EP3653111B1 - Dipositif destinés à fournir des stimuli au cerveau - Google Patents
Dipositif destinés à fournir des stimuli au cerveau Download PDFInfo
- Publication number
- EP3653111B1 EP3653111B1 EP19216447.3A EP19216447A EP3653111B1 EP 3653111 B1 EP3653111 B1 EP 3653111B1 EP 19216447 A EP19216447 A EP 19216447A EP 3653111 B1 EP3653111 B1 EP 3653111B1
- Authority
- EP
- European Patent Office
- Prior art keywords
- auditory
- stimulus
- light
- frequency
- pattern
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Active
Links
- 210000004556 brain Anatomy 0.000 title claims description 11
- 230000000007 visual effect Effects 0.000 claims description 42
- 230000000638 stimulation Effects 0.000 claims description 36
- 208000028173 post-traumatic stress disease Diseases 0.000 claims description 18
- 208000002193 Pain Diseases 0.000 claims description 12
- 238000011282 treatment Methods 0.000 claims description 12
- 208000012902 Nervous system disease Diseases 0.000 claims description 11
- 208000025966 Neurological disease Diseases 0.000 claims description 10
- 208000013738 Sleep Initiation and Maintenance disease Diseases 0.000 claims description 6
- 208000029028 brain injury Diseases 0.000 claims description 6
- 230000000694 effects Effects 0.000 claims description 6
- 206010022437 insomnia Diseases 0.000 claims description 6
- 238000004891 communication Methods 0.000 claims description 5
- 230000003340 mental effect Effects 0.000 claims description 2
- 230000002708 enhancing effect Effects 0.000 claims 2
- 230000002123 temporal effect Effects 0.000 claims 1
- 230000001225 therapeutic effect Effects 0.000 description 30
- 238000000034 method Methods 0.000 description 22
- 238000012549 training Methods 0.000 description 13
- 230000006872 improvement Effects 0.000 description 11
- 210000003128 head Anatomy 0.000 description 10
- 230000001976 improved effect Effects 0.000 description 10
- 208000030886 Traumatic Brain injury Diseases 0.000 description 8
- 230000009529 traumatic brain injury Effects 0.000 description 8
- 208000007333 Brain Concussion Diseases 0.000 description 7
- 210000000988 bone and bone Anatomy 0.000 description 6
- 239000003814 drug Substances 0.000 description 6
- 230000001965 increasing effect Effects 0.000 description 6
- 238000010586 diagram Methods 0.000 description 5
- 230000001939 inductive effect Effects 0.000 description 5
- 238000012360 testing method Methods 0.000 description 5
- 238000002560 therapeutic procedure Methods 0.000 description 5
- 206010016754 Flashback Diseases 0.000 description 4
- 230000008859 change Effects 0.000 description 4
- 210000005069 ears Anatomy 0.000 description 4
- 230000035484 reaction time Effects 0.000 description 4
- 238000011084 recovery Methods 0.000 description 4
- 230000009467 reduction Effects 0.000 description 4
- 238000011285 therapeutic regimen Methods 0.000 description 4
- 238000011269 treatment regimen Methods 0.000 description 4
- 206010029412 Nightmare Diseases 0.000 description 3
- 208000027418 Wounds and injury Diseases 0.000 description 3
- 230000004913 activation Effects 0.000 description 3
- QVGXLLKOCUKJST-UHFFFAOYSA-N atomic oxygen Chemical compound [O] QVGXLLKOCUKJST-UHFFFAOYSA-N 0.000 description 3
- 230000001684 chronic effect Effects 0.000 description 3
- 230000006378 damage Effects 0.000 description 3
- 208000014674 injury Diseases 0.000 description 3
- 230000006996 mental state Effects 0.000 description 3
- 229910052760 oxygen Inorganic materials 0.000 description 3
- 239000001301 oxygen Substances 0.000 description 3
- 230000001953 sensory effect Effects 0.000 description 3
- 230000004936 stimulating effect Effects 0.000 description 3
- 230000001360 synchronised effect Effects 0.000 description 3
- 208000019901 Anxiety disease Diseases 0.000 description 2
- 206010009866 Cold sweat Diseases 0.000 description 2
- 206010010254 Concussion Diseases 0.000 description 2
- 206010010904 Convulsion Diseases 0.000 description 2
- 208000030814 Eating disease Diseases 0.000 description 2
- 208000019454 Feeding and Eating disease Diseases 0.000 description 2
- 206010048533 Hypervigilance Diseases 0.000 description 2
- 208000019022 Mood disease Diseases 0.000 description 2
- 208000028389 Nerve injury Diseases 0.000 description 2
- 206010060860 Neurological symptom Diseases 0.000 description 2
- 208000028017 Psychotic disease Diseases 0.000 description 2
- 230000036506 anxiety Effects 0.000 description 2
- 238000003491 array Methods 0.000 description 2
- 238000004883 computer application Methods 0.000 description 2
- 230000009514 concussion Effects 0.000 description 2
- 235000014632 disordered eating Nutrition 0.000 description 2
- 230000001037 epileptic effect Effects 0.000 description 2
- 230000006870 function Effects 0.000 description 2
- 230000002045 lasting effect Effects 0.000 description 2
- 230000015654 memory Effects 0.000 description 2
- 230000036997 mental performance Effects 0.000 description 2
- 230000008764 nerve damage Effects 0.000 description 2
- 208000022821 personality disease Diseases 0.000 description 2
- 230000010349 pulsation Effects 0.000 description 2
- 230000036632 reaction speed Effects 0.000 description 2
- 230000004044 response Effects 0.000 description 2
- 238000004088 simulation Methods 0.000 description 2
- 239000013589 supplement Substances 0.000 description 2
- 208000024891 symptom Diseases 0.000 description 2
- 238000012935 Averaging Methods 0.000 description 1
- 208000012639 Balance disease Diseases 0.000 description 1
- VYZAMTAEIAYCRO-UHFFFAOYSA-N Chromium Chemical compound [Cr] VYZAMTAEIAYCRO-UHFFFAOYSA-N 0.000 description 1
- 208000000094 Chronic Pain Diseases 0.000 description 1
- YJPIGAIKUZMOQA-UHFFFAOYSA-N Melatonin Natural products COC1=CC=C2N(C(C)=O)C=C(CCN)C2=C1 YJPIGAIKUZMOQA-UHFFFAOYSA-N 0.000 description 1
- 206010053694 Saccadic eye movement Diseases 0.000 description 1
- 208000006011 Stroke Diseases 0.000 description 1
- 241000700605 Viruses Species 0.000 description 1
- 230000009471 action Effects 0.000 description 1
- 230000036626 alertness Effects 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- 230000007177 brain activity Effects 0.000 description 1
- 230000027288 circadian rhythm Effects 0.000 description 1
- 230000001149 cognitive effect Effects 0.000 description 1
- 208000037265 diseases, disorders, signs and symptoms Diseases 0.000 description 1
- 208000035475 disorder Diseases 0.000 description 1
- 230000000792 effect on seizure Effects 0.000 description 1
- 206010015037 epilepsy Diseases 0.000 description 1
- 230000004424 eye movement Effects 0.000 description 1
- 230000010365 information processing Effects 0.000 description 1
- 239000004615 ingredient Substances 0.000 description 1
- 230000033001 locomotion Effects 0.000 description 1
- DRLFMBDRBRZALE-UHFFFAOYSA-N melatonin Chemical compound COC1=CC=C2NC=C(CCNC(C)=O)C2=C1 DRLFMBDRBRZALE-UHFFFAOYSA-N 0.000 description 1
- 229960003987 melatonin Drugs 0.000 description 1
- 230000036651 mood Effects 0.000 description 1
- 201000003152 motion sickness Diseases 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 238000000059 patterning Methods 0.000 description 1
- 230000002093 peripheral effect Effects 0.000 description 1
- 230000036314 physical performance Effects 0.000 description 1
- 238000012545 processing Methods 0.000 description 1
- 239000000047 product Substances 0.000 description 1
- 230000002040 relaxant effect Effects 0.000 description 1
- 230000001020 rhythmical effect Effects 0.000 description 1
- 230000004434 saccadic eye movement Effects 0.000 description 1
- 230000035807 sensation Effects 0.000 description 1
- 210000003625 skull Anatomy 0.000 description 1
- 230000005236 sound signal Effects 0.000 description 1
- 238000006467 substitution reaction Methods 0.000 description 1
- 238000001356 surgical procedure Methods 0.000 description 1
- 208000011580 syndromic disease Diseases 0.000 description 1
- 230000036642 wellbeing Effects 0.000 description 1
- 230000003936 working memory Effects 0.000 description 1
- ZAFYATHCZYHLPB-UHFFFAOYSA-N zolpidem Chemical compound N1=C2C=CC(C)=CN2C(CC(=O)N(C)C)=C1C1=CC=C(C)C=C1 ZAFYATHCZYHLPB-UHFFFAOYSA-N 0.000 description 1
- 229960001475 zolpidem Drugs 0.000 description 1
Images
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M21/00—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis
- A61M21/02—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis for inducing sleep or relaxation, e.g. by direct nerve stimulation, hypnosis, analgesia
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B27/00—Optical systems or apparatus not provided for by any of the groups G02B1/00 - G02B26/00, G02B30/00
- G02B27/01—Head-up displays
- G02B27/017—Head mounted
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M21/00—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis
- A61M2021/0005—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis by the use of a particular sense, or stimulus
- A61M2021/0022—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis by the use of a particular sense, or stimulus by the tactile sense, e.g. vibrations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M21/00—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis
- A61M2021/0005—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis by the use of a particular sense, or stimulus
- A61M2021/0027—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis by the use of a particular sense, or stimulus by the hearing sense
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M21/00—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis
- A61M2021/0005—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis by the use of a particular sense, or stimulus
- A61M2021/0044—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis by the use of a particular sense, or stimulus by the sight sense
- A61M2021/005—Other devices or methods to cause a change in the state of consciousness; Devices for producing or ending sleep by mechanical, optical, or acoustical means, e.g. for hypnosis by the use of a particular sense, or stimulus by the sight sense images, e.g. video
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/35—Communication
- A61M2205/3546—Range
- A61M2205/3569—Range sublocal, e.g. between console and disposable
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/35—Communication
- A61M2205/3576—Communication with non implanted data transmission devices, e.g. using external transmitter or receiver
- A61M2205/3592—Communication with non implanted data transmission devices, e.g. using external transmitter or receiver using telemetric means, e.g. radio or optical transmission
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/50—General characteristics of the apparatus with microprocessors or computers
- A61M2205/502—User interfaces, e.g. screens or keyboards
- A61M2205/505—Touch-screens; Virtual keyboard or keypads; Virtual buttons; Soft keys; Mouse touches
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2205/00—General characteristics of the apparatus
- A61M2205/50—General characteristics of the apparatus with microprocessors or computers
- A61M2205/502—User interfaces, e.g. screens or keyboards
- A61M2205/507—Head Mounted Displays [HMD]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61M—DEVICES FOR INTRODUCING MEDIA INTO, OR ONTO, THE BODY; DEVICES FOR TRANSDUCING BODY MEDIA OR FOR TAKING MEDIA FROM THE BODY; DEVICES FOR PRODUCING OR ENDING SLEEP OR STUPOR
- A61M2209/00—Ancillary equipment
- A61M2209/08—Supports for equipment
- A61M2209/088—Supports for equipment on the body
Definitions
- the present disclosure relates to medical devices and methods.
- the present disclosure relates to providing stimuli to a subject to treat various neurological disorders or conditions and/or to provide performance enhancement.
- Sensory stimulation has been applied to treat various disorders. For example, binaural beats have applied to induce various mental states to encourage sleep, relaxation, meditation, creativity, and other desirable mental states. Combinations of auditory and visual stimuli have been applied to encourage such mental states as well.
- the application of such therapy has been less than ideal in many circumstances.
- Equipment to provide the stimulus can be bulky, expensive, generally inaccessible, and below the critical efficacy threshold for widespread use, typically only helping subsets of the population. Users may find the use of such equipment difficult in many circumstances, such as when trying to sleep in a bedroom or an airplane cabin.
- US 4 892 106 A discloses a multiple afferent sensory stimulation device that is provided to receive a prerecorded tape program upon which the audio stimulation and control signals for the visual stimulation of a subject person's eyes and ears is provided, the invention consisting of a reproducing device to emit the audio and visual control signals on separate left and right channels, the audio stimulation proceeding directly to earphones worn by the subject person, and the visual stimulation control signals processed electronically.
- WO 01/64005 A2 discloses a device for influencing the motor-sensory information processing, whereby visual stimuli for stimulating eye movements may be presented to a user.
- At least one visual channel is provided, arranged in a screen which may be worn on the head of the user and, on viewing the above, in the operating state, at least a first and second extreme eye position within the range of movement may be defined, with at least one optical display element arranged in each of the first and second extreme eye positions and a control module, by means of which the display elements may be alternately activated and deactivated, preferably in a rhythmical pattern.
- US 4 172 406 A discloses a pair of headphones has a right speaker for the right ear and a left speaker for the left ear of the user.
- the speakers are electrically connected to each other and to a sound input source whereby the speakers reproduce sound from the sound input source.
- a pair of goggles is coupled to the headphones and covers the eyes of the user.
- a plurality of lamps are provided in the goggles, visible to the user.
- a microphone is electrically connected in circuit with a source of electrical energy and the lamps whereby sound reproduced by the speakers is picked up by the microphone, converted into electrical energy which varies in intensity with variations in the sound, and varies the energization of the lamps to provide a light pattern which varies in accordance with variations in the sound.
- US 4 315 502 A discloses a device for relaxing, stimulating and/or driving brain wave form function in a human subject.
- the device comprises, in combination, an eye mask having independently controlled left and right eyepieces and a peripheral light array in each eyepiece, an audio headset having independently controlled left and right earpieces and a control panel which controls light and sound signals to the light arrays and earpieces, respectively.
- Various control functions allow simultaneous or alternating light and sound pulsations in the left and right light arrays and earpieces, as well as selective phasing between light and sound pulsations.
- US 2010/323335 A1 discloses a psychotherapeutic device which induces saccadic eye movement of a user in a certain way, thus increasing the efficacy of psychiatric treatment for the user.
- the psychotherapeutic device in accordance with the present invention includes a main body including a frame having an opened portion corresponding to a user's eyes, a support means for placing the frame on the user's face, and a front panel connected to a front surface of the frame and providing light spots moved left and right with respect to the user's eyes; and a control box for controlling the operation of the front panel, wherein the main body and the control box are electrically connected to each other by a wire.
- the present disclosure relates to medical devices and methods which may be used, for example, to provide stimulus to a subject to treat various neurological disorders or conditions, where the stimulus provided may include one or more of an auditory, a visual, or a tactile stimulus.
- neurological disorders which may be treated with devices and methods may include, but are not limited to, insomnia, post-traumatic stress disorder (PTSD), brain injuries including, but not limited to traumatic brain injury (TBI), mild traumatic brain injury (mTBI), or injury from oxygen deprivation of the brain from strokes, depression, anxiety, mood disorders, personality disorders, eating disorders, psychotic disorders, and balance disorders, to name a few.
- the stimulus provided by the medical devices and methods described herein may provide cognitive benefits and/or enhancement, including, but not limited to, improving neuroplasticity, motor skills, coordination, reaction times, alertness, energy, working memory, mood, and feelings of wellbeing.
- stimuli may be provided to the wearer of a headset or sleep mask that may be comfortably worn by a user, such as in bed to induce sleep.
- the wearable headset or sleep mask may be operated by a personal computing device of the user, such as smartphone, having downloaded and active thereon a control application or "app" for the therapy.
- the wearable headset or sleep mask may also concurrently provide tactile stimuli, and the tactile stimuli may be provided from bone conduction transducer that may concurrently provide the auditory stimuli.
- Various patterns of the stimuli to induce different user responses are also disclosed.
- a device that produces an output that may be perceived by a user of the device as a visual, auditory or tactile stimuli at one or more frequencies, or in one or more frequency ranges.
- the stimuli may be turned on and off at frequencies that are believed to induce one or more frequencies of electrical activity in the brain, which are generally accepted as being delta waves (1.0 to 3.0 Hz), theta waves (3.0 to 7.0 Hz), alpha waves (7.0 to 12 Hz), beta waves (12 to 38 Hz), and gamma waves (38 to 42 Hz).
- one embodiment device produces an output that may be perceived by a user of the device as a stimuli at sequential frequencies, such as sequences of alpha waves, theta waves, and delta waves.
- the stimuli is a coordinated auditory and visual stimulation, providing right and left eyes and ears to pulsed light and pulsed auditory in each of the ranges listed above.
- the coordinated stimulation may be: 1) both eyes and both ears being stimulated at the same time; 2) the left eye and ear being stimulated at the same time, followed by the right eye and ear being stimulated at the same time; 3) both eyes being stimulated at the same time, followed by both ears being stimulated at the same time; or 4) the right eye and left ear being stimulated at the same time, followed by the left eye and right eye being stimulated at the same time.
- the stimulation may include, for example, sequentially stimulating in the alpha wave range, followed by the theta wave range, followed by the delta wave range. The stimulation can last for a period of one minute up to an hour.
- the method includes: providing a headset to be worn by the user; applying, with the headset, a left visual stimulus pattern to the left eye of the user; applying, with the headset, a right visual stimulus pattern to the right eye of the user; applying, with the headset, a left auditory stimulus pattern to the left side of a head of the user; and applying, with the headset, a right auditory stimulus pattern to the right side of the head.
- the applications of the left visual stimulus pattern, the right visual stimulus pattern, the left auditory stimulus pattern, and the right auditory stimulus pattern are coordinated with one another.
- the method includes: providing a headset to be worn by the user; applying, with the headset, a left visual stimulus pattern to the left eye of the user; applying, with the headset, a right visual stimulus pattern to the right eye of the user; applying, with the headset, a left auditory stimulus pattern to the left side of a head of the user; and applying, with the headset, a right auditory stimulus pattern to the right side of the head.
- the applications of the left visual stimulus pattern, the right visual stimulus pattern, the left auditory stimulus pattern, and the right auditory stimulus pattern are coordinated with one another.
- the apparatus includes: a frame configured to be worn on a head of a user; a left light source configured to generate a left visual stimulus pattern; a right light source configured to generate a right visual stimulus pattern; a left auditory source configured to generate a left auditory stimulus pattern; a right auditory source configured to generate a right auditory stimulus pattern; and a controller coupled to the left light source, the right light source, the left auditory source, and the right auditory source,
- the applications of the left visual stimulus pattern, the right visual stimulus pattern, the left auditory stimulus pattern, and the right auditory stimulus pattern are independently controlled from one another but coordinated with one another by the controller.
- It is yet another aspect to provide a method to provide stimulation to a user includes: concurrently providing a left-side light stimulus to a left eye of the user, a rightside light stimulus to a right eye of the user, a left-side auditory stimulus to a left side of the user, and a right-side auditory stimulus to a right side of the user for a first time interval; alternating providing the left-side light stimulus and left-side auditory stimulus with providing the right-side light stimulus and right-side auditory stimulus for a second time interval; alternating providing the left-side and right-side light stimuli with providing the left-side and right-side auditory stimuli for a third time interval; and alternating providing the left-side light stimulus and rightside auditory stimulus with providing the right-side light stimulus and left-side auditory stimulus for a fourth time interval.
- It is another aspect to provide a method of providing stimulation to a user includes: providing a headset to be worn by the user; applying, with the headset, a left auditory stimulus pattern to the left side of a head of the user; and applying, with the headset, a right auditory stimulus pattern to the right side of the head,
- the applications of the left auditory stimulus pattern and the right auditory stimulus pattern are coordinated with one another.
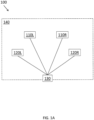
- FIGURE 1A is a schematic diagram of a first embodiment therapeutic system 100.
- Therapeutic system 100 provides one or more outputs that a person wearing the therapeutic system may experience as auditory, visual, and/or tactile stimulus.
- therapeutic system may comprise a left light source 110L, a right light source 110R, a left vibration source 120L, a right vibration source 120R, and a controller 130 for independently controlling and coordinating the action of the light and vibration sources.
- therapeutic system 100 may be positioned on the head of a user with left light source 110L positioned over the left eye to provide a left visual stimuli, right light source 110R positioned over the right eye to provide a right visual stimuli, left vibration source 120L positioned to provide left ear auditory stimuli, and right vibration source 120R positioned to provide right ear auditory stimuli.
- left and right light sources 110L, 110R may each comprise lightemitting diodes, an incandescent light source having a wavelength filter, a fluorescent light source, a backlit LCD panel, or other light source configured to provide to the user light at a desired, predetermined wavelength or wavelength range.
- left and right vibration sources 120L, 120R may each comprise earbuds, miniature speakers, or other vibration sources that can provide auditory stimuli to a user.
- left and right vibration sources 120L, 120R may comprise bone conduction transducers in the audible frequency range to provide vibrations to the user's skull bone that is sensed as auditory by the user's ear.
- one or more of left and right vibration sources 120L, 120R may also produce vibrations that are sensed as tactile stimuli.
- controller 130 may provide first signals to bone conduction transducers that vibrate or oscillate at a first frequency that can be interpreted by the user as auditory stimuli and may provide second signals at a second, lower frequency that can be interpreted as a tactile sensation by the user.
- bone conduction transducers may be adapted to provide both auditory and tactile stimulus to the user.
- left and right vibration sources 120L, 120R provide output at specific one or more frequencies or a range of frequencies, and are turned on and off at a stimulation frequency.
- a vibration source may be programmed to provide an output at an audio frequency of 256 Hz for some period of time, followed by no output for the following period of time.
- the vibration source is the product of an audio frequency and a square wave.
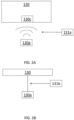
- FIGURE 1B is a schematic diagram of a second embodiment therapeutic system 100'.
- Second embodiment therapeutic system 100' is generally similar to first embodiment therapeutic system 100', except as explicitly noted.
- second embodiment therapeutic system 100' includes a left tactile stimulus source 121L and a right tactile stimulus source 121R, each of which may be individually controlled and coordinated with the controller 130 to provide tactile stimuli to a user of therapeutic system 100'.
- FIGURES 2A and 2B show schematic diagrams of the controller 130 of therapeutic system 100 or 100'.
- therapeutic system 100 or 100' may optionally include an external control unit 130a that may wirelessly communicate with a wireless receiver/transmitter 130c of the controller 130 through a wireless connection 131a.
- the wireless connection 131a may comprise a Bluetooth connection, a Bluetooth LE connection, a WiFi connection, a ZigBee connection, an infrared (IR) connection, a radiofrequency (RF) connection, or an inaudible auditory signal connection, to name a few examples.
- the external control unit 130a may comprise a custom-built, electronic controller.
- the external control unit 130a may comprise a personal computing device of the user that may have downloaded onto and operating, a custom computer application or "app" to operate the system 100 or 100' to provide a therapeutic regimen.
- the personal computing device may comprise a personal computer, a personal laptop computer, a tablet computer (such as an Apple iPad, a Samsung Galaxy Tab, a Microsoft Surface, or an Amazon Fire, to name a few examples), a smartphone (such as an Apple iPhone, a Samsung Galaxy phone, or a Google Nexus phone, to name a few examples), and the custom computer application or "app” may be an application or "app” downloadable from an application distribution platform such as Apple iTunes, Apple Store, Google Play, Google Chrome Web Store, Amazon App Store, or Microsoft Windows Store, to name a few examples.
- an application distribution platform such as Apple iTunes, Apple Store, Google Play, Google Chrome Web Store, Amazon App Store, or Microsoft Windows Store, to name a few examples.
- the application may include one or more therapeutic regimens that the user may select for implementation by the therapeutic system 100 or 100'.
- the application may allow the user to provide feedback information about the efficacy of the therapeutic regimen(s), the feedback may be uploaded and collected by a central server(s) in communication with the application, and the therapeutic regimen(s) may be improved or optimized based on the feedback from the one or more users.
- the system 100 or 100' may further comprise an external control unit 130a, such as a custom-built controller, that may communicate with the controller 130 through a wired connection 131a, for example, a USB, FireWire, or Lightning connection, to name a few examples.
- FIGURE 3A shows one embodiment of the therapeutic system 100 as including therapeutic wearable headset or sleep mask 140 which integrates the light, vibration, and, optionally, tactile sources into a single form factor for presentation to a user.
- therapeutic wearable headset or sleep mask 140 which integrates the light, vibration, and, optionally, tactile sources into a single form factor for presentation to a user.
- left light source 110L is positioned over the left eye to provide a left visual stimuli
- right light source 110R is positioned over the right eye to provide a right visual stimuli
- left vibration source 120L is positioned to provide left ear auditory stimuli
- right vibration source 120R is positioned to provide right ear auditory stimuli.
- the left vibration source 120L and the right vibration source 120R may each comprise bone conduction transducer that may provide both auditory and tactile stimulus.
- wearable headset or sleep mask 140 is therapeutic system 100' which includes left tactile stimulus source 121L and right tactile stimulus source 121R, each of which may be individually controlled and coordinated with the controller 130, as described above regarding FIG. 1B .
- the therapeutic wearable headset or sleep mask 140 may be operated with an external controller 130a (e.g., a smartphone) in communication with the controller 130 through a wireless connection 131a, for example.
- the user US may have an option to turn tactile stimulation on or off, for example.
- FIG. 3B shows a user US wearing the therapeutic wearable headset or sleep mask 140.
- FIGURE 4 shows a flow chart of an exemplary therapeutic method 400 for providing therapeutic auditory, visual, and/or tactile stimulus.
- a subject having a neurological disorder or condition may be identified.
- neurological disorders may include, but are not limited to, insomnia, post-traumatic stress disorder (PTSD), brain injuries such as traumatic brain injury (TBI), mild traumatic brain injury (mTBI), or injuries to the brain due to oxygen deprivation, such as strokes, depression, anxiety, mood disorders, personality disorders, eating disorders, and psychotic disorders.
- PTSD post-traumatic stress disorder
- TBI traumatic brain injury
- mTBI mild traumatic brain injury
- a subject may be selected to undergo a therapeutic method 400 for the purpose of performance enhancement of mental and/or physical tasks for to aid the subject in napping or sleeping.
- the subject may be provided the therapeutic system or headwear, such as the system 100 or 100' described above.
- the subject may wear the therapeutic system or headwear, such as wearable headset or sleep mask 140.
- headset 140 executes programming 450 provided in controller 130 to provide stimuli to the subject.
- the programming provides two or more of auditory, video, and/or tactile stimulus are concurrently provided by headset 140 to the subject, and thus, for example, may provide power to activate left light source 110L, right light source 110R, left vibration source 120L and or right vibration source 120R.
- the left vibration source 120L and the right vibration source 120R may each comprise bone conduction transducer that may provide both auditory and tactile stimulus.
- wearable headset or sleep mask 140 is therapeutic system 100' which includes left tactile stimulus source 121L and right tactile stimulus source 121R, each of which may be individually controlled and coordinated with the controller 130, as described above regarding FIG. 1B .
- providing two or more of auditory, video, and/or tactile stimulus concurrently may provide improved therapeutic benefits as compared to providing only one of auditory, video, or tactile stimulus at one time.
- the two or more auditory, video, and/or tactile stimulus may thus combine to provide the improved therapeutic benefits, for example (i.e., the two or more auditory, video, and/or tactile stimulus may synergize in a way to provide improved results over providing two of the stimuli individually.)
- Exemplary instructions for providing stimuli may be provided, for example, by programming 450, such as a subroutine 450a, which includes the simultaneous activation of all active auditory, video, and/or tactile stimulus sources.
- the activation of all sources may include the activation of tactile stimulation to run throughout all subsequent auditory and/or visual stimulation.
- Another exemplary subroutine 450b may comprise alternating the left auditory, video, and/or tactile stimulus sources with the right auditory, video, and/or tactile stimulus sources (i.e., the left stimuli and right stimuli take turns being active.)
- Another exemplary subroutine 450c may comprise alternating the visual sources with the auditory and/or tactile sources (i.e., the visual stimuli and the auditory/tactile stimuli take turns being active.)
- Another exemplary subroutine 450d may comprise alternating the left auditory and/or tactile source and the right visual source with the right auditory and/or tactile source and the left visual source (i.e., opposite auditory/tactile stimuli take turns being active.)
- Such programming is further described below.
- programming 450 including by not limited to subroutines 450a, 450b, 450c, and 450d, may each be applied one or more times, individually or in combination with one another.
- the programming may, in addition, provide sequences of output in subroutines 450a, 450b, 450c, and 450d at different frequencies and/or timings.
- the subroutines may provide output at specific frequencies that change as the subroutine is repeated.
- subroutine 450a may provide auditory output to vibration source 120R or 120L at a frequency of 256 Hz that is turned on and off, that is it is pulsed, at a pulse frequency of 1 Hz for 2 minutes.
- This square pulse auditory signal thus generates signals at a frequency of 1 Hz in addition to higher harmonics.
- the output at 256 Hz is pulsed at twice the previous pulse frequency for 2 minutes.
- the auditory frequency of 256 Hz may be modulated over a wide range, including frequencies corresponding to brain wave frequencies.
- the brain may be stimulated in a way that it is forced to communicate between the left and right sides of the brain.
- This forced communication for example, can allow PTSD memories to be wired to both sides of the brain, thereby stopping undesirable flashbacks.
- steps show method 400 of treating a patient in accordance with embodiments, a person of ordinary skill in the art will recognize many variations based on the teaching described herein.
- the steps may be completed in a different order. Steps may be added or deleted. Some of the steps may comprise sub-steps. Many of the steps may be repeated as often as beneficial to the treatment.
- circuitry of the controller 130 or the external control unit 130a such as one or more of a processor or logic circuitry such as a central processing unit (CPU) or a programmable array logic for field programmable gate array.
- the circuitry may be programmed to provide one or more of the steps of the method 400, and the program may comprise program instructions stored on a computer readable memory or programmed steps of the logic circuitry such as the programmable array logic or the field programmable gate array, for example.
- the following describes an example of a stimulation pattern that has been found by empirical studies to be effective for inducing sleep, including napping, increasing neuroplasticity, treating brain injuries from strokes, TBI, or mTBI, improving balance, including improving fine motor control and reaction times, and treating PTSD, to name a few indications.
- Light and auditory stimulus at a first frequency may be provided for a first time segment, then at a second lower frequency for a second time segment, and then at a third lower frequency for a third time segment.
- Each time segment may include one or more sub-segments of light and auditory stimulus, each sub-segment comprising one of the subroutines described above, for example.
- the light and auditory stimulus may end after a pre-determined time period, such as 20 minutes.
- the light and auditory stimulus may be ramped back up (i.e., starting from the third frequency, then transitioning to the second frequency, and finally transitioning to the third frequency), such as to wake the user.
- the light and auditory stimulus may be maintained at the second frequency such as to maintain a sleep state of the user.
- tactile stimulus may be provided concurrently with the auditory stimulus.
- the light may be provided at a wavelength of 580 nm and the auditory having a frequency of 256 Hz may be provided, or any of a number of auditory frequencies or combinations thereof that the subject can select as they wish.
- Table 1 below describes an exemplary treatment regimen for this example.
- the stimulation provided in Table 1 first cycles through a block of four Segment A outputs, then cycles through a block of four Segment B outputs, then cycles through seven blocks of four Segment C outputs, and lastly repeats the block of four Segment A outputs.
- Segment A outputs (A1, A2, A3, and A4)
- the auditory and light outputs cycle 115 or 116 times between being on for 0.1277 seconds and then being off for 0.1277 seconds (that is, at a pulse frequency of 3.9 Hz), followed by no output for 0.5 seconds.
- Segment B outputs (B1, B2, B3 and B4)
- Segment C outputs (C1, C2, C3 and C4)
- Segments A1, B1, and C1 pulse the right and left sides of both the light and auditory together, with all outputs are synchronized to be on or off at the same time, as provided by subroutine 450a.
- Segments A2, B2, and C2 synchronize the left side light and auditory output, and the right side light and auditory output to be opposite to one another, as provided by subroutine 450b.
- Segments A3, B3, and C3 synchronize both lights together to be opposite to both auditory outputs, as provided by subroutine 450c.
- Segments A4, B4, and C4 synchronize the right auditory and light to be opposite to the left auditory and light outputs, as provided by subroutine 450d.
- the stimulation pattern of Example 2 includes the part of the treatment regimen shown in Table 1. Specifically, the stimulation first cycles through a block of four Segment A outputs, then cycles through a block of four Segment B outputs, and then cycles through seven blocks of four Segment C outputs. The repetition of the last block of four Segment A outputs is not provided in Example 2.
- the four subroutines described above and herein are applied and repeated for multiple time segments, each at a predetermined stimulation (repetition) frequency.
- the four subroutines may be repeated, such as with each segment of the four subroutines lasting 120 seconds, for example.
- tactile stimulus may be provided concurrently with the auditory stimulus.
- the light may be provided at a wavelength of 580 nm and the auditory having a frequency of 432 Hz may be provided.
- Table 2 below describes an exemplary treatment regimen for this example.
- the stimulation provided in Table 2 cycles through a block of four Segment A outputs 10 times.
- the auditory and light outputs cycle 115 or 116 times between being on for 0.1277 seconds and then being off for 0.1277 seconds, followed by no output for 0.5 seconds.
- Segments A1 pulses the right and left sides of both the light and auditory together, with all outputs are synchronized to be on or off at the same time, as provided by subroutine 450a.
- Segment A2 synchronizes the left side light and auditory output, and the right side light and auditory output to be opposite to one another, as provided by subroutine 450b.
- Segment A3 synchronizes both lights together to be opposite to both auditory outputs, as provided by subroutine 450c.
- Segment A4 synchronizes the right auditory and light to be opposite to the left auditory and light outputs, as provided by subroutine 450d.
- Light and auditory stimulus at a first frequency may be provided for a first time segment, then at a second higher frequency for a second time segment, then back at the first frequency for a subsequent time segment, and so forth.
- Each time segment may include one or more sub-segments of light and auditory stimulus, each sub-segment comprising one of the subroutines described above, for example.
- the light and auditory stimulus may end after a pre-determined time period, such as 20 minutes.
- tactile stimulus may be provided concurrently with the auditory stimulus.
- the light may be provided at a wavelength of 580 nm and the auditory having a frequency of 432 Hz may be provided.
- Table 3 below describes an exemplary treatment regimen for this example.
- the stimulation provided in Table 3 cycles ten times first through a block of four Segment A outputs, then through a block of four Segment D outputs.
- Segment A outputs A1, A2, A3, and A4
- the auditory and light outputs cycle 115 or 116 times between being on for 0.1277 seconds and then being off for 0.1277 seconds, followed by no output for 0.5 seconds.
- Segment D outputs (D1, D2, D3 and D4)
- the auditory and light outputs cycle 44 or 45 times between being on for 0.0667 seconds and then being off for 0. 0667 seconds, followed by no output for 0.5 seconds.
- Segments A1 and D1 pulse the right and left sides of both the light and auditory together, with all outputs are synchronized to be on or off at the same time, as provided by subroutine 450a.
- Segments A2 and D2 synchronize the left side light and auditory output, and the right side light and auditory output to be opposite to one another, as provided by subroutine 450b .
- Segments A3 and D3 synchronize both lights together to be opposite to both auditory outputs, as provided by subroutine 450c.
- Segments A4 and D4 synchronize the right auditory and light to be opposite to the left auditory and light outputs, as provided by subroutine 450d .
- Table 4 lists experimental results for the use of the inventive methods.
- the table lists what was being tested or treated, details of the conditions, the number of subjects, and the results of the tests.
- the stimulation in Example 1 for treating non-sleep related problems and for inducing a short sleep was used for all other treatments.
- Performance Enhancement Marksmanship (rifles and pistols), endurance and speed driving (advanced surveillance, coordination and evasion). 6 hours training each subj ect. 20 Significant improvements in marksmanship in all participants and ease of concentration during speed driving, faster times on endurance trials for 19/20 subjects Performance Enhancement Fine motor skills on bomb disposal personnel 3 hours training with device 3 Improved performance of fine motor skills on bomb disposal VR simulation for all subjects Performance Enhancement Fine motor skills of surgeons- 3 hours training each 3 Improved performance of fine motor skills on surgical procedures VR simulation for all subjects. Performance Enhancement Pistol use and marksmanship.
- Performance Enhancement Soccer player kicking performance 5 days of 1 hour each day 1 Subject went from 5th ranked to highest ranked Stroke Recovery Use on 6 year post stroke subjects. four hours training. 10 Observable balance improvement in 7/10 subjects. 3 subjects had had dramatic improvements in their sleep.
Landscapes
- Health & Medical Sciences (AREA)
- Anesthesiology (AREA)
- Physics & Mathematics (AREA)
- Life Sciences & Earth Sciences (AREA)
- General Health & Medical Sciences (AREA)
- Psychology (AREA)
- Engineering & Computer Science (AREA)
- Biomedical Technology (AREA)
- Heart & Thoracic Surgery (AREA)
- Hematology (AREA)
- Pain & Pain Management (AREA)
- Animal Behavior & Ethology (AREA)
- Acoustics & Sound (AREA)
- Public Health (AREA)
- Veterinary Medicine (AREA)
- General Physics & Mathematics (AREA)
- Optics & Photonics (AREA)
- Radiation-Therapy Devices (AREA)
- Electrotherapy Devices (AREA)
- User Interface Of Digital Computer (AREA)
Claims (14)
- Un appareil (100, 100') pour fournir une stimulation à un utilisateur, l'appareil comprenant:un cadre configuré pour être porté sur la tête de l'utilisateur;une source lumineuse gauche (110L) configurée pour générer un modèle de stimulus visuel gauche;une source lumineuse droite (110R) configurée pour générer un modèle de stimulus visuel droit;une source auditive gauche (120L) configurée pour générer un modèle de stimulus auditif gauche;une source auditive droite (120R) configurée pour générer un modèle de stimulus auditif droit; etun contrôleur (130) couplé à la source lumineuse gauche (110L), à la source lumineuse droite (110R), à la source auditive gauche (120L) et à la source auditive droite (120R),dans lequel le contrôleur (130) est configuré pour contrôler les applications du modèle de stimulus visuel gauche, du modèle de stimulus visuel droit, du modèle de stimulus auditif gauche et du modèle de stimulus auditif droit indépendamment l'un de l'autre mais coordonnés l'un avec l'autre et le contrôleur (130) est configuré pour contrôler la production d'une pluralité de signaux lumineux alternant à gauche et à droite dans le modèle de stimulus visuel gauche et dans le modèle de stimulus visuel droit,dans lequel ledit appareil est configuré pourfournir alternativement le stimulus lumineux du côté gauche et le stimulus auditif du côté droit et fournir le stimulus lumineux du côté droit et le stimulus auditif du côté gauche.
- L'appareil (100, 100') de la revendication 1, dans lequel le contrôleur (130) est en outre configuré pour contrôler la production d'une pluralité de signaux auditifs alternatifs gauche et droit dans le modèle de stimulus auditif gauche et le modèle de stimulus auditif droit.
- L'appareil (100, 100') de l'une des revendications 1 et 2, dans lequel un ou plusieurs des motifs de stimulus visuel gauche ou droit ont une longueur d'onde lumineuse comprise entre 550 nm et 610 nm et/ou dans lequel un ou plusieurs des motifs de stimulus auditif gauche ou droit comprennent une fréquence auditive comprise entre 240 Hz et 480 Hz.
- L'appareil (100, 100') de l'une des revendications précédentes, dans lequel un ou plusieurs des motifs de stimulus visuel gauche comprennent la pulsation répétée d'une lumière à une ou plusieurs des fréquences suivantes : une première fréquence, une deuxième fréquence inférieure à la première fréquence ou une troisième fréquence inférieure à la première et à la deuxième fréquence, en particulier lorsque la première fréquence est comprise entre 3,75 Hz et 4,25 Hz, la deuxième fréquence est comprise entre 1,25 Hz et 1,75 Hz et la troisième fréquence est comprise entre 0,25 Hz et 0,75 Hz.
- L'appareil (100, 100') de la revendication 4, dans lequel l'impulsion répétée de la lumière comprend l'impulsion de la lumière pendant un intervalle de temps prédéterminé, en particulier pendant 25 à 35 secondes.
- L'appareil (100, 100') de l'une des revendications précédentes, dans lequel un ou plusieurs des motifs de stimulus auditifs gauche ou droit comprennent une séquence de motifs de stimulus ayant chacun une fréquence d'impulsion ayant une période d'impulsion, lesdits signaux temporels répétitifs comprenant une partie de la période d'impulsion avec une fréquence auditive de 240 Hz à 480 Hz et une partie de la période d'impulsion.
- L'appareil (100, 100') de la revendication 6, dans lequel cette partie de la période d'impulsion est la moitié de la période d'impulsion.
- L'appareil (100, 100') de l'une des revendications 6 et 7, dans lequel ladite séquence de motifs de stimulation comprend un premier motif de stimulation ayant une première fréquence d'impulsion, un deuxième motif de stimulation ayant une deuxième fréquence d'impulsion inférieure à la première fréquence d'impulsion, et un troisième motif de stimulation ayant une troisième fréquence d'impulsion inférieure à la deuxième fréquence d'impulsion, dans lequel en particulier la première fréquence d'impulsion est comprise entre 3,75 Hz et 4,25 Hz, la deuxième fréquence d'impulsion est comprise entre 1,25 Hz et 1,75 Hz, et la troisième fréquence d'impulsion est comprise entre 0,25 Hz et 0,75 Hz.
- L'appareil (100, 100') de l'une des revendications précédentes, dans lequel la source auditive gauche (120L) est en outre configurée pour générer un modèle de stimulus tactile gauche, et dans lequel la source auditive droite (120R) est en outre configurée pour générer un modèle de stimulus tactile droit.
- L'appareil (100, 100') de l'une des revendications précédentes, dans lequel le contrôleur (130) est configuré pour être en communication et commandé par une unité de commande externe, en particulier dans le cadre d'une communication sans fil.
- L'appareil (100, 100') de l'une des revendications précédentes configuré pour:fournir simultanément un stimulus lumineux du côté gauche à l'œil gauche de l'utilisateur, un stimulus lumineux du côté droit à l'œil droit de l'utilisateur, un stimulus auditif du côté gauche à l'œil gauche de l'utilisateur et un stimulus auditif du côté droit à l'œil droit de l'utilisateur pendant un premier intervalle de temps;alterner la fourniture du stimulus lumineux du côté gauche et du stimulus auditif du côté gauche avec la fourniture du stimulus lumineux du côté droit et du stimulus auditif du côté droit pendant un deuxième intervalle de temps; etalterner la fourniture de stimuli lumineux du côté gauche et du côté droit avec la fourniture de stimuli auditifs du côté gauche et du côté droit pendant un troisième intervalle de temps; etalterner le stimulus lumineux du côté gauche et le stimulus auditif du côté droit avec le stimulus lumineux du côté droit et le stimulus auditif du côté gauche pendant un quatrième intervalle de temps.
- L'appareil (100, 100') de la revendication 1 pour le traitement de la douleur, le traitement d'une maladie ou d'un état neurologique, ou pour l'amélioration des performances.
- L'appareil (100, 100') de la revendication 12, où ladite maladie ou condition neurologique comprend l'insomnie, le syndrome de stress post-traumatique (SSPT) et/ou une lésion cérébrale.
- L'appareil (100, 100') de la revendication 12, dans lequel l'amélioration des performances augmente l'activité des ondes alpha dans le cerveau, améliore le sommeil, les capacités mentales ou les capacités physiques.
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562258965P | 2015-11-23 | 2015-11-23 | |
| EP16869299.4A EP3380000A4 (fr) | 2015-11-23 | 2016-11-23 | Procédés et systèmes destinés à fournir des stimuli au cerveau |
| PCT/US2016/063651 WO2017091758A1 (fr) | 2015-11-23 | 2016-11-23 | Procédés et systèmes destinés à fournir des stimuli au cerveau |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16869299.4A Division EP3380000A4 (fr) | 2015-11-23 | 2016-11-23 | Procédés et systèmes destinés à fournir des stimuli au cerveau |
Publications (3)
| Publication Number | Publication Date |
|---|---|
| EP3653111A1 EP3653111A1 (fr) | 2020-05-20 |
| EP3653111C0 EP3653111C0 (fr) | 2024-01-03 |
| EP3653111B1 true EP3653111B1 (fr) | 2024-01-03 |
Family
ID=58719498
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16869299.4A Withdrawn EP3380000A4 (fr) | 2015-11-23 | 2016-11-23 | Procédés et systèmes destinés à fournir des stimuli au cerveau |
| EP19216447.3A Active EP3653111B1 (fr) | 2015-11-23 | 2016-11-23 | Dipositif destinés à fournir des stimuli au cerveau |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| EP16869299.4A Withdrawn EP3380000A4 (fr) | 2015-11-23 | 2016-11-23 | Procédés et systèmes destinés à fournir des stimuli au cerveau |
Country Status (11)
| Country | Link |
|---|---|
| US (5) | US10328236B2 (fr) |
| EP (2) | EP3380000A4 (fr) |
| JP (1) | JP7269010B2 (fr) |
| KR (1) | KR102349466B1 (fr) |
| CN (1) | CN109152524B (fr) |
| AU (2) | AU2016359154B2 (fr) |
| BR (1) | BR112018010286A2 (fr) |
| CA (1) | CA3029198A1 (fr) |
| EA (1) | EA035285B1 (fr) |
| PH (1) | PH12018501330A1 (fr) |
| WO (1) | WO2017091758A1 (fr) |
Families Citing this family (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US11400252B2 (en) | 2015-11-23 | 2022-08-02 | Sana Heath Inc. | Non-pharmaceutical method of managing pain |
| US11298502B2 (en) | 2015-11-23 | 2022-04-12 | Sana Health, Inc. | Non-pharmaceutical methods of mitigating addiction withdrawal symptoms |
| AU2016359154B2 (en) | 2015-11-23 | 2020-08-06 | Sana Health, Inc. | Methods and systems for providing stimuli to the brain |
| US11524135B2 (en) | 2015-11-23 | 2022-12-13 | Sana Health Inc. | Non-pharmaceutical systems and methods of treating the symptoms of fibromyalgia |
| EP3589193A4 (fr) | 2017-03-02 | 2020-12-30 | Sana Health, Inc. | Procédés et systèmes destinés à moduler des stimuli au cerveau avec des biocapteurs |
| CN111565698A (zh) * | 2017-10-10 | 2020-08-21 | 麻省理工学院 | 用于预防、减轻和/或治疗痴呆的系统和方法 |
| US20190262223A1 (en) * | 2018-02-28 | 2019-08-29 | Bipri, Llc | Therapeutic vibration device and method of use thereof |
| WO2019226656A1 (fr) * | 2018-05-23 | 2019-11-28 | Sana Health, Inc. | Procédé non pharmaceutique de gestion de la douleur |
| CN113164118A (zh) * | 2018-07-20 | 2021-07-23 | S·琼斯 | 双侧刺激装置 |
| US11969556B2 (en) * | 2018-12-21 | 2024-04-30 | Cochlear Limited | Therapeutic sound through bone conduction |
| MX2021010040A (es) * | 2019-02-21 | 2022-01-04 | Sana Health Inc | Metodos no farmaceuticos para mitigar los síntomas de abstinencia de la adicción. |
| KR102299217B1 (ko) * | 2019-03-14 | 2021-09-09 | (주)하스피케어 | 클라우드 기반 암 재활환자 통합 케어 서비스 제공 방법 |
| CN113796096A (zh) * | 2019-05-01 | 2021-12-14 | 唯听助听器公司 | 耳级听觉系统 |
| US11586291B2 (en) * | 2019-05-12 | 2023-02-21 | NeuroHaptics, Inc. | Motion sickness reduction device |
| US11275441B2 (en) * | 2019-05-12 | 2022-03-15 | Neurohaptics, Inc | Motion sickness reduction, directional indication, and neural rehabilitation device |
| WO2021007444A1 (fr) * | 2019-07-10 | 2021-01-14 | Sana Health, Inc. | Systèmes non pharmaceutiques et procédés de traitement des symptômes de la fibromyalgie |
| WO2021236421A1 (fr) * | 2020-05-18 | 2021-11-25 | Sana Health, Inc. | Méthodes et systèmes de traitement de l'anxiété et de la dépression |
| CN114159275A (zh) * | 2020-09-10 | 2022-03-11 | 上海神奕医疗科技有限公司 | 康复训练设备 |
| MY194782A (en) * | 2021-01-22 | 2022-12-15 | Togl Tech Sdn Bhd | Device and method for processing, storing and transmitting a signal in a media file |
| EP4319870A1 (fr) * | 2021-04-07 | 2024-02-14 | Into Technologies Inc. | Systèmes et procédés de traitement d'indications médicales par l'administration de doses de lumière et de son |
| GB2605832A (en) * | 2021-04-15 | 2022-10-19 | Ed Can Help Ltd | Methods and systems for generating sound for use in treating a mental disorder |
| WO2024159210A1 (fr) * | 2023-01-27 | 2024-08-02 | Sana Health, Inc. | Méthodes et systèmes destinés à fournir une stimulation sensorielle à un cerveau afin de traiter un trouble de stress post-traumatique |
Family Cites Families (76)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4172406A (en) * | 1978-10-16 | 1979-10-30 | Martinez Rosa E | Audio-visual headphones |
| US4315502A (en) * | 1979-10-11 | 1982-02-16 | Gorges Denis E | Learning-relaxation device |
| US4892106A (en) * | 1987-10-19 | 1990-01-09 | Gleeson Iii William J | Multiple afferent sensory stimulation device |
| JPH01175867A (ja) | 1987-12-29 | 1989-07-12 | Wako Corp Kk | 耳に装着して治療する装置 |
| US5507716A (en) | 1991-08-21 | 1996-04-16 | The Lucidity Institute, Inc. | Equipment and methods used to induce lucid dreams in sleeping persons |
| US5343261A (en) | 1992-01-09 | 1994-08-30 | Wilson David L | Device for inducing saccadic eye movement |
| JPH07194706A (ja) * | 1993-12-28 | 1995-08-01 | Sateraito Intelligence:Kk | 光信号と音信号とを使用したリラクセーション方法及びリラクセーション機器 |
| US6123661A (en) | 1996-05-28 | 2000-09-26 | Matsushita Electric Works, Ltd. | Relax refresh system |
| US5783909A (en) | 1997-01-10 | 1998-07-21 | Relume Corporation | Maintaining LED luminous intensity |
| US20020198577A1 (en) | 1998-11-30 | 2002-12-26 | Jaillet Peter D. | Apparatus and method for changing critical brain activity using light and sound |
| US6409655B1 (en) * | 1999-03-05 | 2002-06-25 | David L. Wilson | Device for applying stimuli to a subject |
| WO2001064005A2 (fr) * | 2000-02-08 | 2001-09-07 | Bernd Michael Pohlmann | Procede et dispositif pour influer sur le traitement de l'information sensomoteur d'un utilisateur |
| JP2003117001A (ja) * | 2001-10-17 | 2003-04-22 | Kenji Kokama | リラクセーション誘導装置 |
| US20100161010A1 (en) | 2003-12-11 | 2010-06-24 | Thomas David A | Perceptible Apparatus and Methods for Reactive Effect |
| CN2696657Y (zh) * | 2004-04-12 | 2005-05-04 | 王宏 | 多功能脑保健仪 |
| US7645226B2 (en) | 2004-11-12 | 2010-01-12 | Biogenics Ii L.L.C. | Relaxation device and method |
| US20060252978A1 (en) * | 2005-05-09 | 2006-11-09 | Vesely Michael A | Biofeedback eyewear system |
| WO2008131454A1 (fr) * | 2007-04-24 | 2008-10-30 | Altman Mitchell A | Lunettes guide |
| US20080269629A1 (en) * | 2007-04-25 | 2008-10-30 | Robert Howard Reiner | Multimodal therapeutic and feedback system |
| KR20100039287A (ko) * | 2007-05-25 | 2010-04-15 | 토마스 제퍼슨 유니버시티 | 집중력 및/또는 기억력의 향상이 필요한 개체에 집중력 및/또는 기억력을 향상시키는 치료방법 |
| US20090156886A1 (en) | 2007-12-12 | 2009-06-18 | Synapse Research Company | Method and apparatus for providing automatic eye focused therapy |
| US8636640B2 (en) | 2008-04-11 | 2014-01-28 | Brain Symphony LLC | Method and system for brain entertainment |
| US9649469B2 (en) | 2008-04-24 | 2017-05-16 | The Invention Science Fund I Llc | Methods and systems for presenting a combination treatment |
| KR100902610B1 (ko) * | 2008-05-19 | 2009-06-11 | 이인태 | 정신치료장치 |
| US20190030279A1 (en) | 2008-09-22 | 2019-01-31 | Dawn Nowlin | Method of Conditioning a Living Body to Respond Appropriately to Stimuli |
| US8784293B2 (en) | 2008-10-07 | 2014-07-22 | Advanced Brain Monitoring, Inc. | Systems and methods for optimization of sleep and post-sleep performance |
| CN102271757B (zh) * | 2008-12-30 | 2015-11-25 | 皇家飞利浦电子股份有限公司 | 用于施予光疗的系统和方法 |
| WO2010122446A1 (fr) * | 2009-04-16 | 2010-10-28 | Koninklijke Philips Electronics N.V. | Dispositif d'éclairage et procédé de réduction de l'inertie du sommeil ou de régulation de la vigilance |
| US10112029B2 (en) | 2009-06-19 | 2018-10-30 | Integrated Listening Systems, LLC | Bone conduction apparatus and multi-sensory brain integration method |
| US20130090520A1 (en) | 2009-06-19 | 2013-04-11 | Randall Redfield | Bone Conduction Apparatus and Multi-sensory Brain Integration Method |
| US8879745B2 (en) * | 2009-07-23 | 2014-11-04 | Dean Robert Gary Anderson As Trustee Of The D/L Anderson Family Trust | Method of deriving individualized gain compensation curves for hearing aid fitting |
| US8838247B2 (en) | 2010-01-06 | 2014-09-16 | Evoke Neuroscience, Inc. | Transcranial stimulation device and method based on electrophysiological testing |
| US20110213664A1 (en) * | 2010-02-28 | 2011-09-01 | Osterhout Group, Inc. | Local advertising content on an interactive head-mounted eyepiece |
| US8977362B2 (en) | 2010-04-27 | 2015-03-10 | Rhode Island Hospital | Peripheral pain management |
| US9333372B2 (en) * | 2011-02-20 | 2016-05-10 | James Otis | Methods and apparatus for intermittent stimuli |
| BR112013022115A2 (pt) | 2011-03-02 | 2016-12-06 | Koninkl Philips Nv | dispositivo para melhora cognitiva de um usuário, método para melhora cognitiva de um usuário e sistema para uso por um usuário |
| KR20120108488A (ko) * | 2011-03-24 | 2012-10-05 | 유남구 | 수면 보조 장치 및 방법 |
| US8725264B2 (en) | 2011-08-04 | 2014-05-13 | Fundacio Privada Institut de Neurorehabilitacio Guttmann | Method for treating neuropathic pain |
| US8852073B2 (en) | 2012-01-17 | 2014-10-07 | Headwater R&D Inc | Relaxation inducing sleep mask |
| US10342986B2 (en) | 2012-04-06 | 2019-07-09 | Kosivana Holdings Limited | Frequency specific sensory stimulation |
| EP2923501B1 (fr) * | 2012-11-26 | 2020-03-04 | Unyte Health US Inc. | Appareil de conduction osseuse et méthode d'intégration cérébrale multisensorielle |
| US9521976B2 (en) | 2013-01-24 | 2016-12-20 | Devon Greco | Method and apparatus for encouraging physiological change through physiological control of wearable auditory and visual interruption device |
| US9872968B2 (en) * | 2013-04-17 | 2018-01-23 | Sri International | Biofeedback virtual reality sleep assistant |
| WO2015028479A1 (fr) | 2013-08-28 | 2015-03-05 | Koninklijke Philips N.V. | Système stimulant avec stimulation vestibulaire-tactile et auditive |
| US9547175B2 (en) * | 2014-03-18 | 2017-01-17 | Google Inc. | Adaptive piezoelectric array for bone conduction receiver in wearable computers |
| ES2773897T3 (es) | 2014-10-03 | 2020-07-15 | British Columbia Cancer Agency Branch | Aparato para mejorar los resultados de salud de infantes prematuros |
| CN204484476U (zh) * | 2015-01-31 | 2015-07-22 | 东莞市速普得电子科技股份有限公司 | 一种led保健眼罩 |
| US9999835B2 (en) | 2015-02-05 | 2018-06-19 | Sony Interactive Entertainment Inc. | Motion sickness monitoring and application of supplemental sound to counteract sickness |
| CN104546285B (zh) | 2015-02-05 | 2017-03-08 | 京东方科技集团股份有限公司 | 眼罩、睡眠监测装置及方法 |
| USD775260S1 (en) | 2015-02-16 | 2016-12-27 | Cynthia Callendar Gordon | Sleep mask with compartment for wireless speaker system |
| WO2016140408A1 (fr) | 2015-03-05 | 2016-09-09 | 주식회사 프라센 | Dispositif induisant le sommeil et système de gestion du sommeil comprenant le dispositif |
| KR101687321B1 (ko) | 2015-03-05 | 2016-12-16 | 주식회사 프라센 | 수면 유도 장치 및 이를 포함하는 수면 관리 시스템 |
| AU2016359154B2 (en) | 2015-11-23 | 2020-08-06 | Sana Health, Inc. | Methods and systems for providing stimuli to the brain |
| US20220249803A1 (en) | 2015-11-23 | 2022-08-11 | Sana Health, Inc. | Systems and methods for visual cortex targeting and treatment |
| US20220409849A1 (en) | 2015-11-23 | 2022-12-29 | Sana Health, Inc. | Systems and methods for electronic patient stimulation and diagnosis |
| US20220265959A1 (en) | 2015-11-23 | 2022-08-25 | Sana Health, Inc. | Systems and methods for auditory, visual and/or auditory and visual cortex targeting and treatment |
| US10286181B2 (en) | 2015-12-31 | 2019-05-14 | Cigna Intellectual Property, Inc. | Method and apparatus for virtual reality-based mindfulness therapy |
| WO2017149393A1 (fr) | 2016-03-03 | 2017-09-08 | Headwaters, Inc. | Masque oculaire programmé par tonalité audio pour le sommeil et le réveil hygiéniques et thérapeutiques, et ayant plusieurs modes de lever du soleil et de coucher du soleil commandés à distance |
| US10383769B1 (en) | 2016-03-30 | 2019-08-20 | Juanita Miller | Eye cover with audio transmitter |
| US10582908B2 (en) | 2016-04-01 | 2020-03-10 | Intel Corporation | Sleep management device and methods for operation |
| CN205814527U (zh) | 2016-04-07 | 2016-12-21 | 京东方科技集团股份有限公司 | 智能眼罩 |
| CN105640703A (zh) | 2016-04-07 | 2016-06-08 | 京东方科技集团股份有限公司 | 智能眼罩 |
| USD827701S1 (en) | 2016-08-26 | 2018-09-04 | Castar, Inc. | Augmented reality glasses |
| US11850420B2 (en) | 2016-09-19 | 2023-12-26 | Nyx Technologies Ltd. | Multifunctional closed loop neuro feedback stimulating device and methods thereof |
| USD805515S1 (en) | 2016-12-15 | 2017-12-19 | Intel Corporation | Headwear |
| EP3589193A4 (fr) | 2017-03-02 | 2020-12-30 | Sana Health, Inc. | Procédés et systèmes destinés à moduler des stimuli au cerveau avec des biocapteurs |
| WO2019060598A1 (fr) | 2017-09-22 | 2019-03-28 | Theranova, Llc | Procédés et dispositifs de traitement et de gestion de l'addiction |
| WO2019226656A1 (fr) | 2018-05-23 | 2019-11-28 | Sana Health, Inc. | Procédé non pharmaceutique de gestion de la douleur |
| US20190388020A1 (en) | 2018-06-20 | 2019-12-26 | NeuroPlus Inc. | System and Method for Treating and Preventing Cognitive Disorders |
| MX2021010040A (es) | 2019-02-21 | 2022-01-04 | Sana Health Inc | Metodos no farmaceuticos para mitigar los síntomas de abstinencia de la adicción. |
| WO2020219350A1 (fr) | 2019-04-25 | 2020-10-29 | Bose Corporation | Masque de relaxation intelligent |
| US11786694B2 (en) | 2019-05-24 | 2023-10-17 | NeuroLight, Inc. | Device, method, and app for facilitating sleep |
| WO2021007444A1 (fr) | 2019-07-10 | 2021-01-14 | Sana Health, Inc. | Systèmes non pharmaceutiques et procédés de traitement des symptômes de la fibromyalgie |
| US11633565B2 (en) | 2019-07-12 | 2023-04-25 | Bose Corporation | Light diffusers for smart relaxation masks |
| JP7194706B2 (ja) | 2020-03-16 | 2022-12-22 | アンリツ株式会社 | 信号発生装置及び信号発生方法 |
| WO2021236421A1 (fr) | 2020-05-18 | 2021-11-25 | Sana Health, Inc. | Méthodes et systèmes de traitement de l'anxiété et de la dépression |
-
2016
- 2016-11-23 AU AU2016359154A patent/AU2016359154B2/en active Active
- 2016-11-23 WO PCT/US2016/063651 patent/WO2017091758A1/fr active Application Filing
- 2016-11-23 EP EP16869299.4A patent/EP3380000A4/fr not_active Withdrawn
- 2016-11-23 BR BR112018010286-5A patent/BR112018010286A2/pt not_active Application Discontinuation
- 2016-11-23 CN CN201680079663.4A patent/CN109152524B/zh active Active
- 2016-11-23 KR KR1020187017718A patent/KR102349466B1/ko active IP Right Grant
- 2016-11-23 CA CA3029198A patent/CA3029198A1/fr active Pending
- 2016-11-23 EA EA201891224A patent/EA035285B1/ru unknown
- 2016-11-23 US US15/360,808 patent/US10328236B2/en active Active
- 2016-11-23 EP EP19216447.3A patent/EP3653111B1/fr active Active
- 2016-11-23 JP JP2018546406A patent/JP7269010B2/ja active Active
-
2018
- 2018-06-21 PH PH12018501330A patent/PH12018501330A1/en unknown
-
2019
- 2019-05-24 US US16/422,592 patent/US11141559B2/en active Active
-
2020
- 2020-08-27 US US17/005,047 patent/US11679231B2/en active Active
- 2020-08-27 US US17/005,042 patent/US11701487B2/en active Active
- 2020-11-05 AU AU2020264336A patent/AU2020264336B2/en active Active
-
2023
- 2023-01-27 US US18/102,564 patent/US20230173222A1/en active Pending
Also Published As
| Publication number | Publication date |
|---|---|
| AU2016359154A1 (en) | 2018-07-12 |
| EP3653111A1 (fr) | 2020-05-20 |
| US20170143935A1 (en) | 2017-05-25 |
| KR20180103857A (ko) | 2018-09-19 |
| AU2020264336A1 (en) | 2020-11-26 |
| US20190321584A1 (en) | 2019-10-24 |
| US11679231B2 (en) | 2023-06-20 |
| AU2016359154A2 (en) | 2020-02-27 |
| US10328236B2 (en) | 2019-06-25 |
| EA201891224A1 (ru) | 2019-02-28 |
| PH12018501330A1 (en) | 2019-02-11 |
| CN109152524B (zh) | 2021-06-15 |
| JP2019500182A (ja) | 2019-01-10 |
| EP3653111C0 (fr) | 2024-01-03 |
| EP3380000A4 (fr) | 2019-06-19 |
| EA035285B1 (ru) | 2020-05-25 |
| US11701487B2 (en) | 2023-07-18 |
| US20230173222A1 (en) | 2023-06-08 |
| KR102349466B1 (ko) | 2022-01-10 |
| AU2016359154B2 (en) | 2020-08-06 |
| WO2017091758A1 (fr) | 2017-06-01 |
| US20200391000A1 (en) | 2020-12-17 |
| AU2020264336B2 (en) | 2022-12-01 |
| US11141559B2 (en) | 2021-10-12 |
| BR112018010286A2 (pt) | 2018-11-27 |
| CA3029198A1 (fr) | 2017-06-01 |
| EP3380000A1 (fr) | 2018-10-03 |
| NZ743587A (en) | 2021-03-26 |
| CN109152524A (zh) | 2019-01-04 |
| JP7269010B2 (ja) | 2023-05-08 |
| US20200390999A1 (en) | 2020-12-17 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP3653111B1 (fr) | Dipositif destinés à fournir des stimuli au cerveau | |
| EP3551145B1 (fr) | Systèmes et méthodes destinés à traiter des troubles neurologiques | |
| US10799667B2 (en) | Methods and systems for modulating stimuli to the brain with biosensors | |
| ES2899170T3 (es) | Mejora cognitiva mediante retroalimentación | |
| JP2013523357A (ja) | 条件付けを用いて脱同期化をもたらす非侵襲性刺激の装置および方法 | |
| US20220409849A1 (en) | Systems and methods for electronic patient stimulation and diagnosis | |
| US20220265959A1 (en) | Systems and methods for auditory, visual and/or auditory and visual cortex targeting and treatment | |
| US20220249803A1 (en) | Systems and methods for visual cortex targeting and treatment | |
| US11536965B2 (en) | Technologies for multi-randomized audio-visual entrainment | |
| US20230347103A1 (en) | Methods and systems for providing sensory stimulation to a brain to treat post-traumatic stress disorder | |
| NZ743587B2 (en) | Methods and systems for providing stimuli to the brain | |
| WO2024159210A1 (fr) | Méthodes et systèmes destinés à fournir une stimulation sensorielle à un cerveau afin de traiter un trouble de stress post-traumatique | |
| EA039956B1 (ru) | Способ и система для управления стимуляцией головного мозга при помощи биодатчиков |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| PUAI | Public reference made under article 153(3) epc to a published international application that has entered the european phase |
Free format text: ORIGINAL CODE: 0009012 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE APPLICATION HAS BEEN PUBLISHED |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 3380000 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: A1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| RAP1 | Party data changed (applicant data changed or rights of an application transferred) |
Owner name: SANA HEALTH, INC. |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| 17P | Request for examination filed |
Effective date: 20201120 |
|
| RBV | Designated contracting states (corrected) |
Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTG | Intention to grant announced |
Effective date: 20230216 |
|
| GRAJ | Information related to disapproval of communication of intention to grant by the applicant or resumption of examination proceedings by the epo deleted |
Free format text: ORIGINAL CODE: EPIDOSDIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: REQUEST FOR EXAMINATION WAS MADE |
|
| GRAP | Despatch of communication of intention to grant a patent |
Free format text: ORIGINAL CODE: EPIDOSNIGR1 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: GRANT OF PATENT IS INTENDED |
|
| INTC | Intention to grant announced (deleted) | ||
| INTG | Intention to grant announced |
Effective date: 20230720 |
|
| GRAS | Grant fee paid |
Free format text: ORIGINAL CODE: EPIDOSNIGR3 |
|
| GRAA | (expected) grant |
Free format text: ORIGINAL CODE: 0009210 |
|
| STAA | Information on the status of an ep patent application or granted ep patent |
Free format text: STATUS: THE PATENT HAS BEEN GRANTED |
|
| AC | Divisional application: reference to earlier application |
Ref document number: 3380000 Country of ref document: EP Kind code of ref document: P |
|
| AK | Designated contracting states |
Kind code of ref document: B1 Designated state(s): AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO RS SE SI SK SM TR |
|
| REG | Reference to a national code |
Ref country code: GB Ref legal event code: FG4D |
|
| REG | Reference to a national code |
Ref country code: CH Ref legal event code: EP |
|
| REG | Reference to a national code |
Ref country code: DE Ref legal event code: R096 Ref document number: 602016085216 Country of ref document: DE |
|
| REG | Reference to a national code |
Ref country code: IE Ref legal event code: FG4D |
|
| U01 | Request for unitary effect filed |
Effective date: 20240119 |
|
| U07 | Unitary effect registered |
Designated state(s): AT BE BG DE DK EE FI FR IT LT LU LV MT NL PT SE SI Effective date: 20240207 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: ES Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240503 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240404 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240403 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: RS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240403 Ref country code: NO Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240403 Ref country code: IS Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240503 Ref country code: HR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 Ref country code: GR Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240404 Ref country code: CZ Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: PL Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SM Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |
|
| PG25 | Lapsed in a contracting state [announced via postgrant information from national office to epo] |
Ref country code: SK Free format text: LAPSE BECAUSE OF FAILURE TO SUBMIT A TRANSLATION OF THE DESCRIPTION OR TO PAY THE FEE WITHIN THE PRESCRIBED TIME-LIMIT Effective date: 20240103 |