JP2004536783A - シグナル−1/シグナル−2二官能性ペプチド阻害剤 - Google Patents
シグナル−1/シグナル−2二官能性ペプチド阻害剤 Download PDFInfo
- Publication number
- JP2004536783A JP2004536783A JP2002552127A JP2002552127A JP2004536783A JP 2004536783 A JP2004536783 A JP 2004536783A JP 2002552127 A JP2002552127 A JP 2002552127A JP 2002552127 A JP2002552127 A JP 2002552127A JP 2004536783 A JP2004536783 A JP 2004536783A
- Authority
- JP
- Japan
- Prior art keywords
- peptide
- sequence
- signal
- amino acid
- group
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 108090000765 processed proteins & peptides Proteins 0.000 title claims abstract description 381
- 230000001588 bifunctional effect Effects 0.000 title description 3
- 239000003112 inhibitor Substances 0.000 title description 3
- 210000000612 antigen-presenting cell Anatomy 0.000 claims abstract description 87
- 230000028993 immune response Effects 0.000 claims abstract description 62
- 125000000539 amino acid group Chemical group 0.000 claims abstract description 60
- 108700018351 Major Histocompatibility Complex Proteins 0.000 claims abstract description 56
- 230000020382 suppression by virus of host antigen processing and presentation of peptide antigen via MHC class I Effects 0.000 claims abstract description 56
- 239000000816 peptidomimetic Substances 0.000 claims abstract description 32
- 230000029069 type 2 immune response Effects 0.000 claims abstract 4
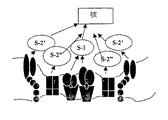
- 210000001744 T-lymphocyte Anatomy 0.000 claims description 132
- 102000016266 T-Cell Antigen Receptors Human genes 0.000 claims description 84
- 210000004027 cell Anatomy 0.000 claims description 84
- 238000000034 method Methods 0.000 claims description 68
- 102000004196 processed proteins & peptides Human genes 0.000 claims description 53
- 102000005962 receptors Human genes 0.000 claims description 52
- 108020003175 receptors Proteins 0.000 claims description 52
- 239000003446 ligand Substances 0.000 claims description 44
- 150000001413 amino acids Chemical class 0.000 claims description 40
- 201000010099 disease Diseases 0.000 claims description 29
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 claims description 29
- SLXKOJJOQWFEFD-UHFFFAOYSA-N 6-aminohexanoic acid Chemical group NCCCCCC(O)=O SLXKOJJOQWFEFD-UHFFFAOYSA-N 0.000 claims description 27
- 238000006243 chemical reaction Methods 0.000 claims description 21
- 230000004044 response Effects 0.000 claims description 21
- 239000000758 substrate Substances 0.000 claims description 18
- 108091008874 T cell receptors Proteins 0.000 claims description 16
- 229960002684 aminocaproic acid Drugs 0.000 claims description 15
- QUBNFZFTFXTLKH-UHFFFAOYSA-N 2-aminododecanoic acid Chemical compound CCCCCCCCCCC(N)C(O)=O QUBNFZFTFXTLKH-UHFFFAOYSA-N 0.000 claims description 10
- DHMQDGOQFOQNFH-UHFFFAOYSA-N Glycine Natural products NCC(O)=O DHMQDGOQFOQNFH-UHFFFAOYSA-N 0.000 claims description 10
- 230000015572 biosynthetic process Effects 0.000 claims description 9
- PBLZLIFKVPJDCO-UHFFFAOYSA-N omega-Aminododecanoic acid Natural products NCCCCCCCCCCCC(O)=O PBLZLIFKVPJDCO-UHFFFAOYSA-N 0.000 claims description 9
- 239000004471 Glycine Substances 0.000 claims description 8
- 125000003630 glycyl group Chemical group [H]N([H])C([H])([H])C(*)=O 0.000 claims description 8
- 230000000903 blocking effect Effects 0.000 claims description 7
- 230000036541 health Effects 0.000 claims description 6
- 230000001404 mediated effect Effects 0.000 claims description 6
- 230000000977 initiatory effect Effects 0.000 claims description 5
- 230000002194 synthesizing effect Effects 0.000 claims description 2
- 125000005647 linker group Chemical group 0.000 claims 11
- 108010075254 C-Peptide Proteins 0.000 claims 2
- 230000001268 conjugating effect Effects 0.000 claims 2
- 230000028996 humoral immune response Effects 0.000 claims 2
- 230000021633 leukocyte mediated immunity Effects 0.000 claims 1
- 108010092262 T-Cell Antigen Receptors Proteins 0.000 description 76
- 241000699670 Mus sp. Species 0.000 description 55
- 108010064593 Intercellular Adhesion Molecule-1 Proteins 0.000 description 42
- 102000015271 Intercellular Adhesion Molecule-1 Human genes 0.000 description 42
- 235000001014 amino acid Nutrition 0.000 description 39
- 230000036039 immunity Effects 0.000 description 33
- 102100022339 Integrin alpha-L Human genes 0.000 description 28
- 108010064548 Lymphocyte Function-Associated Antigen-1 Proteins 0.000 description 28
- 239000000427 antigen Substances 0.000 description 28
- 108091007433 antigens Proteins 0.000 description 27
- 102000036639 antigens Human genes 0.000 description 27
- 206010012601 diabetes mellitus Diseases 0.000 description 25
- 102000004127 Cytokines Human genes 0.000 description 23
- 108090000695 Cytokines Proteins 0.000 description 23
- 102100035857 Glutamate decarboxylase 2 Human genes 0.000 description 23
- 101000873786 Homo sapiens Glutamate decarboxylase 2 Proteins 0.000 description 23
- 102000004388 Interleukin-4 Human genes 0.000 description 23
- 108090000978 Interleukin-4 Proteins 0.000 description 23
- 241000282414 Homo sapiens Species 0.000 description 22
- 238000002474 experimental method Methods 0.000 description 22
- 239000002773 nucleotide Substances 0.000 description 22
- 125000003729 nucleotide group Chemical group 0.000 description 22
- 229940028885 interleukin-4 Drugs 0.000 description 21
- 210000004989 spleen cell Anatomy 0.000 description 21
- 108010002350 Interleukin-2 Proteins 0.000 description 20
- 102000000588 Interleukin-2 Human genes 0.000 description 20
- 102100036011 T-cell surface glycoprotein CD4 Human genes 0.000 description 20
- 238000011161 development Methods 0.000 description 19
- 230000018109 developmental process Effects 0.000 description 19
- DTQVDTLACAAQTR-UHFFFAOYSA-N Trifluoroacetic acid Chemical compound OC(=O)C(F)(F)F DTQVDTLACAAQTR-UHFFFAOYSA-N 0.000 description 18
- 238000012360 testing method Methods 0.000 description 18
- 241000699666 Mus <mouse, genus> Species 0.000 description 17
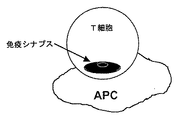
- 210000000225 synapse Anatomy 0.000 description 17
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 16
- 230000004913 activation Effects 0.000 description 16
- 239000011780 sodium chloride Substances 0.000 description 16
- 102100039498 Cytotoxic T-lymphocyte protein 4 Human genes 0.000 description 15
- 102000013462 Interleukin-12 Human genes 0.000 description 15
- 108010065805 Interleukin-12 Proteins 0.000 description 15
- 238000004458 analytical method Methods 0.000 description 15
- 108090000623 proteins and genes Proteins 0.000 description 15
- 108010029697 CD40 Ligand Proteins 0.000 description 14
- 102100032937 CD40 ligand Human genes 0.000 description 14
- 230000000694 effects Effects 0.000 description 14
- 101000914514 Homo sapiens T-cell-specific surface glycoprotein CD28 Proteins 0.000 description 13
- 108010074328 Interferon-gamma Proteins 0.000 description 13
- 102100027213 T-cell-specific surface glycoprotein CD28 Human genes 0.000 description 13
- 229940117681 interleukin-12 Drugs 0.000 description 13
- 238000003114 enzyme-linked immunosorbent spot assay Methods 0.000 description 12
- 230000008595 infiltration Effects 0.000 description 12
- 238000001764 infiltration Methods 0.000 description 12
- 238000002347 injection Methods 0.000 description 12
- 239000007924 injection Substances 0.000 description 12
- 230000003993 interaction Effects 0.000 description 12
- 230000011664 signaling Effects 0.000 description 12
- 208000023275 Autoimmune disease Diseases 0.000 description 11
- 101000889276 Homo sapiens Cytotoxic T-lymphocyte protein 4 Proteins 0.000 description 11
- 239000013566 allergen Substances 0.000 description 11
- 125000003275 alpha amino acid group Chemical group 0.000 description 11
- 230000000295 complement effect Effects 0.000 description 11
- 210000001151 cytotoxic T lymphocyte Anatomy 0.000 description 11
- 230000004069 differentiation Effects 0.000 description 11
- 230000001965 increasing effect Effects 0.000 description 11
- 238000006467 substitution reaction Methods 0.000 description 11
- 206010020751 Hypersensitivity Diseases 0.000 description 10
- 102100031988 Tumor necrosis factor ligand superfamily member 6 Human genes 0.000 description 10
- 206010067584 Type 1 diabetes mellitus Diseases 0.000 description 10
- UCMIRNVEIXFBKS-UHFFFAOYSA-N beta-alanine Chemical compound NCCC(O)=O UCMIRNVEIXFBKS-UHFFFAOYSA-N 0.000 description 10
- 238000001727 in vivo Methods 0.000 description 10
- 210000004698 lymphocyte Anatomy 0.000 description 10
- 239000000463 material Substances 0.000 description 10
- 230000007246 mechanism Effects 0.000 description 10
- 235000018102 proteins Nutrition 0.000 description 10
- 102000004169 proteins and genes Human genes 0.000 description 10
- 238000012546 transfer Methods 0.000 description 10
- 108010039471 Fas Ligand Protein Proteins 0.000 description 9
- 102100037850 Interferon gamma Human genes 0.000 description 9
- OKKJLVBELUTLKV-UHFFFAOYSA-N Methanol Chemical compound OC OKKJLVBELUTLKV-UHFFFAOYSA-N 0.000 description 9
- ZMXDDKWLCZADIW-UHFFFAOYSA-N N,N-Dimethylformamide Chemical compound CN(C)C=O ZMXDDKWLCZADIW-UHFFFAOYSA-N 0.000 description 9
- 206010028980 Neoplasm Diseases 0.000 description 9
- 230000000890 antigenic effect Effects 0.000 description 9
- -1 self-protein Substances 0.000 description 9
- 241001465754 Metazoa Species 0.000 description 8
- 230000007815 allergy Effects 0.000 description 8
- 210000003719 b-lymphocyte Anatomy 0.000 description 8
- 238000000684 flow cytometry Methods 0.000 description 8
- 229920001184 polypeptide Polymers 0.000 description 8
- 230000009258 tissue cross reactivity Effects 0.000 description 8
- 108700028369 Alleles Proteins 0.000 description 7
- 108091022930 Glutamate decarboxylase Proteins 0.000 description 7
- 238000003556 assay Methods 0.000 description 7
- 230000006870 function Effects 0.000 description 7
- 210000002540 macrophage Anatomy 0.000 description 7
- 230000004048 modification Effects 0.000 description 7
- 238000012986 modification Methods 0.000 description 7
- 230000035772 mutation Effects 0.000 description 7
- 244000052769 pathogen Species 0.000 description 7
- 102000040430 polynucleotide Human genes 0.000 description 7
- 108091033319 polynucleotide Proteins 0.000 description 7
- 239000002157 polynucleotide Substances 0.000 description 7
- 230000003827 upregulation Effects 0.000 description 7
- WEVYAHXRMPXWCK-UHFFFAOYSA-N Acetonitrile Chemical compound CC#N WEVYAHXRMPXWCK-UHFFFAOYSA-N 0.000 description 6
- 102100037904 CD9 antigen Human genes 0.000 description 6
- 102100035902 Glutamate decarboxylase 1 Human genes 0.000 description 6
- 230000006044 T cell activation Effects 0.000 description 6
- 201000011510 cancer Diseases 0.000 description 6
- 239000012636 effector Substances 0.000 description 6
- 210000002443 helper t lymphocyte Anatomy 0.000 description 6
- 210000000987 immune system Anatomy 0.000 description 6
- 238000000338 in vitro Methods 0.000 description 6
- 239000012678 infectious agent Substances 0.000 description 6
- 208000015181 infectious disease Diseases 0.000 description 6
- 230000002401 inhibitory effect Effects 0.000 description 6
- 238000004519 manufacturing process Methods 0.000 description 6
- 239000012528 membrane Substances 0.000 description 6
- 210000005087 mononuclear cell Anatomy 0.000 description 6
- 238000010647 peptide synthesis reaction Methods 0.000 description 6
- 230000008569 process Effects 0.000 description 6
- 230000001105 regulatory effect Effects 0.000 description 6
- 208000035473 Communicable disease Diseases 0.000 description 5
- 101001057504 Homo sapiens Interferon-stimulated gene 20 kDa protein Proteins 0.000 description 5
- 101001055144 Homo sapiens Interleukin-2 receptor subunit alpha Proteins 0.000 description 5
- 102100027268 Interferon-stimulated gene 20 kDa protein Human genes 0.000 description 5
- 108010090804 Streptavidin Proteins 0.000 description 5
- 108091023040 Transcription factor Proteins 0.000 description 5
- 102000040945 Transcription factor Human genes 0.000 description 5
- 208000006673 asthma Diseases 0.000 description 5
- 210000004369 blood Anatomy 0.000 description 5
- 239000008280 blood Substances 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 239000013078 crystal Substances 0.000 description 5
- 230000005764 inhibitory process Effects 0.000 description 5
- 238000010172 mouse model Methods 0.000 description 5
- 125000002924 primary amino group Chemical group [H]N([H])* 0.000 description 5
- 239000011347 resin Substances 0.000 description 5
- 229920005989 resin Polymers 0.000 description 5
- 239000000243 solution Substances 0.000 description 5
- IJGRMHOSHXDMSA-UHFFFAOYSA-N Atomic nitrogen Chemical compound N#N IJGRMHOSHXDMSA-UHFFFAOYSA-N 0.000 description 4
- 108010021064 CTLA-4 Antigen Proteins 0.000 description 4
- 229940045513 CTLA4 antagonist Drugs 0.000 description 4
- 102000008186 Collagen Human genes 0.000 description 4
- 108010035532 Collagen Proteins 0.000 description 4
- RTZKZFJDLAIYFH-UHFFFAOYSA-N Diethyl ether Chemical compound CCOCC RTZKZFJDLAIYFH-UHFFFAOYSA-N 0.000 description 4
- WSFSSNUMVMOOMR-UHFFFAOYSA-N Formaldehyde Chemical compound O=C WSFSSNUMVMOOMR-UHFFFAOYSA-N 0.000 description 4
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 4
- 208000001718 Immediate Hypersensitivity Diseases 0.000 description 4
- 241000725643 Respiratory syncytial virus Species 0.000 description 4
- 230000005867 T cell response Effects 0.000 description 4
- 206010045240 Type I hypersensitivity Diseases 0.000 description 4
- 206010053613 Type IV hypersensitivity reaction Diseases 0.000 description 4
- 208000026935 allergic disease Diseases 0.000 description 4
- 230000004075 alteration Effects 0.000 description 4
- RDOXTESZEPMUJZ-UHFFFAOYSA-N anisole Chemical compound COC1=CC=CC=C1 RDOXTESZEPMUJZ-UHFFFAOYSA-N 0.000 description 4
- 208000010216 atopic IgE responsiveness Diseases 0.000 description 4
- 229940000635 beta-alanine Drugs 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 230000001684 chronic effect Effects 0.000 description 4
- 229920001436 collagen Polymers 0.000 description 4
- 210000004443 dendritic cell Anatomy 0.000 description 4
- 230000030609 dephosphorylation Effects 0.000 description 4
- 238000006209 dephosphorylation reaction Methods 0.000 description 4
- 238000013461 design Methods 0.000 description 4
- 239000008103 glucose Substances 0.000 description 4
- 230000002163 immunogen Effects 0.000 description 4
- 210000000428 immunological synapse Anatomy 0.000 description 4
- 230000003834 intracellular effect Effects 0.000 description 4
- 239000006249 magnetic particle Substances 0.000 description 4
- 230000037361 pathway Effects 0.000 description 4
- 230000026731 phosphorylation Effects 0.000 description 4
- 238000006366 phosphorylation reaction Methods 0.000 description 4
- 230000001681 protective effect Effects 0.000 description 4
- 210000003289 regulatory T cell Anatomy 0.000 description 4
- 230000007781 signaling event Effects 0.000 description 4
- 210000001519 tissue Anatomy 0.000 description 4
- 230000005951 type IV hypersensitivity Effects 0.000 description 4
- 208000027930 type IV hypersensitivity disease Diseases 0.000 description 4
- KCXVZYZYPLLWCC-UHFFFAOYSA-N EDTA Chemical compound OC(=O)CN(CC(O)=O)CCN(CC(O)=O)CC(O)=O KCXVZYZYPLLWCC-UHFFFAOYSA-N 0.000 description 3
- 208000009386 Experimental Arthritis Diseases 0.000 description 3
- 241000711549 Hepacivirus C Species 0.000 description 3
- 108010027412 Histocompatibility Antigens Class II Proteins 0.000 description 3
- 102000018713 Histocompatibility Antigens Class II Human genes 0.000 description 3
- 241000282412 Homo Species 0.000 description 3
- 241000713772 Human immunodeficiency virus 1 Species 0.000 description 3
- 241000701806 Human papillomavirus Species 0.000 description 3
- 102000018697 Membrane Proteins Human genes 0.000 description 3
- 108010052285 Membrane Proteins Proteins 0.000 description 3
- 108010018525 NFATC Transcription Factors Proteins 0.000 description 3
- 102000002673 NFATC Transcription Factors Human genes 0.000 description 3
- 108091005804 Peptidases Proteins 0.000 description 3
- 239000004365 Protease Substances 0.000 description 3
- 102100037486 Reverse transcriptase/ribonuclease H Human genes 0.000 description 3
- 230000003213 activating effect Effects 0.000 description 3
- 238000013459 approach Methods 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000001363 autoimmune Effects 0.000 description 3
- 229910052799 carbon Inorganic materials 0.000 description 3
- 230000024245 cell differentiation Effects 0.000 description 3
- 150000001875 compounds Chemical class 0.000 description 3
- 230000021615 conjugation Effects 0.000 description 3
- 230000009089 cytolysis Effects 0.000 description 3
- 238000000432 density-gradient centrifugation Methods 0.000 description 3
- 238000001514 detection method Methods 0.000 description 3
- 238000002050 diffraction method Methods 0.000 description 3
- 230000002068 genetic effect Effects 0.000 description 3
- 201000001421 hyperglycemia Diseases 0.000 description 3
- 210000002865 immune cell Anatomy 0.000 description 3
- 230000002757 inflammatory effect Effects 0.000 description 3
- 230000003278 mimic effect Effects 0.000 description 3
- 238000000302 molecular modelling Methods 0.000 description 3
- 229910052757 nitrogen Inorganic materials 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 210000000496 pancreas Anatomy 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000035755 proliferation Effects 0.000 description 3
- 239000007787 solid Substances 0.000 description 3
- 241000894007 species Species 0.000 description 3
- 230000005945 translocation Effects 0.000 description 3
- 230000003612 virological effect Effects 0.000 description 3
- 125000003088 (fluoren-9-ylmethoxy)carbonyl group Chemical group 0.000 description 2
- NHBKXEKEPDILRR-UHFFFAOYSA-N 2,3-bis(butanoylsulfanyl)propyl butanoate Chemical compound CCCC(=O)OCC(SC(=O)CCC)CSC(=O)CCC NHBKXEKEPDILRR-UHFFFAOYSA-N 0.000 description 2
- KISWVXRQTGLFGD-UHFFFAOYSA-N 2-[[2-[[6-amino-2-[[2-[[2-[[5-amino-2-[[2-[[1-[2-[[6-amino-2-[(2,5-diamino-5-oxopentanoyl)amino]hexanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]pyrrolidine-2-carbonyl]amino]-3-hydroxypropanoyl]amino]-5-oxopentanoyl]amino]-5-(diaminomethylideneamino)p Chemical compound C1CCN(C(=O)C(CCCN=C(N)N)NC(=O)C(CCCCN)NC(=O)C(N)CCC(N)=O)C1C(=O)NC(CO)C(=O)NC(CCC(N)=O)C(=O)NC(CCCN=C(N)N)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(C(=O)NC(CC(C)C)C(O)=O)CC1=CC=C(O)C=C1 KISWVXRQTGLFGD-UHFFFAOYSA-N 0.000 description 2
- 206010003645 Atopy Diseases 0.000 description 2
- 241000894006 Bacteria Species 0.000 description 2
- 101150013553 CD40 gene Proteins 0.000 description 2
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 description 2
- 108010069514 Cyclic Peptides Proteins 0.000 description 2
- 102000001189 Cyclic Peptides Human genes 0.000 description 2
- 238000011510 Elispot assay Methods 0.000 description 2
- 102000013446 GTP Phosphohydrolases Human genes 0.000 description 2
- 108091006109 GTPases Proteins 0.000 description 2
- 101001066288 Gallus gallus GATA-binding factor 3 Proteins 0.000 description 2
- 102000016285 Guanine Nucleotide Exchange Factors Human genes 0.000 description 2
- 108010067218 Guanine Nucleotide Exchange Factors Proteins 0.000 description 2
- WZUVPPKBWHMQCE-UHFFFAOYSA-N Haematoxylin Chemical compound C12=CC(O)=C(O)C=C2CC2(O)C1C1=CC=C(O)C(O)=C1OC2 WZUVPPKBWHMQCE-UHFFFAOYSA-N 0.000 description 2
- 101001018097 Homo sapiens L-selectin Proteins 0.000 description 2
- 101000979342 Homo sapiens Nuclear factor NF-kappa-B p105 subunit Proteins 0.000 description 2
- 101000716102 Homo sapiens T-cell surface glycoprotein CD4 Proteins 0.000 description 2
- 241000725303 Human immunodeficiency virus Species 0.000 description 2
- 206010061218 Inflammation Diseases 0.000 description 2
- 102000008070 Interferon-gamma Human genes 0.000 description 2
- 102000004560 Interleukin-12 Receptors Human genes 0.000 description 2
- 108010017515 Interleukin-12 Receptors Proteins 0.000 description 2
- 102100033467 L-selectin Human genes 0.000 description 2
- OUYCCCASQSFEME-QMMMGPOBSA-N L-tyrosine Chemical compound OC(=O)[C@@H](N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-QMMMGPOBSA-N 0.000 description 2
- 239000000232 Lipid Bilayer Substances 0.000 description 2
- 102000045595 Phosphoprotein Phosphatases Human genes 0.000 description 2
- 108700019535 Phosphoprotein Phosphatases Proteins 0.000 description 2
- 108091000080 Phosphotransferase Proteins 0.000 description 2
- 238000012300 Sequence Analysis Methods 0.000 description 2
- MTCFGRXMJLQNBG-UHFFFAOYSA-N Serine Natural products OCC(N)C(O)=O MTCFGRXMJLQNBG-UHFFFAOYSA-N 0.000 description 2
- XUIMIQQOPSSXEZ-UHFFFAOYSA-N Silicon Chemical compound [Si] XUIMIQQOPSSXEZ-UHFFFAOYSA-N 0.000 description 2
- 230000024932 T cell mediated immunity Effects 0.000 description 2
- AYFVYJQAPQTCCC-UHFFFAOYSA-N Threonine Natural products CC(O)C(N)C(O)=O AYFVYJQAPQTCCC-UHFFFAOYSA-N 0.000 description 2
- 239000004473 Threonine Substances 0.000 description 2
- 102100040245 Tumor necrosis factor receptor superfamily member 5 Human genes 0.000 description 2
- 108091005764 adaptor proteins Proteins 0.000 description 2
- 102000035181 adaptor proteins Human genes 0.000 description 2
- 230000000172 allergic effect Effects 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- 208000010668 atopic eczema Diseases 0.000 description 2
- 230000006472 autoimmune response Effects 0.000 description 2
- 210000004899 c-terminal region Anatomy 0.000 description 2
- 230000003915 cell function Effects 0.000 description 2
- 230000011748 cell maturation Effects 0.000 description 2
- 210000000170 cell membrane Anatomy 0.000 description 2
- 230000006957 competitive inhibition Effects 0.000 description 2
- 238000012790 confirmation Methods 0.000 description 2
- 238000007796 conventional method Methods 0.000 description 2
- 235000018417 cysteine Nutrition 0.000 description 2
- XUJNEKJLAYXESH-UHFFFAOYSA-N cysteine Natural products SCC(N)C(O)=O XUJNEKJLAYXESH-UHFFFAOYSA-N 0.000 description 2
- 230000001461 cytolytic effect Effects 0.000 description 2
- 239000007857 degradation product Substances 0.000 description 2
- 238000012217 deletion Methods 0.000 description 2
- 230000037430 deletion Effects 0.000 description 2
- 238000001212 derivatisation Methods 0.000 description 2
- 238000010790 dilution Methods 0.000 description 2
- 239000012895 dilution Substances 0.000 description 2
- 229940079593 drug Drugs 0.000 description 2
- 239000003814 drug Substances 0.000 description 2
- 230000008278 dynamic mechanism Effects 0.000 description 2
- 201000002491 encephalomyelitis Diseases 0.000 description 2
- 230000017188 evasion or tolerance of host immune response Effects 0.000 description 2
- 230000004907 flux Effects 0.000 description 2
- 238000004108 freeze drying Methods 0.000 description 2
- 230000012010 growth Effects 0.000 description 2
- 230000004727 humoral immunity Effects 0.000 description 2
- 125000001165 hydrophobic group Chemical group 0.000 description 2
- 230000003053 immunization Effects 0.000 description 2
- 238000002649 immunization Methods 0.000 description 2
- 230000020287 immunological synapse formation Effects 0.000 description 2
- 230000004957 immunoregulator effect Effects 0.000 description 2
- 238000010348 incorporation Methods 0.000 description 2
- 238000011534 incubation Methods 0.000 description 2
- 230000006698 induction Effects 0.000 description 2
- 230000002458 infectious effect Effects 0.000 description 2
- 230000004054 inflammatory process Effects 0.000 description 2
- 239000003999 initiator Substances 0.000 description 2
- NOESYZHRGYRDHS-UHFFFAOYSA-N insulin Chemical compound N1C(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(NC(=O)CN)C(C)CC)CSSCC(C(NC(CO)C(=O)NC(CC(C)C)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CCC(N)=O)C(=O)NC(CC(C)C)C(=O)NC(CCC(O)=O)C(=O)NC(CC(N)=O)C(=O)NC(CC=2C=CC(O)=CC=2)C(=O)NC(CSSCC(NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2C=CC(O)=CC=2)NC(=O)C(CC(C)C)NC(=O)C(C)NC(=O)C(CCC(O)=O)NC(=O)C(C(C)C)NC(=O)C(CC(C)C)NC(=O)C(CC=2NC=NC=2)NC(=O)C(CO)NC(=O)CNC2=O)C(=O)NCC(=O)NC(CCC(O)=O)C(=O)NC(CCCNC(N)=N)C(=O)NCC(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC=CC=3)C(=O)NC(CC=3C=CC(O)=CC=3)C(=O)NC(C(C)O)C(=O)N3C(CCC3)C(=O)NC(CCCCN)C(=O)NC(C)C(O)=O)C(=O)NC(CC(N)=O)C(O)=O)=O)NC(=O)C(C(C)CC)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C1CSSCC2NC(=O)C(CC(C)C)NC(=O)C(NC(=O)C(CCC(N)=O)NC(=O)C(CC(N)=O)NC(=O)C(NC(=O)C(N)CC=1C=CC=CC=1)C(C)C)CC1=CN=CN1 NOESYZHRGYRDHS-UHFFFAOYSA-N 0.000 description 2
- 229960003130 interferon gamma Drugs 0.000 description 2
- 230000017307 interleukin-4 production Effects 0.000 description 2
- 238000001990 intravenous administration Methods 0.000 description 2
- 230000009545 invasion Effects 0.000 description 2
- 210000004153 islets of langerhan Anatomy 0.000 description 2
- 230000000670 limiting effect Effects 0.000 description 2
- 210000001165 lymph node Anatomy 0.000 description 2
- 238000004949 mass spectrometry Methods 0.000 description 2
- 239000002609 medium Substances 0.000 description 2
- UZKWTJUDCOPSNM-UHFFFAOYSA-N methoxybenzene Substances CCCCOC=C UZKWTJUDCOPSNM-UHFFFAOYSA-N 0.000 description 2
- 239000000203 mixture Substances 0.000 description 2
- 238000003032 molecular docking Methods 0.000 description 2
- 238000000329 molecular dynamics simulation Methods 0.000 description 2
- 239000013642 negative control Substances 0.000 description 2
- 230000003472 neutralizing effect Effects 0.000 description 2
- 239000012188 paraffin wax Substances 0.000 description 2
- 244000045947 parasite Species 0.000 description 2
- 230000036961 partial effect Effects 0.000 description 2
- 210000003819 peripheral blood mononuclear cell Anatomy 0.000 description 2
- 102000020233 phosphotransferase Human genes 0.000 description 2
- 238000002953 preparative HPLC Methods 0.000 description 2
- 230000002829 reductive effect Effects 0.000 description 2
- 238000004007 reversed phase HPLC Methods 0.000 description 2
- 206010039073 rheumatoid arthritis Diseases 0.000 description 2
- 229910052710 silicon Inorganic materials 0.000 description 2
- 239000010703 silicon Substances 0.000 description 2
- 239000000126 substance Substances 0.000 description 2
- 238000003786 synthesis reaction Methods 0.000 description 2
- HNKJADCVZUBCPG-UHFFFAOYSA-N thioanisole Chemical compound CSC1=CC=CC=C1 HNKJADCVZUBCPG-UHFFFAOYSA-N 0.000 description 2
- 238000004448 titration Methods 0.000 description 2
- 238000002054 transplantation Methods 0.000 description 2
- OUYCCCASQSFEME-UHFFFAOYSA-N tyrosine Natural products OC(=O)C(N)CC1=CC=C(O)C=C1 OUYCCCASQSFEME-UHFFFAOYSA-N 0.000 description 2
- YMXHPSHLTSZXKH-RVBZMBCESA-N (2,5-dioxopyrrolidin-1-yl) 5-[(3as,4s,6ar)-2-oxo-1,3,3a,4,6,6a-hexahydrothieno[3,4-d]imidazol-4-yl]pentanoate Chemical compound C([C@H]1[C@H]2NC(=O)N[C@H]2CS1)CCCC(=O)ON1C(=O)CCC1=O YMXHPSHLTSZXKH-RVBZMBCESA-N 0.000 description 1
- LJRDOKAZOAKLDU-UDXJMMFXSA-N (2s,3s,4r,5r,6r)-5-amino-2-(aminomethyl)-6-[(2r,3s,4r,5s)-5-[(1r,2r,3s,5r,6s)-3,5-diamino-2-[(2s,3r,4r,5s,6r)-3-amino-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-hydroxycyclohexyl]oxy-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxyoxane-3,4-diol;sulfuric ac Chemical compound OS(O)(=O)=O.N[C@@H]1[C@@H](O)[C@H](O)[C@H](CN)O[C@@H]1O[C@H]1[C@@H](O)[C@H](O[C@H]2[C@@H]([C@@H](N)C[C@@H](N)[C@@H]2O)O[C@@H]2[C@@H]([C@@H](O)[C@H](O)[C@@H](CO)O2)N)O[C@@H]1CO LJRDOKAZOAKLDU-UDXJMMFXSA-N 0.000 description 1
- OEBIVOHKFYSBPE-UHFFFAOYSA-N 4-Benzyloxybenzyl alcohol Chemical compound C1=CC(CO)=CC=C1OCC1=CC=CC=C1 OEBIVOHKFYSBPE-UHFFFAOYSA-N 0.000 description 1
- QRXMUCSWCMTJGU-UHFFFAOYSA-N 5-bromo-4-chloro-3-indolyl phosphate Chemical compound C1=C(Br)C(Cl)=C2C(OP(O)(=O)O)=CNC2=C1 QRXMUCSWCMTJGU-UHFFFAOYSA-N 0.000 description 1
- 208000030507 AIDS Diseases 0.000 description 1
- 102000002260 Alkaline Phosphatase Human genes 0.000 description 1
- 108020004774 Alkaline Phosphatase Proteins 0.000 description 1
- 206010027654 Allergic conditions Diseases 0.000 description 1
- 208000032116 Autoimmune Experimental Encephalomyelitis Diseases 0.000 description 1
- 108010037234 BPI peptide Proteins 0.000 description 1
- 206010006448 Bronchiolitis Diseases 0.000 description 1
- 102000000844 Cell Surface Receptors Human genes 0.000 description 1
- 108010001857 Cell Surface Receptors Proteins 0.000 description 1
- 108010062580 Concanavalin A Proteins 0.000 description 1
- QNAYBMKLOCPYGJ-UHFFFAOYSA-N D-alpha-Ala Natural products CC([NH3+])C([O-])=O QNAYBMKLOCPYGJ-UHFFFAOYSA-N 0.000 description 1
- 108020004414 DNA Proteins 0.000 description 1
- 108010009900 Endothelial Protein C Receptor Proteins 0.000 description 1
- 102100030024 Endothelial protein C receptor Human genes 0.000 description 1
- 102000008214 Glutamate decarboxylase Human genes 0.000 description 1
- 208000031886 HIV Infections Diseases 0.000 description 1
- 108010046732 HLA-DR4 Antigen Proteins 0.000 description 1
- 208000005176 Hepatitis C Diseases 0.000 description 1
- 101001002657 Homo sapiens Interleukin-2 Proteins 0.000 description 1
- 101000611023 Homo sapiens Tumor necrosis factor receptor superfamily member 6 Proteins 0.000 description 1
- 102000004877 Insulin Human genes 0.000 description 1
- 108090001061 Insulin Proteins 0.000 description 1
- 108090000174 Interleukin-10 Proteins 0.000 description 1
- 102000003814 Interleukin-10 Human genes 0.000 description 1
- QNAYBMKLOCPYGJ-REOHCLBHSA-N L-alanine Chemical compound C[C@H](N)C(O)=O QNAYBMKLOCPYGJ-REOHCLBHSA-N 0.000 description 1
- 241000699660 Mus musculus Species 0.000 description 1
- 101000924587 Mus musculus Adenomatous polyposis coli protein Proteins 0.000 description 1
- 101001044384 Mus musculus Interferon gamma Proteins 0.000 description 1
- 101001002703 Mus musculus Interleukin-4 Proteins 0.000 description 1
- 102000047918 Myelin Basic Human genes 0.000 description 1
- 101710107068 Myelin basic protein Proteins 0.000 description 1
- 238000005481 NMR spectroscopy Methods 0.000 description 1
- 102100023050 Nuclear factor NF-kappa-B p105 subunit Human genes 0.000 description 1
- 241000283973 Oryctolagus cuniculus Species 0.000 description 1
- 102000004160 Phosphoric Monoester Hydrolases Human genes 0.000 description 1
- 108090000608 Phosphoric Monoester Hydrolases Proteins 0.000 description 1
- 229920001213 Polysorbate 20 Polymers 0.000 description 1
- 239000004793 Polystyrene Substances 0.000 description 1
- 101800001357 Potential peptide Proteins 0.000 description 1
- 102400000745 Potential peptide Human genes 0.000 description 1
- 108020004511 Recombinant DNA Proteins 0.000 description 1
- 238000000692 Student's t-test Methods 0.000 description 1
- 230000006052 T cell proliferation Effects 0.000 description 1
- 206010052779 Transplant rejections Diseases 0.000 description 1
- 108050002568 Tumor necrosis factor ligand superfamily member 6 Proteins 0.000 description 1
- 102100040403 Tumor necrosis factor receptor superfamily member 6 Human genes 0.000 description 1
- 239000003875 Wang resin Substances 0.000 description 1
- NERFNHBZJXXFGY-UHFFFAOYSA-N [4-[(4-methylphenyl)methoxy]phenyl]methanol Chemical compound C1=CC(C)=CC=C1COC1=CC=C(CO)C=C1 NERFNHBZJXXFGY-UHFFFAOYSA-N 0.000 description 1
- 230000005856 abnormality Effects 0.000 description 1
- 238000009825 accumulation Methods 0.000 description 1
- 125000002777 acetyl group Chemical group [H]C([H])([H])C(*)=O 0.000 description 1
- 108010042591 activated protein C receptor Proteins 0.000 description 1
- 235000004279 alanine Nutrition 0.000 description 1
- 125000003368 amide group Chemical group 0.000 description 1
- 230000006229 amino acid addition Effects 0.000 description 1
- 230000003321 amplification Effects 0.000 description 1
- 238000000540 analysis of variance Methods 0.000 description 1
- 230000003042 antagnostic effect Effects 0.000 description 1
- 230000030741 antigen processing and presentation Effects 0.000 description 1
- 230000001640 apoptogenic effect Effects 0.000 description 1
- 230000006907 apoptotic process Effects 0.000 description 1
- 230000004888 barrier function Effects 0.000 description 1
- 210000000227 basophil cell of anterior lobe of hypophysis Anatomy 0.000 description 1
- 230000008901 benefit Effects 0.000 description 1
- 230000033228 biological regulation Effects 0.000 description 1
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N biotin Natural products N1C(=O)N[C@@H]2[C@H](CCCCC(=O)O)SC[C@@H]21 YBJHBAHKTGYVGT-ZKWXMUAHSA-N 0.000 description 1
- 125000003178 carboxy group Chemical group [H]OC(*)=O 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 230000004709 cell invasion Effects 0.000 description 1
- 239000002771 cell marker Substances 0.000 description 1
- 239000006285 cell suspension Substances 0.000 description 1
- 230000001413 cellular effect Effects 0.000 description 1
- 230000007969 cellular immunity Effects 0.000 description 1
- 230000005754 cellular signaling Effects 0.000 description 1
- 239000001913 cellulose Substances 0.000 description 1
- 229920002678 cellulose Polymers 0.000 description 1
- 125000003636 chemical group Chemical group 0.000 description 1
- 238000007385 chemical modification Methods 0.000 description 1
- 239000003795 chemical substances by application Substances 0.000 description 1
- 229910052804 chromium Inorganic materials 0.000 description 1
- 239000011651 chromium Substances 0.000 description 1
- 238000010367 cloning Methods 0.000 description 1
- 238000004590 computer program Methods 0.000 description 1
- 238000010276 construction Methods 0.000 description 1
- 230000001276 controlling effect Effects 0.000 description 1
- 230000004940 costimulation Effects 0.000 description 1
- 230000000139 costimulatory effect Effects 0.000 description 1
- 238000012258 culturing Methods 0.000 description 1
- 230000001186 cumulative effect Effects 0.000 description 1
- 230000016396 cytokine production Effects 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 125000001295 dansyl group Chemical group [H]C1=C([H])C(N(C([H])([H])[H])C([H])([H])[H])=C2C([H])=C([H])C([H])=C(C2=C1[H])S(*)(=O)=O 0.000 description 1
- 230000007123 defense Effects 0.000 description 1
- 238000006731 degradation reaction Methods 0.000 description 1
- 238000000586 desensitisation Methods 0.000 description 1
- 235000012489 doughnuts Nutrition 0.000 description 1
- 230000009977 dual effect Effects 0.000 description 1
- 210000003162 effector t lymphocyte Anatomy 0.000 description 1
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000007613 environmental effect Effects 0.000 description 1
- YQGOJNYOYNNSMM-UHFFFAOYSA-N eosin Chemical compound [Na+].OC(=O)C1=CC=CC=C1C1=C2C=C(Br)C(=O)C(Br)=C2OC2=C(Br)C(O)=C(Br)C=C21 YQGOJNYOYNNSMM-UHFFFAOYSA-N 0.000 description 1
- 150000002148 esters Chemical class 0.000 description 1
- 230000001747 exhibiting effect Effects 0.000 description 1
- 238000013213 extrapolation Methods 0.000 description 1
- 238000005206 flow analysis Methods 0.000 description 1
- 238000000799 fluorescence microscopy Methods 0.000 description 1
- 239000007850 fluorescent dye Substances 0.000 description 1
- 235000013305 food Nutrition 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 230000013632 homeostatic process Effects 0.000 description 1
- 230000007236 host immunity Effects 0.000 description 1
- 244000052637 human pathogen Species 0.000 description 1
- 229940042795 hydrazides for tuberculosis treatment Drugs 0.000 description 1
- 238000010191 image analysis Methods 0.000 description 1
- 230000002519 immonomodulatory effect Effects 0.000 description 1
- 230000008088 immune pathway Effects 0.000 description 1
- 230000002134 immunopathologic effect Effects 0.000 description 1
- 230000001024 immunotherapeutic effect Effects 0.000 description 1
- 238000009169 immunotherapy Methods 0.000 description 1
- 230000006872 improvement Effects 0.000 description 1
- 238000005462 in vivo assay Methods 0.000 description 1
- 230000001939 inductive effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 229940125396 insulin Drugs 0.000 description 1
- 230000019734 interleukin-12 production Effects 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- 238000005304 joining Methods 0.000 description 1
- 210000000265 leukocyte Anatomy 0.000 description 1
- 230000007774 longterm Effects 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 238000001840 matrix-assisted laser desorption--ionisation time-of-flight mass spectrometry Methods 0.000 description 1
- 201000001441 melanoma Diseases 0.000 description 1
- 238000007431 microscopic evaluation Methods 0.000 description 1
- 239000003226 mitogen Substances 0.000 description 1
- 238000010369 molecular cloning Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 201000006417 multiple sclerosis Diseases 0.000 description 1
- 230000000869 mutational effect Effects 0.000 description 1
- 210000000653 nervous system Anatomy 0.000 description 1
- 230000007935 neutral effect Effects 0.000 description 1
- 230000001019 normoglycemic effect Effects 0.000 description 1
- 238000003199 nucleic acid amplification method Methods 0.000 description 1
- 102000039446 nucleic acids Human genes 0.000 description 1
- 108020004707 nucleic acids Proteins 0.000 description 1
- 150000007523 nucleic acids Chemical class 0.000 description 1
- TVMXDCGIABBOFY-UHFFFAOYSA-N octane Chemical compound CCCCCCCC TVMXDCGIABBOFY-UHFFFAOYSA-N 0.000 description 1
- 230000001717 pathogenic effect Effects 0.000 description 1
- 229960001639 penicillamine Drugs 0.000 description 1
- 210000005105 peripheral blood lymphocyte Anatomy 0.000 description 1
- 239000000256 polyoxyethylene sorbitan monolaurate Substances 0.000 description 1
- 235000010486 polyoxyethylene sorbitan monolaurate Nutrition 0.000 description 1
- 229920000136 polysorbate Polymers 0.000 description 1
- 229920002223 polystyrene Polymers 0.000 description 1
- 239000013641 positive control Substances 0.000 description 1
- 230000037452 priming Effects 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000006916 protein interaction Effects 0.000 description 1
- 230000017854 proteolysis Effects 0.000 description 1
- 230000002797 proteolythic effect Effects 0.000 description 1
- 230000008707 rearrangement Effects 0.000 description 1
- 229940045847 receptor mimetic Drugs 0.000 description 1
- 230000006798 recombination Effects 0.000 description 1
- 238000005215 recombination Methods 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 150000003839 salts Chemical class 0.000 description 1
- 239000004576 sand Substances 0.000 description 1
- 238000010187 selection method Methods 0.000 description 1
- 150000003349 semicarbazides Chemical class 0.000 description 1
- 238000000926 separation method Methods 0.000 description 1
- 230000019491 signal transduction Effects 0.000 description 1
- 238000004088 simulation Methods 0.000 description 1
- 239000012453 solvate Substances 0.000 description 1
- 210000000952 spleen Anatomy 0.000 description 1
- 230000002269 spontaneous effect Effects 0.000 description 1
- 238000010186 staining Methods 0.000 description 1
- 238000010561 standard procedure Methods 0.000 description 1
- 230000004936 stimulating effect Effects 0.000 description 1
- 230000008093 supporting effect Effects 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 230000008685 targeting Effects 0.000 description 1
- 239000003104 tissue culture media Substances 0.000 description 1
- 230000009261 transgenic effect Effects 0.000 description 1
- 238000011830 transgenic mouse model Methods 0.000 description 1
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 1
- 230000003442 weekly effect Effects 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K39/0005—Vertebrate antigens
- A61K39/001—Preparations to induce tolerance to non-self, e.g. prior to transplantation
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K14/00—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof
- C07K14/435—Peptides having more than 20 amino acids; Gastrins; Somatostatins; Melanotropins; Derivatives thereof from animals; from humans
- C07K14/705—Receptors; Cell surface antigens; Cell surface determinants
- C07K14/70546—Integrin superfamily
- C07K14/70553—Integrin beta2-subunit-containing molecules, e.g. CD11, CD18
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2821—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against ICAM molecules, e.g. CD50, CD54, CD102
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K16/00—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies
- C07K16/18—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans
- C07K16/28—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants
- C07K16/2803—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily
- C07K16/2833—Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies against material from animals or humans against receptors, cell surface antigens or cell surface determinants against the immunoglobulin superfamily against MHC-molecules, e.g. HLA-molecules
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N9/00—Enzymes; Proenzymes; Compositions thereof; Processes for preparing, activating, inhibiting, separating or purifying enzymes
- C12N9/88—Lyases (4.)
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K39/00—Medicinal preparations containing antigens or antibodies
- A61K2039/57—Medicinal preparations containing antigens or antibodies characterised by the type of response, e.g. Th1, Th2
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07K—PEPTIDES
- C07K2319/00—Fusion polypeptide
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Immunology (AREA)
- Organic Chemistry (AREA)
- Medicinal Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Genetics & Genomics (AREA)
- Molecular Biology (AREA)
- Biochemistry (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Zoology (AREA)
- Biophysics (AREA)
- Engineering & Computer Science (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Microbiology (AREA)
- Wood Science & Technology (AREA)
- Public Health (AREA)
- Animal Behavior & Ethology (AREA)
- Pharmacology & Pharmacy (AREA)
- Veterinary Medicine (AREA)
- Transplantation (AREA)
- Epidemiology (AREA)
- Gastroenterology & Hepatology (AREA)
- Cell Biology (AREA)
- Mycology (AREA)
- Biomedical Technology (AREA)
- Biotechnology (AREA)
- Toxicology (AREA)
- General Engineering & Computer Science (AREA)
- Chemical Kinetics & Catalysis (AREA)
- General Chemical & Material Sciences (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Peptides Or Proteins (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/739,466 US7786257B2 (en) | 2000-12-18 | 2000-12-18 | Signal-1/signal-2 bifunctional peptide inhibitors |
| PCT/US2001/048632 WO2002050250A2 (en) | 2000-12-18 | 2001-12-17 | Signal-1/signal-2 bifunctional peptide inhibitors |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004536783A true JP2004536783A (ja) | 2004-12-09 |
| JP2004536783A5 JP2004536783A5 (enExample) | 2005-06-09 |
Family
ID=24972431
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002552127A Pending JP2004536783A (ja) | 2000-12-18 | 2001-12-17 | シグナル−1/シグナル−2二官能性ペプチド阻害剤 |
Country Status (8)
| Country | Link |
|---|---|
| US (1) | US7786257B2 (enExample) |
| EP (2) | EP1404362B1 (enExample) |
| JP (1) | JP2004536783A (enExample) |
| AT (1) | ATE400293T1 (enExample) |
| AU (1) | AU2002230911A1 (enExample) |
| CA (1) | CA2432767A1 (enExample) |
| DE (1) | DE60134791D1 (enExample) |
| WO (1) | WO2002050250A2 (enExample) |
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017061490A (ja) * | 2011-10-18 | 2017-03-30 | 株式会社アモーレパシフィックAmorepacific Corporation | シリンガレシノールを含むsirt−1活性化剤 |
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8188218B2 (en) | 2006-10-27 | 2012-05-29 | University Of Kansas | Bi-functional peptides for multiple sclerosis treatment and diagnosis |
| US8735154B2 (en) | 2006-10-30 | 2014-05-27 | The University Of Kansas | Templated islet cells and small islet cell clusters for diabetes treatment |
| US8016856B2 (en) * | 2006-11-30 | 2011-09-13 | Cook Medical Technologies Llc | Removable handle for medical device |
| GB0710529D0 (en) | 2007-06-01 | 2007-07-11 | Circassia Ltd | Vaccine |
| ES2402956T3 (es) | 2007-08-15 | 2013-05-10 | Circassia Limited | Péptido con formación de dímeros reducida |
| EP2211889B1 (en) * | 2007-10-25 | 2014-08-20 | The Scripps Research Institute | Antibody-mediated disruption of quorum sensing in bacteria |
| DE102009040716B4 (de) * | 2009-09-10 | 2011-07-14 | Miltenyi Biotec GmbH, 51429 | Verwendung von CD154 zur Identifizierung und Abtrennung von nicht-regulatorischen T-Zellen aus einem Gemisch mit regulatorischen T-Zellen |
| WO2011163568A1 (en) | 2010-06-24 | 2011-12-29 | University Of Kansas | Conjugates comprising an n-oxime bond and associated methods |
| WO2013016544A2 (en) | 2011-07-27 | 2013-01-31 | University Of Kansas | Templated islet cells and small islet cell clusters for diabetes treatment |
| US11919939B2 (en) * | 2017-05-23 | 2024-03-05 | Université De Genève | Beta-2-glycoprotein 1 derived peptide and use thereof for treating antiphospholipid syndrome |
Family Cites Families (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5844095A (en) * | 1991-06-27 | 1998-12-01 | Bristol-Myers Squibb Company | CTLA4 Ig fusion proteins |
| EP0576842B1 (de) * | 1992-06-11 | 2002-10-16 | MERCK PATENT GmbH | Verfahren und Mittel zum Nachweis von Listerien |
| US5540926A (en) | 1992-09-04 | 1996-07-30 | Bristol-Myers Squibb Company | Soluble and its use in B cell stimulation |
| ATE417112T1 (de) | 1997-03-27 | 2008-12-15 | Csl Ltd | Steigerung der immunantwort unter verwendung von zielgerichteten molekülen |
| US7118751B1 (en) * | 1999-10-14 | 2006-10-10 | Trubion Pharmaceuticals, Inc. | DNA vaccines encoding antigen linked to a domain that binds CD40 |
-
2000
- 2000-12-18 US US09/739,466 patent/US7786257B2/en not_active Expired - Fee Related
-
2001
- 2001-12-17 EP EP01991168A patent/EP1404362B1/en not_active Expired - Lifetime
- 2001-12-17 AU AU2002230911A patent/AU2002230911A1/en not_active Abandoned
- 2001-12-17 DE DE60134791T patent/DE60134791D1/de not_active Expired - Fee Related
- 2001-12-17 EP EP07014573A patent/EP1932539A3/en not_active Withdrawn
- 2001-12-17 AT AT01991168T patent/ATE400293T1/de not_active IP Right Cessation
- 2001-12-17 WO PCT/US2001/048632 patent/WO2002050250A2/en not_active Ceased
- 2001-12-17 CA CA002432767A patent/CA2432767A1/en not_active Abandoned
- 2001-12-17 JP JP2002552127A patent/JP2004536783A/ja active Pending
Cited By (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2017061490A (ja) * | 2011-10-18 | 2017-03-30 | 株式会社アモーレパシフィックAmorepacific Corporation | シリンガレシノールを含むsirt−1活性化剤 |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1404362A2 (en) | 2004-04-07 |
| EP1404362A4 (en) | 2005-06-01 |
| ATE400293T1 (de) | 2008-07-15 |
| WO2002050250A2 (en) | 2002-06-27 |
| WO2002050250A3 (en) | 2004-01-08 |
| DE60134791D1 (de) | 2008-08-21 |
| EP1404362B1 (en) | 2008-07-09 |
| EP1932539A2 (en) | 2008-06-18 |
| CA2432767A1 (en) | 2002-06-27 |
| EP1932539A3 (en) | 2008-10-29 |
| US7786257B2 (en) | 2010-08-31 |
| AU2002230911A1 (en) | 2002-07-01 |
| US20050107585A1 (en) | 2005-05-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Chaplin | 1. Overview of the immune response | |
| US8895020B2 (en) | Single chain trimers and uses therefor | |
| US8518697B2 (en) | Single chain trimers and uses therefor | |
| ES2278390T3 (es) | Sistema de presentacion de antigenos de clase ii de cmh y procedimientos de activacion de linfocitos t cd4+. | |
| US10035857B2 (en) | Regulatory T cell mediator proteins and uses thereof | |
| US6984625B2 (en) | Methods and compositions for modulating autoimmunity | |
| US8349320B2 (en) | Compositions and methods for modulating responses mediated or associated with BTLA activity | |
| JP2001508430A (ja) | 免疫抑制因子のエンドサイトーシスによる提示のための化合物、組成物及び方法 | |
| US20230015969A1 (en) | Regulatory t cell mediator proteins and uses thereof | |
| EP1404362B1 (en) | Signal-1/signal-2 bifunctional peptide inhibitors | |
| US11701387B2 (en) | Chimeric antigen receptor specific for BDCA2 antigen | |
| TW202426486A (zh) | 使用針對抑制型kir的促效劑的免疫排斥之迴避方法 | |
| WO2016131911A1 (en) | Methods for the treatment and diagnosis of type 1 diabetes | |
| WO2001026679A2 (en) | T-cells and molecules involved in immune regulation | |
| Miloro | Deciphering the role of the death receptor Fas/CD95 in T cell co-stimulation | |
| Cobbold | T cell tolerance in transplantation: possibilities for therapeutic intervention | |
| Jangalwe | Regulation of Alloreactive CD8 T Cell Responses by Costimulation and Inflammation | |
| Zajac | Antiviral cell-mediated immune responses in beta-2-microglobulin deficient mice | |
| Su | Mechanisms of peptide-IgG gene-transferred tolerance induction: Antigen presentation by tolerogenic B cells and the role of IgG heavy chain | |
| Sutherland | BAFF regulation of peripheral T cell responses | |
| Telander | Clonal anergy: its biochemical basis and functional consequences | |
| AU2005295918A1 (en) | Methods and materials for the inhibition of transplant rejection | |
| Sullivan | Selection and maintenance of a Qa-1-restricted T cell with specificity for insulin | |
| Chen et al. | Specific tolerance induction of allo-Kb-skin grafts by FK506 in the CD8-depleted H-2k recipients required low amounts of Kb-antigen | |
| Lalli | Regulation of T cell Immune Responses by Decay Accelerating Factor |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20041130 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20070827 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20071127 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20071204 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20080227 |
|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A821 Effective date: 20080229 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20090311 |