JP2009525990A - 医薬組成物 - Google Patents
医薬組成物 Download PDFInfo
- Publication number
- JP2009525990A JP2009525990A JP2008553588A JP2008553588A JP2009525990A JP 2009525990 A JP2009525990 A JP 2009525990A JP 2008553588 A JP2008553588 A JP 2008553588A JP 2008553588 A JP2008553588 A JP 2008553588A JP 2009525990 A JP2009525990 A JP 2009525990A
- Authority
- JP
- Japan
- Prior art keywords
- berberine
- blood lipid
- lipid lowering
- functions
- lowering agent
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 239000008194 pharmaceutical composition Substances 0.000 title description 3
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 claims abstract description 157
- 210000004369 blood Anatomy 0.000 claims abstract description 106
- 239000008280 blood Substances 0.000 claims abstract description 106
- YBHILYKTIRIUTE-UHFFFAOYSA-N berberine Chemical compound C1=C2CC[N+]3=CC4=C(OC)C(OC)=CC=C4C=C3C2=CC2=C1OCO2 YBHILYKTIRIUTE-UHFFFAOYSA-N 0.000 claims abstract description 68
- QISXPYZVZJBNDM-UHFFFAOYSA-N berberine Natural products COc1ccc2C=C3N(Cc2c1OC)C=Cc4cc5OCOc5cc34 QISXPYZVZJBNDM-UHFFFAOYSA-N 0.000 claims abstract description 68
- 229940093265 berberine Drugs 0.000 claims abstract description 68
- 235000012000 cholesterol Nutrition 0.000 claims abstract description 58
- 230000007246 mechanism Effects 0.000 claims abstract description 52
- 230000006870 function Effects 0.000 claims abstract description 49
- 150000002632 lipids Chemical class 0.000 claims abstract description 48
- 239000003524 antilipemic agent Substances 0.000 claims abstract description 47
- 108010023302 HDL Cholesterol Proteins 0.000 claims abstract description 37
- 239000000203 mixture Chemical class 0.000 claims abstract description 37
- 230000002829 reductive effect Effects 0.000 claims abstract description 24
- 150000002148 esters Chemical class 0.000 claims abstract description 14
- 108010028554 LDL Cholesterol Proteins 0.000 claims abstract description 12
- 201000010099 disease Diseases 0.000 claims abstract description 12
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims abstract description 12
- 150000003839 salts Chemical class 0.000 claims abstract description 12
- 208000024172 Cardiovascular disease Diseases 0.000 claims abstract description 9
- 201000001320 Atherosclerosis Diseases 0.000 claims abstract description 8
- 208000006011 Stroke Diseases 0.000 claims abstract description 8
- 208000029078 coronary artery disease Diseases 0.000 claims abstract description 8
- 206010003119 arrhythmia Diseases 0.000 claims abstract description 7
- 230000006793 arrhythmia Effects 0.000 claims abstract description 7
- 208000031226 Hyperlipidaemia Diseases 0.000 claims abstract description 5
- 208000008589 Obesity Diseases 0.000 claims abstract description 5
- 206010012601 diabetes mellitus Diseases 0.000 claims abstract description 5
- 235000020824 obesity Nutrition 0.000 claims abstract description 5
- 206010002383 Angina Pectoris Diseases 0.000 claims abstract description 4
- 206010020772 Hypertension Diseases 0.000 claims abstract description 4
- 206010061218 Inflammation Diseases 0.000 claims abstract description 4
- 206010022489 Insulin Resistance Diseases 0.000 claims abstract description 4
- 206010033307 Overweight Diseases 0.000 claims abstract description 4
- 208000026106 cerebrovascular disease Diseases 0.000 claims abstract description 4
- 230000004054 inflammatory process Effects 0.000 claims abstract description 4
- 208000001072 type 2 diabetes mellitus Diseases 0.000 claims abstract description 4
- 210000003169 central nervous system Anatomy 0.000 claims abstract description 3
- 210000001428 peripheral nervous system Anatomy 0.000 claims abstract description 3
- 201000001421 hyperglycemia Diseases 0.000 claims abstract 3
- 241000196324 Embryophyta Species 0.000 claims description 58
- 235000002378 plant sterols Nutrition 0.000 claims description 26
- 241000124008 Mammalia Species 0.000 claims description 19
- 229940121710 HMGCoA reductase inhibitor Drugs 0.000 claims description 18
- 238000000034 method Methods 0.000 claims description 17
- 229940026314 red yeast rice Drugs 0.000 claims description 13
- 230000007423 decrease Effects 0.000 claims description 11
- UFTFJSFQGQCHQW-UHFFFAOYSA-N triformin Chemical compound O=COCC(OC=O)COC=O UFTFJSFQGQCHQW-UHFFFAOYSA-N 0.000 claims description 11
- 235000013361 beverage Nutrition 0.000 claims description 10
- 235000013305 food Nutrition 0.000 claims description 10
- 229930014626 natural product Natural products 0.000 claims description 7
- 239000003814 drug Substances 0.000 claims description 6
- CJWQYWQDLBZGPD-UHFFFAOYSA-N isoflavone Natural products C1=C(OC)C(OC)=CC(OC)=C1C1=COC2=C(C=CC(C)(C)O3)C3=C(OC)C=C2C1=O CJWQYWQDLBZGPD-UHFFFAOYSA-N 0.000 claims description 6
- 235000008696 isoflavones Nutrition 0.000 claims description 6
- 239000002552 dosage form Substances 0.000 claims description 5
- 206010042434 Sudden death Diseases 0.000 claims description 4
- 208000010125 myocardial infarction Diseases 0.000 claims description 4
- 208000011580 syndromic disease Diseases 0.000 claims description 4
- 238000004519 manufacturing process Methods 0.000 claims description 3
- -1 skin Substances 0.000 claims description 3
- 230000000181 anti-adherent effect Effects 0.000 claims description 2
- 239000003963 antioxidant agent Substances 0.000 claims description 2
- 235000006708 antioxidants Nutrition 0.000 claims description 2
- 239000011230 binding agent Substances 0.000 claims description 2
- 239000002775 capsule Substances 0.000 claims description 2
- 235000013339 cereals Nutrition 0.000 claims description 2
- 239000003086 colorant Substances 0.000 claims description 2
- 235000013365 dairy product Nutrition 0.000 claims description 2
- 239000003085 diluting agent Substances 0.000 claims description 2
- 239000007884 disintegrant Substances 0.000 claims description 2
- 239000000839 emulsion Substances 0.000 claims description 2
- 239000000796 flavoring agent Substances 0.000 claims description 2
- 235000003599 food sweetener Nutrition 0.000 claims description 2
- 235000015203 fruit juice Nutrition 0.000 claims description 2
- 239000000314 lubricant Substances 0.000 claims description 2
- 239000000546 pharmaceutical excipient Substances 0.000 claims description 2
- 239000000843 powder Substances 0.000 claims description 2
- 239000003755 preservative agent Substances 0.000 claims description 2
- 239000007901 soft capsule Substances 0.000 claims description 2
- 239000000243 solution Substances 0.000 claims description 2
- 239000002594 sorbent Substances 0.000 claims description 2
- 239000000725 suspension Substances 0.000 claims description 2
- 239000003765 sweetening agent Substances 0.000 claims description 2
- 239000003826 tablet Substances 0.000 claims description 2
- GOMNOOKGLZYEJT-UHFFFAOYSA-N isoflavone Chemical compound C=1OC2=CC=CC=C2C(=O)C=1C1=CC=CC=C1 GOMNOOKGLZYEJT-UHFFFAOYSA-N 0.000 claims 4
- 239000000835 fiber Substances 0.000 claims 2
- 108010073771 Soybean Proteins Proteins 0.000 claims 1
- 239000000853 adhesive Substances 0.000 claims 1
- 230000003078 antioxidant effect Effects 0.000 claims 1
- 208000015114 central nervous system disease Diseases 0.000 claims 1
- 235000013355 food flavoring agent Nutrition 0.000 claims 1
- 208000027232 peripheral nervous system disease Diseases 0.000 claims 1
- 230000002335 preservative effect Effects 0.000 claims 1
- 235000011888 snacks Nutrition 0.000 claims 1
- 229940001941 soy protein Drugs 0.000 claims 1
- 150000003626 triacylglycerols Chemical class 0.000 abstract description 22
- 230000001965 increasing effect Effects 0.000 abstract description 10
- 230000037221 weight management Effects 0.000 abstract description 3
- 229940068065 phytosterols Drugs 0.000 abstract description 2
- VKJGBAJNNALVAV-UHFFFAOYSA-M Berberine chloride (TN) Chemical compound [Cl-].C1=C2CC[N+]3=CC4=C(OC)C(OC)=CC=C4C=C3C2=CC2=C1OCO2 VKJGBAJNNALVAV-UHFFFAOYSA-M 0.000 description 98
- 230000000694 effects Effects 0.000 description 46
- 241000699800 Cricetinae Species 0.000 description 23
- 241001465754 Metazoa Species 0.000 description 22
- 235000020940 control diet Nutrition 0.000 description 22
- 230000000923 atherogenic effect Effects 0.000 description 21
- 210000004185 liver Anatomy 0.000 description 20
- 230000014509 gene expression Effects 0.000 description 19
- 108020004999 messenger RNA Proteins 0.000 description 16
- 230000002195 synergetic effect Effects 0.000 description 16
- 230000037396 body weight Effects 0.000 description 13
- 230000015572 biosynthetic process Effects 0.000 description 11
- 102000004286 Hydroxymethylglutaryl CoA Reductases Human genes 0.000 description 10
- 108090000895 Hydroxymethylglutaryl CoA Reductases Proteins 0.000 description 10
- 239000000463 material Substances 0.000 description 8
- 210000002381 plasma Anatomy 0.000 description 8
- 238000003786 synthesis reaction Methods 0.000 description 8
- 235000005911 diet Nutrition 0.000 description 7
- 239000002471 hydroxymethylglutaryl coenzyme A reductase inhibitor Substances 0.000 description 7
- 230000009467 reduction Effects 0.000 description 7
- 230000002401 inhibitory effect Effects 0.000 description 6
- 239000000047 product Substances 0.000 description 6
- 210000001519 tissue Anatomy 0.000 description 6
- 108010090837 Member 5 Subfamily G ATP Binding Cassette Transporter Proteins 0.000 description 5
- 102000013436 Member 5 Subfamily G ATP Binding Cassette Transporter Human genes 0.000 description 5
- 108010090822 Member 8 Subfamily G ATP Binding Cassette Transporter Proteins 0.000 description 5
- 102000013445 Member 8 Subfamily G ATP Binding Cassette Transporter Human genes 0.000 description 5
- CZMRCDWAGMRECN-UGDNZRGBSA-N Sucrose Chemical compound O[C@H]1[C@H](O)[C@@H](CO)O[C@@]1(CO)O[C@@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO)O1 CZMRCDWAGMRECN-UGDNZRGBSA-N 0.000 description 5
- 229930006000 Sucrose Natural products 0.000 description 5
- 230000001906 cholesterol absorption Effects 0.000 description 5
- 229940079593 drug Drugs 0.000 description 5
- 239000005720 sucrose Substances 0.000 description 5
- CIWBSHSKHKDKBQ-JLAZNSOCSA-N Ascorbic acid Chemical compound OC[C@H](O)[C@H]1OC(=O)C(O)=C1O CIWBSHSKHKDKBQ-JLAZNSOCSA-N 0.000 description 4
- 229920002261 Corn starch Polymers 0.000 description 4
- 102000004190 Enzymes Human genes 0.000 description 4
- 108090000790 Enzymes Proteins 0.000 description 4
- 238000010162 Tukey test Methods 0.000 description 4
- 238000010521 absorption reaction Methods 0.000 description 4
- 239000002253 acid Substances 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- 239000005018 casein Substances 0.000 description 4
- BECPQYXYKAMYBN-UHFFFAOYSA-N casein, tech. Chemical compound NCCCCC(C(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(CC(C)C)N=C(O)C(CCC(O)=O)N=C(O)C(CC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(C(C)O)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=N)N=C(O)C(CCC(O)=O)N=C(O)C(CCC(O)=O)N=C(O)C(COP(O)(O)=O)N=C(O)C(CCC(O)=N)N=C(O)C(N)CC1=CC=CC=C1 BECPQYXYKAMYBN-UHFFFAOYSA-N 0.000 description 4
- 235000021240 caseins Nutrition 0.000 description 4
- 239000001913 cellulose Substances 0.000 description 4
- 229920002678 cellulose Polymers 0.000 description 4
- 235000010980 cellulose Nutrition 0.000 description 4
- 150000001875 compounds Chemical class 0.000 description 4
- 239000008120 corn starch Substances 0.000 description 4
- 230000037213 diet Effects 0.000 description 4
- 230000002440 hepatic effect Effects 0.000 description 4
- 230000000968 intestinal effect Effects 0.000 description 4
- 210000000936 intestine Anatomy 0.000 description 4
- 238000001543 one-way ANOVA Methods 0.000 description 4
- 108090000623 proteins and genes Proteins 0.000 description 4
- 238000012360 testing method Methods 0.000 description 4
- 240000000724 Berberis vulgaris Species 0.000 description 3
- ACTIUHUUMQJHFO-UHFFFAOYSA-N Coenzym Q10 Natural products COC1=C(OC)C(=O)C(CC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)CCC=C(C)C)=C(C)C1=O ACTIUHUUMQJHFO-UHFFFAOYSA-N 0.000 description 3
- 102100038637 Cytochrome P450 7A1 Human genes 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 101000957672 Homo sapiens Cytochrome P450 7A1 Proteins 0.000 description 3
- 101000875401 Homo sapiens Sterol 26-hydroxylase, mitochondrial Proteins 0.000 description 3
- 102100036325 Sterol 26-hydroxylase, mitochondrial Human genes 0.000 description 3
- 238000010171 animal model Methods 0.000 description 3
- 150000003832 berberine derivatives Chemical class 0.000 description 3
- LGJMUZUPVCAVPU-UHFFFAOYSA-N beta-Sitostanol Natural products C1CC2CC(O)CCC2(C)C2C1C1CCC(C(C)CCC(CC)C(C)C)C1(C)CC2 LGJMUZUPVCAVPU-UHFFFAOYSA-N 0.000 description 3
- 239000003613 bile acid Substances 0.000 description 3
- 230000008859 change Effects 0.000 description 3
- KRKNYBCHXYNGOX-UHFFFAOYSA-N citric acid Chemical compound OC(=O)CC(O)(C(O)=O)CC(O)=O KRKNYBCHXYNGOX-UHFFFAOYSA-N 0.000 description 3
- 235000017471 coenzyme Q10 Nutrition 0.000 description 3
- 230000003247 decreasing effect Effects 0.000 description 3
- 230000000378 dietary effect Effects 0.000 description 3
- 230000002526 effect on cardiovascular system Effects 0.000 description 3
- 238000002474 experimental method Methods 0.000 description 3
- 239000000284 extract Substances 0.000 description 3
- 235000021050 feed intake Nutrition 0.000 description 3
- 230000007407 health benefit Effects 0.000 description 3
- 239000010015 huanglian Substances 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 108010022197 lipoprotein cholesterol Proteins 0.000 description 3
- 230000037361 pathway Effects 0.000 description 3
- 210000002966 serum Anatomy 0.000 description 3
- 239000000126 substance Substances 0.000 description 3
- 235000013311 vegetables Nutrition 0.000 description 3
- HSINOMROUCMIEA-FGVHQWLLSA-N (2s,4r)-4-[(3r,5s,6r,7r,8s,9s,10s,13r,14s,17r)-6-ethyl-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1h-cyclopenta[a]phenanthren-17-yl]-2-methylpentanoic acid Chemical compound C([C@@]12C)C[C@@H](O)C[C@H]1[C@@H](CC)[C@@H](O)[C@@H]1[C@@H]2CC[C@]2(C)[C@@H]([C@H](C)C[C@H](C)C(O)=O)CC[C@H]21 HSINOMROUCMIEA-FGVHQWLLSA-N 0.000 description 2
- YUFFSWGQGVEMMI-JLNKQSITSA-N (7Z,10Z,13Z,16Z,19Z)-docosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCCCC(O)=O YUFFSWGQGVEMMI-JLNKQSITSA-N 0.000 description 2
- GVJHHUAWPYXKBD-UHFFFAOYSA-N (±)-α-Tocopherol Chemical compound OC1=C(C)C(C)=C2OC(CCCC(C)CCCC(C)CCCC(C)C)(C)CCC2=C1C GVJHHUAWPYXKBD-UHFFFAOYSA-N 0.000 description 2
- GUBGYTABKSRVRQ-XLOQQCSPSA-N Alpha-Lactose Chemical compound O[C@@H]1[C@@H](O)[C@@H](O)[C@@H](CO)O[C@H]1O[C@@H]1[C@@H](CO)O[C@H](O)[C@H](O)[C@H]1O GUBGYTABKSRVRQ-XLOQQCSPSA-N 0.000 description 2
- 244000161488 Berberis lycium Species 0.000 description 2
- 235000008130 Berberis lycium Nutrition 0.000 description 2
- 235000016068 Berberis vulgaris Nutrition 0.000 description 2
- VTYYLEPIZMXCLO-UHFFFAOYSA-L Calcium carbonate Chemical compound [Ca+2].[O-]C([O-])=O VTYYLEPIZMXCLO-UHFFFAOYSA-L 0.000 description 2
- 244000247747 Coptis groenlandica Species 0.000 description 2
- 235000002991 Coptis groenlandica Nutrition 0.000 description 2
- FBPFZTCFMRRESA-FSIIMWSLSA-N D-Glucitol Natural products OC[C@H](O)[C@H](O)[C@@H](O)[C@H](O)CO FBPFZTCFMRRESA-FSIIMWSLSA-N 0.000 description 2
- FBPFZTCFMRRESA-JGWLITMVSA-N D-glucitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-JGWLITMVSA-N 0.000 description 2
- 235000021294 Docosapentaenoic acid Nutrition 0.000 description 2
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 2
- 208000004930 Fatty Liver Diseases 0.000 description 2
- 206010019708 Hepatic steatosis Diseases 0.000 description 2
- 102000000853 LDL receptors Human genes 0.000 description 2
- 108010001831 LDL receptors Proteins 0.000 description 2
- GUBGYTABKSRVRQ-QKKXKWKRSA-N Lactose Natural products OC[C@H]1O[C@@H](O[C@H]2[C@H](O)[C@@H](O)C(O)O[C@@H]2CO)[C@H](O)[C@@H](O)[C@H]1O GUBGYTABKSRVRQ-QKKXKWKRSA-N 0.000 description 2
- 244000179291 Mahonia aquifolium Species 0.000 description 2
- 235000002823 Mahonia aquifolium Nutrition 0.000 description 2
- PCZOHLXUXFIOCF-UHFFFAOYSA-N Monacolin X Natural products C12C(OC(=O)C(C)CC)CC(C)C=C2C=CC(C)C1CCC1CC(O)CC(=O)O1 PCZOHLXUXFIOCF-UHFFFAOYSA-N 0.000 description 2
- 208000000112 Myalgia Diseases 0.000 description 2
- 241000209094 Oryza Species 0.000 description 2
- 235000007164 Oryza sativa Nutrition 0.000 description 2
- 206010039020 Rhabdomyolysis Diseases 0.000 description 2
- 241000283984 Rodentia Species 0.000 description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N Silicium dioxide Chemical compound O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 description 2
- 229920002472 Starch Polymers 0.000 description 2
- 108010069201 VLDL Cholesterol Proteins 0.000 description 2
- 240000008042 Zea mays Species 0.000 description 2
- 235000005824 Zea mays ssp. parviglumis Nutrition 0.000 description 2
- 235000002017 Zea mays subsp mays Nutrition 0.000 description 2
- 230000002411 adverse Effects 0.000 description 2
- JAZBEHYOTPTENJ-JLNKQSITSA-N all-cis-5,8,11,14,17-icosapentaenoic acid Chemical compound CC\C=C/C\C=C/C\C=C/C\C=C/C\C=C/CCCC(O)=O JAZBEHYOTPTENJ-JLNKQSITSA-N 0.000 description 2
- 230000008901 benefit Effects 0.000 description 2
- ARYTXMNEANMLMU-ATEDBJNTSA-N campestanol Chemical class C([C@@H]1CC2)[C@@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@H](C)CC[C@@H](C)C(C)C)[C@@]2(C)CC1 ARYTXMNEANMLMU-ATEDBJNTSA-N 0.000 description 2
- 229940110767 coenzyme Q10 Drugs 0.000 description 2
- ACTIUHUUMQJHFO-UPTCCGCDSA-N coenzyme Q10 Chemical compound COC1=C(OC)C(=O)C(C\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CC\C=C(/C)CCC=C(C)C)=C(C)C1=O ACTIUHUUMQJHFO-UPTCCGCDSA-N 0.000 description 2
- 235000005822 corn Nutrition 0.000 description 2
- 230000006378 damage Effects 0.000 description 2
- 230000034994 death Effects 0.000 description 2
- 231100000517 death Toxicity 0.000 description 2
- 230000001278 effect on cholesterol Effects 0.000 description 2
- 235000020673 eicosapentaenoic acid Nutrition 0.000 description 2
- JAZBEHYOTPTENJ-UHFFFAOYSA-N eicosapentaenoic acid Natural products CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O JAZBEHYOTPTENJ-UHFFFAOYSA-N 0.000 description 2
- 229960005135 eicosapentaenoic acid Drugs 0.000 description 2
- 238000006911 enzymatic reaction Methods 0.000 description 2
- 208000010706 fatty liver disease Diseases 0.000 description 2
- 230000037406 food intake Effects 0.000 description 2
- 150000002515 isoflavone derivatives Chemical class 0.000 description 2
- 210000003734 kidney Anatomy 0.000 description 2
- 239000008101 lactose Substances 0.000 description 2
- 125000003473 lipid group Chemical group 0.000 description 2
- PCZOHLXUXFIOCF-BXMDZJJMSA-N lovastatin Chemical compound C([C@H]1[C@@H](C)C=CC2=C[C@H](C)C[C@@H]([C@H]12)OC(=O)[C@@H](C)CC)C[C@@H]1C[C@@H](O)CC(=O)O1 PCZOHLXUXFIOCF-BXMDZJJMSA-N 0.000 description 2
- 229960004844 lovastatin Drugs 0.000 description 2
- QLJODMDSTUBWDW-UHFFFAOYSA-N lovastatin hydroxy acid Natural products C1=CC(C)C(CCC(O)CC(O)CC(O)=O)C2C(OC(=O)C(C)CC)CC(C)C=C21 QLJODMDSTUBWDW-UHFFFAOYSA-N 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- LXCFILQKKLGQFO-UHFFFAOYSA-N methylparaben Chemical compound COC(=O)C1=CC=C(O)C=C1 LXCFILQKKLGQFO-UHFFFAOYSA-N 0.000 description 2
- 238000002156 mixing Methods 0.000 description 2
- 210000003205 muscle Anatomy 0.000 description 2
- 208000013465 muscle pain Diseases 0.000 description 2
- 210000000056 organ Anatomy 0.000 description 2
- QELSKZZBTMNZEB-UHFFFAOYSA-N propylparaben Chemical compound CCCOC(=O)C1=CC=C(O)C=C1 QELSKZZBTMNZEB-UHFFFAOYSA-N 0.000 description 2
- 235000009566 rice Nutrition 0.000 description 2
- 239000000600 sorbitol Substances 0.000 description 2
- 235000010356 sorbitol Nutrition 0.000 description 2
- 235000019698 starch Nutrition 0.000 description 2
- 239000008107 starch Substances 0.000 description 2
- 229940032147 starch Drugs 0.000 description 2
- 231100000240 steatosis hepatitis Toxicity 0.000 description 2
- 238000011477 surgical intervention Methods 0.000 description 2
- 238000002560 therapeutic procedure Methods 0.000 description 2
- DCXXMTOCNZCJGO-UHFFFAOYSA-N tristearoylglycerol Chemical compound CCCCCCCCCCCCCCCCCC(=O)OCC(OC(=O)CCCCCCCCCCCCCCCCC)COC(=O)CCCCCCCCCCCCCCCCC DCXXMTOCNZCJGO-UHFFFAOYSA-N 0.000 description 2
- 230000002792 vascular Effects 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- 230000003442 weekly effect Effects 0.000 description 2
- OILXMJHPFNGGTO-UHFFFAOYSA-N (22E)-(24xi)-24-methylcholesta-5,22-dien-3beta-ol Natural products C1C=C2CC(O)CCC2(C)C2C1C1CCC(C(C)C=CC(C)C(C)C)C1(C)CC2 OILXMJHPFNGGTO-UHFFFAOYSA-N 0.000 description 1
- VGSSUFQMXBFFTM-UHFFFAOYSA-N (24R)-24-ethyl-5alpha-cholestane-3beta,5,6beta-triol Natural products C1C(O)C2(O)CC(O)CCC2(C)C2C1C1CCC(C(C)CCC(CC)C(C)C)C1(C)CC2 VGSSUFQMXBFFTM-UHFFFAOYSA-N 0.000 description 1
- DVSZKTAMJJTWFG-SKCDLICFSA-N (2e,4e,6e,8e,10e,12e)-docosa-2,4,6,8,10,12-hexaenoic acid Chemical compound CCCCCCCCC\C=C\C=C\C=C\C=C\C=C\C=C\C(O)=O DVSZKTAMJJTWFG-SKCDLICFSA-N 0.000 description 1
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 1
- FPIPGXGPPPQFEQ-UHFFFAOYSA-N 13-cis retinol Natural products OCC=C(C)C=CC=C(C)C=CC1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-UHFFFAOYSA-N 0.000 description 1
- ARYTXMNEANMLMU-UHFFFAOYSA-N 24alpha-methylcholestanol Natural products C1CC2CC(O)CCC2(C)C2C1C1CCC(C(C)CCC(C)C(C)C)C1(C)CC2 ARYTXMNEANMLMU-UHFFFAOYSA-N 0.000 description 1
- GZJLLYHBALOKEX-UHFFFAOYSA-N 6-Ketone, O18-Me-Ussuriedine Natural products CC=CCC=CCC=CCC=CCC=CCC=CCCCC(O)=O GZJLLYHBALOKEX-UHFFFAOYSA-N 0.000 description 1
- OQMZNAMGEHIHNN-UHFFFAOYSA-N 7-Dehydrostigmasterol Natural products C1C(O)CCC2(C)C(CCC3(C(C(C)C=CC(CC)C(C)C)CCC33)C)C3=CC=C21 OQMZNAMGEHIHNN-UHFFFAOYSA-N 0.000 description 1
- QTBSBXVTEAMEQO-UHFFFAOYSA-M Acetate Chemical compound CC([O-])=O QTBSBXVTEAMEQO-UHFFFAOYSA-M 0.000 description 1
- 206010063659 Aversion Diseases 0.000 description 1
- 241001407783 Berberis sargentiana Species 0.000 description 1
- SGNBVLSWZMBQTH-FGAXOLDCSA-N Campesterol Natural products O[C@@H]1CC=2[C@@](C)([C@@H]3[C@H]([C@H]4[C@@](C)([C@H]([C@H](CC[C@H](C(C)C)C)C)CC4)CC3)CC=2)CC1 SGNBVLSWZMBQTH-FGAXOLDCSA-N 0.000 description 1
- 241000282472 Canis lupus familiaris Species 0.000 description 1
- BVKZGUZCCUSVTD-UHFFFAOYSA-L Carbonate Chemical compound [O-]C([O-])=O BVKZGUZCCUSVTD-UHFFFAOYSA-L 0.000 description 1
- VEXZGXHMUGYJMC-UHFFFAOYSA-M Chloride anion Chemical compound [Cl-] VEXZGXHMUGYJMC-UHFFFAOYSA-M 0.000 description 1
- KRKNYBCHXYNGOX-UHFFFAOYSA-K Citrate Chemical compound [O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O KRKNYBCHXYNGOX-UHFFFAOYSA-K 0.000 description 1
- 241000037740 Coptis chinensis Species 0.000 description 1
- FBPFZTCFMRRESA-KVTDHHQDSA-N D-Mannitol Chemical compound OC[C@@H](O)[C@@H](O)[C@H](O)[C@H](O)CO FBPFZTCFMRRESA-KVTDHHQDSA-N 0.000 description 1
- ZZZCUOFIHGPKAK-UHFFFAOYSA-N D-erythro-ascorbic acid Natural products OCC1OC(=O)C(O)=C1O ZZZCUOFIHGPKAK-UHFFFAOYSA-N 0.000 description 1
- 102100029108 Elongation factor 1-alpha 2 Human genes 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 108700039887 Essential Genes Proteins 0.000 description 1
- 239000001856 Ethyl cellulose Substances 0.000 description 1
- ZZSNKZQZMQGXPY-UHFFFAOYSA-N Ethyl cellulose Chemical compound CCOCC1OC(OC)C(OCC)C(OCC)C1OC1C(O)C(O)C(OC)C(CO)O1 ZZSNKZQZMQGXPY-UHFFFAOYSA-N 0.000 description 1
- 241000282326 Felis catus Species 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 1
- BTEISVKTSQLKST-UHFFFAOYSA-N Haliclonasterol Natural products CC(C=CC(C)C(C)(C)C)C1CCC2C3=CC=C4CC(O)CCC4(C)C3CCC12C BTEISVKTSQLKST-UHFFFAOYSA-N 0.000 description 1
- 101000841231 Homo sapiens Elongation factor 1-alpha 2 Proteins 0.000 description 1
- 241000735429 Hydrastis Species 0.000 description 1
- 241000735432 Hydrastis canadensis Species 0.000 description 1
- 229920002153 Hydroxypropyl cellulose Polymers 0.000 description 1
- PIWKPBJCKXDKJR-UHFFFAOYSA-N Isoflurane Chemical compound FC(F)OC(Cl)C(F)(F)F PIWKPBJCKXDKJR-UHFFFAOYSA-N 0.000 description 1
- XUJNEKJLAYXESH-REOHCLBHSA-N L-Cysteine Chemical compound SC[C@H](N)C(O)=O XUJNEKJLAYXESH-REOHCLBHSA-N 0.000 description 1
- FFEARJCKVFRZRR-BYPYZUCNSA-N L-methionine Chemical compound CSCC[C@H](N)C(O)=O FFEARJCKVFRZRR-BYPYZUCNSA-N 0.000 description 1
- 238000008214 LDL Cholesterol Methods 0.000 description 1
- FYYHWMGAXLPEAU-UHFFFAOYSA-N Magnesium Chemical compound [Mg] FYYHWMGAXLPEAU-UHFFFAOYSA-N 0.000 description 1
- 229930195725 Mannitol Natural products 0.000 description 1
- 241000699670 Mus sp. Species 0.000 description 1
- 102000008934 Muscle Proteins Human genes 0.000 description 1
- 108010074084 Muscle Proteins Proteins 0.000 description 1
- 208000010428 Muscle Weakness Diseases 0.000 description 1
- 208000021642 Muscular disease Diseases 0.000 description 1
- 206010028372 Muscular weakness Diseases 0.000 description 1
- 201000009623 Myopathy Diseases 0.000 description 1
- 229910019142 PO4 Inorganic materials 0.000 description 1
- 241000972673 Phellodendron amurense Species 0.000 description 1
- 229920001131 Pulp (paper) Polymers 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- BUGBHKTXTAQXES-UHFFFAOYSA-N Selenium Chemical compound [Se] BUGBHKTXTAQXES-UHFFFAOYSA-N 0.000 description 1
- LGJMUZUPVCAVPU-JFBKYFIKSA-N Sitostanol Natural products O[C@@H]1C[C@H]2[C@@](C)([C@@H]3[C@@H]([C@H]4[C@@](C)([C@@H]([C@@H](CC[C@H](C(C)C)CC)C)CC4)CC3)CC2)CC1 LGJMUZUPVCAVPU-JFBKYFIKSA-N 0.000 description 1
- 235000021355 Stearic acid Nutrition 0.000 description 1
- 229930182558 Sterol Natural products 0.000 description 1
- QAOWNCQODCNURD-UHFFFAOYSA-L Sulfate Chemical compound [O-]S([O-])(=O)=O QAOWNCQODCNURD-UHFFFAOYSA-L 0.000 description 1
- HZYXFRGVBOPPNZ-UHFFFAOYSA-N UNPD88870 Natural products C1C=C2CC(O)CCC2(C)C2C1C1CCC(C(C)=CCC(CC)C(C)C)C1(C)CC2 HZYXFRGVBOPPNZ-UHFFFAOYSA-N 0.000 description 1
- FPIPGXGPPPQFEQ-BOOMUCAASA-N Vitamin A Natural products OC/C=C(/C)\C=C\C=C(\C)/C=C/C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-BOOMUCAASA-N 0.000 description 1
- 229930003268 Vitamin C Natural products 0.000 description 1
- 229930003427 Vitamin E Natural products 0.000 description 1
- TVXBFESIOXBWNM-UHFFFAOYSA-N Xylitol Natural products OCCC(O)C(O)C(O)CCO TVXBFESIOXBWNM-UHFFFAOYSA-N 0.000 description 1
- DPXJVFZANSGRMM-UHFFFAOYSA-N acetic acid;2,3,4,5,6-pentahydroxyhexanal;sodium Chemical compound [Na].CC(O)=O.OCC(O)C(O)C(O)C(O)C=O DPXJVFZANSGRMM-UHFFFAOYSA-N 0.000 description 1
- 150000007513 acids Chemical class 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000000996 additive effect Effects 0.000 description 1
- 150000001298 alcohols Chemical class 0.000 description 1
- 229930013930 alkaloid Natural products 0.000 description 1
- 150000003797 alkaloid derivatives Chemical class 0.000 description 1
- FPIPGXGPPPQFEQ-OVSJKPMPSA-N all-trans-retinol Chemical compound OC\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C FPIPGXGPPPQFEQ-OVSJKPMPSA-N 0.000 description 1
- 235000020661 alpha-linolenic acid Nutrition 0.000 description 1
- 238000004458 analytical method Methods 0.000 description 1
- 230000003110 anti-inflammatory effect Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 235000010323 ascorbic acid Nutrition 0.000 description 1
- 239000011668 ascorbic acid Substances 0.000 description 1
- 229960005070 ascorbic acid Drugs 0.000 description 1
- 150000003836 berberines Chemical class 0.000 description 1
- 229940076810 beta sitosterol Drugs 0.000 description 1
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 1
- NJKOMDUNNDKEAI-UHFFFAOYSA-N beta-sitosterol Natural products CCC(CCC(C)C1CCC2(C)C3CC=C4CC(O)CCC4C3CCC12C)C(C)C NJKOMDUNNDKEAI-UHFFFAOYSA-N 0.000 description 1
- 210000000941 bile Anatomy 0.000 description 1
- 230000000975 bioactive effect Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- 230000000903 blocking effect Effects 0.000 description 1
- 230000017531 blood circulation Effects 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 238000009395 breeding Methods 0.000 description 1
- 230000001488 breeding effect Effects 0.000 description 1
- 229910000019 calcium carbonate Inorganic materials 0.000 description 1
- 239000001506 calcium phosphate Substances 0.000 description 1
- 229910000389 calcium phosphate Inorganic materials 0.000 description 1
- 235000011010 calcium phosphates Nutrition 0.000 description 1
- 235000000431 campesterol Nutrition 0.000 description 1
- SGNBVLSWZMBQTH-PODYLUTMSA-N campesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CC[C@@H](C)C(C)C)[C@@]1(C)CC2 SGNBVLSWZMBQTH-PODYLUTMSA-N 0.000 description 1
- 239000001768 carboxy methyl cellulose Substances 0.000 description 1
- 230000036996 cardiovascular health Effects 0.000 description 1
- 210000000748 cardiovascular system Anatomy 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 210000004027 cell Anatomy 0.000 description 1
- 230000031154 cholesterol homeostasis Effects 0.000 description 1
- 230000001684 chronic effect Effects 0.000 description 1
- 229940001468 citrate Drugs 0.000 description 1
- 235000015165 citric acid Nutrition 0.000 description 1
- 230000001447 compensatory effect Effects 0.000 description 1
- 239000002299 complementary DNA Substances 0.000 description 1
- 239000000287 crude extract Substances 0.000 description 1
- 238000002425 crystallisation Methods 0.000 description 1
- 230000008025 crystallization Effects 0.000 description 1
- 235000018417 cysteine Nutrition 0.000 description 1
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 235000015872 dietary supplement Nutrition 0.000 description 1
- 235000020669 docosahexaenoic acid Nutrition 0.000 description 1
- KAUVQQXNCKESLC-UHFFFAOYSA-N docosahexaenoic acid (DHA) Natural products COC(=O)C(C)NOCC1=CC=CC=C1 KAUVQQXNCKESLC-UHFFFAOYSA-N 0.000 description 1
- 235000013399 edible fruits Nutrition 0.000 description 1
- 230000002708 enhancing effect Effects 0.000 description 1
- 210000003743 erythrocyte Anatomy 0.000 description 1
- 229920001249 ethyl cellulose Polymers 0.000 description 1
- 235000019325 ethyl cellulose Nutrition 0.000 description 1
- 238000000605 extraction Methods 0.000 description 1
- 239000003925 fat Substances 0.000 description 1
- 235000019197 fats Nutrition 0.000 description 1
- 210000003608 fece Anatomy 0.000 description 1
- 238000000855 fermentation Methods 0.000 description 1
- 230000004151 fermentation Effects 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 235000012631 food intake Nutrition 0.000 description 1
- WIGCFUFOHFEKBI-UHFFFAOYSA-N gamma-tocopherol Natural products CC(C)CCCC(C)CCCC(C)CCCC1CCC2C(C)C(O)C(C)C(C)C2O1 WIGCFUFOHFEKBI-UHFFFAOYSA-N 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 150000004676 glycans Chemical class 0.000 description 1
- 235000005679 goldenseal Nutrition 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 230000037219 healthy weight Effects 0.000 description 1
- 210000002216 heart Anatomy 0.000 description 1
- 239000001863 hydroxypropyl cellulose Substances 0.000 description 1
- 235000010977 hydroxypropyl cellulose Nutrition 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 230000006698 induction Effects 0.000 description 1
- 230000037456 inflammatory mechanism Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 229960002725 isoflurane Drugs 0.000 description 1
- 238000002955 isolation Methods 0.000 description 1
- 229930013397 isoquinoline alkaloid Natural products 0.000 description 1
- 235000021374 legumes Nutrition 0.000 description 1
- 230000003908 liver function Effects 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000011777 magnesium Substances 0.000 description 1
- 229910052749 magnesium Inorganic materials 0.000 description 1
- 239000000845 maltitol Substances 0.000 description 1
- VQHSOMBJVWLPSR-WUJBLJFYSA-N maltitol Chemical compound OC[C@H](O)[C@@H](O)[C@@H]([C@H](O)CO)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O VQHSOMBJVWLPSR-WUJBLJFYSA-N 0.000 description 1
- 235000010449 maltitol Nutrition 0.000 description 1
- 229940035436 maltitol Drugs 0.000 description 1
- 239000000594 mannitol Substances 0.000 description 1
- 235000010355 mannitol Nutrition 0.000 description 1
- 230000010534 mechanism of action Effects 0.000 description 1
- 229940126601 medicinal product Drugs 0.000 description 1
- HEBKCHPVOIAQTA-UHFFFAOYSA-N meso ribitol Natural products OCC(O)C(O)C(O)CO HEBKCHPVOIAQTA-UHFFFAOYSA-N 0.000 description 1
- 230000004060 metabolic process Effects 0.000 description 1
- 235000006109 methionine Nutrition 0.000 description 1
- 229930182817 methionine Natural products 0.000 description 1
- 239000004292 methyl p-hydroxybenzoate Substances 0.000 description 1
- 229960002216 methylparaben Drugs 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 230000002438 mitochondrial effect Effects 0.000 description 1
- 230000009456 molecular mechanism Effects 0.000 description 1
- 235000014571 nuts Nutrition 0.000 description 1
- QIQXTHQIDYTFRH-UHFFFAOYSA-N octadecanoic acid Chemical compound CCCCCCCCCCCCCCCCCC(O)=O QIQXTHQIDYTFRH-UHFFFAOYSA-N 0.000 description 1
- OQCDKBAXFALNLD-UHFFFAOYSA-N octadecanoic acid Natural products CCCCCCCC(C)CCCCCCCCC(O)=O OQCDKBAXFALNLD-UHFFFAOYSA-N 0.000 description 1
- 239000003921 oil Substances 0.000 description 1
- 235000020660 omega-3 fatty acid Nutrition 0.000 description 1
- 229940012843 omega-3 fatty acid Drugs 0.000 description 1
- 239000006014 omega-3 oil Substances 0.000 description 1
- 230000008816 organ damage Effects 0.000 description 1
- 208000033808 peripheral neuropathy Diseases 0.000 description 1
- NBIIXXVUZAFLBC-UHFFFAOYSA-K phosphate Chemical compound [O-]P([O-])([O-])=O NBIIXXVUZAFLBC-UHFFFAOYSA-K 0.000 description 1
- 239000010452 phosphate Substances 0.000 description 1
- 239000000419 plant extract Substances 0.000 description 1
- 229920001282 polysaccharide Polymers 0.000 description 1
- 239000005017 polysaccharide Substances 0.000 description 1
- 239000001267 polyvinylpyrrolidone Substances 0.000 description 1
- 229920000036 polyvinylpyrrolidone Polymers 0.000 description 1
- 235000013855 polyvinylpyrrolidone Nutrition 0.000 description 1
- 230000002265 prevention Effects 0.000 description 1
- 230000001737 promoting effect Effects 0.000 description 1
- 235000010232 propyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000004405 propyl p-hydroxybenzoate Substances 0.000 description 1
- 229960003415 propylparaben Drugs 0.000 description 1
- 239000000700 radioactive tracer Substances 0.000 description 1
- 238000003753 real-time PCR Methods 0.000 description 1
- 230000000384 rearing effect Effects 0.000 description 1
- 230000001105 regulatory effect Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 229920006395 saturated elastomer Polymers 0.000 description 1
- 230000000276 sedentary effect Effects 0.000 description 1
- 235000011649 selenium Nutrition 0.000 description 1
- 239000011669 selenium Substances 0.000 description 1
- 229910052711 selenium Inorganic materials 0.000 description 1
- 239000000377 silicon dioxide Substances 0.000 description 1
- KZJWDPNRJALLNS-VJSFXXLFSA-N sitosterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CC[C@@H](CC)C(C)C)[C@@]1(C)CC2 KZJWDPNRJALLNS-VJSFXXLFSA-N 0.000 description 1
- 229950005143 sitosterol Drugs 0.000 description 1
- 235000009561 snack bars Nutrition 0.000 description 1
- 235000019812 sodium carboxymethyl cellulose Nutrition 0.000 description 1
- 229920001027 sodium carboxymethylcellulose Polymers 0.000 description 1
- 239000001509 sodium citrate Substances 0.000 description 1
- NLJMYIDDQXHKNR-UHFFFAOYSA-K sodium citrate Chemical compound O.O.[Na+].[Na+].[Na+].[O-]C(=O)CC(O)(CC([O-])=O)C([O-])=O NLJMYIDDQXHKNR-UHFFFAOYSA-K 0.000 description 1
- 235000011083 sodium citrates Nutrition 0.000 description 1
- 235000010268 sodium methyl p-hydroxybenzoate Nutrition 0.000 description 1
- 239000008109 sodium starch glycolate Substances 0.000 description 1
- 229940079832 sodium starch glycolate Drugs 0.000 description 1
- 229920003109 sodium starch glycolate Polymers 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 238000013222 sprague-dawley male rat Methods 0.000 description 1
- 239000008117 stearic acid Substances 0.000 description 1
- 150000003432 sterols Chemical class 0.000 description 1
- 235000003702 sterols Nutrition 0.000 description 1
- LGJMUZUPVCAVPU-HRJGVYIJSA-N stigmastanol Chemical compound C([C@@H]1CC2)[C@@H](O)CC[C@]1(C)[C@@H]1[C@@H]2[C@@H]2CC[C@H]([C@H](C)CC[C@@H](CC)C(C)C)[C@@]2(C)CC1 LGJMUZUPVCAVPU-HRJGVYIJSA-N 0.000 description 1
- HCXVJBMSMIARIN-PHZDYDNGSA-N stigmasterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)/C=C/[C@@H](CC)C(C)C)[C@@]1(C)CC2 HCXVJBMSMIARIN-PHZDYDNGSA-N 0.000 description 1
- 235000016831 stigmasterol Nutrition 0.000 description 1
- 229940032091 stigmasterol Drugs 0.000 description 1
- BFDNMXAIBMJLBB-UHFFFAOYSA-N stigmasterol Natural products CCC(C=CC(C)C1CCCC2C3CC=C4CC(O)CCC4(C)C3CCC12C)C(C)C BFDNMXAIBMJLBB-UHFFFAOYSA-N 0.000 description 1
- 235000000346 sugar Nutrition 0.000 description 1
- 239000013589 supplement Substances 0.000 description 1
- 230000009469 supplementation Effects 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
- 208000024891 symptom Diseases 0.000 description 1
- 229920001059 synthetic polymer Polymers 0.000 description 1
- 239000000454 talc Substances 0.000 description 1
- 229910052623 talc Inorganic materials 0.000 description 1
- 239000003784 tall oil Substances 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- QORWJWZARLRLPR-UHFFFAOYSA-H tricalcium bis(phosphate) Chemical compound [Ca+2].[Ca+2].[Ca+2].[O-]P([O-])([O-])=O.[O-]P([O-])([O-])=O QORWJWZARLRLPR-UHFFFAOYSA-H 0.000 description 1
- JFJZZMVDLULRGK-URLMMPGGSA-O tubocurarine Chemical compound C([C@H]1[N+](C)(C)CCC=2C=C(C(=C(OC3=CC=C(C=C3)C[C@H]3C=4C=C(C(=CC=4CCN3C)OC)O3)C=21)O)OC)C1=CC=C(O)C3=C1 JFJZZMVDLULRGK-URLMMPGGSA-O 0.000 description 1
- 235000021122 unsaturated fatty acids Nutrition 0.000 description 1
- 150000004670 unsaturated fatty acids Chemical class 0.000 description 1
- 210000005166 vasculature Anatomy 0.000 description 1
- 235000019871 vegetable fat Nutrition 0.000 description 1
- 239000008158 vegetable oil Substances 0.000 description 1
- 235000019155 vitamin A Nutrition 0.000 description 1
- 239000011719 vitamin A Substances 0.000 description 1
- 235000019154 vitamin C Nutrition 0.000 description 1
- 239000011718 vitamin C Substances 0.000 description 1
- 235000019165 vitamin E Nutrition 0.000 description 1
- 229940046009 vitamin E Drugs 0.000 description 1
- 239000011709 vitamin E Substances 0.000 description 1
- 229940045997 vitamin a Drugs 0.000 description 1
- 239000000811 xylitol Substances 0.000 description 1
- 235000010447 xylitol Nutrition 0.000 description 1
- HEBKCHPVOIAQTA-SCDXWVJYSA-N xylitol Chemical compound OC[C@H](O)[C@@H](O)[C@H](O)CO HEBKCHPVOIAQTA-SCDXWVJYSA-N 0.000 description 1
- 229960002675 xylitol Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K45/00—Medicinal preparations containing active ingredients not provided for in groups A61K31/00 - A61K41/00
- A61K45/06—Mixtures of active ingredients without chemical characterisation, e.g. antiphlogistics and cardiaca
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/105—Plant extracts, their artificial duplicates or their derivatives
- A23L33/11—Plant sterols or derivatives thereof, e.g. phytosterols
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L33/00—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof
- A23L33/10—Modifying nutritive qualities of foods; Dietetic products; Preparation or treatment thereof using additives
- A23L33/14—Yeasts or derivatives thereof
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23L—FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES, NOT OTHERWISE PROVIDED FOR; PREPARATION OR TREATMENT THEREOF
- A23L7/00—Cereal-derived products; Malt products; Preparation or treatment thereof
- A23L7/10—Cereal-derived products
- A23L7/104—Fermentation of farinaceous cereal or cereal material; Addition of enzymes or microorganisms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4741—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having oxygen as a ring hetero atom, e.g. tubocuraran derivatives, noscapine, bicuculline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4738—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems
- A61K31/4745—Quinolines; Isoquinolines ortho- or peri-condensed with heterocyclic ring systems condensed with ring systems having nitrogen as a ring hetero atom, e.g. phenantrolines
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/575—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids substituted in position 17 beta by a chain of three or more carbon atoms, e.g. cholane, cholestane, ergosterol, sitosterol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/06—Fungi, e.g. yeasts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/185—Magnoliopsida (dicotyledons)
- A61K36/48—Fabaceae or Leguminosae (Pea or Legume family); Caesalpiniaceae; Mimosaceae; Papilionaceae
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K36/00—Medicinal preparations of undetermined constitution containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines
- A61K36/18—Magnoliophyta (angiosperms)
- A61K36/88—Liliopsida (monocotyledons)
- A61K36/899—Poaceae or Gramineae (Grass family), e.g. bamboo, corn or sugar cane
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/16—Drugs for disorders of the alimentary tract or the digestive system for liver or gallbladder disorders, e.g. hepatoprotective agents, cholagogues, litholytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- A—HUMAN NECESSITIES
- A23—FOODS OR FOODSTUFFS; TREATMENT THEREOF, NOT COVERED BY OTHER CLASSES
- A23V—INDEXING SCHEME RELATING TO FOODS, FOODSTUFFS OR NON-ALCOHOLIC BEVERAGES AND LACTIC OR PROPIONIC ACID BACTERIA USED IN FOODSTUFFS OR FOOD PREPARATION
- A23V2002/00—Food compositions, function of food ingredients or processes for food or foodstuffs
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- General Health & Medical Sciences (AREA)
- Animal Behavior & Ethology (AREA)
- Medicinal Chemistry (AREA)
- Engineering & Computer Science (AREA)
- Natural Medicines & Medicinal Plants (AREA)
- Epidemiology (AREA)
- Mycology (AREA)
- Botany (AREA)
- Microbiology (AREA)
- Organic Chemistry (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- General Chemical & Material Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Biotechnology (AREA)
- Polymers & Plastics (AREA)
- Food Science & Technology (AREA)
- Nutrition Science (AREA)
- Alternative & Traditional Medicine (AREA)
- Medical Informatics (AREA)
- Heart & Thoracic Surgery (AREA)
- Cardiology (AREA)
- Diabetes (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Vascular Medicine (AREA)
- Biomedical Technology (AREA)
- Rheumatology (AREA)
- Urology & Nephrology (AREA)
- Neurology (AREA)
- Child & Adolescent Psychology (AREA)
- Neurosurgery (AREA)
- Pain & Pain Management (AREA)
- Gastroenterology & Hepatology (AREA)
Abstract
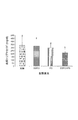
【選択図】 図1
Description
[0001]本出願は2006年2月9日出願の米国仮特許出願第60/771455号明細書についての利益を請求する。
[0002]本発明は医薬組成物に関し、特に心血管の健康を促進するための植物抽出物の組合せに関する。
[0003]冠状動脈性心臓病、アテローム性動脈硬化症、発作、心筋梗塞、サドンデス症候群を含む心血管疾患(CVD)は、世界中の大部分の先進国における死因の第1位である。また、開発途上国では、CVDの有病率は増加傾向にあり、より西洋(北米)化した(高脂肪の)食生活及び(座りがちな)生活様式を取り入れている人々と関係があるように思われる。上昇した循環コレステロール濃度、特に低密度リポタンパク質コレステロール(LDLコレステロール)濃度は、CVDの発現及び進行の主な危険因子の1つとして充分に確立されている。高濃度の循環トリグリセリドも、CVD発生率の増加において決定的な危険因子である。したがって、罹患及び致死の発生率の増加と関係していることが周知であり、また証明されている心血管関連の危険因子を減らすために、リスクの高い患者には総コレステロール及び/又はトリグリセリドの各濃度を低下させることが助言されている。肥満、糖尿病及び高脂血症を有する対象は、高濃度のコレステロール及びトリグリセリドにより悪影響を受ける集団の三大サブグループである。
[0007]ベルベリンと同じ機構を介して機能する血中脂質低下剤及びベルベリンと異なる機構を介して機能する血中脂質低下剤は相乗的に作用して、血中脂質プロファイルを改善することが今や判明した。この改善は、例えば総コレステロール(T−C)、低密度リポタンパク質コレステロール(LDL−C)若しくは非高密度リポタンパク質コレステロール(非HDL−C)、及び/又はトリグリセリド(TG)等の血中脂質の低下、並びに/或いは高密度リポタンパク質コレステロール(HDL−C)とLDL−C又は非HDL−Cとの比の増加において明らかにすることができる。組み合わせて投与した前記2種の血中脂質の調節(例えば低下作用)は、単独で投与した前記2種の合計を超える。
[0047]以下の実施例の目的は、心血管系、脳血管系、血管系、肝臓及び体重にとって明確な健康上の利益のために、BBRとBLLAとの組合せにより、単独でそれぞれ介入するよりも血中脂質プロファイルが相乗的に改善されることを示すことであった。
対照 コレステロール0.15%(w/w)及び脂質5%(w/w)含有準合成カゼインコーンスターチスクロース飼料
BBRCI BBRCI100mg/kg・d含有対照飼料
PS PS1%(w/w)含有対照飼料
BBRCI+PS BBRCI100mg/kg・d及びPS1%(w/w)含有対照飼料
対照 コレステロール0.25%(w/w)含有準合成カゼインコーンスターチスクロース飼料
BBRCI BBRCI100mg/kg・d含有対照飼料
PS PS1%(w/w)含有対照飼料
BBRCI+PS BBRCI100mg/kg・d及びPS1%(w/w)含有対照飼料
対照 コレステロール0.25%(w/w)含有準合成カゼインコーンスターチスクロース飼料
BBRCI BBRCI100mg/kg・d含有対照飼料
PS PS0.5%(w/w)含有対照飼料
BBRCI+PS BBRCI100mg/kg・d及びPS0.5%(w/w)含有対照飼料
対照 コレステロール2%(w/w)及び脂質28%(w/w)含有準合成カゼインコーンスターチスクロース飼料
BBRCI−1 BBRCI100mg/kg・d含有対照飼料
PS PS1%(w/w)含有対照飼料
BBRCI−1+PS BBRCI100mg/kg・d及びPS1%(w/w)含有対照飼料
BBRCI−2 BBRCI200mg/kg・d含有対照飼料
BBRCI−2+PS BBRCI200mg/kg・d及びPS1%(w/w)含有対照飼料
Claims (45)
- ベルベリンと同じ機構を介して機能する血中脂質低下剤と、ベルベリンと異なる機構を介して機能する血中脂質低下剤とを含む血中脂質濃度低下組成物。
- ベルベリンと同じ機構を介して機能する前記血中脂質低下剤が、ベルベリン、1種若しくは複数の薬理学的に許容できるベルベリンの塩又はそれらの混合物を含む、請求項1に記載の組成物。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、スタチン、イソフラボン、それらを1種若しくは複数含有する天然物、それらの誘導体、又はそれらの混合物を含む、請求項1又は2に記載の組成物。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、植物ステロールのエステル、植物スタノールのエステル又はそれらの混合物を含む、請求項1又は2に記載の組成物。
- 紅麹米をさらに含む、請求項4に記載の組成物。
- 接着防止剤、結合剤、剤皮、崩壊剤、賦形剤若しくは希釈剤、香味料、着色料、流動促進剤、滑沢剤、防腐剤、収着剤、甘味料、酸化防止剤又はそれらの混合物をさらに含む、請求項1〜5のいずれか一項に記載の組成物。
- 哺乳動物における血中脂質濃度を低下させるための、ベルベリンと同じ機構を介して機能する血中脂質低下剤及びベルベリンと異なる機構を介して機能する血中脂質低下剤の使用。
- 哺乳動物における血中脂質濃度を低下させる薬剤を製造するための、ベルベリンと同じ機構を介して機能する血中脂質低下剤及びベルベリンと異なる機構を介して機能する血中脂質低下剤の使用。
- 哺乳動物の体重を管理するための、ベルベリンと同じ機構を介して機能する血中脂質低下剤及びベルベリンと異なる機構を介して機能する血中脂質低下剤の使用。
- 低下させる前記血中脂質濃度がトリグリセリド濃度を含む、請求項7又は8に記載の使用。
- 低下させる前記血中脂質濃度が低密度リポタンパク質コレステロール濃度又は非高密度リポタンパク質コレステロール濃度を含む、請求項7、8又は10に記載の使用。
- 低下させる前記血中脂質濃度が総コレステロール濃度を含む、請求項7、8、10又は11に記載の使用。
- 前記の血中脂質濃度の低下により、心血管疾患、高脂血症、アテローム性動脈硬化症、冠状動脈性心臓病、狭心症、脳血管疾患、発作、体重超過若しくは肥満、糖尿病、インシュリン抵抗性、高血糖症、高血圧症、不整脈、中枢神経系の疾患、末梢神経系の疾患又は炎症を治療するか、或いはこれらの疾患にかかる可能性を低下させる、請求項7、8、10、11又は12に記載の使用。
- 前記の血中脂質濃度の低下により、冠状動脈性心臓病、アテローム性動脈硬化症、発作、不整脈、心筋梗塞又はサドンデス症候群を治療するか、或いはこれらの疾患にかかる可能性を低下させる、請求項7、8、10、11又は12に記載の使用。
- ベルベリンと同じ機構を介して機能する前記血中脂質低下剤が、ベルベリン、1種若しくは複数の薬理学的に許容できるベルベリンの塩又はそれらの混合物を含む、請求項7〜14のいずれか一項に記載の使用。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、スタチン、イソフラボン、大豆タンパク、繊維、水溶性繊維、他の脂質低下剤、及びそれらを1種若しくは複数含有する天然物、それらの誘導体又はそれらの混合物を含む、請求項7〜15のいずれか一項に記載の使用。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、植物ステロールのエステル、植物スタノールのエステル又はそれらの混合物を含む、請求項7〜15のいずれか一項に記載の使用。
- 紅麹米も使用される、請求項17に記載の使用。
- 哺乳動物がヒトである、請求項7〜18のいずれか一項に記載の使用。
- 請求項1〜6のいずれか一項に記載の組成物を含む剤形。
- 粉末、錠剤、カプセル、ソフトカプセル、液剤、懸濁剤又は乳剤である、請求項20に記載の剤形。
- 請求項1〜6のいずれか一項に記載の組成物を含む食品又は飲料。
- シリアル、スナックバー、乳製品、果汁、粉末食品又は乾燥粉末飲料ミックスである、請求項22に記載の食品又は飲料。
- 哺乳動物における血中脂質濃度を低下させるための使用説明書と共に、ベルベリンと同じ機構を介して機能する血中脂質低下剤と、ベルベリンと異なる機構を介して機能する血中脂質低下剤とを含む商品パッケージ。
- ベルベリンと同じ機構を介して機能する前記血中脂質低下剤が、ベルベリン、1種若しくは複数の薬理学的に許容できるベルベリンの塩又はそれらの混合物を含む、請求項24に記載の商品パッケージ。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、スタチン、イソフラボン、それらを1種若しくは複数含有する天然物、それらの誘導体、又はそれらの混合物を含む、請求項24又は25に記載の商品パッケージ。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、植物ステロールのエステル、植物スタノールのエステル又はそれらの混合物を含む、請求項24又は25に記載の商品パッケージ。
- 紅麹米をさらに含む請求項27に記載の商品パッケージ。
- 哺乳動物における血中脂質濃度を低下させるための使用説明書と共に、請求項20〜21のいずれか一項に記載の剤形を含む商品パッケージ。
- 哺乳動物における血中脂質濃度を低下させるための使用説明書と共に、請求項22〜23のいずれか一項に記載の食品又は飲料を含む商品パッケージ。
- ボトル、ジャー、ブリスターパック又はボックスである、請求項24〜30のいずれか一項に記載の商品パッケージ。
- 血中脂質濃度を低下させる必要がある哺乳動物に、ベルベリンと同じ機構を介して機能する血中脂質低下剤と、ベルベリンと異なる機構を介して機能する血中脂質低下剤とを投与することを含む方法。
- ベルベリンと同じ機構を介して機能する前記血中脂質低下剤が、ベルベリン、1種若しくは複数の薬理学的に許容できるベルベリンの塩又はそれらの混合物を含む、請求項32に記載の方法。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、スタチン、イソフラボン、それらを1種若しくは複数含有する天然物、それらの誘導体又はそれらの混合物を含む、請求項32又は33に記載の方法。
- ベルベリンと異なる機構を介して機能する前記血中脂質低下剤が、植物ステロール、植物スタノール、植物ステロールのエステル、植物スタノールのエステル又はそれらの混合物を含む、請求項32又は33に記載の方法。
- 紅麹米を投与することをさらに含む請求項35に記載の方法。
- 投与が経口投与である、請求項32〜36のいずれか一項に記載の方法。
- 低下させる前記血中脂質濃度がトリグリセリド濃度を含む、請求項32〜37のいずれか一項に記載の方法。
- 低下させる前記血中脂質濃度が低密度リポタンパク質コレステロール濃度を含む、請求項32〜38のいずれか一項に記載の方法。
- 低下させる前記血中脂質濃度が総コレステロール濃度を含む、請求項32〜39のいずれか一項に記載の方法。
- 哺乳動物における、心血管疾患、高脂血症、アテローム性動脈硬化症、冠状動脈性心臓病、狭心症、脳血管疾患、発作、体重超過若しくは肥満、糖尿病、インシュリン抵抗性、高血糖症、高血圧症、不整脈、中枢神経系の疾患、末梢神経系の疾患又は炎症を治療するか、或いはこれらの疾患にかかる可能性を低下させる、請求項32〜40のいずれか一項に記載の方法。
- 哺乳動物における、冠状動脈性心臓病、アテローム性動脈硬化症、発作、不整脈、心筋梗塞又はサドンデス症候群を治療するか、或いはこれらの疾患にかかる可能性を低下させる、請求項32〜40のいずれか一項に記載の方法。
- 前記哺乳動物がヒトである、請求項32〜42のいずれか一項に記載の方法。
- 哺乳動物の体重を管理するための、ベルベリンと同じ機構を介して機能する血中脂質低下剤の使用。
- ベルベリンと同じ機構を介して機能する前記血中脂質低下剤が、ベルベリン、1種若しくは複数の薬理学的に許容できるベルベリンの塩又はそれらの混合物を含む、請求項44に記載の方法。
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US71145506P | 2006-02-09 | 2006-02-09 | |
| PCT/CA2007/000194 WO2007090289A1 (en) | 2006-02-09 | 2007-02-09 | Combinations of botanical extracts for promoting cardiovascular health |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2009525990A true JP2009525990A (ja) | 2009-07-16 |
| JP2009525990A5 JP2009525990A5 (ja) | 2010-04-02 |
Family
ID=38344842
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2008553588A Pending JP2009525990A (ja) | 2006-02-09 | 2007-02-09 | 医薬組成物 |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US8895079B2 (ja) |
| EP (1) | EP1983989B1 (ja) |
| JP (1) | JP2009525990A (ja) |
| CA (1) | CA2640414C (ja) |
| DK (1) | DK1983989T3 (ja) |
| WO (1) | WO2007090289A1 (ja) |
Cited By (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012524533A (ja) * | 2009-04-23 | 2012-10-18 | エス・アー・コルマン | 薬剤として使用するための減コレステロール酪農製品 |
| JP2022017496A (ja) * | 2015-11-13 | 2022-01-25 | シンセン ハイタイド バイオファーマシューティカル リミテッド | 組成物、ならびにその適用および薬学的調製の方法 |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8003795B2 (en) | 2007-06-22 | 2011-08-23 | Cvi Pharmaceuticals Limited | Compounds and compositions for reducing lipid levels |
| US8716249B2 (en) * | 2008-01-31 | 2014-05-06 | Energy Light Llc | Compositions and methods for improving cardiovascular health |
| CN102076347B (zh) | 2008-05-01 | 2013-07-17 | 阿费克萨生命科学公司 | 用于降低血脂水平的包含黄连和苦丁茶提取物的药物组合物 |
| TWI454221B (en) * | 2008-07-14 | 2014-10-01 | Composition and method for inhibiting formation of body fat | |
| ITMI20081403A1 (it) | 2008-07-29 | 2010-01-30 | Velleja Res Srl | Composizioni comprendenti berberina e/o suoi analoghi o estratti che la contengano, per la prevenzione e il trattamento delle alterazioni del quadro lipidico e glucidico |
| IT1405432B1 (it) * | 2010-12-16 | 2014-01-10 | Funcional Food Res S R L | Preparazione alimentare funzionale ed uso relativo. |
| WO2013016741A1 (en) * | 2011-07-22 | 2013-01-31 | Smith Conrad Anton | Dietary supplement for use in a weight loss program |
| WO2014107657A2 (en) * | 2013-01-04 | 2014-07-10 | Kohn Kenneth I | Cholesterol-lowering compounds in combination with lipid metabolism-altering compounds of non-absorbable sugars, compounds that convert nh3 to nh4+, or hydrogen-generating compounds for the treatment of high cholesterol and inflammation |
| US9221699B2 (en) * | 2013-03-05 | 2015-12-29 | Innovative Environment Technologies, Inc. | Inhibition of methane production during anaerobic reductive dechlorination |
| US9637731B2 (en) | 2013-03-05 | 2017-05-02 | Innovative Environmental Technologies, Inc. | Heavy metal stabilization and methane inhibition during induced or naturally occurring reducing conditions in contaminated media |
| ITUB20150541A1 (it) | 2015-03-03 | 2016-09-03 | Acraf | Composizione comprendente sostanze e/o estratti naturali |
| CA3023648C (en) * | 2016-05-10 | 2023-11-28 | Shenzhen Hightide Biopharmaceutical, Ltd. | Pharmaceutical composition of berberine with epa and dha |
| ES2645028B2 (es) * | 2017-10-13 | 2018-06-04 | Maria D. GÓMEZ GARRE | Preparación farmacéutica única y estable que contiene berberina, en formulación de liberación lenta, una estatina y ubiquinol para el tratamiento de la enfermedad cardiovascular y los factores de riesgo asociados |
| WO2024208983A1 (en) * | 2023-04-06 | 2024-10-10 | Dompe' Farmaceutici S.P.A. | Composition based on plant extracts useful for the treatment of dyslipidaemias |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5589182A (en) * | 1993-12-06 | 1996-12-31 | Tashiro; Renki | Compositions and method of treating cardio-, cerebro-vascular and alzheimer's diseases and depression |
| JPH0995452A (ja) * | 1995-07-21 | 1997-04-08 | Res Inst For Prod Dev | 脂肪分解促進剤 |
| JPH09255570A (ja) * | 1996-03-21 | 1997-09-30 | Fujitsuko Kk | 血中脂質濃度を低減させる薬剤及び可食性組成物 |
| JP2000264842A (ja) * | 1999-03-18 | 2000-09-26 | Medvill Co Ltd | 知母と黄蘖の混合水性抽出物を包含する、抗炎症及び鎮痛用薬剤組成物並びにその製法 |
| WO2001030359A1 (en) * | 1999-10-29 | 2001-05-03 | Triple Crown Ab | Cholesterol lowering and blood lipids lowering composition |
| CN1437973A (zh) * | 2003-01-23 | 2003-08-27 | 北京中医药大学 | 一种治疗缺血性脑中风的药物组合物及其制备方法 |
| JP2004331635A (ja) * | 2003-05-04 | 2004-11-25 | Kaicho Se | 細胞遣伝情報正常化材料及びその製法 |
| JP2008513382A (ja) * | 2004-09-17 | 2008-05-01 | インスティチュート オブ メディシナル バイオテクノロジー、 チャイニーズ アカデミー オブ メディカル サイエンシズ | 高脂血症を治療する方法及び組成物 |
Family Cites Families (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2524783B2 (ja) * | 1987-12-03 | 1996-08-14 | 科研製薬株式会社 | 家畜・家禽の肝機能改善剤 |
| KR20000021073A (ko) | 1998-09-25 | 2000-04-15 | 박원배 | 콜레스테롤 생합성 저해제 |
| US6998501B1 (en) * | 1999-08-30 | 2006-02-14 | Ocean Nutrition Canada Limited | Nutritional supplement for lowering serum triglyceride and cholesterol levels |
| US6436406B1 (en) * | 2000-06-15 | 2002-08-20 | A. Glenn Braswell | Compositions and methods for reducing or controlling blood cholesterol, lipoproteins, triglycerides and atherosclerosis |
| US20060078532A1 (en) | 2004-10-12 | 2006-04-13 | Omoigui Osemwota S | Method of prevention and treatment of Atherosclerosis, Peripheral vascular disease, Coronary artery disease, aging and age-related disorders including osteoporosis, arthritis, type 2 diabetes, dementia and Alzheimer's disease |
| US20060078533A1 (en) | 2004-10-12 | 2006-04-13 | Omoigui Osemwota S | Method of prevention and treatment of aging and age-related disorders including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimer's disease and cancer |
| US20060275294A1 (en) | 2002-08-22 | 2006-12-07 | Omoigui Osemwota S | Method of prevention and treatment of aging, age-related disorders and/or age-related manifestations including atherosclerosis, peripheral vascular disease, coronary artery disease, osteoporosis, arthritis, type 2 diabetes, dementia, alzheimers disease and cancer |
| US20060078531A1 (en) | 2004-10-12 | 2006-04-13 | Osemwota Sota | Method of prevention and treatment of atherosclerosis, peripheral vascular disease, coronary artery disease, and age-related disorders including osteoporosis, arthritis, type 2 diabetes, dementia and Alzheimer's disease |
-
2007
- 2007-02-09 CA CA2640414A patent/CA2640414C/en not_active Expired - Fee Related
- 2007-02-09 DK DK07710609.4T patent/DK1983989T3/da active
- 2007-02-09 US US12/223,297 patent/US8895079B2/en not_active Expired - Fee Related
- 2007-02-09 WO PCT/CA2007/000194 patent/WO2007090289A1/en active Application Filing
- 2007-02-09 EP EP07710609.4A patent/EP1983989B1/en not_active Not-in-force
- 2007-02-09 JP JP2008553588A patent/JP2009525990A/ja active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5589182A (en) * | 1993-12-06 | 1996-12-31 | Tashiro; Renki | Compositions and method of treating cardio-, cerebro-vascular and alzheimer's diseases and depression |
| JPH0995452A (ja) * | 1995-07-21 | 1997-04-08 | Res Inst For Prod Dev | 脂肪分解促進剤 |
| JPH09255570A (ja) * | 1996-03-21 | 1997-09-30 | Fujitsuko Kk | 血中脂質濃度を低減させる薬剤及び可食性組成物 |
| JP2000264842A (ja) * | 1999-03-18 | 2000-09-26 | Medvill Co Ltd | 知母と黄蘖の混合水性抽出物を包含する、抗炎症及び鎮痛用薬剤組成物並びにその製法 |
| WO2001030359A1 (en) * | 1999-10-29 | 2001-05-03 | Triple Crown Ab | Cholesterol lowering and blood lipids lowering composition |
| CN1437973A (zh) * | 2003-01-23 | 2003-08-27 | 北京中医药大学 | 一种治疗缺血性脑中风的药物组合物及其制备方法 |
| JP2004331635A (ja) * | 2003-05-04 | 2004-11-25 | Kaicho Se | 細胞遣伝情報正常化材料及びその製法 |
| JP2008513382A (ja) * | 2004-09-17 | 2008-05-01 | インスティチュート オブ メディシナル バイオテクノロジー、 チャイニーズ アカデミー オブ メディカル サイエンシズ | 高脂血症を治療する方法及び組成物 |
Non-Patent Citations (3)
| Title |
|---|
| JPN6012023680; American Journal of Clinical Nutrition Vol.69, 1999, p.231-236 * |
| JPN7012001704; Nature Medicine Vol.10, No.12, 2004, p.1344-1351 * |
| JPN7012001705; Expert Opinion Investigational Drugs Vol.14, No.5, 2005, p.683-685 * |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2012524533A (ja) * | 2009-04-23 | 2012-10-18 | エス・アー・コルマン | 薬剤として使用するための減コレステロール酪農製品 |
| JP2022017496A (ja) * | 2015-11-13 | 2022-01-25 | シンセン ハイタイド バイオファーマシューティカル リミテッド | 組成物、ならびにその適用および薬学的調製の方法 |
| JP7448511B2 (ja) | 2015-11-13 | 2024-03-12 | シンセン ハイタイド バイオファーマシューティカル リミテッド | 組成物、ならびにその適用および薬学的調製の方法 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1983989B1 (en) | 2013-07-03 |
| DK1983989T3 (da) | 2013-10-14 |
| US20100239603A1 (en) | 2010-09-23 |
| CA2640414A1 (en) | 2007-08-16 |
| EP1983989A1 (en) | 2008-10-29 |
| EP1983989A4 (en) | 2009-07-08 |
| US8895079B2 (en) | 2014-11-25 |
| CA2640414C (en) | 2013-07-09 |
| WO2007090289A1 (en) | 2007-08-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US8895079B2 (en) | Combinations of botanical extracts for promoting cardiovascular health | |
| US9623108B2 (en) | Formulation for oral administration with beneficial effects on the cardiovascular system | |
| KR101840082B1 (ko) | 지질 대사 장애의 치료에 유용한 조성물 | |
| CN1124134C (zh) | 柚苷和柚苷配基作为肝病的预防或治疗剂的应用 | |
| JP5587780B2 (ja) | フコキサンチン又はこれを含有する海藻類抽出物を含有する脂質代謝性疾患の予防又は治療用組成物 | |
| JPH11500725A (ja) | ピペリンのバイオアベイラビリティ増強剤としての使用 | |
| KR20130035530A (ko) | 감귤과피 추출물 또는 나리루틴을 유효성분으로 포함하는 간기능 저해 억제용 조성물 및 감귤과피로부터 나리루틴을 추출하는 방법 | |
| KR101686777B1 (ko) | 감잎 추출물을 포함하는 혈액순환 장애 또는 대사성 질환의 예방 또는 치료용 약학적 조성물 | |
| KR101820096B1 (ko) | 콩 발아배아 추출물을 포함하는 대사성 질환의 예방 또는 치료용 약학 조성물 | |
| TWI359667B (en) | Formulation for oral administration having a healt | |
| KR20100088007A (ko) | 율피 추출물을 포함하는 지방간의 예방 또는 치료용 조성물 | |
| KR20010007625A (ko) | 디오스민을 포함하는 동맥경화증 및 고지혈증의 예방 및치료용 조성물 | |
| KR102568917B1 (ko) | 2S-2`메톡시쿠라리논(2S-2`methoxy-kurarinone)을 유효성분으로 포함하는, 심혈관질환 치료보조용 약학 조성물 | |
| KR101182046B1 (ko) | 오징어젓 또는 오징어를 유효성분으로 함유하는 비만 또는 고지혈증 및 동맥경화성 혈관계 질환의 예방 및 치료용 조성물 | |
| KR20240057723A (ko) | 일리마퀴논을 포함하는 지방간 질환 예방 또는 치료용 약학적 조성물 | |
| KR20070044198A (ko) | 푸마르산 및 푸마르산 유도체를 유효성분으로 함유하는대사증후군 치료제 | |
| US6787162B1 (en) | Method and composition for regulation of blood cholesterol | |
| US20080279968A1 (en) | Composition and method for inducing lipolysis and increasing the metabolism of free fatty acids | |
| KR20160074852A (ko) | 감잎 추출물을 포함하는 혈액순환 장애 또는 대사성 질환의 예방 또는 치료용 약학적 조성물 | |
| TW201300119A (zh) | 用於治療脂質代謝失調之組成物 | |
| KR20120114790A (ko) | 정향 에탄올 추출물의 용매 분획물을 유효성분으로 함유하는 고지혈증 예방 및 치료용 지질개선제 | |
| CA2588436A1 (en) | Composition and method for inducing lipolysis and increasing the metabolism of free fatty acids |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20100208 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20100208 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20120515 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20120815 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20120822 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20120918 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20121106 |