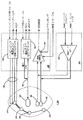
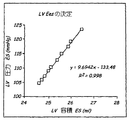
JP2004528864A - 鬱血性心不全を監視する埋め込み可能な医療デバイス - Google Patents
鬱血性心不全を監視する埋め込み可能な医療デバイス Download PDFInfo
- Publication number
- JP2004528864A JP2004528864A JP2002554176A JP2002554176A JP2004528864A JP 2004528864 A JP2004528864 A JP 2004528864A JP 2002554176 A JP2002554176 A JP 2002554176A JP 2002554176 A JP2002554176 A JP 2002554176A JP 2004528864 A JP2004528864 A JP 2004528864A
- Authority
- JP
- Japan
- Prior art keywords
- blood pressure
- heart
- systolic
- chamber
- cardiac cycle
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 206010019280 Heart failures Diseases 0.000 title claims abstract description 123
- 238000012544 monitoring process Methods 0.000 title claims description 31
- 206010007559 Cardiac failure congestive Diseases 0.000 title description 20
- 230000000747 cardiac effect Effects 0.000 claims abstract description 253
- 210000005242 cardiac chamber Anatomy 0.000 claims abstract description 162
- 230000036772 blood pressure Effects 0.000 claims abstract description 154
- 210000002216 heart Anatomy 0.000 claims abstract description 130
- 230000000638 stimulation Effects 0.000 claims abstract description 70
- 230000008602 contraction Effects 0.000 claims abstract description 55
- 230000008859 change Effects 0.000 claims abstract description 51
- 230000000694 effects Effects 0.000 claims abstract description 43
- 238000011084 recovery Methods 0.000 claims abstract description 34
- 230000004044 response Effects 0.000 claims abstract description 21
- 238000000034 method Methods 0.000 claims description 60
- 238000005259 measurement Methods 0.000 claims description 55
- 230000035488 systolic blood pressure Effects 0.000 claims description 50
- 238000009530 blood pressure measurement Methods 0.000 claims description 26
- 238000012545 processing Methods 0.000 claims description 26
- 238000002560 therapeutic procedure Methods 0.000 claims description 21
- 230000035487 diastolic blood pressure Effects 0.000 claims description 20
- 208000000418 Premature Cardiac Complexes Diseases 0.000 claims description 18
- 230000003205 diastolic effect Effects 0.000 claims description 16
- 238000012417 linear regression Methods 0.000 claims description 11
- 230000028161 membrane depolarization Effects 0.000 claims description 10
- 230000002596 correlated effect Effects 0.000 claims description 6
- 238000005070 sampling Methods 0.000 claims description 4
- 230000010247 heart contraction Effects 0.000 claims description 3
- 230000002028 premature Effects 0.000 claims description 3
- 230000000977 initiatory effect Effects 0.000 claims description 2
- 230000003134 recirculating effect Effects 0.000 claims description 2
- 238000009795 derivation Methods 0.000 claims 7
- 230000001939 inductive effect Effects 0.000 claims 5
- 238000005194 fractionation Methods 0.000 claims 4
- 230000003416 augmentation Effects 0.000 claims 3
- 230000003213 activating effect Effects 0.000 claims 2
- 230000001747 exhibiting effect Effects 0.000 claims 2
- 210000005241 right ventricle Anatomy 0.000 description 43
- 230000002861 ventricular Effects 0.000 description 40
- 210000005245 right atrium Anatomy 0.000 description 37
- 238000011282 treatment Methods 0.000 description 28
- 230000006870 function Effects 0.000 description 27
- 230000001746 atrial effect Effects 0.000 description 23
- 210000005240 left ventricle Anatomy 0.000 description 23
- 238000001514 detection method Methods 0.000 description 14
- 210000005246 left atrium Anatomy 0.000 description 14
- 239000004020 conductor Substances 0.000 description 13
- 230000008569 process Effects 0.000 description 12
- 230000033764 rhythmic process Effects 0.000 description 12
- 208000001871 Tachycardia Diseases 0.000 description 11
- 239000003814 drug Substances 0.000 description 11
- 229940079593 drug Drugs 0.000 description 11
- 210000003462 vein Anatomy 0.000 description 11
- 241001465754 Metazoa Species 0.000 description 10
- 210000003748 coronary sinus Anatomy 0.000 description 9
- 230000004217 heart function Effects 0.000 description 9
- 230000005540 biological transmission Effects 0.000 description 8
- 239000008280 blood Substances 0.000 description 7
- 210000004369 blood Anatomy 0.000 description 7
- 230000036471 bradycardia Effects 0.000 description 7
- 208000006218 bradycardia Diseases 0.000 description 7
- 238000013194 cardioversion Methods 0.000 description 7
- 230000004064 dysfunction Effects 0.000 description 7
- 210000002837 heart atrium Anatomy 0.000 description 7
- 230000002107 myocardial effect Effects 0.000 description 7
- 238000004422 calculation algorithm Methods 0.000 description 6
- 238000004364 calculation method Methods 0.000 description 6
- 238000002513 implantation Methods 0.000 description 6
- 230000036279 refractory period Effects 0.000 description 6
- 241000282472 Canis lupus familiaris Species 0.000 description 5
- 206010049447 Tachyarrhythmia Diseases 0.000 description 5
- 230000001684 chronic effect Effects 0.000 description 5
- 230000009977 dual effect Effects 0.000 description 5
- 230000000004 hemodynamic effect Effects 0.000 description 5
- 230000001771 impaired effect Effects 0.000 description 5
- 230000035939 shock Effects 0.000 description 5
- 208000024891 symptom Diseases 0.000 description 5
- 206010037423 Pulmonary oedema Diseases 0.000 description 4
- 206010003119 arrhythmia Diseases 0.000 description 4
- 230000009286 beneficial effect Effects 0.000 description 4
- 230000008901 benefit Effects 0.000 description 4
- 230000000875 corresponding effect Effects 0.000 description 4
- 238000013500 data storage Methods 0.000 description 4
- 230000007423 decrease Effects 0.000 description 4
- 238000010586 diagram Methods 0.000 description 4
- 201000010099 disease Diseases 0.000 description 4
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 4
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 4
- 208000005333 pulmonary edema Diseases 0.000 description 4
- 210000001908 sarcoplasmic reticulum Anatomy 0.000 description 4
- 206010058151 Pulseless electrical activity Diseases 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- 230000001154 acute effect Effects 0.000 description 3
- 230000006793 arrhythmia Effects 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000017531 blood circulation Effects 0.000 description 3
- 230000002999 depolarising effect Effects 0.000 description 3
- 238000011156 evaluation Methods 0.000 description 3
- 230000006872 improvement Effects 0.000 description 3
- 230000033001 locomotion Effects 0.000 description 3
- 230000007246 mechanism Effects 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 230000002269 spontaneous effect Effects 0.000 description 3
- 238000001356 surgical procedure Methods 0.000 description 3
- 230000001360 synchronised effect Effects 0.000 description 3
- 230000006794 tachycardia Effects 0.000 description 3
- JWZZKOKVBUJMES-UHFFFAOYSA-N (+-)-Isoprenaline Chemical compound CC(C)NCC(O)C1=CC=C(O)C(O)=C1 JWZZKOKVBUJMES-UHFFFAOYSA-N 0.000 description 2
- 206010006580 Bundle branch block left Diseases 0.000 description 2
- 206010006582 Bundle branch block right Diseases 0.000 description 2
- 206010007556 Cardiac failure acute Diseases 0.000 description 2
- 206010056370 Congestive cardiomyopathy Diseases 0.000 description 2
- 201000010046 Dilated cardiomyopathy Diseases 0.000 description 2
- 206010015856 Extrasystoles Diseases 0.000 description 2
- 238000004458 analytical method Methods 0.000 description 2
- 210000003157 atrial septum Anatomy 0.000 description 2
- 230000002567 autonomic effect Effects 0.000 description 2
- 206010007625 cardiogenic shock Diseases 0.000 description 2
- 238000000093 extraction electrospray ionisation Methods 0.000 description 2
- 210000002064 heart cell Anatomy 0.000 description 2
- 238000002847 impedance measurement Methods 0.000 description 2
- 208000028867 ischemia Diseases 0.000 description 2
- 229940039009 isoproterenol Drugs 0.000 description 2
- 210000005244 lower chamber Anatomy 0.000 description 2
- 230000003211 malignant effect Effects 0.000 description 2
- 210000004165 myocardium Anatomy 0.000 description 2
- 238000010606 normalization Methods 0.000 description 2
- 230000036284 oxygen consumption Effects 0.000 description 2
- 230000000737 periodic effect Effects 0.000 description 2
- 230000001144 postural effect Effects 0.000 description 2
- 230000036316 preload Effects 0.000 description 2
- 238000000718 qrs complex Methods 0.000 description 2
- 230000034225 regulation of ventricular cardiomyocyte membrane depolarization Effects 0.000 description 2
- 230000002336 repolarization Effects 0.000 description 2
- 238000011160 research Methods 0.000 description 2
- 230000001960 triggered effect Effects 0.000 description 2
- 239000013598 vector Substances 0.000 description 2
- 206010003671 Atrioventricular Block Diseases 0.000 description 1
- OYPRJOBELJOOCE-UHFFFAOYSA-N Calcium Chemical compound [Ca] OYPRJOBELJOOCE-UHFFFAOYSA-N 0.000 description 1
- 206010007558 Cardiac failure chronic Diseases 0.000 description 1
- 208000017667 Chronic Disease Diseases 0.000 description 1
- 206010012335 Dependence Diseases 0.000 description 1
- 241000208011 Digitalis Species 0.000 description 1
- 208000000059 Dyspnea Diseases 0.000 description 1
- 206010013975 Dyspnoeas Diseases 0.000 description 1
- 206010020772 Hypertension Diseases 0.000 description 1
- 206010021143 Hypoxia Diseases 0.000 description 1
- 206010061216 Infarction Diseases 0.000 description 1
- 101100521334 Mus musculus Prom1 gene Proteins 0.000 description 1
- 241000287181 Sturnus vulgaris Species 0.000 description 1
- 206010071436 Systolic dysfunction Diseases 0.000 description 1
- 208000007536 Thrombosis Diseases 0.000 description 1
- 230000001133 acceleration Effects 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000009798 acute exacerbation Effects 0.000 description 1
- 206010000891 acute myocardial infarction Diseases 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000008321 arterial blood flow Effects 0.000 description 1
- 230000004872 arterial blood pressure Effects 0.000 description 1
- 206010003668 atrial tachycardia Diseases 0.000 description 1
- 210000001992 atrioventricular node Anatomy 0.000 description 1
- 230000001042 autoregulative effect Effects 0.000 description 1
- 239000002876 beta blocker Substances 0.000 description 1
- 229940097320 beta blocking agent Drugs 0.000 description 1
- 210000004375 bundle of his Anatomy 0.000 description 1
- 229910052791 calcium Inorganic materials 0.000 description 1
- 239000011575 calcium Substances 0.000 description 1
- 230000003185 calcium uptake Effects 0.000 description 1
- 239000003990 capacitor Substances 0.000 description 1
- 210000004413 cardiac myocyte Anatomy 0.000 description 1
- 238000007675 cardiac surgery Methods 0.000 description 1
- 230000001269 cardiogenic effect Effects 0.000 description 1
- 230000015556 catabolic process Effects 0.000 description 1
- 238000006243 chemical reaction Methods 0.000 description 1
- 230000004087 circulation Effects 0.000 description 1
- 238000004891 communication Methods 0.000 description 1
- 230000008828 contractile function Effects 0.000 description 1
- 230000009989 contractile response Effects 0.000 description 1
- 230000001595 contractor effect Effects 0.000 description 1
- 230000008878 coupling Effects 0.000 description 1
- 238000010168 coupling process Methods 0.000 description 1
- 238000005859 coupling reaction Methods 0.000 description 1
- 239000013078 crystal Substances 0.000 description 1
- 230000034994 death Effects 0.000 description 1
- 230000007547 defect Effects 0.000 description 1
- 230000007812 deficiency Effects 0.000 description 1
- 230000006735 deficit Effects 0.000 description 1
- 230000006866 deterioration Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 230000010339 dilation Effects 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 230000002526 effect on cardiovascular system Effects 0.000 description 1
- 230000009177 electrical depolarization Effects 0.000 description 1
- 239000012530 fluid Substances 0.000 description 1
- 239000007789 gas Substances 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000005003 heart tissue Anatomy 0.000 description 1
- 230000007954 hypoxia Effects 0.000 description 1
- 208000022368 idiopathic cardiomyopathy Diseases 0.000 description 1
- 238000000338 in vitro Methods 0.000 description 1
- 230000007574 infarction Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 239000004041 inotropic agent Substances 0.000 description 1
- 230000010354 integration Effects 0.000 description 1
- 238000001990 intravenous administration Methods 0.000 description 1
- 238000007914 intraventricular administration Methods 0.000 description 1
- WABPQHHGFIMREM-UHFFFAOYSA-N lead(0) Chemical compound [Pb] WABPQHHGFIMREM-UHFFFAOYSA-N 0.000 description 1
- 210000004072 lung Anatomy 0.000 description 1
- 239000003550 marker Substances 0.000 description 1
- 238000012986 modification Methods 0.000 description 1
- 230000004048 modification Effects 0.000 description 1
- 210000003205 muscle Anatomy 0.000 description 1
- 210000000663 muscle cell Anatomy 0.000 description 1
- 230000004118 muscle contraction Effects 0.000 description 1
- 208000010125 myocardial infarction Diseases 0.000 description 1
- 230000010117 myocardial relaxation Effects 0.000 description 1
- 210000000056 organ Anatomy 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000035479 physiological effects, processes and functions Effects 0.000 description 1
- 230000003389 potentiating effect Effects 0.000 description 1
- 230000000750 progressive effect Effects 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 230000000541 pulsatile effect Effects 0.000 description 1
- 230000010349 pulsation Effects 0.000 description 1
- 230000035485 pulse pressure Effects 0.000 description 1
- 238000005086 pumping Methods 0.000 description 1
- 210000003742 purkinje fiber Anatomy 0.000 description 1
- 230000011514 reflex Effects 0.000 description 1
- 230000004213 regulation of atrial cardiomyocyte membrane depolarization Effects 0.000 description 1
- 230000008672 reprogramming Effects 0.000 description 1
- 230000000241 respiratory effect Effects 0.000 description 1
- 230000029058 respiratory gaseous exchange Effects 0.000 description 1
- 238000012552 review Methods 0.000 description 1
- 230000001020 rhythmical effect Effects 0.000 description 1
- 230000000630 rising effect Effects 0.000 description 1
- 230000035945 sensitivity Effects 0.000 description 1
- 208000013220 shortness of breath Diseases 0.000 description 1
- 230000008054 signal transmission Effects 0.000 description 1
- 210000002027 skeletal muscle Anatomy 0.000 description 1
- 230000009163 spontaneous depolarization Effects 0.000 description 1
- 239000000758 substrate Substances 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 238000012360 testing method Methods 0.000 description 1
- 230000001225 therapeutic effect Effects 0.000 description 1
- 210000000115 thoracic cavity Anatomy 0.000 description 1
- 230000017105 transposition Effects 0.000 description 1
- 210000005243 upper chamber Anatomy 0.000 description 1
- 210000002620 vena cava superior Anatomy 0.000 description 1
- 208000021816 ventricular bradycardia Diseases 0.000 description 1
- 210000000596 ventricular septum Anatomy 0.000 description 1
- 206010047302 ventricular tachycardia Diseases 0.000 description 1
- 230000003313 weakening effect Effects 0.000 description 1
- 238000010626 work up procedure Methods 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61N—ELECTROTHERAPY; MAGNETOTHERAPY; RADIATION THERAPY; ULTRASOUND THERAPY
- A61N1/00—Electrotherapy; Circuits therefor
- A61N1/18—Applying electric currents by contact electrodes
- A61N1/32—Applying electric currents by contact electrodes alternating or intermittent currents
- A61N1/36—Applying electric currents by contact electrodes alternating or intermittent currents for stimulation
- A61N1/362—Heart stimulators
- A61N1/365—Heart stimulators controlled by a physiological parameter, e.g. heart potential
- A61N1/36514—Heart stimulators controlled by a physiological parameter, e.g. heart potential controlled by a physiological quantity other than heart potential, e.g. blood pressure
- A61N1/36564—Heart stimulators controlled by a physiological parameter, e.g. heart potential controlled by a physiological quantity other than heart potential, e.g. blood pressure controlled by blood pressure
Landscapes
- Health & Medical Sciences (AREA)
- Cardiology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Life Sciences & Earth Sciences (AREA)
- Biomedical Technology (AREA)
- Physiology (AREA)
- Engineering & Computer Science (AREA)
- Biophysics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Radiology & Medical Imaging (AREA)
- Animal Behavior & Ethology (AREA)
- General Health & Medical Sciences (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- Electrotherapy Devices (AREA)
- Measuring Pulse, Heart Rate, Blood Pressure Or Blood Flow (AREA)
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/751,079 US6438408B1 (en) | 2000-12-28 | 2000-12-28 | Implantable medical device for monitoring congestive heart failure |
| PCT/US2001/049620 WO2002053228A1 (en) | 2000-12-28 | 2001-12-27 | Implantable medical device for monitoring congestive heart failure |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| JP2004528864A true JP2004528864A (ja) | 2004-09-24 |
| JP2004528864A5 JP2004528864A5 (enExample) | 2005-12-22 |
Family
ID=25020375
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| JP2002554176A Pending JP2004528864A (ja) | 2000-12-28 | 2001-12-27 | 鬱血性心不全を監視する埋め込み可能な医療デバイス |
Country Status (6)
| Country | Link |
|---|---|
| US (1) | US6438408B1 (enExample) |
| EP (1) | EP1345651B1 (enExample) |
| JP (1) | JP2004528864A (enExample) |
| CA (1) | CA2433049A1 (enExample) |
| DE (1) | DE60118433T2 (enExample) |
| WO (1) | WO2002053228A1 (enExample) |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006516449A (ja) * | 2003-01-24 | 2006-07-06 | プロテウス バイオメディカル インコーポレイテッド | 心臓ペーシングを改善するための方法および装置 |
| JP2008521578A (ja) * | 2004-12-03 | 2008-06-26 | メドトロニック・インコーポレーテッド | 機械的復元を監視して、高頻度調律の血行力学的耐性を予測する埋め込み可能システム |
| JP2009247812A (ja) * | 2008-04-10 | 2009-10-29 | Nipro Corp | 心電図の表示方法および心電図表示装置 |
| JP2010524636A (ja) * | 2007-05-07 | 2010-07-22 | カーディアック ペースメイカーズ, インコーポレイテッド | 血行動態耐容性を判定するシステムおよび方法 |
| US8509892B2 (en) | 2008-06-19 | 2013-08-13 | Cardiac Pacemakers, Inc. | Method and apparatus for controlling anti-tachyarrhythmia therapy using hemodynamic tolerability |
| JP2015107338A (ja) * | 2007-09-05 | 2015-06-11 | センシブル メディカル イノヴェイションズ リミテッド | 胸部組織液を監視するための方法およびシステム |
| US10506943B2 (en) | 2007-09-05 | 2019-12-17 | Sensible Medical Innovations Ltd. | Methods and systems for monitoring intrabody tissues |
| US10667715B2 (en) | 2008-08-20 | 2020-06-02 | Sensible Medical Innovations Ltd. | Methods and devices of cardiac tissue monitoring and analysis |
Families Citing this family (252)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060064135A1 (en) * | 1997-10-14 | 2006-03-23 | Transoma Medical, Inc. | Implantable pressure sensor with pacing capability |
| US20020120200A1 (en) * | 1997-10-14 | 2002-08-29 | Brian Brockway | Devices, systems and methods for endocardial pressure measurement |
| US20030036746A1 (en) | 2001-08-16 | 2003-02-20 | Avi Penner | Devices for intrabody delivery of molecules and systems and methods utilizing same |
| US7127290B2 (en) * | 1999-10-01 | 2006-10-24 | Cardiac Pacemakers, Inc. | Cardiac rhythm management systems and methods predicting congestive heart failure status |
| US20050154370A1 (en) | 1999-10-29 | 2005-07-14 | Medtronic, Inc. | Methods and systems for providing therapies into the pericardial space |
| US7024248B2 (en) | 2000-10-16 | 2006-04-04 | Remon Medical Technologies Ltd | Systems and methods for communicating with implantable devices |
| US6741885B1 (en) | 2000-12-07 | 2004-05-25 | Pacesetter, Inc. | Implantable cardiac device for managing the progression of heart disease and method |
| US6912420B2 (en) * | 2001-04-10 | 2005-06-28 | Cardiac Pacemakers, Inc. | Cardiac rhythm management system for hypotension |
| US6907288B2 (en) * | 2001-04-10 | 2005-06-14 | Cardiac Pacemakers, Inc. | Cardiac rhythm management system adjusting rate response factor for treating hypotension |
| US6684101B2 (en) * | 2001-04-25 | 2004-01-27 | Cardiac Pacemakers, Inc. | Implantable medical device employing single drive, dual sense impedance measuring |
| US6748271B2 (en) | 2001-07-27 | 2004-06-08 | Cardiac Pacemakers, Inc. | Method and system for treatment of neurocardiogenic syncope |
| US7191000B2 (en) | 2001-07-31 | 2007-03-13 | Cardiac Pacemakers, Inc. | Cardiac rhythm management system for edema |
| US6974460B2 (en) * | 2001-09-14 | 2005-12-13 | Stryker Spine | Biased angulation bone fixation assembly |
| DE10148440A1 (de) * | 2001-10-01 | 2003-04-17 | Inflow Dynamics Inc | Vorrichtung zum Überwachen eines Blutstaus im Herzen |
| US20050137480A1 (en) * | 2001-10-01 | 2005-06-23 | Eckhard Alt | Remote control of implantable device through medical implant communication service band |
| US8457743B2 (en) * | 2001-10-01 | 2013-06-04 | Medtronic, Inc. | Method of vagal stimulation to treat patients suffering from congestive heart failure |
| US7778709B2 (en) * | 2001-10-01 | 2010-08-17 | Medtronic, Inc. | Method and device for using impedance measurements based on electrical energy of the heart |
| US7383088B2 (en) | 2001-11-07 | 2008-06-03 | Cardiac Pacemakers, Inc. | Centralized management system for programmable medical devices |
| US6961615B2 (en) * | 2002-02-07 | 2005-11-01 | Pacesetter, Inc. | System and method for evaluating risk of mortality due to congestive heart failure using physiologic sensors |
| US6645153B2 (en) * | 2002-02-07 | 2003-11-11 | Pacesetter, Inc. | System and method for evaluating risk of mortality due to congestive heart failure using physiologic sensors |
| US20040122487A1 (en) | 2002-12-18 | 2004-06-24 | John Hatlestad | Advanced patient management with composite parameter indices |
| US20040122294A1 (en) | 2002-12-18 | 2004-06-24 | John Hatlestad | Advanced patient management with environmental data |
| US20040122296A1 (en) * | 2002-12-18 | 2004-06-24 | John Hatlestad | Advanced patient management for triaging health-related data |
| US7468032B2 (en) | 2002-12-18 | 2008-12-23 | Cardiac Pacemakers, Inc. | Advanced patient management for identifying, displaying and assisting with correlating health-related data |
| US7043305B2 (en) | 2002-03-06 | 2006-05-09 | Cardiac Pacemakers, Inc. | Method and apparatus for establishing context among events and optimizing implanted medical device performance |
| US8043213B2 (en) | 2002-12-18 | 2011-10-25 | Cardiac Pacemakers, Inc. | Advanced patient management for triaging health-related data using color codes |
| US7983759B2 (en) | 2002-12-18 | 2011-07-19 | Cardiac Pacemakers, Inc. | Advanced patient management for reporting multiple health-related parameters |
| US8391989B2 (en) | 2002-12-18 | 2013-03-05 | Cardiac Pacemakers, Inc. | Advanced patient management for defining, identifying and using predetermined health-related events |
| US7113825B2 (en) * | 2002-05-03 | 2006-09-26 | Cardiac Pacemakers, Inc. | Method and apparatus for detecting acoustic oscillations in cardiac rhythm |
| US7039462B2 (en) * | 2002-06-14 | 2006-05-02 | Cardiac Pacemakers, Inc. | Method and apparatus for detecting oscillations in cardiac rhythm |
| US7096061B2 (en) * | 2002-07-03 | 2006-08-22 | Tel-Aviv University Future Technology Development L.P. | Apparatus for monitoring CHF patients using bio-impedance technique |
| SE0202289D0 (sv) * | 2002-07-22 | 2002-07-22 | St Jude Medical | A congestive heart failure monitor |
| US7344505B2 (en) * | 2002-10-15 | 2008-03-18 | Transoma Medical, Inc. | Barriers and methods for pressure measurement catheters |
| US6945939B2 (en) * | 2002-10-18 | 2005-09-20 | Pacesetter, Inc. | Hemodynamic analysis |
| US7072711B2 (en) | 2002-11-12 | 2006-07-04 | Cardiac Pacemakers, Inc. | Implantable device for delivering cardiac drug therapy |
| US7313434B2 (en) * | 2002-11-25 | 2007-12-25 | Regents Of The University Of Minnesota | Impedance monitoring for detecting pulmonary edema and thoracic congestion |
| AU2003296956A1 (en) | 2002-12-11 | 2004-06-30 | Proteus Biomedical, Inc. | Monitoring and treating hemodynamic parameters |
| US20040111006A1 (en) * | 2002-12-17 | 2004-06-10 | Scout Medical Technologies, Llc | System and method for regulating blood pressure |
| US7378955B2 (en) | 2003-01-03 | 2008-05-27 | Cardiac Pacemakers, Inc. | System and method for correlating biometric trends with a related temporal event |
| WO2004066814A2 (en) | 2003-01-24 | 2004-08-12 | Proteus Biomedical Inc. | Method and system for remote hemodynamic monitoring |
| US7204798B2 (en) * | 2003-01-24 | 2007-04-17 | Proteus Biomedical, Inc. | Methods and systems for measuring cardiac parameters |
| US6915157B2 (en) * | 2003-02-18 | 2005-07-05 | Medtronic, Inc. | Implantable medical device for assessing heart failure state from Mechanical Pulsus Alternans |
| US6887207B2 (en) * | 2003-02-26 | 2005-05-03 | Medtronic, Inc. | Methods and apparatus for estimation of ventricular afterload based on ventricular pressure measurements |
| US7269460B2 (en) * | 2003-02-28 | 2007-09-11 | Medtronic, Inc. | Method and apparatus for evaluating and optimizing ventricular synchronization |
| ATE435670T1 (de) * | 2003-03-31 | 2009-07-15 | Radi Medical Systems | Medizinisches gerät mit druckmessung in einem ventrikel zur steuerung eines herzschrittmachers. |
| US20040220640A1 (en) * | 2003-04-29 | 2004-11-04 | Medtronic, Inc. | Method and apparatus for determining myocardial electrical resitution and controlling extra systolic stimulation |
| US20040220631A1 (en) * | 2003-04-29 | 2004-11-04 | Medtronic, Inc. | Method and apparatus for detecting myocardial electrical recovery and controlling extra-systolic sstimulation |
| US6994676B2 (en) * | 2003-04-30 | 2006-02-07 | Medtronic, Inc | Method and apparatus for assessing ventricular contractile status |
| US7130684B2 (en) * | 2003-04-30 | 2006-10-31 | Medtronic, Inc. | Method and apparatus for improving ventricular status using the force interval relationship |
| US7831303B2 (en) * | 2003-06-17 | 2010-11-09 | Medtronic, Inc. | Cardiac pacing apparatus and method for continuous capture management |
| US7003348B1 (en) | 2003-07-01 | 2006-02-21 | Pacesetter, Inc. | Monitoring cardiac geometry for diagnostics and therapy |
| US7613513B1 (en) | 2003-07-01 | 2009-11-03 | Pacesetter, Inc. | System and method for determining cardiac geometry |
| US20050038478A1 (en) * | 2003-08-11 | 2005-02-17 | Klepfer Ruth N. | Activation recovery interval for classification of cardiac beats in an implanted device |
| US7292888B2 (en) * | 2003-08-11 | 2007-11-06 | Medtronic, Inc. | Cardiac stimulation during a refractory period |
| US8396565B2 (en) | 2003-09-15 | 2013-03-12 | Medtronic, Inc. | Automatic therapy adjustments |
| US20050065555A1 (en) * | 2003-09-24 | 2005-03-24 | Siew Er | Collection and analysis of procedural information |
| US7233824B2 (en) * | 2003-10-07 | 2007-06-19 | Medtronic, Inc. | Secure and efficacious therapy delivery for an extra-systolic stimulation pacing engine |
| US7970466B2 (en) * | 2003-10-07 | 2011-06-28 | Medtronic, Inc. | Method and apparatus for optimization and assessment of response to extra-systolic stimulation (ESS) therapy |
| US7184832B2 (en) * | 2003-10-07 | 2007-02-27 | Medtronic, Inc. | Refractory period tracking and arrhythmia detection |
| US20050075674A1 (en) | 2003-10-07 | 2005-04-07 | Zillmer Glenn C. | Extra-systolic stimulation therapy delivery and sensing via different electrode sets |
| US20050075673A1 (en) * | 2003-10-07 | 2005-04-07 | Warkentin Dwight H. | Method and apparatus for controlling extra-systolic stimulation (ESS) therapy using ischemia detection |
| US7184833B2 (en) * | 2003-10-07 | 2007-02-27 | Medtronic, Inc. | Multiple pacing output channels |
| US7142916B2 (en) * | 2003-10-07 | 2006-11-28 | Medtronic, Inc. | Cardiac pacing modality having improved blanking, timing, and therapy delivery methods for extra-systolic stimulation pacing therapy |
| FR2861996A1 (fr) * | 2003-11-06 | 2005-05-13 | Fred Zacouto | Stimulateur cardiaque orthorythmique inotrope |
| US7184821B2 (en) | 2003-12-03 | 2007-02-27 | Regents Of The University Of Minnesota | Monitoring thoracic fluid changes |
| US6964641B2 (en) * | 2003-12-24 | 2005-11-15 | Medtronic, Inc. | Implantable medical device with sleep disordered breathing monitoring |
| US7232435B2 (en) * | 2004-02-06 | 2007-06-19 | Medtronic, Inc. | Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure |
| US20050187593A1 (en) * | 2004-02-23 | 2005-08-25 | Medtronic, Inc. | Implantable medical device system with communication link to home appliances |
| TW200534827A (en) * | 2004-03-24 | 2005-11-01 | Noninvasive Medical Technologies Llc | Thoracic impedance monitor and electrode array and method of use |
| US7272443B2 (en) * | 2004-03-26 | 2007-09-18 | Pacesetter, Inc. | System and method for predicting a heart condition based on impedance values using an implantable medical device |
| US7505814B2 (en) * | 2004-03-26 | 2009-03-17 | Pacesetter, Inc. | System and method for evaluating heart failure based on ventricular end-diastolic volume using an implantable medical device |
| US20050228693A1 (en) * | 2004-04-09 | 2005-10-13 | Webb James D | Data exchange web services for medical device systems |
| US7676262B1 (en) | 2004-04-20 | 2010-03-09 | Pacesetter, Inc. | Methods and devices for determining exercise compliance diagnostics |
| US7043294B1 (en) | 2004-04-20 | 2006-05-09 | Pacesetter, Inc. | Methods and devices for determining heart rate recovery |
| US7031766B1 (en) | 2004-04-20 | 2006-04-18 | Pacesetter, Inc. | Methods and devices for determining exercise diagnostic parameters |
| DE602004020120D1 (de) * | 2004-04-26 | 2009-04-30 | St Jude Medical | Nachweis von diastolischer herzinsuffizienz |
| US7171271B2 (en) * | 2004-05-11 | 2007-01-30 | Pacesetter, Inc. | System and method for evaluating heart failure using an implantable medical device based on heart rate during patient activity |
| US7416529B2 (en) * | 2004-05-13 | 2008-08-26 | St. Jude Medical Ab | Detection of diastolic heart failure |
| US7333856B1 (en) | 2004-05-17 | 2008-02-19 | Pacesetter, Inc. | Method and system to graphically display programming parameters for multi-chamber devices |
| US7450991B2 (en) * | 2004-05-28 | 2008-11-11 | Advanced Neuromodulation Systems, Inc. | Systems and methods used to reserve a constant battery capacity |
| US7697994B2 (en) * | 2004-06-18 | 2010-04-13 | Medtronic, Inc. | Remote scheduling for management of an implantable medical device |
| US7565197B2 (en) * | 2004-06-18 | 2009-07-21 | Medtronic, Inc. | Conditional requirements for remote medical device programming |
| US7840268B2 (en) | 2004-06-21 | 2010-11-23 | Advanced Neuromodulation Systems, Inc. | System and method of managing medical device historical data |
| US7433853B2 (en) | 2004-07-12 | 2008-10-07 | Cardiac Pacemakers, Inc. | Expert system for patient medical information analysis |
| US7596469B2 (en) * | 2004-07-19 | 2009-09-29 | Baylis Medical Company Inc. | Method and apparatus for prioritizing errors in a medical treatment system |
| US7076399B2 (en) | 2004-07-19 | 2006-07-11 | Baylis Medical Company Inc. | Medical generator with hierarchical error logic |
| US7819909B2 (en) * | 2004-07-20 | 2010-10-26 | Medtronic, Inc. | Therapy programming guidance based on stored programming history |
| WO2006012423A1 (en) * | 2004-07-20 | 2006-02-02 | Medtronic, Inc. | Therapy programming guidance based on stored programming history |
| US7269458B2 (en) | 2004-08-09 | 2007-09-11 | Cardiac Pacemakers, Inc. | Cardiopulmonary functional status assessment via heart rate response detection by implantable cardiac device |
| US7389143B2 (en) | 2004-08-12 | 2008-06-17 | Cardiac Pacemakers, Inc. | Cardiopulmonary functional status assessment via metabolic response detection by implantable cardiac device |
| FR2874331B1 (fr) * | 2004-08-18 | 2006-10-27 | Ela Medical Sa | Dispositif medical implantable actif comprenant des moyens du volume intracardiaque |
| US7387610B2 (en) | 2004-08-19 | 2008-06-17 | Cardiac Pacemakers, Inc. | Thoracic impedance detection with blood resistivity compensation |
| WO2006029090A2 (en) * | 2004-09-02 | 2006-03-16 | Proteus Biomedical, Inc. | Methods and apparatus for tissue activation and monitoring |
| US8271093B2 (en) | 2004-09-17 | 2012-09-18 | Cardiac Pacemakers, Inc. | Systems and methods for deriving relative physiologic measurements using a backend computing system |
| US20060064136A1 (en) * | 2004-09-23 | 2006-03-23 | Medtronic, Inc. | Method and apparatus for facilitating patient alert in implantable medical devices |
| WO2006105474A2 (en) | 2005-03-31 | 2006-10-05 | Proteus Biomedical, Inc. | Automated optimization of multi-electrode pacing for cardiac resynchronization |
| US8755885B2 (en) * | 2004-10-13 | 2014-06-17 | Medtronic, Inc. | Software configurable medical device platform and associated methods |
| US7632235B1 (en) | 2004-11-22 | 2009-12-15 | Pacesetter, Inc. | System and method for measuring cardiac output via thermal dilution using an implantable medical device with an external ultrasound power delivery system |
| US7813808B1 (en) | 2004-11-24 | 2010-10-12 | Remon Medical Technologies Ltd | Implanted sensor system with optimized operational and sensing parameters |
| CA2490858A1 (en) * | 2004-12-07 | 2006-06-07 | Ignis Innovation Inc. | Driving method for compensated voltage-programming of amoled displays |
| US7470233B2 (en) * | 2005-01-26 | 2008-12-30 | Medtronic, Inc. | Method and apparatus for muscle function measurement |
| US7367951B2 (en) * | 2005-01-27 | 2008-05-06 | Medtronic, Inc. | System and method for detecting cardiovascular health conditions using hemodynamic pressure waveforms |
| US20060167361A1 (en) | 2005-01-27 | 2006-07-27 | Bennett Tommy D | Method and apparatus for continuous pulse contour cardiac output |
| US7935062B2 (en) | 2005-01-27 | 2011-05-03 | Medtronic, Inc. | Derivation of flow contour from pressure waveform |
| US7386345B2 (en) * | 2005-01-27 | 2008-06-10 | Cardiac Pacemakers, Inc. | Apparatus and method for temporary treatment of acute heart failure decompensation |
| US7708693B2 (en) | 2005-01-27 | 2010-05-04 | Medtronic, Inc. | System and method for detecting artifactual hemodynamic waveform data |
| US7447543B2 (en) * | 2005-02-15 | 2008-11-04 | Regents Of The University Of Minnesota | Pathology assessment with impedance measurements using convergent bioelectric lead fields |
| US7437192B2 (en) * | 2005-04-05 | 2008-10-14 | Pacesetter, Inc. | System and method for detecting heart failure and pulmonary edema based on ventricular end-diastolic pressure using an implantable medical device |
| US7603170B2 (en) * | 2005-04-26 | 2009-10-13 | Cardiac Pacemakers, Inc. | Calibration of impedance monitoring of respiratory volumes using thoracic D.C. impedance |
| US9089275B2 (en) * | 2005-05-11 | 2015-07-28 | Cardiac Pacemakers, Inc. | Sensitivity and specificity of pulmonary edema detection when using transthoracic impedance |
| US7907997B2 (en) | 2005-05-11 | 2011-03-15 | Cardiac Pacemakers, Inc. | Enhancements to the detection of pulmonary edema when using transthoracic impedance |
| US7340296B2 (en) | 2005-05-18 | 2008-03-04 | Cardiac Pacemakers, Inc. | Detection of pleural effusion using transthoracic impedance |
| US7526338B1 (en) * | 2005-05-23 | 2009-04-28 | Pacesetter, Inc. | Implantable cardiac device for monitoring diastolic heart failure and method of operation and use thereof |
| US7628757B1 (en) | 2005-05-25 | 2009-12-08 | Pacesetter, Inc. | System and method for impedance-based detection of pulmonary edema and reduced respiration using an implantable medical system |
| US20070021678A1 (en) * | 2005-07-19 | 2007-01-25 | Cardiac Pacemakers, Inc. | Methods and apparatus for monitoring physiological responses to steady state activity |
| WO2007021804A2 (en) | 2005-08-12 | 2007-02-22 | Proteus Biomedical, Inc. | Evaluation of depolarization wave conduction velocity |
| US7803121B2 (en) | 2005-08-22 | 2010-09-28 | Scisense Inc. | Implant transmitter |
| US7742815B2 (en) | 2005-09-09 | 2010-06-22 | Cardiac Pacemakers, Inc. | Using implanted sensors for feedback control of implanted medical devices |
| US20070073352A1 (en) * | 2005-09-28 | 2007-03-29 | Euler David E | Method and apparatus for regulating a cardiac stimulation therapy |
| US20070078497A1 (en) * | 2005-10-03 | 2007-04-05 | Vandanacker John P | Remote programming of implantable medical devices |
| US7686768B2 (en) | 2005-11-23 | 2010-03-30 | Vital Sensors Holding Company, Inc. | Implantable pressure monitor |
| US7682313B2 (en) | 2005-11-23 | 2010-03-23 | Vital Sensors Holding Company, Inc. | Implantable pressure monitor |
| US7957797B2 (en) * | 2005-12-02 | 2011-06-07 | Medtronic, Inc. | Closed-loop therapy adjustment |
| US7957809B2 (en) * | 2005-12-02 | 2011-06-07 | Medtronic, Inc. | Closed-loop therapy adjustment |
| US7853322B2 (en) * | 2005-12-02 | 2010-12-14 | Medtronic, Inc. | Closed-loop therapy adjustment |
| US7580746B2 (en) * | 2005-12-07 | 2009-08-25 | Cardiac Pacemakers, Inc. | Implantable medical device for generating cardiac pressure-volume loop and optimizing therapy |
| US7751882B1 (en) | 2005-12-21 | 2010-07-06 | Pacesetter, Inc. | Method and system for determining lead position for optimized cardiac resynchronization therapy hemodynamics |
| US20070156057A1 (en) * | 2005-12-30 | 2007-07-05 | Cho Yong K | Method and system for interpreting hemodynamic data incorporating patient posture information |
| US7581142B2 (en) * | 2006-01-03 | 2009-08-25 | Nec Laboratories America, Inc. | Method and system usable in sensor networks for handling memory faults |
| US8248232B2 (en) * | 2006-01-25 | 2012-08-21 | Greatbatch Ltd. | Hermetically sealed RFID microelectronic chip connected to a biocompatible RFID antenna |
| US9149638B2 (en) * | 2006-01-30 | 2015-10-06 | Medtronic, Inc. | Method and system for controlling pulmonary capillary pressure |
| US7713213B2 (en) * | 2006-03-13 | 2010-05-11 | Cardiac Pacemakers, Inc. | Physiological event detection systems and methods |
| US8712519B1 (en) | 2006-03-31 | 2014-04-29 | Pacesetter, Inc. | Closed-loop adaptive adjustment of pacing therapy based on cardiogenic impedance signals detected by an implantable medical device |
| US7672716B1 (en) | 2006-04-03 | 2010-03-02 | Pacesetter, Inc. | QT-based system and method for detecting and distinguishing dilated cardiomyopathy and heart failure using an implantable medical device |
| US8870742B2 (en) | 2006-04-06 | 2014-10-28 | Ethicon Endo-Surgery, Inc. | GUI for an implantable restriction device and a data logger |
| US7744542B2 (en) * | 2006-04-20 | 2010-06-29 | Cardiac Pacemakers, Inc. | Implanted air passage sensors |
| US20070265666A1 (en) * | 2006-04-27 | 2007-11-15 | Roberts Jonathan P | Implantable sensors having high impedance couplings providing current pathways for improved fault tolerance |
| US20070255353A1 (en) * | 2006-04-27 | 2007-11-01 | Reinke James D | Fault tolerant co-axially wired sensors and methods for implementing same in an implantable medical device |
| US20070265674A1 (en) * | 2006-04-27 | 2007-11-15 | Olson John C | Fault tolerant implantable sensors having redundant electrical grounding connections |
| US20070265662A1 (en) * | 2006-04-27 | 2007-11-15 | Ufford Keith A | Implantable electromagnetic interference tolerant, wired sensors and methods for implementing same |
| US20070265668A1 (en) * | 2006-04-27 | 2007-11-15 | Reinke James D | Fault tolerant sensors and methods for implementing fault tolerance in implantable medical devices |
| US20070255352A1 (en) * | 2006-04-27 | 2007-11-01 | Roline Glen M | Implantable sensors having current-based switches for improved fault tolerance |
| US8005543B2 (en) | 2006-05-08 | 2011-08-23 | Cardiac Pacemakers, Inc. | Heart failure management system |
| US8868181B2 (en) * | 2006-05-15 | 2014-10-21 | Biotronik Crm Patent Ag | Implantable electrostimulator |
| US7955268B2 (en) | 2006-07-21 | 2011-06-07 | Cardiac Pacemakers, Inc. | Multiple sensor deployment |
| US7860567B2 (en) * | 2006-08-31 | 2010-12-28 | Cardiac Pacemakers, Inc. | Sensor for edema |
| US7856260B1 (en) | 2006-09-06 | 2010-12-21 | Pacesetter, Inc. | Implantable cardiac patch for measuring physiologic information |
| US8202224B2 (en) * | 2006-11-13 | 2012-06-19 | Pacesetter, Inc. | System and method for calibrating cardiac pressure measurements derived from signals detected by an implantable medical device |
| US8262581B2 (en) * | 2006-12-11 | 2012-09-11 | National Cerebral And Cardiovascular Center | Solid tissue impedance estimating method, cardiac output calculating method, pulmonary artery wedge pressure calculating method, cardiac output monitoring device, cardiac output monitoring system, pulmonary artery wedge pressure monitoring device, and pulmonary artery wedge pressure monitoring system |
| US8768718B2 (en) * | 2006-12-27 | 2014-07-01 | Cardiac Pacemakers, Inc. | Between-patient comparisons for risk stratification of future heart failure decompensation |
| US9968266B2 (en) | 2006-12-27 | 2018-05-15 | Cardiac Pacemakers, Inc. | Risk stratification based heart failure detection algorithm |
| US7629889B2 (en) | 2006-12-27 | 2009-12-08 | Cardiac Pacemakers, Inc. | Within-patient algorithm to predict heart failure decompensation |
| US9022930B2 (en) * | 2006-12-27 | 2015-05-05 | Cardiac Pacemakers, Inc. | Inter-relation between within-patient decompensation detection algorithm and between-patient stratifier to manage HF patients in a more efficient manner |
| US20080195167A1 (en) * | 2006-12-29 | 2008-08-14 | Ryan Timothy J | Cardiac pacemakers and systems and methods for using them |
| US8894582B2 (en) * | 2007-01-26 | 2014-11-25 | Endotronix, Inc. | Cardiac pressure monitoring device |
| US20080281210A1 (en) * | 2007-01-26 | 2008-11-13 | Nunez Anthony I | Arterial pressure sensing device |
| US8052611B2 (en) | 2007-03-14 | 2011-11-08 | Cardiac Pacemakers, Inc. | Method and apparatus for management of heart failure hospitalization |
| US8208999B2 (en) | 2007-04-04 | 2012-06-26 | Pacesetter, Inc. | System and method for estimating electrical conduction delays from immittance values measured using an implantable medical device |
| US8504153B2 (en) * | 2007-04-04 | 2013-08-06 | Pacesetter, Inc. | System and method for estimating cardiac pressure based on cardiac electrical conduction delays using an implantable medical device |
| US7957799B2 (en) * | 2007-04-30 | 2011-06-07 | Medtronic, Inc. | Non-invasive cardiac potentiation therapy |
| US20090024042A1 (en) * | 2007-07-03 | 2009-01-22 | Endotronix, Inc. | Method and system for monitoring ventricular function of a heart |
| US8706224B1 (en) | 2007-10-30 | 2014-04-22 | Pacesetter, In. | Systems and methods for paired/coupled pacing and dynamic overdrive/underdrive pacing |
| US8818510B2 (en) | 2007-10-30 | 2014-08-26 | Pacesetter, Inc. | Systems and methods for paired/coupled pacing |
| US8838240B2 (en) | 2007-11-21 | 2014-09-16 | Cardiac Pacemakers Inc. | Hemodynamic status assessment during tachycardia |
| US8986253B2 (en) | 2008-01-25 | 2015-03-24 | Tandem Diabetes Care, Inc. | Two chamber pumps and related methods |
| EP2242538B1 (en) | 2008-02-11 | 2016-04-06 | Cardiac Pacemakers, Inc. | Methods of monitoring hemodynamic status for ryhthm discrimination within the heart |
| WO2009102640A1 (en) | 2008-02-12 | 2009-08-20 | Cardiac Pacemakers, Inc. | Systems and methods for controlling wireless signal transfers between ultrasound-enabled medical devices |
| WO2009131749A2 (en) | 2008-02-28 | 2009-10-29 | Proteus Biomedical, Inc. | Integrated circuit implementation and fault control system, device, and method |
| US9320448B2 (en) | 2008-04-18 | 2016-04-26 | Pacesetter, Inc. | Systems and methods for improved atrial fibrillation (AF) monitoring |
| US8401666B2 (en) | 2008-07-11 | 2013-03-19 | Medtronic, Inc. | Modification profiles for posture-responsive therapy |
| US8231556B2 (en) | 2008-07-11 | 2012-07-31 | Medtronic, Inc. | Obtaining baseline patient information |
| US9050471B2 (en) | 2008-07-11 | 2015-06-09 | Medtronic, Inc. | Posture state display on medical device user interface |
| US8504150B2 (en) | 2008-07-11 | 2013-08-06 | Medtronic, Inc. | Associating therapy adjustments with posture states using a stability timer |
| US8249718B2 (en) | 2008-07-11 | 2012-08-21 | Medtronic, Inc. | Programming posture state-responsive therapy with nominal therapy parameters |
| US8708934B2 (en) | 2008-07-11 | 2014-04-29 | Medtronic, Inc. | Reorientation of patient posture states for posture-responsive therapy |
| US8688225B2 (en) | 2008-07-11 | 2014-04-01 | Medtronic, Inc. | Posture state detection using selectable system control parameters |
| US8751011B2 (en) | 2008-07-11 | 2014-06-10 | Medtronic, Inc. | Defining therapy parameter values for posture states |
| US8332041B2 (en) | 2008-07-11 | 2012-12-11 | Medtronic, Inc. | Patient interaction with posture-responsive therapy |
| US8241222B2 (en) * | 2008-07-31 | 2012-08-14 | Medtronic, Inc. | Monitoring hemodynamic status based on intracardiac or vascular impedance |
| US8090443B2 (en) * | 2008-09-15 | 2012-01-03 | Xiaoyi Min | Monitoring HF exacerbation and cardiac resynchronization therapy performance |
| US8408421B2 (en) | 2008-09-16 | 2013-04-02 | Tandem Diabetes Care, Inc. | Flow regulating stopcocks and related methods |
| US8280517B2 (en) | 2008-09-19 | 2012-10-02 | Medtronic, Inc. | Automatic validation techniques for validating operation of medical devices |
| CA2737461A1 (en) | 2008-09-19 | 2010-03-25 | Tandem Diabetes Care, Inc. | Solute concentration measurement device and related methods |
| WO2010039063A1 (en) * | 2008-09-30 | 2010-04-08 | St. Jude Medical Ab | Heart failure detector |
| US8380309B2 (en) * | 2008-09-30 | 2013-02-19 | Medtronic, Inc. | Method and apparatus to optimize pacing heart rate |
| US8591423B2 (en) | 2008-10-10 | 2013-11-26 | Cardiac Pacemakers, Inc. | Systems and methods for determining cardiac output using pulmonary artery pressure measurements |
| WO2010042855A1 (en) | 2008-10-10 | 2010-04-15 | Regents Of The University Of Minnesota | Improved monitor of heart failure using bioimpedance |
| US8062227B2 (en) * | 2008-10-30 | 2011-11-22 | Medtronic, Inc. | Heart failure decompensation determination |
| US8777850B2 (en) * | 2008-10-31 | 2014-07-15 | Medtronic, Inc. | Heart failure patient management using an implantable monitoring system |
| US9301698B2 (en) * | 2008-10-31 | 2016-04-05 | Medtronic, Inc. | Method and apparatus to detect ischemia with a pressure sensor |
| WO2010059291A1 (en) | 2008-11-19 | 2010-05-27 | Cardiac Pacemakers, Inc. | Assessment of pulmonary vascular resistance via pulmonary artery pressure |
| US8428698B2 (en) * | 2009-03-04 | 2013-04-23 | Pacesetter, Inc. | Systems and methods for monitoring DP, IVRT, DiFT, diastolic function and/or HF |
| US20100234906A1 (en) * | 2009-03-16 | 2010-09-16 | Pacesetter, Inc. | System and method for controlling rate-adaptive pacing based on a cardiac force-frequency relation detected by an implantable medical device |
| WO2010126503A1 (en) | 2009-04-29 | 2010-11-04 | Proteus Biomedical, Inc. | Methods and apparatus for leads for implantable devices |
| US9327070B2 (en) | 2009-04-30 | 2016-05-03 | Medtronic, Inc. | Medical device therapy based on posture and timing |
| US9026223B2 (en) | 2009-04-30 | 2015-05-05 | Medtronic, Inc. | Therapy system including multiple posture sensors |
| US8175720B2 (en) | 2009-04-30 | 2012-05-08 | Medtronic, Inc. | Posture-responsive therapy control based on patient input |
| DE102009021492B4 (de) * | 2009-05-15 | 2012-01-12 | Siemens Aktiengesellschaft | Verfahren zur Bestimmung einer MR-Relaxationszeit im Herzmuskel bei einer Magnetresonanzuntersuchung, Magnetresonanzanlage, Computerprogrammprodukt und elektronisch lesbarer Datenträger |
| WO2011011736A2 (en) | 2009-07-23 | 2011-01-27 | Proteus Biomedical, Inc. | Solid-state thin film capacitor |
| CA2921304C (en) | 2009-07-30 | 2018-06-05 | Tandem Diabetes Care, Inc. | Infusion pump system with disposable cartridge having pressure venting and pressure feedback |
| WO2011030287A1 (en) | 2009-09-09 | 2011-03-17 | Guy Dori | Methods and apparatus for optimizing cardiac output, preventing backward heart failure, and minimizing diastolic myocardial wall stress by controlling left ventricular filling |
| US9943236B2 (en) * | 2009-09-30 | 2018-04-17 | Medtronic, Inc. | Methods for guiding heart failure decompensation therapy |
| US20110082376A1 (en) * | 2009-10-05 | 2011-04-07 | Huelskamp Paul J | Physiological blood pressure waveform compression in an acoustic channel |
| US20110106201A1 (en) * | 2009-10-30 | 2011-05-05 | Sourav Bhunia | Implantable heart failure monitor |
| US9462959B2 (en) * | 2009-11-20 | 2016-10-11 | Pacesetter, Inc. | Methods and systems that use implanted posture sensor to monitor left atrial pressure and/or inter-thoracic fluid volume |
| US20110152698A1 (en) | 2009-12-23 | 2011-06-23 | Greenhut Saul E | Method and apparatus for blood pressure waveform baseline estimation and removal |
| US9357949B2 (en) | 2010-01-08 | 2016-06-07 | Medtronic, Inc. | User interface that displays medical therapy and posture data |
| US8758274B2 (en) | 2010-01-08 | 2014-06-24 | Medtronic, Inc. | Automated adjustment of posture state definitions for a medical device |
| US9956418B2 (en) | 2010-01-08 | 2018-05-01 | Medtronic, Inc. | Graphical manipulation of posture zones for posture-responsive therapy |
| US8579834B2 (en) | 2010-01-08 | 2013-11-12 | Medtronic, Inc. | Display of detected patient posture state |
| EP2537107B1 (en) * | 2010-02-16 | 2019-05-15 | Cardiac Pacemakers, Inc. | Kinetics of physiological response to activity during activities of daily living |
| US9566441B2 (en) | 2010-04-30 | 2017-02-14 | Medtronic, Inc. | Detecting posture sensor signal shift or drift in medical devices |
| US8812093B2 (en) | 2010-08-09 | 2014-08-19 | Pacesetter, Inc. | Systems and methods for exploiting near-field impedance and admittance for use with implantable medical devices |
| US8135468B2 (en) | 2010-08-09 | 2012-03-13 | Pacesetter, Inc. | Systems and methods for estimating left atrial pressure (LAP) in patients with acute mitral valve regurgitation for use by an implantable medical device |
| US8670820B2 (en) | 2010-08-09 | 2014-03-11 | Pacesetter, Inc. | Near field-based systems and methods for assessing impedance and admittance for use with an implantable medical device |
| US8718770B2 (en) | 2010-10-21 | 2014-05-06 | Medtronic, Inc. | Capture threshold measurement for selection of pacing vector |
| AU2011349755B2 (en) | 2010-12-20 | 2015-01-22 | Cardiac Pacemakers, Inc. | Physiologic response to posture |
| US9002450B2 (en) | 2010-12-21 | 2015-04-07 | Pacesetter, Inc. | Systems and methods for assessing the sphericity and dimensional extent of heart chambers for use with an implantable medical device |
| US8355784B2 (en) | 2011-05-13 | 2013-01-15 | Medtronic, Inc. | Dynamic representation of multipolar leads in a programmer interface |
| US8768461B2 (en) | 2011-09-06 | 2014-07-01 | Pacesetter, Inc. | Systems and methods for controlling paired pacing interpulse intervals to reduce contractility disequilibrium using an implantable medical device |
| US9248300B2 (en) * | 2011-09-09 | 2016-02-02 | Medtronic, Inc. | Controlling wireless communication in an implanted cardiac device |
| US8818505B2 (en) * | 2011-09-28 | 2014-08-26 | Medtronic, Inc. | Physiological perturbations for measuring heart failure |
| US9907959B2 (en) | 2012-04-12 | 2018-03-06 | Medtronic, Inc. | Velocity detection for posture-responsive therapy |
| US9737719B2 (en) | 2012-04-26 | 2017-08-22 | Medtronic, Inc. | Adjustment of therapy based on acceleration |
| US9180242B2 (en) | 2012-05-17 | 2015-11-10 | Tandem Diabetes Care, Inc. | Methods and devices for multiple fluid transfer |
| US9238100B2 (en) | 2012-06-07 | 2016-01-19 | Tandem Diabetes Care, Inc. | Device and method for training users of ambulatory medical devices |
| US10206592B2 (en) | 2012-09-14 | 2019-02-19 | Endotronix, Inc. | Pressure sensor, anchor, delivery system and method |
| JP6522515B2 (ja) * | 2013-01-22 | 2019-06-05 | デューク ユニバーシティ | 心臓再同期療法(crt)を最適化するためのシステム及び方法 |
| US9174053B2 (en) | 2013-03-08 | 2015-11-03 | Boston Scientific Neuromodulation Corporation | Neuromodulation using modulated pulse train |
| US9173998B2 (en) | 2013-03-14 | 2015-11-03 | Tandem Diabetes Care, Inc. | System and method for detecting occlusions in an infusion pump |
| EP2796156A1 (en) * | 2013-04-24 | 2014-10-29 | ETH Zurich | Biomedical apparatus for pumping blood of a human or an animal patient through a secondary intra- or extracorporeal blood circuit |
| EP3065625A1 (en) * | 2013-11-04 | 2016-09-14 | Cardiac Pacemakers, Inc. | Heart failure detection and risk stratification system |
| WO2015198429A1 (ja) * | 2014-06-25 | 2015-12-30 | 真一 後田 | 胸郭インピーダンスに基づく心機能測定評価装置 |
| JP2017527429A (ja) | 2014-09-15 | 2017-09-21 | ボストン サイエンティフィック ニューロモデュレイション コーポレイション | 神経刺激パルスパターンをプログラムするためのグラフィカルユーザインターフェイス |
| JP6452836B2 (ja) | 2014-11-04 | 2019-01-16 | ボストン サイエンティフィック ニューロモデュレイション コーポレイション | 複合神経刺激パターンをプログラムする方法及び装置 |
| WO2017019191A1 (en) | 2015-07-30 | 2017-02-02 | Boston Scientific Neuromodulation Corporation | User interface for custom patterned electrical stimulation |
| WO2017066187A1 (en) | 2015-10-15 | 2017-04-20 | Boston Scientific Neuromodulation Corporation | User interface for neurostimulation waveform composition |
| EP3399907A4 (en) * | 2016-01-04 | 2019-08-28 | Aventusoft, LLC | SYSTEM AND METHOD FOR MEASURING HEMODYNAMIC PARAMETERS FROM CARDIAC VALVE SIGNALS |
| US12478285B2 (en) | 2016-08-02 | 2025-11-25 | Medtronic, Inc. | Accelerometer signal change as a measure of patient functional status |
| US10952686B2 (en) | 2016-08-02 | 2021-03-23 | Medtronic, Inc. | Mobile application to prompt physical action to measure physiologic response in implantable device |
| US10610132B2 (en) | 2016-08-02 | 2020-04-07 | Medtronic, Inc. | Step detection using accelerometer axis |
| US10835133B2 (en) | 2016-12-20 | 2020-11-17 | Medtronic, Inc. | Hydrostatic offset adjustment for measured cardiovascular pressure values |
| US20180168460A1 (en) | 2016-12-20 | 2018-06-21 | Medtronic, Inc. | Measuring cardiovascular pressure based on patient state |
| US10376159B2 (en) | 2016-12-20 | 2019-08-13 | Medtronic, Inc. | Exercise triggered cardiovascular pressure measurement |
| AU2018254569B2 (en) | 2017-04-20 | 2022-05-12 | Endotronix, Inc. | Anchoring system for a catheter delivered device |
| EP4445834A3 (en) | 2017-04-29 | 2024-12-25 | Cardiac Pacemakers, Inc. | Heart failure event rate assessment |
| EP4656128A2 (en) | 2017-07-19 | 2025-12-03 | Endotronix, Inc. | Physiological monitoring system |
| WO2019046837A1 (en) | 2017-09-02 | 2019-03-07 | Precision Drone Services Intellectual Property, Llc | SEED DISTRIBUTION ASSEMBLY FOR AERIAL VEHICLE |
| US11717186B2 (en) | 2019-08-27 | 2023-08-08 | Medtronic, Inc. | Body stability measurement |
| USD972150S1 (en) | 2019-09-17 | 2022-12-06 | AventuSoft, LLC | Physiological monitor |
| US11602313B2 (en) | 2020-07-28 | 2023-03-14 | Medtronic, Inc. | Determining a fall risk responsive to detecting body position movements |
| US12453859B2 (en) | 2021-02-24 | 2025-10-28 | Medtronic, Inc. | Posture state definition calibration |
Family Cites Families (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3857399A (en) | 1970-03-24 | 1974-12-31 | F Zacouto | Heart pacer |
| FR2248020B1 (enExample) | 1973-10-18 | 1977-05-27 | Pequignot Michel | |
| US4541417A (en) | 1982-06-07 | 1985-09-17 | Krikorian Paul P | Coronary augmenter |
| US4554922A (en) | 1982-09-30 | 1985-11-26 | Prystowsky Eric N | Method of inhibiting cardiac arrhythmias |
| US4674518A (en) | 1985-09-06 | 1987-06-23 | Cardiac Pacemakers, Inc. | Method and apparatus for measuring ventricular volume |
| US5024222A (en) | 1990-02-21 | 1991-06-18 | Siemens-Pacesetter, Inc. | Hemodynamically rate responsive pacemaker and method of automatically adjusting the escape and A-V intervals |
| AU654552B2 (en) | 1991-04-05 | 1994-11-10 | Medtronic, Inc. | Subcutaneous multi-electrode sensing system |
| US6021345A (en) | 1991-05-17 | 2000-02-01 | Cedars-Sinai Medical Center | Methods for detecting propensity for fibrillation using an electrical restitution curve |
| US5213098A (en) | 1991-07-26 | 1993-05-25 | Medtronic, Inc. | Post-extrasystolic potentiation stimulation with physiologic sensor feedback |
| EP0541338B1 (en) | 1991-11-04 | 1996-09-11 | Cardiac Pacemakers, Inc. | Implantable cardiac function monitor and stimulator for diagnosis and therapy delivery |
| US5328442A (en) | 1992-11-20 | 1994-07-12 | Siemens Pacesetter, Inc. | System and method for stimulating a heart having undergone cardiac myoplasty using a single-chamber pacemaker |
| US5564434A (en) | 1995-02-27 | 1996-10-15 | Medtronic, Inc. | Implantable capacitive absolute pressure and temperature sensor |
| AU724404B2 (en) | 1996-01-08 | 2000-09-21 | Impulse Dynamics N.V. | Electrical muscle controller |
| US5626623A (en) | 1996-04-30 | 1997-05-06 | Medtronic, Inc. | Method and apparatus for optimizing pacemaker AV delay |
| WO1998002209A2 (en) | 1996-07-11 | 1998-01-22 | Medtronic, Inc. | Minimally invasive implantable device for monitoring physiologic events |
| US6141586A (en) | 1996-08-19 | 2000-10-31 | Mower Family Chf Treatment Irrevocable Trust | Method and apparatus to allow cyclic pacing at an average rate just above the intrinsic heart rate so as to maximize inotropic pacing effects at minimal heart rates |
| US5800464A (en) | 1996-10-03 | 1998-09-01 | Medtronic, Inc. | System for providing hyperpolarization of cardiac to enhance cardiac function |
| AU5427098A (en) | 1996-11-04 | 1998-05-29 | Johns Hopkins University, The | Assessing cardiac contractility and cardiovascular interaction |
| US6104949A (en) | 1998-09-09 | 2000-08-15 | Vitatron Medical, B.V. | Medical device |
-
2000
- 2000-12-28 US US09/751,079 patent/US6438408B1/en not_active Expired - Fee Related
-
2001
- 2001-12-27 JP JP2002554176A patent/JP2004528864A/ja active Pending
- 2001-12-27 CA CA002433049A patent/CA2433049A1/en not_active Abandoned
- 2001-12-27 EP EP01991449A patent/EP1345651B1/en not_active Expired - Lifetime
- 2001-12-27 DE DE60118433T patent/DE60118433T2/de not_active Expired - Lifetime
- 2001-12-27 WO PCT/US2001/049620 patent/WO2002053228A1/en not_active Ceased
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2006516449A (ja) * | 2003-01-24 | 2006-07-06 | プロテウス バイオメディカル インコーポレイテッド | 心臓ペーシングを改善するための方法および装置 |
| JP2008521578A (ja) * | 2004-12-03 | 2008-06-26 | メドトロニック・インコーポレーテッド | 機械的復元を監視して、高頻度調律の血行力学的耐性を予測する埋め込み可能システム |
| JP2010524636A (ja) * | 2007-05-07 | 2010-07-22 | カーディアック ペースメイカーズ, インコーポレイテッド | 血行動態耐容性を判定するシステムおよび方法 |
| US10506943B2 (en) | 2007-09-05 | 2019-12-17 | Sensible Medical Innovations Ltd. | Methods and systems for monitoring intrabody tissues |
| JP2015107338A (ja) * | 2007-09-05 | 2015-06-11 | センシブル メディカル イノヴェイションズ リミテッド | 胸部組織液を監視するための方法およびシステム |
| US10561336B2 (en) | 2007-09-05 | 2020-02-18 | Sensible Medical Innovations Ltd. | Method and system for monitoring thoracic tissue fluid |
| US10758150B2 (en) | 2007-09-05 | 2020-09-01 | Sensible Medical lnnovations Ltd. | Method, system and apparatus for using electromagnetic radiation for monitoring a tissue of a user |
| US11564586B2 (en) | 2007-09-05 | 2023-01-31 | Sensible Medical Innovations Ltd. | Method and system for monitoring thoracic tissue fluid |
| US11944419B2 (en) | 2007-09-05 | 2024-04-02 | Sensible Medical Innovations Ltd. | Method and system for monitoring thoracic tissue fluid |
| US12059238B2 (en) | 2007-09-05 | 2024-08-13 | Sensible Medical Innovations Ltd. | Method, system and apparatus for using electromagnetic radiation for monitoring a tissue of a user |
| US12303245B2 (en) | 2007-09-05 | 2025-05-20 | Sensible Medical Innovations Ltd. | Method and system for monitoring thoracic tissue fluid |
| JP2009247812A (ja) * | 2008-04-10 | 2009-10-29 | Nipro Corp | 心電図の表示方法および心電図表示装置 |
| US8509892B2 (en) | 2008-06-19 | 2013-08-13 | Cardiac Pacemakers, Inc. | Method and apparatus for controlling anti-tachyarrhythmia therapy using hemodynamic tolerability |
| US10667715B2 (en) | 2008-08-20 | 2020-06-02 | Sensible Medical Innovations Ltd. | Methods and devices of cardiac tissue monitoring and analysis |
| US11529065B2 (en) | 2008-08-20 | 2022-12-20 | Sensible Medical Innovations Ltd. | Methods and devices of cardiac tissue monitoring and analysis |
Also Published As
| Publication number | Publication date |
|---|---|
| US6438408B1 (en) | 2002-08-20 |
| DE60118433T2 (de) | 2006-11-16 |
| DE60118433D1 (de) | 2006-05-18 |
| EP1345651A1 (en) | 2003-09-24 |
| US20020115939A1 (en) | 2002-08-22 |
| WO2002053228A1 (en) | 2002-07-11 |
| CA2433049A1 (en) | 2002-07-11 |
| EP1345651B1 (en) | 2006-03-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| JP4160391B2 (ja) | 電気刺激により機械的心機能障害を処置する埋め込み可能な医療デバイス | |
| EP1345651B1 (en) | Implantable medical device for monitoring congestive heart failure | |
| US7769451B2 (en) | Method and apparatus for optimizing cardiac resynchronization therapy | |
| US6959214B2 (en) | Implantable medical device for measuring mechanical heart function | |
| US7904158B2 (en) | Measurement of coronary sinus parameters to optimize left ventricular performance | |
| JP4170591B2 (ja) | 調整可能心房心室遅延を使った心臓ペーシング | |
| US7184835B2 (en) | Method and apparatus for adjustable AVD programming using a table | |
| CN109640809B (zh) | 使用p波到起搏定时的集成式多装置心脏再同步治疗 | |
| EP1735049B1 (en) | Real-time optimization of right to left ventricular timing sequence in bi-ventircular pacing of heart failure patients | |
| US7286875B1 (en) | Monitoring ventricular contractions using an implantable stimulation device | |
| JP2005507721A (ja) | 心臓血圧および腔寸法を監視する埋め込み可能医療デバイス | |
| US7212861B1 (en) | Monitoring ventricular contractions using an implantable stimulation device | |
| JP2005501617A (ja) | 電気刺激により心臓のメカニカル機能不全を治療するシステム | |
| US8634911B2 (en) | Pacing interval determination for ventricular dyssynchrony | |
| US20060247698A1 (en) | Multi-site PESP with fusion pacing | |
| US7711423B2 (en) | Algorithm for the automatic determination of optimal pacing intervals | |
| US7003348B1 (en) | Monitoring cardiac geometry for diagnostics and therapy | |
| US10874861B2 (en) | Dual chamber pacing without beat-to-beat communication | |
| US7283873B1 (en) | Monitoring and synchronizing ventricular contractions using an implantable stimulation device |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| A521 | Request for written amendment filed |
Free format text: JAPANESE INTERMEDIATE CODE: A523 Effective date: 20041118 |
|
| A621 | Written request for application examination |
Free format text: JAPANESE INTERMEDIATE CODE: A621 Effective date: 20041118 |
|
| A131 | Notification of reasons for refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A131 Effective date: 20070320 |
|
| A601 | Written request for extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A601 Effective date: 20070322 |
|
| A602 | Written permission of extension of time |
Free format text: JAPANESE INTERMEDIATE CODE: A602 Effective date: 20070329 |
|
| A02 | Decision of refusal |
Free format text: JAPANESE INTERMEDIATE CODE: A02 Effective date: 20080115 |