| JP2015121648A Withdrawn JP2015222257A (ja) | 2010-02-24 | 2015-06-17 | 質量スペクトル分析を用いた、治療薬投与のための癌患者のセレクション |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2015121648A Withdrawn JP2015222257A (ja) | 2010-02-24 | 2015-06-17 | 質量スペクトル分析を用いた、治療薬投与のための癌患者のセレクション |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US20110208433A1 (enExample) |
| EP (1) | EP2539704A4 (enExample) |
| JP (2) | JP2013520681A (enExample) |
| KR (1) | KR101556726B1 (enExample) |
| CN (1) | CN102770760A (enExample) |
| AU (2) | AU2011219069C1 (enExample) |
| CA (1) | CA2790928A1 (enExample) |
| TW (1) | TW201142292A (enExample) |
| WO (1) | WO2011106084A1 (enExample) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014505257A (ja) * | 2011-01-28 | 2014-02-27 | バイオデシックス・インコーポレイテッド | ホルモン療法および組み合わせ療法のための転移性乳癌患者の選択の予測試験 |
| JP2014514579A (ja) * | 2011-04-29 | 2014-06-19 | セルジーン コーポレイション | セレブロンを予測因子として使用する癌及び炎症性疾患の治療方法 |
Families Citing this family (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2013180818A2 (en) | 2012-05-29 | 2013-12-05 | Biodesix, Inc. | Deep-maldi tof mass spectrometry of complex biological samples, e.g., serum, and uses thereof |
| SG11201408652SA (en) | 2012-06-26 | 2015-01-29 | Biodesix Inc | Mass-spectral method for selection, and de-selection, of cancer patients for treatment with immune response generating therapies |
| WO2014007859A1 (en) * | 2012-07-05 | 2014-01-09 | Biodesix, Inc. | Method for predicting whether a cancer patient will not benefit from platinum-based chemotherapy agents |
| US9211314B2 (en) | 2014-04-04 | 2015-12-15 | Biodesix, Inc. | Treatment selection for lung cancer patients using mass spectrum of blood-based sample |
| WO2015157109A1 (en) * | 2014-04-08 | 2015-10-15 | Biodesix, Inc. | Egfr and hgf inhibitor therapy for lung cancer |
| WO2016054031A1 (en) * | 2014-10-02 | 2016-04-07 | Biodesix, Inc. | Predictive test for aggressiveness or indolence of prostate cancer from mass spectrometry of blood-based sample |
| US11594403B1 (en) | 2014-12-03 | 2023-02-28 | Biodesix Inc. | Predictive test for prognosis of myelodysplastic syndrome patients using mass spectrometry of blood-based sample |
| US10037874B2 (en) | 2014-12-03 | 2018-07-31 | Biodesix, Inc. | Early detection of hepatocellular carcinoma in high risk populations using MALDI-TOF mass spectrometry |
| US11152197B2 (en) * | 2015-06-24 | 2021-10-19 | City University Of Hong Kong | Method of determining cell cycle stage distribution of cells |
| CN112710723B (zh) * | 2015-07-13 | 2024-11-12 | 佰欧迪塞克斯公司 | 受益于pd-1抗体药物的肺癌患者的预测性测试和分类器开发方法 |
| US11710539B2 (en) | 2016-02-01 | 2023-07-25 | Biodesix, Inc. | Predictive test for melanoma patient benefit from interleukin-2 (IL2) therapy |
| CN106596824A (zh) * | 2016-12-30 | 2017-04-26 | 广州中大南沙科技创新产业园有限公司 | 一种lc‑ms/ms法检测血浆中沙利度胺的方法 |
| CN110383069A (zh) | 2017-01-05 | 2019-10-25 | 佰欧迪塞克斯公司 | 用于鉴定总体不良预后亚组中持久受益于免疫疗法的癌症患者的方法 |
| CN109212042B (zh) * | 2017-06-30 | 2022-03-04 | 齐鲁制药有限公司 | 一种采用液质联用法测定盐酸培唑帕尼基因毒性杂质的分析方法 |
| KR102633621B1 (ko) | 2017-09-01 | 2024-02-05 | 벤 바이오사이언시스 코포레이션 | 진단 및 치료 모니터링용 바이오마커로서의 당펩티드의 식별 및 용도 |
| EP3724885A2 (en) | 2017-12-15 | 2020-10-21 | Iovance Biotherapeutics, Inc. | Systems and methods for determining the beneficial administration of tumor infiltrating lymphocytes, and methods of use thereof and beneficial administration of tumor infiltrating lymphocytes, and methods of use thereof |
| WO2019190732A1 (en) * | 2018-03-29 | 2019-10-03 | Biodesix, Inc. | Apparatus and method for identification of primary immune resistance in cancer patients |
| WO2020019095A1 (es) * | 2018-07-26 | 2020-01-30 | Universidad Católica Del Maule | Proteína rage (receptor de productos finales de glicacíon avanzada) como biomarcador de sensibilidad tumoral y evaluacion de terapia radiologica y radiomimética |
| CN119139478A (zh) * | 2024-10-11 | 2024-12-17 | 首都医科大学附属北京胸科医院 | 一种治疗anxa6+egfr突变的肺腺癌的组合物 |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008057305A2 (en) * | 2006-10-27 | 2008-05-15 | George Mason Intellectual Properties, Inc. | Assay for metastatic colorectal cancer |
| JP2009532673A (ja) * | 2006-03-31 | 2009-09-10 | バイオデシックス・インコーポレイテッド | 疾患を有する患者に薬物が有効かどうかを決定するための方法およびシステム |
| JP2011513728A (ja) * | 2009-01-20 | 2011-04-28 | バイオデシックス・インコーポレイテッド | Egfr経路を標的化する薬物による治療のための頭頸部癌患者の選択 |
| JP2012507033A (ja) * | 2009-01-20 | 2012-03-22 | バイオデシックス・インコーポレイテッド | Egfr経路を標的にする薬物による治療のための結腸直腸癌患者の選択 |
Family Cites Families (41)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4802102A (en) * | 1987-07-15 | 1989-01-31 | Hewlett-Packard Company | Baseline correction for chromatography |
| US5291426A (en) * | 1991-02-27 | 1994-03-01 | The Perkin-Elmer Corporation | Method of correcting spectral data for background |
| ES2070739B1 (es) * | 1993-04-30 | 1997-06-01 | Alcatel Standard Electrica | Dispositivo de conversion de interfaces. |
| US20030108545A1 (en) * | 1994-02-10 | 2003-06-12 | Patricia Rockwell | Combination methods of inhibiting tumor growth with a vascular endothelial growth factor receptor antagonist |
| US6017693A (en) * | 1994-03-14 | 2000-01-25 | University Of Washington | Identification of nucleotides, amino acids, or carbohydrates by mass spectrometry |
| US5672869A (en) * | 1996-04-03 | 1997-09-30 | Eastman Kodak Company | Noise and background reduction method for component detection in chromatography/spectrometry |
| US6253162B1 (en) * | 1999-04-07 | 2001-06-26 | Battelle Memorial Institute | Method of identifying features in indexed data |
| IL153189A0 (en) * | 2000-06-19 | 2003-06-24 | Correlogic Systems Inc | Heuristic method of classification |
| CA2414610A1 (en) * | 2000-07-13 | 2002-01-24 | Medi-Physics, Inc. | Diagnostic procedures using 129xe spectroscopy characteristic chemical shift to detect pathology in vivo |
| SG144731A1 (en) * | 2000-07-18 | 2008-08-28 | Correlogic Systems Inc | A process for discriminating between biological states based on hidden patterns from biological data |
| AU2002241535B2 (en) * | 2000-11-16 | 2006-05-18 | Ciphergen Biosystems, Inc. | Method for analyzing mass spectra |
| US20020119490A1 (en) * | 2000-12-26 | 2002-08-29 | Aebersold Ruedi H. | Methods for rapid and quantitative proteome analysis |
| US20020115056A1 (en) * | 2000-12-26 | 2002-08-22 | Goodlett David R. | Rapid and quantitative proteome analysis and related methods |
| US6829539B2 (en) * | 2001-04-13 | 2004-12-07 | The Institute For Systems Biology | Methods for quantification and de novo polypeptide sequencing by mass spectrometry |
| US6849121B1 (en) * | 2001-04-24 | 2005-02-01 | The United States Of America As Represented By The Secretary Of The Air Force | Growth of uniform crystals |
| US7314717B2 (en) * | 2001-04-30 | 2008-01-01 | Nanogen Inc. | Biopolymer marker indicative of disease state having a molecular weight of 1562 daltons |
| US7113896B2 (en) * | 2001-05-11 | 2006-09-26 | Zhen Zhang | System and methods for processing biological expression data |
| US6675106B1 (en) * | 2001-06-01 | 2004-01-06 | Sandia Corporation | Method of multivariate spectral analysis |
| US7112408B2 (en) * | 2001-06-08 | 2006-09-26 | The Brigham And Women's Hospital, Inc. | Detection of ovarian cancer based upon alpha-haptoglobin levels |
| WO2003006951A2 (en) * | 2001-07-13 | 2003-01-23 | Syngenta Participations Ag | System and method of determining proteomic differences |
| US7016884B2 (en) * | 2002-06-27 | 2006-03-21 | Microsoft Corporation | Probability estimate for K-nearest neighbor |
| WO2004019003A2 (en) * | 2002-08-23 | 2004-03-04 | Efeckta Technologies Corporation | Image processing of mass spectrometry data for using at multiple resolutions |
| US20040147428A1 (en) * | 2002-11-15 | 2004-07-29 | Pluenneke John D. | Methods of treatment using an inhibitor of epidermal growth factor receptor |
| US6906320B2 (en) * | 2003-04-02 | 2005-06-14 | Merck & Co., Inc. | Mass spectrometry data analysis techniques |
| JP2007506442A (ja) * | 2003-05-30 | 2007-03-22 | ゲノミック ヘルス, インコーポレイテッド | Egfr阻害薬への応答に関する遺伝子発現マーカー |
| US20050267689A1 (en) * | 2003-07-07 | 2005-12-01 | Maxim Tsypin | Method to automatically identify peak and monoisotopic peaks in mass spectral data for biomolecular applications |
| US20050048547A1 (en) * | 2003-07-17 | 2005-03-03 | Hongyu Zhao | Classification of disease states using mass spectrometry data |
| SG145705A1 (en) * | 2003-08-01 | 2008-09-29 | Correlogic Systems Inc | Multiple high-resolution serum proteomic features for ovarian cancer detection |
| EP1709442A4 (en) * | 2003-12-11 | 2010-01-20 | Correlogic Systems Inc | METHOD FOR DIAGNOSING BIOLOGICAL CONDITIONS BY USING A CENTRALIZED ADAPTIVE MODEL AND SAMPLE PREPARATION |
| ES2244326B1 (es) * | 2004-04-05 | 2007-02-16 | Laboratorios Del Dr. Esteve, S.A. | Combinacion de substancias activas. |
| JP2008501654A (ja) * | 2004-06-03 | 2008-01-24 | エフ.ホフマン−ラ ロシュ アーゲー | シスプラチンとegfr阻害剤を用いた治療 |
| US20060029574A1 (en) * | 2004-08-06 | 2006-02-09 | Board Of Regents, The University Of Texas System | Biomarkers for diagnosis, prognosis, monitoring, and treatment decisions for drug resistance and sensitivity |
| SI1948180T1 (sl) * | 2005-11-11 | 2013-06-28 | Boehringer Ingelheim International Gmbh | Kombinacijsko zdravljenje raka, ki obsega EGFR/HER2 inhibitorje |
| US7858389B2 (en) * | 2006-03-31 | 2010-12-28 | Biodesix, Inc. | Selection of non-small-cell lung cancer patients for treatment with monoclonal antibody drugs targeting EGFR pathway |
| US7906342B2 (en) * | 2006-03-31 | 2011-03-15 | Biodesix, Inc. | Monitoring treatment of cancer patients with drugs targeting EGFR pathway using mass spectrometry of patient samples |
| CN101201355A (zh) * | 2006-12-15 | 2008-06-18 | 许洋 | 免疫组质谱检测个体化肿瘤生物标志及疗效试剂盒和方法 |
| ES2412432T3 (es) * | 2007-02-27 | 2013-07-11 | Nuclea Biomarkers Llc | Método para predecir la respuesta de pacientes de nsclc al tratamiento mediante un inhibidor de egfr-tk |
| CN101329346A (zh) * | 2007-06-18 | 2008-12-24 | 许洋 | 检测乳腺癌特征蛋白的优化质谱模型及其制备方法和应用 |
| WO2009036123A1 (en) * | 2007-09-11 | 2009-03-19 | Cancer Prevention And Cure, Ltd. | Method of identifying biomarkers in human serum indicative of pathologies of human lung tissues |
| CN101836991B (zh) * | 2009-03-19 | 2013-05-22 | 鼎泓国际投资(香港)有限公司 | 含有索拉非尼、cMet抑制剂与EGFR酪氨酸激酶抑制剂的药物组合物及其应用 |
| WO2014007859A1 (en) * | 2012-07-05 | 2014-01-09 | Biodesix, Inc. | Method for predicting whether a cancer patient will not benefit from platinum-based chemotherapy agents |
-
2011
- 2011-02-22 AU AU2011219069A patent/AU2011219069C1/en not_active Ceased
- 2011-02-22 US US12/932,295 patent/US20110208433A1/en not_active Abandoned
- 2011-02-22 KR KR1020127024976A patent/KR101556726B1/ko not_active Expired - Fee Related
- 2011-02-22 CN CN2011800110326A patent/CN102770760A/zh active Pending
- 2011-02-22 WO PCT/US2011/000323 patent/WO2011106084A1/en not_active Ceased
- 2011-02-22 JP JP2012555001A patent/JP2013520681A/ja not_active Withdrawn
- 2011-02-22 EP EP11747809.9A patent/EP2539704A4/en not_active Withdrawn
- 2011-02-22 CA CA2790928A patent/CA2790928A1/en not_active Abandoned
- 2011-02-24 TW TW100106286A patent/TW201142292A/zh unknown
-
2014
- 2014-05-19 AU AU2014202716A patent/AU2014202716B2/en not_active Expired - Fee Related
- 2014-06-04 US US14/295,783 patent/US20140284468A1/en not_active Abandoned
-
2015
- 2015-06-17 JP JP2015121648A patent/JP2015222257A/ja not_active Withdrawn
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2009532673A (ja) * | 2006-03-31 | 2009-09-10 | バイオデシックス・インコーポレイテッド | 疾患を有する患者に薬物が有効かどうかを決定するための方法およびシステム |
| WO2008057305A2 (en) * | 2006-10-27 | 2008-05-15 | George Mason Intellectual Properties, Inc. | Assay for metastatic colorectal cancer |
| JP2011513728A (ja) * | 2009-01-20 | 2011-04-28 | バイオデシックス・インコーポレイテッド | Egfr経路を標的化する薬物による治療のための頭頸部癌患者の選択 |
| JP2012507033A (ja) * | 2009-01-20 | 2012-03-22 | バイオデシックス・インコーポレイテッド | Egfr経路を標的にする薬物による治療のための結腸直腸癌患者の選択 |
Non-Patent Citations (1)
| Title |
|---|
| JPN5013003688; TAGUCHI F et al.: 'Mass Spectrometry to Classify Non-Small-Cell Lung Cancer Patients for Clinical Outcome After Treatme' J. Natl. Cancer Inst. Vol.99, Issue 11, 20070606, pp.838-846 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014505257A (ja) * | 2011-01-28 | 2014-02-27 | バイオデシックス・インコーポレイテッド | ホルモン療法および組み合わせ療法のための転移性乳癌患者の選択の予測試験 |
| JP2014514579A (ja) * | 2011-04-29 | 2014-06-19 | セルジーン コーポレイション | セレブロンを予測因子として使用する癌及び炎症性疾患の治療方法 |
| JP2017037081A (ja) * | 2011-04-29 | 2017-02-16 | セルジーン コーポレイション | セレブロンを予測因子として使用する癌及び炎症性疾患の治療方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2011219069A1 (en) | 2012-09-13 |
| US20140284468A1 (en) | 2014-09-25 |
| KR101556726B1 (ko) | 2015-10-02 |
| EP2539704A4 (en) | 2015-12-02 |
| EP2539704A1 (en) | 2013-01-02 |
| US20110208433A1 (en) | 2011-08-25 |
| CA2790928A1 (en) | 2011-09-01 |
| JP2015222257A (ja) | 2015-12-10 |
| AU2014202716B2 (en) | 2016-02-25 |
| AU2011219069C1 (en) | 2014-07-17 |
| WO2011106084A1 (en) | 2011-09-01 |
| AU2014202716A1 (en) | 2014-06-12 |
| CN102770760A (zh) | 2012-11-07 |
| TW201142292A (en) | 2011-12-01 |
| KR20130004309A (ko) | 2013-01-09 |
| AU2011219069B2 (en) | 2014-02-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2015222257A (ja) | 質量スペクトル分析を用いた、治療薬投与のための癌患者のセレクション | |
| JP4997345B2 (ja) | Egfr経路を標的にする薬物による治療のための結腸直腸癌患者の選択 | |
| JP5025802B2 (ja) | Egfr経路を標的化する薬物による治療のための頭頸部癌患者の選択 | |
| Park et al. | Role of mass spectrometry-based serum proteomics signatures in predicting clinical outcomes and toxicity in patients with cancer treated with immunotherapy | |
| US8718996B2 (en) | Method for predicting whether a cancer patient will not benefit from platinum-based chemotherapy agents | |
| US20090170215A1 (en) | Selection of non-small-cell lung cancer patients for treatment with monoclonal antibody drugs targeting EGFR pathway | |
| US20120225954A1 (en) | Methods and compositions for the classification of non-small cell lung carcinoma | |
| US20150285817A1 (en) | Method for treating and identifying lung cancer patients likely to benefit from EGFR inhibitor and a monoclonal antibody HGF inhibitor combination therapy | |
| TW201237409A (en) | Predictive test for selection of metastatic breast cancer patients for hormonal and combination therapy | |
| KR102067327B1 (ko) | 최적의 암 치료를 위한 Her2 단백질 정량 | |
| Cremona et al. | Elevated levels of the acute‐phase serum amyloid are associated with heightened lung cancer risk | |
| EP4500177A1 (en) | Thymidine kinase as a marker for immune checkpoint inhibitor efficacy | |
| An et al. | Serum peptide expression and treatment responses in patients with advanced non‑small‑cell lung cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20130513 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20130513 |
|
| A977 | Report on retrieval |
Free format text: JAPANESE INTERMEDIATE CODE: A971007 Effective date: 20140324 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140324 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20140422 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140718 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140728 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20140821 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20140828 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20140919 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20150217 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20150617 |
|
| A911 | Transfer to examiner for re-examination before appeal (zenchi) |
Free format text: JAPANESE INTERMEDIATE CODE: A911 Effective date: 20150731 |
|
| A912 | Re-examination (zenchi) completed and case transferred to appeal board |
Free format text: JAPANESE INTERMEDIATE CODE: A912 Effective date: 20150828 |
|
| RD04 | Notification of resignation of power of attorney |
Free format text: JAPANESE INTERMEDIATE CODE: A7424 Effective date: 20160218 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20160404 |