EP4119668A1 - Aav vectors for treatment of dominant retinitis pigmentosa - Google Patents
Aav vectors for treatment of dominant retinitis pigmentosa Download PDFInfo
- Publication number
- EP4119668A1 EP4119668A1 EP22170988.4A EP22170988A EP4119668A1 EP 4119668 A1 EP4119668 A1 EP 4119668A1 EP 22170988 A EP22170988 A EP 22170988A EP 4119668 A1 EP4119668 A1 EP 4119668A1
- Authority
- EP
- European Patent Office
- Prior art keywords
- rna
- rho
- sequence
- vector
- seq
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Pending
Links
- 208000007014 Retinitis pigmentosa Diseases 0.000 title claims abstract description 23
- 239000013598 vector Substances 0.000 title claims description 68
- 238000011282 treatment Methods 0.000 title description 12
- 101150079354 rho gene Proteins 0.000 claims abstract description 62
- 230000002452 interceptive effect Effects 0.000 claims abstract description 58
- 239000000203 mixture Substances 0.000 claims abstract description 58
- 230000014509 gene expression Effects 0.000 claims abstract description 23
- 108700028369 Alleles Proteins 0.000 claims abstract description 10
- 102100040756 Rhodopsin Human genes 0.000 claims description 183
- 229920002477 rna polymer Polymers 0.000 claims description 163
- 239000004055 small Interfering RNA Substances 0.000 claims description 89
- 108091027967 Small hairpin RNA Proteins 0.000 claims description 61
- 108020004459 Small interfering RNA Proteins 0.000 claims description 51
- 101000611338 Homo sapiens Rhodopsin Proteins 0.000 claims description 19
- 239000002679 microRNA Substances 0.000 claims description 19
- 239000013603 viral vector Substances 0.000 claims description 15
- ISAKRJDGNUQOIC-UHFFFAOYSA-N Uracil Chemical compound O=C1C=CNC(=O)N1 ISAKRJDGNUQOIC-UHFFFAOYSA-N 0.000 claims description 14
- 230000000692 anti-sense effect Effects 0.000 claims description 14
- 108091081021 Sense strand Proteins 0.000 claims description 11
- 108700011259 MicroRNAs Proteins 0.000 claims description 10
- 108091026821 Artificial microRNA Proteins 0.000 claims description 7
- 229940035893 uracil Drugs 0.000 claims description 7
- 230000003247 decreasing effect Effects 0.000 claims description 5
- 230000030279 gene silencing Effects 0.000 claims description 5
- 239000002671 adjuvant Substances 0.000 claims description 4
- 239000000969 carrier Substances 0.000 claims description 4
- 239000013613 expression plasmid Substances 0.000 claims description 3
- 238000000034 method Methods 0.000 abstract description 46
- 150000007523 nucleic acids Chemical class 0.000 abstract description 36
- 102000039446 nucleic acids Human genes 0.000 abstract description 35
- 108020004707 nucleic acids Proteins 0.000 abstract description 35
- 101800001318 Capsid protein VP4 Proteins 0.000 abstract description 11
- 108090000820 Rhodopsin Proteins 0.000 description 173
- NCYCYZXNIZJOKI-IOUUIBBYSA-N 11-cis-retinal Chemical compound O=C/C=C(\C)/C=C\C=C(/C)\C=C\C1=C(C)CCCC1(C)C NCYCYZXNIZJOKI-IOUUIBBYSA-N 0.000 description 158
- 241000702423 Adeno-associated virus - 2 Species 0.000 description 99
- 108090000623 proteins and genes Proteins 0.000 description 88
- 241000282472 Canis lupus familiaris Species 0.000 description 81
- 241000282465 Canis Species 0.000 description 65
- 208000002352 blister Diseases 0.000 description 60
- 238000002347 injection Methods 0.000 description 53
- 239000007924 injection Substances 0.000 description 53
- 239000002245 particle Substances 0.000 description 51
- 102000004169 proteins and genes Human genes 0.000 description 45
- 210000004027 cell Anatomy 0.000 description 44
- 230000003612 virological effect Effects 0.000 description 41
- 210000001525 retina Anatomy 0.000 description 39
- 238000001574 biopsy Methods 0.000 description 36
- 108020004999 messenger RNA Proteins 0.000 description 36
- 241000282414 Homo sapiens Species 0.000 description 34
- 230000002207 retinal effect Effects 0.000 description 24
- 238000001262 western blot Methods 0.000 description 23
- 239000013612 plasmid Substances 0.000 description 19
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 18
- 101100518359 Homo sapiens RHO gene Proteins 0.000 description 17
- 201000007737 Retinal degeneration Diseases 0.000 description 17
- 230000004258 retinal degeneration Effects 0.000 description 17
- 108091032973 (ribonucleotides)n+m Proteins 0.000 description 15
- 102000040650 (ribonucleotides)n+m Human genes 0.000 description 15
- 238000004458 analytical method Methods 0.000 description 15
- 108091008695 photoreceptors Proteins 0.000 description 15
- 108090000565 Capsid Proteins Proteins 0.000 description 12
- 102100023321 Ceruloplasmin Human genes 0.000 description 12
- 238000001727 in vivo Methods 0.000 description 12
- 230000000670 limiting effect Effects 0.000 description 12
- 108091070501 miRNA Proteins 0.000 description 12
- 102000014450 RNA Polymerase III Human genes 0.000 description 11
- 108010078067 RNA Polymerase III Proteins 0.000 description 11
- 201000010099 disease Diseases 0.000 description 11
- 238000003384 imaging method Methods 0.000 description 11
- 238000003364 immunohistochemistry Methods 0.000 description 11
- 238000004519 manufacturing process Methods 0.000 description 11
- 238000004321 preservation Methods 0.000 description 11
- 238000010186 staining Methods 0.000 description 11
- 238000006467 substitution reaction Methods 0.000 description 11
- 230000009452 underexpressoin Effects 0.000 description 11
- 108020004414 DNA Proteins 0.000 description 10
- 239000002299 complementary DNA Substances 0.000 description 10
- 238000003119 immunoblot Methods 0.000 description 10
- 108091026890 Coding region Proteins 0.000 description 9
- 108091028043 Nucleic acid sequence Proteins 0.000 description 9
- 238000002474 experimental method Methods 0.000 description 9
- 239000002773 nucleotide Substances 0.000 description 9
- 125000003729 nucleotide group Chemical group 0.000 description 9
- 239000000243 solution Substances 0.000 description 9
- NYHBQMYGNKIUIF-UUOKFMHZSA-N Guanosine Chemical compound C1=NC=2C(=O)NC(N)=NC=2N1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O NYHBQMYGNKIUIF-UUOKFMHZSA-N 0.000 description 8
- 108010033040 Histones Proteins 0.000 description 8
- 101000785755 Homo sapiens Arrestin-C Proteins 0.000 description 8
- 239000005090 green fluorescent protein Substances 0.000 description 8
- 230000001154 acute effect Effects 0.000 description 7
- 208000035475 disorder Diseases 0.000 description 7
- 230000000694 effects Effects 0.000 description 7
- 239000000463 material Substances 0.000 description 7
- 230000008832 photodamage Effects 0.000 description 7
- 108090000765 processed proteins & peptides Proteins 0.000 description 7
- 230000009467 reduction Effects 0.000 description 7
- 210000001519 tissue Anatomy 0.000 description 7
- 241000972680 Adeno-associated virus - 6 Species 0.000 description 6
- 102100032982 CCR4-NOT transcription complex subunit 9 Human genes 0.000 description 6
- 101150032562 CNOT9 gene Proteins 0.000 description 6
- 241000702421 Dependoparvovirus Species 0.000 description 6
- PEDCQBHIVMGVHV-UHFFFAOYSA-N Glycerine Chemical compound OCC(O)CO PEDCQBHIVMGVHV-UHFFFAOYSA-N 0.000 description 6
- 102000006947 Histones Human genes 0.000 description 6
- 241000124008 Mammalia Species 0.000 description 6
- 241000699666 Mus <mouse, genus> Species 0.000 description 6
- 239000003795 chemical substances by application Substances 0.000 description 6
- 230000001939 inductive effect Effects 0.000 description 6
- 230000035772 mutation Effects 0.000 description 6
- 238000010606 normalization Methods 0.000 description 6
- 102000004196 processed proteins & peptides Human genes 0.000 description 6
- 230000010076 replication Effects 0.000 description 6
- 210000004358 rod cell outer segment Anatomy 0.000 description 6
- 108700026220 vif Genes Proteins 0.000 description 6
- FWMNVWWHGCHHJJ-SKKKGAJSSA-N 4-amino-1-[(2r)-6-amino-2-[[(2r)-2-[[(2r)-2-[[(2r)-2-amino-3-phenylpropanoyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]hexanoyl]piperidine-4-carboxylic acid Chemical compound C([C@H](C(=O)N[C@H](CC(C)C)C(=O)N[C@H](CCCCN)C(=O)N1CCC(N)(CC1)C(O)=O)NC(=O)[C@H](N)CC=1C=CC=CC=1)C1=CC=CC=C1 FWMNVWWHGCHHJJ-SKKKGAJSSA-N 0.000 description 5
- 241001634120 Adeno-associated virus - 5 Species 0.000 description 5
- 238000009010 Bradford assay Methods 0.000 description 5
- 238000001190 Q-PCR Methods 0.000 description 5
- 102000009572 RNA Polymerase II Human genes 0.000 description 5
- 108010009460 RNA Polymerase II Proteins 0.000 description 5
- 238000011529 RT qPCR Methods 0.000 description 5
- FAPWRFPIFSIZLT-UHFFFAOYSA-M Sodium chloride Chemical compound [Na+].[Cl-] FAPWRFPIFSIZLT-UHFFFAOYSA-M 0.000 description 5
- 230000002583 anti-histone Effects 0.000 description 5
- 230000008901 benefit Effects 0.000 description 5
- 230000000903 blocking effect Effects 0.000 description 5
- 239000000872 buffer Substances 0.000 description 5
- 239000007853 buffer solution Substances 0.000 description 5
- 210000000234 capsid Anatomy 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 238000010790 dilution Methods 0.000 description 5
- 239000012895 dilution Substances 0.000 description 5
- 229940042399 direct acting antivirals protease inhibitors Drugs 0.000 description 5
- 238000011156 evaluation Methods 0.000 description 5
- 238000009472 formulation Methods 0.000 description 5
- 238000011068 loading method Methods 0.000 description 5
- 238000012986 modification Methods 0.000 description 5
- 230000004048 modification Effects 0.000 description 5
- 239000002105 nanoparticle Substances 0.000 description 5
- 239000008188 pellet Substances 0.000 description 5
- 239000000137 peptide hydrolase inhibitor Substances 0.000 description 5
- 229920001184 polypeptide Polymers 0.000 description 5
- -1 rRNA Proteins 0.000 description 5
- 239000006228 supernatant Substances 0.000 description 5
- 230000008685 targeting Effects 0.000 description 5
- 230000001225 therapeutic effect Effects 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- 108090000994 Catalytic RNA Proteins 0.000 description 4
- 102000053642 Catalytic RNA Human genes 0.000 description 4
- MIKUYHXYGGJMLM-GIMIYPNGSA-N Crotonoside Natural products C1=NC2=C(N)NC(=O)N=C2N1[C@H]1O[C@@H](CO)[C@H](O)[C@@H]1O MIKUYHXYGGJMLM-GIMIYPNGSA-N 0.000 description 4
- NYHBQMYGNKIUIF-UHFFFAOYSA-N D-guanosine Natural products C1=2NC(N)=NC(=O)C=2N=CN1C1OC(CO)C(O)C1O NYHBQMYGNKIUIF-UHFFFAOYSA-N 0.000 description 4
- 241001465754 Metazoa Species 0.000 description 4
- 239000004480 active ingredient Substances 0.000 description 4
- 150000001413 amino acids Chemical group 0.000 description 4
- 238000001943 fluorescence-activated cell sorting Methods 0.000 description 4
- 229940029575 guanosine Drugs 0.000 description 4
- 150000002632 lipids Chemical class 0.000 description 4
- 239000007788 liquid Substances 0.000 description 4
- 239000013646 rAAV2 vector Substances 0.000 description 4
- 101150066583 rep gene Proteins 0.000 description 4
- 108091092562 ribozyme Proteins 0.000 description 4
- 239000011780 sodium chloride Substances 0.000 description 4
- 241000894007 species Species 0.000 description 4
- 238000011269 treatment regimen Methods 0.000 description 4
- 101150044789 Cap gene Proteins 0.000 description 3
- LFQSCWFLJHTTHZ-UHFFFAOYSA-N Ethanol Chemical compound CCO LFQSCWFLJHTTHZ-UHFFFAOYSA-N 0.000 description 3
- 239000002202 Polyethylene glycol Substances 0.000 description 3
- DNIAPMSPPWPWGF-UHFFFAOYSA-N Propylene glycol Chemical compound CC(O)CO DNIAPMSPPWPWGF-UHFFFAOYSA-N 0.000 description 3
- 241000283984 Rodentia Species 0.000 description 3
- 239000007864 aqueous solution Substances 0.000 description 3
- 230000015572 biosynthetic process Effects 0.000 description 3
- 238000005520 cutting process Methods 0.000 description 3
- 230000007850 degeneration Effects 0.000 description 3
- 238000013461 design Methods 0.000 description 3
- 239000006185 dispersion Substances 0.000 description 3
- 239000003814 drug Substances 0.000 description 3
- 238000001415 gene therapy Methods 0.000 description 3
- 239000004615 ingredient Substances 0.000 description 3
- 238000007918 intramuscular administration Methods 0.000 description 3
- 238000001990 intravenous administration Methods 0.000 description 3
- 239000012528 membrane Substances 0.000 description 3
- 210000000056 organ Anatomy 0.000 description 3
- 210000000608 photoreceptor cell Anatomy 0.000 description 3
- 229920001223 polyethylene glycol Polymers 0.000 description 3
- 238000002360 preparation method Methods 0.000 description 3
- 230000002829 reductive effect Effects 0.000 description 3
- 231100000331 toxic Toxicity 0.000 description 3
- 230000002588 toxic effect Effects 0.000 description 3
- 238000001890 transfection Methods 0.000 description 3
- 230000007704 transition Effects 0.000 description 3
- 238000011144 upstream manufacturing Methods 0.000 description 3
- 239000013607 AAV vector Substances 0.000 description 2
- 241001655883 Adeno-associated virus - 1 Species 0.000 description 2
- 241000202702 Adeno-associated virus - 3 Species 0.000 description 2
- 241000580270 Adeno-associated virus - 4 Species 0.000 description 2
- 241001164825 Adeno-associated virus - 8 Species 0.000 description 2
- 201000004569 Blindness Diseases 0.000 description 2
- 102000004190 Enzymes Human genes 0.000 description 2
- 108090000790 Enzymes Proteins 0.000 description 2
- 241000282326 Felis catus Species 0.000 description 2
- WQZGKKKJIJFFOK-GASJEMHNSA-N Glucose Natural products OC[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-GASJEMHNSA-N 0.000 description 2
- 108010043121 Green Fluorescent Proteins Proteins 0.000 description 2
- 241000699670 Mus sp. Species 0.000 description 2
- 102000010175 Opsin Human genes 0.000 description 2
- 108050001704 Opsin Proteins 0.000 description 2
- 229920002873 Polyethylenimine Polymers 0.000 description 2
- 238000013381 RNA quantification Methods 0.000 description 2
- 241000700605 Viruses Species 0.000 description 2
- 230000009471 action Effects 0.000 description 2
- 239000013543 active substance Substances 0.000 description 2
- 238000010171 animal model Methods 0.000 description 2
- WQZGKKKJIJFFOK-VFUOTHLCSA-N beta-D-glucose Chemical compound OC[C@H]1O[C@@H](O)[C@H](O)[C@@H](O)[C@@H]1O WQZGKKKJIJFFOK-VFUOTHLCSA-N 0.000 description 2
- AIYUHDOJVYHVIT-UHFFFAOYSA-M caesium chloride Chemical compound [Cl-].[Cs+] AIYUHDOJVYHVIT-UHFFFAOYSA-M 0.000 description 2
- 230000015556 catabolic process Effects 0.000 description 2
- 125000002091 cationic group Chemical group 0.000 description 2
- 230000001413 cellular effect Effects 0.000 description 2
- 230000008859 change Effects 0.000 description 2
- HVYWMOMLDIMFJA-DPAQBDIFSA-N cholesterol Chemical compound C1C=C2C[C@@H](O)CC[C@]2(C)[C@@H]2[C@@H]1[C@@H]1CC[C@H]([C@H](C)CCCC(C)C)[C@@]1(C)CC2 HVYWMOMLDIMFJA-DPAQBDIFSA-N 0.000 description 2
- 230000000295 complement effect Effects 0.000 description 2
- 239000002131 composite material Substances 0.000 description 2
- 238000006731 degradation reaction Methods 0.000 description 2
- 239000003085 diluting agent Substances 0.000 description 2
- MWRBNPKJOOWZPW-CLFAGFIQSA-N dioleoyl phosphatidylethanolamine Chemical compound CCCCCCCC\C=C/CCCCCCCC(=O)OCC(COP(O)(=O)OCCN)OC(=O)CCCCCCC\C=C/CCCCCCCC MWRBNPKJOOWZPW-CLFAGFIQSA-N 0.000 description 2
- 239000002612 dispersion medium Substances 0.000 description 2
- 239000003937 drug carrier Substances 0.000 description 2
- 238000013401 experimental design Methods 0.000 description 2
- 238000000684 flow cytometry Methods 0.000 description 2
- 239000012530 fluid Substances 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 239000007943 implant Substances 0.000 description 2
- 239000003112 inhibitor Substances 0.000 description 2
- 238000007912 intraperitoneal administration Methods 0.000 description 2
- 238000005259 measurement Methods 0.000 description 2
- 230000001404 mediated effect Effects 0.000 description 2
- 238000009126 molecular therapy Methods 0.000 description 2
- 239000000178 monomer Substances 0.000 description 2
- 239000003921 oil Substances 0.000 description 2
- 235000019198 oils Nutrition 0.000 description 2
- 239000008194 pharmaceutical composition Substances 0.000 description 2
- 239000000546 pharmaceutical excipient Substances 0.000 description 2
- 230000016732 phototransduction Effects 0.000 description 2
- 239000000843 powder Substances 0.000 description 2
- 239000000047 product Substances 0.000 description 2
- 238000000746 purification Methods 0.000 description 2
- 238000011002 quantification Methods 0.000 description 2
- 230000022532 regulation of transcription, DNA-dependent Effects 0.000 description 2
- 238000004904 shortening Methods 0.000 description 2
- 239000002904 solvent Substances 0.000 description 2
- 238000007920 subcutaneous administration Methods 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000012360 testing method Methods 0.000 description 2
- 230000000699 topical effect Effects 0.000 description 2
- 231100000419 toxicity Toxicity 0.000 description 2
- 230000001988 toxicity Effects 0.000 description 2
- 235000015112 vegetable and seed oil Nutrition 0.000 description 2
- 239000008158 vegetable oil Substances 0.000 description 2
- 239000003981 vehicle Substances 0.000 description 2
- 238000012800 visualization Methods 0.000 description 2
- XLYOFNOQVPJJNP-UHFFFAOYSA-N water Substances O XLYOFNOQVPJJNP-UHFFFAOYSA-N 0.000 description 2
- IIZPXYDJLKNOIY-JXPKJXOSSA-N 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine Chemical compound CCCCCCCCCCCCCCCC(=O)OC[C@H](COP([O-])(=O)OCC[N+](C)(C)C)OC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC IIZPXYDJLKNOIY-JXPKJXOSSA-N 0.000 description 1
- ZOOGRGPOEVQQDX-UUOKFMHZSA-N 3',5'-cyclic GMP Chemical compound C([C@H]1O2)OP(O)(=O)O[C@H]1[C@@H](O)[C@@H]2N1C(N=C(NC2=O)N)=C2N=C1 ZOOGRGPOEVQQDX-UUOKFMHZSA-N 0.000 description 1
- 241001164823 Adeno-associated virus - 7 Species 0.000 description 1
- 241000649045 Adeno-associated virus 10 Species 0.000 description 1
- 101100524317 Adeno-associated virus 2 (isolate Srivastava/1982) Rep40 gene Proteins 0.000 description 1
- 101100524319 Adeno-associated virus 2 (isolate Srivastava/1982) Rep52 gene Proteins 0.000 description 1
- 101100524321 Adeno-associated virus 2 (isolate Srivastava/1982) Rep68 gene Proteins 0.000 description 1
- 101100524324 Adeno-associated virus 2 (isolate Srivastava/1982) Rep78 gene Proteins 0.000 description 1
- 108020005544 Antisense RNA Proteins 0.000 description 1
- 241000282709 Aotus trivirgatus Species 0.000 description 1
- 101100448720 Arabidopsis thaliana GLN1-2 gene Proteins 0.000 description 1
- 241000282672 Ateles sp. Species 0.000 description 1
- 241000894006 Bacteria Species 0.000 description 1
- 241000283690 Bos taurus Species 0.000 description 1
- 102000049320 CD36 Human genes 0.000 description 1
- 108010045374 CD36 Antigens Proteins 0.000 description 1
- 101001086434 Canis lupus familiaris Rhodopsin Proteins 0.000 description 1
- 241000283707 Capra Species 0.000 description 1
- 241000700198 Cavia Species 0.000 description 1
- 241000862448 Chlorocebus Species 0.000 description 1
- 108020004705 Codon Proteins 0.000 description 1
- 241000699800 Cricetinae Species 0.000 description 1
- 102100029140 Cyclic nucleotide-gated cation channel beta-3 Human genes 0.000 description 1
- 101150066038 E4 gene Proteins 0.000 description 1
- 241000283086 Equidae Species 0.000 description 1
- 241000233866 Fungi Species 0.000 description 1
- 102000004437 G-Protein-Coupled Receptor Kinase 1 Human genes 0.000 description 1
- 241000287828 Gallus gallus Species 0.000 description 1
- 241000282575 Gorilla Species 0.000 description 1
- 102000004144 Green Fluorescent Proteins Human genes 0.000 description 1
- 241000282412 Homo Species 0.000 description 1
- 101000771083 Homo sapiens Cyclic nucleotide-gated cation channel beta-3 Proteins 0.000 description 1
- 101000729271 Homo sapiens Retinoid isomerohydrolase Proteins 0.000 description 1
- VSNHCAURESNICA-UHFFFAOYSA-N Hydroxyurea Chemical compound NC(=O)NO VSNHCAURESNICA-UHFFFAOYSA-N 0.000 description 1
- 102000004310 Ion Channels Human genes 0.000 description 1
- 108090000862 Ion Channels Proteins 0.000 description 1
- 241000282553 Macaca Species 0.000 description 1
- 241000282567 Macaca fascicularis Species 0.000 description 1
- 241000282560 Macaca mulatta Species 0.000 description 1
- 241000282579 Pan Species 0.000 description 1
- 241000282520 Papio Species 0.000 description 1
- 235000019483 Peanut oil Nutrition 0.000 description 1
- 241001494479 Pecora Species 0.000 description 1
- 108010039918 Polylysine Proteins 0.000 description 1
- 241000282405 Pongo abelii Species 0.000 description 1
- 229940079156 Proteasome inhibitor Drugs 0.000 description 1
- 102000017143 RNA Polymerase I Human genes 0.000 description 1
- 108010013845 RNA Polymerase I Proteins 0.000 description 1
- 238000012228 RNA interference-mediated gene silencing Methods 0.000 description 1
- 241000700159 Rattus Species 0.000 description 1
- 102100031176 Retinoid isomerohydrolase Human genes 0.000 description 1
- 102000005801 Rod Opsins Human genes 0.000 description 1
- 108010005063 Rod Opsins Proteins 0.000 description 1
- 240000004808 Saccharomyces cerevisiae Species 0.000 description 1
- 241000288961 Saguinus imperator Species 0.000 description 1
- 241000282695 Saimiri Species 0.000 description 1
- 241000282887 Suidae Species 0.000 description 1
- 108020004566 Transfer RNA Proteins 0.000 description 1
- 206010047531 Visual acuity reduced Diseases 0.000 description 1
- 101150063416 add gene Proteins 0.000 description 1
- 230000002411 adverse Effects 0.000 description 1
- 239000010775 animal oil Substances 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 239000012736 aqueous medium Substances 0.000 description 1
- 238000003556 assay Methods 0.000 description 1
- 230000001580 bacterial effect Effects 0.000 description 1
- 229920002988 biodegradable polymer Polymers 0.000 description 1
- 239000004621 biodegradable polymer Substances 0.000 description 1
- 229960000074 biopharmaceutical Drugs 0.000 description 1
- GXJABQQUPOEUTA-RDJZCZTQSA-N bortezomib Chemical compound C([C@@H](C(=O)N[C@@H](CC(C)C)B(O)O)NC(=O)C=1N=CC=NC=1)C1=CC=CC=C1 GXJABQQUPOEUTA-RDJZCZTQSA-N 0.000 description 1
- 229960001467 bortezomib Drugs 0.000 description 1
- 210000004556 brain Anatomy 0.000 description 1
- 210000005056 cell body Anatomy 0.000 description 1
- 238000004113 cell culture Methods 0.000 description 1
- 230000030833 cell death Effects 0.000 description 1
- 235000013330 chicken meat Nutrition 0.000 description 1
- 235000012000 cholesterol Nutrition 0.000 description 1
- 238000004587 chromatography analysis Methods 0.000 description 1
- 238000003776 cleavage reaction Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 238000000576 coating method Methods 0.000 description 1
- 239000003184 complementary RNA Substances 0.000 description 1
- 150000001875 compounds Chemical class 0.000 description 1
- ZOOGRGPOEVQQDX-UHFFFAOYSA-N cyclic GMP Natural products O1C2COP(O)(=O)OC2C(O)C1N1C=NC2=C1NC(N)=NC2=O ZOOGRGPOEVQQDX-UHFFFAOYSA-N 0.000 description 1
- 210000000805 cytoplasm Anatomy 0.000 description 1
- 230000006378 damage Effects 0.000 description 1
- 230000000779 depleting effect Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 230000018109 developmental process Effects 0.000 description 1
- 239000008121 dextrose Substances 0.000 description 1
- 239000002552 dosage form Substances 0.000 description 1
- 229940079593 drug Drugs 0.000 description 1
- 238000002651 drug therapy Methods 0.000 description 1
- 238000005516 engineering process Methods 0.000 description 1
- 230000000925 erythroid effect Effects 0.000 description 1
- 210000003013 erythroid precursor cell Anatomy 0.000 description 1
- 239000000835 fiber Substances 0.000 description 1
- 239000012634 fragment Substances 0.000 description 1
- 238000004108 freeze drying Methods 0.000 description 1
- 230000009368 gene silencing by RNA Effects 0.000 description 1
- 239000008103 glucose Substances 0.000 description 1
- 230000013595 glycosylation Effects 0.000 description 1
- 238000006206 glycosylation reaction Methods 0.000 description 1
- 238000003306 harvesting Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 210000005260 human cell Anatomy 0.000 description 1
- 229960001330 hydroxycarbamide Drugs 0.000 description 1
- 208000015181 infectious disease Diseases 0.000 description 1
- 230000002458 infectious effect Effects 0.000 description 1
- 230000036512 infertility Effects 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 230000005764 inhibitory process Effects 0.000 description 1
- 230000000977 initiatory effect Effects 0.000 description 1
- 238000003780 insertion Methods 0.000 description 1
- 230000037431 insertion Effects 0.000 description 1
- 238000010255 intramuscular injection Methods 0.000 description 1
- 239000007927 intramuscular injection Substances 0.000 description 1
- 238000010253 intravenous injection Methods 0.000 description 1
- NBQNWMBBSKPBAY-UHFFFAOYSA-N iodixanol Chemical compound IC=1C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C(I)C=1N(C(=O)C)CC(O)CN(C(C)=O)C1=C(I)C(C(=O)NCC(O)CO)=C(I)C(C(=O)NCC(O)CO)=C1I NBQNWMBBSKPBAY-UHFFFAOYSA-N 0.000 description 1
- 229960004359 iodixanol Drugs 0.000 description 1
- 229940067606 lecithin Drugs 0.000 description 1
- 239000000787 lecithin Substances 0.000 description 1
- 235000010445 lecithin Nutrition 0.000 description 1
- 231100000518 lethal Toxicity 0.000 description 1
- 230000001665 lethal effect Effects 0.000 description 1
- 244000144972 livestock Species 0.000 description 1
- 238000012423 maintenance Methods 0.000 description 1
- 241001515942 marmosets Species 0.000 description 1
- 230000007246 mechanism Effects 0.000 description 1
- 230000000813 microbial effect Effects 0.000 description 1
- 244000005700 microbiome Species 0.000 description 1
- 235000010446 mineral oil Nutrition 0.000 description 1
- 239000002480 mineral oil Substances 0.000 description 1
- 231100000956 nontoxicity Toxicity 0.000 description 1
- 230000036961 partial effect Effects 0.000 description 1
- 230000001575 pathological effect Effects 0.000 description 1
- 239000000312 peanut oil Substances 0.000 description 1
- 239000003208 petroleum Substances 0.000 description 1
- 230000000144 pharmacologic effect Effects 0.000 description 1
- 239000000049 pigment Substances 0.000 description 1
- 229920000724 poly(L-arginine) polymer Polymers 0.000 description 1
- 108010011110 polyarginine Proteins 0.000 description 1
- 229920000656 polylysine Polymers 0.000 description 1
- 229920005862 polyol Polymers 0.000 description 1
- 150000003077 polyols Chemical class 0.000 description 1
- 108010055896 polyornithine Proteins 0.000 description 1
- 229920002714 polyornithine Polymers 0.000 description 1
- 238000001556 precipitation Methods 0.000 description 1
- 239000002243 precursor Substances 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 230000002035 prolonged effect Effects 0.000 description 1
- 239000003207 proteasome inhibitor Substances 0.000 description 1
- 230000002685 pulmonary effect Effects 0.000 description 1
- 239000002510 pyrogen Substances 0.000 description 1
- 238000003762 quantitative reverse transcription PCR Methods 0.000 description 1
- 239000013608 rAAV vector Substances 0.000 description 1
- 238000002310 reflectometry Methods 0.000 description 1
- 210000000880 retinal rod photoreceptor cell Anatomy 0.000 description 1
- 102200141512 rs104893768 Human genes 0.000 description 1
- 102200141461 rs104893769 Human genes 0.000 description 1
- 230000007017 scission Effects 0.000 description 1
- 230000011218 segmentation Effects 0.000 description 1
- 239000008159 sesame oil Substances 0.000 description 1
- 235000011803 sesame oil Nutrition 0.000 description 1
- 229920000260 silastic Polymers 0.000 description 1
- 230000037432 silent mutation Effects 0.000 description 1
- 239000003549 soybean oil Substances 0.000 description 1
- 235000012424 soybean oil Nutrition 0.000 description 1
- 210000000130 stem cell Anatomy 0.000 description 1
- 230000001954 sterilising effect Effects 0.000 description 1
- 238000004659 sterilization and disinfection Methods 0.000 description 1
- 238000003860 storage Methods 0.000 description 1
- 238000010254 subcutaneous injection Methods 0.000 description 1
- 230000001629 suppression Effects 0.000 description 1
- 239000004094 surface-active agent Substances 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 238000003786 synthesis reaction Methods 0.000 description 1
- 238000007910 systemic administration Methods 0.000 description 1
- 230000009885 systemic effect Effects 0.000 description 1
- 229940124597 therapeutic agent Drugs 0.000 description 1
- 231100001274 therapeutic index Toxicity 0.000 description 1
- 238000011200 topical administration Methods 0.000 description 1
- 238000013518 transcription Methods 0.000 description 1
- 230000035897 transcription Effects 0.000 description 1
- 239000012096 transfection reagent Substances 0.000 description 1
- 241000701447 unidentified baculovirus Species 0.000 description 1
- 238000001291 vacuum drying Methods 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
- C12N15/1138—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing against receptors or cell surface proteins
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/11—DNA or RNA fragments; Modified forms thereof; Non-coding nucleic acids having a biological activity
- C12N15/113—Non-coding nucleic acids modulating the expression of genes, e.g. antisense oligonucleotides; Antisense DNA or RNA; Triplex- forming oligonucleotides; Catalytic nucleic acids, e.g. ribozymes; Nucleic acids used in co-suppression or gene silencing
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N15/00—Mutation or genetic engineering; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification; Use of hosts therefor
- C12N15/09—Recombinant DNA-technology
- C12N15/63—Introduction of foreign genetic material using vectors; Vectors; Use of hosts therefor; Regulation of expression
- C12N15/79—Vectors or expression systems specially adapted for eukaryotic hosts
- C12N15/85—Vectors or expression systems specially adapted for eukaryotic hosts for animal cells
- C12N15/86—Viral vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N7/00—Viruses; Bacteriophages; Compositions thereof; Preparation or purification thereof
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/12—Type of nucleic acid catalytic nucleic acids, e.g. ribozymes
- C12N2310/122—Hairpin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/10—Type of nucleic acid
- C12N2310/14—Type of nucleic acid interfering nucleic acids [NA]
- C12N2310/141—MicroRNAs, miRNAs
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2310/00—Structure or type of the nucleic acid
- C12N2310/50—Physical structure
- C12N2310/53—Physical structure partially self-complementary or closed
- C12N2310/531—Stem-loop; Hairpin
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2330/00—Production
- C12N2330/50—Biochemical production, i.e. in a transformed host cell
- C12N2330/51—Specially adapted vectors
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12N—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA
- C12N2750/00—MICROORGANISMS OR ENZYMES; COMPOSITIONS THEREOF; PROPAGATING, PRESERVING, OR MAINTAINING MICROORGANISMS; MUTATION OR GENETIC ENGINEERING; CULTURE MEDIA ssDNA viruses
- C12N2750/00011—Details
- C12N2750/14011—Parvoviridae
- C12N2750/14111—Dependovirus, e.g. adenoassociated viruses
- C12N2750/14141—Use of virus, viral particle or viral elements as a vector
- C12N2750/14143—Use of virus, viral particle or viral elements as a vector viral genome or elements thereof as genetic vector
Definitions
- adRP Autosomal dominant retinitis pigmentosa
- compositions and methods for treating retinitis pigmentosa relate to compositions and methods for treating retinitis pigmentosa (e.g., dominant retinitis pigmentosa) in a subject (e.g., in a human).
- retinitis pigmentosa e.g., dominant retinitis pigmentosa
- one or both alleles of the rhodopsin gene ( rho gene) of a subject are silenced by administering an interfering RNA molecule to a subject (e.g., to a subject having retinitis pigmentosa, for example to a human having dominant retinitis pigmentosa).
- a replacement rho gene also is administered to the subject to provide a functional RHO protein to restore photoreceptor function to the subject.
- the replacement rho gene has one or more nucleotide substitutions relative to the endogenous gene allele(s) that render the replacement gene resistant to the effects of the interfering RNA.
- the replacement rho gene is a human rho gene (e.g., a wild-type human rho gene) that includes one or more (e.g., 1, 2, 3, 4, 5, or more) substitutions to render the gene resistant (also referred to as "hardened") to degradation mediated by the interfering RNA molecule.
- the one or more nucleotide substitutions are in the coding sequence of the rho gene.
- the one or more nucleotide substitutions are silent (e.g., they do not alter the amino acid sequence of the RHO protein). In some embodiments, the one or more substitutions introduce an amino acid change, but the resulting RHO protein is still sufficiently functional to be therapeutically effective (to restore or maintain at least partial sight, or normal sight).
- an interfering RNA and/or a replacement gene can be delivered to a subject using any suitable technique.
- an interfering RNA is provided to a subject in the form of a gene that encodes the interfering RNA.
- the gene that encodes the interfering RNA is provided to the subject in a recombinant adeno-associated virus (rAAV).
- the replacement gene is provided to the subject in an rAAV.
- the gene that encodes the interfering RNA and the replacement gene are provided in the same rAAV (for example they are both encoded on the same recombinant genome flanked by AAV inverted terminal repeats (ITRs)).
- both genes are under control of the same promoter. In some embodiments, the genes are under the control of two different promoters. In some embodiments, the gene that encodes the interfering RNA and the replacement gene are provided in different rAAVs.
- the interfering RNA is a synthetic ribonucleic acid (RNA) molecule comprising:
- the synthetic RNA molecule is a small interfering RNA (siRNA).
- the interfering RNA is a small hairpin RNA (shRNA).
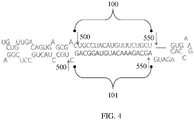
- the shRNA comprises a loop having an RNA of sequence UCAAGAG (SEQ ID NO: 9) or RNA of sequence UGUGCUU (SEQ ID NO: 10).
- the synthetic RNA molecule is an artificial micro RNA (miRNA).
- the artificial miRNA has an RNA sequence of: UGCUGUUGACAGUGAGCGA(X)nUAGUGAAGCCACAGAUGUA(Y)nCUGCCUACUGC CUCGGA (SEQ ID NO: 19), wherein:
- a synthetic RNA described above or elsewhere in this application further comprises an unpaired overhang sequence at the 5' and/or 3' end.
- the unpaired overhang sequence comprises a sequence of repeating bases.
- the sequence of repeating bases comprises repeating uracil (U) bases.
- the unpaired overhang sequence is UU.
- a composition (e.g., a composition for administration to a subject) comprises one or more (e.g., 2, 3, or 4) of the interfering RNAs (e.g., synthetic RNA molecules) described above or elsewhere in this application.
- a composition (e.g., a composition for administration to a subject) comprises a nucleic acid (e.g., a DNA) that encodes one or more (e.g., 2, 3, or 4) of the interfering RNAs (e.g., synthetic RNA molecules) described above or elsewhere in this application.
- a composition also includes one or more physiologically acceptable carriers and/or one or more physiologically acceptable adjuvants.
- a vector encodes one or more (1, 2, 3, or 4, or more) shRNAs and/or artificial miRNAs (e.g., described above or elsewhere herein).
- the shRNAs have a sequence of one or more of SEQ ID NOs: 11-18.
- a vector encodes a replacement rho gene.
- a vector encodes a replacement rho gene and/or one or more shRNAs and/or artificial miRNAs (e.g., described above or elsewhere herein).
- the vector is an expression plasmid. In some embodiments, the vector is a recombinant viral genome (e.g., an rAAV genome). In some embodiments, the vector is a viral vector. In some embodiments, the viral vector comprises an rAAV genome.
- a method of decreasing RHO expression in a subject includes administering to the subject a composition including one or more interfering RNAs and/or one or more vectors each encoding (e.g., capable of expressing) one or more interfering RNAs described above or elsewhere herein.
- a method of treating retinitis pigmentosa (RP) in a subject includes administering to the subject both a composition comprising an interfering RNA or a vector expressing an interfering RNA and a composition comprising a recombinant rho gene (for example a vector encoding the recombinant rho gene), wherein the rho gene is resistant to targeting by the interfering RNA.
- RP retinitis pigmentosa
- the recombinant rho gene is delivered using an rAAV. In some embodiments, the interfering RNA and the recombinant rho gene are delivered using the same rAAV. In some embodiments, the interfering RNA and the recombinant rho gene are both under expression control of a single promoter sequence. In some embodiments, the interfering RNA and the recombinant rho gene are each under expression control of independent promoter sequences (e.g., either constitutive or inducible promoters).
- the interfering RNA and/or modified rho gene are under expression control of (e.g., operatively connected to) a human promoter or a promoter of a different species (e.g., a viral promoter, a prokaryotic promoter, or a eukaryotic promoter, for example, a promoter from a non-human primate, a rodent, a dog, a cat, a pig, or other species).
- a promoter is an RNA polymerase III promoter (e.g., H1 RNA polymerase III promoter) or an RNA polymerase II promoter, or an RNA polymerase I promoter.
- the interfering RNA is shRNA, and the shRNA is under expression control of an RNA polymerase III promoter (e.g., H1 RNA polymerase III promoter).
- the interfering RNA is an artificial miRNA, and the artificial miRNA is under expression control of an RNA polymerase II promoter.
- the recombinant rho gene is under expression control of a constitutive or inducible promoter (e.g ., a human promoter, an eye-specific promoter).
- the constitutive or inducible promoter is a mouse promoter (e.g. , a mouse opsin (MOPS) promoter).
- the subject is a mammal.
- the mammal is a rodent or a dog.
- the mammal is a human (e.g., a human having or known to have, for example diagnosed as having, retinitis pigmentosa, for example dominant retinitis pigmentosa).
- aspects of the application provide methods and compositions that are useful for treating retinitis pigmentosa in a subject (e.g., in a subject having dominant retinitis pigmentosa).
- the expression of endogenous rhodopsin (e.g., mutant and normal) is reduced or suppressed using RNA interference, and the missing protein is replaced by delivering a gene for the normal protein that is engineered to remove the target site for the RNA inhibitor.
- a single adeno-associated virus (AAV) vector is used to deliver both the RNA agent (e.g., small hairpin RNA or artificial microRNA) and the recombinant RHO gene.
- AAV adeno-associated virus
- small hairpin RNAs can be designed to degrade rhodopsin mRNA by targeting sequences that are common in mouse, human, and dog.
- a-miRs artificial microRNAs
- ribozymes RNA enzymes
- Such molecules can be useful to test inhibition in cell culture, in mice, and/or in dogs, to develop inhibitors that can work in human patients.
- small interfering nucleic acids e.g., RNAs
- RNAs small interfering nucleic acids
- four small interfering RNAs that digest human and canine rhodopsin (RHO) mRNA are provided for the purpose of depleting endogenously made rhodopsin in humans and animals.
- these interfering RNAs target rhodopsin expression in subjects having a dominant inherited form of retinitis pigmentosa caused by mutations in RHO. Some of these mutations lead to a toxic form of the protein, the synthesis of which must be silenced to prevent degeneration of the retina.
- an interfering nucleic acid e.g., RNA
- RNA is designed to target a sequence that is specific for a mutant rho gene (e.g., it is complementary to a sequence that is present in the mutant rho gene and not present in a wild-type rho gene).
- an interfering nucleic acid is designed to target a wild-type sequence in a mutant endogenous rho gene (e.g., having one or more mutations at other positions not targeted by the interfering nucleic acid).
- a functional (e.g., wild-type) rho gene that is resistant to the interfering nucleic acid is provided to restore RHO activity in the subject and thereby treat one or more symptoms of a disease or disorder associated with the mutant endogenous rho allele(s) that is/are targeted by the interfering nucleic acid.
- one or more of the interfering RNAs can be delivered using an adeno-associated virus (AAV) vector either as short hairpin RNAs (shRNAs) driven by a promoter (e.g., an RNA polymerase III promoter or other suitable constitutive or inducible promoter) or as artificial microRNAs (miRNAs) driven by a promoter (e.g., using an RNA polymerase II promoter or other suitable constitutive or inducible promoter).
- AAV adeno-associated virus
- the same vector expresses a gene (cDNA) that encodes normal rhodopsin but is resistant to the action of the siRNA expressed by the virus.
- cDNA a gene that encodes normal rhodopsin but is resistant to the action of the siRNA expressed by the virus.
- Non-limiting examples of interfering RNAs are provided in Tables 1-4.
- Table 1 Small interfering RNA (siRNA) SEQ ID NO: Name RNA sequence 1 RHO131-S CUGCCUACAUGUUUCUGCU 2 RHO131-A AGCAGAAACAUGUAGGCAG 3 RHO134-S CCUACAUGUUUCUGCUGAU 4 RHO134-A AUCAGCAGAAACAUGUAGG 5 RHO765-S GCAUGGUCAUCAUCAUGGU 6 RHO765-A ACCAUGAUGAUGACCAUGC 7 RHO820-S GUGGCAUUCUACAUCUUCA 8 RHO820-A UGAAGAUGUAGAAUGCCAC
- Table 2 Hairpin loop RNA SEQ ID NO: RNA sequence 9 UCAAGAG 10 UGUGCUU Table 3: Small hairpin RNA (shRNA) SEQ ID NO: Name RNA sequence 11 RHO131-9 CUGCCUACAUGUUUCUGCUUCAAGAGAGCAGAAACAUGUAGGC
- a normal (e.g., wild-type) rhodopsin ( rho ) gene that is hardened can have a sequence based on the human rho gene (e.g., having a sequence shown in Accession No.
- NG_009115.1 also shown in SEQ ID NO: 41
- the mRNA or a protein coding portion thereof e.g., an mRNA that is encoded by nucleotides 5001-5456 joined to 7238-7406 joined to 8613-8778 joined to 8895-9134 joined to 9970-11706 of SEQ ID NO: 41 or a protein coding portion thereof, for example the coding sequence that consists of nucleotides 5096-5456 joined to 7238-7406 joined to 8613-8778 joined to 8895-9134 joined to 9970-10080 of SEQ ID NO: 41).
- the sequence of a normal rho gene is modified to include one or more (e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10 or more) mutations that render the replacement rho gene resistant to one or more interfering RNAs that are being used as knockdown agent(s) to reduce expression of the mutant endogenous rho gene in a subject being treated.
- one or more e.g., 2, 3, 4, 5, 6, 7, 8, 9, 10 or more
- a recombinant rho gene that has a normal (e.g., wild-type) sequence that is not modified can be used if it has a different sequence than the endogenous rho gene or allele(s) that is/are targeted in the subject being treated, and if the knockdown agents being used are designed to target the endogenous rho gene or allele(s) and not the recombinant rho gene being provided.
- a rho gene from a different species than the subject being treated can be used.
- a the rho gene (e.g., a modified rho gene) can be from the same species as the subject being treated.
- the one or more modifications in the recombinant rho gene alter the nucleic acid sequence of the coding sequence, but do not alter the encoded protein (e.g., they are silent mutations, for example, at the third position of a codon that can include one of two or more different nucleotides without changing the encoded amino acid).
- a recombinant rho gene does not include the intron sequences of a wild-type rho gene.
- a recombinant rho gene encodes an mRNA (or a protein coding portion thereof) of a rho gene that has been modified to be resistant to an interfering RNA.
- a recombinant rho gene includes a wild-type coding sequence that has been modified to be resistant to an interfering RNA.
- the modified wild-type coding sequence is provided along with upstream and/or downstream mRNA sequences that are not derived from the wild-type rho mRNA.
- the recombinant replacement rho gene comprises SEQ ID NO: 42.
- SEQ ID NO: 42 (shown below) encodes a portion of an mRNA sequence that is resistant to an example of an interfering RNA referred to as 820 (e.g., siRNA820 or shRNA820 as described herein).
- SEQ ID NO: 42 includes four substitutions (underlined and in bold below, that correspond to positions 9014, 9017, 9020 and 9023 of SEQ ID NO: 41) that render it resistant to targeting by an 820 interfering RNA.
- the coding sequence in SEQ ID NO: 42 starts at position 88 of SEQ ID NO: 42.
- the recombinant rho gene being delivered can include the coding sequence of SEQ ID NO: 42 (e.g., starting at position 88 of SEQ ID NO: 42) but with a different upstream mRNA sequence.
- a recombinant rho gene can have one or more other sequence modifications (either in addition to or as alternatives) to make it resistant to additional or alternative interfering RNAs (e.g., other interfering RNAs for which sequences are provided herein).
- interfering RNAs e.g., other interfering RNAs for which sequences are provided herein.
- one or more interfering RNAs that target different regions of the endogenous rho coding sequence in a subject can be used.
- SEQ ID NO: 42 non-limiting example of a modified "hardened" recombinant human rho gene
- compositions herein can be administered to a subject in need of treatment.
- the subject has or is suspected of having one or more conditions, diseases, or disorders of the brain and/or eye.
- the subject has or is suspected of having one or more of the conditions, diseases, and disorders disclosed herein.
- the subject has one or more endogenous mutant rho alleles (e.g., associated with or that cause a disease or disorder of the eye or retina).
- the subject has at least one dominant mutant rho allele (e.g., that causes dominant retinitis pigmentosa).
- the subject is a human.
- the subject is a non-human primate.
- Non-limiting examples of non-human primate subjects include macaques (e.g., cynomolgus or rhesus macaques), marmosets, tamarins, spider monkeys, owl monkeys, vervet monkeys, squirrel monkeys, baboons, gorillas, chimpanzees, and orangutans.
- Other exemplary subjects include domesticated animals such as dogs and cats; livestock such as horses, cattle, pigs, sheep, goats, and chickens; and other animals such as mice, rats, guinea pigs, and hamsters.
- the dose of rAAV particles administered to a cell or a subject may be on the order ranging from 10 6 to 10 14 particles/mL or 10 3 to 10 15 particles/mL, or any values therebetween for either range, such as for example, about 10 6 , 10 7 , 10 8 , 10 9 , 10 10 , 10 11 , 10 12 , 10 13 , or 10 14 particles/mL. In one embodiment, rAAV particles of higher than 10 13 particles/mL are be administered.
- the dose of rAAV particles administered to a subject may be on the order ranging from 10 6 to 10 14 vector genomes(vgs)/mL or 10 3 to 10 15 vgs/mL, or any values therebetween for either range, such as for example, about 10 6 , 10 7 , 10 8 , 10 9 , 10 10 , 10 11 , 10 12 , 10 13 , or 10 14 vgs/mL.
- rAAV particles of higher than 10 13 vgs/mL are be administered.
- the rAAV particles can be administered as a single dose, or divided into two or more administrations as may be required to achieve therapy of the particular disease or disorder being treated.
- 0.0001 mL to 10 mLs are delivered to a subject in a dose.
- rAAV viral titers range from 1 ⁇ 10 10 -5 x 10 13 vg/ml. In some embodiments, rAAV viral titers can be 1 ⁇ 10 10 , 2.5 x 10 10 , 5 x 10 10 , 1 ⁇ 10 11 , 2.5 x 10 11 , 5 x 10 11 , 1 ⁇ 10 12 , 2.5 ⁇ 10 12 ,5 ⁇ 10 12 , 1 ⁇ 10 13 , 2.5 x 10 13 , or 5 x 10 13 vg/mL. In some embodiments, viral titers are less than 1 ⁇ 10 10 vg/mL.
- rAAV viral titers are greater than 1 ⁇ 10 15 vg/mL. In one embodiment, rAAV particles are greater than 5 x 10 13 vgs/mL. In some embodiments, rAAV viral titers are administered via methods further described herein (e.g., subretinally or intravitreally).
- the rAAV particles can be administered as a single dose, or divided into two or more administrations as may be required to achieve therapy of the particular disease or disorder being treated.
- from 1 to 500 microliters of a composition described in this application is administered to one or both eyes of a subject.
- about 1, about 10, about 50, about 100, about 200, about 300, about 400, or about 500 microliters can be administered to each eye.
- smaller or larger volumes could be administered in some embodiments.
- the disclosure provides formulations of one or more rAAV-based compositions disclosed herein in pharmaceutically acceptable solutions for administration to a cell or an animal, either alone or in combination with one or more other modalities of therapy, and in particular, for therapy of human cells, tissues, and diseases affecting man.
- rAAV particle or nucleic acid vectors may be administered in combination with other agents as well, such as, e.g., proteins or polypeptides or various pharmaceutically-active agents, including one or more systemic or topical administrations of therapeutic polypeptides, biologically active fragments, or variants thereof.
- agents such as, e.g., proteins or polypeptides or various pharmaceutically-active agents, including one or more systemic or topical administrations of therapeutic polypeptides, biologically active fragments, or variants thereof.
- agents e.g., proteins or polypeptides or various pharmaceutically-active agents, including one or more systemic or topical administrations of therapeutic polypeptides, biologically active fragments, or variants thereof.
- agents e.g., proteins or polypeptides or various pharmaceutically-active agents, including one or more systemic or topical administrations of therapeutic polypeptides, biologically active fragments, or variants thereof.
- the rAAV particles may thus be delivered along with various other agents as required in
- Formulation of pharmaceutically-acceptable excipients and carrier solutions is well-known to those of skill in the art, as is the development of suitable dosing and treatment regimens for using the particular compositions described herein in a variety of treatment regimens, including e.g., oral, parenteral, intravenous, intranasal, intra-articular, and intramuscular administration and formulation.
- these formulations may contain at least about 0.1% of the therapeutic agent (e.g., rAAV particle or host cell) or more, although the percentage of the active ingredient(s) may, of course, be varied and may conveniently be between about 1 or 2% and about 70% or 80% or more of the weight or volume of the total formulation.
- the amount of therapeutic agent(s) (e.g., rAAV particle) in each therapeutically-useful composition may be prepared in such a way that a suitable dosage will be obtained in any given unit dose of the compound.
- an rAAV particle or host cell in suitably formulated pharmaceutical compositions disclosed herein either subcutaneously, intraocularly, intravitreally, parenterally, subcutaneously, intravenously, intracerebro-ventricularly, intramuscularly, intrathecally, orally, intraperitoneally, by oral or nasal inhalation, or by direct injection to one or more cells, tissues, or organs by direct injection.
- the pharmaceutical forms of the rAAV particle or host cell compositions suitable for injectable use include sterile aqueous solutions or dispersions.
- the form is sterile and fluid to the extent that easy syringability exists.
- the form is stable under the conditions of manufacture and storage and is preserved against the contaminating action of microorganisms, such as bacteria and fungi.
- the carrier can be a solvent or dispersion medium containing, for example, water, saline, ethanol, polyol (e.g., glycerol, propylene glycol, and liquid polyethylene glycol, and the like), suitable mixtures thereof, and/or vegetable oils.
- Proper fluidity may be maintained, for example, by the use of a coating, such as lecithin, by the maintenance of the required particle size in the case of dispersion and by the use of surfactants.
- carrier refers to a diluent, adjuvant, excipient, or vehicle with which the rAAV particle or host cell is administered.
- Such pharmaceutical carriers can be sterile liquids, such as water and oils, including those of petroleum oil such as mineral oil, vegetable oil such as peanut oil, soybean oil, and sesame oil, animal oil, or oil of synthetic origin. Saline solutions and aqueous dextrose and glycerol solutions can also be employed as liquid carriers.
- compositions of the present disclosure can be administered to the subject being treated by standard routes including, but not limited to, pulmonary, intranasal, oral, inhalation, parenteral such as intravenous, topical, transdermal, intradermal, transmucosal, intraperitoneal, intramuscular, intracapsular, intraorbital, intravitreal, intracardiac, transtracheal, subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid, intraspinal, epidural and intrasternal injection.
- parenteral such as intravenous, topical, transdermal, intradermal, transmucosal, intraperitoneal, intramuscular, intracapsular, intraorbital, intravitreal, intracardiac, transtracheal, subcutaneous, subcuticular, intraarticular, subcapsular, subarachnoid, intraspinal, epidural and intrasternal injection.
- compositions of the present disclosure can be delivered to the eye through a variety of routes. They may be delivered intraocularly, by topical application to the eye or by intraocular injection into, for example the vitreous (intravitreal injection) or subretinal (subretinal injection) inter-photoreceptor space. In some embodiments, they are delivered to rod photoreceptor cells. Alternatively, they may be delivered locally by insertion or injection into the tissue surrounding the eye. They may be delivered systemically through an oral route or by subcutaneous, intravenous or intramuscular injection.
- they may be delivered by means of a catheter or by means of an implant, wherein such an implant is made of a porous, non-porous or gelatinous material, including membranes such as silastic membranes or fibers, biodegradable polymers, or proteinaceous material. They can be administered prior to the onset of the condition, to prevent its occurrence, for example, during surgery on the eye, or immediately after the onset of the pathological condition or during the occurrence of an acute or protracted condition.
- an implant is made of a porous, non-porous or gelatinous material, including membranes such as silastic membranes or fibers, biodegradable polymers, or proteinaceous material.
- the solution may be suitably buffered, if necessary, and the liquid diluent first rendered isotonic with sufficient saline or glucose.
- aqueous solutions are especially suitable for intravenous, intramuscular, intravitreal, subcutaneous and intraperitoneal administration.
- a sterile aqueous medium that can be employed will be known to those of skill in the art in light of the present disclosure.
- one dosage may be dissolved in 1 ml of isotonic NaCl solution and either added to 1000 ml of hypodermoclysis fluid or injected at the proposed site of infusion, (see for example, " Remington's Pharmaceutical Sciences” 15th Edition, pages 1035-1038 and 1570-1580 ).
- Some variation in dosage will necessarily occur depending on the condition of the subject being treated.
- the person responsible for administration will, in any event, determine the appropriate dose for the individual subject.
- preparations should meet sterility, pyrogenicity, and the general safety and purity standards as required by, e.g., FDA Office of Biologics standards.
- Sterile injectable solutions are prepared by incorporating the rAAV particles or host cells in the required amount in the appropriate solvent with several of the other ingredients enumerated above, as required, followed by filtered sterilization.
- dispersions are prepared by incorporating the various sterilized active ingredients into a sterile vehicle which contains the basic dispersion medium and the required other ingredients from those enumerated above.
- the preferred methods of preparation are vacuum-drying and freeze-drying techniques which yield a powder of the active ingredient plus any additional desired ingredient from a previously sterile-filtered solution thereof.
- rAAV particle, nucleic acid vector, or host cell compositions and time of administration of such compositions will be within the purview of the skilled artisan having benefit of the present teachings. It is likely, however, that the administration of therapeutically-effective amounts of the disclosed compositions may be achieved by a single administration, such as for example, a single injection of sufficient numbers of infectious particles to provide therapeutic benefit to the patient undergoing such treatment. Alternatively, in some circumstances, it may be desirable to provide multiple, or successive administrations of the rAAV particle or host cell compositions, either over a relatively short, or a relatively prolonged period of time, as may be determined by the medical practitioner overseeing the administration of such compositions.
- rod cells remain structurally intact and/or viable upon silencing of cellular rhodopsin gene expression.
- rods cells in which cellular rhodopsin gene expression is silenced have shortened outer segments which would normally contain rhodopsin.
- the length of the outer segments can be maintained or restored (e.g., partially or completely) using the exogenously added (hardened) rhodopsin gene, the expression of which is resistant to silencing using the compositions described in this application.
- the composition may include rAAV particles or host cells, either alone, or in combination with one or more additional active ingredients, which may be obtained from natural or recombinant sources or chemically synthesized.
- rAAV particles are administered in combination, either in the same composition or administered as part of the same treatment regimen, with a proteasome inhibitor, such as Bortezomib, or hydroxyurea.
- compositions described above are typically administered to a subject in an effective amount, that is, an amount capable of producing a desirable result.
- the desirable result will depend upon the active agent being administered.
- an effective amount of a rAAV particle may be an amount of the particle that is capable of transferring a heterologous nucleic acid to a host organ, tissue, or cell.
- Toxicity and efficacy of the compositions utilized in methods of the disclosure can be determined by standard pharmaceutical procedures, using either cells in culture or experimental animals to determine the LD50 (the dose lethal to 50% of the population).
- the dose ratio between toxicity and efficacy the therapeutic index and it can be expressed as the ratio LD50/ED50.
- Those compositions that exhibit large therapeutic indices are preferred. While those that exhibit toxic side effects may be used, care should be taken to design a delivery system that minimizes the potential damage of such side effects.
- the dosage of compositions as described herein lies generally within a range that includes an ED50 with little or no toxicity. The dosage may vary within this range depending upon the dosage form employed and the route of administration utilized.
- aspects of the disclosure relate to recombinant adeno-associated virus (rAAV) particles for delivery of one or more nucleic acid vectors comprising a gene of interest into various tissues, organs, and/or cells.
- the rAAV particles comprise an rAAV capsid protein as described herein, e.g., comprising one or more amino acid substitutions.
- the gene of interest encodes a polypeptide or protein of interest (e.g., a therapeutic polypeptide or protein).
- the gene of interest encodes an RNA of interest (e.g., a therapeutic mRNA, siRNA, shRNA, microRNA, antisense RNA, tRNA, rRNA, or a ribozyme).
- a gene of interest is a replacement gene (e.g., an eye-specific gene, a functional gene, a functional RHO gene).
- a gene of interest comprises or encodes an RNA of interest (e.g ., microRNA, siRNA, shRNA) and a replacement gene of interest (e.g. , an eye-specific gene, a functional gene, a functional RHO gene).
- a functional RHO gene comprises an RHO gene comprising silent nucleotide substitutions that render it incapable of being degraded by an RNA of interest (e.g. , microRNA, siRNA, shRNA).
- an RNA of interest and a replacement gene of interest are under the control of the same promoter.
- an RNA of interest and a replacement gene of interest are under the control of separate promoters. Any suitable promoters can be used, for example, but not limited to a viral promoter (e.g., a CMV or other viral promoter), a microbial (e.g., a yeast or bacterial), or a eukaryotic (e.g., a mammalian) promoter.
- a viral promoter e.g., a CMV or other viral promoter
- a microbial e.g., a yeast or bacterial
- a eukaryotic e.g., a mammalian
- Recombinant AAV (rAAV) particles may comprise at a minimum (a) one or more heterologous nucleic acid regions comprising a sequence encoding a gene of interest (e.g., an RNA of interest and/or a replacement gene of interest) and (b) one or more regions comprising inverted terminal repeat (ITR) sequences (e.g., wild-type ITR sequences or engineered ITR sequences) flanking the one or more heterologous nucleic acid regions.
- the nucleic acid vector is between 4kb and 5kb in size (e.g., 4.2 to 4.7 kb in size).
- This nucleic acid vector may be encapsidated by a viral capsid, such as an AAV1, AAV2, AAV3, AAV4, or AAV5 capsid, which may comprise a modified capsid protein as described herein.
- the nucleic acid vector is circular.
- the nucleic acid vector is single-stranded.
- the nucleic acid vector is double-stranded.
- a double-stranded nucleic acid vector may be, for example, a self-complementary vector that contains a region of the nucleic acid vector that is complementary to another region of the nucleic acid vector, initiating the formation of the double-strandedness of the nucleic acid vector.
- the rAAV particle may be of any AAV serotype, including any derivative or pseudotype (e.g., 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 2/1, 2/5, 2/8, or 2/9).
- the serotype of an rAAV viral vector refers to the serotype of the capsid proteins of the recombinant virus.
- the rAAV particle is not AAV2.
- the rAAV particle is AAV2.
- the rAAV particle is AAV6.
- the rAAV particle is an AAV6 serotype comprising an rAAV capsid protein as described herein.
- Non-limiting examples of derivatives and pseudotypes include rAAV2/1, rAAV2/5, rAAV2/8, rAAV2/9, AAV2-AAV3 hybrid, AAVrh.10,AAVhu.14, AAV3a/3b, AAVrh32.33, AAV-HSC15, AAV-HSC17, AAVhu.37, AAVrh.8, CHt-P6, AAV2.5, AAV6.2, AAV2i8, AAV-HSC15/17, AAVM41, AAV9.45, AAV6(Y445F/Y731F), AAV2.5T, AAV-HAE1/2, AAV clone 32/83, AAVSHHIO, AAV2 (Y->F), AAV8 (Y733F), AAV2.15, AAV2.4, AAVM41, and AAVr3.45.
- the rAAV particle is a pseudotyped rAAV particle, which comprises (a) a nucleic acid vector comprising ITRs from one serotype (e.g., AAV2) and (b) a capsid comprised of capsid proteins derived from another serotype (e.g., AAV1, AAV3, AAV4, AAV5, AAV6, AAV7, AAV8, AAV9, or AAV10).
- a pseudotyped rAAV particle which comprises (a) a nucleic acid vector comprising ITRs from one serotype (e.g., AAV2) and (b) a capsid comprised of capsid proteins derived from another serotype (e.g., AAV1, AAV3, AAV4, AAV5, AAV6, AAV7, AAV8, AAV9, or AAV10).
- rAAV particles and nucleic acid vectors are also known in the art and commercially available (see, e.g., Zolotukhin et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28 (2002) 158-167 ; and U.S. Patent Publication Numbers US20070015238 and US20120322861 , which are incorporated herein by reference; and plasmids and kits available from ATCC and Cell Biolabs, Inc.).
- a plasmid containing the nucleic acid vector may be combined with one or more helper plasmids, e.g., that contain a rep gene (e.g., encoding Rep78, Rep68, Rep52 and Rep40) and a cap gene (e.g., encoding VP1, VP2, and VP3, including a modified VP3 region as described herein), and transfected into a producer cell line such that the rAAV particle can be packaged and subsequently purified.
- helper plasmids e.g., that contain a rep gene (e.g., encoding Rep78, Rep68, Rep52 and Rep40) and a cap gene (e.g., encoding VP1, VP2, and VP3, including a modified VP3 region as described herein), and transfected into a producer cell line such that the rAAV particle can be packaged and subsequently purified.
- helper plasmids e.g., that contain a rep gene (
- the one or more helper plasmids include a first helper plasmid comprising a rep gene and a cap gene (e.g., encoding a rAAV capsid protein as described herein) and a second helper plasmid comprising a E1a gene, a E1b gene, a E4 gene, a E2a gene, and a VA gene.
- the rep gene is a rep gene derived from AAV2 or AAV6 and the cap gene is derived from AAV2 or AAV6 and may include modifications to the gene in order to produce the modified capsid protein described herein.
- Helper plasmids, and methods of making such plasmids are known in the art and commercially available (see, e.g., pDM, pDG, pDP1rs, pDP2rs, pDP3rs, pDP4rs, pDP5rs, pDP6rs, pDG(R484E/R585E), and pDP8.ape plasmids from PlasmidFactory, Bielefeld, Germany; other products and services available from Vector Biolabs, Philadelphia, PA; Cellbiolabs, San Diego, CA; Agilent Technologies, Santa Clara, Ca; and Addgene, Cambridge, MA; pxx6; Grimm et al.
- helper plasmids are produced or obtained, which comprise rep and cap ORFs for the desired AAV serotype and the adenoviral VA, E2A (DBP), and E4 genes under the transcriptional control of their native promoters.
- the cap ORF may also comprise one or more modifications to produce a modified capsid protein as described herein.
- HEK293 cells available from ATCC ® ) are transfected via CaPO4-mediated transfection, lipids or polymeric molecules such as Polyethylenimine (PEI) with the helper plasmid(s) and a plasmid containing a nucleic acid vector described herein.
- PEI Polyethylenimine
- HEK293 cells are then incubated for at least 60 hours to allow for rAAV particle production.
- Sf9-based producer stable cell lines are infected with a single recombinant baculovirus containing the nucleic acid vector.
- HEK293 or BHK cell lines are infected with a HSV containing the nucleic acid vector and optionally one or more helper HSVs containing rep and cap ORFs as described herein and the adenoviral VA, E2A (DBP), and E4 genes under the transcriptional control of their native promoters.
- the HEK293, BHK, or Sf9 cells are then incubated for at least 60 hours to allow for rAAV particle production.
- the rAAV particles can then be purified using any method known the art or described herein, e.g., by iodixanol step gradient, CsCl gradient, chromatography, or polyethylene glycol (PEG) precipitation.
- the disclosure also contemplates host cells that comprise at least one of the disclosed rAAV particles or nucleic acid vectors.
- host cells include mammalian host cells, with human host cells being preferred, and may be either isolated, in cell or tissue culture.
- the transformed host cells may be comprised within the body of a non-human animal itself.
- the host cell is a cell of erythroid lineage, such as a CD36 + burst-forming units-erythroid (BFU-E) cell or a colony-forming unit-erythroid (CFUE-E) progenitor cell.
- BFU-E burst-forming units-erythroid
- CFUE-E colony-forming unit-erythroid
- compositions described herein are formulated in a nanoparticle.
- compositions described herein e.g., siRNA, shRNA, and/or replacement genes of interest
- a lipid nanoparticle e.g., siRNA, shRNA, and/or replacement genes of interest
- compositions described herein are formulated in a lipid-polycation complex, referred to as a cationic lipid nanoparticle.
- the formation of the lipid nanoparticle may be accomplished by methods known in the art and/or as described in U.S. Pub. No.
- the polycation may include a cationic peptide or a polypeptide such as, but not limited to, polylysine, poly ornithine and/or polyarginine and the cationic peptides described in International Pub. No. WO2012013326 or US Patent Pub. No. US20130142818 ; each of which is herein incorporated by reference in its entirety.
- compositions described herein e.g.
- siRNA, shRNA, and/or replacement genes of interest are formulated in a lipid nanoparticle that includes a non-cationic lipid such as, but not limited to, cholesterol or dioleoyl phosphatidylethanolamine (DOPE).
- a non-cationic lipid such as, but not limited to, cholesterol or dioleoyl phosphatidylethanolamine (DOPE).
- DOPE dioleoyl phosphatidylethanolamine
- siRNA short interfering RNA
- siRNAs would be effective as small hairpin RNAs expressed from an RNA polymerase III promoter and delivered by AAV
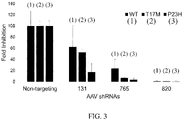
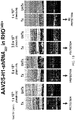
- the DNA sequences for shRNAs were cloned expressing three of the siRNAs ( FIG. 2 ).
- the relative knockdown of RHO using siRNA did not exactly predict relative suppression of RHO using shRNA, since siRNA 131 (SEQ ID NO: 1) was the most effective siRNA, but the least effective of the shRNAs that were tested.
- Simple transfection of siRNAs as RNA may not exactly predict the effectiveness of shRNA designed to produce the same siRNAs by transcription and processing.
- Table 5 lists RNA sequences corresponding to shRNA sequences identified as capable of cleaving RHO, including the sense strand, loop, antisense strand, and overhang. Each sequence is depicted with two alternative loop sequences that are bolded and underlined for emphasis, with overhangs italicized and underlined for emphasis.
- RNA sequence (Sense Strand - Loop - Antisense Strand - Overhang ) 20 RHO131-9 CUGCCUACAUGUUUCUGCU UCAAGA GAGCAGAAACAUGUAGGCAG UU 21 RHO131-10 CUGCCUACAUGUUUCUGCU UGUGCUU AGCAGAAACAUGUAGGCAG UU 22 RHO134-9 CCUACAUGUUUCUGCUGAU UCAAGAG AUCAGCAGAAACAUGUAGG UU 23 RHO134-10 CCUACAUGUUUCUGCUGAU UGUGCUU AUCAGCAGAAACAUGUAGG UU 24 RHO765-9 GCAUGGUCAUCAUCAUGGU UCAAGAG ACCAUGAUGAUGACCAUGC UU 25 RHO765-10 GCAUGGUCAUCAUCAUGGU UCAAGAG ACCAUGAUGAUGACCAUGC UU 25 RHO765-10 GCAUGGUCAUCAUCAUGGU UCAAGAG ACCAUGAUGAUGACCAUGC UU 25 RHO765-10 G
- siRNAs can also be delivered as artificial microRNAs 7 of a structure like that in FIG. 4 .
- the exemplary microRNA of FIG. 4 comprises sense (100) and antisense (101) strands of RHO131 (SEQ ID NOs: 1 and 2, respectively).
- the advantage of this mode of expression is that production of the siRNA can be made cell type specific through the use of a specific promoter sequence. In this case the proximal promoter of the human rhodopsin gene or the human rhodopsin kinase promoter would be used to restrict expression to photoreceptor cells.
- Dog 2190 received subretinal injections of AAV2/5-sc-H1-shRNA-Rho131 at the concentrations listed in Table 6. The dog was terminated 8 weeks post injection. Several 3 mm neuroretinal biopsy punches were collected from each eye from both bleb and non-bleb regions.
- Table 6 Dog 2190 Dog Genotype Sex DOB Age at injection Right Eye (OD) Left Eye (OS) 2190 rcd1 carrier M 8/11/14 1 year AAV2/5-sc-H1-shRNA-Rho 131 ⁇ 4063 5E+12 vg/ml 150 ⁇ l SR AAV2/5-sc-H1-shRNA-Rho 131 ⁇ 4063 1E+12 vg/ml 150 ⁇ l SR
- AAV2/5-sc-H1-shRNA constructs described herein can also be referred to as AAV2/5-sc-MOP500 rGFP-shRNA constructs.
- the AAV2/5 indicates that the nucleic acid encoding the shRNA is flanked by AAV2 ITRs and provided in an rAAV particle comprising AAV5 capsid proteins.
- any interfering RNA described herein and any recombinant RHO gene described herein can be provided on the same AAV nucleic acid (e.g., flanked by AAV2 ITRs) in an rAAV particle (e.g., comprising AAV5 capsid proteins).
- any interfering RNA described herein and any recombinant RHO gene described herein can be provided on different AAV nucleic acids (e.g., each flanked by AAV2 ITRs, or each flanked by ITRs from different AAV serotypes) encapsidated in different rAAV particles (e.g., each comprising AAV5 capsid proteins, or each having capsid proteins from different AAV serotypes).
- Dog 2194 (rcd1 carrier) received subretinal injections of AAV2/5-sc-H1-shRNA-Rho820 at the concentrations listed in Table 7. The dog was terminated 8 weeks post injection. Several 3 mm neuroretinal biopsy punches were collected from each eye from both bleb and non-bleb regions.
- Table 7 Dog 2194 Dog Genotype Sex DOB Age at injection Right Eye (OD) Left Eye (OS) 2194 rcd1 carrier F 8/11/14 1 year AAV2/5-sc-H1-shRNA-Rho820 ⁇ 4064 5E+12 vg/ml 150 ⁇ l SR 50 ⁇ l intravitr AAV2/5-sc-H1-shRNA-Rho820 ⁇ 4064 1E+12 vg/ml Small bleb 50 ⁇ l SR 250 ⁇ l intravitr
- an mRNA for a replacement gene can be modified at one or more positions to "harden” it ( i.e., to make it resistant to degradation by siRNA).
- one or more silent nucleotide substitutions can be included in the replacement gene relative to the siRNA that is provided to knockdown the endogenous gene (e.g., RHO gene).
- FIGs. 9 and 10 provide non-limiting examples of nucleotide substitutions that can be introduced into the replacement RHO mRNA. It should be appreciated that a replacement RHO gene can include one or more of these substitutions.
- FIG. 9 depicts a representation of base pairing that occurs between each shRNA and the target sequence of endogenous human or hardened RHO mRNA. All shRNAs base-pair perfectly with the target sequence of RHO mRNA of dog.
- White box mismatch between shRNA and endogenous dog as well as hardened RHO mRNA.
- Dark gray box a weak wobble base pairing that occurs in RNA between guanosine and uracil.
- Light gray box mismatch between shRNA and hardened RHO mRNA only.
- FIG. 10 depicts a representation of base pairing that occurs between shRNA134 and the target sequence of endogenous human or hardened RHO131 mRNA.
- the proximity of the target sequences of shRNAs 131 and 134 enables the same hardened RHO131 to be employed.
- Dark gray box a weak wobble base pairing that occurs in RNA between guanosine and uracil.
- Light gray box mismatch between shRNA and hardened RHO mRNA only.
- FIGs. 12A-B depict non-limiting examples of siRNA (e.g. , shRNA) encoded in a DNA vector.
- the DNA vector can further encode inverted terminal repeat (ITR) sequences flanking the siRNA (e.g ., shRNA).
- ITR inverted terminal repeat
- a DNA vector encoding siRNA flanked by ITR sequences can be used in the production of recombinant AAV particles comprising the siRNA.
- FIG. 12C depicts a non-limiting example of a replacement RHO mRNA (e.g. , Human RHO) encoded in a DNA vector.
- the DNA vector can further encode ITR sequences flanking the replacement RHO mRNA (e.g ., Human RHO).
- a DNA vector encoding replacement RHO mRNA flanked by ITR sequences can be used in the production of recombinant AAV particles comprising the Human rho.
- a similar DNA vector may be provided that includes both the replacement rho gene and one or more sequences encoding one or more interfering RNAs flanked by ITR sequences.
- the interfering RNAs and/or replacement rho genes are operatively coupled to a promoter (e.g., an RNA polymerase III promoter, or H1 RNA polymerase III promoter, or other promoter as described in this application).
- the interfering RNAs and/or replacement rho genes represented in FIGs. 12A-C are under the control of an H1 RNA polymerase III promoter. The constructs of FIGs.
- MOP500-rGFP a -385/+86 portion of the mouse rod opsin promoter (MOP500) upstream of the reversed sequence of GFP (rGFP) that is therefore not expressed
- MOP500 a -385/+86 portion of the mouse rod opsin promoter upstream of the reversed sequence of GFP (rGFP) that is therefore not expressed
- a plasmid or rAAV nucleic acid can encode an interfering RNA and/or a modified rho gene as described herein, each under the control of the same or a different promoter (e.g., an H1 promoter) without a mouse opsin promoter and/or without any GFP coding sequence (in either orientation).
- a different promoter e.g., an H1 promoter
- a mouse opsin promoter e.g., without a mouse opsin promoter and/or without any GFP coding sequence (in either orientation).
- Example 6 includes the identification of viral titers of AAV2/5 carrying the shRNA820 knockdown reagent that efficiently silence rhodopsin expression after subretinal injection in WT dogs, evidence by in vivo retinal imaging in WT dogs of the preservation of the layer (ONL) that contains the photoreceptors, but reduction of the layers that contain rhodopsin following subretinal injection with AAV215-sc-H1- shRNA820 , identification of viral titers of AAV2/5 carrying the shRNA820 knockdown reagent that efficiently silence rhodopsin expression after subretinal injection in the mutant RHO T4R/+ dog, a naturally-occurring model of RHO-ADRP, identification of viral titers of AAV2/5-sc-H1- shRNA820 that confer protection to photoreceptors from light-induced retinal degeneration in the mutant RHO T4R/+ dogs, evidence that efficient knockdown of rhodopsin expression leads to
- Table 8 RHO KD with shRNA820 in WT RHO +/+ dogs Dog Genotype Sex DOB Age at injection Right Eye (OD) Left Eye (OS) 2194 rcd1 carrier F 8/11/14 1 year AAV2/5-sc-H1-shRNA-Rho820 ⁇ 4064 5E+12 vg/ml 150 ul SR AAV2/5-sc-H1-shRNA-Rho820 ⁇ 4064 1E+12 vg/ml Small bleb 50 ul SR BR442 RPE65 carrier F 12/15/13 1Y+11M AAV2/5-sc-H1-shRNA820 ⁇ 4064 1E+12 vg/ml 150 ul SR AAV2/5-sc-H1-shRNA820 ⁇ 4064 1E+12 vg/ml 150 ul SR GSR2 CNGB3 carrier rcd1 carrier M 8/19/2014 64 wks AAV2/5-
- FIGs. 13A-13E show RNA and protein analysis of rhodopsin knockdown with different viral titers of AAV2/5-sc-H1-shRNA820 injected subretinally in WT RHO +/+ dogs.
- FIG. 13A shows retinal maps showing position of biopsy punches used for western blot analysis and RNA quantitation.
- FIG. 13B shows a bar graph showing remaining canine Rhodopsin RNA in the treated area as a percentage of levels measured in the untreated area of the same retina.
- FIG. 13C shows immunoblot showing the amount of Rhodopsin in biopsy punches taken from treated (Tx) and untreated (UnTx) areas of canine retina. Histone H3 was used for normalization. Bar graphs show remaining canine Rhodopsin protein as a percentage of levels measured in the untreated area of the same retina.
- FIGs. 13D-13E show tables showing numerical values from each experiment (reported as a percentage of RNA or protein remaining, and alternatively as a percent knockdown of RNA or protein, respectively).
- FIGs. 14A-D show the assessment of ONL and ELM/IS/OS integrity following subretinal injection of different titers of AAV2/5-sc-H1-shRNA820 in WT dogs.
- FIG. 14A shows ONL thickness maps;
- FIG. 14B shows ELM/IS/OS maps of normalized intensity;
- FIG. 14C shows ONL thickness values;
- FIG. 14D shows values of normalized intensity of the ELM/IS/OS layers.
- cSLO/OCT was performed at 8 weeks post injection and light exposure (1 min at 1mW/cm 2 ) was performed on OS eye in all dogs to trigger light-induced retinal degeneration. cSLO/OCT was performed again on all dogs 2 weeks post light exposure to assess any rescue effect conferred by the treatment. Dogs were terminated and several 3mm neuroretinal biopsy punches were collected from OD eye from both bleb/treated and non-bleb/untreated regions to measure the level of expression of canine Rhodopsin by western blot and RHO RNA by qPCR analysis. The OS eye was fixed, embedded in optimal cutting temperature media and processed for histology and immunohistochemistry staining.
- the surrounding non bleb/untreated regions were used as an internal control for each eye.
- Two weeks post light-exposure retinas were re-imaged, and showed good preservation of ONL thickness in the bleb/treated area of eyes injected with 1 ⁇ 10 12 and 5 x 10 11 vg/ml titers.
- Very mild rescue was seen at 2.5 ⁇ 10 11 vg/ml and none with 1 x 10 11 vg/ml titers.
- FIGs. 15A-15E show RNA and protein analysis of rhodopsin knockdown with different viral titers of AAV2/5-sc-H1-shRNA820 injected subretinally in mutant RHO T4R/+ dogs.
- FIG. 15A shows retinal maps showing position of biopsy punches used for western blot analysis and RNA quantitation. Paired dark gray, gray and dotted circles indicate the position of biopsy punches in the bleb and non-bleb region for each replication of western blot, whereas the black circles indicate the position of biopsy punches for RNA quantitation.
- FIG. 15B is a bar graph showing the amount of remaining canine Rhodopsin RNA as a percentage of levels measured in the untreated area of the same retina.
- FIG. 15C is an immunoblot showing the amount of canine Rhodopsin in biopsy punches taken from treated (Tx) and untreated (UnTx) areas of canine retina. Histone H3 was used for normalization. Bar graphs show the amount of remaining canine Rhodopsin protein as a percentage of levels measured in the untreated area of the same retina.
- FIGs. 15D-15E are tables showing numerical values from each experiment (reported as a percentage of RNA or protein remaining, and alternatively as a percent knockdown of RNA or protein, respectively).
- FIGs. 16A-16D show OCT B scans encompassing the treated (with different viral titers of AAV2/5-sc-H1-shRNA820) and untreated retinal areas of RHO T4R/+ dogs 2 weeks following exposure to a brief dose of light that causes acute retinal degeneration in mutant RHO T4R/+ dogs.
- FIG. 16A OCT scan of a dog treated with 1 ⁇ 10 12 vg/ml.
- FIG. 16B OCT scan of a dog treated with 5 x 10 11 vg/ml.
- FIG. 16C OCT scan of a dog treated with 2.5 ⁇ 10 11 vg/ml.
- FIGs. 17A-17B show topographic map ONL thickness from a RHO T4R/+ treated with AAV2/5-sc-shRNA820 showing protection from light-induced retinal degeneration.
- FIG. 17A ONL thickness map of an untreated WT control dog (left panel), and ONL thickness map of EM411-OS treated with AAV2/5-sc-shRNA820 at 5E+11 vg/ml and showing preserved ONL thickness in the treated/bleb region weeks after light-induced damage. Black and white curve shows the limits of the bleb as seen immediately after the subretinal injection. Panels below show OCT B scans with ONL colored in darker gray (middle band) for visualization purposes.
- FIG. 17B Loci outside and inside the bleb were selected for ONL thickness measurements.
- FIG. 18 shows histology (H&E stained) and immunohistochemistry (Rhodopsin was stained in green and appears as the lighter staining in the lower panels of FIG. 18 ; human cone arrestin was stained in red and appears as the gray staining in the lower panels of FIG. 18 ) in the treated (Tx) and Untreated (UnTx) areas of mutant RHO T4R/+ retinas injected subretinally with different viral titers AAV2/5-sc-H1-shRNA820.
- cSLO/OCT In vivo retinal imaging (cSLO/OCT) was performed at 11 weeks post injection and light exposure (1 min at 1mW/cm 2 ) was performed on OS eye in both dogs to trigger light-induced retinal degeneration. cSLO/OCT was performed again on all dogs 2 weeks post light exposure to assess any rescue effect conferred by the treatment. Dogs were terminated and several 3mm neuroretinal biopsy punches were collected from OD eye from both bleb/treated and non-bleb/untreated regions to measure the level of expression of Rhodopsin by western blot and canine and human RHO RNA by qPCR analysis. The OS eye was fixed, embedded in optimal cutting temperature media and processed for histology and immunohistochemistry staining.
- mutant T4R rhodopsin can be differentiated from WT (canine or human) RHO protein as it has a lower MW that can be detected on immunoblots.
- RHO immunoblots from heterozygous mutant RHO T4R/+ thus show two bands corresponding to WT (high MW), and mutant T4R (low MW) RHO proteins.
- Anti-histone H3 antibody (Abcam ab1791, diluted 1:3000) was used as a loading control and to normalize the signals.
- FIGs. 19A-19F show RNA and protein analysis of rhodopsin knockdown and replacement with AAV2/5-sc-HOP-RHO820-H1-shRNA820 injected subretinally in mutant RHO T4R/+ dogs at 5 ⁇ 10 11 vg/ml titer.
- FIG. 19A shows retinal maps showing position of biopsy punches used for western blot analysis and RNA quantitation. Paired dark gray, gray and dotted circles indicate the position of biopsy punches in the treated (Tx) and untreated (UnTx) regions for each replication of western blot, whereas the black circles indicate the position of biopsy punches for RNA quantitation.
- FIG. 19A shows retinal maps showing position of biopsy punches used for western blot analysis and RNA quantitation. Paired dark gray, gray and dotted circles indicate the position of biopsy punches in the treated (Tx) and untreated (UnTx) regions for each replication of western blot, whereas the black
- FIG. 19B shows immunoblot showing the amount of total rhodopsin (canine + human RHO820) in biopsy punches taken from treated (Tx) and untreated (UnTx) areas of canine retina. Histone H3 was used for normalization. Bar graphs show the percentage of remaining rhodopsin protein in the Treated and untreated areas. Note the loss of the lower MW band (corresponding to mutant T4R RHO protein) in the treated areas of EM424-OD and EM425-OD.
- FIG. 19C is a table showing numerical values for each pair of punches used for protein quantification.
- FIG. 19D is a bar graph showing remaining canine Rhodopsin RNA in the treated areas as a percentage of canine RHO RNA levels measured in untreated areas.
- FIG. 19E is a bar graph showing levels of human RHO820 in the treated areas as a percentage of canine RHO RNA levels measured in untreated areas.
- FIG. 19F is a table showing numerical values for each pair of punches used for RNA quantification.
- ONL thickness in the bleb/treated area was preserved at all post-injection time points suggesting that this vector construct was not toxic and that there was no loss of photoreceptors during that period.
- the surrounding non bleb/untreated regions were used as an internal control for each eye. Two weeks post light-exposure there was complete preservation of ONL thickness in the bleb/treated area in both injected eyes.
- FIGs. 20A-20C show in vivo retinal imaging showing protection from light-induced retinal degeneration in the region of a mutant RHO T4R/+ retina treated with AAV2/5-sc-HOP-RHO820-H1-shRNA820 at 5 x 10 11 /vg/ml titer.
- FIG. 20A shows en face cSLO composite showing 2 weeks post light exposure the retinal region (border demarcated by white arrows) that was protected from degeneration.
- Light gray arrow indicates the location within the treated area of the OCT B scans shown in FIG. 20B
- dark gray arrow indicates the location within the untreated area of the OCT B scans shown in FIG. 20C.
- FIG. 20A shows en face cSLO composite showing 2 weeks post light exposure the retinal region (border demarcated by white arrows) that was protected from degeneration.
- Light gray arrow indicates the location within the treated area of the OCT B scans shown in FIG. 20B
- FIG. 20B shows OCT B scans within the treated area before injection, 11 weeks post injection, and 13 weeks post injection / 2 weeks post light exposure. ONL thickness is preserved throughout the treated area at both time-points following the injection of the viral vector.
- FIGs. 20B and 30V show OCT B scans within the untreated area before injection, 11 weeks post injection, and 13 weeks post injection / 2 weeks post light exposure. ONL is preserved up to 11 weeks post injection but is completely lost 2 weeks after light exposure.
- FIG. 21 shows topographic maps of ONL thickness from a two RHO T4R/+ treated with AAV2/5-sc-HOP-RHO820-H1-shRNA820 at 5 x 10 11 /vg/ml titer showing protection from light-induced retinal degeneration.
- Immunohistochemistry was used to assess the level of protection conferred by AAV2/5-sc-HOP-RHO820-H1-shRNA820 in the treated/bleb area.
- the surrounding non bleb/untreated regions were used as an internal control for each eye.
- Antibodies directed against rhodopsin (RHO), and human cone arrestin (hCA) were used to assess the integrity of rod outer segments and cone morphology.
- RHO rhodopsin
- hCA human cone arrestin
- FIG. 22 shows immunohistochemistry (Rhodopsin was stained in green and appears as the lighter staining in the panels of FIG. 22 ; human cone arrestin was stained in red appears as the gray staining in the panels of FIG. 22 ) in the treated, transition zone and untreated areas of mutant RHO T4R/+ retinas subretinally injected with AAV2/5-sc-HOP-RHO820-H1-shRNA820 at 5 x 10 11 /vg/ml titer.
- ONL outer nuclear layer
- OS rod outer segments
- inventive embodiments are presented by way of example only and that, within the scope of the appended claims and equivalents thereto, inventive embodiments may be practiced otherwise than as specifically described and claimed.
- inventive embodiments of the present disclosure are directed to each individual feature, system, article, material, kit, and/or method described herein.
- a reference to "A and/or B", when used in conjunction with open-ended language such as “comprising” can refer, in one embodiment, to A only (optionally including elements other than B); in another embodiment, to B only (optionally including elements other than A); in yet another embodiment, to both A and B (optionally including other elements); etc.
- the phrase "at least one,” in reference to a list of one or more elements, should be understood to mean at least one element selected from any one or more of the elements in the list of elements, but not necessarily including at least one of each and every element specifically listed within the list of elements and not excluding any combinations of elements in the list of elements.
- This definition also allows that elements may optionally be present other than the elements specifically identified within the list of elements to which the phrase "at least one" refers, whether related or unrelated to those elements specifically identified.
- At least one of A and B can refer, in one embodiment, to at least one, optionally including more than one, A, with no B present (and optionally including elements other than B); in another embodiment, to at least one, optionally including more than one, B, with no A present (and optionally including elements other than A); in yet another embodiment, to at least one, optionally including more than one, A, and at least one, optionally including more than one, B (and optionally including other elements); etc.
Landscapes
- Health & Medical Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Engineering & Computer Science (AREA)
- Genetics & Genomics (AREA)
- Chemical & Material Sciences (AREA)
- Biomedical Technology (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Organic Chemistry (AREA)
- Zoology (AREA)
- Wood Science & Technology (AREA)
- General Engineering & Computer Science (AREA)
- Biotechnology (AREA)
- Molecular Biology (AREA)
- General Health & Medical Sciences (AREA)
- Microbiology (AREA)
- Biochemistry (AREA)
- Plant Pathology (AREA)
- Biophysics (AREA)
- Physics & Mathematics (AREA)
- Medicinal Chemistry (AREA)
- Virology (AREA)
- Veterinary Medicine (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Pharmacology & Pharmacy (AREA)
- Animal Behavior & Ethology (AREA)
- Public Health (AREA)
- Ophthalmology & Optometry (AREA)
- General Chemical & Material Sciences (AREA)
- Immunology (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
- Medicines Containing Material From Animals Or Micro-Organisms (AREA)
- Pigments, Carbon Blacks, Or Wood Stains (AREA)
- Inorganic Compounds Of Heavy Metals (AREA)
- Micro-Organisms Or Cultivation Processes Thereof (AREA)
- Medicinal Preparation (AREA)
- Paper (AREA)
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201662302122P | 2016-03-01 | 2016-03-01 | |
| US201662398451P | 2016-09-22 | 2016-09-22 | |
| EP17760752.0A EP3423582B1 (en) | 2016-03-01 | 2017-03-01 | Aav vectors for treatment of dominant retinitis pigmentosa |
| PCT/US2017/020289 WO2017151823A1 (en) | 2016-03-01 | 2017-03-01 | Aav vectors for treatment of dominant retinitis pigmentosa |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP17760752.0A Division EP3423582B1 (en) | 2016-03-01 | 2017-03-01 | Aav vectors for treatment of dominant retinitis pigmentosa |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| EP4119668A1 true EP4119668A1 (en) | 2023-01-18 |
Family
ID=59743213
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP22170988.4A Pending EP4119668A1 (en) | 2016-03-01 | 2017-03-01 | Aav vectors for treatment of dominant retinitis pigmentosa |
| EP17760752.0A Active EP3423582B1 (en) | 2016-03-01 | 2017-03-01 | Aav vectors for treatment of dominant retinitis pigmentosa |
Family Applications After (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP17760752.0A Active EP3423582B1 (en) | 2016-03-01 | 2017-03-01 | Aav vectors for treatment of dominant retinitis pigmentosa |
Country Status (9)
Families Citing this family (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2923877T3 (es) | 2016-03-01 | 2022-10-03 | Univ Florida | Vectores VAA para el tratamiento de la retinitis pigmentosa dominante |
| JP7385282B2 (ja) * | 2017-11-07 | 2023-11-22 | ザ・ユニヴァーシティ・オヴ・ノース・キャロライナ・アト・チャペル・ヒル | 環状rna分子のための方法及び組成物 |
| SG11202011151VA (en) * | 2018-05-15 | 2020-12-30 | Univ Washington | Compositions and methods for reducing spliceopathy and treating rna dominance disorders |
| CN118480544A (zh) * | 2018-06-01 | 2024-08-13 | 佛罗里达大学研究基金会有限公司 | 用于治疗显性视网膜色素变性的组合物和方法 |
| GB201817469D0 (en) * | 2018-10-26 | 2018-12-12 | Univ Oxford Innovation Ltd | Gene therapy for retinal disease |
| CN111518813B (zh) * | 2019-02-03 | 2023-04-28 | 武汉纽福斯生物科技有限公司 | 视紫红质的编码序列、其表达载体构建及其应用 |
| JP2022545378A (ja) * | 2019-08-15 | 2022-10-27 | ザ・チルドレンズ・ホスピタル・オブ・フィラデルフィア | SCA1の治療のための、導入遺伝子およびイントロン由来miRNAを組み合わせた治療法 |
| CN113038971B (zh) * | 2020-04-21 | 2022-06-24 | 北京中因科技有限公司 | RHO-adRP基于基因编辑的方法和组合物 |
| CN111926044B (zh) * | 2020-10-12 | 2021-01-22 | 北京大学第三医院(北京大学第三临床医学院) | 结合突变rho基因的核酸分子和试剂盒 |
| MX2023008826A (es) | 2021-02-01 | 2023-09-15 | Regenxbio Inc | Terapia génica para lipofuscinosis neuronal ceroidea. |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20070015238A1 (en) | 2002-06-05 | 2007-01-18 | Snyder Richard O | Production of pseudotyped recombinant AAV virions |
| WO2008125846A2 (en) * | 2007-04-12 | 2008-10-23 | The Provost, Fellows And Scholars Of The College Of The Holy And Undivided Trinity Of Queen Elizabeth | Genetic suppression and replacement |
| WO2010127209A2 (en) * | 2009-04-30 | 2010-11-04 | The Research Foundation Of State University Of New York | Compositions and methods for therapy of macular degeneration |
| WO2012013326A1 (en) | 2010-07-30 | 2012-02-02 | Curevac Gmbh | Complexation of nucleic acids with disulfide-crosslinked cationic components for transfection and immunostimulation |
| US20120178702A1 (en) | 1995-01-23 | 2012-07-12 | University Of Pittsburgh | Stable lipid-comprising drug delivery complexes and methods for their production |
| US20120322861A1 (en) | 2007-02-23 | 2012-12-20 | Barry John Byrne | Compositions and Methods for Treating Diseases |
| WO2015143418A2 (en) * | 2014-03-21 | 2015-09-24 | Genzyme Corporation | Gene therapy for retinitis pigmentosa |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB9606961D0 (en) | 1996-04-02 | 1996-06-05 | Farrar Gwyneth J | Genetic strategy III |
| US8551970B2 (en) | 1996-04-02 | 2013-10-08 | Optigen Patents Limited | Genetic suppression and replacement |
| US20040234999A1 (en) | 1996-04-02 | 2004-11-25 | Farrar Gwenyth Jane | Genetic suppression and replacement |
| US7994305B2 (en) * | 2003-04-18 | 2011-08-09 | The Trustees Of The University Of Pennsylvania | Compositions and methods for siRNA inhibition of angiopoietin 1 and 2 and their receptor Tie2 |
| US20070009899A1 (en) | 2003-10-02 | 2007-01-11 | Mounts William M | Nucleic acid arrays for detecting gene expression in animal models of inflammatory diseases |
| WO2005090572A2 (en) | 2004-03-24 | 2005-09-29 | Oncotherapy Science, Inc. | Compositions and methods for treating pancreatic cancer |
| US20080221057A1 (en) | 2007-02-16 | 2008-09-11 | Wyeth | Secreted protein ccdc80 regulates adipocyte differentiation |
| WO2009035792A1 (en) * | 2007-08-03 | 2009-03-19 | Melba Ketchum | Compositions, methods and systems for the simultaneous determination of parentage, identity, sex, genotype and/or phenotype and breed determination in animals |
| CN102061308B (zh) | 2010-10-29 | 2013-05-08 | 北京未名凯拓作物设计中心有限公司 | 一种用百草枯筛选转基因植物的方法 |
| CN102061305B (zh) * | 2010-12-15 | 2012-07-18 | 深圳市百恩维生物科技有限公司 | 一种多ShRNA高效协同沉默基因方法及载体 |
| US20130064815A1 (en) | 2011-09-12 | 2013-03-14 | The Trustees Of Princeton University | Inducing apoptosis in quiescent cells |
| WO2013175446A1 (en) * | 2012-05-25 | 2013-11-28 | Commissariat A L'energie Atomique Et Aux Energies Alternatives | Vector for the selective silencing of a gene in astrocytes |
| WO2014138792A1 (en) | 2013-03-14 | 2014-09-18 | Commonwealth Scientific And Industrial Research Organisation | Double-stranded rna |
| NZ733844A (en) | 2015-02-26 | 2022-07-01 | Ionis Pharmaceuticals Inc | Allele specific modulators of p23h rhodopsin |
| WO2016176690A2 (en) | 2015-04-30 | 2016-11-03 | The Trustees Of Columbia University In The City Of New York | Gene therapy for autosomal dominant diseases |
| US11096955B2 (en) | 2016-02-09 | 2021-08-24 | Fondazione Telethon | Synthetic promoters and uses thereof |
| US20170348387A1 (en) | 2016-02-29 | 2017-12-07 | The Trustees Of The University Of Pennsylvania | Aav-mediated gene therapy for nphp5 lca-ciliopathy |
| ES2923877T3 (es) | 2016-03-01 | 2022-10-03 | Univ Florida | Vectores VAA para el tratamiento de la retinitis pigmentosa dominante |
-
2017
- 2017-03-01 ES ES17760752T patent/ES2923877T3/es active Active
- 2017-03-01 CA CA3014671A patent/CA3014671A1/en active Pending
- 2017-03-01 IL IL261014A patent/IL261014B2/en unknown
- 2017-03-01 WO PCT/US2017/020289 patent/WO2017151823A1/en not_active Application Discontinuation
- 2017-03-01 CN CN201780014309.8A patent/CN109154002B/zh active Active
- 2017-03-01 EP EP22170988.4A patent/EP4119668A1/en active Pending
- 2017-03-01 JP JP2018545818A patent/JP7272795B2/ja active Active
- 2017-03-01 CN CN202210934990.0A patent/CN115896106A/zh active Pending
- 2017-03-01 IL IL302455A patent/IL302455A/en unknown
- 2017-03-01 EP EP17760752.0A patent/EP3423582B1/en active Active
- 2017-03-01 US US16/081,307 patent/US11118185B2/en active Active
- 2017-03-01 AU AU2017227776A patent/AU2017227776C1/en active Active
-
2021
- 2021-05-21 US US17/327,609 patent/US20210324387A1/en active Pending
-
2022
- 2022-09-29 JP JP2022156496A patent/JP7642211B2/ja active Active
-
2023
- 2023-09-27 AU AU2023237106A patent/AU2023237106B2/en active Active
-
2024
- 2024-09-27 JP JP2024168632A patent/JP2025016451A/ja active Pending
Patent Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20120178702A1 (en) | 1995-01-23 | 2012-07-12 | University Of Pittsburgh | Stable lipid-comprising drug delivery complexes and methods for their production |
| US20070015238A1 (en) | 2002-06-05 | 2007-01-18 | Snyder Richard O | Production of pseudotyped recombinant AAV virions |
| US20120322861A1 (en) | 2007-02-23 | 2012-12-20 | Barry John Byrne | Compositions and Methods for Treating Diseases |
| WO2008125846A2 (en) * | 2007-04-12 | 2008-10-23 | The Provost, Fellows And Scholars Of The College Of The Holy And Undivided Trinity Of Queen Elizabeth | Genetic suppression and replacement |
| WO2010127209A2 (en) * | 2009-04-30 | 2010-11-04 | The Research Foundation Of State University Of New York | Compositions and methods for therapy of macular degeneration |
| WO2012013326A1 (en) | 2010-07-30 | 2012-02-02 | Curevac Gmbh | Complexation of nucleic acids with disulfide-crosslinked cationic components for transfection and immunostimulation |
| US20130142818A1 (en) | 2010-07-30 | 2013-06-06 | Curevac Gmbh | Complexation of nucleic acids with disulfide-crosslinked cationic components for transfection and immunostimulation |
| WO2015143418A2 (en) * | 2014-03-21 | 2015-09-24 | Genzyme Corporation | Gene therapy for retinitis pigmentosa |
Non-Patent Citations (18)
| Title |
|---|
| AURICCHIO ET AL., HUM. MOLEC. GENET., vol. 10, 2001, pages 3075 - 3081 |
| DUAN ET AL., J. VIROL., vol. 75, 2001, pages 7662 - 7671 |
| GORBATYUK MJUSTILIEN VLIU JHAUSWIRTH WWLEWIN AS: "Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery", VISION RES., vol. 47, 2007, pages 1202 - 1208, XP022003534, DOI: 10.1016/j.visres.2006.11.026 |
| GRIMM ET AL.: "Helper Virus-Free, Optically Controllable, and Two-Plasmid-Based Production of Adeno-associated Virus Vectors of Serotypes 1 to 6", MOLECULAR THERAPY, vol. 7, 2003, pages 839 - 850, XP002256403, DOI: 10.1016/S1525-0016(03)00095-9 |
| GRIMM ET AL.: "Novel Tools for Production and Purification of Recombinant Adenoassociated Virus Vectors", HUMAN GENE THERAPY, vol. 9, 1998, pages 2745 - 2760, XP002093963 |
| HALBERT ET AL., J. VIROL., vol. 74, 2000, pages 1524 - 1532 |
| KERN, A. ET AL.: "Identification of a Heparin-Binding Motif on Adeno-Associated Virus Type 2 Capsids", JOURNAL OF VIROLOGY, vol. 77, 2003, pages 11072 - 11081, XP055548734, DOI: 10.1128/JVI.77.20.11072-11081.2003 |
| KHVOROVA AREYNOLDS AJAYASENA SD: "Functional siRNAs and miRNAs exhibit strand bias", CELL, vol. 115, 2003, pages 209 - 216, XP002609792, DOI: 10.1016/S0092-8674(03)00801-8 |
| KRONENBERG ET AL.: "A Conformational Change in the Adeno-Associated Virus Type 2 Capsid Leads to the Exposure of Hidden VP1 N Termini", JOURNAL OF VIROLOGY, vol. 79, 2005, pages 5296 - 5303, XP055470106, DOI: 10.1128/JVI.79.9.5296-5303.2005 |
| MAO HGORBATYUK MSROSSMILLER BHAUSWIRTH WWLEWIN AS: "Long-Term Rescue of Retinal Structure and Function by Rhodopsin RNA Replacement with a Single Adeno-Associated Viral Vector in P23H RHO Transgenic Mice", HUM GENE THER., vol. 23, 2012, pages 356 - 366, XP055197799, DOI: 10.1089/hum.2011.213 |
| MOL THER., vol. 20, no. 4, April 2012 (2012-04-01), pages 1035 - 1038 |
| MOULLIER, P.SNYDER, R.O.: "International efforts for recombinant adeno-associated viral vector reference standards", MOLECULAR THERAPY, vol. 16, 2008, pages 1185 - 1188 |
| REYNOLDS ALEAKE DBOESE QSCARINGE SMARSHALL WSKHVOROVA A: "Rational siRNA design for RNA interference", NAT BIOTECHNOL., vol. 22, 2004, pages 326 - 330, XP002311429, DOI: 10.1038/nbt936 |
| ROSSMILLER BMAO HLEWIN AS: "Gene therapy in animal models of autosomal dominant retinitis pigmentosa", MOL VIS., vol. 18, 2012, pages 2479 - 2496 |
| S. BEHRMAN ET AL: "A CHOP-regulated microRNA controls rhodopsin expression", GENES & DEVELOPMENT, vol. 12, no. 7, 21 March 2011 (2011-03-21), pages 982 - 927, XP055197790, ISSN: 0890-9369, DOI: 10.1083/jcb.201010055 * |
| SUSAN F. MURRAY ET AL: "Allele-Specific Inhibition of Rhodopsin With an Antisense Oligonucleotide Slows Photoreceptor Cell Degeneration", INVESTIGATIVE OPTHALMOLOGY & VISUAL SCIENCE, vol. 56, no. 11, 5 October 2015 (2015-10-05), US, pages 6362, XP055373895, ISSN: 1552-5783, DOI: 10.1167/iovs.15-16400 * |
| ZHOU HXIA XGXU Z: "An RNA polymerase II construct synthesizes short-hairpin RNA with a quantitative indicator and mediates highly efficient RNAi", NUCLEIC ACIDS RES., vol. 33, 2005, pages e62 - e70 |
| ZOLOTUKHIN ET AL.: "Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors", METHODS, vol. 28, 2002, pages 158 - 167, XP002256404, DOI: 10.1016/S1046-2023(02)00220-7 |
Also Published As
| Publication number | Publication date |
|---|---|
| IL302455A (en) | 2023-06-01 |
| CN109154002B (zh) | 2022-08-30 |
| US20190093111A1 (en) | 2019-03-28 |
| IL261014A (en) | 2018-10-31 |
| EP3423582A4 (en) | 2019-11-13 |
| JP2025016451A (ja) | 2025-02-04 |
| AU2017227776C1 (en) | 2024-01-25 |
| JP2019511216A (ja) | 2019-04-25 |
| AU2017227776B2 (en) | 2023-07-06 |
| JP7642211B2 (ja) | 2025-03-10 |
| JP7272795B2 (ja) | 2023-05-12 |
| AU2023237106B2 (en) | 2025-08-28 |
| IL261014B2 (en) | 2023-10-01 |
| US20210324387A1 (en) | 2021-10-21 |
| IL261014B1 (en) | 2023-06-01 |
| CN109154002A (zh) | 2019-01-04 |
| EP3423582A1 (en) | 2019-01-09 |
| CN115896106A (zh) | 2023-04-04 |
| WO2017151823A1 (en) | 2017-09-08 |
| EP3423582B1 (en) | 2022-05-04 |
| US11118185B2 (en) | 2021-09-14 |
| JP2022185052A (ja) | 2022-12-13 |
| CA3014671A1 (en) | 2017-09-08 |
| AU2023237106A1 (en) | 2023-10-19 |
| AU2017227776A1 (en) | 2018-08-23 |
| ES2923877T3 (es) | 2022-10-03 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20210324387A1 (en) | Aav vectors for treatment of dominant retinitis pigmentosa | |
| ES2856403T3 (es) | Composiciones a base de rAAV y procedimientos para el tratamiento de la esclerosis lateral amiotrofica | |
| JP7504967B2 (ja) | MeCP2発現カセット | |
| US12203074B2 (en) | Compositions and methods for treatment of dominant retinitis pigmentosa | |
| CN116568338A (zh) | 改进的aav介导的x连锁视网膜劈裂症治疗 | |
| WO2023122804A1 (en) | Compositions and methods comprising a cardiac-specific promoter | |
| US20240425882A1 (en) | Compositions useful in treatment of cdkl5 deficiency disorder (cdd) | |
| CN114761569A (zh) | 阿尔茨海默氏病的基因疗法 | |
| US11806408B2 (en) | Methods and compositions for treating cone-rod retinal dystrophy | |
| HK40002351A (en) | Aav vectors for treatment of dominant retinitis pigmentosa | |
| HK40002351B (en) | Aav vectors for treatment of dominant retinitis pigmentosa | |
| WO2025090575A9 (en) | Gene therapy for treatment of retinal degeneration | |
| WO2025102034A1 (en) | Gene therapy for barth syndrome | |
| WO2023240177A1 (en) | Products and methods for treating diseases or conditions associated with mutant or pathogenic kcnq3 expression | |
| KR20250096825A (ko) | 목적 유전자용 발현 카세트 및 이의 용도 |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN PUBLISHED |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 3423582 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| P01 | Opt-out of the competence of the unified patent court (upc) registered |
Effective date: 20230523 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20230718 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: HK Ref legal event code: DE Ref document number: 40088240 Country of ref document: HK |