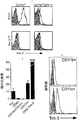
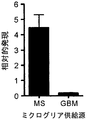
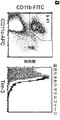
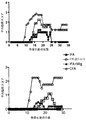
JP2010510223A - Tim−3調節物質の治療的使用 - Google Patents
Tim−3調節物質の治療的使用 Download PDFInfo
- Publication number
- JP2010510223A JP2010510223A JP2009537223A JP2009537223A JP2010510223A JP 2010510223 A JP2010510223 A JP 2010510223A JP 2009537223 A JP2009537223 A JP 2009537223A JP 2009537223 A JP2009537223 A JP 2009537223A JP 2010510223 A JP2010510223 A JP 2010510223A
- Authority
- JP
- Japan
- Prior art keywords
- tim
- agent
- polypeptide
- galectin
- activity
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Withdrawn
Links
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/10—Drugs for disorders of the urinary system of the bladder
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/10—Drugs for genital or sexual disorders; Contraceptives for impotence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/16—Otologicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/02—Nutrients, e.g. vitamins, minerals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2227/00—Animals characterised by species
- A01K2227/10—Mammal
- A01K2227/105—Murine
-
- A—HUMAN NECESSITIES
- A01—AGRICULTURE; FORESTRY; ANIMAL HUSBANDRY; HUNTING; TRAPPING; FISHING
- A01K—ANIMAL HUSBANDRY; AVICULTURE; APICULTURE; PISCICULTURE; FISHING; REARING OR BREEDING ANIMALS, NOT OTHERWISE PROVIDED FOR; NEW BREEDS OF ANIMALS
- A01K2267/00—Animals characterised by purpose
- A01K2267/03—Animal model, e.g. for test or diseases
- A01K2267/035—Animal model for multifactorial diseases
- A01K2267/0387—Animal model for diseases of the immune system
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2317/00—Immunoglobulins specific features
- C07K2317/70—Immunoglobulins specific features characterized by effect upon binding to a cell or to an antigen
- C07K2317/75—Agonist effect on antigen
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Engineering & Computer Science (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pharmacology & Pharmacy (AREA)
- Immunology (AREA)
- Animal Behavior & Ethology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- General Chemical & Material Sciences (AREA)
- Veterinary Medicine (AREA)
- Neurology (AREA)
- Molecular Biology (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Biochemistry (AREA)
- Biophysics (AREA)
- Genetics & Genomics (AREA)
- Biomedical Technology (AREA)
- Neurosurgery (AREA)
- Gastroenterology & Hepatology (AREA)
- Zoology (AREA)
- Toxicology (AREA)
- Cell Biology (AREA)
- Diabetes (AREA)
- Obesity (AREA)
- Orthopedic Medicine & Surgery (AREA)
- Physical Education & Sports Medicine (AREA)
- Urology & Nephrology (AREA)
- Gynecology & Obstetrics (AREA)
- Endocrinology (AREA)
- Reproductive Health (AREA)
- Hematology (AREA)
- Nutrition Science (AREA)
- Psychiatry (AREA)
- Hospice & Palliative Care (AREA)
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US85939106P | 2006-11-15 | 2006-11-15 | |
| US92394507P | 2007-04-17 | 2007-04-17 | |
| PCT/US2007/024067 WO2008060617A2 (en) | 2006-11-15 | 2007-11-15 | Therapeutic uses of tim-3 modulators |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2010510223A true JP2010510223A (ja) | 2010-04-02 |
| JP2010510223A5 JP2010510223A5 (cg-RX-API-DMAC7.html) | 2012-01-26 |
Family
ID=39402274
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2009537223A Withdrawn JP2010510223A (ja) | 2006-11-15 | 2007-11-15 | Tim−3調節物質の治療的使用 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US20100061992A1 (cg-RX-API-DMAC7.html) |
| EP (1) | EP2081961A2 (cg-RX-API-DMAC7.html) |
| JP (1) | JP2010510223A (cg-RX-API-DMAC7.html) |
| AU (1) | AU2007319806A1 (cg-RX-API-DMAC7.html) |
| CA (1) | CA2668693A1 (cg-RX-API-DMAC7.html) |
| WO (1) | WO2008060617A2 (cg-RX-API-DMAC7.html) |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2011155607A1 (ja) * | 2010-06-11 | 2011-12-15 | 協和発酵キリン株式会社 | 抗tim-3抗体 |
| JP2015535259A (ja) * | 2012-10-29 | 2015-12-10 | ザ ボード オブ トラスティーズ オブ ザ ユニバーシティ オブ アーカンソー | 新規粘膜アジュバントおよびデリバリーシステム |
| JP2019507194A (ja) * | 2016-03-04 | 2019-03-14 | ガレクティン・サイエンシーズ・リミテッド・ライアビリティ・カンパニーGalectin Sciences, LLC | ガレクチンに関連する疾患を予防および処置するためのセレノガラクトシド化合物およびその使用 |
| JP2019518787A (ja) * | 2016-05-13 | 2019-07-04 | ユニベルシテ ド ロレーヌUniversite De Lorraine | ガレクチンのcrdのレクチン活性に基づく組換えタンパク質のアフィニティー精製のための方法 |
| WO2021172889A1 (ko) * | 2020-02-25 | 2021-09-02 | 국립암센터 | Cd11b+ 세포에서 tim-3의 발현 수준을 측정하는 암 진단 또는 예후 예측을 위한 정보제공 방법 |
Families Citing this family (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010084999A1 (en) * | 2009-01-26 | 2010-07-29 | Protegene, Inc. | Immunosuppressive agents and prophylactic and therapeutic agents for autoimmune diseases |
| US8883174B2 (en) * | 2009-03-25 | 2014-11-11 | The Board Of Regents, The University Of Texas System | Compositions for stimulation of mammalian innate immune resistance to pathogens |
| US8841418B2 (en) | 2011-07-01 | 2014-09-23 | Cellerant Therapeutics, Inc. | Antibodies that specifically bind to TIM3 |
| US10513540B2 (en) | 2012-07-31 | 2019-12-24 | The Brigham And Women's Hospital, Inc. | Modulation of the immune response |
| US20140120157A1 (en) * | 2012-09-19 | 2014-05-01 | Georgetown University | Targeted liposomes |
| EP3679950A1 (en) | 2012-10-12 | 2020-07-15 | The Brigham and Women's Hospital, Inc. | Enhancement of the immune response |
| EP3049442A4 (en) | 2013-09-26 | 2017-06-28 | Costim Pharmaceuticals Inc. | Methods for treating hematologic cancers |
| JOP20200094A1 (ar) | 2014-01-24 | 2017-06-16 | Dana Farber Cancer Inst Inc | جزيئات جسم مضاد لـ pd-1 واستخداماتها |
| JOP20200096A1 (ar) | 2014-01-31 | 2017-06-16 | Children’S Medical Center Corp | جزيئات جسم مضاد لـ tim-3 واستخداماتها |
| BR112016020919A2 (pt) | 2014-03-12 | 2018-01-23 | Yeda Res & Dev | redução dos níveis ou da atividade sistêmica de células t regulatórias para o tratamento de doença e lesão do snc |
| US10618963B2 (en) | 2014-03-12 | 2020-04-14 | Yeda Research And Development Co. Ltd | Reducing systemic regulatory T cell levels or activity for treatment of disease and injury of the CNS |
| US10519237B2 (en) | 2014-03-12 | 2019-12-31 | Yeda Research And Development Co. Ltd | Reducing systemic regulatory T cell levels or activity for treatment of disease and injury of the CNS |
| UY36032A (es) | 2014-03-14 | 2015-10-30 | Novartis Ag | Moléculas de anticuerpo que se unen a lag-3 y usos de las mismas |
| RS61678B1 (sr) | 2014-05-28 | 2021-05-31 | Agenus Inc | Anti-gitr antitela i postupci za njihovu primenu |
| CN107206071A (zh) | 2014-09-13 | 2017-09-26 | 诺华股份有限公司 | Alk抑制剂的联合疗法 |
| US20180298095A1 (en) * | 2015-04-24 | 2018-10-18 | The Brigham And Women's Hospital, Inc. | Interactions between ceacam and tim family members |
| WO2016179194A1 (en) * | 2015-05-04 | 2016-11-10 | Jounce Therapeutics, Inc. | Lilra3 and method of using the same |
| US10682390B2 (en) | 2015-07-16 | 2020-06-16 | Biokine Therapeutics Ltd. | Compositions and methods for treating cancer |
| IL299072A (en) | 2015-12-02 | 2023-02-01 | Memorial Sloan Kettering Cancer Center | Antibodies and methods of use thereof |
| NZ749355A (en) | 2016-05-27 | 2023-04-28 | Agenus Inc | Anti-tim-3 antibodies and methods of use thereof |
| EP4512829A3 (en) | 2016-07-14 | 2025-06-11 | Bristol-Myers Squibb Company | Antibodies against tim3 and uses thereof |
| BR112019023722A2 (pt) | 2017-05-12 | 2020-05-26 | Galectin Sciences, Llc | Compostos para a prevenção e tratamento de doenças e o uso dos mesmos |
| CN111201030B (zh) * | 2017-07-25 | 2024-11-01 | 真和制药有限公司 | 通过阻断tim-3和其配体的相互作用治疗癌症 |
| CA3127113A1 (en) | 2019-01-30 | 2020-08-06 | Dongxu Sun | Anti-gal3 antibodies and uses thereof |
| EP3725370A1 (en) | 2019-04-19 | 2020-10-21 | ImmunoBrain Checkpoint, Inc. | Modified anti-pd-l1 antibodies and methods and uses for treating a neurodegenerative disease |
| JP2023528797A (ja) | 2020-05-26 | 2023-07-06 | トゥルーバインディング,インコーポレイテッド | ガレクチン-3を遮断することにより炎症性疾患を処置する方法 |
Family Cites Families (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2003063792A2 (en) * | 2002-01-30 | 2003-08-07 | The Brigham And Women's Hospital, Inc. | Compositions and methods related to tim-3, a th1-specific cell surface molecule |
| EP1685159B1 (en) * | 2003-10-03 | 2012-08-01 | Brigham & Women's Hospital | Tim-3 polypeptides |
-
2007
- 2007-11-15 CA CA002668693A patent/CA2668693A1/en not_active Abandoned
- 2007-11-15 EP EP07867488A patent/EP2081961A2/en not_active Withdrawn
- 2007-11-15 WO PCT/US2007/024067 patent/WO2008060617A2/en not_active Ceased
- 2007-11-15 AU AU2007319806A patent/AU2007319806A1/en not_active Abandoned
- 2007-11-15 JP JP2009537223A patent/JP2010510223A/ja not_active Withdrawn
- 2007-11-15 US US12/514,602 patent/US20100061992A1/en not_active Abandoned
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9556270B2 (en) | 2010-06-11 | 2017-01-31 | Kyowa Hakko Kirin Co., Ltd | Anti-TIM-3 antibody |
| US10550181B2 (en) | 2010-06-11 | 2020-02-04 | Kyowa Kirin Co., Ltd | Anti-TIM-3 antibody |
| US8552156B2 (en) | 2010-06-11 | 2013-10-08 | Kyowa Hakko Kirin Co., Ltd | Anti-TIM-3 antibody |
| AU2011262758B2 (en) * | 2010-06-11 | 2014-04-24 | Kyowa Kirin Co., Ltd. | Anti-tim-3 antibody |
| AU2011262758A8 (en) * | 2010-06-11 | 2014-09-04 | Kyowa Kirin Co., Ltd. | Anti-tim-3 antibody |
| JP2017189168A (ja) * | 2010-06-11 | 2017-10-19 | 協和発酵キリン株式会社 | 抗tim−3抗体 |
| WO2011155607A1 (ja) * | 2010-06-11 | 2011-12-15 | 協和発酵キリン株式会社 | 抗tim-3抗体 |
| JPWO2011155607A1 (ja) * | 2010-06-11 | 2013-08-15 | 協和発酵キリン株式会社 | 抗tim−3抗体 |
| JP2015535259A (ja) * | 2012-10-29 | 2015-12-10 | ザ ボード オブ トラスティーズ オブ ザ ユニバーシティ オブ アーカンソー | 新規粘膜アジュバントおよびデリバリーシステム |
| JP7086008B2 (ja) | 2016-03-04 | 2022-06-17 | ガレクティン・サイエンシーズ・リミテッド・ライアビリティ・カンパニー | ガレクチンに関連する疾患を予防および処置するためのセレノガラクトシド化合物およびその使用 |
| JP2019507194A (ja) * | 2016-03-04 | 2019-03-14 | ガレクティン・サイエンシーズ・リミテッド・ライアビリティ・カンパニーGalectin Sciences, LLC | ガレクチンに関連する疾患を予防および処置するためのセレノガラクトシド化合物およびその使用 |
| JP2019518787A (ja) * | 2016-05-13 | 2019-07-04 | ユニベルシテ ド ロレーヌUniversite De Lorraine | ガレクチンのcrdのレクチン活性に基づく組換えタンパク質のアフィニティー精製のための方法 |
| JP7105761B2 (ja) | 2016-05-13 | 2022-07-25 | ユニベルシテ ド ロレーヌ | ガレクチンのcrdのレクチン活性に基づく組換えタンパク質のアフィニティー精製のための方法 |
| WO2021172889A1 (ko) * | 2020-02-25 | 2021-09-02 | 국립암센터 | Cd11b+ 세포에서 tim-3의 발현 수준을 측정하는 암 진단 또는 예후 예측을 위한 정보제공 방법 |
Also Published As
| Publication number | Publication date |
|---|---|
| AU2007319806A1 (en) | 2008-05-22 |
| US20100061992A1 (en) | 2010-03-11 |
| CA2668693A1 (en) | 2008-05-22 |
| WO2008060617A2 (en) | 2008-05-22 |
| WO2008060617A3 (en) | 2008-12-24 |
| EP2081961A2 (en) | 2009-07-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP2010510223A (ja) | Tim−3調節物質の治療的使用 | |
| AU2022200016B2 (en) | Cancer vaccines and methods of treatment using the same | |
| JP4903128B2 (ja) | Tim−1、tim−2、およびtim−4の機能を調節することによって免疫応答を調節する方法 | |
| AU2013312211B2 (en) | VISTA modulators for diagnosis and treatment of cancer | |
| US11207393B2 (en) | Regulatory T cell PD-1 modulation for regulating T cell effector immune responses | |
| US10703817B2 (en) | System for delivery into XCR1 positive cell and uses thereof | |
| JP2022033899A (ja) | 免疫調節剤を併用するがんの治療 | |
| KR20190102267A (ko) | Psgl-1 길항제 및 그의 용도 | |
| ES2939112T3 (es) | Composiciones y métodos de uso de interleucina-10 en combinación con inhibidores de vías de puntos de control inmunitario | |
| JP2017533207A (ja) | Slamf1アンタゴニスト及びその使用 | |
| EP1333850B1 (en) | Use of il-13 inhibitors for the treatment of tumors | |
| WO2016141312A1 (en) | Beta-catenin inhibitors in cancer immunotherapy | |
| JP2022525223A (ja) | sEphB4-HSA融合タンパク質を用いたがんの治療 | |
| EP1615670A2 (en) | Targets for tumor growth inhibition | |
| US20210379147A1 (en) | Method and system for treating cancer utilizing tinagl1 | |
| JP4989850B2 (ja) | TGF−βの遮断により腫瘍再発を防ぐ方法 | |
| JP2022546023A (ja) | Tnfrsf25抗体を用いた癌の治療方法 | |
| US20250367289A1 (en) | Dosage regimen for a combination therapy consisting oftcr-engineered t-cells in combination with a pd-1 axis binding antagonist | |
| US20230340127A1 (en) | Methods and compositions for cancer treatment by inhibition of fbxo44 | |
| CN101027392B (zh) | 通过调节tim-1、tim-2和tim-4功能从而调节免疫应答的方法 | |
| US20100297146A1 (en) | Immune system programming through b7-dc | |
| Winograd | Induction of T Cell Immunity Overcomes Resistance to Pd-1 and Ctla-4 Blockade and Improves Survival in Pancreatic Cancer | |
| Adams | of the International Society for Biological Therapy of Cancer (now the Society for Immunotherapy of Cancer) |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20101112 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20101112 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20111114 |
|
| A761 | Written withdrawal of application |
Free format text: JAPANESE INTERMEDIATE CODE: A761 Effective date: 20120516 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20120516 |